false 0001813814 0001813814 2024-03-07 2024-03-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 7, 2024
Mind Medicine (MindMed) Inc.
(Exact Name of Registrant as Specified in Its Charter)
|
|
|
|
|
| British Columbia, Canada |
|
001-40360 |
|
98-1582538 |
(State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
One World Trade Center, Suite 8500 New York, New York |
|
|
|
10007 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (212) 220-6633
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trading symbol(s) |
|
Name of each exchange on which registered |
| Common Shares, no par value per share |
|
MNMD |
|
The Nasdaq Stock Market LLC |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 |
Regulation FD Disclosure |
On March 7, 2024, Mind Medicine (MindMed) Inc. (the “Company”) issued a press release announcing that the U.S. Food & Drug Administration has granted breakthrough designation to the Company’s MM120 (lysergide d-tartrate) program for the treatment of generalized anxiety disorder (“GAD”) and that the Company’s Phase 2b trial of MM120 in GAD met its key secondary endpoint, demonstrating statistically significant durability of effect through Week 12. A copy of the press release is attached hereto as Exhibit 99.1.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, or the Exchange Act, or subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, or the Securities Act. The information in this Item 7.01, including Exhibit 99.1, shall not be deemed incorporated by reference into any other filing with the U.S. Securities Exchange Commission, or the SEC, made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
On March 7, 2024, the Company delivered written notice to Cantor Fitzgerald & Co. and Oppenheimer & Co. Inc. (the “Agents”) that it was suspending and terminating the prospectus, dated May 16, 2022 (the “ATM Prospectus”), relating to up to $100,000,000 of the Company’s common shares that may be issued and sold pursuant to the Controlled Equity OfferingSM Sales Agreement, dated as of May 3, 2022, by and between the Company and the Agents (the “Sales Agreement”). The Company will not make any sales of its common shares pursuant to the Sales Agreement, unless and until a new prospectus, prospectus supplement or registration statement is filed. Other than the termination of the ATM Prospectus, the Sales Agreement remains in full force and effect.
On March 7, 2024, the Company also posted an updated investor presentation on its website. A copy of the presentation is filed herewith as Exhibit 99.2 and is incorporated by reference herein.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
| Date: March 7, 2024 |
|
|
|
Mind Medicine (MindMed) Inc. |
|
|
|
|
|
|
|
|
By: |
|
/s/ Robert Barrow |
|
|
|
|
Name: |
|
Robert Barrow |
|
|
|
|
Title: |
|
Chief Executive Officer |
Exhibit 99.1
THURSDAY MARCH 7, 2023, 6:00 AM EST
MINDMED RECEIVES FDA BREAKTHROUGH THERAPY DESIGNATION
AND ANNOUNCES POSITIVE 12-WEEK DURABILITY DATA FROM PHASE 2B STUDY OF
MM120 FOR GENERALIZED ANXIETY DISORDER
-A single oral administration of MM120 100 µg met its key secondary endpoint and maintained a clinically and statistically significant
HAM-A reductions compared to placebo at 12 weeks with a 65% clinical response rate and 48% clinical remission rate-
-MindMed plans to hold an End-of-Phase 2 meeting with the
U.S. Food & Drug Administration (FDA) in the first half of 2024 and initiate its Phase 3 clinical program in the second half of 2024-
-MindMed will host a webcast to discuss data from its Phase 2b study at 8:00 am ET-
NEW YORK — (BUSINESS WIRE) — Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (Cboe Canada MMED), (the “Company” or
“MindMed”), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, today announced that FDA has granted breakthrough designation to its MM120 (lysergide d-tartrate) program for the treatment of generalized anxiety disorder (GAD). The Company also announced that its Phase 2b study of MM120 in GAD met its key secondary endpoint, and
12-week topline data demonstrated clinically and statistically significant durability of activity observed through Week 12.
MindMed previously announced rapid, clinically meaningful, and statistically significant improvements on the Hamilton Anxiety rating scale (HAM-A) compared to placebo at Week 4, which was the trial’s primary endpoint. MM120 was administered as a single dose in a monitored clinical setting with no additional therapeutic intervention.
“I’ve conducted clinical research studies in psychiatry for over two decades and have seen studies of many drugs under development for the treatment
of anxiety. That MM120 exhibited rapid and robust efficacy, solidly sustained for 12 weeks after a single dose, is truly remarkable,” stated David Feifel, MD, PhD, Professor Emeritus of Psychiatry at the University of California, San Diego and
Director of the Kadima Neuropsychiatry Institute in La Jolla, California and an investigator in the MM120 study. “These results suggest the potential MM120 has in the treatment of anxiety, and those of us who struggle every day to alleviate
anxiety in our patients look forward to seeing results from future Phase 3 trials.”
MM120 100 µg – the dose with optimal clinical
activity observed in the trial – demonstrated a 7.7-point improvement over placebo at Week 12 (-21.9 MM120 vs. -14.2
placebo; p<0.003 Cohen’s d=0.81), with a 65% clinical response rate and a 48% clinical remission rate sustained to Week 12. Clinical Global Impressions - Severity (CGI-S) scores on average improved
from 4.8 to 2.2 in the 100-µg dose group, representing a two-category shift from ‘markedly ill’ to ‘borderline ill’ at Week 12 (p<0.004).
This clinical activity was rapid, observed as early as study day 2, and durable with further improvements observed in mean HAM-A or CGI-S scores between Weeks 4 and 12.
Based on the significant unmet medical need in the treatment of GAD – especially in patients who do not
respond to or tolerate currently available medications – along with the initial clinical data from Phase 2b and other research conducted by MindMed, the U.S. Food & Drug Administration (FDA) has designated MM120 for GAD as a
breakthrough therapy. The Company plans to hold an End-of-Phase 2 meeting with the FDA in the first half of 2024 and initiate a Phase 3 clinical program in the second
half of 2024.
“The FDA’s decision to designate MM120 as a breakthrough therapy for GAD and the durability data from our Phase 2b study provide
further validation of the important potential role this treatment can play in addressing the huge unmet need among individuals living with GAD,” said Robert Barrow, Chief Executive Officer and Director of MindMed. “We are committed to
bringing MM120 to people living with GAD and delivering on the potential of our pipeline to treat serious brain health disorders.”
In the Phase 2b
study, known as MMED008, MM120 was generally well-tolerated with most adverse events rated as mild to moderate, transient and occurring on dosing day, and being consistent with expected acute effects of the study drug. The most common adverse events
(at least 10% incidence in the high dose groups) on dosing day included illusion, hallucinations, euphoric mood, anxiety, abnormal thinking, headache, paresthesia, dizziness, tremor, nausea, vomiting, feeling abnormal, mydriasis and hyperhidrosis.
Prior to treatment with MM120, study participants were clinically tapered and then washed out from any anxiolytic or antidepressant treatments and did
not receive any form of study-related psychotherapy for the duration of their participation in the study.
“As a clinician and clinical researcher, I
applaud the way this study was designed by MindMed to isolate the effect of MM120 by removing confounding variables like additional medications and psychotherapy,” said Reid Robison, MD, Psychiatrist and Chief Clinical Officer at Numinus
(TSX:NUMI) who has served as adjunct faculty at the University of Utah for the last 12 years and was an investigator in the MM120 study. “It gives me confidence in the data and the positive results give me hope that this may translate into
meaningful benefits for my patients.”
The primary data analyses from MMED008 have been accepted for presentation at the American Psychiatric
Association’s annual meeting, which will be held in New York on May 4-8, 2024. The study is also being submitted for publication in a leading medical journal.
Conference Call and Webcast
MindMed management will host
a webcast at 8:00 am ET today to discuss the Phase 2b results of MM120 in GAD. The webcast and slides will be accessible live under “News & Events” on the Investors page of the Company’s website at https://ir.mindmed.co/ or
by clicking here. A replay of the event will be available on MindMed’s website. The webcast will be archived on the Company’s website for at least 30 days after the conference call.
About Generalized Anxiety Disorder (GAD)
GAD is a common condition associated with significant impairment that adversely affects millions of people. GAD results in fear, persistent anxiety and a
constant feeling of being overwhelmed. It is characterized by excessive, persistent, and unrealistic worry about everyday things. Approximately 10% of U.S. adults, representing around 20 million people, currently suffer from GAD, an
underdiagnosed and underserved indication that is associated with significant impairment, less accomplishment at work and reduced labor force participation. Despite the significant personal and societal burden of GAD, there has been little
innovation in the treatment of GAD in the past several decades, with the last new drug approval occurring in 2004.
About MMED008
MMED008 was a multi-center, parallel, randomized, double-blind, placebo-controlled, dose-optimization study. The trial enrolled 198 participants who were
randomized to receive a single administration of MM120 at a dose of 25, 50, 100 or 200 µg or placebo. The full analysis set (FAS) for the trial included 194 subjects, those that had at least one valid post-baseline Hamilton Anxiety rating
scale (HAM-A) score. Subjects enrolled in the trial presented with severe GAD symptoms (average baseline HAM-A scores of approximately 30). The study’s main
objective was to determine the dose-response relationship of four doses of MM120 versus placebo as measured by the change in HAM-A from Baseline to Week 4. The key secondary objective of the study was to
determine the dose-response relationship of four doses of MM120 versus placebo as measured by the change in HAM-A from Baseline to Week 8. Secondary objectives, measured up to 12 weeks after the single
administration, include assessments of anxiety symptoms, safety and tolerability, and other measures of efficacy and quality of life. More information about the trial is available on the MindMed website (mindmed.co) or on clinicaltrials.gov
(NCT05407064).
About MM120
Lysergide is a synthetic
ergotamine belonging to the group of classic, or serotonergic, psychedelics, which acts as a partial agonist at human serotonin-2A
(5-hydroxytryptamine-2A [5-HT2A]) receptors. MindMed is developing MM120 (lysergide
D-tartrate), the tartrate salt form of lysergide, for GAD and is exploring its potential applications in other serious brain health disorders.
About MindMed
MindMed is a clinical stage
biopharmaceutical company developing novel product candidates to treat brain health disorders. Our mission is to be the global leader in the development and delivery of treatments that unlock new opportunities to improve patient outcomes. We are
developing a pipeline of innovative product candidates, with and without acute perceptual effects, targeting neurotransmitter pathways that play key roles in brain health disorders.
MindMed trades on NASDAQ under the symbol MNMD and on the Cboe Canada (formerly known as the NEO Exchange, Inc.) under the symbol MMED.
Forward-Looking Statements
Certain statements in this
news release related to the Company constitute “forward-looking information” within the meaning of applicable securities laws and are prospective in nature. Forward-looking information is not based on historical facts, but rather on
current expectations and projections
about future events and are therefore subject to risks and uncertainties which could cause actual results to differ materially from the future results expressed or implied by the forward-looking
statements. These statements generally can be identified by the use of forward-looking words such as “will”, “may”, “should”, “could”, “intend”, “estimate”, “plan”,
“anticipate”, “expect”, “believe”, “potential” or “continue”, or the negative thereof or similar variations. Forward-looking information in this news release includes, but is not limited to,
statements regarding anticipated upcoming milestones, and progress of trials and studies; results and timing of and reporting of full data from the Company’s Phase 2b clinical trial of MM120; timing of a potential
End-of-Phase-2 meeting with the FDA; timing of the initiation of a potential Phase 3 clinical trial of MM120; and the potential
benefits of the Company’s product candidates. There can be no guarantees regarding the results of the potential Phase 3 clinical trial or that, following any such trial, MM120 will receive the necessary regulatory approvals. There are numerous
risks and uncertainties that could cause actual results and the Company’s plans and objectives to differ materially from those expressed in the forward-looking information, including history of negative cash flows; limited operating history;
incurrence of future losses; availability of additional capital; lack of product revenue; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened
regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to herein and the risks described
in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, under headings such as “Special Note Regarding Forward-Looking Statements,” “Risk Factors”
and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and other filings and furnishings made by the Company with the securities regulatory authorities in all provinces and territories of Canada
which are available under the Company’s profile on SEDAR at www.sedar.com and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Except as required by law, the Company undertakes no duty or obligation to
update any forward-looking statements contained in this release as a result of new information, future events, changes in expectations or otherwise.
For
Media Inquiries, please contact: media@mindmed.co
For Investor Inquiries, please contact: ir@mindmed.co
Source: Mind Medicine (MindMed) Inc.

