false 0001534133 0001534133 2024-02-13 2024-02-13
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
February 13, 2024
Date of Report (Date of earliest event reported)
CalciMedica, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Delaware |
|
001-39538 |
|
45-2120079 |
| (State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 505 Coast Boulevard South, Suite 307 La Jolla, California |
|
92037 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: (858) 952-5500
Not Applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
CALC |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. |
Regulation FD Disclosure. |
On February 13, 2024, CalciMedica, Inc. (the “Company”) posted an updated corporate presentation under the “Investors and Media” section of the Company’s website. The Company may use the corporate presentation from time to time in conversations with analysts, investors and others. A copy of the corporate presentation is included as Exhibit 99.1 to this report and is incorporated herein by reference.
The information in this Item 7.01, including the attached Exhibit 99.1, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing made by the Company under the Securities Act of 1933, as amended, or the Exchange Act.
FDA Clearance of IND Application for Phase 2 Trial of Auxora for the Treatment of Severe Acute Kidney Injury
On February 13, 2024, the Company announced the clearance of its Investigational New Drug application (“IND”) by the U.S. Food and Drug Administration (“FDA”) for the Company’s lead product candidate, Auxora, a potent and selective small molecule inhibitor of Orai1-containing calcium release-activated calcium channels, to be evaluated in a Phase 2 trial in acute kidney injury (“AKI”) with associated acute hypoxemic respiratory failure (“AHRF”). The Company expects to initiate the trial, named KOURAGE, in the first half of 2024 with data expected in 2025.
AKI is classified as stages 1, 2 and 3 depending on the degree of kidney injury. In the presence of AHRF, stage 2 and stage 3 AKI, both classified as severe, put patients at a 50% or greater risk for death while hospitalized and in the 90 days after discharge. Survivors of severe AKI may develop or progress to chronic kidney disease, leading to an eventual need for dialysis. There are approximately 1.1 million patients in the United States suffering from stage 2 and 3 AKI over half of whom have associated AHRF. There are currently no approved therapies for AKI.
KOURAGE is a randomized, double-blind, placebo-controlled study that will evaluate 150 patients with stage 2 and 3 AKI who have AHRF and are receiving oxygen by non-invasive mechanical ventilation, high flow nasal cannula or intermittent mandatory ventilation (“IMV”). Patients will be stratified by classification of stage of AKI as well as the use of IMV. Patients will receive either a four-hour infusion of Auxora or placebo at 1.25 mL/kg as a first dose, after which they will receive Auxora or placebo at 1.0 mL/kg at hours 24, 48, 72 and 96. The primary endpoint of the trial will be evaluation of patients through day 30 to determine days alive, ventilator-free and dialysis-free. Secondary endpoints will include a composite of all-cause mortality, decrease in estimated glomerular filtration rate (“eGFR”), and the incidence of dialysis over a period of 90 days, also known as MAKE-90 (Major Adverse Kidney Events at 90 days).
AKI is a common consequence of severe COVID-19 pneumonia and in the Company’s CARDEA trial, which studied Auxora in patients with severe and critical COVID-19 pneumonia, results showed a nearly 40% reduction in reported AKI in Auxora-treated patients as compared to placebo-treated patients. In a post-hoc analysis of CARDEA patients with compromised kidney function (eGFR< 60 mL/min/1.73 m2) at enrollment, the drug was well tolerated and there was a survival benefit for patients treated with Auxora compared to those on placebo. Biomarker analysis from blood samples taken from over 190 CARDEA patients showed that Auxora increased Angiopoietin-1 while decreasing Angiopoietin-2, suggesting stabilization of the endothelium and the potential to treat AKI. Finally, published work from others showed that elevated serum IL-17 levels, a CRAC channel-mediated cytokine, were differentially elevated in critically ill patients with stage 2 and 3 AKI when compared to those without AKI, and the elevation was independently associated with both hospital mortality and long-term adverse outcomes.
The Company’s initial pre-clinical studies in an ischemia/reperfusion injury (“IRI”) model of AKI were encouraging. A single dose of Auxora after IRI increased GFR by 61% and decreased mononuclear (inflammatory) cell infiltration by 30%. Further details from this study and results from the Company’s more recent pre-clinical study of multiple doses of Auxora given over several days and initiated after a greater time interval following IRI were also very strong and will be presented at the 29th International AKI & Continuous Renal Replacement Therapy Conference taking place March 12-15, 2024 in San Diego, CA.
Pipeline and Corporate Updates
On February 13, 2024, the Company provided the following updates to its pipeline for its product candidates:
| |
• |
|
data is expected in the first half of 2024 for the Company’s Phase 2b CARPO trial of Auxora in acute pancreatitis (“AP”) with accompanying systemic inflammatory response syndrome (“SIRS”); |
| |
• |
|
data is expected in the second half of 2024 for the investigator-sponsored Phase 1/2 CRSPA trial of Auxora in pediatric patients with asparaginase-induced pancreatic toxicity (“AIPT”) as a side effect of pediatric acute lymphoblastic leukemia treatment with asparaginase; |
| |
• |
|
data is expected in the first half of 2024 for the Company’s Phase 2 biomarker clinical trial of Auxora in mechanically ventilated COVID-19 pneumonia patients with acute respiratory distress syndrome (“ARDS”); |
| |
• |
|
the Company plans to submit an IND to the FDA in the second half of 2024 for the Company’s oral candidate (CM6336) for the treatment of chronic pancreatitis; and |
| |
• |
|
the Company plans to submit an IND to the FDA in the second half of 2024 for the Company’s oral candidate (CM6336) for the treatment of rheumatoid arthritis. |
In addition, following the completion of the previously announced private placement transaction, the Company expects to have a cash runway into the second half of 2025.
Cautionary Statement Regarding Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements which include, but are not limited to, statements regarding the Company’s planned and ongoing clinical trials and the timing, design, expected patient enrollment and timing for the release of data thereof, including its planned Phase 2 clinical trial of Auxora in AKI with associated AHRF, its ongoing Phase 2b CARPO trial of Auxora in AP with accompanying SIRS, its ongoing Phase 1/2 CRSPA trial of Auxora in pediatric patients with AIPT; the potential benefits of Auxora for the treatment of AKI; the estimated patient population in the United States for AKI; plans to present results from the Company’s pre-clinical studies in an ischemia/reperfusion injury model of AKI at the 29th International AKI & Continuous Renal Replacement Therapy Conference; the Company’s development plans for Auxora; the Company’s plans to submit IND applications to the FDA for its oral candidate CM6336 for the treatment of chronic pancreatitis and rheumatoid arthritis and the timing thereof; and the Company’s expectations regarding its cash runway. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. The Company’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the impact of fluctuations in global financial markets on the Company’s business and the actions it may take in response thereto; the Company’s ability to execute its plans and strategies; the ability to obtain and maintain regulatory approval for Auxora; results from clinical trials or preclinical studies may not be indicative of results that may be observed in the future; potential safety and other complications from Auxora; the scope progress and expansion of developing and commercializing Auxora; the size and growth of the market therefor and the rate and degree of market acceptance thereof; economic, business, competitive, and/or regulatory factors affecting the business of the Company generally; the Company’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and elsewhere in the Company’s most recent filings with the SEC, including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and any subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the SEC from time to time.
The forward-looking statements included in this Current Report on Form 8-K are made only as of the date hereof. The Company assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: February 13, 2024 |
|
CalciMedica, Inc. |
|
|
|
|
|
|
|
|
By: |
|
/s/ A. Rachel Leheny, Ph.D. |
|
|
|
|
Name: |
|
A. Rachel Leheny, Ph.D. |
|
|
|
|
Title: |
|
Chief Executive Officer |

