As filed with the Securities and Exchange
Commission on December 27, 2023
Registration No. 333-275316
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
FIRST WAVE BIOPHARMA, INC.
(Exact name of registrant as specified in its
charter)
| Delaware |
2834 |
46-4993860 |
(State or
other jurisdiction of
incorporation or organization) |
(Primary
Standard Industrial
Classification Code Number) |
(I.R.S.
Employer
Identification Number) |
777 Yamato Road, Suite 502
Boca Raton, Florida 33431
(561) 589-7020
(Address, including zip code, and telephone number,
including area code, of registrant’s principal
executive offices)
James Sapirstein, President, Chief Executive
Officer and Chairman
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, Florida 33431
(561) 589-7020
(Name, address, including zip code, and telephone
number,
including area code, of agent for service)
Copies to
Barry
I. Grossman, Esq.
Jessica Yuan, Esq. |
|
M.
Ali Panjwani, Esq. |
Ellenoff
Grossman & Schole LLP
1345 Avenue of the Americas
New York, New York 10105
Telephone: (212) 370-1300 |
|
Pryor
Cashman LLP
7 Times Square, 40th Floor
New York, New York 10036
Telephone: (212) 421-4100 |
Approximate
date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following
box. x
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the same
offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering: ¨
Indicate by check mark whether the registrant
is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company.
See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”
and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
¨ |
|
Accelerated filer |
¨ |
| Non-accelerated filer |
x |
|
Smaller reporting company |
x |
| |
|
|
Emerging growth company |
¨ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ¨
The Registrant hereby amends this Registration
Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which
specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities
Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to
said Section 8(a), may determine.
The information in this preliminary prospectus is not
complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange
Commission is effective. This preliminary prospectus is not an offer to sell these securities, nor is it a solicitation of offers to
buy these securities, in any state where the offer or sale is not permitted.
| PRELIMINARY
PROSPECTUS |
SUBJECT
TO COMPLETION |
DATED
DECEMBER 27, 2023 |

Up to 1,381,215 Shares of Common Stock
1,381,215 Pre-Funded Warrants to Purchase up to 1,381,215 Shares of Common Stock
Up to 2,762,430 Common Warrants to Purchase up to 2,762,430 Shares of Common Stock
Common Stock underlying the Pre-Funded Warrants and
Up to 2,762,430 Shares of Common Stock underlying
the Common Warrants
We are offering up to 1,381,215 shares of
common stock, together with up to 2,762,430 common warrants to purchase up to 2,762,430 shares of common stock at an assumed combined
public offering price of $3.62 per share and common warrants (the last reported sale price per share of our common stock on the Nasdaq
Capital Market, on December 21, 2023). Each share of common stock is being offered together with two common warrants to purchase shares
of common stock. Each common warrant will be exercisable to purchase one share of common stock, will have an exercise price of $
per share, will be exercisable upon issuance and will expire five years from the date of issuance. The shares of common stock and common
warrants will be separately issued. This prospectus also covers the shares of common stock issuable from time to time upon the exercise
of the common warrants.
We are also offering up to 1,381,215 pre-funded
warrants to those purchasers, whose purchase of shares of common stock in this offering would result in the purchaser, together with
its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our
outstanding common stock following the consummation of this offering in lieu of the shares of our common stock that would result in ownership
in excess of 4.99% (or, at the election of the purchaser, 9.99%). Each pre-funded warrant will be exercisable for one share of common
stock at an exercise price of $0.0001 per share. Each pre-funded warrant is being offered together with the same two common warrants
to purchase two shares of common stock described above being offered with each share of common stock. The purchase price of each pre-funded
warrant will equal the combined public offering price per share of common stock and common warrants being sold in this offering, less
the $0.0001 per share exercise price of each such pre-funded warrant. Each pre-funded warrant will be exercisable upon issuance and will
expire when exercised in full. The pre-funded warrants and common warrants will be separately issued. For each pre-funded warrant that
we sell, the number of shares of common stock that we are selling will be decreased on a one-for-one basis. This prospectus also covers
the shares of common stock issuable from time to time upon the exercise of the pre-funded warrants.
There is no established public trading market
for the pre-funded warrants or common warrants, and we do not expect a market to develop. We do not intend to apply for listing of the
pre-funded warrants or common warrants on any securities exchange or other nationally recognized trading system. Without an active trading
market, the liquidity of the pre-funded warrants and common warrants will be limited.
We have engaged Roth Capital Partners, LLC, or
the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use
its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing
or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific
number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth in the table below,
which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to be received in escrow,
trust or similar arrangement. There is no minimum number of shares of securities or minimum aggregate amount of proceeds that is a condition
for this offering to close. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of
proceeds received by us, and investors in this offering will not receive a refund if we do not sell all of the securities offered hereby.
Because there is no escrow account and no minimum number of securities or amount of proceeds, investors could be in a position where
they have invested in us, but we have not raised sufficient proceeds in this offering to adequately fund the intended uses of the proceeds
as described in this prospectus. We will bear all costs associated with the offering. See “Plan of Distribution” on
page 19 of this prospectus for more information regarding these arrangements. This offering will end no later than three trading days
from the date of this prospectus.
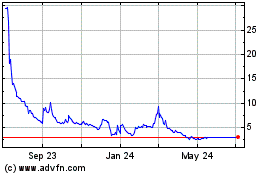
Our common stock is listed on the Nasdaq Capital
Market under the symbol “FWBI.” On December 21, 2023, the last reported sale price of our common stock on the Nasdaq Capital
Market was $3.62 per share. Effective as of 12:01 am Eastern Time on December 18, 2023, we filed an amendment to our Amended and Restated
Certificate of Incorporation to effect a one-for-twenty reverse stock split of our issued and outstanding shares of common stock (the
“Reverse Stock Split”). Unless otherwise indicated, all share and per share prices in this prospectus have been adjusted
to reflect the Reverse Stock Split. However, common stock share and per share amounts in certain of the documents incorporated by reference
herein have not been adjusted to give effect to the Reverse Stock Split.
All share, common warrant and pre-funded warrant
numbers are based on an assumed combined public offering price of $3.62 per share or pre-funded warrant, as applicable, and common warrant.
The actual combined public offering price per share and common warrants and the actual combined public offering price per pre-funded
warrant and common warrants will be determined between us and investors based on market conditions at the time of pricing, and may be
at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout this prospectus
may not be indicative of the final public offering price.
You should read this prospectus, together with
additional information described under the headings “Incorporation of Certain Information By Reference” and “Where
You Can Find More Information,” carefully before you invest in any of our securities.
Investing in our securities involves a high
degree of risk. See the section entitled “Risk Factors” beginning on page 6 of this prospectus and in the documents
incorporated by reference into this prospectus for a discussion of risks that should be considered in connection with an investment in
our securities.
| | |
Per
Share and
Accompanying
Common Warrants | |
Per
Pre-Funded
Warrant and
Accompanying
Common Warrants | |
| Total | |
| Public offering price(1) | |
$ | |
$ | |
| $ | |
| Placement agent fees(2) | |
$ | |
$ | |
| $ | |
| Proceeds to us, before expenses(3) | |
$ | |
$ | |
| $ | |
(1) The public offering price corresponds to
(x)(i) a public offering price per share of $[__] and (ii) a public offering price per common warrant of $[__], and (y)(i)
a public offering price per pre-funded warrant of $[__] and (ii) a public offering price per common warrant of $[__].
(2) See “Plan of Distribution”
for additional information about the compensation payable to the placement agent.
(3) Because there is no minimum number of securities
or amount of proceeds required as a condition to closing in this offering, the actual public offering amount, placement agent fees, and
proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth
above. We estimate the total expenses of this offering payable by us, excluding the placement agent fee, will be approximately $270,000.
The delivery of the shares of common stock and
any pre-funded warrants and common warrants to purchasers is expected to be made no later than ,
2023.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus.
Any representation to the contrary is a criminal offense.
Roth Capital Partners
The date of this prospectus is ,
2023.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
We incorporate by reference important information
into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where
You Can Find More Information.” You should carefully read this prospectus as well as additional information described under
“Incorporation of Certain Information by Reference,” before deciding to invest in our securities.
We have not, and the placement agent has not,
authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free
writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide
no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities
offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus
or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our securities.
Our business, financial condition, results of operations and prospects may have changed since that date.
The information incorporated by reference or
provided in this prospectus contains statistical data and estimates, including those relating to market size and competitive position
of the markets in which we participate, that we obtained from our own internal estimates and research, as well as from industry and general
publications and research, surveys and studies conducted by third parties. Industry publications, studies and surveys generally state
that they have been obtained from sources believed to be reliable. While we believe our internal company research is reliable and the
definitions of our market and industry are appropriate, neither this research nor these definitions have been verified by any independent
source.
For investors outside the United States: We have
not, and the placement agent has not, done anything that would permit this offering or possession or distribution of this prospectus
in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who
come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities
and the distribution of this prospectus outside the United States.
This prospectus and the information incorporated
by reference into this prospectus contain references to our trademarks and to trademarks belonging to other entities. Solely for convenience,
trademarks and trade names referred to in this prospectus and the information incorporated by reference into this prospectus, including
logos, artwork, and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate,
in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor
to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply
a relationship with, or endorsement or sponsorship of us by, any other company.
CAUTIONARY NOTE REGARDING
FORWARD-LOOKING STATEMENTS
This prospectus, and any documents we incorporate
by reference, contain certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in
this prospectus and any documents we incorporate by reference, other than statements of historical facts, are forward-looking statements
including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects,
plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other
important factors that may cause our actual results, performance or achievements to be materially different from any future results,
performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate”, “believe”,
“estimate”, “expect”, “intend”, “may”, “plan”, “predict”, “project”,
“target”, “potential”, “will”, “would”, “could”, “should”, “continue”
and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these
identifying words. These forward-looking statements include, among other things, statements about:
| ● |
our ability to maintain compliance
with the continued listing requirements of the Nasdaq Stock Market LLC; |
| ● |
our ability to satisfy
our payment obligations in connection with the acquisition of First Wave Bio, Inc. and the settlement payments; |
| ● |
statements regarding geopolitical
events, including the wars in Israel and Ukraine and their effects on our operations, access to capital, research and development
and clinical trials and potential disruption in the operations and business of third-party vendors, contract research organizations
(“CROs”), contract development and manufacturing organizations (“CDMOs”), other service providers, and collaborators
with whom we conduct business; |
| ● |
the availability of capital
to satisfy our working capital requirements; |
| ● |
our
current and future capital requirements and our ability to raise additional funds to satisfy our capital needs; |
| ● |
our ability to consummate our proposed acquisition of ImmunogenX, Inc.,
our potential sale of niclosamide, and other strategic transactions; |
| ● |
the
integration and effects of our acquisitions and other strategic transactions; |
| ● |
the accuracy of our estimates
regarding expense, future revenue and capital requirements; |
| ● |
ability to continue operating
as a going concern; |
| ● |
our
plans to develop and commercialize our product candidates, including adrulipase and niclosamide; |
| ● |
our ability to initiate
and complete our clinical trials and to advance our principal product candidates into additional clinical trials, including pivotal
clinical trials, and successfully complete such clinical trials; |
| ● |
regulatory developments
in the U.S. and foreign countries; |
| ● |
the performance of our
third-party vendor(s), CROs, CDMOs and other third-party non-clinical and clinical development collaborators and regulatory service
providers |
| ● |
our ability to obtain and
maintain intellectual property protection for our core assets; |
| ● |
the size of the potential
markets for our product candidates and our ability to serve those markets; |
| ● |
the rate and degree of
market acceptance of our product candidates for any indication once approved; |
| ● |
the success of competing
products and product candidates in development by others that are or become available for the indications that we are pursuing; |
| ● |
the loss of key scientific,
clinical and nonclinical development, and/or management personnel, internally or from one of our third-party collaborators; and |
| ● |
other risks and uncertainties, including those listed
in the “Risk Factors” section of this prospectus and the documents incorporated by reference herein. |
These forward-looking statements are only predictions
and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not
place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and
expectations disclosed in the forward-looking statements we make. We have based these forward-looking statements largely on our current
expectations and projections about future events and trends that we believe may affect our business, financial condition and operating
results. We have included important factors in the cautionary statements included in this prospectus that could cause actual future results
or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential
impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus with the understanding
that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking
statements whether as a result of new information, future events or otherwise, except as required by applicable law.
PROSPECTUS SUMMARY
This summary highlights information contained
elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest
in our securities. You should read this entire prospectus carefully, including the “Risk Factors” section in this prospectus
and under similar captions in the documents incorporated by reference into this prospectus. In this prospectus, unless otherwise stated
or the context otherwise requires, references to “First Wave BioPharma”, “AzurRx”, “Company”, “we”,
“us”, “our” or similar references mean First Wave BioPharma, Inc. and its subsidiaries on a consolidated basis.
References to “First Wave BioPharma” refer to First Wave BioPharma, Inc. on an unconsolidated basis. References to “First
Wave Bio” refer to First Wave Bio, Inc., First Wave BioPharma’s wholly-owned subsidiary.
Overview
We are engaged in the research and development
of targeted, non-systemic therapies for the treatment of patients with gastrointestinal (“GI”) diseases. Non-systemic therapies
are non-absorbable drugs that act locally, i.e. the intestinal lumen, skin or mucosa, without reaching an individual’s systemic
circulation.
We are currently focused on developing our pipeline
of gut-restricted GI clinical drug candidates, including the biologic adrulipase (formerly MS1819), a recombinant lipase enzyme designed
to enable the digestion of fats and other nutrients, and niclosamide, an oral small molecule with anti-viral and anti-inflammatory properties.
Our adrulipase programs are focused on the development of an oral, non-systemic, biologic capsule for the treatment of exocrine pancreatic
insufficiency (“EPI”) in patients with cystic fibrosis (“CF”) and chronic pancreatitis (“CP”). The
Company’s niclosamide programs leverage proprietary oral and topical formulations to address multiple GI conditions, including
inflammatory bowel disease (“IBD”) indications and viral diseases.
We are developing our drug candidates for a host
of GI diseases where there are significant unmet clinical needs and limited therapeutic options, resulting in painful, life threatening
and discomforting consequences for patients.
Recent Developments
Non-Binding Letter of Intent for Potential
Sale of Niclosamide
On December 27, 2023, we announced that we
have entered into a non-binding letter of intent (the “Niclosamide LOI”) for a proposed sale of our niclosamide program,
which is designed to treat inflammatory bowel diseases such as ulcerative colitis and related conditions, to an undisclosed biopharmaceutical
company (the “Niclosamide Sale”). The Niclosamide LOI contemplates a low seven-figure upfront payment to the Company for
the rights to Niclosamide, as well as economics to the Company related to future milestones and royalties.
The Niclosamide LOI only represents a mutual
indication of interest regarding the Niclosamide Sale and the terms of the Niclosamide Sale are subject to a number of contingencies,
including the completion of customary due diligence and the negotiation and execution of definitive agreements. Upon execution of the
definitive agreement, the completion of the transaction will be subject to, among other matters, satisfaction of the conditions negotiated
therein, the buyer having secured adequate financing, and receipt of all third party (including governmental) approvals, licenses, consents,
and clearances, as and when applicable. There can be no assurance that the Niclosamide Sale will be completed on the terms contemplated
in the Niclosamide LOI or otherwise. In particular, the timing of closing of any such transaction and the aggregate consideration that
we may receive may materially differ from that currently contemplated by the Niclosamide LOI.
Non-Binding Letter of Intent for Potential
Merger with ImmunogenX
On December 18, 2023, we announced that we
entered into a non-binding letter of intent (the “IMGX LOI”) for a proposed acquisition of ImmunogenX, Inc. (IMGX), a clinical-stage
biotherapeutics company developing Phase 3-ready latiglutenase, a targeted, oral biotherapeutic for celiac disease. Pursuant to the IMGX
LOI, we would acquire 100% of the outstanding equity of IMGX on a fully diluted basis and, after stockholder approval of the proposed
transaction, the shareholders of IMGX will own a majority of the equity interests of the combined company (the “Acquisition”).
Following the close of the proposed Acquisition, James Sapirstein is expected to continue serving as Chairman and Chief Executive Officer
with Jack Syage, Ph.D., Chief Executive Officer and Co-Founder of ImmunogenX, assuming the role of President and Chief Operating Officer,
with the combined company focused on advancing a GI pipeline comprised of multiple late-stage clinical assets, including latiglutenase
and capeserod. Operational and financial leadership positions will be comprised of current First Wave Biopharma executives, while clinical,
regulatory affairs, and scientific positions will be led by executives of ImmunogenX.
We additionally anticipate concurrent financings
and a strategic licensing agreement for latiglutenase will occur after the close of the Acquisition. Our non-binding term sheet for a licensing agreement with a strategic
global pharmaceutical company provides for a cash payment to the Company from the licensor upon closing of the licensing and certain milestone
and royalty payments as consideration for the exclusive license of latiglutenase within the United States and Canada. The closing of the
strategic licensing agreement and any other financings would be contingent upon the completion of numerous events, including the closing
of the Acquisition, completion of customary due diligence, the negotiation and execution of definitive agreements, and receipt of all
required third party (including governmental) approvals, licenses, consents, and clearances, as and when applicable.
The IMGX LOI is subject to an exclusivity
payment by the Company, upon execution of the IMGX LOI, of $500,000. The LOI only represents a mutual indication of interest regarding
the Acquisition and the terms of the Acquisition are subject to a number of contingencies, including the completion of customary due
diligence and the negotiation and execution of definitive agreements. Any definitive agreement with respect to the Acquisition would
be subject to approval by the respective parties to the IMGX LOI, including approval by our Board of Directors, and would likely include
a number of customary provisions, including without limitation, representations and warranties of IMGX and us, restrictive covenants,
and indemnification provisions, as well as various closing conditions, including without limitation receipt of applicable third party
approvals and receipt of satisfactory financing and licensing arrangements. There can be no assurance that the Acquisition will be completed
on the terms contemplated in the IMGX LOI or otherwise. In particular, the timing of closing of any such transaction and the aggregate
consideration that we may receive may materially differ from that currently contemplated by the LOI.
Reverse Stock Split
On December 12, 2023, we held a special meeting
of stockholders (the “Special Meeting”) where our stockholders approved a proposal granting our Board of Directors the discretion
to effect a reverse stock split of our issued and outstanding common stock through an amendment (the “Amendment”) to our
Amended and Restated Certificate of Incorporation, as amended to date (the “Charter”), at a ratio of not less than 1-for-10
and not more than 1-for-20, with such ratio to be determined by the Board of Directors.
On December 13, 2023, we filed the Amendment
to our Charter with the Secretary of State of the State of Delaware to effect a reverse stock split of our Common Stock at a ratio of
1-for-20. The Reverse Stock Split became effective in accordance with the terms of the Amendment at 12:01 AM Eastern Time on December
18, 2023, and began trading on a split-adjusted basis when the market opened on Monday, December 18, 2023. There was no corresponding
reduction in the number of authorized shares of common stock and no change in the par value per share.
Nasdaq Listing Compliance
On October 26, 2023, we received written notice
(the “Notification Letter”) from the Listing Qualifications Department (the “Staff”) of The Nasdaq Stock Market
LLC (“Nasdaq”) notifying the Company that it was not in compliance with the shareholder approval requirement set forth in
Nasdaq Listing Rule 5635(d), which requires prior shareholder approval for transactions, other than public offerings, involving the issuance
of 20% or more of an issuer’s pre-transaction shares outstanding at less than the applicable Minimum Price (as defined in Listing
Rule 5635(d)(1)(A)).
The Staff’s
determination relates to the offering and issuance by us (the “Offering”) of an aggregate of: (i) 30,500 shares (the “Shares”)
of our common stock, par value $0.0001 per share, (ii) pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to
an aggregate of 133,750 shares of common stock (the “Pre-Funded Warrant Shares”) and (iii) common warrants (the “Warrants”)
to purchase up to an aggregate of 328,500 shares of Common Stock (the “Common Warrant Shares” and, together with the Pre-Funded
Warrant Shares, the “Warrant Shares”). The public offering price for each Share and accompanying Warrants, each to purchase
one share of common stock, was $12.80, and the public offering price for each Pre-Funded Warrant and accompanying Warrants, each to purchase
one share of Common Stock, was $12.798. The Offering was previously disclosed in our Current Report on Form 8-K filed with the Securities
and Exchange Commission on July 21, 2023.
The Staff
determined that the Offering was not a “public offering” under Nasdaq Listing Rule 5635(d) due to the type of offering, a
best efforts offering pursuant to a placement agency agreement, and the fact that one investor purchased a predominant portion of the
Offering. As a result, because the Offering represented greater than 20% of the common stock outstanding and was priced below the Minimum
Price, the Staff determined that we were required to obtain shareholder approval prior to the issuance of common stock in the Offering
under Listing Rule 5635(d).
