0001595097falseNONE00015950972024-01-262024-01-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 26, 2024 |
CORBUS PHARMACEUTICALS HOLDINGS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-37348 |
46-4348039 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
500 River Ridge Drive |
|
Norwood, Massachusetts |
|
02062 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (617) 963-0100 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.0001 per share |
|
CRBP |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On January 26, 2024, Corbus Pharmaceuticals Holdings, Inc. (the “Company”) issued a press release announcing data from the ongoing Phase 1 clinical trial for SYS6002 (CRB-701) conducted by the Company’s development partner, CSPC Pharmaceutical Group, that is being presented at the 2024 American Society of Clinical Oncology Genitourinary Cancers Symposium (the “2024 ASCO GU”) on January 26, 2024. A copy of the press release is attached hereto as Exhibit 99.1.
The Company also updated its presentation used by management to describe its business. A copy of the presentation is furnished as Exhibit 99.2 and is incorporated herein by reference.
The information in this Current Report on Form 8-K under Item 7.01, including the information contained in Exhibits 99.1 and 99.2, is being furnished to the Securities and Exchange Commission (the “SEC”), and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by a specific reference in such filing.
Item 8.01 Other Events.
On January 26, 2024, the Company announced data from the ongoing Phase 1 clinical trial of SYS6002 (CRB-701) conducted by the Company’s development partner, CSPC Pharmaceutical Group, that is being presented at the 2024 ASCO GU on January 26, 2024.
The Phase 1 dose escalation study is being conducted in China and is enrolling patients with metastatic urothelial cancer as well as patients with other solid tumors prospectively confirmed to have nectin-4 positive tumors. The study opened for enrollment in January 2023 and the data presented is through December 2023 from the first eighteen patients reflective of the first six dose cohorts (0.2-3.6 mg/kg).
Safety
•CRB-701 was well-tolerated with the majority of adverse events being grade one or two and reversible.
•No adverse events above grade three were observed.
•There have been no dose discontinuations or reductions in the study to date. There has been a singular participant that experienced a temporary dose interruption.
•The dose escalation is ongoing at cohort 7 (4.5 mg/kg).
•No cases of drug-related peripheral neuropathy or skin rash have been reported to date.
PK
•Single dose PK suggested that TAb, ADC and MMAE increase in a dose proportional manner.
•No obvious accumulation was observed on cycle 3, day 1.
•When compared to the exposures achieved with enfortumab vedotin (EV) at 1.25 mg/kg Q1W x21 days, CRB-701 (SYS6002) consistently demonstrated lower free MMAE concentrations.
Efficacy
•Dose level 5 (2.7 mg/kg and above) represents the predicted therapeutically relevant doses based on allometric scaling.
•A mixed tumor population (n=7) receiving doses of 2.7 mg/kg or 3.6 mg/kg demonstrated an ORR of 43% (3 partial responses - 2 unconfirmed and one non responding participant with no-nectin-4 expression) and a disease control rate of 71%.
•The longest observed response to date is 11 cycles (~10 months) and ongoing.
•All nectin-4 positive mUC and cervical patients at doses ≥ 2.7 mg/kg that were assessable at the time of the December 2023 data-cut off demonstrated levels of disease control and represent the CRB-701 (SYS6002) responsive population to date.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Corbus Pharmaceuticals Holdings, Inc. |
|
|
|
|
Date: |
January 26, 2024 |
By: |
/s/ Yuval Cohen |
|
|
|
Name: Yuval Cohen
Title: Chief Executive Officer |
Exhibit 99.1
CRB-701 (SYS6002) A Next Generation Nectin-4 Targeting Antibody Drug Conjugate Demonstrates Encouraging Safety and Efficacy in Patients with Nectin-4 Positive Tumors in First-In-Human Study Presented at ASCO-GU 2024
•Q3W schedule of CRB-701 (SYS6002) demonstrates a 43% ORR and 71% DCR at predicted therapeutically relevant doses
•All assessable nectin-4 positive study participants with mUC and cervical cancer treated at or above this dose demonstrated some level of disease control
•No dose limiting toxicities (DLTs) have been observed to-date up to 3.6 mg/kg (cohort 6) with further escalation at 4.5 mg/kg ongoing
•No cases of peripheral neuropathy or skin rash have been observed to date
•Cohort 6 is the first cohort selected for dose expansion
Norwood, MA, January 26, 2024 (GLOBE NEWSWIRE) -- Corbus Pharmaceuticals Holdings, Inc. (NASDAQ: CRBP) (“Corbus” or the “Company”), today announced that data from the first-in-human clinical study of CRB-701 (SYS6002) is being presented as a poster by the Company’s development partner CSPC Pharmaceutical Group at the 2024 American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU). The Phase 1 dose escalation study is being conducted in China and is enrolling participants with metastatic urothelial cancer (mUC) as well as participants with other solid tumors prospectively confirmed to have nectin-4 positive tumors. The study opened for enrollment in January 2023 and data through December 2023 from the first eighteen participants reflective of the first six dose cohorts (0.2-3.6mg/kg) will be shared.
The poster is titled Phase 1 Dose Escalation of SYS6002(CRB-701), a Next Generation Nectin-4 Targeting Antibody Drug Conjugate by DingWei Ye, et al and is being presented today at the poster session between 11:30 am-1pm PST. The poster will also be available on the Corbus website at the start of the poster presentation.
Safety
•CRB-701 was well-tolerated with the majority of adverse events being grade one or two and reversible.
•No adverse events above grade three were observed.
•There have been no dose discontinuations or reductions in the study to date. There has been a singular participant that experienced a temporary dose interruption.
•The dose escalation is ongoing at cohort 7 (4.5 mg/kg).
•No cases of drug-related peripheral neuropathy or skin rash have been reported to date.
PK
•Single dose PK suggested that TAb, ADC and MMAE increase in an approximate dose proportional manner.
•No obvious accumulation was observed on cycle 3, day 1.
•When compared to the exposures achieved with enfortumab vedotin (EV) at 1.25 mg/kg Q1W x21 days, CRB-701 (SYS6002) consistently demonstrated lower free MMAE concentrations.
Efficacy
•Dose level 5 (2.7 mg/kg) and above represents the predicted therapeutically relevant doses based on allometric scaling.
•A mixed tumor population (n=7) receiving doses of 2.7 mg/kg or 3.6 mg/kg demonstrated an ORR of 43% (3 partial responses -2 unconfirmed and one non responding participant with no-nectin-4 expression ) and a disease control rate of 71%.
•The longest observed response to date is 11 cycles (~10 months) and ongoing.
•All nectin-4 positive mUC and cervical patients at doses ≥ 2.7 mg/kg that were assessable at the time of the December 2023 data-cut off demonstrated levels of disease control and represent the CRB-701 (SYS6002) responsive population to date.
Dr. Yuval Cohen Chief Executive Office of Corbus commented, “CRB-701 with its novel antibody and next generation linker technology, appears to have a differentiated PK profile compared to EV, with a current safety profile devoid of peripheral neuropathy and skin rash, both dose limiting toxicities for EV. This could translate into meaningful benefits for mUC patients and other nectin-4 positive solid tumors such as cervical cancer.” In reviewing the emerging profile of CRB-701 with one of the preeminent experts in GU cancers, Dr Daniel P. Petrylack M.D., Professor of Medicine and Urology at Yale School of Medicine, Dr. Petrylak shared that “the clinical responses in nectin-4 positive mUC and cervical cancer patients are encouraging and the early clinical safety provides the first evidence that CRB-701 has clinical activity in multiple nectin-4 expressing tumors. This justifies further investigation into the safety and efficacy of this promising compound.” Dr. Cohen concluded, “As the current clinical study continues to progress in China with our partner CSPC, we at Corbus are looking forward to commencing our clinical study in the US in Q1 2024 under an already open IND. We are grateful to CSPC for the work that has gone into conducting this ongoing study and to the clinicians and study participants."
Dose escalation and expansion are ongoing and additional data presentations are planned for later this year.
About CRB-701
CRB-701 (SYS6002) is a next-generation antibody-drug-conjugate (ADC) targeting nectin-4, that contains a site-specific, cleavable linker and a homogenous drug antibody ratio of 2, using MMAE as the payload. Nectin-4 is a clinically validated, tumor-associated antigen in urothelial cancer. The Nectin-4 ADC PADCEV® (enfortumab vedotin-ejfv) is approved for use in late metastatic urothelial cancer and recently received an expanded label under an accelerated approval from the Food and Drug Administration for use in combination with KEYTRUDA® for patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-containing chemotherapy.
About Corbus
Corbus Pharmaceuticals Holdings, Inc. is a precision oncology company with a diversified portfolio and is committed to helping people defeat serious illness by bringing innovative scientific approaches to well understood biological pathways. Corbus’ pipeline includes CRB-701, a next generation antibody drug conjugate that targets the expression of Nectin-4 on cancer cells to release a cytotoxic payload, CRB-601, an anti-integrin monoclonal antibody which blocks the activation of TGFβ expressed on cancer cells, and CRB-913, a highly peripherally restricted CB1 inverse agonist for the treatment of obesity. Corbus is headquartered in Norwood, Massachusetts. For more information on Corbus, visit corbuspharma.com. Connect with us on Twitter, LinkedIn and Facebook.
Forward-Looking Statements
This press release contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company's restructuring, trial results, product development, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statement that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management's current beliefs and assumptions.
These statements may be identified by the use of forward-looking expressions, including, but not limited to, "expect," "anticipate," "intend," "plan," "believe," "estimate," "potential,” "predict," "project," "should," "would" and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors on our operations, clinical development plans and timelines, which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company's filings with the Securities and Exchange Commission. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
All product names, logos, brands and company names are trademarks or registered trademarks of their respective owners. Their use does not imply affiliation or endorsement by these companies.
INVESTOR CONTACT:
Sean Moran
Chief Financial Officer
Corbus Pharmaceuticals
smoran@corbuspharma.com
Bruce Mackle
Managing Director
LifeSci Advisors, LLC
bmackle@lifesciadvisors.com

Connecting Innovation to Purpose Corporate Presentation January 26 2024 NASDAQ: CRBP • CorbusPharma.com • @CorbusPharma Exhibit 99.2

Forward-Looking Statements This presentation contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company’s restructuring, trial results, product development, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statements that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions. These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors, including the potential impact of the recent COVID-19 pandemic and the potential impact of sustained social distancing efforts, on our operations, clinical development plans and timelines, which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the Securities and Exchange Commission. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this presentation. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. All product names, logos, brands and company names are trademarks or registered trademarks of their respective owners. Their use does not imply affiliation or endorsement by these companies.
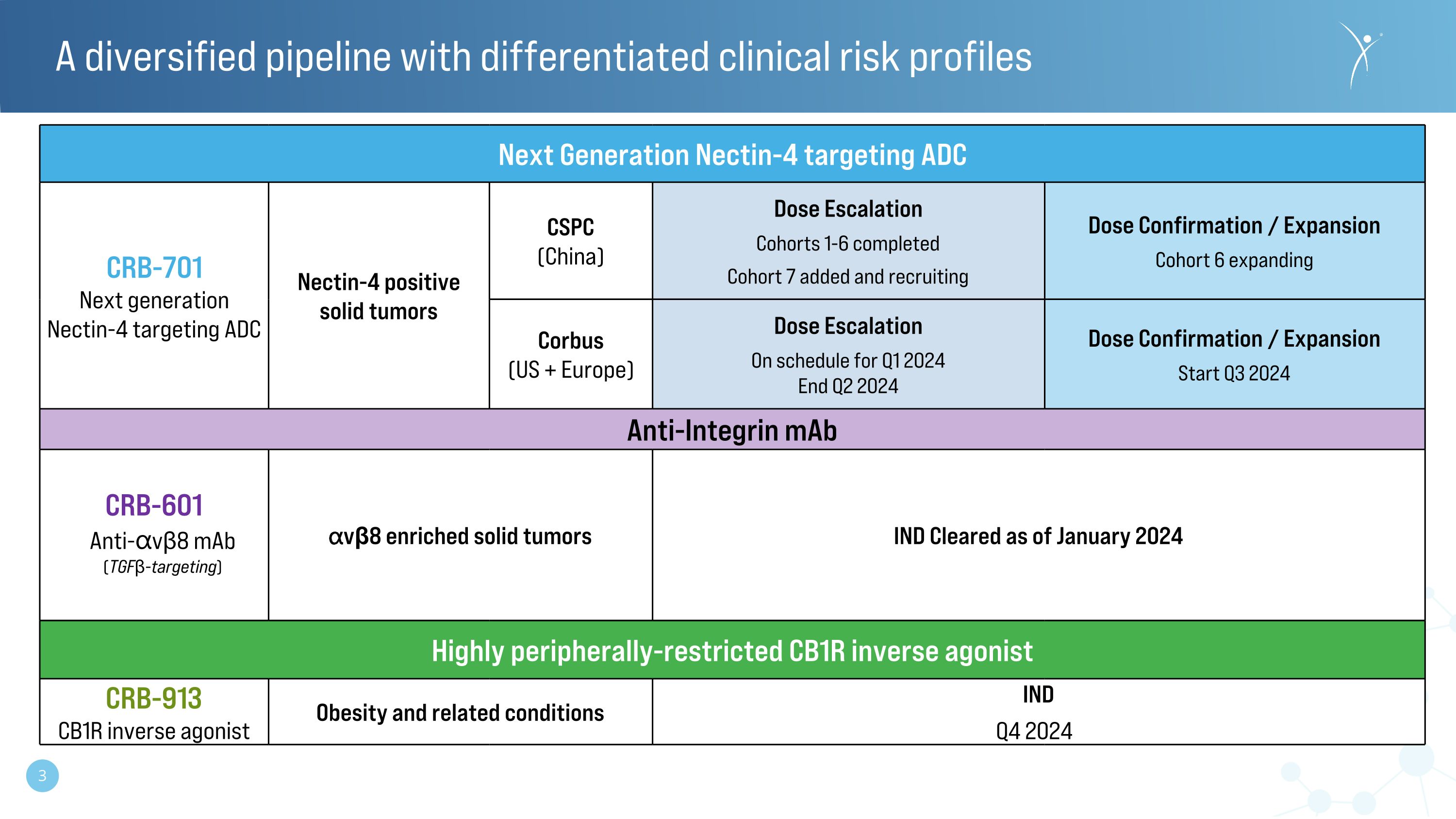
Next Generation Nectin-4 targeting ADC CRB-701 Next generation Nectin-4 targeting ADC Nectin-4 positive�solid tumors CSPC�(China) Dose Escalation Cohorts 1-6 completed Cohort 7 added and recruiting Dose Confirmation / Expansion Cohort 6 expanding Corbus�(US + Europe) Dose Escalation On schedule for Q1 2024 End Q2 2024 Dose Confirmation / Expansion Start Q3 2024 Anti-Integrin mAb CRB-601�Anti-⍺vβ8 mAb�(TGFβ-targeting) ⍺vβ8 enriched solid tumors IND Cleared as of January 2024 Highly peripherally-restricted CB1R inverse agonist CRB-913 CB1R inverse agonist Obesity and related conditions IND Q4 2024) A diversified pipeline with differentiated clinical risk profiles

