0001583771
false
0001583771
2023-07-24
2023-07-24
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported):
July 24, 2023
Hepion
Pharmaceuticals, Inc.
(Exact name of registrant as specified in its
charter)
| Delaware | |
001-36856 | |
46-2783806 |
(State or other jurisdiction
of incorporation or organization) |
|
(Commission
File Number) |
|
IRS Employer
Identification No.) |
399
Thornall Street, First Floor
Edison, NJ 08837
(Address of principal executive
offices)
Registrant’s telephone
number, including area code: (732) 902-4000
(Former name or former address, if changed since
last report)
Securities registered pursuant to Section 12(b) of
the Act:
| Title of each class: |
|
Trading Symbol(s) |
|
Name of each exchange on which registered: |
| Common Stock |
|
HEPA |
|
Nasdaq
Capital Market |
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written
communication pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant
is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter)
or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ¨
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure |
Hepion
Pharmaceuticals, Inc. (the “Company”) intends to conduct meetings with third parties in which its corporate slide presentation
will be presented. A copy of the presentation materials is attached as Exhibit 99.1 to this Current Report on Form 8-K and
is incorporated herein by reference.
The information in this
Item 7.01 and the document attached as Exhibit 99.1 is being furnished and shall not be deemed “filed” for purposes of
Section 18 of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), nor otherwise subject to the
liabilities of that section, nor incorporated by reference in any filing under the Securities Act of 1933 or the Exchange Act, except
as shall be expressly set forth by specific reference in such a filing.
SIGNATURE
Pursuant to the requirements
of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto
duly authorized.
Dated: July 24, 2023
| |
|
| |
HEPION PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Robert Foster |
| |
|
Robert Foster |
| |
|
Chief Executive Officer |
Exhibit 99.1

1 1 Rencofilstat (CRV431): A Novel Drug Candidate for NASH, Fibrosis, and HCC July 2023 Creating a Therapeutic Ecosystem NASDAQ: HEPA

2 This presentation may contain forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Such forward - looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words . You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward - looking information . Such statements are only predictions, and our actual results may differ materially from those anticipated in these forward - looking statements . We believe that it is important to communicate future expectations to investors . However, there may be events in the future that we are not able to accurately predict or control . Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger - scale clinical trials, the risk that we will not obtain approval to market our products, risks associated with delays, increased costs and funding shortages caused by the COVID - 19 pandemic ; the risks associated with dependence upon key personnel and the need for additional financing . We do not assume any obligation to update forward - looking statements as circumstances change . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Hepion Pharmaceuticals or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . Statements Forward - Looking

3 Rencofilstat Anti - Fibrotic Drug Candidate • Novel mechanism - cyclophilin inhibition • Once - daily, oral medication – soft gel capsules • Collagen - specific anti - fibrotic • Targets key pathologies including fibrosis, inflammation, cell injury • All clinical trials to date show rencofilstat to be well tolerated – over 425 subjects dosed • Currently undergoing Phase 2 clinical trials Rencofilstat Highlights

4 NASH Fibrotic Liver Disease Leading Indication for Rencofilstat

5 Program Pre - Clinical Phase 1 Phase 2 Phase 3 NASH Fast Track Designation Hepatocellular Carcinoma Orphan Drug Designation Multiple Myeloma Prostate Cancer Idiopathic Pulmonary Fibrosis Renal Fibrosis Cardiac Fibrosis Biopsy Summary of Rencofilstat Programs – ‘Pipeline within a Product’ Liver Function

6 Image adapted from “From NASH to HCC: current concepts and future challenges”, Anstee et al. (2019) NASH is Driving a Healthcare Crisis The Need and Opportunity NAFLD n on - a lcoholic f atty l iver d isease Approx. 25% of global population Up to 100 million in U.S. “Fatty liver” disease associated with obesity, diabetes, hypertension, etc. 1.5 – 6.5% globally Approx. 20 million in U.S. A more severe form of NAFLD, with inflammation and liver scarring (fibrosis) NASH n on - a lcoholic s teato h ep atitis HCC h epato c ellular c arcinoma Most prevalent type (90%) of liver cancer & liver cancer is 2 most common cancer - related death* >905,000 new cases and >830,000 deaths globally* >30,000 new cases annually in U.S.* with 5 - year survival of 18%** *The Global Cancer Observatory (Globocan), December 2020 **UpToDate, February 2022

