UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of
the
Securities Exchange Act of 1934
(Amendment No. _)
| Filed by the Registrant |
☒ |
| Filed by a Party other than the Registrant |
☐ |
Check the appropriate box:
| ☒ |
Preliminary Proxy Statement |
| ☐ |
Confidential, for Use of the Commission Only (as Permitted by Rule 14a-6(e)(2)) |
| ☐ |
Definitive Proxy Statement |
| ☐ |
Definitive Additional Materials |
| ☐ |
Soliciting Material Pursuant to §240.14a-12 |
ONCONETIX, INC.
(Name of Registrant as Specified in Its Charter)
(Name of Person(s) Filing Proxy Statement,
if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| ☒ |
No fee required. |
| ☐ |
Fee paid previously with preliminary materials. |
| ☐ |
Fee computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11. |
PRELIMINARY COPY
Onconetix, Inc.
201 E. Fifth Street, Suite 1900
Cincinnati, OH 45202
To the Stockholders of Onconetix, Inc.:
You are cordially invited to attend the special
meeting (the “Special Meeting”) of Onconetix, Inc. (“Onconetix” or the “Company”) to be held virtually
via live webcast on [●], 2024, beginning at [●] A.M., Eastern Time.
| |
1. |
To approve and adopt an amendment to the Onconetix Amended and Restated Certificate of Incorporation (the “Charter”), in the form appended to the accompanying proxy statement as Annex A (the “Reverse Stock Split Amendment”), to effect a reverse stock split of all of the outstanding shares of the Company’s common stock, par value $0.00001 per share (“Common Stock”), at a ratio in the range of 1-for-30 to 1-for-60, with such ratio to be determined by the Onconetix Board (the “Board”) (the “Reverse Stock Split Proposal”); |
| |
2. |
To approve, in accordance with Nasdaq Listing Rule 5635, the issuance of up to 5,709,935 shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series A Preferred Stock, par value $0.00001 per share (“Series A Preferred Stock”) (the “Series A Conversion Proposal”); |
| 3. | To approve, in accordance with Nasdaq Listing Rule 5635, the issuance
of: (i) 269,672,900 shares of Common Stock to be issued upon conversion of the Company’s Series B Preferred Stock, par value $0.00001
per share (“Series B Preferred Stock”) and (ii) such number of shares of Common Stock to be issued by the Company in a $5
million private placement financing of units (the “PMX Financing”), which shall initially include 20,000,000 shares of Common
Stock and up to 6,000,000 shares of Common Stock underlying warrants included in the units, subject to adjustment, plus such additional
number of shares of Common Stock to be issuable upon the satisfaction of certain price protection conditions, as described further herein
(the “PMX Issuance Proposal”); |
| 4. | To ratify the appointment by the Board of EisnerAmper LLP (“EisnerAmper”) as the Company’s
independent registered public accounting firm for the fiscal year ending December 31, 2024 (the “Auditor Ratification Proposal”);
and |
| 5. | To approve the adjournment of the Special Meeting, if necessary or appropriate, to solicit additional
proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock
Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal (the “Adjournment
Proposal”). |
The Board has fixed the close of business on [●],
2024 as the record date (the “Record Date”) for the Special Meeting and only stockholders who held Common Stock of Onconetix
as of the Record Date will be entitled to vote at the Special Meeting and at any adjournments and postponements thereof.
The Onconetix Board has unanimously determined and resolved that the Reverse Stock Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal, the Auditor
Ratification Proposal and the Adjournment Proposal are advisable and fair to, and in the best interests of, Onconetix and its stockholders,
and has approved the Reverse Stock Split Amendment, subject to stockholder approval. Accordingly, the Onconetix
Board unanimously recommends that Onconetix stockholders vote “FOR” each of the foregoing proposals.
Your vote is important. More information
about Onconetix and the Special Meeting is contained in the accompanying proxy statement. You are encouraged to read the accompanying
proxy statement in its entirety.
| |
Very truly yours, |
| |
|
| |
/s/ Dr. Ralph Schiess |
| |
Dr. Ralph Schiess |
| |
Interim Chief Executive Officer |
The accompanying proxy statement is dated [●], 2024 and is first
being mailed to the stockholders of Onconetix on or about [●], 2024.
Onconetix, Inc.
201 E. Fifth Street, Suite 1900
Cincinnati, OH 45202
NOTICE OF SPECIAL MEETING
OF STOCKHOLDERS
TO BE HELD ON [●], 2024
TO THE STOCKHOLDERS OF Onconetix,
Inc.:
NOTICE IS HEREBY GIVEN that a special meeting
of stockholders (the “Special Meeting”) of Onconetix, Inc. (“Onconetix” or the “Company”), a Delaware
corporation, will be held virtually via live webcast on [●], 2024, beginning at [●] a.m., Eastern Time. You are cordially invited
to attend the Special Meeting, which will be held for the following purposes:
| |
1. |
To approve and adopt an amendment to the Onconetix Amended and Restated Certificate of Incorporation (the “Charter”), in the form appended to the accompanying proxy statement as Annex A (the “Reverse Stock Split Amendment”), to effect a reverse stock split of all of the outstanding shares of the Company’s common stock, par value $0.00001 per share (“Common Stock”), at a ratio in the range of 1-for-30 to 1-for-60, with such ratio to be determined by the Board (the “Reverse Stock Split Proposal”); |
| |
2. |
To approve, in accordance with Nasdaq Listing Rule 5635, the issuance of up to 5,709,935 shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series A Preferred Stock, par value $0.00001 per share (“Series A Preferred Stock”) (the “Series A Conversion Proposal”); |
| 3. | To approve, in accordance with Nasdaq Listing Rule 5635, the issuance
of: (i) 269,672,900 shares of Common Stock to be issued upon conversion of the Company’s Series B Preferred Stock, par value $0.00001
per share (“Series B Preferred Stock”) and (ii) such number of shares of Common Stock to be issued by the Company in a $5
million private placement financing of units (the “PMX Financing”), which shall initially include 20,000,000 shares of Common
Stock and up to 6,000,000 shares of Common Stock underlying warrants included in the units, subject to adjustment, plus such additional
number of shares of Common Stock to be issuable upon the satisfaction of certain price protection conditions, as described further herein
(the “PMX Issuance Proposal”); |
| 4. | To ratify the appointment by the Board of EisnerAmper LLP (“EisnerAmper”) as the Company’s
independent registered public accounting firm for the fiscal year ending December 31, 2024 (the “Auditor Ratification Proposal”);
and |
| 5. | To approve the adjournment of the Special Meeting, if necessary or appropriate, to solicit additional
proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock
Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal (the “Adjournment
Proposal”). |
The Proposals are described in the accompanying
proxy statement, which we encourage you to read in its entirety before voting. Only holders of record of Common Stock at
the close of business on [●], 2024 are entitled to notice of the Special Meeting and to vote and have their votes counted at the
Special Meeting and any adjournments or postponements of the Special Meeting. A complete list of Onconetix stockholders of record entitled
to vote at the Special Meeting will be available for ten days before the Special Meeting at the principal executive offices of Onconetix
for inspection by stockholders during ordinary business hours for any purpose germane to the Special Meeting.
The Onconetix Board unanimously recommends
that Onconetix stockholders vote “FOR” each of the foregoing proposals.
The existence of any financial and personal
interests of one or more of Onconetix’s directors may be argued to result in a conflict of interest on the part of such director(s) between
what he, she or they may believe is in the best interests of Onconetix and its stockholders and what he, she or they may believe is best
for himself, herself or themselves in determining to recommend that stockholders vote for the proposals. See the section entitled “Interests
of Onconetix’s Directors and Executive Officers in the Proposals” in the accompanying proxy statement for a further discussion
of this issue.
Assuming a quorum is present at the Special Meeting,
(i) approval of the Reverse Stock Split Proposal requires the approval of the affirmative vote of the
holders of a majority of the outstanding shares of Onconetix Common Stock entitled to vote thereon and (ii) the other proposals require
the affirmative vote of the majority of the votes cast by stockholders present virtually or represented by proxy and entitled to vote
on the matter at the Special Meeting. Whether or not you plan to virtually attend the Special Meeting, please vote by proxy over the internet
or telephone using the instructions included with the accompanying proxy card, or promptly complete your proxy card and return it in the
enclosed postage-paid envelope, in order to authorize the individuals named on your proxy card to vote your shares of Onconetix common
stock at the Special Meeting. If you hold your shares through a broker, bank or other nominee in “street name” (instead of
as a registered holder) please follow the instructions on the voting instruction form provided by your bank, broker or nominee to vote
your shares. The list of Onconetix stockholders entitled to vote at the Special Meeting will be available at Onconetix’s headquarters
during regular business hours for examination by any Onconetix stockholder for any purpose germane to the Special Meeting for a period
of at least ten days prior to the Special Meeting. The stockholder list will also be available for examination during the Special
Meeting via the Special Meeting website.
PLEASE VOTE AS PROMPTLY AS POSSIBLE, WHETHER
OR NOT YOU PLAN TO ATTEND THE SPECIAL MEETING, VIA THE SPECIAL MEETING WEBSITE. IF YOU LATER DESIRE TO REVOKE OR CHANGE YOUR PROXY FOR
ANY REASON, YOU MAY DO SO IN THE MANNER DESCRIBED IN THE ACCOMPANYING PROXY STATEMENT. FOR FURTHER INFORMATION CONCERNING THE PROPOSALS
BEING VOTED UPON, THE SHARE EXCHANGE AGREEMENT, THE PMX TRANSACTION, USE OF THE PROXY AND OTHER RELATED MATTERS, YOU ARE URGED TO READ
THE ACCOMPANYING PROXY STATEMENT.
| |
By Order of the Board, |
| |
|
| |
/s/ Dr. Ralph Schiess |
| |
Dr. Ralph Schiess |
| |
Interim Chief Executive Officer |
| |
Onconetix, Inc. |
IF YOU RETURN YOUR PROXY CARD WITHOUT AN INDICATION
OF HOW YOU WISH TO VOTE, YOUR SHARES WILL BE VOTED IN FAVOR OF EACH OF THE PROPOSALS.
This proxy statement is dated [●], 2024 and
is first being mailed to the stockholders of Onconetix on or about [●], 2024.
TABLE OF CONTENTS
REFERENCES TO ADDITIONAL INFORMATION
The accompanying proxy statement incorporates
important business and financial information about Onconetix from other documents that Onconetix has filed with the U.S. Securities
and Exchange Commission (“SEC”) and that are not contained in and are instead incorporated by reference in the accompanying
proxy statement. For a list of documents incorporated by reference in the accompanying proxy statement, see “Where You Can Find
More Information.” This information is available for you, without charge, to review through the SEC’s website at www.sec.gov.
You may request a copy of the accompanying proxy
statement, any of the documents incorporated by reference in the accompanying proxy statement or other information filed with the SEC
by Onconetix, without charge, by written request directed to the following contact:
Onconetix, Inc.
Attention: Bruce Harmon, Chief Financial Officer
Email: bharmon@onconetix.com
201 E. Fifth Street, Suite 1900
Cincinnati, OH 45202
In order for you to receive timely delivery
of the documents in advance of the special meeting of Onconetix stockholders to be held on [●], 2024, which is referred to as the
“Special Meeting,” you must request the information no later than [●], 2024.
If you have any questions about the Special
Meeting or need to obtain a proxy card or other information, please contact Onconetix’s proxy solicitor at:
[●]
The contents of the websites of the SEC, Onconetix,
Proteomedix or any other entity are not incorporated in the accompanying proxy statement. The information about how you can obtain
certain documents that are incorporated by reference in the accompanying proxy statement at these websites is being provided only for
your convenience.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING
STATEMENTS
This proxy statement, and the documents incorporated
by reference into this proxy statement, includes certain “forward-looking statements” within the meaning of, and subject to
the safe harbor created by, Section 27A of the Securities Act, Section 21E of the Exchange Act and the Private Securities
Litigation Reform Act of 1995, which are referred to as the “safe harbor provisions.” Statements contained or incorporated
by reference in this proxy statement that are not historical facts are forward-looking statements, including statements regarding Onconetix’s
or Proteomedix’s business and future financial and operating results, and other aspects of Onconetix’s or Proteomedix’s
operations or operating results. Words such as “may,” “should,” “will,” “believe,” “expect,”
“anticipate,” “target,” “project,” and similar phrases that denote future expectations or intent regarding
Onconetix’s or Proteomedix’s financial results, operations, and other matters are intended to identify forward-looking statements
that are intended to be covered by the safe harbor provisions. Investors are cautioned not to rely upon forward-looking statements as
predictions of future events. The outcome of the events described in these forward-looking statements is subject to known and unknown
risks, uncertainties, and other factors that may cause future events to differ materially from the forward-looking statements in this
proxy statement, including:
| ● | risks relating to fluctuations of the market value of Onconetix Common Stock, including as a result of
uncertainty as to the long-term value of the common stock of Onconetix or as a result of broader stock market movements; |
| ● | Onconetix stockholders who receive shares of Onconetix Common Stock as a result of the conversion of any
Series A or Series B Preferred Stock will have rights as Onconetix common stockholders that differ from their current rights as preferred
stockholders; |
| ● | our ability to commercialize ENTADFI and Proclarix and integrate the assets and commercial operations
acquired into Onconetix’s business; |
| ● | failure to attract, motivate and retain executives and other key employees; |
| |
● |
disruptions in the business of Onconetix or Proteomedix, which could have an adverse effect on their respective businesses and financial results; and |
| ● | the unaudited pro forma condensed
combined financial information in this proxy statement is presented for illustrative purposes only and may not be reflective of the operating
results and financial condition of the combination of Onconetix and Proteomedix. |
The forward-looking statements contained in this proxy statement are
also subject to additional risks, uncertainties, and factors, including those described in financial statements of Onconetix included
in this proxy statement, as well as Onconetix’s most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q
and other documents filed by either of them from time to time with the SEC. See the section titled “Where You Can Find More
Information.”
The forward-looking statements included in this
report are made only as of the date hereof. Onconetix does not undertake to update, alter or revise any forward-looking statements made
in this report to reflect events or circumstances after the date of this report or to reflect new information or the occurrence of unanticipated
events, except as required by law.
FREQUENTLY ASKED QUESTIONS
The following questions and answers briefly
address some questions that you, as an Onconetix stockholder, may have regarding the matters being considered at the Special Meeting.
You are urged to carefully read this proxy statement and the other documents referred to in this proxy statement in their entirety because
this section may not provide all the information that is important to you regarding these matters. See “Summary” for a summary
of important information regarding the Special Meeting. Additional important information is contained in the annexes to, and the documents
incorporated by reference in, this proxy statement. You may obtain the information incorporated by reference in this proxy statement,
without charge, by following the instructions in the section titled “Where You Can Find More Information.”
Why am I receiving this proxy statement?
We sent you this proxy statement because our Board is soliciting your
proxy to vote at the Special Meeting that Onconetix is holding to seek stockholder approval on certain matters described in further detail
herein. This proxy statement summarizes the information you need to vote at the Special Meeting. You do not need to attend the Special
Meeting to vote your shares.
What is being voted on?
You are being asked to vote
on five proposals:
|
1. |
To approve and adopt the Reverse Stock Split Amendment, to effect a reverse stock split of all of the outstanding shares of our Common Stock, at a ratio in the range of 1-for-30 to 1-for-60, with such ratio to be determined by the Board; |
| |
2. |
To approve, in accordance with Nasdaq Listing Rule 5635, the issuance of up to 5,709,935 shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series A Preferred Stock, par value $0.00001 per share; |
| 3. | To approve, in accordance with Nasdaq Listing Rule 5635, the issuance
of: (i) 269,672,900 shares of Common Stock to be issued upon conversion of the Series B Preferred Stock and (ii) such number of shares
of Common Stock to be issued by the Company in the PMX Financing, which shall initially include 20,000,000 shares of Common Stock and
up to 6,000,000 shares of Common Stock underlying warrants included in the units, subject to adjustment, plus such additional number of
shares of Common Stock to be issuable upon the satisfaction of certain price protection conditions, as described further herein; |
| 4. | To ratify the appointment by the Board of EisnerAmper as the Company’s independent registered public
accounting firm for the fiscal year ending December 31, 2024; and |
| 5. | To approve the adjournment of the Special Meeting, if necessary or appropriate, to solicit additional
proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock
Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal. |
When are this proxy statement and the accompanying
materials scheduled to be sent to stockholders?
On or about [●], 2024, we will begin mailing
our proxy materials, including the Notice of the Special Meeting, this proxy statement, and the accompanying proxy card or, for shares
held in street name (i.e., shares held for your account by a broker or other nominee), a voting instruction form.
When and where will the Special Meeting take
place?
The Special Meeting will be held virtually via
a live, audio-only webcast on [●], 2024, beginning at [●] a.m., Eastern Time. There will not be a physical meeting location.
Onconetix stockholders will be able to virtually attend and vote at the Special Meeting by visiting www.[●].com, which is
referred to as the “Special Meeting website.” In order to virtually attend and vote at the Special Meeting, you will need
the 16-digit control number located on your proxy card. If you hold your shares of Common Stock in “street name,” you may
virtually attend and vote at the Special Meeting only if you obtain a specific control number from your brokerage firm, bank, dealer or
other similar organization, trustee, or nominee giving you the right to vote such shares.
When is the record date for the Special Meeting?
The record date for determination of stockholders
entitled to vote at the Special Meeting is the close of business on [●], 2024, which we refer to as the “record date.”
Who is entitled to vote at the Special Meeting?
All holders of record of shares of Onconetix Common
Stock who held shares at the close of business on [●], 2024, the record date, are entitled to receive notice of, and to vote at,
the Special Meeting. Virtual attendance at the Special Meeting via the Special Meeting website is not required to vote. See below and
the section titled “The Special Meeting — Methods of Voting” for instructions on how to vote without
virtually attending the Special Meeting.
Does my vote matter?
Yes, your vote is very important, regardless of
the number of shares that you own.
How does the Onconetix Board recommend that I
vote at the Special Meeting?
The Onconetix Board unanimously recommends that Onconetix stockholders
vote “FOR” each of the proposals.
Why should I vote for the Reverse Stock Split
Proposal?
On September 18, 2023, we received notice from
Nasdaq staff indicating that, based upon the closing bid price of the Common Stock for the prior 30 consecutive business days, we were
not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq, as set forth
in Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”). We have 180 days from September 18, 2023, or through March 16, 2024,
to regain compliance with the Bid Price Rule.
To regain compliance with the Bid Price Rule and
qualify for continued listing on the Nasdaq Capital Market, the closing bid price per share of our common stock must be at least $1.00
for at least 10 consecutive business days on or prior to March 16, 2024. The Nasdaq Staff retains discretion to extend this 10-business
day period to determine that the Company has demonstrated an ability to maintain long-term compliance.
If we fail to regain compliance with the Bid Price Rule before
March 16, 2024 but meet all of the other applicable standards for initial listing on Nasdaq with the exception of the Bid Price Rule,
then we may be eligible to have an additional 180 calendar days, or until September 12, 2024, to regain compliance with the Bid Price Rule.
If we do not regain compliance with the Bid Price Rule by the end of the compliance period (or the second compliance
period, if applicable), our Common Stock will become subject to delisting. In the event that we receive notice that our Common Stock is
being delisted, the Nasdaq listing rules permit us to appeal a delisting determination by Nasdaq to a hearings panel, but there can be
no assurance that the panel would grant the Company’s request for continued listing.
The Board believes that the failure of stockholders
to approve the Reverse Stock Split Amendment could prevent the Company from complying with the Bid Price Rule and could, among other risks,
inhibit our ability to conduct capital raising activities. If the Nasdaq Stock Market delists the Common Stock, then the Common Stock
would likely become traded on an over-the-counter market such as that maintained by OTC Markets Group Inc., which does not have the substantial
corporate governance or quantitative requirements for continued listing that the Nasdaq Stock Market has. In that event, interest in Common
Stock may decline and certain institutions may not have the ability to trade in the Common Stock, all of which could have a material adverse
effect on the liquidity or trading volume of the Common Stock. If the Common Stock becomes significantly less liquid due to delisting
from the Nasdaq Stock Market, the Company’s stockholders may not have the ability to liquidate their investments in the Common Stock
as and when desired, and the Company believes its ability to maintain and obtain analyst coverage, attract investor interest, and have
access to capital may become significantly diminished as a result.
Why should I vote for the Series A Conversion
Proposal?
We are subject to the Nasdaq Rules because our
Common Stock is currently listed on the Nasdaq Capital Market.
Pursuant to Nasdaq Rule 5635(a), stockholder approval
is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for Common Stock) in connection
with the acquisition of the stock or assets of another company if, due to the present or potential issuance of common stock, including
shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into or exercisable for common
stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power equal to or in excess of
20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable for common stock; or (B)
the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding
before the issuance of the stock or securities.
Combined with the shares issued in the PMX Transaction
and those shares that are issuable in the PMX Financing, the shares issuable upon conversion of the Series A Preferred Stock would result
in the issuance of more than 20% of the voting power and the number of shares of Common Stock outstanding as of the issuance of the Series
A Preferred Stock. As a result of the foregoing, in accordance with Nasdaq Rule 5635(a), the Series A Certificate of Designation provides
that the Series A Preferred Stock will not be convertible into Common Stock until such time as we obtain stockholder approval for their
removal, as discussed in “Proposal 2: Series A Conversion Proposal.”
If stockholders do not approve the Series A Conversion Proposal, the
Company will not be able to honor any conversions of Series A Preferred Stock held by Veru Inc. (“Veru”) (see “Information
About the Business of the Combined Company—Recent Acquisitions—ENTADFI”).
Why should I vote for the PMX Issuance Proposal?
As discussed above, pursuant to Nasdaq Rule 5635(a),
stockholder approval is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for
Common Stock) in connection with the acquisition of the stock or assets of another company if, due to the present or potential issuance
of common stock, including shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into
or exercisable for common stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power
equal to or in excess of 20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable
for common stock; or (B) the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of
shares of common stock outstanding before the issuance of the stock or securities.
Combined with the shares issuable upon conversion
of the Series A Preferred Stock and the shares of Common Stock issued in the PMX Transaction, the shares issuable upon conversion of the
Series B Preferred Stock and issuable in the PMX Financing would result in the issuance of more than 20% of the voting power and the number
of shares of Common Stock outstanding as of the issuance of each of (i) shares in the PMX Financing and (ii) the Series B Preferred Stock.
If stockholders do not approve the PMX Issuance Proposal by January 1, 2025, the Company will be obligated to redeem the shares of Series
B Preferred Stock for cash, as discussed in “Proposal 3: PMX Issuance Proposal.”
If stockholders do not approve the PMX Issuance
Proposal, the Company will not be able to complete the PMX Financing.
Why should I vote for the Auditor Ratification
Proposal?
EisnerAmper has served as the Company’s
independent registered public accounting firm since July 2023. Our Audit Committee and Board believe that stability and continuity in
the Company’s auditor is important as we advance our business plan.
Why should I vote for the Adjournment Proposal?
If the Adjournment Proposal is not approved, the
Onconetix Board may not be able to adjourn the Special Meeting to another time and place if necessary or appropriate to permit the solicitation
of additional proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal.
Will stockholders have the ability to unwind
the PMX Transaction if they do not approve the PMX Issuance Proposal?
No, the PMX Transaction closed on December 15,
2023, and stockholder approval of the PMX Issuance Proposal was not a condition to closing the PMX Transaction. If stockholders
have not approved the conversion of the Series B Preferred Stock into Common Stock by January 1, 2025, at
the request of the holder setting forth such holder’s request to cash settle a number of shares of Series B Preferred Stock, the
Company shall pay to such holder an amount in cash equal to (i) the Fair Value (as defined below) of the shares of Series B Preferred
Stock set forth in such request multiplied by (ii) the Conversion Ratio (as defined in the Certificate of Designation of the Series
B Preferred Stock) in effect on the trading day on which the request is delivered to Onconetix.
The “Fair Value” of shares shall be fixed with reference to the last reported closing stock price on the principal trading
market of the Common Stock.
The consummation
of the related PMX Financing is conditioned upon receipt of stockholder approval of the PMX Issuance Proposal.
What is a proxy?
A proxy is a stockholder’s legal designation
of another person to vote shares owned by such stockholder on their behalf. If you are a stockholder of record, you can vote by proxy
over the internet or by mail by following the instructions provided in the enclosed proxy card, or, by telephone if you are Onconetix
stockholder of record. If you hold shares beneficially through a broker, bank or other nominee in “street name,” you should
follow the voting instructions provided by your broker, bank or other nominee.
How many votes do I have at the Special
Meeting?
Each Onconetix stockholder is entitled to one
vote on each proposal for each share of Common Stock held of record at the close of business on the record date. At the close of business
on the record date, there were [●] shares of Common Stock outstanding.
What constitutes a quorum for the Special Meeting?
A quorum is the minimum number of shares required
to be represented, either through virtual attendance or through representation by proxy, to hold a valid meeting.
The holders of one-third of the issued and outstanding
shares of Common Stock entitled to vote at the Special Meeting must be present in person or represented by proxy in order to constitute
a quorum for the transaction of business at the Special Meeting. Virtual attendance at the Special Meeting will constitute presence in
person for the purpose of determining the presence of a quorum for the transaction of business at the Special Meeting. Abstentions will
count as votes present and entitled to vote for the purpose of determining the presence of a quorum for the transaction of business at
the Special Meeting.
Since the Auditor Ratification Proposal is
considered a routine matter, shares held in “street name” through a broker, bank or other nominee will be counted as
present for the purpose of determining the existence of a quorum if such broker, bank or other nominee does not have instructions to
vote on such proposal.
How can I vote my shares at the Special
Meeting?
Shares held directly in your name as an Onconetix
stockholder of record may be voted at the Special Meeting via the Special Meeting website at www.[●].com. You will need the
16-digit control number included on your proxy card in order to access and vote via the Special Meeting website as described in the section
titled “The Special Meeting — Virtually Attending the Special Meeting.”
If you hold your shares through a stockbroker,
nominee, fiduciary or other custodian you may also be able to vote through a program provided through Broadridge Financial Solutions (“Broadridge”)
that offers Internet voting options. If your shares are held in an account at a brokerage firm or bank participating in the Broadridge
program, you are offered the opportunity to elect to vote via the Internet. Votes submitted via the Internet through the Broadridge program
must be received by 11:59 p.m. Eastern Time on [●], 2024. See the section titled “The Special Meeting — Virtually
Attending the Special Meeting.”
Even if you plan to virtually attend the Special
Meeting via the Special Meeting website, Onconetix recommends that you vote by proxy in advance as described below so that your vote will
be counted if you later decide not to or become unable to virtually attend the Special Meeting.
For additional information on virtually attending
the Special Meeting, see the section titled “The Special Meeting.”
How can I vote my shares without virtually
attending the Special Meeting?
Whether you hold your shares directly as a stockholder
of record of Onconetix or beneficially in “street name,” you may direct your vote by proxy without virtually attending the
Special Meeting.
If you are a stockholder of record, you can vote
by proxy:
| ● | by Internet 24 hours a day, seven days a week, until 11:59 p.m. Eastern Time on [●],
2024 (have your proxy card in hand when you visit the website); |
| ● | by telephone in accordance with the instructions on your proxy card, until 11:59 p.m. Eastern Time
on [●], 2024 (have your proxy card in hand when you call); or |
| ● | by completing and mailing your proxy card in accordance with the instructions provided on the proxy card. |
If you hold shares beneficially in “street
name,” you should follow the voting instructions provided by your bank, broker, or other nominee. If you hold your shares through
a stockbroker, nominee, fiduciary or other custodian you may also be able to vote through a program provided through Broadridge that offers
Internet voting options. If your shares are held in an account at a brokerage firm or bank participating in the Broadridge program, you
are offered the opportunity to elect to vote via the Internet. Votes submitted via the Internet through the Broadridge program must be
received by 11:59 p.m. Eastern Time on [●], 2024.
For additional information on voting procedures,
see the section titled “The Special Meeting.”
What stockholder vote is required for the approval
of each proposal at the Special Meeting?
Approval of the Reverse Stock Split Proposal requires the affirmative vote of the holders of Common Stock representing at least a majority of the
outstanding shares of Common Stock entitled to vote thereon.
The Series A Conversion Proposal, the PMX Issuance
Proposal, the Auditor Ratification Proposal and the Adjournment Proposal require the affirmative vote of the majority of the votes cast
by stockholders present virtually or represented by proxy and entitled to vote on the matter at the Special Meeting.
What is a “broker non-vote?”
Under Nasdaq rules, banks, brokers and other nominees
may use their discretion to vote “uninstructed” shares (i.e., shares of record held by banks, brokers or other nominees, but
with respect to which the beneficial owner of such shares has not provided instructions on how to vote on a particular proposal) with
respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. All proposals
other than the Auditor Ratification Proposal are “non-routine” matters.
A “broker non-vote” occurs on a proposal
when (i) a broker, bank or other nominee has discretionary authority to vote on one or more proposals to be voted on at a meeting
of stockholders, but is not permitted to vote on other proposals without instructions from the beneficial owner of the shares, and (ii) the
beneficial owner fails to provide the broker, bank or other nominee with such instructions. The Auditor
Ratification Proposal is the only matter for which Onconetix expects there to be broker non-votes.
What will happen if I fail to vote or
abstain from voting on each proposal at the Special Meeting?
An abstention represents a stockholder’s
affirmative choice to decline to vote on a proposal. If a stockholder indicates on its proxy card that it wishes to abstain from voting
its shares, or if a broker, bank or other nominee holding its customers’ shares of record causes abstentions to be recorded for
shares, these shares will be considered present and entitled to vote at the annual meeting. As a result, abstentions will be counted for
purposes of determining the presence or absence of a quorum and will also count as votes against a proposal in cases where approval of
the proposal requires the affirmative vote of a majority of the shares outstanding or present in person or represented by proxy and entitled
to vote at the annual meeting.
What is the difference between holding shares
as a stockholder of record and as a beneficial owner of shares held in “street name”?
If your shares of Common Stock are registered
directly in your name with the transfer agent of Onconetix, you are considered the stockholder of record with respect to those shares.
As the stockholder of record, you have the right to vote directly at the Special Meeting. You may also grant a proxy directly to Onconetix,
or to a third party to vote your shares at the Special Meeting.
If your shares of Common Stock are held by brokerage
firm, bank, dealer or other similar organization, trustee, or nominee, you are considered the beneficial owner of shares held in “street
name.” Your brokerage firm, bank, dealer or other similar organization, trustee, or nominee will send you, as the beneficial owner,
a package describing the procedures for voting your shares. You should follow the instructions provided by your brokerage firm, bank,
dealer or other similar organization, trustee, or nominee to vote your shares.
In order to virtually attend and vote at the Special
Meeting via the Special Meeting website you should follow the voting instructions provided by your bank, broker or other nominee. If you
hold your shares of Common Stock through a stockbroker, nominee, fiduciary or other custodian you may also be able to vote through a program
provided through Broadridge that offers Internet voting options. If your shares of Common Stock are held in an account at a brokerage
firm or bank participating in the Broadridge program, you are offered the opportunity to elect to vote via the Internet. Votes submitted
via the Internet through the Broadridge program must be received by 11:59 p.m. Eastern Time on [●], 2024.
If my shares of Common Stock are held in “street
name” by my brokerage firm, bank, dealer or other similar organization, trustee, or nominee, will my brokerage firm, bank, dealer
or other similar organization, trustee, or nominee automatically vote those shares for me?
No. Your bank, broker or other nominee will only
be permitted to vote your shares of Common Stock at the Special Meeting if you instruct your bank, broker or other nominee. You should
follow the procedures provided by your bank, broker or other nominee regarding the voting of your shares. Banks, brokers and other nominees
who hold shares of Common Stock in “street name” for their customers have authority to vote on “routine” proposals
when they have not received instructions from beneficial owners. However, banks, brokers and other nominees are prohibited from exercising
their voting discretion with respect to non-routine matters, which includes all proposals other than the Auditor Ratification Proposal. As a result, absent specific instructions from the beneficial owner of such shares, banks, brokers
and other nominees are not empowered to vote such shares on such proposals.
What should I do if I receive more
than one set of voting materials for the Special Meeting?
If you hold shares of Common Stock in “street
name” and also directly in your name as a stockholder of record or otherwise, or if you hold shares of Common Stock in more than
one brokerage account, you may receive more than one set of voting materials relating to the Special Meeting.
Record Holders. For shares held directly,
please vote by proxy over the internet or by telephone, using the instructions included with the accompanying proxy card, or promptly
complete your proxy card and return it in the enclosed postage-paid envelope, in order to ensure that all of your shares of Common Stock
are voted.
Shares Held in “Street Name.”
For shares held in “street name” through a bank, broker or other nominee, you should follow the procedures provided by bank,
broker or other nominee to submit a proxy or vote your shares.
If a stockholder gives a proxy, how are the
shares of Common Stock voted?
Regardless of the method you choose to vote, the
individuals named on the enclosed proxy card will vote your shares of Common Stock in the way that you indicate. For each item before
the Special Meeting, you may specify whether your shares of Common Stock should be voted “for” or “against,” or
abstain from voting.
For more information regarding how your shares
will be voted if you properly sign, date and return a proxy card, but do not indicate how your Common Stock should be voted, see below
“— How will my shares be voted if I return a blank proxy?”
How will my shares be voted if I return
a blank proxy?
If you sign, date and return your proxy and do not indicate how you
want your shares of Common Stock to be voted, then your shares of Common Stock will be voted in accordance with the recommendation of
the Onconetix Board, “FOR” each of the proposals.
Can I change my vote after I have
submitted my proxy?
Any Onconetix stockholder giving a proxy has the
right to revoke the proxy and change their vote before the proxy is voted at the Special Meeting by doing any of the following:
| ● | subsequently submitting a new proxy for the Special Meeting that is received by the deadline specified
on the accompanying proxy card; |
| ● | giving written notice of your revocation to Onconetix’s Corporate Secretary; or |
| ● | virtually attending and voting at the Special Meeting via the Special Meeting website. Note that a proxy
will not be revoked if you attend, but do not vote at, the Special Meeting. |
Execution or revocation of a proxy will not in
any way affect your right to virtually attend and vote at the Special Meeting via the Special Meeting website. See the section titled
“The Special Meeting — Revocability of Proxies.”
If I hold my shares in “street name,”
can I change my voting instructions after I have submitted voting instructions to my bank, broker or other nominee?
If your shares are held in the name of a bank,
broker or other nominee and you previously provided voting instructions to your bank, broker or other nominee, you should follow the instructions
provided by your bank, broker or other nominee to revoke or change your voting instructions.
Where can I find the voting results of
the Special Meeting?
The preliminary voting results for the Special
Meeting are expected to be announced at the Special Meeting. In addition, within four Business Days following certification of the
final voting results, Onconetix will file the final voting results of the Special Meeting (or, if the final voting results have not yet
been certified, the preliminary results) with the SEC on a Current Report on Form 8-K.
Do Onconetix stockholders have dissenters’
or appraisal rights?
The stockholders of Onconetix are not entitled
to appraisal rights in connection with the proposals at the Special Meeting under Delaware law.
What happens if I sell my shares of Common
Stock after the record date but before the Special Meeting?
The record date is earlier than the date of the
Special Meeting. If you sell or otherwise transfer your shares of Common Stock after the record date but before the Special Meeting, you
will, unless special arrangements are made, retain your right to vote at the Special Meeting.
Who will solicit and pay the cost of soliciting
proxies?
Onconetix has engaged [●], which is referred
to as “[●],” to assist in the solicitation of proxies for the Special Meeting. Onconetix estimates that it will pay [●]
a fee of approximately $[●], plus reimbursement for certain out-of-pocket fees and expenses. Onconetix has agreed to indemnify [●]
against various liabilities and expenses that relate to or arise out of its solicitation of proxies (subject to certain exceptions).
Onconetix also may reimburse banks, brokers and
other custodians, nominees and fiduciaries or their respective agents for their expenses in forwarding proxy materials to beneficial owners
of Common Stock. Onconetix directors, officers and employees also may solicit proxies by telephone, by electronic means or in person.
They will not be paid any additional amounts for soliciting proxies.
What should I do now?
You should read this proxy statement carefully
and in its entirety, including the annexes. Then, you may vote by proxy over the internet or by telephone, using the instructions included
with the accompanying proxy card, or promptly complete your proxy card and return it in the enclosed postage-paid envelope, so that your
shares will be voted in accordance with your instructions.
How can I find more information about
Onconetix?
You can find more information about Onconetix
from various sources described in the section titled “Where You Can Find More Information.”
Whom do I call if I have questions
about the Special Meeting?
If you have questions about the Special Meeting,
or desire additional copies of this proxy statement or additional proxies, you may contact Onconetix’s proxy solicitor:
[●]
SUMMARY
For your convenience, provided below is a brief
summary of certain information contained in this proxy statement. This summary highlights selected information from this proxy statement
and does not contain all of the information that may be important to you as an Onconetix stockholder. To understand the PMX Transaction
fully and for a more complete description of the terms of the PMX Transaction, you should read carefully this entire proxy statement,
its annexes and the other documents to which you are referred. You may obtain the information incorporated by reference in this proxy
statement, without charge, by following the instructions under “Where You Can Find More Information.”
The PMX Transaction
On December 15, 2023, Onconetix acquired all
of the issued and outstanding equity interests of Proteomedix (the “Purchased Shares”) in exchange for newly issued shares
of Onconetix Common Stock, and newly issued shares of Series B Preferred Stock, as further described below (the “Share Exchange”
and the other transactions contemplated by the Share Exchange Agreement, the “PMX Transaction”).
The terms and conditions of the PMX Transaction
are contained in the Share Exchange Agreement, a copy of which is attached as Annex B hereto. Onconetix and Proteomedix encourage
you to read the Share Exchange Agreement carefully and in its entirety, as it is the legal document that governs the PMX Transaction.
Further, on December 15, 2023,
Onconetix filed a Certificate of Amendment to its Amended and Restated Certificate of Incorporation with the Delaware Secretary of State.
The amendment changed the name of Onconetix from “Blue Water Biotech, Inc.” to “Onconetix, Inc.,” effective immediately
(the “Name Change”).
The Parties to the PMX Transaction
Onconetix, Inc.
Onconetix is a commercial stage biotechnology company focused on the
research, development, and commercialization of innovative solutions for oncology. It owns ENTADFI, an FDA-approved, once daily pill that
combines finasteride and tadalafil for the treatment of benign prostatic hyperplasia (“BPH”), a disorder of the prostate,
and Proclarix, an in vitro diagnostic test for prostate cancer approved for sale in the European Union under the In Vitro Diagnostic Regulation
(“IVDR”) and a lab developed test currently in the U.S., originally developed by Proteomedix.
Onconetix shares are listed for trading on The
Nasdaq Capital Market under the symbol “ONCO.” For more corporate and product information please visit Onconetix’s website
at http://www.onconetix.com. Onconetix’s principal executive offices are located at 201 E. Fifth Street, Suite 1900, Cincinnati,
Ohio 45202, and its telephone number is (513) 620-4101.
Proteomedix AG (Proteomedix)
Founded in 2010, Proteomedix develops, markets
and sells non-invasive diagnostic tests accompanied by decision support systems to detect and assess the prognosis of cancer. Proteomedix’s
lead product, Proclarix®, is an in vitro diagnostic test for prostate cancer. Proteomedix is working to address all stages
in cancer management by developing tools for both more accurate detection and more efficient treatment of cancer including (i) diagnostic
tests to early detect and define the stage of cancer; (ii) prognostic tools for the identification of patients with aggressive disease;
and (iii) stratification biomarkers to match patients with therapies that are more likely to be safe and effective.
Proclarix addresses the unsolved problem of prostate
cancer overdiagnosis leading to numerous negative prostate biopsies that increase costs for the healthcare system and uncertainty for
patients. Proclarix is approved for sale in the European Union under the IVDR. Clinical studies have confirmed that Proclarix accurately
identifies clinically significant prostate cancer through a risk score derived from a clinical decision support system and could help
avoid many unneeded biopsies. Proclarix as a clinical support system is designed to aggregate multimodal information in an effort to develop
a patient centric diagnostic approach. Proteomedix intends to add more information to the risk score in the future, such as other biomarkers
or magnetic resonance imaging data, to provide an even more powerful tool to guide the patient’s diagnostic journey. The markers
and the bioinformatics algorithm used are patent protected.
The guidelines of the European Association of Urology (“EAU”)
and of the American Urological Association/Society of Urologic Oncology (“AUA/SUO”) both recommend the use of blood-based
biomarker tests, such as Proclarix, to aid in the early detection and evaluation of prostate cancer. Proclarix can be performed in any
laboratory using standard equipment. In Europe, Proteomedix has begun marketing Proclarix to pilot laboratories in selected markets that
are open to self-pay to show initial adoption. In the United States, the development and commercialization of Proclarix is being pursued
by Laboratory Corporation of America Holdings, more commonly called Labcorp, pursuant to an exclusive license agreement entered into between
Proteomedix and Labcorp in 2023.
Onconetix’s Reasons for the PMX Transaction
and Recommendation of the Onconetix Board
For a description of factors considered by the Onconetix Board in reaching
its decision to approve the share exchange agreement and the transactions contemplated thereby, including the PMX Transaction, and additional
information on the recommendation of the Onconetix Board, see the section titled “Background of the PMX Transaction.”
The Special Meeting
The Special Meeting will be held in a virtual
meeting format via live, audio-only webcast on [●], 2024, beginning at [●] a.m., Eastern Time. Onconetix stockholders will be
able to virtually attend and vote at the Special Meeting by visiting the Special Meeting website at www.[●].com.
The purposes of the Special Meeting are as follows:
| |
1. |
To approve and adopt the Reverse Stock Split Amendment, to effect a reverse stock split of all of the outstanding shares of our Common Stock, at a ratio in the range of 1-for-30 to 1-for-60, with such ratio to be determined by the Board; |
| |
2. |
To approve, in accordance with Nasdaq Listing Rule 5635, the issuance of up to 5,709,935 shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series A Preferred Stock, par value $0.00001 per share; |
| 3. | To approve, in accordance with Nasdaq Listing Rule 5635, the issuance
of: (i) 269,672,900 shares of Common Stock to be issued upon conversion of the Series B Preferred Stock and (ii) such number of shares
of Common Stock to be issued by the Company in the PMX Financing, which shall initially include 20,000,000 shares of Common Stock and
up to 6,000,000 shares of Common Stock underlying warrants included in the units, subject to adjustment, plus such additional number of
shares of Common Stock to be issuable upon the satisfaction of certain price protection conditions, as described further herein; |
| 4. | To ratify the appointment by the Board of EisnerAmper as the Company’s independent registered public
accounting firm for the fiscal year ending December 31, 2024; and |
| 5. | To approve the adjournment of the Special Meeting, if necessary or appropriate, to solicit additional
proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock
Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal. |
A quorum of Onconetix stockholders is necessary
to conduct business at the Special Meeting. The presence in person or by proxy of the holders of one-third of the issued and outstanding
shares of Common Stock entitled to vote at the Special Meeting will constitute a quorum. Virtual attendance at the Special Meeting will
constitute presence in person for the purpose of determining the presence of a quorum for the transaction of business at the Special Meeting.
Abstentions will count as votes present and entitled to vote for the purpose of determining the presence of a quorum for the transaction
of business at the Special Meeting. Since the Auditor Ratification Proposal is considered a routine
matter, shares held in “street name” through a broker, bank or other nominee will be counted as present for the purpose of
determining the existence of a quorum if such broker, bank or other nominee does not have instructions to vote on such proposal.
Approval of the Reverse Stock Split Proposal requires the affirmative vote of the holders of Common Stock representing at least a majority of the
outstanding shares of Common Stock entitled to vote thereon. The Series A Conversion Proposal, the PMX Issuance Proposal, the Auditor
Ratification Proposal and the Adjournment Proposal require the affirmative vote of the majority of the votes cast by stockholders present
virtually or represented by proxy and entitled to vote on the matter at the Special Meeting.
An abstention represents a stockholder’s
affirmative choice to decline to vote on a proposal. If a stockholder indicates on its proxy card that it wishes to abstain from voting
its shares, or if a broker, bank or other nominee holding its customers’ shares of record causes abstentions to be recorded for
shares, these shares will be considered present and entitled to vote at the annual meeting. As a result, abstentions will be counted for
purposes of determining the presence or absence of a quorum and will also count as votes against a proposal in cases where approval of
the proposal requires the affirmative vote of a majority of the shares outstanding or present in person or represented by proxy and entitled
to vote at the annual meeting.
Interests of Onconetix Directors and Executive
Officers in the PMX Transaction
As of the
date of this proxy statement, Onconetix directors and executive officers do not have interests in the proposals that are different from,
or in addition to, the interests of other Onconetix stockholders generally, except that:
| |
● |
Dr. Ralph Schiess, our Interim Chief Executive Officer and Chief Science
Officer, is a holder of 269,749 shares of Common Stock and 195,664 shares of Series B Preferred Stock. |
| |
● |
Christian Brühlmann, our Chief Strategy Officer, is a holder of 236,029 shares of Common Stock and 171,204 shares of Series B Preferred Stock. |
Certain Beneficial Owners of Onconetix Common
Stock
At the close of business on [●], 2024, the
latest practicable date prior to the date of this proxy statement, Onconetix directors and executive officers and their affiliates, as
a group, owned and were entitled to vote approximately [●]% of the shares of Onconetix common stock.
DESCRIPTION OF THE PMX TRANSACTION AND RELATED
FINANCING
General Description
of the Share Exchange Agreement
On
December 15, 2023, Onconetix entered into a Share Exchange Agreement (the “Share Exchange Agreement”), by and among (i) Onconetix,
(ii) Proteomedix AG, a Swiss Company (“Proteomedix”), (iii) each of the holders of outstanding capital stock or Proteomedix
convertible securities (other than Proteomedix stock options) named therein (collectively, the “Sellers”) and (iv) Thomas
Meier, in the capacity as the representative of Sellers in accordance with the terms and conditions of the Share Exchange Agreement (the
“Sellers’ Representative”).
Pursuant
to the Share Exchange Agreement, subject to the terms and conditions set forth therein, the Sellers agreed to sell to Onconetix,
and Onconetix agreed to buy, all of the Purchased Shares in exchange for newly issued shares of Common Stock, and newly issued shares
of Series B Preferred Stock, as further described below.
The
consummation (the “Closing”) of the Share Exchange was subject to customary closing conditions and the execution of the Subscription
Agreement (as defined below) entered into with Altos Ventures, a shareholder of Proteomedix prior to the closing of the PMX Transaction
(the “PMX Investor”). The Share Exchange closed on December 15, 2023 (the
“Closing Date”).
Consideration
In
full payment for the Purchased Shares, Onconetix issued shares (the “Exchange Shares”) consisting of: (i) 3,675,414
shares of Common Stock equal to approximately 19.9% of the total issued and outstanding Common Stock and (ii) 2,696,729 shares of
Series B Preferred Stock convertible into 269,672,900 shares of Common Stock. The parties
agreed that the aggregate value of the Exchange Shares at Closing was equal to approximately Seventy-Five Million U.S.
Dollars ($75,000,000) (the “Exchange Consideration”) less the value of the Proteomedix Shares for which the Proteomedix
Stock Options (as defined below) are exercisable immediately prior to the Closing, subject to adjustment for indemnification as
described below. Following the Closing, 22,061,746 shares of Common Stock were issued and outstanding.
Tungsten
Advisors acted as financial advisor to Proteomedix. As part of compensation for services rendered by Tungsten Advisors, $7,500,000 in
Exchange Shares was issued to certain affiliates of Tungsten Advisors (the “Advisor Parties”) out of the total Exchange Consideration
issued by Onconetix.
As a result of the PMX Transaction, Proteomedix became a direct, wholly
owned subsidiary of Onconetix. It is anticipated that, following the Conversion (as defined below) and closing of the investment pursuant
to the Subscription Agreement (as defined below), Sellers will own approximately 87.2% of the outstanding equity interests of Onconetix, the PMX Investor
will own approximately 7.5% of the outstanding equity interests of Onconetix, and the stockholders of Onconetix immediately prior to the
Closing will own approximately 5.3% of the outstanding equity interests of Onconetix.
Each
option to purchase shares of Proteomedix (each, a “Proteomedix Stock Option”) outstanding immediately before the Closing,
whether vested or unvested, remains outstanding until the Conversion unless otherwise terminated in accordance with its terms. At the
Conversion, each outstanding Proteomedix Stock Option, whether vested unvested, shall be assumed by Onconetix and converted into the right
to receive (a) an option to acquire shares of Common Stock (each, an “Assumed Option”) or (b) such other derivative security
as Onconetix and Proteomedix may agree, subject in either case to substantially the same terms and conditions as were applicable to such
Proteomedix Stock Option immediately before the Closing. Each Assumed Option shall: (i) represent the right to acquire a number of shares
of Common Stock equal to the product of (A) the number of Proteomedix Common Shares that were subject to the corresponding Proteomedix
Option immediately prior to the Closing, multiplied by (B) the Exchange Ratio (as defined in the Share Exchange Agreement”); and
(ii) have an exercise price (as rounded down to the nearest whole cent) equal to the quotient of (A) the exercise price of the corresponding
Proteomedix Option, divided by (B) the Exchange Ratio.
Series B Preferred
Stock
Subject to any requirements related
to the Committee on Foreign Investment in the United States (“CFIUS”), upon approval by the requisite vote
of stockholders of Onconetix at the Special Meeting (“Stockholder Approval”), each share of Series B Preferred
Stock shall automatically convert into 100 shares of Common Stock in accordance with the terms of the Certificate of Designation (the
“Conversion”). If Stockholder Approval is not obtained by January 1, 2025, Onconetix shall be obligated to cash settle the
Series B Preferred Stock, as described below.
Representations
and Warranties
Onconetix,
Proteomedix and the Sellers have made customary representations and warranties in the Share Exchange Agreement. The
representations and warranties of Onconetix and Proteomedix shall survive until the Conversion and the representations and warranties
of the Sellers shall survive until the first anniversary of the Closing.
Indemnification
Until
the earlier of (i) Stockholder Approval or (ii) June 30, 2024 (the “Claim Deadline”), Onconetix may assert Claims against
Proteomedix and Sellers for any and all Losses incurred by Onconetix with respect to: (i) any inaccuracy in or breach of any of the representations
or warranties made by Proteomedix contained in the Share Exchange Agreement or (ii) any breach or non-fulfillment of any covenant, agreement
or obligation to be performed by Proteomedix pursuant to the Share Exchange Agreement. Until the Claim Deadline, the Sellers’ Representative,
acting on behalf of the Sellers, may assert Claims against Onconetix for any Loss incurred by the Sellers with respect to: (i) any inaccuracy
in or breach of any of the representations or warranties of Onconetix contained in the Share Exchange Agreement or (ii) any breach or
non-fulfillment of any covenant, agreement or obligation to be performed by Onconetix pursuant to the Share Exchange Agreement.
The
number of shares of Common Stock issued upon Conversion shall be increased or decreased by a number determined by dividing the Net Adjustment
by the ten-day volume-weighted average price (“VWAP”) of the Common Stock for the ten (10)-day period preceding the third
day prior to the Closing Date and rounding down to the nearest whole share; provided, however, that (i) there shall be no adjustment to
the number of shares of Common Stock issued upon Conversion if the Net Adjustment is less than $1,000,000 and (ii) the number of shares
of Common Stock issued upon Conversion shall not be increased or decreased by more than 10% of the number of shares of Common Stock that
would be issuable absent such adjustment. As used herein, “Net Adjustment” means the absolute value of the difference between
the aggregate adjustment in favor of each party with respect to Losses that is agreed by Onconetix and the Sellers’ Representative
or determined by a mutually acceptable dispute resolution firm.
From
and after the Closing and until the first anniversary of the Closing, Sellers, severally and not jointly, are required to indemnify Onconetix
and its affiliates and their respective representatives (collectively, the “Onconetix Indemnitees”) against (i) any inaccuracy
in or breach of any of the representations or warranties of such Seller contained in the Share Exchange Agreement and (ii) breach or non-fulfillment
of any covenant, agreement or obligation to be performed by such Seller pursuant to the Share Exchange Agreement. Any payment due from
any Seller in respect of an indemnification claim by any Onconetix Indemnitee shall solely be satisfied by recourse to the Exchange Shares
and the shares of Common Stock issuable upon the Conversion, with each share of Common Stock valued at the same price per share of Common
Stock used to determine the Exchange Ratio.
Covenants of the Parties
Each
party to the Share Exchange Agreement agreed to use its commercially reasonable efforts to effect the PMX Transaction. Onconetix
agreed to use its commercially reasonable efforts to, as soon as practicable, obtain from each holder of more than five percent (5%) of
Onconetix’s voting stock and each director and executive officer of Onconetix, a duly executed Stockholder Support Agreement (as
defined below).
The
Share Exchange Agreement contains certain covenants by each of the parties, to be observed during the period between Closing and Conversion,
including covenants regarding: (1) the provision of access to properties, books and personnel; (2) delivery of Onconetix’s financial
statements; (3) litigation support; (4) Onconetix’s public filings; (5) no insider trading; (6) further assurances; (7) public announcements;
(8) confidentiality; (9) indemnification of directors and officers and tail insurance; (10) intended tax treatment of the Share Exchange;
(11) Section 16 matters and (12) transfer taxes.
The
parties agreed to take all necessary actions to cause Onconetix’s board of directors immediately after the Stockholder Approval
(the Post-Stockholder Approval Onconetix Board) to consist of five directors, including: (i) two persons who are designated by Onconetix and
reasonably acceptable to Proteomedix; and (ii) three persons who are designated by Proteomedix
and reasonably acceptable to Onconetix.
The
issuance of the Conversion Shares, amendment of Onconetix’s certificate of incorporation to authorize sufficient additional
shares of Common Stock to permit the Conversion (to the extent required to consummate the PMX Transaction) and the appointment of
the Post-Stockholder Approval Onconetix Board requires the approval of Onconetix’s stockholders. Onconetix agreed to prepare
and file with the SEC a proxy statement (a “Proxy Statement”) for the purpose of soliciting proxies from the
stockholders of Onconetix for the matters to be acted on at the special meeting of the stockholders of Onconetix. Onconetix also
agreed to prepare a registration statement on Form S-1 or Form S-4 in connection with the registration under the Securities Act of
1933, as amended (the “Securities Act”), of the issuance of Onconetix Securities to be issued under the Share Exchange
Agreement and prepare a Proxy Statement for the purpose of soliciting proxies from Onconetix stockholders for the matters to be
acted upon at the Special Meeting.
Sellers, Onconetix and Proteomedix agreed
to, at the election of Onconetix (which election it has determined not to exercise) or upon the request of CFIUS, submit to CFIUS a joint
declaration or notice with respect to the PMX Transaction as promptly as practicable, but in no event later than sixty (60) days after
the date of the Share Exchange Agreement. The parties, in cooperation with each other, agreed to use reasonable best efforts to take
all such actions within their respective powers to obtain the approval of CFIUS (“CFIUS Approval”), and, without limiting
the foregoing, the parties agreed to, after reasonable negotiation efforts, agree to such requirements or conditions to mitigate any
national security concerns as may be requested or required by CFIUS in connection with, or as a condition of, CFIUS Approval, including
entering into a mitigation agreement, letter of assurance, or national security agreement, but provided: (1) the parties shall have no
obligation to (A) propose, negotiate, commit to or effect, by consent decree, hold separate order, agreement or otherwise, the sale,
transfer, license, divestiture or other disposition of, any of the businesses, product lines or assets of Onconetix or any of its affiliates
or of the Sellers, (B) terminate existing, or create new, relationships, contractual rights or obligations of Onconetix or its affiliates,
(C) effect any other change or restructuring of Onconetix or its affiliates, or (D) otherwise take or commit to take any actions reasonably
expected to have a material adverse effect on the operation of the business of the Sellers or that interfere with Onconetix’s ability
to control Proteomedix or Onconetix’s ability to direct the management and policies of the business of Proteomedix in any material
respect; and (2) Proteomedix and the Sellers agreed not take or agree to take any of the foregoing actions without the prior written
consent of Onconetix.
The parties agreed
to use commercially reasonable best efforts to (i) ensure that the application for Onconetix’s change of control (“Nasdaq
Change of Control Application”) is filed with The Nasdaq Stock Market LLC (“Nasdaq”) and (ii) to respond to any questions
from Nasdaq with respect to the Nasdaq Change of Control Application promptly following receipt of such questions, but in no event later
than ten (10) business days following receipt of such questions.
During the time between Closing and the Conversion,
Onconetix also agreed, and agreed to cause its Subsidiaries, to conduct their respective businesses in the ordinary course of business
in all material respects and agreed to covenants regarding operation of their respective businesses, including covenants related to (i)
amendments to Onconetix’s organizational documents; (ii) recapitalization of Onconetix’s equity interests; (iii) issuance
of additional securities; (iv) incurrence of additional indebtedness; (v) material changes to tax elections; (vi) amendments or termination
of material contracts; (vii) records and books; (viii) establishment of any Subsidiary or entry into a new line of business; (ix) maintenance
of insurance policies; (x) revaluation of material assets or material changes in accounting methods, principles or policies except to
the extent to comply with U.S. GAAP; (xi) waiver or settlement of any claim, action or proceeding, other than waivers not in excess of
$500,000; (xii) acquisition of equity interests or assets, or any other form of business combination, outside of the ordinary course of
business; (xiii) capital expenditures in excess of $500,000 individually or $1,000,000 in the aggregate; (xiv) adoption of a
plan of liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other reorganization; (xv) voluntary
incurrence of any liability or obligation in excess of $500,000 individually or $1,000,000 in the aggregate other than pursuant to the
terms of a Contract in existence as of the date of the Share Exchange Agreement or entered into in the ordinary course of business, except
in connection with a Permitted Financing; (xvi) sale, lease, license or other disposition of any material portion of Onconetix
properties, assets or rights; (xvii) entry into any agreement, understanding or arrangement with respect to the voting of Common
Stock, except in connection with a Permitted Financing; (xviii) taking any action that would reasonably be expected to significantly
delay or impair the obtaining of any Consents of any Governmental Authority to be obtained in connection with the Share Exchange Agreement;
or (xix) authorizing or agreeing to do any of the foregoing actions.
“Permitted Financing”
means one or more debt or equity financing transactions consummated by and funded into Onconetix during the time between Closing and the
Conversion resulting in aggregate gross proceeds of no greater than $25 million.
Governing Law
The
Share Exchange Agreement is governed by the laws of the State of Delaware.
Terms of the Series B Preferred Stock
The
terms of the Series B Preferred Stock, as described in the Certificate of Designation, are
as follows:
Voting. The
shares of Series B Preferred Stock carry no voting rights except: (i) with respect
to the election of the Proteomedix Director (as described below) and (ii) that the affirmative vote of the holders of a majority
of the outstanding shares of Series B Preferred Stock (the “Majority Holders”),
acting as a single class, shall be necessary to (A) alter or change adversely the powers, preferences or rights given to the Series B
Preferred Stock, (B) alter or amend the Certificate of Designation, or amend or repeal any provision of, or add any provision to, Onconetix’s
certificate of incorporation or bylaws, if such action would adversely alter or change the preferences, rights, privileges or powers of,
or restrictions provided for the benefit of the Series B Preferred Stock, (C) issue
further shares of Series B Preferred Stock or increase or decrease (other than by conversion) the number of authorized shares of Series
B Preferred Stock, or (D) authorize or create any class or series of stock, or issue shares of any class or series of stock, that has
powers, preferences or rights senior to the Series B Preferred Stock.
Proteomedix
Director. The Majority Holders, voting exclusively and as a separate class, shall be entitled to elect one (1) director of Onconetix.
Any director elected as provided in the preceding sentence may be removed without cause by, and only by, the affirmative vote of the holders
of the Series B Preferred Stock. If the holders of Series B Preferred Stock fail to elect a director, then any directorship not so filled
shall remain vacant until such time as the holders of the Series B Preferred Stock elect a person to fill such directorship; and no such
directorship may be filled by stockholders of Onconetix other than by the holders of Series B Preferred Stock. At any meeting held for
the purpose of electing a director, the presence in person or by proxy of the holders of a majority of the outstanding shares of Series
B Preferred Stock shall constitute a quorum for the purpose of electing such director.
Redemption.
The shares of Series B Preferred Stock are not redeemable by Onconetix.
Liquidation
Preference. Upon a liquidation, dissolution or winding-up of Onconetix, whether voluntary or involuntary (a “Liquidation”),
the holders of Series B Preferred Stock shall be entitled to receive out of the assets, whether capital or surplus, of Onconetix the same
amount that a holder of Common Stock would receive if such Holder’s Series B Preferred Stock were fully converted to Common Stock
at the Conversion Ratio (as defined below) plus an additional amount equal to any dividends declared but unpaid to such shares, which
amounts shall be paid pari passu with all holders of Common Stock.
Dividends.
The holders of the Series B Preferred Stock shall be entitled to receive, dividends on shares of Series B Preferred Stock (on an as-if-converted-to-common-stock
basis) equal to and in the same form, and in the same manner, as dividends (other than dividends on shares of the Common Stock payable
in the form of Common Stock) actually paid on shares of the Common Stock when, as and if such dividends (other than dividends payable
in the form of Common Stock) are paid on shares of the Common Stock.
Conversion.
Following Stockholder Approval, each share of Series B Preferred Stock shall be converted into shares of Common Stock (the “Conversion
Shares”) at a ratio of 100 Conversion Shares for each share of Series B Preferred Stock (the “Conversion Ratio”). All
shares of Series B Preferred Stock shall automatically and without any further action required be converted into Conversion Shares at
the Conversion Ratio upon the latest date on which (i) Onconetix has received the Stockholder Approval with respect to the issuance of
all of the shares of Common Stock issuable upon Conversion in excess of 20% of the issued and outstanding Common Stock on the Closing
Date and (ii) Onconetix has effected an increase in the number of shares of Common Stock authorized under its certificate of incorporation,
to the extent required to consummate the PMX Transaction.
Cash
Settlement. If, at any time after the earlier of the date of the Stockholder Approval or January 1, 2025 (the earliest such date,
the “Cash Settlement Date”), Onconetix (x) has obtained the Stockholder Approval but fails to or has failed to deliver
to a holder certificate or certificates representing the Conversion Shares, or deliver documentation of book entry form of (or cause its
transfer agent to electronically deliver such evidence) Conversion Shares on or prior to the fifth business day after the date of the
Stockholder Approval, or (y) has failed to obtain the Stockholder Approval, Onconetix shall, in either case, at the request of the
holder setting forth such holder’s request to cash settle a number of shares of Series B Preferred Stock, pay to such holder an
amount in cash equal to (i) the Fair Value (as defined below) of the shares of Series B Preferred Stock set forth in such request multiplied
by (ii) the Conversion Ratio in effect on the trading day on which the request is delivered to Onconetix, with such payment to be made
within two (2) business days from the date of the request by the holder, whereupon, after payment in full thereon by Onconetix, Onconetix’s
obligations to deliver such shares underlying the request shall be extinguished. “Fair Value” of shares shall be fixed with
reference to the last reported closing stock price on the principal trading market of the Common Stock on which the Common Stock is listed
as of the trading day on which the request is delivered to Onconetix.
Certain Adjustments.
If Onconetix, at any time while the Series B Preferred Stock is outstanding: (A) pays a stock dividend or otherwise makes a
distribution or distributions payable in shares of Common Stock; (B) subdivides outstanding shares of Common Stock into a larger number
of shares; or (C) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares,
then the Conversion Ratio shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock outstanding
immediately after such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately before
such event (excluding any treasury shares of the Corporation). If, at any time while the Series B Preferred Stock is outstanding,
either (A) Onconetix effects any merger or consolidation of Onconetix with or into another person or any stock sale to, or other business
combination with or into another person (other than such a transaction in which Onconetix is the surviving or continuing entity and holds
at least a majority of the Common Stock after giving effect to the transaction and its Common Stock is not exchanged for or converted
into other securities, cash or property), (B) Onconetix effects any sale, lease, transfer or exclusive license of all or substantially
all of its assets in one transaction or a series of related transactions, (C) any tender offer or exchange offer (whether by Onconetix
or another person) is completed pursuant to which more than 50% of the Common Stock not held by Onconetix or such person is exchanged
for or converted into other securities, cash or property, or (D) Onconetix effects any reclassification of the Common Stock or any
compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or
property (in any such case, a “Fundamental PMX Transaction”), then, in connection with any such transaction in (A) through
(D), the holders of Series B Preferred Stock shall receive in such transaction, the same kind and amount of securities, cash or property
that a holder of Common Stock would receive if such holder’s Series B Preferred Stock were fully converted to Common Stock, plus
an additional amount equal to any dividends declared but unpaid to such shares, which amounts shall be paid pari passu with
all holders of Common Stock in the Fundamental PMX Transaction (the “Alternate Consideration”). If holders of Common Stock
are given any choice as to the securities, cash or property to be received in a transaction in (A) through (D), then the holders
of Series B Preferred Stock shall be given the same choice as to the Alternate Consideration it receives in such transaction.
Lock-Up Agreement
Simultaneously
with the execution of the Share Exchange Agreement, the Sellers and the Advisor Parties, as shareholders of Proteomedix, entered into
Lock-Up Agreements (each, a “Lock-Up Agreement”). Pursuant to each Lock-Up Agreement, each signatory thereto will agree not
to, during the period commencing from the Closing Date and ending on the 6-month anniversary of the date of Stockholder Approval: (i)
lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase
any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly,
the Exchange Shares or the Conversion Shares, (ii) enter into any swap or other arrangement that transfers to another, in whole or
in part, any of the economic consequences of ownership of the Exchange Shares or the Conversion Shares, or (iii) publicly disclose
the intention to do any of the foregoing, whether any such transaction described in clauses (i), (ii) or (iii) above is to be settled
by delivery of the Exchange Shares or the Conversion Shares or other securities, in cash or otherwise (subject to certain exceptions).
Non-Competition and Non-Solicitation Agreement
Simultaneously
with the execution of the Share Exchange Agreement, certain executive officers (each, a “Management Shareholder”) of Proteomedix
each entered into a non-competition and non-solicitation agreement (collectively, the “Non-Competition and Non-Solicitation Agreements”)
with Onconetix. Under the Non-Competition and Non-Solicitation Agreements, each Management Shareholder agreed not to compete with Proteomedix,
and after the Closing, Onconetix, and their respective affiliates during the three-year period following the Closing and, during such
three-year restricted period, not to solicit employees or customers of such entities. Each Non-Competition and Non-Solicitation Agreement
also contains customary confidentiality and non-disparagement provisions.
Stockholder Support Agreement
Simultaneously
with the execution of the Share Exchange Agreement, Onconetix, Proteomedix and certain directors of Onconetix who are stockholders of
Onconetix, entered into a Stockholder Support Agreement (the “Stockholder Support Agreement”), pursuant to which, among other
things, each such stockholder of Onconetix has agreed (a) to support the adoption of the Share Exchange Agreement and the approval of
the PMX Transaction, subject to certain customary conditions, and (b) not to transfer any of their subject shares (or enter into any arrangement
with respect thereto), subject to certain customary conditions.
Stockholder Subscription
Agreement and Debenture
In
connection with the PMX Transaction, on December 15, 2023, Onconetix entered into a Subscription Agreement (the “Subscription Agreement”)
with the PMX Investor for a private placement of $5.0 million of units (the “Units”), each Unit comprised of (i) one share
of Common Stock and (ii) one pre-funded warrant (collectively, the “Warrants”) to purchase 0.3 shares of Common Stock at an
exercise price of $0.001 per share, for an aggregate purchase price per Unit of $0.25 (the “Purchase Price”). Additional shares
are issuable to the PMX Investor to the extent the PMX Investor continues to hold Common Stock included in the Units and if the VWAP during
the 270 days following closing is less than the Purchase Price, as set forth in the Subscription Agreement.
Stockholder
approval is a condition to closing the PMX Financing, and the offering is expected to close following stockholder approval of the issuance
of the Conversion Shares. Within 30 days after closing, Onconetix will file a resale registration statement with the SEC registering
the resale of the Common Stock issuable pursuant to the Subscription Agreement and the Warrants.
On
January 23, 2024, the Company issued a non-convertible debenture (the “Debenture”) to the PMX Investor in the principal sum
of $5.0 million, the payment of which shall offset the Aggregate Purchase Price for the Units pursuant to the Subscription Agreement.
The Debenture has an interest
rate of 4.0% per annum, and the principal and accrued interest are repayable in full upon the earlier of (i) the closing under the Subscription
Agreement and (ii) June 30, 2024. Additionally, the $5.0 million subscription amount under the Subscription Agreement shall be increased
by the amount of interest payable under the Debenture. As of February 12, 2024, a total of $5 million of principal was outstanding under
the Debenture.
THE PARTIES TO THE
TRANSACTION
Onconetix, Inc.
Onconetix is a commercial stage biotechnology
company focused on the research, development, and commercialization of innovative solutions for oncology. It owns ENTADFI, an FDA-approved,
once daily pill that combines finasteride and tadalafil for the treatment of BPH, a disorder of the prostate, and Proclarix, an in vitro
diagnostic test for prostate cancer approved for sale in the European Union under the In Vitro Diagnostic Regulation (“IVDR”)
and a lab developed test currently in the U.S., originally developed by Proteomedix.
Onconetix shares are listed for trading on The
Nasdaq Capital Market under the symbol “ONCO.” For more corporate and product information please visit Onconetix’s website
at http://www.onconetix.com. Onconetix’s principal executive offices are located at 201 E. Fifth Street, Suite 1900, Cincinnati,
Ohio 45202, and its telephone number is (513) 620-4101.
Proteomedix AG
Founded in 2010, Proteomedix develops, markets
and sells non-invasive diagnostic tests accompanied by decision support systems to detect and assess the prognosis of cancer. Proteomedix’s
lead product, Proclarix®, is an in vitro diagnostic test for prostate cancer. Proteomedix is working to address all stages
in cancer management by developing tools for both more accurate detection and more efficient treatment of cancer including (i) diagnostic
tests to early detect and define the stage of cancer; (ii) prognostic tools for the identification of patients with aggressive disease;
and (iii) stratification biomarkers to match patients with therapies that are more likely to be safe and effective.
Proclarix addresses the unsolved problem of
prostate cancer overdiagnosis leading to numerous negative prostate biopsies that increase costs for the healthcare system and uncertainty
for patients. Proclarix is approved for sale in the European Union under the IVDR. Clinical studies have confirmed that Proclarix accurately
identifies clinically significant prostate cancer through a risk score derived from a clinical decision support system and could help
avoid many unneeded biopsies. Proclarix as a clinical support system is designed to aggregate multimodal information in an effort to
develop a patient centric diagnostic approach. Proteomedix intends to add more information to the score in the future, such as other
biomarkers or magnetic resonance imaging data, to provide an even more powerful tool to guide the patient’s diagnostic journey.
The markers and the bioinformatics algorithm used are patent protected.
The guidelines of the European Association
of Urology (“EAU”) and of the American Urological Association/Society of Urologic Oncology (“AUA/SUO”) both recommend
the use of blood-based biomarker tests, such as Proclarix, to aid in the early detection and evaluation of prostate cancer. Proclarix
can be performed in any laboratory using standard equipment. In Europe, Proteomedix has begun marketing Proclarix to pilot laboratories
in selected markets that are open to self-pay to show initial adoption. In the United States, the development and commercialization of
Proclarix is being pursued by Laboratory Corporation of America Holdings, more commonly called Labcorp, pursuant to an exclusive license
agreement entered into between Proteomedix and Labcorp in 2023.
INFORMATION ABOUT THE BUSINESS OF THE COMBINED
COMPANY
Our Company
We are a commercial stage biotechnology company
focused on the research, development, and commercialization of innovative solutions for oncology. We own ENTADFI, an FDA-approved, once
daily pill that combines finasteride and tadalafil for the treatment of BPH, a disorder of the prostate, and Proclarix, an in vitro diagnostic
test for prostate cancer approved for sale in the European Union under the In Vitro Diagnostic Regulation (“IVDR”)
and a lab developed test currently in the U.S., originally developed by Proteomedix.
ENTADFI allows men to receive treatment for their
symptoms of BPH without the negative sexual side effects typically seen in patients on finasteride alone. Following a recent business
strategy shift towards the field of oncology and deprioritization of preclinical vaccine programs, we are building additional assets in
therapeutics, diagnostics, and clinician services for oncology. ENTADFI will become the inaugural therapeutic drug in the Company’s
expanding portfolio of oncology therapeutics once launched.
Proclarix is an easy-to-use next generation protein-based
blood test that can be done with the same sample as a patient’s regular Prostate-Specific Antigen (“PSA”) test. The
PSA test is a well-established prostate specific marker that measures the concentration of PSA molecules in a blood sample. A high level
of PSA can be a sign of prostate cancer. However, PSA levels can also be elevated for many other reasons including infections, prostate
stimulation, vigorous exercise or even certain medications. PSA results can be confusing for many patients and even physicians. It is
estimated over 50% of biopsies with elevated PSA are negative or clinically insignificant resulting in an overdiagnosis and overtreatment
that impacts the physician’s routine, our healthcare system, and the quality of patients’ lives. Proclarix helps doctors and
patients with unclear PSA test results through the use of our proprietary Proclarix Risk Score which delivers clear and immediate diagnostic
support for further treatment decisions. No additional intervention is required and results are available quickly. Local diagnostic laboratories
can easily add this affordable multiparametric test to their existing infrastructure.
Prior to the acquisition of ENTADFI, we managed
one distinct business segment, which was research and development. Beginning in the second quarter of 2023, as a result of the acquisition
of ENTADFI, for which we are working towards commercial launch, we operated in two business segments: research and development and commercial.
During the third quarter of 2023, we deprioritized our vaccine discovery and development programs, and accordingly, we now operate in
one segment: commercial. Our recent acquisition of Proteomedix during the fourth quarter of 2023 and its related diagnostic product Proclarix
was determined to be within our commercial segment. The research and development segment was our historical business, and was dedicated
to the research and development of various vaccines to prevent infectious diseases. The commercial segment was new in the second quarter
of 2023 and is dedicated to the commercialization of our products approved for sale, namely ENTADFI in the U.S. and Proclarix in Europe.
Recent key developments affecting our business
include:
| |
● |
Announced Shift in Business Strategy to Focus on the Field of
Oncology: On October 30, 2023, in a letter to stockholders, former President and CEO, Dr. Neil Campbell, announced that
the Company intends to shift its focus toward building a foundation of therapeutic, diagnostic, and service products in the field of
oncology. The Company’s previous activities in acquiring assets from WraSer and Xspire Pharma, including certain commercial
relationships intended for the marketing and sale of these assets, were reassessed and it was decided that they would not meet the Company’s requirements for creating greater shareholder value.
Additionally, the Company conducted a strategic and tactical assessment of its preclinical vaccine programs and, considering the
immense amount of time and resources needed to pursue these programs as well as evolving market dynamics, these programs have been
deprioritized. The Company believes that the strategic shift in business strategy towards the field of oncology, as well as pursuing
the launch of ENTADFI in 2024, will enhance stockholder value and enable the Company to provide leading-edge therapeutics,
diagnostics, and services to clinicians, patients, and caregivers. |
| |
● |
Acquired a Commercial Stage Oncology Company: On December 15, 2023, the Company closed its acquisition of Proteomedix and introduced Onconetix, Inc. as a new name for the combined company. The closing of the acquisition of Proteomedix for all stock consideration provides Proteomedix shareholders with an initial 16.4% ownership stake of Onconetix, and Series B Preferred Stock convertible into 269,672,900 shares of Onconetix
Common Stock, subject to Onconetix stockholder approval of the same. |
| |
● |
Signed Various Agreements to Support the Commercial Launch of ENTADFI: Throughout the third quarter of 2023, the Company signed several agreements and established key relationships to support the commercial launch of ENTADFI. These agreements include the following: |
| |
● |
Marketing and Advertising Support: In July 2023, the Company signed a Master Services Agreement with bfw Advertising Inc. (“bfw”) to generate marketing and advertising material for Onconetix’s commercial stage drug portfolio. Bfw will work to increase awareness for Onconetix’s commercial products through patient-facing materials, website updates, social ads, targeted provider engagement, as well as materials to support Onconetix’s sales team, among other services. |
| |
● |
Healthcare Payer Coverage Support: In July 2023, Onconetix signed an agreement with Advantage Point Solutions, LLC (“APS”) to support Onconetix’s market access strategy for its commercial pharmaceutical portfolio. APS will support market access for ENTADFI, including assistance in formulary negotiations with key healthcare payers and pharmacy benefit managers in the commercial and government sectors. With its robust network of relationships, APS helps commercial stage pharmaceutical companies build long-term relationships with payers with the goal of maximizing access and reimbursement for approved pharmaceutical products. APS also has decades of experience advising companies on product launches across a broad spectrum of therapeutic areas. |
| |
● |
Telemedicine Channel: In July 2023, Onconetix signed an agreement with UpScriptHealth to generate a robust, online telemedicine platform to distribute ENTADFI. Through this platform, UpScriptHealth will help support patients with benign prostatic hyperplasia throughout the prescription and coverage process, as well as provide eligible patients access to ENTADFI mailed directly to their homes. |
| |
● |
Entered into Distribution Agreement: On September 21, 2023, the Company entered into an Exclusive Distribution Agreement to engage Cardinal Health 105, LLC as its exclusive third-party logistics distribution agent for sales of all of the Company’s commercial assets. |
| |
● |
Granted Pharmaceutical Wholesaler License in Ohio and Tennessee: The Ohio State Board of Pharmacy and the Tennessee State Board of Pharmacy, in July 2023 and September 2023, respectively, granted Onconetix a license to operate as a pharmaceutical wholesaler. These licenses allow Onconetix to conduct business in the States of Ohio and Tennessee. |
Since our inception in October 2018 until April
2023, when we acquired ENTADFI, we devoted substantially all of our resources to performing research and development, undertaking preclinical
studies and enabling manufacturing activities in support of our product development efforts, hiring personnel, acquiring and developing
our technology and now deprioritized vaccine candidates, organizing and staffing our company, performing business planning, establishing
our intellectual property portfolio and raising capital to support and expand such activities.
We are currently focusing our efforts on (i) building
out our commercial capabilities to launch ENTADFI in the marketplace and (ii) commercializing Proclarix.
Given ENTADFI is currently
FDA-approved for sale in the United States and Proclarix is CE-marked for sale in the European Union, we expect to generate revenue from
sales of ENTADFI and Proclarix in the near term. Although we anticipate these sales to offset some expenses relating to commercial
scale up and development, we expect our expenses will increase substantially in connection with our ongoing activities, as we:
| |
● |
commercialize and/or launch ENTADFI and Proclarix, and other commercial-stage
products; |
| |
● |
hire additional personnel; |
| |
● |
operate as a public company; and |
| |
● |
obtain, maintain, expand and protect our intellectual property portfolio. |
We rely and will continue to rely on third parties
for the manufacturing of ENTADFI and Proclarix. We have no internal manufacturing capabilities, and we will continue to rely on third
parties, of which the main suppliers are single-source suppliers, for commercial products.
As we have a product in
the commercial stage, we are seeking to build a robust and efficient commercial team to accommodate this development. This includes appropriate
personnel and third-party relationships and contracts to execute our commercialization strategy. We also expect to incur significant
commercialization expenses related to marketing, manufacturing and distribution for those products.
We do not have any products approved for sale,
aside from ENTADFI, from which we have not generated any revenue from product sales, and Proclarix, from which we have generated only
minimal amounts of revenue since its acquisition. To date, we have financed our operations primarily with proceeds from our sale of preferred
securities to seed investors, the IPO, the 2022 Private Placements, the proceeds received from a warrant exercise in August 2023, and
the proceeds received from the issuance of debt in January 2024. We will continue to require significant additional capital to commercialize
ENTADFI and Proclarix and fund operations for the foreseeable future. Accordingly, until such time as we can generate significant
revenue, if ever, we expect to finance our cash needs through public or private equity or debt financings, third-party (including government)
funding and to rely on third-party resources for marketing and distribution arrangements, as well as other collaborations, strategic
alliances and licensing arrangements, or any combination of these approaches, to support our operations.
We have incurred net losses since inception and
expect to continue to incur net losses in the foreseeable future. Our net losses may fluctuate significantly from quarter-to-quarter and
year-to-year, depending in large part on the timing of our preclinical studies, clinical trials and manufacturing activities, our expenditures
on other research and development activities and commercialization activities. As of September 30, 2023, the Company had a working capital
deficit of approximately $8.1 million and an accumulated deficit of approximately $34.4 million. We will need to raise additional capital
within the next 12 months to sustain operations. In addition, if Stockholder Approval is not obtained by January 1, 2025, the Company
may be obligated to cash settle the Series B Preferred Stock.
Until we generate revenue sufficient to support
self-sustaining cash flows, if ever, we will need to raise additional capital to fund our continued operations, including our product
development and commercialization activities related to our current and future products. There can be no assurance that additional capital
will be available to us on acceptable terms, or at all, or that we will ever generate revenue sufficient to provide self-sustaining cash
flows. These circumstances raise substantial doubt about our ability to continue as a going concern. The September 30, 2023 unaudited
condensed financial statements of Onconetix included in this proxy statement do not include any adjustment that might be necessary if
the Company is unable to continue as a going concern.
Because of the numerous risks and uncertainties
associated with our business, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve
or maintain profitability. Additionally, even if we are able to generate revenue from ENTADFI or Proclarix, we may not become profitable.
If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations
at planned levels and may be forced to reduce our operations.
Management and Board Changes
Effective as of August 16, 2023, Joseph Hernandez
resigned as Chairman, Chief Executive Officer, and a member of the Board (the “Board”) of the Company.
Effective August 16, 2023, the Board appointed
Jon Garfield, the Company’s former Chief Financial Officer, to serve as the Company’s interim principal executive officer.
Effective as of October 4, 2023, Jon Garfield resigned as Chief Financial Officer and interim principal executive officer of the Company.
The Company and Mr. Garfield entered into a separation agreement, which provided for two months of severance payment.
Effective as of September 2, 2023, Vuk Jeremic
resigned as a member of the Board of the Company as well as from his positions as a member of the Compensation Committee and Nominating
and Corporate Governance Committee of the Board. Mr. Jeremic’s departure was not the result of any disagreement with management
or the Board on any matter relating to the Company’s operations, policies or practices.
On October 4, 2023, the Company appointed Dr.
Neil Campbell, 63, as President and Chief Executive Officer of the Company and as a member of the Board.
In connection with Dr. Campbell’s appointment,
the Company and Dr. Campbell entered into an employment agreement (the “Campbell Employment Agreement”), pursuant to which
Dr. Campbell would serve as President and Chief Executive Officer of the Company and was paid a signing bonus of $75,000 and an annual
base salary of $475,000. Pursuant to the Campbell Employment Agreement, Dr. Campbell was granted a long-term equity incentive grant
in the form of an option to purchase 532,326 shares of the Company’s common stock. Such award was to vest in quarterly increments
over a period of three years, subject to Dr. Campbell’s continued employment by the Company on the applicable vesting date. Dr.
Campbell’s option grant has an exercise price per share equal to $0.4305, which was the closing price of the Company’s common
stock on Nasdaq on the grant date.
On October 4, 2023, the Company also appointed
Bruce Harmon, 65, as Chief Financial Officer of the Company, effective immediately.
In connection with Mr. Harmon’s appointment,
the Company and Mr. Harmon entered into an employment agreement (the “Harmon Employment Agreement”), pursuant to which Mr. Harmon will
serve as Chief Financial Officer of the Company and will be paid an annual base salary of $325,000. In addition, Mr. Harmon is
entitled to receive, subject to employment by the Company on the applicable date of bonus payout, an annual target discretionary bonus
of up to 30% of his annual base salary, payable at the discretion of the Compensation Committee of the Board. Pursuant to the Harmon Employment
Agreement, Mr. Harmon is also eligible to receive healthcare benefits as may be provided from time to time by the Company to
its employees generally, and to receive paid time off annually. Pursuant to the Harmon Employment Agreement, Mr. Harmon was
granted a long-term equity incentive grant in the form of an option to purchase 177,442 shares of the Company’s common stock. Such
award vests in quarterly increments over a period of three years, subject to Mr. Harmon’s continued employment by the Company
on the applicable vesting date. Mr. Harmon’s option grant has an exercise price per share equal to $0.4305, which was the closing
price of the Company’s common stock on Nasdaq on the grant date.
In connection with the acquisition of Proteomedix,
Christian Brühlmann was appointed as Chief Strategy Officer and Dr. Ralph Schiess was appointed as Chief Science Officer. Mr. Brühlmann
co-founded Proteomedix and served as its Chief Financial and Operations Officer from March 2010 until November 2018. Beginning in December
2018, Mr. Brühlmann served as Proteomedix’s Chief Business Officer. Dr. Schiess co-founded Proteomedix in March 2010 and served
as its Chief Executive Officer from its inception until December 2019. Dr. Schiess then served as Proteomedix’s Chief Scientific
Officer from January 2020 to May 2023. Dr. Schiess returned to his role as Chief Executive Officer in June 2023.
On and effective December
21, 2023, Erin Henderson resigned as Chief Business Officer to pursue other opportunities. On January 17, 2024, the Company entered into
a Separation Agreement and General Release with Ms. Henderson, pursuant to which the Company agreed to engage The Aetos Group, a management
consulting company founded and managed by Ms. Henderson (“Aetos”), to perform certain consulting services for the Company.
On January 17, 2024, the Company entered into a Consulting Agreement with Aetos, pursuant to which Aetos will provide consulting services
to the Company until April 25, 2024, and receive a monthly fee of approximately $27,083.
On and effective January 10, 2024, Dr. Neil Campbell
resigned as Chief Executive Officer, President and Director. The Company entered into a Release of Claims with Dr. Campbell, pursuant
to which Dr. Campbell will receive a one-time severance payment of $158,333. On January 12, 2024, the Board appointed Dr. Ralph Schiess,
the Company’s Chief Science Officer, to serve as the Company’s Interim Chief Executive Officer. As Interim Chief Executive
Officer, Dr. Schiess shall have general supervision and direction of the business and affairs of the Company.
Recent Acquisitions
Proteomedix
See the section entitled “Description
of the PMX Transaction and Related Financing” and “Description of Proteomedix’s Business.”
ENTADFI
On April 19, 2023, the Company entered into
an asset purchase agreement with Veru (the “Veru APA”). Pursuant to, and subject to the terms and conditions of, the Veru
APA, the Company purchased substantially all of the assets related to Veru’s ENTADFI business and assumed certain liabilities
of Veru. The Transaction closed on April 19, 2023.
The Company purchased substantially all of Veru’s
assets, rights and property related to ENTADFI for a total possible consideration of $100.0 million (as described below). The acquisition
of ENTADFI capitalizes on the demonstrable success of the FDA-approved drug ENTADFI for treating benign prostatic hyperplasia and
counteracting negative sexual side effects seen in men on alternative BPH therapies.
Pursuant to the terms of the Veru APA, the Company
agreed to provide Veru with initial consideration totaling $20.0 million, consisting of (i) $6.0 million paid upon the closing of the
Transaction, (ii) an additional $4.0 million in the form of a non-interest bearing note payable due on September 30, 2023, and (iii) an
additional $10.0 million in the form of two equal (i.e. each for $5.0 million) non-interest bearing notes payable, each due on April 19,
2024 and September 30, 2024. On September 29, 2023, the Company entered into an amendment (the “Amendment”) of the Veru APA.
Pursuant to the Veru Amendment, the $4.0 million note payable originally due on September 30, 2023, was deemed paid and fully satisfied
upon (1) the payment to Veru of $1 million in immediately available funds on September 29, 2023, and (2) the issuance to Veru by October
3, 2023 of 3,000 shares of Series A Preferred Stock of the Company.
The terms of the Series A Preferred Stock are
set forth in the Certificate of Designations, which was filed with the State of Delaware on September 29, 2023. Pursuant to the Certificate
of Designations, each share of Series A Preferred Stock will convert one year from the date of issuance of the Series A Preferred Stock
into that number of shares of the Company’s common stock determined by dividing the Stated Value (as defined in the Certificate
of Designations) of $1,000 per share by the Conversion Price (as defined in the Certificate of Designations) of $0.5254 per share, subject
to adjustment as provided in the Certificate of Designations, subject to certain stockholder approval limitations. The Series A Preferred
Stock is entitled to share ratably in any dividends paid on the Company’s common stock (on an as-if-converted-to-common-stock basis),
has no voting rights except as to certain significant matters specified in the Certificate of Designations, and has a liquidation preference
equal to the Stated Value of $1,000 per share plus any accrued but unpaid dividends thereon. The Series A Preferred Stock is redeemable
in whole or in part at the Company’s option at any time. The Certificate of Designations authorized the issuance of up to 10,000
shares of Series A Preferred Stock.
The Series A Preferred Stock issued to Seller
is initially convertible, in the aggregate, into approximately 5,709,935 shares of the Company’s common stock, subject to adjustment
and certain stockholder approval limitations specified in the Certificate of Designations. Pursuant to the Veru Amendment, the Company
agreed to use commercially reasonable efforts to obtain such stockholder approval by December 31, 2023. The Company also agreed to include
the shares of common stock issuable upon conversion of the Series A Preferred Stock in the next resale registration statement filed with
the SEC.
Additionally, the terms of the Veru APA require
the Company to pay Veru up to an additional $80.0 million based on the Company’s net sales from the ENTADFI business after
closing. The Milestone Payments are payable as follows: (i) $10.0 million is payable if the Company’s annual net sales from
the ENTADFI business equal or exceed $100.0 million, (ii) $20.0 million is payable if the Company’s annual net sales from
the ENTADFI business equal or exceed $200.0 million, and (3) $50.0 million is payable if annual net sales from the ENTADFI business
equal or exceed $500.0 million. No more than one Milestone Payment shall be made for the achievement of each net sales milestone. There
can be no assurance that the net sales milestones for payment of any of the Milestone Payments will be reached.
Furthermore, in connection with the Transaction,
the Company assumed royalty and milestone obligations under an asset purchase agreement for tadalafil-finasteride combination entered
into by Veru and Camargo Pharmaceutical Services, LLC on December 11, 2017. The Camargo Obligations assumed by the Company include a 6%
royalty on all sales of tadalafil-finasteride and sales milestone payments of up to $22.5 million as follows: (i) $5.0 million is
payable upon the first time the Company achieves net sales from ENTADFI of $100.0 million during a calendar year, (ii) $7.5 million
is payable upon the first time the Company achieves net sales from ENTADFI of $200.0 million during a calendar year, and (3) $10.0 million
is payable upon the first time the Company achieves net sales from ENTADFI of $300.0 million during a calendar year.
WraSer
On June 13, 2023 (the “Execution Date”),
the Company entered into an asset purchase agreement with WraSer, LLC, a Mississippi limited liability company, Xspire Pharma,
LLC, a Mississippi limited liability company (collectively, the “WraSer Seller”), and Legacy-Xspire Holdings, LLC, a
Delaware limited liability company and the parent company of the WraSer Seller (“Parent”) (the “WraSer APA”).
Pursuant to, and subject to the terms and conditions of, the WraSer APA, on the WraSer Closing Date (as defined below) the Company will
purchase six FDA-approved pharmaceutical assets across several indications, including cardiology, otic infections, and pain management
(the “WraSer Assets”).
Under the terms of the WraSer APA, the Company
will purchase the WraSer Assets for (i) $3.5 million in cash at signing of the WraSer APA (the “Signing Cash”); (ii) $4.5
million in cash on the later of (x) 90 days after the signing of the WraSer APA or (y) the date that all closing conditions under the
WraSer APA are met or otherwise waived (the “WraSer Closing Date”); (iii) 1.0 million shares of the Company’s common
stock (the “Closing Shares”) issuable on the WraSer Closing Date, and (iv) $500,000 in cash one year from the WraSer Closing
Date. The closing of the transaction is subject to certain customary closing conditions and the delivery to the Company of financial statements
of WraSer Seller and Parent for the fiscal years ended December 31, 2022 and 2021 audited by a qualified auditor reasonably acceptable
to the Company.
Within 90 days of the WraSer Closing Date, the
Company will use its best efforts to file with the SEC, (at its sole cost and expense,) a registration statement to register on Form S-3
registering under the Securities Act, the resale of the Closing Shares and will use its best efforts to have the registration statement
declared effective as soon as practicable after filing.
In conjunction with the WraSer APA, the Company
and the WraSer Seller entered into a Management Services Agreement (the “MSA”) on the Execution Date. Pursuant to the terms
of the MSA, the Company was to act as the manager of the WraSer Seller’s business during the period between the Execution Date and
WraSer Closing Date. During this period, the Company was to make advances to WraSer, if needed to sustain operations. The Company’s
involvement as manager of the WraSer Seller’s business ended when WraSer filed for relief under chapter 11 of the U.S. Bankruptcy
Code in the Bankruptcy Court (see below). If, on the WraSer Closing Date, the WraSer Seller’s cash balance is in excess of the target
amount specified in the MSA of $1.1 million (the “Cash Target”), the Company was to apply that excess to the $4.5 million
cash payment due upon closing. Conversely, if there is a shortfall, the Company would have been required to remit the difference to the
WraSer Seller over time. Specifically, as the Company collected accounts receivable generated after the WraSer Closing Date, the Company
would have been required to remit 50% of the collections to the WraSer Seller until the shortfall is paid in full. The MSA terminates
on the WraSer Closing Date.
The WraSer APA can be terminated prior to closing
as follows (i) upon agreement with all parties; (ii) upon breach of contract of either party, uncured within 20 days of notice. If the
WraSer APA is terminated upon agreement with all parties or upon uncured breach of contract by the WraSer Seller, the initial $3.5 million
payment is retained by the WraSer Sellers. If it is determined that there is an uncured breach of contract by the WraSer Seller, and the
WraSer APA is terminated, the Company will have an unsecured claim against WraSer for the $3.5 million payment made by the Company upon
execution of the WraSer APA. The closing of the Transaction is subject to various closing conditions, including submission of the FDA
transfer documentation to transfer ownership of the acquired product regulatory approvals to the Company.
On September 26, 2023, WraSer and its affiliates
filed for relief under chapter 11 of the U.S. Bankruptcy Code in the Bankruptcy Court.
On October 4, 2023, the parties agreed to amend
the WraSer APA. Shortly after its bankruptcy filing, WraSer filed a motion seeking approval of the WraSer APA as amended. The amendment,
among other things, eliminates the $500,000 post-closing payment due June 13, 2024 and staggers the $4.5 million cash payment that the
Company would otherwise have to pay at closing to: (i) $2.2 million to be paid at closing, (ii) $2.3 million, to be paid in monthly installments
of $150,000 commencing January 2024 (the “Post-Closing Payment”) and (iii) 789 shares of Series A Preferred Stock to be paid
at closing. The amendment also reduced the number of products we were acquiring by excluding pain medications and including only (i) Ciprofloxacin
0.3% and Fluocinolone 0.025% Otic Solution, under the trademark OTOVEL and its Authorized Generic Version approved under US FDA NDA No.
208251, (ii) Ciprofloxacin 0.2% Otic solution, under the trademark CETRAXAL, and (iii) Vorapaxar Sulfate tablets under the trademark Zontivity
approved under US FDA NDA N204886.
In October 2023, WraSer alerted us that its sole
manufacturer for the active pharmaceutical ingredient (“API”) for Zontivity, the key driver for the WraSer acquisition, would
no longer manufacture the API for Zontivity. We believe that this development constituted a Material Adverse Effect under the APA enabling
us to terminate the APA and MSA. On October 20, 2023, we filed a motion for relief from the automatic stay in the Bankruptcy Court to
exercise our termination rights under the WraSer APA, as amended. On December 18, 2023, the Bankruptcy Court entered an Agreed Order lifting
the automatic stay to enable us to exercise our rights to terminate the APA and the MSA without prejudice to the parties’ respective
rights, remedies, claims and defenses they had against one another under the APA and MSA. On December 21, 2023, we filed a Notice with
the Bankruptcy Court terminating the APA and MSA. WraSer has advised us that it does not believe that a Material Adverse Event occurred.
Due to the WraSer bankruptcy filing and our status as an unsecured creditor of WraSer, it is also unlikely that we will recover the $3.5
million Signing Cash or any costs and resources in connection with services provided by the Company under the WraSer MSA.
Agreement with Cardinal Health
On September 21, 2023, the Company entered into
an Exclusive Distribution Agreement (the “Exclusive Distribution Agreement”), effective as of September 20, 2023 (the “Effective
Date”), with Cardinal Health 105, LLC (“Cardinal Health”). Pursuant to, and subject to the terms and conditions of,
the Exclusive Distribution Agreement, the Company engaged Cardinal Health as its exclusive third-party logistics distribution agent for
sales of all of the Company’s commercial assets. The term of the Distribution Agreement is three years from the Effective Date and
automatically renews for additional terms of one year each unless terminated pursuant to the terms of the Exclusive Distribution Agreement.
Under the terms of the Exclusive Distribution Agreement, the Company must pay to Cardinal Health a one-time start-up fee of $15,500, and
upon launch of ENTADFI, a monthly account management fee of $7,000, and other fees for various services, including post-launch program
implementation, information systems, warehouse operations, and financial services.
Corporate Name Change and Amendment to Bylaws
On April 21, 2023, the Company filed an amendment
to its Articles of Incorporation with the Secretary of State of Delaware to change its corporate name from “Blue Water Vaccines,
Inc.” to “Blue Water Biotech, Inc.”. The name change was effective as of April 21, 2023. In connection with the name
change, the Company amended the Company’s bylaws to reflect the corporate name Blue Water Biotech, Inc., also effective on April
21, 2023.
On December 15, 2023, the Company filed an amendment
to its certificate of incorporation with the Secretary of State of Delaware to change its corporate name from “Blue Water Biotech,
Inc.” to “Onconetix, Inc.”
In connection with the name change, the Company
also amended the Company’s bylaws to reflect the new corporate name.
On May 31, 2023, the Board amended the Company’s
bylaws to reduce the quorum requirement at meetings of the Company’s stockholders from a majority of the voting power of the outstanding
shares of stock of the Company entitled to vote, to one-third of the voting power of the outstanding shares of stock of the Company entitled
to vote, effective immediately. No other changes were made to the bylaws.
Warrant Inducement
On July 31, 2023, the Company
entered into a common stock preferred investment options exercise inducement offer letter (the “Inducement Letter”) with
a certain holder (the “Holder”) of existing preferred investment options to purchase shares of the Company’s common
stock at the original exercise price of $2.546 per share, issued on August 11, 2022 (the “Existing PIOs”). Pursuant
to the Inducement Letter, the Holder agreed to exercise for cash its Existing PIOs to purchase an aggregate of 2,486,214 shares of the
Company’s common stock, at a reduced exercise price of $1.09 per share, in exchange for the Company’s agreement to issue
new preferred investment options (“Inducement PIOs”) to purchase up to 4,972,428 shares of the Company’s common stock.
The Inducement PIOs have substantially the same terms as the Existing PIOs, except that the Inducement PIOs have an exercise price of
$1.09 per share and a term of five (5) years from the date of issuance. On August 2, 2023, the transaction (the “Warrant Inducement”)
closed. The Company received aggregate net proceeds of approximately $2.3 million from the Warrant Inducement, after deducting placement
agent fees and other offering expenses payable by the Company.
The Company engaged H.C. Wainwright &
Co. LLC (“Wainwright”) to act as its placement agent in connection with the Warrant Inducement and paid Wainwright a
cash fee equal to 7.5% of the gross proceeds received from the exercise of the Existing PIOs as well as a management fee equal to
1.0% of the gross proceeds from the exercise of the Existing PIOs. The Company also agreed to reimburse Wainwright for its expenses
in connection with the exercise of the Existing PIOs and the issuance of the Inducement PIOs, $50,000 for fees and expenses of legal
counsel and other out-of-pocket expenses and agreed to pay Wainwright for non-accountable expenses in the amount of $35,000 and a
clearing fee of $15,950. In addition, the exercise for cash of the Existing PIOs triggered the issuance to Wainwright or its
designees of warrants to purchase 149,173 shares of common stock, which have the same terms as the Inducement PIOs except for an
exercise price equal to $1.3625 per share. The Company also agreed to pay Wainwright a cash fee of 7.5% of any gross proceeds that
the Company may receive from the exercise for cash of the Inducement PIOs and issue warrants to Wainwright or its designees upon any
exercise for cash of the Inducement PIOs, that number of shares of common stock equal to 6.0% of the aggregate number of such shares
of common stock underlying any Inducement PIOs that have been exercised, also with an exercise price of $1.3625. The maximum cash
payable under this provision is $406,496 and the maximum number of warrants issuable under this provision is 298,346.
Nasdaq Compliance
On September 18, 2023, we received notice from
Nasdaq staff indicating that, based upon the closing bid price of the Common Stock for the prior 30 consecutive business days, we were
not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq, as set forth
in the Bid Price Rule. We have 180 days from September 18, 2023, or through March 16, 2024, to regain compliance with the Bid Price Rule.
On August 22, 2023, we received a notice from
Nasdaq that we were not in compliance with Nasdaq Listing Rule 5250(c)(1), which requires listed companies to timely file all required
periodic financial reports with the SEC, given our failure to timely file our quarterly report on Form 10-Q for the quarter ended June
30, 2023. On October 20, 2023, we filed our Form 10-Q for the period ended June 30, 2023, and on November 1, 2023, we announced that
we had regained compliance with Nasdaq Listing Rule 5250(c)(1).
DESCRIPTION
OF PROTEOMEDIX’S BUSINESS
Overview
Founded in 2010, Proteomedix develops, markets
and sells non-invasive diagnostic tests accompanied by decision support systems to detect and assess the prognosis of cancer. Proteomedix’s
lead product, Proclarix®, is an in vitro diagnostic test for prostate cancer. Proteomedix is working to address all stages
in cancer management by developing tools for both more accurate detection and more efficient treatment of cancer including (i) diagnostic
tests to early detect and define the stage of cancer; (ii) prognostic tools for the identification of patients with aggressive disease;
and (iii) stratification biomarkers to match patients with therapies that are more likely to be safe and effective.
Currently, prostate cancer stands as the most
prevalent and second most fatal cancer type affecting men. The widespread utilization of prostate-specific antigen (“PSA”)
screening since it began broadly available in the 1980s helped reduce the occurrence of metastatic prostate cancers by over half, but
also led to a notable increase in overdiagnosis, sometimes resulting in excessive treatment, severe complications, and potential psychological
distress. Worldwide, approximately 100 million PSA tests for prostate cancer diagnosis are conducted annually, with around 10% yielding
heightened PSA readings in a so-called diagnostic “grey zone” where the results of the PSA test are inconclusive. Consequently,
there exists a considerable population of men each year who are notified of their heightened risk for prostate cancer based on elevated
PSA levels, with limited options beyond invasive needle biopsies for managing their cancer risk.
Proclarix addresses the unsolved problem of
prostate cancer overdiagnosis leading to negative prostate biopsies that increase costs for the healthcare system and uncertainty for
patients. Proclarix is approved for sale in the European Union under the In Vitro Diagnostic Regulation (“IVDR”).
Clinical studies have confirmed that Proclarix accurately identifies clinically significant prostate cancer through a risk score derived
from a clinical decision support system and could help avoid many unneeded biopsies. Proclarix as a clinical support system is designed
to aggregate multimodal information in an effort to develop a patient centric diagnostic approach. We intend to add more information
to the risk score in the future, such as other biomarkers or magnetic resonance imaging (“MRI”) data, to provide an even
more powerful tool to guide the patient’s diagnostic journey. The markers and the bioinformatics algorithm used are patent protected.
The guidelines of the European Association of
Urology (“EAU”) and of the American Urological Association/Society of Urologic Oncology (“AUA/SUO”) both recommend
the use of blood-based biomarker tests, such as Proclarix, to aid in the early detection and evaluation of prostate cancer. Proclarix
can be performed in any laboratory using standard equipment. In Europe, Proteomedix has begun marketing Proclarix to pilot laboratories
in selected markets that are open to self-pay to show initial adoption. In the United States, the development and commercialization of
Proclarix is being pursued by Laboratory Corporation of America Holdings, more commonly called Labcorp, pursuant to an exclusive license
agreement entered into between Proteomedix and Labcorp in 2023.
Technology and Intellectual Property
Proteomedix’s biomarkers were discovered
using a genetics-guided discovery approach focusing on the PI3K/PTEN cancer pathway that plays a dominant role in prostate cancer development.
Applying proteomics technology to a disease-relevant mouse model allowed the identification of proteins specifically linked to the molecular
cause of prostate cancer. The biomarkers and the bioinformatics algorithm used in Proclarix are protected by issued and pending patents
in Europe, the United States and other countries.
Team
Proteomedix was founded by a multi-disciplinary
group of scientists and clinicians that include Prof. emeritus Dr. med. Thomas Cerny, president of the Swiss Cancer Research Foundation,
Prof. Ruedi Aebersold, a pioneer in proteomics technology development, and the late Prof. Wilhelm Krek, a leader in cancer research. The
company’s management consists of Dr. Ralph Schiess (Chief Executive Officer), who developed the biomarker technology, and Christian
Brühlmann (Chief Business Officer), with seasoned experience in finance, business development and product management.
Market Potential and Competitive Advantages
The PSA test represents the current standard
of care in prostate cancer diagnosis. It accurately identifies individuals with no sign of disease. Approximately 10% of all men have
elevated PSA levels, commonly referred to as the diagnostic “grey zone”, of which only 20-40% present clinically with cancer.
Proclarix is intended for use in diagnosing these patients where it is difficult to decide if a biopsy is necessary to verify a potential
clinically significant cancer diagnosis. The high unmet need for improved patient stratification or diagnostic triage in this segment
is addressed only by a few tests. Compared to those tests Proclarix has important competitive advantages: (i) it shows superior clinical
performance, (ii) it is blood-based and therefore minimally invasive and (iii) it is highly reproducible in comparison to e.g., urine-based
tests. The use of Proclarix does not require prior prostate massage. Samples are stable and can be shipped at ambient temperature. Proclarix
has a high accuracy and negative predictive value (NPV) and is easy to automate on equipment readily available as well as adaptable to
current laboratory practice and thus clinical routine.
Commercialization Strategy
Proteomedix has started marketing Proclarix to
pilot laboratories in selected markets in Europe that are open to self-pay to show initial adoption, while pursuing reimbursement from
public and private payors in key European markets to secure broad adoption in the longer term. The market introduction of Proclarix has
followed a two-phased approach: first a market preparation phase in which we reach out to key opinion leaders in selected European countries
to solicit their support for Proclarix, followed by a market development phase where we begin commercializing Proclarix in those markets
with focused marketing and sales activities to urologists and general practitioners. We intend to secure access to testing through partnerships
with reference diagnostic labs. We have initiated outreach to commercial laboratories and hospital laboratories that are routinely serving
study sites and academic collaboration partners, and have established pilots with laboratories in Switzerland, Germany, Italy and the
United Kingdom.
In the United States, Proteomedix entered
into an exclusive partnership with Labcorp in 2023 pursuant to which Labcorp has the exclusive right to develop and commercialize Proclarix,
and other products developed by Labcorp using Proteomedix’s intellectual property covered by the license, in the United States
for identification, screening, staging, predisposition, diagnosis, prognosis, monitoring, prevention or treatment selection with respect
to prostate cancer. In consideration for granting Labcorp an exclusive license, Proteomedix received an upfront license fee and is entitled
to royalty and milestone payments based upon sales of licensed products in the United States. Labcorp is wholly responsible for the cost
of research, development and commercialization of licensed products in the United States but has the right to offset a portion of those
costs against future royalty and milestone payments otherwise due to Proteomedix.
Market Opportunity
The
worldwide market for in vitro diagnostic (“IVD”) products was valued at $117.8
billion in 2022. Europe and North America are the largest markets, followed by Asia, mainly
Japan and China, according to MarketsandMarkets.
Proclarix, the first diagnostic product of Proteomedix,
is addressing unmet medical needs related to prostate cancer, which is the second most frequently diagnosed cancer in men, with an estimated
1.4 million new cases and more than 395,000 deaths worldwide in 2020, according to World Cancer Research Fund International. Worldwide,
approximately 150 million PSA tests are carried out per year of which around two-thirds are used for prostate cancer diagnosis in a screening
setting mostly ordered by general practitioners. Approximately 8.5 million patients fall in the diagnostic grey zone. The target application
of Proclarix is to re-measure samples in this diagnostic grey zone.
About two-thirds of prostate cancer diagnoses
occur in countries ranking very high in the Human Development Index, where only 18% of the world’s male population resides, according
to the American Cancer Society. This underscores a significant market demand for improved diagnostic tools, especially in regions with
robust healthcare infrastructure where early detection and treatment are paramount. Our innovative test aims to meet this demand by offering
enhanced accuracy, accessibility, and efficiency, positioning it as a valuable asset in the fight against prostate cancer while also presenting
lucrative commercial opportunities for stakeholders. Figure 1 lists the market potential for the different regions.
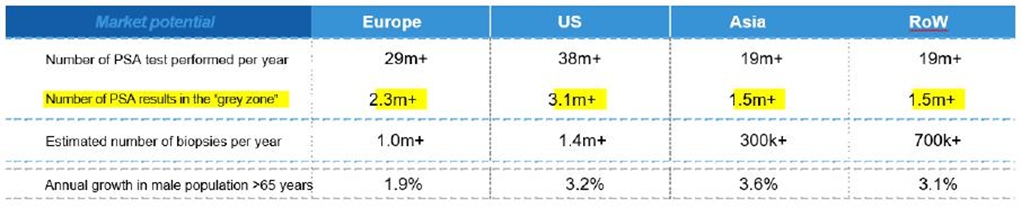
Figure 1: Overview of Global Market Potential.
Currently, standard prostate cancer screening
combines a digital rectal exam (“DRE”) with the measurement of PSA. PSA is not a highly cancer specific marker, meaning it
picks up many benign conditions of raised PSA levels in the blood - such as clinically not significant enlargement of the prostate or
inflammation. The consequences are prostate cancer overdiagnosis, leading to unnecessary prostate biopsies. It is currently estimated
that more than 60% of men that undergo a biopsy have no clinically significant prostate cancer, but due to the biopsy become exposed to
potential side effects such as infections, bleeding and incontinence.
The use of MRI for the diagnosis of prostate
cancer has been rapidly adopted during the last decade. There is clinical evidence that MRI allows clinicians to verify diagnosis and
improve localization, risk stratification and staging of clinically significant prostate cancer over other methods. MRI-guided biopsy
has a higher accuracy than ultrasound-guided biopsy. However, MRI-based diagnosis of prostate cancer is hampered by the relatively high
costs of US$415 – US$900 and limited availability. Still, up to one-third of MRIs are inconclusive. Thus, there is a clear need
for an improved non-invasive diagnostic test with higher specificity for clinically significant prostate cancer to aid in selecting patients
undergoing MRI, MRI-guided biopsy, and biopsy.
Proper classification in clinically significant
cancer and non-significant type or non-cancer conditions such as benign prostate hyperplasia is important to prevent overtreatment and
its associated side-effects and costs. Proteomedix is developing diagnostic tools for disease prognosis and monitoring that are essential
for reliable, patient-friendly and cost-effective disease management. Proteomedix’s biomarkers have shown the potential to distinguish
between those prostate cancer patients who are more likely to respond to certain drug-based interventions. With this information, better
choices for drug therapies can be made to maximize the likelihood of efficacious treatment. Proteomedix’s biomarkers could also
aid in clinical drug development.
Our Product Strategy
Business Model
Proteomedix develops novel diagnostic tests
in a highly regulated field. The company’s core competencies include the development of high-quality immunoassays and management
of regulatory affairs. The company’s expertise in immunoassay development is the result of a highly specialized workforce that,
together with an external software development company, developed the proprietary software integrated in the company’s lead IVD
product, Proclarix. The company’s personnel also have extensive experience in implementing and maintaining a state-of-the-art quality
management system to comply with regulatory requirements, including performing clinical studies and managing key opinion leaders (“KOLs”).
The company’s experience and expertise in these fields was obtained by hiring experienced personnel as well as through key advisors.
Proteomedix will initially focus on marketing (especially medical marketing
towards physicians) and direct sales to laboratories in selected countries. Once the geographical focus is expanded to additional countries,
sales will be through a specialized distributor, but Proteomedix will still provide medical marketing and technical customer support to
laboratories that offer the testing service to physicians. Production capabilities will initially not be built up in-house because this
requires substantial investments in production equipment. Instead, manufacturing is outsourced to a contract manufacturing organization
(“CMO”) in Germany. All of the key reagents used in the company’s IVD kits (i.e., antigens and antibodies) are proprietary
and owned exclusively by Proteomedix. These reagents are produced by an independent supplier in Germany and shipped to the CMO for manufacturing
of the IVD kits.
Products and Pipeline
Proteomedix is seeking to develop diagnostic,
prognostic and predictive tools to enable more efficient cancer management at all stages of disease progression. Proteomedix’s tests
use proprietary protein biomarkers to address the limitations in current cancer detection, prognosis, and therapy prediction. In addition,
Decision Support Systems support the clinical decision-making by integrating different inputs in a risk score (see Figure 2).

Figure 2: Product Pipeline
Proclarix
Clinical Decision Support to Reduce Prostate Cancer Overdiagnosis
Proclarix is used to indicate the risk of clinically significant prostate
cancer through a risk score derived from a clinical decision support system (Figure 3). On the reagent side it is comprised of two quantitative
Enzyme-linked Immunosorbent Assays (“ELISA”) that measure the concentration of thrombospondin 1 (“THBS1”) and
cathepsin D (“CTSD”) in human serum. The clinical decision support system is a web-based software running a proprietary algorithm
that integrates the values for THBS1 and CTSD, the patient’s age and total and free PSA levels from third party providers (e.g.,
Roche Diagnostics, Siemens Healthineers) to calculate a risk score.
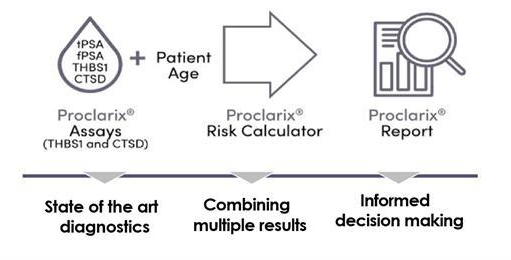
Figure 3: Proclarix: Assays and software algorithm for risk score calculation.
Proclarix is used as an aid in prostate cancer
diagnosis as a second-line test after PSA and DRE testing. It enables a personalized decision for each patient based on objective risk
parameters (4 serum glycoproteins + age) to triage between biopsy or a monitoring approach. Proclarix has been validated and approved
for use in men with elevated total PSA (2.0 to 10.0 ng/mL), a normal DRE not suspicious for cancer and an elevated prostate volume (≥35
mL) (Figure 4). The Proclarix decision support tool returns a risk score that can be used as an aid in discriminating between clinically
significant (grade group 2 or higher [GG2+]) and insignificant prostate cancer or benign prostate disease. The risk score of Proclarix
gives the physician and patient actionable information to confidently make decisions when considering the necessity of a prostate biopsy
which is required for diagnosis of prostate cancer.
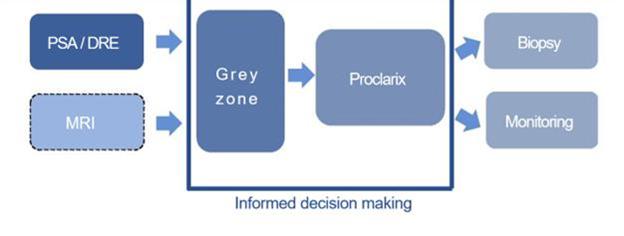
Figure 4: Proclarix: Finding clinically significant prostate
cancer in the diagnostic “grey zone.”
Clinical Studies
Proteomedix’s biomarkers have been tested
in clinical studies including a total of more than 2,000 patient samples from multiple clinical sites, and results have been published
in peer-reviewed journals. We believe these results demonstrate that Proclarix is a valuable test identifying clinically significant prostate
cancer thereby facilitating informed decision making for patients considering a prostate biopsy.
Validation Study. The study leading to
the granting of regulatory approval in Europe included 955 samples collected at two clinical sites, a screening center in Innsbruck, Austria,
as well as a referral center in Hamburg, Germany. The results of this study demonstrated that by using the Proclarix test the burden of
unneeded biopsies could have been lowered by approximately 43% — twice as much compared to clinical comparators percent free PSA
(“%fPSA”) or PSA density. High sensitivity of 90% and a negative predictive value of 95% for clinically significant prostate
cancer indicated that the diagnosis of very few cancers would have been delayed.
PROPOSe Study. The PROPOSe study evaluated the accuracy of Proclarix
in prostate biopsy decision making. Ten clinical sites in Germany, Denmark and Austria prospectively enrolled 457 men presenting for prostate
biopsy. Proclarix detected clinically significant cancer with high sensitivity above 90% and reliably ruled out patients with no or indolent
cancer with a negative predictive value greater than 90%. When the biopsy performed was guided by MRI, both sensitivity (97%) and negative
predictive value (96%) were even higher. Importantly, Proclarix was significantly superior to the current clinical standard, %fPSA, in
ruling out unneeded biopsies (22% vs. 14%) and the primary study endpoint was met (p-value < 0.005).
Naples Study. A two-center study evaluated Proclarix and the
Prostate Health Index (phi) test from Beckman Coulter, Inc. for predicting clinically significant prostate cancer in a total of 344 men.
Both Proclarix and the phi test accurately predicted clinically significant cancer. When using predefined cut-offs recommended by the
manufacturers, Proclarix (cut-off 10) outperformed phi (cut-off 27) in terms of specificity and positive predictive value (p < 0.002)
at similar sensitivities.
