U. S. SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K/A
(Mark One)
[X]
Annual report under Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended August
31, 2014
[_]
Transition report under Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition
period from __________ to __________.
Commission File Number: 0-15482
ONCOLOGIX TECH, INC.
(Name of small business issuer in its
charter)
| Nevada |
|
86-1006416 |
| (State or other jurisdiction of incorporation or organization |
|
(I.R.S. Employer Identification No.) |
| |
|
|
| 1604 W. Pinhook Rd. #200, Lafayette, LA |
|
71360 |
| (Address of principal executive offices) |
|
Zip Code |
| |
|
|
| |
|
|
| (616) 977-9933 |
| (Issuer’s telephone number) |
| |
|
|
| Securities registered under Section 12(b) of the Exchange Act: |
| |
|
|
| Title of Each Class |
|
Name of Each Exchange on Which Registered |
| Common Stock, $.001 par value |
|
OTC:QB |
| |
|
|
| Securities registered under Section 12(g) of the Exchange Act: None |
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. -Yes [_]
No [X]
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes
[_] No [X]
Indicate
by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act of 1934
during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes [X] No [_]
Indicate
by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive
Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (Section 232.405 of this chapter) during the
preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X]
No [_]
Indicate
by check mark if disclosure of delinquent filers in response to Item 405 of Regulation S-K (Section 229.405 of this chapter) is
not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information
statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [_]
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions
of “large accelerated filer”, “accelerated filer”, and “smaller reporting company” in Rule
12b-2 of the Exchange Act.
Large accelerated
Filer [_] Accelerated Filer [_]
Non-accelerated
Filer [_] Smaller Reporting Company [X]
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act) Yes [_]
No [X]
Indicate the number of shares outstanding of each of the
issuer’s classes of common stock, as of the latest practicable date.
| Class |
|
Outstanding at November 19, 2014 |
| Common Stock, $0.001 par value |
|
146,365,004 |
The aggregate number
of shares held by non-affiliates of the registrant at August 31, 2014 was 127,546,434. The aggregate market value of the Common
Stock of the registrant held by non-affiliates as of August 31, 2014 was approximately $535,695 based on closing stock price of
the registrant’s common stock on that date.
Documents Incorporated By Reference
None
Explanatory Note
This amendment to the Current Report
on Form 10-K filed by Oncologix Tech, Inc. (the “Company”) with the Security and Exchange commission on December 11, 2014 is filed solely to update Exhibit 99 – Consent
of Auditor.
| TABLE OF CONTENTS |
| |
|
|
|
| PART I |
| |
|
|
|
| Item 1 |
|
Description of Business |
3 |
| Item 1A |
|
Risk Factors |
19 |
| Item 1B |
|
Unresolved Staff Comments |
23 |
| Item 2 |
|
Properties |
23 |
| Item 3 |
|
Legal Proceedings |
23 |
| Item 4 |
|
Mine Safety Disclosures |
23 |
| |
|
|
|
| PART II |
| |
|
|
|
| Item 5 |
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of |
|
| |
|
Equity Securities |
24 |
| Item 6 |
|
Selected Financial Data |
29 |
| Item 7 |
|
Management's Discussion and Analysis of Financial Condition and Results of Operations |
29 |
| Item 7A |
|
Quantitative and Qualitative Disclosures About Market Risk |
32 |
| Item 8 |
|
Financial Statements and Supplementary Data |
33 |
| Item 9 |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
77 |
| Item 9A(T) |
Controls and Procedures |
77 |
| Item 9B |
|
Other Information |
78 |
| |
|
|
|
| PART III |
| |
|
|
|
| Item 10 |
|
Directors, Executive Officers and Corporate Governance |
78 |
| Item 11 |
|
Executive Compensation |
79 |
| Item 12 |
|
Security Ownership of Certain Beneficial Owners and Management and Related |
|
| |
|
Stockholder Matters |
83 |
| Item 13 |
|
Certain Relationships and Related Transactions, and Director Independence |
83 |
| Item 14 |
|
Principal Accountant Fees and Services |
84 |
| |
|
|
|
| PART IV |
| |
|
|
|
| Item 15 |
|
Exhibits, Financial Statement Schedules |
85 |
| |
|
SIGNATURES |
88 |
PART I
FORWARD LOOKING STATEMENTS
This annual report on Form
10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section
21E of the Securities Exchange Act of 1934, as amended that are based on management’s beliefs and assumptions and on information
currently available to our management. For this purpose any statement contained in this annual report on Form 10-K that is not
a statement of historical fact may be deemed to be forward-looking, including, but not limited to those relating to future demand
for the products and services we offer, changes in the composition of the products and services we offer, future revenues, expenses,
results of operations, liquidity and capital resources or cash flows, the commodity price environment, managing our asset base,
our ability to restructure our existing credit facilities or to obtain additional debt or equity financing, management’s
assessment of internal control over financial reporting, financial results, opportunities, growth, business plans, strategies and
objectives. Without limiting the foregoing, words such as “believe,” “expect,” “project,” “intend,”
“estimate,” “budget,” “plan,” “forecast,” “predict,” “may,”
“will,” “could,” “should,” or “anticipate” or comparable terminology are intended
to identify forward-looking statements. These statements by their nature involve known and unknown risks, uncertainties, and other
factors that may cause our actual results, performance or achievements or the industry to be materially different from any future
results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, but are not
limited to, market factors, market prices and marketing activity, future revenues and costs, unsettled political conditions, civil
unrest and governmental actions, foreign currency fluctuations, and environmental and labor laws and other factors detailed herein
and in our other filings with the U.S. Securities and Exchange Commission (the “Commission”) filings. Additional Factors
that may cause actual results, our performance or achievements, or industry results, to differ materially from those contemplated
by such forward-looking statements include without limitation:
| · | our ability to raise capital when needed and on acceptable terms and conditions; |
| · | our ability to identify and acquire a viable operating business; |
| · | our ability to attract and retain management, and to integrate and maintain technical information
and management information systems; |
| · | the intensity of competition; and |
| · | general economic conditions. |
Forward-looking statements
are predictions and not guarantees of future performance or events. The forward-looking statements are based on current industry,
financial and economic information, which we have assessed but which by its nature, is dynamic and subject to rapid and possibly
abrupt changes. Our actual results could differ materially from those stated or implied by such forward-looking statements due
to risks and uncertainties associated with our business. We hereby qualify all our forward-looking statements by these cautionary
statements. These forward-looking statements speak only as of their dates and should not be unduly relied upon. We undertake no
obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or
otherwise. All written and oral forward-looking statements made in connection with this Annual Report on Form 10-K that are attributable
to us or persons acting on our behalf are expressly qualified in their entirety by these cautionary statements. Given the uncertainties
that surround such statements, you are cautioned not to place undue reliance on such forward-looking statements.
Throughout this report,
unless otherwise indicated by the context, references herein to the “Company”, “Oncologix”, “we”,
our” or “us” means Oncologix Tech, Inc.., a Nevada corporation and its corporate subsidiaries and predecessors.
Item 1. Description of Business
OVERVIEW
Oncologix Tech, Inc. is
a diversified medical holding company that operates and manufactures Class II medical device products, delivers Personal Healthcare
Services and distributes Home Medical and Durable Medical products nationally. For its clients, Oncologix provides FDA approved
medical devices and products as well as state licensed healthcare services. For its shareholders, Oncologix operates profitable
business divisions that build, maintain and nourish shareholder value. The Company’s corporate mission is to be the best
small cap medical holding company delivering
devices, medical products & technologies,
and healthcare services in North America.
We were originally formed
in 1995, and in FY 2000 we changed our name to "BestNet Communications Corp." At that time we provided worldwide long
distance telephone communication and teleconferencing services to commercial and residential consumers through the internet, which
was disposed of in 2007 due to lack of profitability. In July 2006 we changed our business model to develop medical device products.
In July 2006 we acquired JDA Medical Technologies, Inc. ("JDA") and merged this business into Oncologix Corporation,
our wholly owned subsidiary. On January 22, 2007, we changed our name to Oncologix Tech, Inc., to reflect this new business
model. The business at that time was the development of a medical device product for brachytherapy (radiation therapy), called
the “Oncosphere” (or “Oncosphere System”), for the advanced treatment of soft-tissue cancers. Due to a
lack of funding, we suspended those new product development activities on December 31, 2007. On November 1, 2013, due to the development
of the brachytherapy device being several years away, and the indication that the product could not be marketed with no guarantee
of FDA approvals, it was determined that continued financial support required by Oncologix Corporation would cost the Company substantial capital
beyond its means, therefore the Company’s management and Board of Directors disposed of Oncologix Corporation and its
Brachytherapy medical device subsidiary. Furthermore, as part of the disposal, the Company was relieved of over $90,000 in debt.
On March 22, 2013, we acquired
all the outstanding stock of Dotolo Research Corporation (“Dotolo”), an FDA Registered, Class II, medical device manufacturer
with 25 years of product sales in the hydro-colonic irrigation, bowel preparation market. Dotolo Research Corporation began operations
in 1989 and sells hardware and disposable products to a customer base of over 900+ customers both domestically and internationally.
The Company currently operates in a limited, but competitive environment in hydro-colonic irrigation, of which there are only four
(4) companies approved by the FDA to manufacture a Class II medical device for colonic-hydro therapy. Since the acquisition,
we have not had significant revenues from sales of our products due to a lack of operating capital needed to procure raw material
inventory to currently fill customers’ re-supply and new equipment orders.
On August 1, 2013, we acquired
the outstanding stock of Angels of Mercy, Inc. Angels provides non-medical, Personal Care Attendant (PCA) Activities of Daily Living
(ADL) services, Supervised Independent Living (SIL), Long-Term Senior Care, and other approved health service programs performed
by a trained caregiver that meets the health service needs of beneficiaries whose disabilities preclude the performance of certain
independent living skills..
On December 10, 2013, Angels
of Mercy, Inc. acquired the assets of Amian Health Services LLC and Amian Health Services of Alex LLC, herein after referred to
as “Amian”. Amian delivers health-care services that provide routine health and personal care support with
Activities of Daily Living (ADL) to clients with physical impairments or disabilities in private homes, nursing care facilities,
hospice care settings, and other residential settings. Amian holds both PCA-Medicaid Waiver Provider and Residential Rehabilitation/Supervised
Independent Living (SIL), and personal care services for Veterans with licenses issued by the Division of Licensing and Certification
of the Department of Social Services, Veterans Administration Social Services and the Louisiana Department of Health and Hospitals.
All administrative personnel of Amian have been merged into AOM to obtain operational synergies. In August 2014, Angels of
Mercy, Inc. officially changed its name to Amian Angels, Inc. (“Amian Angels”).
On July 21, 2014 we formed
Advanced Medical Products and Technologies Inc. to enter into the Durable Medical and Home Medical Equipment markets. The objective
is to acquire companies in this space to develop our Medical Products and Technologies Segment.
On September 25,
2014, we acquired 100% of the stock of Esteemcare Inc. and its subsidiary Affordable Medical Equipment Solutions, Inc. . Esteemcare,
Inc, was established in 1996, is a Durable and Home Medical equipment and supply distributor for respiratory therapy, and is Accredited
by the “Joint Commission on Healthcare Organizations”. Esteemcare targets patients with sleep obstructive disorders
or related chronic illnesses who are insured by Medicare, Medicaid, third-party insurers, or have the ability to pay for our products
from their own private resources.
BUSINESS SEGMENTS
We identify our reportable
segments based on our management structure, financial data and market. We have identified three business segments: Medical Device
Manufacturing, Personal Care Services, and Medical Products Distribution and New Technologies.
MEDICAL DEVICE MANUFACTURING
Dotolo Research Corporation
(“Dotolo”) designs, manufactures and distributes the Toxygen hardware system with disposables speculums and disposable
tubing. The company has a valid Registration from the FDA as a Class II medical device, is licensed by Health Canada, a CE mark
for Europe, and is in compliance with ISO 9001:2000 and ISO 13485:2003 regulations. Our product’s primary use is for bowel
preparation prior to medical procedures such as a Colonoscopy and OB/GYN medical procedures and for individuals seeking health
and wellness prevention for good colon health. There are currently over 22 million colonoscopy procedures performed annually within
the United States and our strategic mission is to become the preferred choice of colon lavage cleansing and bowel preparation by
both patients and medical professionals. Management believes its primary competitive strengths is its 25 year manufacturing history
in the market of hydro-colonic irrigation, its on-going development of new product technologies, exceptional quality controls,
and world-wide distribution of all hardware and disposable products. The Company utilizes an indirect, re-seller sales force to
promote and sell the company products. Management believes that the Company must expand its manufacturing capacity and its’
distribution sales channels to be able to move into medical markets that management believes is a high growth market. The Management’s
strategy to acquire Dotolo Research into a publicly held company in the United States is to enhance the Company’s capital
raising abilities to fund increased manufacturing capacity, increase current inventory levels, improve payment terms with our core
raw material suppliers to reduce our COGS, execution of new product designs on both hardware and disposable products, maintain
regulatory compliance, expansion of a sales and marketing team and additional acquisitions of companies within the medical device
and healthcare markets.
Marketing
The Company's overall marketing
strategy is to strengthen is current position in the health and wellness markets, leverage its brand reputation and 25 year history
as a quality manufacturer of medical device products, and sell directly into the medical space. The direct sales strategy of providing
products to health and wellness spas has served the Company’s needs to date and has enabled us to obtain orders while avoiding
the costs associated with hiring marketing employees and direct sales representatives. However, future growth will require us to
increase our product and brand awareness in the medical markets, hire a full time Quality Assurance/Regulatory Manager, Director
of Sales and Marketing, establish additional clinical research on bowel preparations and leverage the existing clinical research
performed by Dr. Joseph Fiorito et al. at Danbury Hospital utilizing the Dotolo Research products, increase our brand and marketing,
our web presence, and strengthen our domestic and international reseller distribution channels. The Company’s distribution
model has resulted in the Company not having a single customer account for more than 10% of its total sales. The Company is seeking
to expand its operations so that it can continue to service health and wellness customers but specifically sell products directly
to Out-Patient Endoscopy Centers, hospital systems, Veterans Administration Hospitals, Skilled Nursing Homes, and Sterile kit packing
distributors which will provide more profitable sales orders.
Domestic and International Payment Terms
Dotolo Research Corporation
records revenue both domestically and internationally through indirect sales channels as products are sold to independent distributors
or product re-sellers. Terms on all products sold are 50% of the total order, paid in advance, and the balance due prior to final
shipment (FOB). These sales terms, to date, has allowed our company to experience extremely few collection problems on its domestic
sales. International sales orders are via wire transfer and we have little to no payable receivable issues. Management believes
that once we enter into the medical space we will experience payment terms of Net-45 which will require the company to increase
its inventory levels on disposable products up to 30-day inventory supply levels.
Manufacturing
Dotolo Research Corporation
procures raw materials from various sources and performs on-site manufacturing and final sub-assembly operations. The company does
not outsource its manufacturing processes. Management believes that the skill level and experience of the Company’s work
force as well as the Company’s operations allow it to meet
current order demand but will need to ramp
up skilled bench assembly workers and packaging employees when those orders from the medical markets commence. The Company's principal
raw material is injection-molded plastic, medical grade respiration tubing, water-filtration parts, and hardware components such
as metal housings, fitting and valves. The Company purchases its injection-molding raw materials from two (2) suppliers and hardware
component parts from numerous sources none of which is believed by the Company to be a dominant supplier.
Trademarks, Licenses and Patents
Dotolo Research Corporation
owns numerous patents, with its primary patent on the Toxygen hardware, Patent # 5,788,650, “Colon Hydrotherapy Apparatus”-
Ultra Violet Water Filtration System. This Patent is valid through 2018 and protects this product design both domestically and
internationally.
Management believes that
new patents will be granted on our new speculum and hardware designs and in each instance, the patents will, when granted, be sufficient
for us to pursue our proposed business strategy. The grant of a patent by the U.S. Patent Office does not insure that the
patent will be upheld in litigation seeking to protect the patent or that the patent will not be found to infringe on patents held
by others.
Device Approval Process
It is anticipated that
our new product(s) re-designs will be regulated as a Class II medical device and will be subject to extensive regulation by the
FDA and other regulatory authorities in the US. The Food, Drug, and Cosmetic Act (“FD&C Act”) and
other federal and state statutes and regulations govern the research, design, development, preclinical and clinical testing, manufacturing,
safety, approval or clearance, labeling, packaging, storage, record keeping, servicing, promotion, import and export, and distribution
of medical devices.
Unless an exemption applies,
each medical device we wish to commercially distribute in the U.S. will require either prior premarket notification, or 510(k)
clearance, or premarket approval (PMA) from the FDA. The FDA classifies medical devices into one of three classes. Devices
requiring fewer controls because they are deemed to pose lower risk are placed in Class I or II. Class I devices are subject to
general controls such as labeling, premarket notification, and adherence to the FDA’s Quality System Regulation (a set of
current good manufacturing practice requirements put forth by the FDA which govern the methods used in, and the facilities and
controls used for, the design, manufacture, packaging, labeling, storage, installation and servicing of finished devices) (“QSR”).
Class II devices are subject to special controls such as performance standards, post-market surveillance, FDA guidelines, as well
as general controls. Some Class I and Class II devices are exempted by regulation from the premarket notification, or 510(k),
clearance requirement or the requirement of compliance with certain provisions of the QSR. Devices are placed in Class III, which
requires approval of a PMA application, if insufficient information exists to determine that the application of general controls
or special controls are sufficient to provide reasonable assurance of safety and effectiveness, or they are life-sustaining, life-supporting
or implantable devices, or the FDA deems these devices to be “not substantially equivalent” either
to a previously 510(k) cleared device or to a “pre-amendment” Class III device in commercial distribution
before May 28, 1976, for which PMA applications have not been required. The FDA may classify our products as class III devices,
requiring PMA approval. A PMA application must be supported by valid scientific evidence, which typically requires extensive
data, including technical, pre-clinical, clinical, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction
the safety and effectiveness of the device. A PMA application must include, among other things, a complete description of the device
and its components, a detailed description of the methods, facilities and controls used to manufacture the device, and proposed
labeling. A PMA application also must be accompanied by a user fee, unless exempt. For example, the FDA does not require the submission
of a user fee for a small business’s first PMA. After a PMA application is submitted and found to be sufficiently complete,
the FDA begins an in-depth review of the submitted information. During this review period, the FDA may request additional information,
or clarification of information already provided. Also during the review period, an advisory panel of experts from outside the
FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the
device. In addition, the FDA generally will conduct a pre-approval inspection of the manufacturing facility to ensure compliance
with the QSR, which requires manufacturers to follow design, testing, control, documentation, and other quality assurance procedures.
Our current products are
classified as a Class II device and we do not anticipate we will be required to first seek premarket approval for our products.
The FDA will determine based upon the 510(k) submission and may agree if the
risk is low enough, and safety and effectiveness
can be assured with special controls in place, the PMA will not be required.
If a PMA is required, the
FDA can delay, limit, or deny approval of a PMA application for many reasons, including:
• The product may not
be safe or effective to the FDA’s satisfaction;
• The data from our
pre-clinical studies and clinical trials may be insufficient to support approval;
• The manufacturing
process or facilities we use may not meet applicable requirements; and
• Changes in FDA approval
policies or adoption of new regulations may require additional data.
If the FDA evaluations of
both the PMA application and the manufacturing facilities are favorable, the FDA will either issue an approval letter, or approvable
letter, which usually contains a number of conditions which must be met in order to secure final approval of the PMA. When and
if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter authorizing
commercial marketing of the device for certain indications. If the FDA’s evaluation of the PMA or manufacturing facilities
is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The FDA may also determine that additional
clinical trials are necessary, in which case the PMA approval may be delayed while the trials are conducted and the data acquired
is submitted in an amendment to the PMA. Even with additional trials, the FDA may not approve the PMA application. The PMA process
can be expensive, uncertain, and lengthy and a number of devices for which FDA approval has been sought by other companies have
never been approved for marketing.
New PMA applications or
PMA supplements may be required for modifications to the manufacturing process, labeling and device specifications, materials or
design of a device that is approved through the PMA process. PMA supplements often require submission of the same type of information
as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device
covered by the original PMA application, and may not require as extensive clinical data or the convening of an advisory panel.
Clinical trials are almost always required to support a PMA application, and are sometimes required for a 510(k) clearance. These
trials generally require submission of an application for an Investigational Device Exemption (“IDE”) to the
FDA. If a trial is considered a “Non-Significant Risk” (“NSR”) study subject to
abbreviated IDE regulations, a formal IDE submission is not required by the FDA. An IDE application must be supported by
appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that
the testing protocol is scientifically sound. The IDE application must be approved in advance by the FDA for a specified
number of patients, unless the product is deemed a non-significant risk device and eligible for more abbreviated IDE requirements.
Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the
study protocol and informed consent form are approved by appropriate institutional review boards (“IRBs”) at
the clinical trial sites. The FDA’s approval of an IDE allows clinical testing to go forward, but does not bind the FDA to
accept the results of the trial as sufficient to prove the product’s safety and effectiveness, even if the trial meets its
intended success criteria. All clinical trials must be conducted in accordance with the FDA’s IDE regulations that
govern investigational device labeling, prohibit promotion of the investigational device, and specify an array of recordkeeping,
reporting and monitoring responsibilities of study sponsors and study investigators. . Required records and reports are subject
to inspection by the FDA. The results of clinical testing may be unfavorable or, even if the intended safety and effectiveness
success criteria are achieved, may not be considered sufficient for the FDA to grant approval or clearance of a product.
Although we believe our
clinical trials will provide favorable data to support our PMA application, upon evaluation the FDA may conclude differently. Delays
in receipt of or failure to receive FDA approval, the withdrawal of previously received approvals, or failure to comply with existing
or future regulatory requirements would have a material adverse effect on our business, financial condition, and results of operations.
Even if granted, the approvals may include significant limitations on the intended use and indications for use for which our products
may be marketed.
After a device is approved
or cleared and placed in commercial distribution, numerous regulatory requirements apply. These include:
• establishing registration
and device listing;
• implementing QSR, which requires
manufacturers to follow design, testing, control, documentation and other quality assurance procedures;
• labeling regulations, which
prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions
on labeling;
• medical device reporting regulations,
which require that manufacturers report to the FDA if a device may have caused or contributed to a death or serious injury or malfunctioned
in a way that would likely cause or contribute to a death or serious injury if it were to recur; and
• corrections and removal reporting
regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken
to reduce a risk to health posed by the device or to remedy a violation of the FD&C Act that may present a risk to health.
Also, the FDA may require
us to conduct post market studies or order us to establish and maintain a system for tracking our products through the chain of
distribution to the patient level. The FDA enforces regulatory requirements by conducting periodic, unannounced inspections
and market surveillance.
Failure to comply with applicable
regulatory requirements, including those applicable to the conduct of our clinical trials, can result in enforcement action by
the FDA, which may lead to any of the following sanctions:
• Warning letters;
• Fines and civil penalties;
• Unanticipated expenditures;
• Delays in approving
or refusal to approve our applications, including supplements;
• Withdrawal of FDA
approval;
• Product recall or
seizure;
• Interruption of production;
• Operating restrictions;
• Injunctions; and
• Criminal prosecution.
Our products are manufactured
in compliance with current Good Manufacturing Practices (“GMP”) requirements set forth in the QSR. The QSR requires
a quality system for the design, manufacture, packaging, labeling, storage, installation and servicing of marketed devices and
includes extensive requirements with respect to quality management and organization, device design, equipment, purchase and handling
of components, production and process controls, packaging and labeling controls, device evaluation, distribution, installation,
complaint handling, servicing, and record keeping. The FDA enforces the QSR through periodic unannounced inspections If the FDA
believes that we are not in compliance with QSR, it can shut down the manufacturing operations, require recall of our products,
refuse to approve new marketing applications, institute legal proceedings to detain or seize products, enjoin future violations,
or assess civil and criminal penalties against us or our officers or other employees. Any such action by the FDA would have
a material adverse effect on our business. We cannot assure you that we will be able to comply with all applicable FDA regulations.
Non-FDA Government Regulation
The advertising of our products
will be subject to both FDA and Federal Trade Commission regulations. In addition, the sale and marketing of our products will
be subject to a complex system of federal and state laws and regulations intended to deter, detect, and respond to fraud and abuse
in the healthcare system. These laws and regulations restrict and may prohibit pricing, discounting, commissions and other
commercial practices that may be typical outside of the healthcare business. In particular, anti-kickback and self-referral laws
and regulations will limit our flexibility in crafting promotional programs and other financial arrangements with medical professionals
in connection with the sale of our products and related services, especially with respect to physicians seeking reimbursement through
Medicare or Medicaid. These federal laws include, by way of example, the following:
• the anti-kickback statute prohibits
certain business practices and relationships that might affect the provision and cost of healthcare services reimbursable under
Medicare, Medicaid and other federal healthcare programs, including the payment or receipt of remuneration for the referral of
patients whose care will be paid by Medicare or other federal healthcare programs;
• the physician self-referral
prohibition, commonly referred to as the Stark Law, which prohibits referrals by physicians of Medicare or Medicaid patients to
providers of a broad range of designated healthcare services in which the physicians or their immediate family members have ownership
interests or with which they have certain other financial arrangements;
• the anti-inducement law, which
prohibits providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services
covered by either program;
• the Civil False Claims Act,
which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment by the federal
government, including the Medicare and Medicaid programs; and
• the Civil Monetary Penalties
Law, which authorizes the US Department of Health and Human Services (“HHS”) to impose civil penalties administratively
for fraudulent or abusive acts.
Sanctions for violating
these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, money penalties,
imprisonment, denial of Medicare and Medicaid payments, or exclusion from the Medicare and Medicaid programs, or both. These
laws also impose an affirmative duty on those receiving Medicare or Medicaid funding to ensure that they do not employ or contract
with persons excluded from the Medicare and other government programs.
Many states have adopted
or are considering legislative proposals similar to the federal fraud and abuse laws, some of which extend beyond the Medicare
and Medicaid programs to prohibit the payment or receipt of remuneration for the referral of patients and physician self-referrals
regardless of whether the service was reimbursed by Medicare or Medicaid. Many states have also adopted or are considering
legislative proposals to increase patient protections, such as limiting the use and disclosure of patient-specific health information.
These state laws typically impose criminal and civil penalties similar to the federal laws.
In the ordinary course of
their business, medical device manufacturers and suppliers have been and are subject regularly to inquiries, investigations and
audits by federal and state agencies that oversee these laws and regulations. Recent federal and state legislation has greatly
increased funding for investigations and enforcement actions, which have increased dramatically over the past several years. This
trend is expected to continue. Private enforcement of healthcare fraud also has increased, due in large part to amendments to the
Civil False Claims Act in 1986 that were designed to encourage private persons to sue on behalf of the government. These whistleblower
suits by private persons, known as qui tam relaters, may be filed by almost anyone, including physicians and their employees and
patients, our employees, and even competitors. The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”),
in addition to its privacy provisions, created a series of new healthcare-related crimes.
Competition
The Company currently operates
in a limited, but competitive environment in hydro-colonic irrigation, of which there are only four (4) companies that are approved
by the FDA to manufacture a Class II medical device for colon-hydro therapy. The Company's major competitors are located in Phoenix,
Arizona, Clearwater Florida, San Antonio, Texas, and in Canada. The Company must design and develop a broader range of speculum
and disposable products to maintain and grow its market share in the disposable markets.
In a sense, the Company
competes with traditional methods of bowel preparation prior to colonoscopy procedures such as Sodium Bi-phosphate and Sodium Phosphate
which have general medical acceptance. The Food and Drug Administration (FDA) issued a safety
alert regarding Fleet enemas and other related products in 2008. This alert was due to reports of acute phosphate nephropathy which
is an acute kidney injury occurring in some patients who had used oral sodium phosphate (OSP) products for bowel cleansing prior
to medical procedures. This injury was seen in patients who did not present with risk factors for this condition. We believe that
a hydro-colonic irrigation method of bowel preparation will be highly favored by consumers as it does not require a difficult
24 hour fasting period, risk of renal failure, and an often unpleasant 12 hour, overnight bowel preparation.
Proprietary Rights
We have entered into an
Employment Agreement with our Chief Executive Officer, and Non-Circumvent and Non-Disclosure agreements with key employees that
require them to keep all of our proprietary information confidential. We cannot assure that such protections will prove adequate
should they be challenged in litigation.
Legal Proceedings
The Company is not party
to any legal proceedings.
Inventory Management
The Company's ability to
manage its inventories properly is an important factor in its operations. Inventory shortages can impede the Company's ability
to meet orders on a timely basis. Conversely, excess inventories will result in increased inventory carrying costs that will lower
gross margins. If the Company is unable to effectively manage its inventory, its business, results of operations and financial
condition will be adversely affected. Dotolo Research Corporation currently operates its inventory management system on MAS-90
(SAGE), and inventory is First In- First- Out (FIFO) and we operate a Just in Time (JIT) system. All raw materials are procured
only after receipt of orders and we maintain fifteen (15) day inventory levels to support our top customers with disposable products
with those customers that provide the company with Standing Purchase Orders.
Suppliers and Service Providers
The Company's ability to
competitively price its products depends on the cost of raw material components especially petroleum based products, packaging
costs, cost of fuel for shipping charges, and its ability to maintain its’ fixed and variable overhead. The cost of materials
is subject to annual price increases from our suppliers and the company will enter into long-term pricing contracts to hold pricing
levels firm for one (1) year with future increases based upon the Producer Price Index (PPI).
Customers
The Company's financial
success is directly related to the willingness of our customers to continue to purchase its products. The Company does not typically
have long-term contracts with its customers however once in the medical market the Company hopes to execute standing re-supply
orders with large out-patient clinics and hospital systems. Sales to the Company's customers are generally on an order-by-order
basis and are subject to rights of cancellation and rescheduling by the customers. Failure to fill customers' orders in a timely
manner could harm the Company's relationships with its customers. Furthermore, if any of the Company's major customers experiences
a significant downturn in its business, then these customers may reduce or discontinue purchases from the Company, which could
have an adverse effect on the Company's business, results of operations and financial condition.
The Company sells its products
to our customers from its Published Price List and discounts pricing based on sales volumes. The company does not extend credit.
Financial difficulties of a customer could cause the Company to stop doing business with that customer or reduce its business with
that customer. The Company's inability to collect from its customers or a cessation or reduction of sales to certain customers
because of financial concerns could have an adverse effect on the Company's business, results of operations and financial condition.
The target markets and targeted customers for
our products include:
| · | Health and Wellness Spas |
| · | Endoscopy Out-Patient Clinics |
| · | Hospital Systems- Veterans Hospitals |
| · | Assisted Living Facilities |
| · | Individuals with disabilities such as paraplegic, quadriplegic and obesity |
Implementation of Growth Strategy.
As part of its growth strategy in our health
and wellness and medical device markets, the Company will:
| · | Re-strengthen and expand existing distribution channels s for our medical device products within
the United States. Currently these channels have not been active due to a lack of capital for the production of inventor. |
| · | Re-strengthen our existing international distribution and re-seller agreements that we have previously
established specifically in the United Kingdom, Saudi Arabia (Middle East), Africa, Mexico, Taiwan and Australia. |
| · | Release new product development on Version 2 of the Toxygen hardware system in 2nd qtr
2015 and release new speculum disposable products. |
| · | Expand its factory operations to be able to accept large orders that will involve larger production
runs that management believes will be more profitable. |
These initiatives are
largely conditioned upon the Company obtaining additional capital, of which there can be no assurance. Any such failures could
have an adverse effect on the Company's business, results of operations and financial condition.
PERSONAL CARE SERVICES
Amian Angels, Inc. (“Amian
Angels”) provides non-medical, Personal Care Attendant (PCA) services, Supervised Independent Living (SIL), Long-Term Senior
Care, and other approved programs performed by a trained caregiver that will meet the health service needs of beneficiaries whose
disabilities preclude the performance of certain independent living skills related to the activities of daily living (ADL).
PCA services that are reimbursed to Amian Angels
under the waiver:
| · | Assisting with personal hygiene, dressing, bathing, and grooming |
| · | Performing or assisting in the performance of tasks related to maintaining a safe, healthy and
stable living environment such as: |
| · | Light cleaning tasks in areas of the home used by the recipient, |
| · | Shopping for such items as health and hygiene products, clothing and groceries, |
| · | Performing activities of daily living inside and outside of the home which require attendant care, |
| · | Assisting with or performing beneficiary laundry care chores |
| · | Assisting with bladder and/or bowel requirements, including bed pan routines |
| · | If indicated on the Plan of Care, assisting beneficiary to clinics, physician’s offices,
and other appointments, |
| · | Assisting the beneficiary to receive any service specified in the written Plan of Care, including
leisure skills development, |
| · | Assisting in activities which would enhance the individual employability and improve and enhance
the beneficiary’s quality of life. |
Supervised Independent Living (SIL) is an alternative
program for mentally challenged, developmentally disabled or physically disabled individuals. Amian Angels provides the following
personal health services:
| · | Assistance with safety procedures and emergency contacts within the home environment |
| · | Counseling during the transition period |
| · | Assistance in meeting needs with medical management, employment, financial, budgeting, household
management, personal care, recreation and transportation |
| · | Accessibility to community resources |
| · | Independence and personal growth |
| · | Freedom from abuse, neglect and exploitation |
Personal Care Attendant Providers Licensing
Standards
Pursuant to R.S. 40:2120.2,
all Personal Care Attendant service providers and Home and Community- Based Services must be licensed by the Department of Health
& Hospitals. DHH establishes the minimum licensing standards and the licensing provisions contain the core requirements
for HCBS providers depending upon the services rendered by the HCBS provider. Currently, Angels of Mercy, Inc is in full
compliance and good standing with the licensing requirements set forth and established by the State of Louisiana Department of
Health & Hospitals.
