Current Report Filing (8-k)
June 26 2023 - 6:04AM
Edgar (US Regulatory)
0001434868
false
0001434868
2023-06-24
2023-06-24
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported):
June 24, 2023
Esperion Therapeutics, Inc.
(Exact name of registrant as specified in
its charter)
| Delaware |
|
001-35986 |
|
26-1870780 |
(State or other jurisdiction of
incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification No.) |
3891 Ranchero Drive, Suite 150
Ann Arbor, MI |
|
48108 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (734) 887-3903
Not Applicable
Former name or former address, if changed
since last report
Check the appropriate box below if the Form 8-K filing
is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
¨ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting material pursuant to
Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange
Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of
the Act:
| Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
| Common Stock, par value $0.001 per share |
|
ESPR |
|
NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging
growth company ¨
If an emerging
growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with
any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
¨
Item 7.01. Regulation FD Disclosure.
On June 24, 2023, Esperion
Therapeutics, Inc. (the “Company”) announced the results from its pre-specified, primary prevention CLEAR Outcomes subgroup
analysis, which were presented at the 83rd American Diabetes Association Scientific Sessions and simultaneously published in the Journal
of the American Medical Association. A copy of the press release is being furnished herewith as Exhibit 99.1.
The information contained
in Item 7.01 of this Current Report on Form 8-K and Exhibit 99.1 attached hereto is intended to be furnished and shall not be deemed “filed”
for purposes of Section 18 of the Securities Exchange Act of 1934 or otherwise subject to the liabilities of that section, nor shall
it be deemed incorporated by reference in any filing under the Securities Act of 1933, except as expressly set forth by specific reference
in such filing.
Item 8.01. Other Information.
Business Update
The
Company is filing information for the purpose of supplementing and updating certain aspects of the description of its business from that
described under the heading, “Item 1. Business” in the Company’s Annual Report on Form 10-K for the
year ended December 31, 2022, filed with the U.S. Securities and Exchange Commission (the “SEC”) on February 21,
2023. The updated disclosure is set forth below:
On June 24, 2023, the
Company announced the results from its pre-specified, primary prevention CLEAR Outcomes subgroup analysis. The primary prevention patient
population included 4,206 statin-intolerant patients from the CLEAR Outcomes trial (30% of the total 13,970 patients) who had no prior
cardiovascular events, LDL-C level greater than or equal to 100 mg/dL at the start of the study, and who were at high risk for cardiovascular
events.
Results from this primary
prevention analysis show significant reduction in cardiovascular risk, including a 36% risk reduction of MACE-3 (composite of major adverse
cardiovascular events including non-fatal myocardial infarction, non-fatal stroke and cardiovascular death), and a 30% risk reduction
of MACE-4 (composite of major adverse cardiovascular events including non-fatal myocardial infarction, non-fatal stroke, coronary revascularization
and cardiovascular death) in the primary prevention population.
Forward-Looking Statements
This
Form 8-K contains forward-looking statements that are made pursuant to the safe harbor provisions of the federal securities
laws, including statements regarding expected operational expenses, expected revenue of our commercial products, future operations,
expected milestone payments from partners, commercial products and expected growth, clinical development and regulatory submissions,
and other statements containing the words “anticipate,” “believe,” “estimate,”
“expect,” “intend,” “may,” “plan,” “predict,” “project,”
“suggest,” “target,” “potential,” “will,” “would,” “could,”
“should,” “continue,” and similar expressions. Any express or implied statements contained in this press
release that are not statements of historical fact may be deemed to be forward-looking statements. Forward-looking statements
involve risks and uncertainties that could cause the Company’s actual results to differ significantly from those projected,
including, without limitation, the impact of the ongoing COVID-19 pandemic on our business, revenues, results of operations and
financial condition, the net sales, profitability, and growth of the Company’s commercial products, clinical activities and
results, supply chain, commercial development and launch plans, and the risks detailed in the Company’s filings with the
Securities and Exchange Commission. Any forward-looking statements contained in this press release speak only as of the date hereof,
and Esperion disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this
press release, other than to the extent required by law.
Item 9.01. Financial Statements and Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: June 26, 2023 |
Esperion Therapeutics, Inc. |
| |
|
| |
By: |
/s/ Sheldon L. Koenig |
| |
|
Sheldon L. Koenig |
| |
|
President and Chief Executive Officer |
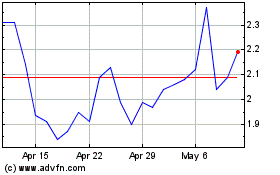
Esperion Therapeutics (NASDAQ:ESPR)
Historical Stock Chart
From May 2024 to Jun 2024
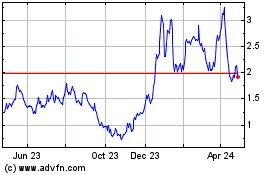
Esperion Therapeutics (NASDAQ:ESPR)
Historical Stock Chart
From Jun 2023 to Jun 2024