false
0001377121
0001377121
2024-01-09
2024-01-09
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section
13 or 15(d)
of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported): January 9, 2024
PROTAGONIST THERAPEUTICS, INC.
(Exact name of registrant as specified
in its charter)
| Delaware |
|
001-37852 |
|
98-0505495 |
(State or other jurisdiction
of incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
7707 Gateway Blvd., Suite 140
Newark, California 94560-1160
(Address of principal executive offices,
including zip code)
(510) 474-0170
(Registrant’s telephone number, including
area code)
Not Applicable
(Former name or former address, if changed
since last report.)
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which
registered |
| Common Stock, par value $0.00001 |
|
PTGX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities
Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ¨
If an emerging
growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any
new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 8.01. Other Events
On January 9, 2024, Protagonist Therapeutics, Inc. (the “Company”)
presented at the 42nd Annual J.P. Morgan Healthcare Conference held in San Francisco, CA. A copy of the presentation is filed as Exhibit 99.1
to this Current Report on Form 8-K and is incorporated by reference herein.
| Item 9.01 |
Financial Statements and Exhibits |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
Protagonist Therapeutics, Inc. |
| |
|
|
| Dated: January 16, 2024 |
|
|
| |
|
|
| |
By: |
/s/
Asif Ali |
| |
|
Asif Ali |
| |
|
Chief Financial Officer |
Exhibit 99.1

1 JP Morgan 42 nd Annual Healthcare Conference Dinesh V. Patel, Ph.D. President & CEO January 09, 2024

Forward - looking Statements 2 This presentation and the accompanying oral presentation contain forward - looking statements made pursuant to the safe harbor pro visions of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this pre sentation, including statements regarding our future results of operations and financial position, business strategy, product candidates, capital res ources, potential markets for our product candidates, our plans related to potential future collaboration arrangements, the impact on our business or p rod uct candidates of actions or determinations of the U.S. Food and Drug Administration (“FDA”), enrollment in our clinical trials, any potential impact o n o ur business related to COVID - 19, our potential receipt of milestone payments and royalties under our Collaboration Agreement with Janssen Biotech, Inc. , are forward - looking statements. In some cases, you can identify forward - looking statements by terminology such as “anticipate,” “believe,” “continue ,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “will” or the negative of these terms or other similar exp ressions. The forward - looking statements made in this presentation involve known and unknown risks, uncertainties and other important fact ors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievemen ts expressed or implied by the forward - looking statements. These forward - looking statements are subject to risks and uncertainties, including those discuss ed in Protagonist’s filings with the Securities and Exchange Commission, including in the “Risk Factors” and “Management’s Discussion and Analysi s o f Financial Condition and Results of Operations” sections of most recently filed periodic reports on Form 10 - K and Form 10 - Q and subsequent filings an d in the documents incorporated by reference therein. Because forward - looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward - looking statements as pre dictions of future events. The events and circumstances reflected in our forward - looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward - looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or othe rwi se. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. They are currently limited by Federal law to investigational use, and no representation is made as to their s afe ty or effectiveness for the purposes for which they are being investigated. The trademarks included herein are the property of the owners thereof and are us ed for reference purposes only. Such use should not be construed as an endorsement of such products. Nothing contained in this presentation i s, or should be construed as, a recommendation, promise or representation by the presenter or Protagonist or any director, employee, agent or ad visor of Protagonist. This presentation does not purport to be all inclusive or to contain all the information you may desire.
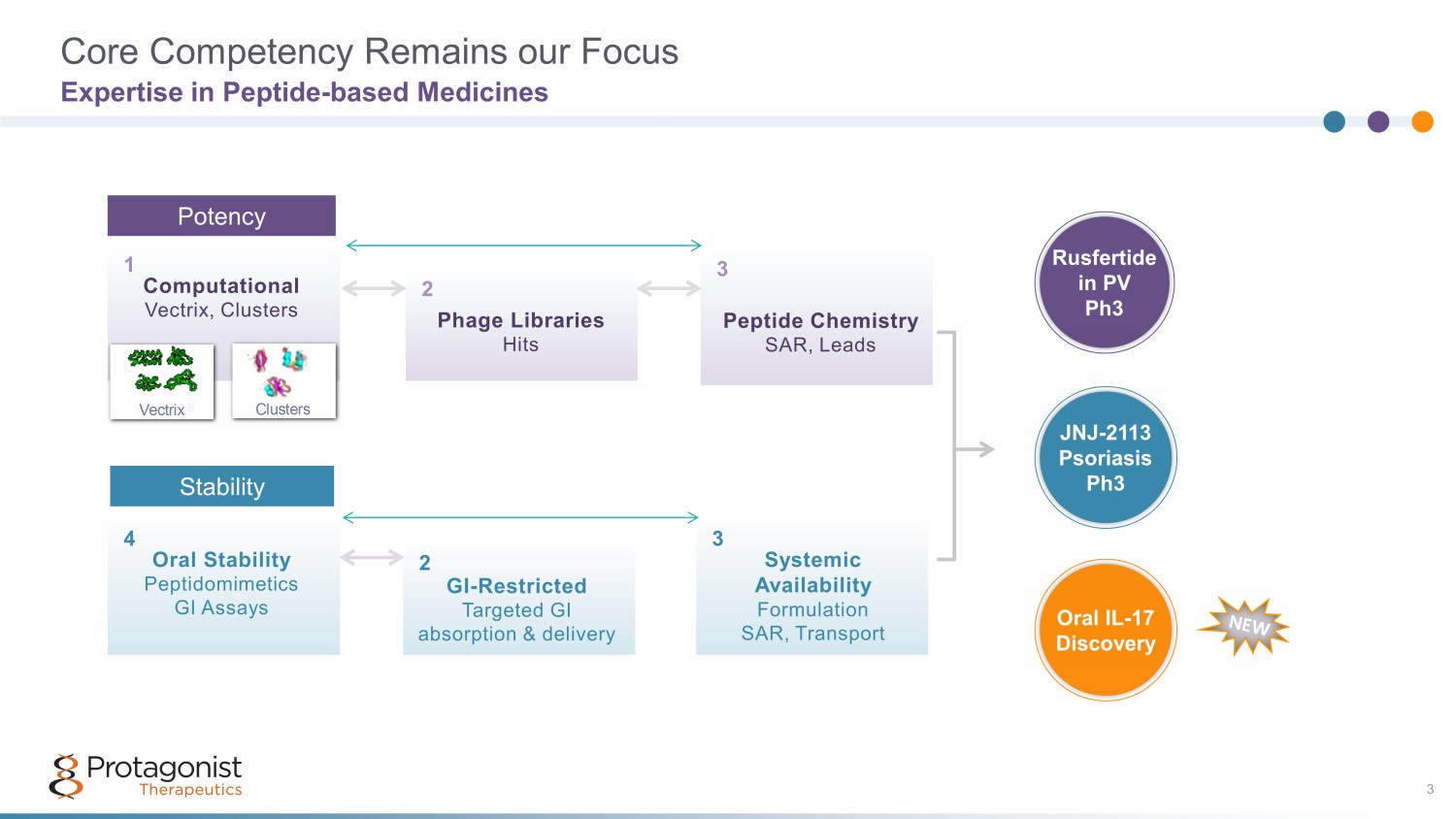
Core Competency Remains our Focus Expertise in Peptide - based Medicines Potency Peptide Chemistry SAR, Leads Phage Libraries Hits Computational Vectrix, Clusters Vectrix ® Clusters 1 2 3 POTENCY Oral Stability Peptidomimetics GI Assays GI-Restricted Targeted GI absorption & delivery Systemic Availability Formulation SAR, Transport 4 5 6 STABILITY Computational Vectrix , Clusters 1 Peptide Chemistry SAR, Leads Phage Libraries Hits Computational Vectrix, Clusters Vectrix ® Clusters 1 2 3 POTENCY Oral Stability Peptidomimetics GI Assays GI-Restricted Targeted GI absorption & delivery Systemic Availability Formulation SAR, Transport 4 5 6 STABILITY Peptide Chemistry SAR, Leads 3 Phage Libraries Hits 2 Stability Oral Stability Peptidomimetics GI Assays 4 Systemic Availability Formulation SAR, Transport 3 GI - Restricted Targeted GI absorption & delivery 2 JNJ - 2113 h3 3 JNJ - 2113 Psoriasis Ph3 Rusfertide in PV Ph3 Oral IL - 17 Discovery

