Report of Foreign Issuer (6-k)
February 14 2018 - 8:35AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
6-K
REPORT OF
FOREIGN PRIVATE ISSUER
PURSUANT TO RULE
13a-16
OR
15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of February 2018
Commission File Number:
001-36697
DBV TECHNOLOGIES S.A.
(Translation of registrant’s name into English)
177-181
avenue Pierre Brossolette
92120 Montrouge France
(Address of principal executive office)
Indicate by check mark whether
the registrant files or will file annual reports under cover of Form
20-F
or Form
40-F:
☒ Form
20-F ☐ Form
40-F
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(7): ☐
EXHIBIT LIST
|
|
|
|
|
Exhibit
|
|
Description
|
|
|
|
|
99.1
|
|
Press Release dated February 14, 2018.
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DBV TECHNOLOGIES S.A.
|
|
|
|
|
|
|
Date: February 14, 2018
|
|
|
|
By:
|
|
/s/ David Schilansky
|
|
|
|
|
|
|
|
Name
|
|
David Schilansky
|
|
|
|
|
|
|
|
Title:
|
|
Deputy Chief Executive Officer
|

Press Release
Montrouge, France, February 14, 2018
DBV
Technologies Provides Update on Regulatory Progress for Viaskin Peanut
DBV Technologies (Euronext: DBV – ISIN: FR0010417345 – Nasdaq Stock
Market: DBVT) today announced that the U.S. Food and Drug Administration (FDA) has agreed that the available efficacy and safety data for Viaskin Peanut supports the submission of a Biologics License Application (BLA) for the treatment of peanut
allergy in children four to 11 years of age.
The FDA provided written responses to the clinical
pre-BLA
meeting
package submitted by the Company, which reflect agreement on the content of the clinical module of the BLA for Viaskin Peanut. DBV remains on track to submit its BLA in the second half of 2018.
“We are pleased with this positive step forward in our progress towards potential approval of Viaskin Peanut, and appreciate the feedback we received
from the FDA supporting submission of our BLA,”
said
Dr.
Pierre-Henri
Benhamou
,
Chairman & Chief Executive Officer of DBV
Technologies
. “There are
approximately one million children in the U.S. diagnosed with this life-threatening disease, and we look forward to continue working with the agency to address this urgent unmet medical need.”
About DBV Technologies
DBV Technologies is developing
Viaskin
®
, a proprietary technology platform with broad potential applications in immunotherapy. Viaskin is based on epicutaneous immunotherapy, or EPIT
®
, DBV’s method of delivering biologically active compounds to the immune system through intact skin. With this new class of self-administered and
non-invasive
product candidates, the company is dedicated to safely transforming the care of food allergic patients, for whom there are no approved treatments. DBV’s food allergies programs include
ongoing clinical trials of Viaskin Peanut and Viaskin Milk, and preclinical development of Viaskin Egg. DBV is also pursuing a human
proof-of-concept
clinical study of
Viaskin Milk for the treatment of Eosinophilic Esophagitis, and exploring potential applications of its platform in vaccines and other immune diseases. DBV Technologies has global headquarters in Montrouge, France and New York, NY. Company shares
are traded on segment A of Euronext Paris (Ticker: DBV, ISIN code: FR0010417345), part of the SBF120 index, and traded on the Nasdaq Global Select Market in the form of American Depositary Shares (each representing
one-half
of one ordinary share) (Ticker: DBVT). For more information on DBV Technologies, please visit our website:
www.dbv-technologies.com
Forward Looking Statements
This press release may
contain forward-looking statements and estimates, including statements regarding the potential of Viaskin Peanut and the regulatory posture of Viaskin Peanut. These forward-looking statements and estimates are not promises or guarantees and involve
substantial risks and uncertainties. At this stage, the products of the Company have not been authorized for sale in any country. Among the factors that could cause actual results to differ materially from those described or projected herein include
uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals and the risk that historical clinical results in one patient population may not be predictive of future clinical trial
results in different patient populations. A further list and description of these risks, uncertainties and other risks can be

found in the Company’s regulatory filings with the French Autorité des Marchés Financiers, the
Company’s Securities and Exchange Commission filings and reports, including in the Company’s Annual Report on Form
20-F
for the year ended December 31, 2016 and future filings and reports by the
Company. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements and estimates, which speak only as of the date hereof. Other than as required by applicable law, DBV Technologies undertakes no
obligation to update or revise the information contained in this Press Release.
DBV Investor Relations Contact
Sara Blum Sherman
Senior Director, Investor
Relations & Strategy
+1 212-271-0740
sara.sherman@dbv-technologies.com
DBV Media Contact
Roberta Di Giorgio
Senior Director, Corporate Communications
+1 917-612-2861
roberta.digiorgio@dbv-technologies.com
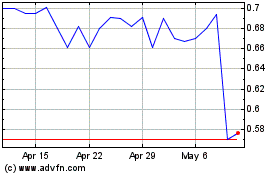
DBV Technologies (NASDAQ:DBVT)
Historical Stock Chart
From Aug 2024 to Sep 2024
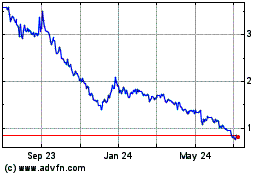
DBV Technologies (NASDAQ:DBVT)
Historical Stock Chart
From Sep 2023 to Sep 2024