As filed with the Securities and Exchange Commission on July 14, 2023
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
under the Securities Act of 1933
Athersys, Inc.
(Exact name of registrant as specified in its charter)
| |
|
|
|
|
|
Delaware
|
|
2834
|
|
20-4864095
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification Number)
|
3201 Carnegie Avenue
Cleveland, Ohio 44115-2634
(216) 431-9900
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Daniel Camardo
Chief Executive Officer
3201 Carnegie Avenue
Cleveland, Ohio 44115-2634
(216) 431-9900
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Faith L. Charles, Esq.
Naveen Pogula, Esq.
Thompson Hine LLP
300 Madison Avenue, 27th Floor
New York, New York 10017-4611
(212) 344-5680
|
|
Leslie Marlow, Esq.
Patrick J. Egan, Esq.
Hank Gracin, Esq.
Blank Rome LLP
1271 Avenue of the Americas
New York, New York 10020
(212) 885-5001
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large Accelerated Filer ☐
|
|
Smaller Reporting Company ☒
|
|
Accelerated Filer ☐
|
|
Emerging Growth Company ☐
|
|
Non-Accelerated Filer ☒
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION, DATED July 14, 2023 |
Up to Shares of Common Stock
Common Warrants to purchase up to shares of Common Stock
Pre-Funded Warrants to purchase up to shares of Common Stock
Up to shares of Common Stock underlying the Common Warrants
Up to shares of Common Stock underlying the Pre-Funded Warrants

We are offering up to $ shares (the “Shares”) of our common stock, par value $0.001 per share, together with common warrants to purchase up to shares of common stock (the “Common Warrants”) at an assumed combined public offering price of $ , which is the last sale price of our common stock as reported by The Nasdaq Capital Market (“Nasdaq” or the "Nasdaq Capital Market") on , 2023. The Shares and Common Warrants are immediately separable and will be issued separately in this offering. Our common stock is currently listed on Nasdaq and trades under the symbol “ATHX.”
We are offering to certain purchasers whose purchase of Shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants (the “Pre-Funded Warrants”), in lieu of Shares that otherwise would result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. Each Pre-Funded Warrant is exercisable for one share of common stock at an exercise price of $0.0001, and the assumed purchase price for a Pre-Funded Warrant and accompanying Common Warrant is $ . The Pre-Funded Warrants and accompanying Common Warrants are immediately separable and will be issued separately in this offering. The Pre-Funded Warrants are immediately exercisable and may be exercised at any time until all of the Pre-Funded Warrants are exercised in full. For each Pre-Funded Warrant we sell, the number of Shares we are offering will be decreased on a one-for-one basis.
There is no established trading market for the Pre-Funded Warrants or Common Warrants, and we do not expect a market to develop. In addition, we do not intend to list the Pre-Funded Warrants or Common Warrants on Nasdaq, any other national securities exchange or any other trading system. Without an active trading market, the liquidity of the Pre-Funded Warrants and Common Warrants may be limited.
We have engaged A.G.P./Alliance Global Partners (whom we refer to herein as the “Placement Agent”) to act as our exclusive placement agent in connection with the securities offered by this prospectus. The Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities but has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. We have agreed to pay the Placement Agent a fee based upon the aggregate gross proceeds raised in this offering as set forth in the table below.
There is no minimum number of securities or minimum aggregate amount of proceeds for this offering to close. We expect this offering to be completed not later than two (2) business days following the commencement of this offering and we will deliver all securities to be issued in connection with this offering delivery versus payment (“DVP”)/receipt versus payment (“RVP”) upon receipt of investor funds received by us. Accordingly, neither we nor the Placement Agent have made any arrangements to place investor funds in an escrow account or trust account since the Placement Agent will not receive investor funds in connection with the sale of the securities offered hereunder.
The final public offering price of the securities sold in this offering will be determined between us, the Placement Agent, and the investors in the offering and may be at a discount to the current market price of our common stock. Therefore, the recent market price used throughout this prospectus will not be indicative of the actual public offering price.
Investing in our securities involves a high degree of risk. See the “Risk Factors” section beginning on page 13 of this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
| |
|
Per Share and
accompanying
Common
Warrant
|
|
Per Pre-
Funded
Warrant and
accompanying
Common
Warrant
|
|
Total
|
|
Public offering price(1)
|
|
$
|
|
|
$
|
|
|
$
|
|
|
Placement Agent’s fees (2)
|
|
$
|
|
|
$
|
|
|
$
|
|
|
Proceeds to us, before expenses(3)
|
|
$
|
|
|
$
|
|
|
$
|
|
|
(1)
|
The combined public offering price is $ per share of common stock and accompanying Common Warrant and $ per Pre-Funded Warrant and accompanying Common Warrant.
|
| |
|
|
(2)
|
We have agreed to pay the Placement Agent a total cash fee equal to 7.0% of the gross proceeds of the offering. We have also agreed to reimburse the Placement Agent for its accountable offering-related legal expenses in an amount up to $80,000 and pay the Placement Agent a non-accountable expense allowance of up to $75,000. See “Plan of Distribution” for a description of the compensation payable to the Placement Agent.
|
|
(3)
|
Does not include potential proceeds from the exercise of the Common Warrants and/or Pre-Funded Warrants for cash, if any.
|
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery of the securities is expected to be made on or about , 2023.
Sole Placement Agent
A.G.P.
The date of this prospectus is , 2023
TABLE OF CONTENTS
|
PROSPECTUS SUMMARY
|
5 |
|
SUMMARY FINANCIAL DATA
|
12 |
|
RISK FACTORS
|
13 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
20 |
|
USE OF PROCEEDS
|
21 |
|
DIVIDEND POLICY
|
22 |
|
CAPITALIZATION
|
23 |
|
DILUTION
|
25 |
|
DESCRIPTION OF CAPITAL STOCK
|
27 |
|
DESCRIPTION OF SECURITIES WE ARE OFFERING
|
28 |
|
PLAN OF DISTRIBUTION
|
31 |
|
LEGAL MATTERS
|
33 |
|
EXPERTS
|
33 |
|
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
|
33 |
|
WHERE YOU CAN FIND MORE INFORMATION
|
34 |
We are responsible for the information contained in this prospectus. We have not, and the Placement Agent has not, authorized anyone to provide you with any other information other than in this prospectus, and we take no responsibility for, and the Placement Agent has not taken responsibility for, any other information others may give you. We are not, and the Placement Agent is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than its date. Our business, financial condition, results of operations and prospects may have changed since that date.
PROSPECTUS SUMMARY
This summary highlights certain information appearing elsewhere in or incorporated by reference into this prospectus. This summary is not complete and does not contain all of the information you should consider prior to investing in the securities offered hereby. After you read this summary, you should read and consider carefully the more detailed information and financial statements and related notes that we include or incorporate by reference in this prospectus, including the information incorporated by reference from our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K. If you invest in our securities, you are assuming a high degree of risk.
References in this prospectus to the terms “we,” “us” or “the Company” or other similar terms mean Athersys, Inc. and its consolidated subsidiaries, unless we state otherwise or the context indicates otherwise.
Company Overview
We are a biotechnology company that is focused primarily in the field of regenerative medicine. We are committed to the discovery and development of best-in-class therapies designed to extend and enhance the quality of human life and have established a portfolio of therapeutic product development programs to address significant unmet medical needs in multiple disease areas. Our MultiStem® (invimestrocel) cell therapy, a patented and proprietary allogeneic stem cell product candidate, is our lead platform product and is currently in late-stage clinical development. Our most advanced therapeutic program is focused on the treatment of ischemic stroke, which is currently being evaluated in a pivotal Phase 3 clinical trial ongoing in the United States under a Special Protocol Assessment agreement (“SPA”), from the U.S. Food and Drug Administration (the “FDA”), in Europe and in certain other international locations. Our current clinical development programs are focused on treating critical care and other conditions where current standard of care is limited or inadequate for many patients. These represent major areas of clinical need, as well as substantial commercial opportunities.
We believe our MultiStem cell therapy product candidate represents a potential breakthrough in the field of regenerative medicine and stem cell therapy, and could be used to treat a range of disease indications. MultiStem received Regenerative Medicine Advanced Therapy (“RMAT”), designation for the treatment of both ischemic stroke and acute respiratory distress syndrome (“ARDS”). MultiStem treatment has shown the potential to enhance tissue repair and healing in multiple ways, including reducing inflammatory damage, protecting tissue that is at risk following acute or ischemic injury, and promoting formation of new blood vessels in regions of ischemic injury. These cells appear to be responsive to the environment in which they are administered, by homing to sites of injury and/or organs involved in injury response and providing active disease response. These cells also produce proteins that may provide benefit in both acute and chronic conditions and regulate other cell types. In contrast to traditional pharmaceutical products or biologics that generally act through a single biological mechanism of action, MultiStem cell therapy may enhance healing and tissue repair through several distinct mechanisms acting in parallel, resulting in a more effective therapeutic response.
We believe the therapeutic and commercial potential for MultiStem cell therapy to be very broad, applying to many areas of significant unmet medical need, and we are pursuing opportunities in several potential multi-billion dollar markets. While traditional pharmaceuticals and biologic therapies typically may be used to treat only a single disease or a narrowly defined set of related conditions, MultiStem cell therapy may have far broader potential and could be developed in different formulations and with different delivery approaches to effectively treat a wide range of disease indications.
The MultiStem product candidate under development may be unique among regenerative medicine approaches because it has the potential to be manufactured on a large scale, can be administered in an “off-the-shelf” manner with minimal processing, and has the potential to augment healing by providing biological potency and therapeutic effects that other cell therapy approaches may not be able to achieve. Additionally, MultiStem treatment has consistently demonstrated good tolerability in both preclinical and clinical studies. Like conventional drugs and biologics, the product candidate is cleared from the body over time, which we believe may enhance product safety relative to other types of stem cell therapy. While the product candidate does not permanently engraft in the patient, the therapeutic effects of treatment with MultiStem cells appear to be durable based on both clinical and preclinical results.
We have evaluated the use of MultiStem cell therapy as a potential treatment in several disease areas. Working with an international network of leading investigators and prominent research and clinical institutions, and through our own internal efforts, we have explored the potential for MultiStem cell therapy to be used as a treatment of acute and chronic forms of neurological conditions or injury, inflammatory and immune disorders, certain pulmonary conditions and cardiovascular disease. We have advanced several MultiStem programs into clinical development, targeting areas of significant medical need and major commercial market opportunities, and have two ongoing clinical trials in the critical care area. We have a collaboration with HEALIOS K.K. (“Healios”), to develop and commercialize MultiStem for the treatment of certain indications in Japan. Among other things, Healios has a license to our technology and is responsible for the development and commercialization of MultiStem for ischemic stroke and ARDS in Japan on an exclusive basis.
Our lead program is our pivotal Phase 3 clinical trial to evaluate the potential for MultiStem treatment of patients who have suffered neurological damage from an ischemic stroke entitled, “MultiStem Administration for Stroke Treatment and Enhanced Recovery Study-2,” or MASTERS-2. The results from our completed Phase 2 study demonstrated favorable tolerability for MultiStem, consistent with the results from prior studies. Though the Phase 2 study did not achieve the primary endpoints for the intent-to-treat population, MultiStem treatment was associated with lower rates of mortality and life-threatening adverse events, infections and pulmonary events, and a reduction in hospitalization and time in the intensive care unit (“ICU”). In addition, analyses show that patients who received MultiStem treatment earlier in the study’s treatment window (24 to 36 hours post-stroke, in accordance with the original study protocol) had better recovery in comparison to placebo. Furthermore, analysis of biomarker data obtained from samples of study subjects indicated that MultiStem treatment reduced post-stroke inflammation compared to placebo, and the results suggest that this effect was more pronounced for subjects who received MultiStem earlier within the treatment window. This effect is consistent with our hypothesis regarding mechanisms of action and related preclinical data, and with the clinical data suggesting faster and improved recovery for MultiStem-treated patients relative to current standard of care.
The one-year follow-up data from the Phase 2 trial demonstrated that MultiStem-treated subjects on average continued to improve through one year and had a significantly higher rate of “Excellent Outcome,” as defined below, compared to placebo subjects at one year when evaluating all of the intent-to-treat subjects enrolled in the study. Achievement of an Excellent Outcome is important because it means that a patient has substantially improved (i.e., receiving an “Excellent” score in each of the three clinical rating scales used to assess patient improvement) and has regained the ability to live and function independently with a high quality of life. The relative improvement in Excellent Outcome was even more pronounced in the study subjects who received MultiStem treatment within 36 hours of the stroke. If the MultiStem cell therapy candidate is proven effective in our ongoing Phase 3 registrational study, and if it receives a marketing authorization from the FDA, this treatment window and its favorable administration profile would make this therapy available to most ischemic stroke patients in contrast to other therapies (e.g., tissue plasminogen activator, or tPA, or mechanical thrombectomy), which have shorter treatment windows or are limited to certain patients.
Our MASTERS-2 trial treating ischemic stroke patients is ongoing in the United States and certain other international locations. We received agreement from the FDA under a SPA that the design and planned analysis of MASTERS-2 adequately address the objectives necessary to support a regulatory submission. It is possible that following MASTERS-2 we will conduct either a post-registry study or a confirmatory study, depending on the strength and robustness of the study outcome. The FDA granted us Fast Track and RMAT designations for our clinical product for the treatment of ischemic stroke. Fast Track is an important designation given to qualified investigational therapies that show promise in providing benefit to patients in areas of significant unmet medical need. Fast Track designation allows for an expedited regulatory review process after the clinical data is submitted to help speed development of promising therapies to the market in order to help patients in areas where current standard of care is limited. Such designation for a new biologic product means that the FDA will take such actions as are appropriate to expedite the development and review of our application to approve the product, and specifically, under Fast Track designation, the program becomes eligible for rolling submission, accelerated approval and priority review, facilitating a timely regulatory review. This program subsequently received the RMAT designation from the FDA that was established under the 21st Century Cures Legislation. The RMAT designation may be obtained for eligible cell therapy and other regenerative medicine and advanced therapies when the FDA agrees that preliminary clinical evidence indicates that the therapy has demonstrated the potential to effectively address unmet medical needs for a serious or life-threatening disease or condition. The RMAT designation is the equivalent of the non-regenerative medicine product’s Breakthrough Therapy designation, and designated products benefit from all Breakthrough Therapy features, in addition to eligibility for filing for registration under accelerated approval path. The designation also enables sponsors to discuss with the FDA multidisciplinary strategic development plans, including expediting manufacturing development plans for commercialization and supporting priority review and rolling submission.
