false000157942800015794282024-01-082024-01-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): January 08, 2024 |
Axsome Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-37635 |
45-4241907 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
One World Trade Center, 22nd Floor |
|
New York, New York |
|
10007 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (212) 332-3241 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, Par Value $0.0001 Per Share |
|
AXSM |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On January 8, 2024, the Company updated its corporate presentation and posted such corporate presentation to the Company’s website. The updated corporate presentation is filed as Exhibit 99.1 hereto and incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
Exhibit No. |
|
Description |
|
|
|
99.1 |
|
Corporate Presentation. |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Axsome Therapeutics, Inc. |
|
|
|
|
Date: |
January 8, 2024 |
By: |
/s/ Herriot Tabuteau, M.D. |
|
|
Name: Title: |
Herriot Tabuteau, M.D.
President and Chief Executive Officer |

Corporate Presentation January 2024

Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the continued commercial success of our Sunosi® and Auvelity® products and the success of our efforts to obtain any additional indication(s) with respect to solriamfetol and/or AXS-05; the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected revenues or expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials, and the number or type of studies or nature of results necessary to support the filing of a new drug application (“NDA”) for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates; whether issues identified by FDA in the complete response letter may impact the potential approvability of the Company’s NDA for AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment for the MOMENTUM clinical trial; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s products and product candidates, if approved; the Company’s anticipated capital requirements, including the amount of capital required for the continued commercialization of Sunosi and Auvelity and for the Company’s commercial launch of its other product candidates, and the potential impact on the Company’s anticipated cash runway; unforeseen circumstances or other disruptions to normal business operations arising from or related to COVID-19; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this press release and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance.. This presentation contains statements regarding the Company’s observations based upon the reported clinical data. