false000133997000013399702023-09-262023-09-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 26, 2023
ATYR PHARMA, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
Delaware |
|
001-37378 |
|
20-3435077 |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
10240 Sorrento Valley Road, Suite 300 San Diego, CA |
|
|
|
92121 |
(Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (858) 731-8389
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
|
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, par value $0.001 per share |
LIFE |
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
aTyr Pharma, Inc. (the Company) intends to use an investor presentation to conduct meetings with investors, stockholders and analysts and at investor conferences, and which the Company intends to place on its website. A copy of the presentation materials is attached hereto as Exhibit 99.1 and is incorporated herein by reference. The Company does not undertake to update the presentation materials.
The information under this Item 7.01, including Exhibit 99.1, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing
Item 8.01. Other Events.
The Company recently initiated its Phase 2 proof-of concept study of efzofitimod (the EFZO-CONNECT study) in patients with systemic sclerosis (SSc, also known as scleroderma)-associated ILD (SSc-ILD) with the activation of a trial site that is actively recruiting patients. The EFZO-CONNECT study is a randomized, double-blind placebo-controlled proof-of-concept study to evaluate the efficacy, safety and tolerability of efzofitimod in patients with SSc-ILD. This will be a 28-week study with three parallel cohorts randomized 2:2:1 to either 270 mg or 450 mg of efzofitimod or placebo dosed intravenously monthly for a total of six doses. The study intends to enroll 25 patients at multiple centers in the United States. The primary objective of the study will be to evaluate the efficacy of multiple doses of intravenous efzofitimod on pulmonary, cutaneous and systemic manifestations in patients with SSc-ILD. Secondary objectives will include safety and tolerability.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