The design of MASTERS-2 has also received a Final Scientific Advice positive opinion from the European Medicines Agency (“EMA”), representing the EMA’s agreement that successful results from the trial could result in registration and marketing approval of the MultiStem cell therapy. We have also received Advanced Therapy Medicinal Product (“ATMP”), Certificate for Quality Data from the EMA. We believe these designations from the FDA and EMA could accelerate the development, regulatory review and subsequent commercialization of products, like MultiStem cell therapy for ischemic stroke and ARDS, if future clinical evaluation demonstrates appropriate safety and therapeutic effectiveness.
Business Strategy
Our principal business objective is to discover, develop and commercialize novel therapeutic products for disease indications that represent significant areas of clinical need and where we believe there is a substantial commercial opportunity. The key elements of our strategy are outlined below:
| |
•
|
Advance our Lead Programs through Clinical Developments to Registration and Commercialization. We are focused on the design and execution of clinical studies (e.g., ischemic stroke and trauma) intended to enable product registration in major markets. We are also engaged in activities intended to enable effective commercialization (e.g., preparation for scaled commercial manufacturing, product branding, product reimbursement and marketing strategies). We may partner with other companies to complete such development and preparation activities, and to market the product upon regulatory approval.
|
| |
•
|
Efficiently Conduct Clinical Development to Establish Clinical Proof-of-Concept and Biological Activity for Other Applications of our Product Candidates. We conduct our clinical studies with the intent to establish safety and efficacy proof-of-concept and/or evidence of biological activity in a number of important disease areas where our cell therapies are expected to have benefit. Our strategy is to conduct well-designed studies beginning early in the clinical development process, thus establishing a robust foundation for later-stage development, partnering activity and expansion into complementary areas. We are committed to a rigorous clinical and regulatory approach, which we believe has helped us to advance our programs efficiently, providing high quality, transparent communications and regulatory submissions.
|
| |
•
|
Enter into Arrangements with Business Partners to Accelerate Development and Create Value. In addition to our internal development efforts, an important part of our strategy is to work with collaborators and partners to accelerate product development, reduce our development costs and broaden our commercial access. We anticipate that this strategy will help us to develop a portfolio of high-quality product development opportunities, enhance our clinical development and commercialization capabilities and increase our ability to generate value from our proprietary technologies. Historically, we have entered into licensing arrangements with companies such as Healios, Chugai Pharmaceutical Co., Ltd., Pfizer Inc., Bristol-Myers Squibb Company, Johnson & Johnson, Wyeth Pharmaceuticals Inc. (now part of Pfizer), RTI Surgical, Inc. and others.
|
| |
•
|
Efficiently Explore New High Potential Therapeutic Applications, Leveraging Third-Party Research Collaborations and our Results from Related Areas. Our MultiStem cell therapy has shown promise in many disease areas, including in treating neurological conditions, inflammatory and immune disorders, certain pulmonary conditions, cardiovascular disease, and other conditions where the current standard of care is limited or inadequate for many patients. We have explored potential clinical indications where our therapies may achieve best-in-class profile and where we believe we can effectively address significant unmet medical needs. In order to achieve this goal, we leveraged collaborative research relationships with investigators from many leading research and clinical institutions across the United States and Europe, including the Cleveland Clinic, Case Western Reserve University, the University of Minnesota, the Medical College of Georgia at Augusta University, the University of Oregon Health Sciences Center, the University of Pittsburgh Medical Center, the Katholieke Universiteit Leuven, and the University of Regensburg, among other institutions, with current research ongoing at UTHealth, Maastricht University, the University of Birmingham, and Newcastle University. Through this network of collaborations, we have evaluated MultiStem cell therapy in a range of preclinical models that reflect various types of human disease or injury and have advanced several indications to a state of Investigational New Drug Application (“IND”), readiness, which could be quickly advanced upon via a future partnership or internally if we elect to move them into the clinical stage.
|
| |
•
|
Continue to Expand our Intellectual Property Portfolio. We have a broad intellectual property estate that covers our proprietary products and technologies, as well as methods of production and methods of use. Our intellectual property is important to our business and we take significant steps to protect its value. We have ongoing research and development efforts through collaborative research activities with others, which aim to develop new technologies, applications and intellectual property and enable us to file patent applications that cover new applications of our existing technologies or product candidates, including MultiStem cell therapy as well as methods of production and method of use and other opportunities. We currently have approximately 360 patents related to our technologies, providing protection in the United States, Europe, Japan and other areas.
|
Risk Factor Summary
Our ability to execute on our business strategy is subject to a number of risks, which are discussed more fully in the section “Risk Factors” of this prospectus and in our Annual Report on Form 10-K for the year ended December 31, 2022 and in any subsequent Quarterly Reports on Form 10-Q, each filed with the SEC and incorporated herein by reference. You should carefully consider these risks before making an investment in our common stock. These risks include, among others, the following:
| |
•
|
Our management will have broad discretion over the use of the proceeds to us from this offering and may apply it to uses that do not improve our operating results or the value of our securities.
|
| |
•
|
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
|
| |
•
|
There is substantial doubt about our ability to continue as a going concern, which may affect our ability to obtain future financing and may require us to curtail our operations. We will need substantial additional funding to develop our products and for our future operations. If we are unable to obtain the funds necessary to do so, we may be required to further delay, scale back or eliminate our product development activities or may be unable to continue our business.
|
| |
•
|
If we are unable to raise capital, we could be forced to eliminate our product development programs, may be unable to continue our business and may need to file for protection under the bankruptcy laws.
|
| |
•
|
We have incurred losses since inception, and we expect to incur significant net losses in the foreseeable future and may never become profitable.
|
| |
•
|
We are heavily dependent on the successful development and commercialization of MultiStem products, and if we encounter delays or difficulties in the development of these product candidates, our business could be harmed.
|
| |
•
|
We have reduced the size of our organization, and we may encounter difficulties in managing our business as a result of this reduction, which could disrupt our operations. In addition, we may not achieve anticipated benefits and savings from the reduction.
|
| |
•
|
Our product candidates are currently in the development stage and we have no therapeutic products approved for sale. If we are unable to develop, obtain regulatory approval or market any of our product candidates, our financial condition will be negatively affected, and we may have to curtail or cease our operations.
|
| |
•
|
We may experience delays in clinical trials and regulatory approval relating to our products that could adversely affect our financial results and our commercial prospects for our pharmaceutical or stem cell products.
|
| |
•
|
Even if we obtain regulatory approval of any of our product candidates, the approved products may be subject to post-approval studies and will remain subject to ongoing regulatory requirements. If we fail to comply, or if concerns are identified in subsequent studies, our approval could be withdrawn, and our product sales could be suspended.
|
| |
•
|
We are not currently in compliance with Nasdaq’s continued listing requirements. If we are unable to comply with Nasdaq’s continued listing requirements, our common stock could be delisted, which could affect the price of our common stock and liquidity and reduce our ability to raise capital.
|
| |
•
|
If we inadvertently violate the guidelines pertaining to promotion and advertising of our clinical candidates or approved products, we may be subject to disciplinary action by the FDA’s Division of Drug Marketing, Advertising, and Communications or other regulatory bodies.
|
| |
•
|
If we inadvertently violate the guidelines pertaining to promotion and advertising of our clinical candidates or approved products, we may be subject to disciplinary action by the FDA’s Division of Drug Marketing, Advertising, and Communications or other regulatory bodies.
|
| |
•
|
Even if we or our collaborators receive regulatory approval for our products, those products may never be commercially successful.
|
| |
•
|
We may not successfully maintain our existing collaborative and licensing arrangements, or establish new ones, which could adversely affect our ability to develop and commercialize our product candidates.
|
| |
•
|
We rely on third parties to manufacture our MultiStem product candidate.
|
| |
•
|
Our ability to compete may decline if we are not successful in adequately protecting our patented and other proprietary technologies.
|
| |
•
|
We may not have adequate protection for our unpatented proprietary information, which could adversely affect our competitive position.
|
| |
•
|
Many potential competitors, including those who have greater resources and experience than we do, may develop products or technologies that make ours obsolete or noncompetitive.
|
| |
•
|
The availability, manner, and amount of reimbursement for our product candidates from government and private payers are uncertain, and our inability to obtain adequate reimbursement for any products could severely limit our product sales.
|
| |
•
|
Increased information technology security threats and more sophisticated and targeted computer crime could pose a risk to our systems, networks, and products.
|
Recent Developments
Forbearance, Restructuring and Settlement Agreement
On May 17, 2023, we entered into a Forbearance, Restructuring and Settlement Agreement (the “Forbearance Agreement”) with a supplier, which amends certain supply agreements between us and the supplier (the “Supply Agreements”). The Forbearance Agreement restructures our matured and unmatured liabilities owed to the supplier under the Supply Agreements, comprised of past due and current due obligations in the approximate amount of $20.9 million and future obligations in the approximate amount of $9.8 million, less (i) approximately $3.9 million in credits applied by the supplier based upon prior agreements. The Forbearance Agreement provides that the supplier shall forbear from exercising rights and remedies available as a result of existing overdue amounts under the Supply Agreements, so long as we pay to the supplier an aggregate of $11.8 million, in monthly payments of $0.25 million, commencing in October 2023.
Under the Forbearance Agreement, we also issued a convertible promissory note, guaranteed by certain of our subsidiaries, to the supplier in the principal amount of $15.0 million (the “Note”). The Note bears interest at a rate of 10.0% per annum, which shall be capitalized and added to the principal amount semi-annually on January 1 and July 1, commencing on July 1, 2023, and must be repaid in full, including accrued and unpaid interest thereunder, on (or before, subject to certain conditions) May 17, 2026 (the “Maturity Date”). If an Event of Default (as defined in the Note) occurs, then (i) the interest on the Note shall accrue at a rate of 14.0% per annum (or, if less, the maximum rate permitted by applicable law) and (ii) the Maturity Date may be accelerated. Subject to a beneficial ownership limitation of 19.99% of our outstanding common stock and any shareholder approval requirements, the supplier may elect, at its sole discretion, to convert any outstanding principal and interest on the Note into shares of our common stock at a conversion price of $1.30 per share.
Nasdaq Compliance
On October 14, 2022, we received a written notice (the “Notice”) from the Listing Qualifications Department of The Nasdaq Stock Market LLC that we are not in compliance with the requirement to maintain a minimum market value of listed securities of $35 million, as set forth in Nasdaq Listing Rule 5550(b)(2) (the “Market Value Standard”) because the market value of the common stock was below $35 million for 30 consecutive business days. The Notice provided that, in accordance with Nasdaq Listing Rule 5810(c)(3)(C), we had a period of 180 calendar days from the date of the Notice, or until April 12, 2023, to regain compliance under the Market Value Standard.
On April 13, 2023, we received notice from Nasdaq that we had not regained compliance with the Market Value Standard. On April 14, 2023, we filed our request for a hearing, which was held before the Nasdaq Hearing Panel (the “Panel”) on May 18, 2023.
On June 26, 2023, the Panel notified us that it had granted our request for an exception through September 15, 2023, to the continued listing requirements, subject to our demonstrating compliance with the Market Value Standard by September 15, 2023. In accordance with the Market Value Standard and Nasdaq Listing Rule 5810(c)(3)(C), compliance with the Market Value Standard may be achieved if at any time during the compliance period the market value of the listed securities closes at a value of at least $35 million for a minimum of ten consecutive business days.
The Panel noted that it reserves the right to reconsider the terms of this exception based on any event, condition or circumstance that exists or develops that would, in the opinion of the Panel, make continued listing of our securities on Nasdaq inadvisable or unwarranted. In addition, the Nasdaq Listing and Hearing Review Council may, on its own motion, determine to review any Panel decision within 45 calendar days after issuance of the written decision. There can be no assurance that we will be able to regain compliance under the Market Value Standard, or will otherwise be in compliance with other Nasdaq listing criteria. While we are exercising diligent efforts to maintain the listing of our common stock on The Nasdaq Capital Market, there can be no assurance that we will be able to regain or maintain compliance with Nasdaq listing criteria.
Corporate Information
We were incorporated in Delaware and our headquarters are located at 3201 Carnegie Avenue, Cleveland, Ohio 44115. Our telephone number is (216) 431-9900. Our website is http://www.athersys.com. The information accessible on or through our website is not part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
THE OFFERING
The summary below describes the principal terms of this offering. The “Description of Capital Stock” section of this prospectus contains a more detailed description of our common stock.
|
Issuer
|
|
Athersys, Inc.
|
| |
|
|
|
Common stock offered by us
|
|
Up to Shares based on an assumed combined public offering price of $ , which is equal to the last sale price of our common stock as reported by Nasdaq on , 2023.
|
| |
|
|
|
Common Warrants offered by us
|
|
Common Warrants to purchase up to shares of our common stock, which will be exercisable during the period commencing on the date of their issuance and ending years from such date at an exercise price per share equal to 100% of the public offering price/assumed exercise price of $ . Each Share (or Pre-Funded Warrant in lieu of a Share) is being sold together with one Common Warrant but are immediately separable and will be issued separately in this offering. This prospectus also relates to the shares of common stock issuable upon exercise of any Common Warrants sold in this offering.
|
| |
|
|
|
Pre-Funded Warrants offered by us
|
|
We are offering to certain purchasers whose purchase of Shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding shares of common stock immediately following the closing of this offering, the opportunity to purchase, if such purchasers so choose, Pre-Funded Warrants, in lieu of Shares that would otherwise result in any such purchaser’s beneficial ownership, together with its affiliates and certain related parties, exceeding 4.99% (or, at the election of such purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering. The purchase price of each Pre- Funded Warrant and accompanying Common Warrant is equal to the purchase price of the Shares and accompanying Common Warrant in this offering minus $0.0001, the exercise price of each Pre-Funded Warrant. Each Pre-Funded Warrant is immediately exercisable and may be exercised at any time until it has been exercised in full. For each Pre-Funded Warrant we sell, the number of Shares we are offering will be decreased on a one- for-one basis. This offering also relates to the shares of common stock issuable upon exercise of any Pre-Funded Warrants sold in this offering.
|
| |
|
|
|
Common stock to be outstanding immediately after this offering
|
|
Up to shares (assuming the sale of the maximum number of Shares in this offering, at the assumed combined public offering price of $ ).
|
| |
|
|
|
Use of proceeds
|
|
We estimate that the net proceeds from the sale of securities in this offering will be approximately $ million, based on the assumed public offering price of $ per share, the last reported sale price of our common stock on Nasdaq on , 2023, and after deducting estimated placement agent fees and estimated offering expenses payable by us. We intend to use the net proceeds of this offering for working capital and general corporate purposes. See “Use of Proceeds.”
|
|
Prior Warrant Amendments
|
|
In connection with this offering, we may enter into privately negotiated agreements with the holders of certain existing outstanding warrants to purchase shares of our common stock (the “Prior Warrants”) to, among other things, reduce the exercise price of such Prior Warrants to that of the Common Warrants being offered and sold in this offering and to extend the current expiration date of the Prior Warrants to the expiration date of the Common Warrants being offered and sold in this offering. In addition, we expect that certain terms of the Prior Warrants will also be amended to be substantially consistent with those of the Common Warrants being offered hereby. There can be no assurance that we will amend the Prior Warrants in connection with this offering or as to the final terms of any amendments to the Prior Warrants.
|
| |
|
|
|
Risk factors
|
|
An investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 13 for a discussion of certain factors that you should consider when evaluating an investment in our securities.
|
| |
|
|
|
Nasdaq trading symbol
|
|
Our common stock is listed on The Nasdaq Capital Market under the symbol “ATHX.”
|
Unless otherwise indicated, all information contained in this prospectus exclude, as of March 31, 2023:
| |
•
|
a total of 1,626,312 shares of common stock authorized and reserved for future issuance under outstanding awards under our equity incentive plans;
|
| |
•
|
a total of 27,491 shares of common stock authorized and reserved for future issuance under our equity incentive plans;
|
| |
•
|
a total of 429,500 shares of common stock authorized and reserved for future issuance under outstanding inducement awards granted outside of our equity incentive plans;
|
| |
•
|
1,920,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued and sold in August 2022;
|
| |
•
|
2,000,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in September 2022;
|
| |
•
|
a total of 9,109,090 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our November 2022 public offering;
|
| |
•
|
a total of 3,685,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our April 2023 public offering;
|
| |
•
|
a total of 11,538,461 shares of common stock authorized and reserved for future issuance upon conversation feature of our May 2023 Convertible Note;
|
| |
•
|
400,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants held by Healios; and
|
| |
•
|
no exercise of the Common Warrants or Pre-Funded Warrants sold in this offering.
|
The information discussed above is illustrative only and will adjust based on the actual public offering price and other terms of this offering determined at pricing. Unless expressly indicated or the context requires otherwise, all information in this prospectus assumes an assumed public offering price of $ per share of common stock, which is the last reported sale price of our common stock on Nasdaq on , 2023.