Exhibit 99.1 Developing Novel Therapies for Acute Inflammatory and
Immunologic Diseases February 2024 1

Forward-Looking Statements This presentation contains forward-looking
statements which include, but are not limited to, statements regarding CalciMedica’s business strategy and clinical development plans; the design and potential benefits of CalciMedica’s product candidates; CalciMedica’s ongoing and
planned clinical trials; the timing for CalciMedica’s receipt and announcement of data from its clinical trials; the estimated patient populations and addressable market for CalciMedica’s product candidates; and expectations regarding
CalciMedica’s cash runway. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. CalciMedica’s expectations and beliefs regarding these matters may not
materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties
related to: the impact of fluctuations in global financial markets on CalciMedica’s business and the actions it may take in response thereto; CalciMedica’s ability to execute its plans and strategies; the ability to obtain and maintain
regulatory approval for CalciMedica’s product candidates; results from clinical trials may not be indicative of results that may be observed in the future; potential safety and other complications from CalciMedica’s product candidates;
economic, business, competitive, and/or regulatory factors affecting the business of CalciMedica generally; CalciMedica’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional
risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in CalciMedica’s most recently filed
periodic report, and subsequent periodic reports filed by CalciMedica, under the Securities Exchange Act of 1934, as amended, from time to time and available at www.sec.gov. These documents can be accessed on CalciMedica’s web page at
calcimedica.com. These forward-looking statements are based on information available to, and expectations of, CalciMedica of the date of this presentation. CalciMedica disclaims any obligation to update these forward-looking statements, except as
may be required by law. 2
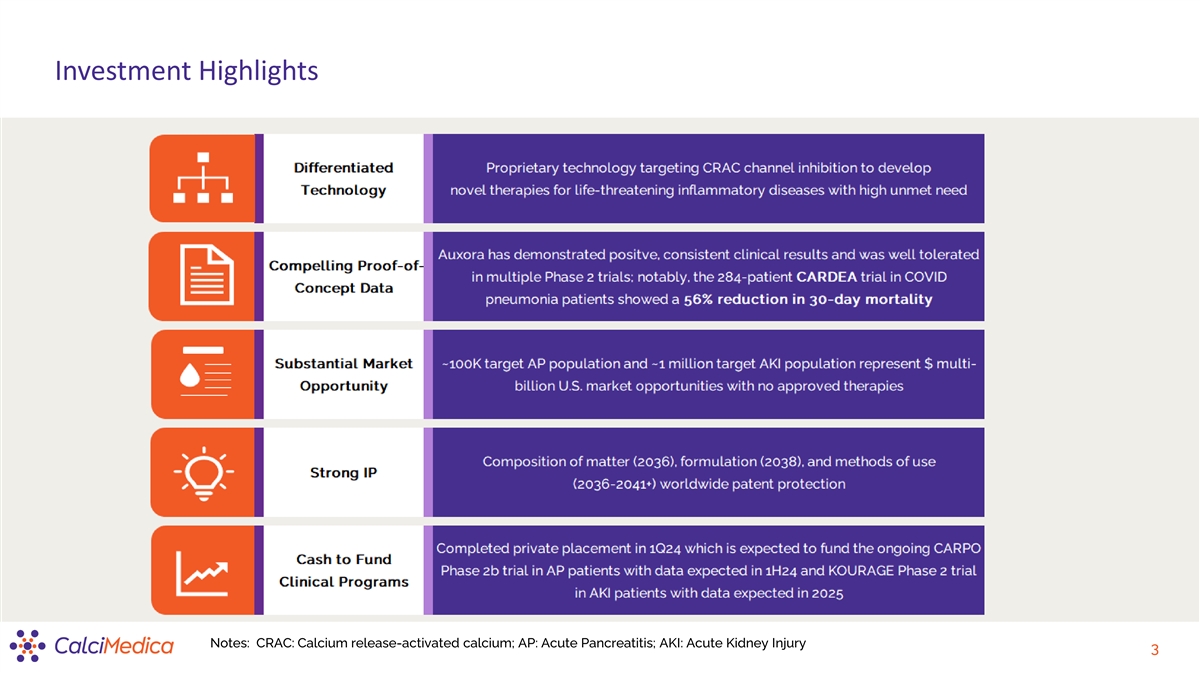
Investment Highlights Notes: CRAC: Calcium release-activated calcium;
AP: Acute Pancreatitis; AKI: Acute Kidney Injury 3
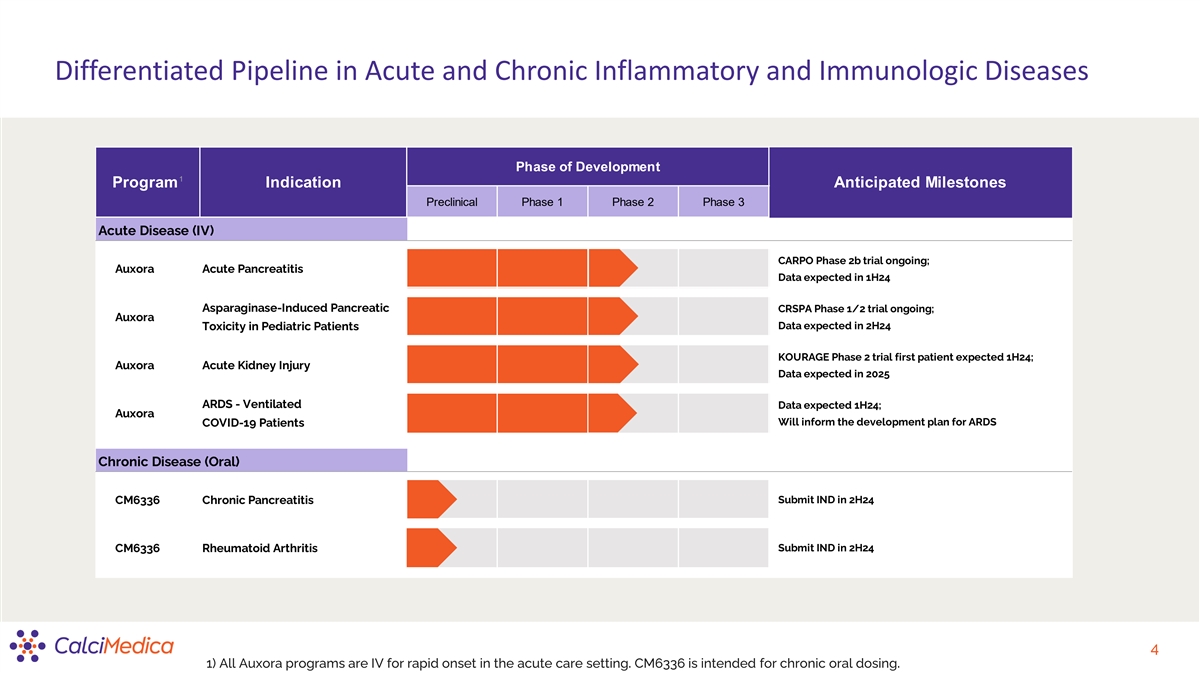
Differentiated Pipeline in Acute and Chronic Inflammatory and
Immunologic Diseases Phase of Development 1 Program Indication Anticipated Milestones Preclinical Phase 1 Phase 2 Phase 3 Acute Disease (IV) CARPO Phase 2b trial ongoing; Auxora Acute Pancreatitis Data expected in 1H24 Asparaginase-Induced
Pancreatic CRSPA Phase 1/2 trial ongoing; Auxora Toxicity in Pediatric Patients Data expected in 2H24 KOURAGE Phase 2 trial first patient expected 1H24; Auxora Acute Kidney Injury Data expected in 2025 ARDS - Ventilated Data expected 1H24; Auxora
Will inform the development plan for ARDS COVID-19 Patients Chronic Disease (Oral) CM6336 Chronic Pancreatitis Submit IND in 2H24 Submit IND in 2H24 CM6336 Rheumatoid Arthritis 4 1) All Auxora programs are IV for rapid onset in the acute care
setting. CM6336 is intended for chronic oral dosing.

