
Advancing medicines. Solving problems. Improving lives. Advancing medicines. Solving problems. Improving lives. Corporate Presentation November 2023

Advancing medicines. Solving problems. Improving lives. Disclaimer This presentation and the accompanying oral commentary has been prepared by Aquestive Therapeutics, Inc. (the “Company”, “our” or “us”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding the advancement and related timing of our product candidate Anaphylm™ (epinephrine) Sublingual Film for the emergency treatment of severe allergic reactions, including anaphylaxis, through clinical development and approval by the U.S. Food and Drug Administration (FDA), including the filing of our pivotal pharmacokinetic (PK) clinical trial and other supporting clinical studies for Anaphylm; regarding the advancement and relating timing through clinical development and approval by the FDA of the Company’s New Drug Application (NDA) for our product candidate Libervant™ (diazepam) Buccal Film for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient’s usual seizure pattern in patients with epilepsy between two and five years of age; regarding the approval for U.S. market access of Libervant for these epilepsy patients aged 12 years and older, and overcoming the orphan drug market exclusivity of an FDA approved nasal spray product of another company extending to January 2027 for this age group of the patient population; regarding the potential benefits our products and product candidates could bring to patients; regarding the potential for licensing to third parties our product candidate Anaphylm outside of the U.S. and Libervant in the U.S. and around the world; regarding the ability to bring our product candidates, including Anaphylm and Libervant, to market and achieve market acceptance of those products; regarding the rate and degree of market acceptance and demand for our licensed products; regarding the potential and related timing for expanding the Company’s manufacturing capabilities and supporting the growth of demand for existing and potential future licensed products in the U.S. and other countries and growing revenue from such activities; regarding the 2023 financial outlook of the Company and its growth and future financial and operating results and financial position; and other statements that are not historical facts. These forward-looking statements are subject to the uncertain impact of global business and macroeconomic conditions, including as a result of inflation, rising interest rates, instability in the global banking system, and geopolitical conflicts, including the wars in Ukraine and Israel, and the impact of the COVID-19 global pandemic on the Company’s business including with respect to its clinical trials including site initiation, enrollment and timing and adequacy of clinical trials; on regulatory submissions and regulatory reviews and approval of Anaphylm and Libervant, pharmaceutical ingredient and other raw materials supply chain, manufacture, and distribution; and ongoing availability of an appropriate labor force and skilled professionals. These forward-looking statements are based on the Company’s current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward- looking statements. Such risks and uncertainties include, but are not limited to, risks associated with the Company’s development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials for Anaphylm, Libervant and our other product candidates; risk of the Company’s ability to generate sufficient data in the clinical trials for FDA approval of Anaphylm and Libervant for epilepsy patients between 2 and 5 years of age; risk of the Company’s ability to address the FDA’s comments on the Company’s pivotal PK study protocol and other concerns identified in the FDA End-of-Phase 2 meeting for Anaphylm; risk that the FDA may require additional clinical studies for approval of Anaphylm and Libervant for patients epilepsy between 2 and 5 years of age; risk of delays in or the failure to receive FDA approval of Anaphylm, Libervant and our other product candidates; risks that the FDA will not approve Libervant for U.S. market access for any age group of patients by overcoming the seven year orphan drug market exclusivity of an FDA approved nasal spray product in effect until January 2027, and there can be no assurance that the Company will be successful in obtaining any of the foregoing FDA approvals for Anaphylm and Libervant, including for U.S. market access for Libervant for any age group of patients; risk that a competing pediatric epilepsy product of Libervant will receive FDA approval prior to the Company’s receipt of FDA approval of the Libervant NDA for these epilepsy patients between 2 and 5 years of age; risk relating to the unpredictability of the FDA’s decisions regarding orphan drug exclusivity; risk of litigation brought by third parties relating to overcoming their orphan drug exclusivity of an FDA approved product should the FDA approve Libervant for U.S. market access for any age group of this epilepsy patient population; risk in obtaining market access for Libervant for other reasons; risks associated with the Company’s development work, including any delays or changes to the timing, cost and success of the Company’s product development activities; risk of the success of any competing products; risk inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks, and regulatory limitations); risk of the rate and degree of market acceptance of our product candidates, including Anaphylm and Libervant, and our licensed products in the U.S. and abroad; risk of insufficient capital and cash resources, including insufficient access to available debt and equity financing and revenues from operations, to satisfy all of the Company’s short-term and longer term liquidity and cash requirements and other cash needs, at the times and in the amounts needed, including to fund future clinical development activities for Anaphylm, Libervant and our other product candidates; risk that our manufacturing capabilities will be sufficient to support demand for existing and potential future licensed products in the U.S. and other countries; risk of achieving growth in our base business; risk of our ability to enter into other commercial transactions with third parties that will support growth of our business and execution of key initiatives; risk of the success of any competing products; risk of eroding market share for Suboxone® and risk as a sunsetting product, which accounts for the substantial part of our current operating revenue; risk of the size and growth of our product markets; risks of compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with intellectual property rights and infringement claims relating to the Company's products; risk of unexpected patent developments; uncertainties related to general economic, political (including acts of war and terrorism), business, industry, regulatory, financial and market conditions and other unusual items; and other risks and uncertainties affecting the Company described in the “Risk Factors” section and in other sections included in the Company’s 2022 Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K filed with the U.S. Securities and Exchange Commission. Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only as of the date made. All subsequent forward-looking statements attributable to the Company or any person acting on its behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update forward-looking statements or outlook or guidance after the date of this presentation whether as a result of new information, future events or otherwise, except as may be required by applicable law. PharmFilm® and the Aquestive logo are registered trademarks of Aquestive Therapeutics, Inc. The trade name for AQST-109 “Anaphylm” has been conditionally approved by the FDA. Final approval of the Anaphylm™ proprietary name is conditioned on FDA approval of the product candidate, AQST-109. All other registered trademarks referenced herein are the property of their respective owners. © 2023 Property of Aquestive Therapeutics, Inc. 2

Advancing medicines. Solving problems. Improving lives. Our Mission Aquestive is a pharmaceutical company advancing medicines to bring meaningful improvement to patients’ lives through innovative science and delivery technologies 3

Advancing medicines. Solving problems. Improving lives. In the next five years, we aim to: • Grow the existing and ex-U.S. collaboration revenue • Secure FDA approval for Anaphylm™ in the U.S. • Launch Libervant™ in the U.S. in or prior to 2027 • Advance product candidates utilizing Adrenaverse™ platform (epinephrine prodrug platform) We Have a Strong Vision for Building the Company 4 1. Estimate is based on an orphan drug market exclusivity block until January of 2027 by an FDA approved nasal spray product.
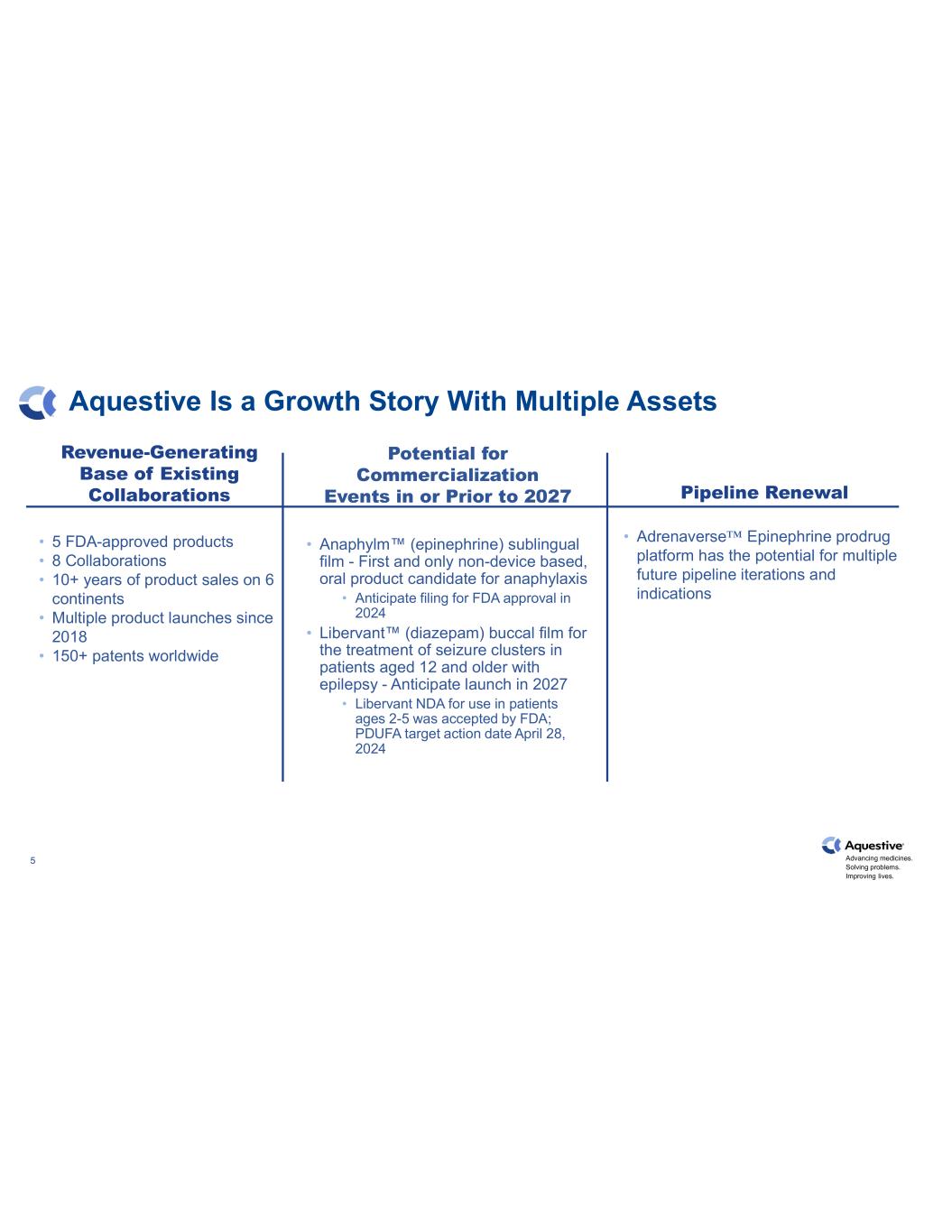
Advancing medicines. Solving problems. Improving lives. Aquestive Is a Growth Story With Multiple Assets 5 Revenue-Generating Base of Existing Collaborations • 5 FDA-approved products • 8 Collaborations • 10+ years of product sales on 6 continents • Multiple product launches since 2018 • 150+ patents worldwide Pipeline Renewal • Adrenaverse™ Epinephrine prodrug platform has the potential for multiple future pipeline iterations and indications Potential for Commercialization Events in or Prior to 2027 • Anaphylm™ (epinephrine) sublingual film - First and only non-device based, oral product candidate for anaphylaxis • Anticipate filing for FDA approval in 2024 • Libervant™ (diazepam) buccal film for the treatment of seizure clusters in patients aged 12 and older with epilepsy - Anticipate launch in 2027 • Libervant NDA for use in patients ages 2-5 was accepted by FDA; PDUFA target action date April 28, 2024