SUMMARY FINANCIAL DATA
The following is a summary of our results of operations and financial position. The summary consolidated financial data set forth below should be read in conjunction with “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and the notes thereto incorporated by reference into this prospectus.
| |
|
Three Months Ended March 31,
|
|
|
Year Ended December 31,
|
|
| |
|
2023
|
|
|
2022
|
|
|
2023
|
|
|
2022
|
|
| |
|
(in thousands, except per share data)
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contract and grant revenues
|
|
|
— |
|
|
|
2,912 |
|
|
|
5,325 |
|
|
|
5,514 |
|
|
Operating expenses
|
|
|
7,334 |
|
|
|
25,289 |
|
|
|
82,334 |
|
|
|
92,571 |
|
|
Loss from operations
|
|
|
(7,334 |
) |
|
|
(22,378 |
) |
|
|
(77,009 |
) |
|
|
(87,058 |
) |
|
Other income (expense), net
|
|
|
(477 |
) |
|
|
162 |
|
|
|
4,475 |
|
|
|
102 |
|
|
Net loss
|
|
|
(7,811 |
) |
|
|
(22,216 |
) |
|
|
(72,534 |
) |
|
|
(86,955 |
) |
|
Net loss per share, basic and diluted
|
|
$ |
(0.43 |
) |
|
$ |
(2.27 |
) |
|
$ |
(6.07 |
) |
|
$ |
(9.69 |
) |
|
Weighted average shares outstanding, basic and diluted
|
|
|
18,292 |
|
|
|
9,768 |
|
|
|
11,945 |
|
|
|
8,971 |
|
Please see Note C to our audited consolidated financial statements incorporated by reference into this prospectus for an explanation of the method used to calculate net loss attributable to common stockholders, basic and diluted net loss per common share, and the number of shares used in the computation of per share amounts.
| |
|
Historical
|
|
|
Pro forma (1) |
|
| |
|
March 31,
|
|
|
March 31,
|
|
| |
|
2023
|
|
|
2023
|
|
| |
|
(in thousands)
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
Cash, cash equivalents and available-for-sale securities
|
|
|
3,121 |
|
|
|
6,366 |
|
|
Working capital
|
|
|
(31,847 |
) |
|
|
(28,601 |
) |
|
Total assets
|
|
|
21,632 |
|
|
|
24,878 |
|
|
Warrant liabilities and note payable
|
|
|
(1,163 |
) |
|
|
(1,163 |
) |
|
Total stockholders’ equity
|
|
|
(31,011 |
) |
|
|
(27,766 |
) |
(1) Pro forma figures give effect to the receipt of $3,380,347 of net proceeds from the issuance of 2,315,000 shares of common stock in the April 2023 Offerings, the receipt of $81 of proceeds upon exercise of 813,000 shares of common stock underlying the pre-funded warrants issued in the April 2023 Offerings, and the vesting of 367,664 restricted stock units. Pro forma figures exclude the effect of the April 2023 amendment to the August 2022 and September 2022 Warrants to, among other things, reduce the exercise price to $0.96 per share with respect to 1,920,000 shares of Common Stock covered by the August 2022 Warrants and 1,760,000 shares of Common Stock covered by the September 2022 Warrants.
RISK FACTORS
Investment in our common stock involves a high degree of risk and uncertainty. You should carefully consider each of the risks and uncertainties described below and discussed under the section captioned “Risk Factors” contained in our Annual Report on Form 10-K for the year ended December 31, 2022, (the “Annual Report”),as updated by our subsequent filings under the Securities Exchange Act of 1934, as amended, each of which is incorporated by reference in this prospectus in their entirety, together with other information in this prospectus and the information and documents incorporated by reference in this prospectus, before you decide to buy our securities. If any of the risks and uncertainties therein or below materializes, our business, financial condition, liquidity and results of operations could be materially and adversely affected. This could cause the trading price of our common stock to decline, and you could lose all or part of your investment. You should not interpret the disclosure of any risk factor to imply that the risk has not already materialized.
Risks Related to Our Business
There is substantial doubt about our ability to continue as a going concern, which may affect our ability to obtain future financing and may require us to curtail our operations. We will need substantial additional funding to develop our products and for our future operations. If we are unable to obtain the funds necessary to do so, we may be required to further delay, scale back or eliminate our product development activities or may be unable to continue our business.
The audited financial statements and accompanying notes presented in our Annual Report include disclosures and an opinion from our independent registered public accounting firm stating that our recurring losses and negative cash flows from operations raise substantial doubt about our ability to continue as a going concern. Our financial statements as of December 31, 2022 and 2021 were prepared under the assumption that we will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty.
The development of our product candidates will require a commitment of substantial funds to conduct the research, which may include preclinical and clinical testing, necessary to obtain regulatory approvals and bring our products to market. Net cash used in our operations was $59.0 million in 2022, $76.2 million in 2021 and $61.8 million in 2020.
At December 31, 2022, we had $9.0 million of cash and cash equivalents. As of March 31, 2023, we had accounts payable of $29.0 million, of which approximately 75% is owed to our primary contract manufacturer, that was subsequently agreed to be settled for a convertible note as described in “Prospectus Summary — Recent Developments,” and we only had cash and cash equivalents of $3.1 million. Accordingly, we will need substantially more funding to advance our product candidates through development and into commercialization, including to put in place manufacturing capacity to support such commercial activity. Our future capital requirements will depend on many factors, including:
| |
•
|
our ability to raise capital to fund our operations;
|
| |
•
|
the progress, scope, costs and results of our clinical and preclinical testing of any current or future product candidates;
|
| |
•
|
the possibility of delays in, adverse events of and excessive costs of the development process;
|
| |
•
|
the cost of manufacturing our product candidates;
|
| |
•
|
the cost of prosecuting, defending and enforcing patent claims and other intellectual property rights;
|
| |
•
|
the time and cost involved in obtaining regulatory approvals;
|
| |
•
|
expenses related to complying with cGMP of therapeutic product candidates;
|
| |
•
|
costs of financing or acquiring additional capital equipment and development technologies;
|
| |
•
|
competing technological and market developments;
|
| |
•
|
our ability to establish and maintain collaborative and other arrangements with third parties to assist in bringing our products to market and the cost of such arrangements;
|
| |
•
|
the amount and timing of payments or equity investments that we receive from collaborators or changes in or terminations of future or existing collaboration and licensing arrangements and the timing and amount of expenses we incur to support these collaborations and license agreements;
|
| |
•
|
costs associated with the integration of any new operation, including costs relating to future mergers and acquisitions with companies that have complementary capabilities;
|
| |
•
|
expenses related to the establishment of sales and marketing capabilities for products awaiting approval or products that have been approved;
|
| |
•
|
expenses related to establishing manufacturing capabilities;
|
| |
•
|
the level of our sales and marketing expenses; and
|
| |
•
|
our ability to introduce and sell new products.
|
We have secured capital historically from grant revenues, collaboration proceeds and debt and equity offerings. We will need to secure substantial additional capital to fund our future operations. We cannot be certain that additional capital will be available on acceptable terms or at all. To the extent we raise additional capital through the sale of equity securities, the ownership position of our existing stockholders could be substantially diluted. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock. Fluctuating interest rates could also increase the costs of any debt financing we may obtain.
Importantly, we expect that the results of our MASTERS-2 clinical trial, will have a significant impact, favorable or unfavorable, on our ability to access capital from potential third-party commercial partners or the equity capital markets. Depending on the nature of these results, we may accelerate or may delay certain programs. In the longer term, we will have to continue to generate additional capital to meet our needs until we would become cash flow positive as a result of the sales of our clinical products, if they are approved for marketing.
Failure to successfully address ongoing liquidity requirements will have a material adverse effect on our business. If we are unable to obtain additional capital on acceptable terms when needed, we may be required to take actions that harm our business and our ability to achieve cash flow in the future, including possibly the surrender of our rights to some technologies or product opportunities, delaying our clinical trials or curtailing or ceasing operations.
If we are unable to raise capital, we could be forced to eliminate our product development programs, may be unable to continue our business and may need to file for protection under the bankruptcy laws.
There is substantial doubt about our ability to continue as a going concern, which may affect our ability to obtain future financing and may require us to curtail our operations. In the near term, we will need substantial additional funding to develop our MultiStem product candidate and to continue our operations. Even if we are able to obtain additional funding in the near term, such funding may not be sufficient to allow us to continue our operations for an extended period of time.
The audited financial statements and accompanying notes presented in the Annual Report on Form 10-K for the year ended December 31, 2022 include disclosures and an opinion from our independent registered public accounting firm stating that our recurring losses and negative cash flows from operations raise substantial doubt about our ability to continue as a going concern.
As of March 31, 2023, we had accounts payable of $29.0 million that is currently due and we only had cash and cash equivalents of $3.1 million. To conserve cash, we have been delaying payments to most of our suppliers and service providers, including our primary contract manufacturer. In the near term, we will need to obtain significant capital funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources to fund our operations. However, there can be no assurance that we will be able to obtain adequate funding on terms acceptable to us, on a timely basis or at all, particularly in light of our current stock price and liquidity. If we are unable to obtain funding, we may be required to further delay, reduce or eliminate our MultiStem product candidate approval efforts, which could adversely affect our business prospects, and we may be unable to continue operations.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, products or product candidates or to grant licenses on terms that may not be favorable to us.
Adequate additional financing may not be available to us on acceptable terms, or at all. There can be no assurance that we will be able to license-out our MultiStem product candidate on a timely basis or on terms that are favorable to us, or at all. Our failure to raise capital through financing or a license as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We could be forced to discontinue the development of our MultiStem product candidate and seek collaborators on terms that are less favorable than might otherwise be available, and relinquish or license, potentially on unfavorable terms, our rights to MultiStem. If we are unable to obtain adequate financing, we would likely have to file for protection under the bankruptcy laws to continue to pursue potential transactions and conduct a wind-down of our Company. If we decide to dissolve and liquidate our assets or to seek protection under the bankruptcy laws, it is unclear to what extent we will be able to pay our obligations, and, accordingly, it is further unclear whether and to what extent any resources will be available for distributions to stockholders.
We have incurred losses since inception, and we expect to incur significant net losses in the foreseeable future and may never become profitable.
Since our inception in 1995, we incurred significant losses and negative cash flows from operations. We incurred net losses of $72.5 million in 2022, $87.0 million in 2021 and $78.8 million in 2020. As of March 31, 2023, we had an accumulated deficit of $663.7 million, and we will not commence sales of our clinical product candidates until they receive regulatory approval for commercialization. We expect to spend significant resources over the next several years to continue our research and product development programs, including clinical trials of our product candidates and to prepare for possible regulatory approval and commercial activities. We expect to continue to incur substantial losses through at least the next several years and may incur losses in subsequent periods, and our ability to commercialize our product candidates is uncertain. To date, substantially all of our revenue has been derived from corporate collaborations, license agreements and government grants. In order to achieve profitability, we must develop products and technologies that can be commercialized by us or through our existing or future collaborations. Our ability to generate revenues and become profitable will depend on our ability, alone or with potential collaborators, to timely, efficiently and successfully complete the development of our product candidates. We have never earned revenue from selling a product and we may never do so, as none of our product candidates have been approved for sale, since they are currently being tested in human studies. We cannot assure you that we will ever earn sales revenue or that we will ever become profitable. If we sustain losses over an extended period, we may be unable to continue our business.
Risks Related to Our Dependence on Third Parties
We may not successfully maintain our existing collaborative and licensing arrangements, or establish new ones, which could adversely affect our ability to develop and commercialize our product candidates.
A key element of our business strategy is to commercialize some of our product candidates through collaborations with other companies. Our strategy includes establishing collaborations and licensing agreements with one or more pharmaceutical, biotechnology or device companies, preferably after we have advanced product candidates through the initial stages of clinical development. However, we may not be able to establish or maintain such licensing and collaboration arrangements necessary to develop and commercialize our product candidates. Even if we are able to maintain or establish licensing or collaboration arrangements, these arrangements may not be on favorable terms and may contain provisions that will restrict our ability to develop, test and market our product candidates. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
Our agreements with our collaborators and licensees may have provisions that give rise to disputes regarding the rights and obligations of the parties. These and other possible disagreements could lead to termination of the agreement or delays in collaborative research, development, supply, or commercialization of certain product candidates, or could require or result in litigation or arbitration. Moreover, disagreements could arise with our collaborators over rights to intellectual property or our rights to share in any of the future revenues of products developed by our collaborators. These kinds of disagreements could result in costly and time-consuming litigation. Any such conflicts with our collaborators could reduce our ability to obtain future collaboration agreements and could have a negative impact on our relationship with existing collaborators.