Overactivation of CRAC Channels: Immune System Activation and Tissue
Cell Injury 5

Acute Inflammation: Underlying Cause Across Many Diseases Acute
Pancreatitis ARDS Acute Kidney Injury Old Paradigm Future Paradigm Treatable Trait A Treatable Trait B Auxora has demonstrated positive clinical results in all 3 of these large, underserved patient populations 1) Sources: Reddy, Kiran, Carolyn S.
Calfee, and Danny F. McAuley. Acute respiratory distress syndrome subphenotypes beyond the 6 syndrome: a step toward treatable traits?. American Journal of Respiratory and Critical Care Medicine 203.12 (2021): 1449-1451.
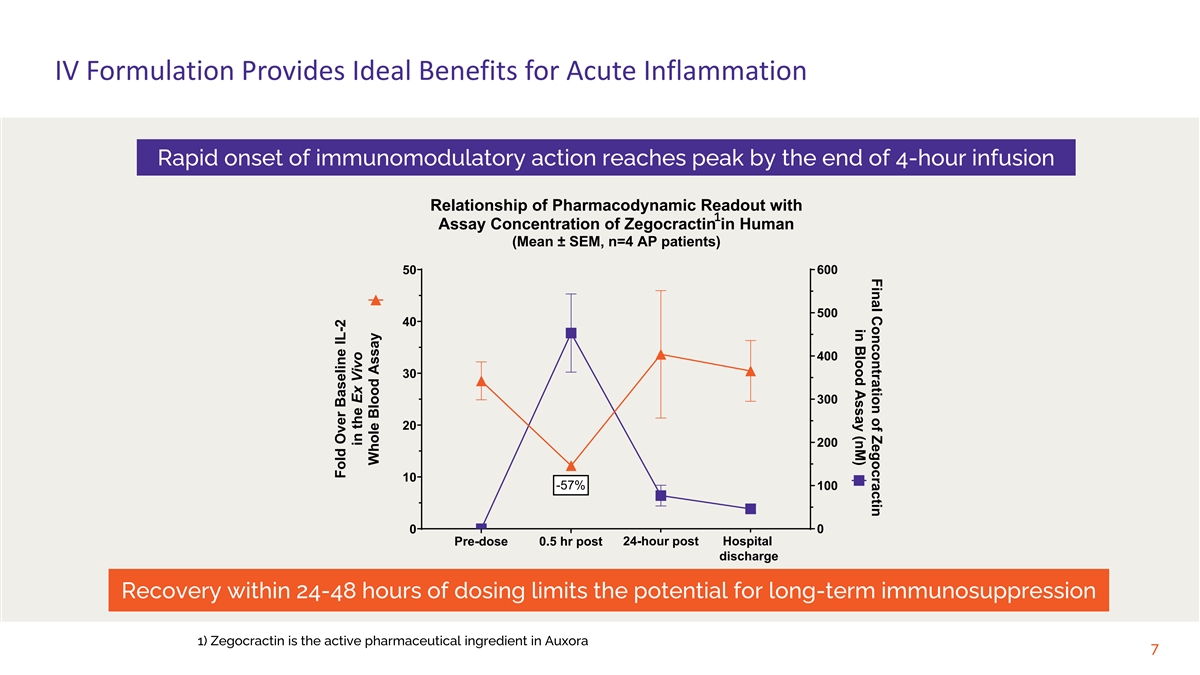
Final Concontration of Zegocractin in Blood Assay (nM) IV Formulation
Provides Ideal Benefits for Acute Inflammation Rapid onset of immunomodulatory action reaches peak by the end of 4-hour infusion Relationship of Pharmacodynamic Readout with 1 Assay Concentration of Zegocractin in Human (Mean ± SEM, n=4 AP
patients) 600 50 500 40 400 30 300 20 200 10 -57% 100 0 0 24-hour post Hospital Pre-dose 0.5 hr post Day 2 Discharge discharge Recovery within 24-48 hours of dosing limits the potential for long-term immunosuppression 1) Zegocractin is the active
pharmaceutical ingredient in Auxora 7 Fold Over Baseline IL-2 in the Ex Vivo Whole Blood Assay