Advancing medicines. Solving problems. Improving lives. 6 Our Core Technology is Branded as PharmFilm®
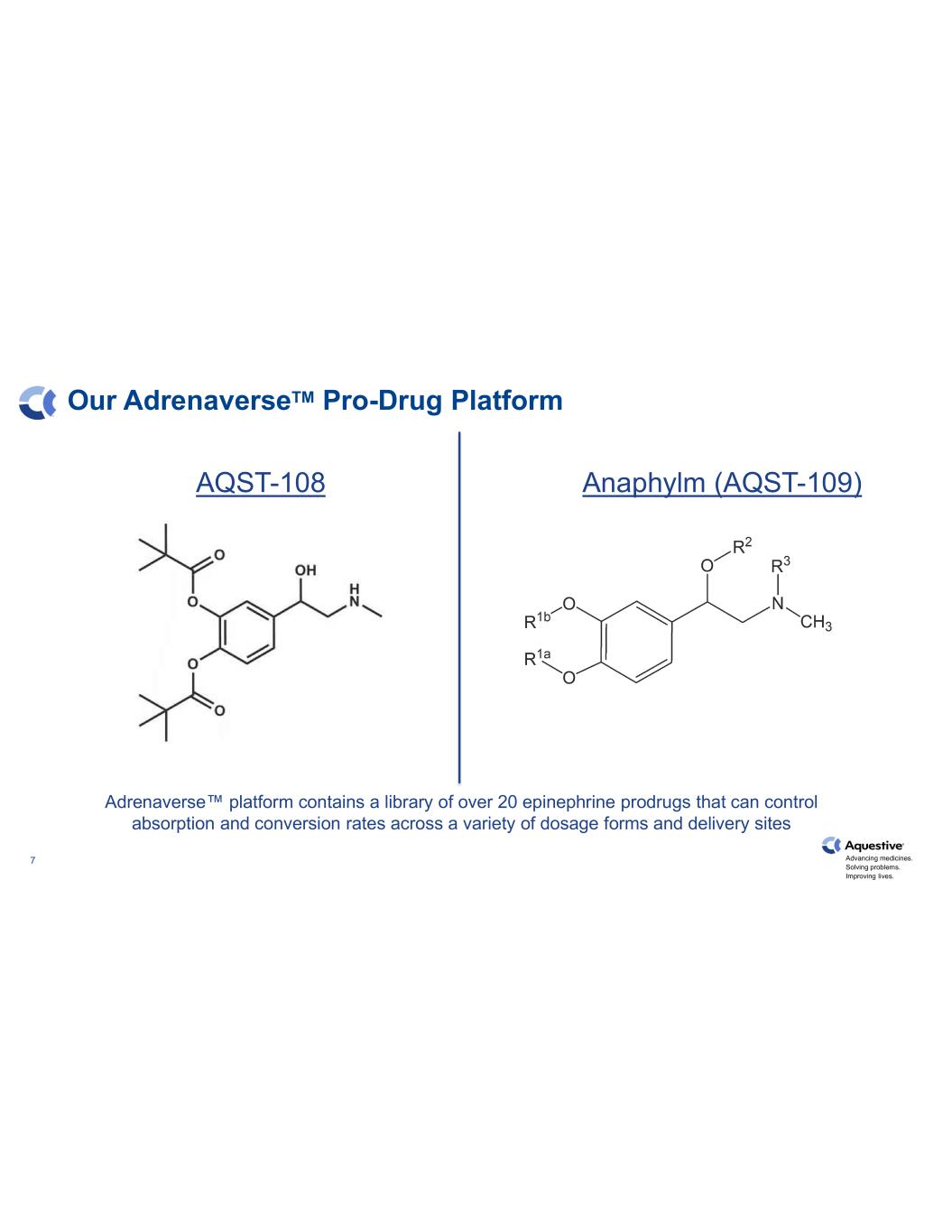
Advancing medicines. Solving problems. Improving lives. 7 Our AdrenaverseTM Pro-Drug Platform AQST-108 Anaphylm (AQST-109) Adrenaverse™ platform contains a library of over 20 epinephrine prodrugs that can control absorption and conversion rates across a variety of dosage forms and delivery sites

Advancing medicines. Solving problems. Improving lives. Product Portfolio & Licensing Opportunities in 2023 and 2024 AQST-108 formulation (epinephrine) (Alternative Indications) Proprietary growth drivers Preclinical Clinical Filed Marketed 8 Hypera – Brazil Haisco - China Libervant™ (diazepam) Buccal Film (Seizure Clusters) Libervant™ (diazepam) buccal film United States Asia South America Anaphylm™ (epinephrine) sublingual film Ex-U.S. Rights Assertio Therapeutics Indivior Kempharm Zevra Mitsubishi Tanabe – U.S. Hypera – Brazil Zambon - EU Haisco - China Pharmanovia Licensing Opportunities Regional Licensing Agreements IP Licenses (Royalty-Only)Global Licensing Agreements (riluzole) oral film (development stage) Anaphylm™ (epinephrine) Sublingual Film (Anaphylaxis)

Advancing medicines. Solving problems. Improving lives. Strong Leadership Team Daniel Barber President, CEO and Director Lori J. Braender SVP, General Counsel Ken Marshall Chief Commercial Officer Mark Schobel Chief Innovation & Technology Officer Ernie Toth Chief Financial Officer 9 Cassie Jung SVP, Operations Steve Wargacki SVP, R&D Carl Kraus Chief Medical Officer Strong Operations & Partnering Team Experienced Science/IP/Development Team Peter Boyd SVP, IT, HR, & Communications

Advancing medicines. Solving problems. Improving lives. We Are Now Focused on the Next Chapter • Commence pivotal study for Anaphylm™ in Q4 2023 • Continue to strengthen the balance sheet • Continue to pursue out-licensing opportunities for Libervant™ and Anaphylm™ • Continue to grow our license and supply business 10

Advancing medicines. Solving problems. Improving lives. Anaphylm™ (epinephrine) Sublingual Film 11

Advancing medicines. Solving problems. Improving lives. Anaphylaxis: A Serious Systemic Hypersensitivity Reaction That is Usually Rapid in Onset And May Be Fatal 12 1. Turner PJ, et al. World Allergy Org J. 2019;12100066. 2. FARE, 2022; https://www.foodallergy.org/resources/facts-and-statistics. 3. Fromer L. The American Journal of Medicine (2016);129, 1244-1250. As many as 32 million people in the United States are at chronic risk for acute anaphylactic episodes 52% of patients in a nationwide patient survey who had previously experienced anaphylaxis had never received an epinephrine auto-injector prescription Direct costs of anaphylaxis have been estimated at $1.2 billion per year 60% of respondents in same patient survey did not have an epinephrine auto-injector currently available 1 2 3 3 3

Advancing medicines. Solving problems. Improving lives. 13 Treatment of Anaphylaxis – Epinephrine • Epinephrine is the first line of treatment for anaphylaxis • Epinephrine is the only medication proven to stop a life-threatening allergic reaction • Epinephrine dosage (current medication delivery systems): • 0.3-0.5mg intramuscularly (IM) or subcutaneously • Children’s dosage is weight based: 1. 0.10mg (for children 16.5 to 33 pounds) — AUVI-Q® brand only 2. 0.15mg (for children under 66 pounds) 3. 0.3mg (for children and adults over 66 pounds) • A second dose of epinephrine can be given as needed 1 1. Epinephrine in the Management of Anaphylaxis. Brown JC, Simons E, Rudders SA. J Allergy Clin Immunol Pract. 2020 Apr;8(4):1186-1195. doi: 10.1016/j.jaip.2019.12.015 PMID: 32276687. 2. EpiPen® Package Insert. 2 2

Advancing medicines. Solving problems. Improving lives. 14 Epinephrine Market Trending towards >4.6M TRx in 2023 1. Symphony Health September 2023, All Market Data is limited to US & Territories The 2022 epinephrine market surpassed 4 million TRx in 2022 and rebounded to historical highs following a downturn due to generics and the Covid-19 pandemic. TRx counts in 2023 have exceeded prior year for 9 consecutive months. 1

Advancing medicines. Solving problems. Improving lives. 15 Generic Market With High Levels of Dissatisfaction and Unmet Need 1. KOL feedback; Aquestive Market Research. 2. Fromer L. The American Journal of Medicine (2016);129, 1244-1250. 3. Warren et al. Ann Allergy Asthma Immunol (2018). 4. Brooks et al. Ann Allergy Asthma Immunol (2017). 5. Asthma and Allergy Foundation of America Patient Survey Report (2019). 6. El Turki et al. EmergMed J (2017). Current Standard of Care = Large, Needle Based Injectors Numerous Studies and Patient Surveys Articulate Significant Dissatisfaction with Current Offerings • Right place, right time • <50% of patients carry their EpiPen® – often due to hassle factor • Refusal of treatment • 25-50% of patients refuse treatment with EpiPen® – often due to needle reluctance • Time to treat post exposure • 60% of patients/caregivers delay treatment – often due to needle reluctance • Failed administration in the field • 23-35% of patients and caregivers fail to dose correctly • Oversized devices • Hard to carry • Medical guidelines recommend always having 2 doses on hand • Needle based • High prevalence of needle phobia (especially in children) • Not always intuitive to use • Even trained health care providers have been shown to incorrectly inject 3,4,5 1 1 6 2
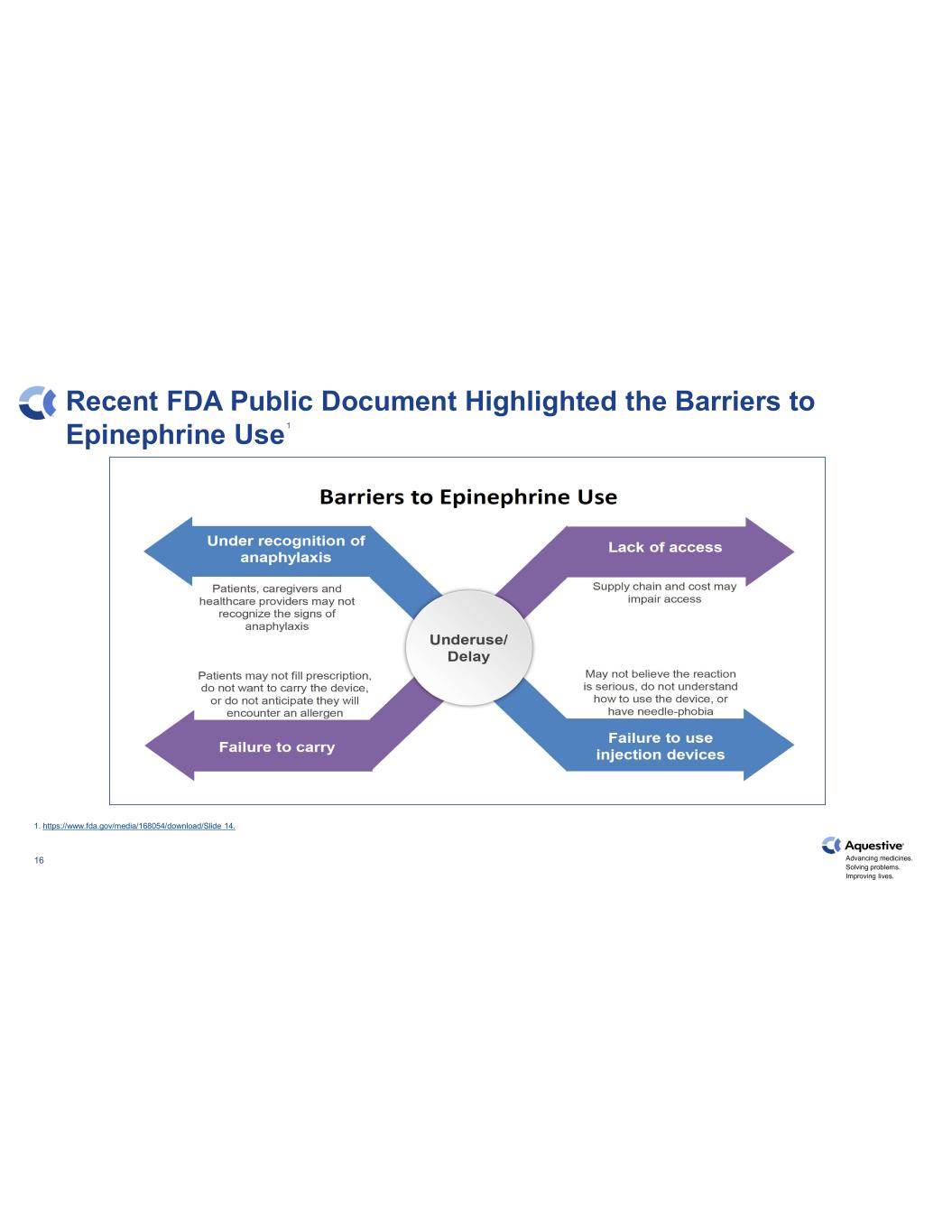
Advancing medicines. Solving problems. Improving lives. 16 Recent FDA Public Document Highlighted the Barriers to Epinephrine Use 1 1. https://www.fda.gov/media/168054/download/Slide 14.
Advancing medicines. Solving problems. Improving lives. CONFIDENTIAL17 Anaphylm™ (epinephrine) Sublingual Film First and only non-device based, orally delivered epinephrine product candidate Fast absorption into the bloodstream Non-device administration Portability + +

