As
filed with the Securities and Exchange Commission on November 8, 2023.
Registration
Statement No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
ADIAL
PHARMACEUTICALS, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
8071 |
|
82-3074668 |
(State
or other jurisdiction of
incorporation or organization) |
|
(Primary
Standard Industrial
Classification Code Number) |
|
(I.R.S.
Employer
Identification No.) |
Adial
Pharmaceuticals, Inc.
1180
Seminole Trail, Suite 495
Charlottesville,
Virginia 22901
(434)
422-9800
(Address,
including zip code, and telephone number, including area code, of registrant’s principal executive office)
Cary
Claiborne
President
and Chief Executive Officer
Adial
Pharmaceuticals, Inc.
1180
Seminole Trail, Suite 495
Charlottesville,
Virginia 22901
(434)
422-9800
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Leslie
Marlow, Esq.
Hank
Gracin, Esq.
Patrick
J. Egan, Esq.
Blank
Rome LLP
1271
Avenue of the Americas
New
York, New York 10020
Telephone:
(212) 885-5000
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If
any of the Securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, as amended, check the following box: ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act Registration Statement number of the earlier effective Registration Statement for the same
offering: ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, please check the following box and list
the Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering: ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act Registration Statement number of the earlier effective Registration Statement for the same offering: ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer ☐ |
Accelerated
filer ☐ |
| Non-accelerated
filer ☒ |
Smaller
reporting company ☒ |
| |
Emerging
Growth Company ☒ |
If
an emerging growth company, indicate by checkmark if the registrant has not elected to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the
Registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective
on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities
and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
SUBJECT
TO COMPLETION |
DATED
NOVEMBER 8, 2023 |
4,340,426
Shares
Common
Stock

This
prospectus relates to the resale from time to time of up to 4,340,426 shares of Common Stock, par value $0.001 per share (the “Common
Stock”), of Adial Pharmaceuticals, Inc. by the selling stockholders identified in this prospectus (the “Selling Stockholders”),
including their pledgees, assignees, donees, transferees or their respective successors-in-interest consisting of (i) 1,418,440 shares
of Common Stock issuable upon the exercise of outstanding pre-funded warrants (the “Pre-Funded Warrants”) to purchase shares
of Common Stock purchased by a Selling Stockholder (the “Investor Selling Stockholder”) in a private placement transaction
that closed on October 24, 2023 (the “Private Placement”); (ii) 1,418,440 shares of Common Stock issuable upon the exercise
of outstanding Series A warrants to purchase shares of Common Stock purchased by the Investor Selling Stockholder in the Private Placement
(the “Series A Common Warrants”); (iii) 1,418,440 shares of Common Stock issuable upon the exercise of outstanding Series
B warrants to purchase shares of Common Stock purchased by the Investor Selling Stockholder in the Private Placement (the “Series
B Common Warrants” and together with the Series A Common Warrants, the “Common Warrants”); and (iv) 85,106 shares of
Common Stock issuable upon the exercise of outstanding warrants (the “Placement Agent Warrants”) issued to designees of H.C.
Wainwright & Co., LLC (“H.C.W.” or the “Placement Agent”) as partial compensation for H.C.W. acting as placement
agent in connection with the Private Placement. The shares of Common Stock issuable upon exercise of the Common Warrants are referred
to as the “Common Warrant Shares,” the shares of Common Stock issuable upon exercise of the Pre-Funded Warrants are referred
to as the “Pre-Funded Warrant Shares” and the shares of Common Stock issuable upon exercise of the Placement Agent Warrants
are referred to as the “Placement Agent Warrant Shares.”
We
are filing the registration statement on Form S-1, of which this prospectus forms a part, to fulfill our contractual obligations with
the Selling Stockholders to provide for the resale by the Selling Stockholders of the shares of Common Stock offered hereby. See “Selling
Stockholders” beginning on page 15 of this prospectus for more information about the Selling Stockholders. The registration
of the shares of Common Stock to which this prospectus relates does not require the Selling Stockholders to sell any of their shares
of our Common Stock.
We
are not offering any shares of Common Stock under this prospectus and will not receive any proceeds from the sale or other disposition
of the shares of our Common Stock covered hereby. See “Use of Proceeds” beginning on page 9 of this prospectus.
The
Selling Stockholders identified in this prospectus, or their pledgees, assignees, donees, transferees or their respective successors-in-interest,
from time to time may offer and sell through public or private transactions at prevailing market prices, at prices related to prevailing
market prices or at privately negotiated prices the shares held by them directly or through underwriters, agents or broker-dealers on
terms to be determined at the time of sale, as described in more detail in this prospectus. See “Plan of Distribution” beginning
on page 17 of this prospectus for more information about how the Selling Stockholders may sell their respective shares of Common
Stock. The Selling Stockholders may be deemed “underwriters” within the meaning of Section 2(a)(11) of the Securities Act
of 1933, as amended.
In
connection with the Private Placement, we have agreed, pursuant to a registration rights agreement that we have entered into with the
Investor Selling Stockholder, to bear all of the expenses in connection with the registration of the Common Warrant Shares, the Pre-Funded
Warrant Shares and the Placement Agent Warrant Shares pursuant to this prospectus. The Selling Stockholders will pay or assume all commissions,
discounts, fees of underwriters, agents, selling brokers or dealer managers and similar expenses, if any, attributable to their respective
sales of the shares of Common Stock.
Our
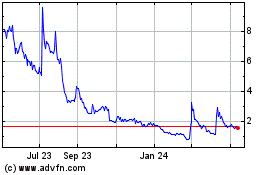
Common Stock is listed on the Nasdaq Capital Market under the symbol “ADIL”. On November 3, 2023, the closing price
of our Common Stock on the Nasdaq Capital Market was $2.26 per share.
We
are an “emerging growth company” under the federal securities laws and, as such, are subject to reduced public company reporting
requirements.
Investing
in our securities involves risks. You should review carefully the risks and uncertainties described under the heading “Risk
Factors” contained in this prospectus and under similar headings in the other documents that are incorporated by reference
into this prospectus, as described beginning on page 6 of this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed
upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense. The securities are not being
offered in any jurisdiction where the offer is not permitted.
The
date of this prospectus is , 2023
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
You
should rely only on the information we have provided or incorporated by reference into this prospectus and any related free writing prospectus.
We have not authorized anyone to provide you with information different from that contained in this prospectus, any applicable prospectus
supplement or any related free writing prospectus. No dealer, salesperson or other person is authorized to give any information or to
represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus. You
must not rely on any unauthorized information or representation. This prospectus is an offer to sell only the shares of Common Stock
offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information
in this prospectus or any related free writing prospectus is accurate only as of the date on the front of the document and that any information
we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of
delivery of this prospectus or any sale of a security.
This
prospectus and the documents incorporated by reference into this prospectus include statistical and other industry and market data that
we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party
research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although
they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications
and third-party research, surveys and studies are reliable. We are ultimately responsible for all disclosure included in this prospectus.
The
Selling Stockholders are offering the shares of Common Stock only in jurisdictions where such issuances are permitted. The distribution
of this prospectus and the issuance of the shares of Common Stock in certain jurisdictions may be restricted by law. Persons outside
the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to,
the issuance of the shares and the distribution of this prospectus outside the United States. This prospectus does not constitute, and
may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, the shares of Common Stock offered by this
prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
This
prospectus is part of a registration statement that we filed with the U.S. Securities and Exchange Commission (the “SEC”),
under which the Selling Stockholders may offer from time-to-time securities described herein in one or more offerings. If required, each
time a Selling Stockholder offers shares, we will provide you with, in addition to this prospectus, a prospectus supplement that will
contain specific information about the terms of that offering. We may also authorize one or more free writing prospectuses to be provided
to you that may contain material information relating to that offering. We may also use a prospectus supplement and any related free
writing prospectus to add, update or change any of the information contained in this prospectus or in documents we have incorporated
by reference. This prospectus, together with any related free writing prospectuses and the documents incorporated by reference into this
prospectus, includes all material information relating to this offering. To the extent that any statement that we make in a prospectus
supplement is inconsistent with statements made in this prospectus, the statements made in this prospectus will be deemed modified or
superseded by those made in a prospectus supplement. Please carefully read both this prospectus and any prospectus supplement before
buying any of the securities offered.
This
prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the
actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some
of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration
statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the sections entitled
“Where You Can Find More Information” and “Incorporation of Certain Information By Reference.”
This
prospectus provides you with a general description of the shares of Common Stock the Selling Stockholders may offer. To the extent that
any statement made in an accompanying prospectus supplement is inconsistent with statements made in this prospectus, the statements made
in this prospectus will be deemed modified or superseded by those made in the accompanying prospectus supplement. You should read both
this prospectus and any accompanying prospectus supplement together with the additional information described under the section “Where
You Can Find More Information” included elsewhere in this prospectus.
Neither
we nor any Selling Stockholder has authorized anyone to provide you with information different from that contained in this prospectus,
any accompanying prospectus supplement or in any related free-writing prospectus filed by us with the SEC. Neither we nor any Selling
Stockholder takes any responsibility for, or provides any assurance as to the reliability of, any information other than the information
in this prospectus, any accompanying prospectus supplement or in any related free-writing prospectus filed by us with the SEC. This prospectus
and any accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other
than the securities described in this prospectus or any accompanying prospectus supplement or an offer to sell or the solicitation of
an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information
appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free-writing prospectus
is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed
materially since those dates.
Except
as otherwise indicated herein or as the context otherwise requires, references in this prospectus to “Adial,” “the
Company,” “we,” “us,” “our” and similar references refer to Adial Pharmaceuticals, Inc., an
entity incorporated under the laws of the State of Delaware.
Smaller
Reporting Company – Scaled Disclosure
Pursuant
to Item 10(f) of Regulation S-K promulgated under the Securities Act of 1933, as amended, as indicated herein, we have elected to comply
with the scaled disclosure requirements applicable to “smaller reporting companies,” including providing two years of audited
financial statements.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains predictive or “forward-looking statements” within the meaning of the Private Securities Litigation Reform
Act of 1995. All statements other than statements of current or historical fact contained in this prospectus, including statements that
express our intentions, plans, objectives, beliefs, expectations, strategies, predictions or any other statements relating to our future
activities or other future events or conditions are forward-looking statements. The words “anticipate,” “believe,”
“continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,”
“predict,” “project,” “will,” “should,” “would” and similar expressions,
as they relate to us, are intended to identify forward-looking statements.
These
statements are based on current expectations, estimates and projections made by management about our business, our industry and other
conditions affecting our financial condition, results of operations or business prospects. These statements are not guarantees of future
performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may
differ materially from what is expressed or forecasted in, or implied by, the forward-looking statements due to numerous risks and uncertainties.
Factors that could cause such outcomes and results to differ include, but are not limited to, risks and uncertainties arising from:
| |
● |
our
projected financial position and estimated cash burn rate; |
| |
● |
our
estimates regarding expenses, future revenues and capital requirements; |
| |
● |
our
need to raise substantial additional capital to fund our operations; |
| |
● |
the
success, cost and timing of our clinical trials; |
| |
● |
our
dependence on third parties in the conduct of our clinical trials; |
| |
● |
our
ability to obtain the necessary regulatory approvals to market and commercialize our product candidates; |
| |
● |
the
potential that results of preclinical and clinical trials indicate our current product candidates or any future product candidates
we may seek to develop are unsafe or ineffective; |
| |
● |
the
results of market research conducted by us or others; |
| |
● |
our
ability to obtain and maintain intellectual property protection for our current product candidates; |
| |
● |
our
ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce
or protect our intellectual property rights; |
| |
● |
the
possibility that a third party may claim we have infringed, misappropriated or otherwise violated their intellectual property rights
and that we may incur substantial costs and be required to devote substantial time defending against these claims; |
| |
● |
our
reliance on third-party suppliers and manufacturers; |
| |
● |
the
success of competing therapies and products that are or become available; |
| |
● |
our
ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; |
| |
● |
the
potential for us to incur substantial costs resulting from product liability lawsuits against us and the potential for these product
liability lawsuits to cause us to limit our commercialization of our product candidates; |
| |
● |
market
acceptance of our product candidates, the size and growth of the potential markets for our current product candidates and any future
product candidates we may seek to develop, and our ability to serve those markets; and |
| |
● |
the
successful development of our commercialization capabilities, including sales and marketing capabilities. |
Our
current product candidate is undergoing clinical development and has not been approved by the Food and Drug Administration (“FDA”)
or the European Commission. This product candidate has not been, nor may it ever be, approved by any regulatory agency or competent authorities
nor marketed anywhere in the world.