Disc./Pre - Clinical Phase 1 Phase 2 Phase 3 Key Milestones ICONIC - LEAD Ph3, n~600 PACIFIC Ph2 Elevated Hct (>48%), n=20 REVIVE Ph2, n=70, 40 wk study + 3 yr OLE RUSFERTIDE Hepcidin Mimetic • Enrollment completion 1Q 24 • Completed; OLE ongoing • Completed 1 See clinicaltrials.gov NCT06095115 2 See clinicaltrials.gov NCT06095102 3 See clinicaltrials.gov NCT06049017 JNJ - 2113 Oral IL - 23R Peptide Antagonist Polycythemia Vera (PV) FRONTIER 1 & 2 Ph2b, n~255 ICONIC - TOTAL Ph3 in special areas of psoriasis, n~300 Psoriasis VERIFY Ph3, n~250 • Completed • Completion ~ May ’25 3 Product Pipeline: Multiple Assets with Multi - Billion Dollar Market Potential Ulcerative Colitis (UC) ANTHEM Ph2b, n~240 HEMATOLOGY • Primary: PASI 90 & IGA 0/1; completion ~ Nov ’24 1 • Primary: IGA 0/1; completion ~ Nov ’24 2 4 THRIVE LTE • For REVIVE patients on years 3 - 5 I & I ICONIC - ADVANCE 1 Ph 3, n~750 ICONIC - ADVANCE 2 Ph 3, n~675 • S uperiority study vs. deucravacitinib ; planned • S uperiority study vs. deucravacitinib ; planned • Oral IL - 17 peptide antagonist program Discovery HEME Oral IL - 17 DISCOVERY • Hits/Leads in heme program

Rusfertide Hepcidin Hormone Mimetic Addressing Unmet Needs in Polycythemia Vera

Myeloproliferative neoplasm characterized by excessive production of red blood cells ( RBCs) 1 Treatment goal is to control HCT <45% to minimize TEs, CV risks and death 3 • Elevated hematocrit ( Hct ) is a hallmark of the disease 2 Serious, chronic disease associated with increased thrombotic and cardiovascular risks 1 - 3 Rare disease with ~100,000 diagnosed and treated patients in US 4 • Diagnosed commonly in individuals 50 - 70 years of age • Median survival ~20 years Polycythemia Vera Disease Background 1. ~160,000 diagnosed patients in USA. NORD Rare Disease Database, Polycythemia Vera. https:// rarediseases.org /rare - diseases/polycythemia - vera/ 2. Spivak JL. Ann Hematol 2018; 19(2):1 - 14. 3. Marchioli R, et al. N Engl J Med 2013; 368:22 - 33 4. Internal estimates based on data on file 6

