0001367644false00013676442024-02-292024-02-290001367644dei:FormerAddressMember2024-02-292024-02-29
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 29, 2024
EMERGENT BIOSOLUTIONS INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | | | | |
| Delaware | | 001-33137 | | 14-1902018 |
| (State or other jurisdiction | | (Commission File Number) | | (IRS Employer |
| of incorporation) | | | | Identification No.) |
300 Professional Drive,
Gaithersburg, Maryland 20879
(Address of principal executive offices, including zip code)
(240) 631-3200
(Registrant’s telephone number, including area code)
400 Professional Drive, Suite 400, Gaithersburg, Maryland 20879
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, Par Value $0.001 per share | EBS | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement
Amendment of Senior Secured Credit Facilities
On February 29, 2024, Emergent BioSolutions Inc. (the “Company”) entered into a Forbearance Agreement and Sixth Amendment to Amended and Restated Credit Agreement (the “Forbearance Agreement and Amendment”), among the Company, as borrower, certain subsidiaries of the Company, as guarantors, Wells Fargo Bank, National Association, as administrative agent (in such capacity, the “Administrative Agent”) and certain lenders party thereto (the “Lenders”). The Forbearance Agreement and Amendment amends the Amended and Restated Credit Agreement, dated as of October 15, 2018, among the Company, the lenders party thereto from time to time and the Administrative Agent (as previously amended, modified and supplemented, the “Existing Credit Agreement”), relating to the Company’s senior secured credit facilities consisting of a senior revolving credit facility (the “Revolving Credit Facility”) and a senior term loan facility (the “Term Loan Facility” and together with the Revolving Credit Facility, the “Senior Secured Credit Facilities”).
The Forbearance Agreement and Amendment amends the Existing Credit Agreement to, among other things, (a) provide that the Administrative Agent and the Lenders forbear from exercising all rights and remedies under the Existing Credit Agreement and the other related loan documents arising from the occurrence and continuation of certain specified events of default during a forbearance period (the “Forbearance Period”) between the forbearance effective date until the earlier to occur of (x) 5:00 p.m. on April 30, 2024 and (y) the occurrence of any event of default (other than the specified events of default) or default under the Forbearance Agreement and Amendment and notice by the Administrative Agent to the Company of the termination of the Forbearance Period and (b) provide consent by the required revolving credit lenders to make further loans to the Company or other extensions of credit to the credit parties during the Forbearance Period, notwithstanding the occurrence of the specified events of default, subject to certain conditions set forth in the Forbearance Agreement and Amendment, including a limit on Revolving Credit Facility indebtedness of $270 million.
The Forbearance Agreement and Amendment also amends (x) the interest rate benchmark in the definition of Applicable Margin from 6.00% per annum to 6.50% per annum with respect to SOFR Loans, Daily Simple SONIA Loans and Eurocurrency Rate Loans, (y) the mandatory prepayment threshold amount for unrestricted cash and cash equivalents from $125,000,000 to $100,000,000, and (z) the mandatory principal prepayment amount from 75% of all milestone payments received by the Company and its subsidiaries from certain project milestone payments to 100%.
Under the Forbearance Agreement and Amendment, the Company and the other guarantors have also agreed to cause Emergent BioSolutions Canada Inc. to (i) become a guarantor under the Senior Secured Credit Facilities and (ii) grant a security lien in all collateral owned by Emergent BioSolutions Canada Inc. (subject to the exclusions and exceptions specified in the Collateral Agreement) to the Administrative Agent. In addition, in connection with the entry into the Forbearance Agreement and Amendment, the Company paid a forbearance fee of approximately $1.2 million.
The foregoing description of the Forbearance Agreement and Amendment does not purport to be complete and is qualified in its entirety by reference to the full text of the Forbearance Agreement and Amendment, a copy of which is filed herewith as Exhibit 10.1 and is incorporated herein by reference. The Forbearance Agreement and Amendment contains representations, warranties and covenants that were made only for purposes of such agreement and as of specific dates, that are solely for the benefit of the parties to such agreement, and that may be subject to limitations agreed upon by the contracting parties. The Forbearance Agreement and Amendment is not intended to provide investors and the public with factual information about the Company’s current state of affairs. Rather, investors and the public should look to other disclosures contained in the Company’s filings with the U.S. Securities and Exchange Commission.
Item 2.02 Results of Operations and Financial Condition.
On March 6, 2024, the Company announced financial and operating results for the period ended December 31, 2023. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 2.03 Creation of a Direct Financial Obligation or an Obligation Under an Off-Balance Sheet Arrangement of a Registrant.
The information contained in Item 1.01 of this Form 8-K under the subheading “Amendment of Senior Secured Credit Facilities” is incorporated into this Item 2.03 by reference.
Item 7.01 Regulation FD Disclosure.
On March 6, 2024, the Company will hold a conference call to discuss its financial and operating results for the period ended December 31, 2023. The Company will use presentation materials in connection with this conference call (the “Earnings Call Slides”), which will be posted on the Company’s website at www.emergentbiosolutions.com. A copy of the Earnings Call Slides is furnished as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| | | | | | | | |
| Exhibit No. | | Description |
| 10.1 | | |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | |
| EMERGENT BIOSOLUTIONS INC. |
| | | |
| Dated: March 6, 2024 | By: | /s/ RICHARD S. LINDAHL |
| | Name: Richard S. Lindahl
Title: Executive Vice President, Chief Financial
Officer and Treasurer |
| | |

EXECUTION VERSION WEIL:99600594143717.0003 FORBEARANCE AGREEMENT AND SIXTH AMENDMENT TO AMENDED AND RESTATED CREDIT AGREEMENT This Forbearance Agreement and Sixth Amendment to Amended and Restated Credit Agreement, dated as of February 29, 2024 (this “Forbearance Agreement and Amendment”), is entered into by and among EMERGENT BIOSOLUTIONS INC., a Delaware corporation (the “Borrower”), the Guarantors (as defined in the Credit Agreement referred to below) party hereto, the Lenders party hereto (the “Lenders”), and WELLS FARGO BANK, NATIONAL ASSOCIATION, as administrative agent (the “Administrative Agent”). Capitalized terms used herein and not otherwise defined herein shall have the meanings ascribed to them in the Credit Agreement. RECITALS WHEREAS, the Borrower, the lenders party thereto from time to time and the Administrative Agent have entered into that certain Amended and Restated Credit Agreement, dated as of October 15, 2018 (as amended by the First Amendment to Amended and Restated Credit Agreement dated as of June 27, 2019, as further amended by the Second Amendment to Amended and Restated Credit Agreement dated as of August 7, 2020, as further amended by the Consent, Limited Waiver, Third Amendment to Amended and Restated Credit Agreement dated as of February 14, 2023, as further amended by the Fourth Amendment to Amended and Restated Credit Agreement, Waiver and First Amendment to Amended and Restated Collateral Agreement dated as of May 15, 2023, and as further amended by the Fifth Amendment to Amended and Restated Credit Agreement dated as of July 14, 2023, and as may further be amended, restated, amended and restated, supplemented or otherwise modified prior to the date hereof, the “Existing Credit Agreement”; the Existing Credit Agreement, as modified by this Forbearance Agreement and Amendment, the “Credit Agreement”); WHEREAS, the Borrower has notified the Administrative Agent that certain Defaults and Events of Defaults have occurred or are anticipated to occur as described in Schedule A attached hereto (collectively, the “Specified Events of Default”); WHEREAS, in accordance with Article X of the Existing Credit Agreement, and but for the forbearance agreed to hereunder, the Administrative Agent, on behalf of the Lenders, is authorized to exercise various rights and remedies arising from the occurrence and continuance of the Specified Events of Default; WHEREAS, the Credit Parties have requested that, during the Forbearance Period (as defined below), Administrative Agent and the Lenders forbear from exercising rights and remedies under the Existing Credit Agreement and the other Loan Documents arising from the occurrence and continuation of the Specified Events of Default; WHEREAS, the undersigned Lenders collectively constitute the Required Lenders under the Credit Agreement; and WHEREAS, the Administrative Agent and the Lenders have agreed, during the Forbearance Period, to forbear from exercising enforcement rights and remedies under the Existing Credit Agreement and the other Loan Documents arising from the Specified Events of Default and to make the amendments to the Existing Credit Agreement set forth in Section 2 hereof, solely to the extent and on the terms, and subject to the conditions, and in reliance on the representations and warranties set forth, in this Forbearance Agreement and Amendment. ACKNOWLEDGMENTS A. Each of the Credit Parties, the Administrative Agent and each of the Lenders hereby acknowledges and agrees that, as of February 29, 2024, the unpaid principal balance of the Obligations was $467,860,035.47 without offset, reduction or counterclaim, but the foregoing amount does not include any Certain identified information has been excluded from the exhibit because it is both (i) not material and (ii) is the type of information that the registrant treats as private or confidential. Double asterisks denote omissions.

Page 2 WEIL:99600594143717.0003 accrued and unpaid interest, costs, fees, and other expenses payable by the Credit Parties to the Administrative Agent and the Lenders under the Existing Credit Agreement that have not been charged to the Obligations as of such date. B. Each of the Credit Parties acknowledges and agrees that (i) this Forbearance Agreement and Amendment is one of the Loan Documents under the Credit Agreement, and (ii) any Loans or other extensions of credit that any Lender makes under the Loan Documents in the future will be (x) “Obligations” under the Credit Agreement and (y) secured by all liens and security interests granted by any Credit Party to secure the Obligations. C. To the extent that there is a conflict between the terms of this Forbearance Agreement and Amendment and the terms of the Credit Agreement, the terms of this Forbearance Agreement and Amendment shall govern. NOW, THEREFORE, in consideration of the premises and for other good and valuable consideration (the receipt and sufficiency of which are hereby acknowledged), the parties hereto hereby agree as follows: Section 1. Forbearance (a) Forbearance Agreement and Amendment. Subject to all of the terms, conditions, and limitations set forth in this Forbearance Agreement and Amendment, the Administrative Agent and the Lenders hereby agree to forbear, solely during the Forbearance Period, from exercising all rights and remedies under the Credit Agreement and the other Loan Documents and applicable law arising solely from the occurrence and the continuation of the Specified Events of Default; provided, that, notwithstanding anything contained herein to the contrary, nothing herein shall limit, restrict, impair or otherwise modify the Lenders’ or the Administrative Agent’s right or ability to take any of the following actions, in each case to the extent permitted under the Credit Agreement and the other Loan Documents: (i) declare and/or send any notices and communications with respect to this Forbearance Agreement and Amendment, the Loan Documents and/or the Obligations (including, without limitation, notices and communications with respect to (A) any Default or Event of Default (including the Specified Events of Default), or any other breach of any Loan Document, (B) any reservation of rights and (C) any terms and provisions of any Loan Documents, and any rights and obligations arising thereunder or relating thereto); or (ii) take any action in order to create, perfect, preserve, protect or evidence (but not enforce its secured creditor rights and remedies with respect to) the Secured Parties’ Lien on any Collateral. (b) Extensions of Credit. (i) The Borrower hereby acknowledges and agrees that as a result of the Specified Events of Default, the Lenders have no obligation to make any further Loans or other Extensions of Credit to Borrower or any other Credit Party. Notwithstanding the foregoing or anything to the contrary in the Existing Credit Agreement and pursuant to Section 12.2(j) thereof, the Required Revolving Credit Lenders have consented to the making of further Loans or other Extensions of Credit to the Credit Parties during the Forbearance Period, subject to satisfaction of the conditions thereto set forth in Section 6.2 of the Credit Agreement other than the failure to satisfy conditions solely as a result of the occurrence of the Specified Events of Default; provided, that, (x) the Revolving Credit Outstandings, in the aggregate, shall not exceed $270,000,000, until and unless consented to in writing by Required Revolving Credit Lenders in accordance with Section 12.2(j) of the Credit Agreement and (y) to the extent the

Page 3 WEIL:99600594143717.0003 Borrower can satisfy such conditions, the obligations of the Lenders pursuant to Section 6.2 of the Credit Agreement shall remain in full force and effect; and (ii) during the Forbearance Period, prior to the Lenders making or participating in any Extensions of Credit and/or any Issuing Lender issuing or extending any Letter of Credit, the Credit Parties shall deliver a certificate of a Responsible Officer of each Credit Party, together with the Notice of Borrowing, certifying (i) that no Default or Event of Default (other than the Specified Events of Default) shall have occurred and be continuing (A) on the borrowing, continuation or conversion date with respect to such Loan or after giving effect to the Loans to be made, continued or converted on such date or (B) on the issuance or extension date with respect to such Letter of Credit or after giving effect to the issuance or extension of such Letter of Credit on such date, (ii) compliance with the financial covenant set forth in Section 9.11(e) of the Credit Agreement (except any non-compliance that results or resulted in any Specified Events of Default) as of the last day of the most recent Measurement Period (together with reasonably detailed calculations), and (iii) compliance with Section 2.4(b)(iii) of the Credit Agreement (together with reasonably detailed calculations). (c) Forbearance Period. As used in this Forbearance Agreement and Amendment, the term “Forbearance Period” means the period beginning on the Forbearance Effective Date (as defined below) and ending upon the occurrence of the earliest to occur of (such earliest event, the “Forbearance Termination Event”): (i) 5:00 p.m. (New York City time) on April 30, 2024 (the “Scheduled Termination Date”) (or such later date as may be requested by the Borrower and consented to in writing (including via e-mail) by the Administrative Agent, on behalf of the Required Lenders); (ii) (x) the occurrence of any Event of Default (other than the Specified Events of Default) or any failure of the Borrower or any other Credit Party to comply timely with any term, condition, or covenant set forth in this Forbearance Agreement and Amendment (each such failure, a “Forbearance Default”) and any such Forbearance Default continues unremedied or unwaived for ten (10) calendar days and (y) notice by the Administrative Agent to the Borrower of termination of the Forbearance Period. From and after the occurrence of the Forbearance Termination Event, the Forbearance Period, and all forbearance obligations of the Lenders and the Administrative Agent hereunder, shall automatically terminate, without any requirement of notice or declaration of any kind. From and after the occurrence of the Forbearance Termination Event, the Administrative Agent and the Lenders shall be entitled to exercise and to enforce any and all rights and remedies available to the Administrative Agent and/or any of the Lenders under the Credit Agreement, any of the other Loan Documents, at law or otherwise against the Borrower or the Guarantors or in relation to the Collateral, including, without limitation, any and all rights and remedies to which the Administrative Agent and/or any of the Lenders is or may become entitled as a consequence of any Events of Default that have occurred prior to, during or after the Forbearance Period (including the Specified Events of Default). For the avoidance of doubt, notwithstanding anything to the contrary in this Forbearance Agreement and Amendment, immediately and automatically upon occurrence of an Event of Default under Section 10.1(f) of the Credit Agreement, all Obligations will become immediately due and payable, and the Lenders and the Administrative Agent shall become entitled to immediately exercise all rights, remedies, powers and privileges with respect to such Event of Default as provided in Article X of the Credit Agreement, in each case, without requirement for any notice, presentment, demand or any other action on the part of any Lender, the Administrative Agent or any other Person. (d) No Other Waivers; Reservation of Rights. Neither the Administrative Agent nor any of the Lenders has waived any of the Specified Events of Default, any other Defaults or Events of Default or any of the liabilities or obligations (including any Obligations) under any of the Loan Documents, and neither the

