Filed Pursuant to Rule
424(b)(3)
Registration No. 333-273308

Marizyme,
Inc.
Up
to 915,071,257 Shares of Common Stock
This
prospectus relates to the offer and resale of up to 915,071,257 shares of common stock, par value $0.001 per share (“common
stock”), of Marizyme, Inc. that may be sold from time to time by the selling stockholders named in this prospectus, which consist
of:
| |
● |
13,971,324
shares of outstanding common stock held by existing
stockholders; |
| |
|
|
| |
● |
221,939,338 shares of common
stock issuable upon the conversion of the Company’s outstanding 10% Secured Convertible
Promissory Notes (the
“Convertible Notes”), assuming that all convertible debts and other liabilities
under the Convertible Notes are converted into shares of common stock, without
regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
380,986,336 shares of common
stock issuable upon the exercise of the Company’s outstanding Class C Common Stock
Purchase Warrants (the
“Class C Warrants”), without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
66,159,434
shares
of common stock issuable upon the conversion of the Company’s outstanding 15% Original
Issue Discount Unsecured Subordinated Convertible Promissory Notes (the “OID Convertible
Notes”), assuming that the OID Convertible Notes are held until maturity and that all
convertible debts and other liabilities under the OID Convertible Notes are converted
into shares of common stock, without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
84,546,202
shares
of common stock issuable upon the exercise of the Company’s outstanding Class E Common
Stock Purchase Warrants (the “Class E Warrants”), without regard to any applicable
limitations or restrictions; |
| |
|
|
| |
● |
80,796,202
shares
of common stock issuable upon the exercise of the Company’s outstanding Class F Common
Stock Purchase Warrants (the “Class F Warrants”), without regard to any
applicable limitations or restrictions; and |
| |
|
|
| |
● |
66,672,421 shares
of common stock issuable upon the exercise of the Company’s Placement Agent Warrants
(collectively, the “Placement Agent Warrants”), without regard to any
applicable limitations or restrictions. |
The
holders of the outstanding shares of common stock, the Convertible Notes, the Class C Warrants, the OID Convertible Notes, the Class
E Warrants, the Class F Warrants, and the Placement Agent Warrants that are identified in the “Selling Stockholders” section of this prospectus are each referred
to herein as a “selling stockholder” and collectively as the “selling stockholders”.
This
prospectus also covers any additional shares of common stock that may become issuable upon any anti-dilution adjustment pursuant to the
terms of the Convertible Notes, the Class C Warrants, the OID Convertible Notes, the Class E Warrants, the Class F Warrants, or the Placement
Agent Warrants issued to the selling stockholders by reason of stock splits, stock dividends, and other events described therein.
We
will not receive any proceeds from the resale of shares of common stock by the selling stockholders. Assuming the full exercise of the
Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants for cash, we will receive gross proceeds
of approximately $70.4 million.
Currently,
a limited public market exists for our common stock. Our common stock is quoted for trading on the OTCQB tier of OTC Markets Group, Inc.
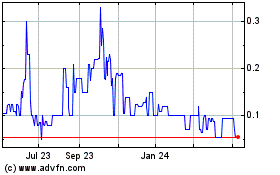
(“OTCQB”) under the symbol “MRZM”. On October 16, 2023, the last reported sale price of our common stock
on the OTCQB was $0.23 per share. We have applied to list our common stock under the symbol “MRZM” on the Nasdaq Capital
Market tier operated by The Nasdaq Stock Market LLC (“Nasdaq”). There can be no guarantee that we will successfully list
our common stock on the Nasdaq Capital Market. The registration of the selling stockholders’ resale of the Company’s common
stock as described in this prospectus is not conditioned upon our successful listing on the Nasdaq Capital Market.
Unlike
an initial public offering, the resale by the selling stockholders is not being underwritten by any investment bank. The selling stockholders
may or may not elect to sell, either prior to, in connection with, or at any point after a listing of our common stock on the Nasdaq
Capital Market, all or any portion of their shares of common stock covered by this prospectus. The selling stockholders may offer and
sell the common stock being offered by this prospectus from time to time in public or private transactions, or both. These sales will
occur at fixed prices, at market prices prevailing at the time of sale, at prices related to prevailing market prices, or at negotiated
prices. The selling stockholders may sell shares to or through underwriters, broker-dealers or agents, who may receive compensation in
the form of discounts, concessions or commissions from the selling stockholders, the purchasers of the shares, or both. Any participating
broker-dealers and any selling stockholders who are affiliates of broker-dealers may be deemed to be “underwriters” within
the meaning of the Securities Act of 1933, as amended (the “Securities Act”), and any commissions or discounts given to any
such broker-dealer or affiliates of a broker-dealer may be regarded as underwriting commissions or discounts under the Securities Act.
The selling stockholders have informed us that they do not have any agreement or understanding with any person
to distribute their common stock. See “Plan of Distribution” for a more complete description of the ways in which
the shares may be sold.
This offering will terminate on the earlier
of the date when all of the securities registered for offer and resale hereunder have been sold pursuant to this prospectus or one
year from the date the registration statement of which this
prospectus forms a part is declared effective, unless we terminate it earlier in accordance with the requirements of certain agreements with the selling stockholders. See “Plan of Distribution”.
Investing
in our shares of common stock involves a high degree of risk. See “Risk Factors” beginning on page 12 for a
discussion of information that should be considered in connection with an investment in our shares of common stock.
We
are a “smaller reporting company” under applicable federal securities laws and, as such, we have elected to comply with certain
reduced public company reporting requirements for this prospectus and future filings.
Neither
the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined
if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The
date of this prospectus is October 17, 2023
TABLE
OF CONTENTS
This
prospectus describes the general manner in which the selling stockholders may offer from time to time up to 915,071,257 shares
of common stock. You should rely only on the information that we have provided or incorporated by reference in this prospectus, any applicable
prospectus supplement and any related free writing prospectus that we may authorize to be provided to you. We have not authorized anyone
to provide you with different information. No dealer, salesperson or other person is authorized to give any information or to represent
anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus that we may authorize
to be provided to you. You must not rely on any unauthorized information or representation. This prospectus is an offer to sell only
the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that
the information in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate only as of
the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the
document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any
related free writing prospectus, or any sale of a security.
If
necessary, the specific manner in which the shares of common stock may be offered and sold will be described in a supplement to this
prospectus, which supplement may also add, update or change any of the information contained in this prospectus. To the extent there
is a conflict between the information contained in this prospectus and any prospectus supplement, you should rely on the information
in such prospectus supplement, provided that if any statement in one of these documents is inconsistent with a statement in another document
having a later date — for example, a document incorporated by reference in this prospectus or any prospectus supplement —
the statement in the document having the later date modifies or supersedes the earlier statement.
Neither
the delivery of this prospectus nor any distribution of shares of common stock pursuant to this prospectus shall, under any circumstances,
create any implication that there has been no change in the information set forth or incorporated by reference into this prospectus or
in our affairs since the date of this prospectus. Our business, financial condition, results of operations and prospects may have changed
since such date.
TRADEMARKS,
TRADE NAMES AND SERVICE MARKS
We
use various trademarks, trade names and service marks in our business. For convenience or readability, we may not include the ℠,
® or ™ symbols, but such omission is not meant to indicate that we would not protect our intellectual
property rights to the fullest extent allowed by law. Any other trademarks, trade names or service marks referred to in this prospectus
are the property of their respective owners.
INDUSTRY
AND MARKET DATA
We
are responsible for the information contained in this prospectus. This prospectus includes industry and market data that we obtained
from periodic industry publications, third-party studies and surveys, filings of public companies in our industry and internal company
surveys. These sources generally state that the information they provide has been obtained from sources believed to be reliable, but
that the accuracy and completeness of the information are not guaranteed. The forecasts and projections are based on historical market
data, and there is no assurance that any of the forecasts or projected amounts will be achieved. Industry and market data could be wrong
because of the method by which sources obtained their data and because information cannot always be verified with complete certainty
due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations
and uncertainties. The market and industry data used in this prospectus involve risks and uncertainties that are subject to change based
on various factors, including the COVID-19 pandemic and those discussed in the section titled “Risk Factors.” These
and other factors could cause results to differ materially from those expressed in, or implied by, the estimates made by independent
parties and by us. Furthermore, we cannot assure you that a third party using different methods to assemble, analyze or compute industry
and market data would obtain the same results.
GLOSSARY
OF LIFE SCIENCE TERMS
The
following is a glossary of certain life science terms that are used in this prospectus.
| 510(k) Application |
|
A 510(k) application is a premarket submission made to FDA to demonstrate that the device to be marketed is as safe and effective as,
that is, substantially equivalent to, a legally marketed device. |
| |
|
|
| ACA |
|
The
Patient Protection and Affordable Care Act. |
| |
|
|
| Autologous |
|
In transplantation, referring
to transfer of an organ or other tissue from one location to another in the same person; or to blood or blood components that the
donor has previously donated and receives at a later time, usually perioperatively (i.e., around the time of surgery). |
| |
|
|
| Biofilm |
|
A collective of one or
more types of microorganisms that can grow on many different surfaces. |
| |
|
|
| Biomarker |
|
A broad subcategory of
medical signs – that is, objective indications of medical state observed from outside the patient – which can be measured
accurately and reproducibly. |
| |
|
|
| Cardiac Surgery |
|
A surgery on the heart
or great vessels performed by cardiac surgeons. |
| |
|
|
| CABG |
|
Coronary artery bypass
surgery, or CABG, also known as coronary artery bypass graft surgery, and colloquially heart bypass or bypass surgery, is a surgical
procedure to restore normal blood flow to an obstructed coronary artery. |
| |
|
|
| CE Marking |
|
The CE marking indicates
that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin. |
| |
|
|
| cGCP |
|
Current Good Clinical Practices,
the standard required by the FDA for conducting clinical trials. |
| |
|
|
| cGMP |
|
Current Good Manufacturing
Processes, which are the standards mandated by the FDA to assure the proper design, monitoring and control of manufacturing processes
and facilities in connection with the production of pharmaceuticals. |
| |
|
|
| CKD |
|
Chronic kidney disease,
or CKD, means your kidneys are damaged and cannot filter blood the way they should. |
| |
|
|
| De Novo Process |
|
An FDA application process
for medical device marketing rights that was instituted by the FDA to address novel medical devices of low to moderate risk that
do not have a valid predicate device (that is, a similar device that has already been approved by the FDA for marketing in the U.S.). |
| |
|
|
| DuraGraft Registry |
|
Marizyme’s European
patient registry of subjects who have undergone CABG surgery utilizing DuraGraft. |
| |
|
|
| EDI |
|
Endothelial damage inhibitor. |
| |
|
|
| eGFR |
|
Estimated glomerular filtration
rate, a key measure of kidney function health and/or stage of kidney disease. |
| |
|
|
| Enzyme |
|
A type of protein that
regulates nearly all chemical reactions in cells. |
| |
|
|
| Fat Grafting |
|
A surgical process by which
fat is transferred from one area of the body to another area. |
| |
|
|
| FDA |
|
The U.S. Food and Drug
Administration. |
| |
|
|
| HHS |
|
The U.S. Department of
Health and Human Services. |
| |
|
|
| HIPAA |
|
The Health Insurance Portability
and Accountability Act of 1996, as amended. |
| |
|
|
| IRB |
|
Institutional Review Board,
tasked with reviewing clinical trial protocols and informed consent information for patients in clinical trials for the FDA. |
| |
|
|
| Ischemic Injury |
|
Injury caused by diminished
or absent blood flow. |
| |
|
|
| Organ |
|
Structure, such as the
heart, made up of different types of tissues that all work together. |
| |
|
|
| Registry |
|
A place to store detailed
information about people with a specific disease or condition, who provide the information on a voluntary basis. |
| |
|
|
| STS Registry |
|
The Society of Thoracis
Surgeons national database which collects data from more than 90% of U.S. based cardiac surgery centers. |
| |
|
|
| Vein |
|
Blood vessel that carries
blood back to the heart and has one-way valves that keep blood moving toward the heart. |
PROSPECTUS
SUMMARY
This
summary highlights selected information contained elsewhere in this prospectus. This summary is not complete and does not contain all
of the information that you should consider before deciding whether to invest in our securities. You should carefully read the entire
prospectus, including the risks associated with an investment in our company discussed in the “Risk Factors” section of this
prospectus, before making an investment decision. Some of the statements in this prospectus are forward-looking statements. See “Cautionary
Note Regarding Forward-Looking Statements.”
In
this prospectus, “we,” “us,” “our,” “our company,” “the Company,” “Marizyme”
and similar references refer to Marizyme, Inc., a Nevada corporation (“Marizyme, Inc.”), and its wholly-owned subsidiaries,
Somahlution, Inc., a Delaware corporation (“Somahlution, Inc.”), Somaceutica, Inc., a Florida corporation (“Somaceutica,
Inc.”), Marizyme Sciences, Inc., a Florida corporation (“Marizyme Sciences”), and My Health Logic Inc., a corporation
incorporated pursuant to the laws of the Province of Alberta, Canada (“My Health Logic”), and (ii) the term “common
stock” refers to the common stock, par value $0.001 per share, of Marizyme, Inc. The financial information included herein is presented
in United States dollars, or U.S. Dollars, the functional currency of our company.
Our
Company
Overview
We
are focused on commercializing or developing three medical technologies and related products – DuraGraft™,
MATLOC® and MAR-FG-001. DuraGraft is a first-in-class De Novo granted and CE marked intra-operative
vascular graft storage and flushing solution used during CABG surgeries. MATLOC is a point-of-care, lab-on-chip digital screening and
diagnostic device platform, initially being developed for quantitative chronic kidney disease, or CKD, assessment. MAR-FG-001 is a technology
in development for use in fat grafting procedures formulated as a tumescent solution base for protecting adipose tissue during adipose
tissue harvesting and storage. DuraGraft, MATLOC and MAR-FG-001 are expected to serve an unmet significant market need in several areas,
including, cardiac surgery, CKD assessment, and fat grafting.
Since October
2023, DuraGraft has been authorized for marketing by the U.S. Food and Drug Administration, or FDA, for use as an intra-operative
vascular conduit storage and flushing solution used during CABG surgeries in the United States, subject to applicable risks,
mitigation requirements, and control provisions. Since August 2014, DuraGraft has also had the CE marking required to be
sold in the EEA, and DuraGraft has therefore been assessed as meeting the EEA safety, health, and environmental protection
requirements.
We
intend to continue the advancement of our other
two primary product technologies, MATLOC and MAR-FG-001. Our MATLOC CKD point-of-care device is being developed toward a functional prototype,
mainly through the development of its lab-on-chip technology under a Sponsored Research Agreement, or SRA. An SRA is an agreement (which
may be classified as a grant, contract or cooperative agreement) under which one party (the “Sponsor”) provides funding to
a second party to support the performance of a specified research project or related activity. The Sponsor may be a foundation, government
agency, for-profit entity, research institute, or another university. We will also continue to ready MAR-FG-001 for viability for development
and manufacturing. We intend to fully develop and market MAR-FG-001 in the U.S. for fat grafting for procedures such as plastic and cosmetic
surgery.
In the near term, we expect to generate revenue
primarily from the sale of DuraGraft through the expansion of our international marketing efforts by our distribution partners in Europe
and in other countries that accept CE marking. We intend to commercialize DuraGraft in the U.S. primarily through
hospital integrated networks using our own direct sales force. We anticipate that once we commence marketing and sales operations
for DuraGraft in the U.S., we will be able to generate sustainable revenue growth, accelerate the development of a functional MATLOC
device prototype, and expedite the development of MAR-FG-001 into medical products.
Our
Corporate History and Structure
Marizyme,
Inc. is a Nevada corporation that was incorporated on March 20, 2007. From 2007 to early 2018, we operated under a number of different
names with different management teams and in different industries. We changed our name to Marizyme, Inc. on March 21, 2018, to reflect
our new life science focus, and at that time we also changed our common stock ticker symbol to “MRZM.”
In
September 2018, we acquired assets relating to Krillase®, a technology intended for the treatment of certain harmful
blockages, plaque and biofilms, from ACB Holding AB, Reg. No. 559119-5762, a company incorporated and organized under the laws of Sweden
(“ACB Holding”). In July 2020, we acquired from Somahlution, Inc., a Delaware corporation (“Somahlution, Inc.”),
Somahlution, LLC, a Delaware limited liability company (“Somahlution, LLC”), and Somaceutica LLC, a Delaware limited
liability company (“Somaceutica LLC”), which we refer to together as “Somahlution,” all of the assets of Somahlution,
including our DuraGraft-related assets, as well as the outstanding capital stock of Somahlution, Inc., which we refer to as the “Somahlution
Assets.” In December 2021, we acquired My Health Logic and assets relating to MATLOC®, a technology intended
for the screening of biomarkers relating to CKD, from Health Logic Interactive Inc. (“HLII”). In connection with the My Health
Logic acquisition, David Barthel, former chief executive officer of HLII and My Health Logic, became our Chief Executive Officer and
a member of our board of directors; George Kovalyov, previously the chief operating officer and a director of HLII, became our Chief
Financial Officer and Treasurer; and Harrison Ross, previously the Chief Financial Officer of HLII, became our Vice President of Finance.
The
Company previously planned to develop and commercialize FDA-approved products based on the Krillase assets. We suspended these plans
due to our determination to prioritize the completion of regulatory processes to obtain FDA authorization for the commercialization
of DuraGraft in the United States and the development of a functional MATLOC device prototype and MAR-FG-001-based viable products. We
intend to maintain the Krillase assets for potential future development and commercialization or disposition. Any determination as to
these matters would be based on a number of factors. See “Management’s Discussion and Analysis of Financial Condition
and Results of Operations – Principal Factors Affecting Our Financial Performance” for a summary of factors that we may
consider in this respect.
There
is no assurance that any of our intellectual property assets will ever be developed and fully commercialized and generate significant
revenues or will ever attract significant interest from potential buyers or investors. See “Risk Factors – Risks Related
to Our Business – We may not be able to monetize intangible assets, which may result in the need to record an impairment charge.”
Recent Developments
For a description of certain
recent developments, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations –
Liquidity and Capital Resources – Recent Developments”.
Our Products and Technologies
DuraGraft™
Through our acquisition of the Somahlution Assets
in July 2020, we acquired key intellectual assets based on a patent-protected cytoprotective platform technology designed to reduce ischemic
injury to organs and tissues in grafting and transplantation surgeries. These assets include DuraGraft, a first-in-class, De Novo
granted and CE marked intra-operative vascular graft storage and flushing solution used during CABG surgeries.
DuraGraft is the first and only medical product
that has received authorization by the FDA for marketing for use as an intra-operative vascular conduit storage and flushing solution
used during CABG surgeries. DuraGraft is also the only product certified for marketing in Europe and other jurisdictions for this indication
as an endothelial damage inhibitor, or EDI. DuraGraft carries CE marking and is approved for marketing in 36 countries outside the
United States on three continents, including all countries in the European Union, or the EU, including Spain, Austria, Ireland, Germany,
and Italy, as well as countries outside the EU including the United Kingdom, or the UK, Turkey, Switzerland, Chile, and the Philippines.
DuraGraft is also the only patented product for this indication. The DuraGraft patent portfolio includes granted patents and pending
applications in over 30 countries throughout the world, including patents granted in the United States, Europe, Australia, India, Argentina,
South Africa, Mexico, and several Asian countries.
Cardiac care is a large
and growing industry. According to the U.S. Centers for Disease Control and Prevention, or CDC, the estimated average annual US cost
of coronary heart disease is $219 billion (Centers for Disease Control and Prevention, Office of Policy, Performance, and Evaluation,
“Health Topics – Heart Disease and Heart Attack.” POLARIS, August 17, 2021). According
to a market analysis report, the size of the CABG procedures market globally was approximately $10.2 billion as of 2022 (Rahul Gotadki,
Market Research Future, “Coronary Artery Bypass Graft Market Research Report Information By Type (Off-Pump, On-Pump, Minimally
Invasive Direct CABG, Endoscopic Vein Harvesting and Others), By Procedure (Single CABG Surgery, Double CABG Surgery, Triple CABG Surgery,
Quadruple CABG Surgery and Others), By End-User (Hospitals, Cardiology Clinics, Research Institutes and Others), and By Region (North
America, Europe, Asia-Pacific, And Rest Of The World) – Market Forecast Till 2030.”, July 2023). The same source reports
that this market is forecast to increase at a compound annual growth rate, or CAGR, of 8.20% between 2023 and 2030. Globally, it is estimated
that more than 1 million CABG procedures are performed worldwide each year (Gaudino, Mario, Weill Cornell Medicine, “Method Using
Artery for Coronary Artery Bypass Linked to Better Long-term Outcomes Than Using Vein,” July 14, 2020), with procedures performed
in the U.S. being a substantial percentage of the total global procedures performed. According to the American Heart Association,
CABG is the most common type of open-heart surgery in the United States with more than 500,000 surgeries performed each year
(The Society of Thoracic Surgeons, “Coronary Artery Bypass Grafting (CABG).” The Patient Guide to Heart, Lung, and
Esophageal Surgery, May 2019).
Having received FDA authorization for marketing
of DuraGraft, we are proceeding with our plans to commercialize DuraGraft in the United States and continue to generate
international revenue growth from sales of DuraGraft. In the U.S. marketplace, we intend
to employ a small direct sales force focusing on marketing and sales to hospital integrated networks. We have also begun the process
of developing the U.S. CABG market for DuraGraft with select clinical studies, the development of known opinion leaders, or KOLs, the
promotion of existing publications, and digital marketing. We
will also seek to develop and commercialize additional applications for the technology underlying DuraGraft.
Our DuraGraft commercialization
plan using its CE marking and existing distribution partners in select European and Asian countries resumed in the second quarter of
2022, with a targeted approach based on market access, existing KOLs, clinical data and revenue penetration. In
Europe and elsewhere, we will continue our DuraGraft marketing efforts relying on our DuraGraft CE marking and our distribution partners.
The CE marking signifies that DuraGraft may be sold in the EEA and that DuraGraft has been assessed as meeting safety, health, and environmental
protection requirements. We are currently working with local distributors of cardiovascular disease-related products, in accordance with
local regulatory requirements, to sell and increase the market share of DuraGraft in Spain, Austria, Switzerland, Germany, Chile, Turkey,
Italy, and the UK among others.
MATLOC®
In
December 2021, we acquired My Health Logic, its lab-on-chip technology platform and its in-development patient-centric, digital point-of-care
screening and diagnostic device, MATLOC.
The
excitement over microfluidics, also known as lab-on-a-chip technology, lies in its potential for producing revolutionary, timely, accessible,
and practical point-of-care devices; devices that are patient-centric (one-to-many, rather than doctor centric, one-to-one) and support
self-care and independence. Microfluidics is a technology for analyzing small volumes of fluids, with the potential to miniaturize complex
laboratory procedures onto a small microchip, hence the term “lab-on-chip”.
Marizyme’s
lab-on-chip technology is currently being developed for screening and diagnosis related to the three leading biomarkers for CKD, a disease
estimated to affect 37 million Americans (National Kidney Foundation, “Chronic kidney disease (CKD),” 2023) – or approximately
one out of every nine Americans. If left untreated, many patients will advance to end stage renal disease, or ESRD, often leading to
kidney transplant, renal failure, or dialysis. Since 90% of those with CKD do not know they have it (Centers for Disease Control and
Prevention, “Chronic Kidney Disease in the United States, 2023,” May 30, 2023), the risk of progression in the disease is
high and this creates massive burdens for CKD patients and healthcare systems (Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney
disease. Lancet. 2017, March 25;389(10075):1238-1252. doi: 10.1016/ S0140-6736(16)32064-5. Epub 2016 Nov 23). In 2018 Medicare alone
spent $130 billion on CKD and ESRD-related costs (United States Renal Data System (“USRDS”). 2020 USRDS annual data report:
epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and
Kidney Diseases, Bethesda, MD, 2020 (“2020 USRDS”), Volume 1 Chronic Kidney Disease; Chapter 6 Healthcare Expenditures for
Persons with CKD; Highlights; Bullet #1: “Medicare fee-for-service (FFS) spending for beneficiaries with CKD who did not have ESRD
exceeded $81 billion in 2018 and represented 22.3% of Medicare FFS spending (Tables 6.1 and 6.2).”; Volume 2; End Stage Renal Disease:
End Stage Renal Disease: Chapter 9 Healthcare Expenditures for Persons with ESRD; Highlights; Bullet #1: “Total Medicare-related
expenditures for beneficiaries with ESRD rose to $49.2B in 2018.”). With the increase of diabetics and hypertension cases in the
U.S., which make up roughly two-thirds of all CKD patients (USRDS 2020 ADR Reference Tables, Vol. 2—ESRD; Ref. Tables A Incidence;
Table A.4 Incident counts of reported ESRD patients, by age and primary diagnosis, 2016–2018 combined. Col. X, Row 38 (All patients)
382,398; Col. X, Row 33 (Diabetes) 179,706; Col. X, Row 34 (Hypertension) 110,807.] [Computed from: (Col. X shows all gender and race.)
All patients (Col. X, Row 38) = 382,398 / DM (Col. X, Row 33) 179,706; (179,706/382,398) = 47% (0.4699); HTN (Col. X, Row 34) 110,807
(110,807/382,398) = 29% (0.2897)), CKD related healthcare costs are expected to increase significantly. Compounding this development
is the fact that less than 50% of diabetic patients are annually screened or tested for kidney damage (Folkerts, K., Petruski-Ivleva,
N., Comerford, E., et al., “Adherence to Chronic Kidney Disease Screening Guidelines Among Patients With Type 2 Diabetes in a US
Administrative Claims Database,” Mayo Clinic Proceedings 2021;96(4):975-986) doi: https://doi.org/10.1016/j.mayocp.2020.07.037).
This creates an unmet need for point-of-care technologies that facilitate CKD screening and diagnosis, which further facilitates earlier
screening and diagnosis and detection to slow down or eliminate the CKD progression. By combining lab-on-chip technology with our MATLOC
device, it will be able to quantitatively read the two urine biomarkers, albumin and creatine, and a blood biomarker, eGFR, necessary
for effective CKD screening and diagnosis at point-of-care with results available instantly on a patient’s smartphone.
The
COVID-19 pandemic has massively accelerated the ongoing transformation in healthcare. Connected consumer electronic devices are enabling
24/7 home-based digital healthcare. We believe that consumers have the desire and are now becoming empowered to manage their own healthcare
and that they will seek to utilize our point-of-care MATLOC device.
With
our lab-on-chip technology and MATLOC device in development, we are striving to achieve earlier detection and slowing of the progression
of CKD, allowing patients and healthcare systems to reduce the enormous costs of kidney failure, transplant, and/or dialysis. After completing
the technology for CKD assessment, we plan to explore the commercial potential of other biomarkers for chronic diseases to be measured
at point-of-care.
We
anticipate that once we commence marketing and sales operations for DuraGraft in the U.S., we will be able to accelerate the development
of a functional MATLOC device prototype.
MAR-FG-001
In November 2021, we reinitiated the development
of MAR-FG-001, our fat grafting technology. MAR-FG-001 is a tumescent solution base for fat grafting procedures that may be used for
plastic and cosmetic surgeries. The Company intends to develop MAR-FG-001 for use during these and other fat grafting procedures.
Fat
grafting is a surgical process used in medical reconstructive and other plastic surgery procedures in which fat is transferred from one
area of the body to another (known as “autologous fat grafting” or simply “fat grafting”) to correct a defect,
replace injured tissue, or to make cosmetic enhancements.
Compared
to standard solutions, we believe that MAR-FG-001 could better protect adipose tissue from ischemic and oxidative injury and increase
adipocyte and stromal cell viability, which is key to improving retention of fat volume thereby improving patient outcomes following
fat grafting procedures.
The
global market for autologous fat grafting was estimated to be $699.96 million in 2021 and was projected to grow at a CAGR of 8.62% until
2028 (“Global Autologous Fat Grafting Market – Industry Trends and Forecast to 2028,” Data Bridge Market Research,
December 2020). Growing preference for the use of non-invasive aesthetic techniques in skin rejuvenation, more rapid recovery with lesser
allergic risks and reduced downtime compared to other procedures are some of the factors contributing to increasing demand. The adoption
rate for autologous fat grafting procedures in the United States was 2.2% of all augmentation and reconstruction procedures as of 2018,
suggesting significant potential for growth of adoption of these procedures (“Autologous Fat Grafting Market Analysis By Product
(Integrated Fat Transfer Systems, Aspiration and Harvesting Systems, Liposuction Systems, Fat Processing Systems, De-epithelialization
Devices), By Application & Region - Global Market Insights 2021 to 2031,” Fact.MR, March 2022). With approximately 22.4 million
plastic surgeries performed in the United States in 2020 (American Society of Plastic Surgeons, “Plastic Surgery Statistics Report
– 2020,” April 27, 2021), there is potential for widespread implementation of innovative fat grafting systems.
MAR-FG-001
is currently in development and not yet available for sale in any markets.
Our
Historical Performance
Our net loss was approximately $20.6
million and $6.9 million for the three months
ended June 30, 2023 and June 30, 2022, respectively, and was approximately $23.1 million and $13.0 million for the six months ended June
30, 2023 and June 30, 2022, respectively. For the fiscal years ended December 31, 2022 and 2021, our net loss was approximately $38.2
million and $11.0 million, respectively. We expect to incur expenses and operating losses over the next several years. Accordingly, we
will need additional financing to support our continuing operations. We will seek to fund our operations through public or private equity
offerings, debt financings, government or other third-party funding, collaborations and licensing arrangements. Adequate additional financing
may not be available to us on acceptable terms, or at all. Our failure to raise capital as and when needed would impact our going concern
status and would have a negative impact on our financial condition and our ability to pursue our business strategy and continue as a
going concern. We will need to generate significant revenues to achieve profitability, and we may never do so.
Our
Competitive Strength
We
believe that the following competitive strength will enable us to compete effectively:
| ● | Superior,
first-in-class vascular graft storage and flushing solution. DuraGraft is
the first and only medical product that has received authorization by the
FDA for marketing for use as an intra-operative vascular conduit storage and flushing solution
used during CABG surgeries. DuraGraft is also the only product certified for marketing
in Europe and other jurisdictions for this indication. |
Our
Growth Strategies
We
will strive to grow our business by pursuing the following key growth strategies:
| ● | Commercialize
DuraGraft. |
| ● | Develop
MATLOC technology and related products. |
| ● | Develop
MAR-FG-001 fat grafting technology and related products. |
| ● | Acquire
more life science assets. |
The
strategic plans described above will require capital. We will not receive any of the required capital in this offering except upon the
exercise of warrants held by the selling stockholders for issuance of the shares of common stock that are being registered for resale
by the registration statement of which this prospectus forms a part. There can be no assurances that we will be able to raise the capital
that we need to execute our plans or that capital, whether through securities offerings, either private or public, will be available
to us on acceptable terms, if at all. An inability to raise sufficient funds could cause us to scale back our development and growth
plans or discontinue them altogether.
COVID-19
Pandemic
The Company has been impacted
by the COVID-19 pandemic and related supply chain shortages and other economic conditions, and some of its earlier plans to diversify
and expand its operations were delayed as a result. Moreover, the impact of the COVID-19 pandemic on the Company’s
supply chain and its ability to produce DuraGraft inventory was a primary reason that we did not generate substantial revenue from sales
of DuraGraft during 2021 and 2022. The Company’s inventory production of DuraGraft returned to its pre-pandemic level at the end
of the second quarter of 2022, but lingering effects of the COVID-19 pandemic continued to depress demand for DuraGraft and cause revenues
from DuraGraft during the first and second quarters of 2023 to be minimal. There can be no assurance that future supply chain
disruptions and other effects of COVID-19 outbreaks will not adversely impact our revenues.
In
addition, the Company is dependent upon certain contract manufacturers and suppliers and their ability to reliably and efficiently fulfill
its orders is critical to the Company’s business success. The COVID-19 pandemic has impacted and may continue to impact certain
of the Company’s manufacturers and suppliers. As a result, the Company has faced and may continue to face delays or difficulty
sourcing certain products, which could negatively affect the Company’s business and financial results.
While it is not possible
at this time to estimate the total impact that COVID-19 could have on our business in the future, the continued spread of COVID-19
and variants of the virus, the rate of vaccinations regionally and globally and the measures taken by the government authorities, and
any future epidemic disease outbreaks, could: Disrupt the supply chain and the manufacture or shipment of products and supplies for use
by us in our research activities and by strategic partners for their distribution and sales activities; delay, limit or prevent us in
our research activities and strategic partners in their distribution and sales activities; impede our negotiations with strategic partners;
impede testing, monitoring, data collection and analysis and other related activities by us; interrupt or delay the operations of the
FDA or other regulatory authorities, which may impact review and approval timelines for initiation of clinical trials or marketing; or
impede the launch or commercialization of any approved products; any of which could delay our strategic partnership plans, increase our
operating costs, and have a material adverse effect on our business, financial condition and results of operations.
For
further discussion of the impact of the COVID-19 pandemic on our business, please see “Management’s Discussion and Analysis
of Financial Condition and Results of Operations”, and “Risk Factors –
Risks Related to Our Business – The COVID-19 pandemic has adversely impacted the Company’s supply chain and could materially
and adversely affect our ability to conduct clinical trials and engage with our third-party vendors and thereby have a material adverse
effect on our financial results.”
Implications
of Being a Smaller Reporting Company
We
are a “smaller reporting company” as defined in Rule 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage
of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our shares held by non-affiliates
equals or exceeds $250 million as of the prior June 30th, or (2) our annual revenues equaled or exceeded $100 million during such completed
fiscal year and the market value of our shares held by non-affiliates equals or exceeds $700 million as of the prior June 30th. Such
reduced disclosure and corporate governance obligations may make it more challenging for investors to analyze our results of operations
and financial prospects.
For
additional information, see “Risk Factors – Risks Related to This Offering and Ownership of Our Securities –
We are a ‘smaller reporting company’ within the meaning of the Exchange Act, and if we take advantage of certain exemptions
from disclosure requirements available to smaller reporting companies, this could make our securities less attractive to investors and
may make it more difficult to compare our performance with other public companies.” and “—As a ‘smaller
reporting company,’ we may at some time in the future choose to exempt our company from certain corporate governance requirements
that could have an adverse effect on our public stockholders.”
Corporate
Information
Our
principal executive office is located at 555 Heritage Drive, Suite 205, Jupiter, Florida 33458 and our telephone number is (561) 935-9955.
We maintain a website at www.marizyme.com. Information available on this website is not incorporated by reference in and is not deemed
a part of this prospectus or the registration statement of which this prospectus forms a part. Our filings with the SEC are available
for inspection through the SEC’s website at http://www.sec.gov.
The
Offering
| Common stock offered by the selling stockholders: |
|
This
prospectus relates to the offer and resale by the selling stockholders of up to 915,071,257 shares of common stock that may
be sold from time to time by the selling stockholders named in this prospectus, which includes: |
| |
|
|
|
|
| |
|
|
● |
13,971,324 shares of outstanding common stock held
by existing stockholders; |
| |
|
|
|
|
| |
|
|
● |
221,939,338
shares
of common stock issuable upon the conversion of the outstanding Convertible Notes, assuming
that all convertible debts and other liabilities under the Convertible Notes are converted
into shares of common stock, without regard to any applicable limitations or restrictions; |
| |
|
|
|
|
| |
|
|
● |
380,986,336
shares of common stock upon the exercise of the outstanding Class C Warrants,
without regard to any applicable limitations or restrictions; |
| |
|
|
|
|
| |
|
|
● |
66,159,434
shares
of common stock upon the conversion of the outstanding OID Convertible Notes, assuming that
the OID Convertible Notes are held until maturity and that all convertible debts and other
liabilities under the OID Convertible Notes are converted into shares of common
stock, without regard to any applicable limitations or restrictions; |
|
|
|
|
|
| |
|
|
● |
84,546,202
shares
of common stock upon the exercise of the outstanding Class E Warrants, without regard to
any applicable limitations or restrictions; |
| |
|
|
|
|
| |
|
|
● |
80,796,202
shares
of common stock upon the exercise of the outstanding Class F Warrants, without regard to
any applicable limitations or restrictions; and |
| |
|
|
|
|
| |
|
|
● |
66,672,421
shares of common
stock upon the exercise of the Placement Agent Warrants, without regard to any applicable
limitations or restrictions. |
| |
|
|
|
|
| Shares
of common stock outstanding at commencement of the offering(1): |
|
46,666,760
shares of common stock. |
| |
|
|
| Use of proceeds: |
|
We
will not receive any proceeds from the resales of shares of common stock by the selling stockholders. Assuming the full exercise
of the Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants for cash, we will receive gross
proceeds of approximately $70.4 million. We plan to use the proceeds for working capital and general corporate purposes. See
“Use of Proceeds.” |
| |
|
|
| Risk factors: |
|
Investing
in our common stock involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment.
You should carefully consider the information set forth in the “Summary of Risk Factors” section beginning on
page 10 and “Risk Factors” section beginning on page 12 before deciding to invest in our common
stock. |
| |
|
|
| Trading market and symbol: |
|
Our common stock is quoted for trading on the OTCQB under the symbol “MRZM”. We have applied to list our common stock under the symbol “MRZM” on the Nasdaq Capital Market. There can be no guarantee that we will successfully list our common stock on the Nasdaq Capital Market. The registration of the selling stockholders’ resale of the Company’s common stock as described in this prospectus is not conditioned upon our successful listing on the Nasdaq Capital Market. |
| (1) |
The
number of shares of common stock outstanding is based on 46,666,760 shares outstanding as
of September 19, 2023, and excludes the following securities as of such
date: |
| |
● |
3,925,943 shares of common
stock that have been approved by our board of directors for issuance upon the exercise of vested and unvested options granted to
our officers, directors, employees and service providers at a weighted average exercise price of $1.33 per share pursuant to our
Amended and Restated 2021 Stock Incentive Plan (the “Stock Incentive Plan” or the “Plan”); |
| |
|
|
| |
● |
350,000 unissued restricted
shares of common stock subject to certain performance-based vesting terms and issuable to corporations controlled by our Chief Financial
Officer and Vice President of Finance under the Plan and that will be granted upon the vesting of such restricted shares under certain
side agreements with these corporations; |
| |
|
|
| |
● |
7,200,000 shares of common
stock reserved for issuance pursuant to the Plan, which is inclusive of the 3,925,943 shares issuable upon the exercise of vested
and unvested options that have been granted under the Plan and 350,000 unissued restricted shares of common stock subject to certain
performance-based vesting terms and that will be granted under the Plan upon the vesting of such restricted shares under the side
agreements referred to above; |
| |
|
|
| |
● |
221,939,338 shares of common
stock issuable upon conversion of outstanding Convertible Notes,
assuming that all convertible debts and other liabilities under the Convertible Notes
are converted into shares of common stock, and that all issuable shares of common
stock upon conversion in full of the Convertible Notes are issued, without regard to any
applicable limitations or restrictions; |
| |
|
|
| |
● |
380,986,336 shares of common
stock issuable upon full exercise of outstanding Class C Warrants at an exercise price of
$0.10 per share, without
regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
66,159,434
shares
of common stock issuable upon conversion of outstanding OID Convertible Notes, assuming that
the OID Convertible Notes are held until maturity, that all convertible debts and other
liabilities under the OID Convertible Notes are converted into shares of common
stock, and that all issuable shares of common stock upon conversion in full of the OID Convertible
Notes are issued, without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
84,546,202
shares
of common stock issuable upon exercise of outstanding Class E Warrants at an exercise price
of $0.10 per share, without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
80,796,202
shares
of common stock issuable upon exercise of outstanding Class F Warrants at an exercise price
of $0.20 per share, without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
280,014
shares of common stock issuable upon exercise of outstanding Placement Agent Warrants at an exercise price of $1.375 per share (the
“2020 Placement Agent Warrants”), without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
48,234,054 shares of common stock issuable upon exercise
of certain outstanding Placement Agent Warrants issued on July 25, 2023 and September 27, 2023 at an exercise price of $0.10 per
share (the “Replacement Placement Agent Warrants”),
without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
11,694,656 shares
of common stock issuable upon exercise of certain Placement Agent Warrants issued on September 13, 2023 at an exercise price of $0.10
per share (collectively, the “OID Notes and Class E Placement Agent Warrants”, and together with the Class F Placement
Agent Warrants, the “OID Units Placement Agent Warrants”), without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
6,463,697 shares
of common stock issuable upon exercise of Placement Agent Warrants issued on September 13, 2023 at an exercise price of $0.20 per
share (collectively, the “Class F Placement Agent Warrants”), without regard to any applicable limitations or restrictions; |
| |
|
|
| |
● |
577,796
shares of common stock issuable upon full exercise of other outstanding warrants at a weighted-average
exercise price of approximately $3.56 per share, without regard to any applicable
limitations or restrictions. |
In
addition, unless otherwise indicated, the information throughout this prospectus, including outstanding share amounts as of September
19, 2023, reflects and assumes no exercise or conversion of any of the securities listed above, and no issuance of any shares of
common stock pursuant to the Plan.
Summary
Financial Information
The
following tables summarize certain financial data regarding our business and should be read in conjunction with our financial statements
and related notes contained elsewhere in this prospectus and the information under “Management’s Discussion and Analysis
of Financial Condition and Results of Operations.”
Our
summary financial data as of and for the six months ended June 30, 2023 and 2022 are derived from the financial statements
included elsewhere in this prospectus. Our summary financial data as of and for the fiscal years ended December 31, 2022 and 2021 are
derived from our audited financial statements included elsewhere in this prospectus. All consolidated financial statements included in
this prospectus are prepared and presented in accordance with generally accepted accounting principles in the United States of America
(“GAAP”). The summary financial information is only a summary and should be read in conjunction with the historical financial
statements and related notes contained elsewhere herein. The consolidated financial statements contained elsewhere fully represent our
financial condition and operations; however, they are not indicative of our future performance.
| | |
Six
Months Ended
June 30, | | |
Year Ended December 31, | | |
Year Ended December 31, | |
| | |
2023 | | |
2022 | | |
2022 | | |
2021 | |
| Statements of Operations Data | |
(Unaudited) | | |
(Unaudited) | | |
| | |
| |
| Revenue | |
$ | 313,713 | | |
$ | 61,809 | | |
$ | 233,485 | | |
$ | 210,279 | |
| Total operating expenses | |
| 7,161,692 | | |
| 8,647,883 | | |
| 37,502,962 | | |
| 8,951,308 | |
| Total operating loss | |
| (6,936,865 | ) | |
| (8,597,099 | ) | |
| (37,323,796 | ) | |
| (8,821,383 | ) |
| Total other income (expense) | |
| (16,194,121 | ) | |
| (4,451,770 | ) | |
| (842,074 | ) | |
| (2,176,546 | ) |
| Net loss | |
$ | (23,130,986 | ) | |
$ | (13,048,869 | ) | |
$ | (38,165,870 | ) | |
$ | (10,997,929 | ) |
| Loss per share – basic and diluted | |
$ | (0.55 | ) | |
$ | (0.32 | ) | |
$ | (0.94 | ) | |
$ | (0.31 | ) |
| Weighted average number of shares of common stock – basic and diluted | |
| 41,979,194 | | |
| 40,728,740 | | |
| 40,727,092 | | |
| 36,041,613 | |
| | |
As of June
30, | | |
As of
December 31, | |
| | |
2023 | | |
2022 | | |
2021 | |
| Balance Sheet Data | |
(unaudited) | | |
| | |
| |
| Cash | |
$ | 205,226 | | |
$ | 510,865 | | |
$ | 4,072,339 | |
| Total current assets | |
| 1,058,577 | | |
| 1,955,303 | | |
| 4,401,818 | |
| Total assets | |
| 36,981,452 | | |
| 38,687,638 | | |
| 65,999,350 | |
| Total current liabilities | |
| 20,821,718 | | |
| 2,921,767 | | |
| 3,133,721 | |
| Total liabilities | |
| 29,711,796 | | |
| 21,265,653 | | |
| 18,309,018 | |
| Total stockholder’s equity | |
| 7,269,656 | | |
| 17,421,985 | | |
| 47,690,332 | |
| Total liabilities and stockholder’s equity | |
| 36,981,452 | | |
| 38,687,638 | | |
| 65,999,350 | |
Summary
of Risk Factors
An
investment in our securities involves a high degree of risk. You should carefully consider the risks summarized below. These risks are
discussed more fully in the “Risk Factors” section immediately following this Prospectus Summary. These risks include,
but are not limited to, the following:
Risks
Related to Our Business
| ● | We
have incurred losses since inception, and we anticipate that we will incur continued losses
for the foreseeable future. Moreover, our independent registered public accounting firm’s
report, contained herein, includes an explanatory paragraph that expresses substantial doubt
about our ability to continue as a going concern, indicating the possibility that we may
not be able to operate in the future. |
| ● | We
defaulted under the Convertible Notes and, as a result, the Convertible Note holders may
accelerate amounts owed under such Convertible Notes and seek to take possession the assets
securing our obligations. Our other indebtedness could also expose us to risks that could
adversely affect our business, financial condition and results of operations. |
| ● | Management
identified a material weakness in our internal controls, and failure to remediate it or any
future ineffectiveness of internal controls could have a material adverse effect on the Company’s
business and the price of its common stock. |
| ● | If
we continue to fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related
to disclosure controls and procedures, or, if we discover additional material weaknesses
and other deficiencies in our internal control and accounting procedures, our securities’
prices could decline significantly and raising capital could be more difficult. |
| ● | Misconduct
of employees, subcontractors, agents and business partners could cause us to lose existing
contracts or customers and adversely affect our ability to obtain new contracts and customers
and could have a significant adverse impact on our business and reputation. |
| ● | We
have negative working capital. |
| ● | We
have a limited operational history. |
| ● | If
we fail to retain current members of our senior management, or to identify, attract, integrate
and retain additional key personnel, our business will be harmed. |
| ● | If
we do not generate sufficient cash flow from operations in the future, we may not be able
to fund our product development efforts and acquisitions or fulfill our future obligations. |
| ● | We
will require substantial additional funding which may not be available to us on acceptable
terms, or at all. Failing to raise the necessary additional capital could force us to delay,
reduce, eliminate or abandon growth initiatives, development or commercialization of our
technologies and products. |
| ● | We
may not be able to monetize intangible assets, which may result in the need to record an
impairment charge. |
| ● | Acquisitions
present many risks, and we may not realize the financial and strategic goals we anticipate
at the time of an acquisition. |
| ● | The
medical device market is highly competitive, and we may not be able to effectively compete
against other providers of medical devices, particularly those with greater resources. |
| ● | Our
future performance may depend on the success of products we have not yet developed or acquired. |
| ● | Our
products may never achieve market acceptance. |
| ● | We
may delay or terminate the development or acquisition of a product at any time if we believe
the perceived market or commercial opportunity does not justify further investment, which
could materially harm our business. |
| ● | Product
liability lawsuits against us could cause us to incur substantial liabilities and to limit
commercialization of any products that we may develop or acquire. |
| ● | We
may not be able to protect or enforce our intellectual property rights, which could impair
our competitive position. |
| ● | Confidentiality
agreements with employees and others may not adequately prevent disclosure of trade secrets
and other proprietary information and may not adequately protect our intellectual property. |
| ● | We
may be subject to intellectual property infringement claims by third parties which could
be costly to defend, divert management’s attention and resources, and may result in
liability. |
| ● | Competitors
may violate our intellectual property rights, and we may bring litigation to protect and
enforce our intellectual property rights, which may result in substantial expense and may
divert our attention from implementing our business strategy. |
| ● | We
may be dependent on third-party manufacturers since we will not initially directly
manufacture our products. |
| ● | We
currently have no marketing and sales organization and have limited experience as a company
in commercializing products, and we may need to invest significant resources to develop these
capabilities. If we are unable to establish marketing and sales capabilities in the United
States, we may not be able to generate substantial product revenue. |
| ● | The
COVID-19 pandemic has adversely impacted the Company’s supply chain and could materially
and adversely affect our ability to conduct clinical trials and engage with our third-party
vendors and thereby have a material adverse effect on our financial results. |
Risks
Related to Government Regulation
| ● | Only
one of our
products has been authorized for marketing in the United States, other
products may not be granted authorization, and any authorization may be subject
to significant limitations. |
| ● | Failure
to obtain regulatory approvals in foreign jurisdictions will prevent us from marketing our
products internationally. |
| ● | Even
if we receive regulatory approval for any product we may develop or acquire, we will be subject
to ongoing regulatory obligations and continued regulatory review, which may result in significant
additional expense and subject us to penalties if we fail to comply with applicable regulatory
requirements. |
| ● | Healthcare
reform measures could hinder or prevent our products’ commercial success. |
| ● | If
we fail to comply with healthcare regulations, we could face substantial penalties and our
business, operations and financial condition could be adversely affected. |
Risks
Related to This Offering and Ownership of Our Securities
| ● | Our
efforts to obtain and maintain a listing of our common stock on the Nasdaq Capital Market
may fail and may not prevent, or may cause, the decline of the value of your common stock. |
| ● | Substantial
future sales or issuances of our securities, or the perception in the public markets that
these sales or issuances may occur, may depress the price of our common stock. Also, future
issuances of our common stock or rights to purchase common stock could result in additional
dilution of the percentage ownership of our stockholders and could cause the prices of our
common stock to fall. |
RISK
FACTORS
An
investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors, together with
the other information contained in this prospectus, before purchasing our common stock. We have listed below (not necessarily in order
of importance or probability of occurrence) what we believe to be the most significant risk factors applicable to us, but they do not
constitute all of the risks that may be applicable to us. Any of the following factors could harm our business, financial condition,
results of operations or prospects, and could result in a partial or complete loss of your investment. Some statements in this prospectus,
including statements in the following risk factors, constitute forward-looking statements. Please refer to the section titled “Cautionary
Note Regarding Forward-Looking Statements”.
Risks
Related to Our Business
We
have incurred losses since inception, and we anticipate that we will incur continued losses for the foreseeable future. Moreover, our
independent registered public accounting firm’s report, contained herein, includes an explanatory paragraph that expresses substantial
doubt about our ability to continue as a going concern, indicating the possibility that we may not be able to operate in the future.
As
of June 30, 2023, December 31, 2022 and December 31, 2021, the Company had an accumulated deficit of approximately $109.1
million, $86.0 million and $47.8 million, respectively, respectively. We expect to incur significant and increasing operating
losses for the next several years as we expand our acquisition efforts, continue clinical trials, acquire, or license technologies, advance
other medical devices into clinical development, complete clinical trials, seek regulatory approval and, if we receive FDA approval,
commercialize our products. Primarily because of our losses incurred to date, our expected continued future losses, and limited cash
balances, our independent registered public accounting firm has included in its report an explanatory paragraph expressing substantial
doubt about our ability to continue as a going concern. Our ability to continue as a going concern is contingent upon, among other factors,
the sale of the shares of common stock or obtaining alternate financing. We cannot provide any assurance that we will be able to raise
additional capital.
If
we are unable to secure additional capital, we may be required to curtail our research and development initiatives and take additional
measures to reduce costs in order to conserve our cash in amounts sufficient to sustain operations and meet our obligations. These measures
could cause significant delays in our clinical and regulatory efforts, which are critical to the realization of our business plan. The
accompanying financial statements do not include any adjustments that may be necessary should we be unable to continue as a going concern.
It is not possible for us to predict currently the potential success of our business. The revenue and income potential of our proposed
business and operations are currently unknown. If we cannot continue as a viable entity, you may lose some or all your investment in
our company.
We
defaulted under the Convertible Notes and, as a result, the Convertible Note holders may accelerate amounts owed under such Convertible
Notes and seek to take possession the assets securing our obligations. Our other indebtedness could also expose us to risks that could
adversely affect our business, financial condition and results of operations.
We
defaulted under the Convertible Notes due to a cross-default provision that was triggered by the non-repayment of principal under a promissory
note on May 7, 2023. Under the terms of the Convertible Notes, the aggregate amount that may be due is $22,193,934, or approximately
$5.7 million more than would otherwise have been due under the Convertible Notes. Under the unit purchase agreement entered into on May
27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s subsidiaries entered into a guaranty to
guarantee the repayment of the Company’s obligations under the Convertible Notes, and the Company and its subsidiaries entered
into security agreements granting security interests in all of their respective assets for up to the dollar value owed under the respective
Convertible Notes. The respective Convertible Notes were issued for aggregate principal of approximately $1.2 million. Under each unit
purchase agreement entered into with respect to subsequent issuances of the Convertible Notes, the Company and its subsidiaries were
obligated to enter into similar security agreements and guaranties, but did not do so.
The
holders of the Convertible Notes have not exercised any remedies applicable to the Convertible Notes or given notice of any intention
to do so as of the date of this prospectus. If Univest Securities, LLC (“Univest”), as the appointed representative of the
Convertible Note holders, remains so appointed, no investor other than Univest may pursue any remedy with respect to the Convertible
Notes. However, if the amount owed due to the default is not repaid upon demand, the holders of the Convertible Notes may nonetheless
seek to remove Univest from this appointed position, take possession of some or all of the Company’s and its subsidiaries’
assets, force the Company and its subsidiaries into bankruptcy proceedings, or seek other legal remedies against the Company and its
subsidiaries. In such event, the Company’s business, operating results and financial condition may be materially adversely affected.
In
addition to the Convertible Notes, we have incurred indebtedness under the OID Convertible Notes in the aggregate principal amount of
$6,163,697. The OID Convertible Notes accrue interest at 10% and will mature on various dates from February 12, 2024 to May 30, 2024.
We have also incurred trade debts to various vendors. In the future, we may incur additional indebtedness.
Even
if the holders of the Convertible Notes do not pursue remedies in response to our default under the Convertible Notes, our indebtedness
could have significant negative consequences for our security holders, business, results of operations and financial condition by, among
other things:
| ● | increasing
our vulnerability to adverse economic and industry conditions; |
| ● | limiting
our ability to obtain additional financing; |
| ● | requiring
the dedication of a substantial portion of our cash flow from operations to service our indebtedness,
which will reduce the amount of cash available for other purposes; |
| ● | limiting
our flexibility to plan for, or react to, changes in our business; and |
| ● | placing
us at a possible competitive disadvantage with competitors that are less leveraged than us
or have better access to capital. |
Our
business may not generate sufficient funds, and we may otherwise be unable to maintain sufficient cash reserves to pay any indebtedness
that we may incur. The OID Convertible Notes contain restrictive covenants, including cross-default provisions, that are similar to the
Convertible Notes. In addition, any future indebtedness that we may incur may also contain financial and other restrictive covenants
that will limit our ability to operate our business, raise capital or make payments under our indebtedness. If we fail to comply with
such covenants or to make payments under any of our indebtedness when due, then we would be in default under that indebtedness, which
could, in turn, result in that indebtedness becoming immediately payable in full and cross-default or cross-acceleration under our other
indebtedness and other liabilities.
Management
has determined that disclosure controls and procedures and internal control over financial reporting were not effective, identified a
material weakness in our internal controls, and a failure to remediate it or any future ineffectiveness of internal controls could have
a material adverse effect on the Company’s business and the price of its securities.
As
previously reported in “Item 9A. Controls and Procedures” of the Company’s Annual Report on Form 10-K for the
fiscal year ended December 31, 2022, our management determined that our disclosure controls and procedures and internal control over
financial reporting, or ICFR, were not effective due to a material weakness in ICFR as of December 31, 2022, and as previously reported
in “Item 4. Controls and Procedures” of the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended
June 30, 2023, our management determined that our disclosure controls and procedures were not effective due to material weaknesses
in ICFR as of June 30, 2023.
A “material weakness” is a deficiency, or a combination of deficiencies, in ICFR, such
that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will
not be prevented or detected on a timely basis. As previously reported, we have taken, and plan to continue to take, measures to remediate
the Company’s internal weaknesses in ICFR. However, the implementation of these measures may not address any control deficiencies
in our ICFR. Our failure to address any control deficiencies could result in inaccuracies in our financial statements and could also
impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. Moreover,
effective ICFR is important to prevent fraud. Failure to report its financial information on an accurate or timely basis may thereby
subject the Company to adverse regulatory consequences, including sanctions by the SEC or violations of applicable securities exchange
or quotation service listing rules. There could also be a negative reaction in the financial markets due to a loss of investor confidence
in the Company and the lack of timeliness or reliability of its financial statements. Confidence in the reliability of the Company’s
financial statements may suffer due to the Company’s reporting of a material weakness in its ICFR. This could materially adversely
affect the Company’s business, financial condition, results of operations and prospects and lead to a decline in the price of its
common stock.
If
we continue to fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or,
if we discover additional material weaknesses and other deficiencies in our internal control and accounting procedures, our securities’
prices could decline significantly and raising capital could be more difficult.
If
we continue to fail to comply with the rules under the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) related to disclosure
controls and procedures, or, if we discover additional material weaknesses and other deficiencies in our internal control and accounting
procedures, the prices of our securities could decline significantly and raising capital could be more difficult. Moreover, effective
internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. If we
cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose
confidence in our reported financial information, and the trading prices of our securities could drop significantly. In addition, we
cannot be certain that additional material weaknesses or significant deficiencies in our internal controls will not be discovered in
the future.
As
a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We
are not an “accelerated filer” or a “large accelerated filer” under the Exchange Act. Rule 12b-2 under the Exchange
Act defines an “accelerated filer” to mean any company that first meets the following conditions at the end of each fiscal
year: The company had a public float of $75 million or more, but less than $700 million, as of the last business day of the company’s
most recently completed second fiscal quarter; the company has been subject to the reporting requirements of the Exchange Act for at
least twelve calendar months; the company has filed at least one annual report under the Exchange Act; the company did not have annual
revenues of less than $100 million and either no public float or a public float of less than $700 million; and, once the company determines
that it does not qualify for “smaller reporting company” status because it exceeded one or more of the current thresholds
for such status, is not eligible to regain “smaller reporting company” status under the test provided under paragraph (3)(iii)(B)
of the “smaller reporting company” definition in Rule 12b-2 of the Exchange Act. Rule 12b-2 under the Exchange Act defines
a “large accelerated filer” in the same way except that the company meeting the definition must have a public float of $700
million or more as of the last business day of the company’s most recently completed second fiscal quarter.
A
non-accelerated filer is not required to file an auditor attestation report on internal control over financial reporting that is otherwise
required under Section 404(b) of the Sarbanes-Oxley Act.
Therefore,
our ICFR does not receive the level of review provided by the process relating to the auditor attestation included in annual reports
of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our securities
less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our securities
less attractive as a result, there may be a less active trading market for our securities and trading prices for our securities may be
negatively affected.
We
are a “smaller reporting company” within the meaning of the Exchange Act, and if we take advantage of certain exemptions
from disclosure requirements available to smaller reporting companies, this could make our securities less attractive to investors and
may make it more difficult to compare our performance with other public companies.
Rule
12b-2 of the Exchange Act defines a “smaller reporting company” as an issuer that is not an investment company, an asset-backed
issuer, or a majority-owned subsidiary of a parent that is not a smaller reporting company and that:
| ● | had
a public float of less than $250 million as of the last business day of its most recently
completed second fiscal quarter, computed by multiplying the aggregate worldwide number of
shares of its voting and non-voting common equity held by non-affiliates by the price at
which the common equity was last sold, or the average of the bid and asked prices of common
equity, in the principal market for the common equity; or |
| ● | in
the case of an initial registration statement under the Securities Act or the Exchange Act
for shares of its common equity, had a public float of less than $250 million as of a date
within 30 days of the date of the filing of the registration statement, computed by multiplying
the aggregate worldwide number of such shares held by non-affiliates before the registration
plus, in the case of a Securities Act registration statement, the number of such shares included
in the registration statement by the estimated public offering price of the shares; or |
| ● | in
the case of an issuer whose public float as calculated under paragraph (1) or (2) of this
definition was zero or whose public float was less than $700 million, had annual revenues
of less than $100 million during the most recently completed fiscal year for which audited
financial statements are available. |
If
a company determines that it does not qualify for smaller reporting company status because it exceeded one or more of the above thresholds,
it will remain unqualified unless when making its annual determination it meets certain alternative threshold requirements which will
be lower than the above thresholds if its prior public float or prior annual revenues exceed certain thresholds.
As
a smaller reporting company, we are not required to include a Compensation Discussion and Analysis section in our proxy statements; we
may provide only two years of financial statements; and we need not provide the table of selected financial data. We also have other
“scaled” disclosure requirements that are less comprehensive than issuers that are not smaller reporting companies which
could make our securities less attractive to potential investors, which could make it more difficult for our securityholders to sell
their securities.
Even
if our common stock is listed on the Nasdaq Capital Market, we may utilize an exemption from certain corporate governance requirements
for “smaller reporting companies” that could have an adverse effect on our public stockholders.
Under
Nasdaq rules, a “smaller reporting company,” as defined in Rule 12b-2 under the Exchange Act, is not subject to certain corporate
governance requirements otherwise applicable to companies listed on the Nasdaq Capital Market. For example, a smaller reporting company
is exempt from the Nasdaq requirement of having a compensation committee comprised solely of directors meeting certain enhanced independence
standards, as long as the compensation committee has at least two members who do meet such standards. Although we have determined not
to avail ourselves of this or other exemptions from Nasdaq requirements that are or may be afforded to smaller reporting companies while
we seek to obtain and maintain the listing of our shares on the Nasdaq Capital Market, in the future we may elect to rely on any or all
of these exemptions. By electing to utilize any such exemptions, our company may be subject to greater risks of poor corporate governance,
poorer management decision-making processes, and reduced results of operations from problems in our corporate organization. Consequently,
if we were to avail ourselves of these exemptions, our stock price might suffer, and there is no assurance that we would be able to continue
to meet all continuing listing requirements of Nasdaq from which we would not be exempt, including minimum stock price requirements.
Misconduct
of employees, subcontractors, agents and business partners could cause us to lose existing contracts or customers and adversely affect
our ability to obtain new contracts and customers and could have a significant adverse impact on our business and reputation.
On
January 28, 2022, the Company filed a Complaint in the Circuit Court of the Fifteenth Judicial Circuit in and for Palm Beach County,
Florida (the “Florida Circuit Court”), case number 50-2022-CA-000859-XXX-MB, against Amy Chandler (the “Chandler
Complaint”). The Chandler Complaint sought damages for breach of fiduciary duty, breach of contract, negligence, conversion, and
civil theft. The Chandler Complaint alleged that, prior to her resignation in September 2021, Ms. Chandler intentionally and recklessly
took actions to cancel the CE certificate required by European Union regulations in order for Marizyme and its subsidiary, Somahlution,
Inc., to ship and distribute certain products to/within the EU. The Chandler Complaint also alleged that Ms. Chandler disregarded her
fiduciary duty to Marizyme and responsibilities as the top regulatory and compliance official of Marizyme. As a result, the Chandler
Complaint alleged that Ms. Chandler’s actions caused significant disruption and damage to Marizyme’s business, including,
but not limited to, financial damages and damage to Marizyme’s reputation and business relationships. The Chandler Complaint further
alleged that prior to her last day, Ms. Chandler stole confidential, proprietary files governing Marizyme’s quality management
system, which related to internal business operations, and that Marizyme incurred significant costs to recreate these files. The Chandler
Complaint alleged damages in excess of thirty thousand dollars ($30,000), exclusive of interest, attorneys’ fees, and costs.
On
February 28, 2022, Ms. Chandler filed an Answer, Affirmative Defenses and Counterclaim with the Florida Circuit Court (the “Answer”).
The Answer denied the claims in the Chandler Complaint and most of the factual allegations regarding Ms. Chandler’s alleged actions.
The Answer also asserted a counterclaim against the Company for defamation per se. The Answer sought to recover monetary damages, attorneys’
fees, and court costs in connection with this litigation. The Answer also demanded a trial by jury on all triable issues.
On
March 18, 2022, the Company filed a Motion to Dismiss Ms. Chandler’s Counterclaim with the Florida Circuit Court (the “Motion
to Dismiss”). The Motion to Dismiss stated that Ms. Chandler’s Counterclaim for defamation per se should be dismissed with
prejudice. On July 13, 2022, the court ruled that the counterclaim of defamation was dismissed with prejudice. Ms. Chandler’s deposition
was taken on September 21, 2022. On November 21, 2022, the Company filed a notice of voluntary dismissal without prejudice of its complaint
and the case was dismissed.
Although
we obtained a CE certificate to market DuraGraft in the EU under the Company’s name in May 2021, prior to the cancellation of the
CE certificate to market DuraGraft in the EU under the name of our subsidiary, we were not able to make the necessary labeling changes
which were required to reinitiate the marketing and distribution of DuraGraft products under the Company’s name until June 2022,
due primarily to supply chain and production interruptions resulting from the COVID-19 pandemic. As a result of having to restore our
quality management system and relabel DuraGraft, we were compelled to hire, at significant cost, two full-time quality consultants in
November 2021. Fulfillment of orders of our DuraGraft product was delayed for more than nine months during the relabeling process. We
believe that at least some of our distributors may have lost trust in our ability to deliver DuraGraft as a result. Although no orders
have been cancelled, we suffered delayed and lost revenue until the relabeling process could be completed. Since June 2022, we have resumed
shipments of correctly-labeled DuraGraft to our distributors, but may not have fully restored the trust of our distributors and recaptured
market momentum.
Future
potential misconduct could include fraud, theft of trade secrets, corporate sabotage, or other improper activities such as falsifying
time or other records and violations of laws. Other examples could include the failure to comply with our policies and procedures or
with foreign, federal, state or local government procurement regulations, regulations regarding the use and safeguarding of classified
or other protected information, legislation regarding the pricing of labor and other costs in government contracts, laws and regulations
relating to environmental, health or safety matters, bribery of foreign government officials, import-export control, lobbying or similar
activities, and any other applicable laws or regulations. Any such misconduct could result in claims, remediation costs, regulatory sanctions
against us, loss of current and future customers or contracts and serious harm to our reputation. Although we have implemented policies,
procedures and controls to prevent and detect these activities, these precautions have not in the past and may not prevent all misconduct,
and as a result, we could face unknown risks or losses. Our failure to comply with applicable laws or regulations as a result of the
misconduct by any of our employees, subcontractors, agents or business partners could damage our reputation, force us to expend significant
resources to address and cure such misconduct, delay, disrupt or fatally undermine our business plans and operations, and subject us
to fines and penalties, restitution or other damages, loss of regulatory clearance, loss of current and future customer contracts, any
of which could irreparably and materially adversely affect our business, reputation and our future results.
We
have negative working capital.
The
Company had negative working capital (current assets less current liabilities) of approximately $(19.8 million) as of June
30, 2023, negative working capital of approximately $1.0 million as of December 31, 2022, and working capital of approximately $1.3
million as of December 31, 2021. Any significant declines in our revenues could result in decreases in our working capital, which would
reduce our cash balances. Our failure to generate sufficient revenues or profits or to obtain additional financing or raise additional
capital could have a material adverse effect on our operations and on our ability to meet our obligations as they become due. The occurrence
of any of the foregoing risks would have a material adverse effect on our financial results, business and prospects.
We
have a limited operational history.
We
have a limited history upon which an evaluation of our prospects and future performance can be made. Our ongoing and proposed operations
are subject to all business risks associated with new enterprises. The likelihood of our success must take into consideration the problems,
expenses, difficulties, complications, and delays frequently encountered in connection with the expansion of a business operation in
an emerging industry, and the continued development of advertising, promotions, and a corresponding customer base. There is a possibility
that we could sustain losses in the future, and there are no assurances that we will ever operate profitably.
We
will need to increase the size of our organization.
We
are a small company with 12 full-time employees and two full-time consultants as of October 12, 2023. To execute our business
plan, including the future conducting of clinical trials and the expected commercialization of our medical devices, we will need to expand
our employee base for managerial, operational, financial, and other resources. Future growth will impose significant added responsibilities
on members of management, including the need to identify, recruit, maintain and integrate additional employees. Over the next 12 months,
depending on the progress of our acquisition efforts and future planned business development and capital raising efforts, we plan to
add additional employees to assist us with our development programs. Our future financial performance and our ability to commercialize
our products and devices and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To
that end, we must be able to:
| ● | manage
development efforts effectively; |
| ● | manage
any future clinical trials effectively; |
| ● | integrate
additional management, administrative, manufacturing and sales and marketing personnel; |
| ● | maintain
sufficient administrative, accounting and management information systems and controls; and |
| ● | hire
and train additional qualified personnel. |
We
may not be able to accomplish these tasks, and our failure to accomplish any of them could harm our financial results and impact our
ability to achieve development milestones.
If
we fail to retain current members of our senior management, or to identify, attract, integrate and retain additional key personnel, our
business will be harmed.
In
order to develop our medical devices, we need to retain or attract certain personnel, consultants or advisors with experience in medical
device development activities that include a number of disciplines, including research and development, clinical trials, medical matters,
government regulation medical devices, manufacturing, formulation and chemistry, business development, accounting, finance, regulatory
affairs, human resources and information systems. We are highly dependent upon our senior management and consultants. The loss of services
of one or more of our members of senior management could delay or prevent the successful completion of our planned product development
or the commercialization of medical devices.
Our
success depends in part on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific
personnel and on our ability to develop and maintain important relationships with leading academic institutions, clinicians, and scientists.
The competition for qualified personnel in medical device field is intense. We will need to hire additional personnel as we expand our
product development and commercial activities. While, generally, we have not had difficulties recruiting qualified individuals, to date,
we may not be able to attract and retain quality personnel on acceptable terms given the competition for such personnel among medical
device and other life science companies. In connection with the acquisition of My Health Logic, we engaged a new Chief Executive Officer,
Chief Financial Officer and Vice President of Finance. If we are not able to retain these individuals in their current functions, we
may not be able to execute our business plan and maximize our growth strategy, to the detriment of our business. Additionally, the Company
does not carry key person life insurance. If we lose any key managers or employees or are unable to attract and retain qualified key
personnel, directors, advisors or consultants, the development of our medical devices could be delayed or terminated, and our business
may be harmed.
If
we do not generate sufficient cash flow from operations in the future, we may not be able to fund our product development efforts and
acquisitions or fulfill our future obligations.
Our
ability to generate sufficient cash flow from operations to fund our operations and product development efforts, including the payment
of cash consideration in acquisitions and the payment of our other obligations, depends on a range of economic, competitive, and business
factors, many of which are outside of our control. We cannot assure you that our business will generate sufficient cash flow from operations,
or that we will be able to liquidate our investments, repatriate cash and investments held in our overseas subsidiaries, sell assets,
or raise equity or debt financings when needed or desirable. An inability to fund our operations or fulfill outstanding obligations could
have a material adverse effect on our business, financial condition, and results of operations. For further information, please refer
to “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital
Resources.”
We
will require substantial additional funding which may not be available to us on acceptable terms, or at all. Failing to raise the necessary
additional capital could force us to delay, reduce, eliminate or abandon growth initiatives, development or commercialization of our
technologies and products.
Our
existing capital resources will not be sufficient to meet our projected operating requirements beyond December 2023, and there will remain
substantial doubt regarding our ability to continue as a going concern unless we receive substantial financing. We expect to significantly
increase our spending to advance the development of our medical devices and launch and commercialize any medical devices for which we
receive regulatory approval. This might include the possibility of building our own marketing and sales organizations to address certain
markets if we fail to identify and engage third-party organizations that can perform these services for us.
We
will also require additional capital to fund our other operating expenses and capital expenditures. Our future capital requirements will
depend on many factors, including:
| ● | the
progress of our product development programs; |
| ● | the
number of technologies and products we pursue; |
| ● | the
time and costs involved in obtaining regulatory approvals; |
| ● | the
costs involved in filing and prosecuting patent applications and enforcing or defending patent
claims; |
| ● | our
plans to establish sales, marketing and/or manufacturing capabilities; |
| ● | the
effect of competing technological and market developments; |
| ● | the
terms and timing of any collaborative, licensing, and other arrangements that we may establish; |
| ● | general
market conditions for offerings from life science companies; |
| ● | our
ability to establish, enforce and maintain selected strategic alliances and activities required
for technology and product commercialization; and |
| ● | our
revenues, if any, from successful development and commercialization of our technologies and
products. |
In
order to carry out our business plans and implement our strategy beyond December 2023, we anticipate that we will need to obtain additional
financing from time to time and may choose to raise additional funds through strategic collaborations, licensing arrangements, additional
public or private equity or debt financing, bank lines of credit, asset sales, government grants, or other arrangements. We cannot be
sure that any additional funding, if needed, will be available on terms favorable to us or at all. Furthermore, any additional equity
or equity-related financing may be dilutive to our stockholders, and debt or equity financing, if available, may subject us to restrictive
covenants and significant interest costs. If we obtain funding through a strategic collaboration or licensing arrangement, we may be
required to relinquish our rights to certain of our medical device or marketing territories. Our inability to raise capital when needed
would harm our business, financial condition, and results of operations, and could cause the price of our common stock to decline or
require that we wind down our operations altogether.
We
may not be able to monetize intangible assets, which may result in the need to record an impairment charge.
As
part of our acquisition of assets from ACB Holding, completed on September 12, 2018, Marizyme acquired all rights, titles, and interest
in the Krillase technology, a group of intangible assets which at that time was valued at $28,600,000. The useful lives of the intangible
assets are based on the life of the patent and related technology. The patents and related technology for Krillase have not been amortized
since the acquisition, as they have not yet been put into operation. At December 31, 2022, management determined that the carrying value
of Krillase exceeded its recoverable amount. Impairment of $24,350,000 was recognized on these intangible assets for the year ended December
31, 2022.
The
Company previously planned to develop and commercialize FDA-approved products based on the Krillase assets. We suspended these plans
due to our determination to prioritize the completion of regulatory processes to obtain FDA authorization for the commercialization
of DuraGraft in the United States and the development of a functional MATLOC device prototype and MAR-FG-001-based viable products. We
intend to maintain the Krillase assets for potential future development and commercialization or disposition. Any determination as to
these matters would be based on a number of factors. See “Management’s Discussion and Analysis of Financial Condition
and Results of Operations – Principal Factors Affecting Our Financial Performance” for a summary of factors that we may
consider in this respect.
There
is no assurance that any of our intellectual property assets will ever be developed and fully commercialized and generate significant
revenues or will ever attract significant interest from potential buyers or investors. See “Risk Factors – Risks Related
to Our Business – We may not be able to monetize intangible assets, which may result in the need to record an impairment charge.”
If the Company is unable to reinitiate development of products based on the Krillase assets before the end of 2023, it is likely that
further impairment to the Krillase assets will be required.
Our
consolidated balance sheet as of June 30, 2023 contains approximately $27.3 million of intangible assets and approximately
$7.2 million in goodwill. The risk of failure to monetize intangible assets and goodwill is significant, and there can be no certainty
that these assets ultimately will yield successful products. The nature of our business is high-risk and requires that we invest in a
large number of projects in an effort to achieve a successful portfolio of approved products. Our ability to realize value on these significant
investments is often contingent upon, among other things, regulatory approvals, availability of resources, and market acceptance. These
intangible and goodwill assets may become impaired and be written off at some time in the future, which can have a material adverse effect
on the financial statements.
Acquisitions
present many risks, and we may not realize the financial and strategic goals we anticipate at the time of an acquisition.
Our
growth is dependent upon market growth, our ability to enhance existing products, and our ability to introduce new products and services
on a timely basis. In recent years, we have addressed and intend to continue to address the need to develop new products and services
and enhance existing products through acquisitions of other companies, product lines and/or technologies. However, acquisitions, including
those of high-technology companies, are inherently risky. We cannot provide any assurance that any of our acquisitions or future acquisitions
will be successful in helping us reach our financial and strategic goals. The risks we commonly encounter in undertaking, managing, and
integrating acquisitions are:
| ● | an
uncertain revenue and earnings stream from the acquired company, which could dilute our earnings; |
| ● | difficulties
and delays in integrating the personnel, operations, technologies, products, and systems
of the acquired companies; |
| ● | our
ongoing business may be disrupted, and our management’s attention may be diverted by
acquisition, transition, or integration activities; |
| ● | the
need to implement controls, procedures, and policies appropriate for a larger public company
at companies that prior to acquisition had lacked such controls, procedures, and policies; |
| ● | difficulties
managing or integrating an acquired company’s technologies or lines of business; |
| ● | potential
difficulties in completing projects associated with purchased in-process research and development; |
| ● | entry
into markets in which we have no or limited direct prior experience and where competitors
have stronger market positions, and which are highly competitive; |
| ● | the
potential loss of key employees of the acquired company; |
| ● | potential
difficulties integrating the acquired products and services into our sales channel; |
| ● | assuming
pre-existing contractual relationships of an acquired company that we would not have otherwise
entered the termination or modification of which may be costly or disruptive to our business; |
| ● | being
subject to unfavorable revenue recognition or other accounting treatment because of an acquired
company’s practices; and |
| ● | intellectual
property claims or disputes. |
Our
failure to manage growth effectively and successfully integrate acquired assets and/or companies due to these or other factors could
have a material adverse effect on our business, results of operations and financial condition. In addition, we may not have the opportunity
to make suitable acquisitions on favorable terms in the future, which could negatively impact the growth of our business. We expect that
other companies in our industry will compete with us to acquire compatible businesses. This competition could increase prices for businesses
and technologies that we would likely pursue, and our competitors may have greater resources than we do to complete these acquisitions.
The
medical device market is highly competitive, and we may not be able to effectively compete against other providers of medical devices,
particularly those with greater resources.
We
expect to face intense competition from companies with dominant market positions in the medical device industry. These competitors have
significantly greater financial, technical, marketing and other resources than we have and may be better able to:
| ● | respond
to new technologies or technical standards; |
| ● | react
to changing customer requirements and expectations; |
| ● | acquire
other companies to gain new technologies or products that may displace our products; |
| ● | manufacture,
market and sell products; |
| ● | acquire,
prosecute, enforce and defend patents and other intellectual property; |
| ● | devote
resources to the development, production, promotion, support and sale of products; and |
| ● | deliver
a broad range of competitive products at lower prices. |
We
expect competition in the markets in which we participate to continue to increase as existing competitors improve or expand their product
offerings.
Our
future performance may depend on the success of products we have not yet developed or acquired.
Technology
is an important component of our business and growth strategy, and our success depends on the development, implementation and acceptance
of our products. Commitments to develop new products must be made well in advance of any resulting sales, and technologies and standards
may change during development, potentially rendering our products outdated or uncompetitive before their introduction. Our ability to
develop products to meet evolving industry requirements and at prices acceptable to our customers will be significant factors in determining
our competitiveness. We may expend considerable funds and other resources on the development of our products without any guarantee that
these products will be successful. If we are not successful in bringing one or more products to market, whether because we fail to address
marketplace demand, fail to develop viable technologies or otherwise, our revenues may decline and our results of operations could be
seriously harmed.
Our
products may never achieve market acceptance.
Our
ability to generate revenues from product sales and to achieve profitability will depend upon our ability to successfully commercialize
our products. Because we have not yet begun to offer any of our products for sale in the U.S. and have limited sales of DuraGraft overseas,
we have no basis to predict whether any of our products will achieve market acceptance. A number of factors may limit the market acceptance
of any of our products, including:
| ● | the
timing of regulatory approvals of our products and market entry compared to competitive products; |
| ● | the
effectiveness of our products, including any potential side effects, as compared to alternative
treatments; |
| ● | the
rate of adoption of our products by hospitals, doctors and nurses and acceptance by the health
care community; |
| ● | the
competitive features of our products, including price, as compared to other similar products; |
| ● | the
availability of insurance or other third-party reimbursement, such as Medicare, for patients
using our products; |
| ● | the
extent and success of our marketing efforts and those of our collaborators; and |
| ● | unfavorable
publicity concerning our products or similar products. |
We
may delay or terminate the development or acquisition of a product at any time if we believe the perceived market or commercial opportunity
does not justify further investment, which could materially harm our business.
Even
though the results of preclinical studies and clinical trials that we have conducted or may conduct in the future may support further
development of one or more of our products, we may delay, suspend or terminate the future development or acquisition of a product at
any time for strategic, business, financial or other reasons, including the determination or belief that the emerging profile of the
product is such that it may not receive FDA approval, gain meaningful market acceptance, generate a significant return to stockholders,
or otherwise provide any competitive advantages in its intended indication or market.
Any
products we may develop or acquire may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare
reform initiatives, thereby harming our business.
The
regulations that govern marketing approvals, pricing and reimbursement for new products vary widely from country to country. Some countries
require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after
marketing approval is granted. In some foreign markets, pricing remains subject to continuing governmental control even after initial
approval is granted. As a result, we might obtain regulatory approval for a product in a particular country, but then be subject to price
regulations that delay our commercial launch of the product and negatively impact the revenue we are able to generate from the sale of
the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more other products
we may develop, even if other products we may develop or acquire obtain regulatory approval. Pressure from social activist groups and
future government regulations, whose goal it is to reduce the cost of medical devices, particularly in less developed nations, also may
result in downward pressure on the prices of our product.
Our
ability to commercialize any products we may develop or acquire successfully also will depend in part on the extent to which reimbursement
for these products and related treatments becomes available from government health administration authorities, private health insurers
and other organizations. Government authorities and third-party payors, such as private health insurers and health maintenance organizations,
decide which treatments they will pay for and establish reimbursement levels. A primary trend in the U.S. healthcare industry and elsewhere
is cost containment. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the
amount of reimbursement for particular treatments. We cannot be sure that reimbursement will be available for any product that we commercialize
and, if reimbursement is available, what the level of reimbursement will be. Reimbursement may impact the demand for, or the price of,
any product for which we obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may
not be able to successfully commercialize any product that we successfully develop.
Moreover,
eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including
research, development, acquisition, manufacture, sale and distribution. Payment rates may vary according to the use of the product and
the clinical setting in which it is used, may be based on payments allowed for lower cost products that are already reimbursed and may
be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required
by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of products
from countries where they may be said at lower prices than in the U.S. Third-party payors often rely upon Medicare coverage policy and
payment limitations in setting their own reimbursement policies. Our inability to promptly obtain coverage and profitable payment rates
from both government funded and private payors could have a material adverse effect on our operating results, our ability to raise capital
needed to commercialize products and our overall financial condition. To obtain reimbursement or pricing approval in some countries,
we may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available therapies. Our
business could be materially harmed if reimbursement of any products we may develop, if any, is unavailable or limited in scope or amount
or if pricing is set at unsatisfactory levels.
Product
liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we
may develop or acquire.
We
face an inherent risk of product liability exposure related to the sale of any products we may develop or acquire. The marketing, sale
and use of any products we may develop or acquire could lead to the filing of product liability claims against us if someone alleges
that our products failed to perform as designed. We may also be subject to liability for a misunderstanding of, or inappropriate reliance
upon, the information we provide. If we cannot successfully defend ourselves against claims that any product we may develop or acquire
caused injuries, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| ● | decreased
demand for our products; |
| ● | injury
to our reputation and significant negative media attention; |
| ● | withdrawal
of patients from clinical studies or cancellation of studies; |
| ● | significant
costs to defend the related litigation and distraction to our management team; |
| ● | substantial
monetary awards to patients; |
| ● | the
inability to commercialize any products that we may develop or acquire. |
In
addition, insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in
an amount adequate to satisfy any liability that may arise.
We
may not be able to protect or enforce our intellectual property rights, which could impair our competitive position.
Our
success depends significantly on our ability to protect our rights to the patents, trademarks, trade secrets, copyrights and all the
other intellectual property rights used, or expected to be used, in our products. Protecting intellectual property rights is costly and
time consuming. We rely primarily on patent protection and trade secrets, as well as a combination of copyright and trademark laws and
nondisclosure and confidentiality agreements to protect our technology and intellectual property rights. However, these legal means afford
only limited protection and may not adequately protect our rights or permit us to gain or maintain any competitive advantage. Despite
our intellectual property rights practices, it may be possible for a third party to copy or otherwise obtain and use our technology without
authorization, develop similar technology independently or design around our patents.
We
cannot be assured that any of our pending patent applications will result in the issuance of a patent to us. The U.S. Patent and Trademark
Office, or PTO, may deny or require significant narrowing of claims in our pending patent applications, and patents issued as a result
of the pending patent applications, if any, may not provide us with significant commercial protection or be issued in a form that is
advantageous to us. We could also incur substantial costs in proceedings before the PTO. Patents that may be issued to or licensed by
us in the future may expire or may be challenged, invalidated or circumvented, which could limit our ability to stop competitors from
marketing related technologies. Upon expiration of our issued or licensed patents, we may lose some of our rights to exclude others from
making, using, selling or importing products using the technology based on the expired patents. There is no assurance that competitors
will not be able to design around our patents. We also rely on unpatented proprietary technology. We cannot assure you that we can meaningfully
protect all our rights in our unpatented proprietary technology or that others will not independently develop substantially equivalent
proprietary products or processes or otherwise gain access to our unpatented proprietary technology.
Further,
we may not be able to obtain patent protection or secure other intellectual property rights in all the countries in which we operate,
and under the laws of such countries, patents and other intellectual property rights may be unavailable or limited in scope. If any of
our patents fails to protect our technology, it would make it easier for our competitors to offer similar products. Our trade secrets
may be vulnerable to disclosure or misappropriation by employees, contractors and other persons. Any inability on our part to adequately
protect our intellectual property may have a material adverse effect on our business, financial condition and results of operations.
We
seek to protect our know-how and other unpatented proprietary technology with confidentiality agreements and/or intellectual property
assignment agreements with our team members, independent distributors and consultants. However, such agreements may not be enforceable
or may not provide meaningful protection for our proprietary information in the event of unauthorized use or disclosure or other breaches
of the agreements or in the event that our competitors discover or independently develop similar or identical designs or other proprietary
information. In addition, we intend to rely on the use of registered and common law trademarks with respect to the brand names of some
of our products. Common law trademarks provide less protection than registered trademarks. Loss of rights in our trademarks could adversely
affect our business, financial condition and results of operations.
Confidentiality
agreements with employees and others may not adequately prevent disclosure of trade secrets and other proprietary information and may
not adequately protect our intellectual property.
We
rely on trade secrets to protect our technology, especially where we do not believe patent protection is obtainable, or prior to us filing
patent applications on inventions we may make from time to time. However, trade secrets are difficult to protect. In order to protect
our proprietary technology and processes, we also rely in part on confidentiality and intellectual property assignment agreements with
our corporate partners, employees, consultants, outside scientific collaborators and sponsored researchers and other advisors. These
agreements may not effectively prevent disclosure of confidential information nor result in the effective assignment to us of intellectual
property and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information or other breaches
of the agreements. In addition, others may independently discover our trade secrets and proprietary information, and in such case, we
could not assert any trade secret rights against such party. Enforcing a claim that a third-party illegally obtained and is using our
trade secrets is difficult, expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the U.S. may
be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the
scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business
position.
We
may be subject to intellectual property infringement claims by third parties which could be costly to defend, divert management’s
attention and resources, and may result in liability.
The
medical device and life science industry is characterized by vigorous protection and pursuit of intellectual property rights. Companies
in the medical device and life science industry have used intellectual property litigation to gain a competitive advantage in the marketplace.
From time to time, third parties may assert against us their patent, copyright, trademark and other intellectual property rights relating
to technologies that are important to our business. Searching for existing intellectual property rights may not reveal important intellectual
property and our competitors may also have filed for patent protection, which is not publicly-available information, or claimed trademark
rights that have not been revealed through our availability searches. We may be subject to claims that our team members have disclosed,
or that we have used, trade secrets or other proprietary information of our team members’ former employers. Our efforts to identify
and avoid infringing on third parties’ intellectual property rights may not always be successful. Any claims that our products
or processes infringe these rights, regardless of their merit or resolution, could be costly, time consuming and may divert the efforts
and attention of our management and technical personnel. In addition, we may not prevail in such proceedings given the complex technical
issues and inherent uncertainties in intellectual property litigation.
Any
claims of patent or other intellectual property infringement against us, even those without merit, could:
| ● | increase
the cost of our products; |
| ● | be
expensive and/or time consuming to defend; |
| ● | result
in our being required to pay significant damages to third parties; |
| ● | force
us to cease making or selling products that incorporate the challenged intellectual property; |
| ● | require
us to redesign, reengineer or rebrand our products and technologies; |
| ● | require
us to enter into royalty or licensing agreements in order to obtain the right to use a third
party’s intellectual property on terms that may not be favorable or acceptable to us; |
| ● | require
us to develop alternative non-infringing technology, which could require significant effort
and expense; |
| ● | require
us to indemnify third parties pursuant to contracts in which we have agreed to provide indemnification
for intellectual property infringement claims; |
| ● | result
in our customers or potential customers deferring or limiting their purchase or use of the
affected products impacted by the claims until the claims are resolved; and |
| ● | otherwise
have a material adverse effect on our business, financial condition and results of operations. |
Any
of the foregoing could affect our ability to compete or have a material adverse effect on our business, financial condition and results
of operations.
Competitors
may violate our intellectual property rights, and we may bring litigation to protect and enforce our intellectual property rights, which
may result in substantial expense and may divert our attention from implementing our business strategy.
We
believe that the success of our business will depend, in significant part, on obtaining patent protection for our products and technologies,
defending our patents and preserving our trade secrets. Our failure to pursue any potential claim could result in the loss of our proprietary
rights and harm our position in the marketplace. Therefore, we may be forced to pursue litigation to enforce our rights. Future litigation
could result in significant costs and divert the attention of our management and key personnel from our business operations and the implementation
of our business strategy.
We
may be dependent on third-party manufacturers since we will not initially directly manufacture our products.
Initially,
we will not directly manufacture our products and will rely on third parties to do so for us. If our manufacturing and distribution agreements
are not satisfactory, we may not be able to develop or commercialize products as planned. In addition, we may not be able to contract
with third parties to manufacture our products in an economical manner. Furthermore, third-party manufacturers may not adequately perform
their obligations, may delay clinical development or submission of products for regulatory approval or otherwise may impair our competitive
position. We may not be able to enter into or maintain relationships with manufacturers who have the capacities to meet our manufacturing
needs, master the manufacturing processes required for our products, and comply with good manufacturing practices. If a product manufacturer
fails to comply with good manufacturing practices, we could experience significant time delays or we may be unable to commercialize or
continue to market the products. Changes in our manufacturers could require costly new product testing and facility compliance inspections.
In the United States, failure to comply with good manufacturing practices or other applicable legal requirements can lead to federal
seizure of violative products, injunctive actions brought by the federal government, and potential criminal and civil liability on the
part of a company and its officers and employees. Because of these and other factors, we may not be able to replace our manufacturing
capacity quickly or efficiently in the event that our manufacturers are unable to manufacture our products at one or more of their facilities.
We
have no experience in large-scale product manufacturing, nor do we have the resources or facilities to manufacture our products. We cannot
guarantee that we or our third-party manufacturers will be able to increase capacity in a timely or cost-effective manner, or at all.
Delays in providing or increasing production or processing capacity could result in additional expense or delays in our clinical trials,
regulatory submissions and commercialization of our products.
As
a result of these factors, the sale and marketing of our products could be delayed or we could be forced to develop our own manufacturing
capacity, which could require substantial additional funds and personnel and compliance with extensive regulations.
We
currently have no marketing and sales organization and have limited experience as a company in commercializing products, and we may need
to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities in the
United States, we may not be able to generate substantial product revenue.
We
have no internal sales, marketing or distribution capabilities, and have only limited experience with commercializing a product. Although
our medical device product DuraGraft has received regulatory authorization in the United States, we must build a marketing and sales
organization with technical expertise and supporting distribution capabilities to commercialize this product in the U.S. market, which
may be expensive or time-consuming. We have only limited experience as a company with the marketing, sale or distribution of biopharmaceutical
products and there are significant risks involved in the building and managing of a sales organization, including our ability to hire,
retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel
and effectively manage a sales and marketing team. Any failure or delay in the development of our internal sales, marketing and distribution
capabilities would adversely impact the commercialization of our products in the United States. If we are not successful in commercializing
our product in the U.S., we may not be able to generate substantial future product revenue and we would incur significant additional
losses.
We
may be dependent on the sales and marketing efforts of third parties, especially internationally, if we choose not to develop
an extensive sales and marketing staff.
Initially,
we will depend to some extent on the efforts of third parties (including sales agents and distributors) to carry out the sales
and marketing of our products, particularly with respect to international sales. We currently have distribution partners internationally
for DuraGraft, which we expect to continue to work with in the future. We anticipate that each third party will control the amount and
timing of resources generally devoted to these activities. However, these third parties may not be able to generate demand for our products.
In addition, there is a risk that these third parties will develop products competitive to ours, which would likely decrease their incentive
to vigorously promote and sell our products. If we are unable to enter into co-promotion agreements or to arrange for third-party distribution
of our products, we will be required to expend time and resources to develop an effective internal sales force. However, it may not be
economical for us to market our own products, or we may be unable to effectively market our products, especially outside the
United States. Therefore, our business could be harmed if we fail to enter into arrangements with third parties for the sales and
marketing of our products or otherwise fail to establish sufficient marketing capabilities.
Security
breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation
to suffer.
In
the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information
and that of our suppliers and business partners, as well as personally identifiable information of clinical trial participants and employees.