CRB-701�Next Gen �Nectin-4 Targeting ADC
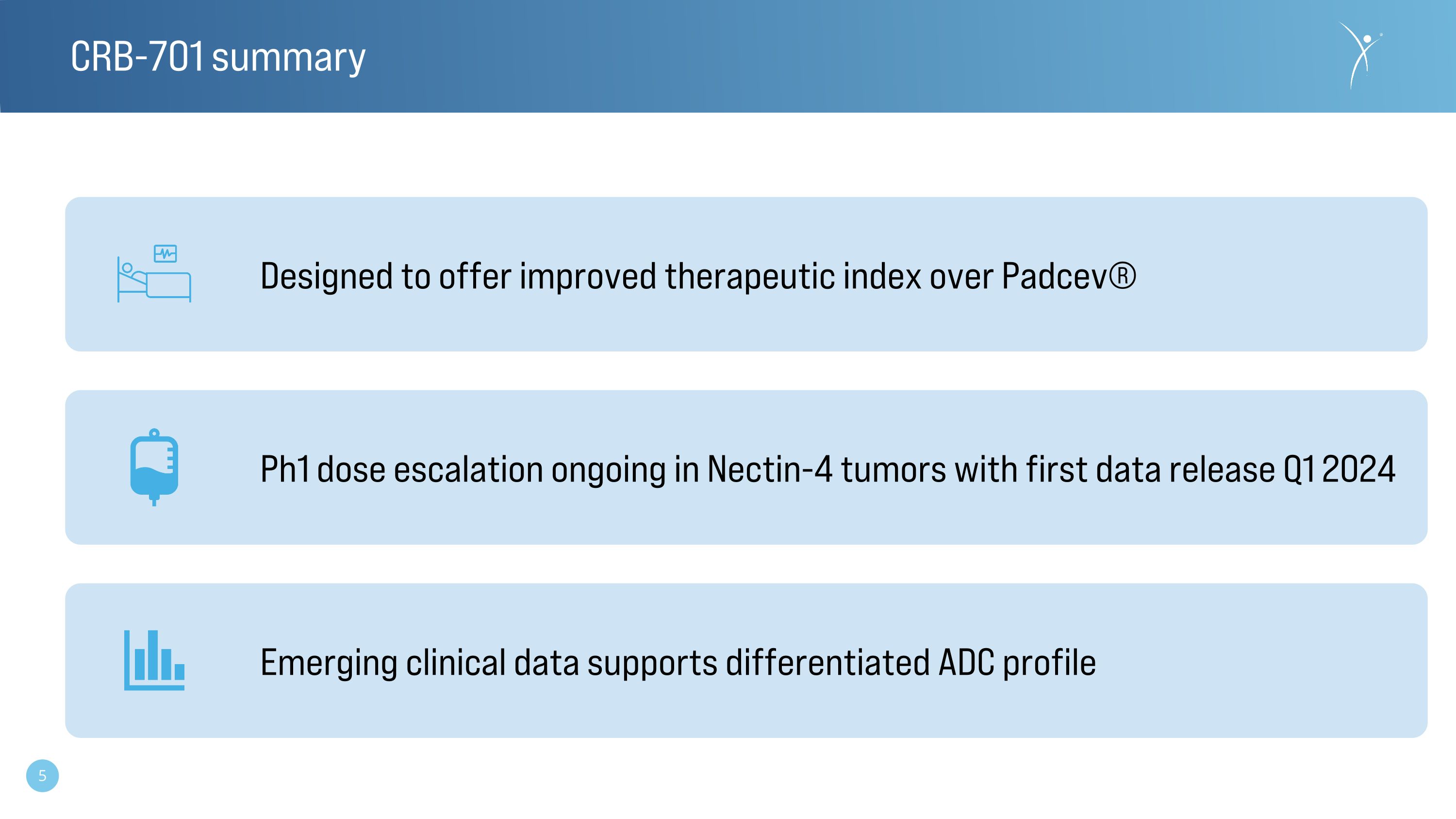
CRB-701 summary Designed to offer improved therapeutic index over Padcev® Ph1 dose escalation ongoing in Nectin-4 tumors with first data release Q1 2024 Emerging clinical data supports differentiated ADC profile
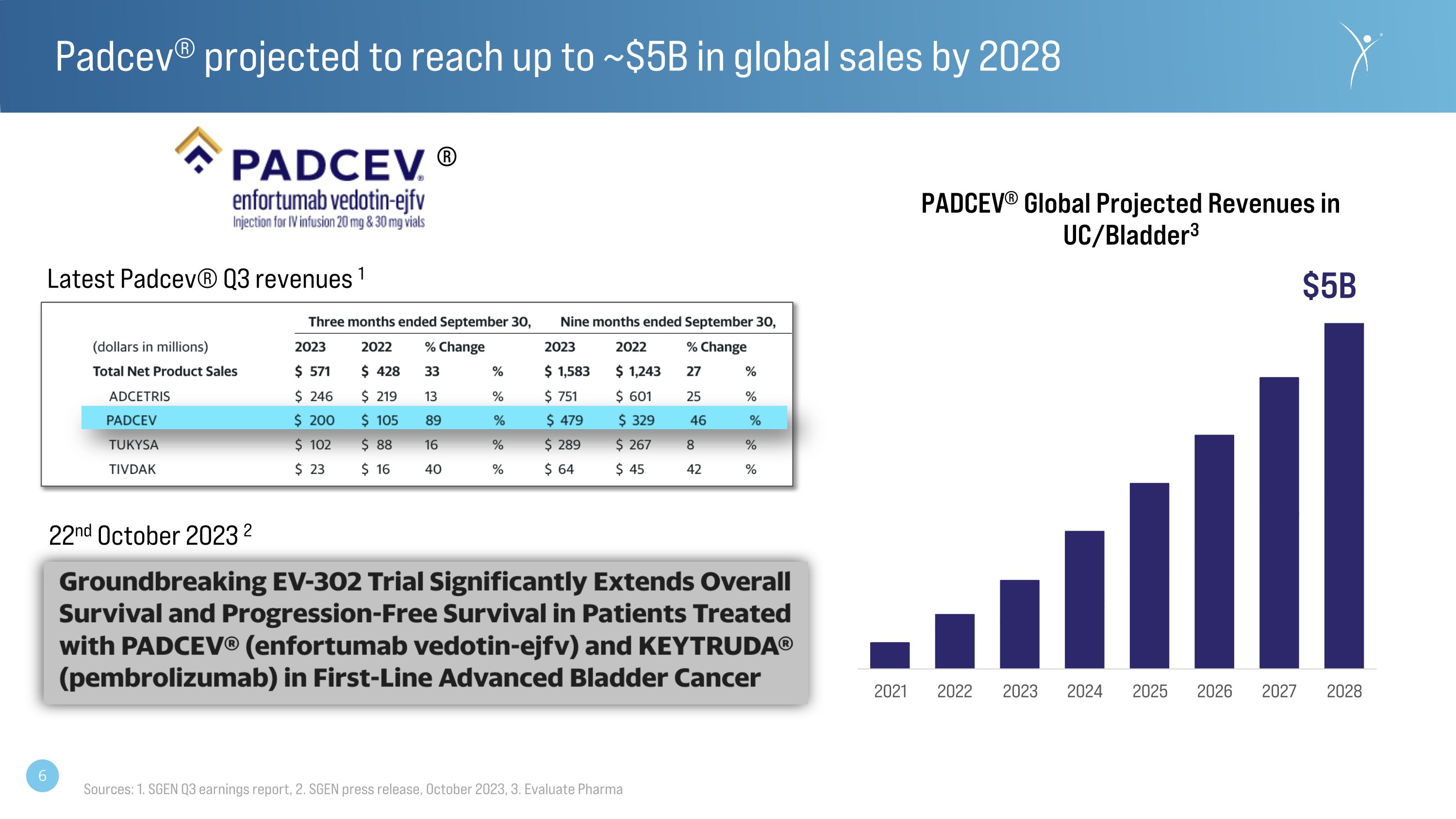
Padcev® projected to reach up to ~$5B in global sales by 2028 $5B ® Latest Padcev® Q3 revenues 1 22nd October 2023 2 Sources: 1. SGEN Q3 earnings report, 2. SGEN press release, October 2023, 3. Evaluate Pharma
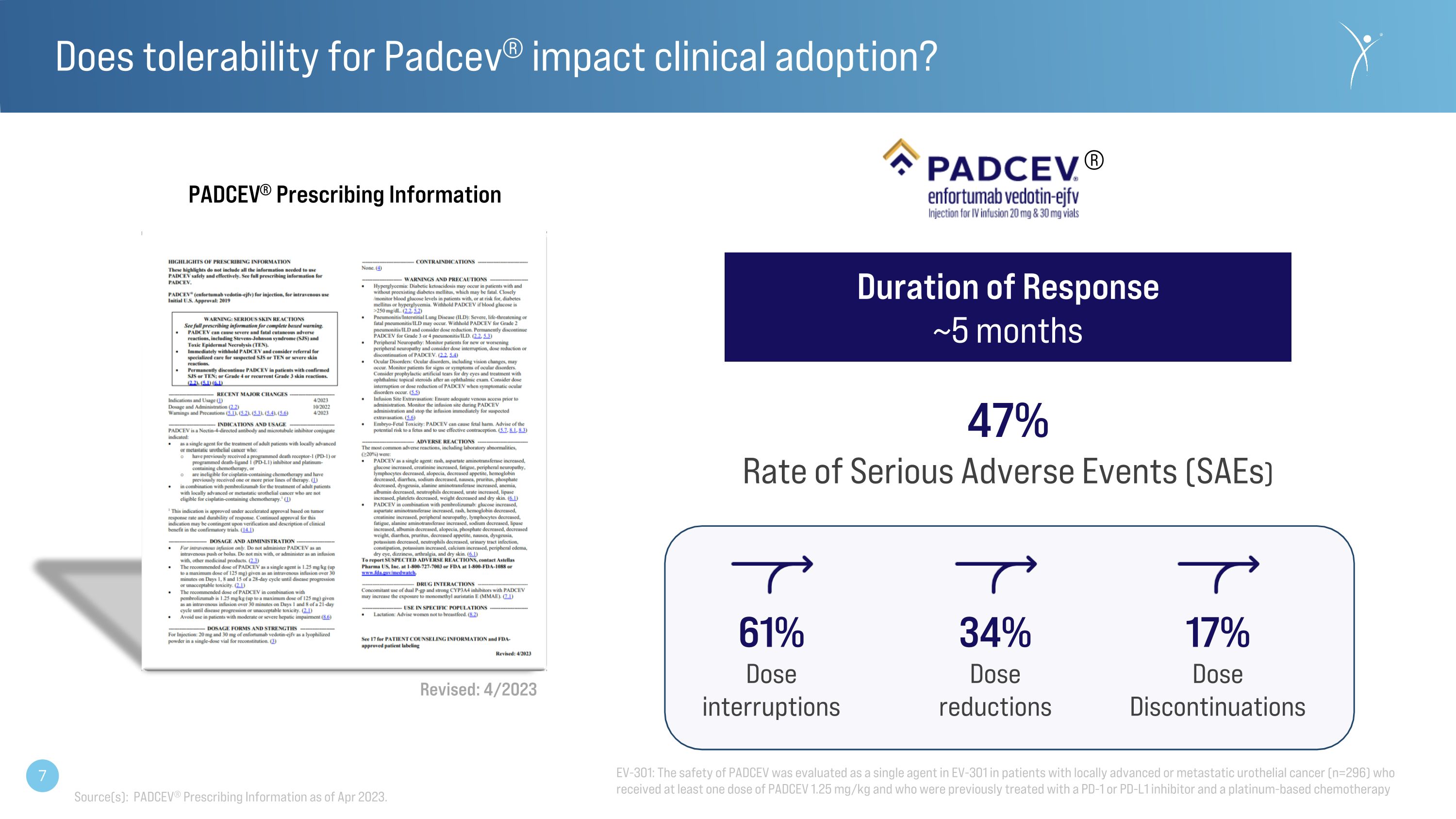
Does tolerability for Padcev® impact clinical adoption? ® Duration of Response ~5 months 47% Rate of Serious Adverse Events (SAEs) 61% Dose interruptions 34% Dose reductions 17% Dose Discontinuations Revised: 4/2023 PADCEV® Prescribing Information Source(s): PADCEV® Prescribing Information as of Apr 2023. EV-301: The safety of PADCEV was evaluated as a single agent in EV-301 in patients with locally advanced or metastatic urothelial cancer (n=296) who received at least one dose of PADCEV 1.25 mg/kg and who were previously treated with a PD-1 or PD-L1 inhibitor and a platinum-based chemotherapy
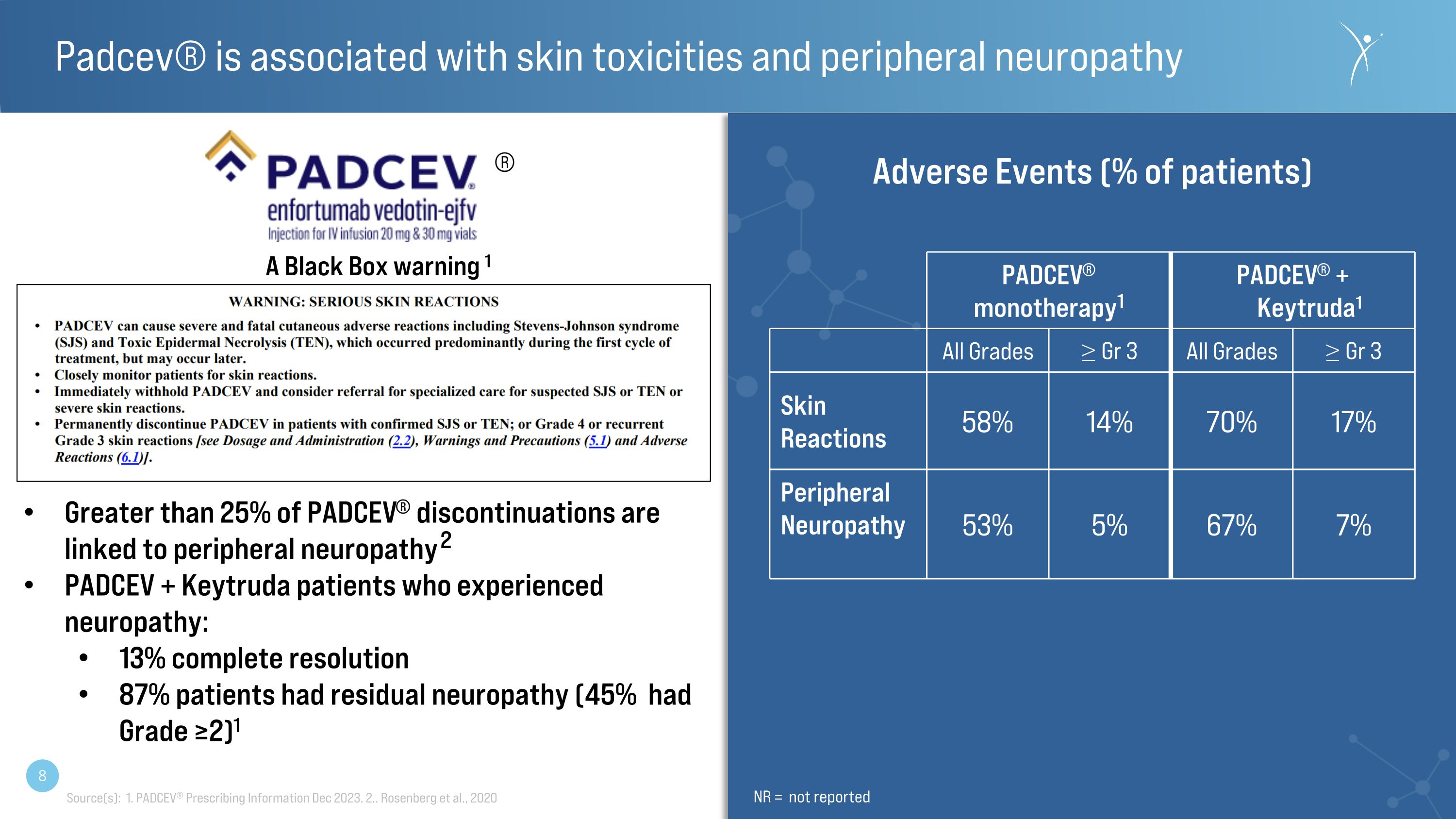
Padcev® is associated with skin toxicities and peripheral neuropathy PADCEV® monotherapy1 PADCEV® + Keytruda1 All Grades ≥ Gr 3 All Grades ≥ Gr 3 Skin�Reactions 58% 14% 70% 17% Peripheral Neuropathy 53% 5% 67% 7% Adverse Events (% of patients) Source(s): 1. PADCEV® Prescribing Information Dec 2023. 2.. Rosenberg et al., 2020 ® A Black Box warning 1 NR = not reported Greater than 25% of PADCEV® discontinuations are linked to peripheral neuropathy 2 PADCEV + Keytruda patients who experienced neuropathy: 13% complete resolution 87% patients had residual neuropathy (45% had Grade ≥2)1
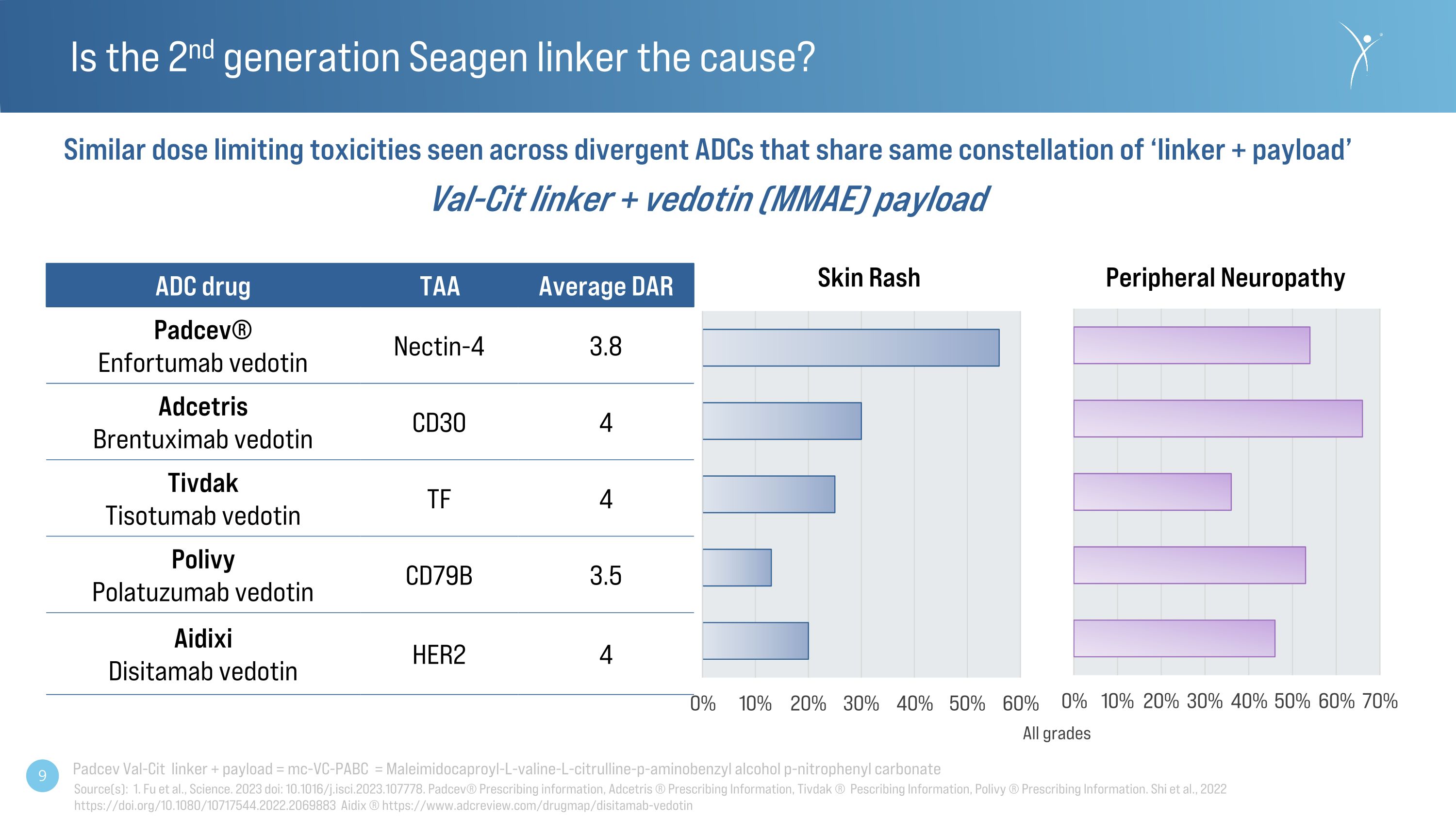
Is the 2nd generation Seagen linker the cause? Skin Rash Peripheral Neuropathy Similar dose limiting toxicities seen across divergent ADCs that share same constellation of ‘linker + payload’ Val-Cit linker + vedotin (MMAE) payload All grades ADC drug TAA Average DAR Padcev® Enfortumab vedotin Nectin-4 3.8 Adcetris�Brentuximab vedotin CD30 4 Tivdak�Tisotumab vedotin TF 4 Polivy Polatuzumab vedotin CD79B 3.5 Aidixi�Disitamab vedotin HER2 4 Source(s): 1. Fu et al., Science. 2023 doi: 10.1016/j.isci.2023.107778. Padcev® Prescribing information, Adcetris ® Prescribing Information, Tivdak ® Pescribing Information, Polivy ® Prescribing Information. Shi et al., 2022 https://doi.org/10.1080/10717544.2022.2069883 Aidix ® https://www.adcreview.com/drugmap/disitamab-vedotin Padcev Val-Cit linker + payload = mc-VC-PABC = Maleimidocaproyl-L-valine-L-citrulline-p-aminobenzyl alcohol p-nitrophenyl carbonate
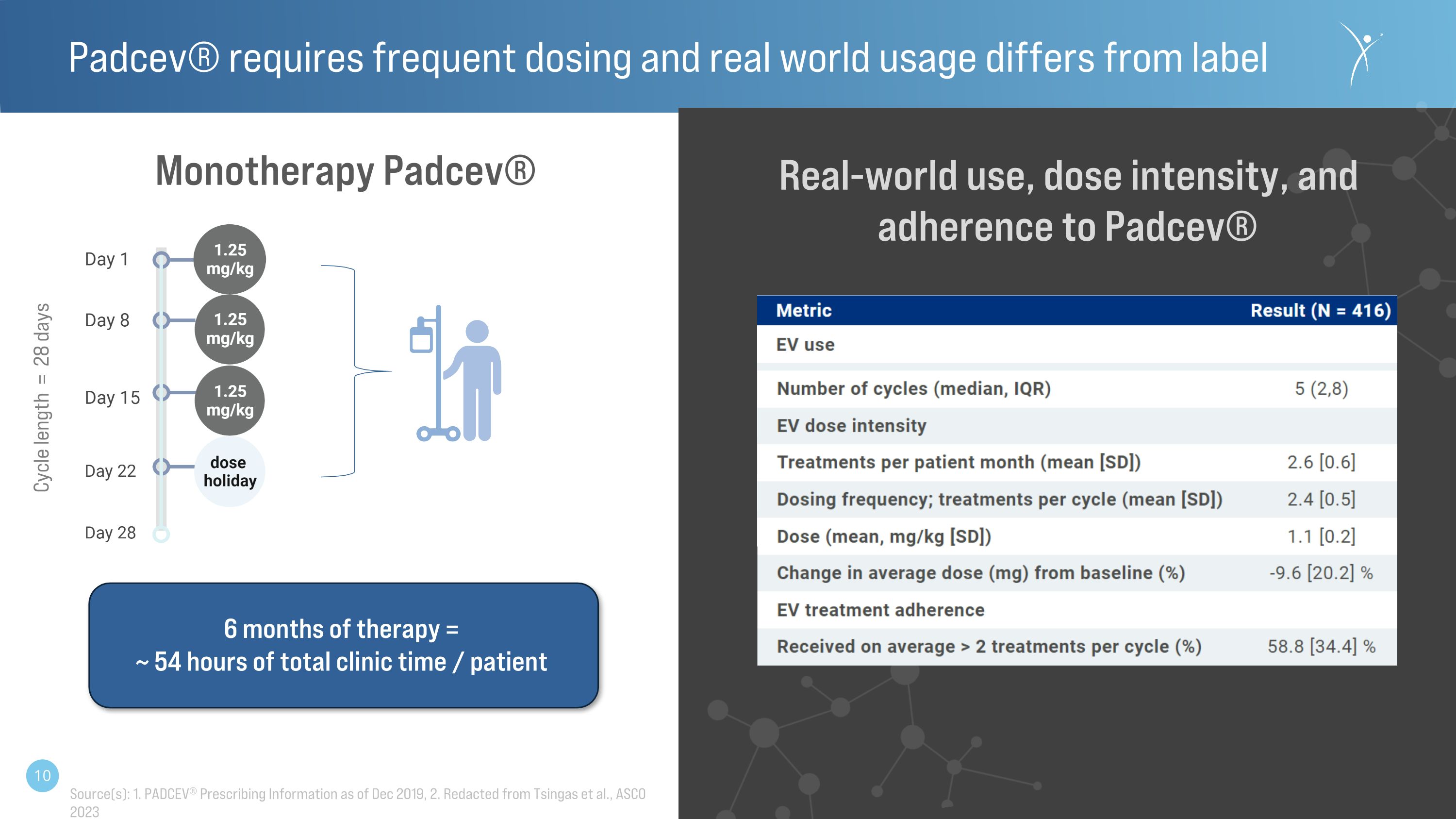
Monotherapy Padcev® Source(s): 1. PADCEV® Prescribing Information as of Dec 2019, 2. Redacted from Tsingas et al., ASCO 2023 6 months of therapy = ~ 54 hours of total clinic time / patient Real-world use, dose intensity, and adherence to Padcev® Padcev® requires frequent dosing and real world usage differs from label Cycle length = 28 days
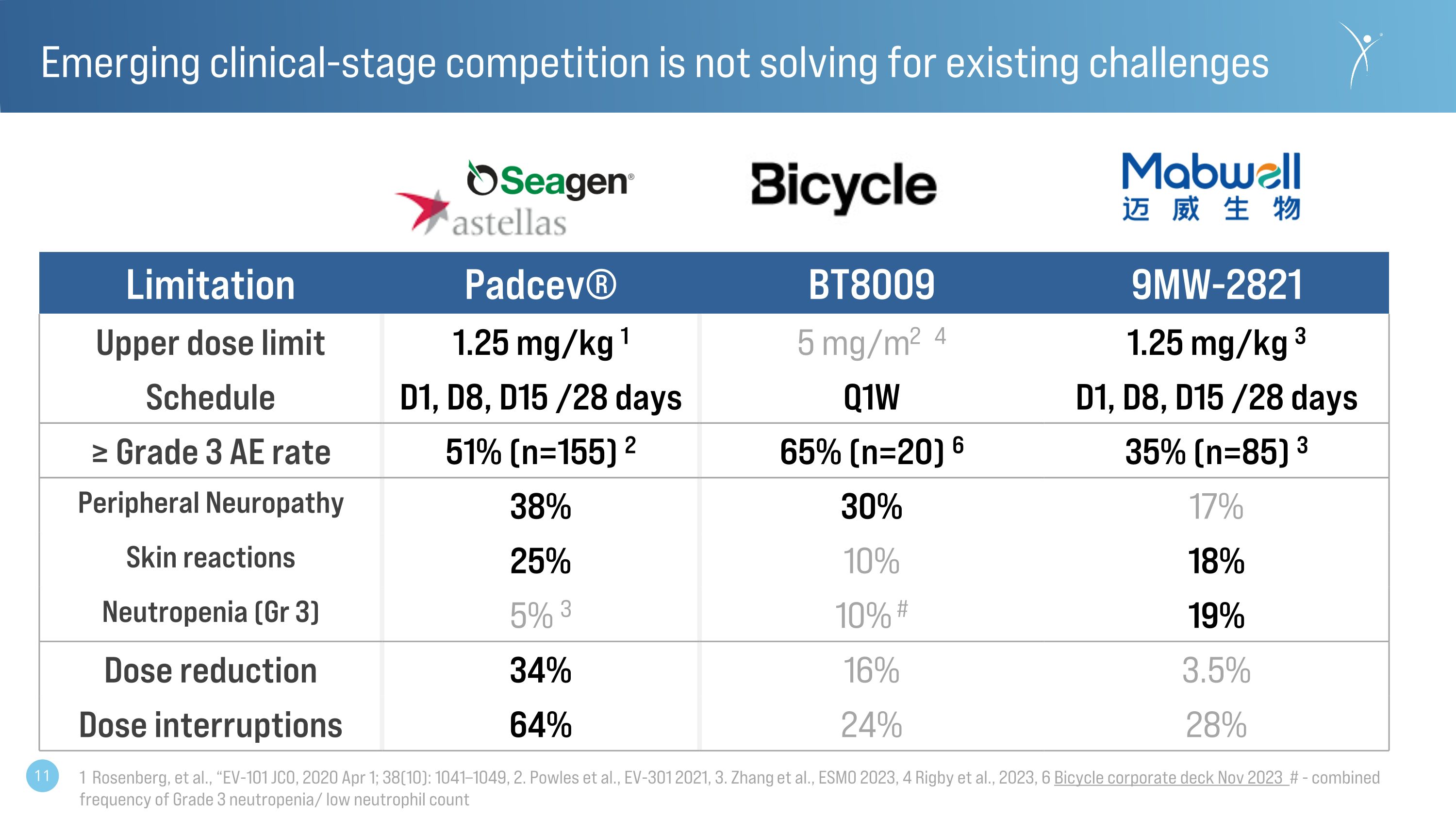
Emerging clinical-stage competition is not solving for existing challenges Limitation Padcev® BT8009 9MW-2821 Upper dose limit 1.25 mg/kg 1 5 mg/m2 4 1.25 mg/kg 3 Schedule D1, D8, D15 /28 days Q1W D1, D8, D15 /28 days ≥ Grade 3 AE rate 51% (n=155) 2 65% (n=20) 6 35% (n=85) 3 Peripheral Neuropathy 38% 30% 17% Skin reactions 25% 10% 18% Neutropenia (Gr 3) 5% 3 10% # 19% Dose reduction 34% 16% 3.5% Dose interruptions 64% 24% 28% 1 Rosenberg, et al., “EV-101 JCO, 2020 Apr 1; 38(10): 1041–1049, 2. Powles et al., EV-301 2021, 3. Zhang et al., ESMO 2023, 4 Rigby et al., 2023, 6 Bicycle corporate deck Nov 2023 # - combined frequency of Grade 3 neutropenia/ low neutrophil count
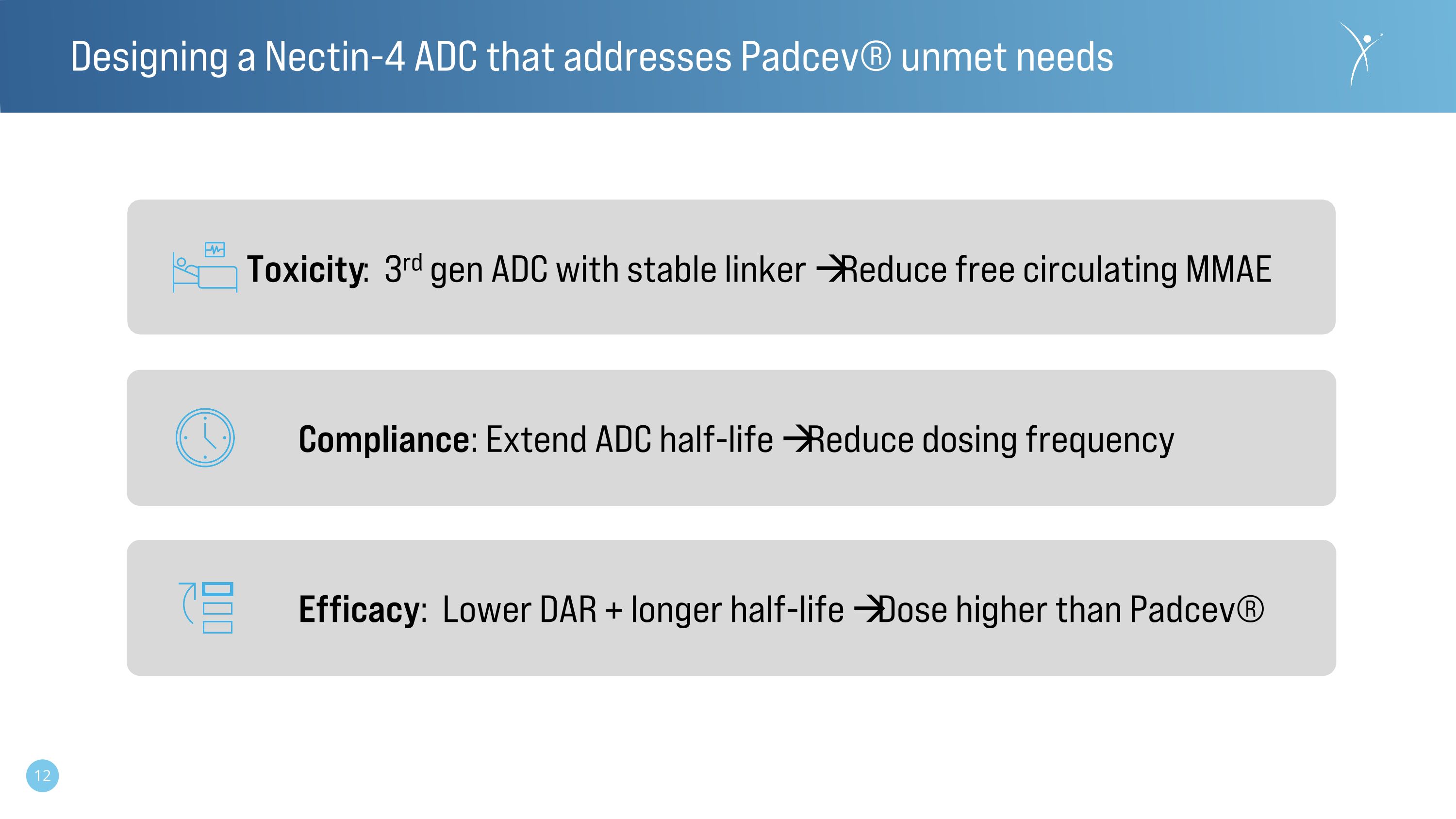
Designing a Nectin-4 ADC that addresses Padcev® unmet needs Toxicity: 3rd gen ADC with stable linker Reduce free circulating MMAE Compliance: Extend ADC half-life Reduce dosing frequency Efficacy: Lower DAR + longer half-life Dose higher than Padcev®
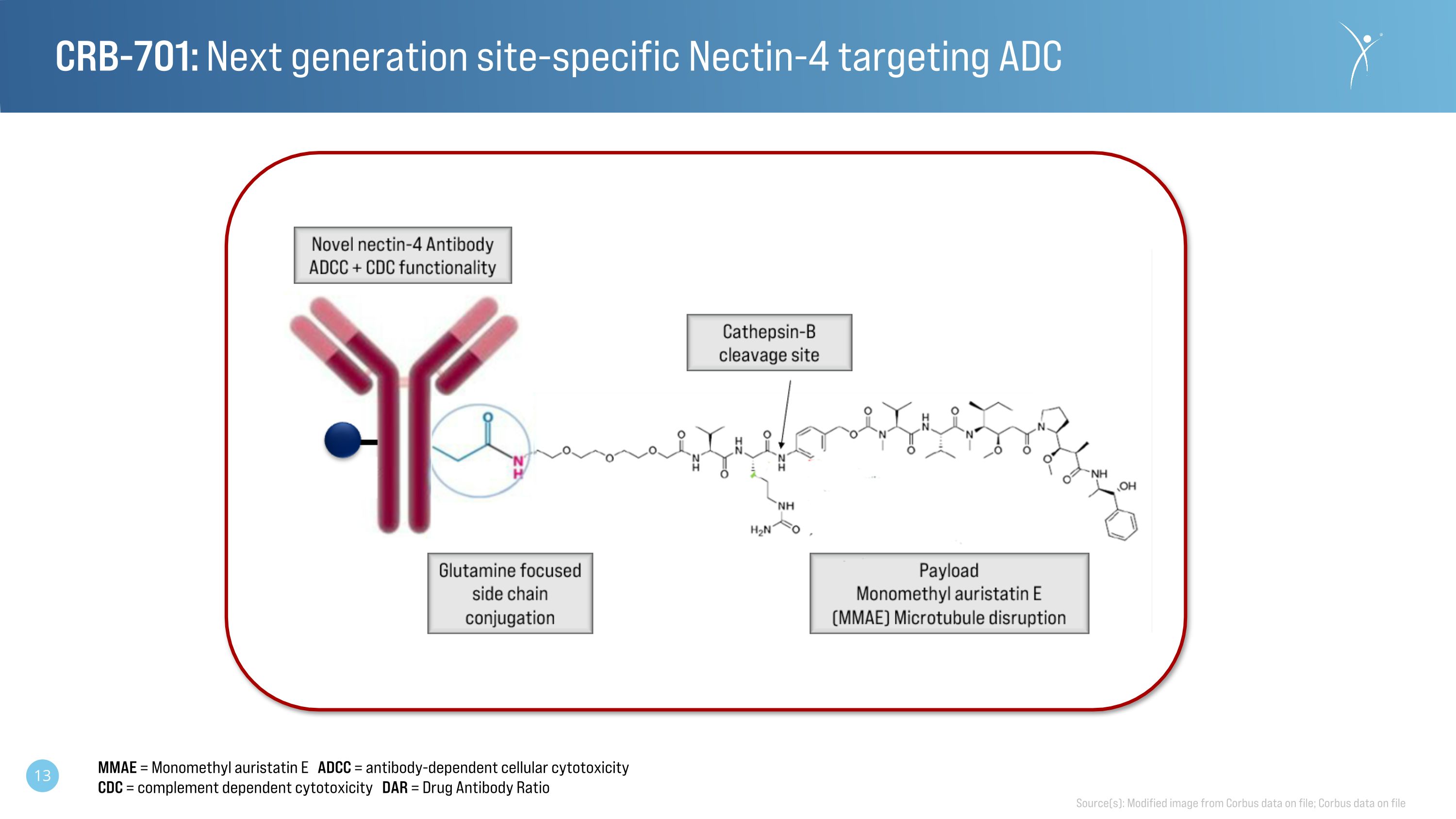
CRB-701: Next generation site-specific Nectin-4 targeting ADC Source(s): Modified image from Corbus data on file; Corbus data on file MMAE = Monomethyl auristatin E ADCC = antibody-dependent cellular cytotoxicity CDC = complement dependent cytotoxicity DAR = Drug Antibody Ratio