4 3 2 Polycythemia Vera 7 • Burdensome symptoms including fatigue and concentration problems 2, 3 A Blood Disorder with Significant Unmet Medical Need 4 Uncontrolled HCT is associated with higher rates of death from cardiovascular causes or thrombotic events 1 Real world data shows that up to 78% of patients have uncontrolled HCT with tests > 45% 4 • Current standard of care (SOC) approaches are inadequate for HCT control and symptoms management 5 There is no available pharmaceutical option with RBC - specific mechanism to target HCT • Rusfertide is a mimetic of natural iron homeostasis & erythrocytosis regulating hormone of Hepcidin 1. Marchioli R, et al. N Engl J Med 2013; 368:22 - 33. 2. Mesa R, et al. BMC Cancer. 2016;16,167. 3. MPN Landmark Survey, PV Report (2017). 4. Verstovsek S, et al. Real - world treatments and thrombotic events in polycythemia vera patients in the USA. Ann Hematol. 2023 Mar;102(3):571 - 581. doi : 10.1007/s00277 - 023 - 05089 - 6. Epub 2023 Jan 13. PMID: 36637474; PMCID: PMC9977710. Maintaining HCT<45% is critical, as per NCCN guidelines 1 Rusfertide , a hepcidin mimetic, could potentially provide an RBC - specific treatment option for PV
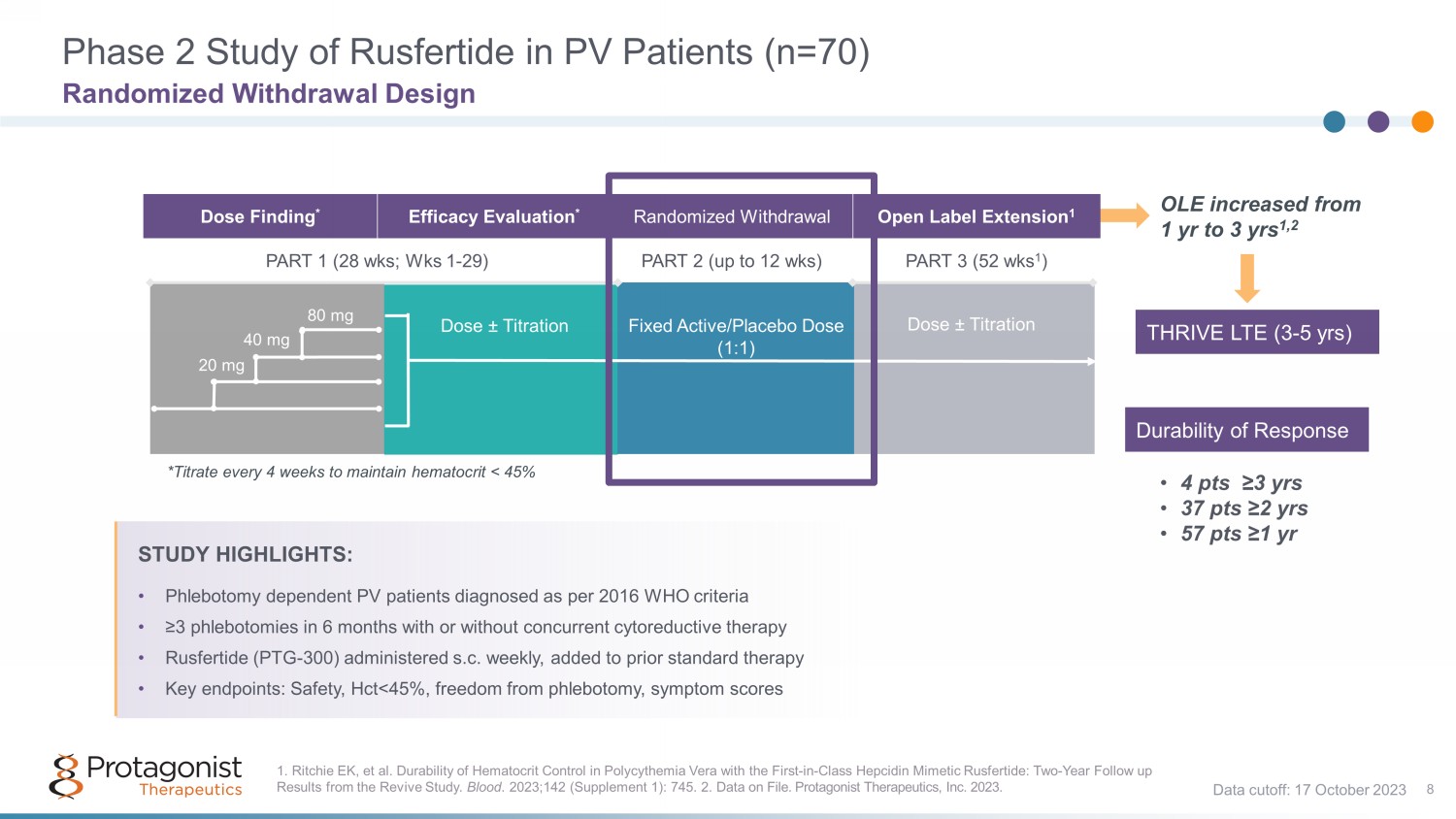
Dose Finding * Efficacy Evaluation * Randomized Withdrawal Open Label Extension 1 PART 1 (28 wks ; Wks 1 - 29) PART 2 (up to 12 wks ) PART 3 (52 wks 1 ) STUDY HIGHLIGHTS: • Phlebotomy dependent PV patients diagnosed as per 2016 WHO criteria • ≥3 phlebotomies in 6 months with or without concurrent cytoreductive therapy • Rusfertide (PTG - 300) administered s.c. weekly, added to prior standard therapy • Key endpoints: Safety, Hct <45%, freedom from phlebotomy, symptom scores Dose ± Titration Fixed Active/Placebo Dose (1:1) Dose ± Titration *Titrate every 4 weeks to maintain hematocrit < 45% 20 mg 40 mg 80 mg OLE increased from 1 yr to 3 yrs 1,2 • 4 pts ≥3 yrs • 37 pts ≥2 yrs • 57 pts ≥1 yr 1. Ritchie EK, et al. Durability of Hematocrit Control in Polycythemia Vera with the First - in - Class Hepcidin Mimetic Rusfertide: T wo - Year Follow up Results from the Revive Study. Blood . 2023;142 (Supplement 1): 745. 2. Data on File. Protagonist Therapeutics, Inc. 2023. Phase 2 Study of Rusfertide in PV Patients (n=70) Randomized Withdrawal Design 8 Data cutoff: 17 October 2023 Durability of Response THRIVE LTE (3 - 5 yrs )