Similarly, our business partners and third-party providers possess certain of our sensitive data. The secure maintenance of this information
is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may
be vulnerable to attacks by hackers or breached due to employee error, malfeasance, or other disruptions. Any such breach could compromise
our networks and the information stored there could be accessed, publicly disclosed, lost, or stolen. Any such access, disclosure, or
other loss of information, including our data being breached at our business partners or third-party providers, could result in legal
claims or proceedings, liability under laws that protect the privacy of personal information, disrupt our operations, and damage our
reputation which could adversely affect our business.
Our
business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Natural
disasters could severely disrupt our operations or the operations of manufacturing facilities and have a material adverse effect on our
business, financial condition, results of operations and prospects. If a natural disaster, power outage or other event occurred that
prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as manufacturing
facilities, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible for us to continue our business
for a substantial period of time. The disaster recovery and business continuity plans that we have in place currently are limited and
may not prove adequate in the event of a serious disaster or similar event. We are in the early stages of constructing an additional
manufacturing facility and establishing a relationship with a third-party contract manufacturer as a back-up supplier for the commercial
supply of our products, if necessary, but there is no assurance that we will establish such a relationship in a timely manner, on acceptable
terms, or at all. We may incur substantial expenses as a result of the limited nature of our disaster recovery and business continuity
plans, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
The
COVID-19 pandemic has adversely impacted the Company’s supply chain and could materially and adversely affect our ability to conduct
clinical trials and engage with our third-party vendors and thereby have a material adverse effect on our financial results.
The
Company has been impacted by the COVID-19 pandemic and related supply chain shortages and other economic conditions, and some of its
earlier plans to diversify and expand its operations were delayed as a result. Moreover, the impact of the COVID-19 pandemic on the Company’s
supply chain and its ability to produce DuraGraft inventory was a primary reason that we did not generate substantial revenue from sales
of DuraGraft during 2021 and 2022. The Company’s inventory production of DuraGraft returned to its pre-pandemic level at the end
of the second quarter of 2022, but lingering effects of the COVID-19 pandemic continued to depress demand for DuraGraft and cause revenues
from DuraGraft during the first and second quarters of 2023 to be minimal. There can be no assurance that future supply chain disruptions
and other effects of COVID-19 outbreaks will not adversely impact our revenues.
In
addition, the Company is dependent upon certain contract manufacturers and suppliers and their ability to reliably and efficiently fulfill
its orders is critical to the Company’s business success. The COVID-19 pandemic has impacted and may continue to impact certain
of the Company’s manufacturers and suppliers, which could result in unavoidable delays and/or increases in our operating costs.
If we are unable to obtain our devices in sufficient quantity and in a timely manner, the development and testing of our medical devices
may be delayed or become infeasible, and regulatory approval or commercial launch of any of our medical devices may be delayed or not
obtained. As a result, the Company has faced and may continue to face additional delays, costs or difficulty sourcing certain products,
which could negatively affect the Company’s business and financial results.
While
it is not possible at this time to estimate the total impact that COVID-19 could have on our business in the future, the continued spread
of COVID-19 and variants of the virus, the rate of vaccinations regionally and globally and the measures taken by the government authorities,
and any future epidemic disease outbreaks, could: Disrupt the supply chain and the manufacture or shipment of products and supplies for
use by us in our research activities and by strategic partners for their distribution and sales activities; delay, limit or prevent us
in our research activities and strategic partners in their distribution and sales activities; impede our negotiations with strategic
partners; impede testing, monitoring, data collection and analysis and other related activities by us; interrupt or delay the operations
of the FDA or other regulatory authorities, which may impact review and approval timelines for initiation of clinical trials or marketing;
or impede the launch or commercialization of any approved products; any of which could delay our strategic partnership plans, increase
our operating costs, and have a material adverse effect on our business, financial condition and results of operations.
Our
business, financial condition and results of operations could be adversely affected by the political and economic conditions of the countries
in which we conduct business.
Our
business, financial condition and results of operations could be adversely affected by the political and economic conditions of the countries
in which we conduct business. These factors include:
| ● | challenges
associated with cultural differences, languages and distance; |
| ● | differences
in clinical practices, needs, products, modalities and preferences; |
| ● | longer
payment cycles in some countries; |
| ● | credit
risks of many kinds; |
| ● | legal
and regulatory differences and restrictions; |
| ● | currency
exchange fluctuations; |
| ● | foreign
exchange controls that might prevent us from repatriating cash earned in certain countries; |
| ● | political
and economic instability and export restrictions; |
| ● | variability
in sterilization requirements for multi-usage surgical devices; |
| ● | potential
adverse tax consequences; |
| ● | higher
cost associated with doing business internationally; |
| ● | challenges
in implementing educational programs required by our approach to doing business; |
| ● | negative
economic developments in economies around the world and the instability of governments, including
the threat of war, terrorist attacks, epidemic or civil unrest; |
| ● | adverse
changes in laws and governmental policies, especially those affecting trade and investment; |
| ● | pandemics,
such as COVID-19, the Ebola virus, the enterovirus and the avian flu, which may adversely
affect our workforce as well as our local suppliers and customers; |
| ● | import
or export licensing requirements imposed by governments; |
| ● | differing
labor standards; |
| ● | differing
levels of protection of intellectual property; |
| ● | the
threat that our operations or property could be subject to nationalization and expropriation; |
| ● | varying
practices of the regulatory, tax, judicial and administrative bodies in the jurisdictions
where we operate; and |
| ● | potentially
burdensome taxation and changes in foreign tax. |
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance
by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and our
financial condition and results of operations.
Actual
events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional
counterparties or other companies in the financial services industry or the financial services industry generally, or concerns or rumors
about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems.
For example, on March 10, 2023, Silicon Valley Bank (“SVB”), was closed by the California Department of Financial Protection
and Innovation, which appointed the FDIC as receiver. Similarly, on March 12, 2023, Signature Bank Corp. (“Signature”), and
Silvergate Capital Corp. were each swept into receivership. Although a statement by the Department of the Treasury, the Federal Reserve
and the FDIC indicated that all depositors of SVB would have access to all of their money after only one business day of closure, including
funds held in uninsured deposit accounts, borrowers under credit agreements, letters of credit and certain other financial instruments
with SVB, Signature or any other financial institution that is placed into receivership by the FDIC may be unable to access undrawn amounts
thereunder. In addition, on May 1, the FDIC announced that First Republic had been closed by the California Department of Financial Protection
and Innovation and its assets seized by the FDIC. JPMorgan Chase eventually won the auction, paying the FDIC $10.6 billion for nearly
all of First Republic’s assets. Although we are not a borrower under or party to any material letter of credit or any other such
instruments with SVB, Signature or any other financial institution currently in receivership, if we enter into any such instruments and
any of our lenders or counterparties to such instruments were to be placed into receivership, we may be unable to access such funds.
In addition, if any of our customers, suppliers or other parties with whom we conduct business are unable to access funds pursuant to
such instruments or lending arrangements with such a financial institution, such parties’ ability to pay their obligations to us
or to enter into new commercial arrangements requiring additional payments to us could be adversely affected. In this regard, counterparties
to credit agreements and arrangements with these financial institutions, and third parties such as beneficiaries of letters of credit
(among others), may experience direct impacts from the closure of these financial institutions and uncertainty remains over liquidity
concerns in the broader financial services industry. Similar impacts have occurred in the past, such as during the 2007-2008 financial
crisis.
Inflation
and rapid increases in interest rates have led to a decline in the trading value of previously-issued government securities with interest
rates below current market interest rates. Although the U.S. Department of Treasury, FDIC and Federal Reserve Board have announced a
program to provide up to $25 billion of loans to financial institutions secured by certain of such government securities held by financial
institutions to mitigate the risk of potential losses on the sale of such instruments, widespread demands for customer withdrawals or
other liquidity needs of financial institutions for immediately liquidity may exceed the capacity of such program.
Our
access to funding sources and other credit arrangements in amounts adequate to finance or capitalize our current and projected future
business operations could be significantly impaired by factors that affect us, any financial institutions with which we enter into credit
agreements or arrangements directly, or the financial services industry or economy in general. These factors could include, among others,
events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity
agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative
expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions
or financial services industry companies with which we have financial or business relationships, but could also include factors involving
financial markets or the financial services industry generally.
The
results of events or concerns that involve one or more of these factors could include a variety of material and adverse impacts on our
current and projected business operations and our financial condition and results of operations. These risks include, but may not be
limited to, the following:
| ● | delayed
access to deposits or other financial assets or the uninsured loss of deposits or other financial
assets; |
| ● | inability
to enter into credit facilities or other working capital resources; |
| ● | potential
or actual breach of contractual obligations that require us to maintain letters of credit
or other credit support arrangements; or |
| ● | termination
of cash management arrangements and/or delays in accessing or actual loss of funds subject
to cash management arrangements. |
In
addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing
terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit
and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available
funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses
or other obligations, financial or otherwise, result in breaches of our financial and/or contractual obligations, or result in violations
of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other
related or similar factors, could have material adverse impacts on our liquidity and our current and/or projected business operations
and financial condition and results of operations.
In
addition, any further deterioration in the economy or financial services industry could lead to losses or defaults by our distribution
partners, sponsors, vendors, or suppliers, which in turn, could have a material adverse effect on our current and/or projected business
operations and results of operations and financial condition. For example, a distribution partner may fail to make payments when due,
default under their agreements with us, become insolvent or declare bankruptcy, or a supplier may determine that it will no longer deal
with us as a customer. In addition, a vendor or supplier could be adversely affected by any of the liquidity or other risks that are
described above as factors that could result in material adverse impacts on us, including but not limited to delayed access or loss of
access to uninsured deposits or loss of the ability to draw on existing credit facilities involving a troubled or failed financial institution.
The bankruptcy or insolvency of any distribution partners, sponsor, vendor or supplier, or the failure of any distribution partner to
make payments when due, or any breach or default by a distribution partner, sponsor, vendor, or supplier, or the loss of any significant
supplier relationships, could cause us to suffer material losses and may have a material adverse impact on our business.
Climate
change impacts including supply chain disruptions, operational impacts, and geopolitical events may impact our business operations.
We
source a large number of raw materials from third-party suppliers globally. These products include both natural and synthetic materials
derived from plants, animal products, and organic and petroleum-based raw materials. Disruptions to the global supply chain due to climate-related
impacts or geopolitical events are possible and exist as external risk factors that the Company can respond to but not control. These
events could limit the supply of key raw materials to the Company, or could have significant impacts to pricing. We work with multiple
raw material suppliers to mitigate lack of availability from a single supplier, however in some cases products with limited numbers of
suppliers may become difficult to obtain.
Some
of our vendors have manufacturing operations in areas vulnerable to coastal storms which may increase in magnitude and impact due to
climate change. Increasingly large and unprecedented weather events may pose a risk to business operations in vulnerable areas. Storms
could cause business interruptions, incur additional restoration costs, and impact product availability and pricing.
Risks
Related to Government Regulation
Only
one of our products has been
authorized for marketing in the United States, other products may not be granted authorization, and any authorization
may be subject to limitations.
The
Company’s medical device, DuraGraft, has been authorized for marketing in the United States by the FDA for use as an intra-operative
vascular conduit storage and flushing solution used during CABG surgeries in the United States, subject to applicable risks, mitigation
requirements, and control provisions. However, none of our other current or future
products have been approved for sale in the United States. Neither we nor any future collaboration partner can commercialize any products
we may develop in the U.S. without first obtaining regulatory approval for the product from the FDA. The regulatory route in the
U.S. for any products we may develop will be through a De Novo process. The De Novo grant process may take several years to complete,
and De Novo grants may never be obtained for most of our products. Before obtaining regulatory approvals for the commercial
sale of any product we may develop in the U.S., we must demonstrate with substantial evidence, gathered in preclinical and well-controlled
clinical studies, that the planned product is safe and effective for use for that target indication. We may not have the resources
or ability to conduct such a trial or may not successfully enroll or complete any such trial. Any products we may develop may not
achieve the required primary endpoint in the clinical trial and may not receive regulatory approval. We must also demonstrate that the
manufacturing facilities, processes and controls for any products we may develop are adequate.
To
the extent that we or any future collaboration partner
has or seeks to successfully obtain a regulatory approval for any product we may develop for sale in the United States, any approval
might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications,
or may be subject to burdensome post-approval study or risk management requirements. If we are unable to obtain regulatory approval for
any products we may develop in the United States, or any approval contains significant limitations, we may not be able to obtain sufficient
revenue to justify commercial launch. Also, any regulatory approval of a product, once obtained, may be withdrawn. If we are unable to
successfully obtain regulatory approval to sell any products we may develop in the U.S., our business, financial condition, results of
operations and growth prospects could be adversely affected.
Failure
to obtain regulatory approvals in foreign jurisdictions will prevent us from marketing our products internationally.
Our
current overseas distribution and marketing partners for DuraGraft, and any other distribution and marketing partners for this and other
products we may seek to market in foreign countries, must comply with the regulations of the foreign countries in which they operate.
The approval procedures vary among countries and can involve additional clinical testing, and the time required to obtain approval may
differ from that required to obtain FDA approval. Moreover, clinical studies or manufacturing processes conducted in one country may
not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities
in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in
other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative
effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining
FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. We may not be able to file for regulatory
approvals and even if we file we may not receive necessary approvals to commercialize our products in any market.
Even
if we receive regulatory approval for any product we may develop or acquire, we will be subject to ongoing regulatory obligations and
continued regulatory review, which may result in significant additional expense and subject us to penalties if we fail to comply with
applicable regulatory requirements.
Once
regulatory approval has been obtained, the approved product and its manufacturer are subject to continual review by the FDA or non-U.S.
regulatory authorities. Our regulatory approval for any products we may develop or acquire may be subject to limitations on the indicated
uses for which the product may be marketed. Future approvals may contain requirements for potentially costly post-marketing follow-up
studies to monitor the safety and efficacy of the approved product. In addition, we are subject to extensive and ongoing regulatory requirements
by the FDA and other regulatory authorities with regard to the labeling, packaging, adverse event reporting, storage, advertising, promotion
and recordkeeping for our products. In addition, we are required to comply with cGMP regulations regarding the manufacture of any products
we may develop or acquire, which include requirements related to quality control and quality assurance as well as the corresponding maintenance
of records and documentation. Further, regulatory authorities must approve these manufacturing facilities before they can be used to
manufacture products, and these facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities
for compliance with cGMP regulations. If we or a third party discover previously unknown problems with a product, such as adverse events
of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory authority may impose
restrictions on that product, the manufacturer or us, including requiring withdrawal of the product from the market or suspension of
manufacturing.
Inadequate
funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent
new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing
normal business functions on which the operation of our business may rely, which could negatively impact our business.
The
ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding
levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes.
Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other
government agencies on which our operations may rely, including those that fund research and development activities, is subject to the
political process, which is inherently fluid and unpredictable.
Disruptions
at the FDA and other agencies may also slow the time necessary for new product candidates to be reviewed and/or approved by necessary
government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut
down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government
employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability
of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further,
future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize
and continue our operations.
If
a prolonged government shutdown or other disruption occurs, it could significantly impact the ability of the FDA to timely
review and process our regulatory submissions, which could have a material adverse effect on our business. Future shutdowns or other
disruptions could also affect other government agencies such as the SEC, which may also impact our business by delaying review of our
public filings, to the extent such review is necessary, and our ability to access the public markets.
Healthcare
reform measures could hinder or prevent our products’ commercial success.
In
the U.S., there have been, and we expect there will continue to be, ongoing legislative and regulatory changes to the healthcare system
which could affect our future revenue and profitability. Federal and state lawmakers regularly propose and, at times, enact legislation
that could result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical
products and services. For example, one of the most significant healthcare reform measures in decades, the Patient Protection and Affordable
Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or “ACA,” was enacted in 2010. The
ACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud
and abuse measures, all of which will impact existing government healthcare programs.
While
the U.S. Supreme Court has repeatedly upheld the constitutionality of most elements of the ACA, other legal challenges are still pending
final adjudication in several jurisdictions. Although efforts in Congress to repeal the ACA have repeatedly fallen short, there are a
number of ongoing legislative initiatives to modify it. At this time, it remains unclear whether there will be any changes made to the
ACA. We cannot assure you that the ACA, as currently enacted or as amended in the future, will not adversely affect our business and
financial results and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform
will affect our business.
In
addition, other legislative changes have been proposed and adopted since the ACA was enacted. There likely will continue to be legislative
and regulatory proposals at the federal and state levels directed at containing or lowering the cost of health care. Medicare reimbursement
for all products and services, including ours, remains highly susceptible to threats of automatic reductions triggered by budgetary shortfalls.
Such payments are subject to recovery of purported overpayment for several years. We cannot predict the initiatives that may be adopted
in the future or their full impact. We cannot predict whether any additional legislative changes will affect our business.
The
continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain
or reduce costs of health care may adversely affect:
| ● | our
ability to set a price that we believe is fair for our products; |
| ● | our
ability to generate revenue and achieve or maintain profitability; and |
| ● | the
availability of capital. |
Further,
changes in regulatory requirements and guidance may occur, both in the United States and in foreign countries, and we may need to amend
clinical study protocols to reflect these changes. Amendments may require us to resubmit our clinical study protocols to an IRB for reexamination,
which may impact the costs, timing or successful completion of a clinical study. In light of widely publicized events concerning the
safety risk of certain drug and medical device products, regulatory authorities, members of Congress, the Governmental Accounting Office,
medical professionals and the general public have all raised concerns about potential safety issues. These events have resulted in the
recall and withdrawal of medical device products, revisions to product labeling that further limit use of products and establishment
of risk management programs that may, for instance, restrict distribution of certain products or require safety surveillance or patient
education. The increased attention to safety issues may result in a more cautious approach by the FDA or other regulatory authorities
to clinical studies and the medical device approval process. Adverse event data from clinical studies may receive greater scrutiny with
respect to product safety, which may make the FDA or other regulatory authorities more likely to terminate or suspend clinical studies
before completion, or require longer or additional clinical studies that may result in substantial additional expense and a delay or
failure in obtaining approval or approval for a more limited indication than originally sought.
Given
the serious public health risks of high profile adverse safety events with certain products, the FDA or other regulatory authorities
may require, as a condition of approval, costly risk evaluation and mitigation strategies, which may include safety surveillance, restricted
distribution and use, patient education, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events,
preapproval of promotional materials and restrictions on direct-to-consumer advertising.
If
we fail to comply with healthcare regulations, we could face substantial penalties and our business, operations and financial condition
could be adversely affected.
Even
though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors,
certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are and will be applicable
to our business. The regulations that may affect our ability to operate include, without limitation:
| ● | the
federal healthcare program Anti-Kickback Statute, which prohibits, among other things, any
person from knowingly and willfully offering, soliciting, receiving or providing remuneration,
directly or indirectly, in exchange for or to induce either the referral of an individual
for, or the purchase, order or recommendation of, any good or service for which payment may
be made under federal healthcare programs, such as the Medicare and Medicaid programs; |
| ● | the
U.S. Foreign Corrupt Practices Act, which prohibits payments or the provision of anything
of value to foreign officials for the purpose of obtaining or keeping business; |
| ● | the
federal False Claims Act (“FCA”), which prohibits, among other things, individuals
or entities from knowingly presenting, or causing to be presented, false claims, or knowingly
using false statements, to obtain payment from the federal government, and which may apply
to entities like us which provide coding and billing advice to customers; |
| ● | federal
criminal laws that prohibit executing a scheme to defraud any healthcare benefit program
or making false statements relating to healthcare matters; |
| ● | the
federal transparency requirements under the ACA requires manufacturers of drugs, devices,
biologics and medical supplies to report to the Department of Health and Human Services information
related to physician payments and other transfers of value and physician ownership and investment
interests; |
| ● | the
federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”),
as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”),
which governs the conduct of certain electronic healthcare transactions and protects the
security and privacy of protected health information, and |
| ● | state
law equivalents of each of the above federal laws, such as anti-kickback and false claims
laws which may apply to items or services reimbursed by any third-party payor, including
commercial insurers. |
The
ACA, among other things, amends the intent requirement of the U.S. federal Anti-Kickback Statute and criminal healthcare fraud statutes.
A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA provides
that the government may assert that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback
Statute constitutes a false or fraudulent claim for purposes of the FCA.
If
our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us,
we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations.
Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business
and our financial results. Any action against us for violation of these laws, even if we successfully defend against it, could cause
us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving
and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
Climate
change and increased focus by governments, stockholders and customers on sustainability issues, including those related to climate change,
may have a material adverse effect on our business and operations.
Foreign,
federal, state and local governments, as well as some of our vendors and customers, are beginning to respond to climate change issues.
This increased focus on sustainability may result in new legislation or regulations and vendor and customer requirements that could negatively
affect us as we may incur additional costs or be required to make changes to our operations in order to comply with any new regulations
or vendor, customer, or stockholder requirements. Legislation or regulations that potentially impose restrictions, caps, taxes, or other
controls on emissions of greenhouse gases such as carbon dioxide, a by-product of burning fossil fuels, may have a material adverse effect
on our business and operation. For example, if the vendors we contract with to produce and ship our products become subject to increasingly
restrictive laws protecting the environment, including those relating to climate change, we expect that they would incur increased costs
and may pass such costs on to us, which could have a material adverse effect on our business. If our customers or stockholders were to
require us to use vendors that source, manufacture, or supply their products in accordance with certain sustainability standards, we
expect that such standards would likewise force us to incur additional costs and we may fail to pass such additional costs on to our
customers, which could also have a material adverse effect on our business.
In
addition, on March 21, 2022, the SEC proposed new rules requiring a range of climate-related disclosure that would be applicable to all
companies that are required to file annual reports or that file registration statements with the SEC, including the Company. The proposed
climate-related disclosure framework is modeled in part on the Task Force on Climate Related Financial Disclosures’ recommendations,
and also draws upon the Greenhouse Gas (“GHG”) Protocol (“GHG Protocol”). In particular, the proposed rules would
require a registrant to disclose information about: The oversight and governance of climate-related risks by the registrant’s board
and management; how any climate-related risks identified by the registrant have had or are likely to have a material impact on its business
and consolidated financial statements, which may manifest over the short-, medium-, or long-term; how any identified climate-related
risks have affected or are likely to affect the registrant’s strategy, business model, and outlook; the registrant’s processes
for identifying, assessing, and managing climate-related risks and whether any such processes are integrated into the registrant’s
overall risk management system or processes; the impact of climate-related events (severe weather events and other natural conditions
as well as physical risks identified by the registrant) and transition activities (including transition risks identified by the registrant)
on the line items of a registrant’s consolidated financial statements and related expenditures, and disclosure of financial estimates
and assumptions impacted by such climate-related events and transition activities; “Scope 1” and “Scope 2” (as
defined by the SEC’s proposed rule) GHG emissions metrics, separately disclosed, expressed both by disaggregated constituent greenhouse
gases and in the aggregate, and in absolute and intensity terms; “Scope 3” (as defined by the SEC’s proposed rule)
GHG emissions and intensity, if material, or if the registrant has set a GHG emissions reduction target or goal that includes its Scope
3 emissions; and the registrant’s climate-related targets or goals, and transition plan, if any. The proposed rules would be subject
to certain accommodations and phase-in periods. For example, companies meeting the definition of “smaller reporting company”
in Rule 12b-2 of the Exchange Act, which currently includes the Company (see below, “—We are a ‘smaller reporting
company’ within the meaning of the Exchange Act, and if we take advantage of certain exemptions from disclosure requirements available
to smaller reporting companies, this could make our securities less attractive to investors and may make it more difficult to compare
our performance with other public companies.” and “As a ‘smaller reporting company,’ we may at some time
in the future choose to exempt our company from certain corporate governance requirements that could have an adverse effect on our public
stockholders.”), would be exempt from the Scope 3 emissions disclosure requirement. The proposed rules would also require an
attestation report provided by a third-party attestation service provider that satisfies a minimum level of attestation services for
a company that meets the definition of “accelerated filer” or “large accelerated filer” in Rule 12b-2 of the
Exchange Act, including: (1) limited assurance for Scopes 1 and 2 emissions disclosure that scales up to reasonable assurance after a
specified transition period; (2) minimum qualifications and independence requirements for the attestation service provider; and (3) minimum
requirements for the accompanying attestation report. A company that is not an “accelerated filer” or “large accelerated
filer”, which currently includes the Company, would not be subject to this attestation requirement (see above, “—As
a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.”).
Although
we cannot predict the costs of implementation or any potential adverse impacts resulting from the proposed rule, the SEC estimated
that compliance costs for a “smaller reporting company” in the first year of compliance would be $490,000 ($140,000 for
internal costs and $350,000 for outside professional costs), while annual costs in the subsequent five years were estimated to be
$420,000 ($120,000 for internal costs and $300,000 for outside professional costs). For non-“smaller reporting
company” registrants, the costs in the first year of compliance were estimated to be $640,000 ($180,000 for internal costs and
$460,000 for outside professional costs), while annual costs in the subsequent five years were estimated to be $530,000 ($150,000
for internal costs and $380,000 for outside professional costs). To the extent that this rule is finalized as proposed, we could
therefore incur significant increased costs relating to the assessment and disclosure of climate-related matters.
These
potential additional costs, forced changes in operations, or loss of revenues may have a material adverse effect on our business and
operations.
Our
ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We
have and may incur again substantial net operating losses (NOLs) during our history. Unused NOLs may carry forward to offset future taxable
income if we achieve profitability in the future, unless such NOLs are limited or expire under applicable tax laws. Prior to 2018, net
operating losses could generally be carried back for a period of two years or carried forward for 20 years. The Tax Cuts and Jobs Act
of 2017 imposed certain limitations on the deduction of NOLs generated in tax years that began on or after January 1, 2018, including
a limitation on the use of NOLs to offset only 80% of taxable income and the general disallowance of NOL carrybacks. The Coronavirus
Aid, Relief, and Economic Security (CARES) Act of 2020 subsequently suspended that provision with respect to NOLs generated in 2018,
2019 and 2020, and allowed NOLs generated in those years to be carried back for a period of five years or carried forward indefinitely.
In
addition, under the rules of Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), if
a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership
over a three-year period, the corporation’s ability to use its NOLs and other pre-change tax attributes to offset its post-change
taxable income or taxes may be limited. The applicable rules generally operate by focusing on changes in ownership among stockholders
considered by the rules as owning, directly or indirectly, 5% or more of the stock of a company, as well as changes in ownership arising
from new issuances of stock by the company. As a result of these rules, in the event that we experience one or more ownership changes,
including as a result of this registered resale offering or future transactions in our stock, then we may be limited in our ability to
use our federal NOL carryforwards to offset our future taxable income, if any.
As
of December 31, 2022 and 2021, the Company had federal NOL carryforwards of $41,733,000 and $34,971,000, respectively. As of December
31, 2022 and 2021, the Company also had Florida state NOL carryforwards of $18,117,000 and $11,354,000, respectively. Under current tax
law, the federal and Florida state NOL carryforwards may be carried forward indefinitely. However, as discussed above, the Company’s
NOL carryforwards may be subject to annual limitations, which could reduce or defer the utilization of the losses, such as in the event
of an “ownership change” as described above.
Risks
Related to This Offering and Ownership of Our Securities
The
market prices of our securities may fluctuate, and you could lose all or part of your investment.
During
and after this offering, the market prices for our securities may be volatile. The price of our common stock has been volatile and has
fluctuated significantly in the past on the OTCQB. Our common stock may fluctuate or continue to fluctuate widely in price during and
after this offering in response to various factors after this offering, even if our common stock is listed on the Nasdaq Capital Market,
which is not assured and which is not a condition to the commencement of this offering. Many of these factors are beyond our control,
including: Technological innovations or new products and services by us or our competitors; additions or departures of key personnel;
sales of our shares of common stock; our ability to integrate operations, technology, products and services; our ability to execute our
business plan; operating results below expectations; loss of any strategic relationships; industry developments; economic and other external
factors; and period-to-period fluctuations in our financial results. Because we have a very limited operating history with limited revenues
to date, you may consider any one of these factors to be material. In addition, the securities markets have from time to time experienced
significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations
may also materially and adversely affect the market price of our common stock. Your investment in our common stock could lose some or
all its value as a result.
We
cannot predict the extent to which an active public trading market for our common stock will develop or be sustained. If an active public
trading market for our common stock does not develop or cannot be sustained, you may be unable to liquidate your investment in our common
stock.
At
present, there is minimal public trading in our common stock. We cannot predict the extent to which an active public market for our common
stock will develop or be sustained due to a number of factors, including the fact that we are a small company that is relatively unknown
to stock analysts, stock brokers, institutional investors, and others in the investment community that generate or influence sales volume,
and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company
such as ours or purchase or recommend the purchase of our securities until such time as we became more seasoned and viable. As a consequence,
there may be periods of several days or more when trading activity in our common stock is minimal or non-existent, as compared to a seasoned
issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect
on market price. We cannot give you any assurance that an active public trading market for our common stock will develop or be sustained.
If such a market cannot be sustained, you may be unable to liquidate your investment in our common stock.
Our
efforts to obtain and maintain a listing of our common stock on the Nasdaq Capital Market may fail and may not prevent, or may cause,
the decline of the value of your common stock.
In
connection with a proposed public offering that we no longer intend to pursue, we previously applied to list our common stock on the
Nasdaq Capital Market tier of Nasdaq. In April 2023, we withdrew the registration statement relating to the proposed public offering
because we no longer intended to pursue the proposed public offering. We intend to resume the listing process with Nasdaq or another
national securities exchange when the Company is able to meet securities exchange listing requirements and standards. We believe that
such a listing may be important to the Company’s efforts to gain sufficient financing to fund its operations in the short-term
and long-term.
In
connection with the proposed listing of our common stock on the Nasdaq Capital Market, we may effect, subject to processing by the Financial
Industry Regulatory Authority, Inc. (“FINRA”), a reverse stock split of our common stock at the ratio we believe necessary
to allow us to obtain listing approval of our common stock. Even if such a reverse stock split achieves the requisite increase in the
market price of our common stock for listing of our common stock, there can be no assurance that the market price of our common stock
following the reverse stock split will remain at the level required for continuing compliance with such requirements. It is not uncommon
for the market price of a company’s common stock to decline in the period following a reverse stock split. On August 3, 2022, January
5, 2023, and January 13, 2023, we made certain filings with the Secretary of State of the State of Nevada (the “Nevada Secretary
of State”) providing for reverse stock splits and stock splits of our common stock that in aggregate would have effected a reverse
stock split of our common stock on a 1-for-15 ratio. From August 3, 2022 to the date of this prospectus, our common stock has continued
to trade on the OTCQB on a pre-split basis pending FINRA’s processing of such a reverse stock split. On May 15, 2023, we made a
filing with the Nevada Secretary of State providing for a 15-for-1 forward stock split, which effectively returned the number of outstanding
shares to the amount existing prior to the initial filing on August 3, 2022. In addition, FINRA did not process any of the reverse or
forward stock splits and they were not reflected in the price or other information relating to our stock quoted on the OTCQB. Nevertheless,
our stock price declined from $2.39 per share based on the last reported price on the OTCQB on August 3, 2022 to $0.23 per share
based on the last reported price on the OTCQB on October 16, 2023. It is possible that, if we effect a new reverse stock split,
even if FINRA does not process it, it may contribute to the further decline of our common stock. In any event, other factors unrelated
to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the
market price of our common stock and thus jeopardize our ability to meet or maintain Nasdaq’s minimum bid price requirement.
If
we are unable to satisfy Nasdaq’s listing requirements or standards, we will not be able to meet Nasdaq’s initial listing
application requirements. We can provide no assurance that any action taken by us would allow our common stock to be listed on the Nasdaq
Capital Market, stabilize the market price or improve the liquidity of our common stock, prevent the price of our common stock from dropping
below Nasdaq’s minimum bid price requirement, or prevent future non-compliance with Nasdaq listing requirements. If we are unable
to list our common stock on the Nasdaq Capital Market, we may experience heightened difficulty in raising sufficient funding to meet
our capital and cash requirements over the 12 months ended June 30, 2024 and any period beyond that date.
Assuming
that our common stock is listed on the Nasdaq Capital Market, we must continue to meet certain financial and liquidity criteria to maintain
such listing. If we violate applicable Nasdaq listing requirements, or fail to meet any applicable listing standards, our common stock
may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on the Nasdaq Capital Market
outweighs the benefits of such listing.
The
Company’s common stock currently trades on the OTCQB, an over-the-counter market. Our common stock will no longer trade on the
OTCQB in the event we list our common stock on the Nasdaq Capital Market. The failure to list our common stock on the Nasdaq Capital
Market, or the delisting of our common stock from the Nasdaq Capital Market, would cause our common stock to continue to, or become able
to, trade in the United States only on the over-the-counter market. The over-the-counter market is a significantly more limited market
than the Nasdaq Capital Market and other national securities exchange markets. The quotation of our common stock on the over-the-counter
market may result in a less liquid market available for existing and potential stockholders to trade shares of our common stock, could
depress the trading prices of our common stock and could have a long-term adverse impact on our ability to raise capital in the future.
As a result, investors may find it difficult to buy or sell or obtain accurate quotations for our common stock, and the liquidity of
our common stock may remain limited. The failure to list or the delisting of our common stock may therefore materially impair our stockholders’
ability to buy and sell our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market
for, our common stock. The failure to list or the delisting of our common stock could also significantly impair our ability to raise
capital and the value of your investment.
In
the event that our common stock is not listed or is delisted from the Nasdaq Capital Market, U.S. broker-dealers may be discouraged from
effecting transactions in shares of our common stock because they may be considered penny stock and thus be subject to the penny stock
rules.
The
SEC has adopted a number of rules to regulate “penny stock” that restricts transactions involving securities which are deemed
to be penny stock. Such rules include Rules 3a51-1, 15g-1, 15g-2, 15g-3, 15g-4, 15g-5, 15g-6, 15g-7, and 15g-9 under the Exchange Act.
These rules may have the effect of reducing the liquidity of “penny stocks”. “Penny stocks” generally are equity
securities with a price of less than $5.00 per share other than securities registered on the Nasdaq Capital Market and certain national
securities exchanges if current price and volume information with respect to transactions in such securities is provided by the exchange
or system. Our shares of common stock have in the past constituted, currently constitute, and in the future may continue to constitute,
“penny stock” within the meaning of the rules. The additional sales practice and disclosure requirements imposed upon U.S.
broker-dealers may discourage such broker-dealers from effecting transactions in shares of our common stock, which could severely limit
the market liquidity of such shares of common stock and impede their sale in the secondary market.
A
U.S. broker-dealer selling “penny stock” to anyone other than an established customer or “accredited investor”
(generally, an individual with a net worth in excess of $1,000,000 or an annual income exceeding $200,000, or $300,000 together with
the individual’s spouse) must make a special suitability determination for the purchaser and must receive the purchaser’s
written consent to the transaction prior to sale, unless the broker-dealer or the transaction is otherwise exempt. In addition, the “penny
stock” regulations require the U.S. broker-dealer to deliver, prior to any transaction involving a “penny stock”, a
disclosure schedule prepared in accordance with SEC standards relating to the “penny stock” market, unless the broker-dealer
or the transaction is otherwise exempt. A U.S. broker-dealer is also required to disclose commissions payable to the U.S. broker-dealer
and the registered representative and current quotations for the securities. Finally, a U.S. broker-dealer is required to submit monthly
statements disclosing recent price information with respect to the “penny stock” held in a customer’s account and information
with respect to the limited market in “penny stocks”.
Stockholders
should be aware that, according to the SEC, the market for “penny stocks” has suffered in recent years from patterns of fraud
and abuse. Such patterns include (i) control of the market for the security by one or a few broker-dealers that are often related to
the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press
releases; (iii) “boiler room” practices involving high-pressure sales tactics and unrealistic price projections by inexperienced
sales persons; (iv) excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and (v) the wholesale dumping
of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, resulting in investor losses.
Our management is aware of the abuses that have occurred historically in the “penny stock” market. Although we do not expect
to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive
within the confines of practical limitations to prevent the described patterns from being established with respect to our common stock.
Substantial
future sales or issuances of our securities, or the perception in the public markets that these sales or issuances may occur, may depress
the price of our common stock. Also, future issuances of our common stock or rights to purchase common stock could result in additional
dilution of the percentage ownership of our stockholders and could cause the price of our common stock to fall.
Sales
of substantial amounts of our securities in the public market in the course of this ongoing offering, or the perception that these sales
could occur, could adversely affect the price of our common stock and could impair our ability to raise capital through the sale of additional
securities. Prior to the commencement of this offering, we will have 46,666,760 shares of common stock outstanding. After this
offering commences, all of the shares of common stock registered for resale for this offering will also be freely tradable without restriction
or further registration under the federal securities laws, which will consist of the following:
| ● | 13,971,324
shares
of outstanding common stock held by existing stockholders; |
| ● | 221,939,338
shares
of common stock issuable upon the conversion of the outstanding Convertible Notes, assuming
that all convertible debts and other liabilities under the Convertible Notes are converted
into shares of common stock, without regard to any applicable limitations or restrictions; |
| ● | 380,986,336
shares
of common stock upon the exercise of the outstanding Class C Warrants at an exercise price
of $0.10 per share, without regard to any applicable limitations or restrictions; |
| ● | 66,159,434
shares
of common stock upon the conversion of the outstanding OID Convertible Notes, without regard
to limitations or restrictions, and assuming that the OID Convertible Notes are held
until maturity and that all convertible debts and other liabilities under the OID
Convertible Notes are converted into shares of common stock; |
| ● | 84,546,202
shares
of common stock upon the exercise of the outstanding Class E Warrants at an exercise price
of $0.10 per share, without regard to any applicable limitations or restrictions; |
| ● | 80,796,202
shares
of common stock issuable upon the exercise of the Class F Warrants at an exercise price
of $0.20 per share, without regard to any applicable limitations or restrictions; |
| ● | 11,694,656
shares
of common stock upon the exercise of the OID Notes and Class E Placement Agent Warrants at
an exercise price of $0.10 per share, without regard to any applicable limitations
or restrictions; |
| ● | 6,463,697
shares
of common stock upon the exercise of the Class F Placement Agent Warrants at an exercise
price of $0.20 per share, without regard to any applicable limitations or restrictions; |
| ● | 48,234,054
shares of common stock issuable upon exercise of the Replacement Placement Agent Warrants
at an exercise price of $0.10 per share, without regard to any applicable limitations
or restrictions; and |
| ● | 280,014
shares of common stock upon the exercise of the 2020 Placement Agent Warrants at an exercise
price of $1.375 per share, without
regard to any applicable limitations or restrictions. |
These
shares include a total of 901,099,931 shares of common stock newly issuable upon conversion or exercise of outstanding convertible
notes or warrants, which, when added to the number of outstanding shares, could result in a total of 915,071,257 shares of common
stock that are freely tradable without restriction or further registration under the federal securities laws, disregarding contractual
limitations on conversion or exercise rights. 288,098,772 of these shares are issuable for no payment upon conversion of outstanding
Convertible Notes and OID Convertible Notes, and 477,227,194 of these shares are issuable upon exercise of Class C Warrants, Class
E Warrants, or OID Notes and Class E Placement Agent Warrants at an exercise price of $0.10 per share, disregarding contractual limitations
on conversion or exercise rights. Since all of the shares issuable upon conversion of the Convertible Notes and OID Convertible Notes
will be issuable for no payment and the shares being offered for resale that are issuable upon exercise of warrants may be issued at
a lower price per share than the price that you pay or have paid for your common stock, the registration for resale of such common stock
upon issuance may result in pronounced dilution of your shares.
We
may also elect to file a registration statement on Form S-8 to register shares, including shares under stock options or other equity
compensation previously granted to our officers, directors, employees and service providers or reserved for future issuance under the
Stock Incentive Plan during or after this offering. Subject to the satisfaction of vesting conditions, shares registered under the registration
statement on Form S-8 will be available for resale immediately in the public market without restriction other than those restrictions
imposed on sales by affiliates pursuant to Rule 144 under the Securities Act (“Rule 144”). As of October 10, 2023,
3,925,943 shares of common stock were issuable upon the exercise of vested and unvested options at a weighted average exercise price
of $1.33 per share; and 2,924,057 shares of common stock remained available for issuance pursuant to the Stock Incentive Plan.
Additionally,
certain of our employees, executive officers, and directors may enter into Rule 10b5-1 trading plans providing for sales of shares of
our common stock from time to time. Under a Rule 10b5-1 trading plan, a broker executes trades pursuant to parameters established by
the employee, director, or officer when entering into the plan, without further direction from the employee, officer, or director. A
Rule 10b5-1 trading plan may be amended or terminated in some circumstances. Our employees, executive officers, and directors also may
buy or sell additional shares outside of a Rule 10b5-1 trading plan when they are not in possession of material, non-public information,
subject to compliance with registration requirements or exemptions from registration requirements under the Securities Act.
We
expect that significant additional capital will be needed in the future to continue our planned operations, including expanding research
and development, funding clinical trials, purchasing of capital equipment, hiring new personnel, commercializing our products, and continuing
activities as an operating public company. To the extent we raise additional capital by issuing equity securities, our stockholders may
experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions
at prices and in a manner we determine from time to time. If we sell common stock, convertible securities, or other equity securities
in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution
to our existing stockholders, and new investors could gain rights superior to our existing stockholders.
In
the event that the market prices of our securities drops significantly when the restrictions on resale by our existing securityholders
lapse, existing stockholders’ dilution might be reduced to the extent that the decline in the prices of our securities impedes
our ability to raise capital through the issuance of additional shares of common stock or other equity securities. However, in the event
that our capital-raising ability is weakened as a result of lower market prices for our securities, we may be unable to continue to fund
our operations, which may further harm the value of the market prices for our securities.
We
have broad discretion in the use of the net proceeds from this offering, and our use of the offering proceeds may not yield a favorable
return on your investment.
We
may receive up to approximately $70.4 million in proceeds upon the exercise of warrants issued to the selling stockholders. While
we currently intend to use these proceeds for working capital and general corporate purposes, we have considerable discretion in the
application of the proceeds. You will not have the opportunity, as part of your investment decision, to assess whether the proceeds are
being used in a manner agreeable to you. You must rely on our judgment regarding the application of these proceeds. The proceeds may
be used for corporate purposes that do not immediately improve our profitability or increase the price of our common stock. See “Use
of Proceeds” for more information.
We
have never paid cash dividends on our stock and do not intend to pay dividends for the foreseeable future.
We
have paid no cash dividends on any class of our stock to date and we do not anticipate paying cash dividends in the near term. For the
foreseeable future, we intend to retain any earnings to finance the development and expansion of our business, and we do not anticipate
paying any cash dividends on our common stock. Accordingly, investors must be prepared to rely on sales of their securities after price
appreciation to earn an investment return, which may never occur. Investors seeking cash dividends should not purchase our common stock.
Any determination to pay dividends in the future will be made at the discretion of our board of directors and will depend on our results
of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our board deems
relevant.
Raising
additional capital may adversely affect your rights as stockholders, restrict our operations or require us to relinquish rights to our
technologies or medical devices.
We
expect to finance our cash needs through a combination of private and public equity offerings, debt financings, government or other third-party
funding, and collaboration arrangements or acquisitions. To the extent that we raise additional capital through the sale of common stock,
convertible securities or other equity securities, the ownership interest of our stockholders may be materially diluted, and the terms
of these securities may include liquidation or other preferences that adversely affect the interests of our stockholders. Debt financing
and preferred equity financing, if available, would result in increased fixed payment obligations and may involve agreements that include
covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures
or declaring dividends, that could adversely impact our ability to conduct our business. Securing additional financing could require
a substantial amount of time and attention from our management and may divert a disproportionate amount of their attention away from
day-to-day activities, which may adversely affect our management’s ability to oversee the development or acquisition of products.
If
we raise additional funds through collaborations, strategic alliances or marketing, distribution, or licensing arrangements with third
parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or medical devices
or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings
when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant
rights to develop and market medical devices that we would otherwise prefer to develop and market ourselves.
We
may be at risk of securities class action litigation.
We
may be at risk of securities class action litigation. In the past, life sciences, biotechnology and pharmaceutical companies have experienced
significant stock price volatility, particularly when associated with binary events such as clinical trials and product approvals. If
we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could
harm our business and results in a decline in the market prices of our securities.
If
securities or industry analysts do not publish research or reports about our business, or if they change their recommendations regarding
our common stock adversely, the market price and trading volume of our common stock could decline.
The
trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about
us or our business. We do not currently have and may never obtain research coverage by industry or financial analysts. If no or few analysts
commence coverage of us, the trading prices of our securities would likely decrease. Even if we do obtain analyst coverage, if one or
more of the analysts who cover us downgrade our securities, the price of our common stock would likely decline. If one or more of these
analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets,
which in turn could cause the price of our common stock or trading volume to decline.
Our
articles of incorporation, bylaws and Nevada law have anti-takeover provisions that could discourage, delay or prevent a change in control,
which may cause the prices of our securities to decline.
Our
articles of incorporation, bylaws and Nevada law contain provisions which could make it more difficult for a third party to acquire us,
even if closing such a transaction would be beneficial to our stockholders. We are currently authorized to issue up to 25,000,000 shares
of “blank check” preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined
at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock
may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion
and redemption rights and sinking fund provisions. No shares of our preferred stock are currently outstanding. The issuance of any preferred
stock could materially adversely affect the rights of the holders of our securities, and therefore, reduce the value of our securities.
In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell
our assets to, a third party and thereby preserve control by current management.
Our
articles of incorporation, bylaws or Nevada law contain provisions that are intended to deter coercive takeover practices and inadequate
takeover bids by making such practices or bids unacceptably expensive to the raider and to encourage prospective acquirers to negotiate
with our board of directors rather than to attempt a hostile takeover. These provisions include, among others:
| ● | the
inability of our stockholders to call a special meeting; |
| ● | rules
regarding how stockholders may present proposals or nominate directors for election at stockholder
meetings; |
| ● | the
right of our board to issue preferred stock without stockholder approval; and |
| ● | the
ability of our directors, and not stockholders, to fill vacancies on our board of directors. |
Provisions
of our articles of incorporation, bylaws or Nevada law also could have the effect of discouraging potential acquisition proposals or
making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions
may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, our articles of incorporation,
our bylaws or Nevada law, as applicable, among other things, may provide our board of directors with the ability to alter our bylaws
without stockholder approval, and provide that vacancies on our board of directors may be filled by a majority of directors in office,
although less than a quorum.
In
addition, we are subject to Nevada’s Combination with Interested Stockholders Statute (Nevada Revised Statutes (“NRS”)
Sections 78.411 – 78.444), which prohibits an interested stockholder from entering into a “combination” with the corporation,
unless certain conditions are met. These provisions are expected to discourage certain types of coercive takeover practices and inadequate
takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with our board of directors. These
provisions may delay or prevent someone from acquiring or merging with us, which may cause the market prices of our securities to decline.
We
have elected out of Nevada’s Acquisition of Controlling Interest Statute (NRS Sections 78.378 – 78.3793), which prohibits
an acquirer, under certain circumstances, from voting shares of a corporation’s stock after crossing specific threshold ownership
percentages. The election out of the Acquisition of Controlling Interest Statute can be reversed by an amendment to our bylaws by the
stockholders or our board of directors, which would also have the effect of discouraging or delaying from acquiring or merging with us.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements that are based on our management’s beliefs and assumptions and on information currently
available to us. All statements other than statements of historical facts are forward-looking statements. The forward-looking statements
are contained principally in, but not limited to, the sections entitled “Prospectus Summary,” “Risk Factors,”
“Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business.”
These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and
other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any
future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking
statements include, but are not limited to, statements about:
| ● | results
and timing of our clinical trials and planned clinical trials; |
| ● | the
timing of, and outcome of, regulatory approvals needed to market and commercialize our products; |
| ● | the
cost, quality, availability, and reliability of the supply of materials and services needed
for products; |
| ● | our
ability to develop our medical technologies into viable products and services; |
| ● | the
marketing success of our distribution partners; |
| ● | demand
for, and market acceptance of, our products; |
| ● | our
goals and strategies; |
| ● | our
future business development, financial condition and results of operations; |
| ● | expected
changes in our revenue, costs or expenditures; |
| ● | growth
of and competition trends in our industry; |
| ● | our
expectations regarding demand for, and market acceptance of, our products; |
| ● | our
expectations regarding our relationships with investors, institutional funding partners and
other parties with whom we collaborate; |
| ● | our
expectation regarding the use of proceeds from this offering; |
| ● | fluctuations
in general economic and business conditions in the markets in which we operate; and |
| ● | relevant
government policies and regulations relating to our industry. |
In
some cases, you can identify forward-looking statements by terms such as “may,” “could,” “will,”
“should,” “would,” “expect,” “plan,” “intend,” “anticipate,”
“believe,” “estimate,” “predict,” “potential,” “project” or “continue”
or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance
on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases,
beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current
expectations include, among other things, those listed under the heading “Risk Factors” and elsewhere in this prospectus.
If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results
may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee
of future performance.
The
forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made
in this prospectus. Although we are a public company and have ongoing disclosure obligations under United States federal securities laws,
we do not intend to update or otherwise revise the forward-looking statements in this prospectus, whether as a result of new information,
future events or otherwise.
USE
OF PROCEEDS
We
will not receive any proceeds from the resale of shares of common stock by the selling stockholders. Assuming the full exercise of the
Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants for cash, we may receive gross proceeds
of approximately $70.4 million.
We
cannot predict when or if the Class C Warrants, the Class E Warrants, the Class F Warrants, or the Placement Agent Warrants will be exercised,
and it is possible that the Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants may expire
and never be exercised. In addition, the Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants
are or may become exercisable on a cashless basis under certain conditions. As a result, we may never receive meaningful,
or any, cash proceeds from the exercise of the Class C Warrants, the Class E Warrants, the Class F Warrant, or the Placement Agent Warrants,
and we cannot plan on any specific uses of any proceeds we may receive beyond the purposes described herein.
We
therefore have no specific plan for any proceeds from any exercise of the Class C Warrants, the Class E Warrants, the Class F Warrants,
or the Placement Agent Warrants except to generate funds for working capital and general corporate purposes. We will have broad discretion
in the way that we use these proceeds.
The
selling stockholders will pay any underwriting discounts and commissions and expenses incurred by them for brokerage, accounting, tax
or legal services or any other expenses incurred by them in disposing of the shares. We will bear all other costs, fees and expenses
incurred in effecting the registration of the shares covered by this prospectus, including, without limitation, all registration and
filing fees and fees and expenses of our counsel and our accountants.
See
“Plan of Distribution” elsewhere in this prospectus for more information.
DIVIDEND
POLICY
We
have never declared or paid cash dividends on our common stock. We currently intend to retain all available funds and any future earnings
for use in the operation of our business and do not anticipate paying any cash dividends on our common stock in the near future. We may
also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash
dividends on our common stock. Any future determination to declare dividends will be made at the discretion of our board of directors
and will depend on our financial condition, operating results, capital requirements, contractual restrictions, general business conditions
and other factors that our board of directors may deem relevant. See also “Risk Factors — Risks Related to This Offering
and Ownership of Our Securities — We have never paid cash dividends on our stock and do not intend to pay dividends for the foreseeable
future.”
MARKET
FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market
Information
Our
common stock is quoted on the OTCQB under the ticker symbol “MRZM.” To date, there has been limited trading for our common
stock. We have applied to list our common stock under the symbol “MRZM” on the Nasdaq Capital Market. There can be no guarantee
that we will successfully list our common stock on the Nasdaq Capital Market. The registration of the selling stockholders’ resale
of the Company’s common stock as described in this prospectus is not conditioned upon our successful listing on the Nasdaq Capital
Market. As of September 19, 2023, a total of 46,666,760 shares of common stock were outstanding. On October 16, 2023, the last
reported price of the common stock on the OTCQB was $0.23 per share.
Number
of Holders of Our Shares of Common Stock
We
had 374 record holders of our common stock as of September 19, 2023. Because brokers and other institutions hold shares
on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The
following discussion and analysis summarizes the significant factors affecting our operating results, financial condition, liquidity
and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction
with our financial statements and the related notes thereto included elsewhere in this prospectus. The discussion contains forward-looking
statements that are based on the beliefs of management, as well as assumptions made by, and information currently available to, our management.
Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors,
including those discussed below and elsewhere in this prospectus, particularly in the sections titled “Risk Factors” and
“Cautionary Note Regarding Forward-Looking Statements”.
Overview
We
are a medical technology company changing the landscape of cardiac care by delivering innovative solutions for CABG surgery. Our principal
products or medical technologies, DuraGraft and MAR-FG-001, and other aspects of our business and strategic priorities, are described
in the “Business” section of this prospectus.
We
have incurred losses for each period from inception. Our net loss was approximately $20.6 million and $6.9 million for
the three months ended June 30, 2023 and June 30, 2022, respectively, and was approximately $23.1 million and $13.0 million
for the six months ended June 30, 2023 and June 30, 2022, respectively. We expect to incur significant expenses and operating losses
over the next several years. Accordingly, we will need additional financing to support our continuing operations. We will seek to fund
our operations through public or private equity offerings, debt financings, government or other third-party funding, collaborations and
licensing arrangements. Adequate additional financing may not be available to us on acceptable terms, or at all. Our failure to raise
capital as and when needed would impact our going concern and would have a negative impact on our financial condition and our ability
to pursue our business strategy and continue as a going concern. We will need to generate significant revenues to achieve profitability,
and we may never do so.
Impact
of COVID-19 Pandemic
The
Company has been impacted by the COVID-19 pandemic and related supply chain shortages and other economic conditions, and some of its
earlier plans to diversify and expand its operations were delayed as a result. Moreover, the impact of the COVID-19 pandemic on the Company’s
supply chain and its ability to produce DuraGraft inventory was a primary reason that we did not generate substantial revenue from sales
of DuraGraft during 2021 and 2022. The Company’s inventory production of DuraGraft returned to its pre-pandemic level at the end
of the second quarter of 2022, but lingering effects of the COVID-19 pandemic continued to depress demand for DuraGraft and cause revenues
from DuraGraft during the first and second quarters of 2023 to be minimal. There can be no assurance that future supply chain
disruptions and other effects of COVID-19 outbreaks will not adversely impact our revenues.
In
addition, the Company is dependent upon certain contract manufacturers and suppliers and their ability to reliably and efficiently fulfill
its orders is critical to the Company’s business success. The COVID-19 pandemic has impacted and may continue to impact certain
of the Company’s manufacturers and suppliers. As a result, the Company has faced and may continue to face delays or difficulty
sourcing certain products, which could negatively affect the Company’s business and financial results.
While
it is not possible at this time to estimate the total impact that COVID-19 could have on our business in the future, the continued spread
of COVID-19 and variants of the virus, the rate of vaccinations regionally and globally and the measures taken by the government authorities,
and any future epidemic disease outbreaks, could: Disrupt the supply chain and the manufacture or shipment of products and supplies for
use by us in our research activities and by strategic partners for their distribution and sales activities; delay, limit or prevent us
in our research activities and strategic partners in their distribution and sales activities; impede our negotiations with strategic
partners; impede testing, monitoring, data collection and analysis and other related activities by us; interrupt or delay the operations
of the FDA or other regulatory authorities, which may impact review and approval timelines for initiation of clinical trials or marketing;
or impede the launch or commercialization of any approved products; any of which could delay our strategic partnership plans, increase
our operating costs, and have a material adverse effect on our business, financial condition and results of operations.
Principal
Factors Affecting Our Financial Performance
Our
operating results are primarily affected by the following factors:
| ● | our
ability to generate revenue from sales of our products; |
| ● | our
ability to obtain FDA approval for our products; |
| ● | our
ability to access additional capital and the size and timing of subsequent financings, if
any; |
| ● | our
ability to become listed on a national securities exchange; |
| ● | the
costs of acquiring and utilizing data, technology, and/or intellectual property to successfully
reach our goals and to remain competitive; |
| ● | personnel
and facilities costs in any region in which we seek to introduce and market our products; |
| ● | the
costs of sales, marketing, and customer acquisition; |
| ● | the
average price for our products that will be paid by consumers; |
| ● | the
number of our products ordered per quarter; |
| ● | costs
to manufacture our products; |
| ● | the
costs of compliance with any unforeseen regulatory obstacles or governmental mandates in
any states or countries in which we seek to operate; and |
| ● | the
costs of any additional clinical studies which are deemed necessary for us to remain viable
and competitive in any region of the world. |
Smaller
Reporting Company
We
are a “smaller reporting company” as defined in Rule 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage
of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We
will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our shares held by non-affiliates
equals or exceeds $250 million as of the prior June 30th, or (2) our annual revenues equaled or exceeded $100 million during
such completed fiscal year and the market value of our shares held by non-affiliates equals or exceeds $700 million as of the prior June
30th.
Summary
of Significant Events During Three Months Ended June 30, 2023
Below
is a summary of certain events relating to the Company’s capital structure and financial condition that occurred during the three
months ended June 30, 2023. For further discussion of these developments, see the related subsections of “—Liquidity and
Capital Resources – Funding Requirements and Other Liquidity Matters” below.
| ● | On
April 21, 2023, the Company filed an application with the SEC for the withdrawal of a registration
statement, as amended, relating to a proposed underwritten public offering for gross proceeds
of approximately $8 million. The application for withdrawal of the registration statement
was subsequently deemed granted. The application for withdrawal was filed to withdraw the
registration statement because the Company no longer intended to pursue the proposed public
offering. |
| |
● |
In April 2023, the Company
agreed to allow certain warrants with an exercise price of $5.00 per share to be exercised during a certain period for $0.10 per share
to purchase a total of 2,652,159 shares of common stock for gross proceeds of approximately $265,216. As a result of this warrant exercise,
pursuant to the terms applicable to the outstanding Convertible Notes and Class C Warrants, the conversion price of the Convertible
Notes adjusted from $1.75 per share to $0.10 per share, the exercise price of the Class C Warrants adjusted from $2.25 per share to
$0.10 per share, and the number of shares that the Class C Warrants may be exercised to purchase was increased proportionately. In
connection with these developments and the lack of sufficient authorized shares of common stock under the Company’s articles
of incorporation to meet its obligations under outstanding convertible or exercisable securities, the Company also obtained additional
conversion and exercise rights waivers from certain holders of the Convertible Notes and Class C Warrants. As a result of these adjustment
provisions and the conversion and exercise terms applicable to the Convertible Notes and Class C Warrants, including the effect of
the applicable waivers, the number of shares into which the Convertible Notes may be converted adjusted from 2,554,944 shares of common
stock to 44,711,770 shares, not including shares convertible from interest under the Convertible Notes, and the number of shares that
the Class C Warrants may be exercised to purchase adjusted from 5,109,904 shares of common stock to 114,973,110 shares. |
| |
● |
On May 15, 2023, the Company
made a state filing which provided for the increase of the Company’s authorized common stock from 20,000,000 shares to 300,000,000
shares and the corresponding change of every one (1) share of the Company’s issued and outstanding common stock to fifteen (15)
shares, such that there were 300,000,000 authorized shares of common stock with no proportional change to the outstanding shares of
common stock, which totaled 43,420,350 shares as of May 15, 2023. |
| ● | In
May 2023, the Company did not repay the amount of the principal under an unsecured promissory
note (the “Walleye Promissory Note”) issued to Walleye Opportunities Master Fund
Ltd (“Walleye”) by its maturity date, and accordingly, the principal under the
note increased from $1,000,000 to $1,250,000. Due to this nonpayment, the Company also cross-defaulted
under the Convertible Notes. As a result, the aggregate amount due under the Convertible
Notes increased to approximately $21.4 million from approximately $16.2 million and became
immediately due upon demand. |
| |
● |
In May 2023, the Company
issued OID Convertible Notes for aggregate principal of $3,781,335 and convertible into up to 40,544,200 shares of common stock if
held to maturity, Class E Warrants, exercisable for the purchase of 54,766,686 shares of common stock at $0.10 per share, and Class
F Warrants, exercisable for the purchase of 51,016,686 shares of common stock at $0.20 per share. The OID Convertible Notes, Class
E Warrants and Class F Warrants provided that they were not convertible or exercisable until the effectiveness of the Capital Event
Amendment (as defined below). Net proceeds from the issuances totaled $870,000. |
| ● | On
June 23, 2023, the Company filed with the SEC and subsequently distributed to certain stockholders
of record a definitive proxy statement relating to a special meeting of stockholders to be
held on August 9, 2023 (the “Special Stockholders Meeting”), to consider and
vote on the following proposals: (i) to approve an amendment to the Company’s articles
of incorporation, as amended to date, to increase the total number of shares of authorized
common stock from 300,000,000 to 2,000,000,000 (the “Capital Event Amendment”),
and (ii) to approve an amendment to the Company’s articles of incorporation, as amended
to date, to provide that holders of any of the Company’s bonds, debentures or other
obligations of the Company may have, at the option of the board of directors of the Company,
any of the rights of a stockholder of the Company, and that the holders of the Convertible
Notes issued between December 21, 2021 and August 12, 2022, shall be entitled, from the date
of the filing with the Nevada Secretary of State providing such amendment, to vote with the
shares of the Company’s common stock, on an as-converted to common stock basis without
actually having converted the Convertible Notes to shares of the Company’s common stock,
with respect to all corporate matters of the Company on which the holders of common stock
are entitled to vote, as provided in of the Convertible Notes (the “Voting Rights Amendment”). |
Results
of Operations
Components
of Results of Operations for Three and Six Months Ended June 30, 2023 and June 30, 2022
The
components of our results of operations during the three and six months ended June 30, 2023 and 2022 were as follows:
Revenue
Revenue
represents gross product sales less service fees and product returns. For our distribution partner channel, we recognize revenue for
product sales at the time of delivery of the product to our distribution partner. As our products have an expiration date, if a product
expires, we will replace the product at no charge. Currently, all of our revenue is generated from the sale of DuraGraft in European
and Asian markets where the product has the required regulatory approvals.
Direct
Cost of Revenue
Direct
cost of revenue include primarily product costs, which include all costs directly related to the purchase of raw materials, charges from
our contract manufacturing organizations, and manufacturing overhead costs, as well as shipping and distribution charges. Direct cost
of revenue also include losses from excess, slow-moving or obsolete inventory and inventory purchase commitments, if any.
Professional
Fees
Professional
fees include legal fees relating to intellectual property development, due diligence and corporate matters, and consulting fees for accounting,
finance, and valuation services. Professional fees paid to a certain related party relate to certain consulting services. Increased expenses
related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with SEC requirements and with securities
exchange rules in future periods are likely to occur, including likely increases in costs of compliance with SEC regulations of
public companies in general, and with securities exchange rules due to the Company’s plans to resume the securities exchange listing
process when it can meet securities exchange listing requirements.
Salaries
and Stock-Based Compensation
Salaries
consist of compensation and related personnel costs. Stock-based compensation represents the fair value of equity-settled share awards
on stock options granted by the Company to its employees, officers, directors, and consultants. The fair value of awards is calculated
using the Black-Scholes option pricing model, which considers the following factors: exercise price, current market price of the underlying
shares, expected life, risk-free interest rate, expected volatility, dividend yield, and forfeiture rate.
Research
and Development
All
research and development costs are expensed in the period incurred and consist primarily of salaries, payroll taxes, and employee benefits,
those individuals involved in research and development efforts, external research and development costs incurred under agreements with
contract research organizations and consultants to conduct and support the Company’s clinical trials of DuraGraft, and costs related
to manufacturing DuraGraft for clinical trials. The Company has entered into various research and development contracts with various
organizations and other companies.
Depreciation
and Amortization
Intangible
assets are recorded at cost less accumulated amortization and accumulated impairment losses. Intangible assets acquired as a result of
an acquisition or in a business combination are measured at fair value at the acquisition date. The useful lives of intangible assets
are assessed as either finite or indefinite. Intangible assets with finite lives are amortized over the estimated useful economic life
and assessed for impairment whenever there is an indication that the intangible asset may be impaired. The estimated useful life and
amortization method are reviewed at the end of each reporting period, with the effect of any changes in estimates being accounted for
on a prospective basis.
Royalty
Expenses
In
connection with the Somahlution Acquisition (as defined in “—Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Exercise of Somahlution Warrants with Reduced Exercise Price”), the Company entered
into the Somahlution Agreement (as defined in “—Liquidity and Capital Resources – Funding Requirements and Other
Liquidity Matters – Exercise of Somahlution Warrants with Reduced Exercise Price”), under which the Company became
legally obligated to pay royalties on all net sales of certain products. Royalty expenses consists of royalty payable accrued on net
sales of DuraGraft product within and outside of the U.S.
Other
General and Administrative Expenses
Other
general and administrative expenses consist principally of marketing and selling expenses, facility costs, administrative and office
expenses, director and officer insurance premiums, and investor relations costs associated with operating a public company.
Other
Income (Expenses)
Other
income and expenses consist of mark-to-market adjustments on contingent liabilities assumed on the acquisition of all of the Somahlution
Assets and interest and accretion expenses related to our Convertible Notes.
Comparison
of the Three Months Ended June
30, 2023 and 2022
The
following table summarizes our results of operations for the three months ended June 30, 2023 and 2022:
| | |
Three Months Ended June 30, | | |
| |
| | |
2023 | | |
2022 | | |
Change | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 184,739 | | |
$ | 61,809 | | |
$ | 122,930 | |
| Cost of goods sold | |
| 49,611 | | |
| 11,025 | | |
| 38,586 | |
| Gross profit | |
| 135,128 | | |
| 50,784 | | |
| 84,344 | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Direct costs of revenue | |
| | | |
| | | |
| | |
| Professional fees | |
| 511,844 | | |
| 873,865 | | |
| (362,021 | ) |
| Salary expenses | |
| 335,004 | | |
| 902,106 | | |
| (567,102 | ) |
| Research and development | |
| 691,393 | | |
| 1,371,470 | | |
| (680,077 | ) |
| Stock-based compensation | |
| 160,762 | | |
| 676,242 | | |
| (515,480 | ) |
| Depreciation and amortization | |
| 210,293 | | |
| 210,361 | | |
| (68 | ) |
| Royalty expense | |
| 162,065 | | |
| - | | |
| 162,065 | |
| Other general and administrative expenses | |
| 2,867,749 | | |
| 618,498 | | |
| 2,249,251 | |
| Total operating expenses | |
| 4,939,110 | | |
| 4,652,542 | | |
| 286,568 | |
| Total operating loss | |
$ | (4,803,982 | ) | |
$ | (4,601,758 | ) | |
$ | (202,224 | ) |
| Other income (expenses): | |
| | | |
| | | |
| | |
| Interest and accretion expense | |
| (13,424,169 | ) | |
| (530,226 | ) | |
| (12,893,943 | ) |
| Change in fair value of contingent liabilities | |
| 714,000 | | |
| (1,792,000 | ) | |
| 2,506,000 | |
| Loss on debt extinguishment | |
| (684,682 | ) | |
| - | | |
| (684,682 | ) |
| Loss on issuance of debt | |
| (2,377,569 | ) | |
| - | | |
| (2,377,569 | ) |
| Net loss | |
$ | (20,576,402 | ) | |
$ | (6,923,984 | ) | |
$ | (13,652,418 | ) |
Revenue
and Direct Cost of Revenue
We
recognized revenue of approximately $0.18 million for the three months ended June 30, 2023 compared to $0.06 million
for the three months ended June 30, 2022. Immaterial revenue was generated in the second quarter of 2022 due
to the impact of the COVID-19 pandemic on the Company’s supply chain and its ability to produce DuraGraft inventory. The Company’s
inventory production of DuraGraft returned to its pre-pandemic level at the end of the second quarter of 2022, but lingering effects
of the pandemic continued to depress demand for DuraGraft and caused revenues from DuraGraft during the second quarter
of 2023 to be minimal.
Professional
Fees
Professional
fees decreased by approximately $0.4 million or 41.4% to approximately $0.5 million in the three months ended June 30, 2023 compared
to approximately $0.9 million in the three months ended June 30, 2022. The decrease was due to reduced legal fees and placement agent
fees and expenses during the quarter ended June 30, 2023 compared to the quarter ended June 30, 2022.
Salary
Expenses
Salary
expenses in the three months ended June 30, 2023 were approximately $0.3 million, an approximately $0.6 million or 62.9%
decrease from the comparative quarter. The decrease can be attributed to reductions in employee salaries as part of efforts to streamline
the Company’s operations.
Research
and Development
Research
and development expenses in the three months ended June 30, 2023 were approximately $0.7 million, approximately a $0.7
million or 49.6% decrease from the comparative period. The decrease in research and development expenses can be mainly attributed
to the Company’s suspension of expenditures required for FDA marketing authorization for the Company’s MATLOC and Krillase-related
assets during the quarter ended June 30, 2023 that were incurred during the quarter ended June 30, 2022.
The
Company previously sought to develop and commercialize FDA-approved products based on its Krillase and MATLOC assets simultaneously with
its DuraGraft products. During the first and second quarters of 2023, the Company prioritized the completion of regulatory processes
to obtain FDA authorization for the commercialization of DuraGraft in the United States and the development of a functional MATLOC device
prototype and MAR-FG-001-based viable products. The Company intends to maintain the Krillase assets for potential future development
and commercialization or disposition. Any determination as to these matters would be based on a number of factors. See “—Principal
Factors Affecting Our Financial Performance” above for a summary of factors that we may consider in this respect. There is
no assurance that any of the Company’s intellectual property assets will ever be developed and fully commercialized and generate
significant revenues or will ever attract significant interest from potential buyers or investors. See “Risk Factors –
Risks Related to Our Business – We may not be able to monetize intangible assets, which may result in the need to record an impairment
charge.”
Stock-Based
Compensation
Stock-based
compensation decreased by $0.5 million or 76.2% to approximately $0.2 million for the three months ended June 30,
2023 from approximately $0.7 million in the comparative quarter ended June 30, 2022. The decrease in stock-based compensation
was due to acceleration of vesting of certain stock options by the Company’s board of directors in the comparative
quarter ended June 30, 2022.
Depreciation
and Amortization
Depreciation
and amortization remained consistent at $0.2 million in the current and comparative period. The Company did not acquire any new tangible
or intangible capital assets in any of the periods indicated.
Royalty
Expense
During
the three months ended June 30, 2023, the Company recorded an aggregate of $0.16 million in royalty expense - $0.01 million
in royalties payable was incurred on sales of DuraGraft outside of the U.S.; the remaining $0.15 million in royalties expense
was recorded as a 50% reduction of the prepaid royalty balance owed to the former beneficial owners of Somahlution. No royalties
were accrued in the comparative period as no sales of DuraGraft occurred in the three months ended June 30, 2022.
Other
General and Administrative Expenses
Other
general and administrative expenses increased approximately $2.2 million or 363.7% to approximately $2.9 million
in the three months ended June 30, 2023. Approximately $0.5 million of the increase can be attributed to the deferred
offering costs expensed as a result of adjustments to the terms of the Convertible Notes and $0.7 million of the deferred offering costs
that were expensed due to the Company’s withdrawal of its registration statement for a public offering in April
2023. Additionally, $1.3 million of other general and administrative expenses can be attributed to the valuation of
a Class E Warrant for the purchase of 7,500,000 shares of common stock at $0.10 per share and a Class F Warrant for the purchase
of 3,750,000 shares of common stock at $0.20 per share that were issued on May 22, 2023 in connection with the First OID Units
Closing (as defined in “—Liquidity and Capital Resources – Funding Requirements and Other Liquidity Matters –
OID Units Private Placement – First Closing”) of the OID Units Private Placement (as defined in “—Liquidity
and Capital Resources – Recent Developments – Third Closing of OID Units Private Placement”) pursuant to the
terms of the Hexin Promissory Note (as defined in the same section).
Other
Income (Expenses)
In
the three months ended June 30, 2023, the
Company incurred approximately $5.7 million of interest and accretion costs associated with certain securities issued at
discount relating to the Units Private Placement (as defined in “—Liquidity and Capital Resources – Recent
Developments – Issuance of Replacement Securities”) and the OID Units Private Placement, compared to $0.5
million of interest and accretion costs recorded in the second quarter of 2022 on the Convertible Notes. Additionally, due to the
non-repayment of the initial principal amount of $1.0 million under the Walleye Promissory Note by its maturity date of May 7, 2023,
the Company also defaulted under the Convertible Notes on the same date, resulting in a default amount of approximately $5.7
million accrued to the principal of the Convertible Notes. This represents a $12.9 million or 2,431.8% increase over
the comparative quarter ended June 30, 2022.
Additionally,
the Company recognized $0.7 million of fair value gain from mark-to-market adjustments on the contingent liabilities assumed on
the acquisition of the Somahlution Assets due to the change of the fair value of the contingent consideration compared to $1.8 million
of fair value loss, an increase of $2.5 million from mark-to-market adjustment on the contingent liability in the comparative
quarter ended June 30, 2022.
In
the three months ended June 30, 2023, due to the substantial reduction of the conversion price of the Convertible Notes and Class C Warrants
in April 2023, the Company recorded a loss of approximately $0.68 million on the extinguishment of the Convertible Notes. The Company
also recorded a loss of $2.4 million on issuance of OID Convertible Notes due to the fair value of Class E Warrants and Class F Warrants
exceeding the value of the debt principal.
Comparison
of the Six Months Ended June 30, 2023 and 2022
The
following table summarizes our results of operations for the six months ended June 30, 2023 and 2022:
| | |
Six Months Ended June 30, | | |
| |
| | |
2023 | | |
2022 | | |
Change | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 313,713 | | |
$ | 61,809 | | |
$ | 251,904 | |
| Cost of goods sold | |
| 88,886 | | |
| 11,025 | | |
| 77,861 | |
| Gross profit | |
| 224,827 | | |
| 50,784 | | |
| 174,043 | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Direct costs of revenue | |
| | | |
| | | |
| | |
| Professional fees | |
| 896,650 | | |
| 1,417,905 | | |
| (521,255 | ) |
| Salary expenses | |
| 601,972 | | |
| 1,817,746 | | |
| (1,215,774 | ) |
| Research and development | |
| 1,297,390 | | |
| 2,589,766 | | |
| (1,292,376 | ) |
| Stock-based compensation | |
| 371,728 | | |
| 1,392,674 | | |
| (1,020,946 | ) |
| Depreciation and amortization | |
| 420,631 | | |
| 420,722 | | |
| (91 | ) |
| Royalty expense | |
| 198,248 | | |
| - | | |
| 198,248 | |
| Other general and administrative expenses | |
| 3,375,073 | | |
| 1,009,070 | | |
| 2,366,003 | |
| Total operating expenses | |
| 7,161,692 | | |
| 8,647,883 | | |
| (1,486,191 | ) |
| Total operating loss | |
$ | (6,936,865 | ) | |
$ | (8,597,099 | ) | |
$ | 1,660,234 | |
| Other income (expenses): | |
| | | |
| | | |
| | |
| Interest and accretion expense | |
| (15,121,870 | ) | |
| (829,770 | ) | |
| (14,292,100 | ) |
| Change in fair value of contingent liabilities | |
| 1,990,000 | | |
| (3,622,000 | ) | |
| 5,612,000 | |
| Loss on debt extinguishment | |
| (684,682 | ) | |
| - | | |
| (684,682 | ) |
| Loss on issuance of debt | |
| (2,377,569 | ) | |
| - | | |
| (2,377,569 | ) |
| Net loss | |
$ | (23,130,986 | ) | |
$ | (13,048,869 | ) | |
$ | (10,082,117 | ) |
Revenue
and Direct Cost of Revenue
We
recognized revenue of approximately $0.31 million for the six months ended June 30, 2023 compared to $0.06 million for the six months
ended June 30, 2022. Immaterial revenue was generated in the six months ended June 30, 2022 due to the impact of the COVID-19 pandemic
on the Company’s supply chain and its ability to produce DuraGraft inventory. The Company’s inventory production of DuraGraft
returned to its pre-pandemic level at the end of the second quarter of 2022, but lingering effects of the pandemic continued to depress
demand for DuraGraft and caused revenues from DuraGraft during the first half of 2023 to be minimal.
Professional
Fees
Professional
fees decreased by approximately $0.5 million or 36.8% to approximately $0.9 million in the six months ended June 30, 2023 compared to
approximately $1.4 million in the six months ended June 30, 2022. The decrease was due to reduced legal fees and placement agent fees
and expenses during the six months ended June 30, 2023 compared to the six months ended June 30, 2022.
Salary
Expenses
Salary
expenses in the six months ended June 30, 2023 were approximately $0.6 million, an approximately $1.2 million or 66.9% decrease from
the comparative period. The decrease is attributable to reductions in employee salaries as part of efforts to streamline the Company’s
operations.
Research
and Development
Research
and development expenses in the six months ended June 30, 2023 were approximately $1.3 million, approximately a $1.3 million or 49.9%
decrease from the comparative period. The decrease in research and development expenses can be mainly attributed to the Company’s
suspension of expenditures required for FDA marketing authorization for the Company’s MATLOC and Krillase-related assets during
the six months ended June 30, 2023 that were incurred during the six months ended June 30, 2022.
The
Company previously sought to develop and commercialize FDA-approved products based on its Krillase and MATLOC assets simultaneously with
its DuraGraft products. During the first and second quarters of 2023, the Company prioritized the completion of regulatory processes
to obtain FDA authorization for the commercialization of DuraGraft in the United States and the development of a functional MATLOC device
prototype and MAR-FG-001-based viable products. The Company intends to maintain the Krillase assets for potential future development
and commercialization or disposition. Any determination as to these matters would be based on a number of factors. See “—Principal
Factors Affecting Our Financial Performance” above for a summary of factors that we may consider in this respect. There is
no assurance that any of the Company’s intellectual property assets will ever be developed and fully commercialized and generate
significant revenues or will ever attract significant interest from potential buyers or investors. See “Risk Factors –
Risks Related to Our Business – We may not be able to monetize intangible assets, which may result in the need to record an impairment
charge.”
Stock-Based
Compensation
Stock-based
compensation decreased by $1.0 million to approximately $0.4 million for the six months ended June 30, 2023 from approximately $1.4 million
in the comparative period ended June 30, 2022, which represents a 73.3% decrease period over period. The decrease in stock-based compensation
was mainly due to acceleration of vesting of certain stock options by the Company’s Board of Directors in the second quarter ended
June 30, 2022.
Depreciation
and Amortization
Depreciation
and amortization remained consistent at $0.4 million in the current and comparative period. The Company did not acquire any new tangible
or intangible capital assets in any of the periods indicated.
Royalty
Expense
During
the six months ended June 30, 2023, the Company recorded an aggregate of $0.2 million in royalty expense - $0.05 million in royalties
payable was incurred on sales of DuraGraft outside of the U.S.; the remaining $0.15 million in royalties expense was recorded as a 50%
reduction of the prepaid royalty balance owed to the former beneficial owners of Somahlution. No royalties were accrued in the comparative
period as minimal sales of DuraGraft occurred in the six months ended June 30, 2022.
Other
General and Administrative Expenses
Other
general and administrative expenses increased approximately $2.4 million or 234.5% to approximately $3.4 million in the six months ended
June 30, 2023. Approximately $0.5 million of the increase can be attributed to the deferred offering costs expensed as a result of adjustments
to the Convertible Notes and $0.7 million of the deferred offering costs that were expensed due to the Company’s withdrawal of
its registration statement for a public offering in April 2023. Additionally, $1.3 million of other general and administrative expenses
can be attributed to the valuation of a Class E Warrant for the purchase of 7,500,000 shares of common stock at $0.10 per share and a
Class F Warrant for the purchase of 3,750,000 shares of common stock at $0.20 per share that were issued on May 22, 2023 in connection
with the First OID Units Closing pursuant to the terms of the Hexin Promissory Note.
Other
Income (Expenses)
In
the six months ended June 30, 2023, the Company incurred approximately $7.9 million of interest and accretion costs associated with certain
securities issued at discount relating to the Units Private Placement and the OID Units Private Placement, compared to $0.8 million of
interest and accretion costs recorded in the first half of 2022 on the Convertible Notes. Additionally, due to the non-repayment of the
initial principal amount of $1.0 million under the Walleye Promissory Note by its maturity date of May 7, 2023, the Company also defaulted
under the Convertible Notes on the same date, resulting in a default amount of $5.7 million accrued to the principle of the Convertible
Notes. This represents a $14.3 million or 1,722.4% increase over the six months ended June 30, 2022.
Additionally,
the Company recognized $2.0 million of fair value gain from mark-to-market adjustments on the contingent liabilities assumed on the acquisition
of the Somahlution Assets due to the change of the fair value of the contingent consideration compared to $3.6 million of fair value
loss, an increase of $5.6 million from mark-to-market adjustment on the contingent liability from the comparative period ended June 30,
2022.
In
the six months ended June 30, 2023, due to the substantial reduction of the conversion price of the Convertible Notes in April 2023,
the Company recorded a loss of approximately $0.68 million on the extinguishment of the Convertible Notes. The Company also recorded
a loss of $2.4 million on issuance of OID Convertible Notes due to the fair value of Class E Warrants and Class F warrants exceeding
the value of the debt principal.
Fiscal
Years Ended December 31, 2022 and 2021
Components
of Results of Operations
The
components of our results of operations during the fiscal years ended December 31, 2022 and 2021 were as follows.
Revenue
Revenue
represents gross product sales less service fees and product returns. For our distribution partner channel, we recognize revenue for
product sales at the time of delivery of the product to our distribution partner. As our products have an expiration date, if a product
expires, we will replace the product at no charge. Currently, all of our revenue is generated from the sale of DuraGraft in European
and Asian markets where the product has the required regulatory approvals.
Direct
Costs of Revenue
Direct
costs of revenue include primarily product costs, which include all costs directly related to the purchase of raw materials, charges
from our contract manufacturing organizations, and manufacturing overhead costs, as well as shipping and distribution charges. Direct
costs of revenue also include losses from excess, slow-moving or obsolete inventory and inventory purchase commitments, if any.
Research
and Development
All
research and development costs are expensed in the period incurred and consist primarily of salaries, payroll taxes, and employee benefits,
those individuals involved in research and development efforts, external research and development costs incurred under agreements with
contract research organizations and consultants to conduct and support the Company’s clinical trials of DuraGraft, and costs related
to manufacturing DuraGraft for clinical trials. The Company has entered into various research and development contracts with various
organizations and other companies.
Professional
Fees
Professional
fees include legal fees relating to intellectual property development, due diligence and corporate matters, and consulting fees for accounting,
finance, and valuation services. Professional fees paid to a certain related party relate to certain consulting services. Increased expenses
related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with SEC requirements and with securities
exchange rules in future periods are likely to occur, due due to likely increases in costs of compliance with SEC regulations
of public companies in general, and with securities exchange rules due to the Company’s plans to resume the securities exchange
listing process when it can meet securities exchange listing requirements.
Salaries
and Stock-Based Compensation
Salaries
consist of compensation and related personnel costs. Stock-based compensation represents the fair value of equity-settled share awards
on stock options granted by the Company to its employees, officers, directors, and consultants. The fair value of awards is calculated
using the Black-Scholes option pricing model, which considers the following factors: exercise price, current market price of the underlying
shares, expected life, risk-free interest rate, expected volatility, dividend yield, and forfeiture rate.
Other
General and Administrative Expenses
Other
general and administrative expenses consist principally of marketing and selling expenses, facility costs, administrative and office
expenses, director and officer insurance premiums, and investor relations costs associated with operating a public company.
Other
Income (Expenses)
Other
income and expenses consist of mark-to-market adjustments on contingent liabilities assumed on the acquisition of the Somahlution Assets,
interest and accretion expenses related to our Convertible Notes, impairment of intangible assets, gain on debt extinguishment, and cancellation
of common stock pursuant to the October 2022 Letter Agreement (as defined in “—Liquidity and Capital Resources –
Recent Developments – Issuance of Replacement Securities”).
The
following table summarizes our results of operations for the fiscal years ended December 31, 2022 and 2021:
| | |
Year Ended December 31, | | |
| |
| | |
2022 | | |
2021 | | |
Change | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 233,485 | | |
$ | 210,279 | | |
$ | 23,206 | |
| Cost of goods sold | |
| 54,319 | | |
| 80,354 | | |
| (26,035 | ) |
| Gross profit | |
| 179,166 | | |
| 129,925 | | |
| 49,241 | |
| Operating expenses: | |
| | | |
| | | |
| | |
| Direct costs of revenue | |
| | | |
| | | |
| | |
| Professional fees (includes related party amounts of $172,800 and $410,400, respectively) | |
| 2,082,079 | | |
| 2,269,756 | | |
| (187,677 | ) |
| Salary expenses | |
| 2,421,969 | | |
| 2,887,309 | | |
| (465,340 | ) |
| Research and development | |
| 3,978,826 | | |
| 1,681,899 | | |
| 2,296,927 | |
| Stock-based compensation | |
| 1,905,948 | | |
| 898,444 | | |
| 1,007,504 | |
| Depreciation and amortization | |
| 841,444 | | |
| 43,871 | | |
| 797,573 | |
| Impairment of intangible assets | |
| 24,350,000 | | |
| - | | |
| 24,350,000 | |
| Other general and administrative expenses | |
| 1,922,696 | | |
| 1,170,029 | | |
| 752,667 | |
| Total operating expenses | |
| 37,502,962 | | |
| 8,951,308 | | |
| 28,551,654 | |
| Total operating loss | |
$ | (37,323,796 | ) | |
$ | (8,821,383 | ) | |
$ | (28,502,413 | ) |
| | |
| | | |
| | | |
| | |
| Other income (expenses): | |
| | | |
| | | |
| | |
| Interest and accretion expense | |
| (2,789,255 | ) | |
| (126,024 | ) | |
| (2,663,231 | ) |
| Change in fair value of contingent liabilities | |
| 1,606,000 | | |
| (1,387,000 | ) | |
| 2,993,000 | |
| Gain/(loss) on debt extinguishment | |
| 338,181 | | |
| (663,522 | ) | |
| 1,001,703 | |
| Other income | |
| 3,000 | | |
| - | | |
| 3,000 | |
| Total other income (expense) | |
| (842,074 | ) | |
| (2,176,546 | ) | |
| 1,334,472 | |
| | |
| | | |
| | | |
| | |
| Net loss | |
$ | (38,165,870 | ) | |
$ | (10,997,929 | ) | |
$ | (27,167,941 | ) |
Revenue
We
recognized revenue of approximately $0.2 million for the year ended December 31, 2022 compared to approximately $0.2 million for the
year ended December 31, 2021. The relatively insignificant change year over year was primarily due to the impact of the COVID-19 pandemic
on the Company’s supply chain and ability to produce DuraGraft inventory in fiscal year 2021. The Company’s inventory production
of DuraGraft returned to its pre-pandemic level at the end of the second quarter of 2022, but lingering effects of the COVID-19 pandemic
continued to depress demand for DuraGraft.
Direct
Costs of Revenue
During
the year ended December 31, 2022, we incurred approximately $0.05 million in direct costs of revenue, representing a decrease of $0.03
million, or 32.4%, compared to approximately $0.08 million in direct costs of revenue incurred during the year ended December 31, 2021.
During the second half of 2022, our executive and management teams’ efforts to re-establish the Company’s business relationships
with its trusted manufacturing and distribution partners, and the loosening of COVID-19 restrictions, led to a decrease in direct costs
of revenue.
Professional
Fees
Professional
fees decreased by $0.2 million, or 8.3%, to approximately $2.1 million for the year ended December 31, 2022, compared to approximately
$2.3 million for the year ended December 31, 2021. The decrease predominantly relates to higher professional fees incurred in the prior
comparative year due to the acquisition of My Health Logic in December 2021 and regulatory and valuation work related to the Somahlution
and My Health Logic acquisitions. The professional fees expense remained elevated, however, due to the Company’s efforts to grow
its business, commercialize its three main products and list its securities on a securities exchange. Related party professional fees
decreased by approximately $0.2 million or 57.8% to approximately $0.2 million during fiscal year 2022 from approximately $0.4 million
during fiscal year 2021 because the Company reduced consulting services expenses from related parties.
Salary
Expenses
Salary
expenses for the year ended December 31, 2022 were approximately $2.4 million, a $0.5 million, or 16.1%, decrease, from approximately
$2.9 million in the comparative year. The decrease is attributable to the restructuring of the Company’s executive and management
teams in 2021.
Research
and Development Expenses
During
the year ended December 31, 2022, we incurred approximately $4.0 million in research and development expenses compared to approximately
$1.7 million in the previous year ended December 31, 2021, or 136.6% more than the previous year. The increase in research and development
expenses can be attributed to the Company’s acquisition of MATLOC assets in late 2021 and its intensified focus on development
and advancement towards commercialization during 2022 of DuraGraft and MATLOC, as well as Krillase, which the Company no longer intends
to commercialize.
Stock-Based
Compensation
Stock-based
compensation increased to approximately $1.9 million in fiscal 2022 from approximately $0.9 million in fiscal year 2021, which represents
112.1% increase year over year. The increase in stock-based compensation was due to a higher weighted average number of options outstanding
and not fully vested in fiscal 2022 compared to fiscal 2021 as well as $0.3 million of compensation cost recognized on restricted share
awards during the year ended December 31, 2022.
Depreciation
and Amortization
Depreciation
and amortization increased by approximately $0.8 million, or 1,818.0%, to approximately $0.8 million in the year ended December 31, 2022
compared to approximately $0.4 million in the year ended December 31, 2021. The increase was due to the initial amortization in 2022
of the intangible capital assets that were acquired in December 2021 with the My Health Logic acquisition, and the increase in amortization
of the Somahlution Assets beginning in 2022 following their valuation in the second half of 2021.
Impairment
of Intangible Assets
During
the year ended December 31, 2022, the Company recognized impairment loss of approximately $24.4 million related to Krillase intangible
assets carrying value exceeding its recoverable amount. If the Company is unable to reinitiate development of products based on the Krillase
assets before the end of 2023, it is likely that further impairment to the Krillase assets will be required.
Other
General and Administrative Expenses
Other
general and administrative expenses increased approximately $0.8 million, or 64.3%, to approximately $1.9 million during the year ended
December 31, 2022 from approximately $1.2 million during the year ended December 31, 2021. The majority of the increase in expenses during
the year ended December 31, 2022 was due to the Company’s non-legal fees related to preparation for a proposed public offering
throughout 2022. As of April 2023, the Company determined that it no longer intended to pursue the proposed public offering. It is likely
that a reduction in other general and administrative expenses will not occur in future periods as any expenses not incurred due to the
determination not to pursue the proposed public offering are likely to be more than offset by fees relating to the filing of the registration
statement of which this prospectus forms a part relating to this proposed resale offering, likely increases in costs of compliance with
SEC regulations of public companies in general, and with securities exchange rules due to the Company’s plans to resume the securities
exchange listing process when it can meet securities exchange listing requirements.
Other
Income (Expenses)
During
the year ended December 31, 2022, the Company incurred approximately $2.8 million of interest and accretion expenses compared to 2021,
or approximately $2.7 million, or 2,113.3%, in greater interest and accretion expenses than the approximately $0.1 million in interest
and accretion expenses incurred in 2021. “Accretion expense” refers to an amount recognized as an expense classified as an
operating item in the statement of income resulting from the increase in the carrying amount of the liability associated with the asset
retirement obligation. During 2022 and 2021, the Convertible Notes were issued with a debt discount of approximately $6.5 million and
approximately $6.8 million, respectively. Debt “discount” is defined as the difference between the net proceeds, after expense,
received upon issuance of debt and the amount repayable at its maturity. Accretion expense tends to increase with the passage of time
while interest expense remains relatively flat year over year.
The
fair value of contingent liabilities changed from approximately $(1.4 million) in 2021 to approximately $1.6 million in 2022. The change
in fair value was due to a change in the valuation assumptions used to calculate the value of royalties, performance warrants, and pediatric
voucher sales liabilities issued in connection with the Somahlution Acquisition.
As
a result of the extinguishment of obligations under certain Convertible Notes and Class C Warrants, the Company recorded
approximately $0.3 million gain on debt extinguishment for the year ended December 31, 2022. During the year ended December 31, 2021,
due to the substantial reduction of the purchase and conversion price terms of the Convertible Notes and related modifications
to the Units Private Placement agreements in December 2021, the Company recorded a loss of approximately $0.7 million on the extinguishment
of the Convertible Notes.
Liquidity
and Capital Resources
To
date, we have incurred significant net losses and negative cash flows from operations. As of June 30, 2023, we had available cash
of approximately $0.2 million and accumulated deficit of approximately $109.1 million. As of December 31, 2022 and 2021,
we had available cash of approximately $0.5 million and $4.1 million, respectively, and accumulated deficit of approximately $86.0 million
and $47.8 million respectively. We have historically funded our operations through capital raises.