2
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
ATYR PHARMA, INC. |
|
|
|
|
|
|
|
By: |
/s/ Jill M. Broadfoot |
|
|
Jill M. Broadfoot |
|
|
Chief Financial Officer |
|
|
|
Date: September 26, 2023 |
|
|
3

The Evolutionary Intelligence Biotech September 2023 Exhibit 99.1

Forward Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements regarding: the potential therapeutic benefits of proteins derived from tRNA synthetase genes and our product candidates and development programs; the ability to successfully advance our product candidates and undertake certain development activities (such as the initiation of clinical trials, clinical trial enrollment, the conduct of clinical trials and announcement of clinical results) and accomplish certain development goals, and the timing of such events; the potential market opportunity for our product candidates; our ability to receive regulatory approvals for, and commercialize, our product candidates; our ability to identify and discover additional product candidates; potential activities and payments under collaboration agreements; and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, our subsequently filed Quarterly Reports on Form 10-Q, and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the uses for which they are being studied. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involved a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
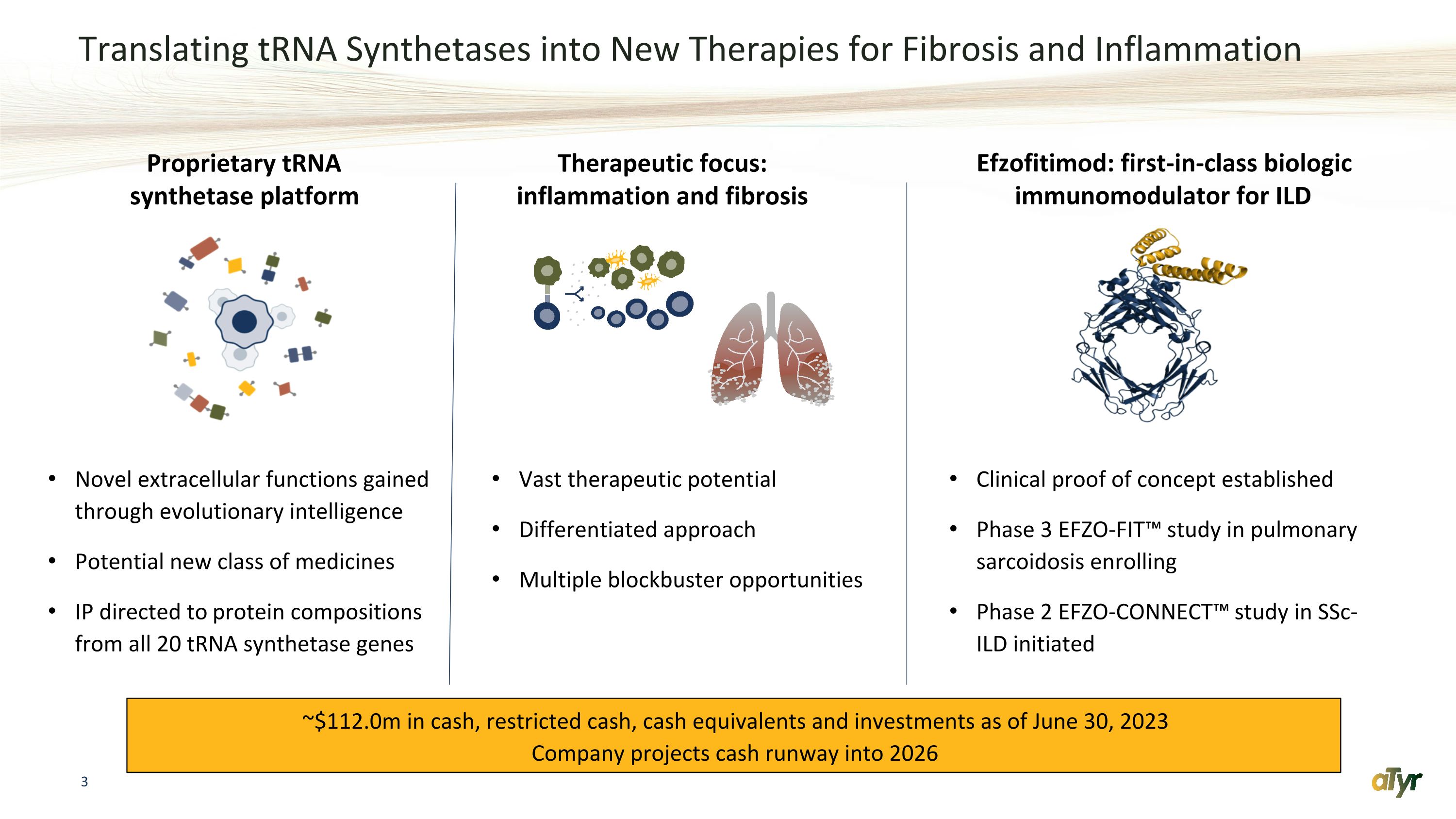