Part 2: Blinded Randomized Withdrawal, Weeks 29 - 41 9 • 69.2% subjects (18/26) responders* – 8 non - responders as per protocol definition - 3 fulfilled the phlebotomy eligibility criteria - 5 discontinued treatment per patient/investigator discretion - 7 out of 8 non - responders continued treatment in OLE • 92.3% subjects (24 out of 26) in rusfertide arm did not receive phlebotomy in the 12 - week randomization part of the study Rusfertide Met the Primary Endpoint of Efficacy (p=0.0003) Placebo (N=27) Rusfertide (N=26) 0 10 20 30 40 50 60 70 80 69.2% 18.5% P e r c e n t o f R e s p o n d e r s p=0.0003 n=5 n=18 * Responder definition as per protocol • Did not receive a phlebotomy • Completed 12 weeks of treatment • Hematocrit control maintained without phlebotomy eligibility, which is defined as - Hematocrit ≥45% that was ≥3% higher than Week 29 pre - randomization hematocrit value or - Hematocrit >48% or - An increase of ≥5% in hematocrit compared to Week 29 pre - randomization hematocrit value Highly Significant Efficacy* in Rusfertide Arm vs. Placebo Adapted from Kremyanskaya et al. EHA2023; Abstract LB2710.

Phase 2 REVIVE Study: Safety and Exposure 10 Rusfertide Was Generally Well Tolerated; No New Safety Signals with Longer Follow - up 1 The nature and extent of the SAEs observed in the REVIVE study is consistent with comorbidities anticipated in the PV population, including vascular events 2 - 5 and skin cancer 6,7 Data cutoff: 17 October 2023 • Median duration of exposure to rusfertide was 105.4 weeks (3 - 182 weeks range) • Majority of TEAEs were Grade 1 or 2 – 77.1% of TEAEs had a maximum grade of 2 – 21.4% of TEAEs were grade 3 – No Grade 4 or 5 TEAEs – The most common TEAEs were injection site reactions, which were localized and grade 1 - 2 in severity and decreased in incidence over time • 14 patients (20%) experienced an SAE – Most SAEs were assessed as being unrelated to rusfertide by investigators 1. Adapte d from Ritchie EK, et al. Blood . 