Recent
Developments
Issuance
of Replacement Securities
As
previously reported in its Annual Report on Form 10-K for the fiscal year ended December 31, 2021 filed on March 31, 2022 and the 2022
Form 10-K, in May 2021, the Company entered into an exclusive Placement Agency Agreement, subsequently modified on December 21, 2021
(the “2021 Placement Agency Agreement”) with Univest pursuant to which Univest agreed to act as the Company’s exclusive
placement agent for a private placement (the “Units Private Placement”) of up to $18,000,000 in units which, subsequent to
certain modifications of the terms of such private placement, consisted of the Convertible Notes, initially convertible into shares of
common stock at a conversion price of $1.75 per share, subject to adjustment, and Class C Warrants, initially exercisable for shares
of common stock at an exercise price of $2.25 per share, subject to adjustment. On January 24, 2022, the Company issued each of Univest
and its designee, Bradley Richmond (“Mr. Richmond”), as a registered representative of Univest entitled to a portion of Univest’s
compensation due to his employment terms, a Convertible Note in the principal amount of $120,000 and $180,000, respectively (collectively,
the “Placement Agent Convertible Notes”), and a Class C Warrant for the purchase of 137,142 and 205,714 shares of common
stock, subject to adjustment, respectively (collectively, the “Placement Agent Class C Warrants”), in exchange for Univest’s
and Mr. Richmond’s services provided to the Company in connection with the Company’s acquisition of My Health Logic Inc.
(“My Health Logic”). In addition, as previously reported in the 2022 Report, on June 26, 2022, pursuant to the 2021 Placement
Agency Agreement, in anticipation of the final closing of the Units Private Placement, in exchange for $100, the Company issued Univest
a warrant for the purchase of 231,239 shares of common stock, and a warrant to Mr. Richmond, as a registered representative of Univest
who is entitled to a portion of Univest’s compensation due to his employment terms, for the purchase of 347,039 shares of common
stock (the “2022 Placement Agent Warrants”), each of which was initially exercisable at $1.75 per share, subject to adjustment.
Separately,
on January 26, 2022 and February 14, 2022, the Company granted Mr. Richmond two warrants (the “Consultant Warrants”) to purchase
up to 150,000 shares of common stock at an exercise price of $0.01 per share as compensation for certain services. Mr. Richmond fully
exercised both of the Consultant Warrants for 300,000 shares of common stock in March 2022 (the “Consultant Shares” and together
with the Placement Agent Convertible Notes, the Placement Agent Class C Warrants, and the 2022 Placement Agent Warrants, the “Cancelled
Securities”).
On
February 14, 2022, the Company filed a registration statement on Form S-1, which was subsequently amended on certain dates, relating
to the Company’s proposed underwritten public offering for gross proceeds of approximately $8 million. Univest was named as the
representative of the underwriters of the offering. As previously reported in the 2022 Report, the staff (“FINRA Staff”)
of the Corporate Financing Department of the Financial Industry Regulatory Authority, Inc. (“FINRA”) determined that the
sales of the Placement Agent Convertible Notes, the Placement Agent Class C Warrants, the 2022 Placement Agent Warrants, the Consultant
Shares, as well as a grant of a stock option to Mr. Richmond constituted underwriting compensation in connection with the Company’s
proposed public offering pursuant to FINRA Rule 5110, based on the FINRA Staff’s interpretation of such rule. Consequently, each
of Univest and Mr. Richmond, pursuant to a letter agreement entered into with the Company, dated October 28, 2022, addressed and submitted
to the FINRA Staff (the “October 2022 Letter Agreement”), agreed to forego their applicable rights to these securities. Pursuant
to the October 2022 Letter Agreement, the parties thereto agreed that the cancellation or disposal of the aforementioned securities shall
be without recourse by either Univest or Mr. Richmond. Accordingly, the Placement Agent Convertible Notes, the Placement Agent Class
C Warrants, the 2022 Placement Agent Warrants, and the Consultant Shares were subsequently cancelled, and the Company and Mr. Richmond
agreed to the transfer of his stock option to a Company designee who is unaffiliated with Mr. Richmond.
The
Convertible Notes including the Placement Agent Convertible Notes prior to their cancellation, Class C Warrants including the Placement
Agent Class C Warrants prior to their cancellation, and the 2022 Placement Agent Warrants contained certain adjustment provisions as
described below under “—Funding Requirements and Other Liquidity Matters – Adjustments to Conversion Price of Convertible
Notes and Exercise Price of Class C Warrants and Number of Underlying Shares”, except that the terms of the January 2023 Letter
Agreement (as defined and described in that section) did not expressly apply to the Placement Agent Class C Warrants or the 2022 Placement
Agent Warrants, which had previously been cancelled as described above.
In
April 2023, as a result of the events described below under “—Funding Requirements and Other Liquidity Matters –
Exercise of Somahlution Warrants at Reduced Exercise Price”, and pursuant to the adjustment provisions described below under
“—Funding Requirements and Other Liquidity Matters – Adjustments to Conversion Price of Convertible Notes and Exercise
Price of Class C Warrants and Number of Underlying Shares”, the conversion price of the Convertible Notes and the exercise
price of the Class C Warrants were both adjusted to $0.10 per share of common stock. As a result of these adjustment provisions and the
conversion or exercise terms of the Convertible Notes and Class C Warrants, the aggregate number of shares into which the Convertible
Notes may be converted or the Class C Warrants may be exercised to purchase adjusted upwards proportionately.
On
April 21, 2023, prior to the effectiveness of the registration statement for its proposed public offering, the Company filed an application
with the SEC for the withdrawal of the registration statement, including all amendments and exhibits to the registration statement. The
application for withdrawal stated that the Company did not intend to pursue the contemplated public offering at that time. Pursuant to
Rule 477 under the Securities Act, the application was deemed granted at the time the application was filed, because it was filed before
the effective date of the registration statement, and the SEC did not notify the Company that the application for withdrawal would not
be granted within 15 calendar days after the Company had filed the application.
On
June 22, 2023, Univest and Mr. Richmond requested through counsel that the Company either reissue the Cancelled Securities or issue them
new securities with equivalent terms and conditions, in light of the fact that the Company was not proceeding with the public offering.
On July 16, 2023, Univest and Mr. Richmond communicated through counsel that they believed that the Cancelled Securities should be reissued
as if they were never canceled, meaning that they should have all of the adjusted terms that may have applied to the Cancelled Securities
had they not been cancelled.
Pursuant
to these requests, on July 25, 2023 and September 29, 2023, the Company approved the grant of the following securities to Univest and
Mr. Richmond: (i) Convertible Notes (the “Replacement Convertible Notes”) with terms and conditions equivalent to or at least
as favorable as those that would have applied to each of the respective Placement Agent Convertible Notes, such that, among other adjustments,
the principal and any accrued interest under the Replacement Convertible Notes is convertible into shares of common stock at $0.10 per
share, subject to adjustment, reflecting adjustments to their terms equivalent to those that applied to the Convertible Notes as a result
of the Somahlution Warrants Exercise, and with principal increased to reflect the amount of interest that would have been incurred under
the respective Placement Agent Convertible Notes up to the date of issuance, such that the adjusted principal under the Replacement Convertible
Notes is $137,983.56 and $206,975.34, respectively, being the original principal under the Placement Agent Convertible Notes plus pre-issuance
interest that would have accrued since the date of the issuance of the Placement Agent Convertible Notes as of July 25, 2023, maturing
on July 25, 2025, and that, taking into account the Convertible Notes’ amended Mandatory Default Amount provisions as described
in “—Letter Agreement to Amend Convertible Notes to Permit Conversion of Mandatory Default Amount” and the default
under the Convertible Notes that occurred as a result of their cross-default provisions as described in “—Funding Requirements
and Other Liquidity Matters – Cross-Default Under Convertible Notes”, approximately $182,772 and $274,157 is due, respectively,
and up to 1,827,715 and 2,741,573 shares of common stock are issuable upon conversion thereof, subject to adjustment and applicable beneficial
ownership limitations and other limitations or restrictions, and having an approximate dollar value of $182,772 and $274,157, respectively;
(ii) Class C Warrants (the “Replacement Class C Warrants”) with terms and conditions equivalent to or at least as favorable
as those that would have applied to each of the respective Placement Agent Class C Warrants, reflecting adjustments to their terms equivalent
to those that applied to the Class C Warrants as a result of the Somahlution Warrants Exercise such that the exercise price per share
of the Replacement Class C Warrants is $0.10 per share, subject to adjustment, and the number of shares of common stock issuable upon
exercise of each of the Replacement Class C Warrants reflecting a proportional increase relative to the number of shares issuable under
the respective Placement Agent Class C Warrants equivalent to the difference between the exercise price per share of the Placement Agent
Class C Warrants and the exercise price of such Replacement Class C Warrants, such that up to 3,548,148 and 5,322,223 shares of common
stock, respectively, are issuable upon exercise thereof, subject to adjustment and applicable beneficial ownership limitations and other
limitations or restrictions; (iii) Placement Agent Warrants (the “Replacement Placement Agent Warrants”) with terms and conditions
equivalent to or at least as favorable as those that would have applied to those that applied to each of the respective 2022 Placement
Agent Warrants, reflecting adjustments to their terms equivalent to those that applied to the Class C Warrants as a result of the Somahlution
Warrants Exercise such that the exercise price per share of the Replacement Placement Agent Warrants is $0.10 per share, subject to adjustment,
and the number of shares of common stock issuable upon exercise of each of the Replacement Placement Agent Warrants reflecting a proportional
increase relative to the number of shares issuable under the respective 2022 Placement Agent Warrants equivalent to the difference between
the exercise price per share of the 2022 Placement Agent Warrants and the exercise price of the Replacement Placement Agent Warrants,
such that up to 19,293,621 and 28,940,432 shares of common stock, respectively, are issuable upon exercise thereof, subject to adjustment
and applicable beneficial ownership limitations and other limitations or restrictions; and (iv) 300,000 shares of common stock to Mr.
Richmond, being the same number of shares as the Consultant Shares (the “Replacement Consultant Shares” and together with
the Replacement Convertible Notes, the Replacement Class C Warrants, and Replacement Placement Agent Warrants, the “Replacement
Placement Agent and Consultant Securities”). In connection with these grants, each of Univest and Mr. Richmond agreed to waivers
of any registration rights that would have been applicable to each of the Replacement Convertible Notes, the Replacement Class C Warrants,
and the Replacement Placement Agent Warrants.
In
May 2023, the event described under “—Funding Requirements and Other Liquidity Matters – Default Under Promissory
Note” and “—Funding Requirements and Other Liquidity Matters – Cross-Default Under Convertible Notes”
occurred. Assuming that the event described would have triggered the cross-default provision in the Placement Agent Convertible Notes
if they had not been cancelled, and assuming that each of the Replacement Placement Agent Convertible Notes are also subject to immediate
payment of the applicable Mandatory Default Amount, the aggregate Mandatory Default Amount that may be due under the Replacement Placement
Agent Convertible Notes would be $456,929, or approximately $0.1 million more than would otherwise have been due under the Placement
Agent Convertible Notes on the date of the default had they been outstanding on that date.
Under
the unit purchase agreement entered into on May 27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s
subsidiaries entered into a guaranty to guarantee the repayment of the Company’s obligations under the Convertible Notes, and the
Company and its subsidiaries entered into security agreements granting security interests in all of their respective assets for up to
the dollar value owed under the respective Convertible Notes. The respective Convertible Notes were issued for aggregate principal of
approximately $1.2 million. Under each unit purchase agreement entered into with respect to subsequent issuances of the Convertible Notes,
including the unit purchase agreement entered into with each of Univest and Mr. Richmond in connection with the issuance of the Placement
Agent Convertible Notes, the Company and its subsidiaries were obligated to enter into similar security agreements and guaranties, but
did not do so.
Univest
and Mr. Richmond have not exercised any remedies applicable to the Replacement Convertible Notes or given notice of any intention to
do so as of the date of this prospectus. In the event that the balance owed under the Replacement Convertible Notes, including the respective
Mandatory Default Amount, is not repaid upon demand, Univest and Mr. Richmond may seek to take possession of some or all of the Company’s
and its subsidiaries’ assets, force the Company and its subsidiaries into bankruptcy proceeds, or seek other legal remedies against
the Company and its subsidiaries. In such event, the Company’s business, operating results and financial condition may be materially
adversely affected.
Letter
Agreement to Amend Convertible Notes to Permit Conversion of Mandatory Default Amount
On
October 3, 2023, under a letter agreement between Univest, as unitholder representative for the investors in the Units Private Placement,
and the Company (the “Convertible Notes Letter Agreement”), the definition of the amount that must be paid upon the first
uncured or uncurable default under the Convertible Notes was amended to equal 135% of the outstanding principal plus accrued interest
as of the date on which the first event of default occurred under the Convertible Notes (the “Mandatory Default Amount”),
and to permit the conversion of the Mandatory Default Amount into shares of common stock at the applicable conversion price. As a result,
at the current conversion price of $0.10 per share, the outstanding Convertible Notes became convertible into an aggregate of 221,939,338
shares of common stock. The Convertible Notes Letter Agreement also amended the Convertible Notes to provide that a full conversion of
the Mandatory Default Amount would also fully extinguish any outstanding principal and accrued and unpaid interest; that non-voluntary
conversion of the Mandatory Default Amount (which would also extinguish any outstanding principal and accrued and unpaid interest as
noted above) under all of the Convertible Notes will occur only if at any time following the 60-day anniversary of the final closing
date or termination of the Units Private Placement, and provided there is an effective registration statement permitting the issuance
or resale of the shares of common stock into which the Convertible Notes may be converted, (A) the common stock is listed on a senior
national securities exchange, (B) the daily volume-weighted average price for the prior twenty (20) consecutive trading days is $6.00
or more (adjusted for splits and similar distributions) and (C) the daily trading volume is at least $1,000,000 during such 20-day period;
and that, for all Convertible Notes, upon the occurrence of a Qualified Financing, the conversion price of the Convertible Notes will
be adjusted to the price per share equal to 75% of the price per equity security in such financing.
In
connection with the Convertible Notes Letter Agreement, additional Replacement Placement Agent Warrants were issued to Univest and Mr.
Richmond on October 3, 2023 to purchase 15,244,839 and 22,867,250 shares of common stock, respectively, which together with their previous
Replacement Placement Agent Warrants issued to purchase 4,048,762 and 6,073,182 shares of common stock, respectively, totaled 8% in aggregate
of the shares issuable upon conversion of the Convertible Notes, including the Mandatory Default Amount, and the Class C Warrants, in
accordance with the 2021 Placement Agency Agreement. The Replacement Placement Agent Warrants are exercisable, in whole or in part, until
October 3, 2028 by payment of cash or on a cashless net exercise basis, and contain certain antidilution provisions and exercise price
adjustment provisions that are substantially identical to equivalent provisions of the Class C Warrants; are currently exercisable for
$0.10 per share; and the holders of such warrants have the rights set forth in the unit purchase agreement dated as of August 12, 2022
with respect to the Convertible Notes and Class C Warrants that were issued in August 2022.
Special
Stockholders Meeting, Change in Authorized Shares, and Approval of Voting Rights for Certain Debtholders
The
Special Stockholders Meeting was held on August 9, 2023 to consider and vote on the following proposals: (i) to approve the Capital Event
Amendment, and (ii) to approve the Voting Rights Amendment. Approval of each proposal required a “quorum” at the meeting
consisting of a majority (more than 50%) of the outstanding voting shares as of the record date, present in person or represented by
proxy, and the affirmative “for” vote of the holders of a majority of the shares represented at the meeting, in person or
by proxy, and entitled to vote.
At
the Special Stockholders Meeting, a quorum was attained, and each of the proposals described above was approved by the required number
of votes of the Company’s stockholders. On August 15, 2023, the board of directors of the Company approved resolutions authorizing
each of the holders of the Convertible Notes to vote with the shares of common stock, on an as-converted to common stock basis, with
respect to all corporate matters of the Company on which the holders of common stock are entitled to vote, subject to any applicable
beneficial ownership limitations, upon the effectiveness of the Certificate of Amendment Pursuant to NRS 78.380 & 78.390 to be filed
with the Nevada Secretary of State providing for the Capital Event Amendment and the Voting Rights Amendment (the “Certificate
of Amendment”). On August 16, 2023, the Company filed the Certificate of Amendment with the Nevada Secretary of State providing
for the Capital Event Amendment and the Voting Rights Amendment, and the Certificate of Amendment became effective upon filing. Accordingly,
upon the filing of the Certificate of Amendment, (i) the Company’s authorized shares of common stock were increased from 300,000,000
to 2,000,000,000 with no corresponding change in the number of issued and outstanding shares of common stock; (ii) the Company’s
board of directors may grant any of the rights of stockholders to any of the holders of any of the Company’s bonds, debentures
or other obligations; and (iii) all of the holders of the Convertible Notes became entitled to vote with the shares of the Company’s
common stock, on an as-converted to common stock basis without actually having converted the Convertible Notes to shares of the Company’s
common stock, with respect to all corporate matters of the Company on which the holders of common stock are entitled to vote, as provided
in the Convertible Notes.
As
a result of the filing of the Certificate of Amendment, the voting rights of the holders of the Company’s common stock may be deemed
to be proportionately reduced to the extent that the voting rights granted to the holders of the Convertible Notes by the Certificate
of Amendment may be exercised at a meeting or with respect to other applicable actions of the stockholders of the Company. As of the
date of this prospectus, the holders of these voting rights have the right to cast up to 214,709,068 votes, compared to 46,666,760 votes
by the holders of shares of the outstanding common stock. These voting rights remain subject to beneficial ownership limitations, including
that holders of Convertible Notes may vote the amount of shares of common stock that that they may convert their Convertible Notes into
subject to the applicable beneficial ownership percentage limitation.
Third
Closing of OID Units Private Placement
On
July 10, 2023, the Company conducted the third closing (the “Third OID Units Closing”) of a private placement (the “OID
Units Private Placement”) of up to $10,000,000 for an aggregate of up to 100,000,000 units (“OID Units”), under a unit
purchase agreement between Hexin Global Ltd. (“Hexin”), which represented that it is an “accredited investor”
as such term is defined by Rule 501(a) of the Securities Act, and the Company (each, an “OID Units Purchase Agreement”),
with each issuance of OID Units to an investor in the OID Units Private Placement consisting of (i) an OID Convertible Note, convertible
into shares of common stock plus additional shares based on accrued interest at $0.10 per share, subject to adjustment, (ii) a Class
E Warrant for the purchase of 125% of the shares of common stock into which the OID Convertible Note may be converted at $0.10 per share,
subject to adjustment, and (iii) a Class F Warrant for the purchase of 125% of the shares of common stock into which the OID Convertible
Note may be converted at $0.20 per share, subject to adjustment. The Class E Warrant and Class F Warrant owned by Hexin or other investors
are collectively sometimes referred to as the “Class E and F Warrants”. Under each OID Units Purchase Agreement, the minimum
investment permitted is $1,000. The Company retained Univest as the Company’s exclusive placement agent in connection with the
sale of the OID Units under a Placement Agency Agreement, dated April 27, 2023 (the “April 2023 PAA”). In addition to the
rights set forth in each OID Units Purchase Agreement as described below under “—Funding Requirements and Other Liquidity
Matters – OID Units Private Placement – OID Units Purchase Agreement”, the OID Convertible Notes as described
below under “—Funding Requirements and Other Liquidity Matters – OID Units Private Placement – OID Convertible
Notes”, and the Class E and F Warrants as described below under “Description of Securities – Securities Issued
in the OID Units Private Placement – Class E Warrants” and “—Class F Warrants”, each investor
party to an OID Units Purchase Agreement and each holder of an OID Convertible Note or Class E and F Warrants has rights under the respective
Registration Rights Agreement with the Company (each, “OID Units Registration Rights Agreement”), as described below under
“—Funding Requirements and Other Liquidity Matters – OID Units Private Placement – OID Units Registration
Rights Agreement”.
In
connection with the Third OID Units Closing, on July 10, 2023, Hexin paid a subscription amount of $1,000,000, and the Company issued
11,764,710 OID Units consisting of (i) an OID Convertible Note in the principal amount of $1,176,471, convertible into 11,764,710 shares
of common stock plus additional shares based on accrued interest at $0.10 per share, subject to adjustment, (ii) a Class E Warrant for
the purchase of 14,705,880 shares of common stock at $0.10 per share, subject to adjustment, and (iii) a Class F Warrant for the purchase
of 14,705,880 shares of common stock at $0.20 per share, subject to adjustment. See below for additional related discussion of these
securities. In connection with the Third OID Units Closing, after deducting Univest’s 8% fee of $80,000 and $50,000 for reimbursement
of Univest’s legal fees pursuant to the April 2023 PAA, and, pursuant to the 2021 Placement Agency Agreement, after deducting Univest’s
outstanding fees of $140,000 or 8% from the prior issuances of the Walleye Promissory Note for the principal amount of $1,000,000 on
February 6, 2023 and an unsecured promissory note (the “Hexin Promissory Note”) to Hexin on December 28, 2022 for the principal
amount of $750,000, the Company received net proceeds from the Third OID Units Closing of $730,000.
Amendment
to May 2023 OID Units Registration Rights Agreements
In
connection with the First OID Units Closing as described below under “—OID Units Private Placement – First
Closing”, which occurred on May 12, 2023, an OID Units Registration Rights Agreement, dated as of May 12, 2023 (the “May
12, 2023 Registration Rights Agreement”), between the Company and Walleye, provided that the Company file a registration statement
within 60 days of such closing, registering the resale of the shares of common stock issuable pursuant to conversion of the 15% original
issue discount unsecured subordinated convertible promissory note and exercise of the Class E Warrant and Class F Warrant issued to that
investor in connection with such closing, and contained other terms and conditions. As previously reported in a Current Report on Form
8-K filed on June 5, 2023, in connection with the second closing of the OID Units Private Placement, which occurred on May 30, 2023,
an OID Units Registration Rights Agreement, dated as of May 30, 2023 (the “May 30, 2023 Registration Rights Agreement” and
each of the May 30, 2023 Registration Rights Agreement and the May 12, 2023 Registration Rights Agreement respectively, the “Amended
OID Units Registration Rights Agreements”), between the Company and Walleye, Hexin and Frank Maresca Jr. (“Mr. Maresca”),
also provided that the Company file a registration statement within 60 days of such closing, registering the resale of the shares of
common stock issuable pursuant to conversion of the OID Convertible Notes and exercise of the Class E Warrants and Class F Warrant issued
to these investors in connection with such closing, and contained other terms and conditions.
Holders
of rights to 67% or more of the shares issuable upon conversion of the OID Convertible Notes and exercise of the Class E Warrants and
the Class F Warrants may agree to amend or waive the requirements of each of the respective Amended OID Units Registration Rights Agreements.
Under an Amendment to Registration Rights Agreement, dated as of July 6, 2023 (“Amendment to Registration Rights Agreement”),
Walleye and Hexin, being holders of such rights, agreed to an amendment to each of the Amended OID Units Registration Rights Agreements
to provide that the initial filing of the registration statement required by such Amended OID Units Registration Rights Agreements shall
be made on or before the date that is 67 days after the date of the respective closing of the OID Units Private Placement.
Fourth
Closing of OID Units Private Placement
On
August 30, 2023, the Company conducted the fourth closing (the “Fourth OID Units Closing”) of the OID Units Private Placement
under an OID Units Purchase Agreement. Each investor represented that it is an “accredited investor” as such term is defined
by Rule 501(a) of the Securities Act. Pursuant to the OID Units Purchase Agreement, the Company issued OID Units to each investor by
delivering to such investor (i) an OID Convertible Note, convertible into shares of common stock plus additional shares based on accrued
interest at $0.10 per share, subject to adjustment, (ii) a Class E Warrant for the purchase of 125% of the shares of common stock into
which the respective OID Convertible Note may be converted at $0.10 per share, subject to adjustment, and (iii) a Class F Warrant for
the purchase of 125% of the shares of common stock into which the respective OID Convertible Note may be converted at $0.20 per share,
subject to adjustment. Univest was the Company’s exclusive placement agent in connection with the sale of the OID Units under the
April 2023 PAA. In addition to the rights set forth in the OID Units Purchase Agreement as described below under “—Funding
Requirements and Other Liquidity Matters – OID Units Private Placement – OID Units Purchase Agreement”,
the OID Convertible Notes as described below under “—Funding Requirements and Other Liquidity Matters – OID Units
Private Placement – OID Convertible Notes”, and the Class E and F Warrants as described below under “Description
of Securities – Securities Issued in the OID Units Private Placement – Class E Warrants” and “—Class
F Warrants”, each investor party to an OID Units Purchase Agreement and each holder of an OID Convertible Note or Class E and
F Warrants has rights under an OID Units Registration Rights Agreement with the Company, as described below under “—Funding
Requirements and Other Liquidity Matters – OID Units Private Placement – OID Units Registration Rights Agreement”.
In
connection with the Fourth OID Units Closing, on August 30, 2023, 12 of the investors paid an aggregate subscription amount of $825,000,
and the Company issued to these investors 9,705,960 OID Units consisting of (i) OID Convertible Notes in the aggregate principal amount
of $970,596, convertible into 9,705,960 shares of common stock plus additional shares based on accrued interest at $0.10 per share, subject
to adjustment, (ii) Class E Warrants for the purchase of 12,132,448 shares of common stock at $0.10 per share, subject to adjustment,
and (iii) Class F Warrants for the purchase of 12,132,448 shares of common stock at $0.20 per share, subject to adjustment. In connection
with the Fourth OID Units Closing, after deducting Univest’s 8% fee of $66,000 and $25,000 for reimbursement of Univest’s
legal fees pursuant to the April 2023 PAA, the Company received net proceeds of $734,000.
Also
in connection with the Fourth OID Units Closing, under a Cancellation and Exchange Agreement, dated as of August 30, 2023, between the
Company and Mr. Maresca (the “August 2023 Maresca Cancellation Agreement”), Mr. Maresca agreed to cancel $200,000 of aggregate
short-term indebtedness to Mr. Maresca, which arose in the ordinary course of business for certain consulting services provided by Mr.
Maresca to the Company, in exchange for: (i) the execution of the August 2023 OID Units Purchase Agreement, (ii) the execution of the
August 2023 OID Units Registration Rights Agreement, and (iii) the issuance of 2,352,950 OID Units consisting of (a) an OID Convertible
Note in the principal amount of $235,295 for a subscription equal to $200,000 (the amount of the indebtedness being cancelled), convertible
into 2,352,950 shares of common stock plus additional shares based on accrued interest at $0.10 per share, subject to adjustment (the
“August 2023 Maresca OID Convertible Note”), (b) a Class E Warrant for the purchase of 2,941,187 shares of common stock at
$0.10 per share, subject to adjustment (the “August 2023 Maresca Class E Warrant”), and (c) a Class F Warrant for the purchase
of 2,941,187 shares of common stock at $0.20 per share, subject to adjustment (the “August 2023 Maresca Class F Warrant”).
The August 2023 Maresca Cancellation Agreement contains a release of claims against the Company relating to the cancelled indebtedness.
The
August 2023 Maresca OID Convertible Note, the August 2023 Maresca Class E Warrant and the August 2023 Maresca Class F Warrant are subject
to the terms and conditions of the August 2023 Maresca Cancellation Agreement. Mr. Maresca’s rights under the OID Units Purchase
Agreement and the August 2023 OID Units Registration Rights Agreement are also subject to the terms and conditions of the August 2023
Maresca Cancellation Agreement.
Univest
did not receive a fee in connection with the transactions conducted pursuant to the August 2023 Maresca Cancellation Agreement because
there were no related gross proceeds.
Issuance
of OID Units Placement Agent Warrants
Under
the April 2023 PAA and the forms of Placement Agent Warrants agreed to in connection with the April 2023 PAA, the Company also agreed,
upon Univest’s payment of $100.00, to issue the OID Units Placement Agent Warrants to Univest and/or its designee(s) at each closing
of the OID Units Private Placement. The OID Units Placement Agent Warrants were required to be issued for the purchase of 8% of the aggregate
number of shares of common stock initially issuable upon conversion of the OID Convertible Notes at $0.10 per share, 8% of the aggregate
number of shares of common stock initially issuable upon exercise of the Class E Warrants at $0.10 per share, and 8% of the aggregate
number of shares of common stock initially issuable upon exercise of the Class F Warrants at $0.20 per share, subject to adjustment.
On September 12, 2023, Univest notified the Company that the OID Units Private Placement’s final closing had occurred, and on September
13, 2023, made a payment to the Company of $100.00. Accordingly, on September 13, 2023, OID Units Placement Agent Warrants were issued
to Univest and its designees, Bradley Richmond, Bartholemew Pan-Kita, and Azem Nesimi, to purchase a total of 4,356,687, 6,695,616, 635,294,
and 7,059 shares of common stock at $0.10 per share, respectively, which in aggregate was equal to 8% of the shares issuable upon conversion
or exercise of the OID Convertible Notes and the Class E Warrants issued in each of the closings of the OID Units Private Placement as
of that date, and a total of 2,585,479, 3,878,218, 0, and 0 shares of common stock at $0.20 per share, respectively, which in aggregate
was equal to 8% of the shares issuable upon exercise of the Class F Warrants issued in each of the closings of the OID Units Private
Placement as of that date.
The
OID Units Placement Agent Warrants are generally exercisable from the date of issuance until August 16, 2028. The exercise right is
subject to the following beneficial ownership limitation: Exercise is permitted only if it would not cause the holder (together with
its Affiliates (as defined by Rule 405 under the Securities Act), and any other persons acting as a group together with the holder
or any of the holder’s Affiliates), to beneficially own in excess of the percentage of the outstanding securities that are
permitted to be beneficially owned (as described below), which, for purposes of the limitation, includes shares issuable upon
exercise of the OID Units Placement Agent Warrants, excludes shares issuable upon exercise of the unexercised portion of the OID
Units Placement Agent Warrants and exercise or conversion of the unexercised or nonconverted portion of any other securities of the
Company subject to an analogous limitation on conversion or exercise beneficially owned by the holder or any of its Affiliates, and
otherwise calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. For
purposes of the OID Units Placement Agent Warrants, the number of outstanding shares of common stock shall be determined after
giving effect to the conversion or exercise of securities of the Company, including the exercised portion of the OID Units Placement
Agent Warrants, by the holder or its Affiliates since the date as of which such number of outstanding shares of Common Stock was
reported. The maximum percentage of beneficial ownership of the Company’s outstanding securities that applies to an exercise
of each OID Units Placement Agent Warrant is 9.99% of the number of shares of the common stock outstanding immediately after giving
effect to the issuance of shares of common stock issuable upon exercise of the OID Units Placement Agent Warrant. The holder, upon
notice to the Company, may increase or decrease the percentage limit, provided that the limitation in no event exceeds 9.99% of the
number of shares of common stock outstanding immediately after giving effect to the issuance of shares of common stock upon exercise
of the OID Units Placement Agent Warrant. Any increase in the limitation will not be effective until the 61st day after such notice
is delivered to the Company. This limitation does not apply to certain provisions of the OID Units Placement Agent
Warrants.
The
OID Units Placement Agent Warrants provide for exercise by payment of cash or on a cashless net exercise basis. In the event the Company
sells, grants, issues, or otherwise disposes of common stock or securities with rights to common stock, or announces an intention to
do so, at a lower price per share than the applicable OID Units Placement Agent Warrants’ exercise price, before the listing of
the common stock on NYSE American LLC (“NYSE American”), Nasdaq, or the New York Stock Exchange (the “NYSE” and
together with NYSE American and Nasdaq, a “National Stock Exchange”), while such OID Units Placement Agent Warrants are outstanding,
then the applicable OID Units Placement Agent Warrants’ exercise price will be reduced to the greater of such lower price or a
floor price as determined by any applicable National Stock Exchange. The OID Units Placement Agent Warrants provide for equivalent registration
rights as provided for under the August 2023 OID Units Registration Rights Agreement. The OID Units Placement Agent Warrants also have
customary antidilution provisions in the event of stock splits, certain changes of control or similar transactions, and rights offerings.
The OID Units Placement Agent Warrants also provide mutual indemnification relating to claims relating to a registration statement under
which shares issuable upon exercise of the OID Units Placement Agent Warrants may be sold to the same or equivalent extent as the indemnification
provision contained in the OID Units Purchase Agreement.
Going Concern
The Company, since its inception,
has incurred recurring operating losses and negative cash flows from operations. As of June 30, 2023, December 31, 2022 and December
31, 2021, the Company had an accumulated deficit of approximately $109.1 million, $86.0 million and $47.8 million, respectively.
Our net loss was approximately $20.6 million and $6.9 million for the three months ended June 30, 2023 and June 30,
2022, respectively, and was approximately $23.1 million and $13.0 million for the six months ended June 30,
2023 and June 30, 2022, respectively. For the fiscal years ended December 31, 2022 and 2021, our net loss was approximately
$38.2 million and $11.0 million, respectively. The Company also had negative working capital as of June 30, 2023 and December
31, 2022 of approximately $(19.8 million) and $(1.0 million), respectively. The Company had $0.2 million and $0.5 million in cash as
of June 30, 2023 and December 31, 2022, respectively. In view of these material trends of increasing accumulated deficits,
repeated net losses, increasingly negative working capital, and reduced cash than was provided by operations, the
Company expects that it will not have sufficient cash to fund operations for the next twelve months without additional financing. Without
further funding, these conditions raise substantial doubt about the Company’s ability to continue as a going concern.
The accompanying consolidated financial statements
have been prepared in conformity with GAAP, which contemplate continuation of the Company as a going concern and the realization
of assets and satisfaction of liabilities in the normal course of business. The ability of the Company to continue its operations is dependent
on the execution of management’s plans, which include the raising of capital through the debt and/or equity markets, until such
time that funds provided by operations are sufficient to fund working capital requirements. If the Company were not to continue as a going
concern, it would likely not be able to realize its assets at values comparable to the carrying value or the fair value estimates reflected
in the balances set out in the preparation of the consolidated financial statements.
There can be no assurances that the Company will be
successful in generating additional cash from the equity/debt markets or other sources to be used for operations. The consolidated financial
statements do not include any adjustments relating to the recoverability of assets and classification of assets and liabilities that might
be necessary. Based on the Company’s current resources, the Company will not be able to continue to operate without additional immediate
funding. Should the Company not be successful in obtaining the necessary financing to fund its operations, the Company would need to curtail
certain or all operational activities and/or contemplate the sale of its assets, if necessary.
Funding
Requirements and Other Liquidity Matters
We
expect to continue to incur expenses and operating losses for the foreseeable future. We anticipate that our expenses will increase as
a result of the following operational and business development efforts:
| ● | Increase
our expertise and knowledge through hiring and retaining qualified operational, financial
and management personnel, who are expected to develop an efficient infrastructure to support
development and commercialization of therapies and devices; |
| ● | Increase
in research and development and legal expenses as we continue to develop our products, conduct
clinical trials and pursue FDA clearances; |
| ● | Expand
our product portfolio through the identification and acquisition of additional life science
assets; and |
| ● | Seek
to increase awareness about our products to boost sales and distributions internationally. |
Until
such time, if ever, as we can generate substantial product revenues to support our cost structure, the Company will continue to have
to raise funds beyond its current working capital balance in order to finance future development of products, potential acquisitions,
and meet its debt obligations until such time as future profitable revenues are achieved.
We
expect to finance our cash needs through a combination of private and public equity offerings, debt financings, government or other third-party
funding, and collaborations, arrangements or acquisitions. On February 14, 2022, we filed
a registration statement on Form S-1, subsequently amended, relating to a proposed underwritten public offering for gross proceeds of
approximately $8 million from the issuance of units consisting of a share of common stock and two warrants to purchase one share of common
stock. On April 21, 2023, prior to the effectiveness of the registration statement, we filed an application with the SEC for the withdrawal
of the registration statement, including all amendments and exhibits to the registration statement. The application for withdrawal was
filed to withdraw the registration statement because the Company no longer intended to pursue the proposed public offering. Pursuant
to Rule 477 under the Securities Act, the application was deemed granted at the time the application was filed, because it was filed
before the effective date of the registration statement, and the SEC did not notify us that the application for withdrawal would not
be granted within 15 calendar days after the Company had filed the application.
We
have not withdrawn the listing application that we submitted to Nasdaq in connection with the proposed public offering. We expect to
resume the securities exchange listing process when we believe that we can meet all applicable securities exchange listing requirements
and standards. We believe that such a listing may be an important factor in the Company’s
efforts to gain sufficient financing to fund its operations in the short-term and long-term. There is no guarantee or assurance that
our common stock will be approved for listing on the Nasdaq Capital Market. If we are unable to list our common stock on the Nasdaq Capital
Market or other national securities exchange, we may experience heightened difficulty in raising sufficient funding to meet our capital
and cash requirements over the 12 months ended June 30, 2024 and any period beyond that date. To the extent that we raise additional
capital through the sale of common stock, convertible securities or other equity securities, the ownership interest of our stockholders
may be materially diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the interests
of our stockholders. Debt financing and preferred equity financing, if available, would result in increased fixed payment obligations
and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional
debt, making capital expenditures or declaring dividends, that could adversely impact our ability to conduct our business. Securing additional
financing could require a substantial amount of time and attention from our management and may divert a disproportionate amount of their
attention away from day-to-day activities, which may adversely affect our management’s ability to oversee the development or acquisition
of product.
If
we raise additional funds through collaborations, strategic alliances or marketing, distribution, or licensing arrangements with third
parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates
or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings
when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant
rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. These factors raise substantial
doubt about the Company’s ability to continue as a going concern. See “—Going Concern” above.
Summary of Cash Flow
The following table provides
a summary of our net cash flow for all financial statement periods presented in this prospectus:
| |
|
Six
Months Ended |
|
|
Fiscal
Year Ended |
|
| |
|
June 30,
2023 |
|
|
June 30,
2022 |
|
|
Change
($) |
|
|
December 31,
2022 |
|
|
December 31,
2021 |
|
|
Change
($) |
|
| Net cash provided by/(used in): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating activities |
|
$ |
(2,318,013 |
) |
|
$ |
(7,055,675 |
) |
|
$ |
4,737,662 |
|
|
$ |
(10,852,148 |
) |
|
$ |
(5,787,095 |
) |
|
$ |
5,065,053 |
|
| Investing activities |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
| Financing activities |
|
|
2,012,374 |
|
|
|
5,028,312 |
|
|
|
(3,015,938 |
) |
|
|
7,290,674 |
|
|
|
6,956,672 |
|
|
|
334,002 |
|
| Net change in cash |
|
$ |
(305,639 |
) |
|
$ |
(2,027,363 |
) |
|
$ |
1,721,724 |
|
|
|
(3,561,474 |
) |
|
$ |
1,169,577 |
|
|
|
(4,731,051 |
) |
Summary of Operating Activities
Net
cash used in operating activities was approximately $2.3 million and $7.1 million for the six months ended June
30, 2023 and 2022, respectively. The net cash used in operating activities for the six months ended June 30, 2023 was
due to approximately $1.3 million spent on research and development, approximately $0.6 million spent on salaries and related
compensation expenses, $3.4 million in other general and administrative expenses and approximately $0.9 million spent on
professional fees. The net cash used in operating activities for the six months ended June 30, 2022 was due to approximately
$2.6 million spent on research and development, approximately $1.42 million spent on professional fees and $1.8
million spent on salaries and related compensation expenses. The decrease in net cash used in operating activities in the six
months ended June 30, 2023 compared to the six months ended June 30, 2022 was primarily due to the decrease in the
Company’s research and development expenses, salaries and other compensation, and professional fees.
Net cash used in operating activities
was approximately $10.85 million and $5.79 million for the years ended December 31, 2022 and 2021, respectively. The net cash used in
operating activities for the year ended December 31, 2022 was due to approximately $3.98 million spent on research and development, approximately
$2.42 million spent on salaries and related compensation expenses, $1.92 million in other general and administrative expenses and approximately
$2.08 million spent on professional fees. The net cash used in operating activities for the year ended December 31, 2021 was due to approximately
$2.27 million spent on professional fees, $2.89 million spent on salaries and related compensation expenses and $1.68 million spent on
research and development activities. The increase in net cash used in operating activities in 2022 compared to 2021 was primarily due
to the increase in the Company’s research and development expenses and other general and administrative expenses.
Summary of Financing Activities
Net
cash provided by financing activities for the six months ended June 30, 2023 was $2.0 million, of which $0.9 million
was due to funds raised from the issuance of an OID Convertible Note. An additional $1.0 million of funds was raised
from the issuance of the Walleye Promissory Note and approximately $0.3 million was received from the exercise of the Somahlution
Warrants (as defined below under “—Funding Requirements and Other Liquidity Matters – Exercise of Somahlution Warrants
at Reduced Exercise Price”). During the six months ended June 30, 2023 the Company also repaid approximately
$0.2 million in notes payable.
Net
cash provided by financing activities for the six months ended June 30, 2022 was due to $5.2 million of funds raised
from the issuance of Convertible Notes. The Company also settled an aggregate of $0.3 million in notes payable as part of
the Units Private Placement during the six months ended June 30, 2022.
Net cash provided by financing activities for the
year ended December 31, 2022 was due to approximately $6.5 million of funds raised from the issuance of convertible notes in the Units
Private Placement, net of issuance costs, and $0.78 million received from the issuance of other promissory notes, net of repayments. During
2022 the Company also repaid approximately $0.1 million in aggregate notes payable in connection with the Units Private Placement issuances
and repaid approximately $0.1 million in notes payable assumed on the acquisition of My Health Logic. Net cash provided by financing activities
for the year ended December 31, 2021 was due to $6.69 million of funds raised from the issuance of convertible notes in the Units Private
Placement, net of issuance costs, and $0.26 million obtained from issuance of promissory notes to related parties. The increase in net
cash provided by financing activities in 2022 compared to 2021 was due to the combined increase in funds raised from the Units Private
Placement and the issuance of other promissory notes.
Exercise
of Somahlution Warrants at Reduced Exercise Price
On
December 15, 2019, the Company entered into an asset purchase agreement (the “Somahlution Agreement”), as amended on March
31, 2020, May 29, 2020, and July 30, 2020, with Somahlution. Pursuant to the terms of the Somahlution Agreement, as amended, the Company
agreed to the purchase of the Somahlution Assets (the “Somahlution Acquisition”). On August 4, 2020, the Somahlution Acquisition
closed. As consideration for the Somahlution Acquisition, the Company issued to Somahlution’s former owners (the “Former
Somahlution Owners”) a total of 10,000,000 restricted shares of common stock (the “Somahlution Purchase Shares”) and
five-year warrants to purchase an additional 2,999,955 shares of common stock with an exercise price of $5.00 per share (the “Somahlution
Warrants”).
On
April 13, 2023, the Company delivered offer letter agreements (the “Somahlution Warrant Offer Letter Agreements”) to the
Former Somahlution Owners, which offered to allow the Former Somahlution Owners to exercise the Somahlution Warrants to purchase the
number of restricted shares of common stock issuable under the Somahlution Warrants at an exercise price reduced by the Company from
$5.00 per share to $0.10 per share, on or prior to April 21, 2023, for maximum total cash proceeds of $299,995.50. As of the conclusion
of this offer period, four of the Former Somahlution Owners had entered into Somahlution Warrant Offer Letter Agreements and had exercised
their Somahlution Warrants to purchase a total of 2,652,159 shares of common stock for gross proceeds of approximately $265,216 (the
“Somahlution Warrants Exercise”). Univest as the Company’s placement agent, which facilitated the Somahlution Warrants
Exercise, waived any fees or reimbursable expenses that would otherwise have been payable with respect to the Somahlution Warrants Exercise
pursuant to the 2021 Placement Agency Agreement.
Adjustments
to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants and Number of Underlying Shares
As
described under “—Convertible Notes” below, during the fiscal years ended December 31, 2021 and 2022, the Company
conducted a private placement of units (the “Units Private Placement”) consisting of the Convertible Notes and the Class
C Warrants, as were modified or amended from time to time, the general terms of which are described in detail in that section. Among
their other terms, the Convertible Notes and Class C Warrants provide that a lower price per share, or more favorable terms, respectively,
under subsequent equity issuances, not including any Qualified Financing (as defined in “—Convertible Notes”)
and certain other exempt issuances, will be applicable to the conversion or exercise rights under the Convertible Notes and Class C Warrants,
respectively. In addition, under a Letter Agreement between the Company and Univest as placement agent for the investors in the Units
Private Placement, dated January 12, 2023 (the “January 2023 Letter Agreement”), the parties agreed that simultaneously with
any adjustment to the exercise price under the Class C Warrants as a result of any equity issuances, not including Qualified Financings
and certain other exempt issuances, the number of shares of common stock that are issuable under the Class C Warrants will be increased
such that the aggregate exercise price of such shares will be the same as the aggregate exercise price in effect immediately prior to
the adjustment (without regard to any limitations on exercise contained in the Class C Warrants, including the beneficial ownership limitation
otherwise provided under the Class C Warrants described in “Description of Securities – Securities Issued in the Units
Private Placement – Class C Warrants”).
At
the time of the Somahlution Warrants Exercise, the balance under the Convertible Notes was convertible at a conversion price of $1.75
per share and the Class C Warrants were exercisable at an exercise price of $2.25 per share. Outstanding Convertible Notes had underlying
principal of $14,471,177, and outstanding Class C Warrants were exercisable to purchase a total of 16,538,473 shares of common stock.
As the Somahlution Warrants Exercise constituted a financing at a lower price per share that the conversion price and exercise price
of the Convertible Notes and Class C Warrants, respectively, and such financing was not a Qualified Financing, as a result of the Somahlution
Warrants Exercise and pursuant to the adjustment provisions described above, the conversion price of the Convertible Notes and the exercise
price of the Class C Warrants adjusted to $0.10 per share, and the number of shares of common stock into which the Convertible Notes
may be converted and the Class C Warrants may be exercised to purchased increased proportionately by 1,750% and 2,225%, respectively.
See “—Recent Developments – Letter Agreement to Amend Convertible Notes to Permit Conversion of Mandatory
Default Amount” for a discussion of a subsequent development which further increased the number of shares of common stock into
which the outstanding Convertible Notes may be converted based on the Mandatory Default Amount.
Default
Under Promissory Note
On
February 6, 2023, under a securities purchase agreement, the Company issued the Walleye Promissory Note to Walleye for $1,000,000 with
a maturity date of May 7, 2023. The Walleye Promissory Note did not carry interest. The principal amount under the Walleye Promissory
Note was required to be repaid in full on the maturity date. In the event that the principal amount was not repaid in full on the maturity
date, the principal amount was to increase to $1,250,000. As of May 8, 2023, the Company had not repaid the Walleye Promissory Note.
Accordingly, the principal outstanding under the Walleye Promissory Note increased to $1,250,000. See “—Cross-Default
under Convertible Notes” and “—OID Units Private Placement – Second Closing” below for related
developments.
Cross-Default
Under Convertible Notes
Each
of the Convertible Notes provides that any default on indebtedness of more than $100,000, other than indebtedness under the respective
Convertible Notes, will also result in default under the Convertible Notes. The Convertible Notes provide that such default is not subject
to any cure period. Due to the non-repayment of the initial principal amount of $1,000,000 under the Walleye Promissory Note by its maturity
date, May 7, 2023, the Company also defaulted under the Convertible Notes on the same date. The Convertible Notes provided that due to
this default, the Company became obligated to pay the Mandatory Default Amount. The Mandatory Default Amount may be declared due by each
holder immediately. The aggregate Mandatory Default Amount that may be due under the Convertible Notes was $21,871,631 on the date of
the default, or approximately $5.3 million more than would otherwise have been due under the Convertible Notes on the date of the default.
Under
the unit purchase agreement entered into on May 27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s
subsidiaries entered into a guaranty to guarantee the repayment of the Company’s obligations under the Convertible Notes, and the
Company and its subsidiaries entered into security agreements granting security interests in all of their respective assets for up to
the dollar value owed under the respective Convertible Notes. The respective Convertible Notes were issued for aggregate principal of
approximately $1.2 million. Under each unit purchase agreement entered into with respect to subsequent issuances of the Convertible Notes,
the Company and its subsidiaries were obligated to enter into similar security agreements and guaranties, but did not do so.
The
holders of the Convertible Notes have not exercised any remedies applicable to the Convertible Notes or given notice of any intention
to do so as of the date of this prospectus. If Univest, as the appointed representative of the Convertible Note holders, remains so appointed,
no investor other than Univest may pursue any remedy with respect to the Convertible Notes. However, if the amount owed due to the default
is not repaid upon demand, the holders of the Convertible Notes may nonetheless seek to remove Univest from this appointed position,
take possession of some or all of the Company’s and its subsidiaries’ assets, force the Company and its subsidiaries into
bankruptcy proceedings, or seek other legal remedies against the Company and its subsidiaries. In such event, the Company’s business,
operating results and financial condition may be materially adversely affected.
Convertible
Notes
During
the years ended December 31, 2022 and 2021, the Company issued 147,711,770 units in the Units Private Placement, consisting of Convertible
Notes and Class C Warrants, at a post-adjustment price per unit of $0.10, giving effect to the adjustments in the terms of the Units
Private Placement resulting from the Somahlution Warrants Exercise as described above under “—Adjustments
to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants and Number of Underlying Shares”,
for gross cash proceeds of approximately $14.2 million. Of the total 147,711,770 units issued, the following were issued for no cash
payment: 2,786,780 units were issued to settle notes payable assumed on acquisition of My Health Logic, 400,000 units were issued to
settle accounts payable, and 3,000,000 units were issued in exchange for services rendered to the Company in the year ended December
31, 2022. The number of units issued in the Units Private Placement corresponds with the number of shares of common stock into
which the principal underlying the Convertible Notes may be converted. The cash proceeds from the Units Private Placement were used to sustain the Company’s growth and meet its capital
obligations.
In connection with the Units Private Placement,
we issued the Convertible Notes, which in aggregate were initially convertible into an aggregate of 8,269,228 shares of
common stock. The principal under outstanding Convertible Notes as of June 30, 2023 was $14,471,177. Each Convertible Note
was initially convertible into common stock of the Company at a price per share of $1.75, subject to adjustment. All outstanding Convertible
Notes provide that a lower price per share under subsequent equity issuances, not including Qualified Financings, will be applied
to the conversion price of the Convertible Notes, and have
customary antidilution provisions. As a result of the adjustments applicable to the Convertible Notes described under
“—Adjustments to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants and Number
of Underlying Shares”, the conversion price of the Convertible Notes was reduced to $0.10 per share. In addition, due to the
event triggering of the cross-default provision under the Convertible Notes described above under “—Cross-Default Under
Convertible Notes”, and the Convertible Notes Letter Agreement, the aggregate Mandatory Default Amount that may be due under
the Convertible Notes was $21,871,631 on the date of the default, or approximately $5.7 million more than would otherwise have been
due under the Convertible Notes on the date of the default. As amended subsequent to June 30, 2023, the Mandatory Default Amount is
convertible into common stock.
The
Convertible Notes mature 24 months after their issuance date and accrue 10% of simple interest per annum on the outstanding principal
amount.
As amended
by the Convertible Notes Letter Agreement, the Convertible Notes provide that the Mandatory Default Amount or the Convertible Notes’
principal and accrued interest may be converted into shares of common stock at any time at the option of each holder at the conversion
price, subject to any applicable beneficial ownership limitations on receiving shares upon such conversion. Any conversion by the holder
will be applied toward the Mandatory Default Amount until it is fully converted. If the Mandatory Default Amount is fully converted,
the Mandatory Default Amount and any outstanding principal and accrued interest will be fully extinguished.
As
amended by the Convertible Notes Letter Agreement, the Convertible Notes provide that if at any time following the 60-day anniversary
of the final closing date or termination of the Units Private Placement, and provided there is an effective registration statement permitting
the issuance or resale of the shares of common stock into which the Convertible Notes may be converted, if (A) the common stock is listed
on a senior national securities exchange, (B) the daily volume-weighted average price for the prior twenty (20) consecutive trading days
is $6.00 or more (adjusted for splits and similar distributions) and (C) the daily trading volume is at least $1,000,000 during such
20-day period, then the Company will have the right to require the Convertible Notes to convert all or any portion of the Mandatory Default
Amount then remaining under the Convertible Notes into shares of common stock at the above conversion price in effect on the mandatory
conversion date.
As
amended by the Convertible Notes Letter Agreement, the Convertible Notes also provide that upon the occurrence of a Qualified Financing,
the conversion price of the Convertible Notes will be adjusted to the price per share equal to 75% of the price per equity security in
such financing.
Under
the Unit Purchase Agreement entered into on May 27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s
subsidiaries entered into a guaranty to guarantee the repayment of the Company’s obligations under the Convertible Notes, and the
Company and its subsidiaries entered into security agreements granting security interests in all of their respective assets for up to
the dollar value owed under the respective Convertible Notes. The respective Convertible Notes were issued for aggregate principal of
approximately $1.2 million. Under each Unit Purchase Agreement entered into with respect to subsequent issuances of the Convertible Notes,
the Company and its subsidiaries were obligated to enter into similar security agreements and guaranties, but did not do so.
Shares
of common stock may not be received pursuant to a conversion of a Convertible Note to the extent that the holder (together
with any other person with which the holder is considered to be part of a “group” under Section 13 of the Exchange Act or
with which the holder otherwise files reports under Section 13 and/or Section 16 of the Exchange Act) would otherwise become the
“beneficial owner” (as such term is defined in the Exchange Act and the rules and regulations thereunder) of in excess of
4.99% of the shares of common stock outstanding, subject to automatic increase to 9.99% if the holder becomes a beneficial
owner of more than 4.99%, subject to a requirement that any increase in the limitation will not be effective until the
61st day after notice is delivered to the Company. This limitation
does not apply to certain provisions of the Convertible Notes.
The
Convertible Notes have certain registration requirements as to the shares of common stock underlying the Convertible Notes upon the final
closing of the Units Private Placement under the respective registration rights agreements between the Company and the purchasers of
the Convertible Notes. By executing certain lock-up agreements with Univest acting as the representative of the underwriters of a proposed
public offering that we no longer intend to pursue or waiver agreements with the Company, the purchasers of the Convertible Notes agreed
that doing so amounted to a waiver of their rights under the respective registration rights agreements, and that any such registration
rights will be afforded to such purchasers in a subsequent registration statement. The assignees of certain Convertible Notes and the
holders of the Replacement Convertible Notes have also entered into a waiver and consent with the Company providing for the waiver and
deferral of all registration rights applicable to the respective Convertible Notes until a date and time that the Company shall determine
in its sole discretion that it may provide for such rights.
Each
Convertible Note provides that its holder may vote with the shares of common stock, on an as-converted to common stock basis, with respect
to all corporate matters of the Company on which the holders of common stock are entitled to vote, subject to any applicable beneficial
ownership limitations. Under the NRS the right to vote is generally a feature of a share of any class or series of stock of a corporation
and, thus, a right granted to stockholders of a corporation. Under the NRS, convertible promissory notes (or any other form of bond,
debenture or other obligation) typically do not provide holders with voting rights. However, Section 78.197 of the NRS provides that
a corporation organized in Nevada “may provide in its articles of incorporation that the holder of a bond, debenture or other obligation
of the corporation may have any of the rights of a stockholder in the corporation.” As described
in “—Recent Developments – Special Stockholder Meeting, Change in Authorized Shares, and Approval
of Voting Rights for Certain Debtholders”, at the Special Stockholders Meeting, the stockholders of the Company approved the
Voting Rights Amendment, and on August 16, 2023, the Company filed the Certificate of Amendment
with the Nevada Secretary of State providing for, among other things, the Voting Rights Amendment, and the Certificate
of Amendment became effective upon filing. Accordingly, upon the filing of the Certificate of Amendment, among other things, (i)
the Company’s board of directors may grant any of the rights of stockholders to any of the holders of any of the Company’s
bonds, debentures or other obligations; and (ii) all of the holders of the Convertible Notes issued between December 21, 2021 and August
12, 2022 became entitled to vote with the shares of the Company’s common stock, on an as-converted to common stock basis without
actually having converted the Convertible Notes to shares of the Company’s common stock, with respect to all corporate matters
of the Company on which the holders of common stock are entitled to vote, as provided in the Convertible Notes. Pursuant to the Certificate
of Amendment, the board of directors extended these voting rights to all other holders of the Convertible Notes issued since August 12,
2022. Accordingly, all holders of the Convertible Notes are entitled to vote with the shares of the Company’s common stock, on
an as-converted to common stock basis without actually having converted the Convertible Notes to shares of the Company’s common
stock, with respect to all corporate matters of the Company on which the holders of common stock are entitled to vote, as provided in
the Convertible Notes.
The
2021 Placement Agency Agreement and form of unit purchase agreement relating to the Units Private Placement provide that up to $18 million
and $17 million of the units containing the Convertible Notes may be sold, respectively. On August 12, 2022, we completed the final closing
of the Units Private Placement.
On
June 14, 2023, a Convertible Note with $119,980 underlying principal, approximately $14,693 accrued interest, and a Mandatory Default
Amount of $180,123 was converted into 1,346,410 shares of common stock at the applicable conversion price of $0.10 per share at the election
of the Convertible Note holder. Pursuant to the Convertible Notes Letter Agreement, the Convertible Note remains convertible into 454,819
shares of common stock.
OID
Units Private Placement
First
Closing
On
May 12, 2023, the Company conducted the first closing (the “First OID Units Closing”) of the OID Units Private Placement
of up to $10,000,000 for an aggregate of up to 100,000,000 OID Units, under an OID Units Purchase Agreement. Each investor represented
that it is an “accredited investor” as such term is defined by Rule 501(a) of the Securities Act. Each issuance of OID Units
to an investor in the OID Units Private Placement consists of (i) an OID Convertible Note, convertible into shares of common stock plus
additional shares based on accrued interest at $0.10 per share, subject to adjustment, (ii) a Class E Warrant for the purchase of 125%
of the shares of common stock into which the respective OID Convertible Note may be converted at $0.10 per share, subject to adjustment,
and (iii) a Class F Warrant for the purchase of 125% of the shares of common stock into which the respective OID Convertible Note may
be converted at $0.20 per share, subject to adjustment. Under the OID Units Purchase Agreement, the minimum investment permitted is $1,000.
The Company retained Univest as its exclusive placement agent in connection with the sale of the OID Units pursuant to the OID Units
Purchase Agreement under the April 2023 PAA. In addition to the rights set forth in each OID Units Purchase Agreement, the OID Convertible
Notes, and the Class E and F Warrants, each investor party to each OID Units Purchase Agreement and each holder of one of the OID Convertible
Notes or the Class E and F Warrants will have rights under an OID Units Registration Rights Agreement, as described below.
In
connection with the First OID Units Closing, on May 12, 2023, Walleye entered into an OID Units Purchase Agreement dated as of such date,
paid a subscription amount of $1,000,000, and the Company issued 11,764,710 OID Units consisting of (i) an OID Convertible Note in the
principal amount of $1,176,471, convertible into 11,764,710 shares of common stock plus additional shares based on accrued interest at
$0.10 per share, subject to adjustment, (ii) a Class E Warrant for the purchase of 14,705,880 shares of common stock at $0.10 per share,
subject to adjustment, and (iii) a Class F Warrant for the purchase of 14,705,880 shares of common stock at $0.20 per share, subject
to adjustment. In connection with the First OID Units Closing, pursuant to the April 2023 PAA and the OID Units Purchase Agreement, the
Company received net proceeds of $870,000 after deducting Univest’s 8% fee and $50,000 for reimbursement of Univest’s legal
fees and expenses. See below for additional related discussion.
Second
Closing
On
May 30, 2023, the Company conducted the second closing (the “Second OID Units Closing”) of the OID Units Private Placement.
Each investor represented that it is an “accredited investor” as such term is defined by Rule 501(a) of the Securities Act.
In connection with the Second OID Units Closing, Hexin and Walleye agreed to the cancellation of the Hexin Promissory Note and the Walleye
Promissory Note, respectively, and Mr. Maresca agreed to the cancellation of certain indebtedness, in exchange for OID Units and related
agreements, as described below.
First,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Hexin (the “Hexin Cancellation Agreement”),
Hexin agreed to cancel the Hexin Promissory Note in exchange for: (i) the execution of an OID Units Purchase Agreement, dated as of May
30, 2023, between the Company and Hexin, Walleye and Mr. Maresca (the “Second OID Units Purchase Agreement”), (ii) the execution
of an OID Units Registration Rights Agreement, dated as of May 30, 2023, between the Company and Hexin, Walleye and Mr. Maresca (the
“Second OID Units Registration Rights Agreement”), and (iii) the issuance of 9,578,040 OID Units consisting of (a) an OID
Convertible Note in the principal amount of $957,804 for a subscription equal to $814,133 (the aggregate amount of principal plus accrued
and unpaid interest under the Hexin Promissory Note as of May 30, 2023), convertible into 9,578,040 shares of common stock plus additional
shares based on accrued interest at $0.10 per share, subject to adjustment (the “Hexin OID Convertible Note”), (b) a Class
E Warrant for the purchase of 11,972,550 shares of common stock at $0.10 per share, subject to adjustment (the “Second Hexin Class
E Warrant”), and (c) a Class F Warrant for the purchase of 11,972,550 shares of common stock at $0.20 per share, subject to adjustment
(the “Second Hexin Class F Warrant”). The Hexin Cancellation Agreement contains a release of claims against the Company relating
to the Hexin Promissory Note.
Second,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Walleye (the “Walleye Cancellation
Agreement”), Walleye agreed to cancel the Walleye Promissory Note in exchange for: (i) the execution of the Second OID Units Purchase
Agreement, (ii) the execution of the Second OID Units Registration Rights Agreement, and (iii) the issuance of 14,705,890 OID Units consisting
of (a) an OID Convertible Note in the principal amount of $1,470,589 for a subscription equal to $1,250,000 (the aggregate amount of
principal under the Walleye Promissory Note as of May 30, 2023), convertible into 14,705,890 shares of common stock plus additional shares
based on accrued interest at $0.10 per share, subject to adjustment (the “Second Walleye OID Convertible Note”), (b) a Class
E Warrant for the purchase of 18,382,362 shares of common stock at $0.10 per share, subject to adjustment (the “Second Walleye
Class E Warrant”), and (c) a Class F Warrant for the purchase of 18,382,362 shares of common stock at $0.20 per share, subject
to adjustment (the “Second Walleye Class F Warrant”). The Walleye Cancellation Agreement contains a release of claims against
the Company relating to the Walleye Promissory Note.
Third,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Mr. Maresca (together with the Hexin Cancellation
Agreement and the Walleye Cancellation Agreement, the “Cancellation and Exchange Agreements”), Mr. Maresca agreed to cancel
$150,000 of the aggregate short-term indebtedness to Mr. Maresca, which arose in the ordinary course of business for certain consulting
services provided by Mr. Maresca to the Company, in exchange for: (i) the execution of the Second OID Units Purchase Agreement, (ii)
the execution of the Second OID Units Registration Rights Agreement, and (iii) the issuance of 1,764,710 Units consisting of (a) an OID
Convertible Note in the principal amount of $176,471 for a subscription equal to $150,000 (the amount of the indebtedness being cancelled),
convertible into 1,764,710 shares of common stock plus additional shares based on accrued interest at $0.10 per share, subject to adjustment
(the “Maresca OID Convertible Note”), (b) a Class E Warrant for the purchase of 2,205,887 shares of common stock at $0.10
per share, subject to adjustment (the “Maresca Class E Warrant”), and (c) a Class F Warrant for the purchase of 2,205,887
shares of common stock at $0.20 per share, subject to adjustment (the “Maresca Class F Warrant”). The Maresca Cancellation
Agreement contains a release of claims against the Company relating to the cancelled indebtedness.
The
Hexin OID Convertible Note, the Second Hexin Class E Warrant and the Second Hexin Class F Warrant are subject to the terms and conditions
of the Hexin Cancellation Agreement. The Second Walleye OID Convertible Note, the Second Walleye Class E Warrant and the Second Walleye
Class F Warrant are subject to the terms and conditions of the Walleye Cancellation Agreement. The Maresca OID Convertible Note, the
Maresca Class E Warrant and the Maresca Class F Warrant are subject to the terms and conditions of the Maresca Cancellation Agreement.
The Second OID Units Purchase Agreement and the Second OID Units Registration Rights Agreement are also subject to the terms and conditions
of each of the Cancellation and Exchange Agreements.
Univest
did not receive a fee in connection with the Second OID Units Closing because there were no gross proceeds.
OID
Units Purchase Agreement
Each
OID Units Purchase Agreement provides a right of first offer to investor parties to any proposed offer or sale of equity securities by
the Company until the first anniversary of the First OID Units Closing (i.e., May 12, 2024). Each OID Units Purchase Agreement
also contains certain liquidated damages provisions, including for any failure of the Company to meet the requirements for the OID Convertible
Notes or the Class E and Class F Warrants to be converted or exercised for non-restricted shares of common stock under Rule 144, and
on every 30th day (pro-rated for periods totaling less than 30 days) thereafter, until cured or such Rule 144 requirements no longer
apply, up to a maximum of 25% of each affected investor’s subscription amount. Each OID Units Purchase Agreement also contains
a most-favored nations clause that provides that in connection with any subsequent equity financing of the Company for consideration
(a “Subsequent Financing”), each investor may accept the securities and terms of the Subsequent Financing in substitution
of the securities and terms of each OID Units Purchase Agreement, upon notice from an investor, subject to the terms and conditions of
each OID Units Purchase Agreement. Each OID Units Purchase Agreement, the OID Convertible Notes and the Class E and F Warrants will be
amended to incorporate the terms and forms of the securities sold in the Subsequent Financing upon the closing of the Subsequent Financing.
Each OID Units Purchase Agreement will terminate upon certain events including mutual written consent, by either party upon notice if
a closing has not occurred within 15 business days of the date of the agreement, an event of default under the OID Convertible Notes,
the full conversion or repayment of the OID Convertible Notes and the non-ownership of any shares of common stock issuable upon conversion
of the OID Convertible Notes or exercise of the Class E and F Warrants. Each OID Units Purchase Agreement also contains indemnification
of the investors relating to claims relating to the transactions under each OID Units Purchase Agreement which will survive termination.
Each investor’s rights under the OID Units Purchase Agreement may be assigned to another “accredited investor” as defined
by Rule 501(a) of the Securities Act.
OID
Convertible Notes
The
OID Convertible Notes will mature nine months after the date of the First OID Units Closing with respect to OID Convertible Notes issued
in the first two closings of the OID Units Private Placement and will mature nine months after the date of issuance for subsequent closings.
The OID Convertible Notes accrue 10% of interest per annum on the outstanding principal amount. The OID Convertible Notes will be unsecured
and subordinated to any senior indebtedness of the Company. The OID Convertible Notes’ principal and accrued interest may generally
be converted at any time at a conversion price of $0.10 per share, subject to adjustment, at the option of the holder, into shares of
common stock, subject to certain limitations on receiving shares upon conversion of an OID Convertible Note, including that such shares
may be received only to the extent that the holder (together with any other person with which the holder is considered to be part of
a “group” under Section 13 of the Exchange Act or with which the holder otherwise files reports under Section 13 and/or Section
16 of the Exchange Act) would not become the “beneficial owner” (as such term is defined in the Exchange Act and the rules
and regulations thereunder) of in excess of 4.99% of the shares of common stock outstanding, subject to automatic increase to 9.99% if
the holder becomes a beneficial owner of more than 4.99%, subject to a 61-day notice requirement. This limitation does not apply to certain
provisions of the OID Convertible Notes. In the event the Company offers, sells, grants, issues, or otherwise disposes of common stock
or securities with rights to common stock, or announces the intention to do one of such things, before the listing of the common stock
on a National Stock Exchange, at a lower price per share than the OID Convertible Notes’ conversion price while the OID Convertible
Notes are outstanding, then generally the conversion price of the OID Convertible Notes will be lowered to such price per share. This
adjustment provision will apply one time only. The OID Convertible Notes also have customary antidilution provisions in the event of
stock splits, certain changes of control or similar transactions, and rights offerings. While the OID Convertible Notes are outstanding
and for 12 months after the Company lists its common stock on a National Stock Exchange, the Company may not exchange or cooperate to
exchange any indebtedness or securities, reduce or change the conversion, exercise or exchange price of any securities convertible, exercisable
or exchangeable for common stock, amend non-convertible debt to convertible debt, issue securities at a price based on or varying with
trading prices or quotations for the common stock or with a price reset term, or agree to sell securities at a future determined price.
Until 30 days after the OID Convertible Notes are converted or repaid in full, the Company may not sell any securities in a capital or
debt raising transaction or series of related transactions which grant to an investor the right to receive additional securities based
upon future transactions of the Company on terms more favorable than those granted to such investor in such transaction or series of
related transactions. The OID Convertible Notes may not be prepaid by the Company. In the event of default under the OID Convertible
Notes, subject to certain cure rights, interest under the OID Convertible Notes will increase to the lower of 18% and the maximum legal
interest rate, and the outstanding balance will become immediately due and payable. The OID Convertible Notes have the registration rights
set forth in the respective OID Units Registration Rights Agreement. See “—OID Units Registration Rights Agreement”
below.
Class
E and F Warrants
The
Class E and F Warrants are generally exercisable from the date of the Capital Event Amendment until five years from the date of issuance.
The exercise right is subject to a beneficial ownership limitation such that shares of common stock may be received upon exercise only
to the extent that the holder (together with any other person with which the holder is considered to be part of a “group”
under Section 13 of the Exchange Act or with which the holder otherwise files reports under Section 13 and/or Section 16 of the Exchange
Act) would not become the “beneficial owner” (as such term is defined in the Exchange Act and the rules and regulations thereunder)
of in excess of 4.99% of the shares of common stock outstanding, subject to automatic increase to 9.99% if the holder becomes a beneficial
owner of more than 4.99%, subject to a 61-day notice requirement. This limitation does not apply to certain provisions of the Class E
and F Warrants. The Class E and F Warrants provide for exercise on a cashless net exercise basis if there is no effective registration
statement registering or current prospectus available for the resale of shares of common stock issuable under the Class E and F Warrants
(a “Registration Default”) after 180 days following the issue date (the “Registration Deadline”). In addition,
for each 30 days following the Registration Deadline, or portion of any 30-day period thereafter in which a Registration Default exists,
the amount of shares issuable under the Class E and F Warrants shall be automatically increased by 5%, prorated for a partial month,
not to exceed in the aggregate an additional 25%. In the event the Company sells, grants, issues, or otherwise disposes of common stock
or securities with rights to common stock, or announces an intention to do so, at a lower effective price per share than the exercise
prices of the Class E and F Warrants, while any such Class E and F Warrants are outstanding, then generally the applicable Class E and
F Warrants’ exercise price(s) will be reduced to the greater of such lower price or a floor price as determined by any applicable
National Stock Exchange. The Class E and F Warrants have the registration rights set forth in the respective OID Units Registration Rights
Agreement. The Class E and F Warrants also have customary antidilution provisions in the event of stock splits, certain changes of control
or similar transactions, and rights offerings. The Class E and F Warrants may be transferred subject to their terms.
OID
Units Registration Rights Agreement
Under
each OID Units Registration Rights Agreement, as amended if applicable, the Company is required to file a registration statement with
the SEC registering the resale of the shares of common stock issuable pursuant to conversion of the OID Convertible Notes and exercise
of the Class E and F Warrants within 67 days of the date of the respective closing of the OID Units Private Placement and to cause the
registration statement to become effective within 120 days after such filing date. The Company must maintain the effectiveness of the
registration statement until the earlier of the first anniversary of its effectiveness date and the date that the shares registered for
resale may be resold without volume or manner-of-sale restrictions pursuant to Rule 144 and without the requirement for the Company to
be in compliance with the current public information requirement under Rule 144. If the Company fails to file the registration statement
by the filing deadline or cause it to become effective by the effectiveness deadline, or the registration statement ceases to be effective
or the related prospectus becomes unavailable for resales for more than 10 consecutive calendar days or more than an aggregate of 15
calendar days during any 12-month period, then on the date of such failure and every 30 calendar days after such date until the failure
is cured, the Company must pay to each investor partial liquidated damages equal to 1% of the aggregate purchase price paid by such investor
pursuant to the respective OID Units Purchase Agreement, up to a maximum of 10% of the aggregate subscription amount paid by the investor.
If the Company fails to pay the partial liquidated damages within seven days of any such failure, the Company will pay interest thereon
at a rate of the lesser of 18% per annum or the maximum amount permitted under applicable law to each investor, accruing daily from the
date such partial liquidated damages are due until such amounts, plus all such interest thereon, are paid in full. Additional liquidated
damages requirements will end when the applicable failure is cured or Rule 144 becomes available for resale of all the shares of common
stock otherwise required to be registered for resale under each OID Units Registration Rights Agreement. Liquidated damages will not
apply to a failure that is due to limits imposed by the SEC’s interpretation of Rule 415 under the Securities Act. In addition,
if there is not an effective registration statement covering all shares of common stock subject to the registration rights under each
OID Units Registration Rights Agreement at any time when required and the Company proposes to file a registration statement to register
certain other offerings, not including an underwritten public offering of its securities for its own account or the account of others
or certain other types of registration statements, then the Company must provide notice to investor parties and include the shares otherwise
required to be registered under each OID Units Registration Rights Agreement within 15 days of such notice, unless they are eligible
for resale pursuant to Rule 144 (without volume restrictions or current public information requirements). Each OID Units Registration
Rights Agreement contains related procedural and filing requirements and investor notice and review rights as to certain events and filings
relating to the registration statement. The Company will be responsible for all fees and expenses relating to compliance with each OID
Units Registration Rights Agreement, as well as up to $10,000 in reasonable attorney fees for the investors’ review of the registration
statement. Each OID Units Registration Rights Agreement contains mutual indemnification provisions for claims relating to a registration
statement. The investor’s rights under the OID Units Purchase Agreement may be assigned to another “accredited investor”
as defined by Rule 501(a) of the Securities Act.
Holders
of rights to 67% or more of the shares issuable upon conversion of the OID Convertible Notes and exercise of the Class E and F Warrants
may agree to amend or waive the requirements of each respective OID Units Registration Rights Agreement. As described under “—Recent
Developments – Amendment to May 2023 OID Units Registration Rights Agreements”, under an Amendment to Registration Rights
Agreement, dated as of July 6, 2023, sufficient holders of such rights agreed to amend each OID Units Registration Rights Agreement then
in effect to provide that the initial filing of the registration statement required by such OID Units Registration Rights Agreement shall
be made on or before the date that is 67 days after the date of the respective closing of the OID Units Private Placement. Each subsequent
OID Units Registration Rights Agreement also contains the amended term.
April
2023 PAA
Under
the April 2023 PAA, Univest acted as the Company’s exclusive placement agent in connection with the OID Units Private Placement.
The Company agreed to pay Univest a cash placement fee equal to 8% of the gross proceeds from the sale of the OID Units, 8% of the gross
proceeds from the exercise of the Class E and F Warrants, and certain Placement Agent Warrants. The Company further agreed to reimburse
Univest for the fees and expenses of its due diligence and legal counsel of up to $200,000. The April 2023 PAA provides indemnification
rights to Univest and its affiliates in the event of certain claims relating to the April 2023 PAA or related transactions. Under the
April 2023 PAA, Univest has the right to act as the Company’s sole placement agent or an underwriter for any future equity financing
occurring during the 18-month period following the consummation of the OID Units Private Placement. The term of the April 2023 PAA continues
until the completion of the OID Units Private Placement, subject to termination after 15 days’ notice after July 31, 2023 or earlier
in the case of termination for cause. Univest will also receive fees and Placement Agent Warrants on the same basis as described above
with respect to any private or public offering or other financing or capital raising transaction of any kind of the Company within 12
months of the termination or expiration of the April 2023 PAA with an investor whom Univest has, directly or indirectly, introduced to
the Company during the term of the April 2023 PAA.
Pursuant
to the April 2023 PAA, in connection with closings of the OID Units Private Placement, as of June 30, 2023, the Company had paid Univest
$80,000 in total fees and $50,000 as reimbursement of Univest’s legal fees.
Other
Contractual Obligations and Commitments
Royalties
and Other Commitments
On
December 15, 2019, the Company entered into the Somahlution Agreement to acquire the Somahlution Assets, including DuraGraft. The Somahlution
Agreement was amended on March 31, 2020 and May 29, 2020 to extend the termination date. On July 30, 2020, the Company and Somahlution
entered into Amendment No. 3 to the Somahlution Agreement (“Amendment No. 3”). Pursuant to the terms of this amendment, it
was agreed that, as part of the Somahlution Acquisition, the Company would acquire the outstanding capital stock of Somahlution, Inc.,
held by Somahlution, LLC, rather than the assets of Somahlution, Inc., in addition to the Somahlution Assets. This change to the Somahlution
Agreement was made to accommodate EU requirements with respect to the future manufacturing under Somahlution, Inc. of CE marked products
for sale in the EU. In Amendment No. 3, the Company agreed to assume certain payables of Somahlution related to clinical and medical expenses.
The parties also orally agreed that the payments on the assumed debts would be recorded as a prepaid royalty against future royalties.
Pursuant to the Somahlution Agreement and in consideration
of the outstanding capital stock of Somahlution, Inc., the Company agreed to pay to the Former Somahlution Owners, among other consideration:
| |
● |
The
following contingent consideration upon receiving FDA final approval and insurance reimbursement approval on the products, and in
the amounts, specified below, subject to certain expiration terms, none of which had been earned or granted as of June 30,
2023: |
| |
|
|
● |
Royalties to be paid on all net sales of the product of 6% on the first $50 million of international net sales (and 5% on the first $50 million of U.S. net sales), 4% for greater than $50 million up to $200 million, and 2% for greater than $200 million; |
| |
|
|
● |
Payment on a pro rata basis of 10% of the cash value of rare pediatric voucher sales following FDA approval and subsequent sale to an unaffiliated third party of a rare pediatric voucher based on DuraGraft; |
| |
|
|
● |
Following FDA approval and subsequent sale to an unaffiliated third party of a rare pediatric voucher based on DuraGraft, grant of warrants on a pro rata basis to purchase an aggregate of 250,000 shares of common stock with a term of five years and a strike price determined based on the average of the closing prices of the common stock for the 30 calendar days following the date of the public announcement of FDA approval; and |
| |
|
|
● |
Upon the sale of DuraGraft products, the Company will pay pro rata the Somahlution designees 15% of the net sale proceeds towards the liquidation preference maximum amount of $20 million described below; |
| |
|
● |
Somahlution derived solid organ transplant products: |
| |
|
|
● |
Royalties to be paid on all net sales of the product of 6% on the first $50 million of international net sales, 4% for greater than $50 million up to $200 million, and 2% for greater than $200 million; |
| |
|
|
● |
Upon the sale of DuraGraft products, the Company will pay pro rata the Somahlution designees 15% of the net sale proceeds towards the liquidation preference maximum amount of $20 million described below; |
| |
|
● |
Somahlution Assets-derived over-the-counter products: |
| |
|
|
● |
Royalties to be paid on all net sales of the product of 6% on the first $50 million of international net sales, 4% for greater than $50 million up to $200 million, and 2% for greater than $200 million; |
| |
|
● |
Other Somahlution Assets-derived products from existing Somahlution pipelines: |
| |
|
|
● |
Royalties to be paid on all net sales of the product of 1%; and |
| |
● |
A liquidation preference, up to a maximum of $20 million, and the Company will pay 15% of the net sale proceeds towards the liquidation preference maximum amount upon the sale by the Company of all or substantially all of the Somahlution Assets. |
In July and August, 2020, the Company
completed the acquisition of the Somahlution Assets. In August 2020, the Company issued the Somahlution Purchase Shares, the Somahlution
Warrants, and prepaid royalties to the Former Somahlution Owners with a total dollar value of $339,091. As of June 30, 2023 and
December 31, 2022, the value of the prepaid royalties was $140,843 and $339,091, respectively. For additional discussion
of this transaction, see “Certain Relationships and
Related Party Transactions – Transactions with Related Persons”.
Office and Laboratories Space Lease
On December 11, 2020, the
Company entered into a five-and-a-half-year lease agreement for approximately 10,300 square feet of administrative office and laboratories
space, which commenced in December 2020 at a monthly rent of approximately $10,800, increasing by 2.5% annually beginning in the second
year of the lease until the end of the term. Additionally, pursuant to the agreement, the Company was required to pay approximately $12,000
per month in operating expenses.
Effective April 1, 2022, the Company amended its lease
agreement for administrative office and laboratories to add 3,053 additional square feet of space. The monthly cost of total expended
lease space is approximately $15,260 increasing to $15,641 in 2023 and will continue to increase by 2.5% annually thereafter until the
end of the term. The monthly operating expenses for total expanded premises have increased from approximately $12,000 to $17,500 per month.
The term of the lease remained unchanged.
The total rent expense for the three
and six months ended June 30, 2023 was $37,674 and $192,502, respectively. Total rent expense for the three and six months ended June 30, 2022 was $110,353 and $221,253, respectively. Total rent expense
under the lease for the years ended December 31, 2022 and 2021 was $370,945 and $118,084, respectively.
As of June 30, 2023, the maturities
of the lease liabilities for the periods ending December 31 are as follows:
| 2023 | |
$ | 211,747 | |
| 2024 | |
| 434,082 | |
| 2025 | |
| 444,934 | |
| 2026 | |
| 266,034 | |
| Total lease payments | |
| 1,356,797 | |
| Less: Present value discount | |
| (62,355 | ) |
| Total | |
$ | 1,294,442 | |
As of December 31, 2022 and 2021, minimum lease payments
in relation to lease commitments were payable as follows:
| | |
2022 | | |
2021 | |
| Within 1 year | |
$ | 423,495 | | |
$ | 277,142 | |
| After 1 year and within 5 years | |
| 1,145,050 | | |
| 962,376 | |
| Total lease commitments | |
$ | 1,568,545 | | |
$ | 1,239,518 | |
We enter into contracts in the normal course of business
for our contract research services, contract manufacturing services, professional services and other services and products for operating
purposes. These contracts generally provide for termination after a notice period, and, therefore, are cancelable contracts and not included
in the discussion above.
Other Promissory Notes
On October 23, 2022, the Company issued
a promissory note to Hub International Limited for $204,050 bearing interest at the annual rate of 6.75% per annum, due September 23,
2023, payable monthly starting November 23, 2022. As of June 30, 2023 and December 31, 2022, the balance due under the promissory
note was $41,886 and $164,729, respectively. During 2021, a promissory note in the principal amount of $127,798 bearing interest
at the annual rate of 5.22% per annum, due August 24, 2022, payable monthly starting November 24, 2021, was issued to the same creditor
and was repaid with interest prior to entry into the note issued on October 23, 2022.
On December 28, 2022, the Company issued
the Hexin Promissory Note for the principal amount of $750,000 bearing interest at the annual rate of 20% per annum, due June 28, 2023.
Under the Hexin Promissory Note, the Company agreed to issue warrants to purchase common stock equal to $1,500,000 with the same terms
as any warrants issued in the Company’s next financing round and which will be immediately exercisable. Default in the payment
of principal or interest or other material covenant under the note would trigger a default penalty equal to 0.666% of $750,000
per month during the period of default, and other lender rights, including the demand of immediate payment of all amounts due including
accrued but unpaid interest, and recovery of all costs, fees including attorney’s fees and disbursements, and expenses relating
to collection and enforcement of the promissory note. In connection with the First OID Units Closing, pursuant to the Hexin Promissory
Note, on May 22, 2023, the Company issued Hexin a Class E Warrant for the purchase of 7,500,000 shares of common stock and
a Class F Warrant for the purchase of 3,750,000 shares of common stock, which provide for registration rights under the May 12, 2023
Registration Rights Agreement. On May 30, 2023, Hexin
agreed to cancel and exchange the Hexin Promissory Note for the
issuance of 11,764,710 OID Units as described above under “—Third
Closing of OID Units Private Placement”.
On
February 6, 2023, the Company issued the Walleye Promissory Note for $1,000,000 with a maturity date of May 7, 2023. The note did not
carry interest and the principal amount was required to be repaid in full on the maturity date. Under the terms of the Walleye
Promissory Note, the principal amount was increased to $1,250,000 because it was not repaid by the maturity date. See “—Funding
Requirements and Other Liquidity Matters – Default Under Promissory Note”. On
May 30, 2023, the Walleye Promissory Note was cancelled and exchanged for OID Units. See “—Funding Requirements and Other Liquidity Matters
– OID Units Private Placement – Second Closing”.
In connection with the My Health Logic
acquisition, Marizyme assumed an aggregate of $468,137 in notes payable. These notes were unsecured, and carried interest at a rate of
9% per annum with no maturity date. The Company settled an aggregate of $278,678 of these notes payable in connection with the Units
Private Placement issuances during the year ended December 31, 2022. As of June 30, 2023, December 31, 2022 and December 31, 2021,
the balance of the remaining note payable was $241,403, $218,100 and $469,252, respectively.
Changes in Capitalization
On
May 15, 2023, the Company filed a Certificate of Change Pursuant to NRS 78.209 with the Nevada Secretary of State, which provided for
the increase of the Company’s authorized common stock from 20,000,000 shares to 300,000,000 shares and the corresponding change
of every one (1) share of the Company’s issued and outstanding common stock to fifteen (15) shares, and which became effective
upon filing at 11:40 AM Pacific Time on May 15, 2023. Upon the effectiveness of this filing, authorized shares of common stock was increased
to 300,000,000 with a proportional increase in the outstanding shares of common stock to 43,420,350
shares of common stock as of May 15, 2023.
On
June 23, 2023, the Company filed with the SEC and subsequently distributed to certain stockholders of record a definitive proxy statement
relating to the Special Stockholders Meeting, to consider and vote on the following proposals: (i) to approve the Capital Event Amendment,
and (ii) to approve the Voting Rights Amendment. See “—Recent Developments – Special Stockholder Meeting, Change
in Authorized Shares, and Approval of Voting Rights for Certain Debtholders” for related recent developments subsequent to
June 30, 2023.
Consulting
and Trade Debt
As
of June 30, 2023, we owed a total of approximately $2.0 million for goods and services from a number of consultants, law
firms, a medical institution, and our independent registered public accounting firm. To our knowledge, none of these parties had given
notice of default or engaged collections services.
Off-Balance
Sheet Arrangements
As
of June 30, 2023, the Company had no off-balance sheet arrangements that have or are reasonably likely to have a current or future
material effect on its financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital
expenditures or capital resources.
Critical
Accounting Policies and Significant Judgments and Estimates
Our
management’s discussion and analysis of our financial condition and results of operations is based on our financial statements,
which have been prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of
our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses
and the disclosure of contingent assets and liabilities in our financial statements and accompanying notes. We evaluate these estimates
and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable
under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities
that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While
our significant accounting policies are more fully described in “Note 1 – Organization, Basis of Presentation and Summary
of Significant Accounting Policies”, included with the audited financial statements included with this prospectus, and “Note
3 – Summary of Significant Account Policies”, included with the unaudited interim financial statements included with
this prospectus, we believe that the following accounting policies are the most critical for fully understanding and evaluating our financial
condition and results of operations.
Deferred
Offering Cost
The
Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process capital
stock financings as deferred offering costs until such financings are consummated. After consummation of the financing, these costs are
recorded in stockholders’ equity (deficit) as a reduction of additional paid-in capital generated as a result of the offering.
Should a planned equity financing be abandoned, the deferred offering costs will be expensed immediately as a charge to operating expenses
in the statements of operations. As of June 30, 2023, the Company had recorded deferred offering costs of $697,202 (December
31, 2022 - $387,412) reported as a prepaid expense on the accompanying balance sheets, and expensed $535,717 of deferred offering
costs in connection with the extinguishment of indebtedness pursuant to exchange agreements in November 2021 and December 2021 which
provided for the cancellation and exchange of certain outstanding convertible promissory notes. See “Note 7 - Convertible Promissory
Notes and Warrants” included in the footnotes to the unaudited interim financial statements accompanying this prospectus.
Fair
Value Measurements
The
Company uses the fair value hierarchy to measure the value of its financial instruments. The fair value hierarchy is based on inputs
to valuation techniques that are used to measure fair value that are either observable or unobservable. Observable inputs reflect assumptions
market participants would use in pricing an asset or liability based on market data obtained from independent sources, while unobservable
inputs reflect a reporting entity’s pricing based upon its own market assumptions. The basis for fair value measurements for each
level within the hierarchy is described below:
| |
● |
Level
1 – Quoted prices for identical assets or liabilities in active markets. |
| |
|
|
| |
● |
Level
2 – Quoted prices for identical or similar assets and liabilities in markets that are not active; or other model-derived valuations
whose inputs are directly or indirectly observable or whose significant value drivers are observable. |
| |
|
|
| |
● |
Level
3 – Valuations derived from valuation techniques in which one or more significant inputs to the valuation model are unobservable
and for which assumptions are used based on management estimates. |
The
Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent
possible and considers counterparty credit risk in its assessment of fair value.
The
carrying amounts of certain accounts and other receivables, accounts payable and accrued expenses, note payable, and amounts due to related
parties approximate fair value due to the short-term nature of these instruments.
The
fair value of lease obligations is determined using discounted cash flows based on the expected amounts and timing of the cash flows
discounted using a market rate of interest adjusted for appropriate credit risk.
The
contingent liabilities assumed on the Somahlution Acquisition consist of present values of royalty payments, performance warrants
and pediatric voucher warrants, future rare pediatric voucher sales, and liquidation preference. Management measured these contingencies
in accordance with Level 3 of the fair value hierarchy.
| |
i. |
The
performance warrants and pediatric vouchers warrants liabilities were valued using a Monte Carlo simulation model utilizing the following
weighted average assumptions: risk free rate of 1.19%, expected volatility of 69.62%, expected dividend of $0, and expected life
of 5.96 years. For the three and six months ended June 30, 2023, changes in these assumptions resulted in a $582,000
and $1,206,000 decrease in fair value of these liabilities, respectively (June 30, 2022 - $2,092,000 and $2,898,000 increase,
respectively). At June 30, 2023, the fair market value of performance warrants and pediatric vouchers warrants liabilities
was $221,000 (December 31, 2022 - $1,427,000). |
| |
ii. |
The
present value of royalty payments was measured using the scenario-based methodology. In assessing the value attributed to the royalty
payments, the estimated future cash flows were discounted to their present value using a pre-tax discount rate that reflects current
market assessments of the time value of money and the risks specific to the revenue from net sales of the product. The cash flows
derived from the Company’s fifteen-year strategic plan are based on management’s expectations of market growth, industry
reports and trends, and past performances. These projections are inherently uncertain due to the evolving impact of the COVID-19
pandemic. The discounted cash flow model included projections surrounding revenue, discount rates, and growth rates. The discount
rates used to calculate the present value of royalty payments reflect specific risks of the Company and market conditions and the
mid-range was estimated at 20.6%. For the three and six months ended June 30, 2023, changes in these assumptions resulted
in a $129,000 and $796,000 decrease in fair value of this liability, respectively (June 30, 2022 - $293,000 decrease and
$772,000 increase, respectively). At June 30, 2023, the fair market value of royalty payments was $4,606,000 (December
31, 2022 - $5,402,000). |
| |
|
|
| |
iii. |
Rare
pediatric voucher sales liability was valued based on the scenario-based methodology where the estimated future cash flows are discounted
to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the
risks specific to the asset – 20.6%. For the three and six months ended June 30, 2023, changes in these assumptions
resulted in a $3,000 decrease and $12,000 increase in fair value of this liability, respectively (June 30, 2022 - $7,000
and $48,000 decrease, respectively). At June 30, 2023, the fair market value of rare pediatric voucher sales liability
was $1,067,000 (December 31, 2022 - $1,055,000). |
| |
|
|
| |
iv. |
The
present value of liquidation preference liability, included in the contingent consideration, was determined using the Black-Scholes
option pricing method and represents the fair value of the maximum payment amount according to the agreement. The following assumptions
were used in the Black-Scholes option pricing model: risk free rate of 0.21%, expected volatility of 78.93%, expected dividend of
$0, and expected life of 5 years. No changes to the fair value of liquidation preference liability were recorded in the three and
six months ended June 30, 2023 and 2022. At June 30, 2023, the fair market value of liquidation preference
was $1,823,000 (December 31, 2022 - $1,823,000). |
The
derivative liabilities consist of optional and automatic conversion features and the share redemption feature attached to the convertible
notes, issued pursuant to certain convertible promissory notes and warrants transactions (see “Note 7 – Convertible Promissory
Notes and Warrants”, included in the footnotes to the unaudited interim financial statements accompanying this prospectus).
The
Company has no financial assets measured at fair value on a recurring basis. None of the Company’s non-financial assets or liabilities
are recorded at fair value on a non-recurring basis. No transfers between levels have occurred during the periods presented.