We
may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place
undue reliance on our forward-looking statements. Forward-looking statements should be regarded solely as our current plans, estimates
and beliefs. We have included important factors in the cautionary statements included in this document and the documents incorporated
by reference in this prospectus, particularly in the section entitled “Risk Factors” that we believe could cause actual results
or events to differ materially from the forward-looking statements that we make. Moreover, we operate in a very competitive and rapidly
changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess
the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ
materially from those contained in any forward-looking statements we may make. Given these risks and uncertainties, readers are cautioned
not to place undue reliance on such forward-looking statements. All forward-looking statements are qualified in their entirety by this
cautionary statement. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions,
joint ventures or investments we may make. You should read this prospectus and the documents that we have filed as exhibits to this prospectus
and incorporated by reference herein completely and with the understanding that our actual future results may be materially different
from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained
in this prospectus and the documents incorporated by reference in this prospectus are made as of the date of this prospectus and the
dates of the documents incorporated by reference in this prospectus and we do not assume any obligation to update any forward-looking
statements, whether as a result of new information, future events or otherwise, except as required by applicable law.. Investors should
evaluate any statements made by us in light of these important factors.
PROSPECTUS
SUMMARY
This
summary highlights information contained in greater detail elsewhere in this prospectus. This summary is not complete and does not contain
all of the information you should consider in making your investment decision. You should read the entire prospectus carefully before
making an investment in our securities. You should carefully consider, among other things, our financial statements and the related notes
and the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and
Results of Operations” included elsewhere in, or incorporated by reference into, this prospectus.
In
this prospectus, unless the context otherwise requires, the terms “we,” “us,” “our,” “Adial”
and the “Company” refer to Adial Pharmaceuticals, Inc.
Overview
We
are a clinical-stage biopharmaceutical company focused on the development of therapeutics for the treatment or prevention of addiction
and related disorders. Our lead investigational new drug product, AD04, is a genetically targeted therapeutic agent being developed for
the treatment of alcohol use disorder (“AUD”). AD04 was recently investigated in a Phase 3 clinical trial, designated the
ONWARD trial, for the potential treatment of AUD in subjects with certain target genotypes, which were identified using our companion
diagnostic genetic test. Based on our analysis of the subgroup data from the ONWARD trial, we are now focused on commercializing AD04
in the U.S. and Europe.
We
continue to explore opportunities to expand our portfolio in the field of addiction and related disorders such as pain reduction, both
through internal development and through acquisitions. Our vision is to create the world’s leading addiction focused pharmaceutical
company.
In
January 2021, we expanded our portfolio in the field of addiction with the acquisition of Purnovate, LLC via a merger into our wholly
owned subsidiary, Purnovate, Inc., (“Purnovate”) and in January 2023, we entered into an option agreement with Adovate LLC
(“Adovate”), pursuant to which we granted to Adovate an exclusive option for a period of one hundred twenty (120) days from
the effective date of the Option Agreement for Adovate or its designated affiliate to acquire all of the assets of Purnovate and to assume
related liabilities and expenses. On May 8, 2023, Adovate sent a letter exercising its option effective May 16, 2023 and made payment
of the $450,000 in fees due on exercise. On August 17, 2023, a Bill of Sale, Assignment and Assumption Agreement (“Bill of Sale”)
was executed between Purnovate and Adovate, transferring the Purnovate assets to Adovate, effective as of June 30, 2023. On August 17,
2023, Purnovate and Adovate also entered into a Letter Agreement which stated that Adovate acquired the assets of Purnovate effective
as of June 30, 2023, pursuant to the Bill of Sale. On September 18, 2023, the parties executed a final acquisition agreement which memorialized
the terms of the sale of the Purnovate assets to Adovate pursuant to the Option Agreement and Bill of Sale.
We
have devoted the vast majority of our resources to development efforts relating to AD04, including preparation for conducting clinical
trials, providing general and administrative support for these operations and protecting our intellectual property.
We
currently do not have any products approved for sale and we have not generated any significant revenue since our inception. From our
inception through the date of this prospectus, we have funded our operations primarily through the private and public placements of debt
and equity securities and equity lines.
We
have incurred net losses in each year since our inception, including net losses of approximately $1.8 million and $6.8 million for the
six months ended June 30, 2023 and 2022, respectively. We had accumulated deficits of approximately $65.4 and $63.7 million as of June
30, 2023 and December 31, 2022, respectively. Substantially all our operating losses resulted from costs incurred in connection with
our research and development programs, from general and administrative costs associated with our operations, and from financing costs.
We
will not generate revenue from product sales unless and until we successfully complete development and obtain marketing approval for
AD04, which we expect will take a number of years and is subject to significant uncertainty. Based our current projections, we do not
believe our current cash and cash equivalents will be sufficient to fund our operations for the next twelve months from the date of this
prospectus.
Until
such time, if ever, as we can generate substantial revenue from product sales, we expect to finance our operating activities through
a combination of equity offerings, debt financings, government or other third-party funding, commercialization, marketing and distribution
arrangements and other collaborations, strategic alliances and licensing arrangements. However, we may be unable to raise additional
funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such
other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop AD04.
Corporate
Information
Adial
Pharmaceuticals, L.L.C. was formed as a Virginia limited liability company in November 2010. Adial Pharmaceuticals, L.L.C. converted
from a Virginia limited liability company into a Virginia corporation on October 3, 2017, and then reincorporated in Delaware on October
11, 2017 by merging the Virginia corporation with and into Adial Pharmaceuticals, Inc., a Delaware corporation that was incorporated
on October 5, 2017 as a wholly owned subsidiary of the Virginia corporation. We refer to this as the corporate conversion/reincorporation.
In connection with the corporate conversion/reincorporation, each unit of Adial Pharmaceuticals, L.L.C. was converted into shares of
common stock of the Virginia corporation and then into shares of common stock of Adial Pharmaceuticals, Inc., the members of Adial Pharmaceuticals,
L.L.C. became stockholders of Adial Pharmaceuticals, Inc. and Adial Pharmaceuticals, Inc. succeeded to the business of Adial Pharmaceuticals,
L.L.C.
Our
principal executive offices are located at 1180 Seminole Trail, Suite 495, Charlottesville, Virginia 22901, and our telephone number
is (434) 422-9800. Our website address is www.adial.com. Information contained on our website is intended for informational purposes
only and is not incorporated by reference into this prospectus, and it should not be considered to be part of this prospectus or the
registration statement of which this prospectus forms a part. The SEC maintains an internet site that contains reports, proxy and information
statements, and other information regarding issuers like us that file documents electronically with the SEC. The address of the SEC website
is www.sec.gov.
Recent
Developments
October
2023 Private Placement
On
October 19, 2023, we entered into a securities purchase agreement (the “Purchase Agreement”) with the Investor Selling Stockholder
pursuant to which we sold to the Investor Selling Stockholder in the Private Placement priced at-the-market consistent with the rules
of the Nasdaq Stock Market LLC, (i) the Pre-Funded Warrants to purchase up to an aggregate of 1,418,440 shares of Common Stock, (ii)
Series A Common Warrants to purchase up to an aggregate of 1,418,440 shares of Common Stock, and (iii) Series B Common Warrants to purchase
up to an aggregate of 1,418,440 shares of Common Stock. The combined purchase price of each Pre-Funded Warrant and accompanying Series
A Common Warrant and Series B Common Warrant was $2.819.
The
Private Placement closed on October 24, 2023. We received aggregate gross proceeds from the Private Placement of approximately $4.0 million,
before deducting the placement agent commissions and estimated offering expenses payable by us. We intend to use the net proceeds from
the Private Placement for working capital purposes. H.C.W. acted as the placement agent in the Private Placement and as part of its compensation
we issued to designees of H.C.W. Placement Agent Warrants to purchase up to 85,106 shares of Common Stock.
See
the section of this prospectus entitled “Description of the Private Placement” for a more detailed description of the Private
Placement.
Implications
of Being an Emerging Growth Company and a Smaller Reporting Company
We
qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS
Act”). As an “emerging growth company,” we may take advantage of specified reduced disclosure and other requirements
that are otherwise applicable generally to public companies. These provisions include, but are not limited to:
| |
● |
requiring
only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly
reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Securities
Act of 1933, as amended (the “Securities Act”), filings; |
| |
● |
reduced
disclosure about our executive compensation arrangements; |
| |
● |
no
non-binding advisory votes on executive compensation or golden parachute arrangements; and |
| |
● |
exemption
from compliance with the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant
to Section 404(b) of the Sarbanes Oxley Act of 2002 (“SOX”). |
We
may take advantage of these exemptions for up to five years or such earlier time that we are no longer an “emerging growth company.”
We will continue to remain an “emerging growth company” until the earliest of the following: (i) the last day of the fiscal
year following the fifth anniversary of the date of the completion of our initial public offering; (ii) the last day of the fiscal year
in which our total annual gross revenue is equal to or more than $1.235 billion; (iii) the date on which we have issued more than $1
billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer
under the rules of the SEC.
We
are also a “smaller reporting company” as defined in the Securities Exchange Act of 1934, as amended (the “Exchange
Act”), and have elected to take advantage of certain of the scaled disclosures available to smaller reporting companies. To the
extent that we continue to qualify as a “smaller reporting company” as such term is defined in Rule 12b-2 under the Exchange
Act, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an “emerging growth
company” may continue to be available to us as a “smaller reporting company,” including exemption from compliance with
the auditor attestation requirements pursuant to SOX and reduced disclosure about our executive compensation arrangements. We will continue
to be a “smaller reporting company” until we have $250 million or more in public float (based on our common stock) measured
as of the last business day of our most recently completed second fiscal quarter or, in the event we have no public float (based on our
common stock) or a public float (based on our common stock) that is less than $700 million and annual revenues of $100 million or more
during the most recently completed fiscal year.
We
may choose to take advantage of some, but not all, of these exemptions. We have taken advantage of reduced reporting requirements in
this prospectus. Accordingly, the information contained herein may be different from the information you receive from other public companies
in which you hold stock. In addition, the JOBS Act provides that an emerging growth company may take advantage of an extended transition
period for complying with new or revised accounting standards, delaying the adoption of these accounting standards until they would apply
to private companies. We have elected to avail ourselves of the extended transition period for complying with new or revised financial
accounting standards. As a result of the accounting standards election, we will not be subject to the same implementation timing for
new or revised accounting standards as other public companies that are not emerging growth companies which may make comparison of our
financials to those of other public companies more difficult.
Additional
Information
For
additional information related to our business and operations, please refer to the reports incorporated herein by reference, including
our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K as filed with the
SEC, as described in the section entitled “Incorporation of Certain Information by Reference” included elsewhere in this
prospectus.
THE
OFFERING
All
share number and exercise price information presented in this prospectus reflects a 1-for-25 reverse stock split of our common stock,
which was effected on August 4, 2023.
Shares
of Common Stock offered by the Selling Stockholders |
|
4,340,426
shares (consisting of: (i) 1,418,440 shares of our Common Stock issuable upon the exercise of the Pre-Funded Warrants, (ii) 1,418,440
shares of our Common Stock issuable upon the exercise of the Series A Common Warrants, (iii) 1,418,440 shares of Common Stock issuable
upon exercise of the Series B Common Warrants; (iv) 85,106 shares of Common Stock issuable upon the exercise of the Placement Agent
Warrants). |
| |
|
|
Common
Stock to be outstanding after this offering(1) |
|
5,558,407
shares of Common Stock, assuming the exercise of all of the Pre-Funded Warrants, the Common
Warrants and the Placement Agent Warrants
|
| Registration
Rights |
|
Under
the terms of the Registration Rights Agreement, we agreed to file this registration statement
with respect to the registration of the resale by the Investor Selling Stockholder of the
Pre-Funded Warrant Shares and the Common Warrant Shares by the 20th calendar
day following the date of the Registration Rights Agreement, and to use commercially reasonable
efforts to have the registration statement declared effective as promptly as practical, and
in any event, no later than the 45th calendar day following the date of the Registration
Rights Agreement or, in the event of a full review by the SEC, 75 days. In addition, we agreed
that, upon the registration statement being declared effective under the Securities Act,
we will use commercially reasonable efforts to maintain the effectiveness of the registration
statement until the date that (i) the Investor Selling Stockholder has sold all of the shares
of Common Stock issuable under the Registration Rights Agreement or (ii) such shares may
be resold by the Investor Selling Stockholder pursuant to Rule 144 of the Securities Act,
without the requirement for us to be in compliance with the current public information required
under such rule and without volume or manner-of-sale restriction.
|
| Use
of Proceeds |
|
The
Selling Stockholders will receive all of the proceeds of the sale of shares of Common Stock
offered from time to time pursuant to this prospectus. Accordingly, we will not receive any
proceeds from the sale of shares of Common Stock that may be sold from time to time pursuant
to this prospectus; however, we will receive proceeds from the any cash exercise of the Pre-Funded
Warrants, the Common Warrants and the Placement Agent Warrants. See “Use of Proceeds.”