Clinical evaluation of Proclarix. Results of multiple clinical
evaluations using Proclarix together with MRI for prostate cancer diagnosis showed that Proclarix can be used in a broad range of patients
without the need for prostate volume restriction. The aim of one such evaluation was the assessment of the diagnostic performance of Proclarix
in combination with MRI. Blood samples from 721 men undergoing MRI followed by biopsy at two clinical centers were analyzed. The combined
Proclarix-MRI score’s specificity (68%) was significantly (p<0.001) better compared to Proclarix (27%) or MRI (28%) alone for
diagnosing clinically significant prostate cancer. Importantly, Proclarix by itself was found to be useful in men with indetermined imaging
results by outperforming PSA density in terms of specificity (25% vs 13%, p=0.004) at 100% sensitivity. In another evaluation of a study
of 517 men with suspected prostate cancer, Proclarix performed well in accurately diagnosing prostate cancer in the overall study population
and in a subset of men with elevated PSA 2 to 10 ng/mL, prostate volume ≥35 mL, and normal DRE (n=281). In addition, a sub-analysis
of was performed specifically analyzing 169 men with an indeterminate MRI result and Proclarix was more accurate in selecting appropriate
candidates for prostate biopsy when compared to PSA density and online risk calculators. A third evaluation describes which patients with
suspected prostate cancer can benefit from Proclarix after MRI and concluded that Proclarix outperformed PSA density in the selection
of candidates for prostate biopsy, especially in men with PI-RADS 1-3. In these studies, Proclarix proved to be effective before, after
and together with MRI assessment to identify men at risk of clinically significant prostate cancer and those who can safely avoid biopsy.
Proclarix in combination with MRI reliably predicted clinically significant prostate cancer and ruled out men with no or indolent cancer.
Clinical Guidelines
Guidelines assist clinicians in making informed
treatment decisions, taking into account the available scientific data. To reduce the number of negative biopsies in asymptomatic men
with a PSA level between 3–10 ng/mL and a normal DRE, the EAU guidelines recommend using an online risk-calculator that is correctly
calibrated to the population prevalence, MRI of the prostate or an additional biomarker test such as Proclarix. The EAU guidelines specifically
state that Proclarix has been correlated with the detection of significant prostate cancer, notably in case of equivocal MRI results.
Proclarix was also included in the 2023 AUA/SUO
clinical practice guideline. The AUA/SUO guideline covers recommendations on the early detection of prostate cancer and provides a framework
to facilitate clinical decision-making in the implementation of prostate cancer screening, biopsy, and follow-up. The AUA/SUO guideline
concludes that the evaluation of prostate cancer risk should be focused on the detection of clinically significant prostate cancer (GG2+).
The AUA/SUO guidelines advice that use of laboratory biomarkers such as Proclarix, prostate MRI, and biopsy techniques may improve detection
and safety when a prostate biopsy is deemed necessary following prostate cancer screening.
The inclusion of Proclarix in the European and
U.S. guidelines is an important recognition of the clinical value of Proclarix. It serves as a validation for the clinical utility and
importance of using Proclarix in the detection of prostate cancer and we believe it will lead to broader acceptance of Proclarix and accelerate
payor adoption.
Research and Development and Pipeline Products
Prosgard
Prosgard as a clinical support
system is designed to aggregate multimodal information in an effort to develop a patient centric diagnostic approach. The vision for Prosgard
is to add more information to the existing Proclarix risk score in the future such as other biomarkers, clinical information or MRI imaging
data to provide an even more powerful tool to guide the patient’s diagnostic journey.
Prognosis (Px)
A subset of Proteomedix’s
protein biomarkers also correlate with prostate cancer prognosis. Radical prostatectomy provides excellent cancer control of clinically
localized prostate cancer. However, approximately 30% of surgically treated men will experience cancer recurrence within 10 years of surgery.
Several clinical parameters and the combination thereof (e.g., the Cancer of the Prostate Risk Assessment (“CAPRA”) score)
have been shown to be reliable predictors of treatment failure. Still, there is a compelling need to identify novel markers that are specifically
linked to the presence of biologically aggressive prostate cancer for improved prediction of outcome in populations with moderately elevated
PSA levels.
A novel serum biomarker quintet that improves disease prognosis
in men with confirmed prostate cancer
A clinical evaluation of a multivariable model
comprising fibronectin 1, galectin-3-binding protein, lumican, matrix metalloprotease 9, thrombospondin-1 and PSA together with clinical
Grade Group (GG) and clinical stage (cT) was created was performed. The prognostic utility of the proposed marker combination was assessed
in serum samples from 557 men with confirmed localized prostate cancer. The analysis showed that the proposed model had a better prediction
for disease progression and thus prostate cancer aggressiveness compared to the “CAPRA” score. This novel biomarker test has
the potential to improve prostate cancer patient management by indicating who needs active treatment. In contrast to the existing biomarker
tests from competitors that all need tissue specimens, the test is non-invasive and can be directly measured in patients’ blood
samples.
Prediction (Rx)
Proteomedix’s protein
biomarkers further have the potential to predict the response of patients treated with drugs that inhibit the PI3K signaling pathway.
Proteomedix analyzed the blood of patients participating in a Phase II trial (SAKK 08/08). The patients were treated with Novartis AG’s
Everolimus, a drug inhibiting the PI3K pathway signaling by blocking mTOR. A subset of 8 serum biomarkers could individually predict reaching
the primary endpoint (progression free survival at 12 weeks) with an accuracy of at least 75%. Early discussions with pharma partners
on the potential use of such markers in personalized medicine studies are ongoing.
Decision Support Systems
Recent initiatives are incorporating
as well as interpreting clinical information from various sources (e.g., biomarker information and other patient data) enabling physicians
to have more comprehensive biochemical insight into each patient’s disease in order to determine the optimal treatment plan for
the patient. Collating multiple data sources in clinical workflows allows precision-medicine resulting in cost-effective diagnostics and
therapies. Proclarix already consists of a decision support system integrating different values in a risk score. In the future, additional
clinical information like the results of an MRI scan can be integrated in the report to provide a complete picture of the diagnostic situation
of the patient to enable effective patient management.
Product Quality and Safety
Proteomedix’s quality management system
is ISO (International Organization for Standardization) 13485:2016 certified for the “Design and development, production and distribution
of in-vitro diagnostic reagents and stand-alone software for prostate cancer management”. Proteomedix is annually audited by TÜV
SÜD Product Service GmbH, an internationally recognized notified body headquartered in Germany. ISO certification is a prerequisite
for obtaining CE-mark, the regulatory clearance requirement for market access, recognized by the European Commission (“EC”)
in the IVDR. Under the IVDR, diagnostic products are categorized under a new system of one of four classifications from class A (low
risk) to class D (highest risk). Proclarix, as class C device, was assessed by TÜV SÜD for conformity resulting in IVDR certification.
The certification of Proclarix under the new IVDR demonstrates compliance to the highest quality standard currently in force for tests
used in screening, diagnosis, or staging of cancer. Proteomedix is marketing Proclarix as one of the first IVDR compliant cancer tests
demonstrating the commitment to highest analytical and clinical performance.
Intellectual Property
Technology
Cancer arises from different
genetic mutations that can be linked to specific signaling pathways often referred to as cancer pathways. Depending on what pathway is
affected in a patient, results in different cancer subtypes that are more or less aggressive and further determines if a patient responds
to a certain drug treatment or not.
Proteomedix’s biomarkers were discovered
by a group of researchers at ETH Zurich using a genetics-guided discovery approach focusing on the PI3K/PTEN cancer pathway that plays
a dominant role in prostate cancer development. Using a mouse model and mass-spectrometry based proteomics technology including a glycoprotein
enrichment technology led to the identification of proteins directly linked to the molecular cause of cancer and therefore correlating
to the disease status in the prostate. Different serum glycoproteins were combined to form multiplexed biomarker signatures predictive
for tissue PI3K/PTEN status as well as diagnosis and prognosis of prostate cancer (Figure 5). The genetic-guided proteomics approach enabled
the fast discovery and validation of several biomarkers which in different combinations correspond to diagnosis, prognosis and potentially
to therapy response.
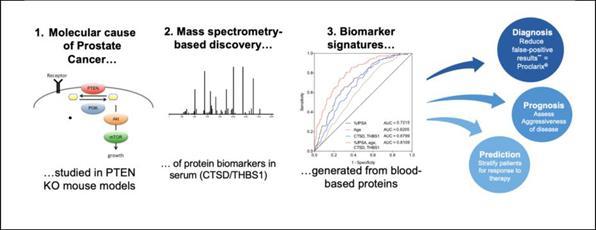
Figure 5: Proteomics approach to improve prostate cancer disease
management.
The biomarker assays were
transferred from a mass spectrometry-based to an immunoassay-based platform. Immunoassay-based measurement offers several advantages compared
to other analytical methods. In general, immunoassays provide a rapid, sensitive, reproducible, cost effective and easily manageable analysis.
The reagents used are stable and the method is established in routine diagnostic laboratories guaranteeing broad compatibility of Proteomedix’s
tests on established automated clinical platforms and thus rapid adoption rates and platform flexibility of the diagnostic tests. The
deep knowledge in selecting novel biomarkers, assay development and clinical development enabled Proteomedix to enable several R&D
partnerships.
In 2021, Proteomedix entered into a research and development partnership
with New Horizon Health Limited, Grand Cayman, Cayman Islands. The partnership builds on complimentary platform and biomarker developments
with utility in cancer patient management.
In 2022, Immunovia AB (Sweden) partnered with Proteomedix to leverage
Proteomedix’s research and development capabilities and advances their research and development efforts. With this partnership,
Immunovia gained a more flexible research and development organization, increased its research and development productivity, and refocused
internal resources on commercial build up, thus further accelerating the roll-out of their proprietary IMMrayTM PanCan-d test.
The partnership capitalizes on the combined expertise of two leading innovators in proteomics-based diagnostics, who have both launched
innovative oncology tests, Immunovia with IMMrayTM PanCan-d in the U.S. and Proteomedix with Proclarix® in Europe.
Patents
Proteomedix has exclusively licensed worldwide
rights to one patent family from ETH Zurich and the State Hospital of St. Gallen, which describes and protects the use of the proprietary
biomarkers for diagnosing and monitoring prostate cancer. The parent international patent application WO 2009138392 A1 was filed on May
14, 2008 (priority date) and was granted in China (CN201027373B), Europe (EP2281201B1), Japan (JP6025607B) and the United States (US10151755B2/
US9377463B2).
Proteomedix has also obtained a non-exclusive
license from ETH Zurich for certain patents pertaining to specific enrichment of glycoproteins, including EP1514107, that ETH Zurich licensed
from the Institute for Systems Biology (ISB), Seattle. The license enables Proteomedix to use the glycoprotein technology for the development
of new diagnostic products.
In addition, a new patent
covering the latest development and clinical results was filed by Proteomedix on July 15, 2016. The patent covers the specific test format
and algorithm contained in Proteomedix’s first product (Proclarix) for the improved diagnosis of prostate cancer. An international
application (WO2018011212A1) was filed and the patent was granted in Europe (EP3270163B1), Japan (JP6979712B2), South Korea (KR102408276B1),
Australia (AU2017294979B2), United States (US11320435B2) and China (CN109477836B) with the application still pending in Canada (CA3028874A1).
A patent application describing
a method combining Proclarix and magnetic resonance imaging to diagnose prostate cancer was filed by Proteomedix on June 29, 2021. The
patent was originally filed in Switzerland and subsequently as PCT application (WO2023274742A1) and as national applications in the United
States and China.
A patent application describing
a method measuring a blood-based protein combination with prognostic utility in prostate cancer patients was filed by Proteomedix on June
29, 2021. The patent was originally filed in Switzerland followed by an international application (WO2018011212A1). National applications
were filed in Europe, United States and China.
Trademarks
The brand “Proteomedix”
was filed on June 4, 2010, and registered under no. 602190 in Switzerland on June 22, 2010. This application served as the basis for the
international trademark application. The product name “Proclarix” was filed on July 1, 2019, and registered under no. 733974
in Switzerland on July 22, 2019. This application served as the basis for the international trademark application. The product name “Prosgard”
was filed on July 1, 2019, and registered under no. 733975 in Switzerland on July 22, 2019.
Competition
The molecular diagnostics field is intensely competitive
and characterized by rapid technological changes, frequent new product introductions, changing customer preferences, emerging competition,
evolving industry standards, reimbursement uncertainty and price competition. Moreover, recent consolidation in the industry permits larger
clinical laboratory service providers to increase cost efficiencies and service levels, resulting in more intense competition.
Proclarix Competition Analysis
The market for assessing men at risk for prostate cancer is large,
with many competitors some of which possess substantially greater financial, selling, logistical and laboratory resources, more experience
in dealing with third-party payors, and greater market penetration, purchasing power and marketing budgets, as well as more experience
in providing diagnostic services. Some companies and institutions are developing liquid biopsy (blood and urine)-based tests and diagnostic
tests based on the detection of proteins, mRNA, nucleic acids or the presence of fragments of mutated genes that are associated with prostate
cancer. These competitors could have technological, financial, reputational, and market access advantages over us.
There are a number of tests already on the market
or in clinical testing or commercial development that are also intended to triage diagnostics in men with moderately elevated PSA levels.
Of these tests the majority also target solely PSA as a biomarker. Certain isoforms of PSA are differentiated or transcript levels (mRNA)
are determined in addition to protein levels. Of these tests the best established is %fPSA, which is also available from all suppliers
of the PSA test, including market leaders Abbott Laboratories, Roche Diagnostics, Siemens Healthineers AG and Beckman Coulter, Inc. However,
the sensitivity and specificity improvements are very modest.
The 4Kscore from OPKO Health, Inc. (Nasdaq:OPK)
and the phi score from Beckman Coulter, Inc. measure additional forms of PSA and related proteins but they do not include additional biomarkers
either. The 4Kscore test is a blood based 4-plex test which combines the results of the blood test with clinical information in an algorithm
that calculates a patient’s percent risk for aggressive prostate cancer prior to an initial or repeat biopsy (no previous diagnosis
of prostate cancer). The 4Kscore test received marketing approval from the FDA in December 2021. The phi score combines the results of
three blood tests to provide information about what elevated PSA levels might mean and the probability of finding prostate cancer on biopsy.
The IsoPSA test of Cleveland Diagnostics, Inc. analyzes structural changes of PSA to detect underlying cancer biology.
Over the last decade, gene-based testing in
urine targeting additional biomarkers became available. The PCA3 test from Gen-Probe Inc. (now a part of Hologic, Inc.) was the
first genetic assay to be introduced to the market. The SelectMDx test from MDxHealth SA measures a combination of two genes and
integrates them together with PSA value, prostate volume, patient age and digital rectal exam to a risk score. The assay targets
mRNA transcripts in the patient’s urine. mRNA is normally not sufficiently shed into urine to allow for direct analysis.
Therefore, this test method requires prostate massage prior to sample collection and the urine samples will be collected in a
specialized practice. The ExoDx IntelliScore from Exosome Diagnostics, Inc., a subsidiary of Bio-Techne Corporation, measures PCA3
as well as other gene transcripts in exosomes harvested from urine. The method does not require prostate massage, but the varying
biomarkers yield a high number (30%) of samples indeterminate. Additionally, because mRNA is relatively unstable, the samples
require cold storage in shipment and relatively rapid testing turn-around.
The Stockholm3 test is part of an academic initiative,
OncoWatch, led by the Karolinska Institute, Sweden and funded by the European Institute of Innovation and Technology Health program. The
Stockholm3 test is a blood-based test that predicts the risk for aggressive prostate cancer at biopsy by analyzing five protein markers,
more than 100 genetic markers and clinical data.
Except for PCA3, Prostate Health Index and 4Kscore,
all of the above-mentioned tests are only available as a testing service through specialized reference laboratories, they are not offered
as commercial products. Testing is performed centrally as a laboratory developed test (“LDT”) by a single diagnostic laboratory.
Uptake of LDTs in the United States has been limited, and in Europe they are mostly not known to urologists. The only country in Europe
where LDTs have gained some acceptance so far is Spain. Combined, approximately 100,000 LDT tests were performed in 2019. Average price
/ reimbursement (if covered) is at US$600.
In recent years, MRI-based diagnosis followed
by targeted biopsy is becoming the standard of choice in specialized centers. As MRI instrumentation is costly and its availability is
still limited, there is a need for diagnostics supporting the decision to perform MRI that Proclarix can fulfill. MRI is not regarded
as competitive to the Proclarix positioning, but complementary.
Competitive
Advantages of Proclarix
Despite
being on the market for some time, none of the competitor tests made it into the standard of care and penetration is very limited. Proclarix
has important competitive advantages:
| |
● |
Blood-based
test |
- |
Minimally
invasive, high reproducibility, no prostate massage required, suitably stable for shipment, the most common sample type in clinical
laboratories and therefore fitting in current lab workflow |
| |
● |
Immunoassay-based |
- |
Compatible
with existing laboratory instrumentation in local laboratory |
| |
● |
Easy
to automate |
- |
Adaptable
to clinical routine, fast time to result |
| |
● |
Objective
result generation |
- |
Comparable
results independent of operator |
| |
● |
Genetics-guided
discovery |
- |
Cancer-related,
highly plausible biomarkers |
Proclarix
can be applied in any diagnostic laboratory, using readily available immunoassay technology platforms. Furthermore, Proclarix fits very
well into the current laboratory workflow, which is important for laboratories that are driven by efficiency and cost.
The
stakeholders benefit in various ways from Proclarix:
Patients:
Gain more certainty whether a biopsy is really needed through a minimally invasive procedure with a fast time to result. This
results in reduced anxiety about prostate cancer diagnosis and less complications and side effects from biopsies.
Physicians:
Focus on relevant patients with clinically significant cancer and increased patient satisfaction by significantly reducing unneeded
prostate biopsies and its accompanying complications. No need for additional training or new logistic processes: Standard blood-drawing
equipment can be used and the blood sample sent to the current laboratory.
Laboratory:
Increase revenue with no additional investment for new equipment because Proclarix is readily applicable in any laboratory.
Payer
(insurance company): Increase profits by saving costs for avoided biopsies (accompanied by risk of complications, discomfort)
and resulting overtreatment.
Sales,
Distribution, Marketing and Advertising
In
clinical diagnostics high throughput assay parameters like PSA typically are performed on closed, fully integrated systems that use proprietary
reagents. Integrated systems are provided by a few mid-sized to large diagnostic companies (e.g., Roche Diagnostics, Abbott Laboratories,
Siemens Healthineers AG, DiaSorin S.p.A.) with a worldwide distribution network. Reagents are provided in a closed-system approach, access
is through collaboration agreements only. Business development discussions with multiple diagnostic companies have already started.
Lower
volume parameters are run on smaller, open systems that are used in laboratories for tests with lower throughput to complement the test
menu. Access to these open systems presents an option for direct commercialization in selected markets during market introduction. First,
the goal is to establish commercial proof of concept and drive initial market adoption.
Market
adoption of a new test is driven by KOLs and clinical urology centers. Publication of clinical studies proving the medical benefit of
the test and KOLs advocating it at scientific conferences will trigger the usage by other physicians. Additionally, demand is created
through urology centers specialized in prostate cancer that cover a large geographical area. Their influence on other urologists and
general practitioners in the region will lead to multiplier effects. Diagnostic testing in clinical urology centers is provided either
by an in-house hospital laboratory or a commercial laboratory where Proclarix will be implemented.
General
practitioners recruit patients for screening and decide whether to refer a patient to a specialist. They have an important gatekeeper
role and Proclarix is a helpful tool for this triage. Marketing outreach of commercial laboratory networks (e.g., Unilabs, Switzerland;
Sonic Healthcare, Australia; Labcorp, U.S.A.) provides an opportunity to directly address the large number of general practitioners and
urologists in private practices through their specialized sales force.
Onconetix’s
Management’s Discussion and Analysis of Financial Condition and
Results of OperationS
Components
of Results of Operations
Research
and Development Expenses
Substantially
all of our research and development expenses have consisted of expenses incurred in connection with the development of our product candidates.
These expenses include fees paid to third parties to conduct certain research and development activities on our behalf, consulting costs,
costs for laboratory supplies, product acquisition and license costs, certain payroll and personnel-related expenses, including salaries
and bonuses, employee benefit costs and stock-based compensation expenses for our research and product development employees and allocated
overheads, including information technology costs and utilities. We expense both internal and external research and development expenses
as they are incurred.
We
do not allocate our internal costs by product candidate, as a significant amount of research and development expenses include costs,
such as payroll and other personnel expenses, laboratory supplies and allocated overhead, and external costs, such as fees paid to third
parties to conduct research and development activities on our behalf, which are not tracked by product candidate.
Predicting
the timing or cost to complete our clinical programs or validation of our commercial manufacturing and supply processes is difficult
and delays may occur because of many factors, including factors outside of our control. For example, if the FDA or other regulatory authorities
were to require us to conduct clinical trials beyond those that we currently anticipate, we could be required to expend significant additional
financial resources and time on the completion of clinical development. Furthermore, we are unable to predict when or if our product
candidates will receive regulatory approval with any certainty.
Selling,
General and Administrative Expenses
Selling,
general and administrative expenses consist principally of payroll and personnel expenses, including salaries and bonuses, benefits and
stock-based compensation expenses, professional fees for legal, consulting, accounting and tax services, and commercialization of ENTADFI,
including information technology costs, and other general operating expenses not otherwise classified as research and development expenses.
We
anticipate that our selling, general and administrative expenses will continue to increase as a result of increased personnel costs,
expanded infrastructure and higher consulting, legal and accounting services costs associated with complying with the applicable stock
exchange and the SEC requirements, investor relations costs and director and officer insurance premiums associated with being a public
company, along with costs for commercialization of products.
Results
of Operations
Comparison
of the Years Ended December 31, 2022 and 2021
The
following table summarizes our statements of operations and comprehensive loss for the periods indicated:
| | |
Year
Ended
December 31,
2022 | | |
Year
Ended
December 31,
2021 | | |
$
Change | | |
%
Change | |
| Operating
expenses | |
| | |
| | |
| | |
| |
| General
and administrative | |
$ | 9,351,552 | | |
$ | 2,092,304 | | |
| 7,259,248 | | |
| 346.9 | % |
| Research
and development | |
| 4,129,688 | | |
| 1,325,030 | | |
| 2,804,658 | | |
| 211.7 | % |
| Total
operating expenses | |
| 13,481,240 | | |
| 3,417,334 | | |
| 10,063,906 | | |
| 294.5 | % |
| Loss
from operations | |
| (13,481,240 | ) | |
| (3,417,334 | ) | |
| (10,063,906 | ) | |
| 294.5 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Other
income | |
| | | |
| | | |
| | | |
| | |
| Change
in fair value of contingent warrant liability | |
| (61,410 | ) | |
| — | | |
| (61,410 | ) | |
| * | |
| Total
other income | |
| (61,410 | ) | |
| — | | |
| (61,410 | ) | |
| * | |
| Net
loss | |
$ | (13,419,830 | ) | |
$ | (3,417,334 | ) | |
| (10,002,496 | ) | |
| 292.7 | % |
General
and Administrative Expenses
For
the year ended December 31, 2022, general and administrative expenses increased by approximately $7.3 million compared to 2021. The increase
was mainly due to an increase in employee and director compensation and benefits, including annual bonus compensation and stock-based
compensation, of approximately $2.4 million, an increase in professional services, which is comprised primarily of audit, accounting,
and legal services, of approximately $1.2 million, increases in various business activities related to company growth and development
such as entering into a new lease, patent-related expenses, franchise taxes, travel, and business advisory services totaling approximately
$0.9 million, and increases in other business activities related to now being a public company of approximately $1.1 million. In addition,
during the year ended December 31, 2022, the Company incurred approximately $1.3 million in expense related to the settlement agreement
with Boustead and approximately $0.3 million for a non-recurring termination fee to the Company’s former underwriter, for early
termination of the agreement with that underwriter.
Research
and Development Expenses
For
the year ended December 31, 2022, research and development expenses increased by approximately $2.8 million compared to 2021. The increase
was primarily attributable to an increase in employee compensation and benefits, including annual bonus compensation and stock-based
compensation, of approximately $1.1 million, an increase in preclinical development activities of approximately $1.5 million mainly related
to BWV-201, and an increase in external research and development personnel costs of approximately $0.4 million, offset by a decrease
in license fees of approximately $0.3 million, primarily related to the one-time license fees incurred pursuant to a license agreement,
dated June 1, 2021, with Children’s Hospital Medical Center, d/b/a Cincinnati Children’s Hospital Medical Center during the
year ended December 31, 2021.
Other
Income
Other
income for the year ended December 31, 2022 relates to the change in fair value of the contingent warrant liability, which was incurred
at the close of private placement issuances in April and August 2022. There was no other income or expense during the year ended December
31, 2021.
Comparison
of the Nine Months Ended September 30, 2023 and 2022
The
following table summarizes our statements of operations for the periods indicated:
| | |
Nine Months
Ended
September 30,
2023 | | |
Nine Months
Ended
September 30,
2022 | | |
$
Change | | |
%
Change | |
| Operating
costs and expenses | |
| | |
| | |
| | |
| |
| Selling,
general and administrative | |
$ | 8,337,615 | | |
$ | 7,311,243 | | |
$ | 1,026,372 | | |
| 14.0 | % |
| Research
and development | |
| 2,148,327 | | |
| 2,924,037 | | |
| (775,710 | ) | |
| (26.5 | )% |
| Impairment
of deposit on asset purchase agreement | |
| 3,500,000 | | |
| - | | |
| 3,500,000 | | |
| 100.0 | % |
| Total
operating expenses | |
| 13,985,942 | | |
| 10,235,280 | | |
| 3,750,662 | | |
| 36.6 | % |
| Loss
from operations | |
| (13,985,942 | ) | |
| (10,235,280 | ) | |
| (3,750,662 | ) | |
| (36.6 | )% |
| Total
other income (expense) | |
| (1,072,880 | ) | |
| 33,375 | | |
| (1,106,255 | ) | |
| (3,314.6 | )% |
| Net
loss | |
$ | (15,058,822 | ) | |
$ | (10,201,905 | ) | |
$ | (4,856,917 | ) | |
| (47.6 | )% |
Selling,
General and Administrative Expenses
For
the nine months ended September 30, 2023, selling, general and administrative expenses increased by approximately $1.0 million
compared to the same period in 2022. The increase was mainly attributable to increased commercialization activities for ENTADFI of
$2.2 million, an increase of $1.1 million in professional fees, a loss on impairment of long-lived assets of $0.3 million, and $0.2 million
incurred for the loss on related party receivable. These increases were offset by a decrease in employee compensation of $1.0 million,
primarily related to lower stock-based compensation expenses and a decrease in executive bonuses and a decrease in various business activities,
such as business advisory services, travel related expenses, and rent expense, totaling $0.2 million. In addition, there was a decrease
of $1.3 million related to a loss contingency and a decrease of $0.3 million for a non-recurring termination penalty to a former underwriter,
for early termination of the agreement with that underwriter, both of which were incurred in the nine months ended September 30, 2022,
with no similar expense in the current period.
Research
and Development Expenses
For
the nine months ended September 30, 2023, research and development expenses decreased by approximately $0.8 million compared to
the same period in 2022. The decrease was primarily due to the Company’s decision to deprioritize its vaccine programs during the
three months ended September 30, 2023.
Impairment
of Deposit on Asset Purchase Agreement
During
the nine months ended September 30, 2023, a $3.5 million impairment loss was recorded on the deposit for the WraSer asset purchase agreement.
Other
Income (Expense)
Other
expense incurred during the nine months ended September 30, 2023, primarily relates to interest expense incurred on new notes payable
issued in April 2023 related to the acquisition of ENTADFI, a loss on extinguishment of a note payable of $0.5 million in connection
with the Veru APA, and the change in fair value of the contingent warrant liability. Other income recorded during the
nine months ended September 30, 2022, relates to the change in fair value of the contingent warrant liability.
Liquidity
and Capital Resources
Since
inception in October 2018 until April 2023, when we acquired ENTADFI, we devoted substantially all of our efforts to research and development,
undertaking preclinical studies and enabling manufacturing activities in support of our product development efforts, hiring personnel,
acquiring and developing our technology and now deprioritized vaccine candidates, organizing and staffing our company, performing business
planning, establishing our intellectual property portfolio, and raising capital to support and expand such activities. Since our April
2023 acquisition of ENTADFI, we have been focusing our efforts on building out our commercial capabilities to launch ENTADFI in
the marketplace and consummation of the December 2023 acquisition of Proteomedix, and its related diagnostic product. We do not have
any products approved for sale, aside from ENTADFI and Proclarix, and have not yet generated significant revenue from product sales.
We
have incurred net losses in each year since inception and expect to continue to incur net losses in the foreseeable future. Our net loss
was $5.3 million and $15.1 million for the three and nine months ended September 30, 2023, respectively. As of September 30, 2023, we
had an accumulated deficit of $34.4 million. We also generated negative operating cash flows of $9.3 million for the nine months
ended September 30, 2023.
These
factors, along with the Company’s forecasted future cash flows, indicate that the Company will be unable to meet its contractual
commitments and obligations as they come due in the ordinary course of business within one year following the issuance of the September
30, 2023 condensed financial statements.
On
January 23, 2024, the Company issued the Debenture in exchange for $4.6 million in net cash proceeds. The Debenture is repayable in full
upon the earlier of (i) the closing under the Subscription Agreement and (ii) June 30, 2024.
We
will require significant amounts of additional capital in the short-term, to continue to fund our continuing operations, satisfy existing
and future obligations and liabilities, including the remaining payments due under the Veru APA, payment due on the Debenture, and other
contracts entered into in support of the Company’s commercialization plans, in addition to funds needed to support our working
capital needs and business activities, including the commercialization of ENTADFI and Proclarix, and the development and commercialization
of our current product candidates and future product candidates. Management’s plans for funding the Company’s operations
include generating product revenue from sales of ENTADFI, which has not yet been successfully commercialized, a process that will require
significant amounts of additional capital to complete. In addition, certain of the commercialization activities are outside of the Company’s
control, including but not limited to, securing contracts with wholesalers and third-party payers, securing contracts with third-party
logistics providers, and obtaining required licensure is various jurisdictions, as well as attempting to secure additional required funding
through equity or debt financings if available. However, we may not be able to obtain additional financing on terms favorable to us,
if at all, which creates significant uncertainty that we will be able to successfully launch ENTADFI and expand commercialization of
Proclarix. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, or if we expend
capital on projects that are not successful, our ability to continue to support our business growth and to respond to business challenges
could be significantly limited, or we may even have to cease our operations. If we raise additional funds through further issuances of
equity or convertible debt securities, our existing stockholders could suffer significant dilution, and any new equity securities we
issue could have rights, preferences and privileges superior to those of holders of our common stock.
Future
Funding Requirements
Our
primary uses of cash to date have been to fund our operations, which consist primarily of
research and development expenditures related to our programs, costs related to acquisitions
and potential acquisitions, and selling, general and administrative expenditures. We anticipate
that we will continue to incur significant expenses for the foreseeable future as we continue
to commercialize ENTADFI and Proclarix and expand our corporate infrastructure, including
the costs associated with being a public company. We are subject to all of the risks typically
related to the development of new drug candidates, and we may encounter unforeseen expenses,
difficulties, complications, delays and other unknown factors that may adversely affect our
business. We anticipate that we will need substantial additional funding in connection with
our continuing operations and in order to execute our long-term business plan.
We
will require significant amounts of additional capital in the short-term, to continue to
fund our continuing operations, satisfy existing and future obligations and liabilities,
including the remaining payments due under the Veru APA and other contracts entered into
in support of the Company’s commercialization plans, in addition to funds needed to
support our working capital needs and business activities, including the commercialization
of ENTADFI and the development and commercialization of our current product candidates and
future product candidates. Until we can generate a sufficient amount of revenue from sales
of ENTADFI or Proclarix, we expect to finance our future cash needs through public or private
equity or debt financings, third-party (including government) funding and marketing and distribution
arrangements, as well as other collaborations, strategic alliances and licensing arrangements,
or any combination of these approaches. The future sale of equity or convertible debt securities
may result in dilution to our stockholders, and, in the case of preferred equity securities
or convertible debt, those securities could provide for rights, preferences or privileges
senior to those of our common stock. Debt financing may subject us to covenant limitations
or restrictions on our ability to take specific actions, such as incurring additional debt,
making capital expenditures or declaring dividends. There can be no assurance that we will
be successful in acquiring additional funding at levels sufficient to fund our operations
or on terms favorable or acceptable to us. If we are unable to obtain adequate financing
when needed or on terms favorable or acceptable to us, we may be forced to delay, reduce
the scope of our business activities.
Our
future capital requirements will depend on many factors, including:
| |
● |
the
cost of building a sales force in anticipation of any product commercialization; |
| |
|
|
| |
● |
the
costs of future commercialization activities, including product manufacturing, marketing, sales, royalties and distribution, for
ENTADFI, Proclarix and other products for which we have received or will receive marketing approval; |
| |
|
|
| |
● |
the
timing, scope, progress, results and costs of research and development, testing, screening, manufacturing, preclinical and non-clinical
studies and clinical trials; |
| |
|
|
| |
● |
the
outcome, timing and cost of seeking and obtaining regulatory approvals from the FDA and comparable foreign regulatory authorities,
including the potential for such authorities to require that we perform field efficacy studies, require more studies than those that
we currently expect or change their requirements regarding the data required to support a marketing application; |
| |
|
|
| |
● |
our
ability to maintain existing, and establish new, strategic collaborations, licensing or other arrangements and the financial terms
of any such agreements, including the timing and amount of any future milestone, royalty or other payments due under any such agreement; |
| |
|
|
| |
● |
any
product liability or other lawsuits related to our products; |
| |
|
|
| |
● |
the
expenses needed to attract, hire and retain skilled personnel; |
| |
|
|
| |
● |
the
revenue, if any, received from commercial sales, or sales to foreign governments, of ENTADFI, Proclarix or other products for which
we may have received or will receive marketing approval; |
| |
|
|
| |
● |
the
costs to establish, maintain, expand, enforce and defend the scope of our intellectual property portfolio, including the amount and
timing of any payments we may be required to make, or that we may receive, in connection with licensing, preparing, filing, prosecuting,
defending and enforcing our patents or other intellectual property rights; and |
| |
|
|
| |
● |
the
costs of operating as a public company. |
A
change in the outcome of any of these or other variables could significantly change the costs and timing associated with our business
activities. Furthermore, our operating plans may change in the future, and we may need additional funds to meet operational needs and
capital requirements associated with such change.
Cash
Flows
The
following table summarizes our cash flows for the periods indicated:
| | |
Nine
Months
Ended
September 30,
2023 | | |
Nine
Months
Ended
September 30,
2022 | |
| Net
cash used in operating activities | |
$ | (9,269,131 | ) | |
$ | (5,875,492 | ) |
| Net
cash used in investing activities | |
| (9,864,613 | ) | |
| (31,488 | ) |
| Net
cash provided by financing activities | |
| 1,035,060 | | |
| 33,115,222 | |
| Net
increase (decrease) in cash | |
$ | (18,098,684 | ) | |
$ | 27,208,242 | |
| | |
Year
Ended
December 31,
2022 | | |
Year
Ended
December 31,
2021 | |
| Net
cash used in operating activities | |
$ | (8,698,860 | ) | |
$ | (2,044,235 | ) |
| Net
cash used in investing activities | |
| (9,339 | ) | |
| (1,924 | ) |
| Net
cash provided by (used in) financing activities | |
| 32,532,384 | | |
| (334,188 | ) |
| Net
increase (decrease) in cash | |
$ | 23,824,185 | | |
$ | (2,380,347 | ) |
Cash
Flows from Operating Activities
Net
cash used in operating activities for the nine months ended September 30, 2023, was
approximately $9.3 million, which primarily resulted from a net loss of $15.1 million.
This was offset by a net change in our operating assets and liabilities of $0.1 million,
an impairment loss of $3.5 million related to the deposit on the WraSer APA, an approximate
$0.5 million loss on the extinguishment of a note payable, noncash stock-based compensation
of approximately $0.6 million, noncash interest expense of approximately $0.5 million, a
$0.3 million loss on impairment of long-lived assets, and the loss on related party receivable
of approximately $0.3 million.
Net
cash used in operating activities for the nine months ended September 30, 2022, was
approximately $5.9 million, which primarily resulted from a net loss of approximately
$10.2 million, which was partially offset by noncash stock-based compensation of approximately
$1.8 million, and a net change in our operating assets and liabilities of approximately $2.6 million.
Net
cash used in operating activities for the year ended December 31, 2022, was $8.7 million, which primarily resulted from a net loss of
$13.4 million, and was partially offset by noncash stock-based compensation of approximately $2.0 million, the fair value of restricted
common stock that was issued of approximately $0.3 million, and a net change in our operating assets and liabilities of $2.5 million.
Net
cash used in operating activities for the year ended December 31, 2021, was $2.0 million, which primarily resulted from a net loss of
$3.4 million, which was partially offset by a net change in our operating assets and liabilities of $1.2 million and stock-based compensation
of $0.1 million.
Cash
Flows from Investing Activities
Net
cash used in investing activities for the nine months ended September 30, 2023, was approximately $9.9 million, of which approximately
$6.1 million was used for the acquisition of ENTADFI, $3.5 million was used for the deposit in connection with the potential WraSer APA,
which closing is pending at September 30, 2023, and $0.3 million is the net change in the receivable from related parties.
Net
cash used in investing activities for the nine months ended September 30, 2022, was approximately $31,000, which resulted from
purchases of property and equipment and the net change in the receivable from related parties.
Net
cash used in investing activities for the years ended December 31, 2022, and 2021 was $9,000 and $2,000, respectively, which resulted
from purchases of property and equipment.
Cash
Flows from Financing Activities
Net
cash provided by financing activities for the nine months ended September 30, 2023, was approximately $1.0 million, and resulted from
net proceeds from the exercise of preferred investment options in connection with the warrant inducement transaction of $2.3 million
offset by $1.0 million in principal payments on a note payable, $59,000 in purchases of treasury shares, and $205,000 of payment in deferred
offering costs.
Net
cash provided by financing activities for the nine months ended September 30, 2022, was approximately $33.1 million, and resulted
primarily from the close of our IPO and the April and August Private Placements.
Net
cash provided by financing activities for the year ended December 31, 2022, was approximately
$32.5 million, and resulted primarily from the close of our IPO and the Private Placements,
which resulted in net proceeds of approximately $33.1 million, offset by approximately $0.6
million in treasury share repurchases. Net cash used in financing activities for the year
ended December 31, 2021, was $0.3 million related to payments of deferred offering costs.
Legal
Contingencies
From
time to time, we may become involved in legal proceedings arising from the ordinary course of business. We record a liability for such
matters when it is probable that future losses will be incurred and that such losses can be reasonably estimated.
Off-Balance
Sheet Arrangements
During
the periods presented we did not have, nor do we currently have, any off-balance sheet arrangements as defined in the rules and regulations
of the SEC.
PROTEOMEDIX
Management’s Discussion and Analysis of Financial Condition and
Results of OperationS
You
should read the following discussion in conjunction with our financial statements and related notes included elsewhere in this proxy
statement. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing
of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors,
including, but not limited to, those set forth under “Risk Factors” and elsewhere in this proxy statement.
Critical
Accounting Policies and Estimates
Basis
of Presentation
Proteomedix’s
financial statements are prepared in accordance with U.S. Generally Accepted Accounting Principles (“U.S GAAP”), which require
the recognition and disclosure of foreign currency translation adjustments resulting from the translation of financial statements denominated
in currencies other than the U.S. Dollar.
The
functional currency of Proteomedix is the Swiss Franc. Transactions denominated in foreign currencies are translated into the functional
currency at the exchange rates prevailing at the date of the transaction. The resulting translation adjustments are recorded as a separate
component of accumulated other comprehensive income (loss).
Cash
and Cash Equivalents
For
purposes of reporting cash flows, Proteomedix has defined cash and cash equivalents as all cash in banks and highly liquid investments
available for current use with an initial maturity of three months or less to be cash equivalents. Proteomedix had no cash equivalents
as of December 31, 2022 or 2021.
Proteomedix
maintains its cash balances at financial institutions that are insured by Swiss Financial Market Supervisory Authority (“FINMA”).
Proteomedix’s cash balances may at times exceed the insurance provided by FINMA. Proteomedix has not experienced any losses on
these accounts and management does not believe that Proteomedix is exposed to any significant risks related to excess deposits.
Collaborative Agreements
Proteomedix
periodically enters into strategic alliance agreements with counterparties to produce products and/or provide services to customers.
Alliances created by such agreements are not legal entities, have no employees, no assets and have no true operations. These arrangements
create contractual rights and Proteomedix accounts for these alliances as a collaborative arrangement by reporting costs incurred and
reimbursements received from transactions within research and development expense within the statements of comprehensive loss.
Share-Based
Compensation
Proteomedix
accounts for equity instruments issued in exchange for the receipt of goods or services from other than employees in accordance with
Financial Accounting Standard Board (“FASB”) Account Standard Codification (“ASC”) 718, “Compensation –
Stock Compensation.” Costs are measured at the estimated fair value of the consideration received or the estimated fair value of
the equity instruments issued, whichever is more reliably measurable. The value of equity instruments issued for consideration other
than employee services is determined on the earliest of a performance commitment or completion of performance by the provider of goods
or services as defined by FASB ASC 718, “Compensation – Stock Compensation.”
Revenue
Recognition
Effective
on January 1, 2021, Proteomedix adopted ASC Topic 606, “Revenue from Contracts with Customers” (“ASC 606”). Pursuant
to ASC 606, revenues are recognized when control of services performed is transferred to customers, in an amount that reflects the consideration
we expect to be entitled to in exchange for those services. ASC 606 provides for a five-step model that includes:
| (i) | identifying
the contract with a customer, |
| (ii) | identifying
the performance obligations in the contract, |
| (iii) | determining
the transaction price, |
| (iv) | allocating
the transaction price to the performance obligations, and |
| (v) | recognizing
revenue when, or as, an entity satisfies a performance obligation. |
Product
Sales
Proteomedix
derives revenue through sales of its products directly to end users and to distributors. Proteomedix sells its products to customers
including laboratories, hospitals, medical centers, doctors and distributors. Proteomedix considers customer purchase orders, which in
some cases are governed by master sales agreements or standard terms and conditions, to be the contracts with a customer. For each contract,
Proteomedix considers the promise to transfer products, each of which are distinct, to be the identified performance obligations. In
determining the transaction price Proteomedix evaluates whether the price is subject to refund or adjustment to determine the net consideration
to which it expects to be entitled. Proteomedix fulfils its performance obligation applicable to product sales once the product is transferred
to the customer.
Development
Services
Proteomedix
provides a range of services to life sciences customers referred to as “Development Services” including testing for biomarker
discovery, assay design and development. These Development Services are performed under individual statement of work (“SOW”)
arrangements with specific deliverables defined by the customer. Development Services are generally performed on a time and materials
basis. During the performance and through completion of the service to the customer in accordance with the SOW, we have the right to
bill the customer for the agreed upon price and we recognize the Development Services revenue over the period estimated to complete the
SOW. We generally identify each SOW as a single performance obligation.
Completion
of the service and satisfaction of the performance obligation under a SOW is typically evidenced by access to the data or test made available
to the customer or any other form or applicable manner of delivery defined in the SOW. However, for certain SOWs under which work is
performed pursuant to the customer’s highly customized specifications, we have the enforceable right to bill the customer for work
completed, rather than upon completion of the SOW. For those SOWs, we recognize revenue over a period of time during which the work is
performed based on the expended efforts (inputs). As the performance obligation under the SOW is satisfied, any amounts earned as revenue
and billed to the customer are included in accounts receivable. Any revenues earned but not yet billed to the customer as of the date
of the financial statements are recorded as contract assets and are included in prepaids and other current assets as of the financial
statement date. Amounts recorded in contract assets are reclassified to accounts receivable in our financial statements when the customer
is invoiced according to the billing schedule in the contract.
In
circumstances where a SOW includes variable consideration component, Proteomedix estimates the amount of variable consideration that
should be included in the transaction price utilizing either the expected value method or the most likely amount method, depending on
which method is expected to better predict the amount of consideration to which Proteomedix will be entitled. The value of variable consideration
is included in the transaction price if, and to the extent, it is probable that a significant reversal of the amount of cumulative revenue
recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. These estimates are
reassessed each reporting period, as required, and any adjustment required is recorded on a cumulative catch-up basis, which would affect
revenue and net income in the period of adjustment.
Licensing
Revenues
license
revenues are determined based on an assessment of whether the license is distinct from any other performance obligations that may be
included in the underlying licensing arrangement. If the customer is able to benefit from the license without provision of any other
performance obligations by Proteomedix and the license is thereby viewed as a distinct or functional license, Proteomedix then determines
whether the customer has acquired a right to use the license or a right to access the license. For functional licenses that do not require
further substantive development or other ongoing activities by Proteomedix, the customer is viewed as acquiring the right to use the
license as, and when, transferred and revenues are generally recorded at a point in time. For symbolic licenses providing substantial
value only in conjunction with other performance obligations to be provided by Proteomedix, revenues are generally recorded over the
term of the license agreement using the inputs based on contractual remaining time for such license. Such other obligations provided
by Proteomedix generally include manufactured products, additional development services or other deliverables that are contracted to
be provided during the license term.
Royalties
associated with licensing arrangements are estimated and recognized when sales under supply agreements with commercial licensees are
recorded, absent any contractual constraints or collectability uncertainties. Royalties which are contingent on meeting certain sales
milestones are recorded when it has become probable that milestones will be met.
Defined
Benefit Pension Plan
Proteomedix
sponsors a defined benefit pension plan (the “Plan”) covering eligible employees. The Plan provides retirement benefits based
on employees’ years of service and compensation levels. Proteomedix recognizes an asset for such plan’s overfunded status
or a liability underfunded status in its balance sheets. Additionally, Proteomedix measures its plan’s assets and obligations that
determine its funded status as of the end of the year and recognizes the changes in the funded status in the year in which the changes
occur. Those changes are reported in ‘accumulated other comprehensive loss. Proteomedix uses actuarial valuations to determine
its pension and postretirement benefit costs and credits. The amounts calculated depend on a variety of key assumptions, including discount
rates and expected return on plan assets. Current market conditions are considered in selecting these assumptions.
Proteomedix’s
pension plans are generally valued using the net asset value (NAV) per share as a practical expedient for fair value provided certain
criteria are met. The NAVs are determined based on the fair values of the underlying investments in the funds. In circumstances where
the criteria are not met, fair is determined based on the underlying market in which the funds are traded which is generally considered
to be an active market.
Components
of Results of Operations
Marketing
and business development
This
classification of expenses includes all efforts related to the marketing and early stage commercialization of Proclarix as well as general
business development expenses related to Proclarix and other business areas (e.g. development services, pipeline products). Such costs
are expensed in the period in which they occur.
We
expect our marketing and business development expenses to increase in the future and we continue to expand our commercialization and
of Proclarix as well as other business areas.
Research
and development
This
classification of expenses includes all costs associated with the development of Proclarix
and pipeline products as well as development activities related to collaborations with partners
(e.g. research and development collaborations for pipeline products or development services).
These expenses include fees paid to third parties to conduct certain research and development
activities on our behalf, consulting costs, costs for clinical samples used in clinical studies,
costs for laboratory supplies, certain payroll and personnel-related expenses, including
salaries. We expense both internal and external research and development expenses as they
are incurred.
We
do not allocate our internal costs by product candidate, as a significant amount of research and development expenses include costs,
such as payroll and other personnel expenses, laboratory supplies and allocated overhead, and external costs, such as fees paid to third
parties to conduct research and development activities on our behalf, which are not tracked by product candidate.
We
expect our research and development expenses to increase. If any regulatory authorities were to require us to conduct clinical trials,
we could be required to expend significant additional financial resources and time on the completion of clinical development. Furthermore,
we are unable to predict with any certainty when or if our pipeline product will be required to obtain regulatory approvals and the magnitude
of any additional development costs required to bring those products to a commercial ready state.
General
and administrative
General
and administrative expense consists principally of payroll and personnel expenses, including salaries and bonuses, benefits and stock-based
compensation expenses, professional fees for legal, consulting, accounting and tax services and other general operating expenses not
otherwise classified as research and development expenses.
We
anticipate that our general and administrative expense will continue to increase as a result of increased personnel costs, expanded infrastructure
and higher consulting, legal and accounting services costs associated with Proteomedix’s continued growth.
Depreciation
Depreciation
relates to the amortization of our certain long-term assets over their estimated useful lives. These costs are expensed in the period
incurred, which is generally the period the asset is in service until the asset has been disposed of by Proteomedix.
Interest
expense
Interest
on our outstanding convertible notes payable is expensed as incurred. Interest expense also includes the costs of converting other currencies
into CHF, our functional currency. We consider this to be a component of our capital financing activities and therefore include this
amount in interest expense in the accompanying statements of comprehensive income (loss).
Foreign
currency translation adjustments
This
balance is the result of the translation of our financial statements from CHF, our functional currency, to United States Dollars, our
reporting currency. Assets and liabilities are translated using the exchange ratio as of the end of the reporting period which was 1.098,
1.082 and 1.093 as of December 31, 2022, December 31, 2021 and September 30, 2023, respectively. Equity is translated using historical
exchange rates relevant to the transactions. Revenues and expenses are translated using the average exchange rate during the reporting
period. The significant factors contributing to the changes in these balances are related to the disparity between CHF and USD as well
as changes in the composition of our assets and liabilities during the period.
Changes
in pension benefit obligations
As
required by Swiss law, we sponsor a defined benefit pension plan for all of our employees. Changes in these balances are related to gains
and losses in the underlying pension assets and liabilities, actuarial gains and losses as well as settlements due to the payment of
benefits.
Results
of Operations
Comparison
of the years ended December 31, 2022 and 2021
| | |
Years
ended | | |
$ | | |
% | |
| | |
2022 | | |
2021 | | |
Change | | |
Change | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 392,460 | | |
$ | 140,600 | | |
$ | 251,860 | | |
| 179 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Cost
of goods sold | |
| 48,429 | | |
| 31,977 | | |
| 16,452 | | |
| 51 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Gross
profit | |
| 344,031 | | |
| 108,623 | | |
| 235,408 | | |
| 217 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Operating
expenses | |
| | | |
| | | |
| | | |
| | |
| Marketing
and business development | |
| 240,298 | | |
| 200,096 | | |
| 40,202 | | |
| 20 | % |
| Research
and development | |
| 393,274 | | |
| 312,586 | | |
| 80,688 | | |
| 26 | % |
| General
and administrative | |
| 1,671,960 | | |
| 1,766,843 | | |
| (94,883 | ) | |
| (5 | )% |
| Depreciation | |
| 17,492 | | |
| 36,866 | | |
| (19,374 | ) | |
| (53 | )% |
| Total
operating expenses | |
| 2,323,024 | | |
| 2,316,391 | | |
| 6,633 | | |
| 0 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Loss
from operations | |
| (1,978,993 | ) | |
| (2,207,768 | ) | |
| 228,775 | | |
| (10 | )% |
| | |
| | | |
| | | |
| | | |
| | |
| Other
expense | |
| | | |
| | | |
| | | |
| | |
| Interest
expense | |
| (63,580 | ) | |
| (41,536 | ) | |
| (22,044 | ) | |
| 53 | % |
| Total
other expense | |
| (63,580 | ) | |
| (41,536 | ) | |
| (22,044 | ) | |
| 53 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Net
loss before provision for income taxes | |
| (2,042,573 | ) | |
| (2,249,304 | ) | |
| 206,731 | | |
| (9 | )% |
| | |
| | | |
| | | |
| | | |
| | |
| Provision
for income taxes | |
| - | | |
| - | | |
| - | | |
| 0 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Net
loss | |
| (2,042,573 | ) | |
| (2,249,304 | ) | |
| 206,731 | | |
| (9 | )% |
| | |
| | | |
| | | |
| | | |
| | |
| Other
comprehensive (loss) income | |
| | | |
| | | |
| | | |
| | |
| Benefit
pension obligation changes | |
| 179,892 | | |
| 397,709 | | |
| (217,817 | ) | |
| (55 | )% |
| | |
| | | |
| | | |
| | | |
| | |
| FX
translation adjustment | |
| (4,986 | ) | |
| 32,837 | | |
| (37,823 | ) | |
| (115 | )% |
| Total
other comprehensive (loss) income | |
| 174,906 | | |
| 430,546 | | |
| (255,640 | ) | |
| (59 | )% |
| Comprehensive
loss | |
$ | (1,867,667 | ) | |
$ | (1,818,758 | ) | |
$ | (48,909 | ) | |
| 3 | % |
Revenue
Revenue
increased by $252 thousand, or 179%, for the year ended December 31, 2022 compared to the year ended December 31, 2021. The increase
was due to Proteomedix entering into a significant development services contract with a customer in the latter part of 2022.
Loss
from Operations
During
the years ended December 31, 2022 and 2021, our comprehensive loss was $1.9 million and $1.8 million, respectively. The decrease
was due to an increase in revenue associated with the provision of development services to third parties.
Marketing
and business development
Marketing
and business development expense increased by $40 thousand, or 20%, for the year ended December 31, 2022 compared to the year ended
December 31, 2021. The increase was due to increased medical marketing efforts in Europe.
Research
and development
Research
and development expense increased by $81 thousand, or 26%, for the year ended December 31, 2022 compared to the year ended December
31, 2021. The increase was due to a collaboration arrangement with a third party under which we recognized certain costs for personnel,
facilities and material.
General
and administrative
General
and administrative expense decreased by $95 thousand, or 5%, for the year ended December 31, 2022 compared to the year ended December
31, 2021. The decrease was due to a reduction to our use of consultants for non-core services.
Depreciation
Depreciation
decreased by $19 thousand, or 53%, for the year ended December 31, 2022 compared to the year ended December 31, 2021. The decrease
was due to existing fixed assets reaching the end of the respective estimated useful lives.
Interest
expense
Interest
expense increased by $22 thousand, or 53%, for the year ended December 31, 2022 compared to the year ended December 31, 2021. The
increase was due to losses associated with the conversion of currencies received from our customers into CHF, our functional currency.
Benefit
pension obligation changes
Benefit
pension obligation changes decreased by $218 thousand, or 55%, for the year ended December 31, 2022 compared to the year ended December
31, 2021. The decrease was due to a non-recurring payment of benefits out of funds held in trust within the pension trust in 2021.
Foreign
currency translation adjustments
Foreign
currency translation adjustments decreased by $38 thousand, or 115%, for the year ended December 31, 2022 compared to the year ended
December 31, 2021. The decrease was due to changes in the exchange rate between CHF and USD as well as additional borrowings during 2021.
Comparison
of the nine months ended September 30, 2023 and 2022
| | |
Nine
months ended | | |
| | |
| |
| | |
September 30,
2023 | | |
September 30,
2022 | | |
$
Change | | |
%
Change | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 2,092,761 | | |
$ | 128,773 | | |
$ | 1,963,988 | | |
| 1525 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Cost
of goods sold | |
| 22,548 | | |
| 28,176 | | |
| (5,628 | ) | |
| -20 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Gross
profit | |
| 2,070,213 | | |
| 100,597 | | |
| 1,969,616 | | |
| 1545 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Operating
expenses | |
| | | |
| | | |
| | | |
| | |
| Marketing
and business development | |
| 151,478 | | |
| 172,478 | | |
| (21,000 | ) | |
| -12 | % |
| Research
and development | |
| 275,020 | | |
| 262,818 | | |
| 12,202 | | |
| 5 | % |
| General
and administrative | |
| 1,240,875 | | |
| 1,633,860 | | |
| (392,985 | ) | |
| -24 | % |
| Depreciation | |
| 9,293 | | |
| 12,966 | | |
| (3,673 | ) | |
| -28 | % |
| Total
operating expenses | |
| 1,676,666 | | |
| 2,082,122 | | |
| (405,456 | ) | |
| -60 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Income
(loss) from operations | |
| 393,547 | | |
| (1,981,525 | ) | |
| 2,375,072 | | |
| 1605 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Other
expense | |
| | | |
| | | |
| | | |
| | |
| Interest
expense | |
| (74,359 | ) | |
| (48,257 | ) | |
| (26,102 | ) | |
| 54 | % |
| Total
other expenses | |
| (74,359 | ) | |
| (48,257 | ) | |
| (26,102 | ) | |
| 54 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Net
income (loss) before provision for income taxes | |
| 319,188 | | |
| (2,029,782 | ) | |
| 2,348,970 | | |
| 1659 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Provision
for income taxes | |
| - | | |
| - | | |
| - | | |
| 0 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Net
income (loss) | |
| 319,188 | | |
| (2,029,782 | ) | |
| 2,348,970 | | |
| 1659 | % |
| | |
| | | |
| | | |
| | | |
| | |
| FX
translation adjustment | |
| 172,351 | | |
| 344,957 | | |
| (172,606 | ) | |
| -50 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Changes
in pension benefit obligation | |
| (168,307 | ) | |
| 369,287 | | |
| (537,594 | ) | |
| -146 | % |
| | |
| | | |
| | | |
| | | |
| | |
| Comprehensive
income | |
$ | 323,232 | | |
$ | (1,315,538 | ) | |
$ | 1,638,770 | | |
| 1464 | % |
Revenue
Revenue
increased by $2 million, or 1,525%, for the nine months ended September 30, 2023 compared to the nine months ended September 30, 2022.
Approximately $1.5 million of the increase was due to the expansion and continued progress of a development services contract with a
single customer and approximately $0.5 million of the increase was due to a one-time licensing contract with a single customer, in each
case during the nine months ended September 30, 2023.
Loss
from Operations
During
the nine months ended September 30, 2023, our comprehensive income was $323 thousand and during the nine months ended September 30, 2022,
our comprehensive loss was $1.3 million. The change was due to increased revenue associated with the provision of development services
and licensing fees.
Marketing
and business development
Marketing
and business development expense decreased by $21 thousand, or 12%, for the nine months ended September 30, 2023 compared to the nine
months ended September 30, 2022. The decrease was due to narrowing our marketing efforts in EMEA and focusing on existing lab partners
already utilizing our Proclarix product.
Research
and development
Research
and development expense increased by $12 thousand, or 5%, for the nine months ended September 30, 2023 compared to the nine months ended
September 30, 2022. The increase was due to a collaboration arrangement with a third party under which we recognized certain costs for
personnel, facilities and material.
General
and administrative
General
and administrative expense decreased by $393 thousand, or 24%, for the nine months ended September 30, 2023 compared to the nine months
ended September 30, 2022. The decrease was due to a reduction to our use of consultants for non-core services as well as reduced personnel
costs.
Depreciation
Depreciation
decreased by $4 thousand, or 28%, for the nine months ended September 30, 2023 compared to the nine months ended September 30, 2022.
The decrease was due to existing fixed assets reaching the end of the respective estimated useful lives.
Interest
expense
Interest
expense increased by $26 thousand, or 54%, for the nine months ended September 30, 2023 compared to the nine months ended September 30,
2022. The increase was due to losses associated with the conversion of currencies received from our customers into CHF.
Benefit
pension obligation changes
Benefit
pension obligation changes decreased by $538 thousand, or 146%, for the nine months ended September 30, 2023 compared to the nine months
ended September 30, 2022. The decrease was due to actuarial gains offset by contributions made by employees during 2023 which did not
occur in 2022.
Foreign
currency translation adjustments
Foreign
currency translation adjustments decreased by $172 thousand, or 50%, for the nine months ended September 30, 2023 compared to the nine
months ended September 30, 2022. The decrease was due to changes in the exchange rate between CHF and USD.
Trend
Information
Other
than as disclosed elsewhere in this proxy statement, we are not aware of any trends, uncertainties, demands, commitments or events that
are reasonably likely to have a material effect on our revenues, net income, profitability, liquidity or capital resources, or that would
cause reported financial information not necessarily to be indicative of future operating results or financial condition.
Liquidity
and Capital Resources
| | |
For
the
nine months
ended
September 30,
2023 | | |
For
the
nine months
ended
September 30,
2022 | | |
Year
ended
December 31,
2022 | | |
Year
ended
December 31,
2021 | |
| Net
cash (used in) provided by | |
| | |
| | |
| | |
| |
| Operating
activities | |
$ | 346,029 | | |
$ | (1,477,904 | ) | |
$ | (1,933,570 | ) | |
$ | (2,239,556 | ) |
| Investing
activities | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Financing
activities | |
$ | - | | |
$ | (50,000 | ) | |
$ | (50,000 | ) | |
$ | 3,277,170 | |
Operating
Activities
Net
cash provided by operating activities for the nine months ended September 30, 2023, was $346 thousand, compared to cash used of $1.5
million for the nine months ended September 30, 2022, a decrease of $1.8 million. The decrease was due to increased revenue from development
services and a one-time licensing fee received during 2023.
Net
cash used in operating activities for the year ended December 31, 2022, was $1.9 million, compared to $2.3 million for the
year ended December 31, 2021, a decrease of $366 thousand. The decrease was due to increased revenue during the period which resulted
in a reduced amount of cash used in operations.
Investing
Activities
We
had no cash used in or provided by investing activities during any of the periods presented above.
Financing
Activities
Net
cash used in financing activities for the nine months ended September 30, 2023, was $0, compared to $50 thousand for the nine months
ended September 30, 2022, a decrease of $50 thousand. The decrease was due to the one-time repayment of a note payable during 2022.
Net
cash used in financing activities for the year ended December 31, 2022, was $50 thousand, compared to net cash provided by financing
activities of $3.4 million for the year ended December 31, 2021. The decrease was due to the one-time repayment of a note payable during
2022.
Liquidity
Outlook
Since
our inception, we have incurred significant operating losses and negative cash flows and have financed our operations primarily through
the issuance of shares and convertible notes to shareholders and directors. Our primary short-term requirements for liquidity and
capital are to fund general working capital and capital expenditures. Our principal long-term working capital uses include the development
of ancillary diagnostic markers and related supporting services to expand our existing intellectual property portfolio.
We
expect to incur significant expenses in connection with our ongoing activities as we continue to implement our business strategy. We
will need additional funding in connection with these activities. Our future funding requirements, both short-term and long-term, will
depend on many factors, including:
| ● | the
cost associated with the anticipated expansion of our distribution of Proclarix in Europe
and launching distribution in the United States; |
| ● | the
costs of future commercialization activities, including product manufacturing, marketing,
sales for Proclarix; |
| ● | the
costs associated with investments in our pipeline to expand our product offering in the future; |
| ● | the
outcome, timing and cost of seeking and obtaining regulatory approvals from regulatory authorities,
including the potential for such authorities to require that we perform field efficacy studies,
require more studies than those that we currently expect or change their requirements regarding
the data required to support a marketing application; |
| ● | our
ability to maintain existing, and establish new, strategic collaborations, licensing or other
arrangements and the financial terms of any such agreements, including the timing and amount
of any future milestone, royalty or other payments due under any such agreement; |
| ● | any
product liability or other lawsuits related to our products; |
| ● | the
expenses needed to attract, hire and retain skilled personnel; and |
| ● | the
costs and timing of preparing, filing and prosecuting patent applications, maintaining and
enforcing our intellectual property rights and defending any intellectual property-related
claims. |
A
change in the outcome of any of these or other variables could significantly change the costs and timing associated with our business
activities. Furthermore, our operating plans may change in the future, and we may need additional funds to meet operational needs and
capital requirements associated with such change.
For
the foreseeable future, we expect to continue financing our operations through capital contributions or loans made by Onconetix. We do
not currently have any committed external source of funds. If we or Onconetix are unable to raise additional funds, we may be required
to delay, reduce, suspend or cease our product development programs or any future commercialization efforts, which would have a negative
impact on our business, prospects, operating results and financial condition.
As
of September 30, 2023, we had an accumulated deficit of $27 million. As of December 31, 2022 and December 31, 2021, we had an accumulated
deficit of $27.2 million and $25.2 million, respectively. As of September 30, 2023, we had cash of $1 million. As of December 31, 2022
and December 31, 2021, we had cash of $470 thousand and $2.5 million, respectively. These matters, among others, raise substantial doubt
about Proteomedix’s ability to continue as a going concern for the 12 months following the issuance of the accompanying consolidated
financial statements.
Management
believes that the actions presently being taken to further implement its business plan and generate revenues provide the opportunity
for Proteomedix to continue as a going concern. While Proteomedix believes in the viability of its strategy to generate revenues and
the ability of its Onconetix to provide additional funds, there can be no assurances to that effect. The ability of Proteomedix to continue
as a going concern is dependent upon Proteomedix’s ability to further implement its business plan and obtaining additional funding
from Onconetix as needed.
Off-Balance
Sheet Arrangements
We
did not have over the past three fiscal years, and we currently do not have, any off-balance sheet arrangements as defined in the rules
and regulations of the SEC. To the extent we have any contingent assets or liabilities, these have been captured and audited within the
accompanying consolidated financial statements.
Background
of the PMX Transaction
Description
of Negotiations between Onconetix and Proteomedix
On
October 5, 2023, Tungsten Advisors (“Tungsten”), on behalf of Proteomedix, submitted a draft letter of intent (“LOI”)
to Onconetix. The LOI terms proposed by Proteomedix included, among other things (i) proposed transaction consideration to Proteomedix
equity holders consisting of newly issued shares of Onconetix common stock with an aggregate value of $75 million, (ii) a mutual exclusivity
period to last until a definitive agreement is executed and (iii) a contingent value right (“CVR”) to additional consideration
for each Onconetix stockholder in the event of any future corporate transaction. The draft LOI also provided that the completion of a
private placement in Onconetix of at least $5 million, to close concurrently with the proposed transaction between Onconetix and Proteomedix,
would be a closing condition to the proposed transaction.
On
October 18, 2023, Tungsten, on behalf of Onconetix, emailed to Proteomedix a mark-up of the proposed LOI. The revised draft of the LOI
proposed by Onconetix, as compared with the initial draft of the LOI, among other things (i) revised the exclusivity term to a period
of sixty days, (ii) added a six-month post-closing lock-up period for Proteomedix equity holders, (iii) provided that Onconetix would
issue to Proteomedix stockholders a number of shares equal to up to 19.9% of Onconetix’s then issued and outstanding shares of
common stock, with the remainder of the consideration to be issued as shares of Onconetix convertible preferred stock, convertible upon
approval by Onconetix stockholders, (iv) deleted the concept of the issuance of CVRs to Onconetix stockholders and (v) added as a closing
condition, if deemed necessary or appropriate by the board of directors of Onconetix (the “Onconetix Board”), the receipt
by the Onconetix Board of a fairness opinion issued by a reputable firm opining that the proposed transaction is fair to Onconetix stockholders.
On
October 20, 2023, Tungsten, on behalf of Proteomedix, emailed to Onconetix a revised draft of the LOI and, after additional communication
among Tungsten, Onconetix and Proteomedix, Proteomedix and Onconetix finalized the LOI. On October 22, 2023, the board of directors of
Proteomedix approved the LOI. On October 24, 2023, the Onconetix Board approved the LOI, following which the parties executed the LOI.
Subsequent
to the execution of the LOI, a “kick-off” meeting was held by videoconference on October 30, 2023, among (i) representatives
of Onconetix, (ii) representatives of Onconetix’s U.S. legal counsel, Ellenoff Grossman & Schole LLP (“EGS”), (iii)
representatives of Tungsten, (iv) representatives of Proteomedix’s Swiss legal counsel, Vischer AG (“Vischer”) and
(v) representatives of Proteomedix’s U.S. legal counsel, Brown Rudnick LLP, to discuss the anticipated terms of the proposed transaction
outlined in the LOI and, at a high level, the anticipated process and timeline to complete the proposed transaction. Following that meeting,
on October 10, 2023, Tungsten provided Onconetix, EGS and Onconetix’s Swiss legal counsel, Wenger Plattner, access to a virtual
data room containing certain financial and legal information of Proteomedix. On November 30, 2023, Onconetix provided Proteomedix, Nelson
Mullins Riley & Scarborough LLP (“NM”), U.S. legal counsel to Proteomedix
replacing Brown Rudnick, and Vischer access to a virtual data room containing certain financial and legal information of Onconetix.
The
parties and their legal counsel discussed and negotiated the terms of the Share Exchange Agreement, an initial draft of which was prepared
and sent by EGS to NM on November 14, 2023. Between November 14, 2023, and December 15, 2023, Onconetix, EGS, Wenger Plattner, Proteomedix,
NM and Vischer exchanged multiple drafts of the Share Exchange Agreement. Numerous calls and virtual meetings between EGS, Wenger Plattner,
NM and Vischer were held during this period to discuss the terms of the Share Exchange Agreement, including meetings on December 5, 2023,
December 7, 2023, December 10, 2023, December 13, 2023, December 14, 2023 and December 15, 2023. Representatives of Onconetix and Proteomedix
participated in many of these calls and meetings. The topics discussed during these calls and virtual meetings included, without limitation,
(i) mechanics of the share exchange, including with respect to outstanding Proteomedix options, (ii) representations, warranties and
covenants of Proteomedix shareholders, Proteomedix and Onconetix, (iii) covenants regarding the operation of Onconetix and Proteomedix
between the Closing and the conversion (the “Conversion”) of the shares of Onconetix Series B Convertible Preferred Stock
and (iv) indemnification.
During
the course of negotiations of the Share Exchange Agreement, the parties also exchanged drafts of, and negotiated the terms of, the Lock-Up
Agreement, the Non-Competition and Non-Solicitation Agreement, the Support Agreement and the Series B Certificate of Designation (collectively,
the “Ancillary Agreements”).
During
the period between execution of the LOI and signing of the Share Exchange Agreement, Onconetix’s legal counsel, Wenger Plattner
and EGS, conducted legal due diligence based on the documents and other information provided by Proteomedix in the virtual data room.
Due diligence efforts focused, among other areas, on Proteomedix’s capitalization, intellectual property and material contracts.
To facilitate legal due diligence efforts, EGS sent to Brown Rudnick and Tungsten a customary legal due diligence request list on November
2, 2023, which requests were responded to by Proteomedix and its counsel in writing, orally during meetings and by Proteomedix periodically
uploading responsive documents and other information to the virtual data room. Over the following weeks and until the Share Exchange
Agreement was signed, Proteomedix, Onconetix and their respective counsel continued to hold supplemental diligence meetings and engage
in related communication.
During
the period between the execution of the LOI and signing of the Share Exchange Agreement, Proteomedix’s legal counsel, NM and Vischer,
conducted legal due diligence based on the documents and other information provided by Onconetix in the virtual data room. Due diligence
efforts focused, among other areas, on Onconetix’s capitalization, intellectual property and material contracts. To facilitate
legal due diligence efforts, Nelson Mullins sent to EGS a customary legal due diligence request list on November 20, 2023, which requests
were responded to by Onconetix and its counsel in writing, orally during meetings and by Onconetix periodically uploading responsive
documents and other information to the virtual data room.
The
execution version of the Share Exchange Agreement and the Ancillary Agreements contain a number of material terms reflecting
negotiations between the parties subsequent to November 14, 2023, including, among other things, that (i) the parties agreed that
the aggregate value of the shares (the “Exchange Shares”) of Onconetix common stock and shares of Series B Convertible
Preferred Stock issued as consideration in the share exchange would be equal to approximately Seventy-Five Million U.S. Dollars
($75,000,000), less the value of Proteomedix shares for which outstanding Proteomedix options are exercisable, subject to adjustment
for indemnification, (ii) Proteomedix options would remain outstanding until the Conversion, at which time outstanding Proteomedix
options would be assumed by Onconetix and converted into the right to receive options to acquire shares of Onconetix common stock or
such other derivative security as Onconetix and Proteomedix agree, (iii) Proteomedix would indemnify Onconetix for any breaches of
Proteomedix’s representations, warranties or covenants contained in the Share Exchange Agreement through an adjustment of the
number of shares of Onconetix common stock issued upon Conversion, (iv) Onconetix would indemnify Proteomedix for any breaches of
Onconetix’s representations, warranties or covenants contained in the Share Exchange Agreement through an adjustment of the
number of shares of Onconetix common stock issued upon Conversion and (v) Proteomedix shareholders would indemnify Onconetix for any
breaches of the representations, warranties and covenants of Proteomedix shareholders contained in the Share Exchange Agreement by
recourse to the Exchange Shares and the shares of Onconetix common stock issuable upon the Conversion.
On
December 13, 2023, the Onconetix Board convened a virtual meeting to consider the proposed transaction between Proteomedix and Onconetix.
EGS gave a brief presentation to the Onconetix Board regarding the terms of the Share Exchange Agreement and the transactions contemplated
thereby. Onconetix management, led by Mr. Campbell, also presented to the Onconetix Board Onconetix’s management’s analysis
of Proteomedix and the business opportunity that Onconetix management believed may be represented by the transaction with Proteomedix,
based on information and materials shared with the Onconetix Board prior to such meeting. After review and discussion, including questions
from members of the Onconetix Board posed to EGS and to Onconetix management, the Onconetix Board adjourned the December 13, 2023 meeting,
and agreed to take action with regard to the Share Exchange Agreement by written consent, a form of which was circulated to the members
of the Onconetix Board on December 14, 2023. On December 15, 2023, the members of the Onconetix Board agreed, by unanimous written consent,
to approve the proposed final version of the Share Exchange Agreement and the transactions contemplated thereby and recommended that
Onconetix’s stockholders adopt and approve in all respects the Share Exchange Agreement and the transactions contemplated thereby.
Onconetix
Board’s Reasons for the Approval of the Business Combination
The
Onconetix Board, in evaluating the PMX Transaction, consulted with Onconetix’s management and its financial and legal advisors.
In reaching its unanimous resolution that the Share Exchange Agreement and the transactions contemplated thereby, including the PMX Transaction
and the issuance of shares of common stock in connection therewith, are advisable and in the best interests of Onconetix, the Onconetix
Board considered a range of factors, including, but not limited to, the factors discussed below. In light of the number and wide variety
of factors considered in connection with its evaluation of the PMX Transaction, the Onconetix Board did not consider it practicable to,
and did not attempt to, quantify or otherwise assign relative weights to the specific factors that it considered in reaching its determination
and supporting its decision. The Onconetix Board viewed its decision as being based on all of the information available and the factors
presented to and considered by it. In addition, individual directors may have given different weight to different factors. This explanation
of Onconetix’s reasons for the PMX Transaction and all other information presented in this section is forward-looking in nature
and, therefore, should be read in light of the factors discussed under “Cautionary Note Regarding Forward-Looking Statements.”
The
Onconetix Board considered a number of factors pertaining to the PMX Transaction as generally supporting its decision to enter into the
Share Exchange Agreement and the transactions contemplated thereby, including, but not limited to, the following material factors:
| ● | Immediate
Revenue Stream. Proteomedix is a commercial-stage business and generating revenue from
Proclarix. |
| | | |
| ● | Strategic
Alignment. Marketing and sales activities for both Onconetix’s ENTADFI and Proteomedix’s
Proclarix are focused on urologists. |
| | | |
| ● | Large
and Expanding Growth Industry: According to Global Industry Analysts, the prostate cancer
diagnostics market is anticipated to grow from approximately $8.5 billion in 2023 to approximately
$13.7 billion in 2030. The industry is experiencing a transformation due to non-invasive
and more precise tests. With its current technology, which incorporates over 10 years of
industry expertise and innovation, Proteomedix is particularly well positioned to benefit
from this growing market. Proteomedix expects strong growth. |
| | | |
| ● | Due
Diligence. Due diligence examinations of Proteomedix and discussions with Proteomedix’s
management team and Onconetix’s legal advisors concerning Onconetix’s due diligence
examination of Proteomedix. |
| | | |
| ● | Financial
Condition. The Board also considered factors such as Proteomedix’s historical financial
results, outlook, financial plan and debt structure as well as mergers and acquisitions activity
for companies in the life science diagnostic industry. In considering these factors, the
Onconetix Board reviewed Proteomedix’s historical growth and its current prospects
for growth if Proteomedix achieves its business plan and various historical and current balance
sheet items of Proteomedix. |
| | | |
| ● | Experienced
Management Team. Proteomedix has a strong management team with significant operating
experience. The senior management of Proteomedix intend to remain with Proteomedix in the
capacity of officers and/or directors, providing helpful continuity in advancing Proteomedix’s
strategic and growth goals. |
| | | |
| ● | Fairness
Opinion. Wainwright provided its opinion to the Onconetix Board that, subject to the
procedures followed, assumptions made, qualifications and limitations on the review undertaken
and the other matters considered by Wainwright in preparing its opinion, the Exchange Consideration
was fair, from a financial point of view, to Onconetix. |
| | | |
| ● | Lock-Up.
Stockholders of Proteomedix have agreed to be subject to a 180-day lockup in respect of their
Company securities subject to certain customary exceptions. |
| | | |
| ● | PIPE
Investment. Third-party investor interest in the PIPE investment served as validation
of the valuation and opportunity represented by a transaction with Proteomedix. |
| | | |
| ● | Other
Alternatives. The Board believed, after a thorough review of other strategic opportunities
reasonably available to Onconetix, that the PMX Transaction represented the best potential
opportunity for Onconetix. |
| | | |
| ● | Negotiated
Transaction. The financial and other terms of the Share Exchange Agreement and the fact
that such terms and conditions are reasonable and were the product of arm’s length
negotiations between Onconetix and Proteomedix. |
The Onconetix Board also considered a variety
of uncertainties and risks and other potentially negative factors concerning the PMX Transaction including, but not limited to, the following:
| |
● |
Business Plan and Projections May Not Be Achieved. The risk that Proteomedix may not be able to execute on the business plan, and realize the financial performance as set forth in the financial projections, in each case, presented to Onconetix’s management team and board of directors. |
| |
● |
Litigation. The possibility of litigation challenging the PMX Transaction or that an adverse judgment granting permanent injunctive relief could indefinitely enjoin consummation of the PMX Transaction. |
| |
● |
Benefits May Not Be Achieved. The risks that the potential benefits of the PMX Transaction may not be fully achieved or may not be achieved within the expected timeframe. |
| |
● |
The Company Stockholders Receiving a Minority Position in Proteomedix. The risk that Onconetix stockholders will hold a minority position in Proteomedix. |
| |
● |
Fees and Expenses. The fees and expenses associated with completing the PMX Transaction. |
| |
● |
Other Risks Factors. Various other risk factors associated with the business of Proteomedix, as described in the section entitled “Risk Factors” appearing elsewhere in this proxy statement. |
The above discussion of the material factors
considered by the Onconetix Board is not intended to be exhaustive, but does set forth the principal factors considered by the Onconetix
Board.
Management Forecasts
The management of Onconetix and Proteomedix prepared certain limited
financial forecasts about their potential future business. These analyses were shared with Wainwright, and were utilized by Wainwright,
without independent verification, in connection with the preparation of its fairness opinion. These financial forecasts and the material
assumptions contained therein are described below.
The financial
forecasts prepared by management of Onconetix and Proteomedix and utilized by Wainwright were not detailed financial projections maintained
by management of Onconetix and Proteomedix in the ordinary course and were not prepared with a view towards public disclosure. Furthermore,
those forecasts are dependent entirely on assumptions about a variety of events and circumstances that may or may not occur and are subject,
in all upon a number of assumptions respects, to actual results and to risks and contingencies, known and unknown, many of which are
outside of Onconetix’s and Proteomedix’s control, in many cases, and which cannot be predicted in advance. Forward-looking information,
including information about future business plans, are inherently subject to significant uncertainties and contingencies. Forward-looking
statements are also susceptible to multiple interpretations and inherently reflect assumptions with respect to general business, economic,
regulatory, market and financial conditions and other future events, all of which are difficult to predict and many of which are beyond
the control of Onconetix and Proteomedix. Investors are encouraged to read carefully the information contained in this proxy statement,
including the financial information and the descriptions about various risks and uncertainties concerning Onconetix’s and Proteomedix’s
business described herein, including under the headings “Risk Factors,” “Onconetix’s Management’s
Discussion and Analysis of Financial Condition and Results of Operations,” “Proteomedix’s Management’s Discussion
and Analysis of Financial Condition and Results of Operations” and “Cautionary Statement Regarding Forward-Looking Statements.”
The financial forecasts that were prepared by management of Onconetix
and Proteomedix and shared with Wainwright comprised probability weighted projections of management of Onconetix and Proteomedix’s
expected future cash flows as shown in the following table. As further described below, Wainwright,
without independent verification, utilized these projections in connection with
the preparation of its fairness opinion.
Onconetix management considered
these projections to be reasonable, based on management’s observations, expertise and industry knowledge, taking into account that
all of Onconetix’s planned business activities, as reflected in the forecasts, are speculative, and the costs and expenses Onconetix
incurs may be different, potentially substantially, from the estimates thereof incorporated in the assumptions. Moreover, Onconetix’s
plans may, and can be expected, to change over the timeline of the forecast periods, potentially materially, as underlying facts and circumstances
specific to Onconetix and more general, change.
Proteomedix management considered
these projections to be reasonable, based on management’s observations, expertise and industry knowledge, taking into account that
all of Proteomedix’s planned business activities, as reflected in the forecasts, are speculative, and the costs and expenses Proteomedix
incurs may be different, potentially substantially, from the estimates thereof incorporated in the assumptions. Moreover, Proteomedix’s
plans may, and can be expected, to change over the timeline of the forecast periods, potentially materially, as underlying facts and circumstances
specific to Proteomedix and more general, change.
Opinion of Onconetix’s Financial Advisor
The Board retained Wainwright on November 6, 2023, to render an opinion
as to the fairness, from a financial point of view, to Onconetix of the Exchange Consideration to be paid by Onconetix pursuant to the
Share Exchange Agreement.
On December 13, 2023, Wainwright rendered its
oral opinion to the board of directors of Onconetix (which was subsequently confirmed in writing by delivery of Wainwright’s written
opinion dated the same date) to the effect that, based upon and subject to the assumptions, factors, qualifications and limitations set
forth in the written opinion described herein, as of December 13, 2023, the Exchange Consideration was fair, from a financial point of
view, to Onconetix.
Wainwright’s opinion was prepared for
the information of the board of directors of Onconetix and only addressed the fairness, from a financial point of view, to Onconetix
of the Exchange Consideration to be paid by Onconetix pursuant to the Share Exchange Agreement. Wainwright was not requested to
opine as to, and Wainwright’s fairness opinion does not address, the relative merits of the Share Exchange or any alternatives
to the Share Exchange, Onconetix’s underlying decision to proceed with or effect the Share Exchange, or any other aspect of
the Share Exchange. Wainwright’s opinion does not address the fairness of the Share Exchange to the holders of any class of
securities, creditors or other constituencies of Onconetix. Wainwright did not express an opinion about the fairness of the amount
or nature of any compensation payable or to be paid to any of the officers, directors or employees of Onconetix, whether or not
relative to the Share Exchange. Wainwright did not express an opinion about the fairness of the Private Placement Investment.
The summary of Wainwright’s opinion
in this proxy statement is qualified in its entirety by reference to the full text of its written opinion, which is included as
Annex C to this proxy statement and sets forth the procedures followed, assumptions made, qualifications and limitations on the
review undertaken and other matters considered by Wainwright in preparing its opinion. Wainwright’s opinion was prepared for
the information of the board of directors of Onconetix for its use in connection with its consideration of the Share Exchange.
Neither Wainwright’s written opinion nor the summary of its opinion and the related analyses set forth in this proxy statement
are intended to be, and they do not constitute, a recommendation to any stockholder of Onconetix as to how such stockholder should
vote with respect to any matter relating to the Share Exchange or any other matter.
The terms of the Share Exchange, the consideration to be paid in the
Share Exchange, and the related transactions were determined through arm’s length negotiations between Onconetix and Proteomedix
and were approved unanimously by Onconetix’s board of directors. Wainwright did not determine the consideration to be paid by Onconetix
in connection with the Share Exchange.
In connection with rendering the fairness
opinion described above and performing its related financial analyses, Wainwright, among other things, reviewed:
| ● | the financial terms of the Share Exchange described in a
draft of the Share Exchange Agreement dated December 13, 2023; |
| ● | certain information, including financial forecasts, relating to the
business, earnings, cash flow, assets, liabilities and prospects of Onconetix and Proteomedix that were furnished to Wainwright by management
of Onconetix and Proteomedix, respectively; |
| ● | relevant market sizing projections for the assets and liabilities that
will be acquired by Onconetix; |
| |
● |
management of Onconetix’s assessment of the strategic rationale for, and the potential benefits of the Share Exchange; |
| |
● |
the reported price and trading activity of Onconetix’s common stock; |
| |
● |
certain publicly available information, including but not limited to, Onconetix’s recent filings with the Securities and Exchange Commission and the financial statements set forth therein; |
| ● | the financial terms, to the extent publicly available, of
certain acquisition and financing transactions that Wainwright deemed relevant; and |
| ● | such other analyses and such other factors as Wainwright
deemed appropriate for the purpose of rendering its opinion. |
For purposes of its opinion, with the approval of the board of directors
of Onconetix and without independent verification, Wainwright assumed that:
| |
● |
the Creditors and the former holders of the Purchased Shares will own 87.1% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion; |
| |
● |
the Private Placement Investors will own 7.6% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion; |
| |
● |
the holders of the outstanding equity of Onconetix immediately prior to the Share Exchange will own 5.3% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion; and |
| |
● |
the total number of shares (after giving effect to the Conversion) of Onconetix common stock to be outstanding immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion is based on $75.0 million of shares outstanding and an assumed 10-day VWAP of $0.249 per share. |
In arriving at its opinion, Wainwright assumed
and relied upon, without verifying independently, the accuracy and completeness of all information that was publicly available or was
furnished, or otherwise made available, to Wainwright, or discussed with or reviewed by or for Wainwright for the purposes of preparing
its opinion, and further assumed that the financial information provided to Wainwright had been prepared by the respective managements
of Onconetix and Proteomedix on a reasonable basis in accordance with industry practice, and that the managements of Onconetix and Proteomedix
were not aware of any information or facts that would make any information provided to Wainwright incomplete or misleading.
With respect to the financial forecasts,
estimates and other forward-looking information reviewed by Wainwright, Wainwright assumed that such information had been reasonably
prepared by the respective managements of Onconetix and Proteomedix based on assumptions reflecting their best currently available
estimates and judgments as to the expected future results of operations and financial condition of Onconetix and Proteomedix,
respectively. Wainwright was not engaged to assess the achievability of any such financial forecasts, estimates or forward-looking
information or the assumptions on which they were based, and Wainwright expressed no opinion as to such information or assumptions.
In addition, Wainwright did not assume any responsibility for, and did not perform, any appraisals or valuations of any specific
assets or liabilities (fixed, contingent or other) of Onconetix or Proteomedix, nor was Wainwright furnished or provided with any
such appraisals or valuations. Without limiting the generality of the foregoing, Wainwright was not engaged to, and did not
undertake, any independent analysis of any pending or threatened litigation, regulatory action, possible unasserted claims or other
contingent liabilities, to which Onconetix, Proteomedix or any of their respective affiliates is a party or may be subject, and at
the direction of the board of directors of Onconetix and with its consent, Wainwright’s fairness opinion made no assumption
concerning, and did not consider, the possible assertion of claims, outcomes or damages arising out of any such matters.
Wainwright relied upon and assumed, without independent
verification, that the representations and warranties of all parties set forth in the Share Exchange Agreement and all related documents
and instruments that are referred to therein are true and correct, that each party to the Share Exchange Agreement will fully and timely
perform all of the covenants and agreements required to be performed by such party, that the Share Exchange will be consummated pursuant
to the terms of the Share Exchange Agreement, without amendment thereto, and that all conditions to the consummation of the Share Exchange
will be satisfied without waiver by any party of any conditions or obligations thereunder. Wainwright further assumed that the Share Exchange
Agreement was in all material respects identical to the draft of the Share Exchange Agreement provided to Wainwright. Finally, Wainwright
also assumed that all the necessary regulatory approvals and consents required for the Share Exchange, including the approval of the stockholders
of Onconetix, will be obtained in a manner that will not adversely affect Proteomedix.
In connection with its opinion, Wainwright assumed
and relied upon, without independent verification, the accuracy and completeness of all of the financial, legal, regulatory, tax, accounting
and other information provided to, discussed with or reviewed by it. Wainwright’s opinion does not address any legal, tax, accounting,
or regulatory matters. Wainwright’s fairness opinion was approved by its fairness opinion committee prior to delivering it to the
board of directors of Onconetix.
Wainwright’s opinion is necessarily based
upon the information available to Wainwright and facts and circumstances as they existed and were subject to evaluation as of December
13, 2023, which is the date of the Wainwright opinion. Although events occurring after the date of the Wainwright opinion could materially
affect the assumptions used in preparing the opinion, Wainwright does not have any obligation to update, revise or reaffirm its opinion
and Wainwright expressly disclaims any responsibility to do so. Wainwright did not express any opinion as to the value of the shares of
Onconetix’s common stock to be issued in the Share Exchange or the prices at which shares of Onconetix’s common stock may
trade following announcement of the Share Exchange or at any future time.
The terms of the Share Exchange, the consideration
to be paid in the Share Exchange, and the related transactions were determined through arm’s length negotiations between Onconetix
and Proteomedix and were approved unanimously by Onconetix’s board of directors. Wainwright did not determine the consideration
to be paid by Onconetix in connection with the Share Exchange. Wainwright’s opinion and its presentation to Onconetix’s board
of directors was one of many factors taken into consideration by the board of directors of Onconetix in deciding to approve, adopt and
authorize the Share Exchange Agreement. Consequently, the analyses as described herein should not be viewed as determinative of the opinion
of Onconetix’s board of directors with respect to the consideration to be paid by Onconetix in the Share Exchange or of whether
Onconetix’s board of directors would have been willing to agree to different consideration.
The following is a summary of the material financial
analyses performed by Wainwright in connection with the preparation of its fairness opinion, which opinion was rendered orally to the
board of directors of Onconetix (and subsequently confirmed in writing by delivery of Wainwright’s written opinion dated the same
date) on December 13, 2023. The preparation of analyses and a fairness opinion is a complex analytic process involving various determinations
as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances
and, therefore, such an opinion is not readily susceptible to summary description and this summary does not purport to be a complete description
of the analyses performed by Wainwright or the delivery of Wainwright’s opinion to the board of directors of Onconetix. This summary
includes information presented in tabular format. In order to fully understand the financial analyses presented by Wainwright, the tables
must be read together with the text of each analysis summary and considered as a whole. The tables alone do not constitute a complete
summary of the financial analyses. Considering any portion of such analyses and of the factors considered, without considering all analyses
and factors, could create a misleading or incomplete view of the process underlying Wainwright’s opinion.
In furnishing its opinion, Wainwright did not
attempt to combine the analyses described herein into one composite valuation range, nor did Wainwright assign any quantitative weight
to any of the analyses, or the other factors considered. Furthermore, in arriving at its opinion, Wainwright did not attribute any particular
weight to any analysis or factor considered by it, but rather made qualitative judgments as to the significance and relevance of each
analysis and factor in light of one another. Accordingly, Wainwright has stated that it believes that its analyses must be considered
as a whole and that considering any portion of its analyses, without considering all of the analyses, could create a misleading or incomplete
view of the process underlying its opinion or the conclusions to be drawn therefrom.
In conducting the analysis as to the fairness, from a financial point of view, to Onconetix of the Exchange Consideration to be paid by Onconetix pursuant to the Share Exchange Agreement, Wainwright evaluated
the stand-alone valuations of Onconetix and Proteomedix. Wainwright then evaluated the potential valuation of the combined company and
compared it to the pro forma ownership of the combined company by the stockholders of Onconetix immediately prior to the Share Exchange
pursuant to the terms of the Share Exchange Agreement.
The results of the application by Wainwright of
each of the valuation methodologies utilized in connection with its fairness opinion are summarized below.
Consideration to be paid in the Share Exchange
As specified in the Share Exchange Agreement, the parties attributed
an enterprise value of $75.0 million to Proteomedix and an enterprise value of $9.9 million to Onconetix representing equity value of
$4.6 million plus net debt of $5.3 million. As noted above, for purposes of its opinion, with the approval of the board of directors of
Onconetix and without independent verification, Wainwright made the following assumptions:
| |
● |
the Creditors and the former holders of the Purchased Shares will own 87.1% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion; |
| |
● |
the Private Placement Investors will own 7.6% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion; |
| |
● |
the holders of the outstanding equity of Onconetix immediately prior to the Share Exchange will own 5.3% of the outstanding equity of Onconetix immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion. |
In analyzing the fairness, from a financial point of view, to Onconetix
of the Exchange Consideration to be paid by Onconetix pursuant to the Share Exchange Agreement, Wainwright evaluated the implied valuation
of Proteomedix on a standalone basis and compared that to the $75.0 million enterprise value attributable to Proteomedix in the Share
Exchange Agreement and the implied valuation of Onconetix on a standalone basis and compared that to the $9.9 million enterprise value
attributable to Onconetix in the Share Exchange Agreement.
Proteomedix Implied Valuation
Wainwright determined a range of implied valuations
for Proteomedix using the following valuation metrics, each of which is described further below.
Discounted Cash Flow Analysis
The discounted cash flow analysis is a “forward
looking” methodology and is based on projected future cash flows to be generated by Proteomedix which are then discounted back to
the present. This methodology has three primary components: (1) the present value of projected unlevered cash flows for a determined period;
(2) the present value of the terminal value of cash flows (representing firm value beyond the time horizon on the projections) or a perpetuity
growth calculation based on terminal free cash flow; and (3) the weighted average cost of capital (WACC) used to discount such future
cash flows and terminal value or perpetuity value back to the present. The future cash flows plus the terminal value or perpetual value
of such cash flows are discounted by the company’s risk-adjusted cost of capital, the WACC, to derive a present value.
Proteomedix management provided to Wainwright
a probability weighted projection of Proteomedix’s expected future cash flows as shown in the following table.
$ in millions
Source: Company Management
Wainwright estimated a perpetuity growth rate
of between 0% and 4.0%. Wainwright also assumed a Weighted Average Cost of Capital (WACC or discount rate) range of 11.1% to 15.1%. Based
on these inputs, Wainwright determined an enterprise value range of between $159.0 million and $356.0 million. The tables provided below
show these calculations and the WACC calculated by Wainwright.
Proteomedix WACC Analysis

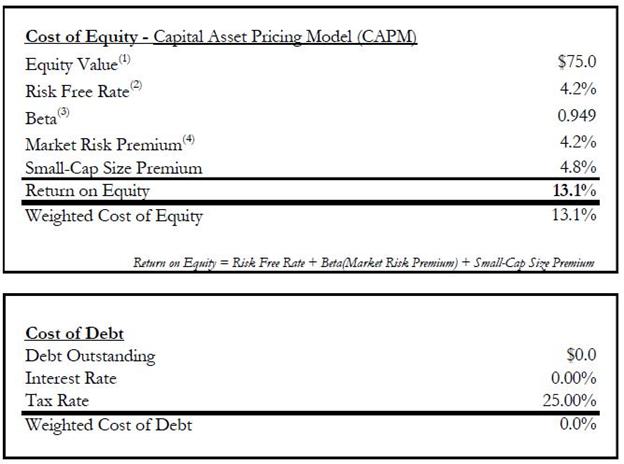
Source: Bloomberg & FactSet; market data as of 12/11/2023
| (1) | Market Capitalization on 12/11/2023, FactSet |
| (2) | Based on yield of 5-year treasury bond as published by
FactSet on 12/11/2023 |
| (3) | Beta determined by relevering average 2-year unlevered
adjusted beta for select public market comparable companies |
| (4) | Long-term U.S.A. ERP as of July 2023 as published by Aswath
Damodaran |
Based on these inputs, Wainwright calculated an
enterprise value range between $185.0 million and $271.0 million using the perpetuity growth methodology, compared to the $75 million
enterprise value attributable to Proteomedix in the Share Exchange Agreement.
Comparable Public Company Analysis
Wainwright also evaluated the implied enterprise
valuation of Proteomedix using a comparable company analysis. The comparable company analysis uses data based on current enterprise values
of public companies that Wainwright viewed as comparable to Proteomedix to develop a measure of current value for Proteomedix. Wainwright
reviewed the total enterprise values of selected publicly traded, commercial-stage medical diagnostic companies that Wainwright viewed
as operating in similar commercial markets to Proteomedix. The selected comparable public companies shown in the table below had an enterprise
valuation range of between $43.0 million (25th percentile) and $255.0 million (75th percentile). Wainwright did
not exclude any companies meeting the criteria described above.
$ in millions

Source: FactSet as of 12/11/2023
Based on the analysis described above, Wainwright
estimated that the enterprise value of Proteomedix ranged between $43.0 million and $255.0 million, compared to the $75 million enterprise
value attributable to Proteomedix in the Share Exchange Agreement.
Precedent M&A Transactions
The precedent M&A analysis uses data based
on the values acquirers have previously placed on comparable companies in a merger or acquisition to develop a measure of current value
for Proteomedix. Wainwright examined precedent transactions, from June 25, 2018 through August 2, 2022, involving publicly traded, commercial-stage
medical diagnostic companies that Wainwright viewed as operating in similar commercial markets to Proteomedix. Wainwright used only the
upfront consideration paid in these transactions and did not consider any contingent value rights or other contingent consideration. The
transactions shown in the table below had upfront consideration values ranging between $30.0 million (25th percentile) and
$432.0 million (75th percentile). Wainwright did not exclude any companies meeting the criteria described above.
$ in millions
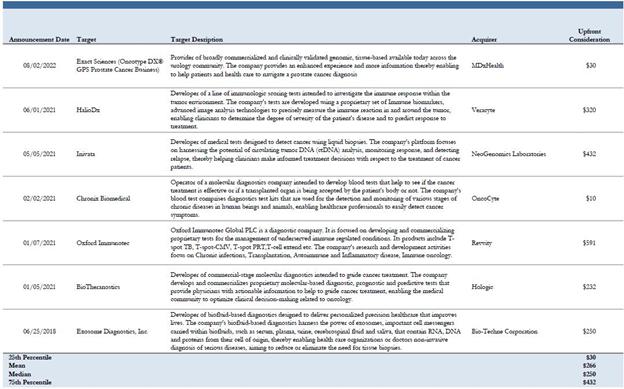
Source: Company SEC Filings, Press Releases, Pitchbook as of 12/11/2023
| (1) | Adjusted for the ~10% stake in Inivata that NeoGenomics
already had before the outright acquisition |
Onconetix Implied Valuation
Wainwright determined a range of implied valuations for Onconetix using
the following valuation metrics, each of which is described further below. Wainwright stated its belief that significant weight should
be applied to the discounted cash flow analysis for Onconetix because Onconetix’s public comparable companies and precedent transaction
analyses did not account for (i) the significant dilution that Onconetix would need to take on to fund future operations, (ii) Onconetix’s
high cost of capital and (iii) Onconetix’s inability to access the capital markets.
Discounted Cash Flow Analysis
The discounted cash flow analysis is a “forward
looking” methodology and is based on projected future cash flows to be generated by Onconetix which are then discounted back to
the present. This methodology has three primary components: (1) the present value of projected unlevered cash flows for a determined period;
(2) the present value of the terminal value of cash flows (representing firm value beyond the time horizon on the projections) or a perpetuity
growth calculation based on terminal free cash flow; and (3) the weighted average cost of capital (WACC) used to discount such future
cash flows and terminal value or perpetuity value back to the present. The future cash flows plus the terminal value or perpetual value
of such cash flows are discounted by the company’s risk-adjusted cost of capital, the WACC, to derive a present value.
Onconetix management provided to Wainwright a probability weighted
projection of Onconetix’s expected future cash flows as shown in the following table.
$ in millions

Source: Company Management
Wainwright estimated a perpetuity growth rate
of between (2.0)% and 2.0%. Wainwright also assumed a Weighted Average Cost of Capital (WACC or discount rate) range of 14.8% to 18.8%.
Based on these inputs, Wainwright determined an enterprise value range of between $5.0 million and $7.0 million. The tables provided below
show these calculations and the WACC calculated by Wainwright.
Onconetix WACC Analysis

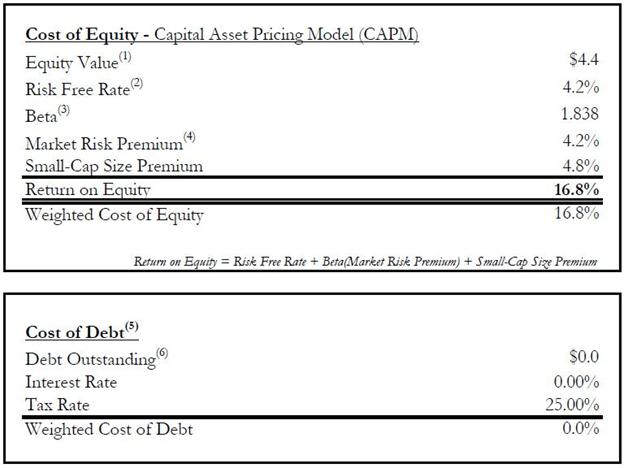
Source: Bloomberg & FactSet; market data as of 12/11/2023
| (1) | Market Capitalization on 12/11/2023, FactSet |
| (2) | Based on yield of 5-year treasury bond as published by FactSet on 12/11/2023 |
| (3) | Two-year historical adjusted beta for BWV per Bloomberg as of 12/11/2023 |
| (4) | Long-term U.S.A. ERP as of July 2023 as published by Aswath Damodaran |
| (5) | All numbers taken from Company 10-Q filed 10/20/2023 representing Q2-23 |
| (6) | Does not include the consideration owed to Veru Inc. since it does not accrue any interest |
Based on these inputs, Wainwright calculated an enterprise value range
between $5.0 million and $6.0 million using the perpetuity growth methodology, compared to the $9.9 million enterprise value attributable
to Onconetix in the Share Exchange Agreement.
Comparable Public Company Analysis
Wainwright also evaluated the implied enterprise valuation of Onconetix
using a comparable company analysis. The comparable company analysis uses data based on current enterprise values of public companies
that Wainwright viewed as comparable to Onconetix to develop a measure of current value for Onconetix. Wainwright reviewed the total enterprise
values of selected publicly traded, specialty pharmaceutical companies that Wainwright viewed operating in similar commercial markets
to Onconetix. The selected comparable public companies shown in the table below had an enterprise valuation range of between $5.0 million
(25th percentile) and $43.0 million (75th percentile). Wainwright did not exclude any companies meeting the criteria
described above.
$ in millions
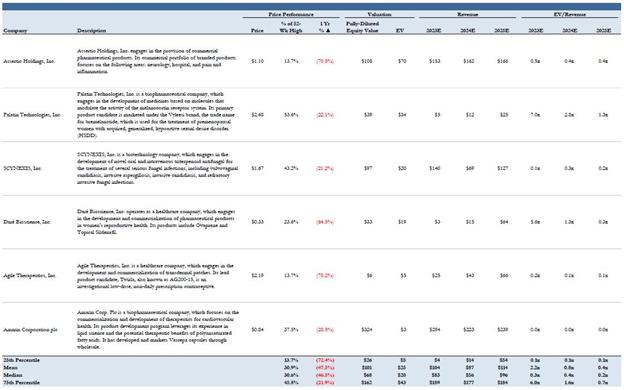
Source: FactSet as of 12/11/2023
Based on the analysis described above, Wainwright estimated that the
enterprise value of Onconetix ranged between $5.0 million and $43.0 million, compared to the $9.9 million enterprise value attributable
to Onconetix in the Share Exchange Agreement.
Precedent M&A Transactions
The precedent M&A analysis uses data based on the values acquirers
have previously placed on comparable companies in a merger or acquisition to develop a measure of current value for Onconetix. Wainwright
examined precedent transactions, from September 6, 2019 through August 31, 2023, involving, specialty pharmaceutical companies that Wainwright
viewed operating in similar commercial markets to Onconetix. Wainwright used only the upfront consideration paid in these transactions
and did not consider any contingent value rights or other contingent consideration. The transactions shown in the table below had upfront
consideration values ranging between $15.0 million (25th percentile) and $79.0 million (75th percentile).
$ in millions
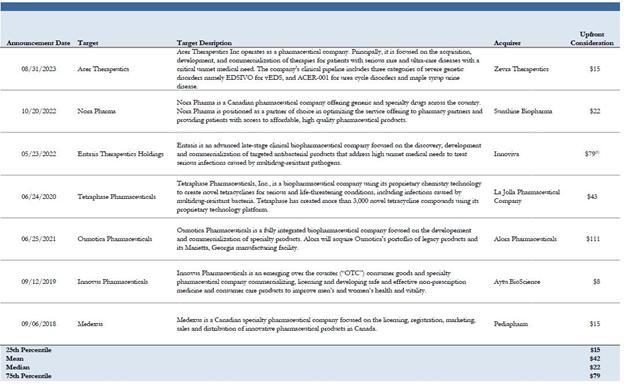
Source: Company SEC Filings, Press Releases, Pitchbook as of 12/11/2023
| (1) | Adjusted consideration for acquisition of remaining ~40%
of outstanding shares to reflect company’s fully diluted enterprise value |
General
Wainwright is a nationally recognized investment banking firm that
provides financial advisory services and is continually engaged in the valuation of businesses and their securities in connection with
mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and
valuations for estate, corporate and other purposes. The Onconetix board of directors retained Wainwright to render an opinion as to the
fairness, from a financial point of view, to Onconetix of the Exchange Consideration to be paid by Onconetix pursuant to the Share Exchange
Agreement based upon the foregoing qualifications, experience and expertise.
Onconetix paid Wainwright a fee of $250,000
for rendering its fairness opinion delivered in connection with the Share Exchange. The opinion fee was not contingent in whole or
in part on the success of the Share Exchange, or on the results of Wainwright’s evaluation and analysis or upon the
conclusions reached in Wainwright’s opinion. In addition, Onconetix agreed to reimburse Wainwright for its reasonable
out-of-pocket expenses, including reasonable fees and disbursements of its counsel. Onconetix has also agreed to indemnify
Wainwright against certain liabilities and other items that may arise out of Onconetix’s engagement of Wainwright.
Onconetix’s board of directors did not limit Wainwright in any way in the investigations it made or the procedures it followed
in rendering its opinion.
Except as described below, Wainwright has not
had a material relationship with, nor otherwise received fees from, Onconetix, Proteomedix or any other parties to the Share Exchange
during the two years preceding the date of Wainwright’s opinion:
| |
● |
In July 2023, Wainwright
acted as Onconetix’s exclusive placement agent in connection with the warrant inducement transaction described under “Information
About the Business of the Combined Business – Warrant Inducement.” Onconetix paid Wainwright a cash fee of approximately
$230,000. The Company also agreed to reimburse Wainwright for its expenses in connection with the exercise of the Existing PIOs and the
issuance of the Inducement PIOs, $50,000 for fees and expenses of legal counsel and other out-of-pocket expenses and agreed to pay Wainwright
for non-accountable expenses in the amount of $35,000 and a clearing fee of $15,950. In addition, the exercise for cash of the Existing
PIOs triggered the issuance to Wainwright or its designees of warrants to purchase 149,173 shares of common stock, which have substantially
the same terms as the Inducement PIOs except for an exercise price equal to $1.3625 per share. The Company also agreed to pay Wainwright
a cash fee of 7.5% of any gross proceeds that the Company may receive from the exercise for cash of the Inducement PIOs and issue warrants
to Wainwright or its designees upon any exercise for cash of the Inducement PIOs, that number of shares of common stock equal to 6.0%
of the aggregate number of such shares of common stock underlying any Inducement PIOs that have been exercised, also with an exercise
price of $1.3625. The maximum cash payable under this provision is $406,496 and the maximum number of warrants issuable under this provision
is 298,346. |
| |
|
|
| |
● |
In March 2023, Onconetix entered into an At The Market Offering Agreement with Wainwright (the “ATM Agreement”) covering the sale of up to $3.9 million of Onconetix’s common stock pursuant to which Onconetix agreed to pay to Wainwright a commission of 3.0% of the gross proceeds from the sale of shares and to reimburse Wainwright for certain expenses. No sales have occurred under the ATM Agreement. |
| |
|
|
| |
● |
In August 2022, Wainwright
acted as Onconetix’s exclusive placement agent in connection with a private placement of securities. Onconetix paid Wainwright
a cash fee of approximately $850,000 and non-accountable expenses of $85,000. In addition, the Company issued to
Wainwright, or its designees, warrants to purchase up to 220,997 shares of common stock (the “August Wainwright
Warrants”). The August Wainwright Warrants have substantially the same terms as the preferred investment options issued in the
private placement, except that the exercise price was $3.3938. Further, upon any exercise for cash of any preferred investment
options, the Company agreed to pay Wainwright a 7.5% cash fee and to issue Wainwright (or its designees) additional warrants to
purchase the number of shares of common stock equal to 6.0% of the aggregate number of shares of common stock underlying the
preferred investment options that have been exercised, also with an exercise price of $3.3938 (the “August Contingent
Warrants”). The maximum cash fee payable upon the cash exercise of the August Wainwright Warrants is approximately $949,485
and the maximum number of shares of Onconetix common stock covered by the August Contingent Warrants issuable to Wainwright under
this provision is 298,346. |
| |
|
|
| |
● |
In April 2022, Wainwright
acted as Onconetix’s exclusive placement agent in connection with a private placement of securities. Onconetix paid Wainwright
a cash fee of approximately $680,000 and reimbursed certain out-of-pocket expenses in an aggregate of $50,000 and non-accountable
expenses of $35,000. In addition, the Company issued to Wainwright or its designees warrants to purchase up to 70,849
shares of common stock (the “April Wainwright Warrants”). The Wainwright Warrants are in substantially the same form as
the preferred investment options issued in the private placement, except that the exercise price is $8.46875. Further, upon any
exercise for cash of any preferred investment options, the Company agreed to pay Wainwright a 7.5% cash fee and issue to Wainwright
(or its designees) additional warrants to purchase the number of shares of common stock equal to 6.0% of the aggregate number of
shares of common stock underlying the preferred investment options that have been exercised, also with an exercise price of $8.46875
(the “April Contingent Warrants”). The maximum cash fee payable upon the cash exercise of the April Wainwright Warrants
is approximately $588,930 and the maximum number of shares of Onconetix common stock covered by the April Contingent Warrants
issuable to Wainwright under this provision is 70,849 and were exchanged in connection with the August 2022 private
placement. |
In the future, Wainwright may provide financial advisory
and investment banking services to Onconetix, Proteomedix or their respective affiliates for which Wainwright would expect to receive
compensation.
Consistent with applicable legal and regulatory requirements, Wainwright
has adopted policies and procedures to establish and maintain the independence of its research departments and personnel. As a result,
Wainwright’s research analysts may hold views, make statements or investment recommendations and/or publish research reports with
respect to Onconetix, Proteomedix and/or the Share Exchange that differ from the views of its investment banking personnel.
RISK FACTORS
Risks Related to Onconetix
There is substantial doubt about our ability
to continue as a “going concern.”
The Company has incurred
substantial operating losses since inception and expects to continue to incur significant operating losses for the foreseeable future.
As of September 30, 2023, the Company had cash of approximately $7.7 million, a working capital deficit of approximately $8.1 million
and an accumulated deficit of approximately $34.4 million.
On January 23, 2024, the Company issued the Debenture
in exchange for $4.6 million in net cash proceeds. The Debenture is repayable in full upon the earlier of (i) the closing under the Subscription
Agreement and (ii) June 30, 2024.
The Company will require significant additional capital to fund its
continuing operations, satisfy existing and future obligations and liabilities, and otherwise support the Company’s working capital
needs and business activities, including making the remaining payments to Veru, the commercialization of ENTADFI and Proclarix, and the
development and commercialization of its current product candidates and future product candidates. In addition, if Stockholder Approval
is not obtained by January 1, 2025, the Company may be obligated to cash settle the Series B Preferred Stock. Management’s
plans include generating product revenue from sales of ENTADFI, which is subject to further successful commercialization activities, and
Proclarix, which may still be subject to further successful commercialization activities within certain jurisdictions. Certain of the
commercialization activities are outside of the Company’s control, including but not limited to, securing contracts with wholesalers
and third-party payers, securing contracts with third-party logistics providers, obtaining required licensure in various jurisdictions,
as well as attempting to secure additional required funding through equity or debt financings if available. However, there are currently
no commitments in place for further financing nor is there any assurance that such financing will be available to the Company on favorable
terms, if at all. If the Company is unable to secure additional capital, it may be required to delay or curtail any future clinical trials,
development and/or commercialization of products and product candidates, and it may take additional measures to reduce expenses in order
to conserve its cash in amounts sufficient to sustain operations and meet its obligations. These conditions raise substantial doubt about
the Company’s ability to continue as a going concern for a period of time within one year after the date of the issuance of the
condensed financial statements included in this proxy statement. If Stockholder Approval is not obtained, the Series B Preferred Stock becomes redeemable by the holders
of the Series B Preferred Stock for cash. The Company does not currently have sufficient cash to redeem the shares of Series
B Preferred Stock.
We entered into an asset purchase agreement
and management services agreement with WraSer, which have been terminated because we believe that a material adverse event has occurred
with respect to the WraSer Assets. However, the termination is subject to WraSer’s right to challenge the termination and assert
claims against us.
On June 13, 2023, we entered into the WraSer APA and MSA with WraSer
in connection with the purchase of the WraSer Assets. Under the WraSer APA, we paid $3.5 million in cash to WraSer at signing. In October
2023, WraSer alerted us that its sole manufacturer for the API for Zontivity, the key driver for the WraSer acquisition, would no longer
manufacture the API for Zontivity. We believe that this development constituted a Material Adverse Effect under the APA enabling us to
terminate the APA and MSA. On October 20, 2023, we filed a motion for relief from the automatic stay in the Bankruptcy Court to exercise
our termination rights under the WraSer APA, as amended. On December 18, 2023, the Bankruptcy Court entered an Agreed Order lifting the
automatic stay to enable us to exercise our rights to terminate the APA and the MSA without prejudice to the parties’ respective
rights, remedies, claims and defenses they had against one another under the APA and MSA. On December 21, 2023, we filed a Notice with
the Bankruptcy Court terminating the APA and MSA. WraSer has advised us that it does not believe that a Material Adverse Event occurred.
Due to the WraSer bankruptcy filing and our status as an unsecured creditor of WraSer, it is also unlikely that we will recover the $3.5
million Signing Cash or any costs and resources in connection with services provided by the Company under the WraSer MSA.
Company stockholders may not realize a
benefit from the ENTADFI or Proteomedix acquisitions commensurate with the ownership dilution they will experience in connection
with the transactions.
If the Company is unable to realize the full strategic and financial
benefits currently anticipated from the recent ENTADFI and Proteomedix acquisitions, our stockholders may experience a dilution of their
ownership interests our Company without receiving any commensurate benefit, or only receiving part of the commensurate benefit to the
extent the Company is able to realize only part of the strategic and financial benefits currently anticipated from the transactions.
The issuance or conversion of securities would
result in significant dilution in the equity interest of existing stockholders and adversely affect the marketplace of the securities.
The issuance or conversion of common shares or other securities convertible
into common shares would result in significant dilution in the equity interest of existing stockholders and adversely affect the market
price of the common shares. We have issued 3,000 shares of Series A Preferred Stock to Veru which are initially convertible one year from
issuance, in the aggregate, into 5,709,935 shares of the Company’s common stock, subject to adjustment and certain shareholder approval
limitations specified in the Certificate of Designations. We have issued 2,696,729 shares
of Series B Preferred Stock to former shareholders of Proteomedix which are initially convertible, in the aggregate, into 269,672,900 shares
of the Company’s common stock, subject to adjustment and certain shareholder approval limitations specified in the Certificate of
Designations. These and other future issuances of or conversions of securities may result in significant dilution to existing stockholders,
which could adversely impact your investment.
We may have violated Section 13(k) of the
Exchange Act (implementing Section 402 of the Sarbanes-Oxley Act of 2002) and may be subject to sanctions as a result.
Section 13(k) of the Exchange
Act provides that it is unlawful for a company that has a class of securities registered under Section 12 of the Exchange Act to, directly
or indirectly, including through any subsidiary, extend or maintain credit in the form of a personal loan to or for any of its directors
or executive officers. In the fiscal year ended December 31, 2022 and the nine months ended September 30, 2023, we paid certain expenses
of our former Chief Executive Officer and Chairman of the Board, which may be deemed to be personal loans made by us to our former Chief
Executive Officer and Chairman of the Board that are not permissible under Section 13(k) of the Exchange Act. Issuers that are found to
have violated Section 13(k) of the Exchange Act may be subject to civil sanctions, including injunctive remedies and monetary penalties,
as well as criminal sanctions. The imposition of any of such sanctions on us could have a material adverse effect on our business, financial
position, results of operations or cash flows.
Misconduct and errors by our current and
former employees and our third-party service providers could cause a material adverse effect on our business and reputation.
Our employees and third-party
service providers are integral to our business operations, including confidential information. If any such information were leaked to
unintended recipients due to human error, theft, malicious sabotage or fraudulent manipulation, we may be subject to liability for loss
of such information. Further, if any of our employees or third-party service providers absconded with our proprietary data or know-how
in order to compete with us, our competitive position may be materially and adversely affected.
Any improper conduct or use
of funds by any of our employees or third-party service providers in contravention of our protocols and policies may lead to regulatory
and disciplinary proceedings involving us. We may be perceived to have facilitated or participated in such conduct and we could be subject
to liability, damages, penalties and reputational damage. It is impossible to completely identify and eradicate all risks of misconduct
or human errors, and our precautionary measures may not be able to effectively detect and prevent such risks from happening.
Occurrence of any of the
above risks could result in a material adverse effect on our business and results of operations, as we are exposed to potential liability
to borrowers and investors, reputational damage, regulatory intervention, financial harm. Our ability to attract new and retain existing
borrowers and investors and operate as an ongoing concern may be impaired.
We may consider strategic alternatives in
order to maximize stockholder value, including financing, strategic alliances, licensing arrangements, acquisitions or the possible sale
of our business. We may not be able to identify or consummate any suitable strategic alternatives and any consummated strategic alternatives
may not be successful.
We may consider all strategic
alternatives that may be available to us to maximize stockholder value, including financings, strategic alliances, licensing arrangements,
acquisitions or the possible sale of our business. Our exploration of various strategic alternatives may not result in any specific action
or transaction. To the extent that this engagement results in a transaction, our business objectives may change depending upon the nature
of the transaction. There can be no assurance that we will enter into any transaction as a result of the engagement. Furthermore, if we
determine to engage in a strategic transaction, we cannot predict the impact that such strategic transaction might have on our operations
or stock price. We also cannot predict the impact on our stock price if we fail to enter into a transaction.
In addition, we face significant
competition in seeking appropriate strategic partners, and the negotiation process is time-consuming and complex. Moreover, we may not
be successful in our efforts to establish a strategic partnership or other alternative arrangements for our business activities because
they may be deemed to be at too early of a stage of development for collaborative effort. Any delays in entering into new strategic partnership
agreements harm our business prospects, financial condition and results of operations.
If we license products or
businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing
operations and company culture. We cannot be certain that, following a strategic transaction or license, we will achieve the results,
revenue or specific net income that justifies such transaction.