Currently, we have a material collaboration and licensing arrangement is with Healios, also a significant holder of our outstanding shares of common stock, to develop and commercialize MultiStem cell therapy for the treatment of ischemic stroke and ARDS in Japan, among other things, and we also have license agreements with third parties pursuant to which we in-license certain aspects of our technologies. These arrangements may not have specific termination dates; rather, each arrangement terminates upon the occurrence of certain events.
Healios has alleged that we are in material breach of our comprehensive framework agreement for commercial manufacturing and ongoing support for, among other things, not meeting our supply obligations and cooperation and assistance obligations. We strongly disagree with Healios’ allegations and will continue to work with Healios to try to resolve this dispute. However, there can be no assurance that we will be able to resolve this dispute without legal proceedings, which could divert management’s attention, be costly and result in damages and/or costly injunctive or other remedies.
Risks Related to Our Common Stock and This Offering
Our management will have broad discretion over the use of the proceeds to us from this offering and may apply it to uses that do not improve our operating results or the value of our securities.
Our management will have broad discretion in the application of the net proceeds from this offering, and investors will be relying solely on such judgment of our management regarding the application of these proceeds. We have not allocated the net proceeds for specific purposes. Investors will not have the opportunity, as part of their investment decision, to assess whether the proceeds are being used appropriately. Our use of the proceeds may not improve our operating results or increase the value of the securities being offered hereby.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase.
Since the price per share of our common stock being offered is substantially higher than the historical net tangible book value per share of our common stock, you will suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. If you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of approximately $ per share in the adjusted net tangible book value of the common stock. See the section entitled “Dilution” in this prospectus for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common stock.
In the future, we may need to issue additional authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our stockholders. In May 2023, we also issued the Note to a supplier in exchange for a reduction in the amount of our liabilities owed to the supplier, and the Note is convertible into a significant amount of our common stock. We also have outstanding options and warrants that are convertible into shares of common stock.We may also issue additional common stock or other securities that are convertible into or exercisable for common stock in connection with hiring or retaining employees, future acquisitions, future sales of securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of common stock may create downward pressure on the trading price of our common stock. There can be no assurance that we will not be required to issue additional shares, warrants, or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the offering price of the shares of common stock in this offering.
We are not currently in compliance with Nasdaq’s continued listing requirements. If we are unable to comply with Nasdaq’s continued listing requirements, our common stock could be delisted, which could affect the price of our common stock and liquidity and reduce our ability to raise capital.
Our common stock is currently listed on The Nasdaq Capital Market. The Nasdaq Capital Market has established certain quantitative criteria and qualitative standards that companies must meet to remain listed for trading on this market.
On October 14, 2022, we received a written notice (the “Notice”) from the Listing Qualifications Department of The Nasdaq Stock Market LLC that we are not in compliance with the requirement to maintain a minimum market value of listed securities of $35 million, as set forth in Nasdaq Listing Rule 5550(b)(2) (the “Market Value Standard”) because the market value of the common stock was below $35 million for 30 consecutive business days. The Notice does not impact the listing of the common stock on The Nasdaq Capital Market at this time.
The Notice provided that, in accordance with Nasdaq Listing Rule 5810(c)(3)(C), the Company had a period of 180 calendar days from the date of the Notice, or until April 12, 2023, to regain compliance under the Market Value Standard.
On April 13, 2023, we received notice from The Nasdaq Stock Market that we had not regained compliance with the Market Value Standard. On April 14, 2023, we filed our request for a hearing, which was held before the Nasdaq Hearing Panel (the “Panel”) on May 18, 2023.
On June 26, 2023, the Panel notified the Company that it had granted our request for an exception through September 15, 2023, to the continued listing requirements, subject to the Company demonstrating compliance with the Market Value Standard by September 15, 2023.
The Panel noted that it reserves the right to reconsider the terms of this exception based on any event, condition or circumstance that exists or develops that would, in the opinion of the Panel, make continued listing of the Company’s securities on Nasdaq inadvisable or unwarranted. In addition, the Nasdaq Listing and Hearing Review Council may, on its own motion, determine to review any Panel decision within 45 calendar days after issuance of the written decision.
There can be no assurance that the Company will be able to regain compliance with the Market Value Standard, or will otherwise be in compliance with other Nasdaq listing criteria. While the Company is exercising diligent efforts to maintain the listing of the Company’s common stock on The Nasdaq Capital Market, there can be no assurance that the Company will be able to regain or maintain compliance with Nasdaq listing criteria. If the Company fails to regain compliance with Nasdaq’s continued listing standards during the exception period granted by the Panel, our common stock will be subject to delisting from Nasdaq. Any delisting of our common stock could adversely affect the market liquidity of our common stock and the market price of our common stock could decrease. Furthermore, if our common stock were delisted it could adversely affect our ability to obtain financing for the continuation of our operations and our ability to attract and retain employees by means of equity compensation and/or result in the loss of confidence by investors.
Broad market and industry factors, as well as economic and political factors, also may materially adversely affect the market price of Athersys’ common stock. The market price of our common stock has been extremely volatile and may continue to be volatile due to numerous circumstances beyond our control.
The market price of our common stock has fluctuated, and may continue to fluctuate, widely, due to many factors, some of which may be beyond our control. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock:
| |
•
|
developments in our clinical trials, particularly our MASTERS-2 trial;
|
| |
•
|
announcements of significant changes in our business or operations, including the decision to implement restructurings such as a reduction in our workforce;
|
| |
•
|
the development status of our MultiStem product candidate, including clinical study results and determinations by regulatory authorities with respect thereto;
|
| |
•
|
the initiation, termination or reduction in the scope of any collaboration arrangements, including with Healios, or any disputes or developments regarding such collaborations;
|
| |
•
|
our inability to obtain additional funding;
|
| |
•
|
disputes or other developments concerning our proprietary rights;
|
| |
•
|
additions or departures of key personnel;
|
| |
•
|
comments by securities analysts or discussions of our business, products, financial performance, prospects or stock price by the financial and scientific press and online investor communities;
|
| |
•
|
changes in, or failure to meet, securities analysts’ or investors’ expectations of our financial performance;
|
| |
•
|
large stockholders exiting their position in our common stock or an increase or decrease in the short interest in our common stock;
|
| |
•
|
public concern as to, and legislative action with respect to, the pricing and availability of prescription drugs or the safety of drugs and drug delivery techniques;
|
| |
•
|
regulatory developments in the United States and in foreign countries;
|
| |
•
|
dilutive effects of sales of shares of common stock by Athersys or Athersys’ stockholders; and
|
| |
•
|
overall general market fluctuations.
|
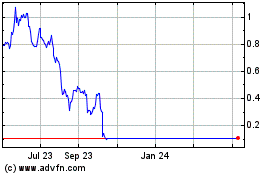
The market prices for securities of biopharmaceutical and biotechnology companies, and early-stage drug discovery and development companies like Athersys in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies and our company. For example, on July 26, 2022 and July 28, 2022, the closing price of our common stock on The Nasdaq Capital Market was $4.25 and $8.25, respectively, and daily trading volume on these days was approximately 0.2 million and 10.7 million shares, respectively.
During this time, we did not release any material information regarding us or our business. These broad market fluctuations may adversely affect the trading price of our common stock. In particular, a proportion of our common stock has been and may continue to be traded by short sellers, which may put pressure on the supply and demand for our common stock, further influencing volatility in its market price. Additionally, these and other external factors have caused and may continue to cause the market price and demand for our common stock to fluctuate, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock.
In addition, in the past, following periods of market volatility in the market price of a company’s securities or the reporting of unfavorable news, securities litigation has often been instituted against these companies. Volatility in the market price of our shares could also increase the likelihood of regulatory scrutiny. Securities litigation, if instituted against us, or any regulatory inquiries or actions that we face could result in substantial costs, diversion of our management’s attention and resources and unfavorable publicity, regardless of the merits of any claims made against us or the ultimate outcome of any such litigation or action.
A “short squeeze” is a sudden increase in demand for shares of our common stock that largely could lead to extreme price volatility in shares of our common stock.
Investors may purchase shares of our common stock to hedge existing exposure or to speculate on the price of our common stock. Speculation on the price of our common stock may involve long and short exposures. To the extent aggregate short exposure exceeds the number of shares of our common stock available for purchase on the open market, investors with short exposure may have to pay a premium to repurchase shares of our common stock for delivery to lenders of our common stock. Those repurchases may, in turn, dramatically increase the price of our common stock until additional shares of our common stock are available for trading or borrowing. This is often referred to as a “short squeeze.” A proportion of our common stock has been and may continue to be traded by short sellers, which may increase the likelihood that our common stock will be the target of a short squeeze. A short squeeze could lead to volatile price movements in shares of our common stock that are unrelated or disproportionate to our operating performance and, once investors purchase the shares of our common stock necessary to cover their short positions, the price of our common stock may rapidly decline. Investors that purchase shares of our common stock during a short squeeze may lose a significant portion of their investment.
There is no public market for the Pre-Funded Warrants or Common Warrant being offered in this offering.
There is no established public trading market for the Pre-Funded Warrants or Common Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants or Common Warrants on Nasdaq or any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the Pre-Funded Warrants and Common Warrants will be limited.
We may not receive any additional funds upon the exercise of the Pre-Funded Warrants or Common Warrants.
Each Pre-Funded Warrant and Common Warrant may be exercised by way of a cashless exercise, meaning that the holder may not pay a cash purchase price upon exercise, but instead would receive upon such exercise the net number of shares of our common stock determined according to the formula set forth in the Pre-Funded Warrant or Common Warrant. Accordingly, we may not receive any additional funds upon the exercise of the Pre-Funded Warrants or Common Warrants.
The Pre-Funded Warrants and Common Warrants are speculative in nature.
The Pre-Funded Warrants and Common Warrants do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the Common Warrants may exercise their right to acquire common stock and pay an exercise price of $ per share, subject to certain adjustments, prior to years from the date on which such warrants were issued, after which date any unexercised Common Warrants will expire and have no further value. Holders of Pre-Funded Warrants have identical rights, except that the Pre-Funded Warrants have an exercise price of $0.0001 and do not expire until exercised in full. Moreover, following this offering, the market value of the Pre-Funded Warrants and Common Warrants, if any, is uncertain and there can be no assurance that the market value of the Pre-Funded Warrants and Common Warrants will equal or exceed their imputed offering price. The Pre-Funded Warrants and Common Warrants will not be listed or quoted for trading on any market or exchange. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the Common Warrants and consequently, whether it will ever be profitable for holders of the Common Warrants to exercise the warrants.
Holders of the Common Warrants and Pre-Funded Warrants offered hereby will have no rights as common stockholders with respect to the shares our common stock underlying the warrants until such holders exercise their warrants and acquire our common stock, except as otherwise provided in the Common Warrants and Pre-Funded Warrants.
Until holders of the Common Warrants and/or Pre-Funded Warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our common stock underlying such warrants, except to the extent that holders of such Common Warrants and Pre-Funded Warrants will have certain rights to participate in distributions or dividends paid on our common stock as set forth in the Common Warrants. Upon exercise of the Common Warrants and Pre-Funded Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
The best efforts structure of this offering may have an adverse effect on our business plan.
The Placement Agent is offering the securities in this offering on a reasonable best efforts basis. The Placement Agent is not required to purchase any securities, but will use its reasonable best efforts to sell the securities offered. As a “best efforts” offering, there can be no assurance that the offering contemplated hereby will ultimately be consummated or will result in any proceeds being made available to us. The success of this offering will impact our ability to use the proceeds to execute our business plan. An adverse effect on the business may result from raising less than anticipated, and from the fact that there is no minimum raise.
Purchasers who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase agreement.
In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement.
Additionally, in connection with this offering, we may agree to amend the terms of certain of our outstanding warrants held by certain significant purchasers in this offering who will enter into the securities purchase agreement. Any such amendments may, among other things, decrease the exercise prices to be the same as the exercise prices of the Common Warrants offered in this offering, or increase the term of exercise of those warrants.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference herein and therein contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties. These forward-looking statements relate to, among other things, the expected timetable for development of our product candidates, our growth strategy and our future financial performance, including our operations, economic performance, financial condition, prospects and other future events. We have attempted to identify forward-looking statements by using such words as “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “should,” “suggest,” “will” or other similar expressions. These forward-looking statements are only predictions and are largely based on our current expectations.
In addition, a number of known and unknown risks, uncertainties and other factors could affect the accuracy of these statements. Some of the more significant known risks that we face are the risk that we will be unable to raise capital to fund our operations in the near term and long term, including our ability to obtain funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources, on terms acceptable to us or at all, and to continue as a going concern and our ability to successfully resolve the payment issues with our primary contract manufacturer and gain access to our clinical product. The following risks and uncertainties may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements:
| |
•
|
our ability to raise capital to fund our operations in the near term and long term, including our ability to obtain funding through public or private equity offerings, debt financings, collaborations and licensing arrangements or other sources, on terms acceptable to us or at all, and to continue as a going concern;
|
| |
•
|
our collaborators’ ability and willingness to continue to fulfill their obligations under the terms of our collaboration agreements and generate sales related to our technologies;
|
| |
•
|
the possibility of unfavorable results from ongoing and additional clinical trials involving our MultiStem® (invimestrocel) cell therapy, or MultiStem;
|
| |
•
|
the risk that positive results in a clinical trial may not be replicated in subsequent or confirmatory trials or success in an early stage clinical trial may not be predictive of results in later stage or large scale clinical trials;
|
| |
•
|
our ability to regain compliance with the requirement to maintain a minimum market value of listed securities of $35 million as set forth in Nasdaq Listing Rule 5550(b)(2);
|
| |
•
|
the timing and nature of results from MultiStem clinical trials, including the MASTERS-2 Phase 3 clinical trial evaluating the administration of MultiStem for the treatment of ischemic stroke;
|
| |
•
|
our ability to meet milestones and earn royalties under our collaboration agreements, including the success of our collaboration with Healios;
|
| |
•
|
the success of our MACOVIA clinical trial evaluating the administration of MultiStem for the treatment of ARDS induced by COVID-19 and other pathogens, and the MATRICS-1 clinical trial being conducted with The University of Texas Health Science Center at Houston evaluating the treatment of patients with serious traumatic injuries;
|
| |
•
|
the availability of product sufficient to meet our clinical needs and potential commercial demand following any approval;
|
| |
•
|
the possibility of delays in, adverse results of, and excessive costs of the development process;
|
| |
•
|
our ability to successfully initiate and complete clinical trials of our product candidates;
|
| |
•
|
the possibility of delays, work stoppages or interruptions in manufacturing by third parties or us, such as due to material supply constraints, contamination, operational restrictions due to COVID-19 or other public health emergencies, labor constraints, regulatory issues or other factors that could negatively impact our trials and the trials of our collaborators;
|
| |
•
|
uncertainty regarding market acceptance of our product candidates and our ability to generate revenues, including MultiStem cell therapy for neurological, inflammatory and immune, cardiovascular and other critical care indications;
|
| |
•
|
changes in external market factors;
|
| |
•
|
changes in our industry’s overall performance;
|
| |
•
|
changes in our business strategy;
|
| |
•
|
our ability to protect and defend our intellectual property and related business operations, including the successful prosecution of our patent applications and enforcement of our patent rights, and operate our business in an environment of rapid technology and intellectual property development;
|
| |
•
|
our possible inability to realize commercially valuable discoveries in our collaborations with pharmaceutical and other biotechnology companies;
|
| |
•
|
the success of our efforts to enter into new strategic partnerships and advance our programs;
|
| |
•
|
our possible inability to execute our strategy due to changes in our industry or the economy generally;
|
| |
•
|
changes in productivity and reliability of suppliers;
|
| |
•
|
the success of our competitors and the emergence of new competitors;
|
| |
•
|
our ability to maintain our listing on Nasdaq and meet Nasdaq’s listing requirements; and
|
| |
•
|
the risks described in our Annual Report on Form 10-K for the year ended December 31, 2022 and our other filings with the SEC.
|
Any forward-looking statement you read in this prospectus or any document incorporated by reference herein reflects our current views with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, operating results, growth strategy and liquidity. Although we currently believe that the expectations reflected in these forward-looking statements are reasonable, we cannot guarantee our future results, levels of activity or performance. You should not place undue reliance on these forward-looking statements because such statements speak only as of the date when made. We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as otherwise required by law. You are advised, however, to consult any further disclosures we make on related subjects in our reports on Forms 10-Q, 8-K and 10-K filed with the SEC. You should understand that it is not possible to predict or identify all risk factors. Consequently, you should not consider any such list to be a complete set of all potential risks or uncertainties.