We intend to use the proceeds from the any cash exercise of the Pre-Funded Warrants, the
Common Warrants and the Placement Agent Warrants for working capital purposes.
|
| Plan
of Distribution |
|
The
Selling Stockholders named in this prospectus, or their pledgees, donees, transferees, distributees, beneficiaries or other successors-in-interest,
may offer or sell the shares of Common Stock offered hereby from time to time through public or private transactions at prevailing
market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Stockholders may also
resell the shares of Common Stock to or through underwriters, broker-dealers or agents, who may receive compensation in the form
of discounts, concessions or commissions. |
| Risk
Factors |
|
See
“Risk Factors” beginning on page 6 of this prospectus and in the documents incorporated by reference in this
prospectus and the other information included in this prospectus for a discussion of factors you should carefully consider before
investing in our securities. |
| |
|
|
| Nasdaq
Capital Market trading symbol |
|
Our
Common Stock is listed on the Nasdaq Capital Market under the symbol “ADIL.” |
| (1) | The
number of shares of Common Stock to be outstanding after this offering is based on 1,217,981 shares of our Common Stock outstanding as
of November 3, 2023, and excludes: |
| ● | 329,022
shares of Common Stock issuable as of the date hereof upon the exercise of Common Stock warrants outstanding at a weighted average exercise
price of $74.44 per share; |
| ● | 204,059
shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted-average exercise price of $52.49 per share;
and |
| ● | 162,994
shares of Common Stock available for future issuance under the 2017 Equity Incentive Plan. |
RISK
FACTORS
Our
business, results of operations and financial condition and the industry in which we operate are subject to various risks. Accordingly,
investing in our securities involves a high degree of risk. This prospectus does not describe all of those risks. You should consider
the risk factors described in this prospectus below, as well as those described under the caption “Risk Factors” in the documents
incorporated by reference herein, including our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q, together
with the other information contained or incorporated by reference in this prospectus.
We
have described below and, in the documents incorporated by reference herein, the most significant risk factors applicable to us, but
they do not constitute all of the risks that may be applicable to us. New risks may emerge from time to time, and it is not possible
for us to predict all potential risks or to assess the likely impact of all risks. Before making an investment decision, you should carefully
consider these risks as well as other information we include or incorporate by reference in this prospectus and any amendment to this
prospectus or any prospectus supplement. This prospectus also contains forward-looking statements that involve risks and uncertainties.
Our actual results could differ materially from those anticipated in the forward-looking statements as a result of a number of factors,
including the risks described below. See the section titled “Cautionary Note Regarding Forward-Looking Statements.”
Risks
Related to Our Financial Position and Need for Capital
We
must raise additional capital to fund our operations in order to continue as a going concern.
Although
we raised net proceeds of approximately $3.5 million in the Private Placement and we believe that, with together with a $350 thousand
in expected expense reimbursements due, we will have sufficient cash and cash equivalents to fund our ongoing operations for a period
of a least 12 months subsequent to the date of this prospectus, we will need to raise additional capital through the sale of additional
equity or debt securities or other debt instruments, strategic relationships or grants, or other arrangements to support our future operations.
Our current business plan includes expansion for our commercialization efforts, which will require additional funding. If we are unable
to improve our liquidity position, we may not be able to continue as a going concern. Our ability to continue as a going concern is dependent
upon our ability to generate revenue and raise capital from financing transactions. Our future is dependent upon its ability to obtain
financing and upon future profitable operations from the development of its new business opportunities. There can be no assurance that
we will be successful in accomplishing these objectives. Without such additional capital, we may be required to curtail or cease operations
and be required to realize our assets and discharge our liabilities other than in the normal course of business which could cause investors
to suffer the loss of all or a substantial portion of their investment. Marcum LLP, our independent registered public accounting firm
for the fiscal year ended December 31, 2022, has included an explanatory paragraph in its opinion that accompanies our audited consolidated
financial statements as of and for the year ended December 31, 2022, indicating that our current liquidity position raises substantial
doubt about our ability to continue as a going concern.
We
have incurred losses from our continuing operations every year and quarter since our inception and anticipate that we will continue to
incur losses from our continuing operations in the future. We anticipate that we will need to raise additional funds even if we sell
the maximum number of securities offered in this offering.
We
are a clinical stage biotechnology pharmaceutical company that is focused on the discovery and development of medications for the treatment
of addictions and related disorders of AUD in patients with certain targeted genotypes. We have a limited operating history. Investment
in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant
risk that any potential product candidate will fail to demonstrate adequate effect or an acceptable safety profile, gain regulatory approval
and become commercially viable. We have no products approved for commercial sale and have not generated any revenue from product sales
to date, and we continue to incur significant research and development and other expenses related to our ongoing operations. To date,
we have not generated positive cash flow from operations, revenues, or profitable operations, nor do we expect to in the foreseeable
future. As of June 30, 2023, we had an accumulated deficit of approximately $65.5 million and as of December 31, 2022, we had an accumulated
deficit of approximately $63.7 million. Even though we raised net proceeds of approximately $3.5 million in the Private Placement and
these proceeds, together with receipt of an additional $350 thousand in expense reimbursements due, are expected to fund our ongoing
operations for at least 12 months from the date of this prospectus, we will need to raise additional funds as our current cash and cash
equivalents to fund operation beyond that point or to fund any additional projects or expenses. We believe that additional equity financings
are the most likely source of capital going forward. There can be no assurance that we will be able to complete any such financing transaction
on acceptable terms or otherwise.
Even
if we succeed in commercializing our product candidate or any future product candidates, we expect that the commercialization of our
product will not begin until 2026 or later, we will continue to incur substantial research and development and other expenditures to
develop and market additional product candidates and will continue to incur substantial losses and negative operating cash flow. We may
encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business.
The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue.
Our prior losses and expected future losses have had and will continue to have an adverse effect on our shareholders’ equity and
working capital.
Even
if we can raise additional funding, we may be required to do so on terms that are dilutive to you.
The
capital markets have been unpredictable in the past for unprofitable companies such as ours. In addition, it is generally difficult for
development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able
to raise often depends on variables that are beyond our control. As a result, we may not be able to secure financing on terms attractive
to us, or at all. If we can consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If
adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition
and our continued viability will be materially adversely affected.
Risks
Related to the Private Placement and Ownership of the Warrants
Our
management will have broad discretion over the use of the net proceeds from the Private Placement and may apply it to uses that do not
improve our operating results or the value of our securities.
Our
management will have broad discretion over the use of proceeds from the Private Placement. We intend to use the net proceeds from the
Private Placement for working capital and other general corporate purposes. Our management will have considerable discretion in the application
of the net proceeds, and shareholders will not have the opportunity, as part of their investment decision, to assess whether the proceeds
are being used appropriately. The net proceeds, if any, may be used for corporate purposes that do not improve our operating results
or enhance the value of our Common Stock. The failure of our management to use these funds effectively could have a material adverse
effect on our business, cause the market price of our Common Stock to decline and impair the commercialization of our products and/or
delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in short-term,
investment-grade, interest-bearing instruments and U.S. government securities. These investments may not yield a favorable return to
our stockholders.
There
is no public market for the Pre-Funded Warrants, Common Warrants or Placement Agent Warrants.
There
is no established public trading market for the Pre-Funded Warrants, Common Warrants or Placement Agent Warrants, and we do not expect
a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants, Common Warrants or Placement Agent Warrants
on the Nasdaq Capital Market or any national securities exchange or other nationally recognized trading system. Without an active market,
the liquidity of the Pre-Funded Warrants, Common Warrants and Placement Agent Warrants will be limited.
We
may not receive any additional funds upon the exercise of the Pre-Funded Warrants, Common Warrants or Placement Agent Warrants.
Each
Pre-Funded Warrant, Common Warrant and Placement Agent Warrant may be exercised by way of a cashless exercise, meaning that the holder
may not pay a cash purchase price upon exercise under certain circumstances, but instead would receive upon such exercise the net number
of shares of our Common Stock determined according to the formula set forth in the Pre-Funded Warrants, Common Warrants or Placement
Agent Warrants. Accordingly, we may not receive any additional funds upon the exercise of the Pre-Funded Warrants, Common Warrants or
Placement Agent Warrants.
The
Pre-Funded Warrants, Common Warrants and Placement Agent Warrants are speculative in nature.
The
Pre-Funded Warrants, Common Warrants and Placement Agent Warrants do not confer any rights of Common Stock ownership on their holders,
such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of Common Stock at a
fixed price for a limited period of time. Specifically, commencing on the earlier of (i) if permissible by the applicable rules and regulations
of the Nasdaq Stock Market LLC (“Nasdaq”), payment by the holder of $0.125 per Common Warrant Share and (ii) the date of
which approval (as may be required by the applicable rules and regulations of Nasdaq (or any successor entity) from our stockholders
with respect to issuance of all of the Common Warrants and the Common Warrant Shares upon the exercise thereof is obtained, holders of
the Common Warrants may exercise their right to acquire shares of our Common Stock and pay an exercise price of $2.82 per share, subject
to certain adjustments, prior to five and one-half years from the issuance date for the Series A Common Warrant and 18 months from the
issuance date for the Series B Common Warrant, after which date any unexercised Common Warrants will expire and have no further value.
Holders of Placement Agent Warrants may exercise their right to acquire our Common Stock and pay an exercise price of $3.525 per share,
subject to certain adjustments, prior to five and one-half years from the date on which such Placement Agent Warrants were issued, after
which date any unexercised Placement Agent Warrants will expire and have no further value. Holders of Pre-Funded Warrants have identical
rights, except that the Pre-Funded Warrants have an exercise price of $0.001 and do not expire until exercised in full. The market value
of the Pre-Funded Warrants, Common Warrants and Placement Agent Warrants, if any, is uncertain and there can be no assurance that the
market value of the Pre-Funded Warrants, Common Warrants and Placement Agent Warrants will equal or exceed their imputed offering price.
The Pre-Funded Warrants, Common Warrants and Placement Agent Warrants will not be listed or quoted for trading on any market or exchange.
There can be no assurance that the market price of the Common Stock will ever equal or exceed the exercise price of the Common Warrants
or Placement Agent Warrants and consequently, whether it will ever be profitable for holders of the Common Warrants or Placement Agent
Warrants to exercise the warrants.
Holders
of the Common Warrants, Pre-Funded Warrants and Placement Agent Warrants will have no rights as common stockholders with respect to the
shares our Common Stock underlying the warrants until such holders exercise their warrants and acquire our Common Stock, except as otherwise
provided in the Common Warrants, and Pre-Funded Warrants and Placement Agent Warrants.
Until
holders of the Common Warrants, Pre-Funded Warrants and/or and Placement Agent Warrants acquire shares of our Common Stock upon exercise
thereof, such holders will have no rights with respect to the shares of our Common Stock underlying such warrants, except to the extent
that holders of such Common Warrants, Pre-Funded Warrants and Placement Agent Warrants will have certain rights to participate in distributions
or dividends paid on our Common Stock as set forth in such warrants. Upon exercise of the Common Warrants, Pre-Funded Warrants and Placement
Agent Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date
occurs after the exercise date.
We
do not intend to pay dividends on our Common Stock, so any returns will be limited to increases, if any, in our Common Stock’s
value. Your ability to achieve a return on your investment will depend on appreciation, if any, in the price of our Common Stock.
We
currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate
declaring or paying any cash dividends for the foreseeable future. Any future determination to declare dividends will be made at the
discretion of our board of directors and will depend on, among other factors, our financial condition, operating results, capital requirements,
general business conditions and other factors that our board of directors may deem relevant. Any return to stockholders will therefore
be limited to the appreciation in the value of their stock, if any.
USE
OF PROCEEDS
The
Selling Stockholders will receive all of the proceeds of the sale of shares of Common Stock offered from time to time pursuant to this
prospectus. Accordingly, we will not receive any proceeds from the sale of shares of Common Stock that may be sold from time to time
pursuant to this prospectus; however, we will receive proceeds from the cash exercise of the Pre-Funded Warrants, Common Warrants and
Placement Agent Warrants.
We
will bear the out-of-pocket costs, expenses and fees incurred in connection with the registration of shares of our Common Stock to be
sold by the Selling Stockholders pursuant to this prospectus. Other than registration expenses, the Selling Stockholders will bear any
underwriting discounts, commissions, placement agent fees or other similar expenses payable with respect to sales of shares of our Common
Stock.
DIVIDEND
POLICY
We
currently intend to retain all available funds and any future earnings to fund the growth and development of our business. We have never
declared or paid any cash dividends on our capital stock. We do not intend to pay cash dividends on our Common Stock in the foreseeable
future. Investors should not purchase our Common Stock with the expectation of receiving cash dividends.
Any
future determination to declare dividends will be made at the discretion of our board of directors and will depend on our financial condition,
operating results, capital requirements, general business conditions, and other factors that our board of directors may deem relevant.
DETERMINATION
OF THE OFFERING PRICE
The
prices at which the shares of Common Stock covered by this prospectus may actually be sold will be determined by the prevailing public
market price for shares of our Common Stock or by negotiations between the Selling Stockholders and buyers of our Common Stock in private
transactions or as otherwise described in “Plan of Distribution.”