On
December 12, 2023, during the Special Meeting, our stockholders’ ratified our entry into the Offering as we received
the affirmative vote of the majority of the votes cast by shares of our common stock present or represented by proxy and entitled to
vote at the Special Meeting.
Initial
Topline Results from Phase 2 SPAN Trial
On July 13, 2023, we announced that we had received
topline results from our Phase 2 SPAN clinical trial investigating an enhanced enteric microgranule delivery formulation of adrulipase
for the treatment of EPI in patients with CF. Initial data from the study indicate the enhanced adrulipase formulation was safe and well
tolerated and demonstrated an improvement over prior formulations of adrulipase. However, the preliminary data also indicate that it
is likely the primary efficacy endpoint was not achieved. We are continuing to assess this data, along with secondary efficacy endpoint
data, and plan to schedule a Type C meeting with the FDA in 2024 to discuss next steps for the Adrulipase program.
The Phase 2 SPAN clinical trial was designed
to investigate the safety, tolerability and efficacy of an enteric microgranule delivery formulation for adrulipase in a titrated dose-escalation
study involving thirteen (13) patients. The primary efficacy endpoint is the coefficient of fat absorption, with secondary endpoints
of stool weight, signs and symptoms of malabsorption and coefficient of nitrogen absorption.
Based on the initial safety and efficacy results,
we plan to pursue an End-of-Phase 2 meeting with the U.S. Food and Drug Administration to review the data and discuss the parameters
for a registrational Phase 3 clinical trial that would satisfy the requirements for a Biologics License Application. We anticipate conducting
the End-of-Phase 2 meeting in 2024.
Sanofi License Agreement
On September 13, 2023, we entered into a License
Agreement (the “License Agreement”) with Sanofi (“Sanofi”), pursuant to which we received a license to obtain
certain exclusive worldwide rights to develop and commercialize Capeserod, a selective 5-HT4 receptor partial agonist which we intend
to repurpose and develop for gastrointestinal indications.
Warrant Reload and Repricing
On June 13, 2023, we entered into warrant exercise
inducement offer letters with certain holders of warrants to purchase shares of our common stock pursuant to which the holders agreed
to exercise for cash their existing warrants to purchase 86,216 shares of our common stock, in the aggregate, at a reduced exercise price
of $23.00 per share, in exchange for our agreement to issue new warrants on substantially the same terms as the existing warrants, to
purchase up to 172,433 shares of our common stock and a cash payment of $2.50 per inducement warrant share which was paid in full upon
the exercise of the existing warrants. We received aggregate gross proceeds of approximately $2.4 million from the exercise of the existing
warrants by the holders and the sale of the inducement warrants.
On September 14, 2023, we entered into warrant
exercise inducement offer letters with a holder of warrants to purchase shares of common stock pursuant to which the holders agreed to
exercise for cash their existing warrants to purchase 294,101 shares of common stock, in the aggregate, at a reduced exercised price
of $8.60 per share, in exchange for new warrants on substantially the same terms as the existing warrants as described below, to purchase
up to 588,203 shares of common stock and a cash payment of $2.50 per warrant share which was paid in full upon the exercise of the existing
warrants. We received aggregate gross proceeds of approximately $4.0 million from the exercise of the existing warrants by the holders
and the sale of the inducement warrants.
Corporate Information
We were incorporated on January 30, 2014 in the
State of Delaware. In June 2014, we acquired 100% of the issued and outstanding capital stock of AzurRx SAS. In September 2021, we acquired
First Wave Bio through a merger transaction, and changed our name to First Wave BioPharma, Inc. Our principal executive offices are located
at 777 Yamato Road, Suite 502, Boca Raton, Florida 33431. Our telephone number is (561) 589-7020. We maintain a website at www.firstwavebio.com.
The information contained on our website is not, and should not be interpreted to be, a part of this prospectus.
The Offering
| Common
Stock to be Offered |
Up
to 1,381,215 shares of our common stock based on an assumed combined public offering price of $3.62 per share of common stock and
accompanying common warrants, which is the last reported sale price of our common stock on December 21, 2023, on the Nasdaq Capital
Market, and no sale of any pre-funded warrants. |
| |
|
| Pre-funded Warrants
to be Offered |
We
are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser,
together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such
purchasers so choose, up to 1,381,215 pre-funded warrants to purchase shares of common stock, in lieu of shares of common stock that
would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser,
9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock. The purchase
price of each pre-funded warrant and accompanying common warrants will equal the price at which the share of common stock and accompanying
common warrants are being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will
be $0.0001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded
warrants are exercised in full. This offering also relates to the shares of common stock issuable upon exercise of any pre-funded
warrants sold in this offering. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be
decreased on a one-for-one basis. Because we will issue two common warrants to purchase two shares of common stock for each share
of our common stock and for each pre-funded warrant to purchase one share of our common stock sold in this offering, the number of
common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded
warrants sold. |
| Common
Warrants to be Offered |
Up
to 2,762,430 common warrants to purchase up to an aggregate of 2,762,430 shares of our common stock, based on the sale of our common
stock at an assumed combined public offering price of $3.62 per share of common stock and accompanying common warrants, which is
the last reported sale price of our common stock on December 21, 2023. Each share of our common stock and each pre-funded warrant
to purchase one share of our common stock is being sold together with two common warrants to purchase two shares of our common stock.
Each common warrant will be exercisable to purchase one share of our common stock, will have an exercise price of $[__] per share
(representing [__]% of the price at which a share of common stock and accompanying common warrant are sold to the public in this
offering), will be immediately exercisable and will expire on the five year anniversary of the original issuance date. The shares
of common stock and pre-funded warrants, and the accompanying common warrants, as the case may be, can only be purchased together
in this offering but will be issued separately and will be immediately separable upon issuance. This prospectus also relates to the
offering of the shares of common stock issuable upon exercise of the common warrants. |
| |
|
| Common
Stock to be Outstanding Immediately After this Offering |
2,061,171
shares, (assuming we sell only shares of common stock and no pre-funded warrants and assuming no exercise of the common warrants). |
| |
|
| Use of Proceeds |
We estimate that
the net proceeds from this offering will be approximately $4.4 million, excluding the proceeds, if any, from the cash exercise of
the common warrants in this offering. We currently intend to use the net proceeds from this offering for working capital and general
corporate purposes, including the further development of our product candidates. See “Use of Proceeds” for additional
information. |
| |
|
| Risk Factors |
An investment in our
securities involves a high degree of risk. See “Risk Factors” beginning on page 6 of this prospectus and the other
information included and incorporated by reference in this prospectus for a discussion of the risk factors you should carefully consider
before deciding to invest in our securities. |
| |
|
| Nasdaq symbol |
Our common stock is
listed on the Nasdaq Capital Market under the symbol “FWBI.” There is no established public trading market for the pre-funded
warrants or common warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded
warrants or common warrants on any national securities exchange or other nationally recognized trading system. Without an active
trading market, the liquidity of the pre-funded warrants and common warrants will be limited. |
The above discussion is based on 673,173 shares of our common stock
outstanding as of September 30, 2023, assumes no sale of pre-funded warrants and excludes, as of that date, the following:
| ● |
36 shares of common stock issuable
upon exercise of stock options, with a weighted average exercise price of $44,952.62 per share, under our Amended and Restated 2014
Omnibus Equity Incentive Plan (the “2014 Plan”); |
| ● |
14 shares of awarded but unissued
restricted stock under our 2014 Plan; |
| ● |
411 shares of common stock
issuable upon exercise of stock options, with a weighted average exercise price of $4,652.79 per share, under our Amended and Restated
2020 Omnibus Equity Incentive Plan (the “2020 Plan”); |
| ● |
3,649 shares of awarded but
unissued restricted stock units under our 2020 Plan; |
| ● |
50,458 shares of common stock
available for future issuance under our 2020 Plan; |
| ● |
898,630 shares of common stock
issuable upon exercise of outstanding warrants, with a weighted average exercise price of $47.44 per share; |
| ● |
154 shares of common stock
issuable upon conversion of Series B Preferred Stock, including in respect of accrued and unpaid dividends of approximately $1.0
million through September 30, 2023; |
| ● |
either (x) if the holders of
Series B Preferred Stock elect to exchange into our registered direct and private placement offering from January 2021, up to 157
additional shares of common stock issuable upon conversion of Series C Convertible Preferred Stock (the “Series C Preferred
Stock”) and up to 157 shares of common stock issuable upon exercise of warrants or (y) if the holders of Series B Preferred
Stock elect to exchange into our sales made on November 30, 2021, at a price of $10,985.94 per share, pursuant to our At The Market
Offering Agreement dated May 26, 2021 (the “ATM Agreement”) (such price being the lowest price per share sold under the
ATM Agreement to date and eligible for the holders of the Series B Preferred Stock to exchange into), up to 454 additional shares
of common stock, in each case that may be issued pursuant to the exchange right in excess of amounts currently underlying Series
B Preferred Stock; and |
| ● |
shares of common stock issuable upon
exercise of the common warrants issued in this offering.
|
Except
as otherwise indicated, the information in this prospectus supplement gives effect to the 1-for-20 Reverse Stock Split of our common
stock, effected on December 18, 2023, and assumes no exercise of options or exercise of warrants and no conversion of any shares of preferred
stock described above.
RISK FACTORS
An investment in our securities involves a
high degree of risk. Before deciding whether to purchase our securities, including the shares of common stock offered by this prospectus,
you should carefully consider the risks and uncertainties described under “Risk Factors” in our Annual
Report on Form 10-K for the fiscal year ended December 31, 2022, any subsequent Quarterly Report on Form 10-Q and our other filings
with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial condition
and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value of our securities
could decline and you could lose some or all of your investment. Additional risks not presently known to us or that we currently believe
are immaterial may also significantly impair our business operations. If any of these risks occur, our business, results of operations
or financial condition and prospects could be harmed. In that event, the market price of our common stock and the value of the warrants
could decline, and you could lose all or part of your investment.
Risks Related to This Offering
Our failure to maintain compliance
with Nasdaq’s continued listing requirements could result in the delisting of our Common Stock.
Our common stock is currently
listed for trading on the Nasdaq Capital Market. We must satisfy the continued listing requirements of Nasdaq, to maintain the listing
of our common stock on the Nasdaq Capital Market.
On
August 17, 2023, we received notice from the Listing Qualifications Staff (the “Staff”) of the Nasdaq Stock Market LLC (“Nasdaq”)
indicating that we were not in compliance with the $2.5 million minimum stockholders’ equity requirement for continued listing
of the Common Stock on Nasdaq, as set forth in Nasdaq Listing Rule 5550(b)(1) (the “Minimum Stockholders’ Equity Rule”).
In that regard, we reported stockholders’ equity of $881,960 in our Quarterly Report on Form 10-Q for the period ended June
30, 2023 (we did not then, and do not now, meet the alternative compliance standards relating to the market value of listed securities
of $35 million or net income from continuing operations of $500,000 in the most recently completed fiscal year or in two of the last
three most recently completed fiscal years).
On October
2, 2023, we submitted a plan to the Staff to regain compliance with the Minimum Stockholders’ Equity Rule.
Additionally,
as we have previously reported, on August 24, 2023, we received a notice (the “Minimum Bid Price Notice”) from the Staff
indicating that, based upon the closing bid price of our Common Stock for the last 30 consecutive business days, we are not currently
in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on the Nasdaq Capital Market,
as set forth in Nasdaq Listing Rule 5550(a)(2) (the “Minimum Bid Price Rule”). We are provided a compliance period of 180
calendar days from the date of the Notice, or until February 20, 2024, to regain compliance with the Minimum Bid Price Rule, pursuant
to Nasdaq Listing Rule 5810(c)(3)(A). If at any time before February 20, 2024, the closing bid price of the Common Stock closes at or
above $1.00 per share for a minimum of 10 consecutive business days, subject to Nasdaq’s discretion to extend this period pursuant
to Nasdaq Listing Rule 5810(c)(3)(G) to 20 consecutive business days, Nasdaq will provide written notification that we have achieved
compliance with the Minimum Bid Price Rule, and the matter would be resolved. If we do not regain compliance during the compliance period
ending February 20, 2024, then Nasdaq may grant us a second 180 calendar day period to regain compliance, provided we meet the continued
listing requirement for market value of publicly-held shares and all other initial listing standards for the Nasdaq Capital Market, other
than the minimum closing bid price requirement, and notify Nasdaq of our intent to cure the deficiency.
We
will continue to monitor the closing bid price of the Common Stock and will seek to regain compliance with all applicable Nasdaq requirements
within the allotted compliance periods. If we do not regain compliance within the allotted compliance periods, including any extensions
that may be granted by Nasdaq, Nasdaq will provide notice that the Common Stock will be subject to delisting. We would then be entitled
to appeal that determination to a Nasdaq hearings panel.
In
addition, on October 26, 2023, we received notice from the Staff of Nasdaq indicating that, in connection with our July 2023 Offering,
we were not in compliance with Nasdaq’s shareholder approval requirements set forth in Listing Rule 5635(d), which requires prior
shareholder approval for transactions, other than public offerings, involving the issuance of 20% or more of the pre-transaction shares
outstanding at less than the Minimum Price. On December 12, 2023, during the Special Meeting, our
stockholders’ ratified our entry into the Offering as we received the affirmative
vote of the majority of the votes cast by shares of our common stock present or represented by proxy and entitled to vote at the Special
Meeting.
There can be no assurance
that we will be able to ultimately regain and sustain compliance with all applicable requirements for continued listing on the Nasdaq
Stock Market LLC. In the event that we are unable to regain and sustain compliance will all the applicable requirements for continued
listing, our common stock may be delisted from Nasdaq.
If our common stock were
delisted from Nasdaq, trading of our common stock would most likely take place on an over-the-counter market established for unlisted
securities, such as the OTCQB or the Pink Market maintained by OTC Markets Group Inc. An investor would likely find it less convenient
to sell, or to obtain accurate quotations in seeking to buy, our common stock on an over-the-counter market, and many investors would
likely not buy or sell our common stock due to difficulty in accessing over-the-counter markets, policies preventing them from trading
in securities not listed on a national exchange or other reasons. In addition, as a delisted security, our common stock would be subject
to SEC rules as a “penny stock,” which impose additional disclosure requirements on broker-dealers. The regulations
relating to penny stocks, coupled with the typically higher cost per trade to the investor of penny stocks due to factors such as broker
commissions generally representing a higher percentage of the price of a penny stock than of a higher-priced stock, would further limit
the ability of investors to trade in our common stock. In addition, delisting would materially and adversely affect our ability to raise
capital on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, suppliers, customers and
employees and fewer business development opportunities. For these reasons and others, delisting would adversely affect the liquidity,
trading volume and price of our common stock, causing the value of an investment in us to decrease and having an adverse effect on our
business, financial condition and results of operations, including our ability to attract and retain qualified employees and to raise
capital.
We have broad discretion in the use of the net proceeds from
this offering and may not use them effectively.
Our management will have broad discretion in the
application of the net proceeds, including for any of the purposes described in the section of this prospectus entitled “Use
of Proceeds.” You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will
not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. The failure
by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our
business, cause the price of our securities to decline and delay the development of our product candidates. Pending the application of
these funds, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
You will experience immediate and substantial dilution in the
net tangible book value of the shares you purchase in this offering and may experience additional dilution in the future.
The combined public offering price per share
of common stock and related common warrants, and the combined public offering price of each pre-funded warrant and related common warrants,
will be substantially higher than the pro forma as adjusted net tangible book value per share of our common stock after giving effect
to this offering. Assuming the sale of 1,381,215 shares of our common stock and 2,762,430 common warrants to purchase up to 2,762,430
shares of common stock at an assumed combined public offering price of $3.62 per share and related common warrants, the closing
sale price per share of our common stock on the Nasdaq Capital Market on December 21, 2023, assuming no sale of any pre-funded
warrants in this offering, no exercise of the common warrants being offered in this offering and after deducting the placement agent
fees and commissions and estimated offering expenses payable by us, you will incur immediate dilution in pro forma as adjusted net tangible
book value of approximately $0.71 per share. As a result of the dilution to investors purchasing securities in this offering, investors
may receive significantly less than the purchase price paid in this offering, if anything, in the event of the liquidation of our company.
See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you participate
in this offering. To the extent shares are issued under outstanding options and warrants at exercise prices lower than the public offering
price of our common stock in this offering, you will incur further dilution.
There is no public market for the common warrants or pre-funded
warrants being offered by us in this offering.
There is no established public trading market
for the common warrants or the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply
to list the common warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system.
Without an active market, the liquidity of the common warrants and pre-funded warrants will be limited.
The common warrants and pre-funded warrants are speculative
in nature.
The common warrants and pre-funded warrants offered
hereby do not confer any rights of share of common stock ownership on their holders, such as voting rights or the right to receive dividends,
but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically, commencing on the date of issuance,
holders of the common warrants may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of
$ per share of common stock, and holders of the pre-funded warrants may acquire the shares of common stock issuable upon exercise of
such warrants at an exercise price of $0.0001 per share of common stock. Moreover, following this offering, the market value of the common
warrants and pre-funded warrants is uncertain and there can be no assurance that the market value of the common warrants or pre-funded
warrants will equal or exceed their respective public offering prices. There can be no assurance that the market price of the shares
of common stock will ever equal or exceed the exercise price of the common warrants or pre-funded warrants, and consequently, whether
it will ever be profitable for holders of common warrants to exercise the common warrants or for holders of the pre-funded warrants to
exercise the pre-funded warrants.
Holders of the warrants offered hereby will have no rights as
common stockholders with respect to the shares our common stock underlying the warrants until such holders exercise their warrants and
acquire our common stock, except as otherwise provided in the warrants.
Until holders of the common warrants and the pre-funded
warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our
common stock underlying such warrants, except to the extent that holders of such warrants will have certain rights to participate in
distributions or dividends paid on our common stock as set forth in the warrants. Upon exercise of the common warrants and the pre-funded
warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs
after the exercise date.
This is a best efforts offering, no minimum amount of securities
is required to be sold, and we may not raise the amount of capital we believe is required for our business plans, including our near-term
business plans.
The placement agent has agreed to use its reasonable
best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities
from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum
number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required
as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently
determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the securities offered
hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund
in the event that we do not sell an amount of securities sufficient to support our continued operations, including our near-term continued
operations. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term and may need to
raise additional funds, which may not be available or available on terms acceptable to us.
Purchasers who purchase our securities in this offering pursuant
to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase
agreement.
In addition to rights and remedies available to
all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement
will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those
investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including, but not
limited to: (i) timely delivery of securities; (ii) agreement to not enter into any financings for 30 days from closing; and (iii) indemnification
for breach of contract.
Risks Related to the Potential Acquisition of IMGX
While we have entered into a non-binding IMGX LOI with IMGX
for the Acquisition, there is no assurance that the Acquisition will be completed on the terms contained in the IMGX LOI or otherwise.
As discussed above, on December 18, 2023, we
announced that we have entered into the non-binding IMGX LOI. See “Prospectus Summary – Recent Developments.”
The amount and structure of the consideration could change as a result of subsequent negotiations, due diligence, or other factors. Any
definitive agreement with respect to the Acquisition would be subject to approval by the respective parties to the IMGX LOI, including
approval by our Board of Directors, and would likely include a number of customary provisions, including without limitation, representations
and warranties of IMGX and us, restrictive covenants, and indemnification provisions, as well as various closing conditions, including
without limitation, receipt of applicable third party approvals, satisfactory financing and licensing arrangements. There can be no assurance
that the parties will ultimately negotiate and enter into definitive transaction agreements on the terms contemplated by the IMGX LOI
or otherwise. In particular, the timing of closing of any such transaction and the aggregate consideration that we may receive may materially
differ from that currently contemplated by the IMGX LOI. In addition, in the event that the Acquisition does not occur, we may, in the
future, enter into other non-binding or binding letters of intent, as well as definitive documentation relating to the acquisition of
IMGX, however there can be no assurance that we will do so. If we do not complete the acquisition of IMGX pursuant to the IMGX LOI, other
letters of intent, and any related transaction documentation, we will have incurred expenses without our stockholders realizing any benefit
therefrom. Additionally, if we fail to consummate such anticipated Acquisition, such failure could result in fluctuations to the market
price of our common stock, and may have a material adverse impact on our financial condition and results of operations.
Risks Related to the Potential Sale of Niclosamide
While we have entered into the non-binding Niclosamide LOI
for the Niclosamide Sale, there is no assurance that the Niclosamide sale will be completed on the terms contained in the Niclosamide
LOI or otherwise.
