As filed with the Securities and Exchange Commission on February 13, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-3
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
VISTAGEN THERAPEUTICS, INC.
(Exact Name Of Registrant As Specified In Its Charter)
|
Nevada
|
|
20-5093315
|
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification Number)
|
| |
|
|
|
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
(650) 577-3600
|
|
Shawn K. Singh, J.D.
Chief Executive Officer
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
(650) 577-3600
|
|
(Address, including zip code, and telephone number,
including area code of Registrant’s principal executive offices)
|
|
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
|
From time to time after the effective date of this Registration Statement
(Approximate date of commencement of proposed sale to public)
Copies of all communications, including all communications sent to the agent for service, should be sent to:
Shawn K. Singh, J.D.
Chief Executive Officer
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
(650) 577-3600
Copies to:
|
Jessica R. Haskell
Associate General Counsel
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, CA 94080
(650) 577-3600
|
|
Christopher D. Lueking
Scott W. Westhoff
Latham & Watkins LLP
300 North Wabash Avenue, Suite 2800
Chicago, IL 60611
(312) 876-7700
|
If the only securities being registered on this form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. ☐
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 of the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. ☒
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, please check the following box. ☐
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, please check the following box. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
|
Smaller reporting company ☒
|
| |
|
Emerging growth company ☐
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This registration statement contains two prospectuses:
| |
•
|
|
a base prospectus covering the offering, issuance and sale by us of up to $350,000,000 in the aggregate of the securities identified above from time to time, subject to market conditions and prices, liquidity objectives and other investment considerations; and
|
| |
•
|
|
a Sales Agreement (defined below) prospectus supplement covering the offering, issuance and sale by us of up to a maximum aggregate offering price of $100,000,000 of our common stock that may be issued and sold under an Open Market Sale AgreementSM (the “Sales Agreement”) entered into with Jefferies LLC (“Jefferies”).
|
The base prospectus immediately follows this explanatory note. The specific terms of any securities to be offered by us pursuant to the base prospectus other than the shares under the Sales Agreement will be specified in a prospectus supplement to the base prospectus. The specific terms of the securities to be issued and sold under the Sales Agreement are specified in the Sales Agreement prospectus supplement that immediately follows the base prospectus. The $100,000,000 of common stock that may be offered, issued and sold under the Sales Agreement prospectus supplement is included in the $350,000,000 of securities that may be offered, issued and sold by us under the base prospectus. Upon termination of the Sales Agreement, any portion of the $100,000,000 included in the Sales Agreement prospectus supplement that is not sold pursuant to the Sales Agreement will be available for sale in other offerings pursuant to the base prospectus and a corresponding prospectus supplement, and if no shares are sold under the Sales Agreement prospectus supplement, the full $350,000,000 of securities may be sold in other offerings by us pursuant to the base prospectus and a corresponding prospectus supplement.
| |
|
|
| |
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
|
| |
|
|
|
SUBJECT TO COMPLETION, DATED FEBRUARY 13, 2024
|
PROSPECTUS
$350,000,000
COMMON STOCK
PREFERRED STOCK
DEBT SECURITIES
WARRANTS
UNITS
We may offer and sell securities from time to time in one or more offerings of up to $350,000,000 in aggregate offering price. This prospectus describes the general terms of these securities and the general manner in which these securities will be offered. We will provide the specific terms of these securities in supplements to this prospectus. The prospectus supplements will also describe the specific manner in which these securities will be offered and may also supplement, update or amend information contained in this document. You should read this prospectus and any applicable prospectus supplement before you invest. In addition, shares of common stock issuable from time to time upon exercise of our pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants are also being offered pursuant to this prospectus. See “Description of Capital Stock – Securities Registered Under Prior Registration Statements” for information regarding the pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants.
We may offer these securities in amounts, at prices and on terms determined at the time of offering. The securities may be sold directly to you, through agents, or through underwriters and dealers. If agents, underwriters or dealers are used to sell the securities, we will name them and describe their compensation in a prospectus supplement.
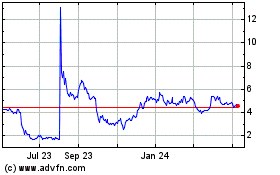
Our common stock is listed on the Nasdaq Capital Market under the symbol “VTGN.” On February 12, 2024, the closing price of our common stock on the Nasdaq Capital Market was $5.15 per share.
Investing in these securities involves certain risks. See “Risk Factors” included in any accompanying prospectus supplement and in the documents incorporated by reference in this prospectus for a discussion of the factors you should carefully consider before deciding to purchase these securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is .
VISTAGEN THERAPEUTICS, INC.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC”) utilizing a “shelf” registration process. Under this shelf registration process, we may from time to time sell any combination of the securities described in this prospectus in one or more offerings for an aggregate initial offering price of up to $350,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide one or more prospectus supplements that will contain specific information about the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus. This prospectus, together with any accompanying prospectus supplement, contains important information you should know before investing in our securities, including important information about us and the securities being offered. You should read both this prospectus and the accompanying prospectus supplement together with the additional information described under the heading “Where You Can Find More Information” below.
You should rely only on the information contained in or incorporated by reference in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. We have not authorized anyone to provide you with different or additional information. If anyone provides you with different, additional or inconsistent information, you should not rely on it. We do not take responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus and any accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in this prospectus or such accompanying prospectus supplement or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free writing prospectus is accurate only as of their respective dates, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any sale of securities. Our business, financial condition, results of operations and prospects may have changed materially since those dates.
Unless the context otherwise indicates, references in this prospectus to the “Company,” “we,” “our” and “us” refer, collectively, to Vistagen Therapeutics, Inc., a Nevada corporation, and its consolidated subsidiaries.
We use our trademarks and our logo in this prospectus and the documents incorporated by reference. Solely for the convenience, trademarks and tradenames referred to in this prospectus appear without the “®” and “™” symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and tradenames.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website at www.vistagen.com. Our website is not a part of this prospectus and is not incorporated by reference in this prospectus.
This prospectus is part of a registration statement on Form S-3 we filed with the SEC. This prospectus omits some information contained in the registration statement in accordance with SEC rules and regulations. You should review the information and exhibits in the registration statement for further information about us and our consolidated subsidiaries and the securities we are offering. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete document to evaluate these statements.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The following documents filed by us with the SEC are incorporated by reference in this prospectus supplement:
| |
●
|
our Annual Report on Form 10-K for the year ended March 31, 2023, filed on June 28, 2023;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended June 30, 2023, filed on August 10, 2022;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended September 30, 2023, filed on November 9, 2023;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended December 31, 2023, filed on February 13, 2024;
|
| |
●
|
our Definitive Proxy Statement on Schedule 14A, filed on July 28, 2023;
|
| |
●
|
our Current Reports on Form 8-K, filed on April 6, 2023, April 19, 2023, June 1, 2023, June 6, 2023, June 7, 2023, June 13, 2023, June 21, 2023, June 23, 2023, July 7, 2023, July 13, 2023, July 18, 2023, August 22, 2023, September 8, 2023, September 13, 2023, September 29, 2023, October 2, 2023, October 4, 2023 (excluding any information furnished in such reports under Item 7.01), October 26, 2023, November 6, 2023 (as amended on November 7, 2023), and January 3, 2024; and
|
| |
●
|
The description of our common stock contained in the Registration Statement on Form 8-A filed pursuant to Section 12(b) of the Securities Exchange Act of 1934, as amended (the Securities Act) on May 3, 2016, including any amendment or report filed with the Commission for the purpose of updating this description.
|
We also incorporate by reference all documents we file pursuant to Section 13(a), 13(c), 14 or 15 of the Exchange Act (other than any portions of filings that are furnished rather than filed pursuant to Items 2.02 and 7.01 of a Current Report on Form 8-K) after the date of the initial registration statement of which this prospectus is a part and prior to effectiveness of such registration statement. All documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of the offering are also incorporated herein by reference and are an important part of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of this registration statement to the extent that a statement contained herein or in any other subsequently filed document which also is or deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this registration statement.
We will provide upon request to each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings, excluding the exhibits to such filings which we have not specifically incorporated by reference in such filings, at no cost, by writing to or calling us at:
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
(650) 577-3600
This prospectus is part of a registration statement we filed with the SEC. You should only rely on the information or representations contained in this prospectus and any accompanying prospectus supplement. We have not authorized anyone to provide information other than that provided in this prospectus and any accompanying prospectus supplement. We are not making an offer of the securities in any state where the offer is not permitted. You should not assume that the information in this prospectus or any accompanying prospectus supplement is accurate as of any date other than the date on the front of the document.
RISK FACTORS
Investing in our securities involves significant risks. Please see the risk factors under the heading “Risk Factors” in our most recent Annual Report on Form 10-K on file with the SEC and our Quarterly Reports on Form 10-Q subsequently filed with the SEC, each of which is incorporated by reference in this prospectus, along with any risk factors identified in a prospectus supplement. Before making an investment decision, you should carefully consider these risks as well as other information we include or incorporate by reference in this prospectus and any prospectus supplement. The risks and uncertainties we have described are not the only ones facing our company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business operations. The occurrence of any of these risks might cause you to lose all or part of your investment in the offered securities. Actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks referenced below and described in the documents incorporated herein by reference. The discussion of risks includes or refers to forward-looking statements. You should read the explanation of the qualifications and limitations on such forward-looking statements discussed elsewhere in this prospectus.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, any prospectus supplement and the documents incorporated by reference herein contain forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this prospectus, any prospectus supplement and the documents incorporated by reference herein, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
| |
●
|
the availability of capital to satisfy our working capital requirements;
|
| |
●
|
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
| |
●
|
our plans to develop and commercialize our product candidates, including, among other things, fasedienol (PH94B) as a potential acute treatment of anxiety for adults with social anxiety disorder (“SAD”), itruvone (PH10) as a potential treatment for adults with major depressive disorder (“MDD”) and other depression-related disorders, PH80 for vasomotor symptoms (hot flashes) due to menopause, premenstrual dysphoric disorder (“PMDD”) or migraine, PH15 for cognition improvement, PH284 for appetite-related disorders or AV-101 as a potential treatment of disorders involving the Central Nervous System (“CNS”);
|
| |
●
|
our ability to initiate and complete necessary preclinical and clinical studies in accordance with applicable regulatory requirements to advance the development of our product candidates and for those studies to generate positive results;
|
| |
●
|
economic, regulatory and political developments in the United States and foreign countries;
|
| |
●
|
the performance of our third-party contractors involved with the manufacture and production of our drug candidates for nonclinical and clinical development activities, contract research organizations, potential commercial activities and other third-party nonclinical and clinical development collaborators and regulatory service providers; |
| |
●
|
our ability to obtain and maintain intellectual property, regulatory and commercial protection for our core assets, including product candidates; |
| |
●
|
the size of the potential markets for our product candidates and our ability to serve those markets;
|
| |
●
|
the rate and degree of market acceptance of our product candidates for any indication once approved;
|
| |
●
|
the success of competing products and product candidates in development by others that are or become available for the indications that we are pursuing in the markets we seek to enter on our own or with collaborators;
|
| |
●
|
the loss of key scientific, clinical and nonclinical development, regulatory, commercial, and/or management personnel, internally or from one of our third-party collaborators; contract manufacturing organizations, contract research organizations or other service providers;
|
| |
●
|
our ability to continue as a going concern;
|
| |
●
|
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
|
| |
●
|
our use of our existing cash and cash equivalents; and
|
| |
●
|
other risks and uncertainties, including those described under Item 1A, “Risk Factors,” in our Annual Report on Form 10-K for the fiscal year ended March 31, 2023, and those described under Part II, Item 1A, “Risk Factors,” in our Quarterly Reports on Form 10-Q for the quarters ended June 30, 2023, September 30, 2023 and December 31, 2023, which risk factors are incorporated herein by reference.
|
These forward-looking statements are only predictions, and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and operating results. Moreover, our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur.
You should read this prospectus, any prospectus supplement and the documents incorporated by reference herein and the documents that we have filed as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus and the documents incorporated by reference herein by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
COMPANY OVERVIEW
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all the information you should consider before buying our securities. You should read the following summary together with the more detailed information appearing in this prospectus and any accompanying prospectus supplement, including the section titled “Risk Factors” on page 3, before deciding whether to purchase our securities.
Overview
We are a clinical-stage biopharmaceutical company pioneering neuroscience to deliver first-in-class therapies for psychiatric and neurological disorders. Five of our six clinical-stage product candidates – fasedienol (PH94B), itruvone (PH10), PH15, PH80 and PH284 – belong to a new class of drugs known as pherines, which have the potential to rapidly deliver meaningful efficacy with a differentiated safety profile. Pherines are investigational neuroactive nasal sprays with innovative proposed mechanisms of action that activate chemosensory neurons in the nasal passages to impact fundamental neural circuits in the brain without the need for systemic absorption or binding to receptors in the brain. Our sixth clinical-stage product candidate, AV-101, is an investigational oral drug candidate with the potential to inhibit, but not block, NMDA receptor activity.
We are passionate about transforming what is possible in the treatment of anxiety, depression, and other neuroscience disorders.
Our Clinical-Stage Neuroscience Product Candidates
Fasedienol Nasal Spray for Social Anxiety Disorder (SAD)
Fasedienol (PH94B) is an odorless, tasteless synthetic investigational pherine nasal spray from the androstane family in Phase 3 clinical development for the acute treatment of anxiety for adults with SAD. When administered intranasally in microgram-level doses, fasedienol activates receptors of peripheral nasal chemosensory neurons connected to subsets of neurons in the olfactory bulbs that, in turn, connect to neurons in the limbic amygdala involved in the pathophysiology of SAD, and potentially other anxiety and mood disorders. Fasedienol is pharmacologically active without requiring apparent systemic absorption or direct activity on neurons in the brain to achieve its rapid-onset and short-duration anxiolytic effects. We believe fasedienol has the potential to achieve these effects with significantly reduced risks of side effects and other safety concerns, such as potential drug-drug interactions, abuse, misuse, and addiction, associated with certain other systemic pharmaceuticals that act directly on neurons in the brain and are sometimes prescribed for anxiety disorders.
The U.S. Food and Drug Administration (the FDA) has granted Fast Track designation for the development of fasedienol as a potential treatment for SAD.
Fasedienol PALISADE Phase 3 Program in SAD
Given how fasedienol’s rapid-onset mechanism of action (MOA) is differentiated from all FDA-approved anxiety drugs, our primary target indication for fasedienol is the acute treatment of anxiety in adults with SAD. Currently, there is no FDA-approved drug therapy for the acute treatment of SAD. For that acute indication, we have aligned with the FDA that the Subjective Units of Distress Scale (SUDS) is an appropriate primary efficacy endpoint because it provides a measure of anxiety on a minute-by-minute basis immediately related to the specific stressor. We believe utilizing a simulated anxiety-provoking public speaking challenge study design provides the most appropriate and efficient path for the Phase 3 clinical development of fasedienol’s potential to become the first FDA-approved acute treatment of anxiety for adults with SAD. Our PALISADE Phase 3 Program currently includes four randomized, double-blind, placebo-controlled, Phase 3 clinical trials designed to evaluate the efficacy, safety, and tolerability of a single dose of fasedienol to relieve anxiety symptoms in adult patients with SAD during a simulated, anxiety-provoking public speaking challenge in a clinical setting, as measured using the patient-reported SUDS, two of which Phase 3 trials (PALISADE-1 and PALISADE-2) have been concluded and two of which Phase 3 trials (PALISADE-3 and PALISADE-4) will be initiated in 2024, each with open-label extension. Our PALISADE Phase 3 Program also includes an open-label safety study concluded in 2022 (PALISADE OLS), a Phase 2 repeat dose study to be initiated in 2024 (Repeat Dose Study), two standard preclinical studies to be initiated in 2024, and a small human factor study planned to be initiated in 2025.
In early August 2023, we received and reported positive topline results from our PALISADE-2 Phase 3 trial of fasedienol in SAD based on the 141 subjects who completed the trial. Our PALISADE-2 Phase 3 trial met its primary efficacy endpoint, the difference in mean SUDS scores during the public speaking challenge at baseline (Visit 2) and treatment (Visit 3) for subjects treated with fasedienol versus placebo at Visit 3. Fasedienol-treated patients demonstrated a greater mean change from baseline (least-squares (LS) mean = -13.8) compared to placebo (LS mean = -8.0), for a statistically significant, and we believe clinically relevant, difference between groups of -5.8 (p=0.015). The trial also met its secondary endpoint, demonstrating a statistically significant difference in the proportion of clinician-assessed responders between fasedienol and placebo as measured by the Clinical Global Impressions – Improvement (CGI-I) scale. Responders were identified as those who were rated ‘very much less anxious’ or ‘much less anxious’ and 37.7% of fasedienol-treated patients were rated as responders, as compared to 21.4% of those treated with placebo (p=0.033). The trial also met the important exploratory endpoint of the difference in the proportion of patient-assessed responders between fasedienol and placebo as measured by the Patient’s Global Impression of Change (PGI-C). Responders were identified as those who self-rated ‘very much less anxious’ or ‘much less anxious’ and 40.6% of fasedienol-treated patients were rated as responders, as compared to 18.6% of those treated with placebo (p=0.003). In addition, our PALISADE-2 trial also met the exploratory endpoint of the difference in the proportion of patients in each treatment group with a 20-point or greater improvement in patient-assessed SUDS score from baseline (Visit 2) to treatment (Visit 3). Of the fasedienol-treated patients, 35.7% demonstrated this statistically significant and clinically meaningful improvement in SUDS score, as compared to 18.6% in the placebo-treated group (p=0.020). Fasedienol was observed to be well-tolerated with no serious adverse events, and the treatment-emergent adverse event (TEAE) profiles were comparable between fasedienol and placebo. Overall, no TEAE, except for pyrexia in the placebo group (2.49%), was more prevalent than 2.0%.
To complement the positive topline results from PALISADE-2, we are preparing to launch PALISADE-3 in the first half of 2024 and PALISADE-4 in the second half of 2024. Like PALISADE-2, both PALISADE-3 and PALISADE-4 will be multi-center, randomized, double-blind, placebo-controlled studies designed to evaluate the efficacy, safety, and tolerability of the acute administration of fasedienol to relieve anxiety symptoms in adult patients with SAD after a single dose of fasedienol during a simulated, anxiety-provoking public speaking challenge in a clinical setting, as measured using the patient-reported SUDS as the primary efficacy endpoint. In addition, both PALISADE-3 and PALISADE-4 will have an open-label extension for a period of up to 12 months. We believe either PALISADE-3 or PALISADE-4, if successful, together with PALISADE-2, may establish substantial evidence of effectiveness of fasedienol in support of a potential fasedienol NDA submission for the acute treatment of anxiety in adults with SAD with the FDA.
We are also planning to initiate the Repeat Dose Study in the second half of 2024. The Repeat Dose Study will be a multi-center, randomized, double-blind, placebo-controlled, clinical trial designed to evaluate repeated dosing of fasedienol in adult patients with SAD during a single simulated, anxiety-provoking public speaking challenge in a clinical setting. The Repeat Dose Study trial will consist of three different dosing arms, with an open-label extension for a period of up to 12 months.
The U.S. FDA has granted Fast Track designation for the investigation of fasedienol for the acute treatment of SAD.
Itruvone Nasal Spray for Major Depressive Disorder (MDD)
Itruvone (PH10) is an odorless, tasteless synthetic investigational neuroactive pherine nasal spray from the pregnane family with an innovative potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for depression disorders. Itruvone neuroactive nasal spray is administered at microgram-level doses and is designed to engage and activate chemosensory neurons in the nasal cavity, which are connected to neural circuits in the brain that produce antidepressant effects. Unlike all currently approved oral antidepressants (ADs) and rapid-onset intravenous and intranasal ketamine-based therapy, we believe itruvone does not require systemic absorption or direct activity on neurons in the brain to produce antidepressant effects without the side effects and safety concerns that may be associated with current antidepressant therapies.
We are currently preparing and planning for potential U.S. Phase 2B clinical development of itruvone for treatment of MDD. In a randomized, double-blind, placebo-controlled parallel design Phase 2A clinical trial of itruvone in MDD and published in the peer-reviewed British Journal of Pharmaceutical and Medical Research, at a 6.4 μg dose administered intranasally twice daily for eight weeks, PH10 significantly reduced depressive symptoms as early as one week based on the 17-item Hamilton Depression Scale (HAM-D-17) scores compared to placebo (p=0.022). Itruvone was well-tolerated and did not cause psychological side effects (such as dissociation or hallucinations) or other safety concerns that may be associated with other approved pharmacological therapies for MDD. The trial was sponsored by Pherin Pharmaceuticals (Pherin), now a wholly-owned subsidiary of Vistagen, and was conducted in Mexico. Positive data from our recent U.S. IND-enabling Phase 1 trial demonstrated that there were no reported treatment-related serious adverse events (SAEs) or discontinuations due to adverse events in the trial. Overall, itruvone was well-tolerated and continued to demonstrate a favorable safety profile consistent with all other itruvone trials completed to date.
The U.S. FDA has granted Fast Track designation for the investigation of itruvone for the treatment of MDD.
PH80 Nasal Spray for Women’s Health Disorders
PH80 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for vasomotor symptoms (hot flashes) due to menopause, premenstrual dysphoric disorder (PMDD), and other women’s health disorders and migraine headaches. PH80 activates chemosensory neurons in the nasal cavity connected to neural circuits that modulate the basal forebrain associated with the control of body temperature.
PH80 for Moderate to Severe Vasomotor Symptoms (hot flashes) due to Menopause. We recently reported positive results from a previously unpublished exploratory randomized, double-blind, placebo-controlled Phase 2A study of PH80 for the acute treatment of vasomotor symptoms (hot flashes) due to menopause sponsored by Pherin, now our wholly-owned subsidiary. This Phase 2A study conducted in a real-world setting demonstrated a statistically significant reduction in the daily number of menopausal hot flashes compared to placebo at the end of the first week of treatment (p=<0.001), and the improvement was maintained through each treatment week until the end of the four-week treatment period (p=<0.001). PH80 treatment also significantly reduced the severity, disruption in function, and sweating related to hot flashes during the treatment period as compared with placebo. PH80 was well-tolerated with no serious adverse events (SAEs), and the adverse event profiles were comparable between PH80 and placebo. All 36 subjects completed four weeks of treatment and no subject discontinued participation in the study as a result of TEAEs.
PH80 for Premenstrual Dysphoric Disorder (PMDD). We also recently reported positive results from a previously unpublished exploratory randomized, double-blind, placebo-controlled Phase 2A clinical study of PH80 for acute management of the symptoms of PMDD, including negative mood and physical and behavioral symptoms, in subjects with a regular menstrual cycle and at least a one-year history of PMDD. This Phase 2A study was sponsored by Pherin, now our wholly-owned subsidiary, and demonstrated a statistically significant improvement versus placebo in acute management of the symptoms of PMDD, including negative mood and physical and behavioral symptoms. The initial study visit occurred after the onset of symptoms. All subjects were administered a placebo nasal spray, and those who showed no symptom improvement were eligible to return for the second visit, which occurred after the onset of symptoms during the next menstrual cycle. At the second study visit, subjects were randomized to receive a single dose of 0.9 µg PH80 nasal spray or placebo in the clinic. PH80 demonstrated statistically and clinically significant improvement versus placebo in symptoms of PMDD using the subject-rated Penn Daily Symptom Report (DSR) as early as Day 4 and continuing to Day 6 (p=0.008). PH80 also demonstrated statistically and clinically significant improvement versus placebo at Day 6 on the clinician-rated Premenstrual Tension Scale (PMTS) total score (p=0.006). PH80 was well-tolerated with no SAEs. The most common TEAE was headache, reported by 17% in the placebo group and 7% in the PH80 group. No other TEAE occurred more than once per subject.
We are preparing to conduct certain nonclinical studies during the 2024 calendar year necessary to submit our U.S. IND for potential Phase 2B clinical development of PH80 in the U.S. for one or more women’s health indications.
PH15 Nasal Spray for Cognitive and Psychomotor Performance and Improvement
PH15 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments to improve cognitive impairment caused by mental fatigue and potentially other disorders. We believe intranasal PH15 has the potential to improve cognitive and psychomotor performance and improvement of reaction time in individuals with mental fatigue. We are currently evaluating the potential path forward for PH15, including an assessment of completed studies and studies we believe are necessary to submit a U.S. IND for potential further Phase 2 clinical development of PH15 in the U.S., including the appropriate indication for demonstrating improvement of cognitive function.
PH284 Nasal Spray for Cachexia
PH284 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for the loss of appetite associated with chronic disorders such as cancer. Cachexia is a serious but under-recognized consequence of many chronic diseases with body mass loss of >10% and a prevalence of 5 to 15 %. We believe PH284 may have therapeutic potential for improving subjective feelings of hunger in patients with cachexia. We are currently evaluating the potential path forward for PH284, including assessment of completed studies and studies we believe are necessary to submit a U.S. IND for potential further Phase 2 clinical development of PH15 for the treatment of cachexia, including the appropriate patient populations for demonstrating an increase in appetite and weight gain.
AV-101 for Neurological Disorders
AV-101 (4-Cl-KYN) is a novel, oral prodrug that targets the NMDAR (N-methyl-D-aspartate receptor), an ionotropic glutamate receptor in the brain. Based on observations and findings from preclinical studies, we believe AV-101 has the potential to become a new oral treatment alternative for multiple neuroscience disorders, including levodopa-induced dyskinesia and neuropathic pain among others. We are currently assessing a path forward for potential Phase 2A clinical development of AV-101, either on our own or with collaborators, as a treatment for levodopa-induced dyskinesia associated with Parkinson’s disease therapy and possibly one or more additional neurological disorders involving the NMDAR receptor.
The U.S. FDA has granted Fast Track designation for the investigation of AV-101 for the treatment of neuropathic pain and for the adjunctive treatment of MDD.
Corporate Information
Vistagen Therapeutics, Inc., a Nevada corporation, is the parent of Pherin Pharmaceuticals, Inc., a Delaware corporation, and Vistastem, Inc., a wholly owned California corporation. Our principal executive offices are located at 343 Allerton Avenue, South San Francisco, California 94080, and our telephone number is (650) 577-3600. Our website address is www.vistagen.com.
USE OF PROCEEDS
We intend to use the net proceeds from the sale of any securities offered under this prospectus for general corporate purposes unless otherwise indicated in the applicable prospectus supplement. General corporate purposes may include expenditures for research and development expenditures, nonclinical and clinical trials, contract manufacturing, pre-commercial and commercial activities, information technology, intellectual property, working capital, the acquisition of companies or businesses and the repayment and refinancing of debt. We have not determined the amount of net proceeds to be used specifically for such purposes. As a result, management will retain broad discretion over the allocation of net proceeds.
SECURITIES THAT MAY BE OFFERED
We may offer under this prospectus shares of our common stock and preferred stock, various series of debt securities, warrants to purchase any of such securities and units comprised of one or more of the other securities that may be offered under this prospectus, either individually or in combination with other securities, with a total value of up to $350,000,000 from time to time at prices and on terms to be determined at the time of any offering. This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities, including, to the extent applicable:
| |
•
|
designation or classification;
|
| |
|
|
| |
•
|
aggregate principal amount or aggregate offering price;
|
| |
|
|
| |
•
|
maturity;
|
| |
|
|
| |
•
|
original issue discount;
|
| |
|
|
| |
•
|
rates and times of payment of interest or dividends;
|
| |
|
|
| |
•
|
redemption, conversion, exercise, exchange or sinking fund terms;
|
| |
|
|
| |
•
|
ranking;
|
| |
|
|
| |
•
|
restrictive covenants;
|
| |
|
|
| |
•
|
voting or other rights;
|
| |
|
|
| |
•
|
conversion or exchange prices or rates and, if applicable, any provisions for changes to or adjustments in the conversion or exchange prices or rates and in the securities or other property receivable upon conversion or exchange; and
|
| |
|
|
| |
•
|
a discussion of material U.S. federal income tax considerations, if any.
|
This prospectus may not be used to offer or sell securities unless accompanied by an applicable prospectus supplement. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change information contained in this prospectus or in documents we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer a security that is not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus is a part.
The following descriptions are not complete and may not contain all the information you should consider before investing in any securities we may offer hereunder; they are summarized from, and qualified by reference to, our restated certificate of incorporation, amended and restated by-laws, as amended, and the other documents referred to in the descriptions, all of which are or will be publicly filed with the SEC, as applicable. See “Where You Can Find More Information.”
DESCRIPTION OF DEBT SECURITIES
This section describes the general terms and provisions of debt securities that we may issue from time to time. We may issue debt securities, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized below will apply generally to any future debt securities we may offer under this prospectus, the applicable prospectus supplement will describe the specific terms of any debt securities offered through that prospectus supplement. The terms of any debt securities we offer under a prospectus supplement may differ from the terms we describe below.
We may offer debt securities which may be senior or subordinated. We refer to the senior debt securities and the subordinated debt securities collectively as debt securities. The following description summarizes the general terms and provisions of the debt securities. We will describe the specific terms of the debt securities and the extent, if any, to which the general provisions summarized below apply to any series of debt securities in the prospectus supplement relating to the series and any applicable free writing prospectus that we authorize to be delivered.
We may issue senior debt securities from time to time, in one or more series under a senior indenture to be entered into between us and a senior trustee to be named in a prospectus supplement, which we refer to as the senior trustee. We may issue subordinated debt securities from time to time, in one or more series under a subordinated indenture to be entered into between us and a subordinated trustee to be named in a prospectus supplement, which we refer to as the subordinated trustee. The forms of senior indenture and subordinated indenture are filed as exhibits to the registration statement of which this prospectus forms a part. Together, the senior indenture and the subordinated indenture are referred to as the indentures and, together, the senior trustee and the subordinated trustee are referred to as the trustees. This prospectus briefly outlines some of the provisions of the indentures. The following summary of the material provisions of the indentures is qualified in its entirety by the provisions of the indentures, including definitions of certain terms used in the indentures. Wherever we refer to particular sections or defined terms of the indentures, those sections or defined terms are incorporated by reference in this prospectus or the applicable prospectus supplement. You should review the indentures that are filed as exhibits to the registration statement of which this prospectus forms a part for additional information.
None of the indentures will limit the amount of debt securities that we may issue. The applicable indenture will provide that debt securities may be issued up to an aggregate principal amount authorized from time to time by us and may be payable in any currency or currency unit designated by us or in amounts determined by reference to an index.
General
The senior debt securities will constitute our unsecured and unsubordinated general obligations and will rank pari passu with our other unsecured and unsubordinated obligations, if any. The subordinated debt securities will constitute our unsecured and subordinated general obligations and will be junior in right of payment to our senior indebtedness (including senior debt securities), as described under the heading “– Certain Terms of the Subordinated Debt Securities – Subordination.” The debt securities will be structurally subordinated to all existing and future indebtedness and other liabilities of our subsidiaries unless such subsidiaries expressly guarantee such debt securities.
The debt securities will be our unsecured obligations. Any secured debt or other secured obligations will be effectively senior to the debt securities to the extent of the value of the assets securing such debt or other obligations.
The applicable prospectus supplement and/or free writing prospectus will include any additional or different terms of the debt securities of any series being offered, including the following terms:
| |
•
|
the title and type of the debt securities;
|
| |
•
|
whether the debt securities will be senior or subordinated debt securities, and, with respect to debt securities issued under the subordinated indenture the terms on which they are subordinated;
|
| |
•
|
the aggregate principal amount of the debt securities;
|
| |
•
|
the price or prices at which we will sell the debt securities;
|
| |
•
|
the maturity date or dates of the debt securities and the right, if any, to extend such date or dates;
|
| |
•
|
the rate or rates, if any, per year, at which the debt securities will bear interest, or the method of determining such rate or rates;
|
| |
•
|
the date or dates from which such interest will accrue, the interest payment dates on which such interest will be payable or the manner of determination of such interest payment dates and the related record dates;
|
| |
•
|
the right, if any, to extend the interest payment periods and the duration of that extension;
|
| |
•
|
the manner of paying principal and interest and the place or places where principal and interest will be payable;
|
| |
•
|
provisions for a sinking fund, purchase fund or other analogous fund, if any;
|
| |
•
|
any redemption dates, prices, obligations and restrictions on the debt securities;
|
| |
•
|
the currency, currencies or currency units in which the debt securities will be denominated and the currency, currencies or currency units in which principal and interest, if any, on the debt securities may be payable;
|
| |
•
|
any conversion or exchange features of the debt securities;
|
| |
•
|
whether and upon what terms the debt securities may be defeased;
|
| |
•
|
any events of default or covenants in addition to or in lieu of those set forth in the indenture;
|
| |
•
|
whether the debt securities will be issued in definitive or global form or in definitive form only upon satisfaction of certain conditions;
|
| |
•
|
whether the debt securities will be guaranteed as to payment or performance;
|
| |
•
|
any special tax implications of the debt securities; and
|
| |
•
|
any other material terms of the debt securities.
|
When we refer to “principal” in this section with reference to the debt securities, we are also referring to “premium, if any.”
We may from time to time, without notice to or the consent of the holders of any series of debt securities, create and issue further debt securities of any such series ranking equally with the debt securities of such series in all respects (or in all respects other than (1) the payment of interest accruing prior to the issue date of such further debt securities or (2) the first payment of interest following the issue date of such further debt securities). Such further debt securities may be consolidated and form a single series with the debt securities of such series and have the same terms as to status, redemption or otherwise as the debt securities of such series.