Exhibit 99.2 MM120 for GAD Investor Presentation March 2024

Disclaimer This presentation (the “Presentation”) has been
prepared by Mind Medicine (MindMed) Inc. (“MindMed”, the “Company”, “we”, “our” or “us) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees,
directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any
representations (expressed or implied) contained in, or for any omissions from, this Presentation. This Presentation does not constitute an offering of, or a solicitation of an offer to purchase, securities of MindMed and under no circumstances is
it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement
of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the Securities and Exchange Commission (the SEC ) or by any state, provincial or other
securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note
Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private
Securities Litigation Reform Act of 1995 and other applicable securities laws. Forward-looking statements can often, but not always, be identified by words such as “plans”, “expects”, “is expected”,
“budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative
variations) of such words and phrases, or statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and
similar references to future periods. Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertaining to: the development and commercialization of any medicine or treatment, or the
efficacy of either of the foregoing, the success and timing of our development activities; the success and timing of our planned clinical trials; our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials
or of obtaining FDA or other regulatory approvals; our cash runway funding operations through key clinical readouts and into 2026; the likelihood of obtaining patents or the efficacy of such patents once granted and the potential for the markets
that MindMed is anticipating to access. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our
business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions as of the date of this Presentation. While MindMed considers these assumptions to be reasonable, the assumptions are
inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of MindMed’s control, and actual results and
financial condition may differ materially from those indicated in the forward- looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and financial condition
to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated
medicines and treatments being developed; MindMed’s history of negative cash flows; MindMed’s limited operating history; incurrence of future losses; compliance with laws and regulations; difficulty associated with research and
development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as
those risk factors discussed or referred to throughout the “Risk Factors” sections of MindMed’s most recently filed Annual Report on Form 10-K filed with the SEC and in other filings we make in the future with the SEC and the
securities regulatory authorities in all provinces and territories of Canada, available under the Company’s profile on SEDAR at www.sedar.com. Any forward-looking statement made by MindMed in this Presentation is based only on information
currently available to the Company and speaks only as of the date on which it is made. MindMed undertakes no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a
result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. MM120 is a proprietary, pharmaceutically optimized
form of lysergide D-tartrate and MM402, or R(-)-MDMA, is our proprietary form of the R-enantiomer of MDMA (3,4-methylenedioxymethamphetamine). Lysergide and MDMA are Schedule I substances under the Controlled Substances Act. While the Company is
focused on programs using psychedelic or hallucinogenic compounds and non-hallucinogenic derivatives of these compounds, including in its MM120, MM402 and other product candidates, the Company does not have any direct or indirect involvement with
the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic or hallucinogenic substances except within
laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company's products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy
for the intended uses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data
is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources
believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to
in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of
the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. Investor Presentation | March 2024 2

Introductory Remarks Robert Barrow Chief Executive Officer
We Aim To Be A Global Leader In Brain Health Pipeline Management
Diversified pipeline of clinical Expertise in drug programs targeting development and significant unmet medical needs commercialization Research Expected Runway Leveraging decades of preclinical and Expected cash runway through key clinical research
with promising results clinical readouts and into 2026* in Phase 2b Market Protection Strategies IP and R&D strategies intended to maximize market exclusivity and protection *The company’s cash and cash equivalents of $99.7 million as of
December 31, 2023 and committed credit facility are expected to fund operations into 2026. Investor Presentation | March 2024 5
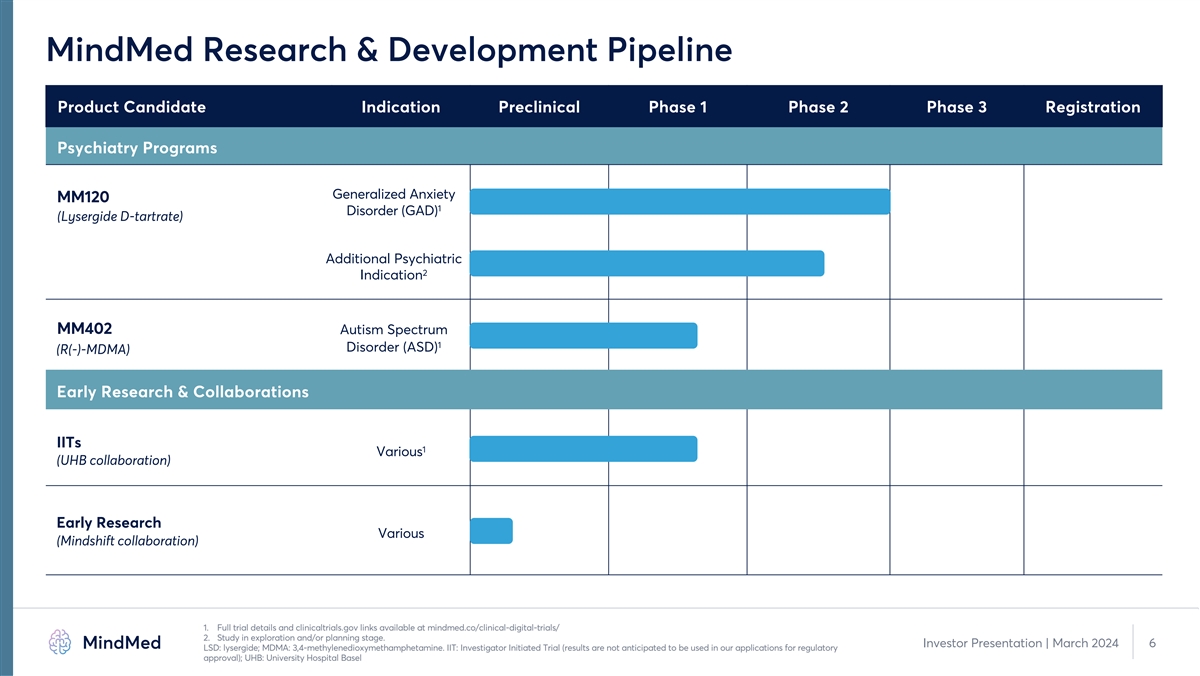
MindMed Research & Development Pipeline Product Candidate Indication
Preclinical Phase 1 Phase 2 Phase 3 Registration Psychiatry Programs Generalized Anxiety MM120 1 Disorder (GAD) (Lysergide D-tartrate) Additional Psychiatric 2 Indication MM402 Autism Spectrum 1 Disorder (ASD) (R(-)-MDMA) Early Research &
Collaborations IITs 1 Various (UHB collaboration) Early Research Various (Mindshift collaboration) 1. Full trial details and clinicaltrials.gov links available at mindmed.co/clinical-digital-trials/ 2. Study in exploration and/or planning stage.
Investor Presentation | March 2024 6 LSD: lysergide; MDMA: 3,4-methylenedioxymethamphetamine. IIT: Investigator Initiated Trial (results are not anticipated to be used in our applications for regulatory approval); UHB: University Hospital
Basel

MM-120 Has the Potential to Address a Large Unmet Need in GAD
Opportunity in Generalized Anxiety Disorder (GAD) 1 • GAD is the 2nd most common mental disorder among adults , Potential Best-in-Class yet there are limited treatment options Therapy with Novel MOA • Symptoms may be debilitating and
treatment inefficacy leads to incomplete remission and intolerable side effects. 1 Large Market 13 million 6.5 million do not respond ~20 million US adults with GAD 3 1 2 to first-line treatment Opportunity receive treatment 77% moderate to severe 1
SSRI/SNRIs : 50% failure rate with often undesirable side effects Benzodiazepines: addiction, tolerance risk; generally used in short-term Significant Need 4 for New Treatments Buspirone : poor efficacy Antipsychotics: short- and long-term risks;
poorly tolerated 1. Mental and Substance Use Disorders Prevalence Study (MDPSU): Findings Report 2023. 2. Kessler RC, Chiu WT, Demler O et al. Prevalence, Severity, and Comorbidity of 12-month DSM-IV Disorders in the National Comorbidity
Survey-Replication. 2005 Arch Gen Psychiatry; 62(6): 617-627. 7 Investor Presentation | March 2024 3. Ansara, Management of Treatment-Resistant Generalized Anxiety Disorder, Ment Health Clin 2020 Nov; 10(6) 326-334) United States Census Bureau,
company calculations. 4. Garakani A, et al., (2020) Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 11:595584. doi: 10.3389/fpsyt.2020.595584
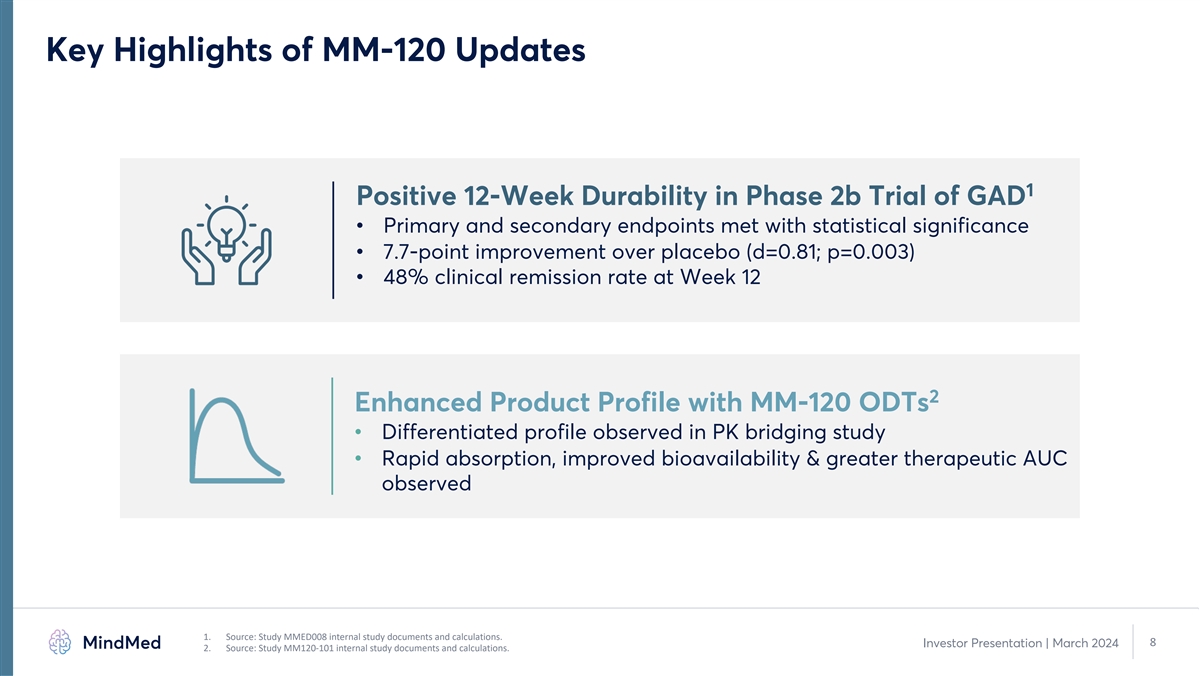
Key Highlights of MM-120 Updates 1 Positive 12-Week Durability in Phase
2b Trial of GAD • Primary and secondary endpoints met with statistical significance • 7.7-point improvement over placebo (d=0.81; p=0.003) • 48% clinical remission rate at Week 12 2 Enhanced Product Profile with MM-120 ODTs •
Differentiated profile observed in PK bridging study • Rapid absorption, improved bioavailability & greater therapeutic AUC observed 1. Source: Study MMED008 internal study documents and calculations. 8 Investor Presentation | March 2024
2. Source: Study MM120-101 internal study documents and calculations.