DESCRIPTION
OF CAPITAL STOCK
The
following description of the material terms of our capital stock and the provisions of our certificate of incorporation (“Certificate
of Incorporation”) and our amended and restated bylaws (“Bylaws”) are summaries and are qualified by reference to copies
of the Certificate of Incorporation and Bylaws, which are filed with the SEC as exhibits to our registration statement of which this
prospectus forms a part.
Authorized
Capital Stock
Our
authorized capital stock consists of 50,000,000 shares of Common Stock, with a par value of $0.001 per share, and 5,000,000 shares of
preferred stock, with a par value of $0.001 per share.
As
of November 3, 2023, there were 1,217,981 shares of our Common Stock outstanding held by 101 record stockholders and no shares
of preferred stock were issued and outstanding.
Common
Stock
Authorized
Shares of Common Stock. We currently have authorized 50,000,000 shares of Common Stock.
Voting
Rights. The holders of Common Stock are entitled to one vote per share on all matters to be voted upon by the stockholders, except
on matters relating solely to terms of preferred stock.
Dividend
Rights. Subject to preferences that may be applicable to any outstanding preferred stock, the holders of Common Stock are entitled
to receive ratably such dividends, if any, as may be declared from time to time by the board of directors out of funds legally available
therefor.
Liquidation
Rights. In the event of our liquidation, dissolution or winding up, the holders of Common Stock are entitled to share ratably in
all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock, if any, then outstanding.
Other
Rights and Preferences. The holders of our Common Stock have no preemptive or conversion rights or other subscription rights. There
are no redemption or sinking fund provisions applicable to our Common Stock.
Listing.
Our Common Stock is listed for trading on the Nasdaq Capital Market under the symbol “ADIL.”
Transfer
Agent and Registrar. The transfer agent and registrar for our Common Stock is VStock Transfer, LLC.
Anti-Takeover
Effects of Delaware Law
The
provisions of Delaware law, our Certificate of Incorporation and our Bylaws described below may have the effect of delaying, deferring
or discouraging another party from acquiring control of us.
Section
203 of the Delaware General Corporation Law
We
are subject to Section 203 of the Delaware General Corporation Law, which prohibits a Delaware corporation from engaging in any business
combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder,
with the following exceptions:
| |
● |
before
such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in
the stockholder becoming an interested stockholder; |
| |
● |
upon
completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned
at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining
the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by
persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to
determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or |
| |
● |
on
or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of
the stockholders, and not by written consent, by the affirmative vote of at least sixty-six and two-thirds percent (66 2/3%) of the outstanding
voting stock that is not owned by the interested stockholder. |
In
general, Section 203 defines business combination to include the following:
| ● | any
merger or consolidation involving the corporation and the interested stockholder; |
| ● | any
sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder; |
| ● | subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to
the interested stockholder; |
| ● | any
transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of
the corporation beneficially owned by the interested stockholder; or |
| ● | the
receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges or other financial benefits by or through
the corporation. |
Certificate
of Incorporation and Bylaws
Our
Certificate of Incorporation and Bylaws provide that:
| ● | our
board of directors is divided into three classes, one class of which is elected each year by our stockholders with the directors in each
class to serve for a three-year term; |
| ● | the
authorized number of directors can be changed only by resolution of our board of directors; |
| ● | directors
may be removed only by the affirmative vote of the holders of at least 60% of our voting stock, whether for cause or without cause; |
| |
● |
our Bylaws may be amended or repealed by our board of directors or by the affirmative vote of sixty-six and two-thirds percent (66 2/3%) of our stockholders; |
| ● | stockholders
may not call special meetings of the stockholders or fill vacancies on the board of directors; |
| ● | our
board of directors will be authorized to issue, without stockholder approval, preferred stock, the rights of which will be determined
at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership
of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
| ● | our
stockholders do not have cumulative voting rights, and therefore our stockholders holding a majority of the shares of Common Stock outstanding
will be able to elect all of our directors; and |
| ● | our
stockholders must comply with advance notice provisions to bring business before or nominate directors for election at a stockholder
meeting. |
Limitations
of Director Liability and Indemnification of Directors, Officers and Employees
Our
Certificate of Incorporation limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides
that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors,
except for liability for any:
| |
● |
breach
of their duty of loyalty to us or our stockholders; |
| |
● |
act
or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| |
● |
unlawful
payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation
Law; or |
| |
● |
transaction
from which the directors derived an improper personal benefit. |
These
limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability
of equitable remedies such as injunctive relief or rescission.
Our
Bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by law and may indemnify employees and
other agents. Our Bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final
disposition of any action or proceeding.
We
have obtained a policy of directors’ and officers’ liability insurance.
We
have entered into separate indemnification agreements with our directors and officers. These agreements, among other things, require
us to indemnify our directors and officers for any and all expenses (including reasonable attorneys’ fees, retainers, court costs,
transcript costs, fees of experts, witness fees, travel expenses, duplicating costs, printing and binding costs, telephone charges, postage,
delivery service fees) judgments, fines and amounts paid in settlement actually and reasonably incurred by such directors or officers
or on his or her behalf in connection with any action or proceeding arising out of their services as one of our directors or officers,
or any of our subsidiaries or any other company or enterprise to which the person provides services at our request provided that such
person follows the procedures for determining entitlement to indemnification and advancement of expenses set forth in the indemnification
agreement. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons
as directors and officers.
The
limitation of liability and indemnification provisions in our Certificate of Incorporation and Bylaws may discourage stockholders from
bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation
against directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results
of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors
and officers pursuant to these indemnification provisions.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us,
we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act
and is therefore unenforceable.
Common
Warrants, Pre-Funded Warrants and Placement Agent Warrants Issued in the Private Placement
See
“Description of the Private Placement” for a description of the Common Warrants, the Pre-Funded Warrants and the Placement
Agent Warrants issued in connection with the Private Placement.
DESCRIPTION
OF THE PRIVATE PLACEMENT
On
October 19, 2023, we entered into the Purchase Agreement with the Investor Selling Stockholder pursuant to which we agreed to issue and
sell to the Investor Selling Stockholder in the Private Placement priced at-the-market consistent with the rules of Nasdaq, securities
consisting of: (i) Pre-Funded Warrants to purchase up to an aggregate of 1,418,440 shares of our Common Stock, (ii) Series A Common Warrants
to purchase up to 1,418,440 shares of Common Stock, and (iii) Series B Common Warrants to purchase up to 1,418,440 shares of Common Stock.
The combined purchase price of each Pre-Funded Warrant and accompanying Common Warrants was $2.819.
The
Private Placement closed on October 24, 2023. We received aggregate gross proceeds from the Private Placement of approximately $4.0 million,
before deducting the Placement Agent commissions and estimated offering expenses payable by us. We intend to use the net proceeds from
the Private Placement for working capital purposes.
Each
Pre-Funded Warrant has an exercise price equal to $0.001 per share. The Pre-Funded Warrants are exercisable at any time after their original
issuance and will not expire until exercised in full. Each Common Warrant has an exercise price equal to $2.82 per share. The Series
A Common Warrants and Series B Common Warrants are exercisable at any time after the earlier of (i) if permissible by the applicable
rules and regulations of Nasdaq, payment by the holder of $0.125 per Common Warrant Share and (ii) the date of which approval as may
be required by the applicable rules and regulations of the Nasdaq (or any successor entity) from our stockholders with respect to
issuance of all of the Common Warrants and the Common Warrant Shares upon the exercise thereof. The Series A Common Warrants will expire
on the five and one-half (5.5) year anniversary of their issuance and the Series B Common Warrants will expire on the 18 month anniversary
of their issuance. The exercise price and number of shares of Common Stock issuable upon exercise of the Common Warrant and Pre-Funded
Warrant are subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events.
The
Pre-Funded Warrants and the Common Warrants issued in the Private Placement provide that a holder of Pre-Funded Warrants or Common Warrants,
as applicable, will not have the right to exercise any portion of its Pre-Funded Warrants or Warrants if such holder, together with its
affiliates, and any other party whose holdings would be aggregated with those of the holder for purposes of Section 13(d) or Section
16 of the Exchange Act would beneficially own in excess of 9.99% for the Pre-Funded Warrants and 4.99% for the Common Warrants of the
number of shares of Common Stock outstanding immediately after giving effect to such exercise (the “Beneficial Ownership Limitation”);
provided, however, that the holder may increase or decrease the Beneficial Ownership Limitation by giving notice to the Company,
with any such increase not taking effect until the sixty-first day after such notice is delivered to the Company but not to any percentage
in excess of 9.99%. The Common Warrants may be exercised on a cashless basis if a registration statement registering Common Warrant Shares
is not effective. The Pre-Funded Warrants may be exercised on a cashless basis.
Subject
to applicable laws, a Common Warrant and Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the applicable
warrant to us together with the appropriate instruments of transfer.
There
is no trading market available for the Common Warrants or the Pre-Funded Warrants on any securities exchange or nationally recognized
trading system. We do not intend to list the Common Warrants or the Pre-Funded Warrants on any securities exchange or nationally recognized
trading system.
In
the event of a fundamental transaction, as described in the Common Warrants and Pre-Funded Warrants and generally including any reorganization,
recapitalization or reclassification of our Common Stock, the sale, transfer or other disposition of all or substantially all of our
properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding Common
Stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding Common Stock, the
holders of the Common Warrants and Pre-Funded Warrants will be entitled to receive upon exercise of the Common Warrants and Pre-Funded
Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Common
Warrants and Pre-Funded Warrants immediately prior to such fundamental transaction. In addition, in certain circumstances, upon a fundamental
transaction, the holder will have the right to require us to repurchase its Common Warrants at the Black Scholes Value; provided, however,
that, if the fundamental transaction is not within our control, including not approved by our board of directors, then the holder shall
only be entitled to receive the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised
portion of the Common Warrant, that is being offered and paid to the holders of Common Stock in connection with the fundamental transaction.
Pursuant
to the terms of the Purchase Agreement, the Company is prohibited from entering into any agreement to issue or announcing the issuance
or proposed issuance of any shares of Common Stock or securities convertible or exercisable into Common Stock for a period commencing
on October 19, 2023 and expiring 60 days from the Effective Date (as defined in the Purchase Agreement). Furthermore, the Company is
also prohibited from entering into any agreement to issue Common Stock or Common Stock Equivalents (as defined in the Purchase Agreement)
involving a Variable Rate Transaction (as defined in the Purchase Agreement), subject to certain exceptions, for a period commencing
on October 19, 2023 and expiring one year from such Effective Date. The Effective Date is defined in the Purchase Agreement as the earliest
of the date that (a) the initial registration statement contemplated by the Registration Rights Agreement (described below) has been
declared effective by the SEC, (b) all of the Common Warrant Shares and Pre-Funded Warrant Shares have been sold pursuant to Rule 144
or may be sold pursuant to Rule 144 without the requirement for the Company to be in compliance with the current public information required
under Rule 144 and without volume or manner-of-sale restrictions, (c) following the one year anniversary of the closing of the Private
Placement provided that the holder of the Common Warrant Shares and the Pre-Funded Warrant Shares is not an affiliate of the Company,
or (d) all of the Common Warrant Shares and Pre-Funded Warrant Shares may be sold pursuant to an exemption from registration under Section
4(a)(1) of the Securities Act without volume or manner-of-sale restrictions and the holders of such shares shall have received an opinion
from our legal counsel reasonably acceptable to them.
The
Purchase Agreement contains customary representations, warranties and agreements by the Company, customary conditions to closing, indemnification
obligations of the Company, including for liabilities arising under the Securities Act, other obligations of the parties and termination
provisions. The representations, warranties and covenants contained in the Purchase Agreement were made only for the purposes of such
agreements and as of the specific dates, were solely for the benefit of the parties to such agreement and may be subject to limitations
agreed upon by the contracting parties.
H.C.W.
served as our exclusive Placement Agent in connection with the Private Placement, pursuant to that certain engagement letter, dated as
of September 27, 2023, as amended October 19, 2023 between us and H.C.W. (the “Engagement Letter”). Pursuant to the Engagement
Letter, we paid H.C.W. (i) a total cash fee equal to 7.0% of the aggregate gross proceeds of the Private Placement, (ii) a management
fee of 1.0% of the aggregate gross proceeds of the Private Placement, (iii) a non-accountable expense allowance of $25,000, and (iv)
$50,000 for legal fees and other out-of-pocket expenses. In addition, we issued to H.C.W. or its designees the Placement Agent Warrants
to purchase up to an aggregate of 85,106 shares of our Common Stock at an exercise price equal to $3.525 per share. The Placement Agent
Warrants are exercisable immediately upon issuance and have a term of exercise equal to five and one-half years from the date of issuance.
On
October 19, 2023, the Company and H.C.W. also entered into an amendment to the Engagement Letter which provides that upon any exercise
for cash of the Series B Common Warrants, the Company shall pay Wainwright (i) a cash fee of 7.0% of the aggregate gross exercise price
paid in cash and (ii) a management fee of 1.0% of the aggregate gross exercise price paid in cash, and further issue to H.C.W (or its
designees) warrants to purchase shares of Common Stock equal to 6.0% of the aggregate number of shares of Common Stock underlying the
Series B Common Warrants that have been exercised having the same terms as the Placement Agent Warrants issued in connection with the
Private Placement.