The
Company measures the following financial instruments at fair value on a recurring basis. As of June 30, 2023 and December 31,
2022, the fair values of these financial instruments were as follows:
| June 30, 2023 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Derivative liabilities | |
$ | - | | |
$ | - | | |
$ | 5,461,702 | |
| Contingent liabilities | |
| - | | |
| - | | |
| 7,717,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 13,178,702 | |
| | |
Fair Value Hierarchy | |
| December 31, 2022 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Liabilities | |
| | |
| | |
| |
| Derivative liabilities | |
$ | - | | |
$ | - | | |
$ | 4,823,725 | |
| Contingent liabilities | |
| - | | |
| - | | |
| 9,707,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 14,530,725 | |
The
following table provides a roll forward of all liabilities measured at fair value using Level 3 significant unobservable inputs:
| Derivative and Contingent Liabilities | |
| |
| Balance at December 31, 2022 | |
$ | 14,530,725 | |
| Change in fair value of contingent liabilities | |
| (1,990,000 | ) |
| Derivative
liabilities extinguished pursuant to Unit Purchase Agreement (Note 7) | |
| (4,823,725 | ) |
| Derivative liabilities issued pursuant to OID Purchase
Agreement (Note 7) | |
| 5,461,702 | |
| Balance at June 30, 2023 | |
$ | 13,178,702 | |
Research
and Development Expenses and Accruals
All
research and development costs are expensed in the period incurred and consist primarily of salaries, payroll taxes, and employee benefits,
for individuals involved in research and development efforts, external research and development costs incurred under agreements with
contract research organizations and consultants to conduct and support the Company’s clinical trials of DuraGraft, and
costs related to manufacturing DuraGraft for clinical trials. The Company has entered into various research and development contracts
with various organizations. Payments of these activities are based on the terms of the individual agreements which matches to the pattern
of costs incurred. Payments made in advance are reflected in the accompanying balance sheets as prepaid expenses. The Company records
accruals for estimated costs incurred for ongoing research and development activities. When evaluating the adequacy of the accrued liabilities,
the Company analyzes progress of the services, including the phase or completion of events, invoices received and contracted costs. Significant
judgments and estimates may be required in determining the prepaid or accrued balances at the end of any reporting period. Actual results
could differ from the Company’s estimates.
Stock-Based
Compensation
Stock-based
compensation expense for employees and directors is recognized in the Condensed Consolidated Statements of Operations based on estimated
amounts, including the grant date fair value and the expected service period. For stock options, the Company estimates the grant date
fair value using a Black-Scholes valuation model, which requires the use of multiple subjective inputs including estimated future volatility,
expected forfeitures and the expected term of the awards. The Company estimates the expected future volatility based on the stock’s
historical price volatility. The stock’s future volatility may differ from the estimated volatility at the grant date. For restricted
stock unit (“RSU”) equity awards, the Company estimates the grant date fair value using its closing stock price on the date
of grant. The Company recognizes the effect of forfeitures in compensation expense when the forfeitures occur. The estimated forfeiture
rates may differ from actual forfeiture rates which would affect the amount of expense recognized during the period. The Company recognizes
the value of the awards over the awards’ requisite service or performance periods. The requisite service period is generally the
time over which share-based awards vest.
Revenue
Recognition
Accounting
Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“Topic 606”)
Pursuant
to Topic 606, the Company recognizes revenue to depict the transfer of promised goods or services to a customer in an amount that reflects
the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this core principle,
Topic 606 outlines a five-step process for recognizing revenue from customer contracts that includes i) identification of the contract
with a customer, ii) identification of the performance obligations in the contract, iii) determining the transaction price, iv) allocating
the transaction price to the separate performance obligations in the contract, and v) recognizing revenue associated with performance
obligations as they are satisfied.
At
contract inception, the Company assesses the goods or services promised within each contract and assess whether each promised good or
service is distinct and determine those that are performance obligations. The Company then recognizes as revenue the amount of the transaction
price that is allocated to the respective performance obligation when the performance obligation is satisfied.
The
Company has identified one performance obligation which is related to DuraGraft product sales. For the Company’s distribution partner
channel, the Company recognizes revenue for product sales at the time of delivery of the product to its distribution partner (customer).
As products have an expiration date, if a product expires, the Company will replace the product at no charge. There were no significant
judgements made in applying this topic.
Intangible
Assets and Goodwill
Intangible
assets are recorded at cost less accumulated amortization and accumulated impairment losses. Intangible assets acquired as a result of
an acquisition or in a business combination are measured at fair value at the acquisition date.
The
useful lives of intangible assets are assessed as either finite or indefinite. Intangible assets with finite lives are amortized over
the estimated useful economic life and assessed for impairment whenever there is an indication that the intangible asset may be impaired.
The estimated useful life and amortization method are reviewed at the end of each reporting period, with the effect of any changes in
estimates being accounted for on a prospective basis.
Goodwill
represents the excess of the purchase price paid for the acquisition of subsidiaries over the fair value of the net assets acquired.
Following the initial recognition, goodwill is measured at cost less any accumulated impairment losses.
In-Process
Research and Development
The
Company evaluates whether acquired intangible assets are a business under applicable accounting standards. Additionally, the Company
evaluates whether the acquired assets have a future alternative use. Intangible assets that do not have future alternative use are considered
acquired in-process research and development (“IPR&D”). When the acquired in-process research and development assets
are not part of a business combination, the value of the consideration paid is expensed on the acquisition date. Future costs to develop
these assets are recorded to research and development expense as they are incurred.
Impairment
| |
● |
Impairment
of long-lived assets: The Company reviews long-lived assets, including property, plant and equipment, for impairment whenever
events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment
loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual
disposition are less than the carrying amount. The impairment loss, if recognized, would be based on the excess of the carrying value
of the impaired asset over its respective fair value. |
| |
● |
Goodwill:
Goodwill is recorded at the time of purchase for the excess of the amount of the purchase price over the fair values of the identifiable
assets acquired and liabilities assumed. The fair value is determined using the estimated discounted future cash flows of the reporting
unit. Goodwill is not amortized and instead is tested at least annually for impairment, or more frequently when events or changes
in circumstances indicate that goodwill might be impaired. This impairment test is performed annually at December 31. Future adverse
changes in market conditions or poor operating results of underlying assets could result in an inability to recover the carrying
value of the goodwill, thereby possibly requiring an impairment charge. |
| |
|
|
| |
● |
In-process
research and development assets: IPR&D assets are reviewed for impairment annually, or sooner if events or changes in circumstances
indicate that the carrying amount of the asset may not be recoverable, and upon establishment of technological feasibility or regulatory
approval. An impairment loss, if any, is calculated by comparing the fair value of the asset to its carrying value. If the asset’s
carrying value exceeds its fair value, an impairment loss is recorded for the difference and its carrying value is reduced accordingly.
Similar to the impairment test for goodwill, the Company may perform a qualitative approach for testing indefinite-lived intangible
assets for impairment. |
Leases
At
the inception of a contractual arrangement, the Company determines whether the contract contains a lease by assessing whether there is
an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration
over a period of time. If both criteria are met, the Company records the associated lease liability and corresponding right-of-use asset
upon commencement of the lease using the implicit rate or a discount rate based on a credit-adjusted secured borrowing rate commensurate
with the term of the lease. The Company additionally evaluates leases at their inception to determine if they are to be accounted for
as an operating lease or a finance lease. A lease is accounted for as a finance lease if it meets one of the following five criteria:
the lease has a purchase option that is reasonably certain of being exercised, the present value of the future cash flows is substantially
all of the fair market value of the underlying asset, the lease term is for a significant portion of the remaining economic life of the
underlying asset, the title to the underlying asset transfers at the end of the lease term, or if the underlying asset is of such a specialized
nature that it is expected to have no alternative uses to the lessor at the end of the term. Leases that do not meet the finance lease
criteria are accounted for as an operating lease. Operating lease assets represent a right to use an underlying asset for the lease term
and operating lease liabilities represent an obligation to make lease payments arising from the lease. Operating lease liabilities with
a term greater than one year and their corresponding right-of-use assets are recognized on the balance sheet at the commencement date
of the lease based on the present value of lease payments over the expected lease term. Certain adjustments to the right-of-use asset
may be required for items such as initial direct costs paid or incentives received. As the Company’s leases do not typically provide
an implicit rate, the Company utilizes the appropriate incremental borrowing rate, determined as the rate of interest that the Company
would have to pay to borrow on a collateralized basis over a similar term and in a similar economic environment. Lease cost is recognized
on a straight-line basis over the lease term and variable lease payments are recognized as operating expenses in the period in which
the obligation for those payments is incurred. Variable lease payments primarily include common area maintenance, utilities, real estate
taxes, insurance, and other operating costs that are passed on from the lessor in proportion to the space leased by the Company. The
Company has elected the practical expedient to not separate between lease and non-lease components.
Recently
Issued Accounting Pronouncements
In
June 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2016-13, Financial Instruments -
Credit Losses (Topic 326). The new standard amends guidance on reporting credit losses for assets held at amortized cost basis and available-for-sale
debt securities. In February 2020, the FASB issued ASU 2020-02, Financial Instruments-Credit Losses (Topic 326) and Leases (Topic 842)
- Amendments to SEC Paragraphs Pursuant to SEC Staff Accounting Bulletin No. 119 and Update to SEC Section on Effective Date Related
to Accounting Standards Update No. 2016-02, Leases (Topic 842), which amends the effective date of the original pronouncement for smaller
reporting companies. ASU 2016-13 and its amendments will be effective for the Company for interim and annual periods in fiscal years
beginning after December 15, 2022. Current expected credit loss estimates of expected credit losses on trade receivables over
their life will be required to be recorded at inception, based on historical information, current conditions, and reasonable and supportable
forecasts. The Company adopted the standard in the first quarter of 2023. There was no material impact on the results of operations.
BUSINESS
Mission
Statement
We
are a medical technology company changing the landscape of cardiac care by delivering innovative solutions for CABG surgery.
Overview
We
are focused on commercializing or developing three medical technologies and related products – DuraGraft™, MATLOC®
and MAR-FG-001. DuraGraft is a first-in-class De Novo granted and CE marked intra-operative vascular graft storage and flushing solution
used during CABG surgeries. MATLOC is a point-of-care, lab-on-chip digital screening and diagnostic device platform, initially being
developed for quantitative CKD assessment. MAR-FG-001 is a technology in development for use in fat grafting procedures formulated as
a tumescent solution base for protecting adipose tissue during adipose tissue harvesting and storage. DuraGraft, MATLOC and MAR-FG-001
are expected to serve an unmet significant market need in several areas, including cardiac surgery, CKD assessment, and fat grafting.
Since
October 2023, DuraGraft has been authorized for marketing by the FDA for use as an intra-operative vascular conduit storage and flushing
solution used during CABG surgeries in the United States, subject to applicable risks, mitigation requirements, and control provisions.
Since August 2014, DuraGraft has also had the CE
marking required to be sold in the EEA, and DuraGraft has therefore been assessed as meeting the EEA safety, health, and environmental
protection requirements. We are pursuing commercialization of DuraGraft in the United States.
We intend to continue the advancement
of our other two primary product technologies, MATLOC and MAR-FG-001. Our MATLOC CKD point-of-care device is being developed toward a
functional prototype, mainly through the development of its lab-on-chip technology under an SRA. An SRA is an agreement (which may be
classified as a grant, contract or cooperative agreement) under which the Sponsor provides funding to a second party to support the performance
of a specified research project or related activity. The Sponsor may be a foundation, government agency, for-profit entity, research
institute, or another university. We will also continue to ready MAR-FG-001 for viability for development and manufacturing. We intend
to fully develop and market MAR-FG-001 in the U.S. for fat grafting for procedures such as plastic and cosmetic surgery.
In
the near term, we expect to generate revenue primarily from the sale of DuraGraft through the expansion of our international marketing
efforts by our distribution partners in Europe and in other countries that accept CE marking. We intend to commercialize
DuraGraft in the U.S. primarily through hospital integrated networks using our own direct sales force. We anticipate that
once we commence marketing and sales operations for DuraGraft in the U.S., we will be able to generate sustainable revenue growth, accelerate the
development of a functional MATLOC device prototype, and expedite the development of MAR-FG-001 into medical products.
DuraGraft™
Through
our acquisition of the Somahlution Assets in July 2020, we acquired key intellectual assets based on a patent-protected cytoprotective
platform technology designed to reduce ischemic injury to organs and tissues in grafting and transplantation surgeries. These assets
include DuraGraft, a first-in-class, De Novo granted and CE marked intra-operative vascular graft storage and flushing solution
used during CABG surgeries.
DuraGraft
is the first and only medical product that has received authorization by the FDA for marketing for use as an intra-operative
vascular conduit storage and flushing solution used during CABG surgeries. DuraGraft is also the only product certified for
marketing in Europe and other jurisdictions for this indication as an EDI. DuraGraft carries CE marking and is approved for
marketing in 36 countries outside the United States on three continents, including all countries in the EU, including Spain,
Austria, Ireland, Germany, and Italy, as well as countries outside the EU including the UK, Turkey, Switzerland, Chile, and the
Philippines. DuraGraft is also the only patented product for this indication. The DuraGraft patent portfolio includes granted
patents and pending applications in over 30 countries throughout the world, including patents granted in the United States, Europe,
Australia, India, Argentina, South Africa, Mexico, and several Asian countries.
Cardiac
care is a large and growing industry. According to the CDC, the estimated average annual U.S. cost of coronary heart disease is $219
billion (Centers for Disease Control and Prevention, Office of Policy, Performance, and Evaluation, “Health Topics – Heart
Disease and Heart Attack.” POLARIS, August 17, 2021). According
to a market analysis report, the size of the CABG procedures market globally was approximately $10.2 billion as of 2022 (Rahul Gotadki,
Market Research Future, “Coronary Artery Bypass Graft Market Research Report Information By Type (Off-Pump, On-Pump, Minimally
Invasive Direct CABG, Endoscopic Vein Harvesting and Others), By Procedure (Single CABG Surgery, Double CABG Surgery, Triple CABG Surgery,
Quadruple CABG Surgery and Others), By End-User (Hospitals, Cardiology Clinics, Research Institutes and Others), and By Region (North
America, Europe, Asia-Pacific, And Rest Of The World) – Market Forecast Till 2030.”, July 2023). The same source reports
that this market is forecast to increase at a CAGR of 2.7% between 2023 and 2028. Globally, it is estimated that more than 1 million
CABG procedures are performed worldwide each year (Gaudino, Mario, Weill Cornell Medicine, “Method Using Artery for Coronary Artery
Bypass Linked to Better Long-term Outcomes Than Using Vein,” July 14, 2020), with procedures performed in the U.S. being a substantial
percentage of the total global procedures performed. According to the American Heart Association, CABG is the most common type
of open-heart surgery in the United States with more than 500,000 surgeries performed each year (The Society of Thoracic Surgeons,
“Coronary Artery Bypass Grafting (CABG).” The Patient Guide to Heart, Lung, and Esophageal Surgery, May 2019).
In
October 2023, DuraGraft was classified as a Class II device and authorized for marketing by the FDA for use and marketing as an intra-operative
vascular conduit storage and flushing solution used during CABG surgeries, subject to applicable risks, mitigation requirements, and
control provisions. The FDA granted this classification and authorization in response to our application under the De Novo request process
of the Food, Drug, and Cosmetic Act (“FD&C Act”). As a Class II device, DuraGraft is subject to special controls to provide
reasonable assurance of the safety and effectiveness of the device type. The FDA identified the following risks and mitigation measures:
risk of adverse tissue reaction requiring mitigation through biocompatibility evaluation; risk of damage to vascular grafts leading to
major adverse cardiac events or vascular injury requiring mitigation through clinical performance data, non-clinical performance testing,
shelf life testing, and labeling; risk of particulate matter contamination leading to vascular occlusion, coronary artery embolization
and occlusion, phlebitis, infarction, and death requiring mitigation through clinical performance data, non-clinical performance testing,
shelf life testing, and labeling; and risk of infection requiring mitigation through sterilization validation.
In
addition to the general controls of the FD&C Act, use of DuraGraft is subject to the following special controls: (1) Clinical data
must evaluate adverse events associated with clinical use of the device. Devices indicated for vascular grafts for coronary artery bypass
graft surgeries must include an evaluation of the incidence of major adverse cardiac events, vein graft occlusion, and mortality. (2)
Non-clinical performance testing must demonstrate that the device performs as intended under anticipated conditions of use. The following
performance characteristics must be tested: (i) Maintenance of cell viability and structural integrity of vascular conduits during storage
at the labeled temperature and storage duration; and (ii) Evaluation of visible and non-visible particulates in the final mixed solution.
(3) Shelf life testing must demonstrate the stability of the device’s chemical components over the identified shelf life. (4) The
device must be demonstrated to be biocompatible. (5) Performance data must demonstrate the sterility of the device. (6) Labeling must
include: (i) The maximum storage duration for vascular autografts in the solution; (ii) A description of all additives or supplements
that are added at the point of care; (iii) The need for visual inspection of the solution for particulate matter prior to use; (iv) A
statement regarding the duration of stability of the final solution after preparation; (v) A summary of the non-clinical performance
testing that supports use of the device as a flushing and storage solution for vascular autografts; and (vi) A summary of the clinical
data that supports use of the device as a flushing and storage solution for vascular autografts. In addition, DuraGraft is a prescription
device and must comply with 21 CFR 801.109. DuraGraft must also comply with applicable requirements under the FD&C Act, including
registration and listing, labeling, medical device reporting (reporting of medical device-related adverse events) for devices or postmarketing
safety reporting for combination products, good manufacturing practice requirements as set forth in the quality systems regulation for
devices or current good manufacturing practices for combination products, and if applicable, the electronic product radiation control
provisions.
In
connection with the FDA’s review of our De Novo request, the FDA did not exempt DuraGraft as a Class II device from the premarket
notification requirements under section 510(k) of the FD&C Act. Future products that use DuraGraft as a predicate device for vascular
graft storage and flushing solutions used during CABG surgery may be required to comply with premarket notification requirements under
Section 510(k) of the FD&C Act.
Marketing
DuraGraft
Having
received FDA authorization for marketing of DuraGraft, we are proceeding with our plans to commercialize DuraGraft in the United States
and continue to generate international revenue growth from sales of DuraGraft. In the U.S. marketplace, we intend to employ a small direct
sales force focusing on marketing and sales to hospital integrated networks. We have also begun the process of developing the U.S. CABG
market for DuraGraft with select clinical studies, the development of KOLs, the promotion of existing publications, and digital marketing.
We will also seek to develop and commercialize additional applications for the technology underlying DuraGraft.
Our
DuraGraft commercialization plan using its CE marking and existing distribution partners in select European and Asian countries resumed
in the second quarter of 2022, with a targeted approach based on market access, existing KOLs, clinical data and revenue penetration.
In Europe and elsewhere, we will continue our DuraGraft marketing efforts relying on our DuraGraft CE marking and our
distribution partners. The CE marking signifies that DuraGraft may be sold in the EEA and that DuraGraft
has been assessed as meeting safety, health,
and environmental protection requirements. We are currently working with local distributors of cardiovascular disease-related
products, in accordance with local regulatory requirements, to sell and increase the market share of DuraGraft in Spain, Austria, Switzerland, Germany, Chile, Turkey, Italy, and the UK among others.
MATLOC®
In
December 2021, we acquired My Health Logic, its lab-on-chip technology platform and its in-development patient-centric, digital point-of-care
screening and diagnostic device, MATLOC.
The
excitement over microfluidics, also known as lab-on-a-chip technology, lies in its potential for producing revolutionary, timely, accessible,
and practical point-of-care devices; devices that are patient-centric (one-to-many, rather than doctor centric, one-to-one) and support
self-care and independence. Microfluidics is a technology for analyzing small volumes of fluids, with the potential to miniaturize complex
laboratory procedures onto a small microchip, hence the term “lab-on-chip”.
Marizyme’s
lab-on-chip technology is currently being developed for screening and diagnosis related to the three leading biomarkers for CKD, a disease
estimated to affect 37 million Americans (National Kidney Foundation, “Chronic kidney disease (CKD),” 2023) – or approximately
one out of every nine Americans. If left untreated, many patients will advance to ESRD, often leading to kidney transplant, renal failure,
or dialysis. Since 90% of those with CKD do not know they have it (Centers for Disease Control and Prevention, “Chronic Kidney
Disease in the United States, 2023,” May 30, 2023), the risk of progression in the disease is high and this creates massive burdens
for CKD patients and healthcare systems (Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017, March 25;389(10075):1238-1252.
doi: 10.1016/ S0140-6736(16)32064-5. Epub 2016 Nov 23). In 2018 Medicare alone spent $130 billion on CKD and ESRD-related costs (United
States Renal Data System (“USRDS”). 2020 USRDS annual data report: epidemiology of kidney disease in the United States. National
Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020 (“2020 USRDS”),
Volume 1 Chronic Kidney Disease; Chapter 6 Healthcare Expenditures for Persons with CKD; Highlights; Bullet #1: “Medicare fee-for-service
(FFS) spending for beneficiaries with CKD who did not have ESRD exceeded $81 billion in 2018 and represented 22.3% of Medicare FFS spending
(Tables 6.1 and 6.2).”; Volume 2; End Stage Renal Disease: End Stage Renal Disease: Chapter 9 Healthcare Expenditures for Persons
with ESRD; Highlights; Bullet #1: “Total Medicare-related expenditures for beneficiaries with ESRD rose to $49.2B in 2018.”).
With the increase of diabetics and hypertension cases in the U.S., which make up roughly two-thirds of all CKD patients (USRDS 2020 ADR
Reference Tables, Vol. 2—ESRD; Ref. Tables A Incidence; Table A.4 Incident counts of reported ESRD patients, by age and primary
diagnosis, 2016–2018 combined. Col. X, Row 38 (All patients) 382,398; Col. X, Row 33 (Diabetes) 179,706; Col. X, Row 34 (Hypertension)
110,807.] [Computed from: (Col. X shows all gender and race.) All patients (Col. X, Row 38) = 382,398 / DM (Col. X, Row 33) 179,706;
(179,706/382,398) = 47% (0.4699); HTN (Col. X, Row 34) 110,807 (110,807/382,398) = 29% (0.2897)), CKD related healthcare costs are expected
to increase significantly. Compounding this development is the fact that less than 50% of diabetic patients are annually screened or
tested for kidney damage (Folkerts, K., Petruski-Ivleva, N., Comerford, E., et al., “Adherence to Chronic Kidney Disease Screening
Guidelines Among Patients With Type 2 Diabetes in a US Administrative Claims Database,” Mayo Clinic Proceedings 2021;96(4):975-986)
doi: https://doi.org/10.1016/j.mayocp.2020.07.037). This creates an unmet need for point-of-care technologies that facilitate CKD screening
and diagnosis, which further facilitates earlier screening and diagnosis and detection to slow down or eliminate the CKD progression.
By combining lab-on-chip technology with our MATLOC device, it will be able to quantitatively read the two urine biomarkers, albumin
and creatine, and a blood biomarker, eGFR, necessary for effective CKD screening and diagnosis at point-of-care with results available
instantly on a patient’s smartphone.
The
COVID-19 pandemic has massively accelerated the ongoing transformation in healthcare. Connected consumer electronic devices are enabling
24/7 home-based digital healthcare. We believe that consumers have the desire and are now becoming empowered to manage their own healthcare
and that they will seek to utilize our point-of-care MATLOC device.
With
our lab-on-chip technology and MATLOC device in development, we are striving to achieve earlier detection and slowing of the progression
of CKD, allowing patients and healthcare systems to reduce the enormous costs of kidney failure, transplant, and/or dialysis. We anticipate
that once we commence marketing and sales operations for DuraGraft in the U.S., we will be able to accelerate the development
of a functional MATLOC device prototype. After completing this technology for CKD assessment, we also plan to explore the commercial
potential of other biomarkers for chronic diseases to be measured at point-of-care using our lab-on-chip technology.
MAR-FG-001
In November 2021, we reinitiated the development
of MAR-FG-001, our fat grafting technology. MAR-FG-001 is a tumescent solution base for fat grafting procedures that may be used for
plastic surgeries. The Company intends to develop MAR-FG-001 for use during these and other fat grafting procedures.
Fat
grafting is a surgical process used in medical reconstructive and other plastic surgery procedures in which fat is transferred from one
area of the body to another (known as “autologous fat grafting” or simply “fat grafting”) to correct a defect,
replace injured tissue, or to make cosmetic enhancements.
Compared
to standard solutions, we believe that MAR-FG-001 could better protect adipose tissue from ischemic and oxidative injury and increase
adipocyte and stromal cell viability, which is key to improving retention of fat volume thereby improving patient outcomes following
fat grafting procedures.
The
global market for autologous fat grafting was estimated to be $699.96 million in 2021 and was projected to grow at a CAGR of 8.62% until
2028 (“Global Autologous Fat Grafting Market – Industry Trends and Forecast to 2028,” Data Bridge Market Research,
December 2020). Growing preference for the use of non-invasive aesthetic techniques in skin rejuvenation, more rapid recovery with lesser
allergic risks and reduced downtime compared to other procedures are some of the factors contributing to increasing demand. The adoption
rate for autologous fat grafting procedures in the United States was 2.2% of all augmentation and reconstruction procedures as of 2018,
suggesting significant potential for growth of adoption of these procedures (“Autologous Fat Grafting Market Analysis By Product
(Integrated Fat Transfer Systems, Aspiration and Harvesting Systems, Liposuction Systems, Fat Processing Systems, De-epithelialization
Devices), By Application & Region - Global Market Insights 2021 to 2031,” Fact.MR, March 2022). With approximately 22.4 million
plastic surgeries performed in the United States in 2020 (American Society of Plastic Surgeons, “Plastic Surgery Statistics Report
– 2020,” April 27, 2021), we anticipate potentially widespread implementation of innovative fat grafting systems.
MAR-FG-001
is currently in development and not yet available for sale in any markets.
Our
Competitive Strength
We
believe that the following competitive strength will enable us to compete effectively:
| |
● |
Superior,
first-in-class vascular graft storage and flushing solution. Management believes
that the DuraGraft platform provides a significant and substantial competitive advantage
over current methods. DuraGraft is the first and only medical
product that has received authorization by the FDA for marketing for use as an intra-operative
vascular conduit storage and flushing solution used during CABG surgeries. DuraGraft is also
the only product certified for marketing in Europe and other jurisdictions for this indication.
DuraGraft carries CE marking and is approved for marketing in 36 countries outside the United
States on three continents, including all countries in the EU, including Spain, Austria,
Ireland, Germany, and Italy, as well as countries outside the EU including the UK, Turkey,
Switzerland, Chile, and the Philippines. DuraGraft is also the only patented product for
this indication. The DuraGraft patent portfolio includes granted patents and pending applications
in over 30 countries throughout the world, including patents granted in the United States,
Europe, Australia, India, Argentina, South Africa, Mexico, and several Asian countries. |
Our
Growth Strategies
We
will strive to grow our business by pursuing the following key growth strategies:
| |
● |
Commercialize
DuraGraft. We intend to commercialize DuraGraft in the United States for use as an intra-operative vascular conduit storage
and flushing solution used during CABG surgeries primarily by employing a small direct sales force focusing on marketing and sales
to hospital integrated networks. We have also begun the process of developing the U.S. CABG market for DuraGraft with select clinical
studies, the development of KOLs, the promotion of existing publications, and digital marketing. We also intend to seek to develop
and commercialize additional applications for the technology underlying DuraGraft. We will continue the distribution of DuraGraft
in Europe and other countries that accept the CE marking and intend to expand this distribution over time. |
| |
|
|
| |
● |
Develop
MATLOC technology and related products. We intend to continue the development of MATLOC toward a functional device prototype. |
| |
|
|
| |
● |
Develop
MAR-FG-001 fat grafting technology and related products. We intend to continue the development of MAR-FG-001 to validate its protective
abilities and its improvements to the retention of fat volume. |
| |
|
|
| |
● |
Acquire
more life science assets. We intend to continue to expand our product portfolio through the identification and acquisition of additional life
science assets. |
We
will not receive any of the required capital in this offering except upon the exercise of warrants held by the selling stockholders for
issuance of the shares of common stock that are being registered for resale by the registration statement of which this prospectus forms
a part. There can be no assurances, however, that we will be able to raise the capital in this offering that we need to execute our plans
or that capital, whether through securities offerings, either private or public, will be available to us on acceptable terms, if at all.
An inability to raise sufficient funds could cause us to scale back our development and growth plans or discontinue them altogether.
Competition
Competition
in the medical device and life science industries is intense. Our competitors include pharmaceutical companies and biotechnology companies,
as well as universities and public and private research institutions. In addition, companies that are active in different but related
fields represent substantial competition for us. Many of our competitors have significantly greater capital resources, larger research
and development staffs and facilities and greater experience in medical device development, regulation, manufacturing and marketing than
we do. These organizations also compete with us to recruit qualified personnel, attract partners for joint ventures or other collaborations,
and license technologies that are competitive with ours. To compete successfully in this industry, we must identify novel and unique
medical devices or methods of treatment and then complete the development of those medical devices as treatments.
The
medical devices that we are attempting to develop will have to compete with existing therapies. In addition, a large number of companies
are pursuing the development of medical devices that target the same conditions that we are targeting, and other companies have existing
products or medical devices in various stages of pre-clinical or clinical development.
Intellectual
Property
We
own, through the acquisitions of Krillase, DuraGraft, MATLOC, and other assets, various patents, trademarks and other intangibles.
Patent
Portfolio
Upon
our acquisition of the Somahlution Assets, we acquired all of the Somahlution intellectual property relating to the Somahlution products,
including patents rights and trademarks relating to DuraGraft. In addition, prior to the closing of the acquisition of the Somahlution
Assets, in certain countries, we paid the costs relating to the filing and registration of patent applications and we were granted ownership
rights to DuraGraft patents issued in those countries.
In
September 2018, we acquired assets relating to Krillase®, a technology intended for the treatment of certain harmful
blockages, plaque and biofilms, including certain patents, patent applications, and trademarks. In December 2021, we acquired assets
relating to MATLOC®, a technology intended for the screening of biomarkers
relating to CKD, including patents rights and trademarks relating to the MATLOC platform and products. The Company previously
planned to develop and commercialize FDA-approved products based on the Krillase assets. We suspended these plans due to our determination
to prioritize the completion of regulatory processes to obtain FDA authorization for the commercialization of DuraGraft in the United
States and the development of a functional MATLOC device prototype and MAR-FG-001-based viable products. We intend to maintain the Krillase
assets for potential future development and commercialization or disposition. Any determination as to these matters would be based on
a number of factors. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations –
Principal Factors Affecting Our Financial Performance” for a summary of factors that we may consider in this respect.
There
is no assurance that any of our intellectual property assets will ever be developed and fully commercialized and generate significant
revenues or will ever attract significant interest from potential buyers or investors. See “Risk Factors – Risks Related
to Our Business – We may not be able to monetize intangible assets, which may result in the need to record an impairment charge.”
As
of October 12, 2023, we own the following patents:
| Product
Type |
|
Country/
Jurisdiction |
|
Official
No. |
|
Status |
|
Title |
| DuraGraft |
|
Argentina |
|
AR098508
B1 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Australia |
|
2014353199 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Austria |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Austria |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Austria |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Belgium |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Belgium |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Belgium |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Brazil |
|
BR112016006512-3 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Canada |
|
2923441 |
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
China |
|
201480024203.2 |
Pending |
|
FORMULATIONS
CONTAINING POLY (0-2-HYDROXYETHYL) STARCH FOR INCREASING THE OXYGEN-CONTENT, STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION
SOLUTION |
| DuraGraft |
|
China |
|
ZL201480061943.3 |
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Czechia |
|
3027016 |
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Denmark |
|
2938186 |
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Denmark |
|
2938187 |
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Denmark |
|
3027016 |
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
EPO |
|
2938186 |
|
EP
Granted |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
EPO |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
EPO |
|
3027016 |
|
EP
Granted |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
France |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
France |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
France |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Germany |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Germany |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Germany |
|
602014048320.8 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
India |
|
5238/DELNP/2015 |
|
Pending |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
India |
|
403699 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
India |
|
201617013141 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Indonesia |
|
P00201603372 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Ireland |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Ireland |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Israel |
|
244834 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Italy |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Italy |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Italy |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Japan |
|
2016-525916 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Jordan |
|
334/2014 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Kuwait |
|
159PA/2014 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Malaysia |
|
MY-181282-A |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Mexico |
|
371723 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Mexico |
|
378411 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Netherlands |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Netherlands |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Netherlands |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
New
Zealand |
|
718991 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Norway |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Norway |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Norway |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Philippines |
|
1-2016-500909 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Republic
of Korea |
|
2428676 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Republic
of Korea |
|
2022-7026130 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Singapore |
|
10201709595W |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Singapore |
|
10202204548W
|
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Singapore |
|
11201604002V |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
South
Africa |
|
2015/04103 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraf |
|
South
Africa |
|
2015/04284 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
South
Africa |
|
2016/02253 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Spain |
|
ES2724238 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Spain |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Spain |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Sri
Lanka |
|
18739 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Sweden |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Sweden |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Sweden |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Switzerland |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
Switzerland |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Switzerland |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Taiwan |
|
TW103115212 |
|
Pending |
|
FORMULATIONS
CONTAINING POLY (0-2-HYDROXYETHYL) STARCH FOR INCREASING THE OXYGEN-CONTENT, STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION
SOLUTION |
| DuraGraft |
|
Taiwan |
|
TW103114889 |
|
Pending |
|
FORMULATIONS
FOR INCREASING THE OXYGEN-CONTENT, STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Taiwan |
|
I540961 |
|
Registered |
|
SOLUTIONS
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Taiwan |
|
I524843 |
|
Registered |
|
SOLUTIONS
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Taiwan |
|
103140510 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN AND TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Thailand |
|
1601002666 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Turkey |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
Turkey |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
United
Kingdom |
|
2938186 |
|
Registered |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
United
Kingdom |
|
2938187 |
|
Registered |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
United
Kingdom |
|
3027016 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
United
States |
|
14/654168 |
|
Petition
to Revive pending |
|
ORGAN
AND TISSUE PRESERVATION FORMULATIONS WITH INCREASED STABILITY AND SHELF LIFE |
| DuraGraft |
|
United
States |
|
14/654170 |
|
Pending |
|
SOLUTION
FOR PRESERVING VASCULAR CONDUITS |
| DuraGraft |
|
United
States |
|
63/285386 |
|
Pending |
|
METHOD
OF PROVIDING POSTOPERATIVE PROTECTION IN PATIENTS UNDERGOING CORONARY ARTERY BYPASS GRAFT SURGERY |
| DuraGraft |
|
United
States |
|
17/684,495 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
United
States |
|
11,291,201 |
|
Registered |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
United
States |
|
63/331,777 |
|
Pending |
|
POWDER
FORMULATIONS AND USE THEREOF IN MEDICAL AND/OR SURGICAL PROCEDURES |
| DuraGraft |
|
United
States |
|
63/333,122 |
|
Pending |
|
FORMULATION
FOR PRESERVATION OF VEINOUS AND ARTERIAL GRAFTS IN DIABETIC PATIENTS |
| DuraGraft |
|
United
States |
|
63/333,123 |
|
Pending |
|
FORMULATION
FOR INHIBITION OF ENDOTHELIAL DAMAGE |
| DuraGraft |
|
United
States |
|
63/333,505 |
|
Pending |
|
FORMULATION
FOR PRESERVING TISSUES OR ORGANS IN PATIENTS WITH AND WITHOUT LEFT MAIN DISEASE UNDERGOING ISOLATED CABG |
| DuraGraft |
|
United
States |
|
63/333,510 |
|
Pending |
|
FORMULATION
FO RMYOCARDIAL PROTECTION DURING GRAFTING |
| DuraGraft |
|
United
States |
|
63/333,514 |
|
Pending |
|
FORMULATION
FOR STORING VEIN GRAFTS |
| DuraGraft |
|
United
States |
|
63/342,370 |
|
Pending |
|
USE
OF CYTOPROTECTANT FORMULATIONS IN CELL OR TISSUE TRANSPORT AND/OR STORAGE |
| DuraGraft |
|
United
States |
|
63/401,486 |
|
Pending |
|
ANTIOXIDANT-CONTAINING
EYE DROP FORMULATIONS AND METHODS OF TREATMENT USING SAME |
| DuraGraft |
|
Vietnam |
|
1-2016-01625 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| DuraGraft |
|
Vietnam |
|
1-2022-04720 |
|
Pending |
|
SOLUTIONS
FOR INCREASING THE STABILITY AND SHELF LIFE OF AN ORGAN TISSUE PRESERVATION SOLUTION |
| Krillase |
|
Australia |
|
2019292557 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Brazil |
|
BR1120210048090 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Canada |
|
3110779 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
China |
|
201980057015.2 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Costa
Rica |
|
2021-0059 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
EAPO |
|
202190494 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
EPO |
|
07865205.4 |
|
Pending |
|
A
CONTROLLED RELEASE ENZYMATIC COMPOSITION AND METHODS OF USE |
| Krillase |
|
EPO |
|
21217552.5 |
|
Pending |
|
A
CONTROLLED RELEASE ENZYMATIC COMPOSITION AND METHODS OF USE |
| Krillase |
|
EPO |
|
13712728.8 |
|
Pending |
|
MIXTURE
OF ENZYMES FROM ANTARCTIC KRILL FOR USE IN THE REMOVAL OF A BIOFILM |
| Krillase |
|
EPO |
|
19824845.2 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Hong
Kong |
|
62021038660.8 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Indonesia |
|
P00202102501 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
Japan |
|
2021-517700 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
South
Africa |
|
2021/00693 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Krillase |
|
United
States |
|
16/457291 |
|
Pending |
|
PHARMACEUTICAL
COMPOSITIONS AND METHODS FOR THE TREATMENT OF THROMBOSIS AND DELIVERY BY MEDICAL DEVICES |
| Somaceutica |
|
United
States |
|
17/261929 |
|
Pending |
|
DERMATOLOGICAL
COMPOSITIONS FOR PROVIDING NUTRIENTS TO SKIN AND METHODS THEREOF |
As
of October 12, 2023, we are the licensee of the following patents:
| Product
Type |
|
Country/
Jurisdiction |
|
Official
No. |
|
Status |
|
Title |
|
Owner/Licensee |
| MATLOC |
|
EPO |
|
19867662.9 |
|
Pending |
|
A
PASSIVE MIXING MICROFLUIDIC URINARY ALBUMIN CHIP (UAL-CHIP) FOR CHRONIC KIDNEY DISEASE ASSESSMENT |
|
Owned
by University of Manitoba. Marizyme is an exclusive licensee. |
| MATLOC |
|
Canada |
|
3,108,813 |
|
Pending |
|
A
PASSIVE MIXING MICROFLUIDIC URINARY ALBUMIN CHIP (UAL-CHIP) FOR CHRONIC KIDNEY DISEASE ASSESSMENT |
|
Owned
by University of Manitoba. Marizyme is an exclusive licensee. |
| MATLOC |
|
United
States |
|
17/266,217 |
|
Pending |
|
A
PASSIVE MIXING MICROFLUIDIC URINARY ALBUMIN CHIP (UAL-CHIP) FOR CHRONIC KIDNEY DISEASE ASSESSMENT |
|
Owned
by University of Manitoba. Marizyme is an exclusive licensee. |
| MATLOC |
|
EPO |
|
19852466.2 |
|
Pending |
|
METHOD
FOR DEVELOPMENT OF MICROFLUIDIC ASSAY DEVICE PROTOTYPE |
|
Owned
by University of Manitoba. Marizyme is exercising its option for exclusive license. |
| MATLOC |
|
Canada |
|
3108991 |
|
Pending |
|
METHOD
FOR DEVELOPMENT OF MICROFLUIDIC ASSAY DEVICE PROTOTYPE |
|
Owned
by University of Manitoba. Marizyme is exercising its option for exclusive license. |
| MATLOC |
|
United
States |
|
17/266204 |
|
Pending |
|
METHOD
FOR DEVELOPMENT OF MICROFLUIDIC ASSAY DEVICE PROTOTYPE |
|
Owned
by University of Manitoba. Marizyme is exercising its option for exclusive license. |
| MATLOC |
|
United
States |
|
63/333,054 |
|
Pending |
|
MICROFLUIDIC
URINE ALBUMIN/CREATININE CHIP (uARC-CHIP) FOR CHRONIC KIDNEY DISEASE EVALUATION |
|
Owned
by University of Manitoba. Marizyme is an exclusive licensee. |
| MATLOC |
|
United
States |
|
63/322,958 |
|
Pending |
|
PAPER-BASED
MICROFLUIDIC CHIP FOR MEASUREMENT OF CYSTATIN C IN PLASMA AND SERUM (CYS-C PAPER CHIP) |
|
Owned
by University of Manitoba. Marizyme is an exclusive licensee. |
Trademarks
As
of October 12, 2023, we own the following trademarks:
| Application
No. |
|
Registration
No. |
|
Country/
Jurisdiction |
|
Case
Status |
|
Trademark |
| 97/401623 |
|
1701131
|
|
United
States |
|
Registered |
|
CYTOPRO |
| 3408723 |
|
2852586 |
|
Argentina |
|
Registered |
|
DURAGRAFT |
| 186456 |
|
186456 |
|
Bangladesh |
|
Registered |
|
DURAGRAFT |
| 5285-2015 |
|
164842C |
|
Bolivia |
|
Registered |
|
DURAGRAFT |
| 909398577 |
|
909398577 |
|
Brazil |
|
Registered |
|
DURAGRAFT |
| 47145 |
|
47145 |
|
Brunei
Darussalam |
|
Registered |
|
DURAGRAFT |
| 1662655 |
|
TMA969008 |
|
Canada |
|
Registered |
|
DURAGRAFT |
| 1156204 |
|
1185551 |
|
Chile |
|
Registered |
|
DURAGRAFT |
| 2015-11331 |
|
265653 |
|
Costa
Rica |
|
Registered |
|
DURAGRAFT |
| 2015-32539 |
|
227843 |
|
Dominican
Republic |
|
Registered |
|
DURAGRAFT |
| 2015-21165 |
|
IEPI/2017/TI/2587 |
|
Ecuador |
|
Registered |
|
DURAGRAFT |
| 2015004632 |
|
212663 |
|
Guatemala |
|
Registered |
|
DURAGRAFT |
| 303632968 |
|
303632968 |
|
Hong
Kong |
|
Registered |
|
DURAGRAFT |
| D002015022001 |
|
IDM000591043 |
|
Indonesia |
|
Registered |
|
DURAGRAFT |
| JO/T/1/120445 |
|
140912 |
|
Jordan |
|
Registered |
|
DURAGRAFT |
| 169368 |
|
169368 |
|
Kuwait |
|
Registered |
|
DURAGRAFT |
| A0040426 |
|
1197989 |
|
Madrid
Protocol (TM) |
|
Registered |
|
DURAGRAFT |
| 2015058165 |
|
2015058165 |
|
Malaysia |
|
Registered |
|
DURAGRAFT |
| 2015-001717 |
|
2015111637 |
|
Nicaragua |
|
Registered |
|
DURAGRAFT |
| 389755 |
|
389755 |
|
Pakistan |
|
Registered |
|
DURAGRAFT |
| 240938 |
|
240938 |
|
Panama |
|
Registered |
|
DURAGRAFT |
| 1567034 |
|
428472 |
|
Paraguay |
|
Registered |
|
DURAGRAFT |
| 619456 |
|
227503 |
|
Peru |
|
Registered |
|
DURAGRAFT |
| 2015/13465 |
|
2015/13465 |
|
South
Africa |
|
Registered |
|
DURAGRAFT |
| 198823 |
|
198823 |
|
Sri
Lanka |
|
Registered |
|
DURAGRAFT |
| AE246475 |
|
246475 |
|
United
Arab Emirates |
|
Registered |
|
DURAGRAFT |
| 469705 |
|
469705 |
|
Uruguay |
|
Registered |
|
DURAGRAFT |
| 97/271792 |
|
1681258
|
|
United
States |
|
Registered |
|
DURAGRAFT |
| 1197989 |
|
UK00801197989 |
|
United
Kingdom |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
African
IPO |
|
Registered |
|
DURAGRAFT
|
| |
|
1616525 |
|
Australia |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Belarus |
|
Registered |
|
DURAGRAFT
|
| 15099005 |
|
534078 |
|
Colombia |
|
Registered |
|
DURAGRAFT
|
| CMA11197989 |
|
CMA11197989 |
|
Cuba |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Egypt |
|
Registered |
|
DURAGRAFT
|
| |
|
1197989 |
|
European
Union |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Finland |
|
Pending |
|
DURAGRAFT
|
| V0095738 |
|
V0095738 |
|
Iceland |
|
Registered |
|
DURAGRAFT
|
| |
|
2728894 |
|
India |
|
Registered |
|
DURAGRAFT
|
| 264333 |
|
264333
|
|
Israel |
|
Registered |
|
DURAGRAFT
|
| 2014-353219 |
|
1197989 |
|
Japan |
|
Registered |
|
DURAGRAFT
|
| |
|
1197989 |
|
Kazakhstan |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Kenya |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Kyrgyzstan |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Liechtenstein |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Morocco |
|
Registered |
|
DURAGRAFT
|
| |
|
995906 |
|
New
Zealand |
|
Registered |
|
DURAGRAFT
|
| |
|
1197989 |
|
Norway |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Oman |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Philippines |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Republic
of Korea |
|
Registered |
|
DURAGRAFT
|
| |
|
1197989 |
|
Russian
Federation |
|
Registered |
|
DURAGRAFT
|
| |
|
T1405531A |
|
Singapore |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Switzerland |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Tunisia |
|
Registered |
|
DURAGRAFT
|
| 2014/35608 |
|
1197989 |
|
Turkey |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Ukraine |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Uzbekistan |
|
Registered |
|
DURAGRAFT
|
| 1197989 |
|
1197989 |
|
Vietnam |
|
Registered |
|
DURAGRAFT
|
| 1571411 |
|
1571411 |
|
Australia |
|
Registered |
|
GALA |
| 840635028 |
|
840635028 |
|
Brazil |
|
Registered |
|
GALA |
| 13200353 |
|
13200353 |
|
China |
|
Registered |
|
GALA |
| 011866902 |
|
011866902 |
|
European
Union |
|
Registered |
|
GALA |
| 258906 |
|
258906 |
|
Israel |
|
Registered |
|
GALA |
| 981751 |
|
981751 |
|
New
Zealand |
|
Registered |
|
GALA |
| 2013/101481 |
|
2013
101481 |
|
Turkey |
|
Registered |
|
GALA |
| 011866902 |
|
UK00911866902 |
|
United
Kingdom |
|
Registered |
|
GALA |
| T1314119B |
|
T1314119B |
|
Singapore |
|
Registered |
|
GALA
|
| 2013-070195 |
|
5645334 |
|
Japan |
|
Registered |
|
GALA
|
| |
|
690472 |
|
Madrid
Protocol (TM) |
|
Registered |
|
KRILLASE |
| 1994/09732 |
|
302346 |
|
Sweden |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
Finland |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
France |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
Germany |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
Norway |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
Portugal |
|
Registered |
|
KRILLASE |
| |
|
690472 |
|
Spain |
|
Registered |
|
KRILLASE |
| |
|
WO0000000690472 |
|
United
Kingdom |
|
Registered |
|
KRILLASE |
| 97/332349 |
|
1690654
|
|
United
States |
|
Registered |
|
KRILLASE |
| 97/332345 |
|
1699350
|
|
United
States |
|
Registered |
|
MARIZYME |
| 90/460461 |
|
1666845 |
|
United
States |
|
Registered |
|
MATLOC |
| 166845 |
|
|
|
Canada |
|
Pending |
|
MATLOC |
| 166845 |
|
|
|
European
Union |
|
Pending |
|
MATLOC |
| 166845 |
|
|
|
United
Kingdom |
|
Pending |
|
MATLOC |
| A0070668 |
|
1379788 |
|
Madrid
Protocol (TM) |
|
Registered |
|
SOMACEUTICA |
| 1863383 |
|
TMA1084929 |
|
Canada |
|
Registered |
|
SOMACEUTICA
|
| |
|
1379788 |
|
European
Union |
|
Registered |
|
SOMACEUTICA
|
| 3713217 |
|
1379788 |
|
India |
|
Registered |
|
SOMACEUTICA
|
| 2017-366542 |
|
1379788 |
|
Japan |
|
Registered |
|
SOMACEUTICA
|
| |
|
1379788 |
|
Liechtenstein |
|
Registered |
|
SOMACEUTICA
|
| |
|
1379788 |
|
Norway |
|
Registered |
|
SOMACEUTICA
|
| 1379788 |
|
UK00801379788 |
|
United
Kingdom |
|
Registered |
|
SOMACEUTICA
|
| 47144 |
|
47144 |
|
Brunei
Darussalam |
|
Registered |
|
SOMAHLUTION |
| 304532067 |
|
304532067 |
|
Hong
Kong |
|
Registered |
|
SOMAHLUTION |
| JO/T/1/142669 |
|
160029 |
|
Jordan |
|
Registered |
|
SOMAHLUTION |
| 2018-4350 |
|
|
|
Kuwait |
|
Pending |
|
SOMAHLUTION |
| A0043179 |
|
1218320 |
|
Madrid
Protocol (TM) |
|
Registered |
|
SOMAHLUTION |
| 2015067387 |
|
2015067387 |
|
Malaysia |
|
Registered |
|
SOMAHLUTION |
| 86/248574 |
|
5027533 |
|
United
States |
|
Registered |
|
SOMAHLUTION |
| 1218320 |
|
UK00801218320 |
|
United
Kingdom |
|
Registered |
|
SOMAHLUTION
|
| |
|
1651327 |
|
Australia |
|
Registered |
|
SOMAHLUTION
|
| 86248574 |
|
1218320 |
|
China |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Egypt |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
European
Union |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Iceland |
|
Registered |
|
SOMAHLUTION
|
| 2859336 |
|
1218320 |
|
India |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Iran |
|
Registered |
|
SOMAHLUTION
|
| 268806 |
|
268806 |
|
Israel |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Japan |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Liechtenstein |
|
Registered |
|
SOMAHLUTION
|
| 1538097 |
|
1544539 |
|
Mexico |
|
Registered |
|
SOMAHLUTION
|
| |
|
1006640 |
|
New
Zealand |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Norway |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Russian
Federation |
|
Registered |
|
SOMAHLUTION
|
| |
|
T1416296G |
|
Singapore |
|
Registered |
|
SOMAHLUTION
|
| |
|
1218320 |
|
Switzerland |
|
Registered |
|
SOMAHLUTION
|
| 2014/84799 |
|
2014/84799 |
|
Turkey |
|
Registered |
|
SOMAHLUTION |
Other
Intellectual Property
We
own the internet domain names www.marizyme.com and www.somahlution.com, which are our primary operating websites. We own additional websites
which are reserved for future operations. The information contained in our websites is not incorporated by reference in this prospectus.
We
generally control access to and use of our proprietary technology and other confidential information through the use of internal and
external controls, including contractual protections with employees, contractors, customers, and partners, and our software is protected
by U.S. and international copyright laws. In this regard, we have signed confidentiality agreements with all of our current and former
employees and consultants. Despite our efforts to protect our trade secrets and proprietary rights through intellectual property rights,
licenses, and confidentiality agreements, unauthorized parties may still copy or otherwise obtain and use our software and technology.
In addition, the laws of some foreign countries in which we sold products do not protect our proprietary rights as fully as do the laws
of the United States. There can be no assurance that our means of protecting our proprietary rights in the United States or abroad were
adequate or that competition will not independently develop similar technology.
Manufacturing,
Distribution and Marketing
We
do not own or operate, and currently have no plans to establish, any manufacturing or distribution facilities. We expect to rely on third
parties for the manufacture and distribution of the medical technology devices that we commercialize.
Having
received FDA authorization for marketing of DuraGraft, we are proceeding with our plans to commercialize DuraGraft in the United States
and continue to generate international revenue growth from sales of DuraGraft. In the U.S. marketplace, we intend to employ a small direct
sales force focusing on marketing and sales to hospital integrated networks. We have also begun the process of developing the U.S. CABG
market for DuraGraft with select clinical studies, the development of KOLs, the promotion of existing publications, and digital marketing.
We will also seek to develop and commercialize additional applications for the technology underlying DuraGraft. In Europe and elsewhere,
we will continue our DuraGraft marketing efforts relying on our DuraGraft CE marking and our distribution partners. The CE marking signifies
that DuraGraft may be sold in the EEA and that DuraGraft has been assessed as meeting safety, health, and environmental protection requirements.
We are currently working with local distributors of cardiovascular disease-related products, in accordance with local regulatory requirements,
to sell and increase the market share of DuraGraft in Spain, Austria, Switzerland, Germany, Chile, Turkey, Italy, and the UK among others.
After
the development of a functional MATLOC prototype and further development of MAR-FG-001, we intend to enter into a commercialization arrangement
with strategic and distribution partners who will be responsible for the marketing and sales of these technology platforms. If we are
not able to find appropriate strategic and distribution partners or our partners are unable to achieve our sales goals, we will need
to develop our own marketing and sales capabilities, which we expect would require more time and resources. As we do not anticipate obtaining
FDA marketing authorization for MATLOC and MAR-FG-001 products within the near-term future, we intend to market MATLOC and MAR-FG-001 in other
jurisdictions in which we may sell related products.
Our
marketing and sales strategies in non-U.S. markets integrate a digital and social marketing campaign, and we also plan to use this type
of marketing in the United States for any product that receives FDA approval.
Facilities
Upon
our acquisition of the Somahlution Assets in July 2020 and continuing through December 2020, we leased approximately 20,000 square feet
in a building located at 225 Chimney Corner Lane, Jupiter, Florida 33458, which is owned by the Chairman of our board of directors and
the former owner of Somahlution. On December 11, 2020, we entered into a five-and-a-half-year lease for approximately 8,500 square feet
at 555 Heritage Drive, Jupiter, Florida 33458, which includes office and laboratory space. Our principal executive office is located
at this Jupiter, Florida address. Effective April 1, 2022, the Company amended the lease agreement to add additional space for administrative
office and laboratories. Under the amended lease, our office and laboratory space increased to 13,535 square feet. The monthly cost of
total expanded lease space during 2022 was approximately $15,260 increasing to $15,641 in 2023 and will increase by 2.5% annually thereafter
until the end of the term. As of April 1, 2022, monthly operating expenses for total expanded premises increased from approximately $12,000
to $17,500 per month. The lease term is currently scheduled to end on July 31, 2026.
Seasonality
and Cyclicality
Our
operating results and operating cash flows have not been subject to significant seasonal variations. We do not expect this pattern to
change in the near term.
Employees
As
of October 12, 2023, the Company had 12 full-time employees and two full-time consultants.
Legal
Proceedings
From
time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However,
litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may
harm our business. Except as described below, we are not aware of any such legal proceedings or claims against us.
DeVito
Litigation and Settlement Agreement
On
June 7, 2022, Nicholas P. DeVito, a former Chairman, Chief Executive Officer, Interim Chief Executive Officer and Interim Chief Financial Officer of the
Company, filed a complaint in the Florida Circuit Court against the Company (the “DeVito Complaint”). The DeVito Complaint
claimed breach of contract, declaratory relief, specific performance, breach of an implied covenant of good faith and fair dealing, and
unjust enrichment against the Company with respect to the Company’s alleged breach of the common stock issuance requirements of
an Incentive Stock Option Agreement between Mr. DeVito and the Company, dated as of July 13, 2019 (the “DeVito ISO”). Under
the DeVito ISO, on July 13, 2019, the Company granted an option to Mr. DeVito to purchase 600,000 shares of common stock at $1.01 per
share, subject to certain vesting terms. The DeVito ISO provided that it would terminate twelve (12) months after the end of Mr. DeVito’s
“Continuous Service,” which was not defined by the DeVito ISO. On August 27, 2020, as part of a Mutual Release of Claims
Agreement between Mr. DeVito and the Company dated as of that date (the “DeVito Release”), the Company agreed to immediately
vest the unvested portion of the DeVito ISO such that the DeVito ISO became fully vested, to pay Mr. DeVito $20,000 to add in the transition
during the month of September 2020, and that Mr. DeVito would retain all of his rights under the DeVito ISO. Under the DeVito Release,
Mr. DeVito agreed to step down from his positions as Interim Chief Executive Officer and Interim Chief Financial Officer on September
1, 2020, to assist Marizyme with its transition to a new Chief Executive Officer and Chief Financial Officer for the month of September
2020, and effective as of September 1, 2020 would no longer act as an officer of Marizyme in any capacity. The DeVito Release also recited
that the Company requested that Mr. DeVito be available for additional consulting going forward as the needs of the business dictate.
The DeVito Release also provided for a general mutual release of claims by the Company and Mr. DeVito apart from continuing employment
obligations and corporate officer indemnification obligations of Marizyme. The DeVito Complaint alleged that Mr. DeVito continued his
role as an advisor and consultant to the Company. Due to the Company’s alleged nonperformance of Mr. DeVito’s exercise rights
under the DeVito ISO, the DeVito Complaint sought declaratory relief, specific performance, direct and consequential damages in an unspecified
amount of more than $30,001.00, damages prescribed by the DeVito ISO, reasonable attorney’s fees and costs, prejudgment interest,
and such other relief as the court deems equitable. On July 21, 2022, the Company filed a motion to dismiss the claims of declaratory
relief, specific performance, and breach of an implied covenant of good faith and fair dealing in the DeVito Complaint. On August 3,
2022, Mr. DeVito filed an amended complaint without a hearing (the “Amended DeVito Complaint”). The Amended DeVito Complaint
included two claims, for breach of contract and unjust enrichment, and otherwise realleged substantially all of the factual allegations
of the DeVito Complaint. On August 26, 2022, the Company filed an Answer to the Amended DeVito Complaint denying substantially all of
the allegations.
Under
a Confidential Settlement Agreement, dated November 18, 2022 (the “DeVito Settlement Agreement”), the Company and Mr. DeVito
agreed that Mr. DeVito would dismiss the DeVito Complaint. The parties also agreed that following an anticipated reverse stock split,
the Company was required to issue Mr. DeVito a certain number of “post-split” shares to be delivered in paper certificate
form within three business days of the reverse stock split. The DeVito Settlement Agreement further provided that the delivered shares
would be subject to normal and customary restrictions pursuant to Rule 144. In the event no reverse stock split had occurred by December
12, 2022, the Company was required to issue Mr. DeVito 240,000 “pre-split” shares. In addition, the parties agreed that no
further continuous service would be required pursuant to Section 2 of the DeVito Release. As of December 12, 2022, the anticipated reverse
stock split of the common stock had not been processed by FINRA. On January 5, 2023, the Company issued 240,000 shares of common stock
to Mr. DeVito.
Chandler
Litigation
On
January 28, 2022, the Company filed a Complaint in the Florida Circuit Court, case number 50-2022-CA-000859-XXX-MB, against Amy Chandler
(the “Chandler Complaint”). The Chandler Complaint sought damages for breach of fiduciary duty, breach of contract, negligence,
conversion, and civil theft. The Chandler Complaint alleged that, prior to her resignation in September 2021, Ms. Chandler intentionally
and recklessly took actions to cancel the CE certificate required by European Union regulations in order for Marizyme and its subsidiary,
Somahlution, Inc., to ship and distribute certain products to/within the EU. The Chandler Complaint also alleged that Ms. Chandler disregarded
her fiduciary duty to Marizyme and responsibilities as the top regulatory and compliance official of Marizyme. As a result, the Chandler
Complaint alleged that Ms. Chandler’s actions caused significant disruption and damage to Marizyme’s business, including,
but not limited to, financial damages and damage to Marizyme’s reputation and business relationships. The Chandler Complaint further
alleged that prior to her last day, Ms. Chandler stole confidential, proprietary files governing Marizyme’s quality management
system, which related to internal business operations, and that Marizyme incurred significant costs to recreate these files. The Chandler
Complaint alleged damages in excess of thirty thousand dollars ($30,000), exclusive of interest, attorneys’ fees, and costs.
On
February 28, 2022, Ms. Chandler filed an Answer, Affirmative Defenses and Counterclaim with the Florida Circuit Court (the “Answer”).
The Answer denied the claims in the Chandler Complaint and most of the factual allegations regarding Ms. Chandler’s alleged actions.
The Answer also asserted a counterclaim against the Company for defamation per se. The Answer sought to recover monetary damages, attorneys’
fees, and court costs in connection with this litigation. The Answer also demanded a trial by jury on all triable issues.
On
March 18, 2022, the Company filed a Motion to Dismiss Ms. Chandler’s Counterclaim with the Florida Circuit Court (the “Motion
to Dismiss”). The Motion to Dismiss stated that Ms. Chandler’s Counterclaim for defamation per se should be dismissed with
prejudice. On July 13, 2022, the court ruled that the counterclaim of defamation was dismissed with prejudice. Ms. Chandler’s deposition
was taken on September 21, 2022. On November 21, 2022, the Company filed a notice of voluntary dismissal without prejudice of its complaint
and the case was dismissed.
Campbell/Harmon
Litigation
On
August 19, 2021, Dr. Neil Campbell, former President, Chief Executive Officer and director of the Company, and Bruce Harmon, former Chief
Financial Officer and Secretary of the Company, each filed a Complaint and Demand for Jury Trial in the Florida Circuit Court, case numbers No. 50-2021-CA-009938 and No. 50-2021-CA-009954,
respectively, against the Company and Insperity Peo Services, L.P., a Delaware limited partnership (“Insperity”), a joint
employer of Dr. Campbell and Mr. Harmon with the Company under a Client Service Agreement, dated November 30, 2020 (collectively, the
“Campbell/Harmon Complaints”). Both Campbell/Harmon Complaints alleged that the Company and Insperity violated Section 448.105
of the Florida Private Whistleblower Act as a result of the constructive terminations of Dr. Campbell and Mr. Harmon after the occurrence
of violations of federal and state law, including federal securities law, at the Company that exposed Dr. Campbell and Mr. Harmon to
civil and criminal forms of liability and that the Company was not addressing to their satisfaction. Both of the Campbell/Harmon Complaints
demanded approximately $30,000-$50,000 in back pay and benefits, interest on back pay, front pay and/or lost earning capacity, compensatory
damages, costs and attorney’s fees, and such other relief as the court deems equitable.
Pursuant
to a Joint Stipulation of Voluntary Dismissal With Prejudice filed in each of these cases, the arbitrator of these cases dismissed Dr.
Campbell and Mr. Harmon’s actions with prejudice on April 18, 2022 and April 14, 2022, respectively, and the court subsequently
dismissed Dr. Campbell and Mr. Harmon’s actions with prejudice on April 22, 2022 and April 14, 2022, respectively.
COVID-19
Pandemic
The
Company has been impacted by the COVID-19 pandemic and related supply chain shortages and other economic conditions, and some of its
earlier plans to diversify and expand its operations were delayed as a result.
In
addition, the Company is dependent upon certain contract manufacturers and suppliers and their ability to reliably and efficiently fulfill
its orders is critical to the Company’s business success. The COVID-19 pandemic has impacted and may continue to impact certain
of the Company’s manufacturers and suppliers. As a result, the Company has faced and may continue to face delays or difficulty
sourcing certain products, which could negatively affect the Company’s business and financial results.
For
a further discussion of the impact of the COVID-19 pandemic on our business, please see “—Revenue”
and “—Direct Costs of Revenue” under “Management’s Discussion and Analysis of Financial Condition
and Results of Operations – Results of Operations – Comparison of the Years Ended December
31, 2022 and 2021”, and “Risk Factors – Risks Related
to Our Business – The COVID-19 pandemic has adversely impacted the Company’s supply chain and could materially and adversely
affect our ability to conduct clinical trials and engage with our third-party vendors and thereby have a material adverse effect on our
financial results.”
Bankruptcy
or Similar Proceedings
There
has been no bankruptcy, receivership or similar proceeding pertaining to the Company.
Environmental
Regulations
We
do not believe that we are or will become subject to any environmental laws or regulations of the United States, Europe or Asia other
than laws or regulations applicable to U.S. publicly-traded companies in general. However, see “Risk Factors – Risks Related
to Government Regulation – Climate change and increased focus by governments, stockholders and customers on sustainability issues,
including those related to climate change, may have a material adverse effect on our business and operations.” for discussion
of material related risks. While we believe that our products and business activities do not currently violate any laws, any regulatory
changes that impose additional restrictions or requirements on us or on our products or potential customers could adversely affect us
by increasing our operating costs or decreasing demand for our products, which could have a material adverse effect on our results of
operations.
Reorganizations,
Purchase or Sale of Assets
Other
than as described above, there have been no other material reclassifications, mergers, consolidations, purchases or sales of a significant
amount of assets not done in the ordinary course of business pertaining to the Company.
Regulation
The
FDA, European Union competent authorities and comparable regulatory authorities in state and local jurisdictions and in other countries
impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution
of medical device products such as those the Company has developed and is developing. These agencies and federal, state and local entities
regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labelling,
storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export
and import of the Company’s medical device medical devices. To comply with the regulatory requirements in each of the jurisdictions
in which the Company is marketing or seeking to market and subsequently sell its products, the Company is establishing processes and
resources to provide oversight of the development, approval processes and launch (including post market surveillance) of its products
and to position those products in order to gain market share.
We
believe that we are and will continue to be in compliance in all material respects with applicable statutes and the regulations passed
in the United States. There are no current orders or directions relating to our company with respect to the foregoing laws and regulations.
U.S.
Government Regulation
In
the United States, the FDA approves and regulates medical devices under the federal FD&C Act, and its implementing
regulations.
The
process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and
regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements
at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative
or judicial sanctions, such as the FDA’s refusal to approve pending Market Authorizations, withdrawal of an approval, imposition
of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution,
injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The
process required by the FDA before a medical device may be marketed in the United States generally involves the following:
| |
● |
completion
of design control activities (including design verification activities such as pre-clinical laboratory tests, engineering tests,
animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or good laboratory practices
(“GLPs”) regulations and 21 CFR part 820 regulations; |
| |
● |
Submission
to the FDA of an investigational device exemption, or IDE, which must become approved before human clinical trials may begin; |
| |
|
|
| |
● |
approval
by an IRB of the study protocol and informed consent forms for the clinical site before each trial may be initiated. Multiple sites
may necessitate the involvement of multiple IRBs and submissions; |
| |
|
|
| |
● |
performance
of adequate and well-controlled human clinical trials in accordance with good clinical practices (“GCPs”), requirements
to establish the safety and efficacy of the proposed medical device product for each indication; |
| |
|
|
| |
● |
submission
to the FDA of a Marketing Application (510(k), De Novo, Premarket Approval (PMA), etc.) which would include the study reports of
the clinical trials, pre-clinical testing, design verification and validation activities, labeling, etc., as well as other
required sections to be included in the Marketing Authorization; |
| |
|
|
| |
● |
satisfactory
completion of an FDA advisory committee review, if applicable; |
| |
|
|
| |
● |
satisfactory
completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance
with current good manufacturing practices (“cGMPs”) or PAI (Pre-approved Inspection) requirements and to assure that
the facilities, methods and controls are adequate to preserve the medical device’s identity and quality; and |
| |
|
|
| |
● |
FDA
approval of the medical device. |
Pre-clinical
Studies and Clinical Trials for Medical Devices
Pre-clinical
studies include laboratory evaluation of the medical device product’s chemistry, engineering testing, stability, biocompatibility
(including toxicity) and shipping (container closure), as well as animal studies to assess potential safety and efficacy. An IDE sponsor
must submit the results of the pre-clinical tests, together with manufacturing information, testing, data and any available clinical
data or literature, among other things, to the FDA as part of an IDE. Some pre-clinical testing may continue even after the IDE is submitted.
An IDE automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions
related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IDE sponsor and
the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IDE may not result in
the FDA allowing clinical trials to commence.
Clinical
trials involve the use of the investigational device to human subjects pursuant to a clinical protocol, under the supervision of qualified
investigators in accordance with GCPs requirements, which include the requirement that all research subjects provide their informed consent
in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things,
the objectives or endpoints of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated.
A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA under the IDE. In addition, an
IRB (central or at each institution participating in the clinical trial) must review and approve the plan for any clinical trial before
it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National
Institutes of Health, or NIH, for public dissemination on their www.clinicaltrials.gov website.
Progress
reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse
events occur. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including
a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval
of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or
if the medical device has been associated with unexpected serious harm to patients.
FDA
Approval of Medical Devices
The
results of the pre-clinical studies and engineering testing, together with detailed information relating to the product’s composition,
manufacture, quality controls and proposed labeling, among other things, and assuming successful completion of clinical testing (if required)
are submitted to the FDA as part of a market approval application, requesting clearance to market the product for one or more indications.
In most cases, the submission of a market approval application is subject to a substantial application user fee. Under the Medical Device
User Fee Act (“MDUFA”), guidelines that are currently in effect are dependent on type of submission, and typically the FDA
has a goal that ranges between 100 – 300 days from the date of “filing” of a standard market approval application for
the substantive review. This total review typically takes longer from the date of submission because the FDA has approximately 15 days
to make a “filing” decision. Additionally, if during the filing decision or the substantive review the FDA determines a sponsor
must provide additional information (AI), the sponsor has 180 days to provide requested information and during such time, the FDA review
clock is halted.
Before
clearing a market approval application, the FDA typically will inspect the facility or facilities where the product is manufactured.
The FDA will not clear an application unless it determines that the manufacturing processes and facilities are in compliance with cGMPs
requirements and adequate to assure consistent production of the product within required specifications. Additionally, before clearing
a market approval application, the FDA may inspect one or more clinical trial sites to assure compliance with GCPs requirements.
After
evaluating the market approval application and all related information, including the advisory committee recommendation, if any, and
inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue clearance in a form consistent
with the type of application. A complete response letter must contain a statement of specific items that prevent the FDA from approving
the application and will also contain conditions that must be met in order to secure final approval of the market approval application
and may require additional clinical or pre-clinical testing in order for FDA to reconsider the application. Even with submission of this
additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If
and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval
letter authorizes clearance to commercially market the medical device product with specific instructions for use for specific indications.
The
FDA De Novo Classification request provides a marketing process to classify novel medical devices for which no legally marketed predicate
device exists and general controls or general and special controls provide reasonable assurance of safety and effectiveness for the intended
use. The De Novo classification is a risk-based classification process and devices that are classified into Class I or Class II through
a De Novo classification request may be marketed and used as predicates for future premarket notification submissions. With the granting
by the FDA of a De Novo request, the new device is authorized to be marketed in the United States and a new classification regulation
for the device type is established.
A
510(k) application is another premarket submission process made available by the FDA which may be used by itself or in combination with
a De Novo classification request to demonstrate that the device to be marketed is at least as safe and effective (substantially equivalent)
to a legally marketed device that is not otherwise subject to pre-market approval requirements. Submitters under a 510(k) application
must compare their device to one or more similar legally marketed devices (predicates) and make and support their substantial equivalency
claims. Until the submitter receives an order declaring a device substantially equivalent, the submitter may not proceed to market the
device. Once the device is determined to be substantially equivalent, it can then be marketed in the United States.
Even
if the FDA clears a product, it may limit the approved indications for use of the product, require that contraindications, warnings or
precautions be included in the product labeling, require that post-approval studies be conducted to further assess safety after approval,
require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution
and use restrictions or other risk management mechanisms which can materially affect the potential market and profitability of the product.
The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs.
After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional
labeling claims, are subject to further testing requirements and FDA review and approval.
U.S.
Post-Approval Requirements for Medical Devices
Medical
device products manufactured or distributed pursuant to FDA clearance are subject to pervasive and continuing regulation by the FDA,
including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising
and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding
new indications or other labeling claims are subject to prior FDA review and approval. There are also continuing, annual user fee requirements
for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental
applications with clinical data.
The
FDA may impose a number of post-approval requirements as a condition of approval of a MA. For example, the FDA may require post-marketing
testing and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In
addition, medical device manufacturers and other entities involved in the design, manufacture and distribution of approved products are
required to register their establishments with the FDA and state agencies and are subject to periodic unannounced inspections by the
FDA and these state agencies for compliance with cGMPs requirements. Changes to the manufacturing process are strictly regulated and
often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations
from cGMPs requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the
sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality
control to maintain cGMPs compliance.
Once
approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or
if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse
events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may
result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials
to assess new safety risks; or imposition of distribution or other restrictions. Other potential consequences include, but are not limited
to:
| ● | restrictions
on the marketing or manufacturing of the product, complete withdrawal of the product from
the market or product recalls; |
| ● | fines,
warning letters or holds on post-approval clinical trials; |
| ● | refusal
of the FDA to approve pending sponsor MAs or supplements to approved MAs, or suspension or
revocation of product approvals; |
| ● | product
seizure or detention, or refusal to permit the import or export of products; or |
| ● | injunctions
or the imposition of civil or criminal penalties. |
The
FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Devices may be promoted
only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce
the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label
uses may be subject to significant liability. In addition, products, if deemed adulterated, can lead to serious consequences as set forth
above as well as civil and criminal penalties.
U.S.
Medical Regulatory Matters Relating to Medical Devices
Manufacturing,
sales, promotion and other activities of medical devices following product approval, where applicable, or commercialization are also
subject to regulation by numerous regulatory authorities in the United States in addition to the FDA, which may include the Centers for
Medicare & Medicaid Services, or CMS, other divisions of the Department of Health and Human Services, or HHS, the Department of Justice,
the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety &
Health Administration, the Environmental Protection Agency and state and local governments and governmental agencies.
Other
U.S. Healthcare Laws Governing Medical Devices
In
addition to FDA restrictions on marketing of medical devices, other U.S. federal and state healthcare regulatory laws restrict business
practices in the medical industry, which include, but are not limited to, state and federal anti-kickback, false claims, data privacy
and security and physician payment and medical device pricing transparency laws.
The
U.S. federal Anti-Kickback Statute prohibits, among other things, any person or entity from knowingly and willfully offering, paying,
soliciting, receiving or providing any remuneration, directly or indirectly, overtly or covertly, to induce or in return for purchasing,
leasing, ordering, or arranging for or recommending the purchase, lease, or order of any good, facility, item or service reimbursable,
in whole or in part, under Medicare, Medicaid or other federal healthcare programs. The term “remuneration” has been broadly
interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between device manufacturers
on the one hand and prescribers, purchasers, formulary managers and beneficiaries on the other. Although there are a number of statutory
exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn
narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases, or recommendations
may be subject to scrutiny if they do not meet the requirements of a statutory or regulatory exception or safe harbor. Failure to meet
all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal
under the U.S. federal Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based
on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to
mean that if anyone purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the
statute has been violated.
Additionally,
the intent standard under the U.S. federal Anti-Kickback Statute was amended by the ACA to a stricter standard such that a person or
entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
In addition, the ACA codified case law that a claim including items or services resulting from a violation of the U.S. federal Anti-Kickback
Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. The majority of states also have
anti-kickback laws, which establish similar prohibitions and, in some cases, may apply to items or services reimbursed by any third-party
payor, including commercial insurers.
The
federal false claims and civil monetary penalties laws, including the civil False Claims Act, prohibit any person or entity from, among
other things, knowingly presenting, or causing to be presented, a false, fictitious or fraudulent claim for payment to, or approval by,
the federal government, knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent
claim to the federal government, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money
to the U.S. federal government. A claim includes “any request or demand” for money or property presented to the U.S. government.
Actions under the civil False Claims Act may be brought by the Attorney General or as a qui tam action by a private individual in the
name of the government. Violations of the civil False Claims Act can result in very significant monetary penalties and treble damages.
Several healthcare companies have been prosecuted under these laws for, among other things, allegedly providing free product to customers
with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing
false claims to be submitted because of the companies’ marketing of products for unapproved, or off-label, uses. Companies also
have been prosecuted for allegedly violating the Anti-Kickback Statute and False Claims Act as a result of impermissible arrangements
between companies and healthcare practitioners or as a result of the provision of remuneration by the companies to the healthcare practitioners.
In addition, the civil monetary penalties statute imposes penalties against any person who is determined to have presented or caused
to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided
as claimed or is false or fraudulent. Many states also have similar fraud and abuse statutes or regulations that apply to items and services
reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Violations
of fraud and abuse laws, including federal and state anti-kickback and false claims laws, may be punishable by criminal and civil sanctions,
including fines and civil monetary penalties, the possibility of exclusion from federal healthcare programs (including Medicare and Medicaid),
disgorgement and corporate integrity agreements, which impose, among other things, rigorous operational and monitoring requirements on
companies. Similar sanctions and penalties, as well as imprisonment, also can be imposed upon executive officers and employees of such
companies. Given the significant size of actual and potential settlements, it is expected that the government authorities will continue
to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud
and abuse laws.
The
federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created additional federal criminal statutes that prohibit,
among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program,
including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing
a criminal investigation of a healthcare offense and knowingly and willfully falsifying, concealing or covering up a material fact or
making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits,
items or services. Similar to the U.S. federal Anti-Kickback Statute, the ACA broadened the reach of certain criminal healthcare fraud
statutes created under HIPAA by amending the intent requirement such that a person or entity does not need to have actual knowledge of
the statute or specific intent to violate it in order to have committed a violation.
In
addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and certain other healthcare
providers. The ACA imposed, among other things, new annual reporting requirements through the Physician Payments Sunshine Act for covered
manufacturers for certain payments and “transfers of value” provided to physicians and teaching hospitals, as well as ownership
and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the
required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties
of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Covered
manufacturers must submit reports by the 90th day of each subsequent calendar year. In addition, certain states require the implementation
of compliance programs and compliance with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance
guidance promulgated by the federal government, impose restrictions on marketing practices and/or tracking and reporting of gifts, compensation
and other remuneration or items of value provided to physicians and other healthcare professionals and entities.
The
Company may also be subject to data privacy and security regulation by both the federal government and the states in which the Company
conducts its business. HIPAA, as amended by HITECH, and their respective implementing regulations, including the Final HIPAA Omnibus
Rule, published on January 25, 2013, impose specified requirements relating to the privacy, security and transmission of individually
identifiable health information held by covered entities and their business associates. Among other things, HITECH made HIPAA’s
security standards directly applicable to “business associates,” defined as independent contractors or agents of covered
entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf
of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates
and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal
courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In
addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other
in significant ways and may not have the same requirements, thus complicating compliance efforts. In the EU, similar privacy requirements
have been implemented under EU Law General Data Protection Regulation (GDPR 2016/679). These requirements include provisions related
to the processing of personal data of individuals within the EEA and also addresses the transfer of personal data outside the EU and
EEA areas.
Coverage
and Reimbursement
Significant
uncertainty exists as to the coverage and reimbursement status of any products for which the Company obtains regulatory approval. In
the United States and markets in other countries, patients who are prescribed treatments for their conditions and providers performing
the prescribed services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are
unlikely to use the Company’s products unless coverage is provided and reimbursement is adequate to cover a significant portion
of the cost of the Company’s products. Sales of any products for which the Company receives regulatory approval for commercial
sale will therefore depend, in part, on the availability of coverage and adequate reimbursement from third-party payors. Third-party
payors include government authorities, managed care plans, private health insurers and other organizations. In the United States, the
process for determining whether a third-party payor will provide coverage for a product typically is separate from the process for setting
the price of such product or for establishing the reimbursement rate that the payor will pay for the product once coverage is approved.
Third-party payors may limit coverage to specific products on an approved list, which might not include all of the FDA-approved products
for a particular indication. A decision by a third-party payor not to cover the Company’s medical devices could reduce physician
utilization of the Company’s products once approved and have a material adverse effect on the Company’s sales, results of
operations and financial condition. Moreover, a third-party payor’s decision to provide coverage for a product does not imply that
an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable the Company to maintain
price levels sufficient to realize an appropriate return on the Company’s investment in product development. Additionally, coverage
and reimbursement for products can differ significantly from payor to payor. One third-party payor’s decision to cover a particular
product or service does not ensure that other payors will also provide coverage for the product or service or will provide coverage at
an adequate reimbursement rate. As a result, the coverage determination process will require the Company to provide scientific and clinical
support for the use of the Company’s products to each payor separately and will be a time-consuming process.
In
the EEA, governments influence the price of products through their pricing and reimbursement rules and control of national health care
systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems
under which products may only be marketed once a reimbursement price has been agreed to by the government. To obtain reimbursement or
pricing approval, some of these countries may require the completion of clinical trials that compare the cost effectiveness of a particular
medical device to currently available therapies. Other member states allow companies to fix their own prices for medicines but monitor
and control company profits. The downward pressure on health care costs in general, particularly prescription products, has become very
intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross
border imports from low-priced markets exert commercial pressure on pricing within a country.
The
containment of healthcare costs has become a priority of federal, state and foreign governments, and the prices of products have been
a focus in this effort. Third-party payors are increasingly challenging the prices charged for medical products, examining the medical
necessity and reviewing the cost-effectiveness of medical products, in addition to questioning safety and efficacy. If these third-party
payors do not consider the Company’s products to be cost-effective compared to other available therapies, they may not cover the
Company’s products after regulatory approval or clearance, or if they do, the level of payment may not be sufficient to allow the
Company to sell its products at a profit.
Healthcare
Reform
A
primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and other third-party payors
have attempted to control costs by limiting coverage and the amount of reimbursement for particular medical products. For example, in
March 2010, the ACA was enacted, which, among other things, increased the minimum Medicaid rebates owed by most manufacturers; created
a new Patient Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness
research, along with funding for such research; creation of the Independent Payment Advisory Board, once empaneled, will have authority
to recommend certain changes to the Medicare program that includes establishment of a Center for Medicare Innovation at the CMS to test
innovative payment and service delivery models to lower Medicare and Medicaid spending. Since its enactment, the U.S. federal government
has delayed or suspended the implementation of certain provisions of the ACA.
The
Company expects that the ACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous
coverage criteria and lower reimbursement and additional downward pressure on the price that the Company receives for any approved product.
Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private
payors. Moreover, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their
marketed products. The implementation of cost containment measures or other healthcare reforms may prevent the Company from being able
to generate revenue, attain profitability or commercialize the Company’s drugs and medical devices.
Additionally,
on August 2, 2011, the Budget Control Act of 2011 created measures for spending reductions by Congress. A Joint Select Committee on Deficit
Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable
to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This included
aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013, and due to subsequent
legislative amendments to the statute, will stay in effect through 2025 unless additional action is taken by Congress. On January 2,
2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several
types of providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period
for the government to recover overpayments to providers from three to five years. More recently, there has been heightened governmental
scrutiny recently over the manner in which manufacturers set prices for their marketed products, which have resulted in several recent
Congressional inquiries and proposed bills designed to, among other things, bring more transparency to product pricing, review the relationship
between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products.
Since
its enactment, there have been judicial, executive and Congressional challenges to certain aspects of the ACA. On June 17, 2021, the
U.S. Supreme Court dismissed the most recent judicial challenge to the ACA brought by several states without specifically ruling on the
constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special
enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA
marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules
that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include
work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid. It
is unclear how other healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace
the ACA will impact our business.
Other
legislative changes have been proposed and adopted since the ACA was enacted. For example, on March 11, 2021, President Biden signed
the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s
average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. Further, in August 2011,
the Budget Control Act of 2011, among other things, included aggregate reductions of Medicare payments to providers of 2% per fiscal
year. These reductions went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect
through 2030, with the exception of a temporary suspension from May 1, 2020 through December 31, 2021, unless additional action is taken
by Congress.
Further,
on May 30, 2018, the Right to Try Act was signed into law. The law, among other things, provides a federal framework for certain patients
to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation
for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without
obtaining FDA permission under the FDA expanded access program. There is no obligation for a pharmaceutical manufacturer to make its
drug products available to eligible patients as a result of the Right to Try Act.
Moreover,
there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products,
which has resulted in several Congressional inquiries, proposed and enacted legislation and executive orders designed to, among other
things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform
government program reimbursement methodologies for drug products. For example, at a federal level, President Biden signed an Executive
Order on July 9, 2021 affirming the administration’s policy to (i) support legislative reforms that would lower the prices of prescription
drugs and biologics, including by allowing Medicare to negotiate drug prices, by imposing inflation caps, and, by supporting the development
and market entry of lower-cost generic drugs and biosimilars; and (ii) support the enactment of a public health insurance option. Among
other things, the Executive Order also directs the HHS to provide a report on actions to combat excessive pricing of prescription drugs,
enhance the domestic drug supply chain, reduce the price that the Federal government pays for drugs, and address price gouging in the
industry; and directs the FDA to work with states and Indian Tribes that propose to develop section 804 Importation Programs in accordance
with the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, and the FDA’s implementing regulations. It is
also possible that additional governmental action is taken in response to the COVID-19 pandemic. Individual states in the United States
have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price
or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency
measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
We
expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could impact the amounts
that federal and state governments and other third-party payors will pay for healthcare products and services.
U.S.
Data Privacy and Security Laws
Numerous
state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access
to, confidentiality, and security of personal information, including health-related information. In the United States, numerous federal
and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA
and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act) that govern the
collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations
of our partners. In addition, certain state and non-U.S. laws, such as the California Consumer Privacy Act, the California Privacy Rights
Act, Australia’s Privacy Act 1988, as amended, and the General Data Protection Regulation, or GDPR, govern the privacy and security
of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and
many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Effective
January 1, 2023, we also became subject to the California Privacy Rights Act, which expands upon the consumer data use restrictions,
penalties and enforcement provisions under the California Consumer Privacy Act, and Virginia’s Consumer Data Protection Act, another
comprehensive data privacy law. In addition, effective July 1, 2023, we may also be subject to the Colorado Privacy
Act and Connecticut’s An Act Concerning Personal Data Privacy and Online Monitoring and regulations promulgated under these
laws, which are also comprehensive consumer privacy laws. Effective December 31, 2023, we may also become subject to the Utah
Consumer Privacy Act, regarding business handling of consumers’ personal data. Effective January 1, 2025, we may also become subject to the Iowa Consumer Privacy Act, a similar consumer data privacy
law. Further, there are several legislative proposals in the United States, at both the federal and state level, that could impose new
privacy and security obligations. Failure to comply with these laws, where
applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security
laws, regulations, and other obligations are constantly evolving, may conflict with each other to make compliance efforts more challenging,
and can result in investigations, proceedings, or actions that lead to significant penalties and restrictions on data processing.
Foreign
Government Regulation
In
order to market the Company’s products in the EEA (which is comprised of the 27 Member States of the EU plus Norway, Iceland and
Liechtenstein) and many other foreign jurisdictions (e.g., in Europe, the UK and Switzerland), a sponsor must obtain separate regulatory
approvals. For example, in the EEA, medical device products can only be commercialized after obtaining a Marketing Authorization, or
MA. As of May 26, 2021, new Marketing Authorizations in the EU must meet the requirements of Regulation (EU) 2017/745. The activities
associated with MA approval are conducted by authorized Notified Bodies (NB) on behalf of the EU competent authorities. Before granting
the MA, the NB makes an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality,
safety and efficacy. In order to make this determination, a sponsor must submit an application for approval.
To
the extent that any of the Company’s medical devices are to be approved and sold in a foreign country other than those countries
comprising the EEA or other countries that accept CE marking, the Company may be subject to similar laws and regulations, which may include,
for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws and implementation of
corporate compliance programs and reporting of payments or other transfers of value to healthcare professionals.
MANAGEMENT
Directors
and Executive Officers
Set
forth below is information regarding our directors and executive officers.
| Name |
|
Age |
|
Position |
| David
Barthel |
|
66 |
|
Chief
Executive Officer, Secretary and Director |
| Dr.
Vithalbhai Dhaduk |
|
70 |
|
Chairman
of the Board and Director |
| George
Kovalyov |
|
38 |
|
Chief
Financial Officer and Treasurer |
| Harrison
Ross |
|
32 |
|
Vice
President of Finance |
| Dr.
Steven Brooks |
|
53 |
|
Chief
Medical Officer |
| Dr.
Catherine Pachuk |
|
66 |
|
Chief
Scientific Officer and Executive Vice President |
| Terry
Brostowin |
|
64 |
|
Director
|
| Dr.
William Hearl |
|
66 |
|
Director
|
| Julie
Kampf |
|
62 |
|
Director
|
| Dr.
Nilesh Patel |
|
59 |
|
Director |
| Michael
Stewart |
|
66 |
|
Director |
David
Barthel. Mr. Barthel has served as our Chief Executive Officer since November 2021, as a director since December 2021, and as
our Secretary since October 2022. From March 2021 to November 2021, Mr. Barthel was Chief Executive Officer of HLII and its former wholly-owned
subsidiary, My Health Logic, a company focused on developing an innovative point-of-care lab-on-chip digital diagnostic device technologies
for CKD. My Health Logic Inc., which holds the MATLOC device technology and all accompanying agreements, was acquired by Marizyme in
December 2021. From July 2019 to February 2021, Mr. Barthel was Managing Director at an affiliate company of Henry Schein, Inc. (Nasdaq:
HSIC). Mr. Barthel founded The SmartPill Corp. in 2002 and led the company as CEO and President until its acquisition in October 2012
by medical device giant, Medtronic plc (Nasdaq: MDT). After the acquisition, Mr. Barthel served as Area Vice President, Southeast Division,
at an affiliate company of Medtronic until July 2019. Mr. Barthel earned a Bachelor of Arts Degree from St. Norbert College in De Pere,
Wisconsin, and an MBA from Lake Forest Graduate School of Management in Lake Forest, Illinois. Our board of directors believes that Mr.
Barthel is qualified to serve as a member of the board of directors due to his 25 years of executive leadership of early-stage and large
corporations in the healthcare and medical devices industries.
Dr.
Vithalbhai Dhaduk. Dr. Dhaduk has been a director since February 2021, and as Chairman since June 2021. From July 2021 to November
2021, Dr. Dhaduk was Interim Chief Executive Officer. Upon Mr. Barthel’s appointment as Chief Executive Officer in November 2021,
Dr. Dhaduk resigned his position as Interim Chief Executive Officer. Dr. Dhaduk also serves as the chairman of our Nominating and Corporate
Governance Committee. Dr. Dhaduk currently serves as President and Chairman at Professional Neurological Associates (since 1987), Chairman
of Dap Dhaduk 1 to 8 (since 1998), Chairman of Caritas International Trading Inc. (since 2011), President and Chairman of Caritas Real
Estate Group (since 2011), President and Chairman of Core Hospitality LLC (since 2011), President and Chairman of Star Real Estate LC
(since 2012), President and Chairman of Coracias Advanced Technology LLC (since 2016), a directors of The Wright Center (since 2017),
and President and Chairman of CorePharma, LLC (since 2018). Previously, Dr. Dhaduk had served as Chairman of Global Pharma Analytics
(2012 – 2019), as President and Chairman of Somahlution, LLC (2012 – 2019), President and Chairman of Apicore LLC (2005 –
2016), President and Chairman of R&D Future Aire Tech (2011 – 2013), President and Chairman of Neuron Biotech (2007 –
2013), President and Chairman of Synerx Pharmaceutical (2007 – 2013), and as a director on the board of directors at FNCB Bancorp,
Inc. (Nasdaq: FNCB). Dr. Dhaduk is a managing trustee of charitable trust and past President and Chairman of Saurashtra Patel Cultural
Samaj. Dr. Dhaduk received a Bachelor of Medicine and Bachelor of Surgery in 1980 from M.P. Shah Medical College in Jamngar, India. Dr.
Dhaduk has a Pennsylvania and New Jersey medical licenses (both since 1985), PGY-1 waiver on basis of excellence in post-graduation from
the Board of Psychiatry and Neurology (1984), FLEX (Federal Licensing Examination (1984) and ECFMG Certification (1981). Dr. Dhaduk has
received the following awards: David Dunn Memorial Award for Outstanding Teaching and Study of Neurology, Medical College of Pennsylvania
(1986 – 1987) and Honors in Medicine, M.P. Shah Medical College, India (1980). Our board of directors believes that Dr. Dhaduk
is qualified to serve as a member of the board due to his experience as a director on various private company boards, and his more than
35 years in the medical profession and as a business owner.
George
Kovalyov. Mr. Kovalyov has been our Chief Financial Officer and Treasurer since December 2021. Since November 2022, Mr. Kovalyov
has been a director of DGTL Holdings Inc. (TSXV:DGTL) (OTCQB:DGTHF). Previously he served as the chief operating officer and director
of HLII from September 2020 to November 2021, and as HLII’s chief financial officer from December 2021 to September 2022. In addition,
Mr. Kovalyov served as a director and audit committee member of Margaret Lake Diamonds Inc. (TSXV:DIA) from January 2021 to August 2022.
From September 2018 to September 2020, Mr. Kovalyov was VP of Finance and director of Phivida Holdings Inc. (CSE:VIDA). From October
2016 to September 2020, he was the principal owner of Schindler and Company, an accounting consulting firm. Mr. Kovalyov is a chartered
accountant and is a member of Chartered Professional Accountants of Canada. He graduated from Kwantlen University College with a Bachelor
of Business Administration (BBA), Accounting.
Harrison
Ross. Mr. Ross has been our Vice President of Finance since December 2021. Previously he had served as the chief financial officer
of HLII from July 2020 to November 2021, and has served as HLII’s chief executive officer and director since December 2021. In
addition, Mr. Ross has served as a director of Duncan Ross Associates Ltd since June 2023 and a director of Cypher Metaverse Inc. (formerly
Codebase Ventures Inc.) (CSE:CODE) (FSE:C5B) (OTCQB:BKLLF) since October 2021. From November 2017 to October 2020, he was CFO of DC Acquisition
Corp., a Canadian Capital Pool Company that completed a reverse takeover of a Canada-focused retail company that currently trades on
the Toronto Stock Exchange. Mr. Ross was simultaneously working part-time for the family-owned company Graymont Limited from January
2017 to December 2019 and before that was an equity analyst at the investment-management firm Duncan Ross and Associates from June 2016
to January 2018. In 2017, Mr. Ross became a CFA Charterholder and in 2013 Mr. Ross received his bachelor’s degree from the University
of Western Ontario in Business and Organizational Studies with a specialization in accounting.