As a result of our failure to timely file
our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023, we are currently ineligible to file new short form registration
statements on Form S-3, which may impair our ability to raise capital on terms favorable to us, in a timely manner or at all.
Form S-3 permits eligible
issuers to conduct registered offerings using a short form registration statement that allows the issuer to incorporate by reference its
past and future filings and reports made under the Securities Exchange Act of 1934, as amended, or the Exchange Act. In addition,
Form S-3 enables eligible issuers to conduct primary offerings “off the shelf” under Rule 415 of the Securities Act of
1933, as amended, or the Securities Act. The shelf registration process, combined with the ability to forward incorporate information,
allows issuers to avoid delays and interruptions in the offering process and to access the capital markets in a more expeditious and efficient
manner than raising capital in a standard registered offering pursuant to a Registration Statement on Form S-1.
As a result of our failure to timely file our
Quarterly Report on Form 10-Q for quarter ended June 30, 2023, we are currently ineligible to file new short form registration
statements on Form S-3 and we will be unable to conduct “off the shelf” offerings under Rule 415 of the Securities
Act using our currently effective Registration Statement on Form S-3 (File No. 333-270383) after we file our annual report for the
fiscal year ended December 31, 2023. As a result, we may be unable to conduct an “at the market” offering pursuant to our
At The Market Offering Agreement with Wainwright after such date. In addition, if we seek to access the capital markets
through a registered offering during the period of time that we are unable to use Form S-3, we may be required to publicly disclose the
proposed offering and the material terms thereof before the offering commences, we may experience delays in the offering process due to
SEC review of a Form S-1 registration statement and we may incur increased offering and transaction costs and other considerations. Disclosing
a public offering prior to the formal commencement of an offering may result in downward pressure on our stock price. In addition, our
inability to conduct an offering “off the shelf” may require us to offer terms that may not be advantageous (or may be less
advantageous) to us or may generally reduce our ability to raise capital in a registered offering. If we are unable to raise capital through
a registered offering, we would be required to conduct our financing transactions on a private placement basis, which may be subject to
pricing, size and other limitations imposed under Nasdaq rules.
If we fail to maintain proper and effective
internal controls, our ability to produce accurate financial statements on a timely basis could be impaired. We have identified weaknesses
in our internal controls, and we cannot provide assurances that these weaknesses will be effectively remediated, or that additional material
weaknesses will not occur in the future.
We are subject to the reporting
requirements of the Exchange Act, the Sarbanes-Oxley Act and Nasdaq rules and regulations. The Sarbanes-Oxley Act requires, among other
things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. Effective internal
control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls
and procedures, is designed to prevent fraud. We must perform system and process evaluation and testing of our internal controls over
financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting in our Annual
Report on Form 10-K for each year, as required by Section 404 of the Sarbanes-Oxley Act (“Section 404”). This requires significant
management efforts and requires us to incur substantial professional fees and internal costs to expand our accounting and finance functions.
Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail
to meet our reporting obligations. In addition, any testing by us, as and when required, conducted in connection with Section 404, or
any subsequent testing by our independent registered public accounting firm, as and when required, may reveal deficiencies in our internal
controls over financial reporting that are deemed to be significant deficiencies or material weaknesses or that may require prospective
or retroactive changes to our financial statements, or may identify other areas for further attention or improvement. Furthermore, we
cannot be certain that our efforts will be sufficient to remediate or prevent future material weaknesses or significant deficiencies from
occurring.
We do not yet have effective
disclosure controls and procedures, or internal controls over all aspects of our financial reporting. Specifically, we have identified
the following control deficiencies which we believe are material weaknesses.
| |
● |
We did not maintain an effective control environment as there was an inadequate segregation of duties with respect to certain cash disbursements. The processing and the approval for payment of credit card transactions and certain bank wires were being handled by the former CEO and an accounting employee, and the accounting employee was responsible for the reconciliation of credit card statements and bank statements. This allowed these individuals to submit unauthorized payments to unauthorized third parties. |
| |
|
|
| |
● |
We did not have an effective risk assessment process over the identification of fraud risks surrounding the authorization, identification, approval and reporting of personal expenses charged to the Company’s corporate credit cards. |
| |
● |
We did not design and maintain effective monitoring of compliance with established accounting policies and procedures. |
| |
|
|
| |
● |
Our controls over the approval and reporting of expenses paid with the Company’s credit cards and certain bank wires were not designed and maintained to achieve the Company’s objectives. |
| |
|
|
| |
● |
We failed to employ a sufficient number of staff to maintain optimal segregation of duties, maintain adequate internal controls surrounding information technology procedures, such as a lack of a written information security policy, maintain adequate controls over the approval and posting of journal entries, and to provide optimal levels of oversight in order to process financial information in a timely manner, analyze and account for complex, non-routine transactions, and prepare financial statements. |
| |
|
|
| |
● |
We do not yet have adequate internal controls in place for the timely identification, approval or reporting of related party transactions. |
We cannot provide assurances that these weaknesses will be effectively
remediated, or that additional material weaknesses will not occur in the future.
As a result of the material
weaknesses in our internal controls over financial reporting described above, and other matters raised or that may in the future be raised
by the SEC, we may face for the prospect of litigation or other disputes which may include, among others, claims invoking the federal
and state securities laws, contractual claims or other claims arising from the material weaknesses in our internal control over financial
reporting and the preparation of our financial statements, any of which claims could result in adverse effects to our business. As of
the date hereof, we have no knowledge of any such litigation or dispute.
CFIUS may delay, prevent or impose conditions
on the Conversion.
CFIUS has authority to review certain direct or
indirect foreign investments in U.S. businesses for national security considerations. Among other things, CFIUS is authorized to require
mandatory filings for certain foreign investments in the United States and to self-initiate national security reviews of certain foreign
direct and indirect investments in U.S. businesses if the parties to such investments choose not to file voluntarily. With respect to
transactions that CFIUS determines present unresolved national security concerns, CFIUS has the power to suspend transactions, impose
mitigation measures or recommend that the President of the United States block pending transactions or order divestitures of completed
transactions when national security concerns cannot be mitigated. Whether CFIUS has jurisdiction to review an acquisition or investment
transaction depends on, among other factors, the nature and structure of the transaction, whether the target company is a U.S. business,
the level of beneficial ownership and voting interests acquired by foreign persons, and the nature of any information, control, access
or governance rights that the transaction affords foreign persons. For example, any transaction that could result in foreign “control”
(as such term is defined in the CFIUS regulations) of a U.S. business is within CFIUS’s jurisdiction. In addition, CFIUS has jurisdiction
over certain investments that do not result in control of a U.S. business by a foreign person but that afford a foreign person certain
access, involvement or governance rights in a “TID U.S. business,” that is, a U.S. business that: (1) produces, designs, tests,
manufactures, fabricates, or develops one or more “critical technologies;” (2) owns, operates, manufactures, supplies or services
certain “critical infrastructure;” or (3) maintains or collects, directly or indirectly, “sensitive personal data”
of U.S. citizens.
Certain entities or individuals associated with
or otherwise involved in the transaction are, are controlled by or have substantial ties with a non-U.S. person. Specifically, each of Dr. Schiess and Dr. Brühlmann is a “foreign person” (as such term is defined in 31 C.F.R. § 800.224).
CFIUS has broad discretion to interpret its regulations,
and we cannot predict whether CFIUS may seek to review the Conversion. If CFIUS reviews the Conversion and identifies an unresolved national
security concern as part of such review, CFIUS could recommend that the President of the United States order one or more foreign persons
to divest all or a portion of the Common Stock that they acquired without first obtaining CFIUS approval. Moreover, should CFIUS determine
that any parties to the Conversion were required to make a filing with CFIUS but failed to do so, CFIUS could impose a civil penalty not
to exceed $250,000 or the value of the relevant transaction, whichever is greater, on the parties it determines were subject to a mandatory
filing requirement.
Onconetix and Proteomedix will submit to
CFIUS a joint declaration or notice with respect to the PMX Transaction upon the request of CFIUS, but Onconetix has determined to
not exercise its right to elect to submit such a joint declaration or notice of its own initiative.
There can be no assurance that we will be
able to comply with the continued listing standards of Nasdaq.
Our continued eligibility for listing on Nasdaq
depends on our ability to comply with Nasdaq’s continued listing requirements.
On September 18, 2023, we received notice from
Nasdaq staff indicating that, based upon the closing bid price of the Common Stock for the prior 30 consecutive business days, we were
not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq, as set forth
in Nasdaq Listing Rule 5550(a)(2). We have 180 days from September 18, 2023, or through March 16, 2024, to regain compliance with the
Bid Price Rule.
If Nasdaq delists our common stock from trading
on its exchange for failure to meet the Bid Price Rule or any other listing standards, we and our stockholders could face significant
material adverse consequences including:
| |
● |
a limited availability of market quotations for our securities; |
| |
|
|
| |
● |
a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our common stock; |
| |
|
|
| |
● |
a limited amount of analyst coverage; and |
| |
|
|
| |
● |
a decreased ability to issue additional securities or obtain additional financing in the future. |
Risks Related to the Commercialization of our Products
We depend entirely on the success of a limited number of products
and/or product candidates, some of which are in preclinical development and have not entered a clinical trial. If we do not obtain regulatory
approval for and successfully commercialize one or more of our products and/or product candidates or we experience significant delays
in doing so, these product candidates may not be profitable.
We have several products that have not received
regulatory approval and may never be able to achieve approval and market such products. We expect that a substantial portion of our efforts
and expenses over the next few years will be devoted to the development of our products and/or product candidates. As a result, our business
currently depends heavily on the successful development, regulatory approval and, if approved, commercialization of these products and/or
product candidates. We cannot be certain that our products and/or product candidates will receive regulatory approval or will be successfully
commercialized even if they receive regulatory approval. The research, testing, manufacturing, safety, efficacy, labeling, approval, sale,
marketing and distribution of our products and/or product candidates are, and will remain, subject to comprehensive regulation by the
FDA and similar foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of any product candidate,
we may need to demonstrate through pre-clinical studies and clinical trials or studies that the product candidate is safe and effective
for use in each target indication. Pharmaceutical, therapeutic, and diagnostic product development is a long, expensive and uncertain
process, and delay or failure can occur at any stage of any of our clinical trials or studies. Failure to obtain regulatory approval for
our products and/or product candidates in the United States will prevent us from commercializing and marketing our products and/or product
candidates. The success of our products and/or product candidates will depend on several additional factors, including:
| |
● |
completing clinical trials and/or studies that demonstrate their efficacy and safety; |
| |
● |
receiving marketing approvals from applicable regulatory authorities; |
| |
● |
completing any post-marketing studies required by applicable regulatory authorities; |
| |
● |
establishing commercial manufacturing capabilities; |
| |
● |
launching commercial sales, marketing and distribution operations; |
| |
● |
the prevalence and severity of adverse events experienced
with our products and/or product candidates; |
| |
● |
acceptance of our products and/or product candidates
by patients, the medical community and third-party payors; |
| |
● |
a continued acceptable safety profile following approval; |
| |
● |
obtaining and maintaining healthcare coverage and
adequate reimbursement for our products and/or product candidates; |
| |
● |
competing effectively with other therapies, including
with respect to the sales and marketing of our products and/or product candidates, if approved; and |
| |
● |
qualifying for, maintaining, enforcing and defending our intellectual property rights and claims. |
Many of these factors are
beyond our control, including the time needed to adequately complete clinical testing, the regulatory submission process, potential threats
to our intellectual property rights and changes in the competitive landscape. It is possible that none of our products and/or product
candidates will ever obtain regulatory approval, even if we expend substantial time and resources seeking such approval. If we do not
achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully
complete clinical trials, obtain regulatory approval or, if approved, commercialize our products and/or product candidates, which would
materially harm our business, financial condition and results of operations.
The marketing approval process of the FDA is lengthy, time consuming
and inherently unpredictable, and if we are ultimately unable to obtain marketing approval for our current product candidates and future
product candidates we intend to develop, our business will be substantially harmed.
We are at a very early stage of development for
some of our products and/or product candidates. The product candidates we intend to develop have not gained marketing approval in the
U.S., and we cannot guarantee that we will ever have marketable products. Our business is substantially dependent on our ability to complete
the development of, obtain marketing approval for, and successfully commercialize our current and future product candidates in a timely
manner. We cannot commercialize our product candidates in the United States without first obtaining approval from the FDA to market each
product candidate. Our product candidates could fail to receive marketing approval for many reasons, including among others:
| |
● |
the FDA may disagree with the design or implementation of our clinical trials; |
| |
● |
Our clinical trials for our product candidate(s) must be successful if we are to seek and obtain regulatory marketing application through the submission of a new Biological License Application (BLA) and or New Drug Application (NDA), and marketing authorization application (MAA) with the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), respectively. Advanced clinical trials are often not successful even if prior trials were successful, and even if we are able to conduct advanced clinical trials and those trials are successful, we may not obtain necessary regulatory approvals for our product candidate(s) or we may be unable to successfully commercialize our products even if we receive the necessary regulatory approvals |
In addition, the process of seeking regulatory
approval to market the product candidates we intend to develop is expensive and time consuming and, notwithstanding the effort and expense
incurred, approval is never guaranteed. If we are not successful in obtaining timely approval of our product candidates from the FDA,
we may never be able to generate significant revenue and may be forced to cease operations. The new BLA, or NDA, process is costly, lengthy
and uncertain. Any BLA or NDA application filed by us will have to be supported by extensive data, including, but not limited to, technical,
pre-clinical, clinical, manufacturing and labelling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the
product for its intended use.
In order to commence a clinical trial in the United
States, we will be required to seek FDA acceptance of an IND for each of our product candidates. We cannot be sure any IND we submit to
the FDA, or any similar clinical trial application we submit in other countries, will be accepted. If we will be required by regulatory
authorities to conduct additional preclinical testing prior to filing an IND or similar application to clinically evaluate any of our
product candidates, this may result in delay in our product candidate development. The results of any such preclinical testing may not
be positive and may not support an application to study any of our product candidates in additional clinical trials.
It is possible that the FDA or EMA will not view
our ongoing or planned trials as providing adequate support for future clinical trials or for an application for marketing approval, for
any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results. If we
are unable to confirm or replicate the results of our trials in larger patient group or if negative results are obtained, we would likely
be further delayed or prevented from advancing further clinical development any of our product candidates.
Additionally, the FDA or EMA may disagree with
the sufficiency of our proposed reliance upon the preclinical, manufacturing or clinical data generated by third-party academic-sponsored
trials, or our interpretation of preclinical, manufacturing or clinical data from our ongoing trials. If so, the FDA or EMA may require
us to obtain and submit additional preclinical, manufacturing or clinical data.
Obtaining approvals from the FDA and from the
regulatory agencies in other countries is an expensive and time-consuming process and is uncertain as to outcome. The FDA and other agencies
could ask us to supplement our submissions, collect non-clinical data, conduct additional clinical trials or engage in other time-consuming
actions, or it could simply deny our applications. In addition, even if we obtain a BLA approval or pre-market approvals in other countries,
the approval could be revoked, or other restrictions imposed if post-market data demonstrate safety issues or lack of effectiveness. We
cannot predict with certainty how, or when, the FDA will act. If we are unable to obtain the necessary regulatory approvals, our financial
condition and cash flow may be adversely affected, and our ability to grow domestically and internationally may be limited. Additionally,
even if cleared or approved, our products may not be approved for the specific indications that are most necessary or desirable for successful
commercialization or profitability.
We may encounter substantial delays in completing our clinical
studies which in turn will require additional costs, or we may fail to demonstrate adequate safety and efficacy to the satisfaction of
applicable regulatory authorities.
It is
impossible to predict if or when our current or future product candidates will prove safe or effective in humans or will receive
regulatory approval. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must
conduct extensive clinical studies to demonstrate the safety and efficacy of the product candidates in humans. Clinical testing is
expensive, time-consuming and uncertain as to the outcome. We cannot guarantee that any clinical studies will be conducted as
planned or completed on schedule, if at all. A failure of one or more clinical studies can occur at any stage of testing. Events
that may prevent successful or timely completion of clinical development include:
| |
● |
delays in reaching, or failing to reach, a consensus with regulatory agencies on study design; |
| |
● |
delays in reaching, or failing to reach, agreement on acceptable terms with a sufficient number of prospective contract research organizations, or CROs, and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| |
● |
delays in recruiting a sufficient number of suitable patients to participate in our clinical studies; |
| |
● |
imposition of a clinical hold by regulatory agencies, after an inspection of our clinical study operations or study sites; |
| |
● |
failure by our CROs, other third parties, or us to adhere to clinical study, regulatory or legal requirements; |
| |
● |
failure to perform in accordance with the FDA’s good clinical practices, or GCPs, or applicable regulatory guidelines in other countries; |
| |
● |
delays in the testing, validation, manufacturing, and delivery of sufficient quantities of our product candidates to the clinical sites; |
| |
● |
delays in having patients complete participation in a study or return for post-treatment follow-up; |
| |
● |
clinical study sites or patients dropping out of a study; |
| |
● |
delay or failure to address any patient safety concerns that arise during the course of a trial; |
| |
● |
unanticipated costs or increases in costs of clinical trials of our product candidates; |
| |
● |
occurrence of serious adverse events associated with the product candidates that are viewed to outweigh its potential benefits; or |
| |
● |
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
We could also encounter delays if a clinical trial
is suspended or terminated by us, by the Institutional Review Board (IRB), or the Ethics Commission of the institutions in which such
trials are being conducted, by an independent Safety Review Board (SRB), for such trial or by the FDA or other regulatory authorities.
Such authorities may suspend or terminate a clinical trial due to a number of factors, including failure to conduct the clinical trial
in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the
FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects,
failure to demonstrate a benefit from using a drug, changes in governmental regulations or administrative actions or lack of adequate
funding to continue the clinical trial.
Any inability to successfully complete pre-clinical
and clinical development could result in additional costs to us or impair our ability to generate revenues from product sales, regulatory
and commercialization milestones and royalties. In addition, if we make manufacturing or formulation changes to our product candidates,
we may need to conduct additional studies to bridge our modified product candidates to earlier versions.
Clinical study delays could also shorten any periods
during which we may have the exclusive right to commercialize our products and/or product candidates or allow our competitors to bring
products to market before we do, which could impair our ability to successfully commercialize our products and/or product candidates.
In addition, any delays in completing our clinical trials will increase our costs, slow down our products and/or product candidates’
development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences
may significantly harm our business, financial condition and prospects. In addition, many of the factors that cause, or lead to, a delay
in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our products and/or
product candidates.
The outcome of pre-clinical studies and early
clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily
predict final results. Further, pre-clinical and clinical data are often susceptible to various interpretations and analyses, and many
companies that have believed their products and/or product candidates performed satisfactorily in pre-clinical studies and clinical trials
have nonetheless failed to obtain marketing approval. If the results of our clinical studies are inconclusive or if there are safety concerns
or adverse events associated with our products and/or product candidates, we may:
| |
● |
be delayed in obtaining marketing approval for our products and/or product candidates, if approved at all; |
| |
● |
obtain approval for indications or patient populations that are not as broad as intended or desired; |
| |
● |
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| |
● |
be required to change the way the product is administered; |
| |
● |
be required to perform additional clinical studies to support approval or be subject to additional post-marketing testing requirements; |
| |
● |
have regulatory authorities withdraw their approval of a product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy; |
| |
● |
experience damage to our reputation. |
Additionally, our products and/or product candidates
could potentially cause other adverse events that have not yet been predicted. The inclusion of ill patients in our clinical studies may
result in deaths or other adverse medical events due to other therapies or medications that such patients may be using. As described above,
any of these events could prevent us from achieving or maintaining market acceptance of our products and/or product candidates and impair
our ability to commercialize our products.
Obtaining and maintaining regulatory approval of our products
or product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval in other jurisdictions.
Obtaining and maintaining regulatory approval
of our products or product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval
in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on
the regulatory approval process in others. For example, even if the FDA grants marketing approval of a pharmaceutical product, comparable
regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product in those countries.
Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater
than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction
may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product must
be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge
for our products is also subject to approval.
We may also submit marketing applications in other
countries. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of pharmaceutical or diagnostic
products with which we must comply prior to marketing in those jurisdictions. Obtaining foreign regulatory approvals and compliance with
foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction
of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or receive applicable
marketing approvals, our target market will be reduced and our ability to realize the full market potential of our vaccine candidates
will be harmed.
Modifications to our products may require new BLA approvals.
Once a particular product receives FDA approval,
expanded uses or uses in new indications of our products may require additional human clinical trials and new regulatory approvals, including
additional IND, BLA and/or NDA, and premarket approvals before we can begin clinical development, and/or prior to marketing and sales.
If the FDA requires new approvals for a particular use or indication, we may be required to conduct additional clinical studies, which
would require additional expenditures and harm our operating results. If the products are already being used for these new indications,
we may also be subject to significant enforcement actions. Conducting clinical trials and obtaining approvals can be a time-consuming
process, and delays in obtaining required future approvals could adversely affect our ability to introduce new or enhanced products in
a timely manner, which in turn would harm our future growth.
Additional delays to the completion of clinical studies may result
from modifications being made to the protocol during the clinical trial, if such modifications are warranted and/or required by the occurrences
in the given trial.
Each modification to the protocol during a clinical
trial has to be submitted to the FDA. This could result in the delay or halt of a clinical trial while the modification is evaluated.
In addition, depending on the quantity and nature of the changes made, the FDA could take the position that the data generated by the
clinical trial are not poolable because the same protocol was not used throughout the trial. This might require the enrollment of additional
subjects, which could result in the extension of the clinical trial and the FDA delaying approval of a product. Any such delay could have
a material adverse effect on our business and results of operations.
There can be no assurance that the data generated from our clinical
trials using modified protocols will be acceptable to the FDA or other regulatory authorities.
There can be no assurance that the data generated
using modified protocols will be acceptable to the FDA or other regulatory authorities or that if future modifications during the trial
are necessary, that any such modifications will be acceptable to the FDA or other regulatory authorities. If the FDA or other regulatory
authorities believe that prior approval is required for a particular modification, they can delay or halt a clinical trial while they
evaluate additional information regarding the change.
Serious injury or death resulting from a failure
of our product candidates during current or future clinical trials could also result in the FDA or other regulatory authority delaying
our clinical trials or denying or delaying approval of a product.
Even though an adverse event may not be the result
of the failure of our product candidate, the FDA or other regulatory authority could delay or halt a clinical trial for an indefinite
period of time while an adverse event is reviewed, and likely would do so in the event of multiple such events.
Any delay or termination of our current or future
clinical trials as a result of the risks summarized above, including delays in obtaining or maintaining required approvals from the FDA
or other regulatory authorities, delays in patient enrollment, the failure of patients to continue to participate in a clinical trial,
and delays or termination of clinical trials as a result of protocol modifications or adverse events during the trials, may cause an increase
in costs and delays in the filing of any product submissions with the FDA or other regulatory authorities, delay the approval and commercialization
of our products or result in the failure of the clinical trial, which could adversely affect our business, operating results and prospects.
We will depend on enrollment and retention of patients in our
clinical trials for our product candidates. If we experience delays or difficulties enrolling or retaining patients in our clinical trials,
our research and development efforts and business, financial condition, and results of operations could be materially adversely affected.
Successful and timely completion of clinical trials
will require that we enroll and retain a sufficient number of patient candidates. Any clinical trials we conduct may be subject to delays
for a variety of reasons, including as a result of patient enrollment taking longer than anticipated, patient withdrawal, or adverse events.
These types of developments could cause us to delay the trial or halt further development.
Our clinical trials will compete with other clinical
trials that are in the same therapeutic areas as our product candidates, and this competition reduces the number and types of patients
available to us, as some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by
one of our competitors. Moreover, enrolling patients in clinical trials for diseases in which there is an approved standard of care is
challenging, as patients will first receive the applicable standard of care. Many patients who respond positively to the standard of care
do not enroll in clinical trials. This may limit the number of eligible patients able to enroll in our clinical trials who have the potential
to benefit from our product candidates and could extend development timelines or increase costs for these programs. Patients who fail
to respond positively to the standard of care treatment will be eligible for clinical trials of unapproved drug candidates. However, these
prior treatment regimens may render our therapies less effective in clinical trials.
Because the number of qualified clinical investigators
and clinical trial sites is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our
competitors use, which will reduce the number of patients who are available for our clinical trials at such clinical trial sites.
Patient enrollment depends on many factors, including:
| |
● |
the size and nature of the patient population; |
| |
● |
the severity of the disease, condition or infection under investigation; |
| |
● |
eligibility criteria for the trial; |
| |
● |
the proximity of patients to clinical sites; |
| |
● |
the design of the clinical protocol; |
| |
● |
the ability to obtain and maintain patient consents; |
| |
● |
perceived risks and benefits of the product candidate under evaluation; |
| |
● |
the ability to recruit clinical trial investigators with the appropriate competencies and experience; |
|
● |
the risk that patients enrolled in clinical trials will drop out of the trials before the administration of our product candidates or trial completion; |
| |
● |
the availability of competing clinical trials; |
| |
● |
the availability of such patients during the COVID-19 pandemic; |
| |
● |
the availability of new drugs approved for the indication the clinical trial is investigating; and |
| |
● |
clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies. |
These factors may make it difficult for us to
enroll enough patients to complete our clinical trials in a timely and cost-effective manner. Delays in the completion of any clinical
trial of our product candidates will increase our costs, slow down our product candidate development and approval process, and delay or
potentially jeopardize our ability to commence product sales and generate revenue. In addition, some of the factors that cause, or lead
to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our
product candidates.
Conducting successful clinical studies may require the enrollment
of large numbers of patients, and suitable patients may be difficult to identify and recruit.
Patient enrollment in clinical trials and completion
of patient participation and follow-up depends on many factors, including the size of the patient population; the nature of the trial
protocol; the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; the availability
of appropriate clinical trial investigators; support staff; and the proximity of patients to clinical sites and ability to comply with
the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged
from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up
to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are
not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to
participate in contemporaneous clinical trials of competitive products.
The results of our future clinical trials may not support our
product candidates’ claims or may result in the discovery of unexpected adverse side effects.
Even if our clinical trials are completed as planned,
we cannot be certain that their results will support our product candidates claims or that the FDA or foreign authorities will agree with
our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will
be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical
trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses. If the FDA concludes
that the clinical trials for any product for which we might seek approval, has failed to demonstrate safety and effectiveness, we would
not receive FDA approval to market that product in the United States for the indications sought.
In addition, such an outcome could cause us to
abandon a product candidate and might delay development of others. Any delay or termination of our clinical trials will delay the filing
of any product submissions with the FDA and, ultimately, our ability to commercialize our product candidates and generate revenues. It
is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of our product
candidates’ profiles.
Adverse events involving our products may lead the FDA or other
regulatory authorities to delay or deny approval for our products or result in product recalls that could harm our reputation, business
and financial results.
Additionally, if any of our products and/or product
candidates receives marketing approval, the FDA could require us to adopt a Risk Evaluation and Mitigation Strategy, or REMS, and other
non-U.S. regulatory authorities could impose other specific obligations as a condition of approval to ensure that the benefits outweigh
its risks, which may include, among other things, a medication guide outlining the risks of the product for distribution to patients,
a communication plan to health care practitioners, and restrictions on how or where the product can be distributed, dispensed or used.
Furthermore, if we or others later identify undesirable side effects caused by any of our products and/or product candidates, several
potentially significant negative consequences could result, including:
| |
● |
regulatory authorities may suspend or withdraw approvals of such a product candidate; |
| |
● |
regulatory authorities may require additional warnings or limitations of use in product labeling; |
| |
● |
we may be required to change the way a product candidate is distributed, dispensed, or administered or conduct additional clinical trials; |
| |
● |
we could be sued and held liable for harm caused to patients; and |
| |
● |
our reputation may suffer. |
Any of these events could prevent us from achieving
or maintaining market acceptance of our products and/or product candidates and could significantly harm our business, prospects, financial
condition and results of operations.
Once a product receives FDA approval, the agency
has the authority to require the recall of commercialized products in the event of adverse side effects, material deficiencies or defects
in design or manufacture. The authority to require a recall must be based on an FDA finding that there is a reasonable probability that
the product would cause serious injury or death. Manufacturers may, under their own initiative, recall a product if any material deficiency
in a product is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of adverse side
effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues.
Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and
results of operations. The FDA requires that certain classifications of recalls be reported to FDA within ten working days after the recall
is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA. We may initiate
voluntary recalls involving our products in the future. A future recall announcement could harm our reputation with customers and negatively
affect our sales. In addition, the FDA and/or other regulatory agencies could take enforcement action for failing to report the recalls
when they were conducted.
Even if we obtain regulatory approval of our products, the products
may not gain market acceptance among regulators, advisory boards, physicians, patients, third-party payors and others in the medical community.
Even if any of our products receive marketing
approval, they may fail to receive recommendations for use by regulators or gain market acceptance by physicians, patients, third-party
payors and others in the medical community. If such products do not achieve an adequate level of acceptance, we may not generate significant
product revenue and may not become profitable. The degree of market acceptance of any product, if approved for commercial sale, will depend
on a number of factors, including but not limited to:
| |
● |
receiving governing/advisory body recommendations for use, as well as recommendations of comparable foreign regulatory and advisory bodies; |
| |
● |
prevalence and severity of the disease targets for which our products are approved; |
| |
● |
physicians, hospitals, third-party payors and patients considering our products as safe and effective; |
| |
● |
the potential and perceived advantages of our products over existing ones, including with respect to treatment of disease; |
| |
● |
the prevalence and severity of any side effects; |
| |
● |
product labeling or product insert requirements of the FDA or comparable foreign regulatory and advisory bodies; |
|
● |
limitations or warnings contained in the labeling approved by the FDA or comparable foreign regulatory and advisory bodies; |
| |
● |
the timing of market introduction of our products and product candidates as well as competitive products; |
| |
● |
the cost of treatment in relation to alternative treatments; |
| |
● |
the availability of coverage and adequate reimbursement and pricing by third-party payors, including government authorities; |
|
● |
the willingness of patients to pay out-of-pocket in the absence of coverage and adequate reimbursement by third-party payors, including government authorities; |
| |
● |
relative convenience and ease of administration, including as compared to competitive products and alternative treatments; and |
| |
● |
the effectiveness of our sales and marketing efforts. |
If our product candidates are approved but fail
to receive recommendations by governing or advisory bodies in either the United States or other countries, or achieve market acceptance
among physicians, healthcare providers, patients, third-party payors or others in the medical community, we will not be able to generate
significant revenue. Even if our products achieve market acceptance, we may not be able to maintain that market acceptance over time if
new products or technologies are introduced that are more favorably received than our products, are more cost effective or render our
products obsolete.
Even if we are able to commercialize our products and/or product
candidates, such products may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform
initiatives, which would harm our business.
The regulations that govern marketing approvals,
pricing, coverage and reimbursement for new drugs vary widely from country to country. In the United States, new and future legislation
may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals.
Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins
after marketing or product-licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject
to continuing governmental control even after initial marketing approval is granted. As a result, we might obtain marketing approval for
a product in a particular country but then be subject to price regulations that delay its commercial launch, possibly for lengthy time
periods, and negatively impact the revenue we are able to generate from the sale of the product in that country. Adverse pricing limitations
may hinder our ability to commercialize and generate revenue from our products and/or product candidates, even if our products and/or
product candidates obtain marketing approval.
Our ability to commercialize our current and any
future products and/or product candidates successfully also will depend in part on the extent to which coverage and adequate reimbursement
for these products and related treatments will be available from government health programs, private health insurers, integrated delivery
networks and other third-party payors. Third-party payors decide which products they will pay for and establish reimbursement levels.
A significant trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and third-party payors have
attempted to control costs by limiting coverage and the amount of payment for particular products. Increasingly, third-party payors are
requiring that pharmaceutical companies provide predetermined discounts from list prices and are challenging the prices charged for medical
products. Coverage and reimbursement may not be available for any product that we commercialize and, if reimbursement is available, the
level of reimbursement may not be sufficient for commercial success. Coverage and reimbursement may impact the demand for, or the price
of, any product candidate for which we obtain marketing approval. If coverage and reimbursement are not available or are available only
to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining coverage
and adequate reimbursement for newly approved products, and coverage may be more limited than the purposes for which the product is approved
by the FDA or similar regulatory authorities outside the United States. Moreover, eligibility for coverage and reimbursement does not
imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture,
sale, and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may
not be made permanent. Coverage and reimbursement rates may vary according to the use of the drug and the medical circumstances under
which it is used may be based on reimbursement levels already set for lower cost products or procedures or may be incorporated into existing
payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs
or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold
at lower prices than in the United States. Commercial third-party payors often rely upon Medicare coverage policies and payment limitations
in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates from both government-funded
programs and private payors for any approved products that we develop could have a material adverse effect on our operating results, our
ability to raise capital needed to commercialize our approved products and our overall financial condition.
Any product candidate for which we obtain marketing approval
could be subject to marketing restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with
regulatory requirements or if we experience unanticipated problems with our products.
Any product candidate for
which we obtain marketing approval, along with the manufacturing processes and facilities, post-approval clinical data, labeling, advertising
and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory
authorities. These requirements include submissions of promotional materials and safety and other post-marketing information and reports,
registration and listing requirements, current Good Manufacturing Practice (“cGMP”) requirements for product facilities,
quality assurance and corresponding maintenance of records and documents and requirements regarding the distribution of samples to physicians
and related recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on
the indicated uses for which the product may be marketed or to the conditions of approval or contain requirements for costly post-marketing
testing and surveillance to monitor the safety or efficacy of the medicine. The FDA closely regulates the post-approval marketing and
promotion of drugs to ensure that they are marketed only for the approved indications and in accordance with the provisions of the approved
labeling. However, companies may share truthful and not misleading information that is otherwise consistent with the product’s
FDA approved labeling. The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we
do not comply with these restrictions, we may be subject to enforcement actions.
In addition, later discovery of previously unknown
problems with our products, manufacturers or manufacturing processes and facilities or failure to comply with regulatory requirements,
may result in, among other things:
| |
● |
restrictions on such products, manufacturers or manufacturing processes or facilities; |
| |
● |
restrictions on the labeling, marketing, distribution or use of a product; |
| |
● |
requirements to conduct post-approval clinical trials, other studies or other post-approval commitments; |
| |
● |
warning or untitled letters; |
| |
● |
withdrawal or recall of the products from the market; |
| |
● |
refusal to approve pending applications or supplements to approved applications that we submit; |
| |
● |
fines, restitution or disgorgement of profits or revenue; |
| |
● |
suspension or withdrawal of marketing approvals; |
| |
● |
refusal to permit the import or export of our products; |
| |
● |
injunctions or the imposition of civil or criminal penalties. |
Failure to obtain regulatory approvals in foreign jurisdictions
will prevent us from marketing our products internationally.
We intend to market future products in international
markets. In order to market our future products in regions such as the European Economic Area, or EEA, Asia Pacific, or APAC, and many
other foreign jurisdictions, we must obtain separate regulatory approvals.
For example, in the EEA, medicinal products can
only be commercialized after obtaining a Marketing Authorization, or MA. Before granting the MA, the European Medicines Agency or the
competent authorities of the member states of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific
criteria concerning its quality, safety and efficacy. In Japan, the Pharmaceuticals and Medical Devices Agency, or the PMDA, of the Ministry
of Health Labor and Welfare, or MHLW, must approve an application under the Pharmaceutical Affairs Act before a new drug product may be
marketed in Japan.
We have had limited interactions with foreign
regulatory authorities. The approval procedures vary among countries and can involve additional clinical testing, and the time required
to obtain approval may differ from that required to obtain FDA approval. Moreover, clinical studies conducted in one country may not be
accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other
countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign
countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the
regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval.
We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory approvals, and
even if we file, we may not receive necessary approvals to commercialize our products in any market.
Legislature, such as the Inflation Reduction
Act, may impact our ability to market and commercialize current or future approved products and reduce our profitability from such assets.
Legislature, either in the
United States or in a foreign country, may impact our ability to market and commercialize currently approved or any products approved
in the future and may reduce our profitability from such assets. For example, the Inflation Reduction Act (“IRA”) was signed
into law in the United States in 2022 and intended to lower out-of-pocket costs associated with pharmaceutical drugs. Key impacts of the
IRA include the following:
| ● | Medicare can now directly negotiate lower prescription drug prices with pharmaceutical manufacturers; |
| ● | the
cost of insulin for Medicare beneficiaries
is now capped at $35; |
| ● | all
recommended adult vaccines are free; |
| ● | drug
companies are required to pay rebates if they raise prices of their products faster than
the rate of inflation. |
Should
we decide to raise prices of our pharmaceutical products post-approval, and raise them higher than the rate of inflation, we may
be exposed to rebates owed to Medicare. This may affect the profitability of our products and reduce revenues associated with them.
We expect to rely on third party manufacturers
for ENTADFI and Proclarix.
For the foreseeable
future, we expect to and do rely on third-party manufacturers and other third parties to produce, package and store sufficient
quantities of ENTADFI and Proclarix to meet demand. ENTADFI and Proclarix are complicated and expensive to manufacture. If our
third-party manufacturers fail to deliver ENTADFI or Proclarix for commercial sale on a timely basis, with sufficient quality, and
at commercially reasonable prices, we may be required to delay or suspend commercial sales and/or production of ENTADFI and
Proclarix. While we may be able to identify replacement third-party manufacturers or develop our own manufacturing capabilities for
ENTADFI and Proclarix, this process would likely cause a delay in the availability of ENTADFI and/or Proclarix and an increase in
costs. In addition, third-party manufacturers may have a limited number of facilities in which ENTADFI and Proclarix can be
produced, and any interruption of the operation of those facilities due to events such as equipment malfunction or failure or damage
to the facility by natural disasters could result in the cancellation of shipments, loss of product in the manufacturing process or
a shortfall in ENTADFI and Proclarix.
In addition, regulatory requirements could pose
barriers to the manufacture of ENTADFI and Proclarix. Third-party manufacturers are required to comply with the FDA’s cGMPs for
ENTADFI. As a result, the facilities used by any manufacturers of ENTADFI, must maintain a compliance status acceptable to the FDA. Holders
of NDAs, or other forms of FDA approvals or clearances, or those distributing a regulated product under their own name, are responsible
for manufacturing even though that manufacturing is conducted by a third-party contract manufacturing organization (“CMO”).
Our third-party manufacturers will be required to produce ENTADFI under FDA cGMPs in order to meet acceptable standards. Our third-party
manufacturers may not perform their obligations under their agreements with us or may discontinue their business before the time required
by us to commercialize our drug candidates. In addition, our manufacturers will be subject to ongoing periodic unannounced inspections
by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. Failure by any
of our manufacturers to comply with applicable cGMPs could result in sanctions being imposed on us, including fines, injunctions, civil
penalties, delays, suspensions or withdrawals of approvals, operating restrictions, interruptions in supply, recalls, withdrawals, issuance
of safety alerts and criminal prosecutions, any of which could have a material adverse effect on our business, financial condition, results
of operations and prospects. Finally, we also could experience manufacturing delays if our CMOs give greater priority to the supply of
other products over ENTADFI or Proclarix or otherwise do not satisfactorily perform according to the terms of their agreements with us.
If any supplier for ENTADFI
or Proclarix experiences any significant difficulties in its manufacturing processes, does not comply with the terms of the agreement
between us or does not devote sufficient time, energy and care to providing our manufacturing needs, we could experience significant interruptions
in the supply of ENTADFI and/or Proclarix, which could impair our ability to supply ENTADFI and/or Proclarix at the levels required for
commercialization and prevent or delay its successful development and commercialization.
Disruptions to or significantly increased
costs associated with transportation and other distribution channels for ENTADFI and/or Proclarix may adversely affect our margins and
profitability.
We expect to rely on the uninterrupted and efficient
operation of third-party logistics companies to transport and deliver ENTADFI and Proclarix. These third-party logistics companies may
experience disruptions to the transportation channels used to distribute our products, including disruptions caused by increased airport
and shipping port congestion, a lack of transportation capacity, increased fuel expenses, and a shortage of manpower or capital or due
to other business interruptions. Disruptions to the transportation channels experienced by our third-party logistics companies may result
in increased costs, including the additional use of airfreight to meet demand. Disruptions to this business model or our relationship
with the third party if, for example, performance fails to meet our expectations, could harm our business.
We may fail or elect not to commercialize
our products.
We may not successfully commercialize
our products. We or our collaboration partners in any potential commercial marketing efforts of our products may not be successful in
achieving widespread patient or physician awareness or acceptance of this product. Also, we may be subject to pricing pressures from competitive
products or from governmental or commercial payors or regulatory bodies that could make it difficult or impossible for us to commercialize
our products. Any failure to commercialize our products could have a material adverse effect on our future revenue and our business.
If we fail to commercialize
our products, our business, financial condition, results of operations and prospects may be materially adversely affected and our reputation
in the industry and in the investment community would likely be damaged.
We may not be able to gain and retain market
acceptance for our products.
Physicians may not prescribe our products, which
would prevent our products from generating revenue. Market acceptance of our products by physicians, patients and payors, will depend
on a number of factors, many of which are beyond our control, including the following:
| |
● |
the clinical indications for which our products are approved, if at all; |
| |
|
|
| |
● |
acceptance by physicians and payors of our products as safe and effective treatment or test; |
| |
|
|
| |
● |
the cost in relation to alternative treatments or tests; |
| |
|
|
| |
● |
the relative convenience and ease of administration of our products for the conditions for which they are intended; |
| |
|
|
| |
● |
the availability and efficacy of competitive drugs or tests; |
| |
|
|
| |
● |
the effectiveness of our sales and marketing efforts; |
| |
|
|
| |
● |
the extent to which our products are approved for inclusion on formularies of hospitals and managed care organizations; |
| |
|
|
| |
● |
the availability of coverage and adequate reimbursement by third parties, such as insurance companies and other health care payors, or by government health care programs, including Medicare and Medicaid; |
| |
|
|
| |
● |
limitations or warnings contained in a product’s FDA or other applicable regulatory agency’s approved labeling; and |
| |
|
|
| |
● |
prevalence and severity of adverse side effects. |
Even if the medical community
accepts that our products are safe and efficacious for its approved indications, physicians may not immediately be receptive to the use
or may be slow to adopt such products as an accepted treatment or test for the conditions for which it is intended. Without head-to-head
comparative data, we will also not be able to promote our products as being superior to competing products. If our products do not achieve
an adequate level of acceptance by physicians and payors, we may not generate sufficient or any revenue from this product. In addition,
our efforts to educate the medical community and third-party payors on the benefits of our product may require significant resources and
may never be successful.
In addition, even if our
products achieve market acceptance, we may not be able to maintain that market acceptance over time if:
| |
● |
new products or technologies are introduced that are more favorably received than our products, are more cost effective or render our products obsolete; |
| |
|
|
| |
● |
Unforeseen complications arise with respect to use of our products or |
| |
|
|
| |
● |
sufficient third-party insurance coverage or reimbursement does not remain available. |
ENTADFI is subject to competition from other
BPH drugs and larger, well-established companies with substantially greater resources than us.
We are engaged in the marketing
of a product in industries, including the pharmaceutical industry, that are highly competitive. The pharmaceutical industry is also characterized
by extensive research and rapid technological progress. Potential competitors with respect to ENTADFI in North America, Europe and elsewhere
include major pharmaceutical companies, specialty pharmaceutical companies and biotechnology firms, universities and other research institutions
and government agencies. Many of our competitors have substantially greater research and development and regulatory capabilities and
experience, and substantially greater management, manufacturing, distribution, marketing and financial resources, than we have. We may
be unable to compete successfully against current and future competitors, and competitive pressures could have a negative effect on our
net revenues and profit margins.
Other parties have developed
and marketed drugs for BPH that have been accepted by the physician, patient and payor communities. Many of these other products have
also reached the point where they are now generic drugs, which means that they are sold at a very low price, a price which ENTADFI may
not be able to meet which could limit the reach of ENTADFI into the physician, patient and payor communities, including government payors.
We may not be able to successfully implement
our strategy to grow sales of ENTADFI in the U.S. market and Proclarix in the European market or, if authorized, in any foreign market.
We may not be able to expand sales of ENTADFI
or Proclarix through partnering with telemedicine or other partners or with commercial diagnostic providers or through our own commercialization
efforts. We may not be able to command a price with private and government payors for ENTADFI or Proclarix that would justify our devotion
of significant resources to attempting to grow sales of ENTADFI or Proclarix. We may not be able to compete efficiently or effectively
in a mature BPH market, which is heavily generic, or the prostate cancer diagnostics market, which is highly competitive. Failure to
grow sales of ENTADFI or Proclarix would have a negative effect on our revenue and future plans.
UNAUDITED PRO FORMA CONSOLIDATED
FINANCIAL INFORMATION
Transaction summary
On December 15, 2023,
Onconetix, Inc, a Delaware corporation f/k/a Blue Water Biotech, Inc. (“Onconetix”), entered into a Share Exchange Agreement
(the “Share Exchange Agreement”), by and among (i) Onconetix, (ii) Proteomedix AG, a Swiss Company (“Proteomedix”),
(iii) each of the holders of outstanding capital stock or convertible securities of Proteomedix (other than Proteomedix Stock Options)
named therein (collectively, the “Sellers”) and (iv) Thomas Meier, in the capacity as the representative of Sellers in accordance
with the terms and conditions of the Share Exchange Agreement (the “Sellers’ Representative”).
Pursuant
to the Share Exchange Agreement, subject to the terms and conditions set forth therein, the Sellers agreed to sell to Onconetix,
and Onconetix agreed to buy, all of the issued and outstanding equity interests of Proteomedix (the “Purchased Shares”) in
exchange for newly issued shares of common stock of Onconetix, par value $0.00001 per share (“Common Stock”), and newly issued
shares of preferred stock of Onconetix, par value $0.00001 per share (“Series B Preferred Stock”), as further described below
(the “Share Exchange” and the other transactions contemplated by the Share Exchange Agreement, the “Transactions”).
The
consummation (the “Closing”) of the Share Exchange was subject to customary closing conditions and the execution of the Subscription
Agreement (as defined below) entered into with an investor (the “PMX Investor”). The Share Exchange closed on
December 15, 2023 (the “Closing Date”).
In
full payment for the Purchased Shares, Onconetix issued shares (the “Exchange Shares”) consisting of: (i) 3,675,414 shares
of Common Stock equal to 19.99% of the total issued and outstanding Common Stock prior to the Closing and (ii) 2,696,729 shares of Series
B Preferred Stock convertible into 269,672,900 shares of Common Stock.
Each option to purchase
shares of Proteomedix (each, a “Proteomedix Stock Option”) outstanding immediately before the Closing, whether vested or unvested,
remains outstanding until the Conversion unless otherwise terminated in accordance with its terms. At the Conversion, each outstanding
Proteomedix Stock Option, whether vested unvested, shall be assumed by Onconetix and converted into the right to receive (a) an option
to acquire shares of Common Stock (each, an “Assumed Option”) or (b) such other derivative security as Onconetix and Proteomedix
may agree, subject in either case to substantially the same terms and conditions as were applicable to such Proteomedix Stock Option immediately
before the Closing. Each Assumed Option shall: (i) represent the right to acquire a number of shares of Common Stock equal to the product
of (A) the number of Proteomedix Common Shares that were subject to the corresponding Proteomedix Option immediately prior to the Closing,
multiplied by (B) the Exchange Ratio; and (ii) have an exercise price (as rounded down to the nearest whole cent) equal to the quotient
of (A) the exercise price of the corresponding Company Option, divided by (B) the Exchange Ratio.
In connection with the
Transactions, on December 15, 2023, Onconetix entered into a Subscription Agreement (the “Subscription Agreement”) with the
PMX Investor for a private placement of $5.0 million of units (the “Units”), each unit comprised of (i) one share of Common
Stock and (ii) one pre-funded warrant (collectively, the “Warrants”) to purchase 0.3 shares of Common Stock at an exercise
price of $0.001 per share, for an aggregate purchase price per Unit of $0.25 (the “Purchase Price”). Additional shares are
issuable to the PMX Investor to the extent the PMX Investor continues to hold Common Stock included in the Units and if the VWAP during
the 270 days following closing is less than the Purchase Price, as set forth in the Subscription Agreement. Onconetix has not concluded on the adjustment(s) required, if any, to reflect the Subscription Agreement in
its consolidated financial statements. Accordingly, the accompanying pro forma information does not include any adjustments that may be
required related to the Subscription Agreement.
The offering is expected
to close following stockholder approval of the issuance of the Conversion Shares. Within 30 days after closing, Onconetix will file a
resale registration statement with the SEC registering the resale of the Common Stock issuable pursuant to the Subscription Agreement
and the Warrants.
Pro forma information
The following unaudited
pro forma consolidated financial statements are based on the Company’s audited and unaudited interim historical consolidated financial
statements and Proteomedix’s audited historical and unaudited interim financial statements as adjusted to give effect to the Company’s
acquisition of Proteomedix. The unaudited pro forma consolidated balance sheet as of September 30, 2023 gives effect to these transactions
as if they occurred on September 30, 2023. The unaudited pro forma consolidated statements of operations for the twelve months ended December
31, 2022 and the nine months ended September 30, 2023 give effect to these transactions as if they occurred on January 1, 2022.
The unaudited pro forma
consolidated financial statements should be read together with the Company’s audited historical financial statements, which are
included in the Company’s most recent Annual Report on Form 10-K, which was filed with the Securities and Exchange Commission on
March 9, 2023, and the most recent Quarterly Report on Form 10-Q, which was filed with the Securities and Exchange Commission on November
17, 2023, and Proteomedix’s audited historical financial statements as of and for the years ended December 31, 2022 and 2021 and
the unaudited condensed financial statements for the period ended September 30, 2023.
The unaudited pro forma
consolidated financial information is provided for informational purposes only and is not intended to represent or be indicative of the
consolidated results of operations or financial position that the Company would have reported had the Proteomedix transaction closed on
the dates indicated and should not be taken as representative of our future consolidated results of operations or financial position.
The pro forma adjustments
related to the Agreement are described in the notes to the unaudited pro forma consolidated financial information and principally include
the following:
| - | Pro forma adjustment to eliminate the Proteomedix liabilities converted at the closing of the merger |
| - | Pro forma adjustment to record the merger of the Company and Proteomedix |
The adjustments to fair
value and the other estimates reflected in the accompanying unaudited pro forma consolidated financial statements may be materially different
from those reflected in the consolidated Company’s consolidated financial statements subsequent to the merger. In addition, the
unaudited pro forma consolidated financial statements do not purport to project the future financial position or results of operations
of the consolidated companies.
These unaudited pro forma
consolidated financial statements do not give effect to any anticipated synergies, operating efficiencies or cost savings that may be
associated with the Agreement. These financial statements also do not include any integration costs the companies may incur related to
the Transactions as part of combining the operations of the companies.
ONCONETIX, INC
PRO FORMA CONSOLIDATED BALANCE SHEET
AS OF SEPTEMBER 30, 2023
| | |
Onconetix, Inc. | | |
Proteomedix AG | | |
Transaction
Adjustments | | |
Notes | | |
Pro-forma
Consolidated | |
| ASSETS | |
| | |
| | |
| | |
| | |
| |
| Current assets | |
| | |
| | |
| | |
| | |
| |
| Cash | |
$ | 7,653,975 | | |
$ | 1,037,425 | | |
$ | - | | |
| | | |
$ | 8,691,400 | |
| Accounts receivable | |
| - | | |
| 116,374 | | |
| - | | |
| | | |
| 116,374 | |
| Inventories | |
| 1,419,272 | | |
| 83,183 | | |
| - | | |
| | | |
| 1,502,455 | |
| Prepaid expenses and other current assets | |
| 467,738 | | |
| 7,304 | | |
| - | | |
| | | |
| 475,042 | |
| Total current assets | |
| 9,540,985 | | |
| 1,244,286 | | |
| - | | |
| | | |
| 10,785,271 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Prepaid expenses, long-term | |
| 55,499 | | |
| - | | |
| - | | |
| | | |
| 55,499 | |
| Right of use asset | |
| - | | |
| 140,588 | | |
| - | | |
| | | |
| 140,588 | |
| Property and equipment, net | |
| 12,503 | | |
| 39,163 | | |
| - | | |
| | | |
| 51,666 | |
| Deferred offering costs | |
| 366,113 | | |
| - | | |
| - | | |
| | | |
| 366,113 | |
| Goodwill | |
| - | | |
| - | | |
| 12,096,903 | | |
| 4b, 4d | | |
| 12,096,903 | |
| Intangible assets | |
| 17,906,771 | | |
| - | | |
| 55,125,000 | | |
| 4b | | |
| 73,031,771 | |
| Total assets | |
$ | 27,881,871 | | |
$ | 1,424,037 | | |
$ | 67,221,903 | | |
| | | |
$ | 96,527,811 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | | |
| | | |
| | | |
| | |
| Current liabilities | |
| | | |
| | | |
| | | |
| | | |
| | |
| Accounts payable | |
$ | 3,176,332 | | |
$ | - | | |
$ | - | | |
| | | |
$ | 3,176,332 | |
| Accrued expenses and deferred revenue | |
| 1,538,544 | | |
| 230,329 | | |
| 582,764 | | |
| 4c, 4e | | |
| 2,351,637 | |
| Convertible notes payable | |
| - | | |
| 5,704,371 | | |
| (5,704,371 | ) | |
| 4c | | |
| - | |
| Notes payable | |
| 12,920,093 | | |
| - | | |
| - | | |
| | | |
| 12,920,093 | |
| Lease liability, current | |
| - | | |
| 62,464 | | |
| - | | |
| | | |
| 62,464 | |
| Contingent warrant liability | |
| 10,461 | | |
| - | | |
| - | | |
| | | |
| 10,461 | |
| Total current liabilities | |
| 17,645,430 | | |
| 5,997,164 | | |
| (5,121,607 | ) | |
| | | |
| 18,520,987 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Notes payable | |
| - | | |
| 109,251 | | |
| - | | |
| | | |
| 109,251.00 | |
| Pension benefit obligation | |
| | | |
| 546,259 | | |
| - | | |
| | | |
| 546,259.00 | |
| Operating lease liability | |
| - | | |
| 78,124 | | |
| - | | |
| | | |
| 78,124 | |
| Total liabilities | |
$ | 17,645,430 | | |
$ | 6,730,798 | | |
$ | (5,121,607 | ) | |
| | | |
$ | 19,254,621 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Temporary stockholders’ equity | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Redeemable preferred stock, Series B | |
| - | | |
| - | | |
| 61,356,274 | | |
| 4a | | |
| 61,356,274 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stockholders’ equity | |
| | | |
| | | |
| | | |
| | | |
| | |
| Preferred stock | |
| - | | |
| - | | |
| - | | |
| | | |
| - | |
| Common stock | |
| 183 | | |
| 466,555 | | |
| (466,518 | ) | |
| 4a, 4c, 4d | | |
| 220 | |
| Additional paid-in-capital | |
| 45,297,371 | | |
| 20,539,478 | | |
| (19,430,696 | ) | |
| 4a, 4c, 4d | | |
| 46,406,153 | |
| Treasury stock, at cost | |
| (625,791 | ) | |
| - | | |
| - | | |
| | | |
| (625,791 | ) |
| Accumulated comprehensive income (loss) | |
| - | | |
| 610,627 | | |
| (610,627 | ) | |
| 4d | | |
| - | |
| Accumulated deficit | |
| (34,435,322 | ) | |
| (26,923,421 | ) | |
| 26,142,119 | | |
| 4d, 4e | | |
| (35,216,624 | ) |
| Total stockholders’ equity of Onconetix, Inc. | |
| 10,236,441 | | |
| (5,306,761 | ) | |
| 5,634,278 | | |
| | | |
| 10,563,958 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Noncontrolling interest in subsidiary | |
| - | | |
| - | | |
| 5,352,958 | | |
| | | |
| 5,352,958 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total stockholders’ equity | |
| 10,236,441 | | |
| (5,306,761 | ) | |
| 10,987,236 | | |
| | | |
| 15,916,916 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total liabilities, temporary stockholders’ equity and stockholders’ equity | |
$ | 27,881,871 | | |
$ | 1,424,037 | | |
$ | 67,221,903 | | |
| | | |
$ | 96,527,811 | |
ONCONETIX, INC
PRO FORMA CONSOLIDATED STATEMENT OF COMPREHENSIVE LOSS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2023
| | |
Onconetix, Inc. | | |
Proteomedix AG | | |
Transaction
Adjustments | | |
Notes | | |
Pro-forma
Consolidated | |
| | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | - | | |
$ | 2,092,761 | | |
$ | - | | |
| | | |
$ | 2,092,761 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cost of goods sold | |
| - | | |
| 22,548 | | |
| - | | |
| | | |
| 22,548 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gross profit | |
| - | | |
| 2,070,213 | | |
| - | | |
| | | |
| 2,070,213 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses | |
| | | |
| | | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 8,337,615 | | |
| 1,392,353 | | |
| - | | |
| | | |
| 9,729,968 | |
| Research and development | |
| 2,148,327 | | |
| 275,020 | | |
| - | | |
| | | |
| 2,423,347 | |
| Depreciation and amortization | |
| - | | |
| 9,293 | | |
| 2,800,081 | | |
| 4b | | |
| 2,809,374 | |
| Impairment of deposit on asset purchase agreement | |
| 3,500,000 | | |
| - | | |
| - | | |
| | | |
| 3,500,000 | |
| Total operating expenses | |
| 13,985,942 | | |
| 1,676,666 | | |
| 2,800,081 | | |
| | | |
| 18,462,689 | |
| Income (loss) from operations | |
| (13,985,942 | ) | |
| 393,547 | | |
| (2,800,081 | ) | |
| | | |
| (16,392,476 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | | |
| | | |
| | | |
| | |
| Loss on extinguishment of note payable | |
| (490,000 | ) | |
| - | | |
| - | | |
| | | |
| (490,000 | ) |
| Interest expense | |
| (483,093 | ) | |
| (74,359 | ) | |
| 74,359 | | |
| 4c | | |
| (483,093 | ) |
| Change in fair value of contingent warrant liability | |
| (99,787 | ) | |
| - | | |
| - | | |
| | | |
| (99,787 | ) |
| Total other income (expense) | |
| (1,072,880 | ) | |
| (74,359 | ) | |
| 74,359 | | |
| | | |
| (1,072,880 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| (15,058,822 | ) | |
| 319,188 | | |
| (2,725,722 | ) | |
| | | |
| (17,465,356 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cumulative preferred stock dividends | |
| - | | |
| - | | |
| - | | |
| | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss applicable to common stockholders | |
| (15,058,822 | ) | |
| 319,188 | | |
| (2,725,722 | ) | |
| | | |
| (17,465,356 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| 172,351 | | |
| - | | |
| | | |
| 172,351 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Changes in pension benefit obligation | |
| - | | |
| (168,307 | ) | |
| - | | |
| | | |
| (168,307 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Total other comprehensive (loss) income | |
| - | | |
| 4,044 | | |
| - | | |
| | | |
| 4,044 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Comprehensive income (loss) applicable to common stockholders | |
$ | (15,058,822 | ) | |
$ | 323,232 | | |
$ | (2,725,722 | ) | |
| | | |
$ | (17,461,312 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss per share attributable to common stockholders, basic and diluted | |
$ | (0.92 | ) | |
$ | - | | |
| | | |
| | | |
$ | (0.87 | ) |
| Weighted average number of common shares outstanding, basic and diluted | |
| 16,452,136 | | |
| - | | |
| 3,675,414 | | |
| 4a | | |
| 20,127,550 | |
ONCONETIX, INC
PRO FORMA CONSOLIDATED STATEMENT OF COMPREHENSIVE LOSS
FOR THE YEAR ENDED DECEMBER 31, 2022
| | |
Onconetix, Inc. | | |
Proteomedix AG | | |
Transaction
Adjustments | | |
Notes | | |
Pro-forma
Consolidated | |
| | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | - | | |
$ | 392,460 | | |
$ | - | | |
| | | |
$ | 392,460 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cost of goods sold | |
| - | | |
| 48,429 | | |
| - | | |
| | | |
| 48,429 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Gross profit | |
| - | | |
| 344,031 | | |
| - | | |
| | | |
| 344,031 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Operating expenses | |
| | | |
| | | |
| | | |
| | | |
| | |
| Selling, general and administrative | |
| 9,351,552 | | |
| 1,912,258 | | |
| 781,302 | | |
| 4e | | |
| 12,045,112 | |
| Research and development | |
| 4,129,688 | | |
| 393,274 | | |
| - | | |
| | | |
| 4,522,962 | |
| Depreciation and amortization | |
| - | | |
| 17,492 | | |
| 3,728,341 | | |
| 4b | | |
| 3,745,833 | |
| Total operating expenses | |
| 13,481,240 | | |
| 2,323,024 | | |
| 4,509,643 | | |
| | | |
| 20,313,907 | |
| Loss from operations | |
| (13,481,240 | ) | |
| (1,978,993 | ) | |
| (4,509,643 | ) | |
| | | |
| (19,969,876 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | | |
| | | |
| | | |
| | |
| Interest expense | |
| - | | |
| (63,580 | ) | |
| 63,580 | | |
| 4c | | |
| - | |
| Change in fair value of contingent warrant liability | |
| 61,410 | | |
| - | | |
| - | | |
| | | |
| 61,410 | |
| Total other income (expense) | |
| 61,410 | | |
| (63,580 | ) | |
| 63,580 | | |
| | | |
| 61,410 | |
| | |
| | | |
| | | |
| - | | |
| | | |
| - | |
| Net loss | |
| (13,419,830 | ) | |
| (2,042,573 | ) | |
| (4,446,063 | ) | |
| | | |
| (19,908,466 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cumulative preferred stock dividends | |
| 96,359 | | |
| - | | |
| - | | |
| | | |
| 96,359 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss applicable to common stockholders | |
| (13,516,189 | ) | |
| (2,042,573 | ) | |
| (4,446,063 | ) | |
| | | |
| (20,004,825 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Benefit pension obligation changes | |
| - | | |
| 179,892 | | |
| - | | |
| | | |
| 179,892 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Foreign currency translation adjustment | |
| - | | |
| (4,986 | ) | |
| - | | |
| | | |
| (4,986 | ) |
| | |
| - | | |
| 174,906 | | |
| - | | |
| | | |
| 174,906 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Comprehensive income (loss) applicable to common stockholders | |
$ | (13,516,189 | ) | |
$ | (1,867,667 | ) | |
$ | (4,446,063 | ) | |
| | | |
$ | (19,829,919 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss per share attributable to common stockholders, basic and diluted | |
$ | (1.10 | ) | |
$ | - | | |
| | | |
| | | |
$ | (1.25 | ) |
| Weighted average number of common shares outstanding, basic and diluted | |
| 12,271,449 | | |
| - | | |
| 3,675,414 | | |
| 4a | | |
| 15,946,863 | |
NOTES TO THE UNAUDITED
PRO FORMA CONSOLIDATED
FINANCIAL INFORMATION
Note 1 — Basis
of Presentation
The audited and unaudited
interim historical consolidated financial statements have been adjusted in the pro forma consolidated financial statements in accordance
with Article 11 of the Securities and Exchange Commission’s Regulation S-X to give effect to pro forma events that are (1) directly
attributable to the business combination, (2) factually supportable and (3) with respect to the pro forma consolidated statements
of operations, expected to have a continuing impact on the consolidated results following the business combination.
The business combination
was accounted for under the acquisition method of accounting in accordance with ASC Topic 805, Business Combinations.
As the acquirer for accounting purposes, the Company has estimated the fair value of Proteomedix’s assets acquired and liabilities
assumed and conformed the accounting policies of Proteomedix to its own accounting policies.
The unaudited pro forma
consolidated financial statements are based on the Company’s audited and unaudited interim historical consolidated financial statements
and Proteomedix’s audited and unaudited interim historical financial statements as adjusted to give effect to the Company’s
acquisition of Proteomedix. The Unaudited Pro Forma Consolidated Balance Sheet as of September 30, 2023 gives effect to these transactions
as if they occurred on September 30, 2023. The Unaudited Pro Forma Consolidated Statements of Comprehensive Loss for the nine months
ended September 30, 2023 and twelve months ended December 31, 2022 gives effect to these transactions as if they occurred
on January 1, 2022.
The
allocation of the purchase price used in the unaudited pro forma consolidated financial statements is based upon a preliminary valuation
by management. The final estimate of the fair values of the assets and liabilities will be determined with the assistance of a third-party valuation
firm. The Company’s preliminary estimates and assumptions are subject to material change upon the finalization of internal studies
and third-party valuations of assets, including property and equipment, intangible assets, and
certain liabilities.
The Unaudited Pro Forma
Consolidated Financial Statements are provided for informational purposes only and is not necessarily indicative of what the consolidated
company’s financial position and results of operations would have actually been had the transactions been completed on the dates
used to prepare these pro forma financial statements. The adjustments to fair value and the other estimates reflected in the accompanying
unaudited pro forma consolidated financial statements may be materially different from those reflected in the consolidated company’s
consolidated financial statements subsequent to the transactions. In addition, the Unaudited Pro Forma Consolidated Financial Statements
do not purport to project the future financial position or results of operations of the consolidated companies. Reclassifications and
adjustments may be required if changes to the Company’s financial presentation are needed to conform Proteomedix accounting policies
to the accounting policies of the Company.
These unaudited pro forma
consolidated financial statements do not give effect to any anticipated synergies, operating efficiencies or cost savings that may be
associated with the transactions. These financial statements also do not include any integration costs the companies may incur related
to the transactions as part of combining the operations of the companies.
Note 2 — Summary
of Significant Accounting Policies
The unaudited pro forma
consolidated financial statements have been prepared in a manner consistent with the accounting policies adopted by the Company. The accounting
policies followed for financial reporting on a pro forma basis are the same as those disclosed in the 2022 Annual Report on Form 10-K and
for Proteomedix, the accounting policies followed for financial reporting on a pro forma basis are the same as those disclosed in the
audited financial statements included in this Proxy Statement. The unaudited pro forma consolidated financial statements do not assume
any differences in accounting policies among the Company and Proteomedix. The Company is reviewing the accounting policies of Proteomedix
to ensure conformity of such accounting policies to those of the Company and, as a result of that review, the Company may identify differences
among the accounting policies of the two companies, that when confirmed, could have a material impact on the consolidated financial statements.
However, at this time, the Company is not aware of any difference that would have a material impact on the unaudited pro forma consolidated
financial statements.
Note 3
— Purchase Price Allocation
On December 15, 2023,
the Company entered into a Share Exchange Agreement with Proteomedix, a Swiss Company, and its shareholders to acquire all outstanding
voting interests of Proteomedix. As consideration for the transfer of the Proteomedix voting interests
the Company issued 3,675,414 shares of common stock and 2,696,729 shares of series B convertible preferred stock.
The
following table summarizes the preliminary allocation of the purchase price based on the estimated fair value of the acquired assets and
assumed liabilities as of September 30, 2023:
| Purchase price | |
$ | 62,465,093 | |
| | |
| | |
| Cash | |
$ | 1,037,425 | |
| Accounts receivable | |
| 116,374 | |
| Inventories | |
| 83,183 | |
| Prepaid expenses and other current assets | |
| 7,304 | |
| Right of use asset | |
| 140,588 | |
| Property and equipment, net | |
| 39,163 | |
| Trade name | |
| 2,399,000 | |
| Customer relations | |
| 1,672,000 | |
| Intellectual property | |
| 51,054,000 | |
| Accrued expenses | |
| (31,791 | ) |
| Operating lease liability | |
| (140,588 | ) |
| Pension benefit obligation | |
| (546,259 | ) |
| Notes payable | |
| (109,251 | ) |
| Non-controlling interest | |
| (5,352,958 | ) |
| Goodwill | |
| 12,096,903 | |
| | |
$ | 62,465,093 | |
This
preliminary purchase price allocation has been used to prepare pro forma adjustments in the pro forma consolidated balance sheet and statements
of comprehensive loss. The final purchase price allocation will be determined when the Company has completed the detailed valuations and
necessary calculations. The final allocation could differ materially from the preliminary allocation used in the pro forma adjustments.
The final allocation may include (1) changes in fair values of property and equipment, (2) changes in allocations to intangible
assets such as trade names and technology, as well as goodwill and (3) other changes to assets and liabilities.
In
accordance with the Agreement, as discussed above, the purchase price includes: (a) $61,356,274 Series B Preferred Shares and (b) $1,108,819
in common stock. For purposes of these pro forma consolidated financial statements, the Company issued 2,696,729 shares
of the Company’s Series B Preferred Stock and 3,675,414 shares of the Company’s
common stock.
Note 4 — Pro
Forma Transaction Accounting Adjustments
The pro forma transaction
accounting adjustments are based on our preliminary estimates and assumptions that are subject to change. The following transaction accounting
adjustments have been reflected in the unaudited pro forma condensed combined financial information:
| a. | This adjustment records (1) the issuance of 2,696,729 Series B
Preferred Stock, valued at $61,356,274 and (2) the issuance of 3,675,414 shares of common stock valued at $1,108,819 based on
a per share price of $0.30, to the sellers and certain creditors of Proteomedix. |
| b. |
As part of the preliminary valuation analysis, the Company separately identified certain intangible assets with an estimated fair value of $55.1 million. The fair value was determined primarily using the ‘income approach’, which requires a forecast of the expected future cash flows. Since all the information required to perform a detailed valuation analysis of Proteomedix’s intangible assets could not be obtained as of the date of this filing, for purposes of these unaudited pro forma consolidated financial statements, the Company used certain assumptions based on publicly available transactions data for the industry. The Company has estimated useful lives of between 5 and 15 years for the identified intangibles, resulting in an adjustment of $2.8 million and $3.7 million of amortization expense to the consolidated statements of comprehensive loss for the nine months ended September 30, 2023 and the twelve months ended December 31, 2022, respectively. These numbers may change significantly when the final allocation of purchase price is calculated. |
The following table depicts the estimated fair value useful life and
amortization of the acquired intangible assets other than goodwill:
| Description | |
Useful life | |
Amortization method | |
Fair Value | |
| Trade name | |
Indefinite | |
None | |
$ | 2,399,000 | |
| Customer relationships | |
5 years | |
Straight-line | |
| 1,672,000 | |
| Internally-developed technology | |
15 years | |
Straight-line | |
| 51,054,000 | |
| Total | |
| |
| |
$ | 55,125,000 | |
Additionally, goodwill
of $12,096,903 was recognized after the recording of the identified assets and liabilities.
| c. | This adjustment records the conversion of the Proteomedix convertible notes payable into 83,114 shares
of common stock of Proteomedix at contract terms. This adjustment also removes accrued interest and interest expense related to these
convertible notes. |
| d. | This adjustment eliminates Proteomedix’s total stockholders’
equity as reported in the unaudited condensed financial statements as of September 30, 2022. |
| e. | This adjustment reflects the accrual of the Company’s
estimated total transaction costs for legal and other professional fees and expenses, which are estimated to be approximately $781,000. |
THE SPECIAL MEETING
This proxy statement is being provided to Onconetix stockholders in
connection with the solicitation of proxies by the Onconetix Board for use at the Special Meeting and at any adjournments or postponements
thereof. Onconetix stockholders are encouraged to read this entire document carefully, including its annexes and the documents incorporated
by reference herein, for more detailed information regarding the share exchange agreement and the transactions contemplated thereby.
Date, Time and Place of the Special Meeting
The Special Meeting is scheduled to be held virtually
via live, audio-only webcast on [●], 2024, beginning at [●] a.m., Eastern Time.
The Special Meeting will be held by means of remote
communication via live webcast. There will not be a physical location. Onconetix stockholders will be able to
virtually attend and vote at the Special Meeting by visiting www.[●].com, which is referred to as the “Special Meeting
website.” Onconetix stockholders will need the 16-digit control number found on their proxy card in order to access the Special
Meeting website.
Matters to Be Considered at the Special Meeting
The purpose of the Special
Meeting is to consider and vote on each of the following proposals, each of which is further described in this proxy statement:
| |
1. |
To approve and adopt the Reverse Stock Split Amendment, to effect a reverse stock split of all of the outstanding shares of our Common Stock, at a ratio in the range of 1-for-30 to 1-for-60, with such ratio to be determined by the Board; |
| |
2. |
To approve, in accordance with Nasdaq Listing Rule 5635, the issuance of up to 5,709,935 shares of Common Stock, subject to adjustment, upon conversion of the Company’s Series A Preferred Stock, par value $0.00001 per share; |
| 3. | To approve, in accordance with Nasdaq Listing Rule 5635, the issuance
of: (i) 269,672,900 shares of Common Stock to be issued upon conversion of the Series B Preferred Stock and (ii) such number of shares
of Common Stock to be issued by the Company in the PMX Financing, which shall initially include 20,000,000 shares of Common Stock and
up to 6,000,000 shares of Common Stock underlying warrants included in the units, subject to adjustment, plus such additional number of
shares of Common Stock to be issuable upon the satisfaction of certain price protection conditions, as described further herein; |
| 4. | To ratify the appointment by the Board of EisnerAmper as
the Company’s independent registered public accounting firm for the fiscal year ending December 31, 2024; and |
| 5. | To approve the adjournment of the Special Meeting, if necessary
or appropriate, to solicit additional proxies if there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock Split Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification
Proposal. |
Only business within the purposes described in
the Special Meeting notice may be conducted at the Special Meeting.
Recommendation of the Onconetix Board
After careful consideration, the Onconetix Board
unanimously recommends that Onconetix’s stockholders vote “FOR” each of the proposals.
Record Date for the Special Meeting and Voting
Rights
The record date to determine Onconetix stockholders
who are entitled to receive notice of and to vote at the Special Meeting or any adjournments or postponements thereof is [●], 2024.
At the close of business on the record date, there were [●] shares of Common Stock issued and outstanding and entitled to vote
at the Special Meeting.
Each Onconetix stockholder is entitled to one
vote on each proposal for each share of Common Stock held of record at the close of business on the record date. Only Onconetix stockholders
of record at the close of business on the record date are entitled to receive notice of and to vote at the Special Meeting and any and
all adjournments or postponements thereof.
A complete list of Onconetix stockholders entitled
to vote at the Special Meeting will be available for inspection at Onconetix’s headquarters during regular business hours for
a period of no less than 10 days before the Special Meeting at 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202. The list
of Onconetix stockholders entitled to vote at the Special Meeting will also be made available for inspection during the Special Meeting
via the Special Meeting website.
Quorum; Abstentions and Broker Non-Votes
A quorum of Onconetix stockholders is necessary
to conduct business at the Special Meeting. The presence in person or by proxy of the holders of one-third of the issued and outstanding
shares of Common Stock entitled to vote at the Special Meeting will constitute a quorum. Shares of Common Stock present at the Special
Meeting by virtual attendance via the Special Meeting website or represented by proxy and entitled to vote, including shares for which
an Onconetix stockholder directs an “abstention” from voting, will be counted for purposes of determining a quorum. Since
the Auditor Ratification Proposal is considered a routine matter, shares held in “street name”
through a broker, bank or other nominee will be counted as present for the purpose of determining the existence of a quorum if such broker,
bank or other nominee does not have instructions to vote on such proposal.
If a quorum is not present, the Special Meeting
will be adjourned or postponed until the holders of the number of shares of Common Stock required to constitute a quorum attend.
Under Nasdaq rules, banks, brokers or other nominees
who hold shares in “street name” on behalf of the beneficial owner of such shares have the authority to vote such shares in
their discretion on certain “routine” proposals when they have not received voting instructions from the beneficial owners.
However, banks, brokers or other nominees are not allowed under Nasdaq rules to exercise their voting discretion with respect to matters
that are “non-routine.” This can result in a “broker non-vote,” which occurs on a proposal when (i) a bank,
broker or other nominee has discretionary authority to vote on one or more “routine” proposals to be voted on at a meeting
of stockholders, but is not permitted to vote on other “non-routine” proposals without instructions from the beneficial owner
of the shares, and (ii) the beneficial owner fails to provide the bank, broker or other nominee with voting instructions on a “non-routine”
matter. All proposals other than the Auditor Ratification Proposal are considered “non-routine”
matters, and banks, brokers or other nominees will not have discretionary authority to vote on such matters before the Special Meeting.
As a result, Onconetix only expects broker non-votes with respect to the Auditor Ratification Proposal.
If you hold your shares of Common Stock in “street name,” your shares will not be voted on any matter other than the Auditor Ratification Proposal unless you affirmatively instruct your bank, broker or other nominee how to vote
your shares in accordance with the voting instructions provided by your bank, broker or other nominee. It is therefore critical that you
cast your vote by instructing your bank, broker or other nominee on how to vote. Brokers will not be able to vote on any of the non-routine
proposals before the Special Meeting unless they have received voting instructions from the beneficial owners.
Required Votes
Assuming a quorum is present at the Special Meeting,
approval of the Reverse Stock Split Proposal requires the affirmative vote of the holders of Common
Stock representing at least a majority of the outstanding shares of Common Stock entitled to vote thereon. If you are an Onconetix stockholder
and fail to vote, fail to instruct your bank, broker or other nominee to vote with respect to the Reverse
Stock Split Proposal, or abstain from voting, it will have the same effect as a vote “AGAINST” the Reverse Stock Split Proposal.
Assuming a quorum is present at the Special Meeting,
the Series A Conversion Proposal, the PMX Issuance Proposal, the Auditor Ratification Proposal and the Adjournment Proposal require the
affirmative vote of the majority of the votes cast by stockholders present virtually or represented by proxy and entitled to vote on the
matter at the Special Meeting. An Onconetix stockholder’s failure to vote by proxy or to vote in person at the Special Meeting will
have no effect on such proposals, provided that a quorum is otherwise present. An abstention or other failure of any shares present or
represented by proxy to vote on such proposals will have no effect on such proposals.
Vote of Onconetix Directors and Executive Officers
As of [●], 2024, the record date, Onconetix directors and executive
officers and their affiliates beneficially owned and were entitled to vote in the aggregate [●] shares of Common Stock, which
represented [●]% of the Common Stock issued and outstanding on the record date. Onconetix currently expects that all Onconetix directors
and executive officers will vote their shares “FOR” each of the proposals. See the section titled “Interests
of Onconetix Directors and Executive Officers” in this proxy statement.
Methods of Voting
Stockholders of Record
If you are an Onconetix stockholder of record,
you may vote at the Special Meeting by proxy over the internet or telephone or by mail, or by virtually attending and voting at the Special
Meeting via the Special Meeting website, as described below.
| ● | By Internet: To vote via the Internet, go to www.proxyvote.com
to complete an electronic proxy card. You will be asked to provide the 16-digit control number from the proxy card you receive. Your
vote must be received by 11:59 p.m. Eastern Time on [●], 2024 to be counted. If you vote via the Internet, you do not need
to return a proxy card by mail. |
| ● | By Telephone: To vote by telephone, dial [●]
(the call is toll-free in the United States and Canada; toll charges apply to calls from other countries) and follow the recorded
instructions. You will be asked to provide the 16-digit control number from the proxy card. Your vote must be received by 11:59 p.m.,
Eastern Time, on [●], 2024 to be counted. If you vote by telephone, you do not need to return a proxy card by mail. |
| ● | By Mail: To vote by mail using the proxy card (if
you requested paper copies of the proxy materials to be mailed to you), you need to complete, date and sign the proxy card and return
it promptly by mail in the envelope provided so that it is received no later than [●], 2024. The persons named in the proxy card
will vote the shares you own in accordance with your instructions on the proxy card you mail. |
| ● | Virtually via the Special Meeting Website: To vote
at the Special Meeting, visit www.[●].com, where you can virtually attend and vote at the Special Meeting. You will be asked
to provide the 16-digit control number from the proxy card you receive in order to access the Special Meeting website. |
Unless revoked, all duly executed proxies representing
shares of Common Stock entitled to vote at the Special Meeting will be voted at the Special Meeting and, where a choice has been specified
on the proxy card, will be voted in accordance with such specification. If you submit an executed proxy without providing instructions
for any proposal, your shares will be voted “FOR” each of the proposals.
Beneficial (Street Name) Stockholders
If you hold your shares of Common Stock through
a bank, broker or other nominee in “street name” instead of as a registered holder, you must follow the voting instructions
provided by your bank, broker or other nominee in order to vote your shares. Your voting instructions must be received by your bank, broker
or other nominee prior to the deadline set forth in the information from your bank, broker or other nominee on how to submit voting instructions.
If you do not provide voting instructions to your bank, broker or other nominee for a proposal, your shares of Common Stock will not be
voted on any proposals other than the Auditor Ratification Proposal because your bank, broker or other
nominee does not have discretionary authority to vote on such proposals. See the section titled “The Special Meeting — Quorum;
Abstentions and Broker Non-Votes.”
If you hold your shares of Common Stock through
a bank, broker or other nominee in “street name” (instead of as a registered holder), you must obtain a specific control number
from your bank, broker or other nominee in order to virtually attend and vote at the Special Meeting via the Special Meeting website.
See the section titled “The Special Meeting — Virtually Attending the Special Meeting.”
Virtually Attending the Special Meeting
If you wish to virtually attend the Special Meeting
via the Special Meeting website, you must (i) be an Onconetix stockholder of record at the close of business on [●], 2024, the
record date, (ii) hold your shares of Common Stock beneficially in the name of a broker, bank or other nominee as of the record date
or (iii) hold a valid proxy for the Special Meeting.
To enter the Special Meeting website and virtually
attend the Special Meeting, you will need the 16-digit control number located on your proxy card. If you hold your shares of Common Stock
in street name beneficially through a broker, bank or other nominee and you wish to virtually attend the Special Meeting via the Special
Meeting website, you will need to obtain your specific control number and further instructions from your bank, broker or other nominee.
The 16-digit control number is also needed to access the list of Onconetix stockholders entitled to vote at the Special Meeting during
the time of the meeting.
If you plan to virtually attend and vote at the
Special Meeting via the Special Meeting website, Onconetix still encourages you to vote in advance by the internet, telephone or (if you
received a paper copy of the proxy materials) by mail so that your vote will be counted even if you later decide not to virtually attend
the Special Meeting via the Special Meeting website. Voting your proxy by the internet, telephone or mail will not limit your right to
virtually attend and vote at the Special Meeting via the Special Meeting website if you later decide to do so.
Revocability of Proxies
Any Onconetix stockholder giving a proxy has the
right to revoke it at any time before the proxy is voted at the Special Meeting. If you are an Onconetix stockholder of record, you may
revoke your proxy by any one of the following actions:
| ● | by sending a signed written notice of revocation to Onconetix’s
Corporate Secretary, provided such notice is received no later than the close of business on [●], 2024; |
| ● | by voting again over the internet or telephone as instructed
on your proxy card before the closing of the voting facilities at 11:59 p.m., Eastern Time, on [●], 2024; |
| ● | by submitting a properly signed and dated proxy card with
a later date that is received by Onconetix’s Corporate Secretary no later than the close of business on [●], 2024; or |
| ● | by virtually attending the Special Meeting via the Special
Meeting website and requesting that your proxy be revoked, or virtually voting via the Special Meeting website as described above. |
Only your last submitted proxy will be considered.
Execution or revocation of a proxy will not in
any way affect an Onconetix stockholder’s right to virtually attend and vote at the Special Meeting via the Special Meeting website.
Written notices of revocation and other communications
relating to the revocation of proxies should be addressed to:
Onconetix, Inc.
Attention: Bruce Harmon, Chief Financial Officer
201 E. Fifth Street, Suite 1900
Cincinnati, Ohio 45202
If your shares of Common Stock are held in “street
name” and you previously provided voting instructions to your broker, bank or other nominee, you should follow the instructions
provided by your broker, bank or other nominee to revoke or change your voting instructions. You may also change your vote by obtaining
your specific control number and instructions from your bank, broker or other nominee and voting your shares at the Special Meeting via
the Special Meeting website.
Proxy Solicitation Costs
Onconetix is soliciting proxies on behalf of the Onconetix Board. Onconetix
will bear the entire cost of soliciting proxies from Onconetix stockholders. Proxies may be solicited on behalf of Onconetix or by Onconetix
directors, officers and other employees in person or by mail, telephone, facsimile, messenger, the internet or other means of communication,
including electronic communication. Onconetix directors, officers and employees will not be paid any additional amounts for their services
or solicitation in this regard.
Onconetix will request that banks, brokers and
other nominee record holders send proxies and proxy material to the beneficial owners of Onconetix common stock and secure their voting
instructions, if necessary. Onconetix may be required to reimburse those banks, brokers and other nominees on request for their reasonable
expenses in taking those actions.
Onconetix has also retained [●] to assist
in soliciting proxies and in communicating with Onconetix stockholders and estimates that it will pay [●] a fee of approximately
$[●], plus reimbursement for certain out-of-pocket fees and expenses. Onconetix also has agreed to indemnify [●] against various
liabilities and expenses that relate to or arise out of its solicitation of proxies (subject to certain exceptions).
Householding
SEC rules permit companies and intermediaries
such as brokers to satisfy delivery requirements for proxy statements and notices with respect to two or more stockholders sharing the
same address by delivering a single proxy statement or a single notice addressed to those stockholders. This process, which is commonly
referred to as “householding,” provides cost savings for companies. Onconetix has previously adopted householding for Onconetix
stockholders of record. As a result, Onconetix stockholders with the same address and last name may receive only one copy of this proxy
statement. Registered Onconetix stockholders (those who hold shares of Common Stock directly in their name with Onconetix’s transfer
agent) may opt out of householding and receive a separate proxy statement or other proxy materials by sending a written request to Onconetix
at the address below.
Some brokers household proxy materials, delivering
a single proxy statement or notice to multiple Onconetix stockholders sharing an address unless contrary instructions have been received
from the affected stockholders. Once you have received notice from your broker that they will be householding materials to your address,
householding will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate
in householding and would prefer to receive a separate proxy statement or notice, or if your receiving multiple copies of these documents
and you wish to request that future deliveries be limited to a single copy, please notify your broker.
Onconetix will promptly deliver a copy of this
proxy statement to any Onconetix stockholder who only received one copy of these materials due to householding upon request in writing
to: Onconetix, Inc., Attn: Bruce Harmon, Chief Financial Officer, at 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202 or by calling
(513) 620-4101.
Adjournments
If a quorum is present at the Special Meeting
but there are insufficient votes at the time of the Special Meeting to approve the Reverse Stock Split
Proposal, the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal, then Onconetix stockholders
may be asked to vote on the Adjournment Proposal. If a quorum is not present, the presiding officer may adjourn the Special Meeting, from
time to time, without notice other than announcement at the meeting of the hour, date and place, if any, to which the meeting is adjourned,
and the means of remote communications, if any, by which Onconetix stockholders and proxyholders may be deemed to be present in person
and vote at such adjourned meeting. The presiding officer may also adjourn the meeting to another hour, date or place, even if a quorum
is present.
At any subsequent reconvening of the Special Meeting
at which a quorum is present, any business may be transacted that might have been transacted at the original meeting, and all proxies
will be voted in the same manner as they would have been voted at the original convening of the Special Meeting, except for any proxies
that have been effectively revoked or withdrawn prior to the time the proxy is voted at the reconvened meeting.
Assistance
If you need assistance voting or completing your
proxy card, or if you have questions regarding the Special Meeting, please contact [●], Onconetix’s proxy solicitor for the
Special Meeting, at:
[●]
ONCONETIX STOCKHOLDERS SHOULD CAREFULLY READ
THIS PROXY STATEMENT IN ITS ENTIRETY FOR MORE DETAILED INFORMATION CONCERNING THE SHARE EXCHANGE AGREEMENT AND THE PMX TRANSACTION. IN
PARTICULAR, ONCONETIX STOCKHOLDERS ARE DIRECTED TO THE SHARE EXCHANGE AGREEMENT, WHICH IS ATTACHED AS ANNEX B HERETO.
PROPOSAL 1: THE REVERSE
STOCK SPLIT PROPOSAL
Reasons for the Reverse Stock Split Proposal
The Board is recommending to the Company’s stockholders for their
approval an amendment that would authorize, but not obligate the Board, to amend the Company’s Certificate of Incorporation to effect
a reverse stock split of the outstanding and treasury shares of Common Stock at a ratio in the range of 1-for-30 to 1-for-60, which ratio
would be subject to the Board’s discretion following stockholder approval (the “Reverse Stock Split”). The Company believes
that the availability of a range of reverse split ratios will provide the Company with the flexibility to implement the Reverse Stock
Split, if effected at all, in a manner designed to maximize the anticipated benefits for the Company and its stockholders. The general
description of the reverse split amendment set forth below is a summary only and is qualified in its entirety by and subject to the full
text of the form of proposed amendment which is attached as Annex A hereto.
The Board’s primary objective in asking
for authority to effect a reverse split is to increase the per-share trading price of our Common Stock. If our Board does not implement
the Reverse Stock Split prior to the one-year anniversary of the date on which the Reverse Stock Split is approved by the Company’s
stockholders at the Special Meeting, the authority granted in this proposal to implement the Reverse Stock Split will terminate and the
Reverse Stock Split Amendment will be abandoned.
On September 18, 2023, we received notice from
Nasdaq staff indicating that, based upon the closing bid price of the Common Stock for the prior 30 consecutive business days, we were
not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq, as set forth
in the Bid Price Rule. We have 180 days from September 18, 2023, or through March 16, 2024, to regain compliance with the Bid Price Rule.
To regain compliance with the Bid Price Rule and
qualify for continued listing on the Nasdaq Capital Market, the closing bid price per share of our common stock must be at least $1.00
for at least 10 consecutive business days on or prior to March 16, 2024. The Nasdaq Staff retains discretion to extend this 10-business
day period to determine that the Company has demonstrated an ability to maintain long-term compliance.
If we fail to regain compliance with the Bid Price Rule before
March 16, 2024 but meet all of the other applicable standards for initial listing on Nasdaq with the exception of the Bid Price Rule,
then we may be eligible to have an additional 180 calendar days, or until September 12, 2024, to regain compliance with the Bid Price Rule.
If we do not regain compliance with the Bid Price Rule by the end of the compliance period (or the second compliance
period, if applicable), our Common Stock will become subject to delisting. In the event that we receive notice that our Common Stock is
being delisted, the Nasdaq listing rules permit us to appeal a delisting determination by Nasdaq to a hearings panel, but there can be
no assurance that the panel would grant the Company’s request for continued listing.
The Board believes that the failure of stockholders
to approve the Reverse Stock Split Amendment could prevent the Company from complying with the Bid Price Rule and could, among other risks,
inhibit our ability to conduct capital raising activities. If the Nasdaq Stock Market delists the Common Stock, then the Common Stock
would likely become traded on an over-the-counter market such as that maintained by OTC Markets Group Inc., which does not have the substantial
corporate governance or quantitative requirements for continued listing that the Nasdaq Stock Market has. In that event, interest in Common
Stock may decline and certain institutions may not have the ability to trade in the Common Stock, all of which could have a material adverse
effect on the liquidity or trading volume of the Common Stock. If the Common Stock becomes significantly less liquid due to delisting
from the Nasdaq Stock Market, the Company’s stockholders may not have the ability to liquidate their investments in the Common Stock
as and when desired, and the Company believes its ability to maintain and obtain analyst coverage, attract investor interest, and have
access to capital may become significantly diminished as a result.
Potential Effects of the Amendment
If the Board decides to implement the Reverse
Stock Split Amendment, the Company would communicate to the public additional details regarding the Reverse Stock Split Amendment (including
the final reverse split ratio, as determined by the Board). By voting in favor of the Reverse Stock Split Amendment, you are also expressly
authorizing the Board to determine not to proceed with, and to defer the timing of, or to abandon, the Reverse Stock Split Amendment,
in the Board’s sole discretion. In determining whether to implement the Reverse Stock Split Amendment following receipt of stockholder
approval of the Reverse Stock Split Amendment, and which reverse split ratio to implement, if any, the Board may consider, among other
things, various factors, such as:
| ● | the Company’s ability to maintain its listing on Nasdaq; |
| ● | the historical trading price and trading volume of the Common
Stock; |
| ● | the then-prevailing trading price and trading volume of the
Common Stock and the expected impact of the reverse stock split on the trading market for the Common Stock in the short and long term; |
| ● | which reverse split ratio would result in the greatest overall
reduction in the Company’s administrative costs; and |
| ● | prevailing general market and economic conditions. |
Principal Reasons for the Reverse Stock Split
The primary objective for effecting the Reverse Stock Split Amendment,
should our Board choose to effect one, would be to increase the per share price of our Common Stock, whether to potentially regain compliance
with the Bid Price Rule or otherwise. Our Board believes that, should the appropriate circumstances arise, effecting the Reverse Stock
Split Amendment, could, among other things, help us to appeal to a broader range of investors, generate greater investor interest in the
Company, improve the perception of our Common Stock as an investment security and could assist in our capital-raising efforts by making
our Common Stock more attractive to a broader range of investors.
A reverse stock split could allow a broader range
of institutions to invest in the Common Stock (namely, funds that are prohibited from buying stocks whose price is below certain thresholds),
potentially increasing trading volume and liquidity of the Common Stock and potentially decreasing the volatility of the Common Stock
if institutions become long-term holders of the Common Stock. A reverse stock split could help increase analyst and broker interest in
the Common Stock as their policies can discourage them from following or recommending companies with low stock prices. Because of the
trading volatility often associated with low-priced stocks, many brokerage houses and institutional investors have internal policies and
practices that either prohibit them from investing in low-priced stocks or tend to discourage individual brokers from recommending low-priced
stocks to their customers. Some of those policies and practices may make the processing of trades in low-priced stocks economically unattractive
to brokers. Additionally, because brokers’ commissions on low-priced stocks generally represent a higher percentage of the stock
price than commissions on higher-priced stocks, a low average price per share of Common Stock can result in individual stockholders paying
transaction costs representing a higher percentage of their total share value than would be the case if the share price were higher. Some
investors, however, may view a reverse stock split negatively since it reduces the number of shares of Common Stock available in the public
market. If the Reverse Stock Split Amendment is approved and the Board believes that effecting the Reverse Stock Split is in the best
interests of the Company and its stockholders, the Board may effect the Reverse Stock Split, regardless of whether the Company’s
stock is at risk of delisting from Nasdaq, trades on the OTC Market, or otherwise for purposes of increasing the per share trading price,
enhancing the liquidity of the Common Stock, and to facilitate capital raising.
Certain Risks Associated with a Reverse Stock
Split
Reducing the number of outstanding shares of the
Common Stock through the Reverse Stock Split Amendment is intended, absent other factors, to increase the per share market price of the
Common Stock. Other factors, however, such as the Company’s financial results, market conditions, the market perception of the Company’s
business and other risks, including those set forth below and in the Company’s SEC filings and reports, including its Annual Report
on Form 10-K for the year ended December 31, 2022, as amended, may adversely affect the market price of the Common Stock. As a result,
there can be no assurance that the Reverse Stock Split, if completed, will result in the intended benefits described above, that the market
price of the Common Stock will increase following the Reverse Stock Split or that the market price of the Common Stock will not decrease
in the future.
The Reverse Stock Split May Not Result in a
Sustained Increase in the Price of the Common Stock. The effect of the Reverse Stock Split upon the market price of the Common Stock
cannot be predicted with any certainty and the Company cannot assure you that the Reverse Stock Split will result in a sustained increase
in the price of the Common Stock for any meaningful period of time, or at all. The Board believes that the Reverse Stock Split has the
potential to increase the market price of the Common Stock, and therefore may help to satisfy the Bid Price Rule, if applicable. However,
the long- and short-term effect of the Reverse Stock Split upon the market price of the Common Stock cannot be predicted with any certainty.
The Reverse Stock Split May Decrease the Liquidity
of the Common Stock. The Board believes that the Reverse Stock Split may result in an increase in the market price of the Common Stock,
which could lead to increased interest in the Common Stock and possibly promote greater liquidity for the Company’s stockholders.
However, the Reverse Stock Split will also reduce the total number of outstanding shares of Common Stock, which may lead to reduced trading
and a smaller number of market makers for the Common Stock. There also can be no assurance the Reverse Stock Split will enhance the Company’s
ability to engage in capital raising activities.
The Reverse Stock Split May Result in Some
Stockholders Owning “Odd Lots” That May Be More Difficult to Sell or Require Greater Transaction Costs per Share to Sell.
If the Reverse Stock Split is implemented, it will increase the number of stockholders who own “odd lots” of less than 100
shares of Common Stock. A purchase or sale of less than 100 shares of Common Stock (an “odd lot” transaction) may result in
incrementally higher trading costs through certain brokers, particularly “full service” brokers. Therefore, those stockholders
who own less than 100 shares of Common Stock following the Reverse Stock Split may be required to pay higher transaction costs if they
sell their Common Stock.
The Reverse Stock Split May Lead to a Decrease
in the Overall Market Capitalization of the Company. The Reverse Stock Split may be viewed negatively by the market and, consequently,
could lead to a decrease in the overall market capitalization of the Company. If the per share market price of the Common Stock does not
increase in proportion to the reverse split ratio, then the value of the Company, as measured by the market capitalization of the Company,
will be reduced.
Impact of a Reverse Stock Split If Implemented
The Reverse Stock Split would affect all holders
of Common Stock uniformly and would not affect any stockholder’s percentage ownership interests or proportionate voting power. The
other principal effects of the Reverse Stock Split Amendment will be that:
| |
● |
the number of issued and outstanding shares of Common Stock (and treasury shares, if any), will be reduced proportionately based on the final reverse split ratio, as determined by the Board; |
| |
|
|
| |
● |
based on the final reverse split ratio, the per share exercise price of all outstanding options and warrants will be increased proportionately and the number of shares of Common Stock issuable upon the exercise of all outstanding options and warrants will be reduced proportionately; and |
| |
|
|
| |
● |
the number of shares reserved for issuance pursuant to any outstanding equity awards and any maximum number of shares with respect to which equity awards may be granted will be reduced proportionately based on the final reverse split ratio. |
The Board does not intend
for a reverse stock split to be the first step in a “going private transaction” within the meaning of Rule 13e-3 of the
Exchange Act. The actual number of shares outstanding after giving effect to the Reverse Stock Split Proposal will depend on the
reverse split ratio that is ultimately selected by the Board. The table below illustrates certain, but not all, possible reverse
stock split ratios, together with the implied number of issued and outstanding shares of the Common Stock resulting from
implementation of the Reverse Stock Split based on [●] shares of the Common Stock outstanding as of [●], 2024. The reverse
stock split will not affect the total number of authorized shares under our certificate of incorporation.
Example
Ratios within Delegated Range of Ratios | |
Number
of Authorized Shares of Common
Stock | | |
Implied
Approximate
Number of Issued
and
Outstanding Shares
of Common Stock
Following the Reverse Stock Split * | |
| 1-for-30 | |
| [●] | | |
| [●] | |
| 1-for-40 | |
| [●] | | |
| [●] | |
| 1-for-50 | |
| [●] | | |
| [●] | |
| 1-for-60 | |
| [●] | | |
| [●] | |
| * | Excludes the effect of fractional share treatment. |
We are currently authorized to issue a maximum
of 250,000,000 shares of our Common Stock. As of the record date, there were [●] shares of our Common Stock issued and outstanding. Although
the number of authorized shares of our Common Stock will not change as a result of the Reverse Stock Split, the number of shares of our
Common Stock issued and outstanding will be reduced in proportion to the ratio selected by the Board. Thus, the Reverse Stock Split will
effectively increase the number of authorized and unissued shares of our Common Stock available for future issuance by the amount of the
reduction effected by the Reverse Stock Split.
Following the Reverse Stock Split, the Board will
have the authority, subject to applicable securities laws, to issue all authorized and unissued shares without further stockholder approval,
upon such terms and conditions as the Board deems appropriate. Although we consider financing opportunities from time to time, other than
shares issuable in connection with the conversion of the Series A Preferred Stock or the Series B Preferred Stock or issuable as a result
of the PMX Financing, we do not currently have any plans, proposals or understandings to issue the additional shares that would be available
if the Reverse Stock Split is approved and effected, but some of the additional shares underlie warrants, which could be exercised or
converted after the Reverse Stock Split Amendment is affected.
Management does not anticipate that the Company’s
financial condition, the percentage ownership of Common Stock by management, the number of the Company’s stockholders or any aspect
of the Company’s business will materially change as a result of the Reverse Stock Split Amendment. Because the Reverse Stock Split
Amendment will apply to all issued and outstanding shares of Common Stock and outstanding rights to purchase Common Stock or to convert
other securities into Common Stock the proposed Reverse Stock Split Amendment will not alter the relative rights and preferences of existing
stockholders, except to the extent the reverse stock split will result in fractional shares, as discussed in more detail below.
The Common Stock is currently registered under
Section 12(b) of the Exchange Act, and the Company is subject to the periodic reporting and other requirements of the Exchange Act. The
Reverse Stock Split Amendment will not affect the registration of the Common Stock under the Exchange Act or the listing of the Common
Stock on Nasdaq to the extent it is still listed for trading on Nasdaq (other than to the extent it may facilitate compliance with Nasdaq
continued listing standards, if applicable). Following the reverse stock split, the Common Stock is expected to continue to be listed
on Nasdaq or OTC Bulletin Board, although it will be considered a new listing with a new Committee on Uniform Securities Identification
Procedures, or CUSIP, number.
The rights of the holders of the Common Stock
will not be affected by the Reverse Stock Split Amendment, other than as a result of the treatment of fractional shares as described below.
For example, a holder of 2% of the voting power of the outstanding shares of the Common Stock immediately prior to the effectiveness of
the Reverse Stock Split Amendment will generally continue to hold 2% of the voting power of the outstanding shares of the Common Stock
immediately after the reverse stock split. The number of stockholders of record will not be affected by the Reverse Stock Split Amendment
(except to the extent any are cashed out as a result of holding fractional shares). If approved and implemented, the Reverse Stock Split
Amendment may result in some stockholders owning “odd lots” of less than 100 shares of the Common Stock. Odd lot shares may
be more difficult to sell, and brokerage commissions and other costs of transactions in odd lots are generally higher than the costs of
transactions in “round lots” of even multiples of 100 shares. The Board believes, however, that these potential effects are
outweighed by the benefits of the Reverse Stock Split Amendment.
Effectiveness of the Reverse Stock Split
The Reverse Stock Split Amendment, if approved by the Company’s
stockholders, would become effective upon the filing and effectiveness (the “Effective Time”) of the Reverse Stock Split Amendment
with the Secretary of State of the State of Delaware, which would take place at the Board’s discretion. The exact timing of the
filing of the Reverse Stock Split Amendment, if filed, would be determined by the Board based on its evaluation as to when such action
will be the most advantageous to the Company and the Company’s stockholders. In addition, the Board reserves the right, notwithstanding
stockholder approval and without further action by the stockholders, to elect not to proceed with the Reverse Stock Split if, at any time
(i) prior to filing the Reverse Stock Split Amendment with the Secretary of State of the State of Delaware and (ii) before the one-year
anniversary of the date on which the Reverse Stock Split is approved by the Company’s stockholders at the Special Meeting, the Board,
in its sole discretion, determines that it is no longer in the Company’s best interests or the best interests of its stockholders
to proceed with the Reverse Stock Split. If our Board does not implement the Reverse Stock Split prior to the one-year anniversary of
the date on which the Reverse Stock Split is approved by the Company’s stockholders at the Special Meeting, the authority granted
in this proposal to implement the Reverse Stock Split will terminate and the Reverse Stock Split Amendment to effect the Reverse Stock
Split will be abandoned.
Effect on Par Value; Reduction in Stated Capital
The proposed Reverse Stock Split Amendment will
not affect the par value of the Company’s stock, which will remain at $0.00001 per share of Common Stock. As a result, the stated
capital on the Company’s balance sheet attributable to its Common Stock, which consists of the par value per share of Common Stock
multiplied by the aggregate number of shares of Common Stock issued and outstanding, will be reduced in proportion to the reverse stock
split ratio selected by the Board. Correspondingly, the Company’s additional paid-in capital account, which consists of the difference
between its stated capital and the aggregate amount paid to the Company upon issuance of all currently outstanding shares of the Common
Stock, will be credited with the amount by which the stated capital is reduced. The Company’s stockholders’ equity, in the
aggregate, will remain unchanged.
Book-Entry Shares
If the Reverse Stock Split is effected, stockholders,
either as direct or beneficial owners, will have their holdings electronically adjusted by the Company’s transfer agent (and, for
beneficial owners, by their brokers or banks that hold in “street name” for their benefit, as the case may be) to give effect
to the reverse stock split. Banks, brokers, custodians or other nominees will be instructed to effect the reverse stock split for their
beneficial holders holding Common Stock in street name. However, these banks, brokers, custodians or other nominees may have different
procedures than registered stockholders for processing the reverse stock split and making payment for fractional shares. If a stockholder
holds shares of Common Stock with a bank, broker, custodian or other nominee and has any questions in this regard, stockholders are encouraged
to contact their bank, broker, custodian or other nominee. The Company does not issue physical certificates to stockholders.
No Appraisal Rights
Under the Delaware General Corporation Law, the
Company’s stockholders are not entitled to dissenter’s rights or appraisal rights with respect to the reverse stock split
described in the Reverse Stock Split Proposal, and the Company will not independently provide its stockholders with any such rights.
Fractional Shares
The Company does not intend to issue fractional
shares in connection with the Reverse Stock Split. The Company currently anticipates that it will cause its exchange agent to aggregate
all fractional share interests following the Reverse Stock Split, sell the aggregated fractional shares interests into the market and
allocate and distribute the net proceeds received from such sale (reduced by any customary brokerage fees, commissions and other expenses)
among the stockholders who would otherwise hold a fractional share interest as a result of the reverse stock split on a pro rata basis.
Stockholders will not be entitled to receive interest for the period of time between the Effective Time and the date payment for their
fractional share interest is received. After the Reverse Stock Split is effected, a stockholder will have no further interest in the Company
with respect to its fractional share interest and persons otherwise entitled to a fractional share will not have any voting, dividend
or other rights with respect thereto, except to receive the above-described cash payment. Although the Company will pay any brokerage
fees, commissions and other expenses related to the exchange agent’s selling in the open market shares that would otherwise be fractional
shares, as described above, such expenses will reduce the cash amounts to be paid to stockholders in lieu of the receipt of fractional
shares. Stockholders should be aware that under the escheat laws of various jurisdictions, sums due for fractional interests that are
not timely claimed after the Effective Time may be required to be paid to the designated agent for each such jurisdiction. Stockholders
otherwise entitled to receive such funds, who have not received them, will have to seek to obtain such funds directly from the jurisdiction
to which they were paid.
Material U.S. Federal Income Tax Considerations
Related to the Reverse Stock Split
The following is a general summary of the material
U.S. federal income tax considerations to U.S. holders (as defined below) of the Reverse Stock Split. This discussion is based upon current
provisions of the Internal Revenue Code of 1986, as amended (the “Code”), existing and proposed Treasury regulations promulgated
under the Code (the “Treasury Regulations”) and judicial authority and administrative interpretations, all as of the date
of this document, and all of which are subject to change, possibly with retroactive effect, and are subject to differing interpretations.
Changes in these authorities may cause the tax consequences to vary substantially from the consequences described below. The Company has
not sought and will not seek an opinion of counsel or any rulings from the Internal Revenue Service (the “IRS”) with respect
to any of the tax considerations discussed below. As a result, there can be no assurance that the IRS will not assert, or that a court
would not sustain, a position contrary to any of the conclusions set forth below.
This discussion is limited to U.S. holders that
hold Common Stock as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment).
This discussion does not address any tax consequences arising under the tax on net investment income or the alternative minimum tax, nor
does it address any tax consequences arising under the laws of any state, local or non-U.S. jurisdiction, U.S. federal estate or gift
tax laws, or any tax treaties. Furthermore, this discussion does not address all aspects of U.S. federal income taxation that may be applicable
to U.S. holders in light of their particular circumstances or to U.S. holders that may be subject to special rules under U.S. federal
income tax laws, including, without limitation:
| |
● |
a bank, insurance company or other financial institution; |
| |
|
|
| |
● |
a tax-exempt or a governmental organization; |
| |
|
|
| |
● |
a real estate investment trust; |
| |
|
|
| |
● |
an S corporation or other pass-through entity (or an investor in an S corporation or other pass-through entity); |
| |
|
|
| |
● |
a regulated investment company or a mutual fund; |
| |
|
|
| |
● |
a dealer or broker in stocks and securities, or currencies; |
| |
● |
a trader in securities that elects mark-to-market treatment; |
| |
|
|
| |
● |
a holder of Common Stock that received such stock through the exercise of an employee option, pursuant to a retirement plan or otherwise as compensation; |
| |
|
|
| |
● |
a person who holds Common Stock as part of a straddle, appreciated financial position, synthetic security, hedge, conversion transaction or other integrated investment or risk reduction transaction; |
| |
|
|
| |
● |
a corporation that accumulates earnings to avoid U.S. federal income tax; |
| |
|
|
| |
● |
a person whose functional currency is not the U.S. dollar; |
| |
|
|
| |
● |
a U.S. holder who holds Common Stock through non-U.S. brokers or other non-U.S. intermediaries; |
| |
|
|
| |
● |
a person subject to Section 451(b) of the Code; or |
| |
|
|
| |
● |
a former citizen or long-term resident of the United States subject to Section 877 or 877A of the Code. |
If a partnership, or any entity (or arrangement)
treated as a partnership for U.S. federal income tax purposes, holds Common Stock, the tax treatment of a partner in such partnership
generally will depend on the status of the partner and the activities of the partnership and upon certain determinations made at the partner
level. A partner in a partnership holding Common Stock should consult its own tax advisor about the U.S. federal income tax consequences
of the Reverse Stock Split.
For purposes of this discussion, a “U.S.
holder” is a beneficial owner of shares of Common Stock that is for U.S. federal income tax purposes:
| |
● |
an individual citizen or resident of the United States; |
| |
|
|
| |
● |
a corporation (or any other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| |
|
|
| |
● |
an estate, whose income is subject to U.S. federal income tax regardless of its source; or |
| |
|
|
| |
● |
a trust (i) the administration of which is subject to the primary supervision of a U.S. court and that has one or more United States persons that have the authority to control all substantial decisions of the trust or (ii) that has made a valid election under applicable Treasury Regulations to be treated as a United States person. |
Tax Consequences of the Reverse Stock Split
Generally
The Reverse Stock Split should constitute a “recapitalization”
for U.S. federal income tax purposes. As a result, a U.S. holder of Common Stock generally should not recognize gain or loss upon the
Reverse Stock Split, except with respect to cash received in lieu of a fractional share of Common Stock, as discussed below. A U.S. holder’s
aggregate tax basis in the shares of Common Stock received pursuant to the reverse stock split should equal the aggregate tax basis of
the shares of Common Stock surrendered (excluding any portion of such basis that is allocated to any fractional share of Common Stock),
and such U.S. holder’s holding period in the shares of Common Stock received should include the holding period in the shares of
Common Stock surrendered. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the shares of
Common Stock surrendered to the shares of Common Stock received in a recapitalization pursuant to the Reverse Stock Split. U.S. holders
of shares of Common Stock acquired on different dates and at different prices should consult their tax advisors regarding the allocation
of the tax basis and holding period of such shares.
Cash in Lieu of Fractional Shares
A U.S. holder of Common Stock that receives cash
in lieu of a fractional share of Common Stock pursuant to the Reverse Stock Split should generally recognize capital gain or loss in an
amount equal to the difference between the amount of cash received and the U.S. holder’s tax basis in the shares of Common Stock
surrendered that is allocated to such fractional share of Common Stock. Such capital gain or loss should be long-term capital gain or
loss if the U.S. holder’s holding period for Common Stock surrendered exceeds one year at the effective time of the Reverse Stock
Split. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
Cash payments received by a U.S. holder of Common
Stock pursuant to the Reverse Stock Split may be subject to information reporting and may be subject to U.S. backup withholding (currently
at 24%) unless such holder provides proof of an applicable exemption or a correct taxpayer identification number and otherwise complies
with the applicable requirements of the backup withholding rules. Any amount withheld under the U.S. backup withholding rules is not
an additional tax and will generally be allowed as a refund or credit against the U.S. holder’s U.S. federal income tax liability
provided that the required information is timely furnished to the IRS.
Required Vote
Assuming a quorum is present at the Special Meeting,
approval of the Reverse Stock Split Proposal requires the affirmative vote of the holders of Common Stock representing at least a majority
of the outstanding shares of Common Stock entitled to vote thereon. If an Onconetix stockholder fails to vote, fails to instruct its bank,
broker, or other nominee to vote with respect to the Reverse Stock Split Proposal, or abstains from voting, it will have the same effect
as a vote “AGAINST” the Reverse Stock Split Proposal.
THE ONCONETIX BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ONCONETIX STOCKHOLDERS VOTE “FOR” THE Reverse Stock Split PROPOSAL.
PROPOSAL 2: SERIES A CONVERSION PROPOSAL
Overview
On September 29, 2023, the Company entered into
an Amendment (the “Veru Amendment”) of the Asset Purchase Agreement (the “Purchase Agreement”), dated as of April
19, 2023, by and between the Company and Veru, Inc., relating to the sale of the product ENTADFI. Pursuant to the Purchase Agreement,
the Company was required to make an installment payment of $4 million by September 30, 2023. Pursuant to the Veru Amendment, the $4 million
installment will be deemed paid and fully satisfied upon (1) the payment to Veru of the sum of $1 million in immediately available funds
on September 29, 2023, and (2) the issuance to Veru by October 3, 2023 of 3,000 shares of Series A Convertible Preferred Stock of the
Company. The Company made such $1 million payment on September 29, 2023.
The Series A Preferred Stock issued to Veru is
initially convertible, in the aggregate, into approximately 5,709,935 shares of the Company’s common stock, subject to adjustment
and assuming approval of this Proposal No. 3.
Purpose of the Series A Conversion Proposal
We are subject to the Nasdaq Rules because our
Common Stock is currently listed on the Nasdaq Capital Market.
Pursuant to Nasdaq Rule 5635(a), stockholder approval
is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for Common Stock) in connection
with the acquisition of the stock or assets of another company if, due to the present or potential issuance of common stock, including
shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into or exercisable for common
stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power equal to or in excess of
20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable for common stock; or (B)
the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding
before the issuance of the stock or securities.
Combined with the shares issued in the PMX Transaction
and those shares that are issuable in the PMX Financing, the shares issuable upon conversion of the Series A Preferred Stock would result
in the issuance of more than 20% of the voting power and the number of shares of Common Stock outstanding as of the issuance of the Series
A Preferred Stock. As a result of the foregoing, in accordance with Nasdaq Rule 5635(a), the Series A Certificate of Designation provides
that the Series A Preferred Stock will not be convertible into Common Stock until such time as we obtain stockholder approval for their
removal, as discussed in “Proposal 2: Series A Conversion Proposal.”
If stockholders do not approve the Series A Conversion Proposal, the
Company will not be able to honor any conversions of Series A Preferred Stock held by Veru.
Description of Series A Preferred Stock
The terms of the Series A Convertible Preferred
Stock are set forth in a Certificate of Designations of Rights and Preferences of Series A Convertible Preferred Stock of the Company
(the “Certificate of Designations”), which was filed with the State of Delaware on September 29, 2023. Pursuant to the Certificate
of Designations, each share of Series A Preferred Stock is convertible by Veru at any time and from time to time from and after one year
from the date of issuance of the Series A Preferred Stock into that number of shares of the Company’s common stock determined by
dividing the Stated Value (as defined in the Certificate of Designations) of $1,000 per share by the Conversion Price (as defined in the
Certificate of Designations) of $0.5254 per share, subject to adjustment as provided in the Certificate of Designations, subject to certain
stockholder approval limitations. The Series A Preferred Stock is entitled to share ratably in any dividends paid on the Company’s
common stock (on an as-if-converted-to-common-stock basis), has no voting rights except as to certain significant matters specified in
the Certificate of Designations, and has a liquidation preference equal to the Stated Value of $1,000 per share plus any accrued but unpaid
dividends thereon. The Series A Preferred Stock is redeemable in whole or in part at the Company’s option at any time.
The Company also agreed to include the shares
of common stock issuable upon conversion of the Series A Preferred Stock in the next resale registration statement filed with the Securities
and Exchange Commission.
Required Vote
Assuming a quorum is present at the Special Meeting,
approval of the Series A Conversion Proposal requires the affirmative vote of the majority of the votes cast by stockholders present virtually
or represented by proxy and entitled to vote on the matter at the Special Meeting. Assuming a quorum is present, if an Onconetix stockholder
fails to vote, fails to instruct its bank, broker, or other nominee to vote with respect to the Series A Conversion Proposal, or abstains
from voting, it will have no effect on the Series A Conversion Proposal.
THE ONCONETIX BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ONCONETIX STOCKHOLDERS VOTE “FOR” THE SERIES A CONVERSION PROPOSAL.
PROPOSAL 3: PMX ISSUANCE PROPOSAL
Overview
As described above, the Company issued 2,696,729
shares of Series B Preferred Stock in the PMX Transaction. Upon conversion of the above-described Series B Preferred Stock,
269,672,900 shares of Common Stock are issuable, assuming approval of this Proposal No.
4.
In connection with the PMX Transaction, on December
15, 2023, Onconetix entered into the Subscription Agreement with the PMX Investor for a private placement of $5.0 million of Units, each
Unit comprised of (i) one share of Common Stock and (ii) one pre-funded warrant to purchase 0.3 shares of Common Stock at an exercise
price of $0.001 per share, for an aggregate purchase price per Unit of $0.25. Additional shares are issuable to the PMX Investor to the
extent the PMX Investor continues to hold Common Stock included in the Units and if the VWAP during the 270 days following closing is
less than the Purchase Price, as set forth in the Subscription Agreement. If the closing of the PMX Financing took place on [●], 2024,
Units containing an aggregate of (i) [●] shares of Common Stock and (ii) Warrants to purchase
[●] shares of Common Stock at an exercise price of $0.001 per share are issuable, assuming approval of this Proposal No. 4.
On January 23, 2024, the Company issued the Debenture
to the PMX Investor in the principal sum of $5.0 million, the payment of which shall offset the Aggregate Purchase Price for the Units
pursuant to the Subscription Agreement. The Debenture has an interest rate of 4.0% per annum, and the principal and accrued interest are
repayable in full upon the earlier of (i) the closing under the Subscription Agreement and (ii) June 30, 2024. Additionally, the $5.0
million subscription amount under the Subscription Agreement shall be increased by the amount of interest payable under the Debenture.