Page 4 WEIL:99600594143717.0003 Administrative Agent nor any of the Lenders has agreed to forbear with respect to any of their respective rights or remedies concerning any Defaults or Events of Default (other than, during the Forbearance Period, the Specified Events of Default solely to the extent expressly set forth herein), which may have occurred or are continuing as of the date hereof or which may occur after the date hereof. Subject to Section 1(a) (solely with respect to the Specified Events of Default and only during the Forbearance Period) above, the Administrative Agent and the Lenders expressly reserve any and all rights, powers, privileges and remedies of the Administrative Agent and the Lenders now or at any time authorized or permitted in accordance with the Credit Agreement and the other Loan Documents or available at law or in equity or otherwise as a result of any Defaults or Events of Default which may be continuing on the date hereof or any Defaults or Events of Default which may occur after the date hereof, including, without limitation, their rights to (a) accelerate the outstanding Obligations, (b) commence any legal or other action to collect any or all of the Obligations from any or all of the Borrower, the other Credit Parties, and any other person liable therefor and/or any Collateral, (c) foreclose or otherwise realize on any or all of the Collateral and/or as appropriate, set- off or apply to the payment of any or all of the Obligations, any or all of the Collateral, (d) take any other enforcement action or otherwise exercise any or all rights and remedies provided for by any or all of the Credit Agreement, the other Loan Documents or applicable law, (e) terminate any commitment to provide Loans or other Extensions of Credit under any or all of the Credit Agreement and other Loan Documents, and (f) reject any forbearance, financial restructuring or other proposal made by or on behalf of Borrower, any other Credit Party or any creditor or equity holder, and the Administrative Agent and the Lenders have not waived any of such rights or remedies, and nothing in this Forbearance Agreement and Amendment, and no delay on any of their part in exercising any such rights or remedies, should be construed as a waiver of any such rights or remedies. Section 2. Amendments to Existing Credit Agreement Subject to and in accordance with the terms and conditions set forth herein, the parties hereto agree that the Existing Credit Agreement is amended as follows: (a) The definition of “Applicable Margin” in Section 1.1 of the Existing Credit Agreement is hereby amended and restated in its entirety to read as follows: “Applicable Margin” means from February 29, 2024, through the Applicable Maturity Date, (i) 5.00% per annum with respect to Base Rate Loans, (ii) 6.50% per annum with respect to SOFR Loans, Daily Simple SONIA Loans and Eurocurrency Rate Loans, and (iii) 0.40% with respect to Commitment Fees. (b) Section 2.4(b)(ii) of the Existing Credit Agreement is hereby amended and restated in its entirety as follows: (iii) if at any time the aggregate amount of Unrestricted Cash and Cash Equivalents (excluding any (x) cash on deposit in accounts the exclusive function of which is to serve as a payroll account and limited to amounts in the aggregate reasonably estimated to cover payroll for a two-week period and (y) to the extent the TSA is in effect, on deposit in accounts held in connection with collecting accounts receivable and processing accounts payable pursuant to the Project Emerald Transaction) exceeds $100,000,000 as of the last day of any week, the Borrower shall on the next Business Day thereafter prepay (without a permanent reduction in the Revolving Credit Commitments) outstanding Revolving Credit Loans in an aggregate principal amount equal to the amount of such excess. (c) Section 4.4(b)(iv) of the Existing Credit Agreement is hereby amended and restated in its entirety as follows:

Page 5 WEIL:99600594143717.0003 (iv) Project Emerald Milestone Payments. Until the outstanding Term Loans are paid in full, the Borrower shall make mandatory principal prepayments of the Term Loans in the manner set forth in clause (vi) below in amounts equal to one hundred percent (100%) of all milestone payments received by the Borrower and its Subsidiaries pursuant to the Project Emerald Transaction within three (3) Business Days after the date of receipt of such payments. (d) Section 8.1(f) of the Existing Credit Agreement is hereby amended and restated in its entirety as follows: (f) Cash Flow Projections. On March 7, 2024, and on the fourth Business Day of each week thereafter, a thirteen-week cash flow projection of the Borrower and its Subsidiaries substantially in the same form delivered in connection with the closing of the Fourth Amendment; provided that, to the extent the TSA is in effect, cash on deposit in accounts held in connection with collecting accounts receivable and processing accounts payable pursuant to the Project Emerald Transaction shall be excluded from such thirteen-week cash flow projection. (e) Section 9.11(e) of the Existing Credit Agreement is hereby amended and restated in its entirety as follows: (e) Minimum Liquidity. Permit Liquidity as of March 8, 2024, and as of the last Business Day of each two-week period thereafter, to be less than $75,000,000. (f) Section 10.1(b) of the Existing Credit Agreement is hereby amended to insert “Section 8.1(f),” immediately before “Section 8.2(a)”. (g) Exhibit G (Form of Assignment and Assumption) to the Existing Credit Agreement is hereby amended and restated such that, after giving effect to all such amendments, Exhibit G to the Credit Agreement shall read in its entirety as set forth on Annex A attached hereto. Section 3. Forbearance Fee On the date hereof, Administrative Agent, for the benefit of the Lenders party hereto, shall have fully earned a fee (the “Forbearance Fee”) equal to $1,245,468.75 for the agreements set forth herein, which Forbearance Fee shall be due and payable in full to Administrative Agent, for the benefit of the Lenders party hereto, on the date hereof. Section 4. Covenants Each of the Credit Parties absolutely and unconditionally agrees, for the benefit of the Lenders and the Administrative Agent, to comply with the following covenants, agreements and obligations at all times while an Event of Default (including a Specified Event of Default) is continuing: (a) once each week (at a time to be mutually agreed and which may be waived by the Administrative Agent) advisors to the Credit Parties shall host a conference call with the Administrative Agent and its advisors to discuss, among other things, updates on sale processes, updates on restructuring efforts, financial operations and performance of the Credit Parties’ business, cash flows, capital raise efforts, regulatory matters, and such other business matters relating to the Credit Parties as the Administrative Agent may reasonably request; (b) upon the reasonable request of the Administrative Agent, advisors to the Credit Parties shall host a conference call or videoconference (at a time to be mutually agreed following the Administrative Agent’s request for such conference call or videoconference) with the Administrative Agent, the Administrative

Page 6 WEIL:99600594143717.0003 Agent’s advisors, and the Lenders, to present and discuss updates on sale processes and restructuring efforts; provided, that the Credit Parties shall not be required to conduct more than one conference call or videoconference during any two week calendar period; (c) with respect to the sale process (which may be consummated in multiple transactions) identified to the Administrative Agent as the “Designated Sale Process”, (i) on or before [**] (or such later date as the Administrative Agent may agree to in its reasonable discretion), the Borrower shall have delivered an [**] with respect to the applicable sale to prospective purchasers and delivered a copy of such [**] to the Administrative Agent’s advisors, (ii) the Borrower shall have requested delivery of [**] of interest from all parties interested in participating in the applicable portion of the Designated Sale Process by [**] (or such later date as the Administrative Agent may agree to in its reasonable discretion) and the Borrower shall promptly deliver copies of any such [**] to the Administrative Agent’s advisors, (iii) on or before [**] (or such later date as the Administrative Agent may agree to in its reasonable discretion), the Borrower shall have selected the [**] for the applicable portion of the Designated Sale Process with whom to proceed to [**] (iv) promptly upon receipt, the Borrower shall provide copies of (x) substantially final versions of the [**]and (y) [**] executed in connection with the applicable portion of the Designated Sale Process, (v) the Borrower shall promptly notify the Administrative Agent upon receipt of a request for, and upon entry into, [**] (vi) on or before [**], the Borrower shall have provided the Administrative Agent’s financial advisor with access to [**] for the applicable portion of the Designated Sale Process, and (vii) on or before [**] (or such later date as the Administrative Agent may agree to in its reasonable discretion), the Borrower shall have delivered a [**] with respect to certain assets disclosed to the Administrative Agent and its advisors to be sold as part of the Designated Sale Process; (d) on or before March 20, 2024 (or such later date as the Administrative Agent may agree to in its reasonable discretion), the Credit Parties shall cause Emergent BioSolutions Canada Inc. to (i) become a Guarantor by delivering to the Administrative Agent a duly executed supplement to the Guaranty Agreement in the form attached thereto as Exhibit A or such other document as the Administrative Agent shall deem reasonably acceptable for such purpose, (ii) grant a security interest in all Collateral (subject to the exclusions and exceptions specified in the Collateral Agreement) owned by such entity by delivering to the Administrative Agent a duly executed supplement to the Collateral Agreement in the form attached thereto as Exhibit A or such other document as the Administrative Agent shall deem reasonably acceptable for such purpose, (iii) deliver to the Administrative Agent such updated Schedules to the Loan Documents as are reasonably requested by the Administrative Agent with respect to such entity, (iv) deliver to the Administrative Agent such other documents as may be reasonably requested by the Administrative Agent, all in form, content and scope reasonably satisfactory to the Administrative Agent, including without limitation, pursuant to Section 8.2(l) of the Credit Agreement, and (v) comply with the provisions of Section 8.15 of the Credit Agreement; provided, that upon request by the Borrower on the basis of a material adverse tax consequence for the Borrower and its Subsidiaries, taken as a whole, the Administrative Agent may in its reasonable discretion waive the requirement that the Credit Parties comply with this Section 4(d); and (e) on or before March 31, 2024, the Borrower shall deliver an annual business plan and budget of the Borrower and its Subsidiaries on a consolidated basis for the remainder of the 2024 fiscal year prepared by management, in form reasonably satisfactory to the Administrative Agent. All of the covenants and obligations contained in this Section 4 and all of the other covenants and obligations of the Credit Parties in this Forbearance Agreement and Amendment, are independent of and in addition to the covenants of the Credit Parties in the Credit Agreement and the other Loan Documents.

Page 7 WEIL:99600594143717.0003 Section 5. Conditions Precedent to the Effectiveness of this Forbearance Agreement and Amendment This Forbearance Agreement and Amendment shall become effective upon the satisfaction (or waiver in writing by Administrative Agent, on behalf of the Required Lenders) of the following conditions precedent (the “Forbearance Effective Date”): (a) the Administrative Agent and each Lender shall have received counterparts of this Forbearance Agreement and Amendment, duly executed and delivered by the Credit Parties, the Administrative Agent, the Required Lenders and the Required Revolving Credit Lenders; (b) the Administrative Agent shall have received payment from the Borrower of (i) the Forbearance Fee required in accordance with the terms and conditions hereof and (ii) reasonable fees, charges and disbursements of the Administrative Agent, McGuireWoods LLP, counsel for the Administrative Agent, and of RPA Advisors, LLC, financial advisor engaged on behalf of the Administrative Agent. Section 6. Representations and Warranties of the Credit Parties To induce the Administrative Agent and the Lenders party hereto to enter into this Forbearance Agreement and Amendment, on and as of the Forbearance Effective Date, each of the Credit Parties hereby represents and warrants to the Administrative Agent and each Lender party hereto as follows: (a) After giving effect to this Forbearance Agreement and Amendment, the representations and warranties of each Credit Party set forth in the Credit Agreement and in each other Loan Document to which it is a party are true and correct in all material respects on and as of the Forbearance Effective Date with the same effect as though made on and as of such date, except to the extent such representations and warranties expressly relate to an earlier date, in which case they shall be true and correct in all material respects as of such earlier date; provided that any representation and warranty that is qualified as to “materiality,” “Material Adverse Effect” or similar language shall be true and correct (after giving effect to any qualification therein) in all respects on such respective dates; (b) except as described in this Forbearance Agreement and Amendment, no Default or Event of Default has occurred and is continuing; (c) it has all requisite power and authority and has taken all necessary corporate and other action to authorize the execution, delivery and performance of this Forbearance Agreement and Amendment and each other document executed in connection herewith to which it is a party in accordance with their respective terms and the transactions contemplated hereby; and (d) this Forbearance Agreement and Amendment and each other document executed in connection herewith has been duly executed and delivered by the duly authorized officers of each Credit Party, and each such document constitutes the legal, valid and binding obligation of each such Credit Party, enforceable in accordance with its terms, subject to applicable bankruptcy, insolvency, reorganization, moratorium or other laws affecting creditors’ rights generally and subject to general principals of equity. Section 7. Release (a) In consideration of, among other things, Administrative Agent’s and the Lenders’ execution and delivery of this Forbearance Agreement and Amendment, each of Borrower and the other Credit Parties, on behalf of itself and its Related Parties, successors and assigns (collectively, “Releasors”), hereby forever agrees and covenants not to sue or prosecute against any Releasee (as hereinafter defined) and hereby forever waives, releases and discharges, to the fullest extent permitted by law, each Releasee from any and all claims (including, without limitation, crossclaims, counterclaims, rights of set-off and recoupment), actions,

Page 8 WEIL:99600594143717.0003 causes of action, suits, debts, accounts, interests, liens, promises, warranties, damages and consequential damages, demands, agreements, bonds, bills, specialties, covenants, controversies, variances, trespasses, judgments, executions, costs, expenses or claims whatsoever, that such Releasor now has or hereafter may have, of whatsoever nature and kind, whether known or unknown, whether now existing or hereafter arising, whether arising at law or in equity (collectively, the “Claims”), against the Administrative Agent (and any subagent thereof), each Lender and each Issuing Lender and their respective Related Parties, and their respective successors and assigns (collectively, the “Releasees”), based in whole or in part on facts, whether or not now known, existing on or before the Forbearance Effective Date, that relate to, arise out of or otherwise are in connection with: (i) any or all of the Loan Documents or transactions contemplated thereby or any actions or omissions in connection therewith, or (ii) any aspect of the dealings or relationships between or among Borrower and the other Credit Parties, on the one hand, and any or all of the Administrative Agent, the Lenders and the Issuing Lenders, on the other hand, relating to any or all of the documents, transactions, actions or omissions referenced in clause (i) hereof. In entering into this Forbearance Agreement and Amendment, Borrower and each other Credit Party consulted with, and has been represented by, legal counsel and expressly disclaims any reliance on any representations, acts or omissions by any of the Releasees and hereby agrees and acknowledges that the validity and effectiveness of the releases set forth above do not depend in any way on any such representations, acts and/or omissions or the accuracy, completeness or validity thereof. (b) Each of Borrower and other Credit Parties, on behalf of itself and its Related Parties and its successors, assigns, hereby absolutely, unconditionally and irrevocably, covenants and agrees with and in favor of each Releasee that it will not sue (at law, in equity, in any regulatory proceeding or otherwise) any Releasee on the basis of any Claim released, remised and discharged by Borrower or any other Credit Party pursuant to Section 7(a) hereof. If Borrower, any other Credit Party or any of its successors, assigns or other legal representatives violates the foregoing covenant, Borrower and other Credit Parties, each for itself and its successors, assigns and legal representatives, agrees to pay, in addition to such other damages as any Releasee may sustain as a result of such violation, all attorneys’ fees and costs incurred by any Releasee as a result of such violation. (c) Each party’s obligations under this Section shall survive the termination of the Loan Documents and payment of the obligations thereunder. Section 8. Reference to and Effect on the Credit Agreement and the Loan Documents Except as expressly provided herein, the Existing Credit Agreement and the other Loan Documents shall remain unmodified and in full force and effect. This Forbearance Agreement and Amendment shall not be deemed (a) to be a waiver of, or consent to, or a modification or amendment of, any other term or condition of the Existing Credit Agreement or any other Loan Document other than as expressly set forth herein, (b) to prejudice any right or rights which the Administrative Agent or the Lenders may now have or may have in the future under or in connection with the Existing Credit Agreement or the other Loan Documents or any of the instruments or agreements referred to therein, as the same may be amended, restated, supplemented or modified from time to time, or (c) to be a commitment or any other undertaking or expression of any willingness to engage in any further discussion with the Borrower, any of its Subsidiaries or any other Person with respect to any other waiver, amendment, modification or any other change to the Existing Credit Agreement or the Loan Documents or any rights or remedies arising in favor of the Lenders or the Administrative Agent, or any of them, under or with respect to any such documents. References in the Credit Agreement to “this Agreement” (and indirect references such as “hereunder”, “hereby”, “herein”, “hereof” or other words of like import) and in any Loan Document to the “Credit Agreement” shall be deemed to be references to the Existing Credit Agreement as modified hereby. Section 9. Further Assurances Each Credit Party agrees to, to the extent required by the Loan Documents, make, execute and deliver all such additional and further acts, things, deeds, instruments and documents as the Administrative Agent

Page 9 WEIL:99600594143717.0003 may reasonably require for the purposes of implementing or effectuating the provisions of this Forbearance Agreement and Amendment and the other Loan Documents. Section 10. Acknowledgement and Reaffirmation Each Credit Party (a) consents to this Forbearance Agreement and Amendment and agrees that the transactions contemplated by this Forbearance Agreement and Amendment shall not limit or diminish the obligations of such Person under, or release such Person from any obligations under, any of the Loan Documents to which it is a party (except to the extent modified by this Forbearance Agreement and Amendment), (b) confirms and reaffirms its obligations under each of the Loan Documents to which it is a party (except to the extent modified by this Forbearance Agreement and Amendment) and (c) agrees that each of the Loan Documents to which it is a party (except to the extent modified by this Forbearance Agreement and Amendment) remains in full force and effect and is hereby ratified and confirmed. Section 11. Costs and Expenses The Borrower hereby reconfirms its obligations pursuant to Section 12.3(a) of the Credit Agreement to pay and reimburse the Administrative Agent in accordance with the terms hereof. Section 12. Execution in Counterparts This Forbearance Agreement and Amendment may be executed in any number of counterparts and by the different parties hereto on separate counterparts, each of which counterparts when executed and delivered shall be an original, but all of which shall together constitute one and the same instrument. Delivery by facsimile or electronic transmission of an executed counterpart of a signature page to this Forbearance Agreement and Amendment shall be effective as delivery of an original executed counterpart of this Forbearance Agreement and Amendment. Section 13. Administrative Agent and Lender Authorization This Forbearance Agreement and Amendment has been duly authorized, executed and delivered by the Administrative Agent and each of the undersigned Lenders and constitutes the legal, valid and binding obligation of such party enforceable against such party in accordance with its terms, subject to bankruptcy, insolvency, reorganization, moratorium or similar laws affecting the enforcement of creditors’ rights generally and by general equitable principles (whether enforcement is sought by proceedings in equity or at law) and an implied covenant of good faith and fair dealing. The undersigned Lenders constitute the Required Lenders and Required Revolving Credit Lenders under the Credit Agreement. Section 14. Governing Law THIS FORBEARANCE AGREEMENT AND AMENDMENT SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK. Section 15. Notices All communications and notices hereunder shall be made in writing and delivered in the manner as provided in Section 12.1 of the Credit Agreement. Section 16. Successors and Assigns This Forbearance Agreement and Amendment shall be binding on and inure to the benefit of the parties hereto and their successors and permitted assigns.