Any document incorporated by reference in this prospectus may also contain statistical data and estimates we obtained from industry publications and reports generated by third parties. Although we believe that such publications and reports are reliable, we have not independently verified their data.
USE OF PROCEEDS
We estimate that the net proceeds to us from the sale of the all securities offered hereby will be approximately $ million, based upon an assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting estimated placement agent fees and estimated offering expenses payable by us.
Each $0.25 increase or decrease in the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting placement agent fees and estimated offering expenses payable by us. Similarly, each increase or decrease of 1,000,000 shares in the number of shares of common stock offered by us would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $ million, assuming the public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, remains the same, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting placement agent fees and estimated offering expenses payable by us. We do not expect that a change in the public offering price or the number of shares by these amounts would have a material effect on our uses of the proceeds from this offering, although it may accelerate the time at which we will need to seek additional capital.
We intend to use the net proceeds from this offering for working capital and general corporate purposes. The amount, timing and nature of specific expenditures of net proceeds from this offering will depend on a number of factors, including the timing, scope, progress and results of our development efforts and the timing and progress of any collaboration efforts. As of the date of this prospectus, we cannot specify with certainty all of the particular uses of the proceeds from this offering. Accordingly, we will retain broad discretion over the use of such proceeds.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock. We would have to rely upon dividends and other payments from our wholly-owned subsidiary, ABT Holding Company, to generate the funds necessary to make dividend payments, if any, on our common stock. ABT Holding Company, however, is legally distinct from us and has no obligation to pay amounts to us. We do not anticipate that we will pay any dividends on our common stock in the foreseeable future. Rather, we anticipate that we will retain earnings, if any, for use in the development of our business. Payment of future cash dividends, if any, will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, the requirements of then-existing debt instruments, and other factors that the board of directors deems relevant.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of March 31, 2023:
| |
• |
on a pro forma basis to give effect to the receipt of $3,380,347 of net proceeds from the issuance of 2,315,000 shares of common stock in the April 2023 Offerings, the receipt of $81 of proceeds upon exercise of 813,000 shares of common stock underlying the pre-funded warrants issued in the April 2023 Offerings, and the vesting of 367,664 restricted stock units; and |
| |
• |
on a pro forma as adjusted basis to give effect to our issuance and sale of shares of our common stock in this offering at the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, and after deducting placement agent fees and estimated offering expenses payable by us. |
The as adjusted information below is illustrative only, and our capitalization following the completion of this offering is subject to adjustment based on the actual public offering price of our common stock and other terms of this offering determined at pricing. You should read the information in this table together with our financial statements and the related notes appearing elsewhere in this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of our most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q.
| |
|
|
As of March 31, 2023
|
|
| |
|
|
Actual
|
|
|
Pro
Forma
|
|
|
As
Adjusted
(1)(2)
|
|
| |
|
|
(in thousands except share and per share data)
|
|
|
Cash and cash equivalents
|
|
$ |
3,121 |
|
|
$ |
6,366 |
|
|
|
|
|
|
Warrant liabilities and note payable
|
|
$ |
(1,163 |
) |
|
$ |
(1,163 |
) |
|
$ |
(1,163 |
) |
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Preferred stock, at stated value; 10,000,000 shares authorized, and no shares issued and outstanding at March 31, 2023, actual and as adjusted
|
|
|
— |
|
|
|
— |
|
|
|
|
|
| |
Common stock, $0.001 par value; 600,000,000 shares authorized, and 18,448,489 shares issued and outstanding at March 31, 2023, actual, and shares issued and outstanding at March 31, 2023, as adjusted
|
|
|
18 |
|
|
|
22 |
|
|
|
|
|
| |
Additional paid-in capital
|
|
|
632,660 |
|
|
|
635,917 |
|
|
|
|
|
| |
Accumulated deficit
|
|
|
(663,689 |
) |
|
|
(663,705 |
) |
|
|
(663,705 |
) |
|
Total stockholders’ equity
|
|
|
(31,011 |
) |
|
|
(27,766 |
) |
|
|
|
|
|
Total capitalization
|
|
$ |
(32,174 |
) |
|
$ |
(28,929 |
) |
|
|
|
|
|
(1)
|
A $0.25 increase (decrease) in the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, would increase (decrease) each of our as adjusted cash and cash equivalents, common stock, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting placement agent’s fees and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1,000,000 shares in the number of shares of common stock offered by us would increase (decrease) each of our as adjusted cash and cash equivalents, common stock, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, remains the same, and after deducting placement agent fees and estimated offering expenses payable by us.
|
|
(2)
|
The foregoing discussion and table above exclude, as of March 31, 2023, the following:
|
| |
•
|
a total of 1,626,312 shares of common stock authorized and reserved for future issuance under outstanding awards under our equity incentive plans;
|
| |
•
|
a total of 27,491 shares of common stock authorized and reserved for future issuance under our equity incentive plans;
|
| |
•
|
a total of 429,500 shares of common stock authorized and reserved for future issuance under outstanding inducement awards granted outside of our equity incentive plans;
|
| |
•
|
1,920,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued and sold in August 2022;
|
| |
•
|
2,000,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in September 2022;
|
| |
•
|
a total of 9,109,090 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our November 2022 public offering;
|
| |
•
|
a total of 3,685,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our April 2023 public offering;
|
| |
•
|
a total of 11,538,461 shares of common stock authorized and reserved for future issuance upon conversation feature of our May 2023 Convertible Note; and
|
| |
•
|
400,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants held by Healios.
|
DILUTION
If you invest in our securities in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
As of March 31, 2023, we had a historical net tangible book value of -$31.01 million, or -$1.681 per share of common stock, based on the 18,448,489 shares of common stock outstanding as of such date. Our historical net tangible book value (deficit) is the amount of our total tangible assets less our total liabilities. Our historical net tangible book value (deficit) per share represents historical net tangible book value (deficit) divided by the number of shares of our common stock outstanding as of March 31, 2023.
After giving effect to our issuance and sale of shares of common stock in this offering at the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting placement agent fees and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of would have been $ million, or $ per share. This amount represents an immediate increase in our pro forma as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution in our pro forma as adjusted net tangible book value of $ per share to investors purchasing shares of our common stock in this offering. We determine dilution per share to investors participating in this offering by subtracting pro forma as adjusted net tangible book value per share after this offering from the assumed initial public offering price per share paid by investors participating in this offering.
The following table illustrates this dilution on a per share basis:
|
Assumed public offering price per share
|
|
|
|
|
|
$ |
|
|
|
Pro forma net tangible book value per share as of March 31, 2023
|
|
$ |
|
|
|
|
|
|
|
Increase in net tangible book value (deficit) per share attributable to equity transactions since March 31, 2023
|
|
$ |
0.42 |
|
|
|
|
|
|
Increase in net tangible book value (deficit) per share attributable to new investors purchasing common stock in this offering
|
|
$ |
|
|
|
|
|
|
|
Pro forma as adjusted net tangible book value (deficit) per share after giving effect to this offering
|
|
|
|
|
|
$ |
|
|
|
Dilution in pro forma as adjusted net tangible book value (deficit) per share to new investors purchasing common stock in this offering
|
|
|
|
|
|
$ |
|
|
The dilution information discussed above is illustrative only and may change based on the actual public offering price and other terms of this offering. Each $0.25 increase or decrease in the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, would increase or decrease, as applicable, our pro forma as adjusted net tangible book value per share after this offering by $ per share and increase or decrease, as applicable, the dilution to investors purchasing shares in this offering by $ per share, in each case assuming the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting placement agent fees and estimated offering expenses payable by us. Similarly, an increase of 1,000,000 in the number of shares of common stock offered by us would increase the pro forma as adjusted net tangible book value after this offering by $ per share and decrease the dilution per share to new investors participating in this offering by $ per share, and a decrease of 1,000,000 shares of common stock offered by us would decrease the pro forma as adjusted net tangible book value by $ per share, and increase the dilution per share to new investors in this offering by $ per share, in each case assuming the assumed public offering price of $ per share, which is the last reported sale price of our common stock on Nasdaq on , 2023, remains the same, assuming no sale of any Pre-Funded Warrants and no exercise of the Common Warrants issued in connection with this offering, and after deducting placement agent fees and estimated offering expenses payable by us.
The as adjusted number of shares of common stock outstanding after this offering includes the following:
| |
•
|
18,448,489 shares of common stock outstanding as of March 31, 2023;
|
| |
• |
on a pro forma basis to give effect to the receipt of $3,380,347 of net proceeds from the issuance of 2,315,000 shares of common stock in the April 2023 Offerings, the receipt of $81 of proceeds upon exercise of 813,000 shares of common stock underlying the pre-funded warrants issued in the April 2023 Offerings, and the vesting of 367,664 restricted stock units; and |
| |
•
|
shares of common stock to be issued at the closing of this offering.
|
The pro forma as adjusted number of shares of common stock outstanding after this offering excludes, as of March 31, 2023, the following:
| |
•
|
a total of 1,626,312 shares of common stock authorized and reserved for future issuance under outstanding awards under our equity incentive plans;
|
| |
•
|
a total of 27,491 shares of common stock authorized and reserved for future issuance under our equity incentive plans;
|
| |
•
|
a total of 429,500 shares of common stock authorized and reserved for future issuance under outstanding inducement awards granted outside of our equity incentive plans;
|
| |
•
|
1,920,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued and sold in August 2022;
|
| |
•
|
2,000,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in September 2022;
|
| |
•
|
a total of 9,109,090 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our November 2022 public offering;
|
| |
•
|
a total of 3,685,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants issued in connection with our April 2023 public offering;
|
| |
•
|
a total of 11,538,461 shares of common stock authorized and reserved for future issuance upon conversation feature of our May 2023 Convertible Note; and
|
| |
•
|
400,000 shares of common stock authorized and reserved for future issuance upon exercise of warrants held by Healios.
|
To the extent that new stock options are issued or any outstanding stock options are exercised, or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
DESCRIPTION OF CAPITAL STOCK
General
The following description of our capital stock and certain provisions of our certificate of incorporation, as amended (“Certificate of Incorporation”), and our bylaws ("Bylaws") is a summary and is qualified in its entirety by reference to our Certificate of Incorporation and our Bylaws, which are filed as exhibits to the registration statement of which this prospectus forms a part.
We are authorized to issue 600,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share.
Common Stock
This section describes the general terms and provisions of our common stock. For more detailed information, you should refer to our Certificate of Incorporation and Bylaws, copies of which have been filed with the SEC. These documents are also incorporated by reference into the registration statement of which this prospectus forms a part.
Holders of shares of our common stock will be entitled to receive dividends if and when declared by the board of directors from funds legally available therefor, and, upon liquidation, dissolution or winding-up of our company, will be entitled to share ratably in all assets remaining after payment of liabilities. The holders of shares of our common stock will not have any preemptive rights, but will be entitled to one vote for each share of common stock held of record. Stockholders will not have the right to cumulate their votes for the election of directors. The shares of our common stock offered hereby, when issued, will be fully paid and nonassessable.
Preferred Stock
This section describes the general terms and provisions of our preferred stock. For more detailed information, you should refer to our Certificate of Incorporation and Bylaws, copies of which have been filed with the SEC. These documents are also incorporated by reference into the registration statement of which this prospectus forms a part.
Our board of directors is authorized, without action by our stockholders, to designate and issue up to 10,000,000 shares of preferred stock, par value $0.001 per share, in one or more series. The board of directors can fix the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions. Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of our common stock. The issuance of preferred stock, while providing flexibility in connection with possible future financings, acquisitions and other corporate purposes could, under certain circumstances, have the effect of delaying, deferring or preventing a change in control of us and could adversely affect the market price of our common stock. We do not have any shares of preferred stock outstanding, and we have no current plans to issue any preferred stock.
Transfer Agent and Registrar
We have appointed Computershare Investor Services as the transfer agent and registrar for our common stock.
Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “ATHX.”
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering up to $ shares (the “Shares”) of our common stock, par value $0.001 per share, together with common warrants to purchase up to shares of common stock (the “Common Warrants”) at an assumed combined public offering price of $ , which is the last sale price of our common stock as reported by The Nasdaq Capital Market (“Nasdaq” or the “Nasdaq Capital Market”) on , 2023. The Shares and Common Warrants are immediately separable and will be issued separately in this offering. We are also offering to certain purchasers whose purchase of Shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if any such purchaser so chooses, pre-funded warrants (the “Pre-Funded Warrants”), in lieu of Shares that otherwise would result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. For each Pre-Funded Warrant we sell, the number of Shares we are offering will be decreased on a one-for-one basis.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Capital Stock” in this prospectus and are incorporated herein by reference.
The following summary of certain terms and provisions of Pre-Funded Warrants and Common Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the forms of such warrants, which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in such forms of warrants for a complete description of the terms and conditions of the Pre-Funded Warrants and Common Warrants. The Pre-Funded Warrants will be issued separately from the accompanying Common Warrants and may be transferred separately immediately thereafter.