Purpose of the PMX Issuance Proposal
Pursuant to Nasdaq Rule 5635(a), stockholder approval
is required prior to the issuance by the Company of Common Stock (or securities convertible into or exercisable for Common Stock) in connection
with the acquisition of the stock or assets of another company if, due to the present or potential issuance of common stock, including
shares issued pursuant to an earn-out provision or similar type of provision, or securities convertible into or exercisable for common
stock, other than a public offering for cash: (A) the common stock has or will have upon issuance voting power equal to or in excess of
20% of the voting power outstanding before the issuance of stock or securities convertible into or exercisable for common stock; or (B)
the number of shares of common stock to be issued is or will be equal to or in excess of 20% of the number of shares of common stock outstanding
before the issuance of the stock or securities. Combined with the shares of Common Stock already issued in the PMX Transaction and the
shares of Common Stock issuable upon conversion of the Series A Preferred Stock, the shares issuable upon conversion of the Series B Preferred
Stock and those shares that are issuable in the PMX Financing would result in the issuance of more than 20% of the voting power and the
number of shares of Common Stock outstanding as of the issuance of the Series B Preferred Stock and the date of the Subscription Agreement
for the PMX Financing.
If stockholders have not approved the PMX Issuance
Proposal by January 1, 2025, at the request of the holder setting forth such holder’s request to cash settle a number of shares
of Series B Preferred Stock, the Company shall pay to such holder an amount in cash equal to (i) the Fair Value (as defined below) of
the shares of Series B Preferred Stock set forth in such request multiplied by (ii) the Conversion Ratio (as defined in the Certificate
of Designation of the Series B Preferred Stock) in effect on the trading day on which the request is delivered to Onconetix. The “Fair
Value” of shares shall be fixed with reference to the last reported closing stock price on the principal trading market of the Common
Stock.
Stockholder approval is a condition to closing
the PMX Financing, and the offering is expected to close following stockholder approval of the issuance of the Conversion Shares. If
stockholders do not approve the PMX Issuance Proposal, the Company will not be able to complete the PMX Financing.
Description of Series B Preferred Stock
Voting. The
shares of Series B Preferred Stock carry no voting rights except: (i) with respect
to the election of the Proteomedix Director (as described below) and (ii) that the affirmative vote of the Majority Holders, acting
as a single class, shall be necessary to (A) alter or change adversely the powers, preferences or rights given to the Series B Preferred
Stock, (B) alter or amend the Certificate of Designation, or amend or repeal any provision of, or add any provision to, Onconetix’s
certificate of incorporation or bylaws, if such action would adversely alter or change the preferences, rights, privileges or powers of,
or restrictions provided for the benefit of the Series B Preferred Stock, (C) issue
further shares of Series B Preferred Stock or increase or decrease (other than by conversion) the number of authorized shares of Series
B Preferred Stock, or (D) authorize or create any class or series of stock, or issue shares of any class or series of stock, that has
powers, preferences or rights senior to the Series B Preferred Stock.
Proteomedix
Director. The Majority Holders, voting exclusively and as a separate class, shall be entitled to elect one (1) director of Onconetix.
Any director elected as provided in the preceding sentence may be removed without cause by, and only by, the affirmative vote of the holders
of the Series B Preferred Stock. If the holders of Series B Preferred Stock fail to elect a director, then any directorship not so filled
shall remain vacant until such time as the holders of the Series B Preferred Stock elect a person to fill such directorship; and no such
directorship may be filled by stockholders of Onconetix other than by the holders of Series B Preferred Stock. At any meeting held for
the purpose of electing a director, the presence in person or by proxy of the holders of a majority of the outstanding shares of Series
B Preferred Stock shall constitute a quorum for the purpose of electing such director.
Redemption.
The shares of Series B Preferred Stock are not redeemable by Onconetix.
Liquidation
Preference. Upon a Liquidation, the holders of Series B Preferred Stock shall be entitled to receive out of the assets, whether
capital or surplus, of Onconetix the same amount that a holder of Common Stock would receive if such Holder’s Series B Preferred
Stock were fully converted to Common Stock at the Conversion Ratio (as defined below) plus an additional amount equal to any dividends
declared but unpaid to such shares, which amounts shall be paid pari passu with all holders of Common Stock.
Dividends.
The holders of the Series B Preferred Stock shall be entitled to receive, dividends on shares of Series B Preferred Stock (on an as-if-converted-to-common-stock
basis) equal to and in the same form, and in the same manner, as dividends (other than dividends on shares of the Common Stock payable
in the form of Common Stock) actually paid on shares of the Common Stock when, as and if such dividends (other than dividends payable
in the form of Common Stock) are paid on shares of the Common Stock.
Conversion.
Following Stockholder Approval, each share of Series B Preferred Stock shall be converted into the Conversion Shares at the Conversion
Ratio. All shares of Series B Preferred Stock shall automatically and without any further action required be converted into Conversion
Shares at the Conversion Ratio upon the latest date on which (i) Onconetix has received the Stockholder Approval with respect to the issuance
of all of the shares of Common Stock issuable upon Conversion in excess of 20% of the issued and outstanding Common Stock on the Closing
Date and (ii) Onconetix has effected an increase in the number of shares of Common Stock authorized under its certificate of incorporation,
to the extent required to consummate the PMX Transaction.
Cash
Settlement. If, at any time after the Cash Settlement Date, Onconetix (x) has obtained the Stockholder Approval but fails to
or has failed to deliver to a holder certificate or certificates representing the Conversion Shares, or deliver documentation of book
entry form of (or cause its transfer agent to electronically deliver such evidence) Conversion Shares on or prior to the fifth business
day after the date of the Stockholder Approval, or (y) has failed to obtain the Stockholder Approval, Onconetix shall, in either
case, at the request of the holder setting forth such holder’s request to cash settle a number of shares of Series B Preferred Stock,
pay to such holder an amount in cash equal to (i) the Fair Value (as defined below) of the shares of Series B Preferred Stock set forth
in such request multiplied by (ii) the Conversion Ratio in effect on the trading day on which the request is delivered to Onconetix, with
such payment to be made within two (2) business days from the date of the request by the holder, whereupon, after payment in full thereon
by Onconetix, Onconetix’s obligations to deliver such shares underlying the request shall be extinguished. “Fair Value”
of shares shall be fixed with reference to the last reported closing stock price on the principal trading market of the Common Stock on
which the Common Stock is listed as of the trading day on which the request is delivered to Onconetix.
Certain Adjustments.
If Onconetix, at any time while the Series B Preferred Stock is outstanding: (A) pays a stock dividend or otherwise makes a
distribution or distributions payable in shares of Common Stock; (B) subdivides outstanding shares of Common Stock into a larger number
of shares; or (C) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares,
then the Conversion Ratio shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock outstanding
immediately after such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately before
such event (excluding any treasury shares of the Corporation). If, at any time while the Series B Preferred Stock is outstanding,
either (A) Onconetix effects any merger or consolidation of Onconetix with or into another person or any stock sale to, or other business
combination with or into another person (other than such a transaction in which Onconetix is the surviving or continuing entity and holds
at least a majority of the Common Stock after giving effect to the transaction and its Common Stock is not exchanged for or converted
into other securities, cash or property), (B) Onconetix effects any sale, lease, transfer or exclusive license of all or substantially
all of its assets in one transaction or a series of related transactions, (C) any tender offer or exchange offer (whether by Onconetix
or another person) is completed pursuant to which more than 50% of the Common Stock not held by Onconetix or such person is exchanged
for or converted into other securities, cash or property, or (D) Onconetix effects any reclassification of the Common Stock or any
compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or
property, then, in connection with any such transaction in (A) through (D), the holders of Series B Preferred Stock shall receive in such
transaction, the same kind and amount of securities, cash or property that a holder of Common Stock would receive if such holder’s
Series B Preferred Stock were fully converted to Common Stock, plus an additional amount equal to any dividends declared but unpaid to
such shares, which amounts shall be paid pari passu with all holders of Common Stock in the Fundamental PMX Transaction (the
“Alternate Consideration”). If holders of Common Stock are given any choice as to the securities, cash or property to
be received in a transaction in (A) through (D), then the holders of Series B Preferred Stock shall be given the same choice as to the
Alternate Consideration it receives in such transaction.
Required Vote
Assuming a quorum is present at the Special Meeting,
approval of the PMX Issuance Proposal requires the affirmative vote of the majority of the votes cast by stockholders present virtually
or represented by proxy and entitled to vote on the matter at the Special Meeting. Assuming a quorum is present, if an Onconetix stockholder
fails to vote, fails to instruct its bank, broker, or other nominee to vote with respect to the PMX Issuance Proposal, or abstains from
voting, it will have no effect on the PMX Issuance Proposal.
THE ONCONETIX BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ONCONETIX STOCKHOLDERS VOTE “FOR” THE PMX ISSUANCE PROPOSAL.
PROPOSAL
4: AUDITOR RATIFICATION PROPOSAL
Introduction
On [●], 2024, the Board recommended
the stockholder ratification of the appointed the firm of EisnerAmper as the Company’s independent registered public accounting
firm for the fiscal year ending December 31, 2024. At the Meeting, our stockholders will be asked to ratify such appointment of EisnerAmper
to serve as our independent registered public accounting firm. The Board, through the Audit Committee, is directly responsible for appointing
the Company’s independent registered public accounting firm. The Board is not bound by the outcome of this vote but will consider
these voting results when selecting the Company’s independent registered public accounting firm for fiscal year 2024. A representative
of EisnerAmper is not expected to be present at the Meeting.
On
June 29, 2023, Mayer Hoffman McCann P.C. (“MHM”), the Company’s independent registered public accounting firm, informed
the Company that it resigned, effective June 29, 2023.
MHM audited the Company’s
financial statements as of and for the years ended December 31, 2022 and 2021. MHM’s audit reports on the Company’s financial
statements as of, and for the fiscal years ended December 31, 2022 and 2021, dated March 8, 2023, did not contain any adverse opinion
or a disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principles.
During the Company’s
fiscal years ended December 31, 2022 and 2021, and the subsequent interim period through July 6, 2023, there were no disagreements between
the Company and MHM on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure,
which disagreements, if not resolved to the satisfaction of MHM, would have caused MHM to make reference to the subject matter of the
disagreements in connection with its audit reports on the Company’s financial statements for such periods.
During the Company’s fiscal years ended
December 31, 2022 and 2021, and the subsequent interim period through July 6, 2023, there were no “reportable events”, as
defined in Regulation S-K Item 304(a)(1)(v), except as previously disclosed in the Company’s Form 10-K for the fiscal year ended
December 31, 2022, MHM identified a material weakness in internal controls in connection with a lack of staff (a) to maintain optimal
segregation of duties and to provide optimal levels of oversight in order to process financial information in a timely manner, analyze
and account for complex, non-routine transactions, and prepare financial statements and (b) to timely identify, approve or report related
party transactions. The Company is taking steps to remediate these material weaknesses.
On July 6, 2023, the
Audit Committee appointed EisnerAmper to serve as the Company’s independent registered public accounting firm for the fiscal year
ending December 31, 2023 and related interim periods. The decision to engage EisnerAmper was approved by the Audit Committee. During the
Company’s two most recent fiscal years and the subsequent interim period through July 6, 2023, the Company did not consult EisnerAmper
with respect to any of the matters or events listed in Regulation S-K Item 304(a)(2).
Fees
The table below presents the
aggregate fees billed for professional services rendered by EisnerAmper for the year ended December 31, 2023.
| | |
| |
| Audit fees | |
$ | 778,568 | |
| Audit-related fees | |
| - | |
| Tax fees | |
| - | |
| All other fees | |
| - | |
| Total fees | |
$ | 778,568 | |
In the above table, “audit fees” are
fees billed for services provided related to the audit of our annual consolidated financial statements, quarterly reviews of our interim
consolidated financial statements, and services normally provided by EisnerAmper in connection with regulatory filings or engagements
for that fiscal period.
Our Audit Committee determined
that the services provided by EisnerAmper were compatible with maintaining the independence of EisnerAmper as our independent registered
public accounting firm.
The table below presents the aggregate fees billed
for professional services rendered by MHM for the years ended December 31, 2023 and 2022.
| | |
2023 | | |
2022 | |
| Audit fees | |
$ | 208,426 | | |
$ | 633,629 | |
| Audit-related fees | |
| - | | |
| - | |
| Tax fees | |
$ | 11,889 | | |
| 9,975 | |
| All other fees | |
| - | | |
| - | |
| Total fees | |
$ | 220,315 | | |
$ | 643,604 | |
In the above table, “audit fees” are
fees billed for services provided related to the audit of our annual financial statements, quarterly reviews of our interim financial
statements, and services normally provided by MHM in connection with regulatory filings or engagements for those fiscal periods. “Tax
fees” consist of amounts billed by an associated entity of MHM for services in connection with the preparation of our federal and
state tax returns.
Our Audit Committee determined that the services
provided by MHM were compatible with maintaining the independence of MHM as our independent registered public accounting firm.
Pre-Approval Policies and Procedures
The formal written charter
for our Audit Committee requires that the Audit Committee pre-approve all audit services to be provided to us, whether provided by
our principal auditor or other firms, and all other services (review, attest and non-audit) to be provided to us by our independent registered
public accounting firm, other than de minimis non-audit services approved in accordance with applicable SEC rules.
The Audit Committee has adopted
a pre-approval policy that sets forth the procedures and conditions pursuant to which audit and non-audit services proposed
to be performed by our independent registered public accounting firm may be pre-approved. This pre-approval policy generally provides
that the Audit Committee will not engage an independent registered public accounting firm to render any audit, audit-related, tax or permissible
non-audit service unless the service is either (i) explicitly approved by the Audit Committee or (ii) entered into pursuant
to the pre-approval policies and procedures described in the pre-approval policy. Unless a type of service to be provided by
our independent registered public accounting firm has received this latter general pre-approval under the pre-approval policy,
it requires specific pre-approval by the Audit Committee.
On an annual basis, the Audit
Committee reviews and generally pre-approves the services (and related fee levels or budgeted amounts) that may be provided by the
Company’s independent registered public accounting firm without first obtaining specific pre-approval from the Audit Committee.
The Audit Committee may revise the list of general pre-approved services from time to time, based on subsequent determinations. Any
member of the Audit Committee to whom the committee delegates authority to make pre-approval decisions must report any such pre-approval decisions
to the Audit Committee at its next scheduled meeting. If circumstances arise where it becomes necessary to engage the independent registered
public accounting firm for additional services not contemplated in the original pre-approval categories or above the pre-approved amounts,
the Audit Committee requires pre-approval for such additional services or such additional amounts.
Our Audit Committee was formed
upon the consummation of our initial public offering. As a result, the Audit Committee did not pre-approve all of the foregoing services,
although any services rendered prior to the formation of our Audit Committee were approved by our Board. Since the formation of our Audit
Committee, and on a going-forward basis, the Audit Committee has and will pre-approve all auditing services and permitted non-audit services
to be performed for us by our auditors, including the fees and terms thereof (subject to the de minimis exceptions for non-audit services
described in the Exchange Act which are approved by the Audit Committee prior to the completion of the audit).
Required Vote
Assuming a quorum is present at the Special Meeting,
approval of the Auditor Ratification Proposal requires the affirmative vote of the majority of the votes cast by stockholders present
virtually or represented by proxy and entitled to vote on the matter at the Special Meeting. Assuming a quorum is present, if an Onconetix
stockholder fails to vote, fails to instruct its bank, broker, or other nominee to vote with respect to the Auditor Ratification Proposal,
or abstains from voting, it will have no effect on the Auditor Ratification Proposal. Since this is a routine matter, brokers may vote
at the Special Meeting on this proposal, provided that they have not received instructions from a beneficial owner.
THE ONCONETIX BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ONCONETIX STOCKHOLDERS VOTE “FOR” THE Auditor Ratification Proposal.
PROPOSAL 5: ADJOURNMENT PROPOSAL
The Special Meeting may be adjourned to another
time and place if necessary or appropriate to permit the solicitation of additional proxies if there are insufficient votes at the time
of the Special Meeting to approve the Reverse Stock Split Proposal, the Series A Conversion Proposal,
the PMX Issuance Proposal or the Auditor Ratification Proposal.
The Company is asking stockholders to authorize
the holder of any proxy solicited by the Board to vote in favor of any adjournment of the Special Meeting, if necessary or appropriate,
to solicit additional proxies if there are insufficient votes to approve the Reverse Stock Split Proposal,
the Series A Conversion Proposal, the PMX Issuance Proposal or the Auditor Ratification Proposal
Required Vote
Assuming a quorum is present at the Special Meeting,
approval of the Adjournment Proposal requires the affirmative vote of the majority of the votes cast by stockholders present virtually
or represented by proxy and entitled to vote on the matter at the Special Meeting. Assuming a quorum is present, if an Onconetix stockholder
fails to vote, fails to instruct its bank, broker, or other nominee to vote with respect to the Adjournment Proposal, or abstains from
voting, it will have no effect on the Adjournment Proposal.
THE ONCONETIX BOARD OF DIRECTORS UNANIMOUSLY
RECOMMENDS THAT ONCONETIX STOCKHOLDERS VOTE “FOR” THE ADJOURNMENT PROPOSAL.
INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS
IN THE PROPOSALS
As of the date of this proxy statement, Onconetix
directors and executive officers do not have interests in the proposals that are different from, or in addition to, the interests of other
Onconetix stockholders generally, except that:
| |
● |
Dr. Ralph Schiess, our Interim Chief Executive Officer and Chief Science Officer, is a holder of 269,749 shares of Common Stock and 195,664 shares of Series B Preferred Stock. |
| |
● |
Christian Brühlmann, our Chief Strategy Officer, is a holder of 236,029 shares of Common Stock and 171,204 shares of Series B Preferred Stock. |
Management
of the combined company
Directors and Executive Officers
The following table provides information regarding our executive officers
and directors as of February 14, 2024:
| Name |
|
Age |
|
Position(s) |
| Executive Officers and Directors |
|
|
|
|
| Dr. Ralph Schiess |
|
45 |
|
Interim Chief Executive Officer and Chief Science Officer |
| Bruce Harmon |
|
65 |
|
Chief Financial Officer |
| Christian Brühlmann |
|
47 |
|
Chief Strategy Officer |
| Non-Employee Directors |
|
|
|
|
| James Sapirstein |
|
62 |
|
Non-Executive Chairman of the Board |
| Simon Tarsh |
|
62 |
|
Director |
| Timothy Ramdeen |
|
32 |
|
Director |
| Thomas Meier, PhD |
|
61 |
|
Director |
| Ajit Singh |
|
60 |
|
Director |
Dr. Ralph Schiess has
been Chief Science Officer since December 2023 and Interim Chief Executive Officer since January 2024. Dr. Schiess worked for 10
years in world-class academic institutions both in the U.S. and Europe and gained over 10 years of experience in the diagnostic
industry successfully developing products from scientific prototypes to commercial products. Dr. Schiess co-founded Proteomedix in
2010 and was CEO until December 2019, then served as Chief Scientific Officer from January 2020 to May 2023. Dr. Schiess returned to
his role as Chief Executive Officer in June 2023. Under his guidance, Proteomedix identified novel biomarker signatures with utility
in prostate cancer diagnosis, prognosis and therapy management. Upon Proteomedix’s acquisition by the Company in December 2023,
Dr. Schiess was appointed Chief Science Officer of Onconetix, and in January 2024, Dr. Schiess was appointed Interim Chief Executive Officer
of Onconetix. Dr. Schiess received his M.S. from the University of Zurich, followed
by an internship at the Institute of System Biology in Seattle. Thereafter, he received a doctorate from the Swiss Federal Institute
of Technology.
Bruce Harmon has more
than 40 years of experience in financial positions with life sciences companies and various other industries. Mr. Harmon has served in
a variety of roles, including chief financial officer, controller, chief executive officer, and audit committee chairman. He has been
an independent consultant since 2008 through his business, Lakeport Business Services, Inc., and served in the outsourced CFO capacity
for multiple publicly traded companies. During this time, Mr. Harmon was CFO of Marizyme, Inc. (OTCMKTS:MRZM) from 2020 to 2021, CFO of
bioAffinity Technologies Inc. (Nasdaq: BIAF) in 2022, a director of Dale Biotech LLC since 2017, and a director of Patriax Industries
since 2023. Mr. Harmon joined as Chief Financial Officer of Onconetix in October 2023. He has extensive experience with fundraising, public
offerings, mergers and acquisitions, and turnarounds. Earlier in his career, he was a member of a team that, at the invitation of the
Environmental Programmé, presented a green building product to delegates at the United Nations. He earned a Bachelor of Science
degree in accounting from Missouri State University.
Christian
Brühlmann has been Chief Strategy Officer since December 2023. He was CBO and co-founder of Proteomedix, which was acquired
by the Company in December 2023. Mr. Brühlmann co-founded Proteomedix and served as its Chief Financial and Operations Officer
from March 2010 until November 2018. Beginning in December 2018, Mr. Brühlmann served as Proteomedix’s Chief Business
Officer. Mr. Brühlmann gained 20 years of experience in public and private companies in the life sciences, information and
communications (ICT) and financial industries. Being responsible for product management, business development, operations and
finance, he was instrumental in Proteomedix’s development from inception to the market introduction of Proclarix. Previously,
he worked for Swisscom, Switzerland’s telecom market leader in several strategic and leadership roles in the area of
digitalization. Mr. Brühlmann received his Bachelor and Master in Business Administration from University of Zurich,
Switzerland and completed executive professional trainings at the Babson College, USA and at the University of St. Gallen,
Switzerland.
James Sapirstein,
one of our directors since February 2022, has over 35 years of experience leading, founding, growing, and selling healthcare companies,
specifically in the pharmaceutical space. Mr. Sapirstein is currently the President, CEO and Chairman of First Wave BioPharma, Inc. (Nasdaq:
FWBI), where he has been since October 2019. His career began in sales at Eli Lilly, eventually rising to Director of International Marketing
at Bristol Myers Squibb from July 1996 to June 2000, and later led the launch of Viread (tenofovir) at Gilead Sciences, Inc. (Nasdaq:
GILD), where he served as Global Marketing Lead from June 2020 to June 2002. From November 2006 to January 2011, he served as founding
CEO of Tobira Therapeutics (Nasdaq: TBRA), then a private company, and later acquired by Allergan (NYSE: AGN). Since then, he has served
as CEO of Alliqua Biomedical (Nasdaq: ALQA) from September 2012 to February 2014 and CEO of Contravir Pharmaceuticals (Nasdaq: CTRV)
from March 2014 to October 2018. He has been part of almost two dozen drug product launches and specifically either led or has been a
key member of several HIV product launches into different new classes of therapeutics at the time. Additionally, Mr. Sapirstein has held
board positions on ZyVersa Therapeutics, Inc. (Nasdaq: ZVSA) since January 2023 and Enochian Biosciences (Nasdaq: ENOB) since April 2018.
He previously served as a director of Marizyme, Inc. (OTCMKTS:MRZM) (Executive Chairman) from December 2018 to June 2021, Leading Biosciences
from 2016 to 2021, BioNJ, an association of biopharma industries in New Jersey, from February 2017 to February 2019, RespireRX (OTCBB:RSPI)
from April 2014 to January 2020, NanoViricides Inc. (NYSE: NNVC) from November 2018 to January 2020, and BWAC from December 2020 until
its business combination with Clarus in September 2021. He is also a Board Director for BIO, the leading Biopharma Industries Organization
promoting public policy and networking in the healthcare space, where he sits on both the Health Section and Emerging Companies Section
Governing Boards. Mr. Sapirstein received a B.S. in Pharmacy from Rutgers University and his MBA from Fairleigh Dickinson University.
He is well qualified to serve on our Board due to his extensive network from decades in the healthcare industry. Mr. Sapirstein brings
to our Board a significant depth of experience in the pharmaceutical and biotechnology industries that will be invaluable to the Company
as we continue to develop biotechnology assets.
Simon Tarsh, one of
our directors since August 2022, has more than 40 years of financial experience, working in both the UK and the U.S. He has recently retired
from Deloitte Consulting LLP, where he was a Senior Managing Director in the Finance and Enterprise Performance Practice, where he had
served global clients since 2007. He led a growing global practice focused around Operational Transformation, including supporting Carve
Out transactions, joint ventures and hybrid structures, both in the US and in international locations, such as India, China, Eastern Europe
and Latin America. He supported high growth companies with their finance operations as they globalized, and was able to advise them on
their expansion, while balancing growth with appropriate controls. Prior to moving to the United States in 2007, Mr. Tarsh’s consulting
career began with PA Consulting Group, London in 1988, where he was elected as a Partner in 1997, and he built ISG’s business process
outsourcing advisory practice in Europe between 2001 and 2006. Mr. Tarsh’s early career was in finance, working with Marathon Oil
and Dow Chemical, and during this period, he qualified as a Chartered Accountant. Mr. Tarsh received a Bachelor of Science undergraduate
degree in Business and Administration from the University of Salford, Manchester, UK in 1981, and an MBA from City University Business
School, London, UK in 1988. He is a Fellow of the Chartered Institute of Management Accountants (1984), which is considered as a CPA equivalent.
Mr. Tarsh’s deep financial experience at Deloitte Consulting LLP for fifteen years offers valuable insights to our Board, particularly
given the enhanced accounting rules and regulations affecting public companies.
Timothy Ramdeen, one
of our directors since January 2023, has nearly a decade of experience in private equity and hedge fund investing, capital markets, and
company formation. Since June 2022, Mr. Ramdeen has been founder and managing partner of Dharma Capital Advisors, an investment and advisory
firm focused on early-stage private and public companies. From March 2021 to March 2022, Mr. Ramdeen was co-founder, chief investment
officer, and portfolio manager at Sixth Borough Capital Management, a multi-stage, event-driven hedge fund focused on both private and
public equities. Since 2022, Mr. Ramdeen has been the co-founder of Amplexd Therapeutics, which is a women’s health/biotechnology
company focused on providing low-cost, effective, safe and accessible treatments for early cervical and HPV-related cancers worldwide.
Mr. Ramdeen also serves as a corporate advisor/board member to multiple early-stage companies and investment funds. Previously, Mr. Ramdeen
was the fifth hire at Altium Capital Management (“Altium”), a healthcare-focused investment firm, where from July 2019 to
March 2021 he served as the sole investment analyst on the private capital markets/special situations desk (privately-negotiated financings,
direct investments, event-driven long/short, and private to public investments in micro and small-cap companies). During his tenure at
Altium, Mr. Ramdeen was instrumental in co-creating the firm’s SPAC and reverse merger investment efforts and establishing extensive
relationships with sell-side constituents, buy-side counterparts, and hundreds of private and publicly traded companies across biotechnology,
therapeutics, healthcare services, medical devices and medtech. From 2017 to 2018, Mr. Ramdeen worked for Brio Capital Management, an
event-driven hedge fund focused on small and micro cap equities. Mr. Ramdeen received his B.S. in Biology from Temple University, where
he conducted scientific research across neurology, oncology, and developmental biology. In addition, Mr. Ramdeen earned his MBA in Finance
from NYU Stern School of Business. Mr. Ramdeen brings to our Board extensive experience in capital advisement and company development,
specifically within the life science industry and for publicly traded companies.
Thomas Meier, one
of our directors since February 1, 2024, has close to 25 years’ experience as a life-science and biotech entrepreneur, executive
manager, and board member. Since June 2022, Dr. Meier has served as Chairman of, and member of the Audit and Compensation Committees of,
Santhera Pharmaceuticals Holding AG (SIX: SANN), a publicly listed Swiss specialty pharmaceutical company focused on the development and
commercialization of innovative medicines for rare neuromuscular and pulmonary diseases. Dr. Meier has served on the board of Santhera
since 2017 and stepped down as the company’s CEO in November 2019 after having served 15 years as executive manager, the last 8
years as CEO. In 2020, Dr. Meier became managing partner of Viopas Venture Consulting GmbH, a Swiss consultancy and advisory firm for
the healthcare industry. Since 2020, Dr. Meier has served as a board member of Novaremed AG, a privately held Swiss company developing
innovative treatment options for the management of chronic pain and alternatives to opioids. Dr. Meier has served on Novaremed’s
Audit Committee since October 2021 and became Executive Chairman of the company in January 2024. Since January 2022, Dr. Meier also serves
on the board of Visgenx Inc. (USA). In September 2021, he co-founded SEAL Therapeutics AG, a privately owned Swiss gene therapy company
for which he also serves as Chairman. Between July 2020 and November 2021, he served as Chairman of privately held Pharmabiome AG (Switzerland).
Dr. Meier has a PhD in Biology and qualified as lecturer in neurosciences at the Biozentrum, University of Basel (Switzerland). Dr. Meier
brings to our board experience as an internationally recognized scientist with track record in clinical research of orphan diseases.
Ajit Singh, one of
our directors since February 7, 2024, is a Partner at Silicon Valley based Artiman Ventures, focused on early-stage technology and life
science investments, with over $1 billion in assets under management. Besides serving on the board of directors of Artiman portfolio
companies, he has served on the boards of Sofie Biosciences, a PET radiopharmaceuticals company focused on Oncology and Neurology, Leo
Cancer Care, focused on radiation oncology since 2013, Artidis, an oncology diagnostics company with nanomechanical biomarkers for cancer,
and Chronus Health, in the area of Point-of-Care diagnostics since 2023. He also serves on the Board of Trustees of American Association
for Cancer Research (AACR) Foundation, the oldest and the largest cancer research organization globally. Dr. Singh is an Adjunct Professor
in the School of Medicine at Stanford where he teaches clinical diagnostics and entrepreneurship. In the past, Dr. Singh has served as
a Lead Director on the Board of Directors of Max Healthcare, and as a Senior Advisor to the Tata Trusts Cancer program, which developed
a “plan centrally, deliver locally” platform for cancer care, and delivered it via comprehensive cancer centers built bespoke
with funding from the Tata Group. Until 2023, he also served on the board of directors of Cadila Pharmaceuticals. Prior to joining Artiman,
Dr. Singh was the President and CEO of BioImagene, a company specializing in AI-based Cancer Diagnostics, based in California. BioImagene
was acquired by Roche Pharmaceuticals in September 2010. Before BioImagene, Dr. Singh spent nearly twenty years at Siemens in various
roles, in the United States and Germany, most recently as the global CEO of Siemens Oncology, and Siemens Digital Imaging Systems. Before
transitioning to these executive responsibilities, Dr. Singh spent several years in R&D at Siemens Research in Princeton, responsible
for research in the areas of artificial intelligence and robotics. During this time, he concurrently served as an adjunct faculty at
Princeton University. Dr. Singh has a Ph.D. in Computer Science from Columbia University, a Master’s degree in Computer Engineering
from Syracuse University, and a Bachelor’s in Electrical Engineering from Indian Institute of Technology (IIT) in Varanasi, India.
He has published two books and numerous refereed articles and holds five patents. His Top-10 Book Review is carried by various blogs
and reading journals in December every year. Mr. Singh brings to our board significant experience in the biotech industry and diagnostic
field, particularly in a commercial execution capacity.
Board of Directors and Corporate Governance
General
Our business and affairs are
organized under the direction of our Board, which currently consists of five members. Our Board is divided into three classes, Class I,
Class II and Class III, with members of each class serving staggered three-year terms. Our directors are divided among the three classes
as follows:
| |
● |
the Class I directors are Simon Tarsh and Thomas Meier, and their term will expire at our 2025 annual meeting of stockholders; |
| |
● |
the Class II director is James Sapirstein, and his term will expire at our 2026 annual meeting of stockholders; and |
| |
● |
the Class III directors are Timothy Ramdeen and Ajit Singh, and their term will expire at our 2024 annual meeting of stockholders. |
Our Amended and Restated Certificate
of Incorporation and our Amended and Restated Bylaws provide that the authorized number of directors may be changed only by resolution
of the Board. Our directors hold office until the earlier of their death, resignation, removal or disqualification, or until their successors
have been elected and qualified. Our board of directors does not have a formal policy on whether the roles of Chief Executive Officer
and Chairman of our Board should be separate. The primary responsibilities of our Board are to provide oversight, strategic guidance,
counselling and direction to our management.
We have no formal policy regarding
board diversity. Our priority in selection of board members is identification of members who will further the interests of our stockholders
through his or her established record of professional accomplishment, the ability to contribute positively to the collaborative culture
among board members, knowledge of our business and understanding of the competitive landscape.
Directors and Executive Officers Qualifications
We believe that the collective
skills, experiences and qualifications of our directors provide our Board with the expertise and experience necessary to advance the interests
of our stockholders. In selecting directors, the Board considers candidates that possess qualifications and expertise that will enhance
the composition of the Board. Nominees for director will be selected on the basis of, among other things, leadership experience, knowledge,
skills, expertise, integrity, diversity, ability to make independent analytical inquiries, understanding of the Company’s business
environment and willingness to devote adequate time and effort to Board responsibilities. The Nominating & Corporate Governance Committee
may require certain skills or attributes, such as financial or accounting experience, to meet specific board needs that arise from time
to time and will also consider the overall experience and makeup of its members to obtain a broad and diverse mix of board members. We
believe that our directors should have the highest professional and personal ethics and values, consistent with our longstanding values
and standards. They should have broad experience at the policy-making level in business, exhibit commitment to enhancing stockholder value
and have sufficient time to carry out their duties and to provide insight and practical wisdom based on their past experience.
Committees of the Board
Our Board has established three
standing committees—audit, compensation and nominating and corporate governance—each of which operates under a charter that
has been adopted by our Board. Copies of each committee’s charter are posted on the “Investor Relations” section of
our website, which is located at https://onconetix.com/corporate-governance/governance-overview. Each committee has the composition
and responsibilities described below. Our Board may from time to time establish other committees.
Audit Committee
Our audit committee (“Audit
Committee”) consists of Simon Tarsh, who is the chair of the committee, Timothy Ramdeen and James Sapirstein. Our Board has determined
that each of the members of our Audit Committee satisfies the Nasdaq Marketplace Rules and SEC independence requirements. The functions
of this committee include, among other things:
| ● | evaluating the performance,
independence and qualifications of our independent auditors and determining whether to retain our existing independent auditors or engage
new independent auditors; |
| ● | reviewing and approving the
engagement of our independent auditors to perform audit services and any permissible non-audit services; |
| ● | reviewing our annual and quarterly
financial statements and reports, including the disclosures contained under the caption “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” and discussing the statements and reports with our independent auditors and management; |
| ● | reviewing with our independent
auditors and management significant issues that arise regarding accounting principles and financial statement presentation and matters
concerning the scope, adequacy and effectiveness of our financial controls; |
| ● | reviewing and approving, in
accordance with the Company’s policies, any related party transaction as defined by applicable rules and regulations |
| ● | reviewing our major financial
risk exposures, including the guidelines and policies to govern the process by which risk assessment and risk management is implemented;
and |
| ● | reviewing and evaluating on
an annual basis the performance of the audit committee, including compliance of the audit committee with its charter. |
The Board has determined that
Simon Tarsh qualifies as an “audit committee financial expert” within the meaning of applicable SEC regulations and meets
the financial sophistication requirements of the Nasdaq Marketplace Rules. In making this determination, the Board has considered Mr.
Tarsh’s extensive financial experience and business background. Both our independent registered public accounting firm and management
periodically meet privately with our Audit Committee.
Compensation Committee
Our compensation committee
(“Compensation Committee”) consists of James Sapirstein, who is the chair of the committee, Simon Tarsh and Timothy Ramdeen.
Our board of directors has determined that each of the members of our Compensation Committee is an outside director, as defined pursuant
to Section 162(m) of the Internal Revenue Code of 1986, as amended, or the Code, and satisfies the Nasdaq Marketplace Rules independence
requirements. The functions of this committee include, among other things:
| ● | reviewing, modifying and approving
(or if it deems appropriate, making recommendations to the full board of directors regarding) our overall compensation strategy and policies; |
| ● | reviewing and approving the
compensation, the performance goals and objectives relevant to the compensation, and other terms of employment of our executive officers; |
| ● | reviewing and approving (or
if it deems appropriate, making recommendations to the full board of directors regarding) the equity incentive plans, compensation plans
and similar programs advisable for us, as well as modifying, amending or terminating existing plans and programs; |
| ● | reviewing and approving the
terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for
our executive officers; |
| ● | reviewing with management and
approving our disclosures under the caption “Compensation Discussion and Analysis” in our periodic reports or proxy statements
to be filed with the SEC; and |
| ● | preparing the report that the
SEC requires in our annual proxy statement. |
Nominating and Corporate Governance Committee
Our nominating and corporate
governance committee (“Nominating Committee”) consists of Timothy Ramdeen, who is the chair of the committee, James Sapirstein
and Simon Tarsh. Our Board has determined that each of the members of this committee satisfies the Nasdaq Marketplace Rules independence
requirements. The functions of this committee include, among other things:
| ● | identifying, reviewing and
evaluating candidates to serve on our board of directors consistent with criteria approved by our board of directors; |
| ● | evaluating director performance
on the board and applicable committees of the board and determining whether continued service on our board is appropriate; |
| ● | evaluating, nominating and
recommending individuals for membership on our board of directors; and |
| ● | evaluating nominations by stockholders
of candidates for election to our board of directors. |
Board Leadership Structure
Our board of directors is
free to select the Chairman of the board of directors and the Chief Executive Officer in a manner that it considers to be in the best
interests of our company at the time of selection. Currently, Dr. Ralph Schiess serves as our Interim Chief Executive Officer. All five
members of our board of directors have been deemed to be “independent” by the board of directors.
Our board of directors, as
a whole and also at the committee level, plays an active role overseeing the overall management of our risks. Our Audit Committee reviews
risks related to financial and operational items with our management and our independent registered public accounting firm. Our board
of directors is in regular contact with our Chief Executive Officer, who reports directly to the board of directors and who supervise
day-to-day risk management.
Role of Board in Risk Oversight Process
We face a number of risks, including those described under the caption
“Risk Factors” contained elsewhere in this proxy statement. Our board of directors believes that risk management is an important
part of establishing, updating and executing on our business strategy. Our board of directors has oversight responsibility relating to
risks that could affect the corporate strategy, business objectives, compliance, operations, and the financial condition and performance
of our company. Our board of directors focuses its oversight on the most significant risks facing us and on our processes to identify,
prioritize, assess, manage and mitigate those risks. Our board of directors receives regular reports from members of our senior management
on areas of material risk to us, including strategic, operational, financial, legal and regulatory risks. While our board of directors
has an oversight role, management is principally tasked with direct responsibility for management and assessment of risks and the implementation
of processes and controls to mitigate their effects on us.
Our board is generally responsible
for the oversight of corporate risk in its review and deliberations relating to our activities. Our principal source of risk falls into
two categories, financial and product commercialization. Our Audit Committee oversees management of financial risks; our board regularly
reviews information regarding our cash position, liquidity and operations, as well as the risks associated with each. The board regularly
reviews plans, results and potential risks related to our product offerings, growth, and strategies. Our Compensation Committee oversees
risk management as it relates to our compensation plans, policies and practices for all employees including executives and directors,
particularly whether our compensation programs may create incentives for our employees to take excessive or inappropriate risks which
could have a material adverse effect on our company.
Scientific Advisory Board
In January 2020, we formally established a Scientific
Advisory Board to advise our management regarding our clinical and regulatory development programs and other customary matters. Our scientific
advisors are experts in various areas of medicine including theoretical epidemiology, vaccine research and development, and biotechnology.
Code of Business Conduct and Ethics
We have adopted a written
code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer,
principal financial officer, principal accounting officer or controller, or persons performing similar functions. The code of business
conduct and ethics is posted on our website at www.onconetix.com. We expect that any amendments or waivers to the code that are
required by law or Nasdaq Marketplace Rules will be disclosed on our website.
Insider Trading Policy
On December 1, 2023, we adopted
insider trading policies and procedures governing the purchase, sale, and/or other dispositions of our securities by directors, officers
and employees, which are reasonably designed to promote compliance with insider trading laws, rules and regulations, and applicable Nasdaq
listing standards.
DESCRIPTION OF SECURITIES OF THE COMBINED COMPANY
Pursuant to our Amended and Restated Certificate
of Incorporation, our authorized capital stock consists of 250,000,000 shares of common stock, and 10,000,000 shares of preferred stock,
$0.00001 par value per share.
Common Stock
Our common stock is listed on the Nasdaq Capital
Market under the symbol “ONCO.”
Under the terms of our Amended and Restated Certificate
of Incorporation, holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders,
including the election of directors, and do not have cumulative voting rights. The holders of outstanding shares of common stock are entitled
to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as our board
of directors from time to time may determine. Our common stock is not entitled to pre-emptive rights and is not subject to conversion
or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to stockholders
are distributable ratably among the holders of our common stock after payment of liquidation preferences, if any, on any outstanding payment
of other claims of creditors. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected
by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Preferred Stock
Under the terms of our Amended and Restated Certificate
of Incorporation, our board of directors is authorized, without further action by the stockholders, to establish one or more class
or series, and fix the relative rights and preferences of the company’s undesignated capital stock.
A summary of the terms of the Series A Preferred
Stock and the Series B Preferred Stock is set forth in the sections entitled “Proposal 2: Series A Conversion Proposal”
and “Proposal 3: PMX Issuance Proposal,” respectively.
Anti-Takeover Effects of Delaware Law and Our
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws
Some provisions of Delaware law, our Amended and
Restated Certificate of Incorporation and our Amended and Restated Bylaws could prohibit or delay mergers or other takeover or change
in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders
the opportunity to sell their stock at a price above the prevailing market price.
These provisions, summarized below, are intended
to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking
to acquire control of us to first negotiate with our board of directors. We believe that the benefits of the increased protection of our
potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages
of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the General Corporation
Law of the State of Delaware, which prohibits persons deemed to be “interested stockholders” from engaging in a “business
combination” with a publicly held Delaware corporation for three years following the date these persons become interested stockholders
unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed
manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates
and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s
voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in
a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions
not approved in advance by the board of directors.
Choice of Forum
Our Amended and Restated Certificate of Incorporation
provides that, unless we consent in writing to an alternative forum, to the fullest extent permitted by law, that derivative actions brought
in our name, actions against directors, officers and employees for breach of fiduciary duty and certain other actions may be brought only
in the Court of Chancery in the State of Delaware, except any action (A) as to which the Court of Chancery in the State of Delaware determines
that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent
to the personal jurisdiction of the Court of Chancery within ten days following such determination), (B) which is vested in the exclusive
jurisdiction of a court or forum other than the Court of Chancery or (C) for which the Court of Chancery does not have subject matter
jurisdiction. If an action is brought outside of Delaware, the stockholder bringing the suit will be deemed to have consented to service
of process on such stockholder’s counsel. Although we believe this provision benefits us by providing increased consistency in the
application of law in the types of lawsuits to which it applies, a court may determine that this provision is unenforceable, and to the
extent it is enforceable, the provision may have the effect of discouraging lawsuits against our directors and officers.
Our Amended and Restated Certificate of Incorporation
provides that the exclusive forum provision will be applicable to the fullest extent permitted by applicable law, subject to certain exceptions.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all suits brought to enforce any duty or liability created
by the Exchange Act or the rules and regulations thereunder. As a result, the exclusive forum provision will not apply to suits brought
to enforce any duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction.
In addition, our Amended and Restated Certificate of Incorporation provides that, unless we consent in writing to the selection of an
alternative forum, the federal district courts of the United States of America shall, to the fullest extent permitted by law, be the exclusive
forum for the resolution of any complaint asserting a cause of action arising under the Securities Act or the rules and regulations promulgated
thereunder. We note, however, that there is uncertainty as to whether a court would enforce this provision and that investors cannot waive
compliance with the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act creates concurrent
jurisdiction for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the
rules and regulations thereunder.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is Continental Stock Transfer & Trust Company. The Transfer Agent’s address is 1 State Street, 30th Floor,
New York, New York 10004.
Listing
Our common stock is traded
on The Nasdaq Capital Market under the trading symbol “ONCO.”
Beneficial
Ownership of Securities
The following table sets
forth certain information concerning the ownership of our common stock, with respect to: (i) each person, or group of affiliated persons,
known to us to be the beneficial owner of more than five percent of our common stock; (ii) each of our directors; (iii) each of our named
executive officers; and (iv) all of our current directors and executive officers as a group.
Applicable percentage ownership
is based on 22,061,746 shares of common stock outstanding as of February 5, 2024.We have determined beneficial ownership in accordance
with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting
or investment power with respect to such securities. In addition, pursuant to such rules, we deemed outstanding shares of common stock
subject to options, restricted stock, or warrants held by that person that are currently exercisable or exercisable within 60 days of
February 5, 2024. We did not deem such shares outstanding, however, for the purpose of computing the percentage ownership of any other
person. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the beneficial owners named
in the table below have sole voting and investment power with respect to all shares of our common stock that they beneficially own, subject
to applicable community property laws.
| | |
Shares of Common Stock Owned | |
| Name and Address of Beneficial Owner(1) | |
Number of Shares | | |
Percentage | |
| Executive Officers and Directors | |
| | |
| |
| Dr. Ralph Schiess | |
| 269,749 | (2) | |
| 1.2 | % |
| Bruce Harmon | |
| 14,787 | (3) | |
| * | |
| Christian Brühlmann | |
| 236,029 | (4) | |
| 1.1 | % |
| Simon Tarsh | |
| 4,073 | (5) | |
| * | |
| Timothy Ramdeen | |
| 2,386 | (6) | |
| * | |
| James Sapirstein | |
| 28,555 | (7) | |
| * | |
| Thomas Meier | |
| - | | |
| - | |
| Ajit Singh | |
| - | | |
| - | |
| | |
| | | |
| | |
| All directors and named executive officers as a group (7 persons) | |
| 555,579 | | |
| 2.5 | % |
| 5% Stockholders | |
| | | |
| | |
| Joseph Hernandez | |
| 2,650,351 | (8) | |
| 12.0 | % |
| Altos Venture AG | |
| 1,103,403 | (9) | |
| 5.0 | % |
| American Financial Group, Inc. | |
| 1,440,927 | (10) | |
| 6.5 | % |
| * |
Represents beneficial ownership of less than 1%. |
| (1) |
Unless otherwise noted, the business address of each of the following entities or individuals is c/o Onconetix, Inc., 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202. |
| (2) |
Consists of 269,749 shares of common stock. |
| (3) |
Consists of 14,787 shares of common stock underlying options that are currently exercisable within 60 days of February 5, 2024. |
| (4) |
Consists of 236,029 shares of common stock. |
| (5) |
Consists of 4,073 shares of common stock underlying options that are currently exercisable within 60 days of February 5, 2024. |
| (6) |
Consists of 2,386 shares of common stock underlying options that are currently exercisable within
60 days of February 5, 2024. |
| (7) |
Consists of 28,555 shares of common stock underlying options that are currently exercisable within
60 days of February 5, 2024. |
| (8) |
Based on a Schedule 13G filed with the SEC on February 14, 2023. The principal business address for Mr. Hernandez was c/o Onconetix, Inc., 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202. |
| (9) |
Based on a Schedule 13D filed with the SEC on December 28, 2023. The principal business address for Altos Venture AG is Obertorweg 64, CH-4123 Allschwil/Switzerland. |
| (10) |
Based on a Schedule 13G/A filed with the SEC on January 26, 2024. The principal business address for AFG is 301 East Fourth Street, Cincinnati, Ohio 45202. |
STOCKHOLDER PROPOSALS
Any stockholder proposals intended to be presented
at the Onconetix 2024 annual meeting and considered for inclusion in Onconetix’s proxy materials must be received by Onconetix
on or before [●], 2024. Such proposals must be submitted in writing to: Onconetix, Inc., Attention: Bruce Harmon, Chief Financial
Officer, 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202. Such proposals must also meet the other requirements and procedures
prescribed by Rule 14a-8 under the Exchange Act relating to stockholder proposals.
HOUSEHOLDING OF PROXY MATERIALS
SEC rules permit companies and intermediaries
such as brokers to satisfy delivery requirements for proxy statements and notices with respect to two or more stockholders sharing the
same address by delivering a single proxy statement or a single notice addressed to those stockholders. This process, which is commonly
referred to as “householding,” provides cost savings for companies.
Onconetix has previously adopted householding
for stockholders of record. As a result, stockholders with the same address and last name may receive only one copy of this proxy statement
from Onconetix. Registered Onconetix stockholders (those who hold shares directly in their name with Onconetix’s transfer agent)
may opt out of householding and receive a separate proxy statement or other proxy materials by sending a written request to Onconetix,
at the address below.
Some brokers also household proxy materials, delivering
a single proxy statement or notice to multiple stockholders sharing an address unless contrary instructions have been received from the
affected stockholders. Once you have received notice from your broker that they will be householding materials to your address, householding
will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in
householding and would prefer to receive a separate proxy statement or notice, or if your household is receiving multiple copies of these
documents and you wish to request that future deliveries be limited to a single copy, please notify your broker.
Requests for additional copies of this proxy statement
should be directed to: Onconetix, Inc., 201 E. Fifth Street, Suite 1900, Cincinnati, Ohio 45202, Attention: Bruce Harmon, Chief Financial
Officer.
WHERE YOU CAN FIND MORE INFORMATION
We are “incorporating by reference”
in this proxy statement certain documents we file with the SEC, which means that we can disclose important information to you by referring
you to those documents. The information in the documents incorporated by reference is considered to be part of this proxy statement. We
have filed the following documents with the SEC and they are incorporated herein by reference as of their respective dates of filing.
| |
(i) |
our Annual Report on Form 10-K for the fiscal year ended December 31, 2022 as filed with the SEC on March 9, 2023; |
| |
|
|
| |
(ii) |
our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023 as filed with the SEC on May 12, 2023; the quarter ended June 30, 2023 as filed with the SEC on October 20, 2023; and the quarter ended September 30, 2023 as filed with the SEC on November 17, 2023; |
| |
|
|
| |
(iii) |
our Current Reports on Form 8-K dated January 17, 2023, March 29, 2023, April 20, 2023, April 24, 2023, June 6, 2023, June 14, 2023, July 6, 2023 (three Current Reports), July 11, 2023, July 21, 2023, July 25, 2023, July 31, 2023 (two Current Reports), August 1, 2023, August 3, 2023, August 10, 2023, August 22, 2023, August 28, 2023, September 8, 2023, September 22, 2023, October 3, 2023, October 10, 2023, October 18, 2023, November 2, 2023, December 18, 2023, December 21, 2023, December 27, 2023, December 28, 2023, January 12, 2024, January 19, 2024, January 29, 2024, February 12, 2024 and February 13, 2024. |
| |
|
|
| |
(iv) |
the description of our securities registered under Section 12 of the Exchange Act as filed as Exhibit 4.2 on Annual Report on Form 10-K for the year ended December 31, 2021 as filed with the SEC on March 31, 2022. |
Any statement contained in a document incorporated or deemed to be
incorporated by reference in this proxy statement shall be deemed modified, superseded or replaced for purposes of this proxy statement
to the extent that a statement contained in this proxy statement, or in any subsequently filed document that also is deemed to be incorporated
by reference in this proxy statement, modifies, supersedes or replaces such statement. Any statement so modified, superseded or replaced
shall not be deemed, except as so modified, superseded or replaced, to constitute a part of this proxy statement. None of the information
that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K or any corresponding information, either furnished under Item
9.01 or included as an exhibit therein, that we may from time to time furnish to the SEC will be incorporated by reference into, or otherwise
included in, this proxy statement, except as otherwise expressly set forth in the relevant document. Subject to the foregoing, all information
appearing in this proxy statement is qualified in its entirety by the information appearing in the documents incorporated by reference.
You may request, orally or
in writing, a copy of these documents, which will be provided to you at no cost (other than exhibits, unless such exhibits are specifically
incorporated by reference), by contacting Bruce Harmon, c/o Onconetix, Inc., at 201 E. Fifth Street, Suite 1900, Cincinnati, OH 45202.
Our telephone number is (513) 620-4101. Information about us is also available at our website at http://www.onconetix.com. However, the
information on our website is not a part of this proxy statement and is not incorporated by reference.
Annex A
Reverse Stock Split Amendment
CERTIFICATE OF AMENDMENT
OF CERTIFICATE OF INCORPORATION
OF ONCONETIX, INC.
Onconetix, Inc., a corporation organized and existing
under the laws of the State of Delaware (the “Corporation”), does hereby certify as follows:
1. The name of the Corporation is Onconetix, Inc.
2. The Certificate of Incorporation of the Corporation
is amended by adding the following new paragraph to the end of Article IV, Section D:
6. Upon the filing and effectiveness
(the “Effective Time”) of this amendment to the Corporation’s Certificate of Incorporation, as amended,
pursuant to the Delaware General Corporation Law, each [*1] ([*]) shares of the Common Stock issued immediately prior to the
Effective Time (the “Old Common Stock”) shall be reclassified and combined into one validly issued, fully paid
and non-assessable share of the Corporation’s Common Stock, $0.001 par value per share (the “New Common Stock”),
without any action by the holder thereof (the “Reverse Stock Split”). No fractional shares of New Common Stock
shall be issued as a result of the Reverse Stock Split and, in lieu thereof, upon surrender after the Effective Time of a book entry position
which formerly represented shares of Old Common Stock that were issued and outstanding immediately prior to the Effective Time, any person
who would otherwise be entitled to a fractional share of New Common Stock as a result of the Reverse Stock Split, following the Effective
Time, shall be entitled to receive a cash payment equal to the fraction of a share of New Common Stock to which such holder would otherwise
be entitled multiplied by the closing price per share of the New Common Stock on The Nasdaq Stock Market LLC at the close of business
on the date prior to the Effective Time. Each book entry position that theretofore represented shares of Old Common Stock shall thereafter
represent that number of shares of New Common Stock into which the shares of Old Common Stock represented by such book entry position
shall have been reclassified and combined; provided, that each person holding of record a book entry position that represented shares
of Old Common Stock shall receive, a new book entry position evidencing and representing the number of shares of New Common Stock to which
such person is entitled under the foregoing reclassification and combination.
3. This Certificate of Amendment has been duly
adopted by the Board of Directors and stockholders of the Corporation in accordance with Section 242 of the General Corporation Law of
the State of Delaware.
4. This Certificate of Amendment shall become
effective as of 9:00 a.m., Eastern Time on [__], 202[__].
IN WITNESS WHEREOF, the Corporation has caused
this Certificate of Amendment to be duly executed in its corporate name as of the [__]th day of [__], 202[__].
| |
By |
|
| |
|
Ralph
Schiess |
| |
|
Interim Chief Executive Officer |
| 1 | Range equals thirty [30] to sixty [60] |
Annex B
Share Exchange Agreement
EXECUTION
VERSION
PRIVATE
& CONFIDENTIAL
SHARE
EXCHANGE AGREEMENT
by
and among
BLUE
WATER BIOTECH, INC.,
PROTEOMEDIX
AG,
THOMAS
MEIER as the sellers’ representative,
and
THE
SHAREHOLDERS OF PROTEOMEDIX AG NAMED HEREIN,
Dated
as of December 15, 2023
TABLE
OF CONTENTS
| |
Page |
| ARTICLE I.
SHARE EXCHANGE |
2 |
| 1.1. |
Exchange of the Company Shares. |
2 |
| 1.2. |
Exchange Consideration |
2 |
| 1.3. |
Surrender of Company Securities and Disbursement of
Exchange Consideration |
2 |
| 1.4. |
Treatment of Company Convertible Securities |
3 |
| 1.5. |
Seller Consent |
3 |
| 1.6. |
Termination of Certain Agreements |
3 |
| |
|
|
| ARTICLE II.
CLOSING |
3 |
| 2.1. |
Closing |
3 |
| 2.2. |
Conditions to Obligations of Buyer |
4 |
| 2.3. |
Conditions to Obligations of the Company |
5 |
| |
|
|
| ARTICLE III.
representations and warranties of BUYER |
5 |
| 3.1. |
Organization and Standing |
5 |
| 3.2. |
Authorization; Binding Agreement |
6 |
| 3.3. |
Governmental Approvals |
6 |
| 3.4. |
Non-Contravention |
6 |
| 3.5. |
Capitalization |
7 |
| 3.6. |
Subsidiaries |
7 |
| 3.7. |
SEC Filings and Buyer Financials |
8 |
| 3.8. |
Absence of Certain Changes |
9 |
| 3.9. |
Compliance with Laws |
9 |
| 3.10. |
Permits |
9 |
| 3.11. |
Litigation |
9 |
| 3.12. |
Material Contracts |
10 |
| 3.13. |
Intellectual Property |
11 |
| 3.14. |
Taxes and Returns |
13 |
| 3.15. |
Real Property |
15 |
| 3.16. |
Personal Property |
15 |
| 3.17. |
Title to and Sufficiency of Assets |
16 |
| 3.18. |
Employee Matters |
16 |
| 3.19. |
Benefit Plans |
17 |
| 3.20. |
Environmental Matters. |
18 |
| 3.21. |
Transactions with Related Persons |
19 |
| 3.22. |
Investment Company Act |
19 |
| 3.23. |
Finders and Brokers |
19 |
| 3.24. |
Certain Business Practices |
20 |
| 3.25. |
Business Insurance |
20 |
| 3.26. |
Top Suppliers |
20 |
| 3.27. |
Independent Investigation |
21 |
| |
|
|
| Article IV.
representations and warranties of THE COMPANY |
21 |
| 4.1. |
Organization and Standing |
21 |
| 4.2. |
Authorization; Binding Agreement |
22 |
| 4.3. |
Capitalization |
22 |
| 4.4. |
Subsidiaries |
23 |
| 4.5. |
Governmental Approvals |
23 |
| 4.6. |
Non-Contravention |
24 |
| 4.7. |
Financial Statements |
24 |
| 4.8. |
Absence of Certain Changes |
25 |
| 4.9. |
Compliance with Laws |
25 |
| 4.10. |
Permits |
25 |
| 4.11. |
Litigation |
26 |
| 4.12. |
Material Contracts |
26 |
| 4.13. |
Intellectual Property |
28 |
| 4.14. |
Taxes and Returns |
29 |
| 4.15. |
Real Property |
32 |
| 4.16. |
Personal Property |
32 |
| 4.17. |
Title to and Sufficiency of Assets |
32 |
| 4.18. |
Employee Matters |
33 |
| 4.19. |
Benefit Plans |
34 |
| 4.20. |
Environmental Matters |
35 |
| 4.21. |
Transactions with Related Persons |
35 |
| 4.22. |
Business Insurance |
36 |
| 4.23. |
Top Customers and Suppliers |
36 |
| 4.24 |
Certain Business Practices. |
37 |
| 4.25 |
Investment Company Act. |
37 |
| 4.26. |
Finders and Brokers. |
37 |
| 4.27. |
No Subsidiaries. |
37 |
| 4.28. |
Information Supplied. |
38 |
| 4.29. |
Independent Investigation |
38 |
| |
|
|
| ARTICLE v.
representations and warranties of THE SELLERS |
38 |
| 5.1. |
Organization and Standing |
38 |
| 5.2. |
Authorization; Binding Agreement |
38 |
| 5.3. |
Ownership |
39 |
| 5.4. |
Governmental Approvals |
39 |
| 5.5. |
Non-Contravention |
39 |
| 5.6. |
No Litigation |
39 |
| 5.7. |
Investment Representations |
40 |
| 5.8. |
Finders and Brokers |
40 |
| 5.9. |
Information Supplied |
40 |
| 5.10. |
Independent Investigation |
41 |
| |
|
|
| ARTICLE VI.
OTHER AGREEMENTS OF the PARTIES |
41 |
| 6.1. |
Access and Information |
41 |
| 6.2. |
Litigation Support |
41 |
| 6.3. |
No Trading |
42 |
| 6.4. |
Efforts |
42 |
| 6.5. |
Further Assurances |
42 |
| 6.6. |
Conduct of Business Prior to Conversion |
42 |
| 6.7. |
Buyer Public Filings |
45 |
| 6.8. |
The Registration Statement |
45 |
| 6.9. |
Nasdaq Change of Control Application |
47 |
| 6.10. |
Public Announcements |
47 |
| 6.11. |
Confidential Information |
48 |
| 6.12. |
Post-Closing Board of Directors and Executive Officers |
48 |
| 6.13. |
Indemnification of Directors and Officers; Tail Insurance |
49 |
| 6.14. |
Transfer Taxes |
49 |
| 6.15. |
Tax Matters |
49 |
| 6.16. |
Section 16 Matters |
49 |
| 6.17. |
Delivery of Audited Company Financial
Statements |
50 |
| 6.18. |
Exchange of Company Stock Options |
50 |
| 6.19. |
CFIUS |
51 |
| |
|
|
| ARTICLE VII.
Survival |
52 |
| 7.1 |
Survival |
52 |
| 7.2. |
Conversion Adjustment |
52 |
| 7.3. |
Sellers’ Indemnification |
53 |
| 7.4. |
Exclusive Remedies |
55 |
| 7.5. |
Share Escrow |
55 |
| |
|
|
| ARTICLE VIII.
WAIVERs And releases |
56 |
| 8.1. |
Release and Covenant Not to Sue |
56 |
| |
|
|
| ARTICLE Ix.
MISCELLANEOUS |
56 |
| 9.1 |
Notices |
56 |
| 9.2. |
Binding Effect; Assignment |
57 |
| 9.3. |
Third Parties |
57 |
| 9.4. |
Governing Law; Jurisdiction |
58 |
| 9.5. |
WAIVER OF JURY TRIAL |
58 |
| 9.6. |
Specific Performance |
58 |
| 9.7. |
Severability |
58 |
| 9.8. |
Amendment |
59 |
| 9.9. |
Waiver |
59 |
| 9.10. |
Entire Agreement |
59 |
| 9.11. |
Interpretation |
60 |
| 9.12. |
Counterparts |
60 |
| 9.13. |
Sellers’ Representative |
60 |
| |
|
|
| ARTICLE X.
DEFINITIONS |
61 |
| 10.1. |
Certain Definitions |
61 |
| 10.2. |
Section References |
70 |
INDEX
OF EXHIBITS
| Exhibit |
Description |
| |
|
| Exhibit A |
Form of Lock-Up Agreement |
| Exhibit B |
Form of Non-Competition and Non-Solicitation Agreement |
| Exhibit C |
Form of Support Agreement |
| Exhibit D |
Form of Series B Certificate of Designation |
| |
|
| Annex I |
Sellers |
SHARE
EXCHANGE AGREEMENT
This
SHARE EXCHANGE AGREEMENT (this “Agreement”) is made and entered into as of December 15, 2023, by and among
(i) Proteomedix AG, a Swiss Company (the “Company”), (ii) Blue Water Biotech, Inc., a Delaware
corporation (“Buyer”), (iii) each of the holders of outstanding capital stock or Company Convertible Securities
(other than Company Stock Options) named on Annex I hereto (collectively, the “Sellers”) and (iv) Thomas
Meier, in the capacity as the representative of Sellers in accordance with the terms and conditions of this Agreement (the “Sellers’
Representative” and together with the Sellers and the Company, the “Seller Parties”). The Company,
Buyer, Sellers’ Representative and the Sellers are sometimes referred to herein individually as a “Party”
and, collectively, as the “Parties”. Capitalized terms used and not otherwise defined herein shall have the
respective meanings ascribed thereto in Article X hereof.
RECITALS:
WHEREAS,
Sellers collectively own 100% of the issued and outstanding equity interests of the Company;
WHEREAS,
subject to and upon the terms and conditions set forth in this Agreement, Buyer desires to purchase from Sellers, and Sellers wish to
sell to Buyer, all of the issued and outstanding Company Shares in exchange for newly issued shares of common stock and newly issued
shares of preferred stock of Buyer, subject to the terms and conditions set forth herein (the “Share Exchange”
and the other transactions contemplated by this Agreement, the “Transactions”);
WHEREAS,
the Parties desire that following the Transactions (i) the Sellers will own a majority of the issued and outstanding shares of Buyer
Common Stock (as defined below) as measured based on the number of shares of Buyer Common Stock outstanding immediately prior to the
Closing (as defined below) and (ii) Buyer will own 100% of the issued and outstanding equity interests of the Company;
WHEREAS,
simultaneously with the execution and delivery of this Agreement, (a) the Sellers are entering into lock-up agreements with Buyer
and the Company, in the form attached hereto as Exhibit A (the “Lock-Up Agreements”), which Lock-Up
Agreements shall become effective as of the Closing and provide that Sellers shall not transfer the Common Shares (as defined below)
and Preferred Shares (as defined below) from the Closing until (6) months following the date of the Stockholder Approval and (b) each
of Dr. Ralph Schiess and Christian Brühlmann (together, the “Management Shareholders”) are entering into
non-competition and non-solicitation agreements in favor of Buyer and the Company, in the form attached hereto as Exhibit B (the
“Non-Competition and Non-Solicitation Agreements”), which Non-Competition and Non-Solicitation Agreements shall
become effective as of the Closing;
WHEREAS,
the board of directors of Buyer (the “Buyer Board”) has (a) determined that it is fair, advisable and in
the best interests of Buyer and its stockholders to enter into this Agreement and consummate the Transactions, (b) approved the execution,
delivery and performance by Buyer of this Agreement and the consummation of the Transactions, all upon the terms and subject to the conditions
set forth herein and (c) determined to recommend to the Buyer’s stockholders a vote in favor of the Stockholder Approval Matters
at a special meeting of Buyer’s stockholders to be called and held for such purpose; and
WHEREAS,
the board of directors of the Company has (a) determined that it is fair, advisable and in the best interests of the Company and
its shareholders to enter into this Agreement and consummate the Transactions, and (b) approved the execution, delivery and performance
by the Company of this Agreement and the consummation of the Transactions, all upon the terms and subject to the conditions set forth
herein.
NOW,
THEREFORE, in consideration of the premises set forth above, and the representations, warranties, covenants and agreements contained
in this Agreement, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending
to be legally bound hereby, the Parties hereto agree as follows:
Article
I
SHARE EXCHANGE
1.1
Exchange of the Company Shares. At the Closing, upon the terms and subject to the conditions of this Agreement, the Sellers shall
sell, transfer, convey, assign and deliver to Buyer, and Buyer shall purchase, acquire and accept from the Sellers, all of the issued
and outstanding equity interests of the Company (collectively, the “Purchased Shares”), free and clear of all
Liens (other than those imposed by applicable securities Laws and those incurred by Buyer).
1.2
Exchange Consideration.
(a)
At the Closing, upon the terms and subject to the conditions of this Agreement, and in full payment for the Purchased Shares,
Buyer shall issue and register: (i) in the name of the creditors of the Company set forth on Annex I hereto (the “Company
Creditors”) such number of shares of Buyer Common Stock and Buyer Preferred Stock set forth opposite each Company Creditor’s
name (the “Creditor Shares”), and (ii) in the name of the Sellers such number of shares of Buyer Common Stock
(the “Common Shares”) and such number of shares of Buyer Preferred Stock (the “Preferred Shares”
and together with the Common Shares and the Creditor Shares, the “Exchange Shares”) set forth opposite each
Seller’s name on Annex I hereto. The aggregate value of the Exchange Shares at the Closing shall be equal to Seventy-Five
Million U.S. Dollars ($75,000,000) (the “Exchange Consideration”) less the value of the Company Shares for
which the Company Stock Options are exercisable immediately prior to the Closing and less the value of the Creditor Shares. Each Company
Share shall be exchanged for the right to receive a number of Exchange Shares determined in accordance with the terms of this Agreement.
Following the Share Exchange, each Seller shall cease to have any other rights in and to the Company.
(b)
The Preferred Shares shall have the rights, preferences and privileges as set forth in a Certificate of Designation, in the form
attached hereto as Exhibit D (the “Series B Certificate of Designation), to be filed with the Secretary of
State of the State of Delaware on or prior to the Closing, and shall automatically convert into shares of Buyer Common Stock as provided
in the Certificate of Designation.
1.3
Surrender of the Company Securities and Disbursement of Exchange Consideration.
(a)
At the Closing, Buyer shall cause the Exchange Shares to be issued to the Sellers in exchange for their Company Shares in accordance
with each Seller’s portion of the Exchange Consideration.
(b)
At the Closing, each Seller will transfer to Buyer their Company Shares, by endorsement in blank of any certificates representing
the Company Shares (“Company Certificates”) or, if there are no Company Certificates, (ii) assignment declarations
reasonable acceptable to Buyer.
(c)
Notwithstanding anything to the contrary contained herein, no fraction of a share of Buyer Common Stock will be issued by Buyer
by virtue of this Agreement or the Transactions, and each Person who would otherwise be entitled to a fraction of a share of Buyer Common
Stock (after aggregating all fractional shares of Buyer Common Stock that would otherwise be received by such Person) shall instead have
the number of shares of Buyer Common Stock issued to such Person rounded down in the aggregate to the nearest whole share of Buyer Common
Stock.
1.4
Treatment of Company Convertible Securities.
(a)
Prior to the Share Exchange, the holders of Company Convertible Securities other than the Company Stock Options shall convert
all of their rights to receive Company Shares pursuant to such Company Convertible Securities for Company Shares at the applicable conversion
ratio as set forth in the Company Convertible Securities (the “Company Convertible Securities Conversion”).
Following the Company Convertible Securities Conversion, all Company Convertible Securities, other than the Company Stock Options shall
be waived, canceled or terminated, as applicable, shall no longer be outstanding and shall cease to exist, no payment or distribution
shall be made with respect thereto and each holder of Company Convertible Securities shall thereafter cease to have any rights with respect
to such securities.
(b)
At the Closing, each Company Stock Option that is outstanding under any of the equity incentive plans of the Company (collectively,
the “Company Equity Plans”) immediately before the Closing, whether vested or unvested, shall remain outstanding
until exchanged pursuant to Section 6.17(b).
1.5
Seller Consent. Each Seller, as a shareholder or other security
holder of the Company, hereby approves, authorizes and consents to the Company’s execution and delivery of this Agreement and the
Ancillary Documents to which it is or is required to be a party or otherwise bound, the performance by the Company of its obligations
hereunder and thereunder and the consummation by the Company of the transactions contemplated hereby and thereby. Each Seller acknowledges
and agrees that the consents set forth herein are intended and shall constitute such consent of the Sellers as may be required (and shall,
if applicable, operate as a written shareholder resolution of the Company) pursuant to the Company’s Organizational Documents,
any other agreement in respect of the Company to which any Seller is a party or bound and all applicable Laws.
1.6
Termination of Certain Agreements. Without limiting the provisions
of Section 8.1, the Company and the Sellers hereby agree that, effective at the Closing, (a) any shareholders’, voting or
similar agreement among the Company and any of the Sellers or among the Sellers with respect to the Company’s share capital, and
(b) any registration rights agreement between the Company and its shareholders, in each case of clauses (a) and (b), shall automatically,
and without any further action by any of the Parties, terminate in full and become null and void and of no further force and effect.
Further, each Seller and the Company hereby waive any obligations of the parties under the Company’s Organizational Documents or
any agreement described in clause (a) above with respect to the Transactions and the Ancillary Documents, and any failure of the Parties
to comply with the terms thereof in connection with the Transactions.
Article
II
CLOSING
2.1
Closing. The consummation of the Transactions (the “Closing”) shall take place at the offices of Ellenoff
Grossman & Schole LLP (“EGS”), 1345 Avenue of the Americas, New York, New York 10105, remotely via the
electronic exchange of signatures, promptly (but in no event later than two (2) Business Days) following the satisfaction or waiver of
the conditions to Closing set forth in this Agreement at 10:00 a.m. local time, or at such other date, time or place as Buyer and the
Company may agree in writing (the date and time at which the Closing is actually held being the “Closing Date”).
Closing signatures may be transmitted by e-mailed PDF files or by facsimile.
2.2
Conditions to Obligations of Buyer. The obligations of Buyer to consummate the transactions contemplated by this Agreement shall
be subject to the fulfillment or Buyer’s waiver, at or prior to the Closing, of each of the following conditions:
(a)
Secretary Certificates. The Company shall have delivered to Buyer a certificate from its secretary or other executive officer
certifying as to the validity and effectiveness of, and attaching, (A) copies of its Organizational Documents as in effect as of the
Closing Date (immediately prior to the Closing), (B) the resolutions of its board of directors authorizing and approving the execution,
delivery and performance of this Agreement and each Ancillary Document to which it is a party or bound, and the consummation of the Transactions,
and (C) the incumbency of its officers authorized to execute this Agreement or any Ancillary Document to which it is or is required to
be a party or otherwise bound.
(b)
Good Standing. The Company shall have delivered to Buyer an extract from the register of commerce of the Caton Zurich,
Switzerland, issued as of a date no earlier than thirty (30) days prior to the Closing Date.
(c)
Lock-Up Agreement. Each Seller shall have delivered to Buyer the Lock-Up Agreement, in the form attached as Exhibit
A hereto, duly executed by such Seller.
(d)
Non-Competition and Non-Solicitation Agreement. The Company shall have delivered to Buyer the Non-Competition and
Non-Solicitation Agreement, in the form attached as Exhibit B hereto, duly executed by the Management Shareholders.
(e)
Consents. The Company shall have delivered to Buyer the required Consents listed in Schedule 2.2(e).
(f)
Assignment Declaration. The Company shall have delivered to Buyer original copies signed in wet ink of the assignment
declarations regarding the Purchased Shares in a form satisfactory to Buyer.
(g)
Board Approval and Shareholder Registration. The Company shall have delivered to Buyer a resolution of the board of directors
approving the transfer of the Purchased Shares to Buyer and the registration of Buyer as shareholder with voting rights with regard to
the Purchased Shares in the share register of the Company.
(h)
Beneficial Ownership Register. The Company shall have delivered to Buyer a copy of the share and beneficial owner register
of the Company showing the Buyer as shareholder with voting rights with regard to the Purchased Shares in the share register of the Company
and the beneficial owner as notified by Buyer to the Company (if any) as the beneficial owner of the Purchased Shares.
2.3
Conditions to Obligations of the Company. The obligations of the Company to consummate the transactions contemplated by this Agreement
shall be subject to the fulfillment or waiver by the Company, at or prior to the Closing, of each of the following conditions:
(a)
Secretary Certificates. Buyer shall have delivered to the Company a certificate from its secretary or other executive officer
certifying as to, and attaching, (A) copies of Buyer’s Organizational Documents as in effect as of the Closing Date (immediately
prior to the Closing), (B) the resolutions of Buyer’s board of directors authorizing and approving the execution, delivery and
performance of this Agreement and each of the Ancillary Documents to which it is a party or by which it is bound, and the consummation
of the transactions contemplated hereby and thereby, and (C) the incumbency of officers authorized to execute this Agreement or any Ancillary
Document to which Buyer is or is required to be a party or otherwise bound.
(b)
Good Standing. Buyer shall have delivered to the Company a good standing certificate (or similar documents applicable for
such jurisdictions) for Buyer certified as of a date no earlier than thirty (30) days prior to the Closing Date from the proper Governmental
Authority of Buyer’s jurisdiction of organization and from each other jurisdiction in which Buyer is qualified to do business as
a foreign entity as of the Closing, in each case to the extent that good standing certificates or similar documents are generally available
in such jurisdictions.
(c)
Listing. Buyer shall have submitted an application for the listing on the Nasdaq of the shares of Buyer Common Stock to
be issued at Closing under this Agreement, shall have been notified that such application has been successfully submitted, and shall
have not been contacted by the Nasdaq with respect to any compliance issues with respect to such application.
(d)
Private Placement. Buyer shall have delivered to the Company Subscription Agreements, in form and substance reasonably
acceptable to the Company (the “Subscription Agreements”), executed by Buyer and the Private Placement Investors,
for a Private Placement Investment for an aggregate amount equal to or greater than five million dollars ($5,000,000).
(e)
Fairness Opinion. Buyer Board shall have received the written opinion (or an oral opinion to be confirmed in writing) from
a reputable independent investment banking firm or other independent financial advisory firm that regularly renders fairness opinions
with respect to the type of business that the Company conducts, to the effect that, as of the date of such opinion and based upon and
subject to the various qualifications and assumptions set forth therein, the consideration to be paid by Buyer pursuant to this Agreement
is fair from a financial point of view to the stockholders of Buyer.
Article
III
REPRESENTATIONS AND WARRANTIES OF BUYER
Except
as set forth in (i) the disclosure schedules delivered by Buyer to the Company and the Sellers on the date hereof (the “Buyer
Disclosure Schedules”), the Section numbers of which are numbered to correspond to the Section numbers of this Agreement
to which they refer, or (ii) the SEC Reports (as defined below) that are available on the SEC’s website through EDGAR, Buyer represents
and warrants to the Seller Parties as follows:
3.1
Organization and Standing. Buyer is a corporation duly incorporated, validly existing and in good standing under the Laws of the
State of Delaware. Buyer has all requisite corporate power and authority to own, lease and operate its properties and to carry on its
business as now being conducted. Buyer is duly qualified or licensed and in good standing to do business in each jurisdiction in which
the character of the property owned, leased or operated by it or the nature of the business conducted by it makes such qualification
or licensing necessary. Schedule 3.1 lists all jurisdictions in which any Buyer Company does Business. Buyer has heretofore made
available to the Company accurate and complete copies of its Organizational Documents, each as currently in effect. Buyer
is not in violation of any provision of its Organizational Documents.
3.2
Authorization; Binding Agreement. Buyer has all requisite corporate power and authority to execute and deliver this Agreement
and each Ancillary Document to which it is a party, to perform its obligations hereunder and thereunder and to consummate the transactions
contemplated hereby and thereby, subject to obtaining the Stockholder Approval (including the Conversion Approval). The execution and
delivery of this Agreement and each Ancillary Document to which it is a party and the consummation of the transactions contemplated hereby
and thereby (a) have been duly and validly authorized by the board of directors of Buyer and (b) other than the Stockholder Approval,
no other corporate proceedings, on the part of Buyer are necessary to authorize the execution and delivery of this Agreement and each
Ancillary Document to which it is a party or to consummate the transactions contemplated hereby and thereby. This Agreement has been,
and each Ancillary Document to which Buyer is a party shall be when delivered, duly and validly executed and delivered by Buyer and,
assuming the due authorization, execution and delivery of this Agreement and such Ancillary Documents by the other parties hereto and
thereto, constitutes, or when delivered shall constitute, the legal, valid and binding obligation of Buyer, enforceable against Buyer
in accordance with its terms, except to the extent that enforceability thereof may be limited by applicable bankruptcy, insolvency, reorganization
and moratorium laws and other laws of general application affecting the enforcement of creditors’ rights generally or by any applicable
statute of limitation or by any valid defense of set-off or counterclaim, and the fact that equitable remedies or relief (including the
remedy of specific performance) are subject to the discretion of the court from which such relief may be sought (collectively, the “Enforceability
Exceptions”).
3.3
Governmental Approvals. Except as otherwise described in Schedule 3.3, no Consent of or with any Governmental Authority
on the part of Buyer is required to be obtained or made in connection with the execution, delivery or performance by Buyer of this Agreement
and each Ancillary Document to which it is a party or the consummation by Buyer of the transactions contemplated hereby and thereby,
other than (a) pursuant to any Laws that are designed to prohibit, restrict or regulate actions having the purpose or effect of monopolization
or restraint of trade (“Antitrust Laws”), (b) such filings as are expressly contemplated by this Agreement,
(c) any filings required with Nasdaq or the SEC with respect to the Transactions, (d) applicable requirements, if any, of the Securities
Act, the Exchange Act, and/or any state “blue sky” securities Laws, and the rules and regulations thereunder, and (e) where
the failure to obtain or make such Consents or to make such filings or notifications would not reasonably be expected to have a Material
Adverse Effect on Buyer.
3.4
Non-Contravention. Except as otherwise described in Schedule 3.4, the execution and delivery by Buyer of this Agreement
and each Ancillary Document to which it is a party, the consummation by Buyer of the transactions contemplated hereby and thereby, and
the compliance by Buyer with any of the provisions hereof and thereof, shall not (a) conflict with or violate any provision of Buyer’s
Organizational Documents, (b) subject to obtaining the Consents from Governmental Authorities referred to in Section 3.3
hereof, and the waiting periods referred to therein having expired, and any condition precedent to such Consent or waiver having been
satisfied, conflict with or violate any Law, Order or Consent applicable to Buyer or any of its properties or assets, or (c) (i) violate,
conflict with or result in a breach of, (ii) constitute a default (or an event which, with notice or lapse of time or both, would constitute
a default) under, (iii) result in the termination, withdrawal, suspension, cancellation or modification of, (iv) accelerate the performance
required by Buyer under, (v) result in a right of termination or acceleration under, (vi) give rise to any obligation to make payments
or provide compensation under, (vii) result in the creation of any Lien upon any of the properties or assets of Buyer under, (viii) give
rise to any obligation to obtain any third party Consent or provide any notice to any Person under or (ix) give any Person the right
to declare a default, exercise any remedy, claim a rebate, chargeback, penalty or change in delivery schedule, accelerate the maturity
or performance, cancel, terminate or modify any right, benefit, obligation or other term under, any of the terms, conditions or provisions
of any Buyer Material Contract, except for any deviations from the foregoing clause (c) that would not reasonably be expected to have
a Material Adverse Effect on Buyer.
3.5
Capitalization.
(a)
Buyer is authorized to issue 250,000,000 shares of common stock, $0.00001 par value per share, and 10,000,000 shares are preferred
stock, par value $0.00001 per share. The issued and outstanding Buyer Securities as of the date of this Agreement are set forth on Schedule
3.5(a). All of the issued and outstanding shares of Buyer Common Stock are duly authorized, validly issued, fully paid and non-assessable
and are not subject to or issued in violation of any purchase option, right of first refusal, preemptive right, subscription right or
any similar right under any provision of applicable Law, Buyer’s Organizational Documents or any Contract to which Buyer is a party.
None of the outstanding Buyer Securities have been issued in violation of any applicable securities Laws.
(b)
Except as set forth in Schedule 3.5(b), there are no (i) outstanding options, warrants, puts, calls, convertible securities,
preemptive or similar rights, (ii) bonds, debentures, notes or other Indebtedness having general voting rights or that are convertible
or exchangeable into securities having such rights or (iii) subscriptions or other rights, agreements, arrangements, Contracts or commitments
of any character (other than this Agreement and the Ancillary Documents), (A) relating to the issued or unissued securities of Buyer
or (B) obligating Buyer to issue, transfer, deliver or sell or cause to be issued, transferred, delivered, sold or repurchased any options
or shares or securities convertible into or exchangeable for such securities, or (C) obligating Buyer to grant, extend or enter into
any such option, warrant, call, subscription or other right, agreement, arrangement or commitment for such capital shares. There are
no outstanding obligations of Buyer to repurchase, redeem or otherwise acquire any shares of Buyer or to provide funds to make any investment
(in the form of a loan, capital contribution or otherwise) in any Person. Except as set forth in Schedule 3.5(b), there are no
stockholders’ agreements, voting trusts or other agreements or understandings to which Buyer is a party with respect to the voting
of any shares of Buyer.
(c)
All Indebtedness of Buyer as of the date of this Agreement is disclosed on Schedule 3.5(c). Except as set forth on Schedule
3.5(c), no Indebtedness of Buyer contains any restriction upon: (i) the prepayment of any such Indebtedness, (ii) the incurrence
of Indebtedness by Buyer, (iii) the ability of Buyer to grant any Lien on its properties or assets, or (iv) the consummation of the Transactions.
3.6
Subsidiaries. Schedule 3.6 sets forth the name of each Subsidiary of Buyer, and with respect to each Subsidiary, (a) its
jurisdiction of organization, (b) its authorized shares or other equity interests (if applicable), and (c) the number of issued and outstanding
shares or other equity interests and the record holders and beneficial owners thereof. All of the outstanding equity securities of each
Subsidiary of Buyer are duly authorized and validly issued, fully paid and non-assessable (if applicable), and were offered, sold and
delivered in compliance with all applicable securities Laws, and owned by one or more of the Buyer Companies, free and clear of all Liens
(other than those, if any, imposed by such Subsidiary’s Organizational Documents). There are no Contracts to which Buyer or any
of its Affiliates is a party or bound with respect to the voting (including voting trusts or proxies) of the equity interests of any
Subsidiary of Buyer other than the Organizational Documents of any such Subsidiary. There are no outstanding or authorized options, warrants,
rights, agreements, subscriptions, convertible securities or commitments to which any Subsidiary of Buyer is a party or which are binding
upon any Subsidiary of Buyer providing for the issuance or redemption of any equity interests of any Subsidiary of Buyer. There are no
outstanding equity appreciation, phantom equity, profit participation or similar rights granted by any Subsidiary of Buyer. No Subsidiary
of Buyer has any limitation, whether by Contract, Order or applicable Law, on its ability to make any distributions or dividends to its
equity holders or repay any debt owed to another Buyer Company. Except for the equity interests of the Subsidiaries listed on Schedule
3.6, Buyer does not own or have any rights to acquire, directly
or indirectly, any equity interests of, or otherwise Control, any Person. Except as set forth on Schedule 3.6, no Buyer Company
is a participant in any joint venture, partnership or similar arrangement. There are no outstanding contractual obligations of a Buyer
Company to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in, any other Person.
3.7
SEC Filings and Buyer Financials.
(a)
Except as set forth on Schedule 3.7, Buyer, since January 1, 2021, has filed all forms, reports, schedules, statements,
registration statements, prospectuses and other documents required to be filed or furnished by Buyer with the SEC under the Securities
Act and/or the Exchange Act, together with any amendments, restatements or supplements thereto, and will file all such forms, reports,
schedules, statements and other documents required to be filed subsequent to the date of this Agreement. Except to the extent available
on the SEC’s web site through EDGAR, Buyer has delivered to the Company copies in the form filed with the SEC of all of the following:
(i) Buyer’s annual reports on Form 10-K for each fiscal year of Buyer beginning with the first year Buyer was required to file
such a form, (ii) Buyer’s quarterly reports on Form 10-Q for each fiscal quarter that Buyer filed such reports to disclose its
quarterly financial results in each of the fiscal years of Buyer referred to in clause (i) above, (iii) all other forms, reports, registration
statements, prospectuses and other documents (other than preliminary materials) filed by Buyer with the SEC since the beginning of the
first fiscal year referred to in clause (i) above (the forms, reports, registration statements, prospectuses and other documents referred
to in clauses (i), (ii) and (iii) above, whether or not available through EDGAR, are referred to herein collectively as the “SEC
Reports”), and (iv) all certifications and statements required by (A) Rules 13a-14 or 15d-14 under the Exchange Act, and
(B) 18 U.S.C. §1350 (Section 906 of SOX) with respect to any report referred to in clause (i) above (collectively, the “Public
Certifications”). The SEC Reports (x) were prepared in all material respects in accordance with the requirements of the
Securities Act and the Exchange Act, as the case may be, and the rules and regulations thereunder, and (y) did not, as of their respective
effective dates (in the case of SEC Reports that are registration statements filed pursuant to the requirements of the Securities Act)
and at the time they were filed with the SEC (in the case of all other SEC Reports) contain any untrue statement of a material fact or
omit to state a material fact required to be stated therein or necessary in order to make the statements made therein, in the light of
the circumstances under which they were made, not misleading. The Public Certifications are each true as of their respective dates of
filing. As used in this Section 3.7, the term “file” shall be broadly construed to include any manner permitted by
SEC rules and regulations in which a document or information is furnished, supplied or otherwise made available to the SEC. As of the
date of this Agreement, (A) the Buyer Common Stock is listed on Nasdaq, (B) there are no Actions pending or, to the Knowledge of Buyer,
threatened, against Buyer by the Financial Industry Regulatory Authority with respect to any intention by such entity to suspend, prohibit
or terminate the quoting of such Buyer Securities on Nasdaq and (C) such Buyer Securities are in compliance with all of the applicable
corporate governance rules of Nasdaq.
(b)
The financial statements and notes of Buyer contained or incorporated by reference in the SEC Reports (the “Buyer
Financials”), fairly present in all material respects the financial position and the results of operations, changes in
shareholders’ equity, and cash flows of Buyer at the respective dates of and for the periods referred to in such financial statements,
all in accordance with (i) GAAP methodologies applied on a consistent basis throughout the periods involved and (ii) Regulation S-X or
Regulation S-K, as applicable (except as may be indicated in the notes thereto and for the omission of notes and audit adjustments in
the case of unaudited quarterly financial statements to the extent permitted by Regulation S-X or Regulation S-K, as applicable).
(c)
Buyer has established and maintains disclosure controls and procedures and internal control over financial reporting (as such
terms are defined in paragraphs (e) and (f), respectively, of Rule 13a-15 and paragraph (e) of Rule 15d-15 under the Exchange Act) as
required by Rules 13a-15 and 15d-15 under the Exchange Act. Buyer’s disclosure controls and procedures are designed to ensure that
all information (both financial and non-financial) required to be disclosed by Buyer in the reports that it files or furnishes under
the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC,
and that all such information is accumulated and communicated to Buyer’s management as appropriate to allow timely decisions regarding
required disclosure and to make the certifications required pursuant to Sections 302 and 906 of SOX. Buyer’s management has completed
an assessment of the effectiveness of Buyer’s disclosure controls and procedures and, to the extent required by applicable Law,
presented in any applicable SEC Report that is a periodic report on Form 10-K or Form 10-Q, or any amendment thereto, its conclusions
about the effectiveness of the disclosure controls and procedures as of the end of the period covered by such report or amendment based
on such evaluation.
3.8
Absence of Certain Changes. Since January 1, 2023, except as set forth on Schedule 3.8, each Buyer Company has conducted
its business only in the ordinary course of business consistent with past practice and not been subject to a Material Adverse Effect.
3.9
Compliance with Laws. Except as set forth on Schedule 3.9, no Buyer Company is or has been in material conflict or material
non-compliance with, or in material default or violation of, nor has any Buyer Company received, since January 1, 2021, any written or,
to the Knowledge of Buyer, oral notice of any material conflict or non-compliance with, or material default or violation of, any applicable
Laws by which it or any of its properties, assets, employees, business or operations are or were bound or affected.
3.10
Permits. Each Buyer Company (and its employees who are legally required to be licensed by a Governmental Authority in order to
perform his or her duties with respect to his or her employment with any Buyer Company) holds all Permits necessary to lawfully conduct
its business as presently conducted and as currently contemplated to be conducted, and to own, lease and operate its assets and properties
(collectively, the “Buyer Permits”) except where the failure to have any of such Buyer Permits has not had,
and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. Buyer has made available to
the Company true, correct and complete copies of all Buyer Permits material to Buyer’s business, all of which material Buyer Permits
are listed on Schedule 3.10. All of the Buyer Permits are in full force and effect, and no suspension or cancellation of any of
the Buyer Permits is pending or, to Buyer’s Knowledge, threatened. No Buyer Company is in violation in any material respect of
the terms of any Buyer Permit, and no Buyer Company has received any written or, to the Knowledge of Buyer, oral notice of any Actions
relating to the revocation or modification of any Buyer Permit.
3.11
Litigation. Except as described on Schedule 3.11, there is no (a) Action of any nature currently pending or, to Buyer’s
Knowledge, threatened, nor is there any reasonable basis for any Action to be made (and no such Action has been brought or, to Buyer’s
Knowledge, threatened since January 1, 2021); or (b) Order now pending or outstanding or that was rendered by a Governmental Authority
since January 1, 2021, in either case of (a) or (b) by or against any Buyer Company, its current or former directors or officers (provided,
that any litigation involving the directors or officers of a Buyer Company must be related to Buyer Company’s business, equity
securities or assets), its business, equity securities or assets. The items listed on Schedule 3.11, if finally determined adverse
to the Buyer Companies, will not have, either individually or in the aggregate, a Material Adverse Effect upon any Buyer Company. Since
January 1, 2021, none of the current or former officers, senior management or directors of any Buyer Company have been charged with,
indicted for, arrested for, or convicted of any felony or any crime involving fraud.
3.12
Material Contracts.
(a)
Schedule 3.12(a) sets forth a true, correct and complete list of, and Buyer has made available to the Company (including
written summaries of oral Contracts, true correct and complete copies of, each Contract to which any Buyer Company is a party or by which
any Buyer Company, or any of its properties or assets are bound or affected (each Contract required to be set forth on Schedule 3.12(a),
a “Buyer Material Contract”) that:
(i)
contains covenants that limit the ability of any Buyer Company (A) to compete in any line of business or with any Person
or in any geographic area or to sell, or provide any service or product or solicit any Person, including any non-competition covenants,
employee and customer non-solicit covenants, exclusivity restrictions, rights of first refusal or most-favored pricing clauses or (B)
to purchase or acquire an interest in any other Person;
(ii)
involves any joint venture, profit-sharing, partnership, limited liability company or other similar agreement or arrangement relating
to the formation, creation, operation, management or control of any partnership or joint venture;
(iii)
involves any exchange-traded, over-the-counter or other swap, cap, floor, collar, futures contract, forward contract, option or other
derivative financial instrument or Contract, based on any commodity, security, instrument, asset, rate or index of any kind or nature
whatsoever, whether tangible or intangible, including currencies, interest rates, foreign currency and indices;
(iv)
evidences Indebtedness (whether incurred, assumed, guaranteed or secured by any asset) of any Buyer Company having an outstanding principal
amount in excess of $1,000,000;
(v)
involves the acquisition or disposition, directly or indirectly (by merger or otherwise), of assets with an aggregate value in
excess of $100,000 (other than in the ordinary course of business consistent with past practice) or shares or other equity interests
of any Buyer Company or another Person;
(vi)
relates to any merger, consolidation or other business combination with any other Person or the acquisition or disposition of any other
entity or its business or material assets or the sale of any Buyer Company, its business or material assets;
(vii)
by its terms, individually or with all related Contracts, calls for aggregate payments or receipts by the Buyer Companies under such
Contract or Contracts of at least $250,000 per year or $500,000 in the aggregate (other than each employment, management, service or
consulting agreement);
(viii)
is with any Buyer Top Vendor;
(ix)
obligates the Buyer Companies to provide continuing indemnification or a guarantee of obligations of a third party after the date hereof
in excess of $100,000;
(x)
is between any Buyer Company and any directors, officers or employees of a Buyer Company (other than at-will employment arrangements
and restrictive covenants agreements with employees entered into in the ordinary course of business consistent with past practice), including
all non-competition, severance and indemnification agreements, or any Related Person;
(xi)
obligates the Buyer Companies to make any capital commitment or expenditure in excess of $250,000 (including pursuant to any joint venture);
(xii)
relates to a material settlement under which any Buyer Company has outstanding obligations (other than customary confidentiality obligations);
or
(xiii)
provides another Person (other than another Buyer Company or any manager, director or officer of any Buyer Company) with a power
of attorney.
(b)
Except as disclosed in Schedule 3.12(b), with respect to each Buyer Material Contract: (i) such Buyer Material Contract
is valid and binding and enforceable in all respects against the Buyer Company party thereto and, to the Knowledge of Buyer, each other
party thereto, and is in full force and effect (except, in each case, as such enforcement may be limited by the Enforceability Exceptions);
(ii) the consummation of the Transactions will not affect the validity or enforceability of any Buyer Material Contract; (iii) no Buyer
Company is in breach or default in any material respect, and no event has occurred that with the passage of time or giving of notice
or both would constitute a material breach or default by any Buyer Company, or permit termination or acceleration by the other party
thereto, under such Buyer Material Contract; (iv) to the Knowledge of Buyer, no other party to such Buyer Material Contract is in
breach or default in any material respect, and no event has occurred that with the passage of time or giving of notice or both would
constitute such a material breach or default by such other party, or permit termination or acceleration by any Buyer Company, under such
Buyer Material Contract; (v) no Buyer Company has received written or, to the Knowledge of Buyer, oral notice of an intention by any
party to any such Buyer Material Contract to terminate such Buyer Material Contract or amend the terms thereof, other than modifications
in the ordinary course of business that do not adversely affect any Buyer Company in any material respect; and (vi) no Buyer Company
has waived any rights under any such Buyer Material Contract.
3.13
Intellectual Property.
(a)
Schedule 3.13(a)(i) sets forth: (i) all Patents and Patent applications, Trademarks and service mark registrations and
applications, Copyright registrations and applications and registered Internet Assets owned or licensed by a Buyer Company or otherwise
used or held for use by a Buyer Company in which a Buyer Company is the owner, applicant or assignee (“Buyer Registered IP”),
specifying as to each item, as applicable: (A) the nature of the item, including the title, (B) the owner of the item, (C) the jurisdictions
in which the item is issued or registered or in which an application for issuance or registration has been filed and (D) the issuance,
registration or application numbers and dates; and (ii) all material unregistered Intellectual Property owned or purported to be owned
by a Buyer Company. Schedule 3.13(a)(ii) sets forth all Intellectual Property licenses, sublicenses and other agreements or permissions
(“Buyer IP Licenses”) (other than “shrink wrap,” “click wrap,” and “off the shelf”
Software agreements and other agreements for Software commercially available on reasonable terms to the public generally (collectively,
“Off-the-Shelf Software”), which are not required to be listed, although such licenses are “Buyer IP
Licenses” as that term is used herein), under which a Buyer Company is a licensee or otherwise is authorized to use or practice
any Intellectual Property. Each Buyer Company owns, free and clear of all Liens (other than Permitted Liens), has valid and enforceable
rights in, and has the unrestricted right to use, sell, license, transfer or assign, all material Intellectual Property currently used
or held for use by such Buyer Company, and previously used by such Buyer Company, except for the Intellectual Property that is the subject
of Buyer IP Licenses. Except as set forth on Schedule 3.13(a)(iii),
all Buyer Registered IP is owned exclusively by the applicable Buyer Company without obligation to pay royalties, licensing fees or other
fees, or otherwise account to any third party with respect to such Buyer Registered IP.
(b)
Each Buyer Company has a valid and enforceable license to use all Intellectual Property that is the subject of the Buyer IP Licenses
applicable to such Buyer Company. Each Buyer Company has performed all material obligations imposed on it in Buyer IP Licenses, has made
all payments required to date, and such Buyer Company is not, nor, to the Knowledge of Buyer, is any other party thereto, in material
breach or material default thereunder, nor, to the Knowledge of Buyer, has any event occurred that with notice or lapse of time or both
would constitute a default thereunder. The continued use by the Buyer Companies of the Intellectual Property that is the subject of Buyer
IP Licenses in the same manner that it is currently being used is not restricted by any applicable license of any Buyer Company. All
registrations for Copyrights, Patents, Trademarks and Internet Assets that are owned by or exclusively licensed to any Buyer Company
are valid and in force, and all applications to register any Copyrights, Patents and Trademarks are pending and in good standing, all
without challenge of any kind.
(c)
No Action is pending or, to Buyer’s Knowledge, threatened against a Buyer Company that challenges the validity, enforceability,
ownership, or right to use, sell, license or sublicense any Intellectual Property currently owned, licensed, used or held for use by
the Buyer Companies. No Buyer Company has received any written notice or claim asserting or suggesting that any infringement, misappropriation,
violation, dilution or unauthorized use of the Intellectual Property of any other Person is or may be occurring or has or may have occurred
(including any demands or offers to license any Intellectual Property rights from a third party), as a consequence of the business activities
of any Buyer Company, nor to the Knowledge of Buyer is there a reasonable basis therefor. There are no Orders to which any Buyer Company
is a party or its otherwise bound that (i) restrict the rights of a Buyer Company to use, transfer, license or enforce any Intellectual
Property owned by a Buyer Company, (ii) restrict the conduct of the business of a Buyer Company in order to accommodate a third Person’s
Intellectual Property, or (iii) grant any third Person any right with respect to any Intellectual Property owned by a Buyer Company.
To Buyer’s Knowledge, no Buyer Company is currently infringing, or has, in the past, infringed, misappropriated or violated any
Intellectual Property of any other Person in any material respect in connection with the ownership, use or license of any Intellectual
Property owned or purported to be owned by a Buyer Company or, to the Knowledge of Buyer, otherwise in connection with the conduct of
the respective businesses of the Buyer Companies. To Buyer’s Knowledge, no third party is infringing upon, has misappropriated
or is otherwise violating any Intellectual Property owned, licensed by, licensed to, or otherwise used or held for use by any Buyer Company
(“Buyer IP”) in any material respect. No Buyer Company has received any opinion of counsel that any product
or service provided or distributed in the operation of the businesses thereof, or the conduct of such business, currently or in the past,
infringes any Intellectual Property right of another Person or any opinion of counsel otherwise regarding the right to practice any product
or service in connection with such businesses.
(d)
All employees and independent contractors of a Buyer Company have assigned to the Buyer Companies all Intellectual Property developed
by such employees and independent contractors in the performance of services for a Buyer Company by such Persons other than to the extent
ownership of such Intellectual Property would otherwise vest in the applicable the Buyer Company by operation of law. No current or former
officers, employees or independent contractors of a Buyer Company have claimed any ownership interest in any Intellectual Property owned
by a Buyer Company. To the Knowledge of Buyer, there has been no violation of a Buyer Company’s policies or practices related to
protection of Buyer IP or any confidentiality or nondisclosure Contract relating to the Intellectual Property owned by a Buyer Company.
To Buyer’s Knowledge, none of the employees of any Buyer Company is obligated under any Contract, or subject to any Order, that
would materially interfere with the use of such employee’s best efforts to promote the interests of the Buyer Companies, or that
would materially conflict with the business of any Buyer as presently conducted or contemplated to be conducted. Each Buyer Company has
taken reasonable security measures in order to protect the secrecy, confidentiality and value of the material Buyer IP to the extent
such Buyer IP derives value from the secrecy and/or confidentiality thereof.
(e)
To the Knowledge of Buyer, no Person has obtained unauthorized access to confidential third-party information and data in the
possession of a Buyer Company, nor has there been any other material compromise of the security, confidentiality or integrity of such
information or data. To Buyer’s Knowledge, each Buyer Company has complied with all applicable Laws relating to privacy, personal
data protection, and the collection, processing and use of personal information and its own privacy policies and guidelines. To Buyer’s
Knowledge, the operation of the business of the Buyer Companies has not and does not violate any right to privacy or publicity of any
third party, or constitute unfair competition or trade practices under applicable Law.
(f)
The consummation of any of the Transactions will not result in the material breach, material modification, cancellation,
termination, suspension of, or acceleration of any payments with respect to, or release of source code because of (i) any Contract providing
for the license or other use of Intellectual Property owned by a Buyer Company, or (ii) any Buyer IP License. Following the Closing,
Buyer shall be permitted to exercise, directly or indirectly through its Subsidiaries, all of the Buyer Companies’ rights under
such Contracts or Buyer IP Licenses to the same extent that the Buyer Companies would have been able to exercise had the Transactions
not occurred, without the payment of any additional amounts or consideration other than ongoing fees, royalties or payments which the
Buyer Companies would otherwise be required to pay in the absence of such Transactions.
3.14
Taxes and Returns. Except as set forth on Schedule 3.14:
(a)
Each Buyer Company has timely filed, or caused to be timely filed, all material Tax Returns required to be filed by it (taking
into account all available extensions). All such Tax Returns are true, accurate, correct and complete in all material respects. All Taxes
required to be paid, collected or withheld, other than such Taxes for which adequate reserves in Buyer Financials have been established,
have been timely paid, collected or withheld. Each Buyer Company has complied in all material respects with all applicable Laws relating
to Tax.
(b)
There is no current pending or, to the Knowledge of Buyer, threatened Action against a Buyer Company by a Governmental Authority
in a jurisdiction where a Buyer Company does not file Tax Returns that it is or may be subject to taxation by that jurisdiction.
(c)
No Buyer Company is being audited by any Tax authority or has been notified in writing or, to the Knowledge of Buyer, orally by
any Tax authority that any such audit is contemplated or pending. To Buyer’s Knowledge, there are no claims, assessments, audits,
examinations, investigations or other Actions pending against a Buyer Company in respect of any Tax, and no Buyer Company has been notified
in writing of any proposed Tax claims or assessments against it (other than, in each case, claims or assessments for which adequate reserves
in Buyer Financials have been established).
(d)
There are no Liens with respect to any Taxes upon any Buyer Company’s assets, other than Permitted Liens.
(e)
No Buyer Company has any outstanding waivers or extensions of any applicable statute of limitations to assess any material amount
of Taxes. There are no outstanding requests by a Buyer Company for any extension of time within which to file any Tax Return or within
which to pay any Taxes shown to be due on any Tax Return (other than an extension resulting from having received an automatic extension
of time to file the applicable Tax Return not requiring the approval of any Governmental Authority).
(f)
No Buyer Company has made any change in accounting method (except as required by a change in Law) or received a ruling from,
or signed an agreement with, any taxing authority that would reasonably be expected to have a material impact on its Taxes following
the Closing.
(g)
No Buyer Company has engaged in any (i) “reportable transaction” as defined in Treasury Regulations Section 1.6011-4(b),
(ii) “listed transaction,” or (iii) transaction, a “significant” purpose of which is the avoidance or evasion
of U.S. federal income Tax, within the meanings of Sections 6662, 6662A, 6011, 6012, 6111 or 6707A of the Code or the Treasury Regulations
promulgated thereunder.
(h)
Each Buyer Company has complied with, and is currently in compliance with, all transfer pricing rules and regulations (including,
to the extent applicable, Section 482 of the Code and any comparable or similar provision of applicable Law). To the extent legally required,
the Buyer Companies have properly and timely documented their transfer pricing methodology in compliance with Sections 482 and 6662 of
the Code and any comparable or similar provision of applicable Law. No Buyer Company is a party to any advance pricing agreement or any
similar Contract or agreement. No Buyer Company is subject to any gain recognition agreement under Section 367 of the Code.
(i)
No Buyer Company has been, in the past five (5) years, a party to a transaction reported or intended to qualify as a reorganization
under Section 368 of the Code.
(j)
No Buyer Company has any Liability for the Taxes of another Person (other than another Buyer Company) (i) under any applicable
Tax Law, (ii) as a transferee or successor, or (iii) by Contract, indemnity or otherwise (excluding commercial agreements entered into
in the ordinary course of business the primary purpose of which was not the sharing of Taxes). No Buyer Company is a party to or bound
by any Tax indemnity agreement, Tax sharing agreement or Tax allocation agreement or similar agreement, arrangement or practice (excluding
commercial agreements entered into in the ordinary course of business the primary purpose of which was not the sharing of Taxes) with
respect to Taxes (including advance pricing agreement, closing agreement or other agreement relating to Taxes with any Governmental Authority)
that will be binding on such Buyer Company with respect to any period following the Closing Date.
(k)
No Buyer Company has requested, or is the subject of or bound by any private letter ruling, technical advice memorandum, closing
agreement or similar ruling, memorandum or agreement with any Governmental Authority with respect to any Taxes, nor is any such request
outstanding.
(l)
No Buyer Company: (i) has constituted either a “distributing corporation” or a “controlled corporation”
(within the meaning of Section 355(a)(1)(A) of the Code) in a distribution of securities (to any Person or entity that is not a member
of the consolidated group of which Buyer is the common parent corporation) qualifying for, or intended to qualify for, Tax-free treatment
under Section 355 of the Code (A) within the two-year period ending on the date hereof or (B) in a distribution which could otherwise
constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e) of the
Code) in conjunction with the Transactions; or (ii) is or has ever been (A) a U.S. real property holding corporation within the meaning
of Section 897(c)(2) of the Code, or (B) a member of any consolidated, combined, unitary or affiliated group of corporations for any
Tax purposes other than a group of which Buyer is or was the common parent corporation.
(m)
To the Knowledge of Buyer, no shareholder of Buyer is subject to a binding commitment or has otherwise agreed to sell, exchange, transfer
by gift or otherwise dispose of any of the shares of Buyer received by it pursuant to this Agreement, or take any other action that would
be reasonably likely to prevent the Share Exchange from qualifying as a transaction described in Section 351 of the Code.
(n)
No Buyer Company, nor any of the respective Affiliates of any such Persons, have taken or have agreed to take any action, or is
aware of any fact or circumstance, that would be reasonably likely to prevent the Share Exchange from qualifying as an exchange described
in Section 351 of the Code.
3.15
Real Property.
(a)
Schedule 3.15(a) contains a complete and accurate list of all premises currently leased or subleased or otherwise used
or occupied by a Buyer Company for the operation of the business of a Buyer Company, and of all current leases, lease guarantees, agreements
and documents related thereto, including all amendments, terminations and modifications thereof or waivers thereto (collectively, the
“Buyer Real Property Leases”), as well as the current annual rent and term under each Buyer Real Property Lease.
Buyer has provided to the Company a true and complete copy of each of the Buyer Real Property Leases, and in the case of any oral Buyer
Real Property Lease, a written summary of the material terms of such Buyer Real Property Lease. The Buyer Real Property Leases are valid,
binding and enforceable in accordance with their terms and are in full force and effect. No event has occurred which (whether with or
without notice, lapse of time or both or the happening or occurrence of any other event) would constitute a default on the part of a
Buyer Company or, to the Knowledge of Buyer, any other party under any of Buyer Real Property Leases, and no Buyer Company has received
notice of any such condition.
(b) Schedule 3.15(b) contains a complete and accurate list of all property owned by a Buyer Company (“Buyer Owned
Real Property”), including the name of the record owner of each Buyer Owned Real Property. No Buyer Company is a lessor,
sublessor or grantor under any lease, sublease, Consent, license or other instrument granting to another Person any right to the possession,
use, occupancy or enjoyment of the Owned Real Property.
(c)
All certificates of occupancy, Permits, licenses, franchises, approvals and authorizations (collectively, the “Real
Property Permits”) of all Governmental Authorities, boards of fire underwriters, associations or any other Person having
jurisdiction over the Owned Real Property that are required or appropriate to use or occupy the Owned Real Property or to operate the
Buyer’s business as currently conducted thereon, have been issued and are in full force and effect. Buyer has not received any
written notice from any Governmental Authorities of any uncured violations of any federal, state, county or municipal Law, ordinance,
Order, regulation or requirement affecting the Buyer Companies, the Leased Real Property or the Owned Real Property or the ability of
the Seller and the Buyer Companies to consummate the Transactions. Buyer Companies have not received any written notice that any insurance
policy held by or on behalf of the Buyer Companies relating to or affecting the Buyer Owned Real Property or Buyer Real Property Leases
is not in full force and effect and the Company has not received any written notice of default that remains uncured or notice terminating
or threatening to terminate any such insurance policy.
3.16
Personal Property. Each item of Personal Property which is currently owned, used or leased by a Buyer Company with a book value
or fair market value of greater than Fifty Thousand Dollars ($50,000) or that is otherwise material to its business is set forth on Schedule
3.16, along with to the extent applicable, a list of lease agreements, lease guarantees, security agreements and other agreements
related thereto, including all amendments, terminations and modifications thereof or waivers thereto (“Buyer Personal Property
Leases”). Except as set forth in Schedule 3.16,
all such items of personal property are in good operating condition and repair (reasonable wear and tear excepted consistent with the
age of such items), and are suitable for their intended use in the business of the Buyer Companies. The operation of each Buyer Company’s
business as it is now conducted is not dependent upon the right to use the Personal Property of Persons other than a Buyer Company, except
for such Personal Property that is owned, leased or licensed by, or otherwise contracted to, a Buyer Company. Buyer has provided to the
Company a true and complete copy of each of the Buyer Personal Property Leases, and in the case of any oral Buyer Personal Property Lease,
a written summary of the material terms of such Buyer Personal Property Lease. The Buyer Personal Property Leases are valid, binding
and enforceable in accordance with their terms and are in full force and effect. To the Knowledge of Buyer, no event has occurred which
(whether with or without notice, lapse of time or both or the happening or occurrence of any other event) would constitute a default
on the part of a Buyer Company or any other party under any of the Buyer Personal Property Leases, and no Buyer Company has received
notice of any such condition.
3.17
Title to and Sufficiency of Assets. Each Buyer Company has good and marketable title to, or a valid leasehold interest in or right
to use, all of its assets, free and clear of all Liens other than (a) Permitted Liens, (b) the rights of lessors under leasehold interests,
(c) Liens specifically identified on the Buyer Interim Balance Sheet and (d) Liens set forth on Schedule 3.17. The assets (including
Intellectual Property rights and contractual rights) of the Buyer Companies constitute all of the assets, rights and properties that
are used in the operation of the businesses of the Buyer Companies as it is now conducted or that are used or held by the Buyer Companies
for use in the operation of the businesses of the Buyer Companies, and, taken together, are adequate and sufficient for the operation
of the businesses of the Buyer Companies as currently conducted.
3.18
Employee Matters.
(a)
Except as set forth in Schedule 3.18(a), no Buyer Company is a party to any collective bargaining agreement or other Contract
covering any group of employees, labor organization or other Representative of any of the employees of any Buyer Company and Buyer has
no Knowledge of any activities or proceedings of any labor union or other party to organize or represent such employees. There has not
occurred or, to the Knowledge of Buyer, been threatened any strike, slow-down, picketing, work-stoppage, or other similar labor activity
with respect to any such Buyer Company employees. There are no unresolved labor Actions (including unresolved grievances and age or other
discrimination claims) that are pending or, to the Knowledge of Buyer, threatened, between any Buyer Company and Persons employed by
or providing services as independent contractors to a Buyer Company. No current officer or employee of a Buyer Company has provided any
Buyer Company written or, to the Knowledge of Buyer, oral notice of his or her plan to terminate his or her employment with any Buyer
Company.
(b)
Except as set forth in Schedule 3.18(a), each Buyer Company (i) is and has been in compliance for the past three (3) years
in all material respects with all applicable Laws respecting employment and employment practices, terms and conditions of employment,
health and safety and wages and hours, and other Laws relating to discrimination, disability, labor relations, hours of work, payment
of wages and overtime wages, pay equity, immigration, workers compensation, working conditions, employee scheduling, occupational safety
and health, family and medical leave, and employee terminations, and has not received written or, to the Knowledge of Buyer, oral notice
that there is any pending Action involving unfair labor practices against a Buyer Company, (ii) is not liable for any material past due
arrears of wages or any material penalty for failure to comply with any of the foregoing, and (iii) is not liable for any material payment
to any Governmental Authority with respect to unemployment compensation benefits, social security or other benefits or obligations for
employees, independent contractors or consultants (other than routine payments to be made in the ordinary course of business and consistent
with past practice). There are no Actions pending or, to the Knowledge of Buyer, threatened against a Buyer Company brought by or on
behalf of any applicant for employment, any current or former employee, any Person alleging to be a current or former employee, or any
Governmental Authority, relating to any such Law or regulation, or alleging breach of any express or implied Contract of employment,
wrongful termination of employment, or alleging any other discriminatory, wrongful or tortious conduct in connection with the employment
relationship. No Buyer Company has received any report of any act or allegation of or relating to sex-based discrimination, sexual harassment,
sexual misconduct, workplace harassment, or breach of any policy of any Buyer Company relating to the foregoing, in each case involving
any employee, former employee or independent contractor or consultant, nor has there been any settlement or similar out-of-court or pre-litigation
arrangement relating to any such matters, nor has any such action, settlement or other arrangement been proposed or, to the Buyer’s
Knowledge, threatened.
(c)
Except as set forth on Schedule 3.18(c), (A) no employee is a party to a written employment Contract with a Buyer Company
and each is employed “at will,” and (B) the Buyer Companies have paid in full to all their employees all wages, salaries,
commission, bonuses and other compensation due to their employees, including overtime compensation, and no Buyer Company has any obligation
or Liability (whether or not contingent) with respect to severance payments to any such employees under the terms of any written or,
to Buyer’s Knowledge, oral agreement, or commitment or any applicable Law, custom, trade or practice. Except as set forth in Schedule
3.18(c), each Buyer Company employee has entered into Buyer’s standard form of employee non-disclosure, inventions and restrictive
covenants agreement with a Buyer Company (whether pursuant to a separate agreement or incorporated as part of such employee’s overall
employment agreement), a copy of which has been made available to the Company by Buyer.
(d)
Except as set forth on Schedule 3.18(d), each such independent contractor has entered into customary covenants regarding
confidentiality, non-competition and assignment of inventions and Copyrights in such Person’s agreement with a Buyer Company, a
copy of which has been provided to the Company by Buyer. For the purposes of applicable Law, including the Code, all independent contractors
who are currently, or within the last six (6) years have been, engaged by a Buyer Company are bona fide independent contractors and not
employees of a Buyer Company. Except as set forth on Schedule 3.18(d), each independent contractor is terminable on fewer than
thirty (30) days’ notice, without any obligation of any Buyer Company to pay severance or a termination fee.
3.19
Benefit Plans.
(a)
Set forth on Schedule 3.19(a) is a true and complete list of each Benefit Plan that is maintained, contributed to, required
to be contributed to, or sponsored by Buyer or any Buyer Company for the benefit of any current or former employee, officer, director
or consultant, or under which Buyer or any Buyer Company has any liability (each, a “Buyer Benefit Plan”).
(b)
With respect to each Buyer Benefit Plan, Buyer has made available to the Company accurate and complete copies, if applicable,
of: (i) the current plan documents and currently effective related trust agreements or annuity Contracts (including any amendments, modifications
or supplements thereto), and written descriptions of the material terms of any Buyer Benefit Plans which are not in writing; (ii) the
most recent actuarial valuation; (iii) the most recent summary plan description and summaries of material modifications thereto; (iv)
a copy of the three (3) most recently filed Form 5500 annual reports and accompanying schedules, (v) copy of the most recently received
IRS determination, opinion or advisory letter; (vi) the three (3) most recent nondiscrimination testing reports, safe-harbor notice,
and automatic enrollment notices, as applicable, and (vii) all material non-routine communications with any Governmental Authority within
the past three (3) years concerning any matter that is still pending or for which a Buyer Company has any outstanding Liability or obligation.
(c)
With respect to each Buyer Benefit Plan: (i) such Buyer Benefit Plan has been administered and enforced in all material respects
in accordance with its terms and the requirements of all applicable Laws, and has been maintained, where required, in good standing with
applicable regulatory authorities and Governmental Authorities; (ii) to the Knowledge of Buyer no breach of fiduciary duty has occurred;
(iii) no Action is pending, or to Buyer’s Knowledge, threatened (other than routine claims for benefits arising in the ordinary
course of administration); and (iv) all contributions, premiums and other payments (including any special contribution, interest or penalty)
required to be made with respect to a Buyer Benefit have in all material respects been timely made.
(d)
No Buyer Company has any commitment to modify, change or terminate any Buyer Benefit Plan, other than with respect to a modification,
change or termination required by ERISA or the Code, or other applicable Law.
(e)
No Buyer Company is a party to any agreement, contract, arrangement or Buyer Benefit Plan that has resulted or could result, separately
or in the aggregate, in the payment of (i) any “excess parachute payment” within the meaning of Section 280G of the Code
(or any corresponding or similar provision of state, local or non-U.S. law) or (ii) any amount that will not be fully deductible as a
result of Section 162(m) of the Code (or any corresponding or similar provision of state, local or non-U.S. law).