Registration
Rights Agreement
In
connection with the Private Placement, we entered into the Registration Rights Agreement with the Investor Selling Stockholder, dated
October 19, 2023, pursuant to which we agreed to register for resale the Common Warrant Shares and the Pre-Funded Warrant Shares held
by the Investor Selling Stockholder (the “Registrable Securities”). Under the Registration Rights Agreement, we agreed to
file a registration statement covering the resale of the Registrable Securities within 20 days following the date of the Registration
Rights Agreement (the “Filing Deadline”). We agreed to use commercially reasonable efforts to cause such registration statement
to become effective (the “Effectiveness Deadline”) as soon as practicable (but no later than the 45th calendar
day following the date of the Registration Rights Agreement or, in the event of a “full review” by the SEC, the 75th
calendar day following the date of the Registration Rights Agreement) and to keep such registration statement effective until the
date the Common Warrant Shares and the Pre-Funded Warrant Shares covered by such registration statement have been sold or may be resold
pursuant to Rule 144 without restriction. We agreed to be responsible for all fees and expenses incurred in connection with the registration
of the Registrable Securities.
In
the event (i) the registration statement has not been filed by the Filing Deadline, including filing the registration statement without
affording the Investor the opportunity to review and common thereon or the Company subsequently withdraws the filing of the registration
statement (ii) the Company fails to file with the SEC a request for acceleration of a registration statement within five (5) trading
days of the date that the Company is notified by the SEC that such Registration Statement will not be “reviewed” or will
not be subject to further review, (iii) prior to the effective date of a registration statement, the Company fails to file a pre-effective
amendment and otherwise respond in writing to comments made by the SEC in respect of such registration statement within twenty (20) calendar
days after the receipt of comments by or notice from the SEC that such amendment is required in order for such registration statement
to be declared effective; (iv) the registration statement has not been declared effective prior to Effectiveness Deadline, or (v) after
the registration statement has been declared effective by the SEC, sales cannot be made pursuant to the registration statement for any
reason including by reason of a stop order or our failure to update such registration statement, subject to certain limited exceptions,
then the Company has agreed to make pro rata payments to the Investor as liquidated damages in an amount equal to 1% of the aggregate
amount invested by the Investor in the Registrable Securities per 30-day period or pro rata for any portion thereof for each such month
during which such event continues, subject to an aggregate maximum cap of 6%.
We
have granted the Investor Selling Stockholder customary indemnification rights in connection with the registration statement. The Investor
Selling Stockholder also granted to us customary indemnification rights in connection with the registration statement.
The
foregoing descriptions of the Purchase Agreement, the Pre-Funded Warrants, the Common Warrants, the Placement Agent Warrants and the
Registration Rights Agreement do not purport to be complete and are qualified in their entirety by reference to such agreements, copies
of which are filed as exhibits to the registration statement of which this prospectus forms a part, and are incorporated by reference
herein.
SELLING
STOCKHOLDERS
The
Common Stock being offered by the Selling Stockholders are those issuable to the Selling Stockholders upon exercise of the Common Warrants,
the Pre-Funded Warrants the Placement Agent Warrants. For additional information regarding the issuances of the Common Warrants, the
Pre-Funded Warrants and the Placement Agent Warrants, see “Description of the Private Placement” above. We are registering
the shares of Common Stock in order to permit the Selling Stockholders to offer the shares of Common Stock for resale from time to time.
Except for the ownership of the Common Warrants, the Pre-Funded Warrants and the Placement Agent Warrants, the Selling Stockholders have
not had any material relationship with us within the past three years, except that: (i) on February 10, 2022, we entered into a securities
purchase agreement with the Investor Selling Stockholder, pursuant to which we issued, in a registered direct offering, 92,890 shares
of Common Stock, pre-funded warrants to purchase up to 74,600 shares of Common Stock, and five and one-half year warrants to purchase
up to 159,116 shares of Common Stock for the aggregate purchase price of approximately $10 million and, in connection with such registered
direct offering, we filed a registration statement on Form S-3, File No. 333-263037, to register the resale of the 159,116 shares of
Common Stock underlying the five and one-half year warrants; and (ii) each of Michael Vasinkevich, Noam Rubinstein, Craig Schwabe and
Charles Worthman are associated persons of H.C.W., which served as our placement agent in connection with the Private Placement for which
H.C.W. received compensation as described in the section of this prospectus entitled “Description of the Private Placement.”
The
table below lists the Selling Stockholders and other information regarding the beneficial ownership of the shares of Common Stock by
each of the Selling Stockholders. The second column lists the number of shares of Common Stock beneficially owned by the Selling Stockholders,
based on its ownership of the shares of Common Stock, the Common Warrants, the Pre-Funded Warrants and the Placement Agent Warrants,
as of November 3, 2023, assuming exercise of the Common Warrants, the Pre-Funded Warrants and the Placement Agent Warrants held
by the Selling Stockholders on that date, without regard to any limitations on exercises.
The
third column lists the maximum number of shares of Common Stock being offered by this prospectus by the Selling Stockholders.
In
accordance with the terms of the Registration Rights Agreement with the Investor Selling Stockholder and an agreement with H.C.W., this
prospectus generally covers the resale of the maximum number of shares of Common Stock issuable upon exercise of the Common Warrants,
the Pre-Funded Warrants and the Placement Agent Warrants, determined as if the outstanding Common Warrants, Pre-Funded Warrants and Placement
Agent Warrants were exercised in full as of the trading day immediately preceding the date this registration statement was initially
filed with the SEC, each as of the trading day immediately preceding the applicable date of determination and all subject to adjustment
as provided in the registration right agreement, without regard to any limitations on the exercise of the Common Warrants, the Pre-Funded
Warrants and the Placement Agent Warrants. The fourth and fifth columns assume the sale of all of the shares offered by the Selling Stockholders
pursuant to this prospectus.
Under
the terms of the Common Warrants, the Pre-Funded Warrants and the Placement Agent Warrants, the Selling Stockholders may not exercise
the Common Warrants, the Pre-Funded Warrants or the Placement Agent Warrants to the extent such exercise would cause such Selling Stockholder,
together with its affiliates and attribution parties, to beneficially own a number of shares of Common Stock that would exceed 4.99%
or 9.99%, as applicable, of our then outstanding Common Stock following such exercise, excluding for purposes of such determination shares
of Common Stock issuable upon exercise of such warrants which have not been exercised. The number of shares in the second and fourth
columns do not reflect this limitation. The Selling Stockholders may sell all, some or none of their shares in this offering. See “Plan
of Distribution.”
| Name of Selling Stockholder | |
Number of
Shares
of Common
Stock
Beneficially
Owned
Prior to
Offering(1) | | |
Maximum
Number of
Shares
of Common
Stock
to be Sold
in this
Offering | | |
Number of
Shares
of Common
Stock
Beneficially
Owned After
Offering | | |
Percentage
of Shares
Beneficially
Owned after
Offering(1) | |
| Armistice Capital, LLC (1)(2) | |
| 4,414,436 | | |
| 4,255,320 | | |
| 159,116 | | |
| 2.8 | % |
| Michael Vasinkevich (3) | |
| 54,574 | | |
| 54,574 | | |
| — | | |
| — | |
| Noam Rubinstein (3) | |
| 26,808 | | |
| 26,808 | | |
| — | | |
| — | |
| Craig Schwabe (3) | |
| 2,872 | | |
| 2,872 | | |
| — | | |
| — | |
| Charles Worthman (3) | |
| 852 | | |
| 852 | | |
| — | | |
| — | |
| (1) |
Consists
of (i) 1,418,440 shares issuable upon exercise of the Pre-Funded Warrants; (ii) 1,418,440 shares issuable upon the exercise of the
Series A Common Warrants; and (iii) 1,418,440 shares issuable upon the exercise of the Series B Common Warrants. The Pre-Funded Warrants
are subject to a beneficial ownership limitation of either 9.99% and the Common Warrants are subject to a beneficial ownership limitation
of 4.99%, which in each case restricts the Investor Selling Stockholder from exercising that portion of the warrants that would result
in the Investor Selling Stockholder and its affiliates owning, after exercise, a number of shares of common stock in excess of the
beneficial ownership limitation. The number of shares set forth in the above table does not reflect the application of this limitation.
|
| (2) |
The
securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”),
and may be deemed to be beneficially owned by: (i) Armistice Capital, LLC (“Armistice Capital”), as the investment manager
of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial ownership
limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of the warrants that would
result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of Common Stock in excess of the
beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. Is c/o Armistice Capital, LLC, 510 Madison Avenue,
7th Floor, New York, NY 10022. |
| (3) |
Each
of these Selling Stockholders is affiliated with H.C. Wainwright & Co., LLC. H.C. Wainwright & Co., LLC is a registered broker
dealer and has a registered address of c/o H.C. Wainwright & Co., LLC 430 Park Ave, 3rd Floor, New York, NY 10022.
H.C. Wainwright & Co., LLC has sole voting and dispositive power over the securities held. The number of shares beneficially
owned prior to this offering consist of shares of common stock issuable upon exercise of Placement Agent Warrants, which were received
as compensation for the Private Placement. H.C. Wainwright & Co., LLC acquired the placement agent warrants in the ordinary course
of business and, at the time the placement agent warrants were acquired, H.C. Wainwright & Co., LLC had no agreement or understanding,
directly or indirectly, with any person to distribute such securities. |
PLAN
OF DISTRIBUTION
Each
Selling Stockholder of the securities and any of its pledgees, assignees and successors-in-interest may, from time to time, sell any
or all of their securities covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which
the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholders may
use any one or more of the following methods when selling securities:
| ● | ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| ● | block
trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as
principal to facilitate the transaction; |
| ● | purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| ● | an
exchange distribution in accordance with the rules of the applicable exchange; |
| ● | privately
negotiated transactions; |
| ● | settlement
of short sales; |
| ● | in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated
price per security; |
| ● | through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| ● | a
combination of any such methods of sale; or |
| ● | any
other method permitted pursuant to applicable law. |
The
Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available,
rather than under this prospectus.
Broker-dealers
engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser)
in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in
excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or
markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers
or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they
assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan
or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option
or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the
delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The
Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters”
within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers
or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts
under the Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding,
directly or indirectly, with any person to distribute the securities.
We
are required to pay certain fees and expenses incurred by us incident to the registration of the securities. We have agreed to indemnify
the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We
agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders
without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for
us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect
or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar
effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state
securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the
common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders
and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including
by compliance with Rule 172 under the Securities Act).
LEGAL
MATTERS
The
validity of the shares of our Common Stock being offered by this prospectus have been passed upon for us by Blank Rome LLP, New York,
New York.
EXPERTS
The
financial statements of Adial Pharmaceuticals, Inc. as of December 31, 2022 and 2021 and for each of the two years in the period ended December 31, 2022, incorporated by reference in this registration statement have been
audited by Marcum LLP, an independent registered public accounting firm, as stated in their report (the report on the financial statements
contains an explanatory paragraph regarding our ability to continue as a going concern). Such financial statements are incorporated by
reference in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
We
file reports and proxy statements with the SEC. These filings include our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q,
Current Reports on Form 8-K and proxy statements on Schedule 14A, as well as any amendments to those reports and proxy statements, which
are available free of charge through our website as soon as reasonably practicable after we file them with, or furnish them to, the SEC.
Our Internet website address is www.adial.com. Our website and the information contained on, or that can be accessed through,
the website will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus. You should not rely
on any such information in making your decision whether to purchase our securities. The SEC also maintains a website at www.sec.gov that
contains reports, proxy and information statements and other information regarding us and other issuers that file electronically with
the SEC.
We
have filed with the SEC a registration statement on Form S-1 under the Securities Act relating to the securities being offered by this
prospectus. This prospectus, which constitutes part of that registration statement, does not contain all of the information set forth
in the registration statement or the exhibits and schedules which are part of the registration statement. For further information about
us and the securities offered, see the registration statement and the exhibits and schedules thereto. Statements contained in this prospectus
regarding the contents of any contract or any other document to which reference is made are not necessarily complete, and, in each instance
where a copy of a contract or other document has been filed as an exhibit to the registration statement, reference is made to the copy
so filed, each of those statements being qualified in all respects by the reference.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The
SEC allows us to “incorporate by reference” information from other documents that we file with it, which means that we can
disclose important information to you by referring you to those documents. The information incorporated by reference is considered to
be part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC
prior to the date of this prospectus.