Advancing medicines. Solving problems. Improving lives. 18 Competitive Product Summary ORAL AUTO INJECTOR INTRA NASAL Company Brand Anaphylm EpiPen/Generic Adrenaclick® Auvi-Q® Symjepi® neffy® Utuly™ N/A Administration Sublingual Auto-Injector Auto-Injector Auto- Injector Syringe Device Nasal Spray Nasal Spray Nasal Spray Dosing (Adult/Jr) TBD 0.3 / 0.15 mg 0.3 / 0.15 mg 0.3 / 0.15 / 0.10 mg 0.3 / 0.15 mg 2 mg 6.6 mg Not Reported Market Position 1st & Only Oral 90%+ Share Negligible <10% Negligible 1 Dose per Device 2 Doses per Device Potentially 3rd Nasal to Market Regulatory Status (FDA) Expected NDA Filing 2024 Approved/Marketed CRL Received – Pending Filing after Study Expected Filing 1H ‘23 Expected NDA Filing 2023 1 2 3 2 2, 4 2 1. The data presented on this slide are based on cross-study comparisons and are not based on any head-to-head trials as a result, comparability may be limited/inaccurate. Cross-study comparisons are inherently limited and may suggest misleading similarities or difference. 2. Pending FDA Review. 3. VIATRIS: Formerly Mylan. 4. US WorldMeds markets for Adamis.

Advancing medicines. Solving problems. Improving lives. 19 Scientific Advisory Board David Bernstein, MD University of Cincinnati Carlos Camargo, MD Harvard Medical School David M. Fleischer, MD Children’s Hospital Colorado David Golden, MD Sinai Hospital, Baltimore Matthew Greenhawt, MD Children’s Hospital Colorado Ruchi Gupta, MD, MPH Northwestern Jay Lieberman, MD University of Tennessee John Oppenheimer, MD University of Medicine and Dentistry of NJ - Rutgers
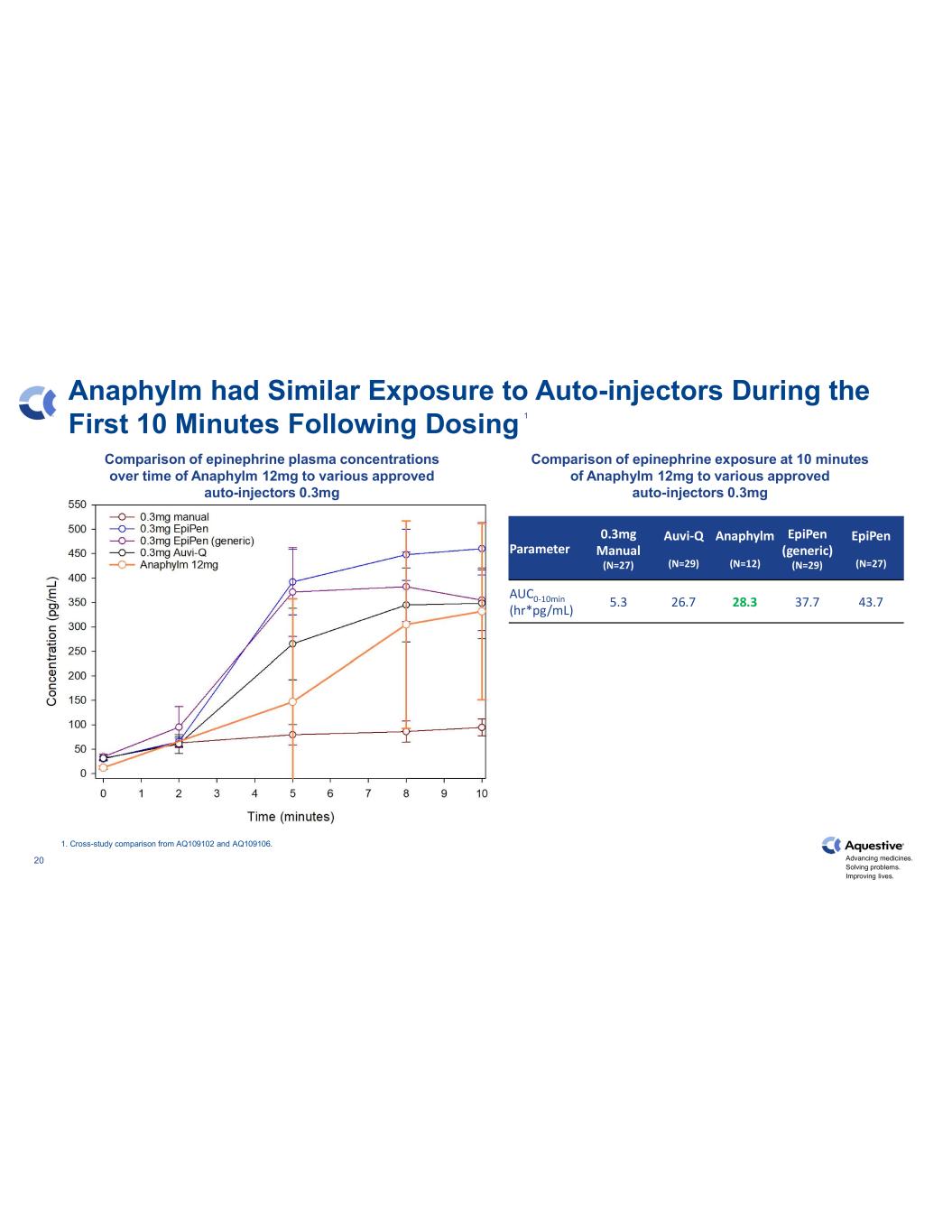
Advancing medicines. Solving problems. Improving lives. 20 Anaphylm had Similar Exposure to Auto-injectors During the First 10 Minutes Following Dosing Parameter 0.3mg Manual (N=27) Auvi-Q (N=29) Anaphylm (N=12) EpiPen (generic) (N=29) EpiPen (N=27) AUC0-10min (hr*pg/mL) 5.3 26.7 28.3 37.7 43.7 1. Cross-study comparison from AQ109102 and AQ109106. Comparison of epinephrine plasma concentrations over time of Anaphylm 12mg to various approved auto-injectors 0.3mg Comparison of epinephrine exposure at 10 minutes of Anaphylm 12mg to various approved auto-injectors 0.3mg 1
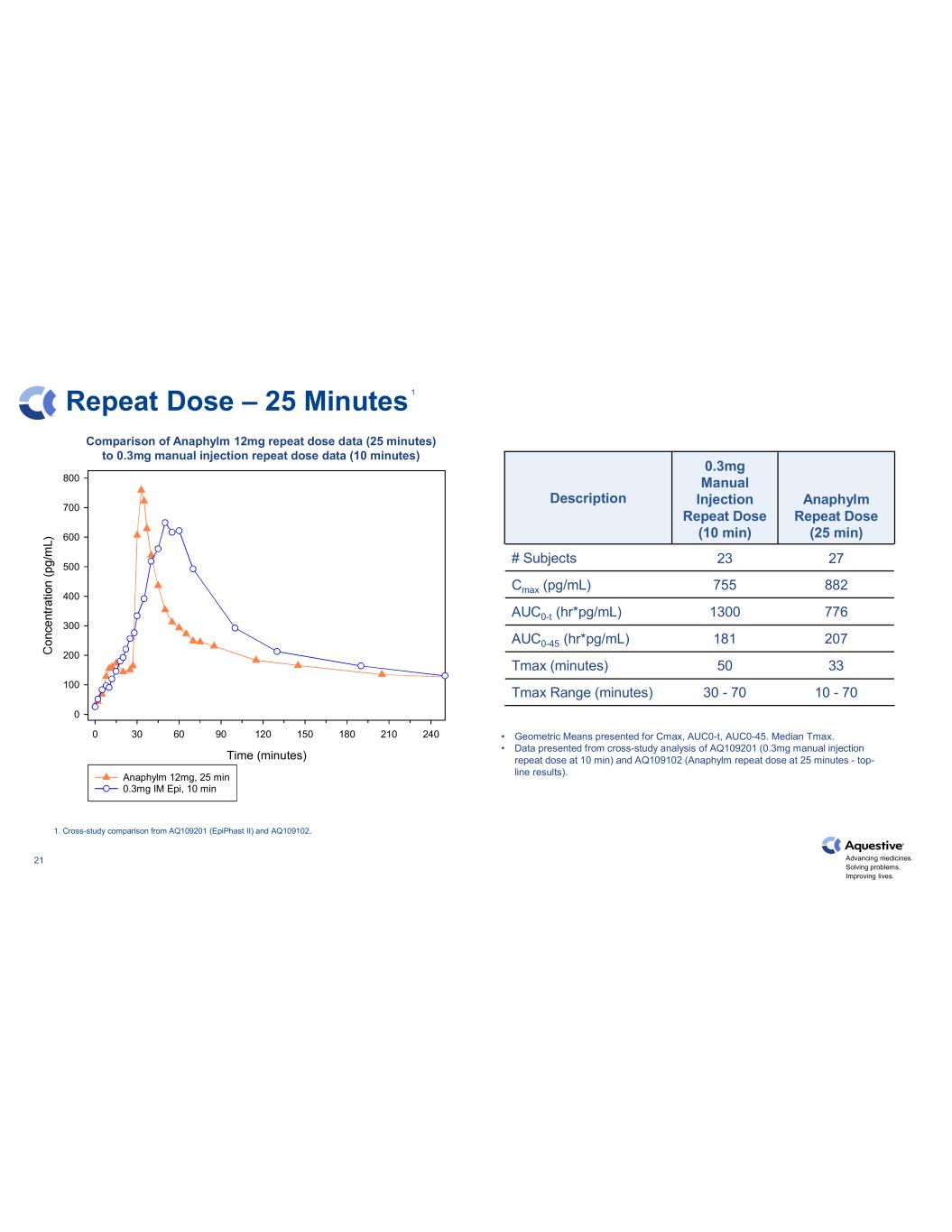
Advancing medicines. Solving problems. Improving lives. 21 Repeat Dose – 25 Minutes Description 0.3mg Manual Injection Repeat Dose (10 min) Anaphylm Repeat Dose (25 min) # Subjects 23 27 Cmax (pg/mL) 755 882 AUC0-t (hr*pg/mL) 1300 776 AUC0-45 (hr*pg/mL) 181 207 Tmax (minutes) 50 33 Tmax Range (minutes) 30 - 70 10 - 70 • Geometric Means presented for Cmax, AUC0-t, AUC0-45. Median Tmax. • Data presented from cross-study analysis of AQ109201 (0.3mg manual injection repeat dose at 10 min) and AQ109102 (Anaphylm repeat dose at 25 minutes - top- line results). 1. Cross-study comparison from AQ109201 (EpiPhast II) and AQ109102. Time (minutes) 0 30 60 90 120 150 180 210 240 0 100 200 300 400 500 600 700 800 Anaphylm 12mg, 25 min 0.3mg IM Epi, 10 min Comparison of Anaphylm 12mg repeat dose data (25 minutes) to 0.3mg manual injection repeat dose data (10 minutes) 1
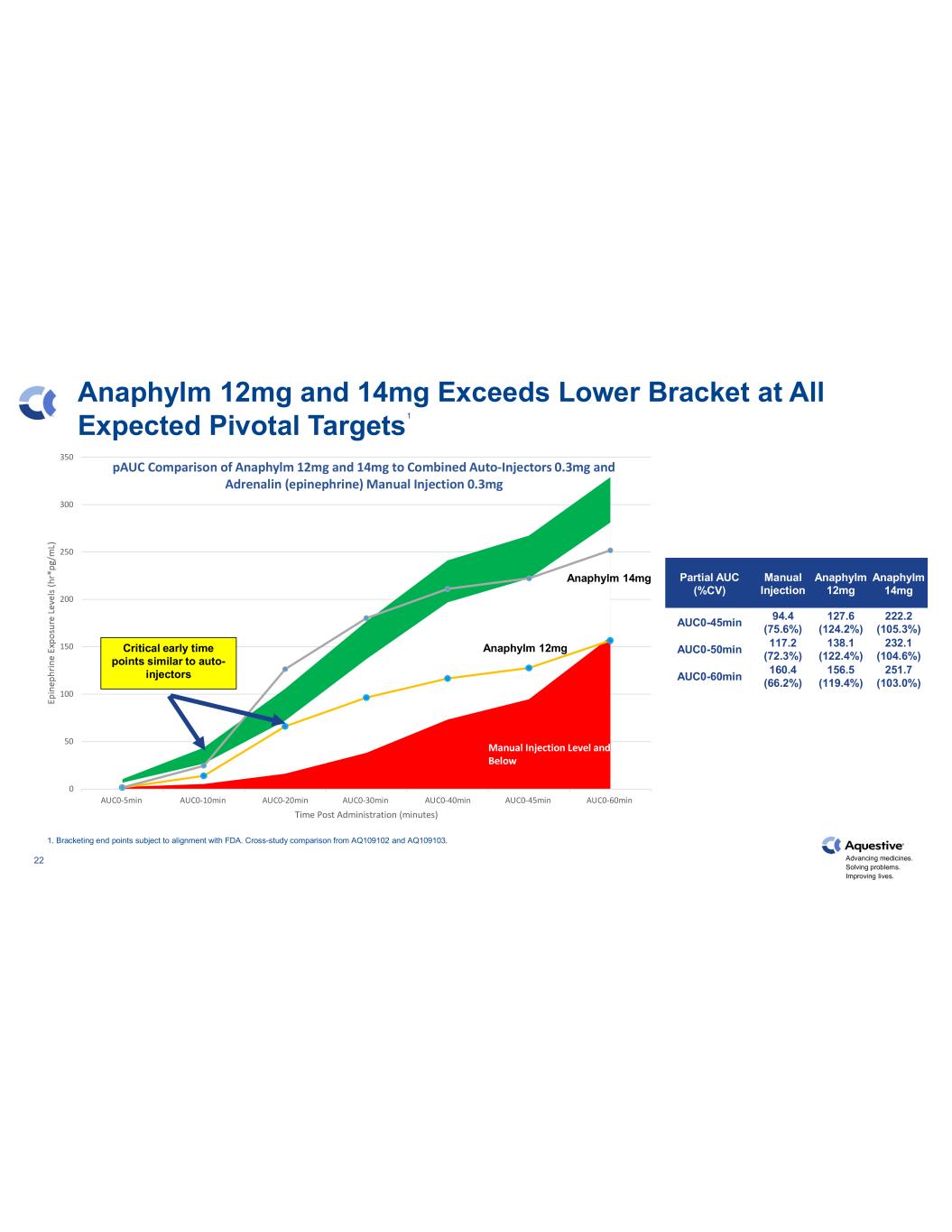
Advancing medicines. Solving problems. Improving lives. 22 0 50 100 150 200 250 300 350 AUC0-5min AUC0-10min AUC0-20min AUC0-30min AUC0-40min AUC0-45min AUC0-60min Time Post Administration (minutes) pAUC Comparison of Anaphylm 12mg and 14mg to Combined Auto-Injectors 0.3mg and Adrenalin (epinephrine) Manual Injection 0.3mg Anaphylm 12mg and 14mg Exceeds Lower Bracket at All Expected Pivotal Targets Manual Injection Level and Below Anaphylm 12mgCritical early time points similar to auto- injectors Partial AUC (%CV) Manual Injection Anaphylm 12mg Anaphylm 14mg AUC0-45min 94.4 (75.6%) 127.6 (124.2%) 222.2 (105.3%) AUC0-50min 117.2 (72.3%) 138.1 (122.4%) 232.1 (104.6%) AUC0-60min 160.4 (66.2%) 156.5 (119.4%) 251.7 (103.0%) 1 1. Bracketing end points subject to alignment with FDA. Cross-study comparison from AQ109102 and AQ109103. Anaphylm 14mg
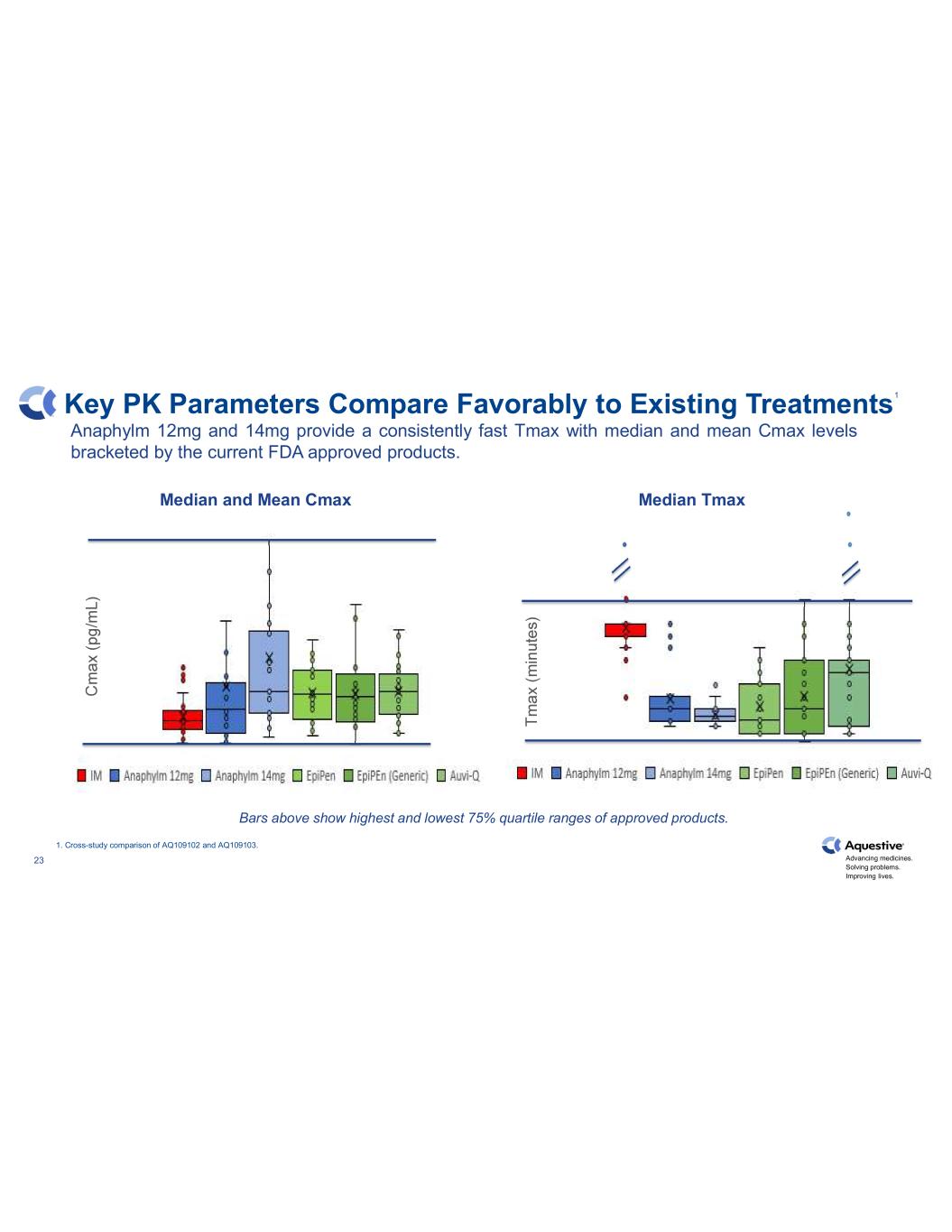
Advancing medicines. Solving problems. Improving lives. 23 Key PK Parameters Compare Favorably to Existing Treatments Bars above show highest and lowest 75% quartile ranges of approved products. 1. Cross-study comparison of AQ109102 and AQ109103. Anaphylm 12mg and 14mg provide a consistently fast Tmax with median and mean Cmax levels bracketed by the current FDA approved products. Median and Mean Cmax Median Tmax 1