Dr.
Steven Brooks. Dr. Brooks has been our Chief Medical Officer since December 2020. Since June 2014, Dr. Brooks has been the principal
of Brooks Medtech, LLC, a medical technology consulting firm. Since January 2021, Dr. Brooks has also been Senior Vice President of Clinical
Affairs of Vita Therapeutics, LLC, a biotechnology company. Dr. Brooks received his MD degree at the University of Pittsburgh School
of Medicine, and Residency, Cardiology and Interventional Cardiology Fellowships at University of Pittsburgh Medical Center. He received
his MBA from the Johns Hopkins Carey School of Business in the Business of Medicine Program, and a B.A at Duke University.
Dr.
Catherine Pachuk. Dr. Pachuk has been our Chief Scientific Officer and Executive Vice President since July 2020. Prior to our
acquisition of the Somahlution Assets in July 2020, Dr. Pachuk had been Somahlution’s Chief Scientific Officer and Executive Vice
President since June 2011. Dr. Pachuk is currently a Scientific Advisory Board member for Ocugen Inc. (NASDAQ: OCGN) where Dr. Pachuk
advises on the development of a COVID-19 vaccine and is currently an invited expert curator for the American Society of Microbiology’s
COVID-19 research registry. Dr. Pachuk is also an adjunct faculty member at both Florida Atlantic University and Baruch Blumberg Institute.
Dr. Pachuk also has a dual Regulatory Affairs Certificate from RAPS (Regulatory Affairs Professional Society) in Medical Devices and
Pharmaceuticals. Dr. Pachuk received her Ph.D. in molecular virology from the University of Pennsylvania where she studied the molecular
biology of coronaviruses, and her BS in Biology from Marywood College.
Terry
Brostowin. Mr. Brostowin has been a member of our board of directors since December 2018. Mr. Brostowin also is a member of its
Audit Committee and Compensation Committee. Mr. Brostowin is an attorney admitted to the Federal Courts in both the Eastern and Southern
Districts of New York and the District of New Jersey. Mr. Brostowin has been President of Brostowin & Associates, PC, since December
2016. Mr. Brostowin has been affiliated with the law firm Brostowin & Associates, PC, since 2009. Mr. Brostowin received his BA in
Education from the State University of New York at Cortland and JD from Touro Law School. Our board of directors believes that Mr. Brostowin
is qualified to serve as a member of the board due to his experience as a practicing attorney for almost 25 years.
Dr.
William Hearl. Dr. Hearl has been a member of our board of directors since October 2020. Dr. Hearl also serves as a member of
the Company’s Audit Committee, Compensation Committee and its Nominating and Corporate Governance Committee. Dr. Hearl is the founder
of Immunomic Therapeutics, Inc. and has served as its chief executive officer, chairman, and president since January 2006. Dr. Hearl
received his Ph.D. in biochemistry from the University of Tennessee and B.S. from East Tennessee State University. Our board of directors
believes that Dr. Hearl is qualified to serve as a member of the board due to his experience as a biotech / biopharma executive having
successfully guided Immuomic Therapeutics to profitability over the last 15 years.
Julie
Kampf. Ms. Kampf has been a member of our board of directors since February 2021. Ms. Kampf serves as chairman of the Company’s
Compensation Committee and also serves as a member of the Nominating and Corporate Governance Committee. Ms. Kampf is currently a director
and the Chief Executive Officer of JBK Associates International, an executive search firm focused on the life science industry, which
she founded in 2003. Since April 2022, Ms. Kampf has also been a director of EOM Pharmaceutical Holdings, Inc. (OTC PINK: IMUC). Since
September 2021, Ms. Kampf has been a director of Jupiter Neurosciences, Inc., which has made filings with the SEC for a proposed underwritten
public offering and listing on Nasdaq under the ticker symbol “JUNS”. Ms. Kampf earned a BA in Political Science from the
University of Rhode Island, where she minored in Marketing. Our board of directors believes that Ms. Kampf is qualified to serve as a
member of the board due to her experience holding executive committee positions on non-profit boards.
Dr.
Nilesh Patel. Dr. Patel has been a member of our board of directors since April 2022. Dr. Patel has been a practicing physician
with and Chairman of Advanced Cardiovascular Specialists LLC since August 2013. Our board of directors believes that Dr. Patel is qualified
to serve as a member of the board due to his experience as a director, ten years of practice in the medical profession, and as a business
owner.
Mr. Michael Stewart. Mr. Stewart has been a member of our board of directors since June 2022. Mr. Stewart also serves as
chairman of the Company’s Audit Committee. Mr. Stewart has served as the Chief Operating Officer and a director of CyDuct, LLC,
a bioinformatics startup, since January 2018. Since May 2022, Mr. Stewart has also served as a director of The Lotus Group Inc., doing
business as GlobalMed Technologies, a health technology company. Since November 2016, Mr. Stewart has also been a consultant for various
clients. From June 2018 to April 2019, Mr. Stewart was a consultant and director of Gadsden Properties, Inc., formerly known as FC Global
Realty Incorporated, a formerly-public real estate development company. From May 2017 to June 2018, Mr. Stewart was the Chief Executive
Officer, Chief Financial Officer, the chairman of the Audit Committee and a member of the Compensation Committee and Governance Committee
of Gadsden Properties. From December 2014 to October 2016, Mr. Stewart was the Chief Executive Officer, President and a director of Strata
Skin Sciences, Inc. (Nasdaq: SSKN), a public medical devices company. From December 2002 to December 2014, Mr. Stewart was Executive
Vice President and Chief Operating Officer of PhotoMedex, Inc., a formerly-public medical device company, now known as Gadsden Properties,
Inc. From October 1999 to December 2002, Mr. Stewart was Chief Executive Officer and President and a director of Surgical Laser Technologies,
Inc., a medical devices company. From October 1990 to December 1999, Mr. Stewart was Vice President and Chief Financial Officer of Surgical
Laser Technologies. From July 1983 to October 1990, Mr. Stewart was a financial manager at Decision One Corporation, a computer products
company. From July 1980 to July 1983, Mr. Stewart was an accountant at Texaco, Inc., now owned by Chevron Corporation (NYSE: CVX), a
public energy company. Mr. Stewart earned a Bachelor of Science degree in Accounting and a Master of Business Administration in Finance
from La Salle University. The board of directors believes that Mr. Stewart is qualified to serve as a member of the board due to his
many years of executive leadership at early-stage and public companies in the healthcare and medical devices industries and financial
expertise.
Family
Relationships
There
are no family relationships between or among any of our current directors, executive officers or persons nominated or charged by the
Company to become directors or executive officers. There are no family relationships among our executive officers and directors and the
executive officers and directors of our direct and indirect subsidiaries.
Arrangements
Between Officers and Directors
Directors
and executive officers are elected until their successors are duly elected and qualified. There are no agreements, arrangements or understandings
known to us pursuant to which any director or executive officer was or is to be selected as a director (or director nominee) or executive
officer.
Involvement
in Certain Legal Proceedings
None
of our directors or executive officers has, during the past ten years:
|
● |
been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor
offences); |
| ● | had
any bankruptcy petition filed by or against the business or property of the person, or of
any partnership, corporation or business association of which he was a general partner or
executive officer, either at the time of the bankruptcy filing or within two years prior
to that time; |
| | | |
| ● | been
subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated,
of any court of competent jurisdiction or federal or state authority, permanently or temporarily
enjoining, barring, suspending or otherwise limiting, his involvement in any type of business,
securities, futures, commodities, investment, banking, savings and loan, or insurance activities,
or to be associated with persons engaged in any such activity; |
| | | |
| ● | been
found by a court of competent jurisdiction in a civil action or by the Securities and Exchange
Commission or the Commodity Futures Trading Commission to have violated a federal or state
securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| | | |
| ● | been
the subject of, or a party to, any federal or state judicial or administrative order, judgment,
decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement
of a civil proceeding among private litigants), relating to an alleged violation of any federal
or state securities or commodities law or regulation, any law or regulation respecting financial
institutions or insurance companies including, but not limited to, a temporary or permanent
injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent
cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting
mail or wire fraud or fraud in connection with any business entity; or |
| | | |
| ● | been
the subject of, or a party to, any sanction or order, not subsequently reversed, suspended
or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange
Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the
Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity
or organization that has disciplinary authority over its members or persons associated with
a member. |
Independent
Directors
Nasdaq’s
rules would generally require that a majority of the Company’s board of directors consist of independent directors. Our board of
directors currently consists of seven members: David Barthel, Dr. Vithalbhai Dhaduk, Dr. William Hearl, Julie Kampf, Terry Brostowin,
Dr. Nilesh Patel, and Michael Stewart. Our board of directors has determined that Dr. Dhaduk, Dr. Hearl, Ms. Kampf, Mr. Brostowin, Dr.
Patel, and Mr. Stewart are independent directors as that term is defined in Nasdaq Listing Rule 5605(a)(2). Each director serves until
our next annual meeting or until his or her successor is duly elected and qualified.
Committees
of the Board of Directors
Our
board of directors has the authority to appoint committees to perform certain management and administration functions. On January 26,
2022, the board restructured its committees and established or reestablished an Audit Committee, a Compensation Committee, and a Nominating
and Corporate Governance Committee, and also formed a Pricing Committee. The board also adopted a committee charter for the Audit Committee,
the Compensation Committee, and the Nominating and Corporate Governance Committee. The committee charters are available on our website
at https://www.marizyme.com.
In
addition, our board of directors may, from time to time, designate one or more additional committees, which shall have the duties and
powers granted to it by our board of directors.
Audit
Committee
Mr.
Stewart, Mr. Brostowin, and Dr. Hearl, each of whom satisfies the “independence” requirements of Rule 10A-3 under the Exchange
Act and Nasdaq’s rules, were selected to serve on our Audit Committee, with Mr. Stewart serving as the chairman. Mr. Stewart has
been determined by our board to be qualified as an “audit committee financial expert” as defined under Item 407(d)(5)(ii)
and (iii) of Regulation S-K.
Compensation
Committee
Ms.
Kampf, Mr. Brostowin, and Dr. Hearl, each of whom satisfies the “independence” requirements of Rule 10C-1 under the Exchange
Act and the Nasdaq Listing Rules, serve on our compensation committee, with Ms. Kampf serving as the chairman. The members of the compensation
committee are also “Non-Employee Directors” within the meaning of Section 16 of the Exchange Act.
Nominating
and Corporate Governance Committee
Dr.
Dhaduk, Dr. Hearl and Ms. Kampf, each of whom satisfies the “independence” requirements of the Nasdaq Listing Rules, serve
on our Nominating and Corporate Governance Committee, with Dr. Dhaduk serving as the chairman.
EXECUTIVE
COMPENSATION
Summary
Compensation Table of Named Executive Officers - Years Ended December 31, 2022 and 2021
The
following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to all individuals
who served as the Company’s principal executive officer or acting in similar capacity during the last two completed fiscal years,
regardless of compensation level, and other individuals as required by Item 402(m)(2) of Regulation S-K, during the noted periods.
| Name and Principal Position | |
Year | | |
Salary
($) | | |
Bonus
($) | | |
Stock Awards
($) | | |
Option Awards
($) | | |
Nonequity Incentive Plan Compensation
($) | | |
Non- qualified Deferred Compensation Earnings
($) | | |
All Other Compensation
($) | | |
Total
($) | |
| David Barthel, | |
| 2022 | | |
$ | 350,000 | | |
$ | - | | |
$ | - | | |
$ | - | (1) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 350,000 | |
| Chief Executive Officer | |
| 2021 | | |
$ | 58,336 | | |
$ | - | | |
$ | - | | |
$ | 503,997 | (2) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 562,333 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Dr. Catherine Pachuk, | |
| 2022 | | |
$ | 325,000 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 325,000 | |
| Chief Scientific Officer and Executive Vice President | |
| 2021 | | |
$ | 298,750 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 298,750 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Dr. Steven Brooks, | |
| 2022 | | |
$ | 300,000 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 300,000 | |
| Chief Medical Officer and Executive Vice President of Medical and Regulatory Affairs | |
| 2021 | | |
$ | 300,000 | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 300,000 | |
(1)
See footnote (2) below. On February 8, 2022, an outstanding option granted to David Barthel to purchase 400,000 shares of common stock
at an exercise price of $2.25 was amended, resulting in the deemed cancellation of the original option, and the grant of a replacement
option for the same number of shares at an exercise price of $1.75 per share. The incremental fair value, computed as of the repricing
or modification date in accordance with FASB ASC Topic 718, with respect to that repriced or modified award, was computed based on the
assumptions described in “Note 1 – Summary of Significant Accounting Policies – Fair Value Measurements –
Stock-Based Compensation” to the Company’s audited financial statements included with this prospectus.
(2)
David Barthel was granted an option to purchase 400,000 shares of common stock at an exercise price of $2.25 per share, subject to vesting
conditions, on October 31, 2021. The grant date fair value was computed in accordance with FASB ASC Topic 718 based on the assumptions
described in “Note 1 – Summary of Significant Accounting Policies – Fair Value Measurements – Stock-Based
Compensation” to the Company’s audited financial statements included with this prospectus.
Outstanding
Equity Awards at Fiscal Year-End of Named Executive Officers
As
of December 31, 2022, the following named executive officers had the following unexercised options, stock that has not vested, and equity
incentive plan awards:
| Option Awards | |
Stock Awards | |
| Name | |
Number of Securities Underlying Unexercised Options (#) Exercisable | | |
Number of Securities
Underlying
Unexercised
Options (#)
Unexercisable | | |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | | |
Option Exercise Price ($) | | |
Option Expiration Date | | |
Number of Shares or Units of Stock Not Vested (#) | | |
Market Value of Shares or Units of Stock That Have Not Vested ($) | | |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | | |
Equity
Incentive
Plan Awards: Market or Payout Value of
Unearned
Shares, Units or Other Rights That Have Not Vested ($) | |
| David Barthel, Chief Executive Officer | |
| 160,000 | (1) | |
| 240,000 | (1) | |
| - | | |
$ | 1.75 | | |
| 10/31/2031 | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Dr. Catherine Pachuk, Chief Scientific Officer and Executive Vice President | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Dr. Steven Brooks, Chief Medical Officer and Executive Vice President of Medical and Regulatory Affairs | |
| 26,666 | (2) | |
| 13,334 | (2) | |
| - | | |
$ | 1.25 | | |
| 11/30/2030 | | |
| - | | |
| - | | |
| - | | |
| - | |
(1)
David Barthel was granted an option to purchase 400,000 shares of common stock on October 31, 2021. The option vested as to 40,000 shares
upon grant, and the balance of 360,000 shares will vest quarterly over a three-year period in equal 30,000-share installments.
(2)
Dr. Steven Brooks was granted an option to purchase 40,000 shares of common stock on December 1, 2020. The option is subject to vesting
in quarterly equal installments over three years.
Executive
Compensation Agreements
David
Barthel
On
October 31, 2021, the Company entered into an executive employment agreement (the “CEO Employment Agreement”), with Mr. Barthel
setting forth the terms of the compensation for his services as Chief Executive Officer of the Company. Pursuant to the CEO Employment
Agreement, Mr. Barthel is entitled to an annual fixed gross salary of $350,000. Upon execution of the CEO Employment Agreement, Mr. Barthel
received incentive stock options, or ISOs, to purchase 400,000 shares of common stock at the agreed upon fair market price of $2.25 per
share. On February 8, 2022, the board of directors determined to approve an amendment to the CEO Employment Agreement to provide that
the fair market price of the ISOs be amended to $1.75 per share of common stock. The board of directors made this determination in part
based on recent arms’-length transactions with certain private placement investors in the Units Private Placement, in which such
investors purchased units comprised of a secured Convertible Note, convertible into common stock at a price per share of $1.75, subject
to adjustment, as well as a Class C Warrant to purchase two shares of common stock with an exercise price equal to the lower of (i) $2.25
per share, subject to adjustment, or (ii) 75% of the cash price per share to be paid by the purchasers in a Qualified Financing. In addition,
Mr. Barthel will be eligible to receive certain equity in the form of ISOs of the Company based upon the attainment of certain metrics,
as follows: Nasdaq listing – 100,000 ISOs; FDA approval of DuraGraft – 75,000 ISOs; FDA approval of MATLOC – 75,000
ISOs; Material 3rd Party Partnerships for DuraGraft – 50,000 ISOs; and Material 3rd Party Partnerships for
MATLOC 1 – 50,000 ISOs. All ISOs listed above will have 10% vesting upon grant and the balance will vest quarterly over a three-year
period in equal installments. Mr. Barthel must be employed with the Company in good standing at the time any such ISOs are awarded according
to the milestones set forth above. In the event that Mr. Barthel’s employment is terminated prior to any milestone being achieved,
he will forfeit his right to receive such ISOs. Mr. Barthel is also eligible to participate in all employee benefit plans, including
health insurance, commensurate with his position. Mr. Barthel will receive $3,000 per month to cover healthcare and related expenses.
Mr. Barthel’s employment is at-will and may be terminated by him or by the Company at any time. Through October 31, 2022, if the
Company had terminated Mr. Barthel’s employment with the Company without Cause (as defined in the CEO Employment Agreement), and
if Mr. Barthel had executed a full release in favor of the Company (in a form acceptable to the Company, which would have included non-competition,
non-solicitation, and non-disparagement provisions), the Company would only have been required to pay Mr. Barthel the total sum equal
to six months of his gross annual salary. Beginning November 1, 2022, if the Company terminates Mr. Barthel’s employment with the
Company without Cause, and if Mr. Barthel executes a full release in favor of the Company, the Company will only pay Mr. Barthel the
total sum equal to 12 months of his annual gross salary. In the event of a termination with Cause, or in the event of a resignation by
Mr. Barthel, Mr. Barthel will not be entitled to any separation payment or any other post-termination severance. The CEO Employment Agreement
contains customary confidentiality provisions and restrictive covenants prohibiting Mr. Barthel from (i) owning or operating a business
that competes with the Company during the term of his employment and for a period of 18 months following the termination of his employment
or (ii) soliciting the Company’s employees for a period of 18 months following the termination of his employment.
On
August 22, 2022, Mr. Barthel executed a limited waiver and consent agreement waiving his option exercise rights. The limited waiver and
consent agreement was to expire on December 31, 2022, or if earlier to occur, upon the approval of an increase in the number of the authorized
shares of common stock under the Company’s articles of incorporation by the Company’s stockholders by majority written consent
or via stockholder vote at the Company’s next stockholder meeting and the implementation of such increase, which were to occur
as soon as practicable after such stockholder approval. On December 27, 2022, we held the Annual Meeting. At the Annual Meeting, stockholders
holding shares of common stock in the Company representing at least a majority of the voting power approved, among other matters, the
Authorized Capital Increase. As a result, the limited waiver and consent agreement expired in accordance with its terms.
George
Kovalyov
On
December 21, 2021, the Company entered into a consulting agreement (the “CFO Consulting Agreement”), with George Kovalyov,
its Chief Financial Officer and Treasurer, and AAT Services Inc., a corporation incorporated pursuant to the laws of British Columbia,
Canada and, together with Mr. Kovalyov, the “CFO Consultant”. Under the CFO Consulting Agreement, the Company engaged the
CFO Consultant for a 12-month term to provide such services as the Company may from time to time request, including, without limiting
the generality of the foregoing, the following: Proper filing with the SEC of quarterly and annual reports; budgeting, allocation of
capital, and payroll processes; assistance in the preparation of a Form S-1 and other Nasdaq listing preparations; assistance with corporate
governance creations and procedures; the creation of valuation reports for the Company’s products and medical devices; preparation
of investment material for current and prospective stockholders; general business development; and seeking potential partners and financing
opportunities.
Under
the CFO Consulting Agreement, the Company will pay the CFO Consultant a monthly base salary of $7,200 and reimburse all reasonable
business-related travel and other business expenses. The Company will also grant the following equity compensation: (i) cashless warrants
to purchase 100,000 shares of common stock of the Company, with an exercise price of $1.26 per share, expiring on December 21, 2026;
(ii) options to purchase 200,000 shares of common stock of the Company, vesting monthly over two years, with an exercise price of $1.75
per share, expiring on December 21, 2031; and (iii) 175,000 restricted shares of common stock of the Company, subject to the following
milestone vesting schedule: (a) 75,000 restricted shares will vest upon the Company successfully listing its common stock on Nasdaq or
the NYSE; 50,000 restricted shares will vest upon any Company financing after January 1, 2022 of debt or equity in which the gross proceeds
equal or exceed $5,000,000; (c) 25,000 restricted shares will vest upon the completion of valuation reports for both Somahlution LLC
and HLII; and (d) 25,000 restricted shares will vest upon a material commercial partnership for MATLOC. The CFO Consultant will not receive
any employee benefits from the Company. The CFO Consultant is subject to a side agreement, under which the granting of restricted
shares under the CFO Consulting Agreement will occur upon vesting. Under a limited waiver and consent agreement, the CFO Consultant
waived any exercise rights it would otherwise have had under the options until certain conditions were met or
December 31, 2022, whichever occurs first. On December 27, 2022, such conditions were met, and as a result, the limited waiver
and consent agreement expired in accordance with its terms.
The
CFO Consulting Agreement automatically renews for additional six-month terms and may be terminated by either party upon 30 days’
notice. If the CFO Consultant is terminated without cause prior to the end of the term, the Company must pay the CFO Consultant $21,429.00
in severance as well as any other due and unpaid compensation. The CFO Consulting Agreement provides for customary confidentiality and
non-solicitation terms.
On
March 3, 2022, we designated Mr. Kovalyov as a recipient of part of the unexercised portion of a fully-vested option to purchase 1,100,000
shares of common stock at $1.01 per share that we and our designees purchased from a former executive. The designated option is exercisable
to purchase 273,750 shares of common stock. The option was granted on March 17, 2022, and expires on March 16, 2024. In connection with
this stock option designation, Mr. Kovalyov paid the former executive $25,000. The Company recorded the change in ownership upon proof
of Mr. Kovalyov’s payment for such option. The Company did not pay or receive cash or other consideration for the repurchase and
designation of the stock option.
Dr.
Steven Brooks
On
December 1, 2020, Dr. Steven Brooks signed an offer letter from us accepting the position as our Chief Medical Officer effective as of
that date. This position provides annual compensation of $300,000, an annual discretionary bonus of potentially 25% of base salary based
upon discretionary objectives to be outlined, and options to purchase 40,000 shares of common stock. We also provided Dr. Brooks a benefit
package to include insurance coverage.
Catherine
Pachuk
Under
an Executive Employment Agreement, dated July 26, 2021, with Dr. Catherine Pachuk, we agreed to appoint Dr. Pachuk as our Chief Scientific
Officer and Executive Vice President. Under the agreement, Dr. Pachuk’s annual salary is $325,000. If terminated without cause
through August 31, 2022, Dr. Pachuk is entitled to eight months’ severance payments. If terminated without cause after August 1,
2022, Dr. Pachuk is entitled to six months’ severance payments. If terminated with cause, Dr. Pachuk is not entitled to any post-termination
or severance compensation. Dr. Pachuk is eligible for all standard employee benefits and certain holiday and leave allowances. The agreement
contains standard intellectual property assignment, non-competition, non-solicitation, non-association, non-disparagement, and confidentiality
provisions. Dr. Pachuk’s employment is at-will, and may be terminated at any time, with or without cause, provided that in the
case of termination with cause based solely on a finding of breach of her agreement, Dr. Pachuk will be given written notice of such
breach and 15 days in which to cure it.
Other
Management Compensation
Bradley
Richmond
Bradley
Richmond was appointed our Acting Vice President of Finance on July 19, 2021. Mr. Richmond resigned from this position upon the appointment
of Harrison Ross as Vice President of Finance on December 21, 2021. Previously, on September 30, 2020, we entered into the Richmond Consulting
Agreement with Mr. Richmond pursuant to which we engaged Mr. Richmond to act as a licensing and market advisor providing services to
us relating to the introduction of licensing, joint venture, and other business development transactions. Under the Richmond Consulting
Agreement, Mr. Richmond specifically agreed that he would not solicit investments, make any recommendations regarding investments or
provide any analysis or advice regarding investments. Under the Richmond Consulting Agreement, Mr. Richmond received a non-refundable
retainer of $50,000 paid as follows: $25,000 in 20,000 shares of common stock issued on December 29, 2020, with an estimated value of
$1.25 per share; $12,500 on October 1, 2020; and $12,500 on November 1, 2020. Also pursuant to the Richmond Consulting Agreement, we
issued Mr. Richmond a warrant to purchase up to 36,364 shares of common stock with an exercise price of $1.375 per share and an expiration
date of October 2, 2026. In addition, we agreed to pay Mr. Richmond a fee on any “revenue or value” to the Company from his
consulting activities outlined as follows, but to be memorialized under a separate written agreement: A cash payment equal to 5% on the
first $10 million of value, 3% on the next $90 million of value, and 1.5% on any value above $90 million. Mr. Richmond was also entitled
to receive 100% warrant coverage on the same scale. “Revenue or value” is defined in the agreement to mean net revenue, which
will include any royalty that is paid out, net of the cost of the product. The Richmond Consulting Agreement terminated according to
its terms on September 30, 2022.
After
Mr. Richmond’s resignation from the position as Acting Vice President of Finance upon the appointment of Harrison Ross as Vice
President of Finance on December 21, 2021, Mr. Richmond continued to serve as an employee of Marizyme until September 24, 2022. During
his employment with us, Mr. Richmond assisted in management of our compliance, personnel and human resources departments as well as our
management team, in filling the gap created by a continuing lack of human and financial resources to enable us to advance our operations.
Mr. Richmond significantly decreased such services by the end of 2021 and ceased providing such services as of September 24, 2022. Mr.
Richmond’s services to us in this capacity were not related to his role as a registered representative of Univest and the services
Univest provides to us in connection with our private placements and proposed offerings. From July 2021 to June 2022, we paid Mr. Richmond
$5,000 per month as employment compensation for his services to us in this capacity and from July 2022 to September 24, 2022, we paid
Mr. Richmond $2,000 per month.
In
addition, as additional compensation for certain services, on each of January 26, 2022 and February 14, 2022, we granted Mr. Richmond
a warrant to purchase up to 150,000 shares of common stock at an exercise price of $0.01 per share, issuable immediately. Mr. Richmond
fully exercised both of the warrants in March 2022 for 300,000 shares. Pursuant to the October 2022 Letter Agreement, Mr. Richmond, a
registered representative of Univest, agreed to forego his rights to such shares of common stock, as the FINRA Staff determined that
such shares constituted underwriting compensation in connection with a proposed public offering based on the FINRA Staff’s interpretation
of FINRA Rule 5110. The shares were subsequently cancelled. Following the withdrawal of the registration statement for the proposed
public offering, the cancelled shares were replaced with new securities. See “Certain Relationships and Related Party Transactions
– Transactions with Related Persons”.
On
March 3, 2022, we designated Mr. Richmond as a recipient of part of the unexercised portion of a fully-vested option to purchase 1,100,000
shares of common stock at $0.01 that we and our designees purchased from a former executive. The designated option is exercisable to
purchase 273,750 shares of common stock. The option was granted on March 17, 2022, and expires on March 16, 2024. In connection with
this stock option designation, Mr. Richmond paid the former executive $25,000 for such option. The Company recorded the change in ownership
upon proof of Mr. Richmond’s payment for such option. The Company did not pay or receive cash or other consideration for the repurchase
and designation of the stock option. Pursuant to the October 2022 Letter Agreement, Mr. Richmond agreed to forego his rights to such
option, and we and Mr. Richmond agreed to the transfer of such option to a Company designee who is unaffiliated with Mr. Richmond, in
accordance with the FINRA Staff’s interpretation of Rule 5110.
Harrison
Ross
On
December 21, 2021, the Company entered into a consulting agreement (the “VP-Finance Consulting Agreement”), with Harrison
Ross, its Vice President of Finance, and Rydra Capital Corp., a corporation incorporated pursuant to the laws of British Columbia, Canada
and, together with Mr. Ross, the “VP-Finance Consultant”. Under the VP-Finance Consulting Agreement, the Company engaged
the VP-Finance Consultant for a 12-month term to provide such services as the Company may from time to time request, including, without
limiting the generality of the foregoing, the following: Budgeting, allocation of capital, and payroll processes; assistance in the preparation
of a Form S-1 and other Nasdaq listing preparations; assistance with corporate governance creations and procedures; the creation of valuation
reports for the Company’s products and medical devices; and assistance in the preparation of investment material for current and
prospective stockholders.
Under
the VP-Finance Consulting Agreement, the Company will pay the VP-Finance Consultant a monthly base salary of $7,200 and reimburse all
reasonable business-related travel and other business expenses. The Company will also grant the following equity compensation: (i) cashless
warrants to purchase 100,000 shares of common stock of the Company, with an exercise price of $1.26 per share, expiring on December 21,
2026; (ii) options to purchase 200,000 shares of common stock of the Company, vesting monthly over two years, with an exercise price
of $1.75 per share, expiring on December 21, 2031; and (iii) 175,000 restricted shares of common stock of the Company, subject to the
following milestone vesting schedule: (a) 75,000 restricted shares will vest upon the Company successfully listing its common stock on
Nasdaq or the NYSE; 50,000 restricted shares will vest upon any Company financing after January 1, 2022 of debt or equity in which the
gross proceeds equal or exceed $5,000,000; (c) 25,000 restricted shares will vest upon the completion of valuation reports for both Somahlution
LLC and HLII; and (d) 25,000 restricted shares will vest upon a material commercial partnership for MATLOC. The VP-Finance Consultant
will not receive any employee benefits from the Company. The VP-Finance Consultant is subject to a side agreement, under which
the granting of the restricted shares will occur upon vesting. Under a limited waiver and consent agreement, the VP-Finance
Consultant waived any exercise rights it would otherwise have had under the options until certain conditions
were met or December 31, 2022, whichever occurs first. On December 27, 2022, such conditions were met, and as a result, the limited waiver
and consent agreement expired in accordance with its terms.
The
VP-Finance Consulting Agreement automatically renews for additional six-month terms and may be terminated by either party upon 30 days’
notice. If the VP-Finance Consultant is terminated without cause prior to the end of the term, the Company must pay the VP-Finance Consultant
$21,600 in severance as well as any other due and unpaid compensation. The VP-Finance Consulting Agreement provides for customary confidentiality
and non-solicitation terms.
On
March 3, 2022, we designated Mr. Ross as a recipient of part of the unexercised portion of a fully-vested option to purchase 1,100,000
shares of common stock at $1.01 per share that we and our designees purchased from a former executive. The designated option is exercisable
to purchase 273,750 shares of common stock. The option was granted on March 17, 2022, and expires on March 16, 2024. In connection with
this stock option designation, Mr. Ross paid the former executive $25,000. The Company recorded the change in ownership upon proof of
Mr. Ross’s payment for such option. The Company did not pay or receive cash or other consideration for the repurchase and designation
of the stock option.
Director
Compensation
The
table below provides information concerning compensation for services as a director paid to all persons who served as members of our
board of directors during the fiscal year ended December 31, 2022, except for those persons who served as a director and are included
in our executive compensation table above:
| Name | |
Fees Earned or Paid in Cash |
| | |
Stock
Awards | | |
Option
Awards | | |
Non-Equity
Incentive Plan
Compensation
Earnings | | |
Nonqualified
Deferred
Compensation Earnings |
| |
All Other
Compensation | | |
Total | |
| Dr. Vithalbhai Dhaduk | |
$ | 24,750 |
(1)(2) | | |
$ | - | | |
$ | 83,175 | (3) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 107,925 | |
| Dr. William Hearl | |
$ | 24,750 |
(4) | | |
$ | - | | |
$ | 83,175 | (3) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 107,925 | |
| Julie Kampf | |
$ | 24,750 |
(5) | | |
$ | - | | |
$ | 83,175 | (3) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 107,925 | |
| Terry Brostowin | |
$ | 21,000 |
(6) | | |
$ | - | | |
$ | 83,175 | (3) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 104,175 | |
| Dr. Nilesh Patel(7) | |
$ | 13,500 |
(8) | | |
$ | - | | |
$ | 249,525 | (9) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 263,025 | |
| Michael Stewart(10) | |
$ | 21,000 |
(11) | | |
$ | - | | |
$ | 249,525 | (9) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | 270,525 | |
(1)
Under an independent director agreement with Dr. Dhaduk, Dr. Dhaduk was entitled to be paid $28,000 annual cash compensation in four
equal quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions
that provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Dr. Dhaduk earned $21,000
under his independent director agreement. However, Dr. Dhaduk was paid $19,000 including the $5,000 payment described in footnote (2)
below. See “—Director Agreements” below.
(2)
On April 22, 2022, the board of directors adopted resolutions that provided for the directors to receive $5,000 as a member of any board
committee. Pursuant to these resolutions, Dr. Dhaduk was entitled to annual compensation of $5,000 as a member of the Pricing Committee
of the board of directors, in addition to the annual and other compensation provided for under his independent director agreement. This
additional annual compensation for 2022 was paid retroactively for the full 2022 calendar year although such compensation was to accrue
beginning as of April 1, 2022 as described in footnote (1) above. See also “—Director Agreements” below.
(3)
Each of Dr. Dhaduk, Dr. Hearl, Ms. Kampf, and Mr. Brostowin held a fully-vested option to purchase 125,000 shares of common stock and
an option to purchase 40,000 shares of common stock subject to vesting conditions as of December 31, 2022.
(4)
Under an independent director agreement with Dr. Hearl, Dr. Hearl was entitled to be paid $33,000 annual cash compensation in four equal
quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions that
provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Dr. Hearl earned $24,750 in
2022 under his independent director agreement. However, Dr. Hearl was paid $16,500 in 2022. See “—Director Agreements”
below.
(5)
Under an independent director agreement with Ms. Kampf, Ms. Kampf was entitled to be paid $33,000 annual cash compensation in four equal
quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions that
provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Ms. Kampf earned $24,750 in
2022 under her independent director agreement. However, Ms. Kampf was paid $16,500 in 2022. See “—Director Agreements”
below.
(6)
Under an independent director agreement with Mr. Brostowin, Mr. Brostowin was entitled to be paid $28,000 annual cash compensation in
four equal quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions
that provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Mr. Brostowin earned $21,000
in 2022 under his independent director agreement. However, Mr. Brostowin was paid $14,000 in 2022. See “—Director Agreements”
below.
(7)
Appointed effective April 22, 2022.
(8)
Under an independent director agreement with Dr. Patel, Dr. Patel was entitled to be paid $18,000 annual cash compensation in four equal
quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions that
provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Dr. Patel earned $13,500 in
2022 under his independent director agreement. However, Dr. Patel was paid $9,000 in 2022. See “—Director Agreements”
below.
(9)
Each of Dr. Nilesh Patel and Michael Stewart held an option to purchase 120,000 shares of common stock subject to vesting conditions
as of December 31, 2022.
(10)
Appointed effective June 1, 2022.
(11)
Under an independent director agreement with Mr. Stewart, Mr. Stewart was entitled to be paid $28,000 annual cash compensation in four
equal quarterly installments beginning in the third quarter of 2022. On September 19, 2022, the board of directors adopted resolutions
that provided for the compensation to be paid to directors to accrue beginning on April 1, 2022. As a result, Mr. Stewart earned $21,000
in 2022 under his independent director agreement. Mr. Stewart was paid $14,000 in 2022. See “—Director Agreements”
below.
In
March 2021, the members of the Company’s board of directors at the time (James Sapirstein, Terry Brostowin, Dr. William Hearl,
Julie Kampf and Dr. Vithalbhai Dhaduk) were each granted 125,000 stock options with an exercise price of $1.25, three-year vesting and
a ten-year term. Black-Scholes was used to determine that each option’s value was $148,750 fully vested. On March 3, 2022, the
Company’s Compensation Committee resolved to accelerate the vesting of these options as to those held by Mr. Brostowin, Dr. Hearl,
Ms. Kampf and Dr. Dhaduk, and such options therefore became fully-vested on that date.
On
August 22, 2022, Mr. Brostowin executed a limited waiver and consent agreement waiving his option exercise rights. The limited waiver
and consent agreement was to expire on December 31, 2022, or if earlier to occur, upon the approval of an increase in the number of the
authorized shares of common stock under the Company’s articles of incorporation by the Company’s stockholders by majority
written consent or via stockholder vote at the Company’s next stockholder meeting and the implementation of such increase, which
were to occur as soon as practicable after such stockholder approval. On December 27, 2022, we held the Annual Meeting. At the Annual
Meeting, stockholders holding shares of common stock in the Company representing at least a majority of the voting power approved, among
other matters, the Authorized Capital Increase. As a result, the limited waiver and consent agreement expired in accordance with its
terms.
Director
Agreements
Our
independent director agreements with our non-executive directors provide that they will receive annual cash fees and equity compensation
in the form of options at fair market value for their service to the board of directors.
Under
their independent director agreements, dated June 7, 2022, each non-executive director will receive an annual cash fee and an annual
award of stock options. We will pay the annual cash compensation fee to each independent director in four equal installments no later
than the fifth business day of each calendar quarter commencing in the quarter ending September 30, 2022. We granted the stock options
to the non-executive directors on July 7, 2022. The annual cash fee paid to each non-executive director will be $28,000 as to Dr. Dhaduk;
$28,000 as to Mr. Brostowin; $33,000 as to Dr. Hearl; $33,000 as to Ms. Kampf; $18,000 as to Dr. Patel; and $28,000 as to Mr. Stewart.
On August 26, 2022, the board adopted resolutions providing that the cash compensation to the independent directors began to accrue as
of April 1, 2022. Under the independent director agreements, the annual award of stock options granted to Dr. Dhaduk, Mr. Brostowin,
Dr. Hearl and Ms. Kampf may be exercised to purchase 40,000 shares of common stock. The exercise price of the initial grant is $2.20
per share. These stock options will vest and become exercisable in 12 equal monthly installments over the first year following the date
of grant, subject to the respective director continuing in service on our board of directors through each such vesting date. The stock
options awarded to Dr. Patel and Mr. Stewart may be exercised to purchase 120,000 shares of common stock. These stock options will vest
and become exercisable in 12 equal quarterly installments over the first three years following the date of grant, subject to the respective
director continuing in service on our board of directors through each such vesting date. The exercise price of the initial grant is $2.20
per share. The term of each stock option is ten years from the date of grant. We will also reimburse each non-executive director for
pre-approved reasonable business-related expenses incurred in good faith in connection with the performance of the director’s duties
for us. As also required under the independent director agreements, we have separately entered into our standard indemnification agreement
for executive officers and directors with each of our non-executive directors.
Pursuant
to a previous director agreement, Terry Brostowin, one of our independent directors, received an option to purchase 140,000 shares of
common stock on December 6, 2018, at an exercise price equal to $1.50 per share, and an option to purchase 250,000 shares of common stock
on July 13, 2019, at an exercise price of $1.01 per share, for service on the board. All of these options are fully vested.
Long-Term
Incentive Plans
There
are no arrangements or plans in which we provide pension, retirement or similar benefits for directors or executive officers. We do not
have any material bonus or profit-sharing plans pursuant to which cash or non-cash compensation is or may be paid to our directors or
executive officers.
Amended
and Restated 2021 Stock Incentive Plan
On
May 18, 2021, our board of directors approved the Marizyme, Inc. Amended and Restated 2021 Stock Incentive Plan (“Stock Incentive
Plan” or the “Plan”), which was ratified by the Company’s stockholders on September 20, 2021. The Stock Incentive
Plan governs stock options issued prior to May 18, 2021. On August 30, 2022 and October 21, 2022, the board approved an amendment to
the Plan to increase the number of shares of common stock reserved for issuance under the Plan, which was ratified by the Company’s
stockholders on December 27, 2022.
Purpose
of the Plan. The purpose of the Plan is to advance our interests and the interests of our stockholders by providing an incentive
to attract, retain and reward persons performing services for us and by motivating such persons to contribute to our growth and profitability.
The maximum number of shares of common stock that may be issued pursuant to awards granted under the Plan is 7,200,000 shares. Cancelled
and forfeited stock options and stock awards may again become available for grant under the Plan. As of October 10, 2023, 2,924,057
shares remained available for issuance under the Plan. We intend that awards granted pursuant to the Plan be exempt from or comply with
Section 409A of the Code (including any amendments or replacements of such section), and the Plan shall be so construed.
The
following summary briefly describes the principal features of the Plan and is qualified in its entirety by reference to the full text
of the Plan.
Awards
that may be granted include: (a) Incentive Stock Options, or ISOs, (b) Nonstatutory Stock Options, (c) Stock Appreciation Rights, or
SARs, (d) Restricted Stock, (e) Restricted Stock Units, or RSUs, (f) Stock granted as a bonus or in lieu of another award, and (g) Performance
Awards. These awards offer us and our stockholders the possibility of future value, depending on the long-term price appreciation of
our common stock and the award holder’s continuing service with us.
Stock
options give the option holder the right to acquire from us a designated number of shares of common stock at a purchase price that is
fixed at the time of the grant of the option. The exercise price generally must not be less than the fair market value of the common
stock on the date of grant. Stock options granted may be either incentive stock options or non-statutory stock options.
SARs,
which may be granted alone or in tandem with options, have an economic value similar to that of options. When an SAR for a particular
number of shares is exercised, the holder receives a payment equal to the difference between the fair market value of the shares on the
date of exercise and the exercise price of the shares under the SAR. The exercise price for SARs normally is the market price of the
shares on the date the SAR is granted. Under the Plan, holders of SARs may receive this payment – the appreciation value –
either in cash or shares of common stock valued at the fair market value on the date of exercise. The form of payment will be determined
by the Company.
Restricted
stock awards represent issued and outstanding shares of common stock subject to vesting criteria. RSUs represent the right to receive
shares of common stock subject to satisfaction of vesting criteria. Restricted stock and the rights under RSUs are forfeitable and non-transferable
until they vest. The vesting date or dates and other conditions for vesting are established when the shares are awarded.
Our
board of directors may grant common stock to any eligible recipient as a bonus, or to grant stock or other awards in lieu of obligations
to pay cash or deliver other property under the Plan or under other plans or compensatory arrangements.
The
Plan also provides for performance awards, representing the right to receive a payment, which may be in the form of cash, shares of common
stock, or a combination, based on the attainment of pre-established goals.
All
of the permissible types of awards under the Plan are described in more detail below.
Administration
of the Plan. The Plan is currently administered by our Compensation Committee. All questions of interpretation of the Plan, of
any award agreement or of any other form of agreement or other document employed by us in the administration of the Plan or of any award
shall be determined by the Compensation Committee, and such determinations shall be final, binding and conclusive upon all persons having
an interest in the Plan or such award, unless fraudulent or made in bad faith. Any and all actions, decisions and determinations taken
or made by the Compensation Committee in the exercise of its discretion pursuant to the Plan or award agreement or other agreement thereunder
(other than determining questions of interpretation pursuant to the preceding sentence) shall be final, binding and conclusive upon all
persons having an interest therein.
Eligible
Recipients. Persons eligible to receive awards under the Plan are employees (including officers or directors who are also treated
as employees); consultants, i.e., persons engaged to provide consulting or advisory services to the Company; and directors.
Shares
Available Under the Plan. The maximum aggregate number of shares of common stock that may be issued under the Plan shall be 7,200,000
shares and shall consist of authorized but unissued or reacquired shares of common stock or any combination thereof, subject to adjustment
for certain corporate changes affecting the shares, such as stock splits, merger, consolidation, reorganization, reincorporation, recapitalization,
reclassification, or stock dividend. Shares subject to an award under the Plan for which the award is canceled, forfeited or expires
again become available for grants under the Plan.
Stock
Options and Stock Appreciation Rights
General.
Stock options and SARs shall be evidenced by award agreements specifying the number of shares of common stock covered thereby, in
such form as the Compensation Committee shall from time to time establish. Each stock option grant will identify the option as an ISO
or Nonstatutory Stock Option. Subject to the provisions of the Plan, the Compensation Committee has the authority to determine all grants
of stock options. That determination will include: (i) the number of shares subject to any option; (ii) the exercise price per share;
(iii) the expiration date of the option; (iv) the manner, time and date of permitted exercise; (v) other restrictions, if any, on the
option or the shares underlying the option; and (vi) any other terms and conditions as the Compensation Committee may determine.
Exercise
Price. The exercise price for each stock option or SAR shall be established in the discretion of the Compensation Committee; provided,
however, that the exercise price per share for the stock option or SAR shall be not less than the fair market value of a share of common
stock on the effective date of grant of the stock option or SAR. Notwithstanding the foregoing, a stock option or SAR may be granted
with an exercise price lower than the minimum exercise price set forth above if such stock option or SAR is granted pursuant to an assumption
or substitution for another option in a manner qualifying under the provisions of Section 424(a) of the Code.
Exercise
Terms. Stock options may be immediately exercisable but subject to repurchase or may be exercisable at such time or times, or upon
such event or events, and subject to such terms, conditions, performance criteria and restrictions as shall be determined by the Compensation
Committee and set forth in the award agreement evidencing such stock option. No stock option or SAR shall be exercisable after the expiration
of ten (10) years after the effective date of grant of such stock option or SAR. Subject to the foregoing, unless otherwise specified
by the Compensation Committee in the grant of a stock option or SAR, any stock option or SAR granted under the Plan shall terminate ten
(10) years after the effective date of grant of the stock option or SAR, unless earlier terminated in accordance with its provisions.
The Compensation Committee may set a reasonable minimum number of shares of common stock that may be exercised at any one time.
Expiration
or Termination. Options, if not previously exercised, will expire on the expiration date established by the Compensation Committee
at the time of grant. In the case of incentive stock options, such term cannot exceed ten years provided that in the case of holders
of more than 10% of our total combined voting stock, such term cannot exceed five years. Options will terminate before their expiration
date if the holder’s service with our company or a subsidiary terminates before the expiration date. The option may remain exercisable
for specified periods after certain terminations of employment, including terminations as a result of death, disability or retirement,
with the precise period during which the option may be exercised to be established by the Compensation Committee and reflected in the
grant evidencing the award.
Incentive
Stock Options. Stock options intending to qualify as ISOs may only be granted to employees, as determined by the board of directors.
No ISO shall be granted to any person if immediately after the grant of such award, such person would own common stock, including common
stock subject to outstanding awards held by him or her under the Plan or any other plan established by the Company, amounting to more
than ten percent (10%) of the total combined voting power or value of all classes of stock of the Company. To the extent that the award
agreement specifies that an option is intended to be treated as an ISO, the option is intended to qualify to the greatest extent possible
as an “incentive stock option” within the meaning of Section 422 of the Code, and shall be so construed; provided, however,
that any such designation shall not be interpreted as a representation, guarantee or other undertaking on the part of the Company that
the option is or will be determined to qualify as an ISO. If and to the extent that any shares of stock are issued under a portion of
any option that exceeds the $100,000 limitation of Section 422 of the Code, such shares of common stock shall not be treated as issued
under an ISO notwithstanding any designation otherwise.
Restricted
Stock Awards. A restricted stock award is a grant of shares of common stock. These awards will be subject to such conditions,
restrictions and contingencies as the Compensation Committee shall determine at the date of grant, including the attainment of one or
more performance criteria. Those may include requirements for continuous service and/or the achievement of specified performance goals.
Restricted stock is forfeitable and generally non-transferable until it vests. The vesting date or dates and other conditions for vesting
are established when the shares are awarded. Holders of restricted stock otherwise generally have the rights of stockholders of the Company,
including voting and dividend rights, to the same extent as other stockholders of the Company.
Restricted
Stock Units. An RSU is a right to receive stock on a future date. The purchase price for shares of stock issuable under each
RSU award shall be established by the board of directors in its discretion. Except as may be required by applicable law or established
by the Compensation Committee, no monetary payment (other than applicable tax withholding) shall be required as a condition of receiving
a RSU award. Shares issued pursuant to any RSU award may (but need not) be made subject to vesting conditions based upon the satisfaction
of such service requirements, conditions, restrictions or performance criteria, as shall be established by the Compensation Committee
and set forth in the award agreement evidencing such award.
Performance
Awards. The Compensation Committee shall designate the terms and conditions of each performance award, including the applicable
performance criteria and performance period. Each performance award shall entitle the participant to a payment in cash or common stock
upon the attainment of performance criteria and other terms and conditions specified by the Compensation Committee. Notwithstanding the
satisfaction of any performance criteria, the amount to be paid under a performance award may be adjusted by the Compensation Committee
on the basis of such further consideration as the Compensation Committee in its sole discretion shall determine. The Compensation Committee
may, in its discretion, substitute actual common stock for the cash payment otherwise required to be made to a participant pursuant to
a performance award.
Performance
Criteria. Under the Plan, performance criteria means business criteria including, but not limited to: revenue; revenue growth;
earnings before interest and taxes; earnings before interest, taxes, depreciation and amortization; earnings per share; operating income;
pre-or after-tax income; net operating profit after taxes; economic value added (or an equivalent metric); ratio of operating earnings
to capital spending; cash flow (before or after dividends); cash-flow per share (before or after dividends); net earnings; net sales;
sales growth; share price performance; return on assets or net assets; return on equity; return on capital (including return on total
capital or return on invested capital); cash flow return on investment; total stockholder return; improvement in or attainment of expense
levels; and improvement in or attainment of working capital levels or performance criteria. Any performance criteria may be used to measure
the Company’s performance as a whole or any of the Company’s business units and may be measured relative to a peer group
or index.
Bonus
Stock and Awards in Lieu of Obligations. The Compensation Committee may grant common stock to any eligible recipient as a bonus,
or to grant common stock or other awards in lieu of obligations to pay cash or deliver other property under the Plan or under other plans
or compensatory arrangements, provided that, in the case of participants subject to Section 16 of the Exchange Act, the amount of such
grants remains within the discretion of the Compensation Committee to the extent necessary to ensure that acquisitions of common stock
or other awards are exempt from liability under Section 16(b) of the Exchange Act. Common stock or awards granted hereunder shall be
subject to such other terms as shall be determined by the Compensation Committee.
Other
Material Provisions. Awards will be evidenced by a written agreement, in such form as may be approved by the Compensation Committee.
In the event of various changes to the capitalization of our company, such as stock splits, stock dividends and similar re-capitalizations,
an appropriate adjustment will be made by the Compensation Committee to the number of shares covered by outstanding awards or to the
exercise price of such awards. The Compensation Committee generally has the power to accelerate the exercise or vesting period of an
award. The Compensation Committee is also permitted to include in the written agreement provisions that provide for certain changes in
the award in the event of a change of control of our company, including acceleration of vesting or payment of the value of the vested
portion or, in its discretion, unvested portion of an award in cash, stock, or property. Except as otherwise determined by the Compensation
Committee at the date of grant, and subject to the terms of the Plan, awards will not be transferable, other than by will or the laws
of descent and distribution. Prior to any award distribution, we are permitted to deduct or withhold amounts sufficient to satisfy any
employee withholding tax requirements. The Compensation Committee has the authority, at any time, to discontinue the granting of awards.
The Compensation Committee also has the authority to alter or amend the Plan or any outstanding award or may terminate the Plan as to
further grants, provided that no amendment may be made without the approval of our stockholders to the extent that such approval is required
by law or applicable rules of a securities exchange, and except as otherwise provided by the Plan, no amendment that would adversely
affect any outstanding award made under the Plan can be made without the consent of the holder of such award.
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions
with Related Persons
The
following includes a summary of transactions since the beginning of our 2020 fiscal year, or any currently proposed transaction, in which
we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or 1% of the average of our total
assets at year-end for the last three completed fiscal years, and in which any related person had or will have a direct or indirect material
interest (other than compensation described under “Executive Compensation” above). We believe the terms obtained or
consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available
or the amounts that would be paid or received, as applicable, in arm’s-length transactions.
| ● | As
described in “Management’s Discussion and Analysis of Financial Condition
and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Exercise of Somahlution Warrants at Reduced Exercise
Price”, on December 15, 2019, the Company entered into the Somahlution Agreement,
as amended on March 31, 2020, May 29, 2020, and July 30, 2020. Under the third amendment,
the Company agreed to assume certain payables of Somahlution related to clinical and medical
expenses. It was also orally agreed by the parties that the payments on the assumed debts
would be recorded as a prepaid royalty against future royalties. |
Pursuant
to the Somahlution Agreement and in consideration of the outstanding capital stock of Somahlution, Inc., the Company agreed to issue
the following securities and pay the following contingent consideration to the former owners of Somahlution:
| ● | the
Somahlution Purchase Shares; |
| ● | the
Somahlution Warrants; |
| ● | The
following contingent consideration upon receiving FDA final approval and insurance reimbursement
approval on the products, and in the amounts, specified below, subject to certain expiration
terms, none of which had been earned or granted as of June 30, 2023 except as otherwise
disclosed below: |
| ● | Grant
of warrants on a pro rata basis for 4,000,000 shares of common stock of the Company, with
a strike price determined based on the average of the closing prices of the common stock
for the 30 calendar days following the date of the acquisition; |
| ● | Royalties
to be paid on all net sales of the product of 6% on the first $50 million of international
net sales (and 5% on the first $50 million of U.S. net sales), 4% for greater than $50 million
up to $200 million, and 2% for greater than $200 million; |
| ● | Payment
on a pro rata basis of 10% of the cash value of rare pediatric voucher sales following FDA
approval and subsequent sale to an unaffiliated third party of a rare pediatric voucher based
on DuraGraft; |
| ● | Following
FDA approval and subsequent sale to an unaffiliated third party of a rare pediatric voucher
based on DuraGraft, grant of warrants on a pro rata basis to purchase an aggregate of 250,000
shares of common stock with a term of five years and a strike price determined based on the
average of the closing prices of the common stock for the 30 calendar days following the
date of the public announcement of FDA approval; and |
| ● | Upon
the sale of DuraGraft products, the Company will pay pro rata the Somahlution designees 15%
of the net sale proceeds towards the liquidation preference maximum amount of $20 million
described below; |
| ● | Somahlution
derived solid organ transplant products: |
| ● | Grant
of warrants on a pro rata basis for 2,000,000 shares of common stock of the Company, with
a strike price determined based on the average of the closing prices of the common stock
for the 30 calendar days following the date of the acquisition; |
| ● | Royalties
to be paid on all net sales of the product of 6% on the first $50 million of international
net sales, 4% for greater than $50 million up to $200 million, and 2% for greater than $200
million; |
| ● | Upon
the sale of DuraGraft products, the Company will pay pro rata the Somahlution designees 15%
of the net sale proceeds towards the liquidation preference maximum amount of $20 million
described below; |
| ● | Somahlution
Assets-derived over-the-counter products: |
| ● | Royalties
to be paid on all net sales of the product of 6% on the first $50 million of international
net sales, 4% for greater than $50 million up to $200 million, and 2% for greater than $200
million; |
| ● | Other
Somahlution Assets-derived products from existing Somahlution pipelines: |
| ● | Royalties
to be paid on all net sales of the product of 1%; |
| ● | If
the Company raised more than $20 million in a fundraising concurrent with the closing of
this transaction, Marizyme was required to issue to the Somahlution designees, pro rata,
a number of additional restricted shares of common stock such that the percentage of the
total number of outstanding common stock of the Company represented by the Somahlution Purchase
Shares prior to such fundraising equals that percentage following the termination of the
fundraising. The Company did not raise more than $20 million in a fundraising concurrent
with the closing of this transaction, and additional restricted shares were not issued to
the Somahlution designees in connection with the Somahlution Agreement; |
| ● | Upon
the sale by the Company of all or substantially all of the Somahlution Assets, a liquidation
preference, up to a maximum of $20 million, to be paid pro rata to the Somahlution designees;
and |
| ● | Upon
closing, Dr. Dhaduk, the Chairman of our board of directors and a former indirect beneficial
owner of Somahlution, and/or his designated family member had the right to appoint two of
the members of the board of directors of the Company, one of whom shall be an independent
director, and both of whom shall be subject to qualifications reasonably determined by the
Company and Company approval, which approval shall not be unreasonably withheld, delayed
or conditioned. At closing, Dr. Dhaduk exercised this right to appoint himself and Dr. Nilesh
Patel to the board of directors. No dollar value has been attributed to these appointments. |
In
July and August, 2020, the Company completed the acquisition of the Somahlution Assets. In August 2020, the Company issued the Somahlution
Purchase Shares, the Somahlution Warrants, and prepaid royalties to the Former Somahlution Owners with a total dollar value of $339,091.
As of June 30, 2023 and December 31, 2022, the value of the prepaid royalties was $140,843 and $339,091. The value of the
prepaid royalties was attributed to Dr. Vithalbhai Dhaduk, our Chairman and a former indirect beneficial owner of Somahlution.
Dr.
Catherine Pachuk, Chief Scientific Officer and Executive Vice President, and one of the Former Somahlution Owners, was issued 268,710
shares having a total value of $499,801 based on the last reported price of the common stock on the OTCQB on July 30, 2020 and a Somahlution
Warrant to purchase 80,613 shares with an aggregate dollar value of $403,065 based on each Somahlution Warrant’s $5.00 per share
exercise price. Ranjanban Dhaduk, Dr. Vithalbhai Dhaduk’s spouse, was issued 3,546,610 shares having a total value of $6,596,695
based on the last reported price of the common stock on the OTCQB on July 30, 2020 and a Somahlution Warrant to purchase 1,063,938 shares
with an aggregate dollar value of $5,319,690 based on each Somahlution Warrant’s $5.00 per share exercise price. As a result of
this acquisition, Ranjanben Dhaduk became a beneficial owner of more than 5% of our outstanding shares. Ranjanben Dhaduk’s husband,
Dr. Vithalbhai Dhaduk, our Chairman, is considered to beneficially own the securities owned by Ranjanben Dhaduk. Each of the above payments
equals the approximate dollar value of the respective transaction and the approximate dollar value of the interest of each investor or
beneficial owner in such transaction.
| ● | On
April 13, 2023, the Company delivered the Somahlution Warrant Offer Letter Agreements to
the Former Somahlution Owners, which offered to allow the Former Somahlution Owners to exercise
the Somahlution Warrants to purchase the number of restricted shares of common stock issuable
under the Somahlution Warrants at an exercise price reduced by the Company from $5.00 per
share to $0.10 per share, on or prior to April 21, 2023, for maximum total cash proceeds
of $299,995.50. As of the conclusion of this offer period, the Somahlution Warrants Exercise
occurred, in which four of the Former Somahlution Owners entered into Somahlution Warrant
Offer Letter Agreements and exercised their Somahlution Warrants to purchase a total of 2,652,159
shares of common stock for gross proceeds of approximately $265,216. Univest, a registered
broker-dealer and member of FINRA, as the Company’s placement agent, which facilitated
the Somahlution Warrant Exercise, waived any fees or reimbursable expenses that would otherwise
have been payable with respect to the Somahlution Warrant Exercise pursuant to the 2021 Placement
Agency Agreement. |
As
a result of the Somahlution Warrants Exercise, the following Former Somahlution Owners became beneficial owners of more than 5% of the
Company’s outstanding shares of common stock: (i) Amar Dhaduk exercised one of the Somahlution Warrants and acquired 529,407 shares
of common stock for $0.10 per share, for a total payment of $52,940.70; (ii) Payal Dhaduk exercised one of the Somahlution Warrants and
acquired 529,407 shares of common stock for $0.10 per share, for a total payment of $52,940.70; and (iii) Darpan Dhaduk exercised one
of the Somahlution Warrants and acquired 529,407 shares of common stock for $0.10 per share, for a total payment of $52,940.70. In addition,
Ranjanben Dhaduk, a beneficial owner of more than 5% of the Company’s outstanding shares of common stock, exercised one of the
Somahlution Warrants and acquired 1,063,938 shares of common stock for $0.10 per share, for a total payment of $106,393.80. Ranjanben
Dhaduk’s husband, Vithalbhai Dhaduk, our Chairman, is considered to beneficially own the shares of common stock owned by Ranjanben
Dhaduk. Each of the above payments equals the approximate dollar value of the respective transaction and the approximate dollar value
of the interest of each investor in such transaction.
| ● | On
December 22, 2021, we closed the acquisition of My Health Logic from HLII. Effective December
21, 2021, George Kovalyov, the former chief operating officer and a former director of HLII,
was appointed our Chief Financial Officer and Treasurer. Also effective December 21, 2021,
Harrison Ross, previously the chief financial officer and a director of My Health Logic,
was appointed our Vice President of Finance. We have entered into consulting agreements with
Mr. Kovalyov and Mr. Ross, as described above under “Executive Compensation –
Executive Compensation Agreements.” As a result of the closing of the acquisition
of My Health Logic, Mr. Kovalyov and Mr. Ross acquired beneficial ownership of 236,386 and
153,678 shares of common stock, respectively. Mr. Kovalyov and Mr. Ross are currently also
serving as the Chief Financial Officer and Chief Executive Officer of HLII, respectively. |
| ● | As
described below, from September 30, 2020 to September 30, 2022, Bradley Richmond was an executive
officer, employee, consultant, and/or services provider to the Company, and, in such capacities,
received certain compensation from the Company. Prior to and during this period, Mr. Richmond
was also a registered representative of Univest, our placement agent since May 2020, and
has been entitled to a portion of Univest’s compensation from us as an investment banking
client pursuant to his employment terms with Univest. Mr. Richmond was also a Managing Director
of Univest from June 2020 to February 2022, has been a Co-Head of Investment Banking at Univest
since February 2022, and is Univest’s Chief Operating Officer. As described below,
Univest was the Company’s placement agent for the 2020 Private Placement, the Units
Private Placement, and the OID Units Private Placement, and would have been the representative
of the underwriters for a proposed public offering that the Company no longer intends to
pursue. Mr. Richmond, in his capacity as registered representative at Univest and pursuant
to his employment terms with Univest, received a percentage of the commissions and a percentage
of the Placement Agent Warrants paid or issued in connection with these private placements,
as described below, and would also have been entitled to receive a percentage of the commissions
and a percentage of the representative’s warrants that would have been earned by Univest
in the Company’s proposed public offering, to the extent that such commissions and
warrants were earned by Univest in accordance with the respective placement agency agreement
or underwriting agreement that we formerly intended to enter into with Univest. |
In
August 2020 and September 2020, prior to Mr. Richmond’s engagement as a consultant, employment, appointment as an executive officer,
or other acts as a provider of services to the Company, we conducted a private placement which was completed on September 25, 2020 in
which we sold an aggregate of 5,600,204 shares of common stock at $1.25 per share for an aggregate of $7,000,255 (the “2020 Private
Placement”). Under an exclusive engagement agreement with Univest, dated May 22, 2020, as amended on August 3, 2020 and August
6, 2020 (the “2020 PAA”), Univest acted as the exclusive placement agent for the 2020 Private Placement. The 2020 PAA provided
for a cash placement fee equal to 8% of the gross proceeds from the sale of securities in the 2020 Private Placement and, in exchange
for a $100 payment, warrants with a cashless exercise provision exercisable for shares of common stock equal to 5% of the aggregate number
of shares issued in the 2020 Private Placement at an exercise price of 110% of the price paid by the investors, terminating five years
from the date of issuance. Univest also agreed to be the underwriter of an underwritten public offering of the Company and related compensation
terms. The Company agreed to pay a total of $37,500 and 10,000 shares of common stock as advisory fees. The Company also agreed to reimburse
Univest for certain expenses, including for due diligence and legal counsel, of up to $300,000 and a non-accountable expense of 1% of
the 2020 Private Placement offering amount. The 2020 PAA provided indemnification rights to Univest and its affiliates in the event of
certain claims relating to Univest’s retention under the 2020 PAA. Univest had the right to act as the Company’s sole placement
agent or an underwriter for any future equity financing occurring during the 12-month period following the date of the 2020 PAA. Under
the 2020 PAA, Univest had the right to appoint a member to the Company’s board of directors from August 3, 2020 to August 3, 2022.
In addition, the 2020 PAA provided for certain other rights and obligations relating to the proposed public offering. The 2020 PAA expired
on November 22, 2021.
Pursuant
to the 2020 PAA, on July 8, 2020, Univest was issued 6,000 shares of common stock, with an approximate dollar value of $14,940 based
on the last reported price of the common stock on July 8, 2020 on the OTCQB of $2.49. On the same date, as a registered representative
of Univest entitled to a portion of Univest’s compensation due to his employment terms, on July 8, 2020, Mr. Richmond was issued
4,000 shares of common stock, with an approximate dollar value of $9,960 based on the last reported price of the common stock on July
8, 2020 on the OTCQB of $2.49. Upon the completion of the 2020 Private Placement, we paid Univest $560,019.20 in cash commissions, of
which Mr. Richmond received from Univest $161,381.53. We also sold to Univest a Placement Agent Warrant to purchase 168,008 shares of
common stock at an exercise price of $1.375 per share, with a total approximate dollar value of $256,667 based on the exercise price
of the warrants. As a registered representative of Univest entitled to a portion of Univest’s compensation due to his employment
terms, Mr. Richmond was issued a Placement Agent Warrant to purchase 112,006 shares of common stock, with a total approximate dollar
value of $154,008 based on the exercise price of the warrants.
On
September 30, 2020, we entered into the Richmond Consulting Agreement with Mr. Richmond, which terminated in accordance with its terms
on September 30, 2022. For a further description of the Richmond Consulting Agreement and compensation terms under the Richmond Consulting
Agreement, see “Executive Compensation – Other Management Compensation – Bradley Richmond” above.
On
July 19, 2021, Mr. Richmond was appointed our Acting Vice President of Finance, which position he held until December 21, 2021. After
Mr. Richmond’s resignation from this position upon the appointment of Harrison Ross as Vice President of Finance on December 21,
2021, Mr. Richmond continued to serve as an employee of the Company until September 24, 2022. For a further description of the services
provided by Mr. Richmond as an employee and related compensation terms, see “Executive Compensation – Other Management
Compensation – Bradley Richmond” above.
As
discussed under “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity
and Capital Resources – Recent Developments – Issuance of Replacement Securities,” on January 26, 2022 and on February
14, 2022, as additional compensation for certain
services, we granted Mr. Richmond the Consultant Warrants to purchase an aggregate of 300,000 shares of common stock
at $0.01 per share, valued at $1.75 per share, for an approximate total dollar value to us and Mr. Richmond of $525,000. In March
2022, Mr. Richmond fully exercised the Consultant Warrants for a total exercise price of $3,000 and was issued 300,000 shares, with
a total approximate dollar value of $582,000 based on the last reported price of the common stock on March 15, 2022 on the OTCQB of $1.94.
Pursuant to the October 2022 Letter Agreement that we entered into with him, Mr. Richmond agreed to forego his rights to such shares
of common stock in accordance with the FINRA Staff’s determination that such shares constituted underwriting compensation in connection
with a proposed public offering based on the FINRA Staff’s interpretation of FINRA Rule 5110. The shares were subsequently cancelled.
As
discussed under “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity
and Capital Resources – Recent Developments – Issuance of Replacement Securities,” in May 2021 and
December 2021, we entered into the 2021 Placement Agency Agreement with Univest pursuant to which Univest agreed to act as our
exclusive placement agent for the Units Private Placement. The 2021 Placement Agency Agreement governed compensation to Univest
for closings of the Units Private Placement that occurred from May 2021 to April 27, 2023 (the date of the April 2023 PAA). Through
the end of 2021, we conducted a number of closings of the Units Private Placement and raised an aggregate gross amount of $7,456,039.
In connection with the 2021 Placement Agency Agreement and our completion of the 2021 closings, we paid Univest $596,483
in cash commissions, of which Mr. Richmond received from Univest $17,473. In 2022, a number of additional closings in the Units Private
Placement occurred, including a final closing on August 12, 2022, in which we raised an aggregate gross amount of $7,315,138, and issued
units in exchange for services or other non-cash consideration valued at approximately $618,678. In connection with these closings, we
paid Univest $415,716.80, of which Mr. Richmond received from Univest $110,693.41, and paid an aggregate of $30,000 to Univest’s
legal counsel. Also, on January 24, 2022, for services in connection with the My Health Logic Acquisition, we issued Univest and Mr.
Richmond the Placement Agent Convertible Notes, valued at a total of $300,000 based on their aggregate principal of $300,000,
and the Placement Agent Class C Warrants, valued at a total of $771,426 based on 342,856 aggregate shares of common stock issuable
upon exercise at an exercise price of $2.25 per share in exchange for Univest’s and Mr. Richmond’s services, provided
to us in connection therewith. On June 26, 2022, in anticipation of the final closing of the Units Private Placement and pursuant to
the 2021 Placement Agency Agreement, we issued Univest, as placement agent, and Mr. Richmond, as a registered representative of Univest
entitled to a portion of Univest’s compensation due to his employment terms, the 2022 Placement Agent Warrants. In accordance
with the 2021 Placement Agency Agreement, the 2022 Placement Agent Warrants were issued in exchange for a $100 payment by Univest,
and were exercisable on a cash or cashless basis, in aggregate, to purchase a number of shares of common stock equal to approximately
8% of the units sold in the Units Private Placement. The 2022 Placement Agent Warrants’ exercise price per share was equal
to the price per unit of the units sold in the Units Private Placement, which, at the time of the 2022 Placement Agent Warrants’
issuance was $1.75 subject to adjustment. The approximate dollar value of the transaction was $1,012,197 based on the exercise price
of the 2022 Placement Agent Warrants. The 2022 Placement Agent Warrants were to expire on June 26, 2027. Pursuant to the
October 2022 Letter Agreement, each of Univest and Mr. Richmond agreed to forego their applicable rights to such securities, because
the FINRA Staff determined that such securities constituted underwriting compensation in connection with a proposed public offering based
on the FINRA Staff’s interpretation of FINRA Rule 5110. The 2022 Placement Agent Warrants were subsequently cancelled.
Between
July 2021 and August 2021, Mr. Richmond made loans to the Company of a total of $691,712 for working capital purposes. These loans, which
were undocumented, carried interest at the annual rate of 1%. The loans were repaid in January 2022, and Mr. Richmond earned and was
paid $6,917.12 in interest on the loans.
In
March 2022, Mr. Richmond, along with several persons, acting individually, purchased a one-fourth portion of the unexercised balance
of a fully-vested option to purchase 1,100,000 shares of common stock at $1.01 per share from one of our former executives. The option
was exercisable to purchase 273,750 shares of common stock, was granted on March 17, 2022, and was to expire on March 16, 2024. Mr. Richmond
paid the former executive $25,000 for such option. The Company recorded the changes in ownership upon proof of Mr. Richmond’s payment
for such option and the Company did not pay or receive cash or other consideration in connection with the purchase of such option. Pursuant
to the October 2022 Letter Agreement, Mr. Richmond agreed to forego his rights to such option, and we and Mr. Richmond agreed to the
transfer of the option to a Company designee who is unaffiliated with Mr. Richmond, in accordance with the FINRA Staff’s interpretation
of Rule 5110.
Subsequent
to the termination of the Richmond Consulting Agreement, Mr. Richmond’s employment with us, other services to us not in his capacity
as a registered representative of Univest, and executive officer position with us, and subsequent to all related compensation paid to
Mr. Richmond in any of these former capacities, we entered into the April 2023 PAA and conducted the OID Units Private Placement.
For a description of the general terms of the April 2023 PAA and the OID Units Placement Agent Warrants, see “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – April 2023 PAA” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent
Developments – OID Units Private Placement – Issuance of OID Units Placement Agent Warrants”, respectively.
Pursuant to the April 2023 PAA, in connection with the closings of the OID Units Private Placement, the Company paid Univest $226,000
in total fees and $125,000 as reimbursement of Univest’s legal fees.
In
connection with the Third OID Units Closing, on July 10, 2023, pursuant to the 2021 Placement Agency Agreement, the Company was required
to pay Univest outstanding fees of $140,000 or 8% of the gross proceeds from the issuance of the Walleye Promissory Note on February
6, 2023 for the principal amount of $1,000,000 and the Hexin Promissory Note on December 28, 2022 for the principal amount of $750,000.
Under
the April 2023 PAA and the forms of Placement Agent Warrants agreed to in connection with the April 2023 PAA, the Company also agreed,
upon Univest’s payment of $100.00, to issue the OID Units Placement Agent Warrants to Univest and/or its designee(s) at each closing
of the OID Units Private Placement. The OID Units Placement Agent Warrants were required to be issued for the purchase of 8% of the aggregate
number of shares of common stock initially issuable upon conversion of the OID Convertible Notes at $0.10 per share, 8% of the aggregate
number of shares of common stock initially issuable upon exercise of the Class E Warrants at $0.10 per share, and 8% of the aggregate
number of shares of common stock initially issuable upon exercise of the Class F Warrants at $0.20 per share, subject to adjustment.
On September 12, 2023, Univest notified the Company that the OID Units Private Placement’s final closing had occurred, and on September
13, 2023, made a payment to the Company of $100.00. Accordingly, on September 13, 2023, OID Units Placement Agent Warrants were issued
to Univest and its designees, Bradley Richmond, Bartholemew Pan-Kita, and Azem Nesimi, to purchase a total of 4,356,687, 6,695,616, 635,294,
and 7,059 shares of common stock at $0.10 per share, respectively, which in aggregate was equal to 8% of the shares issuable upon conversion
or exercise of the OID Convertible Notes and the Class E Warrants issued in each of the closings of the OID Units Private Placement as
of that date, and a total of 2,585,479, 3,878,218, 0, and 0 shares of common stock at $0.20 per share, respectively, which in aggregate
was equal to 8% of the shares issuable upon exercise of the Class F Warrants issued in each of the closings of the OID Units Private
Placement as of that date. Based on their exercise prices, the total approximate dollar value of the OID Units Placement Agent Warrants
issued to each of Univest, Mr. Richmond, Mr. Pan-Kita, and Mr. Nesimi was $952,764, $1,445,205, $63,529, and $706, respectively.
On
June 22, 2023, Univest and Mr. Richmond requested through counsel that the Company either reissue the Cancelled Securities or issue them
new securities with equivalent terms and conditions, in light of the fact that the Company was not proceeding with the public offering.
On July 16, 2023, Univest and Mr. Richmond communicated through counsel that they believed that the Cancelled Securities should be reissued
as if they were never canceled, meaning that they should have all of the adjusted terms that may have applied to the Cancelled Securities
had they not been cancelled.
Pursuant
to these requests, on July 25, 2023 and September 29, 2023, the Company approved the grant of the following securities to Univest and
Mr. Richmond: (i) the Replacement Convertible Notes with terms and conditions equivalent to or at least as favorable as those that would
have applied to each of the respective Placement Agent Convertible Notes, such that, among other adjustments, the principal and any accrued
interest under the Replacement Convertible Notes is convertible into shares of common stock at $0.10 per share, subject to adjustment,
reflecting adjustments to their terms equivalent to those that applied to the Convertible Notes as a result of the Somahlution Warrants
Exercise, and with principal increased to reflect the amount of interest that would have been incurred under the respective Placement
Agent Convertible Notes up to the date of issuance, such that the adjusted principal under the Replacement Convertible Notes is $137,983.56
and $206,975.34, respectively, being the original principal under the Placement Agent Convertible Notes plus pre-issuance interest that
would have accrued since the date of the issuance of the Placement Agent Convertible Notes as of July 25, 2023, maturing on July 25,
2025, and that, taking into account the Convertible Notes’ amended Mandatory Default Amount provisions as described in “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent Developments
– Letter Agreement to Amend Convertible Notes to Permit Conversion of Mandatory Default Amount” and the default
under the Convertible Notes that occurred as a result of their cross-default provisions as described in “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Cross-Default Under Convertible Notes”, approximately $182,772 and $274,157 is due, respectively,
and up to 1,827,715 and 2,741,573 shares of common stock are issuable upon conversion thereof, subject to adjustment and applicable beneficial
ownership limitations and other limitations or restrictions, and having an approximate dollar value of $182,772 and $274,157, respectively;
(ii) the Replacement Class C Warrants with terms and conditions equivalent to or at least as favorable as those that would have applied
to each of the respective Placement Agent Class C Warrants, reflecting adjustments to their terms equivalent to those that applied to
the Class C Warrants as a result of the Somahlution Warrants Exercise such that the exercise price per share of the Replacement Class
C Warrants is $0.10 per share, subject to adjustment, and the number of shares of common stock issuable upon exercise of each of the
Replacement Class C Warrants reflecting a proportional increase relative to the number of shares issuable under the respective Placement
Agent Class C Warrants equivalent to the difference between the exercise price per share of the Placement Agent Class C Warrants and
the exercise price of such Replacement Class C Warrants, such that up to 3,548,148 and 5,322,223 shares of common stock, respectively,
are issuable upon exercise thereof, subject to adjustment and applicable beneficial ownership limitations and other limitations or restrictions,
and having an approximate dollar value of $354,815 and $532,222.30, respectively; (iii) the Replacement Placement Agent Warrants with
terms and conditions equivalent to or at least as favorable as those that would have applied to those that applied to each of the respective
2022 Placement Agent Warrants, reflecting adjustments to their terms equivalent to those that applied to the Class C Warrants as a result
of the Somahlution Warrants Exercise such that the exercise price per share of the Replacement Placement Agent Warrants is $0.10 per
share, subject to adjustment, and the number of shares of common stock issuable upon exercise of each of the Replacement Placement Agent
Warrants reflecting a proportional increase relative to the number of shares issuable under the respective 2022 Placement Agent Warrants
equivalent to the difference between the exercise price per share of the 2022 Placement Agent Warrants and the exercise price of the
Replacement Placement Agent Warrants, such that up to 19,293,621 and 28,940,432 shares of common stock, respectively, are issuable upon
exercise thereof, subject to adjustment and applicable beneficial ownership limitations and other limitations or restrictions, and having
an approximate dollar value of $404,878.20 and $607,318.20, respectively; and (iv) the Replacement Consultant Shares, having an approximate
dollar value of $30,000 based on the last reported price of the common stock on July 25, 2023 on the OTCQB of $0.10. In connection with
these grants, each of Univest and Mr. Richmond agreed to waivers of any registration rights that would have been applicable to each of
the Replacement Convertible Notes, the Replacement Class C Warrants, and the Replacement Placement Agent Warrants.
In
May 2023, the event described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations
– Liquidity and Capital Resources – Funding Requirements and Other Liquidity Matters – Default Under Promissory Note”
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital
Resources – Funding Requirements and Other Liquidity Matters – Cross-Default Under Convertible Notes” occurred.
Assuming that the event described would have triggered the cross-default provision in the Placement Agent Convertible Notes if they had
not been cancelled, and assuming that each of the Replacement Convertible Notes are also subject to immediate payment of the applicable
Mandatory Default Amount (as defined in “Management’s Discussion and Analysis of Financial Condition and Results of Operations
– Liquidity and Capital Resources – Liquidity and Capital Resources – Funding Requirements and Other Liquidity Matters
– Cross-Default Under Convertible Notes”), the Mandatory Default Amount that may be due under the Replacement Convertible
Notes held by Univest and Mr. Richmond would be $182,772 and $274,157, respectively, or approximately $0.1 million more, in aggregate,
than would otherwise have been due under the Placement Agent Convertible Notes on the date of the default had they been outstanding on
that date.
Under
the unit purchase agreement entered into on May 27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s
subsidiaries entered into a guaranty to guarantee the repayment of the Company’s obligations under the Convertible Notes, and the
Company and its subsidiaries entered into security agreements granting security interests in all of their respective assets for up to
the dollar value owed under the respective Convertible Notes. The respective Convertible Notes were issued for aggregate principal of
approximately $1.2 million. Under each unit purchase agreement entered into with respect to subsequent issuances of the Convertible Notes,
including the unit purchase agreement entered into with each of Univest and Mr. Richmond in connection with the issuance of the Placement
Agent Convertible Notes, the Company and its subsidiaries were obligated to enter into similar security agreements and guaranties, but
did not do so.
Univest
and Mr. Richmond have not exercised any remedies applicable to the Replacement Convertible Notes or given notice of any intention to
do so as of the date of this prospectus. In the event that the balance owed under the Replacement Convertible Notes, including the respective
Mandatory Default Amount, is not repaid upon demand, Univest and Mr. Richmond may seek to take possession of some or all of the Company’s
and its subsidiaries’ assets, force the Company and its subsidiaries into bankruptcy proceedings, or seek other legal remedies
against the Company and its subsidiaries. In such event, the Company’s business, operating results and financial condition may
be materially adversely affected.
Promoters
and Certain Control Persons
No
person is deemed to have been a “promoter,” as such term is defined by Rule 405 of the Securities Act, of the Company within
the past five years.
PRINCIPAL
STOCKHOLDERS
The
following table sets forth certain information with respect to the beneficial ownership of our common stock as of September 19, 2023
for (i) each of our named executive officers, other executive officers, and directors; (ii) all of our executive officers and directors
as a group; and (iii) each other person known by us to be the beneficial owner of more than 5% of our outstanding common stock.
Beneficial
ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For
purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock
that such person or any member of such group has the right to acquire within sixty (60) days of September 19, 2023, except as
otherwise disclosed in the footnotes to the table. For purposes of computing the percentage of outstanding shares of common stock held
by each person or group of persons named below, any shares that such person or persons has the right to acquire within sixty (60) days
of September 19, 2023 are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing
the percentage ownership of any other person, except as otherwise disclosed in the footnotes to the table. See the footnotes to the table
below for further discussion of beneficial ownership calculations. The inclusion herein of any shares listed as beneficially owned does
not constitute an admission of beneficial ownership by any person.
| Title of Class | |
Name and Address(1)
of
Beneficial Owner | |
Amount and Nature of Beneficial Ownership(2) | | |
Percent of
Class(2) | |
| | |
| |
Shares | | |
% | |
| Common Stock | |
David Barthel(3)(4) | |
| 280,000 | (5) | |
| 0.6 | |
| Common Stock | |
Dr. Vithalbhai Dhaduk(3)(4) | |
| 4,775,548 | (6) | |
| 10.2 | |
| Common Stock | |
George Kovalyov(4) | |
| 776,802 | (7) | |
| 1.6 | |
| Common Stock | |
Harrison Ross(3) | |
| 694,094 | (8) | |
| 1.5 | |
| Common Stock | |
Dr. Steven Brooks(3) | |
| 56,666 | (9) | |
| 0.1 | |
| Common Stock | |
Dr. Catherine Pachuk(3) | |
| 349,323 | (10) | |
| 0.7 | |
| Common Stock | |
Terry Brostowin(4) | |
| 555,000 | (11) | |
| 1.2 | |
| Common Stock | |
Dr. William Hearl(4) | |
| 165,000 | (12) | |
| * | |
| Common Stock | |
Julie Kampf(4) | |
| 165,000 | (13) | |
| * | |
| Common Stock | |
Dr. Nilesh Patel(4) | |
| 50,000 | (14) | |
| * | |
| Common Stock | |
Michael Stewart(4) | |
| 50,000 | (15) | |
| * | |
| Common Stock | |
All directors and executive officers as a group (11 persons) | |
| 7,917,433 | | |
| 16.1 | |
| Common Stock | |
ESC Holdings LLC(16) | |
| 2,555,640 | | |
| 5.5 | |
| Common Stock | |
Ranjanben V. Dhaduk(17) | |
| 4,610,548 | (17) | |
| 9.9 | |
| Common Stock | |
Univest Securities, LLC(18) | |
| 5,178,769 | (18) | |
| 10.0 | |
| Common Stock | |
Bradley Richmond(19) | |
| 5,140,357 | (19) | |
| 10.0 | |
*
Non-officer director beneficially owning less than 1% of the shares of common stock.
(1)
Unless otherwise indicated in the footnotes below, the address of each beneficial owner listed in the table above is c/o Marizyme, Inc.,
555 Heritage Drive, Suite 205, Jupiter, Florida 33458.
(2)
As of September 19, 2023, a total of 46,666,760 shares of common stock are considered to be outstanding. This number does
not include shares of common stock issuable upon conversion or exercise of outstanding convertible or exercisable securities. Beneficial
ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities.
Except as disclosed in the following footnotes, each of the beneficial owners listed above has direct ownership of and sole voting and
investment power over the shares of common stock of which they are beneficial owners. Pursuant to Exchange Act Rule 13d-3(d)(1), for
each beneficial owner listed above, any options, warrants, convertible notes, or other convertible securities that are exercisable or
convertible within 60 days have also been included for purposes of calculating their percent of class, but not for any other beneficial
owner listed above. Contractual limitations on rights of conversion or exercise of outstanding convertible or exercisable securities
have been assumed to apply for at least 60 days following September 19, 2023, with the effect that such shares of common stock
are not included in the amounts indicated as beneficially owned.
(3)
Executive officer.
(4)
Director.
(5)
Consists of 280,000 shares of common stock issuable upon exercise of an option within 60 days of September 19, 2023.
(6)
Consists of (i) 4,610,548 shares of common stock held indirectly through Ranjanben V. Dhaduk, Dr. Dhaduk’s wife, (ii) 125,000
shares of common stock issuable upon exercise of an option and (iii) 40,000 shares of common stock issuable upon exercise of an option.
(7)
George Kovalyov may be deemed to beneficially own
(i) 111,509 shares of common stock held directly; (ii) 273,750 shares of common stock issuable upon exercise of an option held directly;
(iii) 166,666 shares of common stock issuable upon exercise of an option held directly within 60 days of September 19, 2023; (iv)
124,877 shares of common stock held by AAT Services Inc., of which George Kovalyov is the sole beneficial owner; and (v) 100,000
shares of common stock beneficially owned through AAT Services Inc. issuable upon exercise of a cashless warrant. Mr. Kovalyov
was also granted 175,000 restricted shares of common stock, subject to the following milestone vesting schedule: (A) 75,000 restricted
shares vesting upon the Company successfully listing its common stock on Nasdaq or the NYSE; (B) 50,000 restricted shares vesting upon
any Company financing after January 1, 2022 of debt or equity in which the gross proceeds equal or exceed $5,000,000; (C) 25,000 restricted
shares vesting upon the completion of valuation reports for both Somahlution LLC and HLII; and (D) 25,000 restricted shares vesting
upon a material commercial partnership for MATLOC. AAT Services Inc. and Mr. Kovalyov are subject to a side agreement, under which the
granting of the restricted shares will occur upon vesting, and therefore such restricted shares are not considered to be beneficially
owned on September 19, 2023.
(8)
Harrison Ross may be deemed to beneficially own (i) 141,948 shares of common stock held directly; (ii) 273,750 shares of common stock
issuable upon exercise of an option held directly; (iii)
166,666 shares of common stock issuable upon exercise of an option within 60 days of September 19, 2023; (iii) 11,730 shares of common
stock held by Rydra Capital Corp., of which Harrison Ross is the sole beneficial owner; and (iv) 100,000 shares of common stock beneficially
owned through Rydra Capital Corp. issuable upon exercise of a cashless warrant. Mr. Ross was also granted 175,000 restricted shares
of common stock, subject to the following milestone vesting schedule: (A) 75,000 restricted shares vesting upon the Company successfully
listing its common stock on Nasdaq or the NYSE; (B) 50,000 restricted shares vesting upon any Company financing after January 1, 2022
of debt or equity in which the gross proceeds equal or exceed $5,000,000; (C) 25,000 restricted shares vesting upon the completion of
valuation reports for both Somahlution LLC and HLII; and (D) 25,000 restricted shares vesting upon a material commercial partnership
for MATLOC. Rydra Capital Corp. and Mr. Ross are subject to a side agreement, under which the granting of the restricted shares
will occur upon vesting, and therefore such restricted shares are not considered to be beneficially owned on September 19,
2023.
(9)
Consists of (i) 20,000 shares of common stock, and
(ii) 36,666 shares of common stock issuable upon exercise of an option within 60 days of September 19, 2023.
(10)
Consists of (i) 268,710 shares of common stock,
and (ii) 80,613 shares of common stock issuable upon exercise of a warrant.
(11)
Consists of (i) 140,000 shares of common stock issuable
upon exercise of an option, (ii) 250,000 shares of common stock issuable upon exercise of an option, (iii) 125,000 shares of common stock
issuable upon exercise of an option, and (iv) 40,000 shares of common stock issuable upon exercise of an option.
(12)
Consists of (i) 125,000 shares of common stock issuable
upon exercise of an option and (ii) 40,000 shares of common stock issuable upon exercise of an option.
(13)
Consists of (i) 125,000 shares of common stock issuable
upon exercise of an option and (ii) 40,000 shares of common stock issuable upon exercise of an option.
(14)
Consists of 50,000 shares of common stock
issuable upon exercise of an option within 60 days of September 19, 2023.
(15)
Consists of 50,000 shares of common stock
issuable upon exercise of an option within 60 days of September 19, 2023.
(16)
Emmanuelle Schleipfer-Conley, the owner of ESC Holding
LLC, has sole voting and investment power over the securities held by ESC Holding LLC. Its mailing address is 1 Channel Drive #1706,
Monmouth Beach, NJ 07750.
(17)
Ranjanben V. Dhaduk shares voting and investment
power over her shares of common stock with her husband, Vithalbhai Dhaduk, Chairman of the Company. The mailing address of Ranjanben
V. Dhaduk is 1008 Windemere Circle, Dalton, PA 18414.
(18)
Consists of (i) 6,000 shares of common stock, (ii)
168,008 shares of common stock issuable upon exercise of a 2020 Placement Agent Warrant, (iii) 3,548,148 shares of common stock issuable
upon exercise of a Replacement Class C Warrant, (iv) 19,293,621 shares of common stock issuable upon exercise of Replacement Placement
Agent Warrants, (v) 1,827,715 shares of common stock issuable upon conversion of a Replacement Convertible Note, and (vi) 6,942,166 shares
of common stock issuable upon exercise of OID Units Placement Agent Warrants, which are considered by the Company to be subject to a
beneficial ownership limitation applicable to the 2020 Placement Agent Warrant, the Replacement Class C Warrant, the Replacement Placement
Agent Warrants, the Replacement Convertible Note, and the OID Units Placement Agent Warrants limiting beneficial ownership to 9.99% of
the outstanding shares of common stock. Yi Guo has sole voting and investment power over the securities held by Univest. The business
address of Univest is 375 Park Avenue, 27th Floor, New York, NY 10152.
(19)
Consists of (i) 352,088 shares of common stock,
(ii) 112,006 shares of common stock issuable upon exercise of a 2020 Placement Agent Warrant, (iii) 5,322,223 shares of common stock
issuable upon exercise of a Replacement Class C Warrant, (iv) 28,940,432 shares of common stock issuable upon exercise of Replacement
Placement Agent Warrants, (v) 2,741,573 shares of common stock issuable upon conversion of a Replacement Convertible Note, and (vi) 10,573,834
shares of common stock issuable upon exercise of OID Units Placement Agent Warrants, which are considered by the Company to be subject
to a beneficial ownership limitation applicable to the 2020 Placement Agent Warrant, the Replacement Class C Warrant, the Replacement
Placement Agent Warrants, the Replacement Convertible Note, and the OID Units Placement Agent Warrants limiting beneficial ownership
to 9.99% of the outstanding shares of common stock. The business address of Bradley Richmond is 30 Tintern Lane, Scarsdale, NY 10583.
We
do not currently have any arrangements which if consummated may result in a change of control of our company.
SELLING
STOCKHOLDERS
The
shares of common stock being offered by the selling stockholders are those held by the selling stockholders or issuable to the selling
stockholders upon the exercise of certain warrants to purchase shares of common stock or conversion of certain convertible notes into
shares of common stock held by the selling stockholders. We are registering these shares of common stock for resale in order to comply
with requirements under the terms of certain agreements and securities, as described further below, as well as to permit the selling
stockholders to offer the shares for resale from time to time. Certain selling stockholders include our affiliates and stockholders with
“restricted securities” under the applicable securities laws and regulations who, because of their status as affiliates of
us pursuant to Rule 144 or because they acquired their capital stock from an affiliate or from us within the prior 12 months from the
date of any proposed sale, may otherwise be unable to sell their securities pursuant to Rule 144. See “Shares Eligible for Future
Sale” for further information regarding sales of such “restricted securities” if not sold pursuant to this prospectus.
Except
as disclosed below, the selling stockholders have not had any position, office, or other material relationship with us or any of our
predecessors or affiliates within the past three years other than with respect to the ownership of these securities, and, except as disclosed
below, based on the information provided to us by the selling stockholders, no selling stockholder is a broker-dealer or an affiliate
of a broker-dealer.
The
table below lists the selling stockholders, the securities being offered for resale, and the securities of the Company that are beneficially
owned by each selling stockholder as of September 19, 2023 and that will be beneficially owned after this offering, based on the
beneficial ownership rules of the SEC and certain assumptions as described below.