We
incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents
listed below that we have filed with the SEC (Commission File No. 001-38323):
| |
● |
Our
Annual Report on Form
10-K for the fiscal December 31, 2022 (the “Annual Report”) with the SEC on March 30, 2023 as updated on Current Report on Form 8-K filed with the SEC on September 27, 2023 solely to recast certain financial information and
related disclosures included in the Company’s Annual Report; |
| |
|
|
| |
● |
Our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 filed with the SEC on May 12, 2023, our Quarterly Report on Form 10-Q
for the quarter ended June 30, 2023 filed with the SEC on August 21, 2023, as amended by our Quarterly Report on Form 10-Q/A filed with the SEC on October 30, 2023; |
| |
● |
Our
Current Reports on Form 8-K filed with the SEC on February
1, 2023, February
21, 2023, February
24, 2023, February
27, 2023, March
2, 2023, March
7, 2023, March
21, 2023 April
13, 2023, May 10,
2023, May 24,
2023, June 2,
2023; July 10,
2023, August 4,
2023, August 18,
2023, August 23,
2023, September
21, 2023, October
24, 2023 and November 6, 2023;
|
| |
|
|
| |
● |
Our
Current Report on Form 8-K/A filed with the SEC on October 30, 2023; |
| |
|
|
| |
● |
Our
Definitive Proxy Statement on Schedule 14A filed with the SEC on October 2, 2023; and |
| |
|
|
| |
● |
The
description of our Common Stock set forth in (i) our registration statements on Form 8-A12B, filed with the SEC on December 11, 2017
and Form 8-A12B/A filed with the SEC on July 23, 2018 (File No. 001-38323) and (ii) Exhibit 4.17—Description of Securities
to our Annual Report on Form 10-K for the fiscal year ended December 31, 2022. |
We
also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits
filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant
to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made (i) on or after the date of the initial filing of the
registration statement of which this prospectus forms a part and prior to effectiveness of such registration statement, and (ii) on or
after the date of this prospectus but prior to the termination of the offering (i.e., until the earlier of the date on which all of the
securities registered hereunder have been sold or the registration statement of which this prospectus forms a part has been withdrawn).
Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future
filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is
incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace
such earlier statements.
We
will furnish without charge to each person, including any beneficial owner, to whom a prospectus is delivered, upon written or oral request,
a copy of any or all of the documents incorporated by reference into this prospectus but not delivered with the prospectus, including
exhibits that are specifically incorporated by reference into such documents. You should direct any requests for documents to:
Adial
Pharmaceuticals, Inc.
1180
Seminole Trail, Suite 495
Charlottesville,
VA 22901
Telephone
(434) 422-9800
Attention:
Corporate Secretary
You
may also access these documents, free of charge, on the SEC’s website at www.sec.gov or on our website at https://ir.adial.com/sec-filings.
The information contained in, or that can be accessed through, our website is not incorporated by reference in, and is not part of, this
prospectus or any accompanying prospectus supplement.
In
accordance with Rule 412 of the Securities Act, any statement contained in a document incorporated by reference herein shall be deemed
modified or superseded to the extent that a statement contained herein or in any other subsequently filed document which also is or is
deemed to be incorporated by reference herein modifies or supersedes such statement.
You
should rely only on information contained in, or incorporated by reference into, this prospectus and any prospectus supplement. We have
not authorized anyone to provide you with information different from that contained in this prospectus or incorporated by reference into
this prospectus. We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized
or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such an
offer or solicitation.
4,340,426
Shares of Common Stock

PROSPECTUS
,
2023
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution
The
following table indicates the expenses to be incurred in connection with the offering described in this registration statement, other
than placement agent fees, all of which will be paid by Adial Pharmaceuticals, Inc. (the “Registrant”). All amounts are estimates
except the SEC registration fee and the Financial Industry Authority, Inc. (“FINRA”) filing fee.
| Item | |
Amount | |
| SEC registration fee | |
$ | 1,439 | |
| Legal fees and expenses | |
| 175,000 | |
| Accounting fees and expenses | |
| 5,000 | |
| Miscellaneous fees and expenses | |
| 3,561 | |
| Total | |
$ | 185,000 | |
Item
14. Indemnification of Directors and Officers
The
Registrant is incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law provides that
a Delaware corporation may indemnify any persons who were, are, or are threatened to be made, parties to any threatened, pending or completed
action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such
corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation, or is or
was serving at the request of such corporation as an officer, director, employee or agent of another corporation or enterprise. The indemnity
may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred
by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or
she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or
proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons
who were, are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the
corporation by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or is or was
serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity
may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense
or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in
or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the
officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise
in the defense of any action referred to above, the corporation must indemnify him or her against the expenses (including attorneys’
fees) actually and reasonably incurred.
The
Registrant’s certificate of incorporation and amended and restated bylaws provide for the indemnification of its directors and
officers to the fullest extent permitted under the Delaware General Corporation Law.
Section
102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director
of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary
duties as a director, except for liability for any:
| ● | transaction
from which the director derives an improper personal benefit; |
| ● | act
or omission not in good faith or that involves intentional misconduct or a knowing violation
of law; |
| ● | unlawful
payment of dividends or redemption of shares; or |
| ● | breach
of a director’s duty of loyalty to the corporation or its stockholders. |
The
Registrant’s certificate of incorporation includes such a provision. Expenses incurred by any officer or director in defending
any such action, suit or proceeding in advance of its final disposition shall be paid by the Registrant upon delivery to it of an undertaking,
by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director
or officer is not entitled to be indemnified by the Registrant.
Section
174 of the Delaware General Corporation Law provides, among other things, that a director who willfully or negligently approves of an
unlawful payment of dividends or an unlawful stock purchase or redemption, may be held liable for such actions. A director who was either
absent when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions
to be entered in the books containing minutes of the meetings of the board of directors at the time such action occurred or immediately
after such absent director receives notice of the unlawful acts.
As
permitted by the Delaware General Corporation Law, the Registrant has entered into indemnity agreements with each of its directors and
executive officers, that require the Registrant to indemnify such persons against any and all costs and expenses (including attorneys’,
witness or other professional fees) actually and reasonably incurred by such persons in connection with any action, suit or proceeding
(including derivative actions), whether actual or threatened, to which any such person may be made a party by reason of the fact that
such person is or was a director or officer or is or was acting or serving as an officer, director, employee or agent of the Registrant
or any of its affiliated enterprises. Under these agreements, the Registrant is not required to provide indemnification for certain matters,
including:
| ● | indemnification
beyond that permitted by the Delaware General Corporation Law; |
| ● | indemnification
for any proceeding with respect to the unlawful payment of remuneration to the director or
officer; |
| ● | indemnification
for certain proceedings involving a final judgment that the director or officer is required
to disgorge profits from the purchase or sale of the Registrant’s stock; |
| ● | indemnification
for proceedings involving a final judgment that the director’s or officer’s conduct
was in bad faith, knowingly fraudulent or deliberately dishonest or constituted willful misconduct
or a breach of his or her duty of loyalty, but only to the extent of such specific determination; |
| ● | indemnification
for proceedings or claims brought by an officer or director against us or any of the Registrant’s
directors, officers, employees or agents, except for claims to establish a right of indemnification
or proceedings or claims approved by the Registrant’s board of directors or required
by law; |
| ● | indemnification
for settlements the director or officer enters into without the Registrant’s consent;
or |
| ● | indemnification
in violation of any undertaking required by the Securities Act or in any registration statement
filed by the Registrant. |
The
indemnification agreements also set forth certain procedures that will apply in the event of a claim for indemnification thereunder.
Except
as otherwise disclosed in our filings with the SEC, there is at present no pending litigation or proceeding involving any of the Registrant’s
directors or executive officers as to which indemnification is required or permitted, and the Registrant is not aware of any threatened
litigation or proceeding that may result in a claim for indemnification.
The
Registrant has an insurance policy in place that covers its officers and directors with respect to certain liabilities, including liabilities
arising under the Securities Act or otherwise.
Item
15. Recent Sales of Unregistered Securities
During
the last three years, we have issued unregistered securities to the persons described below. None of these transactions involved any
underwriters, underwriting discounts or commissions, or any public offering. We believe that each transaction was exempt from the registration
requirements of the Securities Act by virtue of Section 4(a)(2) thereof as a transaction not involving a public offering and/or Rule
506 of Regulation D promulgated under the Securities Act. The recipients both had access, through their relationship with us, to information
about us.
On
November 18, 2020, the Registrant entered into a Common Stock Purchase Agreement with Keystone Capital Partners, LLC for the issuance
of up to 113,687 shares of Common Stock including 7,000 commitment shares. The Registrant issued an aggregate of 72,836 shares to Keystone
Capital Partners, LLC pursuant to the terms of the Common Stock Purchase Agreement.
On
January 26, 2021, the Registrant issued an aggregate of 28,000 shares of Common Stock for the purchase of the equity of Purnovate, Inc.
On
March 11, 2021, the Registrant issued in a private placement to three investors the first tranche of an aggregate of 3,880 shares of
Common Stock for gross proceeds of $291,003.
On
June 1, 2021, the Registrant closed the second tranche of its private offering of Common Stock pursuant to which its issued to three
investors 24,120 shares of Common Stock for gross proceeds of $1,809,000.
On
July 6, 2021, the Registrant closed the first tranche of its private placement offering of Common Stock pursuant to which it issued to
three investors an aggregate of 66,666 shares of Common Stock for gross proceeds of $5,000,004.
On
August 3, 2021 and August 4, 2021, the Registrant closed the second tranche of its private offering of Common Stock pursuant to which
the Registrant issued to three investors an aggregate of 60,000 shares of Common Stock for gross proceeds $4,500,000.
On
November 9, 2021, the Registrant issued 800 shares of common stock to Bespoke Growth Partners, Inc. (“Bespoke”) in connection
with a private placement.
On
December 17, 2021, the Registrant, issued 7,200 shares of the Registrant’s common stock to Bespoke in connection with a private
placement.
On
February 10, 2022, the Registrant issued (i) 92,890 shares of Common Stock, (ii) pre-funded warrants to purchase up to 74,600 shares
of Common Stock with an exercise price of $0.025 per share and (iii) warrants with a term of five years and six months from the date
of issuance, to purchase an aggregate of up to 159,116 shares of Common Stock at an exercise price of $63.00 per share, subject to customary
adjustments thereunder. The Registrant issued the shares of Common Stock and pre-funded warrants in a registered direct offering and
the warrants in a concurrent private placement.
On
February 24, 2023, the Registrant issued to the Joseph Gunnar & Co., LLC a warrant to purchase up to an aggregate of 7,317 shares
of common stock.
On
May 31, 2023, the Registrant issued to Alumni Capital LP (“Alumni Capital”) 7,983 shares of Common Stock as commitment shares
pursuant to a purchase agreement with Alumni Capital. Since May 31, 2023, the Registrant has issued and sold 20,555 shares of Common
Stock to Alumni Capital under the purchase agreement.
October
2023 Private Placement
On
October 24, 2023, we issued and sold Pre-Funded Warrants to purchase 1,418,440 shares of Common Stock, Series A Common Warrants to purchase
1,418,440 shares of Common Stock, and Series B Common Warrants to purchase 1,418,440 shares of Common Stock to one institutional investor
for gross proceeds of approximately $4.0 million. The Series A Common Warrants are exercisable for five and one-half (5.5) years and
have an exercise price of $2.82. The Series B Common Warrants are exercisable for 18 months and have an exercise price of $2.82. The
Series A Common Warrants and Series B Common Warrants are exercisable at any time after the earlier of (i) if permissible by the applicable
rules and regulations of the Nasdaq Stock Market (the “Nasdaq”), payment by the holder of $0.125 per Common Warrant Share
and (ii) the date of which approval as may be required by the applicable rules and regulations of Nasdaq (or any successor entity)
from our stockholders with respect to issuance of all of the Common Warrants and the Common Warrant Shares upon the exercise thereof.
The Pre-Funded Warrants have an exercise price of $0.001 and do not expire until exercised in full.
In
connection with the Private Placement, we issued warrants (the “Placement Agent Warrants”) to purchase an aggregate of 85,106
shares of Common Stock to the placement agent, which is equal to 6.0% of the shares of Common Stock sold in the Private Placement. The
Placement Agent Warrants are immediately exercisable for five and one-half (5.5) years and have an exercise price of $3.525.
We
did not pay or give, directly or indirectly, any commission or other remuneration, including underwriting discounts or commissions, in
connection with any of the issuances of securities listed above. The recipients of the securities in each of these transactions represented
their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution
thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate access,
through their employment or other relationship with us or through other access to information provided by us, to information about us.
The sales of these securities were made without any general solicitation or advertising.
Item
16. Exhibits and financial statement schedules.
| (a) | The
exhibits to the registration statement are set forth within the Exhibit Index immediately preceding the signature page hereto. |
| (b) | No
financial statement schedules are provided because the information called for is not required or is shown either in the financial statements
or notes. |
Item
17. Undertakings.
The
undersigned Registrant hereby undertakes:
To
file, during any period in which offers or sales are being made, a post-effective amendment to this Registration Statement:
(i)
to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii)
to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered and any deviation from the low or
high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule
424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering
price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii)
to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement;
provided,
however, that paragraphs (i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment
by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section
15(d) of the Exchange Act that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed
pursuant to Rule 424(b) that is part of the registration statement.