7 Why Develop a Drug for NASH? • No currently approved drugs for the treatment of NASH • Market for NASH is extremely large (estimated as ~20 million adults in U.S. alone) • NAFLD/NASH may be asymptomatic with no convenient diagnostic to identify subjects early in disease progression • Symptoms may only appear when disease has progressed to the point where disease associated fibrosis is well established • Consequences of NASH may be severe (need for liver transplant, cancer, cardiovascular disease, and death)

8 NASH DISEASE PROGRESSION normal liver cells fatty liver cells inflammation, cell injury, and activation of fibrotic cells production of fibrotic matrix WHY? • Generally poor response rates with drugs in development • Many pathologic mechanisms contribute to disease Most failed drugs and drugs in development are "metabolic" drugs e .g. target liver fat Rencofilstat inhibits collagen Multiple T herapeutic A gents T argeting M ultiple S tages of Disease L ikely Required NASH Therapeutic Strategies RENCOFILSTAT directly targets fibrosis and inflammation

9 Phase 2b and 3 NASH Drug Development Landscape ASK1 inhibitor Galectin - 3 inhibitor LOXL2 inhibitor FASN inhibitor FXR agonist CCR2/5 inhibitor THR β agonist SCD1 inhibitor PPAR agonist FGF21 analog Pegbelfermin 2 Pegozafermin 2 Efruxifermin 2 Lanifibranor 1 Elafibranor 1 Seladelpar 1 Saroglitazar 1 Aramchol 1 Resmetirom 1 ASC41 1 VK2809 1 Cenicriviroc 1 Selonsertib 1 Rencofilstat 1 Belapectin 2 (NASH cirrhosis only) Denifanstat 1 Simtuzumab 2 OCA 1 bile acid synthesis cyclophilin inhibitor Phase 3 Phase 2b Discontinued Suspended 1 Oral dosing 2 Injection/infusion Regulation Inhibition Enhanced omega - 3 fatty acids Icosabutate 1 DGAT2 inhibitor ION224 2 FGF19 analog Aldafermin 2 Incretins and combinations Semaglutide 2 BI 456906 2 Efocipegdutide 2 Tirzepatide 2 mRNA inhibitors ALN - HSD 2 AZD2693 2 BMS - 986263 2 GSK4532990 2 Ursodeoxycholic acid and combinations HTD1801 1 Norursodeoxycholic acid 1 Free fatty acids synthesis, SCD1, FASN Lipotoxicity, Metabolic stress Inflammation, Apoptosis Fibrosis dietary fat caspase inhibitor Emricasan 1 MTOT modulator Azemiglitazone 2

10 Rencofilstat Mode of Action

11 Collagen - Core To Fibrotic Diseases Excessive Collagen Production – Universal to Every Fibrotic Disease » Fibrosis is an exaggerated “scarring” of tissue in response to many types of cellular injuries, chronic inflammation, & other tissue insults » Fibrosis impairs normal organ structure & function » Fibrosis contributes to an estimated 45% of deaths in the developed world* » Collagen molecules are the primary constituents of fibrotic scars *Nature Reviews Drug Discovery 14.10 (2015): 693 - 720

12 Rencofilstat Attenuates Collagen Synthesis by Inhibiting Cyclophilin B Enzyme Activity - "normalizing" disease - associated fibrosis Denotes CypB participation Am J Respir Cell Mol Biol 2022 Apr;66(4):363 - 381. Fighting the Fiber: Targeting Collagen in Lung Fibrosis. Claudia A Staab - Weijnitz Cyclophilin B is one of the most active enzymes involved in the proper folding, synthesis, secretion, and eventual crosslinking of collagen that forms the fibrotic matrix. Rencofilstat potently inhibits cyclophilin B and thereby decreases collagen production and fibrosis.