Translating tRNA Synthetases into New Therapies for Fibrosis and Inflammation Novel extracellular functions gained through evolutionary intelligence Potential new class of medicines IP directed to protein compositions from all 20 tRNA synthetase genes Clinical proof of concept established Phase 3 EFZO-FIT™️ study in pulmonary sarcoidosis enrolling Phase 2 EFZO-CONNECT™️ study in SSc-ILD initiated Efzofitimod: first-in-class biologic immunomodulator for ILD Proprietary tRNA synthetase platform Vast therapeutic potential Differentiated approach Multiple blockbuster opportunities Therapeutic focus: inflammation and fibrosis ~$112.0m in cash, restricted cash, cash equivalents and investments as of June 30, 2023 Company projects cash runway into 2026
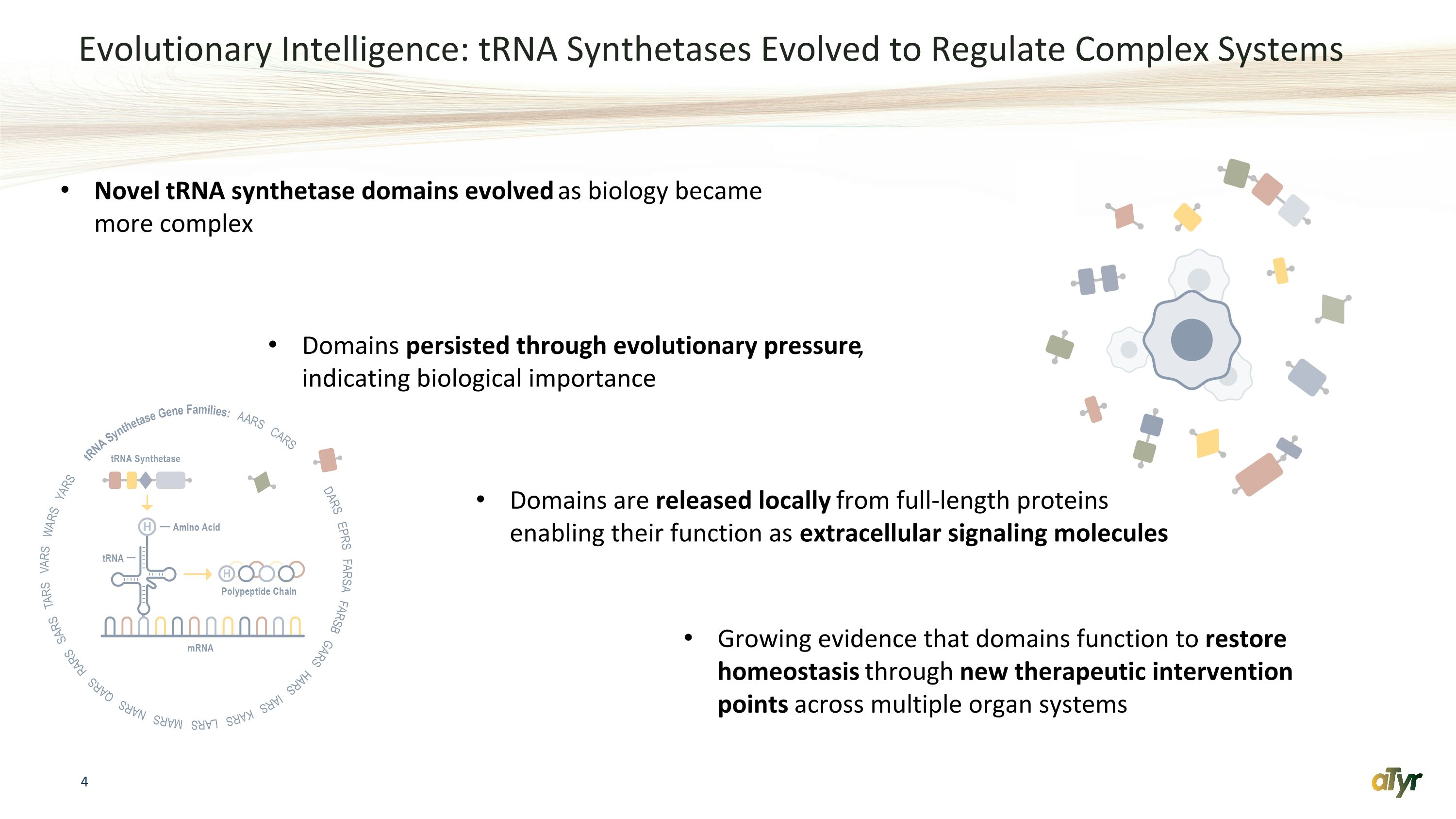
Evolutionary Intelligence: tRNA Synthetases Evolved to Regulate Complex Systems Domains are released locally from full-length proteins enabling their function as extracellular signaling molecules Growing evidence that domains function to restore homeostasis through new therapeutic intervention points across multiple organ systems Novel tRNA synthetase domains evolved as biology became more complex Domains persisted through evolutionary pressure, indicating biological importance

Increasing Validation of aTyr Science in Peer Reviewed Journals “Efzofitimod: a novel anti-inflammatory agent for sarcoidosis” – first major review article for efzofitimod (https://doi.org/10.36141/svdld.v40i1.14396); “Efzofitimod for the treatment of pulmonary sarcoidosis” – Phase 1b/2a data publication (https://doi.org/10.1016/j.chest.2022.10.037); ATYR2810’s target NRP2 biology featured on the cover of Science Translational Medicine (https://www.science.org/doi/10.1126/scitranslmed.adf1128)
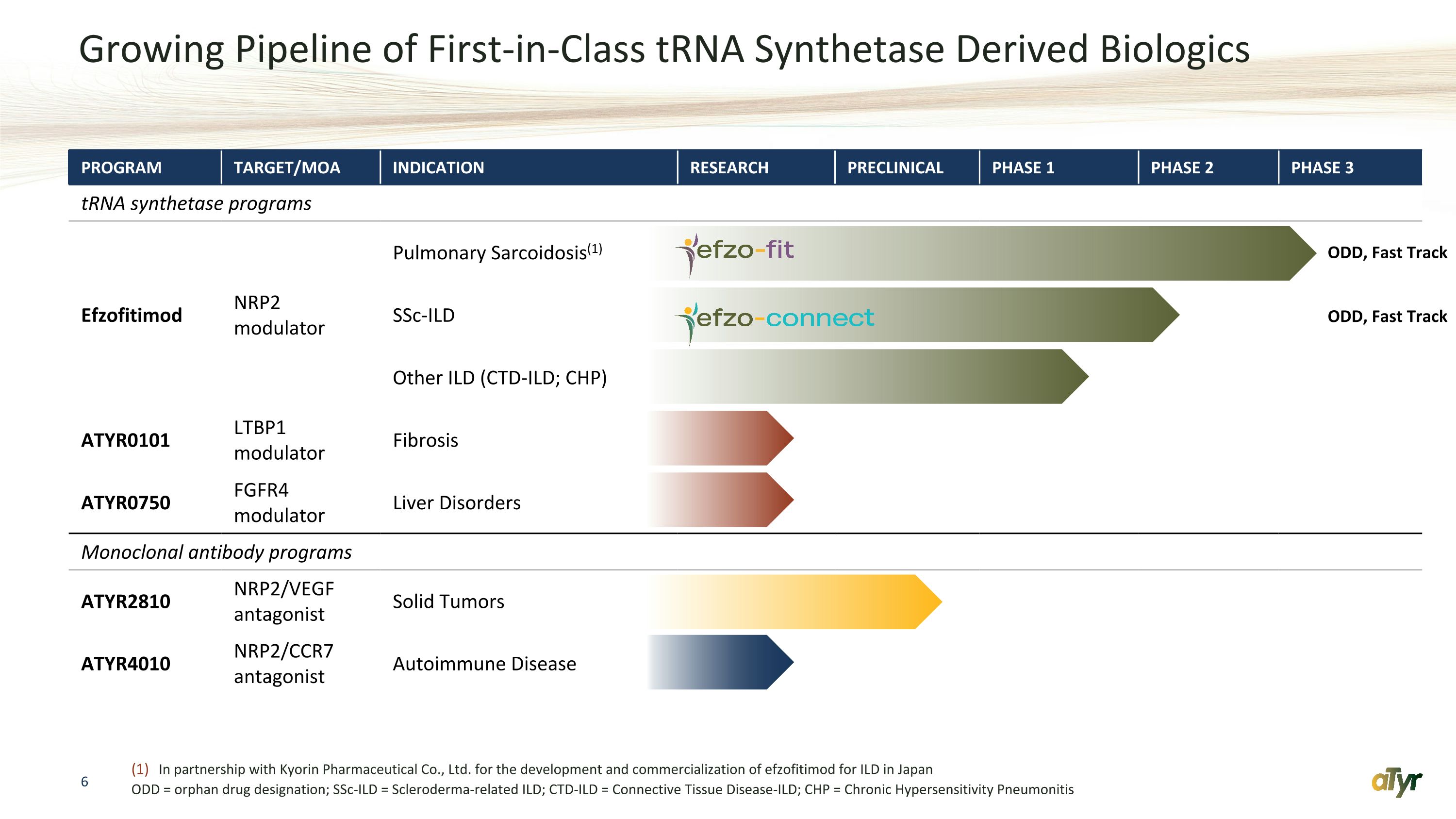
