NEW YORK, July 30, 2024 /PRNewswire/ -- The
global medical device manufacturing outsourcing market
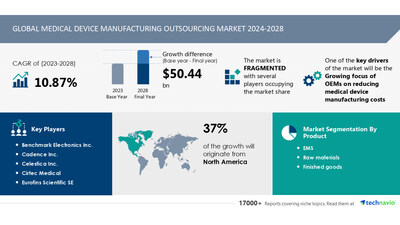
size is estimated to grow by USD 50.44
billion from 2024-2028, according to Technavio. The market
is estimated to grow at a CAGR of 10.87% during the
forecast period. Growing focus of oems on reducing medical
device manufacturing costs is driving market growth, with
a trend towards increasing m and a activities.
However, stringent regulatory environment poses a
challenge. Key market players include Benchmark Electronics Inc.,
Cadence Inc., Celestica Inc., Cirtec Medical, Eurofins Scientific
SE, Flex Ltd., Gerresheimer AG, Heraeus Holding GmbH, Integer
Holdings Corp., Jabil Inc., Kimball Electronics Inc., NN Inc.,
Nortech Systems Inc., Plexus Corp., Sanmina Corp., Tata Sons Pvt.
Ltd., TE Connectivity Ltd., Tecomet Inc., TRICOR Systems Inc., and
West Pharmaceutical Services Inc..
Get a detailed analysis on regions, market
segments, customer landscape, and companies - Click for the
snapshot of this report
|
Forecast
period
|
2024-2028
|
|
Base Year
|
2023
|
|
Historic
Data
|
2018 - 2022
|
|
Segment
Covered
|
Product (EMS, Raw
materials, and Finished
goods), Medical Device Regulatory Classification
(Class II, Class III, and Class I), and Geography
(North America, Asia, Europe, and Rest of World
(ROW))
|
|
Region
Covered
|
North America, Asia,
Europe, and Rest of World
(ROW)
|
|
Key companies
profiled
|
Benchmark Electronics
Inc., Cadence Inc.,
Celestica Inc., Cirtec Medical, Eurofins Scientific
SE, Flex Ltd., Gerresheimer AG, Heraeus Holding
GmbH, Integer Holdings Corp., Jabil Inc., Kimball
Electronics Inc., NN Inc., Nortech Systems Inc.,
Plexus Corp., Sanmina Corp., Tata Sons Pvt. Ltd.,
TE Connectivity Ltd., Tecomet Inc., TRICOR
Systems Inc., and West Pharmaceutical Services
Inc.
|
Key Market Trends Fueling Growth
The medical device manufacturing outsourcing market is
characterized by a high level of competition among numerous global,
regional, and local players. To gain a competitive edge, companies
are increasingly engaging in mergers and acquisitions (M&A) for
inorganic growth. This trend is evident in the medical device
manufacturing outsourcing industry, with a significant number of
transactions taking place recently. Buyers utilize M&A as a
strategic tool to expand their product offerings, enter new
markets, and consolidate their existing businesses. This approach
has led to increased inorganic growth for many companies,
particularly larger players. Large entities view the medical device
industry as an attractive growth market and pursue M&A
strategies to expand their market presence. For instance, Eurofins
Scientific SE's acquisition of Inpac Medizintechnik GmbH and
WESSLING Hungary are recent examples of this trend. These
acquisitions not only help companies gain market share but also
facilitate technological advancements. Companies aim to leverage
M&A for long-term objectives, such as expanding into new
markets and enhancing their capabilities. This dynamic is expected
to drive the growth of the medical device manufacturing outsourcing
market in the forecast period.
The Medical Device Manufacturing Outsourcing market is
experiencing significant growth, particularly in sectors like
Oncology, Coronary stents, and Wound care. Companies are
outsourcing the production of Scientific innovation in devices,
including Orthopedic devices, IVD, Ophthalmic, General &
plastic surgery, Drug delivery, Dental, Endoscopy, Diabetes care,
Cardiovascular devices, and more. Outsourcing allows for Quality
assurance services, Regulatory affairs services, Product
implementation services, Product upgrade services, and Product
maintenance services. Contract manufacturing services cover
electronic components, sensors, assembly, packaging, and
procurement of polymers. Diagnostic Imaging, Orthopedic, and
Cosmetic surgeries are also benefiting from this trend. Age-related
conditions, cardiovascular diseases, orthopaedic issues,
Neurological disorders, and Cardiac disorders are driving demand.
IT outsourcing includes quality control, logistics, and
productivity improvements.
Discover 360° analysis of this market. For
complete information, schedule your consultation- Book
Here!
Market Challenges
- Medical device manufacturing outsourcing is a common practice
among Original Equipment Manufacturers (OEMs). However, regulatory
compliance plays a crucial role in this process. For instance, when
manufacturing is outsourced overseas, each country has unique
regulations for medical products. The US Food and Drug
Administration (FDA) and the European Commission have implemented
stringent regulations for quality control and environmental
requirements. The FDA's 510(k) process for clearing certain medical
devices has faced scrutiny, with Class III devices taking longer
approval times in the US. Adherence to regulations, including
testing, quality control, and documentation procedures, is
mandatory and subjected to continual reviews through inspections
and product field monitoring. Failure to comply with these
regulations can significantly impact business activities, making
regulatory guidelines a significant challenge for the global
medical device manufacturing outsourcing market.
- The Medical Device Manufacturing Outsourcing market encompasses
various sectors such as general & plastic surgery, drug
delivery, dental, endoscopy, diabetes care, cardiovascular devices,
and more. Outsourcing medical device production offers benefits
like access to modern technology and state-of-the-art equipment.
However, challenges exist. For instance, medical practitioners
demand high product quality and stringent adherence to regulations.