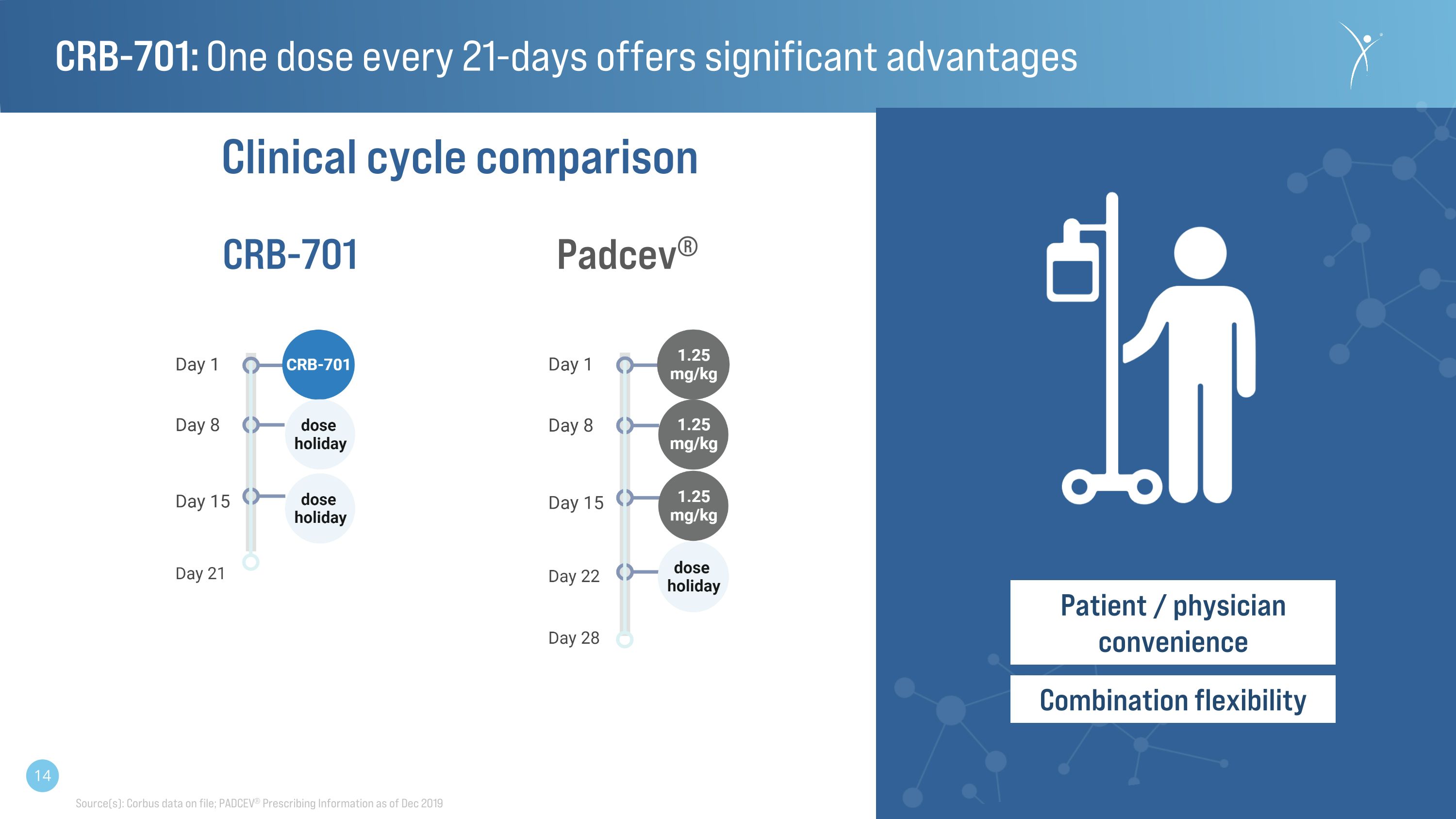
CRB-701: One dose every 21-days offers significant advantages Padcev® CRB-701 Patient / physician convenience Combination flexibility Clinical cycle comparison Source(s): Corbus data on file; PADCEV® Prescribing Information as of Dec 2019
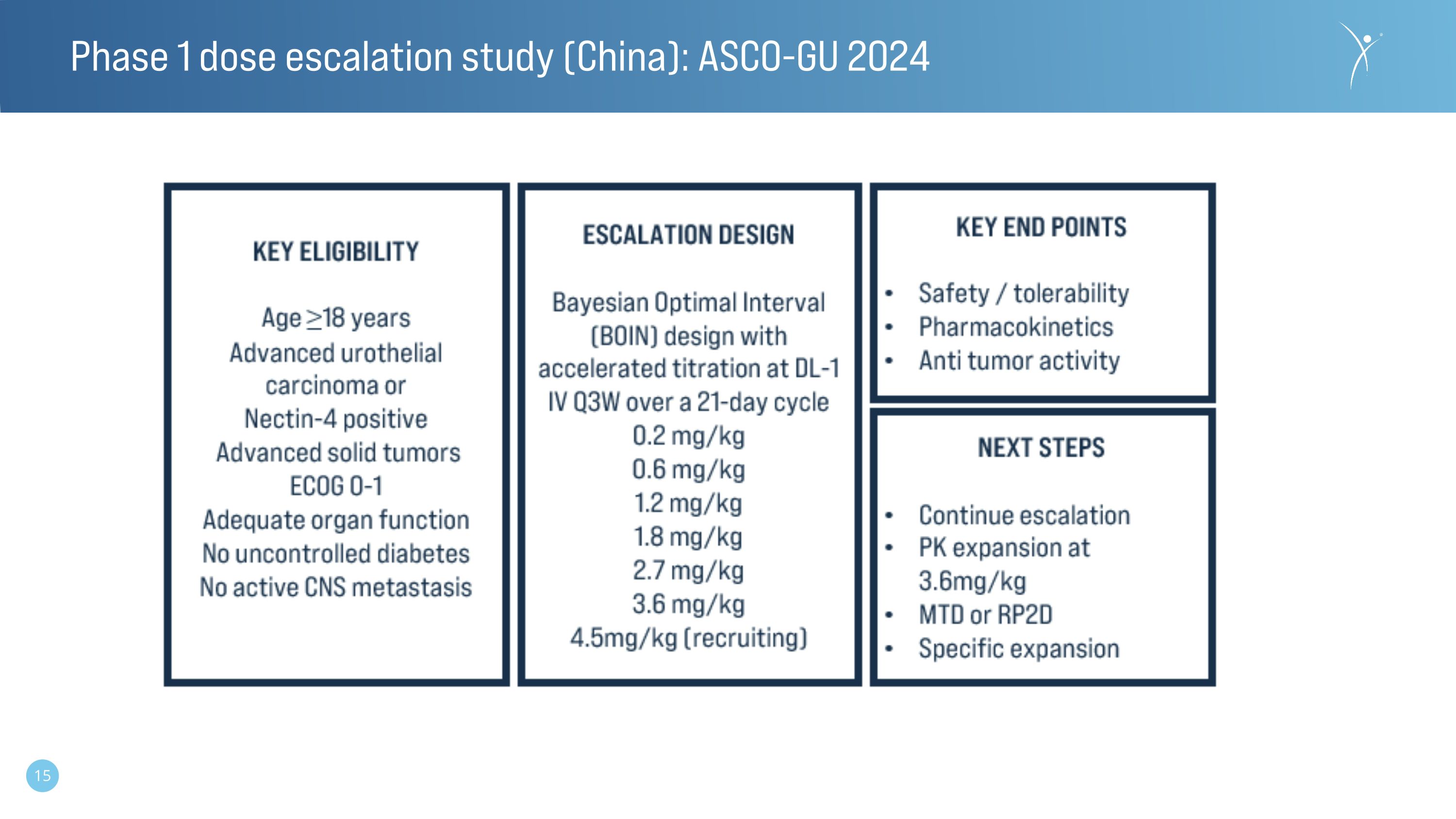
Phase 1 dose escalation study (China): ASCO-GU 2024
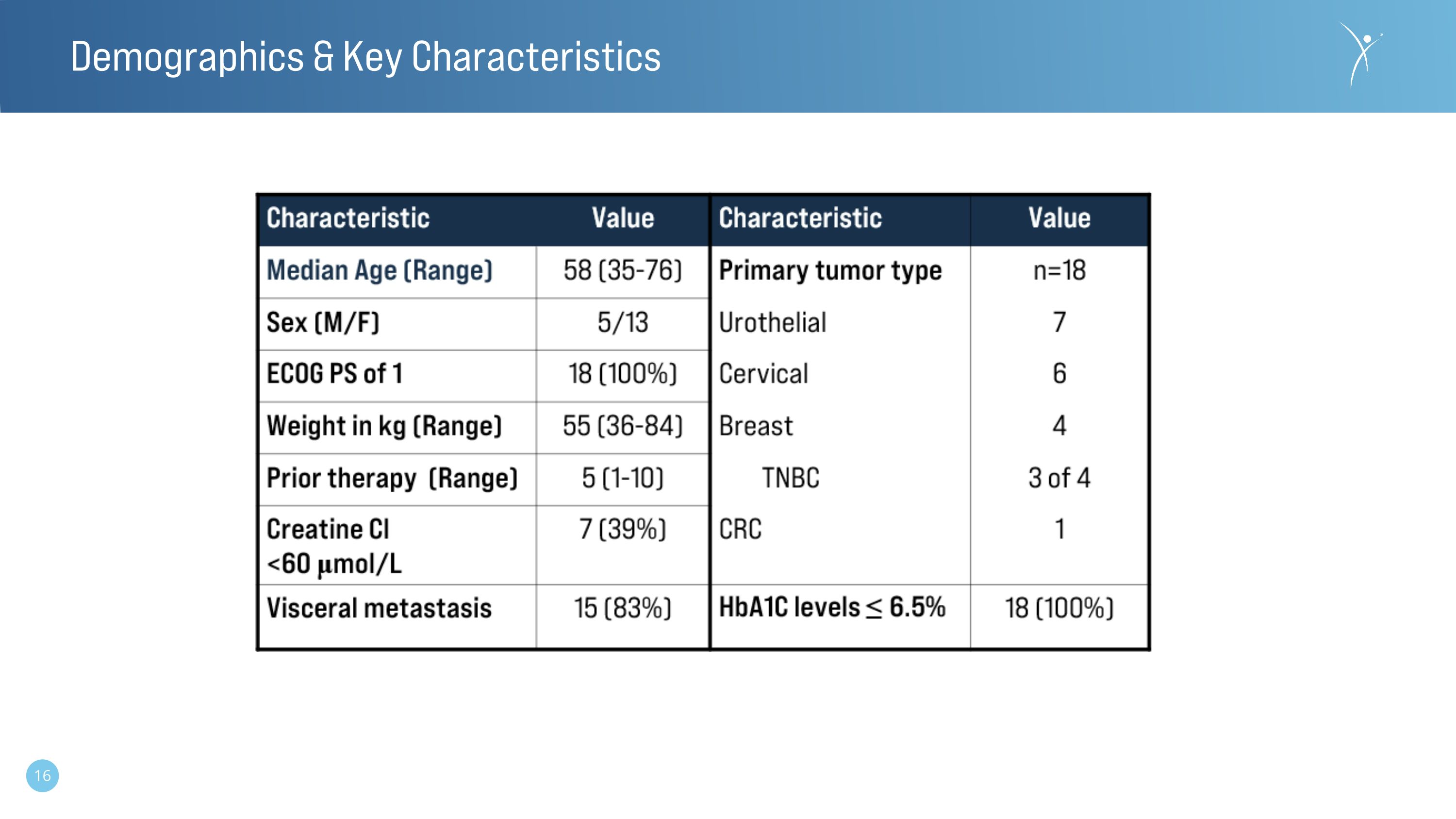
Demographics & Key Characteristics
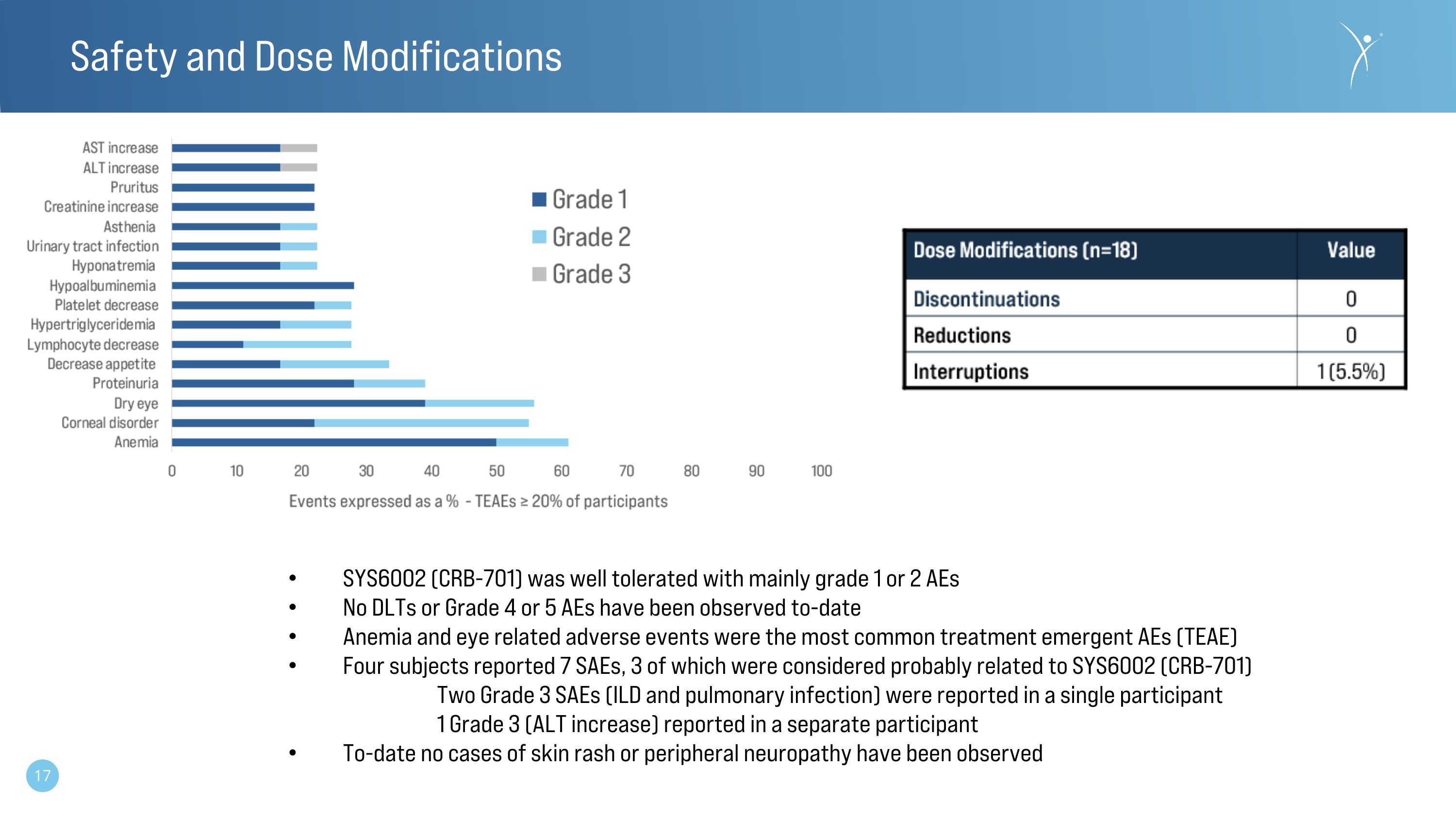
Safety and Dose Modifications SYS6002 (CRB-701) was well tolerated with mainly grade 1 or 2 AEs No DLTs or Grade 4 or 5 AEs have been observed to-date Anemia and eye related adverse events were the most common treatment emergent AEs (TEAE) Four subjects reported 7 SAEs, 3 of which were considered probably related to SYS6002 (CRB-701) Two Grade 3 SAEs (ILD and pulmonary infection) were reported in a single participant 1 Grade 3 (ALT increase) reported in a separate participant To-date no cases of skin rash or peripheral neuropathy have been observed
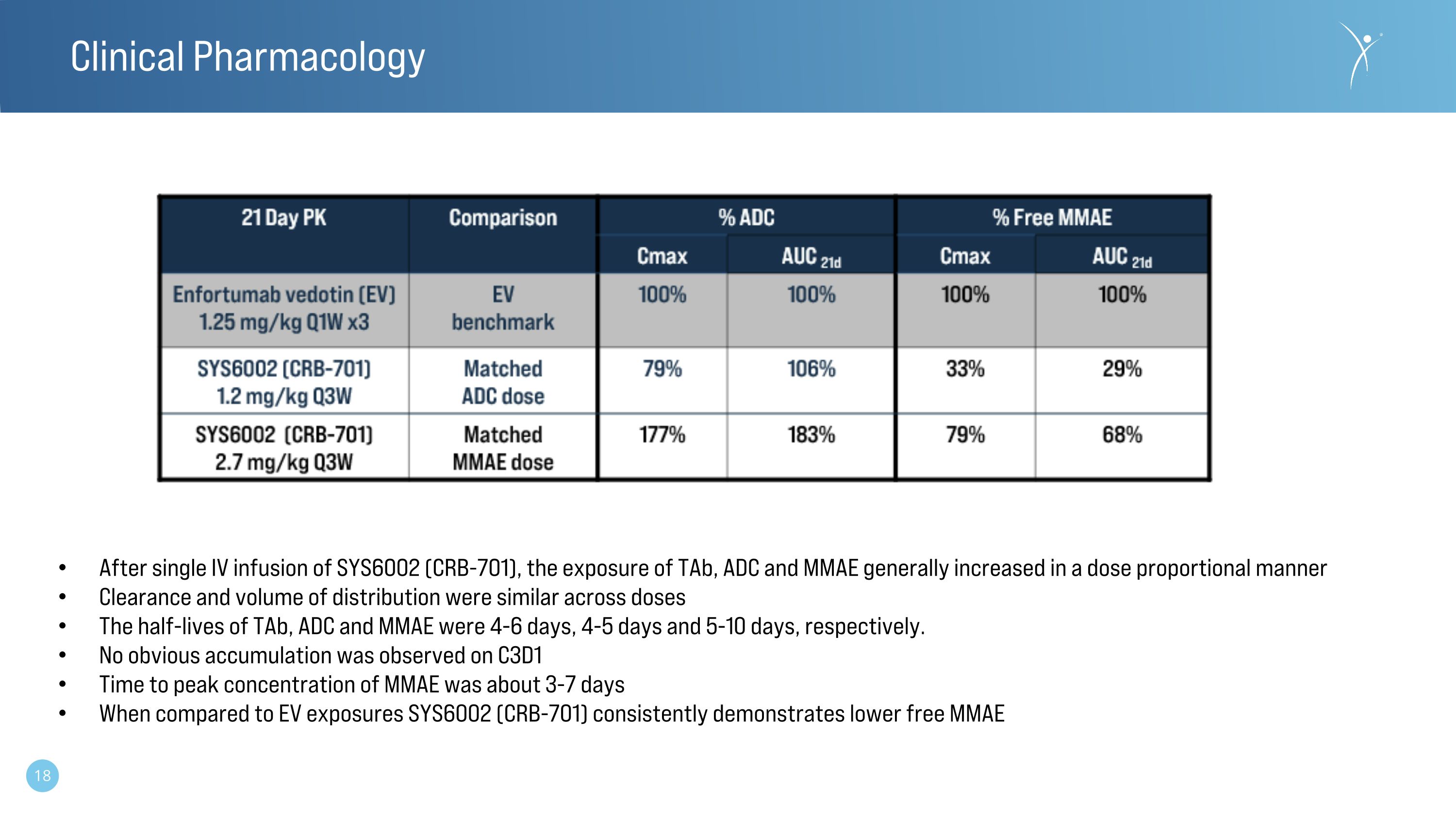
Clinical Pharmacology After single IV infusion of SYS6002 (CRB-701), the exposure of TAb, ADC and MMAE generally increased in a dose proportional manner Clearance and volume of distribution were similar across doses The half-lives of TAb, ADC and MMAE were 4-6 days, 4-5 days and 5-10 days, respectively. No obvious accumulation was observed on C3D1 Time to peak concentration of MMAE was about 3-7 days When compared to EV exposures SYS6002 (CRB-701) consistently demonstrates lower free MMAE
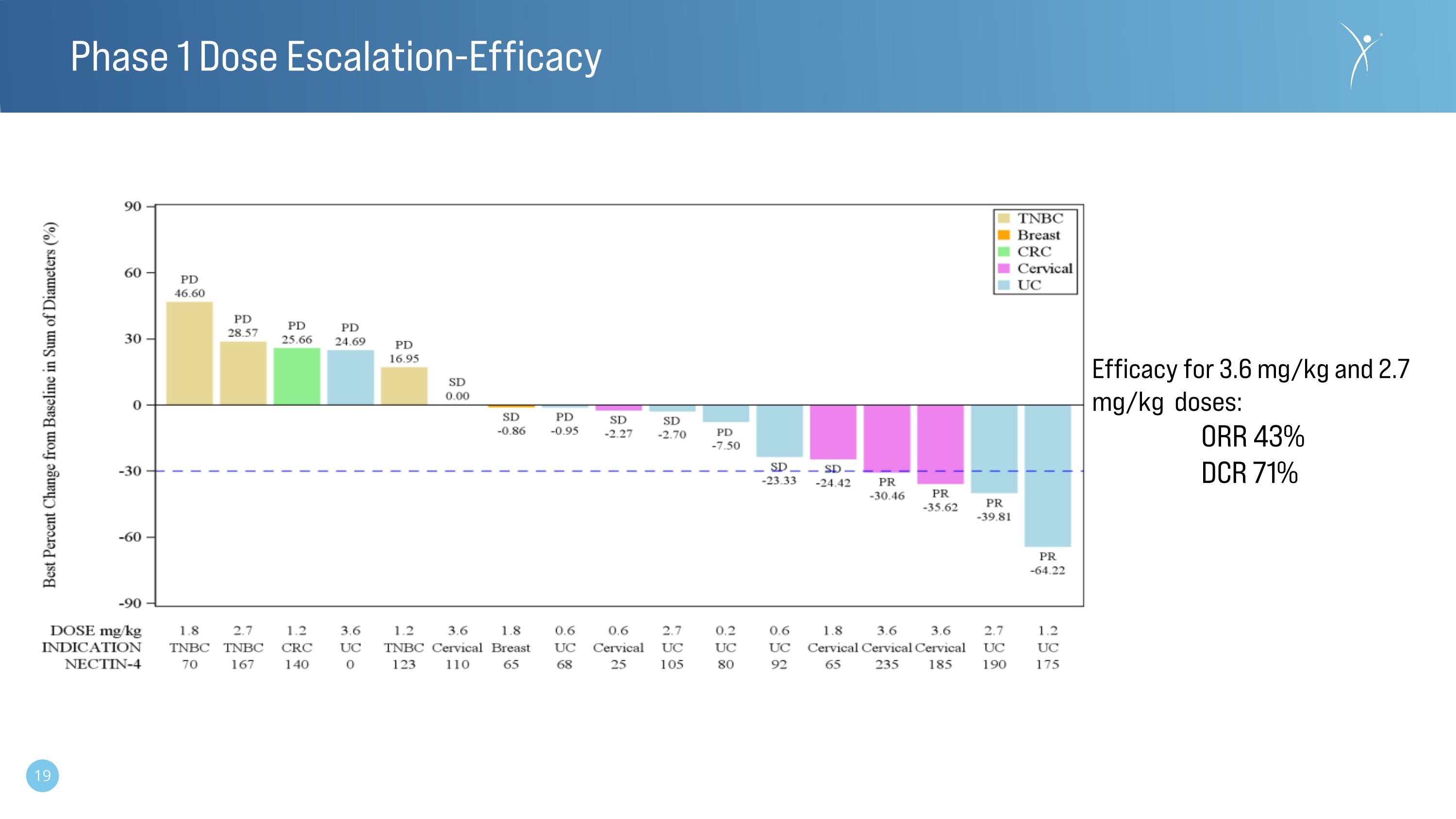
Phase 1 Dose Escalation-Efficacy Efficacy for 3.6 mg/kg and 2.7 mg/kg doses: ORR 43% DCR 71%
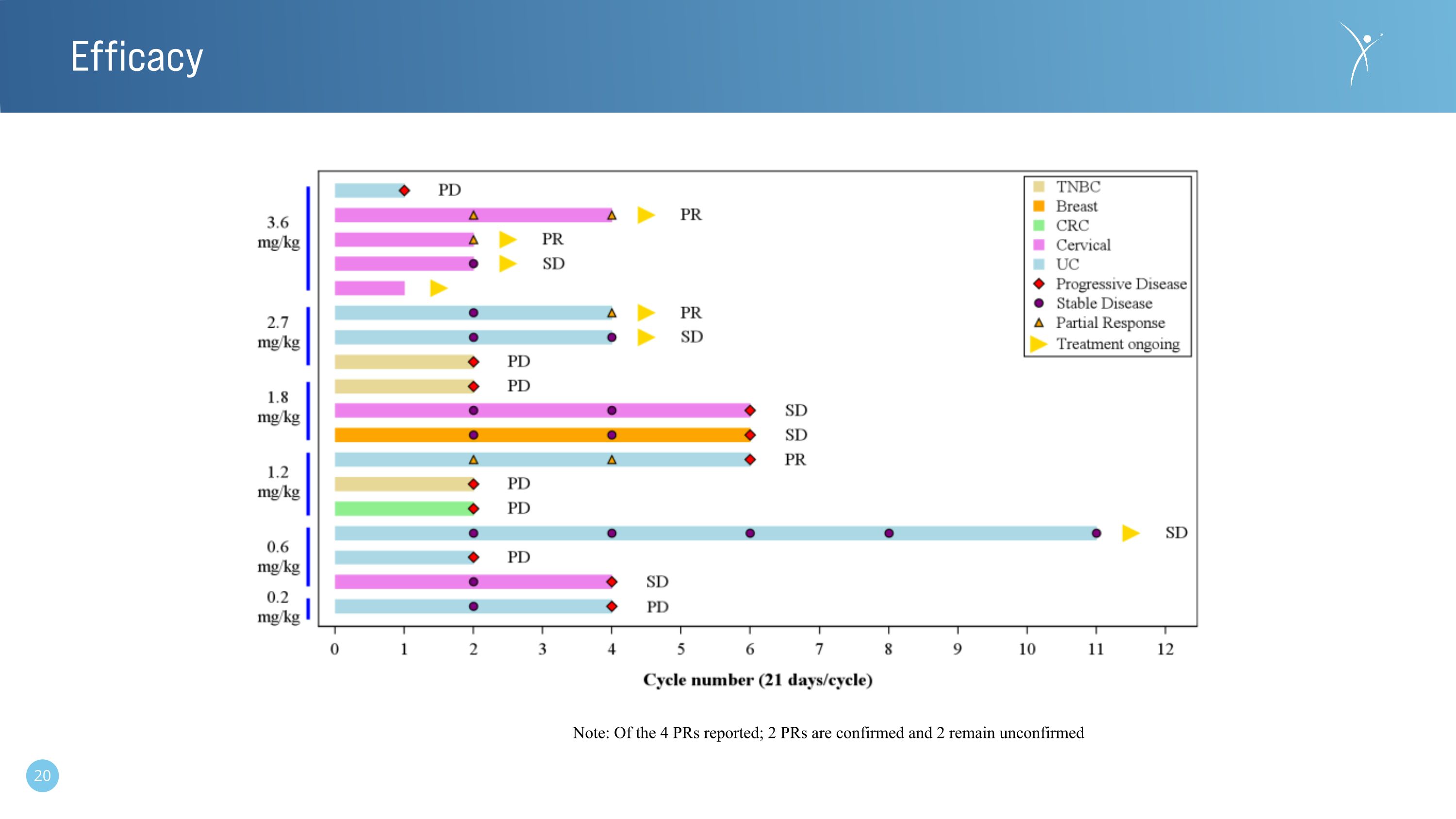
Efficacy Note: Of the 4 PRs reported; 2 PRs are confirmed and 2 remain unconfirmed
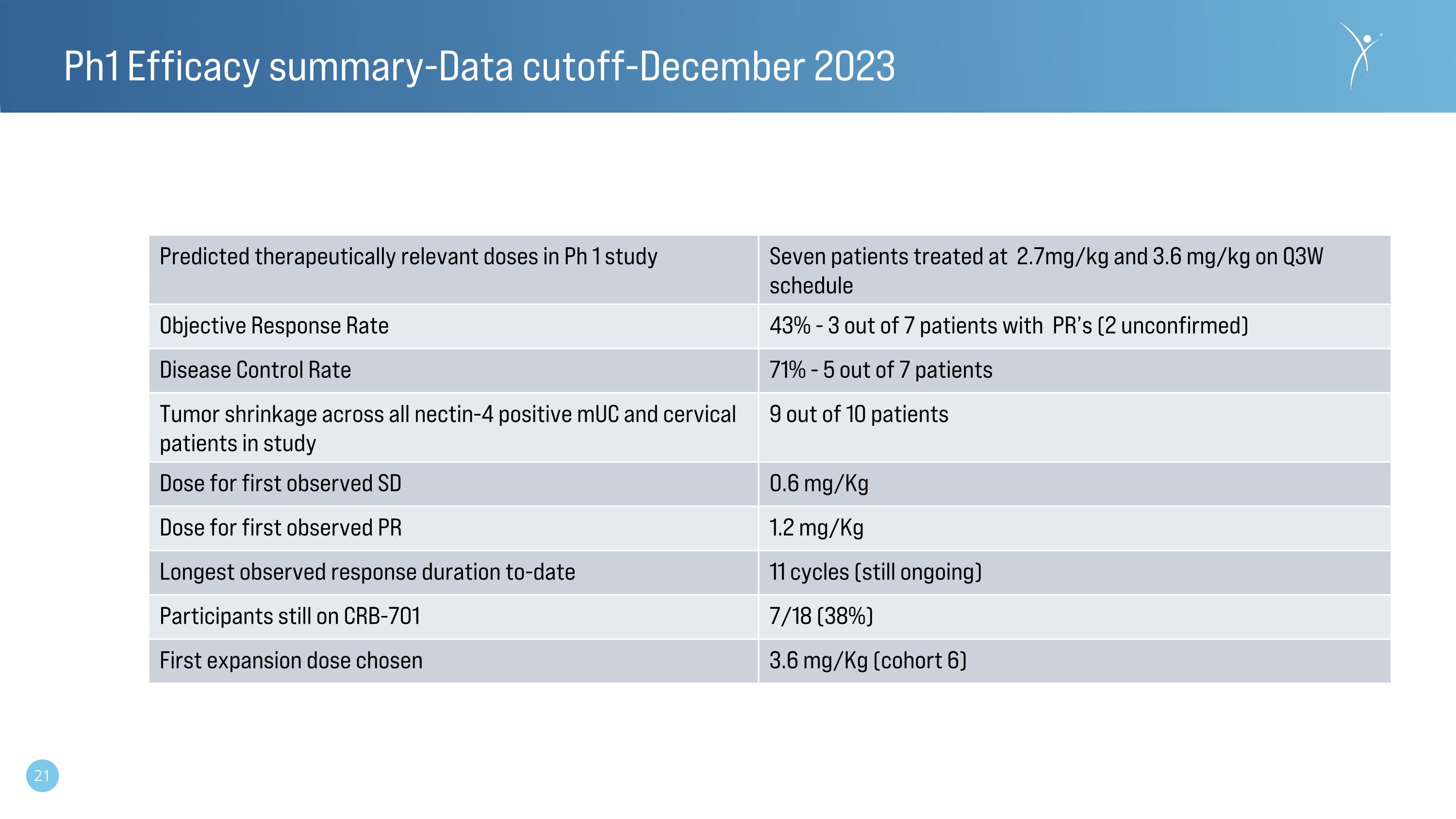
Ph1 Efficacy summary-Data cutoff-December 2023 Predicted therapeutically relevant doses in Ph 1 study Seven patients treated at 2.7mg/kg and 3.6 mg/kg on Q3W schedule Objective Response Rate 43% - 3 out of 7 patients with PR’s (2 unconfirmed) Disease Control Rate 71% - 5 out of 7 patients Tumor shrinkage across all nectin-4 positive mUC and cervical patients in study 9 out of 10 patients Dose for first observed SD 0.6 mg/Kg Dose for first observed PR 1.2 mg/Kg Longest observed response duration to-date 11 cycles (still ongoing) Participants still on CRB-701 7/18 (38%) First expansion dose chosen 3.6 mg/Kg (cohort 6)
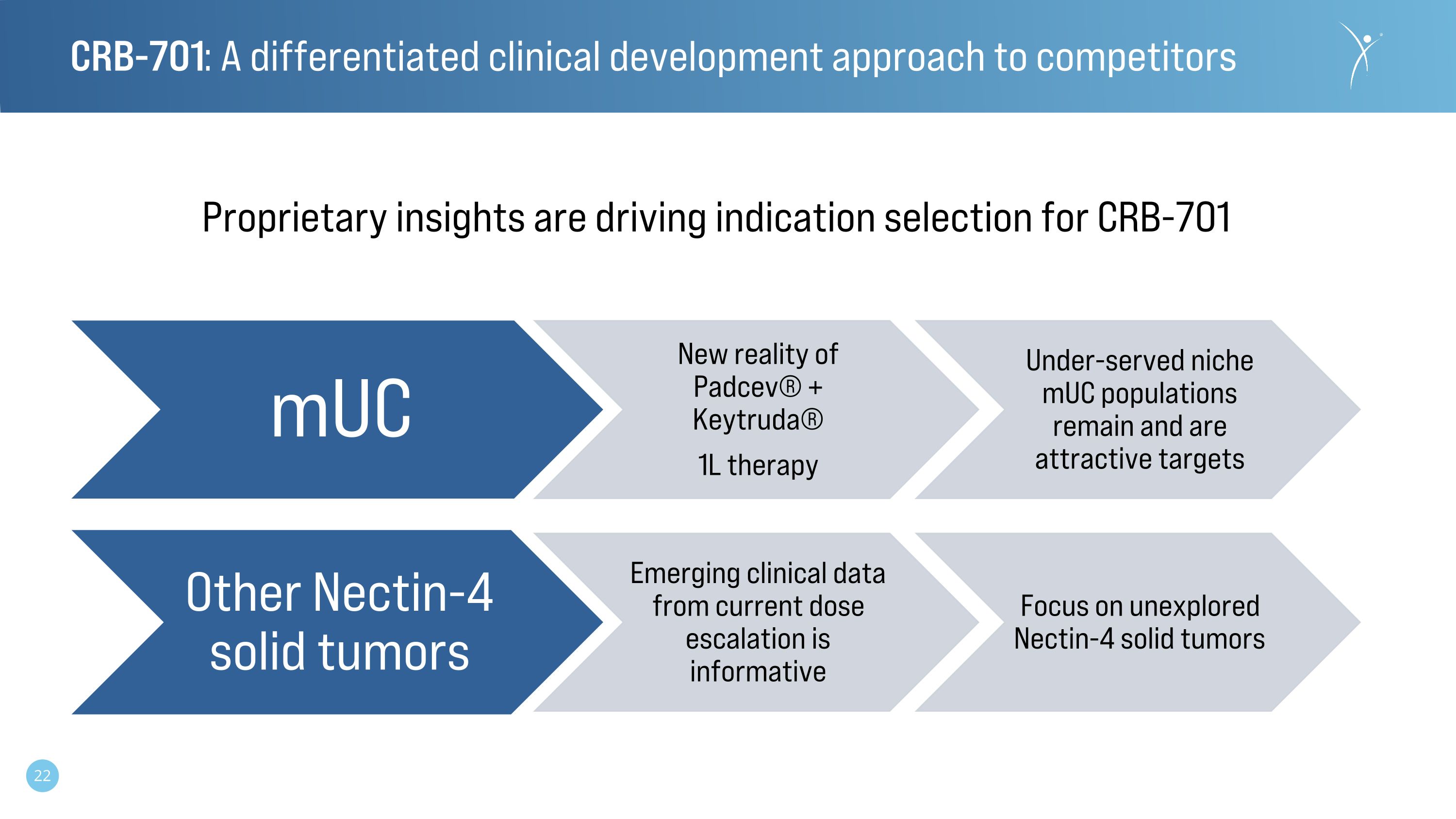
CRB-701: A differentiated clinical development approach to competitors mUC New reality of Padcev® + Keytruda® 1L therapy Under-served niche mUC populations remain and are attractive targets Other Nectin-4 solid tumors Emerging clinical data from current dose escalation is informative Focus on unexplored Nectin-4 solid tumors Proprietary insights are driving indication selection for CRB-701
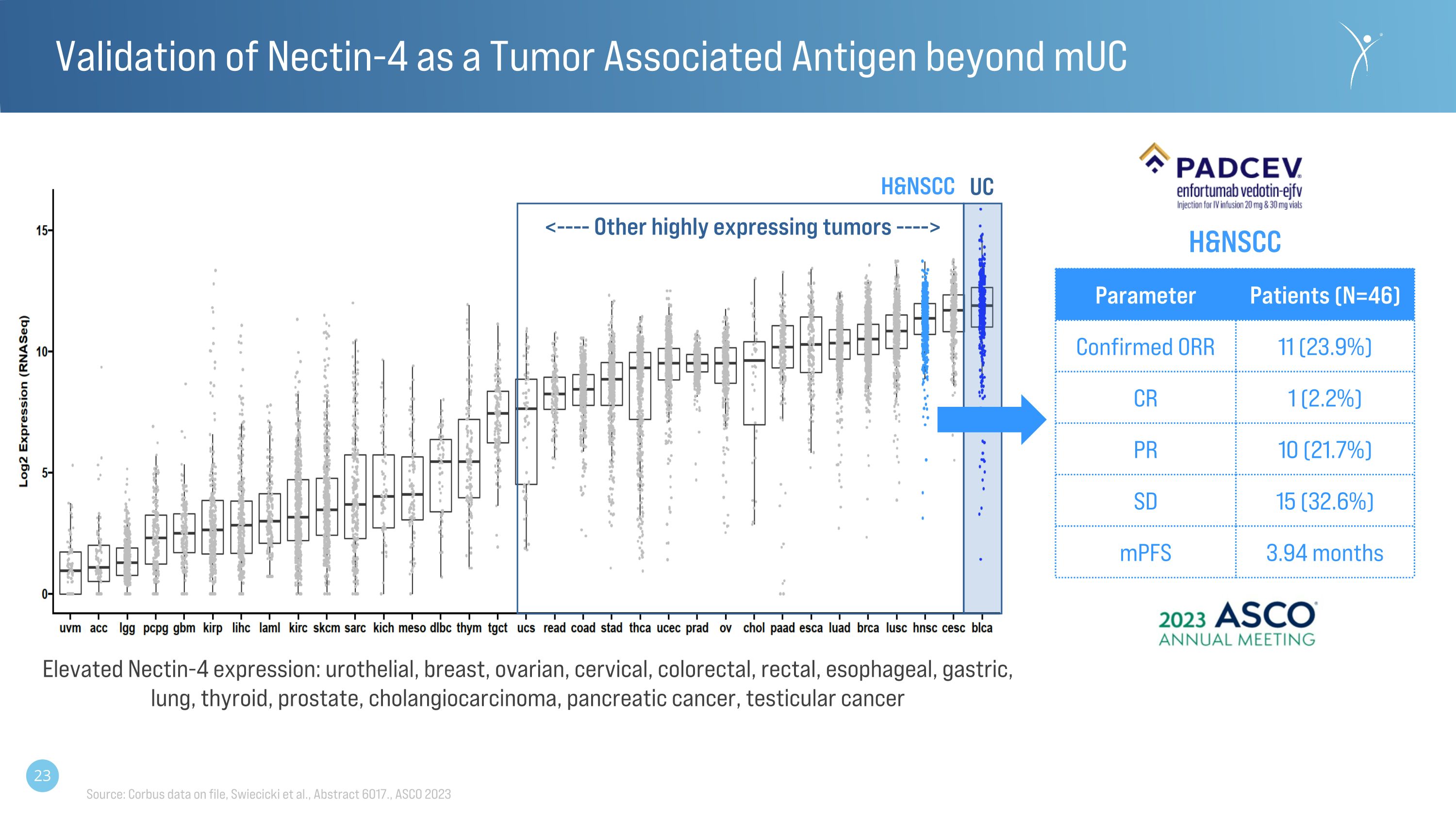
Validation of Nectin-4 as a Tumor Associated Antigen beyond mUC Parameter Patients (N=46) Confirmed ORR 11 (23.9%) CR 1 (2.2%) PR 10 (21.7%) SD 15 (32.6%) mPFS 3.94 months Source: Corbus data on file, Swiecicki et al., Abstract 6017., ASCO 2023 Elevated Nectin-4 expression: urothelial, breast, ovarian, cervical, colorectal, rectal, esophageal, gastric, lung, thyroid, prostate, cholangiocarcinoma, pancreatic cancer, testicular cancer <---- Other highly expressing tumors ----> UC H&NSCC H&NSCC
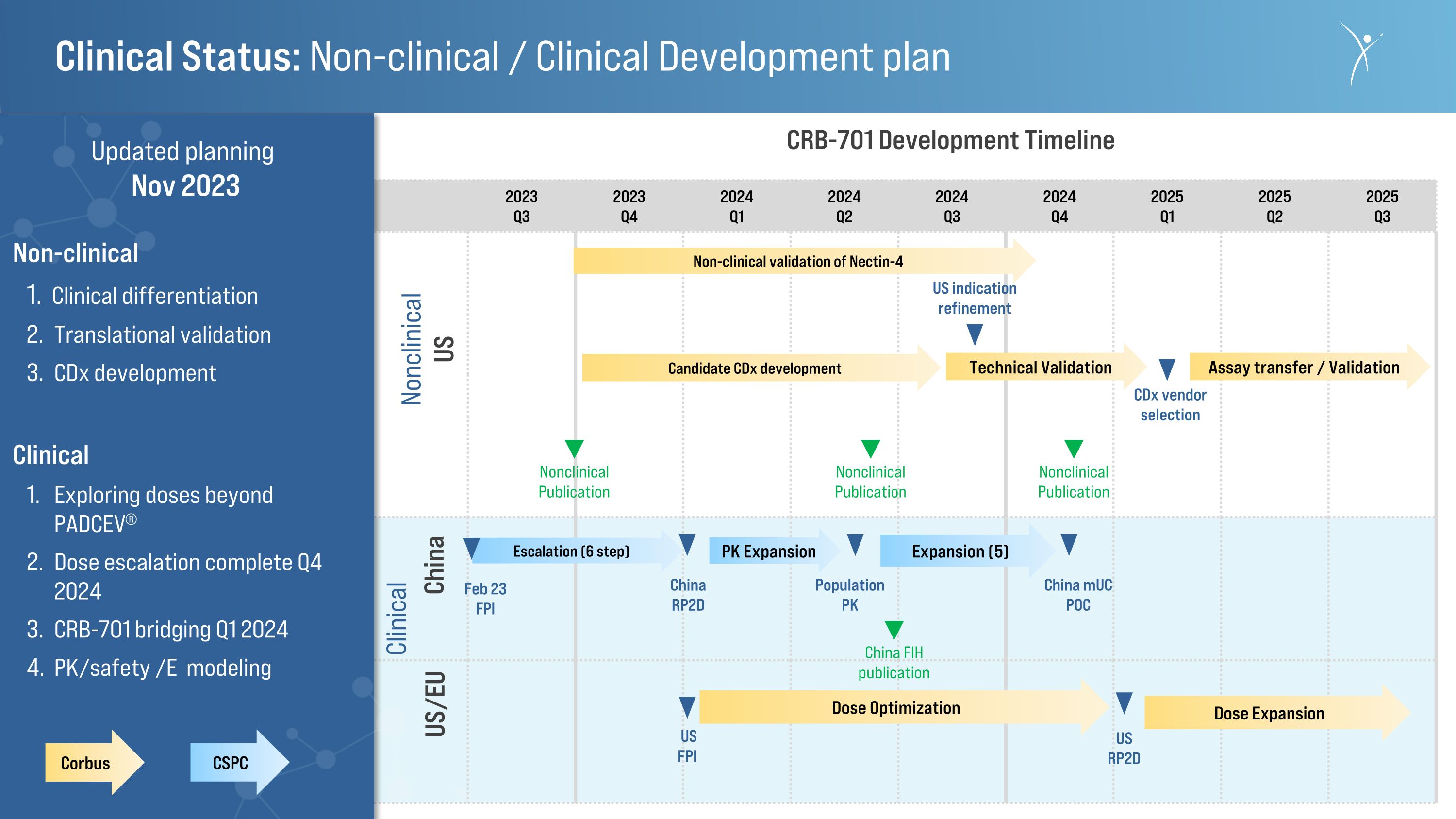
Clinical Status: Non-clinical / Clinical Development plan 2023 Q1 2023 Q2 2023 Q3 2023 Q4 2024�Q1 2024 Q2 2024 Q3 2024�Q4 2025 Q1 2025 Q2 2025�Q3 CRB-701 Development Timeline China US/EU Non-clinical validation of Nectin-4 US RP2D Escalation (6 step) Nonclinical US CDx vendor selection Expansion (5) Feb 23 FPI Population PK China RP2D China mUC POC Assay transfer / Validation Nonclinical Publication US indication refinement Corbus CSPC Dose Optimization Nonclinical Publication Technical Validation Clinical US FPI Updated planning Nov 2023 Non-clinical Clinical differentiation Translational validation CDx development Clinical Exploring doses beyond PADCEV® Dose escalation complete Q4 2024 CRB-701 bridging Q1 2024 PK/safety /E modeling PK Expansion Nonclinical Publication Candidate CDx development China FIH publication Dose Expansion