(f)
None of the Buyer Benefit Plans is or has at any time been, nor does any Buyer Company or any ERISA Affiliate (as defined
below) have or reasonably expect to have any liability or obligation under (i) a multiemployer plan (within the meaning of Section 3(37)
or 4001(a)(3) of ERISA), (ii) a single employer pension plan (within the meaning of Section 4001(a)(15) of ERISA) subject to Section
412 of the Code or Title IV of ERISA, (iii) a multiple employer plan subject to Section 413(c) of the Code, (iv) a multiple employer
welfare arrangement under ERISA, or (v) a voluntary employees’ beneficiary association as defined in Section 501(c)(9) of the Code.
For purposes of this Agreement, “ERISA Affiliate” means any entity that together with any Buyer Company is
a “single employer” for purposes of Section 4001(b)(1) of ERISA or Sections 414(b), (c), (m) or (o) of the Code.
3.20
Environmental Matters. Except as set forth in Schedule 3.20:
(a)
Each Buyer Company is and has been in compliance in all material respects with all applicable Environmental Laws, including obtaining,
maintaining in good standing, and complying in all material respects with all Permits required for its business and operations by Environmental
Laws (“Environmental Permits”), no Action is pending or, to Buyer’s Knowledge, threatened to revoke,
modify, or terminate any such Environmental Permit, and, to Buyer’s Knowledge, no facts, circumstances, or conditions currently
exist that could adversely affect such continued compliance with Environmental Laws and Environmental Permits or require capital expenditures
to achieve or maintain such continued compliance with Environmental Laws and Environmental Permits.
(b)
No Buyer Company is the subject of any outstanding Order or Contract with any Governmental Authority or other Person in respect
of any (i) Environmental Laws, (ii) Remedial Action, or (iii) Release or threatened Release of a Hazardous Material. No Buyer Company
has assumed, contractually or by operation of Law, any Liabilities or obligations under any Environmental Laws.
(c)
No Action has been made or is pending, or to Buyer’s Knowledge, threatened, against any Buyer Company or any assets of a
Buyer Company alleging either or both that a Buyer Company may be in material violation of any Environmental Law or Environmental Permit
or may have any material Liability under any Environmental Law.
(d)
No Buyer Company has manufactured, treated, stored, disposed of, arranged for or permitted the disposal of, generated, handled
or Released any Hazardous Material, or owned or operated any property or facility, in a manner that has given or would reasonably be
expected to give rise to any material Liability or obligation under applicable Environmental Laws. No fact, circumstance, or condition
exists in respect of any Buyer Company or any property currently or formerly owned, operated, or leased by any Buyer Company or any property
to which a Buyer Company arranged for the disposal or treatment of Hazardous Materials that could reasonably be expected to result in
a Buyer Company incurring any material Environmental Liabilities.
(e)
There is no investigation of the business, operations, or currently owned, operated, or leased property of a Buyer Company or,
to Buyer’s Knowledge, previously owned, operated, or leased property of a Buyer Company pending or, to Buyer’s Knowledge,
threatened that could lead to the imposition of any Liens under any Environmental Law or material Environmental Liabilities.
(f)
To the Knowledge of Buyer, there is not located at any of the properties of a Buyer Company any (i) underground storage
tanks, (ii) asbestos-containing material, or (iii) equipment containing polychlorinated biphenyls.
(g)
Buyer has provided to the Company all environmentally related site assessments, audits, studies, reports, analysis and results
of investigations that have been performed in respect of the currently or previously owned, leased, or operated properties of any Buyer
Company.
3.21
Transactions with Related Persons. Except as set forth on Schedule 3.21, no Buyer Company nor any of its Affiliates, nor
any officer, director, manager, employee, trustee or beneficiary of a Buyer Company or any of its Affiliates, nor any immediate family
member of any of the foregoing (whether directly or indirectly through an Affiliate of such Person) (each of the foregoing, a “Related
Person”) is presently, or in the past three (3) years, has been, a party to any transaction with a Buyer Company, including
any Contract or other arrangement (a) providing for the furnishing of services by (other than as officers, directors or employees of
Buyer Company), (b) providing for the rental of real property or Personal Property from or (c) otherwise requiring payments to (other
than for services or expenses as directors, officers or employees of the Buyer Company in the ordinary course of business consistent
with past practice) any Related Person or any Person in which any Related Person has an interest as an owner, officer, manager, director,
trustee or partner or in which any Related Person has any direct or indirect interest (other than the ownership of securities representing
no more than two percent (2%) of the outstanding voting power or economic interest of a publicly traded company). Except as set forth
on Schedule 3.21, no Buyer Company has outstanding any Contract or other arrangement or commitment with any Related Person, and
no Related Person owns any real property or Personal Property, or right, tangible or intangible (including Intellectual Property) which
is used in the business of any Buyer Company. Except as set forth on Schedule 3.21, assets of the Buyer Companies do not include
any receivable or other obligation from a Related Person, and the Liabilities of the Buyer Companies do not include any payable or other
obligation or commitment to any Related Person. Schedule 3.21 specifically identifies all Contracts, arrangements or commitments
set forth on such Schedule 3.21 that cannot be terminated upon sixty (60) days’ notice by the Buyer Companies without cost
or penalty.
3.22
Investment Company Act. Buyer is not an “investment company” or a Person directly or indirectly “controlled”
by or acting on behalf of an “investment company”, in each case within the meaning of the Investment Company Act.
3.23
Finders and Brokers. Except as set forth on Schedule 3.23, no broker, finder or investment banker is entitled to any brokerage,
finder’s or other fee or commission from Buyer, the Buyer Companies or any of their respective Affiliates in connection with the
Transactions based upon arrangements made by or on behalf of Buyer.
3.24
Certain Business Practices.
(a)
No Buyer Company, nor any of their Representatives acting on their behalf, has (i) used any funds for unlawful contributions,
gifts, entertainment or other unlawful expenses relating to political activity, (ii) made any unlawful payment to foreign or domestic
government officials or employees, to foreign or domestic political parties or campaigns or violated any provision of the U.S. Foreign
Corrupt Practices Act of 1977 or any other local or foreign anti-corruption or bribery Law, (iii) made any other unlawful payment or
(iv) since January 1, 2021, directly or indirectly, given or agreed to give any unlawful gift or similar benefit in any material amount
to any customer, supplier, governmental employee or other Person who is or may be in a position to help or hinder any Buyer Company or
assist and Buyer Company in connection with any actual or proposed transaction.
(b)
The operations of each Buyer Company are and have been conducted at all times in compliance with money laundering statutes in
all applicable jurisdictions, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued,
administered or enforced by any Governmental Authority, and no Action involving a Buyer Company with respect to any of the foregoing
is pending or, to the Knowledge of Buyer, threatened.
(c)
No Buyer Company or any of their respective directors or officers, or, to the Knowledge of Buyer, any other Representative acting
on behalf of a Buyer Company, is currently identified on the specially designated nationals or other blocked person list or otherwise
currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department (“OFAC”),
and no Buyer Company has, directly or indirectly, used any funds, or loaned, contributed or otherwise made available such funds to any
Subsidiary, joint venture partner or other Person, in connection with any sales or operations in Cuba, Iran, Syria, Sudan, Myanmar or
any other country sanctioned by OFAC or for the purpose of financing the activities of any Person currently subject to, or otherwise
in violation of, any U.S. sanctions administered by OFAC in the last five (5) fiscal years.
3.25
Business Insurance.
(a)
Schedule 3.25(a) lists all insurance policies (by policy number, insurer, coverage period, coverage amount, annual premium
and type of policy) held by a Buyer Company relating to a Buyer Company or its business, properties, assets, directors, officers and
employees, copies of which have been provided to the Company. All premiums due and payable under all such insurance policies have been
timely paid and the Buyer Companies are otherwise in material compliance with the terms of such insurance policies. Each such insurance
policy (i) is legal, valid, binding, enforceable and in full force and effect and (ii) will continue to be legal, valid, binding, enforceable,
and in full force and effect on identical terms following the Closing. No Buyer Company has any self-insurance or co-insurance programs.
Since January 1, 2021, no Buyer Company has received any notice from, or on behalf of, any insurance carrier relating to or involving
any adverse change or any change other than in the ordinary course of business, in the conditions of insurance, any refusal to issue
an insurance policy or non-renewal of a policy.
(b)
Schedule 3.25(b) identifies each individual insurance claim in excess of $50,000 made by a Buyer Company since January
1, 2021. Each Buyer Company has reported to its insurers all claims and pending circumstances that would reasonably be expected to result
in a claim, except where such failure to report such a claim would not be reasonably likely to be material to the Buyer Companies. To
the Knowledge of Buyer, no event has occurred, and no condition or circumstance exists, that would reasonably be expected to (with or
without notice or lapse of time) give rise to or serve as a basis for the denial of any such insurance claim. No Buyer Company has made
any claim against an insurance policy as to which the insurer is denying coverage.
3.26
Top Suppliers. Schedule 3.26 lists, by dollar volume received or paid, as applicable, for each of (a) the twelve (12) months
ended on December 31, 2022 and (b) the period from January 1, 2023 through September 30, 2023, the ten (10) largest suppliers of goods
or services to the Buyer Companies (the “Buyer Top Vendors”), along with the amounts of such dollar volumes.
The relationships of each Buyer Company with such suppliers are good commercial working relationships and (i) no Buyer Top Vendor within
the last twelve (12) months has cancelled or otherwise terminated, or, to Buyer’s Knowledge, intends to cancel or otherwise terminate,
any material relationships of such Person with a Buyer Company, (ii) no Buyer Top Vendor has during the last twelve (12) months decreased
materially or, to Buyer’s Knowledge, threatened to stop, decrease or limit materially, or intends to modify materially its material
relationships with a Buyer Company or intends to stop, decrease or limit materially its products or services to any Buyer Company or
its usage or purchase of the products or services of any Buyer Company, (iii) to Buyer’s Knowledge, no Buyer Top Vendor intends
to refuse to pay any amount due to any Buyer Company or seek to exercise any remedy against any Buyer Company, (iv) except as set forth
on Schedule 3.26, no Buyer Company has within the past two
(2) years been engaged in any material dispute with any Buyer Top Vendor, and (v) to Buyer’s Knowledge, the consummation of the
Transactions and the Ancillary Documents will not adversely affect the relationship of any Buyer Company with any Buyer Top Vendor.
3.27
Independent Investigation. Buyer has conducted its own independent investigation, review and analysis of the business, results
of operations, condition (financial or otherwise) or assets of the Target Companies and acknowledges that it has been provided adequate
access to the personnel, properties, assets, premises, books and records, and other documents and data of the Target Companies for such
purpose. Buyer acknowledges and agrees that: (a) in making its decision to enter into this Agreement and to consummate the Transactions,
it has relied solely upon its own investigation and the express representations and warranties of the Company and the Sellers set forth
in this Agreement (including the related portions of the Company Disclosure Schedules (as defined below)) and in any certificate delivered
to Buyer pursuant hereto, and the information provided by or on behalf of the Company or the Sellers for the Registration Statement;
and (b) none of the Company or the Sellers or their respective Representatives have made any representation or warranty as to the Target
Companies or the Sellers, except as expressly set forth in this Agreement (including the related portions of the Company Disclosure Schedules)
or in any certificate delivered to Buyer pursuant hereto.
Article
IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except
as set forth in the disclosure schedules delivered by the Company to Buyer on the date hereof (the “Company Disclosure Schedules”),
the Section numbers of which are numbered to correspond to the Section numbers of this Agreement to which they refer, the Company hereby
represents and warrants to Buyer as follows:
4.1
Organization and Standing. The Company is a company duly incorporated, validly existing and in good standing under the laws of
Switzerland and has all requisite corporate power and authority to own, lease and operate its properties and to carry on its business
as now being conducted. Each other Target Company is a corporation or other entity duly formed, validly existing and in good standing
under the Laws of its jurisdiction of organization and has all requisite corporate power and authority to own, lease and operate its
properties and to carry on its business as now being conducted. Each Target Company is duly qualified or licensed and in good standing
in the jurisdiction in which it is incorporated or registered and in each other jurisdiction where it does business or operates to the
extent that the character of the property owned, leased or operated by it or the nature of the business conducted by it makes such qualification
or licensing necessary. Schedule 4.1 lists all jurisdictions in which any Target Company is qualified to conduct business and
all names other than its legal name under which any Target Company does business. The Company has provided to Buyer accurate and complete
copies of the Organizational Documents of each Target Company, each as amended to date and as currently in effect. No Target Company
is in violation of any provision of its Organizational Documents.
4.2
Authorization; Binding Agreement. The Company has all requisite corporate power and authority to execute and deliver this Agreement
and each Ancillary Document to which it is a party, to perform the Company’s obligations hereunder and thereunder and to consummate
the transactions contemplated hereby and thereby. The execution and delivery of this Agreement and each Ancillary Document to which the
Company is a party and the consummation of the transactions contemplated hereby and thereby, (a) have been duly and validly authorized
by the board of directors and shareholders of the Company in accordance with the Company’s Organizational Documents, the laws of
its jurisdiction of incorporation or formation, any other applicable Law and any Contract to which the Company or any of its shareholders
are party or bound and (b) no other corporate proceedings on the part of the Company are necessary to authorize the execution and delivery
of this Agreement and each Ancillary Document to which it is a party or to consummate the transactions contemplated hereby and thereby.
This Agreement has been, and each Ancillary Document to which the Company is a party shall be when delivered, duly and validly executed
and delivered by the Company Party and assuming the due authorization, execution and delivery of this Agreement and any such Ancillary
Document by the other parties hereto and thereto, constitutes, or when delivered shall constitute, the legal, valid and binding obligation
of the Company, enforceable against the Company in accordance with its terms, subject to the Enforceability Exceptions.
4.3
Capitalization.
(a)
The issued shares in the capital of the Company consists of 412,572 Company Shares, and there are no other issued equity interests
of the Company, or rights to acquire equity interests of the Company, except for the Company Convertible Securities. Prior to giving
effect to the Transactions, Sellers are the legal (registered) and beneficial owners of all of the issued and outstanding equity interests
of the Company, with each Seller owning the Company Shares or Convertible Securities set forth on Schedule 4.3(a), all of which
are owned by the Sellers free and clear of any Liens other than those imposed under the Company Organizational Documents and applicable
securities Laws. After giving effect to the Share Exchange, Buyer shall own all of the issued and outstanding Company Shares free and
clear of any Liens other than those imposed under the Company Organizational Documents and applicable securities Laws. All of the issued
Company Shares have been duly authorized, are fully paid and non-assessable (meaning no further payments are due by the respective holder)
and not in violation of any purchase option, right of first refusal, preemptive right, subscription right or any similar right under
any provision of the laws of its jurisdiction of incorporation or formation, any other applicable Law, the Company’s Organizational
Documents or any Contract to which the Company is a party or by which the Company or its securities are bound. The Company does not,
directly or indirectly, hold any of its shares or other equity interests in treasury.
(b)
Schedule 4.3(b) sets forth the beneficial and record owners of all outstanding Company Convertible Securities (if any)
prior to the Share Exchange, and except as set forth on Schedule 4.3(b), there are no Company Convertible Securities or preemptive
rights or rights of first refusal or first offer, nor are there any Contracts, commitments, arrangements or restrictions to which the
Company or, to the Knowledge of the Company, any of its shareholders are a party or bound relating to any equity securities of the Company,
whether or not outstanding. There are no outstanding or authorized equity appreciation, phantom equity or similar rights with respect
to the Company. Except as set forth on Schedule 4.3(b), there are no voting trusts, proxies, shareholder agreements or any other
agreements or understandings with respect to the voting of the Company’s share capital. Except as set forth in the Company’s
Organizational Documents, there are no outstanding contractual obligations of the Company to repurchase,
redeem or otherwise acquire any of its shares or securities, nor has the Company granted any registration rights to any Person with respect
to its shares. All of the issued and outstanding securities of the Company have been granted, offered, sold and issued in compliance
with all applicable securities Laws. Except as set forth on Schedule 4.3(b),
no shares in the capital of the Company are issuable and no rights in connection with any interests, warrants, rights, options or other
securities of the Company accelerate or otherwise become triggered (whether as to vesting, exercisability, convertibility or otherwise)
as a result of the consummation of the Transactions.
(c)
All Indebtedness of the Company as of the date of this Agreement is disclosed in the audited financial statements of the Target
Companies as of December 31, 2022, or on Schedule 4.3(c). No Indebtedness of the Company contains any restriction upon: (i) the
prepayment of any such Indebtedness, (ii) the incurrence of Indebtedness by the Company, (iii) the ability of the Company to grant any
Lien on its properties or assets, or (iv) the consummation of the Transactions.
(d)
Since January 1, 2023, the Company has not declared or paid any distribution or dividend in respect of its shares and has not
repurchased, redeemed or otherwise acquired any shares in the capital of the Company, and the board of directors of the Company has not
authorized any of the foregoing.
4.4
Subsidiaries. Schedule 4.4 sets forth the name of each Subsidiary of the Company, and with respect to each Subsidiary (a)
its jurisdiction of organization, (b) its authorized shares or other equity interests (if applicable), and (c) the number of issued and
outstanding shares or other equity interests and the record holders and beneficial owners thereof. All of the outstanding equity securities
of each Subsidiary of the Company are duly authorized and validly issued, fully paid and non-assessable (if applicable), and were offered,
sold and delivered in compliance with all applicable securities Laws, and owned by one or more of the Target Companies free and clear
of all Liens (other than those, if any, imposed by such Subsidiary’s Organizational Documents). There are no Contracts to which
the Company or any of its Affiliates is a party or bound with respect to the voting (including voting trusts or proxies) of the equity
interests of any Subsidiary of the Company other than the Organizational Documents of any such Subsidiary. There are no outstanding or
authorized options, warrants, rights, agreements, subscriptions, convertible securities or commitments to which any Subsidiary of the
Company is a party or which are binding upon any Subsidiary of the Company providing for the issuance or redemption of any equity interests
of any Subsidiary of the Company. There are no outstanding equity appreciation, phantom equity, profit participation or similar rights
granted by any Subsidiary of the Company. Except as set forth in Schedule 4.4, no Subsidiary of the Company has any limitation,
whether by Contract, Order or applicable Law, on its ability to make any distributions or dividends to its equity holders or repay any
debt owed to another Target Company. Except for the equity interests of the Subsidiaries listed on Schedule 4.4, the Company does
not own or have any rights to acquire, directly or indirectly, any equity interests of, or otherwise Control, any Person. No Target Company
is a participant in any joint venture, partnership or similar arrangement. There are no outstanding contractual obligations of the Target
Company to provide funds to, or make any investment (in the form of a loan, capital contribution or otherwise) in, any other Person.
4.5
Governmental Approvals. No Consent of or with any Governmental Authority on the part of any Target Company is required to be obtained
or made in connection with the execution, delivery or performance by the Company of this Agreement or any Ancillary Documents or the
consummation by the Company of the transactions contemplated hereby or thereby other than (a) such filings as are expressly contemplated
by this Agreement, (b) pursuant to Antitrust Laws and (c) where the failure to obtain or make such Consents or to make such filings or
notifications would not reasonably be expected to have a Material Adverse Effect on the Company.
4.6
Non-Contravention. Except as otherwise described in Schedule 4.6, the execution and delivery by the Company (or any other
Target Company, as applicable) of this Agreement and each Ancillary Document to which any Target Company is a party, the consummation
by any Target Company of the transactions contemplated hereby and thereby, and compliance by any Target Company with any of the provisions
hereof and thereof, shall not (a) conflict with or violate any provision of any Target Company’s Organizational Documents, (b)
subject to obtaining the Consents from Governmental Authorities referred to in Section 4.6 hereof, and the waiting periods referred
to therein having expired, and any condition precedent to such Consent or waiver having been satisfied, conflict with or violate any
Law, Order or Consent applicable to any Target Company or any of its properties or assets, or (c) (i) violate, conflict with or result
in a breach of, (ii) constitute a default (or an event which, with notice or lapse of time or both, would constitute a default) under,
(iii) result in the termination, withdrawal, suspension, cancellation or modification of, (iv) accelerate the performance required by
any Target Company under, (v) result in a right of termination or acceleration under, (vi) give rise to any obligation to make payments
or provide compensation under, (vii) result in the creation of any Lien upon any of the properties or assets of any Target Company under,
(viii) give rise to any obligation to obtain any third party Consent or provide any notice to any Person under or (ix) give any Person
the right to declare a default, exercise any remedy, claim a rebate, chargeback, penalty or change in delivery schedule, accelerate the
maturity or performance, cancel, terminate or modify any right, benefit, obligation or other term under, any of the terms, conditions
or provisions of any Company Material Contract, except for any deviations from the foregoing clause (c) that would not reasonably be
expected to have a Material Adverse Effect on the Company.
4.7
Financial Statements.
(a)
As used herein, the term “Company Financials” means (i) the audited consolidated financial statements
of the Target Companies (including, in each case, any related notes thereto), consisting of the consolidated balance sheet of the Target
Companies as of December 31, 2022 (the “Company Balance Sheet”; such date, the “Company Balance
Sheet Date”), and as of December 31, 2021 and December 31, 2020, and the related consolidated audited income statements,
changes in shareholder equity and statements of cash flows for the years then ended (the “Audited Company Financials”).
The Audited Company Financials (x) were prepared from the books and records of the Target Companies as of the times and for the periods
referred to therein, (y) were prepared in accordance with Swiss GAAP, consistently applied throughout and among the periods involved
(except that the unaudited statements exclude the footnote disclosures and other presentation items required for Swiss GAAP and exclude
year-end adjustments which will not be material in amount), and (z) are complete and correct and fairly present in all material respects
the consolidated financial position of the Target Companies as of the respective dates thereof and the consolidated results of the operations
and cash flows of the Target Companies for the periods indicated. No Target Company has ever been subject to the reporting requirements
of Sections 13(a) and 15(d) of the Exchange Act.
(b)
Each Target Company maintains accurate books and records reflecting its assets and Liabilities and maintains proper and adequate
internal accounting controls that provide reasonable assurance that (i) such Target Company does not maintain any off-the-book accounts
and that such Target Company’s assets are used only in accordance with such Target Company’s management directives, (ii)
transactions are executed with management’s authorization, (iii) transactions are recorded as necessary to permit preparation of
the financial statements of such Target Company and to maintain accountability for such Target Company’s assets, (iv) access to
such Target Company’s assets is permitted only in accordance with management’s authorization, (v) the reporting of such Target
Company’s assets is compared with existing assets at regular intervals and verified for actual amounts, and (vi) accounts, notes
and other receivables and inventory are recorded accurately, and proper and adequate procedures are implemented to effect the collection
of accounts, notes and other receivables on a current and timely basis. All of the financial books and records of the Target Companies
are complete and accurate in all material respects and have been maintained in the ordinary course consistent with past practice and
in accordance with applicable Laws. No Target Company has been subject to or involved in any material fraud that involves management
or other employees who have a significant role in the internal controls over financial reporting of any Target Company. Since January
1, 2021, no Target Company nor its Representatives has received any written complaint, allegation, assertion or claim regarding the accounting
or auditing practices, procedures, methodologies or methods of any Target Company or its internal accounting controls, including any
material written complaint, allegation, assertion or claim that any Target Company has engaged in questionable accounting or auditing
practices.
(c)
The Target Companies do not have any Indebtedness other than the Indebtedness set forth on Schedule 4.7(c), and in such
amounts (including principal and any accrued but unpaid interest or other obligations with respect to such Indebtedness), as set forth
on Schedule 4.7(c). Except as disclosed on Schedule 4.7(c), no Indebtedness of any Target Company contains any restriction
upon (i) the prepayment of any of such Indebtedness, (ii) the incurrence of Indebtedness by any Target Company, or (iii) the ability
of the Target Companies to grant any Lien on their respective properties or assets.
(d)
No Target Company is subject to any Liabilities or obligations (whether or not required to be reflected on a balance sheet prepared
in accordance with Swiss GAAP), including any off-balance sheet obligations or any “variable interest entities” (within the
meaning Accounting Standards Codification 810), except for those that are either (i) adequately reflected or reserved on or provided
for in the consolidated balance sheet of the Company and its Subsidiaries as of the Company Balance Sheet Date contained in the Company
Financials or (ii) not material and that were incurred after the Company Balance Sheet Date in the ordinary course of business consistent
with past practice (other than Liabilities for breach of any Contract or violation of any Law).
(e)
All financial projections with respect to the Target Companies that were delivered by or on behalf of the Company to Buyer or
its Representatives were prepared in good faith using assumptions that the Company believes to be reasonable.
4.8
Absence of Certain Changes. Since January 1, 2023, except as set forth on Schedule 4.8 or for actions expressly contemplated
by this Agreement, each Target Company has (a) conducted its business only in the ordinary course of business consistent with past practice
and (b) not been subject to a Material Adverse Effect.
4.9
Compliance with Laws. No Target Company is or has been in material conflict or material non-compliance with, or in material default
or violation of, nor has any Target Company received, since January 1, 2021, any written or, to the Knowledge of the Company, oral notice
of any material conflict or non-compliance with, or material default or violation of, any applicable Laws by which it or any of its properties,
assets, employees, business or operations are or were bound or affected.
4.10
Permits. Each Target Company (and its employees who are legally required to be licensed by a Governmental Authority in order to
perform his or her duties with respect to his or her employment with any Target Company), holds all Permits necessary to lawfully conduct
its business as presently conducted and as currently contemplated to be conducted, and to own, lease and operate its assets and properties
(collectively, the “Company Permits”) except where the failure to have any of such Company Permits has not
had, and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. The Company has made
available to Buyer true, correct and complete copies of all Company Permits material to the Company’s business, all of which material
Company Permits are listed on Schedule 4.10. All of the Company
Permits are in full force and effect, and no suspension or cancellation of any of the Company Permits is pending or, to the Company’s
Knowledge, threatened. No Target Company is in violation in any material respect of the terms of any Company Permit, and no Target Company
has received any written or, to the Knowledge of the Company, oral notice of any Actions relating to the revocation or modification of
any Company Permit.
4.11
Litigation. Except as described on Schedule 4.11, there is no (a) Action of any nature currently pending or, to the Company’s
Knowledge, threatened, nor is there any reasonable basis for any Action to be made (and no such Action has been brought or, to the Company’s
Knowledge, threatened since January 1, 2021); or (b) Order now pending or outstanding or that was rendered by a Governmental Authority
since January 1, 2021, in either case of (a) or (b) by or against any Target Company, its current or former directors or officers (provided,
that any litigation involving the directors or officers of a Target Company must be related to the Target Company’s business, equity
securities or assets), its business, equity securities or assets. The items listed on Schedule 4.11, if finally determined adverse
to the Target Companies, will not have, either individually or in the aggregate, a Material Adverse Effect upon the Target Companies,
taken as a whole. Since January 1, 2021, none of the current or former officers, senior management or directors of any Target Company
have been charged with, indicted for, arrested for, or convicted of any felony or any crime involving fraud.
4.12
Material Contracts.
(a)
Schedule 4.12(a) sets forth a true, correct and complete list of, and the Company has made available to Buyer (including
written summaries of oral Contracts), true, correct and complete copies of, each Contract to which any Target Company is a party or by
which any Target Company, or any of its properties or assets are bound or affected (each Contract required to be set forth on Schedule
4.12(a), a “Company Material Contract”) that:
(i)
contains covenants that limit the ability of any Target Company (A) to compete in any line of business or with any
Person or in any geographic area or to sell, or provide any service or product or solicit any Person, including any non-competition covenants,
employee and customer non-solicit covenants, exclusivity restrictions, rights of first refusal or most-favored pricing clauses or (B)
to purchase or acquire an interest in any other Person;
(ii)
involves any joint venture, profit-sharing, partnership, limited liability company or other similar agreement or arrangement relating
to the formation, creation, operation, management or control of any partnership or joint venture;
(iii)
involves any exchange-traded, over-the-counter or other swap, cap, floor, collar, futures contract, forward contract, option or other
derivative financial instrument or Contract, based on any commodity, security, instrument, asset, rate or index of any kind or nature
whatsoever, whether tangible or intangible, including currencies, interest rates, foreign currency and indices;
(iv)
evidences Indebtedness (whether incurred, assumed, guaranteed or secured by any asset) of any Target Company having an outstanding principal
amount in excess of $100,000;
(v)
involves the acquisition or disposition, directly or indirectly (by merger or otherwise), of assets with an aggregate value in
excess of $100,000 (other than in the ordinary course of business consistent with past practice) or shares or other equity interests
of any Target Company or another Person;
(vi)
relates to any merger, consolidation or other business combination with any other Person or the acquisition or disposition of any other
entity or its business or material assets or the sale of any Target Company, its business or material assets;
(vii)
by its terms, individually or with all related Contracts, calls for aggregate payments or receipts by the Target Companies under such
Contract or Contracts of at least $100,000 per year or $500,000 in the aggregate (other than each employment, management, service or
consulting agreement);
(viii)
is with any Company Top Customer or Company Top Vendor;
(ix)
obligates the Target Companies to provide continuing indemnification or a guarantee of obligations of a third party after the date hereof
in excess of $100,000;
(x)
is between any Target Company and any directors, officers or employees of a Target Company (other than at-will employment arrangements
and restrictive covenant agreements with employees entered into in the ordinary course of business consistent with past practice, and
loans made to employees in the ordinary course of business in an amount not exceeding $25,000), including all non-competition, severance
and indemnification agreements, or any Related Person;
(xi)
obligates the Target Companies to make any capital commitment or expenditure in excess of $100,000 (including pursuant to any joint venture);
(xii)
relates to a material settlement entered into within three (3) years prior to the date of this Agreement or under which any Target Company
has outstanding obligations (other than customary confidentiality obligations); or
(xiii)
provides another Person (other than another Target Company or any manager, director or officer of any Target Company) with a power
of attorney.
(b)
With respect to each Company Material Contract: (i) such Company Material Contract is valid and binding and enforceable in all
respects against the Target Company party thereto and, to the Knowledge of the Company, each other party thereto, and is in full force
and effect (except, in each case, as such enforcement may be limited by the Enforceability Exceptions); (ii) the consummation of the
Transactions will not affect the validity or enforceability of any Company Material Contract; (iii) no Target Company is in breach or
default in any material respect, and no event has occurred that with the passage of time or giving of notice or both would constitute
a material breach or default by any Target Company, or permit termination or acceleration by the other party thereto, under such Company
Material Contract; (iv) no Target Company is in breach or default in any material respect, and no event has occurred that to the Knowledge
of the Company, no other party to such Company Material Contract is in breach or default in any material respect, and no event has occurred
that with the passage of time or giving of notice or both would constitute such a material breach or default by such other party, or
permit termination or acceleration by any Target Company, under such Company Material Contract; (v) no Target Company has received written
or, to the Knowledge of the Company, oral notice of an intention by any party to any such Company Material Contract to terminate such
Company Material Contract or amend the terms thereof, other than modifications in the ordinary course of business that do not adversely
affect any Target Company in any material respect; and (vi) no Target Company has waived any rights under any such Company Material Contract.
4.13
Intellectual Property.
(a)
Schedule 4.13(a)(i) sets forth: (i) all Patents and Patent applications, Trademarks and service mark registrations and
applications, Copyright registrations and applications and registered Internet Assets owned or licensed by a Target Company or otherwise
used or held for use by a Target Company in which a Target Company is the owner, applicant or assignee (“Company Registered
IP”), specifying as to each item, as applicable: (A) the nature of the item, including the title, (B) the owner of the
item, (C) the jurisdictions in which the item is issued or registered or in which an application for issuance or registration has been
filed and (D) the issuance, registration or application numbers and dates; and (ii) all material unregistered trademarks and software
owned or purported to be owned by a Target Company; Schedule 4.13(a)(ii) sets forth all Intellectual Property licenses, sublicenses
and other agreements or permissions (“Company IP Licenses”) (other than Off-the-Shelf Software), which are
not required to be listed, although such licenses are “Company IP Licenses” as that term is used herein), under which a Target
Company is a licensee or otherwise is authorized to use or practice any Intellectual Property. Each Target Company owns, free and clear
of all Liens (other than Permitted Liens), has valid and enforceable rights in, and has the unrestricted right to use, sell, license,
transfer or assign, all material Intellectual Property currently used or held for use by such Target Company, and previously used by
such Target Company, except for the Intellectual Property that is the subject of the Company IP Licenses. Except as set forth on Schedule
4.13(a)(iii), all Company Registered IP is owned exclusively by the applicable Target Company without obligation to pay royalties,
licensing fees or other fees, or otherwise account to any third party with respect to such Company Registered IP.
(b)
All registration and maintenance fees relating to the Intellectual Property have been paid when due. Each Target Company has a
valid and enforceable license to use all Intellectual Property that is the subject of the Company IP Licenses applicable to such Target
Company. Each Target Company has performed all material obligations imposed on it in the Company IP Licenses, has made all payments required
to date, and such Target Company is not, nor, to the Knowledge of the Company, is any other party thereto, in material breach or material
default thereunder, nor, to the Knowledge of the Company, has any event occurred that with notice or lapse of time or both would constitute
a default thereunder. The continued use by the Target Companies of the Intellectual Property that is the subject of the Company IP Licenses
in the same manner that it is currently being used is not restricted by any applicable license of any Target Company. All registrations
for Copyrights, Patents, Trademarks and Internet Assets that are owned by or exclusively licensed to any Target Company are valid and
in force, and all applications to register any Copyrights, Patents and Trademarks are pending and in good standing, all without challenge
of any kind.
(c)
No Action is pending or, to the Company’s Knowledge, threatened against a Target Company that challenges the validity, enforceability,
ownership, or right to use, sell, license or sublicense any Intellectual Property currently owned, licensed, used or held for use by
the Target Companies. No Target Company has received any written notice or claim asserting or suggesting that any infringement, misappropriation,
violation, dilution or unauthorized use of the Intellectual Property of any other Person is or may be occurring or has or may have occurred
(including any demands or offers to license any Intellectual Property rights from a third party), as a consequence of the business activities
of any Target Company, nor to the Knowledge of the Company is there a reasonable basis therefor. There are no Orders to which any Target
Company is a party or its otherwise bound that (i) restrict the rights of a Target Company to use, transfer, license or enforce any Intellectual
Property owned by a Target Company, (ii) restrict the conduct of the business of a Target Company in order to accommodate a third Person’s
Intellectual Property, or (iii) grant any third Person any right with respect to any Intellectual Property owned by a Target Company.
No Target Company is currently infringing, or has, in the past, infringed, misappropriated or violated any Intellectual Property of any
other Person in any material respect in connection with the ownership, use or license of any Intellectual Property owned or purported
to be owned by a Target Company or, to the Knowledge of the Company, otherwise in connection with the conduct of the respective businesses
of the Target Companies. To the Company’s Knowledge, no third party is infringing upon, has misappropriated or is otherwise violating
any Intellectual Property owned, licensed by, licensed to, or otherwise used or held for use by any Target Company (“Company
IP”) in any material respect. No Target Company has received any opinion of counsel that
any product or service provided or distributed in the operation of the businesses thereof, or the conduct of such business, currently
or in the past, infringes any Intellectual Property right of another Person or any opinion of counsel otherwise regarding the right to
practice any product or service in connection with such businesses.
(d)
All employees and independent contractors of a Target Company have assigned to the Target Companies all Intellectual Property
(including but not limited to inventions, and in each case including the unrestricted right of use) developed by such employees and independent
contractors in the performance of services for a Target Company by such Persons (without further payment or royalty) other than to the
extent ownership of such Intellectual Property would otherwise vest in the applicable the Target Company by operation of law. No current
or former officers, employees or independent contractors of a Target Company have claimed any ownership interest in any Intellectual
Property owned by a Target Company. To the Knowledge of the Company, there has been no violation of a Target Company’s policies
or practices related to protection of the Company IP or any confidentiality or nondisclosure Contract relating to the Intellectual Property
owned by a Target Company. To the Company’s Knowledge, none of the employees of any Target Company is obligated under any Contract,
or subject to any Order, that would materially interfere with the use of such employee’s best efforts to promote the interests
of the Target Companies, or that would materially conflict with the business of any Target Company as presently conducted or contemplated
to be conducted. Each Target Company has taken reasonable security measures in order to protect the secrecy, confidentiality and value
of the material Company IP to the extent such Company IP derives value from the secrecy and/or confidentiality thereof.
(e)
To the Knowledge of the Company, no Person has obtained unauthorized access to confidential third-party information and data in
the possession of a Target Company, nor has there been any other material compromise of the security, confidentiality or integrity of
such information or data. To the Knowledge of the Company, each Target Company has complied with all applicable Laws relating to privacy,
personal data protection, and the collection, processing and use of personal information and its own privacy policies and guidelines.
To the Knowledge of the Company, the operation of the business of the Target Companies has not and does not violate any right to privacy
or publicity of any third party, or constitute unfair competition or trade practices under applicable Law. The Company has taken adequate
precautions to protect, document and safeguard all trade secrets, know-how, confidential information, customer lists, software, technical
information, data and process technology that relate to the business of the Company.
(f)
The consummation of any of the Transactions will not result in the material breach, material modification, cancellation,
termination, suspension of, or acceleration of any payments with respect to, or release of source code because of (i) any Contract providing
for the license or other use of Intellectual Property owned by a Target Company, or (ii) any Company IP License. Following the Closing,
the Company shall be permitted to exercise, directly or indirectly through its Subsidiaries, all of the Target Companies’ rights
under such Contracts or Company IP Licenses to the same extent that the Target Companies would have been able to exercise had the Transactions
not occurred, without the payment of any additional amounts or consideration other than ongoing fees, royalties or payments which the
Target Companies would otherwise be required to pay in the absence of such Transactions.
4.14
Taxes and Returns. Except as set forth on Schedule 4.14:
(a)
Each Target Company has timely filed, or caused to be filed, all material Tax Returns required to be filed by it (taking into
account all available extensions). All such Tax Returns are true, accurate, correct and complete in all material respects. All Taxes
required to be paid, collected or withheld, other than such Taxes for which adequate reserves in the Company Financials have been established,
have been timely paid, collected or withheld. Each Target Company has complied in all material respects
with all applicable Laws relating to Tax.
(b)
There is no current pending or, to the Knowledge of the Company, threatened Action against a Target Company by a Governmental
Authority in a jurisdiction where a Target Company does not file Tax Returns that it is or may be subject to taxation by that jurisdiction.
(c)
No Target Company is being audited by any Tax authority or has been notified in writing or, to the Knowledge of the Company, orally
by any Tax authority that any such audit is contemplated or pending. To the Knowledge of the Company, there are no claims, assessments,
audits, examinations, investigations or other Actions pending against a Target Company in respect of any Tax, and no Target Company has
been notified in writing of any proposed Tax claims or assessments against it (other than, in each case, claims or assessments for which
adequate reserves in the Company Financials have been established).
(d)
There are no Liens with respect to any Taxes upon any Target Company’s assets, other than Permitted Liens.
(e)
No Target Company has any outstanding waivers or extensions of any applicable statute of limitations to assess any material amount
of Taxes. There are no outstanding requests by a Target Company for any extension of time within which to file any Tax Return or within
which to pay any Taxes shown to be due on any Tax Return (other than an extension resulting from having received an automatic extension
of time to file the applicable Tax Return not requiring the approval of any Governmental Authority).
(f)
No Target Company has made any change in accounting method (except as required by a change in Law) or received a ruling
from, or signed an agreement with, any taxing authority that would reasonably be expected to have a material impact on its Taxes following
the Closing.
(g)
No Target Company has engaged in any (i) “reportable transaction” as defined in Treasury Regulations Section 1.6011-4(b),
(ii) “listed transaction,” or (iii) transaction, a “significant” purpose of which is the avoidance or evasion
of U.S. federal income Tax, within the meanings of Sections 6662, 6662A, 6011, 6012, 6111 or 6707A of the Code or the Treasury Regulations
promulgated thereunder.
(h)
Each Target Company has complied with, and is currently in compliance with, all transfer pricing rules and regulations (including,
to the extent applicable, Section 482 of the Code and any comparable or similar provision of applicable Law). To the extent legally required,
the Target Companies have properly and timely documented their transfer pricing methodology in compliance with Sections 482 and 6662
of the Code and any comparable or similar provision of applicable Law. No Target Company is a party to any advance pricing agreement
or any similar Contract or agreement. No Target Company is subject to any gain recognition agreement under Section 367 of the Code.
(i)
No Target Company has been, in the past five (5) years, a party to a transaction reported or intended to qualify as a reorganization
under Section 368 of the Code.
(j)
No Target Company has any Liability for the Taxes of another Person (other than another Target Company) (i) under any applicable
Tax Law, (ii) as a transferee or successor, or (iii) by Contract, indemnity or otherwise (excluding commercial agreements entered into
in the ordinary course of business the primary purpose of which was not the sharing of Taxes). No Target Company is a party to or bound
by any Tax indemnity agreement, Tax sharing agreement or Tax allocation agreement or similar agreement, arrangement or practice (excluding
commercial agreements entered into in the ordinary course of business the primary purpose of which was not the sharing of Taxes) with
respect to Taxes (including advance pricing agreement, closing agreement or other agreement relating to Taxes with any Governmental Authority)
that will be binding on such Target Company with respect to any period following the Closing Date.
(k)
No Target Company has requested, or is the subject of or bound by any private letter ruling, technical advice memorandum, closing
agreement or similar ruling, memorandum or agreement with any Governmental Authority with respect to any Taxes, nor is any such request
outstanding.
(l)
No Target Company: (i) has constituted either a “distributing corporation” or a “controlled corporation”
(within the meaning of Section 355(a)(1)(A) of the Code) in a distribution of securities (to any Person or entity that is not a member
of the consolidated group of which the Company is the common parent corporation) qualifying for, or intended to qualify for, Tax-free
treatment under Section 355 of the Code (A) within the two-year period ending on the date hereof or (B) in a distribution which could
otherwise constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e)
of the Code) in conjunction with the Transactions; or (ii) is or has ever been (A) a U.S. real property holding corporation within the
meaning of Section 897(c)(2) of the Code, or (B) a member of any consolidated, combined, unitary or affiliated group of corporations
for any Tax purposes other than a group of which the Company is or was the common parent corporation.
(m)
No Target Company is treated as a domestic corporation (as such term is defined in Section 7701 of the Code) for U.S. federal income
tax purposes. No Target Company has ever been engaged in a U.S. trade or business (within the meaning of the Code).
(n)
As a result of the Share Exchange, Buyer will satisfy the “active trade or business test” as defined in Treasury Regulation
Section 1.367(a)-3(c)(3), including, without limitation, the requirements that (i) Buyer be engaged, directly or indirectly through a
qualified Subsidiary or qualified partnership, in an active trade or business for the entire thirty-six (36) month period immediately
preceding the Transactions, (ii) Buyer has no intention at the time of the Transactions to dispose of or discontinue such trade or business,
and (iii) the substantiality test (as defined in Treasury Regulation Section 1.367(a)-3(c)(3)(iii)) will be satisfied.
(o)
To the Knowledge of the Company, no Seller is subject to a binding commitment or has otherwise agreed to sell, exchange, transfer
by gift or otherwise dispose of any of the shares of Buyer, or take any other action that would be reasonably likely to prevent the Share
Exchange from qualifying as a transaction described in Section 351 of the Code.
(p)
No Target Company, nor any of the respective Affiliates of any such Persons, have taken or have agreed to take any action, or
is aware of any fact or circumstance, that would be reasonably likely to prevent the Share Exchange from qualifying as an exchange described
in Section 351 of the Code.
(q)
There are no actual or contingent Tax Liabilities of the Company in connection with (i) any acquisition of a company or any merger,
de-merger or similar transaction involving the Company, or (ii) any shareholder loans or other transactions between the Company on the
one hand and any of the Sellers or any of their Affiliates on the other hand.
(r)
The Company has no permanent establishments (Betriebsstätte im steuerrechtlichen Sinn) in Switzerland or in
any other country.
(s)
The Company has not made any hidden profit distributions to shareholders or related parties at any time. No hidden capital
contributions have been made to the Company at any time.
4.15
Real Property. Schedule 4.15 contains a complete and accurate list of all premises currently leased or subleased or otherwise
used or occupied by a Target Company for the operation of the business of a Target Company, and of all current leases, lease guarantees,
agreements and documents related thereto, including all amendments, terminations and modifications thereof or waivers thereto (collectively,
the “Company Real Property Leases”), as well as the current annual rent and term under each Company Real Property
Lease. The Company has provided to Buyer a true and complete copy of each of the Company Real Property Leases, and in the case of any
oral Company Real Property Lease, a written summary of the material terms of such Company Real Property Lease. The Company Real Property
Leases are valid, binding and enforceable in accordance with their terms and are in full force and effect. To the Knowledge of the Company,
no event has occurred which (whether with or without notice, lapse of time or both or the happening or occurrence of any other event)
would constitute a default on the part of a Target Company or any other party under any of the Company Real Property Leases, and no Target
Company has received notice of any such condition. The Company has made no changes to Company Real Property Leases that will require
material payments by the Company at termination of the lease to restore the leased premises to their original conditions. No Target Company
owns any real property or any interest in real property (other than the leasehold interests in the Company Real Property Leases).
4.16
Personal Property. Each item of Personal Property which is currently owned, used or leased by a Target Company with a book value
or fair market value of greater than Fifty Thousand Dollars ($50,000) is set forth on Schedule 4.16, along with, to the extent
applicable, a list of lease agreements, lease guarantees, security agreements and other agreements related thereto, including all amendments,
terminations and modifications thereof or waivers thereto (“Company Personal Property Leases”). Except as set
forth in Schedule 4.16, all such items of Personal Property are in good operating condition and repair (reasonable wear and tear excepted
consistent with the age of such items), and are suitable for their intended use in the business of the Target Companies. The operation
of each Target Company’s business as it is now conducted is not dependent upon the right to use the Personal Property of Persons
other than a Target Company, except for such Personal Property that is owned, leased or licensed by, or otherwise contracted to, a Target
Company. The Company has provided to Buyer a true and complete copy of each of the Company Personal Property Leases, and in the case
of any oral Company Personal Property Lease, a written summary of the material terms of such the Company Personal Property Lease. The
Company Personal Property Leases are valid, binding and enforceable in accordance with their terms and are in full force and effect.
To the Knowledge of the Company, no event has occurred which (whether with or without notice, lapse of time or both or the happening
or occurrence of any other event) would constitute a default on the part of a Target Company or any other party under any of the Company
Personal Property Leases, and no Target Company has received notice of any such condition.
4.17
Title to and Sufficiency of Assets. Each Target Company has good and marketable title to, or a valid leasehold interest in or
right to use, all of its assets, free and clear of all Liens other than (a) Permitted Liens, (b) the rights of lessors under leasehold
interests, and (c) Liens specifically identified on the Company Balance Sheet. The assets (including Intellectual Property rights and
contractual rights) of the Target Companies constitute all of the assets, rights and properties that are used in the operation of the
businesses of the Target Companies as it is now conducted or that are used or held by the Target Companies for use in the operation of
the businesses of the Target Companies, and, taken together, are adequate and sufficient for the operation of the businesses of the Target
Companies as currently conducted.
4.18
Employee Matters.
(a)
No Target Company is a party to any collective bargaining agreement or other Contract covering any group of employees, labor organization
or other Representative of any of the employees of any Target Company and the Company has no Knowledge of any activities or proceedings
of any labor union or other party to organize or represent such employees. There has not occurred or, to the Knowledge of the Company,
been threatened any strike, slow-down, picketing, work-stoppage, or other similar labor activity with respect to any Target Company employees.
There are no unresolved labor Actions (including unresolved grievances and age or other discrimination claims) that are pending or, to
the Knowledge of the Company, threatened, between any Target Company and Persons employed by or providing services as independent contractors
to a Target Company. No current officer or employee of a Target Company has provided any Target Company written or, to the Knowledge
of the Company, oral notice of his or her plan to terminate his or her employment with any Target Company.
(b)
Except as set forth in Schedule 4.18(b), each Target Company (i) is and has been in compliance for the past three
(3) years in all material respects with all applicable Laws respecting employment and employment practices, terms and conditions of employment,
health and safety and wages and hours, and other Laws relating to discrimination, disability, labor relations, hours of work, payment
of wages and overtime wages, pay equity, immigration, workers compensation, working conditions, employee scheduling, occupational safety
and health, family and medical leave, and employee terminations, and has not received written or, to the Knowledge of the Company, oral
notice that there is any pending Action involving unfair labor practices against a Target Company, (ii) is not liable for any material
past due arrears of wages or any material penalty for failure to comply with any of the foregoing, and (iii) is not liable for any
material payment to any Governmental Authority with respect to unemployment compensation benefits, social security or other benefits
or obligations for employees, independent contractors or consultants (other than routine payments to be made in the ordinary course of
business and consistent with past practice). There are no Actions pending or, to the Knowledge of the Company, threatened against a Target
Company brought by or on behalf of any applicant for employment, any current or former employee, any Person alleging to be a current
or former employee, or any Governmental Authority, relating to any such Law or regulation, or alleging breach of any express or implied
Contract of employment, wrongful termination of employment, or alleging any other discriminatory, wrongful or tortious conduct in connection
with the employment relationship. No Target Company has received any report of any act or allegation of or relating to sex-based discrimination,
sexual harassment, sexual misconduct, workplace harassment, or breach of any policy of any Target Company relating to the foregoing,
in each case involving any employee, former employee or independent contractor or consultant, nor has there been any settlement or similar
out-of-court or pre-litigation arrangement relating to any such matters, nor has any such action, settlement or other arrangement been
proposed or, to the Company’s Knowledge, threatened.
(c)
Schedule 4.18(c) hereto sets forth a complete and accurate list as of the date hereof of all employees of the Target Companies
showing for each as of such date the employee’s name, job title or description, employer and location. Except as set forth on Schedule
4.18(c), (A) all employees are parties to a written employment Contract with a Target Company and (B) the Target Companies have paid
in full to all their employees all wages, salaries, commission, bonuses and other compensation due to their employees, including overtime
compensation, and no Target Company has any obligation or Liability (whether or not contingent) with respect to severance payments to
any such employees under the terms of any written or, to the Company’s Knowledge, oral agreement, or commitment or any applicable
Law, custom, trade or practice. Except as set forth in Schedule 4.18(c), each Target Company employee has entered into the Company’s
standard employment agreement with a Target Company (whether pursuant to a separate agreement or incorporated as part of such employee’s
overall employment agreement), a copy of which has been made available to Buyer by the Company.
(d)
Schedule 4.18(d) contains a list of all independent contractors (including consultants) currently engaged by any Target
Company. Except as set forth on Schedule 4.18(c), all of such independent contractors are a party to a written Contract with a
Target Company. Except as set forth on Schedule 4.18(d), each independent contractor is terminable on fewer than thirty (30) days’
notice, without any obligation of any Target Company to pay severance or a termination fee.
4.19
Benefit Plans..
(a)
Set forth on Schedule 4.19(a) is a true and complete list of each Foreign Plan of a Target Company (each, a “Company
Benefit Plan”). No Target Company nor any ERISA Affiliate has ever established, maintained, contributed to, or has or had
any Liability with respect to (or had an obligation to contribute to) any Benefit Plan, whether or not subject to ERISA, which is not
a Foreign Plan.
(b)
With respect to each Company Benefit Plan, the Company has made available to Buyer accurate and complete copies, if applicable,
of: (i) the current plan documents and currently effective related trust agreements or annuity Contracts (including any amendments, modifications
or supplements thereto), and written descriptions of the material terms of any Company Benefit Plans which are not in writing; (ii) the
most recent actuarial valuation; and (iv) all material non-routine communications with any Governmental Authority within the past three
(3) years concerning any matter that is still pending or for which a Target Company has any outstanding Liability or obligation.
(c)
With respect to each Company Benefit Plan: (i) such Company Benefit Plan (1) has been administered and enforced in all material
respects in accordance with its terms and the requirements of all applicable Laws, and (2) has been maintained, where required, in good
standing with applicable regulatory authorities and Governmental Authorities (iii) no Action is pending, or to the Company’s Knowledge,
threatened (other than routine claims for benefits arising in the ordinary course of administration); and (iv) all contributions, premiums
and other payments (including any special contribution, interest or penalty) required to be made with respect to a Company Benefit have
in all material respects been timely made. No Target Company has incurred, or will incur in connection with the Transactions, any material
Liability in connection with termination of, or withdrawal from, any Company Benefit Plan, except for customary administrative charges.
(d)
To the extent applicable, the present value of the accrued benefit Liabilities (whether or not vested) under each Company Benefit
Plan, determined as of the end of the Company’s most recently ended fiscal year on the basis of reasonable actuarial assumptions,
did not exceed the current value of the assets of such Company Benefit Plan allocable to such benefit Liabilities or have been accrued
in all material respects on the Company Financials.
(e)
The Company is not, nor will be, obligated, whether under any Company Benefit Plan or otherwise, to pay separation, severance,
termination or similar benefits to any Person as a result of any Transaction, nor will any Transaction accelerate the time of payment
or vesting, or increase the amount, of any benefit or other compensation due to any Person. The Transactions shall not be the direct
or indirect cause of any amount paid or payable by a Target Company being classified as an “excess parachute payment” under
Section 280G of the Code and no arrangement exists pursuant to which the Company or any Target Company will be required to “gross
up” or otherwise compensate any Person because of the imposition of any excise tax under Section 4999 on a payment to such Person.
4.20
Environmental Matters. Except as set forth in Schedule 4.20:
(a)
Each Target Company is and has been in compliance in all material respects with all applicable Environmental Laws, including obtaining,
maintaining in good standing, and complying in all material respects with all Environmental Permits required for its business and operations,
no Action is pending or, to the Company’s Knowledge, threatened to revoke, modify, or terminate any such Environmental Permit,
and, to the Company’s Knowledge, no facts, circumstances, or conditions currently exist that could adversely affect such continued
compliance with Environmental Laws and Environmental Permits or require capital expenditures to achieve or maintain such continued compliance
with Environmental Laws and Environmental Permits.
(b)
No Target Company is the subject of any outstanding Order or Contract with any Governmental Authority or other Person in respect
of any (i) Environmental Laws, (ii) Remedial Action, or (iii) Release or threatened Release of a Hazardous Material. No Target Company
has assumed, contractually or by operation of Law, any Liabilities or obligations under any Environmental Laws.
(c)
No Action has been made or is pending, or to the Company’s Knowledge, threatened against any Target Company or any assets
of a Target Company alleging either or both that a Target Company may be in material violation of any Environmental Law or Environmental
Permit or may have any material Liability under any Environmental Law.
(d)
No Target Company has manufactured, treated, stored, disposed of, arranged for or permitted the disposal of, generated, handled
or Released any Hazardous Material, or owned or operated any property or facility, in a manner that has given or would reasonably be
expected to give rise to any material Liability or obligation under applicable Environmental Laws. No fact, circumstance, or condition
exists in respect of any Target Company or any property currently or formerly owned, operated, or leased by any Target Company or any
property to which a Target Company arranged for the disposal or treatment of Hazardous Materials that could reasonably be expected to
result in a Target Company incurring any material Environmental Liabilities.
(e)
There is no investigation of the business, operations, or currently owned, operated, or leased property of a Target Company or,
to the Company’s Knowledge, previously owned, operated, or leased property of a Target Company pending or, to the Company’s
Knowledge, threatened that could lead to the imposition of any Liens under any Environmental Law or material Environmental Liabilities.
(f)
To the Knowledge of the Company, there is not located at any of the properties of a Target Company any (i) underground storage
tanks, (ii) asbestos-containing material, or (iii) equipment containing polychlorinated biphenyls.
(g)
The Company has provided to Buyer all environmentally related site assessments, audits, studies, reports, analysis and results
of investigations that have been performed in respect of the currently or previously owned, leased, or operated properties of any the
Target Company.
4.21
Transactions with Related Persons. No Target Company nor any of its Related Persons is presently, or in the past three (3) years,
has been, a party to any transaction with a Target Company, including any Contract or other arrangement (a) providing for the furnishing
of services by (other than as officers, directors or employees of the Target Company), (b) providing for the rental of real property
or Personal Property from or (c) otherwise requiring payments to (other than for services or expenses as directors, officers or employees
of the Target Company in the ordinary course of business consistent with past practice) any Related Person or any Person in which any
Related Person has an interest as an owner, officer, manager, director, trustee or partner or in which any Related Person has any direct
or indirect interest (other than the ownership of securities representing no more than two percent (2%) of the outstanding voting power
or economic interest of a publicly traded company). No Target Company has outstanding any Contract or other arrangement or commitment
with any Related Person, and no Related Person owns any real property or Personal Property, or right, tangible or intangible (including
Intellectual Property) which is used in the business of any Target Company. The assets of the Target Companies do not include any receivable
or other obligation from a Related Person, and the Liabilities of the Target Companies do not include any payable or other obligation
or commitment to any Related Person other than employment agreements entered at arm’s length terms. Schedule 4.21
specifically identifies all Contracts, arrangements or commitments set forth on such Schedule
4.21 that cannot be terminated upon sixty (60) days’ notice by the Target Companies without cost or penalty other than employment
agreements entered at arm’s length terms.
4.22
Business Insurance.
(a)
Schedule 4.22(a) lists all insurance policies (by policy number, insurer, coverage period, coverage amount, annual premium
and type of policy) held by a Target Company relating to a Target Company or its business, properties, assets, directors, officers and
employees, copies of which have been provided to Buyer. All premiums due and payable under all such insurance policies have been timely
paid and the Target Companies are otherwise in material compliance with the terms of such insurance policies. Each such insurance policy
(i) is legal, valid, binding, enforceable and in full force and effect and (ii) will continue to be legal, valid, binding, enforceable,
and in full force and effect on identical terms following the Closing. No Target Company has any self-insurance or co-insurance programs.
Since January 1, 2021, no Target Company has received any notice from, or on behalf of, any insurance carrier relating to or involving
any adverse change or any change other than in the ordinary course of business, in the conditions of insurance, any refusal to issue
an insurance policy or non-renewal of a policy.
(b)
Schedule 4.22(b) identifies each individual insurance claim in excess of $50,000 made by a Target Company since January
1, 2021. Each Target Company has reported to its insurers all claims and pending circumstances that would reasonably be expected to result
in a claim, except where such failure to report such a claim would not be reasonably likely to be material to the Target Companies. To
the Knowledge of the Company, no event has occurred, and no condition or circumstance exists, that would reasonably be expected to (with
or without notice or lapse of time) give rise to or serve as a basis for the denial of any such insurance claim. No Target Company has
made any claim against an insurance policy as to which the insurer is denying coverage.
4.23
Top Customers and Suppliers. Schedule 4.23 lists, by dollar volume received or paid, as applicable, for each of (a) the
twelve (12) months ended on December 31, 2022 and (b) the period from January 1, 2023 through the Company Balance Sheet Date, the ten
(10) largest customers of the Target Companies (the “Company Top Customers”) and the ten largest suppliers
of goods or services to the Target Companies (the “Company Top Vendors”), along with the amounts of such dollar
volumes. The relationships of each Target Company with such suppliers and customers are good commercial working relationships and (i)
no Company Top Vendor or Company Top Customer within the last twelve (12) months has cancelled or otherwise terminated, or, to the Company’s
Knowledge, intends to cancel or otherwise terminate, any material relationships of such Person with a Target Company, (ii) no Company
Top Vendor or Company Top Customer has during the last twelve (12) months decreased materially or, to the Company’s Knowledge,
threatened to stop, decrease or limit materially, or intends to modify materially its material relationships with a Target Company or
intends to stop, decrease or limit materially its products or services to any Target Company or its usage or purchase of the products
or services of any Target Company, (iii) to the Company’s Knowledge, no Company Top Vendor or Company Top Customer intends to refuse
to pay any amount due to any Target Company or seek to exercise any remedy against any Target Company, (iv) no Target Company has within
the past two (2) years been engaged in any material dispute with any Company Top Vendor or Company Top Customer, and (v) to the Company’s
Knowledge, the consummation of the Transactions and the Ancillary Documents will not adversely affect the relationship of any Target
Company with any Company Top Vendor or Company Top Customer.
4.24
Certain Business Practices.
(a)
No Target Company, nor any of their respective Representatives acting on their behalf has (i) used any funds for unlawful contributions,
gifts, entertainment or other unlawful expenses relating to political activity, (ii) made any unlawful payment to foreign or domestic
government officials or employees, to foreign or domestic political parties or campaigns or violated any provision of the U.S. Foreign
Corrupt Practices Act of 1977 or any other local or foreign anti-corruption or bribery Law, (iii) made any other unlawful payment or
(iv) since January 1, 2021, directly or indirectly given or agreed to give any unlawful gift or similar benefit in any material amount
to any customer, supplier, governmental employee or other Person who is or may be in a position to help or hinder any Target Company
or assist any Target Company in connection with any actual or proposed transaction.
(b)
The operations of each Target Company are and have been conducted at all times in compliance with money laundering statutes in
all applicable jurisdictions, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued,
administered or enforced by any Governmental Authority, and no Action involving a Target Company with respect to the any of the foregoing
is pending or, to the Knowledge of the Company, threatened.
(c)
No Target Company or any of their respective directors or officers, or, to the Knowledge of the Company, any other Representative
acting on behalf of a Target Company is currently identified on the specially designated nationals or other blocked person list or otherwise
currently subject to any U.S. sanctions administered by OFAC, and no Target Company has, directly or indirectly, used any funds, or loaned,
contributed or otherwise made available such funds to any Subsidiary, joint venture partner or other Person, in connection with any sales
or operations in Cuba, Iran, Syria, Sudan, Myanmar or any other country sanctioned by OFAC or for the purpose of financing the activities
of any Person currently subject to, or otherwise in violation of, any U.S. sanctions administered by OFAC in the last five (5) fiscal
years.
4.25
Investment Company Act. No Target Company is an “investment company” or a Person directly or indirectly “controlled”
by or acting on behalf of an “investment company”, in each case within the meaning of the Investment Company Act.
4.26
Finders and Brokers. Except as set forth in Schedule 4.26, no broker, finder or investment banker is entitled to any brokerage,
finder’s or other fee or commission from the Company, the Target Companies or any of their respective Affiliates in connection
with the Transactions contemplated hereby based upon arrangements made by or on behalf of any Target Company.
4.27
No Subsidies. No Target Company has received any subsidies, aid or relief from any Governmental Authority or organization (including
but not limited to Tax relief), which will be or may have to be repaid due to the execution or the consummation of the transactions contemplated
by this Agreement or otherwise.
4.28
Information Supplied. None of the information supplied or to be supplied by the Company expressly for inclusion or incorporation
by reference: (a) in any current report on Form 8-K, and any exhibits thereto or any other report, form, registration or other filing
made with any Governmental Authority (including the SEC) with respect to the Transactions or any Ancillary Documents; (b) in the Registration
Statement; or (c) in the mailings or other distributions to Buyer’s stockholders and/or prospective investors with respect to the
consummation of the Transactions or in any amendment to any of documents identified in (a) through (c), will, when filed, made available,
mailed or distributed, as the case may be, contain any untrue statement of a material fact or omit to state any material fact required
to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not
misleading. None of the information supplied or to be supplied by the Company expressly for inclusion or incorporation by reference in
any of the Closing Press Release and the Closing Filing will, when filed or distributed, as applicable, contain any untrue statement
of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein,
in light of the circumstances under which they are made, not misleading. Notwithstanding the foregoing, the Company makes no representation,
warranty or covenant with respect to any information supplied by or on behalf of Buyer or its Affiliates.
4.29
Independent Investigation. The Company has conducted its own independent investigation, review and analysis of the business,
results of operations, condition (financial or otherwise) or assets of Buyer and acknowledges that it has been provided adequate access
to the personnel, properties, assets, premises, books and records, and other documents and data of Buyer for such purpose. The Company
acknowledges and agrees that: (a) in making its decision to enter into this Agreement and to consummate the Transactions, it has relied
solely upon its own investigation and the express representations and warranties of Buyer set forth in this Agreement (including the
related portions of the Buyer Disclosure Schedules) and in any certificate delivered to the Company pursuant hereto, and the information
provided by or on behalf of Buyer for the Registration Statement; and (b) none of Buyer or its Representatives have made any representation
or warranty as to Buyer, except as expressly set forth in this Agreement (including the related portions of the Buyer Disclosure Schedules)
or in any certificate delivered to the Company pursuant hereto.
Article
V
REPRESENTATIONS AND WARRANTIES OF THE SELLERS
Except
as set forth in the Seller Disclosure Schedules, the Section numbers of which are numbered to correspond to the Section numbers of this
Agreement to which they refer, each Seller, severally and not jointly, hereby represents and warrants to the Company and Buyer as follows:
5.1
Organization and Standing. Such Seller, if not an individual, is an entity duly organized, validly existing and in good standing
under the Laws of the jurisdiction of its formation and has all requisite power and authority to own, lease and operate its properties
and to carry on its business as now being conducted.
5.2
Authorization; Binding Agreement. Such Seller has all requisite power, authority and legal right and, if an individual, capacity,
to execute and deliver this Agreement and each Ancillary Document to which it is a party, to perform such Seller’s obligations
hereunder and thereunder and to consummate the transactions contemplated hereby and thereby. This Agreement has been, and each Ancillary
Document to which such Seller is or is required to be a party has been or shall be when delivered, duly and validly executed and delivered
by such Seller and assuming the due authorization, execution and delivery of this Agreement and any such Ancillary Document by the other
parties hereto and thereto, constitutes, or when delivered shall constitute, the legal, valid and binding obligation of such Seller,
enforceable against such Seller in accordance with its terms, subject to the Enforceability Exceptions. No other corporate proceedings,
other than as set forth elsewhere in the Agreement, on the part of such Seller or the Company are necessary to authorize the execution
and delivery by such Seller of this Agreement and each Ancillary Document to which such Seller is a party or to consummate the transactions
contemplated hereby and thereby.
5.3
Ownership. Such Seller owns good, valid and marketable title to the Purchased Shares set forth opposite such Seller’s name
on Annex I attached hereto, free and clear of any and all Liens (other than those imposed by applicable securities Laws or the
Company’s Organizational Documents). There are no proxies, voting rights, shareholders’ agreements or other agreements or
understandings, to which such Seller is a party or by which such Seller is bound, with respect to the voting or transfer of any of such
Seller’s Purchased Shares other than this Agreement. Upon delivery of such Seller’s Purchased Shares to Buyer on the Closing
Date in accordance with this Agreement, the entire legal and beneficial interest in such Purchased Shares and good, valid and marketable
title to such Purchased Shares, free and clear of all Liens (other than those imposed by applicable securities Laws or those incurred
by Buyer), will pass to Buyer.
5.4
Governmental Approvals. No Consent of or with any Governmental Authority on the part of such Seller is required to be obtained
or made in connection with the execution, delivery or performance by such Seller of this Agreement or any Ancillary Documents or the
consummation by such Seller of the transactions contemplated hereby or thereby other than (a) such filings as expressly contemplated
by this Agreement, (b) pursuant to Antitrust Laws, (c) any filings required with Nasdaq or the SEC with respect to the Transactions,
(d) applicable requirements, if any, of the Securities Act, the Exchange Act, and/or any state “blue sky” securities Laws,
and the rules and regulations thereunder, and (e) where the failure to obtain or make such Consents or to make such filings or notifications,
would not reasonably be expected to materially impair or delay the ability of such Seller to consummate the Transactions.
5.5
Non-Contravention. The execution and delivery by such Seller of this Agreement and each Ancillary Document to which it is a party
or otherwise bound and the consummation by such Seller of the transactions contemplated hereby and thereby, and compliance by such Seller
with any of the provisions hereof and thereof, will not, (a) if such Seller is an entity, conflict with or violate any provision of such
Seller’s Organizational Documents, (b) conflict with or violate any Law, Order or Consent applicable to such Seller or any of its
properties or assets or (c) (i) violate, conflict with or result in a breach of, (ii) constitute a default (or an event which, with notice
or lapse of time or both, would constitute a default) under, (iii) result in the termination, withdrawal, suspension, cancellation or
modification of, (iv) accelerate the performance required by such Seller under, (v) result in a right of termination or acceleration
under, (vi) give rise to any obligation to make payments or provide compensation under, (vii) result in the creation of any Lien upon
any of the properties or assets of such Seller under, (viii) give rise to any obligation to obtain any third party Consent or provide
any notice to any Person or (ix) give any Person the right to declare a default, exercise any remedy, claim a rebate, chargeback, penalty
or change in delivery schedule, accelerate the maturity or performance, cancel, terminate or modify any right, benefit, obligation or
other term under, any of the terms, conditions or provisions of, any Contract to which such Seller is a party or such Seller or its properties
or assets are otherwise bound, except for any deviations from the foregoing clause (c) that has not had and would not reasonably be expected
to materially impair or delay the ability of such Seller to consummate the Transactions.
5.6
No Litigation. There is no Action pending or, to the Knowledge of such Seller, threatened, nor any Order is outstanding, against
or involving such Seller, whether at law or in equity, before or by any Governmental Authority, which would reasonably be expected to
materially and adversely affect the ability of such Seller to consummate the transactions contemplated by, and discharge its obligations
under, this Agreement and the Ancillary Documents to which such Seller is or is required to be a party.
5.7
Investment Representations. Such Seller (a) is either not a “U.S. Person,” as such term is defined in Rule 902 of
Regulation S under the Securities Act, or is an “accredited investor,” as such term is defined in Rule 501(a) of Regulation
D under the Securities Act; (b) is acquiring its portion of the Exchange Shares for itself for investment purposes only, and not with
a view towards any resale or distribution of such Exchange Shares; (c) has been advised and understands that the Exchange Shares (i)
are being issued in reliance upon one or more exemptions from the registration requirements of the Securities Act and any applicable
state securities Laws, (ii) have not been registered under the Securities Act or any applicable state securities Laws and, therefore,
must be held indefinitely and cannot be resold unless such Exchange Shares are registered under the Securities Act and all applicable
state securities Laws, unless exemptions from registration are available, and (iii) are subject to additional restrictions on transfer
pursuant to such Seller’s Lock-Up Agreement; and (d) is aware that an investment in Buyer is a speculative investment and is subject
to the risk of complete loss. Such Seller does not have any Contract with any Person to sell, transfer, or grant participations to such
Person, or to any third Person, with respect to the Exchange Shares. Such Seller is capable of evaluating the risks and merits of an
investment in Buyer and of protecting its interests in connection with this investment. Such Seller has carefully read and understands
all materials provided by or on behalf of Buyer or its Representatives to such Seller or such Seller’s Representatives pertaining
to an investment in Buyer and has consulted, as such Seller has deemed advisable, with its own attorneys, accountants or investment advisors
with respect to the investment contemplated hereby and its suitability for such Seller. Such Seller acknowledges that the Exchange Shares
are subject to dilution for events not under the control of such Seller. Such Seller has completed its independent inquiry and has relied
fully upon the advice of its own legal counsel, accountant, financial and other Representatives in determining the legal, tax, financial
and other consequences of this Agreement and the transactions contemplated hereby and the suitability of this Agreement and the transactions
contemplated hereby for such Seller and its particular circumstances, and, except as set forth herein, has not relied upon any representations
or advice by Buyer or its Representatives. Such Seller acknowledges and agrees that, except as set forth in Article III (including
the related portions of the Buyer Disclosure Schedules), no representations or warranties have been made by Buyer or any of its Representatives,
and that such Seller has not been guaranteed or represented to by any Person, (i) any specific amount or the event of the distribution
of any cash, property or other interest in Buyer or (ii) the profitability or value of the Exchange Shares in any manner whatsoever.
Such Seller: (A) has been represented by independent counsel (or has had the opportunity to consult with independent counsel and has
declined to do so); (B) has had the full right and opportunity to consult with such Seller’s attorneys and other advisors and has
availed itself of this right and opportunity; (C) has carefully read and fully understands this Agreement in its entirety and has had
it fully explained to it or him by such counsel; (D) is fully aware of the contents hereof and the meaning, intent and legal effect thereof;
and (E) is competent to execute this Agreement and has executed this Agreement free from coercion, duress or undue influence.
5.8
Finders and Brokers. No broker, finder or investment banker is entitled to any brokerage, finder’s or other fee or commission
from such Seller or any of its Affiliates in connection with the transactions contemplated hereby based upon arrangements made by or
on behalf of such Seller.
5.9
Information Supplied. None of the information supplied or to be supplied by such Seller expressly for inclusion or incorporation
by reference: (a) in any Current Report on Form 8-K, and any exhibits thereto or any other report, form, registration or other filing
made with any Governmental Authority (including the SEC) with respect to the Transactions or any Ancillary Documents; (b) in the Registration
Statement; or (c) in the mailings or other distributions to Buyer’s stockholders and/or prospective investors with respect to the
consummation of the Transactions or in any amendment to any of documents identified in (a) through (c), will, when filed, made available,
mailed or distributed, as the case may be, contain any untrue statement of a material fact or omit to state any material fact required
to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not
misleading. None of the information supplied or to be supplied by such Seller expressly for inclusion or incorporation by reference in
any of the Closing Filing and the Closing Press Release will, when filed or distributed, as applicable, contain any untrue statement
of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein,
in light of the circumstances under which they are made, not misleading. Notwithstanding the foregoing, such Seller does not make any
representation, warranty or covenant with respect to any information supplied by or on behalf of Buyer or its Affiliates.
5.10
Independent Investigation. Such Seller has conducted its own independent investigation, review and analysis of the business,
results of operations, condition (financial or otherwise) or assets of Buyer and acknowledges that it has been provided adequate access
to the personnel, properties, assets, premises, books and records, and other documents and data of Buyer for such purpose. Such Seller
acknowledges and agrees that: (a) in making its decision to enter into this Agreement and to consummate the transactions contemplated
hereby, such Seller has relied solely upon its own investigation and the express representations and warranties of Buyer set forth in
this Agreement (including the related portions of the Buyer Disclosure Schedules) and in any certificate delivered to such Seller pursuant
hereto, and the information provided by or on behalf of Buyer for the Registration Statement; and (b) neither Buyer not its Representatives
have made any representation or warranty as to Buyer, except as expressly set forth in this Agreement (including the related portions
of the Buyer Disclosure Schedules) or in any certificate delivered to such Seller pursuant hereto.
Article
VI
OTHER AGREEMENTS OF the PARTIES
6.1
Access and Information. During the period from the Closing until the Conversion (the “Interim Period”),
Buyer shall give, and shall cause its Representatives to give, the Sellers’ Representatives, at reasonable times during normal
business hours and upon reasonable intervals and notice, reasonable access to all offices and other facilities and to all employees,
properties, Contracts, agreements, commitments, books and records, financial and operating data and other information (including Tax
Returns, internal working papers, client files, client Contracts and director service agreements), of or pertaining to Buyer or its Subsidiaries,
as the Sellers’ Representatives may reasonably request regarding Buyer, its Subsidiaries and their respective businesses, assets,
Liabilities, financial condition, prospects, operations, management, employees and other aspects (including unaudited quarterly financial
statements, including a consolidated quarterly balance sheet and income statement, a copy of each material report, schedule and other
document filed with or received by a Governmental Authority pursuant to the requirements of applicable securities Laws, and independent
public accountants’ work papers (subject to the consent or any other conditions required by such accountants, if any)) and cause
each of Buyer’s Representatives to reasonably cooperate with the Sellers’ Representatives in his investigation; provided,
however, that the Sellers’ Representatives shall conduct any such activities in such a manner as not to unreasonably interfere
with the business or operations of Buyer or any of its Subsidiaries.
6.2
Litigation Support. Following the Closing, in the event that and for so long as any party is actively contesting or defending
against any Action in connection with any fact, situation, circumstance, status, condition, activity, practice, plan, occurrence, event,
incident, action, failure to act or transaction that existing on or prior to the Closing Date involving the Company, each of the other
parties will (i) reasonably cooperate with the contesting or defending party and its counsel in the contest or defense, (ii) make available
its personnel at reasonable times during normal business hours and upon reasonable notice and (iii) provide (A) such testimony and (B)
access to its non-privileged books and records as may be reasonably requested in connection with the contest or defense, at the sole
cost and expense of the contesting or defending party (unless such contesting or defending party is entitled to indemnification therefor
Article VII in which case, the costs and expense will be borne by the parties as set forth in Article VII).
6.3
No Trading. The Company and the Sellers each acknowledge and agree that it is aware, and that their respective Affiliates are
aware (and each of their respective Representatives is aware or, upon receipt of any material nonpublic information of Buyer, will be
advised) of the restrictions imposed by U.S. federal securities laws and the rules and regulations of the SEC and Nasdaq promulgated
thereunder or otherwise (the “Federal Securities Laws”) and other applicable foreign and domestic Laws on a
Person possessing material nonpublic information about a publicly traded company. The Company, Buyer, the Sellers and the Sellers Representative
each hereby agree that, while it is in possession of such material nonpublic information, it shall not purchase or sell any securities
of Buyer, communicate such information to any third party, take any other action with respect to Buyer in violation of such Laws, or
cause or encourage any third party to do any of the foregoing.
6.4
Efforts.
(a)
Subject to the terms and conditions of this Agreement, each Party shall use its commercially reasonable efforts, and shall cooperate
fully with the other Parties, to take, or cause to be taken, all actions and to do, or cause to be done, all things reasonably necessary,
proper or advisable under applicable Laws and regulations to consummate the Transactions (including the receipt of all applicable Consents
of Governmental Authorities) and to comply as promptly as practicable with all requirements of Governmental Authorities applicable to
the Transactions. Without limiting the foregoing, each Party shall use its commercially reasonable efforts, and shall cooperate fully
with the other Parties, to as soon as practicable obtain from each holder of more than five percent (5%) of Buyer’s voting stock
and each director and executive officer of Buyer a duly executed Parent Stockholder Support Agreement in the form attached as Exhibit
C.
6.5
Further Assurances. The Parties hereto shall further cooperate with each other and use their respective commercially reasonable
efforts to take or cause to be taken all actions, and do or cause to be done all things, necessary, proper or advisable on their part
under this Agreement and applicable Laws to consummate the Transactions as soon as reasonably practicable, including preparing and filing
as soon as practicable all documentation to effect all necessary notices, reports and other filings.
6.6
Conduct of Business Prior to Conversion.
(a)
Unless the Sellers’ Representative shall otherwise consent in writing (such consent not to be unreasonably withheld, conditioned
or delayed), during the Interim Period, except as expressly contemplated by this Agreement or the Ancillary Documents or as set forth
on Schedule 6.5 Buyer shall, and shall cause its Subsidiaries to, (i) conduct their respective businesses, in all material respects,
in the ordinary course of business consistent with past practice, (ii) comply with all Laws applicable to Buyer and its Subsidiaries
and their respective businesses, assets and employees, and (iii) take all commercially reasonable measures necessary or appropriate to
preserve intact, in all material respects, their respective business organizations, to keep available the services of their respective
managers, directors, officers, employees and consultants, and to preserve the possession, control and condition of their respective material
assets, all as consistent with past practice.
(b)
Without limiting the generality of Section 6.6(a) and except as contemplated, permitted or required by the terms of this
Agreement or the Ancillary Documents or as required by applicable Law or as set forth on Schedule 6.5, during the Interim Period,
without the prior written consent of the Sellers’ Representative (such consent not to be unreasonably withheld, conditioned or
delayed), Buyer shall not, and shall cause its Subsidiaries to not:
(i)
amend, waive or otherwise change, in any respect, its Organizational Documents except as required by applicable Law except
in connection with a Permitted Financing;
(ii)
(A) authorize for issuance, issue, grant, sell, pledge, dispose of or propose to issue, grant, sell, pledge or dispose of any of its
equity securities or any options, warrants, commitments, subscriptions or rights of any kind to acquire or sell any of its equity securities,
or other securities, including any securities convertible into or exchangeable for any of its equity securities or other security interests
of any class and any other equity-based awards, other than the issuance of the Buyer Common Stock issuable upon conversion of the Preferred
Shares or (B)_engage in any hedging transaction with a third Person with respect to such securities, except, in each case of (A) and
(B), pursuant to a Company Benefit Plan or in connection with a Permitted Financing;
(iii)
split, combine, recapitalize or reclassify any of its shares or other equity interests or issue any other securities in respect thereof
or pay or set aside any dividend or other distribution (whether in cash, equity or property or any combination thereof) in respect of
its shares or other equity interests, or directly or indirectly redeem, purchase or otherwise acquire or offer to acquire any of its
securities;
(iv)
incur, create, assume, prepay or otherwise become liable for any Indebtedness (directly, contingently or otherwise) in excess of $500,000
individually or $1,000,000 in the aggregate, make a loan or advance to or investment in any third party, or guarantee or endorse any
Indebtedness, Liability or obligation of any Person, except in connection with a Permitted Financing;
(v)
make or rescind any material election relating to Taxes, settle any claim, action, suit, litigation, proceeding, arbitration,
investigation, audit or controversy relating to Taxes, file any amended Tax Return or claim for refund, or make any material change in
its accounting or Tax policies or procedures, in each case except as required by applicable Law or in compliance with U.S. GAAP;
(vi)
terminate, waive or assign any material right under any Buyer Material Contract or Company Material Contract other than in the ordinary
course of business;
(vii)
fail to maintain its books, accounts and records in all material respects in the ordinary course of business consistent;
(viii)
establish any Subsidiary or enter into any new line of business;
(ix)
fail to use commercially reasonable efforts to keep in force insurance policies or replacement or revised policies providing insurance
coverage with respect to its assets, operations and activities in such amount and scope of coverage substantially similar to that which
is currently in effect;
(x)
revalue any of its material assets or make any material change in accounting methods, principles or practices, except to the extent
required to comply with U.S. GAAP and after consulting Buyer’s outside auditors;
(xi)
waive, release, assign, settle or compromise any claim, action or proceeding (including any suit, action, claim, proceeding or investigation
relating to this Agreement or the transactions contemplated hereby), other than waivers, releases, assignments, settlements or compromises
that involve only the payment of monetary damages (and not the imposition of equitable relief on, or the admission of wrongdoing by,
Buyer or its Subsidiary) not in excess of $500,000 (individually or in the aggregate), or otherwise pay, discharge or satisfy any Actions,
Liabilities or obligations, unless such amount has been reserved in the Buyer Financials;
(xii)
acquire, including by merger, consolidation, acquisition of equity interests or assets, or any other form of business combination, any
corporation, partnership, limited liability company, other business organization or any division thereof, or any material amount of assets
outside the ordinary course of business;
(xiii)
make capital expenditures in excess of $500,000 individually for any project (or set of related projects) or $1,000,000 in the aggregate;
(xiv)
adopt a plan of complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other reorganization;
(xv)
voluntarily incur any Liability or obligation (whether absolute, accrued, contingent or otherwise) in excess of $500,000 individually
or $1,000,000 in the aggregate other than pursuant to the terms of a Contract in existence as of the date of this Agreement or entered
into in the ordinary course of business or in accordance with the terms of this Section 6.6 during the Interim Period, except
in connection with a Permitted Financing;
(xvi)
sell, lease, license, transfer, exchange or swap, mortgage or otherwise pledge or encumber (including securitizations), or otherwise
dispose of any material portion of its properties, assets or rights;
(xvii)
enter into any agreement, understanding or arrangement with respect to the voting of Buyer Common Stock, except in connection with a
Permitted Transaction or Permitted Financing;
(xviii)
take any action that would reasonably be expected to significantly delay or impair the obtaining of any Consents of any Governmental
Authority to be obtained in connection with this Agreement; or
(xix)
authorize or agree to do any of the foregoing actions.
Buyer
shall notify the Company in writing of any such actions taken in accordance with the foregoing proviso and shall use commercially reasonable
efforts to mitigate any negative effects of such actions on Buyer and its Subsidiaries.
6.7
Buyer Public Filings. During the Interim Period, Buyer will keep current and timely file all of its public filings with the SEC
and otherwise comply in all material respects with applicable securities Laws and shall use its commercially reasonable efforts prior
to the Conversion to maintain the listing of Buyer Common Stock on Nasdaq.
6.8
The Registration Statement .
(a)
As promptly as practicable after the date hereof, Buyer shall prepare with the assistance of the Company and file with the SEC
a registration statement on Form S-1, Form S-4 or similar form (as amended or supplemented from time to time, the “Registration
Statement”) in connection with the registration under the Securities Act of the Buyer Securities to be issued under this
Agreement prior to the Closing, and the resale thereof, as applicable, and the Buyer Common Stock underlying the Buyer Preferred Stock,
and will also prepare a proxy statement of Buyer (as amended, the “Proxy Statement”) for the purpose of soliciting
proxies from Buyer stockholders for the matters to be acted upon at the Special Stockholder Meeting.
(b)
The Proxy Statement shall include proxy materials for the purpose of soliciting proxies from Buyer stockholders to vote, at a
special meeting of Buyer stockholders to be called and held for such purpose (the “Special Stockholder Meeting”),
in favor of resolutions approving (A) the issuance of shares of Buyer Common Stock in connection with the Conversion, by the holders
of Buyer Common Stock in accordance with Buyer’s Organizational Documents and the rules and regulations of the SEC and Nasdaq,
(B) amendment of Buyer’s Certificate of Incorporation to authorize sufficient additional shares of Common Stock to permit the Conversion,
(C) the appointment of the members of the Post-Stockholder Approval Buyer Board, in each case in accordance with Section
6.12 hereof, and (D) such other matters as the Company and Buyer shall hereafter mutually determine to be necessary or appropriate
in order to effect the Transactions (the approvals described in foregoing clauses (A) through (D), collectively, the “Stockholder
Approval Matters”), and (E) the adjournment of the Special Stockholder Meeting, if necessary or desirable in the reasonable
determination of Buyer.
(c)
If, on the date one day immediately preceding the date for which the Special Stockholder Meeting is scheduled, Buyer reasonably believes
that it will not receive proxies representing a sufficient number of shares to obtain the Stockholder Approval, whether or not a quorum
is present, or, Buyer will not have sufficient shares of Buyer common stock to constitute a quorum, Buyer may in its sole discretion
make one or more successive postponements or adjournments of the Special Stockholder Meeting as long as such Special Stockholder Meeting
is not postponed more than five days for each postponement or adjournment or an aggregate of ten days for all such postponements or adjournments.
In connection with the Registration Statement and the Proxy Statement, Buyer shall file with the SEC financial and other information
about the Transactions in accordance with applicable Law and applicable proxy solicitation and registration statement rules set forth
in Buyer’s Organizational Documents and the rules and regulations of the SEC and Nasdaq. Buyer shall cooperate and provide the
Company (and its counsel) with a reasonable opportunity to review and comment on the Registration Statement and the Proxy Statement and
any amendment or supplement thereto prior to filing the same with the SEC. The Company shall provide Buyer with such information concerning
the Target Companies and their equity holders, officers, directors, employees, assets, Liabilities, condition (financial or otherwise),
business and operations that may be required or appropriate for inclusion in the Registration Statement or Proxy Statement, or in any
amendments or supplements thereto, which information provided by the Company shall be true and correct and not contain any untrue statement
of a material fact or omit to state a material fact necessary in order to make the statements made, in light of the circumstances under
which they were made, not materially misleading.
(d)
Buyer shall use commercially reasonable best efforts to have the Proxy Statement filed with the SEC as promptly as reasonably practicable.
Buyer shall take any and all reasonable and necessary actions required to satisfy the requirements of the Securities Act, the Exchange
Act and other applicable Laws in connection with the Proxy Statement and the Special Stockholder Meeting, respectively. Each of Buyer
and the Company shall, and shall cause each of its Subsidiaries to, make their respective directors, officers and employees, upon reasonable
advance notice, available to the Company, Buyer and their respective Representatives in connection with the drafting of the public filings
with respect to the Transactions, including the Registration Statement and the Proxy Statement, and responding in a timely manner to
comments from the SEC. Each Party shall promptly correct any information provided by it for use in the Registration Statement and the
Proxy Statement (and other related materials) if and to the extent that such information is determined to have become false or misleading
in any material respect or as otherwise required by applicable Laws. Buyer shall amend or supplement the Proxy Statement and cause the
Proxy Statement, as so amended or supplemented, to be filed with the SEC and to be disseminated to Buyer’s stockholders to the
extent required by applicable Laws and subject to the terms and conditions of this Agreement and Buyer’s Organizational Documents;
provided, however, Buyer may not amend the Proxy Statement without Buyer’s written consent.
(e)
Buyer, with the assistance of the other Parties, shall promptly respond to any SEC comments on the Registration Statement and Proxy Statement
and shall otherwise use their commercially reasonable efforts to cause the Registration Statement and Proxy Statement to “clear”
comments from the SEC and become effective, as applicable. Buyer shall provide the Company with copies of any written comments, and shall
inform the Company of any material oral comments, that Buyer or their respective Representatives receive from the SEC or its staff with
respect to the Registration Statement and Proxy Statement, the Special Stockholder Meeting promptly after the receipt of such comments
and shall give the Company a reasonable opportunity under the circumstances to review and comment on any proposed written or material
oral responses to such comments. Buyer shall use its commercially reasonable efforts to maintain the effectiveness of the Registration
Statement until such time that all restrictive legends have been removed in respect to the Buyer Securities registered under the Registration
Statement pursuant to this Section 6.8.
(f)
As soon as practicable following the Proxy Statement “clearing” comments from the SEC, Buyer shall distribute the Proxy Statement
to Buyer’s stockholders and, pursuant thereto, shall call the Special Stockholder Meeting. Buyer agrees that: (i) Buyer’s
Board shall recommend that the holders of Buyer Common Stock vote to approve the Stockholder Approval Matters and shall use commercially
reasonable efforts to solicit such approval within the timeframe set forth in this Section 6.8, (ii) the Proxy Statement shall
include a statement to the effect that Buyer’s Board recommends that Buyer’s stockholders vote to approve the Stockholder
Approval Matters.
(g)
Buyer shall comply with all applicable Laws, any applicable rules and regulations of Nasdaq, Buyer’s Organizational Documents and
this Agreement in the preparation, filing and distribution of the Proxy Statement, any solicitation of proxies thereunder, the calling
and holding of the Special Stockholder Meeting.
6.9
Nasdaq Change of Control Application. The Parties shall use commercially reasonable best efforts to ensure that the application
for Buyer’s change of control is filed with Nasdaq (the “Nasdaq Change of Control Application”).
Each of the Parties shall use commercially reasonable best efforts to respond to any questions from Nasdaq with respect to the Nasdaq
Change of Control Application promptly following receipt of such questions, but in no event later than ten (10) Business Days following
receipt of such questions.
6.10
Public Announcements.
(a)
The Parties agree that no public release, filing or announcement concerning this Agreement or the Ancillary Documents or the transactions
contemplated hereby or thereby shall be issued by any Party or any of their Affiliates without the prior written consent (not be unreasonably
withheld, conditioned or delayed) of Buyer and the Company, except as such release or announcement may be required by applicable Law
or the rules or regulations of any securities exchange, in which case the applicable Party shall use commercially reasonable efforts
to allow the other Parties reasonable time to comment on, and arrange for any required filing with respect to, such release or announcement
in advance of such issuance.
(b)
The Parties shall mutually agree upon and, as promptly as practicable after the Closing (but in any event within twenty-four (24) hours
thereafter), issue a press release announcing the consummation of the Transactions (the “Closing Press Release”).
Promptly after the issuance of the Closing Press Release and within four (4) Business Days of execution of this Agreement, Buyer shall
file a current report on Form 8-K (the “Closing Filing”) with the Closing Press Release and a description of
the Closing as required by Federal Securities Laws which Buyer shall review, comment upon and approve (which approval shall not be unreasonably
withheld, conditioned or delayed) prior to filing. In connection with the preparation of the Closing Filing, the Closing Press Release,
or any other report, statement, filing notice or application made by or on behalf of a Party to any Governmental Authority or other third
party in connection with the transactions contemplated hereby, each Party shall, upon request by any other Party, furnish the Parties
with all information concerning themselves, their respective directors, officers and equity holders, and such other matters as may be
reasonably necessary or advisable in connection with the transactions contemplated hereby, or any other report, statement, filing, notice
or application made by or on behalf of a Party to any third party and/ or any Governmental Authority in connection with the transactions
contemplated hereby.
6.11
Confidential Information.
(a)
The Company and the Sellers agree that they shall, and shall cause their respective Representatives to: (i) treat and hold in strict
confidence any Buyer Confidential Information, and will not use for any purpose (except in connection with the consummation of the Transactions
or the Ancillary Documents, performing their obligations hereunder or thereunder or enforcing their rights hereunder or thereunder),
nor directly or indirectly disclose, distribute, publish, disseminate or otherwise make available to any third party any of the Buyer
Confidential Information without Buyer’s prior written consent; and (ii) in the event that the Company, any Seller or any of their
respective Representatives becomes legally compelled to disclose any Buyer Confidential Information, (A) provide Buyer to the extent
legally permitted with prompt written notice of such requirement so that Buyer or an Affiliate thereof may seek, at Buyer’s cost,
a protective Order or other remedy or waive compliance with this Section 6.11(a), and (B) in the event that such protective Order
or other remedy is not obtained, or Buyer waives compliance with this Section 6.11(a), furnish only that portion of such Buyer
Confidential Information which is legally required to be provided as advised by outside counsel and to exercise its commercially reasonable
efforts to obtain assurances that confidential treatment will be accorded such Buyer Confidential Information. In the event that this
Agreement is terminated and the transactions contemplated hereby are not consummated, the Company, Buyer and the Sellers shall, and shall
cause their respective Representatives to, promptly deliver to Buyer or destroy (at Buyer’s election) any and all copies (in whatever
form or medium) of Buyer Confidential Information and destroy all notes, memoranda, summaries, analyses, compilations and other writings
related thereto or based thereon.
(b)
Buyer hereby agrees it shall, and shall cause its Representatives to: (i) treat and hold in strict confidence any Company Confidential
Information, and will not use for any purpose (except in connection with the consummation of the Transactions or the Ancillary Documents,
performing its obligations hereunder or thereunder or enforcing its rights hereunder or thereunder), nor directly or indirectly disclose,
distribute, publish, disseminate or otherwise make available to any third party any of the Company Confidential Information without the
Company’s prior written consent; and (ii) in the event that Buyer or any of its Representatives becomes legally compelled to disclose
any Company Confidential Information, (A) provide the Company to the extent legally permitted with prompt written notice of such requirement
so that the Company may seek, at the Company’s sole expense, a protective Order or other remedy or waive compliance with this Section
6.11(b) and (B) in the event that such protective Order or other
remedy is not obtained, or the Company waives compliance with this Section 6.11(b), furnish only that portion of such the Company
Confidential Information which is legally required to be provided as advised by outside counsel and to exercise its commercially reasonable
efforts to obtain assurances that confidential treatment will be accorded such the Company Confidential Information. In the event that
this Agreement is terminated and the transactions contemplated hereby are not consummated, Buyer shall, and shall cause its Representatives
to, promptly deliver to the Company or destroy (at the Company’s election) any and all copies (in whatever form or medium) of the
Company Confidential Information and destroy all notes, memoranda, summaries, analyses, compilations and other writings related thereto
or based thereon. Notwithstanding the foregoing, Buyer and its Representatives shall be permitted to disclose any and all the Company
Confidential Information to the extent required by the Federal Securities Laws.
6.12
Post-Approval Board of Directors . The Parties shall take all necessary action, including causing the directors of Buyer to resign,
so that following the Stockholder Approval, Buyer’s board of directors (the “Post-Stockholder Approval Buyer Board”)
will consist of five (5) individuals. Immediately after the Stockholder Approval, the Parties shall take all necessary action to designate
and appoint to the Post-Stockholder Approval Buyer Board (i) two (2) individuals that are designated by Buyer prior to
the Closing who will be reasonably acceptable to the Company (the “Buyer Director”); and (ii) three (3) individuals
that are designated by the Company prior to the Closing who will be reasonably acceptable to Buyer (the “Company Directors”).
6.13
Indemnification of Directors and Officers; Tail Insurance.
(a)
The Parties agree that all rights to exculpation, indemnification and advancement of expenses existing in favor of the current or former
directors and officers of Buyer and each Person who served as a director, officer, member, trustee or fiduciary of another corporation,
partnership, joint venture, trust, pension or other employee Benefit Plan or enterprise at the request of Buyer (the “D&O
Indemnified Persons”) as provided in Buyer’s Organizational Documents or under any indemnification, employment or
other similar agreements between any D&O Indemnified Person and Buyer, in each case as in effect on the date of this Agreement, shall
survive the date upon which the Buyer obtains Stockholder Approval and continue in full force and effect in accordance with their respective
terms to the extent permitted by applicable Law. For a period of six (6) years after the date upon which the Buyer obtains Stockholder
Approval, Buyer shall cause the Organizational Documents of Buyer to contain provisions no less favorable with respect to exculpation
and indemnification of and advancement of expenses to D&O Indemnified Persons than are set forth as of the date of this Agreement
in the Organizational Documents of Buyer to the extent permitted by applicable Law. The provisions of this Section 6.13 shall
survive the Closing and are intended to be for the benefit of, and shall be enforceable by, each of the D&O Indemnified Persons and
their respective heirs and Representatives.
(b)
For the benefit of Buyer’s directors and officers, Buyer shall be permitted prior to the date upon which the Buyer obtains Stockholder
Approval to obtain and fully pay the premium for a “tail” insurance policy that provides coverage for up to a six-year period
from and after the Stockholder Approval for events occurring prior to the date upon which the Buyer obtains Stockholder Approval (the
“D&O Tail Insurance”) that is substantially equivalent to and in any event not less favorable in the aggregate
than Buyer’s existing policy or, if substantially equivalent insurance coverage is unavailable, the best available coverage. If
obtained, Buyer shall maintain the D&O Tail Insurance in full force and effect, and continue to honor the obligations thereunder,
and Buyer shall timely pay or cause to be paid all premiums with respect to the D&O Tail Insurance.
6.14
Transfer Taxes. All transfer, documentary, sales, use, stamp, registration, indirect and other substantially similar Taxes (including
any indirect capital gains Taxes) and fees incurred in connection with this Agreement (collectively, “Transfer Taxes”)
shall be borne by the party responsible for such Transfer Taxes. The party responsible for such Transfer Taxes shall, at its own expense,
file all necessary Tax Returns and other documentation with respect to all Transfer Taxes, and the Sellers agree to cause the Company
to cooperate in the filing of such Tax Returns and other documentation, including promptly supplying any information in its possession
that is reasonably necessary to complete such Tax Returns and other documentation.
6.15
Tax Matters. Each of the Parties (together with each of its respective Affiliates) shall use its reasonable best efforts to cause,
taken together, the Share Exchange to qualify as an exchange described in Section 351 of the Code, and shall not take any action or fail
to take any action that could reasonably be expected to impede or prevent, taken together, the Share Exchange from qualifying as an exchange
described in Section 351 of the Code.
6.16
Section 16 Matters. Subject to the following sentence, prior to the Closing, Buyer and the Company will take all such steps as
may be required (to the extent permitted under applicable Laws and no-action letters issued by the SEC) to cause any acquisition of shares
of Buyer Common Stock (including derivative securities with respect to shares of Buyer Common Stock) by each Person (including any director
by deputization) who is or will be subject to the reporting requirements of Section 16(a) of the Exchange Act with respect to Buyer,
to be exempt under Rule 16b-3 under the Exchange Act.
6.17
Delivery of Audited Company Financial Statements.
(a)
As soon as reasonably practicable following the date of this Agreement (and in any event no later than February 5, 2024), Sellers shall
use commercially reasonably best efforts to cause the Company to complete an audit of the financial statements of the Target Companies
as of December 31, 2022, and December 31, 2021, and for the fiscal years then ended which (i) shall be prepared in accordance with GAAP,
applied on a consistent basis throughout the periods indicated (except, in the case of any audited financial statements, as may be specifically
indicated in the notes thereto and subject, in the case of any unaudited financial statements, to normal year-end audit adjustments (none
of which is expected to be, individually or in the aggregate, material) and the absence of notes thereto), (ii) shall fairly present,
in all material respects, the financial position, results of operations, stockholders’ deficit and cash flows of Target Companies,
as applicable, as at the date thereof and for the period indicated therein (subject to, in the case of any unaudited financial statements,
normal year-end audit adjustments (none of which is expected to be, individually or in the aggregate, material)), (iii) in the case of
any audited financial statements, shall be (A) certified as audited in accordance with GAAP and the standards of the PCAOB by a PCAOB
qualified auditor upon the filing of the initial Registration Statement, (B) shall contain an unqualified report of the Target Companies’
auditors and (C) shall be substantially identical in all material respects to the unaudited financial statements from the same period
that have been provided to the Buyer and (iv) shall comply in all material respects with the applicable accounting requirements
and with the rules and regulations of the SEC, the Exchange Act and the Securities Act in effect as of the respective dates of delivery
(including Regulation S-X or Regulation S-K, as applicable). In addition, each Seller shall reasonably cooperate with Buyer and the Company
to cause the Company to use its best efforts to deliver to Buyer any financial statements or similar reports of the Target Companies,
required to be included in the Registration Statement or any other filings to be made with the SEC in connection with the transactions
contemplated by this Agreement or any other Transaction Document.
(b)
The Sellers shall reasonably cooperate with Buyer to cause the Target Companies (i) to assist, upon advance written notice, during normal
business hours and in a manner such as to not unreasonably interfere with the normal operation of the Target Companies in causing to
be prepared in a timely manner any other financial information or statements (including customary pro forma financial statements) that
are required to be included in the Registration Statement and any other filings to be made by Buyer with the SEC in connection with the
Transactions and (ii) to obtain the consents of the Target Companies’ auditors, if applicable, with respect thereto as may be required
by applicable Law or requested by the SEC.
6.18
Exchange of Company Stock Options. At the time of the Conversion, each outstanding Company Stock Option that is outstanding under
any Company Equity Plan, whether vested unvested, shall be assumed by the Buyer and converted into the right to receive (a) an option
to acquire shares of Buyer Common Stock (each, an “Assumed Option”) or (b) such other derivative security as
Buyer and the Company my agree, subject in either case to substantially the same terms and conditions as were applicable to such Company
Stock Option immediately before the Closing (including, without limitation, the vesting and acceleration provisions therein), except
any references therein to the Company or Company Common Shares will instead mean the Buyer and Buyer Common Stock, respectively. Each
Assumed Option shall: (i) represent the right to acquire a number of shares of Buyer Common Stock (as rounded up to the nearest whole
number) equal to the product of (A) the number of Company Common Shares that were subject to the corresponding Company Option immediately
prior to the Closing, multiplied by (B) the Exchange Ratio; and (ii) have an exercise price (as rounded down to the nearest whole cent)
equal to the quotient of (A) the exercise price of the corresponding Company Option, divided by (B) the Exchange Ratio.
6.19
CFIUS.
(a)
Pursuant to this Section 6.19 and in accordance with the DPA, at the election of Buyer, and
unless Buyer notifies the Company otherwise, or upon the request of CFIUS, the Sellers, the Company, and the Buyer shall submit or cause
to be submitted to CFIUS a joint declaration or notice (“CFIUS Filing”) with respect to the Transactions as
promptly as practicable, but in no event later than sixty (60) Days after the date of this Agreement. The Sellers, Company, and/or the
Buyer shall prepare and submit a draft CFIUS Filing, and then work diligently to promptly finalize and file a final CFIUS Filing addressing
any comments or questions received from CFIUS on the draft CFIUS Filing. The Parties shall, and shall cause their respective Affiliates,
to assist with and provide any information and documents need for the preparation of the CFIUS filing and to provide CFIUS with any additional
or supplemental information requested by CFIUS during its assessment, (and, if applicable) review, (and, if applicable, investigation)
process within three (3) Business Days (in the case of a CFIUS Notice) and within two (2) Business Days (in the case of a CFIUS Declaration)
or by the deadline stated in the inquiry from CFIUS, unless an extension is granted in writing by CFIUS. In the case of filing of a CFIUS
Notice with respect to the Transactions, the filing fee paid to CFIUS shall be at Buyer’s expense.
(b)
The Parties shall, and shall cause their respective Affiliates to cooperate in good faith to: (i) promptly inform each other Party,
or its counsel, upon receipt of any substantive communication received by such Party from, or given by such Party to CFIUS regarding
any such filing, submission, proceeding or the Transactions; (ii) permit each other Party or its counsel to review and discuss
reasonably in advance, and consider in good faith the views of each other Party or its counsel in connection with, any proposed
substantive communication to be given by it to CFIUS, (iii) give each other Party or its counsel reasonable advance notice of any
in-person meeting, and any conference call that is initiated by such Party or scheduled in advance with CFIUS or such private party,
and not participate independently therein without first giving each other Party or its counsel reasonable opportunity to attend and
participate therein or, in the event such other Party or its counsel does not attend or participate therein, consulting with such
other Party or its counsel reasonably in advance and considering in good faith the views of such other Party or its counsel in
connection therewith.
(c)
The Parties, in cooperation with each other, shall use reasonable best efforts to take all such actions within their respective powers
to obtain the CFIUS Approval, and, without limiting the foregoing, the Parties shall, after reasonable negotiation efforts, agree to
such requirements or conditions to mitigate any national security concerns as may be requested or required by CFIUS in connection with,
or as a condition of, the CFIUS Approval, including entering into a mitigation agreement, letter of assurance, or national security agreement,
but provided: (1) the Parties shall have no obligation to (A) propose, negotiate, commit to or effect, by consent decree, hold separate
order, agreement or otherwise, the sale, transfer, license, divestiture or other disposition of, any of the businesses, product lines
or assets of Buyer or any of its Affiliates or of the Sellers, (B) terminate existing, or create new, relationships, contractual rights
or obligations of Buyer or its Affiliates, (C) effect any other change or restructuring of Buyer or its Affiliates, or (D) otherwise
take or commit to take any actions reasonably expected to have a material adverse effect on the operation of the business of the Sellers
or that interfere with Buyer’s ability to control the Company or Buyer’s ability to direct the management and policies of
the business of the Company in any material respect; and (2) the Company and the Sellers shall not take or agree to take any of the foregoing
actions without the prior written consent of Buyer.
Article
VII
Survival
7.1
Survival.
(a)
Subject to the limitations and other provisions of this Agreement, the representations and warranties contained in Articles III and
IV herein shall survive the Closing and shall remain in full force and effect until the Conversion and the representations and warranties
contained in Article V herein shall survive the Closing and remain in full force and effect until the first anniversary of the
Closing. Notwithstanding the foregoing, any claims asserted in good faith with reasonable specificity (to the extent known at such time)
and in writing by notice from the non-breaching Party to the breaching Party prior to the expiration date of the applicable survival
period shall not thereafter be barred by the expiration of the relevant representation or warranty and such claims shall survive until
finally resolved.
(b)
The covenants contained in this Agreement shall survive the Closing and remain in full force and effect until the Conversion.
7.2
Conversion Adjustment
(a)
Buyer Claims. Until the earlier of (i) Stockholder Approval or (ii) June 30, 2024 (the “Claim Deadline”),
Buyer may assert Claims against the Company and Sellers for any and all loss, liability, damage, claim, penalty, fine, forfeiture, action,
fee, costs and expense (collectively, “Losses”) incurred or sustained by, or imposed upon, Buyer based upon,
arising out of, with respect to or by reason of: (i) any inaccuracy in or breach of any of the representations or warranties made by
the Company contained in this Agreement or in any certificate or instrument delivered by or on behalf of the Company pursuant to this
Agreement, as of the date such representation or warranty was made or as if such representation or warranty was made on and as of the
Closing Date (except for representations and warranties that expressly relate to a specified date, the inaccuracy in or breach of which
will be determined with reference to such specified date); or (ii) any breach or non-fulfillment of any covenant, agreement or obligation
to be performed by the Company pursuant to this Agreement.
(b)
Sellers’ Claims. Until the Claim Deadline, the Sellers’ Representative, acting on behalf of the Sellers, may assert
Claims against Buyer for any Loss incurred or sustained by, or imposed upon, the Sellers based upon, arising out of, with respect to
or by reason of: (i) any inaccuracy in or breach of any of the representations or warranties of Buyer contained in this Agreement or
in any certificate or instrument delivered by or on behalf of Buyer pursuant to this Agreement, as of the date such representation or
warranty was made or as if such representation or warranty was made on and as of the Closing Date (except for representations and warranties
that expressly relate to a specified date, the inaccuracy in or breach of which will be determined with reference to such specified date);
or (ii) any breach or non-fulfillment of any covenant, agreement or obligation to be performed by Buyer pursuant to this Agreement.
(c)
Adjustment. Subject to the limitations set forth herein, the number of shares of Common Stock issued upon Conversion shall be
increased or decreased by a number determined by dividing the Net Adjustment (as defined herein) by the ten-day VWAP of the Buyer Common
Stock for the ten (10)-day period preceding the third day prior to the Closing Date and rounding down to the nearest whole share; provided,
however, that (i) there shall be no adjustment to the number of shares of Common Stock issued upon Conversion if the Net Adjustment is
less than $1,000,000 and (ii) the number of shares of Common Stock issued upon Conversion shall not be increased or decreased by more
than 10% of the number of shares of Common Stock that would be issuable absent such adjustment.
(d)
Procedure. Buyer and the Sellers’ Representative may assert Claims pursuant to paragraphs (a) or (b), respectively, of this
Section 7.2 by delivering written notice to the other Parties on or prior to the Claim Deadline setting forth in reasonable detail
the basis for the Claim or Claims and a good faith estimate of the Loss arising from each Claim. Within two (2) Business Days of the
Claim Deadline, the Buyer and the Sellers’ Representative shall meet and use reasonable good faith efforts to resolve any disagreements
as to any Claims made in a manner pursuant to this Section 7.2. If they do not obtain a final resolution within five (5) Business
Days of the Claim Deadline, Buyer and the Sellers’ Representative shall jointly retain Mazars USA, LLP or one of its Affiliates,
or another mutually acceptable dispute resolution firm (the “Firm”), to resolve any remaining disagreements.
Buyer and the Sellers’ Representative shall direct the Firm to render a determination as soon as possible, and the Firm shall use
commercially reasonable efforts to render a determination within thirty (30) days after its retention and Buyer, the Sellers’ Representative
and their respective agents shall cooperate with the Firm during its engagement. The Firm may consider only those items and amounts in
the notice of a Claim which Buyer and the Sellers’ Representative are unable to resolve. In resolving any disputed item, the Firm
may not assign a value to any item greater than the greatest value for such item claimed by either party or less than the smallest value
for such item claimed by either party. The Firm’s determination shall be based solely on written submissions or oral presentations
by Buyer and the Sellers’ Representative or their respective agents that are in accordance with the terms and procedures set forth
in this Agreement (i.e., not on independent review) and on the definitions included herein. Without the prior consent of the Sellers’
Representative (in the case of Buyer) or Buyer (in the case of the Sellers’ Representative), no Party (or their respective Representatives)
may have any ex parte conversations or meetings with the Firm, and there may not be any hearings or oral examinations, testimony, depositions,
discovery or other similar proceedings. Each of Buyer and the Sellers’ Representative shall execute a reasonable and customary
engagement letter consistent with the terms of this Agreement, if such letter is required by the Firm. Absent manifest error or fraud,
the determination of the Firm shall be final, conclusive and binding upon Buyer and the Sellers’ Representative and enforceable
as an arbitration award in any court of competent jurisdiction under the terms of the Federal Arbitration Act or its state Law equivalents.
The costs and expenses of the Firm shall be borne equally by Buyer, on the one hand, and the Sellers’ Representative, on the other
hand; provided, that, the Firm shall have the power, in its sole discretion, to allocate costs and expenses between the Sellers’
Representative, on the one hand, and Buyer, on the other hand, based upon the portion of the contested amount not awarded to each party
bears to the contested amount actually claimed by such party. As used herein, “Net Adjustment” means the absolute
value of the difference between the aggregate adjustment in favor of each party with respect to Losses that is agreed by Buyer and the
Sellers’ Representative or determined by the Firm.
(e)
Solely for purposes of calculating the amount of any Losses arising out of or caused by any breach of any representation or warranty
in this Agreement, any references in any such representation or warranty to “material,” or “Material Adverse Effect”
or similar qualifications shall be disregarded.
7.3
Sellers’ Indemnification
(a)
Indemnification by Each Seller. Each Seller, severally and not jointly, shall indemnify and defend each of Buyer and its Affiliates
and their respective Representatives (collectively, the “Buyer Indemnitees”) against, and shall hold each of
them harmless from and against, and shall pay and reimburse each of them for, any and all Losses incurred or sustained by, or imposed
upon, the Buyer Indemnitees based upon, arising out of, with respect to or by reason of: (i) any inaccuracy in or breach of any of the
representations or warranties of such Seller contained in this Agreement or in any certificate or instrument delivered by or on behalf
of such Seller pursuant to this Agreement, as of the date such representation or warranty was made or as if such representation or warranty
was made on and as of the Closing Date (except for representations and warranties that expressly relate to a specified date, the inaccuracy
in or breach of which will be determined with reference to such specified date); (ii) breach or non-fulfillment of any covenant, agreement
or obligation to be performed by such Seller pursuant to this Agreement.
(b)
Limitations. Notwithstanding anything to the contrary contained herein, the Parties expressly acknowledge and agree that any payment
due from any Seller in respect of an indemnification claim by any Buyer Indemnitee hereunder shall solely be satisfied by recourse to
the Exchange Shares and the shares of Buyer Common Stock issuable upon the Conversion, with each share of Buyer Common Stock valued at
the same price per share of Buyer Common Stock used to determine Exchange Ratio.
(c)
Indemnification Procedures.
(i)
Direct Claims. Any Action by a Buyer Indemnitee on account of
a Loss (a “Direct Claim”) subject to indemnification pursuant to this Section 7.3 shall be asserted
by such Buyer Indemnitee giving the Seller reasonably prompt written notice thereof, but in any event not later than thirty (30) days
after the Buyer Indemnitee becomes aware of such Direct Claim. The failure to give such prompt written notice shall not, however, relieve
the Seller of its indemnification obligations, except and only to the extent that the Seller forfeits rights or defenses by reason of
such failure. Such notice by the Buyer Indemnitee shall describe the Direct Claim in reasonable detail, shall include copies of all material
written evidence thereof and shall indicate the estimated amount, if reasonably practicable, of the Loss that has been or may be sustained
by the Buyer Indemnitee. The Seller shall have thirty (30) days after its receipt of such notice to respond in writing to such Direct
Claim. The Buyer Indemnitee shall allow the Seller and its professional advisors to investigate the matter or circumstance alleged to
give rise to the Direct Claim, and whether and to what extent any amount is payable in respect of the Direct Claim and the Buyer Indemnitee
shall assist the Seller’s investigation by giving such information and assistance (including access to the Company’s premises
and personnel and the right to examine and copy any accounts, documents or records) as the Seller or any of its professional advisors
may reasonably request. If the Seller does not so respond within such thirty (30)-day period, the Seller shall be deemed to have rejected
such claim, in which case the Buyer Indemnitee shall be free to pursue such remedies as may be available to the Buyer Indemnitee on the
terms and subject to the provisions of this Agreement.
(ii)
Third Party Claims.
(A)
If any Buyer Indemnitee receives notice of the assertion or commencement
of any Action made or brought by any Person who is not a party to this Agreement or an Affiliate of a party to this Agreement or a Representative
of the foregoing (a “Third Party Claim”) against such Buyer Indemnitee with respect to which the Seller is
obligated to provide indemnification under this Section 7.3, the Buyer Indemnitee shall give the Seller reasonably prompt written
notice thereof, but in any event not later than thirty (30) calendar days after receipt of such notice of such Third Party Claim. The
failure to give such prompt written notice shall not, however, relieve the Seller of its indemnification obligations, except and only
to the extent that the Seller forfeits rights or defenses by reason of such failure. Such notice by the Buyer Indemnitee shall describe
the Third Party Claim in reasonable detail, shall include copies of all material written evidence thereof and shall indicate the estimated
amount, if reasonably practicable, of the Loss that has been or may be sustained by the Buyer Indemnitee. The Seller shall have the right
to participate in, or by giving written notice to the Buyer Indemnitee, to assume the defense of any Third Party Claim at the Seller’s
expense and by the Seller’s own counsel, and the Buyer Indemnitee shall cooperate in good faith in such defense; provided, that
Seller shall not have the right to assume the defense of any such Third Party Claim that (x) is asserted directly by or on behalf of
a Person that is a supplier or customer of the Company, or (y) seeks an injunction or other equitable relief against the Indemnified
Party. In the event that the Seller assumes the defense of any Third Party Claim, subject to this Section 7.3(c), it shall have
the right to take such action as it deems necessary to avoid, dispute, defend, appeal or make counterclaims pertaining to any such Third
Party Claim in the name and on behalf of the Buyer Indemnitee. The Buyer Indemnitee shall have the right to participate in the defense
of any Third Party Claim with counsel selected by it subject to the Seller’s right to assume the defense thereof. The fees and
disbursements of such counsel shall be at the expense of the Buyer Indemnitee, provided, that if in the reasonable opinion of counsel
to the Buyer Indemnitee, a Buyer Indemnitee is a named defendant and (A) there are legal defenses available to such Buyer Indemnitee
that are different from or additional to those available to the Seller; or (B) there exists a conflict of interest between the Seller
and the Buyer Indemnitee that cannot be waived. If the Seller elects not to defend such Third Party Claim, or fails to diligently prosecute
the defense of such Third Party Claim, the Buyer Indemnitee may pay, compromise, and/or defend such Third Party Claim and seek indemnification
for any and all Losses based upon, arising from or relating to such Third Party Claim. Sellers and Buyer shall cooperate with each other
in all reasonable respects in connection with the defense of any Third Party Claim, including making available (subject to the provisions
of Section 6.8) records relating to such Third Party Claim and furnishing, without expense (other than reimbursement of actual
out-of-pocket expenses) to the defending party, management employees of the non- defending party as may be reasonably necessary for the
preparation of the defense of such Third Party Claim.
(B)
Notwithstanding any other provision of this Agreement, the Seller shall
not enter into settlement of any Third Party Claim without the prior written consent of the Buyer Indemnitee, except as provided in this
Section 7.3. If a firm offer is made to settle a Third Party Claim without leading to liability or the creation of a financial
or other obligation on the part of the Buyer Indemnitee and provides, in customary form, for the unconditional release of each Buyer
Indemnitee from all liabilities and obligations in connection with such Third Party Claim and the Seller desires to accept and agree
to such offer, the Seller shall give written notice to that effect to the Buyer Indemnitee. If the Buyer Indemnitee fails to consent
to such firm offer within ten (10) days after its receipt of such notice, the Buyer Indemnitee may continue to contest or defend such
Third Party Claim and, in such event, the maximum liability of the Seller as to such Third Party Claim shall not exceed the amount of
such settlement offer. If the Buyer Indemnitee fails to consent to such firm offer and also fails to assume defense of such Third Party
Claim, the Seller may settle the Third Party Claim upon the terms set forth in such firm offer to settle such Third Party Claim. If the
Buyer Indemnitee has assumed the defense pursuant to Section Seller (which consent shall not be unreasonably withheld, conditioned
or delayed).