The
amount of shares of common stock beneficially owned by the selling stockholders before the offering is listed in the second column of
the table below. The information regarding the beneficial ownership by each of the selling stockholders is based on the selling stockholder’s
beneficial ownership of shares of common stock, shares of common stock issuable upon the conversion of certain Convertible Notes, shares
of common stock issuable upon the exercise of certain Class C Warrants, or shares of common stock issuable upon the exercise of certain
Placement Agent Warrants. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or
investment power with respect to securities. For purposes of the table below and in accordance with such rules, a person or group of
persons is deemed to have “beneficial ownership” of any shares of common stock that such person or any member of such group
has the right to acquire within 60 days of September 19, 2023. For purposes of computing the percentage of outstanding shares
of our common stock held by each person or group of persons named below and in accordance with such rules, any shares that such person
or persons has the right to acquire within 60 days of September 19, 2023 are deemed to be outstanding for such person, but not
deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares
listed as beneficially owned does not constitute an admission of beneficial ownership by any person. In addition to these beneficial
ownership rules, the number of shares beneficially owned by the selling stockholders reflect the following summarized beneficial ownership
limitations (see “Description of Securities” for additional related discussion):
| ● | Each
holder of a Convertible Note or Class C Warrant generally will not have the right to receive
shares of common stock upon conversion of the Convertible Note or exercise of
the Class C Warrant to the extent that the holder would otherwise become the “beneficial
owner” (as such term is defined in the Exchange Act and the rules and regulations thereunder)
of in excess of 4.99% of the shares of common stock outstanding, subject to automatic
increase to 9.99% if the holder becomes a beneficial owner of more than 4.99%,
subject to a 61-day notice requirement. This limitation does not apply to certain provisions
of the Convertible Notes and Class C Warrants. The shares of common stock that may not
be received upon conversion of a Convertible Note or exercise of a Class C Warrant due to
this beneficial ownership limitation are not considered beneficially owned as of September
19, 2023. |
| ● | Each
holder of an OID Convertible Note or Class E Warrant or a Class F Warrant generally will
not have the right to receive shares of common stock upon conversion of the OID Convertible
Note or exercise of the Class E Warrant or Class F Warrant to the extent that the
holder would otherwise become the beneficial owner of in excess of 4.99% of the shares
of common stock outstanding, subject to automatic increase to 9.99% if the holder
becomes a beneficial owner of more than 4.99% of the outstanding shares of common
stock, subject to a 61-day notice requirement. This limitation does not apply to certain
provisions of the OID Convertible Notes and Class E and F Warrants. The shares of common
stock that may not be received upon conversion of a Convertible Note or
exercise of a Class E Warrant or a Class F Warrant due to this
beneficial ownership limitation are not considered beneficially owned as of September 19,
2023. |
| ● | Each
holder of a 2020 Placement Agent Warrant may exercise such warrant only
if it would not cause the holder to beneficially own in excess of 9.99% of
the number of shares of the common stock outstanding immediately after
giving effect to the issuance of shares of common stock issuable upon exercise of the
2020 Placement Agent Warrant. The holder, upon notice to the Company,
may increase or decrease the percentage limit, provided that the limitation in no
event exceeds 9.99% of the number of shares of the common stock outstanding immediately
after giving effect to the issuance of shares of common stock upon exercise of the 2020 Placement
Agent Warrant. Any increase in the limitation will not be effective until the 61st day after
such notice is delivered to the Company. This limitation does not apply to certain provisions
of the 2020 Placement Agent Warrant. The shares of common stock that may not be
purchased upon exercise of a 2020 Placement Agent Warrant due to this
beneficial ownership limitation are not considered beneficially owned as of September
19, 2023. |
| ● | Each
holder of a Replacement
Placement Agent Warrant will generally not have the right to receive shares upon exercise
of a Replacement Placement Agent Warrant to the extent that the holder would otherwise become
a beneficial owner of in excess of 4.99% of the shares of common stock outstanding, subject
to automatic increase to 9.99% if the holder becomes a beneficial owner of more than 4.99%,
subject to a requirement that any increase in the limitation will not be effective until
the 61st day after notice is delivered to the Company. This limitation does not apply to
certain provisions of the Replacement Placement Agent Warrants. The shares of common stock
that may not be issued upon exercise of a Replacement Placement Agent Warrant due to this
beneficial ownership limitation are not considered beneficially owned as of September 19,
2023. |
| ● | Each
holder of an OID Units Placement Agent Warrant may exercise such warrant only if it would
not cause the holder to beneficially own in excess of
9.99% of the number of shares of the common stock outstanding immediately after giving effect
to the issuance of shares of common stock issuable upon exercise of the OID Units
Placement Agent Warrant. The holder, upon notice to the Company, may increase or decrease
the percentage limit, provided that the limitation in no event exceeds 9.99% of the number
of shares of the common stock outstanding immediately after giving effect to the issuance
of shares of common stock upon exercise of the OID Units Placement Agent Warrant.
Any increase in the limitation will not be effective until the 61st day after such notice
is delivered to the Company. This limitation does not apply to certain provisions of the
OID Units Placement Agent Warrant. The shares of common stock that may not be purchased
under an OID Units Placement Agent Warrant due to this beneficial ownership
limitation are not considered beneficially owned as of September 19, 2023. |
In
accordance with the terms of each Units Registration Rights Agreement, each of the Convertible Notes, each OID Units Registration Rights
Agreement, and each of the OID Convertible Notes, the table includes for resale under this prospectus the sum of the maximum number of
shares of common stock issuable upon the conversion of the Convertible Notes and the OID Convertible Notes issued to the selling stockholders,
and assuming that all convertible debts and other liabilities under the Convertible Notes are converted into shares
of common stock, without regard to any applicable limitations or restrictions. In accordance with the terms of each Units
Registration Rights Agreement, each OID Units Registration Rights Agreement, the Registration Rights Letter Agreement (as defined in
“Description of Securities – Placement Agent Warrants – Placement Agent Warrants Issued in Connection with
2020 Private Placement”), the Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants,
the table below includes for resale under this prospectus the sum of the maximum number of shares of common stock issuable upon the exercise
in full of the Class C Warrants, the Class E Warrants, the Class F Warrants, and the Placement Agent Warrants issued to the selling stockholders,
without regard to any applicable limitations or restrictions. The holders of the Convertible Notes and Class C Warrants
have executed waivers of the registration rights under these securities. Notwithstanding these waivers, the shares underlying these securities
are included in the amounts being offered under this prospectus as reflected in the table below. In accordance with the terms of the
Registration Rights Letter Agreement, the table also includes for resale under this prospectus the shares of common stock issued to the
selling stockholders in connection with a private placement completed on September 25, 2020. We have also elected to include certain
other shares held by stockholders for resale as reflected in the table below.
Except
as otherwise indicated in the table below, the selling stockholders may sell all, some or none of the shares of common stock being offered.
See “Plan of Distribution.” We have no way of determining the number of shares of common stock each selling stockholder
will hold after this offering.
The
amount and percentage of shares of common stock beneficially owned by the selling stockholders after the offering is listed in the fourth
and fifth columns of the table below, respectively. For purposes of determining the amount and percentage of shares of common stock beneficially
owned after this offering, we have assumed that any shares of common stock being offered will be sold in this offering, and any shares
of common stock being offered that are issuable upon exercise of a warrant or conversion of a convertible note that are being offered
will be sold immediately following the exercise or conversion of the warrant or convertible note under which such common stock is issuable,
without regard to any applicable limitations or restrictions.
| | |
Amount
of Shares of Common Stock Beneficially Owned Prior
to this | | |
Amount of Shares of Common Stock | | |
Shares of Common Stock Beneficially Owned After this Offering | |
| Name of Selling Stockholder | |
Offering | | |
Being Offered | | |
Amount(1) | | |
Percent(1) | |
| ABSSV Group Inc.(2) | |
| 1,837,405 | (2) | |
| 1,837,405 | (2) | |
| - | | |
| - | |
| Nicholas Carosi III Revocable Living Trust(3) | |
| 734,958 | (3) | |
| 734,958 | (3) | |
| - | | |
| - | |
| Dim Sum Sam Inc.(4) | |
| 2,438,370 | (4) | |
| 4,409,745 | (4) | |
| - | | |
| - | |
| Jerome J. Ginsburg and Carol P. Ginsburg | |
| 367,490 | (5) | |
| 367,490 | (5) | |
| - | | |
| - | |
| Joerg Augsburg | |
| 367,490 | (6) | |
| 367,490 | (6) | |
| - | | |
| - | |
| Jun Capital Holdings Limited(7) | |
| 2,366,942 | (7) | |
| 29,398,302 | (7) | |
| - | | |
| - | |
| Ka Kit Lau | |
| 2,438,370 | (8) | |
| 4,409,745 | (8) | |
| - | | |
| - | |
| Kang Yoo | |
| 2,440,471 | (9) | |
| 3,674,788 | (9) | |
| - | | |
| - | |
| Kristie Chow | |
| 1,102,448 | (10) | |
| 1,102,448 | (10) | |
| - | | |
| - | |
| Li Ling and Adeline Kim | |
| 1,102,448 | (11) | |
| 1,102,448 | (11) | |
| - | | |
| - | |
| Mercurius Capital Limited(12) | |
| 734,958 | (12) | |
| 734,958 | (12) | |
| - | | |
| - | |
| Ouna Naomi Tong | |
| 1,469,629 | (13) | |
| 1,469,629 | (13) | |
| - | | |
| - | |
| Qi Wu | |
| 2,437,845 | (14) | |
| 4,593,490 | (14) | |
| - | | |
| - | |
| Qiaohong Li | |
| 734,958 | (15) | |
| 734,958 | (15) | |
| - | | |
| - | |
| Randel Green | |
| 2,204,873 | (16) | |
| 2,204,873 | (16) | |
| - | | |
| - | |
| Rongpeng Xia | |
| 2,425,765 | (17) | |
| 8,819,490 | (17) | |
| - | | |
| - | |
| T&J Brothers Group LLC(18) | |
| 2,446,773 | (18) | |
| 7,171,699 | (18) | |
| - | | |
| - | |
| Timothy Alpers | |
| 734,958 | (19) | |
| 734,958 | (19) | |
| - | | |
| - | |
| Weifeng Li | |
| 1,102,448 | (20) | |
| 1,102,448 | (20) | |
| - | | |
| - | |
| Xiansong Luo | |
| 734,958 | (21) | |
| 734,958 | (21) | |
| - | | |
| - | |
| Xiao Zhang | |
| 1,837,405 | (22) | |
| 1,837,405 | (22) | |
| - | | |
| - | |
| Xuexin Wang | |
| 1,469,915 | (23) | |
| 1,469,915 | (23) | |
| - | | |
| - | |
| Yiufai Yeung | |
| 734,958 | (24) | |
| 734,958 | (24) | |
| - | | |
| - | |
| Zhuo Yang | |
| 2,442,572 | (25) | |
| 2,939,830 | (25) | |
| - | | |
| - | |
| Eugene Podokshik | |
| 20,000 | | |
| 20,000 | | |
| - | | |
| - | |
| BWL Investments Ltd(26) | |
| 2,427,633 | (26) | |
| 12,052,632 | (26) | |
| 444,432 | | |
| * | |
| Raine Juhani Peltokoski | |
| 2,435,218 | (27) | |
| 5,512,550 | (27) | |
| - | | |
| - | |
| David Moss | |
| 734,958 | (28) | |
| 734,958 | (28) | |
| - | | |
| - | |
| US Strategic Capital LLC(29) | |
| 734,958 | (29) | |
| 734,958 | (29) | |
| - | | |
| - | |
| RCA Capital Partners Inc.(30) | |
| 2,444,504 | (30) | |
| 7,824,103 | (30) | |
| - | | |
| - | |
| Roberto Nascimento | |
| 238,855 | (31) | |
| 238,855 | (31) | |
| - | | |
| - | |
| Nelson E. Wert | |
| 1,469,915 | (32) | |
| 1,469,915 | (32) | |
| - | | |
| - | |
| Richard Klein | |
| 734,958 | (33) | |
| 734,958 | (33) | |
| - | | |
| - | |
| William N. Strawbridge | |
| 10,000 | | |
| 10,000 | | |
| - | | |
| - | |
| John Willis | |
| 367,490 | (34) | |
| 367,490 | (34) | |
| - | | |
| - | |
| Frank Maresca Jr. | |
| 2,402,537 | (35) | |
| 16,446,546 | (35) | |
| 275,269 | | |
| * | |
| Bologna Family Restaurant SpA(36) | |
| 2,450,975 | (36) | |
| 6,428,134 | (36) | |
| - | | |
| - | |
| About Investment Ltd(37) | |
| 2,450,975 | (37) | |
| 43,054,809 | (37) | |
| - | | |
| - | |
| Stanton Ross | |
| 2,065,542 | (38) | |
| 2,065,542 | (38) | |
| - | | |
| - | |
| Thomas Heckman | |
| 929,478 | (39) | |
| 929,478 | (39) | |
| - | | |
| - | |
| Bryce Ferrell | |
| 2,450,975 | (40) | |
| 4,131,085 | (40) | |
| - | | |
| - | |
| Viner Total Investments Fund(41) | |
| 2,450,975 | (41) | |
| 246,425,988 | (41) | |
| - | | |
| - | |
| Bevilacqua PLLC(42) | |
| 1,639,437 | (42) | |
| 1,639,437 | (42) | |
| - | | |
| - | |
| 1319983 BC Ltd.(43) | |
| 2,450,975 | (43) | |
| 3,229,228 | (43) | |
| - | | |
| - | |
| Li Lin | |
| 2,450,975 | (44) | |
| 4,071,684 | (44) | |
| - | | |
| - | |
| Oleg Kovalyov | |
| 2,380,260 | (45) | |
| 4,886,429 | (45) | |
| - | | |
| - | |
| Progressive Automations Inc.(46) | |
| 1,222,624 | (46) | |
| 1,222,624 | (46) | |
| - | | |
| - | |
| Waichun Logistics Technology Ltd(47) | |
| 2,450,975 | (47) | |
| 59,111,507 | (47) | |
| - | | |
| - | |
| Pioneer
Capital Anstalt(48) | |
| 2,450,975 | (48) | |
| 40,727,025 | (48) | |
| - | | |
| - | |
| Li Ben | |
| 1,215,673 | (49) | |
| 1,215,673 | (49) | |
| - | | |
| - | |
| Chiu Hoi Cheung | |
| 2,450,975 | (50) | |
| 8,110,100 | (50) | |
| - | | |
| - | |
| Mibao Xiong | |
| 810,989 | (51) | |
| 810,989 | (51) | |
| - | | |
| - | |
| Qing Fu | |
| 2,450,975 | (52) | |
| 4,054,340 | (52) | |
| - | | |
| - | |
| Yun Zhang | |
| 2,077,069 | (53) | |
| 2,027,069 | (53) | |
| 50,000 | | |
| * | |
| Xiaoqing Wang | |
| 2,450,975 | (54) | |
| 4,115,773 | (54) | |
| - | | |
| - | |
| Yu Deng | |
| 2,028,894 | (55) | |
| 2,028,894 | (55) | |
| - | | |
| - | |
| Xiaoli Duan | |
| 2,450,975 | (56) | |
| 4,054,949 | (56) | |
| - | | |
| - | |
| Jingfeng Chen | |
| 2,450,975 | (57) | |
| 4,054,340 | (57) | |
| - | | |
| - | |
| Rafael Lopes Rogo | |
| 2,450,975 | (58) | |
| 4,054,949 | (58) | |
| - | | |
| - | |
| Honey Tree Trading(59) | |
| 2,450,975 | (59) | |
| 6,082,423 | (59) | |
| - | | |
| - | |
| Jason Douglas Riley | |
| 2,450,975 | (60) | |
| 4,055,152 | (60) | |
| - | | |
| - | |
| Alessandro Solimeo | |
| 2,027,474 | (61) | |
| 2,027,474 | (61) | |
| - | | |
| - | |
| Allesia Solimeo | |
| 2,027,474 | (62) | |
| 2,027,474 | (62) | |
| - | | |
| - | |
| Anthony Cirillo | |
| 793,052 | (63) | |
| 694,958 | (63) | |
| 98,094 | | |
| * | |
| Supereight Capital Ltd.(64) | |
| 2,437,687 | (64) | |
| 2,953,575 | (64) | |
| 253,000 | | |
| * | |
| Xiniue Nie | |
| 2,450,975 | (65) | |
| 2,779,830 | (66) | |
| - | | |
| - | |
| Qinghui Lin | |
| 734,958 | (66) | |
| 694,958 | (67) | |
| 40,000 | | |
| * | |
| Bangrong Li | |
| 2,421,563 | (67) | |
| 9,729,383 | (67) | |
| 560,000 | | |
| * | |
| Walleye Opportunities Master
Fund Ltd(68) | |
| 2,450,975 | (68) | |
| 94,576,187 | (68) | |
| - | | |
| - | |
| Hexin Global Ltd.(69) | |
| 2,450,975 | (69) | |
| 87,513,011 | (69) | |
| - | | |
| - | |
| Univest Securities, LLC(70) | |
| 5,178,769 | (70) | |
| 31,779,659 | (70) | |
| 6,000 | | |
| * | |
| Bradley Richmond(71) | |
| 5,140,357 | (71) | |
| 47,990,068 | (71) | |
| 52,088 | | |
| * | |
| Alta Investments LLC(72) | |
| 2,450,975 | (72) | |
| 4,205,996 | (72) | |
| - | | |
| - | |
| Horberg Enterprises
LP(73) | |
| 2,450,975 | (73) | |
| 2,523,604 | (73) | |
| - | | |
| - | |
| MSS Capital LLC(74) | |
| 2,450,975 | (74) | |
| 4,205,996 | (74) | |
| - | | |
| - | |
| Jacob Boegel | |
| 414,352 | (75) | |
| 420,605 | (75) | |
| - | | |
| - | |
| Ying Liu | |
| 828,707 | (76) | |
| 841,213 | (76) | |
| - | | |
| - | |
| Janice E. Glure Trust(77) | |
| 2,071,732 | (77) | |
| 2,102,998 | (77) | |
| - | | |
| - | |
| Kenneth M. Sutin MD
Revocable Trust(78) | |
| 2,071,732 | (78) | |
| 2,102,998 | (78) | |
| - | | |
| - | |
| Rainforest Partners
LLC(79) | |
| 2,450,975 | (79) | |
| 8,411,956 | (79) | |
| - | | |
| - | |
| Mingyong Huang | |
| 2,450,975 | (80) | |
| 4,205,996 | (80) | |
| - | | |
| - | |
| Todd A. Carpenter | |
| 1,035,866 | (81) | |
| 1,051,499 | (81) | |
| - | | |
| - | |
| Weifang Xu | |
| 2,450,975 | (82) | |
| 3,364,782 | (82) | |
| - | | |
| - | |
| Bartholemew Pan-Kita(83) | |
| 635,294 | (83) | |
| 635,294 | (83) | |
| - | | |
| - | |
| Azem Nesimi(84) | |
| 7,059 | (84) | |
| 7,059 | (84) | |
| - | | |
| - | |
| Alvaro Croquevielle | |
| 220,107 | | |
| 220,107 | | |
| - | | |
| - | |
| Amar Dhaduk(85) | |
| 2,294,097 | | |
| 529,407 | | |
| 1,764,690 | | |
| * | |
| Darpan Dhaduk(86) | |
| 2,294,097 | | |
| 529,407 | | |
| 1,764,690 | | |
| * | |
| Kevin Dunleavy | |
| 15,252 | | |
| 15,252 | | |
| - | | |
| - | |
| Letitia Maresca | |
| 108,500 | | |
| 100,000 | | |
| 8,500 | | |
| * | |
| Payal Dhaduk(87) | |
| 2,294,097 | | |
| 529,407 | | |
| 1,764,690 | | |
| * | |
| Ranjanben V. Dhaduk(88) | |
| 4,610,548 | | |
| 3,546,610 | | |
| 1,063,938 | | |
| * | |
| Raul Ciudad | |
| 142,200 | | |
| 142,200 | | |
| - | | |
| - | |
| Rieux Enterprise Corp.(89) | |
| 1,072,560 | | |
| 1,072,560 | | |
| - | | |
| - | |
| SIF Investment Ltd(90) | |
| 116,760 | | |
| 116,760 | | |
| - | | |
| - | |
* Less than 1%.
| (1) |
The
percentage of beneficial ownership after completion of the offering is based on 947,766,691 shares of common stock outstanding
following the offering, consisting of 46,666,760 shares of common stock outstanding as of September 19, 2023 and 901,099,931
additional shares of common stock being offered under this prospectus that were not outstanding on that date. As noted above,
for purposes of determining the amount and percentage of shares of common stock beneficially owned after this offering, we have assumed
that any shares of common stock being offered will be sold in this offering, and any shares of common stock being offered that are
issuable upon exercise of a warrant or conversion of a convertible note that are being offered will be sold immediately following
the exercise or conversion of the warrant or convertible note under which such common stock is issuable, without regard to any
applicable limitations or restrictions. |
| |
|
| (2) |
Consists
of (i) 100,000 shares of common stock and (ii)
1,737,405 shares of common stock issuable upon exercise of a Class C Warrant. YanBo Huang, President of ABSSV Group Inc., has sole
voting and investment power over the securities held by ABSSV Group Inc. |
| |
|
| (3) |
Consists
of (i) 40,000 shares of common stock and (ii)
694,958 shares of common stock issuable upon exercise of a Class C Warrant. Nicholas Carosi has sole voting and investment power
over the securities held by Nicholas Carosi III Revocable Living Trust. |
| |
|
| (4) |
Beneficially
owned shares of common stock consist of (i)
240,000 shares of common stock and (ii) 4,169,745 shares of common stock issuable upon exercise of a Class C Warrant, subject
to a beneficial ownership limitation applicable to the Class C Warrants and the Convertible Note limiting beneficial ownership to
4.99% of the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 240,000 shares
of common stock and (ii) the 4,169,745 shares of common stock issuable upon exercise of a Class C Warrant. Sam Yan, sole member
of Dim Sum Sam Inc., has sole voting and investment power over the securities held by Dim Sum Sam Inc. |
| |
|
| (5) |
Consists
of (i) 20,000 shares of common stock
and (ii) 347,490 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (6) |
Consists
of (i) 20,000 shares of common stock and (ii)
347,490 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (7) |
Consists
of (i) 1,600,000 shares of common stock and
(ii) 27,798,302 shares of common stock issuable upon exercise of a Class C Warrant, subject to a beneficial ownership limitation
applicable to the Class C Warrant limiting beneficial ownership to 4.99% of the outstanding shares of common stock. The shares of
common stock being offered for resale consist of (i) the 1,600,000 shares of common stock and (ii) the 27,798,302 shares of common
stock issuable upon exercise of a Class C Warrant. Feng Guo, sole member of Jun Capital Holdings Limited, has sole voting and
investment power over the securities held by Jun Capital Holdings Limited. |
| |
|
| (8) |
Beneficially
owned shares of common stock consist of (i) 240,000 shares of common stock held directly, (ii) 4,169,745 shares of common stock issuable
upon exercise of a Class C Warrant held directly, (iii) 40,000 shares of common stock held by Mercurius Capital Limited, and (iv)
694,958 shares of common stock issuable upon exercise of a Class C Warrant held by Mercurius Capital Limited, subject to a beneficial
ownership limitation applicable to the Class C Warrants limiting beneficial ownership to 4.99% of the outstanding shares of common
stock. The shares of common stock being offered for resale consist of (i) the 240,000 shares of common stock held directly and (ii)
the 4,169,745 shares of common stock issuable upon exercise of the Class C Warrant held directly. Ka Kit Lau, Chief Executive Officer
of Mercurius Capital Limited, has sole voting and investment power over the securities held by Mercurius Capital Limited. Mercurius
Capital Limited is a selling stockholder and may offer and sell in this offering the securities described above that are held by
it. See footnote 12. |
| |
|
| (9) |
Beneficially
owned shares of common stock consist
of (i) 200,000 shares of common stock and (ii) 3,474,788 shares of common stock issuable upon exercise of two Class C Warrants,
subject to a beneficial ownership limitation applicable to the Class C Warrants limiting beneficial ownership to
4.99% of the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i)
the 200,000 shares of common stock and (ii) the 3,474,788 shares of common stock issuable upon exercise of the Class C Warrants. |
| (10) |
Consists
of (i) 60,000 shares of common stock and (ii)
1,042,448 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (11) |
Consists
of (i) 60,000 shares of common stock and (ii)
1,042,448 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (12) |
Consists
of (i) 40,000 shares of common stock and (ii)
694,958 shares of common stock issuable upon exercise of a Class C Warrant. Ka Kit Lau, Chief Executive Officer of Mercurius
Capital Limited, has sole voting and investment power over the securities held by Mercurius Capital Limited. |
| |
|
| (13) |
Consists
of (i) 79,984 shares of common stock and (ii)
1,389,645 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (14) |
Beneficially
owned shares of common stock consist
of (i) 250,000 shares of common stock and (ii) 4,343,490 shares of common stock issuable upon exercise of two Class C Warrants,
subject to a beneficial ownership limitation applicable to the Class C Warrants limiting beneficial ownership to 4.99% of
the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 250,000 shares
of common stock held and (ii) the 4,343,490 shares of common stock issuable upon exercise of the Class C Warrants. |
| |
|
| (15) |
Consists
of (i) 40,000 shares of common stock and (ii)
694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (16) |
Consists
of (i) 120,000 shares of common stock and (ii)
2,084,873 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (17) |
Beneficially
owned shares of common stock consist of (i)
480,000 shares of common stock and (ii) 8,339,490 shares of common stock issuable upon exercise of a Class C Warrant, subject
to a beneficial ownership limitation applicable to the Class C Warrants limiting beneficial ownership to 4.99% of the outstanding
shares of common stock. The shares of common stock being offered for resale consist of (i) the 480,000 shares of common stock and
(ii) the 8,339,490 shares of common stock issuable upon exercise of the Class C Warrant. |
| |
|
| (18) |
Beneficially
owned shares of common stock consist of (i) 80,000 shares of common stock, (ii) 4,989,915 shares of common stock issuable upon exercise
of two Class C Warrants, and (iii) 2,101,784 shares of common stock issuable upon conversion of a Convertible Note, subject to a
beneficial ownership limitation applicable to the Class C Warrants and the Convertible Note limiting beneficial ownership to 4.99%
of the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 80,000 shares of
common stock, (ii) the 4,989,915 shares of common stock issuable upon exercise of two Class C Warrants, and (iii) the 2,101,784 shares
of common stock issuable upon conversion of the Convertible Note. Xingyu Yang and Zhonghao Chen, Partners of T&J Brothers Group
LLC, have joint voting and investment power over the securities held by T&J Brothers Group LLC. |
| |
|
| (19) |
Consists
of (i) 40,000 shares of common stock and (ii)
694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (20) |
Consists
of (i) 60,000 shares of common stock
and (ii) 1,042,448 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (21) |
Consists
of (i) 40,000 shares of common stock and (ii)
694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (22) |
Consists
of (i) 100,000 shares of common stock and (ii) 1,737,405 shares of common stock issuable upon exercise of a Class C Warrant. |
| (23) |
Consists
of (i) 80,000 shares of common stock and (ii)
1,389,915 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (24) |
Consists
of (i) 40,000 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (25) |
Beneficially
owned shares of common stock consist of (i)
160,000 shares of common stock and (ii) 2,779,830 shares of common stock issuable upon exercise of a Class C Warrant,
subject to a beneficial ownership limitation applicable to the Class C Warrant limiting beneficial ownership to 4.99% of the
outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 160,000 shares of common
stock and (ii) the 2,779,830 shares of common stock issuable upon exercise of two Class C Warrants. |
| |
|
| (26) |
Beneficially
owned shares of common stock consist of (i) 444,432 shares of common stock, (ii) 8,237,337 shares of common stock issuable upon exercise
of three Class C Warrants, and (iii) 3,815,295 shares of common stock issuable upon conversion of two Convertible Notes, subject
to a beneficial ownership limitation applicable to the Class C Warrants and the Convertible Notes limiting beneficial ownership to
4.99% of the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 8,237,337
shares of common stock issuable upon exercise of three Class C Warrants, and (ii) the 3,815,295 shares of common stock issuable upon
conversion of the two Convertible Notes. Braeden Lichti, Chief Executive Officer of BWL Investments Ltd, has sole voting and investment
power over the securities held by BWL Investments Ltd. |
| |
|
| (27) |
Beneficially
owned shares of common stock consist of (i) 300,020 shares of common stock and (ii) 5,212,530 shares of common stock issuable upon
exercise of a Class C Warrant, subject to a beneficial ownership limitation applicable to the Class C Warrants and the Convertible
Notes limiting beneficial ownership to 4.99% of the outstanding shares of common stock. The shares of common stock being offered
for resale consist of (i) the 300,020 shares of common stock and (ii) the 5,212,530 shares of common stock issuable upon exercise
of a Class C Warrant. |
| |
|
| (28) |
Consists
of (i) 40,000 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (29) |
Consists
of (i) 40,000 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise of a Class C Warrant. Yue Wu
has sole voting and investment power over the securities held by US Strategic Capital LLC. |
| |
|
| (30) |
Beneficially
owned shares of common stock consist of (i) 123,200 shares of common stock, (ii) 4,831,804 shares of common stock issuable upon exercise
of two Class C Warrants, (iii) 1,607,280 shares of common stock issuable upon conversion of a Convertible Note, (iv) 441,187 shares
of common stock issuable upon exercise of a Class E Warrant, (v) 441,187 shares of common stock issuable upon exercise of a Class
F Warrant, and (vi) 360,685 shares of common stock issuable upon conversion of an OID Convertible Note within 60 days of September
19, 2023, subject to a beneficial ownership limitation applicable to the Class C Warrants, the Convertible Note, the Class E Warrant,
the Class F Warrant and the OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 123,200 shares of common stock, (ii) the 4,831,804 shares
of common stock issuable upon exercise of the two Class C Warrants, (iii) the 1,607,280 shares of common stock issuable upon conversion
of the Convertible Note, (iv) 441,187 shares of common stock issuable upon exercise of the Class E Warrant, (v) 441,187 shares of
common stock issuable upon exercise of the Class F Warrant, and (vi) up to 379,445 shares of common stock issuable upon conversion
of the OID Convertible Note upon maturity. Wayne Andrews, President of RCA Capital Partners Inc., has sole voting and investment
power over the securities held by RCA Capital Partners Inc. |
| |
|
| (31) |
Consists
of (i) 13,000 shares of common stock and (ii) 225,855 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (32) |
Consists
of (i) 80,000 shares of common stock and (ii) 1,389,915 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (33) |
Consists
of (i) 40,000 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise of a Class C Warrant. |
| (34) |
Consists
of (i) 20,000 shares of common stock and (ii) 347,490 shares of common stock issuable upon exercise of a Class C Warrant. |
| |
|
| (35) |
Beneficially
owned shares of common stock consist of (i) 922,269 shares of common stock, (ii) 680,168 shares of common stock issuable upon exercise
of a Class C Warrant, (iii) 406,200 shares of common stock issuable upon conversion of a Convertible Note, (iv) 5,147,063
shares of common stock issuable upon exercise of two Class E Warrants, (v) 5,147,063 shares of common stock issuable upon
exercise of two Class F Warrants, and (vi) 4,252,389 shares of common stock issuable upon conversion of two OID Convertible
Notes within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
C Warrants, the Convertible Note, the Class E Warrants, the Class F Warrants and the OID Convertible Notes limiting beneficial
ownership to 4.99% of the outstanding shares of common stock. The shares of common stock being offered for resale consist
of (i) 647,000 shares of common stock, (ii) the 680,168 shares of common stock issuable upon exercise of the Class C Warrant,
(iii) the 406,200 shares of common stock issuable upon conversion of the Convertible Note, (iv) the 5,147,074 shares
of common stock issuable upon exercise of the two Class E Warrants, (v) the 5,147,074 shares of common stock
issuable upon exercise of the two Class F Warrants, and (vi) up to 4,419,030 shares of common stock issuable
upon conversion of the two OID Convertible Notes upon maturity. |
| |
|
| (36) |
Beneficially
owned shares of common stock consist of (i) 4,058,897 shares of common stock issuable upon exercise of two Class C Warrants and (ii)
2,369,237 shares of common stock issuable upon conversion of two Convertible Notes, subject to a beneficial ownership limitation
applicable to the Class C Warrants and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 4,058,897 shares of common
stock issuable upon exercise of the two Class C Warrants and (ii) the 2,369,237 shares of common stock issuable upon conversion
of the two Convertible Notes. Saverio Solimeo, President of Bologna Family Restaurant SpA, has sole voting and investment power over
the securities held by Bologna Family Restaurant SpA. |
| |
|
| (37) |
Beneficially
owned shares of common stock consist of (i) 26,956,337 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
16,098,472 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 26,956,337 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 16,098,472 shares of common stock issuable upon conversion
of the Convertible Note. Jiaming Li, Chief Executive Officer of About Investment Ltd, has sole voting and investment power over the
securities held by About Investment Ltd. |
| |
|
| (38) |
Consists
of (i) 1,293,222 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 772,320 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (39) |
Consists
of (i) 581,940 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 347,538 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (40) |
Beneficially
owned shares of common stock consist of (i) 2,586,445 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,544,640 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,586,445 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,544,640 shares of common stock issuable upon
conversion of the Convertible Note. |
| |
|
| (41) |
Beneficially
owned shares of common stock consist
of (i) 154,285,714 shares of common stock issuable upon exercise of a Class C Warrant and (ii) 92,140,274 shares of common
stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation applicable to the Class C Warrant
and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock. The shares of common stock
being offered for resale consist of (i) the 154,285,714 shares of common stock issuable upon exercise of a Class C Warrant
and (i) the 92,140,274 shares of common stock issuable upon conversion of the Convertible Note. Cheng Wan Wing, Chen Hua,
and Hu Mingyue, the directors of Viner Total Investments Fund, Apollo Asset Management Limited, Viner Total Investments
Fund’s investment manager, and Wong Ho Tim, the sole officer, director or other affiliate of Apollo Asset Management Limited,
currently share voting and investment power over the securities held by Viner Total Investments Fund. |
| (42) |
Beneficially
owned shares of common stock consist of (i) 1,028,571 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
610,866 shares of common stock issuable upon conversion of a Convertible Note. The shares of common stock being offered
for resale consist of (i) the 1,028,571 shares of common stock issuable upon exercise of the Class C Warrant and (ii)
the 610,866 shares of common stock issuable upon conversion of the Convertible Note. Louis A. Bevilacqua has sole voting and
investment power over the securities held by Bevilacqua PLLC. Mr. Bevilacqua disclaims beneficial ownership of such shares except
to the extent of his pecuniary interest, if any, in such shares. See “Legal Matters” for further information about
Bevilacqua PLLC. |
| |
|
| (43) |
Beneficially
owned shares of common stock consist of (i) 2,028,008 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,201,220 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership
limitation applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the
outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 2,028,008 shares
of common stock issuable upon exercise of the Class C Warrant and (ii) the 1,201,220 shares of common stock issuable upon
conversion of the Convertible Note. Lisa Ross, Director of 1319983 BC Ltd., has sole voting and investment power over the securities
held by 1319983 BC Ltd. |
| |
|
| (44) |
Beneficially
owned shares of common stock consist of (i) 2,570,785 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,500,899 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,570,785 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,500,899 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (45) |
Beneficially
owned shares of common stock consist of (i) 1,346,410 shares of common stock, (ii) 3,085,200 shares of common stock issuable
upon exercise of a Class C Warrant, and (iii) 454,819 shares of common stock issuable upon conversion of a Convertible Note, subject
to a beneficial ownership limitation applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to
4.99% of the outstanding shares of common stock. The shares of common stock being offered for resale consist of (i) the 1,346,410
shares of common stock, (ii) the 3,085,200 shares of common stock issuable upon exercise of the Class C Warrant, and (iii) the 454,819
shares of common stock issuable upon conversion of the Convertible Note. |
| |
|
| (46) |
Consists
of (i) 771,942 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 450,682 shares of common stock issuable upon conversion of a Convertible Note.
Vladimir Kovalyov, Director of Progressive Automations Inc., has sole voting and investment power over the securities held by
Progressive Automations Inc. |
| |
|
| (47) |
Beneficially
owned shares of common stock consist of 59,111,507
shares of common stock issuable upon conversion of three Convertible Notes, subject to a beneficial ownership limitation applicable
to the Convertible Notes limiting beneficial ownership to 4.99% of the outstanding shares of common stock. The shares
of common stock being offered for resale consist of the 59,111,507 shares of common stock issuable upon conversion of the
Convertible Notes. Hui Xian, Chief Executive Officer of Waichun Logistics Technology Ltd, has sole voting and investment
power over the securities held by Waichun Logistics Technology Ltd. |
| |
|
| (48) |
Beneficially
owned shares of common stock consist of (i) 25,714,285 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
15,012,740 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common
stock. The shares of common stock being offered for resale consist of (i) the 25,714,285 shares of common stock
issuable upon exercise of the Class C Warrant and (ii) the 15,012,740 shares of common stock issuable upon conversion of the
Convertible Note. Lucas Mair, director of Pioneer Capital Anstalt, has sole voting and investment power over the securities
held by Pioneer Capital Anstalt. The business address of Pioneer Capital Anstalt is Drescheweg 2, 9490 Vaduz, Principality
of Liechtenstein, with an additional mailing address of c/o LH Financial Services, 510 Madison Ave., Suite 1400, New York, NY 10022. |
| (49) |
Consists
of (i) 770,914 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 444,759 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (50) |
Beneficially
owned shares of common stock consist of (i) 5,142,985 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
2,967,115 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 5,142,985 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 2,967,115 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (51) |
Consists
of (i) 514,285 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 296,704 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (52) |
Beneficially
owned shares of common stock consist of (i) 2,571,042 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,483,298 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,571,042 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,483,298 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (53) |
Beneficially
owned shares of common stock consist of (i) 50,000 shares of common stock, (ii) 1,285,457 shares of common stock issuable
upon exercise of a Class C Warrant and (iii) 741,612 shares of common stock issuable upon conversion of a Convertible Note.
The shares of common stock being offered for resale consist of (i) the 1,285,457 shares of common stock issuable
upon exercise of the Class C Warrant and (ii) the 741,612 shares of common stock issuable upon conversion of the Convertible
Note. |
| |
|
| (54) |
Beneficially
owned shares of common stock consist of (i) 2,610,000 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,505,773 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,610,000 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,505,773 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (55) |
Consist
of (i) 1,286,614 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 742,280 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (56) |
Beneficially
owned shares of common stock consist of (i) 2,571,428 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,483,521 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,571,428 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,483,521 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (57) |
Beneficially
owned shares of common stock consist of (i) 2,571,042 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,483,298 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,571,042 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 1,483,298 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (58) |
Consists
of (i) 2,571,428 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 1,483,521 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (59) |
Beneficially
owned shares of common stock consist of (i) 3,857,142 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
2,225,281 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding
shares of common stock. The shares of common stock being offered for resale consist of (i) the 3,857,142 shares of
common stock issuable upon exercise of the Class C Warrant and (ii) the 2,225,281 shares of common stock issuable upon conversion
of the Convertible Note. Barbara Knight, owner of Honey Tree Trading, has sole voting and investment power over the securities held
by Honey Tree Trading. |
| (60) |
Beneficially
owned shares of common stock consist of (i) 2,571,557 shares of common stock issuable upon exercise of a Class C Warrant and (ii)
1,483,595 shares of common stock issuable upon conversion of a Convertible Note, subject to a beneficial ownership limitation
applicable to the Class C Warrant and the Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of (i) the 2,571,557 shares of common
stock issuable upon exercise of the Class C Warrant and (ii) the 1,483,595 shares of common stock issuable upon conversion
of the Convertible Note. |
| |
|
| (61) |
Consists
of (i) 1,285,714 shares of common stock issuable upon exercise of a Class C Warrant and (ii) 741,760 shares of common stock issuable
upon conversion of a Convertible Note. |
| |
|
| (62) |
Consists
of (i) 1,285,714 shares of common stock issuable
upon exercise of a Class C Warrant and (ii) 741,760 shares of common stock issuable upon conversion of a Convertible Note. |
| |
|
| (63) |
Beneficially
owned shares of common stock consist of (i) 98,094 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise
of a Class C Warrant. The shares of common stock
being offered for resale consist of the 694,958 shares of common stock issuable upon exercise of the Class C Warrant. |
| |
|
| (64) |
Beneficially
owned shares of common stock consist of (i)
253,000 shares of common stock and (ii) 2,953,575 shares of common stock issuable upon exercise of a Class C Warrant,
subject to a beneficial ownership limitation applicable to the Class C Warrant limiting beneficial ownership to 4.99%
of the outstanding shares of common stock. The shares of common stock being offered for resale consist of the
2,953,575 shares of common stock issuable upon exercise of the Class C Warrant. Richard Calta, President of Supereight Capital
Ltd., has sole voting and investment power over the securities held by Supereight Capital Ltd. |
| |
|
| (65) |
Beneficially
owned shares of common stock consist
of 2,779,830 shares of common stock issuable upon exercise of a Class C Warrant, subject to a beneficial ownership limitation
applicable to the Class C Warrant limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of the 2,779,830 shares of common stock issuable upon exercise of
the Class C Warrant. |
| |
|
| (66) |
Beneficially
owned shares of common stock consist
of (i) 40,000 shares of common stock and (ii) 694,958 shares of common stock issuable upon exercise of a Class C
Warrant. The shares of common stock being offered for resale consist of the 694,958 shares of common stock issuable upon exercise
of the Class C Warrant. |
| |
|
| (67) |
Beneficially
owned shares of common stock consist of (i)
560,000 shares of common stock and (ii) 9,729,383 shares of common stock issuable upon exercise of a Class C Warrant, subject to
a beneficial ownership limitation applicable to the Class C Warrant limiting beneficial ownership to 4.99% of the outstanding shares
of common stock. The shares of common stock being offered for resale consist of the 9,729,383 shares of common stock issuable
upon exercise of the Class C Warrant. |
| |
|
| (68) |
Beneficially
owned shares of common stock consist
of (i) 33,088,249 shares of common stock issuable upon exercise of two Class E Warrants, (ii) 33,088,249 shares of common stock issuable
upon exercise of two Class F Warrants, and (iii) 27,775,998 shares of common stock issuable upon conversion of two OID Convertible
Notes within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class E Warrants, Class
F Warrants, and OID Convertible Notes limiting beneficial ownership to 4.99% of the outstanding shares of common stock. The shares
of common stock being offered for resale consist of (i) the 33,088,249 shares of common stock issuable upon exercise of the two Class
E Warrants, (ii) the 33,088,249 shares of common stock issuable upon exercise of the two Class F Warrants, and (iii) up to 28,399,689
shares of common stock issuable upon conversion of the two OID Convertible Notes upon maturity. William England has sole voting
and investment power over the securities held by Walleye Opportunities Master Fund Ltd. |
| (69) |
Beneficially
owned shares of common stock consist
of (i) 34,178,431 shares of common stock issuable upon exercise of three Class E Warrants, (ii) 30,428,431 shares of common stock
issuable upon exercise of three Class F Warrants, and (iii) up to 22,216,333 shares of common stock issuable upon conversion of
two OID Convertible Notes within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrants, Class F Warrants, and OID Convertible Notes limiting beneficial ownership to 4.99% of the outstanding shares of common
stock. The shares of common stock being offered for resale consist of (i) the 34,178,431 shares of common stock issuable upon exercise
of the three Class E Warrants, (ii) the 30,428,431 shares of common stock issuable upon exercise of the three Class F Warrants, and
(iii) up to 22,906,149 shares of common stock issuable upon conversion of the two OID Convertible Notes upon maturity.
Chuntao Zhao, sole director of Hexin Global Ltd., has sole voting and investment power over the securities held by Hexin Global Ltd. |
| |
|
| (70) |
Beneficially
owned shares of common stock consist of (i) 6,000 shares of common stock, (ii) 168,008 shares of common stock issuable upon
exercise of a 2020 Placement Agent Warrant, (iii) 3,548,148 shares of common stock issuable upon exercise of a Replacement Class
C Warrant, (iv) 19,293,621 shares of common stock issuable upon exercise of Replacement Placement Agent Warrants, (v) 1,827,715 shares
of common stock issuable upon conversion of a Replacement Convertible Note, and (vi) 6,942,166 shares of common stock issuable upon
exercise of OID Units Placement Agent Warrants, which are considered by the Company to be subject to a beneficial ownership limitation
applicable to the 2020 Placement Agent Warrant, the Replacement Class C Warrant, the Replacement Placement Agent Warrants, the Replacement
Convertible Note, and the OID Units Placement Agent Warrants limiting beneficial ownership to 9.99% of the outstanding shares of
common stock. The shares of common stock being offered for resale consist of (i) the 168,008 shares of common stock issuable
upon exercise of the 2020 Placement Agent Warrant, (ii) the 3,548,148 shares of common stock issuable upon exercise
of the Replacement Class C Warrant, (iii) the 19,293,621 shares of common stock issuable upon exercise of the Replacement
Placement Agent Warrants, (iv) the 1,827,715 shares of common stock issuable upon conversion of the Replacement Convertible Note,
and (v) 6,942,166 shares of common stock issuable upon exercise of the OID Units Placement Agent Warrants. Yi Guo has
sole voting and investment power over the securities held by Univest. Univest served as our placement agent in connection with each
of the 2020 Private Placement, the Units Private Placement, and the OID Units Private Placement, and provided consulting service
in connection with the My Health Logic Acquisition, and was issued the 2020 Placement Agent Warrant, the Replacement Class
C Warrant, the Replacement Convertible Note, the Replacement Placement Agent Warrant, and were issued the OID Units Placement
Agent Warrants, as partial compensation for such services. |
| |
|
| (71) |
Beneficially
owned shares of common stock consist of (i) 352,088 shares of common stock, (ii) 112,006 shares of common stock issuable
upon exercise of a 2020 Placement Agent Warrant, (iii) 5,322,223 shares of common stock issuable upon exercise of a Replacement
Class C Warrant, (iv) 28,940,432 shares of common stock issuable upon exercise of Replacement Placement Agent Warrants, (v) 2,741,573
shares of common stock issuable upon conversion of a Replacement Convertible Note, and (vi) 10,573,834 shares of common stock issuable
upon exercise of OID Units Placement Agent Warrants, which are considered by the Company to be subject to a beneficial ownership
limitation applicable to the 2020 Placement Agent Warrant, the Replacement Class C Warrant, the Replacement Placement Agent Warrants,
the Replacement Convertible Note, and the OID Units Placement Agent Warrants limiting beneficial ownership to 9.99% of the outstanding
shares of common stock. The shares of common stock being offered for resale consist of (i) 300,000 of the shares of common
stock, (ii) the 112,006 shares of common stock issuable upon exercise of the 2020 Placement Agent Warrant, (iii) the 5,322,223
shares of common stock issuable upon exercise of the Replacement Class C Warrant, (iv) the 28,940,432 shares of common stock issuable
upon exercise of the Replacement Placement Agent Warrants, (v) the 2,741,573 shares of common stock issuable upon conversion of the
Replacement Convertible Note, and (vi) the 10,573,834 shares of common stock issuable upon exercise of the OID Units Placement Agent
Warrants. On September 30, 2020, we entered into the Richmond Consulting Agreement with Mr. Richmond, which terminated in accordance
with its terms on September 30, 2022. For a further description of the Richmond Consulting Agreement and compensation terms under
the Richmond Consulting Agreement, see “Executive Compensation – Other Management Compensation – Bradley Richmond”
above. On July 19, 2021, Mr. Richmond was appointed our Acting Vice President of Finance, which position he held until December 21,
2021. After Mr. Richmond’s resignation from this position upon the appointment of Harrison Ross as Vice President of Finance
on December 21, 2021, Mr. Richmond continued to serve as an employee of the Company until September 24, 2022. Bradley
Richmond is a registered representative of Univest and may be considered an “associated person” of Univest. Mr. Richmond,
on behalf of Univest, provided placement agent services in connection with each of the 2020 Private Placement, the Units
Private Placement, and the OID Units Private Placement, and Mr. Richmond provided consulting services in connection with the My
Health Logic Acquisition, and received certain compensation for such services. See “Certain Relationships and Related Party
Transactions – Transactions with Related Persons” for a further description of the services provided by Mr. Richmond
to the Company and the compensation provided to Mr. Richmond by the Company. |
| (72) |
Beneficially
owned shares of common stock consist of (i) 1,470,600 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 1,470,600
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 1,202,265 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 1,470,600 shares of common stock issuable upon exercise of
the Class E Warrant, (ii) the 1,470,600 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 1,264,796
shares of common stock issuable upon conversion of the OID Convertible Note upon maturity. Stephen Funk, Manager and sole member
of Alta Investments LLC, has sole voting and investment power over the securities held by Alta Investments LLC. |
| |
|
| (73) |
Beneficially
owned shares of common stock consist of (i) 882,362 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 882,362
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 721,361 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 882,362 shares of common stock issuable upon exercise of the
Class E Warrant, (ii) the 882,362 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 758,880 shares
of common stock issuable upon conversion of the OID Convertible Note upon maturity. Howard Todd Horberg, President of Horberg Enterprises
LP, has sole voting and investment power over the securities held by Horberg Enterprises LP. |
| |
|
| (74) |
Beneficially
owned shares of common stock consist of (i) 1,470,600 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 1,470,600
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 1,202,265 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 1,470,600 shares of common stock issuable upon exercise of
the Class E Warrant, (ii) the 1,470,600 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 1,264,796
shares of common stock issuable upon conversion of the OID Convertible Note upon maturity. Scott Caputo, Managing Member of MSS Capital
LLC, has sole voting and investment power over the securities held by MSS Capital LLC. |
| |
|
| (75) |
Beneficially
owned shares of common stock consist of (i) 147,062 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 147,062
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 120,228 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 147,062 shares of common stock issuable upon exercise of the
Class E Warrant, (ii) the 147,062 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 126,481 shares
of common stock issuable upon conversion of the OID Convertible Note upon maturity. |
| |
|
| (76) |
Beneficially
owned shares of common stock consist of (i) 294,125 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 294,125
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 240,457 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023. The shares of common stock being offered for resale consist of (i)
the 294,125 shares of common stock issuable upon exercise of the Class E Warrant, (ii) the 294,125 shares of common stock issuable
upon exercise of the Class F Warrant, and (iii) up to 252,963 shares of common stock issuable upon conversion of the OID Convertible
Note upon maturity. |
| (77) |
Beneficially
owned shares of common stock consist of (i) 735,300 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 735,300
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 601,132 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023. The shares of common stock being offered for resale consist of (i)
the 735,300 shares of common stock issuable upon exercise of the Class E Warrant, (ii) the 735,300 shares of common stock issuable
upon exercise of the Class F Warrant, and (iii) up to 632,398 shares of common stock issuable upon conversion of the OID Convertible
Note upon maturity. Janice E. Glure, Trustee of the Janice E. Glure Trust, has sole voting and investment power over the securities
held by the Janice E. Glure Trust. |
| |
|
| (78) |
Beneficially
owned shares of common stock consist of (i) 735,300 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 735,300
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 601,132 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023. The shares of common stock being offered for resale consist of (i)
the 735,300 shares of common stock issuable upon exercise of the Class E Warrant, (ii) the 735,300 shares of common stock issuable
upon exercise of the Class F Warrant, and (iii) up to 632,398 shares of common stock issuable upon conversion of the OID Convertible
Note upon maturity. Kenneth M. Sutin, MD, Trustee of the Kenneth M. Sutin MD Revocable Trust, has sole voting and investment power
over the securities held by the Kenneth M. Sutin MD Revocable Trust. |
| |
|
| (79) |
Beneficially
owned shares of common stock consist of (i) 2,941,187 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 2,941,187
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 2,404,521 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 2,941,187 shares of common stock issuable upon exercise of
the Class E Warrant, (ii) the 2,941,187 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 2,529,582
shares of common stock issuable upon conversion of the OID Convertible Note upon maturity. Mark Weinberger, Managing Member of Rainforest
Partners LLC, has sole voting and investment power over the securities held by the Rainforest Partners LLC. |
| |
|
| (80) |
Beneficially
owned shares of common stock consist of (i) 1,470,600 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 1,470,600
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 1,202,265 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 1,470,600 shares of common stock issuable upon exercise of
the Class E Warrant, (ii) the 1,470,600 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 1,264,796
shares of common stock issuable upon conversion of the OID Convertible Note upon maturity. |
| |
|
| (81) |
Beneficially
owned shares of common stock consist of (i) 367,650 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 367,650
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 300,566 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023. The shares of common stock being offered for resale consist of (i)
the 367,650 shares of common stock issuable upon exercise of the Class E Warrant, (ii) the 367,650 shares of common stock issuable
upon exercise of the Class F Warrant, and (iii) up to 316,199 shares of common stock issuable upon conversion of the OID Convertible
Note upon maturity. |
| |
|
| (82) |
Beneficially
owned shares of common stock consist of (i) 1,176,475 shares of common stock issuable upon exercise of a Class E Warrant, (ii) 1,176,475
shares of common stock issuable upon exercise of a Class F Warrant, and (iii) 961,808 shares of common stock issuable upon conversion
of an OID Convertible Note within 60 days of September 19, 2023, subject to a beneficial ownership limitation applicable to the Class
E Warrant, Class F Warrant, and OID Convertible Note limiting beneficial ownership to 4.99% of the outstanding shares of common stock.
The shares of common stock being offered for resale consist of (i) the 1,176,475 shares of common stock issuable upon exercise of
the Class E Warrant, (ii) the 1,176,475 shares of common stock issuable upon exercise of the Class F Warrant, and (iii) up to 1,011,832
shares of common stock issuable upon conversion of the OID Convertible Note upon maturity. |
| (83) |
Consists
of 635,294 shares of common stock issuable upon exercise of an OID Units Placement Agent Warrant. Bartholemew Pan-Kita is a registered
representative of Univest and may be considered an “associated person” of Univest. Mr. Pan-Kita, on behalf of Univest,
provided placement agent services in connection with the OID Units Private Placement, and was issued the OID Units Placement Agent
Warrant as partial compensation for such services. Mr. Pan-Kita acquired the OID Units Placement Agent Warrant in the ordinary course
of business, and at the time of the acquisition of the OID Units Placement Agent Warrant, Mr. Pan-Kita had no agreements or understandings,
directly or indirectly, with any person to distribute the OID Units Placement Agent Warrant or the shares of common stock issuable
upon exercise of the OID Units Placement Agent Warrant. |
| |
|
| (84) |
Consists
of 7,059 shares of common stock issuable upon exercise of an OID Units Placement Agent Warrant. Azem Nesimi is a registered representative
of Univest and may be considered an “associated person” of Univest. Mr. Nesimi, on behalf of Univest, provided placement
agent services in connection with the OID Units Private Placement, and was issued the OID Units Placement Agent Warrant as partial
compensation for such services. Mr. Nesimi acquired the OID Units Placement Agent Warrant in the ordinary course of business, and
at the time of the acquisition of the OID Units Placement Agent Warrant, Mr. Nesimi had no agreements or understandings, directly
or indirectly, with any person to distribute the OID Units Placement Agent Warrant or the shares of common stock issuable upon exercise
of the OID Units Placement Agent Warrant. |
| |
|
| (85) |
Amar
Dhaduk is an adult child of Vithalbhai Dhaduk, Chairman of the Company. |
| |
|
| (86) |
Darpan
Dhaduk is an adult child of Vithalbhai Dhaduk, Chairman of the Company. |
| |
|
| (87) |
Payal
Dhaduk is an adult child of Vithalbhai Dhaduk, Chairman of the Company. |
| |
|
| (88) |
Ranjanben
V. Dhaduk is the spouse of Vithalbhai Dhaduk, Chairman of the Company. |
| |
|
| (89) |
Fernando
Prieto Domínguez, director of Rieux Enterprise Corp., has sole voting and investment power over the securities held by Rieux
Enterprise Corp. |
| |
|
| (90) |
Jorge
Selume Zaror, director of SIF Investment Ltd, has sole voting and investment power over the securities held by SIF Investment Ltd. |
DESCRIPTION OF SECURITIES
The following is a summary of the rights of our securities
and certain provisions of our articles of incorporation, as amended, and our bylaws. This summary does not purport to be complete and
is qualified in its entirety by the provisions of our articles of incorporation, as amended, and bylaws, each of which is filed as an
exhibit to the registration statement, of which this prospectus forms a part.
General
As of the date of this prospectus,
our authorized capital stock consisted of:
| |
● |
2,000,000,000
shares of common stock, par value $0.001 per
share; and |
| |
|
|
| |
● |
25,000,000 shares of “blank check” preferred stock, par value $0.001 per share. |
As of September 19, 2023, there
were 46,666,760 shares of common stock issued and outstanding and no shares of preferred stock issued and outstanding, not including
shares of common stock issuable upon conversion or exercise of outstanding convertible or exercisable securities.
Common Stock
Holders of our common stock: (i) are entitled to share
ratably in all of our assets available for distribution upon liquidation, dissolution or winding up of our affairs; (ii) do not have preemptive,
subscription or conversion rights, nor are there any redemption or sinking fund provisions applicable thereto; and (iii) are entitled
to one vote per share on all matters on which stockholders may vote at all stockholder meetings. Each stockholder is entitled to receive
the dividends as may be declared by our directors out of funds legally available for dividends. Our directors are not obligated to declare
a dividend. Any future dividends will be subject to the discretion of our directors and will depend upon, among other things, future earnings,
the operating and financial condition of the Company, our capital requirements, general business conditions and other pertinent factors.
The presence of the persons entitled to vote a majority
(more than 50%) of the outstanding voting shares on a matter before the stockholders shall constitute the quorum necessary for the consideration
of the matter at a stockholders meeting.
The vote of the holders of a majority of the votes
cast on the matter at a meeting at which a quorum is present shall constitute an act of the stockholders, except for the election of directors,
who shall be appointed by a plurality of the shares entitled to vote at a meeting at which a quorum is present. The common stock does
not have cumulative voting rights. Accordingly, the holders of a majority of the common stock voting for election of directors can elect
100% of our directors if they choose to do so.
Preferred Stock
Subject to the terms contained in any designation
of a series of preferred stock, the board of directors is expressly authorized, at any time and from time to time, to fix, by resolution
or resolutions, the rights, powers, designations, preferences and relative, participating, optional and other rights and qualifications,
limitations and restrictions for shares of any class or classes of preferred stock, without the consent of the stockholders of the Company.
Somahlution Acquisition Warrants
On August 5, 2020, in connection with
the acquisition of all of the Somahlution Assets, the Company issued the Somahlution Warrants to purchase 2,999,995 shares of common
stock. The Somahlution Warrants were initially exercisable at an exercise price of $5.00 per share and a term of five years. On April
13, 2023, the Company delivered the Somahlution Warrant Offer Letter Agreements to the Former Somahlution Owners, which offered to allow
the Former Somahlution Owners to exercise the Somahlution Warrants to purchase the number of restricted shares of common stock issuable
under the Somahlution Warrants at an exercise price reduced by the Company from $5.00 per share to $0.10 per share, on or prior to April
21, 2023, for maximum total cash proceeds of $299,995.50. As of the conclusion of this offer period, the Somahlution Warrants Exercise
occurred, in which four of the Former Somahlution Owners entered into Somahlution Warrant Offer Letter Agreements and exercised their
Somahlution Warrants to purchase a total of 2,652,159 shares of common stock for gross proceeds of approximately $265,216. Univest, a
registered broker-dealer and member of FINRA, as the Company’s placement agent, which facilitated the Somahlution Warrant Exercise,
waived any fees or reimbursable expenses that would otherwise have been payable with respect to the Somahlution Warrant Exercise pursuant
to the 2021 Placement Agency Agreement. After the Somahlution Warrants Exercise and the conclusion of the offer period provided
in the Somahlution Warrant Offer Letter Agreements, the remaining outstanding Somahlution Warrants reverted to an exercise price per
share of $5.00 and were exercisable for 347,796 shares of common stock.
Securities Issued in the Units Private Placement
Convertible Notes
In connection
with the Units Private Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Convertible Notes,” we issued the Convertible Notes, which in aggregate
were initially convertible into an aggregate of 8,269,228 shares of common stock, prior to the adjustment and amendment described
below. The principal under outstanding Convertible Notes is $14,816,136. Each Convertible Note was initially convertible into
common stock of the Company at a price per share of $1.75, subject to adjustment. All outstanding Convertible Notes provide that a
lower price per share under subsequent equity issuances, not including Qualified Financings, will be applied to the conversion price
of the Convertible Notes, and have customary antidilution
provisions. As a result of the adjustments applicable to the Convertible Notes described under “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Adjustments to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants and
Underlying Shares”, the conversion price of the Convertible Notes was reduced to $0.10 per share, and, as adjusted,
the number of shares into which the Convertible Notes may be converted adjusted upwards proportionately. In addition, as a result
of the amendment to the Convertible Notes described under “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Recent Developments – Letter Agreement to Amend
Convertible Notes to Permit Conversion of Mandatory Default Amount”, each of the Convertible Notes became convertible
into the number of shares equal to the Mandatory Default Amount under each Convertible note divided by the same conversion price.
As a result of the above adjustments and amendment, the aggregate number of shares into which the Convertible Notes may be converted
is currently 221,939,338 shares.
The Convertible Notes mature
24 months after their issuance date and accrue 10% of simple interest per annum on the outstanding principal amount.
As amended,
the Convertible Notes provide that the Mandatory Default Amount or the Convertible Notes’ principal and accrued interest may
be converted into shares of common stock at any time at the option of each holder at the conversion price, subject
to any applicable beneficial ownership limitations upon receiving shares upon conversion. Any conversion by the holder will be applied
toward the Mandatory Default Amount until it is fully converted. If the Mandatory Default Amount is fully converted, the Mandatory Default
Amount and any outstanding principal and accrued interest will be fully extinguished.
As amended,
the Convertible Notes provide that if at any time following the 60-day anniversary of the final closing date or termination of the
Units Private Placement, and provided there is an effective registration statement permitting the issuance or resale of the shares
of common stock into which the convertible notes may be converted, if (A) the common stock is listed on a senior national securities
exchange, (B) the daily volume-weighted average price for the prior twenty (20) consecutive trading days is $6.00 or more (adjusted for
splits and similar distributions) and (C) the daily trading volume is at least $1,000,000 during such 20-day period, then the Company
will have the right to require the Convertible Notes to convert all or any portion of the Mandatory Default Amount, principal or accrued
interest then remaining under the Convertible Notes into shares of common stock at the above conversion price in effect on the mandatory
conversion date.
As amended,
the Convertible Notes also provide that upon the occurrence of a Qualified Financing, the conversion price of the Convertible Notes will
be adjusted to the price per share equal to 75% of the price per equity security in such financing.
Shares of
common stock may not be received pursuant to a conversion of a Convertible Note to the extent that the holder (together with any
other person with which the holder is considered to be part of a “group” under Section 13 of the Exchange Act or with which
the holder otherwise files reports under Section 13 and/or Section 16 of the Exchange Act) would otherwise become the “beneficial
owner” (as such term is defined in the Exchange Act and the rules and regulations thereunder) of in excess of 4.99% of the shares
of common stock outstanding, subject to automatic increase to 9.99% if the holder becomes a beneficial owner of more than
4.99% of the outstanding shares of common stock, subject to a 61-day notice requirement. This limitation does not apply to certain provisions
of the Convertible Notes.
The
Convertible Notes have certain registration requirements as to the shares of common stock underlying the Convertible Notes upon
the final closing of the Units Private Placement under the respective Units Registration Rights Agreements between the Company and the
purchasers of the Convertible Notes. By executing certain lock-up agreements with Univest acting as the representative of the underwriters
of a proposed public offering that we no longer intend to pursue, the initial purchasers of the Convertible Notes agreed that
doing so amounted to a waiver of their rights under the respective Units Registration Rights Agreements, and that any such registration
rights will be afforded to such purchasers in a subsequent registration statement. The assignees of certain Convertible
Notes and the holders of the Replacement Convertible Notes have also entered into a waiver and consent with the Company providing
for the waiver and deferral of all registration rights applicable to the respective Convertible Notes until a date and
time that the Company shall determine in its sole discretion that it may provide for such rights.
Each Convertible
Note provides that its holder may vote with the shares of common stock, on an as-converted to common stock basis, with respect to all
corporate matters of the Company on which the holders of common stock are entitled to vote, subject to any applicable beneficial ownership
limitations. Under the NRS the right to vote is generally a feature of a share of any class or series of stock of a corporation and,
thus, a right granted to stockholders of a corporation. Under the NRS, convertible promissory notes (or any other form of bond, debenture
or other obligation) typically do not provide holders with voting rights. However, Section 78.197 of the NRS provides that a corporation
organized in Nevada “may provide in its articles of incorporation that the holder of a bond, debenture or other obligation of the
corporation may have any of the rights of a stockholder in the corporation.” At the Special Stockholders Meeting, the stockholders
of the Company approved the Voting Rights Amendment, and on August 16, 2023, the Company filed the
Certificate of Amendment with the Nevada Secretary of State providing for, among other things, the Voting Rights Amendment, and
the Certificate of Amendment
became effective upon filing. Accordingly, upon the filing of the Certificate of Amendment, among other things, (i)
the Company’s board of directors may grant any of the rights of stockholders to any of the holders of any of the Company’s
bonds, debentures or other obligations; and (ii) all of the holders of the Convertible Notes issued between December 21, 2021 and August
12, 2022 became entitled to vote with the shares of the Company’s common stock, on an as-converted to common stock basis without
actually having converted the Convertible Notes to shares of the Company’s common stock, with respect to all corporate matters
of the Company on which the holders of common stock are entitled to vote, as provided in the Convertible Notes. Pursuant to the Certificate
of Amendment, the board of directors extended these voting rights to all other holders of the Convertible Notes issued since August 12,
2022. Accordingly, all holders of the Convertible Notes are entitled to vote with the shares of the Company’s common stock, on
an as-converted to common stock basis without actually having converted the Convertible Notes to shares of the Company’s common
stock, with respect to all corporate matters of the Company on which the holders of common stock are entitled to vote, as provided in
the Convertible Notes.
Under the
Unit Purchase Agreement entered into on May 27, 2021 with certain holders of several of the Convertible Notes, each of the Company’s
subsidiaries entered into a guaranty to guarantee the repayment of the Company’s obligations under the Convertible Notes, and the
Company and its subsidiaries entered into security agreements granting security interests in all of their respective assets for up to
the dollar value owed under the respective Convertible Notes. The respective Convertible Notes were issued for aggregate principal of
approximately $1.2 million. Under each Unit Purchase Agreement entered into with respect to subsequent issuances of the Convertible Notes,
the Company and its subsidiaries were obligated to enter into similar security agreements and guaranties, but did not do so.
The 2021
Placement Agency Agreement and form of unit purchase agreement relating to the Units Private Placement provide that up to $18 million
and $17 million of the units containing the Convertible Notes may be sold, respectively. On August 12, 2022, we completed the final closing
of the Units Private Placement.
Class C Warrants
In connection
with the issuance of the Convertible Notes in the Units Private Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Convertible Notes,” we issued Class C Warrants, which in aggregate were
initially exercisable to purchase up to 16,538,486 shares of common stock. Each Class C Warrant is exercisable for two shares of common
stock and had an initial exercise price per share equal to the lower of (i) $2.25 and (ii) 75% of the cash price per share paid by the
other purchasers of next round securities in a
Qualified Financing, subject to certain adjustment provisions. The Class C Warrants provide that more favorable terms under subsequent
equity issuances, not including Qualified Financings, will be applicable to the exercise rights of such Class C Warrants. The Class C
Warrants also have related adjustment rights pursuant to the January 2023 Letter Agreement, as described below. As a result of
these adjustment provisions, and the adjustments applicable to the Class C Warrants described under “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Adjustments to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants and
Underlying Shares”, and the adjustment provisions described in this section, the exercise price of the Class
C Warrants was reduced to $0.10 per share, and, as adjusted, the total number of shares that the Class C Warrants may be
exercised to purchase is 380,986,336 shares. The Class C Warrants are exercisable for a period of five years from issuance.
Each Class C Warrant and related rights under the respective unit purchase agreement and registration rights agreement are transferable
in accordance with their terms.
The
Class C Warrants have certain
registration requirements as to the shares of common stock underlying the Class C Warrants upon the final closing of the Units
Private Placement under the respective Units Registration Rights Agreements between the Company and the purchasers of the Class C Warrants.
By executing certain lock-up agreements with the Univest acting as the representative of the underwriters in a proposed public offering
that we no longer intend to pursue, or by executing other waiver agreements with the Company, the initial purchasers of the Class
C Warrants agreed that doing so amounted to a waiver of their rights under the respective Units Registration Rights Agreements, and that
any such registration rights will be afforded to such purchasers in a subsequent registration statement. In addition, the assignees
of certain Class C Warrants and the holders of the Replacement Class C Warrants have each entered into a waiver and
consent with the Company providing for the waiver and deferral of all registration rights applicable to the respective Class C
Warrants until a date and time that the Company shall determine in its sole discretion that it may provide for such rights.
Each holder
of a Class C Warrant will generally not have the right to receive shares upon exercise of a Class C Warrant to the extent
that the holder (together with any other person with which the holder is considered to be part of a “group” under Section
13 of the Exchange Act or with which the holder otherwise files reports under Section 13 and/or Section 16 of the Exchange Act) would
otherwise become the “beneficial owner” (as such term is defined in the Exchange Act and the rules and regulations
thereunder) of in excess of 4.99% of the shares of common stock outstanding, subject to automatic increase to 9.99% if the holder
becomes a beneficial owner of more than 4.99%, subject to a requirement that any increase in the limitation will
not be effective until the 61st day after notice is delivered to the Company. This limitation does not apply to certain
provisions of the Class C Warrants.
Under the January
2023 Letter Agreement, we agreed that simultaneously with any adjustment to the exercise price under the Class C Warrants as a result
of any equity issuances, not including Qualified Financings, the number of shares of common stock that are issuable under the
Class C Warrants will be increased such that the aggregate exercise price of such shares will be the same as the aggregate exercise price
in effect immediately prior to the adjustment (without regard to any limitations on exercise contained in the Class C Warrants,
including the beneficial ownership limitation otherwise provided under the Class C Warrants described above).
The 2021 Placement
Agency Agreement and form of unit purchase agreement relating to the Units Private Placement provide that up to $18 million and $17
million of the units containing the Class C Warrants may be sold, respectively. On August 12, 2022, we completed the final closing of
the Units Private Placement.
Class D Warrant
On February 6, 2023, the
Company issued a Class D Common Stock Purchase Warrant (the “Class D Warrant”).
The Class D Warrant may be exercised to purchase up to a number of shares of common stock equal to the quotient of 250% of the subscription
amount of $1,000,000 divided by the price per share at which shares are sold under the Registration Statement on Form S-1 of the Company
(File No. 333-262697) registering the sale of units to be issued in a proposed public offering of the Company, at an exercise price equal
to the public offering price per unit at which units are sold under the referenced registration
statement. The Class D Warrant may be exercised from the date that such registration statement is declared effective until the
date that is five years after such date, and may be exercised to purchase.
The
referenced registration statement was withdrawn as of April 21, 2023, and will not be declared effective. As a result, the Company
has determined that the Class D Warrant will not be exercisable.
Securities Issued in the
OID Units Private Placement
OID Convertible Notes
In connection
with the OID Units Private Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement,” we have issued OID Convertible Notes which in
the aggregate may be converted into 49,578,055 shares of common stock at $0.10 per share, subject to adjustment, to purchase up to the
aggregate principal amount of $4,957,806, plus additional shares based on accrued interest at $0.10 per share, subject to adjustment.
See “Management’s Discussion and Analysis of
Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements and Other Liquidity
Matters – OID Units Private Placement – OID Convertible Notes” for a description of other terms and
conditions contained in the OID Convertible Notes. Each of the OID Convertible Notes has the registration rights set forth in the respective
OID Units Registration Rights Agreement and certain other rights under the respective OID Units Purchase Agreement. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding
Requirements and Other Liquidity Matters – OID Units Private Placement – OID Units Registration Rights
Agreement” for a description of the terms and conditions contained in each OID Units Registration Rights Agreement and
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements and Other Liquidity Matters
– OID Units Private Placement – OID Units Purchase Agreement” for a description of the terms
and conditions contained in each OID Units Purchase Agreement. The OID Convertible Notes issued in connection with the Second
OID Units Closing and one OID Convertible Note issued in connection with the Fourth OID Units Closing are also subject to the
terms and conditions of the respective Cancellation and Exchange Agreements. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – Second Closing” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent Developments
– Fourth Closing of OID Units Private Placement” for a description of the terms and conditions contained in
the Cancellation and Exchange Agreements.
Class
E Warrants
In connection with the OID Units Private
Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement” and a requirement under the Hexin Promissory Note,
we have issued Class E Warrants for the aggregate purchase of 84,546,202 shares of common stock at $0.10
per share, subject to adjustment. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – Class E and F Warrants” for a description
of other terms and conditions contained in the Class E Warrants. Each of the Class E Warrants has the registration rights
set forth in the respective OID Units Registration Rights Agreement and certain other rights under the respective OID Units Purchase
Agreement. See “Management’s Discussion and
Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements and
Other Liquidity Matters – OID Units Private Placement – OID Units Registration Rights Agreement”
for a description of the terms and conditions contained in each OID Units Registration Rights Agreement and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – OID Units Purchase Agreement” for
a description of the terms and conditions contained in each OID Units Purchase Agreement. The Class E Warrants issued in connection
with the Second OID Units Closing and one Class F Warrant issued in connection with the Fourth OID Units Closing are also subject
to the terms and conditions of the respective Cancellation and Exchange Agreements. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – Second Closing” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent Developments
– Fourth Closing of OID Units Private Placement” for a description of the terms and conditions contained in
the Cancellation and Exchange Agreements.
Class
F Warrants
In connection
with the OID Units Private Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement” and a requirement under the Hexin Promissory Note,
we issued Class F Warrants for the aggregate purchase of 80,796,202 shares of common stock at $0.20 per share, subject to adjustment.
See “Management’s Discussion and Analysis of
Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements and Other Liquidity
Matters – OID Units Private Placement – Class E and F Warrants” for a description of other terms and conditions
contained in the Class F Warrants. Each of the Class F Warrants has the registration rights set forth in the respective OID Units Registration
Rights Agreement and certain other rights under the respective OID Units Purchase Agreement. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – OID Units Registration Rights Agreement”
for a description of the terms and conditions contained in each OID Units Registration Rights Agreement and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – OID Units Purchase Agreement” for a description
of the terms and conditions contained in each OID Units Purchase Agreement. The Class F Warrants issued in connection with the Second
OID Units Closing and one Class F Warrant issued in connection with the Fourth OID Units Closing are also subject to the terms and conditions
of the respective Cancellation and Exchange Agreements. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement – Second Closing” and “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent Developments
– Fourth Closing of OID Units Private Placement” for a description of the terms and conditions contained in the
Cancellation and Exchange Agreements.
Placement Agent Warrants
Placement
Agent Warrants Issued in Connection with 2020 Private Placement
In connection
with the 2020 Private Placement, we issued the 2020 Placement Agent Warrants for the aggregate purchase of 280,014 shares of common stock
at an exercise price of $1.375 per share. The 2020 Placement Agent Warrants may be exercised from September 25, 2020 to September 25,
2025, by payment of cash or on a cashless net exercise basis, and contain certain antidilution provisions. The exercise rights under
each of the 2020 Placement Agent Warrants are also subject to the following beneficial ownership limitation: Exercise is permitted only
if it would not cause the holder (together with its Affiliates (as defined by Rule 405 under the Securities Act), and any other persons
acting as a group together with the holder or any of the holder’s Affiliates), to beneficially own in excess of the percentage
of the outstanding securities that are permitted to be beneficially owned (as described below), which, for purposes of the limitation,
includes shares issuable upon exercise of the 2020 Placement Agent Warrant, excludes shares issuable upon exercise of the unexercised
portion of the 2020 Placement Agent Warrant and exercise or conversion of the unexercised or nonconverted portion of any other securities
of the Company subject to a limitation on conversion or exercise analogous to the limitation contained herein beneficially owned by the
holder or any of its Affiliates, and otherwise calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations
promulgated thereunder. For purposes of the 2020 Placement Agent Warrants, the number of outstanding shares of common stock shall be
determined after giving effect to the conversion or exercise of securities of the Company, including the 2020 Placement Agent Warrant,
by the holder or its Affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The maximum
percentage of beneficial ownership of the Company’s outstanding securities that applies to an exercise of a 2020 Placement Agent
Warrant is 9.99% of the number of shares of the common stock outstanding immediately after giving effect to the issuance of shares of
common stock issuable upon exercise of the 2020 Placement Agent Warrant. The holder, upon notice to the Company, may increase or decrease
the percentage limit, provided that the limitation in no event exceeds 9.99% of the number of shares of the common stock outstanding
immediately after giving effect to the issuance of shares of common stock upon exercise of the 2020 Placement Agent Warrant. Any increase
in the limitation will not be effective until the 61st day after such notice is delivered to the Company. This limitation does not
apply to certain provisions of the 2020 Placement Agent Warrant.
Each holder is granted all
registration rights set forth in the registration rights letter agreement, dated as of August 5, 2020, by and between the Company and
Univest as placement agent for the investors in the placement (the “Registration Rights Letter Agreement”), which provides
certain “piggyback” registration rights to the investors in the private placement, to the full extent as if the Placement
Agent Warrant holder was a party to such agreement; provided, however that the Placement Agent Warrant holder will determine whether to
include any shares issuable upon exercise of the holder’s Placement Agent Warrant in a registration statement.
Cancelled
2022 Placement Agent Warrants Issued in Connection with Units Private Placement; Replacement with Replacement Placement
Agent Warrants
In connection
with the Units Private Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Convertible Notes,” upon its final closing, we were required to issue
the 2022 Placement Agent Warrants to Univest, as placement agent, and its designee, to purchase an aggregate of 8% of the total
number of units sold in the Units Private Placement for a total payment of $100. On June 26, 2022, in anticipation of the final closing
of the Units Private Placement, in exchange for $100, we issued Univest a 2022 Placement Agent Warrant for the purchase of 231,359
shares of common stock, and a 2022 Placement Agent Warrant to Bradley Richmond, as a registered representative of Univest who
is entitled to a portion of Univest’s compensation due to his employment terms, for the purchase of 347,039 shares of common stock.
The 2022 Placement Agent Warrants had an exercise price equal to the conversion price of the Convertible Notes, which has been
adjusted to $0.10 per share. The 2022 Placement Agent Warrants were exercisable, in whole or in part, until June 26, 2027 by payment
of cash or on a cashless net exercise basis, and contained certain antidilution provisions and exercise price adjustment provisions that
were substantially identical to equivalent provisions of the Class C Warrants.
Pursuant to
the October 2022 Letter Agreement, each of Univest and Mr. Richmond agreed to forego their applicable rights to such warrants, as the
FINRA Staff determined that such securities constituted underwriting compensation in connection with a proposed public offering based
on the FINRA Staff’s interpretation of FINRA Rule 5110. The 2022 Placement Agent Warrants were subsequently cancelled. The Company
issued Replacement Placement Agent Warrants with terms and conditions equivalent to or at least as favorable as those that would have
applied to those that applied to each of the respective 2022 Placement Agent Warrants, reflecting adjustments to their terms equivalent
to those that applied to the Class C Warrants as a result of the Somahlution Warrants Exercise such that the exercise price per share
of the Replacement Placement Agent Warrants is $0.10 per share, subject to adjustment, and the number of shares of common stock issuable
upon exercise of each of the Replacement Placement Agent Warrants reflecting a proportional increase relative to the number of shares
issuable under the respective 2022 Placement Agent Warrants equivalent to the difference between the exercise price per share of the
2022 Placement Agent Warrants and the exercise price of the Replacement Placement Agent Warrants, such that up to 19,293,621 and 28,940,432
shares of common stock, respectively, are issuable upon exercise thereof, subject to adjustment and applicable beneficial ownership limitations
and other limitations or restrictions.
Each holder
of a Replacement Placement Agent Warrant will generally not have the right to receive shares upon exercise of a Replacement Placement
Agent Warrant to the extent that the holder (together with any other person with which the holder is considered to be part of a “group”
under Section 13 of the Exchange Act or with which the holder otherwise files reports under Section 13 and/or Section 16 of the Exchange
Act) would otherwise become the “beneficial owner” (as such term is defined in the Exchange Act and the rules and regulations
thereunder) of in excess of 4.99% of the shares of common stock outstanding, subject to automatic increase to 9.99% if the holder becomes
a beneficial owner of more than 4.99%, subject to a requirement that any increase in the limitation will not be effective until the 61st
day after notice is delivered to the Company. This limitation does not apply to certain provisions of the Replacement Placement Agent
Warrants.
The Replacement Placement Agent Warrants
are assumed to have the same registration rights that would have applied to the respective 2022 Placement Agent Warrants, which provided
that the holders of such warrants have the rights set forth in each Units Registration Rights Agreement.
OID
Units Placement Agent Warrants Issued in Connection with OID Units Private Placement
In connection with the OID Units Private
Placement described above in the section “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent
Developments – Issuance of OID Units Placement Agent Warrants,” under the April 2023 PAA and the forms
of Placement Agent Warrants agreed to in connection with the April 2023 PAA, the Company also agreed, upon Univest’s payment of
$100.00, to issue the OID Units Placement Agent Warrants to Univest and/or its designee(s) at each closing of the
OID Units Private Placement. The OID Units Placement Agent Warrants were required to be issued for the purchase
of 8% of the aggregate number of shares of common stock initially issuable upon conversion of the OID Convertible Notes
at $0.10 per share, 8% of the aggregate number of shares of common stock initially issuable upon exercise of the Class
E Warrants at $0.10 per share, and 8% of the aggregate number of shares of common stock initially issuable upon exercise of the Class
F Warrants at $0.20 per share, subject to adjustment. On September 12, 2023, Univest notified the Company that the OID Units Private
Placement’s final closing had occurred, and on September 13, 2023, made a payment to the Company of $100.00. Accordingly, on September
13, 2023, OID Units Placement Agent Warrants were issued to Univest and its designees, Bradley Richmond, Bartholemew Pan-Kita, and Azem
Nesimi, to purchase a total of 4,356,687, 6,695,616, 635,294, and 7,059 shares of common stock at $0.10 per share, respectively, which
in aggregate was equal to 8% of the shares issuable upon conversion or exercise of the OID Convertible Notes and the Class E Warrants
issued in each of the closings of the OID Units Private Placement as of that date, and a total of 2,585,479, 3,878,218, 0, and 0 shares
of common stock at $0.20 per share, respectively, which in aggregate was equal to 8% of the shares issuable upon exercise of the Class
F Warrants issued in each of the closings of the OID Units Private Placement as of that date. See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Recent
Developments – Issuance of OID Units Placement Agent Warrants” for a description of other terms and conditions
contained in the OID Units Placement Agent Warrants.
Options
On May 18, 2021, our board of directors approved the
Stock Incentive Plan, which was ratified by the Company’s stockholders on September 20, 2021.
The Stock Incentive Plan governs stock options issued prior to May 18, 2021. On August 30, 2022 and October 21, 2022, the board approved
the amendment of the Plan to increase the number of shares of common stock reserved for issuance under the Plan, which was ratified by
the Company’s stockholders on December 27, 2022. The Plan provides for the grant of restricted stock, stock options or other equity
securities to our officers, employees, directors, advisors and consultants. The maximum number of shares of common stock that may be issued
pursuant to awards granted under the Plan is 7,200,000 shares. Cancelled and forfeited stock options and stock awards may again become
available for grant under the Plan. As of the date of this prospectus, a total of 350,000 shares of restricted common stock, and options
to purchase a total of 3,925,943 shares of common stock at a weighted-average price of $1.33 per share, had been granted, and 2,924,057
shares remained available for issuance under the Plan. The Plan will continue in effect until its termination by our board of directors;
provided, however, that all awards under the Plan must be granted by May 18, 2031. For further information about the Plan, see “Executive
Compensation – Amended and Restated 2021 Stock Incentive Plan”.
In August 2022,
certain holders of stock options executed limited waiver and consent agreements waiving their conversion rights. The limited waiver and
consent agreements were to expire on December 31, 2022, or if earlier to occur, upon the approval of an increase in the number of the
authorized shares of common stock under the Company’s articles of incorporation by the Company’s stockholders by majority
written consent or via stockholder vote at the Company’s next stockholder meeting and the implementation of such increase, which
were to occur as soon as practicable after such stockholder approval. On December 27, 2022, we held the Annual Meeting. At the
Annual Meeting, stockholders holding shares of common stock in the Company representing at least a majority of the voting power approved,
among other matters, the Authorized Capital Increase. As a result, the limited waiver and consent agreements expired in accordance with
their terms.
Anti-Takeover Provisions
Provisions of the Nevada Revised Statutes and our
bylaws could have the effect of delaying or preventing a third-party from acquiring us, even if the acquisition would benefit our stockholders.
Such provisions of Nevada law and our bylaws are intended to enhance the likelihood of continuity and stability in the composition of
our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may
involve an actual or threatened change of control of our company. These provisions are designed to reduce our vulnerability to an unsolicited
proposal for a takeover that does not contemplate the acquisition of all our outstanding shares, or an unsolicited proposal for the restructuring
or sale of all or part of our company.
Nevada Anti-Takeover Statutes
We have elected not to be governed by the terms and
provisions of Nevada’s control share acquisition laws (Nevada Revised Statutes 78.378 - 78.3793), which prohibit an acquirer, under
certain circumstances, from voting shares of a corporation’s stock after crossing specific threshold ownership percentages, unless
the acquirer obtains the approval of the issuing corporation’s stockholders. The first such threshold is the acquisition of at least
one-fifth but less than one-third of the outstanding voting power. We may become subject to Nevada’s Control Share Acquisition Act
if the Company has 200 or more stockholders of record, at least 100 of whom are residents of the state of Nevada, does business in the
state of Nevada directly or through an affiliated corporation, and we amend our bylaws to reverse the election to not be governed by these
statutes. Currently, we do not conduct business in the state of Nevada directly or through an affiliated corporation.
We are governed by the terms and provisions of Nevada’s
Combination with Interested Stockholders Statute (Nevada Revised Statutes 78.411 - 78.444), which prohibits an “interested stockholder”
from entering into a “combination” with the corporation, unless certain conditions are met. An “interested stockholder”
is a person who, together with affiliates and associates, beneficially owns (or within the prior three years, did beneficially own) 10%
or more of the corporation’s voting stock, or otherwise has the ability to influence or control such corporation’s management
or policies.
Nevada law also provides that no director may be removed
by less than a two-thirds vote of the issued and outstanding shares entitled to vote on the removal (Nevada Revised Statutes 78.335).
This requirement also has the effect of discouraging change of control of the Company.
Bylaws
In addition, various provisions of our bylaws may
also have an anti-takeover effect. These provisions may delay, defer or prevent a tender offer or takeover attempt of the Company that
a stockholder might consider in his or her best interest, including attempts that might result in a premium over the market price for
the shares held by our stockholders. Our bylaws may be adopted, amended or repealed by the affirmative vote of the holders of at least
a majority of the voting power of our outstanding shares of capital stock, and except as provided by Nevada law, our board of directors
shall have the power to adopt, amend or repeal the bylaws by a vote of not less than a majority of our directors. Any bylaw provision
adopted by the board of directors may be amended or repealed by the holders of a majority of the voting power of our outstanding shares
of capital stock. Our bylaws also contain limitations as to who may call special meetings as well as require advance notice of stockholder
matters to be brought at a meeting.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock
are available for our board of directors to issue without stockholder approval. We may use these additional shares for a variety of corporate
purposes, including raising additional capital, corporate acquisitions and employee stock plans. The existence of our authorized but unissued
shares of common stock could render it more difficult or discourage an attempt to obtain control of the Company by means of a proxy context,
tender offer, merger or other transaction since our board of directors can issue large amounts of capital stock as part of a defense to
a take-over challenge. In addition, we have authorized in our articles of incorporation 25,000,000 shares of preferred stock. However,
the board acting alone and without approval of our stockholders can designate and issue one or more series of preferred stock containing
super-voting provisions, enhanced economic rights, rights to elect directors, or other dilutive features, that could be utilized as part
of a defense to a take-over challenge.
Supermajority Voting Provisions
Nevada law provides generally that the affirmative
vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s articles of incorporation or
bylaws, unless a corporation’s articles of incorporation or bylaws, as the case may be, require a greater percentage. Although our
articles of incorporation and bylaws do not currently provide for such a supermajority vote on any matters, our board of directors can
amend our bylaws and we can, with the approval of our stockholders, amend our articles of incorporation to provide for such a super-majority
voting provision.
Market Listing
Our common stock is quoted on the OTCQB under the
ticker symbol “MRZM.” We have applied to list our common stock under the symbol “MRZM”, on the Nasdaq Capital
Market tier operated by Nasdaq. There can be no guarantee that we will successfully list our common stock on the Nasdaq Capital Market.
The registration of the selling stockholders’ resale of the Company’s common stock as described in this prospectus is not
conditioned upon our successful listing on the Nasdaq Capital Market.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock
is Securities Transfer Corporation, 2901 N Dallas Parkway, Suite 380,
Plano, Texas 75093, telephone (469) 633-0101.
SHARES ELIGIBLE FOR FUTURE SALE
General
Prior to this offering, our common stock has been
quoted on the OTCQB under the symbol “MRZM”. However, there has been limited trading for our common stock. We
have applied to list our common stock under the symbol “MRZM” on the Nasdaq Capital Market. There can be no guarantee
that we will successfully list our common stock on the Nasdaq Capital Market. The registration of the selling stockholders’ resale
of the Company’s common stock as described in this prospectus is not conditioned upon our successful listing on the Nasdaq Capital
Market. Future sales of substantial amounts of common stock in the public market after our offering, or the possibility of these sales
occurring, could cause the prevailing market prices for our securities to fall or impair our ability to raise equity capital in the future.
The common stock sold in this offering will be freely
tradable without restriction or further registration under the Securities Act under an effective registration statement. We have applied
to list our common stock on the Nasdaq Capital Market tier operated by Nasdaq. There can be no guarantee that we will successfully list
our common stock on the Nasdaq Capital Market. The registration of the selling stockholders’ resale of the Company’s common
stock as described in this prospectus is not conditioned upon our successful listing on the Nasdaq Capital Market.
Previously-issued shares of common stock as well as
shares issuable upon the conversion of convertible notes, exercise of warrants, or exercise of stock options, or pursuant to the terms
of other agreements, are, or will be upon issuance, “restricted securities,” as that term is defined in Rule 144 under the
Securities Act, unless registered for sale or resale pursuant to an effective registration statement. These restricted securities are
eligible for public sale only if such public resale is registered under the Securities Act or if the resale qualifies for an applicable
exemption from registration, including under Rule 144, Rule 701, or Regulation S under the Securities Act, which are summarized below.
Rule 144
In general, a person who has beneficially owned restricted
shares of common stock for at least twelve months, or at least six months in the event we have been a reporting company under the Exchange
Act for at least 90 days before the sale, would be entitled to sell such securities, provided that such person is not deemed to be an
affiliate of ours at the time of sale or to have been an affiliate of ours at any time during the 90 days preceding the sale. A person
who is an affiliate of ours at such time would be subject to additional restrictions, by which such person would be entitled to sell within
any three-month period only a number of shares that does not exceed the greater of the following:
| |
● |
1% of the number of shares of common stock then outstanding; or |
| |
|
|
| |
● |
1% of the average weekly trading volume of our common stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale; |
provided that, in each case, we are subject to the
periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Securities Act Rule 144 trades must also comply
with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
Rule 701
In general, Securities Act Rule 701 allows a stockholder
who purchased shares of capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate
of ours during the immediately preceding 90 days to sell those shares in reliance upon Securities Act Rule 144, but without being required
to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. All holders of Rule 701 shares,
however, are required to wait until 90 days after the date of this prospectus before selling shares pursuant to Rule 701.
Regulation S
Regulation S provides generally that sales made in
offshore transactions are not subject to the registration or prospectus-delivery requirements of the Securities Act.
Registration Rights Agreements
2020 Private Placement Registration Rights Letter
Agreement
In connection with a private placement conducted from
August 2020 to September 2020, we entered into the Registration Rights Letter Agreement. Under the Registration Rights Letter Agreement,
the Company provided “piggyback” registration rights to the investors in such private placement, so as to require us to include,
at the option of Univest acting on behalf of the investors, the investors’ shares in a registration statement to register other
shares of common stock that the Company had determined to file after the initial closing date of the private placement, subject to certain
exceptions.