You may present debt securities for exchange and you may present debt securities for transfer in the manner, at the places and subject to the restrictions set forth in the debt securities and the applicable prospectus supplement. We will provide you those services without charge, although you may have to pay any tax or other governmental charge payable in connection with any exchange or transfer, as set forth in the indenture.
Debt securities may bear interest at a fixed rate or a floating rate. Debt securities bearing no interest or interest at a rate that at the time of issuance is below the prevailing market rate (original issue discount securities) may be sold at a discount below their stated principal amount. U.S. federal income tax considerations applicable to any such discounted debt securities or to certain debt securities issued at par which are treated as having been issued at a discount for U.S. federal income tax purposes will be described in the applicable prospectus supplement.
We may issue debt securities with the principal amount payable on any principal payment date, or the amount of interest payable on any interest payment date, to be determined by reference to one or more currency exchange rates, securities or baskets of securities, commodity prices or indices. You may receive a payment of principal on any principal payment date, or a payment of interest on any interest payment date, that is greater than or less than the amount of principal or interest otherwise payable on such dates, depending on the value on such dates of the applicable currency, security or basket of securities, commodity or index. Information as to the methods for determining the amount of principal or interest payable on any date, the currencies, securities or baskets of securities, commodities or indices to which the amount payable on such date is linked and certain related tax considerations will be set forth in the applicable prospectus supplement.
Certain Terms of the Senior Debt Securities
Covenants. Unless we indicate otherwise in a prospectus supplement, the senior debt securities will not contain any financial or restrictive covenants, including covenants restricting either us or our subsidiaries from incurring, issuing, assuming or guaranteeing any indebtedness secured by a lien on any of our or our subsidiaries’ property or capital stock, or restricting either us or our subsidiaries from entering into sale and leaseback transactions.
Consolidation, Merger and Sale of Assets. Unless we indicate otherwise in a prospectus supplement, we may not consolidate with or merge into any other person, in a transaction in which we are not the surviving corporation, or convey, transfer or lease our properties and assets substantially as an entirety to any person, in either case, unless:
| |
•
|
the successor entity, if any, is a U.S. corporation, limited liability company, partnership or trust (subject to certain exceptions provided for in the senior indenture);
|
| |
•
|
the successor entity assumes our obligations on the senior debt securities and under the senior indenture;
|
| |
•
|
immediately after giving effect to the transaction, no default or event of default shall have occurred and be continuing; and
|
| |
•
|
certain other conditions are met.
|
No Protection in the Event of a Change in Control. Unless we indicate otherwise in a prospectus supplement with respect to a particular series of senior debt securities, the senior debt securities will not contain any provisions that may afford holders of the senior debt securities protection in the event we have a change in control or in the event of a highly leveraged transaction (whether or not such transaction results in a change in control).
Events of Default. Unless we indicate otherwise in a prospectus supplement with respect to a particular series of senior debt securities, the following are events of default under the senior indenture for any series of senior debt securities:
| |
•
|
failure to pay interest on any senior debt securities of such series when due and payable, if that default continues for a period of 30 days (or such other period as may be specified for such series);
|
| |
•
|
failure to pay principal on the senior debt securities of such series when due and payable whether at maturity, upon redemption, by declaration or otherwise (and, if specified for such series, the continuance of such failure for a specified period);
|
| |
•
|
default in the performance of or breach of any of our covenants or agreements in the senior indenture applicable to senior debt securities of such series, other than a covenant breach which is specifically dealt with elsewhere in the senior indenture, and that default or breach continues for a period of 90 days after we receive written notice from the trustee or from the holders of 25% or more in aggregate principal amount of the senior debt securities of such series;
|
| |
•
|
certain events of bankruptcy or insolvency, whether or not voluntary; and
|
| |
•
|
any other event of default provided for in such series of senior debt securities as may be specified in the applicable prospectus supplement.
|
The default by us under any other debt, including any other series of debt securities, is not a default under the senior indenture.
If an event of default other than an event of default specified in the fourth bullet point above occurs with respect to a series of senior debt securities and is continuing under the senior indenture, then, and in each such case, either the trustee or the holders of not less than 25% in aggregate principal amount of such series then outstanding under the senior indenture (each such series voting as a separate class) by written notice to us and to the trustee, if such notice is given by the holders, may, and the trustee at the request of such holders shall, declare the principal amount of and accrued interest on such series of senior debt securities to be immediately due and payable, and upon this declaration, the same shall become immediately due and payable.
If an event of default specified in the fourth bullet point above occurs and is continuing, the entire principal amount of and accrued interest on each series of senior debt securities then outstanding shall become immediately due and payable.
Unless otherwise specified in the prospectus supplement relating to a series of senior debt securities originally issued at a discount, the amount due upon acceleration shall include only the original issue price of the senior debt securities, the amount of original issue discount accrued to the date of acceleration and accrued interest, if any.
Upon certain conditions, declarations of acceleration may be rescinded and annulled and past defaults may be waived by the holders of a majority in aggregate principal amount of all the senior debt securities of such series affected by the default, each series voting as a separate class. Furthermore, subject to various provisions in the senior indenture, the holders of a majority in aggregate principal amount of a series of senior debt securities, by notice to the trustee, may waive an existing default or event of default with respect to such senior debt securities and its consequences, except a default in the payment of principal of or interest on such senior debt securities or in respect of a covenant or provision of the senior indenture which cannot be modified or amended without the consent of the holders of each such senior debt security. Upon any such waiver, such default shall cease to exist, and any event of default with respect to such senior debt securities shall be deemed to have been cured, for every purpose of the senior indenture; but no such waiver shall extend to any subsequent or other default or event of default or impair any right consequent thereto.
The holders of a majority in aggregate principal amount of a series of senior debt securities may direct the time, method and place of conducting any proceeding for any remedy available to the trustee or exercising any trust or power conferred on the trustee with respect to such senior debt securities. However, the trustee may refuse to follow any direction that conflicts with law or the senior indenture, that may involve the trustee in personal liability or that the trustee determines in good faith may be unduly prejudicial to the rights of holders of such series of senior debt securities not joining in the giving of such direction and may take any other action it deems proper that is not inconsistent with any such direction received from holders of such series of senior debt securities. A holder may not pursue any remedy with respect to the senior indenture or any series of senior debt securities unless:
| |
•
|
the holder gives the trustee written notice of a continuing event of default;
|
| |
•
|
the holders of at least 25% in aggregate principal amount of such series of senior debt securities make a written request to the trustee to pursue the remedy in respect of such event of default;
|
| |
•
|
the requesting holder or holders offer the trustee indemnity satisfactory to the trustee against any costs, liability or expense;
|
| |
•
|
the trustee does not comply with the request within 60 days after receipt of the request and the offer of indemnity; and
|
| |
•
|
during such 60-day period, the holders of a majority in aggregate principal amount of such series of senior debt securities do not give the trustee a direction that is inconsistent with the request.
|
These limitations, however, do not apply to the right of any holder of a senior debt security to receive payment of the principal of and interest on such senior debt security in accordance with the terms of such debt security, or to bring suit for the enforcement of any such payment in accordance with the terms of such debt security, on or after the due date for the senior debt securities, which right shall not be impaired or affected without the consent of the holder.
The senior indenture requires certain of our officers to certify, on or before a fixed date in each year in which any senior debt security is outstanding, as to their knowledge of our compliance with all covenants, agreements and conditions under the senior indenture.
Satisfaction and Discharge. We can satisfy and discharge our obligations to holders of any series of debt securities if:
| |
•
|
we pay or cause to be paid, as and when due and payable, the principal of and any interest on all senior debt securities of such series outstanding under the senior indenture; or
|
| |
•
|
all senior debt securities of such series have become due and payable or will become due and payable within one year (or are to be called for redemption within one year) and we deposit in trust a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the debt securities of that series on their various due dates.
|
Under current U.S. federal income tax law, the deposit and our legal release from the debt securities would be treated as though we took back your debt securities and gave you your share of the cash and debt securities or bonds deposited in trust. In that event, you could recognize a gain or loss on the debt securities you give back to us. Purchasers of the debt securities should consult their own advisers with respect to the tax consequences to them of such deposit and discharge, including the applicability and effect of tax laws other than the U.S. federal income tax law.
Defeasance. Unless the applicable prospectus supplement provides otherwise, the following discussion of legal defeasance and discharge and covenant defeasance will apply to any series of debt securities issued under the indentures.
Legal Defeasance. We can legally release ourselves from any payment or other obligations on the debt securities of any series (called “legal defeasance”) if certain conditions are met, including the following:
| |
•
|
We deposit in trust for your benefit and the benefit of all other direct holders of the debt securities of the same series a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the debt securities of that series on their various due dates.
|
| |
•
|
There is a change in current U.S. federal income tax law or an Internal Revenue Service (“IRS”) ruling that lets us make the above deposit without causing you to be taxed on the debt securities any differently than if we did not make the deposit and instead repaid the debt securities ourselves when due. Under current U.S. federal income tax law, the deposit and our legal release from the debt securities would be treated as though we took back your debt securities and gave you your share of the cash and debt securities or bonds deposited in trust. In that event, you could recognize gain or loss on the debt securities you give back to us.
|
| |
•
|
We deliver to the trustee a legal opinion of our counsel confirming the tax law change or ruling described above.
|
If we accomplish legal defeasance, as described above, you would have to rely solely on the trust deposit for repayment of the debt securities. You could not look to us for repayment in the event of any shortfall.
Covenant Defeasance. Without any change of current U.S. federal tax law, we can make the same type of deposit described above and be released from some of the covenants in the debt securities (called “covenant defeasance”). In that event, you would lose the protection of those covenants but would gain the protection of having money and securities set aside in trust to repay the debt securities. In order to achieve covenant defeasance, we must do the following (among other things):
| |
•
|
We must deposit in trust for your benefit and the benefit of all other direct holders of the debt securities of the same series a combination of cash and U.S. government or U.S. government agency obligations that will generate enough cash to make interest, principal and any other payments on the debt securities of that series on their various due dates.
|
| |
•
|
We must deliver to the trustee a legal opinion of our counsel confirming that under current U.S. federal income tax law we may make the above deposit without causing you to be taxed on the debt securities any differently than if we did not make the deposit and instead repaid the debt securities ourselves when due.
|
If we accomplish covenant defeasance, you could still look to us for repayment of the debt securities if there were a shortfall in the trust deposit. In fact, if one of the events of default occurred (such as our bankruptcy) and the debt securities become immediately due and payable, there may be such a shortfall. Depending on the events causing the default, you may not be able to obtain payment of the shortfall.
Modification and Waiver. We and the trustee may amend or supplement the senior indenture or the senior debt securities without the consent of any holder:
| |
•
|
to convey, transfer, assign, mortgage or pledge any assets as security for the senior debt securities of one or more series;
|
| |
•
|
to evidence the succession of a corporation, limited liability company, partnership or trust to us, and the assumption by such successor of our covenants, agreements and obligations under the senior indenture or to otherwise comply with the covenant relating to mergers, consolidations and sales of assets;
|
| |
•
|
to comply with requirements of the SEC in order to effect or maintain the qualification of the senior indenture under the Trust Indenture Act of 1939, as amended;
|
| |
•
|
to add to our covenants such new covenants, restrictions, conditions or provisions for the protection of the holders, and to make the occurrence, or the occurrence and continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default;
|
| |
•
|
to cure any ambiguity, defect or inconsistency in the senior indenture or in any supplemental indenture or to conform the senior indenture or the senior debt securities to the description of senior debt securities of such series set forth in this prospectus or any applicable prospectus supplement;
|
| |
•
|
to provide for or add guarantors with respect to the senior debt securities of any series;
|
| |
•
|
to establish the form or forms or terms of the senior debt securities as permitted by the senior indenture;
|
| |
•
|
to evidence and provide for the acceptance of appointment under the senior indenture by a successor trustee, or to make such changes as shall be necessary to provide for or facilitate the administration of the trusts in the senior indenture by more than one trustee;
|
| |
•
|
to add to, delete from or revise the conditions, limitations and restrictions on the authorized amount, terms, purposes of issue, authentication and delivery of any series of senior debt securities;
|
| |
•
|
to make any change to the senior debt securities of any series so long as no senior debt securities of such series are outstanding; or
|
| |
•
|
to make any change that does not adversely affect the rights of any holder in any material respect.
|
Other amendments and modifications of the senior indenture or the senior debt securities issued may be made, and our compliance with any provision of the senior indenture with respect to any series of senior debt securities may be waived, with the consent of the holders of a majority of the aggregate principal amount of the outstanding senior debt securities of all series affected by the amendment or modification (voting together as a single class); provided, however, that each affected holder must consent to any modification, amendment or waiver that:
| |
•
|
extends the final maturity of any senior debt securities of such series;
|
| |
•
|
reduces the principal amount of any senior debt securities of such series;
|
| |
•
|
reduces the rate or extends the time of payment of interest on any senior debt securities of such series;
|
| |
•
|
reduces the amount payable upon the redemption of any senior debt securities of such series;
|
| |
•
|
changes the currency of payment of principal of or interest on any senior debt securities of such series;
|
| |
•
|
reduces the principal amount of original issue discount securities payable upon acceleration of maturity or the amount provable in bankruptcy;
|
| |
•
|
waives an uncured default in the payment of principal of or interest on the senior debt securities (except in the case of a rescission of acceleration as described above);
|
| |
•
|
changes the provisions relating to the waiver of past defaults or changes or impairs the right of holders to receive payment or to institute suit for the enforcement of any payment or conversion of any senior debt securities of such series on or after the due date therefor;
|
| |
•
|
modifies any of the provisions of these restrictions on amendments and modifications, except to increase any required percentage or to provide that certain other provisions cannot be modified or waived without the consent of the holder of each senior debt security of such series affected by the modification; or
|
| |
•
|
reduces the above-stated percentage of outstanding senior debt securities of such series whose holders must consent to a supplemental indenture or modifies or amends or waives certain provisions of or defaults under the senior indenture.
|
It shall not be necessary for the holders to approve the particular form of any proposed amendment, supplement or waiver, but it shall be sufficient if the holders’ consent approves the substance thereof. After an amendment, supplement or waiver of the senior indenture in accordance with the provisions described in this section becomes effective, the trustee must give to the holders affected thereby certain notice briefly describing the amendment, supplement or waiver. Any failure by the trustee to give such notice, or any defect therein, shall not, however, in any way impair or affect the validity of any such amendment, supplemental indenture or waiver.
No Personal Liability of Incorporators, Stockholders, Officers, Directors. The senior indenture provides that no recourse shall be had under any obligation, covenant or agreement of ours in the senior indenture or any supplemental indenture, or in any of the senior debt securities or because of the creation of any indebtedness represented thereby, against any of our incorporators, stockholders, officers or directors, past, present or future, or of any predecessor or successor entity thereof under any law, statute or constitutional provision or by the enforcement of any assessment or by any legal or equitable proceeding or otherwise. Each holder, by accepting the senior debt securities, waives and releases all such liability.
Concerning the Trustee. The senior indenture provides that, except during the continuance of an event of default, the trustee will not be liable except for the performance of such duties as are specifically set forth in the senior indenture. If an event of default has occurred and is continuing, the trustee will exercise such rights and powers vested in it under the senior indenture and will use the same degree of care and skill in its exercise as a prudent person would exercise under the circumstances in the conduct of such person’s own affairs.
The senior indenture and the provisions of the Trust Indenture Act of 1939 incorporated by reference therein contain limitations on the rights of the trustee thereunder, should it become a creditor of ours or our subsidiaries, to obtain payment of claims in certain cases or to realize on certain property received by it in respect of any such claims, as security or otherwise. The trustee is permitted to engage in other transactions, provided that if it acquires any conflicting interest (as defined in the Trust Indenture Act), it must eliminate such conflict or resign.
We may have normal banking relationships with the senior trustee in the ordinary course of business.
Unclaimed Funds. All funds deposited with the trustee or any paying agent for the payment of principal, premium, interest or additional amounts in respect of the senior debt securities that remain unclaimed for two years after the date upon which such principal, premium or interest became due and payable will be repaid to us. Thereafter, any right of any holder of senior debt securities to such funds shall be enforceable only against us, and the trustee and paying agents will have no liability therefor.
Governing Law. The senior indenture and the senior debt securities will be governed by, and construed in accordance with, the internal laws of the State of New York.
Certain Terms of the Subordinated Debt Securities
Other than the terms of the subordinated indenture and subordinated debt securities relating to subordination or otherwise as described in the prospectus supplement relating to a particular series of subordinated debt securities, the terms of the subordinated indenture and subordinated debt securities are identical in all material respects to the terms of the senior indenture and senior debt securities.
Additional or different subordination terms may be specified in the prospectus supplement applicable to a particular series.
Subordination. The indebtedness evidenced by the subordinated debt securities is subordinate to the prior payment in full of all of our senior indebtedness, as defined in the subordinated indenture. During the continuance beyond any applicable grace period of any default in the payment of principal, premium, interest or any other payment due on any of our senior indebtedness, we may not make any payment of principal of or interest on the subordinated debt securities (except for certain sinking fund payments). In addition, upon any payment or distribution of our assets upon any dissolution, winding-up, liquidation or reorganization, the payment of the principal of and interest on the subordinated debt securities will be subordinated to the extent provided in the subordinated indenture in right of payment to the prior payment in full of all our senior indebtedness. Because of this subordination, if we dissolve or otherwise liquidate, holders of our subordinated debt securities may receive less, ratably, than holders of our senior indebtedness. The subordination provisions do not prevent the occurrence of an event of default under the subordinated indenture.
The term “senior indebtedness” of a person means with respect to such person the principal of, premium, if any, interest on, and any other payment due pursuant to any of the following, whether outstanding on the date of the subordinated indenture or incurred by that person in the future:
| |
•
|
all of the indebtedness of that person for money borrowed;
|
| |
•
|
all of the indebtedness of that person evidenced by notes, debentures, bonds or other securities sold by that person for money;
|
| |
•
|
all of the lease obligations that are capitalized on the books of that person in accordance with generally accepted accounting principles;
|
| |
•
|
all indebtedness of others of the kinds described in the first two bullet points above and all lease obligations of others of the kind described in the third bullet point above that the person, in any manner, assumes or guarantees or that the person in effect guarantees through an agreement to purchase, whether that agreement is contingent or otherwise; and
|
| |
•
|
all renewals, extensions or refundings of indebtedness of the kinds described in the first, second or fourth bullet point above and all renewals or extensions of leases of the kinds described in the third or fourth bullet point above;
|
unless, in the case of any particular indebtedness, renewal, extension or refunding, the instrument creating or evidencing it or the assumption or guarantee relating to it expressly provides that such indebtedness, renewal, extension or refunding is not superior in right of payment to the subordinated debt securities. Our senior debt securities constitute senior indebtedness for purposes of the subordinated debt indenture.
DESCRIPTION OF CAPITAL STOCK
General
The following description of our capital stock is intended as a summary only and therefore is not a complete description of our capital stock. This description is based upon, and is qualified by reference to, our Restated and Amended Articles of Incorporation, as amended (our “Charter”), our Amended and Restated Bylaws, as amended (our “Bylaws”), and applicable provisions of Nevada corporate law. You should read our Charter and Bylaws for the provisions that are important to you.
Our authorized capital stock consists of 325,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, $0.001 par value per share, all of which preferred stock is undesignated. The following description of our capital stock and provisions of our Charter and Bylaws are summaries and are qualified by reference to our Charter and Bylaws. Copies of these documents are filed with the SEC as exhibits to our Annual Report on Form 10-K for the year ended March 31, 2023, filed with the SEC on June 28, 2023.
The following is a description of our common stock and certain provisions of our Charter, and our amended and restated bylaws, and certain provisions of Nevada law.
We may elect or be required to amend our Charter to increase the number of shares of common stock authorized for issuance prior to completing sales of shares of our common stock, or securities convertible and/or exchangeable into shares of our common stock described in this prospectus and/or any accompanying prospectus supplement.
Common Stock
This section describes the general terms of our common stock that we may offer from time to time. For more detailed information, a holder of our common stock should refer to our Charter and our Bylaws.
Except as otherwise expressly provided in our Charter, or as required by applicable law, all shares of our common stock have the same rights and privileges and rank equally, share ratably and are identical in all respects as to all matters, including, without limitation, those described below. All outstanding shares of common stock are fully paid and nonassessable.
Voting Rights Each holder of our common stock is entitled to cast one vote for each share of common stock held on all matters submitted to a vote of stockholders. Cumulative voting for election of directors is not allowed under our Charter, which means that a plurality of the shares voted can elect all of the directors then outstanding for election. Except as otherwise provided under Nevada law or our Charter and Bylaws, on matters other than election of directors, action on a matter is approved if the votes cast favoring the action exceed the votes cast opposing the action.
Dividend Rights The holders of outstanding shares of our common stock are entitled to receive dividends out of funds legally available, if our Board of Directors (our “Board”), in its discretion, determines to issue a dividend, and only at the times and in the amounts that our Board may determine. Our Board is not obligated to declare a dividend. We have not paid any dividends on our common stock in the past and we do not intend to pay dividends in the foreseeable future.
Liquidation Rights Upon our liquidation, dissolution or winding-up, the holders of our common stock will be entitled to share equally, identically and ratably in all assets remaining, subject to the prior satisfaction of all outstanding debt and liabilities and the preferential rights and payment of liquidation preferences, if any, on any outstanding shares of preferred stock.
No Preemptive or Similar Rights Our common stock is not subject to conversion, redemption, sinking fund or similar provisions.
Listing on The Nasdaq Capital Market Our common stock is listed on The Nasdaq Capital Market under the symbol “VTGN.”
Authorized but Unissued Shares The authorized but unissued shares of common stock are available for future issuance without stockholder approval, subject to any limitations imposed by applicable listing rules of The Nasdaq Stock Market. These additional shares may be used for a variety of corporate finance transactions, acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock could make it more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Transfer Agent and Registrar The transfer agent and registrar for our common stock is Computershare Trust Company, N.A.
Securities Offered Under Prior Registration Statements
On October 2, 2023, we entered into an underwriting agreement (the “Underwriting Agreement”) with Jefferies LLC, Stifel, Nicolaus & Company, Incorporated, and William Blair & Company, L.L.C., as the representatives of the underwriters listed on Schedule A attached thereto (the “Underwriters”), in connection with the underwritten offering, issuance and sale by us of 15,010,810 shares common stock, pre-funded warrants to purchase up to 3,577,240 shares of common stock (the “Pre-Funded Warrants”), warrants to purchase up to 9,294,022 shares of common stock (or pre-funded warrants to purchase up to 9,294,022 shares of common stock in lieu thereof) (the “Tranche 1 Warrants”) and warrants to purchase 11,265,086 shares of common stock (or pre-funded warrants to purchase up to 11,265,086 shares of common stock in lieu thereof) (the “Tranche 2 Warrants”).
We are also offering shares of common stock issuable from time to time upon exercise of our pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants. The following is a summary of certain terms and conditions of the pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants, which summary is subject in all respect to the provisions contained in the pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants. Forms of each of the pre-funded warrants, Tranche 1 Warrants and Tranche 2 Warrants are filed with the SEC as exhibits to our Current Report on Form 8-K, dated October 4, 2023 and incorporated by reference herein.
Pre-Funded Warrants
Exercisability. The pre-funded warrants are exercisable at any time after their original issuance. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and by payment in full in immediately available funds for the number of shares of common stock purchased upon such exercise. As an alternative to payment in immediately available funds, the holder may, in its sole discretion, elect to exercise the pre-funded warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the pre-funded warrant. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitations. A holder will not have the right to exercise any portion of the pre-funded warrant if the holder (together with its affiliates) would beneficially own in excess of 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. However, any holder may elect, prior to issuance of its pre-funded warrant, to include a provision in such holder’s pre-funded warrant that will permit such holder to elect to increase or decrease such percentage to any other percentage upon at least 61 days’ prior notice from the holder to us.
Exercise Price. The exercise price per whole share of our common stock purchasable upon the exercise of the pre-funded warrants is $0.001 per share of common stock. The exercise price of the pre-funded warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws, the pre-funded warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. The pre-funded warrants are not listed, and we do not intend to list the pre-funded warrants on The Nasdaq Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except by virtue of such holder’s ownership of shares of our common stock, the holder of a pre-funded warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the pre-funded warrant.
Tranche 1 Warrants
Exercisability. The Tranche 1 Warrants are exercisable at any time after their original issuance and will expire 60 days after the later of (i) the date on which we first publicly disclose, whether by press release or Form 8-K filing, the top-line data for our PALISADE-3 study and (ii) the date on which we first publicly disclose, whether by press release or Form 8-K filing, the top-line data for our PALISADE-4 study. The Tranche 1 Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and by payment in full in immediately available funds for the number of shares of common stock or pre-funded warrants purchased upon such exercise. As an alternative to payment in immediately available funds, the holder may if and only if at the time of exercise hereof there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of the common stock or pre-funded warrants to the holder, then in its sole discretion, elect to exercise the Tranche 2 Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock or pre-funded warrants determined according to the formula set forth in the Tranche 1 Warrant. No fractional shares of common stock or pre-funded warrants will be issued in connection with the exercise of a Tranche 1 Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitations. A holder will not have the right to exercise any portion of the Tranche 1 Warrant if the holder (together with its affiliates) would beneficially own in excess of 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Tranche 1 Warrant. However, any holder may elect, prior to issuance of its Tranche 1 Warrant, to include a provision in such holder’s Tranche 1 Warrant that will permit such holder to elect to increase or decrease such percentage to any other percentage upon at least 61 days’ prior notice from the holder to us.
Exercise Price. The exercise price per whole share of our common stock or pre-funded warrants purchasable upon the exercise of the Tranche 1 Warrants is $5.38 per share of common stock or pre-funded warrant. The exercise price of the Tranche 1 Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws, the Tranche 1 Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. The Tranche 1 Warrants are not listed, and we do not intend to list the Tranche 1 Warrants on The Nasdaq Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Tranche 1 Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Tranche 1 Warrants will be entitled to receive upon exercise of the Tranche 1 Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Tranche 1 Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except by virtue of such holder’s ownership of shares of our common stock, the holder of a Tranche 1 Warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Tranche 1 Warrant.
Tranche 2 Warrants
Form. The Tranche 2 Warrants will be issued as individual warrant agreements to the investors. You should review the form of Tranche 2 Warrants, which will be filed as an exhibit to a Current Report on Form 8-K, for a complete description of the terms and conditions applicable to the Tranche 2 Warrants.
Exercisability. The Tranche 2 Warrants are exercisable at any time after their original issuance and will expire on the five year anniversary of the original issuance. The Tranche 2 Warrants will be exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice and by payment in full in immediately available funds for the number of shares of common stock or pre-funded warrants purchased upon such exercise. As an alternative to payment in immediately available funds, the holder may, in its sole discretion, elect to exercise the Tranche 2 Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of shares of common stock or pre-funded warrants determined according to the formula set forth in the Tranche 2 Warrant. No fractional shares of common stock or pre-funded warrants will be issued in connection with the exercise of a Tranche 2 Warrant. In lieu of fractional shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price.
Exercise Limitations. A holder will not have the right to exercise any portion of the Tranche 2 Warrant if the holder (together with its affiliates) would beneficially own in excess of 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Tranche 2 Warrant. However, any holder may elect, prior to issuance of its Tranche 2 Warrant, to include a provision in such holder’s Tranche 2 Warrant that will permit such holder to elect to increase or decrease such percentage to any other percentage upon at least 61 days’ prior notice from the holder to us.
Exercise Price. The exercise price per whole share of our common stock or pre-funded warrant purchasable upon the exercise of the Tranche 2 Warrants is $8.877 per share of common stock or pre-funded warrant. The exercise price of the Tranche 2 Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Transferability. Subject to applicable laws, the Tranche 2 Warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. The Tranche 2 Warrants are not listed, and we do not intend to list the Tranche 2 Warrants on The Nasdaq Capital Market, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions. In the event of a fundamental transaction, as described in the Tranche 2 Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Tranche 2 Warrants will be entitled to receive upon exercise of the Tranche 2 Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Tranche 2 Warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. Except by virtue of such holder’s ownership of shares of our common stock, the holder of a Tranche 2 Warrant does not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the Tranche 2 Warrant.
DESCRIPTION OF PREFERRED STOCK
This section describes the general terms and provisions of our authorized preferred stock, as well as preferred stock that we may offer from time to time. The applicable prospectus supplement will describe the specific terms of the shares of preferred stock offered through that prospectus supplement, which may differ from the terms we describe below. We will file a copy of the certificate of designation that contains the terms of each new series of preferred stock with the SEC each time we issue a new series of preferred stock, and these certificates of designation will be incorporated by reference into the registration statement of which this prospectus is a part. Each certificate of designation will establish the number of shares included in a designated series and fix the designation, powers, privileges, preferences and rights of the shares of each series as well as any applicable qualifications, limitations or restrictions. A holder of our preferred stock should refer to the applicable certificate of designation, our Charter, and the applicable prospectus supplement (and any related free writing prospectus that we may authorize to be provided to you) for more specific information.
We are authorized, subject to limitations prescribed by Nevada law, to issue up to 10.0 million shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers, preferences and rights of the shares of each series and any of its qualifications, limitations or restrictions.
Our Board may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of the Company and may adversely affect the market price of our common stock and the voting and other rights of the holders of our common stock.
Shares of Preferred Stock Issuable Pursuant to this Prospectus
Any preferred stock offered pursuant to this prospectus will have the terms described below unless otherwise provided in the prospectus supplement relating to a particular series of preferred stock. You should read the prospectus supplement relating to the particular series of preferred stock being offered for specific terms, including:
| |
•
|
the designation and stated value per share of the preferred stock and the number of shares offered;
|
| |
•
|
the amount of liquidation preference per share;
|
| |
•
|
the price at which the preferred stock will be issued;
|
| |
•
|
the dividend rate, or method of calculation of dividends, the dates on which dividends will be payable, whether dividends will be cumulative or noncumulative and, if cumulative, the dates from which dividends will commence to accumulate;
|
| |
•
|
any redemption or sinking fund provisions;
|
| |
•
|
if other than the currency of the United States, the currency or currencies including composite currencies in which the preferred stock is denominated and/or in which payments will or may be payable;
|
| |
•
|
any conversion provisions;
|
| |
•
|
any special tax implications of the preferred stock; and
|
| |
•
|
any other rights, preferences, privileges, limitations and restrictions on the preferred stock.
|
The preferred stock will, when issued, be fully paid and nonassessable. Unless otherwise specified in the prospectus supplement, each series of preferred stock will rank equally as to dividends and liquidation rights in all respects with each other series of preferred stock. The rights of holders of shares of each series of preferred stock will be subordinate to those of our general creditors.
Rank. Unless otherwise specified in the prospectus supplement, the preferred stock will, with respect to dividend rights and rights upon our liquidation, dissolution or winding up of our affairs, rank:
| |
•
|
senior to our common stock and to all equity securities ranking junior to such preferred stock with respect to dividend rights or rights upon our liquidation, dissolution or winding up of our affairs;
|
| |
•
|
on a parity with all equity securities issued by us, the terms of which specifically provide that such equity securities rank on a parity with the preferred stock with respect to dividend rights or rights upon our liquidation, dissolution or winding up of our affairs; and
|
| |
•
|
junior to all equity securities issued by us, the terms of which specifically provide that such equity securities rank senior to the preferred stock with respect to dividend rights or rights upon our liquidation, dissolution or winding up of our affairs.
|
The term “equity securities” does not include convertible debt securities.
Dividends. Holders of the preferred stock of each series will be entitled to receive, when, as and if declared by our Board, cash dividends at such rates and on such dates described in the prospectus supplement. Different series of preferred stock may be entitled to dividends at different rates or based on different methods of calculation. The dividend rate may be fixed or variable or both. Dividends will be payable to the holders of record as they appear on our stock books on record dates fixed by our board of directors, as specified in the applicable prospectus supplement.
Dividends on any series of preferred stock may be cumulative or noncumulative, as described in the applicable prospectus supplement. If our Board does not declare a dividend payable on a dividend payment date on any series of noncumulative preferred stock, then the holders of that noncumulative preferred stock will have no right to receive a dividend for that dividend payment date, and we will have no obligation to pay the dividend accrued for that period, whether or not dividends on that series are declared payable on any future dividend payment dates. Dividends on any series of cumulative preferred stock will accrue from the date we initially issue shares of such series or such other date specified in the applicable prospectus supplement.
No dividends may be declared or paid or funds set apart for the payment of any dividends on any parity securities unless full dividends have been paid or set apart for payment on the preferred stock. If full dividends are not paid, the preferred stock will share dividends pro rata with the parity securities.
No dividends may be declared or paid or funds set apart for the payment of dividends on any junior securities unless full dividends for all dividend periods terminating on or prior to the date of the declaration or payment will have been paid or declared and a sum sufficient for the payment set apart for payment on the preferred stock.
Liquidation Preference. Upon any voluntary or involuntary liquidation, dissolution or winding up of our affairs, then, before we make any distribution or payment to the holders of any common stock or any other class or series of our capital stock ranking junior to the preferred stock in the distribution of assets upon any liquidation, dissolution or winding up of our affairs, the holders of each series of preferred stock shall be entitled to receive out of assets legally available for distribution to stockholders, liquidating distributions in the amount of the liquidation preference per share set forth in the prospectus supplement, plus any accrued and unpaid dividends thereon. Such dividends will not include any accumulation in respect of unpaid noncumulative dividends for prior dividend periods. Unless otherwise specified in the prospectus supplement, after payment of the full amount of their liquidating distributions, the holders of preferred stock will have no right or claim to any of our remaining assets. Upon any such voluntary or involuntary liquidation, dissolution or winding up, if our available assets are insufficient to pay the amount of the liquidating distributions on all outstanding preferred stock and the corresponding amounts payable on all other classes or series of our capital stock ranking on parity with the preferred stock and all other such classes or series of shares of capital stock ranking on parity with the preferred stock in the distribution of assets, then the holders of the preferred stock and all other such classes or series of capital stock will share ratably in any such distribution of assets in proportion to the full liquidating distributions to which they would otherwise be entitled.