Sectors like cosmetic surgeries focus on physical appearance, while
age-related conditions, cardiovascular diseases, orthopaedic
issues, neurological disorders, and cardiac disorders require
specialized expertise. Procurement of electronic components,
sensors, polymers, and assembly services are crucial. Logistics, IT
outsourcing, quality control, and supply chain management are
essential for productivity and time-to-market. Non-core services
like testing, intellectual property protection, and data
confidentiality are also critical. Contract manufacturing segments
prioritize patient epidemiology, product quality, and security and
privacy. Incorporating medical device technology while addressing
challenges in areas like electronic components, sensors, and
testing ensures a successful outsourcing partnership.
For more insights on driver and challenges
- Download a Sample Report
Segment Overview
This medical device manufacturing outsourcing market report
extensively covers market segmentation by
- Product
- 1.1 EMS
- 1.2 Raw materials
- 1.3 Finished goods
- Medical Device Regulatory Classification
- 2.1 Class II
- 2.2 Class III
- 2.3 Class I
- Geography
- 3.1 North America
- 3.2 Asia
- 3.3 Europe
- 3.4 Rest of World (ROW)
1.1 EMS- The Electronics Manufacturing Services
(EMS) market in the medical device industry is experiencing
significant growth due to various factors. Major product categories
include patient monitoring, diagnostic imaging, in-vitro
diagnostics, and pacemakers. Notable EMS providers include Jabil
Inc., Celestica Inc., Sanmina Corp., and Flex Ltd. The global EMS
market is anticipated to expand due to increasing healthcare
expenditure, high growth potential in emerging economies, and
technological advancements in medical devices. OEMs are outsourcing
to reduce costs and focus on strategic initiatives. Robotics,
wireless products, and advanced medical software are driving demand
for EMS. EMS providers offer cost savings, improved quality,
innovation, and efficiency, enabling OEMs to bring new products to
market faster and manage their supply chains effectively. Cost
pressures and the need for profitability in low-to-medium volume
manufacturing are key drivers for outsourcing. In the US, the
Affordable Care Act is expected to boost EMS providers, leading to
increased outsourcing and market growth. EMS providers can upgrade
electronic designs within existing medical devices, further fueling
market growth.
For more information on market segmentation with geographical
analysis including forecast (2024-2028) and historic data (2018 -
2022) - Download a Sample Report
Research Analysis
The Medical Device Manufacturing Outsourcing Market encompasses
various medical devices and technologies, including Oncology,
Coronary stents, Orthopedic devices, Wound care, and Diagnostic
Imaging. These devices undergo scientific innovation to improve
patient outcomes and address unmet medical needs. Quality assurance
services and regulatory affairs services ensure compliance with
industry standards and regulations. Product implementation,
upgrade, and maintenance services enable seamless integration and
continuous improvement of devices. Contract manufacturing services
provide economies of scale and expertise in device production,
assembly, packaging, and procurement of components such as
electronic parts, sensors, polymers, and raw materials. Age-related
conditions and cardiovascular diseases are significant markets for
medical device outsourcing, with a growing focus on IVD,
Ophthalmic, and other therapeutic areas. The market is driven by
the increasing prevalence of chronic diseases, technological
advancements, and the need for cost-effective solutions.
Market Research Overview
The Medical Device Manufacturing Outsourcing market encompasses
various medical specialties, including Oncology, Cardiovascular
devices (Coronary stents), Orthopedic devices, Wound care, and
Diagnostic Imaging. Outsourcing services cover scientific
innovation, quality assurance, regulatory affairs, product
implementation, upgrade, and maintenance. Contract manufacturing
services include finished goods assembly, electronics, sensors, and
procurement of polymers and electronic components. Medical
practitioners in fields like General & plastic surgery,
Ophthalmic, IVD, Dental, Endoscopy, Diabetes care, and
Cardiovascular devices increasingly outsource non-core services to
focus on their core competencies. This trend is driven by the need
for modern technology, productivity, and time-to-market advantages.
Key services include IT outsourcing for quality control, logistics,
and supply chain management. Security and privacy, data
confidentiality, product quality, and intellectual property
protection are crucial concerns. The contract manufacturing segment
leverages state-of-the-art equipment to deliver high-quality
products while addressing age-related conditions, cardiovascular
diseases, orthopaedic issues, neurological disorders, and cardiac
disorders.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Product
-
- EMS
- Raw Materials
- Finished Goods
- Medical Device Regulatory Classification
-
- Class II
- Class III
- Class I
- Geography
-
- North America
- Asia
- Europe
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory
company. Their research and analysis focuses on emerging market
trends and provides actionable insights to help businesses identify
market opportunities and develop effective strategies to optimize
their market positions.
With over 500 specialized analysts, Technavio's report library
consists of more than 17,000 reports and counting, covering 800
technologies, spanning across 50 countries. Their client base
consists of enterprises of all sizes, including more than 100
Fortune 500 companies. This growing client base relies on
Technavio's comprehensive coverage, extensive research, and
actionable market insights to identify opportunities in existing
and potential markets and assess their competitive positions within
changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: media@technavio.com
Website: www.technavio.com/
 View original content to download
multimedia:https://www.prnewswire.com/news-releases/medical-device-manufacturing-outsourcing-market-size-is-set-to-grow-by-usd-50-44-billion-from-2024-2028--growing-focus-of-oems-on-reducing-medical-device-manufacturing-costs-to-boost-the-market-growth-technavio-302209483.html
View original content to download
multimedia:https://www.prnewswire.com/news-releases/medical-device-manufacturing-outsourcing-market-size-is-set-to-grow-by-usd-50-44-billion-from-2024-2028--growing-focus-of-oems-on-reducing-medical-device-manufacturing-costs-to-boost-the-market-growth-technavio-302209483.html
SOURCE Technavio