Results for MM120 in GAD Delivered on Target Product Profile after 1,2
Single Dose with Significant Improvement in All Endpoints Fast Acting 1.8-point reduction in CGI-S within 24 hours (p<0.0001) 21.9-point improvement in HAM-A at Week 12 (p=0.003) Durable Activity represents further improvement from Week 4 3
Response / Remission 48% of participants in remission at Week 12 Favorable tolerability profile with most AEs limited to Limited Side Effect Burden dosing day Scalability, Access & Value Results achieved with no additional therapy 1. Source:
Study MMED008 internal study documents and calculations. 100 µg dose group. 2. Represents all analyzed secondary endpoints in week 12 topline analysis, including HAM-A, CGI-S and MADRS. Investor Presentation | March 2024 9 3. p-values not
calculated for remission rates between groups. CGI-S: Clinical Global Impressions – Severity; HAM-A: Hamilton Anxiety Scale.
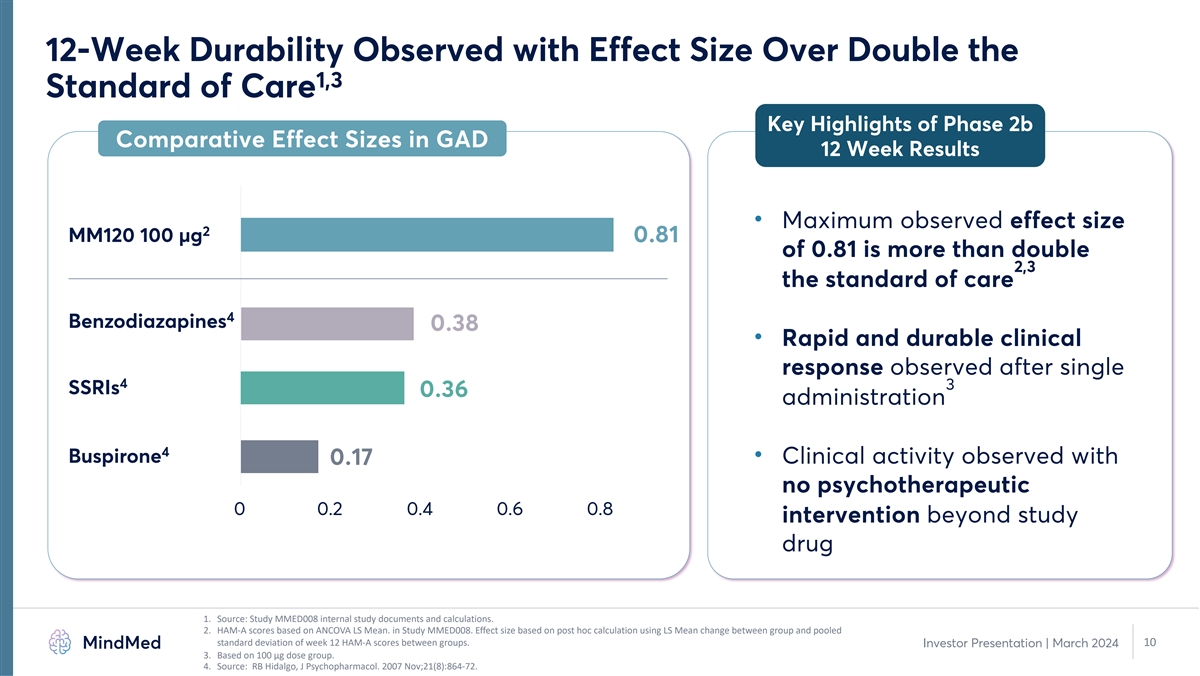
12-Week Durability Observed with Effect Size Over Double the 1,3
Standard of Care Key Highlights of Phase 2b Comparative Effect Sizes in GAD 12 Week Results • Maximum observed effect size 2 MM120 100 µg 0.81 of 0.81 is more than double 2,3 the standard of care 4 Benzodiazapines 0.38 • Rapid and
durable clinical response observed after single 4 3 SSRIs 0.36 administration 4 Buspirone • Clinical activity observed with 0.17 no psychotherapeutic 0 0.2 0.4 0.6 0.8 intervention beyond study drug 1. Source: Study MMED008 internal study
documents and calculations. 2. HAM-A scores based on ANCOVA LS Mean. in Study MMED008. Effect size based on post hoc calculation using LS Mean change between group and pooled standard deviation of week 12 HAM-A scores between groups. 10 Investor
Presentation | March 2024 3. Based on 100 µg dose group. 4. Source: RB Hidalgo, J Psychopharmacol. 2007 Nov;21(8):864-72.

PK Bridging Study Demonstrates Enhanced Product Profile for MM120 ODTs
1 Comparative PK Profile 2.5 MM120 Capsule Differentiated Performance of MM120 ODTs MM120 ODT 2 2 50% faster onset of action 1.5 3 17% improved bioavailability 1 4 0.5 23% increase in AUC at target conc. AUC 0 5 0 2 4 6 8 10 12 14 16 18 20 22 24
Reduced GI side effects Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on
comparison of geometric mean ratio of total area under the curve. Investor Presentation | March 2024 11 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL 5. Based on a
comparison between Phase 2b study of MM120 capsules in GAD versus PK bridging study of MM120 ODTs AUC: area under the curve; GI: gastrointestinal; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)

MM120 LSD-D-tartrate for Generalized Anxiety Disorder (GAD) Summary of
Full Topline Results from Phase 2b Trial Daniel R Karlin, MD, MA Chief Medical Officer

Positive 12-Week Topline Results from Phase 2b Study in GAD: Strong 1
Durability of Effect after Single Dose of MM120 2 • Met the primary and all secondary endpoints with statistical significance • MCP-Mod analysis results support dose-response relationship for MM120 in GAD 3,4 • Large observed
effect size of d=0.81 at 12 weeks is more than double the standard of care o Durability of at least 3 months after a single dose of MM120 observed • Statistically and clinically significant 21.9-point improvement in HAM-A score at week 12
(p=0.0025) 3 represents further improvement from four-week topline data o Rapid and durable clinical activity with continued improvement at week 12 5 o 48% clinical remission rate through 12-week observation period 2 o Clinically and statistically
significant improvements on all analyzed secondary endpoints at week 12 • MM120 was well-tolerated with no related serious adverse events o Mostly transient, mild-to-moderate adverse events consistent with drug class and prior studies 6 o No
drug-related serious adverse event (SAE) and no suicide-related safety signal • Supports long-term durability of single administration MM120 and we believe further supports advancement of 100 µg MM120 into Phase 3 development for GAD 1.
Source: Study MMED008 internal study documents and calculations. Individual group results reported on 100 µg dose group vs. placebo. 2. Represents all analyzed secondary endpoints in week 12 topline analysis, including HAM-A, CGI-S and MADRS.
3. Based on 100 µg dose group; HAM-A scores based on ANCOVA LS Mean. Effect size based on post hoc calculation by study statistician using LS Mean change between group and pooled standard deviation of ending HAM-A scores across groups. 4.
Examination of baseline group assignment for all of the studies (20 studies utilizing the HAM-A (Hamilton Anxiety Scale) and 1 study using the PARS (Pediatric Anxiety Scale) for the primary outcome measurement. Source: RB Hidalgo, J 13 Investor
Presentation | March 2024 Psychopharmacol. 2007 Nov;21(8):864-72. 5. Remission defined as HAM-A score of ≤7. 6. Suicidality assessment based on reported adverse events.