Upon any such liquidation, dissolution or winding up and if we have made liquidating distributions in full to all holders of preferred stock, we will distribute our remaining assets among the holders of any other classes or series of capital stock ranking junior to the preferred stock according to their respective rights and preferences and, in each case, according to their respective number of shares. For such purposes, our consolidation or merger with or into any other corporation, trust or entity, or the sale, lease or conveyance of all or substantially all of our property or assets will not be deemed to constitute a liquidation, dissolution or winding up of our affairs.
Redemption. If so provided in the applicable prospectus supplement, the preferred stock will be subject to mandatory redemption or redemption at our option, as a whole or in part, in each case upon the terms, at the times and at the redemption prices set forth in such prospectus supplement.
The prospectus supplement relating to a series of preferred stock that is subject to mandatory redemption will specify the number of shares of preferred stock that shall be redeemed by us in each year commencing after a date to be specified, at a redemption price per share to be specified, together with an amount equal to all accrued and unpaid dividends thereon to the date of redemption.
Voting Rights. Holders of preferred stock will not have any voting rights, except as required by law or as indicated in the applicable prospectus supplement.
Unless otherwise provided for under the terms of any series of preferred stock, no consent or vote of the holders of shares of preferred stock or any series thereof shall be required for any amendment to our restated certificate of incorporation that would increase the number of authorized shares of preferred stock or the number of authorized shares of any series thereof or decrease the number of authorized shares of preferred stock or the number of authorized shares of any series thereof (but not below the number of authorized shares of preferred stock or such series, as the case may be, then outstanding).
Conversion Rights. The terms and conditions, if any, upon which any series of preferred stock is convertible into our common stock will be set forth in the applicable prospectus supplement relating thereto. Such terms will include the number of shares of common stock into which the shares of preferred stock are convertible, the conversion price, rate or manner of calculation thereof, the conversion period, provisions as to whether conversion will be at our option or at the option of the holders of the preferred stock, the events requiring an adjustment of the conversion price and provisions affecting conversion in the event of the redemption.
Transfer Agent and Registrar. The transfer agent and registrar for the preferred stock will be set forth in the applicable prospectus supplement.
DESCRIPTION OF WARRANTS
The following description, together with the additional information we include in any applicable prospectus supplements or free writing prospectus, summarizes the material terms and provisions of the warrants that we may offer under this prospectus. Warrants may be offered independently or together with common stock or preferred stock and/or debt securities offered by any prospectus supplement or free writing prospectus, and may be attached to or separate from those securities. While the terms we have summarized below will generally apply to any future warrants we may offer under this prospectus, we will describe the particular terms of any warrants that we may offer in more detail in the applicable prospectus supplement or free writing prospectus. The terms of any warrants we offer under a prospectus supplement or free writing prospectus may differ from the terms we describe below.
We may issue warrants to purchase common stock, preferred stock or debt securities. We may offer warrants separately or together with one or more additional warrants, common stock, preferred stock or debt securities, or any combination of those securities in the form of units, as described in the applicable prospectus supplement. If we issue warrants as part of a unit, the accompanying prospectus supplement will specify whether those warrants may be separated from the other securities in the unit prior to the expiration date of the warrants. The applicable prospectus supplement will also describe the following terms of any warrants:
| |
•
|
the specific designation and aggregate number of, and the offering price at which we will issue, the warrants;
|
| |
•
|
the currency or currency units in which the offering price, if any, and the exercise price are payable;
|
| |
•
|
the date on which the right to exercise the warrants will begin and the date on which that right will expire or, if you may not continuously exercise the warrants throughout that period, the specific date or dates on which you may exercise the warrants;
|
| |
•
|
whether the warrants are to be sold separately or with other securities as parts of units;
|
| |
•
|
whether the warrants will be issued in definitive or global form or in any combination of these forms, although, in any case, the form of a warrant included in a unit will correspond to the form of the unit and of any security included in that unit;
|
| |
•
|
any applicable material U.S. federal income tax consequences;
|
| |
•
|
the identity of the warrant agent for the warrants and of any other depositaries, execution or paying agents, transfer agents, registrars or other agents;
|
| |
•
|
the proposed listing, if any, of the warrants or any securities purchasable upon exercise of the warrants on any securities exchange;
|
| |
•
|
the designation and terms of any equity securities purchasable upon exercise of the warrants;
|
| |
•
|
the designation, aggregate principal amount, currency and terms of any debt securities that may be purchased upon exercise of the warrants;
|
| |
•
|
if applicable, the designation and terms of the preferred stock with which the warrants are issued and the number of warrants issued with each security;
|
| |
•
|
if applicable, the date from and after which any warrants issued as part of a unit and the related debt securities, preferred stock, or common stock will be separately transferable;
|
| |
•
|
the number of shares of common stock, preferred stock purchasable upon exercise of a warrant and the price at which those shares may be purchased;
|
| |
•
|
if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
|
| |
•
|
information with respect to book-entry procedures, if any;
|
| |
•
|
the anti-dilution provisions of, and other provisions for changes to or adjustment in the exercise price of, the warrants, if any;
|
| |
•
|
any redemption or call provisions; and
|
| |
•
|
any additional terms of the warrants, including terms, procedures and limitations relating to the exchange or exercise of the warrants.
|
In the event that we issue warrants, we may issue the warrants under a warrant agreement, which, if applicable, we will enter into with a warrant agent to be selected by us. Forms of these warrant agreements and forms of the warrant certificates representing the warrants, and the complete warrant agreements and forms of warrant certificates containing the terms of the warrants being offered, will be filed as exhibits to the registration statement of which this prospectus is a part or will be incorporated by reference from reports that we file with the SEC. We use the term “warrant agreement” to refer to any of these warrant agreements. We use the term “warrant agent” to refer to the warrant agent under any of these warrant agreements. The warrant agent will act solely as an agent of ours in connection with the warrants and will not act as an agent for the holders or beneficial owners of the warrants.
DESCRIPTION OF OUR UNITS
This section outlines some of the provisions of the units and the unit agreements. This information may not be complete in all respects and is qualified entirely by reference to the unit agreement with respect to the units of any particular series. The specific terms of any series of units will be described in the applicable prospectus supplement. If so described in a particular prospectus supplement, the specific terms of any series of units may differ from the general description of terms presented below.
We may issue units comprised of one or more of the other securities that may be offered under this prospectus, in any combination. The following, together with the additional information we may include in the applicable prospectus supplement, summarizes the material terms and provisions of the units that we may offer under this prospectus. While the terms summarized below will apply generally to any units we may offer, we will describe the particular terms of any series of units in more detail in the applicable prospectus supplement.
Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately at any time, or at any time before a specified date.
Any applicable prospectus supplement will describe:
| |
•
|
the material terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
| |
•
|
any material provisions relating to the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units;
|
| |
•
|
any special tax implications of the units; and
|
| |
•
|
any material provisions of the governing unit agreement that differ from those described above.
|
DESCRIPTION OF CERTAIN PROVISIONS OF NEVADA LAW AND
OUR CHARTER AND BYLAWS
Transactions with Interested Persons
Under the Nevada Revised Statutes (the “NRS”) a transaction with the Company (i) in which a Company director or officer has a direct or indirect interest, or (ii) involving another corporation, firm or association in which one or more of the Company’s directors or officers are directors or officers of the corporation, firm or association or have a financial interest in the corporation firm or association, is not void or voidable solely because of the director’s or officer’s interest or common role in the transaction if any one of the following circumstances exists:
| |
●
|
the fact of the common directorship, office or financial interest is known to our Board or a committee of our Board and a majority of disinterested directors on the Board (or on the committee) authorize, approved or ratify the transaction in good faith;
|
| |
●
|
the fact of the common directorship, office or financial interest is known to the stockholders and stockholders holding a majority of the shares, including shares held by the common or interested directors or officers, authorize, approve or ratify the transaction in good faith;
|
| |
●
|
the fact of the common directorship, office or financial interest is not known to the director or officer at the time the transaction is brought to the Board for action; or
|
| |
●
|
the transaction is fair to the Company at the time it is authorized or approved.
|
Anti-Takeover Provisions
Our Charter and Nevada law include certain provisions which may have the effect of delaying or deterring a change in control or in our management or encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include authorized blank check preferred stock, restrictions on business combinations, and the availability of authorized but unissued common stock.
Combination with Interested Stockholders Statute
Sections 78.411 to 78.444 of the NRS, which apply to any Nevada corporation which has at least 200 stockholders of record and is publicly traded, including us, prohibits an “interested stockholder” from entering into specified types of business “combinations” with the Nevada corporation for two years, unless certain conditions are met. A “combination” includes:
| |
●
|
any merger of the corporation or any subsidiary of the corporation with an “interested stockholder,” or any other entity, whether or not itself an “interested stockholder,” which is, or after and as a result of the merger would be, an affiliate or associate of an “interested stockholder;”
|
| |
●
|
any sale, lease, exchange, mortgage, pledge, transfer, or other disposition in one transaction, or a series of transactions, to or with an “interested stockholder” or any affiliate or associate of an “interested stockholder,” of assets of the corporation or any subsidiary:
|
| |
i.
|
having an aggregate market value equal to more than 5% of the aggregate market value of the corporation’s assets, determined on a consolidated basis;
|
| |
ii.
|
having an aggregate market value equal to more than 5% of the aggregate market value of all outstanding voting shares of the corporation; or
|
| |
iii.
|
representing more than 10% of the earning power or net income, determined on a consolidated basis, of the corporation; or
|
| |
●
|
the issuance or transfer by the corporation or any subsidiary, of any shares of the corporation or any subsidiary to an “interested stockholder” or any affiliate or associate of an “interested stockholder,” having an aggregate market value equal to 5% or more of the aggregate market value of all of the outstanding voting shares of the corporation, except under the exercise of warrants or rights to purchase shares offered, or a dividend or distribution paid or made, pro rata to all stockholders of the resident domestic corporation;
|
| |
●
|
the adoption of any plan, or proposal for the liquidation or dissolution of the corporation, under any agreement, arrangement or understanding, with the “interested stockholder,” or any affiliate or associate of the “interested stockholder;”
|
| |
●
|
if any of the following actions occurs:
|
| |
i.
|
a reclassification of the corporation’s securities, including, without limitation, any splitting of shares, share dividend, or other distribution of shares with respect to other shares, or any issuance of new shares in exchange for a proportionately greater number of old shares;
|
| |
ii.
|
recapitalization of the corporation;
|
| |
iv.
|
merger or consolidation of the corporation with any subsidiary; or
|
| |
v.
|
any other transaction, whether or not with or into or otherwise involving the interested stockholder,
|
under any agreement, arrangement or understanding, whether or not in writing, with the interested stockholder or any affiliate or associate of the interested stockholder, which has the immediate and proximate effect of increasing the proportionate share of the outstanding shares of any class or series of voting shares or securities convertible into voting shares of the corporation or any subsidiary of the corporation which is beneficially owned by the interested stockholder or any affiliate or associate of the interested stockholder, except as a result of immaterial changes because of adjustments of fractional shares; or
| |
●
|
any receipt by an “interested stockholder” or any affiliate or associate of an “interested stockholder,” except proportionately as a stockholder of the corporation, of the benefit of any loan, advance, guarantee, pledge or other financial assistance or any tax credit or other tax advantage provided by or through the corporation.
|
An “interested stockholder” is a person who is:
| |
●
|
directly or indirectly, the beneficial owner of 10% or more of the voting power of the outstanding voting shares of the corporation; or
|
| |
●
|
an affiliate or associate of the corporation, which at any time within two years immediately before the date in question was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then outstanding shares of the corporation.
|
A corporation to which the Combinations with Interested Stockholders Statute applies may not engage in a “combination” within two years after the interested stockholder first became an interested stockholder, unless the combination meets all of the requirements of the corporation’s articles of incorporation and (i) the combination or the transaction by which the person first became an interested stockholder is approved by the board of directors before the person first became an interested stockholder, or (ii)(a) the combination is approved by the board of directors and (b) at or after that time, the combination is approved at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of the stockholders representing at least 60% of the outstanding voting power of the corporation not beneficially owned by the interested stockholder or the affiliates or associates of the interested stockholder. If this approval is not obtained, the combination may be consummated after the two year period expires if either (i)(a) the combination or transaction by which the person first became an interested stockholder is approved by the board of directors before such person first became an interested stockholder, (b) the combination is approved by a majority of the outstanding voting power of the corporation not beneficially owned by the interested stockholder or any affiliate or associate of the interested stockholder, or (c) the combination otherwise meets the requirements of the Combination with Interested Stockholders statute. Alternatively, a combination with an interested stockholder engaged in more than 2 years after the date the person first became an interested stockholder may be permissible if the aggregate amount of cash and the market value of consideration other than cash to be received by holders of shares of common stock and holders of any other class or series of shares meets the minimum requirements set forth in the statue, and prior to the completion of the combination, except in limited circumstances, the interested stockholder has not become the beneficial owner of additional voting shares of the corporation.
Acquisition of Controlling Interest Statute
In addition, Nevada’s “Acquisition of Controlling Interest Statute,” prohibits an acquiror, under certain circumstances, from voting shares of a target corporation’s stock after crossing certain threshold ownership percentages, unless the acquiror obtains the approval of the target corporation’s stockholders. Sections 78.378 to 78.3793 of the NRS only apply to Nevada corporations with at least 200 stockholders, including at least 100 record stockholders who are Nevada residents, that do business directly or indirectly in Nevada and whose articles of incorporation or bylaws in effect ten days following the acquisition of a controlling interest by an acquiror do not prohibit its application.
We do not intend to “do business” in Nevada within the meaning of the Acquisition of Controlling Interest Statute. Further, our Bylaws contain a specific opt out from the statute. Therefore, we believe it is unlikely that this statute will apply to us. The statute specifies three thresholds:
| |
●
|
at least one-fifth but less than one-third;
|
| |
●
|
at least one-third but less than a majority; and
|
| |
●
|
a majority or more, of the outstanding voting power.
|
Once an acquiror crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold (or within 90 days preceding the date thereof) become “control shares” which could be deprived of the right to vote until a majority of the disinterested stockholders restore that right. A special stockholders’ meeting may be called at the request of the acquiror to consider the voting rights of the acquiror’s shares. If the acquiror requests a special meeting and gives an undertaking to pay the expenses of said meeting, then the meeting must take place no earlier than 30 days (unless the acquiror requests that the meeting be held sooner) and no more than 50 days (unless the acquiror agrees to a later date) after the delivery by the acquiror to the corporation of an information statement which sets forth the range of voting power that the acquiror has acquired or proposes to acquire and certain other information concerning the acquiror and the proposed control share acquisition.
If no such request for a stockholders’ meeting is made, consideration of the voting rights of the acquiror’s shares must be taken at the next special or annual stockholders’ meeting. If the stockholders fail to restore voting rights to the acquiror, or if the acquiror fails to timely deliver an information statement to the corporation, then the corporation may, if so provided in its articles of incorporation or bylaws, call certain of the acquiror’s shares for redemption at the average price paid for the control shares by the acquiror.
Our Charter and our Bylaws do not currently permit us to redeem an acquiror’s shares under these circumstances. The Acquisition of Controlling Interest Statute also provides that in the event the stockholders restore full voting rights to a holder of control shares that owns a majority of the voting stock, then all other stockholders who do not vote in favor of restoring voting rights to the control shares may demand payment for the “fair value” of their shares as determined by a court in dissenter’s rights proceeding pursuant to Chapter 92A of the NRS.
PLAN OF DISTRIBUTION
We may sell securities:
| |
•
|
directly to purchasers; or
|
| |
•
|
through a combination of any of these methods of sale.
|
We may also sell equity securities covered by this registration statement in an “at the market offering” as defined in Rule 415 under the Securities Act of 1933, as amended (the “Securities Act”). Such offering may be made into an existing trading market for such securities in transactions at other than a fixed price, either:
| |
•
|
on or through the facilities of The Nasdaq Capital Market or any other securities exchange or quotation or trading service on which such securities may be listed, quoted or traded at the time of sale; and/or
|
| |
•
|
to or through a market maker other than on The Nasdaq Capital Market or such other securities exchanges or quotation or trading services.
|
In addition, we may issue the securities as a dividend or distribution or in a subscription rights offering to our existing security holders. This prospectus may be used in connection with any offering of our securities through any of these methods or other methods described in the applicable prospectus supplement.
We may directly solicit offers to purchase securities, or agents may be designated to solicit such offers. We will, in the prospectus supplement relating to such offering, name any agent that could be viewed as an underwriter under the Securities Act, and describe any commissions that we must pay. Any such agent will be acting on a best efforts basis for the period of its appointment or, if indicated in the applicable prospectus supplement, on a firm commitment basis.
The distribution of the securities may be effected from time to time in one or more transactions:
| |
•
|
at a fixed price, or prices, which may be changed from time to time;
|
| |
•
|
at market prices prevailing at the time of sale;
|
| |
•
|
at prices related to such prevailing market prices; or
|
Each prospectus supplement will describe the method of distribution of the securities and any applicable restrictions.
The prospectus supplement with respect to the securities of a particular series will describe the terms of the offering of the securities, including the following:
| |
•
|
the name of the agent or any underwriters;
|
| |
•
|
the public offering or purchase price and the proceeds we will receive from the sale of the securities;
|
| |
•
|
any discounts and commissions to be allowed or re-allowed or paid to the agent or underwriters;
|
| |
•
|
all other items constituting underwriting compensation;
|
| |
•
|
any discounts and commissions to be allowed or re-allowed or paid to dealers; and
|
| |
•
|
any exchanges on which the securities will be listed.
|
If any underwriters or agents are utilized in the sale of the securities in respect of which this prospectus is delivered, we will enter into an underwriting agreement or other agreement with them at the time of sale to them, and we will set forth in the prospectus supplement relating to such offering the names of the underwriters or agents and the terms of the related agreement with them.
If a dealer is utilized in the sale of the securities in respect of which this prospectus is delivered, we will sell such securities to the dealer, as principal. The dealer may then resell such securities to the public at varying prices to be determined by such dealer at the time of resale.
If we offer securities in a subscription rights offering to our existing security holders, we may enter into a standby underwriting agreement with dealers, acting as standby underwriters. We may pay the standby underwriters a commitment fee for the securities they commit to purchase on a standby basis. If we do not enter into a standby underwriting arrangement, we may retain a dealer-manager to manage a subscription rights offering for us.
Remarketing firms, agents, underwriters, dealers and other persons may be entitled under agreements which they may enter into with us to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
If so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in the prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in the prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
| |
•
|
the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under the laws of the jurisdiction to which that institution is subject; and
|
| |
•
|
if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery contracts.
|
Certain agents, underwriters and dealers, and their associates and affiliates may be customers of, have borrowing relationships with, engage in other transactions with, and/or perform services, including investment banking services, for us or one or more of our respective affiliates in the ordinary course of business.
In order to facilitate the offering of the securities, any underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the securities or any other securities the prices of which may be used to determine payments on such securities. Specifically, any underwriters may overallot in connection with the offering, creating a short position for their own accounts. In addition, to cover overallotments or to stabilize the price of the securities or of any such other securities, the underwriters may bid for, and purchase, the securities or any such other securities in the open market. Finally, in any offering of the securities through a syndicate of underwriters, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the securities in the offering if the syndicate repurchases previously distributed securities in transactions to cover syndicate short positions, in stabilization transactions or otherwise. Any of these activities may stabilize or maintain the market price of the securities above independent market levels. Any such underwriters are not required to engage in these activities and may end any of these activities at any time.
Under Rule 15c6-1 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), trades in the secondary market generally are required to settle in two business days, unless the parties to any such trade expressly agree otherwise. The applicable prospectus supplement may provide that the original issue date for your securities may be more than two scheduled business days after the trade date for your securities. Accordingly, in such a case, if you wish to trade securities on any date prior to the second business day before the original issue date for your securities, you will be required, by virtue of the fact that your securities initially are expected to settle more than two scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
The securities may be new issues of securities and may have no established trading market. The securities may or may not be listed on a national securities exchange. We can make no assurance as to the liquidity of or the existence of trading markets for any of the securities.
LEGAL MATTERS
Certain legal matters in connection with this offering will be passed upon for us by Latham & Watkins LLP, Chicago, Illinois. The validity of the securities offered hereby will be passed upon for us by Woodburn and Wedge, Reno, Nevada. Additional legal matters may be passed upon for us or any underwriters, dealers or agents, by counsel that we will name in the applicable prospectus supplement.
EXPERTS
WithumSmith+Brown, PC (“Withum”) our independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended March 31, 2023, as set forth in their report, which is incorporated by reference in this prospectus. The report for Vistagen Therapeutics, Inc. includes an explanatory paragraph about the existence of substantial doubt concerning its ability to continue as a going concern. Our financial statements are incorporated by reference in reliance on Withum’s report, given on their authority as experts in accounting and auditing.
| |
|
|
| |
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
|
| |
|
|
|
SUBJECT TO COMPLETION, DATED FEBRUARY 13, 2024
|
PROSPECTUS SUPPLEMENT
(To Prospectus dated , 2024)
Up to $100,000,000
Common Stock
On May 14, 2021, we entered into an Open Market Sale AgreementSM (the “Sales Agreement”) with Jefferies LLC (“Jefferies”) relating to shares of our common stock, par value $0.001 per share. In accordance with the terms of the Sales Agreement, under this prospectus supplement, we may offer and sell shares of our common stock having an aggregate offering price of up to $100,000,000 from time to time through Jefferies, acting as our sales agent.
Our common stock is listed on The Nasdaq Capital Market under the trading symbol “VTGN.” On February 12, 2024, the last reported sale price of our common stock on The Nasdaq Capital Market was $5.15 per share.
Sales of our common stock, if any, under this prospectus supplement may be made by any method permitted that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended (the “Securities Act”). Jefferies is not required to sell any specific amount of securities, but will act as our sales agent and use commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us, consistent with its normal trading and sales practices, on mutually agreed terms between Jefferies and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Jefferies will be entitled to a commission of up to 3.0% of the gross sales price per share of common stock sold under this Prospectus Supplement. In connection with the sale of our common stock on our behalf, Jefferies will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have also agreed to provide indemnification and contribution to Jefferies with respect to certain liabilities, including liabilities under the Securities Act or the Securities Exchange Act of 1934, as amended (the “Exchange Act”). See “Plan of Distribution” beginning on page S-13 for additional information regarding the compensation to be paid to Jefferies.
Investing in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page S-9 of this prospectus supplement and under similar headings in the documents incorporated by reference into this prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities, or determined if this prospectus supplement and the accompanying base prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Jefferies
The date of this prospectus supplement is , 2024.
TABLE OF CONTENTS
Prospectus Supplement
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement is part of a shelf registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”). Under the shelf registration process, we may offer shares of our common stock having an aggregate offering price of up to $350,000,000. Under this prospectus supplement, we may from time to time sell shares of our common stock having an aggregate offering price of up to $100,000,000 at prices and on terms to be determined by market conditions at the time of the offering. The $100,000,000 of shares of our common stock that may be sold under this prospectus supplement are included in the $350,000,000 of shares of common stock that may be sold under the registration statement.
This prospectus supplement and the accompanying prospectus relate to the offering of our common stock. Before buying any of the common stock that we are offering, we urge you to carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated by reference as described under the heading “Incorporation of Certain Information by Reference” in this prospectus supplement. These documents contain important information that you should consider when making your investment decision.
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of the common stock we are offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein or therein. The second part, the accompanying prospectus, provides more general information. Generally, when we refer to this prospectus, we are referring to both this prospectus supplement and the accompanying prospectus. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus or any document incorporated by reference herein or therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies or supersedes the earlier statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein or in the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreement, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
You should rely only on the information contained in, or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectus that we may authorize for use in connection with this offering. We have not, and Jefferies has not, authorized anyone to provide any information other than that contained or incorporated by reference in this prospectus supplement, in the accompanying prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We and Jefferies take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We are not, and Jefferies is not, making an offer to sell, or soliciting an offer to purchase, the securities offered by this prospectus supplement and the accompanying prospectus in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer or solicitation of an offer in such jurisdiction. The information contained in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein or therein, and in any free writing prospectus prepared by or on behalf of us that we may authorize for use in connection with this filing is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospectus may have changed since those dates. It is important for you to read and consider all information contained in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, and any free writing prospectus prepared by or on behalf of us that we may authorize for use in connection with this offering, in their entirety, before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference” in this prospectus supplement and in the accompanying prospectus.
We are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of the common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of the common stock and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
Unless otherwise stated, all references in this prospectus supplement and the accompanying prospectus to “we,” “us,” “our,” “Vistagen,” “the Company” and similar designations refer, collectively, to Vistagen Therapeutics, Inc., a Nevada corporation, and its consolidated subsidiaries.
We use our trademarks and our logo in this prospectus supplement and the documents incorporated by reference. Solely for the convenience, trademarks and tradenames referred to in this prospectus supplement appear without the “®” and “™” symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights to these trademarks and tradenames.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying base prospectus and the documents incorporated by reference herein and therein contain forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this prospectus, any prospectus supplement and the documents incorporated by reference herein, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
| |
●
|
the availability of capital to satisfy our working capital requirements;
|
| |
●
|
the accuracy of our estimates regarding expenses, future revenues and capital requirements;
|
| |
●
|
our plans to develop and commercialize our product candidates, including, among other things, fasedienol (PH94B) as a potential acute treatment of anxiety for adults with social anxiety disorder (“SAD”), itruvone (PH10) as a potential treatment for adults with major depressive disorder (“MDD”) and other depression-related disorders, PH80 for vasomotor symptoms (hot flashes) due to menopause, premenstrual dysphoric disorder (“PMDD”) or migraine, PH15 for cognition improvement, PH284 for appetite-related disorders or AV-101 as a potential treatment of disorders involving the Central Nervous System (“CNS”);
|
| |
●
|
our ability to initiate and complete necessary preclinical and clinical studies in accordance with applicable regulatory requirements to advance the development of our product candidates and for those studies to generate positive results;
|
| |
●
|
economic, regulatory and political developments in the United States and foreign countries;
|
| |
●
|
the performance of our third-party contractors involved with the manufacture and production of our drug candidates for nonclinical and clinical development activities, contract research organizations, potential commercial activities and other third-party nonclinical and clinical development collaborators and regulatory service providers;
|
| |
●
|
our ability to obtain and maintain intellectual property, regulatory and commercial protection for our core assets, including product candidates;
|
| |
●
|
the size of the potential markets for our product candidates and our ability to serve those markets;
|
| |
●
|
the rate and degree of market acceptance of our product candidates for any indication once approved;
|
| |
●
|
the success of competing products and product candidates in development by others that are or become available for the indications that we are pursuing in the markets we seek to enter on our own or with collaborators;
|
| |
●
|
the loss of key scientific, clinical and nonclinical development, regulatory, commercial, and/or management personnel, internally or from one of our third-party collaborators; contract manufacturing organizations, contract research organizations or other service providers;
|
| |
●
|
our ability to continue as a going concern;
|
| |
●
|
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
|
| |
●
|
our use of our existing cash and cash equivalents; and
|
| |
●
|
other risks and uncertainties, including those described under Item 1A, “Risk Factors,” in our Annual Report on Form 10-K for the fiscal year ended March 31, 2023, and those described under Part II, Item 1A, “Risk Factors,” in our Quarterly Reports on Form 10-Q for the quarters ended June 30, 2023, September 30, 2023 and December 31, 2023, which risk factors are incorporated herein by reference.
|
These forward-looking statements are only predictions, and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and operating results. Moreover, our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur.
You should read this prospectus supplement, the accompanying base prospectus and the documents incorporated by reference herein and therein completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus supplement, the accompanying base prospectus and the documents incorporated by reference herein and therein by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information about us and this offering and does not contain all of the information that you should consider before investing in our securities. Before investing in our common stock, you should carefully read the information contained and incorporated by reference in this prospectus supplement, the accompanying prospectus, any related free-writing prospectus that we have authorized for use in connection with this offering and the documents incorporated by reference herein or therein, including the sections titled “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements” and the financial statements and accompanying notes.
Business Overview
We are a clinical-stage biopharmaceutical company pioneering neuroscience to deliver first-in-class therapies for psychiatric and neurological disorders. Five of our six clinical-stage product candidates – fasedienol (PH94B), itruvone (PH10), PH15, PH80 and PH284 – belong to a new class of drugs known as pherines, which have the potential to rapidly deliver meaningful efficacy with a differentiated safety profile. Pherines are investigational neuroactive nasal sprays with innovative proposed mechanisms of action that activate chemosensory neurons in the nasal passages to impact fundamental neural circuits in the brain without the need for systemic absorption or binding to receptors in the brain. Our sixth clinical-stage product candidate, AV-101, is an investigational oral drug candidate with the potential to inhibit, but not block, NMDA receptor activity.
We are passionate about transforming what is possible in the treatment of anxiety, depression, and other neuroscience disorders.
Our Clinical-Stage Neuroscience Product Candidates
Fasedienol Nasal Spray for Social Anxiety Disorder (SAD)
Fasedienol (PH94B) is an odorless, tasteless synthetic investigational pherine nasal spray from the androstane family in Phase 3 clinical development for the acute treatment of anxiety for adults with SAD. When administered intranasally in microgram-level doses, fasedienol activates receptors of peripheral nasal chemosensory neurons connected to subsets of neurons in the olfactory bulbs that, in turn, connect to neurons in the limbic amygdala involved in the pathophysiology of SAD, and potentially other anxiety and mood disorders. Fasedienol is pharmacologically active without requiring apparent systemic absorption or direct activity on neurons in the brain to achieve its rapid-onset and short-duration anxiolytic effects. We believe fasedienol has the potential to achieve these effects with significantly reduced risks of side effects and other safety concerns, such as potential drug-drug interactions, abuse, misuse, and addiction, associated with certain other systemic pharmaceuticals that act directly on neurons in the brain and are sometimes prescribed for anxiety disorders.
The U.S. Food and Drug Administration (the FDA) has granted Fast Track designation for the development of fasedienol as a potential treatment for SAD.
Fasedienol PALISADE Phase 3 Program in SAD
Given how fasedienol’s rapid-onset mechanism of action (MOA) is differentiated from all FDA-approved anxiety drugs, our primary target indication for fasedienol is the acute treatment of anxiety in adults with SAD. Currently, there is no FDA-approved drug therapy for the acute treatment of SAD. For that acute indication, we have aligned with the FDA that the Subjective Units of Distress Scale (SUDS) is an appropriate primary efficacy endpoint because it provides a measure of anxiety on a minute-by-minute basis immediately related to the specific stressor. We believe utilizing a simulated anxiety-provoking public speaking challenge study design provides the most appropriate and efficient path for the Phase 3 clinical development of fasedienol’s potential to become the first FDA-approved acute treatment of anxiety for adults with SAD. Our PALISADE Phase 3 Program currently includes four randomized, double-blind, placebo-controlled, Phase 3 clinical trials designed to evaluate the efficacy, safety, and tolerability of a single dose of fasedienol to relieve anxiety symptoms in adult patients with SAD during a simulated, anxiety-provoking public speaking challenge in a clinical setting, as measured using the patient-reported SUDS, two of which Phase 3 trials (PALISADE-1 and PALISADE-2) have been concluded and two of which Phase 3 trials (PALISADE-3 and PALISADE-4) will be initiated in 2024, each with open-label extension. Our PALISADE Phase 3 Program also includes an open-label safety study concluded in 2022 (PALISADE OLS), a Phase 2 repeat dose study to be initiated in 2024 (Repeat Dose Study), two standard preclinical studies to be initiated in 2024, and a small human factor study planned to be initiated in 2025.
In early August 2023, we received and reported positive topline results from our PALISADE-2 Phase 3 trial of fasedienol in SAD based on the 141 subjects who completed the trial. Our PALISADE-2 Phase 3 trial met its primary efficacy endpoint, the difference in mean SUDS scores during the public speaking challenge at baseline (Visit 2) and treatment (Visit 3) for subjects treated with fasedienol versus placebo at Visit 3. Fasedienol-treated patients demonstrated a greater mean change from baseline (least-squares (LS) mean = -13.8) compared to placebo (LS mean = -8.0), for a statistically significant, and we believe clinically relevant, difference between groups of -5.8 (p=0.015). The trial also met its secondary endpoint, demonstrating a statistically significant difference in the proportion of clinician-assessed responders between fasedienol and placebo as measured by the Clinical Global Impressions – Improvement (CGI-I) scale. Responders were identified as those who were rated ‘very much less anxious’ or ‘much less anxious’ and 37.7% of fasedienol-treated patients were rated as responders, as compared to 21.4% of those treated with placebo (p=0.033). The trial also met the important exploratory endpoint of the difference in the proportion of patient-assessed responders between fasedienol and placebo as measured by the Patient’s Global Impression of Change (PGI-C). Responders were identified as those who self-rated ‘very much less anxious’ or ‘much less anxious’ and 40.6% of fasedienol-treated patients were rated as responders, as compared to 18.6% of those treated with placebo (p=0.003). In addition, our PALISADE-2 trial also met the exploratory endpoint of the difference in the proportion of patients in each treatment group with a 20-point or greater improvement in patient-assessed SUDS score from baseline (Visit 2) to treatment (Visit 3). Of the fasedienol-treated patients, 35.7% demonstrated this statistically significant and clinically meaningful improvement in SUDS score, as compared to 18.6% in the placebo-treated group (p=0.020). Fasedienol was observed to be well-tolerated with no serious adverse events, and the treatment-emergent adverse event (TEAE) profiles were comparable between fasedienol and placebo. Overall, no TEAE, except for pyrexia in the placebo group (2.49%), was more prevalent than 2.0%.