As discussed above, on December 27, 2023, we
announced that we entered into the Niclosamide LOI. See “Prospectus Supplement Summary – Recent Developments.”
The amount and structure of the consideration could change as a result of subsequent negotiations, due diligence, or other factors. Any
definitive agreement with respect to the Niclosamide Sale would be subject to approval by the respective parties to the Niclosamide LOI,
including approval by our Board of Directors, and would likely include a number of customary provisions, including without limitation,
representations and warranties of the buyer and us, restrictive covenants, and indemnification provisions, as well as various closing
conditions, including without limitation receipt of applicable third party approvals and satisfactory financing arrangements. There can
be no assurance that the Niclosamide LOI parties will ultimately negotiate and enter into definitive transaction agreements on the terms
contemplated by the Niclosamide LOI or otherwise. In particular, the timing and closing of any such transaction and the aggregate consideration
that we may receive may materially differ from that currently contemplated by the Niclosamide LOI. In addition, in the event the Niclosamide
Sale does not occur, we may, in the future, enter into other non-binding or binding letters of intent, as well as definitive documentation
relating to the sale of niclosamide, however there can be no assurance that we will do so. If we do not complete the sale of niclosamide
pursuant to the Niclosamide LOI, other letters of intent, and any related transaction documentation, we will have incurred expenses without
our stockholders realizing any benefit therefrom. Additionally, if we fail to consummate such anticipated Niclosamide Sale, such failure
could result in fluctuations to the market price of our common stock, and may have a material adverse impact on our financial condition
and results of operations.
USE OF PROCEEDS
We estimate that the net proceeds from the
offering will be approximately $4.4 million, after deducting the placement agent fees and estimated offering expenses payable by us,
assuming no sale of any fixed combinations of pre-funded warrants and warrants offered hereunder. If the common warrants are exercised
in full for cash, the estimated net proceeds will increase to $14.4 million. However, because this is a best-efforts offering and there
is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, the placement agent’s
fees and net proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth on the cover
page of this prospectus.
We currently intend to use the net proceeds from
this offering for working capital and general corporate purposes, including the further development of our product candidates. This expected
use of proceeds from this offering represents our intentions based upon our current plans and prevailing business conditions, which could
change in the future as our plans and prevailing business conditions evolve. The amounts and timing of our use of proceeds will vary
depending on a number of factors, including the amount of cash generated or used by our operations. As a result, we will retain broad
discretion in the allocation of the net proceeds of this offering.
DILUTION
If you invest in our securities in this offering,
your interest will be diluted immediately to the extent of the difference between the public offering price paid by the purchasers of
the shares of common stock and common warrants sold in this offering and the as-adjusted net tangible book value per shares of common
stock after this offering.
The net tangible book value of our common stock
as of September 30, 2023, was approximately $1.595 million, or approximately $2.3688 per share of common stock. Net tangible book value
per share represents the amount of our total tangible assets less total liabilities divided by the total number of our shares of common
stock outstanding as of September 30, 2023.
After giving effect to the sale by us in this
offering of 1,381,215 shares of common stock, or up to 1,381,215 pre-funded warrants in lieu of shares of common stock, and 2,762,430
common warrants at a price per share and related common warrants of $3.62, and assuming the exercise in full of pre-funded warrants issued
in this offering, our pro forma as adjusted net tangible book value as of September 30, 2023, would have been approximately $6.0 million,
or approximately $2.9083 per share of common stock. This represents an immediate increase in net tangible book value of approximately
$0.5395 per share of common stock to our existing security holders and an immediate dilution in as adjusted net tangible book value of
approximately $0.7117 per share of common stock to purchasers of common stock in this offering. The final public offering price
will be determined through negotiation between us, the placement agent, and prospective investors in the offering and may be at a discount
to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the
final public offering price. The following table illustrates this per share dilution:
| Assumed combined public offering price per share and accompanying common
warrant | |
| | | |
| | | |
$ | 3.62 | |
| Historical net tangible book value per share as of September 30, 2023 | |
| | | |
$ | 2.3688 | | |
| | |
| Increase in as adjusted net tangible book value per share attributable to this offering | |
| | | |
$ | 0.5395 | | |
| | |
| As adjusted net tangible book value per share after giving
effect to this offering | |
| | | |
| | | |
$ | 2.9083 | |
| Dilution per share to new investors in this offering | |
| | | |
| | | |
$ | 0.7117 | |
Each $0.10 increase or decrease in the assumed
combined public offering price of $3.62 per share and accompanying common warrants, which was the last reported sale price of our
common stock on the Nasdaq Stock Market LLC on December 21, 2023, would increase or decrease the as adjusted net tangible book value
per share by $0.0625 per share and the dilution per share to investors participating in this offering by $0.0375 per share,
assuming that the number of shares and accompanying common warrants offered by us, as set forth on the cover page of this prospectus,
remains the same and after deducting the placement agent fees and commissions and estimated offering expenses payable by us, and excluding
the proceeds, if any, from the cash exercise of the common warrants issued in this offering.
We may also increase or decrease the number
of shares we are offering. A 1.0 million share increase in the number of shares and accompanying common warrants offered by us,
as set forth on the cover page of this prospectus, would increase the as adjusted net tangible book value per share by approximately
$0.1501 and decrease the dilution per share to new investors participating in this offering by approximately $0.1501, based on an assumed
combined public offering price of $3.62 per share and accompanying common warrants, which was the last reported sale price of our common
stock on the Nasdaq Stock Market LLC on December 21, 2023, remaining the same and after deducting the placement agent fees and commissions
and estimated offering expenses payable by us, and excluding the proceeds, if any, from the exercise of the common warrants issued in
this offering. Similarly, a 1.0 million share decrease in the number of shares and accompanying common warrants offered by us, as set
forth on the cover page of this prospectus, would decrease the as adjusted net tangible book value per share by approximately $0.4346
and increase the dilution per share to new investors participating in this offering by approximately $0.4346, based on an assumed combined
public offering price of $3.62 per share and accompanying common warrants, which was the last reported sale price of our common stock
on the Nasdaq Stock Market LLC on December 21, 2023, remaining the same and after deducting the placement agent fees and commissions
and estimated offering expenses payable by us, and excluding the proceeds, if any, from the cash exercise of the common warrants issued
in this offering.
The table and discussion above are based on 673,173
shares of common stock outstanding as of September 30, 2023, and excludes, as of that date, the following:
| ● |
36 shares of common stock issuable
upon exercise of stock options, with a weighted average exercise price of $44,952.62 per share, under our Amended and Restated 2014
Omnibus Equity Incentive Plan (the “2014 Plan”); |
| ● |
14 shares
of awarded but unissued restricted stock under our 2014 Plan; |
| ● |
411 shares of common stock
issuable upon exercise of stock options, with a weighted average exercise price of $4,652.79 per share, under our Amended and Restated
2020 Omnibus Equity Incentive Plan (the “2020 Plan”); |
| ● |
3,649
shares of awarded but unissued restricted stock units under our 2020 Plan; |
| ● |
50,458
shares of common stock available for future issuance under our 2020 Plan; |
| ● |
898,630
shares of common stock issuable upon exercise of outstanding warrants, with a weighted average exercise price of $47.44 per share; |
| ● |
154 shares of common stock
issuable upon conversion of Series B Preferred Stock, including in respect of accrued and unpaid dividends of approximately $1.0
million through September 30, 2023; |
| ● |
either (x) if the holders of
Series B Preferred Stock elect to exchange into our registered direct and private placement offering from January 2021, up to157
additional shares of common stock issuable upon conversion of Series C Preferred Stock and up to 157 shares of common stock issuable
upon exercise of warrants or (y) if the holders of Series B Preferred Stock elect to exchange into our sales made on November 30,
2021, at a price of $10,985.94 per share, pursuant to our ATM Agreement (such price being the lowest price per share sold under the
ATM Agreement to date), up to 454 additional shares of common stock, in each case that may be issued pursuant to the exchange right
in excess of amounts currently underlying Series B Preferred Stock; and |
| ● |
shares of common stock issuable upon exercise of the common warrants issued in this offering. |
The information discussed above is illustrative
only and will adjust based on the actual public offering price, the actual number of shares and common warrants that we offer in this
offering, and other terms of this offering determined at pricing. Except as indicated otherwise, the discussion and table above assumes
(i) no sale of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one
basis, and (ii) no exercise of common warrants accompanying the shares of common stock sold in this offering
DESCRIPTION OF CAPITAL
STOCK
The following summary of the rights of our
capital stock is not complete and is subject to and qualified in its entirety by reference to our Charter and Bylaws, copies of which
are filed as exhibits to our Annual
Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 20, 2023, and the Certificate of Designations
and forms of securities, copies of which are filed as exhibits to the registration statement of which this prospectus forms a part ,
which are incorporated by reference herein.
General
Our authorized capital stock consists of:
| ● |
100,000,000 shares of common stock, par value $0.0001
per share; and |
| ● |
10,000,000 shares of preferred stock, par value $0.0001. |
As of September 30, 2023, there were 50,000,000
shares of Common Stock authorized, and 10,000,000 shares of preferred stock authorized, of which a series of 5,194.81 shares of Series
B Convertible Preferred Stock (the “Series B Preferred Stock”), a series of 75,000 shares of Series C 9.00% Convertible Junior
Preferred Stock (the “Series C Preferred Stock”), a series of 150 shares of Series D Preferred Stock, a series of 150 shares
of Series E Preferred Stock, and a series of 7,000 shares of Series F Preferred Stock have been designated.
On December 12, 2023, shareholders at the Special
Meeting approved a proposal to amend our Charter to increase the authorized shares of common stock from 50,000,000 shares to 100,000,000
shares. On December 13, 2023, we filed an amendment to our Charter with the Secretary of State of the State of Delaware increasing our
authorized shares of common stock to 100,000,000 shares, effective at 12:01 AM on December 18, 2023.
As of September 30, 2023, there were 673,173
shares of Common Stock issued and outstanding, approximately 521.72 shares of Series B Preferred Stock issued and outstanding, no shares
of Series C Preferred Stock issued and outstanding, no shares of Series D Preferred Stock issued and outstanding, no shares of Series
E Preferred Stock issued and outstanding, and no shares of Series F Preferred Stock issued and outstanding.
The additional shares of our authorized capital
stock available for issuance may be issued at times and under circumstances so as to have a dilutive effect on earnings per share and
on the equity ownership of the holders of our common stock. The ability of our board of directors to issue additional shares of stock
could enhance the board’s ability to negotiate on behalf of the stockholders in a takeover situation but could also be used by
the board to make a change of control more difficult, thereby denying stockholders the potential to sell their shares at a premium and
entrenching current management. The following description is a summary of the material provisions of our capital stock. You should refer
to our certificate of incorporation, as amended and restated (the “Charter”), and our bylaws, as amended and restated (the
“Bylaws”), both of which are on file with the SEC as exhibits to previous SEC filings, for additional information. The summary
below is qualified by provisions of applicable law.
Common Stock
Holders of our common stock are entitled to one
vote for each share held of record on all matters on which the holders are entitled to vote (or consent pursuant to written consent).
Directors are elected by a plurality of the votes present in person or represented by proxy and entitled to vote. Our Charter and Bylaws
do not provide for cumulative voting rights.
Holders of our common stock are entitled to receive,
ratably, dividends only if, when and as declared by our board of directors out of funds legally available therefor and after provision
is made for each class of capital stock having preference over the common stock.
In the event of our liquidation, dissolution
or winding-up, the holders of common stock are entitled to share, ratably, in all assets remaining available for distribution after payment
of all liabilities and after provision is made for each class of capital stock having preference over the common stock.
Holders of our common stock have no preemptive,
conversion or subscription rights, and there are no redemption or sinking fund provisions applicable to the common stock. The rights,
preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders
of shares of any series of our preferred stock that we may designate and issue in the future.
Transfer Agent
The transfer agent and registrar for our common
stock is Colonial Stock Transfer Co., Inc., 7840 S. 700 E., Sandy, Utah 84070, Tel: (801) 355-5740.
Preferred Stock
We currently have up to 10,000,000 shares of
preferred stock, par value $0.0001 per share, authorized and available for issuance in one or more series. Our board of directors is
authorized to divide the preferred stock into any number of series, fix the designation and number of each such series, and determine
or change the designation, relative rights, preferences, and limitations of any series of preferred stock. The board of may increase
or decrease the number of shares initially fixed for any series, but no decrease may reduce the number below the shares then outstanding
and duly reserved for issuance. As of September 30, 2023, approximately 5,194.81 shares were designated as Series B Preferred Stock,
of which approximately 521.72 shares were issued and outstanding, 75,000 shares were designated as Series C Preferred Stock, none of
which were issued and outstanding, 150 shares were designated as Series D Preferred Stock, none of which were issued and outstanding,
150 shares were designated as Series E Preferred Stock, none of which were issued and outstanding, and 7,000 shares were designated as
Series F Preferred Stock, none of which were issued and outstanding.
Transfer Agent and Registrar for Preferred Stock
The transfer agent and registrar for any series
or class of preferred stock will be set forth in each applicable prospectus or prospectus supplement when and if any such preferred stock
is offered.
Anti-Takeover Effects of Certain Provisions of Delaware Law and
of Our Charter and Bylaws
Certain provisions of Delaware law, our Charter
and Bylaws discussed below may have the effect of making more difficult or discouraging a tender offer, proxy contest or other takeover
attempt. These provisions are expected to encourage persons seeking to acquire control of our company to first negotiate with our Board
of Directors. We believe that the benefits of increasing our ability to negotiate with the proponent of an unfriendly or unsolicited
proposal to acquire or restructure our company outweigh the disadvantages of discouraging these proposals because negotiation of these
proposals could result in an improvement of their terms.
Delaware Anti-Takeover Law.
We are subject to Section 203 of the Delaware
General Corporation Law (the “DGCL”). Section 203 generally prohibits a public Delaware corporation from engaging in a “business
combination” with an “interested stockholder” for a period of three years after the date of the transaction in which
the person became an interested stockholder, unless:
| ● |
prior to the date of the transaction, the Board of
Directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming
an interested stockholder; |
| ● |
upon consummation of the transaction that resulted
in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation
outstanding at the time the transaction commenced, excluding specified shares; or |
| ● |
at or subsequent to the date of the transaction, the
business combination is approved by the Board of Directors and authorized at an annual or special meeting of stockholders, and not
by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested
stockholder. |
Section 203 defines a “business
combination” to include:
| ● |
any merger or consolidation involving the corporation
and the interested stockholder; |
| ● |
any sale, lease, exchange, mortgage, pledge, transfer
or other disposition of 10% or more of the assets of the corporation to or with the interested stockholder; |
| ● |
subject to exceptions, any transaction that results
in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| ● |
subject to exceptions, any transaction involving the
corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially
owned by the interested stockholder; or |
| ● |
the receipt by the interested stockholder of the benefit
of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an “interested
stockholder” as any person that is:
| ● |
the owner of 15% or more of the outstanding voting
stock of the corporation; |
| ● |
an affiliate or associate of the corporation who was
the owner of 15% or more of the outstanding voting stock of the corporation at any time within three years immediately prior to the
relevant date; or |
| ● |
the affiliates and associates of the above. |
Under specific circumstances, Section 203 makes
it more difficult for an “interested stockholder” to effect various business combinations with a corporation for a three-year
period, although the stockholders may, by adopting an amendment to the corporation’s certificate of incorporation or bylaws, elect
not to be governed by this section, effective 12 months after adoption.
Our Charter and Bylaws do not exclude us from
the restrictions of Section 203. We anticipate that the provisions of Section 203 might encourage companies interested in acquiring us
to negotiate in advance with our Board of Directors since the stockholder approval requirement would be avoided if a majority of the
directors then in office approve either the business combination or the transaction that resulted in the stockholder becoming an interested
stockholder.
Charter and Bylaws.
Provisions of our Charter and Bylaws may delay
or discourage transactions involving an actual or potential change of control or change in our management, including transactions in
which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to
be in their best interests. Therefore, these provisions could adversely affect the price of our common stock.
Stockholder Action by Written Consent
Our Bylaws provide that our stockholders may
take action by written consent or electronic transmission, setting forth the action so taken, signed or e-mailed by the holders of outstanding
stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting for such
purpose.
Potential Effects of Authorized but Unissued Stock
We have shares of common stock and preferred
stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of corporate purposes,
including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as a dividend on the capital
stock.
The existence of unissued and unreserved common
stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred
stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by means of a merger, tender
offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board of directors has the discretion
to determine designations, rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights,
redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest extent permissible under the
DGCL and subject to any limitations set forth in our Charter. The purpose of authorizing the board of directors to issue preferred stock
and to determine the rights and preferences applicable to such preferred stock is to eliminate delays associated with a stockholder vote
on specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings,
acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage
a third party from acquiring, a majority of our outstanding voting stock.
DESCRIPTION OF SECURITIES
WE ARE OFFERING
We are offering up to 1,381,215 shares of our
common stock and up to 2,762,430 common warrants to purchase up to 2,762,430 shares of common stock. Each share of common stock is being
offered together with two common warrants to purchase two shares of common stock. We are also offering up to 1,381,215 pre-funded warrants
to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates
and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares
of common stock following the consummation of this offering in lieu of the shares of common stocks that would result in such excess ownership.
Each pre-funded warrant will be exercisable for one share of common stock. Each pre-funded warrant is being offered together with two
common warrants to purchase two shares of common stock. No warrant for fractional shares of common stock will be issued, rather warrants
will be issued only for whole shares of common stock. We are also registering the shares of common stock issuable from time to time upon
exercise of the pre-funded warrants and common warrants offered hereby.
Common Stock
The material terms and provisions of our common
stock are described under the caption “Description of Capital Stock” in this prospectus and are incorporated herein
by reference.
Common Warrants
The following is a summary of certain terms and
provisions of the common warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by,
the provisions of the common warrant, the form of which will be filed as an exhibit to the registration statement of which this prospectus
forms a part. Prospective investors should carefully review the terms and provisions of the form of common warrant for a complete description
of the terms and conditions of the common warrants.
Duration and Exercise Price
Each common warrant offered hereby will be
exercisable for one share of common stock and will have an exercise price equal to $ per share. The common warrants will be immediately
exercisable and may be exercised until the fifth anniversary of the issuance date. The exercise price and number of shares of common
stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar
events affecting our common stock and the exercise price. The common warrants will be issued separately from the common stock or pre-funded
warrants, respectively, and may be transferred separately immediately thereafter. The common warrants will be issued in certificated
form only.
Exercisability
The common warrants will be exercisable, at the
option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the
number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder
(together with its affiliates) may not exercise any portion of such holder’s common warrants to the extent that the holder would
own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice
from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s common
warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such
percentage ownership is determined in accordance with the terms of the common warrants.
Cashless Exercise
If, at the time a holder exercises its common
warrants, a registration statement registering the issuance of the shares of common stock underlying the common warrants under the Securities
Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated
to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise
(either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the common warrant.
Fundamental Transactions
In the event of any fundamental transaction, as
described in the common warrants and generally including any merger with or into another entity, sale of all or substantially all of
our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent exercise of a common warrant,
the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable
upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor
or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as
a result of such transaction by a holder of the number of shares of our common stock for which the common warrant is exercisable immediately
prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the common warrants have
the right to require us or a successor entity to redeem the common warrants for cash in the amount of the Black-Scholes Value (as defined
in each common warrant) of the unexercised portion of the common warrants concurrently with or within 30 days following the consummation
of a fundamental transaction.
However, in the event of a fundamental transaction
which is not in our control, including a fundamental transaction not approved by our board of directors, the holders of the common warrants
will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the
same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the common
warrant that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that
consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our common stock are given
the choice to receive alternative forms of consideration in connection with the fundamental transaction. If the holders of our common
stock are not offered or paid any consideration in such fundamental transaction, then such holders will be deemed to have received common
stock.
Transferability
Subject to applicable laws, a common warrant may
be transferred at the option of the holder upon surrender of the common warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the common warrants. Rather, the number of shares of common stock to be issued will, at our election, either be
rounded up to the next whole share or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction
multiplied by the exercise price.
Trading Market
There is no established trading market for the
common warrants, and we do not expect an active trading market to develop. We do not intend to apply to list the common warrants on any
securities exchange or other trading market. Without a trading market, the liquidity of the common warrants will be extremely limited.
Right as a Stockholder
Except as otherwise provided in the common warrants
or by virtue of the holder’s ownership of shares of our common stock, such holder of common warrants does not have the rights or
privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s common warrants.
The common warrants will provide that the holders of the common warrants have the right to participate in distributions or dividends
paid on our shares of common stock.
Waivers and Amendments
The common warrant may be modified or amended
or the provisions of the common warrant waived with our and the holder’s written consent.