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about the Company's industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. Axsome, Auvelity, Sunosi, and MoSEIC, are trademarks or registered trademarks of Axsome Therapeutics, Inc. or its affiliates. Except as with respect to Auvelity and Sunosi for their approved indications, the development products referenced herein have not been approved by the FDA. Forward Looking Statements & Safe Harbor
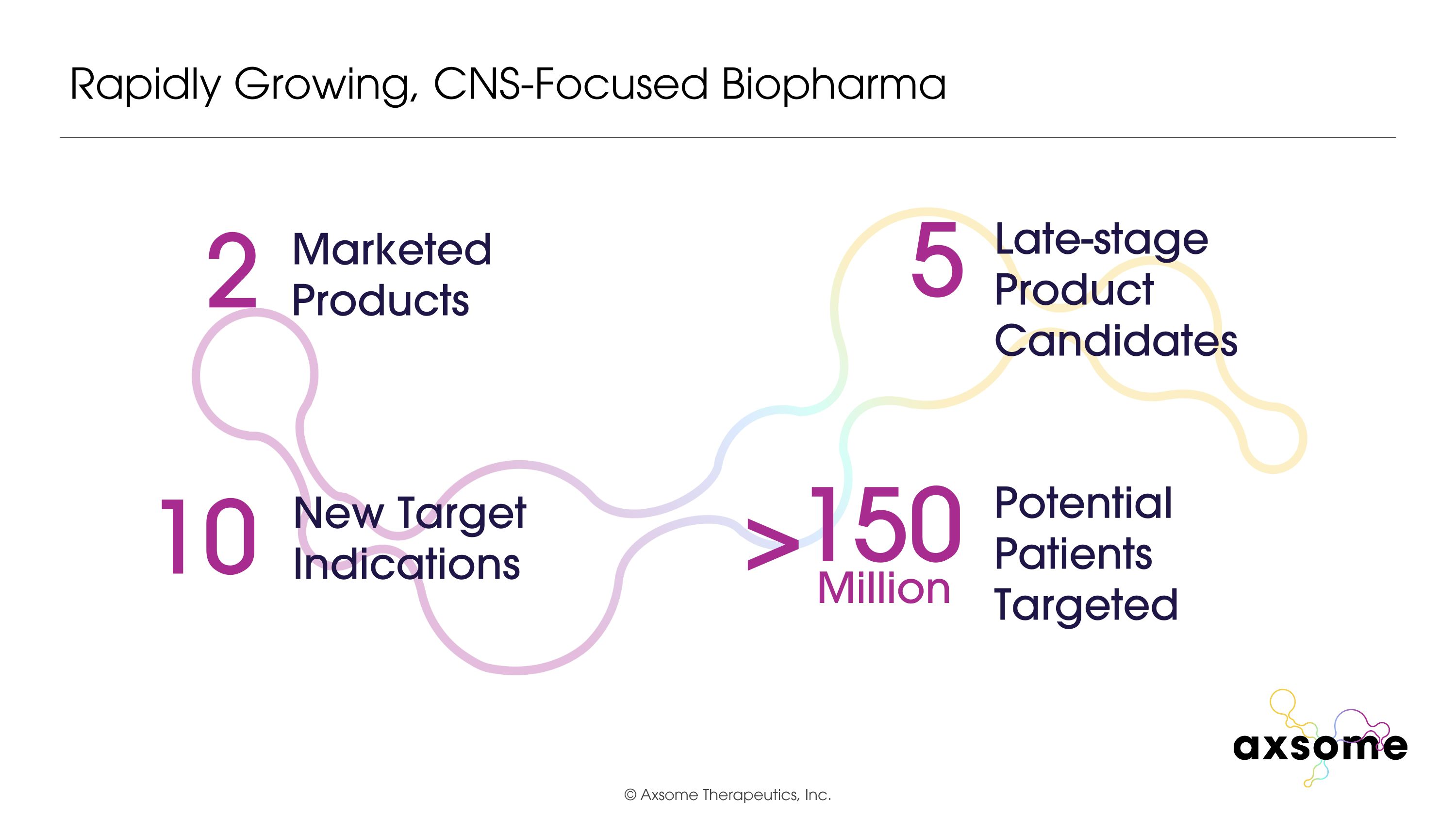
Rapidly Growing, CNS-Focused Biopharma 2 Marketed Products 10 New Target Indications 5 Late-stage Product Candidates Potential Patients Targeted 150 Million >
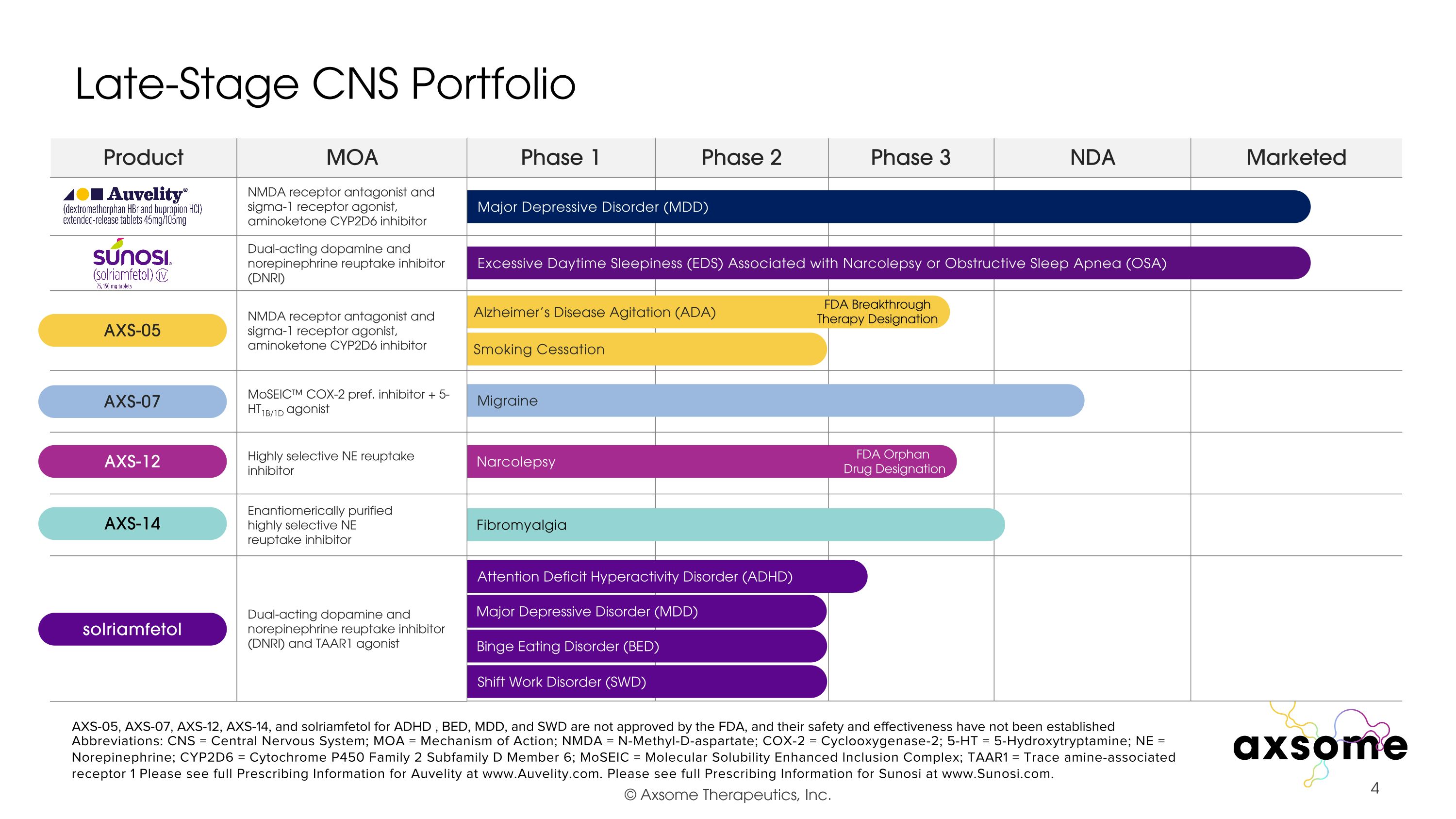
Late-Stage CNS Portfolio Phase 1 Phase 2 Phase 3 NDA Marketed Abbreviations: CNS = Central Nervous System; MOA = Mechanism of Action; NMDA = N-Methyl-D-aspartate; COX-2 = Cyclooxygenase-2; 5-HT = 5-Hydroxytryptamine; NE = Norepinephrine; CYP2D6 = Cytochrome P450 Family 2 Subfamily D Member 6; MoSEIC = Molecular Solubility Enhanced Inclusion Complex; TAAR1 = Trace amine-associated receptor 1 Please see full Prescribing Information for Auvelity at www.Auvelity.com. Please see full Prescribing Information for Sunosi at www.Sunosi.com. Alzheimer’s Disease Agitation (ADA) Smoking Cessation Attention Deficit Hyperactivity Disorder (ADHD) Migraine Narcolepsy Fibromyalgia AXS-05, AXS-07, AXS-12, AXS-14, and solriamfetol for ADHD , BED, MDD, and SWD are not approved by the FDA, and their safety and effectiveness have not been established Major Depressive Disorder (MDD) Excessive Daytime Sleepiness (EDS) Associated with Narcolepsy or Obstructive Sleep Apnea (OSA) Binge Eating Disorder (BED) Shift Work Disorder (SWD) FDA Breakthrough Therapy Designation FDA Orphan Drug Designation Major Depressive Disorder (MDD) Product MOA NMDA receptor antagonist and sigma-1 receptor agonist, aminoketone CYP2D6 inhibitor Dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) NMDA receptor antagonist and sigma-1 receptor agonist, aminoketone CYP2D6 inhibitor MoSEIC™ COX-2 pref. inhibitor + 5-HT1B/1D agonist Highly selective NE reuptake inhibitor Enantiomerically purified highly selective NE �reuptake inhibitor Dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) and TAAR1 agonist AXS-05 AXS-07 AXS-12 solriamfetol AXS-14
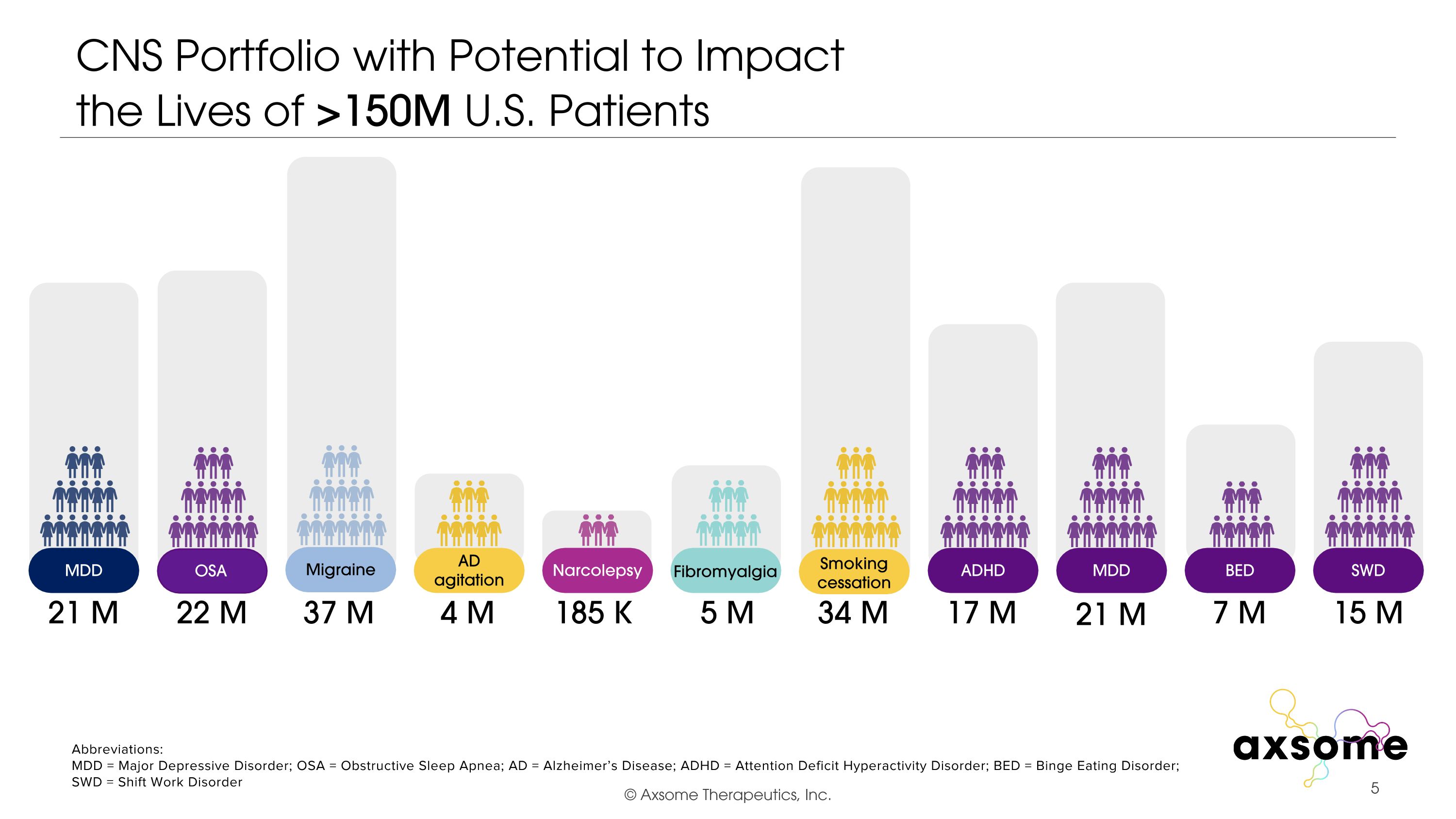