Page 10 WEIL:99600594143717.0003 Section 17. Amendments No amendment, modification or waiver of the terms of this Forbearance Agreement and Amendment shall be effective except in a writing signed by the Credit Parties, the Administrative Agent and the Required Lenders; provided that the Administrative Agent and the Required Lenders may agree to (x) extend the Forbearance Period or (y) include any additional Default or Event of Default occurring after the date hereof as “Defaults” or “Specified Events of Default” hereunder by providing email confirmation of such amendment sent from Administrative Agent and any such email confirmation, to the extent explicitly acknowledged therein, shall be effective as an amendment and modification of the terms set forth herein. [Signature pages follow.]



[Signature Page to Forbearance Agreement and Sixth Amendment] WELLS FARGO BANK, NATIONAL ASSOCIATION, as Administrative Agent, Swingline Lender, Issuing Lender and Lender By: Name: Troy Jefferson Title: Executive Director

[Signature Page to Forbearance Agreement and Sixth Amendment] BMO BANK NATIONAL ASSOCIATION, as Lender By: Name: Title: Drew Feller Vice President, BMO Bank National Association

[Signature Page to Forbearance Agreement and Sixth Amendment] Capital One, N.A., as Lender By: Name: Ryan Guenin Title: Duly Authorized Signatory

[Signature Page to Forbearance Agreement and Sixth Amendment] DNB Capital LLC By: Name: Title: By: Name: Title: Dania Hinedi Senior Vice President Bret Douglas Senior Vice President

[Signature Page to Forbearance Agreement and Sixth Amendment] ROYAL BANK OF CANADA, as Lender By: Name: Juliet K. M. Eck Title: Authorized Signatory

[Signature Page to Forbearance Agreement and Sixth Amendment] VÄRDE INVESTMENT PARTNERS, L.P. By Värde Investment Partners G.P., L.P., Its General Partner By Värde Investment Partners UGP, LLC, Its General Partner By Värde Partners, L.P., Its Managing Member By Värde Partners, Inc., Its General Partner By: Name: Matthew Mach Title: Managing Director

WEIL:99600594143717.0003 SCHEDULE A SPECIFIED EVENTS OF DEFAULT 1. Event of Default under Section 10.1(c) of the Credit Agreement as a result of the Borrower’s delivery of annual financial statements pursuant with Section 8.1(a) of the Credit Agreement for the fiscal year ending December 31, 2023 with a “going concern” or like qualification or exception. 2. Event of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrower’s failure to consummate the Junior Capital Raise by April 30, 2024 pursuant to Section 8.20 of the Credit Agreement. 3. Event of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrower’s failure to comply with the Consolidated Debt Service Coverage Ratio for the fiscal quarter ending March 31, 2024 set forth in Section 9.11(a) of the Credit Agreement. 4. Event of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrower’s failure to comply with the Consolidated Leverage Ratio for the fiscal quarter ending March 31, 2024 set forth in Section 9.11(b) of the Credit Agreement. 5. Events of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrower’s failure to comply with the minimum Consolidated EBITDA for the periods ending November 30, 2023, December 31, 2023, January 31, 2024 and February 29, 2024 set forth in Section 9.11(c) of the Credit Agreement. 6. Event of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrower’s failure to comply with the maximum Capital Expenditures for the period ending February 29, 2024 set forth in Section 9.11(d) of the Credit Agreement. 7. Events of Default under Section 10.1(b) of the Credit Agreement as a result of the Borrowers’ failure to comply with Section 8.3(a) of the Credit Agreement as it relates to any of the foregoing Specified Defaults. 8. Events of Default under Section 10.1(d) of the Credit Agreement as a result of any Specified Event of Default.

WEIL:99600594143717.0003 ANNEX A To Forbearance Agreement and Sixth Amendment to Amended and Restated Credit Agreement

WEIL:99600594143717.0003 EXHIBIT G to Amended and Restated Credit Agreement dated as of October 15, 2018 by and among Emergent BioSolutions Inc., as Borrower, the lenders party thereto, as Lenders, and Wells Fargo Bank, National Association, as Administrative Agent FORM OF ASSIGNMENT AND ASSUMPTION

ASSIGNMENT AND ASSUMPTION This Assignment and Assumption (the “Assignment and Assumption”) is dated as of the Effective Date set forth below and is entered into by and between [INSERT NAME OF ASSIGNOR] (the “Assignor”) and the parties identified on the Schedules hereto and [the] [each]1 Assignee identified on the Schedules hereto as “Assignee” or as “Assignees” (collectively, the “Assignees” and each, an “Assignee”). [It is understood and agreed that the rights and obligations of the Assignees2 hereunder are several and not joint.]3 Capitalized terms used but not defined herein shall have the meanings given to them in the Amended and Restated Credit Agreement identified below (the “Credit Agreement”), receipt of a copy of which is hereby acknowledged by [the] [each] Assignee. The Standard Terms and Conditions set forth in Annex 1 attached hereto are hereby agreed to and incorporated herein by reference and made a part of this Assignment and Assumption as if set forth herein in full. For an agreed consideration, the Assignor hereby irrevocably sells and assigns to the [Assignee] [respective Assignees], and [the] [each] Assignee hereby irrevocably purchases and assumes from the Assignor, subject to and in accordance with the Standard Terms and Conditions and the Credit Agreement, as of the Effective Date inserted by the Administrative Agent as contemplated below (i) all of the Assignor’s rights and obligations in its capacity as a Lender under the Credit Agreement and any other documents or instruments delivered pursuant thereto to the extent related to the amount and percentage interest identified below of all of such outstanding rights and obligations of the Assignor under the respective facilities identified below (including without limitation any letters of credit, guarantees, and swingline loans included in such facilities) and (ii) to the extent permitted to be assigned under Applicable Law, all claims, suits, causes of action and any other right of the Assignor (in its capacity as a Lender) against any Person, whether known or unknown, arising under or in connection with the Credit Agreement, any other documents or instruments delivered pursuant thereto or the loan transactions governed thereby or in any way based on or related to any of the foregoing, including, but not limited to, contract claims, tort claims, malpractice claims, statutory claims and all other claims at law or in equity related to the rights and obligations sold and assigned pursuant to clause (i) above (the rights and obligations sold and assigned to [the] [any] Assignee pursuant to clauses (i) and (ii) above being referred to herein collectively as, [the] [an] “Assigned Interest”). Each such sale and assignment is without recourse to the Assignor and, except as expressly provided in this Assignment and Assumption, without representation or warranty by the Assignor. 1. Assignor: [INSERT NAME OF ASSIGNOR] 2. Assignee(s): See Schedules attached hereto 3. Borrower: Emergent BioSolutions Inc., a Delaware corporation 4. Administrative Agent: Wells Fargo Bank, National Association, as the administrative agent under the Credit Agreement 5. Credit Agreement: The Amended and Restated Credit Agreement dated as of October 15, 2018 among Emergent BioSolutions Inc., a Delaware corporation, as Borrower, the Lenders party thereto, and Wells 1 For bracketed language here and elsewhere in this form relating to the Assignee(s), if the assignment is to a single Assignee, choose the first bracketed language. If the assignment is to multiple Assignees, choose the second bracketed language. 2 Select as appropriate. 3 Include bracketed language if there are multiple Assignees.

Fargo Bank, National Association, as Administrative Agent (as amended, restated, supplemented or otherwise modified) 6. Assigned Interest: See Schedules attached hereto [7. Trade Date: ______________]4 [Remainder of page intentionally left blank; signature page follows] 4 To be completed if the Assignor and the Assignees intend that the minimum assignment amount is to be determined as of the Trade Date.

Effective Date: _____________ ___, 2____ [TO BE INSERTED BY THE ADMINISTRATIVE AGENT AND WHICH SHALL BE THE EFFECTIVE DATE OF RECORDATION OF TRANSFER IN THE REGISTER THEREFOR] The terms set forth in this Assignment and Assumption are hereby agreed to: ASSIGNOR [NAME OF ASSIGNOR] By: Name: Title: ASSIGNEES See Schedules attached hereto

[Consented to and]5 Accepted: WELLS FARGO BANK, NATIONAL ASSOCIATION, as Administrative Agent, Issuing Lender and Swingline Lender By:_________________________________ Name: Title: [Consented to:]6 [APPLICABLE ISSUING LENDER], as Issuing Lender By:________________________________ Name: Title: [Consented to:]7 EMERGENT BIOSOLUTIONS INC. By:________________________________ Name: Title: 5 To be added only if the consent of the Administrative Agent and/or the Swingline Lender and Issuing Lender is required by the terms of the Credit Agreement. 6 To be added only if the consent of Issuing Lender is required by the terms of the Credit Agreement. 7 To be added only if the consent of the Borrower is required by the terms of the Credit Agreement.
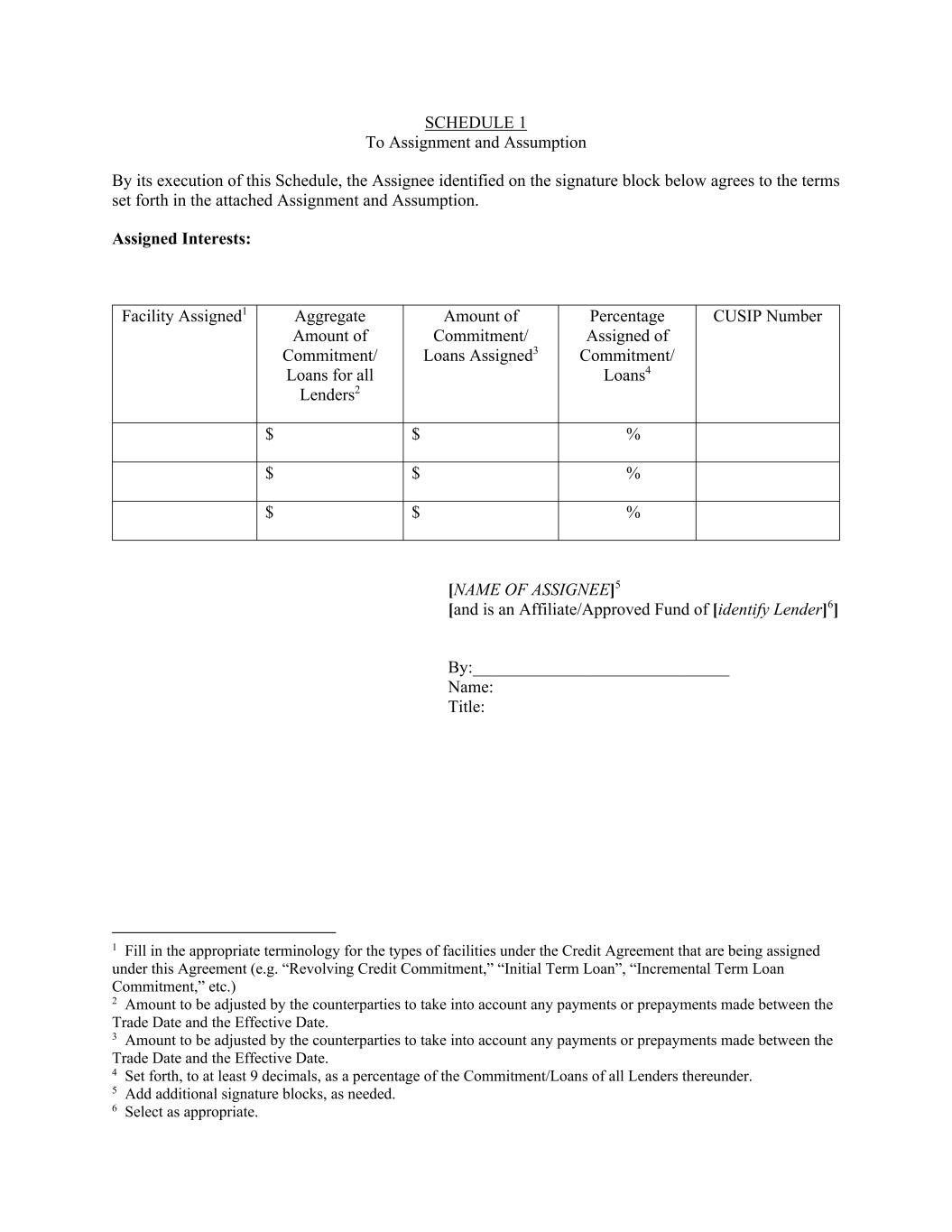
SCHEDULE 1 To Assignment and Assumption By its execution of this Schedule, the Assignee identified on the signature block below agrees to the terms set forth in the attached Assignment and Assumption. Assigned Interests: Facility Assigned1 Aggregate Amount of Commitment/ Loans for all Lenders2 Amount of Commitment/ Loans Assigned3 Percentage Assigned of Commitment/ Loans4 CUSIP Number $ $ % $ $ % $ $ % [NAME OF ASSIGNEE]5 [and is an Affiliate/Approved Fund of [identify Lender]6] By:______________________________ Name: Title: 1 Fill in the appropriate terminology for the types of facilities under the Credit Agreement that are being assigned under this Agreement (e.g. “Revolving Credit Commitment,” “Initial Term Loan”, “Incremental Term Loan Commitment,” etc.) 2 Amount to be adjusted by the counterparties to take into account any payments or prepayments made between the Trade Date and the Effective Date. 3 Amount to be adjusted by the counterparties to take into account any payments or prepayments made between the Trade Date and the Effective Date. 4 Set forth, to at least 9 decimals, as a percentage of the Commitment/Loans of all Lenders thereunder. 5 Add additional signature blocks, as needed. 6 Select as appropriate.