Services and Fee Structure
Amian Angels is reimbursed
for each approved “Unit of Service” provided, as determined by the Health Care Financing Administration (HCFA), the
Department of Social Services and based upon a detailed Case Management, Plan of Care for each beneficiary. A unit of service for
PCA services will be one-half hour. At least fifteen (15) minutes of
service must be provided to the individual
in order for Amian Angels to bill for a unit of service. A maximum of 1,825 hours (3,650 half-hour units) per beneficiary, per
year can be billed under the Medicaid waiver program.
Long
Term Care (LTC) - Older population or anyone whose disability occurred after their 21st birthday. Reimbursement rate: $2.89/quarter
hour
Early
Periodic Screening Diagnostic Treatment (EPSDT) – This service is provided from 3 years of age or older until 21 years
of age. Reimbursement rate: $2.53/quarter hour
Elderly
and Young Adult Waiver (EDA) – This service is provided by age 22 and normally up through death of the client. Reimbursement
rate: $2.83/quarter hour
New
Opportunity Waiver (NOW) The longest running Medicaid Waiver program and has the most resources available for the clients.
Several
programs within the NOW- Title 19.
Reimbursement rates:
IFS day program: $3.61/quarter
hour
IFS night: $2.17quarter
hour
IFS day x2: $2.72/quarter
hour
IFS day x3: 2.36/quarter
hour
IFS night x2: $1.52/quarter
hour
Supervised
Independent Living (SIL) - Clients live independently but with supervision and assistance from Amian Angels trained staff. Activities
of Daily Living skills are taught by staff to clients. Some clients go to day programs, some are employed, and some clients receive
24 hours/day personal care services. Reimbursement rate: 16.93/hour/daily care 24/7
Non-FDA Government
Regulation
The
advertising of our healthcare services and medical device products will be subject to both FDA and Federal Trade Commission regulations.
In addition, the sale and marketing of our products will be subject to a complex system of federal and state laws and regulations
intended to deter, detect, and respond to fraud and abuse in the healthcare system. These laws and regulations restrict and
may prohibit pricing, discounting, commissions and other commercial practices that may be typical outside of the healthcare business.
In particular, anti-kickback and self-referral laws and regulations will limit our flexibility in crafting promotional programs
and other financial arrangements with medical professionals in connection with the sale of our products and related services, especially
with respect to physicians seeking reimbursement through Medicare or Medicaid. These federal laws include, by way of example,
the following:
• the anti-kickback statute
prohibits certain business practices and relationships that might affect the provision and cost of healthcare services reimbursable
under Medicare, Medicaid and other federal healthcare programs, including the payment or receipt of remuneration for the referral
of patients whose care will be paid by Medicare or other federal healthcare programs;
• the physician self-referral
prohibition, commonly referred to as the Stark Law, which prohibits referrals by physicians of Medicare or Medicaid patients to
providers of a broad range of designated healthcare services in which the physicians or their immediate family members have ownership
interests or with which they have certain other financial arrangements;
• the anti-inducement law, which
prohibits providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services
covered by either program;
• the Civil False Claims Act,
which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment by the federal
government, including the Medicare and Medicaid programs; and
• the Civil Monetary Penalties
Law, which authorizes the US Department of Health and Human Services (“HHS”) to impose civil penalties administratively
for fraudulent or abusive acts.
Sanctions for violating
these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, money penalties,
imprisonment, denial of Medicare and Medicaid payments, or exclusion from the Medicare and Medicaid programs, or both. These
laws also impose an affirmative duty on those receiving Medicare or Medicaid funding to ensure that they do not employ or contract
with persons excluded from the Medicare and other government programs.
Many states have adopted
or are considering legislative proposals similar to the federal fraud and abuse laws, some of which extend beyond the Medicare
and Medicaid programs to prohibit the payment or receipt of remuneration for the referral of patients and physician self-referrals
regardless of whether the service was reimbursed by Medicare or Medicaid. Many states have also adopted or are considering
legislative proposals to increase patient protections, such as limiting the use and disclosure of patient-specific health information.
These state laws typically impose criminal and civil penalties similar to the federal laws.
In the ordinary course of
their business, medical device manufacturers and suppliers have been and are subject regularly to inquiries, investigations and
audits by federal and state agencies that oversee these laws and regulations. Recent federal and state legislation has greatly
increased funding for investigations and enforcement actions, which have increased dramatically over the past several years. This
trend is expected to continue. Private enforcement of healthcare fraud also has increased, due in large part to amendments to the
Civil False Claims Act in 1986 that were designed to encourage private persons to sue on behalf of the government. These whistleblower
suits by private persons, known as qui tam relaters, may be filed by almost anyone, including physicians and their employees and
patients, our employees, and even competitors. The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”),
in addition to its privacy provisions, created a series of new healthcare-related crimes.
Competition
The Company currently operates
in a highly competitive environment in Personal Care Attendant (PCA) services. The Company's competitors are located throughout
the State of Louisiana where Personal Care Attendant licenses are authorized by the Department of Health and Hospitals to conduct
business.. To provide a competitive edge on the competition, Amian Angels will continue to hire highly skilled company employees,,
deliver on-going training, and deliver the highest level of personal care services.
Proprietary Rights
We have entered into an
Employment Agreement with our President, Key Managers and have executed Non-Circumvent and Non-Disclosure agreements with key employees
that require them to maintain all of our proprietary information and customers lists confidential. We cannot assure that
such protections will prove adequate should they be challenged in litigation.
Customers
The Company's financial
success is directly related to maintaining the continued excellence of personal care services we provide to our current clients
and the willingness of newly qualified clients to accept our organization as the “Preferred Provider Choice” to provide
their loved ones or themselves with the highest level of personal care.
MEDICAL PRODUCTS AND TECHNOLOGIES
Esteemcare Inc.
Esteemcare, Inc, established in 1996, is a Durable
and Home Medical equipment and supply distributor for respiratory therapy and is Accredited by the “Joint Commission on
Healthcare Organizations”.
Esteemcare - Company Vision:
• Promote excellence in set- up and patient education
of Respiratory Therapy, home medical equipment and sleep therapy services.
• Do so through efficient utilization of resources
by professionally trained Respiratory therapists.
• To operate under the supervision and medical direction
of referring physicians.
Esteemcare targets patients
with sleep obstructive disorders or related chronic illnesses who are insured by Medicare, Medicaid, third-party insurers, or have
the ability to pay for our products from their own private resources. Sleep apnea is a serious sleep disorder that occurs when
a person's breathing is interrupted during sleep. People with untreated sleep apnea stop breathing repeatedly during their sleep,
sometimes hundreds of times. This means the brain -- and the rest of the body -- may not get enough oxygen.
There are two types of sleep apnea:
• Obstructive sleep apnea
(OSA): The more common of the two forms of apnea, it is caused by a blockage of the airway, usually when the soft tissue in the
back of the throat collapses during sleep.
• Central sleep apnea: Unlike
OSA, the airway is not blocked, but the brain fails to signal the muscles to breathe, due to instability in the respiratory control
center.
Sleep apnea can affect anyone at any age, even
children. Risk factors for sleep apnea include:
• Being male
• Being overweight
• Being over age 40
• Having a large neck size
(17 inches or greater in men and 16 inches or greater in women
• Having large tonsils, a
large tongue, or a small jaw bone
• Having a family history
of sleep apnea
• Gastroesophageal reflux,
or GERD
• Nasal obstruction due to
a deviated septum, allergies, or sinus problems
What Are the Effects of Sleep Apnea?
If left untreated, sleep apnea can
result in a growing number of health problems, including:
• High blood pressure
• Stroke
• Heart failure, irregular
heart-beats, and heart attacks
• Diabetes
• Depression
• Worsening of ADHD
• Headaches
Facilities
The Company operates from
two (2) administrative locations: Columbia South Carolina, a 1,800 square foot commercial building and in Charleston, South Carolina,
a 1,200 square foot commercial building. The business leases are on month-to-month agreements with the monthly lease payments below
market average.
Employees
As of September 25, 2014,
Esteemcare has eight (8) full time employees, one (1) Office Manager, three (3) billing personnel, two (2) Respiratory Technicians,
one (1) accounting manager, and one (1) delivery person, None of these employees are covered by any collective bargaining agreement.
The Company presently considers its employee relations to be satisfactory.
Physicians Referrals- Customers
The Company's financial
success is directly related to maintaining the continued relationships with referring physicians and delivering a high excellence
of personal care and services to our clients and the willingness of new physicians and qualified clients to accept our organization
as the “Preferred Choice” to provide the CPAP equipment and respiratory supplies with the highest level of personal
care.
Medicare Competitive Bidding Areas
Esteemcare was awarded
contracts in 46 Competitive - MSA Bid Areas.
Product Category: CPAP
Devices, Respiratory Assist Devices, and Related Supplies
Major MSA Markets Awarded By Company:
Albany-Schenectady-Troy,
NY , Albuquerque, NM ,Asheville, NC ,Atlanta-Sandy Springs-Marietta, GA Augusta-Richmond County, GA-SC ,Baton Rouge, LA , Bridgeport-Stamford-Norwalk,
CT ,Bronx-Manhattan, NY ,Cape Coral-Fort Myers, FL , Charleston-North Charleston-Summerville, SC ,New Haven-Milford, CT ,New Orleans-Metairie-Kenner,
LA ,North East NY CBA Metro ,North Port-Bradenton-Sarasota, FL ,Northern NJ Metro CBA ,Ocala, FL , Oklahoma City, OK ,Omaha-Council
Bluffs, NE-IA , Palm Bay-Melbourne-Titusville, FL ,Phoenix-Mesa-Glendale, AZ ,Poughkeepsie-Newburgh-Middletown, NY Raleigh-Cary,
NC ,Colorado Springs, CO, Columbia, SC ,Deltona-Daytona Beach-Ormond, FL ,Denver-Aurora-Broomfield, CO, Greensboro-High Point,
NC , Greenville-Mauldin-Easley, SC ,Hartford-West Hartford-East Hartford, CT, Jacksonville, FL ,Lakeland-Winter Haven, FL ,Milwaukee-Waukesha-West
Allis, WI, Minneapolis-St. Paul-Bloomington, MN-WI, Nassau-Brooklyn-Queens-Richmond County Metro Richmond, VA ,Seattle-Tacoma-Bellevue,
WA ,Southern NY Metro CBA
Medicare Competitive Bidding Area Awards
Suffolk County, Syracuse,
NY ,Tampa-St. Petersburg-Clearwater, FL ,Tucson, AZ ,Tulsa, OK Virginia Beach-Norfolk-Newport News, VA-NC , Washington-Arlington-Alexandria,
DC-VA-MD-WV
The HME/DME-Respiratory Market
The U.S. domestic healthcare
spending is expected to increase by approximately $2.3 trillion from $2.7 trillion in 2011 to $5.0 trillion in 2022, according
to the Centers for Medicare and Medicaid Services (“CMS”). Revenues for CPAP Therapy to treat sleep disorders are projected
to grow at 15%-20% for the next 5 years. No other product line in undergoing this much consistent growth. Continuous Positive Airwave
Pressure (CPAP) is a treatment in which a mask is worn over the nose and/or mouth while you sleep. The mask is hooked up to a machine
that delivers a continuous flow of air into the nose. This air flow helps keep the airways open so that breathing is regular. CPAP
is considered by many experts to be the most effective treatment for sleep apnea.
As the baby boomer population
ages, CMS estimates that the number of Americans over the age of 65 will increase from an estimated 42.6 million in 2012 to 58.3
million in 2022. The Centers for Disease Control estimates that 80% of older adults have at least one chronic condition and 50%
have at least two. Esteemcare is medical distributor providing Durable and Home Medical CPAP products and related supplies. The
products that we distribute are classified as Durable Medical Equipment (“DME”). CMS estimates that the national expenditures
within the DME market will increase by over $27 billion from $38.9 billion in 2011 to $66.7 billion in 2022. The number of DME
companies billing Medicare less than $300,000 per year has been declining, or consolidating, over the last few years according
to HME News, primarily as a result of increased Medicare accreditation, bonding requirements and Medicare Competitive Bidding.
We have been able to attract new referral physicians and patients looking for new suppliers as a result of consolidation within
the DME market over the last few years.
Small medical distribution
companies can compete against national companies because Home Medical Equipment (HME) and Durable Medical Equipment (DME) pricing
is government regulated. Medical Device-network.com in January of 2012 reports, “The market is highly regulated, however,
transparent and ‘rules-based’, small and medium enterprises (SMEs) have traditionally played a crucial role in the
development of new products in the medical services, biotech and medical device industry. Because of their quick adaptability,
ability to identify market niches and considerable innovative potential, these firms form an important component of the healthcare
industry worldwide. Accounting for more than 50% of all pipeline products, they have a significant role in the future of the healthcare
industry”. According to the U.S. Census Bureau, the generation described as the "Baby Boom" is just now reaching
age 65. The United States' National Institutes of Health, National Institute for Aging projects that in the next fifteen (15) years
the population aged 65 to 84 years old will grow by roughly 50%, peaking in 2025. The combined U.S. market for home healthcare
products is expected to expand annually by nearly 7.0%. The Company anticipates an increased emphasis on medical equipment solutions
that reduce the cost of care and improve patient outcomes. Our medical distribution strategy
is to serve the growing demand for HME by building
a national and international distribution network with exclusive rights to products that are positioned to address the changing
industry requirements.
Key Market Segments Served
Skilled Nursing Facilities:
There are in excess of 17,000 skilled nursing facilities in US with 1.84 million beds which are projected to grow 10% per annum
through 2016. By 2020, a 65-year-old man could be expected to live to the age of 82 and a woman could be expected to live to the
age of 85. For those over the age of 65, there is a 41 percent chance that they will spend an average of 2.5 years in a skilled
nursing facility. A one-year stay in a nursing home can cost between $30,000 and $80,000.
Acute and Critical Care
Facilities: Although there has been a decline in the number of hospitals that provide acute care services in the United States,
there is an estimated 5,200 medical facilities and the total numbers of acute care facilities and out-patient surgery centers have
increased annually. This shift was in part the result of payer pricing pressures, declining physician reimbursements, and this
has t created an incentive for acute care hospitals to lower-costs and to establish physician-owned, out-patient surgery centers.
Assisted Living Facilities:
There are approximately 33,000 Assisted Living Facilities operating in the U.S. today. Nursing home spending is expected to grow
6.6 percent annually by 2018.
Hospitals and Medical Institutions:
There are well over 5,000 hospitals in the U.S. with approximately 1,000,000 beds. Although the short-term outlook for hospitals
is mixed, a common theme is to reduce the total cost of care, improve efficiencies and allow patients to remain in their home with
home based care as long as possible.
Federal Agencies: In fiscal
year 2008, the Department of Veterans Affairs (VA) spent about $4.1 billion on long-term care for veterans. The VA estimated a
budget increase of 165% between fiscal years 2008 and 2015 for institutional and non-institutional care.
Home Medical Care Industry:
It is estimated that in 2010 there were over 30,000 home health care facilities in the United States. Home health care spending
is projected to grow an average of 7.9 percent from 2013 to 2018.
Stroke Centers: There are
an estimated 500 stroke centers throughout the United States.
Burn Centers: There are
an estimated 120 burn centers throughout the United States.
Physical/Occupational Therapy
Centers: There are well over 5,000 physical therapy facilities throughout the United States. The demand for physical therapists
is expected grow by 30% over the next decade.
Esteemcare revenues are
derived from physician referrals, sleep centers, patient referrals, in-service set-ups, and by supplying CPAP products and supplies
to meet the growing requirements to combat severe/chronic sleep disorders. Customers meet with company Respiratory Technicians
and our therapists provide the appropriate CPAP device to the customer. We market our products directly to referral physicians,
insurance payers, and consumers through our direct push marketing efforts. We target consumers with chronic sleep obstructive conditions
and who require a continuous supply of medical products that provide attractive gross margins.
We receive initial contact
from prospective customers in the form of physician prescriptions. These referrals are then qualified and become new customers.
Our qualification efforts primarily involve verifying insurance eligibility, obtaining the required medical documentation from
the customer’s physician, and explaining our billing and collection processes, if applicable. The majority of the new customers
qualified from our process typically place their initial order with us within 24 -48 hours from the time we receive initial contact
from the physician and customer. Since our inception, we have demonstrated our ability to attract and retain customers with our
unique customer service that generates recurring revenues on supply parts that can last for periods of greater than two years.
Accounts Receivable
Our accounts receivable
are generally due from Medicare, Medicaid, private insurance companies, and our private patients. Accounts receivable are reported
net of allowances for contractual adjustments and uncollectible accounts. The collection process is time consuming, complex and
typically involves the submission of claims to multiple
layers of payers whose payment of claims may
be contingent upon the payment of another payer. As a result, our collection efforts may be active for up to 18 to 24 months from
the initial billing date. In accordance with regulatory requirements, we make reasonable and appropriate efforts to collect our
accounts receivable, including deductible and co-payment amounts, in a manner consistent for all classes of payers.
The Company has established
an allowance to account for contractual adjustments that result from differences between the payment amount received and the expected
realizable amount. These adjustments are recorded as a reduction of both gross revenues and accounts receivable. Based on our current
billing system, we are unable to directly compare the aggregate estimated allowance for contractual adjustments to the actual contractual
adjustments recorded. However, we do analyze the aggregate allowance for contractual adjustments as a percentage of net sales compared
to the last twelve months’ actual contractual adjustments as a percentage of net sales to determine that our estimated allowance
for contractual adjustments is a reasonable basis for recording our periodic allowance for contractual adjustments.
As a result of the Budget
Control Act of 2011, or sequestration, effective April 1, 2013, Medicare reduced payments for Part B Medicare claims by 2%, but
did not change the published allowable Medicare rates. Allowances for uncollectible accounts (or bad debts) are recorded as an
operating expense and consist of billed charges that are ultimately deemed uncollectible due to the customer’s or third-party
payer’s inability or refusal to pay. In establishing the appropriate allowance for uncollectible accounts, management makes
assumptions with respect to future collectability. We base our estimates of accounts receivable collectability on our historical
collection and write-off experience, our credit policies, and aging of our accounts receivable. Changes in judgment regarding these
factors will cause the level of accounts receivable allowances to be adjusted.
The typical collection
process begins with the electronic submission of a claim to Medicare, Medicaid, or other primary insurance carriers, for which
a response (and payment) is obtained within 15 to 30 days. Any claim denials are generally acted upon timely following the response
and, where applicable, corrected claims are submitted. A response (and co- payment) for amounts billed to secondary payers, including
Medicaid, private third-party insurance carriers, and patients, generally occurs within 30 to 60 days of submission of the claim.
On a continual basis, the outstanding accounts receivable balances are reviewed by collection personnel, including contacting the
insurance company and/or patient in an attempt to determine why payment has not been remitted and obtain payment from the respective
responsible party. When applicable, corrected claims are submitted to the insurance carrier. Patient statements are generated and
sent out monthly. Outbound calls are continually made to patients with outstanding balances in an attempt to obtain payment. Uncollectible
account balances for all payer classes are written off after remaining unpaid for a period of 24 months. Balances that are determined
to be uncollectible prior to the passage of 24 months from the last billing date are written off at the time of such determination.
We perform eligibility
and insurance verification on patients prior to the shipment of products and submission of a claim. As a result, we do not have
amounts that are pending approval from third-party payers outside of the typical review process for submitted claims.
Accounts receivable are
reported net of allowances for contractual adjustments and uncollectible accounts. Contractual adjustments are recorded against
revenues. Contractual adjustments result from differences between the payment amount received and the expected realizable amount.
Bad debt is recorded as an operating expense and consists of billed charges that are ultimately deemed uncollectible due to the
customer’s or third-party payer’s inability or refusal to pay.
The Company performs analyses
to evaluate the net realizable value of accounts receivable. Specifically, the Company considers historical realization data, accounts
receivable aging trends, other operating trends and relevant business conditions. Because of continuing changes in the health care
industry and third-party reimbursement, it is possible that the Company’s estimates could change, which could have a material
impact on the Company’s results of operations and cash flows. The Company does not accrue interest on its accounts receivable.
Revenue Recognition
We recognize revenue related
to product sales upon delivery to customers provided that we have received and verified any written documentation required to bill
Medicare, other government agencies, third-party payers, and patients. For product shipments for which we have not yet received
the required written documentation, revenue recognition is delayed until the period in which those documents are collected and
verified. We record revenue at the amounts expected
to be collected from government agencies, other
third-party payers, and from patients directly. We record, if necessary, contractual adjustments equal to the difference between
the reimbursement amounts defined in the fee schedule and the revenue recorded per the billing system. These adjustments are recorded
as a reduction of both gross revenues and accounts receivable. We analyze various factors in determining revenue recognition, including
a review of specific transactions, current Medicare regulations and reimbursement rates, historical experience and the credit-worthiness
of patients. Medicare reimburses at 80% of the government-determined prices for reimbursable supplies, and we bill the remaining
balance to either third-party payers or directly to patients.
Esteemcare- Payer Mix:
Medicare- 40%
Private Insurance- 26%
Blue Cross/Blue Shield of South Carolina-
17%
Private Pay 8%
Tricare/VA- 7%
Medicaid- 2%
Product Mix:
Sleep 95%- Oxygen 5%
Shipping and Handling Costs:
Shipping and handling:
Charges/costs are not allowed to be charged to the patients in compliance with Medicare policy.
Equipment Leases
The Company performs a
review of newly acquired equipment leases to determine whether a lease should be treated as a capital or operating lease. Capital
lease assets are capitalized and depreciated over the term of the initial lease. A liability equal to the present value of the
aggregated lease payments is recorded utilizing the stated lease interest rate. If an interest rate is not stated, the Company
will determine an estimated cost of capital and utilize that rate to calculate the present value. For equipment leases, the Company
records rent expense and amortization of leasehold improvements on a straight-line basis over the initial term of the lease.
Operating Leases
The Company leases various
software, and equipment under non-cancelable operating leases that expire at various times that extend through April 2016.
Proprietary Rights
We have entered into an
Employment Agreement with our President, Key Managers and have executed Non-Circumvent and Non-Disclosure agreements with key employees
that require them to keep all of our proprietary information and customers lists confidential. We cannot assure that such protections
will prove adequate should they be challenged in litigation.
Legal Proceedings
From time to time, we are
party to certain legal proceedings that arise in the ordinary course and are incidental to our business. There are currently no
such pending proceedings to which we are a party that our management believes will have a material adverse effect on the Company’s
financial position or results of operations. However, future events or circumstances, currently unknown to management, will determine
whether the resolution of pending or threatened litigation or claims will ultimately have a material effect on our consolidated
financial position, liquidity or results of operations in any future reporting periods.
ONCOLOGIX TECH EMPLOYEES
We currently employ a total
of 191 full and part-time employees. Our Chief Executive Officer operates a business office in Louisiana, our Chief Financial Officer
located in Michigan, our Medical Product Division President located in Arizona and our Health Services Division President in Louisiana.
Our Medical Device manufacturing segment has 1 full-time employee. Our Personal Care Services segment employs seventeen (17) full
time employees and one hundred and sixty-four (164) part-time employees, and Esteemcare Inc has six (6) full-time employees.. None
of these employees are covered by any collective bargaining agreement. The Company presently considers its employee relations to
be satisfactory.
Item 1A. Risk Factors
RISK FACTORS
Those interested in investing
in the Company should carefully consider the following Risk Factors pertaining to Oncologix Tech as well as the risks and uncertainties
that are described in the Company's most recent Annual and Quarterly Reports under the Securities Exchange Act of 1934. These Risk
Factors are not all inclusive.
Going Concern Qualification.
Our Independent Accountants
have expressed doubt about our ability to continue as a going concern. The ability to continue as a going concern is an issue raised
as a result of the material operating losses incurred since inception, and its stockholders' deficit. We expect to continue to
experience net operating losses. Our ability to continue as a going concern is subject to our ability to obtain necessary funding
from outside sources, including obtaining additional funding from the sale of our securities or obtaining loans from various financial
institutions where possible. The going concern increases the difficulty in meeting such goals.
Risk of Issued Series D Convertible Preferred
Stock to Common Shareholders
In March 2013, our Board
of Directors authorized up to 60,000 shares of Series D Convertible Preferred Stock. Each share of Series D Convertible stock has
a par value of $0.001 and is convertible into 1,000 shares of common stock beginning after March 1, 2014. In December 2013, our
Board of Directors increased the shares designated as Series D Convertible Preferred Stock to 200,000. Each share of Series D Convertible
Preferred Stock has a stated value of $80.25. Each shares of Series D Convertible Preferred Stock shall have voting rights as stated
next: March 1, 2013 to February 28, 2014, 400 votes per share; March 1, 2014 to February 28, 2015, 800 votes per share; March 1,
2015 to February 28, 2016, 1,200 votes per share; March 1, 2016 to February 28, 2017, 1,600 votes per share; March 1, 2017 and
after, 2,000 votes per share.
In the event of any liquidation,
dissolution or winding-up of the Corporation, the Series D Preferred Stock then issued and outstanding shall be entitled to be
paid out of the assets of the Corporation available for distribution to its shareholders in a position senior to the Corporation’s
Common Stock shareholders. The effect of these senior securities could affect the value of our common stock.
Our internal control over financial reporting
is not considered effective, our business and stock price could be adversely affected.
Section 404 of the
Sarbanes-Oxley Act of 2002 requires us to evaluate the effectiveness of our internal control over financial reporting as of the
end of each fiscal year, and to include a management report assessing the effectiveness of our internal control over financial
reporting in our annual report on Form 10-K for that fiscal year. Our management, including our chief executive officer and chief
financial officer, does not expect that our internal control over financial reporting will prevent all error and all fraud. As
of August 31, 2014, the Company identified two material weaknesses: a) Oncologix lacks the necessary corporate accounting resources
to maintain adequate segregation of duties; b) In addition, we have a lack of a functioning Audit Committee as we only have one
independent director is not considered a Financial Expert within the meaning of Section 407 of the Sarbanes-Oxley Act. We may experience
a loss of public confidence, which could have an adverse effect on our business and on the market price of our common stock due
to our internal control being ineffective.
Financial Condition of Dotolo Research Corporation (“DOTOLO”)
Dotolo Research Corporation
has limited working capital with little cash on hand at August 31, 2014. Since January 31, 2013, Dotolo has incurred indebtedness
of $50,000 to meet its working capital needs. These two notes bear interest at 18% per annum and require minimum, monthly interest
payments of $750. Additional financing will be required to get Dotolo to cash flow, break even.
Need for Additional Capital
We will need substantial
funds to procure raw materials for inventory, to complete the redesign of new product introductions, manufacturing, and the sales
and marketing of our products at Dotolo. Consequently, we will seek to raise further capital through not only possible public and
private offerings of equity and debt securities, but also collaborative arrangements, strategic alliances, and equity and debt
financings from other sources. Amian Angels operates with positive cash flow sufficient to service the business operations and
debt payment requirements of the acquisition but will need additional funds to complete the audit in addition to the costs for
other required SEC regulatory filings. We estimate the need to raise at least $500,000 of additional funding for working capital
and inventory procurement for Dotolo. Additionally, we estimate the need to raise at least $1,200,000 for overhead of Oncologix
Tech, Inc and debt servicing. We may be unable to raise additional capital on commercially acceptable terms, if at all, and if
we raise capital through additional equity financing, existing shareholders may have their ownership interests diluted. Our failure
to be able to generate adequate funds from operations or from additional sources would harm our business.
Uncertainties Regarding Healthcare Reimbursement
and Reform
Our ability to execute
our strategy in the medical markets depends in part on the extent to which healthcare services and products are paid by governmental
agencies, private health insurers and other organizations, such as health maintenance organizations, for the cost of such products
and related medical treatments. Our business could be harmed if healthcare payers and providers implement cost-containment measures
and if governmental agencies implement cost reduction measures that reduce payment to our customers for their use of our products.
Industry Intensely Competitive.
The medical device and
health services industry is intensely competitive. While we maintain a market share in hardware and disposable products , there
is no guarantee we can maintain or re-gain that market share. We will compete with both public and private medical device and pharmaceutical
companies that have a greater number of products on the market, have greater financial resources and have other competitive advantages.
We cannot be certain that one or more of our competitors will not receive patent protection that dominates, blocks or adversely
affects our product development or business; will benefit from significantly greater sales and marketing capabilities or will not
develop products that are accepted more widely than ours.
Healthcare Service Industry Intensely Competitive.
The healthcare service
industry is very competitive. We will compete with both public and private healthcare service companies that hold licenses within
the State of Louisiana and directly compete with companies that may have greater financial resources and have other competitive
advantages.
Intellectual Property Risk.
Our ability to obtain and
maintain patent and other protection for our products will affect our success. The patent positions of medical device companies
can be highly uncertain and involve complex legal and factual questions. Future patent rights, if granted, may not be upheld in
a court of law if challenged. Our patent rights may not provide competitive advantages for our products and may be challenged,
infringed upon or circumvented by our competitors. We cannot patent our products in all countries or afford to litigate every potential
violation worldwide. Because of the large number of patent filings in medical device, our competitors may have filed applications
or been issued patents and may obtain additional patents and proprietary rights relating to products or processes competitive with
or similar to ours. We cannot be certain that U.S. or foreign patents do not exist or will not issue that would harm our ability
to commercialize our products and product candidates.
Possible Failure to Comply with Government
Regulations.
We, and any prospective
contract manufacturers and suppliers are subject to extensive, complex, costly, and evolving governmental rules, regulations and
restrictions administered by the FDA, by other federal and state agencies, and by governmental authorities in other countries.
In the United States, our products are registered as a Class II device and cannot be marketed until they are approved for market
by the FDA. Obtaining FDA market approval involves the submission, among other information, may require clinical studies on the
product, and requires substantial time, effort and financial resources. The FDA, and other federal and state agencies, as well
as equivalent agencies of other countries with whom we will export our products, will also perform pre-licensing inspections of
our facility, if any, and our contract manufacturers' and suppliers' facilities. Our failure or the failure of our contract manufacturers
or suppliers to meet FDA or other agencies' requirements would delay or preclude our ability to sell our products potentially having
an adverse material effect on our business. Even with FDA market approval, we, as well as our partners, contract manufacturers
and suppliers, are subject to numerous FDA requirements covering, among other things, testing, manufacturing, quality control,
labeling and continuing review of medical products, and to permit government inspection at all times. Failure to meet or comply
with any rules, regulations, or restrictions of the FDA or other agencies could result in fines, unanticipated expenditures, product
delays, non-approval or recall, interruption of production, and criminal prosecution.
Exposure to Product Liability Claims.
Our design, testing, development,
manufacture, and marketing of products involve an inherent risk of exposure to product liability claims and related adverse publicity.
Although we believe that our product liability insurance is adequate, additional insurance coverage is expensive and in the future
we may be unable to obtain additional liability coverage on acceptable terms. If we are unable to obtain sufficient insurance at
an acceptable cost or if a successful product liability claim is made against us, whether fully covered by insurance or not, our
business could be harmed.
Reliance on Key Personnel
Our success will depend,
to a great extent, upon the experience, abilities and continued services of our executive officers and key management personnel.
If we lose the services of any of these officers or key personnel, our business could be harmed. Our success also will depend upon
our ability to attract and retain other highly qualified Regulatory, Marketing, Sales, and manufacturing personnel and our ability
to develop and maintain relationships with key individuals in the industry. Competition to attract qualified personnel and relationships
is intense and we compete with other companies in our industry. We may not be able to continue to attract and retain qualified
personnel.
Uncertainty as to our Ability to Initiate
Operations and Manage Growth.
Our efforts to market our
products will result in new and increased responsibilities for management personnel and will place a strain upon our management,
financial systems, and resources. We may be required to continue to implement and to improve our management, operating and financial
systems, procedures and controls on a timely basis and to expand, train, motivate and manage our employees. There can be no assurance
that our personnel, systems, procedures, and controls will be adequate to support our future operations.
Compliance with Government Regulations.
We, and all healthcare
service companies are subject to extensive, and evolving governmental rules, regulations and restrictions administered by the Department
of Health & Hospitals, the Bureau of Health Services Financing, by other federal and state agencies, and by governmental authorities.
Integration of Newly Acquired Businesses.
The Company may make strategic
acquisitions in the future and cannot assure that it will be able to successfully integrate the operations of newly-acquired businesses
into the Company's current operations. It is Management intent to consolidate various business functions to include Information
Technology, Accounting, legal under a central core operation. The failure to integrate newly acquired businesses or the inability
to make suitable strategic acquisitions in the future could have an adverse effect on the Company's business, results of operations
and financial condition.
Attraction and Retention of Qualified Personnel
The Company is dependent
on the efforts and abilities of its senior executive officers. While the Company believes that its senior management team has significant
experience and depth, appropriate senior management succession plans are in place. The Company's future success also depends on
its ability to identify, attract and retain additional qualified personnel.
Broker-Dealer Requirements May Affect Trading
and Liquidity of Our Common Stock
Section 15(g) of the Securities
Exchange Act of 1934, as amended, and Rule 15g-2 promulgated thereunder by the SEC require broker-dealers dealing in penny stocks
to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written
receipt of the document before effecting any transaction in a penny stock for the investor's account.
Potential investors in
the Registrant's common stock are urged to obtain and read such disclosure carefully before purchasing any shares that are deemed
to be "penny stock." Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor
for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i)
obtain from the investor information concerning his or her financial situation, investment experience and investment objectives;
(ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that
the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions;
(iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in
(ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects
the investor's financial situation, investment experience and investment objectives. Compliance with these requirements may make
it more difficult for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the
market or otherwise.
Item 1B. Unresolved Staff
Comments
None.
Item 2. Properties
Our Chief Executive Officer
operates out of his office in Louisiana and our Chief Financial Officer operates out of his home office in Michigan. Angels operates
from two (2) administrative locations: Lafayette, Louisiana, a 2,800 square foot commercial office and also Alexandria, Louisiana,
a 1,500 square foot residential home. The business lease in Alexandria, Louisiana in on month-to-month lease agreement and the
Lafayette, Louisiana administrative office is on a 5-year lease with both monthly lease payments negotiated below market average.