CNS Portfolio with Potential to Impact �the Lives of >150M U.S. Patients 17 M 34 M 185 K 4 M 37 M 5 M 22 M 21 M Abbreviations: MDD = Major Depressive Disorder; OSA = Obstructive Sleep Apnea; AD = Alzheimer’s Disease; ADHD = Attention Deficit Hyperactivity Disorder; BED = Binge Eating Disorder; SWD = Shift Work Disorder 7 M 15 M Migraine AD agitation Fibromyalgia Smoking cessation Narcolepsy OSA MDD ADHD MDD BED SWD 21 M
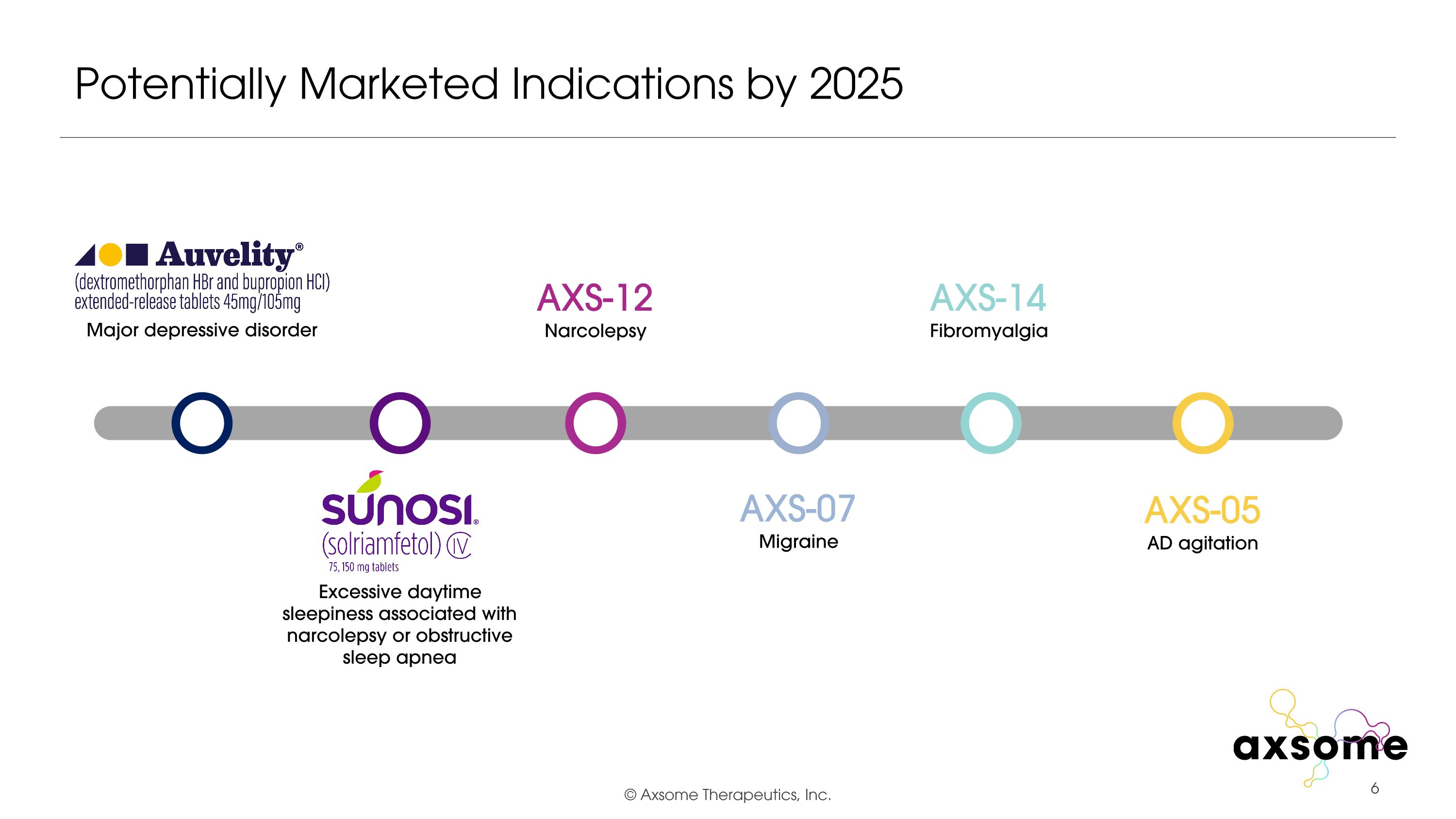
Potentially Marketed Indications by 2025 AXS-14 Fibromyalgia AXS-07 Migraine AXS-05 AD agitation AXS-12 Narcolepsy Major depressive disorder Excessive daytime sleepiness associated with narcolepsy or obstructive sleep apnea

Marketed Products
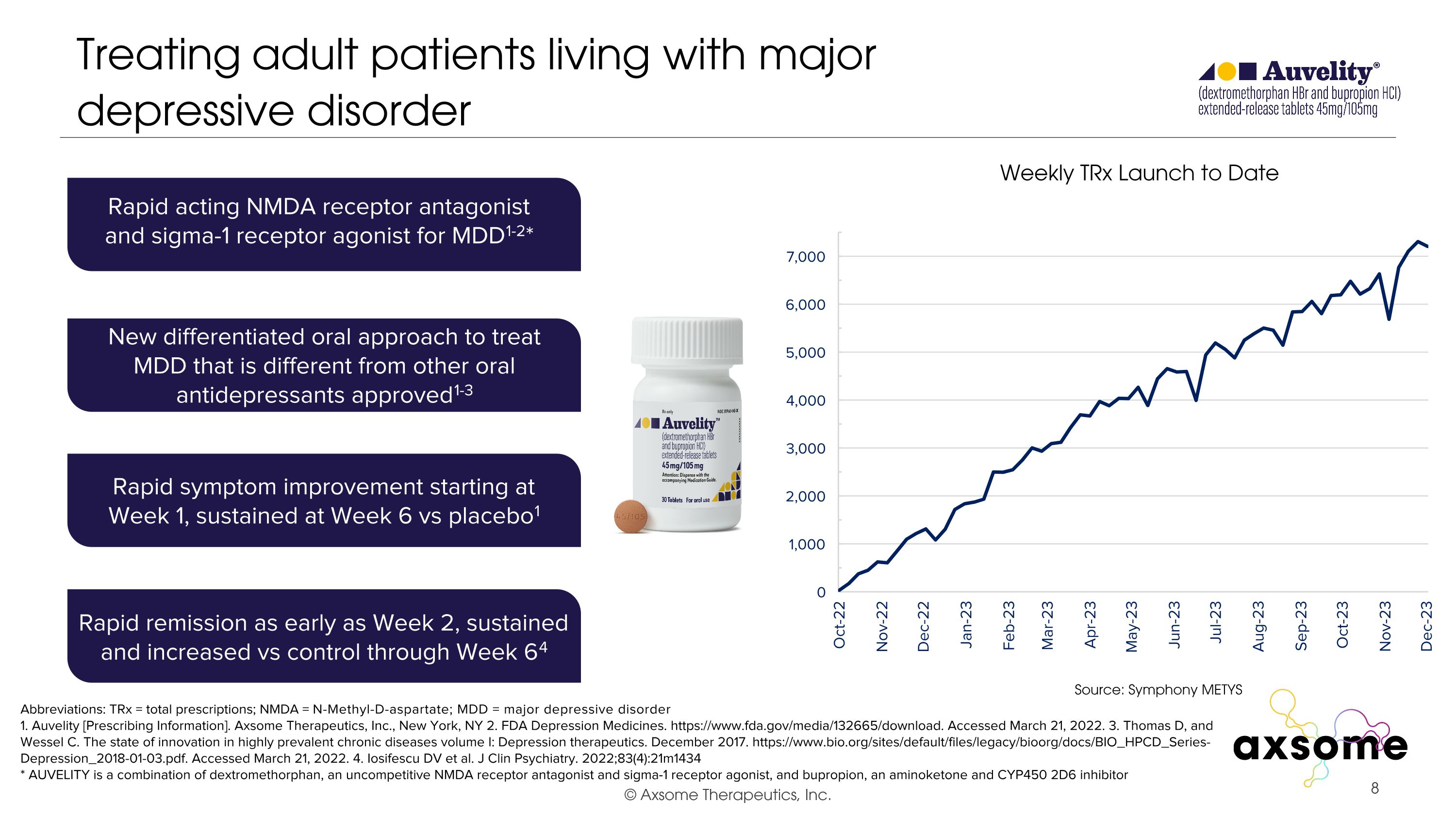
Treating adult patients living with major depressive disorder Abbreviations: TRx = total prescriptions; NMDA = N-Methyl-D-aspartate; MDD = major depressive disorder 1. Auvelity [Prescribing Information]. Axsome Therapeutics, Inc., New York, NY 2. FDA Depression Medicines. https://www.fda.gov/media/132665/download. Accessed March 21, 2022. 3. Thomas D, and Wessel C. The state of innovation in highly prevalent chronic diseases volume I: Depression therapeutics. December 2017. https://www.bio.org/sites/default/files/legacy/bioorg/docs/BIO_HPCD_Series-Depression_2018-01-03.pdf. Accessed March 21, 2022. 4. Iosifescu DV et al. J Clin Psychiatry. 2022;83(4):21m1434 * AUVELITY is a combination of dextromethorphan, an uncompetitive NMDA receptor antagonist and sigma-1 receptor agonist, and bupropion, an aminoketone and CYP450 2D6 inhibitor Rapid acting NMDA receptor antagonist and sigma-1 receptor agonist for MDD1-2* New differentiated oral approach to treat MDD that is different from other oral antidepressants approved1-3 Rapid symptom improvement starting at Week 1, sustained at Week 6 vs placebo1 Rapid remission as early as Week 2, sustained and increased vs control through Week 64 Source: Symphony METYS Weekly TRx Launch to Date
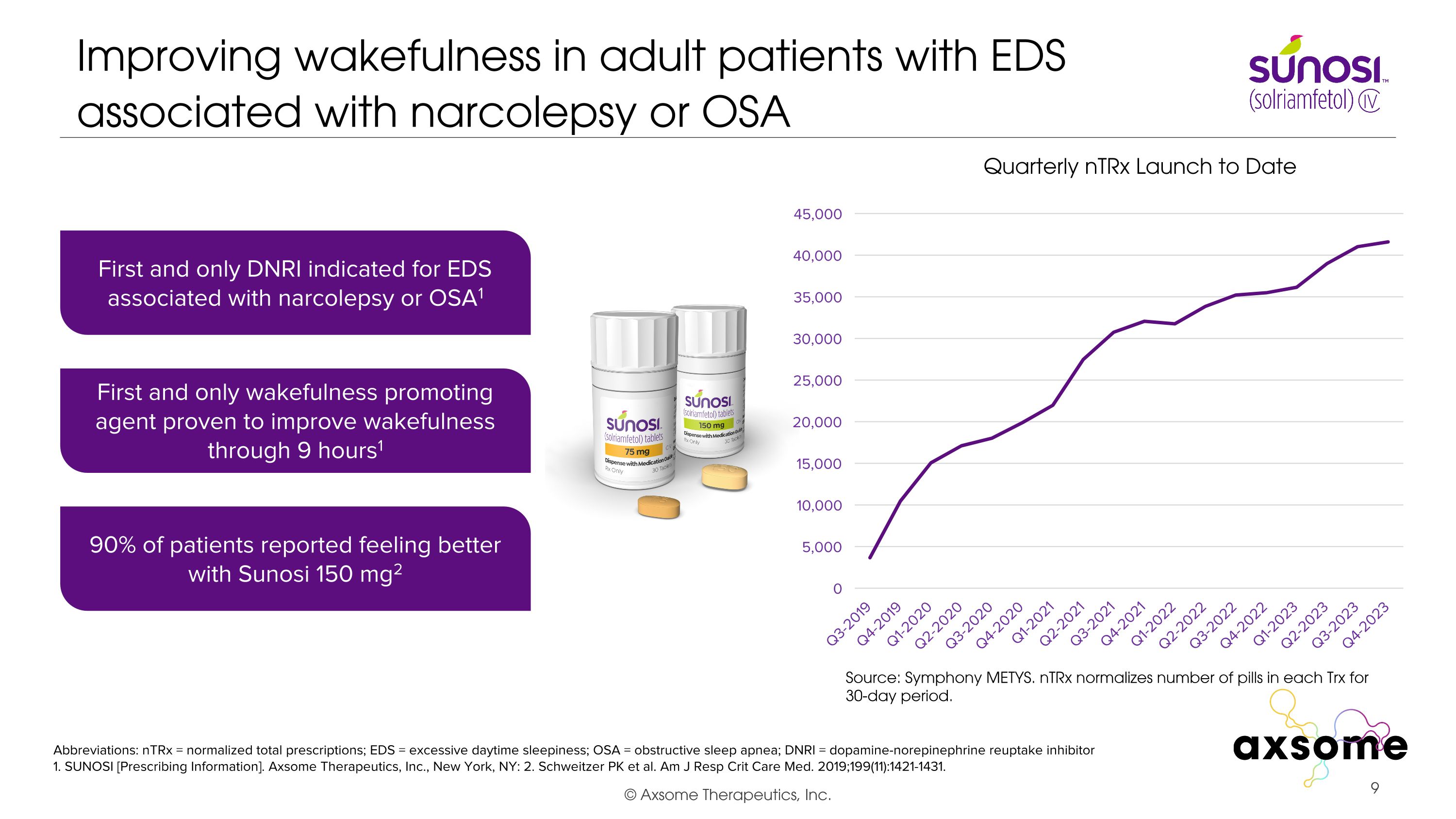
Improving wakefulness in adult patients with EDS associated with narcolepsy or OSA Abbreviations: nTRx = normalized total prescriptions; EDS = excessive daytime sleepiness; OSA = obstructive sleep apnea; DNRI = dopamine-norepinephrine reuptake inhibitor 1. SUNOSI [Prescribing Information]. Axsome Therapeutics, Inc., New York, NY: 2. Schweitzer PK et al. Am J Resp Crit Care Med. 2019;199(11):1421-1431. First and only DNRI indicated for EDS associated with narcolepsy or OSA1 90% of patients reported feeling better with Sunosi 150 mg2 First and only wakefulness promoting agent proven to improve wakefulness through 9 hours1 Quarterly nTRx Launch to Date Source: Symphony METYS. nTRx normalizes number of pills in each Trx for 30-day period.