ANNEX 1 to Assignment and Assumption STANDARD TERMS AND CONDITIONS FOR ASSIGNMENT AND ASSUMPTION 1. Representations and Warranties. 1.1 Assignor. The Assignor (a) represents and warrants that (i) it is the legal and beneficial owner of [the] [the relevant] Assigned Interest, (ii) [the] [such] Assigned Interest is free and clear of any lien, encumbrance or other adverse claim, (iii) it has full power and authority, and has taken all action necessary, to execute and deliver this Assignment and Assumption and to consummate the transactions contemplated hereby and (iv) it is [not] a Defaulting Lender; and (b) assumes no responsibility with respect to (i) any statements, warranties or representations made in or in connection with the Credit Agreement or any other Loan Document, (ii) the execution, legality, validity, enforceability, genuineness, sufficiency or value of the Loan Documents or any collateral thereunder, (iii) the financial condition of [Holdings,] the Borrower, any of [its][their respective] Subsidiaries or Affiliates or any other Person obligated in respect of any Loan Document or (iv) the performance or observance by [Holdings,] the Borrower, any of [its][their respective] Subsidiaries or Affiliates or any other Person of any of their respective obligations under any Loan Document. 1.2. Assignee[s]. [The] [Each] Assignee (a) represents and warrants that (i) it has full power and authority, and has taken all action necessary, to execute and deliver this Assignment and Assumption and to consummate the transactions contemplated hereby and to become a Lender under the Credit Agreement, (ii) it meets the requirements of an Eligible Assignee under the Credit Agreement (subject to such consents, if any, as may be required under Section 12.9(b)(iii) of the Credit Agreement), (iii) from and after the Effective Date, it shall be bound by the provisions of the Credit Agreement as a Lender thereunder and, to the extent of [the] [the relevant] Assigned Interest, shall have the obligations of a Lender thereunder, (iv) it is sophisticated with respect to decisions to acquire assets of the type represented by the Assigned Interest and either it, or the Person exercising discretion in making its decision to acquire [the] [such] Assigned Interest, is experienced in acquiring assets of such type, (v) it has received a copy of the Credit Agreement, and has received or has been accorded the opportunity to receive copies of the most recent financial statements delivered pursuant to Section 8.1 thereof, as applicable, and such other documents and information as it deems appropriate to make its own credit analysis and decision to enter into this Assignment and Assumption and to purchase [the] [such] Assigned Interest, (vi) it has, independently and without reliance upon the Administrative Agent or any other Lender and based on such documents and information as it has deemed appropriate, made its own credit analysis and decision to enter into this Assignment and Assumption and to purchase [the] [such] Assigned Interest, and (vii) if it is a Foreign Lender, attached to the Assignment and Assumption is any documentation required to be delivered by it pursuant to the terms of the Credit Agreement, duly completed and executed by [the] [such] Assignee; and (b) agrees that (i) it will, independently and without reliance upon the Administrative Agent, [the] [any] Assignor or any other Lender, and based on such documents and information as it shall deem appropriate at the time, continue to make its own credit decisions in taking or not taking action under the Loan Documents, and (ii) it will perform in accordance with their terms all of the obligations which by the terms of the Loan Documents are required to be performed by it as a Lender. 2. Payments. From and after the Effective Date, the Administrative Agent shall make all payments in respect of [the] [each] Assigned Interest (including payments of principal, interest, fees and other amounts) to [the] [the relevant] Assignor for amounts which have accrued to but excluding the