Growing Pipeline of First-in-Class tRNA Synthetase Derived Biologics PROGRAM TARGET/MOA INDICATION RESEARCH PRECLINICAL PHASE 1 PHASE 2 PHASE 3 tRNA synthetase programs Efzofitimod NRP2 modulator Pulmonary Sarcoidosis(1) SSc-ILD Other ILD (CTD-ILD; CHP) ATYR0101 LTBP1 modulator Fibrosis ATYR0750 FGFR4 modulator Liver Disorders Monoclonal antibody programs ATYR2810 NRP2/VEGF antagonist Solid Tumors ATYR4010 NRP2/CCR7 antagonist Autoimmune Disease ODD, Fast Track ODD, Fast Track In partnership with Kyorin Pharmaceutical Co., Ltd. for the development and commercialization of efzofitimod for ILD in Japan ODD = orphan drug designation; SSc-ILD = Scleroderma-related ILD; CTD-ILD = Connective Tissue Disease-ILD; CHP = Chronic Hypersensitivity Pneumonitis

Efzofitimod First-in-Class Biologic Immunomodulator for Interstitial Lung Disease (ILD)
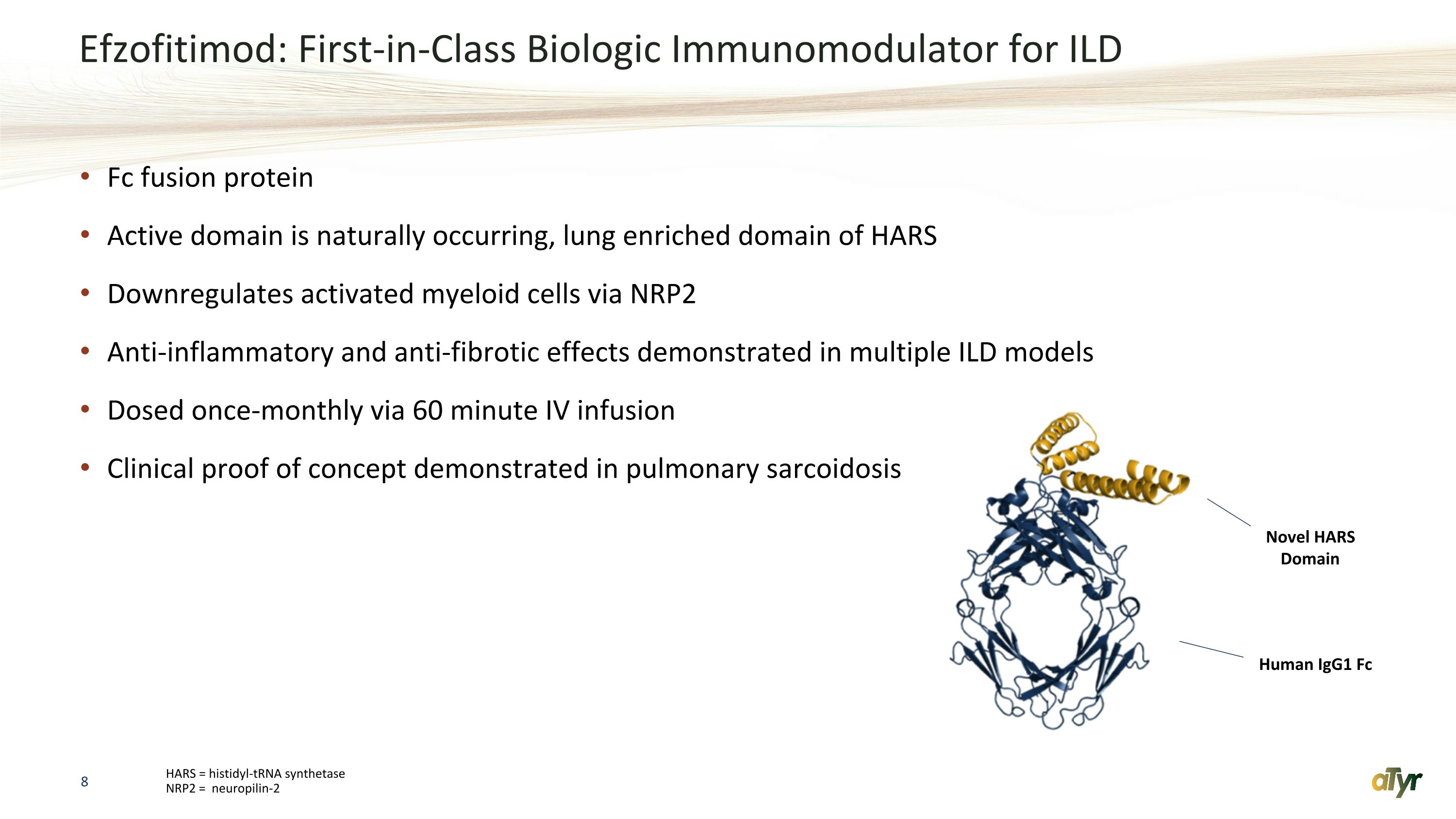
Efzofitimod: First-in-Class Biologic Immunomodulator for ILD Fc fusion protein Active domain is naturally occurring, lung enriched domain of HARS Downregulates activated myeloid cells via NRP2 Anti-inflammatory and anti-fibrotic effects demonstrated in multiple ILD models Dosed once-monthly via 60 minute IV infusion Clinical proof of concept demonstrated in pulmonary sarcoidosis HARS = histidyl-tRNA synthetase �NRP2 = neuropilin-2 Novel HARS Domain Human IgG1 Fc
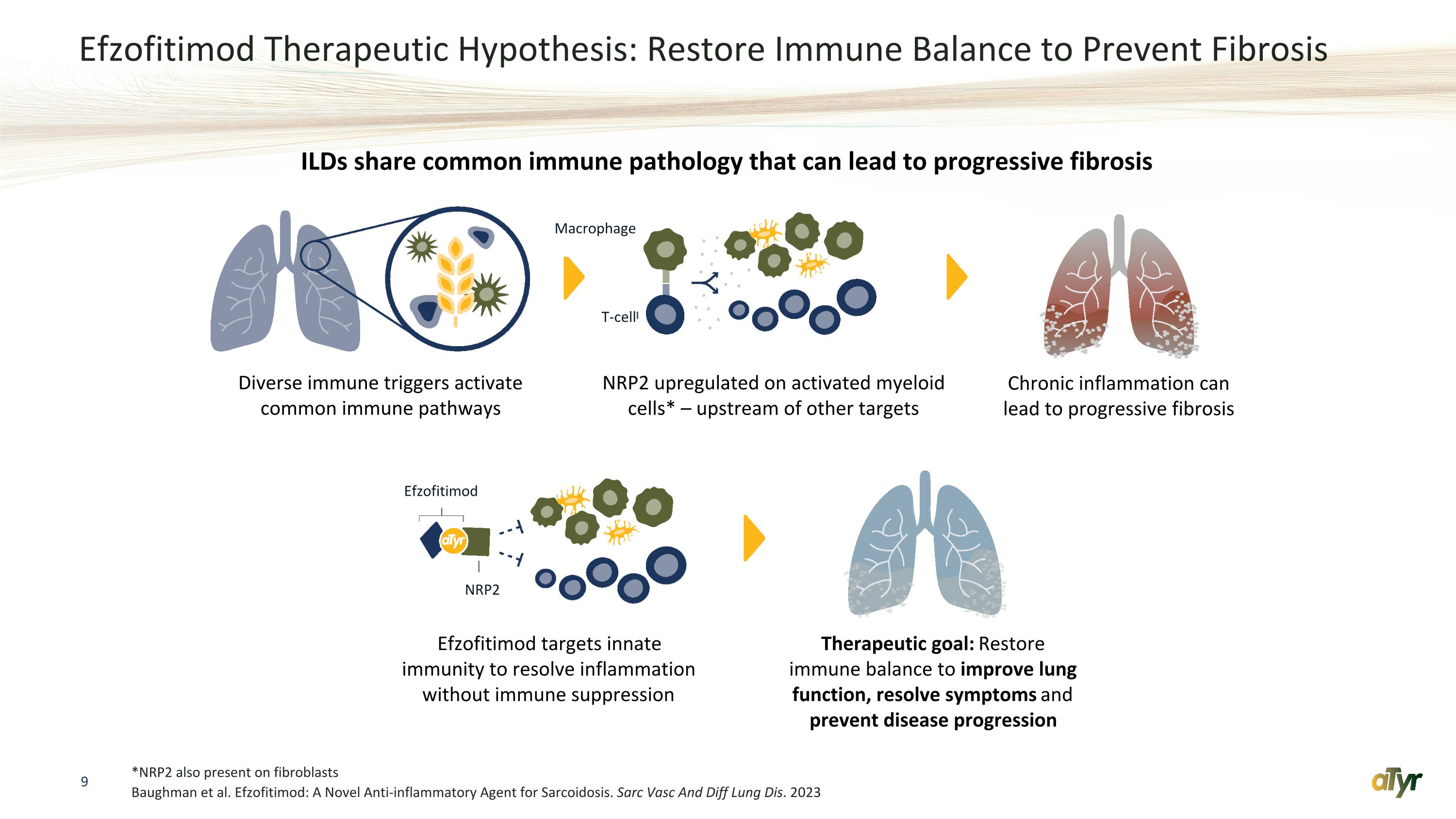
Efzofitimod Therapeutic Hypothesis: Restore Immune Balance to Prevent Fibrosis *NRP2 also present on fibroblasts Baughman et al. Efzofitimod: A Novel Anti-inflammatory Agent for Sarcoidosis. Sarc Vasc And Diff Lung Dis. 2023 Diverse immune triggers activate common immune pathways NRP2 upregulated on activated myeloid cells* – upstream of other targets Chronic inflammation can lead to progressive fibrosis Efzofitimod targets innate immunity to resolve inflammation without immune suppression ILDs share common immune pathology that can lead to progressive fibrosis Therapeutic goal: Restore immune balance to improve lung function, resolve symptoms and prevent disease progression Efzofitimod Macrophage T-cell NRP2
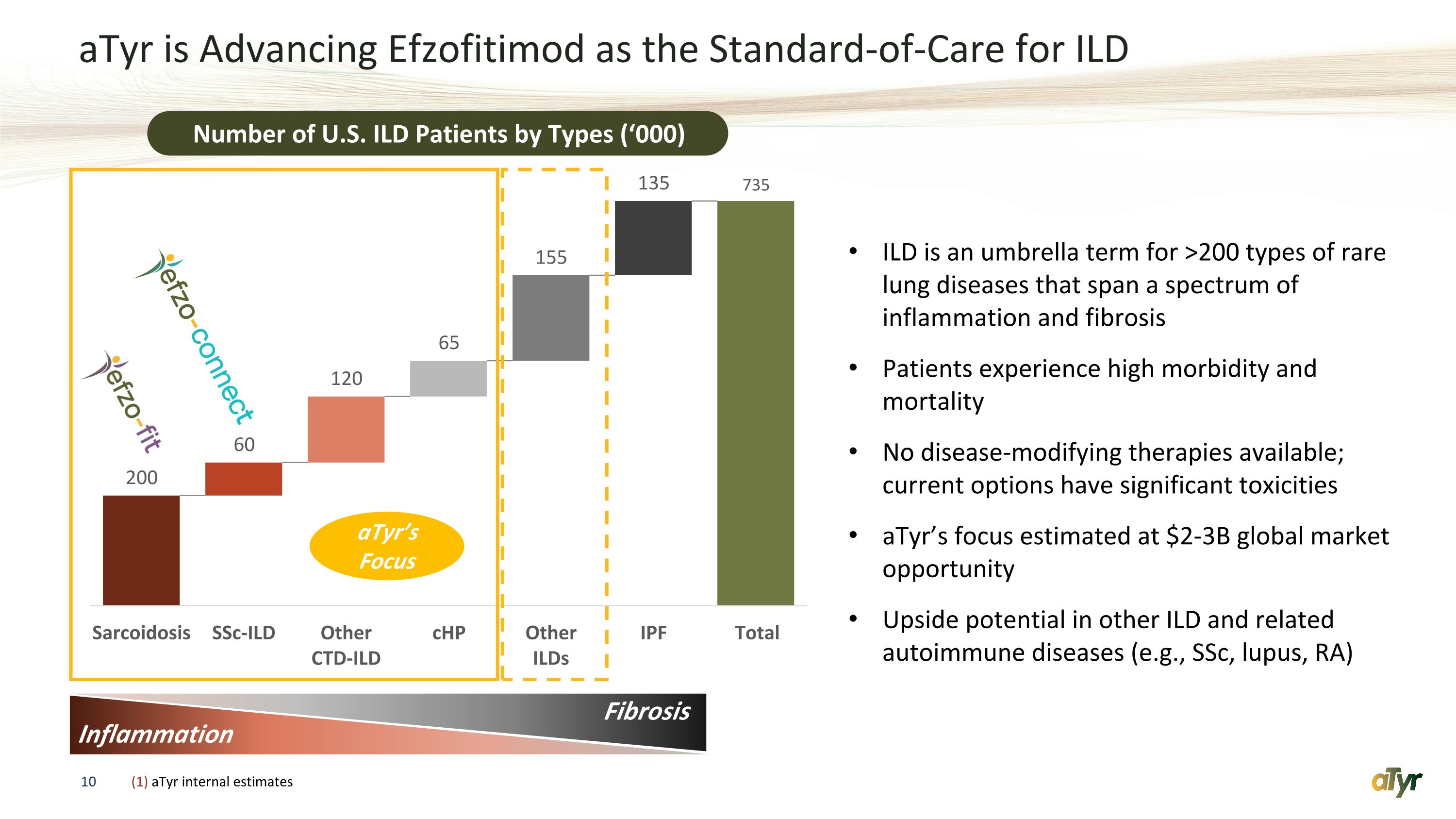