Pre-funded Warrants
The following summary of certain terms and provisions
of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the pre-funded warrant, the form of which will be filed as an exhibit to the registration statement of which this prospectus forms
a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description
of the terms and conditions of the pre-funded warrants.
Duration and Exercise Price
Each pre-funded warrant offered hereby will have
an initial exercise price per share of common stock equal to $0.0001. The pre-funded warrants will be immediately exercisable and will
expire when exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate
adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock and
the exercise price. Subject to the rules and regulations of the applicable trading market, we may at any time during the term of the
pre-funded warrant, subject to the prior written consent of the holders, reduce the then current exercise price to any amount and for
any period of time deemed appropriate by our board of directors. The pre-funded warrants will be issued in certificated form only.
Exercisability
The pre-funded warrants will be exercisable, at
the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for
the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder
(together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than
4.99% of the outstanding shares of common stock immediately after exercise, except that upon at least 61 days’ prior notice from
the holder to us, the holder may increase the amount of beneficial ownership of outstanding shares after exercising the holder’s
pre-funded warrants up to 9.99% of the number of our shares of common stock outstanding immediately after giving effect to the exercise,
as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants
in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99%
of our outstanding shares of common stock.
Cashless Exercise
In lieu of making the cash payment otherwise contemplated
to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise
(either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the pre-funded warrants. Rather, at the Company’s election, the number of shares of common stock to be issued
will be rounded up to the next whole share or the Company will pay a cash adjustment in an amount equal to such fraction multiplied by
the exercise price.
Transferability
Subject to applicable laws, a pre-funded warrant
may be transferred at the option of the holder upon surrender of the pre-funded warrants to us together with the appropriate instruments
of transfer.
Trading Market
There is no trading market available for the pre-funded
warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market to develop. We do
not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading market. Without a trading market,
the liquidity of the pre-funded warrants will be extremely limited. The shares of common stock issuable upon exercise of the pre-funded
warrants are currently traded on the Nasdaq Capital Market.
Right as a Shareholder
Except as otherwise provided in the pre-funded
warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the pre-funded warrants do not have
the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their pre-funded
warrants. The pre-funded warrants will provide that the holders of the pre-funded warrants have the right to participate in distributions
or dividends paid on our shares of common stock.
Fundamental Transaction
In the event of a fundamental transaction, as
described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our shares of
common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group becoming
the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, the holders of the pre-funded
warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property
that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction on
a net exercise basis.
Waivers and Amendments
The pre-funded warrant may be modified or amended
or the provisions of the pre-funded warrant waived with our and the holder’s written consent.
PLAN OF DISTRIBUTION
We engaged Roth Capital Partners, LLC (“Roth”
or the “placement agent”), to act as our exclusive placement agent to solicit offers to purchase the securities offered by
this prospectus on a reasonable best efforts basis. Roth is not purchasing or selling any securities, nor are they required to arrange
for the purchase and sale of any specific number or dollar amount of securities, other than to use their “reasonable best efforts”
to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered. There is no
minimum amount of proceeds that is a condition to closing of this offering. The placement agent does not guarantee that it will be able
to raise new capital in this offering. The terms of this offering were subject to market conditions and negotiations between us and prospective
investors in consultation with the placement agent. The placement agent will have no authority to bind us. This offering will terminate no
later than January 2, 2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that
date. We will have one closing for all the securities purchased in this offering. The combined public offering price per share (or pre-funded
warrant) and accompanying common warrant will be fixed for the duration of this offering. Roth may engage one or more sub-placement agents
or selected dealers to assist with the offering.
We will enter into a securities purchase agreement
directly with the institutional investors, at the investor’s option, who purchase our securities in this offering. Investors who
do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities
in this offering.
Placement Agent Fees and Expenses
The following table shows the per share and accompanying
common warrants, and per pre-funded and accompanying common warrants, and total placement agent fees we will pay in connection with the
sale of the securities in this offering.
| | |
Per Share
and
Accompanying
Common Warrant | | |
Per Pre-Funded
Warrant and
Accompanying
Common Warrant | | |
Total | |
| Public offering price | |
$ | | | |
$ | | | |
$ | | |
| Placement agent fees | |
$ | | | |
$ | | | |
$ | | |
| Proceeds to us, before expenses | |
$ | | | |
$ | | | |
$ | | |
We estimate that the total expenses of the offering,
including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding placement agent fees,
will be approximately $270,000, all of which are payable by us. This figure includes the placement agent’s accountable expenses,
including, but not limited to, legal fees for placement agent’s legal counsel, that we have agreed to pay at the closing of the
offering up to an aggregate expense reimbursement of $100,000.
Other Relationships
The placement agent acted as the placement agent
in connection with the private placement consummated in July 2023 and as financial advisor for the warrant inducement transaction in
September 2023, for each of which it has received customary fees and expenses. The placement agent may, from time to time, engage in
transactions with or perform services for us in the ordinary course of its business and may continue to receive compensation from us
for such services.
Determination of Offering Price
The combined public offering price per share and
common warrants and the combined public offering price per pre-funded warrant and common warrants we are offering and the exercise prices
and other terms of the warrants were negotiated between us and the investors, in consultation with the placement agent based on the trading
of our common stock prior to this offering, among other things. Other factors considered in determining the public offering prices of
the securities we are offering and the exercise prices and other terms of the warrants include the history and prospects of our company,
the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment
of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Lock-up Agreements
Each of our officers and directors have agreed
to be subject to a lock-up period of 30 days following the date of this prospectus. This means that, during the applicable lock-up period,
they may not offer for sale, contract to sell, or sell any shares of our common stock or any securities convertible into, or exercisable
or exchangeable for, shares of our common stock subject to certain customary exceptions. In addition, we have agreed to not issue any
shares of common stock or securities exercisable or convertible into shares of common stock for a period of 60 days following the closing
date of this offering, subject to certain exceptions, and to not issue any securities that are subject to a price reset based on trading
prices of our common stock or upon a specified or contingent event in the future, or enter into an agreement to issue securities at a
future determined price, for a period of 120 days following the closing date of this offering, subject to certain exceptions.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is Colonial Stock Transfer Company, Inc.
The Nasdaq Capital Market Listing
Our common stock is currently listed on the
Nasdaq Stock Market LLC under the symbol “FWBI.” On December 21, 2023, the reported closing price per share of our common
stock was $3.62. The final public offering price will be determined between us, the placement agent and the investors in the offering,
and may be at a discount to the current market price of our common stock. Therefore, the assumed public offering price used throughout
this prospectus may not be indicative of the final public offering price. There is no established public trading market for the common
warrants or pre-funded warrants, and we do not expect such markets to develop. In addition, we do not intend to apply for a listing of
the common warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system.
Indemnification
We have agreed to indemnify the placement agent
against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the placement
agent may be required to make for these liabilities.
Regulation M
The placement agent may be deemed to be an underwriter
within the meaning of Section 2(a)(11) of the Securities Act and any fees received by it and any profit realized on the sale of the securities
by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent
will be required to comply with the requirements of the Securities Act and the Exchange Act of 1934, as amended (the “Exchange
Act”), including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit
the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement agent may
not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities or
attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed
their participation in the distribution.
Electronic Distribution
A prospectus in electronic format may be made
available on a website maintained by the placement agent and the placement agent may distribute prospectuses electronically. Other than
the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of
which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent and should not be relied upon
by investors.
INCORPORATION OF CERTAIN
INFORMATION BY REFERENCE
The following documents filed with the SEC are
incorporated by reference into this prospectus:
| ● |
our
Annual Report on Form 10-K for the year ended December 31, 2022, filed on March 20, 2023; |
| |
|
| ● |
our
Quarterly Reports on Form 10-Q for the period ended March 31, 2023, filed on May 12, 2023, for the period ended June 30, 2023,
filed with the SEC on August 14, 2023, and for the period ended September 30, 2023, filed with the SEC on November
12, 2023. |
| |
|
| ● |
our Current Reports on Form 8-K, filed on January
17, 2023, February 7,
2023, March 15, 2023,
April 7, 2023, April
21, 2023, June 16, 2023,
June 23, 2023, July
13, 2023, July
21, 2023, August
18, 2023, August
25, 2023, September
14, 2023, September
15, 2023, October
10, 2023, October
31, 2023, December
14, 2023, and December 18, 2023
(other than any portions thereof deemed furnished and not filed); |
| |
|
| ● |
our
definitive proxy statements on Schedule 14A, filed on May 15, 2023 and November
13, 2023; and |
| |
|
| ● |
the description of our common stock which is registered
under Section 12 of the Exchange Act, in our registration statement on Form
8-A, filed on August 8, 2016, as supplemented and updated by the description of our capital stock set forth in Exhibit
4.31 of our Annual Report on Form 10-K for the year ended December 31, 2022, filed on March 20, 2023, including any amendment
or reports filed for the purposes of updating this description. |
We also incorporate by reference all documents
we file pursuant to Section 13(a), 13(c), 14 or 15 of the Exchange Act (other than any portions of filings that are furnished rather
than filed pursuant to Items 2.02 and 7.01 of a Current Report on Form 8-K) after the date of the initial registration statement of which
this prospectus is a part and prior to effectiveness of such registration statement. All documents we file in the future pursuant to
Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of the offering
are also incorporated by reference and are an important part of this prospectus.
Any statement contained in a document incorporated
or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of this registration statement
to the extent that a statement contained herein or in any other subsequently filed document which also is or deemed to be incorporated
by reference herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so
modified or superseded, to constitute a part of this registration statement.
WHERE YOU CAN FIND MORE
INFORMATION
This prospectus is part of a registration statement
we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits
to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we
refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely
only on the information contained in this prospectus or incorporated by reference into this prospectus. We have not authorized anyone
else to provide you with different information. We are not making an offer of these securities in any jurisdiction where the offer is
not permitted. You should assume that the information contained in this prospectus, or any document incorporated by reference in this
prospectus, is accurate only as of the date of those respective documents, regardless of the time of delivery of this prospectus or any
sale of our securities.
We are subject to the informational requirements
of the Exchange Act and in accordance therewith we file annual, quarterly, and other reports, proxy statements and other information
with the Commission under the Exchange Act. Such reports, proxy statements and other information, including the Registration Statement,
and exhibits and schedules thereto, are available to the public through the Commission’s website at www.sec.gov.
We make available free of charge on or through
our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports
filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable
after we electronically file such material with or otherwise furnish it to the Commission. The registration statement and the documents
referred to under “Incorporation of Certain Information by Reference” are also available on our website, www.firstwavebio.com/investors/regulatory-filings.
We have not incorporated by reference into this
prospectus the information on our website, and you should not consider it to be a part of this prospectus.
LEGAL MATTERS
The validity of the securities offered hereby
will be passed upon for us by Ellenoff Grossman & Schole LLP, New York, New York. The placement agent is being represented by Pryor
Cashman LLP.
EXPERTS
The consolidated audited financial statements
incorporated by reference in this prospectus and elsewhere in the registration statement have been incorporated by reference in reliance
upon the report of Mazars USA LLP, independent registered public accounting firm, upon the authority of said firm as experts in accounting
and auditing. The 2022 and 2021 audited annual consolidated financial statements of First Wave BioPharma, Inc. (formerly known as AzurRx
BioPharma, Inc.), as of and for the years ended December 31, 2022 and 2021, have been audited by Mazars USA LLP, independent registered
public accounting firm. The audit reports dated March 20, 2023 for the 2022 and March 21, 2022 for the 2021 audited annual consolidated
financial statements includes an explanatory paragraph which states that certain circumstances raise substantial doubt about our ability
to continue as a going concern.
Up to 1,381,215 Shares of Common Stock
1,381,215 Pre-Funded Warrants to Purchase up to 1,381,215 Shares of Common Stock
Up to 2,762,430 Common Warrants to Purchase up to 2,762,430 Shares of Common Stock
Common Stock underlying the Pre-Funded Warrants and
Up to 2,762,430 Shares of Common Stock underlying
the Common Warrants

PRELIMINARY PROSPECTUS
Roth Capital Partners
The date of this prospectus is ,
2023.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. |
Other Expenses of Issuance
and Distribution. |
The following table indicates the expenses to
be incurred in connection with the offering described in this registration statement, other than underwriting discounts and commissions,
all of which will be paid by us. All amounts are estimated except the Securities and Exchange Commission registration fee.
| |
|
Amount |
|
| SEC Registration Fee |
|
$ |
2,214 |
|
| FINRA Filing Fee |
|
|
5,000 |
|
| Legal Fees and Expenses |
|
|
125,000 |
|
| Accounting Fees and Expenses |
|
|
25,000 |
|
| Transfer Agent and Registrar fees and expenses |
|
|
5,000 |
|
| Miscellaneous Expenses |
|
|
5,000 |
|
| Total expenses |
|
$ |
167,214 |
|
| Item 14. |
Indemnification of Directors and Officers. |
Amended and Restated Bylaws
Pursuant to our bylaws, our directors and officers
will be indemnified to the fullest extent allowed under the laws of the State of Delaware for their actions in their capacity as our
directors and officers.
We must indemnify any person made a party to
any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative (“Proceeding”)
by reason of the fact that he is or was a director, against judgments, penalties, fines, settlements and reasonable expenses (including
attorney’s fees) (“Expenses”) actually and reasonably incurred by him in connection with such Proceeding if: (a) he
conducted himself in good faith, and: (i) in the case of conduct in his own official capacity with us, he reasonably believed his conduct
to be in our best interests, or (ii) in all other cases, he reasonably believes his conduct to be at least not opposed to our best interests;
and (b) in the case of any criminal Proceeding, he had no reasonable cause to believe his conduct was unlawful.
We must indemnify any person made a party to
any Proceeding by or in the right of us, by reason of the fact that he is or was a director, against reasonable expenses actually incurred
by him in connection with such proceeding if he conducted himself in good faith, and: (a) in the case of conduct in his official capacity
with us, he reasonably believed his conduct to be in our best interests; or (b) in all other cases, he reasonably believed his conduct
to be at least not opposed to our best interests; provided that no such indemnification may be made in respect of any proceeding in which
such person shall have been adjudged to be liable to us.
No indemnification will be made by unless authorized
in the specific case after a determination that indemnification of the director is permissible in the circumstances because he has met
the applicable standard of conduct.
Reasonable expenses incurred by a director who
is party to a proceeding may be paid or reimbursed by us in advance of the final disposition of such Proceeding in certain cases.
We have the power to purchase and maintain insurance
on behalf of any person who is or was our director, officer, employee, or agent or is or was serving at our request as an officer, employee
or agent of another corporation, partnership, joint venture, trust, other enterprise, or employee benefit plan against any liability
asserted against him and incurred by him in any such capacity or arising out of his status as such, whether or not we would have the
power to indemnify him against such liability under the provisions of the amended and restated bylaws.
Delaware Law
We are incorporated under the laws of the State
of Delaware. Section 145 of the Delaware General Corporation Law provides that a Delaware corporation may indemnify any persons who are,
or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative
or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer,
director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee
or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines
and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided
that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s
best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was
illegal. A Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or
completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee
or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another
corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such
person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he
or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted
without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful
on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses
which such officer or director has actually and reasonably incurred. Our amended and restated certificate of incorporation and amended
and restated bylaws provide for the indemnification of our directors and officers to the fullest extent permitted under the Delaware
General Corporation Law.
Section 102(b)(7) of the Delaware General Corporation
Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable
to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
| ● |
transaction from which the director derives an improper
personal benefit; |
| |
|
| ● |
act or omission not in good faith or that involves
intentional misconduct or a knowing violation of law; |
| |
|
| ● |
unlawful payment of dividends or redemption of shares;
or |
| |
|
| ● |
breach of a director’s duty of loyalty to the
corporation or its stockholders. |
Our amended and restated certificate of incorporation
and amended and restated bylaws include such a provision. Expenses incurred by any officer or director in defending any such action,
suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of
such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not
entitled to be indemnified by us.
Section 174 of the Delaware General Corporation
Law provides, among other things, that a director who willfully or negligently approves of an unlawful payment of dividends or an unlawful
stock purchase or redemption may be held liable for such actions. A director who was either absent when the unlawful actions were approved,
or dissented at the time, may avoid liability by causing his or her dissent to such actions to be entered in the books containing minutes
of the meetings of the board of directors at the time such action occurred or immediately after such absent director receives notice
of the unlawful acts.
Indemnification Agreements
As permitted by the Delaware General Corporation
Law, we have entered, and intend to continue to enter, into separate indemnification agreements with each of our directors and executive
officers, that require us to indemnify such persons against any and all expenses (including attorneys’ fees), witness fees, damages,
judgments, fines, settlements and other amounts incurred (including expenses of a derivative action) in connection with any action, suit
or proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that such person is or
was a director, an officer or an employee of us or any of our affiliated enterprises, provided that such person acted in good faith and
in a manner such person reasonably believed to be in or not opposed to our best interests and, with respect to any criminal proceeding,
had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth certain procedures
that will apply in the event of a claim for indemnification thereunder.
At present, there is no pending litigation or
proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not
aware of any threatened litigation or preceding that may result in a claim for indemnification.
We have an insurance policy covering our officers
and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
Insofar as indemnification for liabilities arising
under the Securities Act may be permitted to our directors, officers or controlling persons, we have been advised that in the opinion
of the SEC this indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
| Item 15. |
Recent Sales of Unregistered
Securities. |
During the three months ended March 31, 2020,
the Company issued an aggregate of 2 shares of its Common Stock to consultants with a grant date fair value of $87,105 for services provided,
that was recorded as part of G&A expense. Such issuance was exempt from registration under 4(a)(2) of the Securities Act.
During the three months ended March 31, 2020,
the Company issued its outside Board members an aggregate of 2 shares of Common Stock for the settlement of accounts payable in the aggregate
amount of $131,137. The aggregate effective settlement price was $52,080 per share, and each individual stock issuance was based on the
closing stock price of the Common Stock on the initial date the payable was accrued. Such issuance was exempt from registration under
4(a)(2) of the Securities Act.
On July 16, 2020, we issued an aggregate of 2,912.583005
shares of Series B Convertible Preferred Stock, at a price of $7,700.00 per share, initially convertible into an aggregate of 693 shares
of our Common Stock at $32,340.00 per share, together with Series B Warrants to purchase an aggregate of 346 shares of Common Stock at
an exercise price of $35,700.00 per share. An aggregate of 1,975.578828 shares of Series B Preferred Stock initially convertible into
470 shares of Common Stock and related 235 Series B Warrants were issued for cash consideration, resulting in aggregate gross proceeds
to us of approximately $15.2 million. In addition, the balance of an aggregate of 937.004221 shares of Series B Preferred Stock initially
convertible into 223 shares of Common Stock and related Series B Warrants to purchase 111 shares of Common Stock was issued to certain
investors in exchange for consideration consisting of approximately $6.9 million aggregate outstanding principal amount, together with
accrued and unpaid interest thereon of approximately $0.3 million, of certain Senior Convertible Promissory Notes issued between December
20, 2019 and January 9, 2020. As additional consideration to the Exchange Investors, we also issued certain additional warrants to purchase
an aggregate of 42 shares of Common Stock at an exercise price of $35,700.00 per share. We issued to the placement agent in the offerings
warrants to purchase up to 7.0% of the aggregate number of shares of Common Stock underlying the Series B Preferred Stock sold for cash
consideration in the Private Placement, or 32 shares. The issuance of these securities was made pursuant to Section 4(a)(2) of the Securities
Act, and the rules promulgated thereunder, to accredited investors.
During the period from April 6, 2020 through May
22, 2020, we sold an aggregate of 32 shares of Common Stock pursuant to an equity line agreement, from which we derived approximately
$869,000 in net proceeds. The sales of these shares under the equity line agreement was exempt from registration under the Securities
Act of 1933, as amended, in reliance upon Section 4(a)(2) (or Regulation D promulgated thereunder).
On January 5, 2021, in a private placement offering
we sold to an investor 5,333.3333 shares of Series C Preferred Stock, which shares are convertible into an aggregate of 126 shares of
Common Stock, together with warrants to purchase up to an aggregate of 253 shares of Common Stock, with an exercise price of $33,600.00
per share and an expiration term of five and one-half years from the date of issuance. The aggregate gross proceeds from the offering,
excluding the net proceeds, if any, from the exercise of the Private Placement Warrants will be approximately $8.0 million. The issuance
of these securities was made pursuant to Section 4(a)(2) of the Securities Act, and the rules promulgated thereunder, to accredited investors.