Item 3. Legal Proceedings
None.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s
Common Equity, Related Stockholder matters and Issuer Purchases of Equity Securities
Market for Common Stock
On May 19, 2011, the Company
affected a one-for-four reverse stock split. All share and per share information have been restated to retroactively show the effect
of this stock split. The reverse split was previously approved by a majority of the Company’s shareholders on March 24, 2011.
Our common stock is traded
on the OTC:QB under the symbol “OCLG”. The high and low close prices of the Company's common stock as reported for
the last two fiscal years, by fiscal quarter (i.e. first quarter = September 1 through November 30) were as follows:
| |
High |
Low |
| FISCAL YEAR ENDED: |
|
|
| August 31, 2014 |
|
|
| First Quarter |
$ 0.0250 |
$ 0.0071 |
| Second Quarter |
$ 0.0270 |
$ 0.0066 |
| Third Quarter |
$ 0.0088 |
$ 0.0055 |
| Fourth Quarter |
$ 0.0090 |
$ 0.0045 |
| |
|
|
| FISCAL YEAR ENDED: |
|
|
| August 31, 2013 |
|
|
| First Quarter |
$ 0.08 |
$ 0.05 |
| Second Quarter |
$ 0.07 |
$ 0.04 |
| Third Quarter |
$ 0.08 |
$ 0.03 |
| Fourth Quarter |
$ 0.05 |
$ 0.01 |
| |
|
|
The closing stock price
of our common stock on November 19, 2014, was $0.0042.
Issuer Repurchases of Equity Securities
We did not repurchase any
of our equity securities during the fourth quarter of the year ended August 31, 2014.
Holders
As of November 13, 2014,
the Company had 90 shareholders of record of its common stock. As of November 13, 2014, 1,342 owners of our common stock held them
in the names of various broker-dealers. As of November 13, 2014, the Company had one Unit holder of record. As of November
13, 2014, the Company had ten owners of Units who held them in the names of various brokers.
Dividend Policy
The Company has never declared
any cash dividends on any of the Company’s equity securities and currently plans to retain future earnings, if any, for business
growth.
Stock Performance Graph
As a smaller reporting
company, as defined in Rule 12b-2 promulgated under of the Securities Exchange Act of 1934, as amended, and in Item 10(f)(1) of
Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information
requested by this Item.
Equity Compensation
Plan Information
The following table sets
forth information regarding the number of shares of our common stock that may be issued pursuant to our equity compensation plans
or arrangements as of the end of fiscal 2014.
2000 Stock Incentive Plan
| |
Number of Securities |
|
|
|
Number of Securities |
| |
To Be Issued Upon |
|
Weighted Average |
|
Remaining Available |
| |
Exercise of Outstanding |
|
Exercise Price of |
|
For Future |
| |
Options |
|
Outstanding Options |
|
Issuance Under Plans |
| |
|
|
|
|
|
| Equity compensation plans |
|
|
|
|
|
| approved by stockholders |
6,173,750 |
|
$0.016 |
|
559,796 |
| |
|
|
|
|
|
| Equity compensation plans |
|
|
|
|
|
| not approved by stockholders |
- |
|
$0.000 |
|
- |
| |
|
|
|
|
|
| TOTAL |
6,173,750 |
|
$0.016 |
|
559,796 |
The Company is authorized
to issue up to 7,500,000 shares of common stock under its 2000 Stock Incentive Plan. Shares may be issued as incentive stock options,
non-statutory stock options, deferred shares or restricted shares. Options are granted at the fair market value of the common stock
on the date of the grant and have terms of up to ten years. The 2000 Stock Incentive Plan also provides for an annual grant of
options to members of our Board of Directors. For fiscal years ended August 31, 2012, 2011, 2010, 2009 and 2008, our Board of Directors
elected to waive the grant of these annual options. We have ,473,293 shares of common available for future issuance under our 2000
Stock Incentive Plan as of August 31, 2014. Under the 2000 Stock Incentive Plan the price of the granted common stock options are
equal to the fair market value of such shares on the date of grant. This plan has been approved by our shareholders.
2013 Omnibus Incentive Plan
| |
Number of Securities |
|
|
|
Number of Securities |
| |
To Be Issued Upon |
|
Weighted Average |
|
Remaining Available |
| |
Exercise of Outstanding |
|
Exercise Price of |
|
For Future |
| |
Options |
|
Outstanding Options |
|
Issuance Under Plans |
| |
|
|
|
|
|
| Equity compensation plans |
|
|
|
|
|
| approved by stockholders |
- |
|
$0.00 |
|
- |
| |
|
|
|
|
|
| Equity compensation plans |
|
|
|
|
|
| not approved by stockholders |
- |
|
$0.00 |
|
8,000,000 |
| |
|
|
|
|
|
| TOTAL |
- |
|
$0.00 |
|
8,000,000 |
The Company is authorized
to issue up to 10,000,000 shares of common stock under its 2013 Omnibus Incentive Plan to employees, officers, directors and consultants.
The issuance adoption of this plan has been approved by the Company’s Board of Directors on May 20, 2013. This plan has not
been approved by the Company’s shareholders and consequently, we cannot issue Incentive Stock Options to employees at this
time. Any options are granted at the fair market value of the common stock on the date of the grant and have terms of up to ten
years. We have 8,000,000 shares of common available for future issuance under our 2013 Omnibus Incentive Plan as of August 31,
2014. Under the 2013 Omnibus Incentive Plan the price of the granted common stock options are equal to the fair market value of
such shares on the date of grant.
For a description of our
2000 Stock Incentive Plan and 2013 Omnibus Incentive Plan, please see the description set forth in Note 8 of our Consolidated Financial
Statements.
Recent Sales of Unregistered
Securities
The following table contains
information regarding our sales of unregistered securities during the past two fiscal years.
| Date |
Securities |
|
Underwriters/ |
|
| Sold |
Sold |
Consideration |
Purchasers * |
Notes |
| |
|
|
|
|
| 10/15/2012 |
1,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 1,000,000 shares of common stock to a non-related accredited investor at $0.02 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 1/6/2013 |
2,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 2,000,000 shares of common stock to a non-related accredited investor at $0.01 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 2/8/2013 |
1,024,164 |
$ - |
Anthony Silverman, former CEO |
Anthony Silverman, our former President and CEO, converted a promissory note in the amount of $10,242 in principal and interest into 1,024,164 shares of common stock at $0.01 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/17/2013 |
2,000,000 |
$ 10,000 |
Accredited Investor |
The Company sold 2,000,000 shares of common stock to a non-related accredited investor at $0.005 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 7/17/2013 |
4,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 4,000,000 shares of common stock to a non-related accredited investor at $0.005 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 8/8/2013 |
6,000,000 |
$ 36,000 |
Accredited Investor |
The Company sold 6,000,000 shares of common stock to a non-related accredited investor at $0.006 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 9/12/2013 |
1,000,000 |
$ - |
Vendor |
The Company issued 1,000,000 S-8 shares to a vendor for consulting work. The Company recorded an expense of $11,500 upon the issuance of those shares. |
| 9/12/2013 |
1,500,000 |
$ 10,000 |
Accredited Investor |
The Company sold 1,500,000 shares of common stock to an affiliated accredited investor at $0.00667 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 10/3/2013 |
4,000,000 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $15,620 in principal and interest into 4,000,000 shares of common stock at $0.00391 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 12/3/2013 |
1,891,123 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $9,380 in principal and interest into 1,891,123 shares of common stock at $0.00496 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/3/2014 |
2,000,000 |
$ - |
Vendor |
The company issued 2,000,000 shares of common stock as consideration for services. The company recorded an expense of $22,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/13/2014 |
3,076,923 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $20,000 in principal and interest into 3,076,923 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/14/2014 |
1,000,000 |
$ - |
Vendor |
The company issued 1,000,000 shares of common stock as consideration for services. The company recorded an expense of $19,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/15/2014 |
117,436 |
$ - |
Accredited Investor |
Additional reset shares were issued to a non-affiliated accredited investor in connection with the prior conversion of $9,380 in principal and interest into 117,436 shares of common stock. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/21/2014 |
3,500,000 |
$ - |
Accredited Investor |
The company issued 3,500,000 shares of common stock as consideration for fees. The company recorded an expense of $45,500 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/21/2014 |
1,500,000 |
$ - |
Accredited Investor |
The company issued 1,500,000 shares of common stock as consideration for fees. The company recorded an expense of $30,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/31/2014 |
3,472,222 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $25,000 in principal and interest into 3,472,222 shares of common stock at $0.0072 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 2/7/2014 |
1,000,000 |
$ - |
Accredited Investor |
The company issued 1,000,000 shares of common stock as consideration for fees. The company recorded an expense of $9,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 2/24/2014 |
4,615,385 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $30,000 in principal and interest into 4,615,385 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 3/12/2014 |
4,615,385 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $30,000 in principal and interest into 4,615,385 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 4/7/2014 |
2,936,314 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $19,086 in principal and interest into 2,936,314 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 4/7/2014 |
5,383,007 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $17,764 in principal and interest into 5,383,007 shares of common stock at $0.0033 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/2/2014 |
5,000,000 |
$ - |
Accredited Investor |
The company issued 5,000,000 shares of common stock as consideration for services. The company recorded an expense of $30,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/25/2014 |
5,138,746 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $25,000 in principal and interest into 5,138,746 shares of common stock at $0.004865 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 7/14/2014 |
2,500,000 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $11,000 in principal and interest into 2,500,000 shares of common stock at $0.0044 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 7/24/2014 |
1,149,425 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,149,425 shares of common stock at $0.00435 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 8/1/2014 |
3,416,764 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $8,456 in principal and interest into 3,416,764 shares of common stock at $0.002475 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 8/26/2014 |
1,200,000 |
$ - |
Accredited Investor |
The Company sold 1,200,000 shares of common stock to an affiliated accredited investor at $0.00833 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 9/10/2014 |
1,058,201 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,058,201 shares of common stock at $0.004725 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 10/28/2014 |
1,473,622 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,473,622 shares of common stock at $0.003383 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 11/3/2014 |
1,508,296 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,508,296 shares of common stock at $0.003315 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 11/10/2014 |
1,724,733 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,724,733 shares of common stock at $0.002899 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 11/12/2014 |
6,000,000 |
|
Vendor |
The company issued 6,000,000 shares of common stock as consideration for services. The company recorded an expense of $24,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| |
|
|
|
|
| |
87,801,746 |
$ 116,000 |
|
|
| |
|
|
|
|
| * There were no underwriters associated with any of our Sales of Unregistered Securities. |
Transfer Agent
Our transfer agent and
registrar are American Stock Transfer and Trust Co., 6201 15th Avenue, Brooklyn, NY 11219, Telephone (718) 921-8200.
Item 6. Selected Financial Data
We are a “smaller
reporting company”, as defined by regulation S-K and as such, are not required to provide the information contained in this
item pursuant to Regulation S-K.
Item 7. Management’s
discussion and Analysis of Financial Condition and Results of Operations
The following discussion
and analysis should be read in conjunction with our Consolidated Financial Statements and notes appearing elsewhere in this Report.
RECENT ACQUISITIONS AND
DIVESTURES
In furthering our strategy
to be the best small cap medical device and healthcare holding company, we acquired Dotolo Research Corporation (“DOTOLO”)
on March 22, 2013, Angels of Mercy, Inc. (“AOM”) on August 1, 2013, Amian Health Services on December 10, 2013, and
Esteemcare Inc. on September 25, 2014. These acquisitions are further described in Note 4 – Acquisition Activities, in the
Notes to Consolidated Financial Statements appearing in Item 8 of this Form 10-K.
RESULTS OF OPERATIONS
Comparison of the fiscal years ended
August 31, 2014 (‘fiscal 2014”) and 2013 (‘fiscal 2013”)
Revenue
Revenues increased to $3,677,475 for fiscal
2014, from $244,246 from the comparable period in fiscal 2013. Revenues were driven by sales from Amian Angels Inc.
Cost of Revenues
Cost of revenues increased
to $3,063,064 for fiscal 2014, from $195,699 during the comparable period in fiscal 2013. Cost of revenues for Dotolo were $46,843
for fiscal 2014 and consist primarily of direct labor and minor purchases of materials for our new product development . Cost of
revenues for Amian Angels were $3,016,221 for fiscal 2014 and consist primarily of wages paid to personal care service employees
who directly provide the PCA and SIL services.
Research and Development Expense
Research and development expense increased to
$30,000 for fiscal 2014, from $0 during the comparable period in fiscal 2013. Research and development expenses consisted of costs
for the development of dies and molds for Dotolo’s Toxygen product..
General and Administrative Expense
General and administrative
expenses primarily include officer and administrative salaries, office rent, utilities, legal and accounting services, insurance,
public filing costs as well as other incidental overhead costs.
General and administrative
expense increased to $1,133,431 during fiscal 2014, from $329,667, an increase of over 100% or $803,764 from the comparable period
in fiscal 2013. The primary reason for the increase is due to the general and administrative expenses associated with full years
expenses of Dotolo and Amian Angels, whose companies were acquired late fiscal 2013. Payroll and related expenses increased to
$305,698 during the fiscal 2014, from $193,029 in the comparable period in fiscal 2013, due primarily to the hiring of our CEO,
President of Amian Angels, President of our Medical Products division and administrative salaries at Amian Angels. Legal expense
increased to $68,675 fiscal
2014, from $16,531 in the comparable
period in fiscal 2013, due primarily to legal fees incurred in fiscal 2014 related to our acquisition of Amian Health Services
and legal fees related to our senior lender borrowing. Rent expense increased to $80,329 during fiscal 2014, from $15,500 in the
comparable period in fiscal 2013, as a result of facilities rented by both Dotolo and Amian Angels which were acquired in late
fiscal 2013. Insurance expense increased to $67,316 during fiscal 2014, from $14,006 in the comparable period in fiscal 2013, due
primarily to workers compensation and facility insurance from Amian Angels. Stock Based Compensation increased to $91,163 during
fiscal 2014, from $0 in the comparable period in fiscal 2013, due to the issuance of stock options to the company’s senior
management. Investor Relation and press release expense increased to $84,870 during fiscal 2014, from $299 during the comparable
period in fiscal 2013 primarily as a result of additional press releases and investor relations contracts entered into during fiscal
2014. Bad debt expense increased to $120,456 during fiscal 2014, from $0 during the comparable period in fiscal 2013 as a result
of write offs of uncollectible receivables from Amian Angels.
Depreciation and Amortization
Depreciation and amortization
increased to $22,819 during fiscal 2014, from $5,455 during fiscal 2013. The increase in depreciation and amortization was the
result of fixed assets acquired in our two fiscal 2013 and one fiscal 2014 acquisitions.
Interest Income
We had no interest income
in fiscal 2014 or fiscal 2013.
Interest and Finance Charges
Interest and finance charges
increased to $805,618 during fiscal 2014 from $34,181, an increase of over 100% from fiscal 2013. The increase is primarily attributable
to the acquisition of additional non-related party debt and convertible debt incurred during fiscal 2014.
Interest and finance charges
– related parties decreased to $3,192 during fiscal 2014, from $152,087, a decrease of 98% or $148,895 for the comparable
period in fiscal 2013. The decrease is primarily attributable to reclassifying former related party debt as non-related during
fiscal 2014.
A summary of interest and finance charges is
as follows:
| |
|
|
|
|
|
For the Year Ended |
| |
|
|
|
|
|
August 31, |
|
August 31, |
| |
|
|
|
|
|
2014 |
|
2013 |
| Interest expense on non-convertible notes |
$ 264,339 |
|
$ 4,734 |
| Interest expense on non-convertible notes - related parties |
3,192 |
|
579 |
| Interest expense on convertible notes payable |
81,474 |
|
10,000 |
| Interest expense on convertible notes payable - related parties |
- |
|
14,102 |
| Amortization of note payable discounts |
108,325 |
|
- |
| Amortization of note payable discounts - related parties |
- |
|
- |
| Other interest and finance charges |
351,480 |
|
156,853 |
| |
|
|
|
|
|
|
|
|
| Total interest and finance charges |
$ 808,810 |
|
$ 186,268 |
Loss on Conversion of Notes Payable
Loss on conversion of notes
payable increased to $137,505 during fiscal 2014, from $10,242, for the comparable period in fiscal 2013. The increase was due
to the issuance of shares of common stock for the conversions of a non-related party convertible promissory notes during the fiscal
2014 at below market value.
Income Taxes
At August 31, 2014, the
Company had federal net operating loss carry forwards totaling approximately $25,100,000. At August 31, 2014, the Company did not
have any state net operating loss carry forwards. The federal net
operating loss carry forwards expire in various
amounts beginning in 2018 and ending in 2035. Due to our history of incurring losses from operations, we have provided a valuation
allowance for our net operating loss carry forward.
LIQUIDITY AND CAPITAL RESOURCES
Since March 2013, we have
acquired three companies. While these acquisitions greatly increased the value of our Company, we are not fully cash flow positive.
Cash flows from the issuances of promissory notes and common stock for cash are not sufficient to meet our working capital requirements
for the foreseeable future or provide for expansion opportunities. We incurred $1,407,571 and $653,459 in net losses, and we used
$629,942 and $105,817 in cash for operations for the years ended August 31, 2014 and 2013, respectively. Investing activities used
$67,232 and $71,226 for the years ended August 31, 2014 and 2013, respectively. Net cash generated from financing activities for
the years ended August 31, 2014 and 2013 was $675,222 and $214,568, respectively. As of August 31, 2014, we had a cash balance
of $17,504. These conditions raise substantial doubt about our ability to continue as a going concern.
We anticipate that we will
require approximately $1,500,000 to operate through August 31, 2015. Approximately $1,000,000 will be required to fund corporate
overhead including debt servicing with the balance to invest into raw material inventory, manufacturing and product revisions at
Dotolo. Additional funding will allow us to meet our current sales demands and expenses of Dotolo, Amian Angels and Oncologix,
while keeping our public filings current.
As of August 31, 2014,
we had total outstanding short-term and long-term debt and liabilities totaling $2,800,176 net of discounts. Please see Note 8
for further information.
CRITICAL ACCOUNTING POLICIES
“The preparation
of financial statements in accordance with accounting standards generally accepted in the United States requires management to
make estimates and assumptions that affect both the recorded values of assets and liabilities at the date of the financial statements
and the revenues recognized and expenses incurred during the reporting period. Our estimates and assumptions affect our recognition
of income taxes, the carrying value of our long-lived assets and our provision for certain contingencies. We evaluate the reasonableness
of these estimates and assumptions continually based on a combination of historical information and other information that comes
to our attention that may vary our outlook for the future. Actual results may differ from these estimates under different assumptions.
We suggest that the Summary
of Significant Accounting Policies, as described in Note 2 of our Consolidated Financial Statements, be read in conjunction with
this Management’s Discussion and Analysis of Financial Condition and Results of Operations. We believe the critical accounting
policies that most impact our consolidated financial statements are described in Note 3 of our Consolidated Financial Statements.
CODE OF ETHICS
Our
Board of Directors has adopted a code of ethics that applies to our principal executive officer, principal financial officer and
to other persons performing similar functions. The code of ethics is designed to deter wrongdoing and to promote honest and ethical
conduct, full, fair, accurate, timely and understandable disclosure, compliance with applicable laws, rules and regulations, prompt
internal reporting of violations of the code and accountability for adherence to the code. We will provide a copy of our code of
ethics, without charge, to any person upon receipt of written request for such delivered to our corporate headquarters. All such
requests should be send to Oncologix Tech, Inc., P.O. Box 8832, Grand Rapids, MI 49518-8832.
OFF-BALANCE SHEET ARRANGEMENTS
We do not maintain off-balance sheet arrangements
nor do we participate in any non-exchange traded contracts requiring fair value accounting treatment. The Company has no off-balance
sheet arrangements as of August 31, 2014 and 2013.
NEW ACCOUNTING STANDARDS
We have evaluated all Accounting
Standards Updates through the date the financial statements were issued and do not believe any will have a material impact. For
details of new accounting standards, please refer to Note 17 of our Consolidated Financial Statements.
Item 7A. Quantitative and Qualitative Disclosures
About Market Risk
As a smaller reporting
company, as defined in Rule 12b-2 promulgated under of the Securities Exchange Act of 1934, as amended, and in Item 10(f)(1) of
Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information
requested by this Item.
Item 8. Financial Statements and Supplementary
Data
Oncologix Tech, Inc. and Subsidiaries
Consolidated Financial Statements
Year Ended August 31, 2014 and 2013
Contents
| Report of Seale and Beers, CPAs, Independent Registered Public Accounting Firm |
F-1 |
| |
|
| Audited Financial Statements |
|
| |
|
| Consolidated Balance Sheets |
F-3 |
| Consolidated Statements of Operations |
F-4 |
| Consolidated Statements of Changes in Stockholders' Deficit |
F-5 |
| Consolidated Statements of Cash Flows |
F-6 |
| Notes to Consolidated Financial Statements |
F-7 |

PCAOB Registered Auditors – www.sealebeers.com
REPORT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
To the Board of Directors and Stockholders
of
Oncologix Tech, Inc.
We have audited the accompanying balance sheets
of Oncologix Tech, Inc.as of August 31, 2013 and 2014, and the related statements of income, stockholders’ equity (deficit),
and cash flows for each of the years in the two-year period ended August 31, 2014. Oncologix’s management is responsible
for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with
the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform
the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company
is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included
consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the
circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over
financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting
the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made
by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable
basis for our opinion.
In our opinion, the financial statements referred
to above present fairly, in all material respects, the financial position of Oncologix Tech, Inc. as of August 31, 2013 and 2014,
and the related statements of income, stockholders’ equity (deficit), and cash flows for each of the years in the two-year
period ended August 31, 2014 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have
been prepared assuming that the Company will continue as a going concern. As discussed in Note 3 to the financial statements, the
Company has negative working capital at August 31, 2014, has incurred recurring losses and recurring negative cash flow from operating
activities, and has an accumulated deficit which raises substantial doubt about its ability to continue as a going concern. Management’s
plans concerning these matters are also described in Note 3. The financial statements do not include any adjustments that might
result from the outcome of this uncertainty.
/s/ Seale and Beers, CPAs
Seale and Beers, CPAs
Las Vegas, Nevada
December 5, 2014
8250
W Charleston Blvd, Suite 100 - Las Vegas, NV 89117 Phone: (888)727-8251 Fax: (888)782-2351
ONCOLOGIX TECH,
INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE
SHEETS
AUGUST 31, 2014 AND 2013
| | |
| |
|
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| | |
| |
|
| ASSETS |
| |
| |
|
| Current Assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 17,504 | | |
$ | 39,456 | |
| Accounts receivable (net of allowance of $9,000) | |
| 213,399 | | |
| 108,319 | |
| Inventory | |
| 31,271 | | |
| 31,271 | |
| Prepaid expenses and other current assets | |
| 9,307 | | |
| 39,176 | |
| Prepaid commissions and finders' fees | |
| 3,152 | | |
| — | |
| | |
| | | |
| | |
| Total current assets | |
| 274,633 | | |
| 218,222 | |
| | |
| | | |
| | |
| Property and equipment (net of accumulated depreciation | |
| | | |
| | |
| of $28,264 and $11,820) | |
| 39,967 | | |
| 78,533 | |
| Deposits and other assets | |
| 14,582 | | |
| 10,050 | |
| Goodwill | |
| 1,781,779 | | |
| 1,696,425 | |
| Patents, registrations (net of amortization of $97,983 and $91,859) | |
| 24,497 | | |
| 30,620 | |
| | |
| | | |
| | |
| Total assets | |
$ | 2,135,458 | | |
$ | 2,033,850 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS' DEFICIT | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | |
| Convertible notes payable (net of discount of $99,491 and $0) | |
$ | 436,308 | | |
$ | 360,025 | |
| Notes payable (net of discount of $18,596 and $0) | |
| 946,104 | | |
| 138,494 | |
| Notes payable - related parties | |
| 51,600 | | |
| 56,200 | |
| Accounts payable and other accrued expenses | |
| 732,934 | | |
| 895,664 | |
| Accrued interest payable | |
| 148,681 | | |
| 66,969 | |
| Accrued interest payable - related parties | |
| 6,342 | | |
| 65,743 | |
| Current portion of long term debt | |
| 82,532 | | |
| — | |
| | |
| | | |
| | |
| Total current liabilities | |
| 2,404,501 | | |
| 1,583,095 | |
| | |
| | | |
| | |
| Long-term liabilities: | |
| | | |
| | |
| Notes payable (net of current portion) | |
| 395,675 | | |
| 811,500 | |
| Convertible notes payable | |
| — | | |
| — | |
| | |
| | | |
| | |
| Total long-term liabilities | |
| 395,675 | | |
| 811,500 | |
| | |
| | | |
| | |
| Total liabilities | |
| 2,800,176 | | |
| 2,394,595 | |
| | |
| | | |
| | |
| Stockholders' Deficit: | |
| | | |
| | |
| Series A Preferred stock, par value $.001 per share; 10,000,000 shares | |
| | | |
| | |
| authorized; 129,062 and 129,062 shares issued and outstanding at | |
| | | |
| | |
| August 31, 2014 and August 31, 2013, respectively | |
| 129 | | |
| 129 | |
| Series D Preferred stock, par value $.001 per share; 10,000,000 shares | |
| | | |
| | |
| authorized; 78,564 and 58,564 shares issued and outstanding at | |
| | | |
| | |
| August 31, 2014 and August 31, 2013, respectively | |
| 79 | | |
| 59 | |
| Common stock, par value $.001 per share; 750,000,000 shares authorized; | |
| | | |
| | |
| 134,600,152 and 74,587,422 shares issued and outstanding at | |
| | | |
| | |
| August 31, 2014 and August 31, 2013, respectively | |
| 134,600 | | |
| 74,587 | |
| Additional paid-in capital | |
| 47,565,869 | | |
| 58,560,265 | |
| Accumulated deficit prior to reentering development stage | |
| (48,370,395 | ) | |
| (58,992,296 | ) |
| Common stock subscribed | |
| 5,000 | | |
| — | |
| Noncontrolling interest | |
| — | | |
| (3,489 | ) |
| | |
| | | |
| | |
| Total stockholders' deficit | |
| (664,718 | ) | |
| (360,745 | ) |
| | |
| | | |
| | |
| Total liabilities and stockholders' deficit | |
$ | 2,135,458 | | |
$ | 2,033,850 | |
The accompanying notes are an integral part
of these consolidated financial statements.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| | |
For the Year Ended |
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| | |
| |
|
| Revenues | |
$ | 3,677,475 | | |
$ | 244,246 | |
| | |
| | | |
| | |
| Cost of revenues | |
| 3,063,064 | | |
| 195,699 | |
| | |
| | | |
| | |
| Gross profit | |
| 614,411 | | |
| 48,547 | |
| | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | |
| General and administrative | |
| 1,133,431 | | |
| 329,667 | |
| Research and development expense | |
| 30,000 | | |
| — | |
| Depreciation and amortization | |
| 22,819 | | |
| 5,455 | |
| | |
| | | |
| | |
| Total operating expenses | |
| 1,186,250 | | |
| 335,122 | |
| | |
| | | |
| | |
| Loss from operations | |
| (571,839 | ) | |
| (286,575 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| Acquisition costs | |
| — | | |
| (173,864 | ) |
| Interest and finance charges | |
| (805,618 | ) | |
| (34,181 | ) |
| Interest and finance charges - related parties | |
| (3,192 | ) | |
| (152,087 | ) |
| Loss on conversion of notes payable - related parties | |
| (137,505 | ) | |
| (10,242 | ) |
| Loss on disposal of assets | |
| (28,748 | ) | |
| — | |
| Other income (expenses) | |
| 139,331 | | |
| 3,490 | |
| | |
| | | |
| | |
| Total other income (expense) | |
| (835,732 | ) | |
| (366,884 | ) |
| | |
| | | |
| | |
| Loss from continuing operations | |
| (1,407,571 | ) | |
| (653,459 | ) |
| | |
| | | |
| | |
| Discontinued operations | |
| | | |
| | |
| Operating loss from discontinued operations | |
| (36 | ) | |
| (14 | ) |
| Gain on disposal of discontinued operations | |
| 95,564 | | |
| — | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Gain from discontinued operations | |
| 95,528 | | |
| (14 | ) |
| | |
| | | |
| | |
| Less loss attributable to noncontrolling interest | |
| — | | |
| — | |
| | |
| | | |
| | |
| Net gain from discontinued operations | |
| 95,528 | | |
| (14 | ) |
| | |
| | | |
| | |
| Net loss before income taxes | |
| (1,312,043 | ) | |
| (653,473 | ) |
| | |
| | | |
| | |
| Income taxes | |
| — | | |
| — | |
| | |
| | | |
| | |
| Net loss attributable to common shareholders | |
$ | (1,312,043 | ) | |
$ | (653,473 | ) |
| | |
| | | |
| | |
| Gain (loss) per common share, basic and diluted: | |
| | | |
| | |
| Continuing operations | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
| Discontinued operations | |
| 0.00 | | |
| (0.00 | ) |
| | |
| | | |
| | |
| | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
| | |
| | | |
| | |
| Weighted average number of shares | |
| | | |
| | |
| outstanding - basic and diluted | |
| 102,556,908 | | |
| 61,864,435 | |
The accompanying notes are an integral part
of these consolidated financial statements.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS
OF CHANGES IN STOCKHOLDERS’ DEFICIT
FOR THE PERIOD SEPTEMBER 1, 2013 THROUGH
AUGUST 31, 2014
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
|
| | |
Series A | |
Series D | |
| |
| |
Additional | |
| |
Non- | |
Common | |
|
| | |
| Preferred Stock | | |
| Preferred Stock | | |
Common Stock | | |
| Paid-in | | |
| Accumulated | |
|
|
Controlling |
|
|
|
Stock |
|
|
|
|
|
| | |
| Shares | | |
| Amount | | |
| Shares | | |
| Amount | | |
| Shares | | |
| Amount | | |
| Capital | | |
| Deficit | | |
| Interest | | |
| Subscribed | | |
| Total | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, August 31, 2012 | |
| 129,062 | | |
$ | 129 | | |
| — | | |
$ | — | | |
| 57,563,258 | | |
$ | 57,563 | | |
$ | 57,697,233 | | |
$ | (58,338,851 | ) | |
$ | (3,475 | ) | |
$ | — | | |
$ | (587,401 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of stock purchased for cash | |
| — | | |
| — | | |
| — | | |
| — | | |
| 15,000,000 | | |
| 15,000 | | |
| 91,000 | | |
| — | | |
| — | | |
| — | | |
| 106,000 | |
| Issuance of stock for fees | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,000,000 | | |
| 1,000 | | |
| 7,000 | | |
| — | | |
| — | | |
| — | | |
| 8,000 | |
| Issuance of preferred stock | |
| — | | |
| — | | |
| 58,564 | | |
| 59 | | |
| | | |
| | | |
| 585,581 | | |
| — | | |
| — | | |
| — | | |
| 585,640 | |
| Conversion of notes payable | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,024,164 | | |
| 1,024 | | |
| 9,217 | | |
| — | | |
| — | | |
| — | | |
| 10,241 | |
| Issuance of warrants for finance charges | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 159,992 | | |
| — | | |
| — | | |
| — | | |
| 159,992 | |
| Loss on conversion of notes payable - | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| related parties | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 10,242 | | |
| — | | |
| — | | |
| — | | |
| 10,242 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (653,445 | ) | |
| (14 | ) | |
| — | | |
| (653,459 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, August 31, 2013 | |
| 129,062 | | |
$ | 129 | | |
| 58,564 | | |
$ | 59 | | |
| 74,587,422 | | |
$ | 74,587 | | |
$ | 58,560,265 | | |
$ | (58,992,296 | ) | |
$ | (3,489 | ) | |
$ | — | | |
$ | (360,745 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Issuance of stock purchased for cash | |
| — | | |
| — | | |
| — | | |
| — | | |
| 2,700,000 | | |
| 2,700 | | |
| 17,300 | | |
| — | | |
| — | | |
| — | | |
| 20,000 | |
| Issuance of stock for fees | |
| — | | |
| — | | |
| — | | |
| — | | |
| 15,000,000 | | |
| 15,000 | | |
| 150,500 | | |
| — | | |
| — | | |
| — | | |
| 165,500 | |
| Stock based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 91,163 | | |
| — | | |
| — | | |
| — | | |
| 91,163 | |
| Issuance of preferred stock for fees | |
| — | | |
| — | | |
| 20,000 | | |
| 20 | | |
| — | | |
| — | | |
| 149,980 | | |
| — | | |
| — | | |
| — | | |
| 150,000 | |
| Acquisition of business assets | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| Conversion of notes payable | |
| — | | |
| — | | |
| — | | |
| — | | |
| 42,312,730 | | |
| 42,313 | | |
| 174,112 | | |
| — | | |
| — | | |
| 5,000 | | |
| 221,425 | |
| Loss on conversion of notes payable | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 137,387 | | |
| — | | |
| — | | |
| — | | |
| 137,387 | |
| Beneficial conversion feature - notes | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| payable | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 140,445 | | |
| — | | |
| — | | |
| — | | |
| 140,445 | |
| Issuance of warrants for fees and | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| finance charges | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| 82,192 | | |
| — | | |
| — | | |
| — | | |
| 82,192 | |
| Disposition of subsidiary | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (11,937,475 | ) | |
| 12,029,472 | | |
| 3,489 | | |
| — | | |
| 95,486 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (1,407,571 | ) | |
| — | | |
| — | | |
| (1,407,571 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, August 31, 2014 | |
| 129,062 | | |
$ | 129 | | |
| 78,564 | | |
$ | 79 | | |
| 134,600,152 | | |
$ | 134,600 | | |
$ | 47,565,869 | | |
$ | (48,370,395 | ) | |
$ | — | | |
$ | 5,000 | | |
$ | (664,718 | ) |
The accompanying notes are an integral part
of these consolidated financial statements
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | |
|
|
|
|
|
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| Operating activities: | |
| | | |
| | |
| Net loss | |
$ | (1,312,043 | ) | |
$ | (653,459 | ) |
| | |
| | | |
| | |
| Net gain from discontinued operations | |
| (95,528 | ) | |
| — | |
| | |
| | | |
| | |
| Net loss from continuing operations | |
| (1,407,571 | ) | |
| (653,459 | ) |
| | |
| | | |
| | |
| Adjustments to reconcile net loss to net cash used | |
| | | |
| | |
| in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 22,819 | | |
| 5,456 | |
| Loss on disposal of property and equipment | |
| 28,748 | | |
| — | |
| Stock based compensation | |
| 91,163 | | |
| — | |
| Amortization of discount on notes payable and warrants | |
| 108,325 | | |
| — | |
| Loss on conversion of notes payable - related parties | |
| 137,387 | | |
| 10,241 | |
| Bad debt expense | |
| 124,231 | | |
| — | |
| Non-cash interest charges | |
| 49,093 | | |
| — | |
| Issuance of debt for fees | |
| — | | |
| 65,000 | |
| Issuance of stock and warrants for fees | |
| 397,691 | | |
| 145,406 | |
| | |
| | | |
| | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (229,311 | ) | |
| 4,031 | |
| Prepaid expenses and other current assets | |
| 29,869 | | |
| 9,566 | |
| Prepaid commissions and finders' fees | |
| (3,152 | ) | |
| — | |
| Inventory | |
| — | | |
| 69,610 | |
| Deposits and other assets | |
| (4,532 | ) | |
| — | |
| Accounts payable and other accrued expenses | |
| (106,466 | ) | |
| 206,591 | |
| Accrued interest payable - related parties | |
| (59,401 | ) | |
| 17,090 | |
| Accrued interest payable | |
| 95,637 | | |
| 14,651 | |
| | |
| | | |
| | |
| Net operating cash flows - continuing operations | |
| (725,470 | ) | |
| (105,817 | ) |
| | |
| | | |
| | |
| Net operating cash flows - discontinued operations | |
| 95,528 | | |
| — | |
| | |
| | | |
| | |
| Net cash used in operating activities | |
| (629,942 | ) | |
| (105,817 | ) |
| | |
| | | |
| | |
| Investing activities: | |
| | | |
| | |
| Purchase of property and equipment | |
| (878 | ) | |
| — | |
| Cash acquired with Amian Health Services assets | |
| 8,646 | | |
| — | |
| Acquisition of Amian Health Services assets | |
| (75,000 | ) | |
| 1,653 | |
| Acquisition of Angels subsidiary | |
| — | | |
| (72,879 | ) |
| | |
| | | |
| | |
| Net cash used in investing activities | |
| (67,232 | ) | |
| (71,226 | ) |
| | |
| | | |
| | |
| Financing activities: | |
| | | |
| | |
| Proceeds from issuance of convertible notes | |
| 250,000 | | |
| — | |
| Proceeds from issuance of notes payable - related parties | |
| — | | |
| 33,361 | |
| Proceeds from issuance of notes payable | |
| 1,451,628 | | |
| 120,000 | |
| Proceeds from the issuance of common stock | |
| 10,000 | | |
| 106,000 | |
| Repayment of notes payable | |
| (1,031,806 | ) | |
| (25,917 | ) |
| Repayment of notes payable - related parties | |
| (4,600 | ) | |
| (18,876 | ) |
| Repayment of convertible notes payable | |
| — | | |
| — | |
| | |
| | | |
| | |
| Net cash provided by financing activities | |
| 675,222 | | |
| 214,568 | |
| | |
| | | |
| | |
| Net increase (decrease) in cash and cash equivalents | |
| (21,952 | ) | |
| 37,525 | |
| | |
| | | |
| | |
| Cash and cash equivalents, beginning of period | |
| 39,456 | | |
| 1,931 | |
| | |
| | | |
| | |
| Cash and cash equivalents, end of period | |
$ | 17,504 | | |
$ | 39,456 | |
The accompanying notes are an integral part
of these consolidated financial statements.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CONSOLIDATED
RESTATED STATEMENTS OF CASH FLOWS (CONTINUED)
Supplemental disclosure
of cash flow information (continued):
| | |
For the Year Ended |
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| | |
| |
|
| Cash paid during the year for: | |
| | | |
| | |
| | |
| | | |
| | |
| Interest | |
$ | 79,930 | | |
$ | 4,822 | |
| Income Taxes | |
$ | — | | |
$ | — | |
The accompanying notes are an integral part
of these consolidated financial statements.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1
- ORGANIZATION AND DESCRIPTION OF THE COMPANY
Oncologix Tech, Inc. is
a diversified medical holding company with operating segments in medical device, healthcare services and medical products and technologies.