Units Private Placement Registration Rights Agreements
In connection with the Units Private
Placement described under “Management’s Discussion
and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – Convertible Notes”, we entered into the Units Registration Rights Agreements
with certain investors which required us to register the sale of the shares of common stock issuable upon conversion of their outstanding
Convertible Notes or upon exercise of their Class C Warrants, and
any additional shares issuable upon the incurrence of interest under the Convertible Notes. The agreements required us to file
a registration statement within 45 calendar days after the final closing date of the Units Private Placement, and the registration statement
was required to become effective within 120 days of its filing date. Under waiver agreements between the Units Private Placement investors
or their assigns and Univest acting as the representative of the underwriters of a proposed public offering that we no longer intend
to pursue, or with the Company, such registration rights have been waived. Certain investors have assigned their Convertible Notes or
Warrants to other investors pursuant to consents from the underwriters, and their assigns have executed agreements with registration
rights waiver provisions.
OID Units Private Placement Registration Rights
Agreements
In connection with the OID Units Private
Placement described under “Management’s Discussion
and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement”, we entered into the OID Units Registration Rights
Agreements and an amendment with respect to certain of such OID Units Registration Rights Agreement with certain investors. As amended,
if applicable, the OID Units Registration Rights Agreements require us to file a registration statement with the SEC registering the
resale of the shares of common stock issuable pursuant to conversion of the OID Convertible Notes and exercise of the Class E and F Warrants
within 67 days of the date of the respective closing of the OID Units Private Placement and to cause the registration statement to become
effective within 120 days after such filing date. We are also required to See “Management’s
Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Funding Requirements
and Other Liquidity Matters – OID Units Private Placement” for additional related discussion.
Placement
Agent Warrants Registration Rights
The holders of the 2020 Placement
Agent Warrants were granted all registration rights set forth in the Registration Rights Letter Agreement. The Replacement Placement
Agent Warrants are assumed to have the same registration rights that would have applied to the respective 2022 Placement Agent Warrants,
which provided that the holders of such warrants have the rights set forth in each Units Registration Rights Agreement. The OID Units
Placement Agent Warrants provide for equivalent registration rights as provided for under the August 2023 Registration Rights Agreement.
Amended and Restated 2021 Stock Incentive Plan
We may file a registration statement on Form S-8 under
the Securities Act to register all shares of our common stock issuable or reserved for issuance under the Stock Incentive Plan. Subject
to the satisfaction of vesting conditions, the registration statement on Form S-8 will permit the resale of such shares by non-affiliates
in the public market without restriction under the Securities Act and the sale by affiliates in the public market, subject to compliance
with the resale provisions of Rule 144 under the Securities Act.
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS
FOR NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material U.S. federal
income and estate tax consequences of the ownership and disposition of our common stock. This summary is limited to Non-U.S. Holders (as
defined below) that hold our common stock as a capital asset (generally, property held for investment) for U.S. federal income tax purposes.
This summary does not discuss all of the aspects of U.S. federal income and estate taxation that may be relevant to a non-U.S. Holder
in light of the Non-U.S. Holder’s particular investment or other circumstances. Accordingly, all prospective Non-U.S. Holders should
consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the ownership and disposition
of our common stock.
This summary is based on provisions of the Code, applicable
U.S. Treasury regulations, and administrative and judicial interpretations, all as in effect or in existence on the date of this prospectus.
Subsequent developments in U.S. federal income or estate tax law, including changes in law or differing interpretations, which may be
applied retroactively, could alter the U.S. federal income and estate tax consequences of owning and disposing of our common stock as
described in this summary. There can be no assurance that the Internal Revenue Service, or IRS, will not take a contrary position with
respect to one or more of the tax consequences described herein and we have not obtained, nor do we intend to obtain, a ruling from the
IRS with respect to the U.S. federal income or estate tax consequences of the ownership or disposition of our common stock.
As used in this summary, the term “Non-U.S.
Holder” means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
| |
● |
an individual who is a citizen or resident of the United States; |
| |
|
|
| |
● |
a corporation (or other entity treated as a corporation) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia; |
| |
|
|
| |
● |
an entity or arrangement treated as a domestic partnership; |
| |
|
|
| |
● |
an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
| |
|
|
| |
● |
a trust, if (1) a U.S. court is able to exercise primary supervision over the trust’s administration and one or more “United States persons” (within the meaning of the Code) has the authority to control all of the trust’s substantial decisions, or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a United States person. |
If an entity or arrangement treated as a partnership
for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in such a partnership generally will depend
upon the status of the partner, the activities of the partnership and certain determinations made at the partner level. Partnerships,
and partners in partnerships, that hold our common stock should consult their own tax advisors as to the particular U.S. federal income
and estate tax consequences of owning and disposing of our common stock that are applicable to them.
This summary does not consider any specific facts
or circumstances that may apply to a Non-U.S. Holder and does not address any special tax rules that may apply to particular Non-U.S.
Holders, such as:
| |
● |
a Non-U.S. Holder that is a financial institution, insurance company, tax-exempt organization, pension plan, broker, dealer or trader in securities, dealer in currencies, U.S. expatriate, controlled foreign corporation or passive foreign investment company; |
| |
|
|
| |
● |
a non-U.S. Holder holding our common stock as part of a conversion transaction, constructive sale, wash sale or other integrated transaction or a hedge, straddle or synthetic security; |
| |
|
|
| |
● |
a Non-U.S. Holder that holds or receives our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; or |
| |
|
|
| |
● |
a non-U.S. Holder that at any time owns, directly, indirectly or constructively, 5% or more of our outstanding common stock. |
In addition, this summary does not address any U.S.
state or local, or non-U.S. or other tax consequences, or any U.S. federal income or estate tax consequences for beneficial owners of
a Non-U.S. Holder, including stockholders of a controlled foreign corporation or passive foreign investment company that holds our common
stock.
Each Non-U.S. Holder should consult its own tax
advisor regarding the U.S. federal, state, local and non-U.S. income and other tax consequences of owning and disposing of our common
stock.
Distributions on Our Common Stock
We do not currently expect to pay any cash dividends
on our common stock. If we make distributions of cash or property (other than certain pro rata distributions of our common stock) with
respect to our common stock, any such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent
paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax rules. If a distribution exceeds
our current and accumulated earnings and profits, the excess will be treated as a nontaxable return of capital to the extent of the Non-U.S.
Holder’s adjusted tax basis in our common stock and will reduce (but not below zero) such Non-U.S. Holder’s adjusted tax basis
in our common stock. Any remaining excess will be treated as gain from a disposition of our common stock subject to the tax treatment
described below in “— Dispositions of Our Common Stock.”
Distributions on our common stock that are treated
as dividends and that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United States will
be taxed on a net income basis at the regular graduated rates and in the manner applicable to United States persons. An exception may
apply if the Non-U.S. Holder is eligible for, and properly claims, the benefit of an applicable income tax treaty and the dividends are
not attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States. In such case, the
Non-U.S. Holder may be eligible for a lower rate under an applicable income tax treaty between the United States and its jurisdiction
of tax residence. Dividends that are effectively connected with a Non-U.S. Holder’s conduct of a trade or business in the United
States will not be subject to U.S. withholding tax if the Non-U.S. Holder provides to the applicable withholding agent a properly executed
IRS Form W-8ECI (or other applicable form) in accordance with the applicable certification and disclosure requirements. A Non-U.S. Holder
treated as a corporation for U.S. federal income tax purposes may also be subject to a “branch profits tax” at a 30% rate
(unless the Non-U.S. Holder is eligible for a lower rate under an applicable income tax treaty) on the Non-U.S. Holder’s earnings
and profits (attributable to dividends on our common stock or otherwise) that are effectively connected with the Non-U.S. Holder’s
conduct of a trade or business within the United States. The amount of taxable earnings and profits is generally reduced by amounts reinvested
in the operations of the U.S. trade or business and increased by any decline in its equity.
The certifications described above must be provided
to the applicable withholding agent prior to the payment of dividends and must be updated periodically. A Non-U.S. Holder may obtain a
refund or credit of any excess amounts withheld by timely filing an appropriate claim for a refund with the IRS. Non-U.S. Holders should
consult their own tax advisors regarding their eligibility for benefits under any relevant income tax treaty and the manner of claiming
such benefits.
The foregoing discussion is subject to the discussions
below under “—Backup Withholding and Information Reporting” and “—FATCA Withholding.”
Dispositions of Our Common Stock
A Non-U.S. Holder generally will not be subject to
U.S. federal income tax (including U.S. withholding tax) on gain recognized on any sale or other disposition of our common stock unless:
| |
● |
the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base maintained by the Non-U.S. Holder in the United States); in such case, the gain would be subject to U.S. federal income tax on a net income basis at the regular graduated rates and in the manner applicable to United States persons (unless an applicable income tax treaty provides otherwise) and, if the Non-U.S. Holder is treated as a corporation for U.S. federal income tax purposes, the “branch profits tax” described above may also apply; |
| |
● |
the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of the disposition and meets certain other requirements; in such case, except as otherwise provided by an applicable income tax treaty, the gain, which may be offset by certain U.S. source capital losses, generally will be subject to a flat 30% U.S. federal income tax, even if the Non-U.S. Holder is not treated as a resident of the United States under the Code; or |
| |
|
|
| |
● |
we are or have been a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes at any time during the shorter of (i) the five-year period ending on the date of disposition and (ii) the period that the Non-U.S. Holder held our common stock. |
Generally, a corporation is a USRPHC, if the fair
market value of its “United States real property interests” equals or exceeds 50% of the sum of the fair market value of its
worldwide real property interests plus its other assets used or held for use in a trade or business. We believe that we are not currently,
and we do not anticipate becoming in the future, a USRPHC. However, because the determination of whether we are a USRPHC is made from
time to time and depends on the relative fair market values of our assets, there can be no assurance in this regard. If we were a USRPHC,
the tax relating to disposition of stock in a USRPHC generally will not apply to a Non-U.S. Holder whose holdings, direct, indirect and
constructive, constituted 5% or less of our common stock at all times during the applicable period, provided that our common stock is
“regularly traded on an established securities market” (as provided in applicable U.S. Treasury regulations) at any time during
the calendar year in which the disposition occurs. However, no assurance can be provided that our common stock will be regularly traded
on an established securities market for purposes of the rules described above. Non-U.S. Holders should consult their own tax advisors
regarding any possible adverse U.S. federal income tax consequences to them if we are, or were to become, a USRPHC.
The foregoing discussion is subject to the discussions
below under “—Backup Withholding and Information Reporting” and “—FATCA Withholding.”
Federal Estate Tax
Any shares of our common stock that are owned (or
treated as owned) by an individual who is not a U.S. citizen or resident of the United States (as specially defined for U.S. federal estate
tax purposes) at the time of his or her death will be included in that individual’s gross estate for U.S. federal estate tax purposes,
unless an applicable estate tax or other treaty provides otherwise, and therefore may be subject to U.S. federal estate tax.
Backup Withholding and Information Reporting
Backup withholding (currently at a rate of 24%) may
apply to dividends paid by U.S. corporations in some circumstances, but will not apply to payments of dividends on our common stock to
a Non-U.S. Holder if the Non-U.S. Holder provides to the applicable withholding agent a properly executed IRS Form W-8BEN or W-8BEN-E
(or other applicable form) certifying under penalties of perjury that the Non-U.S. Holder is not a United States person or is otherwise
entitled to an exemption. However, the applicable withholding agent generally will be required to report to the IRS (and to such Non-U.S.
Holder) payments of dividends on our common stock and the amount of U.S. federal income tax, if any, withheld from those payments. In
accordance with applicable treaties or agreements, the IRS may provide copies of such information returns to the tax authorities in the
country in which the Non-U.S. Holder resides.
The gross proceeds from sales or other dispositions
of our common stock may be subject, in certain circumstances discussed below, to U.S. backup withholding and information reporting. If
a Non-U.S. Holder sells or otherwise disposes of any of our common stock outside the United States through a non-U.S. office of a non-U.S.
broker and the disposition proceeds are paid to the Non-U.S. Holder outside the United States, the U.S. backup withholding and information
reporting requirements generally will not apply to that payment. However, U.S. information reporting, but not U.S. backup withholding,
will apply to a payment of disposition proceeds, even if that payment is made outside the United States, if a Non-U.S. Holder sells our
common stock through a non-U.S. office of a broker that is a United States person or has certain enumerated connections with the United
States, unless the broker has documentary evidence in its files that the Non-U.S. Holder is not a United States person and certain other
conditions are met or the Non-U.S. Holder otherwise qualifies for an exemption.
If a Non-U.S. Holder receives payments of the proceeds
of a disposition of our common stock to or through a U.S. office of a broker, the payment will be subject to both U.S. backup withholding
and information reporting unless the Non-U.S. Holder provides to the broker a properly executed IRS Form W-8BEN or W-8BEN-E (or other
applicable form) certifying under penalties of perjury that the Non-U.S. Holder is not a United States person, or the Non-U.S. Holder
otherwise qualifies for an exemption.
Backup withholding is not an additional tax. Any amounts
withheld under the backup withholding rules may be credited against the Non-U.S. Holder’s U.S. federal income tax liability (which
may result in the Non-U.S. Holder being entitled to a refund), provided that the required information is timely furnished to the IRS.
FATCA Withholding
The Foreign Account Tax Compliance Act and related
Treasury guidance (commonly referred to as FATCA) impose U.S. federal withholding tax at a rate of 30% on payments to certain foreign
entities of (i) U.S.-source dividends (including dividends paid on our common stock) and (ii) the gross proceeds from the sale or other
disposition of property that produces U.S.-source dividends (including sales or other dispositions of our common stock). This withholding
tax applies to a foreign entity, whether acting as a beneficial owner or an intermediary, unless such foreign entity complies with (i)
certain information reporting requirements regarding its U.S. account holders and its U.S. owners and (ii) certain withholding obligations
regarding certain payments to its account holders and certain other persons. Accordingly, the entity through which a Non-U.S. Holder holds
its common stock will affect the determination of whether such withholding is required. While withholding under FATCA would have also
applied to payments of gross proceeds from the sale or other disposition of our common stock on or after January 1, 2019, U.S. Treasury
regulations proposed in December, 2018 eliminate such withholding on payments of gross proceeds entirely. Taxpayers generally may rely
on these proposed U.S. Treasury regulations until final U.S. Treasury regulations are issued. Non-U.S. Holders are encouraged to consult
their tax advisors regarding the possible application of FATCA in their particular circumstances.
PLAN OF DISTRIBUTION
Each selling stockholder of the securities of the
Company, and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their Company securities
covered hereby on the OTCQB or any stock exchange, market or trading facility on which such securities are traded or in private transactions.
These sales may be at fixed or negotiated prices. A selling stockholder may use any one or more of the following methods when selling
securities:
| |
● |
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
● |
block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| |
● |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
● |
an exchange distribution in accordance with the rules of the applicable exchange; |
| |
● |
privately negotiated transactions; |
| |
● |
settlement of short sales entered into after the effective date of the registration statement of which this prospectus forms a part; |
| |
● |
in transactions through broker-dealers that agree with the selling stockholders to sell a specified number of such securities at a stipulated price per security; |
| |
● |
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
● |
a combination of any such methods of sale; or |
| |
● |
any other method permitted pursuant to applicable law. |
The selling stockholders may also sell securities
under Rule 144, if available, rather than under this prospectus.
Broker-dealers engaged by the selling stockholders
may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders
(or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except
as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission
in compliance with FINRA Rule 2121; and in the case of a principal transaction a markup or markdown in compliance with FINRA Rule 2121.
In connection with the sale of the securities or interests
therein, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in
turn engage in short sales of the securities in the course of hedging the positions they assume. The selling stockholders may also sell
securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that
in turn may sell these securities. The selling stockholders may also enter into option or other transactions with broker-dealers or other
financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial
institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant
to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholders and any broker-dealers or
agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities
Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale
of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each
selling stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly,
with any person to distribute the securities. Each selling stockholder with rights under any Units Registration Rights Agreement or OID
Units Registration Rights Agreement has agreed that in no event shall any broker-dealer receive fees, commissions and markups which, in
the aggregate, would exceed eight percent (8%).
The Company is required to pay certain fees and expenses
incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the selling stockholders against
certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
Because selling stockholders may be deemed to be “underwriters”
within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act including
Rule 172 thereunder. The selling stockholders that have rights under any Units Registration Rights Agreement or OID Units Registration
Rights Agreement have advised us that there is no underwriter or coordinating broker acting in connection with the proposed sale of the
resale securities by the selling stockholders.
With respect to the selling stockholders that have
rights under any Units Registration Rights Agreement or OID Units Registration Rights Agreement, we agreed to keep this prospectus effective
until the earliest of (i) one year from the date the registration statement of which this prospectus forms a part is declared effective
by the SEC, (ii) the date on which the securities may be resold by the selling stockholders without registration and without regard to
any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current
public information under Rule 144 under the Securities Act or any other rule of similar effect or (iii) the date on which all of the securities
have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. With respect to the
selling stockholders that have rights under the Registration Rights Letter Agreement, we agreed to keep this prospectus effective for
one year from the date the registration statement of which this prospectus forms a part is declared effective by the SEC. The resale securities
will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in
certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable
state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange
Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect
to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In
addition, the selling stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder,
including Regulation M, which may limit the timing of purchases and sales of the securities by the selling stockholders or any other person.
We will make copies of this prospectus available to the selling stockholders and have informed them of the need to deliver a copy of this
prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL MATTERS
Bevilacqua PLLC has acted as our counsel
in connection with the preparation of this prospectus. Certain legal matters as to Nevada law in connection with this offering have
been passed upon by Sherman & Howard L.L.C.
As of the date
of this prospectus, Bevilacqua PLLC owns units consisting of a Convertible Note in the principal amount of $40,000 and a Mandatory
Default Amount of $61,072 convertible into 610,866 shares of common stock, and a Class C Warrant for the purchase of 1,028,571
shares of common stock at $0.10 per share. Bevilacqua PLLC received these securities as partial consideration for legal services previously
provided to us. Bevilacqua PLLC has waived all rights to the security interest provided in the Convertible Note.
EXPERTS
The consolidated audited financial statements of Marizyme,
Inc. as of and for the years ended December 31, 2022 and 2021 appearing in this prospectus and registration statement have been audited
by WithumSmith+Brown, PC, an independent registered public accounting firm, as stated in their report appearing herein, which report contains
an explanatory paragraph regarding the ability of the Company to continue as a going concern. These consolidated financial statements
have been so included in reliance upon the report of such firm given upon its authority as an expert in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement
on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, which constitutes
a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is
contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with
respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration
statement. Statements contained in this prospectus concerning the contents of any contract or any other document are not necessarily complete.
If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document
that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects
by the filed exhibit.
You may read and copy the registration statement,
including the related exhibits and schedules, and any document we file with the SEC without charge on the SEC’s website. The SEC
maintains an internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our
filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
We file periodic reports, proxy statements and other
information with the SEC. These periodic reports, proxy statements and other information are available for inspection on the SEC’s
website. We also maintain a website at www.marizyme.com. You may access our annual reports on Form 10-K, quarterly reports on Form 10-Q,
current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act
with the SEC free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information
contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual
reference only.
FINANCIAL STATEMENTS
| Unaudited Condensed Consolidated Financial
Statements for the Three and Six Months Ended June 30, 2023 and 2022 |
|
| Condensed
Consolidated Balance Sheets as of June 30, 2023 and December 31, 2022 |
F-2 |
| Condensed
Consolidated Statements of Operations for the Three and Six Months Ended June 30, 2023 and 2022 |
F-3 |
| Condensed
Consolidated Statements of Changes in Stockholders’ Equity for the Three and Six Months Ended June 30, 2023 and
2022 |
F-4 |
| Condensed
Consolidated Statements of Cash Flows for the Six Months Ended June 30, 2023 and 2022 |
F-5 |
| Notes
to Condensed Consolidated Financial Statements for the Three and Six Months Ended June 30, 2023 and 2022 |
F-6 |
| |
|
| Audited Consolidated Financial Statements for the
Years Ended December 31, 2022 and 2021 |
|
| Report of Independent Registered Public Accounting Firm (PCAOB ID No. 100) |
F-22 |
| Consolidated Balance Sheets as of December 31, 2022 and 2021 |
F-24 |
| Consolidated Statements of Operations for the Years Ended December 31, 2022 and 2021 |
F-25 |
| Consolidated Statements of Changes in Stockholders’ Equity for the Years Ended December 31, 2022 and 2021 |
F-26 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2022 and 2021 |
F-27 |
| Notes to Consolidated Financial Statements for the Years Ended December 31, 2022 and 2021 |
F-28 |
MARIZYME,
INC.
Condensed
Consolidated Balance Sheets
| | |
June
30, 2023 | | |
December
31, 2022 | |
| | |
(unaudited) | | |
| |
| ASSETS: | |
| | | |
| | |
| Current | |
| | | |
| | |
| Cash | |
$ | 205,226 | | |
$ | 510,865 | |
| Accounts receivable | |
| 72,975 | | |
| 87,801 | |
| Other receivables | |
| 34,306 | | |
| 15,310 | |
| Prepaid expenses | |
| 606,347 | | |
| 1,125,761 | |
| Inventory | |
| 139,723 | | |
| 215,566 | |
| Total current assets | |
| 1,058,577 | | |
| 1,955,303 | |
| Non-current | |
| | | |
| | |
| Property, plant and equipment,
net | |
| 12,500 | | |
| 12,545 | |
| Operating lease right-of-use
assets, net | |
| 1,294,442 | | |
| 1,485,023 | |
| Intangible assets, net | |
| 27,254,434 | | |
| 27,675,020 | |
| Prepaid royalties, non-current | |
| 140,843 | | |
| 339,091 | |
| Deposits | |
| 30,000 | | |
| 30,000 | |
| Goodwill | |
| 7,190,656 | | |
| 7,190,656 | |
| Total
non-current assets | |
| 35,922,875 | | |
| 36,732,335 | |
| Total
assets | |
$ | 36,981,452 | | |
$ | 38,687,638 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’
EQUITY: | |
| | | |
| | |
| Current | |
| | | |
| | |
| Accounts payable and accrued
expenses | |
$ | 2,038,005 | | |
$ | 1,365,443 | |
| Notes payable | |
| 283,290 | | |
| 1,132,829 | |
| Due to related parties | |
| 143,351 | | |
| - | |
| Convertible notes- Units
Private Placement | |
| 12,316,495 | | |
| - | |
| Convertible notes - OID | |
| 150,086 | | |
| - | |
| Derivative liabilities | |
| 5,461,702 | | |
| - | |
| Operating
lease obligations | |
| 428,789 | | |
| 423,495 | |
| Total current liabilities | |
| 20,821,718 | | |
| 2,921,767 | |
| Non-current | |
| | | |
| | |
| Operating lease obligations,
net of current portion | |
| 865,653 | | |
| 1,061,528 | |
| Note payable, net of current portion | |
| | | |
| | |
| Convertible notes– Units Private Placement | |
| 307,425 | | |
| 2,751,633 | |
| Derivative liabilities | |
| - | | |
| 4,823,725 | |
| Contingent
liabilities | |
| 7,717,000 | | |
| 9,707,000 | |
| Total
non-current liabilities | |
| 8,890,078 | | |
| 18,343,886 | |
| Total liabilities | |
| 29,711,796 | | |
| 21,265,653 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 10) | |
| - | | |
| - | |
| | |
| | | |
| | |
| Stockholders’
equity: | |
| | | |
| | |
| Preferred stock, $0.001 par value, 25,000,000
shares authorized, no shares issued and outstanding as of June 30, 2023 and December 31, 2022 | |
| - | | |
| - | |
| Common stock, par value
$0.001, 300,000,000 shares authorized, issued and outstanding shares - 45,366,760 at June 30, 2023 and 40,528,191 at December 31,
2022 | |
| 45,366 | | |
| 40,528 | |
| Additional paid-in capital | |
| 116,344,709 | | |
| 103,370,890 | |
| Accumulated
deficit | |
| (109,120,419 | ) | |
| (85,989,433 | ) |
| Total stockholders’
equity | |
| 7,269,656 | | |
| 17,421,985 | |
| Total liabilities and
stockholders’ equity | |
$ | 36,981,452 | | |
$ | 38,687,638 | |
The
accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MARIZYME,
INC.
Condensed
Consolidated Statements of Operations
(Unaudited)
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
Three
Months Ended June 30, | | |
Six
Months Ended June 30, | |
| | |
2023 | | |
2022 | | |
2023 | | |
2022 | |
| | |
| | |
| | |
| | |
| |
| Revenue | |
$ | 184,739 | | |
$ | 61,809 | | |
$ | 313,713 | | |
$ | 61,809 | |
| Direct cost of revenue | |
| 49,611 | | |
| 11,025 | | |
| 88,886 | | |
| 11,025 | |
| Gross profit | |
| 135,128 | | |
| 50,784 | | |
| 224,827 | | |
| 50,784 | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Professional fees (includes
related party amounts of $161,000, $163,200, $322,000 and $266,400 respectively) | |
| 511,844 | | |
| 873,865 | | |
| 896,650 | | |
| 1,417,905 | |
| Salary expenses | |
| 335,004 | | |
| 902,106 | | |
| 601,972 | | |
| 1,817,746 | |
| Research and development | |
| 691,393 | | |
| 1,371,470 | | |
| 1,297,390 | | |
| 2,589,766 | |
| Stock-based compensation | |
| 160,762 | | |
| 676,242 | | |
| 371,728 | | |
| 1,392,674 | |
| Depreciation and amortization | |
| 210,293 | | |
| 210,361 | | |
| 420,631 | | |
| 420,722 | |
| Impairment of intangible assets | |
| | | |
| | | |
| | | |
| | |
| Royalty expense | |
| 162,065 | | |
| - | | |
| 198,248 | | |
| - | |
| Other
general and administrative expenses | |
| 2,867,749 | | |
| 618,498 | | |
| 3,375,073 | | |
| 1,009,070 | |
| Total operating expenses | |
| 4,939,110 | | |
| 4,652,542 | | |
| 7,161,692 | | |
| 8,647,883 | |
| Total operating loss | |
| (4,803,982 | ) | |
| (4,601,758 | ) | |
| (6,936,865 | ) | |
| (8,597,099 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | | |
| | | |
| | |
| Interest and accretion
expenses | |
| (13,424,169 | ) | |
| (530,226 | ) | |
| (15,121,870 | ) | |
| (829,770 | ) |
| Change in fair value of
contingent liabilities | |
| 714,000 | | |
| (1,792,000 | ) | |
| 1,990,000 | | |
| (3,622,000 | ) |
| Loss
on debt extinguishment | |
| (684,682 | ) | |
| - | | |
| (684,682 | ) | |
| - | |
| Loss on issuance of debt | |
| (2,377,569 | ) | |
| - | | |
| (2,377,569 | ) | |
| - | |
| Other income | |
| | | |
| | | |
| | | |
| | |
| Total other income (expense) | |
| (15,772,420 | ) | |
| (2,322,226 | ) | |
| (16,194,121 | ) | |
| (4,451,770 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss | |
$ | (20,576,402 | ) | |
$ | (6,923,984 | ) | |
$ | (23,130,986 | ) | |
$ | (13,048,869 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Loss per share –
basic and diluted | |
$ | (0.48 | ) | |
$ | (0.17 | ) | |
$ | (0.55 | ) | |
$ | (0.32 | ) |
| Loss per share –
basic | |
$ | (0.48 | ) | |
$ | (0.17 | ) | |
$ | (0.55 | ) | |
$ | (0.32 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Weighted average number
of shares of common stock outstanding – basic and diluted | |
| 43,187,439 | | |
| 40,828,188 | | |
| 41,979,194 | | |
| 40,728,740 | |
| Weighted average number
of shares of common stock outstanding – basic | |
| 43,187,439 | | |
| 40,828,188 | | |
| 41,979,194 | | |
| 40,728,740 | |
The
accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MARIZYME,
INC.
Condensed
Consolidated Statements of Changes in Stockholders’ Equity
Three
and Six Months Ended June 30, 2023 and 2022
(Unaudited)
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| | |
Common
Stock | | |
Additional
Paid-in | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, December 31, 2021 | |
| 40,528,191 | | |
$ | 40,528 | | |
$ | 95,473,367 | | |
$ | (47,823,563 | ) | |
$ | 47,690,332 | |
| Stock-based compensation
expense | |
| - | | |
| - | | |
| 716,432 | | |
| - | | |
| 716,432 | |
| Issuance of warrants | |
| - | | |
| - | | |
| 2,969,916 | | |
| - | | |
| 2,969,916 | |
| Exercise of warrants | |
| 300,000 | | |
| 300 | | |
| 2,700 | | |
| - | | |
| 3,000 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| (6,124,885 | ) | |
| (6,124,885 | ) |
| Balance, March 31, 2022 | |
| 40,828,191 | | |
| 40,828 | | |
| 99,162,415 | | |
| (53,948,448 | ) | |
| 45,254,795 | |
| Stock-based compensation
expense | |
| - | | |
| - | | |
| 676,242 | | |
| - | | |
| 676,242 | |
| Issuance of warrants | |
| - | | |
| - | | |
| 2,341,659 | | |
| - | | |
| 2,341,659 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| (6,923,984 | ) | |
| (6,923,984 | ) |
| Balance, June 30, 2022 | |
| 40,828,191 | | |
$ | 40,828 | | |
$ | 102,180,316 | | |
$ | (60,872,432 | ) | |
$ | 41,348,712 | |
| | |
Common
Stock | | |
Additional
Paid-in | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, December 31, 2022 | |
| 40,528,191 | | |
$ | 40,528 | | |
$ | 103,370,890 | | |
$ | (85,989,433 | ) | |
$ | 17,421,985 | |
| Stock-based compensation
expense | |
| - | | |
| - | | |
| 210,966 | | |
| - | | |
| 210,966 | |
| Issuance of shares | |
| 240,000 | | |
| 240 | | |
| 153,360 | | |
| - | | |
| 153,600 | |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| (2,554,584 | ) | |
| (2,554,584 | ) |
| Balance, March 31, 2023 | |
| 40,768,191 | | |
| 40,768 | | |
| 103,735,216 | | |
| (88,544,017 | ) | |
| 15,231,967 | |
| Stock-based compensation
expense | |
| - | | |
| - | | |
| 160,762 | | |
| - | | |
| 160,762 | |
| Issuance of shares | |
| 1,946,410 | | |
| 1,946 | | |
| 221,540 | | |
| - | | |
| 223,486 | |
| Exercise of warrants | |
| 2,652,159 | | |
| 2,652 | | |
| 262,564 | | |
| - | | |
| 265,216 | |
| Issuance of warrants on debt
extinguishment | |
| - | | |
| - | | |
| 19,058,426 | | |
| - | | |
| 19,058,426 | |
| Issuance of warrants on promissory note | |
| - | | |
| - | | |
| 1,333,128 | | |
| - | | |
| 1,333,128 | |
| Warrants cancelled in debt
extinguishment | |
| - | | |
| - | | |
| (8,426,927 | ) | |
| - | | |
| (8,426,927 | ) |
| Net
loss | |
| - | | |
| - | | |
| - | | |
| (20,576,402 | ) | |
| (20,576,402 | ) |
| Balance, June 30, 2023 | |
| 45,366,760 | | |
$ | 45,366 | | |
$ | 116,344,709 | | |
$ | (109,120,419 | ) | |
$ | 7,269,656 | |
The
accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MARIZYME,
INC.
Condensed
Consolidated Statements of Cash Flows
(Unaudited)
| | |
2023 | | |
2022 | |
| | |
Six
Months Ended June 30, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Cash flows from operating
activities: | |
| | | |
| | |
| Net loss | |
$ | (23,130,986 | ) | |
$ | (13,048,869 | ) |
| Adjustments to reconcile
net loss to net cash used in operating activities: | |
| | | |
| | |
| Depreciation and amortization | |
| 420,631 | | |
| 420,722 | |
| Stock-based compensation | |
| 371,728 | | |
| 1,392,674 | |
| Stock-based compensation - restricted common stock | |
| | | |
| | |
| Interest and accretion
on convertible notes and notes payable | |
| 15,121,870 | | |
| 829,770 | |
| Issuance of warrants for
services | |
| - | | |
| 1,850,533 | |
| Impairment of intangible assets | |
| | | |
| | |
| Change in fair value of
contingent liabilities | |
| (1,990,000 | ) | |
| 3,622,000 | |
| Loss on debt extinguishment | |
| 684,682 | | |
| - | |
| Other income | |
| | | |
| | |
| Loss on issuance of debt | |
| 2,377,569 | | |
| - | |
| Shares issued as part of
the Confidential Settlement Agreement | |
| 153,600 | | |
| - | |
| Shares issued for services | |
| 138,000 | | |
| - | |
| Warrants issued as part
of promissory note agreement | |
| 1,333,128 | | |
| - | |
| Change in operating assets
and liabilities: | |
| | | |
| | |
| Accounts and other receivable | |
| (4,171 | ) | |
| (15,715 | ) |
| Prepaid expenses | |
| 519,414 | | |
| 21,787 | |
| Inventory | |
| 75,843 | | |
| (246,060 | ) |
| Accounts payable and accrued
expenses | |
| 1,467,328 | | |
| (973,544 | ) |
| Due
to related parties | |
| 143,351 | | |
| (908,973 | ) |
| Net
cash used in operating activities | |
| (2,318,013 | ) | |
| (7,055,675 | ) |
| | |
| | | |
| | |
| Cash flows from financing
activities: | |
| | | |
| | |
| Proceeds from issuance of Convertible Notes - OID,
net of issuance cost | |
| 870,000 | | |
| 5,120,743 | |
| Proceeds from financing, net of issuance cost | |
| | | |
| | |
| Proceeds from shares issued
for exercise of warrants | |
| 265,216 | | |
| 3,000 | |
| Proceeds
from promissory notes, net of repayments | |
| 877,158 | | |
| (95,431 | ) |
| Net
cash provided by financing activities | |
| 2,012,374 | | |
| 5,028,312 | |
| | |
| | | |
| | |
| Net change in cash | |
| (305,639 | ) | |
| (2,027,363 | ) |
| | |
| | | |
| | |
| Cash at beginning of period | |
| 510,865 | | |
| 4,072,339 | |
| | |
| | | |
| | |
| Cash at end of period | |
$ | 205,226 | | |
$ | 2,044,976 | |
| | |
| | | |
| | |
| Supplemental disclosure
of cash flow information: | |
| | | |
| | |
| Cash
paid for interest | |
$ | - | | |
$ | - | |
| Cash
paid for taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash investing and financing
activities: | |
| | | |
| | |
| Derivative
liabilities and debt discount issued in connection with convertible notes | |
$ | 5,461,702 | | |
$ | 1,938,379 | |
| Warrants
and debt discount issued in connection with convertible notes | |
$ | 19,058,426 | | |
$ | 3,461,042 | |
| Settlement
of notes payable with convertible notes | |
$ | 2,064,133 | | |
$ | 278,678 | |
| Warrants cancelled in debt extinguishment | |
$ | 8,426,927 | | |
$ | - | |
| Shares issued on conversion of convertible notes | |
$ | 84,140 | | |
$ | - | |
The
accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
MARIZYME,
INC.
NOTES
TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
JUNE
30, 2023
NOTE
1 – DESCRIPTION OF BUSINESS
Marizyme,
Inc. (the “Company” or “Marizyme”) is a Nevada corporation originally incorporated on March 20, 2007, under the
name SWAV Enterprises, Ltd. On September 6, 2010, the Company name was changed to GBS Enterprises Inc. and from 2010, to September 2018,
the Company was in the software products and advisory services business for email and instant messaging applications. The Company divested
that business between December 2016 and September 2018, and focused on the acquisition of life science technologies.
On
March 21, 2018, the Company’s name was changed to Marizyme, Inc., to reflect the new life sciences focus. Marizyme’s
common stock is currently quoted on the OTCQB tier of OTC Markets Group, Inc. under the symbol “MRZM”.
NOTE
2 – GOING CONCERN
The
Company’s unaudited condensed consolidated financial statements are prepared using accounting principles generally accepted in
the United States of America applicable to a going concern, which contemplates the realization of assets and liquidation of liabilities
in the normal course of business. However, the Company does not have an established source of revenues sufficient to cover its operating
costs, which require the Company to rely on investing and financing activities in order to continue as a going concern. The Company,
since its inception, has incurred recurring operating losses and negative cash flows from operations and has an accumulated deficit of
$109,120,419 at June 30, 2023 (December 31, 2022 - $85,989,433). At June 30, 2023, the Company has negative working capital of $19,763,141
(December 31, 2022 - $966,464) and $205,226 (December 31, 2022 - $510,865) of cash on hand, which may not be sufficient to fund operations
for the next twelve months. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Under
the going concern assumption, an entity is ordinarily viewed as continuing its business for the foreseeable future with neither the intention
or necessity of liquidation, ceasing trading, or seeking protection from creditors pursuant to the laws and regulations. Accordingly,
assets and liabilities are recorded on the basis that the entity will be able to realize its assets and discharge its liabilities in
the normal course of business.
The
ability of the Company to continue as a going concern is dependent upon its ability to continue to successfully develop its intangible
assets and receive an approval from the U.S. Food and Drug Administration (“FDA”) to extend the selling of its products into
the U.S. market which may result in the Company attaining profitable operations.
During
the next twelve months from the date the unaudited condensed consolidated financial statements were issued, the Company’s foreseeable
cash requirements will relate to continuous operations of its business, maintaining its good standing and making the required filing
with the Securities and Exchange Commission (“SEC”), and the payment of expenses associated with its product development.
The Company may experience a cash shortfall and be required to raise additional capital. Management intends to raise additional funds
by way of a private or public offering. While the Company believes in the viability of its strategy to continue to develop and expand
its products and generate sufficient revenue and in its ability to raise additional funds, there can be no assurances to that effect.
The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business
plan and generate sufficient revenue and its ability to raise additional funds by way of a public or private offering.
The
unaudited condensed consolidated financial statements do not include any adjustments related to the recoverability and classification
of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue
as a going concern.
NOTE
3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis
of Presentation and Principles of Consolidation
The accompanying unaudited condensed consolidated financial statements
include the consolidated accounts of the Company and its wholly owned subsidiaries: My Health Logic Inc. (“My Health Logic”
or “MHL”), Somahlution, Inc. (“Somahlution”), Somaceutica, Inc. (“Somaceutica”), and Marizyme Sciences,
Inc. (“Marizyme Sciences”). All intercompany transactions have been eliminated on consolidation.
The
accompanying unaudited condensed consolidated financial statements included in this Quarterly Report on Form 10-Q have been prepared
in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The unaudited
condensed consolidated financial statements presented in this Quarterly Report should be read in conjunction with the consolidated financial
statements and accompanying notes included in the Company’s Annual Report on Form 10-K filed with the SEC on March 24, 2023 (the
“2022 Form 10-K”). The condensed consolidated balance sheet as of December 31, 2022 was derived from audited consolidated
financial statements included in the 2022 Form 10-K but does not include all disclosures required by U.S. GAAP for complete financial
statements. The Company’s significant accounting policies are described in Note 1 to those consolidated financial statements.
Interim
results may not be indicative of the results that may be expected for the full year or any future periods. Certain information and footnote
disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted from these
interim financial statements. The unaudited condensed consolidated financial statements reflect all adjustments which in the opinion
of management are necessary to fairly present the results of operations, financial condition, cash flows and stockholders’ equity
for the periods indicated. Except as otherwise disclosed, all such adjustments are of a normal recurring nature.
Deferred
Offering Cost
The
Company capitalizes certain legal, professional accounting and other third-party fees that are directly associated with in-process capital
stock financings as deferred offering costs until such financings are consummated. After consummation of the financing, these costs are
recorded in stockholders’ equity (deficit) as a reduction of additional paid-in capital generated as a result of the offering.
Should a planned equity financing be abandoned, the deferred offering costs will be expensed immediately as a charge to operating expenses
in the condensed consolidated statements of operations. As of June 30, 2023, the Company had deferred offering costs of $Nil (December
31, 2022 - $387,412)
reported as a prepaid expense on the accompanying condensed consolidated balance sheets, and expensed an aggregate of $1,203,537
of
deferred offering costs - $535,717
of
which was related to the extinguishment of 2021 Unit Purchase Agreement (see Note 7) and $667,820
related
to a planned uplisting, which was not pursued due to a management’s decision to seek different means to obtain financing.
Use
of Estimates
The
preparation of the unaudited condensed consolidated financial statements in accordance with U.S. GAAP requires management to make use
of certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and
liabilities as of the date of the unaudited condensed consolidated financial statements and the reported amounts of revenue and expenses
during the reported periods. The Company bases its estimates on historical experience and on various other assumptions that management
believes are reasonable under the circumstances, the results of which form the basis for making judgments about carrying values of assets
and liabilities that are not readily apparent from other sources. Actual results could differ from those estimates. Significant estimates
are related to the recoverability of long-term assets including intangible assets and goodwill, amortization expense, valuation of warrants,
stock-based compensation, derivative liabilities and contingent liabilities.
Fair
Value Measurements
The
Company uses the fair value hierarchy to measure the value of its financial instruments. The fair value hierarchy is based on inputs
to valuation techniques that are used to measure fair value that are either observable or unobservable. Observable inputs reflect assumptions
market participants would use in pricing an asset or liability based on market data obtained from independent sources, while unobservable
inputs reflect a reporting entity’s pricing based upon its own market assumptions. The basis for fair value measurements for each
level within the hierarchy is described below:
| ● |
Level
1 – Quoted prices for identical assets or liabilities in active markets. |
| ● |
Level
2 – Quoted prices for identical or similar assets and liabilities in markets that are not active; or other model-derived valuations
whose inputs are directly or indirectly observable or whose significant value drivers are observable. |
| ● |
Level
3 – Valuations derived from valuation techniques in which one or more significant inputs to the valuation model are unobservable
and for which assumptions are used based on management estimates. |
The
Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent
possible and considers counterparty credit risk in its assessment of fair value.
The
carrying amounts of certain accounts and other receivables, accounts payable and accrued expenses, notes payable, and amounts due to
related parties approximate fair value due to the short-term nature of these instruments.
The
fair value of lease obligations is determined using discounted cash flows based on the expected amounts and timing of the cash flows
discounted using a market rate of interest adjusted for appropriate credit risk.
The
contingent liabilities assumed on the acquisition of Somahlution in 2020 consist of present values of royalty payments, performance warrants
and pediatric voucher warrants, future rare pediatric voucher sales, and liquidation preference. Management measured these contingencies
in accordance with Level 3 of the fair value hierarchy.
| |
i. |
The
performance warrants and pediatric vouchers warrants liabilities were valued using a Monte Carlo simulation model utilizing the following
weighted average assumptions: risk free rate of 1.19%, expected volatility of 69.62%, expected dividend of $0, and expected life
of 5.96 years. For the three and six months ended June 30, 2023, changes in these assumptions resulted in a $582,000 and $1,206,000
decrease in fair value of these liabilities, respectively (June 30, 2022 – $2,092,000 and $2,898,000 increase, respectively).
At June 30, 2023, the fair market value of performance warrants and pediatric vouchers warrants liabilities was $221,000 (December
31, 2022 – $1,427,000). |
| |
|
|
| |
ii. |
The
present value of royalty payments was measured using the scenario-based methodology. In assessing the value attributed to the royalty
payments, the estimated future cash flows were discounted to their present value using a pre-tax discount rate that reflects current
market assessments of the time value of money and the risks specific to the revenue from net sales of the product. The cash flows
derived from the Company’s fifteen-year strategic plan are based on managements’ expectations of market growth, industry
reports and trends, and past performances. These projections are inherently uncertain due to the evolving impact of the COVID-19
pandemic. The discounted cash flow model included projections surrounding revenue, discount rates, and growth rates. The discount
rates used to calculate the present value of royalty payments reflect specific risks of the Company and market conditions and the
mid-range was estimated at 20.6%. For the three and six months ended June 30, 2023, changes in these assumptions resulted in a $129,000
and $796,000 decrease in fair value of this liability, respectively (June 30, 2022 – $293,000 decrease and $772,000 increase,
respectively). At June 30, 2023, the fair market value of royalty payments was $4,606,000 (December 31, 2022 – $5,402,000). |
| |
|
|
| |
iii. |
Rare
pediatric voucher sales liability was valued based on the scenario-based methodology where the estimated future cash flows are discounted
to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the
risks specific to the asset – 20.6%. For the three and six months ended June 30, 2023, changes in these assumptions resulted
in a $3,000 decrease and $12,000 increase in fair value of this liability, respectively (June 30, 2022 – $7,000 and $48,000
decrease, respectively). At June 30, 2023, the fair market value of rare pediatric voucher sales liability was $1,067,000 (December
31, 2022 – $1,055,000). |
| |
|
|
| |
iv. |
The
present value of liquidation preference liability, included in the contingent consideration, was determined using the Black-Scholes
option pricing method and represents the fair value of the maximum payment amount according to the agreement. The following assumptions
were used in the Black-Scholes option pricing model: risk free rate of 0.21%, expected volatility of 78.93%, expected dividend of
$0, and expected life of 5 years. No changes to the fair value of liquidation preference liability were recorded in the three and
six months ended June 30, 2023 and 2022. At June 30, 2023, the fair market value of liquidation preference was $1,823,000 (December
31, 2022 – $1,823,000). |
The
derivative liabilities consist of optional and automatic conversion features and the share redemption feature attached to the convertible
notes, issued pursuant to the convertible promissory notes and warrants transactions as described in Note 7.
The
Company has no financial assets measured at fair value on a recurring basis. None of the Company’s non-financial assets or liabilities
are recorded at fair value on a non-recurring basis. No transfers between levels have occurred during the periods presented.
Marizyme
measures the following financial instruments at fair value on a recurring basis. As of June 30, 2023, and December 31, 2022, the fair
values of these financial instruments were as follows:
SCHEDULE OF FAIR VALUES OF FINANCIAL INSTRUMENTS
June 30, 2023
| |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair
Value Hierarchy | |
| June 30,
2023 | |
Level
1 | | |
Level
2 | | |
Level
3 | |
| Liabilities | |
| | | |
| | | |
| | |
| Derivative
liabilities | |
$ | - | | |
$ | - | | |
$ | 5,461,702 | |
| Contingent
liabilities | |
| - | | |
| - | | |
| 7,717,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 13,178,702 | |
| December 31, 2022 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair
Value Hierarchy | |
| December
31, 2022 | |
Level
1 | | |
Level
2 | | |
Level
3 | |
| Liabilities | |
| | | |
| | | |
| | |
| Derivative
liabilities | |
$ | - | | |
$ | - | | |
$ | 4,823,725 | |
| Contingent
liabilities | |
| - | | |
| - | | |
| 9,707,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 14,530,725 | |
The
following table provides a roll forward of all liabilities measured at fair value using Level 3 significant unobservable inputs:
SCHEDULE
OF LIABILITIES FAIR VALUE MEASURED
| Derivative and Contingent
Liabilities | |
| |
| Balance at December 31, 2022 | |
$ | 14,530,725 | |
| Change in fair value of
contingent liabilities | |
| (1,990,000 | ) |
| Derivative liabilities
extinguished pursuant to Unit Private Placement (Note 7) | |
| (4,823,725 | ) |
| Derivative
liabilities issued pursuant to OID Purchase Agreement (Note 7) | |
| 5,461,702 | |
| Balance at June 30, 2023 | |
$ | 13,178,702 | |
Research
and Development Expenses and Accruals
All
research and development costs are expensed in the period incurred and consist primarily of salaries, payroll taxes, and employee benefits,
for individuals involved in research and development efforts, external research and development costs incurred under agreements with
contract research organizations and consultants to conduct and support the Company’s ongoing clinical trials of DuraGraft, and
costs related to manufacturing DuraGraft for clinical trials. The Company has entered into various research and development contracts
with various organizations. Payments of these activities are based on the terms of the individual agreements which matches to the pattern
of costs incurred. Payments made in advance are reflected in the accompanying balance sheets as prepaid expenses. The Company records
accruals for estimated costs incurred for ongoing research and development activities. When evaluating the adequacy of the accrued liabilities,
the Company analyzes progress of the services, including the phase or completion of events, invoices received and contracted costs. Significant
judgments and estimates may be required in determining the prepaid or accrued balances at the end of any reporting period. Actual results
could differ from the Company’s estimates.
Stock-Based
Compensation
Stock-based
compensation expense for employees and directors is recognized in the condensed consolidated statements of operations based on estimated
amounts, including the grant date fair value and the expected service period. For stock options, the Company estimates the grant date
fair value using a Black-Scholes valuation model, which requires the use of multiple subjective inputs including estimated future volatility,
expected forfeitures and the expected term of the awards. The Company estimates the expected future volatility based on the stock’s
historical price volatility. The stock’s future volatility may differ from the estimated volatility at the grant date. For restricted
stock unit (“RSU”) equity awards, the Company estimates the grant date fair value using its closing stock price on the date
of grant. The Company recognizes the effect of forfeitures in compensation expense when the forfeitures occur. The estimated forfeiture
rates may differ from actual forfeiture rates which would affect the amount of expense recognized during the period. The Company recognizes
the value of the awards over the awards’ requisite service or performance periods. The requisite service period is generally the
time over which share-based awards vest.
New
Accounting Standards and Updates from the Securities and Exchange Commission
In
June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-13,
“Financial Instruments - Credit Losses (Topic 326)”. The new standard amends guidance on reporting credit losses for assets
held at amortized cost basis and available-for-sale debt securities. In February 2020, the FASB issued ASU 2020-02, “Financial
Instruments-Credit Losses (Topic 326)” and “Leases (Topic 842)” - Amendments to SEC Paragraphs Pursuant to SEC Staff
Accounting Bulletin No. 119 and Update to SEC Section on Effective Date Related to Accounting Standards Update No. 2016-02, Leases (Topic
842), which amends the effective date of the original pronouncement for smaller reporting companies. ASU 2016-13 and its amendments will
be effective for the Company for interim and annual periods in fiscal years beginning after December 15, 2022. Current Expected Credit
Losses estimates of expected credit losses on trade receivables over their life will be required to be recorded at inception, based on
historical information, current conditions, and reasonable and supportable forecasts. The Company adopted the standard in its first quarter
of 2023. There was no material impact on the results of operations.
NOTE
4 – LEASES
On
December 11, 2020, the Company entered into a five-and-a-half-year lease agreement for approximately 10,300 square feet of administrative
office and laboratories space, which commenced in December 2020 at a monthly rent of approximately $10,800, increasing by 2.5% annually
beginning in the second year of the lease until the end of the term. Additionally, pursuant to the agreement, the Company would pay approximately
$12,000 per month in operating expenses.
Effective
April 1, 2022, the Company amended its lease agreement for administrative office and laboratories to add an additional 3,053 square feet
of space. The monthly cost of total expended lease space is approximately $15,260 increasing to $15,641 in 2023 and will continue to
increase by 2.5% annually thereafter until the end of the term. The monthly operating expenses for the total expanded premises have increased
from approximately $12,000 to $17,500 per month. The term of the lease remains unchanged. As of June 30, 2023, the remaining lease term
was 3.08 years. The lease has been classified as an operating lease.
The
assets and liabilities from the lease were recognized at the lease commencement date based on the present value of remaining lease payments
over the lease term using the discount rate of 3.95%, which is the average commercial interest available at the time.
The
total rent expense for the three and six months ended June 30, 2023 was $37,674 and $192,502, respectively (June 30, 2022 – $110,353
and $221,253, respectively).
The
following table summarizes supplemental condensed consolidated balance sheet information related to the operating leases as of June 30,
2023, and December 31, 2022:
SCHEDULE OF RIGHT-OF-USE ASSET AND RELATED LEASE LIABILITIES
| | |
June
30, 2023 | | |
December
31, 2022 | |
| Right-of-use
assets | |
$ | 1,294,442 | | |
$ | 1,485,023 | |
| | |
| | | |
| | |
| Operating lease liabilities, current | |
$ | 428,789 | | |
$ | 423,495 | |
| Operating lease liabilities,
non-current | |
| 865,653 | | |
| 1,061,528 | |
| Total operating lease
liabilities | |
$ | 1,294,442 | | |
$ | 1,485,023 | |
As
of June 30, 2023, the maturities of the lease liabilities for the periods ending December 31 are as follows:
SCHEDULE OF MATURITIES OF THE LEASE LIABILITIES
| | |
| | |
| 2023 | |
$ | 211,747 | |
| 2024 | |
| 434,082 | |
| 2025 | |
| 444,934 | |
| 2026 | |
| 266,034 | |
| Total lease payments | |
| 1,356,797 | |
| Less: Present value
discount | |
| (62,355 | ) |
| Total | |
$ | 1,294,442 | |
NOTE
5 – INTANGIBLE ASSETS
Krillase
As
part of the asset acquisition of ACB Holding AB, Reg. No. 559119-5762, completed on September 12, 2018, Marizyme acquired all rights,
titles, and interest in the Krillase technology, a group of intangible assets worth $28,600,000. Krillase is a naturally occurring enzyme
that acts to break protein bonds and has applications in wound debridement, wound healing, dental care and thrombosis. The useful lives
of the intangible assets are based on the life of the patent and related technology. The patents and related technology for Krillase
have not been amortized since the acquisition, as they have not yet been put into operations.
At
December 31, 2022, management determined that the carrying value of Krillase exceeded its recoverable amount. Impairment of $24,350,000
was recognized on Krillase intangible assets and recorded in the impairment of intangible assets in the consolidated statements of operations
for the year ended December 31, 2022. However, for the three and six months ended June 30, 2023 and 2022, no impairment has been recognized
on Krillase intangible assets.
DuraGraft
As
part of Somahlution acquisition in 2020, Marizyme purchased $18,170,000 of intangible assets related to the DuraGraft® technology.
No impairment has been recognized on DuraGraft intangible assets for the three and six months ended June 30, 2023 and 2022.
My
Health Logic
As
part of My Health Logic acquisition completed on December 22, 2021, Marizyme purchased MHL’s lab-on-chip technology platform and
its patient-centric, digital point-of-care diagnostic device, MATLOC, fair valued at an aggregate amount of $6,600,000. No impairment
has been recognized on My Health Logic intangible assets for the three and six months ended June 30, 2023 and 2022.
SCHEDULE OF INTANGIBLE ASSETS AMORTIZATION EXPENSE
| | |
June
30, 2023 | |
| | |
Gross
Carrying Amount | | |
Accumulated
Amortization | | |
Net
Carrying Amount | |
| Krillase intangible assets | |
$ | 4,250,000 | | |
$ | - | | |
$ | 4,250,000 | |
| Patents in process | |
| 122,745 | | |
| - | | |
| 122,745 | |
| DuraGraft patent | |
| 5,256,000 | | |
| (1,179,230 | ) | |
| 4,076,770 | |
| DuraGraft - Distributor relationship | |
| 308,000 | | |
| (89,833 | ) | |
| 218,167 | |
| DuraGraft IPR&D - Cyto Protectant Life
Sciences | |
| 12,606,000 | | |
| - | | |
| 12,606,000 | |
| My Health Logic - Trade name | |
| 450,000 | | |
| (49,018 | ) | |
| 400,982 | |
| My Health Logic - Biotechnology | |
| 4,600,000 | | |
| (412,647 | ) | |
| 4,187,353 | |
| My Health Logic - Software | |
| 1,550,000 | | |
| (157,583 | ) | |
| 1,392,417 | |
| Total intangibles | |
$ | 29,142,745 | | |
$ | (1,888,311 | ) | |
$ | 27,254,434 | |
| | |
December
31, 2022 | |
| | |
Gross
Carrying
Amount | | |
Accumulated
Amortization | | |
Impairment | | |
Net
Carrying
Amount | |
| Krillase intangible assets | |
$ | 28,600,000 | | |
$ | - | | |
$ | (24,350,000 | ) | |
$ | 4,250,000 | |
| Patents in process | |
| 122,745 | | |
| - | | |
| - | | |
| 122,745 | |
| DuraGraft patent | |
| 5,256,000 | | |
| (977,076 | ) | |
| - | | |
| 4,278,924 | |
| DuraGraft - Distributor relationship | |
| 308,000 | | |
| (74,433 | ) | |
| - | | |
| 233,567 | |
| DuraGraft IPR&D - Cyto Protectant Life
Sciences | |
| 12,606,000 | | |
| - | | |
| - | | |
| 12,606,000 | |
| My Health Logic - Trade name | |
| 450,000 | | |
| (32,947 | ) | |
| - | | |
| 417,053 | |
| My Health Logic - Biotechnology | |
| 4,600,000 | | |
| (277,353 | ) | |
| - | | |
| 4,322,647 | |
| My Health Logic - Software | |
| 1,550,000 | | |
| (105,916 | ) | |
| - | | |
| 1,444,084 | |
| Total intangibles | |
$ | 53,492,745 | | |
$ | (1,467,725 | ) | |
$ | (24,350,000 | ) | |
$ | 27,675,020 | |
SCHEDULE OF GOODWILL
| Goodwill | |
DuraGraft | | |
My
Health Logic | | |
Total | |
| Balance, June 30, 2023 and December 31, 2022 | |
$ | 5,416,000 | | |
$ | 1,774,656 | | |
$ | 7,190,656 | |
The
following changes to the Company’s intangible assets had taken place in the periods indicated:
SCHEDULE OF INTANGIBLE ASSETS
| Balance, December 31, 2021 | |
$ | 52,866,192 | |
| Impairment | |
| (24,350,000 | ) |
| Amortization expense | |
| (841,172 | ) |
| Balance, December 31, 2022 | |
| 27,675,020 | |
| Amortization expense | |
| (420,586 | ) |
| Balance, June 30, 2023 | |
$ | 27,254,434 | |
Future
amortizations for DuraGraft and My Health Logic intangible assets for the remaining six month of the fiscal 2023 will be
$420,586, for the next five years from 2024 through 2028 - $841,172 for
each year, and for 2029 and thereafter- $6,069,827. Amortization related to in-process research and development will be
determined upon the Company achieving commercialization.
NOTE
6 – NOTES PAYABLE
a)
On October 23, 2022, the Company issued a note payable to Hub International for $204,050 bearing interest at the annual rate of 6.75%
per annum, due September 23, 2023, payable monthly starting November 23, 2022. As of June 30, 2023, the balance of note payable due was
$41,887 (December 31, 2022 - $164,729).
b)
On December 28, 2022, the Company issued a promissory note to Hexin for the principal amount of $750,000 bearing interest at the annual
rate of 20% per annum, due June 28, 2023 (the “Hexin Promissory Note”). For the three and six months ended June 30, 2023,
the Company accrued $25,914 and $64,133 in interest on the promissory note, respectively (June 30, 2022 - $Nil and $Nil, respectively).
Hexin agreed to cancel the Hexin Promissory Note with the outstanding balance of $814,133 (the aggregate amount of principal plus accrued
and unpaid interest as of May 30, 2023) in exchange for the issuance of 9,578,040 OID Units (see Note 7), which the Company issued to
Hexin on May 30, 2023.
c)
On February 2, 2023, the Company issued an unsecured promissory note to Walleye Opportunities Master Fund Ltd. (the “Walleye Promissory
Note”) for $1,000,000 with a maturity date of May 7, 2023. The note carried no interest and the principal amount was required to
be repaid in full on the maturity date. In the event that the principal amount was not repaid in full on maturity date, the principal
amount required to be increased to $1,250,000. As of the maturity date of the note, the principal amount was not repaid and therefore
increased from $1,000,000 to $1,250,000. Walleye agreed to cancel the promissory note, with the outstanding balance of $1,250,000 in
exchange for the issuance of 14,705,890 OID Units (see Note 7), which were issued to Walleye on May 30, 2023.
d)
As part of the My Health Logic Inc. acquisition, completed in November 2021, Marizyme assumed an aggregate of $468,137
in notes payable, the notes were unsecured, bore
interest at a rate of 9%
per annum with no maturity date. The Company settled an aggregate of $278,678
of these notes payable as part of Unit Purchase
Agreement issuances during the year ended December 31, 2022 (see Note 7). For the three and six months ended June 30, 2023, the Company
accrued $5,298
and $18,366
in interest on the notes payable, respectively
(June 30, 2022 - $4,501
and $10,586,
respectively). As of June 30, 2023, balance of the remaining note payable was $241,403
(December 31, 2022 - $218,100).
NOTE
7 - CONVERTIBLE PROMISSORY NOTES AND WARRANTS
2021
Convertible Notes and Warrants
From
May 2021 to August 2022, the Company conducted a private placement (the “Units Private Placement”) of units (the “Units”)
consisting of 10% secured convertible promissory notes (the “Convertible Notes”) and accompanying warrants (the “Class
C Warrants”), as were modified or amended from time to time. The most recent terms of the Convertible Notes and Class C Warrants
were as follows:
Convertible
Notes Terms
The
Convertible Notes mature in 24 months from the initial closing date and accrue 10% of simple interest per annum on the outstanding principal
amount. The Convertible Notes principal and accrued interest can be converted at any time at the option of the holder at a conversion
price of $1.75 per share. In the event the Company consummates, while the Convertible Note is outstanding, an equity financing with a
gross aggregate amount of securities sold of not less than $10,000,000 (“Qualified Financing”), then all outstanding principal,
together with all unpaid accrued interest under the Convertible Notes, shall automatically convert into shares of the equity financing
at the lesser of (i) 75% of the cash price per share paid in the financing and the conversion price of $1.75 per unit.
In
the event that the Company consummates, while the Convertible Note is outstanding, an equity financing with a gross aggregate amount
of securities sold less than $10,000,000 (“Unqualified Financing”), then the conversion price of the Convertible Notes of $1.75 per share and the exercise price of Class C warrants of $2.25
per share will change to the equity offering price. Moreover, in that event, the Class C Warrant
holders may also apply a most-favored-nations clause in their warrants to request that their Class C Warrants provide that their warrant
exercise rights should be further adjusted to allow them to purchase a proportionately higher number of additional shares equal to the
initial number of shares which may be purchased by exercise of their Class C Warrants, multiplied by a fraction equal to the current
exercise price divided by the adjusted exercise price, which would allow such Class C Warrant holders to purchase the same aggregate
dollar amount of shares as initially provided such Class C Warrants. If the Convertible Notes and Class C Warrants’ conversion
or exercise rights become so adjusted as a result of such an equity financing, then the Company would be required to register the additional
shares of common stock that these securities may be converted into or exercised to purchase for resale. The Convertible Notes are secured
by a first priority security interest in all assets of the Company.
Class
C Warrants Terms
| |
● |
Exercise
price is the lower of (i) $2.25 per share, or (ii) the Automatic Conversion Price (the lesser of (i) 75% of the cash price per share
paid by the other purchasers of next round securities in the Qualified Financing and (ii) the Conversion Price ($2.25, subject to
Customary Antidilution Adjustments). |
| |
● |
Exercisable
for a period of 5 years from issuance. |
| |
● |
Warrant
Coverage: 200%. |
The
Company determined that the optional and automatic conversion feature and the share redemption feature attached to the Convertible Notes
met the definition of derivative liabilities and that the detachable warrants issued did not meet the definition of a liability and therefore
was accounted for as an equity instrument.
The
fair value of the warrants issued and the fair value of derivative liabilities issued have been recorded as debt discount and are being
amortized to interest and accretion expense using the effective interest method over the term of the Convertible Notes.
In
2021, the Company issued an aggregate of 4,260,594 Units for the proceeds of $6,692,765 net of issuance costs. In 2022, the Company issued
additional 4,180,071 units for the proceeds of $6,500,743 net of issuance cost.
Adjustment
to Conversion Price of Convertible Notes and Exercise Price of Class C Warrants
On
April 13, 2023, the Company obtained exercise and conversion rights waivers and amendments from holders of the Convertible Notes and
Class C Warrants (“Original Securities”), pursuant to which, the conversion price of the Convertible Notes and the exercise
price of the Class C Warrants was adjusted to $0.10 per share (“New Securities”) and all other terms remained the same. On
April 13, 2023, outstanding Convertible Notes had underlying principle of $14,432,996, and outstanding Class C Warrants were exercisable
to purchase a total of 16,538,486 shares of common stock.
The
Company determined that the terms of the New Securities were substantially different from the Original Securities, and, as such the exchange
of the Original Securities for the New Securities was accounted for as an extinguishment of debt on April 13, 2023, and the New Securities
accounted for as a new debt issuance.
As
a result of this substantial modification, a total of 8,269,237
Units outstanding were replaced with an aggregate
of 190,584,260
pro rata Units, and a loss on debt extinguishment
of $684,682 was
recorded in condensed consolidated statements of operations for the six months ended June 30, 2023 (June 30, 2022 - $Nil).
The
Company determined that the optional conversion feature attached to the Convertible Notes did not meet the definition of derivative
liabilities and that the detachable warrants issued did not meet the definition of a liability and therefore was accounted for as an
equity instrument. The fair value of $19,058,426
of the warrants issued has been recorded as a component of equity and a debt discount and is being amortized to interest and
accretion expense using the effective interest method over the term of the Convertible Notes.
During
the three and six months ended June 30, 2023, the Company recognized interest and accretion expense of $5,730,714 and $7,286,891, respectively,
on the Original and New Securities in aggregate (June 30, 2022 - $524,884 and $816,881, respectively) in the condensed consolidated statements
of operations.
Each of the Convertible Notes
provides that any default on indebtedness of more than $100,000,
other than indebtedness under the respective Convertible Notes, will also result in default under the Convertible Notes. Due to the
non-repayment of the initial principal amount of $1,000,000
under the Walleye Promissory Note by its maturity date of May
7, 2023 (see Note 6), the Company also defaulted under the Convertible Notes on the same date. The Convertible Notes provide
that due to this default, the Company became obligated to pay 135%
of the outstanding principal amount under each of the Convertible Notes on the date on which the default occurred (the
“Mandatory Default Amount”). The Mandatory Default Amount may be declared due by each holder immediately. The
aggregate Mandatory Default Amount that may be due under the Convertible Notes was $27,996,132
on the date of the default, or $7,258,256 more than would otherwise have been due under the Convertible Notes on the date of the
default. Therefore, as a result of the event of the default, the Company accreted $7,258,256 to
the value of the Convertible Notes and included this amount in interest and accretion expenses on the condensed consolidated
statement of operations. The holders of the Convertible Notes have not exercised any remedies applicable to the Convertible
Notes as of the date of this report. In the event that the balance owed under the Convertible Notes, including the respective
Mandatory Default Amount, is not repaid upon demand, the holders of the Convertible Notes may seek to take possession of some or all
of the Company’s and its subsidiaries’ assets, force the Company and its subsidiaries into bankruptcy proceedings, or seek
other legal remedies against the Company and its subsidiaries. In such an event, the Company’s business, operating results and
financial condition may be materially adversely affected.
The
following table summarizes supplemental balance sheet information related to the convertible notes, net of debt discount outstanding,
as of June 30, 2023, and December 31, 2022:
SCHEDULE
OF CONVERTIBLE NOTES
| Convertible Notes,
Net of Debt Discount | |
| |
| Balance, December 31, 2021 | |
$ | 26,065 | |
| Principal of Convertible Notes issued - Original
securities | |
| 7,315,138 | |
| Issuance costs | |
| (535,717 | ) |
| Debt discount | |
| (6,479,421 | ) |
| Debt accretion | |
| 2,763,749 | |
| Debt extinguishment | |
| (338,181 | ) |
| Balance, December 31, 2022 | |
| 2,751,633 | |
| Debt accretion on Original securities | |
| 1,835,741 | |
| Debt extinguishment | |
| (4,587,374 | ) |
| Principal of Convertible Notes issued - New securities | |
| 19,058,426 | |
| Debt discount | |
| (19,058,426 | ) |
| Debt accretion on New Securities | |
| 5,451,150 | |
| Debt accretion associated with Mandatory Default Amount | |
| 7,258,256 | |
| Conversion of debt | |
| (85,486 | ) |
| Balance, June 30, 2023 | |
$ | 12,623,920 | |
SCHEDULE
OF CONVERTIBLE NOTES NET OF DEBT DISCOUNT
| | |
June
30, 2023 | | |
December
31, 2022 | |
| Convertible notes - total principal | |
$ | 18,824,730 | | |
$ | 14,432,996 | |
| Mandatory Default Amount | |
| 7,258,256 | | |
| - | |
| Unamortized issuance
costs and discount | |
| (13,459,066 | ) | |
| (11,681,363 | ) |
| Convertible Notes, Net
of Debt Discount | |
$ | 12,623,920 | | |
$ | 2,751,633 | |
| | |
June
30, 2023 | | |
December
31, 2022 | |
| Current portion | |
$ | 12,316,495 | | |
$ | - | |
| Non-current portion | |
| 307,425 | | |
| 2,751,633 | |
| Convertible Notes, Net
of Debt Discount | |
$ | 12,623,920 | | |
$ | 2,751,633 | |
2023
Convertible Notes and Warrants
On
May 12, 2023, the Company conducted the initial closing (the “OID Units Initial Closing”) of a private placement of up to
$10,000,000 for an aggregate of up to 100,000,000 units (the “OID Units”) under a Unit Purchase Agreement, dated as of the
same date, with accredited investors (the “OID Purchase Agreement”), each consisting of (i) a 15% original issue discount
unsecured subordinated convertible promissory note (each, a “OID Convertible Note” and collectively, the “OID Convertible
Notes”), convertible into shares of common stock plus additional shares based on accrued interest at $0.10 per share, subject to
adjustment, (ii) a warrant for the purchase of 125% of the shares of common stock into which the related OID Convertible Notes may be
converted at $0.10 per share, subject to adjustment (the “Class E Warrant”), and (iii) a warrant for the purchase of 125%
of the shares of common stock into which the related OID Convertible Note may be converted at $0.20 per share, subject to adjustment
(each, a “Class F Warrant,” and each Class F Warrant together with each Class E Warrant collectively, the “OID Warrants”).
As
part of the OID Units Initial Closing and on the same date, Walleye Opportunities Master Fund Ltd. (“Walleye”) paid a subscription
amount of $1,000,000 and the Company issued Walleye 11,764,710 OID Units consisting of (i) an OID Convertible Note in the principal amount
of $1,176,471, convertible into 11,764,710 shares of common stock plus additional shares based on accrued interest at $0.10 per share,
(ii) a Class E Warrant for the purchase of 14,705,880 shares of common stock, and (iii) a Class F Warrant for the purchase of 14,705,880
shares of common stock at $0.20 per share.
On
May 30, 2023, the Company conducted the second closing (the “OID Units Second Closing”) of the OID Units Private Placement.
In the OID Units Second Closing, Hexin Global Ltd. (“Hexin”) and Walleye agreed to the cancellation of the Hexin Promissory
Note and the Walleye Promissory Note (see Note 6), respectively, and Frank Maresca (“Maresca”), a consultant to the Company,
agreed to the cancellation of certain indebtedness, in exchange for OID Units and related agreements, as described below.
First,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Hexin (the “Hexin Cancellation Agreement”),
Hexin agreed to cancel the Hexin Promissory Note (see Note 6) in exchange for the issuance of 9,578,040 OID Units consisting of (a) an
OID Convertible Note in the principal amount of $957,804 for a subscription equal to $814,133, convertible into 9,578,040 shares of common
stock plus additional shares based on accrued interest at $0.10 per share, (b) a Class E Warrant for the purchase of 11,972,550 shares
of common stock at $0.10 per share, and (c) a Class F Warrant for the purchase of 11,972,550 shares of common stock at $0.20 per share.
Second,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Walleye (the “Walleye Cancellation
Agreement”), Walleye agreed to cancel the Walleye Promissory Note (see Note 6) in exchange for the issuance of 14,705,890 Units
consisting of (a) an OID Convertible Note in the principal amount of $1,470,589 for a subscription equal to $1,250,000, convertible into
14,705,890 shares of common stock plus additional shares based on accrued interest at $0.10 per share, (b) a Class E Warrant for the
purchase of 18,382,362 shares of common stock at $0.10 per share, and (c) a Class F Warrant for the purchase of 18,382,362 shares of
common stock at $0.20 per share.
Third,
under a Cancellation and Exchange Agreement, dated as of May 30, 2023, between the Company and Maresca (the “Maresca Cancellation
Agreement” and together with the Hexin Cancellation Agreement and the Walleye Cancellation Agreement, the “Cancellation and
Exchange Agreements”), Maresca agreed to cancel $150,000 of the aggregate short-term indebtedness to Maresca, which arose in the
ordinary course of business for certain consulting services provided by Maresca to the Company, in exchange for the issuance of 1,764,710
Units consisting of (a) an OID Convertible Note in the principal amount of $176,471 for a subscription equal to $150,000 (the amount
of the indebtedness being cancelled), convertible into 1,764,710 shares of common stock plus additional shares based on accrued interest
at $0.10 per share, (b) a Class E Warrant for the purchase of 2,205,887 shares of common stock at $0.10 per share, and (c) a Class F
Warrant for the purchase of 2,205,887 shares of common stock at $0.20 per share.
The
Company determined that the optional conversion feature attached to the OID Convertible Notes did not meet the definition of derivative
liability and that the detachable warrants issued met the definition of a liability and therefore was accounted for as a derivative liability
instrument. The warrants were fair valued at $5,461,702 and recorded as derivative liabilities on the condensed consolidated
balance sheets at June 30, 2023. As a result of the warrant liability
exceeding the value of the debt principal, the
Company recorded a $2,377,569 loss on issuance of debt that was recorded in condensed consolidated statements of operations for the six
months ended June 30, 2023 (June 30, 2022 - $Nil).
During
the three and six months ended June 30, 2023, the Company recognized interest and accretion expense of $150,086 and $150,086, respectively
(June 30, 2022 - $Nil and $Nil, respectively), in the condensed consolidated statements of operations.
The
following table summarizes supplemental balance sheet information related to the OID Convertible Notes, net of debt discount outstanding,
as of June 30, 2023, and December 31, 2022:
SCHEDULE
OF CONVERTIBLE NOTES
| OID Convertible Notes,
Net of Debt Discount | |
| |
| Balance, December 31, 2022 | |
$ | - | |
| Issuance of convertible notes | |
| 3,781,335 | |
| Issuance cost | |
| (697,202 | ) |
| Debt discounts | |
| (3,084,133 | ) |
| Debt accretion | |
| 150,086 | |
| Balance, June 30, 2023 | |
$ | 150,086 | |
SCHEDULE
OF CONVERTIBLE NOTES NET OF DEBT DISCOUNT
| | |
June
30, 2023 | | |
December
31, 2022 | |
| OID Convertible Notes - total principal | |
$ | 3,781,335 | | |
$ | - | |
| Unamortized issuance
costs and discount | |
| (3,631,249 | ) | |
| - | |
| OID Convertible Notes,
Net of Debt Discount | |
$ | 150,086 | | |
$ | - | |
| | |
June
30, 2023 | | |
December
31, 2022 | |
| Current portion | |
$ | 150,086 | | |
$ | - | |
| Non-current portion | |
| - | | |
| - | |
| OID Convertible Notes,
Net of Debt Discount | |
$ | 150,086 | | |
$ | - | |
2023
Convertible Notes Terms
The
OID Convertible Notes will mature in nine months from the date of the OID Units Initial Closing and accrue 10% of interest per annum
on the outstanding principal amount. The OID Convertible Notes will be unsecured and subordinated to any senior indebtedness of the Company.
The OID Convertible Notes’ principal and accrued interest may generally be converted at any time at a conversion price of $0.10
per share, subject to adjustment, at the option of the holder, into shares of common stock, subject to certain limitations: (i) conversion
would not cause the holder to beneficially own more than 4.99% of the Company’s common stock, or more than 9.99% if the holder
beneficially owns more than 4.99% of common stock based on ownership of equity securities of the Company other than the OID Convertible
Notes or the respective warrants; and (ii) the Company’s articles of incorporation have been amended to increase the number of
authorized shares of common stock to a sufficient amount to permit the full conversion of the OID Convertible Notes (the “Capital
Event Amendment”).
2023
Warrants Terms
The
Class E Warrants and Class F Warrants are generally exercisable for a period from the date of the Capital Event Amendment until five
years from the date of issue. The exercise right is subject to a similar beneficial ownership limitation that applies to conversion of
the OID Convertible Notes above, i.e., exercise is permitted only if it would not cause the holder to beneficially own more than 4.99%
of the Company’s common stock, or more than 9.99% if the holder beneficially owns more than 4.99% of common stock based on ownership
of equity securities of the Company other than the OID Convertible Notes or the Class E Warrants and Class F Warrants.
NOTE
8 – STOCKHOLDERS’ EQUITY
The
Company is authorized to issue a total number of 25,000,000 shares of “blank check” preferred stock with a par value of $0.001.
As of June 30, 2023, and December 31, 2022, there were no shares of preferred stock issued or outstanding.
The
Company is authorized to issue a total number of 300,000,000 shares of common stock with a par value of $0.001.
As
of June 30, 2023, there were 45,366,760 (December 31, 2022 - 40,528,191) shares of common stock issued and outstanding.
During
the six months ended June 30, 2023, the Company completed the following issuances:
| ● | The
Company issued 240,000 shares of common stock to Nicholas DeVito as part of the Confidential
Settlement Agreement, dated November 18, 2022 (see Note 10). |
| ● | The
Company issued 2,652,159 shares of common stock on exercise of warrants granted as part of
the Somahlution acquisition, completed on July 30, 2020. |
| ● | The
Company issued 600,000 shares to Mr. Frank Maresca in order to reimburse Mr. Maresca for
the shares of Common Stock that were previously cancelled. |
| ● | The
Company issued 1,346,410 shares on conversion of the debt (see Note 7). |
On
May 18, 2021, the Company’s Board of Directors approved the Marizyme, Inc. Amended and Restated 2021 Stock Incentive Plan (“SIP”).
The SIP incorporates stock options issued prior to May 18, 2021. The SIP authorized 5,300,000 options for issuance. On December 27, 2022,
the Board of Directors requested that stockholders ratify an amendment to the SIP to increase the maximum number of shares of common
stock available for issuance pursuant to awards granted under the SIP by 1,900,000 to 7,200,000, which was approved by the stockholders.
As of June 30, 2023, there remains 2,924,057 options available for issuance (December 31, 2022 – 2,924,057).
During
the three and six months ended June 30, 2023, the Company granted Nil (December 31, 2022 – 400,000) share purchase options to directors
of the Company.
The
summary of option activity for the six months ended June 30, 2023, is as follows:
SCHEDULE
OF STOCK OPTION ACTIVITY
| | |
Number
of Options | | |
Weighted
Average
Exercise Price | | |
Weighted
Average
Contractual
Life | | |
Total
Intrinsic Value | |
| Outstanding at December 31, 2021 | |
| 3,650,943 | | |
$ | 1.24 | | |
| 8.34 | | |
| | |
| Granted | |
| 400,000 | | |
| 2.20 | | |
| | | |
| | |
| Expired | |
| (62,502 | ) | |
| 1.25 | | |
| | | |
| | |
| Forfeited | |
| (62,498 | ) | |
| 1.25 | | |
| | | |
| | |
| Outstanding at December 31, 2022 | |
| 3,925,943 | | |
$ | 1.33 | | |
| 6.06 | | |
$ | - | |
| Granted/forfeited | |
| - | | |
| - | | |
| - | | |
| - | |
| Outstanding at June 30, 2023 | |
| 3,925,943 | | |
| 1.33 | | |
| 5.56 | | |
| - | |
| Exercisable at June 30, 2023 | |
| 3,479,831 | | |
$ | 1.26 | | |
| 5.17 | | |
$ | - | |
As
of June 30, 2023, the Company had the following options outstanding:
SCHEDULE OF OPTIONS OUTSTANDING AND EXERCISABLE
| Exercise
Price | | |
Number
of Options Outstanding | | |
Number
of Options Exercisable | | |
Weighted
Average Remaining Contractual Years | | |
Intrinsic
Value | |
| $ | 1.01 | | |
| 1,985,943 | | |
| 1,985,943 | | |
| 3.00 | | |
$ | - | |
| | 1.25 | | |
| 540,000 | | |
| 533,888 | | |
| 7.66 | | |
| - | |
| | 1.37 | | |
| 200,000 | | |
| 200,000 | | |
| 7.14 | | |
| - | |
| | 1.75 | | |
| 800,000 | | |
| 520,000 | | |
| 8.41 | | |
| - | |
| | 2.20 | | |
| 400,000 | | |
| 240,000 | | |
| 8.95 | | |
| - | |
| $ | 1.33 | | |
| 3,925,943 | | |
| 3,479,831 | | |
| 5.56 | | |
| - | |
| d) |
Restricted
Share Units |
During
the year ended December 31, 2021, the Company granted restricted share awards for an aggregate of 350,000 shares of common stock to directors,
senior officers and consultants of the Company, with underlying performance conditions. As of June 30, 2023, only two out of four performance
conditions have been achieved. Compensation cost of $Nil for the restricted share awards was recognized in stock-based compensation for
the three and six months ended June 30, 2023 (June 30, 2022 - $Nil and $295,750, respectively).
As
of June 30, 2023 and December 31, 2022, there were 478,757,133 and 20,048,487 warrants outstanding, respectively.
SCHEDULE OF WARRANTS OUTSTANDING
| | |
Number | | |
Weighted
Average Price | |
| Balance, December 31, 2021 | |
| 12,144,834 | | |
$ | 2.90 | |
| Issued pursuant to Unit Purchase Agreement | |
| 8,360,147 | | |
| 2.25 | |
| Issued | |
| 878,398 | | |
| 1.16 | |
| Exercised | |
| (300,000 | ) | |
| 0.01 | |
| Expired | |
| (113,637 | ) | |
| 3.00 | |
| Cancelled pursuant to FINRA | |
| (578,398 | ) | |
| 1.75 | |
| Cancelled as part of
debt extinguishment | |
| (342,857 | ) | |
| 2.25 | |
| Balance, December 31, 2022 | |
| 20,048,487 | | |
$ | 2.64 | |
| Warrants modified pursuant to debt extinguishment
(Note 7) | |
| 355,577,447 | | |
| 0.10 | |
| Issued pursuant to debt agreements (Note 7) | |
| 94,533,358 | | |
| 0.15 | |
| Issued pursuant to Hexin Promissory Note (Note 6) | |
| 11,250,000 | | |
| 0.13 | |
| Exercised | |
| (2,652,159 | ) | |
| 0.10 | |
| Balance, June 30, 2023 | |
| 478,757,133 | | |
$ | 0.12 | |
On
April 13, 2023, the Company delivered offer letter agreements (the “Somahlution Warrant Offer Letter Agreements”) to the
Former Somahlution Owners, which offered to allow the Former Somahlution Owners to exercise the Somahlution Warrants to purchase the
number of restricted shares of common stock issuable under the Somahlution Warrants at an exercise price reduced by the Company from
$5.00 per share to $0.10 per share, on or prior to April 21, 2023, for maximum total cash proceeds of $299,996. As of the conclusion
of this offer period, four of the Former Somahlution Owners had entered into Somahlution Warrant Offer Letter Agreements and had exercised
their Somahlution Warrants to purchase a total of 2,652,159 shares of common stock for gross proceeds of approximately $265,216 (the
“Somahlution Warrants Exercise”).
As
a result of this warrant exercise, pursuant to the terms applicable to the Convertible Notes and the Class C Warrants (see Note 7), the
conversion price of the Convertible Notes adjusted from $1.75 per share to $0.10 per share, the exercise price of the Class C Warrants
adjusted from $2.25 per share to $0.10 per share, and the number of shares that the Class C Warrants may be exercised to purchase was
increased proportionately. In connection with these developments and the lack of sufficient authorized shares of common stock under the
Company’s articles of incorporation to meet its obligations under outstanding convertible or exercisable securities, the Company
also obtained additional conversion and exercise rights waivers from Convertible Note and Class C Warrant holders. As a result of these
adjustment provisions and the conversion and exercise terms applicable to the Class C Warrants, including the effect of the applicable
waivers, the number of shares that the Class C Warrants may be exercised to purchase adjusted from 5,109,904 shares of common stock to
114,973,110 shares.
In
May 2023, the Company issued OID Convertible Notes for aggregate principal of $3,781,335 and convertible into up to 37,813,350 shares
of common stock not including shares convertible from interest under the Convertible Notes, Class E Warrants exercisable for the purchase
of 47,266,679 shares of common stock at $0.10 per share, and Class F Warrants for the purchase of 47,266,679 shares of common stock at
$0.20 per share. The OID Convertible Notes, Class E Warrants and Class F Warrants will not be convertible or exercisable until the Capital
Event Amendment becomes effective. As described in Note 7, Class E and Class F warrants were accounted for as a liability, were fair
valued at an aggregate amount of $5,461,702 and were recorded as a short-term derivative liability on the condensed consolidated balance
sheets at June 30, 2023.
Pursuant
to the Hexin Promissory Note (see Note 6), on May 22, 2023, the Company issued Hexin a Class E Warrant that may be exercised to purchase
7,500,000 shares of common stock for $0.10 per share, and a Class F Warrant that may be exercised to purchase 3,750,000 shares of common
stock for $0.20 per share (collectively, the “Hexin Warrants”). The Hexin Warrants will be exercisable in accordance with
the exercise terms and conditions of the other Class E and Class F Warrants described above (see Note 7). The warrants issued had an
average term of 5 years, vested immediately, and were fair valued at $1,333,127 and were recorded in other general and administrative
expenses in the condensed consolidated statements of operations for the three and six months ended June 30, 2023.
| f) |
Stock-based
compensation |
During
the three and six months ended June 30, 2023, the Company recorded $160,762 and $371,728 in non-cash share-based compensation, respectively
(June 30, 2022 - $676,242 and $1,392,674, respectively).
NOTE
9 – RELATED PARTY TRANSACTIONS
As
at June 30, 2023, the Company owed an aggregate of $143,351 (December 31, 2022 - $Nil) to related parties of the Company.
For
the three and six months ended June 30, 2023, the Company incurred and settled $72,000 and $144,000, respectively (June 30, 2022 -$24,400
and $86,400, respectively), in professional service rendered by related parties of the Company and incurred and settled $12,621 of various
expenses incurred by these parties in relation to their services rendered to the Company. The services were provided by entities which
are controlled by management and are pursuant to various consulting agreements.
During
the three and six months ended June 30, 2023, the Company also incurred $332,750 and $665,500 in compensation to Directors and Executive
Officers for their services rendered, respectively (June 30, 2022 – $43,200 and $86,400, respectively), and settled $387,750 and
$631,500 in compensation, respectively.
Additionally,
as part of the Somahlution acquisition in 2020, the Company recorded a prepaid royalty to the shareholders of Somahlution. The former
primary beneficial owner is Dr. Vithal Dhaduk, currently a director, and significant shareholder of the Company. During the three and
six months ended June 30, 2023, the Company accrued $11,065 and $47,248 in royalties payable incurred on sales of the DuraGraft product
outside of the U.S., respectively. This amount was offset against the prepaid royalty receivable.
During
the six months ended June 30, 2023, the Company and shareholders of Somahlution agreed to reduce the prepaid royalty balance by 50% or
by $151,000. As at June 30, 2023, the Company had $140,843 in prepaid royalties (December 31, 2022 - $339,091) which had been classified
as non-current in the condensed consolidated balance sheets.
NOTE
10 – COMMITMENTS AND CONTINGENCIES
Legal
Matters
Under
a Confidential Settlement Agreement, dated November 18, 2022, the Company and Nicholas DeVito agreed that Mr. DeVito would dismiss a
Complaint that Mr. DeVito filed on June 7, 2022 in the Circuit Court of the Fifteenth Judicial Circuit in and for Palm Beach County,
Florida, Case No. 50-2022-CA-005437. The parties also agreed that following an anticipated reverse split, the Company was required to
issue Mr. DeVito 16,000 “post-split” shares to be delivered in paper certificate form within three (3) business days of the
reverse split. The settlement agreement further provided that the delivered shares would be subject to normal and customary restrictions
pursuant to Rule 144 of the SEC. In the event no split occurred by December 12, 2022, the Company was required to issue Mr. DeVito 60,000
“pre-split” shares. In addition, the parties agreed that no further continuous service is required pursuant to Section 2
of the Mutual Release of Claims Agreement between Mr. DeVito and the Company dated as of August 27, 2020. Pursuant to the agreement,
on January 5, 2023, the Company issued 240,000 shares of common stock to Mr. DeVito (see Note 8b).
Contingencies
| |
a. |
On
July 13, 2019, the Company signed a consulting agreement, whereby the individual will receive: |
| |
● |
$30,000
per month through July 13, 2022, |
| |
● |
Option
to purchase 250,000 shares of common stock at a strike price of $1.50, which vest monthly through July 13, 2021. The vesting of these
options was accelerated by the Board on September 2, 2020. |
| |
● |
Royalties
based on sales of Krillase assets, equal to 10% of net sales of the product. During the six months ended June 30, 2023, no revenues
were derived from sales of Krillase product. |
| |
b. |
As
part of the DuraGraft Acquisition, completed on July 31, 2020, the Company entered into the Agreement with Somahlution stockholders,
whereby Marizyme is legally obligated to pay royalties on all net sales for Somahlution, Inc. The royalties associated with the Agreement
are calculated as follows: |
Royalties
on U.S. sales equal to:
| |
● |
5%
on the first $50,000,000 of net sales, |
| |
● |
4%
on net sales of $50,000,001 up to $200,000,000, and |
| |
● |
2%
on net sales over $200,000,000. |
Royalties
on sales outside of the U.S.:
| |
● |
6%
on the first $50,000,000 of net sales, |
| |
● |
4%
on net sales of $50,000,001 up to $200,000,000, and |
| |
● |
2%
on net sales over $200,000,000. |
The
royalties are in perpetuity. During the three and six months ended June 30, 2023, the Company had not earned any revenues from Krillase,
however the Company did incur sales of the DuraGraft products outside of the U.S., on which $11,065 and $47,248 in royalties have been
accrued and offset against the prepaid royalties receivable, respectively (see Note 9).
Upon
receiving FDA clearance for the DuraGraft product, the Company will:
| |
● |
Issue
performance warrants with a strike price determined based on the average of the closing prices of the Company’s common stock
for the 30 calendar days following the date of the public announcement of the FDA approval; and |
| |
● |
Upon
liquidation of all or substantially all of the assets relating to DuraGraft, the Company will pay 15% of the net sale proceeds up
to $20 million. |
| |
c. |
The
Company has entered into arrangements for office and laboratories spaces. As of June 30, 2023, minimum lease payments in relation
to lease commitments are payable as described in Note 4. |
NOTE
11 - SUBSEQUENT EVENTS
Third
Closing of OID Private Placement
On
July 10, 2023, the Company conducted the third closing (“OID Units Third Closing”) of the OID Unit Private Placement of up
to $10,000,000 for an aggregate of up to 100,000,000 OID Units under a Unit Purchase Agreement between Hexin and the Company (see Note
7).
In
connection with the OID Units Third Closing, on July 10, 2023, Hexin paid a subscription amount of $1,000,000, and the Company issued
11,764,710 OID Units consisting of (i) an OID Convertible Note in the principal amount of $1,176,471, convertible into 11,764,710 shares
of common stock plus additional shares based on accrued interest at $0.10 per share, subject to adjustment, (ii) a Class E Warrant for
the purchase of 14,705,880 shares of common stock at $0.10 per share, subject to adjustment, and (iii) a Class F Warrant for the purchase
of 14,705,880 shares of common stock at $0.20 per share, subject to adjustment. In connection with the OID Units Third Closing, the Company
received net proceeds of $730,000.
The
OID Convertible Note issued in the OID Units Third Closing will mature nine months after issuance and accrue 10% of interest per annum
on the outstanding principal amount (see Note 7).
Special
Stockholder Meeting, Change in Capitalization
On
August 9, 2023 the Company held a special meeting of stockholders where an amendment to the Company’s articles of incorporation
to increase the total number of shares of authorized common stock from 300,000,000 to 2,000,000,000 (the “Capital Event Amendment”)
was proposed, voted on and approved.
Reinstatement
of Compensation to Univest Securities, LLC and Mr. Richmond
On
February 14, 2022, the Company filed a registration statement on Form S-1, which was subsequently amended on certain dates (the “Withdrawn
Registration Statement”), relating to the Company’s proposed underwritten public offering (the “Offering”). Univest
was named as the representative of the underwriters of the offering. The staff (“FINRA Staff”) of the Corporate Financing
Department of the Financial Industry Regulatory Authority, Inc. (“FINRA”) determined that the sales of Units, securities,
as well as a grant of a stock option to Mr. Richmond constituted underwriting compensation in connection with the Company’s proposed
public offering pursuant to FINRA Rule 5110, based on the FINRA Staff’s interpretation of such rule. Consequently, each of Univest
and Mr. Richmond, pursuant to a letter agreement entered into with the Company, dated October 28, 2022, addressed and submitted to the
FINRA Staff (the “October 2022 Letter Agreement”), agreed to forego their applicable rights to these securities. Pursuant
to the October 2022 Letter Agreement, the parties thereto agreed that the cancellation or disposal of the aforementioned securities shall
be without recourse by either Univest or Mr. Richmond. Accordingly, all issued Units and shares were subsequently cancelled, and the
Company and Mr. Richmond agreed to the transfer of his stock option to a Company designee who is unaffiliated with Mr. Richmond.
On
June 22, 2023, Univest and Mr. Richmond requested that the Company either reissue the cancelled securities or issue them new securities
with equivalent terms and conditions, in light of the fact that the Company was not proceeding with the offering.