Phase 2b Trial of MM120 Utilized Standard GAD Design and Endpoints 1
and was Aligned with FDA Draft Guidance for Drug Class • Standard GAD study design with endpoints that have supported registration for approved drugs • Randomized, double-blind, placebo-controlled, 12-week trial o Single administration
of MM-120 or placebo o No psychotherapeutic intervention 2 o Trial design closely aligned with subsequently issued FDA 2023 Draft Guidance o Patients washed out of anxiety pharmacotherapy prior to randomization • Enrolled 198 patients with GAD
• Five-arm dose optimization design with 1:1:1:1:1 randomization • Primary endpoint: change in Hamilton Anxiety Scale (HAM-A) at week 4 o Assessed by central rater blinded to treatment assignment and visit number 1. Source: Study MMED008
internal study documents and calculations. 14 Investor Presentation | March 2024 2. FDA 2023 Draft Guidance: Psychedelic Drugs: Considerations for Clinical Investigations.
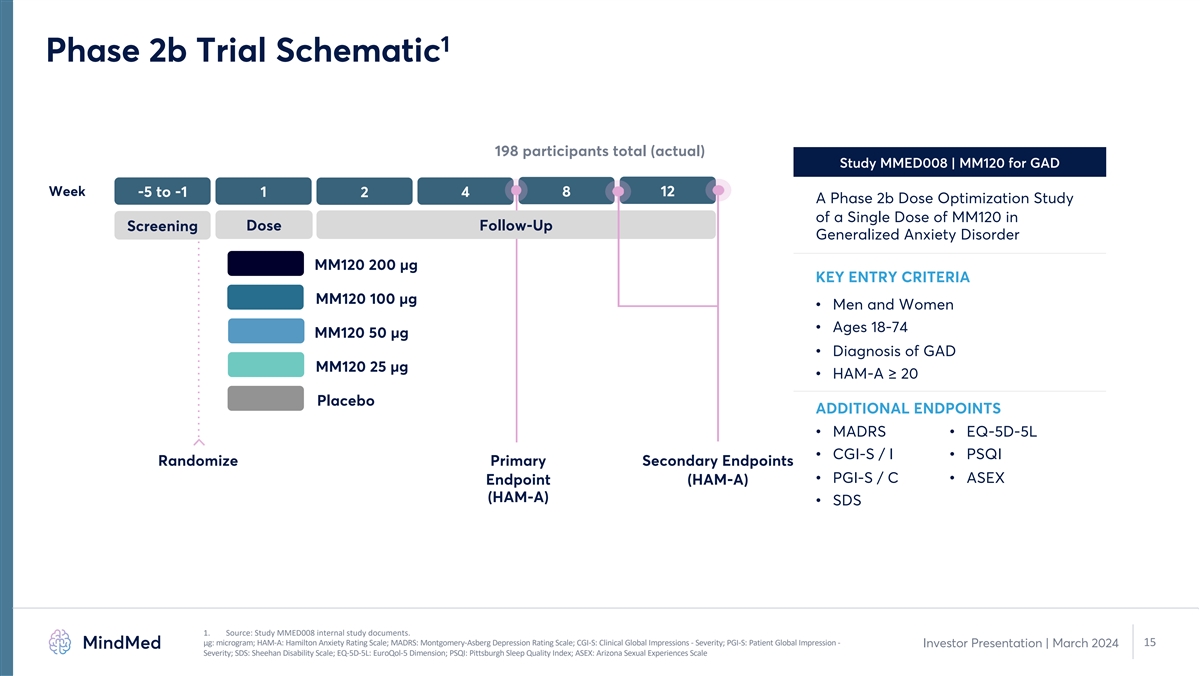
1 Phase 2b Trial Schematic 198 participants total (actual) Study
MMED008 | MM120 for GAD Week -5 to -1 1 4 8 12 2 A Phase 2b Dose Optimization Study of a Single Dose of MM120 in Dose Follow-Up Screening Generalized Anxiety Disorder MM120 200 µg KEY ENTRY CRITERIA MM120 100 µg • Men and Women
• Ages 18-74 MM120 50 µg • Diagnosis of GAD MM120 25 µg • HAM-A ≥ 20 Placebo ADDITIONAL ENDPOINTS • MADRS • EQ-5D-5L • CGI-S / I • PSQI Randomize Primary Secondary Endpoints • PGI-S / C
• ASEX Endpoint (HAM-A) (HAM-A) • SDS 1. Source: Study MMED008 internal study documents. μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impressions -
Severity; PGI-S: Patient Global Impression - 15 Investor Presentation | March 2024 Severity; SDS: Sheehan Disability Scale; EQ-5D-5L: EuroQol-5 Dimension; PSQI: Pittsburgh Sleep Quality Index; ASEX: Arizona Sexual Experiences Scale
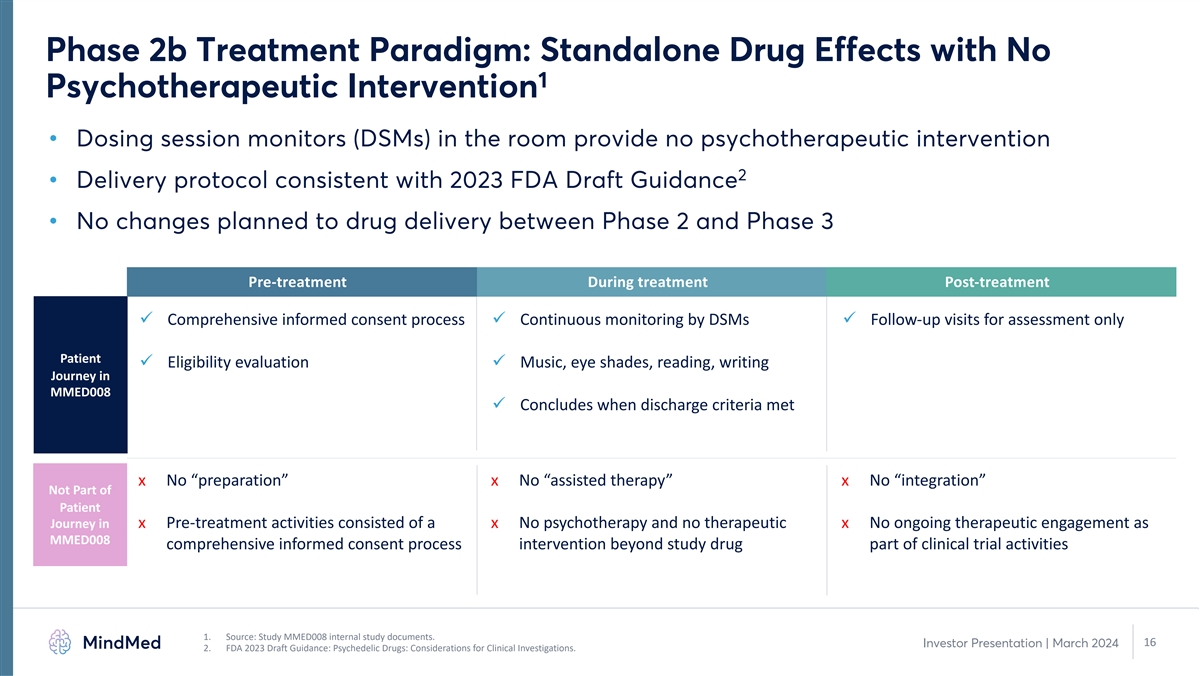
Phase 2b Treatment Paradigm: Standalone Drug Effects with No 1
Psychotherapeutic Intervention • Dosing session monitors (DSMs) in the room provide no psychotherapeutic intervention 2 • Delivery protocol consistent with 2023 FDA Draft Guidance • No changes planned to drug delivery between Phase
2 and Phase 3 Pre-treatment During treatment Post-treatment ü Comprehensive informed consent process ü Continuous monitoring by DSMs ü Follow-up visits for assessment only Patient ü Eligibility evaluation ü Music, eye
shades, reading, writing Journey in MMED008 ü Concludes when discharge criteria met x No “preparation” x No “assisted therapy” x No “integration” Not Part of Patient Journey in x Pre-treatment activities
consisted of a x No psychotherapy and no therapeutic x No ongoing therapeutic engagement as MMED008 comprehensive informed consent process intervention beyond study drug part of clinical trial activities 1. Source: Study MMED008 internal study
documents. 16 Investor Presentation | March 2024 2. FDA 2023 Draft Guidance: Psychedelic Drugs: Considerations for Clinical Investigations.
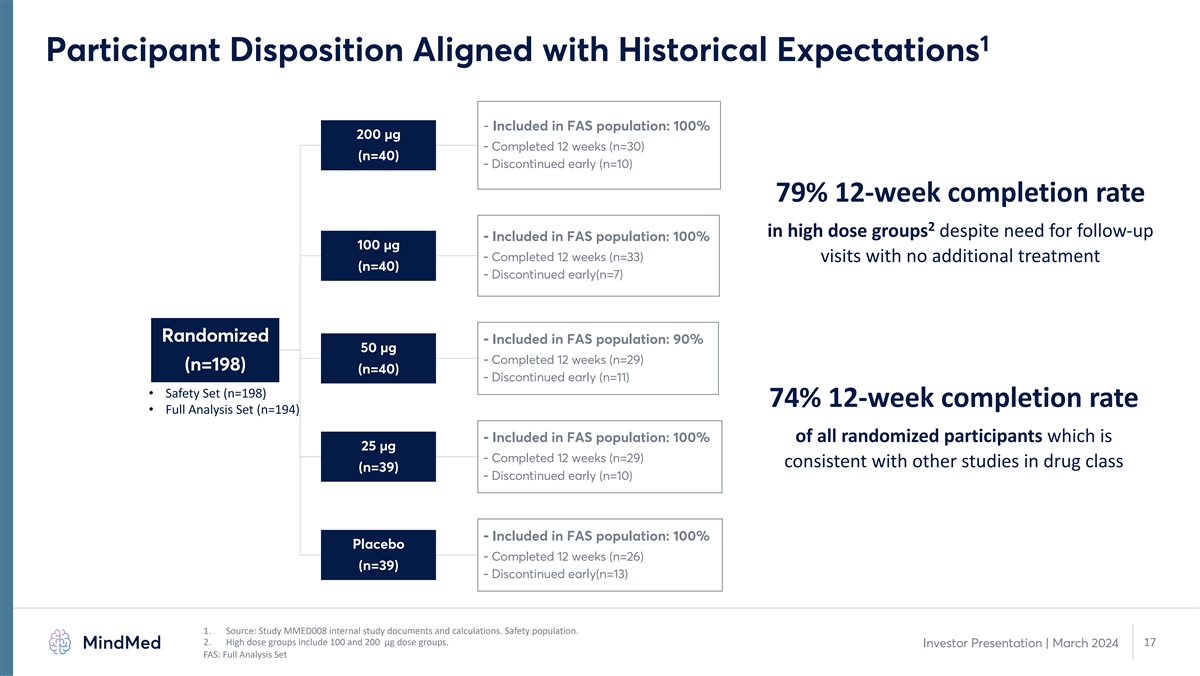
1 Participant Disposition Aligned with Historical Expectations -
Included in FAS population: 100% 200 µg - Completed 12 weeks (n=30) (n=40) - Discontinued early (n=10) 79% 12-week completion rate 2 in high dose groups despite need for follow-up - Included in FAS population: 100% 100 µg - Completed 12
weeks (n=33) visits with no additional treatment (n=40) - Discontinued early(n=7) Randomized - Included in FAS population: 90% 50 µg - Completed 12 weeks (n=29) (n=198) (n=40) - Discontinued early (n=11) • Safety Set (n=198) 74% 12-week
completion rate • Full Analysis Set (n=194) - Included in FAS population: 100% of all randomized participants which is 25 µg - Completed 12 weeks (n=29) consistent with other studies in drug class (n=39) - Discontinued early (n=10) -
Included in FAS population: 100% Placebo - Completed 12 weeks (n=26) (n=39) - Discontinued early(n=13) 1. Source: Study MMED008 internal study documents and calculations. Safety population. 2. High dose groups include 100 and 200 µg dose
groups. 17 Investor Presentation | March 2024 FAS: Full Analysis Set
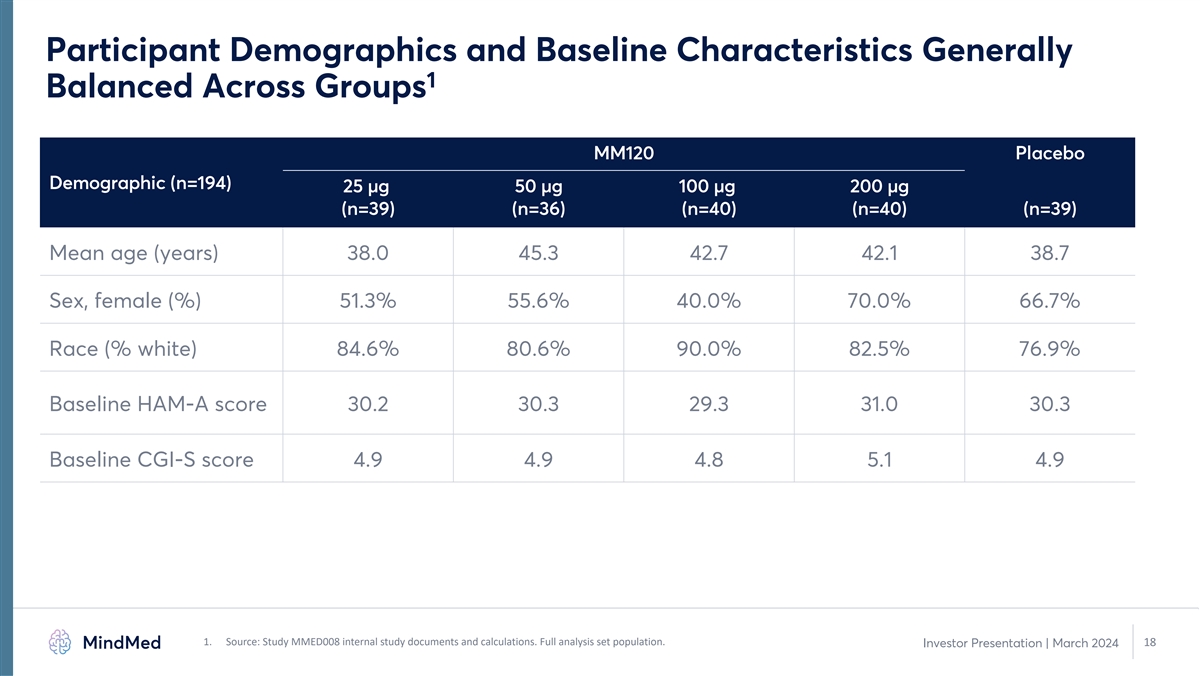
Participant Demographics and Baseline Characteristics Generally 1
Balanced Across Groups MM120 Placebo Demographic (n=194) 25 µg 50 µg 100 µg 200 µg (n=39) (n=36) (n=40) (n=40) (n=39) Mean age (years) 38.0 45.3 42.7 42.1 38.7 Sex, female (%) 51.3% 55.6% 40.0% 70.0% 66.7% Race (% white) 84.6%
80.6% 90.0% 82.5% 76.9% Baseline HAM-A score 30.2 30.3 29.3 31.0 30.3 Baseline CGI-S score 4.9 4.9 4.8 5.1 4.9 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 18 Investor Presentation | March
2024
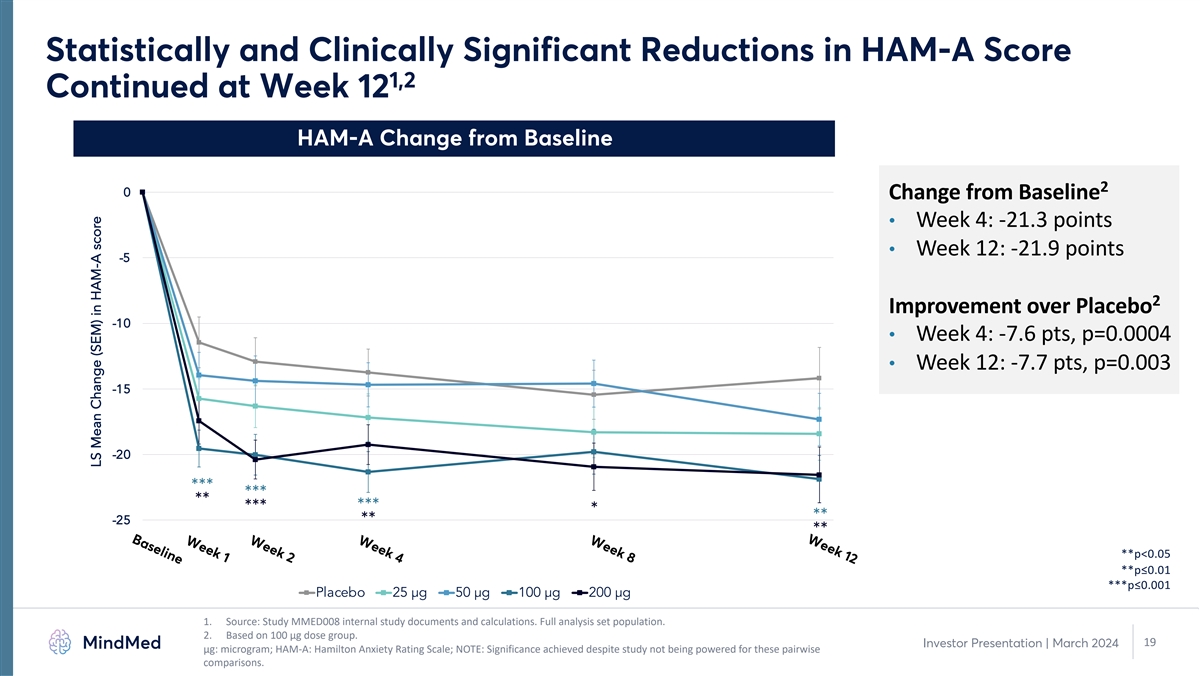
Week 12 Week 8 Week 4 Week 2 Week 1 Baseline Statistically and
Clinically Significant Reductions in HAM-A Score 1,2 Continued at Week 12 HAM-A Change from Baseline 2 0 Change from Baseline • Week 4: -21.3 points • Week 12: -21.9 points -5 2 Improvement over Placebo -10 • Week 4: -7.6 pts,
p=0.0004 • Week 12: -7.7 pts, p=0.003 -15 -20 *** *** ** *** *** * ** -25 ** ** **p<0.05 **p≤0.01 ***p≤0.001 Placebo 25 µg 50 µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and
calculations. Full analysis set population. 2. Based on 100 µg dose group. 19 Investor Presentation | March 2024 μg: microgram; HAM-A: Hamilton Anxiety Rating Scale; NOTE: Significance achieved despite study not being powered for these
pairwise comparisons. LS Mean Change (SEM) in HAM-A score