2023;142 (Supplement 1): 745. 2. Goyal RK, et al. Blood . 2014;124:4840. 3. Griesshammer M, et al. Ann Hematol . 2019;98:1071 - 82. 4. Pemmaraju N, et al. Leuk Res . 2022;115:106809. 5. Kuykendall A, et al. Blood 2023; 142 (Supplement 1): 137. 6. Landtblom AR, et al. Leukemia . 2018;32:2203 - 10. 7 . Pemmaraju N, et al., Blood 2023; 142 (Supplement 1): 3190.
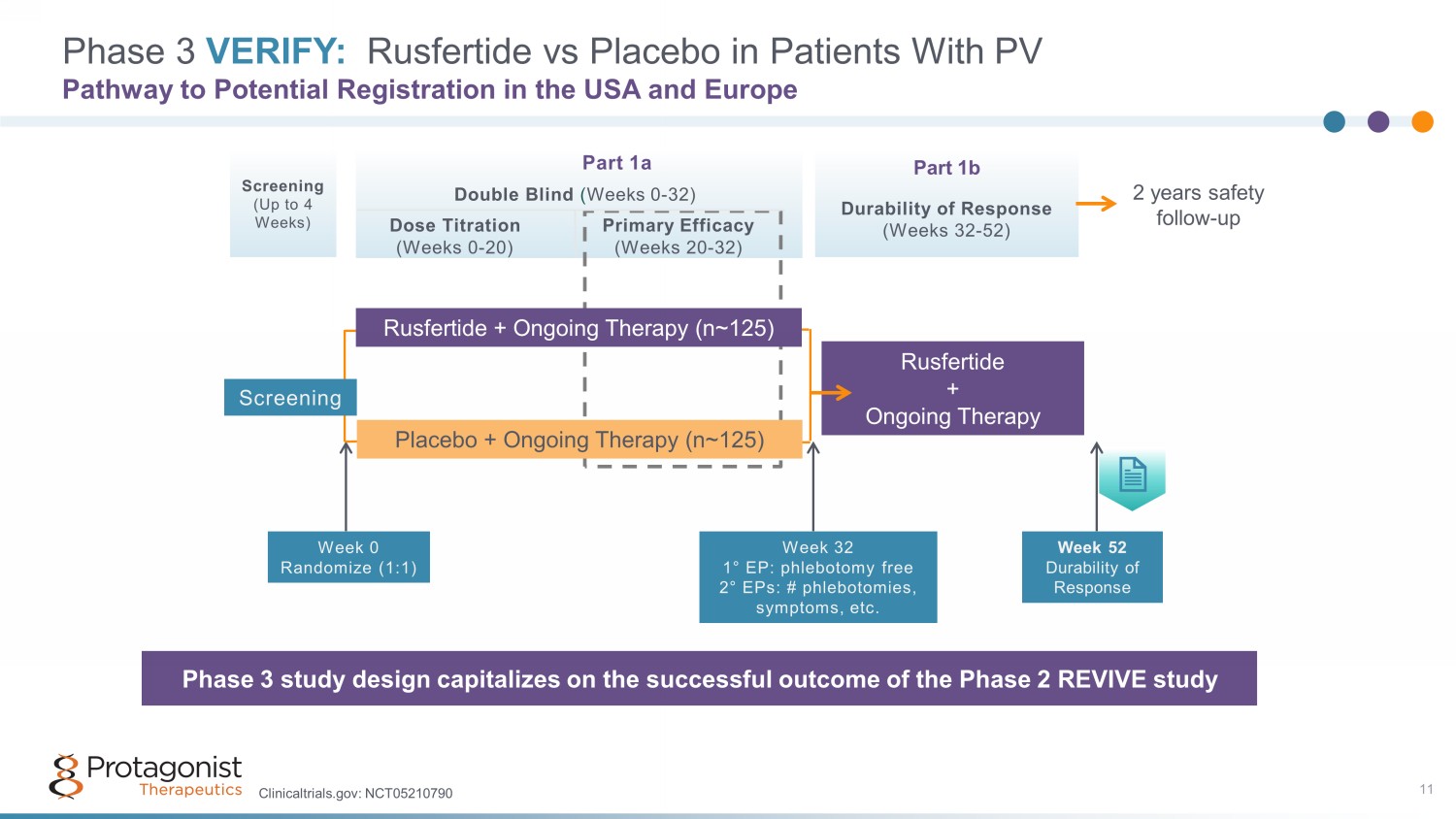
Part 1a Double Blind ( Weeks 0 - 32) Dose Titration Primary Efficacy (Weeks 0 - 20) (Weeks 20 - 32) Part 1b Durability of Response (Weeks 32 - 52) Screening (Up to 4 Weeks) Phase 3 VERIFY: Rusfertide vs Placebo in Patients With PV Pathway to Potential Registration in the USA and Europe Phase 3 study design capitalizes on the successful outcome of the Phase 2 REVIVE study Week 32 1 ° EP: phlebotomy free 2 ° EPs: # phlebotomies, symptoms, etc. Placebo + Ongoing Therapy (n~ 125 ) Rusfertide + Ongoing Therapy Rusfertide + Ongoing Therapy (n~ 125 ) Week 52 Durability of Response Week 0 Randomize (1:1) Screening 2 years safety follow - up 11 Clinicaltrials.gov: NCT05210790
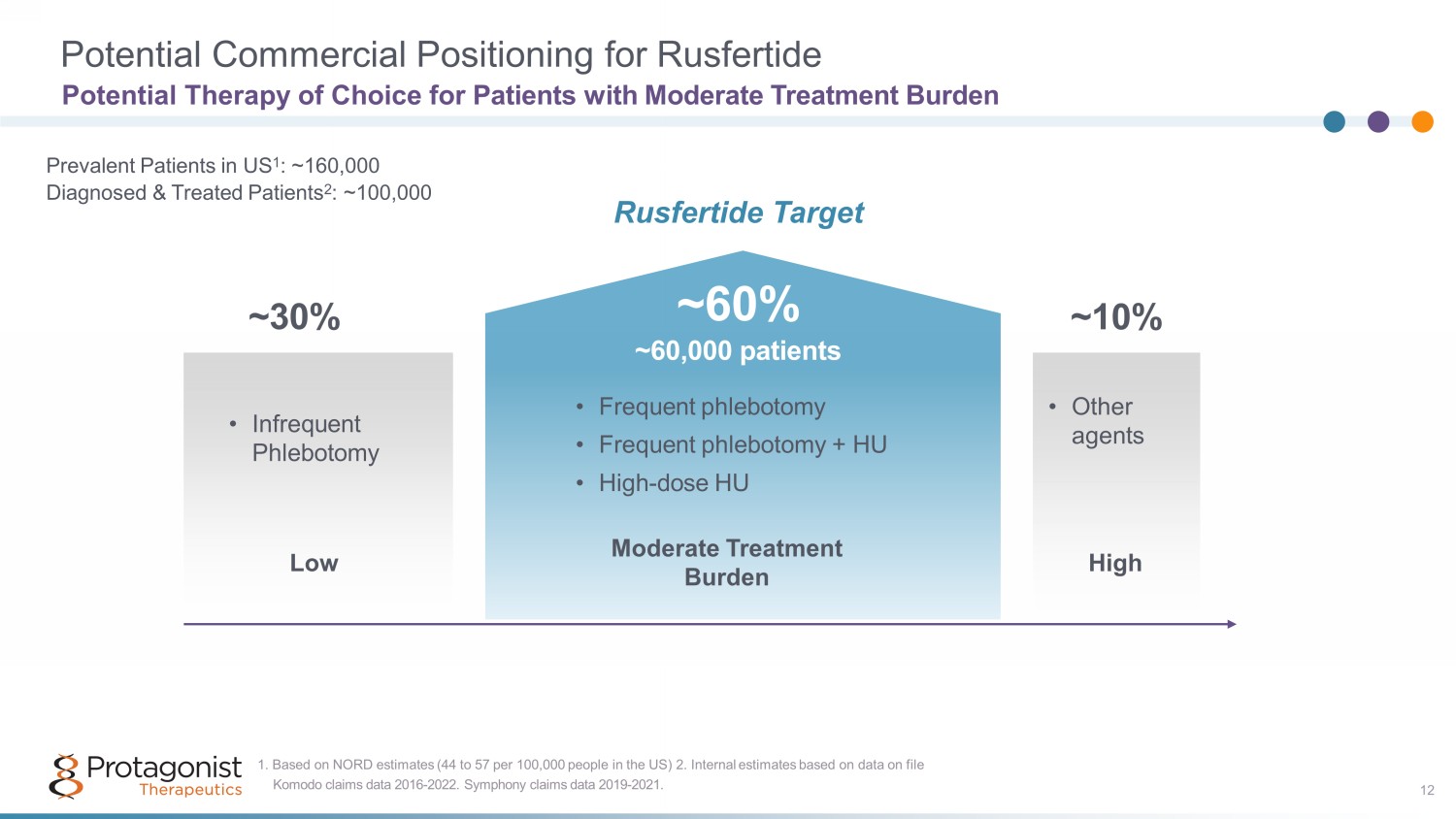
Potential Commercial Positioning for Rusfertide Potential Therapy of Choice for Patients with Moderate Treatment Burden 12 Prevalent Patients in US 1 : ~160,000 Diagnosed & Treated Patients 2 : ~100,000 ~30% ~60% ~60,000 patients ~10% Low Moderate Treatment Burden High Komodo claims data 2016 - 2022. Symphony claims data 2019 - 2021. 1. Based on NORD estimates (44 to 57 per 100,000 people in the US) 2. Internal estimates based on data on file • Infrequent Phlebotomy • Frequent phlebotomy • Frequent phlebotomy + HU • High - dose HU • Other agents Rusfertide Target