That,
for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new
registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to
be the initial bona fide offering thereof.
To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination
of the offering.
That,
for the purpose of determining liability under the Securities Act to any purchaser:
(A)
Each prospectus filed by a Registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date
the filed prospectus was deemed part of and included in the registration statement; and
(B)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on
Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required
by Section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of
the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering
described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter,
such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement
to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide
offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the
registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus
that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede
or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made
in any such document immediately prior to such effective date.
That,
for purposes of determining any liability under the Securities Act, each filing of Registrant’s annual report pursuant to Section
13(a) or 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to
Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of
the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such
indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim
for indemnification against such liabilities (other than the payment by a Registrant of expenses incurred or paid by a director, officer
or controlling person of a Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer
or controlling person in connection with the securities being registered, that Registrant will, unless in the opinion of its counsel
the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
EXHIBIT
INDEX
| Exhibit Number |
|
Description of Exhibit |
| 2.1* |
|
Option Agreement for the Acquisition of Purnovate, Inc. by Adenomed, LLC dated as of January 27, 2023 (Incorporated by reference to Exhibit 2.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 1, 2023) |
| 2.2 |
|
Option Exercise Agreement, dated May 8, 2023, by and between Adovate LLC and Adial Pharmaceuticals, Inc. (Incorporated by reference Exhibit 2.2 to the Current Report on Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on May 10, 2023) |
| 2.3 |
|
Final Acquisition Agreement, dated September 18, 2023, by and between Adovate LLC and Adial Pharmaceuticals, Inc. (Incorporated by reference Exhibit 2.3 to the Current Report on Form 8-K as filed by the Registrant with the Securities and Exchange Commission on September 21, 2023 (File No. 001-38323) |
| 3.1 |
|
Certificate of Incorporation of Adial Pharmaceuticals, Inc. (Incorporated by reference to Exhibit 3.3 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 3.2 |
|
Amended and Restated Bylaws of Adial Pharmaceuticals, Inc., dated February 22, 2022 (Incorporated by reference to Exhibit 3.3 to the Registrant’s Annual Report on Form 10-K (File No. 001-38323), filed with the Securities and Exchange Commission on March 28, 2022) |
| 3.3 |
|
Certificate of Amendment to Certificate of Incorporation of Adial Pharmaceuticals, Inc. (Incorporated by reference to Exhibit 3.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on August 4, 2023) |
| 4.1 |
|
Specimen Common Stock Certificate (Incorporated by reference to Exhibit 4.1 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on October 25, 2017) |
| 4.2+ |
|
Option Agreement between ADial Pharmaceuticals, L.L.C and Tony Goodman, effective July 1, 2017 (Incorporated by reference to Exhibit 4.9 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 4.3+ |
|
Grant Incentive Plan (Incorporated by reference to Exhibit 4.10 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on April 16, 2018) |
| 4.4+ |
|
Form of Adial Pharmaceuticals, Inc. 2017 Equity Incentive Plan (Incorporated by reference to Exhibit 4.11 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 4.5+ |
|
Form of Stock Option Grant Notice, Option Agreement (Incentive Stock Option or Nonstatutory Stock Option) and Notice of Exercise under the 2017 Equity Incentive Plan (Incorporated by reference to Exhibit 4.12 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 4.6 |
|
Description of Securities (Incorporated by reference to Exhibit 4.19 to the Registrant’s Annual Report on Form 10-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 22, 2021) |
| 4.7 |
|
Form of Common Stock Purchase Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on June 12, 2020) |
| 4.8 |
|
Form of Pre-Funded Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 14, 2022) |
| 4.9 |
|
Form of Common Stock Purchase Warrant (Incorporated by reference to Exhibit 4.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 14, 2022) |
| 4.10 |
|
Form of Placement Agent Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 24, 2023) |
| 4.11 |
|
Form of Pre-Funded Warrant (Incorporated by reference to Exhibit 4.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 4.12 |
|
Form of Series A Common Stock Purchase Warrant (Incorporated by reference to Exhibit 4.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 4.13 |
|
Form of Series B Common Stock Purchase Warrant (Incorporated by reference to Exhibit 4.3 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 4.14 |
|
Form of Placement Agent Warrant (Incorporated by reference to Exhibit 4.4 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 5.1# |
|
Opinion of Blank Rome LLP |
| 10.1 |
|
License Agreement between the University of Virginia Patent Foundation and ADial Pharmaceuticals, L.L.C. effective January 21, 2011 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.2 |
|
Amendment #1 to License Agreement between University of Virginia Patent Foundation and ADial Pharmaceuticals, L.L.C effective October 21, 2013 (Incorporated by reference to Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.3 |
|
Amendment #2 to License Agreement between University of Virginia Patent Foundation and ADial Pharmaceuticals, L.L.C effective May 18, 2016 (Incorporated by reference to Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.4 |
|
Amendment #3 to License Agreement between University of Virginia Patent Foundation and ADial Pharmaceuticals, L.L.C effective March 27, 2017 (Incorporated by reference to Exhibit 10.4 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.5+ |
|
Form of Employment Agreement between the Registrant and William B. Stilley, III (Incorporated by reference to Exhibit 10.15 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.6+ |
|
Form of Employment Agreement between the Registrant and Joseph Truluck (Incorporated by reference to Exhibit 10.16 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.7 |
|
Form of Indemnification Agreement (Incorporated by reference to Exhibit 10.18 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.8 |
|
Amendment #4 to License Agreement between University of Virginia Patent Foundation and ADial Pharmaceuticals, L.L.C effective August 15, 2017 (Incorporated by reference to Exhibit 10.20 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on September 7, 2017) |
| 10.9 |
|
Amendment #5 to License Agreement between University of Virginia Patent Foundation and Adial Pharmaceuticals, Inc., dated as of December 14, 2017 (Incorporated by reference to Exhibit 10.23 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on April 16, 2018) |
| 10.10 |
|
Security Agreement dated June 6, 2018 (Incorporated by reference to Exhibit 10.31 to the Registrant’s Registration Statement on Form S-1, File No. 333-220368, filed with the Securities and Exchange Commission on June 11, 2018) |
| 10.11 |
|
Amendment No. 6 to License Agreement between the Registrant, University of Virginia Patent Foundation d/b/a the University of Virginia Licensing and Ventures Group dated as of December 18, 2018 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 19, 2018) |
| 10.12+ |
|
Amendment to Employment Agreement between Adial Pharmaceuticals, Inc. and William B. Stilley, III, dated as of March 11, 2019 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 14, 2019) |
| 10.13+ |
|
Amendment to Employment Agreement between Adial Pharmaceuticals, Inc. and Joseph Truluck, dated as of March 11, 2019 (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 14, 2019) |
| 10.14+ |
|
Consulting Agreement between Adial Pharmaceuticals, Inc. and Dr. Bankole Johnson, dated March 24, 2019 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 26, 2019) |
| 10.15 |
|
Master Services Agreement and related statement of work, dated July 5, 2019, by and between Adial Pharmaceuticals, Inc. and Psychological Education Publishing Company (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on July 8, 2019) |
| 10.16 |
|
Amendment No. 1 to the Adial Pharmaceuticals, Inc. 2017 Equity Incentive Stock Plan (Incorporated by reference to Exhibit 4.2 to the Registrant’s Form S-8, File No. 333-226884, filed with the Securities and Exchange Commission on September 13, 2019) |
| 10.17+ |
|
Form of Stock Option Grant Notice, Option Agreement (Incentive Stock Option or Nonstatutory Stock Option) and Notice of Exercise under the 2017 Equity Incentive Plan (Incorporated by reference to Exhibit 4.3 to the Registrant’s Form S-8, File No. 333-226884, filed with the Securities and Exchange Commission on September 13, 2019) |
| 10.18 |
|
Amendment to Statement of Work under Master Services Agreement dated December 12, 2019, by and between Adial Pharmaceuticals, Inc. and Psychological Education Publishing Company (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 16, 2019) |
| 10.19 |
|
Guaranty, dated December 12, 2019, executed by Dr. Bankole Johnson (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 16, 2019) |
| 10.20 |
|
Pledge and Security Agreement, dated December 12, 2019 (Incorporated by reference to Exhibit 10.3 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 16, 2019) |
| 10.21 |
|
Lock-Up Agreement, dated December 12, 2019 between Adial Pharmaceuticals, Inc., Bankole A. Johnson and certain entities controlled by Bankole A. Johnson (Incorporated by reference to Exhibit 10.4 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 16, 2019) |
| 10.22 |
|
Amendment No. 7 to License Agreement by and between the University of Virginia Patent Foundation d/b/a the University of Virginia Licensing and Ventures Group and Adial Pharmaceuticals, Inc. (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 31, 2019) |
| 10.23+ |
|
Amendment to Employment Agreement between Adial Pharmaceuticals, Inc. and Joseph Truluck, dated as of March 3, 2020 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 6, 2020) |
| 10.24 |
|
Lock-Up Agreement Extension and Right of First Refusal dated August 19, 2020 to Lock-Up Agreement (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on August 25, 2020) |
| 10.25+ |
|
Amendment No. 2 to the Adial Pharmaceuticals, Inc. 2017 Equity Incentive Plan (Incorporated by reference to Appendix A to the Registrant’s Definitive Proxy Statement on Schedule 14A, File No. 001-38323, filed with the Securities and Exchange Commission on July 21, 2020) |
| 10.26 |
|
Common Stock Purchase Agreement, dated as of November 18, 2020, by and between Adial Pharmaceuticals, Inc. and Keystone Capital Partners, LLC (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on November 24, 2020) |
| 10.27 |
|
Registration Rights Agreement, dated as of November 18, 2020, by and between Adial Pharmaceuticals, Inc. and Keystone Capital Partners, LLC (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on November 24, 2020) |
| 10.28 |
|
Equity Purchase Agreement, dated December 7, 2020, by and among Adial Pharmaceuticals, Inc., Purnovate, LLC, the members of Purnovate, LLC and Robert D. Thompson, as member representative (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 10, 2020) |
| 10.29+ |
|
Offer Letter, dated December 14, 2020 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 17, 2020) |
| 10.30 |
|
Amendment, dated January 25, 2021, by and among Adial Pharmaceuticals, Inc., Purnovate, Inc., a wholly owned subsidiary of Adial, PNV Conversion Corp. as successor-in-interest to Purnovate, LLC, and Robert D. Thompson, as member representative, to the Equity Purchase Agreement, dated December 7, 2020. (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 1, 2021) |
| 10.31 |
|
Form of Securities Purchase Agreement (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 15, 2021) |
| 10.32 |
|
Form of Registration Rights Agreement (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 15, 2021) |
| 10.33+ |
|
Amendment to Executive Employment Agreement with William B. Stilley, III, effective as of February 12, 2021 (Incorporated by reference to Exhibit 10.37 to the Registrant’s Annual Report on Form 10-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 22, 2021) |
| 10.34+ |
|
Amendment to Executive Employment Agreement with Joseph Truluck, effective as of February 12, 2021 (Incorporated by reference to Exhibit 10.38 to the Registrant’s Annual Report on Form 10-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 22, 2021) |
| 10.35+ |
|
Amendment to Executive Employment Agreement with William B. Stilley, III, effective as of March 17, 2021 (Incorporated by reference to Exhibit 10.39 to the Registrant’s Annual Report on Form 10-K, File No. 001-38323, filed with the Securities and Exchange Commission on March 22, 2021) |
| 10.36 |
|
Lockup Agreement Extension executed Dr. Bankole Johnson, dated April 5, 2021. (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on April 9, 2021) |
| 10.37 |
|
Form of Stock Purchase Agreement (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on July 9, 2021) |
| 10.38 |
|
Form of Registration Rights Agreement (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on July 9, 2021) |
| 10.39+ |
|
Amendment No. 3 to the Adial Pharmaceuticals, Inc. 2017 Equity Incentive Plan (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on September 29, 2021) |
| 10.40 |
|
Form of Stock Purchase Agreement (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on November 12, 2021) |
| 10.41 |
|
Form of Registration Rights Agreement (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on November 12, 2021) |
| 10.42+ |
|
Employment Agreement between Adial Pharmaceuticals, Inc. and Cary Claiborne, dated as of December 7, 2021 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on December 9, 2021) |
| 10.43 |
|
Form of Securities Purchase Agreement (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 14, 2022) |
| 10.44 |
|
Placement Agency Agreement (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 14, 2022) |
| 10.45+ |
|
Amendment, dated March 22, 2022, to Consulting Agreement between Adial Pharmaceuticals, Inc. and Dr. Bankole Johnson, dated March 24, 2019 (Incorporated by reference to Exhibit 10.45 to the Registrant’s Annual Report on Form 10-K (File No. 001-38323), filed with the Securities and Exchange Commission on March 28, 2022) |
| 10.46 |
|
Amendment to Employment Agreement, dated as of August 22, 2022, between Adial Pharmaceuticals, Inc. and Cary J. Claiborne (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on August 23, 2022) |
| 10.47 |
|
Amendment to Employment Agreement, dated as of August 22, 2022, between Adial Pharmaceuticals, Inc. and William B. Stilley (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on August 23, 2022) |
| 10.48* |
|
Amendment, dated September 8, 2022, to Consulting Agreement between Adial Pharmaceuticals, Inc. and Dr. Bankole A. Johnson, dated March 24, 2019, as amended on March 22, 2022 (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on September 13, 2022) |
| 10.49 |
|
Amendment No. 4 to the Adial Pharmaceuticals, Inc. 2017 Equity Incentive Plan (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 13, 2022) |
| 10.50 |
|
Amendment 6 to Employment Agreement effective as of the January 27, 2023 by and between Adial Pharmaceuticals, Inc. and William B. Stilley, III (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on February 1, 2023) |
| 10.51 |
|
Purchase Agreement, dated as of May 31, 2023, by and between Adial Pharmaceuticals, Inc. and Alumni Capital LP (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on June 2, 2023) |
| 10.52 |
|
Form of Securities Purchase Agreement, dated as of October 19, 2023, by and among Adial Pharmaceuticals, Inc. and the purchaser signatory thereto (Incorporated by reference to Exhibit 10.1 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 10.53 |
|
Form of Registration Rights Agreement, dated as of October 19, 2023, by and among Adial Pharmaceuticals, Inc. and the purchaser signatory thereto (Incorporated by reference to Exhibit 10.2 to the Registrant’s Form 8-K, File No. 001-38323, filed with the Securities and Exchange Commission on October 24, 2023) |
| 23.1# |
|
Consent of Marcum LLP |
| 23.2# |
|
Consent of Blank Rome LLP (See Exhibit 5.1 above) |
| 24.1# |
|
Power of Attorney (included on signature page of this Registration Statement) |
| 107# |
|
Filing Fee Table |
| # |
Filed
herewith |
| + |
Management
contract or compensatory plan or arrangement required to be identified pursuant to Item 15(a)(3) of this report. |
| * |
Certain
portions of this Exhibit have been redacted pursuant to Item 601(b)(10) of Regulation S-K. The redacted information has been marked
by brackets as [***]. The Registrant agrees to furnish supplementally an unredacted copy of this Exhibit to the SEC upon request. |
SIGNATURES
Pursuant
to the requirements of the Securities Act, the Registrant has duly caused this Registration Statement to be signed on its behalf by the
undersigned, thereunto duly authorized, in the City of Charlottesville, State of Virginia, on November 8, 2023.