Advancing medicines. Solving problems. Improving lives. 24 Both 12mg and 14mg Anaphylm Resulted in Clinically Favorable Pharmacodynamic (PD) Effects Anaphylm demonstrates a rapid increase in systolic blood pressure (SBP), pulse and diastolic blood pressure (DBP) within 2 minutes. Minimal impact to PD from increased exposure provided by Anaphylm 14mg. Mean Baseline Adjusted Changes in Systolic Blood Pressure Following Administration Mean Baseline Adjusted Changes in Pulse Following Administration 1. Cross-study comparison of AQ109102 and AQ109103. 1 1

Advancing medicines. Solving problems. Improving lives. 25 Anaphylm Safety and Tolerability • In the clinical program to date, treatment emergent adverse events (TEAEs) were assessed by both incidence and severity o Vast majority of reported TEAEs were mild or moderate in severity o Majority of TEAEs were within the standard of care (SOC) of general disorders and administrative site conditions o No serious adverse events (SAEs) reported and most TEAEs resolved without additional intervention • Cardiovascular adverse event (AE) profile of Anaphylm appears similar to the AE profile of the approved comparators o No severe cardiac events have been observed following Anaphylm dosing, and all TEAEs have required no or minimal intervention o BP elevations have generally been minimal to moderate in degree; no episodes of malignant hypertension (SBP>180mmHg) were observed o Heart rate elevations have generally been minimal to moderate in degree; transient palpitations and tachycardia have frequently been reported, but ventricular tachyarrythmias were not observed

Advancing medicines. Solving problems. Improving lives. 26 Anaphylm 2023-2024 Critical Path 2023 2024 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec Jan Feb Mar Apr May Jun Jul Aug Sept Oct Nov Dec Pilot Studies Pivotal Study Pediatric Study Pre-NDA Meeting NDA Submission