aTyr is Advancing Efzofitimod as the Standard-of-Care for ILD Number of U.S. ILD Patients by Types (‘000) ILD is an umbrella term for >200 types of rare lung diseases that span a spectrum of inflammation and fibrosis Patients experience high morbidity and mortality No disease-modifying therapies available; current options have significant toxicities aTyr’s focus estimated at $2-3B global market opportunity Upside potential in other ILD and related autoimmune diseases (e.g., SSc, lupus, RA) Inflammation Fibrosis aTyr’s Focus (1) aTyr internal estimates
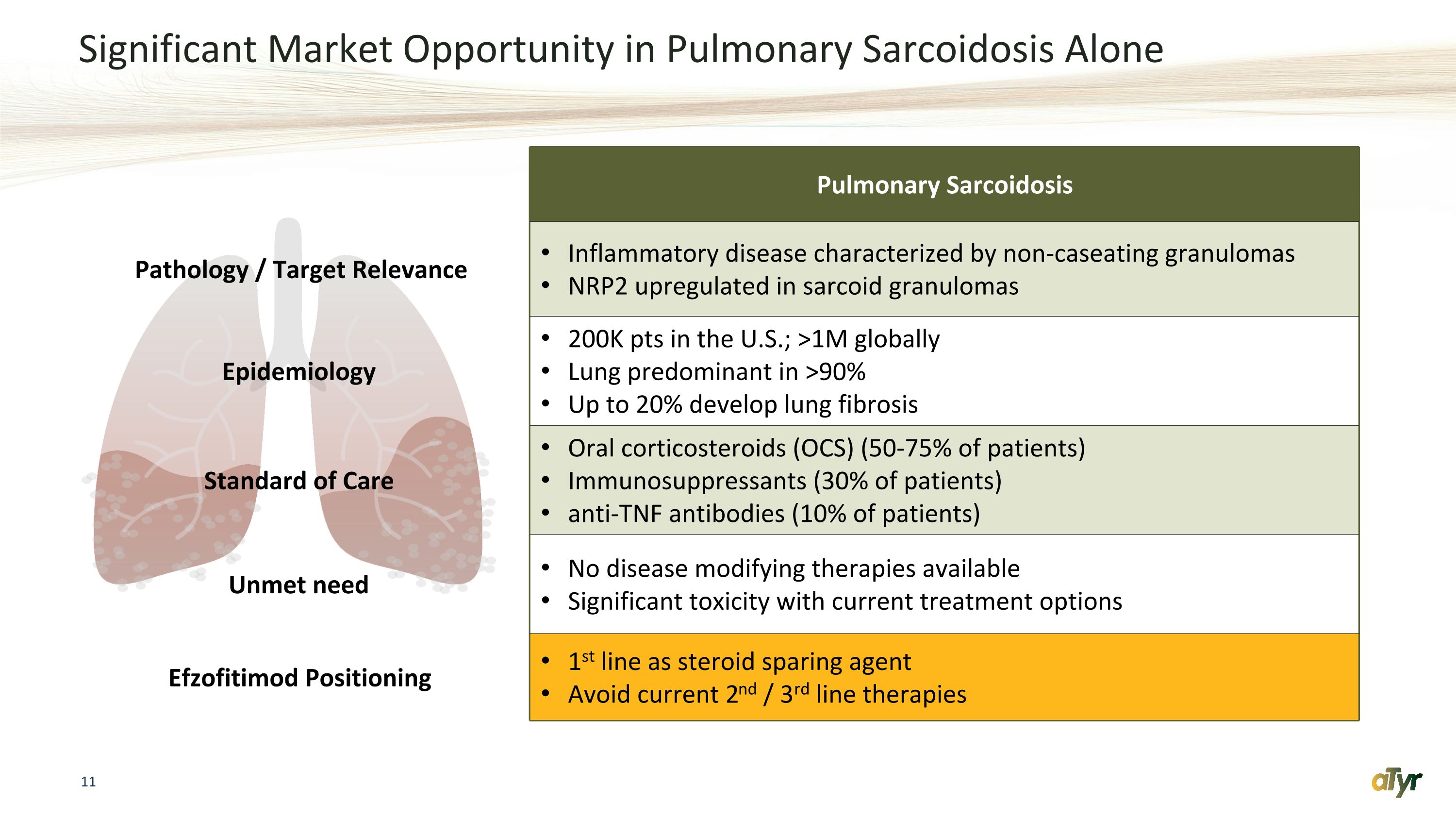
Significant Market Opportunity in Pulmonary Sarcoidosis Alone Pulmonary Sarcoidosis Pathology / Target Relevance Inflammatory disease characterized by non-caseating granulomas NRP2 upregulated in sarcoid granulomas Epidemiology 200K pts in the U.S.; >1M globally Lung predominant in >90% Up to 20% develop lung fibrosis Standard of Care Oral corticosteroids (OCS) (50-75% of patients) Immunosuppressants (30% of patients) anti-TNF antibodies (10% of patients) Unmet need No disease modifying therapies available Significant toxicity with current treatment options Efzofitimod Positioning 1st line as steroid sparing agent Avoid current 2nd / 3rd line therapies
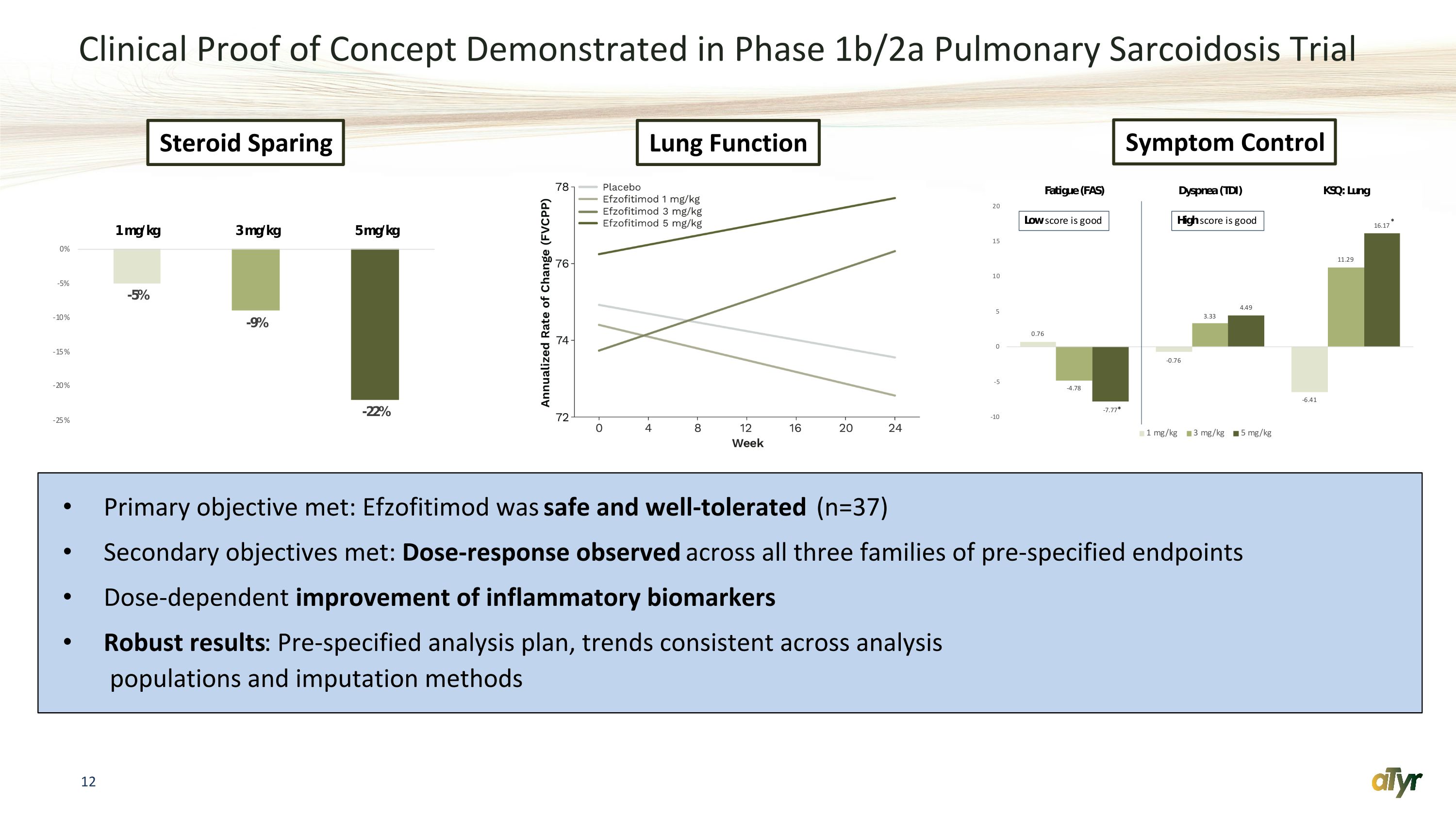
Clinical Proof of Concept Demonstrated in Phase 1b/2a Pulmonary Sarcoidosis Trial Primary objective met: Efzofitimod was safe and well-tolerated (n=37) Secondary objectives met: Dose-response observed across all three families of pre-specified endpoints Dose-dependent improvement of inflammatory biomarkers Robust results: Pre-specified analysis plan, trends consistent across analysis� populations and imputation methods Steroid Sparing Lung Function Symptom Control
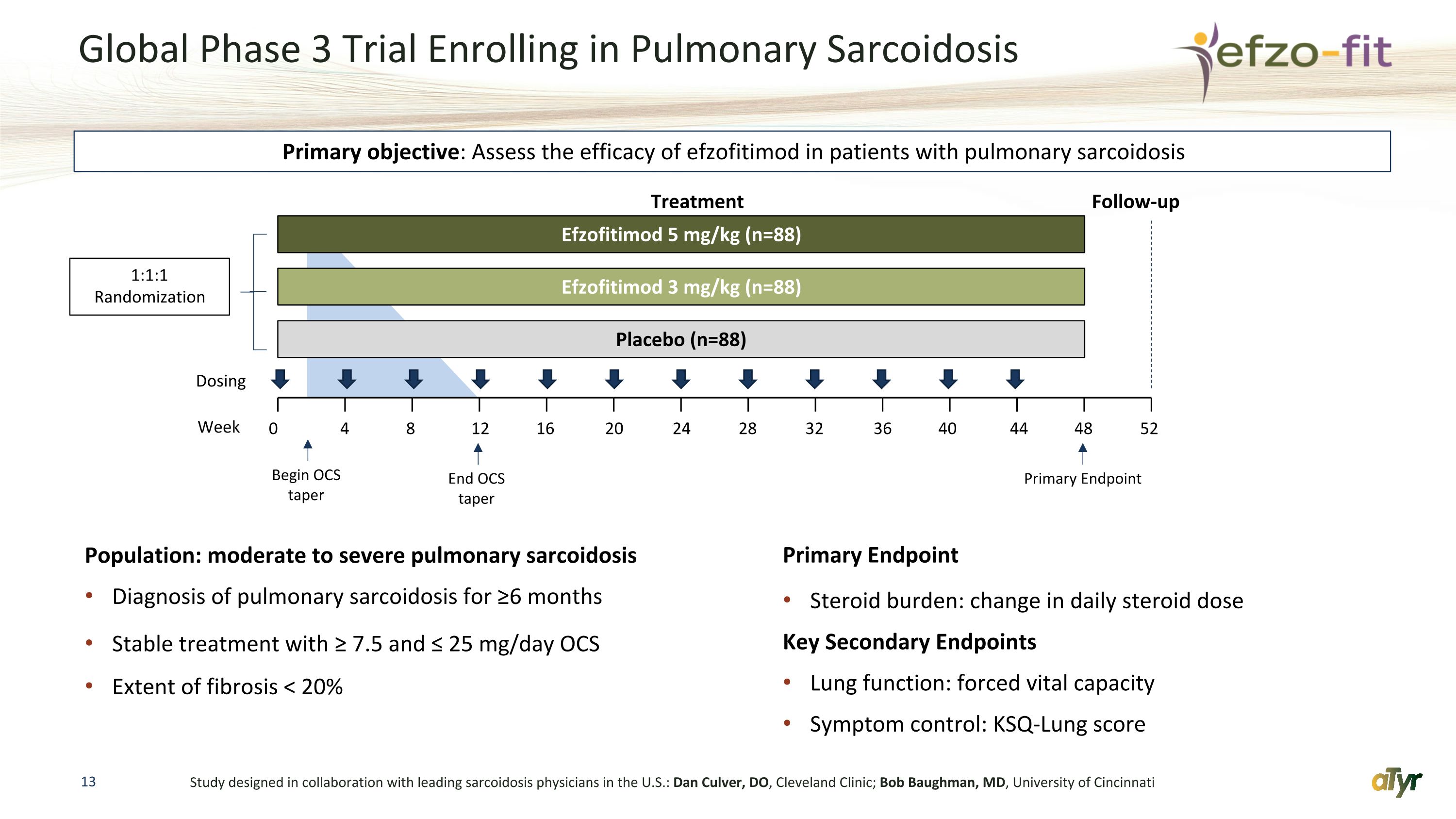