Pre-Funded Warrants Being Offered in this Offering
Duration and Exercise Price
Each Pre-Funded Warrant offered hereby will have an initial exercise price equal to $0.0001 per share of common stock. The Pre-Funded Warrants will be immediately exercisable and may be exercised at any time until the Pre-Funded Warrants are exercised in full. The exercise price and number of shares issuable upon exercise is subject to appropriate proportional adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice and, within the earlier of (i) two trading days and (ii) the number of trading days comprising the standard settlement period with respect to the common stock as in effect on the date of delivery of the notice of exercise thereafter, payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder may not exercise any portion of the Pre-Funded Warrant to the extent that the holder, together with its affiliates and any other persons acting as a group together with any such persons, would own more than 4.99% (or, at the election of the purchaser, 9.99%) of the number of shares of common stock outstanding immediately after exercise (the “Beneficial Ownership Limitation”); provided that a holder with a Beneficial Ownership Limitation of 4.99%, upon notice to us and effective sixty-one (61) days after the date such notice is delivered to us, may increase the Beneficial Ownership Limitation so long as it in no event exceeds 9.99% of the number of shares of common stock outstanding immediately after exercise.
Cashless Exercise
If, at the time a holder exercises its Pre-Funded Warrants, a registration statement registering the issuance of the shares of common stock underlying the Pre-Funded Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may only exercise its Pre-Funded Warrants (either in whole or in part), at such time by means of a cashless exercise in which the holder shall be entitled to receive upon such exercise the net number of shares of common stock determined according to a formula set forth in the Pre-Funded Warrants, which generally provides for a number of shares equal to (A) (1) the volume weighted average price on (x) the trading day preceding the notice of exercise, if the notice of exercise is executed and delivered on a day that is not a trading day or prior to the opening of “regular trading hours” on a trading day or (y) the trading day of the notice of exercise, if the notice of exercise is executed and delivered after the close of “regular trading hours” on such trading day, or (2) the bid price on the day of the notice of exercise, if the notice of exercise is executed during “regular trading hours” on a trading day and is delivered within two hours thereafter, less (B) the exercise price, multiplied by (C) the number of shares of common stock the Pre-Funded Warrant was exercisable into, with such product then divided by the number determined under clause (A) in this sentence.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Pre-Funded Warrants. Rather, we will, at our election, and in lieu of the issuance of such fractional share, either (i) pay cash in an amount equal to such fraction multiplied by the exercise price or (ii) round up to the next whole share issuable upon exercise of the Pre-Funded Warrant.
Transferability
Subject to applicable laws, a Pre-Funded Warrant may be transferred at the option of the holder upon surrender of the Pre-Funded Warrant to us together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon such transfer.
Trading Market
There is no trading market available for the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system. The shares of common stock issuable upon exercise of the Pre-Funded Warrants are currently listed on Nasdaq under the symbol “ATHX.”
Rights as a Stockholder
Except as otherwise provided in the Pre-Funded Warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the Pre-Funded Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Pre-Funded Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction.
Common Warrants Being Offered in this Offering
Dilution and Exercise Price
Each Common Warrant offered hereby will have an initial exercise price equal to $ per share of common stock. The Common Warrants will be immediately exercisable and will expire on the anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate proportional adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
The Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice and, within the earlier of (i) two trading days and (ii) the number of trading days comprising the standard settlement period with respect to the common stock as in effect on the date of delivery of the notice of exercise thereafter, payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder may not exercise any portion of the Common Warrant to the extent that the holder, together with its affiliates and any other persons acting as a group together with any such persons, would own more than 4.99% (or, at the election of the purchaser, 9.99%) of the number of shares of common stock outstanding immediately after exercise (the “Beneficial Ownership Limitation”); provided that a holder with a Beneficial Ownership Limitation of 4.99%, upon notice to us and effective sixty-one (61) days after the date such notice is delivered to us, may increase the Beneficial Ownership Limitation so long as it in no event exceeds 9.99% of the number of shares of common stock outstanding immediately after exercise.
Cashless Exercise
If, at the time a holder exercises its Common Warrants, a registration statement registering the issuance of the shares of common stock underlying the Common Warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may only exercise its Common Warrants (either in whole or in part), at such time by means of a cashless exercise in which the holder shall be entitled to receive upon such exercise the net number of shares of common stock determined according to a formula set forth in the Common Warrants, which generally provides for a number of shares of common stock equal to (A) (1) the volume weighted average price on (x) the trading day preceding the notice of exercise, if the notice of exercise is executed and delivered on a day that is not a trading day or prior to the opening of “regular trading hours” on a trading day or (y) the trading day of the notice of exercise, if the notice of exercise is executed and delivered after the close of “regular trading hours” on such trading day, or (2) the bid price on the day of the notice of exercise, if the notice of exercise is executed during “regular trading hours” on a trading day and is delivered within two hours thereafter, less (B) the exercise price, multiplied by (C) the number of shares of common stock the Common Warrant was exercisable into, with such product then divided by the number determined under clause (A) in this sentence.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the Common Warrants. Rather, we will, at our election, and in lieu of the issuance of such fractional share, either (i) pay cash in an amount equal to such fraction multiplied by the exercise price or (ii) round up to the next whole share issuable upon exercise of the Common Warrant.
Transferability
Subject to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together with the appropriate instruments of transfer and funds sufficient to pay any transfer taxes payable upon such transfer.
Trading Market
There is no trading market available for the Common Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Common Warrants on any securities exchange or nationally recognized trading system. The shares of common stock issuable upon exercise of the Common Warrants are currently listed on Nasdaq under the symbol “ATHX.”
Rights as a Stockholder
Except as otherwise provided in the Common Warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the Common Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Common Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described in the Common Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, the holders of the Common Warrants will be entitled to receive upon exercise of the Common Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Common Warrants immediately prior to such fundamental transaction. Additionally, in the event of a fundamental transaction, we or any successor entity will, at the option of the holder of a Common Warrant exercisable at any time concurrently with or within 30 days after the consummation of the fundamental transaction (or, if later, the date of the public announcement thereof), purchase the Common Warrant from the holder by paying to the holder an amount of consideration equal to the value of the remaining unexercised portion of such Common Warrant on the date of consummation of the fundamental transaction based on the Black-Scholes option pricing model, determined pursuant to a formula set forth in the Common Warrants. The consideration paid to the holder will be the same type or form of consideration that was offered and paid to the holders of shares of common stock in connection with the fundamental transaction; provided that if no such consideration was offered or paid, the holders of common stock will be deemed to have received common stock of the successor entity in such fundamental transaction for purposes of this provision of the Common Warrants.
Amendment to the Prior Warrants
In connection with this offering, we may enter into privately negotiated agreements with the holders the Prior Warrants to, among other things, reduce the exercise price of such Prior Warrants to that of the Common Warrants being offered and sold in this offering and to extend the current expiration date of the Prior Warrants to the expiration date of the Common Warrants being offered and sold in this offering. In addition, we expect that certain terms of the Prior Warrants will also be amended to be substantially consistent with those of the Common Warrants being offered hereby. There can be no assurance that we will amend the Prior Warrants in connection with this offering or as to the final terms of any amendments to the Prior Warrants.
PLAN OF DISTRIBUTION
A.G.P./Alliance Global Partners has agreed to act as our exclusive placement agent in connection with this offering subject to the terms and conditions of the placement agency agreement dated , 2023. The Placement Agent is not purchasing or selling any of the securities offered by this prospectus, nor is it required to arrange the purchase or sale of any specific number or dollar amount of securities, but has agreed to use its reasonable best efforts to arrange for the sale of the securities offered hereby. Therefore, we may not sell the entire amount of securities offered pursuant to this prospectus. We will enter into a securities purchase agreement directly with certain investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus and the documents incorporated by reference herein in connection with the purchase of our securities in this offering.
We will deliver the securities being issued to the investors upon receipt of such investor’s funds for the purchase of the securities offered pursuant to this prospectus. We will deliver the securities being offered pursuant to this prospectus upon closing. We expect this offering to be completed not later than two (2) business days following the commencement of this offering and we will deliver all securities to be issued in connection with this offering delivery versus payment (“DVP”)/receipt versus payment (“RVP”) upon receipt of investor funds received by us. We expect to deliver the securities being offered pursuant to this prospectus on or about , 2023.
We have agreed to indemnify the Placement Agent and specified other persons against specified liabilities, including liabilities under the Securities Act of 1933, as amended (the “Securities Act”), and to contribute to payments the Placement Agent may be required to make in respect thereof.
Fees and Expenses
We have engaged A.G.P./Alliance Global Partners as our exclusive placement agent in connection with this offering. This offering is being conducted on a reasonable “best efforts” basis and the Placement Agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay the Placement Agent a fee based on the aggregate proceeds as set forth in the table below:
| |
|
Per share of Common
Stock and
Accompanying Common
Warrant
|
|
|
Per Pre-Funded
Warrant and
Accompanying Common
Warrant
|
|
|
Total
|
|
|
Public offering price(1)
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
Placement agent fees(2)
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
Proceeds to us, before expenses(3)
|
|
$ |
|
|
|
$ |
|
|
|
|
|
|
|
(1)
|
The combined public offering price is $ per share of common stock and accompanying Common Warrant and $ per Pre-Funded Warrant and accompanying Common Warrant.
|
|
(2)
|
We have agreed to pay the Placement Agent a total cash fee equal to 7.0% of the gross proceeds of the offering. We have also agreed to reimburse the Placement Agent for its accountable offering-related legal expenses in an amount up to $80,000 and pay the Placement Agent a non-accountable expense allowance of up to $75,000.
|
|
(3)
|
Does not include potential proceeds from the exercise of the Common Warrants and/or Pre-Funded Warrants for cash, if any.
|
As stated in the table above, we have also agreed to reimburse the Placement Agent at closing (i) for legal and other expenses incurred by them in connection with the offering in an aggregate amount up to $80,000, and (ii) non-accountable expenses payable to the Placement Agent of up to $75,000. We estimate the total expenses payable by us for this offering, excluding the Placement Agent fees and expenses, will be approximately $ .
The Placement Agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and any profit realized on the resale of the shares sold by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. As an underwriter, the Placement Agent would be required to comply with the requirements of the Securities Act and the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including, without limitation, Rule 415(a)(4) under the Securities Act and Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of shares by the Placement Agent acting as principal. Under these rules and regulations, the Placement Agent:
| |
•
|
may not engage in any stabilization activity in connection with our securities; and
|
| |
•
|
may not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until it has completed its participation in the distribution.
|
Lock-Up Agreements
Our directors and officers have entered into lock-up agreements. Under these agreements, these individuals agreed, subject to specified exceptions, not to sell or transfer any shares of common stock or securities convertible into, or exchangeable or exercisable for, common stock during a period ending days after the completion of this offering, without first obtaining the written consent of the Placement Agent. Specifically, these individuals agreed, in part, subject to certain exceptions, not to:
| |
•
|
offer for sale, sell, pledge, or otherwise transfer or dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) any shares of common stock or securities convertible into or exercisable or exchangeable for common stock;
|
| |
•
|
enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of common stock; or
|
| |
•
|
make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any of our securities.
|
No Sales of Similar Securities
We have agreed, subject to certain exceptions, not to issue, enter into any agreement to issue or announce the issuance or proposed issuance of, any shares of common stock (or securities convertible into or exercisable for common stock) or, subject to certain exceptions, file any registration statement, including any amendments or supplements thereto (other than the prospectus supplement, registration statement or amendment to the registration statement relating to the securities offered hereunder and a registration statement on Form S-8), until 45 days after the completion of this offering. We have also agreed not to enter into a variable rate transaction (as defined in the securities purchase agreement) for 180 days after the completion of this offering.
Discretionary Accounts
The Placement Agent does not intend to confirm sales of the securities offered hereby to any accounts over which it has discretionary authority.
Listing
Our common stock is listed on The Nasdaq Capital Market under the trading symbol “ATHX.” We do not plan to list the Pre-Funded Warrants or Common Warrants on Nasdaq or any other securities exchange or trading market.
Transfer Agent and Registrar
We have appointed Computershare Investor Services as the transfer agent and registrar for our common stock.
Other Activities and Relationships
The Placement Agent and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The Placement Agent and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the Placement Agent and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the Placement Agent or its affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The Placement Agent and its affiliates may hedge such exposure by entering into transactions that consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The Placement Agent and certain of its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
As stated above, the Placement Agent and its affiliates have and may in the future provide, from time to time, investment banking and financial advisory services to us in the ordinary course of business, for which they may receive customary fees and commissions.
For example, on April 18, 2023, we entered into a securities purchase agreement with each purchaser identified on the signature pages thereto, pursuant to which we agreed to issue and sell, in a public offering, (i) an aggregate of 2,315,000 shares of our common stock, and (ii) pre-funded warrants exercisable for an aggregate of 1,370,000 shares of our common stock, together with warrants exercisable for an aggregate of 3,685,000 shares of our common stock in a concurrent private placement (the “April 2023 Offerings”), which transactions closed on April 19, 2023. We received gross proceeds of approximately $3.6 million from the April 2023 Offerings. A.G.P./Alliance Global Partners served as exclusive Placement Agent for the issuance and sale of the securities in the April 2023 Offerings and received an aggregate cash fee equal to 6.5% of the gross proceeds received by us from the sale of the securities in these offerings. We also reimbursed A.G.P./Alliance Global Partners $65,000 for fees and expenses of its legal counsel and other out-of-pocket expenses and agreed to indemnify A.G.P./Alliance Global Partners and specified other persons against specified liabilities, including liabilities under the Securities Act.
The foregoing does not purport to be a complete statement of the terms and conditions of the placement agency agreement or the securities purchase agreement entered into in connection with this offering, copies of which have been filed as exhibits to the registration statement of which this prospectus is a part. See “Where You Can Find More Information.”
LEGAL MATTERS
The validity of the securities offered by this prospectus will be passed upon for us by Thompson Hine LLP, New York, New York. Blank Rome LLP, New York, New York, is acting as counsel to the Placement Agent in connection with certain legal matters related to this offering.
EXPERTS
The consolidated financial statements of Athersys, Inc. at December 31, 2022 and 2021, and for each of the three years in the period ended December 31, 2022, incorporated by reference in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company's ability to continue as a going concern as described in Note B to the consolidated financial statements) included therein, and incorporated herein by reference. Such financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC, which means that we can disclose important information to you by referring you to those documents. Any information referenced this way is considered to be part of this prospectus, and any information that we file later with the SEC will automatically update and, where applicable, supersede this information. We incorporate by reference the following documents that we have filed with the SEC (other than, in each case, documents or information deemed to have been furnished and not filed in accordance with the SEC’s rules):
| |
•
|
our Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the SEC on April 3, 2023;
|
| |
•
|
our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, as filed with the SEC on May 15, 2023;
|
| |
•
|
our Current Reports on Form 8-K filed on January 5, 2023, January 25, 2023, April 14, 2023, April 18, 2023, May 23, 2023, June 28, 2023, and July 5, 2023; and
|
| |
•
|
the description of our common stock set forth in the registration statement on Form 8-A filed on December 6, 2007, as updated by Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 24, 2022, and all amendments and reports filed for the purpose of updating that description.
|
Additionally, all documents filed by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after (i) the date of the initial filing of the registration statement to which this prospectus relates and prior to effectiveness of such registration statement, and (ii) the date of this prospectus and before the termination or completion of any offering hereunder, shall be deemed to be incorporated by reference into this prospectus from the respective dates of filing of such documents, except that we do not incorporate any document or portion of a document that is “furnished” to the SEC, but not deemed “filed.”