To complement the positive topline results from PALISADE-2, we are preparing to launch PALISADE-3 in the first half of 2024 and PALISADE-4 in the second half of 2024. Like PALISADE-2, both PALISADE-3 and PALISADE-4 will be multi-center, randomized, double-blind, placebo-controlled studies designed to evaluate the efficacy, safety, and tolerability of the acute administration of fasedienol to relieve anxiety symptoms in adult patients with SAD after a single dose of fasedienol during a simulated, anxiety-provoking public speaking challenge in a clinical setting, as measured using the patient-reported SUDS as the primary efficacy endpoint. In addition, both PALISADE-3 and PALISADE-4 will have an open-label extension for a period of up to 12 months. We believe either PALISADE-3 or PALISADE-4, if successful, together with PALISADE-2, may establish substantial evidence of effectiveness of fasedienol in support of a potential fasedienol NDA submission for the acute treatment of anxiety in adults with SAD with the FDA.
We are also planning to initiate the Repeat Dose Study in the second half of 2024. The Repeat Dose Study will be a multi-center, randomized, double-blind, placebo-controlled, clinical trial designed to evaluate repeated dosing of fasedienol in adult patients with SAD during a single simulated, anxiety-provoking public speaking challenge in a clinical setting. The Repeat Dose Study trial will consist of three different dosing arms, with an open-label extension for a period of up to 12 months.
The U.S. FDA has granted Fast Track designation for the investigation of fasedienol for the acute treatment of SAD.
Itruvone Nasal Spray for Major Depressive Disorder (MDD)
Itruvone (PH10) is an odorless, tasteless synthetic investigational neuroactive pherine nasal spray from the pregnane family with an innovative potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for depression disorders. Itruvone neuroactive nasal spray is administered at microgram-level doses and is designed to engage and activate chemosensory neurons in the nasal cavity, which are connected to neural circuits in the brain that produce antidepressant effects. Unlike all currently approved oral antidepressants (ADs) and rapid-onset intravenous and intranasal ketamine-based therapy, we believe itruvone does not require systemic absorption or direct activity on neurons in the brain to produce antidepressant effects without the side effects and safety concerns that may be associated with current antidepressant therapies.
We are currently preparing and planning for potential U.S. Phase 2B clinical development of itruvone for treatment of MDD. In a randomized, double-blind, placebo-controlled parallel design Phase 2A clinical trial of itruvone in MDD and published in the peer-reviewed British Journal of Pharmaceutical and Medical Research, at a 6.4 μg dose administered intranasally twice daily for eight weeks, PH10 significantly reduced depressive symptoms as early as one week based on the 17-item Hamilton Depression Scale (HAM-D-17) scores compared to placebo (p=0.022). Itruvone was well-tolerated and did not cause psychological side effects (such as dissociation or hallucinations) or other safety concerns that may be associated with other approved pharmacological therapies for MDD. The trial was sponsored by Pherin Pharmaceuticals (Pherin), now a wholly-owned subsidiary of Vistagen, and was conducted in Mexico. Positive data from our recent U.S. IND-enabling Phase 1 trial demonstrated that there were no reported treatment-related serious adverse events (SAEs) or discontinuations due to adverse events in the trial. Overall, itruvone was well-tolerated and continued to demonstrate a favorable safety profile consistent with all other itruvone trials completed to date.
The U.S. FDA has granted Fast Track designation for the investigation of itruvone for the treatment of MDD.
PH80 Nasal Spray for Women’s Health Disorders
PH80 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for vasomotor symptoms (hot flashes) due to menopause, premenstrual dysphoric disorder (PMDD), and other women’s health disorders and migraine headaches. PH80 activates chemosensory neurons in the nasal cavity connected to neural circuits that modulate the basal forebrain associated with the control of body temperature.
PH80 for Moderate to Severe Vasomotor Symptoms (hot flashes) due to Menopause. We recently reported positive results from a previously unpublished exploratory randomized, double-blind, placebo-controlled Phase 2A study of PH80 for the acute treatment of vasomotor symptoms (hot flashes) due to menopause sponsored by Pherin, now our wholly-owned subsidiary. This Phase 2A study conducted in a real-world setting demonstrated a statistically significant reduction in the daily number of menopausal hot flashes compared to placebo at the end of the first week of treatment (p=<0.001), and the improvement was maintained through each treatment week until the end of the four-week treatment period (p=<0.001). PH80 treatment also significantly reduced the severity, disruption in function, and sweating related to hot flashes during the treatment period as compared with placebo. PH80 was well-tolerated with no serious adverse events (SAEs), and the adverse event profiles were comparable between PH80 and placebo. All 36 subjects completed four weeks of treatment and no subject discontinued participation in the study as a result of TEAEs.
PH80 for Premenstrual Dysphoric Disorder (PMDD). We also recently reported positive results from a previously unpublished exploratory randomized, double-blind, placebo-controlled Phase 2A clinical study of PH80 for acute management of the symptoms of PMDD, including negative mood and physical and behavioral symptoms, in subjects with a regular menstrual cycle and at least a one-year history of PMDD. This Phase 2A study was sponsored by Pherin, now our wholly-owned subsidiary, and demonstrated a statistically significant improvement versus placebo in acute management of the symptoms of PMDD, including negative mood and physical and behavioral symptoms. The initial study visit occurred after the onset of symptoms. All subjects were administered a placebo nasal spray, and those who showed no symptom improvement were eligible to return for the second visit, which occurred after the onset of symptoms during the next menstrual cycle. At the second study visit, subjects were randomized to receive a single dose of 0.9 µg PH80 nasal spray or placebo in the clinic. PH80 demonstrated statistically and clinically significant improvement versus placebo in symptoms of PMDD using the subject-rated Penn Daily Symptom Report (DSR) as early as Day 4 and continuing to Day 6 (p=0.008). PH80 also demonstrated statistically and clinically significant improvement versus placebo at Day 6 on the clinician-rated Premenstrual Tension Scale (PMTS) total score (p=0.006). PH80 was well-tolerated with no SAEs. The most common TEAE was headache, reported by 17% in the placebo group and 7% in the PH80 group. No other TEAE occurred more than once per subject.
We are preparing to conduct certain nonclinical studies during the 2024 calendar year necessary to submit our U.S. IND for potential Phase 2B clinical development of PH80 in the U.S. for one or more women’s health indications.
PH15 Nasal Spray for Cognitive and Psychomotor Performance and Improvement
PH15 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments to improve cognitive impairment caused by mental fatigue and potentially other disorders. We believe intranasal PH15 has the potential to improve cognitive and psychomotor performance and improvement of reaction time in individuals with mental fatigue. We are currently evaluating the potential path forward for PH15, including an assessment of completed studies and studies we believe are necessary to submit a U.S. IND for potential further Phase 2 clinical development of PH15 in the U.S., including the appropriate indication for demonstrating improvement of cognitive function.
PH284 Nasal Spray for Cachexia
PH284 is an odorless, tasteless synthetic investigational pherine nasal spray with a novel, rapid-onset potential MOA that is fundamentally differentiated from the MOA of all currently approved treatments for the loss of appetite associated with chronic disorders such as cancer. Cachexia is a serious but under-recognized consequence of many chronic diseases with body mass loss of >10% and a prevalence of 5 to 15 %. We believe PH284 may have therapeutic potential for improving subjective feelings of hunger in patients with cachexia. We are currently evaluating the potential path forward for PH284, including assessment of completed studies and studies we believe are necessary to submit a U.S. IND for potential further Phase 2 clinical development of PH15 for the treatment of cachexia, including the appropriate patient populations for demonstrating an increase in appetite and weight gain.
AV-101 for Neurological Disorders
AV-101 (4-Cl-KYN) is a novel, oral prodrug that targets the NMDAR (N-methyl-D-aspartate receptor), an ionotropic glutamate receptor in the brain. Based on observations and findings from preclinical studies, we believe AV-101 has the potential to become a new oral treatment alternative for multiple neuroscience disorders, including levodopa-induced dyskinesia and neuropathic pain among others. We are currently assessing a path forward for potential Phase 2A clinical development of AV-101, either on our own or with collaborators, as a treatment for levodopa-induced dyskinesia associated with Parkinson’s disease therapy and possibly one or more additional neurological disorders involving the NMDAR receptor.
The U.S. FDA has granted Fast Track designation for the investigation of AV-101 for the treatment of neuropathic pain and for the adjunctive treatment of MDD.
Corporate Information
Vistagen Therapeutics, Inc., a Nevada corporation, is the parent of Pherin Pharmaceuticals, Inc., a Delaware corporation, and Vistastem, Inc., a wholly owned California corporation. Our principal executive offices are located at 343 Allerton Avenue, South San Francisco, California 94080, and our telephone number is (650) 577-3600. Our website address is www.vistagen.com. The information contained on our website is not part of this prospectus supplement or the accompanying prospectus. We have included our website address as a factual reference and do not intend it to be an active link to our website.
THE OFFERING
|
Common Stock Offered by Us
|
Shares of our common stock having an aggregate offering price of up to $100,000,000.
|
| |
|
|
Plan of Distribution
|
“At the market offering” that may be made from time to time through our sales agent, Jefferies. See “Plan of Distribution” on page S-13 of this prospectus supplement.
|
| |
|
|
Use of Proceeds
|
Our management will retain broad discretion regarding the allocation and use of the net proceeds. We intend to use the net proceeds from this offering to advance the clinical development of our product candidates and for working capital and other general corporate purposes. See “Use of Proceeds” on page S-11.” |
|
Risk Factors
|
Investing in our common stock involves a high degree of risk. See the information contained under the heading “Risk Factors” beginning on page S-9 of this prospectus supplement and under similar headings in the accompanying prospectus and in the other documents that are incorporated by reference herein and therein, including specifically under “Item 1A: Risk Factors” and elsewhere in our Annual Report on Form 10-K for the fiscal year ended March 31, 2023 and in our Quarterly Reports on Form 10-Q for the periods ended June 30, 2023, September 30, 2023 and December 31, 2023.
|
|
Nasdaq Capital Market symbol
|
“VTGN”
|
RISK FACTORS
Investing in our securities involves a high degree of risk. Before making an investment decision, you should carefully consider the risks described below and in our Annual Report on Form 10-K for the fiscal year ended March 31, 2023 and our Quarterly Reports on Form 10-Q for the periods ended June 30, 2023, September 30, 2023 and December 31, 2023 incorporated by reference into this prospectus supplement and the accompanying prospectus, any amendment or update thereto reflected in our subsequent filings with the SEC, any related free writing prospectus and all of the other information contained in this prospectus supplement, the accompanying prospectus, any related free writing prospectus and the documents incorporated by reference herein or therein, including our financial statements and related notes. If any of these risks is realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the trading price of our common stock could decline, and you could lose part or all of your investment. Additional risks and uncertainties that are not yet identified or that we currently believe to be immaterial may also materially harm our business, operating results and financial condition and could result in a complete loss of your investment.
Risks Relating to this Offering
Purchasers will experience immediate dilution in the book value per share of the common stock purchased in the offering.
The shares sold in this offering, if any, will be sold from time to time at various prices. However, we expect that the offering price of our common stock will be substantially higher than the net tangible book value per share of our outstanding common stock. After giving effect to the sale of shares of our common stock in the aggregate amount of $100,000,000 at an assumed offering price of $4.90 per share, the last sale price of our common stock on February 9, 2024 on The Nasdaq Capital Market, and after deducting commissions and estimated offering expenses, our as adjusted net tangible book value as of December 31, 2023 would have been approximately $220.0 million or approximately $4.64 per share. This represents an immediate increase in net tangible book value of approximately $0.10 per share to our existing stockholders and an immediate dilution in as adjusted net tangible book value of approximately $0.26 per share to purchasers of our common stock in this offering. See “Dilution” for more information.
In addition to this offering, subject to market conditions and other factors, we may pursue additional equity financings in the future, including future public offerings or future private placements of equity securities or securities convertible into or exchangeable for equity securities at prices that may be higher or lower than the price per share in this offering. Further, the exercise of outstanding options or warrants could result in further dilution to investors and any additional shares issued in connection with acquisitions, collaborations, licensing transactions or other similar transactions will result in dilution to investors. In addition, the market price of our common stock could fall as a result of resales of any of these shares of common stock due to an increased number of shares available for sale in the market.
The common stock offered hereby will be sold in “at the market offerings,” and investors who buy shares at different times will likely pay different prices.
Investors who purchase shares in this offering at different times will likely pay different prices, and accordingly may experience different levels of dilution and different outcomes in their investment results. We will have discretion, subject to market demand and the terms of the Sales Agreement, to vary the timing, prices and number of shares sold in this offering. In addition, subject to the final determination by our board of directors or any restrictions we may place in any applicable placement notice, there is no minimum or maximum sales price for shares to be sold in this offering. Investors may experience a decline in the value of the shares they purchase in this offering as a result of sales made at prices lower than the prices they paid.
Sales of a substantial number of shares of our common stock, or the perception that such sales may occur, may adversely impact the price of our common stock.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception that such sales may occur, may adversely impact the price of our common stock, even if there is no relationship between such sales and the performance of our business. As of December 31, 2023, we had 27,025,209 shares of common stock outstanding, as well as outstanding options to purchase an aggregate of 729,500 shares of our common stock at a weighted average exercise price of $36.48 per share, and outstanding warrants to purchase up to an aggregate of 24,182,034 shares of our common stock at a weighted average exercise price of $6.24 per share. The exercise and/or conversion of such outstanding derivative securities may result in further dilution of your investment.
Our management will have broad discretion in the use of the net proceeds from this offering and may allocate such net proceeds in ways that you and other stockholders may not approve.
Our management will have broad discretion in the use of the net proceeds from this offering, including for any of the purposes described in the section titled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure of our management to use these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government. These investments may not yield a favorable return to our stockholders.
The actual number of shares we will issue under the Sales Agreement with Jefferies, at any one time or in total, is uncertain.
Subject to certain limitations in the Sales Agreement with Jefferies and compliance with applicable law, we have the discretion to deliver placement notices to Jefferies at any time throughout the term of the Sales Agreement. The number of shares that are sold by Jefferies after delivering a placement notice will fluctuate based on the market price of the common stock during the sales period and limits we set with Jefferies.
Because we have no current plans to pay cash dividends on our common stock for the foreseeable future, you may not receive any return on investment unless you sell your common stock for a price greater than that which you paid for it.
We intend to retain future earnings, if any, for future operations and expansion of our business and have no current plans to pay any cash dividends for the foreseeable future. The declaration, amount and payment of any future dividends on shares of common stock will be at the sole discretion of our Board of Directors. Our Board of Directors may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions, implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as our Board of Directors may deem relevant. In addition, our ability to pay dividends may be limited by covenants in connection with any indebtedness we or our subsidiaries may incur. As a result, you may not receive any return on an investment in our common stock unless you sell our common stock for a price greater than that which you paid for it.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. We currently have research coverage by four securities and industry analysts. If one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
We may be subject to securities litigation, class action and derivative lawsuits, which could result in substantial costs and could divert management attention away from other business concerns.
The market price of our common stock may be volatile and, in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Even if the lawsuits are without merit, defending against these claims can result in substantial costs and divert management time and resources from other business concerns, which could seriously harm our business. An adverse judgment could result in monetary damages, which could have a negative impact on our liquidity and financial condition.
The market price of our common stock may be adversely affected by market conditions affecting the stock markets in general, including price and trading fluctuations on Nasdaq.
Market conditions may result in volatility in the level of, and fluctuations in, market prices of stocks generally and, in turn, our common stock and sales of substantial amounts of our common stock in the market, in each case being unrelated or disproportionate to changes in our operating performance. A weak global economy or other circumstances, such as changes in tariffs and trade, could also contribute to extreme volatility of the markets, which may have an effect on the market price of our common stock.
USE OF PROCEEDS
We may issue and sell shares of our common stock having aggregate sales proceeds of up to $100,000,000 from time to time. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will sell any shares under or fully utilize our Sales Agreement with Jefferies.
We intend to use the net proceeds from this offering to advance the clinical development of our product candidates and for working capital and other general corporate purposes. Pending other uses, we intend to invest our proceeds from this offering in short-term investments or hold them as cash. We cannot predict whether the proceeds invested will yield a favorable return. Our management will have broad discretion in the use of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds.
DILUTION
Purchasers of our common offered by this prospectus supplement and the accompanying prospectus will suffer immediate and substantial dilution in the net tangible book value per share of common stock. Net tangible book value is total assets minus the sum of liabilities and intangible assets. Net tangible book value per share is net tangible book value divided by the total number of shares of common stock outstanding. As of December 31, 2023, our net tangible book value was approximately $122.7 million, or approximately $4.54 per share.
After giving effect to the assumed sale of 20,408,163 shares of common stock in the aggregate amount of $100,000,000 at an assumed public offering price of $4.90 per share, the last reported price of our common stock on The Nasdaq Capital Market on February 9, 2024, after deducting the underwriting discount and commissions, and estimated offering expenses payable by us, our pro forma net tangible book value as of December 31, 2023 would have been approximately $220.0 million or approximately $4.64 per share. This amount represents an immediate increase in net tangible book value of approximately $0.10 per share to existing stockholders and an immediate dilution in net tangible book value of approximately $0.26 per share to purchasers of our common stock in this offering.
The following table illustrates the dilution in net tangible book value per share to new investors:
|
Assumed Public offering price per share
|
|
|
|
|
|
$ |
4.90 |
|
|
Net tangible book value per share as of December 31, 2023
|
|
$ |
4.54 |
|
|
|
|
|
|
Increase in pro forma, net tangible book value per share after this offering
|
|
$ |
0.10 |
|
|
|
|
|
|
Pro forma net tangible book value per share after this offering
|
|
|
|
|
|
$ |
4.64 |
|
|
Dilution in pro forma net tangible book value per share to new investors in this offering
|
|
|
|
|
|
$ |
0.26 |
|
The foregoing discussion and table do not take into account further dilution to new investors that could occur upon the exercise of outstanding options or warrants having a per share exercise price less than the assumed public offering price in this offering. To the extent that we raise additional capital through the sale of equity or convertible debt securities after this offering, the issuance of those securities could result in further dilution to our stockholders.
The above discussion and table are based on 27,025,209 shares of common stock outstanding as of December 31, 2023 and excludes the following securities:
| |
●
|
24,182,034 shares of common stock that have been reserved for issuance upon exercise of outstanding warrants, with a weighted average exercise price of $6.24 per share;
|
| |
●
|
729,500 shares of common stock reserved for issuance upon exercise of outstanding stock options under our 2019 Omnibus Equity Incentive Plan, as amended (the “2019 Plan”), with a weighted average exercise price of $36.48 per share;
|
| |
●
|
552,295 shares of common stock reserved for future issuance in connection with future grants under the 2019 Plan; and |
| |
●
|
19,480 shares of common stock reserved for future issuance in connection with our 2019 Employee Stock Purchase Plan (the “ESPP”). |
PLAN OF DISTRIBUTION
We have previously entered into the Sales Agreement with Jefferies, under which we may offer and sell shares of common stock from time to time through Jefferies acting as agent. Pursuant to this prospectus supplement, we may offer and sell up to $100,000,000 of our shares of common stock. Sales of our shares of common stock, if any, under this prospectus supplement and the accompanying base prospectus will be made by any method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4) under the Securities Act.
Each time we wish to issue and sell shares of common stock under the Sales Agreement, we will notify Jefferies of the number of shares to be issued, the dates on which such sales are anticipated to be made, any limitation on the number of shares to be sold in any one day and any minimum price below which sales may not be made. Once we have so instructed Jefferies, unless Jefferies declines to accept the terms of such notice, Jefferies has agreed to use its commercially reasonable efforts consistent with its normal trading and sales practices to sell such shares up to the amount specified on such terms. The obligations of Jefferies under the Sales Agreement to sell our shares of common stock are subject to a number of conditions that we must meet.
The settlement of sales of shares between us and Jefferies is generally anticipated to occur on the second trading day following the date on which the sale was made. Sales of our shares of common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and Jefferies may agree upon. Pursuant to recent amendments to Rule 15c6-1 of the Exchange Act, settlement for any securities offered under this prospectus supplement on or after May 28, 2024, may occur on the first business day that is also a trading day following the date on which any sales were made in return for payment of the net proceeds to us. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
We will pay Jefferies a commission up to 3.0% of the aggregate gross proceeds we receive from each sale of our shares of common stock. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. In addition, we have agreed to reimburse Jefferies for the fees and disbursements of its counsel in an amount not to exceed $75,000, in addition to certain ongoing disbursements of its legal counsel, unless we and Jefferies otherwise agree. We estimate that the total expenses for the offering, excluding any commissions or expense reimbursement payable to Jefferies under the terms of the Sales Agreement, will be approximately $215,000. The remaining sale proceeds, after deducting any other transaction fees, will equal our net proceeds from the sale of such shares.
Jefferies will provide written confirmation to us before the open on The Nasdaq Capital Market on the day following each day on which shares of common stock are sold under the sales agreement. Each confirmation will include the number of shares sold on that day, the aggregate gross proceeds of such sales and the proceeds to us.
In connection with the sale of the shares of common stock on our behalf, Jefferies will be deemed to be an “underwriter” within the meaning of the Securities Act, and the compensation of Jefferies will be deemed to be underwriting commissions or discounts. We have agreed to indemnify Jefferies against certain civil liabilities, including liabilities under the Securities Act. We have also agreed to contribute to payments Jefferies may be required to make in respect of such liabilities.
The offering of our shares of common stock pursuant to the Sales Agreement will terminate as permitted therein.
This summary of the material provisions of the Sales Agreement does not purport to be a complete statement of its terms and conditions. A copy of the Sales Agreement is filed as an exhibit to the registration statement of which this prospectus supplement forms a part.
Jefferies and its affiliates may in the future provide various investment banking, commercial banking, financial advisory and other financial services for us and our affiliates, for which services they may in the future receive customary fees. In the course of its business, Jefferies may actively trade our securities for its own account or for the accounts of customers, and, accordingly, Jefferies may at any time hold long or short positions in such securities.
A prospectus supplement and the accompanying base prospectus in electronic format may be made available on a website maintained by Jefferies, and Jefferies may distribute the prospectus supplement and the accompanying prospectus electronically.
LEGAL MATTERS
Certain legal matters in connection with this offering will be passed upon for us by Latham & Watkins LLP, Chicago, Illinois. The validity of the securities offered hereby will be passed upon for us by Woodburn and Wedge, Reno, Nevada. Jefferies LLC is being represented in connection with this offering by Cooley LLP, New York, New York.
EXPERTS
WithumSmith+Brown, PC (“Withum”) our independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended March 31, 2023, as set forth in their report, which is incorporated by reference in this prospectus. The report for Vistagen Therapeutics, Inc. includes an explanatory paragraph about the existence of substantial doubt concerning its ability to continue as a going concern. Our financial statements are incorporated by reference in reliance on Withum’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities covered by this prospectus supplement. This prospectus supplement, which is a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the securities covered by this prospectus, please see the registration statement and the exhibits filed with the registration statement. The SEC maintains an Internet website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the website is www.sec.gov.
We are subject to the information and periodic reporting requirements of the Exchange Act and, in accordance therewith, we file periodic reports, proxy statements and other information with the SEC. Such periodic reports, proxy statements and other information are available free of charge at our website, www.vistagen.com, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. Our website and the information contained on that site, or connected to that site, are not incorporated into and are not a part of this prospectus supplement.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The following documents filed by us with the SEC are incorporated by reference in this prospectus supplement:
| |
●
|
our Annual Report on Form 10-K for the year ended March 31, 2023, filed on June 28, 2023;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended June 30, 2023, filed on August 10, 2022;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended September 30, 2023, filed on November 9, 2023;
|
| |
●
|
our Quarterly Report on Form 10-Q for the period ended December 31, 2023, filed on February 13, 2024;
|
| |
●
|
our Definitive Proxy Statement on Schedule 14A, filed on July 28, 2023;
|
| |
●
|
our Current Reports on Form 8-K, filed on April 6, 2023, April 19, 2023, June 1, 2023, June 6, 2023, June 7, 2023, June 13, 2023, June 21, 2023, June 23, 2023, July 7, 2023, July 13, 2023, July 18, 2023, August 22, 2023, September 8, 2023, September 13, 2023, September 29, 2023, October 2, 2023, October 4, 2023 (excluding any information furnished in such reports under Item 7.01), October 26, 2023, November 6, 2023(as amended on November 7, 2023), and January 3, 2024; and
|
| |
●
|
The description of our common stock contained in the Registration Statement on Form 8-A filed pursuant to Section 12(b) of the Securities Exchange Act of 1934, as amended (the Securities Act) on May 3, 2016, including any amendment or report filed with the Commission for the purpose of updating this description.
|
We also incorporate by reference all documents we file pursuant to Section 13(a), 13(c), 14 or 15 of the Exchange Act (other than any portions of filings that are furnished rather than filed pursuant to Items 2.02 and 7.01 of a Current Report on Form 8-K) after the date of the initial registration statement of which this prospectus is a part and prior to effectiveness of such registration statement. All documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the termination of the offering are also incorporated herein by reference and are an important part of this prospectus.
Any statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the purposes of this registration statement to the extent that a statement contained herein or in any other subsequently filed document which also is or deemed to be incorporated by reference herein modifies or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this registration statement.
We will provide upon request to each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in the prospectus but not delivered with the prospectus. You may request a copy of these filings, excluding the exhibits to such filings which we have not specifically incorporated by reference in such filings, at no cost, by writing to or calling us at:
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
(650) 577-3600
This prospectus is part of a registration statement we filed with the SEC. You should only rely on the information or representations contained in this prospectus and any accompanying prospectus supplement. We have not authorized anyone to provide information other than that provided in this prospectus and any accompanying prospectus supplement. We are not making an offer of the securities in any state where the offer is not permitted. You should not assume that the information in this prospectus or any accompanying prospectus supplement is accurate as of any date other than the date on the front of the document.
Up to $100,000,000
Common Stock
PROSPECTUS SUPPLEMENT
Jefferies
, 2024
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 14. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth an estimate of the fees and expenses, other than the underwriting discounts and commissions, payable by us in connection with the issuance and distribution of the securities being registered.
| |
|
Amount
|
|
|
SEC registration fee
|
|
$ |
39,857 |
|
|
Legal fees and expenses
|
|
|
(1)
|
|
|
Accounting fees and expenses
|
|
|
(1)
|
|
| Blue Sky, qualification fees and expenses |
|
|
(1) |
|
| Transfer agent fees and expenses |
|
|
(1) |
|
| Trustee fees and expenses |
|
|
(1) |
|
| Warrant agent fees and expenses |
|
|
(1) |
|
|
Printing and miscellaneous fees and expenses
|
|
|
(1)
|
|
|
Total
|
|
|
(1)
|
|
| |
(1)
|
These fees are calculated based on the securities offered and the number of issuances and accordingly cannot be estimated at this time.
|
ITEM 15. INDEMNIFICATION OF OFFICERS AND DIRECTORS
Section 78.7502 of the NRS permits a corporation to indemnify any person who was, is or is threatened to be made a party in a completed, pending or threatened proceeding, whether civil, criminal, administrative or investigative (except an action by or in the right of the corporation), by reason of being or having been an officer, director, employee or agent of the corporation or serving in certain capacities at the request of the corporation. Indemnification may include attorneys' fees, judgments, fines and amounts paid in settlement. The person to be indemnified must have acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action, such person must have had no reasonable cause to believe his conduct was unlawful.
With respect to actions by or in the right of the corporation, indemnification may not be made for any claim, issue or matter as to which such a person has been finally adjudged by a court of competent jurisdiction to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action was brought or other court of competent jurisdiction determines upon application that in view of all circumstances the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
Unless indemnification is ordered by a court, the determination to pay indemnification must be made by the stockholders, by a majority vote of a quorum of the Board of Directors who were not parties to the action, suit or proceeding, or in certain circumstances by independent legal counsel in a written opinion. Section 78.751 of the NRS permits the articles of incorporation or bylaws to provide for payment to an indemnified person of the expenses of defending an action as incurred upon receipt of an undertaking to repay the amount if it is ultimately determined by a court of competent jurisdiction that the person is not entitled to indemnification.
Section 78.7502 also provides that to the extent a director, officer, employee or agent has been successful on the merits or otherwise in the defense of any such action, he must be indemnified by the corporation against expenses, including attorneys' fees, actually and reasonably incurred in connection with the defense.
Article X of our Charter, entitled “Indemnification,” provides that we shall indemnify, and shall advance or reimburse the reasonable expenses incurred in advance of final disposition of the proceeding of, any individual made a party to a proceeding because that individual is or was a director of the corporation to the full extent and under all circumstances permitted by applicable law. Article X of our Bylaws, entitled “Indemnification,” provides that we shall indemnify our directors and officers to the fullest extent not prohibited by the NRS, except in the case of proceedings initiated by such officers or directors unless certain other factors are present.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and certain employees pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable.
There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification.
ITEM 16. EXHIBITS
|
1.1*
|
Form of Underwriting Agreement
|
|
1.2*
|
Form of Placement Agent Agreement
|
|
1.3
|
Open Market Sale AgreementSM, dated May 14, 2021, by and between Vistagen Therapeutics, Inc. and Jefferies LLC, incorporated by reference from Exhibit 1.1 to the Company’s Current Report on Form 8-K, filed on May 14, 2021.
|
|
3.1
|
Restated Articles of Incorporation of Vistagen Therapeutics, Inc., dated August 16, 2016, incorporated by reference from Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed on August 17, 2016
|
|
3.2
|
Second Amended and Restated Bylaws of Vistagen Therapeutics, Inc., dated August 16, 2016, incorporated by reference from Exhibit 3.2 to the Company’s Current Report on Form 8-K, filed on August 17, 2016
|
|
4.1*
|
Form of specimen certificate representing preferred stock
|
|
4.2
|
Form of indenture
|
|
4.3*
|
Form of debt security
|
|
4.4*
|
Form of warrant
|
|
4.5*
|
Form of warrant agreement
|
|
4.6*
|
Form of unit agreement
|
|
5.1
|
Opinion of Woodburn and Wedge
|
|
23.1
|
Consent of Woodburn and Wedge (included in Exhibit 5.1)
|
|
23.2
|
Consent of Independent Registered Public Accounting Firm – WithumSmith+Brown, PC
|
|
24
|
Power of Attorney (located on signature page)
|
|
25.1**
|
Statement of Eligibility on Form T-1 under the Trust Indenture Act of 1939, as amended, of Debt Trustee (to be filed prior to any issuance of debt securities)
|
|
107
|
Filing Fees Exhibit
|
|
*
|
To be filed, if necessary, by an amendment to this registration statement or incorporation by reference pursuant to a Current Report on Form 8-K in connection with an offering of securities.
|
|
**
|
To be filed in accordance with the requirements of Section 305(b)(2) of the Trust Indenture Act of 1939.
|
ITEM 17. UNDERTAKINGS
(a) The undersigned Registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
provided, however, that paragraphs (i), (ii) and (iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 or Section 15(d) of the Exchange Act of 1934 that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(5) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i) Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and
(ii) Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required by section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
(6) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) That, for purposes of determining any liability under the Securities Act, each filing of the Registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(h) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of each Registrant pursuant to the foregoing provisions, or otherwise, each Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by a Registrant of expenses incurred or paid by a director, officer or controlling person of a Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, that Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(j) The undersigned registrant hereby undertakes to file an application for the purpose of determining the eligibility of the trustee to act under subsection (a) of Section 310 of the Trust Indenture Act (the “Act”) in accordance with the rules and regulations prescribed by the SEC under section 305(b)(2) of the Act.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of South San Francisco, California, on February 13, 2024.
| |
Vistagen Therapeutics, Inc.
|
| |
|
|
| |
By:
|
/s/ Shawn K. Singh
|
|
| |
|
Shawn K. Singh, J.D.
|
| |
|
Chief Executive Officer
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature below constitutes and appoints Shawn K. Singh as attorney-in-fact, with power of substitution, for him or her in any and all capacities, to sign any amendments, including post-effective amendments, to this Registration Statement on Form S-3 and any other registration statement for the same offering that is to be effective under Rule 462(b) of the Securities Act of 1933, and file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
| |
|
|
|
/s/ Shawn K. Singh
Shawn K. Singh
|
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
|
February 13, 2024 |
| |
|
|
|
/s/ Cynthia L. Anderson
Cynthia L. Anderson
|
|
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
|
February 13, 2024 |
| |
|
|
|
/s/ Margaret M. FitzPatrick
Margaret M. FitzPatrick
|
|
Chair of the Board of Directors
|
|
February 13, 2024 |
| |
|
|
|
|
|
/s/ Jerry B. Gin
Jerry B. Gin
|
|
Director
|
|
February 13, 2024 |
| |
|
|
|
|
|
/s/ Ann M. Cunningham
Ann M. Cunningham
|
|
Director
|
|
February 13, 2024 |
| |
|
|
|
|
|
/s/ Joanne Curley
Joanne Curley
|
|
Director
|
|
February 13, 2024 |
| |
|
|
|
|
|
/s/ Mary L. Rotunno
Mary L. Rotunno
|
|
Director
|
|
February 13, 2024 |
| |
|
|
|
|
|
/s/ Jon S. Saxe
Jon S. Saxe
|
|
Director
|
|
February 13, 2024 |
Exhibit 4.2
VISTAGEN THERAPEUTICS, INC.
