Alcon’s Latest Equipment Breakthrough Technologies, Unity VCS and Unity CS, Receive U.S. FDA 510(k) Clearance
June 24 2024 - 1:00AM
Business Wire
- Combined vitreoretinal-cataract system (VCS) and standalone
cataract system (CS) are cleared for use in the U.S.
- New, proprietary technologies designed to deliver
transformative surgical innovation
- Alcon to immediately begin collecting real-world user
experience in the U.S. prior to broad commercialization in
2025
- First innovations to be introduced from Alcon’s cutting-edge
Unity portfolio of surgical equipment
Ad Hoc Announcement Pursuant to Art. 53 LR
Alcon (SIX/NYSE: ALC), the global leader in eye care dedicated
to helping people see brilliantly, today announced that UNITY®
Vitreoretinal Cataract System (VCS) and UNITY® Cataract System (CS)
have received U.S. Food and Drug Administration (FDA) 510(k)
clearance. These innovations are the first to be introduced from
Alcon’s highly anticipated Unity portfolio.
“At Alcon, we have a long legacy of involving our customers
throughout the research and development process to deliver bold
innovation in ophthalmology, and we would like to thank those who
helped us arrive at today’s milestone,” said Franck Leveiller, Head
of Global R&D and Chief Scientific Officer, Alcon. “We are
excited to introduce the next generation of equipment solutions and
consumables—in cataract and vitreoretinal surgery—and deliver
meaningful impact for Eye Care Professionals and patients.”
Unity VCS and Unity CS introduce significant workflow
efficiencies over Alcon’s current market-leading systems,
CONSTELLATION® Vision System for vitreoretinal procedures and
CENTURION® Vision System with ACTIVE SENTRY® for cataract
surgery.
“I have been closely involved in the development of Unity VCS
and Unity CS; this truly innovative system is a significant upgrade
of Alcon’s best-in-class technologies,” said Steve Charles, MD,
FACS, FICS, FASRS. “It is a proud moment to be able to celebrate
this clearance.”
Worldwide, there will be an estimated 31 million cataract
surgeries in 2024, and that number is expected to increase to 37
million by 2029.1 There will be approximately 2.2 million
vitrectomy procedures in 2024 across the globe.2 Alcon is the
global market leader for cataract and retina procedural packs
(consumables used in each surgery).2,3
Today, there are more than 28,000 Centurion and Constellation
devices in the market that will be targeted for upgrade to the
Unity platform over the next decade. In addition to the system,
Unity VCS and Unity CS bring first-to-market technologies and
consumables that are designed to drive significant benefits for the
surgeon, staff and patients.
Alcon has tested Unity VCS and Unity CS during investigational
advisory wet lab sessions with more than 200 highly experienced
surgeons from 30+ countries. Now with 510(k) clearance, Alcon will
begin a thorough program to secure real-world feedback before
commercial launch in 2025. Regulatory submissions will continue
later this year in markets across the globe. CE Mark is expected in
early 2025.
Unity VCS and Unity CS are the latest innovations from the Alcon
Vision Suite—a portfolio of innovative products designed to help
Eye Care Professionals increase clinic and OR efficiency, and
deliver exceptional patient experiences. The Alcon Vision Suite
will continue to grow with cutting-edge Unity products expected to
be introduced over the coming years, adding to our market-leading
legacy products which will continue to be available and
serviceable. Unity VCS and Unity CS will be supported by Alcon’s
training, product maintenance and Services teams.
Cautionary Note Regarding
Forward-Looking Statements
This press release contains “forward-looking statements” within
the meaning of the safe harbor provisions of the United States
Private Securities Litigation Reform Act of 1995. Forward-looking
statements can be identified by words such as: “anticipate,”
“intend,” “commitment,” “look forward,” “maintain,” “plan,” “goal,”
“seek,” “target,” “assume,” “believe,” “project,” “estimate,”
“expect,” “strategy,” “future,” “likely,” “may,” “should,” “will”
and similar references to future periods.
Forward-looking statements are neither historical facts nor
assurances of future performance. Instead, they are based only on
our current beliefs, expectations and assumptions regarding the
future of our business, future plans and strategies, and other
future conditions. Because forward-looking statements relate to the
future, they are subject to inherent uncertainties and risks that
are difficult to predict. Some of these factors are discussed in
our filings with the United States Securities and Exchange
Commission, including our Form 20-F. Should one or more of these
uncertainties or risks materialize, or should underlying
assumptions prove incorrect, actual results may vary materially
from those anticipated. Therefore, you should not rely on any of
these forward-looking statements.
Forward-looking statements in this press release speak only as
of the date they are made, and we assume no obligation to update
forward-looking statements as a result of new information, future
events or otherwise.
About Alcon
Alcon helps people see brilliantly. As the global leader in eye
care with a heritage spanning over 75 years, we offer the broadest
portfolio of products to enhance sight and improve people’s lives.
Our Surgical and Vision Care products touch the lives of more than
260 million people in over 140 countries each year living with
conditions like cataracts, glaucoma, retinal diseases and
refractive errors. Our more than 25,000 associates are enhancing
the quality of life through innovative products, partnerships with
Eye Care Professionals and programs that advance access to quality
eye care. Learn more at www.alcon.com.
About UNITY VCS and UNITY
CS
Indications / Intended Use: The UNITY® VCS
(Vitreoretinal Cataract System) console, when used with compatible
devices, is indicated for use during anterior segment (i.e.
phacoemulsification and removal of cataracts) and posterior segment
(i.e. vitreoretinal) ophthalmic surgery. In addition, with the
optional laser this system is indicated for photocoagulation (i.e.
vitreoretinal and macular pathologies), iridotomy and
trabeculoplasty procedures. The UNITY® CS (Cataract System)
console, when used with compatible devices, is indicated for use
during anterior segment (i.e. phacoemulsification and removal of
cataracts) ophthalmic surgery. Refer to the Directions for Use for
the accessories/consumables and User Manual for a complete listing
of indications, warnings, cautions and notes.
References
- Market Scope 2024 IOL Market Report, 2024.
- Market Scope 2024 Retinal Surgical Device Market Report,
2024.
- Market Scope 2024 Cataract Surgical Equipment Market Report,
2024.
Connect with us on
Facebook LinkedIn
View source
version on businesswire.com: https://www.businesswire.com/news/home/20240622004445/en/
Investor Relations Dan
Cravens Allen Trang + 41 589 112 110 (Geneva) + 1 817 615 2789
(Fort Worth) investor.relations@alcon.com
Media Relations Steven Smith
+ 41 589 112 111 (Geneva) + 1 817 551 8057 (Fort Worth)
globalmedia.relations@alcon.com
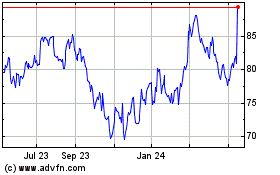
Alcon (NYSE:ALC)
Historical Stock Chart
From Oct 2024 to Nov 2024
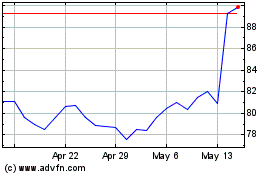
Alcon (NYSE:ALC)
Historical Stock Chart
From Nov 2023 to Nov 2024