Advancing medicines. Solving problems. Improving lives. Libervant™ (diazepam) Buccal Film 27
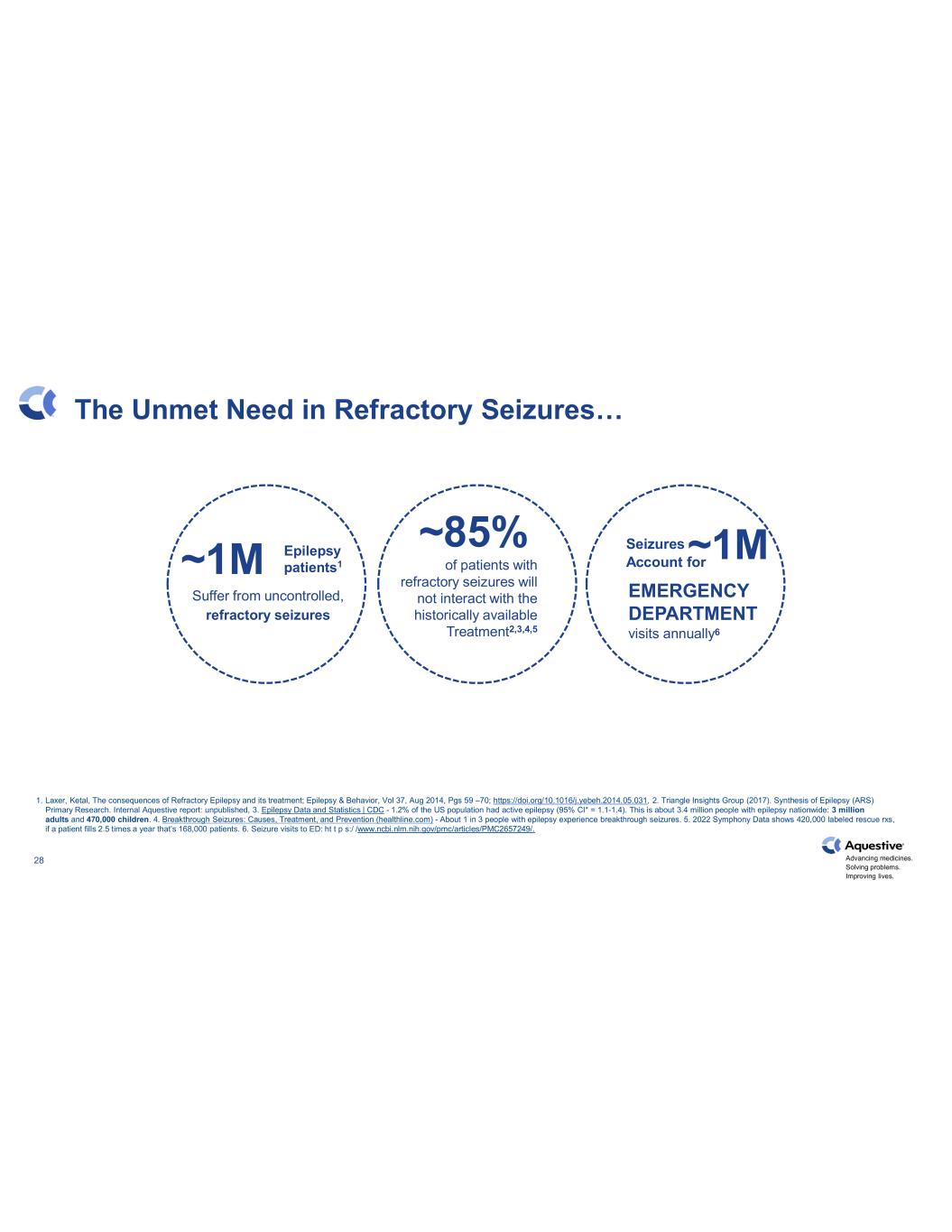
Advancing medicines. Solving problems. Improving lives. 28 1. Laxer, Ketal, The consequences of Refractory Epilepsy and its treatment; Epilepsy & Behavior, Vol 37, Aug 2014, Pgs 59 –70; https://doi.org/10.1016/j.yebeh.2014.05.031, 2. Triangle Insights Group (2017). Synthesis of Epilepsy (ARS) Primary Research. Internal Aquestive report: unpublished, 3. Epilepsy Data and Statistics | CDC - 1.2% of the US population had active epilepsy (95% CI* = 1.1-1.4). This is about 3.4 million people with epilepsy nationwide: 3 million adults and 470,000 children. 4. Breakthrough Seizures: Causes, Treatment, and Prevention (healthline.com) - About 1 in 3 people with epilepsy experience breakthrough seizures. 5. 2022 Symphony Data shows 420,000 labeled rescue rxs, if a patient fills 2.5 times a year that’s 168,000 patients. 6. Seizure visits to ED: ht t p s:/ /www.ncbi.nlm.nih.gov/pmc/articles/PMC2657249/. The Unmet Need in Refractory Seizures… ~1M Suffer from uncontrolled, refractory seizures Epilepsy patients1 Seizures Account for EMERGENCY DEPARTMENT visits annually6 of patients with refractory seizures will not interact with the historically available Treatment2,3,4,5 ~85% ~1M

Advancing medicines. Solving problems. Improving lives. 29 Current Treatments are Either Rectal or Intra Nasal Options
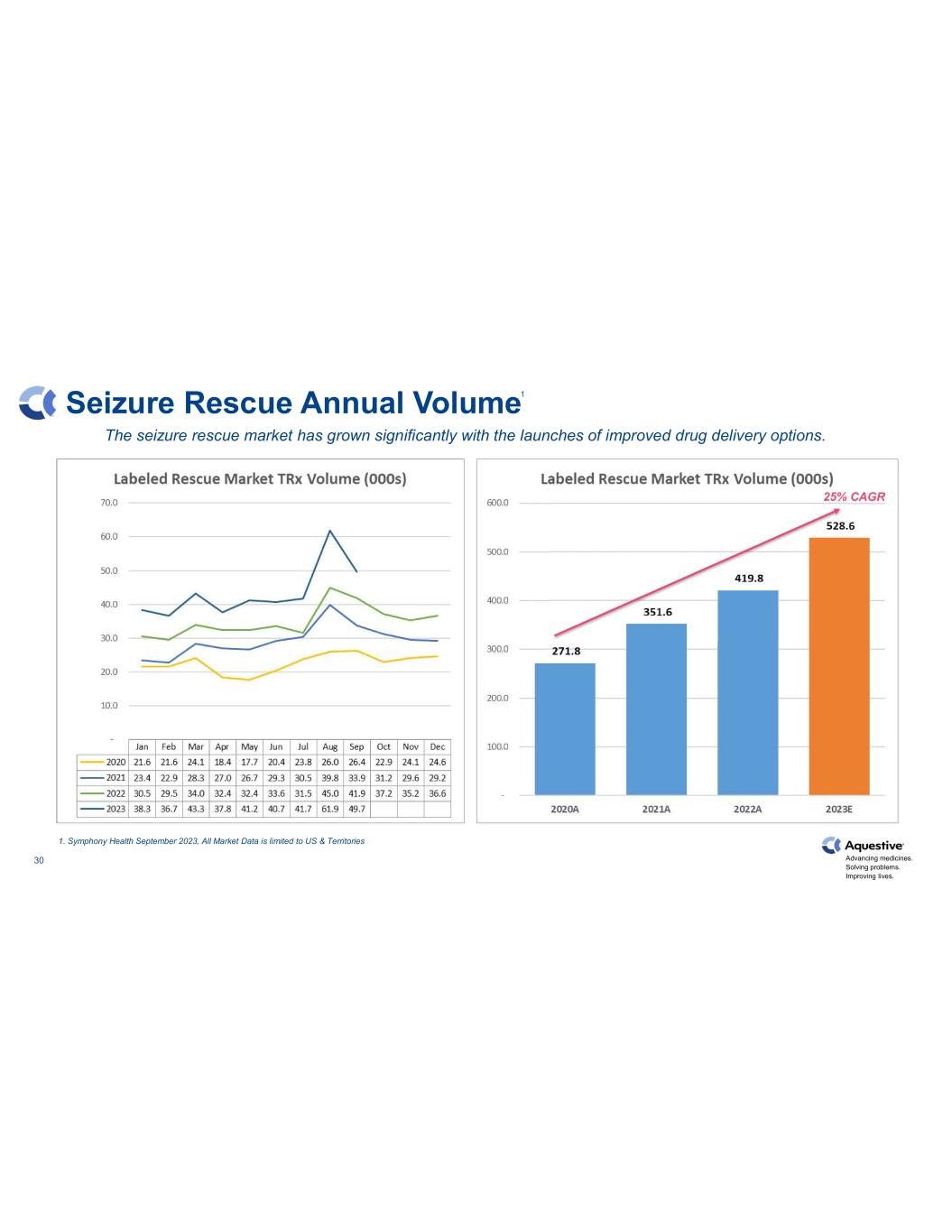
Advancing medicines. Solving problems. Improving lives. 30 Seizure Rescue Annual Volume The seizure rescue market has grown significantly with the launches of improved drug delivery options. 1. Symphony Health September 2023, All Market Data is limited to US & Territories 25% CAGR 1
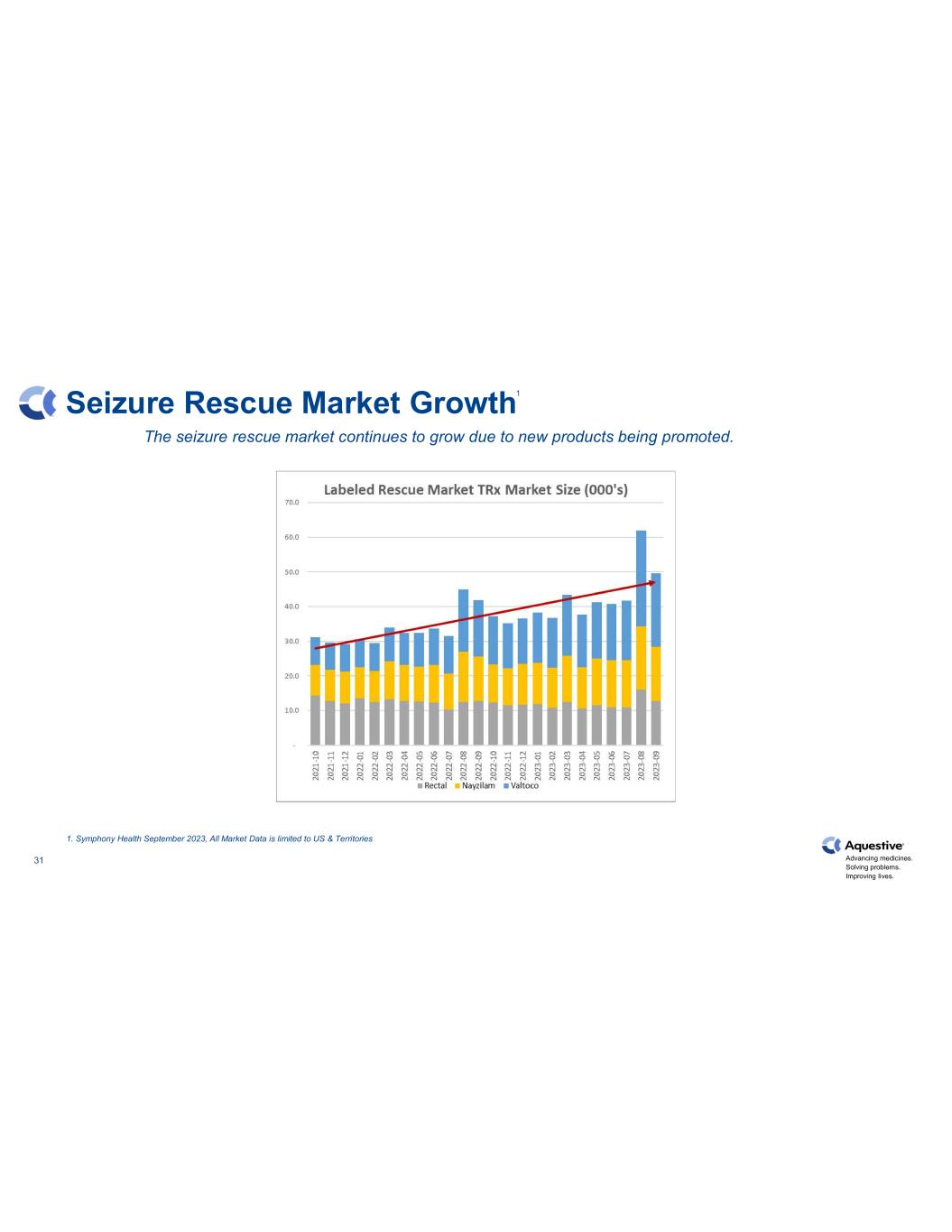
Advancing medicines. Solving problems. Improving lives. 31 Seizure Rescue Market Growth The seizure rescue market continues to grow due to new products being promoted. 1. Symphony Health September 2023, All Market Data is limited to US & Territories 1
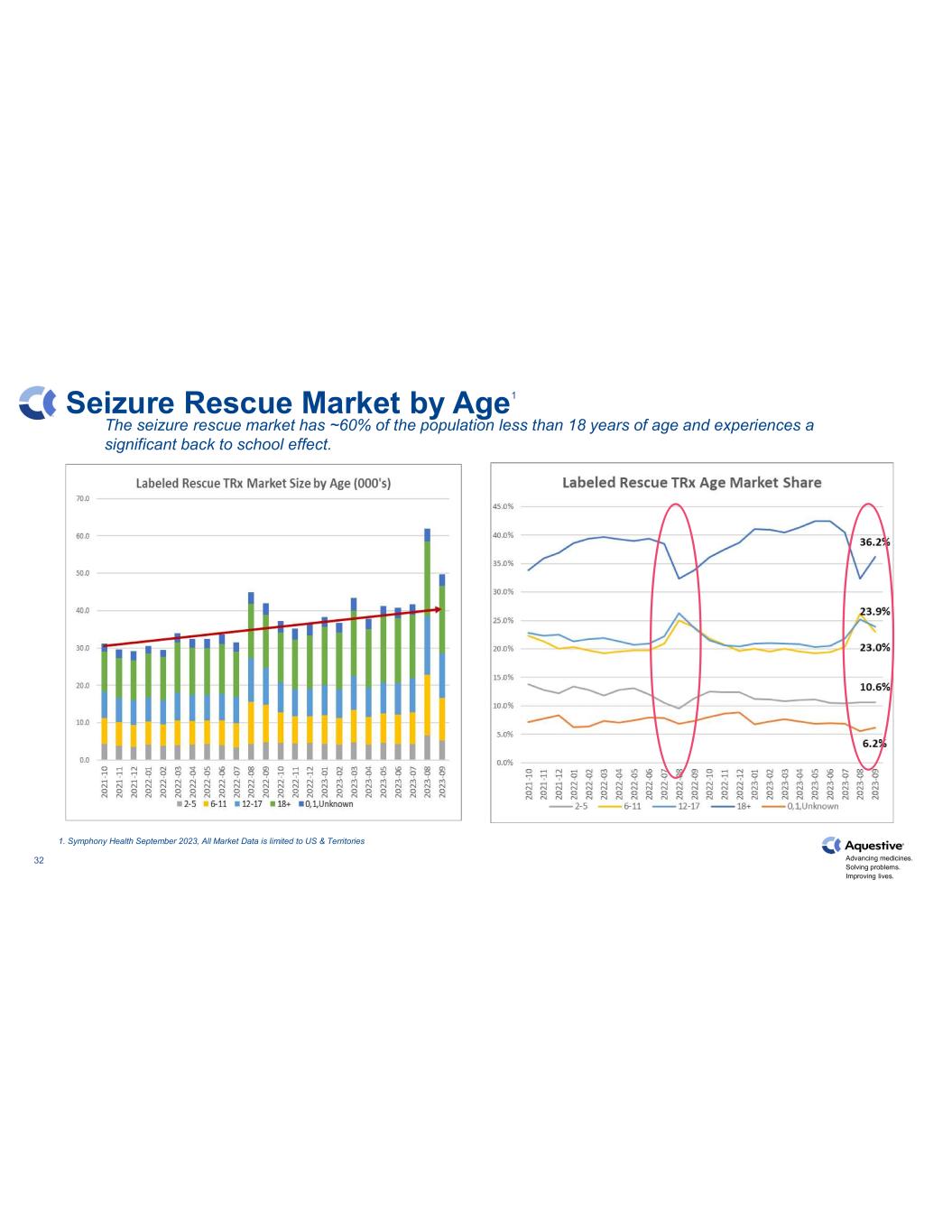
Advancing medicines. Solving problems. Improving lives. 32 Seizure Rescue Market by Age The seizure rescue market has ~60% of the population less than 18 years of age and experiences a significant back to school effect. 1. Symphony Health September 2023, All Market Data is limited to US & Territories 1