JNJ - 2113: Oral IL - 23 Receptor Antagonist Peptide Targeted Investigational Therapy for Psoriasis & Other IL - 23 Mediated Diseases

Protagonist - Janssen Oral, IL - 23R Antagonist Collaboration 14 JNJ - 2113 highlighted as f irst - and best - in class targeted oral IL - 23 peptide antagonist 2 • "Unprecedented potential" across multiple indications: plaque psoriasis, psoriatic arthritis, IBD • $5B+ p otential peak year sales projection 2 Collaboration overview • Initiated in 2017 with I&I market leader Janssen 1 • JNJ - 2113 (formerly PN - 235) jointly discovered using Protagonist’s proprietary peptide discovery platform - Protagonist completed pre - clinical and first Phase 1 study - Janssen responsible for further development and commercialization Positive data from Phase 2 FRONTIER 1 psoriasis study Comprehensive JNJ - 2113 Phase 3 registrational program (ICONIC) in psoriasis • Four Phase 3 studies • PASI 90 as high - bar primary endpoint • Two head - to - head trials vs. deucravacitinib Phase 2b study in ulcerative colitis ongoing (ANTHEM) 1. Stelara® generated $9.7B in sales, and Tremfya® generated $2.7B in sales in 2022, per Johnson & Johnson 2022 Annual Report. Ste lara® and Tremfaya ® not part of Protagonist - Janssen collaboration. 2. JNJ Innovative Medicines Enterprise Business Review, Dec 5, 2023.

JNJ - 2113: Oral, IL - 23R Peptide Antagonist 15 Preclinical, Phase 1 and Phase 2b Data Supportive of a Robust Clinical Development Program 1 1. ISID – Fourie A, et al. First - in - Class Oral Peptide Systemically Targeting the IL - 23 Pathway. Abstract presented at the Inter national Societies for Investigative Dermatology; May 2023 WCD – Bissonnette R, et al. A Phase 2, Randomized, Placebo - controlled, Dose Ranging Study of Oral JNJ - 77242113 for the Treatment of Moderate - to - Severe Plaque Psoriasis: FRONTIER 1. Late Breaking Abstract presented at the World Congress of Dermatology; July 2023 • >24hr half - life in feces (human, cyno , and rat) • >25% fecal recovery after 24hrs in cynos • Rat ear skin inflammation model • Rat TNBS colitis model Pre - clinical Proof - of - Concept High O ral Stability Highly Potent O ral IL - 23R A ntagonist • Picomolar potency Similar or better target affinity vs. IL - 23 mAbs Phase 1 studies in NHVs Phase 2b FRONTIER1 study in Psoriasis • PD based PoC: Inhibition of IL - 23 biomarkers • Potential for best - in - class oral agent for psoriasis Protagonist – JNJ Innovative Medicines Discovery & Development Partnership

JNJ - 2113 FRONTIER 1 Phase 2b Plaque Psoriasis ( PsO ) Study Adult Patients with PP N=255 Eligibility • Moderate – Severe PP Inclusion • BSA > 10% • PASI > 12 Primary endpoint • PASI > 75 at Week 16 Screening Treatment Safety Follow - up (4 Weeks) (Up to 4 Weeks) (Weeks 0 - 16) 25 mg QD LTE (FRONTIER 2) or 4 weeks safety follow - up 50 Mg QD 100 mg QD 50 mg BID 100 mg BID Placebo Week 0 Randomize Week 16 Primary Endpoint Randomize 2 1 3 4 5 6 16

