Table of Contents
Filed Pursuant to Rule 253(g)(1)
File No. 024-12157

Greene Concepts, Inc.
700,000,000 Units
Each Unit Consisting of 3 Shares of Common Stock
and 2 Warrants to Purchase
One Share Each of Common Stock Exercisable at
$0.01 Per Warrant
This Post-Qualification Offering Circular Amendment
No. 3 (the “PQA”) amends the Offering Circular of Greene Concepts, Inc., a New York corporation (“we” or the
“Company”), dated March 30, 2023, and qualified on April 3, 2023, Post-Qualification Offering Circular Amendment No. 1 dated
October 23, 2023, and Post-Qualification Offering Circular Amendment No. 2 dated January 26, 2024, and qualified on February 28, 2024,
as may be amended and supplemented from time to time, to: (a) extend the expiration date of this offering to October 11, 2025; (b) add
200,000,000 Units, for a revised maximum of 700,000,000 Units; and (c) revise the offering price of the 424,666,667 Units that remain
unsold (the “Remaining Units”) to $0.002 per Remaining Unit.
We are offering 700,000,000 units of our securities
(the “Units” or the “Offered Units”), with each Unit consisting of 3 Shares of Common Stock, par value $0.0001
(the “Common Stock”), and 2 warrants (each a “Warrant”) to purchase one share each of Common Stock (each, a “Warrant
Share”) exercisable at $0.01 per Warrant, of which 275,333,333 Units have been sold for cash in the total amount of $1,112,000
and of which 424,666,667 Units, the Remaining Units, are being offered at a fixed price $0.002 per Remaining Unit. The Units are
being offered by our company on a best-efforts basis with no minimum offering required, pursuant to Tier 1 of Regulation A of the United
States Securities and Exchange Commission (the “SEC”).
Through the sale of the Offered Units, the Company
is offering a maximum of 3,500,000,000 shares of Common Stock, including the Warrant Shares. Once a Unit is purchased by an investor,
such investor may separately transfer the Common Stock and the Warrant comprising the Units, at such investor’s discretion. The
Warrants are exercisable upon purchase. Once the Units offered by the Company are qualified by the SEC, the Common Stock and Warrants
comprising the Units, including the Warrant Shares, will have been qualified.
Please be advised that due to the ownership
of super voting rights by our management team in the form of Preferred Shares, your voting rights as a common shareholder will be substantially
limited.
No Escrow
The proceeds of this offering will not be placed
into an escrow account. We will offer the Units, including the Remaining Units, on a best-efforts basis. As there is no minimum offering,
upon the acceptance of any subscription received pursuant to this PQA, the Company shall immediately deposit said proceeds into the bank
account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
Subscriptions are irrevocable and the purchase
price is non-refundable as expressly stated in this PQA. All proceeds received by the Company from subscribers for this Offering will
be available for use by the Company upon acceptance of subscriptions for the Units, including the Remaining Units, by the Company.
The Company, by determination of the Board of
Directors, in its sole discretion, may issue the Units, including the Remaining Units, in this offering for cash, promissory notes, services,
and/or other consideration without notice to subscribers. The aggregate offering price will be based on the price at which the Units are
offered for cash. Any portion of the aggregate offering price or aggregate sales attributable to cash received in a foreign currency will
be translated into United States currency at a currency exchange rate in effect on, or at a reasonable time before, the date of the sale
of the Offered Units. If Offered Units are not sold for cash, the aggregate offering price or aggregate sales will be based on the value
of the consideration as established by bona fide sales of that consideration made within a reasonable time, or, in the absence of sales,
on the fair value as determined by an accepted standard. Valuations of non-cash consideration will be reasonable at the time made.
This offering commenced on April 3, 2023, and
will terminate as indicated herein. This offering will be a continuous offering pursuant to Rule 251(d)(3)(i)(F).
This Offering will be conducted on a “best-efforts”
basis by our company, which means our officers will use their commercially reasonable best efforts in an attempt to offer and sell the
Units. Our officers will not receive any commission or any other remuneration for these sales. In offering the Units, including the Remaining
Units, on our behalf, the officers will rely on the safe harbor from broker-dealer registration set out in Rule 3a4-1 under the Securities
Exchange Act of 1934, as amended.
This PQA shall not constitute an offer to sell
or the solicitation of an offer to buy, nor shall there be any sales of these securities in any state or jurisdiction in which such offer,
solicitation or sale would be unlawful, prior to registration or qualification under the laws of any such state.
Our
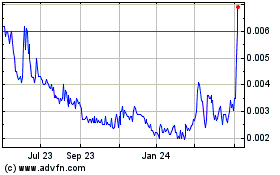
Common Stock is traded in the OTCMarket Pink Open Market under the stock symbol “INKW.”
On October 22, 2024, the closing price of our Common Stock was $0.00205 per share.
Investing in our Common Stock involves a
high degree of risk. See “Risk Factors” beginning on page 4 for a discussion of certain risks that
you should consider in connection with an investment in our Common Stock.
Title
of class of
Securities offered |
|
Total
Number
of Units
Offered |
|
Number
of
Units Sold
to Date |
|
Proceeds
to
Company
to Date(1) |
|
Number
of
Remaining
Units to
Be Sold |
|
Price
to
Public of
Remaining
Units to
Be Sold |
|
Proceeds
to
Company
from
Remaining
Units(1) |
|
Commissions(2) |
|
Total
Proceeds
to
Company(3) |
| Units(4) |
|
700,000,000 |
|
275,333,333 |
|
$1,112,000 |
|
424,666,667 |
|
$0.002 |
|
$849,333 |
|
$-0- |
|
$1,961,333 |
(1) Does not reflect payment of expenses of this
offering, which are estimated to be $100,000 and which include, among other things, legal fees, accounting costs, reproduction expenses,
due diligence, marketing, consulting, administrative services other costs of Blue Sky compliance, and actual out-of-pocket expenses incurred
by our company selling the Offered Units.
(2) Our company is offering the Offered Units
without an underwriter and will not pay any commissions for the sale of Offering Units in this offering.
(3) Assuming the sale of all 424,666,667 Remaining Units; does not
include the exercise of any Warrants. See “Use of Proceeds.”
Investing in the Offered Units involves risks. Greene Concepts,
Inc. currently has limited operations, limited income, and limited assets, is in unsound financial condition, and you should not invest
unless you can afford to lose your entire investment. See “Risk Factors” beginning on page 4. Neither the Securities and
Exchange Commission nor any state securities commission has approved or disapproved of the Offered Units or determined if this PQA is
truthful or complete. Any representation to the contrary is a criminal offense.
Our Board of Directors used its business judgment
in establishing the offering price of $0.002 per Remaining Unit. The offering price per share bears no relationship to our book
value or any other measure of our current value or worth.
No sale may be made to you in this offering
if the aggregate purchase price you pay is more than 10% of the greater of your annual income or your net worth. Different rules apply
to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds,
we encourage you to review Rule 251(d)(2)(i)(C) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov.
THIS PQA DOES NOT CONSTITUTE AN OFFER OR SOLICITATION
IN ANY JURISDICTION IN WHICH SUCH AN OFFER OR SOLICITATION WOULD BE UNLAWFUL. NO PERSON HAS BEEN AUTHORIZED TO GIVE ANY INFORMATION OR
TO MAKE ANY REPRESENTATIONS CONCERNING THE COMPANY OTHER THAN THOSE CONTAINED IN THIS PQA, AND IF GIVEN OR MADE, SUCH OTHER INFORMATION
OR REPRESENTATION MUST NOT BE RELIED UPON.
THE U.S. SECURITIES AND EXCHANGE COMMISSION
DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE
ACCURACY OR COMPLETENESS OF ANY PQA OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION
WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
This PQA is following the offering circular format
described in Part II (a)(1)(ii) of Form 1-A.
Post-Qualification Amendment Offering Circular
No. 3 dated October 14, 2024.
TABLE OF CONTENTS
THIS PQA MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE.
We are offering to sell, and seeking offers to
buy, our securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained
in this PQA offering circular. We have not authorized anyone to provide you with any information other than the information contained
in this PQA. The information contained in this PQA is accurate only as of its date, regardless of the time of its delivery or of any sale
or delivery of our securities. Neither the delivery of this PQA nor any sale or delivery of our securities shall, under any circumstances,
imply that there has been no change in our affairs since the date of this PQA. This PQA will be updated and made available for delivery
to the extent required by the federal securities laws.
SUMMARY
This summary highlights information contained
elsewhere in this PQA. This summary does not contain all of the information that you should consider before deciding to
invest in our securities. You should read this entire PQA carefully, including the “Risk Factors” section, our
historical financial statements and the notes thereto, each included elsewhere herein.
Our Company
Overview
Our company name is Greene Concepts, Inc. We are
headquartered in Marion, North Carolina. We are a New York corporation that was incorporated on August 18, 1952 and previously operated
as Tech-OHM Resistor Corporation, Tech-OHM Electronics, Inc., International Citrus Corporation, Princeton Commercial Holdings, Inc., Eurowind
Energy, Inc., First Petroleum and Pipeline Inc., and Luke Entertainment, Inc. Since our inception, we have operated different businesses
under these different names before changing our name to Greene Concepts, Inc. and engaging in our current business line. Through our wholly-owned
subsidiary, Mammoth Ventures Inc., (“Mammoth”), we are now a bottling and beverage company committed to providing the world
with high quality, healthy, and enhanced beverage choices. Our beverage and bottling facility is located in Marion, North Carolina. The
facility is a 55,000 square foot bottling and beverage plant that is located within the boundaries of the Pisgah National Forest. The
bottling facility has as its water sources a combination of seven (7) spring and artesian wells that are fed from a natural aquifer that
is located deep below the Pisgah National Forest. We are focused on producing spring and artesian water, Additionally, we expect that
Mammoth will act as a third-party producer and bottler of "white label" beverage and water products. White label bottling services
are provided for clients that desire to market their own product formulations, brand name and labeling while outsourcing the production
and bottling of their products to Mammoth.
Before acquiring Mammoth on February 6, 2019,
we operated our legacy business, which was the manufacture and distribution of a line of 25 high quality consumer focused inkjet kits.
On April 30, 2019, our board of directors made a determination to wind down our legacy business and to transition into the beverage and
bottling business.
On February 6, 2019, we entered into a Stock Purchase
Acquisition Agreement and Merger Agreement and Promissory Note Agreement with BNL Capital LLC (“BNL Capital”). Pursuant to
the terms of the agreement, BNL Capital agreed to sell 100% of the outstanding shares of Mammoth to us for a purchase price of $1,350,000.
Mammoth acquired certain assets of the defunct business formerly referred to as “North Cove Springs Bottling and Beverage,”
which includes the Marion, North Carolina bottling facility and related assets. We financed the acquisition through a secured promissory
note in the amount of $1,350,000 in favor of BNL Capital. The promissory note was secured by 100% of the outstanding shares of Mammoth
that are owned by our company. See “Description of Business – Terms of Acquisition of Mammoth Ventures, Inc.” for a
description of the terms of our acquisition of Mammoth.
On March 5, 2021, the Company paid $200,000 to
BNL Capital to satisfy the remaining obligations owed to BNL Capital. BNL Capital also cancelled 7,500,000 shares of Series A Preferred
Stock of the Company owned by BNL Capital.
Upon acquiring Mammoth, we began the process of
performing required maintenance to revitalize all the equipment and facility infrastructure in order to relaunch production at the plant.
At the time of the acquisition all of the plant equipment was in good condition although the equipment had not operated for several years
and it did require a thorough inspection and light maintenance to assure proper operation when the bottling lines are relaunched. At the
time of the acquisition, we hired, Kenneth Porter, a 30+ year veteran of the beverage and bottling industry, as plant manager to oversee
operations as well as the revitalization and expected relaunch of the facility.
The Food and Drug Administration, or FDA, requires
adherence to current good manufacturing practice (“CGMP”), regulations for the processing and bottling of bottled drinking
water, which includes facility inspection and documentation of corrective measures and reporting requirements, as well as new requirements
for hazard assessments and food safety, or HACCP, plans mandated by the Food Safety and Modernization Act (“FSMA”). Final
preparations for inspection are underway, including building and facility maintenance such as pressure washing, painting, general cleaning,
and minor building repairs.
In addition to complete cleaning and maintenance
of the 55,000 square foot facility, standard operating policies and procedures must be documented in accordance with federal legislation.
This documentation includes conducting and reporting of microbial testing of source water and any finished product, which must be completed
prior to initiating filling and packaging of bottles for shipment from our production lines.
As of April 6, 2020, the Company’s, through
its wholly owned subsidiary Mammoth, production facility is fully operational after the Company spent 16 months restoring the production
facility in Marion, North Carolina (the “Marion Facility”). The Marion Facility currently has the capacity to produce 192
million bottles or 8 million cases annually (with current equipment). The Marion Facility has space for additional capacity.
On February 17, 2021, Mammoth paid off all mortgage
liens and obligations for the Marion Facility, equipment and property and has received a certificate of satisfaction from the lien holder.
The certificate of satisfaction is being filed with the McDowell County Registry of Deeds which will remove all liens or encumbrances
from the Marion Facility deed.
On or about June 14, 2021, the Company amended
its Certificate of Incorporation to increase the number of authorized shares to ten billion (10,000,000,000) and create the Series B Convertible
Preferred Stock with one thousand (1,000) shares authorized. Each share of Series B Convertible Preferred Stock will be convertible into
one hundred million (100,000,000) shares of the Corporation’s common stock.
The Company has launched its CBD infused drink
“Happy Mellow” beverage. The product is produced by our co-packers.
Disaster recovery efforts led by the federal government
through Federal Emergency Management Agency, state government emergency response programs, and city or county emergency response programs
require entities to be registered and cleared thru the System for Award Management (SAM). The Company successfully completed validation
requirements and currently is awaiting issuance of Commercial and Government Entity Codes (“CAGE codes”). CAGE codes are unique
identifier assigned to suppliers to various government or defense agencies and provide a standardized method of identifying a given facility
at a specific location. Greene Concepts Inc. expedited application efforts in order to become registered and available to provide assistance
for any disaster or emergency relief efforts. The Company’s Marion bottling facility is a strategically important asset by possessing
a total of seven (7) operational wells. The Marion Facility is located within the Pisgah National Forest with an enormous source of pure
spring water accessed from the aquifer located deep below the national forest. While the bottling facility can operate at full capacity
with a single primary well the Company’s facility has the unique strategic advantage of having six additional operational wells
as backup in the unlikely event of any system failures with the primary or any other well.
Corporate Information
Our principal executive offices are located at
13195 U.S. Highway 221 N, Marion, North Carolina, 28752 and our telephone number is (844) 889-2837. We maintain a website at www.greeneconcepts.com.
Information available on our website is not incorporated herein.
The offering
| Securities being offered: |
|
We
are offering 700,000,000 units of our securities (the Units), with each Unit consisting of 3 Shares of Common Stock, par value $0.0001
(the Common Stock), and 2 warrants (the Warrants) to purchase one share each of Common Stock (the Warrant Shares) exercisable at
$0.01 per Warrant, of which 275,333,333 Units have been sold for cash in the total amount of $1,112,000 and of which 424,666,667
Units (the Remaining Units) are being offered at a fixed price $0.002 per Remaining Unit. |
| |
|
|
| Minimum subscription: |
|
There is no minimum subscription. |
| |
|
|
| Shares outstanding before the offering: |
|
As of the date of this PQA, there are 2,940,667,506 shares of Common Stock
issued and outstanding, 888,390 shares of Preferred Class A Stock issued and outstanding and 60 shares of Preferred Class B Stock issued
and outstanding. |
| |
|
|
Shares outstanding after the offering:(1) |
|
Assuming
the sale of all of the Remaining Units and the exercise of all Warrants
included in the Units, including in the Remaining Units, there will be 5,064,000,841 shares of Common Stock issued and outstanding, 888,390
shares of Preferred Class A Stock issued and outstanding and 60 shares of Preferred Class B Stock issued and outstanding. |
| |
|
|
Number of Warrants to be
outstanding after the offering:
|
|
Assuming the sale of all of the Remaining Units, there will 1,400,000,000 Warrants
issued and outstanding.
|
| |
|
|
Number of shares of Common
Stock underlying the Units:
|
|
A total of 2,100,000,000 shares of Common Stock are included in the Offered
Units, including the Remaining Units, and an additional 1,400,000,000 shares of Common Stock underlie the Warrants, if the maximum amount
of Offered Units are sold and all the Warrants are exercised. The total number of shares of our common stock outstanding assumes that
the maximum number of units each containing shares of our common stock and warrants is sold in this offering and that all the warrants
are exercised.
|
| |
|
|
| Price per unit: |
|
$0.002 |
| |
|
|
| Maximum offering amount: |
|
We are offering a total 700,000,000 Offered Units, including the 424,666,667
Remaining Units, at $0.002 per Unit, for a total of $1,961,333 in proceeds from sales of the Offered Units, with up
to an additional $14,000,000 in proceeds from the exercise of Warrants (See “Distribution”). We
will not raise more than $15,961,333 in gross proceeds in this offering. |
| |
|
| Best efforts offering: |
|
The Offered Units are being offered on a “best efforts”
basis through our Chief Executive Officer, Leonard M. Greene, who will not receive any discounts or commissions for selling the Offered
Units. There is no minimum number of Offered Shares that must be sold in order to close this offering. |
| |
|
|
| Restrictions on investment amount: |
|
Generally, no sale may be made to you in this offering if the aggregate purchase price you pay is more than 10% of the greater of your annual income or net worth. Different rules apply to accredited investors and non-natural persons. Before making any representation that your investment does not exceed applicable thresholds, we encourage you to review Rule 251(d)(2)(i)(c) of Regulation A. For general information on investing, we encourage you to refer to www.investor.gov. |
| |
|
|
| No Escrow account: |
|
We
have not engaged an escrow agent for this offering. Funds invested will be deposited directly into our company’s operating account
and immediately available for our use. |
| |
|
|
| Termination of the offering: |
|
This
offering commenced on April 3, 2023, and will terminate at the earlier
of: (1) the date on which all of the Remaining Shares have been sold, (2) October 11, 2025, or (3) the date on which this offering is
earlier terminated by us in our sole discretion. |
| |
|
|
| Use of proceeds: |
|
If we
sell all of the Offered Units, including the Remaining Units, and assuming the exercise of all of the Warrants, our net proceeds in
this offering (after our estimated offering expenses) will be approximately $$15,472,967-15,961,333. We will use these net proceeds for working
capital and other general corporate purposes. (See “Use of Proceeds”). |
| |
|
| Market for our Common Stock: |
|
Our Common Stock is currently quoted on the OTC Pink Market under the trading symbol “INKW”. |
| |
|
|
| Risk factors: |
|
Investing in our securities involves risks. See the section entitled “Risk Factors” in this PQA and other information included in this PQA for a discussion of factors
you should carefully consider before deciding to invest in our Offered Units. |
RISK FACTORS
Investing in our shares involves a significant
degree of risk. In evaluating our company and an investment in the shares, careful consideration should be given to the following risk
factors, in addition to the other information included in this PQA. Each of these risk factors could materially adversely affect our
business, operating results or financial condition, as well as adversely affect the value of an investment in our shares. The following
is a summary of the most significant factors that make this offering speculative or substantially risky. We are still subject to all
the same risks that all companies in our industry, and all companies in the economy, are exposed to. These include risks relating to
economic downturns, political and economic events and technological developments (such as cyber-security). Additionally, early-stage
companies are inherently riskier than more developed companies. You should consider general risks as well as specific risks when deciding
whether to invest.
Risks Related to our Business, Operating Results and Industry
We
have a history of operating losses and there is a substantial doubt about our ability to continue as a going concern.
For the
fiscal years ended July 31, 2024 and 2023, we reported net losses of $241,886 (unaudited) and $1,399,672 (unaudited), respectively, and
negative cash flow from operating activities of $732,897 (unaudited) and $1,313,051 (unaudited), respectively. We anticipate that we will
continue to report losses and negative cash flow. As a result of these net losses and cash flow deficits, as well as our dependence
on private equity and financings, there is a substantial doubt about our ability to continue as a going concern.
Our consolidated financial
statements do not include any adjustments that might result from the outcome of this uncertainty. These adjustments would likely include
substantial impairment of the carrying amount of our assets and potential contingent liabilities that may arise if we are unable to fulfill
various operational commitments. In addition, the value of our securities, including common stock issued in this offering, would be greatly
impaired. Our ability to continue as a going concern is dependent upon generating sufficient cash flow from operations and obtaining
additional capital and financing, including funds to be raised in this offering. If our ability to generate cash flow from operations
is delayed or reduced and we are unable to raise additional funding from other sources, we may be unable to continue in business even
if this offering is successful. For further discussion about our ability to continue as a going concern and our plan for future liquidity,
see “Management’s Discussion and Analysis of Financial Condition and Results of Operations-Ability to Continue as a Going
Concern.”
We will need additional financing to execute
our business plan which we may not be able to secure on acceptable terms, or at all.
We will require additional financing in the near
and long term to fully execute our business plan. Our success depends on our ability to raise such additional financing on reasonable
terms and on a timely basis. Conditions in the economy and the financial markets may make it more difficult for us to obtain necessary
additional capital or financing on acceptable terms, or at all. If we cannot secure sufficient additional financing, we may be forced
to forego strategic opportunities or delay, scale back or eliminate further development of our goals and objectives, operations and investments
or employ internal cost savings measures.
In order for us to compete and grow, we
must attract, recruit, retain and develop the necessary personnel who have the needed experience.
Recruiting and retaining highly qualified personnel
is critical to our success. These demands may require us to hire additional personnel and will require our existing management personnel
to develop additional expertise. We face intense competition for personnel. The failure to attract and retain personnel or to develop
such expertise could delay or halt the sales and licensing of our product. If we experience difficulties in hiring and retaining personnel
in key positions, we could suffer from delays in our development, loss of customers and sales and diversion of management resources, which
could adversely affect operating results. Our future consultants and advisors may be employed by third parties and may have commitments
under consulting or advisory contracts with third parties that may limit their availability to us.
Quality management plays an essential role
in determining and meeting customer requirements, preventing defects, improving our products and services and maintaining the integrity
of the data that supports the safety and efficacy of our products.
Our future success depends on our ability to maintain
and continuously improve our quality management program. An inability to address a quality or safety issue in an effective and timely
manner may also cause negative publicity, a loss of customer confidence in us or our current or future products, which may result in the
loss of sales and difficulty in successfully launching new products. In addition, a successful claim brought against us in excess of available
insurance or not covered by indemnification agreements, or any claim that results in significant adverse publicity against us, could have
an adverse effect on our business and our reputation.
Our success depends on the services of our Chief Executive Officer,
the loss of whom could disrupt our business.