We operate and manufacture Class II medical device products, delivers Personal Healthcare Services nationally and provides Home
Medical Equipment (HME) and Durabable Medical Equipment (DME) sales in licensed markets. For its clients, Oncologix provides FDA
approved medical devices, State licensed healthcare services and medical product sales. For its shareholders, Oncologix operates
profitable business divisions that build, maintain and nourish shareholder value. The Company’s corporate mission is to be
the best small cap medical device and healthcare services holding company in North America.
We were originally formed
in 1995 and in 2000 we changed our name to "BestNet Communications Corp." At that time we provided worldwide long distance
telephone communication and teleconferencing services to commercial and residential consumers through the internet, which we disposed
of in 2007 due to lack of profitability. In July 2006 we changed our business model to medical device products. In July 2006
we acquired JDA Medical Technologies, Inc. ("JDA") and merged this business into Oncologix Corporation, our wholly owned
subsidiary. On January 22, 2007, we changed our name to Oncologix Tech, Inc., to reflect this new business model. Our business
at this time was the development of a medical device for brachytherapy (radiation therapy), called the “Oncosphere”
(or “Oncosphere System”), for the advanced medical treatment of soft tissue cancers. Due to a lack of funding, we suspended
these development activities on December 31, 2007. On November 1, 2013, due to the development of the brachytherapy device being
several years away, indication that the product could not be marketed and no guarantee of FDA approvals, it was determined that
continued financial support of this product by Oncologix Corporation would cost the Company substantial capital beyond its
means and the Company’s management and Board of Directors disposed of Oncologix Corporation and its Brachytherapy medical
device subsidiary. Furthermore, as part of the disposal, the Company was relieved of over $90,000 in debt.
On March 22, 2013, we acquired
all the outstanding stock of Dotolo Research Corporation (“Dotolo”), a FDA Registered, Class II, medical device manufacturer
with 25 years of product sales in the hydro-colonic irrigation, bowel preparation market. Dotolo Research Corporation began operations
in 1989 and sells hardware and disposable products to a customer base of over 900+ customers both domestically and internationally.
The Company currently operates in a limited, but competitive environment in hydro-colonic irrigation, of which there are only four
(4) companies approved by the FDA to manufacture a Class II medical device for colonic-hydro therapy. Since the acquisition,
we have not had significant revenues from sales of our products, including sales to medical facilities due to a lack of operating
capital needed to procure raw material inventory to currently fill customers’ orders.
On August 1, 2013, we acquired
the outstanding stock of Angels of Mercy, Inc. (“AOM”). Angels provides non-medical, Personal Care Attendant (PCA)
services, Supervised Independent Living (SIL), Long-Term Senior Care, and other approved health service programs performed by a
trained caregiver that will meet the health service needs of beneficiaries whose disabilities preclude the performance of certain
independent living skills related to the activities of daily living (ADL).
On December 10, 2013, Angels
of Mercy, Inc. acquired the assets of Amian Health Services LLC and Amian Health Services of Alex LLC, herein after referred to
as “Amian”. Amian delivers health-care care-services who provide routine health and personal care support
with Activities of Daily Living (ADL) to clients with physical impairments or disabilities in private homes, nursing care facilities,
hospice care settings, and other residential settings. Amian holds both PCA-Medicaid Waiver Provider and Residential Rehabilitation/Supervised
Independent Living (SIL), and personal care services for Veterans with licenses issued by the Division of Licensing and Certification
of the Department of Social Services, Veterans Administration Social Services and the Louisiana Department of Health and Hospitals.
All administrative personnel of Amian have been merged into to gain operating synergies. This company changed its name to
Amian Angels, Inc. (“Amian Angels”) in August 2014.
On July 21, 2014 we formed
Advanced Medical Products and Technologies Inc. to enter into the Durable Medical and Home Medical Equipment markets. We anticipate
acquiring active companies in this area to develop our Medical Products and Technologies Segment.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING
POLICIES
BASIS OF PRESENTATION
In the opinion of management,
the accompanying balance sheets and related interim statements of income, cash flows, and stockholders' equity include all adjustments,
consisting only of normal recurring items, necessary for their fair presentation in conformity with accounting principles generally
accepted in the United States of America ("U.S. GAAP"). Preparing financial statements requires management to make estimates
and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses. Actual results and outcomes may
differ from management's estimates and assumptions. Interim results are not necessarily indicative of results for a full year.
PRINCIPLES OF CONSOLIDATION
The consolidated financial
statements for the fiscal years ended August 31, 2014 and 2013 include the accounts of Oncologix Tech, Inc. and its wholly owned
subsidiaries, Dotolo Research Corporation, Amian Angels, Inc., Advanced Medical Products & Technologies Inc. Dotolo Research
Corporation is a Louisiana Corporation. Angels of Mercy, Inc. is a Louisiana Corporation. Advanced Medical Products & Technologies
is a Nevada corporation. All significant intercompany accounts and transactions have been eliminated in consolidation.
USE OF ESTIMATES
The preparation of financial
statements in accordance with accounting principles generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities
at the date of the financial statements and the reportable amounts of revenues and expenses during the reporting period. Actual
results could differ from those estimates.
REVENUE RECOGNITION
Revenue is recognized by
the Company in accordance with Accounting Standards Codification Topic (“ASC”) 605. Accordingly, revenue is recognized
when all the following criteria are met: persuasive evidence of an arrangement exists; delivery has occurred; the seller’s
price to the buyer is fixed and determinable; and collectability is reasonably assured. Currently, the primary revenue for the
Company is derived from its sales in its Personal Care Services Segment.
Amian Angels is reimbursed
for each approved “Unit of Service” provided, as determined by the Health Care Financing Administration (HCFA), the
Department of Health & Hospitals and the Department of Social Services and based upon a detailed Case Management, Plan of Care
for each beneficiary. A unit of service for PCA services will be one-half hour. At least fifteen (15) minutes of service must be
provided to the individual in order for Amian Angels to bill for a unit of service. A maximum of 1,825 hours (3,650 half-hour units)
per beneficiary, per year can be billed under the Medicaid waiver program. Our primary payor sources is the State of Louisiana,
the Department of Veterans Administration and Private Pay individuals who reimburse us for the services we provide. We currently
experience a two percent claims rejection rate. With the acquisition of Amian, Amian Angels now has private pay clients as well
as Veterans Administration Social Services clients.
CASH AND CASH EQUIVALENTS
The Company considers all
highly liquid instruments, with an initial maturity of three (3) months or less to be cash equivalents.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
ACCOUNTS RECEIVABLE
The Company’s receivables
in its medical device segment are subject to credit risk, and the Company typically does not require collateral on its accounts
receivable. Receivables are generally due within 30 days. The Company maintains an allowance for uncollectable receivables that
reduces the receivables to amounts that are expected to be collected. .
The lead time for account
receivables in our Personal Care service divisions ranges from 14 to 90 days. The majority of the Company’s receivables,
approximately 90%, are collected within 14 days. We bill the State of Louisiana on a weekly basis and are reimbursed two weeks
later via electronic funds transfer. We are able to resubmit any rejected claims an additional two times to Molina Healthcare,
the EDI payment provider for payments within the next twelve months. Currently we maintain an allowance for uncollectible receivables
at a rejection rate of 2% of outstanding receivables. We analyze our claim rejection rate on a quarterly basis and make quality
improvements to reduce the number of rejected claims. Private pay customers are billed semi-monthly. Generally collections occur
within 30 days. Veterans Administration (VA) customers are billed monthly. Generally collections occur within 45 to 60 days. Due
to the recent governmental shutdown, the current lead time for payments is approximately 90 days. Upon final rejection of any resubmitted
claims, the claims are resubmitted and after twelve months the receivables are written off to bad debt expense.
INVENTORY
Inventories are stated
at costs and are held on a first-in, first-out basis. Currently our inventory consists primarily of miscellaneous parts.
PROPERTY AND EQUIPMENT
Property and equipment
is recorded at cost. Depreciation is provided for on the straight-line method over the estimated useful lives of the related assets
as follows:
| Furniture and fixtures |
5 to 10 years |
| Computer equipment |
5 years |
| Equipment |
5 to 10 years |
| Software |
3 to 5 years |
The cost of maintenance
and repairs is charged to expense in the period incurred. Expenditures that increase the useful lives of assets are capitalized
and depreciated over the remaining useful lives of the assets. When items are retired or disposed of, the cost and accumulated
depreciation are removed from the accounts and any gain or loss is included in income.
LONG-LIVED ASSETS
ASC
360 – Property, Plant and Equipment addresses financial accounting and reporting for the impairment or disposal of long-lived
assets. The Company periodically evaluates whether events and circumstances have occurred that may warrant revision of the estimated
useful life of property and equipment or whether the remaining balance of property and equipment, or other long-lived assets, should
be evaluated for possible impairment. Instances that may lead to an impairment include: (i) a significant decrease in the
market price of a long-lived asset group; (ii) a significant adverse change in the extent or manner in which a long-lived
asset or asset group is being used or in its physical condition; (iii) a significant adverse change in legal factors or in
the business climate that could affect the value of a long-lived asset or asset group, including an adverse action or assessment
by a regulatory agency; (iv) an accumulation of costs significantly in excess of the amount originally expected for the acquisition
or construction of a long-lived asset or asset group; (v) a current-period operating or cash flow loss combined with a history
of operating or cash flow losses or a projection or forecast that demonstrates continuing losses associated with the use of a long-lived
asset or asset group; or (vi) a current expectation that, more likely than not, a long-lived asset or asset group will be
sold or otherwise disposed of significantly before the end of its previously estimated useful life.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
An estimate of the related
undiscounted cash flows, excluding interest, over the remaining life of the property and equipment and long-lived assets is used
in assessing recoverability. Impairment loss is measured by the amount which the carrying amount of the asset(s) exceeds the fair
value of the asset(s). The Company primarily employs two methodologies for determining the fair value of a long-lived asset: (i) the
amount at which the asset could be bought or sold in a current transaction between willing parties or (ii) the present value
of estimated expected future cash flows grouped at the lowest level for which there are identifiable independent cash flows.
GOODWILL AND OTHER INTANGIBLE ASSETS
The
Company adopted Accounting Standards Update 2011-08 “Intangibles – Goodwill and Other (Topic 350): Testing Goodwill
for Impairment (“ASU 2011-08”) in the fourth quarter of fiscal 2014 due to its recent acquisition of Dotolo Research
Corporation and Angels of Mercy, Inc. ASU 2011-08 permits an entity to first assess qualitative factors to determine whether it
is more likely that not that the fair value of a reporting unit is less than its carrying amount. Goodwill represents the excess
of the cost of a business combination over the fair value of the net assets acquired and these costs are subject to annual impairment
tests.
We
accounted for the acquisition of Dotolo Research Corporation and Angels of Mercy, Inc. using the acquisition method of accounting
under ASC 805 and ASC 810-10-65. The purchase price was allocated first to identifiable current then fixed assets as well as liabilities
assumed. We then earmarked identifiable intangibles, with the remainder to goodwill. We identified patents as our identifiable
asset for Dotolo Research Corporation. Amounts allocated to Goodwill for the acquisition of Dotolo are based on expanding our product
into the medical market and the potential upside of the sale with a FDA medical device product with a reimbursement code. Dotolo
is one of four companies worldwide with this FSA approved medical device product. Amounts allocated to goodwill for Angels of Mercy,
Inc. are based on increased clients and future revenues.
The
Company evaluates the recoverability of its indefinite lived intangible assets, which consist of Dotolo Research Corporation and
Goodwill in Angels of Mercy, Inc., based on estimates of future royalty payments that are avoided through its ownership of the
intangibles and patents, discounted to their present value. In determining the estimated fair value of the intangibles and patents,
management considers current and projected future levels of revenue based on its plans for Dotolo, business trends, prospects and
market and economic conditions. See Note 4 – Acquisitions for further information on the acquisition of Dotolo.
Pursuant
to ASC 350-20-35, we have identified three reporting units in our business, our medical device segment which consists of Dotolo
Research Corporation, our personal care segment which consists of Angels of Mercy, Inc., and our medical products and technologies
segment which consists of Advanced Medical Products & Technologies, Inc. We follow the two step process in ASC 350-20-35 for
impairment testing. In the first step we compare the fair value of the reporting unit as a whole to its carrying value, including
goodwill. For both reporting units, we have determined that the reporting units’ fair value exceeds its carrying value. We
also compare the carrying value of goodwill by itself for both reporting units.
The following explains
the results of our impairment testing. We have allocated $564,075 of goodwill to the Angels of Mercy, Inc. reporting unit. As of
August 31, 2014 the fair value exceeds the carrying value of goodwill by 50%. We have allocated $1,217,704 of goodwill to the reporting
unit Dotolo Research Corporation. As of August 31, 2014 the fair value exceeds the carrying value of goodwill by 38%. In calculating
the valuation, we used a discounted cash flow method based on the future 5 years cash flows of each reporting unit. We used a discount
rate of 8% which is currently higher that the current long term interest rate. An increase in the overall national interest rate
could have a negative impact on our valuation. An additional risk is the possibility of cash flow projections falling short of
our 5 year estimate amount.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NONCONTROLLING INTEREST
ASC 810 - Consolidation
addresses the accounting and reporting standards for ownership interest in subsidiaries held by parties other than the parent,
the amount of consolidated net income attributable to the parent and to the non-controlling interest, changes in a parent’s
ownership interest, and the valuation of retained non-controlling equity investments when a subsidiary is deconsolidated. During
fiscal 2009, the Company issued a ten percent interest in its subsidiary, Oncologix Corporation, to IUTM as required in a technology
agreement. The Company valued this interest at $212. Through August 31, 2014, the Company has allocated $3,734 losses to its non-controlling
interest. With the disposition of Oncologix Corporation, the Company no longer will have to recognize a non-controlling interest
in its subsidiary.
ADVERTISING COSTS
Advertising costs included
with selling, general and administrative expenses in the accompanying consolidated statements of operations were minimal for fiscal
2014 and fiscal 2013. Such costs are expensed when incurred.
INCOME TAXES
The Company adopted the
provisions of FASB ASC 740 - Income Taxes provides detailed guidance for the financial statement recognition, measurement and disclosure
of uncertain tax positions recognized in the financial statements. Income taxes are determined using the asset and liability method.
This method gives consideration to the future tax consequences associated with temporary differences between the carrying amounts
of assets and liabilities for financial statement purposes and the amounts used for income tax purposes.
FAIR VALUE OF FINANCIAL INSTRUMENTS
The estimated fair values
for financial instruments are determined at discrete points in time based on relevant market information. These estimates involve
uncertainties and cannot be determined with precision. The carrying amounts of accounts payable, accrued expenses, and notes payable
approximate fair value.
STOCK-BASED COMPENSATION
The Company has a stock-based
compensation plan, which is described more fully in Note 9. The Company accounts for stock-based compensation in accordance with
ASC 718. Under the fair value recognition provisions of this statement, share-based compensation cost is measured at the grant
date based on the fair value of the award and is recognized as expense over the vesting period. The Company estimates the fair
value of stock options granted using the Black-Scholes option valuation model. The fair value of all awards is amortized on a straight-line
basis over the vesting periods. The expected term of awards granted represent the period of time they are expected to be outstanding.
The Company determines the expected term based on historical experience with similar awards, giving consideration to the contractual
terms and vesting schedules. The Company estimates the expected volatility of its common stock at the date of grant based on the
historical volatility of its common stock. The risk-free interest rate is based on the U.S. treasury security rate estimated for
the expected life of the options at the date of grant. If actual results differ significantly from estimates, stock-based compensation
could be impacted.
CONVERTIBLE DEBT
Interest on convertible
debt is calculated using the simple interest method. The company recognizes a beneficial conversion feature to the extent the conversion
price is less than the closing stock price on the issuance of the convertible notes. The Company also follows ASC 470-50 and ASC
470-20 regarding changes in the terms of the convertible notes and the induced conversion of its convertible debt.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
RECLASSIFICATIONS
Certain prior year amounts
have been reclassified to conform to the current year presentation.
STOCK INCENTIVE PLANS
Share based payment compensation
costs for equity-based awards are measured on the grant date based on the fair value of the award on that date and is recognized
over the required service period. The fair-value of stock option awards are estimated using the Black-Scholes model. Fair value
of restricted stock awards is based upon the quoted market price of the common stock on the date of grant.
NET LOSS PER COMMON SHARE
Basic earnings (loss) per
share is calculated under the provisions of ASC 260 which provides for calculation of “basic” and “diluted”
earnings per share. Basic earnings per share includes no dilution and is calculated by dividing income (loss) available to
common shareholders by the weighted average number of common shares outstanding for the period. Diluted earnings (loss) per
share is calculated based on the weighted average number of common shares outstanding during the period plus the dilutive effect
of common stock purchase warrants and stock options using the treasury stock method and the dilutive effects of convertible notes
payable and convertible preferred stock using the if-converted method. On Basic and diluted earnings per share for the years ended
August 31, 2014 and 2013 are as follows:
| | |
For the year ended |
|
|
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| | |
| |
|
| | |
| |
|
| Net gain (loss) attributable to common shareholders | |
| | | |
| | |
| Continuing operations | |
$ | (1,407,571 | ) | |
$ | (653,459 | ) |
| Discontinued operations | |
| 95,528 | | |
| (14 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | |
| | |
$ | (1,312,043 | ) | |
$ | (653,473 | ) |
| | |
| | | |
| | |
| Weighted average shares outstanding | |
| 102,556,908 | | |
| 61,864,435 | |
| | |
| | | |
| | |
| Loss per common shares, basis and diluted | |
| | | |
| | |
| Continuing operations | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
| Discontinued operations | |
| 0.00 | | |
| (0.00 | ) |
| | |
| | | |
| | |
| | |
| | | |
| | |
| | |
$ | (0.01 | ) | |
$ | (0.01 | ) |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Due to the net losses during
the years ended August 31, 2014 and 2013, basic and diluted loss per share was the same, as the effect of potentially dilutive
securities would have been anti-dilutive. Shares attributable to convertible notes, stock options, preferred stock and warrants
not included the diluted loss per share calculation. Below lists all dilutive securities as of August 31, 2014 and 2013:
| |
|
|
|
|
|
As of |
| |
|
|
|
|
|
August 31, |
|
August 31, |
| |
|
|
|
|
|
2014 |
|
2013 |
| |
|
|
|
|
|
Underlying |
|
Underlying |
| Description |
Common Shares |
|
Common Shares |
| Convertible preferred stock…………………………………………………………. |
78,564 |
|
58,628,531 |
| Convertible notes payable………………………………………………………………………….. |
98,383,460 |
|
1,383,460 |
| Options……………………………………………………………………….. |
6,173,750 |
|
217,085 |
| Warrants……………………………………………………………………….. |
30,583,333 |
|
7,000,000 |
| |
|
|
|
|
|
|
|
|
| Total potentially dilutive securities……………………………………………………….. |
135,219,107 |
|
67,229,076 |
SEGMENT INFORMATION
ASC 280-10 defines operating
segments as components of a company about which separate financial information is available that is evaluated regularly by the
Company’s Chief Executive Officer and Chief Financial Officer in deciding how to allocate resources and in assessing performance.
The Company currently has three business segments; medical device manufacturing, personal care services and medical products and
technologies.
RECENT ACCOUNTING PRONOUNCEMENTS
We have evaluated all Accounting
Standards Updates through the date the financial statements were issued and do not believe any will have a material impact.
New Accounting Standard
In July 2012, the Financial
Accounting Standards Board (“FASB”) issued Accounting Standards Update 2012-02 “Intangibles – Goodwill
and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment” (“ASU 2012-02”). ASU 2012-02
permits entities to first assess qualitative factors to determine whether it is more likely than not that the fair value of an
indefinite-lived intangible asset is impaired as a basis for determining whether it is necessary to perform the quantitative impairment
test. Under the amendments in ASU 2012-02, an entity is not required to calculate the fair value of an indefinite-lived intangible
asset unless it determines that it is more likely than not that the fair value of the asset is less than its carrying amount. An
entity also will have the option to bypass the qualitative assessment for any indefinite-lived intangible asset in any period and
proceed directly to performing the quantitative impairment test. ASU 2012-02 is effective for interim and annual indefinite-lived
intangible asset impairment tests performed for fiscal years beginning on or after September 15, 2012, with early adoption permitted.
The Company’s adoption of ASU 2012-02 is not expected to have an impact on its consolidated financial statements.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 - GOING CONCERN
The accompanying consolidated
financial statements have been prepared assuming the Company will continue as a going concern. The Company has incurred losses
from operations over the past several years and anticipates additional losses in fiscal 2015 and prior to achieving breakeven.
During the year ended August
31, 2013 we acquired Dotolo Research Corporation and Angels of Mercy, Inc. In December 2013, through Amian Angels, we also acquired
the assets of Amian Health Services. While these acquisitions greatly increase the value of our Company, the combined operations
of OCLG are not cash flow positive at this time. Amian Angels, as a stand-alone business unit, is currently cash flow positive
but alone is unable to support all the corporate overhead or needs of our other subsidiary, Dotolo. We anticipate that we will
require approximately $1,500,000 to operate through December 31, 2014. Approximately $500,000 will be required to fund corporate
overhead including debt servicing with the balance to invest into raw material inventory, manufacturing and product revisions at
Dotolo Research. Additional funding will allow us to meet our current sales demands and expenses of Dotolo, Amian Angels and Oncologix,
while keeping our public filings current.
Our Company is not profitable
and we have to rely on debt and equity financings to fund operations. There is no assurance that the business activities of Dotolo
will achieve breakeven status by the end of 2014. Significant delays in achieving break-even status could affect the ability to
obtain future debt and equity funding. These factors raise substantial doubt about the Company’s ability to continue as a
going concern. After auditing our financial statements, our independent auditor issued a going concern opinion and our ability
to continue is dependent on our ability to raise additional capital. Currently there is a substantial doubt in the Company’s
ability to continue as a going concern.
NOTE 4 – ACQUISITIONS
Dotolo Research Corporation
On March 22, 2013, the
Company acquired all of the outstanding shares of common stock of Dotolo Research Corporation (“Dotolo”), a medical
device company. With this recent acquisition, the company continued on its mission to facilitate the controlling interests and
acquisition of medical device, health care service, medical distribution and emerging health care technology companies. This business
model creates a complete business solution of unlimited marketing and revenues opportunities. Our model combines certain natural
relationships of medical device products with related but distinct products, services, markets and opportunities. The combined
sales, marketing, and operational synergies will enable the Company and our business units to provide a wide variety of complete
technology solutions at significant cost savings.
While operations have contnued
with Dotolo, the revenues have not been significant since the acquisition. This is primarily due to a lack of monies available
to invest into raw material inventory for Dotolo.
The acquisition was accounted
for using the acquisition method of accounting and the purchase price was allocated to the assets acquired and liabilities assumed
based upon their estimated fair values at the date of acquisition. Identifiable intangible assets include patents, trade name and
customer list. The purchase price consisted of the issuance 58,564 shares of a newly created Series D Convertible Preferred Stock
(60,000 shares of Series D Preferred Stock designated). On March 22, 2013, the issued shares had a fair market value of $585,640
based on the fair market value of the underlying common stock shares. The issued Series D Convertible Preferred Stock have a liquidation
value of approximately $4,700,000 and are convertible any time after March 1, 2014 into 1,000 shares of common stock each. Please
see Note 8 for a further description of the Series D Convertible Preferred Stock.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The purchase price was
allocated to assets acquired and liabilities assumed as follows:
| | |
| | |
| Cash and cash equivalents | |
$ | 1,653 | |
| Accounts receivable (net) | |
| 769 | |
| Inventory | |
| 100,881 | |
| Prepaid expenses and other current assets | |
| 31,750 | |
| Property and equipment | |
| 22,957 | |
| Deposits and other assets | |
| 10,050 | |
| Purchased goodwill | |
| 1,217,704 | |
| Patents, registrations | |
| 33,172 | |
| | |
| | |
| Total assets acquired | |
$ | 1,418,936 | |
| | |
| | |
| Accounts payable and other accrued expenses | |
$ | 507,589 | |
| Customer deposits | |
$ | 78,807 | |
| Notes payable | |
| 177,763 | |
| Notes payable - related parties | |
| 58,600 | |
| Accrued interest payable | |
| 9,743 | |
| Accrued interest payable - related parties | |
| 794 | |
| | |
| | |
| Total liabilities assumed | |
$ | 833,296 | |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Angels of Mercy, Inc.
On August 1, 2013, the
Company acquired all the outstanding shares of Common Stock of Angels of Mercy, Inc. Pursuant to the Agreement, the Owners sold
all of the Common Stock of Amian Angels for $650,000 represented by a down payment of $100,000 at closing and a four year Secured
Promissory Note for $550,000. The Company also issued the Owners 1,000,000 four year warrants with an exercise price of $0.015
that possesses a cashless exercise option and agreed to pay $65,000 in broker fees related to this transaction.
The acquisition was accounted
for using the acquisition method of accounting and the purchase price was allocated to the assets acquired and liabilities assumed
based upon their estimated fair values at the date of acquisition. Identifiable intangible assets include patents and purchased
goodwill.
The purchase price was
allocated to assets acquired and liabilities assumed as follows:
| | |
| | |
| Cash and cash equivalents | |
$ | 27,121 | |
| Accounts receivable (net) | |
| 111,581 | |
| Prepaid expenses and other current assets | |
| 7,851 | |
| Property and equipment | |
| 57,000 | |
| Purchased goodwill | |
| 478,721 | |
| | |
| | |
| Total assets acquired | |
$ | 682,274 | |
| | |
| | |
| Accounts payable and other accrued expenses | |
$ | 9,688 | |
| | |
| | |
| Total liabilities assumed | |
$ | 9,688 | |
Amian Health Services
On December 10, 2013, our
subsidiary Angels of Mercy acquired the assets of Amian Health Services. Pursuant to the Agreement, the Owners sold all the assets
for $100,000 represented by a down payment of $75,000 at closing and a one year Secured Promissory Note for $25,000.
The acquisition was accounted
for using the acquisition method of accounting and the purchase price was allocated to the assets acquired and liabilities assumed
based upon their estimated fair values at the date of acquisition. Identifiable intangible assets include patents and purchased
goodwill.
The purchase price was
allocated to assets acquired and liabilities assumed as follows:
| | |
| | |
| Cash and cash equivalents | |
$ | 8,646 | |
| Property and equipment | |
| 6,000 | |
| Purchased goodwill | |
| 85,354 | |
| | |
| | |
| Total assets acquired | |
$ | 100,000 | |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – DISCONTINUED
OPERATIONS
During October 2013 the
Company’s management and Board of Directors determined to dispose of Oncologix Corporation its Brachytherapy medical device
subsidiary. On November 1, 2013, the company entered into a settlement agreement with Firetag, Stoss & Dowdell, PC., our former
attorneys. Per the terms of the settlement agreement, we exchanged our 90% ownership and executed a $50,000 promissory note payable
to Firetag in exchange for the forgiveness by Firetag of $145,522 in prior legal billings. The promissory note bears interest at
4% and requires 12 monthly payments of $4,257.49 beginning on December 1, 2013. Detailed below are the income and expenses related
to these discontinued operations:
| | |
For the year ended |
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| Operating expenses: | |
| | | |
| | |
| General and administrative | |
$ | 36 | | |
$ | 14 | |
| Depreciation and amortization | |
| — | | |
| — | |
| | |
| | | |
| | |
| Total operating expenses | |
| 36 | | |
| 14 | |
| | |
| | | |
| | |
| Loss from operations | |
| (36 | ) | |
| (14 | ) |
| | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | |
| | |
| | | |
| | |
| Total other income (expense) | |
| — | | |
| — | |
| | |
| | | |
| | |
| Loss from discontinued operations | |
| (36 | ) | |
| (14 | ) |
| Gain on disposal of discontinued operations | |
| 95,564 | | |
| — | |
| | |
| | | |
| | |
| Loss from discontinued operations | |
| 95,528 | | |
| (14 | ) |
| | |
| | | |
| | |
| Less loss attributable to noncontrolling interest | |
| — | | |
| — | |
| | |
| | | |
| | |
| Net loss from discontinued operations | |
$ | 95,528 | | |
$ | (14 | ) |
NOTE 6 – INVENTORY
We have inventory, on hand
in the amounts of $31,271 and $31,271 as of August 31, 2014 and 2013, respectively, as it relates to our medical device manufacturing
segment. We do not maintain any inventory for our personal service care segment or our medical products division. Due to a lack
of operating capital for procurement of raw material inventory, we have currently suspended manufacturing of our Toxygen hardware
system and disposable products and have begun a product redesign to take our Toxygen product into new markets. Currently, inventory
on hand is made up of miscellaneous Toxygen hardware parts.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 - PROPERTY AND EQUIPMENT
Property and equipment
is composed of the following at August 31, 2014 and 2013:
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| Furniture | |
$ | 12,688 | | |
$ | 10,388 | |
| Office Equipment | |
| 12,962 | | |
| 10,800 | |
| Computers | |
| 22,321 | | |
| 19,905 | |
| Software | |
| 3,497 | | |
| 3,497 | |
| Leasehold improvements | |
| — | | |
| 29,000 | |
| Equipment | |
| 16,763 | | |
| 16,763 | |
| | |
| | | |
| | |
| Total property and equipment at cost | |
| 68,231 | | |
| 90,353 | |
| | |
| | | |
| | |
| Less: accumulated depreciation and amortization | |
| (28,264 | ) | |
| (11,820 | ) |
| | |
| | | |
| | |
| | |
$ | 39,967 | | |
$ | 78,533 | |
NOTE 8
- LEASES
The Company leases office
space in Alexandria and Lafayette Louisiana and in Phoenix, Az. and the Alexandria office lease is on a month to month basis and
the Lafayette office location is on a 5-year lease. On March 28, 2014, Dotolo Research moved from its current manufacturing location
in Phoenix AZ into E&R Engineer manufacturing facilities located in Tempe, Az. Currently we hold our equipment in storage until
a new facility is located. Rent expense for the year ended August 31, 2014 and 2013 were $80,329 and $15,500, respectively. Following
are the minimum lease payments:
| | 2015 | | |
$ | 51,312 | |
| | 2016 | | |
| 51,312 | |
| | 2017 | | |
| 51,312 | |
| | 2018 | | |
| 51,312 | |
| | 2019 | | |
| 8,552 | |
| | | | |
| | |
| | | | |
| | |
| | Totals | | |
$ | 213,800 | |
NOTE 9 – GOODWILL, PATENTS AND OTHER
INTANGIBLE ASSETS
We currently carry our patents and registrations
net of amortization. As of August 31, 2014 and 2013, the Company has a capitalized cost of patents and registrations in the amount
of $122,479 and accumulated amortization of 97,983. Our patents and registrations are amortized over a 20 year period. Amortization
for each of the next 4 fiscal years, assuming no impairment, will be $6,124 per year.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 — NOTES PAYABLE
CONVERTIBLE NOTES PAYABLE:
Convertible notes payable
consist of the following as of August 31, 2014 and 2013:
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| | |
| | | |
| | |
| 8.0% convertible note due August 31, 2014 | |
$ | 100,000 | | |
$ | 125,000 | |
| 6.0% convertible note due September 2014 | |
| 189,025 | | |
| 235,025 | |
| 12% convertible note due March 19, 2015 (net of discount) | |
| 11,980 | | |
| | |
| 12% convertible note due April 8, 2015 (net of discount) | |
| 19,863 | | |
| | |
| 12% convertible note due October 25, 2015 (net of discount) | |
| 21,260 | | |
| | |
| 10% convertible note due February 21, 2015 (net of discount) | |
| 90,840 | | |
| | |
| 12% convertible note due July 16, 2015 (net of discount) | |
| 3,340 | | |
| | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| | |
| | | |
| | |
| Total unsecured convertible notes payable | |
| 436,308 | | |
| 360,025 | |
| Less: Long-Term portion | |
| — | | |
| — | |
| | |
| | | |
| | |
| Current portion | |
$ | 436,308 | | |
$ | 360,025 | |
The following is a summary
of future minimum payments on convertible notes payable as of August
31, 2014:
| |
Convertible |
| Fiscal Year Ending August 31, |
Notes Payable |
| 2015 |
$ 436,308
|
During May and June 2007,
we issued nine Convertible Promissory Notes in an aggregate principal amount of $700,000. Eight of these notes we-+re converted
into common stock in fiscal 2009. The remaining Convertible Promissory Note, in the principal amount of $125,000, was extended
on January 28, 2010 initially to March 31, 2012, where the conversion rate was reduced to $.60, and then extended to September
30, 2013. In October 2013, the investor sold $25,000 of principal in the note to another accredited investor and currently holds
a note representing the remaining $100,000 in principal. At that time, the note was extended to August 31, 2014 and was further
extended to February 28, 2015. We are currently working with the investor to extend the note. As of August 31, 2014, the Company
has accrued interest in the amount of $60,723.
