Calliditas Therapeutics Shares Rise 33% After FDA Approves Tarpeyo
December 20 2023 - 5:33PM
Dow Jones News
By Stephen Nakrosis
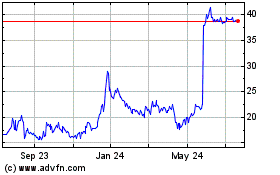
Shares of Calliditas Therapeutics were topping previous 52-week
highs in Wednesday's late-trading session after the company said
the U.S. Food and Drug Administration approved Tarpeyo to treat
certain patients with primary immunoglobulin A nephropathy.
At 4:54 p.m. ET, the company's stock had risen 33%, to $29.96 a
share. The stock hit a 52-week high of $25.64 on May 8.
The FDA approved Tarpeyo, or budesonide, delayed release
capsules "to reduce the loss of kidney function in adults with
primary immunoglobulin A nephropathy at risk for disease
progression," Calliditas said.
Tarpeyo, the first fully FDA-approved treatment for IgAN based
on a measure of kidney function, was first approved in December
2021 under accelerated approval, the company said.
Write to Stephen Nakrosis at stephen.nakrosis@wsj.com
(END) Dow Jones Newswires
December 20, 2023 17:18 ET (22:18 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Calliditas Therapeutics AB (NASDAQ:CALT)
Historical Stock Chart
From Apr 2024 to May 2024
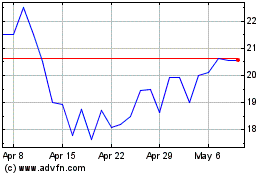
Calliditas Therapeutics AB (NASDAQ:CALT)
Historical Stock Chart
From May 2023 to May 2024