We depend to a large extent on the
services of our CEO, Mr. Leonard Greene. Given his knowledge and experience, he is important to our future prospects and development as
we rely on his expertise in developing our business strategies and maintaining our operations. The loss of the service of Mr. Greene and
the failure to find timely replacements with comparable experience and expertise could disrupt and adversely affect our business.
Our current CEO and Director, Leonard
Greene beneficially owns approximately 33% of the outstanding shares of Series B Preferred Stock, which accounts for approximately 26.6% of the total voting
rights of the Common Stock. The holders of the Series B Preferred Stock control, as a group, 80.0% of the voting rights of all shareholders.
As a result, the holders of the Series B Preferred Stock have substantial voting power in all matters submitted to our stockholders for
approval including:
| |
· |
Election of our board of directors; |
| |
|
|
| |
· |
Removal of any of our directors; |
| |
|
|
| |
· |
Amendment of our Certificate of Incorporation or bylaws; |
| |
|
|
| |
· |
Adoption of measures that could delay or prevent a change in control or impede a merger, takeover or other business combination involving us. |
Leonard Greene, the Company’s Chief
Executive Officer and member of the Company’s Board of Directors own approximately 33% of the Company’s Series B Preferred
Stock. Each share of Series B Preferred stock is entitled to one hundred million (100,000,000) votes per share on all matters to be voted
at any annual or special meeting of the shareholders of the Corporation or action by written consent of shareholders. The Series B Preferred
Stockholders together control 80.0% of the voting rights of all shareholders. As a result, the Series B Preferred Stockholders have substantial
voting power in all matters submitted to our stockholders. The Series B Preferred Stockholders together are able to substantially influence
all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. In
addition, the future prospect of sales of significant amounts of shares held by them could affect the market price of our Common Stock
if the marketplace does not orderly adjust to the increase in shares in the market and the value of your investment in our company may
decrease. The Series B Preferred Holders’ stock ownership may discourage a potential acquirer from making a tender offer or otherwise
attempting to obtain control of us, which in turn could reduce our stock price or prevent our stockholders from realizing a premium over
our stock price.
Although dependent on certain key personnel,
we do not have any key person life insurance policies on any such people.
We are dependent on Leonard Greene in order to
conduct our operations and execute our business plan, however, we have not purchased any insurance policies with respect to him in the
event of his death or disability. Therefore, if Leonard Greene dies or becomes disabled, we will not receive any compensation to assist
with his absence. The loss of Leonard Greene could negatively affect us and our operations.
We face significant
competition for our beverage and bottling business.
The commercial beverage and bottling industry
is highly competitive and we compete with a number of other companies that provide similar products. Our ability to compete successfully
in the commercial beverage industry and to manage our planned growth will depend primarily upon the following factors:
| · | maintaining continuity in our management and
key personnel; |
| · | ability to react to competitive product and pricing
pressures; |
| · | the strength of our brand; |
| · | the ability to expand into specialized bottling,
including carbonated beverages, unique sizes and shapes; |
| · | increasing the productivity of our future sales
employees; |
| · | effectively marketing and selling our products; |
| · | acquiring new customers for our products; |
| · | ability to respond to complaints if necessary;
|
| · | developing and improving our operational, financial
and management controls; |
| · | developing and improving our information reporting
systems and procedures; and |
| · | the design and functionality of our products. |
Many of our competitors
have greater financial, technical, product development, marketing and other resources than we do. These organizations may be better known
than we are and may have more customers or users than we do. We cannot provide assurance that we will be able to compete successfully
against these organizations, which may lead to lower customer satisfaction, decreased demand for our solutions, loss of market share or
reduction of operating profits.
Changes in the non-alcoholic beverage business
environment and retail landscape could adversely impact our financial results.
The non-alcoholic beverage business environment
is rapidly evolving as a result of, among other things, changes in consumer preferences, including changes based on health and nutrition
considerations and obesity concerns; shifting consumer tastes and needs; changes in consumer lifestyles; and competitive product and pricing
pressures. In addition, the non-alcoholic beverage retail landscape is very dynamic and constantly evolving, not only in emerging and
developing markets, where modern trade is growing at a faster pace than traditional trade outlets, but also in developed markets, where
discounters and value stores, as well as the volume of transactions through e-commerce, are growing at a rapid pace. If we are unable
to successfully adapt to the rapidly changing environment and retail landscape, our share of sales, volume growth and overall financial
results could be negatively affected.
A failure to introduce new products or product
extensions into new marketplaces successfully could prevent us from achieving long-term profitability.
We compete in an
industry characterized by rapid changes in consumer preferences, so our ability to continue developing new products to satisfy our consumers'
changing preferences will determine our long-term success. A failure to introduce new products or product extensions into new marketplaces
successfully could prevent us from achieving long-term profitability. In addition, customer preferences are also affected by factors other
than taste, such as the publicity. If we do not adjust to respond to these and other changes in customer preferences, our sales may be
adversely affected. In addition, a failure to obtain any required regulatory approvals for our proposed products could have a material
adverse effect on our business, operating results and financial condition.
Our business is sensitive to public perception.
If any product proves to be harmful to consumers or if scientific studies provide unfavorable findings regarding their safety or effectiveness,
then our image in the marketplace would be negatively impacted.
Our results of operations may be significantly
affected by the public's perception of our company and similar companies. Our business could be adversely affected if any of our products
or similar products distributed by other companies proves to be harmful to consumers or if scientific studies provide unfavorable findings
regarding the safety or effectiveness of our products or any similar products. If our products suffer from negative consumer perception,
it is likely to adversely affect our business and results of operations.
Consumers may have preconceptions about
the health benefits of spring water; such health benefits are not guaranteed or proven.
Health benefits of spring water are not guaranteed
and have not been proven. Although we do not market our products as having any potential health benefits, there is a consumer perception
that drinking spring water has beneficial health effects. Consequently, negative changes in consumers' perception of the benefits of spring
water or negative publicity surrounding spring water may result in loss of market share or potential market share and hence, loss of your
investment. We are also prohibited from touting unconfirmed health benefits in our advertising and promotional activities for the products,
both directly and indirectly through claims made by third-party endorsers when those endorsers have a material connection to our company.
Water scarcity and poor quality could negatively
impact our production costs and capacity.
Water is the main ingredient in our products.
It is also a limited resource, facing unprecedented challenges from overexploitation, increasing pollution, poor management, and climate
change. As demand for water continues to increase, as water becomes scarcer, and as the quality of available water deteriorates, we may
incur increasing production costs or face capacity constraints that could adversely affect our profitability or net operating revenues
in the long run.
Adverse weather conditions could reduce
the demand for our products.
The sales of our products are influenced to some
extent by weather conditions in the markets in which we operate. Unusually cold or rainy weather during the summer months may have a temporary
effect on the demand for our products and contribute to lower sales, which could have an adverse effect on our results of operations for
such periods.
Product contamination or tampering or issues
or concerns with respect to product quality, safety and integrity could adversely affect our business, reputation, financial condition
or results of operations.
Product contamination or tampering, the failure
to maintain high standards for product quality, safety and integrity, including with respect to raw materials and ingredients obtained
from suppliers, or allegations (whether or not valid) of product quality issues, mislabeling, misbranding, spoilage, allergens, adulteration
or contamination with respect to products in our portfolio may reduce demand for such products, and cause production and delivery disruptions
or increase costs, each of which could adversely affect our business, reputation, financial condition or results of operations. If any
of the products in our portfolio are mislabeled or become unfit for consumption or cause injury, illness or death, or if appropriate resources
are not devoted to product quality and safety (particularly as we expand our portfolio into new categories) or to comply with changing
food safety requirements, we could decide to, or be required to, recall products or withdraw from the marketplace and/or we may be subject
to liability or government action, which could result in payment of damages or fines, cause certain products in our portfolio to be unavailable
for a period of time, result in destruction of product inventory, or result in adverse publicity (whether or not valid), which could reduce
consumer demand and brand equity. Moreover, even if allegations of product contamination or tampering or suggestions that our products
were not fit for consumption are meritless, the negative publicity surrounding assertions against us or products in our portfolio or processes
could adversely affect our reputation or brands. Our business could also be adversely affected if consumers lose confidence in product
quality, safety and integrity generally, even if such loss of confidence is unrelated to products in our portfolio. Any of the foregoing
could adversely affect our business, reputation, financial condition or results of operations. In addition, if we do not have adequate
insurance, if we do not have enforceable indemnification from suppliers, bottlers, distributors or other third parties or if indemnification
is not available, the liability relating to such product claims or disruption as a result of recall efforts could materially adversely
affect our business, financial condition or results of operations.
We regularly evaluate potential expansion
into international markets, and any expansion into such international operations could subject us to risks and expenses that could adversely
impact our business, financial condition and results of operations.
To date, we have not undertaken substantial commercial
activities outside of the United States. We have evaluated, and continue to evaluate, potential expansion into certain other international
markets. If and when we seek to expand internationally in the future, our sales and operations would be subject to a variety of risks,
including fluctuations in currency exchange rates, tariffs, import restrictions and other trade barriers, unexpected changes in legal
and regulatory requirements, longer accounts receivable payment cycles, potentially adverse tax consequences, and difficulty in complying
with foreign laws and regulations, as well as U.S. laws and regulations that govern foreign activities. Economic uncertainty in some of
the geographic regions in which we might operate could result in the disruption of commerce and negatively impact our operations in those
areas. Also, if we choose to pursue international expansion efforts, it may be necessary or desirable to contract with third parties,
and we may not be able to enter into such agreements on commercially acceptable terms or at all. Further, such arrangements may not perform
to our expectations, and we may be exposed to various risks as a result of the activities of our partners.
The forecasts of market growth included
in this PQA may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, we cannot assure you
our business will grow at similar rates, if at all.
Growth forecasts are subject to significant uncertainty and are based
on assumptions and estimates that may not prove to be accurate. The forecasts contained in this PQA may prove to be inaccurate. Even if
these markets experience the forecasted growth described in this PQA, we may not grow our business at similar rates, or at all. Our growth
is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties.
Accordingly, the forecasts of market growth included in this PQA should not be taken as indicative of our future growth.
Reductions in future sales of our products
will have an adverse effect on our profitability and ability to generate cash to fund our business plan.
The following factors, among others, could affect
future market acceptance and profitability of our products:
| · | the introduction of additional competitive or
alternative beverage products or white label bottlers; |
| · | changes in consumer preferences among commercial
beverages products; |
| · | changes in awareness of the environmental impact
of commercial beverages products; |
| · | the level and effectiveness of our sales and
marketing efforts; |
| · | any unfavorable publicity regarding our products
or services; |
| · | any unfavorable publicity regarding our future
brands; |
| · | litigation or threats of litigation with respect
to our future products or services; |
| · | the price of our products or services compared
to those of our competitors; |
| · | price increases resulting from rising commodity
costs; |
| · | regulatory developments affecting the manufacturing
or marketing of our products; |
| · | any changes in government policies and practices
related to our products; and |
Adverse developments with respect to the manufacturing
or sale of our products would significantly reduce our net sales and profitability and have a material adverse effect on our ability to
maintain profitability and achieve our business plan.
We will rely on other companies to provide
materials for our products.
We will depend on suppliers, co-packers, and
subcontractors to meet our contractual obligations to our future customers and conduct our operations. Our ability to meet our obligations
to our customers may be adversely affected if suppliers or subcontractors do not provide the agreed-upon supplies or perform the agreed-upon
services in compliance with customer requirements and in a timely and cost-effective manner. Likewise, the quality of our future products
may be adversely impacted if companies from whom we acquire such items do not provide materials which meet required specifications and
perform to our and our customers’ expectations. Our distributors and suppliers may be less likely than us to be able to quickly
recover from natural disasters and other events beyond their control and may be subject to additional risks such as financial problems
that limit their ability to conduct their operations. The risk of these adverse effects may be greater in circumstances where we may
rely on only one or two distributors or suppliers for a particular material.
We plan to source certain materials from a number of third-party
suppliers and, in some cases, single-source suppliers.
Although we believe that alternative suppliers
will be available, the loss of any of our future material suppliers could adversely affect our results of operations and financial condition.
Our inability to preserve the expected economics of these agreements could expose us to significant cost increases in future years.
Substantial disruption to a future distributors’
or suppliers’ manufacturing facilities could occur.
A disruption in production at a future distributors’
or suppliers’ manufacturing facilities could have an adverse effect on our business. The disruption could occur for many reasons,
including fire, natural disasters, weather, water scarcity, manufacturing problems, disease, strikes, transportation or supply interruption,
government regulation, cybersecurity attacks or terrorism. Alternative facilities with sufficient capacity or capabilities may not be
available, may cost substantially more or may take a significant amount of time to start production, each of which could negatively affect
our business and results of operations.
Increased costs could affect our company.
An increase in the cost of raw materials could
affect our profitability. Commodity and other price changes may result in unexpected increases in the cost of raw materials and other
materials used by us. We may also be adversely affected by shortages of raw materials. In addition, energy cost increases could result
in higher transportation, freight and other operating costs. We may not be able to increase our prices to offset these increased costs
without suffering reduced volume, sales and operating profit, and this could have an adverse effect on your investment.
Any disruption in our information systems
could disrupt our future operations and could adversely impact our business and results of operations.
We plan to depend on various information systems
to support our customers’ requirements and to successfully manage our business, including managing orders, supplies, accounting
controls and payroll. Any inability to successfully manage the procurement, development, implementation or execution of our information
systems and back-up systems, including matters related to system security, reliability, performance and access, as well as any inability
of these systems to fulfill their intended purpose within our business, could have an adverse effect on our business and results of operations.
Such disruptions may not be covered by our future business interruption insurance, which is insurance that we plan to, but have not yet,
obtained.
Manufacturing or design defects, unanticipated
use of our products, or inadequate disclosure of risks relating to the use of the products can lead to injury or other adverse events.
These events could lead to recalls or safety alerts
relating to our products (either voluntary or required by governmental authorities) and could result, in certain cases, in the removal
of a product from the market. Any recall could result in significant costs as well as negative publicity that could reduce demand for
our products. Personal injuries relating to the use of our products can also result in product liability claims being brought against
us. In some circumstances, such adverse events could also cause delays in new product approvals. Similarly, negligence in performing our
services can lead to injury or other adverse events.
We will need to increase brand awareness.
Due to a variety of factors, our opportunity to
achieve and maintain a significant market share may be limited. Developing and maintaining awareness of our brand name, among other factors,
is critical. Further, the importance of brand recognition will increase as competition in our market increases. Successfully promoting
and positioning our brand, products and services will depend largely on the effectiveness of our marketing efforts. Therefore, we may
need to increase our financial commitment to creating and maintaining brand awareness. If we fail to successfully promote our brand name
or if we incur significant expenses promoting and maintaining our brand name, it would have a material adverse effect on our results of
operations.
Our future advertising and marketing efforts
may be costly and may not achieve desired results.
We plan to incur substantial expense in connection
with our advertising and marketing efforts. Although we plan to target our advertising and marketing efforts on current and potential
customers who we believe are likely to be in the market for the products we plan to sell, we cannot assure you that our advertising and
marketing efforts will achieve our desired results. In addition, we will periodically adjust our advertising expenditures in an effort
to optimize the return on such expenditures. Any decrease in the level of our advertising expenditures, which may be made to optimize
such return could adversely affect our sales.
We expect our future intellectual property
rights will be critical to our success, and the loss of such rights may materially adversely affect our business.
We expect to own trademarks as we launch new products
in the future. We expect that these trademarks will be very important to our business. We may also own copyright in, and to, the content
on the packaging of our products. We view these future intellectual property rights as very important to our potential success and plan
to protect such intellectual property through registration and enforcement actions. However, there can be no assurance that other parties
will not infringe or misappropriate our future trademarks, copyrights and similar proprietary rights. If we lose some or all of our future
intellectual property rights, our business may be materially adversely affected.
We plan to obtain insurance that may not
provide adequate levels of coverage against claims.
We have obtained commercial product liability
insurance that covers us for up to $1,400,000 in overall damages and $1,000,000 per occurrence. This policy also covers us for general
liability for up to $500,000 for damages to equipment and property. However, there are types of losses we may incur that cannot be insured
against or that we believe are not economically reasonable to insure. Such losses could have a material adverse effect on our business
and results of operations.
Changes in laws and regulations relating
to beverage containers and packaging could increase our costs and reduce demand for our products.
We expect that our initial products will involve
non-refillable recyclable containers in the United States. Legal requirements have been enacted in various jurisdictions in the United
States and overseas requiring that deposits or certain eco-taxes or fees be charged in connection with the sale, marketing and use of
certain beverage containers. Other proposals relating to beverage container deposits, recycling, tethered bottle caps, eco-tax and/or
product stewardship have been introduced in various jurisdictions in the United States and overseas, and we anticipate that similar legislation
or regulations may be proposed in the future at local, state and federal levels, both in the United States and elsewhere. Consumers' increased
concerns and changing attitudes about solid waste streams and environmental responsibility and the related publicity could result in the
adoption of such legislation or regulations. If these types of requirements are adopted and implemented on a large scale in any of the
major markets in which we operate, they could affect our costs or require changes in our distribution model, which could reduce our net
operating revenues and profitability.
Significant additional labeling or warning
requirements or limitations on the marketing or sale of our products may inhibit sales of affected products.
Various jurisdictions may seek to adopt significant
additional product labeling or warning requirements or limitations on the marketing or sale of our products as a result of what they contain
or allegations that they cause adverse health effects. If these types of requirements become applicable to one or more of our major products
under current or future environmental or health laws or regulations, they may inhibit sales of such products.
For example, under one such law in California,
known as Proposition 65, if the state has determined that a substance causes cancer or harms human reproduction, a warning must be provided
for any product sold in the state that exposes consumers to that substance, unless the exposure falls under an established safe harbor
level. If we were required to add Proposition 65 warnings on the labels of one or more of our beverage products produced for sale in California,
the resulting consumer reaction to the warnings and possible adverse publicity could negatively affect our sales both in California and
in other markets.
We are subject to income taxes as well as
non-income-based taxes, such as payroll, sales, use, value-added, net worth, property and goods and services taxes, in the U.S.
Significant judgment is required in determining
our provision for income taxes and other tax liabilities. In the ordinary course of our business, there are many transactions and calculations
where the ultimate tax determination is uncertain. Although we believe that our tax estimates are reasonable: (i) there is no assurance
that the final determination of tax audits or tax disputes will not be different from what is reflected in our income tax provisions,
expense amounts for non-income-based taxes and accruals and (ii) any material differences could have an adverse effect on our financial
position and results of operations in the period or periods for which the determination is made.
We are not subject to Sarbanes-Oxley regulations
and lack the financial controls and safeguards required of public companies.
We do not have the internal infrastructure necessary,
and are not required, to complete an attestation about our financial controls that would be required under Section 404 of the Sarbanes-Oxley
Act of 2002. There can be no assurances that there are no significant deficiencies or material weaknesses in the quality of our financial
controls. We expect to incur additional expenses and diversion of management’s time when it becomes necessary to perform the system
and process evaluation, testing and remediation required to comply with the management certification and auditor attestation requirements.
Risks Related to this Offering and Ownership
of our Securities
The
outstanding shares of our Series B Preferred Stock represent potential significant future dilution in ownership of our Common Stock, including
the shares of Common Stock included in the Units and the Warrant Shares.
The
outstanding shares of Series B Preferred Stock are convertible, at any time, into six billion shares of our Common Stock. At such time
as these shares of Series B Preferred Stock are converted into shares of Common Stock, holders of our Common Stock, including the shares
of Common Stock included in the Units and the Warrant Shares, will incur significant dilution in their ownership of our company. The effect
of the conversion rights of the Special 2021 Series A Preferred Stock is that upon conversion, were such conversion to occur immediately
following the sale of all Offered Units and the issuance of all Warrant Shares, the then-holder(s) of the Series B Preferred Stock, as
a group, will be issued a number of shares of common stock equal to approximately 57% of the then-outstanding issued and outstanding shares
of our Common Stock, as measured after such conversion. We are unable to predict the effect that any such conversion event would have
on the market price of our Common Stock. (See “Dilution – Ownership Dilution”).
We have not engaged a third-party
bank or financial institution to act as escrow agent. Your funds will be deposited directly into our operating account. Since there is
no minimum amount required to be raised by us before we can accept funds, there is no guarantee that any funds other than your own will
be invested in this offering.
We have not currently engaged a third-party
bank or financial institution to act as escrow agent. Your funds will be placed in our general corporate bank account and immediately
available for our use. We are not required to raise any minimum amount in this offering before we may utilize the funds received in this
offering. Potential investors should be aware that there is no assurance that any monies beside their own will be invested in this offering.
We will be subject to penny stock
regulations and restrictions and you may have difficulty selling shares of our Common Stock.
The SEC has adopted regulations which generally
define so-called “penny stocks” to be an equity security that has a market price less than $5.00 per share or an exercise
price of less than $5.00 per share, subject to certain exemptions. We anticipate that our Common Stock will become a “penny
stock”, and we will become subject to Rule 15g-9 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”),
or the “Penny Stock Rule.” This rule imposes additional sales practice requirements on broker-dealers that sell such securities
to persons other than established customers. For transactions covered by Rule 15g-9, a broker-dealer must make a special suitability determination
for the purchaser and have received the purchaser’s written consent to the transaction prior to sale. As a result, this rule may
affect the ability of broker-dealers to sell our securities and may affect the ability of purchasers to sell any of our securities in
the secondary market.
For any transaction involving a penny stock, unless
exempt, the rules require delivery, prior to any transaction in a penny stock, of a disclosure schedule prepared by the SEC relating to
the penny stock market. Disclosure is also required to be made about sales commissions payable to both the broker-dealer and the registered
representative and current quotations for the securities. Finally, monthly statements are required to be sent disclosing recent price
information for the penny stock held in the account and information on the limited market in penny stock.
We do not anticipate that our Common Stock will
qualify for exemption from the Penny Stock Rule. In any event, even if our Common Stock were exempt from the Penny Stock Rule, we would
remain subject to Section 15(b)(6) of the Exchange Act, which gives the SEC the authority to restrict any person from participating in
a distribution of penny stock, if the SEC finds that such a restriction would be in the public interest.
This offering is being conducted on a self-underwritten
“best efforts” basis and we may not be able to execute our growth strategy if the $6 million maximum is not sold.
If you invest in the Common Stock and less than
all of the offered shares are sold, the risk of losing your entire investment will be increased. We are offering our Common Stock on a
self-underwritten “best efforts” basis, and we can give no assurance that all of the offered Common Stock will be sold. If
less than $6 million of Common Stock shares offered are sold, we may be unable to fund all the intended uses described in this PQA from the net proceeds anticipated from this offering without obtaining funds from alternative sources or using working capital
that we generate. Alternative sources of funding may not be available to us at what we consider to be a reasonable cost, and the working
capital generated by us may not be sufficient to fund any uses not financed by offering net proceeds. No assurance can be given to you
that any funds will be invested in this offering other than your own.