On April 1, 2009, we issued
to Ms. Lindstrom, our former Chief Executive Officer, a convertible promissory note in lieu of payment of $235,025 in accrued salary
owed to Ms. Lindstrom. This note accrues interest at a rate of 6% per annum and was originally due on March 31, 2012. On March
16, 2012, Ms. Lindstrom agreed to extend the due date of the note to September 30, 2013. There was no beneficial conversion feature
recognized upon the issuance of this note. This note is currently past due. As of August 31, 2014, the Company has accrued interest
in the amount of $75,927. An outside party has entered into an assignment and settlement agreement with Ms. Lindstrom to purchase
the note. In connection with this Assignment agreement, the purchaser has agreed to extend the note to December 1, 2016. During
fiscal 2014, the Assignee has converted $46,000 of principal into 8,788,171 shares of stock.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On October 2, 2013 we issued
a convertible promissory note in the principal amount of $25,000. This promissory note bears interest at a rate of 8% per annum
and is due on October 2, 2013. The note is convertible at a 45% discount of the average of the three lowest closing bid prices
in the twenty days preceding the date of conversion. At no time may the holder of the note convert the note into shares exceeding
4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion feature of $25,000. On April
6, 2014 the holder elected to convert $17,074 in principal plus $690 in accrued interest into 5,383,007 shares of common stock
at a conversion price of $.0033. In August 1, 2014 the remaining principal of $7,926 plus accrued interest of $530 was converted
into 3,416,764 shares of common stock at a conversion rate of $.002475.
On October 2, 2013, the
Company entered into a securities transfer agreement with an accredited investor as well as a current convertible note holder.
The agreement called for the accredited investor to purchase $25,000 of the current convertible note holder note. The Company issued
to the accredited investor a convertible promissory note bearing interest at 8% and convertible at a 45% discount into shares of
the Company’s common stock using a three-day average of the lowest closing bid prices for the twenty trading days immediately
preceding the conversion date. On October 3, 2013, the investor converted $15,620 into 4,000,000 shares of the Company’s
common stock at a rate of $.003905 per share. On December 3, 2013, the investor converted the remaining principal of $9,380 into
2,008,559 shares of the Company’s common stock at a rate of $0.00467 per share.
On March 19, 2014 we issued
a convertible promissory note in the principal amount of $26,500 to an unrelated accredited investor. This promissory note bears
interest at a rate of 12% per annum and is due on March 19, 2015. The note is convertible at a 38% discount of the lowest closing
bid prices in the thirty days preceding the date of conversion. At no time may the holder of the note convert the note into shares
exceeding 4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion feature of $26,500.
As of August 31, 2014, the Company has accrued interest in the amount of $958.
On April 8, 2014 we issued
a convertible promissory note in the principal amount of $50,000 to an unrelated accredited investor. This promissory note bears
interest at a rate of 12% per annum and is due on April 8, 2015. The note is convertible at a 35% discount of the average 4 lowest
closing bid prices in the twenty days preceding the date of conversion. At no time may the holder of the note convert the note
into shares exceeding 4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion feature
of $50,000. As of August 31, 2014, the Company has accrued interest in the amount of $2,384. We paid off this note on October 2,
2014.
On April 25, 2014 we issued
a convertible promissory note in the principal amount of $25,000 to an unrelated accredited investor. This promissory note bears
interest at a rate of 12% per annum and is due on October 25, 2015. The note is convertible at a 35% discount of the average 4
lowest closing bid prices in the thirty days preceding the date of conversion. At no time may the holder of the note convert the
note into shares exceeding 4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion
feature of $25,000. As of August 31, 2014, the Company has accrued interest in the amount of $1,052.
On May 21, 2014 we issued
a convertible promissory note in the principal amount of $115,000 to an unrelated accredited investor. This promissory note bears
interest at a rate of 10% per annum. This principal includes a 10% OID in the amount of $10,000, which is being amortized over
the term of the note. The note is due in 4 equal installments beginning on the 180th day after the execution of the
note. The company may make the payments in common stock. The note is convertible at a $.009 per share. As additional consideration,
the Company issued 9,583,333 5 year warrants with an exercise price of $.009. At no time may the holder of the note convert the
note into shares exceeding 4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion
feature of $38,322 related to the issuance of the warrants. As of August 31, 2014, the Company has accrued interest in the amount
of $3,239.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On July 16, 2014 we issued
a convertible promissory note in the principal amount of $26,500 to an unrelated accredited investor. This promissory note bears
interest at a rate of 12% per annum and is due on July 26, 2015. The note is convertible at a 38% discount of the lowest closing
bid prices in the thirty days preceding the date of conversion. At no time may the holder of the note convert the note into shares
exceeding 4.99% of the Company’s then outstanding common stock shares. We recorded a beneficial conversion feature of $26,500.
As of August 31, 2014, the Company has accrued interest in the amount of $267.
CONVERTIBLE RELATED PARTY NOTES PAYABLE:
As of August 31, 2014,
there are currently no related party convertible notes payable outstanding. The note related to our former CEO is now classified
as non-related convertible debt for all comparable periods.
RELATED PARTY
NOTES PAYABLE:
| | |
August 31, | |
August 31, |
| | |
2014 | |
2013 |
| 6.0% line of credit (2) | |
$ | 51,600 | | |
$ | 56,200 | |
| | |
| | | |
| — | |
| | |
| | | |
| | |
| Outstanding unsecured related party notes payable | |
$ | 51,600 | | |
$ | 56,200 | |
| | |
| | | |
| | |
| (1) Note payable to current CEO. | |
| | | |
| | |
During the last two years,
Wayne Erwin, our President and CEO, has advanced a total of $51,600 directly to Dotolo in an open advance account. Interest is
being accrued at a rate of 6% per annum. As of August 31, 2014 we have accrued interest in the amount of $6,342. There is no specific
due date on this note.
During April 2013, Wayne
Erwin, our President and CEO, had advanced a total of $10,675 to Oncologix Tech, Inc. This note bore interest at 6%. This note
was paid in full in September 2013 together with accrued interest of $223.
The following is a summary
of future minimum payments on related party notes payable as of August 31, 2014:
| |
Related Conv. |
| Fiscal Year Ending August 31, |
Notes Payable |
| 2015 |
$ 51,600
|
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
OTHER NOTES
PAYABLE:
| | |
August 31, | |
August 31, |
|
| | |
2014 | |
2013 |
|
| 12% note payable due May 2014 | |
$ | — | | |
$ |
15,000 |
| 6% note payable due August 2015 | |
| — | | |
|
111,500 |
| Time Lease Payment due January 2014 | |
| — | | |
|
3,311 |
| Note payable | |
| 15,600 | | |
|
60,600 |
| Note payable - fee reimbursement | |
| — | | |
|
59,583 |
| 18% note payable due January 2015 | |
| 30,000 | | |
|
30,000 |
| 18% note payable due January 2015 | |
| 20,000 | | |
|
20,000 |
| 18% note payable due January 2015 | |
| 63,640 | | |
|
100,000 |
| 6% note payable due October 2017 | |
| 478,207 | | |
|
550,000 |
| 14.5% note payable due January 2015 | |
| 328,028 | | |
|
- |
| Bank line of credit loan | |
| 43,885 | | |
|
- |
| Merchant Loan due December 2014 | |
| 82,737 | | |
|
- |
| Merchant Loan due January 2015 | |
| 78,400 | | |
|
- |
| Merchant Loan due January 2015 | |
| 150,545 | | |
|
- |
| 18% note payable due November 2014 (net of discount) | |
| 8,617 | | |
|
- |
| 18% note payable due November 2014 (net of discount) | |
| 8,648 | | |
|
- |
| 4% note payable due November 2014 | |
| 8,473 | | |
|
- |
| 18% note payable due December 2014 (net of discount) | |
| 71,348 | | |
|
- |
| 6% note payable due December 2014 | |
| 8,500 | | |
|
- |
| 10% note payable due December 2014 (net of discount) | |
| 9,644 | | |
|
- |
| 6% note payable due February 2015 (net of discount) | |
| 2,742 | | |
|
- |
| 6% note payable due August 2015 (net of discount) | |
| 15,297 | |
|
|
| | |
| | | |
|
| |
| | |
| | | |
|
| |
| Subtotal | |
| 1,424,311 | | |
|
949,994 | |
|
| | |
| | | |
|
| |
| Less: Long-Term portion | |
| (395,675 | ) | |
|
(811,500) |
| | |
| | | |
|
| |
| Current portion | |
$ | 1,028,636 | | |
$ |
138,494 |
On May 23, 2013, the Company
issued a one year note in the amount of $20,000. The note bore interest at a rate of 12% per annum. The Company is required to
repay the note at a rate of $1,867 per month, which includes interest, on the 15th day of each month. The note is secured
by certain collateral of our CEO. This note was paid in full in May 2014.
On August 1, 2011 our subsidiary
Dotolo, entered into a note payable agreement to provide funding to its subsidiary in the principal amount of $111,500. In December
2013, this note was assumed by Oncologix Tech, Inc. The note bore interest at 6% and matures on August 31, 2015. During January
through August 2014, the Board of Directors authorized the conversions of the entire principal and accrued interest amount. During
that time frame, the $111,500 in principal was converted into 18,716,229 shares of the Company’s common stock and is considered
paid in full.
On September 16, 2013,
the Company obtained a merchant loan for additional working capital in the amount of $80,000. The merchant loan bores interest
at a rate of 15% and calls for 130 daily payments of $861 for a total repayment amount of $112,000. Out of the net proceeds, the
company also paid $20,000 in broker fees and loan fees of $750. This loan was paid in full on January 3, 2014.
On October 1, 2013, the
Company borrowed 10,000 in principal from an unrelated investor. The note was due January 2, 2014 and bore interest at 22%. Monthly
interest payments of $183.33 are due on the first of each month beginning on November 1, 2013. This note was paid in full on January
3, 2014.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On November 27, 2013, the
Company obtained a merchant loan for additional working capital in the amount of $51,000. This loan requires 180 daily payments
in the amount of $306 for a total repayment amount of $55,021. We netted gross proceeds of $46,032 after paying loan fees. This
note was paid off March 11, 2014.
On December 18, 2013, the
Company obtained a merchant loan for additional working capital in the amount of $72,000. This loan requires 82 daily payments
in the amount of $888 for a total repayment amount of $72,500. We netted gross proceeds of $49,301 after fees of $699. This note
was paid off March 11, 2014.
During April 2012, our
subsidiary Dotolo, entered into a financing agreement to provide up to $150,000 in funding for the subsidiary. The financing agreement
was due in January 2013. We entered into a settlement agreement whereby we paid $45,000 from amounts held in reserve by our senior
lender and are required to make 10 monthly payments of $1,500.
On August 1, 2013, in connection
with our acquisition of Angels of Mercy, Inc. we entered into a promissory note to pay $65,000 of broker’s fees incurred
in the acquisition. Monthly payments of $5,417 are due and payable beginning on August 15, 2013. This note bears no interest. This
was paid in full in July 2014.
On February 27, 2013 our
subsidiary Dotolo, entered into a note payable agreement to provide funding to its subsidiary in the principal amount of $30,000.
The note bears interest at 18% payable monthly on the 15th and is due in full in January 2015. For the year ended August 31, 2014,
we made interest payments in the amount of $5,400. As of August 31, 2014, we have accrued interest of $1,365.
On March 17, 2013 our subsidiary
Dotolo, entered into a note payable agreement to provide funding to its subsidiary in the principal amount of $20,000. The note
bears interest at 18% payable monthly on the 15th and is due in full in January 2015. For the year ended August 31, 2014, we made
interest payments in the amount of $3,600. As of August 31, 2014, we have accrued interest of $990.
On July 26, 2013 the Company
issued an 18 month promissory note in the principal amount of $100,000. These funds were used for the cash down payment for the
Angels acquisition. The note bears interest at 18% and requires monthly interest payments of $1,200 beginning on September 26,
2013. In December 2013, we modified the loan agreement to make monthly payments of $6,200. As of August 31, 2014 the outstanding
balance was $63,640.
On August 1, 2013, in connection
with our acquisition of Angels of Mercy, Inc. we entered into a promissory note to pay $550,000 for the purchase of Angels of Mercy,
Inc. Monthly payments of $9,115 are due and payable beginning on November 1, 2013 with a final balloon payment of $205,705 due
on October 1, 2017. This note bears interest at a rate of 6%. As of August 31, 2014, the outstanding balance of the note is $478,207.
On January 3, 2014, the
Company closed on a 4 million dollar line of credit facility, with an initial draw of $500,000. The Company must meet specific
monthly reporting and collateral requirements to further draw on the revolving credit facility. The $500,000 initial draw is secured
by a 14.5% promissory note, which is convertible ONLY upon default by the Company. In July 2014, we borrowed an additional $75,000
from the principal we repaid. This note is due in six months with an automatic option to renew after six months. As of August 31,
2014, the outstanding balance is $328,028.
During fiscal 2014 we borrowed
$45,000 from our line of open line of credit with our bank. As of August 31, 2014 the outstanding balance of the line of credit
loan was $43,885.
On March 11, 2014, the
Company obtained a merchant loan for additional working capital in the amount of $150,000. This loan requires 209 daily payments
in the amount of $940 for a total repayment amount of $196,500. We netted gross proceeds of $146,750 after paying loan fees.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On April 18, 2014, the
Company obtained a merchant loan for additional working capital in the amount of $120,000. This loan requires 189 daily payments
in the amount of $800 for a total repayment amount of $151,200. We netted gross proceeds of $119,301 after paying loan fees.
On July 10, 2014, the Company
obtained a merchant loan for additional working capital in the amount of $150,000. This loan requires 132 daily payments in the
amount of $1,568 for a total repayment amount of $207,000. We netted gross proceeds of $149,120 after paying loan fees.
On November 5, 2013 and
November 8, 2013, the Company entered into two, one-year promissory notes with accredited investors to borrow a total principal
amount of $20,000. Each promissory note is $10,000 in principal balance, bears interest at 18% and requires monthly interest payments
of $150 each. The company also issued 3,000,000 in cashless warrants as finder’s fees for these funds. The Company recorded
a discount of $14,805 for the issuance of the warrants.
On November 1, 2013, the
Company entered into a Settlement Agreement with its former legal counsel. The current balance owed to prior counsel is $145,523.
Pursuant to the settlement agreement, the Company agreed to pay $50,000 in the form of a one year promissory note and transfer
its 90% ownership interest and all marketing rights of Oncologix Corporation, one of its subsidiaries as full settlement of the
current balance owed. The promissory note bears interest of 4% and requires monthly payment of $4,257 beginning on December 1,
2013. As of August 31, 2014 the outstanding balance was $8,473.
On December 3, 2013, The
Company entered into a twelve month promissory note with an accredited investor to borrow a total principal amount of $75,000.
The note bears interest of 18% per annum and calls for monthly payments of principal and interest of $1,375 beginning on January
15, 2014 with a balloon payment due December 15, 2014. The Company also issued as additional finders’ fees to the investor,
3,500,000 shares of common stock and 1,000,000 cashless warrants with an exercise price of $.025. As of August 31, 2014, the balance
was $72,892. The Company recorded a discount of $5,992 for the issuance of the warrants.
In connection with the
acquisition of Amian Health Services, the Company entered into a twelve month promissory note in the total principal amount of
$25,000. The note bears interest at $6% and requires monthly payments of $2,152. As of August 31, 2014, the balance is $8,500.
On December 20, 2013, the
Company issued a 1-year promissory note to a non-related accredited investor. This note bears interest at 10% per annum and matures
in December 2014. As additional consideration for the operating capital loan, the company issued 3,000,000 cashless two-year warrants
with an exercise price of $0.02. The Company recorded a discount of $7,746 for the issuance of the warrants. As of August 31, 2014
the Company has accrued interest of $837.
On February 7, 2014, the
Company issued a 1-year promissory note in the principal amount of $15,000 to a non-related accredited investor. This note bears
interest at 6% per annum and matures in February 2015. As additional consideration for the operating capital loan, the company
issued 1,500,000 two-year warrants with an exercise price of $0.15 and 1,000,000 shares of common stock. The Company recorded an
expense of $9,000 for the issuance of the common stock. The Company recorded a discount of $5,151 for the issuance of the warrants.
On August 1, 2014 this investor used $10,000 to purchase 1,200,000 shares of common stock. As of August 31, 2014 the Company has
accrued interest of $453.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On August 15, 2014 the
Company issued a 1-year promissory note to a non-related accredited investor. This note bears interest at 10% per annum and matures
in August 2015. As additional consideration for the operating capital loan, the company issued 4,000,000 cashless two-year warrants
with an exercise price of $0.065. The Company recorded a discount of $10,177 for the issuance of the warrants. As of August 31,
2014 the Company has accrued interest of $118.
The following is a summary of future minimum
payments on r notes payable as of August 31, 2014:
| |
Related Conv. |
| Fiscal Year Ending August 31, |
Notes Payable |
| 2015 |
1,038,997 |
| 2016 |
87,623 |
| 2017 |
314,743 |
NOTE 11 — STOCKHOLDERS EQUITY
PREFERRED STOCK:
Series A Convertible Preferred Stock.
The Company is authorized
to issue up to 10,000,000 shares of preferred stock, in one or more series, and to determine the price, rights, preferences and
privileges of the shares of each such series without any further vote or action by the stockholders. The rights of the holders
of common stock will be subject to, and may be adversely affected by, the rights of the holders of any shares of preferred stock
that may be issued in the future.
In January 2003, our Board
of Directors authorized up to 4,500,000 shares of Series A Convertible Preferred Stock. Each share of Series A Convertible
Preferred stock has a par value of $0.001 and is convertible into one-half share of common stock in upon a cash payment by the
holder to the Company of $0.40 per common share. The Series A Convertible Preferred Stock is entitled to receive, in
preference to the common stock, of noncumulative dividends, if declared by the Board of Directors, and a claim on the Company's
assets upon any liquidation of the Company senior to the common stock. These preferred shares are not entitled to voting
rights. There are presently outstanding 129,062 shares of Series A Preferred Stock.
On March 30, 2003, the
Company completed the private placement of Units pursuant to the terms of a Unit Purchase Agreement (the “Units”) with
accredited investors. Each Unit consists of the following underlying securities: (i) three shares of the Company’s common
stock; (ii) one share of Series A Convertible Preferred Stock, par value $.001 per share; and (iii) one three-year warrant to purchase
one share of common stock at a per share price of $0.30. The warrants expired on March 31, 2006. Each share of Series A Convertible
Preferred Stock is convertible into one half share of the Company’s common stock in exchange for $0.40 per common share ($.20
for each Series A Convertible Preferred share converted). The securities underlying the Units are not to be separately tradable
or transferable apart from the Units until such time as determined by the Company’s Board of Directors. A total of 4,032,743
Units were issued. As of August 31, 2014 and August 31, 2013, there were 129,062 and 129,062 Units outstanding that had not been
separated, respectively. These units are presented as their underlying securities on our balance sheet and consist of 64,531 shares
of Series A Preferred Stock and 96,797 shares of common stock which is included in the issued and outstanding shares.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
Below is a table detailing
the outstanding Series A Convertible Preferred Stock shares outstanding during the last two fiscal years:
| | | | |
| Preferred | | |
| Number of | | |
| | | |
| Weighted Avg. | |
| | | | |
| Shares | | |
| Common Shares | | |
| Proceeds if | | |
| Per Common Sh. | |
| | | | |
| Outstanding | | |
| Convertible | | |
| Converted | | |
| Exercise Price | |
| | Outstanding, August 31, 2012 | | |
| 129,062 | | |
| 64,531 | | |
$ | 25,812 | | |
$ | 0.40 | |
| | | | |
| | | |
| | | |
| | | |
| | |
| | Expired/Retired | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | Converted | | |
| — | | |
| — | | |
| — | | |
$ | 0.40 | |
| | Issued | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | Outstanding, August 31, 2013 | | |
| 129,062 | | |
| 64,531 | | |
$ | 25,812 | | |
$ | 0.40 | |
| | | | |
| | | |
| | | |
| | | |
| | |
| | Expired/Retired | | |
| — | | |
| — | | |
| — | | |
$ | 0.40 | |
| | Converted | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | Issued | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| | Outstanding, August 31, 2014 | | |
| 129,062 | | |
| 64,531 | | |
$ | 25,812 | | |
$ | 0.40 | |
Series D Convertible Preferred
Stock
In March 2013, our Board
of Directors authorized up to 60,000 shares of Series D Convertible Preferred Stock. Each share of Series D Convertible stock has
a par value of $0.001 and is convertible into 1,000 shares of common stock beginning after March 1, 2014. Each share of Series
D Convertible Preferred Stock has a stated liquidation value of $80.25. Each shares of Series D Convertible Preferred Stock shall
have voting rights as stated below:
March 1, 2013 to February
28, 2014, 400 votes per share;
March 1, 2014 to February
28, 2015, 800 votes per share;
March 1, 2015 to February
28, 2016, 1,200 votes per share;
March 1, 2016 to February
28, 2017, 1,600 votes per share;
March 1, 2017 and after,
2,000 votes per share;
On March 22, 2013, the
Company issued 58,564 shares of Series D Convertible Preferred Stock to acquire 100% of the outstanding common stock of Dotolo.
On March 22, 2013 the issued shares had a fair market value of $585,640 based on the fair market value of the underlying common
stock shares.
On January 3, 2014, as
payment for $150,000 of banking fees associated with our $4 million line of credit, we issued 20,000 shares of Series D Convertible
Preferred Stock.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
Below is a table detailing
the outstanding Series D Convertible Preferred Stock shares outstanding during the last two fiscal years:
| | |
Preferred | |
Number of | |
| |
Weighted Avg. |
| | |
Shares | |
Common Shares | |
Proceeds if | |
Per Common Sh. |
| | |
Outstanding | |
Convertible | |
Converted | |
Exercise Price |
| Outstanding, August 31, 2012 | |
|
— |
| |
|
— |
| |
$ |
— |
| |
$ |
— |
|
| | |
|
|
| |
|
| |
|
|
| |
|
|
|
| Expired/Retired | |
|
— |
| |
|
— |
| |
|
— |
| |
$ |
— |
|
| Converted | |
|
— |
| |
|
— |
| |
|
— |
| |
$ |
— |
|
| Issued | | |
| 58,564 | | |
| 58,564,000 | | |
| — | | |
$ | 80.25 | |
| Outstanding, August 31, 2013 | | |
| 58,564 | | |
| 58,564,000 | | |
$ | — | | |
$ | — | |
| | | |
| | | |
| | | |
| | | |
| | |
| Expired/Retired | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| Converted | | |
| — | | |
| — | | |
| — | | |
$ | — | |
| Issued | | |
| 20,000 | | |
| 20,000,000 | | |
| — | | |
$ | 80.25 | |
| | Outstanding, August 31, 2014 | | |
| 78,564 | | |
| 78,564,000 | | |
$ | — | | |
$ | 80.25 | |
SUBSCRIBED COMMON STOCK:
Below is a table detailing
the Common Stock Subscribed during the last two fiscal years:
| For the period Ended August 31, 2014 |
| | |
Shares | |
Amount |
| Shares issuable upon conversion of convertible notes payable | |
| 1,058,201 | | |
$ | 5,000 | |
| | |
| | | |
| | |
| Total subscribed stock | |
| 1,058,201 | | |
$ | 5,000 | |
| | |
| | | |
| | |
| For the year Ended August 31, 2013 |
| | |
| Shares | | |
| Amount | |
| Shares issuable upon conversion of convertible notes payable | |
| — | | |
$ | — | |
| | |
| | | |
| | |
| Total subscribed stock | |
| — | | |
$ | — | |
COMMON STOCK:
On March 7, 2014, the Company
increased its authorized shares of common stock to 750,000,000. The increase was approved by a majority of the Company’s
shareholders on January 27, 2014.
Below are recent sales
of unregistered securities:
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
| Date |
Securities |
|
Underwriters/ |
|
| Sold |
Sold |
Consideration |
Purchasers * |
Notes |
| |
|
|
|
|
| 10/15/2012 |
1,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 1,000,000 shares of common stock to a non-related accredited investor at $0.02 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 1/6/2013 |
2,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 2,000,000 shares of common stock to a non-related accredited investor at $0.01 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 2/8/2013 |
1,024,164 |
$ - |
Anthony Silverman, former CEO |
Anthony Silverman, our former President and CEO, converted a promissory note in the amount of $10,242 in principal and interest into 1,024,164 shares of common stock at $0.01 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/17/2013 |
2,000,000 |
$ 10,000 |
Accredited Investor |
The Company sold 2,000,000 shares of common stock to a non-related accredited investor at $0.005 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 7/17/2013 |
4,000,000 |
$ 20,000 |
Accredited Investor |
The Company sold 4,000,000 shares of common stock to a non-related accredited investor at $0.005 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 8/8/2013 |
6,000,000 |
$ 36,000 |
Accredited Investor |
The Company sold 6,000,000 shares of common stock to a non-related accredited investor at $0.006 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 9/12/2013 |
1,000,000 |
$ - |
Vendor |
The Company issued 1,000,000 S-8 shares to a vendor for consulting work. The Company recorded an expense of $11,500 upon the issuance of those shares. |
| 9/12/2013 |
1,500,000 |
$ 10,000 |
Accredited Investor |
The Company sold 1,500,000 shares of common stock to an affiliated accredited investor at $0.00667 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| 10/3/2013 |
4,000,000 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $15,620 in principal and interest into 4,000,000 shares of common stock at $0.00391 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 12/3/2013 |
1,891,123 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $9,380 in principal and interest into 1,891,123 shares of common stock at $0.00496 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/3/2014 |
2,000,000 |
$ - |
Vendor |
The company issued 2,000,000 shares of common stock as consideration for services. The company recorded an expense of $22,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/13/2014 |
3,076,923 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $20,000 in principal and interest into 3,076,923 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/14/2014 |
1,000,000 |
$ - |
Vendor |
The company issued 1,000,000 shares of common stock as consideration for services. The company recorded an expense of $19,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/15/2014 |
117,436 |
$ - |
Accredited Investor |
Additional reset shares were issued to a non-affiliated accredited investor in connection with the prior conversion of $9,380 in principal and interest into 117,436 shares of common stock. These shares were exempt from registration under Section 4(2) of the Securities Act. |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
| 1/21/2014 |
3,500,000 |
$ - |
Accredited Investor |
The company issued 3,500,000 shares of common stock as consideration for fees. The company recorded an expense of $45,500 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/21/2014 |
1,500,000 |
$ - |
Accredited Investor |
The company issued 1,500,000 shares of common stock as consideration for fees. The company recorded an expense of $30,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 1/31/2014 |
3,472,222 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $25,000 in principal and interest into 3,472,222 shares of common stock at $0.0072 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 2/7/2014 |
1,000,000 |
$ - |
Accredited Investor |
The company issued 1,000,000 shares of common stock as consideration for fees. The company recorded an expense of $9,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 2/24/2014 |
4,615,385 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $30,000 in principal and interest into 4,615,385 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 3/12/2014 |
4,615,385 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $30,000 in principal and interest into 4,615,385 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 4/7/2014 |
2,936,314 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $19,086 in principal and interest into 2,936,314 shares of common stock at $0.0065 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 4/7/2014 |
5,383,007 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $17,764 in principal and interest into 5,383,007 shares of common stock at $0.0033 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/2/2014 |
5,000,000 |
$ - |
Accredited Investor |
The company issued 5,000,000 shares of common stock as consideration for services. The company recorded an expense of $30,000 in connection with this issuance. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 6/25/2014 |
5,138,746 |
$ - |
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $25,000 in principal and interest into 5,138,746 shares of common stock at $0.004865 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 7/14/2014 |
2,500,000 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $11,000 in principal and interest into 2,500,000 shares of common stock at $0.0044 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 7/24/2014 |
1,149,425 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $5,000 in principal and interest into 1,149,425 shares of common stock at $0.00435 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 8/1/2014 |
3,416,764 |
|
Accredited Investor |
A non-affiliated accredited investor converted a promissory note in the amount of $8,456 in principal and interest into 3,416,764 shares of common stock at $0.002475 per share. These shares were exempt from registration under Section 4(2) of the Securities Act. |
| 8/26/2014 |
1,200,000 |
$ - |
Accredited Investor |
The Company sold 1,200,000 shares of common stock to an affiliated accredited investor at $0.00833 per share. These shares were exempt from registration under Section 4(2) of the Securities Act |
| |
76,036,894 |
$ 116,000 |
|
|
| |
|
|
|
|
| * There were no underwriters associated with any of our Sales of Unregistered Securities. |
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
NON-CONTROLLING INTEREST
On February 27, 2009, in
connection with the Technology Agreement we entered into with Institut für Umwelttechnologien GmbH, a German Company (“IUT”)
whereunder the parties have agreed that the Company’s marketing rights have been transferred to its subsidiary, Oncologix
Corporation and have issued IUTM 10% of the equity ownership of that subsidiary. As of February 27, 2009, the value of the non-controlling
interest was $212. It was determined at August 31, 2010 the value of the investment in IUTM was impaired. Accordingly, we recorded
an impairment loss in the amount of $3,186 for the year ended August 31, 2010. As of August 31, 2014, as a result of the disposition
of Oncologix Corporation, we do not have to recognize a non-controlling interest.
WARRANTS:
The following table summarizes
warrant activity in fiscal 2014 and 2013:
| |
|
|
|
Weighted Avg. |
| |
|
Number |
|
Exercise Price |
| Outstanding, August 31, 2012 |
|
- |
|
- |
| Expired/Retired |
|
- |
|
- |
| Exercised |
|
- |
|
- |
| Issued |
|
7,000,000 |
|
0.012 |
| Outstanding, August 31, 2013 |
|
7,000,000 |
|
- |
| |
|
|
|
|
| Expired/Retired |
|
- |
|
- |
| Exercised |
|
- |
|
- |
| Issued |
|
23,583,333 |
|
0.011 |
| Outstanding, August 31, 2014 |
|
30,583,333 |
|
0.011 |
The fair value of warrants
granted is estimated using the Black-Scholes option pricing model. This model utilizes the following factors to calculate the fair
value of options granted: (i) annual dividend yield, (ii) weighted-average expected life, (iii) risk-free interest rate and (iv)
expected volatility. The warrants were expensed and accounted for under ASC 718.