We will not, however, incorporate by reference in this prospectus any documents or portions thereof that are not deemed “filed” with the SEC, including any information furnished pursuant to Item 2.02 or Item 7.01 of our current reports on Form 8-K unless, and except to the extent, specified in such current reports.
We will provide you with a copy of any of these filings (other than an exhibit to these filings, unless the exhibit is specifically incorporated by reference into the filing requested) at no cost, if you submit a request to us by writing or telephoning us at the following address and telephone number:
Athersys, Inc.
3201 Carnegie Avenue
Cleveland, Ohio 44115-2634
(216) 431-9900
Attn: Secretary
Any statements contained in a document incorporated by reference in this prospectus shall be deemed to be modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus (or in any other subsequently filed document which also is incorporated by reference in this prospectus) modifies, supersedes or replaces such statement. Any statement so modified, superseded or replaced shall not be deemed, except as so modified, superseded or replaced, to constitute a part of this prospectus. Statements contained in this prospectus and any document incorporated by reference as to the contents of any contract, agreement or other document referred to are not necessarily complete, and in each instance, reference is made to the copy of the contract, agreement or other document filed as an exhibit to the registration statement or any incorporated document, each statement being so qualified by this reference.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock we are offering pursuant to this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information included in the registration statement. For further information pertaining to us and our common stock, you should refer to the registration statement and to its exhibits. Whenever we make reference in this prospectus to any of our contracts, agreements or other documents, the references are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract, agreement or other document.
We are subject to the informational requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains an internet website that contains reports and other information about issuers, like us, that file electronically with the SEC, and you can read our SEC filings, including the registration statement, at the SEC’s website at www.sec.gov. We also maintain a website at www.athersys.com and you may access, free of charge, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and any amendments to those reports, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of, and is not incorporated into, this prospectus.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our directors and officers are indemnified to the fullest extent permitted under Delaware law. We also maintain insurance which protects our officers and directors against any liabilities incurred in connection with their service in such a capacity.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of ours in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
Up to Shares of Common Stock
Common Warrants to purchase up to shares of Common Stock
Pre-Funded Warrants to purchase up to shares of Common Stock
Up to shares of Common Stock underlying the Common Warrants
Up to shares of Common Stock underlying the Pre-Funded Warrants

Sole Placement Agent
A.G.P.
, 2023
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following are the estimated expenses of the issuance and distribution of the securities being registered, all of which are payable by us. All of the items below, except for the SEC registration fee and FINRA filing fee, are estimates.
| |
|
Amounts to be Paid
|
|
|
SEC registration fee
|
|
|
|
|
|
FINRA filing fee
|
|
$ |
2,644.80 |
|
|
Printing and engraving fee
|
|
|
* |
|
|
Legal fees and expenses
|
|
|
* |
|
|
Accounting fees and expenses
|
|
|
* |
|
|
Transfer agent’s fees
|
|
|
* |
|
|
Miscellaneous fees and expenses
|
|
|
* |
|
|
Total
|
|
|
2,644.80* |
|
|
*
|
Fees and expenses (other than the SEC registration fee to be paid upon the filing of this registration statement) will depend on the number and nature of any offerings of securities made pursuant to this registration statement, and cannot be estimated at this time. An estimate of the aggregate expenses in connection with the distribution of securities being offered will be included in any applicable amendment to this registration statement.
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Delaware law provides that directors of a company will not be personally liable for monetary damages for breach of their fiduciary duty as directors, except for liabilities:
| |
•
|
for any breach of their duty of loyalty to the company or its stockholders;
|
| |
•
|
for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
|
| |
•
|
for unlawful payment of dividend or unlawful stock repurchase or redemption, as provided under Section 174 of the General Corporation Law of the State of Delaware (the “DGCL”); or
|
| |
•
|
for any transaction from which the director derived an improper personal benefit.
|
The provisions of Delaware law that relate to indemnification expressly state that the rights provided by the statute are not exclusive and are in addition to any rights provided in bylaws, by agreement, or otherwise. Our certificate of incorporation also provides that if Delaware law is amended to further eliminate or limit the liability of directors, then the liability of our directors shall be eliminated or limited, without further stockholder action, to the fullest extent permissible under Delaware law as so amended.
Our certificate of incorporation requires us to indemnify, to the fullest extent permitted by the DGCL, any and all persons we have the power to indemnify under the DGCL from and against any and all expenses, liabilities or other matters covered by the DGCL. Additionally, our certificate of incorporation requires us to indemnify each of our directors and officers in each and every situation where the DGCL permits or empowers us (but does not obligate us) to provide such indemnification, subject to the provisions of our bylaws. Our bylaws requires us to indemnify our directors to the fullest extent permitted by the DGCL, and permits us, to the extent authorized by the board of directors, to indemnify our officers and any other person we have the power to indemnify against liability, reasonable expense or other matters.
Under our certificate of incorporation, indemnification may be provided to directors and officers acting in their official capacity, as well as in other capacities. Indemnification will continue for persons who have ceased to be directors, officers, employees or agents, and will inure to the benefit of their heirs, executors and administrators. Additionally, under our certificate of incorporation, except under certain circumstances, our directors are not personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director. At present, there is no pending litigation or proceeding involving any of our directors, officers, or employees in which indemnification is sought, nor are we aware of any threatened litigation that may result in claims for indemnification.
Our bylaws also permit us to secure insurance on behalf of any officer, director, employee, or agent for any liability arising out of actions in his or her capacity as an officer, director, employee, or agent. We have obtained an insurance policy that insures our directors and officers against losses, above a deductible amount, from specified types of claims. Finally, we have entered into indemnification agreements with most of our directors and executive officers, which agreements, among other things, require us to indemnify them and advance expenses to them relating to indemnification suits to the fullest extent permitted by law.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
The following list sets forth information regarding all unregistered securities sold by us over the three year period preceding the date of the prospectus that forms a part of this registration statement.
| |
•
|
On May 17, 2023, we entered into a Forbearance, Restructuring and Settlement Agreement with a supplier, pursuant to which we issued a convertible promissory note to the supplier in the principal amount of $15.0 million (the “Note”). Subject to a beneficial ownership limitation of 19.99% of our outstanding common stock and any shareholder approval requirements, the supplier may elect, at its sole discretion, to convert any outstanding principal and interest on the Note into shares of our common stock at a conversion price of $1.30 per share.
|
| |
•
|
On April 18, 2023, we entered into a securities purchase agreement with certain accredited investors, pursuant to which we agreed to sell shares of our common stock and pre-funded warrants, in a registered direct offering, for an aggregate offering price of approximately $3.6 million. We agreed to pay A.G.P./Alliance Global Partners, the placement agent for the offering, a cash fee equal to 6.5% of the aggregate gross proceeds received from the offering. In a concurrent private placement, we issued warrants exercisable for an aggregate of 3,685,000 shares of our common stock.
|
| |
•
|
On April 18, 2023, we amended (i) common stock purchase warrants exercisable for an aggregate of 1,920,000 shares of our common stock that we issued to an accredited investor on September 22, 2022 (the “September Warrants”), and (ii) common stock purchase warrants exercisable for an aggregate of 2,000,000 shares that we issued to an accredited investor on August 17, 2022, as amended on September 22, 2022 (the “August Warrants”). The September Warrants and the August Warrants were issued in a concurrent private placement in connection with a registered direct offering of our securities under that securities purchase agreement, dated August 15, 2022, as amended on September 22, 2022, for an aggregate offering price of approximately $12 million. We agreed to pay A.G.P./Alliance Global Partners, the placement agent for the offering, a cash fee equal to 7.0% of the aggregate gross proceeds received from the offering.
|
| |
•
|
On November 5, 2019, we entered into a common stock purchase agreement with Aspire Capital Fund, LLC (“Aspire Capital”), pursuant to which Aspire Capital committed to purchase from us up to an aggregate of $100 million of shares of our common stock, subject to the terms and conditions specified in such common stock purchase agreement, over the 36-month term of the common stock purchase agreement. From July 1, 2020 through August 11, 2021, the Company sold 1,186,000 shares of its common stock under such common stock purchase agreement, generating proceeds of $54,828,034.
|
| |
•
|
On June 24, 2021, we entered into a common stock purchase agreement with Aspire Capital, pursuant to which Aspire Capital committed to purchase from us up to an aggregate of $100 million of shares of our common stock, subject to the terms and conditions specified in such common stock purchase agreement, over the 36-month term of the common stock purchase agreement. From June 24, 2021 through June 23, 2022, the Company sold 1,600,000 shares of its common stock under such common stock purchase agreement, generating proceeds of $31,068,740.
|
| |
•
|
On May 12, 2022, we entered into a common stock purchase agreement with Aspire Capital, pursuant to which Aspire Capital committed to purchase from us up to an aggregate of $100 million of shares of our common stock, subject to the terms and conditions specified in such common stock purchase agreement, over the 24-month term of the common stock purchase agreement. On July 6, 2022, Aspire Capital delivered notice to us, terminating such common stock purchase agreement, effective immediately. From May 12, 2022 through the date of termination, the Company sold 274,800 shares of its common stock under such common stock purchase agreement, generating proceeds of $1,859,529.
|
We believe the offers, sales and issuances of the above securities by us were exempt from registration under the Securities Act by virtue of Section 4(a)(2) of the Securities Act and Rule 506 thereunder as transactions not involving a public offering. All of the investors were accredited investors as such term is defined in Rule 501 under the Securities Act. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates, notes and warrants issued in these transactions, as applicable. All recipients had adequate access, through their relationships with us, to information about our Company. The sales of these securities were made without any general solicitation or advertising.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) Exhibits:
|
Exhibit No.
|
|
Description of Exhibit
|
|
1.1#
|
|
Form of Placement Agency Agreement
|
| |
|
|
|
3.1
|
|
Certificate of Incorporation of Athersys, Inc., as amended as of June 20, 2013 (incorporated herein by reference to Exhibit 3.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on May 8, 2019)
|
| |
|
|
|
3.2
|
|
Certificate of Amendment to Certificate of Incorporation of Athersys, Inc., as amended as of June 7, 2017 (incorporated herein by reference to Exhibit 3.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 9, 2017)
|
| |
|
|
|
3.3
|
|
Bylaws of Athersys, Inc., as amended and restated as of March 13, 2019 (incorporated herein by reference to Exhibit 3.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on March 14, 2019)
|
| |
|
|
|
3.4
|
|
Certificate of Amendment to Certificate of Incorporation of Athersys, Inc., as amended, effective as of June 16, 2021 (incorporated herein by reference to Exhibit 3.3 to the registrant’s Registration Statement on Form S-3 (Commission No. 333-257409) filed with the Commission on June 25, 2021)
|
| |
|
|
|
3.5
|
|
Certificate of Amendment to Certificate of Incorporation of Athersys, Inc., as amended, effective as of August 26, 2022 (incorporated herein by reference to Exhibit 3.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on August 29, 2022)
|
| |
|
|
|
4.1
|
|
Description of the Registrant's Securities Registered Pursuant to Section 12 of the Securities Exchange Act of 1934 (incorporated herein by reference to Exhibit 4.3 to the registrant's Annual Report on Form 10-K (Commission No. 001-33876) filed with the Commission on March 16, 2020)
|
| |
|
|
|
4.2
|
|
Common Stock Purchase Warrant (ARDS) issued to HEALIOS K.K. by Athersys, Inc. dated August 5, 2021 (incorporated herein by reference to Exhibit 4.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 15, 2021)
|
| |
|
|
|
4.3
|
|
Common Stock Purchase Warrant (Ischemic Stroke) issued to HEALIOS K.K. by Athersys, Inc. dated August 5, 2021 (incorporated herein by reference to Exhibit 4.2 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 15, 2021)
|
|
4.4
|
|
Form of Pre-Funded Warrant (incorporated herein by reference to Exhibit 4.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on August 17, 2022)
|
| |
|
|
|
4.5
|
|
Form of Warrant (incorporated herein by reference to Exhibit 4.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on August 17, 2022)
|
| |
|
|
|
4.6
|
|
Form of Warrant Amendment (incorporate herein by reference to Exhibit 4.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on September 22, 2022)
|
| |
|
|
|
4.7
|
|
Form of New Warrant (incorporate herein by reference to Exhibit 4.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on September 22, 2022)
|
| |
|
|
|
4.8
|
|
Form of Pre-Funded Warrant (incorporated herein by reference to Exhibit 4.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on November 10, 2022)
|
| |
|
|
|
4.9
|
|
Form of Warrant (incorporated herein by reference to Exhibit 4.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on November 10, 2022)
|
| |
|
|
|
4.10
|
|
Form of Pre-Funded Warrant (incorporated herein by reference to Exhibit 4.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on April 18, 2023)
|
| |
|
|
|
4.11
|
|
Form of Warrant (incorporated herein by reference to Exhibit 4.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on April 18, 2023)
|
| |
|
|
|
4.12
|
|
Form of Warrant Amendment No. 1 to Common Stock Purchase Warrant (incorporated herein by reference to Exhibit 4.3 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on April 18, 2023)
|
| |
|
|
|
4.13
|
|
Form of Warrant Amendment No. 2 to Common Stock Purchase Warrant (incorporated herein by reference to Exhibit 4.4 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on April 18, 2023)