13 Cyclophilin Inhibition Produces Consistent Antifibrotic Effects Preclinical Models: Proof of Concept » Liver: 8 NASH - related models Human liver slices » Lung Chronic fibrosis Acute injury Human lung slices » Kidney Acute injury » Heart » Skin Antifibrotic Activity Observed in Every Preclinical Model
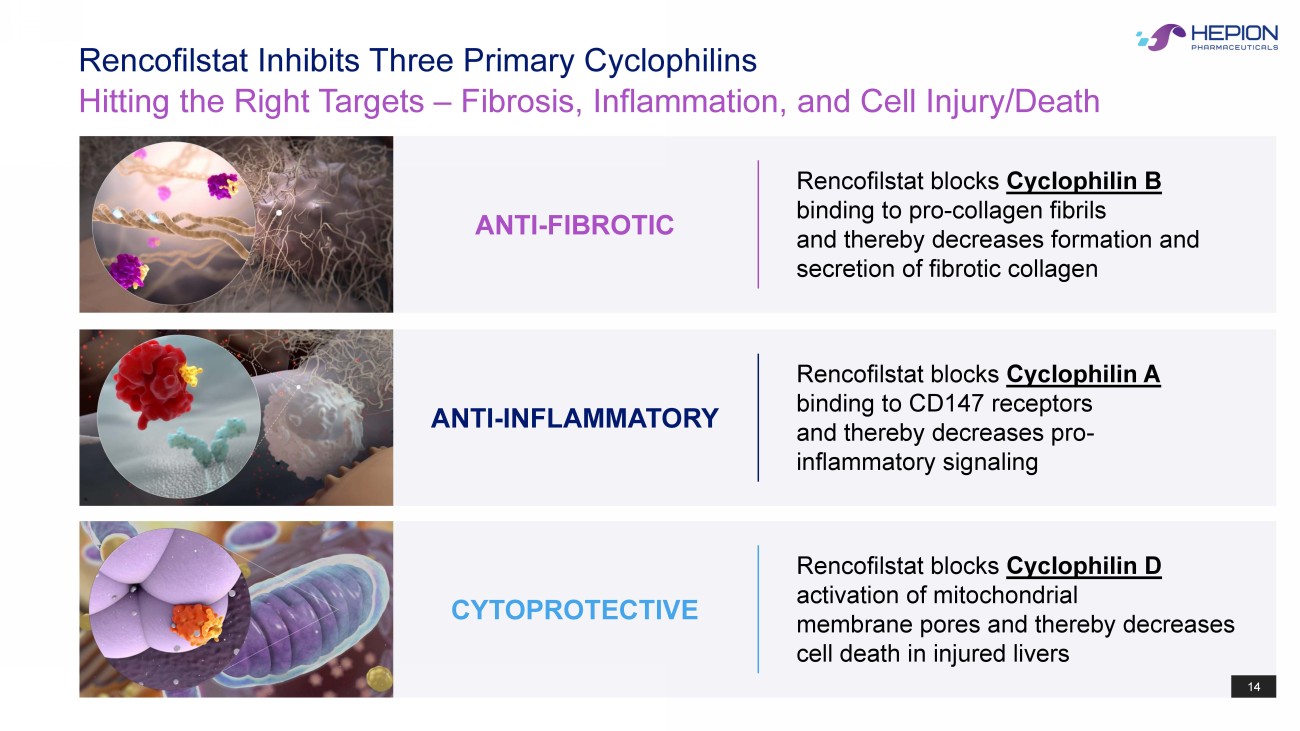
14 Hitting the Right Targets – Fibrosis, Inflammation, and Cell Injury/Death Rencofilstat Inhibits Three Primary Cyclophilins Rencofilstat blocks Cyclophilin D activation of mitochondrial membrane pores and thereby decreases cell death in injured livers CYTOPROTECTIVE 14 Rencofilstat blocks Cyclophilin A binding to CD147 receptors and thereby decreases pro - inflammatory signaling ANTI - INFLAMMATORY Rencofilstat blocks Cyclophilin B binding to pro - collagen fibrils and thereby decreases formation and secretion of fibrotic collagen ANTI - FIBROTIC

15 Overview of Phase 1 Studies (Completed)

16 Key Findings Phase 1 Studies Completed – D emonstrated a Favorable S afety P rofile » No serious adverse events » No adverse events with dose response » Effective t 1/2 ~ 30 hours » Tmax ss ~ 1 – 2 hours » Ketoconazole increased rencofilstat concentrations ~ 5 - fold » Rencofilstat had no effect on midazolam exposure » Rencofilstat absorption was not decreased with high fat meal (AUC increased 18%) Single Ascending Dose (75 - 525mg) Multiple Ascending Dose ( 75 – 375mg) Drug - Drug Interaction (midazolam & ketoconazole) Food Effect (high fat meal)