Effective Date and to [the] [the relevant] Assignee for amounts which have accrued from and after the Effective Date. 3. General Provisions. This Assignment and Assumption shall be binding upon, and inure to the benefit of, the parties hereto and their respective successors and assigns. This Assignment and Assumption may be executed in any number of counterparts, which together shall constitute one instrument. Delivery of an executed counterpart of a signature page of this Assignment and Assumption by telecopy shall be effective as delivery of a manually executed counterpart of this Assignment and Assumption. This Assignment and Assumption shall be governed by, and construed in accordance with, the law of the State of New York.
EMERGENT BIOSOLUTIONS REPORTS FOURTH QUARTER 2023 FINANCIAL RESULTS
•Fourth Quarter 2023 Total Revenues of $277 million, which aligned our Full Year to the mid-point of guidance
•Full Year 2023 Total Revenues of $1.05 billion, which was the mid-point of guidance
•Fourth Quarter 2023 Net Loss of $50 million and Adjusted EBITDA of $3 million
•Issues Q1 2024 and FY 2024 guidance
GAITHERSBURG, Md., March 6, 2024—Emergent BioSolutions Inc. (NYSE: EBS) today reported financial results for the quarter and year ended December 31, 2023.
"Emergent has a long history of helping protect people around the world from opioid overdose emergencies and chemical, biological and radiological threats. This commitment to public health, together with Emergent's leadership, give me confidence in the long-term future of the company," said Joe Papa, President and CEO at Emergent. "Emergent faces some short-term challenges, which we are addressing head on. At the same time, we are making decisions and putting strategies in place for Emergent to add value for customers, patients and investors. Emergent is a company with a bright future, and I am excited to help lead it forward."
FINANCIAL HIGHLIGHTS (1)
Q4 2023 vs. Q4 2022
| | | | | | | | | | | |
| ($ in millions, except per share amounts) | Q4 2023 | Q4 2022 | % Change |
| Total Revenues | $ | 276.6 | | $ | 330.2 | | (16) | % |
| Net Loss | $ | (49.5) | | $ | (67.0) | | 26 | % |
| Net Loss per Diluted Share | $ | (0.95) | | $ | (1.34) | | 29 | % |
Adjusted Net Income (Loss) (2) | $ | (40.0) | | $ | 5.9 | | * |
Adjusted Net Income (Loss) per Diluted Share (2) | $ | (0.77) | | $ | 0.11 | | * |
Adjusted EBITDA (2) | $ | 3.4 | | $ | 44.0 | | (92) | % |
Total Segment Gross Margin % (2) | 31 | % | 32 | % | |
Total Segment Adjusted Gross Margin % (2) | 32 | % | 48 | % | |
| | | |
| * % change is greater than +/- 100% | | | |
Full Year 2023 vs. Full Year 2022
| | | | | | | | | | | |
| ($ in millions, except per share amounts) | 2023 | 2022 | % Change |
| Total Revenues | $ | 1,049.3 | | $ | 1,117.5 | | (6) | % |
| Net Loss | $ | (760.5) | | $ | (211.6) | | * |
| Net Loss per Diluted Share | $ | (14.85) | | $ | (4.22) | | * |
Adjusted Net Loss (2) | $ | (319.0) | | $ | (99.7) | | * |
Adjusted Net Loss per Diluted Share (2) | $ | (6.23) | | $ | (1.98) | | * |
Adjusted EBITDA (2) | $ | (22.3) | | $ | 28.8 | | * |
Total Segment Gross Margin % (2) | 31 | % | 36 | % | |
Total Segment Adjusted Gross Margin % (2) | 33 | % | 41 | % | |
| | | |
| * % change is greater than +/- 100% | | | |
SELECT Q4 2023 AND FULL YEAR BUSINESS UPDATES
•Launched NARCAN® Naloxone HCl Nasal Spray 4 mg Over-The-Counter (“OTC NARCAN®”), broadening our customer base and sales channels to retail pharmacies and digital commerce websites as well as through physician-directed or standing order prescriptions at retail pharmacies, health departments, local law enforcement agencies, community-based organizations, substance abuse centers and other federal agencies
•Announced U.S. Food and Drug Administration (“FDA”) approval of CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), previously known as AV7909, a two-dose anthrax vaccine for post-exposure prophylaxis use
•Awarded a $75 million option to Emergent's existing contract for the acquisition of the newly approved CYFENDUS®
•Awarded a 10-year contract by the Biomedical Advanced Research and Development Authority (“BARDA”) for advanced development, manufacturing scale-up, and procurement of EbangaTM (ansuvimab-zykl) product, a treatment for Ebola
•Awarded a $379.6 million U.S. Department of Defense contract for RSDL®
•The FDA closed out its inspection of the Company’s Camden facility and issued a “close-out letter” of its Warning Letter issued in August 2022
•Continued progress on strengthening our fundamentals with key focus on our Medical Countermeasure (“MCM”) and NARCAN® products
•Implemented organizational and resource changes resulting in $160 million of annual savings
•Amended and extended maturity of our secured credit facility to May 2025
•Divested the Travel Health business valued at $380 million
FOURTH QUARTER 2023 FINANCIAL PERFORMANCE (1)
Revenues
Beginning in 2023, the Company revised the categories used in discussing product/service level revenues. The new categories are:
•NARCAN® — comprises contributions from NARCAN® Nasal Spray
•Other Commercial Products - comprises contributions from Vaxchora® and Vivotif®, which we sold to Bavarian Nordic as part of our travel health business in May 2023
•Anthrax MCM — comprises potential contributions from CYFENDUS®, previously known as AV7909, BioThrax®, Anthrasil® and Raxibacumab
•Smallpox MCM — comprises potential contributions from ACAM2000®, VIGIV and TEMBEXA®
•Other Products — comprises potential contributions from BAT®, RSDL® and Trobigard®
•Bioservices — comprises service and lease revenues from the Bioservices business
| | | | | | | | | | | |
| ($ in millions) | Q4 2023 | Q4 2022 | % Change |
Product sales, net (3): | | | |
NARCAN® | $ | 111.0 | | $ | 91.1 | | 22 | % |
| Other Commercial Products | — | | 4.9 | | NM |
| Anthrax MCM | 111.6 | | 56.4 | | 98 | % |
| Smallpox MCM | 11.5 | | 144.6 | | (92) | % |
| Other Products | 15.0 | | 8.7 | | 72 | % |
| Total Product sales, net | $ | 249.1 | | $ | 305.7 | | (19) | % |
| | | |
| Bioservices: | | | |
| Services | $ | 20.6 | | $ | 17.2 | | 20 | % |
| Leases | 0.2 | | 0.2 | | — | % |
| Total Bioservices revenues | $ | 20.8 | | $ | 17.4 | | 20 | % |
| | | |
| Contracts and grants | $ | 6.7 | | $ | 7.1 | | (6) | % |
| | | |
| Total revenues | $ | 276.6 | | $ | 330.2 | | (16) | % |
| | | |
| | | |
| NM - Not Meaningful |
|
Products Revenue, net
NARCAN®
For Q4 2023, revenues from NARCAN® (naloxone HCl) Nasal Spray increased $19.9 million, or 22%, as compared with Q4 2022. The increase was primarily driven by higher OTC NARCAN® sales to U.S. public interest channels and retailers, partially offset by a decrease in sales of prescription based NARCAN® due to the launch of OTC NARCAN® in 2023 and the cessation of authorized generic NARCAN® sales related to the termination of the Company’s relationship with Sandoz.
Other Commercial Products
For Q4 2023, revenues from Other Commercial Products decreased $4.9 million as compared with Q4 2022. The decrease was driven by no sales of our Vaxchora® and Vivotif® products during the current year quarter, which we sold to Bavarian Nordic as part of our travel health business in May 2023.
Anthrax MCM
For Q4 2023, revenues from Anthrax MCM increased $55.2 million, or 98%, as compared with Q4 2022. The increase reflects the impact of timing of sales related to CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), Anthrasil® (Anthrax Immune
Globulin Intravenous (human)) and BioThrax® (Anthrax Vaccine Adsorbed). Anthrax vaccine product sales are primarily made under annual purchase options exercised by the U.S. Government ("USG"). Fluctuations in revenues result from the timing of the exercise of annual purchase options, the timing and amount of USG purchases, the availability of governmental funding and timing of Company delivery of orders that follow.
Smallpox MCM
For Q4 2023, revenues from Smallpox MCM decreased $133.1 million, or 92%, as compared with Q4 2022. The decrease was due to no current quarter sales of TEMBEXA® and timing of VIGIV deliveries. Fluctuations in revenues from Smallpox MCM result from the timing of the exercise of annual purchase options in existing procurement contracts, the timing of USG purchases, the availability of governmental funding and timing of Company delivery of orders that follow.
Other Products
For Q4 2023, revenues from other product sales increased $6.3 million, or 72%, as compared with Q4 2022. The increase was primarily due to higher BAT® product sales, partially offset by lower sales of RSDL®.
Bioservices Revenues
Services
For Q4 2023, revenues from Bioservices services increased $3.4 million, or 20%, as compared with Q4 2022. The increase was primarily driven by resolution of a customer's outstanding obligation at our Bayview facility and increased production at our Camden facility, partially offset by a decrease in production at our Winnipeg facility. In the prior year, there was a reversal of revenue related to the halt in manufacturing under the Janssen Agreement.
Leases
For Q4 2023, revenues from Bioservices leases were consistent with Q4 2022.
Contracts and Grants
For Q4 2023, revenues from contracts and grants decreased $0.4 million, or 6%, as compared with Q4 2022. The decrease was primarily attributable to changes in the mix and timing of various development initiatives.
Operating Expenses
| | | | | | | | | | | |
| ($ in millions) | Q4 2023 | Q4 2022 | % Change |
| Cost of Commercial product sales | $ | 50.1 | | $ | 40.2 | | 25 | % |
Cost of MCM product sales | 97.2 | | 127.6 | | (24) | % |
| Cost of Bioservices | 37.8 | | 52.7 | | (28) | % |
| Goodwill impairment | — | | 6.7 | | NM |
| | | |
| Research and development (“R&D”) | 29.4 | | 47.0 | | (37) | % |
| Selling, general and administrative (“SG&A”) | 89.7 | | 93.4 | | (4) | % |
| Amortization of intangible assets | 16.2 | | 17.9 | | (9) | % |
| Total operating expenses | $ | 320.4 | | $ | 385.5 | | (17) | % |
| | | |
| | | |
| NM - Not Meaningful | | | |
Cost of Commercial Product Sales
For Q4 2023, cost of Commercial product sales increased $9.9 million, or 25%, as compared with Q4 2022. The increase was primarily due to higher sales of OTC NARCAN® and Branded NARCAN®, partially offset by no sales of Vivotif® and Vaxchora® or related expenses during the current quarter due to the sale of our travel health business to Bavarian Nordic in May 2023.
Cost of MCM Product Sales
For Q4 2023, cost of MCM product sales decreased $30.4 million, or 24%, as compared with Q4 2022. The decrease was primarily due to lower sales of TEMBEXA® and lower shutdown costs, partially offset by increases due to higher sales of CYFENDUS®, Anthrasil® and BioThrax®, inventory write-offs and additional allocations of cost of goods sold to MCM Products from Bioservices.
Cost of Bioservices
For Q4 2023, cost of Bioservices decreased $14.9 million, or 28%, as compared with Q4 2022. The decrease was primarily due to higher allocations to MCM Product cost of goods sold and reduced production activities related to the halt in manufacturing under the Janssen Agreement at our Bayview facility, coupled with decreases in production at the Company’s Camden and Winnipeg facilities.
Research and Development Expenses
For Q4 2023, R&D expenses decreased $17.6 million, or 37%, as compared with Q4 2022. The decrease was primarily due to the sale of the Company’s development program for CHIKV VLP to Bavarian Nordic, which was a significant contributor to prior period R&D expense, as well as a decrease in funded R&D across various development initiatives and reduction in related overhead costs driven by headcount reductions, partially offset by write-offs related to program terminations during the period and an increase in Ebanga™ and TEMBEXA® funded R&D.
Selling, General and Administrative Expenses
For Q4 2023, SG&A expenses decreased $3.7 million, or 4%, as compared with Q4 2022. The decrease was primarily due to decreases in consulting and contracted services and decreases in compensation and other employee costs related to the restructuring initiatives taken during 2023. The decrease was partially offset by an increase in marketing expenses related to the launch of OTC NARCAN® and higher professional services fees related to legal remediation services.
Goodwill Impairment
For Q4 2023, goodwill impairment decreased $6.7 million as compared with Q4 2022. The decrease was related to the Q4 2022 $6.7 million non-cash impairment charge to Goodwill in the Bioservices reporting unit, which reduced the reporting unit's goodwill balance to zero.
ADDITIONAL FINANCIAL INFORMATION (1)
Capital Expenditures
| | | | | | | | | | | |
| ($ in millions) | Q4 2023 | Q4 2022 | % Change |
| Capital expenditures | $ | 11.4 | | $ | 23.6 | | (52) | % |
| Less: capital expenditures reimbursed | — | | 2.5 | | NM |
| Net capital expenditures | $ | 11.4 | | $ | 21.1 | | (46) | % |
| Capital expenditures as a % of total revenues | 4 | % | 7 | % | |
| Net capital expenditures as a % of total revenues | 4 | % | 6 | % | |
| | | |
| NM - Not Meaningful |
For Q4 2023, capital expenditures decreased largely due to lower product development activities across the Company’s facilities.
SEGMENT INFORMATION
In the fourth quarter of 2023, we realigned our reportable operating segments to reflect recent changes in our internal operating and reporting process. The Company now manages the business with a focus on three reportable segments: (1) a Commercial Products segment consisting of our NARCAN® and other commercial products which were sold as part of our travel health business in the second quarter of 2023; (2) a MCM Products segment consisting of the Anthrax - MCM, Smallpox - MCM and Other products and (3) a services segment (“Services”) consisting of our Bioservices. The Company evaluates the performance of these reportable segments based on revenue and segment adjusted gross margin, which is a non-GAAP financial measure. Segment revenue includes external customer sales, but does not include inter-segment services. The Company does not allocate contracts and grants, R&D, SG&A, amortization of intangible assets, interest and other income (expense) or taxes to its evaluation of the performance of these segments.
FOURTH QUARTER 2023 SEGMENT RESULTS
| | | | | | | | | | | | | | |
| ($ in millions) | Commercial Products |
| Quarter Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 111.0 | | $ | 96.0 | | $ | 15.0 | | 16 | % |
| Cost of sales | 50.1 | | 40.2 | | 9.9 | | 25 | % |
| Gross margin ** | $ | 60.9 | | $ | 55.8 | | $ | 5.1 | | 9 | % |
| Gross margin % ** | 55 | % | 58 | % | | |
| | | | |
| | | | |
Segment adjusted gross margin (2) | $ | 60.9 | | $ | 55.8 | | $ | 5.1 | | 9 | % |
Segment adjusted gross margin % (2) | 55 | % | 58 | % | | |
| | | | |
| | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
|
Commercial Products gross margin increased $5.1 million, or 9%, to $60.9 million in the quarter, as compared with $55.8 million in the prior year quarter. Commercial Products gross margin percentage decreased 3 percentage points to 55% for the quarter ended December 31, 2023. The decrease was primarily due to an increase in royalty expense on OTC NARCAN® compared to Q4 2022, partially offset by a favorable change in product mix related to the sale of our travel health products. Commercial Products segment adjusted gross margin was consistent with gross margin.
| | | | | | | | | | | | | | |
| ($ in millions) | MCM Products |
| Quarter Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 138.1 | | $ | 209.7 | | $ | (71.6) | | (34) | % |
| Cost of sales | 97.2 | | 127.6 | | (30.4) | | (24) | % |
| Gross margin ** | $ | 40.9 | | $ | 82.1 | | $ | (41.2) | | (50) | % |
| Gross margin % ** | 30 | % | 39 | % | | |
| Add back: | | | | |
| Changes in fair value of contingent consideration | $ | 0.6 | | $ | 0.2 | | $ | 0.4 | | * |
| Inventory step-up provision | 2.0 | | 51.4 | | (49.4) | | (96) | % |
| Restructuring costs | (1.4) | | — | | (1.4) | | NM |
Segment adjusted gross margin (2) | $ | 42.1 | | $ | 133.7 | | $ | (91.6) | | (69) | % |
Segment adjusted gross margin % (2) | 30 | % | 64 | % | | |
| | | | |
| * % change is greater than +/- 100% | | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
| NM - Not Meaningful |
MCM Products gross margin decreased $41.2 million, or 50%, to $40.9 million in the quarter, as compared with $82.1 million in the prior year quarter. MCM Products gross margin percentage decreased 9 percentage points to 30% for the quarter ended December 31, 2023. The decrease was largely due to lower sales volumes and inventory write-offs, coupled with an unfavorable product revenue mix which was weighted more heavily to lower margin products compared with the prior year quarter. MCM Product segment adjusted gross margin in the current year period excludes the impact of non-cash items related to the changes in the fair value of contingent consideration of $0.6 million, the inventory step-up provision of $2.0 million and the impact of restructuring costs of $(1.4) million.
| | | | | | | | | | | | | | |
| ($ in millions) | Services |
| Quarter Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 20.8 | | $ | 17.4 | | $ | 3.4 | | 20 | % |
| Cost of services | 37.8 | | 52.7 | | (14.9) | | (28) | % |
| Gross margin ** | $ | (17.0) | | $ | (35.3) | | $ | 18.3 | | 52 | % |
| Gross margin % ** | (82) | % | (203) | % | | |
| Add back: | | | | |
| Restructuring costs | $ | 0.3 | | $ | — | | $ | 0.3 | | NM |
Segment adjusted gross margin (2) | $ | (16.7) | | $ | (35.3) | | $ | 18.6 | | 53 | % |
Segment adjusted gross margin % (2) | (80) | % | (203) | % | | |
| | | | |
| | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
| NM - Not Meaningful | | | | |
Services gross margin increased $18.3 million, or 52%, to $(17.0) million in the quarter, as compared with $(35.3) million in the prior year quarter. Services gross margin percentage increased 121 percentage points to (82)% for the quarter ended December 31, 2023. The increase was primarily due to the resolution of a customer's outstanding obligation at our Bayview facility coupled with one-time costs and reserves related to the Janssen Agreement in the prior year quarter, partially offset by additional investments in quality enhancement and improvement initiatives at the Company's Camden facility and decreased production at the Company's Winnipeg facility in the current year. Services segment adjusted gross margin in the current year period excludes the impact of restructuring costs of $0.3 million.
FULL YEAR 2023 SEGMENT RESULTS
| | | | | | | | | | | | | | |
| ($ in millions) | Commercial Products |
| Year Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 497.3 | | $ | 386.6 | | $ | 110.7 | | 29 | % |
| Cost of sales | 210.3 | | 160.3 | | 50.0 | | 31 | % |
| Gross margin ** | $ | 287.0 | | $ | 226.3 | | $ | 60.7 | | 27 | % |
| Gross margin % ** | 58 | % | 59 | % | | |
| | | | |
| | | | |
Segment adjusted gross margin (2) | $ | 287.0 | | $ | 226.3 | | $ | 60.7 | | 27 | % |
Segment adjusted gross margin % (2) | 58 | % | 59 | % | | |
| | | | |
| | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
|
Commercial Products gross margin increased $60.7 million, or 27%, to $287.0 million in 2023, as compared with $226.3 million in the prior year. Commercial Products gross margin percentage decreased 1 percentage point to 58% in 2023. The decrease was largely due to a decrease in the per unit selling price in response to increased competition for generic NARCAN®, partially offset by a decrease in royalty expense. Commercial Products segment adjusted gross margin was consistent with gross margin.
| | | | | | | | | | | | | | |
| ($ in millions) | MCM Products |
| Year Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 447.2 | | $ | 579.6 | | $ | (132.4) | | (23) | % |
| Cost of sales | 305.6 | | 264.3 | | 41.3 | | 16 | % |
| Gross margin ** | $ | 141.6 | | $ | 315.3 | | $ | (173.7) | | (55) | % |
| Gross margin % ** | 32 | % | 54 | % | | |
| Add back: | | | | |
| Changes in fair value of contingent consideration | $ | 0.2 | | $ | 2.6 | | $ | (2.4) | | (92) | % |
| Inventory step-up provision | 3.9 | | 51.4 | | (47.5) | | (92) | % |
| Restructuring costs | 5.6 | | — | | 5.6 | | NM |
Segment adjusted gross margin (2) | $ | 151.3 | | $ | 369.3 | | $ | (218.0) | | (59) | % |
Segment adjusted gross margin % (2) | 34 | % | 64 | % | | |
| | | | |
| | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
| NM - Not Meaningful |
MCM Products gross margin decreased $173.7 million, or 55%, to $141.6 million in 2023, as compared with $315.3 million in the prior year. MCM Products gross margin percentage decreased 22 percentage points to 32% for the year ended December 31, 2023. The decrease was largely due to lower sales volumes and higher shutdown related costs and inventory write-offs, coupled with an unfavorable product revenue mix which was weighted more heavily to lower margin products compared with the prior year. MCM Product segment adjusted gross margin in 2023 excludes the impact of restructuring costs of $5.6 million, non-cash items related to the changes in the fair value of contingent consideration of $0.2 million and the inventory step-up provision of $3.9 million.
| | | | | | | | | | | | | | |
| ($ in millions) | Services |
| Year Ended December 31, |
| 2023 | 2022 | $ Change | % Change |
| Revenues | $ | 78.5 | | $ | 109.9 | | $ | (31.4) | | (29) | % |
| Cost of services | 189.5 | | 268.5 | | (79.0) | | (29) | % |
| Gross margin ** | $ | (111.0) | | $ | (158.6) | | $ | 47.6 | | 30 | % |
| Gross margin % ** | (141) | % | (144) | % | | |
| Add back: | | | | |
| Restructuring costs | $ | 8.4 | | $ | — | | $ | 8.4 | | NM |
Segment adjusted gross margin (2) | $ | (102.6) | | $ | (158.6) | | $ | 56.0 | | 35 | % |
Segment adjusted gross margin % (2) | (131) | % | (144) | % | | |
| | | | |
| | | | |
| ** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues. |
|
| NM - Not Meaningful | | | | |
Services gross margin increased $47.6 million, or 30%, to $(111.0) million in 2023, as compared with $(158.6) million in the prior year. Services gross margin percentage increased 3 percentage points to (141)% for the year ended December 31, 2023. The increase was primarily driven by one-time costs and reserves related to the Janssen Agreement in the prior year. Services segment adjusted gross margin in 2023 excludes the impact of restructuring costs of $8.4 million.
2024 FINANCIAL FORECAST
The Company provides the following financial forecast for full year 2024 and Q1 2024, in both instances reflecting management's expectations based on the most current information available.
Full Year 2024
| | | | | | | | |
($ in millions) METRIC | Full Year 2023 Actual | Full Year 2024 Forecast |
| Total revenues | $1,049.3 | $900 - $1,100 |
| Net loss | $(760.5) | $(183) - $(133) |
Adjusted net loss (2) | $(319.0) | $(130) - $(80) |
Adjusted EBITDA (2) | $(22.3) | $50 - $100 |
Total segment adjusted gross margin % (2) | 33% | 40% - 45% |
| | |
Segment Level Revenue (4) | | |
| Commercial Products | $497.3 | $460 - $500 |
| MCM Products | $447.2 | $340 - $490 |
| Services | $78.5 | $70 - $80 |
Q1 2024
| | | | | |
($ in millions) METRIC | Q1 2024 Forecast |
| Total revenues | $200 - $250 |
FOOTNOTES
(1) All financial information included in this release is unaudited.
(2) See “Non-GAAP Financial Measures” and the "Reconciliation of Non-GAAP Financial Measures" tables for the definitions and reconciliations of these non-GAAP financial measures to the most closely related GAAP financial measures.
(3) Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles.
(4) Other Commercial products, which includes Vivotif® and Vaxchora®, which were sold to Bavarian Nordic as part of our travel health business in May 2023, are not included in the 2024 forecast.
CONFERENCE CALL, PRESENTATION SUPPLEMENT AND WEBCAST INFORMATION
Company management will host a conference call at 5:00 pm eastern time today, March 6, 2024, to discuss these financial results. The conference call and presentation supplement can be accessed from the Company's website or through the following:
By phone
Advance registration is required.
Visit https://register.vevent.com/register/BI4568368bc7f24d88b225baf83e5fa29c to register and receive an email with the dial-in number, passcode and registrant ID.
By webcast
Visit https://edge.media-server.com/mmc/p/zaanahs2.
A replay of the call can be accessed from the Emergent website.
ABOUT EMERGENT BIOSOLUTIONS INC.
At Emergent, our mission is to protect and enhance life. We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 20 years, we have been at work defending people from things we hope will never happen—so that we are prepared just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. In working together, we envision protecting or enhancing 1 billion lives by 2030. For more information, visit our website and follow us on LinkedIn, Twitter, and Instagram.