7.4
Exclusive Remedies. Subject to Section 9.6, the Parties acknowledge and agree that their
sole and exclusive remedy with respect to any and all claims (other than claims arising from fraud, criminal activity or willful misconduct
on the part of a Party hereto in connection with the transactions contemplated by this Agreement) for any breach of any representation,
warranty, covenant, agreement or obligation set forth herein or otherwise relating to the subject matter of this Agreement, shall be
pursuant to the provisions set forth in this Article VII. In furtherance of the foregoing, each Party hereby waives, to the fullest
extent permitted under Law, any and all rights, claims and causes of action for any breach of any representation, warranty, covenant,
agreement or obligation set forth herein or otherwise relating to the subject matter of this Agreement it may have against the other
Parties hereto and their Affiliates and each of their respective Representatives arising under or based upon any Law, except pursuant
to the provisions set forth in this Article VII. Nothing in this Section 7.4 shall limit any Person’s right to seek
and obtain any equitable relief to which any Person shall be entitled or to seek any remedy on account of any Party’s fraudulent,
criminal or intentional misconduct.
7.5
Share Escrow. In the event that at the time of the Conversion a Claim that has been timely asserted by Buyer or its Affiliates
remains unresolved pursuant to the provisions of this Article VII, subject to the limitations and other provisions of this Agreement,
Buyer shall issue all shares of Buyer Common Stock valued in excess of the aggregate amount of all unresolved Claims by Buyer or its
Affiliates and shall deposit the remaining shares in the amount of the unresolved Claim into an escrow account with a third party escrow
agent to be agreed to between Buyer and the Seller Representative. In the event that at the time of the Conversion a Claim that has been
timely asserted by the Sellers’ Representative remains unresolved pursuant to the provisions of this Article VII, Buyer
shall also deposit additional shares of Buyer Common Stock in the amount of the unresolved Claim into an escrow account with a third
party escrow agent to be agreed to between Buyer and the Seller Representative.
Article
VIII
WAIVERS AND Releases
8.1
Release and Covenant Not to Sue. Effective as of the Closing, to the fullest extent permitted by applicable Law, each Seller,
on behalf of itself and its Affiliates that owns any share or other equity interest in or of such Seller (the “Releasing
Persons”), hereby releases and discharges the Target Companies and the Buyer from and against any and all Actions, obligations,
agreements, debts and Liabilities whatsoever, whether known or unknown, both at law and in equity, which such Releasing Person now has,
has ever had or may hereafter have against the Target Companies arising on or prior to the Closing Date or on account of or arising out
of any matter occurring on or prior to the Closing Date, including any rights to indemnification or reimbursement from a Target Company,
whether pursuant to its Organizational Documents, Contract or otherwise, and whether or not relating to Claims pending on, or asserted
after, the Closing Date. From and after the Closing, each Releasing Person hereby irrevocably covenants to refrain from, directly or
indirectly, asserting any Action, or commencing or causing to be commenced, any Action of any kind against the Target Companies or their
respective Affiliates, based upon any matter purported to be released hereby. Notwithstanding anything herein to the contrary, the releases
and restrictions set forth herein shall not apply to any Claims a Releasing Person may have against any party pursuant to the terms and
conditions of this Agreement or any Ancillary Document or any of the other matters set forth on Schedule 8.1.
Article
IX
MISCELLANEOUS
9.1
Notices. All notices, consents, waivers and other communications hereunder shall be in writing and shall be deemed to have been
duly given when delivered (i) in person, (ii) if sent by email on a Business Day before 11:59 p.m. (recipient’s time), when transmitted;
(iii) if sent by email on a day other than a Business Day, or if sent by email after 11:59 p.m. (recipient’s time), on the Business
Day following the date when transmitted; (iv) one Business Day after being sent, if sent by reputable, nationally recognized overnight
courier service or (v) three (3) Business Days after being mailed, if sent by registered or certified mail, pre-paid and return receipt
requested, in each case to the applicable Party at the following addresses (or at such other address for a Party as shall be specified
by like notice):
If
to Buyer prior to or after the Closing, to:
Blue
Water Biotech, Inc.
201 East Fifth Street, Suite 1900
Cincinnati, Ohio 45202
Attn: Dr. Neil Campbell, CEO
Telephone No.: (301) 792-4345
E-mail: ncampbell@bwbioinc.com
|
with
a copy (which will not constitute notice) to:
Ellenoff
Grossman & Schole LLP
1251 Avenue of the Americas
New York, New York 10020
Attn: Barry I. Grossman, Esq.
David
Landau, Esq.
Telephone No.: (212) 370-1300
E-mail: bigrossman@egsllp.com and
dlandau@egsllp.com
|
If
to the Company prior to the Conversion, to:
Proteomedix
AG
Wagistrasse 23
8952 Schlieren
Switzerland
Attn: Ralph Schiess, CEO
Telephone No.: +41 44 733 40 90
E-mail: schiess@proteomedix.com
|
with
a copy (which will not constitute notice) to:
Nelson
Mullins Riley & Scarborough LLP
One Financial Center
Boston, MA 02111
Attn:
Benjamin M. Hron
Telephone No.: (617) 217-4607
E-mail: ben.hron@nelsonmullins.com |
If
to any Seller, to:
the address
of such Seller as set forth underneath such Seller’s signature on the signature page hereto
|
with
a copy (which will not constitute notice) to:
VISCHER
AG
Aeschenvorstadt 4
P.O. Box, CH-4010 Basel
Attn: Dr. Matthias Staehelin
Telephone No.: +41 58 211 33 53
E-mail: mstaehelin@vischer.com
|
If
to the Sellers’ Representative:
Thomas
Meier
c/o Viopas Venture Consulting GmbH
Thiersteinerallee 17; CH-4053 Basel, Switzerland
Telephone: +41 78 756 34 05
Email: thomas@viopasventure.ch
|
with
a copy (which will not constitute notice) to:
VISCHER
AG
Aeschenvorstadt 4
P.O. Box, CH-4010 Basel
Attn: Dr. Matthias Staehelin
Telephone No.: +41 58 211 33 53
E-mail: mstaehelin@vischer.com
|
If
to the Company after the Conversion, to:
Blue
Water Biotech, Inc.
201 East Fifth Street, Suite 1900
Cincinnati, Ohio 45202
Attn: Dr. Neil Campbell, CEO
Telephone No.: (301) 792-4345
E-mail: ncampbell@bwbioinc.com |
with
a copy (which will not constitute notice) to:
VISCHER
AG
Aeschenvorstadt 4
P.O. Box, CH-4010 Basel
Attn: Dr. Matthias Staehelin
Telephone No.: +41 58 211 33 53
E-mail: mstaehelin@vischer.com
|
9.2
Binding Effect; Assignment. This Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of
the Parties hereto and their respective successors and permitted assigns. This Agreement shall not be assigned by operation of Law or
otherwise without the prior written consent of Buyer and the Company (and after the Closing, the Sellers’ Representative), and
any assignment without such consent shall be null and void; provided that no such assignment shall relieve the assigning Party
of its obligations hereunder.
9.3
Third Parties. Except for the rights of the D&O Indemnified Persons set forth in Section 6.13, which the Parties acknowledge
and agree are express third party beneficiaries of this Agreement, nothing contained in this Agreement or in any instrument or document
executed by any party in connection with the transactions contemplated hereby shall create any rights in, or be deemed to have been executed
for the benefit of, any Person that is not a Party hereto or thereto or a successor or permitted assign of such a Party.
9.4
Governing Law; Jurisdiction. This Agreement shall be governed by, construed and enforced in accordance with the Laws of the State
of Delaware without regard to the conflict of laws principles thereof except for the transfer of the Purchased Shares. including ownership,
any related rights and the items to be delivered in connection therewith under Section 2.2(f) to (i), which shall be governed
by and construed in accordance with the substantive law of Switzerland, excluding the principles of international private law. All Actions
arising out of or relating to this Agreement shall be heard and determined exclusively in any state or federal court located in Delaware
(or in any appellate court thereof) (the “Specified Courts”). Each Party hereto hereby (a) submits to
the exclusive jurisdiction of any Specified Court for the purpose of any Action arising out of or relating to this Agreement brought
by any Party hereto and (b) irrevocably waives, and agrees not to assert by way of motion, defense or otherwise, in any such Action,
any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from
attachment or execution, that the Action is brought in an inconvenient forum, that the venue of the Action is improper, or that this
Agreement or the transactions contemplated hereby may not be enforced in or by any Specified Court. Each Party agrees that a final judgment
in any Action shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided
by Law. Each Party irrevocably consents to the service of the summons and complaint and any other process in any other Action relating
to the Transactions, on behalf of itself, or its property, by personal delivery of copies of such process to such Party at the applicable
address set forth in Section 9.1. Nothing in this Section 9.4 shall affect the right of any Party to serve legal process
in any other manner permitted by Law.
9.5 WAIVER
OF JURY TRIAL. EACH OF THE PARTIES HERETO HEREBY WAIVES TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW ANY RIGHT IT MAY HAVE
TO A TRIAL BY JURY WITH RESPECT TO ANY ACTION DIRECTLY OR INDIRECTLY ARISING OUT OF, UNDER OR IN CONNECTION WITH THIS AGREEMENT OR
THE TRANSACTIONS CONTEMPLATED HEREBY. EACH PARTY HERETO (A) CERTIFIES THAT NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED,
EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF ANY ACTION, SEEK TO ENFORCE THAT FOREGOING WAIVER AND
(B) ACKNOWLEDGES THAT IT AND THE OTHER PARTIES HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS,
THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 9.5.
9.6
Specific Performance. Each Party acknowledges that the rights of each Party to consummate the transactions contemplated hereby
are unique, recognizes and affirms that in the event of a breach of this Agreement by any Party, money damages may be inadequate and
the non-breaching Parties may have not adequate remedy at law, and agree that irreparable damage would occur in the event that any of
the provisions of this Agreement were not performed by an applicable Party in accordance with their specific terms or were otherwise
breached. Accordingly, each Party shall be entitled to seek an injunction or restraining order to prevent breaches of this Agreement
and to seek to enforce specifically the terms and provisions hereof, without the requirement to post any bond or other security or to
prove that money damages would be inadequate, this being in addition to any other right or remedy to which such Party may be entitled
under this Agreement, at law or in equity.
9.7
Severability. In case any provision in this Agreement shall be held invalid, illegal or unenforceable in a jurisdiction, such
provision shall be modified or deleted, as to the jurisdiction involved, only to the extent necessary to render the same valid, legal
and enforceable, and the validity, legality and enforceability of the remaining provisions hereof shall not in any way be affected or
impaired thereby nor shall the validity, legality or enforceability of such provision be affected thereby in any other jurisdiction.
Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties will substitute
for any invalid, illegal or unenforceable provision a suitable and equitable provision that carries out, so far as may be valid, legal
and enforceable, the intent and purpose of such invalid, illegal or unenforceable provision.
9.8
Amendment. This Agreement may be amended, supplemented or modified only by execution of a written instrument signed by Buyer,
the Company and the Sellers’ Representative; provided that no amendment, supplementation or modification shall affect a Seller
in a manner materially and adversely disproportionate to the other Sellers without the prior written consent of such Seller, provided,
that, after approval of the Transactions by Buyer stockholders, as applicable, no amendment may be made which by Law requires further
approval by such stockholders without such further approval.
9.9
Waiver. Each of Buyer and the Company on behalf of itself and its Affiliates, and each Seller on its behalf, may in its sole discretion
(i) extend the time for the performance of any obligation or other act of any other non-Affiliated Party hereto, (ii) waive any
inaccuracy in the representations and warranties by such other non-Affiliated Party contained herein or in any document delivered pursuant
hereto and (iii) waive compliance by such other non-Affiliated Party with any covenant or condition contained herein. Any such extension
or waiver shall be valid only if set forth in an instrument in writing signed by the Party or Parties to be bound thereby (including
the Sellers’ Representative in lieu of such Party to the extent provided in this Agreement). Notwithstanding the foregoing, no
failure or delay by a Party in exercising any right hereunder shall operate as a waiver thereof nor shall any single or partial exercise
thereof preclude any other or further exercise of any other right hereunder. Notwithstanding the foregoing, any waiver of any provision
of this Agreement after the Closing shall also require the prior written consent of the Sellers’ Representative.
9.10
Entire Agreement. This Agreement and the documents or instruments referred to herein, including any exhibits, annexes and schedules
attached hereto, which exhibits, annexes and schedules are incorporated herein by reference, together with the Ancillary Documents, embody
the entire agreement and understanding of the Parties hereto in respect of the subject matter contained herein. There are no restrictions,
promises, representations, warranties, covenants or undertakings, other than those expressly set forth or referred to herein or the documents
or instruments referred to herein, which collectively supersede all prior agreements and the understandings among the Parties with respect
to the subject matter contained herein.
9.11
Interpretation. The table of contents and the Article and Section headings contained in this Agreement are solely for the purpose
of reference, are not part of the agreement of the Parties and shall not in any way affect the meaning or interpretation of this Agreement.
In this Agreement, unless the context otherwise requires: (a) any pronoun used in this Agreement shall include the corresponding masculine,
feminine or neuter forms, and words in the singular, including any defined terms, include the plural and vice versa; (b) reference to
any Person includes such Person’s successors and assigns but, if applicable, only if such successors and assigns are permitted
by this Agreement, and reference to a Person in a particular capacity excludes such Person in any other capacity; (c) any accounting
term used and not otherwise defined in this Agreement or any Ancillary Document has the meaning assigned to such term in accordance with
GAAP, as applicable, based on the accounting principles used by the applicable Person; (d) “including” (and with correlative
meaning “include”) means including without limiting the generality of any description preceding or succeeding such term and
shall be deemed in each case to be followed by the words “without limitation”; (e) the words “herein,” “hereto,”
and “hereby” and other words of similar import in this Agreement shall be deemed in each case to refer to this Agreement
as a whole and not to any particular Section or other subdivision of this Agreement; (f) the word “if” and other words of
similar import when used herein shall be deemed in each case to be followed by the phrase “and only if”; (g) the term “or”
means “and/or”; (h) any reference to the term “ordinary course” or “ordinary course of business”
shall be deemed in each case to be followed by the words “consistent with past practice”; (i) any agreement, instrument,
insurance policy, Law or Order defined or referred to herein or in any agreement or instrument that is referred to herein means such
agreement, instrument, insurance policy, Law or Order as from time to time amended, modified or supplemented, including (in the case
of agreements or instruments) by waiver or consent and (in the case of statutes, regulations, rules or orders) by succession of comparable
successor statutes, regulations, rules or orders and references to all attachments thereto and instruments incorporated therein; (j)
except as otherwise indicated, all references in this Agreement to the words “Section,” “Article”, “Schedule”,
“Annex” and “Exhibit” are intended to refer to Sections, Articles, Schedules, Annexes and Exhibits to this Agreement;
and (k) the term “Dollars” or “$” means United States dollars. Any reference in this Agreement to a Person’s
directors shall include any member of such Person’s governing body and any reference in this Agreement to a Person’s officers
shall include any Person filling a substantially similar position for such Person. Any reference in this Agreement or any Ancillary Document
to a Person’s shareholders or stockholders shall include any applicable owners of the equity interests of such Person, in whatever
form. The Parties have participated jointly in the negotiation and drafting of this Agreement. Consequently, in the event an ambiguity
or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties hereto, and no
presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any provision of this Agreement.
To the extent that any Contract, document, certificate or instrument is represented and warranted to by the Company to be given, delivered,
provided or made available by the Company, in order for such Contract, document, certificate or instrument to have been deemed to have
been given, delivered, provided and made available to Buyer or its Representatives, such Contract, document, certificate or instrument
shall have been posted to the electronic data site maintained on behalf of the Company for the benefit of Buyer and its Representatives
at least two (2) Business Days prior to the date of this Agreement and Buyer and its Representatives have been given access to the electronic
folders containing such information. To the extent that any Contract, document, certificate or instrument is represented and warranted
to by Buyer to be given, delivered, provided or made available by Buyer, in order for such Contract, document, certificate or instrument
to have been deemed to have been given, delivered, provided and made available to the Company or its Representatives, such Contract,
document, certificate or instrument shall have been (i) filed publicly or (ii) posted to the electronic data site maintained on behalf
of Buyer for the benefit of the Company and its Representatives at least two (2) Business Days prior to the date of this Agreement and
the Company and its Representatives have been given access to the electronic folders containing such information.
9.12
Counterparts. This Agreement may be executed and delivered (including by facsimile or other electronic transmission) in one or
more counterparts, and by the different Parties hereto in separate counterparts, each of which when executed shall be deemed to be an
original but all of which taken together shall constitute one and the same agreement.
9.13
Sellers’ Representative.
(a)
Each Seller, on behalf of itself and its successors and assigns, appoints Thomas Meier as its agent, proxy, attorney-in-fact and representative
under this Agreement (in such capacity, the “Sellers’ Representative”) and authorizes and directs the
Sellers’ Representative to take any and all actions in the name and on behalf of such Seller as may be necessary or appropriate
to exercise or perform the rights, powers and obligations of such Seller under this Agreement or any other Ancillary Document and to
consummate the transactions contemplated hereby or thereby, with full power of substitution to act in the name, place and stead of such
Seller, including exercising such rights, power and authority, as are authorized, delegated and granted to the Sellers’ Representative
on behalf of Sellers pursuant to this Agreement (including the right to receive notices and other documentation pursuant to the terms
of this Agreement on behalf of Sellers). By its execution hereof, each Seller hereby authorizes, delegates and grants to the Sellers’
Representative authority to take all actions that this Agreement and any Ancillary Document provide are to be taken by such Seller. All
decisions and actions by the Sellers’ Representative, including any agreement between the Sellers’ Representative and Buyer
relating to the defense or settlement of any Claims for which a Seller may be required to indemnify under this Agreement shall be binding
upon all of the Sellers, and no Seller shall have the right to object, dissent, protest or otherwise contest the same, and Buyer is entitled
to rely upon the same in all respects and shall have no liability to any individual Seller for any action taken by the Seller’s
Representative on behalf of the Sellers in accordance with this Section. The provisions of this Section are irrevocable and coupled with
an interest.
(i)
Each Seller agrees that the Sellers’ Representative (i) shall not
be liable for any actions taken or omitted to be taken under or in connection with this Agreement or any Ancillary Document or the transactions
contemplated hereby or thereby and (ii) shall not owe any fiduciary duty or have any fiduciary responsibility to any Seller or the Company
as a result of its actions taken as the Sellers’ Representative pursuant to this Agreement or any Ancillary Document.
(ii)
Each Seller shall, up to the amount of its Sellers Percentage, indemnify
the Sellers’ Representative and hold it harmless against any Losses incurred on the part of the Sellers’ Representative and
arising out of or in connection with the acceptance or administration of the Sellers’ Representative’s duties under this
Agreement, including the reasonable fees and expenses of any legal counsel retained by the Sellers’ Representative. In no event
shall the Sellers’ Representative in such capacity be liable hereunder or in connection herewith for any indirect, punitive, special
or consequential damages. The Sellers’ Representative shall be fully protected against the Sellers in relying upon any written
notice, demand, certificate or document that it in good faith believes to be genuine, including facsimiles or copies thereof. In connection
with the performance of its rights and obligations hereunder, the Sellers’ Representative shall have the right at any time and
from time to time to select and engage, at the cost and expense of the Sellers, attorneys, accountants, investment bankers, advisors,
consultants and clerical personnel and obtain such other professional and expert assistance, maintain such records and incur other out-of-pocket
expenses, as the Sellers’ Representative may deem necessary or desirable from time to time. All of the indemnities,
immunities,
releases and powers granted to the Sellers’ Representative under this Section shall survive the Closing.
Article
X
DEFINITIONS
10.1
Certain Definitions. For purpose of this Agreement, the following capitalized terms have the following meanings:
“Action”
means any notice of noncompliance or violation, or any claim, demand, charge, action, suit, litigation, audit, settlement, complaint,
stipulation, assessment or arbitration, or any request (including any request for information), inquiry, hearing, proceeding or investigation,
by or before any Governmental Authority.
“Affiliate”
means, with respect to any Person, any other Person directly or indirectly Controlling, Controlled by, or under common Control with such
Person.
“Aggregate
Buyer Common Stock” means the quotient obtained by dividing (a) the Exchange Consideration by (b) the ten-day VWAP of the
Buyer Common Stock for the ten (10)-day period preceding the third day prior to the Closing Date.
“Ancillary
Documents” means each agreement, instrument or document attached hereto as an Exhibit, including the Non-Competition and
Non-Solicitation Agreements, the Subscription Agreements, the Series B Certificate of Designation, the Lock-Up Agreements and the other
agreements, certificates and instruments to be executed or delivered by any of the Parties hereto in connection with or pursuant to this
Agreement.
“Benefit
Plans” of any Person means any and all deferred compensation, executive compensation, incentive compensation, equity purchase
or other equity-based compensation plan, employment or consulting, severance or termination pay, holiday, vacation or other bonus plan
or practice, hospitalization or other medical, life or other insurance, supplemental unemployment benefits, profit sharing, pension,
or retirement plan, program, agreement, commitment or arrangement, and each other employee benefit plan, program, agreement or arrangement,
including each “employee benefit plan” as such term is defined under Section 3(3) of ERISA or any similar law in Switzerland,
maintained or contributed to or required to be contributed to by a Person for the benefit of any employee or terminated employee of such
Person, or with respect to which such Person has any Liability, whether direct or indirect, actual or contingent, whether formal or informal,
and whether legally binding or not.
“Business
Day” means any day other than a Saturday, Sunday or a legal holiday on which commercial banking institutions in New York,
New York are authorized to close for business; provided that banks shall not be deemed to be authorized or obligated to be closed
due to a “shelter in place” or similar closure of physical branch locations at the direction of any Governmental Authority
if such banks’ electronic funds transfer systems (including for wire transfers) are open for use by customers on such day.
“Buyer
Common Stock” means the shares of common stock, par value $0.00001 per share, of Buyer.
“Buyer
Company” means each of Buyer and its direct and indirect Subsidiaries.
“Buyer
Confidential Information” means all confidential or proprietary documents and information concerning Buyer or any of its
Affiliates; provided, however, that Buyer Confidential Information shall not include any information which, (i) at the
time of disclosure by the Company, any Seller or any of their respective Representatives, is generally available publicly and was not
disclosed in breach of this Agreement or (ii) at the time of the disclosure by Buyer or its Representatives to by the Company, any Seller
or any of their respective Representatives, was previously known by such receiving party without violation of Law or any confidentiality
obligation by the Person receiving such Buyer Confidential Information.
“Buyer
Interim Balance Sheet” means the balance sheet of Buyer included in its Quarterly Report on Form 10-Q for the quarter ended
September 30, 2023, filed with the SEC on October 20, 2023.
“Buyer
Preferred Stock” means shares of Series B Preferred Stock, par value $0.00001 par value per share, of Buyer.
“Buyer
Securities” means the Buyer Common Stock and the Buyer Preferred Stock and the Buyer Stock Options, collectively.
“Buyer
Stock Options” means options to purchase shares of Buyer Common Stock.
“CFIUS”
means the Committee on Foreign Investment in the United States and each member agency acting on its behalf.
“CFIUS
Approval” means that the Parties have received a written
notice from CFIUS to the effect that: (a) the Transactions are not subject to the DPA; (b) CFIUS has determined that there are no unresolved
national security concerns with respect to the Transactions and has concluded all action under the DPA; (c) if CFIUS has sent a report
to the President of the United States either (i) the President of the United States shall have determined not to use his powers pursuant
to the DPA to suspend, condition, or prohibit the consummation of the Transactions or (ii) the period allotted for presidential action
in the DPA shall have passed without any determination by the President of the United States.
“Code”
means the Internal Revenue Code of 1986, as amended, and any successor statute thereto, as amended. Reference to a specific section of
the Code shall include such section and any valid treasury regulation promulgated thereunder.
“Company
Confidential Information” means all confidential or proprietary documents and information concerning the Target Companies
or the Sellers or any of their respective Affiliates, furnished in connection with this Agreement or the transactions contemplated hereby;
provided, however, that the Company Confidential Information shall not include any information which, (i) at the time of
disclosure by the Company or its Representatives, is generally available publicly and was not disclosed in breach of this Agreement or
(ii) at the time of the disclosure by the Company, the Sellers’ Representative, the Sellers or their respective Representatives
to Buyer or its Representatives was previously known by such receiving party without violation of Law or any confidentiality obligation
by the Person receiving such the Company Confidential Information.
“Company
Convertible Securities” means, collectively, any options, restricted stock units, warrants or rights to subscribe for or
purchase any capital shares of the Company or securities convertible into or exchangeable for, or that otherwise confer on the holder
any right to acquire any capital shares of the Company.
“Company
Fully Diluted Share Amount” means, as of immediately prior to the Closing, the sum of (a) the aggregate number of
Company Outstanding Shares and (b) the aggregate number of Company Shares issuable upon the exercise of outstanding Company Stock
Options calculated using the treasury method of accounting.
“Company
Outstanding Shares” means the total number of shares of the Company Shares issued and outstanding immediately prior to
the Closing after giving effect to the Company Convertible Securities Conversion.
“Company
Securities” means, collectively, the Company Shares, any Company Stock Options and any other the Company Convertible Securities.
“Company
Shares” means the ordinary shares, par value CHF 1.00 per share, of the Company.
“Company
Stock Options” means options to purchase Company Shares.
“Consent”
means any consent, approval, waiver, authorization or Permit of, or notice to or declaration or filing with any Governmental Authority
or any other Person.
“Contracts”
means all contracts, agreements, binding arrangements, bonds, notes, indentures, mortgages, debt instruments, purchase order, licenses
(and all other contracts, agreements or binding arrangements concerning Intellectual Property), franchises, leases and other instruments
or obligations of any kind, written or oral (including any amendments and other modifications thereto).
“Control”
of a Person means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies
of such Person, whether through the ownership of voting securities, by contract, or otherwise. “Controlled”, “Controlling”
and “under common Control with” have correlative meanings. Without limiting the foregoing a Person (the “Controlled
Person”) shall be deemed Controlled by (a) any other Person (i) owning beneficially, as meant in Rule 13d-3 under the Exchange
Act, securities entitling such Person to cast ten percent (10%) or more of the votes for election of directors or equivalent governing
authority of the Controlled Person or (ii) entitled to be allocated or receive ten percent (10%) or more of the profits, losses, or distributions
of the Controlled Person; (b) an officer, director, general partner, partner (other than a limited partner), manager, or member (other
than a member having no management authority that is not a Person described in clause (a) above) of the Controlled Person; or (c) a spouse,
parent, lineal descendant, sibling, aunt, uncle, niece, nephew, mother-in-law, father-in-law, sister-in-law, or brother-in-law of an
Affiliate of the Controlled Person or a trust for the benefit of an Affiliate of the Controlled Person or of which an Affiliate of the
Controlled Person is a trustee.
“Conversion
Approval” means the Conversion shall have been approved by the requisite vote of the Buyer Stockholders (including
any separate class or series vote that is required, whether pursuant to the Buyer’s Organizational Documents, any stockholder agreement
or otherwise) at a meeting of Buyer stockholders, held in accordance with the Delaware General Corporation Law, as amended, and Buyer’s
Organizational Documents.
“Conversion” means
the conversion of all of the total issued and outstanding shares of Series B Preferred Stock into shares of Buyer Common Stock.
“Copyrights”
means any works of authorship, mask works and all copyrights therein, including all renewals and extensions, copyright registrations
and applications for registration and renewal, and non-registered copyrights.
“COVID-19”
means SARS-CoV-2 or COVID-19, and any evolutions thereof or any other related or associated epidemics, pandemics or disease outbreaks.
“COVID-19
Measures” means any quarantine, “shelter in place,” “stay at home,” workforce reduction, social
distancing, shut down, closure, sequester or any other Law, directive, guidelines or recommendations by any Governmental Authority (including
the Centers for Disease Control and the World Health Organization) in each case in connection with, related to or in response to COVID-19,
including the Coronavirus Aid, Relief, and Economic Security Act (CARES) or any changes thereto.
“DPA”
means Section 721 of the Defense Production Act of 1950, as amended, 50 U.S.C. §4565, and all interim and final rules and regulations
issued and effective thereunder.
“Environmental
Law” means any Law in any way relating to (a) the protection of human health and safety, (b) the protection, preservation
or restoration of the environment and natural resources (including air, water vapor, surface water, groundwater, drinking water supply,
surface land, subsurface land, plant and animal life or any other natural resource), or (c) the exposure to, or the use, storage, recycling,
treatment, generation, transportation, processing, handling, labeling, production, Release or disposal of Hazardous Materials.
“Environmental
Liabilities” means, in respect of any Person, all Liabilities, obligations, responsibilities, Remedial Actions, Actions,
Orders, losses, damages, costs, and expenses (including all reasonable fees, disbursements, and expenses of counsel, experts, and consultants
and costs of investigation and feasibility studies), fines, penalties, sanctions, and interest incurred as a result of any claim or demand
by any other Person or in response to any violation of Environmental Law, whether known or unknown, accrued or contingent, whether based
in contract, tort, implied or express warranty, strict liability, criminal or civil statute, to the extent based upon, related to, or
arising under or pursuant to any Environmental Law, Environmental Permit, Order, or Contract with any Governmental Authority or other
Person, that relates to any environmental, health or safety condition, violation of Environmental Law, or a Release or threatened Release
of Hazardous Materials.
“ERISA”
means the U.S. Employee Retirement Income Security Act of 1974, as amended.
“Exchange
Act” means the U.S. Securities Exchange Act of 1934, as amended.
“Exchange
Ratio” means the quotient (rounded to four decimal places) obtained by dividing (a) the Aggregate Buyer Common Stock less
the number of Creditor Shares (on an as converted basis) by (b) the Company Fully Diluted Share Amount.
“Foreign
Plan” means any plan, fund (including any superannuation fund) or other similar program or arrangement established or maintained
outside the United States by the Company or any one or more of its Subsidiaries primarily for the benefit of employees of the Company
or such Subsidiaries residing outside the United States, which plan, fund or other similar program or arrangement provides, or results
in, retirement income, a deferral of income in contemplation of retirement or payments to be made upon termination of employment, and
which plan is not subject to ERISA or the Code.
“GAAP”
means generally accepted accounting principles as in effect in the United States of America.
“Governmental
Authority” means any federal, state, local, foreign or other governmental, quasi-governmental or administrative body, instrumentality,
department or agency or any court, tribunal, administrative hearing body, arbitration panel, commission, or other similar dispute-resolving
panel or body.
“Hazardous
Material” means any waste, gas, liquid or other substance or material that is defined, listed or designated as a “hazardous
substance”, “pollutant”, “contaminant”, “hazardous waste”, “regulated substance”,
“hazardous chemical”, or “toxic chemical” (or by any similar term) under any Environmental Law, or any other
material regulated, or that could result in the imposition of Liability or responsibility, under any Environmental Law, including petroleum
and its by-products, asbestos, polychlorinated biphenyls, radon, mold, and urea formaldehyde insulation.
“Indebtedness”
of any Person means, without duplication, (a) all indebtedness of such Person for borrowed money (including the outstanding principal
and accrued but unpaid interest), whether contingent or otherwise, including the principal amount thereof and all fees and interest accrued
thereon, (b) all obligations for the deferred purchase price of property or services (other than trade payables incurred in the ordinary
course of business), (c) any other indebtedness of such Person that is evidenced by a note, bond, debenture, credit agreement or similar
instrument, minority interests, preferred shares, or other debt security, including all interest accrued thereon, (d) all obligations
of such Person under leases that should be classified as capital leases in accordance with GAAP or Swiss GAAP (as applicable to such
Person), (e) all obligations of such Person for the reimbursement of any obligor on any line or letter of credit, banker’s acceptance,
guarantee or similar credit transaction, (f) all obligations of such Person in respect of acceptances issued or created, (g) all interest
rate and currency swaps, caps, collars and similar agreements or hedging devices under which payments are obligated to be made by such
Person, whether periodically or upon the happening of a contingency, (h) all obligations secured by an Lien on any property of such Person,
(i) any premiums, prepayment fees or other penalties, fees, costs or expenses associated with payment of any Indebtedness of such Person
and (j) all guarantees, pledges or similar assurances by any member of such Person to pay another Person’s debt or to perform another
Person’s obligation in the case of default, (k) all off-balance sheet Liabilities of such Person; and (l) all obligations described
in clauses (a) through (k) above of any other Person which is directly or indirectly guaranteed by such Person or which such Person has
agreed (contingently or otherwise) to purchase or otherwise acquire or in respect of which it has otherwise assured a creditor against
loss.
“Intellectual
Property” means all of the following as they exist in any jurisdiction throughout the world: Patents, Trademarks, Copyrights,
Trade Secrets, Internet Assets, Software and other intellectual property, and all licenses, sublicenses and other agreements or permissions
related to the preceding property.
“Internet
Assets” means any all domain name registrations, web sites and web addresses and related rights, items and documentation
related thereto, and applications for registration therefor.
“Investment
Company Act” means the U.S. Investment Company Act of 1940, as amended.
“Knowledge”
means, with respect to (a) the Company, the actual knowledge of each of Christian Brühlmann or Ralph Schiess, after reasonable inquiry
with his direct reports responsible for the applicable subject matter and any relevant books and records; (b) Buyer, the actual knowledge
of each of Neil Campbell and Bruce Harmon, after reasonable inquiry with his direct reports responsible for the applicable subject matter
and any relevant books and records; and (c) any other Party, (i) if an entity, the actual knowledge of its directors and executive officers,
after reasonable inquiry of their direct reports responsible for the applicable subject matters and any relevant books and records, or
(ii) if a natural person, the actual knowledge of such Party.
“Law”
means any federal, state, local, municipal, foreign or other law, statute, legislation, principle of common law, ordinance, code, edict,
decree, proclamation, treaty, convention, rule, regulation, directive, requirement, writ, injunction, settlement, Order or Consent that
is or has been issued, enacted, adopted, passed, approved, promulgated, made, implemented or otherwise put into effect by or under the
authority of any Governmental Authority.
“Liabilities”
means any and all liabilities, Indebtedness, Actions or obligations of any nature (whether absolute, accrued, contingent or otherwise,
whether known or unknown, whether direct or indirect, whether matured or unmatured, whether due or to become due and whether or not required
to be recorded or reflected on a balance sheet under GAAP, or other applicable accounting standards), including Tax liabilities due or
to become due.
“Lien”
means any mortgage, pledge, security interest, attachment, right of first refusal, option, proxy, voting trust, encumbrance, lien or
charge of any kind (including any conditional sale or other title retention agreement or lease in the nature thereof), restriction (whether
on voting, sale, transfer, disposition or otherwise), any subordination arrangement in favor of another Person, or any filing or agreement
to file a financing statement as debtor under the Uniform Commercial Code or any similar Law.
“Material
Adverse Effect” means, with respect to any specified Person, any fact, event, occurrence, change or effect that has had,
or would reasonably be expected to have, individually or in the aggregate, a material adverse effect upon (a) the business, assets, Liabilities,
results of operations, prospects or condition (financial or otherwise) of such Person and its Subsidiaries, taken as a whole, or (b)
the ability of such Person or any of its Subsidiaries on a timely basis to consummate the Transactions or the Ancillary Documents to
which it is a party or bound or to perform its obligations hereunder or thereunder; provided, however, that for purposes
hereof, any facts, events, occurrences, changes or effects directly or indirectly attributable to, resulting from, relating to or arising
out of the following (by themselves or when aggregated with any other, changes or effects) shall not be deemed to be, constitute, or
be taken into account when determining whether there has or may, would or could have occurred a Material Adverse Effect: (i) general
changes in the financial or securities markets (including changes in the credit, debt, securities and capital markets) or general economic
or political conditions in the country or region in which such Person or any of its Subsidiaries do business; (ii) changes, conditions
or effects that generally affect the industries in which such Person or any of its Subsidiaries principally operate; (iii) changes in
applicable Laws (including COVID-19 Measures) or GAAP or other applicable accounting principles or mandatory changes in the regulatory
accounting requirements applicable to any industry in which such Person and its Subsidiaries principally operate; (iv) conditions caused
by acts of God, terrorism, war (whether or not declared), natural disaster or any outbreak or continuation of an epidemic or pandemic
(including, without limitation, COVID-19) or the worsening thereof, including the effects of any Governmental Authority or other third-party
responses thereto; (v) any failure in and of itself by such Person and its Subsidiaries to meet any internal or published budgets, projections,
forecasts or predictions of financial performance for any period (provided that the underlying cause of any such failure may be considered
in determining whether a Material Adverse Effect has occurred or would reasonably be expected to occur to the extent not excluded by
another exception herein); (vi) the announcement or pendency of the Transactions (including the Share Exchange) (provided that this clause
(vi) shall not apply to any representation or warranty to the extent such representation or warranty relates to the consequences resulting
from the execution, announcement, performance or existence of this Agreement); and (vii) in the case of the Company, the ability of the
Company to make any of the representations and warranties contained in this Agreement as of the date hereof ; provided, further,
however, that any event, occurrence, fact, condition, or change referred to in clauses (i) through (iv) immediately above shall
be taken into account in determining whether a Material Adverse Effect has occurred or could reasonably be expected to occur to the extent
that such event, occurrence, fact, condition, or change has a disproportionate effect on such Person or any of its Subsidiaries compared
to other participants in the industries in which such Person or any of its Subsidiaries primarily conducts its businesses. Notwithstanding
the foregoing, with respect to Buyer, the failure to obtain the Stockholder Approval shall not be deemed
to be a Material Adverse Effect on or with respect to Buyer.
“Nasdaq”
means the Nasdaq Capital Market.
“Order”
means any order, decree, ruling, judgment, injunction, writ, determination, binding decision, verdict, judicial award or other action
that is or has been made, entered, rendered, or otherwise put into effect by or under the authority of any Governmental Authority.
“Organizational
Documents” means, with respect to any Person, its certificate of incorporation and bylaws, statutory books, articles of
association memorandum and articles of association or similar organizational documents, in each case, as amended.
“Patents”
means any patents, patent applications and the inventions, designs and improvements described and claimed therein, patentable inventions,
and other patent rights (including any divisionals, provisionals, continuations, continuations-in-part, substitutions, or reissues thereof,
whether or not patents are issued on any such applications and whether or not any such applications are amended, modified, withdrawn,
or refiled).
“Permits”
means all federal, state, local or foreign or other third-party permits, grants, easements, consents, approvals, authorizations, exemptions,
licenses, franchises, concessions, ratifications, permissions, clearances, confirmations, endorsements, waivers, certifications, designations,
ratings, registrations, qualifications or Orders of any Governmental Authority or any other Person.
“Permitted
Financing” means one or more debt or equity financing transactions consummated by and funded into Buyer during the time
between Closing and the Conversion resulting in aggregate gross proceeds of no greater than $25 million.
“Permitted
Liens” means (a) Liens for Taxes or assessments and similar governmental charges or levies, which either are (i) not delinquent
or (ii) being contested in good faith and by appropriate proceedings, and adequate reserves (as determined in accordance with GAAP) have
been established with respect thereto, (b) other Liens imposed by operation of Law arising in the ordinary course of business for amounts
which are not due and payable and as would not in the aggregate materially adversely affect the value of, or materially adversely interfere
with the use of, the property subject thereto, (c) Liens incurred or deposits made in the ordinary course of business in connection with
social security, (d) Liens on goods in transit incurred pursuant to documentary letters of credit, in each case arising in the ordinary
course of business, or (e) Liens arising under this Agreement or any Ancillary Document.
“Person”
means an individual, corporation, exempted company, partnership (including a general partnership, limited partnership, exempted limited
partnership or limited liability partnership), limited liability company, association, trust or other entity or organization, including
a government, domestic or foreign, or political subdivision thereof, or an agency or instrumentality thereof.
“Personal
Property” means any machinery, equipment, tools, vehicles, furniture, leasehold improvements, office equipment, plant,
parts and other tangible personal property.
“Private
Placement Investment” means a private equity investment in Buyer pursuant to which certain investors (“Private
Placement Investor”) agree to subscribe for and Buyer will agree to issue to each such Private Placement Investor, equity
securities of Buyer pursuant to a Subscription Agreement.
“Release”
means any release, spill, emission, leaking, pumping, injection, deposit, disposal, discharge, dispersal, or leaching into the indoor
or outdoor environment, or into or out of any property.
“Remedial
Action” means all actions to (i) clean up, remove, treat, or in any other way address any Hazardous Material, (ii) prevent
the Release of any Hazardous Material so it does not endanger or threaten to endanger public health or welfare or the indoor or outdoor
environment, (iii) perform pre-remedial studies and investigations or post-remedial monitoring and care, or (iv) correct a condition
of noncompliance with Environmental Laws.
“Representatives”
means, as to any Person, such Person’s Affiliates and the respective managers, directors, officers, employees, independent contractors,
consultants, advisors (including financial advisors, counsel and accountants), agents and other legal representatives of such Person
or its Affiliates.
“SEC”
means the U.S. Securities and Exchange Commission (or any successor Governmental Authority).
“Securities
Act” means the U.S. Securities Act of 1933, as amended.
“Sellers
Percentage” means the percentage of Purchased Shares owned by such Seller as compared to the total number of Purchased
Shares owned by all Sellers as provided on Annex I.
“Sellers’
Representative” has the meaning set forth in the preamble to this Agreement.
“Software”
means any computer software programs, including all source code, object code, and documentation related thereto and all software modules,
tools and databases.
“SOX”
means the U.S. Sarbanes-Oxley Act of 2002, as amended.
“Stockholder
Approval” means the approval by the requisite vote of stockholders of Buyer at the Special Stockholder Meeting of the Stockholder
Approval Matters.
“Subsidiary”
means, with respect to any Person, any corporation, partnership, association or other business entity of which (i) if a corporation,
a majority of the total voting power of capital shares entitled (without regard to the occurrence of any contingency) to vote in the
election of directors, managers or trustees thereof is at the time owned or Controlled, directly or indirectly, by that Person or one
or more of the other Subsidiaries of that Person or a combination thereof, or (ii) if a partnership, association or other business entity,
a majority of the partnership or other similar ownership interests thereof is at the time owned or Controlled, directly or indirectly,
by any Person or one or more Subsidiaries of that Person or a combination thereof. For purposes hereof, a Person or Persons will be deemed
to have a majority ownership interest in a partnership, association or other business entity if such Person or Persons will be allocated
a majority of partnership, association or other business entity gains or losses or will be or Control the managing director, managing
member, general partner or other managing Person of such partnership, association or other business entity. A Subsidiary of a Person
will also include any variable interest entity which is consolidated with such Person under applicable accounting rules.
“Swiss
GAAP” means the accounting principles as in effect pursuant to the Swiss Code of Obligations.
“Target
Company” means each of the Company and its direct and indirect Subsidiaries.
“Tax
Return” means any return, declaration, report, claim for refund, information return or other documents (including any related
or supporting schedules, statements or information) filed or required to be filed in connection with the determination, assessment or
collection of any Taxes or the administration of any Laws or administrative requirements relating to any Taxes.
“Taxes”
means (a) all direct or indirect federal, state, local, foreign and other net income, gross income, gross receipts, sales, use, value-added,
ad valorem, transfer, real property, Personal Property, franchise, profits, license, lease, service, service use, withholding, payroll,
employment, social security and related contributions due in relation to the payment of compensation to employees, excise, severance,
stamp, occupation, premium, property, windfall profits, alternative minimum, estimated, customs, duties or other Taxes, fees, assessments
or charges of any kind whatsoever, together with any interest and any penalties, additions to tax or additional amounts with respect
thereto, (b) any Liability for payment of amounts described in clause (a) whether as a result of being a member of an affiliated, consolidated,
combined or unitary group for any period or otherwise through operation of law, (c) liability under any abandonment or unclaimed property,
escheat or similar Law and (d) any Liability for the payment of amounts described in clauses (a), (b) or (c) of this sentence as a result
of any tax sharing, tax group, tax indemnity or tax allocation agreement with, or any other express or implied agreement to indemnify,
any other Person.
“Trade
Secrets” means any trade secrets, confidential business information, concepts, ideas, designs, research or development
information, processes, procedures, techniques, technical information, specifications, operating and maintenance manuals, engineering
drawings, methods, know-how, data, mask works, discoveries, inventions, modifications, extensions, improvements, and other proprietary
rights (whether or not patentable or subject to Copyright, Trademark, or trade secret protection).
“Trademarks”
means any trademarks, service marks, trade dress, trade names, brand names, internet domain names, designs, logos, or corporate names
(including, in each case, the goodwill associated therewith), whether registered or unregistered, and all registrations and applications
for registration and renewal thereof.
“VWAP”
means, for any security as of any date(s), the dollar volume-weighted average price for such security on the principal securities exchange
or securities market on which such security is then traded during the period beginning at 9:30:01 a.m., New York time, and ending at
4:00:00 p.m., New York time, as reported by Bloomberg through its “HP” function (set to weighted average) or, if the foregoing
does not apply, the dollar volume-weighted average price of such security in the over-the-counter market on the electronic bulletin board
for such security during the period beginning at 9:30:01 a.m., New York time, and ending at 4:00:00 p.m., New York time, as reported
by Bloomberg, or, if no dollar volume-weighted average price is reported for such security by Bloomberg for such hours, the average of
the highest closing bid price and the lowest closing ask price of any of the market makers for such security as reported by OTC Markets
Group Inc. If the VWAP cannot be calculated for such security on such date(s) on any of the foregoing bases, the VWAP of such security
on such date(s) shall be the fair market value as determined reasonably and in good faith by a majority of the disinterested independent
directors of the board of directors (or equivalent governing body) of the applicable issuer. All such determinations shall be appropriately
adjusted for any stock dividend, stock split, stock combination, recapitalization or other similar transaction during such period.
10.2
Section References. The following capitalized terms, as used in this Agreement, have the respective meanings given to them in
the Section as set forth below adjacent to such terms:
| Term |
|
Section |
| Agreement |
|
Preamble |
| Antitrust Laws |
|
3.3 |
| Assumed Option |
|
6.18 |
| Audited Company Financials |
|
4.7(a) |
| Buyer |
|
Preamble |
| Buyer Benefit Plan |
|
3.19(a) |
| Buyer Board |
|
Recitals |
| Buyer Director |
|
6.12 |
| Buyer Disclosure Schedules |
|
Article III |
| Buyer Financials |
|
3.7(b) |
| Buyer fundamental Representations |
|
7.3(a) |
| Buyer Indemnitees |
|
7.2(a) |
| Buyer IP |
|
3.13(c) |
| Buyer IP Licenses |
|
3.13(a) |
| Buyer Material Contract |
|
3.12(a) |
| Buyer Owned Real Property |
|
3.15(b) |
| Buyer Permits |
|
3.10 |
| Buyer Personal Property Leases |
|
3.16 |
| Buyer Real Property Leases |
|
3.15(a) |
| Buyer Registered IP |
|
3.13(a) |
| Buyer Top Vendors |
|
3.26 |
| CFIUS Filing |
|
6.19 |
| Closing |
|
2.1 |
| Closing Date |
|
2.1 |
| Closing Filing |
|
6.10(b) |
| Closing Press Release |
|
6.10(b) |
| Common Shares |
|
1.2(a) |
| Company |
|
Preamble |
| Company Balance Sheet |
|
4.7(a) |
| Company Balance Sheet Date |
|
4.7(a) |
| Company Benefit Plan |
|
4.19(a) |
| Company Certificates |
|
1.3(b) |
| Company Convertible Securities Conversion |
|
1.4(a) |
| Company Creditors |
|
1.2(a) |
| Company Directors |
|
6.12 |
| Company Disclosure Schedules |
|
Article IV |
| Company Equity Plans |
|
1.4(b) |
| Company Financials |
|
4.7(a) |
| Company Fundamental Representations |
|
7.3(a) |
| Company IP |
|
4.13(c) |
| Company IP Licenses |
|
4.13(a) |
| Term |
|
Section |
| Company Material Contract |
|
4.12(a) |
| Company Permits |
|
4.10 |
| Company Personal Property Leases |
|
4.16 |
| Company Real Property Leases |
|
4.15 |
| Company Registered IP |
|
4.13(a) |
| Company Top Customers |
|
4.23 |
| Company Top Vendors |
|
4.23 |
| Creditor Shares |
|
1.2(a) |
| D&O Indemnified Persons |
|
6.13(a) |
| D&O Tail Insurance |
|
6.13(b) |
| Direct Claims |
|
7.4(a) |
| EGS |
|
2.1 |
| Enforceability Exceptions |
|
3.2 |
| Environmental Permit |
|
3.20(a) |
| ERISA Affiliate |
|
3.19(f) |
| Exchange Consideration |
|
1.2(a) |
| Exchange Shares |
|
1.2(a) |
| Exchange Share Value |
|
1.2(a) |
| Federal Securities Laws |
|
6.3 |
| Interim Period |
|
6.1 |
| Loss |
|
8.2 |
| Lock-Up Agreement |
|
Recitals |
| Management Shareholders |
|
Recitals |
| Non-Competition and Non-Solicitation Agreement |
|
Recitals |
| OFAC |
|
3.24(c) |
| Off-the-Shelf Software |
|
3.13(a) |
| Party(ies) |
|
Preamble |
| Post-Stockholder Approval Buyer Board |
|
6.12 |
| Preferred Shares |
|
1.2(a) |
| Public Certifications |
|
3.7(a) |
| Purchased Shares |
|
1.1 |
| Real Property Permits |
|
3.15(c) |
| Registration Statement |
|
6.8(a) |
| Related Person |
|
3.21 |
| Releasing Persons |
|
8.1 |
| SEC Reports |
|
3.7(a) |
| Sellers |
|
Preamble |
| Seller Indemnitees |
|
7.2(b) |
| Seller Parties |
|
Preamble |
| Sellers’ Representative |
|
Preamble |
| Series B Certificate of Designation |
|
1.2(a) |
| Share Exchange |
|
Recitals |
| Special Stockholder Meeting |
|
6.8(b) |
| Specified Courts |
|
9.4 |
| Stockholder Approval Matters |
|
6.8(b) |
| Subscription Agreements 1 |
|
2.3(d) |
| Transactions |
|
Recitals |
| Transfer Taxes |
|
6.14 |
[REMAINDER
OF PAGE INTENTIONALLY LEFT BLANK; SIGNATURE PAGE FOLLOWS]
IN
WITNESS WHEREOF, each Party hereto has caused this Agreement to be signed and delivered by its respective duly authorized officer as
of the date first written above.
| |
The Company: |
| |
|
|
| |
PROTEOMEDIX AG |
| |
|
|
| |
By: |
/s/
Ralph Schiess |
| |
|
Name: |
Ralph Schiess |
| |
|
Title: |
CEO |
| |
|
|
|
| |
Buyer: |
| |
|
|
| |
BLUE WATER BIOTECH, INC. |
| |
|
|
| |
By: |
/s/
Dr. Neil Campbel |
| |
|
Name: |
Dr. Neil Campbell |
| |
|
Title: |
CEO |
| |
|
|
|
| |
Sellers’ Representative: |
| |
|
|
| |
By: |
/s/
Thomas Meier |
| |
|
Thomas Meier, solely in the capacity as
the Sellers’
Representative hereunder |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
| |
Name of Seller: Dr. Ralph Scheiss |
| |
|
| |
By: |
/s/
Dr. Ralph Scheiss |
| |
The Sellers: |
| |
|
| |
Name of Seller: Christian Briihlmann |
| |
|
| |
By: |
/s/
Christian Briihlmann |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Thomas Cerny |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Rudolf Aenersold |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Corinne Krek |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: ETC Zurich |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Altos Venture AG |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Dr. Jurg Geigy |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller:The Habs W. Schoepflin Trust |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Zurcher Kantonalbank |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: W.A. de Vidier Stiftung |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Labrador Trust |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Andre J. Mueller |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Davent Holding AG |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Scalis AG |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Dr. Werner Schafer |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
| |
Name of Seller: Harry Welten |
| |
|
| |
/s/
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Isaac Kobrin |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: Dr. Helge Lubenow |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
| |
The Sellers: |
| |
|
|
| |
Name of Seller: New Horizon Health Ltd |
| |
|
|
| |
By proxy: |
/s/
Dr. Ralph Schiess |
| |
Name: |
Dr. Ralph Schiess |
| |
|
|
| |
By proxy: |
/s/
Harry Welten |
| |
Name: |
Harry Welten |
[Signature
Page to Share Exchange Agreement]
ANNEX
I
List of Sellers
| Seller
Name |
Number
of
Company
Shares
Held by
Seller
(Includes
Conversion
of Notes) |
Number
of
Common
Shares to be
Issued at
Closing |
Number
of
Preferred
Shares to be
Issued at
Closing |
Aggregate
Number of
Common
Shares Post-
Conversion |
| Ralph
Schiess |
|
|
|
|
| Christian
Brühlmann |
|
|
|
|
| Thomas
Cerny |
|
|
|
|
| Rudolf
Aebersold |
|
|
|
|
| Corinne
Krek |
|
|
|
|
| ETH
Zürich |
|
|
|
|
| Altos
Venture AG |
|
|
|
|
| Dr.
Jürg Geigy |
|
|
|
|
| Schoepflin
Trust |
|
|
|
|
| Zürcher
Kantonalbank |
|
|
|
|
| W.A.
de Vigier Stiftung |
|
|
|
|
| Labrador
Trust |
|
|
|
|
| Andre
J. Mueller |
|
|
|
|
| Davent
Holding |
|
|
|
|
| Scablis
AG |
|
|
|
|
| Werner
Schäfer |
|
|
|
|
| Harry
Welten |
|
|
|
|
| Isaac
Kobrin |
|
|
|
|
| Helge
Lubenow |
|
|
|
|
| New
Horizon Health Limited |
|
|
|
|
| TOTAL |
|
|
|
|
List
of Creditors
| Name
of Creditor |
Number
of
Common
Shares to be
Issued at
Closing |
Number
of
Preferred
Shares to be
Issued at
Closing |
Aggregate
Number of
Common
Shares Post-
Conversion |
| Lacarya
Scott |
|
|
|
| Romy
Seth |
|
|
|
| Finalis
Securities LLC |
|
|
|
| TOTAL
|
|
|
|
Annex C
Fairness Opinion

December
13, 2023
Board of Directors
Blue
Water Biotech, Inc.
201
E. Fifth Street, Suite 1900
Cincinnati,
Ohio 25202
Ladies
and Gentlemen:
You
have requested our opinion as to the fairness, from a financial point of view, to Blue Water Biotech, Inc. (“Buyer”) of the
Exchange Consideration (as defined below) to be paid by Buyer pursuant to the proposed Share Exchange Agreement (the “Agreement”)
to be entered into among Buyer, Proteomedix AG, a Swiss Company (the “Company”), the Sellers’ Representative named therein
(the “Sellers’ Representative”) and the Sellers party to the Agreement. Capitalized terms used herein have the respective meanings
ascribed thereto in the December 13, 2023 draft of the Agreement provided to us by Buyer (the “Draft Agreement”).
As
more specifically set forth in the Agreement, and subject to the terms, conditions and adjustments set forth therein, the Agreement provides
that the Buyer will acquire the Company pursuant to a share exchange (the “Share Exchange”) pursuant to which the Sellers
will sell to Buyer and Buyer will purchase from the Sellers all of the Purchased Shares in exchange for the Exchange Shares having an
aggregate value equal to $75,000,000 (the “Exchange Consideration”) less the value of the Company Shares for which Company
Stock Options are exercisable immediately prior to Closing and less the Creditor Shares being issued to the creditors of the Company.
Each Company Share will be exchanged for the right to receive a number of Exchange Shares determined pursuant to the terms of the Agreement.
The Preferred Shares issued in the Share Exchange will be convertible into shares of Buyer Common Stock on the date Buyer receives Stockholder
Approval.
The
obligation of the Company to effect the Share Exchange is subject to a number of conditions, including a condition that the Buyer shall
have delivered to the Company executed Subscription Agreements from the Private Placement Investors for a Private Placement Investment
in an aggregate amount equal to or greater than $5.0 million. For purposes of this opinion, with your approval, we have assumed that
the Private Placement Investment is consummated in accordance with its terms and that Buyer receives proceeds of $5.0 million pursuant
thereto for a per share purchase price equal to the ten-day VWAP of the Buyer Common Stock for the ten (10)-day period preceding the
third day prior to the Closing Date, or $0.249 per share.

Subject
to certain conditions and limitations, the number of shares of Buyer Common Stock issuable upon the Conversion is subject to adjustment
as a result of claims made by either the Sellers against the Buyer or by the Buyer against the Company and the Sellers prior to the Claim
Deadline. For purposes of this opinion, with your approval we have assumed that no such adjustment will occur. In addition, the Sellers
are obligated to indemnify the Buyer for certain breaches or non-fulfilments and under certain circumstances a portion of the shares
of Buyer Common Stock may be paid into an escrow pending the resolution of such claims. For purposes of this opinion, with your approval,
we have assumed that no indemnification payments will be made to the Buyer and no shares of Buyer Common Stock will be paid into escrow.
Further,
for purposes of this opinion, with your approval and without independent verification, we have assumed that (i) the Creditors and the
former holders of the Purchased Shares will own 87.1% of the outstanding equity of Buyer immediately following the Closing and after
giving effect to the Private Placement Investment and the Conversion, (ii) the Private Placement Investors will own 7.6% of the outstanding
equity of Buyer immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion, and
(iii) the holders of the outstanding equity of Buyer immediately prior to the Share Exchange own 5.3% of the outstanding equity of Buyer
immediately following the Closing and after giving effect to the Private Placement Investment and the Conversion.
In
connection with our review of the proposed Share Exchange, and in arriving at our opinion, we have reviewed: (i) the financial terms
of the Share Exchange described in the Draft Agreement; (ii) certain information, including financial forecasts, relating to the business,
earnings, cash flow, assets, liabilities and prospects of the Company that were furnished to us by management of the Company; (iii) financial
forecasts, relating to the business, earnings, assets, liabilities, cash flow, and prospects of the Buyer, furnished to us by the Buyer’s
management; (iv) relevant market sizing projections for the assets and liabilities that will be acquired by the Buyer; (v) management
of the Buyer’s assessment of the strategic rationale for, and the potential benefits of the Share Exchange; (vi) the past and current
operations and financial condition and future prospects of the Buyer; (vii) the reporting price and trading activity for the Buyer’s
common stock; (viii) certain publicly available information, including, but not limited to, the Buyer’s recent filings with the Securities
and Exchange Commission and the financial statements set forth therein; (ix) the financial terms, to the extent publicly available, of
certain acquisition and financing transactions that we deemed to be relevant; and (x) such other analyses and such other factors as we
deemed appropriate for the purpose of rendering our opinion.
We
have assumed and relied upon, without verifying independently, the accuracy and completeness of all information that was publicly
available or was furnished, or otherwise made available, to us or discussed with or reviewed by or for us for purposes of preparing
this opinion. We have further assumed that the financial information provided has been prepared by the respective managements of
Buyer and the Company on a reasonable basis in accordance with industry practice, and that the managements of Buyer and the Company
are not aware of any information or facts that would make any information provided to us incomplete or misleading. Without limiting
the generality of the foregoing, for the purpose of this opinion, we have assumed that the respective managements of Buyer and the
Company prepared reasonably the financial forecasts, estimates and other forward-looking information reviewed by us, based on
assumptions reflecting their best currently available estimates and judgments as to the expected future results of operations and
financial condition of Buyer and the Company, respectively. We express no opinion as to any such financial forecasts, estimates or
forward-looking information or the assumptions on which they were based.

In
connection with our opinion, we have assumed and relied upon, without independent verification, the accuracy and completeness of all
of the financial, legal, regulatory, tax, accounting and other information provided to, discussed with or reviewed by us. Our opinion
does not address any legal, regulatory, tax or accounting issues.
In
arriving at our opinion, we have assumed that the executed Agreement will be in all material respects identical to the Draft Agreement
reviewed by us. We have relied upon and assumed, without independent verification, that (i) the representations and warranties of all
parties set forth in the Agreement and all related documents and instruments that are referred to therein are true and correct, (ii)
each party to the Agreement will fully and timely perform all of the covenants and agreements required to be performed by such party,
(iii) the Share Exchange will be consummated pursuant to the terms of the Agreement without amendments thereto, and (iv) all conditions
to the consummation of the Share Exchange will be satisfied without waiver by any party of any conditions or obligations thereunder.
Additionally, we have assumed that all the necessary regulatory approvals and consents required for the Share Exchange will be obtained
in a manner that will not adversely affect the Company.
In
arriving at our opinion, we have not performed any appraisals or valuations of any specific assets or liabilities (fixed, contingent
or other) of Buyer or the Company, and have not been furnished or provided with any such appraisals or valuations. Without limiting the
generality of the foregoing, we have undertaken no independent analysis of any pending or threatened litigation, regulatory action, possible
unasserted claims or other contingent liabilities, to which Buyer, the Company or any of their respective affiliates is a party or may
be subject, and at your direction and with your consent, our opinion makes no assumption concerning, and therefore does not consider,
the possible assertion of claims, outcomes or damages arising out of any such matters.
This
opinion is necessarily based upon the information available to us and facts and circumstances as they exist and are subject to evaluation
on the date hereof; events occurring after the date hereof could materially affect the assumptions used in preparing this opinion. We
are not expressing any opinion herein as to the value of the shares of Buyer Common Stock to be issued in the Share Exchange or the prices
at which shares of Buyer Common Stock may trade following announcement of the Share Exchange or at any future time, nor are we expressing
any opinion regarding the fairness, from a financial point of view, to Buyer of the Private Placement Investment. We have not undertaken
to reaffirm or revise this opinion or otherwise comment upon any events occurring after the date hereof and do not have any obligation
to update, revise or reaffirm this opinion.

We
have been engaged by Buyer to render this opinion. We will receive a fee in the amount of $250,000 for the provision of this opinion,
which fee is not contingent on the successful completion of the Share Exchange. The Buyer has also agreed to indemnify us against certain
liabilities and to reimburse us for certain expenses in connection with our services. In the ordinary course of business, we and our
affiliates may acquire, hold or sell, for our and our affiliates’ own accounts and for the accounts of customers, equity, debt and other
securities and financial instruments (including bank loans and other obligations) of Buyer and the Company, and, accordingly, may at
any time hold a long or a short position in such securities. Except as described below, we have not had a material relationship with,
nor otherwise received fees from, Buyer or the Company during the two years preceding the date hereof. On July 31, 2023, we acted as
the Buyer’s exclusive placement agent in connection with a warrant inducement transaction for which we received a cash fee of $230,348,
reimbursement of our expenses, a non-accountable expense allowance of $35,000, and 149,173 warrants to purchase shares of Buyer Common
Stock at an exercise price of $1.3625 per share and a six-month right of first refusal commencing on July 31, 2023 offering to act as
the sole book running manager, sole underwriter or sole placement agent on any public offering (including at the market facility) or
a private placement or any other capital raising financing of equity, equity linked or debt securities for the Buyer. We also have the
right to receive additional warrants to purchase shares of Buyer Common Stock at an exercise price of $1,3625 per share in an a amount
equal to 6% of the shares issued upon the cash exercise of any inducement preferred investment options. On March 23, 2023, we entered
into an ATM program with Buyer under which we acted as the exclusive sales agent but have received no fees in connection therewith. On
August 9, 2022, we acted as the Buyer’s exclusive placement agent in connection with a private placement of shares of Buyer Common Stock
and preferred investment rights for which we received a cash fee of $680,000, reimbursement of our expenses and warrants to purchase
220,997 warrants to purchase shares of Buyer Common Stock at an exercise price of $3.3938 per share. On April 13, 2022, we acted as the
Buyer’s exclusive placement agent in connection with a private placement of shares of Buyer’s Common Stock and preferred investment options
for which we received a cash fee of $850,009, reimbursement of our expenses and warrants to purchase 70,489 shares of Buyer Common Stock
at an exercise price of $8.46875 per share. In the future, we may provide financial advisory and investment banking services to Buyer,
the Company or their respective affiliates for which we would expect to receive compensation.
Consistent
with applicable legal and regulatory requirements, H.C. Wainwright & Co., LLC has adopted policies and procedures to establish
and maintain the independence of our research departments and personnel. As a result, our research analysts may hold views, make
statements or investment recommendations and/or publish research reports Page with respect to Buyer, the Company and/or the Share
Exchange that differ from the views of our investment banking personnel.

This
opinion has been prepared for the information of the Board of Directors of Buyer for its use in connection with its consideration of
the Share Exchange and is not intended to be and does not constitute a recommendation to any stockholder of Buyer as to how such stockholder
should vote on any matter relating to the Share Exchange or any other matter. This opinion shall not be disclosed, referred to or published
(in whole or in part), nor shall any public references to us be made, without our prior written approval. This opinion has been approved
for issuance by the H.C. Wainwright & Co., LLC Fairness Opinion Committee.
This
opinion addresses only the fairness, from a financial point of view, to Buyer of the proposed Exchange Consideration and does not address
the relative merits of the Share Exchange or any alternatives to the Share Exchange, Buyer’s underlying decision to proceed with or effect
the Share Exchange, or any other aspect of the Share Exchange. This opinion does not address the fairness of the Merger to the holders
of any class of securities, creditors or other constituencies of Buyer. We are not experts in, nor do we express an opinion on, legal,
tax, accounting or regulatory issues. We do not express an opinion about the fairness of the amount or nature of any compensation payable
or to be paid to any of the officers, directors or employees of Buyer, whether or not relative to the Share Exchange.
Based
upon and subject to the foregoing, it is our opinion that, as of the date hereof, the Exchange Consideration is fair from a financial
point of view to Buyer.
Sincerely,
| /s/
H.C. wainwright & Co.’ LLC |
|
| H.C.
Wainwright & Co., LLC |
|
INDEX TO FINANCIAL STATEMENTS
ONCONETIX INC.
PROTEOMEDIX AG
ONCONETIX, INC.
Condensed Balance Sheets
| | |
September 30,
2023 | | |
December 31,
2022 | |
| |
(Unaudited) | | |
| |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash | |
$ | 7,653,975 | | |
$ | 25,752,659 | |
| Inventories | |
| 1,419,272 | | |
| — | |
| Prepaid expenses and other current assets | |
| 467,738 | | |
| 469,232 | |
| Receivable from related parties, net | |
| — | | |
| 35,850 | |
| Total current assets | |
| 9,540,985 | | |
| 26,257,741 | |
| | |
| | | |
| | |
| Prepaid expenses, long-term | |
| 55,499 | | |
| 38,617 | |
| Property and equipment, net | |
| 12,503 | | |
| 14,089 | |
| Deferred offering costs | |
| 366,113 | | |
| — | |
| Intangible asset | |
| 17,906,771 | | |
| — | |
| Total assets | |
$ | 27,881,871 | | |
$ | 26,310,447 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable | |
$ | 3,176,332 | | |
$ | 1,499,296 | |
| Accrued expenses | |
| 1,538,544 | | |
| 2,409,128 | |
| Notes payable, net of debt discount of $569,907 | |
| 12,920,093 | | |
| — | |
| Contingent warrant liability | |
| 10,461 | | |
| 14,021 | |
| Total current liabilities | |
| 17,645,430 | | |
| 3,922,445 | |
| | |
| | | |
| | |
| Commitments and Contingencies (see Note 9) | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ equity | |
| | | |
| | |
| Preferred stock, $0.00001 par value, 10,000,000 shares authorized at September 30, 2023 and December 31, 2022; 10,000 and 0 shares designated as Series A convertible preferred stock at September 30, 2023 and December 31, 2022, respectively; 0 shares issued and outstanding at September 30, 2023 and December 31, 2022 | |
| — | | |
| — | |
| Common stock, $0.00001 par value, 250,000,000 shares authorized at September 30, 2023 and December 31, 2022; 18,336,597 and 15,724,957 shares issued at September 30, 2023 and December 31, 2022, respectively; 17,819,198 and 15,265,228 shares outstanding at September 30, 2023 and December 31, 2022, respectively | |
| 183 | | |
| 157 | |
| Additional paid-in-capital | |
| 45,297,371 | | |
| 42,331,155 | |
| Treasury stock, at cost; 517,399 and 459,729 shares of common stock at September 30, 2023 and December 31, 2022, respectively | |
| (625,791 | ) | |
| (566,810 | ) |
| Accumulated deficit | |
| (34,435,322 | ) | |
| (19,376,500 | ) |
| Total stockholders’ equity | |
| 10,236,441 | | |
| 22,388,002 | |
| Total liabilities and stockholders’ equity | |
$ | 27,881,871 | | |
$ | 26,310,447 | |
The accompanying notes are an integral part
of these unaudited condensed financial statements.
ONCONETIX, INC.
Condensed Statements of Operations
(Unaudited)
| | |
Three Months
Ended
September 30,
2023 | | |
Three Months
Ended
September 30,
2022 | | |
Nine Months
Ended
September 30,
2023 | | |
Nine Months
Ended
September 30,
2022 | |
| Operating expenses | |
| | |
| | |
| | |
| |
| Selling, general and administrative | |
$ | 4,268,845 | | |
$ | 2,694,254 | | |
$ | 8,337,615 | | |
$ | 7,311,243 | |
| Research and development | |
| 219,238 | | |
| 1,175,480 | | |
| 2,148,327 | | |
| 2,924,037 | |
| Impairment of deposit on asset purchase agreement | |
| — | | |
| — | | |
| 3,500,000 | | |
| — | |
| Total operating expenses | |
| 4,488,083 | | |
| 3,869,734 | | |
| 13,985,942 | | |
| 10,235,280 | |
| Loss from operations | |
| (4,488,083 | ) | |
| (3,869,734 | ) | |
| (13,985,942 | ) | |
| (10,235,280 | ) |
| Other income (expense) | |
| | | |
| | | |
| | | |
| | |
| Loss on extinguishment of note payable | |
| (490,000 | ) | |
| — | | |
| (490,000 | ) | |
| — | |
| Interest expense | |
| (269,097 | ) | |
| — | | |
| (483,093 | ) | |
| — | |
| Change in fair value of contingent warrant liability | |
| (99,728 | ) | |
| 3,072 | | |
| (99,787 | ) | |
| 33,375 | |
| Total other income (expense) | |
| (858,825 | ) | |
| 3,072 | | |
| (1,072,880 | ) | |
| 33,375 | |
| Net loss | |
$ | (5,346,908 | ) | |
$ | (3,866,662 | ) | |
$ | (15,058,822 | ) | |
$ | (10,201,905 | ) |
| Cumulative preferred stock dividends | |
| — | | |
| — | | |
| — | | |
| 96,359 | |
| Net loss applicable to common stockholders | |
$ | (5,346,908 | ) | |
$ | (3,866,662 | ) | |
$ | (15,058,822 | ) | |
$ | (10,298,264 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per share attributable to common stockholders, basic and diluted | |
$ | (0.31 | ) | |
$ | (0.27 | ) | |
$ | (0.92 | ) | |
$ | (0.94 | ) |
| Weighted average number of common shares outstanding, basic and diluted | |
| 17,521,562 | | |
| 14,338,379 | | |
| 16,452,136 | | |
| 10,949,265 | |
The accompanying notes are an integral part
of these unaudited condensed financial statements.
ONCONETIX, INC.
Condensed Statements of Stockholders’ Equity
(Unaudited)
| | |
Preferred Stock | | |
Common Stock | | |
Additional
Paid-in | | |
Treasury Stock | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Shares | | |
Amount | | |
Deficit | | |
Equity | |
| Balance at December 31, 2022 | |
| — | | |
$ | — | | |
| 15,724,957 | | |
$ | 157 | | |
$ | 42,331,155 | | |
| (459,729 | ) | |
$ | (566,810 | ) | |
$ | (19,376,500 | ) | |
$ | 22,388,002 | |
| Exercise of pre-funded warrants | |
| — | | |
| — | | |
| 646,640 | | |
| 7 | | |
| (7 | ) | |
| — | | |
| — | | |
| — | | |
| — | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 185,578 | | |
| — | | |
| — | | |
| — | | |
| 185,578 | |
| Purchase of treasury shares | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (32,638 | ) | |
| (33,454 | ) | |
| — | | |
| (33,454 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (2,846,644 | ) | |
| (2,846,644 | ) |
| Balance at March 31, 2023 | |
| — | | |
$ | — | | |
| 16,371,597 | | |
$ | 164 | | |
$ | 42,516,726 | | |
| (492,367 | ) | |
$ | (600,264 | ) | |
$ | (22,223,144 | ) | |
$ | 19,693,482 | |
| Exercise of stock options | |
| — | | |
| — | | |
| 45,920 | | |
| — | | |
| 459 | | |
| — | | |
| — | | |
| — | | |
| 459 | |
| Issuance of restricted stock | |
| — | | |
| — | | |
| 512,940 | | |
| 5 | | |
| (5 | ) | |
| — | | |
| — | | |
| — | | |
| — | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 272,781 | | |
| — | | |
| — | | |
| — | | |
| 272,781 | |
| Purchase of treasury shares | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (25,032 | ) | |
| (25,527 | ) | |
| — | | |
| (25,527 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (6,865,270 | ) | |
| (6,865,270 | ) |
| Balance at June 30, 2023 | |
| — | | |
$ | — | | |
| 16,930,457 | | |
$ | 169 | | |
$ | 42,789,961 | | |
| (517,399 | ) | |
$ | (625,791 | ) | |
$ | (29,088,414 | ) | |
$ | 13,075,925 | |
| Issuance of common stock from exercise of preferred investment options | |
| — | | |
| — | | |
| 1,575,000 | | |
| 16 | | |
| 2,272,822 | | |
| — | | |
| — | | |
| — | | |
| 2,272,838 | |
| Issuance of warrants for settlement of contingent warrants | |
| — | | |
| — | | |
| — | | |
| — | | |
| 129,184 | | |
| — | | |
| — | | |
| — | | |
| 129,184 | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 105,402 | | |
| — | | |
| — | | |
| — | | |
| 105,402 | |
| Restricted stock forfeitures | |
| — | | |
| — | | |
| (168,860 | ) | |
| (2 | ) | |
| 2 | | |
| — | | |
| — | | |
| — | | |
| — | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (5,346,908 | ) | |
| (5,346,908 | ) |
| Balance at September 30, 2023 | |
| — | | |
$ | — | | |
| 18,336,597 | | |
$ | 183 | | |
$ | 45,297,371 | | |
| (517,399 | ) | |
$ | (625,791 | ) | |
$ | (34,435,322 | ) | |
$ | 10,236,441 | |
| | |
| | |
| | |
| | |
| | |
Additional | |
| | |
Total | |
| | |
Preferred Stock | | |
Common Stock | | |
Paid-in | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | |
Deficit | | |
Equity | |
| Balance at December 31, 2021 | |
| 1,146,138 | | |
$ | 11 | | |
| 3,200,000 | | |
$ | 32 | | |
$ | 7,403,204 | |
$ | (5,956,670 | ) | |
$ | 1,446,577 | |
| Issuance of common stock in initial public offering, net of $2.9 million of offering costs | |
| — | | |
| — | | |
| 2,222,222 | | |
| 22 | | |
| 17,138,818 | |
| — | | |
| 17,138,840 | |
| Conversion of convertible preferred stock to common stock upon initial public offering | |
| (1,146,138 | ) | |
| (11 | ) | |
| 5,626,365 | | |
| 56 | | |
| (45 | ) |
| — | | |
| — | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 19,332 | |
| — | | |
| 19,332 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (2,070,661 | ) | |
| (2,070,661 | ) |
| Balance at March 31, 2022 | |
| — | | |
$ | — | | |
| 11,048,587 | | |
$ | 110 | | |
$ | 24,561,309 | |
$ | (8,027,331 | ) | |
$ | 16,534,088 | |
| Issuance of common stock and warrants in private placement, net of $1.1 million of offering costs | |
| — | | |
| — | | |
| 590,406 | | |
| 6 | | |
| 6,858,322 | |
| — | | |
| 6,858,328 | |
| Exercise of pre-funded warrants | |
| — | | |
| — | | |
| 590,406 | | |
| 6 | | |
| (6 | ) |
| — | | |
| — | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,447,127 | |
| — | | |
| 1,447,127 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (4,264,582 | ) | |
| (4,264,582 | ) |
| Balance at June 30, 2022 | |
| — | | |
$ | — | | |
| 12,229,399 | | |
$ | 122 | | |
$ | 32,866,752 | |
$ | (12,291,913 | ) | |
$ | 20,574,961 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | |
| | | |
| | |
| Issuance of common stock and warrants in private placement, net of $2.2 million of offering costs | |
| — | | |
| — | | |
| 1,350,000 | | |
| 14 | | |
| 8,689,302 | |
| — | | |
| 8,689,316 | |
| Exercise of stock options | |
| — | | |
| — | | |
| 165,452 | | |
| 2 | | |
| 1,653 | |
| — | | |
| 1,655 | |
| Exercise of pre-funded warrants | |
| — | | |
| — | | |
| 945,000 | | |
| 9 | | |
| 936 | |
| — | | |
| 945 | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 329,809 | |
| — | | |
| 329,809 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | |
| (3,866,662 | ) | |
| (3,866,662 | ) |
| Balance at September 30, 2022 | |
| — | | |
$ | — | | |
| 14,689,851 | | |
$ | 147 | | |
$ | 41,888,452 | |
$ | (16,158,575 | ) | |
$ | 25,730,024 | |
The accompanying notes are an integral part
of these unaudited condensed financial statements.
ONCONETIX, INC.
Condensed Statements of Cash Flows
(Unaudited)
| | |
Nine Months Ended
September 30,
2023 | | |
Nine Months Ended
September 30,
2022 | |
| Cash flows from operating activities | |
| | |
| |
| Net loss | |
$ | (15,058,822 | ) | |
$ | (10,201,905 | ) |
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | |
| | | |
| | |
| Impairment of deposit on asset purchase agreement | |
| 3,500,000 | | |
| — | |
| Stock-based compensation | |
| 563,761 | | |
| 1,796,268 | |
| Amortization of debt discount | |
| 483,093 | | |
| — | |
| Loss on extinguishment of note payable | |
| 490,000 | | |
| — | |
| Loss on impairment of other long-lived assets | |
| 267,019 | | |
| — | |
| Loss on related party receivable | |
| 265,648 | | |
| — | |
| Depreciation expense | |
| 4,886 | | |
| 4,906 | |
| Change in fair value of contingent warrant liability | |
| 99,787 | | |
| (33,375 | ) |
| Changes in assets and liabilities: | |
| | | |
| | |
| Inventories | |
| (299,272 | ) | |
| — | |
| Prepaid expenses and other current assets | |
| (9,526 | ) | |
| (469,278 | ) |
| Prepaid expenses, long-term | |
| (16,882 | ) | |
| (66,357 | ) |
| Deposit | |
| — | | |
| (27,588 | ) |
| Accounts payable | |
| 1,418,048 | | |
| 361,103 | |
| Accrued expenses | |
| (976,871 | ) | |
| 2,760,734 | |
| Net cash used in operating activities | |
| (9,269,131 | ) | |
| (5,875,492 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities | |
| | | |
| | |
| Acquisition of assets, including transaction costs of $79,771 | |
| (6,079,771 | ) | |
| — | |
| Deposit made in connection with asset purchase agreement | |
| (3,500,000 | ) | |
| — | |
| Purchases of other long-lived assets | |
| (51,744 | ) | |
| — | |
| Net advances to related parties | |
| (229,798 | ) | |
| (22,149 | ) |
| Purchases of property and equipment | |
| (3,300 | ) | |
| (9,339 | ) |
| Net cash used in investing activities | |
| (9,864,613 | ) | |
| (31,488 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| Purchase of treasury shares | |
| (58,981 | ) | |
| — | |
| Payment of deferred offering costs | |
| (205,093 | ) | |
| (51,304 | ) |
| Principal payment of note payable | |
| (1,000,000 | ) | |
| — | |
| Proceeds from exercise of preferred investment options, net | |
| 2,298,675 | | |
| — | |
| Proceeds from exercise of stock options | |
| 459 | | |
| 1,655 | |
| Proceeds from issuance of common stock in initial public offering, net of underwriting discount | |
| — | | |
| 18,400,000 | |
| Payments of initial public offering costs | |
| — | | |
| (926,972 | ) |
| Proceeds from issuance of common stock and warrants in private placements, net of placement agent discount | |
| — | | |
| 16,468,123 | |
| Payment of private placement issuance costs | |
| | | |
| (777,225 | ) |
| Proceeds from exercise of pre-funded warrants | |
| — | | |
| 945 | |
| Net cash provided by financing activities | |
| 1,035,060 | | |
| 33,115,222 | |
| Net increase (decrease) in cash | |
| (18,098,684 | ) | |
| 27,208,242 | |
| Cash, beginning of period | |
| 25,752,659 | | |
| 1,928,474 | |
| Cash, end of period | |
$ | 7,653,975 | | |
$ | 29,136,716 | |
| | |
| | | |
| | |
| Noncash investing and financing activities: | |
| | | |
| | |
| Inventory and intangible assets acquired through issuance of notes payable | |
$ | 12,947,000 | | |
$ | — | |
| Incremental fair value of exchanged preferred investment options | |
$ | 2,613,011 | | |
$ | 860,204 | |
| Deferred offering costs included in accounts payable and accrued expenses | |
$ | 150,000 | | |
$ | 125,000 | |
| Recognition of contingent warrant liability | |
$ | 25,837 | | |
$ | 75,431 | |
| Warrants issued for settlement of contingent warrants | |
$ | 129,184 | | |
$ | — | |
| Deferred offering costs previously included in prepaid expenses | |
$ | (11,020 | ) | |
$ | — | |
| Exercise of pre-funded warrants | |
$ | 7 | | |
$ | 6 | |
| Issuance of restricted stock | |
$ | 5 | | |
$ | — | |
| Restricted stock forfeitures | |
$ | (2 | ) | |
$ | — | |
| Payment of accrued bonus through related party receivable | |
$ | — | | |
$ | 140,000 | |
| Private placement offering costs included in accounts payable | |
$ | — | | |
$ | 67,823 | |
| Conversion of convertible preferred stock to common stock upon initial public offering | |
$ | — | | |
$ | 45 | |
The accompanying notes are an integral part
of these unaudited condensed financial statements.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 1 — Organization and Basis of Presentation
Organization and Nature of Operations
Onconetix, Inc. (formerly known as Blue
Water Vaccines, Inc.) (the “Company”) was formed on October 26, 2018. Historically, the Company’s focus was on the research
and development of transformational vaccines to prevent infectious diseases worldwide. In April 2023, the Company acquired ENTADFI®,
with plans to commercialize it. ENTADFI® is a Food and Drug Administration (“FDA”)-approved, once daily pill that combines
finasteride and tadalafil for the treatment of benign prostatic hyperplasia. This combination allows men to receive treatment for their
symptoms of benign prostatic hyperplasia without the negative sexual side effects typically seen in patients on finasteride alone. During
the third quarter of 2023, the Company deprioritized its efforts on vaccine development activities to pursue and focus on commercialization
activities for ENTADFI®. On October 30, 2023, the Company announced a new business strategy of focusing
its efforts on building a foundation of therapeutic, diagnostic, and service products in the field of oncology that will bolster and enrich
the practice of medicine for clinicians. ENTADFI® will become the inaugural therapeutic drug in the Company’s expanding
portfolio of oncology therapeutics once launched.
On April 21, 2023, the Company filed an amendment
to its Articles of Incorporation with the Secretary of State of Delaware to change its corporate name from “Blue Water Vaccines,
Inc.” to “Blue Water Biotech, Inc.”. The name change was effective as of April 21, 2023. In connection with the name
change, the Company amended the Company’s bylaws to reflect the corporate name Blue Water Biotech, Inc., also effective on April
21, 2023. No other changes were made to the bylaws.
On May 31, 2023, the board of directors of the
Company (the “Board”) amended the Company’s bylaws to reduce the quorum requirement at meetings of the Company’s
stockholders from a majority of the voting power of the outstanding shares of stock of the Company entitled to vote, to one-third of the
voting power of the outstanding shares of stock of the Company entitled to vote, effective immediately. No other changes were made to
the bylaws.
Initial Public Offering
On February 23, 2022, the Company completed its
initial public offering (“IPO”) in which the Company issued and sold 2,222,222 shares of its common stock, par value $0.00001
per share (“common stock”), at a price to the public of $9.00 per share. Proceeds from the IPO, net of underwriting discounts,
commissions, and offering costs of $2.9 million, were $17.1 million. In connection with the completion of the IPO, all outstanding shares
of the Company’s convertible preferred stock were converted into 5,626,365 shares of common stock.
Basis of Presentation
The Company’s unaudited condensed financial
statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Unaudited Interim Financial Statements
The accompanying condensed balance sheet as of September 30, 2023,
and the condensed statements of operations and the condensed statements of changes in stockholders’ equity for the three and nine
months ended September 30, 2023 and 2022, and the condensed statements of cash flows for the nine months ended September 30, 2023 and
2022 are unaudited. These unaudited interim financial statements have been prepared on the same basis as the audited financial statements,
and in management’s opinion, include all adjustments, consisting of only normal recurring adjustments, necessary for the fair statement
of the Company’s financial position as of September 30, 2023 and its results of operations for the three and nine months ended September
30, 2023 and 2022, and its cash flows for the nine months ended September 30, 2023 and 2022. The financial data and the other financial
information disclosed in the notes to these condensed financial statements related to the three and nine-month periods are also unaudited.
Operating results for the three and nine months ended September 30, 2023, are not necessarily indicative of the results that may be expected
for the year ending December 31, 2023, any other interim periods, or any future year or period. The unaudited condensed financial
statements included in this Report should be read in conjunction with the audited financial statements and notes thereto included
in the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, which includes a broader discussion of the Company’s
business and the risks inherent therein.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 2 — Going Concern and Management’s Plans
The Company’s operating activities to date
have been devoted to seeking licenses, engaging in research and development activities, and potential asset and business acquisitions.
The Company’s product candidates currently under development will require significant additional research and development efforts
prior to commercialization. The Company has financed its operations since inception primarily using proceeds received from seed investors,
and proceeds received from its IPO and private placement issuances in April and August 2022 (the “Private Placements”, see
Note 8). During 2022, the Company completed its IPO and the Private Placements in which the Company received an aggregate of approximately
$33.1 million in net cash proceeds, after deducting placement agent fees and other offering expenses. During the three and nine months
ended September 30, 2023, the Company received net proceeds of approximately $2.3 million in connection with the exercise of preferred
investment options by an investor (see Note 8).
The Company has incurred substantial operating
losses since inception and expects to continue to incur significant operating losses for the foreseeable future. As of September 30, 2023,
the Company had cash of approximately $7.7 million, a working capital deficit of approximately $8.1 million and an accumulated
deficit of approximately $34.4 million.
During June 2023, the Company entered into an
asset purchase agreement with WraSer (the “WraSer APA”) for the acquisition of a significant portion of other assets that
requires the Company to pay consideration of $8.5 million and one million shares of common stock, of which $3.5 million was paid upon
execution of the agreement, $4.5 million of the remainder and common stock was to be paid at closing, and the remaining $500,000 was to
be due June 13, 2024. On September 26, 2023, WraSer and its affiliates filed for relief under chapter 11 of the U.S. Bankruptcy Code in
the Bankruptcy Court. On October 4, 2023, the parties agreed to amend the WraSer APA. The amendment, which is subject to court approval,
seeks, among other things, to eliminate the $500,000 post-closing payment due June 13, 2024 and stagger the $4.5 million cash payment
that the Company would otherwise have to pay at closing to: (i) $2.2 million to be paid at closing, (ii) $2.3 million, to be paid in monthly
installments of $150,000 commencing January 2024 and (iii) 789 shares of Series A Preferred Stock to be paid at closing. On October 6,
2023, the Company was alerted to certain developments in WraSer’s operations relating to WraSer’s inability to manufacture
the active pharmaceutical ingredient for one of the key WraSer Assets, which development the Company believes constitutes a Material Adverse
Effect (as such term is defined in the WraSer APA) that will prevent the Company from closing the transaction. On October 20, 2023, the
Company filed a motion for relief from the automatic stay in the Bankruptcy Court so that the Company can exercise the termination rights
under the WraSer APA, as amended. WraSer has advised the Company that it does not believe that a Material Adverse Event occurred. If the
Bankruptcy Court does not grant the Company’s motion and requires it to complete the transaction, the Company will not be able to
execute its commercialization strategy for the WraSer Assets because there is no manufacturer for the active pharmaceutical ingredient
for Zontivity, the key driver for the WraSer acquisition. Due to the WraSer bankruptcy filing and the Company’s status as an unsecured
creditor of WraSer, it is also unlikely that the Company will recover the $3.5 million initial payment made or any costs and resources
in connection with services provided by the Company under the WraSer Management Services Agreement. In addition, if the WraSer APA is
terminated, the Company will not be able to recover any such costs.
These factors, along with the Company’s
forecasted future cash flows, indicate that the Company will be unable to meet its contractual commitments and obligations as they come
due in the ordinary course of business within one year following the issuance of these condensed financial statements. The Company will
require significant additional capital in the short-term to fund its continuing operations, satisfy existing and future obligations and
liabilities, including the remaining payments due for the acquisition of assets described in Note 5 and other contracts entered into in
support of the Company’s commercialization plans, in addition to funds needed to support the Company’s working capital needs
and business activities. These business activities include the commercialization of ENTADFI®, and the development and commercialization
of the Company’s current product candidates and future product candidates. Management’s plans include generating product revenue
from sales of ENTADFI®, which has not yet been successfully commercialized, a process that will require significant amounts of additional
capital to complete. In addition, certain of the commercialization activities are outside of the Company’s control, including but
not limited to, securing contracts with wholesalers and third party payers, securing contracts with third-party logistics providers, and
obtaining required licensure in various jurisdictions, as well as attempting to secure additional required funding through equity or debt
financings if available; however, there are currently no commitments in place for further financing nor is there any assurance that such
financing will be available to the Company on favorable terms, if at all, which creates significant uncertainty that the Company will
be able to successfully launch ENTADFI®. If the Company is unable to secure additional capital, it may be required to defer any future
clinical trials, should the Company decide to resume these activities, development and/or commercialization of products and product candidates,
and it may take additional measures to reduce expenses in order to conserve its cash in amounts sufficient to sustain operations and meet
its obligations.
Because of historical and expected operating losses
and net operating cash flow deficits, there is substantial doubt about the Company’s ability to continue as a going concern for
one year from the issuance of the condensed financial statements, which is not alleviated by management’s plans. The condensed financial
statements have been prepared assuming the Company will continue as a going concern. These condensed financial statements do not include
any adjustments that might be necessary from the outcome of this uncertainty.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 3 — Summary of Significant Accounting Policies
During the nine months ended September
30, 2023, there were changes to the Company’s significant accounting policies described in the Company’s Annual Report on
Form 10-K for the year ended December 31, 2022, as follows:
Use of Estimates
The preparation of financial statements in conformity
with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and
disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the
reporting periods. The most significant estimates in the Company’s financial statements relate to valuation of inventory, useful
life of the amortizable intangible assets, estimates of future cash flows used to evaluate impairment, accrued research and development
expenses, stock-based compensation, the valuation of preferred stock, and the valuation allowance of deferred tax assets resulting from
net operating losses. These estimates and assumptions are based on current facts, historical experience and various other factors believed
to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets
and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ materially and
adversely from these estimates. To the extent there are material differences between the estimates and actual results, the Company’s
future results of operations will be affected.
Segment
Information
Operating segments are defined as components of
an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker (“CODM”),
or decision-making group, in deciding how to allocate resources and in assessing performance. Prior to the acquisition of ENTADFI®
during the quarter ended June 30, 2023, the Company managed one distinct business segment, which was vaccine discovery and development.
During the second quarter of 2023, as a result of the acquisition of ENTADFI® for which the Company is working towards commercial
launch, the Company operated in two business segments: research and development and commercial. During the third quarter of 2023, the
Company deprioritized its vaccine discovery and development programs, and accordingly, as of September 30, 2023, the Company
was operating in one segment: commercial. Management’s determination that the Company operated as two segments during the second
quarter of 2023 and one segment during the third quarter of 2023, was consistent with the financial information regularly reviewed by
the CODM for purposes of evaluating performance, allocating resources, setting incentive compensation targets, and planning and forecasting
for future periods.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 3 — Summary of Significant
Accounting Policies (cont.)
Inventories
Inventories consist of raw materials, packaging
materials, and work-in-process. Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in,
first-out basis, aside from inventory acquired in an asset acquisition, which is recorded at fair value. The Company periodically reviews
the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise non-saleable items taking into account anticipated
future sales compared with quantities on hand, and the remaining shelf life of goods on hand. If non-saleable items are observed and there
are no alternate uses for the inventory, the Company records a write-down to net realizable value in the period that the decline in value
is first recognized. The Company had no inventory reserves at September 30, 2023 and December 31, 2022.
Acquisitions
The Company evaluates acquisitions to first
determine whether a set of assets acquired constitutes a business and should be accounted for as a business combination. If the assets
acquired are not a business, the transaction is accounted as an asset acquisition in accordance with Accounting Standards Codification
(“ASC”) 805-50, Asset Acquisitions (“ASC 805-50”), which requires the acquiring entity to recognize
assets acquired and liabilities assumed based on the cost to the acquiring entity on a relative fair value basis, except for non-qualifying assets including
financial assets such as inventory. Further, the cost of the acquisition includes the fair value of consideration transferred and direct
transaction costs attributable to the acquisition. Goodwill is not recognized in an asset acquisition and any excess consideration transferred
over the fair value of the net assets acquired is allocated to the identifiable assets based on relative fair values. Contingent consideration
payments in asset acquisitions are recognized when the contingency is determined to be probable and reasonably estimable. If the assets
acquired are a business, the Company accounts for the transaction as a business combination. Business combinations are accounted for by
using the acquisition method of accounting. Under the acquisition method, assets acquired, and liabilities assumed are recorded at their
respective fair values. The excess of the fair value of consideration transferred over the fair value of the net assets acquired is recorded
as goodwill. Contingent consideration obligations are recorded at their fair values on the acquisition date and remeasured at their fair
values each subsequent reporting period until the related contingencies are resolved. The resulting changes in fair values are recorded
in earnings.
Intangible Assets
Intangible assets are reported at cost, less accumulated
amortization. Intangible assets with finite lives are amortized over their estimated useful lives, starting when sales for the related
product begin. Amortization is calculated using the straight-line method.
During the ordinary course of business, the Company
has entered into certain license and asset purchase agreements. Potential milestone payments for development, regulatory, and commercial
milestones are recorded when the milestone is probable of achievement. Upon a milestone being achieved, the associated milestone payment
is capitalized and amortized over the remaining useful life for approved products, or expensed as research and development expense for
milestones relating to products whose FDA approval has not yet been obtained.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including
intangible assets with finite useful lives, for impairment whenever events or changes in business circumstances indicate that the carrying
amount of the assets may not be fully recoverable (a “triggering event”). Factors that the Company considers in deciding when
to perform an impairment review include significant underperformance of the long-lived asset in relation to expectations, significant
negative industry or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is
performed to evaluate a long-lived asset for recoverability, the Company compares forecasts of undiscounted cash flows expected to result
from the use and eventual disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated
undiscounted future cash flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would
be based on the excess of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows. During
the three and nine months ended September 30, 2023, the Company recorded an impairment loss of approximately $267,000 related to $267,000
of implementation costs incurred under cloud computing hosting arrangements that were capitalized during the three and nine months ended September 30, 2023. There were no other
impairment losses on long-lived assets.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 3 — Summary of Significant
Accounting Policies (cont.)
New Accounting Pronouncements
The Company’s management does not believe
that any recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the accompanying
condensed financial statements.
Note 4 — Balance Sheet Details
Inventories
Inventories relate to ENTADFI® product and consisted of the following
as of September 30, 2023, and December 31, 2022:
| | |
September 30,
2023 | | |
December 31,
2022 | |
| Raw materials | |
$ | 329,780 | | |
$ | - | |
| Work-in-process | |
| 1,089,492 | | |
| - | |
| Total | |
$ | 1,419,272 | | |
$ | - | |
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following
as of September 30, 2023, and December 31, 2022:
| | |
September 30,
2023 | | |
December 31,
2022 | |
| Prepaid insurance | |
$ | 277,058 | | |
$ | 148,789 | |
| Prepaid research and development | |
| 89,195 | | |
| 231,981 | |
| Prepaid other | |
| 101,485 | | |
| 88,462 | |
| Total | |
$ | 467,738 | | |
$ | 469,232 | |
Accrued Expenses
Accrued expenses consisted of the following as of September 30, 2023,
and December 31, 2022:
| | |
September 30,
2023 | | |
December 31,
2022 | |
| Accrued research and development | |
$ | 548,287 | | |
$ | 847,747 | |
| Accrued compensation | |
| 386,087 | | |
| 1,132,859 | |
| Accrued deferred offering costs | |
| 125,000 | | |
| 125,000 | |
| Accrued professional fees | |
| 216,000 | | |
| — | |
| Accrued implementation fees | |
| 106,287 | | |
| — | |
| Other accrued expenses | |
| 156,883 | | |
| 125,922 | |
| Accrued franchise taxes | |
| — | | |
| 177,600 | |
| Total | |
$ | 1,538,544 | | |
$ | 2,409,128 | |
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 5 — Acquisitions
ENTADFI®
On April 19, 2023, the Company entered into
an Asset Purchase Agreement (the “ENTADFI® APA”) with Veru Inc. (“Veru”), the seller of the assets. Pursuant
to, and subject to the terms and conditions of, the ENTADFI® APA, the Company purchased substantially all of the assets related to
Veru’s ENTADFI® product (“ENTADFI®”) and assumed certain liabilities of Veru of a trivial amount, (the
“Transaction”) for a total possible consideration of $100 million.
In accordance with the ENTADFI® APA, the Company
agreed to provide Veru with initial consideration totaling $20.0 million, consisting of (i) $6.0 million paid upon the closing of the
Transaction on April 19, 2023, (ii) an additional $4.0 million in the form of a non-interest bearing note payable due on September 30,
2023, and (iii) an additional $10.0 million in the form of two $5.0 million non-interest bearing notes payable, each due on April 19,
2024 and September 30, 2024.
Additionally, the terms of the ENTADFI® APA
require the Company to pay Veru up to an additional $80.0 million based on the Company’s net sales of ENTADFI® after closing
(the “Milestone Payments”). The Milestone Payments are payable as follows: (i) $10.0 million is payable upon the first
time the Company achieves net sales from ENTADFI® of $100.0 million during a calendar year, (ii) $20.0 million is payable upon
the first time the Company achieves net sales from ENTADFI® of $200.0 million during a calendar year, and (3) $50.0 million is payable
upon the first time the Company achieves net sales from ENTADFI® of $500.0 million during a calendar year.
In connection with the Transaction, the Company
also assumed royalty and milestone obligations under an asset purchase agreement for tadalafil-finasteride combination entered into
by Veru and Camargo Pharmaceutical Services, LLC on December 11, 2017 (the “Camargo Obligations”). The Camargo Obligations
assumed by the Company include a 6% royalty on all sales of tadalafil-finasteride and sales milestone payments of up to $22.5 million,
payable to Camargo as follows: (i) $5.0 million is payable upon the first time the Company achieves net sales from ENTADFI® of
$100.0 million during a calendar year, (ii) $7.5 million is payable upon the first time the Company achieves net sales from ENTADFI®
of $200.0 million during a calendar year, and (3) $10.0 million is payable upon the first time the Company achieves net sales from ENTADFI®
of $300.0 million during a calendar year.
On September 29, 2023, the Company entered into an amendment to the
ENTADFI® APA (the “ENTADFI® APA Amendment”), which provides that the $4.0 million note payable originally due on September
30, 2023, was deemed paid and fully satisfied upon (1) the payment to the Seller of $1.0 million in cash on September 29, 2023, and (2)
the issuance to the Seller by October 3, 2023 of 3,000 shares of Series A Convertible Preferred Stock (the “Series A Preferred Stock”)
of the Company (see Note 8). Pursuant to the ENTADFI®APA Amendment, the Series A Preferred Stock will convert to common stock of the
Company one year from the date of issuance, if the required stockholder approval is obtained. The Series A Preferred Stock, which was
issued to the Seller subsequent to September 30, 2023, is initially convertible, in the aggregate, into 5,709,935 shares of the Company’s
common stock, subject to adjustment and certain shareholder approval limitations specified in the Certificate of Designations. Pursuant
to the ENTADFI® APA Amendment, the Company agreed to use commercially reasonable efforts to obtain such shareholder approval by December
31, 2023. The Company also agreed to include the shares of common stock issuable upon conversion of the Series A Preferred Stock in the
next resale registration statement filed with the SEC.
Also, in connection with the Transaction, and
pursuant to the ENTADFI® APA, the Company entered into non-competition and non-solicitation agreements (the “Non-Competition
Agreements”) with two of Veru’s key stockholders and employees (the “Restricted Parties”). The Non-Competition
Agreements generally prohibit the Restricted Parties from either directly or indirectly engaging in the Restricted Business (as such term
is defined in the ENTADFI® APA) for a period of five years from the closing of the Transaction.
The acquisition of ENTADFI® has been
accounted for as an asset acquisition in accordance with ASC 805-50 because substantially all of the fair value of the assets acquired
is concentrated in a single asset, the ENTADFI® product rights. The ENTADFI® products rights consist of trademarks, regulatory
approvals, and other records, and are considered a single asset as they are inextricably linked.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 5 — Acquisitions (cont.)
The following table summarizes the aggregate consideration transferred
for the assets acquired by the Company in connection with the ENTADFI® APA:
| | |
Consideration
Transferred | |
| Consideration transferred at closing | |
$ | 6,000,000 | |
| Fair value of notes payable issued | |
| 12,947,000 | |
| Transaction costs | |
| 79,771 | |
| Total consideration transferred | |
$ | 19,026,771 | |
The fair value of the non-interest bearing notes
payable was estimated using a net present value model using discount rates averaging 8.2%. The resulting fair value is being accreted
to the face value of the notes, through the respective maturity dates. Management evaluated the Milestone Payments and determined that
at the close of the Transaction, they are not considered probable, and as such, the Company did not recognize any amount related to the
Milestone Payments in the consideration transferred.
The following table summarizes the assets acquired with the ENTADFI®
APA:
| | |
Assets Recognized | |
| Inventory | |
$ | 1,120,000 | |
| ENTADFI® Intangible | |
| 17,906,771 | |
| Total fair value of identifiable assets acquired | |
$ | 19,026,771 | |
In accordance with ASC 805-50, the acquired inventory
was recorded at fair value. The remaining consideration transferred was allocated to the ENTADFI® intangible asset, which will be
amortized over its estimated useful life, starting when ENTADFI® sales begin. The Company originally estimated the useful life to
be five years, but during the three months ended September 30, 2023, the useful life was reevaluated and changed to seven years. This
change in estimate has no accounting impact as the amortization period has not yet begun. Acquired inventory is comprised of
work-in-process and raw materials. The fair value of work-in-process inventory was determined based on an estimated sales price of the
finished goods, adjusted for costs to complete the manufacturing process, costs of the selling effort, a reasonable profit allowance
for the remaining manufacturing and selling effort, and an estimate of holding costs. The fair value of raw materials was determined
to approximate replacement cost. The inventory fair value adjustment was approximately $0.3 million and will be amortized as inventory
turns over, which is expected to approximate 1.5 years.
Management evaluated the Camargo Obligations and
determined that at the close of the Transaction, the related sales milestone payments are not considered probable, and as such, the Company
did not recognize any related liability at the date of the Transaction. In addition, royalties under the Camargo Obligations will be recorded
as cost of sales, as the related sales are generated and recognized.
WraSer:
On June 13, 2023 (the “Execution Date”),
the Company entered into an asset purchase agreement with WraSer, LLC, and affiliates (the “Seller”) (the “WraSer
APA”). Pursuant to, and subject to the terms and conditions of, the WraSer APA, on the Closing Date (as defined below) the Company
was to purchase six FDA-approved pharmaceutical assets across several indications, including cardiology, otic infections, and pain management
(the “WraSer Assets”).
Under the terms of the WraSer APA, the
Company was to purchase the WraSer Assets for (i) $3.5 million in cash at signing of the WraSer APA; (ii) $4.5 million in cash on
the later of (x) 90 days after the signing of the WraSer APA or (y) the date that all closing conditions under the WraSer APA are
met or otherwise waived (the “Closing Date”); (iii) 1.0 million shares of the Company’s common stock (the
“Closing Shares”) issuable on the Closing Date, and (iv) $500,000 in cash one year from the Closing Date. On October 4,
2023, the Company and WraSer agreed to amend the WraSer APA (“WraSer APA Amendment”), to modify the payment terms of the
transaction, which amendment is currently pending court approval (see Note 14).
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 5 — Acquisitions (cont.)
Within 90 days of the Closing Date, the Company
will use its best efforts to file with the SEC, (at its sole cost and expense,) a registration statement to register, on Form S-3 registering
under the Securities Act of 1933, as amended (the “Securities Act”), the resale of the Closing Shares and will use its best
efforts to have the registration statement declared effective as soon as practicable after filing.
In conjunction with the WraSer APA, the Company
and the Seller entered into a Management Services Agreement (the “MSA”) on the Execution Date. Pursuant to the terms of the
MSA, the Company will act as the manager of the Seller’s business during the period between the Execution Date and Closing Date.
During this period, the Company will make advances to WraSer, if needed. If, on the Closing Date, the Seller’s cash balance is in
excess of the target amount (“Cash Target”) specified in the MSA, the Company will apply that excess to the $4.5 million cash
payment due upon closing. Conversely, if there is a shortfall, the Company will be required to remit the difference to the Seller over
time.
The WraSer APA can be terminated prior to closing
as follows (i) upon agreement with all parties; (ii) upon breach of contract of either party, uncured within 20 days of notice. If the
WraSer APA is terminated upon agreement with all parties or upon uncured breach of contract by the Company, the initial $3.5 million payment
is retained by the Sellers. If it is determined that there is an uncured breach of contract by the Seller, and the WraSer APA is terminated,
the Company will have an unsecured claim against WraSer for the $3.5 million payment made by the Company upon execution of the WraSer
APA. The closing of the transaction is subject to certain customary closing conditions, including submission of the FDA transfer documentation
to transfer ownership of the acquired product regulatory approvals to the Company.
Management evaluated the terms of the WraSer APA and MSA, and determined
that, at the Execution Date, control under the provisions of ASC 805, Business Combinations, did not transfer to the Company; if
the transaction closes, control will transfer then, and the acquisition date will be the closing date. Management further evaluated the
requirements pursuant to ASC 810, Consolidations, and determined based on the terms of the MSA, and the Company’s involvement
in the Seller’s business, that the Seller is a variable interest entity (“VIE”) to the Company. Management determined
that the Company is not the primary beneficiary of the VIE as the WraSer APA and MSA do not provide the Company with the power to direct
the activities of the VIE that most significantly impact the VIE’s economic performance. While the Company was involved in the day-to-day
business activities of the VIE until WraSer filed for relief under Chapter 11 of the U.S. Bankruptcy Court (see below), the Seller had
to approve substantially all business activities and transactions that significantly impact the economic performance of WraSer during
the term of the MSA. Additionally, the Company is not required to absorb the losses of WraSer if the WraSer APA does not close. As such,
the Company is not required to consolidate WraSer in the Company’s financial statements as of September 30, 2023. The Company recorded
the initial $3.5 million payment as a deposit. The Company does not have any liabilities recorded as of September 30, 2023 associated
with its variable interest in the Seller, and its exposure to the Seller’s losses is limited to no more than the shortfall, if any,
of the Cash Target amount of approximately $1.1 million compared to the Seller’s cash balance on the Closing Date.
On September 26, 2023, WraSer and its affiliates
filed for relief under chapter 11 of the U.S. Bankruptcy Code in the United States Bankruptcy Court for the Middle District of Florida
(the “Bankruptcy Court”), and on October 6, 2023, the Company was alerted to certain issues in WraSer’s operations that
the Company believes constitutes a Material Adverse Effect (as such term is defined in the WraSer APA) that will prevent the Company from
closing the transaction. On October 20, 2023, the Company filed a motion for relief from the automatic stay in the Bankruptcy Court so
that the Company can exercise the termination rights under the WraSer APA, as amended. WraSer has advised the Company that it does
not believe that a Material Adverse Event occurred. If the Bankruptcy Court does not grant the Company’s motion and requires
it to complete the transaction, the Company will not be able to execute its commercialization strategy for the WraSer Assets because there
is no manufacturer for the active pharmaceutical ingredient for Zontivity, the key driver for the WraSer acquisition. Due to the Company’s
status as an unsecured creditor of WraSer, it is unlikely that the Company will recover the $3.5 million initial payment made or any costs
and resources in connection with services provided by the Company under the WraSer MSA and therefore the deposit recorded is impaired.
Management determined that the conditions resulting in impairment existed at September 30, 2023, and accordingly, the Company recorded
a loss on impairment for the $3.5 million deposit during the nine months ended September 30, 2023.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 6 — Significant Agreements
Oxford University Innovation Limited
In December 2018, the Company entered into an option agreement with
Oxford University Innovation (“OUI”), which was a precursor to a license agreement (the “OUI Agreement”), dated
July 16, 2019. Under the terms of the OUI Agreement, the Company holds an exclusive, worldwide license to certain specified patent rights
and biological materials relating to the use of epitopes of limited variability and virus-like particle products and practice processes
that are covered by the licensed patent rights and biological materials for the purpose of developing and commercializing a vaccine product
candidate for influenza. The Company is obligated to use its best efforts to develop and market Licensed Products, as defined in the OUI
Agreement, in accordance with its development plan, report to OUI on progress, achieve the following milestones and must pay OUI nonrefundable
milestone fees when it achieves them: initiation of first Phase I study; initiation of first Phase II study; initiation of first Phase
III/pivotal registration studies; first submission of application for regulatory approval (BLA/NDA); marketing authorization in the United
States; marketing authorization in any EU country; marketing authorization in Japan; first marketing authorization in any other country;
first commercial sale in Japan; first commercial sale in any ROW country; first year that annual sales equal or exceed certain thresholds.
The OUI Agreement also requires the Company to pay certain milestone and royalty payments in the future, as the related contingent events
occur. See Note 9. The OUI Agreement will expire upon ten (10) years from the expiration of the last patent contained in the licensed
patent rights, unless terminated earlier. During the year ended December 31, 2021, the U.S. Patent related to immunogenic composition
was issued to OUI. This patent expires in August 2037. No additional patents have been issued during the three and nine months ended September
30, 2023. Either party may terminate the OUI Agreement for an uncured material breach. The Company was able to terminate the OUI
Agreement for any reason at any time upon six months’ written notice until July 16, 2022, which was the third anniversary of the
OUI Agreement. OUI may terminate immediately if the Company has a petition presented for its winding-up or passes a resolution for winding
up other than for a bona fide amalgamation or reconstruction or compounds with its creditors or has a receiver or administrator appointed.
OUI may also terminate if the Company opposes or challenges the validity of any of the patents or applications in the Licensed Technology,
as defined in the OUI Agreement; raises the claim that the know-how of the Licensed Technology is not necessary to develop and market
Licensed Products; or in OUI’s reasonable opinion, is taking inadequate or insufficient steps to develop or market Licensed Products
and does not take any further steps that OUI requests by written notice within a reasonable time. Subsequent to September 30, 2023, the
Company terminated the license agreement with Oxford University Innovation (see Note 14). The amounts due upon termination of the license agreement were not
significant.
St. Jude Children’s Hospital
The Company entered into a license agreement
(the “St. Jude Agreement”), dated January 27, 2020, with St. Jude Children’s Research Hospital (“St.
Jude”). Under the terms of the St. Jude Agreement, the Company holds an exclusive, worldwide license to certain specified
patent rights and biological materials relating to the use of live attenuated streptococcus pneumoniae and practice processes that
are covered by the licensed patent rights and biological materials for the purpose of developing and commercializing a vaccine
product candidate for streptococcus pneumoniae. The St. Jude Agreement requires the Company to pay certain milestone and royalty
payments in the future, as the related contingent events occur. See Note 9. The St. Jude Agreement will expire upon the expiration
of the last valid claim contained in the licensed patent rights, unless terminated earlier. The Company is obligated to use
commercially reasonable efforts to develop and commercialize the licensed product(s). The milestones include the following events:
(i) complete IND enabling study; (ii) initiate animal toxicology study; (iii) file IND; (iv) complete Phase I Clinical Trial; (v)
commence Phase II Clinical Trial; (vi) commence Phase III Clinical Trial; and (vii) regulatory approval, U.S. or foreign equivalent.
If the Company fails to achieve the development milestones contained in the St. Jude Agreement, and if the Company and St. Jude fail
to agree upon a mutually satisfactory revised timeline, St. Jude will have the right to terminate the St. Jude Agreement. Either
party may terminate the St. Jude Agreement in the event the other party (a) files or has filed against it a petition under the
Bankruptcy Act (among other things) or (b) fails to perform or otherwise breaches its obligations under the St. Jude Agreement and
has not cured such failure or breach within sixty (60) days. The Company may terminate for any reason on thirty (30) days written
notice. On May 11, 2022, the Company entered into an amendment to the St. Jude Agreement, whereby the royalty terms, milestone
payments and licensing fees were amended, and a revised development milestone timeline was agreed to. On March 22, 2023, the Company
entered into another amendment to the St. Jude Agreement, whereby the development milestone timeline was further revised, and which
had no financial impact. Subsequent to September 30, 2023, the Company terminated the license agreement with St. Jude (see Note
14). The amounts due upon termination of the license agreement were not significant.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 6 — Significant Agreements (cont.)
Cincinnati Children’s Hospital Medical Center
The Company entered into a license agreement
(the “CHMC Agreement”), dated June 1, 2021, with Children’s Hospital Medical Center, d/b/a Cincinnati Children’s
Hospital Medical Center (“CHMC”). Under the terms of the CHMC Agreement, the Company holds an exclusive, worldwide license
(other than the excluded field of immunization against, and prevention, control, or reduction in the severity of gastroenteritis caused
by rotavirus and norovirus in China and Hong Kong) to certain specified patent and biological materials relating to the use of norovirus
nanoparticles and practice processes that are covered by the licensed patent rights and biological materials for the purpose of developing
and commercializing CHMC patents and related technology directed to a virus-like particle vaccine platform that utilizes nanoparticle
delivery technology that may have potential broad application to develop vaccines for multiple infectious diseases. The term of the CHMC
Agreement begins on the effective date and extends on a jurisdiction by jurisdiction and product by product basis until the later of:
(i) the last to expire licensed patent; (ii) ten (10) years after the first commercial sale; or (iii) entrance onto the market of a biosimilar
or interchangeable product. The Company is obligated to use commercially reasonable efforts to bring licensed products to market through
diligent research and development, testing, manufacturing and commercialization, to use best efforts to make all necessary regulatory
filings and obtain all necessary regulatory approvals, to achieve milestones relating to development and sales, and report to CHMC on
progress. See Note 9. The Company will also be obligated to pay agreed upon development milestone payments and royalty payment to CHMC,
as the related contingent events occur. The Company may terminate the CHMC Agreement for convenience at any time prior to first commercial
sale of a product or process by providing one hundred and eighty (180) days’ written notice to CHMC. It may also terminate for a
CHMC uncured material breach. CHMC may terminate the CHMC Agreement for an uncured Company material breach or insolvency or bankruptcy.
Pursuant to the terms of the CHMC Agreement, if the Company fails to achieve the milestones, and cannot mutually agree with CHMC on an
amendment to the milestones, then CHMC will have the option of converting any and all of such exclusive licenses to nonexclusive licenses,
to continue developing indications that have already entered development at any stage or in which the Company has invested in developing.
CHMC may also terminate the CHMC Agreement to the fullest extent permitted by law in the countries of the worldwide territory, in the
event the Company or its affiliates challenge or induce others set up challenges to the validity or enforceability of any of the Licensed
Patents, as defined in the CHMC Agreement, and the Company will be obligated to reimburse CHMC for its costs, including reasonable attorneys’
fees.
Ology Bioservices, Inc. (which was later acquired
by National Resilience, Inc.)
The Company entered into a Master Services
Agreement (“Ology MSA”), dated July 19, 2019, with Ology, Inc. (“Ology”) to provide services from time to time,
including but not limited to technology transfer, process development, analytical method optimization, current good manufacturing practice
(“cGMP”) manufacture, regulatory affairs, and stability studies of biologic products. Pursuant to the Ology MSA, the Company
and Ology shall enter into a Project Addendum for each project to be governed by the terms and conditions of the Ology MSA.
The Company has entered into two Project Addendums
as of December 31, 2022. The initial Project Addendum was executed on October 18, 2019 and the Company was required to pay Ology an aggregate
of approximately $4 million. Due to unforeseen delays associated with COVID-19, the Company and Ology entered into a letter agreement
dated January 9, 2020 to stop work on the project, at which point the Company had paid Ology $100,000 for services to be provided.
The second Project Addendum was executed on May 21, 2021 and the Company is obligated to pay Ology an aggregate amount of approximately
$2.8 million, plus reimbursement for materials and outsourced testing, which will be billed at cost plus 15%.
During 2022, the Company entered into three amendments
to the Ology MSA to adjust the scope of work defined in the second Project Addendum. The amendments resulted in a net increase to
the Company’s obligations under the second Project Addendum of $154,000. On March 27, 2023, the second Project Addendum to the Ology
MSA was further amended to increase the scope of the project, resulting in an increase to the Company’s obligations of $180,000
under the Ology MSA.
During the three and nine months ended September 30, 2023, the Company
incurred related research and development expense (net gain) of approximately ($56,000) and $231,000, respectively, and at September 30,
2023, the Company had approximately $900,000 recorded as related accounts payable and accrued expenses. During the three and nine months
ended September 30, 2022, the Company incurred related research and development expenses of approximately $496,000 and $988,000,
respectively.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 6 — Significant Agreements (cont.)