17 Overview of Phase 2a ‘AMBITION’ NASH Trial (Completed)
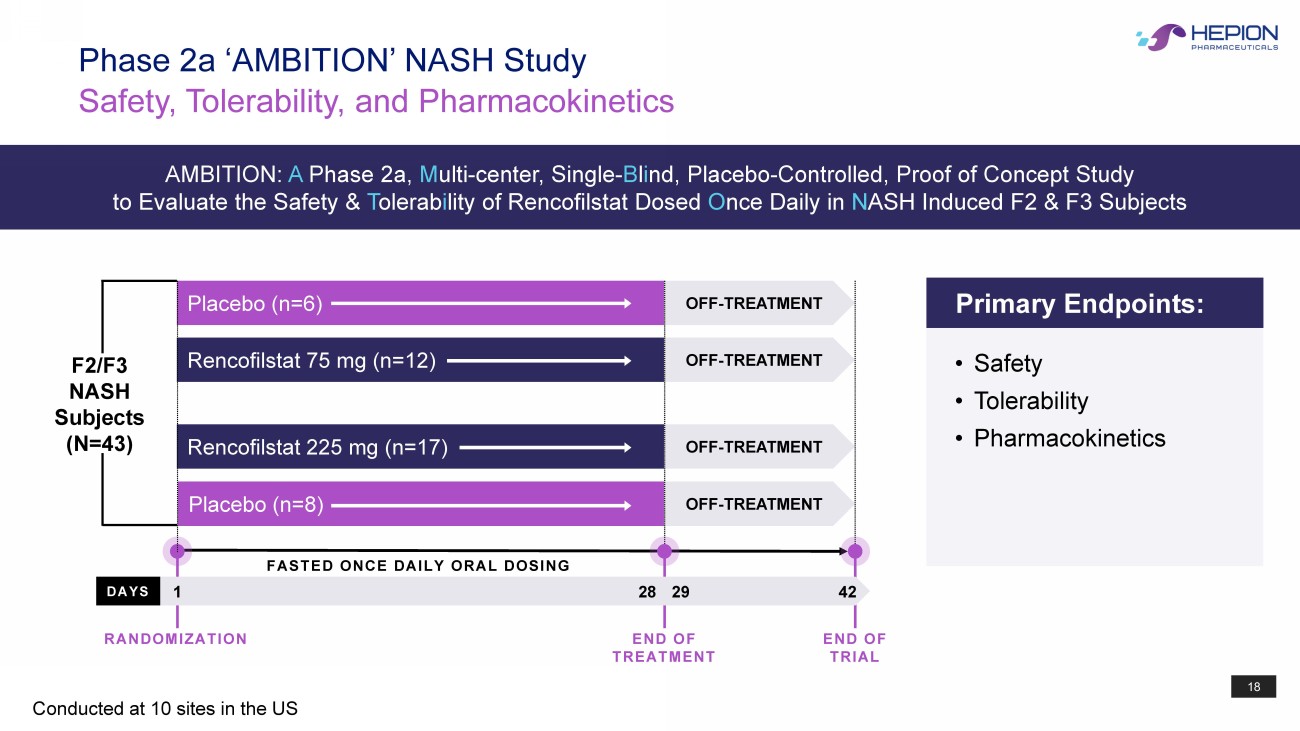
18 F2/F3 NASH Subjects (N=43) Rencofilstat 225 mg (n=17) Placebo (n=8) Placebo (n=6) Rencofilstat 75 mg (n=12) OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT DAYS FASTED ONCE DAILY ORAL DOSING Safety, Tolerability, and Pharmacokinetics Phase 2a ‘AMBITION’ NASH Study Conducted at 10 sites in the US 1 28 42 Primary Endpoints: • Safety • Tolerability • Pharmacokinetics RANDOMIZATION END OF TREATMENT END OF TRIAL 29 AMBITION: A Phase 2a, M ulti - center, Single - B l i nd, Placebo - Controlled, Proof of Concept Study to Evaluate the Safety & T olerab i lity of Rencofilstat Dosed O nce Daily in N ASH Induced F2 & F3 Subjects