Development Pipeline

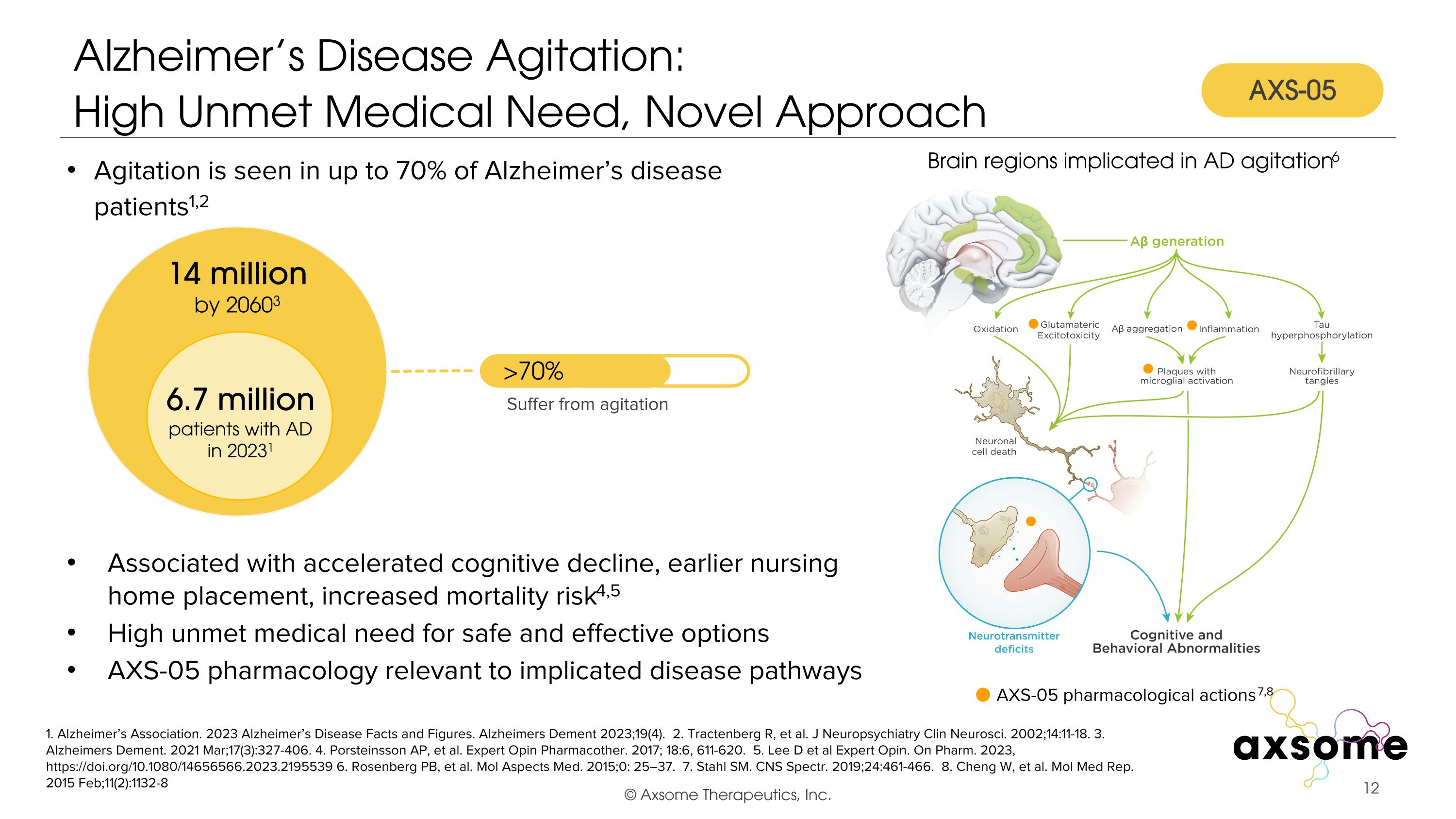
Alzheimer’s Disease Agitation:�High Unmet Medical Need, Novel Approach 1. Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimers Dement 2023;19(4). 2. Tractenberg R, et al. J Neuropsychiatry Clin Neurosci. 2002;14:11-18. 3. Alzheimers Dement. 2021 Mar;17(3):327-406. 4. Porsteinsson AP, et al. Expert Opin Pharmacother. 2017; 18:6, 611-620. 5. Lee D et al Expert Opin. On Pharm. 2023, https://doi.org/10.1080/14656566.2023.2195539 6. Rosenberg PB, et al. Mol Aspects Med. 2015;0: 25–37. 7. Stahl SM. CNS Spectr. 2019;24:461-466. 8. Cheng W, et al. Mol Med Rep. 2015 Feb;11(2):1132-8 Agitation is seen in up to 70% of Alzheimer’s disease patients1,2 Associated with accelerated cognitive decline, earlier nursing home placement, increased mortality risk4,5 High unmet medical need for safe and effective options AXS-05 pharmacology relevant to implicated disease pathways AXS-05 pharmacological actions7,8 Brain regions implicated in AD agitation6 6.7 million patients with AD in 20231 14 million by 20603 >70% Suffer from agitation AXS-05
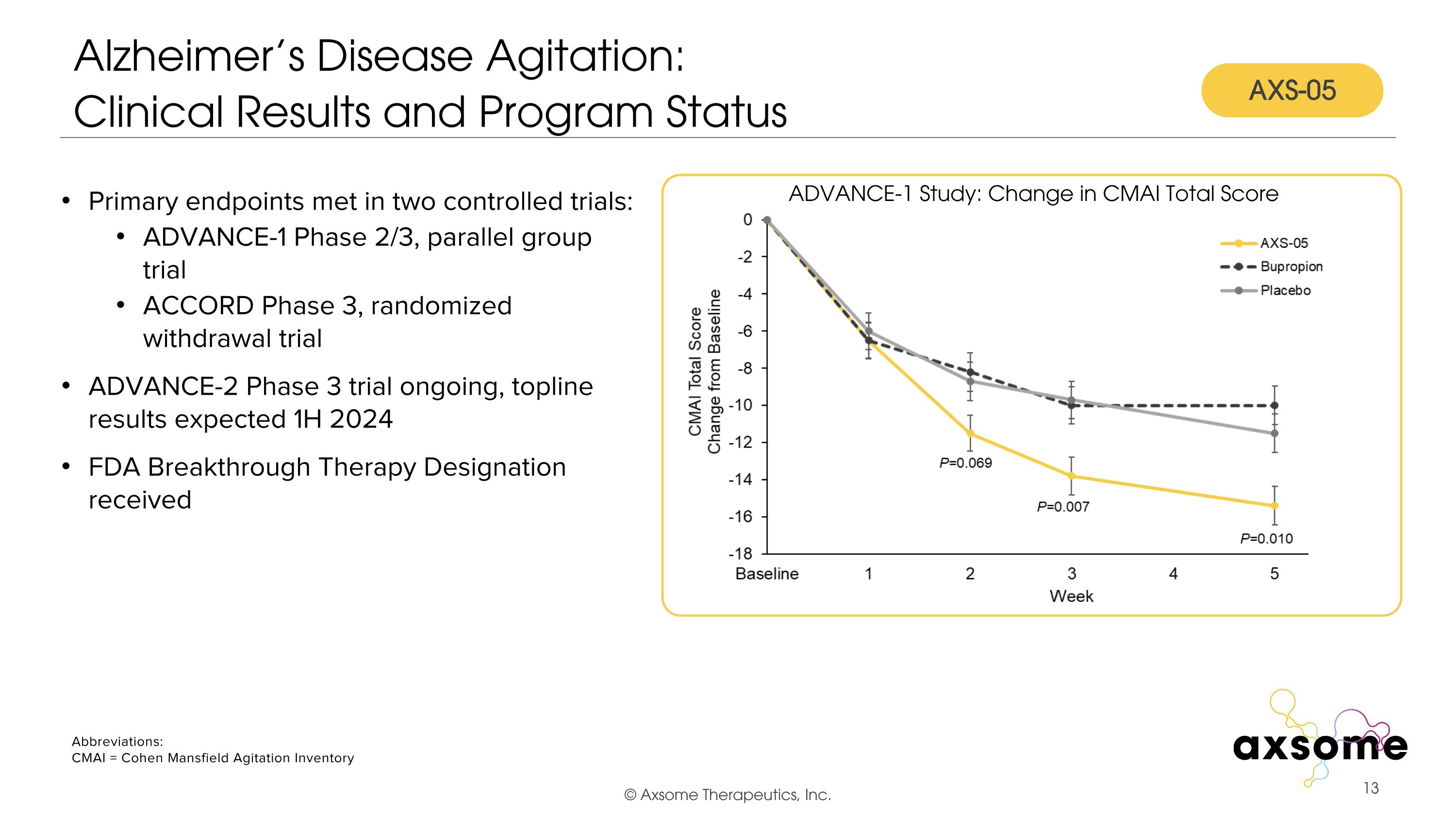
Alzheimer’s Disease Agitation:�Clinical Results and Program Status Primary endpoints met in two controlled trials: ADVANCE-1 Phase 2/3, parallel group trial ACCORD Phase 3, randomized withdrawal trial ADVANCE-2 Phase 3 trial ongoing, topline results expected 1H 2024 FDA Breakthrough Therapy Designation received ADVANCE-1 Study: Change in CMAI Total Score AXS-05 Abbreviations: CMAI = Cohen Mansfield Agitation Inventory
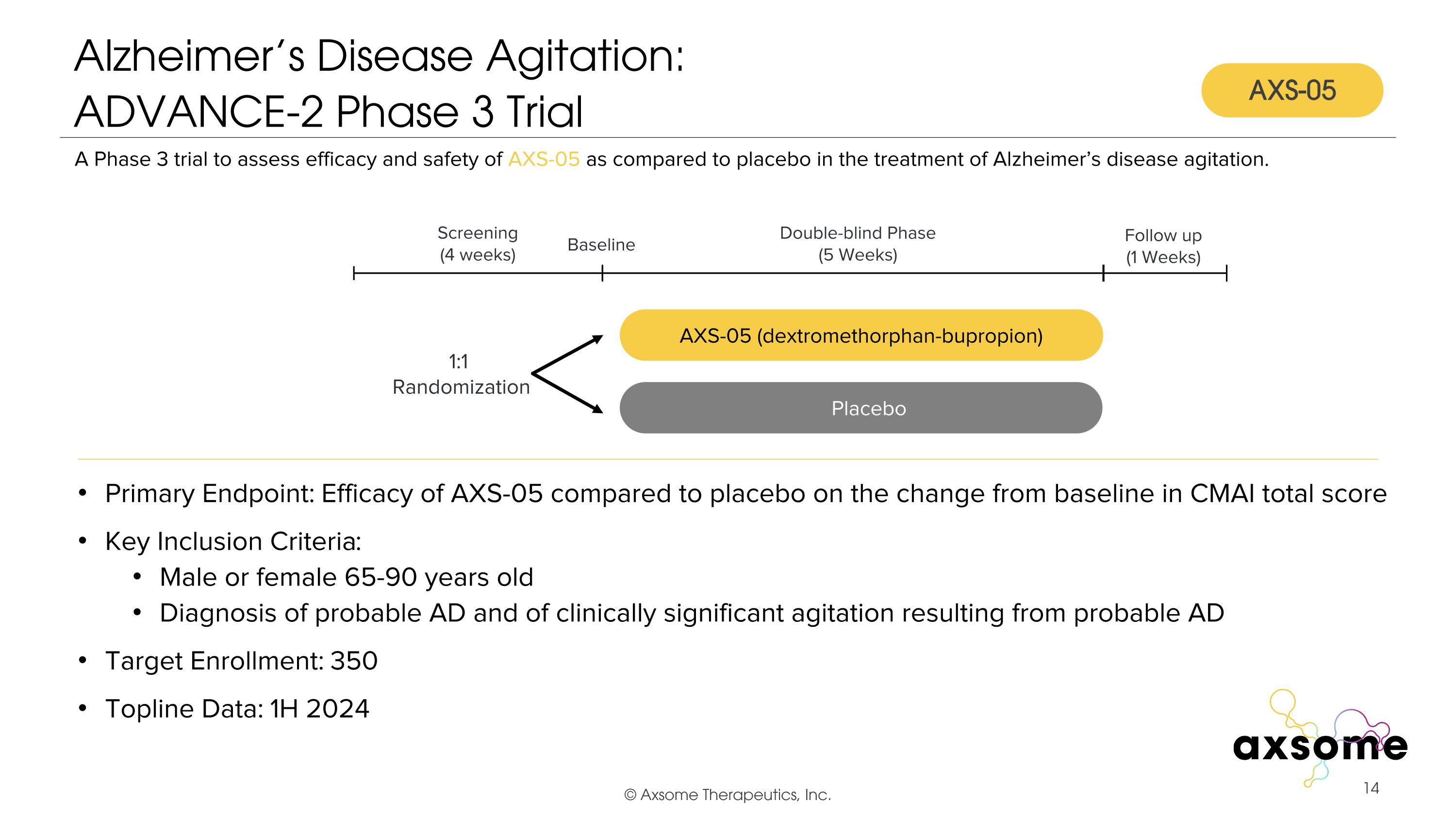
Alzheimer’s Disease Agitation: �ADVANCE-2 Phase 3 Trial Primary Endpoint: Efficacy of AXS-05 compared to placebo on the change from baseline in CMAI total score Key Inclusion Criteria: Male or female 65-90 years old Diagnosis of probable AD and of clinically significant agitation resulting from probable AD Target Enrollment: 350 Topline Data: 1H 2024 AXS-05 twice daily AXS-05 (dextromethorphan-bupropion) Placebo A Phase 3 trial to assess efficacy and safety of AXS-05 as compared to placebo in the treatment of Alzheimer’s disease agitation. Double-blind Phase (5 Weeks) Screening (4 weeks) 1:1 Randomization Follow up (1 Weeks) Baseline AXS-05
