Lantern Pharma to Participate and Present at Multiple Upcoming Conferences
July 17 2023 - 8:00AM
Business Wire
Lantern Pharma Inc. (NASDAQ: LTRN), an artificial intelligence
(“AI”) company developing targeted and transformative cancer
therapies using its proprietary RADR® AI and machine learning
(“ML”) platform with multiple clinical stage drug programs, today
announced that the Company will participate and present at four
upcoming conferences:
- Biotech CEO Summit USA in La Jolla, CA from July 17-19,
2023. Panna Sharma, Lantern’s CEO and President will be
participating. Conference Website:
https://www.biotechceosummit.com
- Cancer Molecular Therapeutics Research Association
Conference, in Watkins Glen, NY from July 23-27, 2023. Kishor
Bhatia Ph.D., Lantern’s Chief Scientific Officer, will be
participating, presentation details TBA. Conference
Website/Registration:
https://www.cancermoleculartherapeutics.org
- Society of Neuro-Oncology/American Society of Clinical
Oncology CNS Cancer Conference in San Francisco, CA, on August
10th, 2023 from 5:30-7:30 p.m. PT. Presentation Title:
LP-184, a novel acylfulvene-derived tumor site activated small
molecule inhibits adult and pediatric CNS tumor cell growth
Conference Website/Registration:
https://www.soc-neuro-onc.org/WEB/Event_Content/2023_SNO_ASCO_CNS_Cancer_Conference.aspx
- International Conference on Drug Conjugates for Directed
Therapy in Darmstadt, Germany on Thursday, August 24th, 2023
from 9:45-10:15 a.m. CEST. Presentation Title: In-silico
Approach for the Identification of ADC Targets with Improved Tumor
Selectivity Conference Website/Registration:
https://www.uni-bielefeld.de/fakultaeten/chemie/projects/magicbulletreloaded/conference/index.xml
Further details on the data and findings presented at the
scientific conferences will be announced following the conference
presentations.
About Lantern Pharma:
Lantern Pharma (NASDAQ: LTRN) is an AI company transforming the
cost, pace, and timeline of oncology drug discovery and
development. Our proprietary AI and machine learning (ML) platform,
RADR®, leverages over 25 billion oncology-focused data points and a
library of 200+ advanced ML algorithms to help solve
billion-dollar, real-world problems in oncology drug development.
By harnessing the power of AI and with input from world-class
scientific advisors and collaborators, we have accelerated the
development of our growing pipeline of therapies including eleven
cancer indications and an antibody-drug conjugate (ADC) program. On
average, our newly developed drug programs have been advanced from
initial AI insights to first-in-human clinical trials in 2-3 years
and at approximately $1.0-2.0 million per program.
Our lead development programs include two Phase 2 clinical
programs and multiple upcoming Phase 1 clinical trials anticipated
for 2023. We have also established a wholly-owned subsidiary,
Starlight Therapeutics Inc., to focus exclusively on the clinical
execution of our promising therapies for CNS and brain cancers,
many of which have no effective treatment options. Our AI-driven
pipeline of innovative product candidates is estimated to have a
combined annual market potential of over $15 billion USD and have
the potential to provide life-changing therapies to hundreds of
thousands of cancer patients across the world.
Please find more information at: Website: www.lanternpharma.com
LinkedIn: https://www.linkedin.com/company/lanternpharma/ Twitter:
@lanternpharma Lantern Pharma Newsletter – The Spark: Sign-up
here
Forward Looking Statements:
This press release contains forward-looking statements within
the meaning of Section 27A of the Securities Act of 1933, as
amended, and Section 21E of the Securities Exchange Act of 1934, as
amended. These forward-looking statements include, among other
things, statements relating to: future events or our future
financial performance; the potential advantages of our RADR®
platform in identifying drug candidates and patient populations
that are likely to respond to a drug candidate; our strategic plans
to advance the development of our drug candidates and antibody drug
conjugate (ADC) development program; estimates regarding the
development timing for our drug candidates and ADC development
program; expectations and estimates regarding clinical trial timing
and patient enrollment; our research and development efforts of our
drug discovery and ADC programs and the utilization of our RADR®
platform to streamline the drug development process; our intention
to leverage artificial intelligence, machine learning and genomic
data to streamline and transform the pace, risk and cost of
oncology drug discovery and development and to identify patient
populations that would likely respond to a drug or ADC candidate;
estimates regarding patient populations, potential markets and
potential market sizes; sales estimates for our drug and ADC
candidates and our plans to discover and develop drug and ADC
candidates and to maximize their commercial potential by advancing
such candidates ourselves or in collaboration with others. Any
statements that are not statements of historical fact (including,
without limitation, statements that use words such as "anticipate,"
"believe," "contemplate," "could," "estimate," "expect," "intend,"
"seek," "may," "might," "plan," "potential," "predict," "project,"
"target," "model," "objective," "aim," "upcoming," "should,"
"will," "would," or the negative of these words or other similar
expressions) should be considered forward-looking statements. There
are a number of important factors that could cause our actual
results to differ materially from those indicated by the
forward-looking statements, such as (i) the impact of the COVID-19
pandemic, (ii) the risk that our research and the research of our
collaborators may not be successful, (iii) the risk that none of
our product candidates has received FDA marketing approval, and we
may not be able to successfully initiate, conduct, or conclude
clinical testing for or obtain marketing approval for our product
candidates, (iv) the risk that no drug product based on our
proprietary RADR® AI platform has received FDA marketing approval
or otherwise been incorporated into a commercial product, and (v)
those other factors set forth in the Risk Factors section in our
Annual Report on Form 10-K for the year ended December 31, 2022,
filed with the Securities and Exchange Commission on March 20,
2023. You may access our Annual Report on Form 10-K for the year
ended December 31, 2022 under the investor SEC filings tab of our
website at www.lanternpharma.com or on the SEC's website at
www.sec.gov. Given these risks and uncertainties, we can give no
assurances that our forward-looking statements will prove to be
accurate, or that any other results or events projected or
contemplated by our forward-looking statements will in fact occur,
and we caution investors not to place undue reliance on these
statements. All forward-looking statements in this press release
represent our judgment as of the date hereof, and, except as
otherwise required by law, we disclaim any obligation to update any
forward-looking statements to conform the statement to actual
results or changes in our expectations.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20230717087662/en/
Nicole Leber Investor Relations Associate
ir@lanternpharma.com
Lantern Pharma (NASDAQ:LTRN)
Historical Stock Chart
From Jun 2024 to Jul 2024
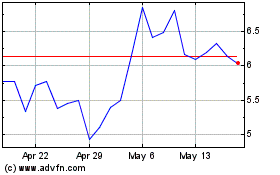
Lantern Pharma (NASDAQ:LTRN)
Historical Stock Chart
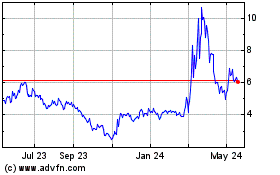
From Jul 2023 to Jul 2024