JNJ - 2113 Phase 2B Frontier 1 Data 37.2% 51.2% 58.1% 65.1% 78.6% 9.3% 25.6% 26.8% 51.2% 46.5% 59.5% 2.3% 11.6% 9.8% 25.6% 23.3% 40.5% 0.0% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% 80.0% 90.0% 25 mg daily (n= 43) 25 mg twice daily (n= 41) 50 mg daily (n=43) 100 mg daily (n=43) 100 mg twice daily (n=42) Placebo % response rate PASI 75 (%) PASI 90 (%) PASI 100 (%) Dose Response • 200 mg once daily oral dose selected for all four phase 3 psoriasis studies • PASI 90 as a high - bar primary endpoint in phase 3 studies 17 PASI 90 PASI 75 PASI 100 Qd more efficacious vs bid

79% 60% 41% 41% 11% NR 69% 44% 9% 67% 46% 33% 67% 44% 16% 81% 57% 29% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% % response rate JNJ-2113 Otezla Sotyktu TAK-279 Stelara Tremfya 1. Cross trial (not head - to - head) comparisons 2. J NJ2113 100 mg bid dose. Wk 16 endpoint (Placebo: PASI 75: 9.3%, PASI 90: 2.3%, PASI 100: 0%) 3. Otezla 30 mg qd approved dose. Week 16 primary endpoint. Papp K et al. Lancet 2012; 380: 738 – 46. (Placebo: PASI 75: 5.7%, PASI 90: 1.1%, PASI 100: NR) 4. Sotyktu 3 mg bid dose (6 mg qd dose approved). Wk 12 primary endpoint. Papp K et al. N Engl J Med 2018; 379:1313 - 1321. (Placebo: PASI 75: 7%, PASI 90: 2%, PASI 100: 0%) 5. TAK - 279 30 mg qd dose (Expected phase 3 dose). Wk 12 primary endpoint. AAD 2023. (Placebo: PASI 75: 5.8%, PASI 90: 0%, PASI 100: 0%) 6. Stelara 45 mg wkly x 4 (~approved 90 mg week 0 and 2 approved dose). Wk 12 primary endpoint. Krueger et al. N Engl J Med 2007 ;35 6:580 - 92. (Placebo: PASI 75: 2%, PASI 90: 2%, PASI 100: 0%) 7. Tremfya 200 mg wk 0, 4, then q 8 wks (approved dose 100 mg wk 0, 4 then q 8 wks). Wk 16 primary endpoint. Gordon KB et al. N Eng l J Med 2015;373:136 - 44. (Placebo: PASI 75: 5%, PASI 90: 2%, PASI 100: 0%) (IL - 23R) 2 (PDE4) 3 (TYK2) 4 (TYK2) 5 (IL - 12/23) 6 (IL - 23) 7 Oral agents I njectable antibodies PASI 90 PASI 100 Oral agents Oral agents PASI 75 18 JNJ - 2113: Cross - study Comparison to Clinically Relevant Benchmarks 1 Potential for First - , Best - , and Only - in - Class Oral Peptide Targeted Therapy for Psoriasis
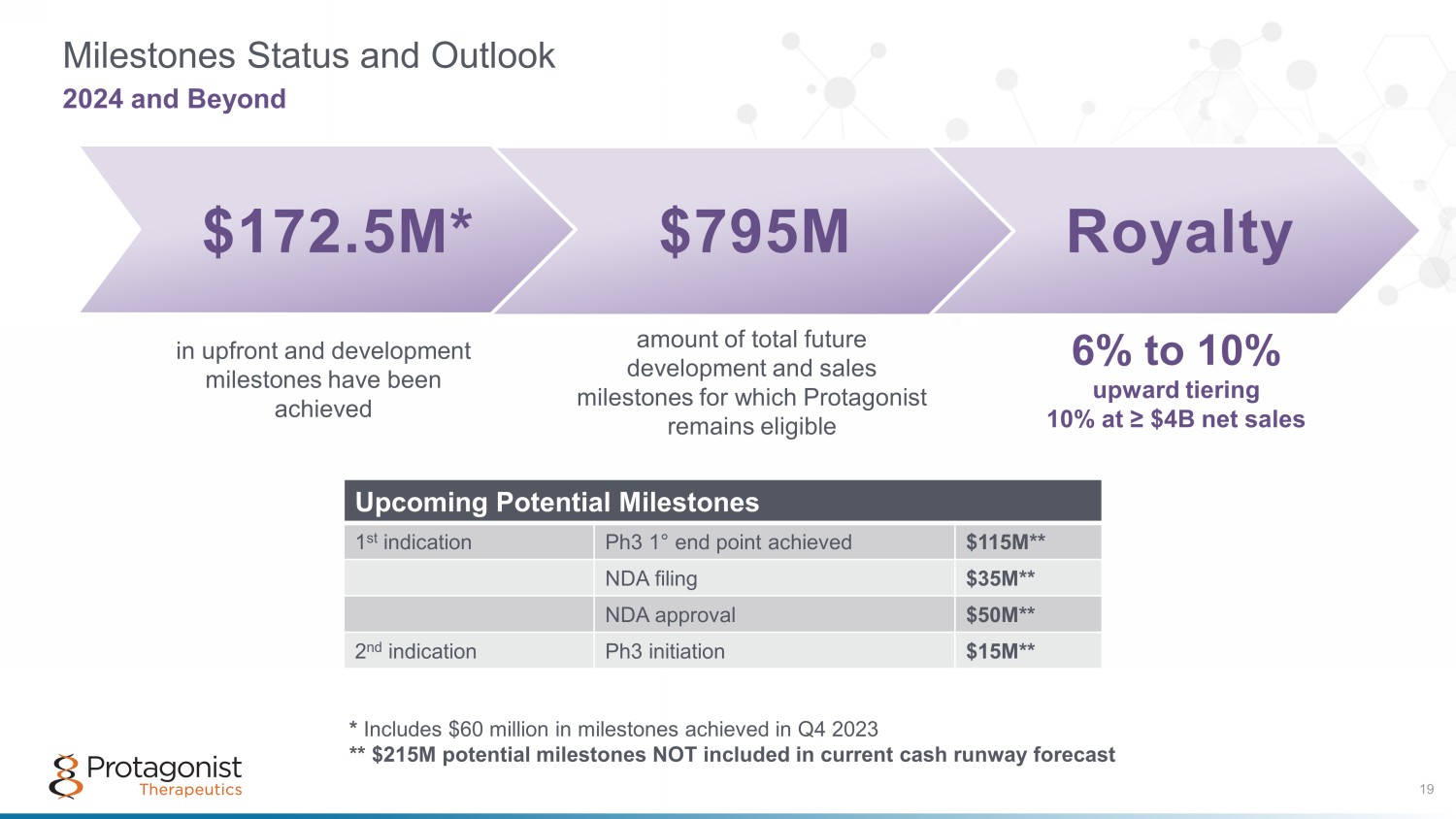
Royalty Milestones Status and Outlook 2024 and Beyond $172.5M* $795M amount of total future development and sales milestones for which Protagonist remains eligible in upfront and development milestones have been achieved 6% to 10% upward tiering 10% at ≥ $4B net sales Upcoming Potential Milestones 1 st indication Ph3 1 ° end point achieved $115M** NDA filing $35M** NDA approval $50M** 2 nd indication Ph3 initiation $15M** * Includes $60 million in milestones achieved in Q4 2023 ** $215M potential milestones NOT included in current cash runway forecast 19

