First Wave BioPharma and Celiac Journey to Host Nasdaq Event in Support of Celiac Disease Awareness Month
May 09 2024 - 7:00AM

First Wave BioPharma, Inc., (NASDAQ: FWBI), (“First Wave BioPharma”
or the “Company”), a clinical-stage biopharmaceutical company
specializing in the development of targeted, non-systemic therapies
for gastrointestinal (GI) diseases, today announced that in
honor of Celiac Disease Awareness Month in May, it will partner
with Celiac Journey, a celiac disease patient advocacy
organization, to share an awareness digital display on the Nasdaq
Tower in Times Square on Thursday, May 16, at 4:25 p.m. ET. The
digital display will also be shown at night as part of the
international “Shine a Light on Celiac Disease” initiative to
illuminate famous landmarks in green. First Wave BioPharma will
also host a photo event at the Nasdaq Market Site featuring celiac
disease advocates and experts who will share their lived
experiences with celiac disease.
“Celiac disease is a topical issue at the
forefront of many people’s lives,” said James Sapirstein, Chairman
and Chief Executive Officer of First Wave BioPharma. “Often
overlooked is the lack of treatment options for this debilitating
chronic autoimmune disorder. We are proud to partner with the
father-son duo, Jon and Jax Bari, of Celiac Journey, to raise
awareness, advocate for research and extend support to the
estimated 3.3 million individuals in the U.S. who are impacted by
celiac disease. As a company dedicated to developing innovative
therapies for GI diseases, we are motivated by the commitment of
advocates to provide a meaningful benefit for those with celiac
disease who struggle to maintain a strict gluten-free diet and
experience drastic symptoms and medical complications as a
result.”
Jax Bari, age 11 and Co-founder of Celiac
Journey, said, “Food insecurity happens every day for celiacs
because of the constant threat of cross contact with gluten, 80% of
foods have gluten in them, the high price of gluten-free food, and
gluten is not required to be labeled on packaged foods in the U.S.
I'm determined to make a difference to create a world where people
like me can eat without fear. I’m grateful to First Wave BioPharma
and Nasdaq for helping us shine a light on celiac disease this
May.”
“As a parent of a child living with celiac
disease, I understand firsthand the daily challenges and fears
associated with this condition,” said Jon Bari, Co-founder of
Celiac Journey. “The need for increased research funding and the
development of alternative treatments beyond a strict gluten-free
diet cannot be overstated. Until there’s a treatment other than a
gluten-free diet, we believe that requiring the labeling of gluten
grains will have the greatest impact on improving quality of life
and safety for celiacs given life’s daily activities that involve
food.”
About Celiac
Disease Celiac disease is a chronic, hereditary
autoimmune and inflammatory disease triggered by gluten
consumption. Celiac disease is characterized by damage to the
lining of the small intestine, causing malabsorption,
gastrointestinal dysfunction, and debilitating symptoms. Over the
course of a lifetime, untreated or poorly managed celiac disease is
often associated with deteriorating general health, multiple
serious intestinal and extra-intestinal medical complications, and
increased morbidity and mortality. Celiac disease is a global
disease and affects approximately 1% of the population worldwide
and is increasing in prevalence with improved diagnostic tools and
improved awareness.
About Celiac JourneyCeliac
Journey, founded by the Bari family, advocates for increased
government funding for celiac disease research and requiring the
labeling of gluten as a top 10 Major Food Allergen on all packaged
foods in the U.S., just as gluten must be declared on all food
labels in 87 other countries. Motivated by their son Jax's
diagnosis with celiac disease, the organization strives to support
advancements in treatments, aiming for a future where 3.3 million
Americans with celiac like Jax can eat without fear. For more
information visit: www.celiacjourney.com.
About First Wave BioPharma,
Inc. First Wave BioPharma is a clinical-stage
biopharmaceutical company specializing in the development of
targeted, non-systemic therapies for gastrointestinal (GI)
diseases. The Company is currently advancing a therapeutic
development pipeline with multiple late-stage clinical programs
built around three proprietary technologies: latiglutenase, a Phase
3-ready, potentially first-in-class, targeted, oral biotherapeutic
for celiac disease; capeserod, a selective 5-HT4 receptor partial
agonist being developed for gastroparesis; and adrulipase, a
recombinant lipase enzyme designed to enable the digestion of fats
and other nutrients in cystic fibrosis and chronic pancreatitis
patients with exocrine pancreatic insufficiency. First Wave
BioPharma is headquartered in Boca Raton, Florida. For more
information visit www.firstwavebio.com.
Forward-Looking StatementsThis
press release may contain certain statements relating to future
results which are forward-looking statements. It is possible that
the Company’s actual results and financial condition may differ,
possibly materially, from the anticipated results and financial
condition indicated in these forward-looking statements, depending
on factors including whether any financing or licensing transaction
may be completed, completed with different terms, in an untimely
manner, or not at all; whether the Company will be able to realize
the expected benefits of its acquisition of ImmunogenX; the
Company’s ability to integrate the assets and contemplated
commercial operations acquired from ImmunogenX into the Company’s
business; whether results obtained in preclinical and nonclinical
studies and clinical trials will be indicative of results obtained
in future clinical trials; whether preliminary or interim results
from a clinical trial will be indicative of the final results of
the trial; whether the Company will be able to maintain compliance
with Nasdaq’s continued listing criteria and the effect of a
delisting from Nasdaq on the market for the Company’s securities;
the size of the potential markets for the Company’s drug candidates
and its ability to service those markets; the effects of the First
Wave Bio, Inc. acquisition, the related settlement and their effect
on the Company’s business, operating results and financial
prospects; and the Company’s current and future capital
requirements and its ability to raise additional funds to satisfy
its capital needs. Additional information concerning the Company
and its business, including a discussion of factors that could
materially affect the Company’s financial results are contained in
the Company’s Annual Report on Form 10-K for the year ended
December 31, 2022, under the heading “Risk Factors,” as well as the
Company’s subsequent filings with the Securities and Exchange
Commission. All forward-looking statements included in this press
release are made only as of the date of this press release, and we
do not undertake any obligation to publicly update or correct any
forward-looking statements to reflect events or circumstances that
subsequently occur or of which we hereafter become aware.
For more information: First Wave
BioPharma, Inc. 777 Yamato Road, Suite 502 Boca Raton, FL
33431 Phone: (561)
589-7020 info@firstwavebio.com
Media contact: Russo PartnersDavid Schull
or Liz Phillips(347)
956-7697david.schull@russopartnersllc.comelizabeth.phillips@russopartnersllc.com
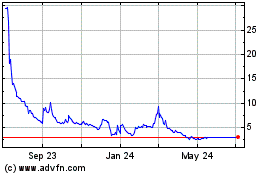
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Dec 2024 to Jan 2025
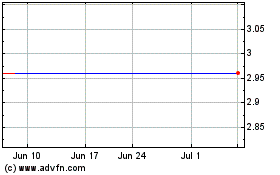
First Wave BioPharma (NASDAQ:FWBI)
Historical Stock Chart
From Jan 2024 to Jan 2025