This is a fixed price offering and the fixed
offering price may not accurately represent the current value of us or our assets at any particular time. Therefore, the purchase price
you pay for our shares may not be supported by the value of our assets at the time of your purchase.
This is a fixed price offering, which means that
the offering price for our shares is fixed. Therefore, the fixed offering price established for our shares may not be supported by
the current value of our company or our assets at any particular time.
We may, in the
future, issue additional shares of Common Stock, which would reduce investors’ percent of ownership and may dilute our share value.
Our certificate of incorporation authorizes the
issuance of 10,000,000,000 shares of Common Stock and 20,000,000 shares of Preferred Stock. As of the date of this PQA,
we had 2,940,667,506 shares of Common Stock outstanding. The future issuance of Common Stock, including in this offering and including
the Warrant Shares, may result in substantial dilution in the percentage of our Common Stock held by our then existing shareholders. We
may value any Common Stock issued in the future on an arbitrary basis. The issuance of Common Stock for future services or acquisitions
or other corporate actions may have the effect of diluting the value of the shares held by our investors and might have an adverse effect
on any trading market for our Common Stock.
We have broad discretion in the use of the
net proceeds from this offering, and our use of the offering proceeds may not yield a favorable return on your investment.
We intend
to use the net proceeds of this offering for working capital. However, our management has broad discretion over how these proceeds
are to be used and based on unforeseen technical, commercial or regulatory issues could spend the proceeds in ways with which you may
not agree. Moreover, the proceeds may not be invested effectively or in a manner that yields a favorable or any return, and consequently,
this could result in financial losses that could have a material adverse effect on our business, financial condition and results of operations.
We have never paid cash dividends on our
stock and we do not intend to pay dividends for the foreseeable future.
We have paid no cash dividends on any class of
our stock to date and we do not anticipate paying cash dividends in the near term. For the foreseeable future, we intend to retain any
earnings to finance the development and expansion of our business, and we do not anticipate paying any cash dividends on our stock. Accordingly,
investors must be prepared to rely on sales of their shares after price appreciation to earn an investment return, which may never occur.
Investors seeking cash dividends should not purchase our shares. Any determination to pay dividends in the future will be made at the
discretion of our board of directors and will depend on our results of operations, financial condition, contractual restrictions, restrictions
imposed by applicable law and other factors our Board deems relevant.
Certain provisions of our certificate of
incorporation may make it more difficult for a third party to effect a change-of-control.
Our certificate of incorporation authorizes our
board of directors to issue up to 20,000,000 shares of Preferred Stock. The Preferred Stock may be issued in one or more series, the terms
of which may be determined at the time of issuance by our board of directors without further action by the stockholders. These terms may
include voting rights including the right to vote as a series on particular matters, preferences as to dividends and liquidation, conversion
rights, redemption rights and sinking fund provisions. The issuance of any Preferred Stock could diminish the rights of holders of existing
shares, and therefore could reduce the value of such shares.
In addition, specific rights granted to future
holders of Preferred Stock could be used to restrict our ability to merge with, or sell assets to, a third party. The ability of our board
of directors to issue Preferred Stock could make it more difficult, delay, discourage, prevent or make it costlier to acquire or effect
a change-in control, which in turn could prevent our stockholders from recognizing a gain in the event that a favorable offer is extended
and could materially and negatively affect the market price of our Common Stock.
Market for Warrants
There is currently no market through which
the Warrants may be sold. The Company does not plan to apply to list the Warrants on the Nasdaq Stock Market or other nationally recognized
trading system, including the QTCQB. There can be no assurance that an active or liquid trading market will develop for the Warrants
after the Offering, or if developed, that such a market will be sustained.
Holders of Warrants have no rights as
a shareholder.
Until a holder of Warrants acquires warrant
shares upon exercise of Warrants, such holder will have no rights with respect to the warrant shares underlying such Warrants. Upon exercise
of such Warrants, such holder will be entitled to exercise the rights of a common shareholder only as to matters for which the record
date occurs after the exercise date.
Risks Related to Controlled Substances
Risks Related to the Regulation of Our
Business and Products
We and our co-packers and suppliers
are subject to extensive governmental regulation and may be subject to enforcement if we are not in compliance with applicable requirements.
We and our co-packers and suppliers are subject
to a broad range of federal, state, and local laws and regulations that govern, among other issues, the testing, development, production,
distribution, marketing, and post-market reporting of foods, including those that contain CBD. These include laws administered by the
U.S. Food and Drug Administration (“FDA”), the U.S. Federal Trade Commission (“FTC”), the U.S. Department of
Agriculture (“USDA”), and other federal, state, and local regulatory authorities. Because we market products that are regulated
as food, we and the companies that co-pack our products are subject to the requirements of the Federal Food, Drug, and Cosmetic Act (“FDCA”)
and regulations promulgated thereunder by the FDA. The statute and regulations govern, among other things, the production, composition,
ingredients, packaging, labeling, and safety of beverages. The FDA requires that facilities that produce food products comply with a
range of requirements, including hazard analysis and preventative controls regulations, current good manufacturing practices (“cGMPs”),
and supplier verification requirements. Production facilities are subject to periodic inspection by federal, state, and local authorities.
If we cannot successfully contract with co-packers for our products and if they cannot conform to our specifications and the strict regulatory
requirements of the FDA and applicable state and local laws, they may be subject to adverse inspectional findings or enforcement actions,
which could materially impact our ability to market our products, could result in their inability to continue to co-pack for us, or could
result in a recall of our products that have already been distributed. If the FDA or other regulatory authority determines that we or
they have not complied with the applicable regulatory requirements, our business, financial condition, and results of operations may
be materially adversely impacted. If we do not comply with labeling requirements, including making unlawful claims about our products,
we could be subject to public warning letters and possible further enforcement actions (which other companies distributing CBD products
have faced).
Failure by us, our co-packers, or our suppliers
to comply with applicable laws and regulations or to obtain and maintain necessary permits, licenses, and registrations relating to our
operations could subject us to administrative and civil penalties, including fines, injunctions, recalls or seizures, warning letters,
restrictions on the production or marketing of our products, or refusals to permit the import or export of products, as well as potential
criminal sanctions, any or all of which could result in increased operating costs resulting in a material effect on our operating results
and business.
The FDA has stated that it interprets
the FDCA to prohibit the sale of food products that contain CBD. The FDA is currently evaluating a potential regulatory pathway for CBD
products pursuant to its current authority; but, unless and until such changes are promulgated, the FDA and other federal and state regulatory
authorities could take enforcement action to prevent us from marketing beverages with CBD, which could adversely impact our business,
financial condition, and results of operations or cause us to halt product sales altogether.
Although hemp and CBD are no longer controlled
substances subject to regulation by the U.S. Drug Enforcement Agency (the “DEA”), the FDA has stated publicly that it is
nonetheless unlawful under the FDCA to introduce food containing CBD into interstate commerce. The FDCA prohibits the introduction or
delivery for introduction into interstate commerce of any food that contains an approved drug or a drug for which substantial clinical
investigations have been instituted and made public, unless a statutory exemption applies. The FDA has publicly stated its conclusion
that none of the statutory exceptions has been met for CBD.
On May 31, 2019, the FDA held a public hearing
to obtain scientific data and information about the safety, production, product quality, marketing, labeling, and sale of products containing
cannabis or cannabis-derived compounds (such as CBD) to provide the FDA with information as it considers policy options related to the
regulation of these products, particularly in light of the changes to the legal status of hemp enacted in the Agriculture Improvement
Act of 2018 (the “2018 Farm Bill”). The FDA has also formed an internal working group to evaluate the potential pathways
to market for CBD products, which could include seeking statutory changes from Congress or promulgating new regulations. If legislative
action is necessary, such legislative changes could take years to finalize and may not include provisions that would enable us to produce,
market, and/or sell our CBD products, and the FDA could similarly take years to promulgate new regulations. Additionally, while the FDA’s
enforcement focus to date has primarily been on CBD products that are associated with therapeutic claims, the agency has recently issued
warning letters to companies that market CBD products without such claims. There is an unquantifiable risk that the FDA could take enforcement
action against us, our co-packers, or our suppliers, or those marketing similar products to us, which could limit or prevent us from
marketing our products and have a material adverse impact on our business, financial condition, and results of operations. While the
FDA announced on March 5, 2020 that it is currently evaluating a risk-based enforcement policy for CBD to provide more clarity to the
industry and the public while the agency takes potential steps to establish a clear regulatory pathway, it remains unclear whether or
when the FDA will ultimately issue such an enforcement policy.
Moreover, local, state, federal, and international
CBD, hemp, and cannabis laws and regulations are rapidly changing and subject to evolving interpretations, which could require us to
incur substantial costs associated with compliance requirements or alteration of certain aspects of our business plan in the event that
our CBD products become subject to new restrictions. In addition, violations of these laws, or allegations of such violations, could
disrupt our business and result in a material adverse effect on our operations. It is also possible that regulations may be enacted in
the future that will be directly applicable to our products. We cannot predict the nature of any future laws, regulations, interpretations,
or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures, when
and if promulgated, could have on our activities in the hemp and CBD industry. The constant evolution of laws and regulations may require
us to incur substantial costs associated with legal and compliance fees and ultimately require us to alter our business plan.
Our products contain CBD derived from
hemp. The 2018 Farm Bill enacted a number of changes to the legal status of hemp and hemp products, including removal from the statutory
list of controlled substances. However, implementation of the 2018 Farm Bill is ongoing, and there is still significant uncertainty regarding
the legal status of hemp and hemp-based products under U.S. law.
Our products that contain CBD are subject
to various state and federal laws regarding the production and sale of hemp-based products. Historically, the DEA had interpreted CBD
to be subject to the Controlled Substances Act (the “CSA”) under the definition for “marijuana,” a Schedule I
controlled substance. However, the 2018 Farm Bill removed “hemp,” from the definition of “marijuana.” “Hemp”
is defined as the plant, Cannabis sativa L., and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids,
isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol (“THC”) concentration
of not more than 0.3% on a dry weight basis. As a result of the enactment of the 2018 Farm Bill, and since we believe that the CBD contained
in our products and the hemp from which it is derived meet the definition of “hemp,” we believe that our CBD products and
the hemp from which they are derived are not Schedule I controlled substances under the CSA. However, there is a risk that we could be
subject to enforcement action, including prosecution, if any of our products are determined not to meet the definition of “hemp”
and to constitute “marijuana” under the CSA based on THC levels or other violations, which would have a negative impact on
our business and operations.
In addition, the 2018 Farm Bill contained
provisions that require the USDA, among other things, to promulgate a new regulatory framework that will govern the growth and cultivation
of hemp, where hemp grown in compliance with the framework would be permitted in interstate commerce throughout the United States. On
October 31, 2019, the USDA issued an interim final rule (“IFR”) establishing the regulations necessary for domestic hemp
production, including provisions for the USDA to approve plans submitted by states and Native American tribes for the monitoring and
regulation of hemp production at the state level. Effective March 22, 2021, The USDA issued the final rule, which creates the U.S. Domestic
Hemp Production Program. The program provides requirements for maintaining records about the land where hemp is produced, testing the
levels of total delta-9 tetrahydrocannabinol, disposing of non-compliant plants, licensing hemp producers, and ensuring compliance under
the new program. The Agricultural Marketing Service (AMS), has been delegated authority to administer the U.S. Domestic Hemp Production
Program. While the 2018 Farm Bill requires state and tribal plans to meet certain basic requirements as outlined in the IFR, nothing
preempts or limits state or tribal laws that are more stringent than the 2018 Farm Bill, and the requirements for lawful hemp production
will vary from state to state. We and our co-packers and suppliers must expend resources to monitor the evolving federal and state legal
landscape for hemp production, and any violations of these laws, or alleged violations, could disrupt our business and result in a material
adverse effect on our operations.
Within the United States, we and our
co-packers and suppliers face a variety of state and local restrictions on the cultivation of hemp, and if state or local regulatory
authorities take enforcement action to prevent us from selling our products, our business, financial condition, and results of operations
could be materially adversely impacted.
The growth and cultivation of hemp is subject
to a complex regulatory framework that is implemented and affected by multiple federal agencies, as well as state and local authorities.
In 2014, four years prior to enacting the 2018 Farm Bill, Congress enacted the Agricultural Act of 2014 (the “2014 Farm Bill”)
to allow for the limited growth and cultivation of industrial hemp under federal law. This statute allowed institutions of higher education
and state departments of agriculture to grow and cultivate industrial hemp for agricultural or other academic research purposes, or for
hemp to be grown under the auspices of a state agricultural pilot program, in states where such growth and cultivation is legal under
state law. While the 2014 Farm Bill will be repealed after October 31, 2020, and although the 2018 Farm Bill created a pathway under
which hemp and its derivatives, including CBD, would no longer be a Schedule I controlled substance under the CSA and would be protected
from interference in interstate commerce, the USDA only recently issued the IFR that contains the regulatory framework that will govern
the growth and cultivation of hemp, and, currently, several states continue to operate under the 2014 Farm Bill. Alongside the current
federal regulatory developments, state and local authorities have enacted their own restrictions on the cultivation or sale of hemp or
hemp-derived CBD, including laws that ban the cultivation or possession of hemp or any other plant of the cannabis genus and derivatives
thereof, such as CBD. Currently, several states ban the cultivation and possession of hemp or CBD, while others have taken enforcement
action against human food products that contain CBD, and states may enact new laws or regulations that prohibit or limit the sale of
such products at any time. In the event of a change in federal or state laws and regulations that are adverse to our CBD products, we
may be restricted or limited with respect to sale or distribution of those products, which could adversely impact our intended business
plan with respect to such products.
The USDA has only recently issued the IFR
and started accepting state and tribal hemp production plans for review, and it remains to be seen which states will submit their own
regulatory plans for the cultivation of hemp and which will become subject to the USDA framework. The timing and content of state regulatory
plans may impact our ability to obtain sufficient quantities of CBD at an acceptable price and on a timely basis. If our current co-packers
and suppliers were to face increased regulation or be unable to continue to supply our business, we may be unable to fulfill orders for
our products or find a suitable replacement co-packers and suppliers in a timely fashion or with comparable pricing. If our current co-packers
or suppliers or any future co-packers or suppliers fail to comply with the applicable regulatory requirements, our business may suffer.
Changes in existing laws or regulations,
including how such existing laws or regulations are enforced by federal, state, and local authorities, or the adoption of new laws or
regulations may increase our costs and otherwise adversely affect our business, financial condition, and results of operations.
In addition to the legal framework applicable
to hemp and CBD, the production and marketing of food products is highly regulated, and we and our co-packers and suppliers are subject
to a variety of federal and state laws and regulations applicable to food. These laws and regulations apply to many aspects of our business,
including the co-packing, packaging, labeling, distribution, advertising, sale, quality, and safety of our products. We could incur costs,
including fines, penalties, and third-party claims, in the event of any violations of, or liabilities under, such requirements, including
any competitor or consumer challenges relating to compliance with such requirements. For example, in connection with the marketing and
advertising of our products, we could be the target of claims relating to false or deceptive advertising, including under the auspices
of the FTC and state consumer protection statutes.
The regulatory environment in which we operate
could change significantly and adversely in the future. The laws and regulations that apply to our products and business may change in
the future and we may incur (directly or indirectly through our co-packers or suppliers) material costs to comply with current or future
laws and regulations or any required product recalls. Any change in production, labeling, or marketing requirements for our products
may lead to an increase in costs or interruptions in our production or raw material supply, either or both of which could adversely affect
our operations and financial condition. For example, recent federal and state attention to the sale of CBD-containing products, and specifically
food products that contain CBD, could result in standards or requirements that mandate changes to our current product ingredients, labeling,
or marketing. New or revised government laws and regulations could significantly limit our ability to operate our business as it is currently
being conducted, result in additional compliance costs, and, in the event of noncompliance, could lead to administrative or civil remedies,
including fines, injunctions, withdrawals, recalls, or seizures and confiscations, as well as potential criminal sanctions. Any such
changes or actions by the FDA or other regulatory agencies could have a material adverse effect on our co-packers, our suppliers, and
our business, financial condition, and results of operations.
Government scrutiny, warnings, and public
perception could increase our costs of production and increase our legal and regulatory expenses, and if we are unable to comply with
the applicable requirements for marketing beverages, we could face substantial civil and criminal penalties.
Producing, processing, labeling, packaging,
storing, and distributing food products are activities that are subject to extensive federal, state, and local regulation. In the United
States, these operations are regulated by the FDA and various state and local public health and agricultural agencies. The FDA Food Safety
Modernization Act of 2011 provides direct recall authority to the FDA for food products and includes a number of other provisions that
are designed to enhance food safety, including increased inspections by the FDA of domestic food facilities. Compliance with government
regulation can be costly or may otherwise adversely affect our business. Moreover, failure to comply with applicable laws and regulations
could subject us to civil remedies, including fines, injunctions, recalls, or seizures, as well as potential criminal sanctions, which
could in turn have a material adverse effect on our business, financial condition, and results of operations.
We operate in a highly regulated environment
with constantly evolving legal and regulatory frameworks. Consequently, we are subject to heightened risk of legal claims, government
investigations, or regulatory enforcement actions. Although we have implemented policies and procedures designed to ensure compliance
with existing laws and regulations, there can be no assurance that our employees, temporary workers, contractors, or agents might not
violate our policies and procedures. Moreover, a failure to maintain effective regulatory compliance policies and procedures could lead
to violations, unintentional or otherwise, of laws and regulations. Legal claims, government investigations, or regulatory enforcement
actions arising out of our failure or alleged failure to comply with applicable laws and regulations could subject us to civil and criminal
penalties that could materially and adversely affect our product sales, reputation, financial condition, and operating results. In addition,
the costs and other effects of defending potential and pending litigation and administrative actions against us may be difficult to determine
and could materially adversely affect our business, financial condition, and results of operations.
Because there has been limited study
on the effects of CBD, future nonclinical and clinical research studies and analysis of such studies by third parties, including government
agencies, may lead to conclusions that dispute or conflict with our understandings and beliefs regarding the benefits, viability, safety,
dosing, and social acceptance of CBD.
Research in the United States and internationally
regarding the benefits, viability, safety, and dosing of isolated cannabinoids (such as CBD or THC) remains in relatively early stages.
There have been few clinical trials on the benefits of CBD conducted on humans or animals, including studies focused on the consumption
of CBD in foods.
Future research and clinical trials may draw
opposing conclusions to statements contained in current articles, reports, and studies regarding CBD or could reach different or negative
conclusions regarding the medical benefits, viability, safety, dosing, or other facts and perceptions related to CBD, which could adversely
affect acceptance of CBD in foods and the demand for such products. Future research may also cause regulatory authorities to change how
they enforce regulatory restrictions applicable to hemp and CBD. We cannot predict any negative research and clinical trial findings
in the future that may have a material adverse impact on our business, financial condition, and results of operation.
Negative publicity from being in the
hemp and CBD space could have a material adverse effect on our business, financial condition, and results of operations.
Hemp and marijuana are both varieties of the
plant, Cannabis sativa L., except that hemp, as defined by federal law for exemption from Schedule I of the CSA, has a delta-9 THC concentration
of not more than 0.3% on a dry weight basis. The same plant with a higher THC content is considered to be marijuana, which is legal for
medical and recreational use under certain state laws, but which is not legal under federal law. The similarities between these plants
can cause confusion, and our activities with hemp may be incorrectly perceived that we are involved in federally illegal marijuana activities.
Further, despite growing support for the cannabis
industry and the legalization of marijuana in certain U.S. states, many individuals and businesses remain opposed to the cultivation
and sale of cannabis and cannabis-derived products. Any negative publicity resulting from an incorrect perception that we operate in
the marijuana space could result in a loss of current or future business. It could also adversely affect the public’s perception
of us or our Common Stock and could lead to reluctance by new parties to do business with or to invest in us. We cannot assure you that
additional business partners, including, but not limited to, financial institutions and distributors and resellers, will not attempt
to end or curtail their relationships with us. Any such negative press or impacts to our business relationships could have a material
adverse effect on our business, financial condition, and results of operations.
Our ability to deduct certain business
expenses for income tax purposes is subject to uncertainty.
Section 280E of the Internal Revenue Code
of 1986, as amended (the “Code”), prohibits the deduction of certain otherwise ordinary business expenses from carrying on
any trade or business that consists of “trafficking” Schedule I or II controlled substances, as defined by the CSA. Under
existing Internal Revenue Service guidance, the bulk of operating costs and general administrative costs of trades or businesses that
are subject to Section 280E of the Code are not permitted to be deducted. Although the 2018 Farm Bill created a pathway under which hemp
and its derivatives, including CBD, would no longer be a Schedule I controlled substance under the CSA, until the USDA implements regulations
pursuant to the 2018 Farm Bill, we believe our ability to deduct certain ordinary business expenses requires compliance with the 2014
Farm Bill. We do not believe that Section 280E of the Code currently forbids our deduction of otherwise ordinary business expenses because
we believe that we are in compliance with the 2014 Farm Bill and/or the products we sell are from co-packers and suppliers that are compliant
with the 2014 Farm Bill. However, until the USDA promulgates regulations under the 2018 Farm Bill, governmentally determined non-compliance
with the 2014 Farm Bill by us, our co-packers, or their suppliers may have a material adverse tax effect on us.
DILUTION
Ownership Dilution
The information under “Investment Dilution”
below does not take into account the potential conversion of the outstanding shares of Series B Preferred Stock into 600,000,000 shares
of Common Stock. These shares of Series B Preferred Stock may be converted into shares of Common Stock at any time.
The conversion of the outstanding shares of Series
B Preferred Stock into shares of Common Stock would cause holders of our Common Stock, including the shares of Common Stock included in
the Offered Units and the Warrant Shares, to incur significant dilution in their ownership of our company. (See “Risk Factors,”
“Description of Securities” and “Security Ownership of Management and Certain Securityholders”).
Investment Dilution
Dilution in net tangible book
value per share to purchasers of our Common Stock in this offering represents the difference between the amount per share paid by purchasers
of the Offered Units in this offering for the shares of Common Stock included in the Units and the shares of Common Stock underlying the
Warrants included in the Units (the Warrant Shares), and the net tangible book value per share immediately after completion of this offering.
In this offering, dilution is attributable primarily to the offering price of the Common Stock included in the Units and the exercise
price of the Warrants being higher than our net tangible book value per share.
If you purchase Offered Units
in this offering, your investment in the shares of Common Stock included in the Units and the shares of Common Stock underlying the Warrants
included in the Units (the Warrant Shares) (the exercise of which is assumed in this section) will be diluted to the extent of the difference
between your purchase price per share of Common Stock and the net tangible book value of our Common Stock after this offering. Our net
tangible book value as of July 31, 2024, was $3,430,855 (unaudited), or $0012 per share. Net tangible book value per share is equal to
total assets minus the sum of total liabilities and intangible assets divided by the total number of shares outstanding.
