MONTVALE, N.J., June 1, 2018 /PRNewswire/ -- PENTAX
Medical Company, a healthcare industry leader in diagnostic and
therapeutic endoscopy, announces the introduction of the C2
CryoBalloon™ Ablation System for the treatment of
Barrett's esophagus, a condition in which the lining of the
esophagus develops abnormal lesions primarily as a result of
chronic gastroesophageal reflux disease (GERD).,1 An
estimated 3.3 million people in the U.S. are living with Barrett's
esophagus,2 which makes an individual 30 to 40 times
more likely to develop esophageal cancer – a disease with,
unfortunately, a 19.2% five-year survival rate.3
PENTAX Medical will feature the next-generation endoscopic
cryoablation device at Digestive Disease
Week® (DDW) in Washington, DC from June 2–5. DDW is the
largest international gathering of physicians, researchers and
academics in the fields of gastroenterology, hepatology, endoscopy
and gastrointestinal surgery. Separately, the company announced
that beginning June 1, the developer
of the C2 CryoBalloon, C2 Therapeutics, which it acquired last
year, will operate under the PENTAX Medical name.
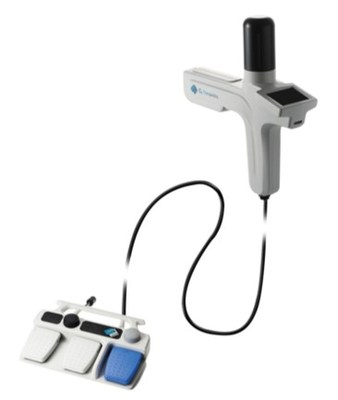
The C2 CryoBalloon Ablation System is used to destroy unwanted
tissue by application of extreme cold. A balloon probe, which is
connected to a catheter, comes in contact with the wall of the
target tissue. Upon activation by a physician, the balloon probe is
simultaneously cooled and inflated with nitrous oxide. The extreme
cold eradicates the targeted precancerous or abnormal lesions along
the esophageal lining while sparing healthy tissue. The system
allows physicians complete control of the procedure under direct
visualization of the treatment area during the procedure, which
lasts between 15 to 30 minutes.4,5
The new C2 CryoBalloon Ablation System offers several key
advantages including the ability to ablate a wide range of
patients, enhanced visualization and control, and a new level of
efficiency. "With the C2 Cryoballoon, you can promote your practice
as a more comprehensive Barrett's center with the latest and best
treatment options," said Harshit S.
Khara, MD, FACG, FASGE, Clinical Associate Professor of
Medicine, Geisinger Medical Center.
According to clinical studies, 95 percent of patients treated
with the C2 CryoBalloon achieve complete eradication of dysplasia
(CED) and 90 percent have complete eradication of intestinal
metaplasia (CEIM) through two years.6 Additionally, one
week following treatment, no patients required narcotic pain
medication.7
"We are committed to advancing the treatment of Barrett's
esophagus with a new generation of ablation devices that are easy
to use and improve clinical outcomes and patient satisfaction,"
said David Woods, Global Chief
Marketing Officer, PENTAX Medical. "The C2 CryoBalloon strengthens
our therapeutic endoscopy portfolio and is an important technology
that enables endoscopists to improve the quality of care they
provide to their patients."
Indications
US ONLY: The C2 CryoBalloon Ablation
System is intended for use as a cryosurgical tool in the field of
general surgery, specifically for endoscopic applications, to
include ablation of Barrett's esophagus with
dysplasia.8
About PENTAX Medical
PENTAX Medical is a division of
Hoya Group. Its mission is to improve the standard of patient care
and quality of healthcare delivery by providing the best endoscopy,
informatics, speech, voice, and swallowing assessment products and
services with a focus on QUALITY, CLINICALLY RELEVANT INNOVATION,
and SIMPLICITY. Through leading edge R&D and manufacturing,
PENTAX Medical provides endoscopic imaging devices and solutions to
the global medical community. Headquartered in Japan, PENTAX
Medical has a worldwide focus and presence with R&D, regional
sales, service, and in-country facilities around the globe. PENTAX
Medical employees represent the diverse countries where we do
business, allowing us to provide innovative solutions tailored to
meet local needs. For more information, visit
www.pentaxmedical.com.
The third-party trademarks used herein
are trademarks of their respective owners.
________________________________
1
https://www.asge.org/home/for-patients/patient-information/understanding-barrett-39-s-esophagus
2 Stuart J Spechler, MD, Berta M and Cecil O Patterson
- Barrett's esophagus: Epidemiology, clinical
manifestations, and
diagnosis http://www.uptodate.com/contents/barretts-esophagus-epidemiology-clinical-manifestations-and-diagnosis
3 SEER stat fact sheets: Esophageal cancer. National
Cancer Institute Web site.
http://seer.cancer.gov/statfacts/html/esoph.html Published date
unknown. Accessed March 1, 2016.
4 Canto MI, Shaheen NJ, Almario JA, Voltaggio L,
Montgomery E, Lightdale CJ. Multifocal Nitrous Oxide Cryoballoon
Ablation with or without EMR for Treatment of Neoplastic Barrett's
Esophagus. Gastrointest Endosc. 2018 Apr 4; pii: S0016-5107(18)30259-1. doi:
10.1016/j.gie.2018.03.024. PMID: 29626424
5 Künzli HT, Schölvinck DW, Meijer SL, Seldenrijk
KA, Bergman JGHM, Weusten BLAM. Efficacy of the CryoBalloon
Focal Ablation System for the Eradication of Dysplastic Barrett's
Esophagus Islands. Endoscopy. 2017 Feb;49(2): 169-175. doi:
10.1055/s-0042-120117. Epub 2016 Dec 20. PMID: 27997963
6 Canto MI. Safety, Efficacy, and Durability of
Endoscopic Nitrous Oxide CryoBalloon Ablation for Eradication of
Barrett's Neoplasia. To be presented via poster presentation
during DDW 2018.
7 Canto MI, Shaheen NJ, Almario JA, Voltaggio
L, Montgomery E, Lightdale CJ. Multifocal Nitrous Oxide Cryoballoon
Ablation with or without EMR for Treatment of Neoplastic Barrett's
Esophagus. Gastrointest Endosc. 2018 Apr 4; pii: S0016-5107(18)30259-1. doi:
10.1016/j.gie.2018.03.024. PMID: 29626424
8 In the European Union, The CryoBalloon Ablation System
is indicated to ablate unwanted tissue in the gastrointestinal
tract, including treatment of Barrett's Esophagus and squamous
dysplasia with application of extreme cold. In the United States, the device is indicated for
the field of general surgery, specifically for endoscopic
applications, to include ablation of Barrett's Esophagus with
dysplasia.
 View original content with
multimedia:http://www.prnewswire.com/news-releases/pentax-medical-introduces-next-generation-c2-cryoballoon-ablation-system-for-treatment-of-barretts-esophagus-300658152.html
View original content with
multimedia:http://www.prnewswire.com/news-releases/pentax-medical-introduces-next-generation-c2-cryoballoon-ablation-system-for-treatment-of-barretts-esophagus-300658152.html
SOURCE PENTAX Medical Company