Seqirus Announces Major Advances in Pandemic Preparedness
August 07 2017 - 4:03PM
Business Wire
- Accelerated development of cell-based
technology quadruples influenza vaccine output in just two years,
strengthening U.S. preparedness against pandemic threats.
- Announcement made on the 10th
anniversary of pandemic partnership with the U.S. Government,
marked by a visit from Health and Human Services Secretary, Dr. Tom
Price.
- Seqirus is leveraging its global
manufacturing network to supply pandemic stockpiles and other
preparedness services to governments around the world.
Seqirus, a global leader in the prevention of influenza,
announced today that the accelerated development of cell-based
manufacturing technology at its state-of-the-art manufacturing
facility in Holly Springs, North Carolina, has delivered a
four-fold increase in seasonal influenza vaccine output in just two
years, strengthening the United States’ capacity to respond to
pandemic threats.
This Smart News Release features multimedia.
View the full release here:
http://www.businesswire.com/news/home/20170807005950/en/
Seqirus marked the 10th anniversary of
their pandemic partnership with the U.S. Government by hosting
Health and Human Services Secretary Dr. Tom Price at the Holly
Springs, N.C. production site. From left to right: Dr. Rick Bright,
Director, Biomedical Advanced Research and Development Authority
(BARDA); Dr. John Anderson, Vice President, Operations, Seqirus;
Dr. Tom Price, Secretary, U.S. Department of Health and Human
Services; Gordon Naylor, President, Seqirus. (Photo: Seqirus)
The announcement was made on the 10th anniversary of a pandemic
partnership with the U.S. Government, which was marked by a visit
to the Holly Springs site by Health and Human Services Secretary,
Dr. Tom Price. The partnership was established in 2007 in response
to the emergence of the H5N1 avian influenza virus as a serious
pandemic threat.
The Holly Springs facility was completed in 2012 and promptly
designated as critical national infrastructure for pandemic
preparedness in the U.S. It harnesses promising cell-based
technology as a modern, efficient and highly scalable alternative
to traditional egg-based manufacturing that also offers the
potential to avoid possible mismatch scenarios in influenza
seasons. Additionally, the facility produces the Seqirus
proprietary adjuvant, MF59, which can have a dose-sparing effect,
thereby further boosting the output of influenza vaccine during a
pandemic emergency.
Seqirus acquired the Holly Springs facility in 2015 and has
successfully accelerated development of the technology, quadrupling
seasonal influenza vaccine output from 5 million trivalent
(three-strain) doses to around 20 million quadrivalent
(four-strain) doses in just two years.
At this capacity, Seqirus is able to produce 150 million doses
of adjuvanted pandemic vaccine within the first six months of a
pandemic declaration, and is also well advanced in delivering surge
capacity programs to increase output to 200 million pandemic
doses.
“With a global influenza pandemic remaining a real and constant
threat, Seqirus is committed to continuing to work with the U.S.
Government to realize the full potential of our cell-based
manufacturing technology at the Holly Springs site,” said Gordon
Naylor, President of Seqirus.
“We’re proud of the advances we’ve made in a relatively short
period of time, and are confident in our technical ability to
deliver significant additional influenza vaccine capacity that can
be rapidly utilized during a pandemic emergency.”
During his visit to the Holly Springs site, Dr. Price met with
Seqirus executives, spoke with scientists and engineers and toured
production areas. Following an address to Seqirus staff, the
Secretary said, “The Holly Springs facility plays a critical role
in our national pandemic strategy and is a real asset in U.S.
biosecurity. We continue to appreciate and are reassured by the
progress being made by companies like Seqirus that contribute to
the nation’s preparedness capabilities.”
Seqirus was established in 2015 when parent company, CSL
Limited, acquired the influenza vaccine business of Novartis. It
has a rich heritage in influenza and operates major manufacturing
facilities in the U.S., U.K. and Australia. Today, Seqirus is the
world’s second largest influenza company and a global leader in
pandemic preparedness and response.
“With the emergence of H7N9 and other avian influenza viruses
with pandemic potential, we are seeing renewed focus on pandemic
preparedness by governments around the world,” said Mr. Naylor. “As
a result, we are leveraging our global manufacturing network to
supply pandemic stockpiles and other preparedness services to
governments in multiple countries.”
Seqirus also recently committed to the World Health Organization
in supplying 10% of its influenza vaccine output in real time for
use in developing countries in the event of a pandemic.
About Seqirus
Seqirus is part of CSL Limited (ASX:CSL),
headquartered in Melbourne, Australia. The CSL Group of companies
employs more than 20,000 people and has operations in more than 60
countries.
Seqirus was established on 31 July 2015 following CSL’s
acquisition of the Novartis influenza vaccines business and its
subsequent integration with bioCSL. As the second largest influenza
vaccine provider in the world, Seqirus is a major contributor to
the prevention of influenza globally and a transcontinental partner
in pandemic preparedness.
Seqirus operates state-of-the-art production facilities in the
U.S., the U.K. and Australia, and manufactures influenza vaccines
using both egg-based and cell-based technologies. It has leading
R&D capabilities, a broad portfolio of differentiated products
and a commercial presence in more than 20 countries.
For more information, visit www.seqirus-US.com
View source
version on businesswire.com: http://www.businesswire.com/news/home/20170807005950/en/
PadillaDani Jurisz, +1
612-455-1726Dani.Jurisz@PadillaCo.comorSeqirusDave Minella,
+1 919-802-7641David.Minella@seqirus.com
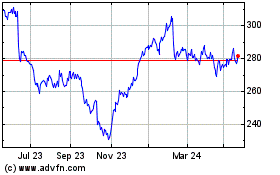
CSL (ASX:CSL)
Historical Stock Chart
From Nov 2024 to Dec 2024
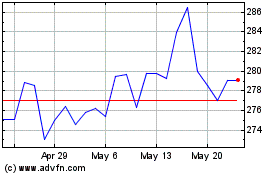
CSL (ASX:CSL)
Historical Stock Chart
From Dec 2023 to Dec 2024