19 Rencofilstat demonstrated a favorable safety profile Efficacy signals were observed in only 28 days including: • Reduction in ALT (marker of inflammation & fibrosis) • Reduction in Pro - C3 (marker of fibrosis) • Downregulation of collagen genes • Upregulation of genes associated with liver recovery and favorable lipid dynamics Early evidence of a concentration - effect relationship was observed with both ALT and Pro - C3 Rencofilstat concentrations are not significantly altered by NASH Rencofilstat concentrations expected to be effective in NASH endpoints (ALT and Pro - C3) were achieved All Primary Endpoints Met Phase 2a ‘AMBITION’ NASH Study

20 Hepion’s Proprietary Artificial Intelligence

21 Right Patient Right Dose The Hepion AI - POWR Proprietary Clinical Process All Data Generative Neural Net Deep Convoluted Neural Net INPUT OUTPUT

22 The Hepion AI - POWR Proprietary Clinical Process Pre - Clinical Data Informs AI/ML for Clinical Development • Biomarkers • Patient Selection • Simulation 1. AI/ML pop PKPD 2. AI/ML QSP 3. AI/ML PBPK AI - POWR PKPD/QSP/PBPK + Multi - omics + QoL + Safety + Deep Learning AI = Digital Biomarkers Responders • Responder Identification • Phase III Enrichment • Predict Clinical Outcome | | | | | | | | | | | | | Deep Learning Individual Response Model Drug - Disease Interaction Model • Digital Biomarkers • Eliminate Biopsy • Patient Specific Dosing • Clinical Monitoring • Reimbursement Identify New Therapeutics via Multi - Omic Data Base Mining Generate Synthetic Data for Patient & Outcome Simulation AI - POWR Œ Allows for Validation Comparisons 1. AI/ML included within developmental processes 2. AI/ML overarching input to outcome Preclinical Phase I Phase II Phase III Clinical Practice

23 Illustration of Hepion’s AI - POWR w for Rencofilstat (RCF) * Genes (number) Predictive Genes (number) AUROC Comment 1733 Statistically Significant 25 0.97 Highly Predictive for ProC3 Response Lipids (number) Predictive Lipids (number) AUROC Comment 443 Statistically Significant 25 0.74 Highly Predictive for ProC3 Response Clinical Labs AUROC Comment 443 Statistically Significant 0.56 Poorly Predictive for ProC3 Response * Key genes identified demonstrate RCF – CypA and B interaction in NASH subjects ProC3 reduction (analogous to Fibrosis Score Response) associated with RCF blood concentrations of: • 964.2 ng/mL (trough) • 1160 ng/mL (2 - hour) Efficacious blood concentrations attained by day 14 and day 1 for 75 and 225 mg RCF, respectively, suggesting a third dosing cohort of 150 mg in future trials