Smoking is single largest cause of preventable death in the U.S.1 70% of smokers want to quit2 Only 3-5% who attempt to quit without assistance are successful for 6-12 months2 Smoking Cessation Abbreviations: NMDA = N-methyl D-aspartate U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. 2014. Hughes JR, et al. Addiction. 2004;99(1):29-38 AXS-05 represents a potentially new mechanism of action for smoking cessation Positive FDA Pre-IND meeting guidance received from the FDA – can proceed to pivotal Phase 2/3 trial Planned trial initiation in 2024 Fast Facts AXS-05 Key Updates AXS-05

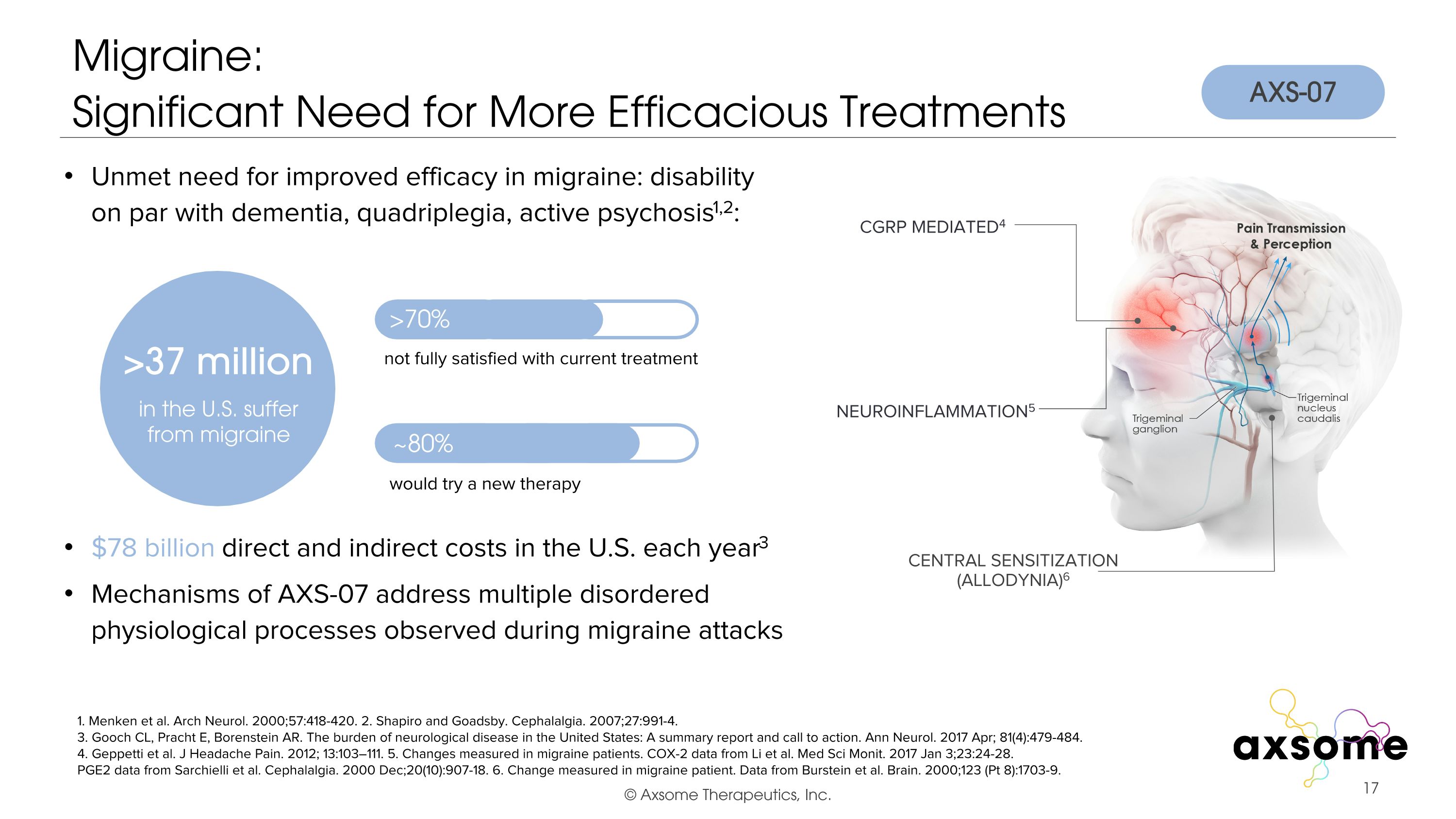
Migraine:�Significant Need for More Efficacious Treatments 1. Menken et al. Arch Neurol. 2000;57:418-420. 2. Shapiro and Goadsby. Cephalalgia. 2007;27:991-4. 3. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: A summary report and call to action. Ann Neurol. 2017 Apr; 81(4):479-484. 4. Geppetti et al. J Headache Pain. 2012; 13:103–111. 5. Changes measured in migraine patients. COX-2 data from Li et al. Med Sci Monit. 2017 Jan 3;23:24-28. PGE2 data from Sarchielli et al. Cephalalgia. 2000 Dec;20(10):907-18. 6. Change measured in migraine patient. Data from Burstein et al. Brain. 2000;123 (Pt 8):1703-9. $78 billion direct and indirect costs in the U.S. each year3 Unmet need for improved efficacy in migraine: disability on par with dementia, quadriplegia, active psychosis1,2: in the U.S. suffer from migraine >70% ~80% not fully satisfied with current treatment would try a new therapy CGRP Mediated4 Central Sensitization (Allodynia)6 AXS-07 Mechanisms of AXS-07 address multiple disordered�physiological processes observed during migraine attacks NEUROINFLAMMATION5 >37 million
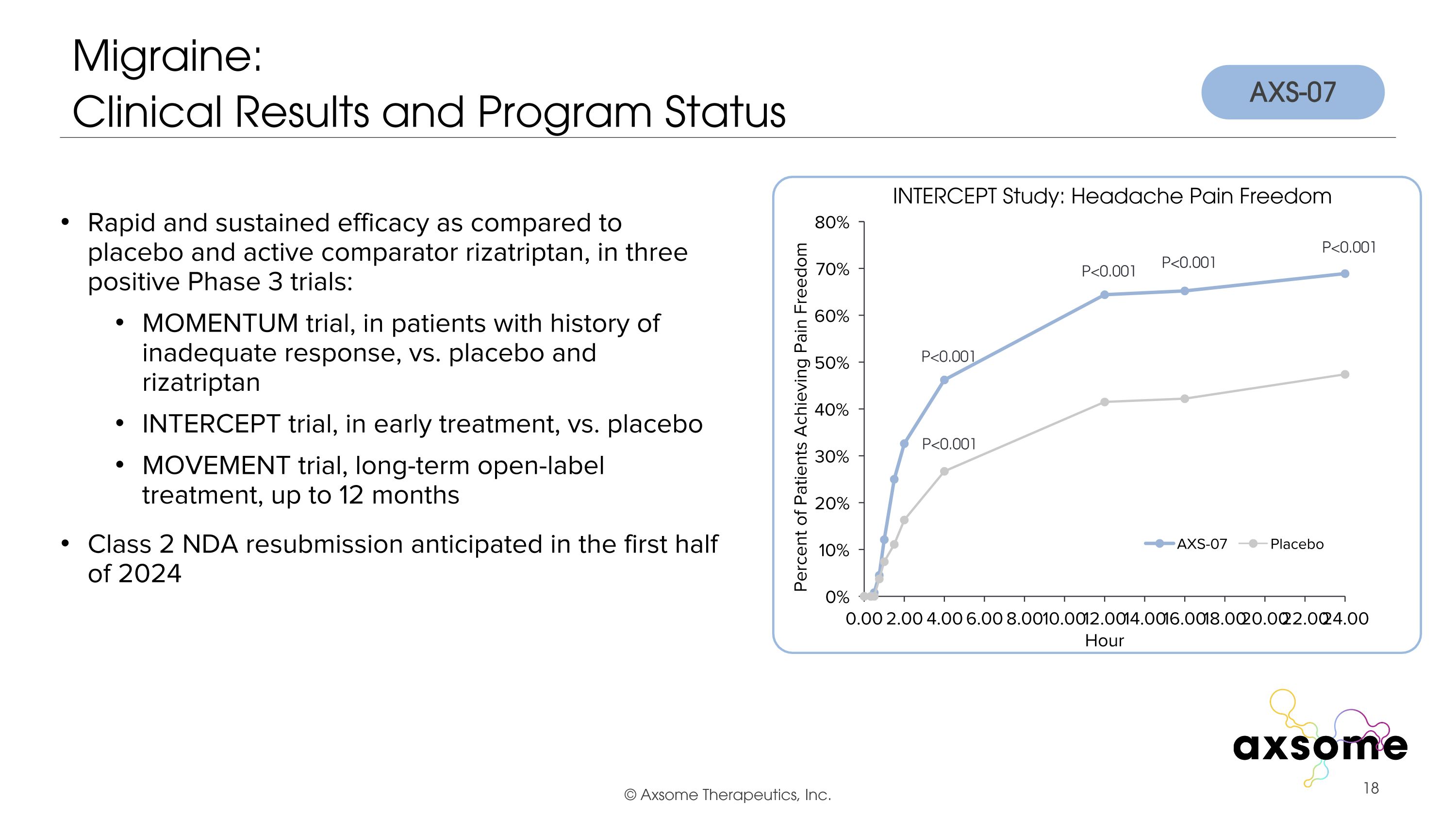
Migraine: �Clinical Results and Program Status P<0.001 P<0.001 P<0.001 P<0.001 P<0.001 Rapid and sustained efficacy as compared to placebo and active comparator rizatriptan, in three positive Phase 3 trials: MOMENTUM trial, in patients with history of inadequate response, vs. placebo and rizatriptan INTERCEPT trial, in early treatment, vs. placebo MOVEMENT trial, long-term open-label treatment, up to 12 months Class 2 NDA resubmission anticipated in the first half of 2024 AXS-07 INTERCEPT Study: Headache Pain Freedom

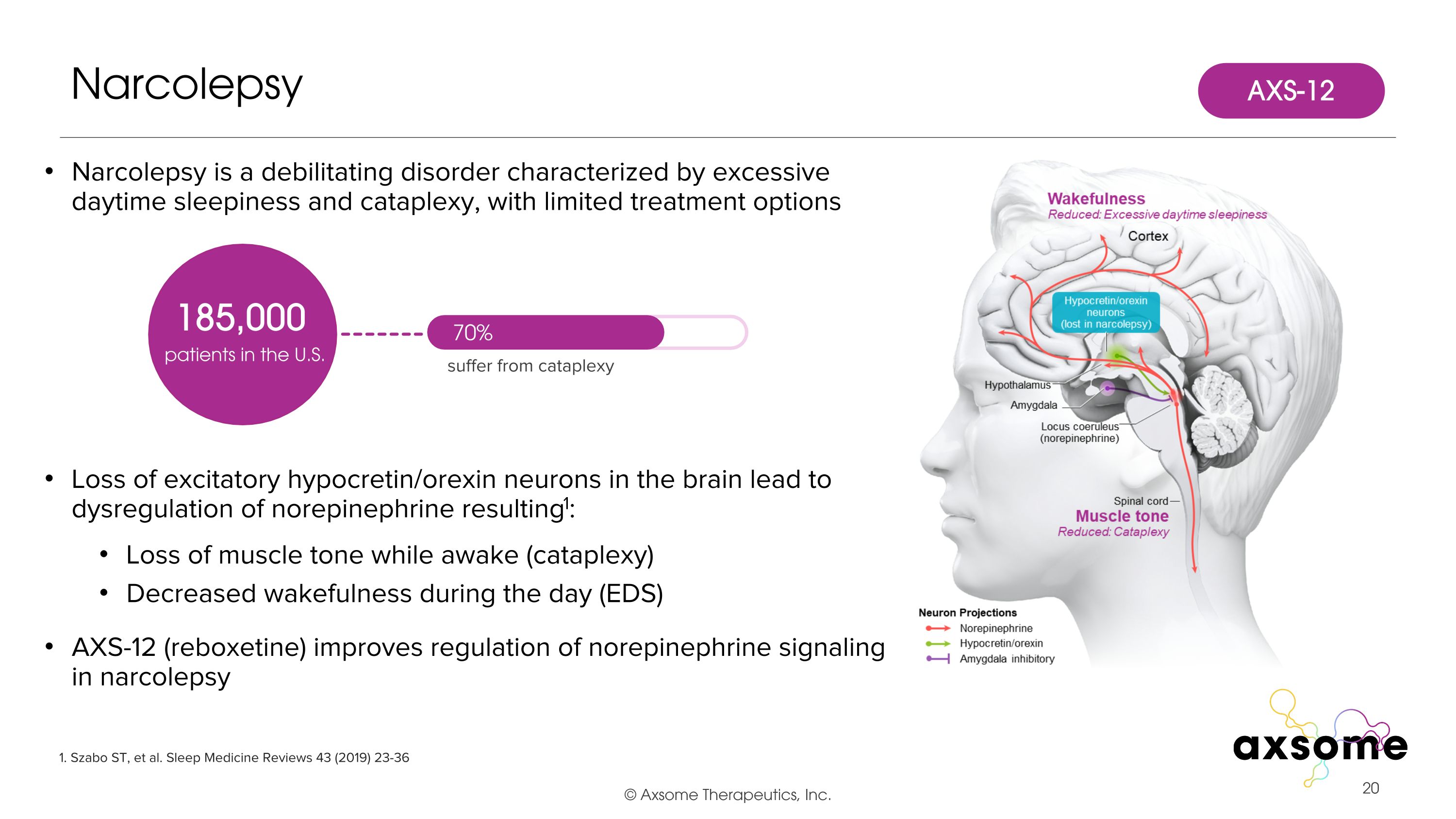
Narcolepsy is a debilitating disorder characterized by excessive daytime sleepiness and cataplexy, with limited treatment options Loss of excitatory hypocretin/orexin neurons in the brain lead to dysregulation of norepinephrine resulting1: Loss of muscle tone while awake (cataplexy) Decreased wakefulness during the day (EDS) AXS-12 (reboxetine) improves regulation of norepinephrine signaling in narcolepsy Narcolepsy 1. Szabo ST, et al. Sleep Medicine Reviews 43 (2019) 23-36 185,000 patients in the U.S. 70% suffer from cataplexy AXS-12