On January 8, 2021, in connection with entering
into a license agreement with a third party, we entered into a securities purchase agreement where we issued 3,290.1960 shares of Series
C Preferred Stock, initially convertible into an aggregate of 78 shares of Common Stock, at an initial stated value of $750.00 per share
and a conversion price of $31,500.00 per share. The Series C Preferred Stock issued, together with any Common Stock issuable upon conversion,
were issued without registration under the Securities Act in reliance on the exemptions provided by Section 4(a)(2) of the Securities
Act as transactions not involving a public offering.
On September 13, 2021, in connection with the
merger with First Wave Bio, Inc., we issued the former stockholders of First Wave Bio, Inc. approximately 148 shares of common stock.
The common stock was issued without registration under the Securities Act in reliance on exemptions provided by Section 4(a)(2) of the
Securities Act as transactions not involving a public offering.
During the three months ended March 31, 2022,
we issued an aggregate of 21 shares of common stock to consultants with a grant date fair value of approximately $119,000 for investor
relations services provided. Such issuance was exempt from registration under 4(a)(2) of the Securities Act.
On July 15, 2022, in a private placement offering,
we sold to investors 150 shares of Series D Preferred Stock, which is convertible into an aggregate of 238 shares of common stock, 150
shares of Series E Preferred Stock, convertible into an aggregate of 238 shares of common stock, Series D Warrants to purchase up to
an aggregate of 476 shares of common stock, with an exercise price of $630.00 and an expiration term of five years from its initial exercise
date. The aggregate gross proceeds from the offering, excluding the net proceeds, if any from the exercise of the Series D Warrants was
approximately $300,000. The issuance of these securities was made pursuant to Section 4(a)(2) of the Securities Act, and the rules promulgated
thereunder, to accredited investors.
On November 20, 2022, in a private placement offering,
we sold to an investor pre-funded warrants to purchase up to an aggregate of 29,761 shares of common stock and common warrants to purchase
up to an aggregate of 59,523 shares of common stock at a purchase price of $83.986 per pre-funded warrant and accompanying common warrant.
The common warrants had an exercise price of $107.59 per share and will expire five and one-half years from the initial exercise date.
The aggregate gross proceeds from the offering, excluding the net proceeds, if any, from the exercise of the common warrants was approximately
$2.5 million. The issuance of these securities was made pursuant to Section 4(a)(2) of the Securities Act, and the rules promulgated
thereunder, to an accredited investor.
On March 12, 2023, in a private placement offering,
we sold to an investor 6,400 shares of common stock, pre-funded warrants to purchase up to an aggregate of 44,750 shares of common stock
and common warrants to purchase up to an aggregate of 102,301 shares of common stock at a purchase price of $78.20 per share and accompanying
common warrant. The common warrants had an exercise price of $73.20 per share and will expire five years from the initial exercise date.
The aggregate gross proceeds from the offering, excluding the net proceeds, if any, from the exercise of the common warrants was approximately
$4.0 million. The issuance of these securities was made pursuant to Section 4(a)(2) of the Securities Act, and the rules promulgated
thereunder, to an accredited investor.
On June 13, 2023, we entered into warrant exercise
inducement offer letters with certain holders of warrants to purchase shares of common stock pursuant to which the holders agreed to
exercise for cash their existing warrants to purchase 86,216 shares of common stock, in the aggregate, at a reduced exercise price of
$23.00 per share, in exchange for new warrants issued in a private placement offering to purchase up to 172,433 shares of common stock
and a cash payment of $2.50 per warrant share which was paid in full upon the exercise of the existing warrants. The aggregate gross
proceeds from the offering, excluding the net proceeds, if any, from the exercise of the new warrants was approximately $2.4 million.
The issuance of the new warrants was made pursuant to Section 4(a)(2) of the Securities Act, and the rules promulgated thereunder, to
an accredited investor.
On July 3, 2023, we issued 2,500 shares of restricted
common stock to a consultant with a grant date fair value of approximately $76,000 for investor relations services provided. Such issuance
was exempt from registration under 4(a)(2) of the Securities Act.
On September 14, 2023, we entered into warrant
exercise inducement offer letters with a holder of warrants to purchase shares of common stock pursuant to which the holders agreed to
exercise for cash their existing warrants to purchase 294,101 shares of common stock, in the aggregate, at a reduced exercised price
of $8.60 per share, in exchange for new warrants on substantially the same terms as the existing warrants as described below, to purchase
up to 588,203 shares of common stock and a cash payment of $2.50 per warrant share which was paid in full upon the exercise of the existing
warrants. The aggregate gross proceeds from the offering, excluding the net proceeds, if any, from the exercise of the new warrants was
approximately $4.0 million. The issuance of the new warrants was made pursuant to Section 4(a)(2) of the Securities Act, and the rules
promulgated thereunder, to an accredited investor.
During the period from July 16, 2020 to date,
pursuant to the exchange right in the Series B Preferred Stock Certificate of Designations, we issued 596 shares of common stock and
warrants to purchase up to 498 of common stock to holders of Series B Preferred Stock exercising such right. The issuance of these securities
was made pursuant to exemptions provided by Section 3(a)(9) under the Securities Act.
The list of exhibits following the signature page
of this registration statement is incorporated by reference herein.
(1) The undersigned registrant hereby undertakes:
(a) To file, during any period in which offers
or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3)
of the Securities Act;
(ii) To reflect in the prospectus any
facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding
the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed
that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the
form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more
than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in
the effective registration statement; and
(iii) To include any material information
with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information
in the registration statement;
provided,
however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective
amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant
pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration
statement.
(b) That, for the purpose of determining any liability
under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities
offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) To remove from registration by means of a
post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(d) That, for the purpose of determining liability
under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement
relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule
430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided,
however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a
document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration
statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was
made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately
prior to such date of first use.
(e) That, for the purpose of determining liability
of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant
hereby undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless
of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means
of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer
or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus
of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter);
(ii) Any free writing prospectus relating
to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free
writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided
by or on behalf of the undersigned registrant; and
(iv) Any other communication that is
an offer in the offering made by the undersigned registrant to the purchaser.
(2) The undersigned registrant hereby undertakes that, for purposes
of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section 13(a)
or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to
Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof.
(3) The undersigned registrant hereby undertakes that: a
(a) For purposes of determining any liability
under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance
on Rule 430A and contained in a form of prospectus filed by the undersigned registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under
the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective; and
(b) For the purpose of determining any liability
under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona
fide offering thereof.
(4) Insofar as indemnification for liabilities arising under the Securities
Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise,
the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities
Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment
by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense
of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being
registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to
a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities
Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly
authorized in the City of Boca Raton, State of Florida, on December 27, 2023.
| FIRST
WAVE BIOPHARMA, INC. |
|
| |
|
|
| By: |
/s/ James Sapirstein |
|
| |
Name: |
James Sapirstein |
|
| |
Title: |
President, Chief Executive
Officer and Chairman of the Board
(Principal Executive Officer) |
|
| |
|
|
|
| By: |
/s/ Sarah Romano |
|
| |
Name: |
Sarah Romano |
|
| |
Title: |
Chief Financial Officer
(Principal Financial and Accounting Officer) |
|
Pursuant to the requirements of the Securities
Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date
|
| |
|
|
|
|
| /s/
James Sapirstein |
|
President,
Chief Executive Officer and Chairman of the Board
(Principal Executive Officer) |
|
December
27, 2023 |
| James Sapirstein |
|
| |
|
|
|
|
| /s/
Sarah Romano |
|
Chief
Financial Officer
(Principal Financial Officer and Principal Accounting Officer) |
|
December
27, 2023 |
| Sarah Romano |
|
| |
|
|
|
|
| * |
|
Director
|
|
December
27, 2023 |
| Edward J. Borkowski
|
|
| |
|
|
|
|
| * |
|
Director
|
|
December
27, 2023 |
| Charles Casamento |
|
| |
|
|
|
|
| * |
|
Director
|
|
December
27, 2023 |
| Terry Coelho |
|
| |
|
|
|
|
| * |
|
Director
|
|
December
27, 2023 |
| Alastair Riddell |
|
| * By |
/s/
James Sapirstein |
|
| James Sapirstein |
|
| Attorney-in-fact |
|
Exhibit
No. |
|
Description |
| 1.1 |
|
Form of Placement Agency Agreement (incorporated by reference to Exhibit
1.1 of the Company’s Amendment No. 1 to Form S-1 filed with the SEC on December 18, 2023). |
| 2.1# |
|
Agreement
and Plan of Merger dated September 13, 2021, by and among the Company, Alpha Merger Sub, Inc., and Fortis Advisors LLC, as shareholder
representative (incorporated by reference to Exhibit 2.1 of the Company’s Current Report on Form 8-K filed with the SEC on
September 13, 2021). ## |
| 3.1 |
|
Amended
and Restated Certificate of Incorporation of the Registrant, as amended to date (incorporated by reference to Exhibit 3.1 of the
Company’s Annual Report on Form 10-K filed with the SEC on March 20, 2023). |
| 3.2 |
|
Certificate
of Amendment to the Amended and Restated Certificate of Incorporation, as amended, of First Wave BioPharma, Inc., dated December
13, 2023 (incorporated by reference to Exhibit 3.1 of the Company’s Current Report on Form 8-K filed with the SEC on December
14, 2023). |
| 3.3 |
|
Amended
and Restated Bylaws, as amended to date (incorporated by reference to Exhibit 3.2 of the Company’s Quarterly Report on Form
10-Q filed with the SEC on August 15, 2022). |
| 4.1 |
|
Form
of Common Stock Certificate (incorporated by reference to Exhibit 4.1 filed with Amendment No 1. To Registration Statement on Form
S-1, filed July 29, 2016). |
| 4.2 |
|
Form
of Investor Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Registration Statement on Form S-1 filed with
the SEC on July 13, 2016). |
| 4.3 |
|
Form
of Underwriter Warrant (incorporated by reference to Exhibit 4.3 to the Company’s Registration Statement on Form S-1, filed
with the SEC on July 29, 2016). |
| 4.4 |
|
Form
of Series A Warrant, dated April 11, 2017 between the Company and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit
10.3 filed with the Company’s Current Report on Form 8-K filed with the SEC on April 12, 2017). |
| 4.5 |
|
Form
of Series A Warrant, dated June 5, 2017 (incorporated by reference to Exhibit 10.3 filed with the Company’s Current Report
on Form 8-K filed with the SEC on June 9, 2017). |
| 4.6 |
|
Form
of Series A-1 Warrant, dated June 5, 2017 (incorporated by reference to Exhibit 10.4 filed with the Company’s Current Report
on Form 8-K filed with the SEC on June 9, 2017). |
| 4.7 |
|
Form
of Underwriter Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on May 4, 2018). |
| 4.8 |
|
Form
of Selling Agent Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on April 3, 2019). |
| 4.9 |
|
Form
of Selling Agent Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on May 14, 2019). |
| 4.10 |
|
Form
of Wainwright Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on July 22, 2019). |
| 4.11 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC on July
20, 2020). |
| 4.12 |
|
Form
of Warrant for Convertible Notes Offering (incorporated by reference to Exhibit 4.2 of the Company’s Registration Statement
on Form S-3 filed with the SEC on July 27, 2020). |
| 4.13 |
|
Form
of Pre-funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on January 4, 2021). |
| 4.14 |
|
Form
of Private Placement Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with
the SEC on January 4, 2021). |
| 4.15 |
|
Form
of Wainwright Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on January 8, 2021). |
| 4.16 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on March 10, 2021). |
| 4.17 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on March
10, 2021). |
| 4.18 |
|
Form
of Wainwright Warrant (incorporated by reference to Exhibit 4.3 of the Company’s Current Report on Form 8-K filed with the
SEC on March 10, 2021). |
| 4.19 |
|
Description
of Capital Stock (incorporated by reference to Exhibit 4.31 of the Company’s Annual Report on Form 10-K filed with the SEC
on March 20, 2023). |
| 4.20 |
|
Form
of Wainwright Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the
SEC on July 27, 2021). |
| 4.21 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on March 1, 2022). |
| 4.22 |
|
Form
of Series C Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on
March 1, 2022). |
| 4.23 |
|
Form
of Placement Agent Warrant (incorporated by reference to Exhibit 4.3 of the Company’s Current Report on Form 8-K filed with the
SEC on March 1, 2022). |
| 4.24 |
|
Form
of Warrant Amendment Agreement (incorporated by reference to Exhibit 4.4 of the Company’s Current Report on Form 8-K filed with
the SEC on March 1, 2022). |
| 4.25 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.1 of the Current Report on Form 8-K filed with the SEC on July 18, 2022). |
| 4.26 |
|
Form
of Placement Agent Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the
SEC on July 18, 2022). |
| 4.27
|
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on October 12, 2022). |
| 4.28 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on October
12, 2022). |
| 4.29 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on November 22, 2022). |
| 4.30 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on November
22, 2022). |
| 4.31 |
|
Form
of Warrant Amendment Agreement (incorporated by reference to Exhibit 4.3 of the Company’s Current Report on Form 8-K filed with
the SEC on November 22, 2022). |
| 4.32 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on March 15, 2023). |
| 4.33 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on March 15,
2023). |
| 4.34 |
|
Form
of Inducement Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on June 16, 2023). |
| 4.35 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.1 of the Company’s Current Report on Form 8-K filed with the SEC
on July 21, 2023). |
| 4.36 |
|
Form
of Warrant (incorporated by reference to Exhibit 4.2 of the Company’s Current Report on Form 8-K filed with the SEC on July 21,
2023). |
| 4.37 |
|
Form
of Inducement Warrant (incorporated by reference to Exhibit 4.1 of the Current Report on Form 8-K filed with the SEC on September 15,
2023). |
| 4.38 |
|
Form
of Pre-Funded Warrant (incorporated by reference to Exhibit 4.36 of the Company’s Amendment No. 1 to Form S-1 filed with the
SEC on December 18, 2023). |
| 4.39 |
|
Form
of Common Warrant (incorporated by reference to Exhibit 4.37 of the Company’s Amendment No. 1 to Form S-1 filed with the
SEC on December 18, 2023). |
| 4.40* |
|
Form of Warrant Agency Agreement |
| 5.1* |
|
Opinion
of Ellenoff Grossman & Schole LLP. |
| 10.1 |
|
Stock
Purchase Agreement dated May 21, 2014 between the Registrant, Protea Biosciences Group, Inc. and its wholly-owned subsidiary, Protea
Biosciences, Inc (incorporated by reference to Exhibit 10.1 of the Company’s Registration Statement on Form S-1 filed with the
SEC on July 13, 2016). |
| 10.2† |
|
Amended
and Restated AzurRx BioPharma, Inc. 2014 Omnibus Equity Incentive Plan (incorporated by reference to Exhibit 10.3 of the Company’s
Registration Statement on Form S-1 filed with the SEC on July 13, 2016). |
| 10.3 |
|
Securities
Purchase Agreement dated April 11, 2017 between the Registrant and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit
10.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 12, 2017). |
| 10.4 |
|
Registration
Rights Agreement dated April 11, 2017 between the Registrant and Lincoln Park Capital Fund, LLC (incorporated by reference to Exhibit
10.4 of the Company’s Current Report on Form 8-K filed with the SEC on April 12, 2017). |
| 10.5 |
|
Form
of Securities Purchase Agreement dated June 5, 2017 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report
on Form 8-K filed with the SEC on June 9, 2017). |
| 10.6 |
|
Form
of Registration Rights Agreement dated June 5, 2017 (incorporated by reference to Exhibit 10.2 of the Company’s Current Report
on Form 8-K filed with the SEC on April 12, 2017). |
| 10.7 |
|
Sublicense
Agreement dated August 7, 2017 by and between the Registrant and TransChem, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s
Current Report on Form 8-K filed with the SEC on August 11, 2017). |
| 10.8 |
|
Asset
Sale and Purchase Agreement, dated December 7, 2018, by and between Protea Biosciences Group, Inc., Protea Biosciences, Inc. and AzurRx
Biopharma, Inc. (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on December
13, 2018). |
| 10.9 |
|
Registration
Rights Agreement, dated February 14, 2019 (incorporated by reference to Exhibit 10.6 of the Company’s Current Report on Form 8-K
filed with the SEC on February 20, 2019). |
| 10.10 |
|
Asset
Purchase Agreement, by and between AzurRx BioPharma, Inc., AzurRx BioPharma SAS and Laboratoires Mayoly Spindler SAS, dated March
27, 2019 (incorporated by reference to Exhibit 10.25 of the Company’s Annual Report on Form 10-K filed with the SEC on April
1, 2019). |
| 10.11 |
|
Patent
License Agreement, by and between AzurRx BioPharma, Inc. and Laboratoires Mayoly Spindler SAS, dated March 27, 2019 (incorporated
by reference to Exhibit 10.26 of the Company’s Annual Report on Form 10-K filed with the SEC on April 1, 2019). |
| 10.12† |
|
Employment
Agreement by and between AzurRx BioPharma, Inc. and James Sapirstein, dated October 8, 2019 (incorporated by reference to Exhibit
10.1 of the Company’s Current Report on Form 8-K filed with the SEC on October 11, 2019). |
| 10.13 |
|
Securities
Purchase Agreement, dated November 13, 2019 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form
8-K filed with the SEC on November 14, 2019). |
| 10.14 |
|
Registration
Rights Agreement, dated November 13, 2019 (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form
8-K filed with the SEC on November 14, 2019). |
| 10.15 |
|
Form
of Note Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with
the SEC on December 30, 2019). |
| 10.16 |
|
Form
of Warrant (incorporated by reference to Exhibit 10.3 of the Company’s Current Report on Form 8-K filed with the SEC on December
30, 2019). |
| 10.17 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.4 of the Company’s Current Report on Form 8-K filed
with the SEC on December 30, 2019). |
| 10.18† |
|
Employment
Agreement by and between AzurRx BioPharma, Inc. and Daniel Schneiderman, dated January 1, 2020 (incorporated by reference to Exhibit
10.1 of the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2020). |
| 10.19 |
|
Form
of Purchase Agreement, by and among the Company and the investors set forth on the signature pages thereto, including the form of
Exchange Addendum (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC
on July 20, 2020). |
| 10.20 |
|
Form
of Registration Rights Agreement, by and among the Company and the investors set forth on the signature page thereto (incorporated
by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the SEC on July 20, 2020). |
| 10.21† |
|
First
Amendment to 2014 Omnibus Equity Incentive Plan (incorporated by reference as Exhibit 10.3 of the Company’s Current Report
on Form 8-K filed with the SEC on July 20, 2020). |
| 10.22† |
|
2020
Omnibus Equity Incentive Plan (incorporated by reference to Exhibit 10.4 of the Company’s Quarterly Report on Form 10-Q filed
with the SEC on November 16, 2020). |
| 10.23† |
|
Amendment
to the 2020 Omnibus Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form
8-K filed with the SEC on June 23, 2023) |
| 10.24 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on January 4, 2021). |
| 10.25 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed
with the SEC on January 4, 2021). |
| 10.26 |
|
First
Wave Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on January 8, 2021). |
| 10.27# |
|
First
Wave License Agreement (incorporated by reference to Exhibit 10.1 filed with the Company’s Current Report on Form 8-K filed
with the SEC on January 13, 2021). |
| 10.28 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 filed with the Company’s Current Report on Form 8-K filed
with the SEC on March 10, 2021). |
| 10.29 |
|
At
The Market Offering Agreement, dated May 26, 2021, by and between AzurRx BioPharma, Inc. and H.C. Wainwright & Co., LLC (incorporated
by reference to Exhibit 1.2 of the Company’s Registration Statement on Form S-3 filed with the SEC on May 26, 2021). |
| 10.30 |
|
Settlement
Agreement, by and between the Company and Fortis Advisors LLC, dated November 15, 2021 (incorporated by reference to Exhibit 10.1
of the Company’s Current Report on Form 8-K filed with the SEC on November 16, 2021). |
| 10.31 |
|
Form
of Waiver (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on February
7, 2022). |
| 10.32† |
|
Employment
Agreement by and between First Wave BioPharma, Inc. and Sarah Romano, dated February 14, 2022 (incorporated by reference to Exhibit
10.1 of the Company’s Current Report on Form 8-K filed with the SEC on February 17, 2022). |
| 10.33 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on March 1, 2022). |
| 10.34 |
|
Form
of Indemnification Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with
the SEC on May 5, 2022). |
| 10.35 |
|
Form
of Waiver Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC
on May 12, 2022). |
| 10.36 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on July 18, 2022). |
| 10.37 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed
with the SEC on July 18, 2022). |
| 10.38# |
|
Form
of Term Sheet, by and between the Representative and the Company, dated July 29, 2022. (incorporated by reference to Exhibit 10.1
of the Company’s Current Report on Form 8-K filed with the SEC on July 29, 2022). |
| 10.39
|
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on October 12, 2022). |
| 10.40 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on November 22, 2022). |
| 10.41 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed
with the SEC on November 22, 2022). |
| 10.42 |
|
Form
of Settlement Agreement, by and between the Representative and the Company, dated November 30, 2022) (incorporated by reference to
Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on December 2, 2022). |
| 10.43 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on March 15, 2023). |
| 10.44 |
|
Form
of Registration Rights Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed
with the SEC on March 15, 2023). |
| 10.45 |
|
Form
of Inducement Letter (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on June 16, 2023). |
| 10.46† |
|
Amendment
to the 2020 Omnibus Equity Incentive Plan (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form
8-K filed with the SEC on June 23, 2023). |
| 10.47 |
|
Form
of Purchase Agreement (incorporated by reference to Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the
SEC on July 21, 2023). |
| 10.48 |
|
Form
of Inducement Letter (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the
SEC on September 15, 2023). |
| 10.49 |
|
License
Agreement, dated September 13, 2023 (incorporated by reference to Exhibit 10.1 of the Company’s Current Report on Form 8-K
filed with the SEC on October 10, 2023). |
| 10.50 |
|
Form of Securities Purchase
Agreement (incorporated by reference to Exhibit 10.50 of the Company’s Amendment No. 1 to Form S-1 filed with the
SEC on December 18, 2023). |
| 16.1 |
|
Letter
from Mazars USA LLP to the U.S. Securities and Exchange Commission, dated May 3, 2022 (incorporated by reference to Exhibit 16.1
of the Company’s Current Report on Form 8-K filed with the SEC on May 3, 2022). |
| 16.2 |
|
Letter
from Marcum LLP to the U.S. Securities and Exchange Commission, dated July 7, 2022 (incorporated by reference to Exhibit 16.1 of
the Company’s Current Report on Form 8-K filed with the SEC on July 7, 2022). |
| 21.1 |
|
Subsidiaries
of the Registrant (incorporated by reference to Exhibit 21.1 of the Company’s Annual Report on Form 10-K filed with the SEC
on March 20, 2023). |
| 23.1* |
|
Consent
of Ellenoff Grossman & Schole LLP (included in Exhibit 5.1). |
| 23.2* |
|
Consent
of Independent Registered Public Accounting Firm – Mazars USA LLP. |
| 24.1* |
|
Power
of Attorney (included in signature page). |
| 107* |
|
Filing
Fee Table. |
| * |
Filed herewith. |
| # |
Certain portions of this exhibit (indicated by “[*****]”)
have been omitted as we have determined (1) it is not material and (2) is the type that the Company treats as private or confidential. |
| ## |
Schedules and exhibits have been omitted pursuant to
Item 601(b)(2) of Regulation S-K. The Company hereby undertakes to furnish supplementally copies of any of the omitted schedules
upon request by the SEC. |
| † |
Indicates a management contract or compensation plan,
contract or arrangement. |
Exhibit 4.40
FIRST WAVE BIOPHARMA, INC.