Global Phase 3 Trial Enrolling in Pulmonary Sarcoidosis Population: moderate to severe pulmonary sarcoidosis Diagnosis of pulmonary sarcoidosis for ≥6 months Stable treatment with ≥ 7.5 and ≤ 25 mg/day OCS Extent of fibrosis < 20% Primary Endpoint Steroid burden: change in daily steroid dose Key Secondary Endpoints Lung function: forced vital capacity Symptom control: KSQ-Lung score Study designed in collaboration with leading sarcoidosis physicians in the U.S.: Dan Culver, DO, Cleveland Clinic; Bob Baughman, MD, University of Cincinnati 0 4 8 12 16 20 24 28 Efzofitimod 5 mg/kg (n=88) Efzofitimod 3 mg/kg (n=88) Placebo (n=88) Week Dosing 1:1:1 Randomization Treatment Follow-up Primary objective: Assess the efficacy of efzofitimod in patients with pulmonary sarcoidosis 32 36 40 44 48 52 Begin OCS taper End OCS taper Primary Endpoint
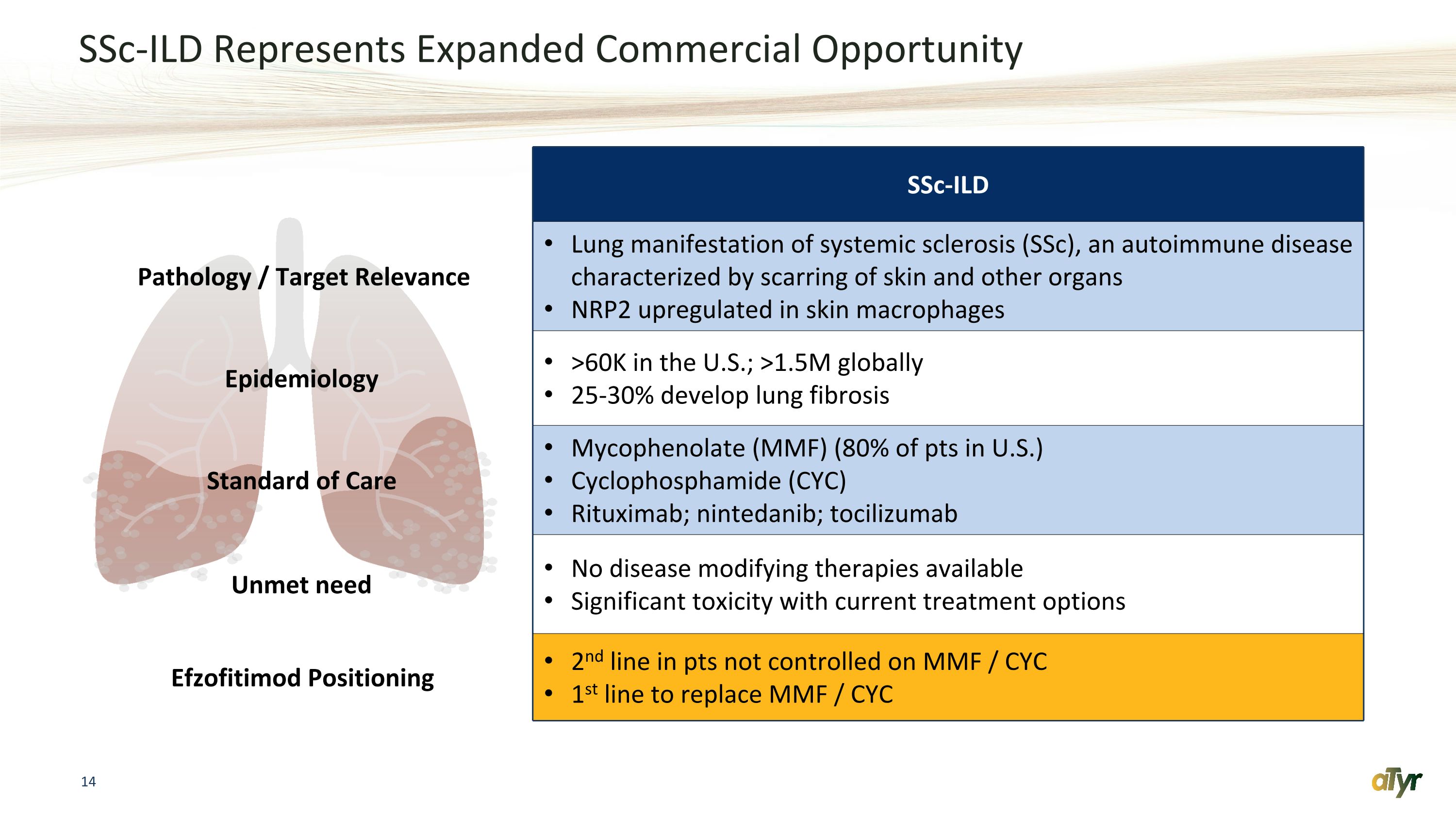
SSc-ILD Represents Expanded Commercial Opportunity SSc-ILD Pathology / Target Relevance Lung manifestation of systemic sclerosis (SSc), an autoimmune disease characterized by scarring of skin and other organs NRP2 upregulated in skin macrophages Epidemiology >60K in the U.S.; >1.5M globally 25-30% develop lung fibrosis Standard of Care Mycophenolate (MMF) (80% of pts in U.S.) Cyclophosphamide (CYC) Rituximab; nintedanib; tocilizumab Unmet need No disease modifying therapies available Significant toxicity with current treatment options Efzofitimod Positioning 2nd line in pts not controlled on MMF / CYC 1st line to replace MMF / CYC
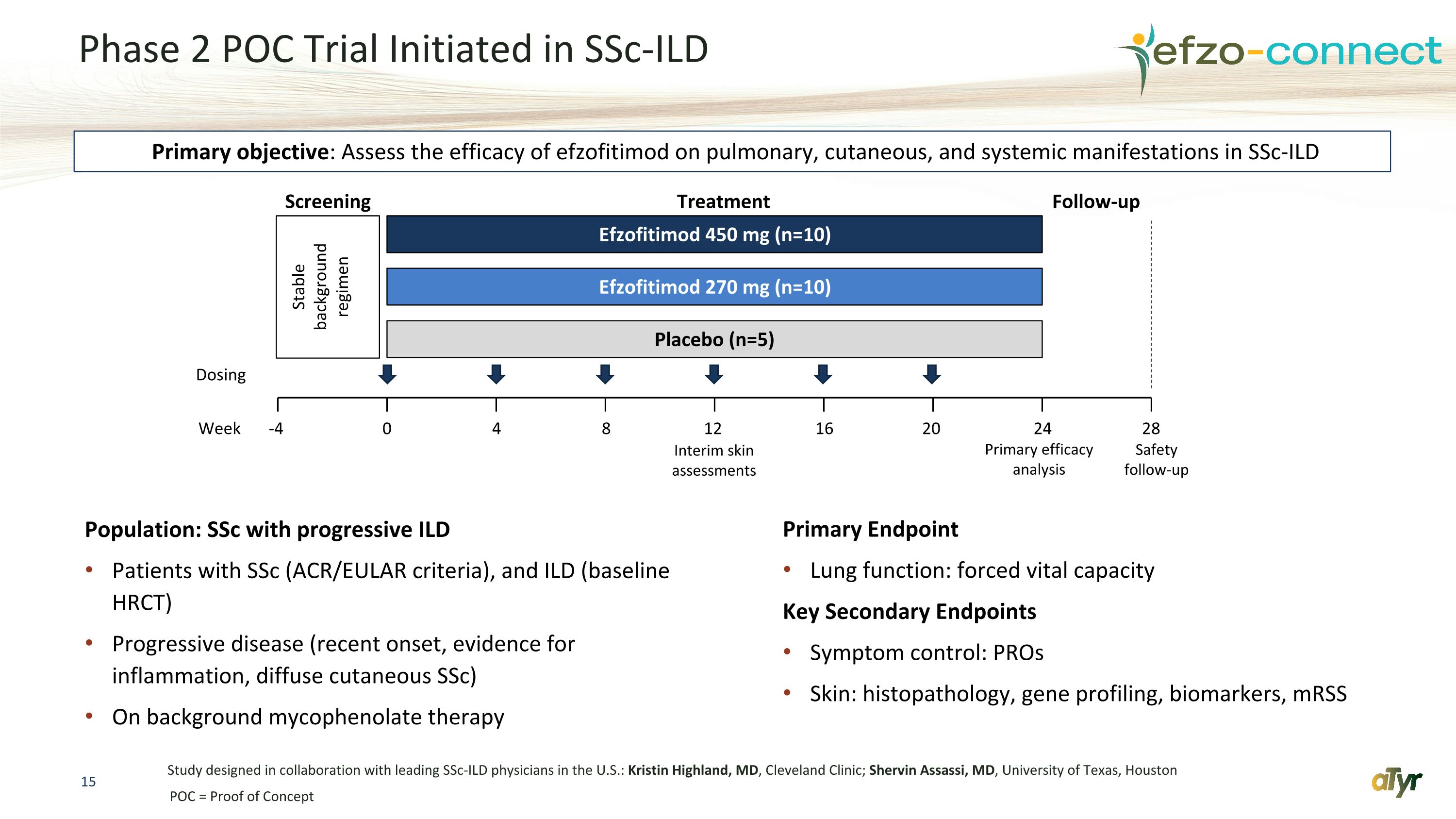
Phase 2 POC Trial Initiated in SSc-ILD Population: SSc with progressive ILD Patients with SSc (ACR/EULAR criteria), and ILD (baseline HRCT) Progressive disease (recent onset, evidence for inflammation, diffuse cutaneous SSc) On background mycophenolate therapy Primary Endpoint Lung function: forced vital capacity Key Secondary Endpoints Symptom control: PROs Skin: histopathology, gene profiling, biomarkers, mRSS Study designed in collaboration with leading SSc-ILD