CRB-701: Summary Emerging clinical safety and efficacy-potential for superior therapeutic index Dose expansion has started (China); dose escalation in US Q-1 2024 3rd generation ADC with improved linker stability-reduces MMAE in circulation

CRB-913: oral cannabinoid type-1 inverse agonist for superior incretin therapy in obesity NASDAQ: CRBP • CorbusPharma.com • @CorbusPharma
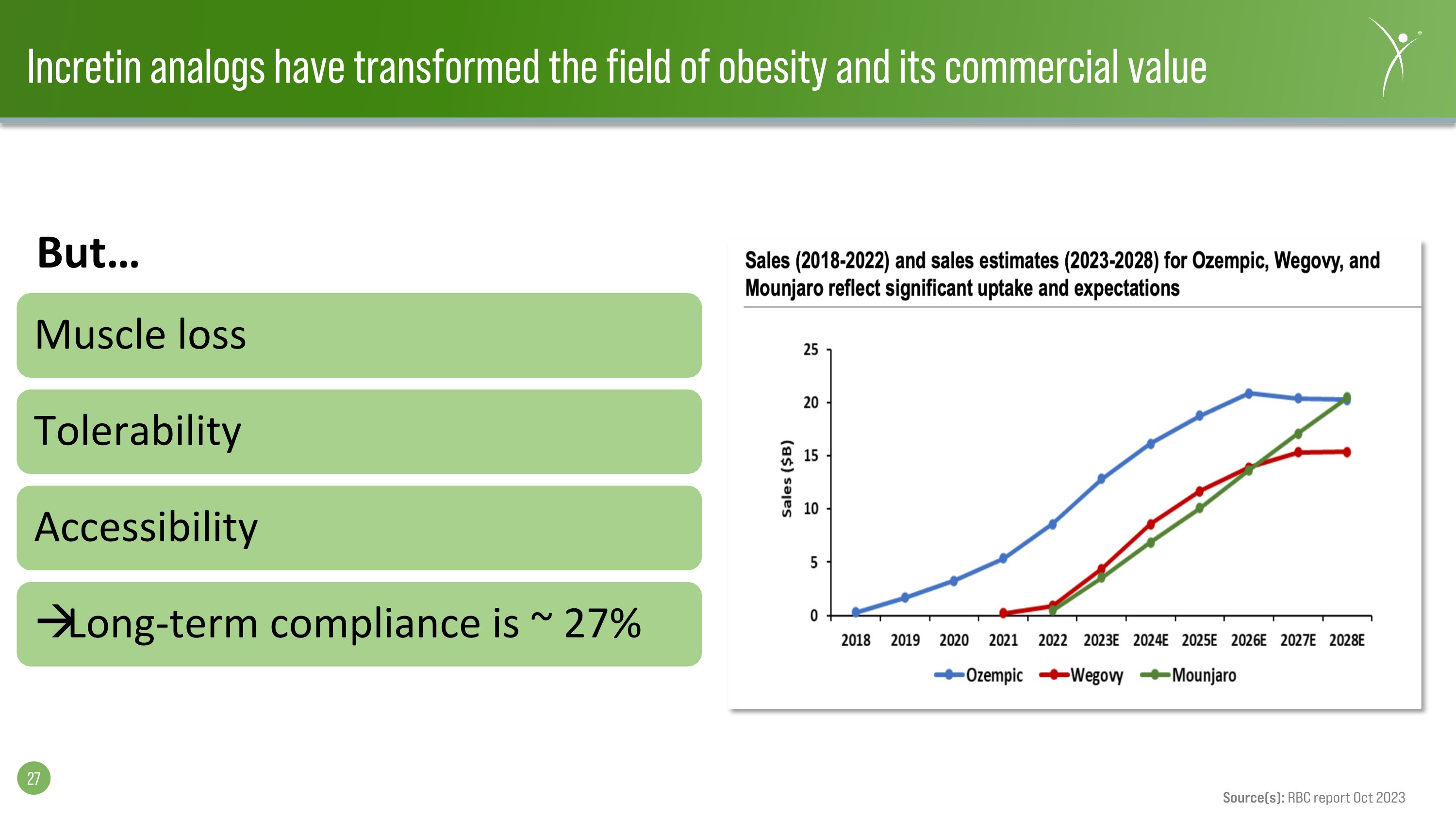
Incretin analogs have transformed the field of obesity and its commercial value Muscle loss Tolerability Accessibility Long-term compliance is ~ 27% Source(s): RBC report Oct 2023 But…
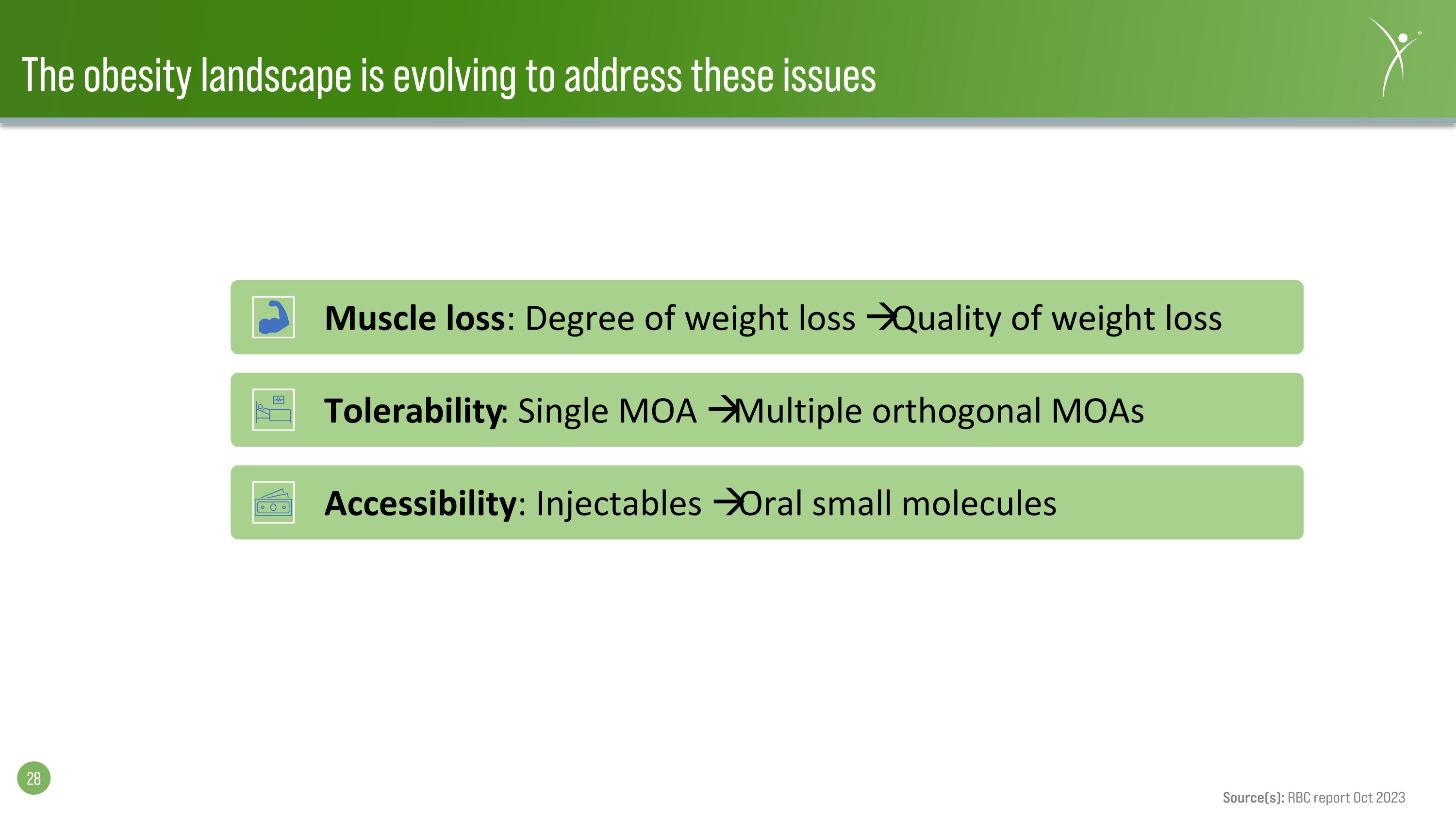
The obesity landscape is evolving to address these issues Muscle loss: Degree of weight loss Quality of weight loss Tolerability: Single MOA Multiple orthogonal MOAs Accessibility: Injectables Oral small molecules Source(s): RBC report Oct 2023