Continued Response and Remission through Week 12 with 65% 1 Clinical
Responder Rate and 48% Clinical Remission Rate 2 2 HAM-A Response Rate at Week 12 HAM-A Remission Rate at Week 12 50 48 70 65 45 63 60 56 40 33 50 44 30 28 40 21 31 20 30 20 10 10 0 0 Placebo 25 µg 50 µg 100 µg 200 µg Placebo 25
µg 50 µg 100 µg 200 µg p-values not calculated p-values not calculated 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on
HAM-A score; Remission is defined as a HAM-A score of ≤ 7. 20 Investor Presentation | March 2024 μg: microgram; HAM-A: Hamilton Anxiety Rating Scale % Responders % Remitters

Primary & Key Secondary Analysis (MCP-Mod) Support Dose Response 1
Relationship for MM120 in GAD 2 Key Takeaways from MCP-Mod Analysis • Statistically significant dose response relationship with multiple model fits • Supports dose selection of 100 µg for subsequent studies in GAD •
Pre-specified model estimates and observed responses drive dose selection for Phase 3 studies Dose 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Source: Novartis. “The MCP-Mod methodology
– A statistical methodology for dose-response. 21 Investor Presentation | March 2024 ` Model Means

Rapid and Sustained Improvements in Clinical Global Impressions –
1 Severity (CGI-S) Starting on Day 2 and Continuing through Week 12 2 CGI-S Improvement in 100 µg Group CGI-S Scores at Week 12 6 – Severely Ill • Statistically and clinically significant improvement by Day 2 5 – Markedly Ill
and maintained through Week 12 4 – Moderately Ill • Greater than 2-unit improvement in CGI-S score through Week 12 **** 3 – Mildly Ill *** ** • Participants on average only 2 – Borderline Ill borderline-to-mildly ill at
Week 12 1 – Normal, not ill at all Baseline Day 2 Week 4 Week 12 Placebo 100 µg *p<0.05 **p≤0.01 ***p≤0.001 ****p≤0.0001 1. Source: Study MMED008 internal study documents and calculations. Full analysis set
population. 2. Significance achieved despite study not being powered for these pairwise comparisons. 22 Investor Presentation | March 2024 μg: microgram; CGI-S: Clinical Global Impressions - Severity

Week 12 Week 8 Week 4 Week 2 Week 1 Baseline Statistically and
Clinically Significant Reductions in Comorbid Depression 1,2 (MADRS) at All Timepoints through Week 12 3 MADRS Change from Baseline 0 2,3 Change from Baseline • Week 4: -18.1 points • Week 12: -18.7 points -5 2,3 Improvement over Placebo
-10 • Week 4: -5.7 points, p<0.05 • Week 12: -6.4 points, p<0.01 -15 ** -20 ** * * ** ** ** * * ** 25 µg 50 µg 100 µg 200 µg Placebo *p<0.05 **p≤0.01 1. Source: MindMed internal study documents and
calculations. Full analysis set population. 2. Based on 100 µg dose group. 23 Investor Presentation | March 2024 3. Significance achieved despite study not being powered for these pairwise comparisons. Based on observed MADRS score at each
timepoint. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale LS Mean Change (SEM) in MADRS score
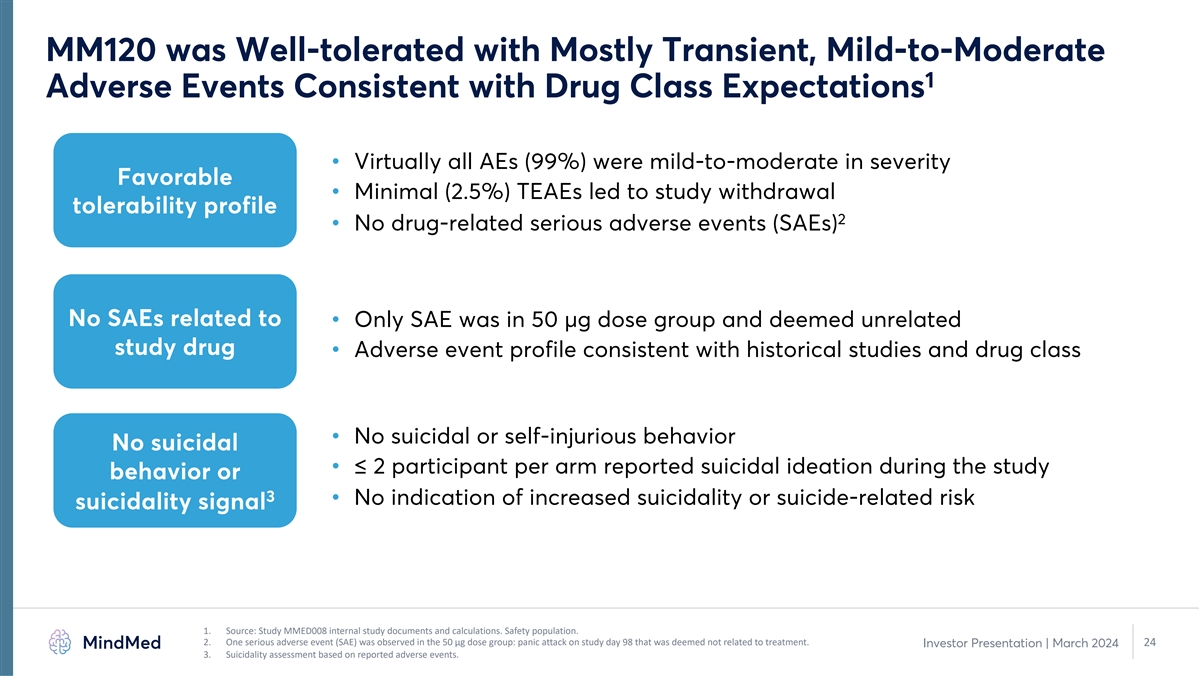
MM120 was Well-tolerated with Mostly Transient, Mild-to-Moderate 1
Adverse Events Consistent with Drug Class Expectations • Virtually all AEs (99%) were mild-to-moderate in severity Favorable • Minimal (2.5%) TEAEs led to study withdrawal tolerability profile 2 • No drug-related serious adverse
events (SAEs) No SAEs related to • Only SAE was in 50 µg dose group and deemed unrelated study drug • Adverse event profile consistent with historical studies and drug class • No suicidal or self-injurious behavior No suicidal
• ≤ 2 participant per arm reported suicidal ideation during the study behavior or 3 • No indication of increased suicidality or suicide-related risk suicidality signal 1. Source: Study MMED008 internal study documents and
calculations. Safety population. 2. One serious adverse event (SAE) was observed in the 50 µg dose group: panic attack on study day 98 that was deemed not related to treatment. 24 Investor Presentation | March 2024 3. Suicidality assessment
based on reported adverse events.
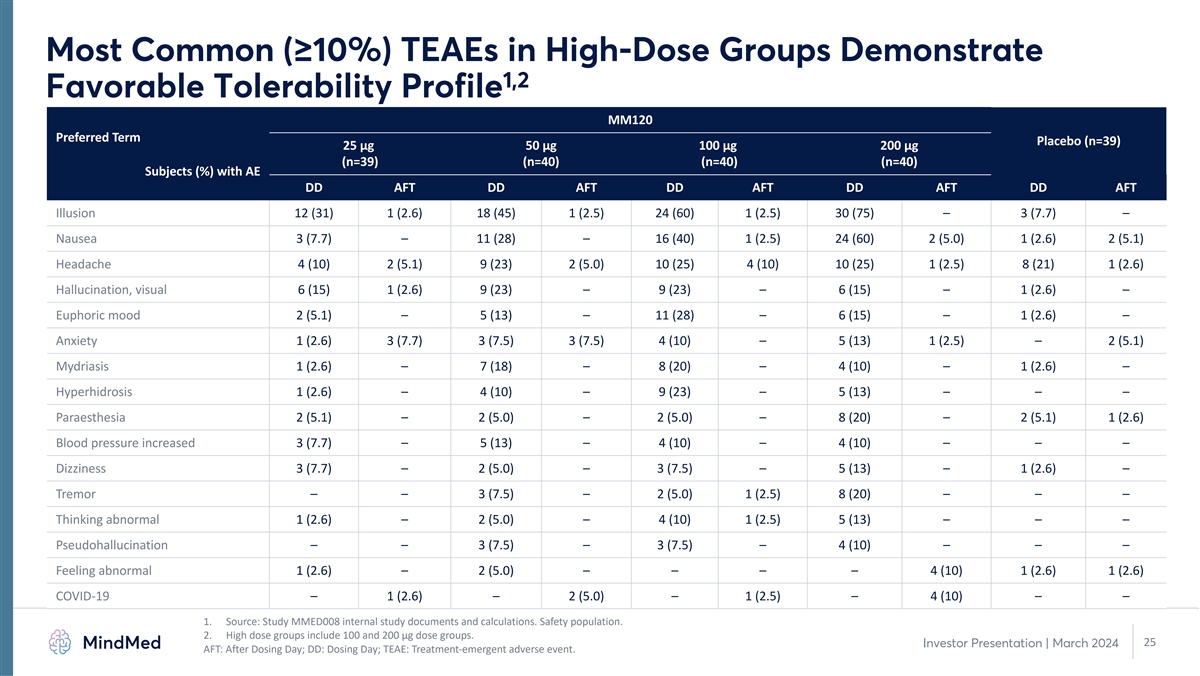
Most Common (≥10%) TEAEs in High-Dose Groups Demonstrate 1,2
Favorable Tolerability Profile MM120 Preferred Term Placebo (n=39) 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD AFT DD AFT Illusion 12 (31) 1 (2.6) 18 (45) 1 (2.5) 24 (60) 1
(2.5) 30 (75) – 3 (7.7) – Nausea 3 (7.7) – 11 (28) – 16 (40) 1 (2.5) 24 (60) 2 (5.0) 1 (2.6) 2 (5.1) Headache 4 (10) 2 (5.1) 9 (23) 2 (5.0) 10 (25) 4 (10) 10 (25) 1 (2.5) 8 (21) 1 (2.6) Hallucination, visual 6 (15) 1 (2.6) 9
(23) – 9 (23) – 6 (15) – 1 (2.6) – Euphoric mood 2 (5.1) – 5 (13) – 11 (28) – 6 (15) – 1 (2.6) – Anxiety 1 (2.6) 3 (7.7) 3 (7.5) 3 (7.5) 4 (10) – 5 (13) 1 (2.5) – 2 (5.1) Mydriasis 1
(2.6) – 7 (18) – 8 (20) – 4 (10) – 1 (2.6) – Hyperhidrosis 1 (2.6) – 4 (10) – 9 (23) – 5 (13) – – – Paraesthesia 2 (5.1) – 2 (5.0) – 2 (5.0) – 8 (20) – 2 (5.1)
1 (2.6) Blood pressure increased 3 (7.7) – 5 (13) – 4 (10) – 4 (10) – – – Dizziness 3 (7.7) – 2 (5.0) – 3 (7.5) – 5 (13) – 1 (2.6) – Tremor – – 3 (7.5) – 2 (5.0) 1
(2.5) 8 (20) – – – Thinking abnormal 1 (2.6) – 2 (5.0) – 4 (10) 1 (2.5) 5 (13) – – – Pseudohallucination – – 3 (7.5) – 3 (7.5) – 4 (10) – – – Feeling abnormal
1 (2.6) – 2 (5.0) – – – – 4 (10) 1 (2.6) 1 (2.6) COVID-19 – 1 (2.6) – 2 (5.0) – 1 (2.5) – 4 (10) – – 1. Source: Study MMED008 internal study documents and calculations. Safety
population. 2. High dose groups include 100 and 200 µg dose groups. 25 Investor Presentation | March 2024 AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse event.