INDENTURE
Dated as of ___________, 20___
[TRUSTEE]
Trustee
TABLE OF CONTENTS
Page
|
ARTICLE I. DEFINITIONS AND INCORPORATION BY REFERENCE
|
1 |
|
Section 1.1.
|
Definitions.
|
1
|
|
Section 1.2.
|
Other Definitions.
|
4
|
|
Section 1.3.
|
Incorporation by Reference of Trust Indenture Act.
|
4
|
|
Section 1.4.
|
Rules of Construction.
|
5
|
| |
|
|
ARTICLE II. THE SECURITIES
|
5 |
|
Section 2.1.
|
Issuable in Series.
|
5
|
|
Section 2.2.
|
Establishment of Terms of Series of Securities.
|
6
|
|
Section 2.3.
|
Execution and Authentication.
|
8
|
|
Section 2.4.
|
Registrar, Paying Agent and Notice Agent.
|
9
|
|
Section 2.5.
|
Paying Agent to Hold Money in Trust.
|
10
|
|
Section 2.6.
|
Holder Lists.
|
10
|
|
Section 2.7.
|
Transfer and Exchange.
|
11
|
|
Section 2.8.
|
Mutilated, Destroyed, Lost and Stolen Securities.
|
11
|
|
Section 2.9.
|
Outstanding Securities.
|
12
|
|
Section 2.10.
|
Treasury Securities.
|
12
|
|
Section 2.11.
|
Temporary Securities.
|
12
|
|
Section 2.12.
|
Cancellation.
|
13
|
|
Section 2.13.
|
Defaulted Interest.
|
13
|
|
Section 2.14.
|
Global Securities.
|
13
|
|
Section 2.15.
|
CUSIP Numbers.
|
15
|
| |
|
|
ARTICLE III. REDEMPTION
|
15 |
|
Section 3.1.
|
Notice to Trustee.
|
15
|
|
Section 3.2.
|
Selection of Securities to be Redeemed.
|
16
|
|
Section 3.3.
|
Notice of Redemption.
|
16
|
|
Section 3.4.
|
Effect of Notice of Redemption.
|
17
|
|
Section 3.5.
|
Deposit of Redemption Price.
|
17
|
|
Section 3.6.
|
Securities Redeemed in Part.
|
17
|
| |
|
|
ARTICLE IV. COVENANTS
|
17 |
|
Section 4.1.
|
Payment of Principal and Interest.
|
17
|
|
Section 4.2.
|
SEC Reports.
|
18
|
|
Section 4.3.
|
Compliance Certificate.
|
18
|
|
Section 4.4.
|
Stay, Extension and Usury Laws.
|
18
|
| |
|
|
ARTICLE V. SUCCESSORS
|
19 |
|
Section 5.1.
|
When Company May Merge, Etc.
|
19
|
|
Section 5.2.
|
Successor Corporation Substituted.
|
19
|
| |
|
|
ARTICLE VI. DEFAULTS AND REMEDIES
|
20 |
|
Section 6.1.
|
Events of Default.
|
20
|
|
Section 6.2.
|
Acceleration of Maturity; Rescission and Annulment.
|
21
|
|
Section 6.3.
|
Collection of Indebtedness and Suits for Enforcement by Trustee.
|
21
|
|
Section 6.4.
|
Trustee May File Proofs of Claim.
|
22
|
|
Section 6.5.
|
Trustee May Enforce Claims Without Possession of Securities.
|
23
|
|
Section 6.6.
|
Application of Money Collected.
|
23
|
|
Section 6.7.
|
Limitation on Suits.
|
23
|
|
Section 6.8.
|
Unconditional Right of Holders to Receive Principal and Interest.
|
24
|
|
Section 6.9.
|
Restoration of Rights and Remedies.
|
24
|
|
Section 6.10.
|
Rights and Remedies Cumulative.
|
24
|
|
Section 6.11.
|
Delay or Omission Not Waiver.
|
25
|
|
Section 6.12.
|
Control by Holders.
|
25
|
|
Section 6.13.
|
Waiver of Past Defaults.
|
25
|
|
Section 6.14.
|
Undertaking for Costs.
|
26
|
| |
|
|
ARTICLE VII. TRUSTEE
|
26 |
|
Section 7.1.
|
Duties of Trustee.
|
26
|
|
Section 7.2.
|
Rights of Trustee.
|
27
|
|
Section 7.3.
|
Individual Rights of Trustee.
|
28
|
|
Section 7.4.
|
Trustee’s Disclaimer.
|
29
|
|
Section 7.5.
|
Notice of Defaults.
|
29
|
|
Section 7.6.
|
Reports by Trustee to Holders.
|
29
|
|
Section 7.7.
|
Compensation and Indemnity.
|
29
|
|
Section 7.8.
|
Replacement of Trustee.
|
30
|
|
Section 7.9.
|
Successor Trustee by Merger, Etc.
|
31
|
|
Section 7.10.
|
Eligibility; Disqualification.
|
31
|
|
Section 7.11.
|
Preferential Collection of Claims Against Company.
|
31
|
| |
|
|
ARTICLE VIII. SATISFACTION AND DISCHARGE; DEFEASANCE
|
32 |
|
Section 8.1.
|
Satisfaction and Discharge of Indenture.
|
32
|
|
Section 8.2.
|
Application of Trust Funds; Indemnification.
|
33
|
|
Section 8.3.
|
Legal Defeasance of Securities of any Series.
|
33
|
|
Section 8.4.
|
Covenant Defeasance.
|
35
|
|
Section 8.5.
|
Repayment to Company.
|
36
|
|
Section 8.6.
|
Reinstatement.
|
36
|
| |
|
|
|
ARTICLE IX. AMENDMENTS AND WAIVERS
|
36 |
|
Section 9.1.
|
Without Consent of Holders.
|
36
|
|
Section 9.2.
|
With Consent of Holders.
|
37
|
|
Section 9.3.
|
Limitations.
|
38
|
|
Section 9.4.
|
Compliance with Trust Indenture Act.
|
38
|
|
Section 9.5.
|
Revocation and Effect of Consents.
|
38
|
|
Section 9.6.
|
Notation on or Exchange of Securities.
|
39
|
|
Section 9.7.
|
Trustee Protected.
|
39
|
| |
|
|
|
ARTICLE X. MISCELLANEOUS
|
39 |
|
Section 10.1.
|
Trust Indenture Act Controls.
|
39
|
|
Section 10.2.
|
Notices.
|
40
|
|
Section 10.3.
|
Communication by Holders with Other Holders.
|
41
|
|
Section 10.4.
|
Certificate and Opinion as to Conditions Precedent.
|
41
|
|
Section 10.5.
|
Statements Required in Certificate or Opinion.
|
41
|
|
Section 10.6.
|
Rules by Trustee and Agents.
|
42
|
|
Section 10.7.
|
Legal Holidays.
|
42
|
|
Section 10.8.
|
No Recourse Against Others.
|
42
|
|
Section 10.9.
|
Counterparts.
|
42
|
|
Section 10.10.
|
Governing Law; Waiver of Jury Trial; Consent to Jurisdiction.
|
43
|
|
Section 10.11.
|
No Adverse Interpretation of Other Agreements.
|
43
|
|
Section 10.12.
|
Successors.
|
43
|
|
Section 10.13.
|
Severability.
|
43
|
|
Section 10.14.
|
Table of Contents, Headings, Etc.
|
43
|
|
Section 10.15.
|
Securities in a Foreign Currency.
|
44
|
|
Section 10.16.
|
Judgment Currency.
|
44
|
|
Section 10.17.
|
Force Majeure.
|
45
|
|
Section 10.18.
|
U.S.A. Patriot Act.
|
45
|
| |
|
|
ARTICLE XI. SINKING FUNDS
|
45 |
|
Section 11.1.
|
Applicability of Article.
|
45
|
|
Section 11.2.
|
Satisfaction of Sinking Fund Payments with Securities.
|
46
|
|
Section 11.3.
|
Redemption of Securities for Sinking Fund.
|
46
|
VISTAGEN THERAPEUTICS, INC.
Reconciliation and tie between Trust Indenture Act of 1939 and
Indenture, dated as of ____________, 20__
| § 310(a)(1) |
|
7.10 |
|
(a)(2)
|
|
7.10
|
|
(a)(3)
|
|
Not Applicable
|
|
(a)(4)
|
|
Not Applicable
|
|
(a)(5)
|
|
7.10
|
|
(b)
|
|
7.10
|
|
§ 311(a)
|
|
7.11
|
|
(b)
|
|
7.11
|
|
(c)
|
|
Not Applicable
|
|
§ 312(a)
|
|
2.6
|
|
(b)
|
|
10.3
|
|
(c)
|
|
10.3
|
|
§ 313(a)
|
|
7.6
|
|
(b)(1)
|
|
7.6
|
|
(b)(2)
|
|
7.6
|
|
(c)(1)
|
|
7.6
|
|
(d)
|
|
7.6
|
|
§ 314(a)
|
|
4.2, 10.5
|
|
(b)
|
|
Not Applicable
|
|
(c)(1)
|
|
10.4
|
|
(c)(2)
|
|
10.4
|
|
(c)(3)
|
|
Not Applicable
|
|
(d)
|
|
Not Applicable
|
|
(e)
|
|
10.5
|
|
(f)
|
|
Not Applicable
|
|
§ 315(a)
|
|
7.1
|
|
(b)
|
|
7.5
|
|
(c)
|
|
7.1
|
|
(d)
|
|
7.1
|
|
(e)
|
|
6.14
|
|
§ 316(a)
|
|
2.10
|
|
(a)(1)(A)
|
|
6.12
|
|
(a)(1)(B)
|
|
6.13
|
|
(b)
|
|
6.8
|
|
§ 317(a)(1)
|
|
6.3
|
|
(a)(2)
|
|
6.4
|
|
(b)
|
|
2.5
|
|
§ 318(a)
|
|
10.1
|
Note: This reconciliation and tie shall not, for any purpose, be deemed to be part of the Indenture.
Indenture dated as of __________, 20__ between Vistagen Therapeutics, Inc., a company incorporated under the laws of the State of Nevada (“Company”), and [TRUSTEE] (“Trustee”).
Each party agrees as follows for the benefit of the other party and for the equal and ratable benefit of the Holders of the Securities issued under this Indenture.
ARTICLE I.
DEFINITIONS AND INCORPORATION BY REFERENCE
Section 1.1. Definitions.
“Affiliate” of any specified person means any other person directly or indirectly controlling or controlled by or under common control with such specified person. For the purposes of this definition, “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with respect to any person, shall mean the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such person, whether through the ownership of voting securities or by agreement or otherwise.
“Agent” means any Registrar, Paying Agent or Notice Agent.
“Board of Directors” means the board of directors of the Company or any duly authorized committee thereof.
“Board Resolution” means a copy of a resolution certified by the Secretary or an Assistant Secretary of the Company to have been adopted by the Board of Directors or pursuant to authorization by the Board of Directors and to be in full force and effect on the date of the certificate and delivered to the Trustee.
“Business Day” means any day except a Saturday, Sunday or a legal holiday in the City of New York, New York (or in connection with any payment, the place of payment) on which banking institutions are authorized or required by law, regulation or executive order to close.
“Capital Stock” means any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock.
“Company” means the party named as such above until a successor replaces it and thereafter means the successor.
“Company Order” means a written order signed in the name of the Company by an Officer and delivered to the Trustee.
“Corporate Trust Office” means the office of the Trustee at which at any particular time its corporate trust business related to this Indenture shall be principally administered.
“Default” means any event which is, or after notice, passage of time or both would be, an Event of Default.
“Depositary” means, with respect to the Securities of any Series issuable or issued in whole or in part in the form of one or more Global Securities, the person designated as Depositary for such Series by the Company, which Depositary shall be a clearing agency registered under the Exchange Act; and if at any time there is more than one such person, “Depositary” as used with respect to the Securities of any Series shall mean the Depositary with respect to the Securities of such Series.
“Discount Security” means any Security that provides for an amount less than the stated principal amount thereof to be due and payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2.
“Dollars” and “$” means the currency of the United States of America.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Foreign Currency” means any currency or currency unit issued by a government other than the government of the United States of America.
“Foreign Government Obligations” means, with respect to Securities of any Series that are denominated in a Foreign Currency, direct obligations of, or obligations guaranteed by, the government that issued or caused to be issued such currency for the payment of which obligations its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof.
“GAAP” means generally accepted accounting principles in the United States of America set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as have been approved by a significant segment of the accounting profession, which are in effect as of the date of determination.
“Global Security” or “Global Securities” means a Security or Securities, as the case may be, in the form established pursuant to Section 2.2 evidencing all or part of a Series of Securities, issued to the Depositary for such Series or its nominee, and registered in the name of such Depositary or nominee.
“Holder” means a person in whose name a Security is registered on the Registrar’s books.
“Indenture” means this Indenture as amended or supplemented from time to time and shall include the form and terms of particular Series of Securities established as contemplated hereunder.
“interest” with respect to any Discount Security which by its terms bears interest only after Maturity, means interest payable after Maturity.
“Maturity” when used with respect to any Security, means the date on which the principal of such Security becomes due and payable as therein or herein provided, whether at the Stated Maturity or by declaration of acceleration, call for redemption or otherwise.
“Officer” means the Chief Executive Officer, President, the Chief Financial Officer, the Treasurer or any Assistant Treasurer, the Secretary or any Assistant Secretary and any Vice President of the Company.
“Officer’s Certificate” means a certificate signed by any Officer that meets the requirements of this Indenture.
“Opinion of Counsel” means a written opinion of legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Company. The opinion may contain customary limitations, conditions and exceptions.
“person” means any individual, corporation, partnership, joint venture, association, limited liability company, joint-stock company, trust, unincorporated organization or government or any agency or political subdivision thereof.
“principal” of a Security means the principal of the Security plus, when appropriate, the premium, if any, on, the Security.
“Responsible Officer” means any officer of the Trustee in its Corporate Trust Office having responsibility for administration of this Indenture and also means, with respect to a particular corporate trust matter, any other officer to whom any corporate trust matter is referred because of his or her knowledge of and familiarity with a particular subject.
“SEC” means the Securities and Exchange Commission.
“Security” or “Securities” means the debentures, notes or other debt instruments of the Company of any Series authenticated and delivered under this Indenture.
“Series” or “Series of Securities” means each series of debentures, notes or other debt instruments of the Company created pursuant to Sections 2.1 and 2.2 hereof.
“Stated Maturity” when used with respect to any Security, means the date specified in such Security as the fixed date on which the principal of such Security or interest is due and payable.
“Subsidiary” of any specified person means any corporation, association or other business entity of which more than 50% of the total voting power of shares of Capital Stock entitled (without regard to the occurrence of any contingency) to vote in the election of directors, managers or trustees thereof is at the time owned or controlled, directly or indirectly, by such person or one or more of the other Subsidiaries of that person or a combination thereof.
“TIA” means the Trust Indenture Act of 1939 (15 U.S. Code §§ 77aaa-77bbbb) as in effect on the date of this Indenture; provided, however, that in the event the Trust Indenture Act of 1939 is amended after such date, “TIA” means, to the extent required by any such amendment, the Trust Indenture Act as so amended.
“Trustee” means the person named as the “Trustee” in the first paragraph of this instrument until a successor Trustee shall have become such pursuant to the applicable provisions of this Indenture, and thereafter “Trustee” shall mean or include each person who is then a Trustee hereunder, and if at any time there is more than one such person, “Trustee” as used with respect to the Securities of any Series shall mean the Trustee with respect to Securities of that Series.
“U.S. Government Obligations” means securities which are direct obligations of, or guaranteed by, the United States of America for the payment of which its full faith and credit is pledged and which are not callable or redeemable at the option of the issuer thereof and shall also include a depositary receipt issued by a bank or trust company as custodian with respect to any such U.S. Government Obligation or a specific payment of interest on or principal of any such U.S. Government Obligation held by such custodian for the account of the holder of a depositary receipt, provided that (except as required by law) such custodian is not authorized to make any deduction from the amount payable to the holder of such depositary receipt from any amount received by the custodian in respect of the U.S. Government Obligation evidenced by such depositary receipt.
Section 1.2. Other Definitions.
|
TERM
|
DEFINED IN SECTION
|
| |
|
| |
|
|
“Agent Member”
|
2.14.6
|
|
“Bankruptcy Law”
|
6.1
|
|
“Custodian”
|
6.1
|
|
“Event of Default”
|
6.1
|
|
“Judgment Currency”
|
10.16
|
|
“mandatory sinking fund payment”
|
11.1
|
|
“New York Banking Day”
|
10.16
|
|
“Notice Agent”
|
2.4
|
|
“optional sinking fund payment”
|
11.1
|
|
“Paying Agent”
|
2.4
|
|
“Registrar”
|
2.4
|
|
“Required Currency”
|
10.16
|
|
“Specified Courts”
|
10.10
|
|
“successor person”
|
5.1
|
Section 1.3. Incorporation by Reference of Trust Indenture Act.
Whenever this Indenture refers to a provision of the TIA, the provision is incorporated by reference in and made a part of this Indenture. The following TIA terms used in this Indenture have the following meanings:
“Commission” means the SEC.
“indenture securities” means the Securities.
“indenture security holder” means a Holder.
“indenture to be qualified” means this Indenture.
“indenture trustee” or “institutional trustee” means the Trustee.
“obligor” on the indenture securities means the Company and any successor obligor upon the Securities.
All other terms used in this Indenture that are defined by the TIA, defined by TIA reference to another statute or defined by SEC rule under the TIA and not otherwise defined herein are used herein as so defined.
Section 1.4. Rules of Construction.
Unless the context otherwise requires:
(a) a term has the meaning assigned to it;
(b) an accounting term not otherwise defined has the meaning assigned to it in accordance with GAAP;
(c) "or” is not exclusive;
(d) words in the singular include the plural, and in the plural include the singular;
(e) provisions apply to successive events and transactions;
(f) in the computation of periods of time from a specified date to a later specified date, the word “from” means “from and including,” and the words “to” and “until” each mean “to but excluding”; and
(g) the phrase “in writing” as used herein shall be deemed to include PDFs, e-mails and other electronic means of transmission, unless otherwise indicated.
ARTICLE II.
THE SECURITIES
Section 2.1. Issuable in Series.
The aggregate principal amount of Securities that may be authenticated and delivered under this Indenture is unlimited. The Securities may be issued in one or more Series. All Securities of a Series shall be identical except as may be set forth or determined in the manner provided in a Board Resolution, a supplemental indenture or an Officer’s Certificate detailing the adoption of the terms thereof pursuant to authority granted under a Board Resolution. In the case of Securities of a Series to be issued from time to time, the Board Resolution, Officer’s Certificate or supplemental indenture detailing the adoption of the terms thereof pursuant to authority granted under a Board Resolution may provide for the method by which specified terms (such as interest rate, maturity date, record date or date from which interest shall accrue) are to be determined. Securities may differ between Series in respect of any matters, provided that all Series of Securities shall be equally and ratably entitled to the benefits of the Indenture.
Section 2.2. Establishment of Terms of Series of Securities.
At or prior to the issuance of any Securities within a Series, the following shall be established (as to the Series generally, in the case of Subsection 2.2.1 and either as to such Securities within the Series or as to the Series generally in the case of Subsections 2.2.2 through 2.2.23) by or pursuant to a Board Resolution, and set forth or determined in the manner provided in a Board Resolution, supplemental indenture hereto or Officer’s Certificate:
2.2.1. the title (which shall distinguish the Securities of that particular Series from the Securities of any other Series) and ranking (including the terms of any subordination provisions) of the Series;
2.2.2. the price or prices (expressed as a percentage of the principal amount thereof) at which the Securities of the Series will be issued;
2.2.3. any limit upon the aggregate principal amount of the Securities of the Series which may be authenticated and delivered under this Indenture (except for Securities authenticated and delivered upon registration of transfer of, or in exchange for, or in lieu of, other Securities of the Series pursuant to Section 2.7, 2.8, 2.11, 3.6 or 9.6);
2.2.4. the date or dates on which the principal of the Securities of the Series is payable;
2.2.5. the rate or rates (which may be fixed or variable) per annum or, if applicable, the method used to determine such rate or rates (including, but not limited to, any commodity, commodity index, stock exchange index or financial index) at which the Securities of the Series shall bear interest, if any, the date or dates from which such interest, if any, shall accrue, the date or dates on which such interest, if any, shall commence and be payable and any regular record date for the interest payable on any interest payment date;
2.2.6. the place or places where the principal of and interest, if any, on the Securities of the Series shall be payable, where the Securities of such Series may be surrendered for registration of transfer or exchange and where notices and demands to or upon the Company in respect of the Securities of such Series and this Indenture may be delivered, and the method of such payment, if by wire transfer, mail or other means;
2.2.7. if applicable, the period or periods within which, the price or prices at which and the terms and conditions upon which the Securities of the Series may be redeemed, in whole or in part, at the option of the Company;
2.2.8. the obligation, if any, of the Company to redeem or purchase the Securities of the Series pursuant to any sinking fund or analogous provisions or at the option of a Holder thereof and the period or periods within which, the price or prices at which and the terms and conditions upon which Securities of the Series shall be redeemed or purchased, in whole or in part, pursuant to such obligation;
2.2.9. the dates, if any, on which and the price or prices at which the Securities of the Series will be repurchased by the Company at the option of the Holders thereof and other detailed terms and provisions of such repurchase obligations;
2.2.10. if other than denominations of $1,000 and any integral multiple thereof, the denominations in which the Securities of the Series shall be issuable;
2.2.11. the forms of the Securities of the Series and whether the Securities will be issuable as Global Securities;
2.2.12. if other than the principal amount thereof, the portion of the principal amount of the Securities of the Series that shall be payable upon declaration of acceleration of the maturity thereof pursuant to Section 6.2;
2.2.13. the currency of denomination of the Securities of the Series, which may be Dollars or any Foreign Currency, and if such currency of denomination is a composite currency, the agency or organization, if any, responsible for overseeing such composite currency;
2.2.14. the designation of the currency, currencies or currency units in which payment of the principal of and interest, if any, on the Securities of the Series will be made;
2.2.15. if payments of principal of or interest, if any, on the Securities of the Series are to be made in one or more currencies or currency units other than that or those in which such Securities are denominated, the manner in which the exchange rate with respect to such payments will be determined;
2.2.16. the manner in which the amounts of payment of principal of or interest, if any, on the Securities of the Series will be determined, if such amounts may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index;
2.2.17. the provisions, if any, relating to any security provided for the Securities of the Series;
2.2.18. any addition to, deletion of or change in the Events of Default which applies to any Securities of the Series and any change in the right of the Trustee or the requisite Holders of such Securities to declare the principal amount thereof due and payable pursuant to Section 6.2;
2.2.19. any addition to, deletion of or change in the covenants applicable to Securities of the Series;
2.2.20. any Depositaries, interest rate calculation agents, exchange rate calculation agents or other agents with respect to Securities of such Series if other than those appointed herein;
2.2.21. the provisions, if any, relating to conversion or exchange of any Securities of such Series, including if applicable, the conversion or exchange price, the conversion or exchange period, provisions as to whether conversion or exchange will be mandatory, at the option of the Holders thereof or at the option of the Company, the events requiring an adjustment of the conversion price or exchange price and provisions affecting conversion or exchange if such Series of Securities are redeemed;
2.2.22. any other terms of the Series (which may supplement, modify or delete any provision of this Indenture insofar as it applies to such Series), including any terms that may be required under applicable law or regulations or advisable in connection with the marketing of Securities of that Series; and
2.2.23. whether any of the Company’s direct or indirect Subsidiaries will guarantee the Securities of that Series, including the terms of subordination, if any, of such guarantees.
All Securities of any one Series need not be issued at the same time and may be issued from time to time, consistent with the terms of this Indenture, if so provided by or pursuant to the Board Resolution, supplemental indenture hereto or Officer’s Certificate referred to above.
Section 2.3. Execution and Authentication.
An Officer shall sign the Securities for the Company by manual, facsimile or electronic signature.
If an Officer whose signature is on a Security no longer holds that office at the time the Security is authenticated, the Security shall nevertheless be valid.
A Security shall not be valid until authenticated by the manual signature of the Trustee or an authenticating agent. The signature shall be conclusive evidence that the Security has been authenticated under this Indenture.
The Trustee shall at any time, and from time to time, authenticate Securities for original issue in the principal amount provided in the Board Resolution, supplemental indenture hereto or Officer’s Certificate, upon receipt by the Trustee of a Company Order. Each Security shall be dated the date of its authentication.
The aggregate principal amount of Securities of any Series outstanding at any time may not exceed any limit upon the maximum principal amount for such Series set forth in the Board Resolution, supplemental indenture hereto or Officer’s Certificate delivered pursuant to Section 2.2, except as provided in Section 2.8.
Prior to the issuance of Securities of any Series, the Trustee shall have received and (subject to Section 7.2) shall be fully protected in relying on: (a) the Board Resolution, supplemental indenture hereto or Officer’s Certificate establishing the form of the Securities of that Series or of Securities within that Series and the terms of the Securities of that Series or of Securities within that Series, (b) an Officer’s Certificate complying with Sections 10.4 and 10.5, and (c) an Opinion of Counsel complying with Sections 10.4 and 10.5.
The Trustee shall have the right to decline to authenticate and deliver any Securities of such Series: (a) if the Trustee, being advised by counsel, determines that such action may not be taken lawfully; or (b) if the Trustee in good faith determines that such action may expose the Trustee to personal liability.
The Trustee may appoint an authenticating agent acceptable to the Company to authenticate Securities. An authenticating agent may authenticate Securities whenever the Trustee may do so. Each reference in this Indenture to authentication by the Trustee includes authentication by such agent. An authenticating agent has the same rights as an Agent to deal with the Company or an Affiliate of the Company.
Section 2.4. Registrar, Paying Agent and Notice Agent.
The Company shall maintain, with respect to each Series of Securities, at the place or places specified with respect to such Series pursuant to Section 2.2, an office or agency where Securities of such Series may be presented or surrendered for payment (“Paying Agent”), where Securities of such Series may be surrendered for registration of transfer or exchange (“Registrar”) and where notices and demands to or upon the Company in respect of the Securities of such Series and this Indenture may be delivered (“Notice Agent”). The Registrar shall keep a register with respect to each Series of Securities and to their transfer and exchange. The Company will give prompt written notice to the Trustee of the name and address, and any change in the name or address, of each Registrar, Paying Agent or Notice Agent. If at any time the Company shall fail to maintain any such required Registrar, Paying Agent or Notice Agent or shall fail to furnish the Trustee with the name and address thereof, such presentations, surrenders, notices and demands may be made or served at the Corporate Trust Office of the Trustee, and the Company hereby appoints the Trustee as its agent to receive all such presentations, surrenders, notices and demands; provided, however, that any appointment of the Trustee as the Notice Agent shall exclude the appointment of the Trustee or any office of the Trustee as an agent to receive the service of legal process on the Company.
The Company may also from time to time designate one or more co-registrars, additional paying agents or additional notice agents and may from time to time rescind such designations; provided, however, that no such designation or rescission shall in any manner relieve the Company of its obligations to maintain a Registrar, Paying Agent and Notice Agent in each place so specified pursuant to Section 2.2 for Securities of any Series for such purposes. The Company will give prompt written notice to the Trustee of any such designation or rescission and of any change in the name or address of any such co-registrar, additional paying agent or additional notice agent. The term “Registrar” includes any co-registrar; the term “Paying Agent” includes any additional paying agent; and the term “Notice Agent” includes any additional notice agent. The Company or any of its Affiliates may serve as Registrar or Paying Agent.
The Company hereby appoints the Trustee the initial Registrar, Paying Agent and Notice Agent for each Series unless another Registrar, Paying Agent or Notice Agent, as the case may be, is appointed prior to the time Securities of that Series are first issued. The rights, powers, duties, obligations and actions of each Agent under this Indenture are several and not joint or joint and several, and the Agents shall only be obliged to perform those duties expressly set out in this Indenture and shall have no implied duties.
Section 2.5. Paying Agent to Hold Money in Trust.
The Company shall require each Paying Agent other than the Trustee to agree in writing that the Paying Agent will hold in trust, for the benefit of Holders of any Series of Securities or the Trustee, all money held by the Paying Agent for the payment of principal of or interest on the Series of Securities and will notify the Trustee in writing of any default by the Company in making any such payment. While any such default continues, the Trustee may require a Paying Agent to pay all money held by it to the Trustee. The Company at any time may require a Paying Agent to pay all money held by it to the Trustee. Upon payment over to the Trustee, the Paying Agent (if other than the Company or a Subsidiary of the Company) shall have no further liability for the money. If the Company or a Subsidiary of the Company acts as Paying Agent, it shall segregate and hold in a separate trust fund for the benefit of Holders of any Series of Securities all money held by it as Paying Agent. Upon any bankruptcy, reorganization or similar proceeding with respect to the Company, the Trustee shall serve as Paying Agent for the Securities. For the avoidance of doubt, a Paying Agent and the Trustee shall be held harmless and have no liability with respect to payments or disbursements (including to the Holders) until they have confirmed receipt of funds sufficient to make the relevant payment. No money held by an Agent needs to be segregated except as is required by law.
Section 2.6. Holder Lists.
If it is serving as Registrar, the Trustee shall preserve in as current a form as is reasonably practicable the most recent list available to it of the names and addresses of Holders of each Series of Securities and shall otherwise comply with TIA § 312(a). If the Trustee is not the Registrar, the Company shall furnish to the Trustee at least ten days before each interest payment date and at such other times as the Trustee may request in writing a list, in such form and as of such date as the Trustee may reasonably require, of the names and addresses of Holders of each Series of Securities.
Every Holder, by receiving and holding Securities, agrees with the Company and the Trustee that neither the Company nor the Trustee or any agent of either of them shall be held accountable by reason of the disclosure of any such information as to the names and addresses of the Holders in accordance with TIA § 312, regardless of the source from which such information was derived, and that the Trustee shall not be held accountable by reason of mailing any material pursuant to a request made under TIA § 312(b).
Section 2.7. Transfer and Exchange.
Where Securities of a Series are presented to the Registrar or a co-registrar with a request to register a transfer or to exchange them for an equal principal amount of Securities of the same Series, the Registrar shall register the transfer or make the exchange if its requirements for such transactions are met. To permit registrations of transfers and exchanges, the Trustee shall authenticate Securities at the Registrar’s request. No service charge shall be made for any registration of transfer or exchange (except as otherwise expressly permitted herein), but the Company may require payment of a sum sufficient to cover any transfer tax or similar governmental charge payable in connection therewith (other than any such transfer tax or similar governmental charge payable upon exchanges pursuant to Sections 2.11, 3.6 or 9.6).
Neither the Company nor the Registrar shall be required (a) to issue, register the transfer of or exchange Securities of any Series for the period beginning at the opening of business 15 days immediately preceding the sending of a notice of redemption of Securities of that Series selected for redemption and ending at the close of business on the day such notice is sent, (b) to register the transfer of or exchange Securities of any Series selected, called or being called for redemption as a whole or the portion being redeemed of any such Securities selected, called or being called for redemption in part or (c) to register the transfer of or exchange Securities of any Series between a record date and payment date for such Series of Securities.
Section 2.8. Mutilated, Destroyed, Lost and Stolen Securities.
If any mutilated Security is surrendered to the Trustee, the Company shall execute and the Trustee shall authenticate and deliver in exchange therefor a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
If there shall be delivered to the Company and the Trustee (i) evidence to their satisfaction of the destruction, loss or theft of any Security and (ii) such security or indemnity bond as may be required by each of them to hold itself and any of its agents harmless, then, in the absence of notice to the Company or the Trustee that such Security has been acquired by a bona fide purchaser, the Company shall execute and upon receipt of a Company Order the Trustee shall authenticate and make available for delivery, in lieu of any such destroyed, lost or stolen Security, a new Security of the same Series and of like tenor and principal amount and bearing a number not contemporaneously outstanding.
In case any such mutilated, destroyed, lost or stolen Security has become or is about to become due and payable, the Company in its discretion may, instead of issuing a new Security, pay such Security.
Upon the issuance of any new Security under this Section, the Company may require the payment of a sum sufficient to cover any tax or other governmental charge that may be imposed in relation thereto and any other expenses (including the fees and expenses of the Trustee) connected therewith.
Every new Security of any Series issued pursuant to this Section in lieu of any destroyed, lost or stolen Security shall constitute an original additional contractual obligation of the Company, whether or not the destroyed, lost or stolen Security shall be at any time enforceable by anyone, and shall be entitled to all the benefits of this Indenture equally and proportionately with any and all other Securities of that Series duly issued hereunder.
The provisions of this Section are exclusive and shall preclude (to the extent lawful) all other rights and remedies with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities.
Section 2.9. Outstanding Securities.
The Securities outstanding at any time are all the Securities authenticated by the Trustee except for those canceled by it, those delivered to it for cancellation, those reductions in the interest on a Global Security effected by the Trustee in accordance with the provisions hereof and those described in this Section as not outstanding.
If a Security is replaced pursuant to Section 2.8, it ceases to be outstanding until the Trustee receives proof satisfactory to it that the replaced Security is held by a bona fide purchaser.
If the Paying Agent (other than the Company, a Subsidiary of the Company or an Affiliate of the Company) holds on the Maturity of Securities of a Series money sufficient to pay such Securities payable on that date, then on and after that date such Securities of the Series cease to be outstanding and interest on them ceases to accrue.