NON-GAAP FINANCIAL MEASURES
In the accompanying analysis of financial information, we sometimes use information derived from consolidated and segment financial information that may not be presented in our financial statements or prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). Certain of these financial measures are considered not in conformity with GAAP (“non-GAAP financial measures”) under the United States Securities and Exchange Commission (“SEC”) rules. Specifically, we have referred to the following non-GAAP financial measures:
•Adjusted Net Income (Loss)
•Adjusted Net Income (Loss) per Diluted Share
•Adjusted EBITDA
•Total Segment Revenues
•Total Segment Gross Margin
•Total Segment Gross Margin %
•Total Segment Adjusted Gross Margin
•Total Segment Adjusted Gross Margin %
•Segment Adjusted Gross Margin
•Segment Adjusted Gross Margin %
We define Adjusted Net Income (Loss) and Adjusted Net Income (Loss) per Diluted Share, which are non-GAAP financial measures, as net loss and net loss per diluted share, respectively, excluding the impact of changes in fair value of contingent consideration, acquisition and divestiture-related costs, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, inventory step-up provisions, and non-cash amortization charges. We use Adjusted Net Income (Loss) for the purpose of calculating Adjusted Net Income (Loss) per Diluted Share. Management uses Adjusted Net Income (Loss) per Diluted Share to assess total Company operating performance on a consistent basis. We believe that these non-GAAP financial measures, when considered together with our GAAP financial results and GAAP financial measures, provide management and investors with an additional understanding of our business operating results, including underlying trends.
We define Adjusted EBITDA, which is a non-GAAP financial measure, as consolidated net loss before income tax provision (benefit), interest expense, net, depreciation, amortization of intangible assets, changes in fair value of contingent consideration, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, acquisition and divestiture-related costs and inventory step-up provisions. We believe that this non-GAAP financial measure, when considered together with our GAAP financial results and GAAP financial measures, provides management and investors with a more complete understanding of our operating results, including underlying trends. In addition, EBITDA is a common alternative measure of operating performance used by many of our competitors. It is used by investors, financial analysts, rating agencies and others to value and compare the financial performance of companies in our industry, although it may be defined differently by different companies. Therefore, we also believe that this non-GAAP financial measure, considered along with corresponding
GAAP financial measures, provides management and investors with additional information for comparison of our operating results with the operating results of other companies.
We have included the definitions of segment gross margin and segment gross margin %, which are GAAP financial measures, below in order to more fully define the components of certain non-GAAP financial measures presented in this press release. We define Segment Gross Margin, as a segment's revenues, less a segment's cost of sales or services. We define Segment Gross Margin %, as Segment Gross Margin as a percentage of a segments revenues. We define Segment Adjusted Gross Margin, which is a non-GAAP financial measure as Segment Gross Margin excluding the impact of restructuring costs and non-cash items related to changes in fair value of contingent consideration and inventory step-up provision. We define Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Segment Adjusted Gross Margin as a percentage of a segment's revenues.
We define Total Segment Revenues, which is a non-GAAP financial measure, as our Total Revenues, less contracts and grants revenue, which is also equal to the sum of the revenues of our operating segments. We define Total Segment Gross Margin, which is a non-GAAP financial measure, as Total Segment Revenues less our aggregate cost of sales or services. We define Total Segment Gross Margin %, which is a non-GAAP financial measure, as Total Segment Gross Margin as a percentage of Total Segment Revenues. We define Total Segment Adjusted Gross Margin, which is a non-GAAP financial measure, as Total Segment Gross Margin, excluding the impact of restructuring costs, inventory step-up provision and the fair value of contingent consideration. We define Total Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Total Segment Adjusted Gross Martin as a percentage of Total Segment Revenues.
Non-GAAP financial measures are not defined in the same manner by all companies and may not be comparable with other similarly titled measures of other companies. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Non-GAAP financial measures should be considered in addition to, but not as a substitute for or superior to, the information contained in our Consolidated Statements of Operations and Consolidated Statements of Cash Flows. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial tables accompanying this press release.
SAFE HARBOR STATEMENT
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances.
There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our MCM products, including CYFENDUS® (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), BioThrax® (Anthrax Vaccine Adsorbed), ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including EbangaTM (ansuvimab-zykl), BAT® (Botulism Antitoxin Heptavalent) and RSDL® (Reactive Skin Decontamination Lotion Kit); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of the generic marketplace on NARCAN® (naloxone HCI) Nasal Spray and future NARCAN® sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; our ability to provide Bioservices for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial
manufacturing under our existing Bioservices contracts; our ability to collect reimbursement for raw materials and payment of services fees from our Bioservices customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and the amended and restated credit agreement relating to such facilities, and our 3.875% Senior Unsecured Notes due 2028; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic; the impact of the organizational changes we announced in January 2023 and August 2023; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements.
Trademarks
Emergent®, BioThrax®, BaciThrax®, RSDL®, BAT®, Trobigard®, Anthrasil®, CNJ-016®, ACAM2000®, NARCAN®, CYFENDUS®, TEMBEXA® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners.
| | | | | |
Investor Contact Rich Lindahl Executive Vice President, Chief Financial Officer lindahlr@ebsi.com
| Media Contact Assal Hellmer Vice President, Communications mediarelations@ebsi.com
|
Emergent BioSolutions Inc.
Consolidated Balance Sheets
(unaudited, in millions, except per share data)
| | | | | | | | | | | |
| December 31, | | December 31, |
| 2023 | | 2022 |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 111.7 | | | $ | 642.6 | |
| | | |
| Accounts receivable, net | 191.0 | | | 159.2 | |
| Inventories, net | 328.9 | | | 350.7 | |
| Prepaid expenses and other current assets | 47.9 | | | 57.9 | |
| Total current assets | 679.5 | | | 1,210.4 | |
| | | |
| Property, plant and equipment, net | 382.8 | | | 817.6 | |
| Intangible assets, net | 566.6 | | | 728.8 | |
| Goodwill | — | | | 218.2 | |
| Other assets | 194.3 | | | 191.3 | |
| Total assets | $ | 1,823.2 | | | $ | 3,166.3 | |
| | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 112.2 | | | $ | 103.5 | |
| Accrued expenses | 18.6 | | | 34.9 | |
| Accrued compensation | 74.1 | | | 87.3 | |
| Debt, current portion | 413.7 | | | 957.3 | |
| Other current liabilities | 32.7 | | | 45.9 | |
| Total current liabilities | 651.3 | | | 1,228.9 | |
| | | |
| Debt, net of current portion | 446.5 | | | 448.5 | |
| Deferred tax liability | 47.2 | | | 59.7 | |
| Other liabilities | 28.9 | | | 41.5 | |
| Total liabilities | $ | 1,173.9 | | | $ | 1,778.6 | |
| | | |
| Stockholders’ equity: | | | |
Preferred stock, par value $0.001 per share; 15.0 shares authorized, no shares issued and outstanding | — | | | — | |
Common stock, par value $0.001 per share; 200.0 shares authorized, 57.8 and 55.7 shares issued; 52.2 and 50.1 shares outstanding, respectively. | 0.1 | | | 0.1 | |
Treasury stock, at cost, 5.6 and 5.6 common shares, respectively | (227.7) | | | (227.7) | |
| Additional paid-in capital | 904.4 | | | 873.5 | |
| Accumulated other comprehensive income (loss), net | (5.7) | | | 3.1 | |
| Retained earnings (accumulated deficit) | (21.8) | | | 738.7 | |
| Total stockholders’ equity | $ | 649.3 | | | $ | 1,387.7 | |
| Total liabilities and stockholders’ equity | $ | 1,823.2 | | | $ | 3,166.3 | |
Emergent BioSolutions Inc.
Consolidated Statements of Operations
(unaudited, in millions, except per share data)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended December 31, | | Year Ended December 31, |
| 2023 | | 2022 | | 2023 | | 2022 |
| Revenues: | | | | | | | |
| Commercial Product sales | $ | 111.0 | | | $ | 96.0 | | | $ | 497.3 | | | $ | 386.6 | |
| MCM Product sales | 138.1 | | | 209.7 | | | 447.2 | | | 579.6 | |
| Total Product sales, net | 249.1 | | | 305.7 | | | 944.5 | | | 966.2 | |
| Bioservices: | | | | | | | |
| Services | 20.6 | | | 17.2 | | | 72.8 | | | 105.0 | |
| Leases | 0.2 | | | 0.2 | | | 5.7 | | | 4.9 | |
| Total Bioservices revenues | 20.8 | | | 17.4 | | | 78.5 | | | 109.9 | |
| Contracts and grants | 6.7 | | | 7.1 | | | 26.3 | | | 41.4 | |
| Total revenues | 276.6 | | | 330.2 | | | 1,049.3 | | | 1,117.5 | |
| | | | | | | |
| Operating expenses: | | | | | | | |
| Cost of Commercial Product sales | 50.1 | | | 40.2 | | | 210.3 | | | 160.3 | |
| Cost of MCM Product sales | 97.2 | | | 127.6 | | | 305.6 | | | 264.3 | |
| Cost of Bioservices | 37.8 | | | 52.7 | | | 189.5 | | | 268.5 | |
| Goodwill impairment | — | | | 6.7 | | | 218.2 | | | 6.7 | |
| Impairment of long-lived assets | — | | | — | | | 306.7 | | | — | |
| Research and development | 29.4 | | | 47.0 | | | 111.4 | | | 188.3 | |
| Selling, general and administrative | 89.7 | | | 93.4 | | | 368.4 | | | 339.5 | |
| Amortization of intangible assets | 16.2 | | | 17.9 | | | 65.6 | | | 59.9 | |
| | | | | | | |
| Total operating expenses | 320.4 | | | 385.5 | | | 1,775.7 | | | 1,287.5 | |
| | | | | | | |
| Loss from operations | (43.8) | | (55.3) | | (726.4) | | (170.0) |
| | | | | | | |
| Other income (expense): | | | | | | | |
| Interest expense | (21.7) | | | (12.8) | | | (87.9) | | | (37.3) | |
| Gain on sale of business | — | | | — | | | 74.2 | | | — | |
| Other, net | 11.0 | | | 6.7 | | | 8.9 | | | (11.7) | |
| Total other income (expense), net | (10.7) | | | (6.1) | | | (4.8) | | | (49.0) | |
| | | | | | | |
| Loss before income taxes | (54.5) | | | (61.4) | | | (731.2) | | | (219.0) | |
| Income tax provision (benefit) | (5.0) | | | 5.6 | | | 29.3 | | | (7.4) | |
| Net loss | $ | (49.5) | | | $ | (67.0) | | | $ | (760.5) | | | $ | (211.6) | |
| | | | | | | |
| Net loss per common share | | | | | | | |
| Basic | $ | (0.95) | | | $ | (1.34) | | | $ | (14.85) | | | $ | (4.22) | |
| Diluted | $ | (0.95) | | | $ | (1.34) | | | $ | (14.85) | | | $ | (4.22) | |
| | | | | | | |
| Shares used in computing net loss per common share | | | | | | | |
| Basic | 51.9 | | 49.9 | | 51.2 | | 50.1 |
| Diluted | 51.9 | | 49.9 | | 51.2 | | 50.1 |
| | | | | | | |
Emergent BioSolutions Inc.
Consolidated Statements of Cash Flows
(unaudited, in millions) | | | | | | | | | | | |
| Year Ended December 31, |
| 2023 | | 2022 |
| Operating Activities | | | |
| Net income (loss) | $ | (760.5) | | | $ | (211.6) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | |
| Share-based compensation expense | 23.1 | | | 45.1 | |
| Depreciation and amortization | 125.1 | | | 143.3 | |
| Change in fair value of contingent obligations, net | 0.2 | | | 2.6 | |
| Amortization of deferred financing costs | 21.3 | | | 4.1 | |
| Deferred income taxes | (8.9) | | | (28.6) | |
| Gain on sale of travel health business | (74.2) | | | — | |
| Goodwill impairment | 218.2 | | | 6.7 | |
| | | |
| Impairment of long-lived assets | 306.7 | | | — | |
| Other | 13.0 | | | 6.4 | |
| Changes in operating assets and liabilities: | | | |
| Accounts receivable | (21.6) | | | 118.1 | |
| Inventories | 0.6 | | | (57.1) | |
| Prepaid expenses and other assets | 11.7 | | | (19.9) | |
| Accounts payable | 10.6 | | | (14.0) | |
| Accrued expenses and other liabilities | (55.7) | | | (66.7) | |
| Accrued compensation | (10.4) | | | (0.8) | |
| Long-term incentive plan accrual | 4.8 | | | — | |
| Income taxes receivable and payable, net | (16.2) | | | 28.7 | |
| Contract liabilities | 5.9 | | | 9.6 | |
| Net cash used in operating activities | (206.3) | | | (34.1) | |
| Investing Activities | | | |
| Purchases of property, plant and equipment | (51.6) | | | (115.8) | |
| Royalty settlement payment | — | | | (21.8) | |
| Milestone payment from prior asset acquisition | (6.3) | | | — | |
| Asset acquisitions | — | | | (243.7) | |
| Proceeds from sale of travel health business, net | 270.2 | | | — | |
| Net cash provided by (used in) investing activities | 212.3 | | | (381.3) | |
| Financing Activities | | | |
| Purchases of treasury stock | — | | | (82.1) | |
| Proceeds from revolving credit facility | 20.0 | | | 598.0 | |
| Principal payments on revolving credit facility | (398.8) | | | — | |
| | | |
| | | |
| Principal payments on term loan facility | (164.6) | | | (33.8) | |
| Proceeds from stock-based compensation activity | 1.8 | | | 5.0 | |
| Taxes paid for stock-based compensation activity | (2.5) | | | (5.9) | |
| | | |
| | | |
| Proceeds from at-the-market sale of stock, net of commissions and expenses | 8.4 | | | — | |
| Net cash provided by (used in) financing activities: | (535.7) | | | 481.2 | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | (1.2) | | | 0.5 | |
| Net change in cash, cash equivalents and restricted cash | (530.9) | | | 66.3 | |
| | | |
| Cash, cash equivalents and restricted cash, beginning of period | 642.6 | | | 576.3 | |
| Cash, cash equivalents and restricted cash, end of period | $ | 111.7 | | | $ | 642.6 | |
| Supplemental cash flow disclosures: | | | |
| Cash paid for interest | $ | 68.3 | | | $ | 33.0 | |
| Cash paid for income taxes | $ | 52.8 | | | $ | 6.2 | |
| Non-cash investing and financing activities: | | | |
| Purchases of property, plant and equipment unpaid at period end | $ | 5.7 | | | $ | 9.4 | |
| | | |
| Gain on extinguishment of debt | $ | 2.5 | | | $ | — | |
| | | |
| | | |
| | | |
| | | |
| | | |
Emergent BioSolutions, Inc.
Reconciliation of Non-GAAP Financial Measures
Reconciliation of Net Loss and Net Loss per Diluted Share to Adjusted Net Income (Loss) and Adjusted Net Income (Loss) per Diluted Share(1)
| | | | | | | | | | | | | | | | | | | | |
| ($ in millions, except per share value) | Three Months Ended December 31, | | Year Ended December 31, | |
| 2023 | 2022 | | 2023 | 2022 | Source |
| Net loss | $ | (49.5) | | $ | (67.0) | | | $ | (760.5) | | $ | (211.6) | | |
| Adjustments: | | | | | | |
| Non-cash amortization charges | $ | 22.0 | | $ | 18.8 | | | $ | 86.8 | | $ | 64.0 | | Intangible Asset ("IA") Amortization, Other Income |
| Changes in fair value of contingent consideration | 0.6 | | 0.2 | | | 0.2 | | 2.6 | | MCM Product COGS |
| | | | | | |
| Impairments | — | | 6.7 | | | 524.9 | | 6.7 | | Goodwill and Long-lived Asset Impairment |
| Severance and restructuring costs | (1.1) | | — | | | 33.4 | | — | | COGS, SG&A and R&D |
| Inventory step-up provision | 2.0 | | 51.4 | | | 3.9 | | 51.4 | | MCM Product COGS |
| Acquisition and divestiture costs | 1.9 | | 0.7 | | | 4.7 | | 1.8 | | SG&A |
| Exit and disposal costs | 6.4 | | — | | | 12.5 | | — | | Other Income (Expense) |
| Gain on sale of business | — | | — | | | (74.2) | | — | | Other Income (Expense) |
| Other income (expense), net item | (2.5) | | — | | | (2.5) | | — | | Other Income (Expense) |
| Tax effect | (19.8) | | (4.9) | | | (148.2) | | (14.6) | | |
| Total adjustments: | $ | 9.5 | | $ | 72.9 | | | $ | 441.5 | | $ | 111.9 | | |
| Adjusted net income (loss) | $ | (40.0) | | $ | 5.9 | | | $ | (319.0) | | $ | (99.7) | | |
| Net loss per diluted share | $ | (0.95) | | $ | (1.34) | | | $ | (14.85) | | $ | (4.22) | | |
| Adjustments: | | | | | | |
| Non-cash amortization charges | $ | 0.42 | | $ | 0.38 | | | $ | 1.70 | | $ | 1.28 | | IA Amortization, Other Income |
| Changes in fair value of contingent consideration | 0.01 | | — | | | — | | 0.05 | | MCM Product COGS |
| | | | | | |
| Impairments | — | | 0.13 | | | 10.25 | | 0.13 | | Goodwill and Long-lived Asset Impairment |
| Severance and restructuring costs | (0.02) | | — | | | 0.65 | | — | | COGS, SG&A and R&D |
| Inventory step-up provision | 0.04 | | 1.03 | | | 0.08 | | 1.03 | | MCM Product COGS |
| Acquisition and divestiture costs | 0.04 | | 0.01 | | | 0.09 | | 0.04 | | SG&A |
| Exit and disposal costs | 0.12 | | — | | | 0.24 | | — | | Other Income (Expense) |
| Gain on sale of business | — | | — | | | (1.45) | | — | | Other Income (Expense) |
| Other income (expense), net item | (0.05) | | — | | | (0.05) | | — | | Other Income (Expense) |
| Tax effect | (0.38) | | (0.10) | | | (2.89) | | (0.29) | | |
| Total adjustments: | $ | 0.18 | | $ | 1.45 | | | $ | 8.62 | | $ | 2.24 | | |
| Adjusted net income (loss) per diluted share | $ | (0.77) | | $ | 0.11 | | | $ | (6.23) | | $ | (1.98) | | |
| Diluted shares used in computing Adjusted net income (loss) per diluted share | 51.9 | | 49.9 | | | 51.2 | | 50.1 | | |
Emergent BioSolutions, Inc.
Reconciliation of Net Loss to Adjusted EBITDA (1)
| | | | | | | | | | | | | | | | | |
| ($ in millions) | Three Months Ended December 31, | | Year Ended December 31, |
| 2023 | 2022 | | 2023 | 2022 |
| Net loss | $ | (49.5) | | $ | (67.0) | | | $ | (760.5) | | $ | (211.6) | |
| Adjustments: | | | | | |
| Depreciation & amortization | $ | 29.6 | | $ | 35.6 | | | $ | 125.1 | | $ | 143.3 | |
| Income taxes | (5.0) | | 5.6 | | | 29.3 | | (7.4) | |
| Total interest expense, net | 21.0 | | 10.8 | | | 80.9 | | 34.0 | |
| Impairments | — | | 6.7 | | | 524.9 | | 6.7 | |
| Inventory step-up provision | 2.0 | | 51.4 | | | 3.9 | | 51.4 | |
| Changes in fair value of contingent consideration | 0.6 | | 0.2 | | | 0.2 | | 2.6 | |
| Severance and restructuring costs | (1.1) | | — | | | 33.4 | | — | |
| Exit and disposal costs | 6.4 | | — | | | 12.5 | | — | |
| Acquisition and divestiture costs | 1.9 | | 0.7 | | | 4.7 | | 1.8 | |
| Gain on sale of business | — | | — | | | (74.2) | | — | |
| Other income (expense), net item | (2.5) | | — | | | (2.5) | | 8.0 | |
| Total adjustments | $ | 52.9 | | $ | 111.0 | | | $ | 738.2 | | $ | 240.4 | |
| Adjusted EBITDA | $ | 3.4 | | $ | 44.0 | | | $ | (22.3) | | $ | 28.8 | |
Emergent BioSolutions, Inc.
Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin %
to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
| | | | | | | | | | | | | | | | | | | | | | | |
Three Months Ended December 31, 2023 (unaudited, in millions) | Commercial Products | MCM Products | Services | | Total Segment | Contracts & Grants | Total Revenues |
| Revenues | $ | 111.0 | | $ | 138.1 | | $ | 20.8 | | | $ | 269.9 | | $ | 6.7 | | $ | 276.6 | |
| | | | | | | |
| Cost of sales or services | 50.1 | | 97.2 | | 37.8 | | | 185.1 | | | |
| | | | | | | |
| Gross margin | $ | 60.9 | | $ | 40.9 | | $ | (17.0) | | | $ | 84.8 | | | |
| Gross margin % | 55 | % | 30 | % | (82) | % | | 31 | % | | |
| | | | | | | |
| Add back: | | | | | | | |
| Changes in fair value of contingent consideration | $ | — | | $ | 0.6 | | $ | — | | | $ | 0.6 | | | |
| Inventory step-up provision | — | | 2.0 | | — | | | 2.0 | | | |
| Restructuring costs | — | | (1.4) | | 0.3 | | | (1.1) | | | |
| | | | | | | |
| Adjusted gross margin | $ | 60.9 | | $ | 42.1 | | $ | (16.7) | | | $ | 86.3 | | | |
| Adjusted gross margin % | 55 | % | 30 | % | (80) | % | | 32 | % | | |
| | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Three Months Ended December 31, 2022 (unaudited, in millions) | Commercial Products | MCM Products | Services | | Total Segment | Contracts & Grants | Total Revenues |
| Revenues | $ | 96.0 | | $ | 209.7 | | $ | 17.4 | | | $ | 323.1 | | $ | 7.1 | | $ | 330.2 | |
| | | | | | | |
| Cost of sales or services | 40.2 | | 127.6 | | 52.7 | | | 220.5 | | | |
| | | | | | | |
| Gross margin | $ | 55.8 | | $ | 82.1 | | $ | (35.3) | | | $ | 102.6 | | | |
| Gross margin % | 58 | % | 39 | % | (203) | % | | 32 | % | | |
| | | | | | | |
| Add back: | | | | | | | |
| Changes in fair value of contingent consideration | $ | — | | $ | 0.2 | | $ | — | | | $ | 0.2 | | | |
| Inventory step-up provision | — | | 51.4 | | — | | | 51.4 | | | |
| | | | | | | |
| | | | | | | |
| Adjusted gross margin | $ | 55.8 | | $ | 133.7 | | $ | (35.3) | | | $ | 154.2 | | | |
| Adjusted gross margin % | 58 | % | 64 | % | (203) | % | | 48 | % | | |
Emergent BioSolutions, Inc.
Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin %
to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
| | | | | | | | | | | | | | | | | | | | | | | |
Year Ended December 31, 2023 (unaudited, in millions) | Commercial Products | MCM Products | Services | | Total Segment | Contracts & Grants | Total Revenues |
| Revenues | $ | 497.3 | | $ | 447.2 | | $ | 78.5 | | | $ | 1,023.0 | | $ | 26.3 | | $ | 1,049.3 | |
| | | | | | | |
| Cost of sales or services | 210.3 | | 305.6 | | 189.5 | | | 705.4 | | | |
| | | | | | | |
| Gross margin | $ | 287.0 | | $ | 141.6 | | $ | (111.0) | | | $ | 317.6 | | | |
| Gross margin % | 58 | % | 32 | % | (141) | % | | 31 | % | | |
| | | | | | | |
| Add back: | | | | | | | |
| Changes in fair value of contingent consideration | $ | — | | $ | 0.2 | | $ | — | | | $ | 0.2 | | | |
| Inventory step-up provision | — | | 3.9 | | — | | | 3.9 | | | |
| Restructuring costs | — | | 5.6 | | 8.4 | | | 14.0 | | | |
| | | | | | | |
| Adjusted gross margin | $ | 287.0 | | $ | 151.3 | | $ | (102.6) | | | $ | 335.7 | | | |
| Adjusted gross margin % | 58 | % | 34 | % | (131) | % | | 33 | % | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Year Ended December 31, 2022 (in millions) | Commercial Products | MCM Products | Services | | Total Segment | Contracts & Grants | Total Revenues |
| Revenues | $ | 386.6 | | $ | 579.6 | | $ | 109.9 | | | $ | 1,076.1 | | $ | 41.4 | | $ | 1,117.5 | |
| | | | | | | |
| Cost of sales or services | 160.3 | | 264.3 | | 268.5 | | | 693.1 | | | |
| | | | | | | |
| Gross margin | $ | 226.3 | | $ | 315.3 | | $ | (158.6) | | | $ | 383.0 | | | |
| Gross margin % | 59 | % | 54 | % | (144) | % | | 36 | % | | |
| | | | | | | |
| Add back: | | | | | | | |
| Changes in fair value of contingent consideration | $ | — | | $ | 2.6 | | $ | — | | | $ | 2.6 | | | |
| Inventory step-up provision | — | | 51.4 | | — | | | 51.4 | | | |
| | | | | | | |
| | | | | | | |
| Adjusted gross margin | $ | 226.3 | | $ | 369.3 | | $ | (158.6) | | | $ | 437.0 | | | |
| Adjusted gross margin % | 59 | % | 64 | % | (144) | % | | 41 | % | | |
Emergent BioSolutions, Inc.
Reconciliation of Net Loss Forecast to Adjusted Net Loss Forecast
| | | | | | | | |
| ($ in millions) | 2024 Full Year Forecast | Source |
| Net loss | $(183) - $(133) | |
| Adjustments: | | |
| Non-cash amortization charges | $65 | IA Amortization Other Income |
| Changes in fair value of contingent consideration | 2 | MCM Product COGS |
| | |
| | |
| | |
| | |
| Tax effect | (14) | |
| Total adjustments: | $53 | |
| Adjusted net loss | $(130) - $(80) | |
Reconciliation of Net Loss Forecast to Adjusted EBITDA Forecast
| | | | | |
| ($ in millions) | 2024 Full Year Forecast |
| Net loss | $(183) - $(133) |
| Adjustments: | |
| Depreciation & amortization | $111 |
| Income taxes | 59 |
| Total interest expense, net | 61 |
| |
| |
| Changes in fair value of contingent consideration | 2 |
| |
| |
| Total adjustments | $233 |
| Adjusted EBITDA | $50 - $100 |
Reconciliations of Forecasted Total Revenues to Forecasted Total Segment Revenues and of Forecasted Segment and Total Segment Gross Margin and Gross Margin % to Forecasted Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
| | | | | |
(in millions) | 2024 Full Year Forecast |
| Total revenues | $900 - $1,100 |
| Contracts & Grants | (30) |
| Total segment revenues | $870 - $1070 |
| |
| Cost of sales or services | $524 - $590 |
| Total segment gross margin | $346 - $480 |
| Total segment gross margin % | 40% - 45% |
| Add back: | |
| Changes in fair value of contingent consideration | $2 |
| |
| |
| Total segment adjusted gross margin | $348 - $482 |
| Total segment adjusted gross margin % | 40% - 45% |