and
COLONIAL STOCK TRANSFER CO., INC. as
Warrant Agent
Warrant Agency Agreement
Dated as of December [ ], 2023
WARRANT AGENCY AGREEMENT
WARRANT AGENCY AGREEMENT,
dated as of December [ ], 2023 (“Agreement”), between First Wave BioPharma, Inc., a Delaware corporation
(the “Company”), and Colonial Stock Transfer Co., Inc. (the “Warrant Agent”).
WITNESSETH
WHEREAS, pursuant to an offering
by the Company of Warrants (as defined below), the Company wishes to issue Warrants in book entry form entitling the respective holders
of the Warrants (the “Holders”, which term shall include a Holder’s transferees, successors and assigns and “Holder”
shall include, if the Warrants are held in “street name”, a Participant (as defined below) or a designee appointed by such
Participant) to purchase an aggregate of up to 2,762,430 shares of Common Stock (the “Common Stock”) underlying the
Warrants (as defined below) upon the terms and subject to the conditions hereinafter set forth (the “Offering”);
WHEREAS, the Company wishes
the Warrant Agent to act on behalf of the Company, and the Warrant Agent is willing so to act, in connection with the issuance, registration,
transfer, exchange, exercise and replacement of the Warrants.
NOW, THEREFORE, in consideration
of the premises and the mutual agreements herein set forth, the parties hereby agree as follows:
Section 1. Certain
Definitions. For purposes of this Agreement, the following terms have the meanings indicated:
(a) “Affiliate”
has the meaning ascribed to it in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
(b) “Business
Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day
on which the Nasdaq Stock Market is authorized or required by law or other governmental action to close.
(c) “Close
of Business” on any given date means 5:00 p.m., New York City time, on such date; provided, however, that if such
date is not a Business Day it means 5:00 p.m., New York City time, on the next succeeding Business Day.
(d) “Person”
means an individual, corporation, association, partnership, limited liability company, joint venture, trust, unincorporated organization,
government or political subdivision thereof or governmental agency or other entity.
(e) “Warrants”
means certain Common Stock purchase warrants of the Company issued in connection with the Warrant Certificate with a term of exercise
of five years following the Initial Exercise Date.
(f) “Warrant
Certificate” means a certificate in substantially the form attached as Exhibit 1-A hereto, representing such number
of Warrant Shares (as defined below) as is indicated therein, provided that any reference to the delivery of a Warrant Certificate in
this Agreement shall include delivery of notice from the Depositary or a Participant (each as defined below) of the transfer or exercise
of the Warrant in the form of a Global Warrant (as defined below).
(g) “Warrant
Shares” means the shares of Common Stock underlying the Warrants and issuable upon exercise of the Warrants.
All other capitalized terms
used but not otherwise defined herein shall have the meaning ascribed to such terms in the Warrant Certificates.
Section 2. Appointment
of Warrant Agent. The Company hereby appoints the Warrant Agent to act as agent for the Company in accordance with the express terms
or conditions hereof (and no implied terms and conditions), and the Warrant Agent hereby accepts such appointment. The Company may from
time to time appoint such Co-Warrant Agents as it may, in its sole discretion, deem necessary or desirable upon ten (10) calendar
days’ prior written notice to the Warrant Agent. The Warrant Agent shall have no duty to supervise, and shall in no event be liable
for, the acts or omissions of any such Co-Warrant Agent. In the event the Company appoints one or more co-Warrant Agents, the respective
duties of the Warrant Agent and any Co-Warrant Agent shall be as the Company shall reasonably determine, provided that such duties and
determination are consistent with the terms and provisions of this Agreement.
Section 3. Global
Warrants.
(a) The
Warrants shall be issuable in book entry form (the “Global Warrants” and, each, a “Global Warrant”).
All of the Warrants shall initially be represented by one or more Global Warrants, deposited with the Warrant Agent and registered in
the name of Cede & Co., a nominee of The Depository Trust Company (the “Depositary”), or as otherwise directed
by the Depositary. Ownership of beneficial interests in the Warrants shall be shown on, and the transfer of such ownership shall be effected
through, records maintained by (i) the Depositary or its nominee for each Global Warrant or (ii) institutions that have accounts
with the Depositary (such institution, with respect to a Warrant in its account, a “Participant”). For purposes of
Regulation SHO, a holder whose interest in a Global Warrant is a beneficial interest in certificate(s) representing such Warrant
held in book-entry form through the Depositary shall be deemed to have exercised its interest in such Warrant upon instructing its broker
that is a Participant to exercise its interest in such Warrant, provided that in each such case payment of the applicable aggregate Exercise
Price (other than in the case of a Cashless Exercise or alternative cashless exercise) is received within the earlier of (i) two
(2) Trading Days (as defined in the Warrant Certificate and (ii) the number of Trading Days comprising the Standard Settlement
Period, in each case following such instruction. As used herein, “Standard Settlement Period” means the standard settlement
period, expressed in a number of Trading Days, on the Company's primary trading market with respect to the Common Stock as in effect on
the date of delivery of the Exercise Notice.
(b) If
the Depositary subsequently ceases to make its book-entry settlement system available for the Warrants, the Company may instruct the
Warrant Agent regarding other arrangements for book-entry settlement. In the event that the Warrants are not eligible for, or it is no
longer necessary to have the Warrants available in, book-entry form, the Warrant Agent shall provide written instructions to the Depositary
to deliver to the Warrant Agent for cancellation each Global Warrant, and the Company shall instruct the Warrant Agent in writing to
deliver to each Holder a Warrant Certificate.
(c) A
Holder has the right to elect at any time or from time to time a Warrant Exchange (as defined below) pursuant to a Warrant Certificate
Request Notice (as defined below). Upon written notice by a Holder to the Warrant Agent for the exchange of some or all of such Holder's
Global Warrants for a Warrant Certificate, evidencing the same number of Warrants, which request shall be in the form attached hereto
as Annex A (a “Warrant Certificate Request Notice” and the date of delivery of such Warrant Certificate Request
Notice by the Holder, the “Warrant Certificate Request Notice Date” and the deemed surrender upon delivery by the Holder
of a number of Global Warrants for the same number of Warrants evidenced by a Warrant Certificate, a “Warrant Exchange”),
the Warrant Agent shall promptly effect the Warrant Exchange and shall promptly issue and deliver, at the expense of the Company, to the
Holder a Warrant Certificate, for such number of Warrants in the name set forth in the Warrant Certificate Request Notice. Such Warrant
Certificate shall be dated the original issue date of the Warrants, shall be executed by manual signature by an authorized signatory of
the Company, shall be in the form attached hereto as Exhibit 1-A. In connection with a Warrant Exchange, the Company agrees
to deliver, or to direct the Warrant Agent to deliver, the Warrant Certificate to the Holder within three (3) Business Days of the
Warrant Certificate Request Notice pursuant to the delivery instructions in the Warrant Certificate Request Notice (“Warrant
Certificate Delivery Date”). If the Company fails for any reason to deliver, or cause the delivery by the Warrant Agent, to
the Holder the Warrant Certificate subject to the Warrant Certificate Request Notice by the Warrant Certificate Delivery Date, the Company
shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Warrant Shares evidenced by such Warrant
Certificate (based on the VWAP (as defined in the Warrants) of the Common Stock on the Warrant Certificate Request Notice Date), $10 per
Trading Day (increasing to $20 per Trading Day on the fifth Trading Day after such liquidated damages begin to accrue) for each Trading
Day after such Warrant Certificate Delivery Date until such Warrant Certificate, as applicable, is delivered or, prior to delivery of
such Warrant Certificate, the Holder rescinds such Warrant Exchange. In no event shall the Warrant Agent be obligated to pay any amounts
pursuant to this Section 3(c), such obligations shall be solely that of the Company and not that of the Warrant Agent. The Company
covenants and agrees that, upon the date of delivery of the Warrant Certificate Request Notice, the Holder shall be deemed to be the holder
of the Warrant Certificate, as applicable, and, notwithstanding anything to the contrary set forth herein, the Warrant Certificate shall
be deemed for all purposes to contain all of the terms and conditions of the Warrants, evidenced by such Warrant Certificate, and the
terms of this Agreement, other than Sections 3(c) and 9 herein, shall not apply to the Warrants evidenced by the Warrant Certificate.
In the event a beneficial owner requests a Warrant Exchange, upon issuance of the paper Warrant Certificate, the Warrant Agent shall act
as warrant agent and the terms of the paper Warrant Certificate so issued shall exclusively govern in respect thereof. If there is any
conflict between the express terms of this Agreement and a Warrant Certificate, the terms of the Warrant Certificate shall govern and
control.
Section 4.
Form of Warrant Certificates. The Warrant Certificate, together with the form of election to purchase Common Stock (“Exercise
Notice”) and the form of assignment to be printed on the reverse thereof, shall be in the form of Exhibit 1-A hereto.
Section 5. Countersignature
and Registration. The Warrant Certificates shall be executed on behalf of the Company by its Chief Executive Officer or Chief Financial
Officer, either manually or by facsimile signature which shall be attested by the Secretary or an Assistant Secretary of the Company,
either manually or by facsimile signature. The Warrant Certificates shall be countersigned by the Warrant Agent either manually or by
facsimile signature and shall not be valid for any purpose unless so countersigned. In case any officer of the Company who shall have
signed any of the Warrant Certificates shall cease to be such officer of the Company before countersignature by the Warrant Agent and
issuance and delivery by the Company, such Warrant Certificates, nevertheless, may be countersigned by the Warrant Agent, issued and delivered
with the same force and effect as though the person who signed such Warrant Certificate had not ceased to be such officer of the Company;
and any Warrant Certificate may be signed on behalf of the Company by any person who, at the actual date of the execution of such Warrant
Certificate, shall be a proper officer of the Company to sign such Warrant Certificate, although at the date of the execution of this
Agreement any such person was not such an officer.
The Warrant Agent will keep
or cause to be kept, at its office located at the address provided in Section 19 hereof, books for registration and transfer of the
Warrant Certificates issued hereunder. Such books shall show the names and addresses of the respective Holders of the Warrant Certificates,
the number of warrants evidenced on the face of each of such Warrant Certificate and the date of each of such Warrant Certificate. The
Warrant Agent will create a special account for the issuance of Warrant Certificates.
Section 6. Transfer,
Split Up, Combination and Exchange of Warrant Certificates; Mutilated, Destroyed, Lost or Stolen Warrant Certificates. With respect
to the Global Warrant, subject to the provisions of the Warrant Certificate, and the last sentence of this first paragraph of Section 6
and subject to applicable law, rules or regulations, or any “stop transfer” instructions the Company may give to the
Warrant Agent, at any time after the closing date of the Offering, and at or prior to the Close of Business on the Termination Date (as
such term is defined in the Warrant Certificate), any Warrant Certificate or Warrant Certificates or Global Warrant or Global Warrants
may be transferred, split up, combined or exchanged for another Warrant Certificate or Warrant Certificates or Global Warrant or Global
Warrants, entitling the Holder to purchase a like number of shares of Common Stock as the Warrant Certificate or Warrant Certificates
or Global Warrant or Global Warrants surrendered then entitled such Holder to purchase. Any Holder desiring to transfer, split up, combine
or exchange any Warrant Certificate or Global Warrant shall make such request in writing delivered to the Warrant Agent, and shall surrender
the Warrant Certificate or Warrant Certificates, together with the required form of assignment and certificate duly executed and properly
completed and such other documentation as the Warrant Agent may reasonably request, to be transferred, split up, combined or exchanged
at the office of the Warrant Agent located at the address provided in Section 19 hereof, provided that no such surrender of a Warrant
Certificate is applicable to the Holder of a Global Warrant. Any requested transfer of Warrants, whether in book-entry form or certificate
form, shall be accompanied by evidence of authority of the party making such request that may be reasonably required by the Warrant Agent.
Thereupon the Warrant Agent shall, subject to the last sentence of this first paragraph of Section 6, countersign and deliver to
the Person entitled thereto a Warrant Certificate or Warrant Certificates, as the case may be, as so requested. The Company may require
payment from the Holder of a sum sufficient to cover any tax or governmental charge that may be imposed in connection with any transfer,
split up, combination or exchange of Warrant Certificates. The Warrant Agent shall not have any duty or obligation to take any action
under any section of this Agreement that requires the payment of taxes and/or charges unless and until it is satisfied that all such payments
have been made.
Upon
receipt by the Warrant Agent of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of a Warrant Certificate,
which evidence shall include an affidavit of loss, or in the case of mutilated certificates, the certificate or portion thereof remaining,
and, in case of loss, theft or destruction, of indemnity or security reasonably acceptable to the Company and the Warrant Agent, and satisfaction
of any other reasonable requirements established by Section 8-405 of the Uniform Commercial Code as in effect in the State of Delaware,
and reimbursement to the Company and the Warrant Agent of all reasonable expenses incidental thereto, and upon surrender to the Warrant
Agent and cancellation of the Warrant Certificate if mutilated, the Company will make and deliver a new Warrant Certificate of like tenor
to the Warrant Agent for delivery to the Holder in lieu of the Warrant Certificate so lost, stolen, destroyed or mutilated.
Section 7. Exercise
of Warrants; Exercise Price; Termination Date.
(a) The
Warrants shall be exercisable commencing on the Initial Exercise Date. The Warrants shall cease to be exercisable and all rights to exercise
the Warrants thereunder and under this Agreement shall cease, at the Close of Business on the Termination Date (as such term is defined
in the Warrant Certificate). Subject to the foregoing and to Section 7(b) below, the Holder of a Warrant may exercise the Warrant,
in whole or in part in accordance with the provisions of Section 2 of the Warrant Certificate. In the case of the Holder of a Global
Warrant, the Holder shall deliver the duly executed Exercise Notice and the payment of the Exercise Price as described herein. Notwithstanding
any other provision in this Agreement, a holder whose interest in a Global Warrant is a beneficial interest in a Global Warrant held in
book-entry form through the Depositary (or another established clearing corporation performing similar functions), shall effect exercises
by delivering to the Depositary (or such other clearing corporation, as applicable) the appropriate instruction form for exercise, complying
with the procedures to effect exercise that are required by the Depositary (or such other clearing corporation, as applicable). The Company
acknowledges that the bank accounts maintained by the Warrant Agent in connection with the services provided under this Agreement will
be in its name and that the Warrant Agent may receive investment earnings in connection with the investment at Warrant Agent risk and
for its benefit of funds held in those accounts from time to time. Neither the Company nor the Holders will receive interest on any deposits
or Exercise Price. No ink-original Exercise Notice shall be required, nor shall any medallion guarantee (or other type of guarantee or
notarization) of any Exercise Notice be required.
(b) Upon
receipt of an Exercise Notice for a Cashless Exercise or alternative cashless exercise, the Warrant Agent shall deliver a copy of the
Exercise Notice to the Company and request from the Company and the Company shall promptly calculate and transmit to the Warrant Agent
in writing the number of Warrant Shares issuable in connection with such Cashless Exercise or alternative cashless exercise. The Warrant
Agent shall have no obligation under this Agreement to calculate, the number of Warrant Shares issuable in connection with a Cashless
Exercise or alternative cashless exercise nor shall the Warrant agent have any duty or obligation to investigate or confirm whether the
Company's determination of the number of Warrant Shares issuable upon such exercise, pursuant to this Section 7, is accurate or correct.
(c) Upon
exercise of a Warrant pursuant to Section 7(a) above, at or prior to the Close of Business on the Termination Date set forth
in such Warrant Certificate, the Warrant Agent shall cause the Warrant Shares underlying such Warrant Certificate, or Global Warrant,
to be delivered to or upon the order of the Holder of such Warrant Certificate, or Global Warrant, registered in such name or names as
may be designated by such Holder, no later than the Warrant Share Delivery Date (as such term is defined in the Warrant Certificate).
If the Company is then a participant in the DWAC system of the Depositary and either (A) there is an effective registration statement
permitting the issuance of the Warrant Shares to or resale of the Warrant Shares by Holder or (B) the Warrant is being exercised
via Cashless Exercise or alternative cashless exercise, then the Warrant Shares shall be transmitted by the Warrant Agent to the Holder
by crediting the account of the Holder's broker with the Depositary through its DWAC system. For the avoidance of doubt, if the Company
becomes obligated to pay any amounts to any Holders pursuant to Section 2(d)(i) or 2(d)(iv) of the Warrant Certificate,
such obligation shall be solely that of the Company and not that of the Warrant Agent. Notwithstanding anything else to the contrary
in this Agreement, except in the case of a Cashless Exercise or alternative cashless exercise, if any Holder fails to duly deliver payment
to the Warrant Agent of an amount equal to the aggregate Exercise Price of the Warrant Shares to be purchased upon exercise of such Holder's
Warrant as set forth in Section 7(a) hereof by the Warrant Share Delivery Date, the Warrant Agent will not obligated to deliver
such Warrant Shares (via DWAC or otherwise) until following receipt of such payment, and the applicable Warrant Share Delivery Date shall
be deemed extended by one day for each day (or part thereof) until such payment is delivered to the Warrant Agent.
(d) The
Warrant Agent shall deposit all funds received by it in payment of the Exercise Price for all Warrants in the account of the Company maintained
with the Warrant Agent for such purpose (or to such other account as directed by the Company in writing) and shall advise the Company
via email at the end of each day on which exercise notices are received or funds for the exercise of any Warrant are received of the amount
so deposited to its account.
(e) In
case the Holder of any Warrant Certificate shall exercise fewer than all Warrants evidenced thereby, upon the request of the Holder, a
new Warrant Certificate evidencing the number of Warrants equivalent to the number of Warrants remaining unexercised may be issued by
the Warrant Agent to the Holder of such Warrant Certificate or to his duly authorized assigns in accordance with Section 2(d)(ii) of
the Warrant Certificate, subject to the provisions of Section 6 hereof.