Demonstrated Biological Activity and was well tolerated in Multiple
Phase 2 Trials Population Results Pancreas Asparaginase- Inducted • Trial ongoing, preliminary results show rapid resolution of pain and food Pancreatic Toxicity tolerance Acute Pancreatitis • Trial ongoing With SIRS 1 Acute Pancreatitis
• Target engagement of CRAC channels in peripheral lymphocytes 1 Acute Pancreatitis • Rapid increase in patients tolerating solid diet (potential trial pivotal endpoint) Accompanied by SIRS and • >2-day reduction in hospital
stay and 50% reduction SIRS Hypoxemia Lung • 56% statistically significant decrease in mortality at Day 30 COVID-19 with Respiratory • 33% reduction in ventilation 2 3 Failure on LFO and HFNC • >2-day shorter hospital stay 2
• ~40% reduction in reported acute kidney injury COVID-19 with Respiratory • Open-label trial with varying doses showing pharmacodynamic response 4 Failure on IMV 8 1) Completed Phase 1 trials in healthy volunteers showed no evidence of
dose-dependent safety or tolerability findings through 365 days 2) LFO : Low Flow Oxygen; 3) HFNC: High-Flow Nasal Cannula; 4) IMV: Invasive Mechanical Ventilation 2

Large U.S. Market Opportunity in Acute Inflammatory Diseases 1 AP with
SIRS AKI Stages 2 & 3 AIPT 100K 1.1M 300 Patients Patients Patients • No approved therapies • No approved therapies • No approved therapies 2 • SOC primarily supportive care • SOC primarily supportive care •
Ultra-orphan pediatric indication • Disease progression: • Disease progression: • Accelerated approval opportunity Ø Severe APØ Chronic kidney disease • Disease progression: Ø Pancreatic necrosisØ End
stage renal diseaseØ Pancreatic necrosis in 50% Ø MortalityØ Mortality 3 Patient figures represent estimated numbers of annual U.S. cases 1) SIRS: Systemic Inflammatory Response Syndrome; 2) SOC: Standard of Care; 3) Sources: Primary
Market Research, KOLs, Healthcare Cost and Utilization Project, Pancreatitis Foundation, and https://www.hcup-us.ahrq.gov/reports/statbriefs/sb231-Acute-Renal-Failure-Hospitalizations.pdf 9 Criteria: Based on RIFLE staging criteria for AKI
classification; Serum creatinine increase over baseline

Auxora for Acute Pancreatitis (AP) 10

AP Population: Significant Unmet Need U.S. Hospitalizations per Year
from AP: ~275,000 ~40% of patients have SIRS at presentation High risk for moderate to severe disease Patients with SIRS+: ~110,000 Small percentage of patients missed Misdiagnosis, timing constraint, or other Target Patients: ~100,000 Target
population is in-hospital patients with SIRS; currently no approved therapy 11 1) Source: Fletcher Spaght Market Research Report (2016)

Patient Journey in Severe AP Disease Progression 48-72 hours 2 Weeks 4
Weeks 6 Weeks Transient organ failure and moderately severe AP 100K US Mortality <5% Patients with SIRS (Predicted Severe 70% Sterile necrosis; Mortality 10% Walled-off Persistent organ failure and severe AP Pancreatitis) pancreatic Mortality
15-20% necrosis 30% Infected necrosis; Mortality 30% 12 1) Source: Adapted from N Engl J Med 2016;375:1972-81. DOI: 10.1056/NEJMra1505202
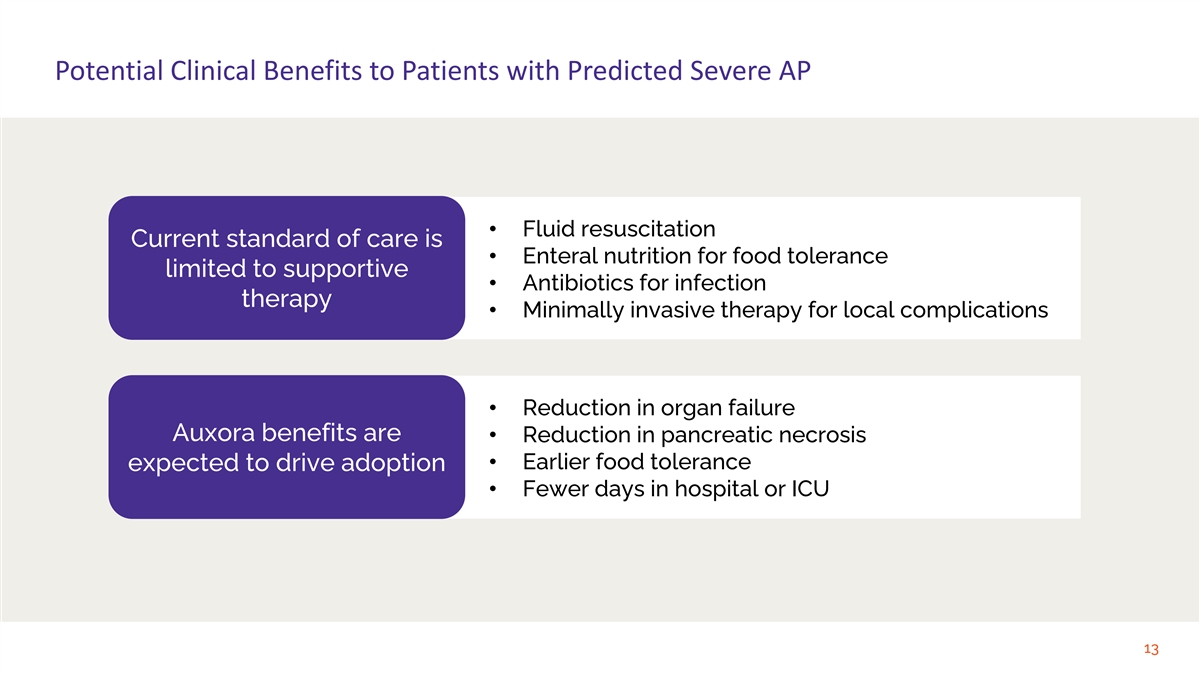
Potential Clinical Benefits to Patients with Predicted Severe AP
• Fluid resuscitation Current standard of care is • Enteral nutrition for food tolerance limited to supportive • Antibiotics for infection therapy • Minimally invasive therapy for local complications • Reduction in
organ failure Auxora benefits are • Reduction in pancreatic necrosis • Earlier food tolerance expected to drive adoption • Fewer days in hospital or ICU 13