physicians in the U.S.: Kristin Highland, MD, Cleveland Clinic; Shervin Assassi, MD, University of Texas, Houston -4 0 4 8 12 16 20 24 28 Efzofitimod 450 mg (n=10) Efzofitimod 270 mg (n=10) Placebo (n=5) Interim skin assessments Primary efficacy analysis Safety follow-up Week Dosing Stable background regimen Screening Treatment Follow-up Primary objective: Assess the efficacy of efzofitimod on pulmonary, cutaneous, and systemic manifestations in SSc-ILD POC = Proof of Concept
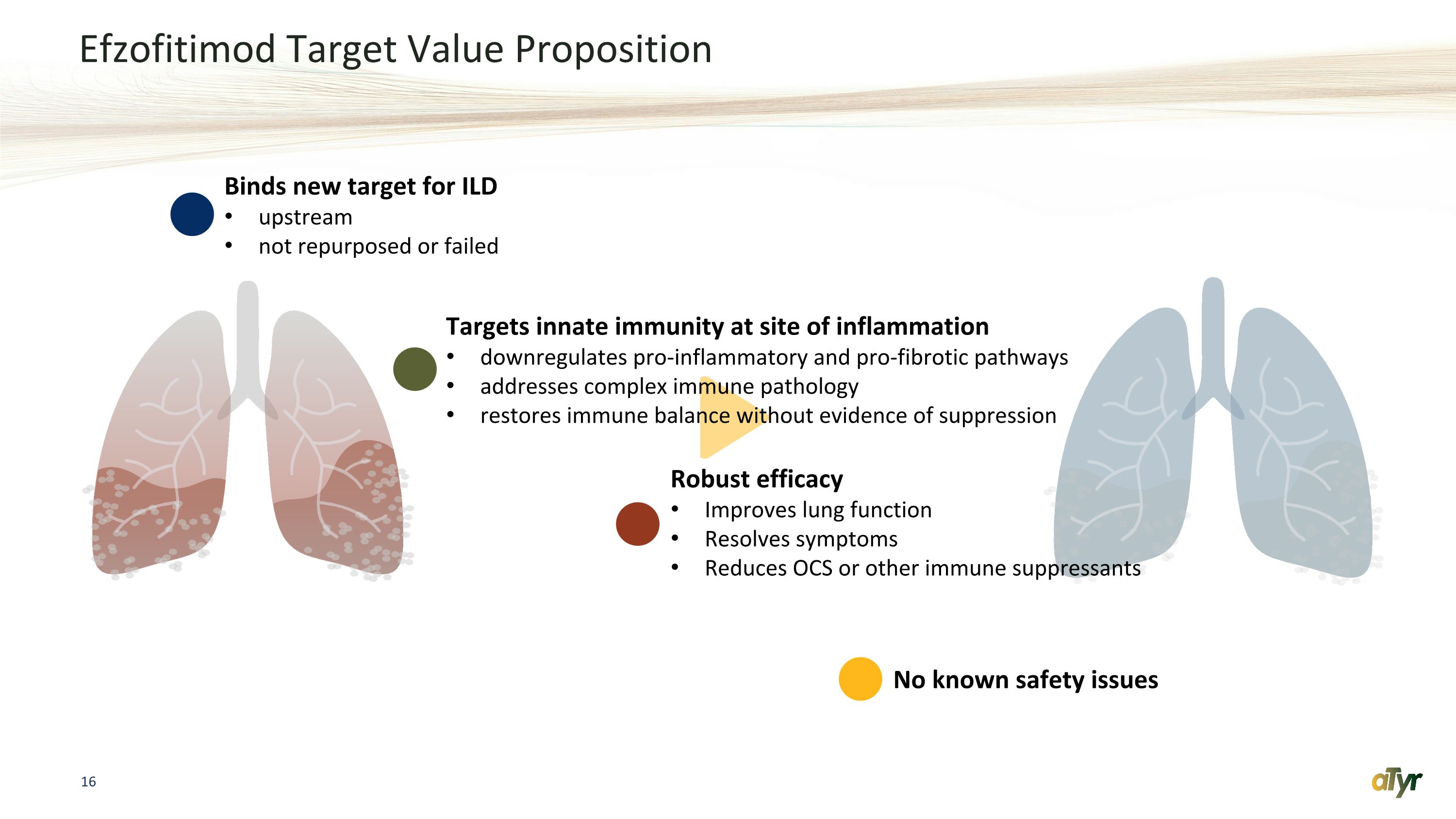
Efzofitimod Target Value Proposition Binds new target for ILD upstream not repurposed or failed Targets innate immunity at site of inflammation downregulates pro-inflammatory and pro-fibrotic pathways addresses complex immune pathology restores immune balance without evidence of suppression Robust efficacy Improves lung function Resolves symptoms Reduces OCS or other immune suppressants No known safety issues

Pre-Clinical Pipeline Generating New Treatments for Inflammation and Fibrosis
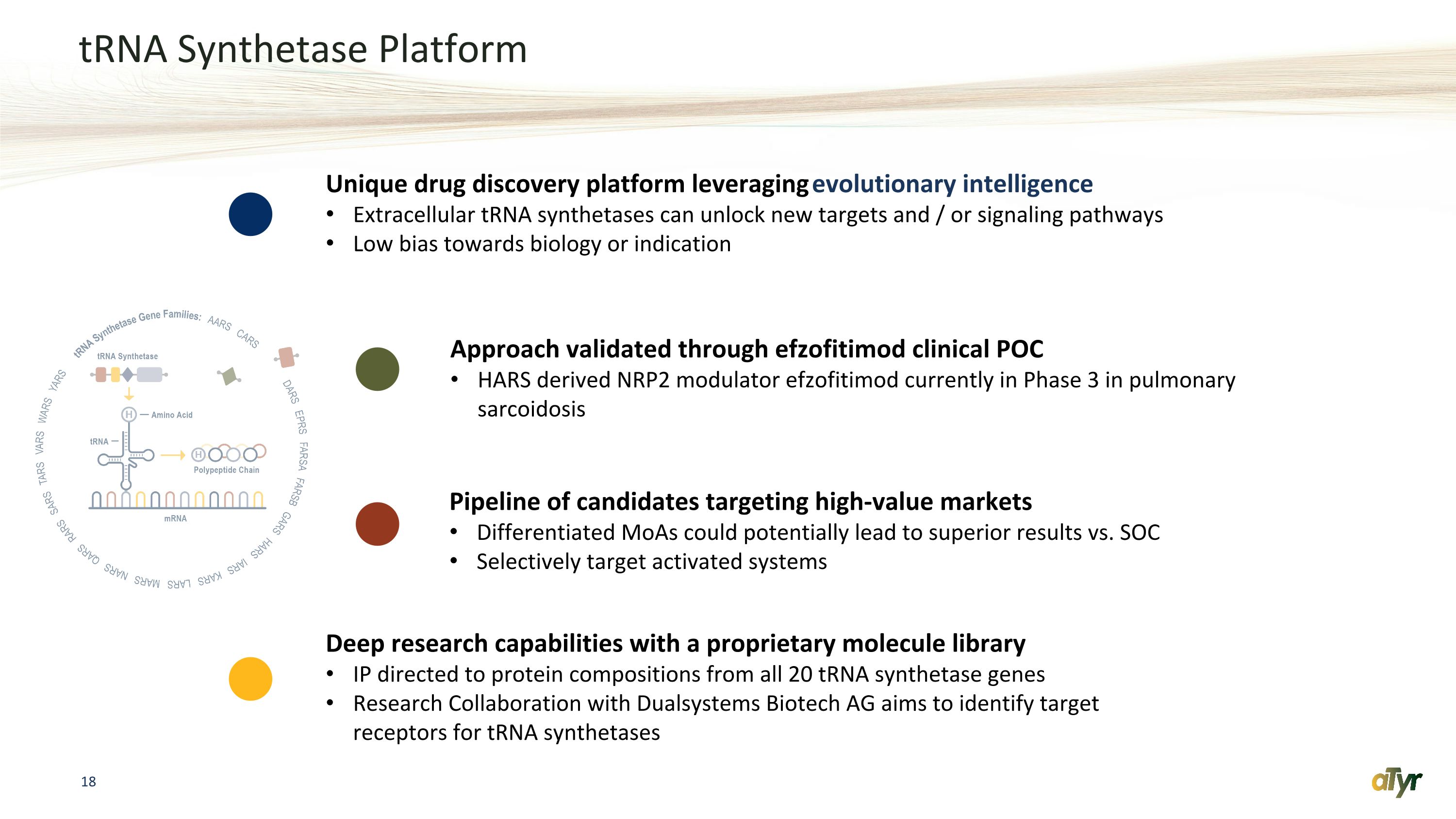
tRNA Synthetase Platform Approach validated through efzofitimod clinical POC HARS derived NRP2 modulator efzofitimod currently in Phase 3 in pulmonary sarcoidosis Unique drug discovery platform leveraging evolutionary intelligence Extracellular tRNA synthetases can unlock new targets and / or signaling pathways Low bias towards biology or indication Pipeline of candidates targeting high-value markets Differentiated MoAs could potentially lead to superior results vs. SOC Selectively target activated systems Deep research capabilities with a proprietary molecule library IP directed to protein compositions from all 20 tRNA synthetase genes Research Collaboration with Dualsystems Biotech AG aims to identify target receptors for tRNA synthetases

Translating tRNA Synthetase Biology into New Therapies for Inflammation and Fibrosis