Section 8. Cancellation
and Destruction of Warrant Certificates. All Warrant Certificates surrendered for the purpose of exercise, transfer, split up, combination
or exchange shall, if surrendered to the Company or to any of its agents, be delivered to the Warrant Agent for cancellation or in canceled
form, or, if surrendered to the Warrant Agent, shall be canceled by it, and no Warrant Certificates shall be issued in lieu thereof except
as expressly permitted by any of the provisions of this Agreement. The Company shall deliver to the Warrant Agent for cancellation and
retirement, and the Warrant Agent shall so cancel and retire, any other Warrant Certificate purchased or acquired by the Company otherwise
than upon the exercise thereof. The Warrant Agent shall destroy such canceled Warrant Certificates in accordance with its procedures,
subject to any applicable law, rule or regulation requiring the Warrant Agent to retain such canceled certificates.
Section 9.
Certain Representations; Reservation and Availability of Shares of Common Stock or Cash.
(a) This
Agreement has been duly authorized, executed and delivered by the Company and, assuming due authorization, execution and delivery hereof
by the Warrant Agent, constitutes a valid and legally binding obligation of the Company enforceable against the Company in accordance
with its terms, and the Warrants have been duly authorized, executed and issued by the Company and, assuming due execution thereof by
the Warrant Agent pursuant hereto and payment therefor by the Holders, constitute valid and legally binding obligations of the Company
enforceable against the Company in accordance with their terms and entitled to the benefits hereof; in each case except as enforceability
may be limited by bankruptcy, insolvency, reorganization, moratorium and other similar laws relating to or affecting creditors' rights
generally or by general equitable principles (regardless of whether such enforceability is considered in a proceeding in equity or at
law).
(b) As
of the date hereof, the authorized capital stock of the Company consists of (i) 100,000,000 shares of Common Stock, of which as of
the date hereof, 679,956 shares are issued and outstanding, 428 shares are reserved for issuance upon exercise of outstanding options
issued under the Company’s stock option plans, 4 shares are reserved for earned restricted stock not yet physically issued, 1,831
shares are reserved for unearned restricted stock and restricted stock units subject to milestone and time-based vesting, 898,444 shares
are reserved for issuance upon exercise of outstanding warrants to purchase Company Stock, 143 shares of Common Stock reserved for issuance
upon conversion of Series B Preferred Stock (the “Series B Preferred Stock”), including in respect of accrued and
unpaid dividends of approximately $1.0 million through as of the date hereof, and (ii) either (x) if the holders of Series B
Preferred Stock elect to exchange into our registered direct and private placement offering from January 2021, up to 157 additional
shares of Common Stock issuable upon conversion of Series C Convertible Preferred Stock and up to 157 shares of Common Stock issuable
upon exercise of warrants or (y) if the holders of Series B Preferred Stock elect to exchange into our sales made on November 30,
2021, at a price of $10,985.94 per share, pursuant to our At The Market Offering Agreement dated May 26, 2021 (the “ATM Agreement”)
(such price being the lowest price per share sold under the ATM Agreement to date and eligible for the holders of the Series B Preferred
Stock to exchange into), up to 454 additional shares of Common Stock, in each case that may be issued pursuant to the exchange right in
excess of amounts currently underlying Series B Preferred Stock. There are no other outstanding obligations, warrants, options or
other rights to subscribe for or purchase from the Company any class of capital stock of the Company except as set forth in reports filed
by the Company with the Securities and Exchange Commission.
(a) The
Company covenants and agrees that it will cause to be reserved and kept available out of its authorized and unissued shares of Common
Stock or its authorized and issued shares of Common Stock held in its treasury, free from preemptive rights, the number of shares of Common
Stock that will be sufficient to permit the exercise in full of all outstanding Warrants.
(b) The
Company further covenants and agrees that it will pay when due and payable any and all federal and state transfer taxes and charges which
may be payable in respect of the original issuance or delivery of the Warrant Certificates or certificates evidencing Common Stock upon
exercise of the Warrants. The Company shall not, however, be required to pay any tax or governmental charge which may be payable in respect
of any transfer involved in the transfer or delivery of Warrant Certificates or the issuance or delivery of certificates for Common Stock
in a name other than that of the Holder of the Warrant Certificate evidencing Warrants surrendered for exercise or to issue or deliver
any certificate for shares of Common Stock upon the exercise of any Warrants until any such tax or governmental charge shall have been
paid (any such tax or governmental charge being payable by the Holder of such Warrant Certificate at the time of surrender) or until
it has been established to the Company's and the Warrant Agent's reasonable satisfaction that no such tax or governmental charge is due.
Section 10. Common
Stock Record Date. Each Person in whose name any certificate for shares of Common Stock is issued (or to whose broker's account is
credited shares of Common Stock through the DWAC system) upon the exercise of Warrants shall for all purposes be deemed to have become
the holder of record for the Common Stock represented thereby on, and such certificate shall be dated, the date on which submission of
the Exercise Notice was made, provided that the Warrant Certificate evidencing such Warrant was duly surrendered (but only if required
herein) and payment of the Exercise Price (and any applicable transfer taxes) was received on or prior to the Warrant Share Delivery Date;
provided, however, that, if the date of submission of the Exercise Notice is a date upon which the Common Stock transfer
books of the Company are closed, such Person shall be deemed to have become the record holder of such shares on, and such certificate
shall be dated, the next succeeding day on which the Common Stock transfer books of the Company are open.
Section 11. Adjustment
of Exercise Price, Number of Shares of Common Stock or Number of the Company Warrants. The Exercise Price, the number of shares covered
by each Warrant and the number of Warrants outstanding are subject to adjustment from time to time as provided in Section 3 of the
Warrant Certificate. In the event that at any time, as a result of an adjustment made pursuant to Section 3 of the Warrant Certificate,
the Holder of any Warrant thereafter exercised shall become entitled to receive any shares of capital stock of the Company other than
shares of Common Stock, thereafter the number of such other shares so receivable upon exercise of any Warrant shall be subject to adjustment
from time to time in a manner and on terms as nearly equivalent as practicable to the provisions with respect to the shares contained
in Section 3 of the Warrant Certificate, and the provisions of Sections 7, 9 and 13 of this Agreement with respect to the shares
of Common Stock shall apply on like terms to any such other shares. All Warrants originally issued by the Company subsequent to any adjustment
made to the Exercise Price pursuant to the Warrant Certificate shall evidence the right to purchase, at the adjusted Exercise Price, the
number of shares of Common Stock purchasable from time to time hereunder upon exercise of the Warrants, all subject to further adjustment
as provided herein.
Section 12. Certification
of Adjusted Exercise Price or Number of Shares of Common Stock. Whenever the Exercise Price or the number of shares of Common Stock
issuable upon the exercise of each Warrant Certificate is adjusted as provided in Section 11 or 13, the Company shall (a) promptly
prepare a certificate setting forth the Exercise Price of each Warrant Certificate, as so adjusted, and a brief, reasonably detailed statement
of the facts accounting for such adjustment, (b) promptly file with the Warrant Agent and with each transfer agent for the Common
Stock a copy of such certificate and (c) instruct the Warrant Agent, at the Company's expense, to send a brief summary thereof to
each Holder of a Warrant Certificate. The Warrant Agent shall be fully protected in relying on such certificate and on any adjustment
or statement therein contained and shall have no duty or liability with respect to and shall not be deemed to have knowledge of any such
adjustment or any such event unless and until it shall have received such certificate.
Section 13.
Fractional Shares of Common Stock. The Company shall not issue fractions of shares of Common Stock upon exercise of Warrants or
distribute stock certificates which evidence fractional shares of Common Stock. Whenever any fraction of a share of Common Stock would
otherwise be required to be issued or distributed, the actual issuance or distribution in respect thereof shall be made in accordance
with Section 2(d)(v) of the Warrant Certificate.
Section 14. Concerning
the Warrant Agent.
(a) The
Warrant Agent accepts its obligations herein set forth upon the terms and conditions hereof. The Company agrees to pay to the Warrant
Agent, pursuant to the fee schedule mutually agreed upon by the parties hereto and attached hereto as Schedule 1, for all services rendered
by it hereunder and, from time to time, its reasonable expenses and counsel fees and other disbursements incurred in the preparation,
delivery, negotiation, amendment, administration and execution of this Agreement and the exercise and performance of its duties hereunder.
(b) The
Company covenants and agrees to indemnify and to hold the Warrant Agent harmless against any costs, expenses (including reasonable fees
and expenses of its legal counsel), losses or damages, which may be paid, incurred or suffered by or to which it may become subject, arising
from or out of, directly, any claims or liability resulting from its actions or omissions as Warrant Agent pursuant hereto; provided,
that such covenant and agreement does not extend to, and the Warrant Agent shall not be indemnified with respect to, such costs, expenses,
losses and damages incurred or suffered by the Warrant Agent as a result of, or arising out of, its violation of law or this Agreement,
gross negligence, bad faith, or willful misconduct (each as determined by a final non-appealable court of competent jurisdiction). Notwithstanding
anything in this Agreement to the contrary, any liability of the Warrant Agent under this Agreement will be limited to the amount of annual
fees paid by the Company to the Warrant Agent during the twelve (12) months immediately preceding the event for which recovery from the
Warrant Agent is being sought. The costs and expenses incurred by the Warrant Agent in enforcing this right of indemnification shall be
paid by the Company.
(c) Upon
the assertion of a claim for which the Company may be required to indemnify the Warrant Agent, the Warrant Agent shall promptly notify
the Company of such assertion, and shall keep the other party reasonably advised with respect to material developments concerning such
claim. However, failure to give such notice shall not affect the Warrant Agent's right to and the Company's obligations for indemnification
hereunder unless such failure to give a prompt notice puts the Company at disadvantage.
(d) Neither
party to this Agreement shall be liable to the other party for any consequential, indirect, punitive, special or incidental damages under
any provisions of this Agreement or for any consequential, indirect, punitive, special or incidental damages arising out of any act or
failure to act hereunder even if that party has been advised of or has foreseen the possibility of such damages.
(e) Notwithstanding
anything contained herein to the contrary, the rights and obligations of the parties set forth in this Section 14 shall survive termination
of this Agreement, the expiration of the Warrants or the resignation, removal or replacement of the Warrant Agent.
Section 15. Purchase
or Consolidation or Change of Name of Warrant Agent. Any Person into which the Warrant Agent or any successor Warrant Agent may be
merged or with which it may be consolidated, or any Person resulting from any merger or consolidation to which the Warrant Agent or any
successor Warrant Agent shall be party, or any Person succeeding to the stock transfer or other shareholder services business of the Warrant
Agent or any successor Warrant Agent, shall be the successor to the Warrant Agent under this Agreement without the execution or filing
of any paper or any further act on the part of any of the parties hereto, provided that such Person would be eligible for appointment
as a successor Warrant Agent under the provisions of Section 17. In case at the time such successor Warrant Agent shall succeed to
the agency created by this Agreement any of the Warrant Certificates shall have been countersigned but not delivered, any such successor
Warrant Agent may adopt the countersignature of the predecessor Warrant Agent and deliver such Warrant Certificates so countersigned;
and in case at that time any of the Warrant Certificates shall not have been countersigned, any successor Warrant Agent may countersign
such Warrant Certificates either in the name of the predecessor Warrant Agent or in the name of the successor Warrant Agent; and in all
such cases such Warrant Certificates shall have the full force provided in the Warrant Certificates and in this Agreement.
In case at any time the name
of the Warrant Agent shall be changed and at such time any of the Warrant Certificates shall have been countersigned but not delivered,
the Warrant Agent may adopt the countersignature under its prior name and deliver Warrant Certificates so countersigned; and in case at
that time any of the Warrant Certificates shall not have been countersigned, the Warrant Agent may countersign such Warrant Certificates
either in its prior name or in its changed name; and in all such cases such Warrant Certificates shall have the full force provided in
the Warrant Certificates and in this Agreement.
Section 16. Duties
of Warrant Agent. The Warrant Agent undertakes the duties and obligations imposed by this Agreement upon the following express terms
and conditions (and no implied terms and conditions), by all of which the Company, by its acceptance hereof, shall be bound and shall
not assume any obligations or relationship of agency or trust with any of the Holders of the Warrants or any other Person:
(a) The
Warrant Agent may consult with legal counsel selected by it (who may be legal counsel for the Company), and the opinion and advice of
such counsel shall be full and complete authorization and protection to the Warrant Agent as to any action taken or omitted by it in good
faith and in accordance with such opinion or advice.
(b) Whenever
in the performance of its duties under this Agreement the Warrant Agent shall deem it necessary or desirable that any fact or matter
be proved or established by the Company prior to taking or suffering any action hereunder, such fact or matter (unless other evidence
in respect thereof be herein specifically prescribed) may be deemed to be conclusively proved and established by a certificate signed
by the Chief Executive Officer or Chief Financial Officer of the Company; and such certificate shall be full authorization and protection
to the Warrant Agent and the Warrant Agent shall incur no liability for or in respect of any action taken, suffered in good faith or
omitted to be taken by it under the provisions of this Agreement in reliance upon such certificate. The Warrant Agent shall have no duty
to act without such a certificate as set forth in this Section 16(b).
(c) Subject
to the limitation set forth in Section 14, the Warrant Agent shall be liable hereunder only for its violation of law or this Agreement,
its own gross negligence, bad faith or willful misconduct (each as determined in a final, non-appealable judgment of a court of competent
jurisdiction).
(d) The
Warrant Agent shall not be liable for or by reason of any of the statements of fact or recitals contained in this Agreement or in the
Warrant Certificates (including in the case of any notation in book entry form to reflect ownership), except its countersignature thereof,
by the Company or be required to verify the same, but all such statements and recitals are and shall be deemed to have been made by the
Company only.
(e) The
Warrant Agent shall not have any liability or be under any responsibility in respect of the validity of this Agreement or the execution
and delivery hereof (except the due execution hereof by the Warrant Agent) or in respect of the validity or execution of any Warrant Certificate
(except its countersignature thereof); nor shall it be responsible for any breach by the Company of any covenant or condition contained
in this Agreement or in any Warrant Certificate; nor shall it be responsible for the adjustment of the Exercise Price or the making of
any change in the number of shares of Common Stock required under the provisions of Section 11 or 13 or responsible for the manner,
method or amount of any such change or adjustment or the ascertaining of the existence of facts that would require any such adjustment
or change (except with respect to the exercise of Warrants evidenced by Warrant Certificates after actual notice of any adjustment of
the Exercise Price); nor shall it by any act hereunder be deemed to make any representation or warranty as to the authorization or reservation
of any shares of Common Stock to be issued pursuant to this Agreement or any Warrant Certificate or as to whether any shares of Common
Stock will, when issued, be duly authorized, validly issued, fully paid and nonassessable.
(f) Each
party hereto agrees that it will perform, execute, acknowledge and deliver or cause to be performed, executed, acknowledged and delivered
all such further and other acts, instruments and assurances as may reasonably be required by the other party hereto for the carrying out
or performing by any party of the provisions of this Agreement.
(g) The
Warrant Agent is hereby authorized to accept instructions with respect to the performance of its duties hereunder from the Chief Executive
Officer or Chief Financial Officer of the Company, and to apply to such officers for advice or instructions in connection with its duties,
and it shall not be liable and shall be indemnified and held harmless for any action taken or suffered to be taken by it in good faith
in accordance with instructions of any such officer, provided Warrant Agent carries out such instructions without violation of law, breach
of this Agreement, gross negligence, bad faith or willful misconduct.
(h) The
Warrant Agent and any shareholder, director, officer or employee of the Warrant Agent may buy, sell or deal in any of the Warrants or
other securities of the Company or become pecuniarily interested in any transaction in which the Company may be interested, or contract
with or lend money to the Company or otherwise act as fully and freely as though it were not Warrant Agent under this Agreement. Nothing
herein shall preclude the Warrant Agent from acting in any other capacity for the Company or for any other Person.
(i) The
Warrant Agent may execute and exercise any of the rights or powers hereby vested in it or perform any duty hereunder either itself or
by or through its attorney or agents, and the Warrant Agent shall not be answerable or accountable for any act, default, neglect or misconduct
of any such attorney or agents or for any loss to the Company resulting from any such act, default, neglect or misconduct, absent a violation
of law, breach of this agreement, gross negligence or bad faith in the selection and continued employment thereof (which gross negligence
and bad faith must be determined by a final, non-appealable judgment of a court of competent jurisdiction).
(j) The
Warrant Agent shall not be obligated to expend or risk its own funds or to take any action that it believes would expose or subject it
to expense or liability or to a risk of incurring expense or liability, unless it has been furnished with assurances of repayment or indemnity
satisfactory to it.
(k) The
Warrant Agent shall not be liable or responsible for any failure of the Company to comply with any of its obligations relating to any
registration statement filed with the Securities and Exchange Commission or this Agreement, including without limitation obligations under
applicable regulation or law.
(l) The
Warrant Agent may rely on and be fully authorized and protected in acting or failing to act upon (a) any guaranty of signature by
an “eligible guarantor institution” that is a member or participant in the Securities Transfer Agents Medallion Program or
other comparable “signature guarantee program” or insurance program in addition to, or in substitution for, the foregoing;
or (b) any law, act, regulation or any interpretation of the same even though such law, act, or regulation may thereafter have been
altered, changed, amended or repealed.
(m) In
the event the Warrant Agent believes any ambiguity or uncertainty exists hereunder or in any notice, instruction, direction, request or
other communication, paper or document received by the Warrant Agent hereunder, the Warrant Agent, may, in its sole discretion, refrain
from taking any action, and shall be fully protected and shall not be liable in any way to Company, the holder of any Warrant or any other
Person for refraining from taking such action, unless the Warrant Agent receives written instructions signed by the Company which eliminates
such ambiguity or uncertainty to the satisfaction of Warrant Agent.
This Section 16 shall
survive the expiration of the Warrants, the termination of this Agreement and the resignation, replacement or removal of the Warrant Agent.
The costs and expenses incurred in enforcing this right of indemnification shall be paid by the Company.
Section 17.
Change of Warrant Agent. The Warrant Agent may resign and be discharged from its duties under this Agreement upon 30 days’
notice in writing sent to the Company and, in the event that the Warrant Agent or one of its Affiliates is not also the transfer agent
for the Company, to each transfer agent of the Common Stock. In the event the transfer agency relationship in effect between the Company
and the Warrant Agent terminates, the Warrant Agent will be deemed to have resigned automatically and be discharged from its duties under
this Agreement as of the effective date of such termination, and the Company shall be responsible for sending any required notice thereunder.
The Company may remove the Warrant Agent or any successor Warrant Agent upon 30 days’ notice in writing, sent to the Warrant Agent
or successor Warrant Agent, as the case may be, and to each transfer agent of the Common Stock, and to the Holders of the Warrant Certificates.
If the Warrant Agent shall resign or be removed or shall otherwise become incapable of acting, the Company shall appoint a successor to
the Warrant Agent. If the Company shall fail to make such appointment within a period of 30 days after such removal or after it has been
notified in writing of such resignation or incapacity by the resigning or incapacitated Warrant Agent or by the Holder of a Warrant Certificate
(who shall, with such notice, submit his Warrant Certificate for inspection by the Company), then the Holder of any Warrant Certificate
may apply to any court of competent jurisdiction for the appointment of a new Warrant Agent, provided that, for purposes of this Agreement,
the Company shall be deemed to be the Warrant Agent until a new warrant agent is appointed. Any successor Warrant Agent, whether appointed
by the Company or by such a court, shall be a Person, other than a natural person, organized and doing business under the laws of the
United States or of a state thereof, in good standing, which is authorized under such laws to exercise stock transfer powers and is subject
to supervision or examination by federal or state authority and which has at the time of its appointment as Warrant Agent a combined capital
and surplus of at least $50,000,000. After appointment, the successor Warrant Agent shall be vested with the same powers, rights, duties
and responsibilities as if it had been originally named as Warrant Agent without further act or deed; but the predecessor Warrant Agent
shall deliver and transfer to the successor Warrant Agent any property at the time held by it hereunder, and execute and deliver any further
assurance, conveyance, act or deed necessary for the purpose but such predecessor Warrant Agent shall not be required to make any additional
expenditure (without prompt reimbursement by the Company) or assume any additional liability in connection with the foregoing. Not later
than the effective date of any such appointment, the Company shall file notice thereof in writing with the predecessor Warrant Agent and
each transfer agent of the Common Stock, and mail a notice thereof in writing to the Holders of the Warrant Certificates. However, failure
to give any notice provided for in this Section 17, or any defect therein, shall not affect the legality or validity of the resignation
or removal of the Warrant Agent or the appointment of the successor Warrant Agent, as the case may be.