Discovery Pipeline Financial Outlook Major Catalysts Ahead 2024 is a year of pipeline execution and strategic evolution for Protagonist

Oral IL - 17 Peptide Antagonists 21 • IL - 17 antagonists expected to be a leader in the I&I space – Global sales expected to increase significantly from ~$29B (2021) to >$50B (2031) for IL - 17 mediated indications 1,2 • Leveraging our oral peptide technology platform • Target product profile (TPP) – Oral peptide, first - in - class – Similar/better potency vs. approved mAbs 3 – Tri - specific (IL - 17 AA, AF & FF) • Development candidate in 2024 4 New Discovery Program PsO PsA Oral IL - 17 Peptide Antagonist JNJ - 2113 Oral IL - 23R Antagonist IBD HS SpA HS: Hidradenitis Suppurativa SpA : Spondyloarthritis PsO : Plaque Psoriasis PsA: Psoriatic Arthritis IBD: Inflammatory Bowel Diseases (Crohn’s and Ulcerative Colitis) 1. JNJ Innovative Medicines Enterprise Business Review, Dec 5 th , 2023 and Clarivate DRG Disease Landscape and Forecast; 2. JNJ Innovative Medicines Enterprise Business Review, Dec 5 th , 2023 and IQVIA claims YTD extrapolated for FY 2023 and projected to 2036; 3. Approved IL - 17 mAbs: COSENTYX ( secukinamab ), TALTZ ( ixekinumba ); 4. Development candidate definition: Ready for IND - enabling studies.

Financial Highlights Financial Resources Forecast Extends Through Q1 2026 CASH, CASH EQUIVALENTS & MARKETABLE SECURITIES provide forecast cash runway through Q1 2026* SHARES OUTSTANDING as of September 30, 2023 Q1 2026 ~57.6M CASH, CASH EQUIVALENTS & MARKETABLE SECURITIES as of September 30, 2023 22 * Includes $60 million in milestones achieved in Q4 2023 * $215M potential Janssen milestones NOT included in current cash runway forecast

2023 2024 2025 2026 Q4 Q1 Q2 Q3 Q4 ASH JNJ - 2113 Janssen Major Catalysts Ahead A Transformative Path Forward for Protagonist, from Discovery to Development to Commercialization Program Rusfertide in PV JNJ - 2113 Janssen Discovery & Pre - Clinical 23 VERIFY Ph3 Enrollment Completion EHA 2 - yr CARC completion VERIFY Ph3 32 wk 1 ° EP (Q1) NDA Filing (Q4) Product launch • Ph3 ICONIC - LEAD psoriasis • Ph3 ICONIC - TOTAL psoriasis • Ph2b ANTHEM UC • Ph3 ICONIC ADVANCE - 1 • Ph3 ICONIC ADVANCE - 2 Ph2b ANTHEM UC completion Anticipated NDA filing for psoriasis • Ph3 ICONIC - LEAD completion • Ph3 ICONIC - TOTAL completion I & I Hematology Oral IL - 17 Program Pre - clinical PoC Oral IL - 17 Peptide Antagonist Development Candidate ASH

Thank you
v3.23.4
Cover
|
Jan. 09, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 09, 2024
|
| Entity File Number |
001-37852
|
| Entity Registrant Name |
PROTAGONIST THERAPEUTICS, INC.
|
| Entity Central Index Key |
0001377121
|
| Entity Tax Identification Number |
98-0505495
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
7707 Gateway Blvd.
|
| Entity Address, Address Line Two |
Suite 140
|
| Entity Address, City or Town |
Newark
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
94560-1160
|
| City Area Code |
510
|
| Local Phone Number |
474-0170
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.00001
|
| Trading Symbol |
PTGX
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Protagonist Therapeutics (NASDAQ:PTGX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Protagonist Therapeutics (NASDAQ:PTGX)
Historical Stock Chart
From Apr 2023 to Apr 2024