The fair value for these
warrants was estimated as of the date of grant using a Black-Scholes option-pricing model with the following assumptions:
| |
|
|
|
For the Year Ended August 31, |
| |
|
|
|
2014 |
|
2013 |
| Volatility |
104% - 444% |
|
97.2% to 97.9% |
| Risk free rate |
0.25% |
|
0.38% |
| Expected dividends |
None |
|
None |
| Expected term (in years) |
2 to 5 years |
|
3 to 4 years |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
Details relative to the 30,583,333 immediately exercisable outstanding
warrants at August 31, 2014 are as follows:
| |
|
|
|
Weighted |
|
|
|
|
|
| |
|
|
|
Average |
|
|
|
|
|
| Date of |
|
Number |
|
Exercise |
|
Remaining |
|
Expiration |
|
| Grant |
|
of Shares |
|
Price |
|
Exercise Life |
|
Date |
|
| |
|
|
|
|
|
|
|
|
|
| Fourth quarter of fiscal 2013 |
|
7,000,000 |
|
$ 0.012 |
|
3 to 4 years |
|
July 2017 |
|
| |
|
|
|
|
|
|
|
|
|
| Outstanding, August 31, 2013 |
|
7,000,000 |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
| First quarter of fiscal 2014 |
|
4,500,000 |
|
$ 0.012 |
|
3 years |
|
November 2017 |
|
| Second quarter of fiscal 2014 |
|
5,500,000 |
|
$ 0.017 |
|
2 to 3 years |
|
Dec 2015 to Dec 2016 |
| Third quarter of fiscal 2014 |
|
9,583,333 |
|
$ 0.012 |
|
5 years |
|
May 2019 |
|
| Fourth quarter of fiscal 2014 |
|
4,000,000 |
|
$ 0.007 |
|
2 years |
|
August 2016 |
|
| |
|
|
|
|
|
|
|
|
|
| Outstanding, August 31, 2014 |
|
30,583,333 |
|
|
|
|
|
|
|
On August 1, 2013, the
company issued 1,000,000 four-year cashless warrants as additional consideration for the acquisition of Amian Angels. These warrants
expire four years after the date of issuance and have an exercise price of $.015.
On August 5, 2013, the
company issued 6,000,000 three-year cashless warrants, to a related party, as finder’s fees related to a working capital
investment. These warrants expire three years after the date of issuance and have an exercise price of $.012.
On September 11, 2013,
the company issued 1,500,000 three-year cashless warrants, to a related party, as finder’s fees related to a working capital
investment. These warrants expire three years after the date of issuance and have an exercise price of $.015.
On November 8, 2013, the
company issued 3,000,000 three-year cashless warrants, to an unrelated party, as finder’s fees related to a working capital
investment. These warrants expire three years after the date of issuance and have an exercise price of $.01.
On December 3, 2014, the
company issued 1,000,000 three-year cashless warrants, to an unrelated party, as finder’s fees related to a working capital
investment. These warrants expire three years after the date of issuance and have an exercise price of $.025.
On December 20, 2014, the
company issued 3,000,000 two-year cashless warrants, to an unrelated party, as finder’s fees related to a working capital
investment. These warrants expire two years after the date of issuance and have an exercise price of $.016.
On February 7, 2014, the
company issued 1,000,000 two-year cashless warrants, to an unrelated party, as finder’s fees related to a working capital
investment. These warrants expire two years after the date of issuance and have an exercise price of $.015.
On May 21, 2014, the company
issued 9,583,333 five-year cashless warrants, to an unrelated party, as finder’s fees related to a working capital investment.
These warrants expire five years after the date of issuance and have an exercise price of $.009.
On August 15, 2014, the
company issued 4,000,000 two-year warrants, to an unrelated party, as finder’s fees related to a working capital investment.
These warrants expire two years after the date of issuance and have an exercise price of $.0065.
The remaining contractual
life of warrants outstanding as of August 31, 2014 was 2.80 years. Warrants for the purchase of 30,583,333 and nil shares were
immediately exercisable on August 31, 2014 and 2013, respectively with a weighted-average price of $0.011 and $0.012 per share,
respectively.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
STOCK OPTIONS:
ASC 718 requires the estimation
of forfeitures when recognizing compensation expense and that this estimate of forfeitures be adjusted over the requisite service
period should actual forfeitures differ from such estimates. Changes in estimated forfeitures are recognized through a cumulative
adjustment, which is recognized in the period of change and which impacts the amount of unamortized compensation expense to be
recognized in future periods.
ASC 718 requires that modification
of the terms or conditions of an equity award is to be treated as an exchange of the original award for a new award. This event
is accounted for as if the entity repurchases the original instrument by issuing a new instrument of equal or greater value, incurring
additional compensation cost for any incremental value.
2000 Stock Incentive Plan
The Company is authorized
to issue up to 7,500,000 shares of common stock under its 2000 Stock Incentive Plan. Shares may be issued as incentive stock options,
non-statutory stock options, deferred shares or restricted shares. Options are granted at the fair market value of the common stock
on the date of the grant and have terms of up to ten years. The 2000 Stock Incentive Plan also provides for an annual grant of
options to members of our Board of Directors. For fiscal years ended August 31, 2008 through 2012, our Board of Directors elected
to waive the grant of these annual options.
On December 13, 2013, the
Board of directors authorized the granting of 6,100,000 options to its three officers; 2,400,000 options to Wayne Erwin, our CEO;
2,100,000 options to Michael Kramarz, our CFO; and 1,600,000 options to Vickie Hart, President of Amian Angels. These options vest
immediately and have an exercise price $.015, the closing stock price on December 13, 2013.
On December 20, 2014, the
Company issued 20,000 options as part of its annual grant program to its two directors. These options vest in 1 year and have an
exercise price of $.016, the closing stock price on December 20, 2013.
We have 485,253 shares
of common stock available for future issuance under our 2000 Stock Incentive Plan as of August 31, 2014. This plan has been approved
by our shareholders.
During the years ended
August 31, 2014 and 2013, we granted 6,120,000 and nil options from the stock incentive plan described above, respectively. During
the years ended August 31, 2014 and 2013, nil and nil options were exercised, respectively. During the years ended August 31, 2014
and 2013, 163,335 and 80,000 options expired, respectively. During the years ended August 31, 2014 and 2013, $91,163 and $0 was
expensed as stock based compensation, respectively.
| | | | |
| | | |
| | | |
| Weighted Average | |
| | | | |
| Number of | | |
| Option Price | | |
| Exercise Price | |
| | | | |
| Options Granted | | |
| Per Share | | |
| Per Share | |
| | | | |
| | | |
| | | |
| | |
| | Outstanding, August 31, 2012 | | |
| 297,085 | | |
| $0.12 - $5.16 | | |
$ | 1.430 | |
| | Granted | | |
| — | | |
| — | | |
$ | — | |
| | Exercised | | |
| — | | |
| — | | |
$ | — | |
| | Cancelled | | |
| (80,000 | ) | |
| $1.60 - $5.16 | | |
$ | 2.420 | |
| | Outstanding, August 31, 2013 | | |
| 217,085 | | |
| $0.12 - $2.00 | | |
$ | 1.120 | |
| | Granted | | |
| 6,120,000 | | |
| $0.015 - $0.016 | | |
$ | 0.020 | |
| | Exercised | | |
| — | | |
| — | | |
$ | — | |
| | Cancelled | | |
| (163,335 | ) | |
| $1.04 - $2.00 | | |
$ | 1.380 | |
| | Outstanding, August 31, 2014 | | |
| 6,173,750 | | |
| $0.12 - $2.00 | | |
$ | 0.016 | |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
The aggregate intrinsic
value in the table above represents the total pre-tax intrinsic value (the difference between our closing stock price on the last
trading day of the third quarter of fiscal 2014 and the exercise price, multiplied by the number of in-the-money options) that
would have been received by the option holders had all option holders exercised their options on August 31, 2014.
Expected volatility is
based primarily on historical volatility. Historical volatility is computed using weekly average pricing observations for an applicable
historic period. We believe this method produces an estimate that is representative of our expectations of the future volatility
over the expected term of our options. We currently have no reason to believe future volatility over the expected life of these
options is likely to differ materially from historical volatility. The weighted-average expected life is based upon share option
exercises, pre and post vesting terminations and share option term expirations. The risk-free interest rate is based on the U.S.
treasury security rate estimated for the expected life of the options at the date of grant.
| | |
Options | |
|
Options | |
|
| | |
Outstanding | |
|
Exercisable | |
|
| Number of options | |
| 6,173,750 | | |
|
6,173,750 |
| Aggregate intrinsic value of options | |
$ | — | | |
$ |
— |
| Weighted average remaining contractual term (years) | |
| 9.23 | | |
|
9.23 |
| Weighted average exercise price | |
$ | 0.016 | | |
$ |
0.016 |
2013 Omnibus Incentive
Plan
The Company is authorized
to issue up to 10,000,000 shares of common stock under its 2013 Omnibus Incentive Plan to employees, officers, directors and consultants.
The issuance adoption of this plan has been approved by the Company’s Board of Directors on May 20, 2013 and was approved
by our shareholders on January 27, 2014. Any options are granted at the fair market value of the common stock on the date of the
grant and have terms of up to ten years. Under the 2013 Omnibus Incentive Plan the price of the granted common stock options are
equal to the fair market value of such shares on the date of grant.
On September 11, 2013,
we issued 1,000,000 S-8 shares to a consultant in payment for investor relations work for the Company. On January 3, 2014, we issued
1,000,000 S-8 shares to a consultant in payment for services to be provided for the Company. We have 8,000,000 shares of common
stock available for future issuance under our 2013 Omnibus Incentive Plan as of August 31, 2014.
NOTE 12
- RELATED PARTY TRANSACTIONS AND CONTINGENCIES:
FINANCING WITH RELATED PARTIES:
During fiscal 2014 and
2013, the Company entered into financing agreements with related parties of the Company. Please see Note 10 – Notes Payable
for further descriptions of these transactions.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
NOTE 13 – BUSINESS
SEGMENTS
We identify our reportable
segments based on our management structure, financial data and market. We have identified three business segments: Personal Care
Services and Medical Device Manufacturing and Medical Products & Technologies
Our Personal Care Service
segment consists of the services of Angels of Mercy, Inc. This segment provides non-medical, Personal Care Attendant (PCA) services,
Supervised Independent Living (SIL), Long-Term Senior Care, and other approved programs performed by a trained caregiver that will
meet the health service needs of beneficiaries whose disabilities preclude the performance of certain independent living skills
related to the activities of daily living (ADL).
Our Medical Device Manufacturing
segment consists of the products of Dotolo Research Corporation. This segment designs, develops, manufactures and distributes the
Toxygen hardware system with disposable speculums and medical grade tubing.
Our Medical Products and
Technologies segment will consist of the products of Advanced Medical Products and Technologies and future acquisitions.
The accounting policies
of the segments are the same as those described, or referred to, in Note 2 - Summary of Significant Accounting Policies. Assets
and related depreciation expense in the column labeled “Corporate Overhead” pertain to capital assets maintained at
the corporate level. Segment loss from operations in the “Corporate Overhead” column contains corporate related expenses
not allocable to the operating segments. Intercompany transactions between operating segments were immaterial in all periods presented.
Below are the segment assets
as of August 31, 2014.
| As of August 31, 2014 |
| | |
Personal Care | |
Medical Device | |
Med. Products | |
Corporate | |
|
| | |
Segment | |
Segment | |
Segment | |
Overhead | |
Totals |
| | |
(Unaudited) | |
(Unaudited) | |
(Unaudited) | |
(Unaudited) | |
|
| ASSETS |
| |
| |
| |
| |
| |
|
| Current Assets: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 9,336 | | |
$ | (110 | ) | |
$ | 1,000 | | |
$ | 7,278 | | |
$ | 17,504 | |
| Accounts receivable (net) | |
| 213,399 | | |
| — | | |
| — | | |
| — | | |
| 213,399 | |
| Inventory | |
| — | | |
| 31,271 | | |
| — | | |
| — | | |
| 31,271 | |
| Prepaid expenses and other current assets | |
| — | | |
| — | | |
| — | | |
| 9,307 | | |
| 9,307 | |
| Prepaid commissions and finders' fees | |
| — | | |
| — | | |
| — | | |
| 3,152 | | |
| 3,152 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total current assets | |
| 222,735 | | |
| 31,161 | | |
| 1,000 | | |
| 19,737 | | |
| 274,633 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Property and equipment (net) | |
| 21,287 | | |
| 17,893 | | |
| — | | |
| 787 | | |
| 39,967 | |
| Deposits and other assets | |
| 2,082 | | |
| 12,500 | | |
| — | | |
| — | | |
| 14,582 | |
| Goodwill | |
| 564,075 | | |
| 1,217,704 | | |
| — | | |
| — | | |
| 1,781,779 | |
| Patents, registrations (net of amortization) | |
| — | | |
| 24,497 | | |
| — | | |
| — | | |
| 24,497 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total assets | |
$ | 810,179 | | |
$ | 1,303,755 | | |
$ | 1,000 | | |
$ | 20,524 | | |
$ | 2,135,458 | |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
Below are the segment assets
as of August 31, 2013.
| As of August 31, 2013 |
| | |
Personal Care | |
Medical Device | |
Med. Products | |
Corporate | |
|
| | |
Segment | |
Segment | |
Segment | |
Overhead | |
Totals |
| | |
| |
| |
| |
| |
|
| ASSETS |
| |
| |
| |
| |
| |
|
| Current Assets: | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 41,985 | | |
$ | (2,742 | ) | |
$ | — | | |
$ | 213 | | |
$ | 39,456 | |
| Accounts receivable (net) | |
| 104,544 | | |
| 3,775 | | |
| — | | |
| — | | |
| 108,319 | |
| Inventory | |
| — | | |
| 31,271 | | |
| — | | |
| — | | |
| 31,271 | |
| Prepaid expenses and other current assets | |
| 7,851 | | |
| 30,050 | | |
| — | | |
| 1,275 | | |
| 39,176 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total current assets | |
| 154,380 | | |
| 62,354 | | |
| — | | |
| 1,488 | | |
| 218,222 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Property and equipment (net) | |
| 55,950 | | |
| 21,461 | | |
| — | | |
| 1,122 | | |
| 78,533 | |
| Deposits and other assets | |
| — | | |
| 10,050 | | |
| — | | |
| — | | |
| 10,050 | |
| Goodwill | |
| 478,721 | | |
| 1,217,704 | | |
| — | | |
| — | | |
| 1,696,425 | |
| Patents, registrations (net of amortization) | |
| — | | |
| 30,620 | | |
| — | | |
| — | | |
| 30,620 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total assets | |
$ | 689,051 | | |
$ | 1,342,189 | | |
$ | — | | |
$ | 2,610 | | |
$ | 2,033,850 | |
Below are the statements of operations for the reporting periods
presented.
| For the Year Ended August 31, 2014 |
| | |
| Personal Care | | |
| Medical Device | | |
| Medical Products | | |
| Corporate | | |
| | |
| | |
| Segment | | |
| Segment | | |
| Segment | | |
| Overhead | | |
| Totals | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Revenues | |
$ | 3,677,475 | | |
$ | — | | |
$ | — | | |
$ | — | | |
$ | 3,677,475 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cost of revenues | |
| 3,016,221 | | |
| 46,843 | | |
| — | | |
| — | | |
| 3,063,064 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gross profit | |
| 661,254 | | |
| (46,843 | ) | |
| — | | |
| — | | |
| 614,411 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
| 487,509 | | |
| 25,371 | | |
| 329 | | |
| 620,222 | | |
| 1,133,431 | |
| Research and development expense | |
| — | | |
| 30,000 | | |
| — | | |
| — | | |
| 30,000 | |
| Depreciation and amortization | |
| 12,793 | | |
| 9,691 | | |
| — | | |
| 335 | | |
| 22,819 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total operating expenses | |
| 500,302 | | |
| 65,062 | | |
| 329 | | |
| 620,557 | | |
| 1,186,250 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| 160,952 | | |
| (111,905 | ) | |
| (329 | ) | |
| (620,557 | ) | |
| (571,839 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | | |
| | |
| Interest and finance charges | |
| (232,573 | ) | |
| (11,337 | ) | |
| — | | |
| (561,708 | ) | |
| (805,618 | ) |
| Other income (expenses) | |
| 73,282 | | |
| 66,049 | | |
| — | | |
| — | | |
| 139,331 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total other income (expense) | |
| (188,039 | ) | |
| 51,573 | | |
| — | | |
| (699,266 | ) | |
| (835,732 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
$ | (27,087 | ) | |
$ | (60,332 | ) | |
$ | (329 | ) | |
$ | (1,319,823 | ) | |
$ | (1,407,571 | ) |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL
STATEMENTS
Below are the statements
of operations for the reporting periods presented.
| For the Year Ended August 31, 2013 |
| | |
| Personal Care | | |
| Medical Device | | |
| Medical Products | | |
| Corporate | | |
| | |
| | |
| Segment | | |
| Segment | | |
| Segment | | |
| Overhead | | |
| Totals | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Revenues | |
$ | 215,248 | | |
$ | 28,998 | | |
$ | — | | |
$ | — | | |
$ | 244,246 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cost of revenues | |
| 156,708 | | |
| 38,991 | | |
| — | | |
| — | | |
| 195,699 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gross profit | |
| 58,540 | | |
| (9,993 | ) | |
| — | | |
| — | | |
| 48,547 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | | |
| | |
| General and administrative | |
| 48,439 | | |
| 49,431 | | |
| — | | |
| 231,653 | | |
| 329,523 | |
| Depreciation and amortization | |
| 1,049 | | |
| 4,048 | | |
| — | | |
| 358 | | |
| 5,455 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total operating expenses | |
| 49,488 | | |
| 53,479 | | |
| — | | |
| 232,011 | | |
| 334,978 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
| 9,052 | | |
| (63,472 | ) | |
| — | | |
| (232,011 | ) | |
| (286,431 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense): | |
| | | |
| | | |
| | | |
| | | |
| | |
| Interest and finance charges | |
| (10,000 | ) | |
| (1,877 | ) | |
| — | | |
| (22,304 | ) | |
| (34,181 | ) |
| Other income (expenses) | |
| — | | |
| 126 | | |
| — | | |
| 3,364 | | |
| 3,490 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total other income (expense) | |
| (10,000 | ) | |
| (1,751 | ) | |
| — | | |
| (355,133 | ) | |
| (366,884 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss from operations | |
$ | (948 | ) | |
$ | (65,223 | ) | |
$ | — | | |
$ | (587,144 | ) | |
$ | (653,315 | ) |
NOTE 14 - JOINT VENTURE
Institut für Umwelttechnologien GmbH
(IUT)
In February 2009, we entered
into a Technology Agreement with Institut für Umwelttechnologien GmbH, a German Company (“IUT”). On September
23, 2010, the Company signed a Memorandum of Understanding with Institut für Umwelttechnologien GmbH and IUT Medical GMBH
confirming certain understandings among the parties with respect to their future relationships and business activities as originally
contemplated in their Technology Agreement of February 27, 2009, which was reaffirmed. On November 1, 2013, with the disposal of
the Company’s subsidiary Oncologix Corporation, the company also ended its relationship with IUT and IUTM.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 15
- RETIREMENT PLAN
Currently, the Company does not have a retirement
plan in place.
NOTE 16 - INCOME TAXES
As of August 31, 2014,
the Company has federal net operating loss carry-forwards totaling approximately $25,100,000 and general business credit carry-forwards
of approximately $140,000. As a result of the acquisition of Angels of Mercy, Inc.(now “Amian Angels Inc.”), the general
business credit carry-forwards are limited to approximately $9,000 per year due to a Section 383 limitation. Currently there are
no state net operating loss carry-forwards. The federal net operating loss carry-forwards expire in various amounts beginning in
2019 and ending in 2035. The Company does not have any current state net operating loss carry-forwards. Certain of the Company's
net operating loss carry-forwards may be subject to annual restrictions limiting their utilization in accordance with Internal
Revenue Code Section 382, which include limitations based on changes in control. Due to our history of losses from operations,
we have provided a valuation allowance for our net operating loss carry-forwards and deferred tax assets, net of certain deferred
tax liabilities.
ASC 740 requires a company
to determine whether it is more likely than not that a tax position will be sustained upon examination based upon the technical
merits of the position. If the more-likely-than-not threshold is met, a company must measure the tax position to determine the
amount to recognize in the financial statements. As a result of the implementation of ASC 740 – Income Taxes, the Company
performed a review of its material tax positions. At the adoption date of ASC 740, the Company had no unrecognized tax benefit
which would affect the effective tax rate. As of August 31, 2014 and 2013, the Company had no accrued interest and penalties
related to uncertain tax positions. The Company is primarily subject to U.S. and Louisiana income taxes. The tax years 2013 to
current remain open to examination by U.S. federal and state tax authorities.
A reconciliation of the
beginning and ending accrual for uncertain tax positions is as follows:
| | |
| For the Year Ended August 31, | |
| | |
| 2014 | | |
| 2013 | |
| | |
| | | |
| | |
| Balance, beginning of year | |
$ | — | | |
$ | — | |
| Decreases in tax positions for prior years | |
| — | | |
| — | |
| Increase in tax positions for prior years | |
| — | | |
| — | |
| Increases in tax positions for current year | |
| — | | |
| — | |
| Settlements | |
| — | | |
| — | |
| Lapse in statute of limitations | |
| — | | |
| — | |
| | |
| | | |
| | |
| Balance, end of year | |
$ | — | | |
$ | — | |
The income tax benefit
for the years ended August 31, 2014 and 2013 is comprised of the following amounts:
| | |
| 2014 | | |
| 2013 | |
| Current: | |
$ | — | | |
$ | — | |
| | |
| | | |
| | |
| Deferred: | |
| | | |
| | |
| Federal | |
| (956,000 | ) | |
| (296,000 | ) |
| State | |
| — | | |
| — | |
| | |
| (956,000 | ) | |
| (296,000 | ) |
| Valuation Allowance | |
| 956,000 | | |
| 296,000 | |
| | |
$ | — | | |
$ | — | |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company's tax benefit
differs from the benefit calculated using the federal statutory income tax rate for the following reasons:
| | |
| 2014 | | |
| 2013 | |
| Statutory tax rate | |
| 35.0 | % | |
| 35.0 | % |
| State income taxes | |
| — | | |
| — | |
| Change in valuation allowance | |
| (35.0 | )% | |
| (35.0 | )% |
| Effective tax rate | |
| 0.0 | % | |
| 0.0 | % |
The components of the net
deferred tax assets (liabilities) are as follows:
| | |
| 2014 | | |
| 2013 | |
| Deferred tax assets (liabilities): | |
| | | |
| | |
| Property and equipment | |
$ | (1,000 | ) | |
$ | (1,000 | ) |
| Intangible assets | |
| 156,000 | | |
| 167,000 | |
| General business credits | |
| 140,000 | | |
| 140,000 | |
| Net operating loss carryforward | |
| 8,785,000 | | |
| 9,730,000 | |
| | |
| 9,080,000 | | |
| 10,036,000 | |
| Valuation allowance | |
| (9,080,000 | ) | |
| (10,036,000 | ) |
| | |
$ | — | | |
$ | — | |
ASC 740 - Income Taxes,
requires a valuation allowance to reduce the deferred tax assets if, based on the weight of the evidence, it is more likely than
not that some or all of the deferred tax assets will not be realized. After consideration of all the evidence, both positive and
negative, management has determined that an $9,080,000 valuation allowance as of August 31, 2014 is necessary to reduce the net
deferred tax assets to the amount that will more likely than not be realized. The decrease in the valuation allowance for the current
year is $956,000.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 17 - RECENT ACCOUNTING PRONOUNCEMENTS
We have evaluated all Accounting
Standards Updates through the date the financial statements were issued and do not believe any will have a material impact on our
financial condition or results of operations.
NEW ACCOUNTING STANDARD
In July 2012, the Financial
Accounting Standards Board (“FASB”) issued Accounting Standards Update 2012-02 “Intangibles – Goodwill
and Other (Topic 350): Testing Indefinite-Lived Intangible Assets for Impairment” (“ASU 2012-02”). ASU 2012-02
permits entities to first assess qualitative factors to determine whether it is more likely than not that the fair value of an
indefinite-lived intangible asset is impaired as a basis for determining whether it is necessary to perform the quantitative impairment
test. Under the amendments in ASU 2012-02, an entity is not required to calculate the fair value of an indefinite-lived intangible
asset unless it determines that it is more likely than not that the fair value of the asset is less than its carrying amount. An
entity also will have the option to bypass the qualitative assessment for any indefinite-lived intangible asset in any period and
proceed directly to performing the quantitative impairment test. ASU 2012-02 is effective for interim and annual indefinite-lived
intangible asset impairment tests performed for fiscal years beginning on or after September 15, 2012, with early adoption permitted.
The Company’s adoption of ASU 2012-02 is not expected to have an impact on its consolidated financial statements.
NOTE 18 – STATEMENT OF CASH FLOWS
For the year ended August
31, 2014, these supplemental non-cash investing and financing activities are summarized as follows:
Amount
| On September 11, 2013, the Company issued 1,000,000 S-8 shares of common stock in payment for a investor relations consulting contract. |
11,500 |
| |
|
| On October 2, 2013, the Company issued a $25,000 convertible promissory note to a non-related party. We recorded a beneficial conversion feature the in amount of $25,000 related to that transaction. |
25,000 |
| |
|
| On October 3, 2013, the Company recorded a loss on conversion of a convertible promissory note in the amount of $15,620. |
15,620 |
| |
|
| On November 5, 2013 and November 8, 2013, the Company issued a total of 3,000,000 warrants to a non-related party as additional compensation for an operating capital investment. |
14,805 |
| |
|
| On December 3, 2013, the Company recorded a loss on conversion of a convertible promissory note in the amount of $12,069. |
12,069 |
| |
|
| On December 3, 2013, the Company recorded a loss on conversion of a convertible promissory note in the amount of $9,720. |
9,720 |
| |
|
| On December 3, 2013, the Company issued a total of 1,000,000 warrants as additional compensation. |
5,992 |
| |
|
| On December 20, 2013, the Company issued a total of 3,000,000 warrants as additional compensation. |
7,746 |
| |
|
| |
|
| |
|
| |
|
| |
|
|
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
| On January 3, 2014, the Company issued 2,000,000 shares of common stock in payment for a services contract. |
22,000 |
| |
|
| On January 13, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $26,154. |
26,154 |
| |
|
| On January 14, 2014, the Company issued 1,000,000 shares of common stock as partial compensation for a investor relations contract. |
19,000 |
| |
|
| On January 21, 2014, the Company issued 3,500,000 shares of common stock as additional compensation for finder’s fees. |
45,500 |
| |
|
| On January 21, 2014, the Company issued 1,500,000 shares of common stock as additional compensation for finder’s fees. |
30,000 |
| |
|
| On January 31, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $16,667. |
16,667 |
| |
|
| On February 7, 2014, the Company issued 1,000,000 shares of common stock as additional compensation for finder’s fees. |
9,000 |
| On February 7, 2014, the Company issued 3,000,000 shares of common stock as additional compensation for finder’s fees. |
5,151 |
| |
|
| On February 24, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $2,308. |
2,308 |
| |
|
| On March 19, 2014, the Company recorded a beneficial conversion feature on the issuance of a convertible note. |
26,500 |
| |
|
| On March 31, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $30,000. |
30,000 |
| |
|
| On April 6, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $1,468. |
1,468 |
| |
|
| On April 6, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $30,683 |
30,683 |
| |
|
| On April 8, 2014, the Company recorded a beneficial conversion feature on the issuance of a convertible note. |
50,000 |
| |
|
| On April 25, 2014, the Company recorded a beneficial conversion feature on the issuance of a convertible note. |
12,445 |
| |
|
| On May 21, 2014 the Company recorded a discount for the issuance of 9,583,333 warrants in connection with the issuance of a convertible note. |
38,322 |
| |
|
| On June 2, 2014, the Company issued 5,000,000 shares of common stock as payments on a services contract. |
28,500 |
| |
|
| On July 14, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $1,500. |
1,500 |
| |
|
| |
|
| |
|
|
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
| On July 24, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $4,885. |
4,885 |
| |
|
| On July 16, 2014, the Company recorded a beneficial conversion feature on the issuance of a convertible note. |
26,500 |
| |
|
| On August 1, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $16,828. |
16,828 |
| |
|
| On August 26, 2014, the Company recorded a loss on conversion of a convertible promissory note in the amount of $1,784. |
1,784 |
| |
|
| On May 21, 2014 the Company recorded a discount for the issuance of 4,000,000 warrants in connection with the issuance of a convertible note. |
10,177 |
| |
|
| Total non-cash transactions from investing and financing activities. |
$ 557,824 |
| |
|
For the year ended August
31, 2013, these supplemental non-cash investing and financing activities are summarized as follows:
| On October 31, 2012, the Company entered into a note payable agreement to finance $10,404 of directors and officer’s insurance premiums. The note bears interest at a rate of 9.27% per annum and was due in ten monthly installments of $1,085, including principal and interest, beginning on November 30, 2012. |
$ 10,404 |
| |
|
| On February 8, 2013, the Company recognized a loss on the conversion of a related party convertible note payable in the amount of $10,241. |
10,241 |
| |
|
| On May 13, 2013 the Company issued 1,000,000 shares as additional compensation for the issuance of a one year promissory note. These shares were valued at $8,000, the closing price |
8,000 |
| |
|
| On August 1, 2013, the Company issued 1,000,000 four year warrants as additional consideration for the purchase of Angels of Mercy, Inc. The value of these options, calculated using the Black-Scholes method were included in the purchase price of Angels of Mercy, Inc. |
22,586 |
| |
|
| On August 5, 2013 the Company issued 6,000,000 three year warrants for finder’s fees in connection with funds raised through a stock purchase agreement. The value of these options calculated using the Black-Scholes method were recorded and interest and finance charges in our financial statements. |
137,406 |
| |
|
| Total non-cash transactions from investing and financing activities. |
$ 188,637 |
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
NOTE 19 - EMPLOYMENT AGREEMENTS
On March 22, 2013, Wayne
Erwin, the Company’s Chief Executive Officer, signed a three year employment agreement. The agreement provides for an annual
salary of $120,000 along with a monthly auto allowance and health insurance allowance totaling $1,250. Annual increases are to
be approved by the Company’s Board of Directors or Compensation Committee. During fiscal 2014 and 2013, $120,000 and 52,500
was expensed as salary, respectively.
On April 1, 2013, Michael
Kramarz, the Company’s Chief Financial Officer, signed a three year employment agreement. The agreement provides for an annual
salary of $58,000 along with a monthly auto allowance and health insurance allowance totaling $500. Annual increases are to be
approved by the Company’s Board of Directors or Compensation Committee. On October 1, 2013, the Company’s Board of
Directors approved a salary increase to $80,000 per year. During fiscal 2014 and 2013, $80,225 and 80,066 was expensed as salary,
respectively.
On August 1, 2013, Vickie
Hart, the President of Amian Angels Inc., signed a three year employment agreement. The agreement provides for an annual salary
of $52,000 along with a monthly health insurance allowance totaling $400. Annual increases are to be approved by the Company’s
Board of Directors or Compensation Committee. During fiscal 2014 and 2013, $59,615 and nil was expensed as salary, respectively.
On July 16, 2014, Harold
Halman, the President of our Medical Products Segment, signed a three year employment agreement. The agreement provides for an
annual salary of $85,000, along with a monthly auto allowance and health insurance allowance totaling $1,300 plus bonus allowances.
Annual increases are to be approved by the Company’s Board of Directors or Compensation Committee. During fiscal 2014 and
2013, $10,625 and nil was expensed as salary, respectively.
NOTE 20 – COMMITMENTS AND CONTINGENCIES
On June 6, 2014, the Company
and its subsidiary Dotolo entered into a services agreement with E & R Industries to provide the Company with retooling and
redesign of some of Dotolo’s parts. The contract calls for periodic cash payments totaling $60,000 along with the issuance
of 5,000,000 common stock shares upon meeting certain milestones.
On June 6, 2014, the Company
and its subsidiary Dotolo entered into a services agreement with Schmitt to provide the Company with redesign of its Toxygen hardware
system. The contract calls for periodic cash payments totaling $30,000 along with the issuance of 3,000,000 common stock shares
upon meeting certain milestones.
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
NOTES TO
CONSOLIDATED FINANCIAL STATEMENTS
NOTE 21
- SUBSEQUENT EVENTS
On September 11, 2014,
the Company obtained a merchant loan for additional working capital in the amount of $100,000. This loan requires 75 daily payments
in the amount of $1,999 for a total repayment amount of $149,900. We netted gross proceeds of $99,295 after paying loan fees.
On September 25, 2014,
the Company took down a second draw from its 4 million dollar line of credit facility in the amount of $1,200,000. The Company
must meet specific monthly reporting and collateral requirements to further draw on the revolving credit facility. The outstanding
balance at the time of the draw was $1,528,029 which is secured by twelve month 14.5% promissory note, which is convertible ONLY
upon default by the Company. This note is automatically renewable for an additional twelve months. The company is required to pay
interest and fees only for the initial 3 months.
On September 25, 2014,
a closing was held pursuant to a Stock Purchase Agreement, dated as of September 25, 2014, (the “Agreement”) by and
among Oncologix Tech Inc. (“OCLG” or “Company”), and Madhu Mathew Mammen and Imad Siddiqui (collectively
the “Owners”), for Company to acquire 100 shares of Common Stock of Esteemcare Inc. and its wholly owned subsidiary
Affordable Medical Equipment Solutions Inc. (“Esteem”), which represents all of the issued and outstanding shares of
Esteem. Pursuant to the Agreement, the Owners sold all of the Common Stock of Esteem for $500,000 represented by a down payment
of $400,000 at closing and a one year Secured Promissory Note for $100,000. The Owners own all of the shares of Esteem, a corporation
organized under the laws of the State of South Carolina, a medical products and technology company.
On October 2, 2014, the
company paid off a $50,000 convertible note borrowed from an accredited investor. The Company paid $71,436 which included a payment
for principal, accrued interest and a 40% prepayment penalty.
Between October 28, 2014
and November 10, 2014, an accredited investor converted $15,000 of principal of a convertible note into 4,706,651 shares of common
stock.
On November 12, 2014, the
Company entered into a consulting contract with a vendor and issued 6,000,000 shares of its common stock. The Company recorded
an expense of $24,000 for this transaction.