AP Phase 2a Clinical Trial Safety, tolerability, and efficacy trial for
various doses of Auxora compared to standard of care 14
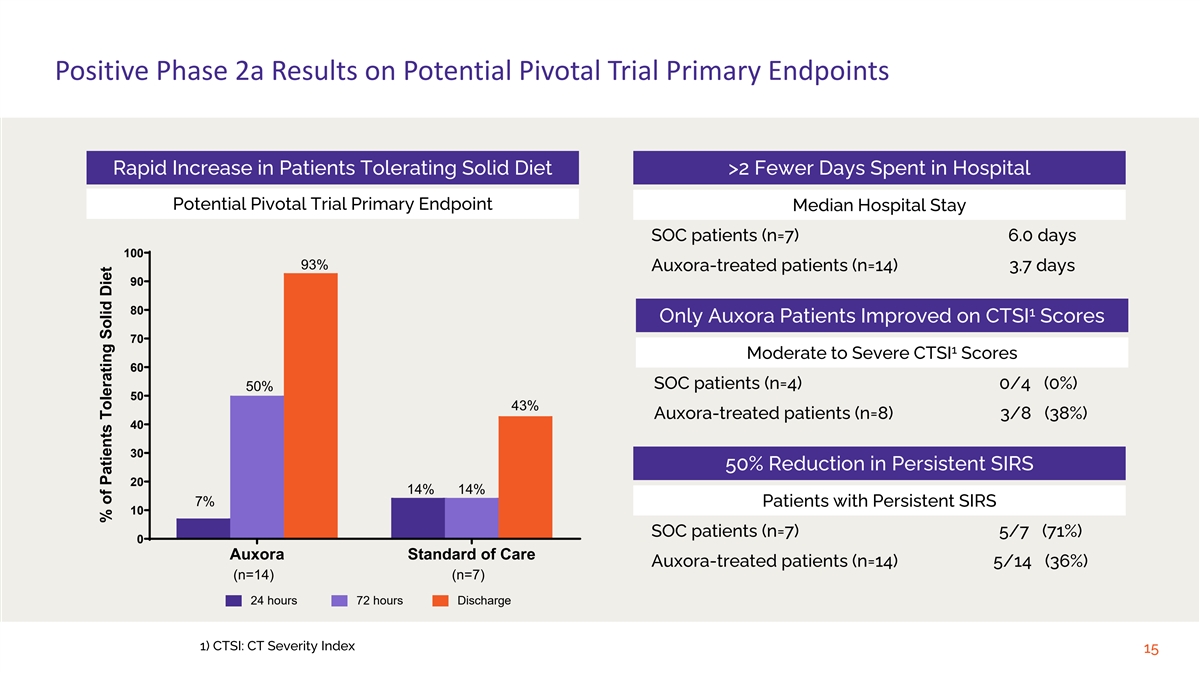
Positive Phase 2a Results on Potential Pivotal Trial Primary Endpoints
Rapid Increase in Patients Tolerating Solid Diet >2 Fewer Days Spent in Hospital Potential Pivotal Trial Primary Endpoint Median Hospital Stay SOC patients (n=7) 6.0 days 100 93% Auxora-treated patients (n=14) 3.7 days 90 80 1 Only Auxora
Patients Improved on CTSI Scores 70 1 Moderate to Severe CTSI Scores 60 SOC patients (n=4) 0/4 (0%) 50% 50 43% Auxora-treated patients (n=8) 3/8 (38%) 40 30 50% Reduction in Persistent SIRS 20 14% 14% 7% Patients with Persistent SIRS 10 SOC patients
(n=7) 5/7 (71%) 0 Auxora Standard of Care Auxora-treated patients (n=14) 5/14 (36%) (n=14) (n=7) 24 hours 72 hours Discharge 1) CTSI: CT Severity Index 15 % of Patients Tolerating Solid Diet

CARPO Phase 2b Clinical Trial in AP Ongoing with Data Expected 1H 2024
Primary End Point Secondary Endpoints Responder analysis planned to validate food tolerance endpoint with FDA 16
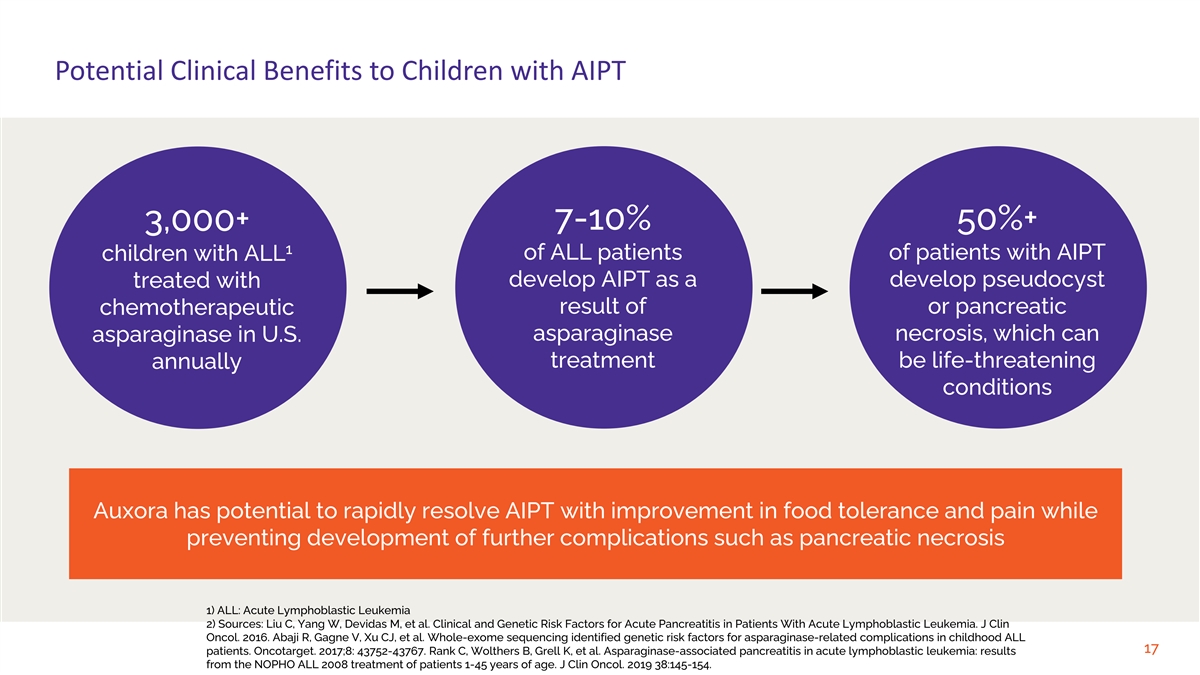
Potential Clinical Benefits to Children with AIPT 7-10% 50%+ 3,000+ 1
of ALL patients of patients with AIPT children with ALL treated with develop AIPT as a develop pseudocyst result of or pancreatic chemotherapeutic asparaginase necrosis, which can asparaginase in U.S. treatment be life-threatening annually
conditions Auxora has potential to rapidly resolve AIPT with improvement in food tolerance and pain while preventing development of further complications such as pancreatic necrosis 1) ALL: Acute Lymphoblastic Leukemia 2) Sources: Liu C, Yang W,
Devidas M, et al. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol. 2016. Abaji R, Gagne V, Xu CJ, et al. Whole-exome sequencing identified genetic risk factors for
asparaginase-related complications in childhood ALL 17 patients. Oncotarget. 2017;8: 43752-43767. Rank C, Wolthers B, Grell K, et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL 2008 treatment of
patients 1-45 years of age. J Clin Oncol. 2019 38:145-154.