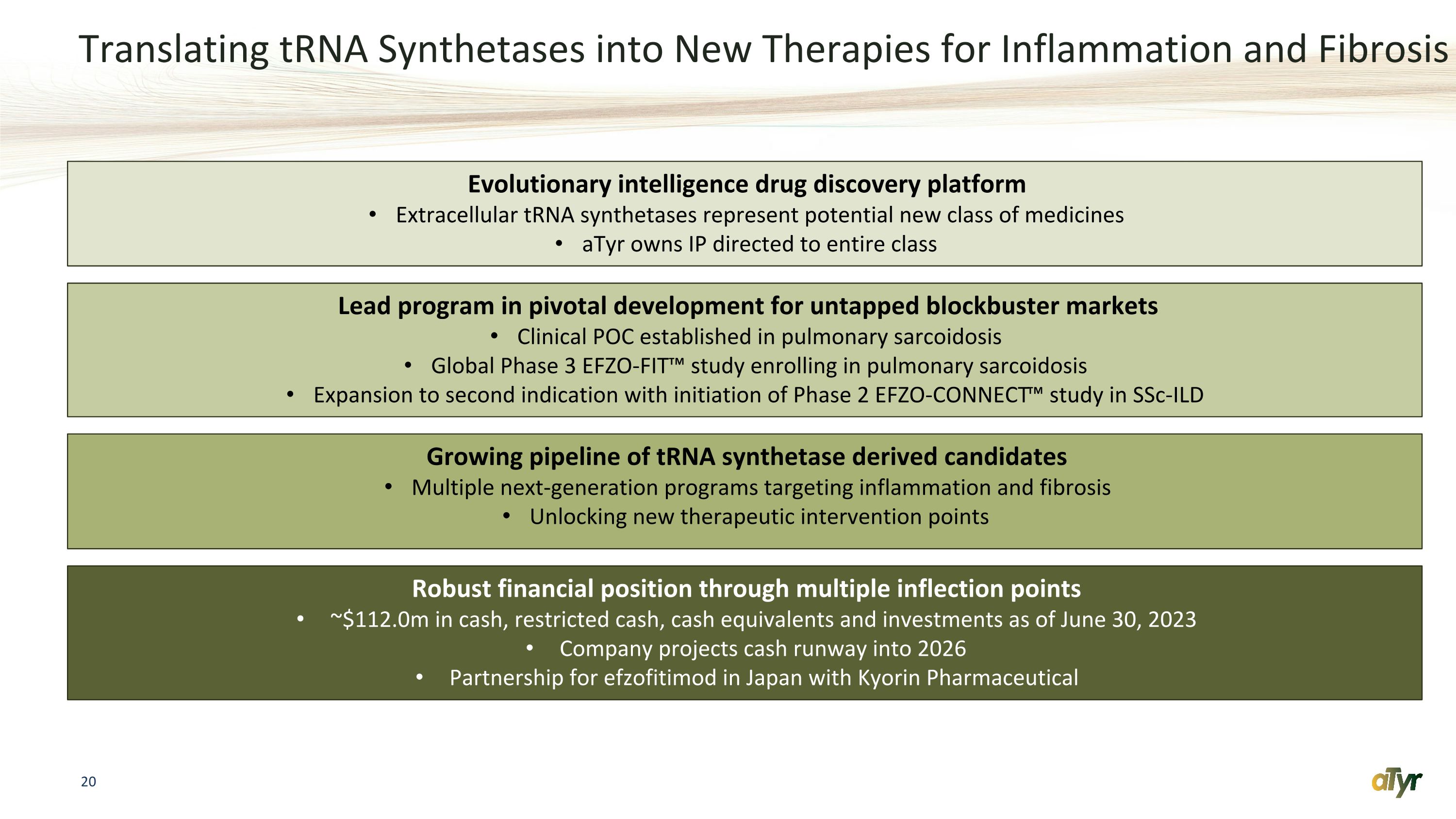
Translating tRNA Synthetases into New Therapies for Inflammation and Fibrosis Evolutionary intelligence drug discovery platform Extracellular tRNA synthetases represent potential new class of medicines aTyr owns IP directed to entire class Lead program in pivotal development for untapped blockbuster markets Clinical POC established in pulmonary sarcoidosis Global Phase 3 EFZO-FIT™️ study enrolling in pulmonary sarcoidosis Expansion to second indication with initiation of Phase 2 EFZO-CONNECT™️ study in SSc-ILD Growing pipeline of tRNA synthetase derived candidates Multiple next-generation programs targeting inflammation and fibrosis Unlocking new therapeutic intervention points Robust financial position through multiple inflection points ~$112.0m in cash, restricted cash, cash equivalents and investments as of June 30, 2023 Company projects cash runway into 2026 Partnership for efzofitimod in Japan with Kyorin Pharmaceutical

Thank You
v3.23.3
Document and Entity Information
|
Sep. 26, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Sep. 26, 2023
|
| Entity Registrant Name |
ATYR PHARMA, INC.
|
| Entity Central Index Key |
0001339970
|
| Entity Emerging Growth Company |
false
|
| Securities Act File Number |
001-37378
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
20-3435077
|
| Entity Address, Address Line One |
10240 Sorrento Valley Road
|
| Entity Address, Address Line Two |
Suite 300
|
| Entity Address, City or Town |
San Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92121
|
| City Area Code |
858)
|
| Local Phone Number |
731-8389
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, par value $0.001 per share
|
| Trading Symbol |
LIFE
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
aTyr Pharma (NASDAQ:LIFE)
Historical Stock Chart
From Mar 2024 to Apr 2024
aTyr Pharma (NASDAQ:LIFE)
Historical Stock Chart
From Apr 2023 to Apr 2024