CB1 inverse agonism: �The return of a clinically-validated obesity drug class 29
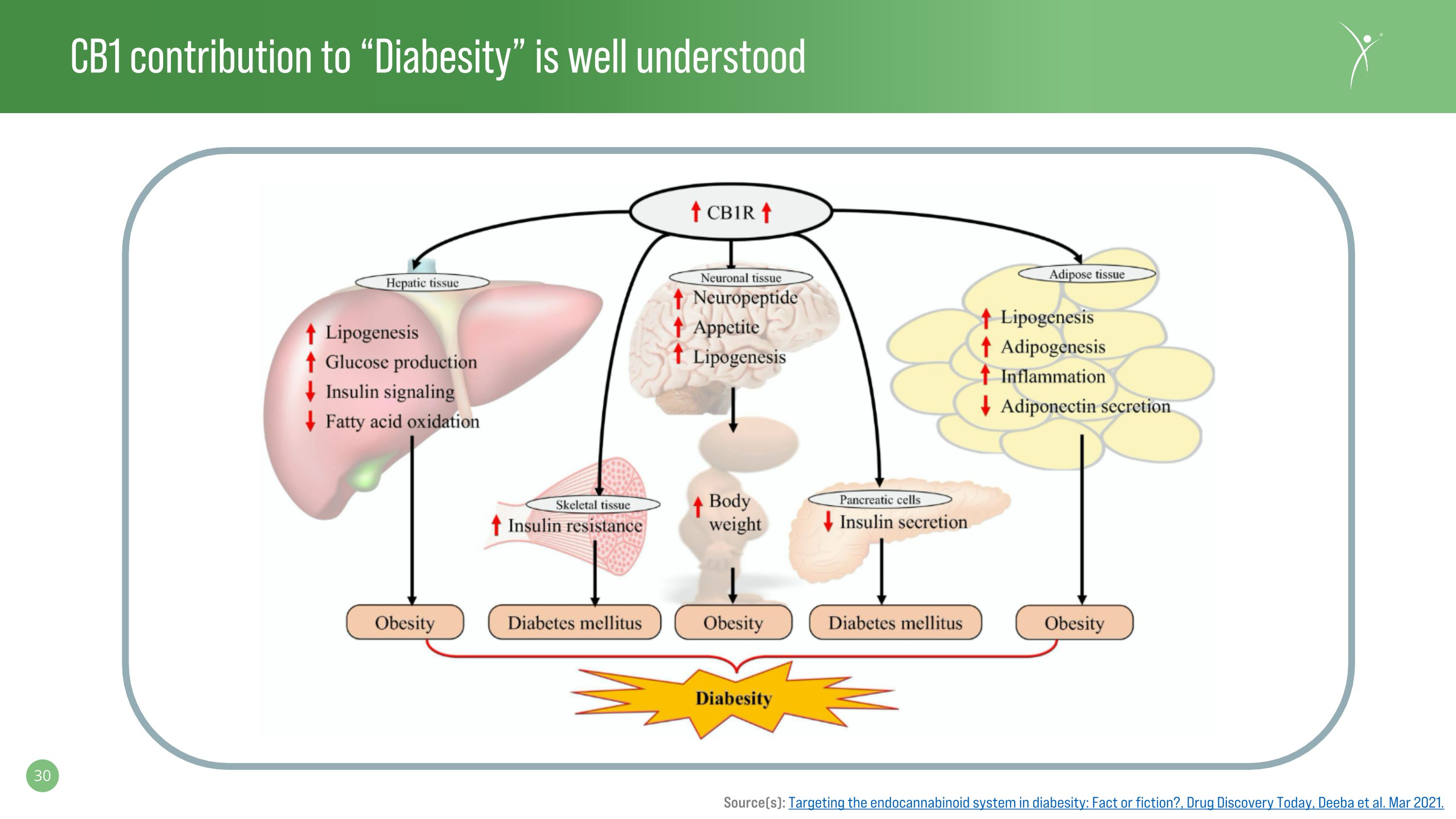
CB1 contribution to “Diabesity” is well understood Source(s): Targeting the endocannabinoid system in diabesity: Fact or fiction?, Drug Discovery Today, Deeba et al. Mar 2021.
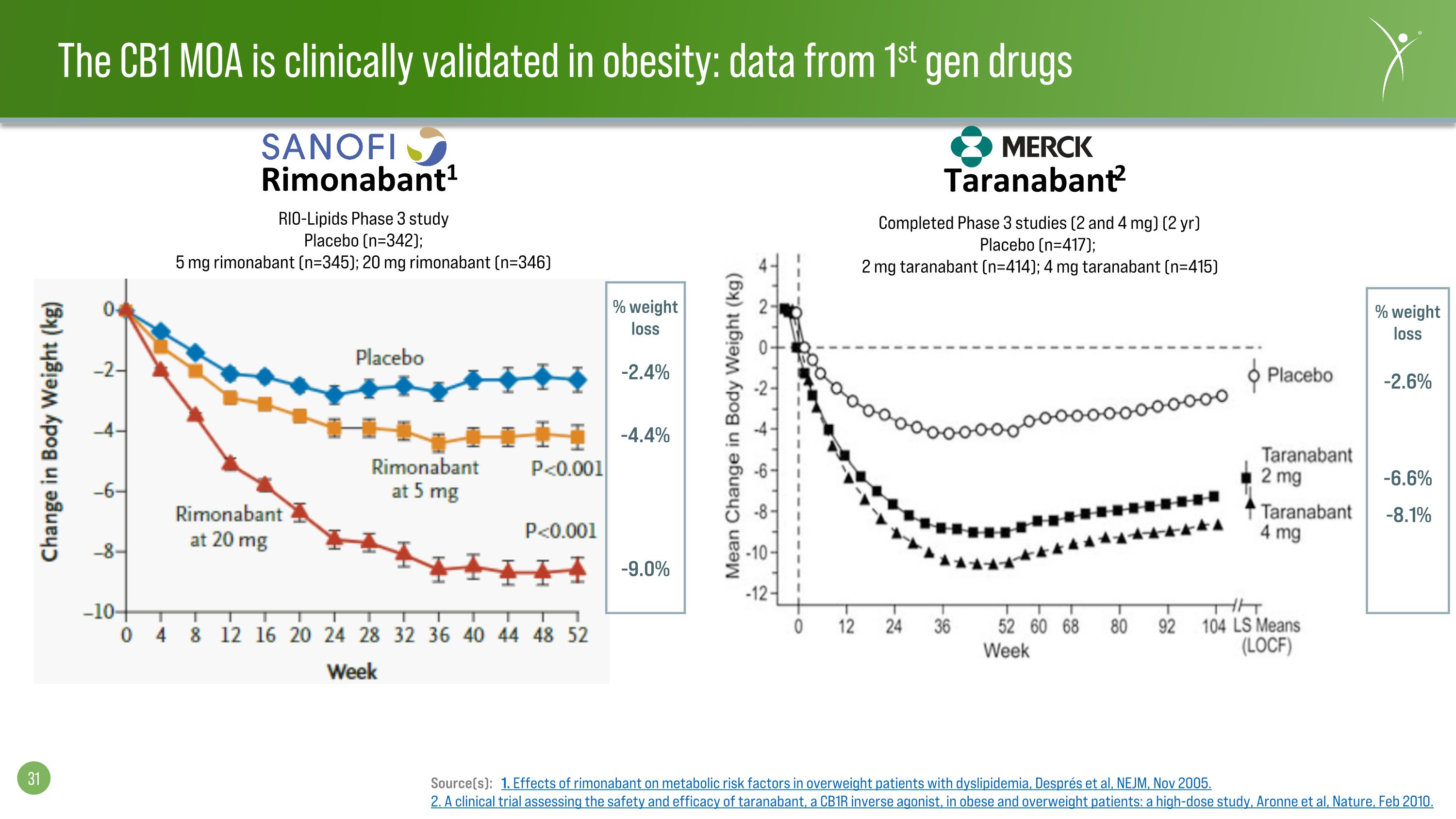
-9.0% -4.4% -2.4% % weight loss RIO-Lipids Phase 3 study Placebo (n=342); 5 mg rimonabant (n=345); 20 mg rimonabant (n=346) Completed Phase 3 studies (2 and 4 mg) (2 yr) Placebo (n=417); 2 mg taranabant (n=414); 4 mg taranabant (n=415) % weight loss -8.1% -6.6% -2.6% Rimonabant1 Taranabant2 Source(s): 1. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia, Després et al, NEJM, Nov 2005. 2. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study, Aronne et al, Nature, Feb 2010. The CB1 MOA is clinically validated in obesity: data from 1st gen drugs
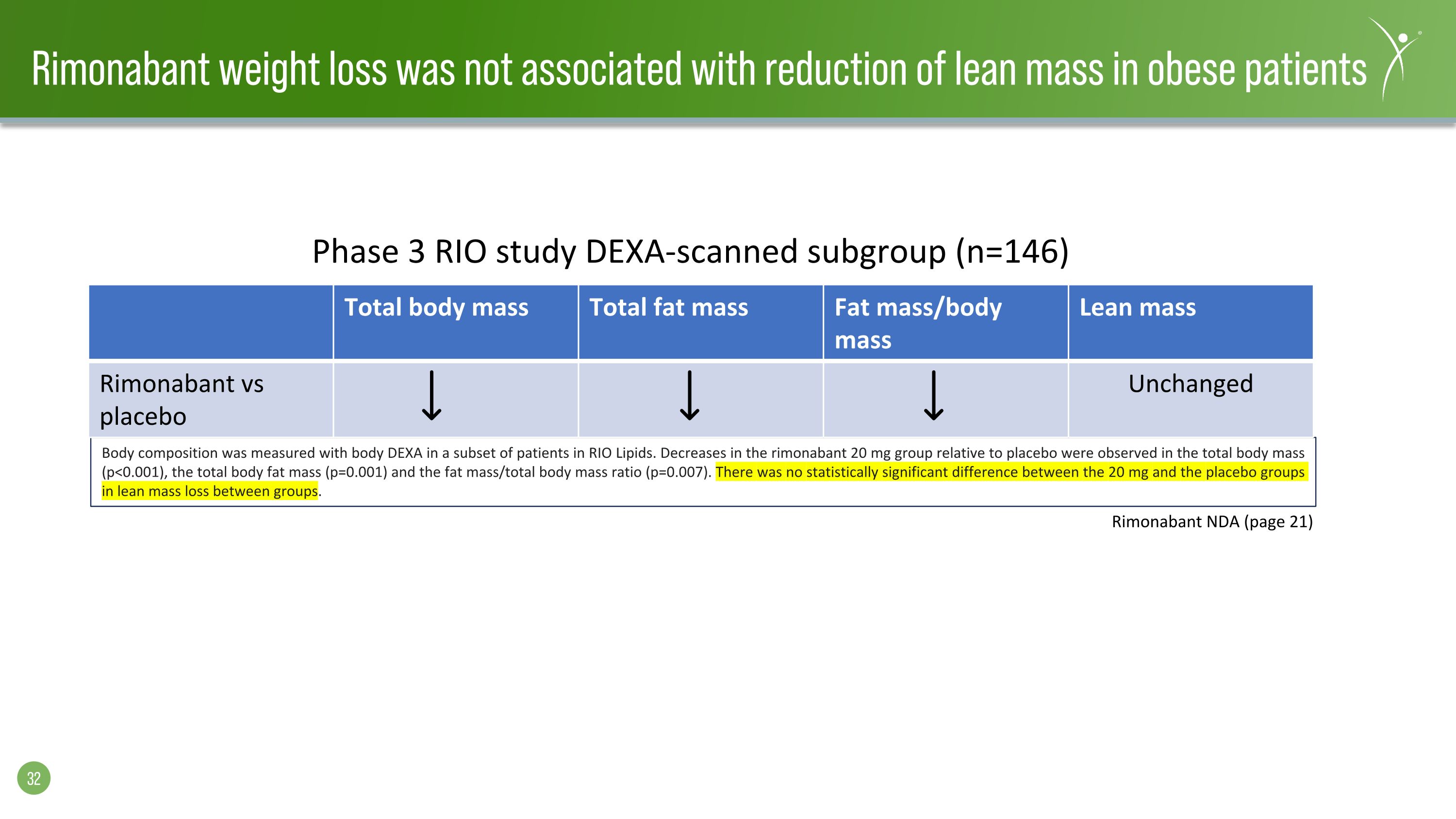
Rimonabant weight loss was not associated with reduction of lean mass in obese patients Body composition was measured with body DEXA in a subset of patients in RIO Lipids. Decreases in the rimonabant 20 mg group relative to placebo were observed in the total body mass (p<0.001), the total body fat mass (p=0.001) and the fat mass/total body mass ratio (p=0.007). There was no statistically significant difference between the 20 mg and the placebo groups in lean mass loss between groups. Rimonabant NDA (page 21) Total body mass Total fat mass Fat mass/body mass Lean mass Rimonabant vs placebo Unchanged Phase 3 RIO study DEXA-scanned subgroup (n=146)
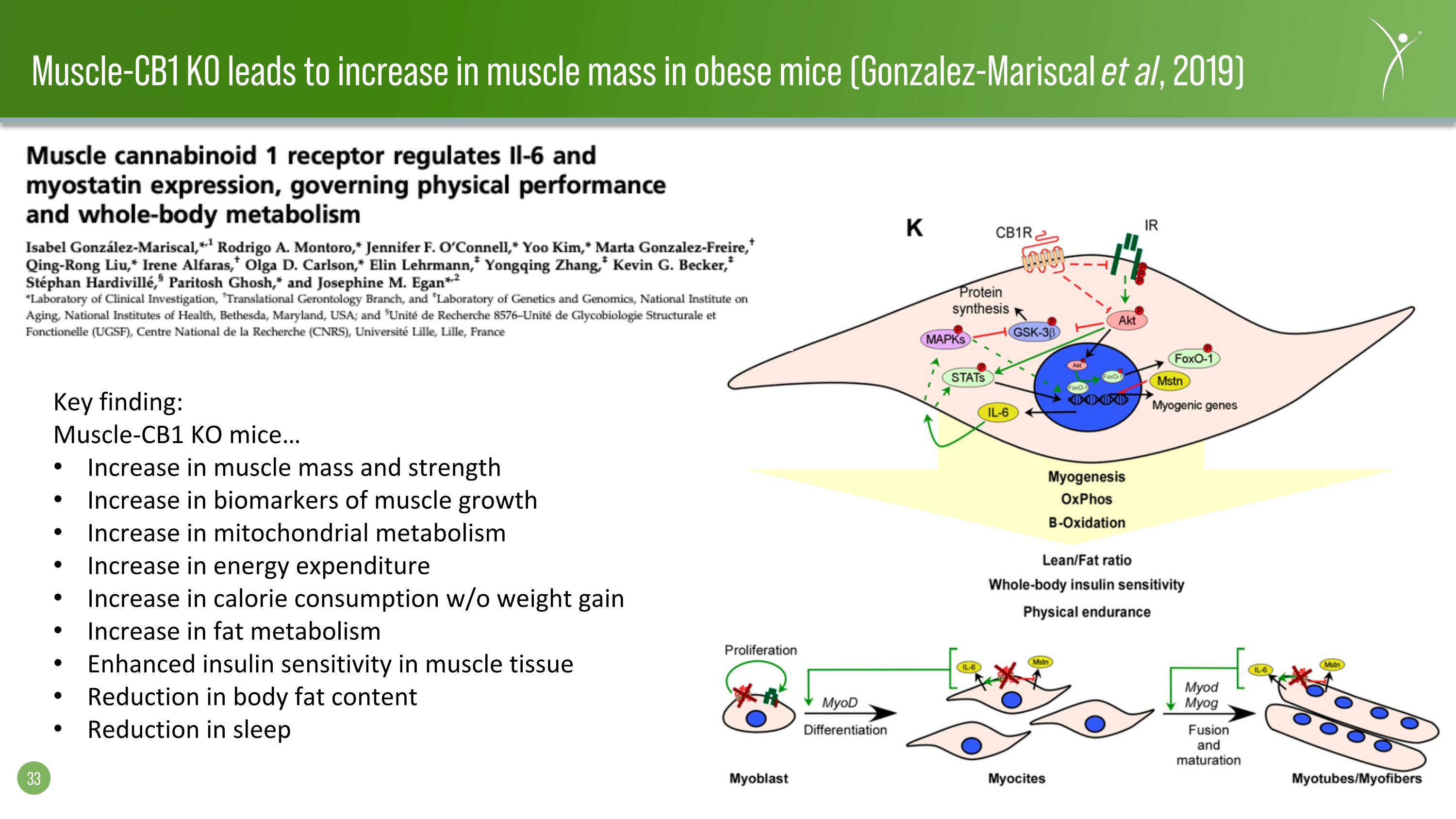
Muscle-CB1 KO leads to increase in muscle mass in obese mice (Gonzalez-Mariscal et al, 2019) Key finding: Muscle-CB1 KO mice… Increase in muscle mass and strength Increase in biomarkers of muscle growth Increase in mitochondrial metabolism Increase in energy expenditure Increase in calorie consumption w/o weight gain Increase in fat metabolism Enhanced insulin sensitivity in muscle tissue Reduction in body fat content Reduction in sleep
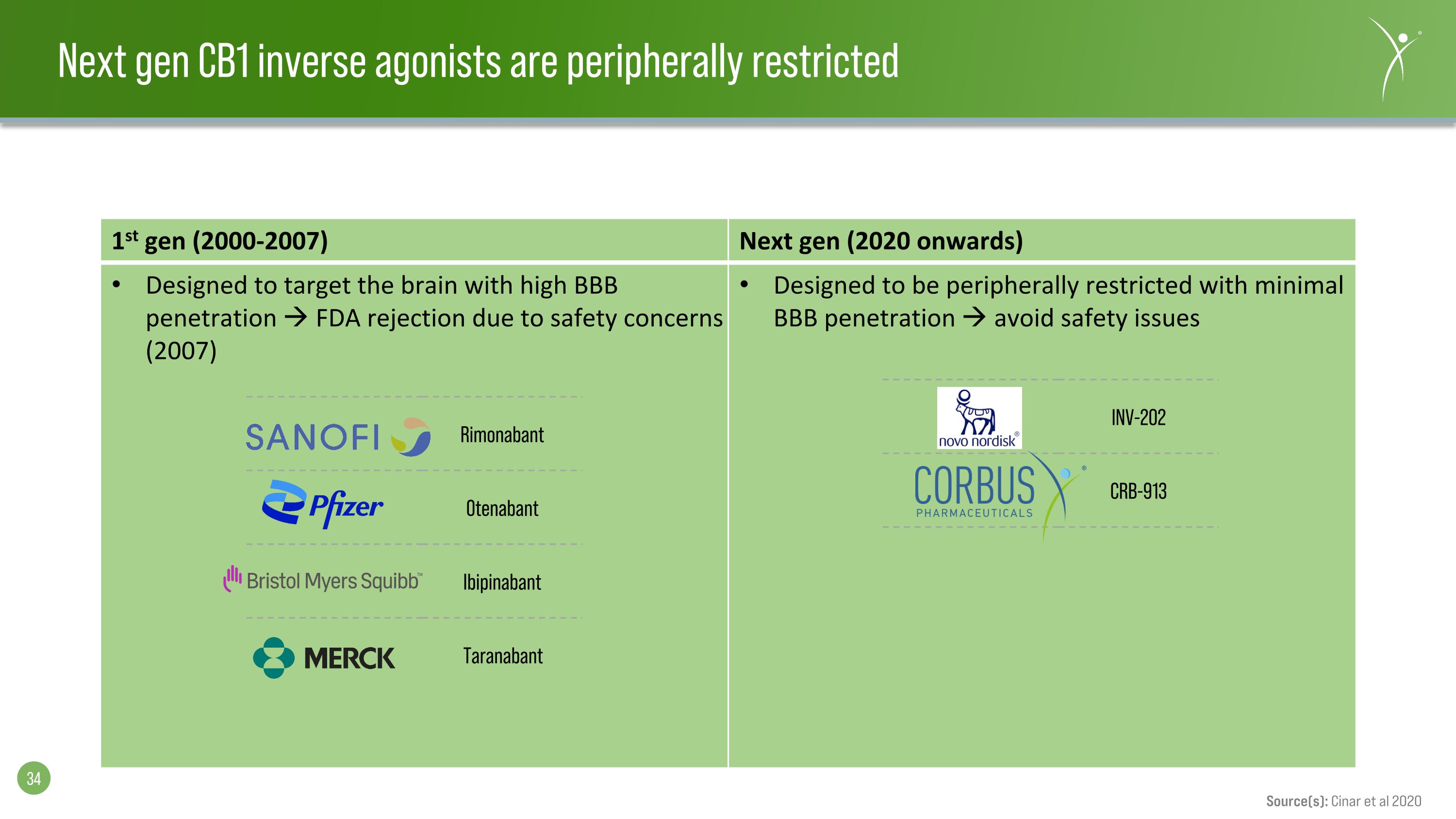
Next gen CB1 inverse agonists are peripherally restricted 1st gen (2000-2007) Next gen (2020 onwards) Designed to target the brain with high BBB penetration FDA rejection due to safety concerns (2007) Designed to be peripherally restricted with minimal BBB penetration avoid safety issues Rimonabant Otenabant Ibipinabant Taranabant INV-202 CRB-913 Source(s): Cinar et al 2020
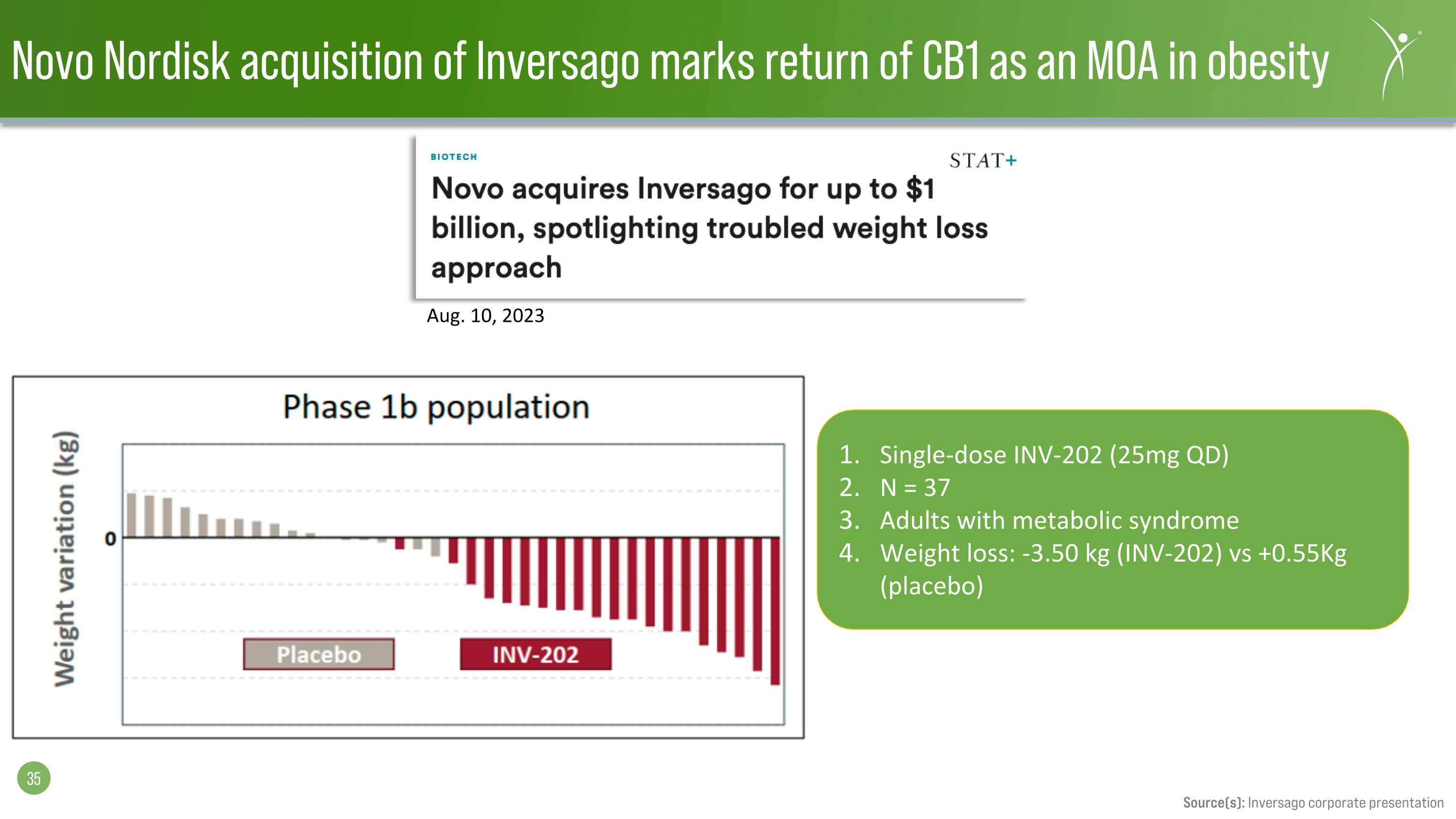
Novo Nordisk acquisition of Inversago marks return of CB1 as an MOA in obesity Single-dose INV-202 (25mg QD) N = 37 Adults with metabolic syndrome Weight loss: -3.50 kg (INV-202) vs +0.55Kg (placebo) Aug. 10, 2023 Source(s): Inversago corporate presentation

CRB-913: oral CB1 inverse agonist for combination therapy with incretins 36 Nov. 2023