University of Texas Health Science Center at
San Antonio
The Company entered into a patent and
technology license agreement (the “UT Health Agreement”), dated November 18, 2022, with the University of Texas Health
Science Center at San Antonio (“UT Health”). Under the terms of the UT Health Agreement, the Company holds an exclusive,
worldwide license (other than the excluded field of vectors, as defined in the UT Health Agreement) to certain specified patent
rights relating to the development of a live attenuated, oral Chlamydia vaccine candidate. An initial non-refundable license
fee of $100,000 was due upon execution of the agreement and subsequent annual license fees are due as follows: $20,000 per year for
each of the four years ending on December 31, 2026; $40,000 per year for each of the two years ending on December 31, 2028, and
$60,000 per year for the year ending December 31, 2029 and each year thereafter until expiration or termination of the UT Health
agreement. The UT Health Agreement also requires the Company to pay certain milestone and royalty payments in the future, as
the related contingent events occur. The UT Health Agreement will expire upon the expiration of the last date of expiration or
termination of the patent rights, unless terminated earlier. The Company may terminate the UT Health Agreement for convenience, by
providing 90 days’ written notice to UT Health. UT Health may terminate the UT Health Agreement in the event the Company (a)
becomes arrears in payment due and does not make payment within 30 days after notification from UT Health or (b) is in breach of any
non-payment provision and does not cure such breach within 60 days after notification from UT Health or (c) UT Health delivers
notice to the Company of three or more actual material breaches of the UT Health Agreement in any 12-month period or (d) in the
event the Company or its affiliates initiates any proceeding or action to challenge the validity, enforceability, or scope of any of
the licensed patents.
Co-development Agreement with AbVacc, Inc.
On February 1, 2023, the Company entered into
a co-development agreement (the “Co-Development Agreement”) with AbVacc, Inc. (“AbVacc”), for the purpose of conducting
research aimed at co-development of specific vaccine candidates, including monkeypox and Marburg virus disease with the potential to expand
to others using the Norovirus nanoparticle platform (“Co-Development Project”), and to govern the sharing of materials and
information, as defined in the Co-Development Agreement, for the Co-Development Project. Under the Co-Development Agreement, AbVacc and
the Company will collaborate, through a joint development committee, to establish and implement a development plan or statement of work
for each Co-Development Project targeted product. Under the Co-Development Agreement, either the Company or AbVacc, whichever party
is the primary sponsor of any resulting product (as defined in the Co-Development Agreement), will be obligated to compensate the other
party for certain milestone payments that would range between $2.1 million and $4.75 million, plus royalties of between 2% to 4%. There
is no fixed obligation for either party, and each party will be responsible for their own costs. The term of the Co-Development Agreement
is three years from the effective date, unless previously terminated by either party, in accordance with the Co-Development
Agreement. During the three and nine months ended September 30, 2023, the Company incurred approximately $2,000 and $21,000, respectively,
in costs for research and development related to the Co-Development Agreement. As of September 30, 2023, the Company evaluated the likelihood
of the Company achieving the specified milestones and generating product sales and determined that the likelihood is not yet probable
and as such no accrual of these payments is required as of September 30, 2023.
Services Agreement
On July 21, 2023, the Company, entered into a Licensing and Services
Master Agreement (“Master Services Agreement”) and a related statement of work with a vendor, pursuant to which the vendor
will provide to the Company commercialization services for the Company’s products, including recruiting, managing, supervising and
evaluating sales personnel and providing sales-related services for such products, for fees totaling up to $29.1 million over the term
of the statement of work. The statement of work had a term through September 6, 2026, unless earlier terminated in accordance with the
Master Services Agreement and the statement of work. On July 29, 2023, a second statement of work was entered into with the same vendor
for certain subscription services providing prescription market data access to the Company. The fees under this statement of work totaled
approximately $800,000, and the term was through July 14, 2025. As of September 30, 2023, the Company prepaid approximately $865,000 for
commercialization services under the first statement of work. The Company also had approximately $898,000 recorded in related
accounts payable as of September 30, 2023. As the prepayment can be applied against amounts invoiced
from the vendor, the prepayment of approximately $865,000 has been offset against accounts payable in the accompanying condensed balance
sheet. On October 12, 2023, the Company terminated the Master Services Agreement and statements of work (see Note 14).
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 7
— Notes Payable
In connection with the ENTADFI® APA (see Note
5), the Company executed three non-interest bearing notes payable (the “Notes”) in the principal amounts of $4.0 million,
$5.0 million and $5.0 million with maturity dates of September 30, 2023, April 19, 2024, and September 30, 2024, respectively. No principal
payments are due until maturity; however, the Company may voluntarily prepay the Notes with no penalty. Additionally, in an Event of Default,
as defined in the Notes, the unpaid principal amount of the Notes will accrue interest at a rate of 10.0% per annum.
On September 29, 2023, the Company and the note holder entered into
an amendment to the ENTADFI® APA, which provided that the $4.0 million note payable originally due on September 30, 2023, was deemed
paid and fully satisfied upon (1) the payment to the Seller of $1.0 million in cash on September 29, 2023, and (2) the issuance to the
Seller by October 3, 2023 of 3,000 shares of Series A Preferred Stock of the Company (see Notes 5 and 14). In connection with the ENTADFI®
APA Amendment, the Company recorded an extinguishment loss on the note payable of approximately $490,000, which represents the difference
between the fair value of the Series A Preferred Stock that will be issued to settle the debt and the carrying value of the note payable
as of September 29, 2023. The balance of the note payable included in the accompanying condensed balance sheet at September 30, 2023,
has been adjusted to the fair value of the Series A Preferred Stock that was issued and relieved the obligation on October 3, 2023. The
extinguishment loss is recognized in other income (expense) in the accompanying condensed statements of operations for the three and nine
months ended September 30, 2023.
To determine the fair value of the Series A Preferred
Stock, the Company first derived the business enterprise value (“BEV”) using a discounted cash flow method. The BEV
was adjusted to an equity value assuming $3.0 million of debt converted to Series A Preferred Stock, which was then allocated across the
Company’s securities. The concluded value for the Series A Preferred Stock utilized the Black-Scholes option pricing model, which
was classified as level 3 in the valuation hierarchy due to the presence of significant unobservable inputs. The following key assumptions
were used in the model: volatility rate of 100%, risk free interest rate of 4.6%, 5.0 year expected term, and the Company’s
aggregate equity value. The volatility was based on the historical and implied volatility of a peer group and the risk-free interest rate
was based on the implied yield available on U.S. Treasury securities with a term commensurate with the estimated expected term.
The Company imputed interest on the Notes using
an average discount rate of 8.2% and recorded a debt discount of approximately $1.1 million at the issuance date. The debt discount is
reflected as a reduction in the carrying amount of the Notes and amortized to interest expense through the respective maturity dates,
using the effective interest method. The Company recorded approximately $0.3 million and $0.5 million of associated interest expense during
the three and nine months ended September 30, 2023, respectively. The unamortized debt discount as of September 30, 2023 was approximately
$0.6 million.
Future minimum principal payments on the Notes as of September 30,
2023 includes $3.0 million in principal that was settled through issuance of Series A Preferred Stock in October 2023 and $10 million
in principal payments that are due in 2024.
Note 8 — Stockholders’ Equity
Authorized Capital
On February 23, 2022, in connection with the closing
of the IPO, the Company filed with the Secretary of State of the State of Delaware its second amended and restated certificate of incorporation
(the “A&R COI”), which became effective immediately. There was no change to the Company’s authorized shares of common
stock and preferred stock of 250,000,000 shares and 10,000,000 shares, respectively, or the par value, which is $0.00001 for both common
and preferred stock.
Preferred Stock
On September 29, 2023, the Company filed a Certificate of Designations
of Rights and Preferences of Series A Convertible Preferred Stock of the Company (the “Certificate of Designations”) with
the State of Delaware to designate and authorize the issuance of up to 10,000 shares of Series A Preferred Stock. Pursuant to the Certificate
of Designations, each share of Series A Preferred Stock is convertible into that number of shares of the Company’s common stock
determined by dividing the Stated Value (as defined in the Certificate of Designations) of $1,000 per share by the Conversion Price (as
defined in the Certificate of Designations) of $0.5254 per share, subject to adjustment as provided in the Certificate of Designations,
subject to certain shareholder approval limitations. The Series A Preferred Stock is entitled to share ratably in any dividends paid on
the Company’s common stock (on an as-if-converted-to-common-stock basis), has no voting rights except as to certain significant
matters specified in the Certificate of Designations, and has a liquidation preference equal to the Stated Value of $1,000 per share plus
any accrued but unpaid dividends thereon. The Series A Preferred Stock is redeemable in whole or in part at the Company’s option
at any time. The Certificate of Designations authorized the issuance of up to 10,000 shares of Series A Preferred Stock. As of September
30, 2023, there were no shares of Series A Convertible Preferred Stock outstanding (see Notes 5 and 14).
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8 — Stockholders’
Equity (cont.)
Common Stock
As of September 30, 2023, and December 31, 2022,
there were 18,336,597 and 15,724,957 shares of common stock issued, respectively, and 17,819,198 and 15,265,228 shares of common stock
outstanding, respectively.
Holders of the Company’s common stock are
entitled to one vote for each share held of record and are entitled upon liquidation of the Company to share ratably in the net assets
of the Company available for distribution after payment of all obligations of the Company and after provision has been made with respect
to each class of stock, if any, having preference over the common stock. The shares of common stock are not redeemable and have no preemptive
or similar rights.
On February 17, 2022, the Company entered
into an underwriting agreement (the “Underwriting Agreement”) with Boustead Securities, LLC, acting as representative of the
underwriters (“Boustead”), in relation to the Company’s IPO, pursuant to which the Company agreed to sell to the underwriters
an aggregate of 2,222,222 shares of the Company’s common stock, at a price of $9.00 per share. The IPO closed on February 23,
2022 and resulted in net proceeds to the Company, after deducting the 8% underwriting discount, and other offering costs, of approximately
$17.1 million.
Treasury Stock
On November 10, 2022, the Board approved a stock
repurchase program (the “Repurchase Program”) to allow the Company to repurchase up to 5.0 million shares of common stock
with a maximum price of $1.00 per share, with discretion to management to make purchases subject to market conditions. On November 18,
2022, the Board approved an increase to the maximum price to $2.00 per share. There is no expiration date for this program.
During the nine months ended September 30, 2023, the Company repurchased
57,670 shares of common stock, for an aggregate of approximately $59,000, at an average price of $1.02 per share. There were no repurchases
of common stock during the three months ended September 30, 2023. Shares that are repurchased are classified as treasury stock pending
future use and reduce the number of shares outstanding used in calculating earnings per share. As of September 30, 2023, there are approximately
4.5 million shares remaining, that can be repurchased under the Repurchase Program.
Private Investments in Public Equity
April 2022 Private Placement
On April 19, 2022, the Company consummated the
closing of a private placement (the “April 2022 Private Placement”), pursuant to the terms and conditions of a securities
purchase agreement, dated as of April 13, 2022. At the closing of the April 2022 Private Placement, the Company issued 590,406 shares
of common stock, pre-funded warrants to purchase an aggregate of 590,406 shares of common stock and preferred investment options to purchase
up to an aggregate of 1,180,812 shares of common stock. The purchase price of each share of common stock together with the associated
preferred investment option was $6.775, and the purchase price of each pre-funded warrant together with the associated preferred investment
option was $6.774. The aggregate net cash proceeds to the Company from the April 2022 Private Placement were approximately $6.9 million,
after deducting placement agent fees and other offering expenses. The pre-funded warrants had an exercise price of $0.001 per share and
were exercised in full on May 24, 2022. The preferred investment options, which had an exercise price of $6.65 per share, were exchanged
in connection with the August 2022 Private Placement, as discussed below.
H.C. Wainwright & Co., LLC (“Wainwright”)
acted as the exclusive placement agent for the April 2022 Private Placement. The Company agreed to pay Wainwright a placement agent fee
and management fee equal to 7.5% and 1.0%, respectively, of the aggregate gross proceeds from the April 2022 Private Placement and reimburse
certain out-of-pocket expenses up to an aggregate of $85,000. In addition, the Company issued warrants to Wainwright (the “April
Wainwright Warrants”) to purchase up to 70,849 shares of common stock. The Wainwright Warrants are in substantially the same form
as the preferred investment options, except that the exercise price is $8.46875. The form of the preferred investment options is a warrant,
and as such the preferred investment options, the pre-funded warrants, and the Wainwright Warrants are collectively referred to as the
“April 2022 Private Placement Warrants”. Further, upon any exercise for cash of any preferred investment options, the Company
agreed to issue to Wainwright additional warrants to purchase the number of shares of common stock equal to 6.0% of the aggregate number
of shares of common stock underlying the preferred investment options that have been exercised, also with an exercise price of $8.46875
(the “April Contingent Warrants”). The maximum number of April Contingent Warrants issuable under this provision of 70,849
were exchanged in connection with the August 2022 Private Placement, as discussed below.
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8
— Stockholders’ Equity (cont.)
The Company evaluated the terms of the April 2022
Private Placement Warrants and determined that they should be classified as equity instruments based upon accounting guidance provided
in ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815-40, Derivatives and Hedging – Contracts
in Entity’s Own Equity (“ASC 815-40”). Since the Company determined that the April 2022 Private Placement Warrants
were equity-classified, the Company recorded the proceeds from the April 2022 Private Placement, net of issuance costs, within common
stock at par value and the balance of the net proceeds to additional paid in capital.
The Company evaluated the terms of
the April Contingent Warrants and determined that they should be classified as a liability based upon accounting guidance provided in
ASC 815-40. Since the April Contingent Warrants are a form of compensation to Wainwright, the Company recorded the value of the liability
as a reduction of additional paid in capital, with subsequent changes in the value of the liability recorded in other income (expense)
in the accompanying condensed statements of operations.
August 2022 Private Placement
On August 11, 2022, the Company consummated the
closing of a private placement (the “August 2022 Private Placement”), pursuant to the terms and conditions of a securities
purchase agreement, dated as of August 9, 2022. At the closing of the August 2022 Private Placement, the Company issued 1,350,000 shares
of common stock, pre-funded warrants to purchase an aggregate of 2,333,280 shares of common stock and preferred investment options to
purchase up to an aggregate of 4,972,428 shares of common stock. The purchase price of each share of common stock together with the associated
preferred investment option was $2.715, and the purchase price of each pre-funded warrant together with the associated preferred investment
option was $2.714. The aggregate net cash proceeds to the Company from the August 2022 Private Placement were approximately $8.7 million,
after deducting placement agent fees and other offering expenses. In addition, the investors in the August 2022 Private Placement, who
are the same investors from the April 2022 Private Placement, agreed to cancel preferred investment options to purchase up to an aggregate
of 1,180,812 shares of the Company’s common stock issued in April 2022. The pre-funded warrants had an exercise price of $0.001
per share. During 2022, an aggregate of 1,686,640 of the pre-funded warrants were exercised. The remaining 646,640 of pre-funded warrants
were exercised during the nine months ended September 30, 2023. The preferred investment options are exercisable at any time on or after
August 11, 2022 through August 12, 2027, at an exercise price of $2.546 per share, subject to certain adjustments as defined in the agreement.
During the three and nine months ended September 30, 2023, 2,486,214 of these preferred investment options were exercised at a reduced
exercise price of $1.09, in connection with the warrant inducement transaction discussed below. As of September 30, 2023, 2,486,214 preferred
investment options are outstanding.
Wainwright acted as the exclusive placement agent
for the August 2022 Private Placement. The Company agreed to pay Wainwright a placement agent fee and management fee equal to 7.5% and
1.0%, respectively, of the aggregate gross proceeds from the August 2022 Private Placement and reimburse certain out-of-pocket expenses
up to an aggregate of $85,000. In addition, the Company issued warrants to Wainwright (the “August Wainwright Warrants”)
to purchase up to 220,997 shares of common stock. The August Wainwright Warrants are in substantially the same form as the preferred
investment options, except that the exercise price is $3.3938. The form of the preferred investment options is a warrant, and as such
the preferred investment options, the pre-funded warrants, and the August Wainwright Warrants are collectively referred to as the “August
2022 Private Placement Warrants”. Further, upon any exercise for cash of any preferred investment options, the Company agreed to
issue to Wainwright additional warrants to purchase the number of shares of common stock equal to 6.0% of the aggregate number of shares
of common stock underlying the preferred investment options that have been exercised, also with an exercise price of $3.3938 (the “August
Contingent Warrants”). The maximum number of August Contingent Warrants issuable under this provision is 298,346, which includes
70,849 of April Contingent Warrants that were modified in connection with the August 2022 Private Placement.
The Company evaluated the terms of the August
2022 Private Placement Warrants and determined that they should be classified as equity instruments based upon accounting guidance provided
in ASC 480 and ASC 815-40. Since the Company determined that the August 2022 Private Placement Warrants were equity-classified, the Company
recorded the proceeds from the August 2022 Private Placement, net of issuance costs, within common stock at par value and the balance
of the net proceeds to additional paid in capital.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8
— Stockholders’ Equity (cont.)
The investors in the April 2022 Private Placement
agreed to cancel the aggregate of 1,180,812 preferred investment options issued in the April 2022 Private Placement, as part of their
participation in the August 2022 Private Placement. The preferred investment options that were cancelled were effectively exchanged for
1,289,148 new preferred investment options in the August 2022 Private Placement, and accordingly have been accounted for as a modification
or exchange of equity-linked instruments. In accordance with ASC 815-40, as the preferred investment options were classified as equity
instruments before and after the exchange, and as the exchange is directly attributable to an equity offering, the Company recognized
the effect of the exchange as an equity issuance cost.
The Company evaluated the terms of the August
Contingent Warrants and determined that they should be classified as a liability based upon accounting guidance provided in ASC 815-40.
As a result of the exchange of the preferred investment options issued in the April 2022 Private Placement, the underlying equity-linked
instruments that would trigger issuance of the April Contingent Warrants was replaced, and therefore the 70,849 of April Contingent Warrants
were exchanged for 70,849 of the August Contingent Warrants. The value of the April Contingent Warrant liability was adjusted to fair
value on the date of modification, using a Monte Carlo simulation, with the change in fair value recognized in the accompanying condensed
statements of operations. The remaining 227,497 August Contingent Warrants were measured as a liability upon the close of the August 2022
Private Placement. Since the Contingent Warrants are a form of compensation to the placement agent, the Company recorded the value of
the liability as a reduction of additional paid in capital.
During the three and nine months ended September 30, 2023, in connection
with the warrant inducement transaction, the Company issued to Wainwright as settlement of the contingent warrant liability associated
with 149,173 of the August Contingent Warrants, which was triggered upon exercise of the underlying preferred investment options. See
Warrant Inducement below for further discussion.
At the Market Offering Agreement
On March 29, 2023, the Company entered into an
At The Market Offering Agreement (the “ATM Agreement”) with H.C. Wainwright & Co., LLC, as sales agent (the “Agent”),
to create an at-the-market equity program under which it may sell up to $3,900,000 of shares of the Company’s common stock (the
“Shares”) from time to time through the Agent (the “ATM Offering”). Under the ATM Agreement, the Agent will be
entitled to a commission at a fixed rate of 3.0% of the gross proceeds from each sale of Shares under the ATM Agreement. The Company has
no obligation to sell, and the Agent is not obligated to buy or sell, any of the Shares under the Agreement and may at any time suspend
offers under the Agreement or terminate the Agreement. The ATM Offering will terminate upon the termination of the ATM Agreement
as permitted therein.
Deferred offering costs associated with the ATM
Agreement are reclassified to additional paid in capital on a pro-rata basis when the Company completes offerings under the ATM Agreement.
Any remaining deferred costs will be expensed to the statements of operations should the planned offering be abandoned.
As of September 30, 2023, no shares have been
sold under the ATM Offering.
Warrant Inducement
On July 31, 2023, the Company entered into a common
stock preferred investment options exercise inducement offer letter (the “Inducement Letter”) with a certain holder (the “Holder”)
of existing preferred investment options (“PIOs”) to purchase shares of the Company’s common stock at the original exercise
price of $2.546 per share, issued on August 11, 2022 (the “Existing PIOs”). Pursuant to the Inducement Letter, the Holder
agreed to exercise for cash its Existing PIOs to purchase an aggregate of 2,486,214 shares of the Company’s common stock (the “Inducement
PIO Shares”), at a reduced exercised price of $1.09 per share, in exchange for the Company’s agreement to issue new preferred
investment options (the “Inducement PIOs”) to purchase up to 4,972,428 shares of the Company’s common stock. The Inducement
PIOs have substantially the same terms as the Existing PIOs.
On August 2, 2023, the Company consummated the
transactions contemplated by the Inducement Letter (the “Warrant Inducement”). The Company received aggregate net proceeds
of approximately $2.3 million from the Warrant Inducement, after deducting placement agent fees and other offering expenses payable by
the Company.
Upon the close of the transaction,
the Company issued the Holder 1,575,000 of the 2,486,214 shares of common stock that were issuable upon exercise of the Existing PIOs.
Due to the beneficial ownership limitation provisions in the Inducement Letter, the remaining 911,214 shares were initially unissued,
and held in abeyance for the benefit of the Holder until notice from the Holder that the shares may be issued in compliance with such
limitation is received. These shares were issued to the Holder in October 2023.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8
— Stockholders’ Equity (cont.)
The Company agreed to file a registration statement covering the resale
of the Inducement PIO Shares issued or issuable upon the exercise of the Inducement PIOs (the “Resale Registration Statement”),
as soon as practicable, and to use commercially reasonable efforts to have such Resale Registration Statement declared effective by the
SEC within 90 days following the date of the Inducement Letter, and to keep the Resale Registration Statement effective at all times until
there are no Inducement PIO Shares. The provision to register the underlying shares in the Warrant Inducement does not require payment
related to the registration rights provided. As such, while the shares were not registered within 90 days of the date of the Inducement
Letter, there is no accounting impact for this provision.
The Company engaged Wainwright to act as its placement
agent in connection with the Warrant Inducement and paid Wainwright a cash fee equal to 7.5% of the gross proceeds received from the exercise
of the Existing PIOs as well as a management fee equal to 1.0% of the gross proceeds from the exercise of the Existing PIOs. The Company
also agreed to reimburse Wainwright for its expenses in connection with the exercise of the Existing PIOs and the issuance of the Inducement
PIOs, up to $50,000 for fees and expenses of legal counsel and other out-of-pocket expenses and agreed to pay Wainwright for non-accountable
expenses in the amount of $35,000. In addition, the exercise for cash of the Existing PIOs triggered the issuance to Wainwright or its
designees, warrants to purchase 149,173 shares of common stock (“Wainwright Inducement Warrants”), which were issuable in
accordance with the terms of the August Contingent Warrants, and have the same terms as the Inducement PIOs except for an exercise price
equal to $1.3625 per share. The Company also agreed to issue warrants to Wainwright upon any exercise for cash of the Inducement PIOs,
that number of shares of common stock equal to 6.0% of the aggregate number of such shares of common stock underlying the Inducement PIOs
that have been exercised, also with an exercise price of $1.3625 (the “Inducement Contingent Warrants”). The maximum number
of Inducement Contingent Warrants issuable under this provision is 298,346.
The Company evaluated the terms of the
Inducement PIOs and the Wainwright Inducement Warrants (collectively, the “August 2023 Inducement Warrants”), and
determined that they should be classified as equity instruments based upon accounting guidance provided in ASC 480 and ASC 815-40.
The Company also evaluated the unissued shares held in abeyance, which represent a prepaid forward contract, and determined that it
is an equity instrument based on the guidance provided in ASC 480 and ASC 815-40.
The Warrant Inducement, which resulted in the
lowering of the exercise price of the Existing PIOs and the issuance of the Inducement PIOs, is considered a modification of the Existing
PIOs under the guidance of Accounting Standards Update (“ASU”) No. 2021-04, Issuer’s Accounting for Certain Modifications
or Exchanges of Equity Classified Written Call Options. The modification is consistent with the “Equity Issuance” classification
under that guidance as the reason for the modification was to induce the holders of the Existing PIOs to cash exercise their warrants,
resulting in the imminent exercise of the Existing PIOs, which raised equity capital and generated net proceeds for the Company of approximately
$2.3 million. As the Existing PIOs and the Inducement PIOs were classified as equity instruments before and after the exchange, and as
the exchange is directly attributable to an equity offering, the Company recognized the effect of the modification of approximately $2.6
million as an equity issuance cost.
In addition, the change in fair value
of the contingent warrant liability associated with 149,173 of the August Contingent Warrants that were settled through issuance of the
Wainwright Inducement Warrants, of approximately $122,000, was recognized in other income (expense) in the accompanying condensed statements
of operations, and the fair value of the contingent warrant liability of approximately $129,000 was derecognized as of the settlement
date. The corresponding amount, representing the fair value of the Wainwright Inducement Warrants, was recognized as additional paid in
capital.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8 — Stockholders’ Equity
(cont.)
The Company evaluated the terms of
the Inducement Contingent Warrants and determined that they should be classified as a liability based upon accounting guidance provided
in ASC 815-40. Since the Inducement Contingent Warrants are a form of compensation to Wainwright, the Company recorded the value of the
liability of approximately $26,000 as a reduction of additional paid in capital, with subsequent changes in the value of the liability
recorded in other income (expense) in the accompanying condensed statements of operations.
Warrants
The following summarizes activity related to the
Company’s outstanding warrants, excluding contingent warrants issuable upon exercise of the preferred investment options, for the
nine months ended September 30, 2023:
| | |
| | |
| | |
Weighted | |
| | |
| | |
| | |
Average | |
| | |
| | |
Weighted | | |
Remaining | |
| | |
| | |
Average | | |
Contractual | |
| | |
Number of | | |
Exercise | | |
Life | |
| | |
Shares | | |
Price | | |
(in years) | |
| Outstanding as of December 31, 2022 | |
| 5,910,914 | | |
$ | 2.37 | | |
| 4.7 | |
| Granted | |
| 5,121,601 | | |
| 1.10 | | |
| | |
| Exercised | |
| (3,132,854 | ) | |
| 0.865 | | |
| | |
| Cancelled | |
| — | | |
| — | | |
| | |
| Outstanding as of September 30, 2023 | |
| 7,899,661 | | |
| 1.68 | | |
| 4.6 | |
| Warrants vested and exercisable as of September 30, 2023 | |
| 7,899,661 | | |
$ | 1.68 | | |
| 4.6 | |
As of September 30, 2023, the outstanding warrants
include 70,849 April 2022 Private Placement Warrants, 2,707,211 August 2022 Private Placement Warrants, and 5,121,601 August 2023 Inducement
Warrants, which are exercisable into 7,899,661 shares of common stock which had a fair value of $0.51 per share, based on the closing
trading price on that day.
Additionally,
as of September 30, 2023, and December 31, 2022, the value of the August Contingent Warrants and the Inducement Contingent Warrants (collectively
the “Contingent Warrants”) was approximately $10,000 and $14,000, respectively. The maximum number of warrants issuable upon
settlement of the Contingent Warrants as of September 30, 2023 and December 31, 2023 was 447,519 and 298,346, respectively.
Equity Incentive Plans
The
Company’s 2019 Equity Incentive Plan (the “2019 Plan”) was adopted by the Board and by its stockholders on July 1,
2019. The Company has reserved 1,400,000 shares of common stock for issuance pursuant to the 2019 Plan.
On February 23, 2022, and in connection with the
closing of the IPO, the Board adopted the Company’s 2022 Equity Incentive Plan (the “2022 Plan”), which is the successor
and continuation of the Company’s 2019 Plan. Under the 2022 Plan, the Company may grant stock options, restricted stock, restricted
stock units, stock appreciation rights, and other forms of awards to employees, directors and consultants of the Company. Upon its effectiveness,
a total of 1,600,000 shares of common stock were reserved for issuance under the 2022 Plan. In August 2022, the number of shares of common
stock reserved for issuance under the 2022 Plan was increased to 2,600,000 and in May 2023, the number of shares of common stock reserved
for issuance under the 2022 Plan was increased to 3,150,000. The stock options and restricted stock granted during the nine months ended
September 30, 2023 were all granted under the 2022 Plan. As of September 30, 2023, there are 1,146,878 shares available for issuance under
the 2022 Plan.
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8
— Stockholders’ Equity (cont.)
Stock Options
The following summarizes activity related to the
Company’s stock options under the 2019 Plan and the 2022 Plan for the nine months ended September 30, 2023:
| | |
| | |
| | |
| | |
Weighted | |
| | |
| | |
| | |
| | |
Average | |
| | |
| | |
Weighted | | |
| | |
Remaining | |
| | |
| | |
Average | | |
Total | | |
Contractual | |
| | |
Number of | | |
Exercise | | |
Intrinsic | | |
Life | |
| | |
Shares | | |
Price | | |
Value | | |
(in years) | |
| Outstanding as of December 31, 2022 | |
| 1,392,654 | | |
$ | 3.30 | | |
$ | 670,161 | | |
| 8.2 | |
| Granted | |
| 102,386 | | |
| 1.19 | | |
| — | | |
| — | |
| Forfeited / cancelled | |
| (70,265 | ) | |
| 3.35 | | |
| — | | |
| — | |
| Exercised | |
| (45,920 | ) | |
| 0.01 | | |
| 45,920 | | |
| — | |
| Outstanding as of September 30, 2023 | |
| 1,378,855 | | |
| 3.25 | | |
| 274,299 | | |
| 7.8 | |
| Options vested and exercisable as of September 30, 2023 | |
| 1,094,563 | | |
$ | 2.95 | | |
$ | 269,994 | | |
| 7.5 | |
The fair value of options granted in 2023 was estimated using the following
assumptions:
| |
|
For the
Nine Months
Ended
September 30, |
|
| |
|
2023 |
|
| Exercise price |
|
$ |
1.05 – 1.29 |
|
| Term (years) |
|
|
5.00 – 10.00 |
|
| Expected stock price volatility |
|
|
113.1% – 119.5 |
% |
| Risk-free rate of interest |
|
|
3.5% – 3.6 |
% |
The weighted average grant date fair value of
stock options granted during the nine months ended September 30, 2023 was $1.08. The aggregate fair value of stock options that vested
during the three and nine months ended September 30, 2023 was approximately $137,000 and $616,000, respectively.
Restricted Stock
On May 9, 2023, the Board’s Compensation
Committee approved the issuance of restricted stock, granted under the Company’s 2022 Plan, to the Company’s executive officers,
employees, and certain of the Company’s consultants. The restricted shares granted totaled 487,500, of which 150,000, 75,000, and
150,000 were granted to the Company’s Chief Executive Officer (“CEO”), Chief Financial Officer, and Chief Business Officer,
respectively. All of the restricted shares granted vest as follows: 50% in January 2024, 25% in August 2024, and 25% in August 2025. In
addition, on May 31, 2023, the Board’s Compensation Committee approved the issuance of 25,440 shares of restricted stock, granted
to the Company’s non-executive Board members, with full vesting on May 31, 2024.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 8 — Stockholders’ Equity
(cont.)
On August 16, 2023, upon his resignation, the
Company’s former Chief Executive Officer forfeited 150,000 shares of unvested restricted stock.
| | |
| | |
Weighted | |
| | |
| | |
Average | |
| | |
| | |
Weighted | |
| | |
| | |
Average | |
| | |
Number of | | |
Grant Date | |
| | |
Shares | | |
Fair Value | |
| Nonvested as of December 31, 2022 | |
| — | | |
$ | — | |
| Granted | |
| 512,940 | | |
| 1.01 | |
| Forfeited / cancelled | |
| (168,860 | ) | |
| 1.02 | |
| Vested | |
| — | | |
| — | |
| Nonvested as of September 30, 2023 | |
| 344,080 | | |
$ | 1.01 | |
Stock-Based Compensation
Stock-based compensation expense related to stock
options and restricted stock, for the three and nine months ended September 30, 2023, and 2022 was as follows:
| | |
For the Three Months Ended
September 30, | | |
For the Nine Months Ended
September 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| Selling, general and administrative | |
$ | 89,832 | | |
$ | 255,115 | | |
$ | 362,191 | | |
$ | 1,193,743 | |
| Research and development | |
| 15,570 | | |
| 74,694 | | |
| 201,570 | | |
| 602,525 | |
| Total | |
$ | 105,402 | | |
$ | 329,809 | | |
$ | 563,761 | | |
$ | 1,796,268 | |
As of September 30, 2023, unrecognized
stock-based compensation expense relating to outstanding stock options and unvested restricted stock is approximately $394,000 and $143,000,
respectively, which is expected to be recognized over a weighted-average period of 1.67 years and 1.49 years, respectively.
ONCONETIX, INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 9 — Commitments and Contingencies
Office Leases
The Company entered into a short-term lease
in Palm Beach, Florida with an unrelated party, with a commencement date of May 1, 2022, for approximately $14,000 per month. The
lease, which was personally guaranteed by the Company’s former CEO, ended on April 30, 2023. During the nine months ended
September 30, 2023, the Company incurred rent expense on this lease of approximately $51,000, and variable lease expense of
approximately $4,000. The Company incurred rent expense for the three and nine months ended September 30, 2022 of approximately
$53,000 and $89,000, respectively.
Litigation
From
time to time, the Company may be subject to various legal proceedings and claims that arise in the ordinary course of its business activities.
As of September 30, 2023, the Company is not a party to any material legal proceedings and is not aware of any pending or threatened claims.
Registration Rights Agreements
In connection with the April 2022 Private Placement
(see Note 8), the Company entered into a Registration Rights Agreement with the purchasers, dated as of April 13, 2022 (the “April
Registration Rights Agreement”). The April Registration Rights Agreement provides that the Company shall file a registration statement
covering the resale of all of the registrable securities (as defined in the April Registration Rights Agreement) with the SEC. The registration
statement on Form S-1 required under the April Registration Rights Agreement was filed with the SEC on May 3, 2022, and became effective
on May 20, 2022. A post-effective amendment to the Form S-1 on Form S-3 relating to such registration statement was filed with the SEC
on April 28, 2023.
In connection with the August 2022 Private Placement
(see Note 8), the Company entered into a Registration Rights Agreement with the purchasers, dated as of August 9, 2022 (the “August
Registration Rights Agreement”). The August Registration Rights Agreement provides that the Company shall file a registration statement
covering the resale of all of the registrable securities (as defined in the August Registration Rights Agreement) with the SEC. The registration
statement on Form S-1 required under the August Registration Rights Agreement was filed with the SEC on August 29, 2022, and became effective
on September 19, 2022. A post-effective amendment to the Form S-1 on Form S-3 relating to such registration statement was filed with the
SEC on April 28, 2023.
Upon the occurrence of any Event (as
defined in the April Registration Rights Agreement and the August Registration Rights Agreement), which, among others, prohibits the purchasers
from reselling the securities for more than ten consecutive calendar days or more than an aggregate of fifteen calendar days during any
12-month period, and should the registration statement cease to remain continuously effective, the Company would be obligated to pay to
each purchaser, on each monthly anniversary of each such Event, an amount in cash, as partial liquidated damages and not as a penalty,
equal to the product of 2.0% multiplied by the aggregate subscription amount paid by such purchaser in the Private Placement. As of September
30, 2023, the Company determined that the likelihood of the Company incurring liquidated damages pursuant to the April Registration Rights
Agreement and the August Registration Rights Agreement is remote, and as such, no accrual of these payments is required as of September
30, 2023.
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 9 — Commitments and Contingencies
(cont.)
Milestone and Royalty Obligations
The Company has entered into various license agreements
with third parties that obligate the Company to pay certain development, regulatory, and commercial milestones, which aggregate to $115.1
million, as well as royalties based on product sales (see Note 6). As of September 30, 2023, the Company evaluated the likelihood of the
Company achieving the specified milestones and generating product sales and determined that the likelihood is not yet probable and as
such no accrual of these payments is required as of September 30, 2023. Subsequent to the balance sheet date, the Company terminated two
of these license agreements (see Note 14).
Indemnification
In the normal course of business, the Company
enters into contracts and agreements that contain a variety of representations and warranties and provide for general indemnifications.
The Company’s exposure under these agreements is unknown because it involves claims that may be made against the Company in the
future but have not yet been made. To date, the Company has not paid any claims related to its indemnification obligations. However, during
the third quarter of 2023, the Company received a claim from its former CEO and a former accounting employee requesting advancement of
certain expenses, which may result in related expenses in the future. As of September 30, 2023, the Company recorded a related accrual
of approximately $91,000, which is included in accrued expenses in the accompanying condensed balance sheet. The maximum potential amount
of future payments the Company could be required to make under these indemnification agreements is not estimable at this
time.
Note 10 — Related Party Transactions
The Company originally engaged the former CEO, who was also the Board
Chairman and prior to the close of the IPO, sole common stockholder of the Company, pursuant to a consulting agreement commencing October
22, 2018, which called for the Company to pay for consulting services performed on a monthly basis. Upon the close of the Company’s
IPO, the consulting agreement was terminated, and the former CEO’s employment agreement became effective. During the nine months
ended September 30, 2022, the Company incurred approximately $63,000 in fees under the consulting agreement, which is recognized in general
and administrative expenses in the accompanying condensed statement of operations.
During 2022 the Company entered into a lease agreement
that was personally guaranteed by the Company’s former CEO. The lease expired on April 30, 2023 (see Note 9).
During the year ended December 31, 2022, the
Company’s compensation committee approved one-time bonus awards of $140,000 and $100,000 to the Company’s former CEO and
Chief Business Officer (“CBO”), respectively, in recognition of their efforts in connection with the Company’s
IPO. These bonuses were recognized during the nine months ended September 30, 2022, as selling, general and administrative expenses
in the accompanying condensed statement of operations.
During the three and nine months ended September
30, 2023, the Company’s Audit Committee completed a review of the Company’s expenses due to certain irregularities identified
with regards to the related party balance. Based on the results of the review, it was determined that the Company paid and recorded within
selling, general and administrative expense personal expenditures of the Company’s former CEO and an accounting employee who was
also the former CEO’s assistant, during 2022 and during the first three quarters of 2023. The Company evaluated the receivable,
which aggregates to approximately $522,000 as of September 30, 2023, and which represents the total of the items identified as personal
in nature for which the Company does not anticipate recovery from the related party. The Company recorded a corresponding reserve for
the full amount, resulting in a net related party receivable balance of $0 as of September 30, 2023. As the Company concluded that the
amounts are not likely to be recovered, this would not cause an adjustment to previously issued financial statements.
Further, the credit card misuse described above resulted in a loss
on related party receivable of approximately $100,000 and $266,000 during the three and nine months ended September 30, 2023, respectively,
which was recorded in selling, general, and administrative expenses in the accompanying statements of operations.
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 10
— Related Party Transactions (cont.)
As of December 31, 2022, the Company had a receivable
from related party of approximately $36,000, consisting of miscellaneous payments made by the Company on the behalf of the Company’s
former CEO, and which was paid in full during the first quarter of 2023.
A former director of the Company, who currently
serves on the Company’s Scientific Advisory Board, serves on the Advisory Board for the Cincinnati Children’s Hospital Medical
Center Innovation Fund, which is affiliated with CHMC. The Company has an exclusive license agreement with CHMC as disclosed in Note 6.
This director resigned from the Board upon the close of the IPO, and also resigned from the Scientific Advisory Board on August 28, 2023.
Note 11 — Income Taxes
No provision for federal, state or foreign income
taxes has been recorded for the three and nine months ended September 30, 2023, and 2022. The Company has incurred net operating losses
for all of the periods presented and has not reflected any benefit of such net operating loss carryforwards in the accompanying condensed
financial statements due to uncertainty around utilizing these tax attributes within their respective carryforward periods. The Company
has recorded a full valuation allowance against all of its deferred tax assets as it is not more likely than not that such assets will
be realized in the near future. The Company’s policy is to recognize interest expense and penalties related to income tax matters
as income tax expense. For the three and nine months ended September 30, 2023, and 2022, the Company has not recognized any interest or
penalties related to income taxes.
Note 12 — Net Loss Per Share
Basic net loss per share is computed by dividing the net income or
loss applicable to common shares by the weighted average number of common shares outstanding during the period. The weighted average number
of shares of common stock outstanding includes (i) pre-funded warrants because their exercise requires only nominal consideration for
delivery of shares, and (ii) the shares held in abeyance because there is no consideration required for delivery of the shares; it does
not include any potentially dilutive securities or any unvested restricted shares of common stock. Certain restricted shares, although
classified as issued and outstanding at September 30, 2023, are considered contingently returnable until the restrictions lapse and will
not be included in the basic net loss per share calculation until the shares are vested. Unvested shares of the Company’s restricted
stock do not contain non-forfeitable rights to dividends and dividend equivalents. Diluted earnings per share is computed using the weighted
average number of common shares and, if dilutive, potential common shares outstanding during the period. Potential common shares consist
of the Company’s warrants, options, and restricted shares. Diluted net loss per share is computed by giving effect to all potential
shares of common stock, including warrants, stock options, and unvested restricted shares, to the extent they are dilutive.
The two-class method is used to determine earnings
per share based on participation rights of participating securities in any undistributed earnings. Each share of preferred stock that
includes rights to participate in distributed earnings is considered a participating security and the Company uses the two-class method
to calculate net income available to the Company’s common stockholders per common share — basic and diluted.
The following securities were excluded from the
computation of diluted shares outstanding due to the losses incurred in the periods presented, as they would have had an anti-dilutive
impact on the Company’s net loss:
| | |
Three and Nine Months Ended
September 30, | |
| | |
2023 | | |
2022 | |
| Unvested shares of restricted stock | |
| 344,080 | | |
| — | |
| Options to purchase shares of common stock | |
| 1,378,855 | | |
| 1,383,801 | |
| Warrants | |
| 7,899,661 | | |
| 5,375,385 | |
| Total | |
| 9,622,596 | | |
| 6,759,186 | |
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 13 — Retirement Plan
On May 31, 2023, the Board voted to adopt a 401(k)
Safe Harbor Non-Elective Plan (the “401(k) Plan”). The 401(k) Plan was an employee savings and retirement plan to which substantially
all employees could have contributed, including the Company’s named executive officers, effective July 1, 2023. Pursuant to the
401(k) Plan, employee and Company contributions would vest immediately, subject to a three-month waiting period for new hires. The Company
was required to contribute 3% of gross pay to eligible employees’ 401(k) Plans. On November 16, 2023, the 401(k) Plan was terminated.
Note 14 — Subsequent Events
Veru APA Amendment
On October 3, 2023, in accordance with the terms of the Veru APA Amendment,
the Company issued 3,000 shares of Series A Preferred Stock to Veru as settlement for the $3,000,000 note payable that had an original
maturity date of September 30, 2023 (see Note 5).
WraSer APA Amendment
On October 4, 2023, the parties agreed to
amend the WraSer APA, which is subject to court approval. Shortly after its bankruptcy filing, WraSer filed a motion seeking
approval of the WraSer APA as amended. The amendment, among other things, eliminates the $500,000 post-closing payment due June 13,
2024 and staggers the $4.5 million cash payment that the Company would otherwise have to pay at closing to: (i) $2.2 million to be
paid at closing, (ii) $2.3 million, to be paid in monthly installments of $150,000 commencing January 2024 and (iii) 789 shares of
Series A Preferred Stock to be paid at closing. The amendment also reduced the number of products we were acquiring by excluding
pain medications and including only (i) Ciprofloxacin 0.3% and Fluocinolone 0.025% Otic Solution, under the trademark OTOVEL and its
Authorized Generic Version approved under US FDA NDA No. 208251, (ii) Ciprofloxacin 0.2% Otic solution, under the trademark
CETRAXAL, and (iii) Vorapaxar Sulfate tablets under the trademark Zontivity approved under US FDA NDA N204886.
On October 6, 2023, the Company was alerted to certain issues in WraSer’s
operations relating to WraSer’s inability to manufacture the active pharmaceutical ingredient for one of the key WraSer Assets,
which development, the Company believes, constitutes a Material Adverse Effect (as such term is defined in the WraSer APA) that will prevent
the Company from closing the transaction. On October 20, 2023, the Company filed a motion for relief from the automatic stay in the Bankruptcy
Court so that we can exercise our termination rights under the WraSer APA, as amended. WraSer has advised the Company that it does not
believe that a Material Adverse Event occurred. If the Bankruptcy Court does not grant the Company’s motion and requires it to complete
the transaction, the Company will not be able to execute its commercialization strategy for the WraSer Assets because there is no manufacturer
for the active pharmaceutical ingredient for Zontivity, the key driver for the WraSer acquisition. Due to the WraSer bankruptcy filing
and the Company’s status as an unsecured creditor of WraSer, it is unlikely that the Company will recover the $3.5 million initial
payment made or any costs and resources in connection with services provided by the Company under the WraSer MSA. If the Company does
not complete the purchase of the WraSer Assets and the WraSer APA is terminated, the Company will not be able to recover any such costs.
Any claims the Company makes for such payment will be general unsecured claims (see Note 5).
ONCONETIX,
INC.
Notes to Condensed Financial Statements
September 30, 2023
(Unaudited)
Note 14 — Subsequent Events (cont.)
Chief Financial Officer Separation Agreement
Effective as of October 4, 2023, Jon Garfield
resigned as Chief Financial Officer of the Company. The Company and Mr. Garfield entered into a separation agreement, which provides for
a two-month severance payment.
Equity Incentive Plan Activity
On October 4, 2023, the Company’s Board
granted stock options to the Company’s newly hired Chief Executive Officer and Chief Financial Officer. The options granted to the
Chief Executive Officer and Chief Financial Officer totaled 532,326 and 177,442, respectively, have an exercise price of $0.4305 per share,
and vest quarterly over a three-year period.
On October 4, 2023, upon his resignation, the
Company’s former Chief Financial Officer forfeited 75,000 shares of unvested restricted stock, and 50,000 stock options.
Services Agreement
On October 12, 2023, the Company terminated a
Master Services Agreement and statements of work with a vendor, resulting in early termination fees of approximately $2.3 million. The
Company will record these fees during the quarter ending December 31, 2023 (see Note 6).
License Terminations
On November 15, 2023, the Company informed Oxford
University Innovation and St. Jude of its termination of the respective license agreements (see Note 6). The amounts due upon termination
of these license agreements were not significant.
ONCONETIX, INC.
INDEX
TO FINANCIAL STATEMENTS
Report
of Independent Registered Public Accounting Firm
To
the Board of Directors
and
Stockholders of Onconetix, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets
of Onconetix, Inc. (formerly known as Blue Water Vaccines, Inc.) (“Company”) as of December 31, 2022 and 2021, and the related
statements of operations, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2022,
and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements
present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of
its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles
generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our
audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial
statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged
to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
We served as the Company’s auditors from 2021 to 2023.
/s/ Mayer Hoffman McCann P.C.
Los Angeles, California
March 8, 2023
ONCONETIX,
INC.
Balance Sheets
| | |
December 31, 2022 | | |
December 31, 2021 | |
| ASSETS | |
| | |
| |
| Current assets | |
| | |
| |
| Cash | |
$ | 25,752,659 | | |
$ | 1,928,474 | |
| Prepaid expenses and other current assets | |
| 469,232 | | |
| 234,551 | |
| Deferred offering costs | |
| — | | |
| 757,646 | |
| Receivable from related parties | |
| 35,850 | | |
| 152,524 | |
| Total current assets | |
| 26,257,741 | | |
| 3,073,195 | |
| | |
| | | |
| | |
| Prepaid expenses, long-term | |
| 38,617 | | |
| — | |
| Property and equipment, net | |
| 14,089 | | |
| 11,502 | |
| Total assets | |
$ | 26,310,447 | | |
$ | 3,084,697 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |
| | | |
| | |
| Current liabilities | |
| | | |
| | |
| Accounts payable | |
$ | 1,499,296 | | |
$ | 582,605 | |
| Accrued expenses | |
| 2,409,128 | | |
| 1,055,515 | |
| Contingent warrant liability | |
| 14,021 | | |
| — | |
| Total current liabilities | |
| 3,922,445 | | |
| 1,638,120 | |
| | |
| | | |
| | |
| Total liabilities | |
| 3,922,445 | | |
| 1,638,120 | |
| | |
| | | |
| | |
| Commitments and Contingencies (see Note 7) | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ equity | |
| | | |
| | |
| Preferred stock, $0.00001 par value, 10,000,000 shares authorized at December 31, 2022 and 2021 | |
| | | |
| | |
| Series Seed: 0 and 1,150,000 shares designated at December 31, 2022 and 2021, respectively; 0 and 1,146,138 shares issued and outstanding at December 31, 2022 and 2021, respectively; $0 and $15.4 million aggregate liquidation preference at December 31, 2022 and 2021, respectively | |
| — | | |
| 11 | |
| Common stock, $0.00001 par value, 250,000,000 shares authorized at December 31, 2022 and 2021; 15,724,957 and 3,200,000 shares issued at December 31, 2022 and 2021, respectively; 15,265,228 and 3,200,000 shares outstanding at December 31, 2022 and 2021, respectively | |
| 157 | | |
| 32 | |
| Additional paid-in-capital | |
| 42,331,155 | | |
| 7,403,204 | |
| Treasury stock, at cost; 459,729 and 0 shares of common stock at December 31, 2022 and 2021, respectively | |
| (566,810 | ) | |
| — | |
| Accumulated deficit | |
| (19,376,500 | ) | |
| (5,956,670 | ) |
| Total stockholders’ equity | |
| 22,388,002 | | |
| 1,446,577 | |
| Total liabilities and stockholders’ equity | |
$ | 26,310,447 | | |
$ | 3,084,697 | |
The
accompanying notes are an integral part of these financial statements.
ONCONETIX,
INC.
Statements of Operations
| | |
Year Ended December 31, 2022 | | |
Year Ended December 31, 2021 | |
| Operating expenses | |
| | |
| |
| General and administrative | |
$ | 9,351,552 | | |
$ | 2,092,304 | |
| Research and development | |
| 4,129,688 | | |
| 1,325,030 | |
| Total operating expenses | |
| 13,481,240 | | |
| 3,417,334 | |
| Loss from operations | |
| (13,481,240 | ) | |
| (3,417,334 | ) |
| | |
| | | |
| | |
| Other income | |
| | | |
| | |
| Change in fair value of contingent warrant liability | |
| (61,410 | ) | |
| — | |
| Total other income | |
| (61,410 | ) | |
| — | |
| Net loss | |
$ | (13,419,830 | ) | |
$ | (3,417,334 | ) |
| Cumulative preferred stock dividends | |
| 96,359 | | |
| 627,391 | |
| Net loss applicable to common stockholders | |
$ | (13,516,189 | ) | |
$ | (4,044,725 | ) |
| | |
| | | |
| | |
| Net loss per share attributable to common stockholders, basic and diluted | |
$ | (1.10 | ) | |
$ | (1.26 | ) |
| | |
| | | |
| | |
| Weighted average number of common shares outstanding, basic and diluted | |
| 12,271,449 | | |
| 3,200,000 | |
The
accompanying notes are an integral part of these financial statements.
ONCONETIX,
INC.
Statements of Stockholders’ Equity
For
the Years Ended December 31, 2022 and 2021
| | |
| | |
| | |
| | |
| | |
Additional | | |
| | |
| | |
| | |
Total | |
| | |
Preferred Stock | | |
Common Stock | | |
Paid-in | | |
Treasury Stock | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Shares | | |
Amount | | |
Deficit | | |
Equity | |
| Balance at December 31, 2020 | |
| 1,146,138 | | |
$ | 11 | | |
| 3,200,000 | | |
$ | 32 | | |
$ | 7,273,063 | | |
| — | | |
$ | — | | |
$ | (2,539,336 | ) | |
$ | 4,733,770 | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 130,141 | | |
| — | | |
| | | |
| — | | |
| 130,141 | |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (3,417,334 | ) | |
| (3,417,334 | ) |
| Balance at December 31, 2021 | |
| 1,146,138 | | |
$ | 11 | | |
| 3,200,000 | | |
$ | 32 | | |
$ | 7,403,204 | | |
| — | | |
$ | — | | |
$ | (5,956,670 | ) | |
$ | 1,446,577 | |
| Issuance of common stock in initial public offering, net of $2.9 million of offering costs | |
| — | | |
| — | | |
| 2,222,222 | | |
| 22 | | |
| 17,138,818 | | |
| — | | |
| — | | |
| — | | |
| 17,138,840 | |
| Conversion of convertible preferred stock to common stock upon initial public offering | |
| (1,146,138 | ) | |
| (11 | ) | |
| 5,626,365 | | |
| 56 | | |
| (45 | ) | |
| — | | |
| — | | |
| — | | |
| — | |
| Issuance of common stock and warrants in April private placement, net of $1.1 million of offering costs | |
| — | | |
| — | | |
| 590,406 | | |
| 6 | | |
| 6,858,322 | | |
| — | | |
| — | | |
| — | | |
| 6,858,328 | |
| Issuance of common stock and warrants in August private placement, net of $2.2 million of offering costs | |
| — | | |
| — | | |
| 1,350,000 | | |
| 14 | | |
| 8,689,302 | | |
| — | | |
| — | | |
| — | | |
| 8,689,316 | |
| Exercise of stock options | |
| — | | |
| — | | |
| 165,452 | | |
| 2 | | |
| 1,653 | | |
| — | | |
| — | | |
| — | | |
| 1,655 | |
| Exercise of pre-funded warrants | |
| — | | |
| — | | |
| 2,277,046 | | |
| 22 | | |
| 1,414 | | |
| — | | |
| — | | |
| — | | |
| 1,436 | |
| Issuance of restricted common stock | |
| — | | |
| — | | |
| 293,466 | | |
| 3 | | |
| 263,921 | | |
| — | | |
| — | | |
| — | | |
| 263,924 | |
| Stock-based compensation | |
| — | | |
| — | | |
| — | | |
| — | | |
| 1,974,566 | | |
| — | | |
| — | | |
| — | | |
| 1,974,566 | |
| Purchase of treasury shares | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (459,729 | ) | |
| (566,810 | ) | |
| | | |
| (566,810 | ) |
| Net loss | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| — | | |
| (13,419,830 | ) | |
| (13,419,830 | ) |
| Balance at December 31, 2022 | |
| — | | |
$ | — | | |
| 15,724,957 | | |
$ | 157 | | |
$ | 42,331,155 | | |
| (459,729 | ) | |
$ | (566,810 | ) | |
$ | (19,376,500 | ) | |
$ | 22,388,002 | |
The
accompanying notes are an integral part of these financial statements.
ONCONETIX,
INC.
Statements of Cash Flows
| | |
Year Ended
December 31,
2022 | | |
Year Ended
December 31,
2021 | |
| Cash flows from operating activities | |
| | |
| |
| Net loss | |
$ | (13,419,830 | ) | |
$ | (3,417,334 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Stock-based compensation | |
| 1,974,566 | | |
| 130,141 | |
| Issuance of restricted common stock | |
| 263,924 | | |
| — | |
| Change in fair value of contingent warrant liability | |
| (61,410 | ) | |
| — | |
| Depreciation expense | |
| 6,752 | | |
| 4,890 | |
| Write off of receivable from related party | |
| — | | |
| 22,242 | |
| Loss on disposal of property and equipment | |
| — | | |
| 1,199 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Prepaid expenses and other current assets | |
| (234,681 | ) | |
| 8,302 | |
| Receivable from related parties | |
| (23,326 | ) | |
| (114,961 | ) |
| Prepaid expenses, long-term | |
| (38,617 | ) | |
| 184,934 | |
| Accounts payable | |
| 1,093,913 | | |
| 336,715 | |
| Accrued expenses | |
| 1,739,849 | | |
| 809,279 | |
| Deferred rent | |
| — | | |
| (9,642 | ) |
| Net cash used in operating activities | |
| (8,698,860 | ) | |
| (2,044,235 | ) |
| | |
| | | |
| | |
| Cash flows from investing activities | |
| | | |
| | |
| Purchase of property and equipment | |
| (9,339 | ) | |
| (1,924 | ) |
| Net cash used in investing activities | |
| (9,339 | ) | |
| (1,924 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities | |
| | | |
| | |
| Payment of deferred offering costs | |
| — | | |
| (334,188 | ) |
| Proceeds from issuance of common stock in initial public offering, net of underwriting discount | |
| 18,400,000 | | |
| — | |
| Payments of initial public offering costs | |
| (926,972 | ) | |
| — | |
| Proceeds from issuance of common stock and warrants in private placements, net of placement agent discount | |
| 16,468,123 | | |
| — | |
| Payments of private placement issuance costs | |
| (845,048 | ) | |
| — | |
| Purchase of treasury shares | |
| (566,810 | ) | |
| — | |
| Proceeds from exercise of stock options | |
| 1,655 | | |
| — | |
| Proceeds from exercise of pre-funded warrants | |
| 1,436 | | |
| — | |
| Net cash provided by (used in) financing activities | |
| 32,532,384 | | |
| (334,188 | ) |
| Net increase (decrease) in cash | |
| 23,824,185 | | |
| (2,380,347 | ) |
| Cash, beginning of period | |
| 1,928,474 | | |
| 4,308,821 | |
| Cash, end of period | |
$ | 25,752,659 | | |
$ | 1,928,474 | |
| | |
| | | |
| | |
| Noncash investing and financing activities: | |
| | | |
| | |
| Deferred offering costs included in accounts payable and accrued expenses | |
$ | — | | |
$ | 423,458 | |
| Conversion of convertible preferred stock to common stock upon initial public offering | |
$ | 45 | | |
$ | — | |
| Recognition of contingent warrant liability upon issuance of common stock in private placements | |
$ | 75,431 | | |
$ | — | |
| Incremental fair value of preferred investment options exchanged in connection with August private placement | |
$ | 860,204 | | |
$ | — | |
| Payment of accrued bonus through related party receivable | |
$ | 140,000 | | |
$ | — | |
| Exercise of pre-funded warrants | |
$ | 6 | | |
$ | — | |
The
accompanying notes are an integral part of these financial statements.
ONCONETIX,
INC.
Notes to Financial Statements
Note
1 — Organization and Basis of Presentation
Organization
and Nature of Operations
Onconetix, Inc. (formerly known as Blue
Water Vaccines, Inc.) (the “Company”) was formed on October 26, 2018, to focus on the research and development of
transformational vaccines to prevent infectious diseases worldwide. The Company’s lead vaccine candidate, BWV-201, is a live
attenuated, intranasally delivered, serotype independent Streptococcus pneumoniae vaccine targeting S. pneumo-induced acute otitis
media and pneumococcal pneumonia. BWV’s influenza vaccine candidates, BWV-101 and BWV-102, are being investigated as a
universal influenza vaccine with the potential to protect against all influenza strains and a pre-pandemic H1 influenza vaccine,
respectively. In addition to exploratory analysis for applications in flu vaccines, the Company’s virus-like particle platform
is being utilized to investigate and develop vaccine candidates against norovirus, rotavirus, malaria, monkeypox, and Marburg virus
disease. Finally, the Company is developing a live attenuated, orally delivered Chlamydia vaccine. All of the Company’s
vaccine candidates are in the pre-clinical developmental stage.
Stock
Split
On
November 24, 2021, the Company effected a 4-for-1 (4:1) stock split (the “Stock Split”) of the Company’s common stock
without any change to its par value, which became effective on November 24, 2021. All references to share and per share amounts for all
periods presented in these financial statements have been retrospectively restated to reflect the Stock Split and proportional adjustment
of the preferred stock conversion ratio.
Initial
Public Offering
On
February 23, 2022, the Company completed its initial public offering (“IPO”) in which the Company issued and sold 2,222,222
shares of its common stock, at a price to the public of $9.00 per share. Proceeds from the IPO, net of underwriting discounts, commissions,
and offering costs of $2.9 million, were $17.1 million. In connection with the completion of the IPO, all outstanding shares of convertible
preferred stock were converted into 5,626,365 shares of common stock. See Note 6.
Basis
of Presentation
The
Company’s financial statements have been prepared in conformity with accounting principles generally accepted in the United States
of America (“U.S. GAAP”).
Note
2 — Liquidity and Financial Condition
The
Company’s operating activities to date have been devoted to seeking licenses and engaging in research and development activities.
The Company’s product candidates currently under development will require significant additional research and development efforts
prior to commercialization. The Company has financed its operations since inception primarily using proceeds received from seed investors,
and proceeds received from its IPO and two private placement issuances (the “Private Placements”). During 2022, the Company
completed its IPO and the Private Placements in which the Company received an aggregate of approximately $33.1 million in net cash proceeds,
after deducting placement agent fees and other offering expenses, see Note 6.
The
Company has incurred substantial operating losses since inception and expects to continue to incur significant operating losses for the
foreseeable future. As of December 31, 2022, the Company had cash of approximately $25.8 million, working capital of approximately $22.3
million and an accumulated deficit of approximately $19.4 million.
The
Company believes the existing cash at December 31, 2022 will be sufficient to continue operations, satisfy its obligations and fund the
future expenditures that will be required to conduct the clinical and regulatory work to develop its product candidates for at least
one year following the date that these financial statements were issued.
The
Company will require significant additional capital to make the investments it needs to execute its long-term business plan. The Company
expects a significant increase in cash outflows as compared to its historical spend for its planned pre-clinical development and clinical
trial activities, and as such, it will need to raise additional capital to sustain operations and meet its long-term operating requirements
beyond the one-year period following the date that these financial statements were issued. The Company expects to seek additional funding
through additional debt or equity financings; however, there are currently no commitments in place for further financing nor is there
any assurance that such financing will be available to the Company on favorable terms, if at all. If the Company is unable to secure
additional capital, it may be required to curtail any clinical trials and development of products and take additional measures to reduce
expenses in order to conserve its cash in amounts sufficient to sustain operations and meet its obligations in the long-term.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the
reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements
and the reported amounts of expenses during the reporting periods. The most significant estimates in the Company’s financial statements
relate to the valuation of common stock (for transactions incurred prior to the consummation of the IPO), stock-based compensation, accrued
research and development expenses and the valuation allowance of deferred tax assets resulting from net operating losses. These estimates
and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the circumstances,
the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses
that are not readily apparent from other sources. Actual results may differ materially and adversely from these estimates. To the extent
there are material differences between the estimates and actual results, the Company’s future results of operations will be affected.
Concentration
of Credit Risk
Financial
instruments that potentially subject the Company to concentrations of credit risk consist of cash accounts in a financial institution,
which, at times, may exceed the Federal Depository Insurance Coverage limit of $250,000. As of December 31, 2022 and 2021, the Company
has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Property
and Equipment
Property
and equipment consists of computers and office furniture and fixtures, all of which are recorded at cost. Depreciation is recorded using
the straight-line method over the respective useful lives of the assets ranging from three to seven years. Long-lived assets are reviewed
for impairment whenever events or circumstances indicate that the carrying amount of these assets may not be recoverable.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
Fair
Value Measurements
Fair
value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction
between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs
used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets
or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
| ● | Level
1, defined as observable inputs such as quoted prices (unadjusted) for identical instruments in active markets; |
| ● | Level
2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices
for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and |
| ● | Level
3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions,
such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable. |
In
some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In
those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input
that is significant to the fair value measurement. Financial instruments, including cash, prepaid expenses, deferred offering costs,
receivables from related party, accounts payable and accrued liabilities are carried at cost, which management believes approximates
fair value due to the short-term nature of these instruments. As of December 31, 2022, the contingent warrant liability that became issuable
upon the closing of the Private Placements is valued on a recurring basis utilizing a Monte Carlo simulation which includes Level 3 inputs.
See Note 6. As of December 31, 2022 and 2021, none of the Company’s non-financial assets or liabilities were recorded at fair value
on a non-recurring basis. No transfers between levels have occurred during the periods presented.
The
following assumptions were used for the valuation of the contingent warrant liability upon the various commitment dates, as discussed
in Note 6, and at December 31, 2022:
| | |
April 19, 2022 | | |
August 11, 2022 | | |
December 31,
2022 | |
| Exercise price | |
$ | 8.46875 | | |
$ | 3.3938 | | |
$ | 3.3938 | |
| Term (years) | |
| 4.00 | | |
| 5.00 | | |
| 4.61 | |
| Expected stock price volatility | |
| 117.0 | % | |
| 127.8 | % | |
| 120.8 | % |
| Risk-free rate of interest | |
| 2.86 | % | |
| 2.98 | % | |
| 4.03 | % |
The
fair value of financial instruments measured on a recurring basis is as follows:
| | |
As of December 31, 2022 | |
| Description | |
Total | | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Liabilities: | |
| | |
| | |
| | |
| |
| Contingent warrant liability | |
$ | 14,021 | | |
| — | | |
| — | | |
$ | 14,021 | |
ONCONETIX,
INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
The
following table summarizes the change in fair value, as determined by Level 3 inputs, for the contingent warrant liability using unobservable
Level 3 inputs for the year ended December 31, 2022:
| | |
Contingent
Warrant
Liability | |
| Balance at December 31, 2021 | |
$ | — | |
| Fair value at issuance | |
| 75,431 | |
| Change in fair value | |
| (61,410 | ) |
| Balance at December 31, 2022 | |
$ | 14,021 | |
Deferred
Offering Costs
The
Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process equity
financings as deferred offering costs until such financings are consummated. After consummation of the equity financing, these costs
are recorded in stockholders’ equity as a reduction of proceeds generated as a result of the offering. Should the in-process equity
financing be abandoned, the deferred offering costs will be expensed immediately as a charge to operating expenses in the statements
of operations. As of December 31, 2022, all previously deferred offering costs related to the IPO, totaling approximately $0.8 million,
and of which $0.3 million were paid during 2021, were netted against the proceeds received upon the closing of the IPO, which occurred
on February 23, 2022.
Research
and Development
The
Company expenses the cost of research and development as incurred. Research and development expenses include costs incurred in funding
research and development activities, license fees, and other external costs. Advance payments for goods and services that will be used
in future research and development activities are expensed when the activity has been performed or when the goods have been received
rather than when the payment is made. Upfront and milestone payments due to third parties that perform research and development services
on the Company’s behalf will be expensed as services are rendered or when the milestone is achieved. When billing terms under research
and development contracts do not coincide with the timing of when the work is performed, the Company is required to make estimates of
outstanding obligations as of period end to those third parties. Accrual estimates are based on several factors, including the Company’s
knowledge of the progress towards completion of the research and development activities, invoicing to date under the contracts, communication
from the research institution or other companies of any actual costs incurred during the period that have not yet been invoiced, and
the costs included in the contracts. Significant judgments and estimates may be made in determining the accrued balances at the end of
any reporting period. Actual results could differ from the estimates made by the Company. The historical accrual estimates made by the
Company have not been materially different from the actual costs. See Notes 5 and 7.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
In
accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic
730-10-25-1, Research and Development, costs incurred in obtaining licenses and patent rights are charged to research and development
expense if the technology licensed has not reached commercial feasibility and has no alternative future use. The licenses purchased by
the Company (see Note 5) require substantial completion of research and development, regulatory and marketing approval efforts to reach
commercial feasibility and have no alternative future use. Accordingly, the total purchase price for the licenses acquired is reflected
as research and development on the Company’s statements of operations.
Contingencies
Accruals
are recorded for loss contingencies when it is probable that a liability has been incurred and the amount of the related loss can be
reasonably estimated. The Company evaluates, on a quarterly basis, developments in legal proceedings and other matters that could cause
an increase or decrease in the amount of the liability that has been accrued previously. Considering facts known at the time of the assessment,
the Company determines whether potential losses are considered reasonably possible or probable and whether they are estimable. Based
upon this assessment, the Company carries out an evaluation of disclosure requirements and considers possible accruals in the financial
statements.
Stock-Based
Compensation
The
Company expenses stock-based compensation to employees and non-employees over the requisite service period based on the estimated grant-date
fair value of the awards. Stock-based awards to employees with graded-vesting schedules are recognized, using the accelerated attribution
method, on a straight-line basis over the requisite service period for each separately vesting portion of the award.
The
Company estimates the fair value of stock option grants using the Black-Scholes option pricing model and the assumptions used in calculating
the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application
of management’s judgment.
Expected
Term — The expected term of options represents the period that the Company’s stock-based awards are expected to be outstanding
based on the simplified method, which is the half-life from vesting to the end of its contractual term.
Expected
Volatility — Volatility is a measure of the amount by which the Company’s share price has historically fluctuated or
is expected to fluctuate (i.e., expected volatility) during a period. Due to the lack of an adequate history of a public market for the
trading of the Company’s common stock and a lack of adequate company-specific historical and implied volatility data, the Company
computes stock price volatility over expected terms based on comparable companies’ historical common stock trading prices. For
these analyses, the Company has selected companies with comparable characteristics, including enterprise value, risk profiles, and position
within the industry.
Common
Stock Fair Value — Due to the absence of an active market for the Company’s common stock prior to the IPO, the fair value
of the common stock underlying the Company’s stock options granted prior to the IPO was estimated at each grant date and was determined
with the assistance of an independent third-party valuation expert. The assumptions underlying these valuations represented management’s
best estimates, which involved inherent uncertainties and the application of significant levels of management judgment. After the completion
of the IPO, the fair value of each share of common stock is based on the closing price of the Company’s common stock, as reported
by the Nasdaq Capital Market, on the grant date of the award.
Risk-Free
Interest Rate — The Company bases the risk-free interest rate on the implied yield available on U.S. Treasury securities with
a remaining term commensurate with the estimated expected term.
Expected
Dividend — The Company has never declared or paid any cash dividends on its shares of common stock and does not plan to pay
cash dividends in the foreseeable future, and, therefore, uses an expected dividend yield of zero in its valuation models.
The
Company recognizes forfeitures of equity awards as they occur.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
Fair
Value of Common Stock
In
order to determine the fair value of shares of common stock of the Company when issuing stock options prior to the IPO, the Company’s
board of directors considered with input from third party valuations, among other things, contemporaneous valuations of the Company’s
common stock. Given the absence of a public trading market of the Company’s capital stock prior to the IPO, the Company’s
board of directors exercised reasonable judgment and considered a number of objective and subjective factors to determine the best estimate
of the fair value of the Company common stock, including:
| ● | the
prices, rights, preferences and privileges of the Company’s preferred stock relative to the Company’s common stock; |
| ● | the
Company’s business, financial condition and results of operations, including related industry trends affecting the Company’s
operations; |
| ● | the
likelihood of achieving a liquidity event, such as an IPO, or sale of the Company, given prevailing market conditions; |
| ● | the
lack of marketability of the Company’s common stock; |
| ● | the
market performance of comparable publicly traded companies; |
| ● | U.S.
and global economic and capital market conditions and outlook; and |
| ● | common
stock valuation methodology. |
In
estimating the fair market value of common stock of the Company, its board of directors first determined the equity value of its business
using accepted valuation methods.
The
Company engaged a third-party valuation specialist to conduct a valuation, which used its most recent preferred stock financing as a
starting point and determined the equity value of the Company based on the Backsolve method using an Option Pricing Method (OPM) to calculate
the implied value based on a market approach. The Company’s equity value was allocated using OPM to estimate the fair market value
of the Company’s classes of equity.
After
the completion of the IPO, the fair value of each share of common stock is based on the closing price of the Company’s common stock
on the grant date of the award, as reported by the Nasdaq Capital Market.
Income
Taxes
Income
taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences
attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective
tax bases and operating loss and tax credit carryforwards.
Deferred
tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary
differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rate is recognized
in operations in the period that includes the enactment date. Deferred tax assets are reduced to estimated amounts expected to be realized
by the use of a valuation allowance.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
Comprehensive
Income (Loss)
The
Company is required to report all components of comprehensive income (loss), including net income (loss), in the accompanying financial
statements in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period
from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments and
foreign currency translation adjustments. Net loss and comprehensive loss were the same for all periods presented.
Warrants
The
Company determines the accounting classification of warrants that are issued, as either liability or equity, by first assessing whether
the warrants meet liability classification in accordance with ASC 480-10, Distinguishing Liabilities from Equity, (“ASC
480-10”), and then in accordance with ASC 815-40, Derivatives and Hedging - Contracts in Entity’s Own Equity (“ASC
815-40”). Under ASC 480-10, warrants are considered liability-classified if the warrants are mandatorily redeemable, obligate the
issuer to settle the warrants or the underlying shares by paying cash or other assets, or must or may require settlement by issuing a
variable number of shares.
If
the warrants do not meet liability classification under ASC 480-10, the Company assesses the requirements under ASC 815-40, which states
that contracts that require or may require the issuer to settle the contract for cash are liabilities recorded at fair value, irrespective
of the likelihood of the transaction occurring that triggers the net cash settlement feature. If the warrants do not require liability
classification under ASC 815-40, in order to conclude equity classification, the Company assesses whether the warrants are indexed to
its common stock and whether the warrants are classified as equity under ASC 815-40 or other applicable GAAP. After all relevant assessments
are made, the Company concludes whether the warrants are classified as liability or equity. Liability-classified warrants are required
to be accounted for at fair value both on the date of issuance and on subsequent accounting period ending dates, with all changes in
fair value after the issuance date recorded as a component of other income (expense), net in the statements of operations. Equity-classified
warrants are accounted for at fair value on the issuance date with no changes in fair value recognized after the issuance date. As of
December 31, 2022, all of the Company’s outstanding warrants are equity-classified warrants, except for the contingent warrants
that became issuable upon the close of the Private Placements. See Note 6.
Treasury
Stock
The
Company records treasury stock activities under the cost method whereby the cost of the acquired stock is recorded as treasury stock.
Net
Loss Per Share
Basic
loss per share is computed by dividing the net income or loss applicable to common shares by the weighted average number of common shares
outstanding during the period, including pre-funded warrants because their exercise requires only nominal consideration for delivery
of shares. Diluted earnings per share is computed using the weighted average number of common shares and, if dilutive, potential common
shares outstanding during the period. Potential common shares consist of the Company’s preferred stock, warrants, and options.
Diluted loss per share excludes the shares issuable upon the conversion of preferred stock, as well as common stock options and warrants,
from the calculation of net loss per share if their effect would be anti-dilutive.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
The
two-class method is used to determine earnings per share based on participation rights of participating securities in any undistributed
earnings. Each preferred stock that includes rights to participate in distributed earnings is considered a participating security and
the Company uses the two-class method to calculate net income available to the Company’s common stockholders per common share —
basic and diluted.
The
following securities were excluded from the computation of diluted shares outstanding for the periods presented, as they would have had
an anti-dilutive impact on the Company’s net loss:
| | |
Years Ended
December 31, | |
| | |
2022 | | |
2021 | |
| Options to purchase shares of common stock | |
| 1,392,654 | | |
| 780,640 | |
| Warrants | |
| 5,264,274 | | |
| — | |
| Series Seed Preferred Stock | |
| — | | |
| 4,584,552 | |
| Total | |
| 6,656,928 | | |
| 5,365,192 | |
New
Accounting Pronouncements
In
April 2012, the Jump-Start Our Business Startups Act (the “JOBS Act”) was signed into law. The JOBS Act contains provisions
that, among other things, reduce certain reporting requirements for an emerging growth company. As an emerging growth company, the Company
may elect to adopt new or revised accounting standards when they become effective for non-public companies, which typically is later
than when public companies must adopt the standards. The Company has elected to take advantage of the extended transition period afforded
by the JOBS Act and, as a result, unless the Company elects early adoption of any standards, will adopt the new or revised accounting
standards on the relevant dates on which adoption of such standards is required for non-public companies.
In
August 2020, the FASB issued Accounting Standards Update (“ASU”) No. 2020-06, Debt — Debt with Conversion and Other
Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for
Convertible Instruments and Contracts in an Entity’s Own Equity, which simplifies accounting for convertible instruments by
removing major separation models required under current GAAP. The ASU also removes certain settlement
conditions that are required for equity contracts to qualify for the derivative scope exception and it also simplifies the diluted earnings
per share calculation in certain areas. This guidance is effective for public business entities except for smaller reporting companies
for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2021. For all other entities, the standard
will be effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Early adoption
is permitted. The Company early adopted ASU 2020-06 on January 1, 2022, using the modified retrospective method, and the adoption of
the ASU did not impact the Company’s financial position, results of operations, cash flows or net loss per share.
ONCONETIX, INC.
Notes
to Financial Statements
Note
3 — Summary of Significant Accounting Policies (cont.)
In
October 2020, the FASB issued ASU 2020-10, Codification Improvements, which updates various codification topics by clarifying
or improving disclosure requirements to align with the SEC’s regulations. The Company adopted ASU 2020-10 as of the reporting period
beginning January 1, 2022. The adoption of this update did not have a material effect on the Company’s financial statements.
In
May 2021, the FASB issued ASU 2021-04, Earnings Per Share (Topic 260), Debt — Modifications and Extinguishments (Subtopic 470-50),
Compensation — Stock Compensation (Topic 718), and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic
815-40): Issuer’s Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options (a consensus
of the FASB Emerging Issues Task Force). The ASU clarifies and reduces diversity in an issuer’s accounting for modifications or
exchanges of freestanding equity-classified written call options (for example, warrants) that remain equity classified after modification
or exchange. The ASU provides guidance that will clarify whether an issuer should account for a modification or an exchange of a freestanding
equity-classified written call option that remains equity classified after modification or exchange as (1) an adjustment to equity and,
if so, the related earnings per share (EPS) effects, if any, or (2) an expense and, if so, the manner and pattern of recognition. The
new guidance is effective for all entities for annual and interim periods beginning after December 15, 2021, and early adoption is permitted,
including adoption in an interim period. The Company adopted ASU 2021-04 on January 1, 2022, and the adoption of the ASU did not impact
the Company’s financial position, results of operations, cash flows or net loss per share.
In
June 2022, the FASB issued ASU No. 2022-03, Fair Value Measurement (Topic 820): Fair Value Measurement of Equity Securities Subject
to Contractual Sale Restrictions (“ASU 2022-03”), which applies to all equity securities measured at fair value that
are subject to contractual sale restrictions. This change prohibits entities from taking into account contractual restrictions on the
sale of equity securities when estimating fair value and introduces required disclosures for such transactions. This guidance is effective
for public business entities beginning after December 15, 2023, including interim periods within those fiscal years. For all other entities,
the standard will be effective for fiscal years beginning after December 15, 2024, including interim periods within those fiscal years.
Early adoption is permitted. The Company early adopted ASU 2022-03 effective July 1, 2022, and the adoption of the ASU did not impact
the Company’s financial position, results of operations, cash flows, or net loss per share.
The
Company’s management does not believe that any other recently issued, but not yet effective, accounting standards if currently
adopted would have a material effect on the accompanying financial statements.
Note
4 — Balance Sheet Details
Prepaid
Expenses and Other Current Assets
Prepaid
expenses and other current assets consisted of the following as of December 31, 2022 and 2021:
| | |
As of
December 31,
2022 | | |
As of
December 31,
2021 | |
| Prepaid research and development | |
$ | 231,981 | | |
$ | 203,910 | |
| Prepaid insurance | |
| 148,789 | | |
| 4,842 | |
| Prepaid other | |
| 88,462 | | |
| 25,799 | |
| Total | |
$ | 469,232 | | |
$ | 234,551 | |
ONCONETIX, INC.
Notes
to Financial Statements
Note
4 — Balance Sheet Details (cont.)
Accrued
Expenses
Accrued
expenses consisted of the following as of December 31, 2022 and 2021:
| | |
As of
December 31,
2022 | | |
As of
December 31,
2021 | |
| Accrued license fees | |
$ | 15,000 | | |
$ | 225,000 | |
| Accrued research and development | |
| 847,747 | | |
| 300,182 | |
| Accrued deferred offering costs | |
| 125,000 | | |
| 246,236 | |
| Accrued compensation | |
| 1,132,859 | | |
| 234,265 | |
| Accrued franchise taxes | |
| 177,600 | | |
| — | |
| Accrued director fees | |
| 38,750 | | |
| — | |
| Accrued other | |
| 72,172 | | |
| 49,832 | |
| Total | |
$ | 2,409,128 | | |
$ | 1,055,515 | |
Note
5 — Significant Agreements
Oxford
University Innovation Limited
In
December 2018, the Company entered into an option agreement with Oxford University Innovation (“OUI”), which was a precursor
to a license agreement (the “OUI Agreement”), dated July 16, 2019. Under the terms of the OUI Agreement, the Company holds
an exclusive, worldwide license to certain specified patent rights and biological materials relating to the use of epitopes of limited
variability and virus-like particle products and practice processes that are covered by the licensed patent rights and biological materials
for the purpose of developing and commercializing a vaccine product candidate for influenza. The Company is obligated to use its best
efforts to develop and market Licensed Products, as defined in the OUI Agreement, in accordance with its development plan, report to
OUI on progress, achieve the following milestones and must pay OUI nonrefundable milestone fees when it achieves them: initiation of
first Phase I study; initiation of first Phase II study; initiation of first Phase III/pivotal registration studies; first submission
of application for regulatory approval (BLA/NDA); marketing authorization in the United States; marketing authorization in any EU country;
marketing authorization in Japan; first marketing authorization in any other country; first commercial sale in Japan; first commercial
sale in any ROW country; first year that annual sales equal or exceed certain thresholds. See Note 7 for additional information on the
milestone payments as well as royalty obligations required under the OUI Agreement. The OUI Agreement will expire upon ten (10) years
from the expiration of the last patent contained in the licensed patent rights, unless terminated earlier. During the year ended December
31, 2021, the U.S. Patent related to immunogenic composition was issued to OUI. This patent expires in August 2037. No additional patents
have been issued as of December 31, 2022. Either party may terminate the OUI Agreement for an uncured material breach. The Company was
able to terminate the OUI Agreement for any reason at any time upon six months’ written notice until July 16, 2022, which was the
third anniversary of the OUI Agreement. OUI may terminate immediately if the Company has a petition presented for its winding-up or passes
a resolution for winding up other than for a bona fide amalgamation or reconstruction or compounds with its creditors or has a receiver
or administrator appointed. OUI may also terminate if the Company opposes or challenges the validity of any of the patents or applications
in the Licensed Technology, as defined in the OUI Agreement; raises the claim that the know-how of the Licensed Technology is not necessary
to develop and market Licensed Products; or in OUI’s reasonable opinion, is taking inadequate or insufficient steps to develop
or market Licensed Products and does not take any further steps that OUI requests by written notice within a reasonable time.
For
the years ended December 31, 2022 and 2021, the Company did not incur any licensing fee payments for intellectual property licenses.
See Note 7.
ONCONETIX, INC.
Notes
to Financial Statements
Note
5 — Significant Agreements (cont.)
St.
Jude Children’s Hospital
The
Company entered into a license agreement (the “St. Jude Agreement”), dated January 27, 2020, with St. Jude Children’s
Research Hospital (“St. Jude”). Under the terms of the St. Jude Agreement, the Company holds an exclusive, worldwide license
to certain specified patent rights and biological materials relating to the use of live attenuated streptococcus pneumoniae and practice
processes that are covered by the licensed patent rights and biological materials for the purpose of developing and commercializing a
vaccine product candidate for streptococcus pneumoniae. The St. Jude Agreement will expire upon the expiration of the last valid claim
contained in the licensed patent rights, unless terminated earlier. The Company is obligated to use commercially reasonable efforts to
develop and commercialize the licensed product(s). The milestones include the following events: (i) complete IND enabling study; (ii)
initiate animal toxicology study; (iii) file IND; (iv) complete Phase I Clinical Trial; (v) commence Phase II Clinical Trial; (vi) commence
Phase III Clinical Trial; and (vii) regulatory approval, U.S. or foreign equivalent. If the Company fails to achieve the development
milestones contained in the St. Jude Agreement, and if the Company and St. Jude fail to agree upon a mutually satisfactory revised timeline,
St. Jude will have the right to terminate the St. Jude Agreement. Either party may terminate the St. Jude Agreement in the event the
other party (a) files or has filed against it a petition under the Bankruptcy Act (among other things) or (b) fails to perform or otherwise
breaches its obligations under the St. Jude Agreement, and has not cured such failure or breach within sixty (60) days. The Company may
terminate for any reason on thirty (30) days written notice. On May 11, 2022, the Company entered into an amendment to the St. Jude Agreement,
whereby the royalty terms, milestone payments and licensing fees were amended, and a revised development milestone timeline was agreed
to. See Note 7 for more information on this amendment.
For
the years ended December 31, 2022 and 2021, the Company recognized $15,000 and $11,000, respectively, for intellectual property licenses,
which is recorded as research and development expenses. See Note 7 for additional information on the milestone payments as well as royalty
obligations required under the St. Jude Agreement.
Cincinnati
Children’s Hospital Medical Center
The
Company entered into a license agreement (the “CHMC Agreement”), dated June 1, 2021, with Children’s Hospital Medical
Center, d/b/a Cincinnati Children’s Hospital Medical Center (“CHMC”). Under the terms of the CHMC Agreement, the Company
holds an exclusive, worldwide license (other than the excluded field of immunization against, and prevention, control, or reduction in
the severity of gastroenteritis caused by rotavirus and norovirus in China and Hong Kong) to certain specified patent and biological
materials relating to the use of norovirus nanoparticles and practice processes that are covered by the licensed patent rights and biological
materials for the purpose of developing and commercializing CHMC patents and related technology directed to a virus-like particle vaccine
platform that utilizes nanoparticle delivery technology that may have potential broad application to develop vaccines for multiple infectious
diseases. The term of the CHMC Agreement begins on the effective date and extends on a jurisdiction by jurisdiction and product by product
basis until the later of: (i) the last to expire licensed patent; (ii) ten (10) years after the first commercial sale; or (iii) entrance
onto the market of a biosimilar or interchangeable product. The Company is obligated to use commercially reasonable efforts to bring
licensed products to market through diligent research and development, testing, manufacturing and commercialization, to use best efforts
to make all necessary regulatory filings and obtain all necessary regulatory approvals, to achieve milestones relating to development
and sales, and report to CHMC on progress. The Company will also be obligated to pay the agreed upon development milestone payments to
CHMC, as well as royalty payments, see Note 7 for additional information. The Company may terminate the CHMC Agreement for convenience,
at any time prior to first commercial sale of a product or process by providing one hundred and eighty (180) days’ written notice
to CHMC. It may also terminate for a CHMC uncured material breach. CHMC may terminate the CHMC Agreement for an uncured Company material
breach or insolvency or bankruptcy. Pursuant to the terms of the CHMC Agreement, if the Company fails to achieve the milestones, and
cannot mutually agree with CHMC on an amendment to the milestones, then CHMC will have the option of converting any and all of such exclusive
licenses to nonexclusive licenses, to continue developing indications that have already entered development at any stage or in which
the Company has invested in developing. CHMC may also terminate the CHMC Agreement to the fullest extent permitted by law in the countries
of the worldwide territory, in the event the Company or its affiliates challenge or induce others set up challenges to the validity or
enforceability of any of the Licensed Patents, as defined in the CHMC Agreement, and the Company will be obligated to reimburse CHMC
for its costs, including reasonable attorneys’ fees.
ONCONETIX, INC.
Notes
to Financial Statements
Note
5 — Significant Agreements (cont.)
For
the years ended December 31, 2022 and 2021, the Company recognized an aggregate of approximately $38,000 and $402,000, respectively,
for intellectual property licenses and patent reimbursements, which are recorded as research and development expenses and included in
accounts payable as of December 31, 2022 and accrued expenses as of December 31, 2021. See Note 7.
Ology
Bioservices, Inc. (which was later acquired by National Resilience, Inc.)
The
Company entered into a Master Services Agreement (“Ology MSA”), dated July 19, 2019, with Ology, Inc. (“Ology”)
to provide services from time to time, including but not limited to technology transfer, process development, analytical method optimization,
cGMP manufacture, regulatory affairs, and stability studies of biologic products. Pursuant to the Ology MSA, the Company and Ology shall
enter into a Project Addendum for each project to be governed by the terms and conditions of the Ology MSA.
The
Company has entered into two Project Addendums as of December 31, 2022. The initial Project Addendum was executed on October 18, 2019
and the Company was required to pay Ology an aggregate of approximately $4 million. Due to unforeseen delays associated with COVID-19,
the Company and Ology entered into a letter agreement dated January 9, 2020 to stop work on the project, at which point the Company had
paid Ology $100,000 for services to be provided. The second Project Addendum was executed on May 21, 2021 and the Company is obligated
to pay Ology an aggregate amount of approximately $2.8 million, plus reimbursement for materials and outsourced testing, which will be
billed at cost plus 15%.
During
2022, the Company entered into three amendments to the Ology MSA, to adjust the scope of work defined in the second Project Addendum.
The amendments resulted in a net increase to the Company’s obligations under the second Project Addendum of $154,000.
During
the years ended December 31, 2022 and 2021, the Company incurred related research and development expenses of approximately $1,329,000
and $328,000, respectively, and had approximately $476,000 and $669,000 recorded as related accounts payable and accrued expenses, respectively,
at December 31, 2022, and approximately $164,000 and $115,000 recorded as related accounts payable and accrued expenses, respectively,
at December 31, 2021.
University
of Texas Health Science Center at San Antonio
The
Company entered into a patent and technology license agreement (the “UT Health Agreement”), dated November 18, 2022, with
the University of Texas Health Science Center at San Antonio (“UT Health”). Under the terms of the UT Health Agreement, the
Company holds an exclusive, worldwide license (other than the excluded field of vectors, as defined in the UT Health Agreement) to certain
specified patent rights relating to the development of a live attenuated, oral Chlamydia vaccine candidate. An initial non-refundable
license fee of $100,000 was due upon execution of the agreement and subsequent annual license fees of $20,000 per year for each of the
four years ending on December 31, 2026; $40,000 per year for each of the two years ending on December 31, 2028, and $60,000 per year
for the year ending December 31, 2029 and each year thereafter until expiration or termination of the UT Health agreement. See Note 7
for information on milestone payments as well as royalty obligations required under the UT Health Agreement. The UT Health Agreement
will expire upon the expiration of the last date of expiration or termination of the patent rights, unless terminated earlier. The Company
may terminate the UT Health Agreement for convenience, by providing 90 days’ written notice to UT Health. UT Health may terminate
the UT Health Agreement in the event the Company (a) becomes arrears in payment due and does not make payment within 30 days after notification
from UT Health or (b) is in breach of any non-payment provision and does not cure such breach within 60 days after notification from
UT Health or (c) UT Health delivers notice to the Company of three or more actual material breaches of the UT Health Agreement in any
12-month period or (d) in the event the Company or its affiliates initiates any proceeding or action to challenge the validity, enforceability,
or scope of any of the licensed patents.
For
the year ended December 31, 2022, the Company recognized an aggregate of $100,000 for intellectual property licenses, which are recorded
as research and development expenses and included in accounts payable as of December 31, 2022.
ONCONETIX,
INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity
Authorized
Capital
On
February 23, 2022, in connection with the closing of the IPO, the Company filed with the Secretary of State of the State of Delaware
an amended and restated certificate of incorporation (the “A&R COI”), which became effective immediately. There was no
change to the Company’s authorized shares of common stock and preferred stock of 250,000,000 shares and 10,000,000 shares, respectively,
or the par value, which is $0.00001 for both common and preferred stock. Prior to this amendment, the Company had designated 1,150,000
shares of preferred stock, with par value $0.00001 per share. In addition, on February 23, 2022 and in connection with the closing of
the IPO, the Company’s board of directors adopted Amended and Restated Bylaws.
Common
Stock
As
of December 31, 2022 and 2021, there were 15,724,957 and 3,200,000 shares of common stock issued, respectively, and 15,265,228 and 3,200,000
shares of common stock outstanding, respectively.
Holders
of the Company’s common stock are entitled to one vote for each share held of record, and are entitled upon liquidation of the
Company to share ratably in the net assets of the Company available for distribution after payment of all obligations of the Company
and after provision has been made with respect to each class of stock, if any, having preference over the common stock, currently including
the Company’s preferred stock. The shares of common stock are not redeemable and have no preemptive or similar rights.
On
February 17, 2022, the Company entered into an underwriting agreement (the “Underwriting Agreement”) with Boustead Securities,
LLC, acting as representative of the underwriters (“Boustead”), in relation to the Company’s IPO, pursuant to which
the Company agreed to sell to the underwriters an aggregate of 2,222,222 shares of the Company’s common stock, at a price of $9.00
per share. The IPO closed on February 23, 2022, and resulted in net proceeds to the Company, after deducting the 8% underwriting discount,
and other offering costs, of approximately $17.1 million. Pursuant to the Underwriting Agreement, the Company issued to Boustead warrants
to purchase 111,111 shares of common stock, exercisable for five years at the option of the holder, at a per share exercise price equal
to $10.35.
The
Company evaluated the terms of the warrants issued at the close of the IPO and determined that they should be classified as equity instruments
based upon accounting guidance provided in ASC 480 and ASC 815-40. Since the Company determined that the warrants were equity-classified,
the Company recorded the proceeds from the IPO, net of issuance costs, within common stock at par value and the balance of the net proceeds
to additional paid in capital.
During
October 2022, in connection with a settlement agreement that was entered into with Boustead, these warrants were exchanged for 93,466
shares of restricted common stock (“the Warrant Exchange”). See Note 7. The Warrant Exchange was accounted for as a modification
of the warrant, with an incremental fair value of approximately $10,000, which was recorded as general and administrative expense in
the accompanying statements of operations. In addition, 200,000 restricted shares of common stock were issued to Boustead upon execution
of an advisory agreement, which was entered into concurrent with the settlement agreement. The fair value of the restricted shares of
common stock, which had no vesting provisions, was valued at $254,000, and was recorded as general and administrative expense in the
accompanying statements of operations. See Note 7.
The
restricted shares of common stock issued under the settlement and advisory agreements was valued based on the closing trading price on
the date the agreements were executed, adjusted to reflect the effect of the restriction on the sale of the common stock. The value of
the restriction was measured using the Black-Scholes model to measure the discount for lack of marketability, using the following assumptions:
expected term of 0.5 years, expected volatility of 96.36%, risk-free interest rate of 4.09% and dividend yield of 0.0%.
Treasury
Stock
On
November 10, 2022, the board of directors approved a stock repurchase program (the “Repurchase Program”) to allow the Company
to repurchase up to 5 million shares of common stock with a maximum price of $1.00 per share, with discretion to management to make purchases
subject to market conditions. On November 18, 2022, the board of directors approved an increase to the maximum price to $2.00 per share.
There is no expiration date for this program.
During
2022, the Company repurchased 459,729 shares of common stock at an average price of $1.23 per share, for approximately $0.6 million.
Shares that are repurchased are classified as treasury stock pending future use and reduce the number of shares outstanding used in calculating
earnings per share. As of December 31, 2022, there are approximately 4.5 million shares remaining, that can be repurchased under the
Repurchase Program.
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
Private
Investments in Public Equity
April
Private Placement
On
April 19, 2022, the Company consummated the closing of a private placement (the “April Private Placement”), pursuant to
the terms and conditions of a securities purchase agreement, dated as of April 13, 2022. At the closing of the April Private
Placement, the Company issued 590,406 shares of common stock, pre-funded warrants to purchase an aggregate of 590,406 shares of
common stock and preferred investment options to purchase up to an aggregate of 1,180,812 shares of common stock. The purchase price
of each share of common stock together with the associated preferred investment option was $6.775, and the purchase price of each
pre-funded warrant together with the associated preferred investment option was $6.774. The aggregate net cash proceeds to the
Company from the April Private Placement were approximately $6.9 million, after deducting placement agent fees and other offering
expenses. The pre-funded warrants had an exercise price of $0.001 per share, were exercisable on or after April 19, 2022, and were
exercisable until the pre-funded warrants were exercised in full. The pre-funded warrants were exercised in full on May 24, 2022,
and as such the Company issued 590,406 shares of common stock on that date. The preferred investment options were exercisable at any
time on or after April 19, 2022 through April 20, 2026, at an exercise price of $6.65 per share, subject to certain adjustments as
set forth in the agreement.
H.C.
Wainwright & Co., LLC (“Wainwright”) acted as the exclusive placement agent for the April Private Placement. The Company
agreed to pay Wainwright a placement agent fee and management fee equal to 7.5% and 1.0%, respectively, of the aggregate gross proceeds
from the April Private Placement and reimburse certain out-of-pocket expenses up to an aggregate of $85,000. In addition, the Company
issued warrants to Wainwright (the “April Wainwright Warrants”) to purchase up to 70,849 shares of common stock. The Wainwright
Warrants are in substantially the same form as the preferred investment options, except that the exercise price is $8.46875. The form
of the preferred investment options is a warrant, and as such the preferred investment options, the pre-funded warrants, and the Wainwright
Warrants are collectively referred to as the “April Private Placement Warrants”. Further, upon any exercise for cash of any
preferred investment options, the Company agreed to issue to Wainwright additional warrants to purchase the number of shares of common
stock equal to 6.0% of the aggregate number of shares of common stock underlying the preferred investment options that have been exercised,
also with an exercise price of $8.46875 (the “April Contingent Warrants”). The maximum number of April Contingent Warrants
issuable under this provision is 70,849.
In
connection with the April Private Placement, the Company entered into a Registration Rights Agreement with the purchasers, dated as of
April 13, 2022 (the “April Registration Rights Agreement”). The April Registration Rights Agreement provides that the Company
shall file a registration statement covering the resale of all of the registrable securities (as defined in the April Registration Rights
Agreement) with the Securities and Exchange Commission (the “SEC”) no later than the 20th calendar day following the date
of the April Registration Rights Agreement and have the registration statement declared effective by the SEC as promptly as possible
after the filing thereof, but in any event no later than the 45th calendar day following April 13, 2022 or, in the event of a full review
by the SEC, the 75th day following April 13, 2022. The registration statement on Form S-1 required under the April Registration Rights
Agreement was filed with the SEC on May 3, 2022, and became effective on May 20, 2022.
Upon
the occurrence of any Event (as defined in the April Registration Rights Agreement), which, among others, prohibits the purchasers from
reselling the securities for more than ten consecutive calendar days or more than an aggregate of fifteen calendar days during any 12-month
period, and should the registration statement cease to remain continuously effective, the Company would be obligated to pay to each purchaser,
on each monthly anniversary of each such Event, an amount in cash, as partial liquidated damages and not as a penalty, equal to the product
of 2.0% multiplied by the aggregate subscription amount paid by such purchaser in the April Private Placement. As of December 31, 2022,
the Company determined that the likelihood of the Company incurring liquidated damages pursuant to the April Registration Rights Agreement
is remote, and as such, no accrual of these payments is required as of December 31, 2022.
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
The
Company evaluated the terms of the April Private Placement Warrants and determined that they should be classified as equity instruments
based upon accounting guidance provided in ASC 480 and ASC 815-40. Since the Company determined that the April Private Placement Warrants
were equity-classified, the Company recorded the proceeds from the April Private Placement, net of issuance costs, within common stock
at par value and the balance of the net proceeds to additional paid in capital.
The
Company evaluated the terms of the April Contingent Warrants and determined that they should be classified as a liability based upon
accounting guidance provided in ASC 815-40. Since the April Contingent Warrants are a form of compensation to Wainwright, the Company
recorded the value of the liability of approximately $36,000, as a reduction of additional paid in capital, with subsequent changes in
the value of the liability recorded in other income in the accompanying statements of operations. The Company measured the liability
upon the close of the April Private Placement using a Monte Carlo simulation. See Note 3.
On
August 11, 2022, the investors in the April Private Placement agreed to cancel the aggregate of 1,180,812 preferred investment options
issued in the April Private Placement, as part of their participation in the August Private Placement. Concurrent with the cancellation
of the April preferred investment options, which was accounted for as an exchange of equity-linked financial instruments, the April Contingent
Warrants, which were issuable only upon exercise of the preferred investment options, were also modified. See ‘August Private Placement’
below for further detail.
August
Private Placement
On
August 11, 2022, the Company consummated the closing of a private placement (the “August Private Placement”), pursuant to
the terms and conditions of a securities purchase agreement, dated as of August 9, 2022. At the closing of the August Private Placement,
the Company issued 1,350,000 shares of common stock, pre-funded warrants to purchase an aggregate of 2,333,280 shares of common stock
and preferred investment options to purchase up to an aggregate of 4,972,428 shares of common stock. The purchase price of each share
of common stock together with the associated preferred investment option was $2.715, and the purchase price of each pre-funded warrant
together with the associated preferred investment option was $2.714. The aggregate net cash proceeds to the Company from the August Private
Placement were approximately $8.7 million, after deducting placement agent fees and other offering expenses. In addition, the investors
in the August Private Placement, who are the same investors from the April Private Placement, agreed to cancel preferred investment options
to purchase up to an aggregate of 1,180,812 shares of the Company’s common stock issued in April 2022. The pre-funded warrants
have an exercise price of $0.001 per share, are exercisable on or after August 11, 2022, and are exercisable until the pre-funded warrants
are exercised in full. The preferred investment options are exercisable at any time on or after August 11, 2022 through August 12, 2027,
at an exercise price of $2.546 per share, subject to certain adjustments as defined in the agreement. During 2022, an aggregate of 1,686,640
of the pre-funded warrants were exercised, and as such the Company issued 1,686,640 shares of common stock. The remaining 646,640 of
pre-funded warrants were exercised subsequent to December 31, 2022. See Note 11.
Wainwright
acted as the exclusive placement agent for the August Private Placement. The Company agreed to pay Wainwright a placement agent fee and
management fee equal to 7.5% and 1.0%, respectively, of the aggregate gross proceeds from the August Private Placement and reimburse
certain out-of-pocket expenses up to an aggregate of $85,000. In addition, the Company issued warrants to Wainwright (the “August
Wainwright Warrants”) to purchase up to 220,997 shares of common stock. The August Wainwright Warrants are in substantially the
same form as the preferred investment options, except that the exercise price is $3.3938. The form of the preferred investment options
is a warrant, and as such the preferred investment options, the pre-funded warrants, and the August Wainwright Warrants are collectively
referred to as the “August Private Placement Warrants”. Further, upon any exercise for cash of any preferred investment options,
the Company agreed to issue to Wainwright additional warrants to purchase the number of shares of common stock equal to 6.0% of the aggregate
number of shares of common stock underlying the preferred investment options that have been exercised, also with an exercise price of
$3.3938 (the “August Contingent Warrants”). The maximum number of August Contingent Warrants issuable under this provision
is 298,346, which includes 70,849 of April Contingent Warrants that were modified in connection with the August Private Placement.
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
In
connection with the August Private Placement, the Company entered into a Registration Rights Agreement with the purchasers, dated as
of August 9, 2022 (the “August Registration Rights Agreement”). The August Registration Rights Agreement provides that the
Company shall file a registration statement covering the resale of all of the registrable securities (as defined in the August Registration
Rights Agreement) with the SEC no later than the 30th calendar day following the date of the August Registration Rights Agreement and
have the registration statement declared effective by the SEC as promptly as possible after the filing thereof, but in any event no later
than the 45th calendar day following August 9, 2022 or, in the event of a full review by the SEC, the 80th day following August 9, 2022.
The registration statement on Form S-1 required under the Registration Rights Agreement was filed with the SEC on August 29, 2022, and
became effective on September 19, 2022.
Upon
the occurrence of any Event (as defined in the August Registration Rights Agreement), which, among others, prohibits the purchasers from
reselling the securities for more than ten consecutive calendar days or more than an aggregate of fifteen calendar days during any 12-month
period, and should the registration statement cease to remain continuously effective, the Company would be obligated to pay to each purchaser,
on each monthly anniversary of each such Event, an amount in cash, as partial liquidated damages and not as a penalty, equal to the product
of 2.0% multiplied by the aggregate subscription amount paid by such purchaser in the August Private Placement. As of December 31, 2022,
the Company determined that the likelihood of the Company incurring liquidated damages pursuant to the August Registration Rights Agreement
is remote, and as such, no accrual of these payments is required as of December 31, 2022.
The
Company evaluated the terms of the August Private Placement Warrants and determined that they should be classified as equity instruments
based upon accounting guidance provided in ASC 480 and ASC 815-40. Since the Company determined that the August Private Placement Warrants
were equity-classified, the Company recorded the proceeds from the August Private Placement, net of issuance costs, within common stock
at par value and the balance of the net proceeds to additional paid in capital.
As
discussed above, the investors in the Private Placements agreed to cancel the aggregate of 1,180,812 preferred investment options issued
in the April Private Placement, as part of their participation in the August Private Placement. The preferred investment options that
were cancelled were effectively exchanged for 1,289,148 new preferred investment options in the August Private Placement, and accordingly
have been accounted for as a modification or exchange of equity-linked instruments. In accordance with ASC 815-40, as the preferred investment
options were classified as equity instruments before and after the exchange, and as the exchange is directly attributable to an equity
offering, the Company recognized the effect of the exchange as an equity issuance cost. The increase in the fair value of the preferred
investment options as a result of the exchange was approximately $860,000, and was determined using the Black-Scholes option pricing
model, with the following assumptions:
| | |
Original | | |
Exchanged | |
| Exercise price | |
$ | 6.65 | | |
$ | 2.546 | |
| Term (years) | |
| 3.67 | | |
| 5.0 | |
| Expected stock price volatility | |
| 116.2 | % | |
| 120.2 | % |
| Risk-free rate of interest | |
| 3.16 | % | |
| 2.98 | % |
The
Company evaluated the terms of the August Contingent Warrants and determined that they should be classified as a liability based upon
accounting guidance provided in ASC 815-40. As a result of the exchange of the preferred investment options issued in the April Private
Placement, the underlying equity-linked instruments that would trigger issuance of the April Contingent Warrants was replaced, and therefore
the 70,849 of April Contingent Warrants were exchanged for 70,849 of the August Contingent Warrants. The value of the April Contingent
Warrant liability was adjusted to fair value on the date of modification, using a Monte Carlo simulation, with the change in fair value
of approximately $8,000 recognized in the accompanying statements of operations. The remaining 227,497 August Contingent Warrants were
measured as a liability upon the close of the August Private Placement. Since the Contingent Warrants are a form of compensation to the
placement agent, the Company recorded the value of the liability of approximately $39,000, as a reduction of additional paid in capital.
The entire 298,346 of August Contingent Warrants were remeasured at December 31, 2022, using a Monte Carlo simulation, with the change
in the value of the liability recorded in other income (expense) in the accompanying statements of operations. See Note 3.
ONCONETIX,
INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
Warrants
The
following summarizes activity related to the Company’s outstanding warrants as discussed above, excluding contingent warrants issuable
upon exercise of the preferred investment options, for the year ended December 31, 2022:
| | |
| | |
| | |
Weighted | |
| | |
| | |
| | |
Average | |
| | |
| | |
Weighted | | |
Remaining | |
| | |
| | |
Average | | |
Contractual | |
| | |
Number of | | |
Exercise | | |
Life | |
| | |
Shares | | |
Price | | |
(in years) | |
| Outstanding as of December 31, 2021 | |
| — | | |
$ | — | | |
| — | |
| Granted | |
| 9,479,883 | | |
| 2.43 | | |
| | |
| Exercised | |
| (2,277,046 | ) | |
| 0.001 | | |
| | |
| Cancelled | |
| (1,291,923 | ) | |
| 6.97 | | |
| | |
| Outstanding as of December 31, 2022 | |
| 5,910,914 | | |
| 2.37 | | |
| 4.7 | |
| Warrants vested and exercisable as of December 31, 2022 | |
| 5,910,914 | | |
$ | 2.37 | | |
| 4.7 | |
As
of December 31, 2022, the outstanding warrants include 70,849 April Private Placement Warrants and 5,840,065 August Private Placement
Warrants, which are exercisable into 5,910,914 shares of common stock which had a fair value of $1.10 per share, based on the closing
trading price on that day.
Additionally,
as of December 31, 2022, the value of the April Contingent Warrants and the August Contingent Warrants (collectively the “Contingent
Warrants”) was approximately $14,000, and none of the Contingent Warrants have been issued, as no preferred investment options
have been exercised.
Preferred
Stock
Prior
to the close of the IPO, the Company had designated 1,150,000 shares of preferred stock as Series Seed Preferred Stock (“Series
Seed”), with an original issue price of $6.09 per share (the “Original Issue Price”). As of December 31, 2022 and 2021,
there were 0 and 1,146,138 shares of Series Seed issued and outstanding, respectively.
Conversion
Each
share of the Series Seed was convertible, at the option of the holder, at any time and from time to time, and without the payment of
additional consideration by the holder, at a conversion price of $1.52 per share, subject to certain adjustments for stock splits, stock
dividends, recapitalizations, and similar corporate transactions, into fully paid and non-assessable shares of the Company’s common
stock. Each Series Seed share was automatically convertible into common stock of the Company, at the then-effective conversion price,
upon the closing of a firmly underwritten public offering netting proceeds of at least $50 million with an offering price of at least
three hundred percent (300%) of the Original Issue Price of the Series Seed. On February 18, 2022, the majority of the holders of the
Series Seed approved the automatic conversion of the outstanding shares of the Series Seed and all related accrued and unpaid dividends,
upon the closing of the IPO. The number of shares of Common Stock to be issued upon the closing of the IPO pursuant to the conversion
were to be calculated in accordance with the original conversion terms provided by the Company’s Amended and Restated Certificate
of Incorporation (“COI”) dated July 1, 2019. This conversion occurred on February 23, 2022, upon the closing of the Company’s
IPO.
ONCONETIX,
INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
Dividends
Holders
of the Series Seed were entitled to receive cumulative dividends at a per share rate of 8% per annum, compounded annually, on the initial
investment amount commencing on the date of issue. Dividends were payable only when, as, and if declared by the board of directors or
upon a Liquidation Event (as defined below). Dividends on Series Seed shares were in preference to any dividend on the Company’s
common stock. As of December 31, 2021, aggregate cumulative dividends totaled $1,489,803, or $1.30 per Series Seed share, and upon the
close of the IPO in 2022, aggregate cumulative dividends of $1,586,162, or $1.38 per Series Seed share, were automatically converted
into shares of common stock.
Liquidation
Preference
In
the event of certain voluntary or involuntary acquisition or sale transactions or upon the liquidation, dissolution or winding up of
the Company (each, a “Liquidation Event”), the holders of Series Seed were entitled to receive out of the
proceeds or assets of the Company legally available for distribution to its stockholders (the “Proceeds”), prior and in
preference to any distribution of the Proceeds of such Liquidation Event to the holders of shares of common stock by reason of their
ownership thereof, an amount (“the Liquidation Preference Amount”) determined based on the provisions of the
Company’s COI. The COI provided that the Liquidation Preference Amount be calculated upon the occurrence of a Liquidation
Event, based on the Company’s achievement of a Pre-Clinical Milestone and a Qualified Financing, both as defined in the COI.
Per the provisions of the COI, if a Liquidation Event occurred before a Pre-Clinical Milestone was achieved, the Liquidation
Preference Amount would be equal to two times the Series Seed Original Issue price per share, plus unpaid cumulative dividends. If a
Liquidation Event occurred after a Pre-Clinical Milestone was achieved, and after a Qualified Financing was completed, then the
Liquidation Preference Amount would be equal to one times the Series Seed Original Issue price, plus unpaid cumulative dividends. If
a Liquidation Event occurred after a Pre-Clinical Milestone was achieved and before a Qualified Financing was completed, the
Liquidation Preference Amount would be equal to the greater of (a) such amount per share as such holder would have been entitled to
receive after a Qualified Financing or (b) two times the Series Seed Original Issue price, plus unpaid cumulative dividends.
As
of December 31, 2021, and all other prior historical periods, the Liquidation Preference Amount was equal to two times the Series Seed
Original Issue Price per share, plus unpaid cumulative dividends. In the event that the Proceeds were insufficient to enable the distribution
in full of the Liquidation Preference Amount to the holders of the Series Seed for all of the preferred shares held by them, all of the
Proceeds were to be distributed among the holders
of Series Seed on a pro rata basis. Upon completion of the distribution required to the holders of Series Seed, all of the remaining
Proceeds available for distribution to stockholders were to be distributed among the holders of common shares and preferred shares, on
an as-converted basis, pro rata based on the number of common shares held by each such holder. However, if upon the occurrence of a Liquidation
Event, the Liquidation Preference Amount the Series Seed stockholders were entitled to receive is two times the Original Issue Price
per share, plus unpaid cumulative dividends, after such distribution is made, then the remaining Proceeds available for distribution
to stockholders were to be distributed among the holders of common shares, pro rata based on the number of common shares held by each
such holder.
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
Voting
On
any matter presented to the stockholders of the Company for their action or consideration at any meeting of stockholders of the Company
(or by written consent of stockholders in lieu of meeting), each holder of outstanding shares of Series Seed was entitled to cast the
number of votes equal to the number of whole shares of common stock into which the shares of Series Seed held by such holder were convertible
as of the record date for determining stockholders entitled to vote on such matter. Holders of Series Seed were to vote together with
the holder of common stock as a single class. Holders of Series Seed shares were entitled to nominate two out of five of the Company’s
directors.
Equity
Incentive Plans
The
Company’s 2019 Equity Incentive Plan (the “2019 Plan”) was adopted by its board of directors and by its stockholders
on July 1, 2019. The Company has reserved 1,400,000 shares of common stock for issuance pursuant to the 2019 Plan. There were no share-based
awards granted under the 2019 Plan during the years ended December 31, 2022 and 2021.
In
addition, on February 23, 2022 and in connection with the closing of the IPO, the Company’s board of directors adopted the Company’s
2022 Equity Incentive Plan (the “2022 Plan”), which is the successor and continuation of the Company’s 2019 Plan. Under
the 2022 Plan, the Company may grant stock options, restricted stock, restricted stock units, stock appreciation rights, and other forms
of awards to employees, directors and consultants of the Company. Upon its effectiveness, a total of 1,600,000 shares of common stock
were reserved for issuance under the 2022 Plan. In August 2022, the number of shares of common stock reserved for issuance under the
2022 Plan was increased to 2,600,000. The stock options granted during the year ended December 31, 2022 were all granted under the 2022
Plan. As of December 31, 2022, there were 1,041,894 options available for issuance under the 2022 Plan.
Stock
Options
The
following summarizes activity related to the Company’s stock options under the 2019 Plan and the 2022 Plan for the year ended December
31, 2022:
| | |
| | |
| | |
| | |
Weighted | |
| | |
| | |
| | |
| | |
Average | |
| | |
| | |
Weighted | | |
| | |
Remaining | |
| | |
| | |
Average | | |
Total | | |
Contractual | |
| | |
Number of | | |
Exercise | | |
Intrinsic | | |
Life | |
| | |
Shares | | |
Price | | |
Value | | |
(in years) | |
| Outstanding as of December 31, 2021 | |
| 780,640 | | |
$ | 0.01 | | |
$ | 532,787 | | |
| 8.1 | |
| Granted | |
| 797,223 | | |
| 5.92 | | |
| — | | |
| — | |
| Forfeited / cancelled | |
| (19,757 | ) | |
| 6.45 | | |
| — | | |
| — | |
| Exercised | |
| (165,452 | ) | |
| 0.01 | | |
| 573,465 | | |
| — | |
| Outstanding as of December 31, 2022 | |
| 1,392,654 | | |
$ | 3.30 | | |
$ | 670,161 | | |
| 8.2 | |
| Options vested and exercisable as of December 31, 2022 | |
| 933,888 | | |
$ | 2.99 | | |
$ | 534,006 | | |
| 8.0 | |
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
The
fair value of options granted in 2022 was estimated using the following assumptions:
| |
|
For the Year
Ended December 31, |
|
| |
|
2022 |
|
| Exercise price |
|
$1.06 –
6.45 |
|
| Term (years) |
|
5.00 – 10.00 |
|
| Expected stock price volatility |
|
112.6% – 121.2% |
|
| Risk-free rate of interest |
|
2.9% – 4.3% |
|
The
weighted average grant date fair value of stock options granted during the year ended December 31, 2022 was $3.40. The aggregate fair
value of stock options that vested during the years ended December 31, 2022 and 2021 was approximately $2.1 million and $0.1 million,
respectively.
Of
the total stock options granted during the year ended December 31, 2022, 200,000 stock options were granted to the Company’s Chief
Executive Officer (“CEO”), Chairman, and significant stockholder, 200,000 stock options were granted to the Company’s
Chief Business Officer (“CBO”), and 100,000 stock options were granted to the Company’s Chief Financial Officer (“CFO”).
The aggregate grant-date fair value of the stock options granted to the CEO, CBO, and CFO was approximately $1.8 million, of which approximately
$1.5 million was recognized as stock-based compensation expense during the year ended December 31, 2022. Additionally, during the year
ended December 31, 2022, the Company granted an aggregate of 72,223 stock options to non-executive directors. The grant-date fair value
of the stock options granted to the non-executive directors was approximately $0.2 million, of which approximately $0.2 million was recognized
as stock-based compensation expense during the year ended December 31, 2022.
During
the year ended December 31, 2022, the Company’s board of directors approved the accelerated vesting of an aggregate of 32,517 stock
options to a former director and a former advisor, in connection with their separation from the Company. The Company recognized stock-based
compensation expense of approximately $0.1 million related to these modifications during the year ended December 31, 2022.
ONCONETIX, INC.
Notes
to Financial Statements
Note
6 — Stockholders’ Equity (cont.)
Stock-Based
Compensation
Stock-based
compensation expense for the years ended December 31, 2022 and 2021 was as follows:
| | |
For the Years Ended
December 31, | |
| | |
2022 | | |
2021 | |
| General and administrative | |
$ | 1,309,687 | | |
$ | 41,061 | |
| Research and development | |
| 664,879 | | |
| 89,080 | |
| Total | |
$ | 1,974,566 | | |
$ | 130,141 | |
As
of December 31, 2022, unrecognized stock-based compensation expense relating to outstanding stock options is approximately $0.7 million,
which is expected to be recognized over a weighted-average period of 1.89 years.
Note
7 — Commitments and Contingencies
Office
Leases
Starting
in 2018, the Company leased office space for approximately $5,500 a month from a related party. The Company was required to pay a $15,000
rental deposit. The Company terminated the related party lease in May 2021. Rent expense related to this lease for the years ended December
31, 2022 and 2021 was approximately $0 and $26,000, respectively. The Company entered into a month-to-month lease in Cincinnati, Ohio,
with an unrelated party in April 2021 with monthly payments of approximately $500 per month.
The
Company entered into a short-term lease in Palm Beach, Florida with an unrelated party, with a commencement date of May 1, 2022, for
approximately $14,000 per month. The lease term ends on April 30, 2023 and is personally guaranteed by the Company’s CEO. During
the year ended December 31, 2022, the Company incurred rent expense on this lease of approximately $129,000, and variable lease expense
of approximately $12,000.
Litigation
From
time to time, the Company may be subject to various legal proceedings and claims that arise in the ordinary course of its business activities.
As of December 31, 2022, the Company is not a party to any material legal proceedings and is not aware of any pending or threatened claims.