The Company may purchase or otherwise acquire the Securities, whether by open market purchases, negotiated transactions or otherwise. A Security does not cease to be outstanding because the Company or an Affiliate of the Company holds the Security (but see Section 2.10 below).
In determining whether the Holders of the requisite principal amount of outstanding Securities have given any request, demand, authorization, direction, notice, consent or waiver hereunder, the principal amount of a Discount Security that shall be deemed to be outstanding for such purposes shall be the amount of the principal thereof that would be due and payable as of the date of such determination upon a declaration of acceleration of the Maturity thereof pursuant to Section 6.2.
Section 2.10. Treasury Securities.
In determining whether the Holders of the required principal amount of Securities of a Series have concurred in any request, demand, authorization, direction, notice, consent or waiver, Securities of a Series owned by the Company or any Affiliate of the Company shall be disregarded, except that for the purposes of determining whether the Trustee shall be protected in relying on any such request, demand, authorization, direction, notice, consent or waiver only Securities of a Series that a Responsible Officer of the Trustee knows are so owned shall be so disregarded.
Section 2.11. Temporary Securities.
Until definitive Securities are ready for delivery, the Company may prepare and the Trustee shall authenticate temporary Securities upon a Company Order. Temporary Securities shall be substantially in the form of definitive Securities but may have variations that the Company considers appropriate for temporary Securities. Without unreasonable delay, the Company shall prepare and the Trustee upon receipt of a Company Order shall authenticate definitive Securities of the same Series and date of maturity in exchange for temporary Securities. Until so exchanged, temporary securities shall have the same rights under this Indenture as the definitive Securities.
Section 2.12. Cancellation.
The Company at any time may deliver Securities to the Trustee for cancellation. The Registrar and the Paying Agent shall forward to the Trustee any Securities surrendered to them for registration of transfer, exchange or payment. The Trustee shall cancel all Securities surrendered for transfer, exchange, payment, replacement or cancellation and shall destroy such canceled Securities (subject to the record retention requirements of the Exchange Act and the Trustee) and deliver a certificate of such cancellation to the Company upon written request of the Company. The Company may not issue new Securities to replace Securities that it has paid or delivered to the Trustee for cancellation.
Section 2.13. Defaulted Interest.
If the Company defaults in a payment of interest on a Series of Securities, it shall pay the defaulted interest, plus, to the extent permitted by law, any interest payable on the defaulted interest, to the persons who are Holders of the Series on a subsequent special record date. The Company shall fix the record date and payment date. At least ten days before the special record date, the Company shall send to the Trustee and to each Holder of the Series a notice that states the special record date, the payment date and the amount of interest to be paid. The Company may pay defaulted interest in any other lawful manner.
Section 2.14. Global Securities.
2.14.1. Terms of Securities. A Board Resolution, a supplemental indenture hereto or an Officer’s Certificate shall establish whether the Securities of a Series shall be issued in whole or in part in the form of one or more Global Securities and the Depositary for such Global Security or Securities.
2.14.2. Transfer and Exchange. Notwithstanding any provisions to the contrary contained in Section 2.7 of the Indenture and in addition thereto, any Global Security shall be exchangeable pursuant to Section 2.7 of the Indenture for Securities registered in the names of Holders other than the Depositary for such Security or its nominee only if (i) such Depositary notifies the Company that it is unwilling or unable to continue as Depositary for such Global Security or if at any time such Depositary ceases to be a clearing agency registered under the Exchange Act, and, in either case, the Company fails to appoint a successor Depositary registered as a clearing agency under the Exchange Act within 90 days of such event or (ii) the Company executes and delivers to the Trustee an Officer’s Certificate to the effect that such Global Security shall be so exchangeable. Any Global Security that is exchangeable pursuant to the preceding sentence shall be exchangeable for Securities registered in such names as the Depositary shall direct in writing in an aggregate principal amount equal to the principal amount of the Global Security with like tenor and terms.
Except as provided in this Section 2.14.2, a Global Security may not be transferred except as a whole by the Depositary with respect to such Global Security to a nominee of such Depositary, by a nominee of such Depositary to such Depositary or another nominee of such Depositary or by the Depositary or any such nominee to a successor Depositary or a nominee of such a successor Depositary.
None of the Trustee or any Agent shall have any obligation or duty to monitor, determine or inquire as to compliance with any restrictions on transfer imposed under this Indenture or under applicable law with respect to any transfer of any interest in any Security (including any transfers between or among Depositary participants, members or beneficial owners in any Global Security) other than to require delivery of such certificates and other documentation or evidence as are expressly required by, and to do so if and when expressly required by the terms of, this Indenture, and to examine the same to determine substantial compliance as to form with the express requirements hereof.
None of the Trustee or any Agent shall have any responsibility or obligation to any beneficial owner of a Global Security, a member of, or a participant in the Depositary or other Person with respect to the accuracy of the records of the Depositary or its nominee or of any participant or member thereof, with respect to any ownership interest in any Security or with respect to the delivery to any participant, member, beneficial owner or other Person (other than the Depositary) of any notice (including any notice of optional redemption) or the payment of any amount, under or with respect to such Security.
2.14.3. Legends. Any Global Security issued hereunder shall bear a legend in substantially the following form:
“THIS SECURITY IS A GLOBAL SECURITY WITHIN THE MEANING OF THE INDENTURE HEREINAFTER REFERRED TO AND IS REGISTERED IN THE NAME OF THE DEPOSITARY OR A NOMINEE OF THE DEPOSITARY. THIS SECURITY IS EXCHANGEABLE FOR SECURITIES REGISTERED IN THE NAME OF A PERSON OTHER THAN THE DEPOSITARY OR ITS NOMINEE ONLY IN THE LIMITED CIRCUMSTANCES DESCRIBED IN THE INDENTURE, AND MAY NOT BE TRANSFERRED EXCEPT AS A WHOLE BY THE DEPOSITARY TO A NOMINEE OF THE DEPOSITARY, BY A NOMINEE OF THE DEPOSITARY TO THE DEPOSITARY OR ANOTHER NOMINEE OF THE DEPOSITARY OR BY THE DEPOSITARY OR ANY SUCH NOMINEE TO A SUCCESSOR DEPOSITARY OR A NOMINEE OF SUCH A SUCCESSOR DEPOSITARY.”
In addition, so long as the Depository Trust Company (“DTC”) is the Depositary, each Global Security registered in the name of DTC or its nominee shall bear a legend in substantially the following form:
“UNLESS THIS GLOBAL SECURITY IS PRESENTED BY AN AUTHORIZED REPRESENTATIVE OF THE DEPOSITORY TRUST COMPANY, A NEW YORK CORPORATION (“DTC”), TO THE COMPANY OR ITS AGENT FOR REGISTRATION OF TRANSFER, EXCHANGE OR PAYMENT, AND ANY GLOBAL SECURITY ISSUED IS REGISTERED IN THE NAME OF CEDE & CO. OR IN SUCH OTHER NAME AS IS REQUESTED BY AN AUTHORIZED REPRESENTATIVE OF DTC (AND ANY PAYMENT IS MADE TO CEDE & CO. OR TO SUCH OTHER ENTITY AS IS REQUESTED BY AN AUTHORIZED REPRESENTATIVE OF DTC), ANY TRANSFER, PLEDGE OR OTHER USE HEREOF FOR VALUE OR OTHERWISE BY OR TO ANY PERSON IS WRONGFUL INASMUCH AS THE REGISTERED OWNER HEREOF, CEDE & CO., HAS AN INTEREST HEREIN.”
2.14.4. Acts of Holders. The Depositary, as a Holder, may appoint agents and otherwise authorize participants to give or take any request, demand, authorization, direction, notice, consent, waiver or other action which a Holder is entitled to give or take under the Indenture.
2.14.5. Payments. Notwithstanding the other provisions of this Indenture, unless otherwise specified as contemplated by Section 2.2, payment of the principal of and interest, if any, on any Global Security shall be made to the Holder thereof.
2.14.6. Agent Members. The registered Holder of a Security will be treated as the owner of such Security for all purposes and only registered Holders shall have rights under this Indenture and the Securities. Members of, or participants in, the Depositary (“Agent Members”) and persons who hold beneficial interests in a Global Security through an Agent Member shall have no rights under this Indenture with respect to any Global Security held on their behalf by the Depositary. The Depositary may be treated by the Company, the Trustee, the Paying Agent, the Registrar and any agent of the foregoing as the absolute owner of the Global Securities for all purposes whatsoever. Notwithstanding the foregoing, nothing herein shall prevent the Company, the Trustee, the Paying Agent, the Registrar or any agent of the foregoing from giving effect to any written certification, proxy or other authorization furnished by the Depositary or impair, as between the Depositary and its Agent Members, the operation of customary practices of such Depositary governing the exercise of the rights of a Holder of a beneficial interest in any Global Security.
Section 2.15. CUSIP Numbers.
The Company in issuing the Securities may use “CUSIP” numbers (if then generally in use), and, if so, the Trustee shall use “CUSIP” numbers in notices of redemption as a convenience to Holders; provided that any such notice may state that no representation is made as to the correctness of such numbers either as printed on the Securities or as contained in any notice of a redemption and that reliance may be placed only on the other elements of identification printed on the Securities, and any such redemption shall not be affected by any defect in or omission of such numbers.
ARTICLE III.
REDEMPTION
Section 3.1. Notice to Trustee.
The Company may, with respect to any Series of Securities, reserve the right to redeem and pay the Series of Securities or may covenant to redeem and pay the Series of Securities or any part thereof prior to the Stated Maturity thereof at such time and on such terms as provided for in such Securities. If a Series of Securities is redeemable and the Company wants or is obligated to redeem prior to the Stated Maturity thereof all or part of the Series of Securities pursuant to the terms of such Securities, it shall notify the Trustee in writing of the redemption date and the principal amount of the Series of Securities to be redeemed. The Company shall give the notice at least 15 days before the redemption date (or such shorter period as may be acceptable to the Trustee).
Section 3.2. Selection of Securities to be Redeemed.
Unless otherwise indicated for a particular Series by a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, if less than all the Securities of a Series are to be redeemed, the Securities of the Series to be redeemed will be selected as follows: (a) if the Securities are in the form of Global Securities, in accordance with the procedures of the Depositary, (b) if the Securities are listed on any national securities exchange, in compliance with the requirements of the principal national securities exchange, if any, on which the Securities are listed or (c) if not otherwise provided for under clause (a) or (b) in the manner that the Trustee deems fair and appropriate, including by lot or other method, unless otherwise required by law or applicable stock exchange requirements, subject, in the case of Global Securities, to the applicable rules and procedures of the Depositary. The Securities to be redeemed shall be selected from Securities of the Series outstanding not previously called for redemption. Portions of the principal of Securities of the Series that have denominations larger than $1,000 may be selected for redemption. Securities of the Series and portions of them it selected for redemption shall be in amounts of $1,000 or whole multiples of $1,000 or, with respect to Securities of any Series issuable in other denominations pursuant to Section 2.2.10, the minimum principal denomination for each Series and the authorized integral multiples thereof. Provisions of this Indenture that apply to Securities of a Series called for redemption also apply to portions of Securities of that Series called for redemption. Neither the Trustee nor the Paying Agent shall be liable for any selection made by it in accordance with this paragraph (including the procedures of the Depositary).
Section 3.3. Notice of Redemption.
Unless otherwise indicated for a particular Series by Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, at least 15 days but not more than 60 days before a redemption date, the Company shall send or cause to be sent by first-class mail or electronically, in accordance with the procedures of the Depositary, a notice of redemption to each Holder whose Securities are to be redeemed.
The notice shall identify the Securities of the Series to be redeemed and shall state:
(a) the redemption date;
(b) the redemption price;
(c) the name and address of the Paying Agent;
(d) if any Securities are being redeemed in part, the portion of the principal amount of such Securities to be redeemed and that, after the redemption date and upon surrender of such Security, a new Security or Securities in principal amount equal to the unredeemed portion of the original Security shall be issued in the name of the Holder thereof upon cancellation of the original Security;
(e) that Securities of the Series called for redemption must be surrendered to the Paying Agent to collect the redemption price;
(f) that interest on Securities of the Series called for redemption ceases to accrue on and after the redemption date unless the Company defaults in the deposit of the redemption price;
(g) the “CUSIP” number, if any; and
(h) any other information as may be required by the terms of the particular Series or the Securities of a Series being redeemed.
At the Company’s request, the Trustee shall give the notice of redemption in the Company’s name and at its expense, provided, however, that the Company has delivered to the Trustee, at least 10 days (unless a shorter time shall be acceptable to the Trustee) prior to the notice date, an Officer’s Certificate requesting that the Trustee give such notice and setting forth the information to be stated in such notice and the form of such notice.
Section 3.4. Effect of Notice of Redemption.
Once notice of redemption is sent as provided in Section 3.3, Securities of a Series called for redemption become due and payable on the redemption date and at the redemption price. Except as otherwise provided in the supplemental indenture, Board Resolution or Officer’s Certificate for a Series, a notice of redemption may not be conditional. Upon surrender to the Paying Agent, such Securities shall be paid at the redemption price plus accrued interest to the redemption date.
Section 3.5. Deposit of Redemption Price.
On or before 11:00 a.m., New York City time, on the redemption date, the Company shall deposit with the Paying Agent money sufficient to pay the redemption price of and accrued interest, if any, on all Securities to be redeemed on that date.
Section 3.6. Securities Redeemed in Part.
Upon surrender of a Security that is redeemed in part, the Trustee shall authenticate for the Holder a new Security of the same Series and the same maturity equal in principal amount to the unredeemed portion of the Security surrendered.
ARTICLE IV.
COVENANTS
Section 4.1. Payment of Principal and Interest.
The Company covenants and agrees for the benefit of the Holders of each Series of Securities that it will duly and punctually pay the principal of and interest, if any, on the Securities of that Series in accordance with the terms of such Securities and this Indenture. On or before 11:00 a.m., New York City time, on the applicable payment date, the Company shall deposit with the Paying Agent money sufficient to pay the principal of and interest, if any, on the Securities of each Series in accordance with the terms of such Securities and this Indenture.
Section 4.2. SEC Reports.
To the extent any Securities of a Series are outstanding, the Company shall deliver to the Trustee within 15 days after it files them with the SEC copies of the annual reports and of the information, documents and other reports (or copies of such portions of any of the foregoing as the SEC may by rules and regulations prescribe) which the Company is required to file with the SEC pursuant to Section 13 or 15(d) of the Exchange Act. The Company also shall comply with the other provisions of TIA § 314(a). Reports, information and documents filed with the SEC via the EDGAR system will be deemed to be delivered to the Trustee as of the time of such filing via EDGAR for purposes of this Section 4.2.
Delivery of reports, information and documents to the Trustee under this Section 4.2 is for informational purposes only and the Trustee’s receipt of the foregoing shall not constitute constructive or actual notice of any information contained therein or determinable from information contained therein, including the Company’s compliance with any of the covenants hereunder (as to which the Trustee is entitled to rely exclusively on Officer’s Certificates). All such reports, information or documents referred to in this Section 4.2 that the Company files with the SEC via the SEC’s EDGAR system shall be deemed to be filed with the Trustee and transmitted to Holders at the time such reports, information or documents are filed via the EDGAR system (or any successor system).
Section 4.3. Compliance Certificate.
To the extent any Securities of a Series are outstanding, the Company shall deliver to the Trustee, within 120 days after the end of each fiscal year of the Company, an Officer’s Certificate stating that a review of the activities of the Company and its Subsidiaries during the preceding fiscal year has been made under the supervision of the signing Officer with a view to determining whether the Company has kept, observed, performed and fulfilled its obligations under this Indenture, and further stating, as to such Officer signing such certificate, that to the best of his/her knowledge the Company has kept, observed, performed and fulfilled each and every covenant contained in this Indenture and is not in default in the performance or observance of any of the terms, provisions and conditions hereof (or, if a Default or Event of Default shall have occurred, describing all such Defaults or Events of Default of which the Officer may have knowledge).
Section 4.4. Stay, Extension and Usury Laws.
The Company covenants (to the extent that it may lawfully do so) that it will not at any time insist upon, plead or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law wherever enacted, now or at any time hereafter in force, which may affect the covenants or the performance of this Indenture or the Securities; and the Company (to the extent it may lawfully do so) hereby expressly waives all benefit or advantage of any such law and covenants that it will not, by resort to any such law, hinder, delay or impede the execution of any power herein granted to the Trustee, but will suffer and permit the execution of every such power as though no such law has been enacted.
ARTICLE V.
SUCCESSORS
Section 5.1. When Company May Merge, Etc.
The Company shall not consolidate with or merge with or into, or convey, transfer or lease all or substantially all of its properties and assets to, any person (a “successor person”) unless:
(a) the Company is the surviving entity or the successor person (if other than the Company) is a corporation, partnership, trust or other entity organized and validly existing under the laws of any U.S. domestic jurisdiction and expressly assumes by supplemental indenture the Company’s obligations on the Securities and under this Indenture; and
(b) immediately after giving effect to the transaction, no Default or Event of Default, shall have occurred and be continuing.
The Company shall deliver to the Trustee prior to the consummation of the proposed transaction an Officer’s Certificate to the foregoing effect and an Opinion of Counsel stating that the proposed transaction and any supplemental indenture comply with this Indenture.
Notwithstanding the above, any Subsidiary of the Company may consolidate with, merge into or transfer all or part of its properties to the Company. Neither an Officer’s Certificate nor an Opinion of Counsel shall be required to be delivered in connection therewith.
Section 5.2. Successor Corporation Substituted.
Upon any consolidation or merger, or any sale, lease, conveyance or other disposition of all or substantially all of the assets of the Company in accordance with Section 5.1, the successor corporation formed by such consolidation or into or with which the Company is merged or to which such sale, lease, conveyance or other disposition is made shall succeed to, and be substituted for, and may exercise every right and power of, the Company under this Indenture with the same effect as if such successor person has been named as the Company herein; provided, however, that the predecessor Company in the case of a sale, conveyance or other disposition (other than a lease) shall be released from all obligations and covenants under this Indenture and the Securities.
ARTICLE VI.
DEFAULTS AND REMEDIES
Section 6.1. Events of Default.
“Event of Default,” wherever used herein with respect to Securities of any Series, means any one of the following events, unless in the establishing Board Resolution, supplemental indenture or Officer’s Certificate it is provided that such Series shall not have the benefit of said Event of Default:
(a) default in the payment of any interest on any Security of that Series when it becomes due and payable, and continuance of such default for a period of 30 days (unless the entire amount of such payment is deposited by the Company with the Trustee or with a Paying Agent prior to 11:00 a.m., New York City time, on the 30th day of such period);
(b) default in the payment of principal of any Security of that Series at its Maturity;
(c) default in the performance or breach of any covenant or warranty of the Company in this Indenture (other than defaults pursuant to paragraph (a) or (b) above or pursuant to a covenant or warranty that has been included in this Indenture solely for the benefit of a Series of Securities other than that Series), which default continues uncured for a period of 60 days after there has been given, by registered or certified mail, to the Company by the Trustee or to the Company and the Trustee by the Holders of at least 25% in principal amount of the outstanding Securities of that Series a written notice specifying such default or breach and requiring it to be remedied and stating that such notice is a “Notice of Default” hereunder;
(d) the Company pursuant to or within the meaning of any Bankruptcy Law:
(i) commences a voluntary case,
(ii) consents to the entry of an order for relief against it in an involuntary case,
(iii) consents to the appointment of a Custodian of it or for all or substantially all of its property,
(iv) makes a general assignment for the benefit of its creditors, or
(v) generally is unable to pay its debts as the same become due;
(e) a court of competent jurisdiction enters an order or decree under any Bankruptcy Law that:
(i) is for relief against the Company in an involuntary case,
(ii) appoints a Custodian of the Company or for all or substantially all of its property, or
(iii) orders the liquidation of the Company,
and the order or decree remains unstayed and in effect for 60 days; or
(f) any other Event of Default provided with respect to Securities of that Series, which is specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate, in accordance with Section 2.2.18.
The term “Bankruptcy Law” means title 11, U.S. Code or any similar Federal or State law for the relief of debtors. The term “Custodian” means any receiver, trustee, assignee, liquidator or similar official under any Bankruptcy Law.
The Company will provide the Trustee written notice of any Default or Event of Default within 30 days of becoming aware of the occurrence of such Default or Event of Default, which notice will describe in reasonable detail the status of such Default or Event of Default and what action the Company is taking or proposes to take in respect thereof.
Section 6.2. Acceleration of Maturity; Rescission and Annulment.
If an Event of Default with respect to Securities of any Series at the time outstanding occurs and is continuing (other than an Event of Default referred to in Section 6.1(d) or (e)) then in every such case the Trustee or the Holders of not less than 25% in principal amount of the outstanding Securities of that Series may declare the principal amount (or, if any Securities of that Series are Discount Securities, such portion of the principal amount as may be specified in the terms of such Securities) of and accrued and unpaid interest, if any, on all of the Securities of that Series to be due and payable immediately, by a notice in writing to the Company (and to the Trustee if given by Holders), and upon any such declaration such principal amount (or specified amount) and accrued and unpaid interest, if any, shall become immediately due and payable. If an Event of Default specified in Section 6.1(d) or (e) shall occur, the principal amount (or specified amount) of and accrued and unpaid interest, if any, on all outstanding Securities shall ipso facto become and be immediately due and payable without any declaration or other act on the part of the Trustee or any Holder.
At any time after such a declaration of acceleration with respect to any Series has been made and before a judgment or decree for payment of the money due has been obtained by the Trustee as hereinafter in this Article provided, the Holders of a majority in principal amount of the outstanding Securities of that Series, by written notice to the Company and the Trustee, may rescind and annul such declaration and its consequences if all Events of Default with respect to Securities of that Series, other than the non-payment of the principal and interest, if any, of Securities of that Series which have become due solely by such declaration of acceleration, have been cured or waived as provided in Section 6.13.
No such rescission shall affect any subsequent Default or impair any right consequent thereon.
Section 6.3. Collection of Indebtedness and Suits for Enforcement by Trustee.
The Company covenants that if:
(a) default is made in the payment of any interest on any Security when such interest becomes due and payable and such default continues for a period of 30 days,
(b) default is made in the payment of principal of any Security at the Maturity thereof, or
(c) default is made in the deposit of any sinking fund payment, if any, when and as due by the terms of a Security,
then, the Company will, upon demand of the Trustee, pay to it, for the benefit of the Holders of such Securities, the whole amount then due and payable on such Securities for principal and interest and, to the extent that payment of such interest shall be legally enforceable, interest on any overdue principal and any overdue interest at the rate or rates prescribed therefor in such Securities, and, in addition thereto, such further amount as shall be sufficient to cover the costs and expenses of collection, including the compensation, reasonable expenses, disbursements and advances of the Trustee, its agents and counsel.
If the Company fails to pay such amounts forthwith upon such demand, the Trustee, in its own name and as trustee of an express trust, may institute a judicial proceeding for the collection of the sums so due and unpaid, may prosecute such proceeding to judgment or final decree and may enforce the same against the Company or any other obligor upon such Securities and collect the moneys adjudged or deemed to be payable in the manner provided by law out of the property of the Company or any other obligor upon such Securities, wherever situated.
If an Event of Default with respect to any Securities of any Series occurs and is continuing, the Trustee, subject to Article VII hereof, may in its discretion proceed to protect and enforce its rights and the rights of the Holders of Securities of such Series by such appropriate judicial proceedings as the Trustee shall deem most effectual to protect and enforce any such rights, whether for the specific enforcement of any covenant or agreement in this Indenture or in aid of the exercise of any power granted herein, or to enforce any other proper remedy.
Section 6.4. Trustee May File Proofs of Claim.
In case of the pendency of any receivership, insolvency, liquidation, bankruptcy, reorganization, arrangement, adjustment, composition or other judicial proceeding relative to the Company or any other obligor upon the Securities or the property of the Company or of such other obligor or their creditors, the Trustee (irrespective of whether the principal of the Securities shall then be due and payable as therein expressed or by declaration or otherwise and irrespective of whether the Trustee shall have made any demand on the Company for the payment of overdue principal or interest) shall be entitled and empowered, by intervention in such proceeding or otherwise,
(a) to file and prove a claim for the whole amount of principal and interest owing and unpaid in respect of the Securities and to file such other papers or documents as may be necessary or advisable in order to have the claims of the Trustee (including any claim for the compensation, reasonable expenses, disbursements and advances of the Trustee, its agents and counsel) and of the Holders allowed in such judicial proceeding, and
(b) to collect and receive any moneys or other property payable or deliverable on any such claims and to distribute the same,
and any custodian, receiver, assignee, trustee, liquidator, sequestrator or other similar official in any such judicial proceeding is hereby authorized by each Holder to make such payments to the Trustee and, in the event that the Trustee shall consent to the making of such payments directly to the Holders, to pay to the Trustee any amount due it for the compensation, reasonable expenses, disbursements and advances of the Trustee, its agents and counsel and any other amounts due the Trustee under Section 7.7.
Nothing herein contained shall be deemed to authorize the Trustee to authorize or consent to or accept or adopt on behalf of any Holder any plan of reorganization, arrangement, adjustment or composition affecting the Securities or the rights of any Holder thereof or to authorize the Trustee to vote in respect of the claim of any Holder in any such proceeding.
Section 6.5. Trustee May Enforce Claims Without Possession of Securities.
All rights of action and claims under this Indenture or the Securities may be prosecuted and enforced by the Trustee without the possession of any of the Securities or the production thereof in any proceeding relating thereto, and any such proceeding instituted by the Trustee shall be brought in its own name as trustee of an express trust, and any recovery of judgment shall, after provision for the payment of the compensation, reasonable expenses, disbursements and advances of the Trustee, its agents and counsel, be for the ratable benefit of the Holders of the Securities in respect of which such judgment has been recovered.
Section 6.6. Application of Money Collected.
Any money or property collected by the Trustee pursuant to this Article shall be applied in the following order, at the date or dates fixed by the Trustee and, in case of the distribution of such money or property on account of principal or interest, upon presentation of the Securities and the notation thereon of the payment if only partially paid and upon surrender thereof if fully paid:
First: To the payment of all amounts due the Trustee under Section 7.7; and
Second: To the payment of the amounts then due and unpaid for principal of and interest on the Securities in respect of which or for the benefit of which such money has been collected, ratably, without preference or priority of any kind, according to the amounts due and payable on such Securities for principal and interest, respectively; and
Third: To the Company.
Section 6.7. Limitation on Suits.
No Holder of any Security of any Series shall have any right to institute any proceeding, judicial or otherwise, with respect to this Indenture, or for the appointment of a receiver or trustee, or for any other remedy hereunder, unless
(a) such Holder has previously given written notice to the Trustee of a continuing Event of Default with respect to the Securities of that Series;
(b) the Holders of not less than 25% in principal amount of the outstanding Securities of that Series shall have made written request to the Trustee to institute proceedings in respect of such Event of Default in its own name as Trustee hereunder;
(c) such Holder or Holders have offered to the Trustee indemnity or security satisfactory to the Trustee against the costs, expenses and liabilities which might be incurred by the Trustee in compliance with such request;
(d) the Trustee for 60 days after its receipt of such notice, request and offer of indemnity has failed to institute any such proceeding; and
(e) no direction inconsistent with such written request has been given to the Trustee during such 60-day period by the Holders of a majority in principal amount of the outstanding Securities of that Series;
it being understood, intended and expressly covenanted by the Holder of every Security with every other Holder and the Trustee that no one or more of such Holders shall have any right in any manner whatever by virtue of, or by availing of, any provision of this Indenture to affect, disturb or prejudice the rights of any other of such Holders, or to obtain or to seek to obtain priority or preference over any other of such Holders or to enforce any right under this Indenture, except in the manner herein provided and for the equal and ratable benefit of all such Holders of the applicable Series.
Section 6.8. Unconditional Right of Holders to Receive Principal and Interest.
Notwithstanding any other provision in this Indenture, the Holder of any Security shall have the right, which is absolute and unconditional, to receive payment of the principal of and interest, if any, on such Security on the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date) and to institute suit for the enforcement of any such payment, and such rights shall not be impaired without the consent of such Holder.
Section 6.9. Restoration of Rights and Remedies.
If the Trustee or any Holder has instituted any proceeding to enforce any right or remedy under this Indenture and such proceeding has been discontinued or abandoned for any reason or has been determined adversely to the Trustee or to such Holder, then and in every such case, subject to any determination in such proceeding, the Company, the Trustee and the Holders shall be restored severally and respectively to their former positions hereunder and thereafter all rights and remedies of the Trustee and the Holders shall continue as though no such proceeding had been instituted.
Section 6.10. Rights and Remedies Cumulative.
Except as otherwise provided with respect to the replacement or payment of mutilated, destroyed, lost or stolen Securities in Section 2.8, no right or remedy herein conferred upon or reserved to the Trustee or to the Holders is intended to be exclusive of any other right or remedy, and every right and remedy shall, to the extent permitted by law, be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at law or in equity or otherwise. The assertion or employment of any right or remedy hereunder, or otherwise, shall not, to the extent permitted by law, prevent the concurrent assertion or employment of any other appropriate right or remedy.
Section 6.11. Delay or Omission Not Waiver.
No delay or omission of the Trustee or of any Holder of any Securities to exercise any right or remedy accruing upon any Event of Default shall impair any such right or remedy or constitute a waiver of any such Event of Default or an acquiescence therein. Every right and remedy given by this Article or by law to the Trustee or to the Holders may be exercised from time to time, and as often as may be deemed expedient, by the Trustee or by the Holders, as the case may be.
Section 6.12. Control by Holders.
The Holders of a majority in principal amount of the outstanding Securities of any Series shall have the right to direct the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred on the Trustee, with respect to the Securities of such Series, provided that
(a) such direction shall not be in conflict with any rule of law or with this Indenture,
(b) the Trustee may take any other action deemed proper by the Trustee which is not inconsistent with such direction,
(c) subject to the provisions of Section 7.1, the Trustee shall have the right to decline to follow any such direction if the Trustee in good faith shall, by a Responsible Officer of the Trustee, determine that the proceeding so directed would involve the Trustee in personal liability, and
(d) prior to taking any action as directed under this Section 6.12, the Trustee shall be entitled to indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
Section 6.13. Waiver of Past Defaults.
The Holders of not less than a majority in principal amount of the outstanding Securities of any Series may on behalf of the Holders of all the Securities of such Series, by written notice to the Trustee and the Company, waive any past Default hereunder with respect to such Series and its consequences, except a Default in the payment of the principal of or interest on any Security of such Series (provided, however, that the Holders of a majority in principal amount of the outstanding Securities of any Series may rescind an acceleration and its consequences, including any related payment default that resulted from such acceleration). Upon any such waiver, such Default shall cease to exist, and any Event of Default arising therefrom shall be deemed to have been cured, for every purpose of this Indenture; but no such waiver shall extend to any subsequent or other Default or impair any right consequent thereon.
Section 6.14. Undertaking for Costs.
All parties to this Indenture agree, and each Holder of any Security by his acceptance thereof shall be deemed to have agreed, that any court may in its discretion require, in any suit for the enforcement of any right or remedy under this Indenture, or in any suit against the Trustee for any action taken, suffered or omitted by it as Trustee, the filing by any party litigant in such suit of an undertaking to pay the costs of such suit, and that such court may in its discretion assess reasonable costs, including reasonable attorneys’ fees, against any party litigant in such suit, having due regard to the merits and good faith of the claims or defenses made by such party litigant; but the provisions of this Section shall not apply to any suit instituted by the Company, to any suit instituted by the Trustee, to any suit instituted by any Holder or group of Holders, holding in the aggregate more than 10% in principal amount of the outstanding Securities of any Series, or to any suit instituted by any Holder for the enforcement of the payment of the principal of or interest on any Security on or after the Maturity of such Security, including the Stated Maturity expressed in such Security (or, in the case of redemption, on the redemption date).
ARTICLE VII.
TRUSTEE
Section 7.1. Duties of Trustee.
(a) If an Event of Default has occurred and is continuing, the Trustee shall exercise the rights and powers vested in it by this Indenture and use the same degree of care and skill in their exercise as a prudent person would exercise or use under the circumstances in the conduct of such person’s own affairs.
(b) Except during the continuance of an Event of Default:
(i) The Trustee need perform only those duties that are specifically set forth in this Indenture and no others, and no implied covenants or obligations will be read into this Indenture against the Trustee.
(ii) In the absence of bad faith on its part, the Trustee may conclusively rely, as to the truth of the statements and the correctness of the opinions expressed therein, upon Officer’s Certificates or Opinions of Counsel furnished to the Trustee and conforming to the requirements of this Indenture; however, in the case of any such Officer’s Certificates or Opinions of Counsel which by any provisions hereof are specifically required to be furnished to the Trustee, the Trustee shall examine such Officer’s Certificates and Opinions of Counsel to determine whether or not they conform to the form requirements of this Indenture.
(c) The Trustee may not be relieved from liability for its own negligent action, its own negligent failure to act or its own willful misconduct, except that:
(i) This paragraph does not limit the effect of paragraph (b) of this Section.
(ii) The Trustee shall not be liable for any error of judgment made in good faith by a Responsible Officer, unless it is proved that the Trustee was negligent in ascertaining the pertinent facts.
(iii) The Trustee shall not be liable with respect to any action taken, suffered or omitted to be taken by it with respect to Securities of any Series in good faith in accordance with the direction of the Holders of a majority in principal amount of the outstanding Securities of such Series relating to the time, method and place of conducting any proceeding for any remedy available to the Trustee, or exercising any trust or power conferred upon the Trustee, under this Indenture with respect to the Securities of such Series in accordance with Section 6.12.