CRB-913: designed to be a best-in-class next gen CB1 inverse agonist Design goals: Best-in-class peripheral restriction Protect lean mass (muscle) Retain 1st gen efficacy Enhance efficacy of incretin analogs
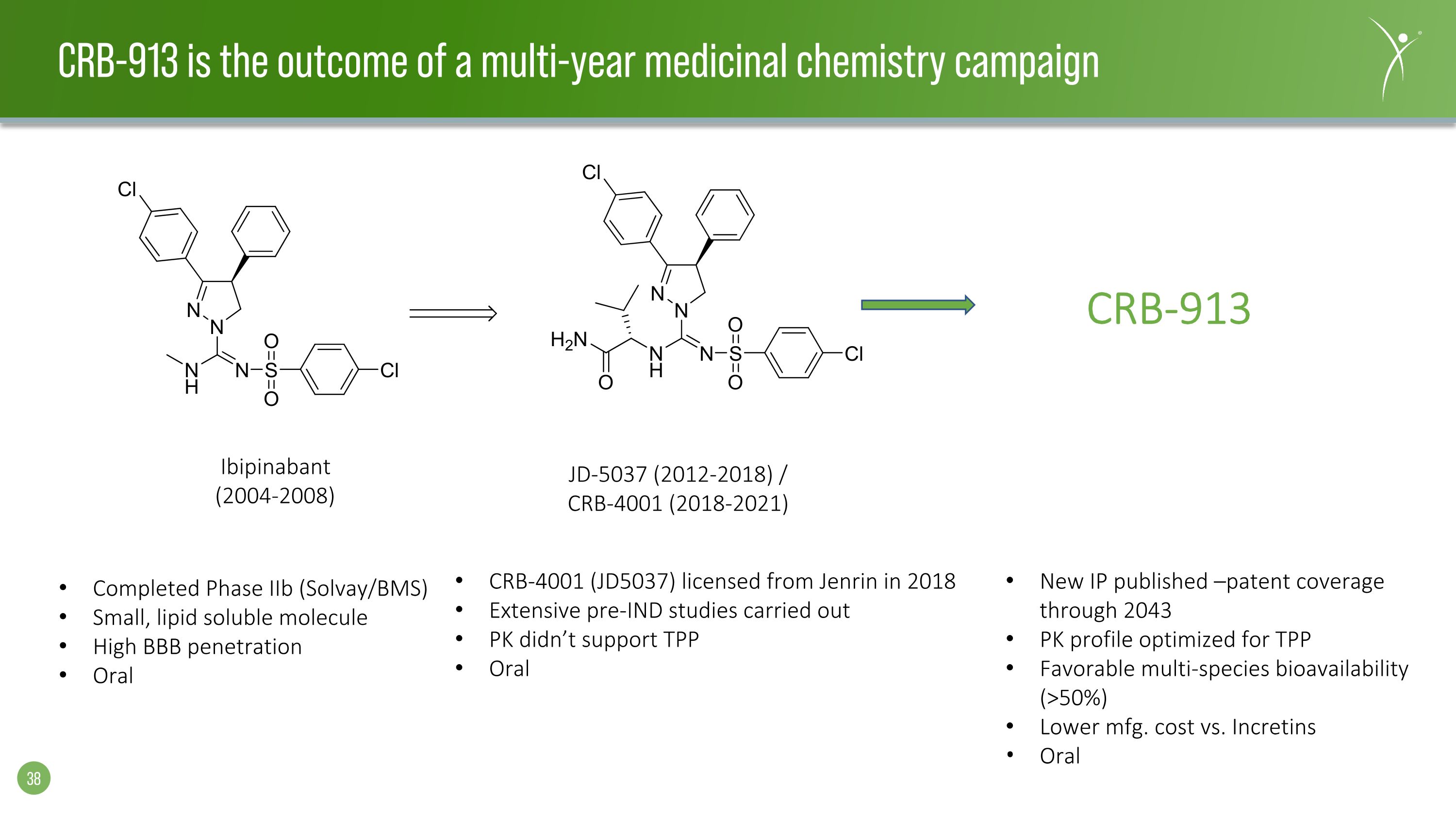
Ibipinabant (2004-2008) JD-5037 (2012-2018) / CRB-4001 (2018-2021) Completed Phase IIb (Solvay/BMS) Small, lipid soluble molecule High BBB penetration Oral CRB-4001 (JD5037) licensed from Jenrin in 2018 Extensive pre-IND studies carried out PK didn’t support TPP Oral CRB-913 New IP published –patent coverage through 2043 PK profile optimized for TPP Favorable multi-species bioavailability (>50%) Lower mfg. cost vs. Incretins Oral CRB-913 is the outcome of a multi-year medicinal chemistry campaign
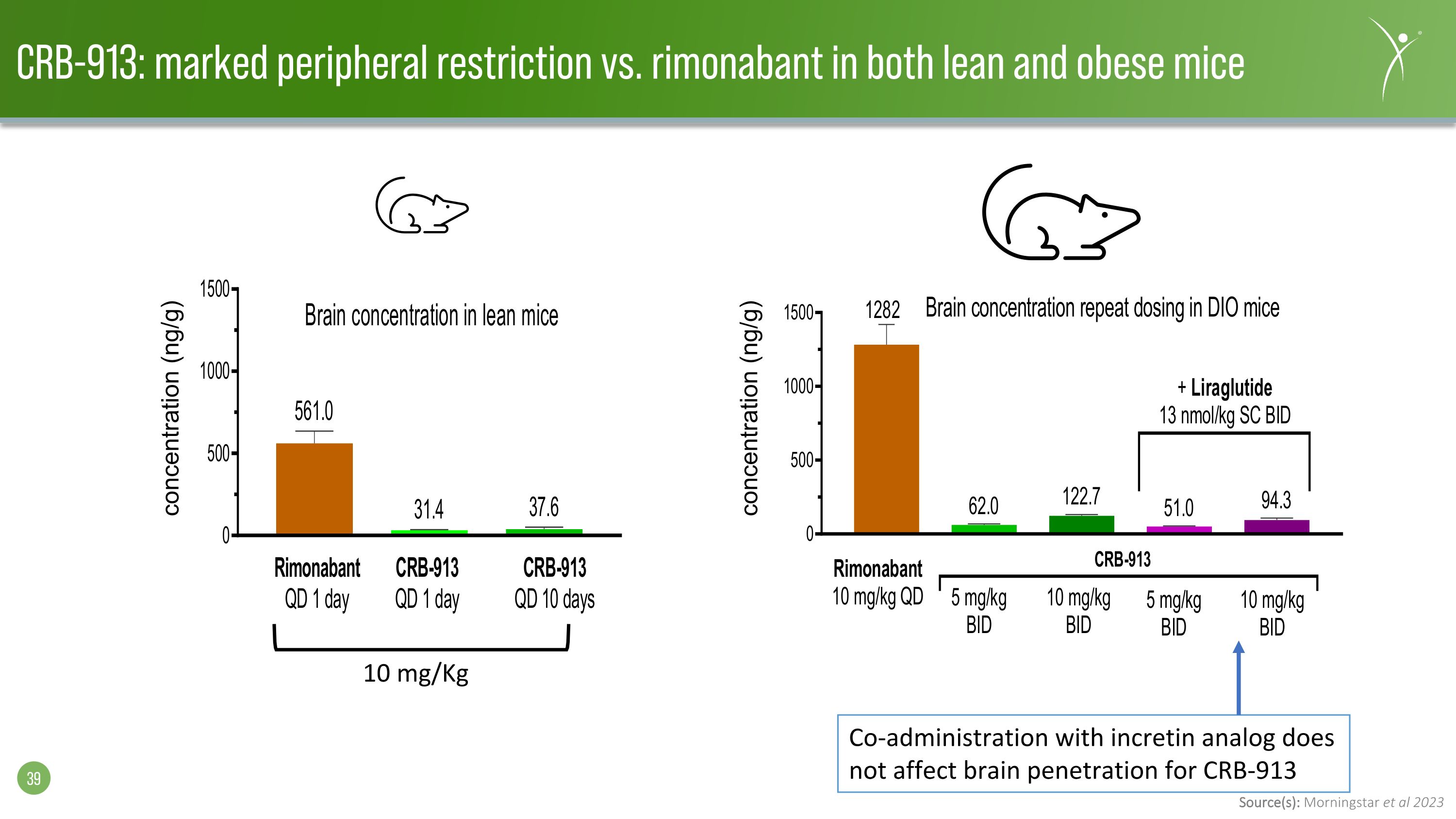
Source(s): Morningstar et al 2023 CRB-913: marked peripheral restriction vs. rimonabant in both lean and obese mice concentration (ng/g) concentration (ng/g) 10 mg/Kg Co-administration with incretin analog does not affect brain penetration for CRB-913
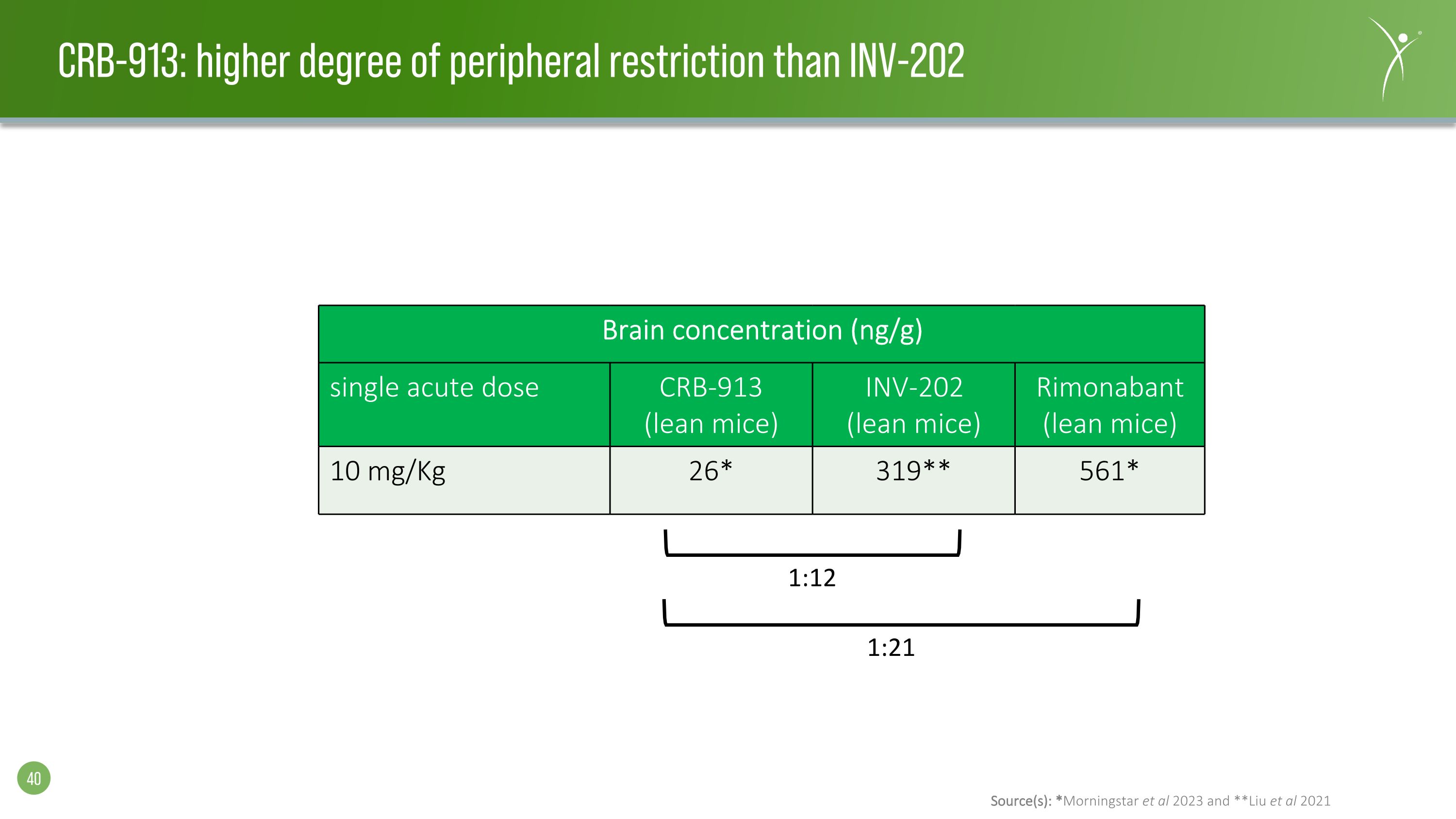
Source(s): *Morningstar et al 2023 and **Liu et al 2021 CRB-913: higher degree of peripheral restriction than INV-202 Brain concentration (ng/g) single acute dose CRB-913 (lean mice) INV-202 (lean mice) Rimonabant (lean mice) 10 mg/Kg 26* 319** 561* 1:12 1:21
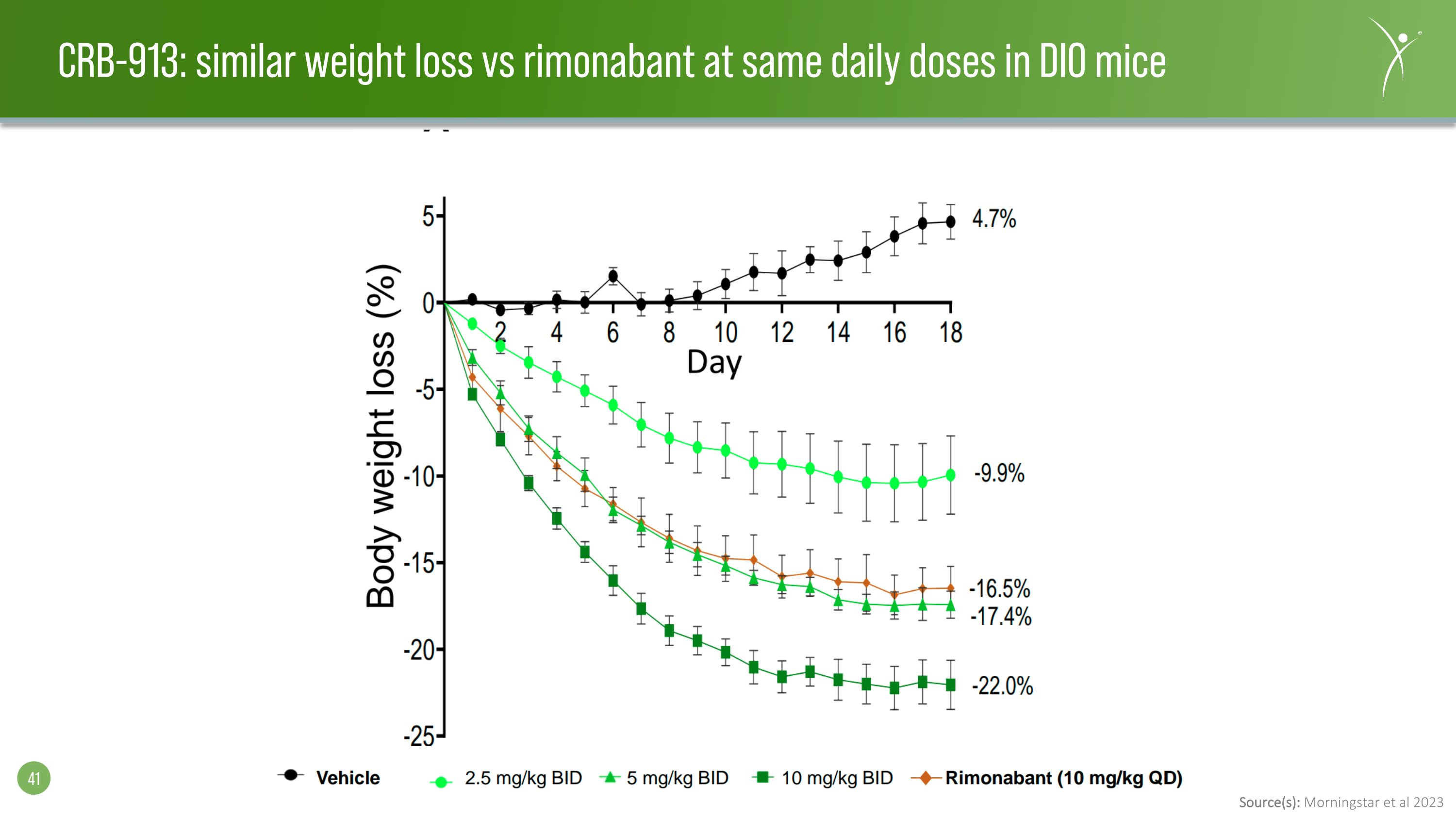
Source(s): Morningstar et al 2023 CRB-913: similar weight loss vs rimonabant at same daily doses in DIO mice
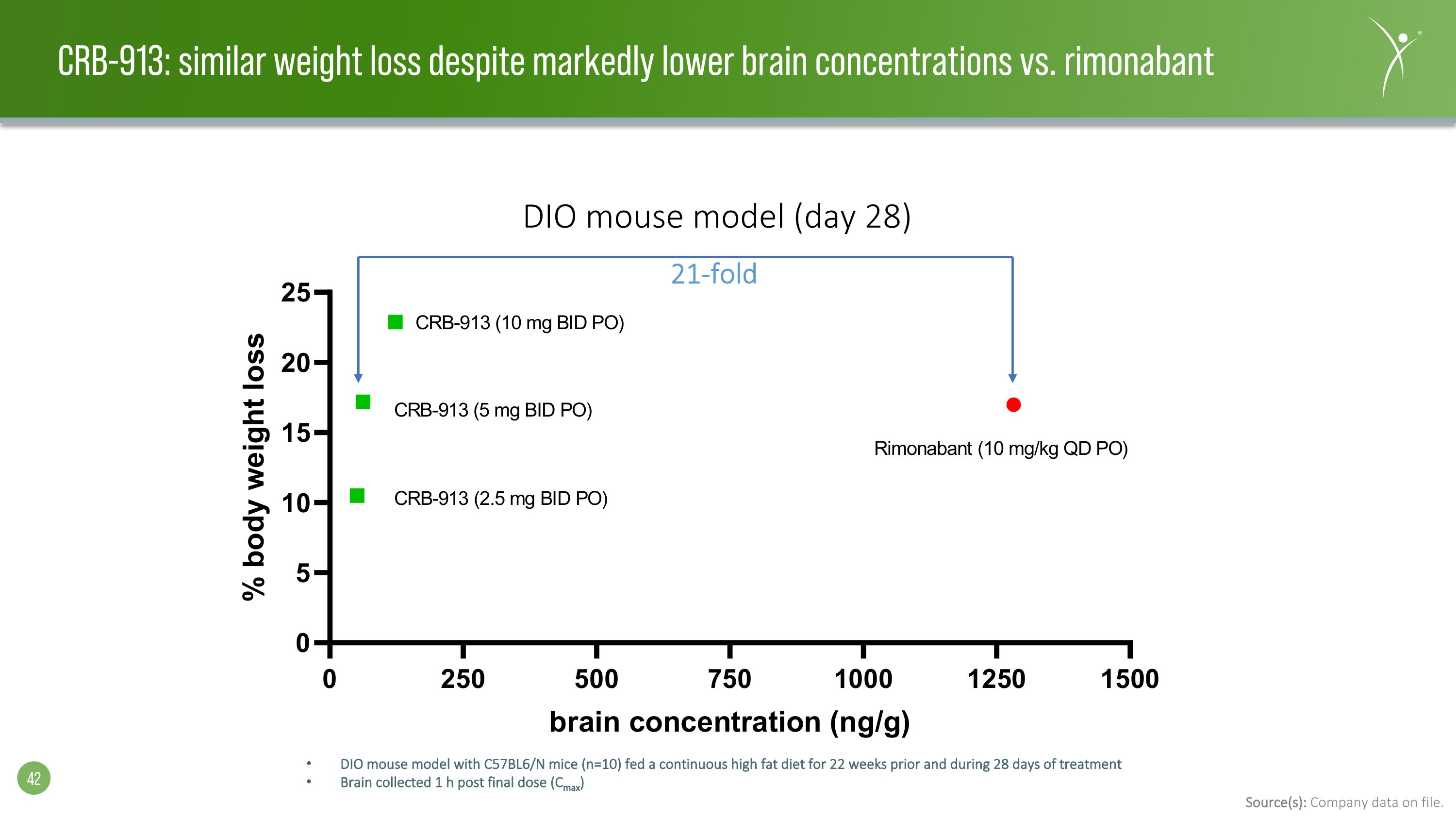
21-fold DIO mouse model (day 28) DIO mouse model with C57BL6/N mice (n=10) fed a continuous high fat diet for 22 weeks prior and during 28 days of treatment Brain collected 1 h post final dose (Cmax) Source(s): Company data on file. CRB-913: similar weight loss despite markedly lower brain concentrations vs. rimonabant
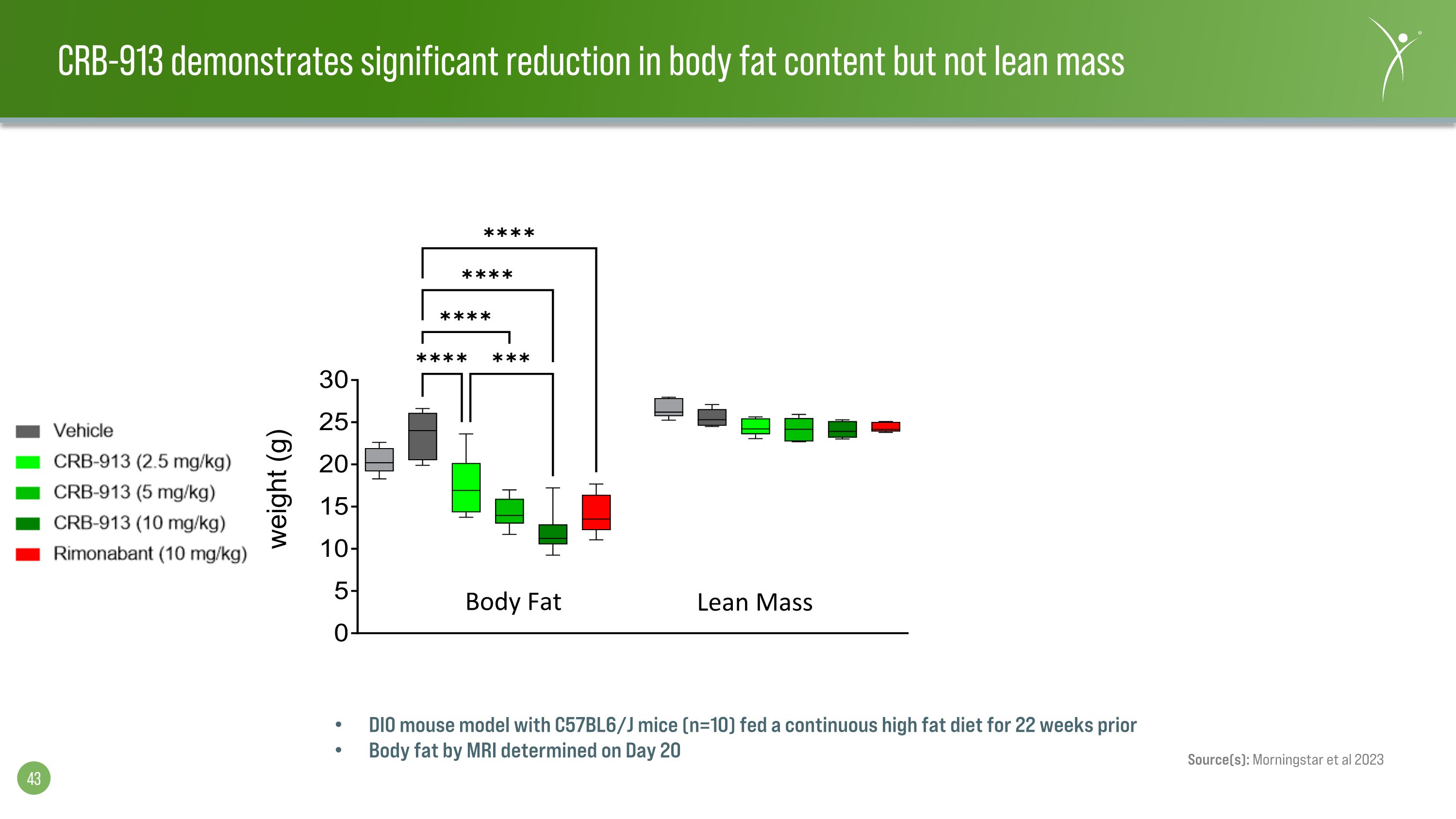
DIO mouse model with C57BL6/J mice (n=10) fed a continuous high fat diet for 22 weeks prior Body fat by MRI determined on Day 20 Source(s): Morningstar et al 2023 CRB-913 demonstrates significant reduction in body fat content but not lean mass weight (g) Body Fat Lean Mass
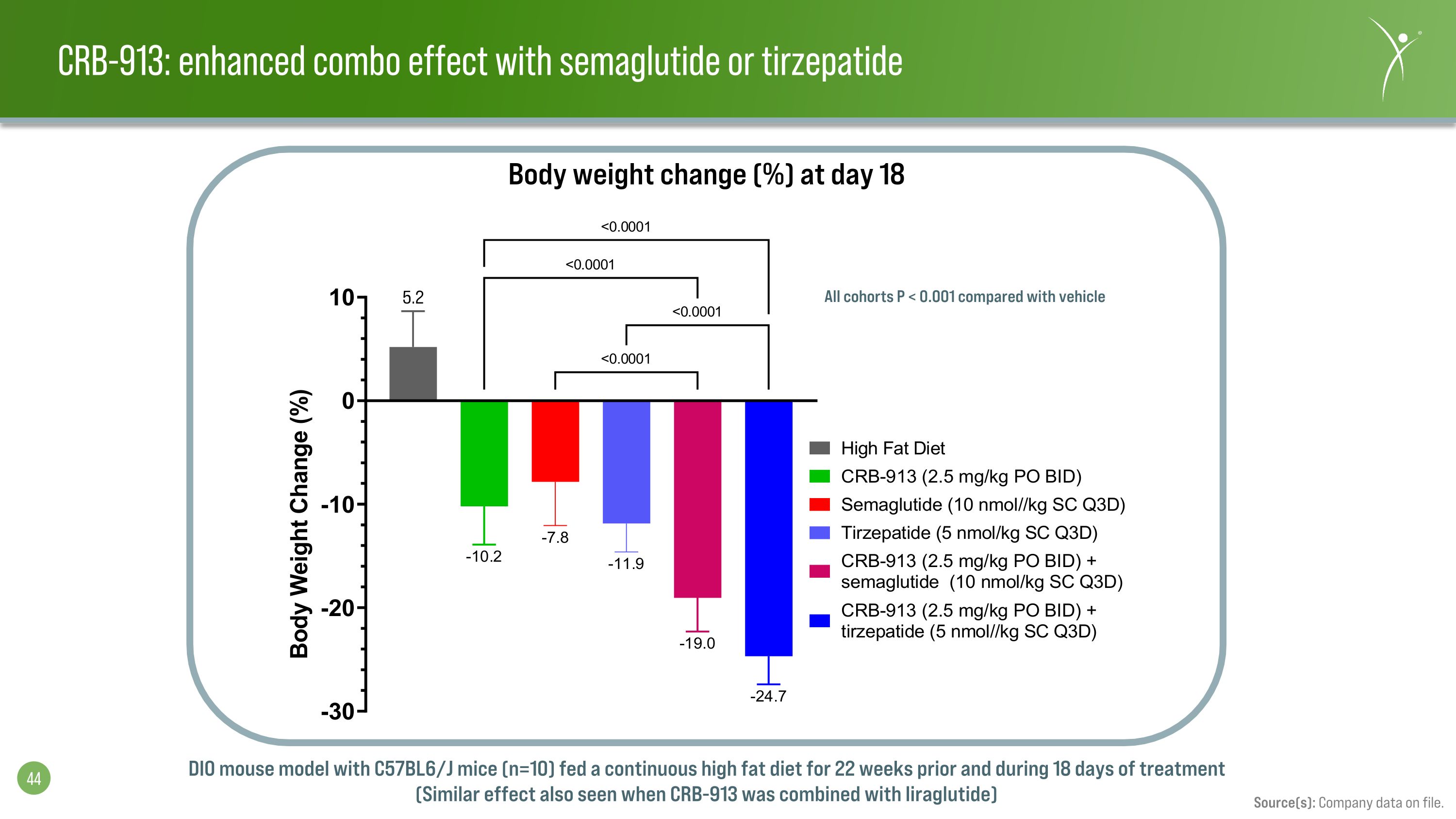
CRB-913: enhanced combo effect with semaglutide or tirzepatide Source(s): Company data on file. DIO mouse model with C57BL6/J mice (n=10) fed a continuous high fat diet for 22 weeks prior and during 18 days of treatment (Similar effect also seen when CRB-913 was combined with liraglutide) Body weight change (%) at day 18 All cohorts P < 0.001 compared with vehicle 5.2
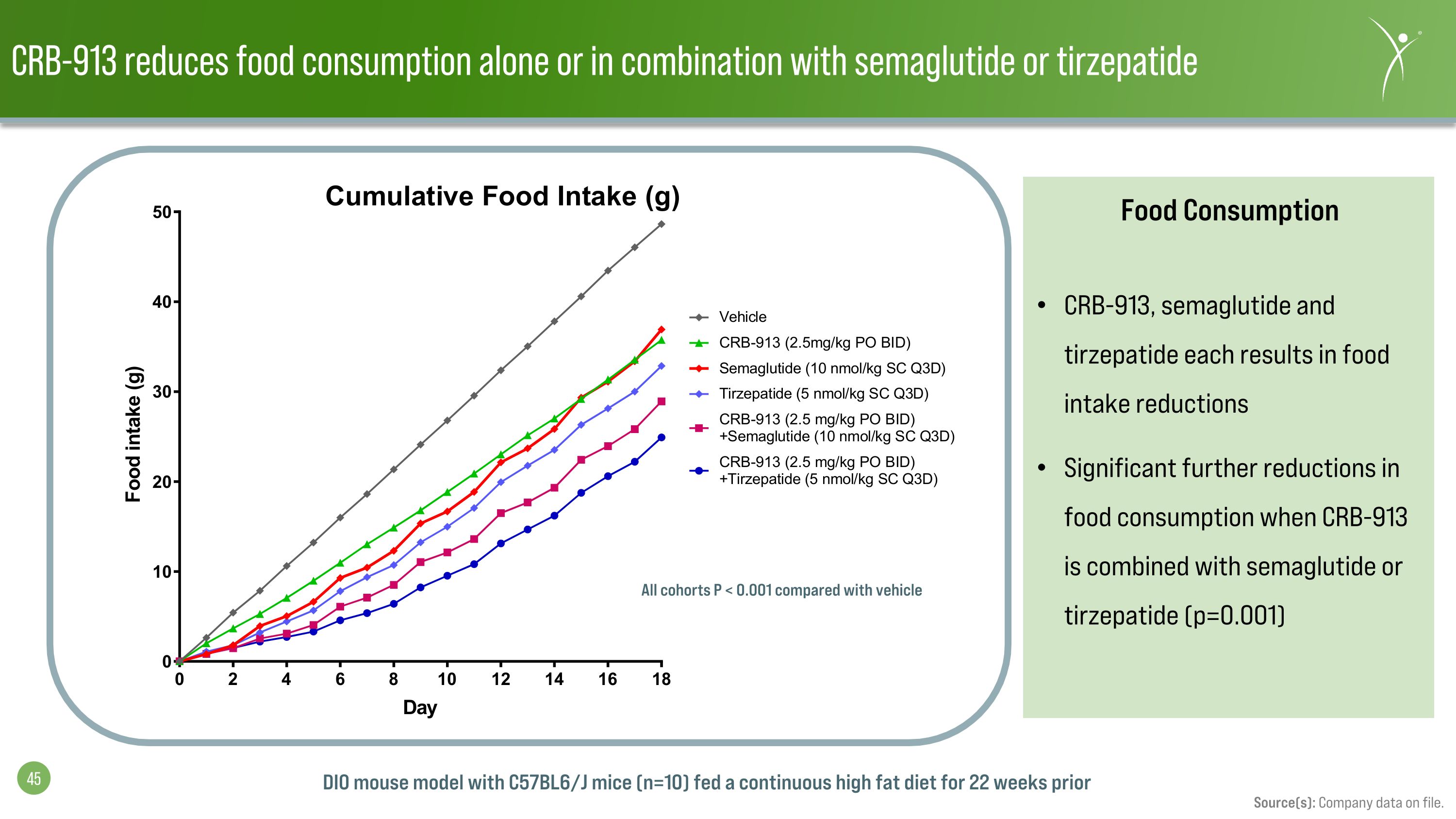
CRB-913 reduces food consumption alone or in combination with semaglutide or tirzepatide Source(s): Company data on file. Food Consumption CRB-913, semaglutide and tirzepatide each results in food intake reductions Significant further reductions in food consumption when CRB-913 is combined with semaglutide or tirzepatide (p=0.001) All cohorts P < 0.001 compared with vehicle DIO mouse model with C57BL6/J mice (n=10) fed a continuous high fat diet for 22 weeks prior
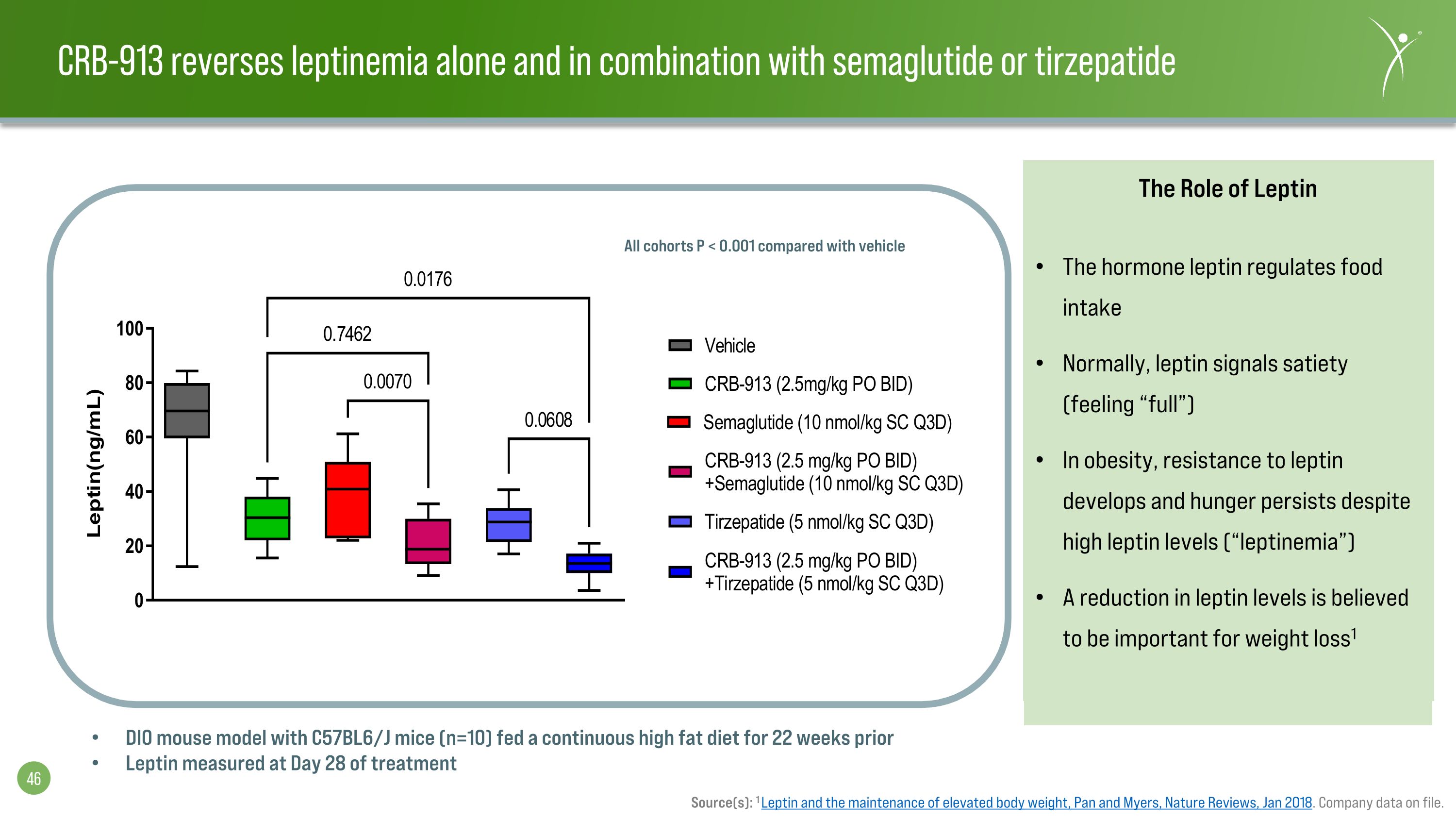
CRB-913 reverses leptinemia alone and in combination with semaglutide or tirzepatide Source(s): 1 Leptin and the maintenance of elevated body weight, Pan and Myers, Nature Reviews, Jan 2018. Company data on file. The Role of Leptin The hormone leptin regulates food intake Normally, leptin signals satiety (feeling “full”) In obesity, resistance to leptin develops and hunger persists despite high leptin levels (“leptinemia”) A reduction in leptin levels is believed to be important for weight loss1 DIO mouse model with C57BL6/J mice (n=10) fed a continuous high fat diet for 22 weeks prior Leptin measured at Day 28 of treatment All cohorts P < 0.001 compared with vehicle
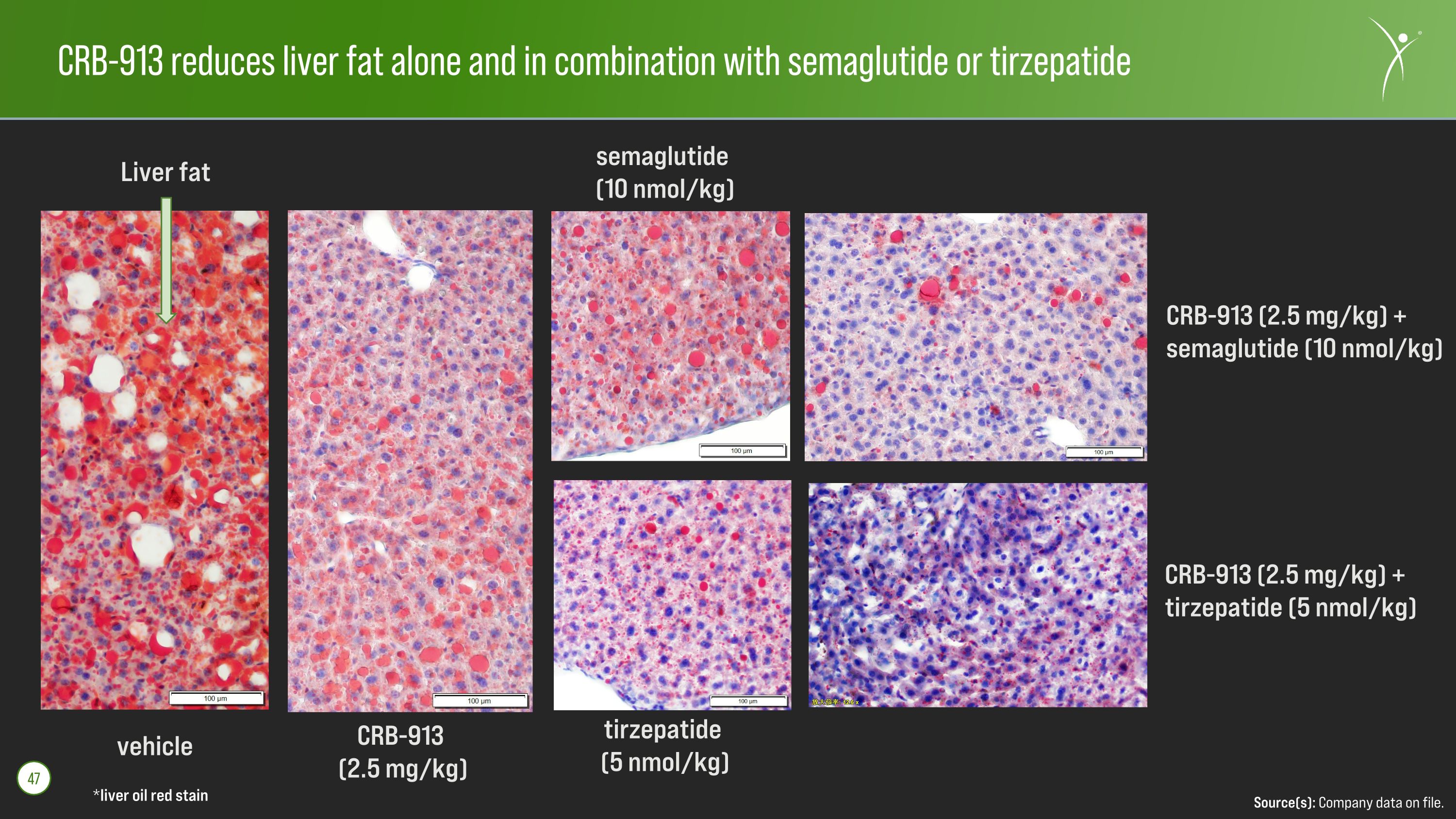
CRB-913 reduces liver fat alone and in combination with semaglutide or tirzepatide *liver oil red stain Source(s): Company data on file. 47 vehicle CRB-913 (2.5 mg/kg) semaglutide (10 nmol/kg) CRB-913 (2.5 mg/kg) + semaglutide (10 nmol/kg) tirzepatide (5 nmol/kg) CRB-913 (2.5 mg/kg) + tirzepatide (5 nmol/kg) Liver fat
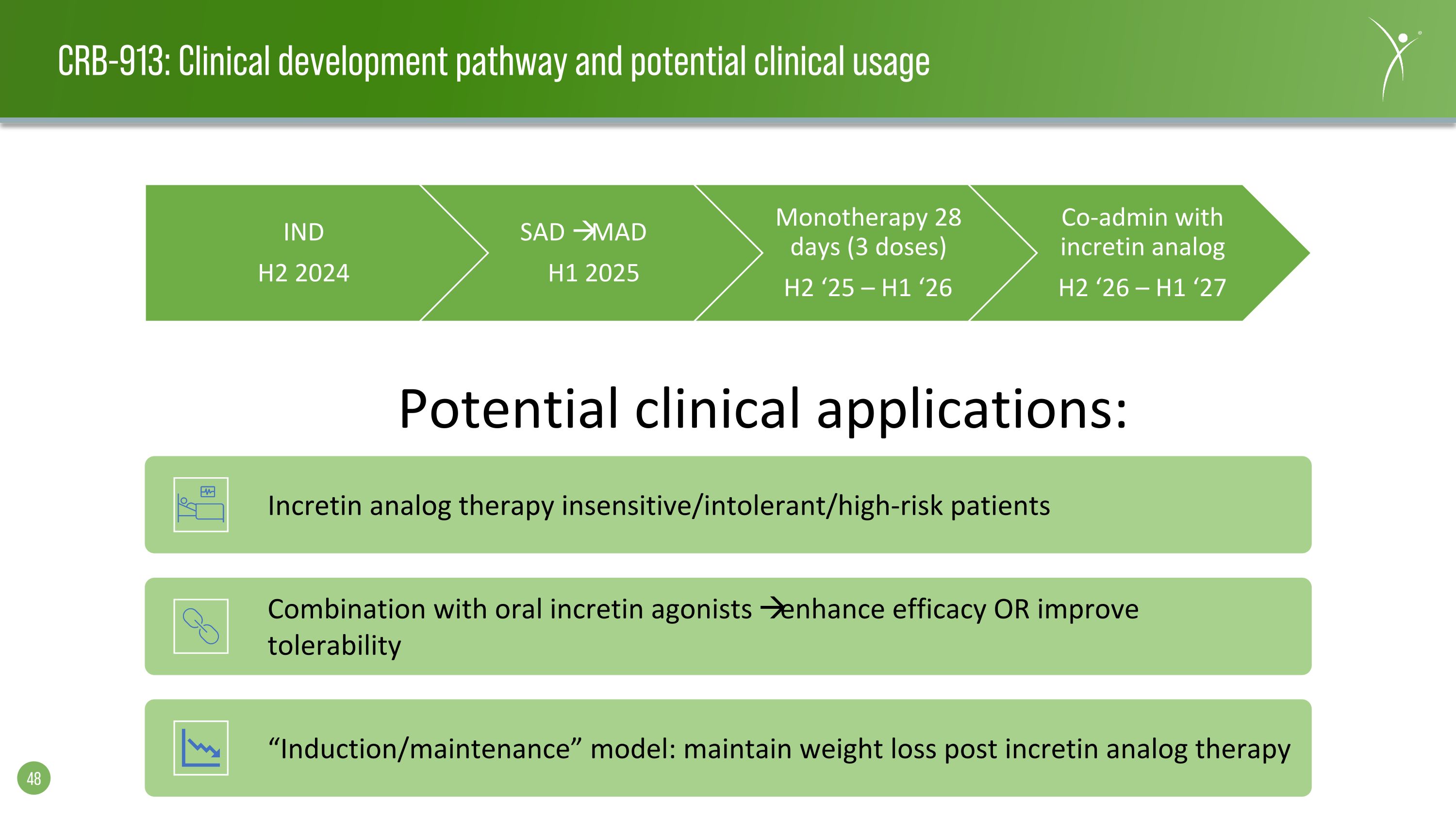
CRB-913: Clinical development pathway and potential clinical usage IND H2 2024 SAD MAD H1 2025 Monotherapy 28 days (3 doses) H2 ‘25 – H1 ‘26 Co-admin with incretin analog H2 ‘26 – H1 ‘27 Potential clinical applications: Incretin analog therapy insensitive/intolerant/high-risk patients Combination with oral incretin agonists enhance efficacy OR improve tolerability “Induction/maintenance” model: maintain weight loss post incretin analog therapy