Advancing medicines. Solving problems. Improving lives. 33 Strong Patient Preference – What Patients Want % Indicating 1: Not at all Important 2 3: Somewhat Important 4 5: Highly Important Top 2 Box Ability to have the repetitive seizure medicine with me at all times 3% 7% 20% 26% 45% 71% Ability to take the medicine as quickly as I possibly can when I need to 3% 4% 14% 28% 51% 79% Ability to take the medicine in a way that is simple for me 2% 2% 13% 23% 60% 83% Ability to take the medicine no matter where I am and what I am doing 3% 2% 14% 23% 58% 81% Ability for me to take the medicine myself, versus someone else having to give it to me 5% 3% 22% 28% 43% 71% 1. Aquestive Therapeutics sponsored preference study (N=101 Patients), data on file. 1
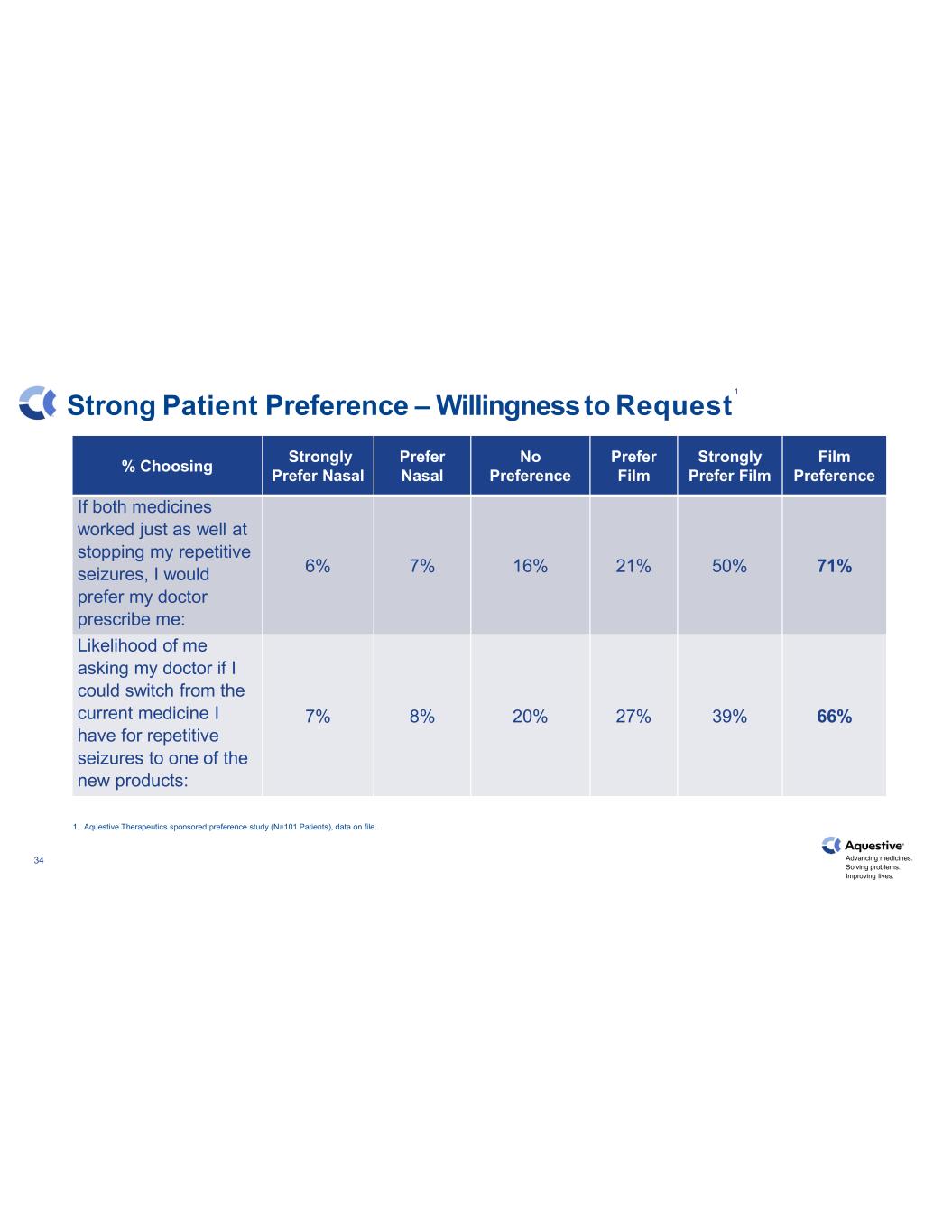
Advancing medicines. Solving problems. Improving lives. 34 Strong Patient Preference – Willingnessto Request % Choosing Strongly Prefer Nasal Prefer Nasal No Preference Prefer Film Strongly Prefer Film Film Preference If both medicines worked just as well at stopping my repetitive seizures, I would prefer my doctor prescribe me: 6% 7% 16% 21% 50% 71% Likelihood of me asking my doctor if I could switch from the current medicine I have for repetitive seizures to one of the new products: 7% 8% 20% 27% 39% 66% 1. Aquestive Therapeutics sponsored preference study (N=101 Patients), data on file. 1
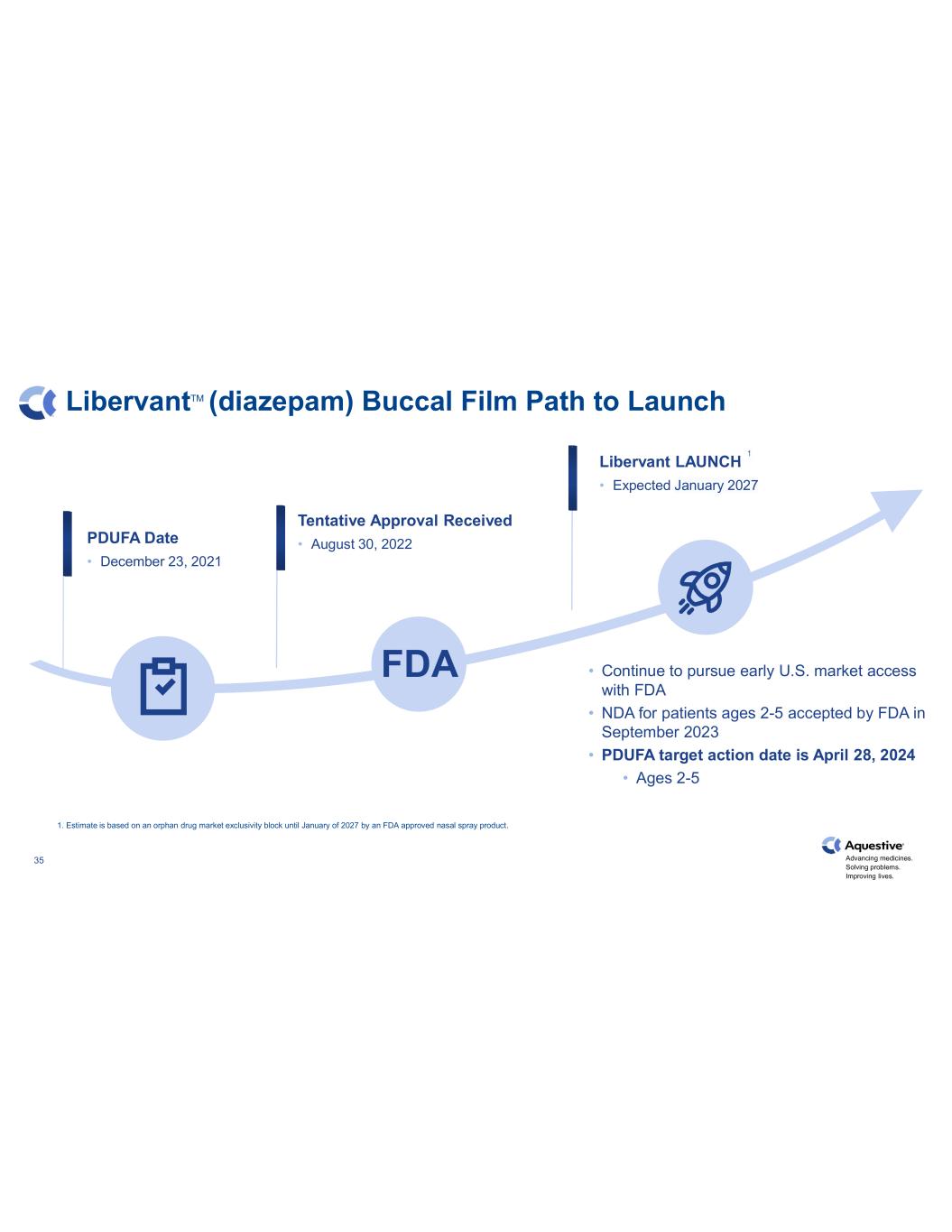
Advancing medicines. Solving problems. Improving lives. 35 LibervantTM (diazepam) Buccal Film Path to Launch Libervant LAUNCH • Expected January 2027 PDUFA Date • December 23, 2021 Tentative Approval Received • August 30, 2022 • Continue to pursue early U.S. market access with FDA • NDA for patients ages 2-5 accepted by FDA in September 2023 • PDUFA target action date is April 28, 2024 • Ages 2-5 1. Estimate is based on an orphan drug market exclusivity block until January of 2027 by an FDA approved nasal spray product. FDA