The tables below illustrate
the dilution (relative to the Common Stock) to purchasers of the Remaining Units in this offering, on a pro forma basis, assuming (A)
100%, 75%, 50% and 25% of the Remaining Units are sold at a per Unit price of $0.002 per Unit (a $0.00067 per share price) and (B)
all of the Warrants included in the Offered Units are exercised.
| Assuming the Sale of 100% of the Remaining Units |
|
| Assumed offering price per share included in the Units |
$0.002 |
| Net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0012 |
| Increase in net tangible book value per share after giving effect to this offering |
$0.0022 |
| Pro forma net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0034 |
| Dilution in net tangible book value per share to purchasers included in the Units in this offering |
$0.0000 |
| |
|
| Assuming the Sale of 75% of the Remaining Units |
|
| Assumed offering price per share included in the Units |
$0.002 |
| Net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0012 |
| Increase in net tangible book value per share after giving effect to this offering |
$0.0019 |
| Pro forma net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0031 |
| Dilution in net tangible book value per share to purchasers included in the Units in this offering |
$0.0000 |
| |
|
| Assuming the Sale of 50% of the Remaining Units |
|
| Assumed offering price per share included in the Units |
$0.002 |
| Net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0012 |
| Increase in net tangible book value per share after giving effect to this offering |
$0.0015 |
| Pro forma net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0027 |
| Dilution in net tangible book value per share to purchasers included in the Units in this offering |
$0.0000 |
| |
|
| Assuming the Sale of 25% of the Remaining Units |
|
| Assumed offering price per share included in the Units |
$0.002 |
| Net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0012 |
| Increase in net tangible book value per share after giving effect to this offering |
$0.0009 |
| Pro forma net tangible book value per share as of July 31, 2024 (unaudited) |
$0.0021 |
| Dilution in net tangible book value per share to purchasers included in the Units in this offering |
$0.0000 |
PLAN OF DISTRIBUTION
This offering relates to the sale of 700,000,000
Offered Units, including the 424,666,667 Remaining Units at an offering price of $0.002 per Offered Unit. Each Offered Unit is
comprised of three shares of common stock, par value $0.0001 and two warrants with an exercise price of $0.01.
Initially, we do not plan to use underwriters
or pay any commissions. We will be selling our Units using our best efforts and no one has agreed to buy any of our Units. This PQA permits
our CEO to sell Offered Units directly to the public, with no commission or other remuneration payable to him for any Offered Units he
may sell. Currently, there is no plan or arrangement to enter into any contracts or agreements to sell the Offered Units through a broker
or dealer, although this may change in the future. Our CEO will sell Offered Units, and intends to offer them to friends, family members
and business acquaintances.
There is no minimum number of Offered Units we
must sell so no money raised from the sale of our Offered Units will go into escrow, trust or another similar arrangement.
We are offering securities in the states of New
York and Indiana. We may decide to offer securities in other states in the future, in our sole discretion.
Initially, the Offered Units are being offered
by Mr. Leonard Greene, the company’s Chief Executive Officer and Mr. Greene will be relying on the safe harbor in Rule 3a4-1 of
the Securities Exchange Act to sell the Offered Units. No sales commission will be paid for Offered Units sold by Mr. Greene. Mr. Greene
is not subject to a statutory disqualification and is not an associated person of a broker or dealer.
Additionally, Mr. Greene primarily performs substantial
duties on behalf of the registrant otherwise than in connection with transactions in securities. Mr. Greene has not been a broker or dealer
or an associated person of a broker or dealer within the preceding 12 months and he has not participated in selling an offering of securities
for any issuer more than once every 12 months other than in reliance on paragraph (a)4(i) or (a)4(iii) of Rule 3a4-1 of the Securities
Exchange Act.
Under the rules of the SEC, our Common Stock is within the definition
of a “penny stock” because the price of our Common Stock is below $5 per share. As a result, our Common Stock will be subject
to the “penny stock” rules and regulations. Broker-dealers who sell penny stocks to certain types of investors are required
to comply with the SEC’s regulations concerning the transfer of penny stock. These regulations require broker-dealers to:
| |
· |
Make a suitability determination prior to selling penny stock to the purchaser; |
| |
|
|
| |
· |
Receive the purchaser’s written consent to the transaction; and |
| |
|
|
| |
· |
Provide certain written disclosures to the purchaser. |
These requirements may restrict the ability
of broker-dealers to sell our Units, and may affect the ability to resell our Units.
OTC Market Considerations
Our Common Stock is eligible for quotation on the OTC Pink Market under
the symbol “INKW.”
The OTC Pink Market is separate and distinct from
the NASDAQ stock market. NASDAQ has no business relationship with issuers of securities quoted on the OTC Pink Market. The SEC’s
order handling rules, which apply to NASDAQ-listed securities, do not apply to securities quoted on the OTC Pink Market.
Although the NASDAQ stock market has rigorous
listing standards to ensure the high quality of its issuers, and can delist issuers for not meeting those standards, the OTC Pink Market
has no listing standards. Rather, it is the market maker who chooses to quote a security on the system, files the application, and is
obligated to comply with keeping information about the issuer in its files. FINRA cannot deny an application by a market maker to quote
the stock of a company. The only requirement for inclusion in this marketplace is that the issuer be current in its reporting requirements
with the SEC or its alternative reporting requirements with OTC Markets Group.
Investors may have greater difficulty in getting
orders filled on the OTC Pink Market. Investors’ orders may be filled at a price much different than expected when an order is placed.
Trading activity in general is not conducted as efficiently and effectively as with NASDAQ-listed securities.
Investors must contact a broker-dealer to trade OTC Pink Market securities.
Investors do not have direct access to the marketplace’s service.
For these securities, there only has to be one market maker.
These transactions are conducted almost entirely
manually. In times of heavy market volume, the limitations of this process may result in a significant increase in the time it takes to
execute investor orders. Therefore, when investors place market orders - an order to buy or sell a specific number of shares at the current
market price - it is possible for the price of a stock to go up or down significantly during the lapse of time between placing a market
order and getting execution.
Because these stocks are usually not followed by analysts, there may
be lower trading volume than for NASDAQ-listed securities.
Blue Sky Law Considerations
The holders of our Common Stock and persons
who desire to purchase them in the OTC Pink Market should be aware that there may be significant state law restrictions upon the
ability of investors to resell our securities. Accordingly, investors should consider any secondary market for the company's
securities to be a limited one. We intend to seek coverage and publication of information regarding the company in an accepted
publication which permits a "manual exemption”. This manual exemption permits a security to be distributed in a
particular state without being registered if the company issuing the security has a listing for that security in a securities manual
recognized by the state. However, it is not enough for the security to be listed in a recognized manual. The listing entry must
contain (1) the names of issuers, officers, and directors, (2) an issuer's balance sheet, and (3) a profit and loss statement for
either the fiscal year preceding the balance sheet or for the most recent fiscal year of operations. We may not be able to secure a
listing containing all of this information. Furthermore, the manual exemption is a non-issuer exemption restricted to secondary
trading transactions, making it unavailable for issuers selling newly issued securities. Most of the accepted manuals are those
published in Standard and Poor's, Moody's Investor Service, Fitch's Investment Service, and Best's Insurance Reports, and many
states expressly recognize these manuals. A smaller number of states declare that they “recognize securities manuals”
but do not specify the recognized manuals. The following states do not have any provisions and therefore do not expressly recognize
the manual exemption: AL, CA, IL, KY, LA, MT, NH, NY, PA, TN and VA.
We currently do not intend to and may not be able
to qualify securities for resale in other states which require shares to be qualified before they can be resold by our shareholders.
Offering Period and Expiration Date
This offering commenced on April 3, 2023, and
will terminate at the earlier of: (1) the date on which all of the Remaining Shares have been sold, (2) October 11, 2025, or (3) the date
on which this offering is earlier terminated by us in our sole discretion.
Procedures for Subscribing
General
If you decide to subscribe for any Units in this offering, you
must:
| |
1. |
Receive, review, execute and deliver to us a Subscription Agreement; and |
| |
2. |
Deliver a check or wire transfer (in accordance with the instructions contained in the Subscription Agreement) for the amount set forth in the Subscription Agreement. |
You must pay for the Units at the time of
your subscription. Subscription agreements may be submitted in paper form, or electronically by email or other means made available to
investors by us. All checks should be made payable to Greene Concepts, Inc. Completed subscription agreements should be sent to us at
the address set forth in the subscription agreement and payments should be sent to us or wired according to the instructions in the subscription
agreement.
Any potential investor will have ample time to
review the Subscription Agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such
Subscription Documents upon request after a potential investor has had ample opportunity to review this PQA. Further, we will not accept
any money until the SEC declares this PQA qualified.
Right to Reject Subscriptions. After
we receive your complete, executed subscription agreement, we have the right to review and accept or reject your subscription in whole
or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest
or deduction.
Acceptance of Subscriptions. Upon
our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the shares subscribed. Once you submit
the subscription agreement, you may not revoke or change your subscription or request your subscription funds. All accepted subscription
agreements are irrevocable.
Under Rule 251 of Regulation A, non-accredited,
non-natural investors are subject to the investment limitation and may only invest funds which do not exceed 10% of the greater of the
purchaser’s revenue or net assets (as of the purchaser’s most recent fiscal year end). As a result, a non-accredited, natural
person may only invest funds which do not exceed 10% of the greater of the purchaser’s annual income or net worth (please see below
on how to calculate your net worth).
How to Calculate Net Worth. For
the purposes of calculating your net worth, it is defined as the difference between total assets and total liabilities. This calculation
must exclude the value of your primary residence and may exclude any indebtedness secured by your primary residence (up to an amount equal
to the value of your primary residence). In the case of fiduciary accounts, net worth and/or income suitability requirements may be satisfied
by the beneficiary of the account or by the fiduciary, if the fiduciary directly or indirectly provides funds for the purchase of the
shares in this offering.
In order to purchase the shares in this offering
and prior to the acceptance of any funds from an investor, an investor will be required to represent, to our satisfaction, that he is
either an accredited investor or is in compliance with the 10% of net worth or annual income limitation on investment in this offering.
USE
OF PROCEEDS
As of the date of this PQA, we have sold a total
of 275,333,333 Offered Units, for an aggregate of $1,112,000 in proceeds. We have applied such proceeds for business expansion efforts, administrative
expenses and working capital.
The
table below sets forth the manner in which we intend to apply the net proceeds (after deducting
$100,000 in estimated offered expenses) derived by us in this offering, assuming (a) the
sale of 25%, 50%, 75% and 100% of the Remaining Units are sold at a per Unit price of
$0.002 (a $0.00067 per share price) and (b) the exercise of all of the Warrants included
in all of the Offered Units (a total of 1,400,000,000 Warrant Shares at an exercise price
of $0.01 per share). All amounts set forth below are estimates.
| |
|
25% Remaining Units Sold and Included Warrants Exercised |
|
|
50% Remaining Units Sold and Included Warrants Exercised |
|
|
75% Remaining Units Sold and Included Warrants Exercised |
|
|
100% Remaining Units Sold and Included Warrants Exercised |
|
| Engineering and Prototyping |
|
$ |
300,000 |
|
|
$ |
600,000 |
|
|
$ |
1,000,000 |
|
|
$ |
1,400,000 |
|
| Marketing |
|
|
200,000 |
|
|
|
400,000 |
|
|
|
700,000 |
|
|
|
1,000,000 |
|
| Legal & Accounting |
|
|
200,000 |
|
|
|
400,000 |
|
|
|
500,000 |
|
|
|
500,000 |
|
| Production and Inventory |
|
|
900,000 |
|
|
|
1,900,000 |
|
|
|
3,000,000 |
|
|
|
4,000,000 |
|
| Administrative and Corporate Expenses |
|
|
100,000 |
|
|
|
200,000 |
|
|
|
300,000 |
|
|
|
400,000 |
|
| Professional Fees and Compensation |
|
|
200,000 |
|
|
|
400,000 |
|
|
|
400,000 |
|
|
|
600,000 |
|
| Working Capital Reserves |
|
|
435,666 |
|
|
|
771,333 |
|
|
|
1,106,000 |
|
|
|
1,842,666 |
|
| TOTAL |
|
$ |
2,335,666 |
|
|
$ |
4,671,333 |
|
|
$ |
7,006,000 |
|
|
$ |
9,342,666 |
|
The expected use of net proceeds from this
offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans
and business conditions evolve and change. The amounts and timing of our actual expenditures, specifically with respect to working capital,
may vary significantly depending on numerous factors. The precise amounts that we will devote to each of the foregoing items, and the
timing of expenditures, will vary depending on numerous factors. As a result, our management will retain broad discretion over the allocation
of the net proceeds from this offering.
In the event we do not sell all of the shares
being offered, we may seek additional financing from other sources in order to support the intended use of proceeds indicated above.
If we secure additional equity funding, investors in this offering would be diluted. In all events, there can be no assurance that additional
financing would be available to us when wanted or needed and, if available, on terms acceptable to us.
The Company reserves the right to change
the use of proceeds set out herein based on the needs of the ongoing business of the Company and the discretion of the Company’s
management. The Company may reallocate the estimated use of proceeds among the various categories or for other uses if management deems
such a reallocation to be appropriate.
DESCRIPTION OF BUSINESS
Our Company
Our company name is Greene Concepts, Inc. We are
headquartered in Marion, North Carolina. We are a New York corporation that was incorporated on August 18, 1952 and previously operated
as Tech-OHM Resistor Corporation, Tech-OHM Electronics, Inc., International Citrus Corporation, Princeton Commercial Holdings, Inc., Eurowind
Energy, Inc., First Petroleum and Pipeline Inc., and Luke Entertainment, Inc. Since our inception, we have operated different businesses
under these different names before changing our name to Greene Concepts, Inc. and engaging in our current business line. Through our wholly-owned
subsidiary, Mammoth Ventures Inc., or Mammoth, we are now a bottling and beverage company committed to providing the world with high quality,
healthy, and enhanced beverage choices. Our beverage and bottling facility is located in Marion, North Carolina. The facility is a 55,000
square foot bottling and beverage plant that is located within the boundaries of the Pisgah National Forest. The bottling facility has
as its water sources a combination of seven (7) spring and artesian wells that are fed from a natural aquifer that is located deep below
the Pisgah National Forest. We are focused on producing spring and artesian water. Additionally, we expect that Mammoth will act as a
third-party producer and bottler of "white label" beverage and water products. White label bottling services are provided for
clients that desire to market their own product formulations, brand name and labeling while outsourcing the production and bottling of
their products to Mammoth.
Before acquiring Mammoth on February 6, 2019,
we operated our legacy business, which was the manufacture and distribution of a line of 25 high quality consumer focused inkjet kits.
On April 30, 2019, our board of directors made a determination to wind down our legacy business and to transition into the beverage and
bottling business.
On February 6, 2019, we entered into a Stock Purchase
Acquisition Agreement and Merger Agreement and Promissory Note Agreement with BNL Capital LLC, or BNL Capital. Pursuant to the terms of
the agreement, BNL Capital agreed to sell 100% of the outstanding shares of Mammoth to us for a purchase price of $1,350,000. Mammoth
acquired certain assets of the defunct business formerly referred to as “North Cove Springs Bottling and Beverage,” which
includes the Marion, North Carolina bottling facility and related assets. We financed the acquisition through a secured promissory note
in the amount of $1,350,000 in favor of BNL Capital. The promissory note was secured by 100% of the outstanding shares of Mammoth that
are owned by our company. See “Description of Business – Terms of Acquisition of Mammoth Ventures, Inc.” for a description
of the terms of our acquisition of Mammoth.
On March 5, 2021, the Company paid $200,000 to
BNL Capital to satisfy the remaining obligations owed to BNL Capital. BNL Capital also cancelled 7,500,000 shares of Series A Preferred
Stock of the Company owned by BNL Capital.
Upon acquiring Mammoth, we began the process of
performing required maintenance to revitalize all the equipment and facility infrastructure in order to relaunch production at the plant.
At the time of the acquisition all of the plant equipment was in good condition although the equipment had not operated for several years
and it did require a thorough inspection and light maintenance to assure proper operation when the bottling lines are relaunched. At the
time of the acquisition, we hired, Kenneth Porter, a 30+ year veteran of the beverage and bottling industry, as plant manager to oversee
operations as well as the revitalization and expected relaunch of the facility.
The Food and Drug Administration, or FDA, requires
adherence to current good manufacturing practice (“CGMP”) regulations for the processing and bottling of bottled drinking
water, which includes facility inspection and documentation of corrective measures and reporting requirements, as well as new requirements
for hazard assessments and food safety(“ HACCP”) plans mandated by the Food Safety and Modernization Act (“FSMA”).
The Company’s facility has successfully passed all required inspections.
In addition to complete cleaning and maintenance
of the 55,000 square foot facility, standard operating policies and procedures must be documented in accordance with federal legislation.
This documentation includes conducting and reporting of microbial testing of source water and any finished product, which must be completed
prior to initiating filling and packaging of bottles for shipment from our production lines.
The plant facility became fully operational on
April 6, 2020.
Terms of Acquisition of Mammoth Ventures, Inc.
On November 29, 2018, Mammoth was incorporated
by BNL Capital, whose principals are Robert Levit and Loren Brown in the State of Florida for the purpose of acquiring the North Carolina
Facility from North Cove Springs Bottling and Beverage, Inc. and its owner Chris Mencis, or North Cove. The acquisition of the assets
constituting the North Carolina Facility by Mammoth closed on February 5, 2019. For at least six years prior to the acquisition of the
assets of the North Carolina Facility, North Cove had no operations whatsoever, had no employees and generated no revenue from any source.
The North Carolina Facility was dormant during that entire period. Thereafter, on the next day, February 6, 2019, we acquired all of the
issued and outstanding capital stock of Mammoth from BNL Capital. Mammoth had no operations other than the ownership of the assets constituting
the North Carolina Facility, which did not operate, from the date of its acquisition of the North Carolina Facility on February 5, 2019
to the date that BNL Capital sold all of the outstanding capital stock of Mammoth to the Company on the next day, February 6, 2019.
We acquired the North Carolina Facility pursuant
to a Stock Purchase Acquisition Agreement and Merger Agreement and Promissory Note Agreement with BNL Capital. Pursuant to the terms of
the agreement, BNL Capital agreed to sell 100% of the outstanding shares of Mammoth to us for a purchase price of $1,350,000. We financed
the acquisition through a secured promissory note in the amount of $1,350,000 in favor of BNL Capital. The promissory note was secured
by 100% of the outstanding shares of Mammoth that are owned by our company. Payments under the note are due in 60 monthly installments
of $5,062.50 each. The first 59 months of the repayment period are interest only. On the 60th month of the repayment period, the entire
remaining outstanding principal balance will become due. As an inducement for BNL Capital entering into the agreement, we also issued
2,000,000 shares of Series A Preferred Stock to BNL Capital.
The Company considered the provisions of FASB
Accounting Standards Codification Topic 805, Business Combinations, or Topic 805, to determine whether an acquisition of all of the outstanding
capital stock of Mammoth should be treated as business combination or an asset acquisition in accordance with GAAP. If the acquisition
of Mammoth does not meet the definition of a “business” under Topic 805, the Company accounts for the transaction as an asset
acquisition rather than a business combination. On the other hand, if the acquisition of Mammoth meets the definition of a business under
Topic 805, the Company applies the acquisition method of accounting for a business combination as provided for in Topic 805. The Company
concluded that the acquisition of the capital stock of Mammoth from BNL Capital does not represent the acquisition of a business and that
the Company is not required to include financial statements or pro forma financial information for Mammoth/North Cove in accordance with
Rule 11-01 of Regulation S-X.
On February 17, 2021, Monmouth announced that
it has paid off all mortgage liens and obligations for the building, equipment and property of the Marion, North Carolina facility and
has received a certificate of satisfaction from the lien holder with a payment of $719,823.56.
Agreements with Camping World, Gander Outdoors
and Overton’s
On or about April 15, 2021, the Company entered
into a Participation Agreement, Vendor Information Form and New Store Contribution Agreement (together, the “CWI Agreements”)
with CWI, Inc. The CWI Agreements will make the Company’s Be Water available at over 175 Camping World retail locations covering
38 states.
Stay Hemp 4 Life
Greene Concepts, Inc. acquired 100% of the membership interests
of Stay Hemp 4 Life LLC (“STAY”) as a wholly owned subsidiary of Greene Concepts, Inc. for a purchase price of $275,000.00
and a royalty of $0.04 per product sold. Stay Hemp 4 Life, a manufacturer and distributor of all-natural hemp infused sports drinks.
Each 20 FL OZ Stay Hemp drink will consist of 20 MG of Greene Concepts’ proprietary blended broad spectrum hemp extract containing
essential vitamins and minerals designed to help support revitalization, focus and relaxation of the body. Each flavor profile delivers
a unique experience to include:
Stay Detoxed Charcoal Berry – Detoxify (100 MG of Activated
Charcoal)
Stay Focused Plum Punch – Mental Clarity (120 MG of Caffeine)
Stay Strong Blood Orange Acai – Strength (200 MG of Vitamin C)
Stay Well Tropical Turmeric – Wellness (5% Turmeric Juice)
Stay Energized Lemon Lime – Energy (120 MG Caffeine)
Stay Calm Pineapple Mint – Calmness (Made with Fresh Mint) cape with its quality hemp extract beverages.
These products are being bottled and shipped from a contracted Colorado facility.
The Company plans to rebrand the Stay Hemp 4 Life brand under the
“Happy Mellow” trademark.
A2M Bio Inc.
On February 9, 2022, the Company entered into a Fee Sharing and
Noncircumvention Agreement (the “A2M Agreement”) with A2M Bio, Inc. (“A2M Bio”). Pursuant to the terms of the
A2M Agreement, A2M Bio will provide brokerage services to the Company to place the Companies products in Walmart and other retailers.
The Company shall pay A2M Bio a referral fee of 6% of Net actual cash receipts from purchasers that have been acquired through A2M Bio.
Happy Mellow
On October 5, 2021, the Company acquired the website www.happymellow.com
and the rights to all associated trademarks of the Happy Mellow brand in exchange for $18,500.00 from Tom Blakeley. The Company is in
the process of registering a trademark for “Happy Mellow”.
Cram Racing Sponsorship
On August 29, 2021, the Company entered into the Cram Racing Enterprises
Sponsorship Agreement with Cram Racing Enterprises, LLC (“Cram Racing”). Pursuant to the terms of the Cram Racing Enterprises
Sponsorship Agreement, the Company paid Cram Racing $65,000.00 to promote “Be Water” in three car races.