CRB-913: designed to be a best-in-class next gen CB1 inverse agonist Best-in-class peripheral restriction Protect lean mass (muscle) Retain 1st gen efficacy Enhance efficacy of incretin analogs

Leadership�Upcoming catalysts Financials

Management Team Yuval Cohen, PhD Chief Executive Officer, Director Corbus co-founder and Chief Executive Officer since 2014. Previously the President and co-founder of Celsus Therapeutics from 2005. Sean Moran, CPA, MBA Chief Financial Officer Corbus co-founder and Chief Financial Officer since 2014. Prior senior financial management experience in emerging biotech and medical device companies. Rachael Brake, PhD Chief Scientific Officer Expert in developing and executing innovative drug discovery and clinical development oncology programs at several leading pharmaceutical companies. Christina Bertsch Head of Human Resources Accomplished senior human resource executive providing strategic HR consulting services to both large and small businesses across a variety of industries

Board of Directors Amb. Alan Holmer Ret. �Chairman of the Board More than two decades of public service in Washington, D.C. including Special Envoy to China; Former CEO of PhRMA. Avery W. (Chip) Catlin �Director More than 25 years of senior financial leadership experience in life science companies; Former CFO and Secretary �of Celldex Therapeutics. Yuval Cohen, PhD Chief Executive Officer, Director Corbus co-founder and Chief Executive Officer since 2014. Previously the President and co-founder of Celsus Therapeutics from 2005. Rachelle Jacques �Director More than 25-year professional career, experience in U.S. and global biopharmaceutical commercial leadership, including multiple �high-profile product launches in rare diseases; CEO of Akari Therapeutics. (NASDAQ: AKTX) John K. Jenkins, MD �Director Distinguished 25-year career serving at the U.S. FDA, including 15 years of senior leadership in CDER and OND. Pete Salzmann, MD, MBA �Director 20 years of industry experience and currently serves as Chief Executive Officer of Immunovant (NASDAQ: IMVT), a biopharmaceutical company focused on developing therapies for patients with autoimmune diseases. Anne Altmeyer, PhD, MBA, MPH�Director 20 years of experience advancing oncology R&D programs and leading impactful corporate development transactions; currently President & CEO of TigaTx. Yong (Ben) Ben, MD, MBA �Director 25 years of oncology R&D experience across industry and academia. Held two industry CMO positions, most recently at BeiGene (BGNE).
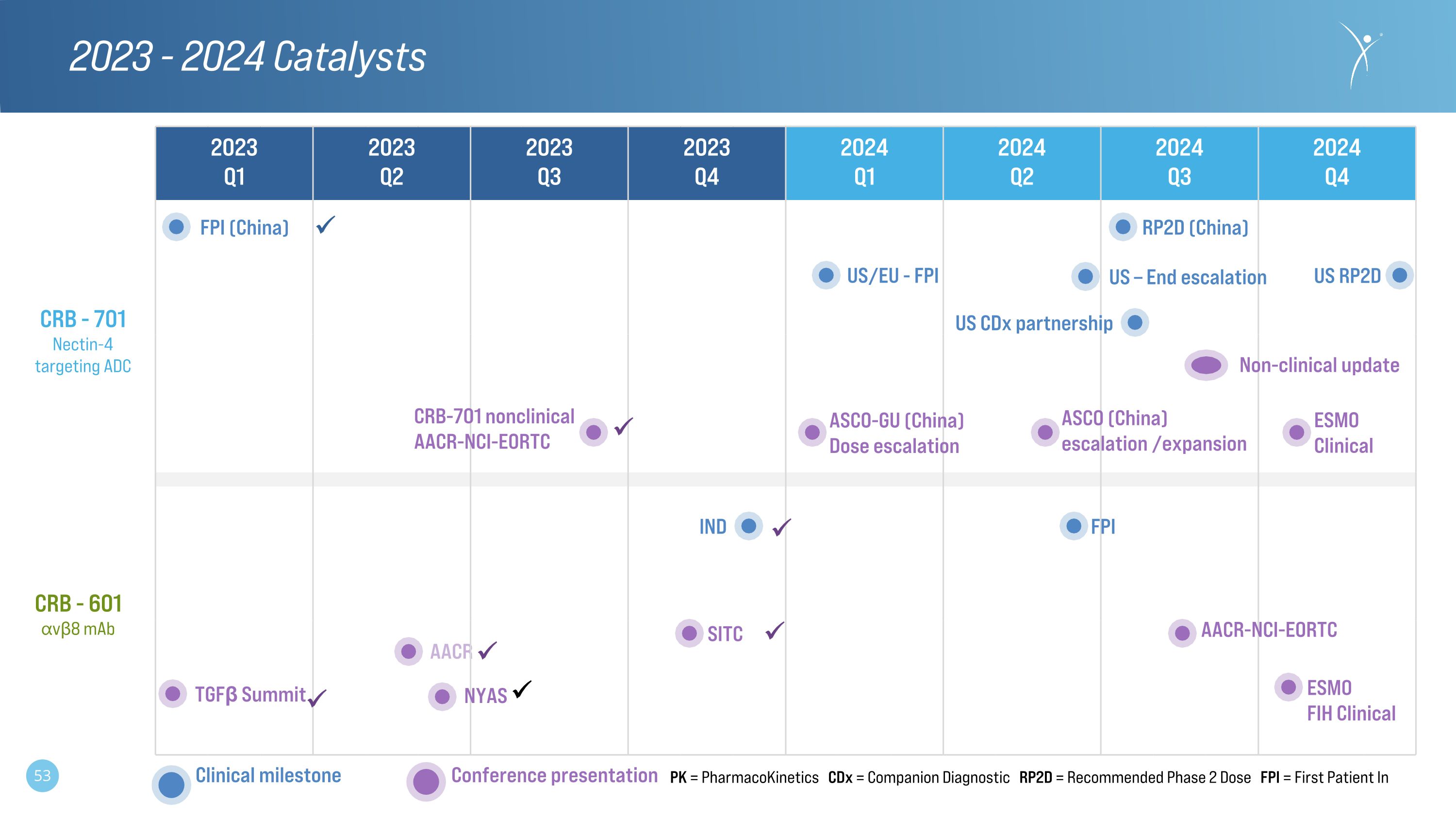
AACR 2023 - 2024 Catalysts 2023 Q1 2023 Q2 2023 Q3 2023 Q4 2024�Q1 2024 Q2 2024 Q3 2024�Q4 CRB - 601�⍺vβ8 mAb CRB - 701�Nectin-4 targeting ADC IND FPI AACR-NCI-EORTC TGFβ Summit NYAS Conference presentation Clinical milestone SITC PK = PharmacoKinetics CDx = Companion Diagnostic RP2D = Recommended Phase 2 Dose FPI = First Patient In ESMO FIH Clinical US CDx partnership CRB-701 nonclinical AACR-NCI-EORTC Non-clinical update ASCO (China) escalation /expansion FPI (China) RP2D (China) ASCO-GU (China) Dose escalation US/EU - FPI US – End escalation US RP2D ESMO Clinical
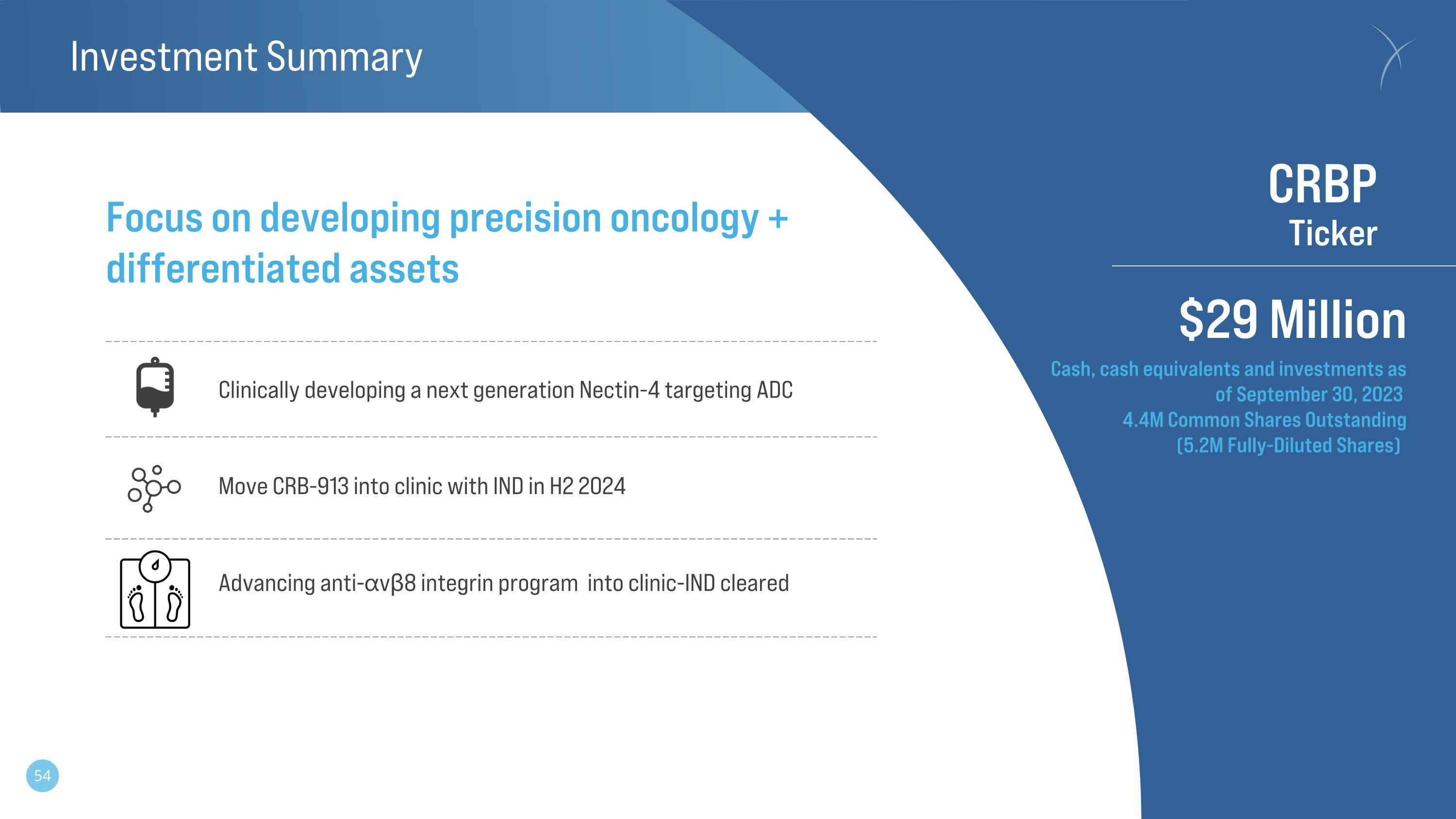
Investment Summary Advancing anti-⍺vβ8 integrin program into clinic-IND cleared CRBP�Ticker $29 Million Focus on developing precision oncology + differentiated assets Clinically developing a next generation Nectin-4 targeting ADC Cash, cash equivalents and investments as �of September 30, 2023 4.4M Common Shares Outstanding�(5.2M Fully-Diluted Shares) Move CRB-913 into clinic with IND in H2 2024