MM120 LSD-D-tartrate for Generalized Anxiety Disorder (GAD) MM120 ODT
PK Bridging Study Daniel R Karlin, MD, MA Chief Medical Officer

PK Bridging Study Demonstrates Enhanced Product Profile for MM120 ODTs
1 Comparative PK Profile 2.5 MM120 Capsule Differentiated Performance of MM120 ODTs MM120 ODT 2 2 50% faster onset of action 1.5 3 17% improved bioavailability 1 4 0.5 23% increase in AUC at target conc. AUC 0 5 0 2 4 6 8 10 12 14 16 18 20 22 24
Reduced GI side effects Time (hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve.
Investor Presentation | March 2024 27 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL 5. Based on a comparison between Phase 2b study of MM120 capsules in GAD versus PK
bridging study of MM120 ODTs AUC: area under the curve; GI: gastrointestinal; ODT: orally dissolving tablet; PK: pharmacokinetics MM120 Concentration (ng/mL)

1 MM120 ODT PK Bridging Study Schematic Study MM120-101 | ODT-PK
Bridging 29 participants total (actual) A Phase 1, Open-label Study to Compare the Pharmacokinetics of Day -21 to -1 1 2-13 14 15-29 Two Formulations of MM120 in Washout Dose 2 Follow-up Screening Dose 1 Healthy Volunteers MM120 MM120 ENTRY CRITERIA
Zydis ODT Zydis ODT • Men and Women (100 µg) (100 µg) • Ages 18-55 • Healthy volunteers • No prohibited medications MM120 MM120 Capsule Capsule (100 µg) (100 µg) Enrollment 1. Based on internal study
documents for Study MM120-101 Investor Presentation | March 2024 28 ODT: orally dissolving tablet

Comparative PK of MM120 ODT vs Capsule Demonstrates Favorable 1 Profile
of MM120 ODTs 2.5 MM120 Capsule MM120 ODT MM120 MM120 1 2 PK Parameter Capsule ODT 1.5 T (hr) 2.25 2.0 max C (ng/mL) 2.63 2.68 max 1 AUC (ng*hr/mL) 15.7 18.7 0-∞ 0.5 AUC (ng*hr/mL) 9.7 12.0 >1ng/mL 0 0 2 4 6 8 10 12 14 16 18 20 22 24 Time
(hours) 1. Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. Investor Presentation | March 2024 29 AUC: area under the curve; C : maximum achieved concentration;
ODT: orally dissolving tablet; PK: pharmacokinetics; T : time to maximum concentration max max MM120 Concentration (ng/mL)

MM120 ODT Demonstrates Faster Absorption and Shorter Time to Reach
Target Concentrations 1.2 MM120 Capsule 1 MM120 ODT Differentiated PK Profile of MM120 ODTs 1 2 50% faster onset of action 0.8 0.6 3 17% improved bioavailability 0.4 4 0.2 23% increased AUC above target conc. AUC 0 0 10 20 30 Time (minutes) 1.
Company analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of
total area under the curve. Investor Presentation | March 2024 30 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; ODT: orally dissolving tablet;
PK: pharmacokinetics MM120 Concentration (ng/mL)
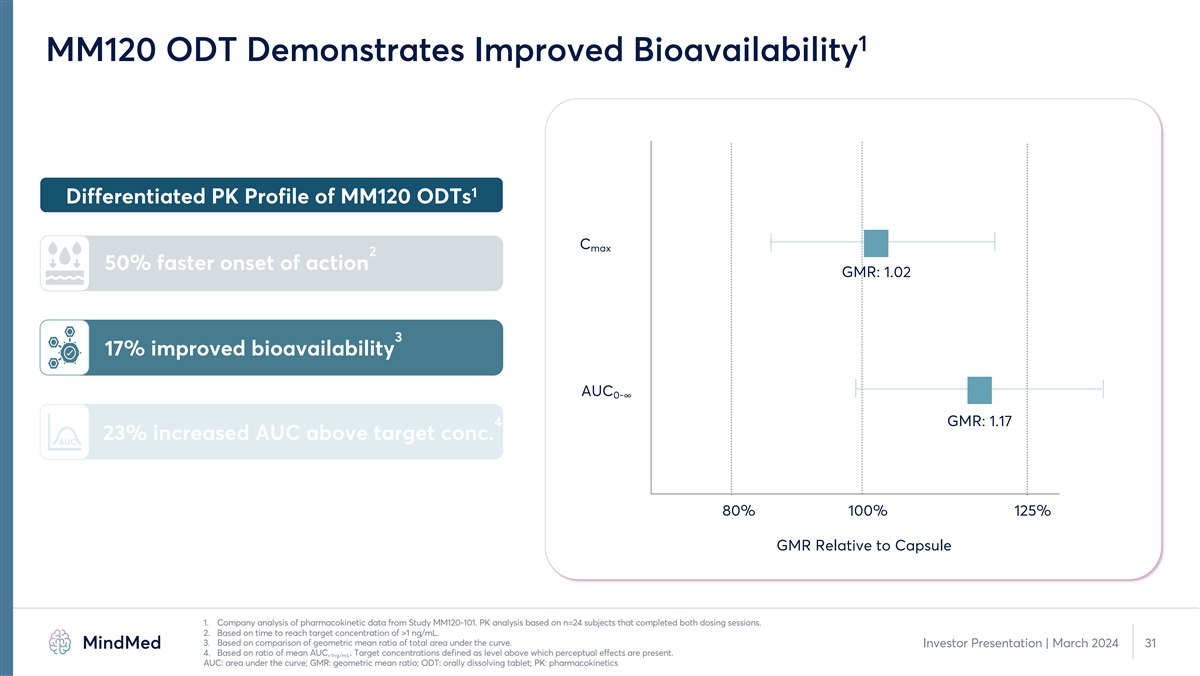
1 MM120 ODT Demonstrates Improved Bioavailability 1 Differentiated PK
Profile of MM120 ODTs C max 2 50% faster onset of action GMR: 1.02 3 17% improved bioavailability AUC 0-∞ 4 GMR: 1.17 23% increased AUC above target conc. AUC 80% 100% 125% GMR Relative to Capsule 1. Company analysis of pharmacokinetic data
from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total area under the curve. Investor
Presentation | March 2024 31 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; GMR: geometric mean ratio; ODT: orally dissolving tablet; PK:
pharmacokinetics
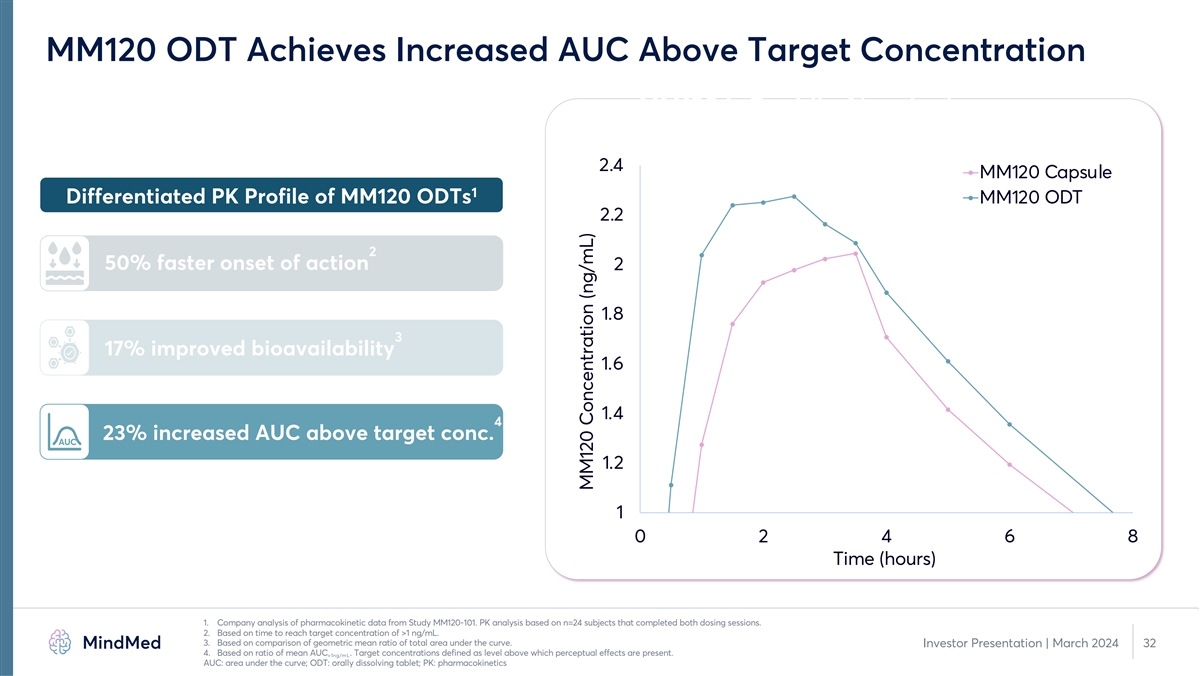
MM120 ODT Achieves Increased AUC Above Target Concentration MM120 is
Rapidly Absorbed 2.4 MM120 Capsule 1 Differentiated PK Profile of MM120 ODTs MM120 ODT 2.2 2 50% faster onset of action 2 1.8 3 17% improved bioavailability 1.6 1.4 4 23% increased AUC above target conc. AUC 1.2 1 0 2 4 6 8 Time (hours) 1. Company
analysis of pharmacokinetic data from Study MM120-101. PK analysis based on n=24 subjects that completed both dosing sessions. 2. Based on time to reach target concentration of >1 ng/mL. 3. Based on comparison of geometric mean ratio of total
area under the curve. Investor Presentation | March 2024 32 4. Based on ratio of mean AUC . Target concentrations defined as level above which perceptual effects are present. >1ng/mL AUC: area under the curve; ODT: orally dissolving tablet; PK:
pharmacokinetics MM120 Concentration (ng/mL)

Summary Comments for MM120 Development Plan Robert Barrow Chief
Executive Officer