24 Phase 2 ‘ALTITUDE - NASH’ Trial (Clinically Complete)

25 • Subjects identified from historical biopsy or by meeting AGILE 3+ criteria for F3 • Subjects who complete study will be considered for enrollment into ASCEND - NASH 2b Phase 2 ‘ALTITUDE - NASH’ (Liver Function Trial) Primary Objective: Evaluate the change in hepatic function with once daily (QD) 75 mg, 150 mg, and 225 mg doses of rencofilstat in subjects with NASH F3 fibrosis using the HepQuant SHUNT test. Rencofilstat 225 mg qd (n=20) Rencofilstat 75 mg qd (n=20) Rencofilstat 150 mg qd (n=20) RANDOMIZED 1:1:1 OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT DAYS 1 120 134 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | F3 NASH Patients (N=60) 60 | | | | | | | | | | | | | | | Endpoints: • Efficacy: - HepQuant SHUNT - NASH NIMs • Safety • Tolerability • Pharmacokinetics
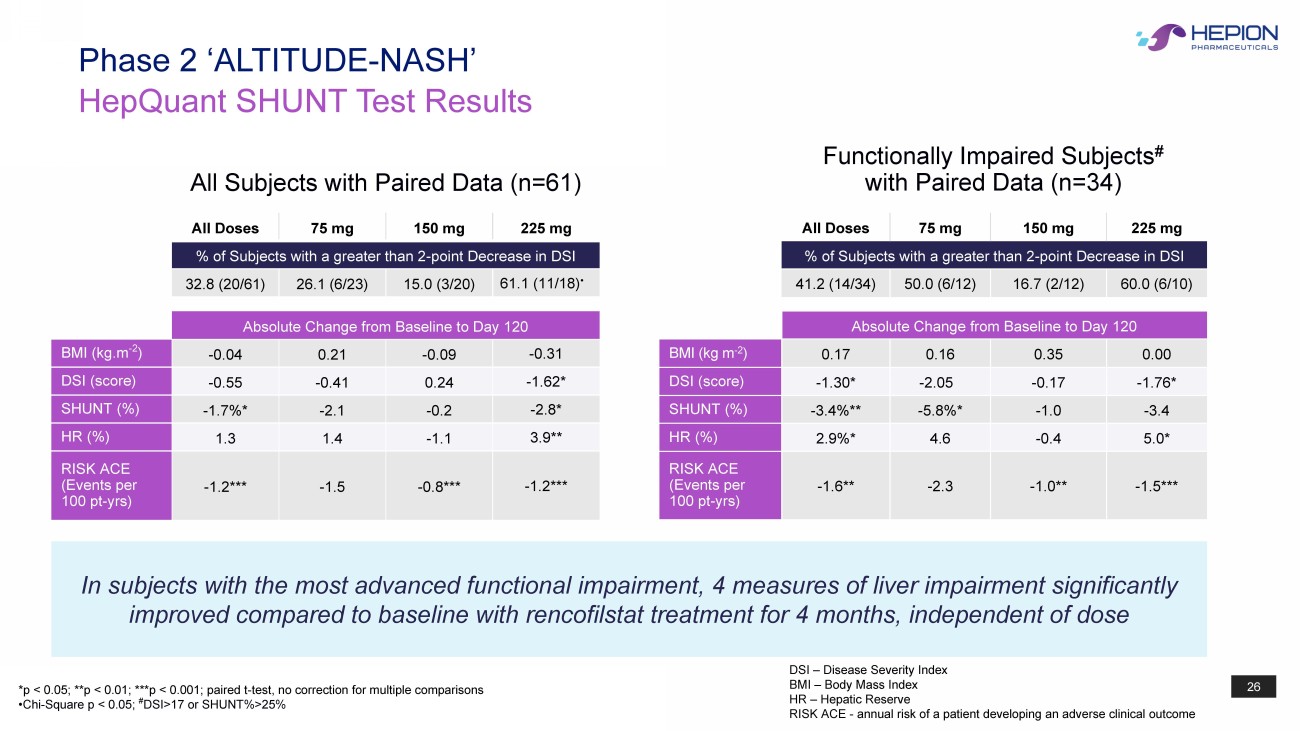
26 HepQuant SHUNT Test Results Phase 2 ‘ALTITUDE - NASH’ All Doses 75 mg 150 mg 225 mg % of Subjects with a greater than 2 - point Decrease in DSI 32.8 (20/61) 26.1 (6/23) 15.0 (3/20) 61.1 (11/18) • Absolute Change from Baseline to Day 120 BMI (kg.m - 2 ) - 0.04 0.21 - 0.09 - 0.31 DSI (score) - 0.55 - 0.41 0.24 - 1.62* SHUNT (%) - 1.7%* - 2.1 - 0.2 - 2.8* HR (%) 1.3 1.4 - 1.1 3.9** RISK ACE (Events per 100 pt - yrs ) - 1.2*** - 1.5 - 0.8*** - 1.2*** All Doses 75 mg 150 mg 225 mg % of Subjects with a greater than 2 - point Decrease in DSI 41.2 (14/34) 50.0 (6/12) 16.7 (2/12) 60.0 (6/10) Absolute Change from Baseline to Day 120 BMI (kg m - 2 ) 0.17 0.16 0.35 0.00 DSI (score) - 1.30* - 2.05 - 0.17 - 1.76* SHUNT (%) - 3.4%** - 5.8%* - 1.0 - 3.4 HR (%) 2.9%* 4.6 - 0.4 5.0* RISK ACE (Events per 100 pt - yrs ) - 1.6** - 2.3 - 1.0** - 1.5*** In subjects with the most advanced functional impairment, 4 measures of liver impairment significantly improved compared to baseline with rencofilstat treatment for 4 months, independent of dose *p < 0.05; **p < 0.01; ***p < 0.001; paired t - test, no correction for multiple comparisons •Chi - Square p < 0.05; # DSI>17 or SHUNT%>25% All Subjects with Paired Data (n=61) Functionally Impaired Subjects # with Paired Data (n=34) DSI – Disease Severity Index BMI – Body Mass Index HR – Hepatic Reserve RISK ACE - annual risk of a patient developing an adverse clinical outcome