Pursuant
to these requests, on July 25, 2023, the Company approved the grant of replacement securities to Univest and Mr. Richmond: (i) Convertible
Notes, convertible into shares of common stock at $0.10 per share, such that the adjusted principal is $137,984 and $206,975, respectively,
maturing on July 25, 2025, such that up to 1,683,131 and 2,524,697 shares of common stock are issuable upon conversion thereof if held
to maturity; (ii) Class C Warrants with the exercise price of $0.10 per share, exercisable into up to 3,548,148 and 5,322,223 shares
of common stock, respectively; (iii) placement agent warrants with the exercise price per share of $0.10, exercisable into up to 4,048,782
and 6,073,182 shares of common stock, respectively; and (iv) 300,000 shares of common stock to Mr. Richmond.
Report
of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders,
Marizyme, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance
sheets of Marizyme, Inc. (the “Company”) as of December 31, 2022 and 2021, and the related consolidated statements of operations,
changes in stockholders’ equity, and cash flows, for the years then ended, and the related notes (collectively referred to as the “consolidated
financial statements”). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects,
the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for the years
then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying consolidated financial statements
have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements,
the Company has suffered recurring losses from operations, has experienced cash used from operations in excess of its current cash position,
and has an accumulated deficit, that raise substantial doubt about its ability to continue as a going concern. Management’s plans
in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might
result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These consolidated financial statements are the responsibility
of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements
based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards
of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated
financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we
engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding
of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s
internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess
the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures
that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the
consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by
management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide
a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are
matters arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated
to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and
(2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter
in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit
matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Impairment Evaluation of Intangible Assets and
Goodwill
Critical Audit Matter Description
As described in Note 1 to the consolidated financial
statements, the Company reviews long-lived intangible assets and goodwill, for impairment whenever events or changes in business circumstances
indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss would be recognized when estimated undiscounted
future cash flows expected to result from the use of the asset and its eventual disposition are less than the carrying amount. In order
to estimate the fair value of the assets, management is required to make significant estimates and assumptions related to the potential
future benefits of these intangible assets. Changes in these assumptions could have a significant impact on the fair value of the reporting
unit, the amount of any intangible asset impairment charge, or both. As a result of management’s assessments, the Company recognized
impairment charges of $24,350,000 during the year ended December 31, 2022, all of which was related to the Krillase technology originally
acquired on September 12, 2018.
We identified the impairment evaluation of intangible
assets and goodwill as a critical audit matter because of the inherent subjectivity involved in management’s estimates and assumptions
related to the future potential benefits attributable to intangible assets. The audit procedures to evaluate the reasonableness of management’s
estimates and assumptions related to the potential future benefits of these intangible assets required a high degree of auditor judgment
and an increased extent of effort, including the need to involve our fair value specialists.
How the Critical Matter was Addressed in the Audit
We evaluated the reasonableness of management’s
forecasts by comparing the forecasts to historical results, internal communications to management and those charged with governance of
Marizyme, and forecasted information included in analyst and industry reports for the Company and its peer companies. With the assistance
of our valuation specialists, we evaluated the reasonableness of the impairment by evaluating methodologies used by management, determining
the completeness and accuracy of information, sensitivity analyses, testing the source information underlying the determination of the
impairment, testing the mathematical accuracy of the calculations, and developing a range of independent estimates and comparing those
to the impairment amount enacted by management.
Fair Value of the Contingent Liabilities for Duragraft
Acquisition
Critical Audit Matter Description
As described in Note 1 to the consolidated financial
statements, the Company had $9,707,000 of contingent liabilities as of December 31, 2022 related to the 2019 acquisition of Duragraft.
The contingent consideration payments are subject to the Company’s achievement of certain revenue targets and achievement of certain
FDA milestones. Management determines the estimated fair value of the contingent consideration liability using a combination of monte
carlo and scenario-based valuation methods. The unobservable inputs used by management to determine the fair value of the contingent consideration
liability include projected revenues, volatility, cost of debt, discount rates and various holding periods and exit terms.
The principal considerations for our determination
that performing procedures relating to the fair value of the contingent liabilities as a critical audit matter are (i) the significant
judgment by management when determining the fair value, (ii) a high degree of auditor judgment, subjectivity and effort in performing
procedures and evaluating audit evidence relating to the monte carlo and scenario-based valuation methods and evaluating management’s
significant assumptions related to the projected revenues, volatility, and discount rates, and (iii) the audit effort involved the use
of professionals with specialized skill and knowledge.
How the Critical Matter was Addressed in the Audit
To test the estimated fair value of contingent consideration
liabilities at year end, our audit procedures included evaluating methodologies used by management, determining the completeness and accuracy
of information, sensitivity analyses, inspecting the terms of the executed agreement, assessing the Monte Carlo and Scenario-Based simulation
models used and testing the key contractual inputs and significant assumptions discussed above. We evaluated the assumptions and judgments
considering observable industry and economic trends and standards, external data sources and regulatory factors. Estimated amounts of
future revenues were evaluated for reasonableness in relation to internal and external analyses, clinical development progress and timelines,
probability of success benchmarks, and regulatory notices. Our procedures included evaluating the data sources used by management in determining
its assumptions and, where necessary, included an evaluation of available information that either corroborated or contradicted management’s
conclusions. We also assessed the professional competence, experience, and objectivity of the Company’s external valuation specialist.
We involved a valuation specialist to assess the Company’s Monte Carlo and Scenario Based simulation models and to perform corroborative
fair value calculations.
/s/ WithumSmith+Brown, PC
We have served as the Company’s auditor since 2020.
Whippany, New Jersey
March 24, 2023
PCAOB ID Number 100
MARIZYME,
INC.
Consolidated
Balance Sheets
| | |
December 31, 2022 | | |
December 31, 2021 | |
| ASSETS: | |
| | | |
| | |
| Current | |
| | | |
| | |
| Cash | |
$ | 510,865 | | |
$ | 4,072,339 | |
| Accounts receivable | |
| 87,801 | | |
| 8,650 | |
| Other receivables | |
| 15,310 | | |
| 41,307 | |
| Prepaid expenses | |
| 1,125,761 | | |
| 257,169 | |
| Inventory | |
| 215,566 | | |
| 22,353 | |
| Total current assets | |
| 1,955,303 | | |
| 4,401,818 | |
| Non-current | |
| | | |
| | |
| Property, plant and equipment, net | |
| 12,545 | | |
| 12,817 | |
| Operating lease right-of-use assets, net | |
| 1,485,023 | | |
| 1,158,776 | |
| Intangible assets, net | |
| 27,675,020 | | |
| 52,866,192 | |
| Prepaid royalties, non-current | |
| 339,091 | | |
| 339,091 | |
| Deposits | |
| 30,000 | | |
| 30,000 | |
| Goodwill | |
| 7,190,656 | | |
| 7,190,656 | |
| Total non-current assets | |
| 36,732,335 | | |
| 61,597,532 | |
| Total assets | |
$ | 38,687,638 | | |
$ | 65,999,350 | |
| | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY: | |
| | | |
| | |
| Current | |
| | | |
| | |
| Accounts payable and accrued expenses | |
$ | 1,365,443 | | |
$ | 1,596,147 | |
| Note payable | |
| 1,132,829 | | |
| 127,798 | |
| Due to related parties | |
| - | | |
| 1,132,634 | |
| Operating lease obligations | |
| 423,495 | | |
| 277,142 | |
| Total current liabilities | |
| 2,921,767 | | |
| 3,133,721 | |
| Non-current | |
| | | |
| | |
| Operating lease obligations, net of current portion | |
| 1,061,528 | | |
| 881,634 | |
| Note payable, net of current portion | |
| - | | |
| 469,252 | |
| Convertible notes | |
| 2,751,633 | | |
| 26,065 | |
| Derivative liabilities | |
| 4,823,725 | | |
| 2,485,346 | |
| Contingent liabilities | |
| 9,707,000 | | |
| 11,313,000 | |
| Total non-current liabilities | |
| 18,343,886 | | |
| 15,175,297 | |
| Total liabilities | |
| 21,265,653 | | |
| 18,309,018 | |
| | |
| | | |
| | |
| Commitments and contingencies (Note 10) | |
| - | | |
| - | |
| | |
| | | |
| | |
| Stockholders’ equity: | |
| | | |
| | |
| Preferred stock, $0.001 par value, 25,000,000 shares authorized, no shares issued and outstanding as of December 31, 2022 and 2021 | |
| - | | |
| - | |
| Preferred stock, value | |
| - | | |
| - | |
| Common stock, par value $0.001, 75,000,000 shares authorized, issued and outstanding shares - 40,528,191 at December 31, 2022 and 2021 | |
| 40,528 | | |
| 40,528 | |
| Common stock, value | |
| 40,528 | | |
| 40,528 | |
| Additional paid-in capital | |
| 103,370,890 | | |
| 95,473,367 | |
| Accumulated deficit | |
| (85,989,433 | ) | |
| (47,823,563 | ) |
| Total stockholders’ equity | |
| 17,421,985 | | |
| 47,690,332 | |
| Total liabilities and stockholders’ equity | |
$ | 38,687,638 | | |
$ | 65,999,350 | |
The
accompanying notes are an integral part of these consolidated financial statements.
MARIZYME,
INC.
Consolidated
Statements of Operations
| | |
| | |
| |
| | |
Years ended December 31, | |
| | |
2022 | | |
2021 | |
| | |
| | |
| |
| Revenue | |
$ | 233,485 | | |
$ | 210,279 | |
| Direct cost of revenue | |
| 54,319 | | |
| 80,354 | |
| Gross profit | |
| 179,166 | | |
| 129,925 | |
| Operating expenses: | |
| | | |
| | |
| Professional fees (includes related party amounts of $172,800 and $410,400, respectively) | |
| 2,082,079 | | |
| 2,269,756 | |
| Salary expenses | |
| 2,421,969 | | |
| 2,887,309 | |
| Research and development | |
| 3,978,826 | | |
| 1,681,899 | |
| Stock-based compensation | |
| 1,905,948 | | |
| 898,444 | |
| Depreciation and amortization | |
| 841,444 | | |
| 43,871 | |
| Impairment of intangible assets | |
| 24,350,000 | | |
| - | |
| Other general and administrative expenses | |
| 1,922,696 | | |
| 1,170,029 | |
| Total operating expenses | |
| 37,502,962 | | |
| 8,951,308 | |
| Total operating loss | |
| (37,323,796 | ) | |
| (8,821,383 | ) |
| | |
| | | |
| | |
| Other income (expense) | |
| | | |
| | |
| Interest and accretion expenses | |
| (2,789,255 | ) | |
| (126,024 | ) |
| Change in fair value of contingent liabilities | |
| 1,606,000 | | |
| (1,387,000 | ) |
| Gain/(loss) on debt extinguishment | |
| 338,181 | | |
| (663,522 | ) |
| Other income | |
| 3,000 | | |
| - | |
| Total other income (expense) | |
| (842,074 | ) | |
| (2,176,546 | ) |
| | |
| | | |
| | |
| Net loss | |
$ | (38,165,870 | ) | |
$ | (10,997,929 | ) |
| | |
| | | |
| | |
| Loss per share – basic and diluted | |
$ | (0.94 | ) | |
$ | (0.31 | ) |
| Loss per share – basic | |
$ | (0.94 | ) | |
$ | (0.31 | ) |
| | |
| | | |
| | |
| Weighted average number of shares of common stock outstanding – basic and diluted | |
| 40,727,092 | | |
| 36,041,613 | |
| Weighted average number of shares of common stock outstanding – basic | |
| 40,727,092 | | |
| 36,041,613 | |
The
accompanying notes are an integral part of these consolidated financial statements.
MARIZYME,
INC.
Consolidated
Statements of Stockholders’ Equity
| | |
| | |
| | |
| | |
| | |
| |
| | |
Common Stock | | |
Additional Paid-in | | |
Accumulated | | |
Total Stockholders’ | |
| | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Equity | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance, December 31, 2020 | |
| 35,928,191 | | |
$ | 35,928 | | |
$ | 82,077,334 | | |
$ | (36,825,634 | ) | |
$ | 45,287,628 | |
| Warrants issued for acquisition | |
| - | | |
| - | | |
| (732,300 | ) | |
| - | | |
| (732,300 | ) |
| Issuance of common stock for acquisition | |
| 4,600,000 | | |
| 4,600 | | |
| 7,769,400 | | |
| - | | |
| 7,774,000 | |
| Warrants issued | |
| - | | |
| - | | |
| 5,493,821 | | |
| - | | |
| 5,493,821 | |
| Stock-based compensation expense | |
| - | | |
| - | | |
| 865,112 | | |
| - | | |
| 865,112 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (10,997,929 | ) | |
| (10,997,929 | ) |
| Balance, December 31, 2021 | |
| 40,528,191 | | |
| 40,528 | | |
| 95,473,367 | | |
| (47,823,563 | ) | |
| 47,690,332 | |
| Balance | |
| 40,528,191 | | |
| 40,528 | | |
| 95,473,367 | | |
| (47,823,563 | ) | |
| 47,690,332 | |
| Stock-based compensation expense | |
| - | | |
| - | | |
| 1,905,948 | | |
| - | | |
| 1,905,948 | |
| Issuance of warrants | |
| - | | |
| - | | |
| 6,191,575 | | |
| - | | |
| 6,191,575 | |
| Exercise of warrants | |
| 300,000 | | |
| 300 | | |
| 2,700 | | |
| - | | |
| 3,000 | |
| Common stock repurchased and cancelled | |
| (300,000 | ) | |
| (300 | ) | |
| (2,700 | ) | |
| - | | |
| (3,000 | ) |
| Warrants repurchased and retired | |
| - | | |
| - | | |
| (200,000 | ) | |
| - | | |
| (200,000 | ) |
| Net loss | |
| - | | |
| - | | |
| - | | |
| (38,165,870 | ) | |
| (38,165,870 | ) |
| Balance, December 31, 2022 | |
| 40,528,191 | | |
$ | 40,528 | | |
$ | 103,370,890 | | |
$ | (85,989,433 | ) | |
$ | 17,421,985 | |
| Balance | |
| 40,528,191 | | |
$ | 40,528 | | |
$ | 103,370,890 | | |
$ | (85,989,433 | ) | |
$ | 17,421,985 | |
The
accompanying notes are an integral part of these consolidated financial statements.
MARIZYME,
INC.
Consolidated
Statements of Cash Flows
| | |
| | |
| |
| | |
Years Ended December 31, | |
| | |
2022 | | |
2021 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (38,165,870 | ) | |
$ | (10,997,929 | ) |
| Adjustments to reconcile net loss to net cash used in operations: | |
| | | |
| | |
| Depreciation and amortization | |
| 841,444 | | |
| 43,871 | |
| Stock-based compensation | |
| 1,905,948 | | |
| 865,112 | |
| Stock-based compensation - restricted common stock | |
| - | | |
| 33,332 | |
| Interest and accretion on convertible notes and notes payable | |
| 2,789,255 | | |
| 126,024 | |
| Issuance of warrants for services | |
| 1,850,533 | | |
| 368,287 | |
| Impairment of intangible assets | |
| 24,350,000 | | |
| - | |
| Change in fair value of contingent liabilities | |
| (1,606,000 | ) | |
| 1,387,000 | |
| (Gain) loss on debt extinguishment | |
| (338,181 | ) | |
| 663,522 | |
| Other income | |
| (3,000 | ) | |
| - | |
| Change in operating assets and liabilities: | |
| | | |
| | |
| Accounts and other receivables | |
| (53,154 | ) | |
| 36,298 | |
| Prepaid expenses | |
| (868,592 | ) | |
| (184,112 | ) |
| Inventory | |
| (193,213 | ) | |
| 33,987 | |
| Accounts payable and accrued expenses | |
| (228,684 | ) | |
| 968,788 | |
| Due to related parties | |
| (1,132,634 | ) | |
| 868,725 | |
| Net cash used in operating activities | |
| (10,852,148 | ) | |
| (5,787,095 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from financing, net of issuance cost | |
| 6,500,743 | | |
| 6,692,765 | |
| Proceeds from promissory notes, net of repayments | |
| 786,931 | | |
| 263,907 | |
| Proceeds from exercise of warrants | |
| 3,000 | | |
| - | |
| Net cash provided by financing activities | |
| 7,290,674 | | |
| 6,956,672 | |
| | |
| | | |
| | |
| Net change in cash | |
| (3,561,474 | ) | |
| 1,169,577 | |
| | |
| | | |
| | |
| Cash at beginning of year | |
| 4,072,339 | | |
| 2,902,762 | |
| | |
| | | |
| | |
| Cash at end of year | |
$ | 510,865 | | |
$ | 4,072,339 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Cash paid for taxes | |
$ | - | | |
$ | - | |
| | |
| | | |
| | |
| Non-cash investing and financing activities: | |
| | | |
| | |
| Derivative liabilities and debt discount issued in connection with convertible notes | |
$ | 2,338,379 | | |
$ | 2,485,346 | |
| Warrants and debt discount issued in connection with convertible notes | |
$ | 4,341,042 | | |
$ | 5,125,534 | |
| Settlement of notes payable with convertible notes | |
$ | 278,678 | | |
$ | - | |
| Warrants repurchased and retired | |
$ | 200,000 | | |
$ | - | |
| Contingent liabilities | |
$ | - | | |
$ | 9,926,000 | |
| Issuance of common stock in connection with business combination | |
$ | - | | |
$ | 7,774,000 | |
| Notes payable assumed in connection with business combination | |
$ | - | | |
$ | 468,137 | |
The
accompanying notes are an integral part of these consolidated financial statements.
MARIZYME,
INC.
Notes
to the Consolidated Financial Statements
December
31, 2022
1.
Organization, Basis of Presentation and Summary of Significant Accounting Policies
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization
Marizyme,
Inc. (the “Company” or “Marizyme”) is a Nevada corporation originally incorporated on March 20, 2007, under the
name SWAV Enterprises, Ltd. On September 6, 2010, the Company name was changed to GBS Enterprises Inc. and from 2010 to September 2018
the Company was in the software products and advisory services business for email and instant messaging applications. The Company divested
that business between December 2016 and September 2018 and focused on the acquisition of life science technologies.
On
March 21, 2018, the Company’s name was changed to Marizyme, Inc., to reflect the new life sciences focus. Marizyme’s common
stock is currently quoted on the OTCQB tier of OTC Markets Group, Inc. under the symbol “MRZM”.
Basis
of Presentation and Principles of Consolidation
The
Company’s consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United
States of America (“GAAP”). The accompanying consolidated financial statements include the consolidated accounts of the Company
and its wholly owned subsidiaries, Somahlution, Inc. (“Somahlution”), Somaceutica, Inc. (“Somaceutica”), Marizyme
Sciences, Inc. (“Marizyme Sciences”), and My Health Logic, Inc. (“My Health Logic”). All intercompany transactions
have been eliminated on consolidation.
Going
Concern
The
Company’s consolidated financial statements are prepared using GAAP as applicable to a going concern, which contemplates the realization
of assets and liquidation of liabilities in the normal course of business. However, the Company does not have an established source of
revenues sufficient to cover its operating costs and to allow it to continue as a going concern. The Company, since its inception, has
incurred recurring operating losses and negative cash flows from operations and has an accumulated deficit of $85,989,433 at December
31, 2022. Additionally, the Company has negative working capital of $966,464 and $510,865 of cash on hand, which may not be sufficient
to fund operations for the next twelve months. These factors raise substantial doubt about the Company’s ability to continue as
a going concern.
Under
the going concern assumption, an entity is ordinarily viewed as continuing its business for the foreseeable future with neither the intention
or necessity of liquidation, ceasing trading, or seeking protection from creditors pursuant to the laws and regulations. Accordingly,
assets and liabilities are recorded on the basis that the entity will be able to realize its assets and discharge its liabilities in
the normal course of business.
The
ability of the Company to continue as a going concern is dependent upon its ability to continue to successfully develop its intangible
assets, receive an approval from the U.S. Food and Drug Administration (“FDA”) to extend the selling of the products into
the U.S. market which may result in the Company attaining profitable operations.
During
the next twelve months from the date the consolidated financial statements were issued, the Company’s foreseeable cash requirements
will relate to continuous operations of its business, maintaining its good standing and making the required filing with the SEC, and
the payment of expenses associated with its product development. The Company may experience a cash shortfall and be required to raise
additional capital. Management intends to raise additional funds by way of a private or public offering. On February 14, 2021, Marizyme
completed a preliminary prospectus with intention to raise up to $17,250,000. Since then, the prospectus has been amended with the most
recent amendment made on February 13, 2023 with intention to raise up to $9,600,000. As of the date of this annual report, the prospectus
remains preliminary. The proceeds from the offering will be used by the Company (i) to develop its DuraGraft, MATLOC, and Krillase platforms;
(ii) to commercialize and produce its products, (iii) to repay promissory notes issued to Walleye Opportunities Master Fund Ltd and Hexin
Global Ltd., and (iv) for general working capital and other corporate purposes. While the Company believes in the viability
of its strategy to continue to develop and expand its products and generate sufficient revenue and in its ability to raise additional
funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s
ability to further implement its business plan and generate sufficient revenue and its ability to raise additional funds by way of a
public or private offering.
The
consolidated financial statements do not include any adjustments related to the recoverability and classification of recorded asset amounts
or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Use
of Estimates
The
preparation of the consolidated financial statements in accordance with GAAP requires management to make use of certain estimates and
assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the
date of the consolidated financial statements and the reported amounts of revenue and expenses during the reported periods. The Company
bases its estimates on historical experience and on various other assumptions that management believes are reasonable under the circumstances,
the results of which form the basis for making judgments about carrying values of assets and liabilities that are not readily apparent
from other sources. Actual results could differ from those estimates. Significant estimates are related to the allocation of the purchase
price in a business combination to the underlying assets and liabilities, recoverability of long-term assets including intangible assets
and goodwill, amortization expense, valuation of warrants, stock-based compensation, derivative liabilities, contingent liabilities,
and deferred tax valuations.
Fair
Value Measurements
The
Company uses the fair value hierarchy to measure the value of its financial instruments. The fair value hierarchy is based on inputs
to valuation techniques that are used to measure fair value that are either observable or unobservable. Observable inputs reflect assumptions
market participants would use in pricing an asset or liability based on market data obtained from independent sources, while unobservable
inputs reflect a reporting entity’s pricing based upon its own market assumptions. The basis for fair value measurements for each
level within the hierarchy is described below:
| |
● |
Level
1 – Quoted prices for identical assets or liabilities in active markets. |
| |
● |
Level
2 – Quoted prices for identical or similar assets and liabilities in markets that are not active; or other model-derived valuations
whose inputs are directly or indirectly observable or whose significant value drivers are observable. |
| |
● |
Level
3 – Valuations derived from valuation techniques in which one or more significant inputs to the valuation model are unobservable
and for which assumptions are used based on management estimates. |
The
Company utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent
possible as well as considers counterparty credit risk in its assessment of fair value.
The
carrying amounts of certain cash, accounts receivable, other receivables, accounts payable and accrued expenses, notes payable, and amounts
due to related parties approximate fair value due to the short-term nature of these instruments.
The
fair value of lease obligations is determined using discounted cash flows based on the expected amounts and timing of the cash flows
discounted using a market rate of interest adjusted for appropriate credit risk.
The
contingent liabilities assumed on the acquisition of Somahlution consist of present values of royalty payments, performance warrants
and pediatric voucher warrants, future rare pediatric voucher sales, and liquidation preference. Management measured these contingencies
in accordance with Level 3 of the fair value hierarchy.
| |
i. |
The
performance warrants and pediatric vouchers warrants liabilities were valued using a Monte Carlo simulation model utilizing the following
weighted average assumptions: risk free rate of 1.19%, expected volatility of 69.62%, expected dividend of $0, and expected life
of 6.21 years. For the year ended December 31, 2022, changes in these assumptions resulted in $2,925,000 decrease in fair value of
these liabilities. The decrease was driven by decrease in the Company’s stock price year over year. At December 31, 2022
the fair market value of performance warrants and pediatric vouchers warrants liabilities was $1,427,000 (2021 - $4,352,000). |
| |
|
|
| |
ii. |
The
present value of royalty payments was measured using the scenario-based methodology. In assessing the value attributed to the royalty
payments, the estimated future cash flows were discounted to their present value using a pre-tax discount rate that reflects current
market assessments of the time value of money and the risks specific to the revenue from net sales of the product. The cash flows
derived from the Company’s fifteen-year strategic plan are based on managements’ expectations of market growth, industry
reports and trends, and past performances. These projections are inherently uncertain due to the evolving impact of the COVID-19
pandemic. The discounted cash flow model included projections surrounding revenue, discount rates, and growth rates. The discount
rates used to calculate the present value of royalty payments reflect specific risks of the Company and market conditions and the
mid-range was estimated at 20.6%. For the year ended December 31, 2022, changes in these assumptions resulted in $1,414,000 increase
in fair value of these liabilities. At December 31, 2022, the fair market value of royalty payments was $5,402,000 (2021 - $3,988,000). |
| |
|
|
| |
iii. |
Rare
pediatric voucher sales liability was valued based on the scenario-based methodology where the estimated future cash flows are discounted
to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the
risks specific to the asset – 20.6%. For the year ended December 31, 2022, changes in these assumptions resulted in $95,000
decrease in fair value of this liability. At December 31, 2022 the fair market value of rare pediatric vouchers was $1,055,000 (2021
- $1,150,000). |
| |
|
|
| |
iv. |
The
present value of liquidation preference liability, included in the contingent consideration, was determined using the Black-Scholes
option pricing method and represents the fair value of the maximum payment amount according to the Agreement. The following assumptions
were used in the Black-Scholes option pricing model: risk free rate of 0.21%, expected volatility of 78.93%, expected dividend of
$0, and expected life of 5 years. No changes to the fair value of liquidation preference liability were recorded in the year ended
December 31, 2022. At December 31, 2022 and 2021, the fair market value of liquidation preference was $1,823,000. |
The
derivative liabilities consisted of optional and automatic conversion features and the share redemption feature attached to the convertible
notes, issued pursuant to the Unit Purchase Agreement (see Note 7).
The
Company has no financial assets measured at fair value on a recurring basis. None of the Company’s non-financial assets or liabilities
are recorded at fair value on a non-recurring basis. No transfers between levels have occurred during the periods presented.
Marizyme
measures the following financial instruments at fair value on a recurring basis. At December 31, 2022 and 2021, the fair values of these
financial instruments were as follows:
SCHEDULE OF FAIR VALUES OF FINANCIAL
INSTRUMENTS
| December 31, 2022 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair Value Hierarchy | |
| December 31, 2022 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Liabilities | |
| | | |
| | | |
| | |
| Derivative liabilities | |
$ | - | | |
$ | - | | |
$ | 4,823,725 | |
| Contingent liabilities | |
| - | | |
| - | | |
| 9,707,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 14,530,725 | |
| December 31, 2021 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| | |
Fair Value Hierarchy | |
| December 31, 2021 | |
Level 1 | | |
Level 2 | | |
Level 3 | |
| Liabilities | |
| | | |
| | | |
| | |
| Derivative liabilities | |
$ | - | | |
$ | - | | |
$ | 2,485,346 | |
| Contingent liabilities | |
| - | | |
| - | | |
| 11,313,000 | |
| Total | |
$ | - | | |
$ | - | | |
$ | 13,798,346 | |
The
following table provides a roll forward of all liabilities measured at fair value using Level 3 significant unobservable inputs:
SCHEDULE OF LIABILITIES FAIR VALUE MEASURED
| Derivative and Contingent Liabilities | |
| |
| Balance at December 31, 2021 | |
$ | 13,798,346 | |
| Change in fair value of contingent liabilities | |
| (1,606,000 | ) |
| Derivative liabilities issued pursuant to Unit Purchase Agreement | |
| 2,338,379 | |
| Balance at December 31, 2022 | |
$ | 14,530,725 | |
Cash
Cash
includes cash in readily available checking accounts.
Concentrations
of Credit Risk
The
Company has significant cash balances at financial institutions which throughout the year regularly exceed the federally insured limit
of $250,000. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial
condition, results of operations, and cash flows.
Inventory
Inventory
consisted of primarily finished goods and is valued at the lower of cost and net realizable value. Cost is determined using the first-in,
first out method. The Company decreases the value of inventory for estimated obsolescence equal to the difference between the cost of
inventory and the estimated market value, based upon an aging analysis of the inventory on hand, specifically known inventory-related
risks, and assumptions about future demand and market conditions. The Company has determined that no inventory reserve was necessary
as of December 31, 2022 and 2021.
Accounts
Receivable
Trade
receivables are amounts due from customers for goods sold in the ordinary course of business. Accounts receivable are non-interest bearing
and are due for settlement in full within 30 days. Trade receivables are shown net of allowance for bad or doubtful debts.
Allowance
for Doubtful Accounts
The
Company establishes an allowance for doubtful accounts to ensure trade and other receivables are not overstated due to non-collectability.
The Company’s allowance is based on a variety of factors, including age of the receivable, significant one-time events, historical
experience, and other risk considerations. The Company did not have an allowance at December 31, 2022 or 2021. The Company did not record
any bad debt expense in each of the years ended December 31, 2022 and 2021.
Property,
Plant, and Equipment, Net
Property,
plant and equipment are recorded at cost, less accumulated depreciation. Depreciation expense is recognized using the straight-line method
over the useful life of the asset. Machinery, computer equipment and related software are depreciated over five to seven years. Furniture
and fixtures are depreciated over four to seven years. Leasehold improvements are amortized over the lesser of the lease term or the
estimated useful lives of the related assets. Expenditures for repairs and maintenance of assets are charged to expense as incurred.
Upon retirement or sale, the cost and related accumulated depreciation of assets disposed of are removed from the accounts and any resulting
gain or loss is included in loss from operations.
Intangible
Assets and Goodwill
Intangible
assets are recorded at cost less accumulated amortization and accumulated impairment losses. Intangible assets acquired as a result of
an acquisition or in a business combination are measured at fair value at the acquisition date.
The
useful lives of intangible assets are assessed as either finite or indefinite. Intangible assets with finite lives are amortized over
the estimated useful economic life and assessed for impairment whenever there is an indication that the intangible asset may be impaired.
The estimated useful life and amortization method are reviewed at the end of each reporting period, with the effect of any changes in
estimates being accounted for on a prospective basis.
Goodwill
represents the excess of the purchase price paid for the acquisition of subsidiaries over the fair value of the net assets acquired.
Following the initial recognition, goodwill is measured at cost less any accumulated impairment losses.
In-Process
Research and Development
The
Company evaluates whether acquired intangible assets are a business under applicable accounting standards. Additionally, the Company
evaluates whether the acquired assets have a future alternative use. Intangible assets that do not have future alternative use are considered
acquired in-process research and development (“IPR&D”). When the acquired in-process research and development assets
are not part of a business combination, the value of the consideration paid is expensed on the acquisition date. Future costs to develop
these assets are recorded to research and development expense as they are incurred.
Impairment
| |
● |
Impairment
of long-lived assets: The Company reviews long-lived assets, including property, plant and equipment, for impairment whenever
events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment
loss would be recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual
disposition are less than the carrying amount. The impairment loss, if recognized, would be based on the excess of the carrying value
of the impaired asset over its respective fair value. |
| |
|
|
| |
● |
Goodwill:
Goodwill is recorded at the time of purchase for the excess of the amount of the purchase price over the fair values of the identifiable
assets acquired and liabilities assumed. The fair value is determined using the estimated discounted future cash flows of the reporting
unit. Goodwill is not amortized and instead is tested at least annually for impairment, or more frequently when events or changes
in circumstances indicate that goodwill might be impaired. This impairment test is performed annually at December 31. Future adverse
changes in market conditions or poor operating results of underlying assets could result in an inability to recover the carrying
value of the goodwill, thereby possibly requiring an impairment charge. |
| |
|
|
| |
● |
In-process
research and development assets: IPR&D assets are reviewed for impairment annually, or sooner if events or changes in circumstances
indicate that the carrying amount of the asset may not be recoverable, and upon establishment of technological feasibility or regulatory
approval. An impairment loss, if any, is calculated by comparing the fair value of the asset to its carrying value. If the asset’s
carrying value exceeds its fair value, an impairment loss is recorded for the difference and its carrying value is reduced accordingly.
Similar to the impairment test for goodwill, the Company may perform a qualitative approach for testing indefinite-lived intangible
assets for impairment. |
Leases
At
the inception of a contractual arrangement, the Company determines whether the contract contains a lease by assessing whether there is
an identified asset and whether the contract conveys the right to control the use of the identified asset in exchange for consideration
over a period of time. If both criteria are met, the Company records the associated lease liability and corresponding right-of-use asset
upon commencement of the lease using the implicit rate or a discount rate based on a credit-adjusted secured borrowing rate commensurate
with the term of the lease. The Company additionally evaluates leases at their inception to determine if they are to be accounted for
as an operating lease or a finance lease. A lease is accounted for as a finance lease if it meets one of the following five criteria:
the lease has a purchase option that is reasonably certain of being exercised, the present value of the future cash flows is substantially
all of the fair market value of the underlying asset, the lease term is for a significant portion of the remaining economic life of the
underlying asset, the title to the underlying asset transfers at the end of the lease term, or if the underlying asset is of such a specialized
nature that it is expected to have no alternative uses to the lessor at the end of the term. Leases that do not meet the finance lease
criteria are accounted for as an operating lease. Operating lease assets represent a right to use an underlying asset for the lease term
and operating lease liabilities represent an obligation to make lease payments arising from the lease. Operating lease liabilities with
a term greater than one year and their corresponding right-of-use assets are recognized on the balance sheet at the commencement date
of the lease based on the present value of lease payments over the expected lease term. Certain adjustments to the right-of-use asset
may be required for items such as initial direct costs paid or incentives received. As the Company’s leases do not typically provide
an implicit rate, the Company utilizes the appropriate incremental borrowing rate, determined as the rate of interest that the Company
would have to pay to borrow on a collateralized basis over a similar term and in a similar economic environment. Lease cost is recognized
on a straight-line basis over the lease term and variable lease payments are recognized as operating expenses in the period in which
the obligation for those payments is incurred. Variable lease payments primarily include common area maintenance, utilities, real estate
taxes, insurance, and other operating costs that are passed on from the lessor in proportion to the space leased by the Company. The
Company has elected the practical expedient to not separate between lease and non-lease components.
Revenue
Recognition
Pursuant
to Topic 606, the Company recognizes revenue to depict the transfer of promised goods or services to a customer in an amount that reflects
the consideration to which the entity expects to be entitled in exchange for those goods or services. To achieve this core principle,
Topic 606 outlines a five-step process for recognizing revenue from customer contracts that includes i) identification of the contract
with a customer, ii) identification of the performance obligations in the contract, iii) determining the transaction price, iv) allocating
the transaction price to the separate performance obligations in the contract, and v) recognizing revenue associated with performance
obligations as they are satisfied.
At
contract inception, the Company assesses the goods or services promised within each contract and assess whether each promised good or
service is distinct and determine those that are performance obligations. The Company then recognizes as revenue the amount of the transaction
price that is allocated to the respective performance obligation when the performance obligation is satisfied.
The
Company has identified one performance obligation which is related to DuraGraft product sales. For the Company’s distribution partner
channel, the Company recognizes revenue for product sales at the time of delivery of the product to its distribution partner (customer).
As products have an expiration date, if a product expires, the Company will replace the product at no charge. There were no significant
judgements made in applying this topic.
Direct
Cost of Revenue
Cost
of sales includes the actual cost of merchandise sold, and the cost of transportation of merchandise from the Company’s third-party
vendor to its distributer.
Research
and Development Expenses and Accruals
All
research and development costs are expensed in the period incurred and consist primarily of salaries, payroll taxes, and employee benefits,
those individuals involved in research and development efforts, external research and development costs incurred under agreements with
contract research organizations and consultants to conduct and support the Company’s ongoing clinical trials of DuraGraft, and
costs related to manufacturing DuraGraft for clinical trials. The Company has entered into various research and development contracts
with various organizations and other companies. Payments of these activities are based on the terms of the individual agreements which
matches to the pattern of costs incurred. Payments made in advances are reflected in the accompanying balance sheets as prepaid expenses.
The Company records accruals for estimated costs incurred for ongoing research and development activities. When evaluating the adequacy
of the accrued liabilities, the Company analyzes progress of the services, including the phase or completion of events, invoices received
and contracted costs. Significant judgments and estimates may be made in determining the prepaid or accrued balances at the end of any
reporting period. Actual results could differ from the Company’s estimates.
Stock-Based
Compensation
Share-based
compensation expense for employees and directors is recognized in the Consolidated Statement of Operations based on estimated amounts,
including the grant date fair value and the expected service period. For stock options, the Company estimates the grant date fair value
using a Black-Scholes valuation model, which requires the use of multiple subjective inputs including estimated future volatility, expected
forfeitures and the expected term of the awards. The Company estimates the expected future volatility based on the stock’s historical
price volatility. The stock’s future volatility may differ from the estimated volatility at the grant date. For restricted stock
unit (“RSU”) equity awards, the Company estimates the grant date fair value using its closing stock price on the date of
grant. The Company recognizes the effect of forfeitures in compensation expense when the forfeitures occur. The estimated forfeiture
rates may differ from actual forfeiture rates which would affect the amount of expense recognized during the period. The Company recognizes
the value of the awards over the awards’ requisite service or performance periods. The requisite service period is generally the
time over which the Company’s share-based awards vest.
Income
Taxes
The
Company accounts for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and liabilities
for the expected future tax consequences of events that have been included in the financial statements. Under this method, deferred tax
assets and liabilities are determined on the basis of the differences between the consolidated financial statements and tax basis of
assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect of
a change in tax rates on deferred tax assets and liabilities is recognized in the consolidated statements of operations in the period
that includes the enactment date.
The
Company recognizes net deferred tax assets to the extent that the Company believes these assets are more likely than not to be realized.
In making such a determination, management considers all available positive and negative evidence, including future reversals of existing
taxable temporary differences, projected future taxable income, tax-planning strategies, and results of recent operations. If management
determines that the Company would be able to realize its deferred tax assets in the future in excess of their net recorded amount, management
would make an adjustment to the deferred tax asset valuation allowance, which would reduce the provision for income taxes.
The
Company records uncertain tax positions on the basis of a two-step process whereby (i) management determines whether it is more likely
than not that the tax positions will be sustained on the basis of the technical merits of the position and (ii) for those tax positions
that meet the more-likely-than-not recognition threshold, management recognizes the largest amount of tax benefit that is more than 50
percent likely to be realized upon ultimate settlement with the related tax authority. The Company recognizes interest and penalties
related to unrecognized tax benefits within income tax expense. Any accrued interest and penalties are included within the related tax
liability. There was no interest or penalties as of December 31, 2022 and 2021.
Segment
Reporting
Operating
segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation
by the chief operating decision maker in making decisions on how to allocate resources and assess performance. The Company views its
operations and manages its business as one operating segment.
Net
Loss Per Share
Basic
net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period, without
consideration for potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss by the weighted-average
number of common shares and dilutive common stock equivalents outstanding for the period determined using the treasury stock and if-converted
methods. Dilutive common stock equivalents are comprised of unvested common stock, options and warrants. For all periods presented, there
is no difference in the number of shares used to calculate basic and diluted shares outstanding as inclusion of the potentially dilutive
securities (warrants, stock options, and common shares subject to repurchase) would be antidilutive.
Recently
Issued Accounting Pronouncements
New Accounting Standards and Updates from the Securities and Exchange Commission
The
Company assesses the adoption impacts of recently issued accounting standards by the Financial Accounting Standards Board or other standard
setting bodies on the Company’s consolidated financial statements as well as material updates to previous assessments. There were
no new material accounting standards issued or adopted in year of 2022 that impacted the Company.
2.
Acquisitions
ACQUISITIONS
DuraGraft®
On
December 15, 2019, the Company entered into a contingent asset purchase agreement (the “Agreement”), as amended
on March 31, 2020 and May 29, 2020, with Somahlution, LLC, Somahlution, Inc., and Somaceutica, LLC (collectively, “Somahlution”
or “Seller”) to acquire all of the assets and none of the liabilities of Somahlution (the “Acquisition”), including
DuraGraft®, a one-time intraoperative vascular graft treatment for use in vascular and bypass surgeries that maintains endothelial
function and structure, and other related properties.
On
July 30, 2020, the Company and Somahlution entered into Amendment No. 3 to the Agreement and the Agreement was finalized. Pursuant to
the terms of this amendment, it was agreed that, as part of the Acquisition, the Company would acquire the outstanding capital stock
of Somahlution, Inc., held by Somahlution, LLC, rather than the assets of Somahlution, Inc. This change to the Agreement was made to
accommodate the European Union (“EU”) requirements with respect to the future manufacturing under Somahlution, Inc. of CE
marked products for sale in the EU. In Amendment No. 3, the Company agreed to assume certain payables of Somahlution related to clinical
and medical expenses. It was agreed that the payments on the assumed debts would be recorded as a prepaid royalty against future royalties.
As of December 31, 2022 and 2021, prepaid royalties were $339,091 and were recorded as a non-current asset.
Pursuant
to the Agreement and in consideration of the outstanding capital stock of Somahlution, Inc., the Company agreed to issue to Somahlution:
| |
● |
10,000,000
restricted shares of common stock of the Company; |
| |
● |
Warrants
to purchase 3,000,000 restricted common shares of the Company with a strike price of $5.00 per common share and a term of five years; |
| |
● |
The
Company, on a pro rata basis, shall grant the Seller the following contingent consideration upon receiving the FDA final approval
and insurance reimbursement approval on the product: |
| |
◌ |
Grant
of performance warrants for 4,000,000 restricted common shares of the Company, with a strike price determined based on the average
of the closing prices of the common shares for the 30 calendar days following the date of the public announcement of FDA approval; |
| |
◌ |
Royalties
to be paid on all net sales of the product acquired from Somahlution of 6% on the first $50 million of international net sales (and
5% on the first $50 million of U.S. net sales), 4% for greater than $50 million up to $200 million, and 2% for greater than $200
million; |
| |
◌ |
Payment
of 10% of cash value of the rare pediatric voucher sales following the FDA approval and subsequent sale to an unaffiliated third
party of a rare pediatric voucher based on Somahlution’s DuraGraft product; |
| ◌ | Grant
of rare pediatric voucher warrants to purchase an aggregate of 250,000 commons shares with
a term of five years and a strike price determined based on the average of the closing prices
of the common shares for the 30 calendar days following the date of the public announcement
of FDA approval, and |
| ◌ | Liquidation
preference, up to a maximum of $20 million upon the sale by the Company of all or substantially
all of the assets relating to the Somahlution products. Upon the sale of either or both of
the DuraGraft or Somahlution derived solid organ transplant products, the Company will pay
15% of the net sale proceeds towards the liquidation preference maximum amount. |
On
July 30, 2020, the Company completed the acquisition of Somahlution Assets (the “Somahlution Transaction”). The
acquisition of Somahlution provided the Company with access to DuraGraft and other related intangible assets, which upon approval by
FDA, will further the Company’s continued growth and international-wide product rollout.
Accounting
Standards Codification (“ASC”) 805-10 the substance of a transaction constitutes a business combination as the business of
Somahlution meets the definition of a business under the standard. Accordingly, the transaction was accounted for in accordance with
the acquisition method of accounting, and the assets acquired, and the liabilities assumed have been recorded at their respective estimated
fair values as of the acquisition date. The purchase price is based on management’s estimate of fair value of the common shares
and warrants issued as well as contingent consideration and liquidation preference given up. The final allocation of the purchase price
consideration to the assets acquired and liabilities assumed has been completed and finalized.
Details
of the carrying amount and the fair value of identifiable assets and liabilities acquired and purchase consideration paid are as follows:
SCHEDULE
OF PRELIMINARY ALLOCATION OF CONSIDERATION
| Consideration | |
| | |
| Common shares | |
$ | 12,500,000 | |
| Warrants | |
| 1,200,000 | |
| Contingent consideration | |
| 9,926,000 | |
| Total consideration | |
$ | 23,626,000 | |
| | |
| | |
| Fair value of identifiable assets acquired, and liabilities assumed | |
| | |
| Net working capital | |
$ | 30,908 | |
| Property, plant, and equipment | |
| 9,092 | |
| Intangible assets | |
| 18,170,000 | |
| Goodwill | |
| 5,416,000 | |
| Total identifiable assets | |
$ | 23,626,000 | |
The
intangible assets acquired include:
| ● | DuraGraft
patent, with estimated remaining economic life of 13 years, |
| ● | “Distribution
relationships” intangible asset related to DuraGraft products, with estimated remaining
economic life of 10 years, and |
| ● | In-process
research and development intangible asset – “Cyto Protectant Life Sciences”
with indefinite economic life. |
Goodwill
is attributed to the workforce and profitability of the acquired business and is not deductible for tax purposes. A residual method methodology
was used to estimate the fair market value goodwill. A pre-tax discount rate based on weighted average cost of capital of 33.8% was used
in the fair value assumptions for the assembled workforce acquired.
Pro
forma revenue, net income, and earnings per share are not presented for this acquisition as they are not material.
My
Health Logic Inc.
On
November 1, 2021, Marizyme entered into a definitive arrangement agreement with HLII pursuant to which the Company will acquire all of
the issued and outstanding common shares of My Health Logic Inc. (“My Health Logic” or “MHL”), a wholly owned
subsidiary of HLII, in exchange for common shares of Marizyme (the “Marizyme Shares”).
Marizyme
is dedicated to the acceleration, development and commercialization of medical technologies that promote patient health, therefore a
strategic decision was made during the year ended December 31, 2021 to acquire My Health Logic, which have provided Marizyme with access
to MHL’s lab-on-chip technology platform and its patient-centric, digital point-of-care diagnostic device, MATLOC 1; and allowed
for further growth and development of Marizyme’s portfolio of medical products.
On
December 22, 2021, Marizyme received the necessary regulatory, court and stock exchange approval to complete the acquisition of MHL resulting
in a total of 4,600,000 Common Shares issued to HLII; 230,000 of these shares are being held and administered by Marizyme to be released
to HLII, less any amounts claimed by Marizyme or its affiliates for any losses arising out of certain breaches as set out in the acquisition
agreement. This resulted in HLII holding approximately 11.35% of the total number of issued and outstanding Marizyme Shares (based on
40,528,191 Marizyme Shares issued and outstanding immediately after closing).
In
accordance with ASC 805-10 the substance of a transaction constitutes a business combination as the business of My Health Logic Inc.
meets the definition of a business under the standard. Accordingly, the transaction was accounted for in accordance with the acquisition
method of accounting, and the assets acquired, and the liabilities assumed have been recorded at their respective estimated fair values
as of the acquisition date. The purchase price is based on management’s estimate of fair value of the common shares issued. The
final allocation of the purchase price consideration to the assets acquired and liabilities assumed has been completed and finalized.
Details
of the carrying amount and the fair value of identifiable assets and liabilities acquired and purchase consideration paid are as follows:
SCHEDULE
OF ASSETS AND LIABILITIES ACQUIRED AND PURCHASE CONSIDERATION
| Consideration given up | |
| | |
| Common shares | |
$ | 7,774,000 | |
| Total consideration given up | |
$ | 7,774,000 | |
| | |
| | |
| Fair value of identifiable assets acquired, and liabilities assumed | |
| | |
| Net working deficit | |
$ | (613,156 | ) |
| Property, plant, and equipment | |
| 12,500 | |
| Intangible assets | |
| 6,600,000 | |
| Goodwill | |
| 1,774,656 | |
| Total identifiable assets | |
$ | 7,774,000 | |
As
a result of the My Health Logic acquisition, the Company acquired its lab-on-chip technology platform, its patient-centric, digital point-of-care
diagnostic device - MATLOC 1, as well as patents rights and trademarks relating to it. In addition, the Company acquired ownership rights
to MATLOC patents issued in the European Union, Canada, and the United States.
The
intangible assets acquired include:
| ● | Trade
name, with estimated remaining economic life of 14 years, |
| ● | Software,
which enables customers to track and update their test results, with economic life of 15
years, and |
| ● | Biotechnology
intangible assets related to lab-on-chip technology, with estimated remaining economic life
of 17 years. |
As
part of the acquisition, Marizyme assumed an aggregate of $468,137 in notes payable, the notes were unsecured, bore interest at a rate
of 9% per annum with no maturity date. For the years ended December 31, 2022 and 2021, Marizyme recognized $19,662 and $1,115 of interest
expense on the notes payable, respectively. The Company settled an aggregate of $278,678 of these notes payable as part of Unit Purchase
Agreement issuances during the year ended December 31, 2022 (see Note 7). As of December 31, 2022, balance of the remaining note payable
was $218,100 (2021 - $469,252).
Goodwill
is attributed to the workforce and profitability of the acquired business and is not deductible for tax purposes. A residual method methodology
was used to estimate the fair market value goodwill. A pre-tax discount rate based on weighted average cost of capital of 37.5% was used
in the fair value assumptions for the assembled workforce acquired.
Pro
forma revenue, net income, and earnings per share are not presented for this acquisition as they are not material.
3.
Leases
LEASES
On
December 11, 2020, the Company entered into a five-and-a-half-year lease agreement for approximately 10,300 square feet of administrative
office and laboratories space, which commenced in December 2020 at a monthly rent of approximately $10,800, increasing by 2.5% annually
beginning in the second year of the lease until the end of the term. Additionally, pursuant to the agreement, the Company would pay approximately
$12,000 per month in operating expenses.
Effective
April 1, 2022, the Company amended its lease agreement for administrative office and laboratories to add additional 3,053 square feet
of space. The monthly cost of total expended lease space is approximately $15,260 increasing to $15,641 in 2023 and will continue to
increase by 2.5% annually thereafter until the end of the term. The monthly operating expenses for total expanded premises have increased
from approximately $12,000 to $17,500 per month. The term of the lease remains unchanged. As of December 31, 2022, the remaining lease
term was 3.42 years. The lease has been classified as an operating lease.
The
assets and liabilities from the lease were recognized at the lease commencement date based on the present value of remaining lease payments
over the lease term using the discount rate of 3.95%, which is the average commercial interest available at the time.
The
total rent expense for the years ended December 31, 2022 and 2021 was $370,945 and $118,084, respectively.
The
following table summarizes supplemental balance sheet information related to the operating lease as of December 31, 2022 and 2021:
SCHEDULE
OF RIGHT-OF-USE ASSET AND RELATED LEASE LIABILITIES
| | |
December 31, 2022 | | |
December 31, 2021 | |
| Right-of-use asset | |
$ | 1,485,023 | | |
$ | 1,158,776 | |
| | |
| | | |
| | |
| Operating lease liabilities, current | |
$ | 423,495 | | |
$ | 277,142 | |
| Operating lease liabilities, non-current | |
| 1,061,528 | | |
| 881,634 | |
| Total operating lease liabilities | |
$ | 1,485,023 | | |
$ | 1,158,776 | |
As
of December 31, 2022, the maturities of the lease liabilities for years ended December 31 are as follows:
SCHEDULE
OF MATURITIES OF LEASE LIABILITIES
| | |
| | |
| 2023 | |
$ | 423,495 | |
| 2024 | |
| 434,082 | |
| 2025 | |
| 444,934 | |
| 2026 | |
| 266,034 | |
| Total lease payments | |
$ | 1,568,545 | |
| Less: Present value discount | |
| (83,522 | ) |
| Total | |
| $ 1,485,023 | |
4.
Intangible Assets and Goodwill
INTANGIBLE ASSETS
Krillase
As
part of the asset acquisition of ACB Holding AB, Reg. No. 559119-5762, completed on September 12, 2018, Marizyme acquired all rights,
titles, and interest in the Krillase technology, a group of intangible assets worth $28,600,000. Krillase is a naturally occurring enzyme
that acts to break protein bonds and has applications in wound debridement, wound healing, dental care and thrombosis. The useful lives
of the intangible assets are based on the life of the patent and related technology. The patents and related technology for Krillase
have not been amortized since the acquisition, as they have not yet been put into operations.
At
December 31, 2022, management determined that the carrying value of Krillase exceeded its recoverable amount. Impairment of $24,350,000
was recognized on Krillase intangible assets and recorded in the impairment of intangible assets in the consolidated statements of operations
for the year ended December 31, 2022 (2021 - $Nil).
DuraGraft
As
part of the Somahlution acquisition (see Note 2), Marizyme purchased $18,170,000 of intangible assets related to the DuraGraft® technology.
No impairment has been recognized on DuraGraft intangible assets for the years ended December 31, 2022 and 2021.
My
Health Logic
As
part of the My Health Logic acquisition (see Note 2), Marizyme purchased MHL’s lab-on-chip technology platform and its patient-centric,
digital point-of-care diagnostic device, MATLOC, fair valued at an aggregate amount of $6,600,000. No impairment has been recognized
on My Health Logic intangible assets for the years ended December 31, 2022 and 2021.
SCHEDULE
OF INTANGIBLE ASSETS AMORTIZATION EXPENSE
| | |
| | |
December 31, 2022 | | |
December 31, 2021 | |
| | |
Gross Carrying Amount | | |
Accumulated Amortization | | |
Impairment | | |
Net Carrying Amount | | |
Gross Carrying Amount | | |
Accumulated Amortization | | |
Net Carrying Amount | |
| Krillase intangible assets | |
$ | 28,600,000 | | |
$ | - | | |
$ | (24,350,000 | ) | |
$ | 4,250,000 | | |
$ | 28,600,000 | | |
$ | - | | |
$ | 28,600,000 | |
| Patents in process | |
| 122,745 | | |
| - | | |
| - | | |
| 122,745 | | |
| 122,745 | | |
| - | | |
| 122,745 | |
| DuraGraft patent | |
| 5,256,000 | | |
| (977,076 | ) | |
| - | | |
| 4,278,924 | | |
| 5,256,000 | | |
| (572,768 | ) | |
| 4,683,232 | |
| DuraGraft - Distributor relationship | |
| 308,000 | | |
| (74,433 | ) | |
| - | | |
| 233,567 | | |
| 308,000 | | |
| (43,633 | ) | |
| 264,367 | |
| DuraGraft IPR&D - Cyto Protectant Life Sciences | |
| 12,606,000 | | |
| - | | |
| - | | |
| 12,606,000 | | |
| 12,606,000 | | |
| - | | |
| 12,606,000 | |
| My Health Logic - Trade name | |
| 450,000 | | |
| (32,947 | ) | |
| - | | |
| 417,053 | | |
| 450,000 | | |
| (804 | ) | |
| 449,196 | |
| My Health Logic - Biotechnology | |
| 4,600,000 | | |
| (277,353 | ) | |
| - | | |
| 4,322,647 | | |
| 4,600,000 | | |
| (6,765 | ) | |
| 4,593,235 | |
| My Health Logic - Software | |
| 1,550,000 | | |
| (105,916 | ) | |
| - | | |
| 1,444,084 | | |
| 1,550,000 | | |
| (2,583 | ) | |
| 1,547,417 | |
| Total intangibles | |
$ | 53,492,745 | | |
$ | (1,467,725 | ) | |
$ | (24,350,000 | ) | |
$ | 27,675,020 | | |
$ | 53,492,745 | | |
$ | (626,553 | ) | |
$ | 52,866,192 | |
SCHEDULE
OF GOODWILL
| Goodwill | |
DuraGraft | | |
My Health Logic | | |
Total | |
| Balance, December 31, 2021 | |
$ | 5,416,000 | | |
$ | 1,774,656 | | |
$ | 7,190,656 | |
| Additions on acquisitions | |
| - | | |
| - | | |
| - | |
| Impairment | |
| - | | |
| - | | |
| - | |
| Balance, December 31, 2022 | |
$ | 5,416,000 | | |
$ | 1,774,656 | | |
$ | 7,190,656 | |
The
following changes to the Company’s intangible assets had taken place in the periods indicated:
SCHEDULE
OF INTANGIBLE ASSETS
| Balance, December 31, 2020 | |
$ | 42,278,211 | |
| Acquired in Somahlution Transaction | |
| 4,022,271 | |
| Acquired in MHL Transaction | |
| 6,600,000 | |
| Additions | |
| 2,775 | |
| Amortization expense | |
| (37,065 | ) |
| Balance, December 31, 2021 | |
| 52,866,192 | |
| Impairment | |
| (24,350,000 | ) |
| Amortization expense | |
| (841,172 | ) |
| Balance, December 31, 2022 | |
$ | 27,675,020 | |
Future
amortizations for DuraGraft and My Health Logic intangible assets for the next five years will be $841,172 for each year from 2023 through
2027 and $6,490,413 for 2028 and thereafter. Amortization related to in process research and development will be determined upon the
Company achieving commercialization.
5.
Accounts Payable and Accrued Expenses
ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts
payable and accrued expenses, summarized by major category, as of December 31, 2022 and 2021 consist of the following:
SCHEDULE
OF ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
| | |
December 31, 2022 | | |
December 31, 2021 | |
| Trade accounts payable | |
$ | 1,224,542 | | |
$ | 1,465,860 | |
| Accrued expenses | |
| 697 | | |
| 8,215 | |
| Accrued compensation expenses | |
| 140,204 | | |
| 122,072 | |
| Total accounts payable and accrued expenses | |
$ | 1,365,443 | | |
$ | 1,596,147 | |
6.
Notes Payable
NOTES PAYABLE
On
October 23, 2022, the Company issued a note payable to Hub International for $204,050 bearing interest at the annual rate of 6.75% per
annum, due September 23, 2023, payable monthly starting November 23, 2022. As at December 31, 2022, the balance of note payable due was
$164,729 (2021 - $127,798).
On
December 28, 2022, the Company issued a promissory note for $750,000 bearing interest at the annual rate of 20% per annum, due
June 28, 2023. The Company agreed to issue to the lender warrants to purchase common stock equal to $1,500,000 with the same terms as
any warrants issued in the Company’s next financing round and will be immediately exercisable. Default in the payment of principal
or interest or other material covenant under the note triggers a default penalty equal to 0.666% of $750,000 per month during the period
of default, and other lender rights, including the demand of immediate payment of all amounts due including accrued but unpaid interest,
and recovery of all costs, fees including attorney’s fees and disbursements, and expenses relating to collection and enforcement
of the promissory note. No liability has been recognized for the warrants as they have not been issued and their terms remain contingent
upon the next financing.
As
referenced in Note 2, as part of the Somahlution acquisition, Marizyme assumed an aggregate of $468,137 in notes payable, the notes were
unsecured, bore interest at a rate of 9% per annum with no maturity date. The Company settled an aggregate of $278,678 of these notes
payable as part of Unit Purchase Agreement issuances during the year ended December 31, 2022 (see Note 7). As of December 31, 2022, balance
of the remaining note payable was $218,100 (2021 - $469,252).
7.
Convertible Promissory Notes and Warrants
CONVERTIBLE PROMISSORY NOTES AND WARRANTS
May
2021 Unit Purchase Agreement
On
May 27, 2021, Marizyme entered into a Unit Purchase Agreement to sell up to 4,000,000 units (the “Units”) at a price per
Unit of $2.50. Each Unit is comprised of (i) a convertible promissory note convertible into common stock of the Company, (ii) a warrant
to purchase one share of common stock of the Company (the “Class A Warrant”); and (iii) a second warrant to purchase common
stock of the Company (the “Class B Warrant”).
In
May 2021, the Company issued and sold 29,978 Units at a price of $2.50 per Unit for gross proceeds of $74,945, consisting of Notes of
$74,945, Class A Warrants for the purchase of 29,978 shares of common stock and Class B Warrants for the purchase of 29,978 shares of
common stock. The Company incurred related issuance costs of $6,745 which will be amortized over the term of the Notes.
In
July 2021, the Company issued and sold 440,000 Units under the Unit Purchase Program for gross proceeds of $1,100,000. The Units included
Notes for $1,100,000, Class A Warrants for 440,000 shares of common stock and Class B Warrants for 440,000 shares of common stock.
September
2021 Amended Unit Purchase Agreement
On
September 29, 2021, due to a lower common stock price, the Company, with the consent of all Unit holders, amended the May 2021 Unit Agreements.
By rescinding their investment, the Unit holders agreed to amend the Unit Purchase Agreement resulted in the following significant changes
to the offering:
| (i) | Decreased
the offering price under the Unit Purchase Agreement from $2.50 per Unit to $2.25 per Unit
for all future sales under the Unit Purchase Agreement. No proceeds from the initial investment
were returned. |
| (ii) | Decreased
the conversion price from $2.50 per share to $2.25 per share for all current Unit holders
and all future investors. |
| (iii) | Cancelled
all Class A Warrants and Class B Warrants and replaced them with Class C Warrants. |
December
2021 Unit Purchase Agreement
On
December 21, 2021, the Company entered into a Unit Purchase Agreement (the “December UPA”) to sell up to 9,714,286 Units
at a price per unit of $1.75. Each Unit is comprised of (i) a convertible promissory note convertible into common stock of the Company
at an initial conversion price of $1.75 and, (ii) a warrant to purchase two shares of Common Stock at an initial purchase price of $2.25
per share (the new Class C Warrant). Under this December UPA, the Company issued and sold 3,438,572 Units at a per unit purchase price
of $1.75, for gross proceeds of $6,000,000. Coinciding with this December UPA, the Company also entered into an Exchange Agreement with
the existing Unit holders (the December 2021 Exchange Agreements, as further described below).
December
2021 Exchange Agreements
On
December 21, 2021, in conjunction with a $6,000,000 investment, the Company and the existing Unit holders agreed to exchange the original
securities (“Old Securities”) held by the current investors/unit holders for New Securities, consisting of (i) a New Note
in the principal amount equal to the original principal amount of the Original Note, plus all accrued interest through the day prior
to December 21, 2021, and (ii) a new warrant (“New Class C Warrants”) in exchange for the original Class C Warrants (“Original
Class C Warrants”). The Exchange of the Original Securities for the New Securities included the following significant changes:
| (i) | Decreased
the offering price under the Unit Purchase Agreement from $2.25 per Unit to $1.75 per Unit.
Outstanding principal and accrued interest were used to purchase Units at the new per unit
price. |
| (ii) | Extended
the maturity date of the notes to December 21, 2023 for all existing notes. |
| (iii) | Decreased
the conversion price from $2.25 per share to $1.75 per share for the New Units. |
| (iv) | Original
Class C Warrants were exchanged for New Class C Warrants with an exercise price of $2.25
per share (unchanged) and a five-year life measured from the date of the Exchange Agreement.
The decrease in the Unit price also resulted in additional number of New Class C Warrants
being issued in exchange for the Original Class C Warrants due to the 200% warrant coverage
provided for in the Unit Purchase Agreement. |
The
Company determined that the terms of the New Securities were substantially different from the Original Securities, and, as such the exchange
of the Original Securities for the New Securities was accounted for as an extinguishment of debt on December 21, 2021, and the New Securities
accounted for as a new debt issuance.
As
a result of this substantial modification, the total of 621,087 Units previously issued was replaced with an aggregate of 832,022 pro
rata Units and a loss on debt extinguishment of $663,522 was recorded in consolidated statement of operations for the year ended December
31, 2021.
During
the year ended December 31, 2022, the Company issued additional 4,180,071 units under the New Securities agreement for the proceeds of
$6,500,743, net of issuance cost. Of the total 4,180,071 Units issued (i) 159,245 Units were issued to settle notes payable assumed on
acquisition of My Health Logic (see Note 4), (ii) 22,857 Units were issued to settle accounts payable, and (iii) 171,428 Units were issued
in exchange for services rendered to the Company in the year ended December 31, 2022.
Additionally,
on October 28, 2022, following a letter agreement entered into between the Company, Bradley Richmond, and Univest Securities, LLC (“Univest”),
dated October 28, 2022, addressed and submitted to the Corporate Financing Department of the Financial Industry Regulatory Authority,
Inc. (the “October 2022 Letter Agreement”), the Company extinguished convertible promissory notes held by Univest and Mr.
Richmond, as well as Class C Warrants, attached to them. The parties agreed to forgo compensation previously received for no consideration
in exchange. As the result of extinguishment of these obligations, the Company recorded $338,181 gain on debt extinguishment in the consolidated
statements of operations for the year ended December 31, 2022.
The
Company determined that the optional and automatic conversion feature and the share redemption feature attached to the convertible notes
meet the definition of derivative liabilities and that the detachable warrants issued do not meet the definition of a liability and therefore
will be accounted for as an equity instrument.
The
fair value of the warrants of $4,341,042 (2021 - $4,299,649) and the fair value of derivative liabilities of $2,438,379 (2021 - $2,485,346)
issued have been recorded as debt discount and are being amortized to interest and accretion expense using the effective interest method
over the term of the Convertible Notes.
During
the year ended December 31, 2022, the Company recognized interest and accretion expense of $2,763,749 (2021 - $116,676) in the
consolidated statements of operations.
SCHEDULE
OF CONVERTIBLE NOTES
| Balance, December 31, 2020 | |
$ | - | |
| Convertible notes issued - original securities | |
| 1,174,945 | |
| Issuance costs | |
| (105,745 | ) |
| Debt discount | |
| (964,153 | ) |
| Debt accretion | |
| 90,611 | |
| Extinguishment of debt in connection with December 2021 Exchange Agreements | |
| (195,658 | ) |
| Convertible notes issued - new securities | |
| 7,456,039 | |
| Issuance costs | |
| (671,044 | ) |
| Debt discount | |
| (6,784,995 | ) |
| Debt accretion | |
| 26,065 | |
| Balance, December 31, 2021 | |
$ | 26,065 | |
| Convertible notes issued - new securities | |
| 7,315,138 | |
| Issuance costs | |
| (535,717) | |
| Debt discount | |
| (6,479,421 | ) |
| Debt accretion | |
| 2,763,749 | |
| Debt extinguishment | |
| (338,181 | ) |
| Balance, December 31, 2022 | |
$ | 2,751,633 | |
SCHEDULE
OF CONVERTIBLE NOTES NET OF DEBT DISCOUNT
| | |
December 31, 2022 | | |
December 31, 2021 | |
| Convertible notes - total principal | |
$ | 14,432,996 | | |
$ | 7,482,104 | |
| Unamortized issuance costs and discount | |
| (11,681,363 | ) | |
| (7,456,039 | ) |
| Convertible Notes, Net of Debt Discount | |
$ | 2,751,633 | | |
$ | 26,065 | |
Convertible
Notes Terms
The
Convertible Notes mature in 24 months from the initial closing date and accrue 10% of simple interest per annum on the outstanding principal
amount. The Convertible Notes principal and accrued interest can be converted at any time at the option of the holder at a conversion
price of $1.75 per share (previously $2.25 per the September 2021 Amendment and originally $2.50 per the May Unit Purchase Agreement).
In the event the Company consummates, while the Convertible Note is outstanding, an equity financing with a gross aggregate amount of
securities sold of not less than $10,000,000 (“Qualified Financing”), then all outstanding principal, together with all unpaid
accrued interest under the Convertible Notes, shall automatically convert into shares of the equity financing at the lesser of (i) 75%
of the cash price per share paid in the financing and the conversion price of $1.75 per unit.
In
the event that the Company consummates, while the Convertible Note is outstanding, an equity financing with a gross aggregate amount
of securities sold less than $10,000,000 (“Unqualified Financing”), then the equity offering price will be applicable to
the conversion price and exercise price of such Convertible Notes and Class C Warrants. Moreover, in that event, the Class C Warrant
holders may also apply a most-favored-nations clause in their warrants to request that their Class C Warrants provide that their warrant
exercise rights should be further adjusted to allow them to purchase a proportionately higher number of additional shares equal to the
initial number of shares which may be purchased by exercise of their Class C Warrants, multiplied by a fraction equal to the current
exercise price divided by the adjusted exercise price, which would allow such Class C Warrant holders to purchase the same aggregate
dollar amount of shares as initially provided such Class C Warrants. If the Convertible Notes and Class C Warrants’ conversion
or exercise rights become so adjusted as a result of such an equity financing, then the Company would be required to register the additional
shares of common stock that these securities may be converted into or exercised to purchase for resale.
The
Convertible Notes are secured by a first priority security interest in all assets of the Company.
New
Class C Warrants Terms
| ● | Exercise
price is the lower of (i) $2.25 per share, or (ii) the Automatic Conversion Price (the lesser
of (i) 75% of the cash price per share paid by the other purchasers of next round securities
in the Qualified Financing and (ii) the Conversion Price ($2.25, subject to Customary Antidilution
Adjustments). |
| ● | Exercisable
for a period of 5 years from issuance. |
| ● | Warrant
Coverage: 200%. |
8.
Stockholders’ Equity
STOCKHOLDERS’ EQUITY
The
Company is authorized to issue a total number of 25,000,000 shares of “blank check” preferred stock with a par value of $0.001.
As of December 31, 2022 and 2021, there were no shares of preferred stock issued or outstanding.
The
Company is authorized to issue a total number of 75,000,000 shares of common stock with a par value of $0.001.
As
of December 31, 2022 and 2021 there were 40,528,191
shares of common stock issued and outstanding.
During the year ended December 31, 2022, the Company had the following share issuances and cancellations:
| ● | On
March 1, 2022, the Company issued 300,000 upon exercise of warrants. |
| ● | On
October 29, 2022, the Company repurchased and cancelled 300,000 shares of common stock. |
During
the year ended December 31, 2021, the Company had the following share issuances:
| ● | On
December 22, 2021, the Company issued 4,600,000 pursuant to the My Health Logic transaction
completion (see Note 2). |
On
May 18, 2021, the Company’s Board of Directors approved the Marizyme, Inc. Amended and Restated 2021 Stock Incentive Plan (“SIP”).
The SIP incorporates stock options issued prior to May 18, 2021. The SIP authorized 5,300,000 options for issuance. On December 27, 2022,
the Board of Directors requested that stockholders ratify an amendment to the SIP to increase the maximum number of shares of common
stock available for issuance pursuant to awards granted under the SIP by 1,900,000 to 7,200,000, which was approved by the stockholders.
As of December 31, 2022, there remains 2,924,057 options available for issuance.
During
the year ended December 31, 2022, the Company granted 400,000 (2021 – 1,532,500) share purchase options to directors, officers,
employees, and consultants of the Company. The weighted-average assumptions used to estimate the fair value of stock options using the
Black-Scholes option valuation model were as follows:
SCHEDULE OF SHARE BASED STOCK OPTION VALUATION ASSUMPTIONS
| | |
2022 | | |
2021 | |
| Risk-free interest rate | |
| 2.98 | % | |
| 1.09 | % |
| Volatility | |
| 117.58 | % | |
| 252.08 | % |
| Exercise price | |
$ | 2.20 | | |
$ | 1.51 | |
| Dividend yield | |
| 0 | % | |
| 0 | % |
| Forfeiture rate | |
| 0 | % | |
| 0 | % |
| Expected life (years) | |
| 9.95 | | |
| 6.38 | |
The
Company recognizes forfeitures as they occur.
The
summary of option activity for the years ended December 31, 2022 and 2021 was as follows:
SCHEDULE
OF STOCK OPTION ACTIVITY
| | |
Number of Options | | |
Weighted Average Exercise Price | | |
Weighted Average Contractual Life | | |
Total Intrinsic Value | |
| Outstanding at December 31, 2020 | |
| 3,800,943 | | |
$ | 1.36 | | |
| 8.82 | | |
| | |
| Granted | |
| 1,532,500 | | |
| 1.51 | | |
| | | |
| | |
| Forfeited | |
| (1,682,500 | ) | |
| 1.36 | | |
| | | |
| | |
| Outstanding at December 31, 2021 | |
| 3,650,943 | | |
$ | 1.24 | | |
| 8.34 | | |
| | |
| Granted | |
| 400,000 | | |
| 2.20 | | |
| | | |
| | |
| Expired | |
| (62,502 | ) | |
| 1.25 | | |
| | | |
| | |
| Forfeited | |
| (62,498 | ) | |
| 1.25 | | |
| | | |
| | |
| Outstanding at December 31, 2022 | |
| 3,925,943 | | |
$ | 1.33 | | |
| 6.06 | | |
$ | - | |
| Exercisable at December 31, 2022 | |
| 3,193,720 | | |
$ | 1.20 | | |
| 5.36 | | |
$ | - | |
As
of December 31, 2022, the Company had the following options issued and outstanding:
SCHEDULE OF OPTIONS OUTSTANDING AND EXERCISABLE
Exercise
Price | | |
Number of Options Outstanding | | |
Number of Options Exercisable | | |
Weighted Average Remaining Contractual Years | | |
Intrinsic Value | |
| $ | 1.01 | | |
| 1,985,943 | | |
| 1,985,943 | | |
| 3.49 | | |
$ | - | |
| | 1.25 | | |
| 540,000 | | |
| 527,777 | | |
| 8.16 | | |
| - | |
| | 1.37 | | |
| 200,000 | | |
| 200,000 | | |
| 7.63 | | |
| - | |
| | 1.75 | | |
| 800,000 | | |
| 360,000 | | |
| 8.91 | | |
| - | |
| | 2.20 | | |
| 400,000 | | |
| 120,000 | | |
| 9.44 | | |
| - | |
| $ | 1.33 | | |
| 3,925,943 | | |
| 3,193,720 | | |
| 6.06 | | |
$ | - | |
During
the year ended December 31, 2021, the Company granted restricted share awards for an aggregate of 350,000 shares of common stock to directors,
senior officers and consultants of the Company, with underlying performance conditions. As of December 31, 2022, the Company determined
that the following performance conditions attached to the restricted share awards were achieved:
| |
● |
The
Company will raise financing for the gross proceeds that equal or exceed $5,000,000, and |
| |
● |
The
Company will complete valuation reports for acquisition of Somahlution and My Health Logic. |
Therefore,
compensation cost of $295,750 for the restricted share awards was recognized in stock-based compensation for the year ended December
31, 2022 (2021 - $Nil).
SCHEDULE OF WARRANTS OUTSTANDING
| | |
Number | | |
Weighted Average Price | |
| December 31, 2020 | |
| 3,393,651 | | |
$ | 4.63 | |
| Issued pursuant to Unit Purchase Agreement | |
| 8,521,183 | | |
| 2.25 | |
| Issued | |
| 230,000 | | |
| 1.39 | |
| December 31, 2021 | |
| 12,144,834 | | |
| $ 2.90 | |
| Issued pursuant to Unit Purchase Agreement | |
| 8,360,147 | | |
| 2.25 | |
| Issued | |
| 878,398 | | |
| 1.16 | |
| Exercised | |
| (300,000 | ) | |
| 0.01 | |
| Expired | |
| (113,637 | ) | |
| 3.00 | |
| Cancelled pursuant to FINRA | |
| (578,398 | ) | |
| 1.75 | |
| Cancelled as part of debt extinguishment | |
| (342,857 | ) | |
| 2.25 | |
| December 31, 2022 | |
| 20,048,487 | | |
$ | 2.64 | |
During
the year ended December 31, 2022, the Company issued the following:
Unit
Purchase Agreements Warrants
During
the year ended December 31, 2022, pursuant to the applicable Unit Purchase Agreement, the Company issued an aggregate of 8,360,159 additional
New Class C Warrants with an exercise price of $2.25 share and a term of five years. However, on October 28, 2022, following the October
2022 Letter Agreement, the Company extinguished convertible promissory notes held by Univest and Mr. Richmond (see Note 7), as well as
Class C Warrants, attached to them. As the result the total of 342,857 Class C Warrants were cancelled.
Other
Warrants
On
January 26 and February 14, 2022, in exchange for services of Mr. Richmond, the Company granted him 300,000 warrants to purchase an aggregate
300,000 shares of Marizyme’s common stock at an exercise price of $0.01 per share. The warrants issued had an average term of 5
years, vested immediately, and were fair valued at $568,677 and recorded in salary expense in the consolidated statement of operations
for the year ended December 31, 2022. On March 15, 2022, Mr. Richmond exercised 300,000 warrants issued to him.
On
June 26, 2022, the Company issued an additional 347,039 warrants to Mr. Richmond and 231,359 warrants to Univest to purchase an aggregate
578,398 shares of Marizyme’s common stock at an exercise price of $1.75 per share. The warrants issued had an average term of 5
years, vested immediately, and were fair valued at $1,281,854, of which $769,113 was recorded in salary expense and $512,741 in professional
fees in the consolidated statements of operations for the year ended December 31, 2022. On October 28, 2022, following the October 2022
Letter Agreement, the aggregate 578,398 warrants granted to Mr. Richmond and Univest were cancelled.
During
the year ended December 31, 2021, the Company issued the following:
Unit
Purchase Agreements Warrants
Pursuant
to the May Unit Purchase Agreement (see Note 7) the Company issued (i) Class A Warrants for the purchase an aggregate of 469,978 shares
of common stock, with a strike price of $3.13 per share and a term of five years, and (ii) Class B Warrants for the purchase an aggregate
of 469,978 shares of common stock with a strike price of $5.00 per share and a term of five years.
On
September 29, 2021, pursuant to the September 2021 Amended Unit Purchase Agreement, all Class A and Class B warrants were replaced with
an aggregate of 1,045,549 pro rata Class C Warrants. The warrants had a strike price of 2.25 per share and a term of five years.
On
December 2, 2021, the Company issued additional 197,777 Class C Warrants with the terms and conditions stipulated in the September 2021
Amended Unit Purchase Agreement.
On
December 21, 2021, pursuant to the December 2021 Exchange Agreements (Note 7) all previously issued Original Class C Warrants were replaced
with an aggregate of 1,664,044 pro rata New Class C Warrants with an exercise price of $2.25 per share (unchanged) and a five-year life
measured from the date of the December 2021 Exchange Agreements. The decrease in the Unit price also resulted in additional number of
New Class C Warrants being issued in exchange for the Original Class C Warrants due to the 200% warrant coverage provided for in the
Unit Purchase Agreement.
On
December 21, 2021, pursuant to the December 2021 Unit Purchase Agreement the Company issued additional 6,857,143 New Class C Warrants
with an exercise price of $2.25 per share and a term of five years.
The
detachable warrants issued were accounted for as an equity instrument and were ascribed an aggregate fair market value of $4,447,982
using the residual fair value allocation method.
Other
Warrants
During
the year ended December 2021, the Company issued warrants for the purchase of an aggregate of 230,000 shares of common stock for a settlement
and services rendered. The warrants issued have an average strike price of $1.39 per share and an average term of 4.74 years, were fair
valued at $368,287 and recorded in professional fees and salary expense in the consolidated statements of operations for the year ended
December 31, 2021.
| f) | Stock-based
compensation |
During
the year ended December 31, 2022, the Company recorded $1,905,948 in non-cash share-based compensation (2021 - $898,444).
9.
Related Party Transactions
RELATED PARTY TRANSACTIONS
As
of December 31, 2022, the Company owed an aggregate of $Nil (2021 - $1,132,634) to related parties of the
Company
During
the year ended December 31, 2022, the Company incurred and settled $172,800 in professional services rendered by related parties of the
Company and incurred and settled $76,390 of various expenses incurred by these parties in relation to their services rendered to the
Company. The services were provided by entities which are controlled by management and are pursuant to various consulting agreements.
Additionally,
as part of the Somahlution transaction in 2020 (Note 2), the Company recorded a prepaid royalty to the stockholders of Somahlution. The
primary beneficial owner is Dr. Vithal Dhaduk, a director and significant stockholder of the Company. As at December 31, 2022, the Company
had $339,091 in prepaid royalties (2021 - $339,091) which had been classified as non-current in the consolidated balance sheets.
10.
Commitments and Contingencies
COMMITMENTS AND CONTINGENCIES
Legal
Matters
Under
a Confidential Settlement Agreement, dated November 18, 2022, the Company and Nicholas DeVito agreed that Mr. DeVito would dismiss a
Complaint that Mr. DeVito filed on June 7, 2022 in the Circuit Court of the Fifteenth Judicial Circuit in and for Palm Beach County,
Florida, Case No. 50-2022-CA-005437. The parties also agreed that following an anticipated reverse split, the Company was required to
issue Mr. DeVito 16,000 “post-split” shares to be delivered in paper certificate form within three (3) business days of the
reverse split. The settlement agreement further provided that the delivered shares would be subject to normal and customary restrictions
pursuant to Rule 144 of the SEC. In the event no split occurred by December 12, 2022, the Company was required to issue Mr. DeVito 60,000
“pre-split” shares. In addition, the parties agreed that no further continuous service is required pursuant to Section 2
of the Mutual Release of Claims Agreement between Mr. DeVito and the Company dated as of August 27, 2020. Pursuant to the agreement,
on January 4, 2023, the Company issued 16,000 shares of common stock to Mr. DeVito.
On
August 19, 2021, Dr. Neil Campbell, former President, Chief Executive Officer and director of the Company, and Bruce Harmon, former Chief
Financial Officer and Secretary of the Company, each filed a Complaint and Demand for Jury Trial against the Company and Insperity Peo
Services, L.P., a Delaware limited partnership (“Insperity”), a joint employer of Dr. Campbell and Mr. Harmon with the Company
under a Client Service Agreement, dated November 30, 2020 (collectively, the “Campbell/Harmon Complaints”). Both Campbell/Harmon
Complaints allege that the Company and Insperity violated Section 448.105 of the Florida Private Whistleblower Act as a result of the
constructive terminations of Dr. Campbell and Mr. Harmon after the occurrence of violations federal and state law, including federal
securities law, at the Company that exposed Dr. Campbell and Mr. Harmon to civil and criminal forms of liability and that the Company
was not addressing to their satisfaction. Both Campbell/Harmon Complaints demand approximately $30,000 - $50,000 in back pay and benefits,
interest on back pay, front pay and/or lost earning capacity, compensatory damages, costs and attorney’s fees, and such other relief
as the court deems equitable. In the year ended December 31, 2022, both cases were dismissed with prejudice and without any financial
impact on the Company.
Contingencies
| a. | On
July 13, 2019, the Company signed a consulting agreement, whereby the individual will receive: |
| ● | $30,000
per month through July 13, 2022. |
| ● | Option
to purchase 250,000 shares of common stock at a strike price of $1.50, which vest monthly
through July 13, 2021. The vesting of these options was accelerated by the Board of Directors
on September 2, 2020. |
| ● | Royalties
based on sales of Krillase assets, equal to 10% of net sales of the product. During the year
ended December 31, 2022, no revenues were derived from sales of Krillase product. |
| b. | As
part of the DuraGraft Acquisition, completed on July 30, 2020 (see Note 2), the Company entered
into the Agreement with Somahlution stockholders, whereby Marizyme is legally obligated to
pay royalties on all net sales for Somahlution, Inc. The royalties associated with the Agreement
are calculated as follows: |
Royalties
on U.S. sales equal to:
| ● | 5%
on the first $50,000,000 of net sales, |
| ● | 4%
on net sales of $50,000,001 up to $200,000,000, and |
| ● | 2%
on net sales over $200,000,000. |
Royalties
on sales outside of the U.S.:
| ● | 6%
on the first $50,000,000 of net sales, |
| ● | 4%
on net sales of $50,000,001 up to $200,000,000, and |
| ● | 2%
on net sales over $200,000,000. |
The
royalties are in perpetuity. As at December 31, 2022, the Company had not earned any revenues from Krillase and did not have any sales
of the DuraGraft products in U.S., therefore no royalties have been accrued or paid in the year.
Upon
receiving FDA approval for the DuraGraft product, the Company will:
| ● | Issue
performance warrants with a strike price determined based on the average of the closing prices
of the Company’s common stock for the 30 calendar days following the date of the public
announcement of the FDA approval; and |
| ● | Upon
liquidation of all or substantially all of the assets relating to DuraGraft, the Company
will pay 15% of the net sale proceeds up to $20 million. |
| c. | The
Company has entered into arrangements for office and laboratories spaces. As at December
31, 2022, minimum lease payments in relation to lease commitments are payable as described
in Note 3. |
Risks
and Uncertainties
Starting
in late 2019, a novel strain of the coronavirus, or COVID-19, began to rapidly spread around the world and every state in the United
States. At this time, there continues to be significant volatility and uncertainty relating to the full extent to which the COVID-19
pandemic and the various responses to it will impact the Company’s business, operations and financial results.
Most
states and cities have at various times instituted quarantines, restrictions on travel, “stay at home” rules, social distancing
measures and restrictions on the types of businesses that could continue to operate, as well as guidance in response to the pandemic
and the need to contain it. As a result, the COVID-19 pandemic may affect the operations of the FDA and other health authorities, including
such authorities in Europe, which could result in delays of reviews and approvals. While there have been no specific notices of delay
from federal or foreign government authorities, potential interruptions, delays, or changes to the operations of the FDA, or of any foreign
authority with which the Company might interact, might impact the approval of any applications the Company plans and will need to file
in the future.
In
addition, the Company is dependent upon certain contract manufacturers and suppliers and their ability to reliably and efficiently fulfill
its orders is critical to the Company’s business success. The COVID-19 pandemic has impacted and may continue to impact certain
of the Company’s manufacturers and suppliers. As a result, the Company has faced and may continue to face delays or difficulty
sourcing certain products, which could negatively affect the Company’s business and financial results.
The
spread of COVID-19 has also adversely impacted global economic activity and has contributed to significant volatility and negative pressure
in financial markets. The pandemic has resulted, and may continue to result, in a significant disruption of global financial markets,
which may reduce the Company’s ability to access capital in the future, which could negatively affect the Company’s liquidity.
If
the COVID-19 pandemic does not continue to slow and the spread of COVID-19 is not contained, the Company’s business operations,
including those of contract manufacturers, could be further delayed or interrupted. The duration of any business disruption cannot be
reasonably estimated at this time but may materially affect the Company’s ability to operate the Company’s business and result
in additional costs. It is not possible to reliably measure or quantify the impact COVID-19 has had on the financial results of the Company.
If the COVID-19 pandemic continues for an extended period, it may materially adversely impact business operations and, consequently,
future financial results.
11.
Income Taxes
INCOME TAXES
As
of December 31, 2022, and 2021, the Company has federal net operating loss carry forwards of $41,733,000
and $34,971,000,
respectively. As of December 31, 2022, and 2021, the Company has Florida state net operating loss carry forwards of $18,117,000
and $11,354,000, respectively. The federal and Florida state net operating loss carryforwards are carried forward
indefinitely. The Company’s net operating loss carry forwards may be subject to annual limitations, which could reduce or
defer the utilization of the losses as a result of an ownership change as defined in Section 382 of the Internal Revenue
Code.
The
Company’s tax expense differs from the “expected” tax expense for Federal income tax purposes (computed by applying
the United States Federal tax rate of 21% to loss before taxes for fiscal year 2022 and 2021), as follows:
SCHEDULE
OF INCOME TAX EXPENSE
| | |
December 31, 2022 | | |
December 31, 2021 | |
| Tax expense (benefit) at the statutory rate | |
$ | (8,014,646 | ) | |
$ | (2,309,565 | ) |
| State taxes | |
| (1,658,268 | ) | |
| - | |
| Non-deductible items | |
| 702,460 | | |
| 620,855 | |
| Deferred true-ups | |
| 40,912 | | |
| (199,782 | ) |
| Other | |
| (121,202 | ) | |
| - | |
| Change in valuation allowance | |
| 9,050,744 | | |
| 1,888,492 | |
| Total | |
$ | - | | |
$ | - | |
The
tax effects of the temporary differences between reportable financial statement income and taxable income are recognized as deferred
tax assets and liabilities.
The
tax years 2022 and 2021 remain open to examination by federal agencies and other jurisdictions in which it operates.
The
following is a reconciliation of the U.S. federal statutory rate to the effective income tax rates for the years ended December 31, 2022
and 2021:
SCHEDULE
OF EFFECTIVE INCOME TAX RATES
| | |
December 31, 2022 | | |
December 31, 2021 | |
| U.S. statutory federal rate | |
| 21.0 | % | |
| 21.0 | % |
| State taxes | |
| 4.3 | % | |
| 0.0 | % |
| Non-deductible / non-taxable items | |
| -1.8 | % | |
| -5.6 | % |
| Deferred true-up | |
| -0.1 | % | |
| 1.8 | % |
| Other | |
| 0.3 | % | |
| 0.0 | % |
| Valuation allowance | |
| -23.7 | % | |
| -17.2 | % |
| Total provision | |
| 0.0 | % | |
| 0.0 | % |
The
tax effect of significant components of the Company’s deferred tax assets and liabilities at December 31, 2022 and 2021, are as
follows:
SCHEDULE
OF DEFERRED TAX ASSETS AND LIABILITIES
| | |
December 31, 2022 | | |
December 31, 2021 | |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss carry forward | |
$ | 9,551,121 | | |
$ | 8,758,337 | |
| Lease liability | |
| 376,379 | | |
| 277,142 | |
| Intangible assets | |
| 3,618,128 | | |
| (3,665,013 | ) |
| Capitalized research and development costs | |
| 974,819 | | |
| - | |
| Deferred
tax assets | |
| 14,520,448 | | |
| 7,315,601 | |
| Deferred tax liabilities: | |
| | | |
| | |
| Fixed assets | |
| (376,379 | ) | |
| (277,142 | ) |
| Deferred
tax liabilities | |
| (376,379 | ) | |
| (277,142 | ) |
| Total gross deferred tax assets | |
| 14,144,068 | | |
| 5,093,324 | |
| Less: Deferred tax asset valuation allowance | |
| (14,144,068 | ) | |
| (5,093,324 | ) |
| Total net deferred taxes | |
$ | - | | |
$ | - | |
In
assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all
of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of
future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal
of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment.
Because
of the historical earnings history of the Company, the net deferred tax assets for 2022 and 2021 were fully offset by a 100% valuation
allowance. The valuation allowance for the remaining net deferred tax assets was $14,144,068 and $5,093,324 as of December 31, 2022 and
2021, respectively. The Company is evaluating the foreign reporting requirements as it relates to revenue from foreign sources and has
determined that any accrual would not be material.
Effective
for tax years beginning after December 31, 2021, taxpayers are required to capitalize any expenses incurred that are considered incidental
to research and experimentation (R&E) activities under IRC Section 174. While taxpayers historically had the option of deducting
these expenses under IRC Section 174, the December 2017 Tax Cuts and Jobs Act mandates capitalization and amortization of R&E expenses
for tax years beginning after December 31, 2021. Expenses incurred in connection with R&E activities in the US must be amortized
over a 5-year period if incurred, and R&E expenses incurred outside the US must be amortized over a 15-year period. R&E activities
are broader in scope than qualified research activities considered under IRC Section 41 (relating to the research tax credit). For the
year ended December 31, 2022, the Company performed an analysis based on available guidance and determined that it will continue to be
in a loss position even after the required capitalization and amortization of its R&E expenses. The Company will continue to monitor
this issue for future developments, but it does not expect R&E capitalization and amortization to require it to pay cash taxes now
or in the near future.
12.
Subsequent Events
SUBSEQUENT EVENTS
Pursuant
to Unit Purchase Agreement (see Note 7), on January 12, 2023, the Company and Univest has signed a Letter for the investors in the Units
Private Placement, dated January 12, 2023 (the “Letter Agreement”), where the parties agreed that simultaneously with any
adjustment to the exercise price under the Class C Warrants as a result of any equity issuances, not including qualified financings and
certain other exempt issuances, the number of shares of Common Stock that may be purchased under the Class C Warrants will be increased
such that the aggregate exercise price of such shares will be the same as the same as the aggregate exercise price in effect immediately
prior to the adjustment, without regard to any limitations on exercise contained in the Class C Warrants, including the beneficial ownership
limitation described above. The Letter Agreement was approved by the Board on January 18, 2022.
On
February 6, 2023, the Company entered into a securities purchase agreement with Walleye Opportunities Master Fund Ltd (“Investor”)
pursuant to which the Company issued to the Investor an Unsecured Subordinated Convertible Promissory Note (the “Note”) in
the aggregate principal amount of $1,000,000 (the “Subscription Amount”) and a Class D Common Stock Purchase Warrant (the
“Warrant”) to purchase up to a number of shares of the Company’s common stock equal to the quotient of 250% of the
Subscription Amount divided by the price per share at which shares are sold in the Public Offering (as defined below) (the “Warrant
Shares”).
The
principal amount of the Note must be repaid in full by the Company to the holder of the Note on or before the date that is 90 days following
the issuance of the Note, or May 7, 2023 (the “Maturity Date”). If all obligations arising under the Note are not paid or
otherwise satisfied in full on the Maturity Date, then the principal amount of the Note shall be increased from $1,000,000 to $1,250,000.
The Note bears no interest. If an event constituting an event of default under the Note occurs, including non-payment, defaults of covenants,
an adverse judgment for payment of $500,000 or more, defaults on certain other indebtedness, bankruptcy-type events, or failure to maintain
directors and officers insurance coverage of at least $1,000,000, and such event of default is not cured with the period specified, the
obligations of the Company under the Note will become subject to immediate repayment obligations. As of March 24, 2023 the Note has not
been repaid by the Company.

MARIZYME,
INC.
Up to 915,071,257 Shares of Common Stock
PROSPECTUS
October
17, 2023
Marizyme (QB) (USOTC:MRZM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Marizyme (QB) (USOTC:MRZM)
Historical Stock Chart
From Apr 2023 to Apr 2024