On September 8, 2021, the Company entered into the Cram Racing
Enterprises Sponsorship Agreement with Cram Racing. Pursuant to the terms of the Cram Racing Enterprises Sponsorship Agreement, the Company
paid Cram Racing $25,000.00 to promote “Be Water” in one car race.
Our Products
BeWater

Domestically sourced in the United States of America from springs
located in the Blue Ridge Mountains and bottled at our bottling plant nestled in the rolling hills of Marion, North Carolina. Be Water™
is a fresh-tasting artesian spring water that optimizes hydration. The brand naturally contains a rich concentration of the minerals
calcium and magnesium giving the water a soft, refreshing taste. The brand seeks to invoke mindfulness, generosity, kindness, strength,
goodness, courage, awareness, patience, integrity, and adaptability from the virtues of water deeply rooted in Taoism. It encourages
introspection and a connection to one’s inner self.

Our flagship artesian spring bottled water line is currently delivered
in durable single-serve containers in six (6) and twenty-four (24) packs is being produced from our spring and artesian wells that are
fed from a natural aquifer that is located deep below the Pisgah National Forest. Designed around the concepts of quality, product durability,
availability, and offering a smooth, crisp, and flavorful taste will help us captivate and retain consumers with loyalty.
 |
We have trademarked the Be Water™ name and logo to cover the
category of bottled water through the United States Patent and Trademark Office (USPTO): https://uspto.report/TM/90645587. The mark
consists of an outline of a water droplet with the words "Be Water™" enclosed in it, with another outline of a water
droplet within the letter "B". |
The Company sells BE WATER through wholesalers and distributors
throughout the country. The Company also markets and sells BE WATER on Amazon.com.
Disaster recovery efforts led by the federal
government through FEMA, state government emergency response programs, and city or county emergency response programs require entities
to be registered and cleared through the System for Award Management (SAM). The Company successfully completed validation requirements
and has received issuance of Commercial and Government Entity Codes (“CAGE codes”). CAGE codes are unique identifier assigned
to suppliers to various government or defense agencies and provide a standardized method of identifying a given facility at a specific
location. The Company expedited application efforts in order to become registered and available to provide assistance for any disaster
or emergency relief effort. The Company’s bottling facility is a strategically important asset by possessing a total of seven (7)
operational wells. The facility is located within the Pisgah National Forest with an enormous source of pure spring water accessed from
the aquifer located deep below the national forest.
Our Production
We are producing BE WATER at our 55,000 square
foot bottling and beverage plant that is located within the boundaries of the Pisgah National Forest in Marion, North Carolina. The bottling
facility has as its water source a combination of seven (7) spring and artesian wells that are fed from a natural aquifer that is located
deep below the Pisgah National Forest. We are focused on producing spring and artesian water.
Since we do not rely on independent third-party
bottlers to manufacture and market our water products, we believe we can more effectively manage quality control and consumer appeal
while responding quickly to changing market conditions.
Happy Mellow

Happy Mellow uses water-soluble transport technology
to deliver nutrients quickly into the body’s circulatory system (also known as bioavailability). This can support a healthy immune
system, balance, stress relief, relaxation, an ease of muscle tension, and a more peaceful and healthier mind and body.
Following significant research and development, the Happy
Mellow beverage line has been formulated to include fast-acting CBD, along with other vitamins. Happy Mellow is low in sugar and contains
all-natural ingredients. Each delicious beverage is infused with 20 mg of broad-spectrum CBD to include:
10 mg CBD
"Cannabidiol” 5 mg CBN “Cannabinol” 5 mg CBG “Cannabigerol”
The current Happy Mellow product line consists
of:
| · | Happy
Mellow Lemon Lime (120 MG of Caffeine) |
| · | Happy
Mellow Plum Punch (Vitamin B12) |
| · | Happy
Mellow Blood Orange Acai (200 MG of Vitamin C) |

Mission: Provide consumers with an all-natural, CBD-infused
healthy beverage alternative.
Vision: Create exceptional drinks that offer a happy,
mellow experience for consumers.
Purpose: Preserve and restore balance to your body through
healthy beverage offerings.
Co-Packing
We do not directly produce our hemp-infused
drinks, Happy Mellow, but instead outsource the production of our products with our specifications to third-party bottlers and co-packers.
We use co-packers to produce our beverage products. The co-packers are responsible for the production and packaging of the finished products,
including the procurement of all required ingredients and packaging materials. We have partnered with multiple co-packers in the United
States to provide fulfillment of our products from quality, low-cost sources. These partners are integral to our success, providing,
we believe, the ability to scale as needed. We store all of our products in our warehouses located in Marion, North Carolina.
Our ability to estimate demand for our products
is imprecise, particularly with new products, and may be less precise during periods of rapid growth, particularly in new markets. If
we materially underestimate demand for our products, are unable to secure sufficient ingredients or raw materials, including, but not
limited to, aluminum cans, plastic bottles, caps, labels, flavors, juice concentrates, coffee, tea, dietary ingredients, other ingredients,
and certain sweeteners, are unable to procure adequate packing arrangements, or are unable adequately or timely to ship our products,
we might not be able to satisfy demand on a short-term basis. That short-term supply inability may also result in a longer-term reduction
in orders for our products.
Our Industry
Consumers are consistently switching from carbonated
drinks to water and healthy energy drinks. As a result, the beverage market has seen increased demand for enhanced water and other functional
drinks. Overall, rapid urbanization, along with widening base of the middle-class population, has increased the demand for a variety of
healthy beverages.
This higher demand for water and functional beverages
has benefited the packaging industry. Moreover, increasing consumer demand for quality products, made from organic and naturally sourced
ingredients, requires science-based formulations of effective products in the beverage industry. These factors have created growth potential
for beverage packaging service providers who have the expertise and knowledge required to create successful products in an increasingly
discerning market.
Modor Intelligence predicts the global beverage
packaging market to grow at a compounded annual growth rate, or CAGR, of 4.17% and reach a value of $142.28 billion by 2023. Currently,
North America accounts for the largest market share, poised to reach $28.84 billion by 2023. According to Statistica, revenue in the US
bottled water segment alone amounts to US$67.5 billion in 2019 and is expected to grow annually by 5.5% (CAGR 2019-2023).
The International Bottled Water Association (IBWA),
and the Beverage Marketing Corporation (BMC), reports bottled water volume grew to 13.2 billion gallons in 2017 to 13.8 gallons in 2018,
an almost five percent increase over the previous year (as compared to more than 6% growth in 2017). This growth is fueled in large part
by increased numbers of consumers choosing bottled water instead of soda. Carbonated soft drink sales decreased for the thirteenth consecutive
year, according to the most recent numbers from BMC. BMC statistics show per capita consumption exceeded 42 gallons of bottled water,
a 6.2 percent increase and the average annual intake of carbonated soft drinks has declined to 37.5 gallons. BMC foresees bottled water
consumption will climb higher than 50 gallons per capita within just a few years. According to BMC, nearly all Americans (94 percent)
believe that bottled water is a healthier choice than soft drinks, and 93 percent say bottled water should be available wherever drinks
are sold.
The shift away from sugary drinks is having a
dramatic impact on sales of functional beverages. The global functional drinks market size is expected to reach USD 93.68 billion
by 2019, according to a new study by Grand View Research, Inc., progressing at a CAGR of 6.1% during the forecast period. Per Grand View
Research, the global functional drink market is anticipated to reach $93.68 billion in 2019, at a CAGR of 6.1%. According to the Grand
View Report, four players hold 55.2% of this market. Functional beverages include energy drinks and sports drinks, and nutritional drinks.
In 2014, energy drinks represented almost 56% of all functional beverage sales. Ibis World reports, over the past five years, the US energy
drink production industry has grown by 5.2% to reach revenue of $9 billion in 2018. The energy drinks market is forecast to register a
CAGR of 3.6% till 2023, whereas, the sports drinks market will grow at a CAGR of 4.3% during the same period.
We believe that the growth in bottled water and
other healthy beverages and away from carbonated or sugary beverages will create opportunities for our company.
Raw Materials and Suppliers
The raw materials used in the production of
our products are obtained by our co-packers and consist primarily of materials such as the flavors, caffeine, sugars or sucralose, taurine,
vitamins, CBD, and hemp seed protein contained in our beverages, the bottles in which our beverages are packaged, and the labeling on
the outside of our beverages. These principal raw materials are subject to price and availability fluctuations. We currently rely on
a few key co-packers, which in turn rely on a few key suppliers. We continually endeavor to have back-up co-packers, which co-packers
would in turn depend on their third-party suppliers to supply certain of the flavors and concentrates that are used in our beverages.
We are also dependent on these co-packers to negotiate arrangements with their existing suppliers that would enable us to obtain access
to certain of such concentrates or flavor formulas under certain extraordinary circumstances. Additionally, in a limited number of cases,
our co-packers may have contractual restrictions with their suppliers or our co-packers may need to obtain regulatory approvals and licenses
that may limit our co-packers’ ability to enter into agreements with alternative suppliers. Contractual restrictions in the agreements
we have with certain distributors may also limit our ability to enter into agreements with alternative distributors. We believe that
a satisfactory supply of co-packers will continue to be available at competitive prices, although there can be no assurance in this regard.
We believe that a satisfactory supply of vendors will continue to be available at competitive prices, although there can be no assurance
in this regard.
Quality Control
All quality control is handled by our co-packers.
To date, we have not had any quality issues with our products. In the future, if any quality issues were to arise, we expect that we
would resolve them with the specific co-packer involved or engage a new co-packer for our products.
Distribution
Distribution patterns in the CBD drink and
water industries are such that large buying groups dictate what products are used in their channels. Working with these large buying
groups could open large distribution channels that could potentially supply our product offerings in several market segments. We have
distribution agreements with each distributor with which we partner that distributes our products. Our distribution agreements are for
a one-year term and typically automatically renews unless the distributor or we terminate the agreement. We ship product directly from
our distribution center in South Carolina, our warehouse in Florida, or directly from our co-packers to our distribution partners in
the United States. Our distributors consist of state-wide tier 1 distributors and regional small-to-medium size distributors.
Market Demand & Condition
Bottled Water
The bottled water market is expected to witness substantial growth in the coming years due to a change in lifestyle patterns of consumers
as they are more inclined towards the most convenient sources of water and food to save time. The key factor driving the growth of the
market has been the increasing consumer demand for functional beverages. Functional beverages are nonalcoholic drinks providing health
benefits beyond their nutritional value, positively affecting or functional focusing on the body or mind to promote the state of
health and well-being. The ease of consumption and its effect on human nutrition are the factors driving the market's demand.
Functional water's rising popularity among the working
class and millennial populations is expected to enhance the market growth. With growing health consciousness, consumers are opting for
packaged water and reducing the consumption of sugary drinks. The consumption of still drinking water has increased in food outlets and
restaurants, which is boosting the market growth. The major opportunities identified for the bottled water industry are innovative products
with minimum environmental and health hazards and a penetration into developing markets.
Source - https://tandobeverage.com/functional-beverage/
According to Grand View Research, in January, 2021,
the global bottled water market size was valued at USD 217.66 billion in 2020 and is expected to expand at a compound annual growth rate
(CAGR) of 11.1% from 2021 to 2028. The global bottled water market size is expected to reach USD $505.19 billion by 2028 and $850.2 billion
by 2030.
91% of consumers expect bottled water to be available
wherever other drinks are sold. 44% of bottled water’s growth comes from people choosing bottled water over soda and juice. PET
single-served plastic bottled water containers are the number 1 packaging type among consumers. When bottled water is not available,
74% of people choose other packaged drinks such as soda, coffee, or tea.
Source - https://issuu.com/ibwa/docs/2021_ibwa_progress_report_final
Source - https://bottledwater.org/statistics/
Growth of the global bottled water market can be attributed
to the declining quality of tap water and increasing health consciousness among consumers. Changing consumption patterns and increasing
demand for bottled water in the hospitality and tourism sector also favor the growth of the market.
Source - https://www.blueweaveconsulting.com/report/global-bottled-water-market
CBD/Hemp Infused Beverages
The mainstream beverage market is seeing new growth
thanks to an influx of demand for innovative CBD-infused and hemp-infused beverage choices. These beverages display robust popularity
in the market as consumers seek healthier, less sugary, and low-alcohol beverages which have influenced consumer product consumption
due to their health-benefiting attributes. This includes bioavailability/water solubility which is the rate at which positive attributes
in the drink is absorbed into the bloodstream to maximize total body health. Hemp infused cannabis beverages is estimated to be the fast-growing
growing CBD segment due to the non-psychoactive property of CBD.
CBD and hemp are the hottest health-and-wellness ingredients
in the $2 trillion consumer packaged goods (CPG) market. With consumers moving away from soft drinks, high sugar beverages and artificial
flavors, we expect our Happy Mellow beverage line to meet the gap once filled by soft drinks.
Consideration has been given to current FDA regulations
so we enter the CBD market responsibly.
According to Cannyx Markets in August 2020,
CBD-infused beverages offer many functional benefits that account for its rapid growth expected in the U.S. during the forecast period
(2018 – 2025).
Our Distribution
Our primary distribution systems utilize the fresh,
natural and organic wholesale food distributors and representatives. Distribution will be targeted to independent natural food retailers
and large box stores through both independent warehouse distribution system and direct-store delivery system. ‘White label’
products utilize customer shipping and qualified independent shipping companies for direct delivery to the convenience channels.
Seasonality
We expect that our operating results will
not be materially affected by seasonal factors, including fluctuations in costs of raw materials, holiday and seasonal programming and
weather conditions.
Our Competition and Competitive Strengths
Our products will compete with many varieties
of liquid refreshment, including water products, soft drinks, juices, fruit drinks, energy drinks and sports drinks, as well as powdered
drinks, coffees, teas, dairy-based drinks, functional beverages and various other nonalcoholic beverages. We will also compete with bottlers
and distributors of national, regional and private label products. Several competitors, including those that dominate the beverage industry,
such as Nestlé S.A., PepsiCo and The Coca-Cola Company, have greater financial resources than we have and aggressive promotion
of their products may adversely affect sales of our brands.
The principal methods of competition in the beverage
industry are price and promotional activity, advertising and marketing programs, point-of-sale merchandising, retail space management,
customer service, product differentiation, packaging innovations and distribution methods. We believe we will be able to differentiate
ourselves in the following ways:
| |
· |
Formulations of products for specific, targeted audiences who have unique health and exercise requirements |
| |
· |
Regulatory capacity that ensures brands and stores are selling product that meets the standards of states and governments |
| |
· |
Access to industry stakeholders, influencers and high-profile consumers who can provide third-party endorsement of our products. |
Intellectual Property
We currently own the trademark for BeWater.
We intend to maintain all registrations of our future significant trademarks and use our future trademarks in the operation of our businesses.
We also own the URL www.greeneconcepts.com and www.happymellow.com.
We are currently applying for a trademark for “Happy Mellow”.
Governmental Regulation
Water and Beverages
The operation of our facility and the production,
distribution and sale of our future products in the United States are subject to the Federal Food, Drug and Cosmetic Act; the Dietary
Supplement Health and Education Act of 1994; the Occupational Safety and Health Act; the Lanham Act; various environmental statutes; and
various other federal, state and local statutes regulating the production, transportation, sale, safety, advertising, labeling and ingredients
of such products. We believe that we are in compliance, in all material respects, with such existing legislation and that prior to the
operation of our facility, we will have all required licenses to operate.
The Food and Drug Administration (FDA) is responsible
for the safety of bottled drinking water. The FDA has set Current Good Manufacturing Practices (CGMPs) specifically for bottled water.
The FDA requires bottled water producers, like us, to:
| |
· |
Process, bottle, hold and transport bottled water under sanitary conditions; |
| |
· |
Protect water sources from bacteria, chemicals and other contaminants; |
| |
· |
Use quality control processes to ensure the bacteriological and chemical safety of the water; |
| |
· |
Sample and test both source water and the final product for contaminants. |
The FDA monitors and inspects bottled water products
and processing plants under its food safety program. When FDA inspects plants, the FDA verifies that the plant's product water and operational
water supply are obtained from an approved source; inspects washing and sanitizing procedures; inspects bottling operations; and determines
whether companies analyze their source water and product water for contaminants.
If we begin to incorporate dietary supplements
into our water to create nutritionally-enhanced beverages, we will become subject to additional certification and regulatory compliance
by the FDA. Compliance efforts relating to dietary supplements to be included in our water are contained in Section 403(r)(6) of the Federal
Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. 343(r)(6)). Section 403(r)(6) of the Federal Food, Drug, and Cosmetic Act (the FD&C
Act) (21 U.S.C. 343(r)(6)) requires that a manufacturer of a dietary supplement making a nutritional deficiency, structure/function, or
general well-being claim have substantiation that the claim is truthful and not misleading. Under section 403(r)(6)(A) of the FD&C
Act, such a statement is one that “claims a benefit related to a classical nutrient deficiency disease and discloses the prevalence
of such disease in the United States, describes the role of a nutrient or dietary ingredient intended to affect the structure or function
in humans, characterizes the documented mechanism by which a nutrient or dietary ingredient acts to maintain such structure or function,
or describes general well-being from consumption for a nutrient or dietary ingredient.” We will only need to collect information
to substantiate our product's nutritional deficiency, structure/function, or general well-being claim if we choose to place a claim on
our product's label.
Certain states and localities require a deposit
or tax on the sale of certain beverages. These requirements vary by each jurisdiction. Similar legislation has been proposed in certain
other states and localities, as well as by Congress. We are unable to predict whether such legislation will be enacted or what impact
its enactment would have on our business, financial condition or results of operations.
Our facility is subject to federal, state and
local environmental laws and regulations. We do not expect that compliance with these provisions will have any material adverse effect
on our financial or competitive position. We believe our future practices and procedures for the control and disposition of toxic or hazardous
substances will comply in all material respects with applicable law.
Government Regulation and Compliance (Hemp
and CBD)
The production, distribution, and sale in
the United States of many of our products are subject to various U.S. federal and state regulations, including, but not limited to: the
FDCA; the Occupational Safety and Health Act; various environmental statutes; the Safe Drinking Water and Toxic Enforcement Act of 1986
(“California Proposition 65”); and a number of other federal, state, and local statutes and regulations applicable to the
production, transportation, sale, safety, advertising, marketing, labeling, and ingredients of such products.
Further, the regulation of food products in
the United States, including products containing CBD, is complex, multi-faceted, and currently undergoing significant change. The FDA,
the FTC, the USDA, and other regulatory authorities at the federal, state, and local levels extensively regulate, among other things,
the research, development, testing, composition, production, import, export, labeling, storage, distribution, promotion, marketing, and
post-market reporting of foods, including those that contain CBD. We, along with our third-party suppliers, co-packers, and third-party
bottlers, are required to navigate a complex regulatory framework. The various federal, state, and local regulations regarding foods
containing CBD are evolving, and we continue to monitor those developments. However, we cannot predict the timing, scope, or terms of
any new or revised state, federal or local regulations relating to animal foods containing CBD.
Regulation of Hemp and CBD
Historically, the DEA regulated CBD, pursuant
to the CSA, which establishes a framework of controls over certain substances, depending on whether they are classified in one of five
risk-based schedules. Schedule I substances are the most stringently controlled, as they have been determined to have a high potential
for abuse, there are no currently accepted medical uses in the United States, and there is a lack of accepted safety for use of the substance
under medical supervision. The CSA classifies “marijuana” as a Schedule I controlled substance and previously defined “marijuana”
to include all parts of the cannabis plant, whether growing or not; the seeds of the plant; the resin extracted from any part
of the plant; and every compound mixture, salt, derivative, mixture, or preparation of the plant, its seeds, or its resin (with
a few exceptions, such as mature stalks of the plant and seeds incapable of germination). Pursuant to this definition, the DEA interpreted
CBD to fall within the statutory definition of “marijuana” as a compound or derivative of the cannabis plant.
In February 2014, Congress enacted the 2014
Farm Bill to allow for the limited growth and cultivation of industrial hemp, which was defined as including all parts of the cannabis
plant, whether growing or not, with a delta-9 THC concentration of not more than 0.3% on a dry weight basis. This statute also allowed,
as permitted by state law, the growing and cultivating of industrial hemp under the auspices of a state agricultural pilot program and
by institutions of higher education and state departments of agriculture.
In December 2018, Congress enacted the 2018
Farm Bill to allow more broadly for the production of hemp pursuant to state and tribal plans overseen by the USDA. The 2018 Farm Bill
amended the statutory definition of “marijuana” under the CSA specifically to exclude “hemp”, which is defined
as any part of the cannabis plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and
salts of isomers, whether growing or not, with a delta-9 THC concentration of not more than 0.3% on a dry weight basis. Under this definition,
as long as CBD meets the statutory definition of “hemp,” then it is no longer a Schedule I controlled substance under the
CSA. However, the 2018 Farm Bill did not modify the FDCA and specifically preserved the FDA’s authority to regulate products containing
cannabis or cannabis-derived compounds, such as CBD, pursuant to the FDCA.
Under the 2018 Farm Bill framework, states
and Native American tribes may submit to the USDA, through the relevant state department of agriculture, a plan under which the state
or Native American tribe will monitor and regulate the production of industrial hemp. For those states that do not have an approved state
plan, the production of hemp will be subject to a USDA established plan, although states retain the ability to prohibit hemp production
within their borders. On October 31, 2019, the USDA issued the IFR to implement the 2018 Farm Bill, which established the required regulatory
framework governing commercial hemp production in the United States. The USDA has begun reviewing hemp production plans submitted by
state and tribal governments, although several states have informed the USDA that they will continue to operate under their 2014 Farm
Bill pilot programs for the time being. Pursuant to the 2018 Farm Bill, the 2014 Farm Bill will remain effective until October 31, 2020
(one year after the date of publication of the IFR). In addition, no state or Native American tribe may prohibit the transportation or
shipment of hemp or hemp products produced in accordance with the 2018 Farm Bill through the state or territory, as applicable. The USDA
has interpreted this provision also to apply to interstate transportation of hemp that complies with the 2014 Farm Bill through October
31, 2020. Effective March 22, 2021, The USDA issued the final rule, which creates the U.S. Domestic Hemp Production Program. The program
provides requirements for maintaining records about the land where hemp is produced, testing the levels of total delta-9 tetrahydrocannabinol,
disposing of non-compliant plants, licensing hemp producers, and ensuring compliance under the new program. The Agricultural Marketing
Service (AMS), has been delegated authority to administer the U.S. Domestic Hemp Production Program.
FDA Regulation of Foods
The FDA regulates foods under the FDCA and
its implementing regulations. The FDCA defines “food” as articles used for food or drink for people or animals, which includes
products that are intended primarily for nutritional use, taste, or aroma and the components of such products. The FDA also imposes certain
requirements on foods relating to their composition, production, labeling, and marketing. Among other items, the facilities in which
our products and ingredients are produced must register with the FDA, comply with cGMPs and comply with a range of food safety requirements.