|
| |
|
|
|
4.14
|
|
Form of Convertible Promissory Note, dated May 17, 2023 (incorporated herein by reference to Exhibit 4.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on May 23, 2023)
|
| |
|
|
|
4.15#
|
|
Form of Pre-Funded Warrant
|
| |
|
|
|
4.16#
|
|
Form of Common Warrant
|
| |
|
|
|
5.1#
|
|
Opinion of Thompson Hine LLP
|
| |
|
|
|
10.1**
|
|
Exclusive License Agreement, dated as of May 17, 2002, by and between Regents of the University of Minnesota and MCL LLC, assumed by ReGenesys, LLC through operation of merger on November 4, 2003 (incorporated herein by reference to Exhibit 10.34 to the registrant’s Current Report on Form 8-K (Commission No. 000-52108) filed with the Commission on June 14, 2007)
|
|
10.2
|
|
Form Indemnification Agreement for Directors, Officers and Directors and Officers (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 000-52108) filed with the Commission on August 6, 2007)
|
| |
|
|
|
10.3
|
|
Form of Incentive Stock Option Agreement (incorporated herein by reference to Exhibit 10.47 to the registrant’s Annual Report on Form 10-K for the year ended December 31, 2010 (Commission No. 001-33876) filed with the Commission on March 25, 2011)
|
| |
|
|
|
10.4*
|
|
Form of Nonqualified Stock Option Agreement for Non-Employee Directors (incorporated herein by reference to Exhibit 10.48 to the registrant’s Annual Report on Form 10-K for the year ended December 31, 2010 (Commission No. 001-33876) filed with the Commission on March 25, 2011)
|
| |
|
|
|
10.5*
|
|
Athersys, Inc. Amended and Restated 2007 Long-Term Incentive Plan (incorporated herein by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-8 (Registration No. 333-212119) filed with the Commission on June 20, 2016)
|
| |
|
|
|
10.6*
|
|
Form of Nonqualified Stock Option Agreement for Non-Employee Directors pursuant to the Athersys, Inc. Amended and Restated 2007 Long-Term Incentive Plan (Amended and Restated Effective June 16, 2011) (incorporated herein by reference to Exhibit 10.49 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on May 6, 2011)
|
| |
|
|
|
10.7*
|
|
Form of Restricted Stock Unit Agreement (incorporated herein by reference to Exhibit 10.2 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2011)
|
| |
|
|
|
10.8*
|
|
Form of Restricted Stock Unit Agreement (incorporated herein by reference to Exhibit 10.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on June 20, 2013)
|
| |
|
|
|
10.9
|
|
License Agreement by and between ABT Holding Company and HEALIOS K.K., dated as of January 8, 2016 (incorporated herein by reference to Exhibit 10.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on May 5, 2016)
|
| |
|
|
|
10.10
|
|
First Amendment to License Agreement, dated as of July 21, 2017, by and between ABT Holding Company and HEALIOS K.K. (incorporated herein by reference to Exhibit 10.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 8, 2017)
|
| |
|
|
|
10.11
|
|
Second Amendment to License Agreement, dated as of September 19, 2017, by and between ABT Holding Company and HEALIOS K.K. (incorporated herein by reference to Exhibit 10.2 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 8, 2017)
|
| |
|
|
|
10.12
|
|
Investor Rights Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of March 13, 2018 (incorporated herein by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on May 10, 2018)
|
| |
|
|
|
10.13**
|
|
Collaboration Expansion Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of June 6, 2018 (incorporated herein by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 9, 2018)
|
|
10.14
|
|
Amendment No. 1 to Collaboration Expansion Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of August 31, 2018 (incorporated herein by reference to Exhibit 10.1 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 6, 2018)
|
| |
|
|
|
10.15
|
|
Amendment No. 2 to Collaboration Expansion Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of December 6, 2018 (incorporated herein by reference to Exhibit 10.44 to the registrant's Annual Report on Form 10-K (Commission No. 001-33876) filed with the Commission on March 15, 2019)
|
| |
|
|
|
10.16
|
|
Amendment No. 3 to Collaboration Expansion Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of December 14, 2018 (incorporated herein by reference to Exhibit 10.45 to the registrant's Annual Report on Form 10-K (Commission No. 001-33876) filed with the Commission on March 15, 2019)
|
| |
|
|
|
10.17*
|
|
Summary of Athersys, Inc. 2022 Cash Bonus Incentive Plan (incorporated herein by reference to Exhibit 10.27 to the registrant's Annual Report on Form 10-K (Commission No. 001-33876) filed with the Commission on March 15, 2022
|
| |
|
|
|
10.18*
|
|
Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 4.4 to the registrant's Registration Statement on Form S-8 (Registration No. 333-232075) filed with the Commission on June 12, 2019)
|
| |
|
|
|
10.19*
|
|
Form of Incentive Stock Option Agreement pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.2 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 7, 2019)
|
| |
|
|
|
10.20*
|
|
Form of Non-Qualified Stock Option Agreement pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.3 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 7, 2019)
|
| |
|
|
|
10.21*
|
|
Form of Non-Qualified Stock Option Agreement (Directors) pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.4 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 7, 2019)
|
| |
|
|
|
10.22*
|
|
Form of Restricted Stock Unit Agreement (Executives) pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.5 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 7, 2019)
|
| |
|
|
|
10.23*
|
|
Form of Restricted Stock Unit Agreement (Non-Executive) pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan (incorporated herein by reference to Exhibit 10.6 to the registrant's Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 7, 2019)
|
| |
|
|
|
10.24*
|
|
Form of Incentive Stock Option Agreement pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan for grants beginning in 2020 (incorporated herein by reference to Exhibit 10.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2020)
|
|
10.25*
|
|
Form of Non-Qualified Stock Option Agreement pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan for grants beginning in 2020 (incorporated herein by reference to Exhibit 10.2 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2020)
|
| |
|
|
|
10.26*
|
|
Form of Non-Qualified Stock Option Agreement (Directors) pursuant to the Athersys, Inc. 2019 Equity and Incentive Compensation Plan for grants beginning in 2020 (incorporated herein by reference to Exhibit 10.3 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2020)
|
| |
|
|
|
10.27*
|
|
Form of Notice of Amendment to Option Rights for Employees (incorporated herein by reference to Exhibit 10.4 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2020)
|
| |
|
|
|
10.28*
|
|
Form of Notice of Amendment to Option Rights for Directors (incorporated herein by reference to Exhibit 10.5 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on August 10, 2020)
|
| |
|
|
|
10.29
|
|
Cooperation Agreement, dated as of February 16, 2021, by and among Athersys, Inc. and HEALIOS K.K. and Dr. Tadahisa Kagimoto (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on February 16, 2021)
|
| |
|
|
|
10.30
|
|
Separation Letter, dated as of February 15, 2021, by and between Athersys, Inc. and Dr. Gil Van Bokkelen (incorporated herein by reference to Exhibit 10.2 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on February 16, 2021)
|
| |
|
|
|
10.31†
|
|
Comprehensive Framework Agreement for Commercial Manufacturing and Ongoing Support, by and between Athersys, Inc. and HEALIOS K.K., dated as of August 5, 2021 (incorporated herein by reference to Exhibit 10.1 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 15, 2021)
|
| |
|
|
|
10.32
|
|
Amendment to Cooperation Agreement, dated as of August 5, 2021, by and among Athersys, Inc. and HEALIOS K.K. and Dr. Tadahisa Kagimoto (incorporated herein by reference to Exhibit 10.2 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 15, 2021)
|
| |
|
|
|
10.33
|
|
Amendment to Investor Rights Agreement, by and between Athersys, Inc. and HEALIOS K.K., dated as of August 5, 2021 (incorporated herein by reference to Exhibit 10.3 to the registrant’s Quarterly Report on Form 10-Q (Commission No. 001-33876) filed with the Commission on November 15, 2021)
|
| |
|
|
|
10.34
|
|
Form of Securities Purchase Agreement, dated as of August 15, 2022, between Athersys, Inc. and each purchaser named in the signature pages thereto (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on August 17, 2022)
|
| |
|
|
|
10.35
|
|
Form of Securities Purchase Agreement Amendment, dated as of September 22, 2022, between Athersys, Inc. and each purchaser named in the signature pages thereto (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on September 22, 2022)
|
|
10.36
|
|
Form of Securities Purchase Agreement, dated as of November 10, 2022, between Athersys, Inc. and each purchaser named in the signature pages thereto (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on November 10, 2022)
|
| |
|
|
|
10.37
|
|
Form of Securities Purchase Agreement, dated as of April 18, 2023, between the Company and each purchaser named in the signature pages thereto (incorporated herein by reference to Exhibit 10.1 to the registrant’s Current Report on Form 8-K (Commission No. 001-33876) filed with the Commission on April 18, 2023)
|
| |
|
|
|
10.38#
|
|
Form of Securities Purchase Agreement
|
| |
|
|
|
21.1
|
|
List of Subsidiaries (incorporated herein by reference to Exhibit 21.1 to the registrant’s Annual report on Form 10-K (Commission No. 001-33876) filed with the Commission on April 3, 2023)
|
| |
|
|
|
23.1
|
|
Consent of Independent Registered Public Accounting Firm
|
| |
|
|
|
23.2#
|
|
Consent of Thompson Hine LLP (included in Exhibit 5.1)
|
| |
|
|
|
24.1
|
|
Power of Attorney (included on signature page)
|
| |
|
|
|
107
|
|
Filing Fee Table.
|
# To be filed by amendment.
* Indicates management contract or any compensatory plan, contract or arrangement.
** Confidential treatment requested as to certain portions, which portions have been filed separately with the SEC.
† Portions of this exhibit (indicated by bracketed asterisks) are omitted in accordance with the rules of the SEC.
(b) Financial Statements Schedules:
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
ITEM 17. UNDERTAKINGS.
The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
The undersigned registrant hereby undertakes that:
| |
(1)
|
For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
|
| |
(2)
|
For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Cleveland, State of Ohio, on July 14, 2023.
|
|
ATHERSYS, INC.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ Daniel A. Camardo |
|
|
|
|
Name: Daniel A. Camardo
|
|
|
|
|
Title: Chief Executive Officer
|
|
POWER OF ATTORNEY AND SIGNATURES
Each individual whose signature appears below hereby constitutes and appoints Daniel A. Camardo as such person’s true and lawful attorney-in-fact and agent, with full power of substitution and revocation, for such person in such person’s name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this Registration Statement (or any Registration Statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, and any substitute or substitutes, full power and authority to do and perform each and every act and thing requisite, necessary and/or advisable to be done in connection therewith, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that any said attorney-in-fact and agent, or any substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement on Form S-1 has been signed by the following persons in the capacities and on the dates indicated.
|
Name
|
|
Title
|
|
Date
|
| |
|
|
|
/s/ Daniel A. Camardo
|
|
Chief Executive Officer
|
|
July 14, 2023
|
|
Daniel A. Camardo
|
|
(Principal Executive Officer)
|
|
|
| |
|
|
|
|
|
/s/ Kasey Rosado
|
|
Interim Chief Financial Officer
|
|
July 14, 2023
|
|
Kasey Rosado
|
|
(Principal Financial and Accounting Officer)
|
|
|
| |
|
|
|
|
|
/s/ Ismail Kola
|
|
Chairman of the Board of Directors
|
|
July 14, 2023
|
|
Ismail Kola
|
|
|
|
|
| |
|
|
|
|
|
/s/ Joseph Nolan
|
|
Director
|
|
July 14, 2023
|
|
Joseph Nolan
|
|
|
|
|
| |
|
|
|
|
|
/s/ Jane Wasman
|
|
Director
|
|
July 14, 2023
|
|
Jane Wasman
|
|
|
|
|
| |
|
|
|
|
|
/s/Jack L. Wyszomierski
|
|
Director
|
|
July 14, 2023
|
|
Jack L. Wyszomierski
|
|
|
|
|
| |
|
|
|
|
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the reference to our firm under the caption "Experts" in the Registration Statement on Form S-1 and related Prospectus of Athersys, Inc. for the registration of shares of its common stock, common warrants and pre-funded warrants and to the incorporation by reference therein of our report dated March 31, 2023, with respect to the consolidated financial statements of Athersys, Inc. included in its Annual Report (Form 10-K) for the year ended December 31, 2022, filed with the Securities and Exchange Commission.
Cleveland, Ohio
July 14, 2023
Exhibit 107
Calculation of Filing Fee Tables
Form S-1
(Form Type)
Athersys, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| |
|
Security
Type
|
|
Security
Class
Title
|
|
Fee
Calculation
or Carry
Forward
Rule
|
|
Amount
Registered
|
|
Proposed
Maximum
Offering
Price
Per
Unit
|
|
Maximum
Aggregate
Offering
Price(1)
|
|
Fee Rate
|
|
Amount of
Registration
Fee
|
|
Carry
Forward
Form
Type
|
|
Carry
Forward
File
Number
|
|
Carry
Forward
Initial
effective
date
|
|
Filing Fee
Previously
Paid In
Connection
with
Unsold
Securities
to
be
Carried
Forward
|
|
|
Newly Registered Securities
|
|
|
Fees to Be Paid
|
|
Equity
|
|
Common stock, par value $0.001 per share (2)
|
|
Rule 457(o)
|
|
— |
|
— |
|
$ |
12,000,000 |
|
|
0.00011020 |
|
$ |
1,322.40 |
(4) |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Other
|
|
Pre-Funded Warrants to purchase shares of common stock(3)(5)
|
|
Other
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Equity
|
|
Common Stock underlying the Pre-Funded Warrants(3)
|
|
Rule 457(o)
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Other
|
|
Common Warrants to purchase shares of common stock(5)
|
|
Other
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Equity
|
|
Common Stock underlying the Common Warrants to purchase Common Stock
|
|
Rule 457(o)
|
|
— |
|
— |
|
$ |
12,000,000 |
|
|
0.00011020 |
|
$ |
1,322.40 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fees Previously Paid
|
|
|
— |
|
|
— |
|
|
— |
|
— |
|
— |
|
|
— |
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carry Forward Securities
|
|
|
Carry Forward Securities
|
|
|
— |
|
|
— |
|
|
— |
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| |
|
Total Offering Amounts
|
|
|
|
|
|
|
|
|
$ |
24,000,000 |
|
|
|
|
$ |
2,644.80 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Fees Previously Paid
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Total Fee Offsets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Net Fee Due
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
2.644.80 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
(1)
(2)
|
Estimated solely for the purpose of determining the amount of the registration fee in accordance with Rule 457(o) under the Securities Act.
Pursuant to Rule 416 under the Securities Act, this registration statement shall also cover any additional shares of the registrant’s securities that become issuable by reason of any share splits, share dividends or similar transactions.
|
| |
(3)
|
The proposed maximum aggregate offering price of the shares of common stock proposed to be sold in the offering will be reduced on a dollar-for-dollar basis based on the aggregate offering price of the Pre-Funded Warrants offered and sold in the offering (plus the aggregate exercise price of the shares of common stock issuable upon exercise of the Pre-Funded Warrants), and as such the proposed aggregate maximum offering price of the shares of common stock and Pre-Funded Warrants (including shares of common stock issuable upon exercise of the Pre-Funded Warrants), if any, is $12,000,000.
|
| |
(4)
|
Calculated pursuant to Rule 457(o) under the Securities Act based on an estimate of the proposed maximum aggregate offering price.
|
| |
(5)
|
No fee due pursuant to Rule 457(g) under the Securities Act.
|
Athersys (NASDAQ:ATHX)
Historical Stock Chart
From Apr 2024 to May 2024
Athersys (NASDAQ:ATHX)
Historical Stock Chart
From May 2023 to May 2024