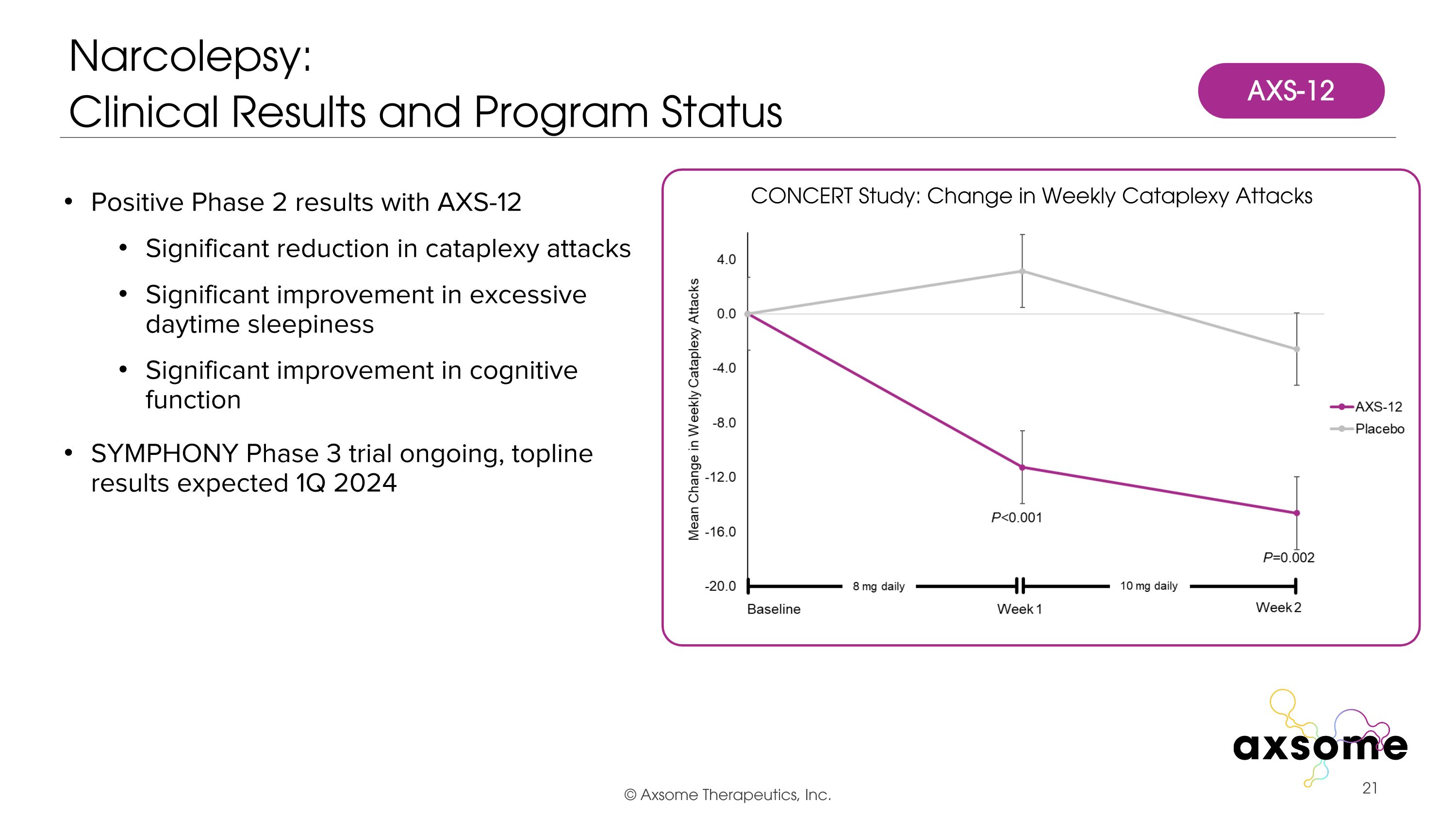
Positive Phase 2 results with AXS-12 Significant reduction in cataplexy attacks Significant improvement in excessive daytime sleepiness Significant improvement in cognitive function SYMPHONY Phase 3 trial ongoing, topline results expected 1Q 2024 Narcolepsy:�Clinical Results and Program Status CONCERT Study: Change in Weekly Cataplexy Attacks AXS-12

Primary Endpoint: Change in the frequency of cataplexy attacks Key Inclusion Criteria: Male or female 15-75 years old Primary diagnosis of narcolepsy with cataplexy Topline Data: 1Q 2024 A Phase 3 trial to assess efficacy and safety of AXS-12 as compared to placebo in the treatment of cataplexy in narcolepsy. Double-blind Phase (5 weeks) Screening 1:1 randomization AXS-12 (reboxetine) Placebo AXS-12 Narcolepsy:�SYMPHONY Phase 3 Trial

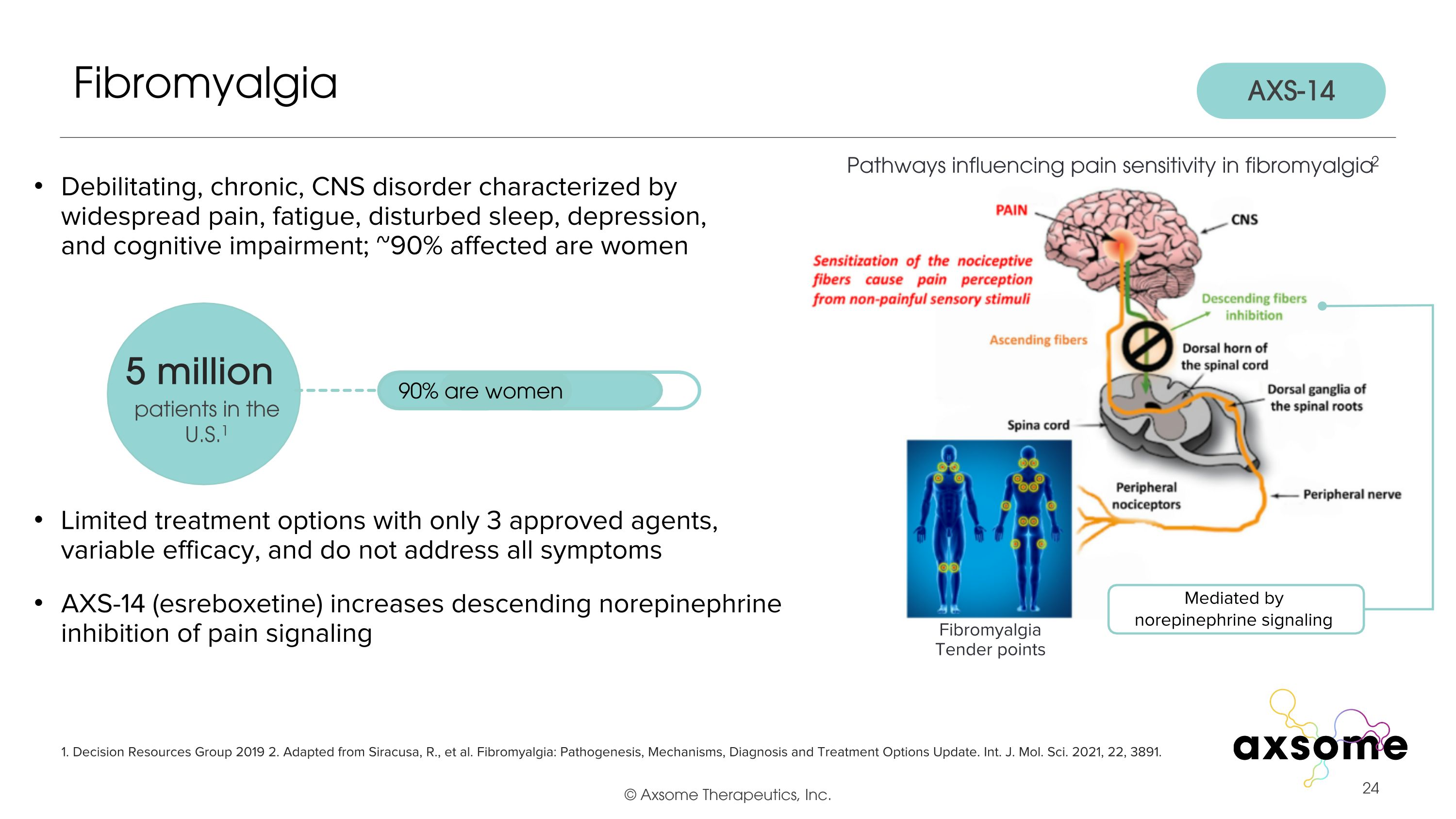
Debilitating, chronic, CNS disorder characterized by widespread pain, fatigue, disturbed sleep, depression, and cognitive impairment; ~90% affected are women Limited treatment options with only 3 approved agents, variable efficacy, and do not address all symptoms AXS-14 (esreboxetine) increases descending norepinephrine inhibition of pain signaling Fibromyalgia 1. Decision Resources Group 2019 2. Adapted from Siracusa, R., et al. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. 5 million patients in the U.S.1 90% are women Pathways influencing pain sensitivity in fibromyalgia2 Mediated by norepinephrine signaling Fibromyalgia Tender points AXS-14
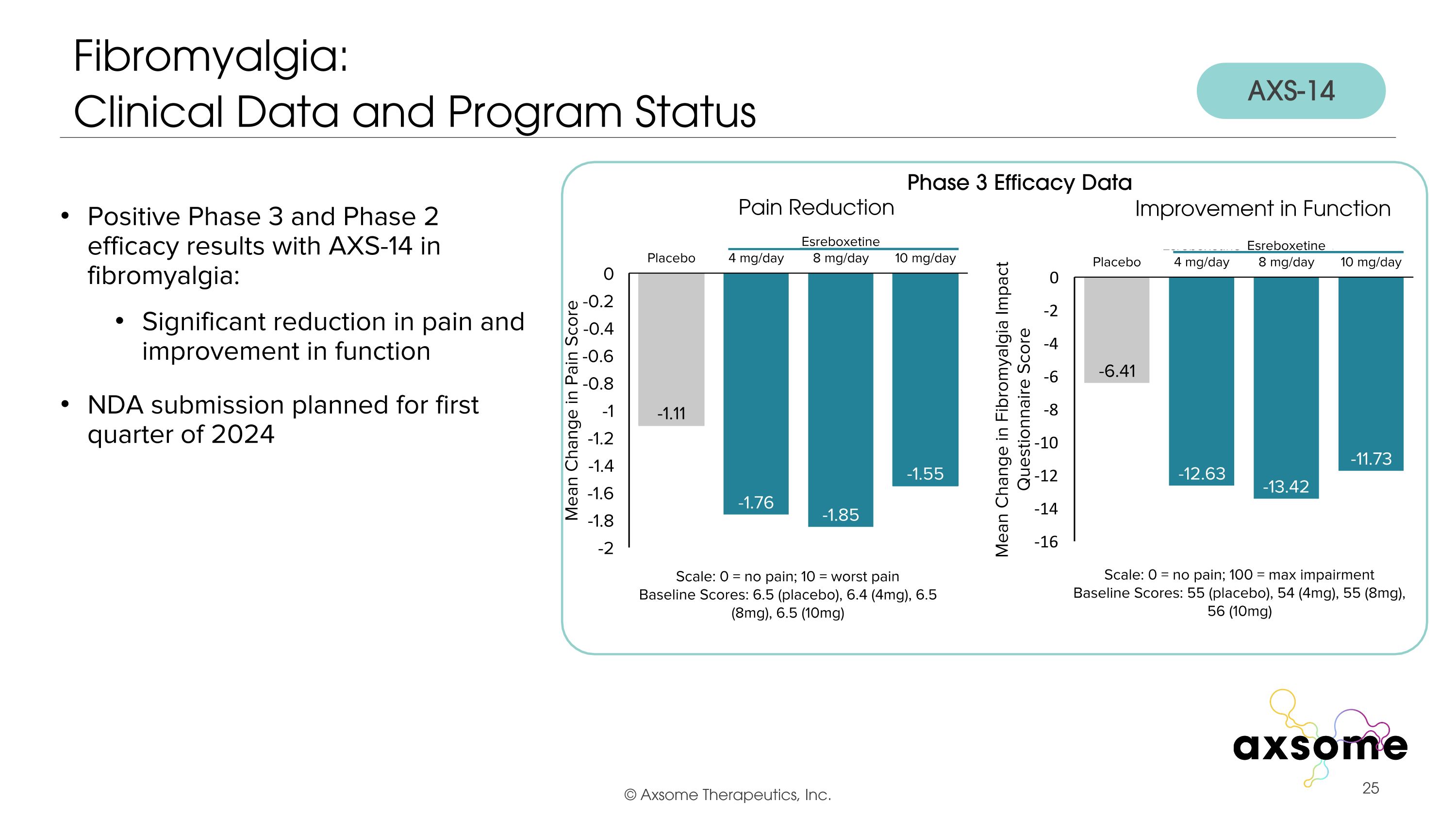
Positive Phase 3 and Phase 2 efficacy results with AXS-14 in fibromyalgia: Significant reduction in pain and improvement in function NDA submission planned for first quarter of 2024 Fibromyalgia: �Clinical Data and Program Status AXS-14 Phase 3 Efficacy Data
Solriamfetol a potentially differentiated option for the treatment of CNS disorders
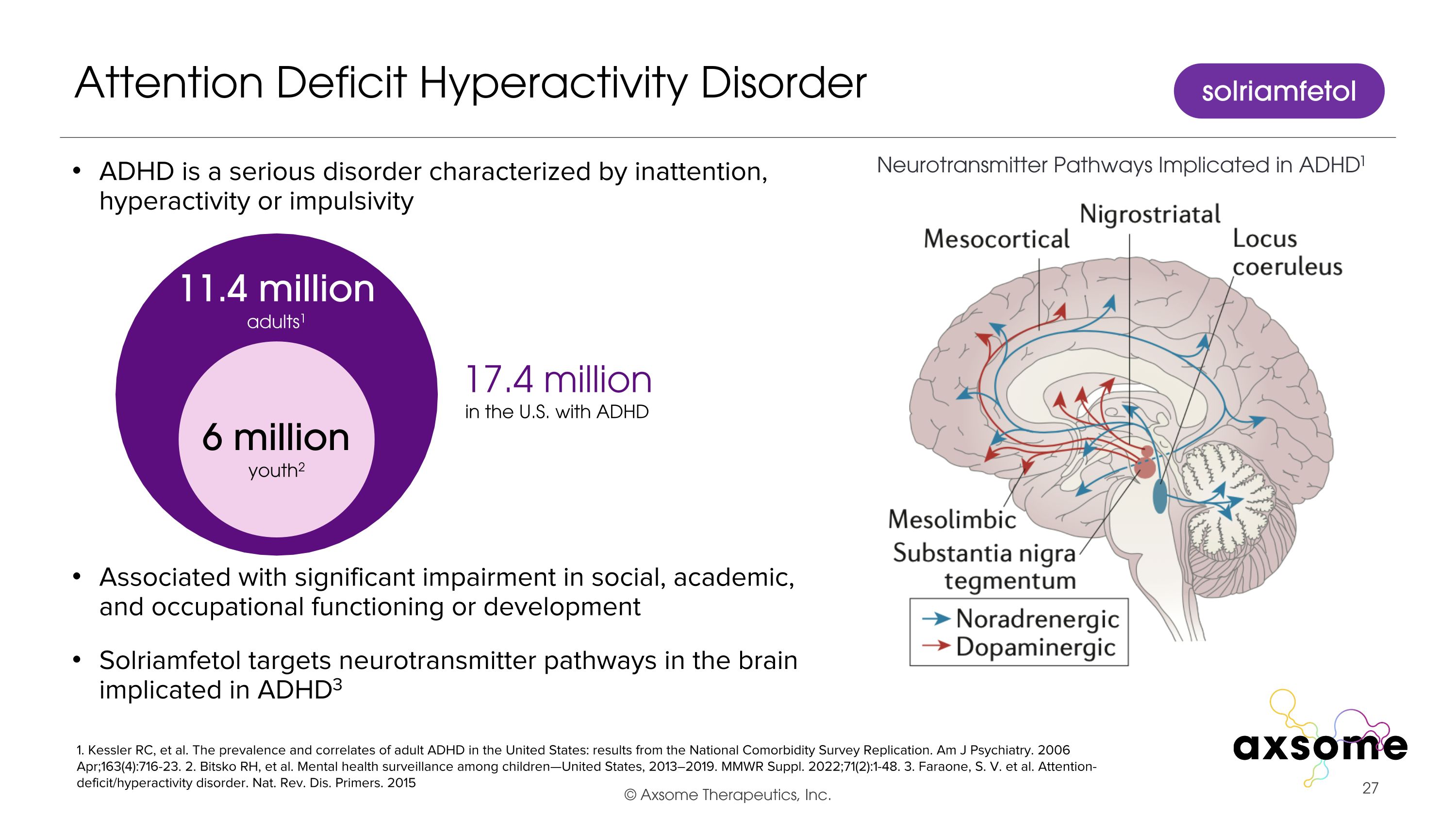
ADHD is a serious disorder characterized by inattention, hyperactivity or impulsivity Associated with significant impairment in social, academic, and occupational functioning or development Solriamfetol targets neurotransmitter pathways in the brain implicated in ADHD3 Attention Deficit Hyperactivity Disorder 1. Kessler RC, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006 Apr;163(4):716-23. 2. Bitsko RH, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Suppl. 2022;71(2):1-48. 3. Faraone, S. V. et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015 17.4 million in the U.S. with ADHD 6 million youth2 11.4 million adults1 Neurotransmitter Pathways Implicated in ADHD1 solriamfetol