Multiple Studies Support Phase 3 Development of MM120 1 •
Achieved goals of Phase 2 development o Characterized dose-response to inform dose selection in GAD o Large, statistically significant and clinically meaningful effect in GAD o Rapid and durable therapeutic benefits on validated endpoint o
Standalone drug effect in absence of psychotherapeutic intervention • Multiple double-blind, placebo-controlled studies supporting activity of MM120 o Phase 2b randomized, placebo-controlled dose optimization trial in GAD (Study MMED008) o One
prior modern, randomized, placebo-controlled IIT of lysergide in anxiety disorders o Over twenty legacy studies of lysergide in anxiety and other neurotic disorders • Phase 2b data supports dose selection and advancement into Phase 3
development 1. Source: Study MMED008 internal study documents and calculations. 34 Investor Presentation | March 2024
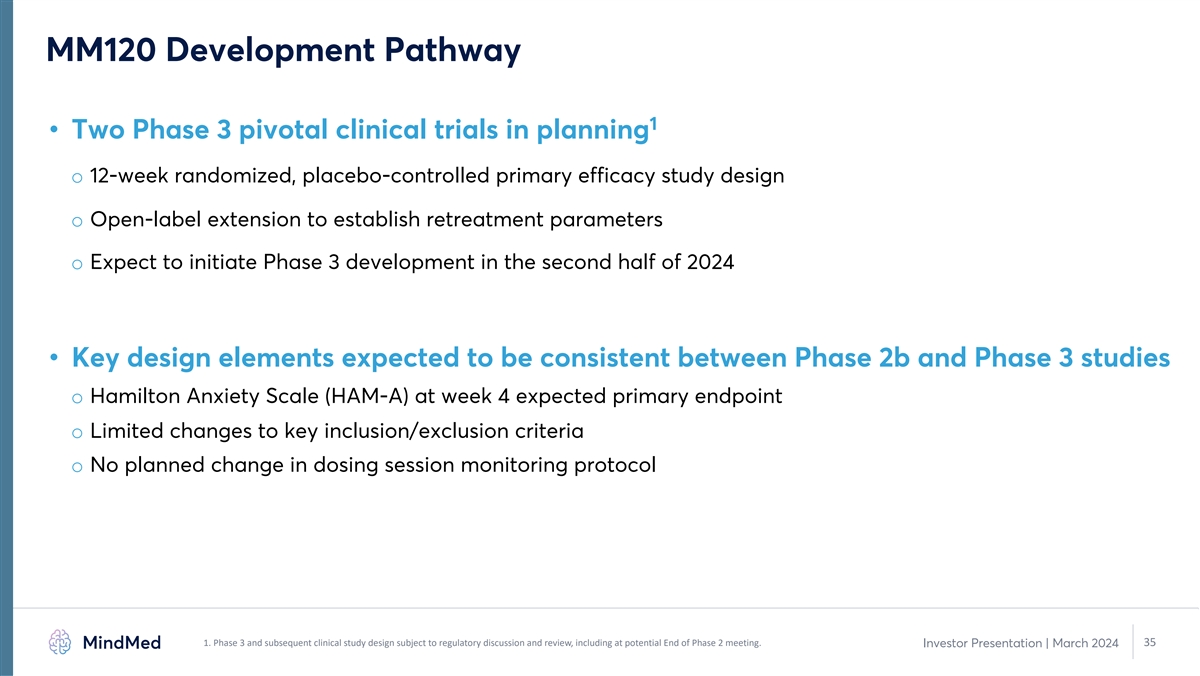
MM120 Development Pathway 1 • Two Phase 3 pivotal clinical trials
in planning o 12-week randomized, placebo-controlled primary efficacy study design o Open-label extension to establish retreatment parameters o Expect to initiate Phase 3 development in the second half of 2024 • Key design elements expected to
be consistent between Phase 2b and Phase 3 studies o Hamilton Anxiety Scale (HAM-A) at week 4 expected primary endpoint o Limited changes to key inclusion/exclusion criteria o No planned change in dosing session monitoring protocol 1. Phase 3 and
subsequent clinical study design subject to regulatory discussion and review, including at potential End of Phase 2 meeting. 35 Investor Presentation | March 2024
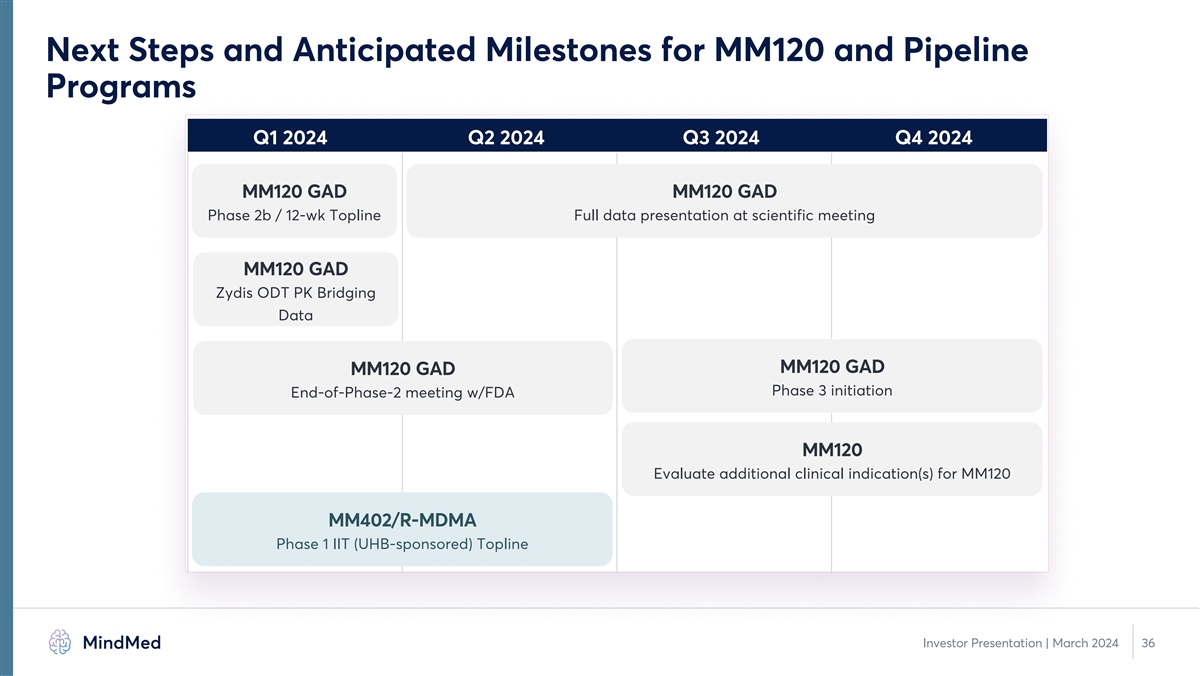
Next Steps and Anticipated Milestones for MM120 and Pipeline Programs
Q1 2024 Q2 2024 Q3 2024 Q4 2024 MM120 GAD MM120 GAD Phase 2b / 12-wk Topline Full data presentation at scientific meeting MM120 GAD Zydis ODT PK Bridging Data MM120 GAD MM120 GAD Phase 3 initiation End-of-Phase-2 meeting w/FDA MM120 Evaluate
additional clinical indication(s) for MM120 MM402/R-MDMA Phase 1 IIT (UHB-sponsored) Topline Investor Presentation | March 2024 36

Q&A 37 Investor Presentation | March 2024

Appendix
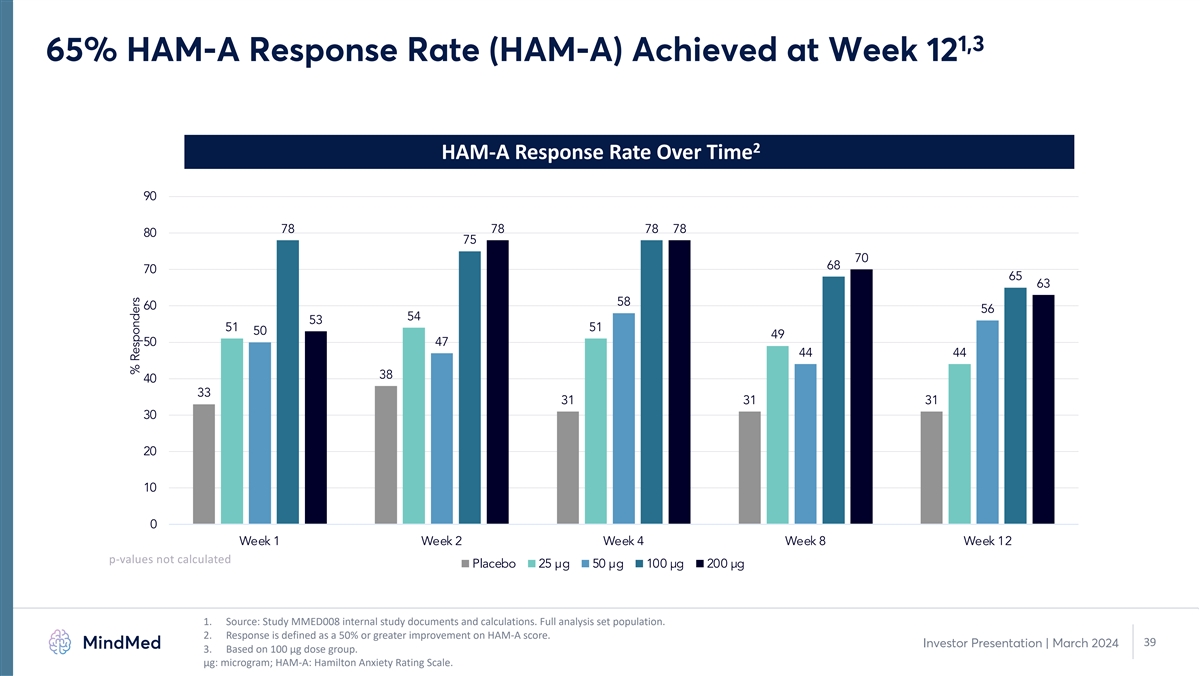
1,3 65% HAM-A Response Rate (HAM-A) Achieved at Week 12 2 HAM-A
Response Rate Over Time 90 78 78 78 78 80 75 70 68 70 65 63 58 60 56 54 53 51 51 50 49 50 47 44 44 38 40 33 31 31 31 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1.
Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on HAM-A score. 39 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg:
microgram; HAM-A: Hamilton Anxiety Rating Scale. % Responders