Proof-of-Concept Ongoing in AIPT Pediatric Patients Had Rapid
Resolution of Pain and Food Intolerance CRSPA Phase 1/2 Trial in Pediatric AIPT • Investigator-initiated open-label trial being conducted at St. Jude Children’s Research Hospital • Assess the safety in pediatric patients with ALL
who have developed AIPT • Estimate the efficacy of Auxora to prevent pseudocyst or necrotizing pancreatis in pediatric patients with AIPT Trial Status • Cohort 1 complete (9 patients) • 8 patients received four daily infusions of
Auxora and had rapid resolution of pain and food intolerance • 1 patient received less than a single infusion of Auxora and developed pancreatic necrosis • Blinded matched, historical control comparison for Cohort 1 completed •
Cohort 1 dosing selected as recommended dose for patients • Expanding to additional sites to complete trial (24 patients) with data expected in 2H24 Results for First Cohort Compared to Blinded, Matched Historical Controls Presented at ASH
2023 18
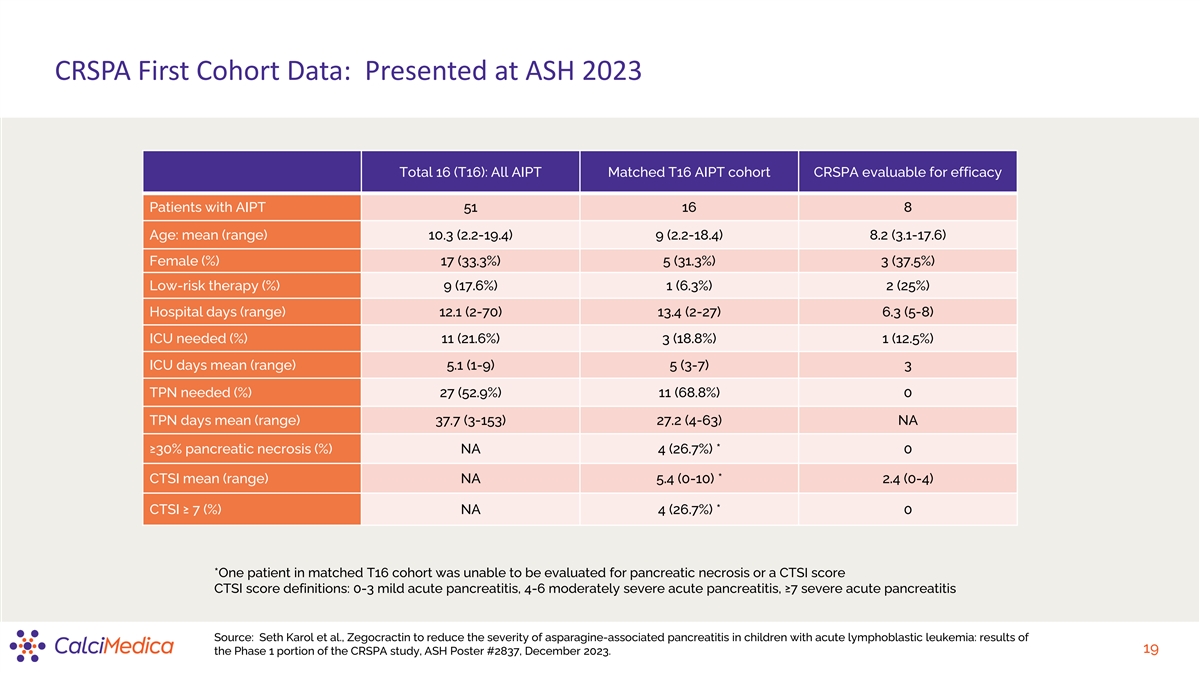
CRSPA First Cohort Data: Presented at ASH 2023 Total 16 (T16): All AIPT
Matched T16 AIPT cohort CRSPA evaluable for efficacy Patients with AIPT 51 16 8 Age: mean (range) 10.3 (2.2-19.4) 9 (2.2-18.4) 8.2 (3.1-17.6) Female (%) 17 (33.3%) 5 (31.3%) 3 (37.5%) Low-risk therapy (%) 9 (17.6%) 1 (6.3%) 2 (25%) Hospital days
(range) 12.1 (2-70) 13.4 (2-27) 6.3 (5-8) ICU needed (%) 11 (21.6%) 3 (18.8%) 1 (12.5%) ICU days mean (range) 5.1 (1-9) 5 (3-7) 3 TPN needed (%) 27 (52.9%) 11 (68.8%) 0 TPN days mean (range) 37.7 (3-153) 27.2 (4-63) NA ≥30% pancreatic necrosis
(%) NA 4 (26.7%) * 0 CTSI mean (range) NA 5.4 (0-10) * 2.4 (0-4) CTSI ≥ 7 (%) NA 4 (26.7%) * 0 *One patient in matched T16 cohort was unable to be evaluated for pancreatic necrosis or a CTSI score CTSI score definitions: 0-3 mild acute
pancreatitis, 4-6 moderately severe acute pancreatitis, ≥7 severe acute pancreatitis Source: Seth Karol et al., Zegocractin to reduce the severity of asparagine-associated pancreatitis in children with acute lymphoblastic leukemia: results of
19 the Phase 1 portion of the CRSPA study, ASH Poster #2837, December 2023.