(d) Every provision of this Indenture that in any way relates to the Trustee is subject to paragraph (a), (b) and (c) of this Section.
(e) The Trustee may refuse to perform any duty or exercise any right or power unless it receives indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in performing such duty or exercising such right or power.
(f) The Trustee shall not be liable for interest on any money received by it except as the Trustee may agree in writing with the Company. Money held in trust by the Trustee need not be segregated from other funds except to the extent required by law.
(g) No provision of this Indenture shall require the Trustee to risk its own funds or otherwise incur any financial liability in the performance of any of its duties or in the exercise of any of its rights or powers, if adequate indemnity against such risk is not assured to the Trustee in its satisfaction.
(h) The Paying Agent, the Notice Agent, the Registrar, any authenticating agent and the Trustee when acting in any other capacity hereunder shall be entitled to the protections and immunities as are set forth in this Article VII.
(i) The rights, privileges, protections, immunities and benefits given to the Trustee, including its right to be indemnified, are extended to, and will be enforceable by, the Trustee in each of its capacities under this Indenture.
Section 7.2. Rights of Trustee.
(a) The Trustee may rely on and shall be protected in acting or refraining from acting upon any document (whether in its original or facsimile form) believed by it to be genuine and to have been signed or presented by the proper person. The Trustee need not investigate any fact or matter stated in the document.
(b) Before the Trustee acts or refrains from acting, it may require an Officer’s Certificate or an Opinion of Counsel or both. The Trustee shall not be liable for any action it takes or omits to take in good faith in reliance on such Officer’s Certificate or Opinion of Counsel.
(c) The Trustee may act through agents and shall not be responsible for the misconduct or negligence of any agent appointed with due care. No Depositary shall be deemed an agent of the Trustee, and the Trustee shall not be responsible for any act or omission by any Depositary.
(d) The Trustee shall not be liable for any action it takes or omits to take in good faith which it believes to be authorized or within its rights or powers.
(e) The Trustee may consult with counsel and the advice of such counsel or any Opinion of Counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by it hereunder in good faith and in reliance thereon.
(f) The Trustee shall be under no obligation to exercise any of the rights or powers vested in it by this Indenture at the request or direction of any of the Holders of Securities unless such Holders shall have offered to the Trustee security or indemnity satisfactory to it against the costs, expenses and liabilities which might be incurred by it in compliance with such request or direction.
(g) The Trustee shall not be bound to make any investigation into the facts or matters stated in any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order, bond, debenture, note, other evidence of indebtedness or other paper or document, but the Trustee, in its discretion, may make such further inquiry or investigation into such facts or matters as it may see fit.
(h) The Trustee shall not be deemed to have notice of any Default or Event of Default unless a Responsible Officer of the Trustee has actual knowledge thereof or unless written notice of any event which is in fact such a default is received by a Responsible Officer at the Corporate Trust Office of the Trustee, and such notice references the Securities generally or the Securities of a particular Series and this Indenture.
(i) In no event shall the Trustee be liable to any person for special, punitive, indirect, consequential or incidental loss or damage of any kind whatsoever (including but not limited to lost profits), even if the Trustee has been advised of the likelihood of such loss or damage.
(j) The permissive right of the Trustee to take the actions permitted by this Indenture shall not be construed as an obligation or duty to do so.
(k) The Trustee will not be required to give any bond or surety in respect of the execution of this Indenture or otherwise.
Section 7.3. Individual Rights of Trustee.
The Trustee in its individual or any other capacity may become the owner or pledgee of Securities and may otherwise deal with the Company or an Affiliate of the Company with the same rights it would have if it were not Trustee. Any Agent may do the same with like rights. The Trustee is also subject to Sections 7.10 and 7.11.
Section 7.4. Trustee’s Disclaimer.
The Trustee makes no representation as to the validity or adequacy of this Indenture or the Securities. The Trustee shall not be accountable for the Company’s use of the proceeds from the Securities and shall not be responsible for any statement in the Securities other than its certificate of authentication.
Section 7.5. Notice of Defaults.
If a Default or Event of Default occurs and is continuing with respect to the Securities of any Series and if it is known to a Responsible Officer of the Trustee, the Trustee shall send to each Holder of the Securities of that Series notice of a Default or Event of Default within 90 days after it occurs or, if later, after a Responsible Officer of the Trustee has knowledge of such Default or Event of Default. Except in the case of a Default or Event of Default in payment of principal of or interest on any Security of any Series, the Trustee may withhold the notice if and so long as its corporate trust committee or a committee of its Responsible Officers in good faith determines that withholding the notice is in the interests of Holders of that Series. The Trustee will not be deemed to have notice or be charged with knowledge of any Default or Event of Default unless written notice thereof has been received by a Responsible Officer, and such notice references the applicable Series of Securities and this Indenture and states on its face that a Default or Event of Default has occurred.
Section 7.6. Reports by Trustee to Holders.
Within 60 days after each anniversary of this Indenture, the Trustee shall transmit by mail to all Holders, as their names and addresses appear on the register kept by the Registrar, a brief report dated as of such anniversary date, in accordance with, and to the extent required under, TIA § 313.
A copy of each report at the time of its mailing to Holders of any Series shall be filed with the SEC and each national securities exchange on which the Securities of that Series are listed. The Company shall promptly notify the Trustee in writing when Securities of any Series are listed on any national securities exchange.
Section 7.7. Compensation and Indemnity.
The Company shall pay to the Trustee from time to time compensation for its services as the Company and the Trustee shall from time to time agree upon in writing. The Trustee’s compensation shall not be limited by any law on compensation of a trustee of an express trust. The Company shall reimburse the Trustee upon request for all reasonable out-of-pocket expenses incurred by it. Such expenses shall include the reasonable compensation and expenses of the Trustee’s agents and counsel.
The Company shall indemnify each of the Trustee and any predecessor Trustee (including for the cost of defending itself) against any cost, expense or liability, including taxes (other than taxes based upon, measured by or determined by the income of the Trustee) incurred by it except as set forth in the next paragraph in the performance of its duties under this Indenture as Trustee or Agent. The Trustee shall notify the Company promptly of any claim for which it may seek indemnity. Failure by the Trustee to so notify the Company shall not relieve the Company of its obligations hereunder, unless and to the extent that the Company is materially prejudiced thereby. The Company shall defend the claim and the Trustee shall cooperate in the defense. The Trustee may have separate counsel, and the Company shall pay the reasonable fees and expenses of such counsel. The Company need not pay for any settlement made without its consent, which consent will not be unreasonably withheld. This indemnification shall apply to officers, directors, employees, shareholders and agents of the Trustee.
The Company need not reimburse any expense or indemnify against any loss or liability incurred by the Trustee or by any officer, director, employee, shareholder or agent of the Trustee through willful misconduct or negligence, as determined by a final decision of a court of competent jurisdiction.
To secure the Company’s payment obligations in this Section, the Trustee shall have a lien prior to the Securities of any Series on all money or property held or collected by the Trustee, except that held in trust to pay principal of and interest on particular Securities of that Series.
When the Trustee incurs expenses or renders services after an Event of Default specified in Section 6.1(d) or (e) occurs, the expenses and the compensation for the services are intended to constitute expenses of administration under any Bankruptcy Law.
The provisions of this Section shall survive the termination of this Indenture and the resignation or removal of the Trustee.
Section 7.8. Replacement of Trustee.
A resignation or removal of the Trustee and appointment of a successor Trustee shall become effective only upon the successor Trustee’s acceptance of appointment as provided in this Section.
The Trustee may resign with respect to the Securities of one or more Series by so notifying the Company at least 30 days prior to the date of the proposed resignation. The Holders of a majority in principal amount of the Securities of any Series may remove the Trustee with respect to that Series by so notifying the Trustee and the Company. The Company may remove the Trustee with respect to Securities of one or more Series if:
(a) the Trustee fails to comply with Section 7.10;
(b) the Trustee is adjudged a bankrupt or an insolvent or an order for relief is entered with respect to the Trustee under any Bankruptcy Law;
(c) a Custodian or public officer takes charge of the Trustee or its property; or
(d) the Trustee becomes incapable of acting.
If the Trustee resigns or is removed or if a vacancy exists in the office of Trustee for any reason, the Company shall promptly appoint a successor Trustee. Within one year after the successor Trustee takes office, the Holders of a majority in principal amount of the then outstanding Securities may appoint a successor Trustee to replace the successor Trustee appointed by the Company.
If a successor Trustee with respect to the Securities of any one or more Series does not take office within 60 days after the retiring Trustee resigns or is removed, the retiring Trustee, the Company or the Holders of at least a majority in principal amount of the Securities of the applicable Series may petition any court of competent jurisdiction for the appointment of a successor Trustee.
A successor Trustee shall deliver a written acceptance of its appointment to the retiring Trustee and to the Company. Immediately after that, the retiring Trustee shall transfer all property held by it as Trustee to the successor Trustee subject to the lien provided for in Section 7.7, the resignation or removal of the retiring Trustee shall become effective, and the successor Trustee shall have all the rights, powers and duties of the Trustee with respect to each Series of Securities for which it is acting as Trustee under this Indenture. A successor Trustee shall send a notice of its succession to each Holder of each such Series. Notwithstanding replacement of the Trustee pursuant to this Section 7.8, the Company’s obligations under Section 7.7 hereof shall continue for the benefit of the retiring Trustee with respect to expenses and liabilities incurred by it for actions taken or omitted to be taken in accordance with its rights, powers and duties under this Indenture prior to such replacement.
Section 7.9. Successor Trustee by Merger, Etc.
Any organization or entity into which the Trustee may be merged or converted or with which it may be consolidated, or any organization or entity resulting from any merger, conversion or consolidation to which the Trustee shall be a party, or any organization or entity succeeding to all or substantially all of the corporate trust business of the Trustee, shall be the successor of the Trustee hereunder, provided such organization or entity shall be otherwise qualified and eligible under Section 7.10, without the execution or filing of any paper or any further act on the part of any of the parties hereto.
Section 7.10. Eligibility; Disqualification.
This Indenture shall always have a Trustee who satisfies the requirements of TIA § 310(a)(1), (2) and (5). The Trustee shall always have a combined capital and surplus of at least $25,000,000 as set forth in its most recent published annual report of condition. The Trustee shall comply with TIA § 310(b).
Section 7.11. Preferential Collection of Claims Against Company.
The Trustee is subject to TIA § 311(a), excluding any creditor relationship listed in TIA § 311(b). A Trustee who has resigned or been removed shall be subject to TIA § 311(a) to the extent indicated.
ARTICLE VIII.
SATISFACTION AND DISCHARGE; DEFEASANCE
Section 8.1. Satisfaction and Discharge of Indenture.
This Indenture shall upon Company Order be discharged with respect to the Securities of any Series and cease to be of further effect as to all Securities of such Series (except as hereinafter provided in this Section 8.1), and the Trustee, at the expense of the Company, shall execute instruments acknowledging satisfaction and discharge of this Indenture, when
(a) either
(i) all Securities of such Series theretofore authenticated and delivered (other than Securities that have been destroyed, lost or stolen and that have been replaced or paid) have been delivered to the Trustee for cancellation; or
(ii) all such Securities of such Series not theretofore delivered to the Trustee for cancellation:
(1) have become due and payable by reason of sending a notice of redemption or otherwise,
(2) will become due and payable at their Stated Maturity within one year,
(3) have been called for redemption or are to be called for redemption within one year under arrangements satisfactory to the Trustee for the giving of notice of redemption by the Trustee in the name, and at the expense, of the Company, or
(4) are deemed paid and discharged pursuant to Section 8.3, as applicable;
and the Company, in the case of (1), (2) or (3) above, has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust an amount of money or U.S. Government Obligations, which amount shall be sufficient for the purpose of paying and discharging each installment of principal (including mandatory sinking fund payments or analogous payments) of and interest on all the Securities of such Series on the dates such installments of principal or interest are due;
(b) the Company has paid or caused to be paid all other sums payable hereunder by the Company; and
(c) the Company has delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for relating to the satisfaction and discharge contemplated by this Section have been complied with.
Notwithstanding the satisfaction and discharge of this Indenture, (x) the obligations of the Company to the Trustee under Section 7.7, (y) if money shall have been deposited with the Trustee pursuant to clause (a) of this Section, the provisions of Sections 2.4, 2.7, 2.8, 8.2 and 8.5, and (z) the rights, powers, trusts and immunities of the Trustee hereunder and the Company’s obligations in connection therewith shall survive.
Section 8.2. Application of Trust Funds; Indemnification.
(a) Subject to the provisions of Section 8.5, all money and U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.1, 8.3 or 8.4 and all money received by the Trustee in respect of U.S. Government Obligations or Foreign Government Obligations deposited with the Trustee pursuant to Section 8.1, 8.3 or 8.4, shall be held in trust and applied by it, in accordance with the provisions of the Securities and this Indenture, to the payment, either directly or through any Paying Agent (including the Company acting as its own Paying Agent) as the Trustee may determine, to the persons entitled thereto, of the principal and interest for whose payment such money has been deposited with or received by the Trustee or to make mandatory sinking fund payments or analogous payments as contemplated by Sections 8.1, 8.3 or 8.4.
(b) The Company shall pay and shall indemnify the Trustee (which indemnity shall survive termination of this Indenture) against any tax, fee or other charge imposed on or assessed against U.S. Government Obligations or Foreign Government Obligations deposited pursuant to Sections 8.1, 8.3 or 8.4 or the interest and principal received in respect of such obligations other than any payable by or on behalf of Holders.
(c) The Trustee shall deliver or pay to the Company from time to time upon Company Order any U.S. Government Obligations or Foreign Government Obligations or money held by it as provided in Sections 8.3 or 8.4 which, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, are then in excess of the amount thereof which then would have been required to be deposited for the purpose for which such U.S. Government Obligations or Foreign Government Obligations or money were deposited or received. This provision shall not authorize the sale by the Trustee of any U.S. Government Obligations or Foreign Government Obligations held under this Indenture.
Section 8.3. Legal Defeasance of Securities of any Series.
Unless this Section 8.3 is otherwise specified, pursuant to Section 2.2, to be inapplicable to Securities of any Series, the Company shall be deemed to have paid and discharged the entire indebtedness on all the outstanding Securities of any Series on the 91st day after the date of the deposit referred to in subparagraph (d) hereof, and the provisions of this Indenture, as it relates to such outstanding Securities of such Series, shall no longer be in effect (and the Trustee, at the expense of the Company, shall, upon receipt of a Company Order, execute instruments acknowledging the same), except as to:
(a) the rights of Holders of Securities of such Series to receive, from the trust funds described in subparagraph (d) hereof, (i) payment of the principal of and each installment of principal of and interest on the outstanding Securities of such Series on the Maturity of such principal or installment of principal or interest and (ii) the benefit of any mandatory sinking fund payments applicable to the Securities of such Series on the day on which such payments are due and payable in accordance with the terms of this Indenture and the Securities of such Series;
(b) the provisions of Sections 2.4, 2.5, 2.7, 2.8, 7.7, 8.2, 8.3, 8.5 and 8.6; and
(c) the rights, powers, trusts and immunities of the Trustee hereunder and the Company’s obligations in connection therewith;
provided that, the following conditions shall have been satisfied:
(d) the Company shall have irrevocably deposited or caused to be deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds specifically pledged as security for and dedicated solely to the benefit of the Holders of such Securities (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms, will provide (and without reinvestment and assuming no tax liability will be imposed on such Trustee), not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal of and interest, on and any mandatory sinking fund payments in respect of all the Securities of such Series on the dates such installments of principal or interest and such sinking fund payments are due;
(e) such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(f) no Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit or during the period ending on the 91st day after such date;
(g) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel to the effect that (i) the Company has received from, or there has been published by, the Internal Revenue Service a ruling or (ii) since the date of execution of this Indenture, there has been a change in the applicable Federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel shall confirm that, the Holders of the Securities of such Series will not recognize income, gain or loss for Federal income tax purposes as a result of such deposit, defeasance and discharge and will be subject to Federal income tax on the same amount and in the same manner and at the same times as would have been the case if such deposit, defeasance and discharge had not occurred;
(h) the Company shall have delivered to the Trustee an Officer’s Certificate stating that the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(i) the Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent provided for relating to the defeasance contemplated by this Section have been complied with.
Section 8.4. Covenant Defeasance.
Unless this Section 8.4 is otherwise specified pursuant to Section 2.2 to be inapplicable to Securities of any Series, the Company may omit to comply with respect to the Securities of any Series with any term, provision or condition set forth under Sections 4.2, 4.3, 4.4 and 5.1 and, unless otherwise specified therein, any additional covenants specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2 (and the failure to comply with any such covenants shall not constitute a Default or Event of Default with respect to such Series under Section 6.1) and the occurrence of any event specified in a supplemental indenture for such Series of Securities or a Board Resolution or an Officer’s Certificate delivered pursuant to Section 2.2 and designated as an Event of Default shall not constitute a Default or Event of Default hereunder, with respect to the Securities of such Series, but, except as specified above, the remainder of this Indenture and such Securities will be unaffected thereby; provided that the following conditions shall have been satisfied:
(a) with reference to this Section 8.4, the Company has irrevocably deposited or caused to be irrevocably deposited (except as provided in Section 8.2(c)) with the Trustee as trust funds in trust for the purpose of making the following payments specifically pledged as security for, and dedicated solely to, the benefit of the Holders of such Securities (i) in the case of Securities of such Series denominated in Dollars, cash in Dollars and/or U.S. Government Obligations or (ii) in the case of Securities of such Series denominated in a Foreign Currency (other than a composite currency), money and/or Foreign Government Obligations, which through the payment of interest and principal in respect thereof in accordance with their terms, will provide (and without reinvestment and assuming no tax liability will be imposed on such Trustee), not later than one day before the due date of any payment of money, an amount in cash, sufficient, in the opinion of a nationally recognized firm of independent certified public accountants or investment bank expressed in a written certification thereof delivered to the Trustee, to pay and discharge each installment of principal (including mandatory sinking fund payments or analogous payments) of and interest on all the Securities of such Series on the dates such installments of principal or interest are due;
(b) such deposit will not result in a breach or violation of, or constitute a default under, this Indenture or any other agreement or instrument to which the Company is a party or by which it is bound;
(c) no Default or Event of Default with respect to the Securities of such Series shall have occurred and be continuing on the date of such deposit;
(d) the Company shall have delivered to the Trustee an Officers’ Certificate and an Opinion of Counsel to the effect that the Holders of the Securities of such Series will not recognize income, gain or loss for Federal income tax purposes as a result of such deposit and covenant defeasance and will be subject to Federal income tax on the same amount and in the same manner and at the same times as would have been the case if such deposit and covenant defeasance had not occurred;
(e) The Company shall have delivered to the Trustee an Officer’s Certificate stating the deposit was not made by the Company with the intent of defeating, hindering, delaying or defrauding any other creditors of the Company; and
(f) The Company shall have delivered to the Trustee an Officer’s Certificate and an Opinion of Counsel, each stating that all conditions precedent herein provided for relating to the covenant defeasance contemplated by this Section have been complied with.
Section 8.5. Repayment to Company.
Subject to applicable abandoned property law, the Trustee and the Paying Agent shall pay to the Company upon request any money held by them for the payment of principal and interest that remains unclaimed for two years. After that, Holders entitled to the money must look to the Company for payment as general creditors unless an applicable abandoned property law designates another person.
Section 8.6. Reinstatement.
If the Trustee or the Paying Agent is unable to apply any money deposited with respect to Securities of any Series in accordance with Section 8.1 by reason of any legal proceeding or by reason of any order or judgment of any court or governmental authority enjoining, restraining or otherwise prohibiting such application, the obligations of the Company under this Indenture with respect to the Securities of such Series and under the Securities of such Series shall be revived and reinstated as though no deposit had occurred pursuant to Section 8.1 until such time as the Trustee or the Paying Agent is permitted to apply all such money in accordance with Section 8.1; provided, however, that if the Company has made any payment of principal of or interest on any Securities because of the reinstatement of its obligations, the Company shall be subrogated to the rights of the Holders of such Securities to receive such payment from the money or U.S. Government Obligations held by the Trustee or Paying Agent after payment in full to the Holders.
ARTICLE IX.
AMENDMENTS AND WAIVERS
Section 9.1. Without Consent of Holders.
The Company and the Trustee may amend or supplement this Indenture or the Securities of one or more Series without the consent of any Holder:
(a) to cure any ambiguity, defect or inconsistency;
(b) to comply with Article V;
(c) to provide for uncertificated Securities in addition to or in place of certificated Securities;
(d) to add guarantees with respect to Securities of any Series or secure Securities of any Series;
(e) to surrender any of the Company’s rights or powers under this Indenture;
(f) to add covenants or events of default for the benefit of the holders of Securities of any Series;
(g) to comply with the applicable procedures of the applicable depositary;
(h) to make any change that does not adversely affect the rights of any Holder;
(i) to provide for the issuance of and establish the form and terms and conditions of Securities of any Series as permitted by this Indenture;
(j) to evidence and provide for the acceptance of appointment hereunder by a successor Trustee with respect to the Securities of one or more Series and to add to or change any of the provisions of this Indenture as shall be necessary to provide for or facilitate the administration of the trusts hereunder by more than one Trustee; or
(k) to comply with requirements of the SEC in order to effect or maintain the qualification of this Indenture under the TIA.
Section 9.2. With Consent of Holders.
Subject to Section 9.3, the Company and the Trustee may enter into a supplemental indenture with the written consent of the Holders of at least a majority in principal amount of the outstanding Securities of each Series affected by such supplemental indenture (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series), for the purpose of adding any provisions to or changing in any manner or eliminating any of the provisions of this Indenture or of any supplemental indenture or of modifying in any manner the rights of the Holders of each such Series. Except as provided in Section 6.13, and subject to Section 9.3, the Holders of at least a majority in principal amount of the outstanding Securities of any Series by notice to the Trustee (including consents obtained in connection with a tender offer or exchange offer for the Securities of such Series) may waive compliance by the Company with any provision of this Indenture or the Securities with respect to such Series.
It shall not be necessary for the consent of the Holders of Securities under this Section 9.2 to approve the particular form of any proposed supplemental indenture or waiver, but it shall be sufficient if such consent approves the substance thereof. After a supplemental indenture or waiver under this section becomes effective, the Company shall send to the Holders of Securities affected thereby, a notice briefly describing the supplemental indenture or waiver. Any failure by the Company to send such notice, or any defect therein, shall not, however, in any way impair or affect the validity of any such supplemental indenture or waiver.
Section 9.3. Limitations.
Without the consent of each Holder affected, an amendment or waiver may not:
(a) reduce the principal amount of Securities whose Holders must consent to an amendment, supplement or waiver;
(b) reduce the rate of or extend the time for payment of interest (including default interest) on any Security;
(c) reduce the principal or change the Stated Maturity of any Security or reduce the amount of, or postpone the date fixed for, the payment of any sinking fund or analogous obligation;
(d) reduce the principal amount of Discount Securities payable upon acceleration of the maturity thereof;
(e) waive a Default or Event of Default in the payment of the principal of or interest, if any, on any Security (except a rescission of acceleration of the Securities of any Series by the Holders of at least a majority in principal amount of the outstanding Securities of such Series and a waiver of the payment default that resulted from such acceleration);
(f) make the principal of or interest, if any, on any Security payable in any currency other than that stated in the Security;
(g) make any change in Sections 6.8, 6.13 or 9.3 (this sentence); or
(h) waive a redemption payment with respect to any Security, provided that such redemption is made at the Company’s option.
Section 9.4. Compliance with Trust Indenture Act.
Every amendment to this Indenture or the Securities of one or more Series shall be set forth in a supplemental indenture hereto that complies with the TIA as then in effect.
Section 9.5. Revocation and Effect of Consents.
Until an amendment is set forth in a supplemental indenture or a waiver becomes effective, a consent to it by a Holder of a Security is a continuing consent by the Holder and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security, even if notation of the consent is not made on any Security. However, any such Holder or subsequent Holder may revoke the consent as to his Security or portion of a Security if the Trustee receives the notice of revocation before the date of the supplemental indenture or the date the waiver becomes effective.
Any amendment or waiver once effective shall bind every Holder of each Series affected by such amendment or waiver unless it is of the type described in any of clauses (a) through (h) of Section 9.3. In that case, the amendment or waiver shall bind each Holder of a Security who has consented to it and every subsequent Holder of a Security or portion of a Security that evidences the same debt as the consenting Holder’s Security.
The Company may, but shall not be obligated to, fix a record date for the purpose of determining the Holders entitled to give their consent or take any other action described above or required or permitted to be taken pursuant to this Indenture. If a record date is fixed, then notwithstanding the second immediately preceding paragraph, those Persons who were Holders at such record date (or their duly designated proxies), and only those Persons, shall be entitled to give such consent or to revoke any consent previously given or take any such action, whether or not such Persons continue to be Holders after such record date. No such consent shall be valid or effective for more than 120 days after such record date.
Section 9.6. Notation on or Exchange of Securities.
The Company or the Trustee may, but shall not be obligated to, place an appropriate notation about an amendment or waiver on any Security of any Series thereafter authenticated. The Company in exchange for Securities of that Series may issue and the Trustee shall authenticate upon receipt of a Company Order in accordance with Section 2.3 new Securities of that Series that reflect the amendment or waiver.
Section 9.7. Trustee Protected.
In executing, or accepting the additional trusts created by, any supplemental indenture permitted by this Article or the modifications thereby of the trusts created by this Indenture, the Trustee shall be entitled to receive, upon request, an Officer’s Certificate and/or an Opinion of Counsel complying with Sections 10.4 and 10.5 and (subject to Section 7.1) shall be fully protected in relying upon such Officer’s Certificate and/or Opinion of Counsel. The Trustee shall sign all supplemental indentures upon delivery of such an Officer’s Certificate or Opinion of Counsel or both, except that the Trustee need not sign any supplemental indenture that adversely affects its rights, duties, liabilities or immunities under this Indenture.
ARTICLE X.
MISCELLANEOUS
Section 10.1. Trust Indenture Act Controls.
If any provision of this Indenture limits, qualifies or conflicts with another provision which is required or deemed to be included in this Indenture by the TIA, such required or deemed provision shall control.
Section 10.2. Notices.
Any notice or communication by the Company or the Trustee to the other, or by a Holder to the Company or the Trustee, is duly given if in writing and delivered in person or mailed by first‑class mail (registered or certified, return receipt requested), email or overnight air courier guaranteeing next day delivery, to the others’ address:
if to the Company:
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94080
Attention: Chief Executive Officer
Telephone: (650) 577-3600
with a copy to:
Latham & Watkins LLP
300 North Wabash Avenue, Suite 2800
Chicago, Illinois 60611
Attention: Christopher D. Lueking and Scott W. Westhoff
Telephone: (312) 876-7700
if to the Trustee:
[TRUSTEE]
[_____]
Attention: [____]
Telephone: [____]
with a copy to:
[_____]
Attention: [____]
Telephone: [____]
The Company or the Trustee by notice to the other may designate additional or different addresses for subsequent notices or communications.
Any notice or communication to a Holder shall be sent electronically or by first-class mail or overnight air courier to his, her or its address shown on the register kept by the Registrar, in accordance with the procedures of the Depositary. Failure to send a notice or communication to a Holder of any Series or any defect in it shall not affect its sufficiency with respect to other Holders of that or any other Series.
If a notice or communication is sent or published in the manner provided above, within the time prescribed, it is duly given, whether or not the Holder receives it.
If the Company sends a notice or communication to Holders, it shall send a copy to the Trustee and each Agent at the same time.
The Trustee shall not have any duty to confirm that the person sending any notice, instruction or other communication by electronic transmission (including by e-mail, facsimile transmission, web portal or other electronic methods) is, in fact, a person authorized to do so. Electronic signatures believed by the Trustee to comply with the ESIGN Act of 2000 or other applicable law (including electronic images of handwritten signatures and digital signatures provided by DocuSign, Orbit, Adobe Sign or any other digital signature provider acceptable to the Trustee) shall be deemed original signatures for all purposes. The Company assumes all risks arising out of the use of electronic signatures and electronic methods to send communications to the Trustee, including without limitation the risk of the Trustee acting on an unauthorized communication, and the risk of interception or misuse by third parties.
Notwithstanding any other provision of this Indenture or any Security, where this Indenture or any Security provides for notice of any event (including any notice of redemption) to a Holder of a Global Security (whether by mail or otherwise), such notice shall be sufficiently given to the Depositary for such Security (or its designee) pursuant to the customary procedures of such Depositary.
Section 10.3. Communication by Holders with Other Holders.
Holders of any Series may communicate pursuant to TIA § 312(b) with other Holders of that Series or any other Series with respect to their rights under this Indenture or the Securities of that Series or all Series. The Company, the Trustee, the Registrar and anyone else shall have the protection of TIA § 312(c).
Section 10.4. Certificate and Opinion as to Conditions Precedent.
Upon any request or application by the Company to the Trustee to take any action under this Indenture, the Company shall furnish to the Trustee:
(a) an Officer’s Certificate stating that, in the opinion of the signers, all conditions precedent, if any, provided for in this Indenture relating to the proposed action have been complied with; and
(b) an Opinion of Counsel stating that, in the opinion of such counsel, all such conditions precedent have been complied with.
Section 10.5. Statements Required in Certificate or Opinion.
Each certificate or opinion with respect to compliance with a condition or covenant provided for in this Indenture (other than a certificate provided pursuant to TIA § 314(a)(4)) shall comply with the provisions of TIA § 314(e) and shall include:
(a) a statement that the person making such certificate or opinion has read such covenant or condition;
(b) a brief statement as to the nature and scope of the examination or investigation upon which the statements or opinions contained in such certificate or opinion are based;
(c) a statement that, in the opinion of such person, such person has made such examination or investigation as is necessary to enable such person to express an informed opinion as to whether or not such covenant or condition has been complied with; and
(d) a statement as to whether or not, in the opinion of such person, such condition or covenant has been complied with.
Section 10.6. Rules by Trustee and Agents.
The Trustee may make reasonable rules for action by or a meeting of Holders of one or more Series. Any Agent may make reasonable rules and set reasonable requirements for its functions.
Section 10.7. Legal Holidays.
If a payment date for any payment made under this Indenture is not a Business Day, payment may be made on the next succeeding Business Day, and no interest shall accrue for the intervening period.
Section 10.8. No Recourse Against Others.
A director, officer, employee or stockholder (past or present), as such, of the Company shall not have any liability for any obligations of the Company under the Securities or the Indenture or for any claim based on, in respect of or by reason of such obligations or their creation. Each Holder by accepting a Security waives and releases all such liability. The waiver and release are part of the consideration for the issue of the Securities.
Section 10.9. Counterparts.
This Indenture may be executed in any number of counterparts and by the parties hereto in separate counterparts, each of which when so executed shall be deemed to be an original and all of which taken together shall constitute one and the same agreement. The exchange of copies of this Indenture and of signature pages by facsimile or electronic format (e.g., “.pdf” or “.tif”) transmission shall constitute effective execution and delivery of this Indenture as to the parties hereto and may be used in lieu of the original Indenture for all purposes. Signatures of the parties hereto transmitted by facsimile or electronic format (e.g., “.pdf” or “.tif”) shall be deemed to be their original signatures for all purposes.
Unless otherwise provided herein or in any other Securities, the words “execute”, “execution”, “signed” and “signature” and words of similar import used in or related to any document to be signed in connection with this Indenture, any Securities or any of the transactions contemplated hereby (including amendments, waivers, consents and other modifications) shall be deemed to include electronic signatures and the keeping of records in electronic form, each of which shall be of the same legal effect, validity or enforceability as a manually executed signature in ink or the use of a paper-based recordkeeping system, as applicable, to the fullest extent and as provided for in any applicable law, including the Federal Electronic Signatures in Global and National Commerce Act, the New York State Electronic Signatures and Records Act and any other similar state laws based on the Uniform Electronic Transactions Act.
Section 10.10. Governing Law; Waiver of Jury Trial; Consent to Jurisdiction.
THIS INDENTURE AND THE SECURITIES, INCLUDING ANY CLAIM OR CONTROVERSY ARISING OUT OF OR RELATING TO THE INDENTURE OR THE SECURITIES, SHALL BE GOVERNED BY THE LAWS OF THE STATE OF NEW YORK.
THE COMPANY, THE TRUSTEE AND THE HOLDERS (BY THEIR ACCEPTANCE OF THE SECURITIES) EACH HEREBY IRREVOCABLY WAIVE, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF OR RELATING TO THIS INDENTURE, THE SECURITIES OR THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY.
Any legal suit, action or proceeding arising out of or based upon this Indenture or the transactions contemplated hereby may be instituted in the federal courts of the United States of America located in the City of New York or the courts of the State of New York in each case located in the City of New York (collectively, the “Specified Courts”), and each party irrevocably submits to the non-exclusive jurisdiction of such courts in any such suit, action or proceeding. Service of any process, summons, notice or document by mail (to the extent allowed under any applicable statute or rule of court) to such party’s address set forth above shall be effective service of process for any suit, action or other proceeding brought in any such court. The Company, the Trustee and the Holders (by their acceptance of the Securities) each hereby irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or other proceeding in the Specified Courts and irrevocably and unconditionally waive and agree not to plead or claim any such suit, action or other proceeding has been brought in an inconvenient forum.
Section 10.11. No Adverse Interpretation of Other Agreements.
This Indenture may not be used to interpret another indenture, loan or debt agreement of the Company or a Subsidiary of the Company. Any such indenture, loan or debt agreement may not be used to interpret this Indenture.