1,3 48% Remission Rate (HAM-A) Achieved through Week 12 2 HAM-A
Remission Rate Over Time 60 50 50 48 45 45 45 43 40 38 35 33 33 33 33 33 33 33 31 30 28 28 26 25 21 21 21 20 18 15 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100 µg 200 µg 1. Source:
Study MMED008 internal study documents and calculations. Full analysis set population. 2. Remission is defined as a HAM-A score of ≤ 7. 40 Investor Presentation | March 2024 3. Based on 100 µg dose group. μg: microgram; HAM-A:
Hamilton Anxiety Rating Scale. % Remitters
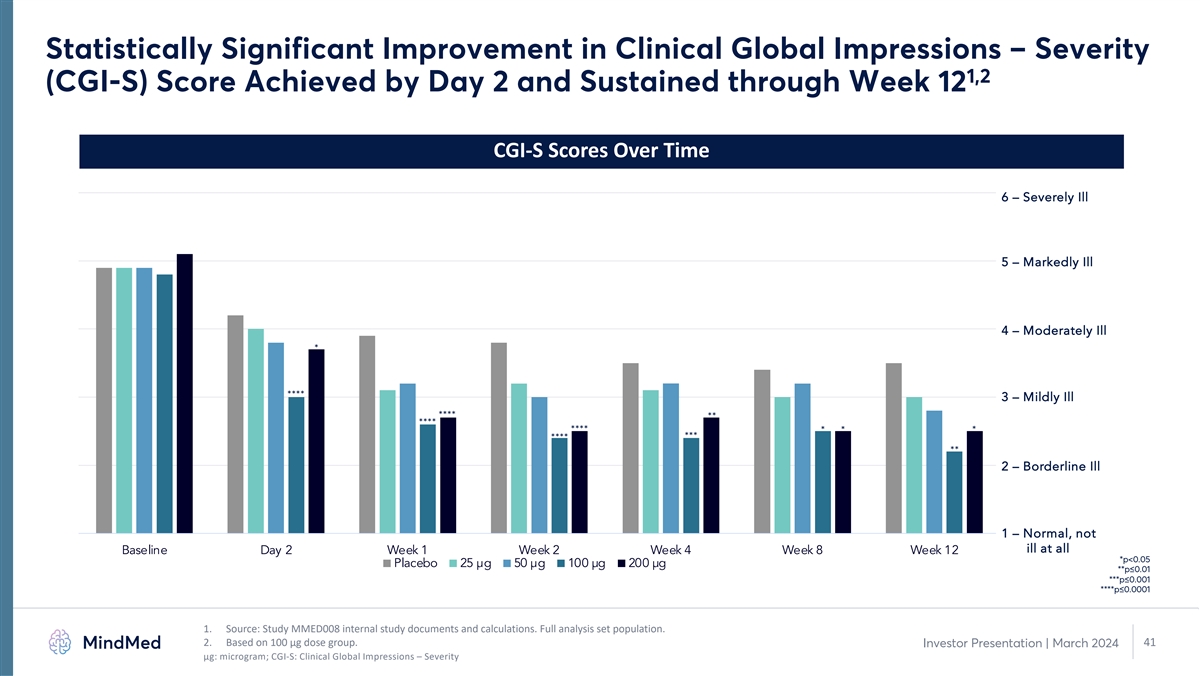
Statistically Significant Improvement in Clinical Global Impressions
– Severity 1,2 (CGI-S) Score Achieved by Day 2 and Sustained through Week 12 CGI-S Scores Over Time 6 – Severely Ill 5 – Markedly Ill 4 – Moderately Ill * **** 3 – Mildly Ill **** ** **** **** * * * *** **** ** 2
– Borderline Ill 1 – Normal, not ill at all Baseline Day 2 Week 1 Week 2 Week 4 Week 8 Week 12 *p<0.05 Placebo 25 µg 50 µg 100 µg 200 µg **p≤0.01 ***p≤0.001 ****p≤0.0001 1. Source: Study MMED008
internal study documents and calculations. Full analysis set population. 2. Based on 100 µg dose group. 41 Investor Presentation | March 2024 μg: microgram; CGI-S: Clinical Global Impressions – Severity
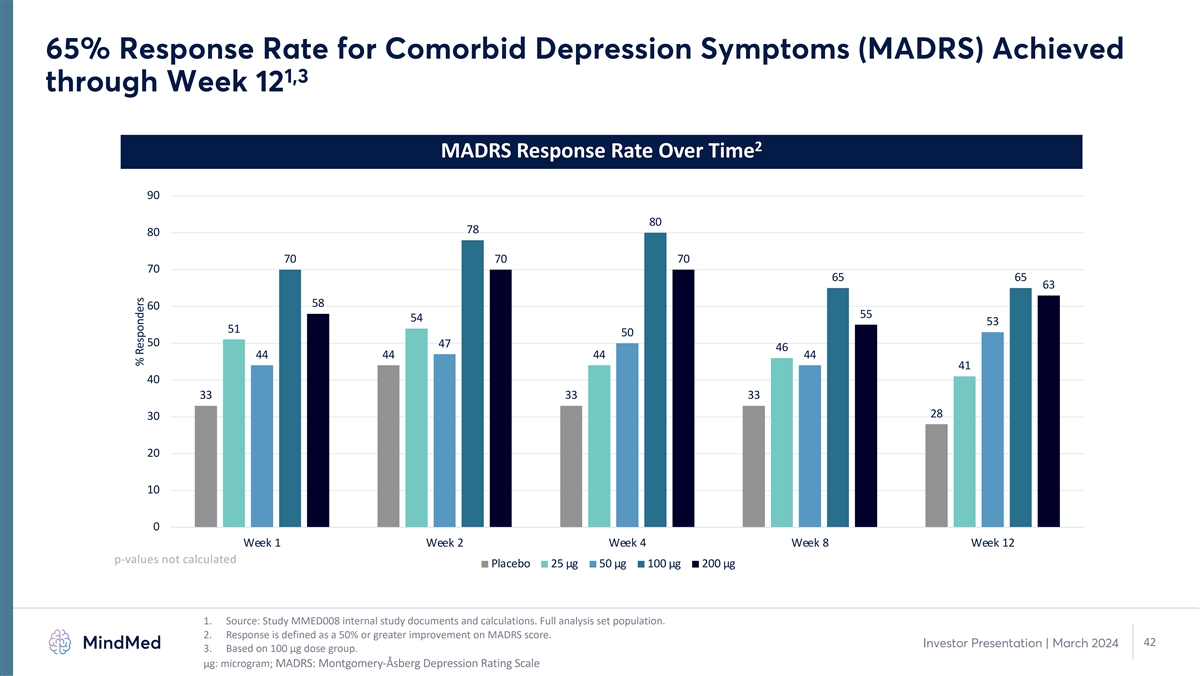
65% Response Rate for Comorbid Depression Symptoms (MADRS) Achieved 1,3
through Week 12 2 MADRS Response Rate Over Time 90 80 78 80 70 70 70 70 65 65 63 58 60 55 54 53 51 50 50 47 46 44 44 44 44 41 40 33 33 33 28 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50 µg 100
µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Response is defined as a 50% or greater improvement on MADRS score. 42 Investor Presentation | March 2024 3. Based on 100
µg dose group. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale % Responders
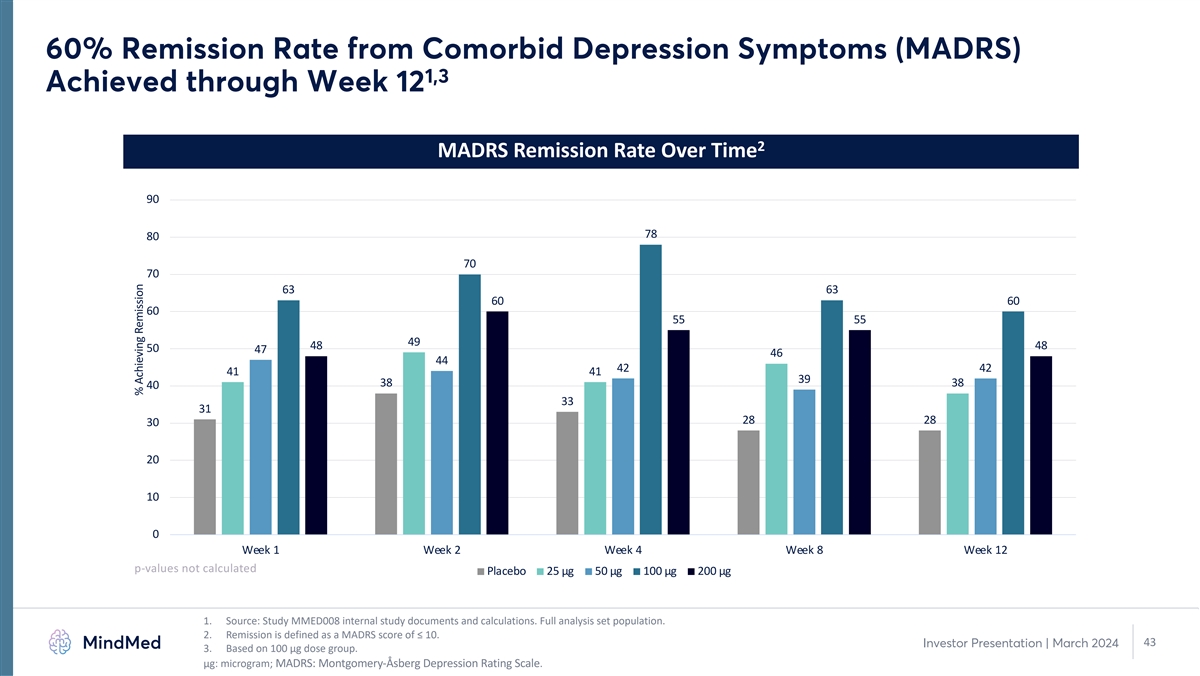
60% Remission Rate from Comorbid Depression Symptoms (MADRS) 1,3
Achieved through Week 12 2 MADRS Remission Rate Over Time 90 78 80 70 70 63 63 60 60 60 55 55 49 48 48 50 47 46 44 42 42 41 41 39 38 38 40 33 31 28 28 30 20 10 0 Week 1 Week 2 Week 4 Week 8 Week 12 p-values not calculated Placebo 25 µg 50
µg 100 µg 200 µg 1. Source: Study MMED008 internal study documents and calculations. Full analysis set population. 2. Remission is defined as a MADRS score of ≤ 10. 43 Investor Presentation | March 2024 3. Based on 100 µg
dose group. μg: microgram; MADRS: Montgomery-Åsberg Depression Rating Scale. % Achieving Remission

1 Most Common (≥10%) TEAEs Across All Groups MM120 was well
tolerated across all dose groups with mostly transient, mild to moderate adverse events MM120 Placebo (n=39) Preferred Term 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD AFT DD
AFT Illusion 12 (31) 1 (2.6) 18 (45) 1 (2.5) 24 (60) 1 (2.5) 30 (75) – 3 (7.7) – Nausea 3 (7.7) – 11 (28) – 16 (40) 1 (2.5) 24 (60) 2 (5.0) 1 (2.6) 2 (5.1) Headache 4 (10) 2 (5.1) 9 (23) 2 (5.0) 10 (25) 4 (10) 10 (25) 1 (2.5)
8 (21) 1 (2.6) Hallucination, visual 6 (15) 1 (2.6) 9 (23) – 9 (23) – 6 (15) – 1 (2.6) – Euphoric mood 2 (5.1) – 5 (13) – 11 (28) – 6 (15) – 1 (2.6) – Anxiety 1 (2.6) 3 (7.7) 3 (7.5) 3 (7.5) 4
(10) – 5 (13) 1 (2.5) – 2 (5.1) Mydriasis 1 (2.6) – 7 (18) – 8 (20) – 4 (10) – 1 (2.6) – Hyperhidrosis 1 (2.6) – 4 (10) – 9 (23) – 5 (13) – – – Fatigue 2 (5.1) – 6
(15) 2 (5.0) 3 (7.5) 1 (2.5) 3 (7.5) 1 (2.5) – 1 (2.6) Paraesthesia 2 (5.1) – 2 (5.0) – 2 (5.0) – 8 (20) – 2 (5.1) 1 (2.6) Blood pressure increased 3 (7.7) – 5 (13) – 4 (10) – 4 (10) – –
– Dizziness 3 (7.7) – 2 (5.0) – 3 (7.5) – 5 (13) – 1 (2.6) – Tremor – – 3 (7.5) – 2 (5.0) 1 (2.5) 8 (20) – – – Thinking abnormal 1 (2.6) – 2 (5.0) – 4 (10) 1 (2.5) 5
(13) – – – 1. Source: Study MMED008 internal study documents and calculations. Safety population. AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse event. 44 Investor Presentation | March 2024
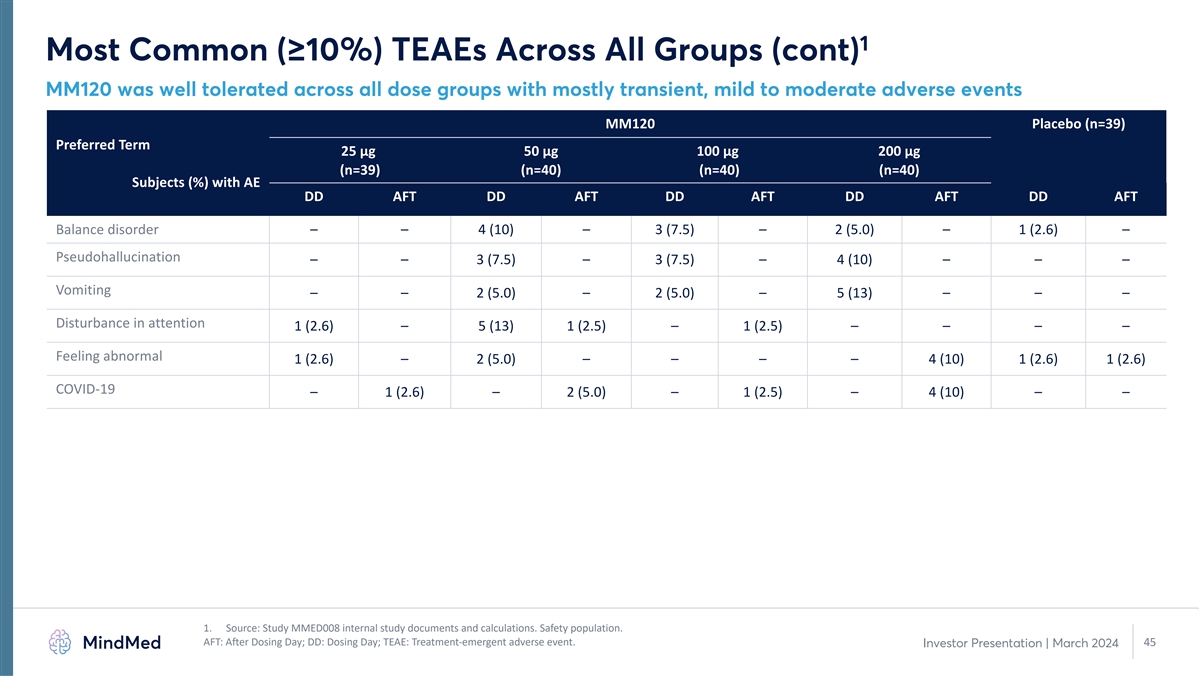
1 Most Common (≥10%) TEAEs Across All Groups (cont) MM120 was
well tolerated across all dose groups with mostly transient, mild to moderate adverse events MM120 Placebo (n=39) Preferred Term 25 µg 50 µg 100 µg 200 µg (n=39) (n=40) (n=40) (n=40) Subjects (%) with AE DD AFT DD AFT DD AFT DD
AFT DD AFT Balance disorder – – 4 (10) – 3 (7.5) – 2 (5.0) – 1 (2.6) – Pseudohallucination – – 3 (7.5) – 3 (7.5) – 4 (10) – – – Vomiting – – 2 (5.0) – 2
(5.0) – 5 (13) – – – Disturbance in attention 1 (2.6) – 5 (13) 1 (2.5) – 1 (2.5) – – – – Feeling abnormal 1 (2.6) – 2 (5.0) – – – – 4 (10) 1 (2.6) 1 (2.6)
COVID-19 – 1 (2.6) – 2 (5.0) – 1 (2.5) – 4 (10) – – 1. Source: Study MMED008 internal study documents and calculations. Safety population. AFT: After Dosing Day; DD: Dosing Day; TEAE: Treatment-emergent adverse
event. 45 Investor Presentation | March 2024
v3.24.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Mind Medicine MindMed (NASDAQ:MNMD)
Historical Stock Chart
From Mar 2024 to Apr 2024
Mind Medicine MindMed (NASDAQ:MNMD)
Historical Stock Chart
From Apr 2023 to Apr 2024