Although foods are not required to obtain
premarket approval from the FDA, any substance that is added to or is expected to become a component of food must be used in accordance
with a food additive regulation, unless it is generally recognized as safe (“GRAS”) under the conditions of its intended
use. A food may be adulterated if it uses an ingredient that is neither GRAS nor an approved food additive, and that food may not be
legally marketed in the United States. The FDA has confirmed that the use of cannabis or cannabis-derived compounds in food products
is subject to these food additive requirements. At this time, there are no approved food additive petitions or regulations for any cannabis-derived
food additive and, while the FDA has issued a “no questions” response to certain GRAS notifications for hemp seed products,
these GRAS determinations do not encompass hemp and CBD products more generally.
Additionally, the FDCA prohibits the introduction
or delivery for introduction into interstate commerce of any food that contains an approved drug for which substantial clinical investigations
have been instituted and made public (unless certain exceptions apply). Under this prohibition, the FDA has stated that foods that contain
CBD are adulterated because CBD is an active ingredient in an FDA-approved drug that was the subject of substantial clinical investigations
before it was marketed as a food, and that none of the exceptions applies.
Although the FDA has stated that it interprets
the FDCA to prohibit the introduction or delivery for introduction into interstate commerce of any food into which CBD has been added
and has taken enforcement action against marketers of certain CBD products (some in collaboration with the FTC), the FDA is in the process
of evaluating its regulatory approach to products containing cannabis and cannabis-derived compounds. The FDA has formed an internal
working group to evaluate the issue and on May 31, 2019 held a public hearing to obtain scientific data and information about the safety,
producing, product quality, marketing, labeling, and sale of products containing cannabis or cannabis-derived compounds. The hearing
featured extensive discussion from a variety of stakeholders regarding the use of hemp and CBD in FDA-regulated products, including foods.
At the hearing, FDA stated that, while it does not have a policy of enforcement discretion with respect to any CBD products, the agency’s
biggest concern is the marketing of products that puts the health and safety of consumers at risk, such as those claiming to prevent,
diagnose, mitigate, treat, or cure serious diseases in the absence of requisite drug approvals.
Further, on March 5, 2020, the FDA issued
a report to Congress that was required under the 2018 Farm Bill in which the agency announced that it is currently evaluating a risk-based
enforcement policy for CBD to provide more clarity to the industry and the public while the agency takes potential steps to establish
a clear regulatory pathway. Although it is unclear whether or when the FDA will ultimately issue such an enforcement policy, the agency
reemphasized that it will continue to take action against unlawful CBD products that pose a risk of harm to the public, including products
with therapeutic claims; products that include contaminants such as heavy metals, THC, and other harmful substances; products
associated with false statements, such as omitted ingredients and incorrect statements about the amount of CBD; and products marketed
to vulnerable populations, such as infants and children.
The labeling of foods is regulated by both
the FDA and state regulatory authorities. FDA regulations require proper identification of the product, a net quantity statement, a statement
of the name and place of business of the producer or distributor, and proper listing of all of the ingredients in order of predominance
by weight. The FDA may classify some of our products differently than we do and may impose more stringent regulations, which could lead
to possible enforcement action.
Under the FDCA, the FDA may require the recall
of a food product if there is a reasonable probability that the product is adulterated or misbranded, and the use of or exposure to the
product will cause serious adverse health consequences or death. In addition, food producers may voluntarily recall or withdraw their
products from the market. If the FDA believes that our products are adulterated, misbranded, or otherwise marketed in violation of the
FDCA, the agency may take further enforcement action, including:
| |
· |
restrictions on the
production or marketing of a product; |
| |
|
|
| |
· |
required modification
of promotional materials or issuance of corrective marketing information; |
| |
|
|
| |
· |
issuance of safety alerts,
press releases, or other communications containing warnings or other safety information about a product; |
| |
|
|
| |
· |
warning or untitled
letters; |
| |
|
|
| |
· |
product seizure or detention; |
| |
|
|
| |
· |
refusal to permit the
import or export of products; |
| |
|
|
| |
· |
fines, injunctions,
or consent decrees; and |
| |
|
|
| |
· |
imposition of civil
or criminal penalties. |
Legislation may be introduced in the United
States at the federal, state, and municipal level in respect of each of the subject areas. Public health officials and health advocates
are increasingly focused on the public health consequences associated with obesity, especially as it affects children, and are seeking
legislative change to reduce the consumption of sweetened beverages. There also has been an increased focus on caffeine content in beverages.
The FDA revised regulations with respect to
serving size information and nutrition labeling on food and beverage products, including requirements to disclose the amount of added
sugars in such products. In December 2018, the USDA promulgated regulations requiring that, by January 1, 2022, the labels of certain
bioengineered foods must include a disclosure that the food is bioengineered. We may incur significant costs to alter our existing packaging
materials to comply with these and other new regulations. Additionally, these new regulations may impact, reduce, or otherwise affect
the purchase and consumption of our products by consumers.
Proposals to limit or restrict the sale and/or
advertising of energy drinks to minors or persons below a specified age, to restrict the venues in which energy drinks can be sold, or
to restrict the use of the Supplemental Nutrition Assistance Program (formerly food stamps) to purchase energy drinks have been raised
or enacted in certain states, counties, and municipalities throughout the United States. Any such limitations or restrictions could adversely
affect our business, financial condition, or results of operations.
We also may in the future be affected by other
existing, proposed, and potential future regulations or regulatory actions, any of which could adversely affect our business, financial
condition, and results of operations. Changes in government regulation, or failure to comply with existing regulations, could adversely
affect our business, financial condition, and results of operations.
Employees and Contractors
We currently have two employees, Leonard Greene,
our CEO, and Kenneth Porter, our plant manager. Mr. Porter is located in North Carolina. Mr. Greene operates out of a home office in Fresno,
California, but travels to our facility in North Carolina as needed. In addition, we use independent contractors for management, legal,
accounting and administrative support.
Health and Safety
The health and safety of our associates is our highest priority, and
this is consistent with our operating philosophy. We have implemented crisis management teams at the enterprise level to ensure our operations
are aligned with global health standards and that we continue to enable the ongoing safe manufacturing and distribution of our products,
as well as the safety of our customers and associates throughout the coronavirus (“COVID-19”) pandemic and beyond.
As the COVID-19 pandemic evolved, we have taken multiple steps to prevent
the potential spread of the virus, to equip our associates to provide an essential service in our communities, and to ensure the ongoing,
safe manufacturing and delivery of our products:
| | · | Adding work from home flexibility; |
| | · | Increasing cleaning protocols across all locations; |
| | · | Initiating regular communication regarding impacts of the COVID-19
pandemic, including health and safety protocols and procedures; |
| | · | Implementing temperature screening of associates at our locations; |
| | · | Establishing new physical distancing procedures for associates
who need to be onsite; |
| | · | Providing personal protective equipment and cleaning supplies; |
| | · | Modifying office work stations with Plexiglas dividers; |
| | · | Implementing protocols to address actual and suspected COVID-19
cases and potential exposure; |
| | · | Suspending all domestic and international non-essential air travel
for all associates; and |
| | · | Requiring masks to be worn in all locations. |
Our products and services were deemed essential and as a result, all
of our production sites continued operating during the COVID-19 pandemic. As such, we have invested in creating a physically safe work
environment for our associates. Our frontline associates have gone above and beyond to perform an essential service for customers and
communities during this global crisis, and our priority is to support them and keep them safe. That’s why we are reinforcing the
availability of “no contact” delivery with our customers, reminding them to communicate any updates to their delivery and
if they leave their empty bottles outside, we will replace them accordingly. We are also encouraging associates to live our safety principles
and stop unsafe work if there is an identified or perceived risk at any delivery location.
Serving Our Communities
We are strongly committed not only to the communities we serve, but
also to the world at large. After all, our families live, work and play in our local communities and around the globe.
It is part of our mission to promote hydration and wellness via sponsorships
and in-kind donations, and to provide aid in the times of need. We provide bottled water products for local sporting events, culinary
and hospitality programs, fundraisers, and associate-supported efforts and have contributed time and resources to many regional causes.
We also donated water to medical centers and first responders, as well as supported hospitality partners feeding front-line workers during
the ongoing COVID-19 pandemic.
Legal Proceedings
We know of no existing or pending
legal proceedings against us, nor are we involved as a plaintiff in any proceeding or pending litigation. There are no proceedings in
which any of our directors, officers or any of their respective affiliates, or any beneficial stockholder, is an adverse party or has
a material interest adverse to our interest.
Recent Developments
On January 11, 2020, we cancelled 225,000,000
shares of our common stock that were registered in the name of Andy Greider. The shares were cancelled pursuant an understanding between
us and Mr. Greider that dated back to October 2018 and is evidenced by a written letter from Mr. Greider to our transfer agent. Mr. Greider
previously provided consulting services to our company and agreed to forfeit these shares when he ceased providing such services.
On November 4, 2020, the Company exchanged 284,516,225 shares of Company
common shares for 3,145,890 Company Preferred Class A shares (the “Share Exchange”).
As part of the Share Exchange, the Company exchanged:
| (a) | 51,927,302 shares of common stock owned by Leonard M. Greene for 520,000 Preferred Class A shares. |
| (b) | 220,000,000 shares of common stock owned by Madeline Kaye and issued 2,200,000 Preferred Class A Shares to Leonard M. Greene |
| (c) | 5,588,923 shares of common stock owned by Amy McNally for 55,890 Preferred Class A Shares |
| (d) | 7,000,000 shares of common stock owned by David Johnson for 70,000 Preferred Class A Shares |
On February 17, 2021, Monmouth announced that
it has paid off all mortgage liens and obligations for the building, equipment and property of the Marion, North Carolina facility and
has received a certificate of satisfaction from the lien holder with a payment of $719,823.56.
On or about June 14, 2021, the Company amended
its Certificate of Incorporation to create a new Preferred Class of Stock, the Company’s Preferred Class B Stock, par value $0.0001.
The Company’s Preferred Class B Stock can be converted into 100,000,000 (100 million) shares of the Company’s Common Stock.
Each share of the Company’s Preferred Class B Stock can also vote as 100,000,000 (100 million) shares of the Company’s Common
Stock.
Three shareholders of the Company’s
Preferred Class A Common Stock converted their shares into 20 shares each of the Company’s Class B Preferred Stock.
Effective July 31, 2024, all of our then-outstanding
outstanding convertible promissory notes were cancelled, a total cancellation amount of $313,996.
DESCRIPTION OF PROPERTY
Currently, our principal
executive offices are located at 13195 U.S. Highway 221 N, Marion, North Carolina, 28752, which is where our 55,000 sq. ft. beverage and
bottling facility is located.
We believe that all our
properties have been adequately maintained, are generally in good condition, and are suitable and adequate for our business.
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and
analysis of our financial condition and results of our operations together with our financial statements and related notes appearing
at the end of this PQA. This discussion contains forward-looking statements reflecting our current expectations that involve risks and
uncertainties. Actual results and the timing of events may differ materially from those contained in these forward-looking statements
due to a number of factors, including those discussed in the section entitled “Risk Factors” and elsewhere
in this PQA.
Overview
Since our inception we operated different businesses under different
names before changing our name to Greene Concepts, Inc. and engaging in our current business. Through our wholly owned subsidiary, Mammoth,
we are now a bottling and beverage company committed to providing the world with high quality, healthy, and enhanced beverage choices.
Our beverage and bottling facility is located in Marion, North Carolina.
As of April 6, 2020, the Company’s production
facility is fully operational after the Company spent 16 months restoring the production facility in Marion, North Carolina (the “Marion
Facility”). The Marion Facility currently has the capacity to produce 192 million bottles or 8 million cases annually (with current
equipment). The Marion Facility has space for additional capacity.
Additionally, Mammoth will act as a third-party
producer and bottler of "white label" beverage and water products. White label bottling services are provided for clients that
desire to market their own product formulations, brand name and labeling while outsourcing the production and bottling of their products
to Mammoth.
Before acquiring Mammoth on February 6, 2019,
we operated our legacy business, which was the manufacture and distribution of a line of 25 high quality consumer focused inkjet kits.
On April 30, 2019, our board of directors made a determination to wind down our legacy business and to transition into the beverage and
bottling business. During the same period, Mammoth began the process of performing required maintenance to revitalize all the equipment
and facility infrastructure in order to relaunch production at the plant. At the time of the acquisition all of the plant equipment was
in good condition although the equipment had not operated for several years and it did require a thorough inspection and light maintenance
to assure proper operation when the bottling lines are relaunched. At the time of the acquisition, we hired, Kenneth Porter, a 30+ year
veteran of the beverage and bottling industry, as plant manager to oversee operations as well as the revitalization and expected relaunch
of the facility.
The Food and Drug Administration, or FDA, requires
adherence to current good manufacturing practice, or CGMP, regulations for the processing and bottling of bottled drinking water, which
includes facility inspection and documentation of corrective measures and reporting requirements, as well as new requirements for hazard
assessments and food safety, or HACCP, plans mandated by the Food Safety and Modernization Act, or FSMA. Final preparations for inspection
are underway, including building and facility maintenance such as pressure washing, painting, general cleaning, and minor building repairs.
Happy Mellow
Happy Mellow uses water-soluble transport
technology to deliver nutrients quickly into the body’s circulatory system (also known as bioavailability). This can support a
healthy immune system, balance, stress relief, relaxation, an ease of muscle tension, and a more peaceful and healthier mind and body.
Following significant research and development,
the Happy Mellow beverage line has been formulated to include fast-acting CBD, along with other vitamins. Happy Mellow is low in sugar
and contains all-natural ingredients. Each delicious beverage is infused with 20 mg of broad-spectrum CBD to include:
10 mg CBD
"Cannabidiol” 5 mg CBN “Cannabinol” 5 mg CBG “Cannabigerol”
The current Happy Mellow product line consists of:
| · | Happy
Mellow Lemon Lime (120 MG of Caffeine) |
| · | Happy
Mellow Plum Punch (Vitamin B12) |
| · | Happy
Mellow Blood Orange Acai (200 MG of Vitamin C) |
Results of Operations
Years Ended July 31, 2024 and 2023.
Gross Sales. For the years ended July 31, 2024
and 2023, we reported gross sales of $856,545 (unaudited) and $719,732 (unaudited), respectively. Our higher gross sales for the 2024
period are attributable to the carry-on effects of our 2023 marketing program.
Cost of Sales. For the years ended July 31,
2024 and 2023, we reported cost of sales of $491,904 (unaudited) and $568,349 (unaudited), or approximately 57%
and 79% of gross sales, respectively. Our higher sales volumes in 2024 allowed us to achieve certain economies of scale in the
production of our product, thereby reducing our cost of sales as a percentage of gross sales.
Gross Margin. For the years ended July 31,
2024 and 2023, we reported a gross margin $364,641 (unaudited) and $151,383 (unaudited), respectively.
Operating Expenses. For the years ended July
31, 2024 and 2023, we reported total operating expenses of $915,857 and (unaudited) $1,308,329 (unaudited).
Administrative Expenses. For the years ended
July 31, 2024 and 2023, we reported administrative expenses of $61,091 (unaudited) and $94,771 (unaudited), respectively.
Professional Fees. For the years ended July
31, 2024 and 2023, we reported professional fees of $452,025 and $712,509 (unaudited), respectively.
Depreciation. For the years ended July 31,
2024 and 2023, we reported depreciation of $68,962 (unaudited) and $88,678 (unaudited), respectively.
Marketing. For the years ended July 31, 2024
and 2023, we reported marketing expenses of $11,653 (unaudited) and $319,341 (unaudited), respectively. The increase in marketing expenses
is attributable primarily to our greater levels of cash with which to market our products.
Brand Consulting. For the years ended July
31, 2024 and 2023, we reported brand consulting expenses of $130,653 (unaudited) and $322,995 (unaudited), respectively.
Plant Operations. For the years ended July
31, 2024 and 2023, we reported plant operations expenses of $30,272 (unaudited) and $19,021 (unaudited), respectively. The decrease in
plant operations expenses is attributable primarily to our lower level of sales activities.
Taxes. For the years ended July 31, 2024 and
2023, we reported tax expenses of $11,553 (unaudited) and $1,394 (unaudited), respectively.
Other Income/Expense. For the year ended July
31, 2024, other income was $309,330 (unaudited), which was comprised of $211,873 (unaudited) in gain on convertible debt write-off, $97,049
(unaudited) in finance and interest fees and $408 in interest income. For the year ended July 31, 2023, other expense was $242,726 (unaudited),
which was comprised of $243,726 (unaudited) in finance and interest fees that was offset by $451 in interest income.
Net Loss. For the years ended July 31, 2024
and 2023, we reported a net loss of $241,886 (unaudited) and $1,399,672 (unaudited), respectively.
Plan of Operations
In order for us to implement our business plan
over the next 12 months, we have identified the following milestones that we expect to achieve:
| |
· |
Expansion
of Broker Network - We expect to continue to develop our working relationship with our national broker network. We continually meet,
train, and go on sales call with our national broker network in order to take advantage of the momentum currently being created by their
efforts. We anticipate a considerable amount of travel and ongoing expenses to be incurred as part of this expansion. |
| |
|
|
| |
· |
Increase
Manufacturing Capacity - BE WATER product: we expect to add one to two new co-packer facilities, strategically located to reduce freight
costs and meet current volumes and future growth objectives. |
| |
|
|
| |
· |
Expand
Retail Distribution - We continue to expand our retail presence. |
| |
|
|
| |
· |
Addition
of Support Staff - In order to support expansion efforts and to continue the training and support of our broker network, we anticipate
that we will need to hire approximately two to three more people on the corporate level for the specific purpose of supporting the broker,
distributor and retailers and their logistical and accounting requirements. We continue to seek and interview candidates to fill our growing
need for additional staffing. |
The milestones set forth above reflect our current
judgment and belief regarding the direction of our business. Actual events, expenditures and results will almost always vary, sometimes
materially, from any estimates, predictions, projections or assumptions suggested herein.
If our own financial resources and future cash-flows
from operations are insufficient to satisfy our capital requirements, we may seek to sell additional equity or debt securities or obtain
additional credit facilities. The sale of additional equity securities will result in dilution to our stockholders. The incurrence of
indebtedness will result in increased debt service obligations and could require us to agree to operating and financial covenants that
could restrict our operations or modify our plans to grow the business. Financing may not be available in amounts or on terms acceptable
to us, if at all. Any failure by us to raise additional funds on terms favorable to us, or at all, will limit our ability to expand our
business operations and could harm our overall business prospects.
Liquidity and Capital Resources
July 31, 2024. At July 31, 2024, our
company had $238,182 (unaudited) in cash and a working capital deficit of $1,699,698 (unaudited), compared to $195,540 (unaudited) in
cash and a working capital deficit of $1,980,173 (unaudited) at July 31, 2023. The change in our working capital position from July 31,
2023, to July 31, 2024, is attributable primarily to our inability to increase sales of our products without achieving a corresponding
reduction in our fixed expenses.
Our company’s current cash position of approximately
$200,000 is adequate for our company to maintain its present level of operations through July 31, 2025.
Cancellation of Convertible Notes. Effective
July 31, 2024, all of our then-outstanding outstanding convertible promissory notes were cancelled, a total cancellation amount of $313,996.
Financing Source. To date in this offering,
we have sold 275,333,333 Units for a total of $1,112,000 in cash. We require additional funding, whether from this offering or from another
source, to increase sales of our products. There is no assurance that we will be successful, in this regard.
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT
EMPLOYEES
The following table sets forth the name and position
of each of our current executive officers, director and significant employees.
| Name |
|
Position |
|
Age |
|
Term of Office |
|
Approximate hours per week for part-time employees |
| Leonard Greene |
|
Chief Executive Officer; Director |
|
68 |
|
1 Year |
|
40
|
| Kenneth Porter |
|
Plant Manager |
|
60 |
|
1 Year |
|
N/A |
Leonard Greene, Chief Executive Officer
Lenny M. Greene is the current Chief Executive
Officer, President and Director of the Company and has held that position since November 19, 2019. On November 19, 2019 Mr. Greene resumed
the role of company CEO and President after having served in both roles from 2003 - 2018. During his previous tenure the company manufactured
and distributed high-quality ink technology formulations for wide format, narrow format and industrial printing applications. Mr. Greene
laid the groundwork to build the organization to an elevated level of competitiveness and professionalism while taking the company public
(OTC: INKW) thereby magnifying expansion opportunities toward near-term growth and long-term value. He brings over thirty years of experience
to Greene Concepts with an impressive acumen of negotiating and closing deals with Fortune 500 corporate accounts. Mr. Greene is an expert
dealing with corporate executives and purchasing agents. His resume includes spearheading sales and service and repair contracts as CEO
for Comservco, a personal computing and Wide Area Network infrastructure business, from $0 to $15 million over a three-year period. Mr.
Greene has managed over 200 nationwide company accounts to include: ABC, CBS, Exxon Corporation, New York City (All City Agencies), New
York Telephone Company, Western Electric, Citibank and Bank of New York. Since February 2023, Mr. Greene has served as a director of Tocca Life
Holdings, Inc., a publicly traded company (symbol: TLIF) engaged in the operation of indoor rock climbing centers.
Kenneth Porter, Plant Manager
Mr. Porter has 36 years of experience in high-speed food and beverage production, managing multiple plants, the maintenance, equipment & processes. Mr. Porter has worked
as a manager for Pepsi Bottling Group, Universal Food & Beverage, Summit Beverage Group and prior to joining Greene Concepts, Ice
River Springs, a bottling company with an annual volume of 25 million cases and 125 employees.
His career began in 1983 working as Production
Manager for Coca-Cola Bottling Co Consolidated. In 1994 he was promoted to Plant Manager for a facility with an annual volume of
20 MM cases, and managed 105 hourly employees and 12 managers.
Mr. Porter’s skills include PM systems, PLCs, Predictive maintenance,
inferred & vibration analysis, Strong people/ coaching skills, Strong problem solving skills, P&L and budgets, Costing of products,
Cost analysis, Capital Appropriations, Managed multi-million dollar projects, Removed and installed complete bottling lines, Personally
installed over 20 pieces of major bottling, blow molding and support equipment, Master sanitation programs, Warehouse management,
inventory control & Union Plants. He has training/certification in Computers, T.Q.M, S.P.C, T.P.M, Team Building, Self-directed
work teams, M.R.P, E.R.P, OSHA & GMP regulations, USDA, FDA, HACCP, SQF, NSF, Six Sigma Black Belt and lean manufacturing champion,
Safety Committees and Pepsi CQV process.
Directors are elected until their successors are
duly elected and qualified.
Family Relationships
There are no family relationships between any
director, executive officer, person nominated or chosen to become a director or executive officer or any significant employee.