Auxora for Acute Kidney Injury (AKI) 20
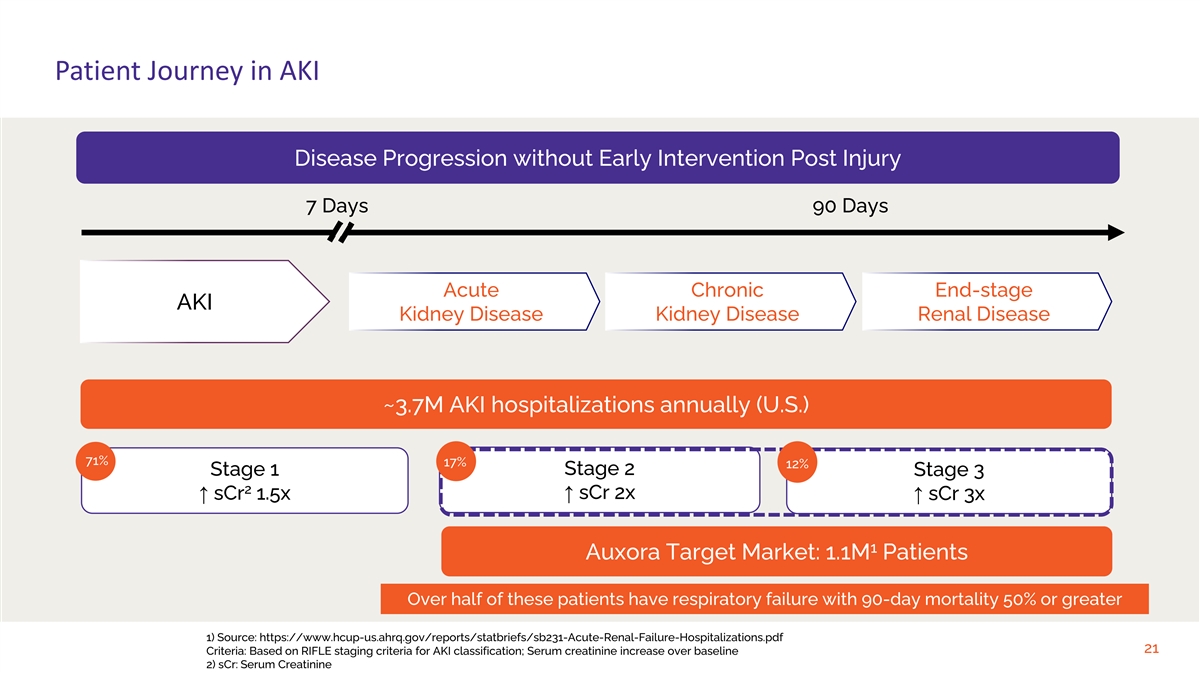
Patient Journey in AKI Disease Progression without Early Intervention
Post Injury 7 Days 90 Days Acute Chronic End-stage AKI Kidney Disease Kidney Disease Renal Disease ~3.7M AKI hospitalizations annually (U.S.) 71% 17% 12% Stage 2 Stage 1 Stage 3 2 ↑ sCr 2x ↑ sCr 1.5x↑ sCr 3x 1 Auxora Target Market:
1.1M Patients Over half of these patients have respiratory failure with 90-day mortality 50% or greater 1) Source: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb231-Acute-Renal-Failure-Hospitalizations.pdf 21 Criteria: Based on RIFLE staging
criteria for AKI classification; Serum creatinine increase over baseline 2) sCr: Serum Creatinine

Potential Clinical Benefits to Patients with AKI Current standard of
care is • Fluid resuscitation / Diuretics • Nutrition limited to supportive • Correction of underlying cause therapy • Reduced need for dialysis Auxora benefits are • Reduced risk of mortality expected to drive adoption
• Greater recovery of renal function 22

1 Improved GFR and Decreased Inflammatory Cell Infiltrates Within 24
Hours in AKI Model A single dose of Auxora or placebo was administered 30 min after bilateral kidney ischemia/reperfusion Mononuclear Cells in Kidney of GFR in Rats After Rats after Ischemia/Reperfusion Kidney Ischemia/Reperfusion 7 1.5×10 0.6
*p<0.042 by t test *p=0.0053 by t test 7 1×10 0.4 6 0.2 5×10 0.0 0 Placebo Auxora Placebo Auxora Recent studies with 3-day Auxora or Placebo dosing initiated 6 hours following ischemia/reprofusion will be th presented in March 2024 at
the 29 International AKI and CRRT Conference 1) GFR: Glomerular filtration rate 23 2) Data courtesy of David Basile, PhD, Indiana University GFR (ml/min/100g BW) Mononuclear Cells/Gm Kidney

Phase 2 CARDEA Trial: Evidence of Renal Protection Ang-2/Tie2 results
in endothelial inflammation with Ang-1/Tie2 signaling maintains vascular integrity increased endothelial permeability Angiopoietin-2 Levels Angiopoietin-1 Levels Decrease Significantly with Auxora Increase Significantly with Auxora (Means ±
SEM) (Means ± SEM) 250 8000 1 ns* p=0.01 1 p=0.01 0 6000 -250 4000 ns* -500 2000 -750 0 -1000 -2000 Placebo Auxora Placebo Auxora Clinical Observations • Mortality benefit with Auxora vs Placebo observed in patients with compromised
kidney function (low GFR) at time of enrollment • ~40% reduction in reported AKI with Auxora vs Placebo 24 Change in Angiopoietin-1 from Screening to 96 hours (pg/mL) Change in Angiopoietin-2 from Screening to 96 hours (pg/mL)