On November 24, 2014, the
Company paid its first installment of $34,779 for a convertible note, which included $$6,029 of accrued interest. This convertible
note was originally issued May 23, 2014.
Item 9. Changes
in and Disagreements with Accountants on Accounting and Financial Disclosure
None
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management,
under the supervision and with the participation of our Chief Executive Officer and our Chief Financial Officer, evaluated the
effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e)
under the Securities Exchange Act of 1934, as amended (the “Exchange Act.”)) and based upon this evaluation, and the
engagement of a qualified outside third party review of our disclosure controls and procedures, concluded that as of August 31,
2014, our disclosure controls and procedures were not effective in ensuring that information required to be disclosed by us in
the reports filed or submitted by us under the Exchange Act is (i) recorded, processed, summarized and reported, within the time
periods specified in the Commission’s rules and forms and (ii) accumulated and communicated to our management, including
our principal executive and financial officers, or persons performing similar functions, as appropriate to allow timely decisions
regarding required disclosure.
Management’s Report on Internal Control over Financial
Reporting
Our
management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control
over financial reporting, defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Exchange Act, is a process designed by,
or under the supervision of, the company’s principal executive officer who is also our principal financial officer and effected
by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability
of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted
accounting principles in the United States and includes those policies and procedures that:
| · | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect
the transactions and dispositions of the assets of the Company; |
| · | provide reasonable assurance that transactions are recorded as necessary to permit preparation
of financial statements in accordance with generally accepted accounting principles in the United States, and that receipts and
expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and |
| · | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition,
use or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because
of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement
of our financial statements would be prevented or detected. Also, projections of any evaluation of effectiveness to future periods
are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate. To mitigate the current limited resources and limited employees, we rely heavily
on direct management oversight of transactions, along with the use of legal and accounting professionals. As we grow, we expect
to increase our number of employees and engage outsourced accounting professionals, which will enable us to implement adequate
segregation of duties within the internal control framework.
Management
of the Company conducted an assessment of the effectiveness of our internal control over financial reporting based on the framework
set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control - Integrated
Framework. As part of this assessment management has taken into consideration that we are a small company, and due to the fact
that we have a limited number of employees, we are not able to have proper segregation of duties and have limited technical accounting
research capabilities. Based on this assessment, management concluded that as of August 31, 2014, we had a material weakness in
our internal control over financial reporting because of the lack of segregation of duties and the limited technical accounting
capabilities. The board of directors, as part of their review of the quarterly and annual financial statements, has complete access
to the detailed
financial information
of the Company for further review and verification of all financial transactions during the reporting periods. Over the next few
months, our plan is to increase the number of independent board members including adding a financial expert. Management believes
these changes and detailed review by the board of directors enhance our effectiveness over financial reporting to provide reasonable
assurance regarding the reliability of financial reporting and preparation of financial statements for external purposes in accordance
with generally accepted accounting principles in the United States.
Attestation Report
of Independent Registered Public Accounting Firm
This
annual report on Form 10-K does not include an attestation report of our independent registered public accounting firm regarding
internal control over financial reporting. Management’s report was not subject to attestation by our independent registered
public accounting firm pursuant to an exemption for non-accelerated filers set forth in Section 989G of the Dodd-Frank Wall Street
Reform and Consumer Protection Act.
Changes in Internal Control Over Financial
Reporting
There were no changes in
our internal controls over financial reporting that occurred during the three months ended August 31, 2014 that have materially
affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
Item 9B. Other Information
None
PART III
Item 10. Directors,
Executive Officers and Corporate Governance
The following table sets
forth our directors, and executive officers, their ages and all offices and positions held. Directors are elected and serve thereafter
until their successors are duly elected by the stockholders. Officers and other employees serve at the will of the Board of Directors.
The information is presented as of October 20, 2014:
| Name |
Age |
Position
held with Company |
| Roy Wayne Erwin |
56 |
Chief Executive Officer, President and Director |
| Michael Kramarz |
45 |
Chief Financial Officer, Secretary |
| Victoria Hart |
60 |
President of Angels of Mercy, Inc. Subsidiary. |
| Harold Halman |
|
|
| Barry Griffith |
46 |
Director |
Roy Wayne Erwin-
Chairman and CEO, has served as the Chairman/CEO of Oncologix Tech Inc. since March 2013, and played an important role in the acquisitions
of Dotolo Research Corporation and Amian Angels Inc. Since 2010, Wayne Erwin has been the Chief Executive Officer of Deep South
Capital, LLC., a company with very limited operations. From 2007 to 2010, Mr. Erwin was the co-founder and Chief Operations Officer
of Electronic Health Network, a leader in Healthcare and Medical Information Technology. From 2007 to 2004 he was the Chief Operating
Officer and Director of New Business Development at Crossroads Regional Hospital, a 68-bed, acute care, in-patient and outpatient
psychiatric and Substance Abuse Facility. From 1995 to 2004, Mr. Erwin was the Regional Director of Sales for Centerpulse Orthopedics,
Inc, a Division of Sulzer Corporation, a world-wide, $ 3.0 billion Swiss conglomerate specializing in orthopedic total joint reconstruction
of hip, knees and shoulder products. Prior to 1995, Mr. Erwin was employed by Valley Lab, Inc., Ball Aerospace and Texas Instruments
in various senior management capacities. He served in the US Army, with the rank of Captain, at the 101st Airborne Division, with
overseas assignments in Panama and Honduras and has advanced military training in Air Borne, Air Assault, and Jungle Warfare training.
Wayne graduated with a Bachelor of Science from Louisiana College- Pineville, Louisiana.
Michael A. Kramarz-
has served as Chief Financial Officer of the Company since July 15, 2004. Mr. Kramarz was first employed by the Company in September
2002, as its Controller and now has over 18 combined years working for public companies. Mr. Kramarz is responsible for all financial
statement, accounting, SEC compliance, payroll and tax functions. From 1995 to 2002, Mr. Kramarz was employed as Accounting Manager
for Assurant Group, a 6 billion dollar
insurance
company. Mr. Kramarz was responsible for the accounting, budgeting and payroll functions of one of Assurant Group’s 20+ million
dollar call center subsidiaries with offices in Michigan and Oklahoma. In addition, Mr. Kramarz was responsible for quarterly consolidations
into the parent company to assist with its SEC and tax filings. From 1992 to 1995, Mr. Kramarz was a staff accountant at VandenToorn
& Associates CPA firm where he was responsible for compilations and reviews of financial statements, as well as tax return
preparation. Mr. Kramarz holds a Certified Management Accountant Designation (CMA) and a Certified Public Accountant Designation
(CPA). Mr. Kramarz holds a Bachelor of Science and Business Administration in Accounting from Aquinas College and a Masters in
the Science of Taxation from
Grand Valley
State University.
Barry Griffith-,
Board of Director, Barry been a Director of the company since December of 2004. Mr. Griffith brings 20 years of early stage and
upstart medical device company experience to Oncologix. Mr. Griffith has been involved in the introduction of novel medical devices
in the Orthopedic, Vascular, Neurological and Cancer markets for companies such as Mitek, Schneider, Novoste and Medtronic. His
present position is founder and principal of The Bench which is and executive search firm within the medical device industry based
out of Newport Beach Ca. Prior to that, he was Director of Sales with Cardiovascular Systems, Inc., Director of Sales for Calypso
Medical Technologies and held the Western Area Director roles with Novoste and Isoray.
Vickie Hart-
President- Amian Angels Services Division, Ms. Hart is the President of Angels of Mercy and brings over 35 years of senior management
and healthcare services experience. Since 2011 through 2013, she was the Owner and President of Triple E Healthcare Services, a
healthcare services consulting firm located in Alexandria, Louisiana. From 1992 through 2010, Ms. Hart was an Assistant Principal
and teacher in Elementary and Secondary education for the Rapides Parish School Systems. Ms. Hart is active in civic and charitable
organizations, recently served on the D.A.R.E. Board of Directors, Central Louisiana Lions Club, and the local United Way. Vickie
Hart holds a Bachelor of Science Degree from Louisiana State University and a Master Degree in Management from Northwestern State
University, Natchitoches, Louisiana.
Harold Halman-
President/COO- Medical Product & Technology Division- Mr. Halman brings 20+ years of new business development, medical sales,
marketing and operations experience to OCLG. Prior to joining the company, Harold was the Chief Executive Officer for Global Medical
located in Phoenix, Arizona, a national provider for home medical equipment. Mr Halman has vast knowledge and direct industry experience
in medical devices and home medical products that will accelerate expansion within the company’s Medical Products and Technology
Division both domestically and internationally. Prior to Global Medical, Mr. Halman was CEO of his management consulting firm involved
in M&A, business turnarounds and business development projects. His direct experience includes bringing forth breakthrough
medical products and technologies to the market with aggressive, multi-tiered distribution strategies.
Compliance with Section 16(a) of the Exchange
Act
Directors, executive officers
and holders of more than 10% of our outstanding common stock are required to comply with Section 16(a) of the Securities Exchange
Act of 1934, which requires generally that such persons file reports regarding ownership of transactions in securities of the Company
on Forms 3, 4, and 5. Based solely on its review of such forms received by it, or written representations from certain reporting
persons, the Company believes that all Section 16(a) filing requirements applicable to its officers and directors were complied
with during the fiscal year ended August 31, 2013.
Code of Ethics
Our Board of Directors
has adopted a code of ethics that applies to our principal executive officer, principal financial officer and to other persons
performing similar functions. The code of ethics is designed to deter wrongdoing and to promote honest and ethical conduct, full,
fair, accurate, timely and understandable disclosure, compliance with applicable laws, rules and regulations, prompt internal reporting
of violations of the code and accountability for adherence to the code. We will provide a copy of our code of ethics, without charge,
to any person upon receipt of written request for such delivered to our corporate headquarters. All such requests should be send
to Oncologix Tech, Inc., P.O. Box 8832, Grand Rapids, MI 49518-8832.
Item 11. Executive Compensation
The following table summarizes
all compensation paid for services rendered to Oncologix for the fiscal years ended August 31, 2014 and 2013 by our Principal
Executive Officer and our two most highly compensated executive officers other than our principal executive officer. None of the
Company's other employees received compensation in excess of $100,000 during the last completed fiscal year.
| SUMMARY COMPENSATION TABLE |
| Name |
|
|
|
|
|
|
Non-qualified |
|
|
| and |
|
|
|
|
|
Nonequity |
deferred |
|
|
| Principal |
|
|
|
Stock |
Option |
incentive plan |
compensation |
All Other |
All Other |
| Position |
Year |
Salary |
Bonus ($) |
awards($) |
awards($) |
compensation($) |
earnings($) |
compensation($) |
Total($) |
| |
|
|
|
|
|
|
|
|
|
| Roy Wayne Erwin |
2014 |
$ 120,000 |
$ - |
$ - |
$ - |
$ - |
$ - |
$ - |
$ 120,000 |
| Chief Executive Officer (1) |
2013 |
$ 52,500 |
$ - |
$ - |
$ - |
$ - |
$ - |
$ - |
$ 52,500 |
| |
|
|
|
|
|
|
|
|
|
| Michael A. Kramarz |
2014 |
$ 80,225 |
$ - |
$ - |
$ - |
$ - |
$ - |
$ - |
$ 80,225 |
| Chief Financial Officer (2) |
2013 |
$ 80,066 |
$ - |
$ - |
$ - |
$ - |
$ - |
$ - |
$ 80,066 |
| (1) | On March 22, 2013, Wayne Erwin, was elected the Company’s Chief Executive Officer, signed
a three year employment agreement. The agreement provides for an annual salary of $120,000 along with a monthly auto allowance
and health insurance allowance totaling $1,250. Annual increases are to be approved by the Company’s Board of Directors or
Compensation Committee. |
| (2) | Mr. Kramarz was hired by Oncologix as our Controller on September 16, 2002. Mr. Kramarz was appointed
Chief Financial Officer on July 15, 2004. Mr. Kramarz salary was set at $76,000 annually on June 25, 2007. From January 1, 2008
to March 31, 2013, Mr. Kramarz has been serving as Chief Financial Officer and was being compensated on a consulting basis. On
April 1, 2013, Mr. Kramarz signed a three year employment agreement. The agreement provides for an annual salary of $58,000 along
with a monthly auto allowance and health insurance allowance totaling $500. On October 1, 2013 the Board of Directors increased
the salary to $80,000. |
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR END |
| |
|
|
|
|
|
|
|
|
|
|
|
| |
Option awards |
|
Stock awards |
| |
|
|
Equity incentive |
|
|
|
|
|
|
|
|
| |
|
|
plan awards: |
|
|
|
|
|
Equity incentive |
Equity incentive |
|
| |
Number of |
Number of |
number of |
|
|
|
|
|
plan awards: |
plan awards: |
|
| |
securities |
securities |
securities |
|
|
|
|
|
number of |
market or payout |
|
| |
underlying |
underlying |
underlying |
|
|
|
Number of |
Market value |
unearned shares, |
value of unearned |
|
| |
unexercised |
unexercised |
unexercised |
|
|
|
shares or units of |
of shares or units |
units or other |
shares, units or |
|
| Name and Principal |
options |
options |
unearned |
Option exercise |
Option expiration |
|
stock that have |
of stock that have |
rights that have |
other rights that |
|
| Position |
(#) Exercisable |
(#) Unexercisable |
options (#) |
price($) |
date |
|
not vested (#) |
not vested ($) |
not vested (#) |
have not vested ($) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
| Roy Wayne Erwin |
2,400,000 |
- |
- |
$ 0.015 |
12/13/23 |
|
- |
$ - |
- |
$ - |
|
| |
10,000 |
|
|
$ 0.016 |
12/20/23 |
|
|
|
|
|
|
| Chief Executive Officer |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
| Michael A. Kramarz |
12,750 |
- |
- |
$ 0.120 |
01/07/18 |
|
- |
$ - |
- |
$ - |
|
| Chief Financial Officer |
2,100,000 |
- |
- |
$ 0.015 |
12/13/23 |
|
|
|
|
|
|
Options Grants
On December 13, 2013, the
Board of Directors Granted 2,400,000 and 2,100,000 options to our CEO and CFO, respectively. These options were granted at $.015,
the closing stock price on December 13, 2013.
Option Exercise
There were no other options
exercised by the Named Executive Officers and the Company did not amend or adjust the exercise price of any stock options during
fiscal 2014 or 2013.
| DIRECTOR COMPENSATION |
| |
|
|
|
|
|
|
|
| Name |
|
|
|
|
Non-qualified |
|
|
| and |
Fees earned |
|
|
Nonequity |
deferred |
|
|
| Principal |
or paid in |
Stock |
Option |
incentive plan |
compensation |
All Other |
All Other |
| Position |
cash ($) |
awards($) |
awards($) |
compensation($) |
earnings($) |
compensation($) |
Total($) |
| |
|
|
|
|
|
|
|
| Roy Wayne Erwin |
$ - |
$ - |
$ 160 |
$ - |
$ - |
$ - |
$ 160 |
| |
|
|
|
|
|
|
|
| Barry Griffith |
$ - |
$ - |
$ 160 |
$ - |
$ - |
$ - |
$ 160 |
All directors are reimbursed
for their reasonable out-of-pocket expenses incurred in connection with attendance of board meetings and advising and consulting
with the officers and management from time to time. In addition, each director receives options to purchase 20,000 shares of common
stock upon election to the board and annual grants of 10,000 options for each year of service thereafter. On December 20, 2013,
the board of directors granted each board member 10,000 options at $.016 as part of the annual option program. The board of directors
elected to waive the annual options due for fiscal years 2012, 2011 and 2010. The options vest one year from the date of the grant
and terminate upon the earlier of 10 years from the date of grant or six months after the director ceases to be a member of the
Board.
No compensation has been
paid to any members of the Company’s Board of Directors for fiscal 2014 or 2013.
Item 12. Security Ownership of Certain Beneficial
Owners and Management and Related Stockholder Matters
The following table sets
forth as of October 20, 2014, certain information with regard to the beneficial ownership of our common stock held by (i) each
shareholder known by us to beneficially own 5% or more of our outstanding common stock, (ii) each director individually, (iii)
the named executive officers and (iv) all of our officers and directors as a group:
| |
Name and Address |
Amount and Nature |
|
Percent |
| Title of Class |
of Beneficial Owner (2) |
of Beneficial Owner (1) |
|
of Class (1)(3) |
| |
|
|
|
|
| Common Stock |
Roy Wayne Erwin |
58,474,000 |
(4) |
28.55% |
| |
|
|
|
|
| Common Stock |
Michael Kramarz |
2,112,750 |
(5) |
1.42% |
| |
|
|
|
|
| Common Stock |
Vickie Hart |
1,600,000 |
(6) |
1.08% |
| |
|
|
|
|
| Common Stock |
Harold Halman |
0 |
|
0.00% |
| |
|
|
|
|
| Common Stock |
Barry Griffith |
235,000 |
(7) |
0.16% |
| |
|
|
|
|
| |
|
|
|
|
| Common Stock |
Anthony Silverman |
6,222,395 |
(8) |
4.25% |
| |
7625 E Via Del Reposo |
|
|
|
| |
Scottsdale, AZ 85028 |
|
|
|
| |
|
|
|
|
| Common Stock |
Donald Schreifels |
26,318,570 |
(9) |
17.10% |
| |
6900 Wedgewood Drive, #340 |
|
|
|
| |
Minneapolis, MN 55311 |
|
|
|
| |
|
|
|
|
| Common Stock |
All directors and executive officers |
62,421,750 |
|
29.93% |
| |
as a group |
|
|
|
Less than 1%
| (1) | Unless otherwise noted, the address of each holder is P.O. Box 8832, Grand Rapids, MI 49518-8832. |
| (2) | A person is deemed to be the beneficial owner of securities that can be acquired within 60 days
from October 20, 2014 through the exercise of any option, warrant or other right. Shares of Common Stock subject to options, warrants
or rights which are currently exercisable or exercisable within 60 days are deemed outstanding solely for computing the percentage
of the person holding such options, warrants or rights, but are not deemed outstanding for computing the percentage of any other
person. |
| (3) | The amounts and percentages in the table are based upon 135,658,353 shares of Common Stock outstanding
as of November 8, 2013. |
| (4) | Includes 56,064 shares of Series D Convertible Preferred Stock, each share convertible into 1,000
shares of the Company’s common stock, and 2,410,000 shares subject to vested options. |
| (5) | Includes 2,112,750 shares subject to vested options. |
| (6) | Includes 1,600,000 shares subject to vested options. |
| (7) | Includes 35,000 shares subject to vested options, direct ownership of 200,000 shares of stock underlying
units held. |
| (8) | Includes 3,500 shares subject to vested options, direct ownership of 6,218,895 shares |
| (9) | Includes direct ownership of 18,818,750 shares of common stock and direct ownership of 7,500,000
three-year warrants to purchase common stock. |
Item 13. Certain Relationships and Related
Transactions, and Director Independence
On September 14, 2012,
the Company issued a 90-Day promissory note to Anthony Silverman, its President and CEO, in the principal amount of $10,000. The
note bore interest at 6% per annum. This note was further extended to February 11, 2013. On February 8, 2013, this note was paid
off together with accrued interest of $242 by the issuance of a convertible promissory note. Please see Convertible Related Party
Notes Payable for further information.
On October 11, 2012, the
Company issued a 30-Day promissory note to Anthony Silverman, its President and CEO, in the principal amount of $5,000. The note
bore an interest rate of 6%. This note was paid off, together with accrued interest of $5 on October 17, 2012.
On November 23, 2012 the
Company issued a 90-Day promissory note to Anthony Silverman, its President and CEO, in the principal amount of $5,000. The note
bore interest at 6% per annum. This note was paid off, together with accrued interest of $39 on January 9, 2013.
On March 8, 2013, the Company
issued a 60-Day promissory note to Anthony Silverman, its former President and CEO, in the principal amount of $2,686. The note
bore interest rate of 6% and the due date has been extended to August 31, 2013. This note was paid off on August 16, 2013 together
with accrued interest of $71.
During the last two years,
Wayne Erwin, our President and CEO, has advanced a total of $51,600 directly to Dotolo in an open advance account. Interest is
being accrued at a rate of 6% per annum. As of August 31, 2014 we have accrued interest in the amount of $6,342. There is no specific
due date on this note.
During April 2013, Wayne
Erwin, our President and CEO, had advanced a total of $10,675 to Oncologix Tech, Inc. This note bore interest at 6%. This note
was paid in full in September 2013 together with accrued interest of $223.
Director Independence
The board has determined
that one of the current directors would qualify as independent director as that term is defined in the listing standards of the
NYSE Amex. Such independence definition includes a series of objective tests, including that the director is not an employee of
the company and has not engaged in various types of business dealings with the company.
Item 14. Principal Accountant Fees and Services
The
following table sets forth approximate fees billed to us by our auditors during the fiscal years ended August 31, 2014 and 2013
for: (i) services rendered for the audit of our annual financial statements and the review of our quarterly financial statements;
(ii) services by our auditor that are reasonably related to the performance of the audit or review of our financial statements
and that are not reported as Audit Fees; (iii) services rendered in connection with tax compliance, tax advice and tax planning;
and (iv) all other fees for services rendered.
| |
August 31, 2014 |
August 31, 2013 |
| |
|
|
| (i) Audit Fees |
$ 50,799 |
$ 14,046 |
| (ii) Audit Related Fees |
$ -- |
$ -- |
| (iii) Tax Fees |
$ -- |
$ -- |
| (iv) All Other Fees |
$ -- |
$ -- |
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a)(1) The financial statements listed in the
index set forth in Item 7 of this Form 10-K is filed as part of this report.
(a)(2) Exhibits
| Number |
Description of Filing |
Method |
| 2.1 |
Agreement of Merger and Plan of Reorganization between BestNet Communications Corp, Oncologix Corporation and JDA Medical Technologies, Inc. |
(9) |
| 3.1 |
Articles of Incorporation, as originally filed with the Nevada Secretary of State on February 19, 1998, and as amended to date |
(1) |
| 3.3 |
Certificate of Amendment to Articles of Incorporation, as originally filed with the Nevada Secretary of State. |
(6) |
| 3.4 |
Amended Certificate of Designations, Rights, Preferences and Limitations of Series A Convertible Preferred Stock, as originally filed with the Nevada Secretary of State on November 19, 2003. |
(6) |
| 3.5 |
Amended Certificate of Designations, Rights, Preferences and Limitations of Series B Convertible Preferred Stock, as originally filed with the Nevada Secretary of State on November 19, 2003. |
(6) |
| 3.6 |
Amended Certificate of Designations, Rights, Preferences and Limitations of Series C Convertible Preferred Stock, as originally filed with the Nevada Secretary of State on November 19, 2003. |
(6) |
| 3.7 |
Amended and Restated Bylaws of BestNet Communications Corp. |
(8) |
| 3.8 |
Certificate of Amendment of Articles of Incorporation, as originally filed with the Nevada Secretary of State. |
(11) |
| 3.9 |
Certificate of Designations, Rights, Preferences and Limitations of Series D Convertible Preferred Stock, as originally filed with the Nevada Secretary of State on April 22, 2013 |
(13) |
| 4 |
2000 Incentive Stock Plan |
(2) |
| 4.1 |
Form of Unit Purchase Agreement |
(7) |
| 10.1 |
Securities Purchase Agreement between Wavetech and the investor and the Placement Agent |
(3) |
| 10.2 |
Registration Rights Agreement between Wavetech, the Investor and the Placement Agent |
(3) |
| 10.3 |
Registration Right Agreement |
(3) |
| 10.4 |
Securities Purchase Agreement |
(3) |
| 10.5 |
Product Customization Agreement |
(4) |
| 10.6 |
Purchase Agreement by and among Softalk, Inc., Interpretel (Canada) Inc. and Wavetech International, Inc. dated October 25, 1999 |
(5) |
| 10.7 |
Amendment No. 1 to Amended and Restated License Agreement |
(5) |
| 10.8 |
Amended and Restated License Agreement |
(5) |
| 10.9 |
Share Exchange Agreement by and among Wavetech International, Inc., Interpretel (Canada) Inc. and Softalk, Inc. dated November 13, 1999 |
(5) |
| 10.10 |
Minutes of Settlement between BestNet Communications Corp. and Softalk, Inc. |
(8) |
| 10.11 |
Lease Agreement Dated November 1, 2005, by and between Noto’s Properties LLC. and BestNet |
(10) |
| 10.12 |
Lease Agreement Dated July 25, 2006, by and between R & J Ventures LLC. and Oncologix Corporation |
(10) |
| 10.13 |
Lease Agreement Dated July 12, 2006, by and between Office Suites Plus and Oncologix Corporation |
(10) |
| 10.14 |
Form of Note and Warrant Purchase Agreement between BestNet and Mountainview Opportunistic Growth Fund LP |
(10) |
| 10.15 |
Form of Note Purchase Agreement Issued July 7, 2006 |
(10) |
| 10.16 |
Franco Consulting Agreement |
(9) |
| 10.17 |
Kennedy Employment Agreement |
(9) |
| 10.18 |
Green Employment Agreement |
(9) |
| 10.19 |
Lowe Employment Agreement |
(9) |
| 10.20 |
License to Fountain Pharmaceuticals, Inc. |
(11) |
| 10.21 |
Erwin Employment Agreement |
(12) |
| 10.22 |
Kramarz Employment Agreement |
(13) |
| 10.23 |
Hart Employment Agreement |
(13) |
| 10.24 |
Halman Employment Agreement |
* |
| |
|
|
| 14.1 |
Oncologix Tech, Inc. Code of Ethics |
(6) |
| 21 |
Subsidiaries of the Registrant |
* |
| 32.1 |
Section 906 Certification of Roy Wayne Erwin |
* |
| 32.2 |
Section 906 Certification of Michael A. Kramarz |
* |
| 31.1 |
Certification of Chief Executive Officer |
* |
| 31.2 |
Certification of Chief Financial Officer |
* |
| 99 |
Consent of Seale and Beers, CPAs |
* |
| (1) | Incorporated by reference to the like numbered exhibit to Form 10-QSB for the quarter ended February
28, 1998. |
| (2) | Incorporated by reference to the like numbered exhibit to Form S-8 as filed on May 29, 2001. |
| (3) | Incorporated by reference to exhibit 4.2 to Form 8-K filed on May 16, 2000. |
| (4) | Incorporated by reference to exhibit 10.1 to the Form 10-K for the fiscal year ended August 31,
2000. |
| (5) | Incorporated by reference to the Form 10-KSB for the fiscal year ended August 31, 1999, exhibits
10.6, 10.7, 10.8 and 10.9 were numbered exhibits 10.1, 10.2, 10.3 and 10.4 respectively in the Form 10-KSB for the year ended August
31, 1999. |
| (6) | Incorporated by reference to the Form 10-KSB for the fiscal year ended August 31, 2003. |
| (7) | Incorporated by reference to the Form 10-QSB for the quarter ended May 31, 2003, exhibit 4.4 was exhibit 10.1 in the Form 10-QSB
for the quarter ended May 31, 2003. |
| (8) | Incorporated by reference to the Form 10-KSB for the fiscal year ended August 31, 2004. |
| (9) | Incorporated by reference to the Current Report on Form 8-K, dated July 26, 2006. |
| (10) | Incorporated by reference to the Form 10-KSB for the fiscal year ended August 31, 2006. |
| (11) | Incorporated by reference to the Form 10-KSB for the fiscal year ended August 31, 2007 |
| (12) | Incorporated by reference to the Form 8-K filed on March 27, 2013 |
| (13) | Incorporated by reference to the Form 10-K for the fiscal year ended August 31, 2013. |
| (b) | Reports on Form 8-K Filed during
the Last Quarter of The Period Covered by This Report are as Follows: |
None
SIGNATURES
In accordance with Section
13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly
authorized.
| |
ONCOLOGIX TECH, INC. |
| |
|
| |
|
| Date: December 15, 2014 |
By: /s/ Roy Wayne Erwin |
| |
Name: Roy Wayne Erwin |
| |
Title: Chief Executive Officer and President |
| Date: December 15, 2014 |
By: /s/ Michael A. Kramarz |
| |
Name: Michael A. Kramarz |
| |
Title: Chief Financial Officer |
| |
|
In accordance with the
Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and
on the dates indicated.
| By: /s/ Roy Wayne Erwin |
Date: December 15, 2014 |
| Roy Wayne Erwin, Principal Executive Officer |
|
| |
|
| By: /s/ Michael A. Kramarz |
Date: December 15, 2014 |
| Michael A. Kramarz, Principal Financial Officer |
|
| |
|
| By: /s/ Michael A. Kramarz |
Date: December 15, 2014 |
| Michael A. Kramarz, Principal Accounting Officer |
|
| |
|
| By: /s/ Barry Griffith |
Date: December 15, 2014 |
| Barry Griffith, Director |
|
Exhibit
10.24
EXECUTIVE EMPLOYMENT
AGREEMENT
This EXECUTIVE EMPLOYMENT AGREEMENT
("Agreement") is made and entered into as of July 16, 2014, by and between Oncologix Tech Inc, (Company), a company duly
incorporated and validly existing under the laws of Nevada and Mr. Harold Halman ("Executive"), with principal office
located at 2640 West Adventure Court Anthem Arizona 85086
RECITALS
A. WHEREAS, the Company
wishes to ensure that it will have the benefits and knowledge of Employee's services and expertise on the terms and conditions
hereinafter set forth; and
C. WHEREAS, Oncologix Tech
is a Publically Traded Holding Company engaged in the business of medical device manufacturing, healthcare services and medical
products sales and distribution; and.
D. WHEREAS, the Company
desires to engage the Executive as its President of Company Medical Products Division and the Executive desires to provide employment
services to the Company on all of the terms and conditions herein set forth.
E. WHEREAS, the Company
desires to provide the Executive with compensation in recognition of the Executive's valuable skills and services.
F. WHEREAS, this agreement
replaces and terminates the Consulting Agreement with Harold Halman dated June 10, 2014 effective this Commencement Date of July
16, 2014.
NOW, THEREFORE, in consideration
of the mutual covenants and conditions herein contained, the parties hereto agree as follows:
ARTICLE I. DEFINITION
In this Agreement, unless the context
otherwise requires, the following words shall have the following meaning:
"Company" means Oncologix,
a company duly incorporated and validly existing under the laws of the Nevada;
"Commencement Date" means
July 16, 2014, the date of commencement of Employment;
"Company Division/Group"
means the Company and all of its divisions and subsidiaries;
"Employment" means the
employment of the Executive under the terms herein;
"Executive" means Harold
Halman, a citizen of the United States of America;
Confidentiality and Non-Competition
Agreement" means the Confidentiality and Non-competition Agreement to be executed by and between the Company and Executive;
ARTICLE II. POSITION AND DUTIES
2.1 Employment. The
Company hereby employs the Executive as its President of the Company’s Medical Products & Technology Division and the
Executive hereby accepts such engagement with the Company, in accordance with and subject to all of the terms, conditions and covenants
set forth in this Agreement.
2.2 Scope of Duties.
The Executive shall be the President of the Company Medical Products Division & Technology Division and shall have such other
or additional offices or positions with the Company as the Chief Executive Officer and the Board of Directors (the "Board")
shall determine from time to time. The Executive shall have responsibility for the following duties operating within such established
guidelines plans or policies as may be established or approved by the Chief Executive Officer and its Board of Directors from time
to time:
(a) Responsible for the Medical
Products & Technology Division business revenue growth, cost/expense controls and reductions, increase business division profitability,
develop strategic plans, financial plans, and is directly responsible for the financial viability of the Company Medical Products
Division, working in conjunction with the Company Chief Executive Officer and the Chief Financial Officer in the financial and
accounting matters of the Division and its subsidiaries, including organizing, managing and supervising the work of the business
unit subsidiaries Managers, officers, employees, sales and marketing , and to include full oversight of the manufacturing/operations
and sales and marketing of the Medical Product Division;
(b) assist the Chief Financial
Officer in preparing the financial statements of the Company Medical Product Division and its subsidiaries in accordance with accounting
principles generally accepted in the United States ("USGAAP"), or such other accounting principles as the Chief Executive
Officer and Chief Financial Officer may direct, and recommending appropriate accounting treatment in accordance with USGAAP or
such other principles;
(c) assist the company CEO/CFO
with the Company's independent accountants in their financial audits and review of the Company's Medical Products division or its
subsidiaries financial statements;
(d) participate in Company new
product developments, perform/assist due diligence on company potential acquisition candidates, improve, document, test, certify
and ensuring all Medical Products subsidiaries keep all licenses and certifications current, implement the Company's internal controls
over financial reporting; assisting and cooperating with any consulting firm engaged by the Company in connection with such projects;
(e) supporting the Company CEO/CFO
and the Board of Directors and the Company legal representative in such matters as preparation and presentation as may be requested
from time to time;
(f) participating in the preparation
and filing of Company press releases, annual and quarterly reports (8-K, 10-K, 10-Q );
(g) participating in investor communications,
including analyst conference calls, road shows and others; and
(h) such other responsibilities
and duties customarily performed by the President in the same industry and listed companies.
2.3 Other Business Affiliations.
The Executive agrees that, without the approval of the Company CEO, the Executive shall not, during the period of employment with
the Company, devote, at any time, to any other business affiliation which would interfere with or derogate from Executive's obligations
under this Agreement. The Executive represents and warrants that his service as such Executive does not create any conflict of
interest in relation to his duties and obligations to the Company hereunder, and agrees that in the event such a conflict arises,
he will promptly report it to the Company CEO.
2.4 No Breach of Duty.
The Executive represents that the Executive's performance of this Agreement and as an employee of the Company does not and will
not breach any agreement or duty to keep in confidence proprietary information acquired by the Executive in confidence or in trust
(i) prior to employment with the Company or (ii) pursuant to his service referred to in Section 2.3. The Executive
has not and will not enter into any agreement either written or oral in conflict with this Agreement. The Executive is not presently
restricted from being employed by the Company or entering into this Agreement.
ARTICLE III. DURATION
3.1 Commencement Date.
The Executive's first day of employment shall be July 16, 2014 ("Commencement Date").