Legal Proceedings
To the best of our knowledge, none of our directors
or executive officers has, during the past five years:
| |
· |
been convicted in a criminal proceeding (excluding traffic violations
and other minor offences); or |
| |
|
|
| |
· |
had any petition under the federal bankruptcy laws or any state insolvency
law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such
person, or any partnership in which he was general partner at or within two years before the time of such filing, or any corporation or
business association of which he was an executive officer at or within two years before the time of such filing. |
COMPENSATION OF DIRECTORS
AND EXECUTIVE OFFICERS
In General
As of the date of this PQA,
there are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of our company, pursuant
to any presently existing plan provided by, or contributed to, our company.
Compensation Summary
The following table summarizes information concerning
the compensation awarded, paid to or earned by, our executive officer.
| Name and Principal Position |
Year
Ended
7/31 |
Salary
($) |
Bonus
($) |
Stock
Awards
($) |
Option
Awards
($) |
Non-Equity Incentive Plan Compensation
($) |
Non-qualified
Deferred
Compensation
Earnings
($) |
All Other Compensation
($) |
Total
($) |
| Leonard M. Greene |
2024 |
84,000 |
– |
– |
– |
– |
– |
– |
84,000 |
| Chief Executive Officer |
2023 |
84,000 |
– |
– |
– |
– |
– |
– |
84,000 |
Outstanding Option Awards
The following table provides certain information
regarding unexercised options to purchase Common Stock, stock options that have not vested and equity-incentive plan awards outstanding
as of the date of this PQA, for the named executive officer.
| |
Option Awards |
Stock Awards |
| Name |
Number of
Securities
Underlying
Unexercised
Options (#)
Exercisable |
Number of
Securities
Underlying
Unexercised
Options (#)
Unexercisable |
Equity
Incentive
Plan
Awards:
Number of
Securities
Underlying
Unexercised
Unearned
Options (#) |
Option
Exercise
Price ($) |
Option
Expiration
Date |
Number of
Shares or
Units of
Stock That
Have Not
Vested (#) |
Market
Value of
Shares or
Units of
Stock That
Have Not
Vested ($) |
Equity
Incentive
Plan Awards:
Number of
Unearned
Shares, Units
or Other
Rights That
Have Not
Vested (#) |
Equity
Incentive
Plan Awards:
Market or
Payout Value
of Unearned
Shares, Units
or Other
Rights That
Have Not
Vested ($) |
| Leonard M. Greene |
– |
– |
– |
N/A |
N/A |
– |
– |
– |
– |
Employment Agreements
The Company has entered into the following employment
agreement that we believe is material to our business:
Leonard Greene
Effective as of November 19, 2019, we entered
into an employment offer letter with Mr. Leonard Greene. Pursuant to the terms of the offer letter, Mr. Greene is appointed as the Chief
Executive Officer of our company. His duties include the general management of the affairs of our company, together with the powers and
duties usually incident to the office of chief executive officer. His monthly compensation is $7,000. He is eligible to participate in
the standard benefits plans offered to similarly situated employees of our company. He is also eligible for annual bonuses at the sole
discretion of the Board of Directors. The agreement may be terminated at any time by either party with or without cause or advance notice.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN
SECURITYHOLDERS
The table below does not give effect to certain
events, as follows:
Series B Preferred Stock Conversion. The table below
does not give effect to the issuance of shares of our Common Stock upon conversion of the outstanding shares of Series B Preferred Stock.
At any time, the holders of the Series B Preferred Stock have the right to convert the shares of Series B Preferred Stock into a total
of six billion shares of our Common Stock. (See “Risk Factors” and “Dilution-Ownership Dilution”).
In light of the caveat stated in the foregoing
paragraph, the following table sets forth information regarding beneficial ownership of our voting stock as of the date of this PQA (i)
by each of our officers and directors who beneficially own more than 5% of our voting stock; (ii) by all of our officers and directors
as a group; and (iii) by each person who is known by us to beneficially own more than 5% of each class of our voting stock. Unless otherwise
specified, the address of each of the persons set forth below is in care of the company at 13195 U.S. Highway 221 N, Marion, North Carolina,
28752.
| Name of Beneficial Owner |
Amount of Beneficial Ownership(1) |
Percent of Common Stock(2) |
Percent of Series B Preferred Stock(3) |
Percent of Total Voting Stock(4)(5) |
| Common Stock |
Series B Preferred Stock |
|
Lucky Pony LLC(6)
633 Gaines Way
Winter Park, FL 32789
|
0 |
20 |
0 |
33.3% |
26.6% |
|
The Leonard and Elizabeth Greene Family Trust(7)
1865 Herndon Ave, Suite K
Clovis, CA 93611
|
0 |
20 |
0 |
33.3% |
26.6% |
|
High Hopes Holdings, LLC(8)
219 Sykes Point Lane
Merritt Island, FL 32953 |
0 |
20 |
0 |
33.3% |
26.6% |
| All directors and officers as a group (1 person) |
0 |
20 |
0.0% |
33.3% |
26.6% |
*Less than 1%.
| (1) |
Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has direct ownership of and sole voting power and investment power with respect to the shares. For each beneficial owner above, any securities acquirable within 60 days have been included in the denominator in accordance with SEC Rule 13d-3(d)(1). |
| (2) |
Based on 2,940,667,506 shares of our Common Stock outstanding as of October 11, 2024. |
| (3) |
Based on 60 shares of our Series B Preferred Stock outstanding as of the date of this PQA. Shares of Series B Preferred Stock are convertible into shares of Common Stock on the basis of 100,000,000 shares of Common Stock for every share of Series B Preferred Stock. Each share of Series B Preferred Stock is entitled to one hundred million (100,000,000) votes per share on all matters to which they are so entitled to vote. |
|
(4) |
Percentage of Total Voting Stock represents
total ownership with respect to all shares of our Common Stock, Series A Preferred Stock and Series B Preferred Stock as a single class
and giving effect to the 1,000 for 1 vote of the Series A Preferred Stock and 100,000,000 for 1 vote of Series B Preferred Stock.
|
| (5) |
Holders of Series B Preferred Stock have
voting rights equal to four times the sum of: (a) the total number of shares of Common Stock which are issued and outstanding at the
time of voting; plus (b) the total number of votes holders of Series A Preferred Stock are entitled. |
| (6) |
Stephen W. Carnes is the managing member of this
entity. |
| (7) |
Leonard Greene, the sole officer and director of our company, is a trustee of this trust. |
| (8) |
Robert Levit is the managing member of this entity. |
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN
TRANSACTIONS
Except as set forth below, since
the beginning of our 2017 fiscal year, we have not entered into any transactions with any related persons in which the amount involved
exceeded the lesser of $120,000 and one percent of the average of our total assets at year-end for the last two completed fiscal years.
As of July 31, 2024, the Company owed $215,312
to Leonard Greene, the Company’s Chief Executive Officer. The loan is non-interest-bearing and unsecured, and due upon demand.
On February 8, 2019,
we issued to BNL Capital, LLC, a significant shareholder at that time, a total of 2 million shares of our Preferred Class A Stock as compensation
for services that BNL Capital, LLC rendered to us in connection with our acquisition of Mammoth Ventures Inc.
SECURITIES BEING OFFERED
We are offering 700,000,000 Offered Units, with
each Unit consisting of 3 shares of Common Stock. and 2 Warrants, with the Remaining Units being offered at an offering price of $__0.00085-0.002
per Unit. Through the sale of Offered Units, the Company is offering a maximum of 3,500,000,000 shares, including the Warrant Shares.
Our authorized capital stock consists of 10,000,000,000
shares of Common Stock, $0.0001 par value per share, and 20,000,000 shares of Preferred Class Stock, par value $0.0001. As of the date
of this Offering Circular, there are 2,940,667,506 shares of our Common Stock issued and outstanding, 888,390 shares of our Series A Preferred
Stock (the “Series A Preferred Stock”) issued and outstanding and 60 shares of Series B Convertible Preferred Stock (the “Series
B Preferred Stock”) issued and outstanding.
The following is a summary of the rights of our
capital stock as provided in our certificate of incorporation and bylaws. For more detailed information, please see our certificate of
incorporation and bylaws, which have been filed as exhibits to the offering statement of which this offering circular is a part.
Common Stock
Voting Rights. The holders of the Common
Stock are entitled to one vote for each share held of record on all matters submitted to a vote of the stockholders. Under our certificate
of incorporation and bylaws, any corporate action to be taken by vote of stockholders other than for election of directors shall be authorized
by the affirmative vote of the majority of votes cast. Directors are elected by a plurality of votes. Stockholders do not have cumulative
voting rights.
Dividend Rights. Subject to preferences
that may be applicable to any then-outstanding holders of our preferred stock, holders of our Common Stock are entitled to receive ratably
dividends, if any, as may be declared from time to time by the board of directors out of legally available funds.
Liquidation Rights. In the event of our
liquidation, dissolution or winding up, holders of Common Stock will be entitled to share ratably in the net assets legally available
for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference
granted to the holders of any then-outstanding shares of Preferred Stock.
Other Rights. Holders of Common Stock have
no preemptive, conversion or subscription rights and there are no redemption or sinking fund provisions applicable to the Common Stock.
The rights, preferences and privileges of the holders of Common Stock are subject to, and may be adversely affected by, the rights of
the holders of shares of any series of Preferred Stock.
Series A Preferred Stock
Voting Rights. Holders of shares of Series
A Preferred Stock vote together with the holders of Common Stock. Each share of Series A Preferred Stock is entitled to one thousand (1,000)
votes per share on all matters. Except as provided by law, the holders of shares of Series A Preferred Stock vote together with the holders
of shares of Common Stock as a single class.
In addition, so long as any shares of Series A
Preferred Stock remains outstanding, in addition to any other vote or consent of stockholders required by our certificate of incorporation,
the company will not, without the affirmative vote or consent of the holders of a majority of the outstanding shares of Series A Preferred
Stock: (i) effect a sale of all or substantially all of the company’s assets or which results in the holders of the company’s
capital stock owning less than fifty percent (50%) of the voting power of the company, (ii) alter or change the rights, preference, or
privileges of the Preferred Class A Stock, (iii) increase or decrease the number of authorized shares of Series A Preferred Stock, (iv)
authorize the issuance of securities having preference over or on par with the Series A Preferred Stock, (v) effectuate a forward or reverse
stock split or dividend of the company’s Common Stock, or (vi) increase the maximum number of directors constituting the board of
directors to a number greater than seven (7); with holders of Series A Preferred Stock having the right, but not the obligation, to fill
four (4) of such board seats.
Dividend Rights. We are not required to
pay dividends at any specific rate on the Series A Preferred Stock; provided, however, that if any dividend is paid on the outstanding
Common Stock, the Series A Preferred Stock would participate in such dividend on a pari passu basis with the holders
of Common Stock on an as converted to Common Stock basis.
Liquidation Rights. In the event of any
voluntary or involuntary liquidation, dissolution or winding up of the company, whether voluntary or involuntary,, before any payment
shall be made to the holders of Common Stock by reason of their ownership thereof, the holders of shares of Series A Preferred Stock then
outstanding shall be entitled to be paid out of the funds and assets available for distribution to its stockholders, an amount per share
equal to the amount that would be paid to one hundred shares of Common Stock (subject to adjustment), plus any dividends declared but
unpaid thereon.
Conversion Rights. Each share of Series
A Preferred Stock is convertible at any time, after one year from the issuance of such share, at the option of the holder into one hundred
(100) shares of Common Stock for each share of Series A Preferred Stock, subject to adjustment in the event of any stock splits, stock
combinations, recapitalizations and similar transactions.
Other Rights. Holders of Series A Preferred
Stock have no preemptive or subscription rights and there are no redemption or sinking fund provisions applicable to our Series A Preferred
Stock.
Series B Convertible Preferred Stock
Voting Rights. Each outstanding share of Series B Preferred Stock shall
be entitled to one hundred million (100,000,000) votes per share on all matters to which the shareholders of the Company are entitled
or required to vote. Further, holders of Series B Preferred Stoc shall have voting rights equal to four times the sum of: (a) the total
number of shares of Common Stock which are issued and outstanding at the time of voting; plus (b) the total number of votes to which holders
of Series A Preferred Stock are entitled. At no time can the combination of votes by holders of Common Stock and Series A Preferred Stock
be equal to or greater than the number of votes to which holders of Series B Preferred Stock shall be entitled.
Dividend Rights. We are not required to
pay dividends at any specific rate on the Series B Preferred Stock; provided, however, that if any dividend is paid on the outstanding
Common Stock, the Series B Preferred Stock would participate in such dividend on a pari passu basis with the holders of Common
Stock on an as converted to Common Stock basis.
Conversion Rights. Each share of Series
B Preferred Stock is convertible at any time, at the option of the holder into one hundred million (100,000,000) shares of Common Stock
for each share of Series B Preferred Stock, subject to adjustment in the event of any stock splits, stock combinations, recapitalizations
and similar transactions.
Transfer Agent and Registrar
The company has engaged Pacific Stock
Transfer Company, Inc. as its transfer agent and registrar. Pacific Stock Transfer’s address is 6725 Via Austi Parkway, Suite 300, Las
Vegas, NV 89119 and its telephone number is (800) 785-7782.
Shares Eligible for
Future Sale
Assuming the sale of all of the Offered Units,
and assuming the exercise of all of the Warrants, we will have 5,064,000,841 shares of Common Stock outstanding. The 3,500,000,000 shares
of Common Stock, including the Warrant Shares, sold in this offering will be freely transferable without restriction or further registration
under the Securities Act, subject to the limitations on ownership set forth in our charter.
LEGAL MATTERS
Certain legal matters with respect to the Units offered by this Offering
Circular will be passed upon by Newlan Law Firm, PLLC, Flower Mound, Texas.
INTERESTS OF NAMED EXPERTS AND COUNSEL
Newlan Law Firm, PLLC owns no securities of our
company.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC an offering statement
on Form 1-A under the Securities Act with respect to the Common Stock offered by this offering circular. This offering circular does not
contain all of the information included in the offering statement, portions of which are omitted as permitted by the rules and regulations
of the SEC. For further information pertaining to us and the Common Stock to be sold in this offering, you should refer to the offering
statement and its exhibits. Whenever we make reference in this offering circular to any of our contracts, agreements or other documents,
the references are not necessarily complete, and you should refer to the exhibits attached to the offering statement for copies of the
actual contract, agreement or other document filed as an exhibit to the offering statement or such other document, each such statement
being qualified in all respects by such reference. Upon the closing of this offering, we will be subject to the informational requirements
of Tier 1 of Regulation A and will be required to file annual reports, semi-annual reports, current reports and other information with
the SEC. We anticipate making these documents publicly available, free of charge, on our website as soon as reasonably practicable after
filing such documents with the SEC.
You can read the offering statement and our future
filings with the SEC over the Internet at the SEC’s website at www.sec.gov. You may also read and copy any document we file with
the SEC at its public reference facility at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents
at prescribed rates by writing to the Public Reference Section of the SEC. Please call the SEC at 1-800-SEC-0330 for further information
on the operation of the public reference facilities.
GREENE CONCEPTS, INC.
CONSOLIDATED
UNAUDITED FINANCIAL STATEMENTS
GREENE CONCEPTS, INC.
CONSOLIDATED BALANCE SHEETS
ON JULY 31, 2024 AND JULY 31,
2023
(UNAUDITED)
| | |
2024 | | |
2023 | |
| ASSETS | |
| | | |
| | |
| | |
| | | |
| | |
| CURRENT ASSETS | |
| | | |
| | |
| | |
| | | |
| | |
| Cash | |
$ | 238,182 | | |
$ | 195,450 | |
| | |
| | | |
| | |
| Accounts Receivable net of allowance of doubtful accounts | |
| 305,635 | | |
| 34,764 | |
| | |
| | | |
| | |
| Inventory | |
| 445,353 | | |
| 325,750 | |
| | |
| | | |
| | |
| Securities | |
| 33 | | |
| 342 | |
| | |
| | | |
| | |
| TOTAL CURRENT ASSETS | |
| 989,203 | | |
| 556,306 | |
| | |
| | | |
| | |
| FIXED ASSETS-NET | |
| 4,771,426 | | |
| 4,591,642 | |
| | |
| | | |
| | |
| OTHER ASSETS | |
| | | |
| | |
| | |
| | | |
| | |
| Investment in Subsidiary | |
| – | | |
| – | |
| | |
| | | |
| | |
| Due from subsidiary | |
| – | | |
| 703 | |
| | |
| | | |
| | |
| Subscription Programs | |
| 359,127 | | |
| 349,369 | |
| | |
| | | |
| | |
| TOTAL ASSETS | |
$ | 6,119,756 | | |
$ | 5,498,020 | |
| | |
| | | |
| | |
| LIABILITIES | |
| | | |
| | |
| | |
| | | |
| | |
| Accounts Payable | |
$ | 29,794 | | |
$ | 71,725 | |
| | |
| | | |
| | |
| Accrued Interest Payable | |
| – | | |
| 123,461 | |
| | |
| | | |
| | |
| Other Liabilities | |
| 196,998 | | |
| – | |
| | |
| | | |
| | |
| Notes Payable (Note 2) | |
| – | | |
| 211,873 | |
| | |
| | | |
| | |
| Note Payable Shareholder | |
| 2,462,109 | | |
| 2,129,420 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES | |
| 2,688,901 | | |
| 2,536,479 | |
| | |
| | | |
| | |
| STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | |
| | |
| | | |
| | |
| Preferred A Stock $.0001 par value 20,000,000 Authorized 888,390 issued & outstanding on July 31, 2024, and 888,390 issued & outstanding on July 31, 2023 | |
| 89 | | |
| 89 | |
| | |
| | | |
| | |
| Preferred B Stock $.001 par value 1,000 Authorized 60 issued & outstanding on July 31, 2024, and 60 issued & outstanding on July 31, 2023 | |
| – | | |
| – | |
| | |
| | | |
| | |
| Common Stock, $.0001 par value 10,000,000,000 Authorized 2,890,667,508 issued & outstanding at July 31, 2024, and 2,270,667,515 issued & outstanding at July 31, 2023 | |
| 289,067 | | |
| 227,067 | |
| | |
| | | |
| | |
| Additional paid-in-capital | |
| 11,206,736 | | |
| 10,557,536 | |
| | |
| | | |
| | |
| Retained earnings/(deficit) | |
| (8,065,037 | ) | |
| (7,823,151 | ) |
| | |
| | | |
| | |
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | |
| 3,430,855 | | |
| 2,961,541 | |
| | |
| | | |
| | |
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
$ | 6,119,756 | | |
$ | 5,498,020 | |
The accompanying notes are
an integral part of the consolidated financial statements.
GREENE CONCEPTS, INC.
CONSOLIDATED STATEMENT OF OPERATIONS
FOR THE THREE AND TWELVE MONTHS ENDED JULY 31,
2024, AND JULY 31, 2023
(UNAUDITED)
| | |
Three Months Ended July 31 | | |
Twelve Months Ended July 31 | |
| | |
2024 | | |
2023 | | |
2024 | | |
2023 | |
| REVENUES: | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Sales | |
$ | 236,099 | | |
$ | 124,250 | | |
$ | 856,545 | | |
$ | 719.732 | |
| | |
| | | |
| | | |
| | | |
| | |
| TOTAL REVENUE | |
| 236,099 | | |
| 124,250 | | |
| 856,545 | | |
| 719,732 | |
| | |
| | | |
| | | |
| | | |
| | |
| COST OF SALES | |
| 192,792 | | |
| 128,974 | | |
| 491,904 | | |
| 568,349 | |
| | |
| | | |
| | | |
| | | |
| | |
| GROSS MARGIN | |
| 43,307 | | |
| (4,724 | ) | |
| 364,641 | | |
| 151,383 | |
| | |
| | | |
| | | |
| | | |
| | |
| OPERATING EXPENSES: | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Administrative expenses | |
| 1,446 | | |
| 30,549 | | |
| 61,091 | | |
| 94,771 | |
| | |
| | | |
| | | |
| | | |
| | |
| Advertising | |
| 77,453 | | |
| – | | |
| 149,647 | | |
| – | |
| | |
| | | |
| | | |
| | | |
| | |
| Professional Fees | |
| 103,146 | | |
| 269,202 | | |
| 452,025 | | |
| 712,509 | |
| | |
| | | |
| | | |
| | | |
| | |
| Depreciation | |
| 17,241 | | |
| 17,241 | | |
| 68,962 | | |
| 88,678 | |
| | |
| | | |
| | | |
| | | |
| | |
| Marketing | |
| 2,719 | | |
| 19,079 | | |
| 11,653 | | |
| 319,341 | |
| | |
| | | |
| | | |
| | | |
| | |
| Brand Consulting | |
| 33,879 | | |
| 73,253 | | |
| 130,653 | | |
| 322,995 | |
| | |
| | | |
| | | |
| | | |
| | |
| Plant operations | |
| 17,914 | | |
| 2,636 | | |
| 30,272 | | |
| 19,021 | |
| | |
| | | |
| | | |
| | | |
| | |
| Taxes | |
| – | | |
| – | | |
| 11,553 | | |
| 1,394 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total Operating expenses | |
| 253,798 | | |
| 411,960 | | |
| 915,857 | | |
| 1,308,329 | |
| | |
| | | |
| | | |
| | | |
| | |
| NET OPERATING INCOME/ (LOSS) | |
| (210,491 | ) | |
| (416,684 | ) | |
| (551,216 | ) | |
| (1,156,946 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| OTHER INCOME/(EXPENSES) | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | |
| Write off of Convertible Debt | |
| 211,873 | | |
| – | | |
| 211,873 | | |
| – | |
| | |
| | | |
| | | |
| | | |
| | |
| Finance and interest fees | |
| 112,268 | | |
| (25,881 | ) | |
| 97,049 | | |
| (243,177 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Interest income | |
| 135 | | |
| 149 | | |
| 408 | | |
| 451 | |
| | |
| | | |
| | | |
| | | |
| | |
| Total Other Income/(Expenses) | |
| 324,276 | | |
| (25,732 | ) | |
| 309,330 | | |
| (242,726 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| NET INCOME/ (LOSS) | |
$ | 113,785 | | |
$ | (442,416 | ) | |
$ | (241,886 | ) | |
$ | (1,399,672 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Basic and Diluted Loss per Common Share | |
$ | .00004 | | |
$ | (.0002 | ) | |
$ | (.00008 | ) | |
$ | (.000625 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted Average Number of Common Shares Outstanding | |
| 2,890,667,508 | | |
| 2,270,667,515 | | |
| 2,890,667,508 | | |
| 2,270,667,515 | |
The accompanying notes are an integral part of
the consolidated financial statements.