| |
ADIAL
PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/
Cary J. Claiborne |
| |
Name: |
Cary
J. Claiborne |
| |
Title: |
President
and Chief Executive Officer |
POWER
OF ATTORNEY
KNOW
ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Cary J. Claiborne and Joseph
Truluck, and each and either of them, his true and lawful agent, proxy and attorney-in-fact, with full power of substitution
and resubstitution, for him and in his name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities
and Exchange Commission any and all amendments (including post-effective amendments) to this registration statement together with all
schedules and exhibits thereto and any subsequent registration statement filed pursuant to Rule 462(b) under the Securities Act, together
with all schedules and exhibits thereto, (ii) act on, sign and file such certificates, instruments, agreements and other documents
as may be necessary or appropriate in connection therewith, (iii) act on and file any supplement to any prospectus included in this
registration statement or any such amendment or any subsequent registration statement filed pursuant to Rule 462(b) under the Securities
Act and (iv) take any and all actions which may be necessary or appropriate to be done, as fully for all intents and purposes as
he might or could do in person, hereby approving, ratifying and confirming all that such agent, proxy and attorney-in-fact or
any of his substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant
to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Cary J. Claiborne |
|
Chief
Executive Officer and President |
|
|
| Cary
J. Claiborne |
|
(Principal
Executive Officer) and Member of the Board of Directors |
|
November
8, 2023 |
| |
|
|
|
|
| /s/
Joseph M. Truluck |
|
Chief
Operating Officer and Chief Financial Officer |
|
|
| Joseph
M. Truluck |
|
(Principal
Financial Officer and Principal Accounting Officer) |
|
November
8, 2023 |
| |
|
|
|
|
| /s/
J. Kermit Anderson |
|
Member
of the Board of Directors |
|
November
8, 2023 |
| J.
Kermit Anderson |
|
|
|
|
| |
|
|
|
|
| /s/
Roberson H. Gilliland |
|
Member
of the Board of Directors |
|
November
8, 2023 |
| Robertson
H. Gilliland |
|
|
|
|
| |
|
|
|
|
| /s/
Tony Goodman |
|
Member
of the Board of Directors |
|
November
8, 2023 |
| Tony
Goodman |
|
|
|
|
| |
|
|
|
|
| /s/
James W. Newman |
|
Member
of the Board of Directors |
|
November
8, 2023 |
| James
W. Newman, Jr. |
|
|
|
|
| |
|
|
|
|
| /s/
Kevin Schuyler |
|
Chairman
of the Board of Directors |
|
November
8, 2023 |
| Kevin
Schuyler, CFA |
|
|
|
|
| |
|
|
|
|
II-9
Exhibit 5.1

1271 Avenue of the Americas | New York, NY 10020
blankrome.com
November 8,
2023
The Board of Directors
Adial Pharmaceuticals, Inc.
1180 Seminole Trail, Suite 495
Charlottesville, Virginia 22901
Ladies and Gentlemen:
This opinion is furnished
to you in connection with a Registration Statement on Form S-1 (the “Registration Statement”) filed with the
Securities and Exchange Commission (the “Commission”) under the Securities Act of 1933, as amended (the “Securities
Act”), for the registration of the resale of an aggregate of 4,340,426 shares (the “Securities”)
of common stock, par value $0.001 per share (the “Common Stock”), of Adial Pharmaceuticals, Inc., a Delaware
corporation (the “Company”). All of the Securities are being registered on behalf of certain stockholders of
the Company (each a “Selling Stockholder” and collectively, the “Selling Stockholders”).
The Securities consist of (i) 1,418,440 shares of Common Stock issuable upon the exercise of outstanding pre-funded warrants (the “Pre-Funded
Warrants”) to purchase shares of Common Stock purchased by the Selling Stockholders in the Private Placement (the “Pre-Funded
Warrant Shares”); (ii) 1,418,440 shares of Common Stock (the “Series A Common Warrant Shares”)
issuable upon the exercise of outstanding Series A common warrants (the “Series A Common Warrants”) to purchase
shares of Common Stock purchased by the Selling Stockholders in the Private Placement; (iii) 1,418,440 shares of Common Stock (the “
Series B Common Warrant Shares” and together with the Series A Common Warrant Shares, the “Common Warrant Shares”)
issuable upon the exercise of outstanding Series B common warrants (the “Series B Common Warrants” and together
with the Series B Common Warrants, the “Common Warrants) to purchase shares of Common Stock purchased by the Selling
Stockholders in the Private Placement; and (iv) 85,106 shares of Common Stock issuable upon the exercise of outstanding warrants issued
to designees of H.C. Wainwright & Co., LLC (“H.C.W.”) in the Private Placement as partial compensation for
acting as placement agent (the “Placement Agent Warrant Shares”) in the Private Placement.
As counsel to the Company,
we have examined the Registration Statement, the Pre-Funded Warrants, the Common Warrants and the Placement Agent Warrants and the originals
or copies, certified or otherwise identified to our satisfaction, of such other documents, corporate records, certificates of public officials
and other instruments as we have deemed necessary for the purposes of rendering this opinion and we are familiar with the proceedings
taken and proposed to be taken by the Company in connection with the filing of the Registration Statement as it relates to the Pre-Funded
Warrant Shares, the Common Warrant Shares and the Placement Agent Warrant Shares. In our examination, we have assumed the genuineness
of all signatures, the authenticity of all documents submitted to us as originals and the conformity with the originals of all documents
submitted to us as copies.
We have assumed that, at
or prior to the time of the delivery of any of the shares of Common Stock, there will not have occurred any change in the law or the facts
affecting the validity of the shares of Common Stock.
Based upon and subject to
the foregoing, we are of the opinion that:
| 1. | The Pre-Funded Warrant Shares have been duly authorized for
issuance and, when issued, delivered and paid for in accordance with the terms of the Pre-Funded Warrants, including the payment of the
exercise price therefor, and the Registration Statement will be validly issued, fully paid and nonassessable. |
| 2. | The Common Warrant Shares have been duly authorized for issuance and, when issued, delivered and paid
for in accordance with the terms of the Common Warrants, including the payment of the exercise price therefor, and the Registration Statement
will be validly issued, fully paid and nonassessable. |
| 3. | The Placement Agent Warrant Shares have been duly authorized for issuance and, when issued, delivered
and paid for in accordance with the terms of the Placement Agent Warrants, including the payment of the exercise price therefor, and the
Registration Statement will be validly issued, fully paid and nonassessable. |
We express no opinion as
to matters governed by any laws other than the General Corporation Law of the State of Delaware (including all related provisions of the
Delaware Constitution and all reported judicial decisions interpreting the General Corporation Law of the State of Delaware and the Delaware
Constitution) and the federal laws of the United States of America, as in effect on the date hereof.

Board of Directors
Adial Pharmaceuticals, Inc.
November 8, 2023
Page 2
We hereby consent to the
filing of this opinion as Exhibit 5.1 to the Registration Statement and to the reference to our firm under the caption “Legal Matters”
in the Registration Statement. In giving our consent, we do not thereby admit that we are in the category of persons whose consent is
required under Section 7 of the Securities Act or the rules and regulations of the Commission thereunder.
| |
Very truly yours, |
| |
|
| |
/s/ Blank Rome LLP |
| |
BLANK ROME LLP |
Exhibit 23.1
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM’S CONSENT
We consent to the incorporation by reference in this Registration Statement
of Adial Pharmaceuticals, Inc. on Form S-1 of our report dated September 26, 2023, which includes an explanatory paragraph as to the Company’s
ability to continue as a going concern, with respect to our audits of the consolidated financial statements of Adial Pharmaceuticals,
Inc. as of and for the years ended December 31, 2022 and 2021 appearing in the Current Report on Form 8-K of Adial Pharmaceuticals, Inc.
filed with the Securities and Exchange Commission on September 27, 2023. We also consent to the reference to our firm under the heading
“Experts” in the Prospectus, which is part of this Registration Statement.
/s/ Marcum LLP
Marcum LLP
Marlton, New Jersey
November 8, 2023
Exhibit 107
Calculation of Filing
Fee Tables
Form S-1
(Form Type)
Adial Pharmaceuticals,
Inc.
(Exact Name of Registrant
as Specified in its Charter)
Table1 – Newly Registered Securities
Security
Type | |
Security Class Title | |
Fee
Calculation
Rule | |
Amount
Registered(1) | | |
Proposed
Maximum
Offering
Price per
Unit(2) | | |
Maximum
Aggregate
Offering
Price | | |
Fee Rate | | |
Amount of
Registration
Fee | |
| | |
| |
| |
| | |
| | |
| | |
| | |
| |
| Equity | |
Common
stock, par value $0.001 per share | |
Rule 457(c)(2) | |
| 4,340,426 | | |
$ | 2.2458 | | |
$ | 9,747,729 | | |
$147.60 per $1,000,000 | | |
$ | 1,439 | |
| Total Offering Amounts | |
| |
| 4,340,426 | | |
| | | |
$ | 9,747,729 | | |
$147.60 per $1,000,000 | | |
$ | 1,439 | |
| Total Fee Offsets(3) | |
| |
| | | |
| | | |
| | | |
| | |
| — | |
| Net Fee Due | |
| |
| | | |
| | | |
| | | |
| | |
$ | 1,439 | |
|
(1)
|
Pursuant to Rule 416(a)
under the Securities Act of 1933, as amended (the “Securities Act”), this registration statement also covers any additional
securities of Adial Pharmaceuticals, Inc. (the “Registrant”) that may be offered or issued in connection with any stock split,
stock dividend or similar transaction. |
| (2) |
Calculated pursuant to Rule 457(c) of the Securities
Act solely for purposes of calculating the registration fee. The price for these shares is based upon the average of the high and low
sale prices of the Registrant’s common stock, par value $0.001 per share, reported on the Nasdaq Capital Market on November 3,
2023.
|
| (3) |
The Registrant does not have any fee offsets to claim. |
Adial Pharmaceuticals (NASDAQ:ADIL)
Historical Stock Chart
From Oct 2024 to Nov 2024
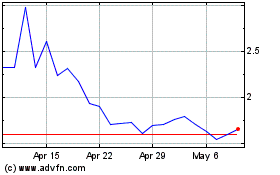
Adial Pharmaceuticals (NASDAQ:ADIL)
Historical Stock Chart
From Nov 2023 to Nov 2024