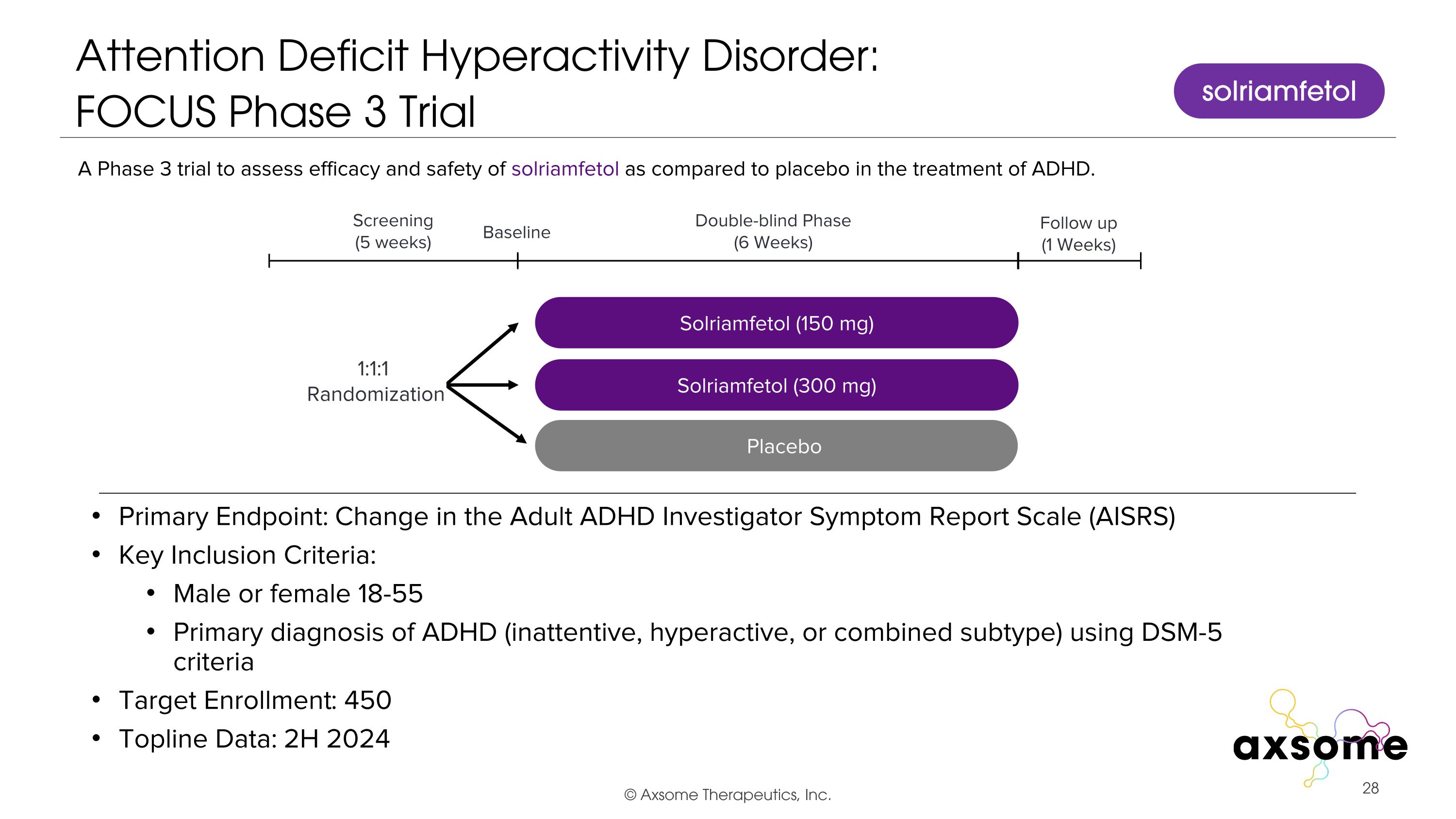
Attention Deficit Hyperactivity Disorder: �FOCUS Phase 3 Trial Primary Endpoint: Change in the Adult ADHD Investigator Symptom Report Scale (AISRS) Key Inclusion Criteria: Male or female 18-55 Primary diagnosis of ADHD (inattentive, hyperactive, or combined subtype) using DSM-5 criteria Target Enrollment: 450 Topline Data: 2H 2024 AXS-05 twice daily Solriamfetol (150 mg) Placebo Double-blind Phase (6 Weeks) Screening (5 weeks) 1:1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of ADHD. Solriamfetol (300 mg) solriamfetol
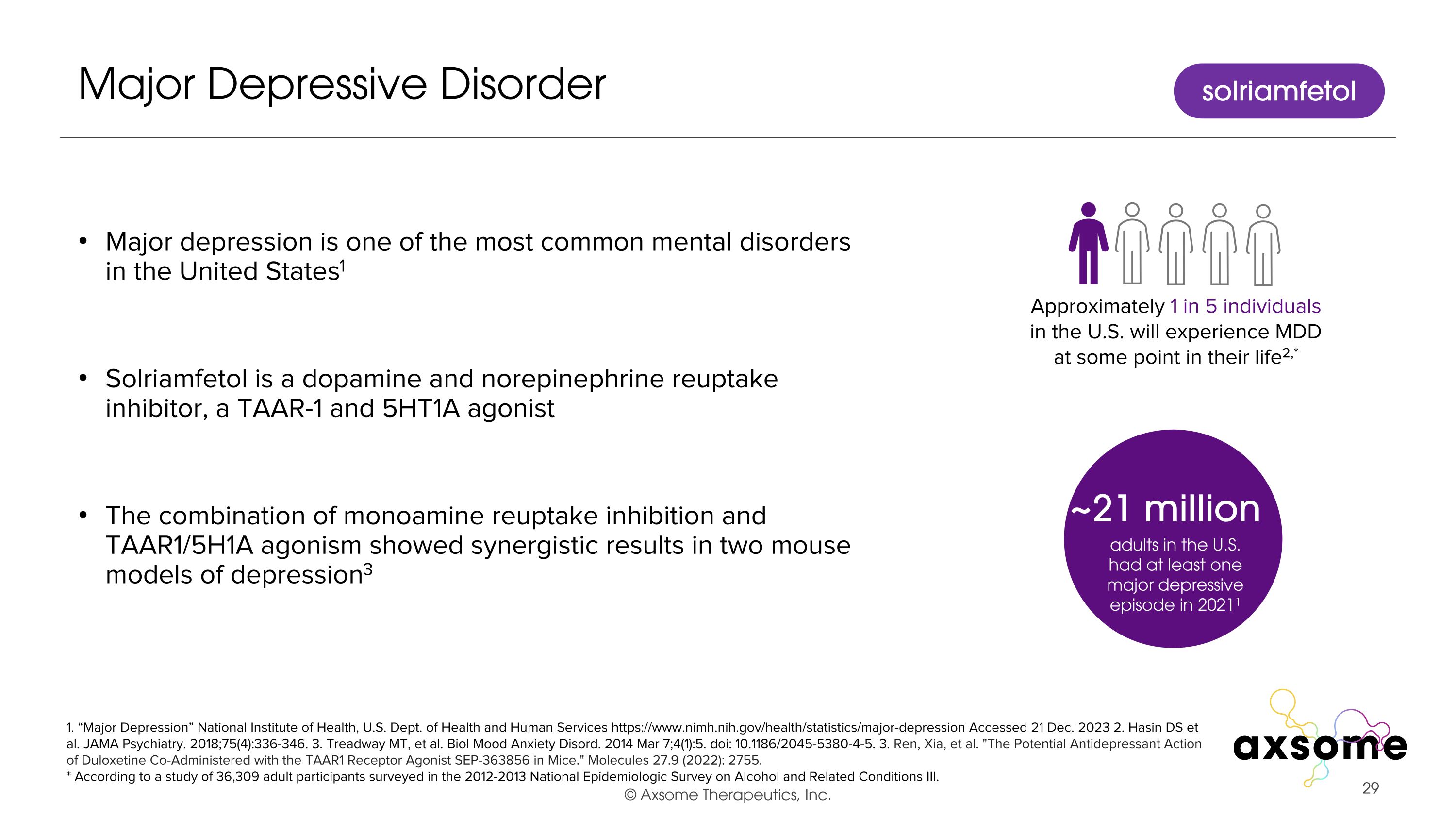
Major Depressive Disorder Major depression is one of the most common mental disorders in the United States1 Solriamfetol is a dopamine and norepinephrine reuptake inhibitor, a TAAR-1 and 5HT1A agonist The combination of monoamine reuptake inhibition and TAAR1/5H1A agonism showed synergistic results in two mouse models of depression3 1. “Major Depression” National Institute of Health, U.S. Dept. of Health and Human Services https://www.nimh.nih.gov/health/statistics/major-depression Accessed 21 Dec. 2023 2. Hasin DS et al. JAMA Psychiatry. 2018;75(4):336-346. 3. Treadway MT, et al. Biol Mood Anxiety Disord. 2014 Mar 7;4(1):5. doi: 10.1186/2045-5380-4-5. 3. Ren, Xia, et al. "The Potential Antidepressant Action of Duloxetine Co-Administered with the TAAR1 Receptor Agonist SEP-363856 in Mice." Molecules 27.9 (2022): 2755. * According to a study of 36,309 adult participants surveyed in the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III. solriamfetol ~21 million adults in the U.S. had at least one major depressive episode in 20211 Approximately 1 in 5 individuals in the U.S. will experience MDD at some point in their life2,*
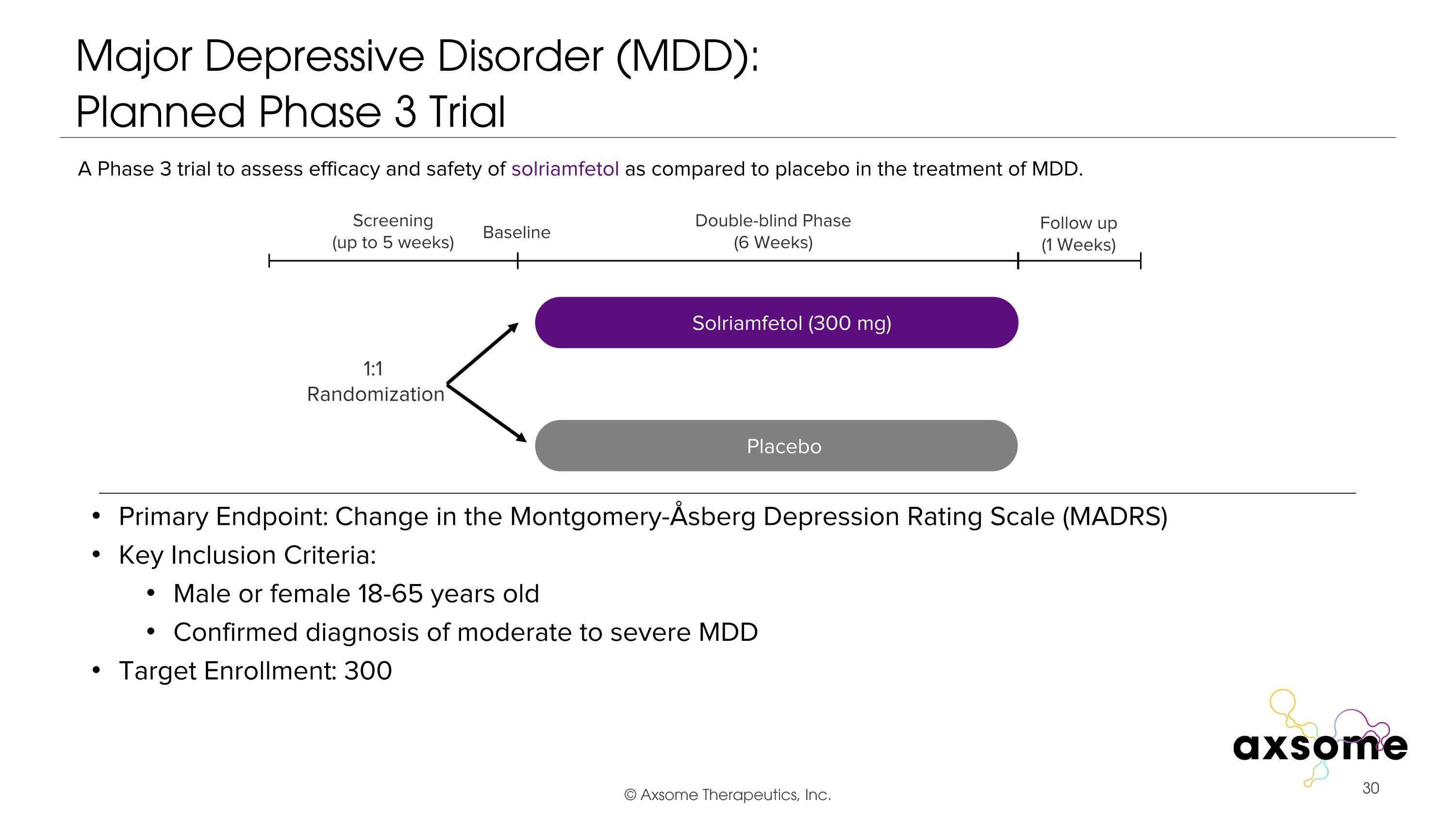
Major Depressive Disorder (MDD): �Planned Phase 3 Trial Primary Endpoint: Change in the Montgomery-Åsberg Depression Rating Scale (MADRS) Key Inclusion Criteria: Male or female 18-65 years old Confirmed diagnosis of moderate to severe MDD Target Enrollment: 300 AXS-05 twice daily Placebo Double-blind Phase (6 Weeks) Screening (up to 5 weeks) 1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of MDD. Solriamfetol (300 mg)