GREEN CONCEPTS, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’
DEFICIT
FOR THE TWELVE MONTHS ENDED JULY 31, 2023
(UNAUDITED)
| | |
PREFERRED | | |
COMMON | | |
ADDITIONAL PAID | | |
ACCUMULATED EQUITY | | |
TOTAL SHAREHOLDERS EQUITY | |
| | |
SHARES | | |
VALUE | | |
SHARES | | |
VALUE | | |
IN CAPITAL | | |
(DEFICIT) | | |
(DEFICIT) | |
| BALANCE
JULY 31, 2022 | |
| 888,450 | | |
$ | 89 | | |
| 2,084,667,515 | | |
$ | 208,467 | | |
$ | 9,987,136 | | |
$ | (6,419,241 | ) | |
$ | 3,776,451 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARE FOR REG
A | |
| – | | |
| – | | |
| 30,000,000 | | |
| 3,000 | | |
| 222,000 | | |
| – | | |
| 225,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET INCOME/(LOSS)
OCTOBER 31, 2022 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (408,219 | ) | |
| (408,219 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE OCTOBER
31, 2022 | |
| 888,450 | | |
$ | 89 | | |
| 2,114,667,515 | | |
$ | 211,467 | | |
$ | 10,209,136 | | |
$ | (6,827,460 | ) | |
$ | 3,593,231 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET INCOME/(LOSS)
JANUARY 31, 2023 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (257,127 | ) | |
| (257,127 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE JANUARY
31, 2023 | |
| 888,450 | | |
$ | 89 | | |
| 2,114,667,515 | | |
$ | 211,467 | | |
$ | 10,209,136 | | |
$ | (7,116,587 | ) | |
$ | 3,304,103 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARE FOR REG
A | |
| – | | |
| – | | |
| 24,000,000 | | |
| 2,400 | | |
| 53,600 | | |
| – | | |
| 56,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS APRIL
30, 2023 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (296,906 | ) | |
| (296,906 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE APRIL
30, 2023 | |
| 888,450 | | |
$ | 89 | | |
| 2,138,667,515 | | |
$ | 213,867 | | |
$ | 10,262,736 | | |
$ | (7,380,735 | ) | |
$ | 3,095,956 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARES FOR REG
A | |
| – | | |
| – | | |
| 132,000,000 | | |
| 13,200 | | |
| 294,000 | | |
| – | | |
| 308,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS JULY
31, 2023 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (442,416 | ) | |
| (442,416 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE JULY
31, 2023 | |
| 888,450 | | |
$ | 89 | | |
| 2,270,667,515 | | |
$ | 227,067 | | |
$ | 10,570,736 | | |
$ | (7,823,151 | ) | |
$ | 2,974,740 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARES FOR REG
A | |
| – | | |
| – | | |
| 90,000,000 | | |
| 9,000 | | |
| 159,000 | | |
| – | | |
| 168,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS OCTOBER
31,2023 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (233,831 | ) | |
| (233,831 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE OCTOBER
31, 2023 | |
| 884,450 | | |
$ | 89 | | |
| 2,270,667,515 | | |
$ | 236,067 | | |
$ | 10,729,736 | | |
$ | (8,056,982 | ) | |
$ | 2,908,910 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS JANUARY
31, 2024 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (20,704 | ) | |
| (20,704 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE JANUARY
31, 2024 | |
| 888,450 | | |
$ | 89 | | |
| 2,360,667,515 | | |
$ | 236,067 | | |
$ | 10,729,736 | | |
$ | (8,077,686 | ) | |
$ | 2,888,205 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARES FOR REG
A | |
| – | | |
| – | | |
| 119,999,996 | | |
| 12,000 | | |
| 108,000 | | |
| – | | |
| 120,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET LOSS APRIL 30, 2024 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| (101,137 | ) | |
| (101,137 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE JANUARY 31, 2024 | |
| 888,450 | | |
| 89 | | |
| 2,480,667,511 | | |
| 248,067 | | |
| 10,837,736 | | |
| (8,178,824 | ) | |
| 2,907,068 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| ISSUANCE OF COMMON SHARES FOR REG
A | |
| – | | |
| – | | |
| 409,999,997 | | |
| 41,000 | | |
| 369,000 | | |
| – | | |
| 41,000 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| NET INCOME JULY
31 2024 | |
| – | | |
| – | | |
| – | | |
| – | | |
| – | | |
| 113,785 | | |
| 113,785 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| BALANCE JULY
31, 2024 | |
| 888,450 | | |
$ | 89 | | |
| 2,890,667,515 | | |
$ | 289,067 | | |
$ | 11,206,736 | | |
$ | (8,065,038 | ) | |
$ | 3,430,853 | |
The accompanying notes are an integral part of the consolidated financial
statements.
GREENE CONCEPTS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE TWELVE MONTHS ENDED JULY 31, 2023 &
JULY 31, 2022
(UNAUDITED)
| | |
2024 | | |
2023 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | |
| | | |
| | |
| | |
| | | |
| | |
| Net Income / (Loss) | |
$ | (241,886 | ) | |
$ | (1,399,672 | ) |
| | |
| | | |
| | |
| Adjustments to reconcile net income to net cash provided by operating activities: | |
| | | |
| | |
| | |
| | | |
| | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| | |
| | | |
| | |
| Write off of Debt | |
| (211,873 | ) | |
| – | |
| | |
| | | |
| | |
| Depreciation and amortization | |
| 68,962 | | |
| 68,962 | |
| | |
| | | |
| | |
| (Increase)/decrease in Due from subsidiary | |
| – | | |
| 45,923 | |
| | |
| | | |
| | |
| (Increase)/decrease in accounts receivable | |
| (270,871 | ) | |
| (24,714 | ) |
| | |
| | | |
| | |
| Increase/ (decrease) in accounts payable | |
| (41,931 | ) | |
| (57,354 | ) |
| | |
| | | |
| | |
| Increase/ (decrease) in accrued interest payable | |
| (123,461 | ) | |
| 66,718 | |
| | |
| | | |
| | |
| Increase/(decrease) in other current liabilities | |
| 196,998 | | |
| (10,260 | ) |
| | |
| | | |
| | |
| (Increase)/decrease in other current assets | |
| 10,768 | | |
| 2,000 | |
| | |
| | | |
| | |
| (Increase)/decrease in inventory | |
| (119,603 | ) | |
| 5,346 | |
| | |
| | | |
| | |
| NET CASH PROVIDED (USED) BY OPERATING ACTIVITIES | |
| (732,897 | ) | |
| (1,313,051 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM INVESTING ACTIVITIES | |
| | | |
| | |
| | |
| | | |
| | |
| Investment in Fixed Assets | |
| (248,748 | ) | |
| (119,655 | ) |
| | |
| | | |
| | |
| NET CASH PROVIDED (USED) BY INVESTING ACTIVITIES | |
| (248,748 | ) | |
| (119,655 | ) |
| | |
| | | |
| | |
| CASH FLOWS FROM FINANCING ACTIVITIES | |
| | | |
| | |
| | |
| | | |
| | |
| Sale of branding rights | |
| – | | |
| – | |
| | |
| | | |
| | |
| (Decrease)/Increase in notes payable | |
| 326,377 | | |
| 133,645 | |
| | |
| | | |
| | |
| (Decrease)/Increase in REG A Equity Investment | |
| 698,000 | | |
| 589,000 | |
| | |
| | | |
| | |
| NET CASH PROVIDED (USED) BY FINANCING ACTIVITIES | |
| 1,024,377 | | |
| 722,645 | |
| | |
| | | |
| | |
| NET INCREASE/ (DECREASE) IN CASH | |
| 42,732 | | |
| (700,641 | ) |
| | |
| | | |
| | |
| CASH AND EQUIVALENTS, BEGINNING OF PERIOD | |
| 195,450 | | |
| 895,511 | |
| | |
| | | |
| | |
| CASH AND EQUIVALENTS, END OF PERIOD | |
$ | 238,182 | | |
$ | 195,450 | |
| | |
| | | |
| | |
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION | |
| | | |
| | |
The accompanying notes are an integral part of
the consolidated financial statements.
GREENE CONCEPTS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
JULY 31, 2023
(UNAUDITED)
NOTE 1 – ORGANIZATION AND OPERATIONS
Greene Concepts, Inc. is headquartered in the
City of Fresno, California and has been in service for fifty-eight years. The Company manufactured and distributed a line of 25 high quality
consumer focused inkjet kits. The Company has recently divested itself of these operations and have acquired a facility that will be focused
on production of a variety of beverage product lines including, but not limited to CBD infused beverages, spring and artesian water, as
well as enhanced athletic drinks in addition to other product offerings The Company has prepared these financial statements on the accrual
basis of accounting.
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES
A. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies
of Greene Concepts, Inc. (the Company) is presented to assist in understanding the Company’s financial statements. The financial
statements and notes are representations of the Company’s management who is responsible for the integrity and objectivity of the
financial statements. These accounting policies conform to generally accepted accounting principles and have been consistently applied
in the preparation of financial statements.
B. BASIS OF ACCOUNTING
The Company utilizes the accrual method of accounting,
whereby revenue is recognized when earned and expenses when incurred. The unaudited financial statements have been prepared in accordance
with generally accepted accounting principles for interim financial information. As such, the financial statements do not include all
of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion
of management, all adjustments considered necessary for a fair presentation have been included and these adjustments are of a normal recurring
nature.
C. USE OF ESTIMATES
The preparation of financial statements in conformity
with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of
assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts
of revenues and expenses during the period. Actual results could differ from those estimates.
D. CASH AND CASH EQUIVALENTS
Cash and cash
equivalents include cash on hand; cash in banks and any highly liquid investments with a maturity of three months or less at the time
of purchase. The Company maintains cash and cash equivalent balances at several financial institutions, which are insured by the Federal
Deposit Insurance Corporation for up to $250,000.
E. FIXED ASSETS
Fixed assets are carried
at cost. Depreciation is computed using the straight-line method of depreciation over the assets estimated useful lives. Maintenance
and repairs are charged to expense as incurred; major renewals and improvements are capitalized. When items of fixed assets are sold
or retired, the related cost and accumulated depreciation is removed from the accounts and any gain or loss is included in the income.
In February 2019 the Company acquired Mammoth Ventures Inc. which included all assets owned
by Mammoth including the Marion, North Carolina facility and all bottling equipment and other assets formerly known as the North Cove
Springs Bottling and Beverage from BNL Capital LLC
F. COMPUTATION OF EARNINGS PER SHARE
Net income per share is computed by dividing the
net income by the weighted average number of common shares outstanding during the period.
G. INCOME TAXES
In February 1992, the Financial Accounting Standards
Board issued Statement on Financial Accounting Standards 109 of “Accounting for Income Taxes.” Under Statement 109, deferred
tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial
statement carrying amounts of existing assets and liabilities and their respective tax bases.
| | |
As of
July 31, 2024 | | |
As of
July 31, 2023 | |
| Net operating loss carryforward | |
$ | (8,065,038 | ) | |
$ | (7,380,735 | ) |
| Valuation allowance | |
| (8,065,038 | ) | |
| (7,380,725 | ) |
| | |
| | | |
| | |
| Net deferred tax assets | |
$ | – | | |
$ | – | |
A reconciliation of the statutory federal income
tax rate to the Company’s effective tax rate is as follows:
| | |
For the Three Months ended
July 31, 2024 | | |
For the Three Months ended
July 31, 2023 | |
| Expected federal statutory rate | |
| (21)% | | |
| (21)% | |
| State Effect on tax rate, net of federal benefit | |
| (4.35)% | | |
| (4.35)% | |
| Change in valuation allowance | |
| 25.35% | | |
| 25.35% | |
| | |
| | | |
| | |
| Income tax provision (benefit) | |
| 1,778,151 | | |
| 1,627,278 | |
The Company, after considering all available evidence,
fully reserved its deferred tax assets since it is more likely than not that such benefits may be realized in future periods. The Company
has not yet established that it can generate taxable income. The Company will continue to evaluate its deferred tax assets to determine
whether any changes in circumstances could affect the realization of their future benefit. If it is determined in future periods that
portions of the Company’s deferred tax assets satisfy the realization standards, the valuation allowance will be reduced accordingly.
G. REVENUE RECOGNITION
Revenue for license fees is recognized upon the
execution and closing of the contract for the amount of the contract. Contract fees are generally due based upon various progress milestones.
Revenue from contract payments are estimated and accrued as earned. Any adjustments between actual contract payments and estimates are
made to current operations in the period they are determined.
H. FAIR VALUE OF FINANCIAL INSTRUMENTS
Statement of Financial Accounting Standards No.
107, “Disclosures about Fair Value of Financial Instruments”, requires disclosures of information about the fair value of
certain financial instruments for which it is practicable to estimate the value. For purpose of this disclosure, the fair value of a financial
instrument is the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a
forced sale or liquidation. The carrying amounts reported in the balance sheet for cash, accounts receivable, inventory, accounts payable
and accrued expenses, and loans payable approximate their fair market value based on the short-term maturity of these instruments.
NOTE 2 – NOTES AND OTHER LOANS PAYABLE
Convertible Notes.
| Date |
|
Name |
|
Principal |
|
Interest Rate |
|
Maturity Date |
| October 1, 2018 |
|
Bradley Wilson |
|
$ |
6,000.00 |
|
|
12.00% APR |
|
October 1,2019 |
| October 5, 2018 |
|
Bradley Wilson |
|
$ |
1,150.00 |
|
|
12.00% APR |
|
October 5, 2019 |
| October 26, 2018 |
|
Bradley Wilson |
|
$ |
12,000.00 |
|
|
12.00% APR |
|
October 26, 2019 |
| October 26, 2018 |
|
Bradley Wilson |
|
$ |
9,223.00 |
|
|
12.00% APR |
|
October 26, 2019 |
| November 15, 2018 |
|
Bradley Wilson |
|
$ |
10,000,00 |
|
|
12.00% APR |
|
November 15, 2019 |
| December 11, 2018 |
|
Bradley Wilson |
|
$ |
10,600.00 |
|
|
12.00% APR |
|
December 11,2019 |
| December 17, 2018 |
|
CDN Associates, LLC |
|
$ |
10,000.00 |
|
|
8.00% APR |
|
December 18, 2019 |
| January 16,2019 |
|
CDN Associates, LLC |
|
$ |
5,000.00 |
|
|
8.00% APR |
|
January 16, 2020 |
| February 6, 2019 |
|
Nuemark Group LLC |
|
$ |
25,000.00 |
|
|
8.00% APR |
|
February 6,2020 |
| February 8, 2019 |
|
Nuemark Group LLC |
|
$ |
15,000.00 |
|
|
8.00% APR |
|
February 8,2020 |
| February 22, 2019 |
|
Nuemark Group LLC |
|
$ |
15,000.00 |
|
|
8.00% APR |
|
February 22,2020 |
| March 6, 2019 |
|
Shaun Diedrich |
|
$ |
2,000.00 |
|
|
8.00% APR |
|
March 6, 2020 |
| January 24, 2020 |
|
Bradley Wilson |
|
$ |
44,400.00 |
|
|
12.00% APR |
|
January 24, 2021 |
| February 19, 2020 |
|
Bradley Wilson |
|
$ |
25,000.00 |
|
|
12.00% APR |
|
February 19, 2021 |
| March 26, 2020 |
|
Bradley Wilson |
|
$ |
10,000.00 |
|
|
12.00% APR |
|
March 26, 2021 |
The Company wrote off the principal and accumulated interest of the
above listed Notes in the fourth Quarter of the fiscal year.
The Principal of the Notes written off was Notes $211, 873.00 and accumulated
interest $102,067.54.
Total Notes with Accumulated Interest written off $ 313,940.55
NOTE 3 – REGULATION A OFFERING
The Company has sold 82,000,000 units of its securities,
with each unit being comprised of three shares of common stock and two warrants to purchase a share of common stock at an exercise price
of $.01 per share, in its current Regulation A offering (SEC File No. 024-12157), for a total of $532,000 in cash.
NOTE 4 – BUSINESS DEVELOPMENTS DURING
FISCAL 2024
Increased Retail Locations
Greene Concepts, Inc. (GCI) continues its year of exponential growth
through 2024 to include significant retail expansion through its sale of its flagship BE WATER brand throughout Walmart stores in North
and South Carolina along with a Walmart blanket purchase order agreement through the remainder of 2024 and beyond. GCI also hired a merchandiser
company (Anderson Merchandisers) to ensure optimal in-store placement, inventory management and performance tracking within North and
South Carolina Walmart stores.
The company began placement of its flagship BE WATER on Walmart shelves
in North and South Carolina beginning May of 2024 following its showcase and approval of the brand by Walmart at Walmart’s corporate
headquarters in Bentonville, Arkansas during the October 2023 Walmart Open Call event.
Additional sales continue through multiple online marketplaces throughout
the U.S. and multiple retail outlets (to include new national vendors). The number of retail outlets continues to rise nationally along
with consumer awareness. The current list of retailers carrying GCI brands includes Walmart, Walmart Marketplace, Camping World, Vitamin
Outlet, Bashas, Amazon, Piggly Wiggly and one hundred other business locations near and around the Marion, NC bottling plant.
Increased Marketing
The Company signed an agreement with New to the Street for a 6-part
media series initiative showcasing current INKW happenings to the masses. This includes digital ads, billboards, videos, and commercials
along with sponsored programming on Bloomberg TV and FOX Business Network.
GCI launched a media campaign with FMW Media’s New to the Street
broadcast platform to expand nationwide awareness for both BE WATER. The interview carries a reach of up to 224 million national and international
households weekly.
GCI’s brand ambassador, Pancho Alvarez, continues to effectively
promote and grow the BE WATER brand regionally within 75 miles of the Company’s bottling plant and also on a national level through
social media platforms. Mr. Alvarez also verifies product placement within each store to ensure proper display and branding. The Company
is also working with many retailers to create planograms to add new BE WATER products as they plan product display layouts in an effort
to be proactive in its approach with Walmart and a number of other retailers toward continued national expansion in 2024.
Increased Partnerships
The Company announced a partnership with Tocca Life Holdings Incorporated
(“Tocca Life”) to include Tocca Life Holding's subsidiaries, Be Climbing Inc. and Aiguille Rock Climbing Center. The partnership
provides BE WATER awareness, sales, and sponsorship support to the climbing centers (indoor rock climbing is a $2.91 billion market).
The partnership also increases opportunities for health and wellness within the $4.5 trillion U.S. healthcare industry while escalating
brand awareness for BE WATER among Florida rock climbing enthusiasts.
Business Plan Objectives
GCI’s growth to retailers nationwide, including its ongoing Walmart
distribution, remains in-line with its business plan objectives:
| · Expand its National Broker Network |
| · Increase its Manufacturing Capacity |
| · Expand Retail Distribution |
| · Add Additional Support Staff (supports the broker network, distributors, and retailers) |
| · Increase its Marketing and Influencer Campaigns |
Increase Retail Growth Streamlining and Preparation
To fully support the Company’s advent into Walmart, GCI engaged
in internal training and production preparation to satisfy Walmart’s logistical requirements long-term. In addition to GCI’s
warehouse staff completing Walmart Academy Training, GCI hired SPS Commerce for Electronic Data Exchange (EDI) integration, automation,
and compliance. A world leader in EDI, SPS Commerce serves as a strategic full-service partner to set up, train and support GCI staff
on all Walmart EDI vendor compliance procedures to include automated invoice, shipping, and order processing. These actions allow the
Company to get BE WATER onto more Walmart shelves faster and ultimately into the hands of more customers.
Company Expansion and Product Line Increase
GCI continued its sales increase through the growth of its product
lines on Walmart’s Online Marketplace with the availability of both 6-pack and four 6-pack (24 total bottles) configurations for
purchase on www.walmart.com. GCI also announced new six-pack delivery configurations of its BE WATER brand to Camping World beyond its
historical 24-pack configuration for easier shelf placement/display and consumer transport for purchasing. This offers Camping World store
managers multiple selection options between six-packs and the legacy 24-packs of BE WATER.
The Company is also preparing to maximize sales by presenting its gallon-size
configuration at the September 24 – 25, 2024 Open Call event at Walmart’s corporate headquarters in Bentonville, AK. If approved,
Walmart will begin selling GCI’s gallon-size bottles at both Walmart’s physical stores and on Walmart Marketplace. While at
Walmart’s corporate headquarters, the Company was invited to mentor new and potential Walmart vendors along with offering BE WATER
bottles to all Open Call participants and several hundred Walmart associates.
GCI has also received multiple Co-pack pre-orders resulting in multiple
monthly truckloads of gallon-sized bottles with four (4) one-gallon sized bottles included in each branded box.
GCI completed the installation of the gallon-sized production line
to fulfill large-scale purchase orders to ensure clean, sustainable, and eco-friendly bottled artesian spring water.
To proliferate BE WATER expansion, GCI is producing and distributing
production to meet the needs of national accounts and expanded customer reach.
1. 24–Pack Cases: Ideal for high demand and bulk
availability.
2. 6–Pack Cases: Smaller size for easy shelf placement,
portability, and convenience.
3. Four 6-Pack Cases: Combines flexibility and scale
with 24 bottles across four packs.
4. Single Bottle: Perfect for cooler displays and single
shelf placements.
5. Gallon-Size: Cost-effective, versatile, and ideal
for families, parties, and larger gatherings.
6. Private (Third-Party) Label: Customized manufacturing
solutions with scalable production, market adaptability, and product diversification.
GCI continues preparing to expand its manufacturing facility by 20,000
sq ft to make it a 75,000 sq ft beverage and bottling facility positioned to serve larger regional and national accounts along with white
label manufacturing for outside entities. The building expansion allows for operational growth while affording GCI the space needed to
install a high-speed line and also the ability to blow its own bottles which will bring down the cost of goods considerably thereby
providing a higher profit margin for GCI.
Bottled Water Industry Connection
Bottled Water continues its expansion as a staple item in the current
U.S. economy. Current growth initiatives are occurring within a thriving bottled water industry currently valued at $318 billion in 2023
with a projected reach of $538.58 billion by 2033 at a compound annual growth rate of 5.4% according to Precedence Research with the U.S.
being the largest consumer of bottled water in the world. Key factors driving this growth include water scarcity in certain regions, the
easy availability of bottled water in retail outlets and increasing consumer health consciousness.
NOTE 5 - SUBSEQUENT EVENTS
Management has evaluated subsequent events through
the date these financial statements were available to be issued. Based on such evaluation, there are no material events that have occurred
that require further disclosure.
Greene Concepts (PK) (USOTC:INKW)
Historical Stock Chart
From Feb 2025 to Mar 2025
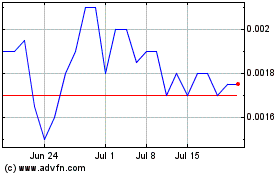
Greene Concepts (PK) (USOTC:INKW)
Historical Stock Chart
From Mar 2024 to Mar 2025