Q4 2023 Financial Results Update March 6, 2024 1

PROPRIETARY AND CONFIDENTIAL 2 Q4 2023 Update Introduction

This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our MCM products, including CYFENDUS® (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), BioThrax® (Anthrax Vaccine Adsorbed), ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including EbangaTM (ansuvimab-zykl), BAT® (Botulism Antitoxin Heptavalent) and RSDL® (Reactive Skin Decontamination Lotion Kit); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of the generic marketplace on NARCAN® (naloxone HCI) Nasal Spray and future NARCAN® sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; our ability to provide Bioservices for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial manufacturing under our existing Bioservices contracts; our ability to collect reimbursement for raw materials and payment of services fees from our Bioservices customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and the amended and restated credit agreement relating to such facilities, and our 3.875% Senior Unsecured Notes due 2028; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic; the impact of the organizational changes we announced in January 2023 and August 2023; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements. Trademarks Emergent®, BioThrax®, RSDL®, BAT®, ACAM2000®, NARCAN®, CYFENDUS® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners. 3 Safe Harbor Statement/Trademarks INTRODUCTION

Agenda 4 Q4 2023 Update INTRODUCTION Item Presenter Topic(s) 1 Joseph C. Papa President and CEO • Focus and Priorities 2 Paul Williams SVP, Products Business • NARCAN® Update 3 Rich Lindahl EVP, CFO and Treasurer • 2023 Q4 & Full Year Financial Review • FY 2024 Guidance 4 Q&A

PROPRIETARY AND CONFIDENTIAL Q4 2023 Update Focus & Priorities Joseph C. Papa President and Chief Executive Officer 55

Focus & Priorities 6 Focus and Priorities • Emergent is uniquely positioned to address public health crises • Quality & Compliance are key value drivers • Near-term priority is to build creditability across all stakeholders, de-risk our balance sheet, and reduce our debt Multi-year plan to transform the business: Expect the next 3-6 months we will strengthen engagement across internal and external stakeholders around our mission to protect and enhance lives. 2024 & 2025 focused on profitable growth and improved operating performance. 2026+ with strategic transformation for long- term growth and profitability.

NARCAN® Nasal Spray Update Paul Williams SVP, Head of Products Business 7

State of the Opioid Crisis & Our Response 8 • The opioid epidemic is one of the country’s biggest public health issues. • Recent data shows 105,000+ Americans died of a drug overdose during the 12-month period from August 2022 to July 2023 – of which eight in 10 deaths were opioid related.¹ • Approximately one life was lost every six minutes due to opioid overdose. ¹ • Leading cause of accidental death in the U.S. given the recent rise in synthetic opioids, such as fentanyl. • Demand for naloxone is expected to increase as the epidemic continues and federal/state programs continue to combat the crisis. How Emergent has responded: • Added NARCAN® Nasal Spray to its portfolio in 2018. ◦ 64 million+ doses have been distributed in the U.S. and Canada since FDA approval in 2016. • Built nationwide logistics network, NARCAN Direct™, to service customers like our public interest partners. • Received FDA approval for OTC designation of NARCAN® Nasal Spray in March 2023. • Drove widespread availability across 32,000 locations, including major retailers and e-commerce sites at OTC launch. • Pursuing additional channels to expand access, e.g. businesses, workplaces. • Increased awareness through Ready to Rescue public education campaign. • In 2023, distributed ~22 million doses / ~11 million two-dose boxes in U.S. and Canada. 1. Centers for Disease Control and Prevention. Provisional Drug Overdose Death Counts. Available at: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Updated February 15, 2023. Accessed August 25, 2023.

9 Rich Lindahl Executive Vice President and Chief Financial Officer Q4 2023 Update Financials

Product & Business Highlights 10 FINANCIALS Strengthened Portfolio • FDA approval of NARCAN® Nasal Spray as the first over- the-counter opioid overdose emergency treatment; launched in retail channel in under 6 months • Awarded $379.6 million U.S. DOD contract for RSDL® • Awarded $75 million option to Emergent’s existing contract for the acquisition of newly licensed CYFENDUS® vaccine • Awarded $238.5 million contract to supply Biothrax® • Awarded a 10-year contract by the BARDA valued at $704 million for advanced development, manufacturing scale- up, and procurement of Ebanga™, a treatment for Ebola • FDA approval of CYFENDUS® (formerly AV7909), a two- dose anthrax vaccine for post-exposure prophylaxis use • Health Canada regulatory approvals for our smallpox products, ACAM2000® vaccine and TEMBEXA® drug • Submitted sBLA to FDA for ACAM2000® vaccine to include immunization against Mpox virus Improved Financial Position • Implemented organizational changes resulting in $60 million in annual savings • Shift in resource deployment resulting in $100 million in annual savings • Amended and extended maturity of our secured credit facility to May 2025 • Travel Health business divestiture – valued at up to $380 million

Key Financial Performance Metrics Q4 2023 vs. Q4 20221 11 Total Revenues Adjusted EBITDA2 Adjusted Net Income (Loss)2 FINANCIALS ($ in millions) Q4 2022 Q4 2023 1. All financial information incorporated within this presentation is unaudited. 2. See "End Notes: Non-GAAP Financial Measures" and "Appendix" for the definitions of non-GAAP terms and reconciliations to the most directly comparable GAAP financial measures. $330.2 $276.6 Q4 2022 Q4 2023 Q4 2022 Q4 2023 Q4 2022 Q4 2023 Q4 2022 Q4 2023 $44.0 $3.4 $5.9 $(40.0) $154.2 $86.3 48% 32% Total Segment Adjusted Gross Margin2 --- Total Segment Adjusted Gross Margin %2

Notable Revenue Elements Q4 2023 vs. Q4 20221 12 FINANCIALS 1.All financial information incorporated within this presentation is unaudited. 2. Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles ($ in millions) Q4 2023 Q4 2022 % Change Product sales, net (2): NARCAN® $ 111.0 $ 91.1 22 % Other Commercial Products — 4.9 NM Anthrax MCM 111.6 56.4 98 % Smallpox MCM 11.5 144.6 (92) % Other Products 15.0 8.7 72 % Total Product sales, net $ 249.1 $ 305.7 (19) % Bioservices: Services $ 20.6 $ 17.2 20 % Leases 0.2 0.2 — % Total Bioservices revenues $ 20.8 $ 17.4 20 % Contracts and grants $ 6.7 $ 7.1 (6) % Total revenues $ 276.6 $ 330.2 (16) % NM - Not Meaningful

Key Financial Performance Metrics Q4 2023 vs. Q4 2022 1 13 SG&A $ --- SG&A Margin %2 FINANCIALS ($ in millions) 1.All financial information incorporated within this presentation is unaudited. 2. SG&A Margin is calculated as Gross SG&A Expense divided by total revenues. Q4 2022 Q4 2022 Q4 2022 Q4 2022 Q4 2022 Q4 2023 Q4 2023 Q4 2023 Q4 2023 Q4 2023 $47.0 $29.4 $93.4 $89.7 28% 32% Cost of Commercial Product Sales Cost of MCM Product Sales Cost of Bioservices R&D $ Q4 2023 Q4 2022 $40.2 $50.1 $127.6 $97.2 $52.7 $37.8

Segment Reporting Q4 2023 vs. Q4 20221 14 Revenue Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 Revenue FINANCIALS ($ in millions) COMMERCIAL PRODUCTS SEGMENT MCM PRODUCTS SEGMENT 1.All financial information incorporated within this presentation is unaudited. 2. See "End Notes: Non-GAAP Financial Measures" and "Appendix" for the definitions of non-GAAP terms and reconciliations to the most directly comparable GAAP financial measures. Q4 2022 Q4 2023 $96.0 $111.0 Q4 2022 $55.8 $60.9 Q4 2022 Q4 2022 Q4 2022 Q4 2023 Q4 2023 Q4 2023 $209.7 $138.1 Q4 2023 58% 55% $133.7 $42.1 64% 30% Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 Revenue SERVICES SEGMENT $17.4 $20.8 Q4 2022 Q4 2022 Q4 2023 Q4 2023 $(35.3) $(16.7) (203)% (44)%