Section 10.12. Successors.
All agreements of the Company in this Indenture and the Securities shall bind its successor. All agreements of the Trustee in this Indenture shall bind its successor.
Section 10.13. Severability.
In case any provision in this Indenture or in the Securities shall be invalid, illegal or unenforceable, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
Section 10.14. Table of Contents, Headings, Etc.
The Table of Contents, Cross Reference Table, headings of the Articles and Sections of this Indenture have been inserted for convenience of reference only, are not to be considered a part hereof and shall in no way modify or restrict any of the terms or provisions hereof.
Section 10.15. Securities in a Foreign Currency.
Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, whenever for purposes of this Indenture any action may be taken by the Holders of a specified percentage in aggregate principal amount of Securities of all Series or all Series affected by a particular action at the time outstanding and, at such time, there are outstanding Securities of any Series which are denominated in more than one currency, then the principal amount of Securities of such Series which shall be deemed to be outstanding for the purpose of taking such action shall be determined by converting any such other currency into a currency that is designated upon issuance of any particular Series of Securities. Unless otherwise specified in a Board Resolution, a supplemental indenture hereto or an Officer’s Certificate delivered pursuant to Section 2.2 of this Indenture with respect to a particular Series of Securities, such conversion shall be at the spot rate for the purchase of the designated currency as published in The Financial Times in the “Currency Rates” section (or, if The Financial Times is no longer published, or if such information is no longer available in The Financial Times, such source as may be selected in good faith by the Company) on any date of determination. The provisions of this paragraph shall apply in determining the equivalent principal amount in respect of Securities of a Series denominated in currency other than Dollars in connection with any action taken by Holders of Securities pursuant to the terms of this Indenture.
All decisions and determinations provided for in the preceding paragraph shall, in the absence of manifest error, to the extent permitted by law, be conclusive for all purposes and irrevocably binding upon the Trustee and all Holders.
Section 10.16. Judgment Currency.
The Company agrees, to the fullest extent that it may effectively do so under applicable law, that (a) if for the purpose of obtaining judgment in any court it is necessary to convert the sum due in respect of the principal of or interest or other amount on the Securities of any Series (the “Required Currency”) into a currency in which a judgment will be rendered (the “Judgment Currency”), the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in the City of New York the Required Currency with the Judgment Currency on the day on which final unappealable judgment is entered, unless such day is not a New York Banking Day, then the rate of exchange used shall be the rate at which in accordance with normal banking procedures the Trustee could purchase in the City of New York the Required Currency with the Judgment Currency on the New York Banking Day preceding the day on which final unappealable judgment is entered and (b) its obligations under this Indenture to make payments in the Required Currency (i) shall not be discharged or satisfied by any tender, any recovery pursuant to any judgment (whether or not entered in accordance with subsection (a)), in any currency other than the Required Currency, except to the extent that such tender or recovery shall result in the actual receipt, by the payee, of the full amount of the Required Currency expressed to be payable in respect of such payments, (ii) shall be enforceable as an alternative or additional cause of action for the purpose of recovering in the Required Currency the amount, if any, by which such actual receipt shall fall short of the full amount of the Required Currency so expressed to be payable, and (iii) shall not be affected by judgment being obtained for any other sum due under this Indenture. For purposes of the foregoing, “New York Banking Day” means any day except a Saturday, Sunday or a legal holiday in the City of New York on which banking institutions are authorized or required by law, regulation or executive order to close.
Section 10.17. Force Majeure.
In no event shall the Trustee be responsible or liable for any failure or delay in the performance of its obligations hereunder arising out of or caused by, directly or indirectly, forces beyond its control, including, without limitation, strikes, work stoppages, accidents, acts of war or terrorism, civil or military disturbances, nuclear or natural catastrophes, pandemics, epidemics or other public health emergencies, or acts of God, and interruptions, loss or malfunctions of utilities, communications or computer (software and hardware) services, it being understood that the Trustee shall use reasonable best efforts which are consistent with accepted practices in the banking industry to resume performance as soon as practicable under the circumstances.
Section 10.18. U.S.A. Patriot Act.
The parties hereto acknowledge that in accordance with Section 326 of the U.S.A. Patriot Act, the Trustee is required to obtain, verify and record information that identifies each person or legal entity that establishes a relationship or opens an account with the Trustee. The parties to this Indenture agree that they will provide the Trustee with such information as it may request in order for the Trustee to satisfy the requirements of the U.S.A. Patriot Act.
ARTICLE XI.
SINKING FUNDS
Section 11.1. Applicability of Article.
The provisions of this Article shall be applicable to any sinking fund for the retirement of the Securities of a Series if so provided by the terms of such Securities pursuant to Section 2.2, except as otherwise permitted or required by any form of Security of such Series issued pursuant to this Indenture.
The minimum amount of any sinking fund payment provided for by the terms of the Securities of any Series is herein referred to as a “mandatory sinking fund payment” and any other amount provided for by the terms of Securities of such Series is herein referred to as an “optional sinking fund payment.” If provided for by the terms of Securities of any Series, the cash amount of any sinking fund payment may be subject to reduction as provided in Section 11.2. Each sinking fund payment shall be applied to the redemption of Securities of any Series as provided for by the terms of the Securities of such Series.
Section 11.2. Satisfaction of Sinking Fund Payments with Securities.
The Company may, in satisfaction of all or any part of any sinking fund payment with respect to the Securities of any Series to be made pursuant to the terms of such Securities (1) deliver outstanding Securities of such Series to which such sinking fund payment is applicable (other than any of such Securities previously called for mandatory sinking fund redemption) and (2) apply as credit Securities of such Series to which such sinking fund payment is applicable and which have been repurchased by the Company or redeemed either at the election of the Company pursuant to the terms of such Series of Securities (except pursuant to any mandatory sinking fund) or through the application of permitted optional sinking fund payments or other optional redemptions pursuant to the terms of such Securities, provided that such Securities have not been previously so credited. Such Securities shall be received by the Trustee, together with an Officer’s Certificate with respect thereto, not later than 15 days prior to the date on which the Trustee begins the process of selecting Securities for redemption and shall be credited for such purpose by the Trustee at the price specified in such Securities for redemption through operation of the sinking fund and the amount of such sinking fund payment shall be reduced accordingly. If as a result of the delivery or credit of Securities in lieu of cash payments pursuant to this Section 11.2, the principal amount of Securities of such Series to be redeemed in order to exhaust the aforesaid cash payment shall be less than $100,000, the Trustee need not call Securities of such Series for redemption, except upon receipt of a Company Order that such action be taken, and such cash payment shall be held by the Trustee or a Paying Agent and applied to the next succeeding sinking fund payment, provided, however, that the Trustee or such Paying Agent shall from time to time upon receipt of a Company Order pay over and deliver to the Company any cash payment so being held by the Trustee or such Paying Agent upon delivery by the Company to the Trustee of Securities of that Series purchased by the Company having an unpaid principal amount equal to the cash payment required to be released to the Company.
Section 11.3. Redemption of Securities for Sinking Fund.
Not less than 45 days (unless otherwise indicated in the Board Resolution, supplemental indenture hereto or Officer’s Certificate in respect of a particular Series of Securities) prior to each sinking fund payment date for any Series of Securities, the Company will deliver to the Trustee an Officer’s Certificate specifying the amount of the next ensuing mandatory sinking fund payment for that Series pursuant to the terms of that Series, the portion thereof, if any, which is to be satisfied by payment of cash and the portion thereof, if any, which is to be satisfied by delivering and crediting of Securities of that Series pursuant to Section 11.2, and the optional amount, if any, to be added in cash to the next ensuing mandatory sinking fund payment, and the Company shall thereupon be obligated to pay the amount therein specified. Not less than 30 days (unless otherwise indicated in the Board Resolution, Officer’s Certificate or supplemental indenture in respect of a particular Series of Securities) before each such sinking fund payment date the Securities to be redeemed upon such sinking fund payment date will be selected in the manner specified in Section 3.2, and the Company shall send or cause to be sent a notice of the redemption thereof to be given in the name of and at the expense of the Company in the manner provided in and in accordance with Section 3.3. Such notice having been duly given, the redemption of such Securities shall be made upon the terms and in the manner stated in Sections 3.4, 3.5 and 3.6.
IN WITNESS WHEREOF, the parties hereto have caused this Indenture to be duly executed as of the day and year first above written.
|
|
VISTAGEN THERAPEUTICS, INC.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
|
|
|
|
|
Name:
|
|
|
|
|
Its:
|
|
|
|
[TRUSTEE], as Trustee
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
|
|
|
|
|
Name:
|
|
|
|
|
Its:
|
|
Exhibit 5.1
February 13, 2024
Vistagen Therapeutics, Inc.
343 Allerton Avenue
South San Francisco, California 94090
Ladies and Gentlemen:
We have acted as special Nevada counsel to Vistagen Therapeutics, Inc., a Nevada corporation (the “Company”), in connection with the preparation of the Registration Statement on Form S-3 (the “Registration Statement”), including a base prospectus (the “Base Prospectus”), to be filed on the date hereof by the Company and the Company with the Securities and Exchange Commission (the “Commission”). The Registration Statement relates to, among other things, the issuance and sale from time to time by the Company, pursuant to Rule 415 of the General Rules and Regulations promulgated under the Securities Act of 1933, as amended (the “Securities Act”), of up to $350,000,000.00 of the following securities: (i) shares of common stock of the Company, $0.001 par value per share (the “Common Stock”); (ii) shares of preferred stock of the Company, $0.001 par value per share (the “Preferred Stock”), in one or more series; (iii) senior debt securities of the Company, in one or more series, consisting of notes, debentures or other evidences of indebtedness (the “Senior Debt Securities”) pursuant to a senior indenture (the “Senior Indenture”), to be entered into between the Company and a senior trustee to be named in a prospectus supplement; (iv) subordinated debt securities of the Company, in one or more series, consisting of notes, debentures or other evidences of indebtedness (the “Subordinated Debt Securities,” and together with the Senior Debt Securities, the “Debt Securities”) pursuant to an subordinated indenture (the “Subordinated Indenture,” and together with the Senior Indenture, the “Indentures”), to be entered into between the Company and a subordinated trustee to be named in a prospectus supplement (v) warrants to purchase equity securities of the Company (“Warrants”); and (vi) units consisting of one or more Warrants, Debt Securities, Preferred Stock, Common Stock or any combination of such securities (“Units”) (items (i) through (vi) above are collectively referred to herein as the “Securities”).
We have also acted as special counsel to the Company in connection with the sale through Jefferies LLC as the sales agent (the “Sales Agent”) from time to time by the Company of shares of Common Stock (the “ATM Shares”) having an aggregate offering price of up to $100,000,000 pursuant to the Registration Statement, the Base Prospectus and the related prospectus supplement for the sale of the ATM Shares included in the Registration Statement (the Base Prospectus and such prospectus supplement, collectively, the “Sales Agreement Prospectus”), and an Open Market Sale AgreementSM, dated as of May 14, 2021 (the “Sales Agreement”).
Vistagen Therapeutics, Inc.
February 13, 2024
Page 2
In connection with this opinion, we have examined originals or copies, certified, or otherwise identified to our satisfaction, of:
| |
(i)
|
the Restated Articles of Incorporation of the Company, as filed with the Nevada Secretary of State on August 11, 2016;
|
| |
(ii)
|
Certificate of Amendment to Articles of Incorporation of the Company, as filed with the Nevada Secretary of State on September 15, 2017;
|
| |
(iii)
|
Certificate of Amendment to Articles of Incorporation of the Company, as filed with the Nevada Secretary of State on September 6, 2019;
|
| |
(iv)
|
Certificate of Amendment to Articles of Incorporation of the Company, as filed with the Nevada Secretary of State on March 5, 2021;
|
| |
(v)
|
Certificate of Amendment to Articles of Incorporation of the Company, as filed with the Nevada Secretary of State on June 5, 2023;
|
| |
(vi)
|
Second Amended and Restated Bylaws of the Company, adopted August 16, 2016, as amended through the date hereof, and certified to us to be currently in effect;
|
| |
(vii)
|
a Certificate of Good Standing for the Company issued by the Nevada Secretary of State on February 13, 2024;
|
| |
(viii)
|
the Sales Agreement;
|
| |
(ix)
|
the Registration Statement;
|
| |
(xi)
|
the Sales Agreement Prospectus;
|
| |
(xii)
|
Resolutions adopted by the Board of Directors of the Company (the “Board of Directors”) dated May 11, 2021, authorizing and approving the Sales Agreement;
|
| |
(xiii)
|
Resolutions adopted by the Board of Directors dated February 13, 2024, authorizing and approving the Registration Statement, the issuance and sale of the Securities, including the issuance of the ATM Shares described in the Registration Statement and the Sales Agreement Prospectus, authorizing and approving the Sales Agreement Prospectus, constituting the ATM Pricing Committee, authorizing the Company to reserve an additional 20,400,000 shares of Common Stock for issuance as ATM Shares, and authorizing the ATM Pricing Committee to act with respect to the ATM Shares described in the Sales Agreement Prospectus; and
|
| |
(xiv)
|
a certificate, dated February 13, 2024, from an Officer of the Company as to certain factual matters, including, the incumbency of the officers of the Company (the “Officer's Certificate”).
|
In addition to the foregoing, we have examined such other instruments, documents and records that we deemed relevant and necessary for the basis of our opinion hereinafter expressed. We have assumed the authenticity of all records, documents and instruments submitted to us as originals, the genuineness of all signatures, the legal capacity of natural persons and the conformity to the originals of all records, documents and instruments submitted to us as copies.
In rendering the opinions contained herein, we have, with your permission, made the following assumptions: (i) all documents submitted to or reviewed by us, including all amendments and supplements thereto, are accurate and complete and, if not originals, are true, correct, and complete copies of the originals; (ii) the signatures on each of such documents by the parties thereto are genuine; (iii) each individual who signed such documents had the legal capacity to do so; (iv) all persons who signed such documents on behalf of a business entity were duly authorized to do so; (v) all statements in certificates of public officials and officers of the Company that we reviewed were and are accurate, and (vi) all representations made by the Company as to matters of fact in the documents that we reviewed were and are accurate. We have assumed that there are no amendments, modifications, or supplements to such documents other than those amendments, modifications, and supplements that are known to us.

Vistagen Therapeutics, Inc.
February 13, 2024
Page 3
This opinion is limited to the Nevada Revised Statutes, and we disclaim any opinion as to the laws of any other jurisdiction. We further disclaim any opinion as to any other statute, rule, regulation, ordinance, order or other promulgation of any other jurisdiction or any regional or local governmental body or as to any related judicial or administrative opinion.
In rendering the opinions set forth below, we have assumed (i) that at the time of offer, issuance and sale of any Securities, the Registration Statement, and any other required post-effective amendments thereto, will be effective under the Securities Act, (ii) any required prospectus supplement with respect to such Securities will have been delivered and filed with the Commission and no stop order suspending its effectiveness will have been issued and remain in effect; (iii) that the Board of Directors of the Company duly authorizes by proper corporate action the terms and issuance of the Securities, (iv) the qualification of the Indentures under the Trust Indenture Act of 1939, as amended (the “TIA”), with respect to the Debt Securities (v) the due execution, authentication, issuance and delivery of the Debt Securities by the Company upon payment of the consideration therefor as provided in the applicable purchase, underwriting or similar agreements duly approved by the requisite corporate action by the Company and otherwise, if applicable, in accordance with the provisions of the Indenture governing the Debt Securities (vi) upon the issuance of Common Stock, including Common Stock that may be issued upon conversion or exercise of any other Securities convertible into or exercisable for Common Stock, the total number of shares of Common Stock issued and outstanding will not exceed the total number of shares of Common Stock that the Company is then authorized to issue under the Restated Articles of Incorporation, as amended; and (vii) upon the issuance of Preferred Stock, including Preferred Stock that may be issued upon conversion or exercise of any other Securities convertible into or exercisable for Preferred Stock, the total number of shares of Preferred Stock issued and outstanding, and the total number of issued and outstanding shares of the applicable class or series of Preferred Stock designated pursuant to the Restated Articles of Incorporation, will not exceed the total number of shares of Preferred Stock or the number of shares of such class or series of Preferred Stock that the Company is then authorized to issue under the Restated Articles of Incorporation, as amended.
In rendering the opinions expressed in paragraphs 4 through 6 below with respect to the Securities referred to therein, we have additionally assumed that: (i) the Trustee identified in the Indenture will have all requisite power and authority to execute, deliver, and perform its obligations under the Indenture in question; (ii) at the time of execution of the Indenture in question, the execution and delivery thereof and the performance of such obligations will have been duly authorized by all necessary action on the Trustee’s part, and the Indenture in question will have been duly delivered by it; (iii) at the time of execution of the Indenture, the Indenture in question will be enforceable in accordance with the terms thereof; (iv) any supplemental indenture to an Indenture and any Board Resolution (as defined in the Indenture in question) and/or Officer’s Certificate (as defined in the Indenture in question) executed and delivered pursuant to the Indenture in question, in any such case, pursuant to which any Debt Securities are issued, will comply with the Indenture in question as theretofore supplemented, and the form and terms of such Debt Securities will comply with the Indenture in question as then supplemented (including by such supplemental indenture) and any such Board Resolution and/or Officer’s Certificate; (v) The Company is and at all times material hereto will be a corporation duly organized and validly existing under the laws of the State of Nevada; and (vi) the Indenture in question actually entered into by the Company and Trustee will not deviate in any material or substantial respect from the Indenture terms described in the Registration Statement, such that any deviation would alter our opinions contained herein.

Vistagen Therapeutics, Inc.
February 13, 2024
Page 4
Based upon the foregoing and our examination of such questions of law as we have deemed necessary or appropriate for the purpose of this opinion, it is our opinion that
| |
1.
|
The Company is validly existing as a corporation in good standing under the laws of the State of Nevada.
|
| |
2.
|
With respect to any offering of Common Stock (the “Offered Common Stock”), when (i) the Registration Statement, as finally amended (including all necessary post-effective amendments), has become effective under the Securities Act; (ii) an appropriate prospectus supplement (or term sheet) with respect to the Offered Common Stock has been prepared, delivered, and filed in compliance with the Securities Act and the applicable rules and regulations thereunder; (iii) if the Offered Common Stock is to be sold pursuant to a firm commitment underwritten offering, an underwriting agreement with respect to the Offered Common Stock will have been duly authorized, executed, and delivered by the Company and the other parties thereto; (iv) the Board of Directors, including any appropriate committee appointed thereby, and appropriate officers of the Company have taken all necessary corporate action to approve the issuance of the Offered Common Stock and related matters; (v) the terms of the issuance and sale of the Offered Common Stock have been duly established in conformity with the Restated Articles of Incorporation and the Bylaws of the Company, each as amended and then in effect, so as not to (A) violate any applicable law, (B) violate the Restated Articles of Incorporation or the Bylaws of the Company, each as amended and then in effect, or (C) result in a default under or breach of any agreement or instrument binding upon the Company and so as to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; and (vi) a certificate or certificates representing the Offered Common Stock are duly executed, countersigned, registered and delivered upon payment of the agreed upon consideration therefor, the Offered Common Stock (including any Common Stock duly issued upon conversion, exchange, or exercise of any Preferred Stock), when issued and sold in accordance with the applicable underwriting agreement, with respect to the Offered Common Stock, or any other duly authorized, executed and delivered valid and binding purchase or agency agreement, will be duly authorized, validly issued, fully paid and nonassessable, provided that the consideration therefor is not less than the par value thereof.
|
| |
3.
|
With respect to any offering of a series of Preferred Stock (the “Offered Preferred Stock”), when (i) the Registration Statement, as finally amended (including all necessary post-effective amendments), has become effective under the Securities Act; (ii) an appropriate prospectus supplement (or term sheet) with respect to the Offered Preferred Stock has been prepared, delivered, and filed in compliance with the Securities Act and the applicable rules and regulations thereunder; (iii) if the Offered Preferred Stock is to be sold pursuant to a firm commitment underwritten offering, an underwriting agreement with respect to the Offered Preferred Stock will have been duly authorized, executed, and delivered by the Company and the other parties thereto; (iv) the Board of Directors, including any appropriate committee appointed thereby, and appropriate officers of the Company have taken all necessary corporate action to approve the issuance and terms of the Offered Preferred Stock and related matters, including the adoption of a Certificate of Designation for the Offered Preferred Stock in accordance with the applicable provisions of Nevada law (the “Certificate of Designation”); (v) the filing of the Certificate of Designation with the Secretary of State of the State of Nevada has duly occurred; (vi) the terms, as well as the terms of the issuance and sale, of the Offered Preferred Stock have been duly established in conformity with the Company’s Restated Articles of Incorporation, including the Certificate of Designation relating to the Offered Preferred Stock, and the Bylaws of the Company, each as amended and then in effect, so as not to (A) violate any applicable law, (B) violate the Restated Articles of Incorporation or the Bylaws of the Company, each as amended and then in effect, or (C) result in a default under or breach of any agreement or instrument binding upon the Company and so as to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; and (vii) a certificate or certificates representing the Offered Preferred Stock are duly executed, countersigned, registered and delivered upon payment of the agreed upon consideration therefor, the Offered Preferred Stock, when issued and sold in accordance with the applicable underwriting agreement, with respect to the Offered Preferred Stock, or any other duly authorized, executed, and delivered valid and binding purchase or agency agreement, will be duly authorized, validly issued, fully paid, and nonassessable, provided that the consideration therefor is not less than the par value thereof.
|

Vistagen Therapeutics, Inc.
February 13, 2024
Page 5
| |
4.
|
With respect to any offering of any series of Debt Securities (the “Offered Debt Securities”), when (i) the Registration Statement, as finally amended (including all necessary post-effective amendments), has become effective under the Securities Act and the applicable Indenture (as supplemented) has been qualified under the Trust Indenture Act; (ii) an appropriate prospectus supplement (or term sheet) with respect to the Offered Debt Securities has been prepared, delivered, and filed in compliance with the Securities Act and the applicable rules and regulations thereunder; (iii) if the Offered Debt Securities are to be sold pursuant to a firm commitment underwritten offering, an underwriting agreement with respect to the Offered Debt Securities will have been duly authorized, executed, and delivered by the Company and the other parties thereto; (iv) the Board of Directors, including any appropriate committee appointed thereby, and appropriate officers of the Company have taken all necessary corporate action to approve the issuance and terms of the Offered Debt Securities and related matters; (v) the terms, as well as the terms of the issuance and sale, of the Offered Debt Securities have been duly established in conformity with the applicable Indenture (as supplemented) so as not to (A) violate any applicable law, (B) violate the Restated Articles of Incorporation or the Bylaws of the Company, each as amended and then in effect, or (C) result in a default under or breach of any agreement or instrument binding upon the Company and so as to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; and (vi) the Offered Debt Securities are duly executed and authenticated in accordance with the provisions of the applicable Indenture (as supplemented) and duly delivered to the purchasers thereof upon payment of the agreed upon consideration therefor, the Offered Debt Securities (including any Debt Securities duly issued upon conversion, exchange, or exercise of any Debt Securities or Preferred Stock), when issued and sold in accordance with the applicable Indenture (as supplemented) and the applicable underwriting agreement, if any, or any other duly authorized, executed, and delivered valid and binding purchase or agency agreement, will be valid and binding obligations of the Company, to the extent Nevada law governs such issues.
|
| |
5.
|
With respect to any offering of Warrants to be issued under a Warrant Agreement (the “Offered Warrants”), when (i) the Registration Statement, as finally amended (including all necessary post-effective amendments), has become effective under the Securities Act; (ii) an appropriate prospectus supplement (or term sheet) with respect to the Offered Warrants has been prepared, delivered, and filed in compliance with the Securities Act and the applicable rules and regulations thereunder; (iii) the Board of Directors, including any appropriate committee appointed thereby, and appropriate officers of the Company have taken all necessary corporate action to approve the issuance and terms of the Offered Warrants and related matters, (iv) the Warrant Agreement has been duly authorized and validly executed and delivered by the Company and the warrant agent under the Warrant Agreement; (v) the terms of the issuance and sale of the Offered Warrants have been duly established in conformity with the Restated Articles of Incorporation and the Bylaws of the Company, each as amended and then in effect, so as not to (A) violate any applicable law, (B) violate the Restated Articles of Incorporation or the Bylaws of the Company, each as amended and then in effect, or (C) result in a default under or breach of any agreement or instrument binding upon the Company and so as to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; and (vi) the Offered Warrants are duly executed, issued, and delivered in accordance with the terms of the Warrant Agreement and the applicable definitive purchase, underwriting, or similar agreement approved by the Board of Directors of the Company, upon payment (or delivery) of the consideration therefor provided for therein, the Offered Warrants will be valid and binding obligations of the Company, to the extent that Nevada law governs such issues.
|

Vistagen Therapeutics, Inc.
February 13, 2024
Page 6
| |
6.
|
With respect to any offering of Units (the “Offered Units”), when (i) the Registration Statement, as finally amended (including all necessary post-effective amendments), has become effective under the Securities Act; (ii) an appropriate prospectus supplement (or term sheet) with respect to the Offered Units has been prepared, delivered, and filed in compliance with the Securities Act and the applicable rules and regulations thereunder; (iii) the Board of Directors, including any appropriate committee appointed thereby, and appropriate officers of the Company have taken all necessary corporate action to authorize and approve (A) the issuance and terms of the Offered Units, (B) the issuance and terms of any Warrants which are a component of the Units, the terms of the offering thereof and related matters, and the execution and delivery of any related Warrant Agreement, and (C) the issuance and terms of any Preferred Stock or Common Stock which are a component of the Units, the terms of the offering thereof and related matters, (iv) the terms of the issuance and sale of the Offered Units have been duly established in conformity with the Restated Articles of Incorporation and the Bylaws of the Company, each as amended and then in effect, so as not to (A) violate any applicable law, (B) violate the Restated Articles of Incorporation or the Bylaws of the Company, each as amended and then in effect, or (C) result in a default under or breach of any agreement or instrument binding upon the Company and so as to comply with any requirement or restriction imposed by any court or governmental body having jurisdiction over the Company; and (v) all of the parties duly execute and deliver (A) the applicable Offered Units, (B) such Warrants and Warrant Agreement, and (C) such Preferred Stock and Common Stock, and each such Security is issued, in each case upon payment of the consideration therefor provided for in the applicable definitive purchase, underwriting, or similar agreement approved by the Board of Directors of the Company and otherwise in accordance with the provisions of the applicable Warrant Agreement, in the case of the Warrants, or the Company’s Restated Articles of Incorporation and Bylaws (as amended to such date and then in effect), in the case of such Preferred Stock and Common Stock, such Offered Units will be valid and binding obligations of the Company, to the extent Nevada law governs such issues.
|
| |
7.
|
When (a) the Registration Statement and the Sales Agreement Prospectus, as finally amended (including all necessary post-effective amendments) have become effective under the Securities Act, (b) if the ATM Shares are to be certificated, certificates in the form required under the NRS representing the ATM Shares are duly executed and countersigned, (c) the Pricing Committee of the Board of Directors has taken all necessary corporate action to approve the issuance of the ATM Shares and related matters, and (d) the ATM Shares are registered in the Company’s share registry and delivered upon payment of the agreed upon consideration therefor, the ATM Shares will be duly authorized by all necessary corporate action of the Company, and when issued and sold in accordance with the provisions of the Sales Agreement, will be validly issued, fully paid and nonassessable, provided that the consideration therefor is not less than $0.001 per share of Common Stock.
|
Vistagen Therapeutics, Inc.
February 13, 2024
Page 7
In rendering the opinions expressed above, we have assumed that, at or prior to the time of the delivery of any such Security, there shall not have occurred any change in law affecting the validity or enforceability of such Security.
With respect to any agreement or instrument reviewed by us, that by its terms or otherwise is governed by the law of any jurisdiction other than the laws of the State of Nevada, our opinion herein is based solely on our understanding of the plain language of such agreement or instrument and we do not express our opinion with respect to the interpretation, validity, binding nature, or enforceability of any such agreement or instrument, and we do not assume any responsibility with respect to the effect on the opinions or statements set forth herein of any interpretation thereof inconsistent with such understanding. We note that the Registration Statement provides that any Indenture shall be governed by the laws of the State of New York. We therefore assume that each Indenture will be enforceable under the laws of the State of New York.
This opinion is rendered to you in connection with the Registration Statement and is not to be relied upon for any other purpose. We disclaim any obligation to advise you of any change of law that occurs, or any facts of which we may become aware, after the date of this opinion.
This opinion is based upon our knowledge of the law and facts relevant to the transactions herein referenced as of the date hereof. We assume no duty to update or supplement this opinion to reflect any facts or circumstances that may hereafter come to our attention or to reflect any changes in any law that may hereafter occur or become effective.
We hereby consent to the filing of this opinion as an exhibit to the Registration Statement. In giving this consent, we do not thereby admit that we are included in the category of persons whose consent is required under Section 7 of the Securities Act or the rules and regulations of the Securities and Exchange Commission.
|
|
Very truly yours,.
WOODBURN AND WEDGE
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
By:
|
/s/ Shawn G. Pearson
|
|
|
|
|
Shawn G. Pearson
|
|
Exhibit 23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Prospectus constituting a part of this Registration Statement on Form S-3 of our report dated June 28, 2023, which includes an explanatory paragraph regarding Vistagen Therapeutics, Inc.’s ability to continue as a going concern, relating to the consolidated financial statements of Vistagen Therapeutics, Inc. as of and for the years ended March 31, 2023 and 2022, which is incorporated by reference in that Prospectus.
We also consent to the reference to us under the caption “Experts” in the Prospectus.
/s/ WithumSmith+Brown, PC
San Francisco, California
February 13, 2024
Exhibit 107
Calculation of Filing Fee Tables
Form S-3
(Form Type)
Vistagen Therapeutics, Inc.
(Exact Name of Registrant as Specified in its Charter)
Table 1: Newly Registered and Carry Forward Securities
| |
Security Type
|
|
Security
Class
Title
|
|
Fee
Calculation
or Carry
Forward
Rule
|
|
Amount
Registered
(a)
|
|
Proposed
Maximum
Offering
Price
Per
Unit
|
|
|
Maximum
Aggregate
Offering
Price
|
|
|
Fee Rate
|
|
|
Amount of
Registration
Fee
|
|
Carry
Forward
Form
Type
|
|
Carry
Forward
File
Number
|
|
Carry
Forward
Initial
effective
date
|
|
Filing Fee
Previously
Paid
In
Connection
with
Unsold
Securities
to be
Carried
Forward
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Newly Registered Securities
|
|
|
Fees to Be
Paid
|
Equity
|
|
Common Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Equity
|
|
Preferred Stock
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Other
|
|
Debt Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Other
|
|
Warrants
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Other
|
|
Units
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Unallocated (Universal)
Shelf
|
|
|
|
Rule 457(o)
|
|
(b)
|
|
(b)
|
|
|
$350,000,000(c)
|
|
|
|
0.00014760 |
|
|
$ |
51,660.00 |
|
|
|
|
|
|
|
|
|
|
|
|
Fees
Previously
Paid
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carry Forward Securities
|
|
|
Carry
Forward
Securities
|
Unallocated (Universal)
Shelf
|
|
Unallocated (Universal)
Shelf
|
|
Rule
415(a)(6)
|
|
(d)
|
|
|
$ |
250,000,000 |
|
|
|
0.0001091 |
|
|
$ |
27,275.00 |
|
S-3
|
|
|
333-254299 |
|
03/26/2021
|
|
$ |
11,803.07 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
Total Offering Amounts
|
|
|
|
|
|
|
|
|
|
|
$ |
51,660.00 |
|
|
|
|
|
|
|
|
|
|
|
| |
Total Fees Previously Paid
|
|
|
|
|
|
|
|
|
|
|
$ |
11,803.07 |
|
|
|
|
|
|
|
|
|
|
|
| |
Total Fee Offsets
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
| |
Net Fee Due
|
|
|
|
|
|
|
|
|
|
|
$ |
39,856.93 |
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
In accordance with Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), this registration statement shall be deemed to cover an indeterminate number of additional shares of Common Stock to be offered or issued from stock splits, stock dividends or similar transaction.
|
| |
|
|
(b)
|
Pursuant to Instruction 2.A.iii.b. of Item 16(b) of Form S-3, this information is not specified as to each class of securities to be registered. There is being registered hereby such indeterminate number of the securities of each identified class as may from time to time be issued at indeterminate prices. Securities registered hereunder may be sold separately, together or in units with other securities registered hereunder.
|
| |
|
|
(c)
|
The proposed maximum aggregate offering price has been estimated solely to calculate the registration fee in accordance with Rule 457(o) under the Securities Act.
|
| |
|
|
(d)
|
On March 15, 2021, the Registrant filed a registration statement on Form S-3 (File No. 333-254299), or the Prior Registration Statement, to register securities with an aggregate maximum offering price of $250,000,000, and paid a registration fee of $27,257.00 in connection therewith. As of the date of this registration statement, an aggregate of $108,185,806.37 of securities registered on the Prior Registration Statement are unsold. Pursuant to Rule 415(a)(6), the Registrant is allowed to apply $11,803.07 toward the registration fee for this registration statement because the unsold securities are being moved from the Prior Registration Statement to this registration statement, and the registration fee previously paid by the Registrant relating to the unsold securities included on this registration statement will continue to be applied to such unsold securities.
|
VistaGen Therapeutics (NASDAQ:VTGN)
Historical Stock Chart
From Mar 2024 to Apr 2024
VistaGen Therapeutics (NASDAQ:VTGN)
Historical Stock Chart
From Apr 2023 to Apr 2024