3.2 Term. This Agreement
shall continue for an initial term of three (3) years from the Commencement Date unless otherwise terminated in accordance
with Article 8 below. Upon the expiration of the initial term, this Agreement shall be not automatically extended but an extension
is to be approved by the CEO and the Board of Directors for successive periods unless terminated by either party upon sixty (60) days'
notice prior to the expiration of the initial term or any subsequent terms.
ARTICLE IV. COMPENSATION
4.1 Salary. The Executive
shall be paid an initial base annual salary of Eighty Five Thousand ($85,000.00) dollars per annum, less deductions required by
law, which shall be paid in equal monthly installments in the U.S. dollar equivalent with the Company's normal and customary payroll
practices. Such salary shall be increased at a minimum of 5% per year, reviewed annually, and any base salary increases or decreases
will be approved by the Company CEO and Company Board of Directors, Compensation Committee. If inadequate funds are available,
the salary and other benefits and expenses not paid to Executive, shall be accrued per quarter. At the end of the quarter, at
the Executive’s option, the accrued salary may be converted into Company stock at a price equivalent to the lowest ten (10)
day average bid price during the preceding quarter.
4.2 PROFESSIONAL FEES.
The Company shall have exclusive authority to determine the fees, or a procedure for establishing the fees, to be charged by
the Company. All sums paid to the Executive or the Company in the way of fees or otherwise for other services of the Executive,
shall, except as otherwise specifically agreed by the Company, be and remain the property of the Company and shall be included
in the Company's name in such checking account or accounts as the Company may from time to time designate.
4.3 CLIENTS AND CLIENT
RECORDS. The Company shall have the authority to determine who will be accepted as clients of the Company, and the
Executive recognizes that such clients accepted are clients of the Company and not the Executive. The Company shall have the
authority to designate, or to establish a procedure for designating which professional Executive of the Company will handle
each such client. All client records and files of any type concerning clients of the Company shall belong to and remain the
property of the Company, notwithstanding the subsequent termination of this Agreement.
4.4 POLICIES AND PROCEDURES.
The Company shall have the authority to establish from time to time the policies and procedures to be followed by the Executive
in performing services for the Company. Executive shall abide by the provisions of any contract entered into by the Company under
which the Executive provides services. Executive shall comply with the terms and conditions of any and all contracts entered by
the Company.
4.5 Bonus. The Executive
will be eligible for quarterly, performance-based cash bonus, at 2% for all new business generated exclusively by Executive based
upon the following parameters:
| |
A. |
Top line Gross revenue – If Executive exclusively secures $ 5million of new revenues over Medical Products Division base line revenues |
| |
B. |
EBITDA Target – Company will establish an Executive bonus program based on the Medical Division EBITDA on an annual basis. Executive will be qualified to earn a 2% quarterly bonus based “Net Income” for any qualified performance period as reported in the audited financial statements for the Division that period. |
4.6 Reimbursable Expenses.
The Company shall reimburse the Executive for all reasonable business expenses incurred in the performance of the Executive's
duties hereunder on behalf of the Company, subject to submission of expense reports to the Company CFO and final approval according
to the Company's financial regulations.
4.7 Automobile.
During his employment, the Executive will be provided a Not to Exceed, flat rate of $500.00 per month as an automobile allowance
by the Company payable within ten (10) days after the 1st of every month.
4.8 Income Taxes.
The payment of tax, social security and similar payments arising out of this Agreement shall be dealt with by the parties in accordance
with applicable laws and regulations.
The Executive agrees to any withholdings
that must be made by the Company pursuant to such laws and regulations. Furthermore, the Executive undertakes to promptly discharge
any payments that are payable by him pursuant to such laws and regulations and agrees to indemnity the Company and hold the Company
harmless from any and all claims made by any entity on account of an alleged failure by the Executive to satisfy such obligations.
ARTICLE V. OTHER BENEFITS, VACATION
, HOLIDAYS, INSURANCE
5.1 Vacation. The Executive
shall be entitled in each calendar year to ten (10) working days' vacation with full salary (in addition to statutory holidays)
to be taken at such reasonable time or times as approved in advance by the Company CEO. The Executive may not accumulate and carry
forward unused vacation to the following calendar year. The entitlement to vacation and, on termination of employment, vacation
pay in lieu of vacation, shall accrue pro rata throughout each calendar year of the period of employment.
5.2 Holidays. The Executive
shall be entitled to the all statutory public holidays observed.
5.3 Benefits. Employee shall
be entitled to participate in any and all medical insurance, group health care programs (including dental, vision and prescription
drug programs), disability insurance, pension and other benefit plans which are made generally available by the Company to other
similarly situated senior level employees performing similar functions as Executive. The Company,
in its sole discretion, at any time may amend or terminate its benefit plans or programs; provided, however, that the Company shall
not do so except to the extent that such amendment or termination is in good faith and applies generally to all employees of the
Company.
If the Company does not have an
Executive Benefit program available, the company will provide a monthly fixed amount of $800.00 to secure Health/Dental and Long
Term Disability insurance.
5.4 Key Man Insurance.
The Company may maintain, at its expense, Key Man Life Insurance (the "Policy") on the life of Employee for the benefit
of the Company with a benefit of One Million Dollars ($1,000,000). Employee's signature to this Agreement constitutes Employee's
written consent to being insured under the Policy and that the Company may continue such life insurance coverage after Employee's
employment with the Company terminates, regardless of the cause of such termination. Employee shall make all necessary applications,
submit to physical examinations and otherwise cooperate with the Company with respect to the purchase of the policy.
5.5 Death of Executive.
In the event the Executive shall die during the term hereof, the Company shall pay to the Executive's surviving spouse, or
if the Executive shall leave no surviving spouse, then to the Executive's estate, only such amounts as may have been earned by
the Executive prior to the Executive's date of death, but which were unpaid at date of death.
5.6 Life Insurance. Company
shall provide a term life policy to Executive in the face value amount of 3x annual base salary.
ARTICLE VI. CONFIDENTIALITY,
NONSOLICITATION AND NONCOMPETITION
6.1 Confidentiality.
During his employment, the Executive will have access to, will become acquainted with various trade secrets, confidential and proprietary
information relating to the business of the Company and its subsidiaries, including but not limited to customer, employee, supplier,
and distributor lists, contacts, addresses, information about employees and employee relations, training manuals and procedures,
recruitment methods and procedures, business plans and projections, employment contracts, employee handbooks, information about
customers and suppliers, price lists, costs and expenses, documents, budgets, proposals, financial information, inventions, patterns,
processes, formulas, data bases, know how, developments, experiments, improvements, computer programs, manufacturing, recruitment
and distribution techniques, specifications, tapes, and compilations of information, all of which are owned by the Company and
its subsidiaries, other parties with which the Company and its subsidiaries do business ("Third Parties") or customers
of the Company and its subsidiaries, and which are used in the operation of the business of the Company and its subsidiaries, or
such Third Parties or customers. The Executive agrees at all times during the term of his employment and thereafter to hold in
strictest confidence, and not to use, except for the benefit of the Company, or to disclose directly or indirectly to any person,
firm or corporation without written authorization of the Board, any trade secrets or confidential information. In addition, the
Executive understands "trade secret" or "confidential information" means all information concerning the Company
and its subsidiaries, Third Parties and/or customers (including but not limited to information regarding the particularities, preferences
and manner of doing business) that is (i) not generally known to the public and (ii) cannot be discovered or replicated
by a third party without substantial expense and effort.
6.2 Non-Solicitation.
The Executive agrees that during the Executive's employment and for a period of the later of two (2) years after the termination
of employment hereunder where the Company does business, the Executive shall not:
(i) directly call upon or solicit
any of the customers of the Company or its subsidiaries that were or became customers during the term of the Executive's employment
(as used herein "Customer" shall mean any person or company as listed as such on the books of Company or its subsidiaries);
or
(ii) induce its subsidiaries or
any employee, agent or consultant of the Company or its subsidiaries to terminate his or her association with the Company or its
subsidiaries.
6.3 Non-Competition.
The Executive agrees that during the Executive's employment and for a period of two (2) years , subject to Section 2.3,
devote full time to the business of the Company and will not directly or indirectly, engage, individually or as an officer, director,
employee, consultant, advisor, partner or co-venture, or as a stockholder or other proprietor owning an interest in any firm, corporation,
partnership or other organization in the business of manufacturing, selling or distributing products in competition with the products
and/or services of the Company and its subsidiaries. The Executive shall, during the term of the Executive's employment and the
term of the non-competition restriction, furnish to the Company CEO a detailed statement of any outside employment or consulting
services in which the Executive seeks to engage or invest, and, as from time to time requested by the Company CEO, resubmit for
approval a detailed statement thereof. In the event the Company CEO determines in good faith that such violation or conflict exists,
the Executive shall refrain from such employment, consulting services or investment.
6.4 Enforceability.
The Executive agrees that, having regard to all the circumstances, the restrictions in this Article 6 are reasonable and necessary
but no more than sufficient for the protection of the Company. The Company and Executive agree that:
(i) each restriction in this Article
6 shall be read and construed independently of the other restrictions so that if one or more are found to be void or unenforceable
as an unreasonable restraint of trade or for any other reason, the remaining restrictions shall not be affected; and
(ii) if any restriction is found
to be void but would be valid and enforceable if some part of it were deleted or the period thereof were deleted or the range
of activities or area dealt with thereby were reduced in scope, the restriction shall apply with such modifications as may be
necessary to make it valid and enforceable.
6.5 Injunctive Relief.
In the event of the breach or threatened breach by the Executive of this Article 6, the Company, in addition to all other remedies
available to it at law or in equity, will be entitled to seek injunctive relief for a minimum amount of one million dollars ($
1,000,000), and/or specific performance to enforce this Article 6 in any court of competent jurisdiction.
6.6 Assignment and Transfer.
Executive’s rights and obligations under this Agreement shall not be transferable by assignment or otherwise, and any purported
assignment, transfer or delegation thereof shall be void. This Agreement shall inure to the benefit of, and be binding upon and
enforceable by, any purchaser of substantially all of Company’s assets, any corporate successor to Company or any assignee
thereof.
ARTICLE VII. ASSIGNMENT OF INVENTIONS
AND INTELLECTUAL PROPERTY
7.1 The parties foresee that the
Executive has created and may create business plans, strategies, customer lists, locate new medical products, product designs or
other intellectual property in the course of his duties hereunder and agree that in this respect the Executive has a special responsibility
to further the interests of the Company.
7.2 Any invention, production,
improvement or design made or process or information discovered or copyright work or trade mark or trade name or source code, business
plans, strategy, marketing plans, or any other intellectual property created by the Executive during the continuance of his Employment
hereunder (whether before or after the date hereof or whether capable of being patented or registered or not and whether or not
made or discovered in the course of his employment hereunder) in conjunction with or in any way affecting or relating to the business
of any company in the Company or capable of being used or adapted for use therein or in connection therewith shall forthwith be
disclosed to the Company and shall belong to and be the absolute property of such company in the Company Division as the Company
may direct.
7.3 The Executive if and whenever
required to do by the Company shall at the expense of the Company apply or join in applying for letters patent or other protection
or registration for any such invention, improvement design process information work trade mark name or get-up source code or other
intellectual property rights as aforesaid which belongs to such company and shall at the expense of such company execute and do
all instruments and things necessary for vesting the said letters patent or other protection or registration when obtained and
all right title and interest to and in the same in such company absolutely and as sole beneficial owner or in such other person
as the Company may specify.
7.4 The Executive hereby irrevocably
appoints the Company to be his attorney in his name and on his behalf to execute and do any such instrument or thing and generally
to use his name for the purpose of giving to the Company the full benefit of this Article and in favor of any third party a certificate
in writing signed by any Executive or by the secretary of the Company that any instrument or act falls within the authority hereby
conferred shall be conclusive evidence that such is the case.
ARTICLE VIII. TERMINATION
8.1 Except as otherwise provided
in Sections 3.3 and 8.2, the Executive employment may be terminated by either party giving the other not less than sixty (60) days'
notice in writing provided that the Company shall have the option to pay salary (pro-rated) in lieu of any required period of notice.
Notwithstanding the sixty (60) days' notice requirement in this Section 8.1, the Executive agrees to continue to perform
his duties hereunder until a new President of the Company Medical Product Division is appointed (if required by the Company) and
to ensure a smooth transition thereafter, and the terms of this Agreement shall continue to apply; provided that any such extension
shall not exceed ninety (90) days. During such ninety (90) days' notice period and any extension thereof pursuant to
this Section 8.1, if required, the Company shall use its diligent efforts to recruit a new President of the Company Medical
Product Division.
8.2 Notwithstanding the other provisions
of this Agreement, the Company may terminate the Employment with cause forthwith without prior notice (but without prejudice to
the rights and remedies of the Executive and Company) for any breach of this Agreement in any of the following cases:
(i) if the Executive fails or neglects
efficiently and diligently to carry out his duties to the reasonable satisfaction of the CEO and the Company Board;
(ii)
if the Executive is guilty of serious misconduct in connection with the Employment;
(iii) if the Executive is
convicted of any criminal offense, which might reasonably be thought to adversely affect the performance of his duties;
(iii) if the Executive does any
act or thing which may bring serious discredit on the Company or the Company Division/Group;
(iv) if the Executive commits any
serious breach of the Company's By-Laws and operating procedures (as laid down by the Company and communicated to the Executive
from time to time) and has caused serious financial loss to the Company;
(v) if the Executive fails to observe
and perform any of the duties and responsibilities imposed by this Agreement or which are imposed by law, or is in breach of any
representation, warranty or covenant made by the Executive under this Agreement;
(vi) if the Executive becomes unsound
of mind or suffers from a mental disorder; or
(vii) if the Executive otherwise
acts in breach of this Agreement so as materially to prejudice the business of the Company or the Company Division/Group.
8.3 The Executive shall not, at
any time after termination of the Employment for whatever reason, represent himself as being in any way connected with the business
of the Company.
8.4 Upon termination of the Employment
for whatever reason, the Executive shall forthwith deliver to the Company or its authorized representative such of the following
as are in his possession or control:
(i) All keys, security and computer
passes, plans statistics, documents, records, papers, magnetic disks, tapes or other software storage media including all copies,
records and memoranda (whether or not recorded in writing or on computer disk or tape) made by the Executive of any confidential
or proprietary information relating to the business of the Company and its subsidiaries;
(ii) All credit cards and charge
cards provided for the Executive's use by the Company; and
(iii) All other property of the
Company not previously referred to in this Article; and
(iv) any breach of this Agreement,
which breach is not cured within fifteen (15) days following written notice of such breach; and
8.5 Termination With-Out Cause.
The Company may not terminate Executive’s employment hereunder at any time without cause, provided, however, that upon
termination, Executive shall be entitled to severance pay for the remaining months in that fiscal year, the full base salary as
stated in this Agreement in addition to accrued but unpaid salary and accrued vacation, less deductions required by law, but only
if, Executive executes a valid and comprehensive release of any and all claims that the Executive may have against the Company
in a form provided by the Company and Executive executes such form within ten (10) days of tender.
8.6 Resignation. Upon termination
by reason of “For Cause “of employment, Executive shall be deemed to have resigned as President of the Company Medical
Products Division.
8.7 DISABILITY OF EXECUTIVE
The Company may terminate this
Agreement without liability if Executive shall be permanently prevented from properly performing his essential duties hereunder
with reasonable accommodation by reason of illness or other physical or mental incapacity for a period of more than ninety (90)
consecutive days. Upon such termination, Executive shall be entitled to all accrued but unpaid Base Salary and vacation as stated
in 8.5 above.
(i) Definitions - For purposes
of this Agreement, whenever used in this Section 8.3:
"Total disability" shall
mean that the Executive is unable, mentally or physically, whether it’s due to sickness, accident, age or other infirmity,
to engage in any aspect of the Executive's normal duties as set forth in this Agreement.
"Partial disability"
shall mean that the Executive is able to perform, to some extent, on behalf of the Company, the particular services in which the
Company specializes, and which the Executive previously performed for the Company, but that the Executive is unable, mentally or
physically, to devote the same amount of time to such services as was devoted prior to the occurrence of such sickness or accident.
"Normal monthly salary"
shall mean the salary which the Executive is being paid by the Company per month as of the commencement date of the period of disability,
as specified hereinabove or as determined by the CEO and the Company Board of Directors pursuant to the terms hereof.
(ii) Total Disability
During a single period of total
disability of the Executive, the Executive shall be entitled to receive from the Company, the Executive's normal monthly salary
for the shorter of first three (3) months of disability or until any disability insurance policy available through the Executive’s
employment begins to pay benefits. If the single period of disability should continue beyond three (3) months, the Executive shall
receive only such amount as the Executive shall be entitled to receive under disability insurance coverage on the Executive, if
any.
(iii) Partial Disability
During a period of partial disability
of the Executive, the Executive shall receive an amount of compensation computed as follows:
That portion of the Executive's
normal monthly basic compensation which bears the same ratio to the Executive's normal monthly basic compensation as the amount
of time which the Executive is able to devote to the usual performance of services on behalf of the Company during such period
bears to the total time the Executive devoted to performing such services prior to the commencement date of the single period of
disability, and such amount shall be calculated by multiplying the Executive’s basic compensation by a fraction, the numerator
of which shall be the percentage of normal services that the Executive is able to perform and the denominator which shall be the
total services that the Executive is able to perform absent the partial disability.
(iv) Combination of Total and
Partial Disability
If a single period of disability
of the Executive consists of a combination of total disability and partial disability, the maximum total disability compensation
to which the Executive shall be entitled from the Company under this disability provision shall not exceed an amount equal to one
(1) times the Executive's normal monthly basic compensation.
(V) Broken Periods of Disability
A period of disability may be continuous
or broken. If broken into partial periods of disability which are separated by intervening periods of work, there shall be aggregated
together all of such successive partial periods of disability except any period prior to the time when any single period of work
extends for three months or longer; and such aggregated periods of disability shall be treated as a single period in determining
the amount of disability compensation to which an Executive shall be entitled under any provision of this Section.
8.6 Termination Due to Disability
If and when the period of total
or partial disability of the Executive totals twelve (12) months, the Executive's employment with the Company shall automatically
terminate. Notwithstanding the foregoing, if the disabled Executive and the Company agree, the disabled Executive may thereafter
be employed by the Company in another management function upon such terms as may be mutually agreeable.
8.7 Commencement Date of Disability
The commencement date of a period
of disability, whether it’s a continuous period or the aggregate of successive partial periods, shall be the first day on
which the Executive is disabled.
8.8 Dispute Regarding Existence
of Disability
Any dispute regarding the existence,
extent or continuance of the disability shall be resolved by the determination of a majority of three (3) competent physicians,
one (1) of whom shall be selected by the Company, one (1) of whom shall be selected by the Executive
and the third (3rd) of whom shall be selected by the other two (2) physicians so selected.
ARTICLE IX- MUTUAL INDEMNIFICATION
9.1 The Executive hereby agrees
to indemnify and hold the Company and its officers, directors, shareholders and Executives harmless from and against any loss,
claim, damage or expense, and/or all costs of prosecution or defense of their rights hereunder, whether in judicial proceedings,
including appellate proceedings, or whether out of court, including without limiting the generality of the foregoing, attorneys'
fees, and all costs and expenses of litigation, arising from or growing out of the Executive's breach or threatened breach of any
covenant contained herein.
9.2 The Company hereby agrees to
indemnify and hold the Executive harmless from and against any loss, claim, damage or expense, and/or all costs of prosecution
or defense of their rights hereunder, whether in judicial proceedings, including appellate proceedings, or whether out of court,
including without limiting the generality of the foregoing, attorneys' fees, and all costs and expenses of litigation, arising
from or growing out of the Company’s breach or threatened breach of any covenant contained herein.
ARTICLE X. MISCELLANEOUS PROVISIONS/JURISDICTION
10.1 Severability.
I If any term or provision of this Agreement shall be held to be invalid or unenforceable for any reason, such term or provision
shall be ineffective to the extent of such invalidity or unenforceability without invalidating the remaining terms and provisions
hereof, and this Agreement shall be construed as if such invalid or unenforceable term or provision had not been contained herein.
10.2 Rights Cumulative.
The rights and remedies provided by this Agreement are cumulative, and the exercise of any right or remedy by either party
hereto (or by its successor), whether pursuant to this Agreement, to any other agreement, or to law, shall not preclude or waive
its right to exercise any or all other rights and remedies.
10.3 Nonwaiver.
No failure or neglect of either party hereto in any instance to exercise any right, power or privilege hereunder or under law
shall constitute a waiver of any other right, power or privilege or of the same right, power or privilege in any other instance.
All waivers by either party hereto must be contained in a written instrument signed by the party to be charged and, in the case
of the Company, by an officer of the Company (other than Executive) or other person duly authorized by the Company.
10.4 Assistance in Litigation.
Executive shall, during and after termination of employment, upon reasonable notice, furnish such information and proper assistance
to the Company as may reasonably be required by the Company in connection with any litigation in which it or any of its subsidiaries
or affiliates is, or may become a party; provided, however, that such assistance following termination shall be furnished at mutually
agreeable times and for mutually agreeable compensation. The company shall pay all legal expenses incurred by Executive with regard
to any/all litigation.
10.5 Survival. Articles
6 and 7 and Section 9.7 shall survive the termination of this Agreement.
10.6 Entire Agreement.
This Agreement, together with all award agreements and long-term incentive agreements is the entire agreement between the parties
hereto concerning the subject matter hereof and supersedes and replaces all prior or contemporaneous agreements or understandings
between the parties.
10.7 Employment Amendments.
This Agreement may not be amended or modified in any manner, except by an instrument in writing signed by duly authorized members
of the Company Board of Directors.
10.8 Notices. Unless
otherwise provided herein, any notice required or permitted under this Agreement shall be given in writing and shall be deemed
to have been duly given upon personal delivery to the party to be notified or five (5) business days after being emailed,
fax, mailed by United States certified or registered mail, postage prepaid, return receipt requested or three (3) days after being
sent by Federal Express or other recognized overnight delivery to the following respective addresses, or at such other address
as each may specify by notice to the other:
Notice to the Executive:
Harold Halman
President- Medical Products Division
& Technology Division
2640 W Adventure Ct
Anthem AZ 85086
10.9 Governing Law.
This Agreement shall be governed by and construed in all respects in accordance with the laws of the State of Louisiana.
11.1 Jurisdiction.
Unless otherwise provided for in this Agreement, the courts within the Parish of Rapides, and the City of Alexandria, Louisiana
shall have exclusive jurisdiction to adjudicate any dispute arising out of this Agreement and/or employment relationship or termination
thereof and the Executive consents to such jurisdiction and venue.
11.2 Construction.
The headings and captions of this Agreement are provided for convenience only and are intended to have no effect in construing
or interpreting this Agreement. The language in all parts of this Agreement shall be in all cases construed according to its fair
meaning and not strictly for or against the Company or Executive.
11.3 Nonwaiver. No
failure or neglect of either party hereto in any instance to exercise any right, power or privilege hereunder or under law shall
constitute a waiver of any other right, power or privilege or of the same right, power or privilege in any other instance. All
waivers by either party hereto must be contained in a written instrument signed by the party to be charged and, in the case of
the Company, by an officer of the Company (other than Executive) or other person duly authorized by the Company.
11.4 Arbitration. Any controversy,
claim or dispute arising out of or relating to this Agreement or the employment relationship, either during the existence of the
employment relationship or afterwards, between the parties hereto, their assignees, their affiliates, their attorneys, or agents,
shall be settled by Binding Arbitration in the State of Louisiana, Alexandria, Louisiana. Such arbitration shall be conducted in
accordance with the then prevailing commercial arbitration rules of the State of Louisiana but the arbitration shall be in front
of an arbitrator, with the following exceptions if in conflict: (a) one arbitrator shall be chosen by Harold Halman and one
arbitrator chosen by the Company; (b) each party to the arbitration will pay its pro rata share of the expenses and fees of
the arbitrator(s), together with other expenses of the arbitration incurred or approved by the arbitrator(s); and (c) arbitration
may proceed in the absence of any party if written notice of the proceedings has been given to such party. The parties agree to
abide by all decisions and awards rendered in such proceedings. Such decisions and awards rendered by the arbitrator shall be final
and conclusive and may be entered in any court having jurisdiction thereof as a basis of judgment and of the issuance of execution
for its collection. All such controversies, claims or disputes shall be settled in this manner in lieu of any action at law or
equity; provided however, that nothing in this subsection shall be construed as precluding the Company from bringing an action
for injunctive relief or other equitable relief or relief under the Confidential Information and Invention Assignment Agreement.
The arbitrator shall not have the right to award punitive damages, consequential damages, lost profits or speculative damages to
either party. The parties shall keep confidential the existence
of the claim, controversy or disputes from third parties (other than the arbitrator), and the determination thereof, unless otherwise
required by law or necessary for the business of the Company. The arbitrator(s) shall be required to follow applicable law.
IF FOR ANY REASON THIS ARBITRATION
CLAUSE BECOMES NOT APPLICABLE, THEN EACH PARTY, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, HEREBY IRREVOCABLY WAIVES ALL
RIGHT TO TRIAL BY JURY AS TO ANY ISSUE RELATING HERETO IN ANY ACTION, PROCEEDING, OR COUNTERCLAIM ARISING OUT OF OR RELATING TO
THIS AGREEMENT OR ANY OTHER MATTER INVOLVING THE PARTIES HERETO.
12.0 Acknowledgment. The
Executive acknowledges that when this Agreement is concluded, the Executive will be able to earn a living without violating the
foregoing restrictions and that the Executive's recognition and representation of this fact is a material inducement to the execution
of this Agreement and to Executive's continued relationship with the Company.
12.1 Survival of Covenants
. All restrictive covenants contained in this Agreement shall survive the termination of this Agreement.
12.2 Limitations on Authority
. Without the express written consent from the Company CEO, and if required, the Company Board of Directors, the Executive shall
have no apparent or implied authority to: (i) Pledge the credit of the Company or any of its other Executives; (ii) Bind the Company
under any contract, agreement, promissory note, letter of intent, note, mortgage or otherwise; (iii) Release or discharge any debt
due the Company unless the Company has received the full amount thereof; or (iv) sell, mortgage, transfer or otherwise dispose
of any assets of the Company.
12.3 Representation and Warranty
of Executive. The Executive acknowledges and understands that the Company has extended employment opportunities to Executive
based upon Executive's representation and warranty that Executive has the skills and knowledge required, is in good health and
able to perform the work contemplated by this Agreement for the term hereof.
12.4 Invalid Provision; Severability
. The invalidity or unenforceability of a particular provision of this Agreement shall not affect the other provisions hereof,
and the Agreement shall be construed in all respects as if such invalid or unenforceable provisions were omitted.
12.5 Entire Agreement. This
Agreement contains the entire agreement and supersedes all prior agreements and understandings, oral or written, with respect to
the subject matter hereof. This Agreement may be changed only by an agreement in writing signed by the party against whom any waiver,
change, amendment, modification, or discharge is sought.
IN WITNESS WHEREOF, the
parties hereto have caused this Agreement to be duly executed and delivered as of the day and year first above written.
| Harold Halman |
|
Oncologix Tech, Inc. |
|
| BY: |
|
|
BY: |
|
|
| Name: |
Harold Halman |
|
Name: |
Roy Wayne Erwin |
|
| Title: |
- |
|
Title: |
Chairman and CEO |
|
Exhibit 21
Subsidiaries of Oncologix Tech, Inc.
Subsidiaries of Registrant
|
Subsidiary |
State of Incorporation or Jurisdiction |
Percent of Ownership of Oncologix Tech, Inc. |
| Dotolo Research Corporation |
Louisiana |
100% |
| Amian Angels, Inc. |
Louisiana |
100% |
| Advanced Medical Products & Technologies, Inc. |
Nevada |
90% |
EXHIBIT 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO SECTION
302 OF THE SARBANES-OXLEY ACT OF 2002
I, Roy Wayne Erwin,
certify that:
| (1) | I have reviewed this Annual Report on Form 10-K of Oncologix Tech, Inc.; |
| (2) | Based on my knowledge, the Report does not contain any untrue statement of a material fact or omit
to state a material fact necessary in order to make the statements made, in light of the circumstances under which such statements
were made, not misleading with respect to the period covered by the Report; |
| (3) | Based on my knowledge, the financial statements, and other financial information included in the
Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant
as of, and for, the periods represented in this report; |
| (4) | The registrant’s other certifying officer and I are responsible for establishing and maintaining
disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) and internal control over financial
reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial
reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented
in Report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered
by this Report based on such evaluation; and |
| (d) | Disclosed in this Report any change in the registrant’s
internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s
fourth fiscal quarter in case of an annual report) that has materially affected, or is reasonably likely to materially affect,
the registrant’s internal control over financial reporting. |
| (5) | The registrant’s other certifying officer and I have disclosed, based on our most recent
evaluation of internal control over financial reporting, to the registrant’s auditors
and the audit committee of the Registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in
the design or operation of internal control over financial reporting which are
reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial
information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant
role in the registrant’s internal control over financial reporting. |
Dated: December 15, 2014
By: /s/ Roy Wayne Erwin
Roy Wayne Erwin
Chief Executive Officer
and President
EXHIBIT 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
PURSUANT TO SECTION
302 OF THE SARBANES-OXLEY ACT OF 2002
I, Michael Kramarz,
certify that:
| (1) | I have reviewed this Annual Report on Form 10-K of Oncologix Tech, Inc.; |
| (2) | Based on my knowledge, the Report does not contain any untrue statement of a material fact or omit
to state a material fact necessary in order to make the statements made, in light of the circumstances under which such statements
were made, not misleading with respect to the period covered by the Report; |
| (3) | Based on my knowledge, the financial statements, and other financial information included in the
Report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant
as of, and for, the periods represented in this report; |
| (4) | The registrant’s other certifying officer and I are responsible for establishing and maintaining
disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e) and internal control over financial
reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have: |
| (b) | Designed such internal control over financial reporting, or caused such internal control over financial
reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting
and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles; |
| (c) | Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented
in Report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered
by this Report based on such evaluation; and |
| (d) | Disclosed in this Report any change in the registrant’s
internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s
fourth fiscal quarter in case of an annual report) that has materially affected, or is reasonably likely to materially affect,
the registrant’s internal control over financial reporting. |
| (6) | The registrant’s other certifying officer and I have disclosed, based on our most recent
evaluation of internal control over financial reporting, to the registrant’s auditors
and the audit committee of the Registrant’s board of directors (or persons performing the equivalent functions): |
| (a) | All significant deficiencies and material weaknesses in
the design or operation of internal control over financial reporting which are
reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial
information; and |
| (b) | Any fraud, whether or not material, that involves management or other employees who have a significant
role in the registrant’s internal control over financial reporting. |
Dated: December
15, 2014
By: /s/ Michael A. Kramarz
Michael A. Kramarz
Chief Financial and Accounting
Officer
Exhibit 32.1
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CERTIFICATION OF PRINCIPAL
EXECUTIVE OFFICER PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF
2002
In connection with
the Annual Report of Oncologix Tech, Inc. (the “Company”) on Form 10-K for the fiscal year ended August 31, 2014, as
filed with the Securities and Exchange Commission on the date hereof (the “Report”), I Roy Wayne Erwin, Chief Executive
Officer and President of the Company, certify, pursuant to 18 U.S.C. ss. 1350, as adopted pursuant to ss. 906 of the Sarbanes-Oxley
Act of 2002, that:
| 1) | The report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange
Act of 1934; and |
| 2) | The information contained in the Report fairly presents, in all material respects, the financial
condition and result of operations of the Company. |
/s/ Roy Wayne Erwin
Roy Wayne Erwin
Chief Executive Officer and President
December 15, 2014
A signed original of this
written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to
the Securities and Exchange Commission or its staff upon request.
The foregoing certification
is being furnished to the Securities and Exchange Commission as an exhibit to the Report and shall not be considered filed as part
of the Report.
Exhibit 32.2
ONCOLOGIX TECH, INC. AND SUBSIDIARIES
CERTIFICATION OF PRINCIPAL
FINANCIAL OFFICER PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF
2002
In connection with
the Annual Report of Oncologix Tech, Inc. (the “Company”) on Form 10-K for the fiscal year ended August 31, 2014, as
filed with the Securities and Exchange Commission on the date hereof (the “Report”), I Michael A. Kramarz, Chief Financial
Officer of the Company, certify, pursuant to 18 U.S.C. ss. 1350, as adopted pursuant to ss. 906 of the Sarbanes-Oxley Act of 2002,
that:
| 1) | The report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange
Act of 1934; and |
| 2) | The information contained in the Report fairly presents, in all material respects, the financial
condition and result of operations of the Company. |
/s/ Michael A. Kramarz
Michael A. Kramarz
Chief Financial Officer
December 15, 2014
A signed original of this
written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to
the Securities and Exchange Commission or its staff upon request.
The foregoing certification
is being furnished to the Securities and Exchange Commission as an exhibit to the Report and shall not be considered filed as part
of the Report.
Exhibit
99

CONSENT OF INDEPENDENT REGISTERED PUBLIC
ACCOUNTING FIRM
We consent to the use, in the statement on
Form 10K ofa Oncologix Tech, Inc., of our report dated December 5, 2014 on our audit of the financial statements of Oncologix Tech,
Inc as of August 31, 2014, and the related statements of operations, stockholders’ equity and cash flows for the years ended
August 31, 2014 and 2013, and the reference to us under the caption “Experts”.
/s/
Seale and Beers, CPAs
Seale and Beers, CPAs
Las Vegas, Nevada
December 5, 2014
Oncologix Tech (CE) (USOTC:OCLG)
Historical Stock Chart
From Nov 2024 to Dec 2024
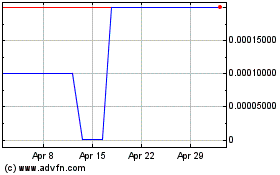
Oncologix Tech (CE) (USOTC:OCLG)
Historical Stock Chart
From Dec 2023 to Dec 2024