Appendix

CRB-601�Potential “best-in-class” �⍺vβ8 mAb
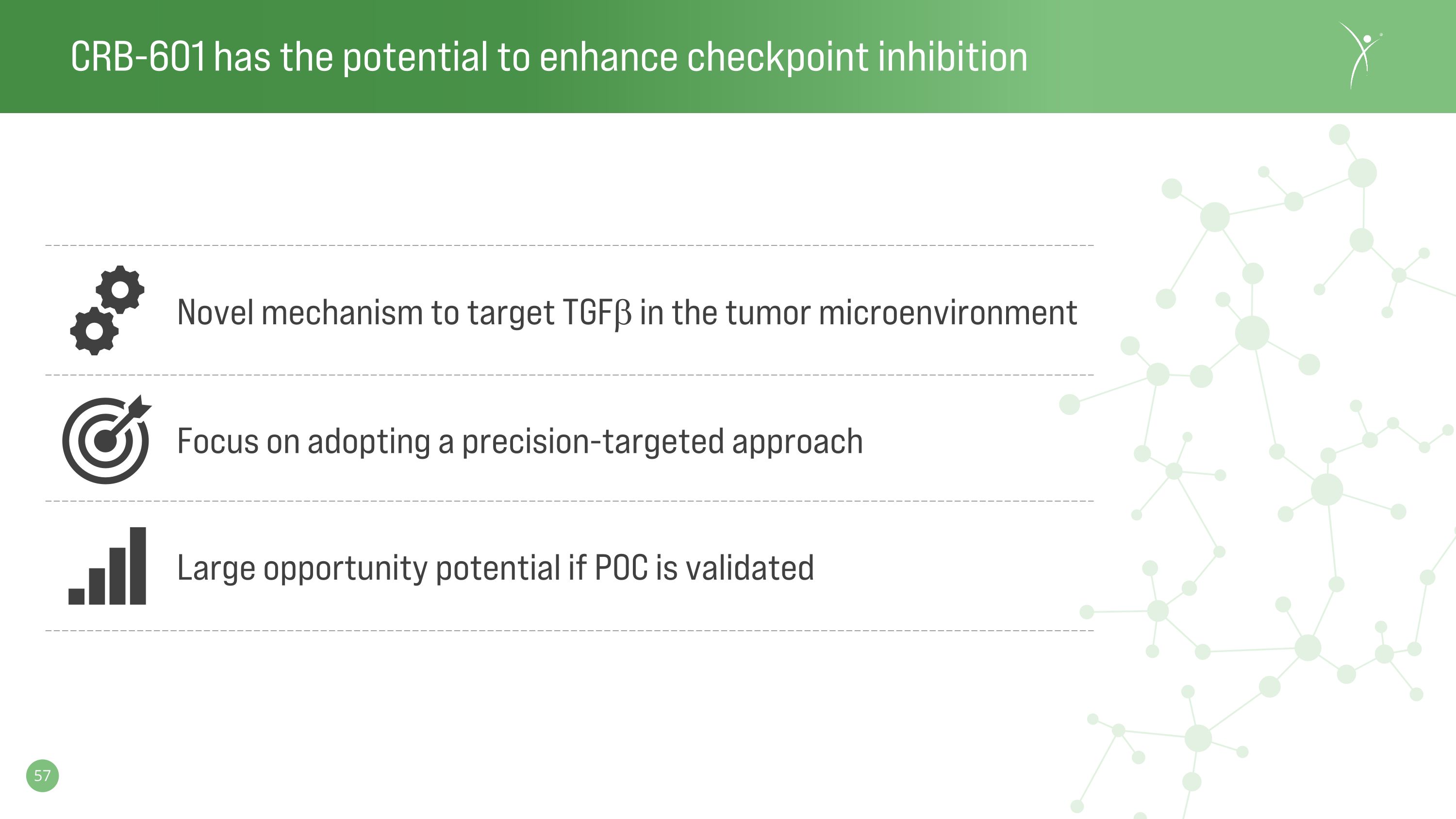
CRB-601 has the potential to enhance checkpoint inhibition Focus on adopting a precision-targeted approach Novel mechanism to target TGFb in the tumor microenvironment Large opportunity potential if POC is validated
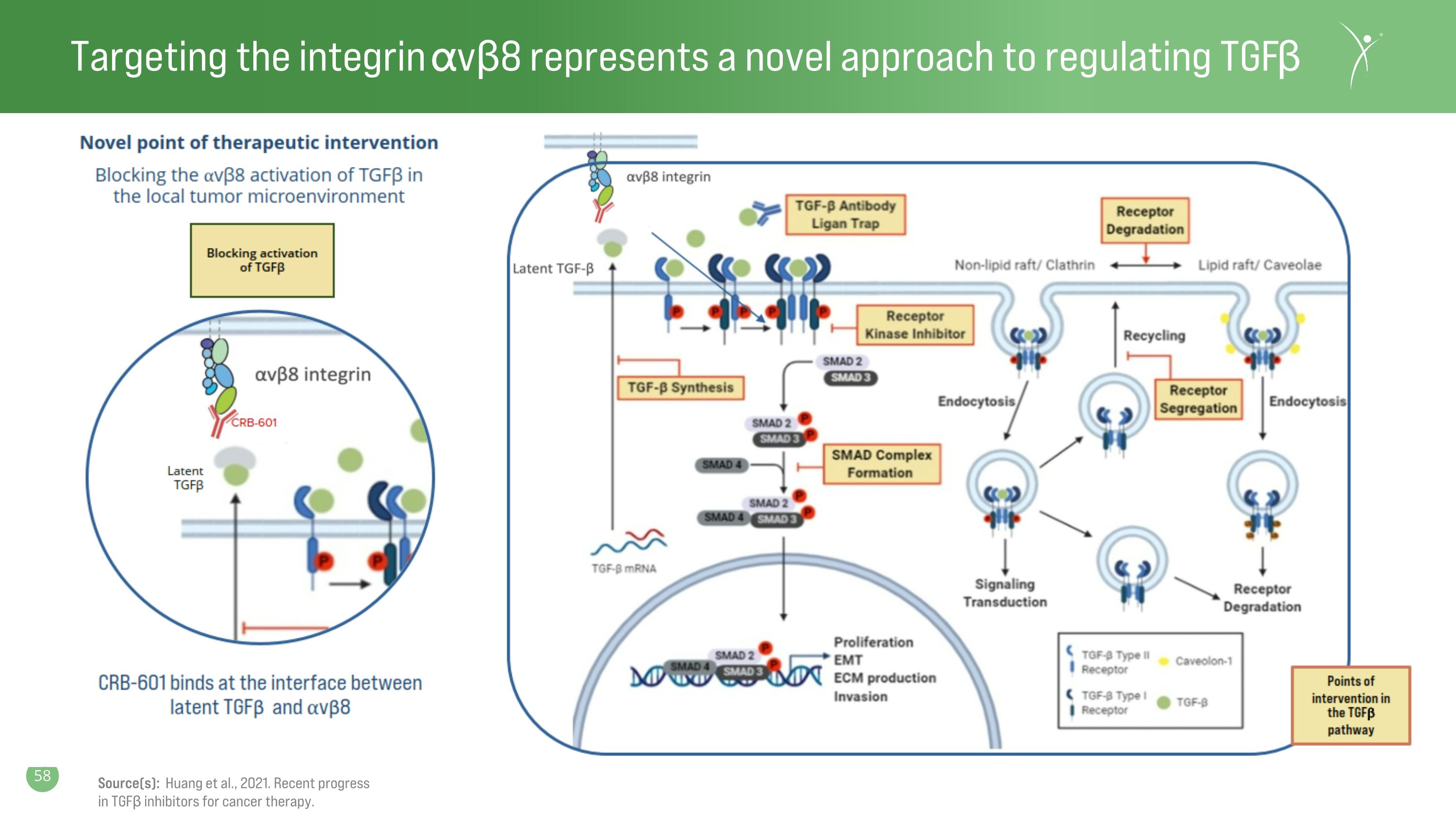
Targeting the integrin ⍺vβ8 represents a novel approach to regulating TGFβ Source(s): Huang et al., 2021. Recent progress in TGFβ inhibitors for cancer therapy.
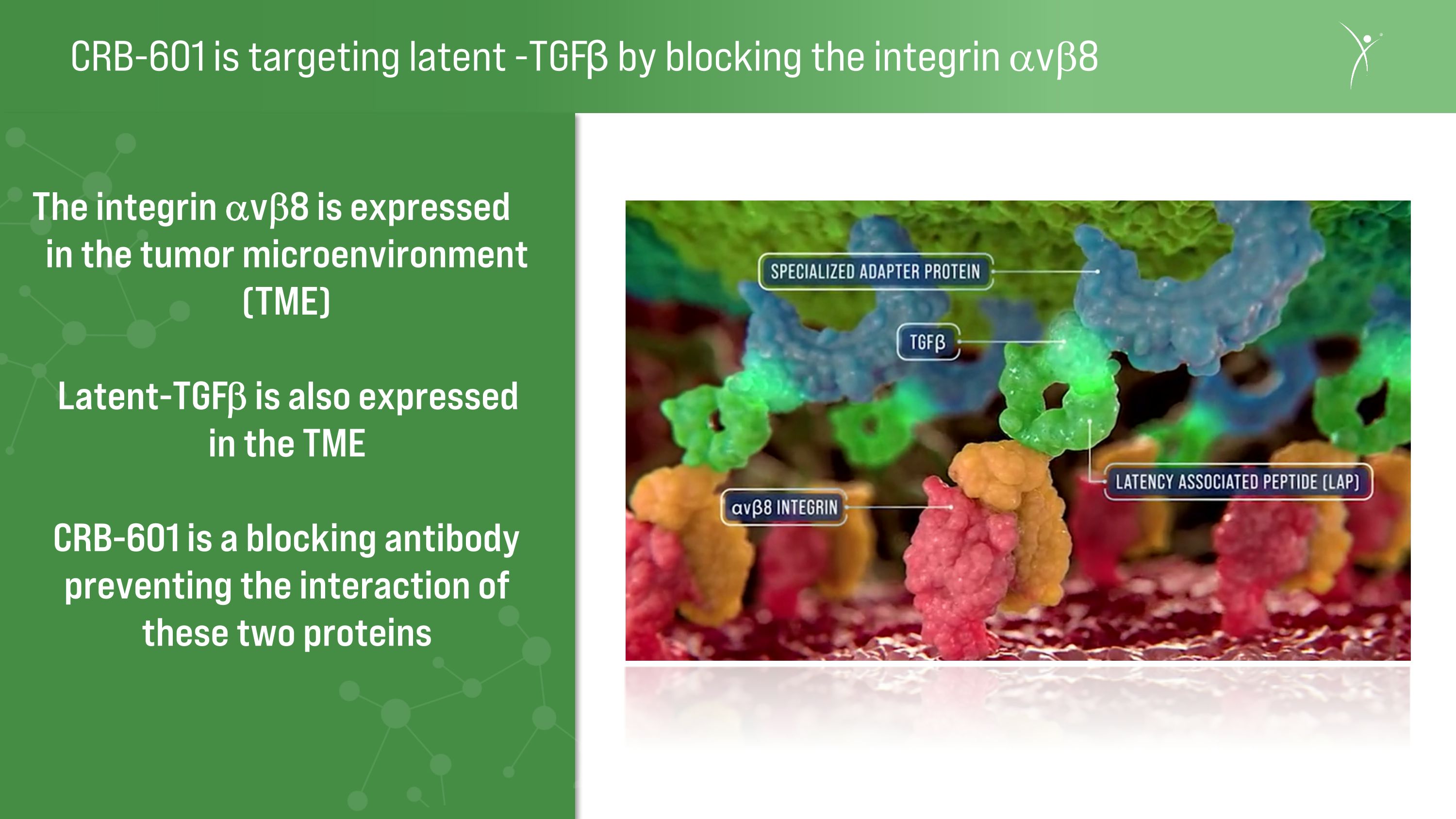
CRB-601 is targeting latent -TGFβ by blocking the integrin avb8 The integrin avb8 is expressed in the tumor microenvironment (TME) Latent-TGFb is also expressed�in the TME CRB-601 is a blocking antibody preventing the interaction of these two proteins
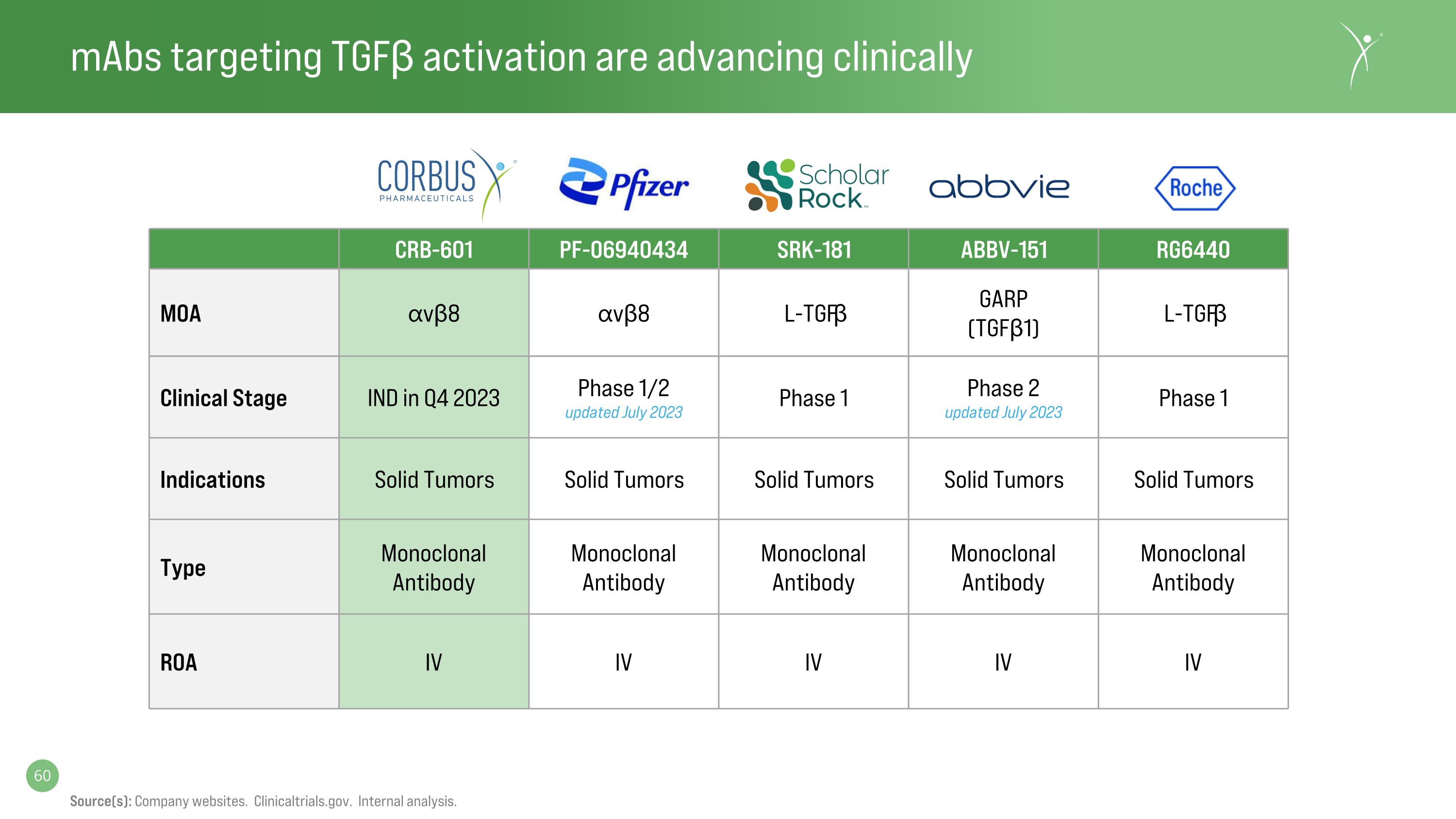
mAbs targeting TGFβ activation are advancing clinically Source(s): Company websites. Clinicaltrials.gov. Internal analysis. CRB-601 PF-06940434 SRK-181 ABBV-151 RG6440 MOA ⍺vβ8 ⍺vβ8 L-TGFβ GARP�(TGFβ1) L-TGFβ Clinical Stage IND in Q4 2023 Phase 1/2�updated July 2023 Phase 1 Phase 2�updated July 2023 Phase 1 Indications Solid Tumors Solid Tumors Solid Tumors Solid Tumors Solid Tumors Type Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody Monoclonal Antibody ROA IV IV IV IV IV
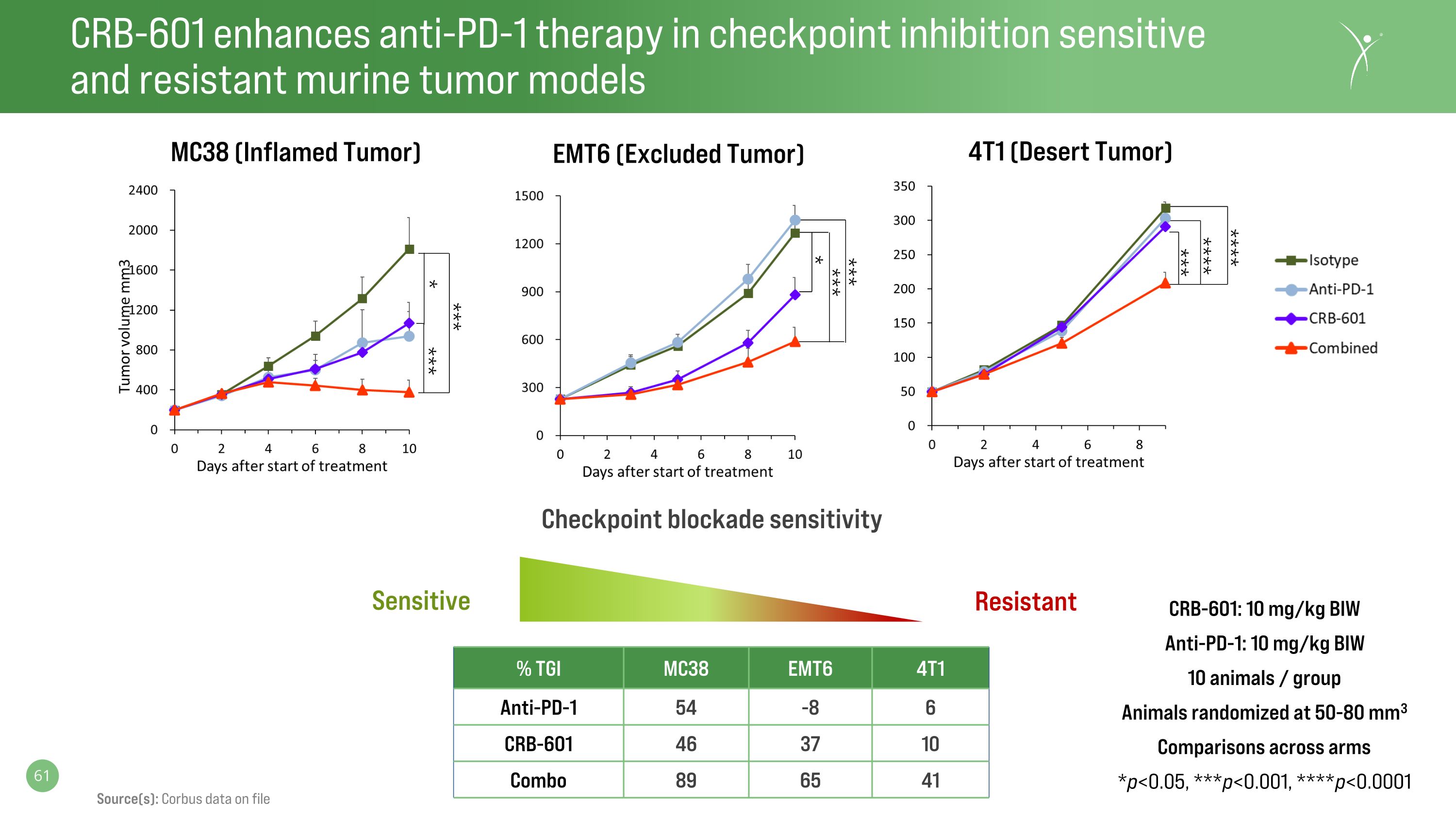
CRB-601 enhances anti-PD-1 therapy in checkpoint inhibition sensitive�and resistant murine tumor models CRB-601: 10 mg/kg BIW Anti-PD-1: 10 mg/kg BIW 10 animals / group Animals randomized at 50-80 mm3 Comparisons across arms *p<0.05, ***p<0.001, ****p<0.0001 % TGI MC38 EMT6 4T1 Anti-PD-1 54 -8 6 CRB-601 46 37 10 Combo 89 65 41 Resistant Checkpoint blockade sensitivity Sensitive MC38 (Inflamed Tumor) EMT6 (Excluded Tumor) 4T1 (Desert Tumor) *** **** **** *** * *** *** *** * Source(s): Corbus data on file
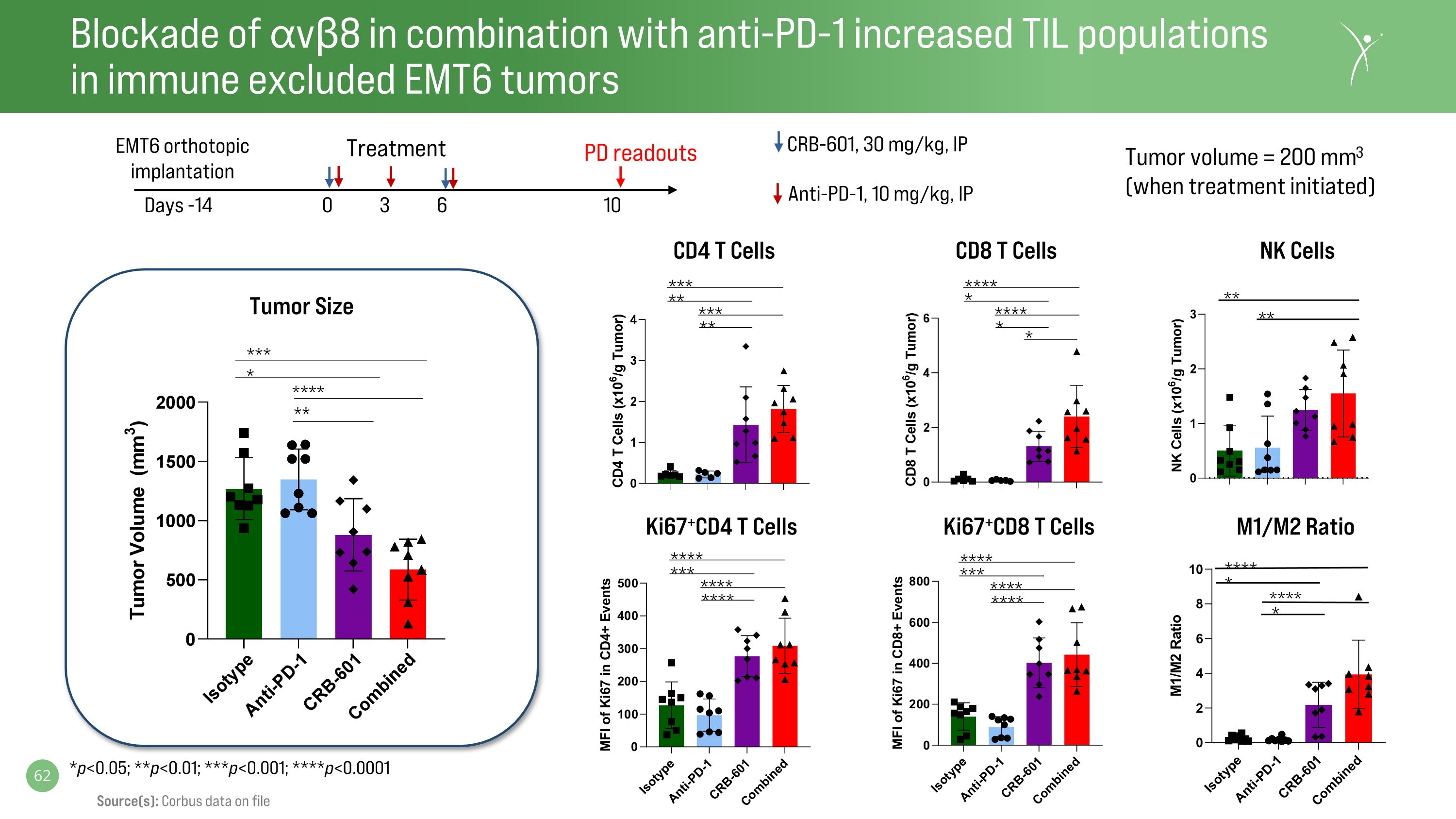
Blockade of ⍺vβ8 in combination with anti-PD-1 increased TIL populations�in immune excluded EMT6 tumors **** *** **** **** **** *** **** **** Ki67+CD4 T Cells Ki67+CD8 T Cells CD4 T Cells CD8 T Cells Tumor Size * *** * **** ** *** ** *** ** **** * **** * Treatment Days -14 0 3 6 10 Anti-PD-1, 10 mg/kg, IP CRB-601, 30 mg/kg, IP EMT6 orthotopic implantation PD readouts Tumor volume = 200 mm3 (when treatment initiated) *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001 M1/M2 Ratio NK Cells ** ** **** **** * * Source(s): Corbus data on file
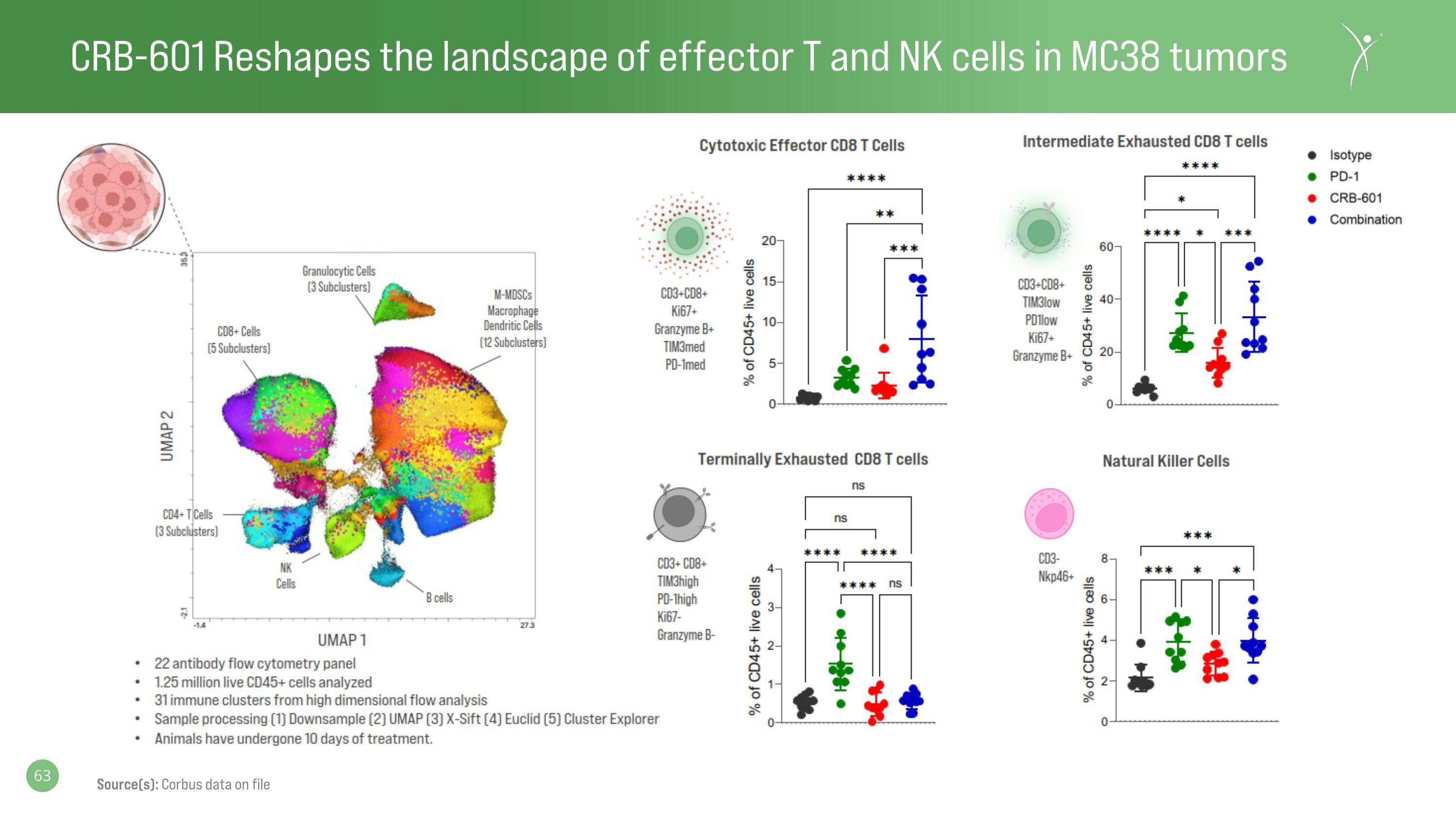
CRB-601 Reshapes the landscape of effector T and NK cells in MC38 tumors Source(s): Corbus data on file
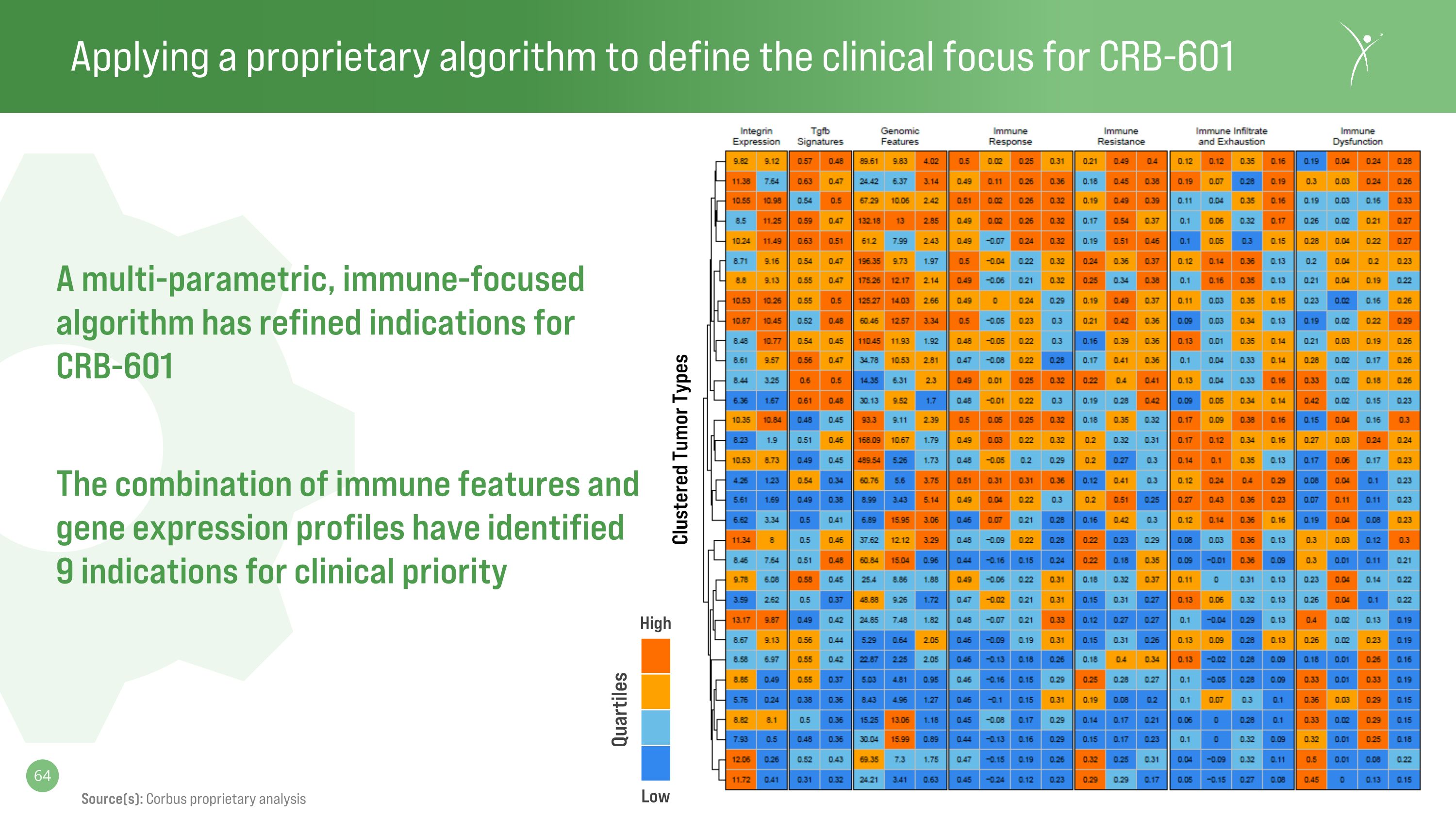
Applying a proprietary algorithm to define the clinical focus for CRB-601 A multi-parametric, immune-focused algorithm has refined indications for�CRB-601 The combination of immune features and gene expression profiles have identified�9 indications for clinical priority High Low Quartiles Source(s): Corbus proprietary analysis Clustered Tumor Types
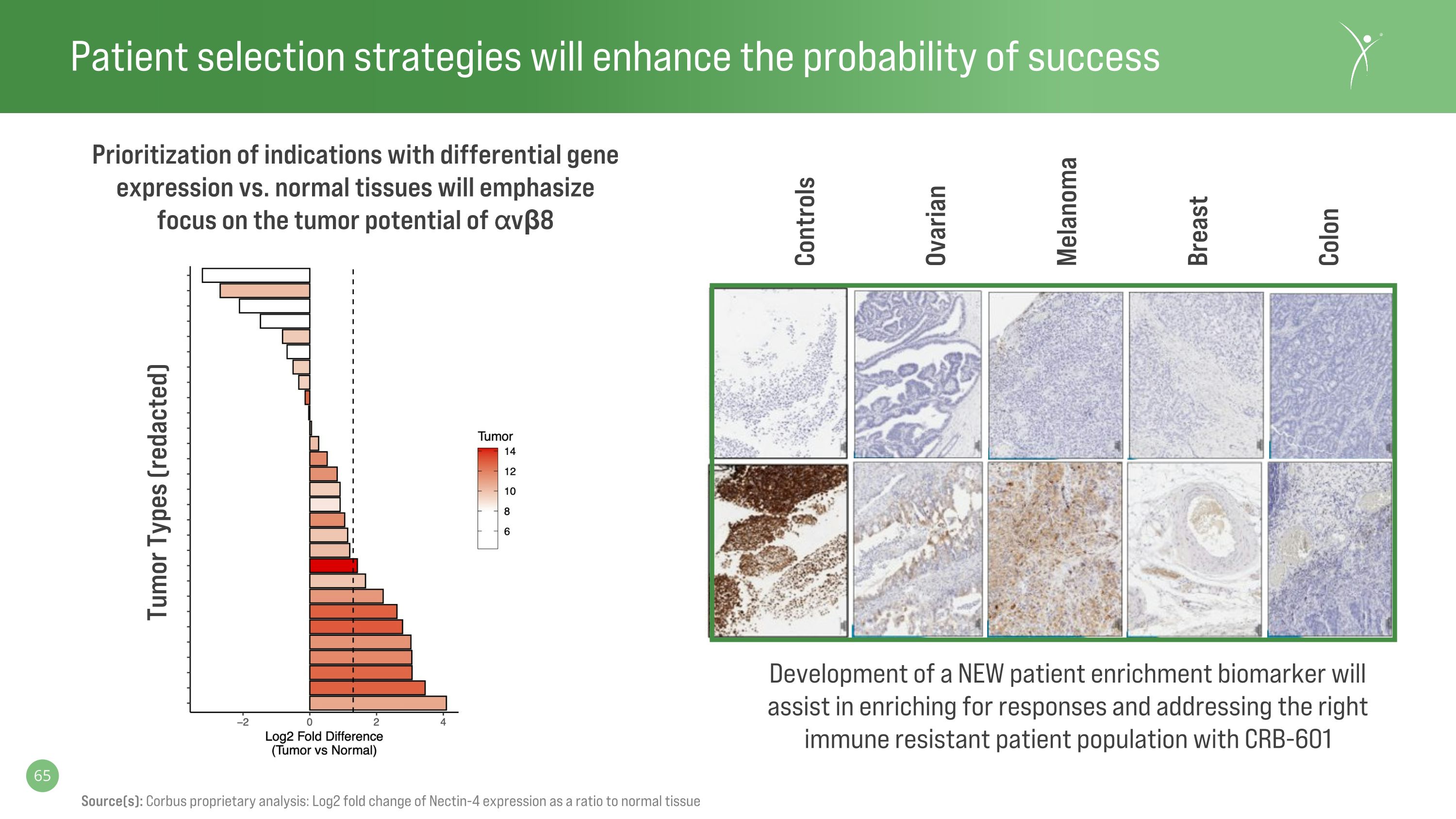
Patient selection strategies will enhance the probability of success Source(s): Corbus proprietary analysis: Log2 fold change of Nectin-4 expression as a ratio to normal tissue Controls Ovarian Melanoma Breast Colon Prioritization of indications with differential gene expression vs. normal tissues will emphasize focus on the tumor potential of ⍺vβ8 Development of a NEW patient enrichment biomarker will assist in enriching for responses and addressing the right immune resistant patient population with CRB-601 Tumor Types (redacted)

CRB-601 Next Steps IND cleared in January-2024 FPI expected H1-2024 Non-clinical validation of a potential patient selection biomarker in 2023 Dose escalation and confirmation will be the focus through 2024
v3.23.4
Document And Entity Information
|
Jan. 26, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 26, 2024
|
| Entity Registrant Name |
CORBUS PHARMACEUTICALS HOLDINGS, INC.
|
| Entity Central Index Key |
0001595097
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37348
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
46-4348039
|
| Entity Address, Address Line One |
500 River Ridge Drive
|
| Entity Address, City or Town |
Norwood
|
| Entity Address, State or Province |
MA
|
| Entity Address, Postal Zip Code |
02062
|
| City Area Code |
(617)
|
| Local Phone Number |
963-0100
|
| Entity Information, Former Legal or Registered Name |
Not Applicable
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.0001 per share
|
| Trading Symbol |
CRBP
|
| Security Exchange Name |
NONE
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Corbus Pharmaceuticals (NASDAQ:CRBP)
Historical Stock Chart
From Mar 2024 to Apr 2024
Corbus Pharmaceuticals (NASDAQ:CRBP)
Historical Stock Chart
From Apr 2023 to Apr 2024