Advancing medicines. Solving problems. Improving lives. Existing Collaborations 36
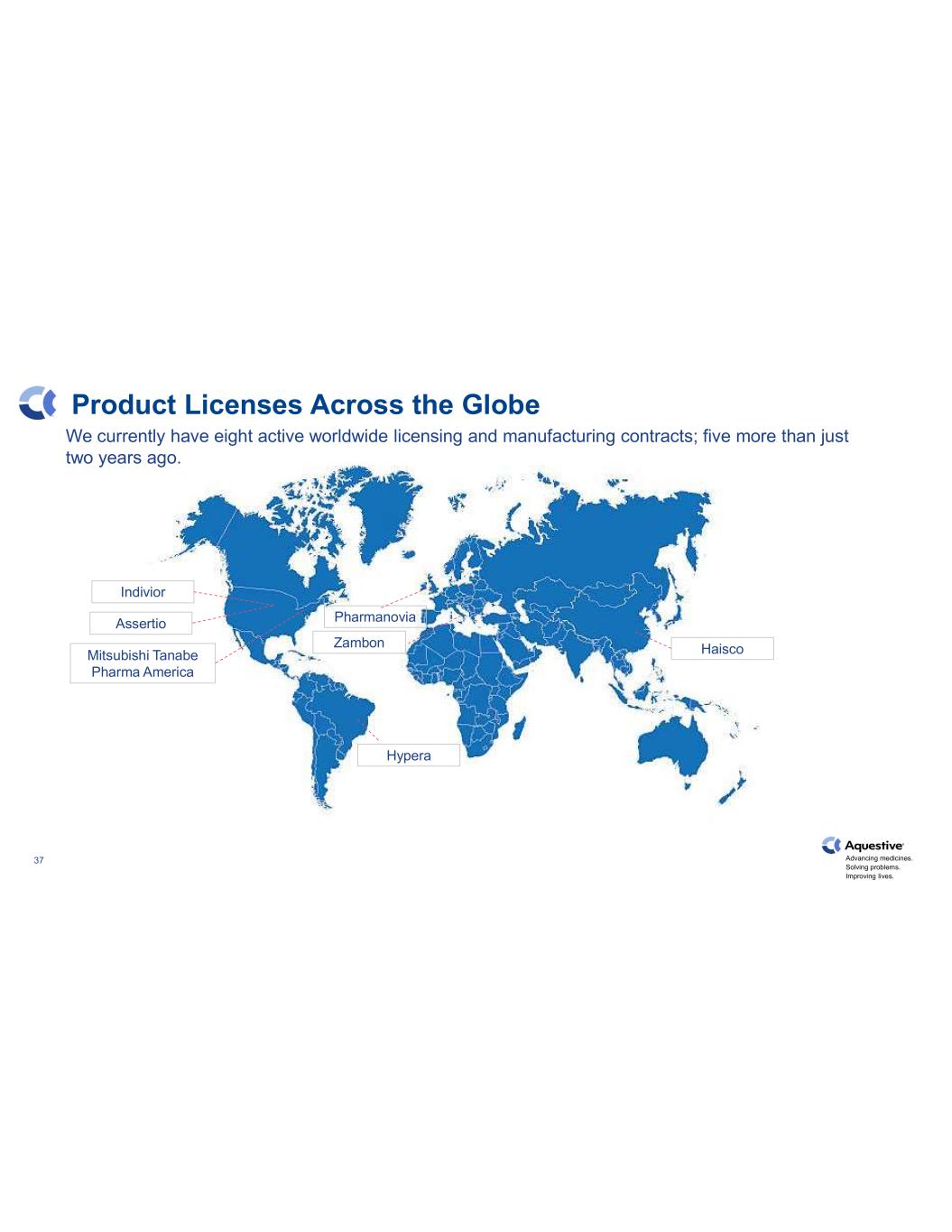
Advancing medicines. Solving problems. Improving lives. 37 Product Licenses Across the Globe Indivior Assertio Hypera Haisco Pharmanovia Zambon Mitsubishi Tanabe Pharma America We currently have eight active worldwide licensing and manufacturing contracts; five more than just two years ago.
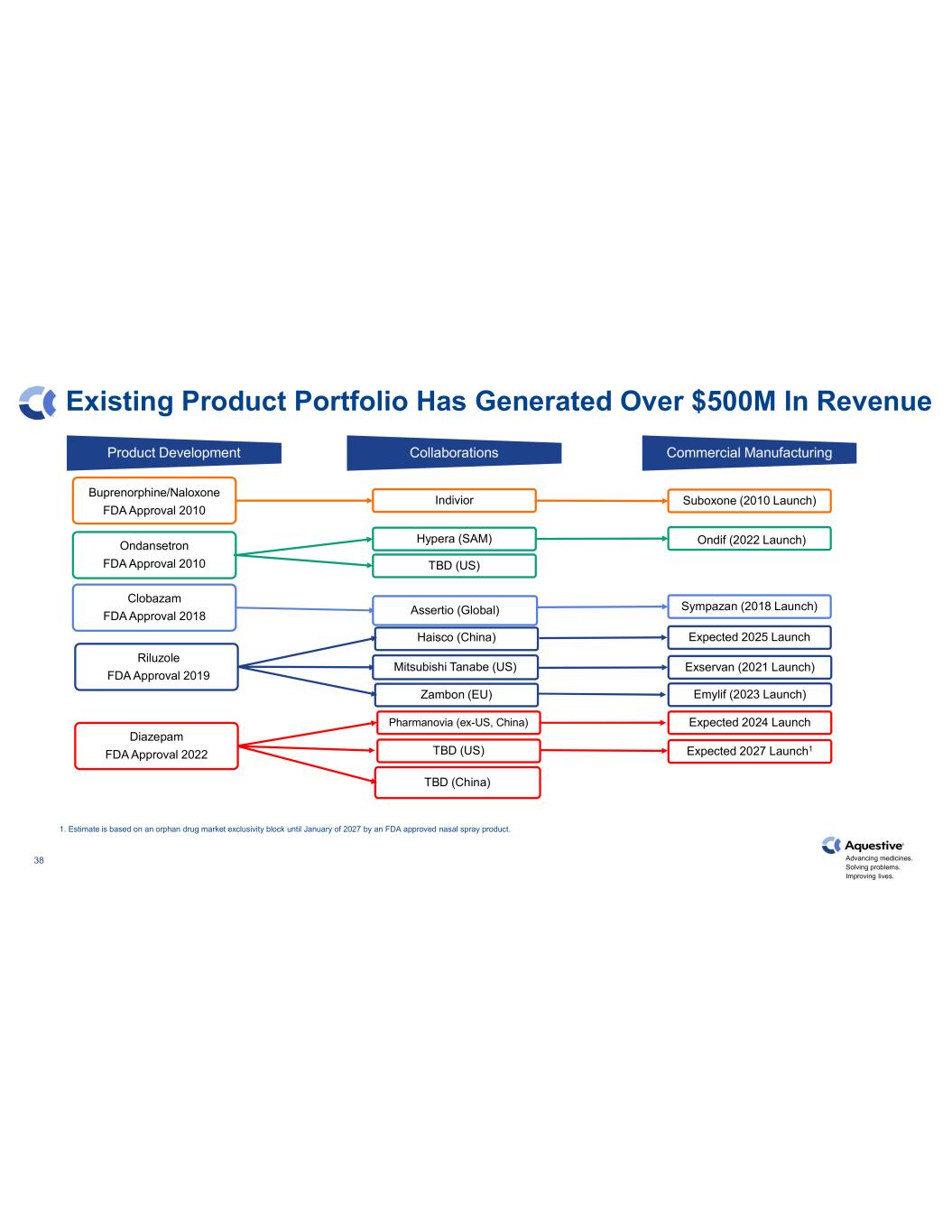
Advancing medicines. Solving problems. Improving lives. 38 Existing Product Portfolio Has Generated Over $500M In Revenue Pharmanovia (ex-US, China) TBD (US) Riluzole FDA Approval 2019 Diazepam FDA Approval 2022 Haisco (China) Zambon (EU) TBD (China) Clobazam FDA Approval 2018 Ondansetron FDA Approval 2010 Buprenorphine/Naloxone FDA Approval 2010 Mitsubishi Tanabe (US) Expected 2025 Launch Indivior Suboxone (2010 Launch) Hypera (SAM) TBD (US) Ondif (2022 Launch) Assertio (Global) Sympazan (2018 Launch) Product Development Collaborations Commercial Manufacturing 1. Estimate is based on an orphan drug market exclusivity block until January of 2027 by an FDA approved nasal spray product. Exservan (2021 Launch) Emylif (2023 Launch) Expected 2024 Launch Expected 2027 Launch1
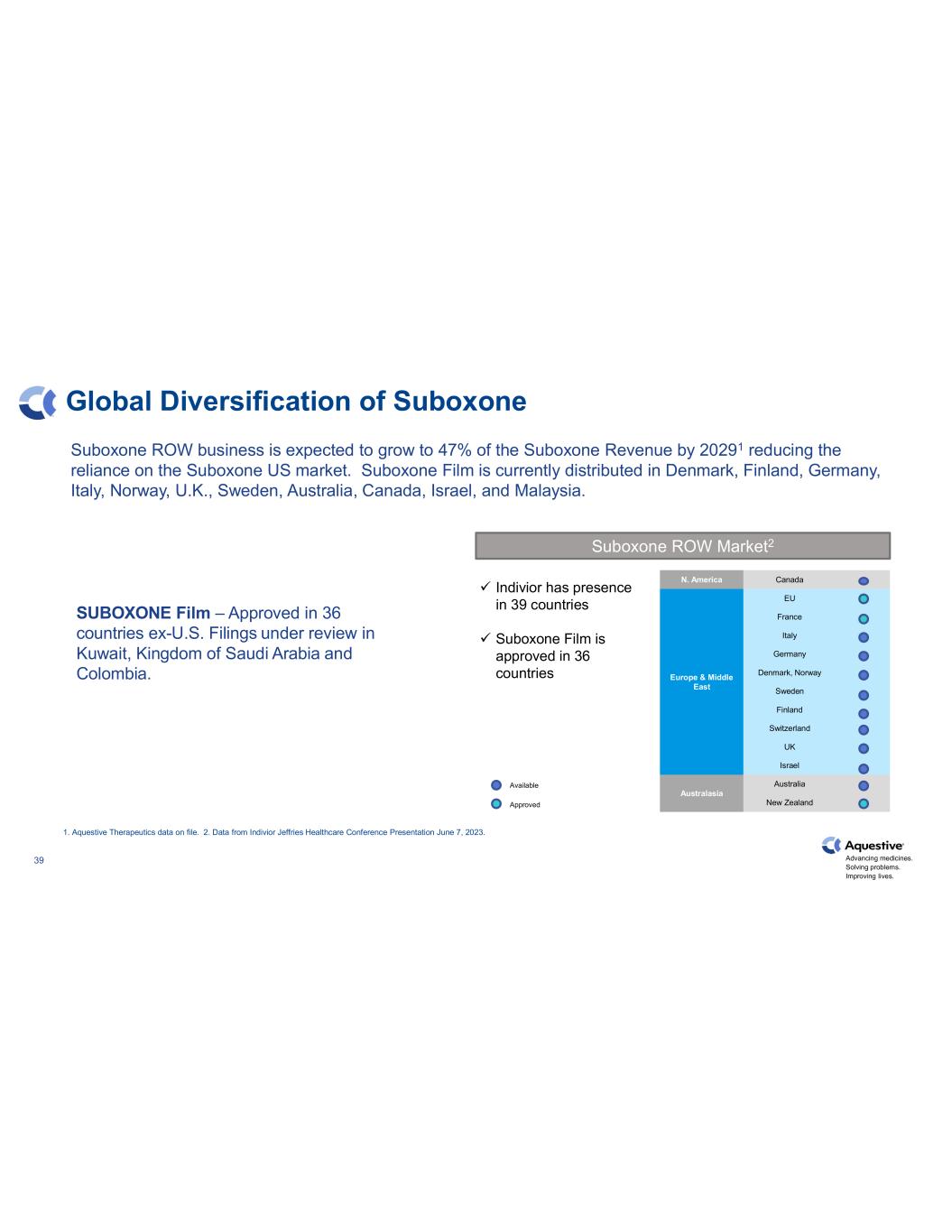
Advancing medicines. Solving problems. Improving lives. 39 Global Diversification of Suboxone 1. Aquestive Therapeutics data on file. 2. Data from Indivior Jeffries Healthcare Conference Presentation June 7, 2023. Suboxone ROW Market2 N. America Canada Europe & Middle East EU France Italy Germany Denmark, Norway Sweden Finland Switzerland UK Israel Australasia Australia New Zealand Available Approved Indivior has presence in 39 countries Suboxone Film is approved in 36 countries Suboxone ROW business is expected to grow to 47% of the Suboxone Revenue by 20291 reducing the reliance on the Suboxone US market. Suboxone Film is currently distributed in Denmark, Finland, Germany, Italy, Norway, U.K., Sweden, Australia, Canada, Israel, and Malaysia. SUBOXONE Film – Approved in 36 countries ex-U.S. Filings under review in Kuwait, Kingdom of Saudi Arabia and Colombia.
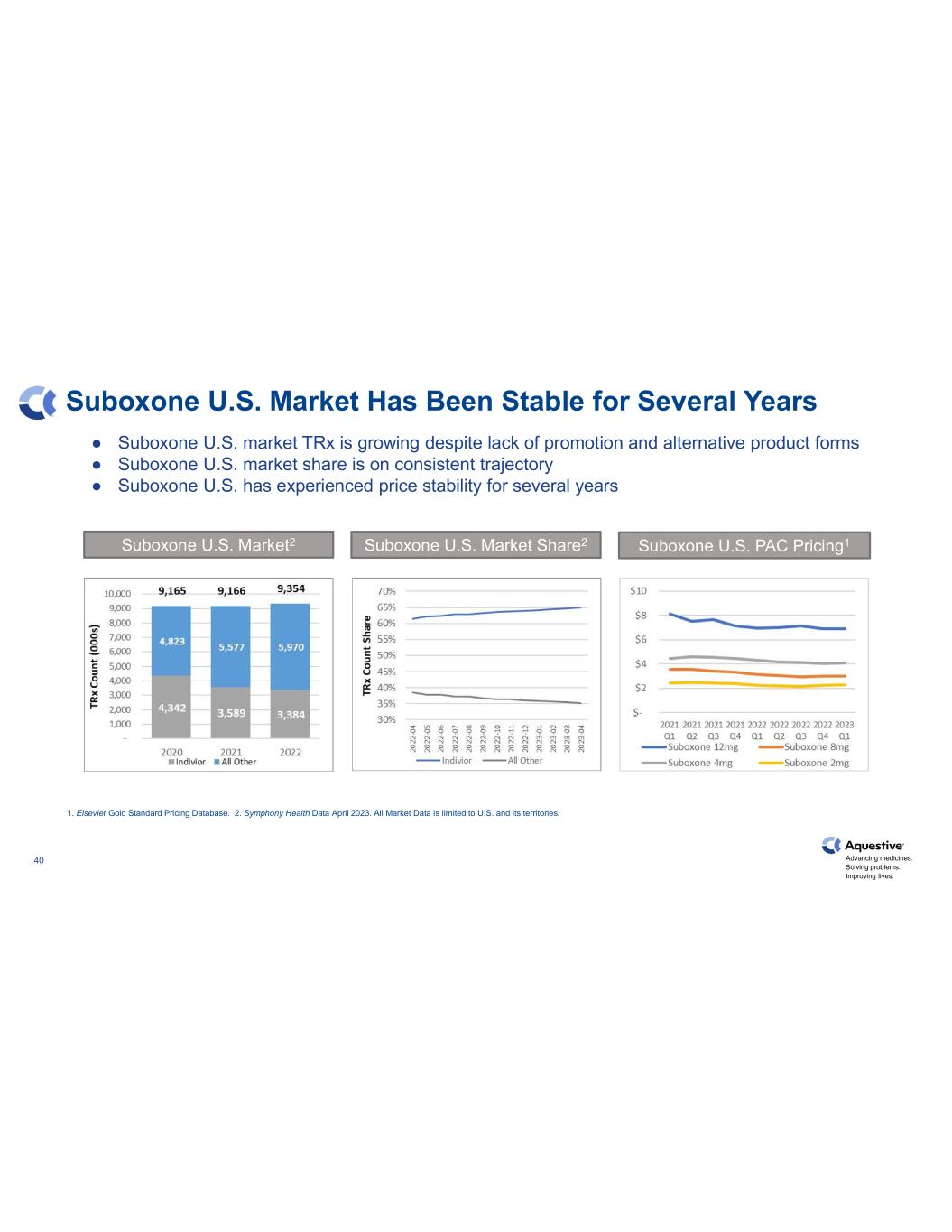
Advancing medicines. Solving problems. Improving lives. 40 Suboxone U.S. Market Has Been Stable for Several Years Suboxone U.S. market TRx is growing despite lack of promotion and alternative product forms Suboxone U.S. market share is on consistent trajectory Suboxone U.S. has experienced price stability for several years 1. Elsevier Gold Standard Pricing Database. 2. Symphony Health Data April 2023. All Market Data is limited to U.S. and its territories. Suboxone U.S. Market2 Suboxone U.S. Market Share2 Suboxone U.S. PAC Pricing1