Section 18. Issuance
of New Warrant Certificates. Notwithstanding any of the provisions of this Agreement or of the Warrants to the contrary, the Company
may, at its option, issue new Warrant Certificates evidencing Warrants in such form as may be approved by its Board of Directors to reflect
any adjustment or change in the Exercise Price per share and the number or kind or class of shares of stock or other securities or property
purchasable under the several Warrant Certificates made in accordance with the provisions of this Agreement.
Section 19. Notices.
Notices or demands authorized by this Agreement to be given or made (i) by the Warrant Agent or by the Holder of any Warrant Certificate
to or on the Company, (ii) by the Company or by the Holder of any Warrant Certificate to or on the Warrant Agent or (iii) by
the Company or the Warrant Agent to the Holder of any Warrant Certificate, shall be deemed given when in writing (a) on the date
delivered, if delivered personally, (b) on the first Business Day following the deposit thereof with Federal Express or another recognized
overnight courier, if sent by Federal Express or another recognized overnight courier, (c) on the fourth Business Day following the
mailing thereof with postage prepaid, if mailed by registered or certified mail (return receipt requested), and (d) the time of transmission,
if such notice or communication is delivered via facsimile or e-mail attachment at or prior to 5:30 p.m. (New York City time) on
a Business Day and (e) the next Business Day after the date of transmission, if such notice or communication is delivered via facsimile
or e-mail attachment on a day that is not a Business Day or later than 5:30 p.m. (New York City time) on any Business Day, in each
case to the parties at the following addresses (or at such other address for a party as shall be specified by like notice):
(a) If
to the Company, to:
First Wave BioPharma, Inc.
777 Yamato Road,
Suite 502
Boca Raton, Florida
33431
Attn: James Sapirstein
Email: jsapirstein@firstwavebio.com
With a copy (which
shall not constitute notice) to:
Ellenoff Grossman &
Schole LLP
1345 Avenue of
the Americas, 11th Fl.
New York, NY 10105
Attn: Barry I.
Grossman, Esq.
Jessica Yuan, Esq.
Email:
bgrossman@egsllp.com
jyuan@egsllp.com
(b) If
to the Warrant Agent, to:
Colonial Stock
Transfer Co., Inc.
7840 S 700 E
Sandy, UT 84070
For any notice delivered by email to be deemed
given or made, such notice must be followed by notice sent by overnight courier service to be delivered on the next Business Day following
such email, unless the recipient of such email has acknowledged via return email receipt of such email.
(c) If
to the Holder of any Warrant Certificate, to the address of such Holder as shown on the registry books of the Company. Any notice required
to be delivered by the Company to the Holder of any Warrant may be given by the Warrant Agent on behalf of the Company. Notwithstanding
any other provision of this Agreement, where this Agreement provides for notice of any event to a Holder of any Warrant, such notice shall
be sufficiently given if given to the Depositary (or its designee) pursuant to the procedures of the Depositary or its designee.
Section 20.
Supplements and Amendments.
(a) The
Company and the Warrant Agent may from time to time supplement or amend this Agreement without the approval of any Holders of Global Warrants
in order to (i) add to the covenants and agreements of the Company for the benefit of the Holders of the Global Warrants, (ii) to
surrender any rights or power reserved to or conferred upon the Company in this Agreement, (iii) to cure any ambiguity, (iv) to
correct or supplement any provision contained herein which may be defective or inconsistent with any other provisions herein, or (v) to
make any other provisions with regard to matters or questions arising hereunder which the Company and the Warrant Agent may deem necessary
or desirable, provided that such addition, correction, supplement, cure or surrender shall not adversely affect the interests of the Holders
of the Global Warrants or Warrant Certificates in any material respect.
(b) In
addition to the foregoing, with the consent of Holders of Warrants entitled, upon exercise thereof, to receive not less than a majority
of the shares of Common Stock issuable thereunder, the Company and the Warrant Agent may modify this Agreement for the purpose of adding
any provisions to or changing in any manner or eliminating any of the provisions of this Agreement or modifying in any manner the rights
of the Holders of the Global Warrants; provided, however, that no modification of the terms (including but not limited to
the adjustments described in Section 11) upon which the Warrants are exercisable or reducing the percentage required for consent
to modification of this Agreement may be made without the consent of the Holder of each outstanding warrant certificate affected thereby;
provided further, however, that no amendment hereunder shall affect any terms of any Warrant Certificate issued in a Warrant
Exchange. As a condition precedent to the Warrant Agent's execution of any amendment, the Company shall deliver to the Warrant Agent a
certificate from a duly authorized officer of the Company that states that the proposed amendment complies with the terms of this Section 20.
No supplement or amendment to this Agreement shall be effective unless duly executed by the Warrant Agent.
Section 21. Successors.
All covenants and provisions of this Agreement by or for the benefit of the Company or the Warrant Agent shall bind and inure to the benefit
of their respective successors and assigns hereunder.
Section 22. Benefits
of this Agreement. Nothing in this Agreement shall be construed to give any Person other than the Company, the Holders of Warrant
Certificates and the Warrant Agent any legal or equitable right, remedy or claim under this Agreement; but this Agreement shall be for
the sole and exclusive benefit of the Company, the Warrant Agent and the Holders of the Warrant Certificates.
Section 23. Governing
Law; Jurisdiction. This Agreement and each Warrant Certificate issued hereunder shall be governed by, and construed in accordance
with, the laws of the State of New York without giving effect to the conflicts of law principles thereof. The Company hereby agrees that
any action, proceeding or claim against it arising out of or relating in any way to this Agreement shall be brought and enforced in the
courts of the State of New York or the United States District Court for the Southern District of New York, and irrevocably submits to
such jurisdiction, which jurisdiction shall be exclusive. The Company hereby waives any objection to such exclusive jurisdiction and that
such courts represent an inconvenience forum.
Section 24.
Counterparts. This Agreement may be executed in any number of counterparts and each of such counterparts shall for all purposes
be deemed to be an original, and all such counterparts shall together constitute but one and the same instrument. A signature to this
Agreement transmitted electronically shall have the same authority, effect and enforceability as an original signature.
Section 25. Captions.
The captions of the sections of this Agreement have been inserted for convenience only and shall not control or affect the meaning or
construction of any of the provisions hereof.
Section 26. Severability.
Wherever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable
law, but if any provision of this Agreement shall be prohibited by or invalid under applicable law, such provision shall be ineffective
to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of
this Agreement; provided, however, that if such prohibited and invalid provision shall adversely affect the rights, immunities, liabilities,
duties or obligations of the Warrant Agent, the Warrant Agent shall be entitled to resign immediately upon written notice to the Company.
Section 27. Force
Majeure. Notwithstanding anything to the contrary contained herein, the Warrant Agent will not be liable for any delays or failures
in performance resulting from acts beyond its reasonable control including, without limitation, acts of God, terrorist acts, shortage
of supply, breakdowns or malfunctions, interruptions or malfunction of computer facilities, or loss of data due to power failures or mechanical
difficulties with information storage or retrieval systems, labor difficulties, war, or civil unrest.
Section 28. Entire
Agreement. The parties hereto acknowledge that there are no agreements or understandings, written or oral, between them with respect
to matters contemplated hereunder other than as set forth herein and the Warrant Certificates, that this Agreement and the Warrant Certificates
contain the entire agreement between them with respect to the subject matter hereof and thereof
Section 29. Fees;
Expenses. As consideration for the services provided by the Warrant Agent (the “Services”), the Company shall pay
to the Warrant Agent the fees set forth on Schedule 1 hereto (the "Fees"). If the Company requests that the Warrant
Agent provide additional services not contemplated hereby, the Company shall pay to the Warrant Agent fees for such services at the Warrant
Agent's reasonable and customary rates, such fees to be governed by the terms of a separate agreement to be mutually agreed to and entered
into by the Parties at such time (the “Additional Service Fee”; together with the Fees, the “Service Fees”)
(a) The
Company shall reimburse the Warrant Agent for all reasonable and documented expenses incurred by the Warrant Agent (including, without
limitation, reasonable and documented fees and disbursements of counsel) in connection with the Services (the “Expenses”);
provided, however, that the Warrant Agent reserves the right to request advance payment for any out-of-pocket expenses.
The Company agrees to pay all Service Fees and Expenses within thirty (30) days following receipt of an invoice from the Warrant Agent.
(b) The
Company agrees and acknowledges that the Warrant Agent may adjust the Service Fees annually, on or about each anniversary date of this
Agreement, by the annual percentage of change in the latest Consumer Price Index of All Urban Consumers United States City Average, as
published by the U.S. Department of Labor, Bureau of Labor Statistics.
(c) Upon
termination of this Agreement for any reason, the Warrant Agent shall assist the Company with the transfer of records of the Company held
by the Warrant Agent. The Warrant Agent shall be entitled to reasonable additional compensation and reimbursement of any Expenses for
the preparation and delivery of such records to the successor agent or to the Company, and for maintaining records and/or Stock Certificates
that are received after the termination of this Agreement (the “Record Transfer Services”).
[remainder of page intentionally left blank;
signature page follows]
IN
WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the day and year first above written.
| |
FIRST WAVE BIOPHARMA, INC. |
| |
|
| |
By: |
|
| |
|
Name: |
| |
|
Title: |
| |
|
| |
COLONIAL STOCK TRANSFER CO., INC. |
| |
|
| |
By: |
|
| |
|
Name: |
| |
|
Title: |
Annex
A: Form of Warrant Certificate Request Notice
WARRANT CERTIFICATE REQUEST NOTICE
To: Colonial Stock Transfer Company, Inc.,
as Warrant Agent for First Wave BioPharma, Inc. (the “Company”)
The undersigned Holder of First Wave BioPharma, Inc.
Common Stock Purchase Warrants (“Warrants”) in the form of [ ]
[ ] Warrants issued by the Company hereby elects to receive a Warrant
Certificate evidencing
the Warrants held by the Holder
as specified below:
1. Name
of Holder of [ ] [ ]
Warrants in form of Global Warrants: _____________
2. Name
of Holder in Warrant Certificate (if different from name of Holder of Warrants in form of Global Warrants): ___________________
3. Number
of Warrants in name of Holder in form of Global Warrants:
4. Number
of Warrants for which Warrant Certificate shall be issued:
5. Number
of Warrants in name of Holder in form of Global Warrants after issuance of Warrant Certificate, if any:
6. [ ]
[ ] Warrant Certificate shall be delivered to the following address:
The undersigned hereby acknowledges and agrees
that, in connection with this Warrant Exchange and the issuance of the Warrant Certificate, the Holder is deemed to have surrendered the
number of Warrants in form of Global Warrants in the name of the Holder equal to the number of Warrants evidenced by the Warrant Certificate.
[SIGNATURE OF HOLDER]
Name of Investing Entity:
Signature of Authorized Signatory of Investing
Entity:
Name of Authorized Signatory:
Title of Authorized Signatory:
Date:
Exhibit 1-A:
Form of Warrant Certificate
Schedule
1
Fees
| Monthly Warrant Administration Fee (per Warrant Issue) | |
| $[•] | |
| EXCHANGE OF WARRANTS INTO COMMON SHARES | |
| $[•] | |
| Per Manual Exercise of Warrants (until established on DTC WARR System) | |
| | |
| | |
| $[•] | |
SPECIAL SERVICES
Services not included herein (including, without
limitation, trustee and custodial services, exchange/tender offer services and stock dividend disbursement services) but requested by
the Company may be subject to additional charges.
OUT-OF-POCKET EXPENSES
All customary out-of-pocket expenses will be billed
in addition to the foregoing fees. These charges include, but are not limited to, printing and stationery, freight and materials delivery,
postage and handling.
The foregoing fees apply to services ordinarily
rendered by the Warrant Agent and are subject to reasonable adjustment based on final review of documents.
Exhibit 5.1
 | | 1345 AVENUE OF THE AMERICAS, 11th FLOOR
NEW YORK, NEW YORK 10017
TELEPHONE: (212) 370-1300
FACSIMILE: (212) 370-7889
www.egsllp.com |
December 27, 2023
First Wave BioPharma, Inc.
777 Yamato Road, Suite 502
Boca Raton, Florida 33431
Re: Registration
Statement on Form S-1
Gentlemen:
We
have acted as counsel to First Wave BioPharma, Inc., a Delaware corporation (the “Company”), in a public offering
pursuant to the Registration Statement on Form S-1 (Registration No. 333-275316) initially filed with the Securities and Exchange
Commission (the “Commission”) pursuant to the Securities Act of 1933, as amended (the “Act”), on
November 3, 2023 (as amended, the “Registration Statement”), and the related prospectus contained therein (the
“Prospectus”). We are rendering this opinion in connection with the filing by the Company of the Registration Statement
relating to the offer and sale by the Company (the “Offering”) consisting of (a) (i) [___] shares (the “Shares”)
of the Company’s common stock, par value $0.0001 per share (the “Common Stock”) or (ii) [___] Pre-Funded
Warrants (the “Pre-Funded Warrants”) to purchase shares of Common Stock (the “Pre-Funded Warrant Shares”)
in lieu thereof of Shares and (b) accompanying [___] Common Stock Warrants (the “Common Warrants”, and together
with the Pre-Funded Warrants, the “Warrants”) to purchase [___] shares of Common Stock (the “Common Warrant
Shares”, and together with the Pre-Funded Share Warrants, the “Warrant Shares”). The Shares, Warrants and
Warrant Shares are covered by the Registration Statement and we understand that the Shares, Warrants and Warrant Shares are to be offered
and sold in the manner described in the Prospectus. This opinion is being delivered at the request of the Company and in accordance with
the requirements of Item 601(b)(5) of Regulation S-K promulgated by the Commission.
For purposes of rendering
the opinions set forth below, we have examined such documents and reviewed such questions of law as we have considered necessary and appropriate
for the purposes of our opinion including (i) the Registration Statement, including the exhibits filed therewith, (ii) the Prospectus,
(iii) the Company’s amended and restated certificate of incorporation and amended and restated bylaws, as currently in effect,
(iv) the form of Securities Purchase Agreement, (v) the form of Placement Agency Agreement (vi) the form of Common Warrant,
(vii) the form of Pre-Funded Warrant, (viii) the form of Warrant Agency Agreement, and (ix) the corporate resolutions and
other actions of the Company that authorize and provide for the filing of the Registration Statement, and we have made such other investigation
as we have deemed appropriate. We have not independently established any of the facts so relied on.
We have also assumed that
all of the shares of Common Stock issuable or eligible for issuance pursuant to exercise of the Warrants following the date hereof will
be issued for not less than par value.
1. Common
Stock. When the Registration Statement becomes effective under the Act and when the offering is completed as contemplated
by the Registration Statement, the Shares will be validly issued, fully paid and non-assessable.
2. Common
Warrants and Pre-Funded Warrants. When the Registration Statement becomes effective under the Act and when the Common Warrants
and Pre-Funded Warrants are issued, delivered and paid for, as contemplated by the Registration Statement, such Common Warrants and Pre-Funded
Warrants will be legally binding obligations of the Company enforceable in accordance with their terms except: (a) as such enforceability
may be limited by bankruptcy, insolvency, reorganization or similar laws affecting creditors’ rights generally and by general equitable
principles (regardless of whether enforceability is considered in a proceeding in equity or at law); (b) as enforceability of any
indemnification or contribution provision may be limited under the federal and state securities laws; (c) that the remedy of specific
performance and injunctive and other forms of equitable relief may be subject to the equitable defenses and to the discretion of the court
before which any proceeding therefor may be brought; and(d) we express no opinion as to whether a state court outside of the State
of New York or a federal court of the United States would give effect to the choice of New York law provided for in the Warrant.
3. Common
Warrant Shares and Pre-Funded Warrant Shares: When the Registration Statement becomes effective under the Act and when
the offering is completed as contemplated by the Registration Statement, the Common Warrant Shares issuable upon exercise of the Common
Warrants will be validly issued, fully paid and non-assessable and the Pre-Funded Warrant Shares issuable upon exercise of the Pre-Funded
Warrants will be validly issued, fully paid and non-assessable.
The opinions expressed in
this opinion letter are limited to the applicable provisions of the General Corporation Law of the State of Delaware, laws of the State
of New York and the federal laws of the United States of America, as in effect on the date hereof. We are not opining on, and we assume
no responsibility for, the applicability or effect on any of the matters covered herein of: (a) any other laws; (b) the laws
of any other jurisdiction; or (c) the laws of any country, municipality or other political subdivision or local government agency
or authority. The opinions set forth below are rendered as of the date of this opinion letter. We assume no obligation to update or supplement
such opinions to reflect any change of law or fact that may occur.
We hereby consent to the filing
of this opinion as Exhibit 5.1 to the Registration Statement and to the reference to our firm under the caption “Legal Matters”
in the prospectus constituting a part of the Registration Statement. In giving such consent, we do not thereby admit that we are included
in the category of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations of the
Commission promulgated thereunder.
| |
Very truly yours, |
| |
|
| |
/s/ Ellenoff Grossman & Schole LLP |
Exhibit 23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
We hereby consent
to the incorporation by reference in the Preliminary Prospectus of First Wave BioPharma, Inc. on Form S-1 Amendment No. 2 (File No.333-275316)
of our report dated March 20, 2023, on the consolidated financial statements of First Wave BioPharma, Inc. as of December 31, 2022 and
2021 and for each of the two years in the period ended December 31, 2022, which appears in the Annual Report on Form 10-K of First
Wave BioPharma, Inc. for the year ended December 31, 2022. The report for First Wave BioPharma, Inc. includes an explanatory paragraph
about the existence of substantial doubt concerning its ability to continue as a going concern. We also consent to the reference to our
Firm under the caption “Experts” in the Registration Statement.
/s/ Mazars USA LLP
New York, New York
December 27, 2023
Exhibit 107
Calculation of Filing Fee Tables
S-1
(Form Type)
First Wave BioPharma, Inc.
(Exact Name of Registrant as Specified in its
Charter)
Table 1: Newly Registered and Carry Forward
Securities
| |
|
Security
Type |
|
Security
Class Title |
|
Fee
Calculation
or Carry
Forward
Rule |
|
Amount
Registered |
|
Proposed
Maximum
Offering
Price Per
Unit |
|
Maximum
Aggregate
Offering
Price(1) |
|
Fee
Rate |
|
Amount
of
Registration
Fee |
Fees to Be
Paid |
|
Equity
|
|
Common
Stock, par value $0.0001 per share (“Common Stock”)(2) |
|
|
|
457(o) |
|
|
|
|
|
$5,000,000 |
|
0.00014760 |
|
$738.00 |
| |
|
Other
|
|
Pre-funded
Warrants to purchase Common Stock(3) |
|
|
|
457(g) |
|
|
|
|
|
— |
|
|
|
(3)(4) |
| |
|
Equity
|
|
Common
Stock underlying the Pre-Funded Warrants(3) |
|
|
|
457(o) |
|
|
|
|
|
— |
|
|
|
(3) |
| |
|
Other
|
|
Warrants
to purchase Common Stock |
|
|
|
457(g) |
|
|
|
|
|
— |
|
|
|
(4) |
| |
|
Equity
|
|
Common
Stock underlying the Warrants to purchase Common Stock |
|
|
|
457(o) |
|
|
|
|
|
$10,000,000 |
|
0.00014760 |
|
$1,476.00 |
Fees
Previously Paid |
|
— |
|
— |
|
|
|
— |
|
— |
|
— |
|
— |
|
|
|
$4,428.00 |
Carry
Forward Securities |
|
— |
|
— |
|
|
|
— |
|
— |
|
|
|
— |
|
|
|
|
| |
|
Total
Offering Amounts |
|
|
|
$15,000,000 |
|
0.00014760 |
|
$2,214.00 |
| |
|
Total
Fees Previously Paid |
|
|
|
|
|
|
|
$4,428.00 |
| |
|
Total
Fee Offsets |
|
|
|
|
|
|
|
— |
| |
|
Net
Fee Due |
|
|
|
|
|
|
|
$0.00 |
| (1) |
Estimated solely for the purpose
of calculating the amount of the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended (the
“Securities Act”). |
| (2) |
Pursuant to Rule 416 under
the Securities Act, this registration statement shall also cover any additional shares of the registrant’s securities that
become issuable by reason of any share splits, share dividends or similar transactions. |
| (3) |
The proposed maximum aggregate
offering price of the Common Stock will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded warrants
issued in the offering, and the proposed maximum aggregate offering price of the pre-funded warrants to be issued in the offering
will be reduced on a dollar-for-dollar basis based on the offering price of any Common Stock issued in the offering. Accordingly,
the proposed maximum aggregate offering price of the Common Stock and pre-funded warrants (including the Common Stock issuable upon
exercise of the pre-funded warrants), if any, is $5,000,000. |
| (4) |
No separate registration fee
is payable pursuant to Rule 457(g) under the Securities Act. |
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Mar 2024 to Apr 2024
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Apr 2023 to Apr 2024