On
April 15, 2022, the Company received a demand letter (the “Demand Letter”) from Boustead. The Demand Letter alleged that
the Company breached the Underwriting Agreement entered into between Boustead and the Company, dated February 17, 2022, in connection
with the Company’s initial public offering. The Demand Letter alleged that, by engaging Wainwright as placement agent in the April
Private Placement, the Company breached Boustead’s right of first refusal (“ROFR”) to act as placement agent granted
to Boustead under the Underwriting Agreement and, as a result of selling securities in the April Private Placement, breached the Company’s
obligation under the Underwriting Agreement not to offer, sell, issue, agree or contract to sell or issue or grant or modify the terms
of any option for the sale of, any securities prior to February 17, 2023 (the “Standstill”).
ONCONETIX,
INC.
Notes
to Financial Statements
Note
7 — Commitments and Contingencies (cont.)
On
October 9, 2022, the Company and Boustead entered into a Settlement Agreement and Release (the “Settlement Agreement”), pursuant
to which Boustead agreed to waive the ROFR and the Standstill, and to release the Company from certain claims with respect to the April
Private Placement, the August Private Placement, and all future private, public equity or debt offerings of the Company. As consideration
for such waiver and termination of the Underwriting Agreement, the Company paid Boustead a cash fee of $1,000,000, $50,000 in legal expenses,
and released Boustead from all claims, subject to certain exceptions. In addition, the Company issued to Boustead 93,466 shares of restricted
common stock in exchange for the cancellation of 111,111 warrants issued to Boustead in connection with the IPO (see Note 6). Concurrent
with the execution of the Settlement Agreement, the Company and Boustead Capital Markets, LLP (“Boustead Capital”) entered
into a three-month Advisory Agreement (the “Advisory Agreement”) for which consideration equal to 200,000 shares of restricted
common stock, with no vesting provisions, was issued to Boustead Capital upon execution of the Advisory Agreement. The restricted common
stock issued in connection with these agreements had an aggregate fair value of approximately $264,000. See Note 6.
The
Company determined that all consideration due by the Company under the Settlement Agreement and the Advisory Agreement relates to the
settlement of a liability that was incurred in 2022, and accordingly, recorded a related expense of approximately $1.3 million for the
year ended December 31, 2022, which is included in general and administrative expenses in the accompanying statements of operations.
Registration
Rights Agreements
See
Note 6, Private Investments in Public Equity.
Significant
Agreements
Oxford
University Innovation Limited
Pursuant
to the OUI Agreement, as disclosed in Note 5, the Company is obligated to pay certain milestone and royalty payments in the future, as
the related contingent events occur. Specifically, the Company is obligated to pay a 6% royalty on all net sales of licensed products,
as defined in the OUI Agreement, with an annual minimum royalty payment of $250,000 starting post-product launch, until the expiration
of the OUI Agreement or revocation of the last valid claim covering a licensed product, at which point a royalty rate of 3% will apply.
An annual maintenance fee of $10,000 and $20,000 is required in the pre-phase III year and Phase III year, respectively, and as defined
in the OUI Agreement. The Company is also obligated to pay a 25% royalty on any sums received by the Company from
any sublicensee (including all up-front, milestone and other one-off payments received by the Company from any sub-licenses or other
contracts granted by the Company with respect to the licensed technology). In addition, the Company is required to pay OUI milestone
payments of up to an aggregate of $51.25 million; specifically, upon the achievement of specified development milestones of approximately
$2.25 million, regulatory milestones of approximately $9.5 million, and commercial milestones of approximately $39.5 million. The annual
maintenance fee and milestone fees are indexed to the RPI (Retail Prices index for all items which is published in the United Kingdom
by the Office for National Statistics, or any replacement of it) and will be increased or decreased as appropriate as set forth in the
OUI Agreement. As of December 31, 2022, the Company evaluated the likelihood of the Company achieving the specified milestones and generating
product sales, and determined the likelihood is not yet probable and as such, no accrual of these payments is required as of December
31, 2022.
ONCONETIX, INC.
Notes
to Financial Statements
Note
7 — Commitments and Contingencies (cont.)
Oxford
University Research Agreement
Pursuant
to the terms of the OUI Agreement, as disclosed in Note 5, the Company entered into a sponsored research agreement dated December 18,
2019 with Oxford University for research related to the OUI Agreement for a period of three years for a total of £420,000. The
Company prepaid the full amount to Oxford of $554,802 for the services in January 2020, of which approximately $0.1 and $0.2 million
remains as a prepaid expense as of December 31, 2022 and 2021, respectively. On May 16, 2022, the Company entered into an amendment to
the Oxford University Research Agreement, whereby the Oxford University Research Agreement was extended until June 30, 2024, with an
option to extend another 12 months, for a fee of £53,500 (or approximately $56,000).
During
the years ended December 31, 2022 and 2021, the Company incurred research and development expenses related to the sponsored research
agreement with Oxford of approximately $51,000 and $185,000, respectively.
St.
Jude Children’s Hospital
Pursuant
to the St. Jude Agreement, as disclosed in Note 5, the Company is obligated to pay certain milestone and royalty payments in the future,
as the related contingent events occur. On May 11, 2022, the Company entered into an amendment to the St. Jude Agreement, whereby the
royalty terms, milestones payments and licensing fees were amended. Specifically, pursuant to the terms of the St. Jude Agreement, as
amended, the Company is obligated to make 5% royalty payments for each licensed product(s) sold by the Company or its affiliates, based
on the net sales for the duration of the St. Jude Agreement, and also pay 15% of consideration received for any sublicenses. The Company
is also required to pay an additional one-time $5,000 license fee, and an annual maintenance fee of $10,000 beginning on the first anniversary
of the Effective Date (which is waived if all of the developmental milestones scheduled for completion before such annual fee is due
have been achieved). In addition, the Company is required to pay St. Jude milestone payments of up to an aggregate of $1.9 million; specifically,
upon the achievement of specified development milestones of $0.3 million, regulatory milestones of $0.6 million, and commercial milestones
of $1.0 million. As of December 31, 2022, the Company evaluated the likelihood of the Company achieving the specified milestones and
generating product sales, and determined the likelihood is not yet probable and as such, no accrual of these payments is required as
of December 31, 2022.
St.
Jude Children’s Sponsored Research Agreement
In
addition to the St. Jude Agreement, the Company also entered into a sponsored research agreement dated May 3, 2021 with St. Jude for
research related to the St. Jude Agreement (the “St. Jude SRA”). Pursuant to the St. Jude SRA, the Company is obligated to
pay St. Jude an aggregate amount of $73,073 in two parts, Phase I for $57,624 and Phase II for $15,449. This sponsored research project
began during 2021.
The
Company entered into a second sponsored research agreement with St. Jude, dated August 29, 2022, pursuant to which the Company is obligated
to pay St. Jude an amount of $75,603 which is due within 30 days of the effective date of the agreement.
During
the years ended December 31, 2022 and 2021, the Company incurred related research and development expenses related to the sponsored research
agreements with St. Jude of approximately $27,000 and $65,000, respectively.
Cincinnati
Children’s Hospital Medical Center
Pursuant
to the CHMC Agreement, as disclosed in Note 5, the Company is obligated to pay certain milestone and royalty payments in the future,
as the related contingent events occur. Specifically, the Company is obligated to pay CHMC a single-digit royalty on net sales, being
5%, 4% or 2% depending on the product, until the last valid claim covering a licensed product exists, at which point the royalty rates
decrease by 50%. The Company is also obligated
to pay up to a 25% royalty on any non-royalty sublicense revenue paid to the Company by any sublicensee. The CHMC Agreement also provides
the Company with an option to license any CHMC or jointly patented modification, alteration or improvement of any invention claimed in
a Licensed Patent (“CHMC Improvement” and “Joint Improvement, respectively”), with a $50,000 option fee for each
Improvement that the Company elects to include in the license grant of the CHMC Agreement. In addition, the Company is required to pay
CHMC milestone payments of up to an aggregate of $59.75 million; specifically, upon the achievement of specified development milestones
of approximately $0.5 million, regulatory milestones of approximately $1.25 million, and commercial milestones of approximately $58 million.
As of December 31, 2022, the Company evaluated the likelihood of the Company achieving the specified milestones and generating product
sales, and determined the likelihood is not yet probable and as such, no accrual of these payments is required as of December 31, 2022.
ONCONETIX, INC.
Notes
to Financial Statements
Note
7 — Commitments and Contingencies (cont.)
CHMC
Sponsored Research Agreement
In
addition to the CHMC Agreement, the Company also entered into a sponsored research agreement dated June 30, 2022 with CHMC for research
related to the CHMC Agreement (the “CHMC SRA”). Pursuant to this research agreement, the Company is obligated to pay CHMC
an aggregate amount not-to-exceed $247,705. The CHMC SRA has a term of one year, and is cancelable upon 60 days written notice by either
party for convenience. In addition, either party may terminate the CHMC SRA in the event the other party (a) files or has filed against
it a petition under the Bankruptcy Act (among other things) or (b) fails to perform or otherwise breaches its obligations under the agreement,
and has not cured such failure or breach within 30 days of notice of material breach.
During
the year ended December 31, 2022, the Company incurred related research and development expenses of approximately $111,000, which was
included in accrued expenses at December 31, 2022. There were no such expenses incurred during the year ended December 31, 2021.
Ology
Bioservices, Inc. (which was later acquired by National Resilience, Inc.)
See
Note 5.
University
of Texas Health Science Center at San Antonio
Pursuant
to the UT Health Agreement, as disclosed in Note 5, the Company is obligated to pay certain milestone and royalty payments in the future,
as the related contingent events occur. Specifically, the Company is obligated to pay UT a single-digit royalty on net sales, being 5%
or 3% depending on whether the product is covered by a valid claim or not, as defined in the agreement. The Company is also obligated
to pay a 20% royalty on any sums received by the Company from any sublicensee. In addition, the Company is required to pay UT Health
milestone payments of up to an aggregate of approximately $2.2 million; specifically, upon the achievement of specified development milestones
of approximately $0.7 million and regulatory milestones of approximately $1.5 million. As of December 31, 2022, the Company evaluated
the likelihood of the Company achieving the specified milestones and generating product sales, and determined the likelihood is not yet
probable and as such, no accrual of these payments is required as of December 31, 2022.
Underwriter
Termination Agreement
On
February 7, 2022, the Company and its former underwriter, Maxim Group (“Maxim”), entered into a termination agreement, whereby
the parties agreed to terminate their engagement of Maxim as the Company’s lead managing underwriter and book runner in connection
with the Company’s IPO. Per the terms of the termination agreement, the Company agreed to pay Maxim a termination fee of $300,000,
due upon the close of the Company’s IPO. The termination fee was recorded as general and administrative expense, and paid, during
the year ended December 31, 2022.
Indemnification
In
the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties
and provide for general indemnifications. The Company’s exposure under these agreements is unknown because it involves claims that
may be made against the Company in the future but have not yet been made. To date, the Company has not paid any claims or been required
to defend any action related to its indemnification obligations. However, the Company may incur charges in the future as a result of
these indemnification obligations.
Risks
and Uncertainties — COVID-19
Management
continues to evaluate the impact of the COVID-19 pandemic on the industry and has concluded that while it is reasonably possible that
the virus could have a negative effect on the Company’s financial position, results of its operations and/or search for drug candidates,
the specific impact is not readily determinable as of the date of these financial statements. The financial statements do not include
any adjustments that might result from the outcome of this uncertainty.
ONCONETIX, INC.
Notes
to Financial Statements
Note
8 — Related Party Transactions
The
Company originally engaged the CEO, who is also the Board Chairman and prior to the close of the IPO, sole common stockholder of the
Company, pursuant to a consulting agreement commencing October 22, 2018, which called for the Company to pay for consulting services
performed on a monthly basis. Upon the close of the Company’s IPO, the consulting agreement was terminated and the CEO’s
employment agreement became effective. During the years ended December 31, 2022 and 2021, the Company incurred approximately $63,000
and $435,000, respectively, in fees under the consulting agreement, which are recognized in general and administrative expenses in the
accompanying statements of operations.
During
2022 the Company entered into a lease agreement that is personally guaranteed by the Company’s CEO. See Note 7.
The
Company also leased office space from a related party, through common ownership. The lease is further described in Note 7 of these financial
statements. The lease was terminated in May 2021, and the related deposit was reclassified to the receivable from related party balance.
During the fourth quarter of 2021, the amounts due from this related party were determined to be uncollectible and were written off.
The total amount written off, which related to the lease deposit, overpaid rent, and utility expenses, was approximately $22,000, and
is recognized in general and administrative expenses in the accompanying statements of operations.
During
the year ended December 31, 2022, the Company’s compensation committee approved one-time bonus awards of $140,000 and $100,000
to the Company’s CEO and CBO, respectively, in recognition of their efforts in connection with the Company’s IPO. These bonuses
were recognized during the year ended December 31, 2022 as general and administrative expenses in the accompanying statements of operations.
During the year ended December 31, 2021, the Company’s board of directors approved a bonus of approximately $200,000 to the CEO,
which is also recognized in general and administrative expenses in the statements of operations. In addition, during the year ended December
31, 2022, the Company’s compensation committee approved stock option grants under the Company’s 2022 Equity Incentive Plan
to certain of the Company’s executive officers. See Note 6.
As
of December 31, 2022 and 2021, the Company has a receivable from related party of approximately $36,000 and $153,000, respectively. The
balance as of December 31, 2022 consists of miscellaneous payments made by the Company on the behalf of the Company’s CEO. Subsequent
to December 31, 2022, the CEO paid the Company the receivable balance. The balance as of December 31, 2021, consists primarily of consulting
fee prepayments to the Company’s CEO, in the amount of $140,000. These consulting fee prepayments were repaid to the Company in
lieu of a bonus payout due to the CEO during May 2022. The remaining balance as of December 31, 2021 consists of miscellaneous payments
made by the Company on the behalf of the CEO.
A
former director of the Company, who currently serves on the Company’s Scientific Advisory Board, serves on the Advisory Board for
the Cincinnati Children’s Hospital Medical Center Innovation Fund, which is affiliated with CHMC. The Company has an exclusive
license agreement with CHMC as disclosed in Note 5. This director resigned from the Company’s board upon the close of its IPO.
Note
9 — Income Taxes
The Company’s major tax jurisdictions are
the United States and various state jurisdictions, and the Company does not have any pending tax audits. Generally, the Company’s federal
returns from 2019 on and state returns from 2018 on, are subject to examination by the United States and state tax authorities; however,
to the extent allowed by law, tax authorities have the ability to adjust the Company’s carryforwards of unutilized net operating losses
and research and development credits for all years.
At December 31, 2022, the Company had a net operating
loss (“NOL”) carryforward for federal and state income tax purposes totaling approximately $12.5 million and $12.1 million,
respectively, available to reduce future taxable income. The federal NOL and certain state NOLs of $8.5 million are carried forward indefinitely
subject to a limitation of 80% of taxable income. State NOLs of approximately $3.7 million will begin to expire in 2024 if not utilized.
The NOL carry forward is subject to review and
possible adjustment by the Internal Revenue Service and state tax authorities. Under the Internal Revenue Code (“IRC”) Sections
382 and 383, annual use of the Company’s net operating loss carryforwards and research credit carryforwards to offset taxable income
and tax, respectively, may be limited based on cumulative changes in ownership. The Company has not completed an analysis to determine
whether any such limitations have been triggered as of December 31, 2022. The amount of the annual limitation, if any, will be determined
based on the value of the Company immediately prior to the ownership change. Subsequent ownership changes may further affect the limitation
in future years.
ONCONETIX, INC.
Notes
to Financial Statements
Note
9 — Income Taxes (cont.)
The
tax effects of the temporary differences and carryforwards that give rise to deferred tax assets consist of the following:
| | |
As of
December 31, | |
| | |
2022 | | |
2021 | |
| Deferred tax assets: | |
| | |
| |
| Net-operating loss carryforward | |
$ | 2,986,738 | | |
$ | 1,120,155 | |
| Capitalized research and development | |
| 885,176 | | |
| — | |
| Stock-based compensation | |
| 308,552 | | |
| 106,171 | |
| Accrued compensation | |
| 186,573 | | |
| 55,500 | |
| License agreement | |
| 82,626 | | |
| 59,685 | |
| Other accrued expenses | |
| 65,886 | | |
| 14,636 | |
| Gross deferred tax assets | |
| 4,515,551 | | |
| 1,356,147 | |
| Valuation allowance | |
| (4,512,546 | ) | |
| (1,353,673 | ) |
| Deferred tax assets, net of allowance | |
$ | 3,005 | | |
$ | 2,474 | |
| Deferred tax liabilities: | |
| | | |
| | |
| Fixed assets | |
| (3,005 | ) | |
| (2,474 | ) |
| Total deferred tax liabilities | |
$ | (3,005 | ) | |
$ | (2,474 | ) |
| Net deferred tax assets | |
$ | — | | |
$ | — | |
The Company has evaluated the positive and negative evidence bearing
upon the realizability of its deferred tax assets. Based on the Company’s history of operating losses since inception, the Company
has concluded that it is more likely than not that the benefit of its deferred tax assets will not be realized. Accordingly, the Company
has provided a full valuation allowance for deferred tax assets as of December 31, 2022 and 2021. During the year ended December 31, 2022,
the valuation allowance increased by approximately $3.2 million.
The
provision for income taxes on earnings subject to income taxes differs from the statutory Federal rate at December 31, 2022 and 2021,
due to the following:
| | |
For the Years Ended
December 31, | |
| | |
2022 | | |
2021 | |
| Expected income tax benefit at Federal statutory tax rate | |
$ | (2,818,164 | ) | |
$ | (717,640 | ) |
| State and local taxes, net of Federal tax benefit | |
| (501,277 | ) | |
| (91,233 | ) |
| Research credits | |
| (16,477 | ) | |
| — | |
| Permanent items | |
| 194,705 | | |
| 9,501 | |
| State rate adjustment | |
| 19,600 | | |
| 119,414 | |
| Other | |
| (37,260 | ) | |
| 4,228 | |
| Change in valuation allowance | |
| 3,158,873 | | |
| 675,730 | |
| Provision for income taxes | |
$ | — | | |
$ | — | |
Under U.S. GAAP, the impact of an uncertain income
tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit
by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being
sustained. Additionally, U.S. GAAP provides guidance on derecognition, classification, interest and penalties, accounting for interim
periods, disclosure and transition.
A reconciliation of the beginning and ending amount
of unrecognized tax benefits is as follows:
| | |
For the Years Ended
December
31, | |
| | |
2022 | | |
2021 | |
| Beginning balance | |
$ | - | | |
$ | - | |
| Increases related to prior year tax positions | |
| 11,517 | | |
| - | |
| Increases related to current year tax positions | |
| 5,493 | | |
| - | |
| Ending balance | |
$ | 17,010 | | |
$ | - | |
At December 31, 2022 and 2021, the Company’s unrecognized
tax benefits were $17,010 and $0, respectively. Due to the existence of the valuation allowance, future changes in the Company’s unrecognized
tax benefits will not impact the effective tax rate. The Company does not expect its unrecognized tax benefits to change significantly
over the next 12 months.
The Company’s policy is to recognize interest
and penalties related to uncertain tax positions in income tax expense. As of December 31, 2022 and 2021, there were no accrued interest
and penalties associated with uncertain tax positions.
ONCONETIX, INC.
Notes
to Financial Statements
Note
10 — Retirement Plan
Effective
January 1, 2022, the Company adopted a defined contribution savings plan pursuant to Section 401(k) of the Internal Revenue Code (“the
401(k) Plan”). The 401(k) Plan is for the benefit of all qualifying employees and permits voluntary contributions by employees
of up to 100% of eligible compensation, subject to the maximum limits imposed by the Internal Revenue Service. The terms of the 401(k)
Plan allow for discretionary employer contributions. No expenses were incurred related to the 401(k) Plan during the year ended December
31, 2022, and the 401(k) Plan lapsed during 2022 due to inactivity.
Note
11 — Subsequent Events
During
January 2023, an aggregate of 646,640 of the Pre-Funded Warrants issued in connection with the August Private Placement were exercised,
at an exercise price of $0.001 per share, and the Company issued 646,640 shares of common stock in accordance with such exercise.
On
January 26, 2023, the Company’s board of directors appointed a new director to replace a director who resigned from the board on
January 13, 2023. The new director was granted 2,386 stock options, with an exercise price of $1.28. In addition, the Company’s
board of directors approved the accelerated vesting of an aggregate of 11,504 stock options to the former director.
On
February 1, 2023, the Company entered into a co-development agreement with AbVacc, Inc. (“AbVacc”), for the purpose of conducting
research aimed at co-development of specific vaccine candidates, including monkeypox and Marburg virus disease with the potential to
expand to others using the Norovirus nanoparticle platform (“Co-Development Project”), and to govern the sharing of materials
and information, as defined in the agreement, for the Co-Development Project. Under the agreement, AbVacc and the Company will collaborate,
through a joint development committee, to establish and implement a development plan or statement of work for each Co-Development Project
targeted product. Under the co-development agreement, either the Company or AbVacc, whichever party is the primary sponsor of any resulting
product (as defined in the agreement), will be obligated to compensate the other party for certain milestone payments that would range
between $2.1 million and $4.75 million, plus royalties of between 2% to 4%. The term of the agreement is three years from the effective
date, unless previously terminated by either party, in accordance with the agreement.
Proteomedix AG
Condensed Balance Sheets
(unaudited)
| | |
September 30, | | |
December 31, | |
| ASSETS | |
2023 | | |
2022 | |
| Current assets | |
| | |
| |
| Cash and cash equivalents | |
$ | 1,037,425 | | |
$ | 470,156 | |
| Accounts receivable | |
| 116,374 | | |
| 236,683 | |
| Inventory | |
| 83,183 | | |
| 95,810 | |
| Prepaid expenses and other current assets | |
| 7,304 | | |
| 26,280 | |
| Total current assets | |
| 1,244,286 | | |
| 828,929 | |
| | |
| | | |
| | |
| Property and equipment | |
| 39,163 | | |
| 40,130 | |
| Right of use asset | |
| 140,588 | | |
| 202,739 | |
| Total assets | |
$ | 1,424,037 | | |
$ | 1,071,798 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCHOLDERS’ DEFICIT | |
| | | |
| | |
| Current Liabilities | |
| | | |
| | |
| Convertible notes payable | |
$ | 5,704,371 | | |
$ | 4,241,942 | |
| Accrued expenses | |
| 230,329 | | |
| 510,578 | |
| Lease liability, current | |
| 62,464 | | |
| 67,546 | |
| Total current liabilities | |
| 5,997,164 | | |
| 4,820,066 | |
| | |
| | | |
| | |
| Non-current liabilities | |
| | | |
| | |
| Convertible notes payable | |
| - | | |
| 1,406,289 | |
| Note payable | |
| 109,251 | | |
| 108,176 | |
| Pension benefit obligation | |
| 546,259 | | |
| 393,640 | |
| Operating lease liability | |
| 78,124 | | |
| 135,193 | |
| Total liabilities | |
| 6,730,798 | | |
| 6,863,364 | |
| | |
| | | |
| | |
| Stockholders’ deficit | |
| | | |
| | |
| Common stock par value 1 CHF, authorized 466,555 shares, outstanding at September 30, 2023 and December 31, 2022 | |
| 466,555 | | |
| 466,555 | |
| Additional paid-in-capital | |
| 20,539,478 | | |
| 20,377,905 | |
| Accumulated comprehensive income | |
| 610,627 | | |
| 606,583 | |
| Accumulated deficit | |
| (26,923,421 | ) | |
| (27,242,609 | ) |
| Total stockholders’ deficit | |
| (5,306,761 | ) | |
| (5,791,566 | ) |
| | |
| | | |
| | |
| Total liabilities and stockholders’ deficit | |
$ | 1,424,037 | | |
$ | 1,071,798 | |
The
accompanying notes are an integral part of these condensed financial statements.
Proteomedix AG
Condensed Statements of Comprehensive Income (Loss)
For the Nine Months Ended September 30, 2023 and 2022
(unaudited)
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Revenue | |
$ | 2,092,761 | | |
$ | 128,773 | |
| | |
| | | |
| | |
| Cost of goods sold | |
| 22,548 | | |
| 28,176 | |
| | |
| | | |
| | |
| Gross profit | |
| 2,070,213 | | |
| 100,597 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Marketing and business development | |
| 151,478 | | |
| 172,478 | |
| Research and development | |
| 275,020 | | |
| 262,818 | |
| General and administrative expenses | |
| 1,240,875 | | |
| 1,633,860 | |
| Depreciation | |
| 9,293 | | |
| 12,966 | |
| Total operating expenses | |
| 1,676,666 | | |
| 2,082,122 | |
| | |
| | | |
| | |
| Income (loss) from operations | |
| 393,547 | | |
| (1,981,525 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Interest expense | |
| (74,359 | ) | |
| (48,257 | ) |
| | |
| | | |
| | |
| Total other income (expenses) | |
| (74,359 | ) | |
| (48,257 | ) |
| | |
| | | |
| | |
| Net income (loss) before provision for income taxes | |
| 319,188 | | |
| (2,029,782 | ) |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net income (loss) | |
| 319,188 | | |
| (2,029,782 | ) |
| | |
| | | |
| | |
| Other comprehensive income (loss) | |
| | | |
| | |
| | |
| | | |
| | |
| Foreign currency translation adjustment | |
| 172,351 | | |
| 344,957 | |
| Changes in pension benefit obligation | |
| (168,307 | ) | |
| 369,287 | |
| | |
| | | |
| | |
| Total other comprehensive income (loss) | |
| 4,044 | | |
| 714,244 | |
| | |
| | | |
| | |
| Comprehensive income (loss) | |
$ | 323,232 | | |
$ | (1,315,538 | ) |
The
accompanying notes are an integral part of these condensed financial statements.
Proteomedix AG
Condensed Statement of Stockholders’ Deficit
For the Nine Months Ended September 30, 2023 and 2022
(unaudited)
| | |
Common Stock | | |
Additional
Paid In | | |
Accumulated
Comprehensive
(Loss) | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Par Value | | |
Capital | | |
Income | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2021 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 20,000,916 | | |
$ | 431,677 | | |
$ | (25,200,036 | ) | |
$ | (4,300,888 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| FX translation adjustment | |
| - | | |
| - | | |
| - | | |
| 344,957 | | |
| - | | |
| 344,957 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock based compensation | |
| - | | |
| - | | |
| 282,742 | | |
| - | | |
| - | | |
| 282,742 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Changes in pension benefit obligation | |
| - | | |
| - | | |
| - | | |
| 369,287 | | |
| - | | |
| 369,287 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,029,782 | ) | |
| (2,029,782 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at September 30, 2022 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 20,283,658 | | |
$ | 1,145,921 | | |
$ | (27,229,818 | ) | |
$ | (5,333,684 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2022 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 20,377,905 | | |
$ | 606,583 | | |
$ | (27,242,609 | ) | |
$ | (5,791,566 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| FX translation adjustment | |
| - | | |
| - | | |
| - | | |
| 172,351 | | |
| - | | |
| 172,351 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock based compensation | |
| - | | |
| - | | |
| 161,573 | | |
| - | | |
| - | | |
| 161,573 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Changes in pension benefit obligation | |
| - | | |
| - | | |
| - | | |
| (168,307 | ) | |
| - | | |
| (168,307 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net income | |
| - | | |
| - | | |
| - | | |
| - | | |
| 319,188 | | |
| 319,188 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at September 30, 2023 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 20,539,478 | | |
$ | 610,627 | | |
$ | (26,923,421 | ) | |
$ | (5,306,761 | ) |
The accompanying notes are
an integral part of these condensed financial statements.
Proteomedix AG
Condensed Statements of Cash Flows
For the Nine Months Ended September 30, 2023 and 2022
(unaudited)
| | |
2023 | | |
2022 | |
| Operating activities | |
| | |
| |
| Net income (loss) | |
$ | 319,188 | | |
$ | (2,029,782 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 9,293 | | |
| 12,966 | |
| Stock based compensation | |
| 161,573 | | |
| 282,742 | |
| Changes in pension benefit obligation | |
| (15,688 | ) | |
| 47,042 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 120,309 | | |
| 46,462 | |
| Inventory | |
| 12,627 | | |
| 10,177 | |
| Prepaid expenses and other current assets | |
| 18,976 | | |
| 63,107 | |
| Accrued expenses | |
| (280,249 | ) | |
| 89,382 | |
| Cash (used in) provided by operating activities | |
| 346,029 | | |
| (1,477,904 | ) |
| | |
| | | |
| | |
| Investing activities: | |
| | | |
| | |
| | |
| - | | |
| - | |
| Cash used in investing activities | |
| - | | |
| - | |
| | |
| | | |
| | |
| Financing activities: | |
| | | |
| | |
| Repayment of notes payable | |
| - | | |
| (50,000 | ) |
| Cash used in financing activities | |
| - | | |
| (50,000 | ) |
| | |
| | | |
| | |
| FX effect on cash | |
| 221,240 | | |
| (91,064 | ) |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| 567,269 | | |
| (1,618,968 | ) |
| | |
| | | |
| | |
| Cash and cash equivalents - beginning of the year | |
| 470,156 | | |
| 2,546,801 | |
| | |
| | | |
| | |
| Cash and cash equivalents - end of year | |
$ | 1,037,425 | | |
$ | 927,833 | |
| | |
| | | |
| | |
| Supplemental cash flow disclosures | |
| | | |
| | |
| Interest paid | |
$ | - | | |
$ | 1,965 | |
| Income taxes paid | |
$ | - | | |
$ | - | |
The accompanying notes are
an integral part of these financial statements.
Proteomedix AG
Notes to Condensed Financial Statements
Note 1 – Organization and Nature of Business
Proteomedix AG (the “Company”) is
a healthcare company whose mission is to transform prostate cancer diagnosis. Proteomedix has identified novel biomarker signatures with
utility in prostate cancer diagnosis, prognosis and therapy management. The lead product Proclarix® is a blood-based
prostate cancer test panel and risk score currently available in Europe and expected to be available in the U.S. in the near future. Proteomedix
is located in the Bio-Technopark of Zurich-Schlieren, Switzerland.
On December 15, 2023, the Company was acquired
by Onconetix, Inc. (formerly Blue Water Biotech, Inc) (the “Parent”). The Parent issued stock of its common stock in exchange
for 100% of the outstanding voting equity of the Company. See Note 10.
Note 2 – Going Concern
The accompanying condensed financial statements
have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets
and satisfaction of liabilities in the normal course of business. For the nine months ended September 30, 2023, the Company had an accumulated
deficit of approximately $27,000,000 and a working capital deficit of approximately $4,800,000, and a lack of profitable operational history.
These matters, among others, raise substantial doubt about the Company’s ability to continue as a going concern.
While the Company is attempting to generate greater
revenues, the Company’s cash position may not be significant enough to support the Company’s daily operations. Management
intends to raise additional funds from its Parent to sustain operations until such time as revenues are sufficient to support the Company’s
operations. Management believes that the actions presently being taken to further implement its business plan and generate revenues provide
the opportunity for the Company to continue as a going concern. While the Company believes in the viability of its strategy to generate
revenues and the ability of its Parent to provide additional funds, there can be no assurances to that effect. The ability of the Company
to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and obtain additional
funding from its Parent as needed.
Note 3 – Summary of Significant Accounting
Policies
Basis of Presentation
The accompanying condensed financial statements
of the Company have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S.
GAAP”) and interim reporting rules of the Securities and Exchange Commission (“SEC”) and should be read in conjunction
with the audited financial statements for the years ended December 31, 2022 and 2021, and notes thereto. In the opinion of management,
all adjustments, consisting of normal recurring adjustments (unless otherwise indicated), necessary for a fair presentation of the financial
position and the results of operations for the interim periods presented have been reflected herein. The results of operations for interim
periods are not necessarily indicative of the results to be expected for the full year.
The functional currency of the Company is the
Swiss Franc and the Company’s condensed financial statements are presented in United States Dollars (USD). Transactions denominated
in foreign currencies are translated into the functional currency at the exchange rates prevailing at the date of the transaction. The
resulting translation adjustments are recorded as a separate component of accumulated other comprehensive income (loss).
Segment
Information
Operating
segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief
operating decision maker (“CODM”), or decision-making group, in deciding how to allocate resources and in assessing performance.
The Company operates in one segment which is consistent with the financial information regularly reviewed by the CODM for purposes of
evaluating performance, allocating resources, setting incentive compensation targets, and planning and forecasting for future periods.
Proteomedix AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
Use of Estimates
The preparation
of condensed financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the condensed financial
statements and the reported amounts of expenses during the reporting periods. The most significant estimates in the Company’s condensed
financial statements relate to valuation of inventory, stock-based compensation, pension benefit obligations, and the valuation allowance
of deferred tax assets resulting from net operating losses. These estimates and assumptions are based on current facts, historical experience
and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments
about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual
results may differ materially and adversely from these estimates. To the extent there are material differences between the estimates and
actual results, the Company’s future results of operations will be affected.
Cash and Cash Equivalents
For purposes
of reporting cash flows, the Company has defined cash and cash equivalents as all cash in banks and highly liquid investments available
for current use with an initial maturity of three months or less to be cash equivalents. The Company had no cash equivalents as of September
30, 2023 and December 31, 2022.
The Company
maintains its cash balances at financial institutions that are insured by Swiss Financial Market Supervisory Authority (“FINMA”).
The Company’s cash balances may at times exceed the insurance provided by FINMA. The Company has not experienced any losses on these
accounts and management does not believe that the Company is exposed to any significant risks related to excess deposits.
Accounts Receivable
The Company
performs periodic credit evaluations of its customers’ financial condition and extends credit to virtually all of its customers
on an uncollateralized basis. Credit losses to date have been insignificant and within management’s expectations. The Company provides
an allowance for doubtful accounts that is based upon a review of outstanding receivables, historical collection information, expected
future losses, and existing economic conditions. Normal accounts receivable are due 30 days after the issuance of the invoice. Receivables
are considered delinquent based on management’s assessment of the individual balance. Delinquent receivables are evaluated for collectability
based on individual credit evaluation and specific circumstances of the customer. As of September 30, 2023, and December 31, 2022, the
Company’s allowance for doubtful accounts was nil, respectively. The Company did not write off any accounts receivable against the
allowance for doubtful accounts during the periods ended September 30, 2023, and 2022. As of September 30, 2023 and December 31, 2022,
substantially all accounts receivable are due from a single customer.
Inventories
Inventories consist of raw materials and finished
goods. Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company
periodically reviews the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise non-saleable items taking
into account anticipated future sales compared with quantities on hand, and the remaining shelf life of goods on hand. If non-saleable
items are observed and there are no alternate uses for the inventory, the Company records a write-down to net realizable value in the
period that the decline in value is first recognized. The Company had no inventory reserves as of September 30, 2023, and December 31,
2022.
Proteomedix AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
Impairment
of Long-Lived Assets
The Company
reviews long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the
assets may not be fully recoverable (a “triggering event”). Factors that the Company considers in deciding when to perform
an impairment review include significant underperformance of the long-lived asset in relation to expectations, significant negative industry
or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate
a long-lived asset for recoverability, the Company compares forecasts of undiscounted cash flows expected to result from the use and eventual
disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated undiscounted future cash
flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would be based on the excess
of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows. During the periods ended September
30, 2023, and 2022, the Company did not identify any impairments related to its long-lived assets.
Property and Equipment
Property and equipment consists of computers and
office furniture and fixtures, all of which are recorded at cost. Depreciation is recorded using the straight-line method over the respective
useful lives of the assets ranging from two to ten years. Long-lived assets are reviewed for impairment whenever events or circumstances
indicate that the carrying amount of these assets may not be recoverable. Upon the retirement or other disposition of property and equipment,
the related cost and accumulated depreciation are charged to operations.
Research and Development Costs
Research and development expenses are those costs
incurred in the discovery, design, and development of new products, processes, or services, as well as the enhancement of existing products.
Research and development costs are expensed as incurred unless such costs have an alternative future use. These costs include, but are
not limited to, salaries, wages, benefits, materials, equipment, and overhead directly attributable to the research and development activities.
Collaborative Agreements
The Company periodically enters into strategic
alliance agreements with counterparties to produce products and/or provide services to customers. Alliances created by such agreements
are not legal entities, have no employees, no assets and have no true operations. These arrangements create contractual rights and the
Company accounts for these alliances as a collaborative arrangement by reporting costs incurred and reimbursements received from transactions
within research and development expense within the statements of comprehensive loss.
Commitments And
Contingencies
Liabilities
for loss contingencies arising from claims, assessments, litigation, fines, penalties and other sources are recorded when management assesses
that it is probable that a liability has been incurred and the amount can be reasonably estimated.
Share Based Compensation
The Company
accounts for equity instruments issued in exchange for the receipt of goods or services from other than employees in accordance with Financial
Accounting Standard Board (“FASB”) Account Standard Codification (“ASC”) 718, “Compensation – Stock
Compensation”. Costs are measured at the estimated fair value of the consideration received or the estimated fair value of the equity
instruments issued, whichever is more reliably measurable. The value of equity instruments issued for consideration other than employee
services is determined on the earliest of a performance commitment or completion of performance by the provider of goods or services as
defined by ASC 718.
Proteomedix AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
Income Taxes
In accordance
with ASC 740, “Income Taxes,” the Company provides for the recognition of deferred tax assets if realization of such assets
is more likely than not. Deferred income tax assets and liabilities are computed for differences between the financial statement and tax
basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable
to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to
reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus
or minus the change during the period in deferred tax assets and liabilities.
In addition,
the Company’s management performs an evaluation of all uncertain income tax positions taken or expected to be taken in the course
of preparing the Company’s income tax returns to determine whether the income tax positions meet a “more likely than not”
standard of being sustained under examination by the applicable taxing authorities. This evaluation is required to be performed for all
open tax years, as defined by the various statutes of limitations, for federal and state purposes. If the Company has interest or penalties
associated with insufficient taxes paid, such expenses are reported in income tax expense.
Revenue Recognition
The Company recognized revenue when control of
goods or services performed is transferred to customers, in an amount that reflects the consideration we expect to be entitled to in exchange
for those services. ASC 606 provides for a five-step model that includes:
| (i) | identifying the contract with a customer, |
| (ii) | identifying the performance obligations in the contract, |
| (iii) | determining the transaction price, |
| (iv) | allocating the transaction price to the performance obligations,
and |
| (v) | recognizing revenue when, or as, an entity satisfies a performance
obligation. |
Product
Sales
The Company derives revenue through sales of its
products directly to end users and to distributors. The Company sells its products to customers including laboratories, hospitals, medical
centers, doctors and distributors. The Company considers customer purchase orders, which in some cases are governed by master sales agreements
or standard terms and conditions, to be the contracts with a customer. For each contract, the Company considers the promise to transfer
products, each of which are distinct, to be the identified performance obligations. In determining the transaction price the Company evaluates
whether the price is subject to refund or adjustment to determine the net consideration to which it expects to be entitled. The Company
fulfils its performance obligation applicable to product sales once the product is transferred to the customer.
Development
Services
The Company
provides a range of services to life sciences customers referred to as “Development Services” including testing for biomarker
discovery, assay design and development. These Development Services are performed under individual statement of work (“SOW”) arrangements
with specific deliverables defined by the customer. Development Services are generally performed on a time and materials basis. During
the performance and through completion of the service to the customer in accordance with the SOW, we have the right to bill the customer
for the agreed upon price and we recognize the Development Services revenue over the period estimated to complete the SOW. We generally
identify each SOW as a single performance obligation.
Completion
of the service and satisfaction of the performance obligation under a SOW is typically evidenced by access to the data or test made available
to the customer or any other form or applicable manner of delivery defined in the SOW. However, for certain SOWs under which work is performed
pursuant to the customer’s highly customized specifications, we have the enforceable right to bill the customer for work completed,
rather than upon completion of the SOW. For those SOWs, we recognize revenue over a period of time during which the work is performed
based on the expended efforts (inputs). As the performance obligation under the SOW is satisfied, any amounts earned as revenue and billed
to the customer are included in accounts receivable. Any revenues earned but not yet billed to the customer as of the date of the condensed
financial statements are recorded as contract assets and are included in prepaids and other current assets as of the condensed financial
statement date. Amounts recorded in contract assets are reclassified to accounts receivable in our financial statements when the customer
is invoiced according to the billing schedule in the contract.
Proteomedix AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
In circumstances where a SOW includes variable
consideration component, the Company estimates the amount of variable consideration that should be included in the transaction price utilizing
either the expected value method or the most likely amount method, depending on which method is expected to better predict the amount
of consideration to which the Company will be entitled. The value of variable consideration is included in the transaction price if, and
to the extent, it is probable that a significant reversal of the amount of cumulative revenue recognized will not occur when the uncertainty
associated with the variable consideration is subsequently resolved. These estimates are reassessed each reporting period, as required,
and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
Licensing
Revenues
license revenues are determined based on an assessment
of whether the license is distinct from any other performance obligations that may be included in the underlying licensing arrangement.
If the customer is able to benefit from the license without provision of any other performance obligations by the Company and the license
is thereby viewed as a distinct or functional license, the Company then determines whether the customer has acquired a right to use the
license or a right to access the license. For functional licenses that do not require further substantive development or other ongoing
activities by the Company, the customer is viewed as acquiring the right to use the license as, and when, transferred and revenues are
generally recorded at a point in time. For symbolic licenses providing substantial value only in conjunction with other performance obligations
to be provided by the Company, revenues are generally recorded over the term of the license agreement using the inputs based on contractual
remaining time for such license. Such other obligations provided by the Company generally include manufactured products, additional development
services or other deliverables that are contracted to be provided during the license term.
Royalties associated with licensing arrangements
are estimated and recognized when sales under supply agreements with commercial licensees are recorded, absent any contractual constraints
or collectability uncertainties. Royalties which are contingent on meeting certain sales milestones are recorded when it has become probable
that milestones will be met.
The following
table disaggregates the Company’s revenues by type for the periods ended September 30, 2023 and 2022.
| | |
Recognition
Method | |
2023 | | |
2022 | |
| Product sales | |
Point in time | |
$ | 40,237 | | |
$ | 74,390 | |
| Licensing revenues | |
Point in time | |
| 516,359 | | |
| - | |
| Development services | |
Over time | |
| 1,536,165 | | |
| 54,383 | |
| | |
| |
$ | 2,092,761 | | |
$ | 128,773 | |
Financial Instruments
The Company’s
financial instruments include cash and cash equivalents, accounts receivable and accounts payable, and are accounted for under the provisions
of ASC Topic 825, “Financial Instruments”. The carrying amount of these financial instruments, as reflected in the condensed
financial statements approximates fair value.
Fair Value Measurement
ASC Topic
820, “Fair Value Measurement”, requires that certain financial instruments be recognized at their fair values at our balance
sheet dates. However, other financial instruments, such as debt obligations, are not required to be recognized at their fair values, but U.S.
GAAP provides an option to elect fair value accounting for these instruments. U.S. GAAP requires the disclosure of the fair values of
all financial instruments, regardless of whether they are recognized at their fair values or carrying amounts in our balance sheets. For
financial instruments recognized at fair value, U.S. GAAP requires the disclosure of their fair values by type of instrument, along with
other information, including changes in the fair values of certain financial instruments recognized in income or other comprehensive income.
For financial instruments not recognized at fair value, the disclosure of their fair values is provided below under “Financial Instruments.”
Nonfinancial
assets, such as property and equipment, and nonfinancial liabilities are recognized at their carrying amounts in the Company’s balance
sheets. U.S. GAAP does not permit nonfinancial assets and liabilities to be remeasured at their fair values. However, U.S. GAAP requires
the remeasurement of such assets and liabilities to their fair values upon the occurrence of certain events, such as the impairment of
property, plant and equipment. In addition, if such an event occurs, U.S. GAAP requires the disclosure of the fair value of the asset
or liability along with other information, including the gain or loss recognized in income in the period the remeasurement occurred.
Proteomedix
AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
The Company
did not have any assets or liabilities at September 30, 2023 and December 31, 2022 which required remeasurement at the respective reporting
periods.
Convertible Instruments
The Company
evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging
Activities”.
U.S. GAAP
requires companies to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial
instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the
embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b)
the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under
other GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded
derivative instrument would be considered a derivative instrument.
The Company
accounts for convertible instruments as follows: Debt discounts under these arrangements are amortized over the term of the related debt
to their stated date of redemption. Proceeds from these convertible notes are reported under the financing section of the statements of
cash flows. Changes to the fair value of the derivative liability are reported as adjustments to reconcile net loss to net cash used in
operating activities in the accompanying statement of cash flows. During the nine months ended September 30, 2023 the Company did not
have any conversion options which required bifurcation from the host instrument.
Defined
Benefit Pension Plan
The Company sponsors
a defined benefit pension plan (the “Plan”) covering eligible employees. The Plan provides retirement benefits based on employees’
years of service and compensation levels. The Company recognizes an asset for such plan’s overfunded status or a liability underfunded
status in its balance sheets. Additionally, the Company measures its plan’s assets and obligations that determine its funded status
as of the end of the year and recognizes the changes in the funded status in the year in which the changes occur. Those changes are reported
in ‘accumulated other comprehensive loss. The Company uses actuarial valuations to determine its pension and postretirement benefit
costs and credits. The amounts calculated depend on a variety of key assumptions, including discount rates and expected return on plan
assets. Current market conditions are considered in selecting these assumptions.
The Company’s pension plans are generally
valued using the net asset value (NAV) per share as a practical expedient for fair value provided certain criteria are met. The NAVs are
determined based on the fair values of the underlying investments in the funds. In circumstances where the criteria are not met, fair
is determined based on the underlying market in which the funds are traded which is generally considered to be an active market.
Recently Issued
Accounting Standards
During the
period ended September 30, 2023, and subsequently, there were several new accounting pronouncements issued by the FASB. Each of these
pronouncements, as applicable, has been or will be adopted by the Company. Management does not believe the adoption of any of these accounting
pronouncements has had or will have a material impact on the Company’s condensed financial statements.
Proteomedix AG
Notes to Condensed Financial Statements
Note 3 – Summary of Significant Accounting
Policies (cont.)
In June
2016, the FASB issued ASU No. 2016-13, “Financial Instruments — Credit Losses (Topic 326): Measurement of Credit Losses on
Financial Instruments,” which requires the Company to measure and recognize expected credit losses for financial assets held and
not accounted for at fair value through net income. In November 2018, April 2019 and May 2019, the FASB issued ASU No. 2018-19, “Codification
Improvements to Topic 326, Financial Instruments — Credit Losses,” “ASU No. 2019-04, Codification Improvements to Topic
326, Financial Instruments — Credit Losses,” “Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments,”
and “ASU No. 2019-05, Financial Instruments — Credit Losses (Topic 326): Targeted Transition Relief,” which provided
additional implementation guidance on the previously issued ASU. The ASU is effective for fiscal years beginning after Dec. 15, 2019 for
public business entities that meet the definition of an SEC filer, excluding entities eligible to be SRCs as defined by the SEC. All other
entities, ASU No. 2016-13 is effective for fiscal years beginning after December 15, 2022. The adoption of this guidance did not have
a material impact on the Company’s condensed financial statements.
Subsequent Events
The Company
has evaluated all transactions through the date the condensed financial statements were issued for subsequent event disclosure consideration.
See Note 10.
Note 4 – Debt
On March 3, 2010, the Company received a loan
from Venture Kick in the amount of 100,000 CHF. This loan bears no interest, is unsecured and may be cancelled by the Company at its discretion.
This loan is subordinated to the Company’s other unsubordinated debt. The loan was to be used solely for business development and
the Company may, at its sole discretion, contribute funds back to Venture Kick to enable that organization to continue its efforts. As
of September 30, 2023 and December 31, 2022 the balance outstanding was approximately $109,000 and $108,000, respectively.
On June 23, 2020, Company entered a convertible
note payable with a financial institution and shareholder of the Company for CHF 550,000 with an interest rate of 0.50% per annum and
a maturity of September 30, 2024. The note provides the holder with an optional conversion feature in the event of an equity financing
of the Company. The conversion price in the event of an equity financing is at a 20% discount the share price from the financing. The
holder is also entitled to convert the note upon the occurrence of a sale of the Company or at maturity of the note in both cases without
a discount. This note was unsubordinated until January 10, 2023, at which point it was also subordinated to all other unsubordinated debts.
The interest rate was changed to 2.50% as of May 1, 2023. As of September 30, 2023 and December 31, 2022, the outstanding balance on this
note was approximately $601,000 and $541,000, respectively.
On June 23, 2020, Company entered into a series
of convertible notes payable with certain shareholders of the Company for CHF 800,000 with an interest rate of 0.50% per annum and a maturity
of September 30, 2024. The note provides the holder with an optional conversion feature in the event of an equity financing greater than
CHF 1,000,000. The conversion price in the event of an equity financing is at a 20% discount the share price from the financing. The holder
is also entitled to convert the note upon the occurrence of a sale of the Company or at maturity of the note in both cases without a discount.
These notes payable are subordinated to the Company’s other unsubordinated debt. As of September 30, 2023 and December 31, 2022,
the outstanding balance on these notes was approximately $874,000 and $865,000, respectively.
On October 26, 2020, Company entered into a series
of convertible notes payable with certain members of the board of directors (Note 8) in the total amount of CHF 161,250 with an interest
rate of 0.25% and a maturity of December 31, 2023. The note provides the holder with an optional conversion feature at a discount of 20%
in the event of an equity financing greater than CHF 1,000,000. The holder is also entitled to convert the note upon the occurrence of
a sale of the Company or at maturity of the note in both cases without a discount. These notes payable are subordinated to the Company’s
other unsubordinated debt. As of September 30, 2023 and December 31, 2022, the outstanding balance on these notes was approximately $177,000
and $174,000, respectively.
Proteomedix AG
Notes to Condensed Financial Statements
Note 4 – Debt (cont.)
On November 23, 2020, Company entered into a series
of convertible notes payable with certain shareholders of the Company in the total amount of CHF 760,080 with an interest rate of 5% and
a maturity of December 31, 2023. The note provides the holder with an optional conversion feature at a discount of 30% in the event of
an equity financing greater than CHF 1,000,000. The holder is also entitled to convert the note upon the occurrence of a sale of the Company
or at maturity of the note in both cases without a discount. These notes payable are subordinated to the Company’s other unsubordinated
debt. As of September 30, 2023 and December 31, 2022, the outstanding balance on these notes was approximately $831,000 and $822,000,
respectively.
On July 19, 2021, Company entered into a convertible
note payable in the total amount of CHF 3,000,000 with an interest rate of 0.5% and an original maturity of September 30, 2023 which was
extended to September 30, 2024. The note provides the holder with a mandatory conversion requirement in the event of an equity financing
greater than CHF 1,000,000. The note is also mandatorily converted in the event certain milestones are achieved related to an R&D
collaboration project entered separately none of which have been met as of December 31, 2022. The holder is also entitled to convert the
note upon the occurrence of a sale of the Company or at maturity of the note in both cases without a discount. These notes payable are
subordinated to the Company’s other unsubordinated debt. As of September 30, 2023 and December 31, 2022, the outstanding balance
on this note was approximately $3,278,000 and $3,245,000, respectively.
The Company did not issue any new notes during
the nine months ended September 30, 2023, all the changes in the above balances are solely due to changes exchange rates between USD and
CHF. All outstanding convertible notes as of December 31, 2022 were converted upon the closing of the acquisition of the Company by the
Parent. See Note 10.
Note 5 – Commitments and Contingencies
Leases
The Company leases it’s primary office and
lab space at a rate of 5,077 CHF per month. The lease began on February 1, 2012 with an initial period ending on January 31, 2015. This
rental agreement can be terminated at the end of March, June and September of a given year with a notice of 12 months. If the Company
wishes to terminate the lease without adhering to the agreed dates, it is liable for the rent and the other tenant obligations until the
rental is continued, but the latest until the next contractual termination date. If the rental agreement is not terminated in writing
by either party after the fixed contract period has expired, while observing the notice period, it will be extended by two years. As of
September 30, 2023 the remaining period of the lease is approximately 21 months.
The rent expense for the periods ended September
30, 2023 and 2022 was $57,582 and $54,653 respectively and was included in ‘general and administrative’ expenses in the accompanying
statements of comprehensive loss. The Company paid $57,582 and $54,653 respectively, in lease payments during the periods ended September
30, 2023 and 2022, and are included in the Company’s operating cash flows for both periods. The change in lease expense and lease
cash payments. The change in lease expense from period to period is due to changes in exchange rate between USD and CHF as the Company’s
minimum monthly lease payments are fixed for the term of the lease.
Switzerland social security obligations
The Company issued certain stock options during periods prior to December
31, 2022. If the recipients exercise these stock options it may result in the recognition of additional social security tax due to the
Switzerland taxing authority. Management assessed the likelihood of this liability having been incurred as of December 31, 2022 and 2021
in accordance with ASC 450, Contingencies, and determined the likelihood was reasonably possible. Accordingly, no accrual for this
contingent obligation has been recognized in the accompanying condensed financial statements. Additionally, management is unable to estimate
an amount or range of amounts related to any amount that may be owed should a recipient exercise a stock option.
Proteomedix AG
Notes to Condensed Financial Statements
Note 6 – Stockholders’ Deficit
Share Capital
The Company has several series of common stock
providing the following provisions. In the event of a bankruptcy or liquidation or winding up of the Company, the holders of Series B3
Common Stock will be entitled to receive, in advance of the holders of Series B2 Common Stock, Series B Common Stock and Series A Stock
and Ordinary Stock, CHF 65 for each Series B3 Common Share they own.
Thereafter, the holders of Series B2 Common Stock
will be entitled to receive, in advance of the holders of Series B Common Stock and Series A Stock and Ordinary Stock, CHF 60 for each
Series B2 Common Share they own.
Thereafter, the holders of Series B Common Stock
will be entitled to receive, in advance of the holders of Series A Common Stock and Ordinary Stock, CHF 50 for each Series B Common Share
they own.
Thereafter, the holders of Series A Common Stock
will be entitled to receive, in advance of the holders of Ordinary Stock, CHF 40 for each Series A Common Share they own.
Thereafter, the other Ordinary Shareholders will
be entitled to receive CHF 40 per Ordinary Share they own and then any remaining assets or proceeds will be distributed pro rata among
all Shareholders.
If there are insufficient assets or proceeds to
pay such amount to the holders of Series B3 Common Stock, the amount available will be paid on a pro rata basis between the holders of
the Series B3 Common Stock.
If, after the full payment of Series B3 Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series B2 Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series B2 Common Stock.
If, after the full payment of Series B2 Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series B Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series B Common Stock.
If, after the full payment of Series B Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series A Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series A Common Stock.
The Company and all Shareholders shall use best
efforts to ensure that any sale, liquidation, disposal of material assets or the entire Company shall be effectuated so as to be tax efficient,
particularly as regards any applicable withholding tax, and fair with regard to the Shareholders.
If in later financing rounds additional preference
rights are granted, then the holders of Series A Common Stock, Series B Common Stock and Series B2 Common Stock shall receive mutatis
mutandis behind the new stock the same rights (taking into account the respective price).”
The Series B3 Common Stock shall have the same
rights and obligations under the Shareholder’s Agreement and the Organizational Rules as the Series B Common Stock and the Series
B2 Common Stock, and thus have the same legal status as the Series B Common Stock and the Series B2 Common Stock.
Proteomedix AG
Notes to Condensed Financial Statements
Note 6 – Stockholders’ Deficit
(cont.)
As of September 30, 2023 and December 31, 2022
the following number of stock for each series was outstanding:
| Share Class | |
Stock | |
| Ordinary | |
| 100,000 | |
| Series A | |
| 65,000 | |
| Series B | |
| 84,200 | |
| Series B2 | |
| 83,334 | |
| Series B3 | |
| 80,038 | |
| Total Outstanding stock | |
| 412,572 | |
Stock options
The Company has granted various stock options
primarily to employees as incentive-based compensation. During the nine months ended September 30, 2023 and 2022, the Company granted
5,307 and -0-, respectively, stock options and recognized $161,573 and $282,742, respectively, in expense related to the vesting of outstanding
stock option grants.
Accumulated other comprehensive loss
The following tables details the amounts reclassified
from other comprehensive loss and the related affected line items within the accompanying statements of comprehensive loss for the periods
ended September 30, 2022 and 2021.
| | |
2023 | | |
2022 | | |
Financial statement |
| Item description | |
Amount | | |
Amount | | |
line item |
| | |
| | |
| | |
|
| Amortization of gains (losses) | |
$ | (24,876 | ) | |
$ | (4,743 | ) | |
General and administrative |
| | |
| | | |
| | | |
|
| | |
$ | (24,876 | ) | |
$ | (4,743 | ) | |
|
The table below details the components and the
Company’s accumulated other comprehensive loss for the periods ended September 30, 2023 and 2022.
| | |
Defined | | |
| | |
| |
| | |
Benefit | | |
Foreign | | |
| |
| | |
Pension | | |
Currency | | |
| |
| | |
Items | | |
Items | | |
Total | |
| Balance as of December 31, 2021 | |
$ | 397,709 | | |
$ | 33,968 | | |
$ | 431,677 | |
| | |
| | | |
| | | |
| | |
| Other comprehensive income before reclassifications | |
| 374,030 | | |
| 344,957 | | |
| 718,987 | |
| | |
| | | |
| | | |
| | |
| Amounts reclassified from accumulated other comprehensive income (loss) | |
| (4,743 | ) | |
| - | | |
| (4,743 | ) |
| | |
| | | |
| | | |
| | |
| Net current period other comprehensive income | |
| 369,287 | | |
| 344,957 | | |
| 714,244 | |
| | |
| | | |
| | | |
| | |
| Balance as of September 30, 2022 | |
$ | 766,996 | | |
$ | 378,925 | | |
$ | 1,145,921 | |
Proteomedix AG
Notes to Condensed Financial Statements
Note 6 – Stockholders’ Deficit
(cont.)
| Balance as of December 31, 2022 | |
$ | 577,601 | | |
$ | 28,982 | | |
$ | 606,583 | |
| | |
| | | |
| | | |
| | |
| Other comprehensive income (loss) before reclassifications | |
| (143,431 | ) | |
| 172,351 | | |
| 28,920 | |
| | |
| | | |
| | | |
| | |
| Amounts reclassified from accumulated other comprehensive income (loss) | |
| (24,876 | ) | |
| - | | |
| (24,876 | ) |
| | |
| | | |
| | | |
| | |
| Net current period other comprehensive income (loss) | |
| (168,307 | ) | |
| 172,351 | | |
| 4,044 | |
| | |
| | | |
| | | |
| | |
| Balance as of September 30, 2023 | |
$ | 409,294 | | |
$ | 201,333 | | |
$ | 610,627 | |
Note 7 – Defined Benefit Pension Plan
The Company sponsors a defined benefit pension
plan covering certain eligible employees. The plan provides retirement benefits based on years of service and compensation levels.
The value of the pension obligation is determined
using the Projected Unit Credit (PUC) method. This method sees each period of service as giving rise to an additional unit of benefit
entitlements/employee benefits. The value of the Company’s employee benefit obligations for active employees, or the Projected Benefit
Obligation (PBO), on the reporting date is the same as the present value of the degree of entitlement existing on this date, in terms
of future salary and pension increases and turnover rates. The valuation of pension obligations of pensioners is made on the basis of
the present value of current pensions taking into account future increases in pensions. The service costs (SC) are calculated using the
present value of the entitlements to employee benefits earned during the year for which calculations are made.
The following significant actuarial assumptions
were used in calculating the benefit obligation and the net periodic benefit cost as of September 30, 2023 and December 31, 2022:
| | |
2023 | | |
2022 | |
| Discount rate | |
| 1.90 | % | |
| 2.30 | % |
| Expected long-term rate of return on plan assets | |
| 1.20 | % | |
| 2.30 | % |
| Rate of compensation increase | |
| 3.00 | % | |
| 3.00 | % |
Changes in these assumptions may have a material
impact on the plan’s obligations and costs.
The components of net periodic benefit cost for
the periods ended September 30, 2023 and 2022 are as follows:
| | |
2023 | | |
2022 | |
| Service cost | |
$ | 69,358 | | |
$ | 118,310 | |
| Interest cost | |
| 31,506 | | |
| 8,080 | |
| Expected return on plan assets | |
| (25,640 | ) | |
| (6,166 | ) |
| Amortization of net (gain)/loss | |
| (24,876 | ) | |
| (4,743 | ) |
| Total | |
$ | 50,348 | | |
$ | 115,481 | |
Proteomedix AG
Notes to Condensed Financial Statements
Note 8 – Related Parties
As of September 30, 2023 and December 31, 2022,
the Company has outstanding convertibles notes of approximately $2,422,000 and $2,422,000, respectively, due to certain shareholders and
directors.
During the periods ended September 30, 2023 and
2022, the Company paid approximately $127,500 and $183,400 to entities owned by members of the board of directors and executive management
for professional services. These amounts are included within ‘general and administrative’ expenses in the accompanying statements
of comprehensive loss.
Note 9 – Subsequent Events
On December 15, 2023,
the Parent and the Company entered into a Share Exchange Agreement which resulted in the Company becoming a wholly owned subsidiary of
the Parent. The consummation of the Share Exchange was subject to customary closing conditions
and closed on December 15, 2023.
Concurrently with the
closing of the Share Exchange Agreement, all outstanding convertibles notes as of December 31, 2022 were converted into 83,114 common
stock of the Company and were then purchased by the Parent.
Proteomedix AG
Financial Statements
and
Independent Auditors’ Report
For the Years Ended December 31, 2022 and 2021
TABLE OF CONTENTS
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Proteomedix AG
Schlieren, Zurich
Switzerland
Opinion on the Financial
Statements
We have audited the accompanying
balance sheets of Proteomedix AG (the “Company”) as of December 31, 2022 and 2021, the related statements of loss and comprehensive
loss, stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2022, and the related notes
(collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all
material respects, the financial position of the Company at December 31, 2022 and 2021, and the results of its operations and its cash
flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted
in the United States of America.
Going Concern Uncertainty
The accompanying financial statements
have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the
Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability
to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements
do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are
the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements
based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the
standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require
that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement,
whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over
financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but
not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly,
we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond
to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements.
Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating
the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
| Zurich, Switzerland, February 14, 2024 |
|
|
| |
|
|
| BDO AG |
|
|
| |
|
|
| /s/ Christoph Tschumi |
|
/s/ Marc Furlato |
| |
|
|
| We have served as the Company’s auditor since 2023. |
|
Proteomedix AG
Balance Sheets
As of December 31, 2022 and 2021
| ASSETS | |
2022 | | |
2021 | |
| Current assets | |
| | |
| |
| Cash and cash equivalents | |
$ | 470,156 | | |
$ | 2,546,801 | |
| Accounts receivable | |
| 236,683 | | |
| 96,211 | |
| Inventory | |
| 95,810 | | |
| 110,584 | |
| Prepaid expenses and other current assets | |
| 26,280 | | |
| 85,632 | |
| Total current assets | |
| 828,929 | | |
| 2,839,228 | |
| | |
| | | |
| | |
| Property and equipment | |
| 40,130 | | |
| 54,003 | |
| Right of use asset | |
| 202,739 | | |
| - | |
| Total assets | |
$ | 1,071,798 | | |
$ | 2,893,231 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCHOLDERS’ DEFICIT | |
| | | |
| | |
| Current Liabilities | |
| | | |
| | |
| Convertible notes payable | |
$ | 4,241,942 | | |
$ | - | |
| Accrued expenses | |
| 510,578 | | |
| 504,766 | |
| Operating lease liability, current | |
| 67,546 | | |
| - | |
| Total current liabilities | |
| 4,820,066 | | |
| 504,766 | |
| | |
| | | |
| | |
| Non-current liabilities | |
| | | |
| | |
| Convertible notes payable | |
| 1,406,289 | | |
| 5,726,368 | |
| Note payable | |
| 108,176 | | |
| 164,509 | |
| Pension benefit obligation | |
| 393,640 | | |
| 798,476 | |
| Operating lease liability | |
| 135,193 | | |
| - | |
| Total liabilities | |
| 6,863,364 | | |
| 7,194,119 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 5) | |
| | | |
| | |
| | |
| | | |
| | |
| Stockholders’ deficit | |
| | | |
| | |
| Common stock par value 1 CHF, authorized 590,951 shares, outstanding 412,572 and 412,572 as of December 31, 2022 and 2021, respectively | |
| 466,555 | | |
| 466,555 | |
| Additional paid-in-capital | |
| 20,377,905 | | |
| 20,000,916 | |
| Accumulated comprehensive (loss) income | |
| 606,583 | | |
| 431,677 | |
| Accumulated deficit | |
| (27,242,609 | ) | |
| (25,200,036 | ) |
| Total stockholders’ deficit | |
| (5,791,566 | ) | |
| (4,300,888 | ) |
| Total liabilities and stockholders’ deficit | |
$ | 1,071,798 | | |
$ | 2,893,231 | |
The accompanying notes are an integral part of
these financial statements.
Proteomedix AG
Statements of Comprehensive Loss
For the years ended December 31, 2022 and
2021
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenue | |
$ | 392,460 | | |
$ | 140,600 | |
| | |
| | | |
| | |
| Cost of goods sold | |
| 48,429 | | |
| 31,977 | |
| | |
| | | |
| | |
| Gross profit | |
| 344,031 | | |
| 108,623 | |
| | |
| | | |
| | |
| Operating expenses | |
| | | |
| | |
| Marketing and business development | |
| 240,298 | | |
| 200,096 | |
| Research and development | |
| 393,274 | | |
| 312,586 | |
| General and administrative | |
| 1,671,960 | | |
| 1,766,843 | |
| Depreciation | |
| 17,492 | | |
| 36,866 | |
| Total operating expenses | |
| 2,323,024 | | |
| 2,316,391 | |
| | |
| | | |
| | |
| Loss from operations | |
| (1,978,993 | ) | |
| (2,207,768 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Interest expense | |
| (63,580 | ) | |
| (41,536 | ) |
| Total other income (expenses) | |
| (63,580 | ) | |
| (41,536 | ) |
| | |
| | | |
| | |
| Net loss before provision for income taxes | |
| (2,042,573 | ) | |
| (2,249,304 | ) |
| | |
| | | |
| | |
| Provision for income taxes | |
| - | | |
| - | |
| | |
| | | |
| | |
| Net loss | |
| (2,042,573 | ) | |
| (2,249,304 | ) |
| | |
| | | |
| | |
| Other comprehensive (loss) income | |
| | | |
| | |
| | |
| | | |
| | |
| Benefit pension obligation changes | |
| 179,892 | | |
| 397,709 | |
| | |
| | | |
| | |
| Foreign currency translation adjustment | |
| (4,986 | ) | |
| 32,837 | |
| | |
| | | |
| | |
| Total other comprehensive (loss) income | |
| 174,906 | | |
| 430,546 | |
| | |
| | | |
| | |
| Comprehensive loss | |
$ | (1,867,667 | ) | |
$ | (1,818,758 | ) |
The accompanying notes are an integral
part of these financial statements.
Proteomedix AG
Statement of Stockholders’ Deficit
For the years ended December 31, 2022 and
2021
| | |
| | |
| | |
Additional | | |
Accumulated | | |
| | |
Total | |
| | |
Common Stock | | |
Paid In | | |
Comprehensive | | |
Accumulated | | |
Stockholders’ | |
| | |
Shares | | |
Par Value | | |
Capital | | |
(Loss) Income | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2020 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 19,928,271 | | |
$ | 1,131 | | |
$ | (22,950,732 | ) | |
$ | (2,554,775 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Change in pension benefit obligation | |
| - | | |
| - | | |
| - | | |
| 397,709 | | |
| - | | |
| 397,709 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock based compensation | |
| - | | |
| - | | |
| 72,645 | | |
| - | | |
| - | | |
| 72,645 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| FX translation adjustment | |
| - | | |
| - | | |
| - | | |
| 32,837 | | |
| - | | |
| 32,837 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,249,304 | ) | |
| (2,249,304 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2021 | |
| 412,572 | | |
| 466,555 | | |
| 20,000,916 | | |
| 431,677 | | |
| (25,200,036 | ) | |
| (4,300,888 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Change in pension benefit obligation | |
| - | | |
| - | | |
| - | | |
| 179,892 | | |
| - | | |
| 179,892 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Stock based compensation | |
| - | | |
| - | | |
| 376,989 | | |
| - | | |
| - | | |
| 376,989 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| FX translation adjustment | |
| - | | |
| - | | |
| - | | |
| (4,986 | ) | |
| - | | |
| (4,986 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,042,573 | ) | |
| (2,042,573 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2022 | |
| 412,572 | | |
$ | 466,555 | | |
$ | 20,377,905 | | |
$ | 606,583 | | |
$ | (27,242,609 | ) | |
$ | (5,791,566 | ) |
The accompanying notes are an integral part of
these financial statements.
Proteomedix AG
Statements of Cash Flows
For the years ended December 31, 2022 and
2021
| | |
2022 | | |
2021 | |
| Operating activities | |
| | |
| |
| Net Loss | |
$ | (2,042,573 | ) | |
$ | (2,249,304 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and Amortization | |
| 17,492 | | |
| 36,866 | |
| Stock based compensation | |
| 376,989 | | |
| 72,645 | |
| Net periodic benefit cost | |
| (224,944 | ) | |
| (40,881 | ) |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| (140,472 | ) | |
| (32,009 | ) |
| Inventory | |
| 14,774 | | |
| 19,522 | |
| Prepaid expenses and other current assets | |
| 59,352 | | |
| (16,734 | ) |
| Accrued expenses | |
| 5,812 | | |
| (29,661 | ) |
| Cash used in operating activities | |
| (1,933,570 | ) | |
| (2,239,556 | ) |
| | |
| | | |
| | |
| Investing activities: | |
| | | |
| | |
| | |
| | | |
| | |
| Cash used in investing activities | |
| - | | |
| - | |
| | |
| | | |
| | |
| Financing activities: | |
| | | |
| | |
| Issuance (repayment) of notes payable | |
| (50,000 | ) | |
| - | |
| Issuance of convertible notes payable | |
| - | | |
| 3,277,170 | |
| Cash (used in) provided by financing activities | |
| (50,000 | ) | |
| 3,277,170 | |
| | |
| | | |
| | |
| FX effect on cash | |
| (93,075 | ) | |
| (26,488 | ) |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| (2,076,645 | ) | |
| 1,011,126 | |
| | |
| | | |
| | |
| Cash and cash equivalents-Beginning of the year | |
| 2,546,801 | | |
| 1,535,675 | |
| Cash and cash equivalents-End of year | |
$ | 470,156 | | |
$ | 2,546,801 | |
| | |
| | | |
| | |
| Supplemental cash flow disclosures | |
| | | |
| | |
| | |
| | | |
| | |
| Interest paid | |
$ | 2,621 | | |
$ | 2,735 | |
| Income taxes paid | |
$ | - | | |
$ | - | |
The accompanying notes are an integral
part of these financial statements.
Proteomedix AG
Notes to Financial Statements
Note 1 – Organization and Nature of Business
Proteomedix AG (the “Company”) is
a healthcare company whose mission is to transform prostate cancer diagnosis. Proteomedix has identified novel biomarker signatures with
utility in prostate cancer diagnosis, prognosis and therapy management. The lead product Proclarix® is a blood-based
prostate cancer test panel and risk score currently available in Europe and expected to be available in the U.S. in the near future. Proteomedix
is located in the Bio-Technopark of Zurich-Schlieren, Switzerland.
On December 15, 2023, the Company was acquired
by Onconetix, Inc. (formerly Blue Water Biotech, Inc) (the “Parent”). The Parent issued stock of its common stock in exchange
for 100% of the outstanding voting equity of the Company. See Note 10.
Note 2 – Going Concern
The accompanying financial statements have been
prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and
satisfaction of liabilities in the normal course of business. For the year ended December 31, 2022, the Company had an accumulated deficit
of approximately $27,200,000, a net loss of approximately $2,042,000, and net cash used in operating activities of approximately $1,934,000,
with approximately $392,000 in revenue recognized, and a lack of profitable operational history. These matters, among others, raise substantial
doubt about the Company’s ability to continue as a going concern for the 12 months following the issuance of these financial statements.
While the Company is attempting to generate greater
revenues, the Company’s cash position may not be significant enough to support the Company’s daily operations. Management
intends to raise additional funds from its Parent to sustain operations until such time as revenues are sufficient to support the Company’s
operations. Management believes that the actions presently being taken to further implement its business plan and generate revenues provide
the opportunity for the Company to continue as a going concern. While the Company believes in the viability of its strategy to generate
revenues and the ability of its Parent to provide additional funds, there can be no assurances to that effect. The ability of the Company
to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and obtaining additional
funding from its Parent as needed.
Note 3 – Summary of Significant Accounting
Policies
Basis of Presentation
The Company’s financial statements are prepared
in accordance with U.S. Generally Accepted Accounting Principles (“U.S GAAP”), which require the recognition and disclosure
of foreign currency translation adjustments resulting from the translation of financial statements denominated in currencies other than
the U.S. Dollar.
The functional currency of the Company is the
Swiss Franc. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing
at the date of the transaction. The resulting translation adjustments are recorded as a separate component of accumulated other comprehensive
income (loss).
Use of Estimates
The preparation
of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported
amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the
reported amounts of expenses during the reporting periods. The most significant estimates in the Company’s financial statements
relate to valuation of inventory, stock-based compensation, pension benefit obligations, and the valuation allowance of deferred tax assets
resulting from net operating losses. These estimates and assumptions are based on current facts, historical experience and various other
factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying
values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results may differ
materially and adversely from these estimates. To the extent there are material differences between the estimates and actual results,
the Company’s future results of operations will be affected.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Segment
Information
Operating
segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief
operating decision maker (“CODM”), or decision-making group, in deciding how to allocate resources and in assessing performance.
The Company operates in one segment which is consistent with the financial information regularly reviewed by the CODM for purposes of
evaluating performance, allocating resources, setting incentive compensation targets, and planning and forecasting for future periods.
Cash and Cash Equivalents
For purposes
of reporting cash flows, the Company has defined cash and cash equivalents as all cash in banks and highly liquid investments available
for current use with an initial maturity of three months or less to be cash equivalents. The Company had no cash equivalents as of December
31, 2022 or 2021.
The Company
maintains its cash balances at financial institutions that are insured by Swiss Financial Market Supervisory Authority (“FINMA”).
The Company’s cash balances may at times exceed the insurance provided by FINMA. The Company has not experienced any losses on these
accounts and management does not believe that the Company is exposed to any significant risks related to excess deposits.
Accounts Receivable
The Company
performs periodic credit evaluations of its customers’ financial condition and extends credit to virtually all of its customers
on an uncollateralized basis. Credit losses to date have been insignificant and within management’s expectations. The Company provides
an allowance for doubtful accounts that is based upon a review of outstanding receivables, historical collection information, and existing
economic conditions. Normal accounts receivable are due 30 days after the issuance of the invoice. Receivables are considered delinquent
based on management’s assessment of the individual balance. Delinquent receivables are evaluated for collectability based on individual
credit evaluation and specific circumstances of the customer. As of December 31, 2022 and 2021, the Company’s allowance for doubtful
accounts was nil, respectively. The Company did not write off any accounts receivable against the allowance for doubtful accounts during
the years ended December 31, 2022 and 2021. As of December 31, 2022 and 2021, substantially all of the Company’s accounts receivable
are due from a single customer.
Inventories
Inventories consist of raw materials and finished
goods. Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company
periodically reviews the composition of inventory in order to identify excess, obsolete, slow-moving or otherwise non-saleable items taking
into account anticipated future sales compared with quantities on hand, and the remaining shelf life of goods on hand. If non-saleable
items are observed and there are no alternate uses for the inventory, the Company records a write-down to net realizable value in the
period that the decline in value is first recognized. The Company had no inventory reserves as of December 31, 2022 and 2021.
The Company’s inventory consisted of the
following at the respective balance sheet dates:
| | |
2022 | | |
2021 | |
| Raw materials | |
$ | 48,408 | | |
$ | 52,942 | |
| Finished goods | |
| 47,402 | | |
| 57,641 | |
| Total | |
$ | 95,810 | | |
$ | 110,583 | |
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Impairment
of Long-Lived Assets
The Company
reviews long-lived assets for impairment whenever events or changes in business circumstances indicate that the carrying amount of the
assets may not be fully recoverable (a “triggering event”). Factors that the Company considers in deciding when to perform
an impairment review include significant underperformance of the long-lived asset in relation to expectations, significant negative industry
or economic trends, and significant changes or planned changes in the use of the assets. If an impairment review is performed to evaluate
a long-lived asset for recoverability, the Company compares forecasts of undiscounted cash flows expected to result from the use and eventual
disposition of the long-lived asset to its carrying value. An impairment loss would be recognized when estimated undiscounted future cash
flows expected to result from the use of an asset are less than its carrying amount. The impairment loss would be based on the excess
of the carrying value of the impaired asset over its fair value, determined based on discounted cash flows. During the years ended December
31, 2022 and 2021, the Company did not identify any impairments related to its long-lived assets.
Property and Equipment
Property and equipment consists of computers and
office furniture and fixtures, all of which are recorded at cost. Depreciation is recorded using the straight-line method over the respective
useful lives of the assets ranging from two to ten years. Long-lived assets are reviewed for impairment whenever events or circumstances
indicate that the carrying amount of these assets may not be recoverable. Upon the retirement or other disposition of property and equipment,
the related cost and accumulated depreciation are charged to operations. A summary of the estimated useful lives is a s follows:
| Description | |
Estimated
Useful Life |
| Computers | |
3 years |
| Office furniture and fixtures | |
2 to 10 years |
The following table summarizes the Company’s
property and equipment, net of accumulated depreciation, as of December 31, 2022 and 2021, by significant class.
| Class | |
2022 | | |
2021 | |
| Computers | |
$ | 79,199 | | |
$ | 75,311 | |
| Office furniture and fixtures | |
| 341,318 | | |
| 346,040 | |
| Less: accumulated depreciation | |
| (380,387 | ) | |
| (367,348 | ) |
| Total | |
$ | 40,130 | | |
$ | 54,003 | |
Depreciation expense for the years ended December
31, 2022 and 2021, was $17,492 and $36,866, respectively.
Lease Accounting.
The Company regularly evaluates whether a contract
meets the definition of a lease whenever a contract grants it the right to control the use of an identified asset for a period in exchange
for consideration. The Company’s lease agreement consists of office space. This lease generally contains an initial term of two
years and with renewals options. If the Company’s lease agreement includes renewal option periods, the Company includes such renewal
options in its calculation of the estimated lease term when it determines the options are reasonably certain to be exercised. When such
renewal options are deemed to be reasonably certain, the estimated lease term determined under ASC 842 will be greater than the non-cancelable
term of the contractual arrangement.
The Company classifies its lessee arrangements
at inception as either operating leases or financing leases. A lease is classified as a financing lease if at least one of the following
criteria is met: (1) the lease transfers ownership of the underlying asset to the lessee, (2) the lease grants the lessee an option to
purchase the underlying asset that the lessee is reasonably certain to exercise, (3) the lease term is for a major part of the remaining
economic life of the underlying asset, (4) the present value of the sum of the lease payments equals or exceeds substantially all of the
fair value of the underlying asset, or (5) the underlying asset is of such a specialized nature that it is expected to have no alternative
use to the lessor at the end of the lease term. A lease is classified as an operating lease if none of the five criteria described above
for financing lease classification is met. The Company has no financing leases as of December 31, 2022 or 2021.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
ROU assets associated with operating leases are
included in “Right of Use Asset” on the Company’s balance sheets. Current and long-term portions of lease liabilities
related to operating leases are included in ‘operating lease liability, current’ and ‘operating lease liability’
on the Company’s balance sheets as of December 31, 2022 and 2021. ROU assets represent the Company’s right to use an underlying
asset for the estimated lease term and lease liabilities represent the Company’s present value of its future lease payments. In
assessing its lease and determining its lease liability at lease commencement or upon modification, the Company was not able to readily
determine the rate implicit for its lessee arrangements, and thus has used its incremental borrowing rate on a collateralized basis to
determine the present value of the lease payments. The Company’s ROU asset is measured as the balance of the lease liability plus
or minus any prepaid or accrued lease payments and any unamortized initial direct costs. Operating lease expenses are recognized on a
ratable basis, regardless of whether the payment terms require the Company to make payments annually, quarterly, monthly, or for the entire
term in advance. If the payment terms include fixed escalator provisions, the effect of such increases is recognized on a straight-line
basis. The Company calculates the straight-line expense over the contract’s estimated lease term, including any renewal option periods
that the Company deems reasonably certain to be exercised and recognizes this as lease expense within ‘general and administrative’
in the accompanying statements of comprehensive loss. See Note 5 for further information regarding the Company’s lease.
Research and Development Costs
Research and development expenses are those costs
incurred in the discovery, design, and development of new products, processes, or services, as well as the enhancement of existing products.
Research and development costs are expensed as incurred unless such costs have an alternative future use. These costs include, but are
not limited to, salaries, wages, benefits, materials, equipment, and overhead directly attributable to the research and development activities.
Collaborative Agreements
The Company periodically enters into strategic
alliance agreements with counterparties to produce products and/or provide services to customers. Alliances created by such agreements
are not legal entities, have no employees, no assets and have no true operations. These arrangements create contractual rights and the
Company accounts for these alliances as a collaborative arrangement by reporting costs incurred and reimbursements received from transactions
within research and development expense within the statements of comprehensive loss.
Commitments and
Contingencies
Liabilities
for loss contingencies arising from claims, assessments, litigation, fines, penalties and other sources are recorded when management assesses
that it is probable that a liability has been incurred and the amount can be reasonably estimated.
Share Based Compensation
The Company
accounts for equity instruments issued in exchange for the receipt of goods or services from other than employees in accordance with Financial
Accounting Standard Board (“FASB”) Account Standard Codification (“ASC”) 718, “Compensation – Stock
Compensation”. Costs are measured at the estimated fair value of the consideration received or the estimated fair value of the equity
instruments issued, whichever is more reliably measurable. The value of equity instruments issued for consideration other than employee
services is determined on the earliest of a performance commitment or completion of performance by the provider of goods or services as
defined by FASB ASC 718, “Compensation – Stock Compensation”.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Income Taxes
In accordance
with ASC 740, “Income Taxes,” the Company provides for the recognition of deferred tax assets if realization of such assets
is more likely than not. Deferred income tax assets and liabilities are computed for differences between the financial statement and tax
basis of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable
to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to
reduce deferred tax assets to the amount expected to be realized. Income tax expense is the tax payable or refundable for the period plus
or minus the change during the period in deferred tax assets and liabilities.
In addition,
the Company’s management performs an evaluation of all uncertain income tax positions taken or expected to be taken in the course
of preparing the Company’s income tax returns to determine whether the income tax positions meet a “more likely than not”
standard of being sustained under examination by the applicable taxing authorities. This evaluation is required to be performed for all
open tax years, as defined by the various statutes of limitations, for federal and state purposes. If the Company has interest or penalties
associated with insufficient taxes paid, such expenses are reported in income tax expense.
Revenue Recognition
Effective on January 1, 2021, the Company adopted
ASC Topic 606, “Revenue from Contracts with Customers” (“ASC 606”). Pursuant to ASC 606, revenues are recognized
when control of services performed is transferred to customers, in an amount that reflects the consideration we expect to be entitled
to in exchange for those services. ASC 606 provides for a five-step model that includes:
| (i) | identifying the contract with a customer, |
| (ii) | identifying the performance obligations in the contract, |
| (iii) | determining the transaction price, |
| (iv) | allocating the transaction price to the performance obligations,
and |
| (v) | recognizing revenue when, or as, an entity satisfies a performance
obligation. |
Product
Sales
The Company derives revenue through sales of its
products directly to end users and to distributors. The Company sells its products to customers including laboratories, hospitals, medical
centers, doctors and distributors. The Company considers customer purchase orders, which in some cases are governed by master sales agreements
or standard terms and conditions, to be the contracts with a customer. For each contract, the Company considers the promise to transfer
products, each of which are distinct, to be the identified performance obligations. In determining the transaction price the Company evaluates
whether the price is subject to refund or adjustment to determine the net consideration to which it expects to be entitled. The Company
fulfils its performance obligation applicable to product sales once the product is transferred to the customer.
Development
Services
The Company
provides a range of services to life sciences customers referred to as “Development Services” including testing for biomarker
discovery, assay design and development. These Development Services are performed under individual statement of work (“SOW”) arrangements
with specific deliverables defined by the customer. Development Services are generally performed on a time and materials basis. During
the performance and through completion of the service to the customer in accordance with the SOW, we have the right to bill the customer
for the agreed upon price and we recognize the Development Services revenue over the period estimated to complete the SOW. We generally
identify each SOW as a single performance obligation.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Completion
of the service and satisfaction of the performance obligation under a SOW is typically evidenced by access to the data or test made available
to the customer or any other form or applicable manner of delivery defined in the SOW. However, for certain SOWs under which work is performed
pursuant to the customer’s highly customized specifications, we have the enforceable right to bill the customer for work completed,
rather than upon completion of the SOW. For those SOWs, we recognize revenue over a period of time during which the work is performed
based on the expended efforts (inputs). As the performance obligation under the SOW is satisfied, any amounts earned as revenue and billed
to the customer are included in accounts receivable. Any revenues earned but not yet billed to the customer as of the date of the financial
statements are recorded as contract assets and are included in prepaids and other current assets as of the financial statement date. Amounts
recorded in contract assets are reclassified to accounts receivable in our financial statements when the customer is invoiced according
to the billing schedule in the contract.
In circumstances where a SOW includes variable
consideration component, the Company estimates the amount of variable consideration that should be included in the transaction price utilizing
either the expected value method or the most likely amount method, depending on which method is expected to better predict the amount
of consideration to which the Company will be entitled. The value of variable consideration is included in the transaction price if, and
to the extent, it is probable that a significant reversal of the amount of cumulative revenue recognized will not occur when the uncertainty
associated with the variable consideration is subsequently resolved. These estimates are reassessed each reporting period, as required,
and any adjustment required is recorded on a cumulative catch-up basis, which would affect revenue and net income in the period of adjustment.
The following
table disaggregates the Company’s revenues by type for the years ended December 31, 2022 and 2021.
| | |
Recognition
Method | |
2022 | | |
2021 | |
| Product sales | |
Point in time | |
$ | 79,085 | | |
$ | 55,311 | |
| Development services | |
Over time | |
| 313,375 | | |
| 85,289 | |
| | |
| |
$ | 392,460 | | |
$ | 140,600 | |
Fair Value Measurement
ASC Topic
820, “Fair Value Measurement”, requires that certain financial instruments be recognized at their fair values at our balance
sheet dates. However, other financial instruments, such as debt obligations, are not required to be recognized at their fair values, but U.S.
GAAP provides an option to elect fair value accounting for these instruments. U.S. GAAP requires the disclosure of the fair values of
all financial instruments, regardless of whether they are recognized at their fair values or carrying amounts in our balance sheets. For
financial instruments recognized at fair value, U.S. GAAP requires the disclosure of their fair values by type of instrument, along with
other information, including changes in the fair values of certain financial instruments recognized in income or other comprehensive income.
For financial instruments not recognized at fair value, the disclosure of their fair values is provided below under “Financial Instruments”.
Nonfinancial
assets, such as property and equipment, and nonfinancial liabilities are recognized at their carrying amounts in the Company’s balance
sheets. GAAP does not permit nonfinancial assets and liabilities to be remeasured at their fair values. However, GAAP requires the remeasurement
of such assets and liabilities to their fair values upon the occurrence of certain events, such as the impairment of property, plant and
equipment. In addition, if such an event occurs, GAAP requires the disclosure of the fair value of the asset or liability along with other
information, including the gain or loss recognized in income in the period the remeasurement occurred.
The Company
did not have any assets or liabilities at December 31, 2022 and 2021 which required remeasurement at the respective reporting periods.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Financial Instruments
The Company’s
financial instruments include cash and cash equivalents, accounts receivable and accounts payable, and are accounted for under the provisions
of ASC Topic 825, “Financial Instruments”. The carrying amount of these financial instruments, as reflected in the financial
statements approximates fair value.
Convertible Instruments
The Company
evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging
Activities”.
U.S. GAAP
requires companies to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial
instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the
embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b)
the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under
other GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded
derivative instrument would be considered a derivative instrument.
The Company
accounts for convertible instruments as follows: Debt discounts under these arrangements are amortized over the term of the related debt
to their stated date of redemption. Proceeds from these convertible notes are reported under the financing section of the statements of
cash flows. During the years ended December 31, 2022 and 2021, the Company did not have any conversion options which required bifurcation
from the host instrument.
Defined
Benefit Pension Plan
The Company sponsors
a defined benefit pension plan (the “Plan”) covering eligible employees. The Plan provides retirement benefits based on employees’
years of service and compensation levels. The Company recognizes an asset for such plan’s overfunded status or a liability underfunded
status in its balance sheets. Additionally, the Company measures its plan’s assets and obligations that determine its funded status
as of the end of the year and recognizes the changes in the funded status in the year in which the changes occur. Those changes are reported
in ‘accumulated other comprehensive loss. The Company uses actuarial valuations to determine its pension and postretirement benefit
costs and credits. The amounts calculated depend on a variety of key assumptions, including discount rates and expected return on plan
assets. Current market conditions are considered in selecting these assumptions.
The Company’s pension plans are generally
valued using the net asset value (NAV) per share as a practical expedient for fair value provided certain criteria are met. The NAVs are
determined based on the fair values of the underlying investments in the funds. In circumstances where the criteria are not met, fair
is determined based on the underlying market in which the funds are traded which is generally considered to be an active market.
Recently Issued
Accounting Standards
During the
period ended December 31, 2022, and subsequently, there were several new accounting pronouncements issued by the FASB. Each of these pronouncements,
as applicable, has been or will be adopted by the Company. Management does not believe the adoption of any of these accounting pronouncements
has had or will have a material impact on the Company’s financial statements.
In August
2020, the FASB issued ASU 2020-06, “Debt - Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging
- Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s
Own Equity” (“ASU 2020-06”), which simplifies the accounting for certain financial instruments with characteristics
of liabilities and equity. This ASU: (1) simplifies the accounting for convertible debt instruments and convertible preferred stock by
removing the existing guidance in ASC 470-20, “Debt: Debt with Conversion and Other Options,” that requires entities to account
for beneficial conversion features and cash conversion features in equity, separately from the host convertible debt or preferred stock;
(2) revises the scope exception from derivative accounting in ASC 815-40 for freestanding financial instruments and embedded features
that are both indexed to the issuer’s own stock and classified in stockholders’ equity, by removing certain criteria required
for equity classification; and (3) revises the guidance in ASC 260, “Earnings Per Share,” to require entities to calculate
diluted EPS for convertible instruments by using the if-converted method. In addition, entities must presume share settlement for purposes
of calculating diluted EPS when an instrument may be settled in cash or shares. For SEC filers, excluding smaller reporting companies,
ASU 2020-06 is effective for fiscal years beginning after December 15, 2021 including interim periods within those fiscal years. Early
adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020. For all other entities, ASU 2020-06 is effective
for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. Entities should adopt the guidance
as of the beginning of the fiscal year of adoption and cannot adopt the guidance in an interim reporting period. The Company adopted
the ASU 2020-06 on January 1, 2021. The adoption of this standard did not have a material impact on the Company’s financial statements.
Proteomedix AG
Notes to Financial Statements
Note 3
– Summary of Significant Accounting Policies (cont.)
Subsequent Events
The Company
has evaluated all transactions through the date the financial statements were issued for subsequent event disclosure consideration. See
Note 10.
Note 4 – Debt
On March 3, 2010, the Company received a loan
from Venture Kick in the amount of 100,000 CHF. This loan bears no interest, is unsecured and may be cancelled by the Company at its discretion.
This loan is subordinated to the Company’s other unsubordinated debt. The loan was to be used solely for business development and
the Company may, at its sole discretion, contribute funds back to Venture Kick to enable that organization to continue its efforts. As
of December 31, 2022 and 2021 the balance outstanding was approximately $108,000 and $115,000, respectively.
On June 23, 2020, Company entered a convertible
note payable with a financial institution and shareholder of the Company for CHF 550,000 with an interest rate of 0.50% per annum and
a maturity of September 30, 2024. The note provides the holder with an optional conversion feature in the event of an equity financing
of the Company. The conversion price in the event of an equity financing is at a 20% discount the share price from the financing. The
holder is also entitled to convert the note upon the occurrence of a sale of the Company or at maturity of the note in both cases without
a discount. This note was unsubordinated until January 10, 2023, at which point it was also subordinated to all other unsubordinated debts.
The interest rate was changed to 2.50% as of May 1, 2023. As of December 31, 2022 and 2021, the outstanding balance on this note was approximately
$541,000 and $548,000, respectively. The Company additionally obtained a COVID-19 loan with such financial institution on April 16, 2020,
in the amount of CHF 50,000 with an interest rate of 0%. As of December 31, 2021, the balance outstanding was approximately $50,000. Such
loan was subsequently fully repaid as of April 2022.
On June 23, 2020, Company entered into a series
of convertible notes payable with certain shareholders of the Company for CHF 800,000 with an interest rate of 0.50% per annum and a maturity
of September 30, 2024. The note provides the holder with an optional conversion feature in the event of an equity financing greater than
CHF 1,000,000. The conversion price in the event of an equity financing is at a 20% discount the share price from the financing. The holder
is also entitled to convert the note upon the occurrence of a sale of the Company or at maturity of the note in both cases without a discount.
These notes payable are subordinated to the Company’s other unsubordinated debt. As of December 31, 2022 and 2021, the outstanding
balance on these notes was approximately $865,000 and $877,000, respectively.
On October 26, 2020, Company entered into a series
of convertible notes payable with certain members of the board of directors (Note 8) in the total amount of CHF 161,250 with an interest
rate of 0.25% and a maturity of December 31, 2023. The note provides the holder with an optional conversion feature at a discount of 20%
in the event of an equity financing greater than CHF 1,000,000. The holder is also entitled to convert the note upon the occurrence of
a sale of the Company or at maturity of the note in both cases without a discount. These notes payable are subordinated to the Company’s
other unsubordinated debt. As of December 31, 2022 and 2021, the outstanding balance on these notes was approximately $174,000 and $177,000,
respectively.
Proteomedix AG
Notes to Financial Statements
Note 4
– Debt (cont.)
On November 23, 2020, Company entered into a series
of convertible notes payable with certain shareholders of the Company in the total amount of CHF 760,080 with an interest rate of 5% and
a maturity of December 31, 2023. The note provides the holder with an optional conversion feature at a discount of 30% in the event of
an equity financing greater than CHF 1,000,000. The holder is also entitled to convert the note upon the occurrence of a sale of the Company
or at maturity of the note in both cases without a discount. These notes payable are subordinated to the Company’s other unsubordinated
debt. As of December 31, 2022 and 2021, the outstanding balance on these notes was approximately $822,000 and $834,000, respectively.
On July 19, 2021, Company entered into a convertible
note payable in the total amount of CHF 3,000,000 with an interest rate of 0.5% and a maturity of September 30, 2023. The note provides
the holder with a mandatory conversion requirement in the event of an equity financing greater than CHF 1,000,000. The note is also mandatorily
converted in the event certain milestones are achieved related to an R&D collaboration project entered separately none of which have
been met as of December 31, 2022. The holder is also entitled to convert the note upon the occurrence of a sale of the Company or at maturity
of the note in both cases without a discount. These notes payable are subordinated to the Company’s other unsubordinated debt. As
of December 31, 2022 and 2021, the outstanding balance on this note was approximately $3,245,000 and $3,290,000, respectively. Subsequent
to December 31, 2022, the maturity date for this note was extended to September 30, 2024.
All outstanding convertible notes as of December
31, 2022 were converted upon the closing of the acquisition of the Company by the Parent. See Note 10.
Note 5 – Commitments and Contingencies
Leases
The Company leases its primary office and lab
space at a rate of 5,077 CHF per month. The lease began on February 1, 2012 with an initial period ending on January 31, 2015. This rental
agreement can be terminated at the end of March, June and September of a given year with a notice of 12 months. If the Company wishes
to terminate the lease without adhering to the agreed dates, it is liable for the rent and the other tenant obligations until the rental
is continued, but the latest until the next contractual termination date. If the rental agreement is not terminated in writing by either
party after the fixed contract period has expired, while observing the notice period, it will be extended by two years. As of December
31, 2022 the remaining period of the lease is approximately 30 months.
The Company temporarily expanded the above lease
to include additional space beginning on January 1, 2020 and ending on April 30, 2021. This space had a month lease payment of 2,843 CHF.
The Company appropriately exercised its termination rights for this lease and has no further obligation to the lessor.
The Company adopted ASC Topic 842, “Leases”,
on January 1, 2022. ASC 842 establishes principles for recognizing, measuring, presenting, and disclosing leases to ensure that lessees
and lessors provide relevant information about their leasing transactions. The Company has adopted ASC 842 using the modified retrospective
approach and elected to use the effective method to apply this standard on the effective date to all remaining leases meeting the criteria
for recognition. Comparative prior periods are not restated and are presented under ASC 840. In applying the modified retrospective approach,
the Company elected the package of practical expedients permitted by ASC 842, which includes:
| - | Existing Leases: The Company did not reassess whether existing contracts are or contain leases. |
| | | |
| - | Initial Direct Costs: The Company did not reassess initial direct costs for existing leases. |
| | | |
| - | Non-lease components: The Company combined lease and non-lease components. |
As a result of the adoption of ASC 842, the Company
recognized right-of-use asset and lease liability of approximately $250,000 on the balance sheet for its lease that was classified as
an operating lease under the previous guidance. The adoption did not have a material impact on the Company’s statement of comprehensive
loss or cash flows.
Proteomedix AG
Notes to Financial Statements
Note 5
– Commitments and Contingencies (cont.)
Initially, the Company measure the right of use
asset and liability associated with its office lease using the following inputs:
| Remaining lease term (in years) | |
| 4 | |
| Discount rate | |
| 0.05 | % |
The Company
records rent on straight-line basis over the terms of the underlying lease. Estimated future minimum lease payments under the lease as
of December 31, 2022 are as follows:
| Year Ending December 31, | |
Amount | |
| 2023 | |
$ | 67,632 | |
| 2024 | |
| 67,632 | |
| 2025 | |
| 67,632 | |
| Total remaining lease payments | |
| 202,896 | |
| Less: imputed interest | |
| 157 | |
| Present value of remaining lease payments | |
$ | 202,739 | |
The rent expense for the years ended December
31, 2022 and 2021 was $65,535 and $68,409 respectively, and was included in ‘general and administrative’ expenses in the accompanying
statements of comprehensive loss. The Company paid $65,535 and $68,409 respectively, in lease payments during the years ended December
31, 2022 and 2021 and are included in the Company’s operating cash flows for both periods. The change in lease expense and lease
cash payments from period to period is due to changes in exchange rate between USD and CHF as the Company’s minimum monthly lease
payments are fixed for the term of the lease.
Switzerland social security obligations
The Company issued certain stock options during
periods prior to December 31, 2022. If the recipients exercise these stock options it may result in the recognition of additional social
security tax due to the Switzerland taxing authority. Management assessed the likelihood of this liability having been incurred as of
December 31, 2022 and 2021 in accordance with ASC 450, Contingencies, and determined the likelihood was reasonably possible. Accordingly,
no accrual for this contingent obligation has been recognized in the accompanying financial statements. Additionally, management is unable
to estimate an amount or range of amounts related to any amount that may be owed should a recipient exercise a stock option.
Federal COVID-19 assistance
During the year ended December 31, 2021, the Company,
as well as many other entities, received payroll assistance from the government of Switzerland as a result of the COVID-19 pandemic. The
total amount received by the Company approximated $171,000 and was used to reduce wages and salaries primarily within ‘general and
administrative’ and ‘research and development’ expenses in the accompanying statements of comprehensive loss.
Note 6 – Stockholders’ Deficit
Share Capital
The Company has several series of common stock
providing the following provisions. In the event of a bankruptcy or liquidation or winding up of the Company, the holders of Series B3
Common Stock will be entitled to receive, in advance of the holders of Series B2 Common Stock, Series B Common Stock and Series A Stock
and Ordinary Stock, CHF 65 for each Series B3 Common Share they own.
Proteomedix AG
Notes to Financial Statements
Note 6
– Stockholders’ Deficit (cont.)
Thereafter, the holders of Series B2 Common Stock
will be entitled to receive, in advance of the holders of Series B Common Stock and Series A Stock and Ordinary Stock, CHF 60 for each
Series B2 Common Share they own.
Thereafter, the holders of Series B Common Stock
will be entitled to receive, in advance of the holders of Series A Common Stock and Ordinary Stock, CHF 50 for each Series B Common Share
they own.
Thereafter, the holders of Series A Common Stock
will be entitled to receive, in advance of the holders of Ordinary Stock, CHF 40 for each Series A Common Share they own.
Thereafter, the other Ordinary Shareholders will
be entitled to receive CHF 40 per Ordinary Share they own and then any remaining assets or proceeds will be distributed pro rata among
all Shareholders.
If there are insufficient assets or proceeds to
pay such amount to the holders of Series B3 Common Stock, the amount available will be paid on a pro rata basis between the holders of
the Series B3 Common Stock.
If, after the full payment of Series B3 Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series B2 Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series B2 Common Stock.
If, after the full payment of Series B2 Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series B Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series B Common Stock.
If, after the full payment of Series B Shareholders,
there are insufficient assets or proceeds to pay such amount to the holders of Series A Common Stock, the amount available will be paid
on a pro rata basis between the holders of the Series A Common Stock.
The Company and all Shareholders shall use best
efforts to ensure that any sale, liquidation, disposal of material assets or the entire Company shall be effectuated so as to be tax efficient,
particularly as regards any applicable withholding tax, and fair with regard to the Shareholders.
If in later financing rounds additional preference
rights are granted, then the holders of Series A Common Stock, Series B Common Stock and Series B2 Common Stock shall receive mutatis
mutandis behind the new shares the same rights (taking into account the respective price).”
The Series B3 Common Stock shall have the same
rights and obligations under the Shareholder’s Agreement and the Organizational Rules as the Series B Common Stock and the Series
B2 Common Stock, and thus have the same legal status as the Series B Common Stock and the Series B2 Common Stock.
As of December 31, 2022 and 2021 the following
number of common stock for each series was outstanding:
| Share Class | |
Stock | |
| Ordinary | |
| 100,000 | |
| Series A | |
| 65,000 | |
| Series B | |
| 84,200 | |
| Series B2 | |
| 83,334 | |
| Series B3 | |
| 80,038 | |
| Total Outstanding stock | |
| 412,572 | |
Proteomedix AG
Notes to Financial Statements
Note 6
– Stockholders’ Deficit (cont.)
Stock options
The Company sponsors a stock option plan (the
“Plan”) which provides common stock option grants to be granted to certain individuals as determined by the board of directors.
All employees and consultants of the Company are eligible to receive awards under the Plan. The terms of each option are determined by
the board of directors and are evidenced by a grant notice provided to the grantee after approval by the board of directors. Generally,
options issued under the Plan have a term of less than 11 years and provide for a four-year vesting period during which the grantee must
remain in the service of the Company. Options are generally granted on either January 1 or July 1 annually and the exercise price is determined
at each respective time by the board of directors. Upon exercise by a grantee, the Company issues new shares of common stock from its
authorized capital to satisfy the exercise.
The Company has granted various stock
options primarily to employees as incentive-based compensation. Stock issued under this plan are measure at fair value using the
Black-Scholes option pricing model as further described below. Upon exercise, the Company issues new stock from its authorized
capital. The following summarizes activity related to the Company’s stock options for the years ended December 31, 2022 and
2021:
| | |
| | |
| | |
| | |
Weighted | |
| | |
| | |
| | |
| | |
Average | |
| | |
| | |
Weighted | | |
| | |
Remaining | |
| | |
| | |
Average | | |
| | |
Contractual | |
| | |
Number of | | |
Exercise | | |
Intrinsic | | |
Life | |
| | |
Stock | | |
Price | | |
Value | | |
(in years) | |
| Outstanding as of December 31, 2020 | |
| 37,573 | | |
$ | 4.54 | | |
$ | 18.11 | | |
| 5.99 | |
| Granted | |
| 23,084 | | |
| 1.10 | | |
| 33.14 | | |
| 10 | |
| Forfeited / cancelled | |
| (7,792 | ) | |
| 1.41 | | |
| 26.50 | | |
| 9.56 | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding as of December 31, 2021 | |
| 52,865 | | |
| 3.40 | | |
| 24.57 | | |
| 8.60 | |
| Granted | |
| - | | |
| - | | |
| - | | |
| - | |
| Forfeited / cancelled | |
| - | | |
| - | | |
| - | | |
| - | |
| Exercised | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding as of December 31, 2022 | |
| 52,865 | | |
$ | 3.35 | | |
$ | 24.62 | | |
| 7.89 | |
| Options vested and exercisable as of December 31, 2022 | |
| 42,459 | | |
$ | 3.52 | | |
$ | 34.34 | | |
| 6.52 | |
The fair value of options granted
during the years ended December 31, 2022 and 2021 was estimated using the following range of assumptions:
| | |
2022 | | |
2021 | |
| Exercise price | |
$ | 1.08 to $27.04 | | |
$ | 1.10 to $27.42 | |
| Term (years) | |
| 3 | | |
| 3 | |
| Expected stock price volatility | |
| 70% | | |
| 70% | |
| Risk-free rate of interest | |
| 1.15% | | |
| -0.73% | |
The weighted average grant date
fair value of stock options granted during the years ended December 31, 2022 and 2021 was $0 and $33.14, respectively. The Company estimates
forfeitures based on the historical pattern of forfeitures for grantees and are recognized as they occur. The Company uses the straight-line
method of measuring compensation cost related to stock option grants which provides that the grants are measured at fair value on the
date of issuance and the related cost is measure over the requisite service period as the options vest with each vesting period being
treated as a single grant over which compensation is recognized. As of December 31, 2022, approximately 16,800 options remain unvested
having a fair value $940,702 which will be recognized in future periods as the options vest. The aggregate fair value of stock options
that vested during the years ended December 31, 2022 and 2021 was approximately $329,000 and $68,000, respectively.
Proteomedix AG
Notes to Financial Statements
Note 6
– Stockholders’ Deficit (cont.)
Accumulated other comprehensive loss
The table below details the components and the
Company’s accumulated other comprehensive loss as of December 31, 2022 and 2021.
| | |
Defined | | |
| | |
| |
| | |
Benefit | | |
Foreign | | |
| |
| | |
Pension | | |
Currency | | |
| |
| | |
Items | | |
Items | | |
Total | |
| Balance as of December 31, 2020 | |
$ | - | | |
$ | 1,131 | | |
$ | 1,131 | |
| | |
| | | |
| | | |
| | |
| Other comprehensive income before reclassifications | |
| 562,461 | | |
| 32,837 | | |
| 595,298 | |
| | |
| | | |
| | | |
| | |
| Amounts reclassified from accumulated other comprehensive
income | |
| (164,752 | ) | |
| - | | |
| (164,752 | ) |
| | |
| | | |
| | | |
| | |
| Net current period other comprehensive income | |
| 397,709 | | |
| 32,837 | | |
| 430,546 | |
| | |
| | | |
| | | |
| | |
| Balance as of December 31, 2021 | |
| 397,709 | | |
| 33,968 | | |
| 431,677 | |
| | |
| | | |
| | | |
| | |
| Other comprehensive income before reclassifications | |
| 475,487 | | |
| (4,986 | ) | |
| 470,501 | |
| | |
| | | |
| | | |
| | |
| Amounts reclassified from accumulated other comprehensive
income | |
| (295,595 | ) | |
| - | | |
| (295,595 | ) |
| | |
| | | |
| | | |
| | |
| Net current period other comprehensive income | |
| 179,892 | | |
| (4,986 | ) | |
| 174,906 | |
| | |
| | | |
| | | |
| | |
| Balance as of December 31, 2022 | |
$ | 577,601 | | |
$ | 28,982 | | |
$ | 606,583 | |
The following tables details the amounts reclassified
from other comprehensive loss and the related affected line items within the accompanying statements of comprehensive loss for the years
ended December 31, 2022 and 2021.
| | |
2022 | | |
2021 | | |
Financial statement |
| Item description | |
Amount | | |
Amount | | |
line item |
| | |
| | |
| | |
|
| Amortization of gains (losses) | |
$ | 6,303 | | |
$ | - | | |
General and administrative |
| | |
| | | |
| | | |
|
| Settlements | |
| 289,292 | | |
| 164,752 | | |
General and administrative |
| | |
| | | |
| | | |
|
| | |
$ | 295,595 | | |
$ | 164,752 | | |
|
Proteomedix AG
Notes to Financial Statements
Note 7 – Defined Benefit Pension Plan
The Company sponsors a defined benefit pension
plan covering certain eligible employees. The plan provides retirement benefits based on years of service and compensation levels.
The value of the pension obligation is determined
using the Projected Unit Credit (PUC) method. This method sees each period of service as giving rise to an additional unit of benefit
entitlements/employee benefits. The value of the Company’s employee benefit obligations for active employees, or the Projected Benefit
Obligation (PBO), on the reporting date is the same as the present value of the degree of entitlement existing on this date, in terms
of future salary and pension increases and turnover rates. The valuation of pension obligations of pensioners is made on the basis of
the present value of current pensions taking into account future increases in pensions. The service costs (SC) are calculated using the
present value of the entitlements to employee benefits earned during the year for which calculations are made.
The following significant actuarial assumptions
were used in calculating the benefit obligation and the net periodic benefit cost as of December 31, 2022 and 2021:
| | |
2022 | | |
2021 | |
| Discount rate | |
| 2.30 | % | |
| 0.35 | % |
| Expected long-term rate of return on plan assets | |
| 2.30 | % | |
| 0.35 | % |
| Rate of compensation increase | |
| 3.00 | % | |
| 3.00 | % |
Changes in these assumptions may have a material
impact on the plan’s obligations and costs.
The components of net periodic benefit cost for
the years ended December 31, 2022 and 2021 are as follows:
| | |
2022 | | |
2021 | |
| Service cost | |
$ | 157,225 | | |
$ | 218,298 | |
| Interest cost | |
| 10,737 | | |
| 3,563 | |
| Expected return on plan assets | |
| (8,195 | ) | |
| (2,366 | ) |
| Amortization of net (gain)/loss | |
| (6,303 | ) | |
| - | |
| Settlements (gain)/loss | |
| (289,292 | ) | |
| (164,752 | ) |
| Total | |
$ | (135,828 | ) | |
$ | 54,743 | |
The components of accumulated comprehensive loss
attributable to the Company’s pension plan for the years ended December 31, 2022 and 2021 are as follows:
| | |
2022 | | |
2021 | |
| Net loss (gain) | |
$ | (475,487 | ) | |
$ | (562,461 | ) |
| Amortization of net gain | |
| 6,303 | | |
| - | |
| Effect of settlement | |
| 289,292 | | |
| 164,752 | |
| Total recorded during the period | |
| (179,892 | ) | |
| (397,709 | ) |
| | |
| | | |
| | |
| Total | |
$ | (577,601 | ) | |
$ | (397,709 | ) |
As of December 31, 2022 and 2021, the funded status
of the plan and the amounts recognized in the balance sheets are as follows:
| | |
2022 | | |
2021 | |
| Projected benefit obligation | |
$ | 1,981,655 | | |
$ | 3,321,683 | |
| Fair value of plan assets | |
| 1,588,015 | | |
| 2,523,207 | |
| Overfunded (underfunded) status | |
$ | (393,640 | ) | |
$ | (798,476 | ) |
Company contributions to the plan during the years
ended December 31, 2022 and 2021 amounted to $89,192 and $95,527, respectively.
Proteomedix AG
Notes to Financial Statements
Note 7 – Defined Benefit Pension Plan (cont.)
A reconciliation of the beginning and ending balances
of the accumulated benefit obligation is provided in the table below:
| As of December 31, 2020 | |
$ | 3,681,625 | |
| Service cost | |
| 218,298 | |
| Interest cost | |
| 3,563 | |
| Actuarial (gain) loss | |
| (365,169 | ) |
| Benefits paid | |
| (22,148 | ) |
| Contributions | |
| 1,131,779 | |
| Settlements | |
| (1,326,265 | ) |
| Projected benefit obligation as of December 31, 2021 | |
| 3,321,683 | |
| Actuarial (gain)/loss due to assumption changes | |
| (173,094 | ) |
| Actuarial (gain)/loss due to plan experience | |
| (192,074 | ) |
| Accumulated benefit obligation as of December 31, 2021 | |
| 2,956,515 | |
| | |
| | |
| As of December 31, 2021 | |
| 3,321,683 | |
| Service cost | |
| 157,225 | |
| Interest cost | |
| 10,737 | |
| Actuarial (gain) loss | |
| (817,009 | ) |
| Benefits paid | |
| (20,470 | ) |
| Contributions | |
| 220,604 | |
| Settlements | |
| (891,115 | ) |
| Projected benefit obligation as of December 31, 2022 | |
| 1,981,655 | |
| Actuarial (gain)/loss due to assumption changes | |
| (594,309 | ) |
| Actuarial (gain)/loss due to plan experience | |
| (222,700 | ) |
| Accumulated benefit obligation as of December 31, 2022 | |
$ | 1,164,646 | |
A reconciliation of the beginning and ending balances
of the plan assets is provided in the table below:
| As of December 31, 2020 | |
$ | 2,444,559 | |
| Actual return on plan asset | |
| 199,755 | |
| Contributions paid by employer | |
| 95,527 | |
| Ordinary contributions paid by employees | |
| 95,527 | |
| Contributions paid by plan participants | |
| 1,036,252 | |
| Benefits paid | |
| (22,148 | ) |
| Settlements | |
| (1,326,265 | ) |
| As of December 31, 2021 | |
| 2,523,207 | |
| Actual return on plan asset | |
| (333,403 | ) |
| Contributions paid by employer | |
| 89,192 | |
| Ordinary contributions paid by employees | |
| 89,192 | |
| Contributions paid by plan participants | |
| 131,412 | |
| Benefits paid | |
| (20,470 | ) |
| Settlements | |
| (891,115 | ) |
| As of December 31, 2022 | |
$ | 1,588,015 | |
Proteomedix AG
Notes to Financial Statements
Note 7 – Defined Benefit Pension Plan (cont.)
Projected benefit payments for the next five years
as of December 31, 2023 are as follows:
| Years ending December 31, | |
| |
| 2023 | |
$ | - | |
| 2024 | |
| - | |
| 2025 | |
| 87,623 | |
| 2026 | |
| 88,704 | |
| 2027 | |
| 89,786 | |
| Thereafter | |
| 627,421 | |
| Total | |
$ | 893,534 | |
Note 8 – Related Parties
As described in Note 4, the Company has several
borrowings from shareholders and members of its board of directors.
During the years ended December 31, 2022 and 2021,
the Company paid approximately $319,000 and $289,000 to entities owned by a member of executive management and two members of the board
of directors for professional services. These amounts are included within ‘general and administrative’ expenses in the accompanying
statements of comprehensive loss.
Note 9 – Income Taxes
The Company has established deferred tax assets
and liabilities for the recognition of future deductions or taxable amounts and operating loss carry forwards. Deferred federal income
tax expense or benefit is recognized as a result of the change in the deferred tax asset or liability during the year using the currently
enacted tax laws and rates that apply to the period in which they are expected to affect taxable income. Valuation allowances are established,
if necessary, to reduce deferred tax assets to the amounts that will more likely than not be realized.
During the years ended December 31, 2022 and 2021,
a reconciliation of income tax expense at the statutory rate of 24.85% to income tax expense at the Company’s effective tax rate
is as follows:
| | |
2022 | | |
2021 | |
| Income tax benefit at statutory rate | |
$ | (507,647 | ) | |
| (24.85 | )% | |
$ | (559,026 | ) | |
| (24.85 | )% |
| Temporary differences | |
| - | | |
| 0 | % | |
| - | | |
| 0 | % |
| Permanent differences | |
| 59,955 | | |
| 2.94 | % | |
| 41,796 | | |
| 1.85 | % |
| Valuation allowance | |
| 447,692 | | |
| 21.92 | % | |
| 517,230 | | |
| 23.00 | % |
| Provision for federal income taxes | |
$ | - | | |
| 0 | % | |
$ | - | | |
| 0 | % |
At December 31, 2022, the Company had approximately
$18,361,000 of unused net operating loss carry forwards for federal purposes which may be carried forward for up to seven years. Unused
net operating loss carry forwards may provide future tax benefits, although there can be no assurance that these net operating losses
will be realized in the future. The tax benefits of these loss carryforward have been fully offset by a valuation allowance. These losses
may be used to offset future taxable income and, if not fully utilized, begin to expire in the year 2023. The Company’s only significant
deferred tax assets are those related to its net operating loss carryforwards and pension fund obligations. The Company has no significant
deferred tax liabilities as of December 31, 2022 and 2021.
Proteomedix AG
Notes to Financial Statements
Note 9 – Income Taxes (cont.)
The following table details the Company’s
net operating loss carry forwards and the related expected expiration dates as of December 31, 2022.
| Years ending December 31, | |
| |
| 2023 | |
$ | 2,126,000 | |
| 2024 | |
| 2,647,000 | |
| 2025 | |
| 2,928,000 | |
| 2026 | |
| 3,356,000 | |
| 2027 | |
| 3,416,000 | |
| 2028 | |
| 2,240,000 | |
| 2029 | |
| 1,648,000 | |
| Total | |
$ | 18,361,000 | |
The Company’s taxes remain open to review
by the relevant taxing authorities generally for five years after the end of the applicable fiscal year end. As of December 31, 2022 the
only open year subject to examination by taxing authorities is the year ended December 31, 2022.
Note 10 – Subsequent Events
On December 15, 2023,
the Parent and the Company entered into a Share Exchange Agreement which resulted in the Company becoming a wholly owned subsidiary of
the Parent. The consummation of the Share Exchange was subject to customary closing conditions
and closed on December 15, 2023.
Concurrently with the
closing of the Share Exchange Agreement, all outstanding convertibles notes as of December 31, 2022 were converted into 83,114 common
stock of the Company and were then purchased by the Parent.
F-102
Onconetix (NASDAQ:ONCE)
Historical Stock Chart
From Mar 2024 to Apr 2024
Onconetix (NASDAQ:ONCE)
Historical Stock Chart
From Apr 2023 to Apr 2024