Moderna Says Health Canada Authorizes Second Omicron-Targeting Bivalent Booster
November 04 2022 - 5:45PM
Dow Jones News
By Stephen Nakrosis
Moderna Inc. on Friday said Health Canada has approved its
BA.4/BA.5 Omicron-targeting bivalent Covid-19 booster vaccine,
mRNA-1273.222.
The company said the authorization is for use as "a booster dose
for active immunization against Covid-19 caused by the SARS-CoV-2
virus in individuals 18 years of age and older."
Moderna said mRNA-1273.222 is its second bivalent Covid-19
vaccine approved by Health Canada, following the approval of
mRNA-1273.214, or SPIKEVAXBivalent, in September.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
November 04, 2022 17:30 ET (21:30 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
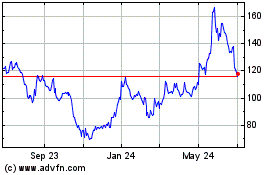
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Aug 2024 to Sep 2024
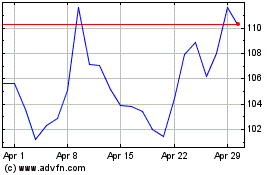
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Sep 2023 to Sep 2024