KOURAGE: Acute Kidney Injury with associated AHRF Phase 2 Trial Design
25

Auxora for Acute Respiratory Distress Syndrome (ARDS) 26
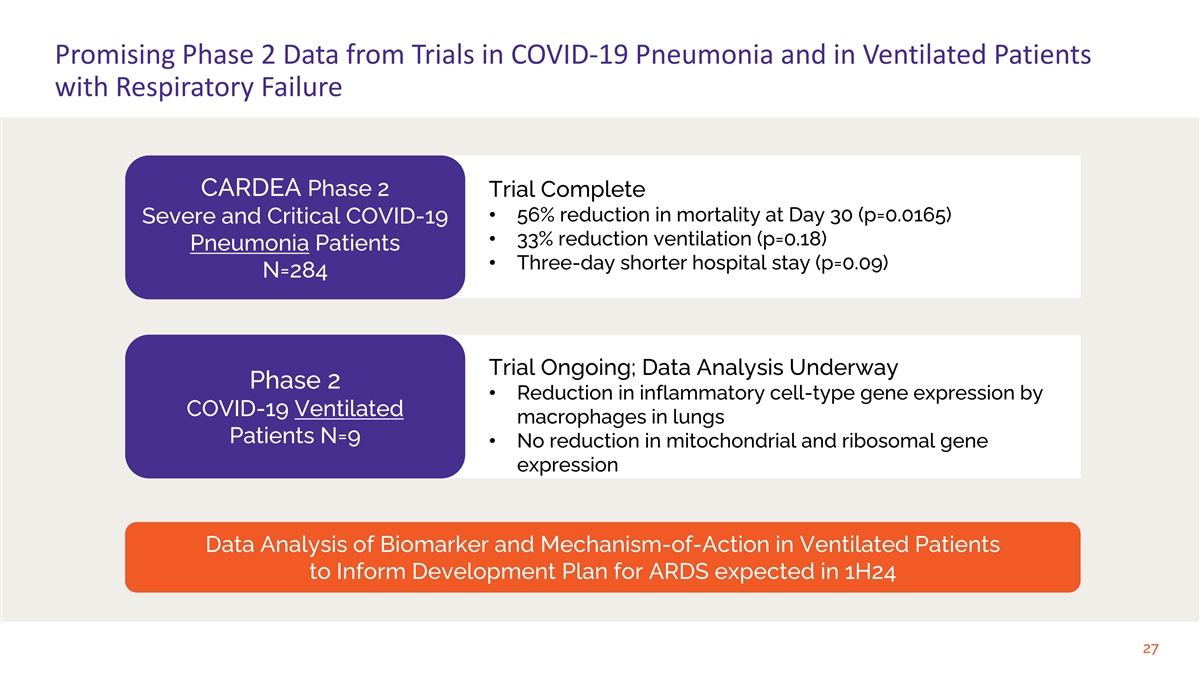
Promising Phase 2 Data from Trials in COVID-19 Pneumonia and in
Ventilated Patients with Respiratory Failure CARDEA Phase 2 Trial Complete • 56% reduction in mortality at Day 30 (p=0.0165) Severe and Critical COVID-19 • 33% reduction ventilation (p=0.18) Pneumonia Patients • Three-day shorter
hospital stay (p=0.09) N=284 Trial Ongoing; Data Analysis Underway Phase 2 • Reduction in inflammatory cell-type gene expression by COVID-19 Ventilated macrophages in lungs Patients N=9 • No reduction in mitochondrial and ribosomal gene
expression Data Analysis of Biomarker and Mechanism-of-Action in Ventilated Patients to Inform Development Plan for ARDS expected in 1H24 27

Platform Application for CRAC Channel Inhibition 28
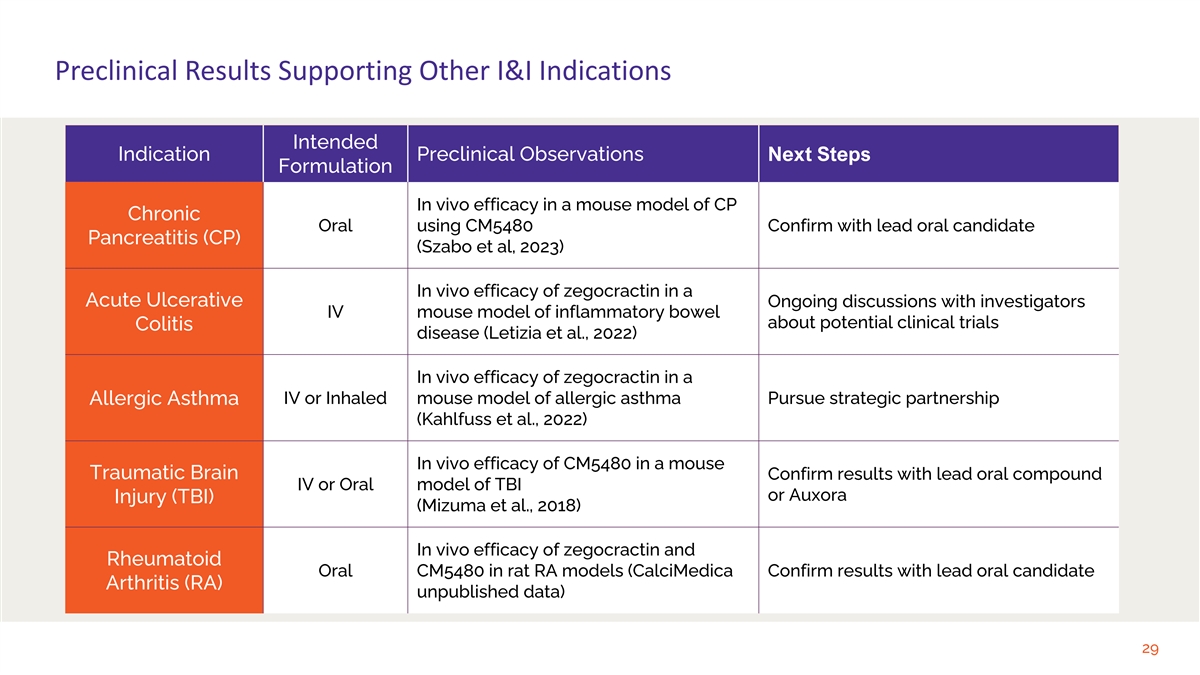
Preclinical Results Supporting Other I&I Indications Intended
Indication Preclinical Observations Next Steps Formulation In vivo efficacy in a mouse model of CP Chronic Oral using CM5480 Confirm with lead oral candidate Pancreatitis (CP) (Szabo et al, 2023) In vivo efficacy of zegocractin in a Acute Ulcerative
Ongoing discussions with investigators IV mouse model of inflammatory bowel about potential clinical trials Colitis disease (Letizia et al., 2022) In vivo efficacy of zegocractin in a IV or Inhaled mouse model of allergic asthma Pursue strategic
partnership Allergic Asthma (Kahlfuss et al., 2022) In vivo efficacy of CM5480 in a mouse Traumatic Brain Confirm results with lead oral compound IV or Oral model of TBI or Auxora Injury (TBI) (Mizuma et al., 2018) In vivo efficacy of zegocractin
and Rheumatoid Oral CM5480 in rat RA models (CalciMedica Confirm results with lead oral candidate Arthritis (RA) unpublished data) 29

Anticipated Milestones 30

Anticipated Milestones CARPO Phase 2b Data Expected in 1H24 AP Phase 3
Initiation Expected in 2025 CRSPA Initial First Cohort Data Released at ASH 2023 AIPT Trial Expansion Underway; Completion Expected 2H24 KOURAGE First Patient Enrolled Expected in 1H24 AKI Data Expected in 2025 Phase 2 Data in Ventilated COVID
Patients Publication Expected in 1H24 ARDS Will Inform the Development Plan for ARDS Cash Runway Current Cash Runway into 2H25 31
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Graybug Vision (NASDAQ:GRAY)
Historical Stock Chart
From Oct 2024 to Nov 2024
Graybug Vision (NASDAQ:GRAY)
Historical Stock Chart
From Nov 2023 to Nov 2024