27 All Subjects % Change From Baseline Subjects with Baseline ProC3 ≥ 37.5 ng/ml % Change From Baseline 75 mg rencofilstat n=23 150 mg rencofilstat n=21 225 mg rencofilstat n=21 75 mg rencofilstat n=10 150 mg rencofilstat n=7 225 mg rencofilstat n=6 ALT - 3.37 *,**** - 13.01 *,** - 21.63 *,** - 13.24 *,*** - 32.24 * - 37.78 *,** AST 4.54 *,** - 8.64 *,** 4.68 * 6.73 *,**** - 30.72 **** - 11.34 *,** ProC3 - 6.47 - 11.12 * - 9.58 *,**** - 3.39 **** - 17.05 **** - 16.23 *,**** PIIINP 2.75 - 0.47 * - 5.6 * - 1.22 **** - 7.36 *,** - 21.48 *,** TIMP1 3.76 30.5 - 3.9 2.4 - 6.69 * - 4.77 * Hyaluronic acid 11.67 - 13.18 * - 10.67 * - 4.56 *,**** 6.99 *,** - 19.66 *,** ELF score 1.03 **** 3.85 *,** - 2.51 *,** - 0.95 *,**** - 1.64 *,** - 5.31 *,** Key NASH Non - Invasive Markers (NIMs) Results Phase 2 ‘ALTITUDE - NASH’ Rencofilstat 225 mg after 4 months of treatment in the high - risk population led to the greatest improvements in NASH biomarkers *Different from Baseline p < 0.001, Friedman ANOVA; **Different from 75 mg Dose p< 0.01; ***Different from 150 mg Dose; ****All Doses p < 0.001. ALT – alanine transferase AST – aspartate transaminase ProC3 – procollagen 3 C - terminal peptide PIIINP – procollagen 3 N - terminal peptide TIMP1 – tissue inhibitor of metalloproteinase – 1 ELF – enhanced liver fibrosis

28 Phase 2b ‘ASCEND - NASH’ Trial (Enrolling)

29 Phase 2b ASCEND - NASH (Biopsy Trial) Primary Objective: Evaluate the efficacy and safety of once - daily 75mg, 150 mg, and 225 mg doses of Rencofilstat compared to placebo in subjects with biopsy proven NASH and stage 2 liver fibrosis (F2) / stage 3 liver fibrosis (F3) Rencofilstat 225 mg qd (n=84) Placebo (n=84) Rencofilstat 75 mg qd (n=84) Rencofilstat 150 mg qd (n=84) RANDOMIZED 1:1:1:1 OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT OFF - TREATMENT MONTHS 1 12 13 | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | INTERIM ANALYSIS | | | | | | | | | | | | | | | | | RANDOMIZATION Data to inform phase 3 trial patient selection and biomarkers for analysis. AI - POWR identification of biomarkers of Rencofilstat response F2/F3 NASH Patients (N=336 ) • Power = 90% to distinguish outcomes at each dosing level • FDA feedback obtained – study design endorsed • Blinded interim analyses when 34 subjects in each cohort reach day 180 and when 56 subjects in each cohort reach day 365

30 Phase 2b ASCEND - NASH Primary Efficacy Endpoint: Superiority of rencofilstat compared to placebo on liver histology at month 12 relative to the screening biopsy, by assessing the proportion of subjects with improvement in fibrosis by at least 1 stage (NASH CRN system) OR NASH resolution without worsening of fibrosis Secondary Efficacy Endpoints: Superiority of rencofilstat compared to placebo on histology at month 12 relative to screening by assessing the proportion of subjects with improvement in fibrosis by: • At least 1 stage regardless of effect on NASH • At least 2 stages regardless of effect on NASH • At least 2 stages AND no worsening of NASH.