Key Financial Performance Metrics FY 2023 vs. FY 20221 15 Total Revenues Adjusted Net Loss2Adjusted EBITDA2 FINANCIALS ($ in millions 1.All financial information incorporated within this presentation is unaudited. 2. See "End Notes: Non-GAAP Financial Measures" and "Appendix" for the definitions of non-GAAP terms and reconciliations to the most directly comparable GAAP financial measures. $1,117.5 $1,049.3 FY 2022 FY 2023 $28.8 FY 2022 FY 2023 FY 2022 FY 2023 FY 2022 FY 2023 FY 2022 FY 2023 $(22.3) $(319.0)$(99.7) Total Segment Adjusted Gross Margin2 --- Total Segment Adjusted Gross Margin %2 $437.0 $335.7 41% 33%

16 SG&A $ --- SG&A Margin %2 FINANCIALS ($ in millions) 1.All financial information incorporated within this presentation is unaudited. 2. SG&A Margin is calculated as Gross SG&A Expense divided by total revenues. $188.3 $96.8 $339.5 $368.4 30% 35% Cost of Commercial Product Sales Cost of MCM Product Sales Cost of Bioservices R&D $ $160.3 $210.3 $264.3 $305.6 $268.5 $189.5 FY 2022 FY 2022 FY 2022 FY 2022 FY 2022 FY 2022 Key Financial Performance Metrics FY 2023 vs. FY 2022 (Continued)1 FY 2023 FY 2023 FY 2023 FY 2023 FY 2023 FY 2023

Segment Reporting FY 2023 vs. FY 20221 17 Revenue Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 Revenue FINANCIALS ($ in millions) COMMERCIAL PRODUCTS SEGMENT SERVICES SEGMENT 62% 56% 1.All financial information incorporated within this presentation is unaudited. 2. See "End Notes: Non-GAAP Financial Measures" and "Appendix" for the definitions of non-GAAP terms and reconciliations to the most directly comparable GAAP financial measures. $386.6 $497.3 $226.3 $287.0 $579.6 $447.2 59% 58% FY 2022 FY 2023 FY 2023 FY 2022 FY 2022 FY 2023 FY 2022 FY 2023 FY 2022 FY 2023 $369.3 $151.3 34% 64% Revenue Segment Adjusted Gross Margin 2 --- Segment Adjusted Gross Margin % 2 MCM PRODUCTS SEGMENT FY 2023 FY 2023 FY 2022 FY 2022 $109.9 $78.5 $(158.6) $(102.6) (131)% (144)%
Balance Sheet & Cash Flow Metrics 18 FINANCIALS As of December 31, 2023 For the Year Ended December 31, 2023 CASH $111.7 ACCOUNTS RECEIVABLE, NET $191.0 TOTAL DEBT $868.4 NET DEBT1,2 $756.7 OPERATING CASH FLOW $(206.3) CAPITAL EXPENDITURES $51.6 ($ in millions) 1.Debt amount indicated on the Company’s balance sheet is net of unamortized debt issuance costs of $8.2M. 2.Net Debt is calculated as Total Debt minus Cash ($868.4M - $111.7M = $756.7M).

2024 Forecast – Updated as of 3/06/2024 19 FINANCIALS 1. See "End Notes: Non-GAAP Financial Measures" and "Appendix" for the definitions of non-GAAP terms and reconciliations to the most directly comparable GAAP financial measures. 2.Other Commercial products, which includes Vivotif® and Vaxchora®, which were sold to Bavarian Nordic as part of our travel health business in May 2023, are not included in the 2024 forecast. ($ in millions) METRIC Full Year 2023 Actual Full Year 2024 Forecast Total revenues $1,049.3 $900 - $1,100 Net loss $(760.5) $(183) - $(133) Adjusted net loss (1) $(319.0) $(130) - $(80) Adjusted EBITDA (1) $(22.3) $50 - $100 Total segment adjusted gross margin % (1) 33% 40% - 45% Segment Level Revenue (2) Commercial Products $497.3 $460 - $500 MCM Products $447.2 $340 - $490 Services $78.5 $70 - $80 ($ in millions) METRIC Q1 2024 Forecast Total revenues $200 - $250

Summary 20 FINANCIALS • 2023 Revenue in-line with mid-point of Guidance • New contract awards and orders from the U.S. Government & DOD throughout 2023 • Notable progress on strengthening our business fundamentals • Positioned for success, driven by our unique focus on protecting communities and addressing global health threats • Near-term priority is to focus on stabilizing the business

PROPRIETARY AND CONFIDENTIAL 21 Q&A

In this presentation, we sometimes use information derived from consolidated and segment financial information that may not be presented in our financial statements or prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). Certain of these financial measures are considered not in conformity with GAAP (“non-GAAP financial measures”) under the United States Securities and Exchange Commission (“SEC”) rules. Specifically, we have referred to the following non-GAAP financial measures: • Adjusted Net Income (Loss) • Adjusted EBITDA • Total Segment Revenues • Total Segment Gross Margin • Total Segment Gross Margin % • Total Segment Adjusted Gross Margin • Total Segment Adjusted Gross Margin % • Segment Adjusted Gross Margin • Segment Adjusted Gross Margin % We define Adjusted Net Income (Loss), which is a non-GAAP financial measure, as net loss excluding the impact of changes in fair value of contingent consideration, acquisition and divestiture-related costs, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, inventory step-up provisions, and non-cash amortization charges. We use Adjusted Net Income (Loss) for the purpose of calculating Adjusted Net Income (Loss) per Diluted Share. We believe that these non-GAAP financial measures, when considered together with our GAAP financial results and GAAP financial measures, provide management and investors with an additional understanding of our business operating results, including underlying trends. We define Adjusted EBITDA, which is a non-GAAP financial measure, as consolidated net loss before income tax provision (benefit), interest expense, net, depreciation, amortization of intangible assets, changes in fair value of contingent consideration, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, acquisition and divestiture-related costs and inventory step-up provisions. We believe that this non-GAAP financial measure, when considered together with our GAAP financial results and GAAP financial measures, provides management and investors with a more complete understanding of our operating results, including underlying trends. In addition, EBITDA is a common alternative measure of operating performance used by many of our competitors. It is used by investors, financial analysts, rating agencies and others to value and compare the financial performance of companies in our industry, although it may be defined differently by different companies. Therefore, we also believe that this non-GAAP financial measure, considered along with corresponding GAAP financial measures, provides management and investors with additional information for comparison of our operating results with the operating results of other companies. We have included the definitions of segment gross margin and segment gross margin %, which are GAAP financial measures, below in order to more fully define the components of certain non-GAAP financial measures presented in this presentation. We define Segment Gross Margin, as a segment's revenues, less a segment's cost of sales or services. We define Segment Gross Margin %, as Segment Gross Margin as a percentage of a segments revenues. We define Segment Adjusted Gross Margin, which is a non-GAAP financial measure as Segment Gross Margin excluding the impact of restructuring costs and non-cash items related to changes in fair value of contingent consideration and inventory step-up provision. We define Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Segment Adjusted Gross Margin as a percentage of a segment's revenues. We define Total Segment Revenues, which is a non-GAAP financial measure, as our Total Revenues, less contracts and grants revenue, which is also equal to the sum of the revenues of our operating segments. We define Total Segment Gross Margin, which is a non-GAAP financial measure, as Total Segment Revenues less our aggregate cost of sales or services. We define Total Segment Gross Margin %, which is a non-GAAP financial measure, as Total Segment Gross Margin as a percentage of Total Segment Revenues. We define Total Segment Adjusted Gross Margin, which is a non-GAAP financial measure, as Total Segment Gross Margin, excluding the impact of restructuring costs, inventory step-up provision and the fair value of contingent consideration. We define Total Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Total Segment Adjusted Gross Martin as a percentage of Total Segment Revenues. Non-GAAP financial measures are not defined in the same manner by all companies and may not be comparable with other similarly titled measures of other companies. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial measures exclude the effect of items that will increase or decrease the Company’s reported results of operations, management strongly encourages investors to review the Company’s consolidated financial statements and publicly filed reports in their entirety. For additional information on the non-GAAP financial measures noted here, please refer to the reconciliation tables provide in the Appendix to this presentation as well as the associated press release which can be found on the Company’s website at www.emergentbiosolutions.com. 22 End Notes: Non-GAAP Financial Measures

23 Appendix 23

Reconciliation of Net Loss to Adjusted Net Income (Loss) 24 APPENDIX (unaudited, $ in millions) Three Months Ended December 31, Year Ended December 31, 2023 2022 2023 2022 Source Net loss $ (49.5) $ (67.0) $ (760.5) $ (211.6) Adjustments: Non-cash amortization charges $ 22.0 $ 18.8 $ 86.8 $ 64.0 Intangible Asset ("IA") Amortization, Other Income Changes in fair value of contingent consideration 0.6 0.2 0.2 2.6 MCM Product COGS Impairments — 6.7 524.9 6.7 Goodwill and Long-lived Asset Impairment Severance and restructuring costs (1.1) — 33.4 — COGS, SG&A and R&D Inventory step-up provision 2.0 51.4 3.9 51.4 MCM Product COGS Acquisition and divestiture costs 1.9 0.7 4.7 1.8 SG&A Exit and disposal costs 6.4 — 12.5 — Other Income (Expense) Gain on sale of business — — (74.2) — Other Income (Expense) Other income (expense), net item (2.5) — (2.5) — Other Income (Expense) Tax effect (19.8) (4.9) (148.2) (14.6) Total adjustments: $ 9.5 $ 72.9 $ 441.5 $ 111.9 Adjusted net income (loss) $ (40.0) $ 5.9 $ (319.0) $ (99.7)

Reconciliation of Net Loss to Adjusted EBITDA 25 APPENDIX (unaudited, $ in millions) Three Months Ended December 31, Year Ended December 31, 2023 2022 2023 2022 Net loss $ (49.5) $ (67.0) $ (760.5) $ (211.6) Adjustments: Depreciation & amortization $ 29.6 $ 35.6 $ 125.1 $ 143.3 Income taxes (5.0) 5.6 29.3 (7.4) Total interest expense, net 21.0 10.8 80.9 34.0 Impairments — 6.7 524.9 6.7 Changes in fair value of contingent consideration 0.6 0.2 0.2 2.6 Severance and restructuring costs (1.1) — 33.4 — Acquisition and divestiture costs 1.9 0.7 4.7 1.8 Gain on sale of business — — (74.2) — Other income (expense), net item (2.5) — (2.5) 8.0 Total adjustments $ 52.9 $ 111.0 $ 738.2 $ 240.4 Adjusted EBITDA $ 3.4 $ 44.0 $ (22.3) $ 28.8

Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin % to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % 26 APPENDIX Three Months Ended December 31, 2023 (unaudited, in millions) Commercial Products MCM Products Services Total Segment Contracts & Grants Total Revenues Revenues $ 111.0 $ 138.1 $ 20.8 $ 269.9 $ 6.7 $ 276.6 Cost of sales or services 50.1 97.2 37.8 185.1 Gross margin $ 60.9 $ 40.9 $ (17.0) $ 84.8 Gross margin % 55 % 30 % (82) % 31 % Add back: Changes in fair value of contingent consideration $ — $ 0.6 $ — $ 0.6 Inventory step-up provision — 2.0 — 2.0 Restructuring costs — (1.4) 0.3 (1.1) Adjusted gross margin $ 60.9 $ 42.1 $ (16.7) $ 86.3 Adjusted gross margin % 55 % 30 % (80) % 32 % Three Months Ended December 31, 2022 (unaudited, in millions) Commercial Products MCM Products Services Total Segment Contracts & Grants Total Revenues Revenues $ 96.0 $ 209.7 $ 17.4 $ 323.1 $ 7.1 $ 330.2 Cost of sales or services 40.2 127.6 52.7 220.5 Gross margin $ 55.8 $ 82.1 $ (35.3) $ 102.6 Gross margin % 58 % 39 % (203) % 32 % Add back: Changes in fair value of contingent consideration $ — $ 0.2 $ — $ 0.2 Inventory step-up provision — 51.4 — 51.4 Adjusted gross margin $ 55.8 $ 133.7 $ (35.3) $ 154.2 Adjusted gross margin % 58 % 64 % (203) % 48 %
Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin % to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % 27 APPENDIX Year Ended December 31, 2023 (unaudited, in millions) Commercial Products MCM Products Services Total Segment Contracts & Grants Total Revenues Revenues $ 497.3 $ 447.2 $ 78.5 $ 1,023.0 $ 26.3 $ 1,049.3 Cost of sales or services 210.3 305.6 189.5 705.4 Gross margin $ 287.0 $ 141.6 $ (111.0) $ 317.6 Gross margin % 58 % 32 % (141) % 31 % Add back: Changes in fair value of contingent consideration $ — $ 0.2 $ — $ 0.2 Inventory step-up provision — 3.9 — 3.9 Restructuring costs — 5.6 8.4 14.0 Adjusted gross margin $ 287.0 $ 151.3 $ (102.6) $ 335.7 Adjusted gross margin % 58 % 34 % (131) % 33 % Year Ended December 31, 2022 (in millions) Commercial Products MCM Products Services Total Segment Contracts & Grants Total Revenues Revenues $ 386.6 $ 579.6 $ 109.9 $ 1,076.1 $ 41.4 $ 1,117.5 Cost of sales or services 160.3 264.3 268.5 693.1 Gross margin $ 226.3 $ 315.3 $ (158.6) $ 383.0 Gross margin % 59 % 54 % (144) % 36 % Add back: Changes in fair value of contingent consideration $ — $ 2.6 $ — $ 2.6 Inventory step-up provision — 51.4 — 51.4 Adjusted gross margin $ 226.3 $ 369.3 $ (158.6) $ 437.0 Adjusted gross margin % 59 % 64 % (144) % 41 %

Reconciliation of Net Loss to Adjusted Net Loss – FY 2024 Forecast 28 APPENDIX ($ in millions) 2024 Full Year Forecast Source Net loss $(183) - $(133) Adjustments: Non-cash amortization charges $65 IA Amortization Other Income Changes in fair value of contingent consideration 2 MCM Product COGS Tax effect (14) Total adjustments: $53 Adjusted net loss $(130) - $(80)

Reconciliation of Net Loss to Adjusted EBITDA – FY 2024 Forecast 29 APPENDIX ($ in millions) 2024 Full Year Forecast Net loss $(183) - $(133) Adjustments: Depreciation & amortization $111 Income taxes 59 Total interest expense, net 61 Changes in fair value of contingent consideration 2 Total adjustments $233 Adjusted EBITDA $50 - $100

Reconciliations of Forecasted Total Revenues to Forecasted Total Segment Revenues and of Forecasted Segment and Total Segment Gross Margin and Gross Margin % to Forecasted Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % 30 APPENDIX (in millions) 2024 Full Year Forecast Total revenues $900 - $1,100 Contracts & Grants (30) Total segment revenues $870 - $1070 Cost of sales or services $524 - $590 Total segment gross margin $346 - $480 Total segment gross margin % 40% - 45% Add back: Changes in fair value of contingent consideration $2 Total segment adjusted gross margin $348 - $482 Total segment adjusted gross margin % 40% - 45%

www.emergentbiosolutions.com 31
v3.24.0.1
Cover Page Cover Page
|
Feb. 29, 2024 |
| Entity Addresses [Line Items] |
|
| Document Type |
8-K
|
| Document Period End Date |
Feb. 29, 2024
|
| Entity Registrant Name |
EMERGENT BIOSOLUTIONS INC.
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity File Number |
001-33137
|
| Entity Tax Identification Number |
14-1902018
|
| Entity Address, Address Line One |
300 Professional Drive
|
| Entity Address, City or Town |
Gaithersburg
|
| Entity Address, State or Province |
MD
|
| Entity Address, Postal Zip Code |
20879
|
| City Area Code |
240
|
| Local Phone Number |
631-3200
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
false
|
| Title of 12(b) Security |
Common Stock, Par Value $0.001 per share
|
| Trading Symbol |
EBS
|
| Security Exchange Name |
NYSE
|
| Entity Central Index Key |
0001367644
|
| Amendment Flag |
false
|
| Former Address |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
400 Professional Drive
|
| Entity Address, Address Line Two |
Suite 400
|
| Entity Address, City or Town |
Gaithersburg
|
| Entity Address, State or Province |
MD
|
| Entity Address, Postal Zip Code |
20879
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024