Advancing medicines. Solving problems. Improving lives. Updated Guidance 41
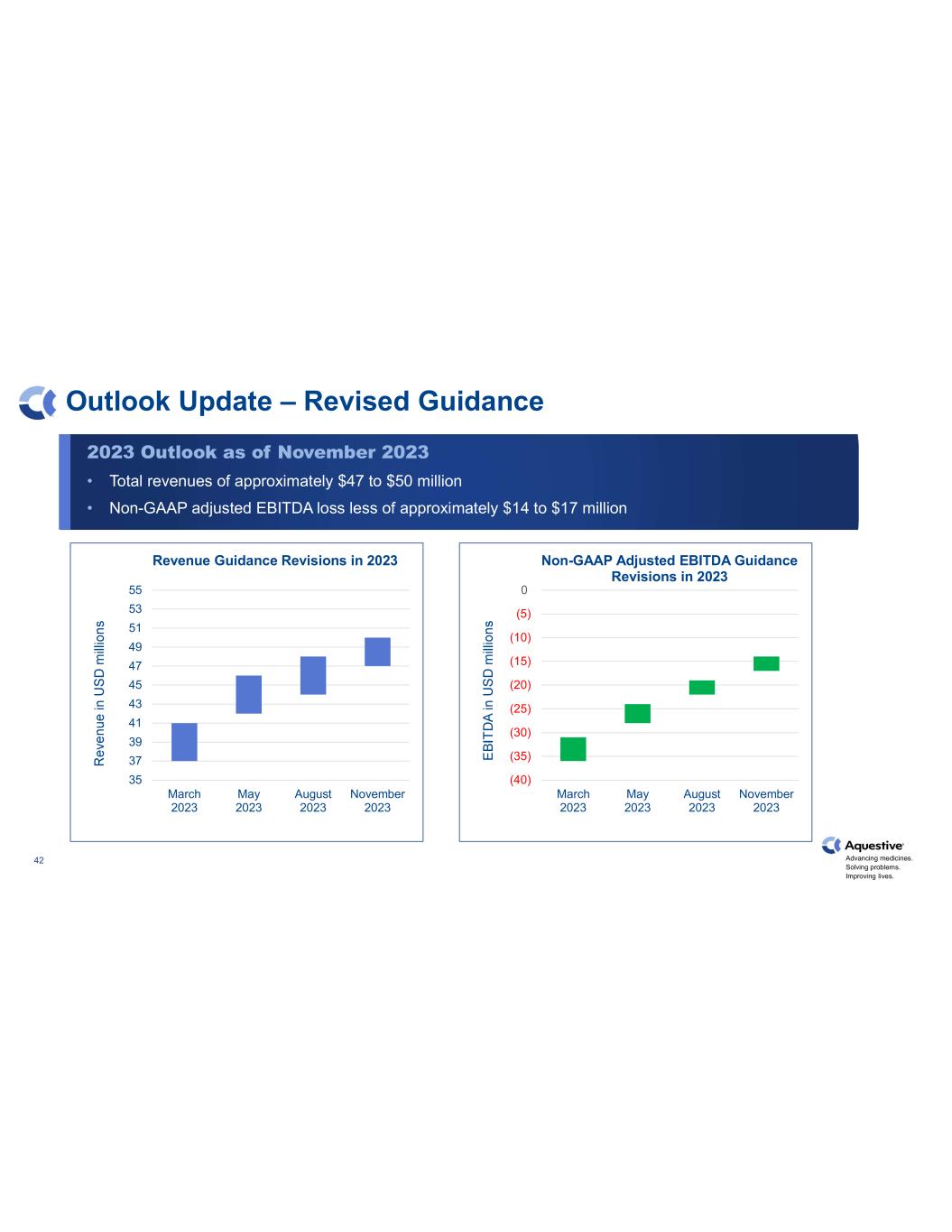
Advancing medicines. Solving problems. Improving lives. 42 Outlook Update – Revised Guidance 35 37 39 41 43 45 47 49 51 53 55 March 2023 May 2023 August 2023 November 2023 Revenue Guidance Revisions in 2023 (40) (35) (30) (25) (20) (15) (10) (5) 0 March 2023 May 2023 August 2023 November 2023 Non-GAAP Adjusted EBITDA Guidance Revisions in 2023 2023 Outlook as of November 2023 • Total revenues of approximately $47 to $50 million • Non-GAAP adjusted EBITDA loss less of approximately $14 to $17 million

Advancing medicines. Solving problems. Improving lives. Thank You 43