31 Phase 2a HCC Trial (Pending)

32 Objectives: » Safety & tolerability » Efficacy: » Disease Control Rate » Duration of response » Overall survival » Objective response rate » 4 - month progression free survival » Pharmacokinetics Safety, Tolerability, Pharmacokinetics, and Efficacy PHASE 2a: Advanced Hepatocellular Carcinoma (HCC) A Phase 2a, Open - Label, Multi - Center, Simon 2 - Stage Study to Assess Preliminary Efficacy, Safety, and Pharmacokinetics of 2 Dosage Levels in Advanced Metastatic Resistant or Refractory HCC Subjects *Administration of r encofilstat may continue until disease progression according to RECIST version 1.1 WEEKS 1 16* 52 END OF STAGE 1 END OF TRIAL RANDOMIZATION Once Daily Oral Dosing Efficacy & Safety Analyses • Tumour measurements • Incidence rates • Safety • Tolerability Rencofilstat 150 mg (n= 21 ) Rencofilstat 225mg (n=21) Stage 2: HCC Subjects (N=42 total) Interim Analysis & Safety Review If ≤ 1 subject achieves DCR: May stop for futility OR If ≥ 2 subjects achieves DCR: Stage 2 Rencofilstat 150 mg (n=8) Rencofilstat 225 mg (n= 8 ) | | | | | | | | | | | | | | | | | | | | | | | | | |

33 Intellectual Property

34 Long Patent Life with Patent Term Extensions (PTE) Intellectual Property Position Family Status Notes Composition of Matter 54 US & International Issued Patents December 2031 Expiry (December 2036 Expiry with PTE) Assuming 2028 and 2029 NDA submission and approval Composition of Matter (Solid State) Filed Formulation 23 US & International Applications Filed; EU Granted (28 countries) November 2039 Expiry (May 2043 Expiry with PTE) Assuming 2023 patent grant and 2028 and 2029 NDA submission and approval Method of Use, Treating Fibrosis 23 US & International Applications Filed February 2041 Expiry + PTE Method of Use, Treating Cancer 23 US & International Applications Filed February 2041 Expiry + PTE Method of Use, Antithrombotic Agents US & PCT Applications Filed December 2041 Expiry + PTE Manufacturing Filed

35 Summary

36 $ 43.0 M Cash as of 03/31/23 Financials Two Value Drivers Tackling Fibrotic Diseases • Rencofilstat , once - daily oral, targeting key drivers of pathology, tested in over 425 subjects • Two Phase 2 NASH trials: • ALTITUDE - NASH – Clinically Complete • ASCEND - NASH Phase 2b – Recruiting • Upcoming Phase 2 for HCC • Developing companion A.I. for clinical development and commercialization strategy • Core scientific team discovered and developed voclosporin (currently marketed) • Robust IP 3.8 M Common Shares Outstanding 36

37 Patrick Mayo, PhD SVP, Clinical Pharmacology and Analytics Joined Isotechnika ( Aurinia ) in 2002 and joined HEPA in 2019. Robert Foster, PharmD, PhD CEO Founded Isotechnika ( Aurinia ) in 1993, and served mostly as CEO & Chairman, until 2014. Joined HEPA in 2016 as CSO and became CEO in 2018. John Cavan, MBA CFO Formerly of Pine Hill, Stemline , Aegerion, AlgoRx , Alpharma , Sony, American Express and International Specialty Products, joined HEPA in 2016. Todd Hobbs, MD CMO Formerly Chief Medical Officer of Novo Nordisk, joined HEPA in 2021. Launa Aspeslet , PhD COO Formerly COO of Isotechnika ( Aurinia ) from 1996 - 2013. Was CEO of an oncology CRO from 2013 until joining HEPA in 2022. Daren Ure , PhD CSO Joined Isotechnika ( Aurinia ) in 2003 and joined HEPA in 2016. Daniel Trepanier, PhD SVP, Drug Development Joined Isotechnika ( Aurinia ) in 1997 and joined HEPA in 2016. Core R&D team has collectively > 120 yrs of experience with cyclophilin inhibition drug development, most notably voclosporin ( Lupkynis ®) for lupus nephritis while at Isotechnika ( Aurinia , NASDAQ:AUPH) Experienced Team

CONTACT US Hepion Pharmaceuticals Inc. 399 Thornall Street, First Floor Edison, New Jersey, USA, 08837 Email: info@hepionpharma.com Phone: 732 - 902 - 4000 www.hepionpharma.com
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Hepion Pharmaceuticals (NASDAQ:HEPA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Hepion Pharmaceuticals (NASDAQ:HEPA)
Historical Stock Chart
From Apr 2023 to Apr 2024