Binge eating disorder (BED) is the most common eating disorder and is thought to involve issues with food reward processing, impulse control, and appetite regulation1,2 Associated with a 2- to 3-fold increased risk of psychiatric and medical comorbidities3 Solriamfetol’s dopamine, norepinephrine, and TAAR1 mechanisms appear relevant to the pathophysiology of BED4-6 Binge Eating Disorder 1. Kessler RM, et al. Neurosci. Biobehav. Rev., vol. 63, pp. 223–238, Apr. 2016 2. Hudson JI, et al. Biol. Psychiatry, vol. 61, no. 3, pp. 348–358, Feb. 2007 3. McElroy SL et al. J. Clin. Psychiatry, vol. 81, no. 5, Sep. 2020 4. Giel KL et al. Nat. Rev. Dis. Primer, vol. 8, no. 1, Art. no. 1, Mar. 2022 5. Bello NT et al. Pharmacol. Biochem. Behav., vol. 97, no. 1, pp. 25–33, Nov. 2010 6. Pruccoli et al. Int. J. Mol. Sci., vol. 22, no. 20, p. 11086, Oct. 2021 solriamfetol Abbreviations: OFC = orbital frontal cortex; vmPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex ~7 million people in the U.S. have BED2 BED is 1.75x more common in women than in men2
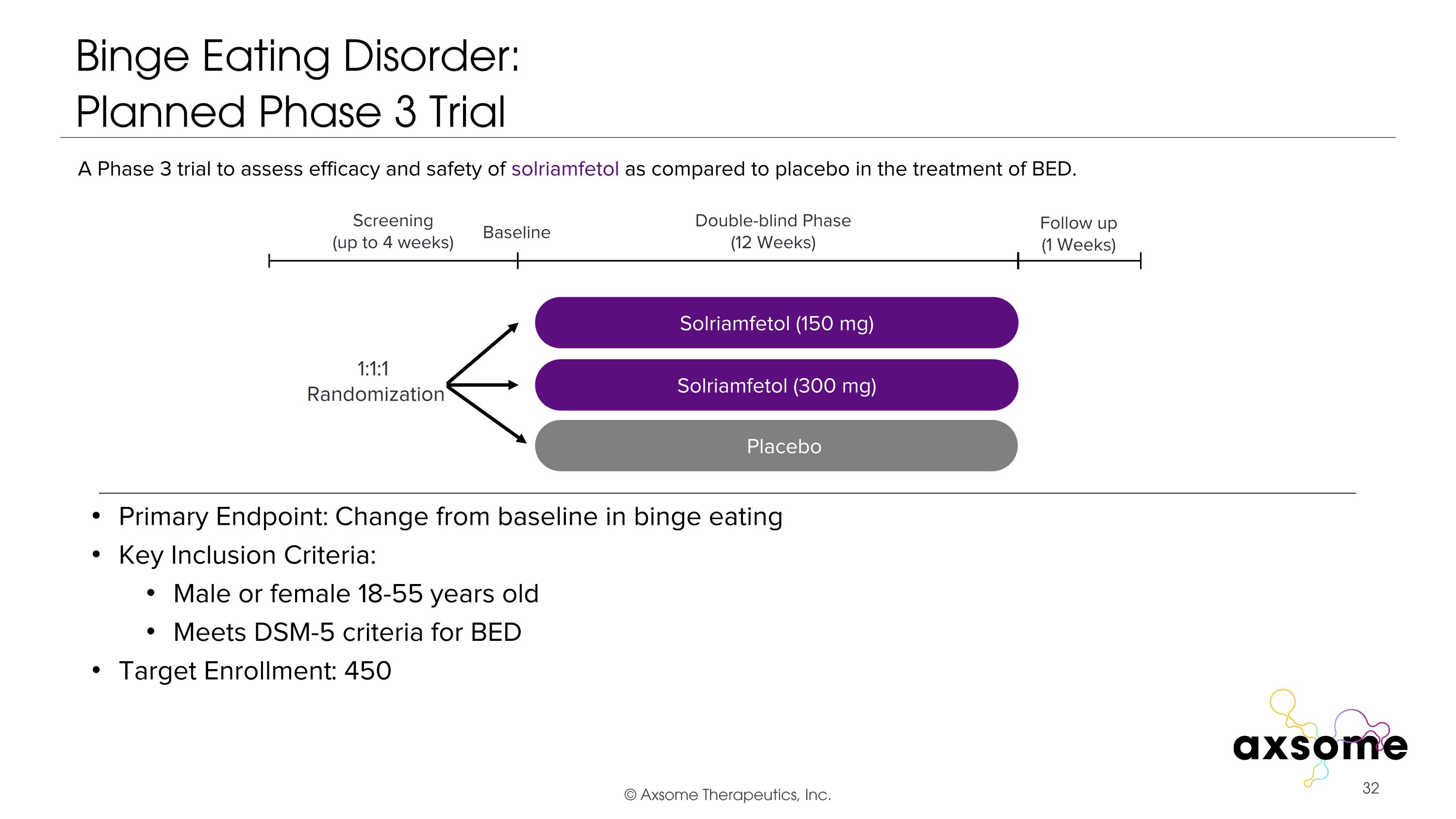
Binge Eating Disorder: �Planned Phase 3 Trial Primary Endpoint: Change from baseline in binge eating Key Inclusion Criteria: Male or female 18-55 years old Meets DSM-5 criteria for BED Target Enrollment: 450 AXS-05 twice daily Solriamfetol (150 mg) Placebo Double-blind Phase (12 Weeks) Screening (up to 4 weeks) 1:1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of BED. Solriamfetol (300 mg)
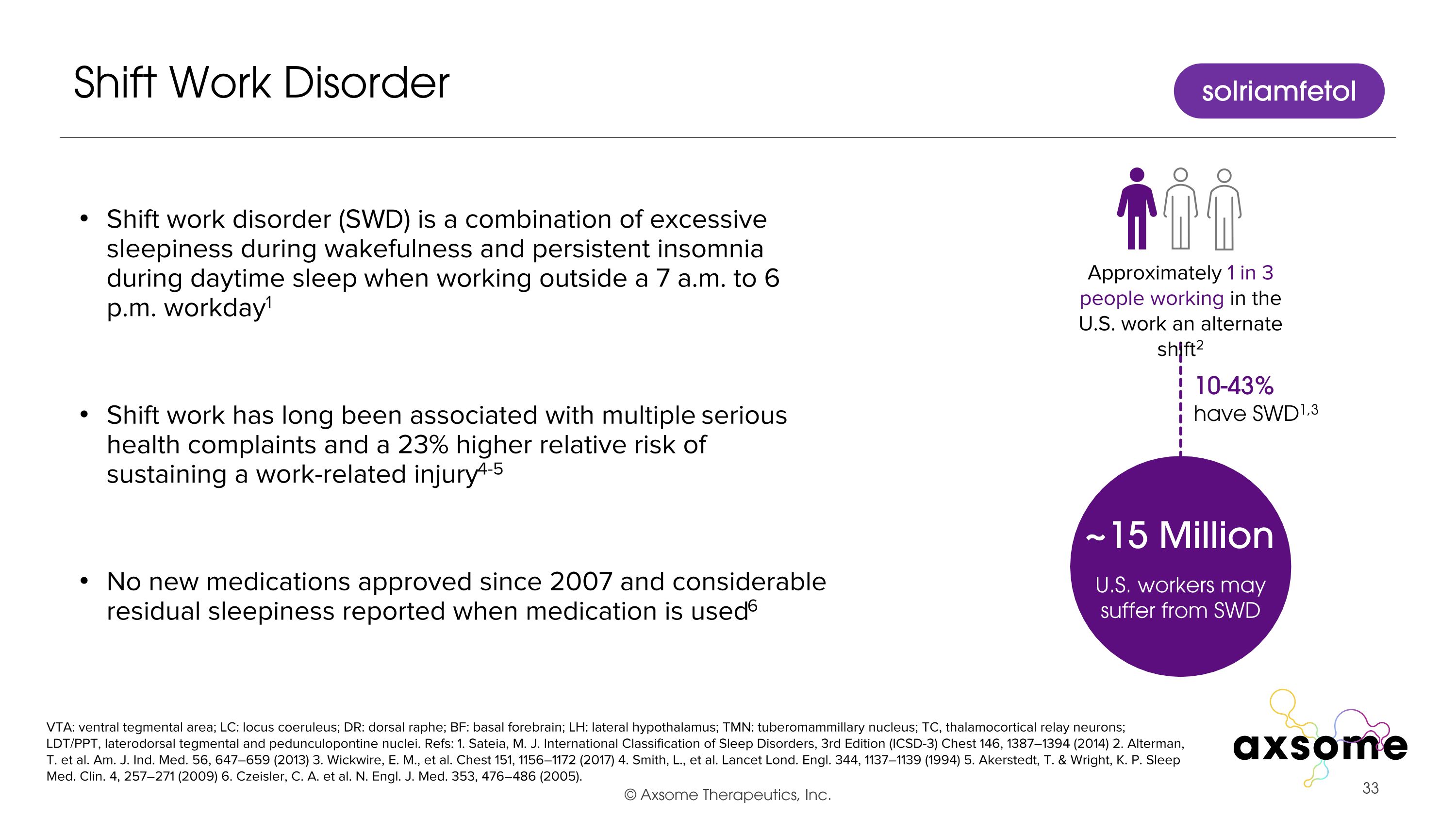
Shift work disorder (SWD) is a combination of excessive sleepiness during wakefulness and persistent insomnia during daytime sleep when working outside a 7 a.m. to 6 p.m. workday1 Shift work has long been associated with multiple serious health complaints and a 23% higher relative risk of sustaining a work-related injury4-5 No new medications approved since 2007 and considerable residual sleepiness reported when medication is used6 Shift Work Disorder VTA: ventral tegmental area; LC: locus coeruleus; DR: dorsal raphe; BF: basal forebrain; LH: lateral hypothalamus; TMN: tuberomammillary nucleus; TC, thalamocortical relay neurons; LDT/PPT, laterodorsal tegmental and pedunculopontine nuclei. Refs: 1. Sateia, M. J. International Classification of Sleep Disorders, 3rd Edition (ICSD-3) Chest 146, 1387–1394 (2014) 2. Alterman, T. et al. Am. J. Ind. Med. 56, 647–659 (2013) 3. Wickwire, E. M., et al. Chest 151, 1156–1172 (2017) 4. Smith, L., et al. Lancet Lond. Engl. 344, 1137–1139 (1994) 5. Akerstedt, T. & Wright, K. P. Sleep Med. Clin. 4, 257–271 (2009) 6. Czeisler, C. A. et al. N. Engl. J. Med. 353, 476–486 (2005). solriamfetol 10-43% have SWD1,3 ~15 Million U.S. workers may suffer from SWD Approximately 1 in 3 people working in the U.S. work an alternate shift2
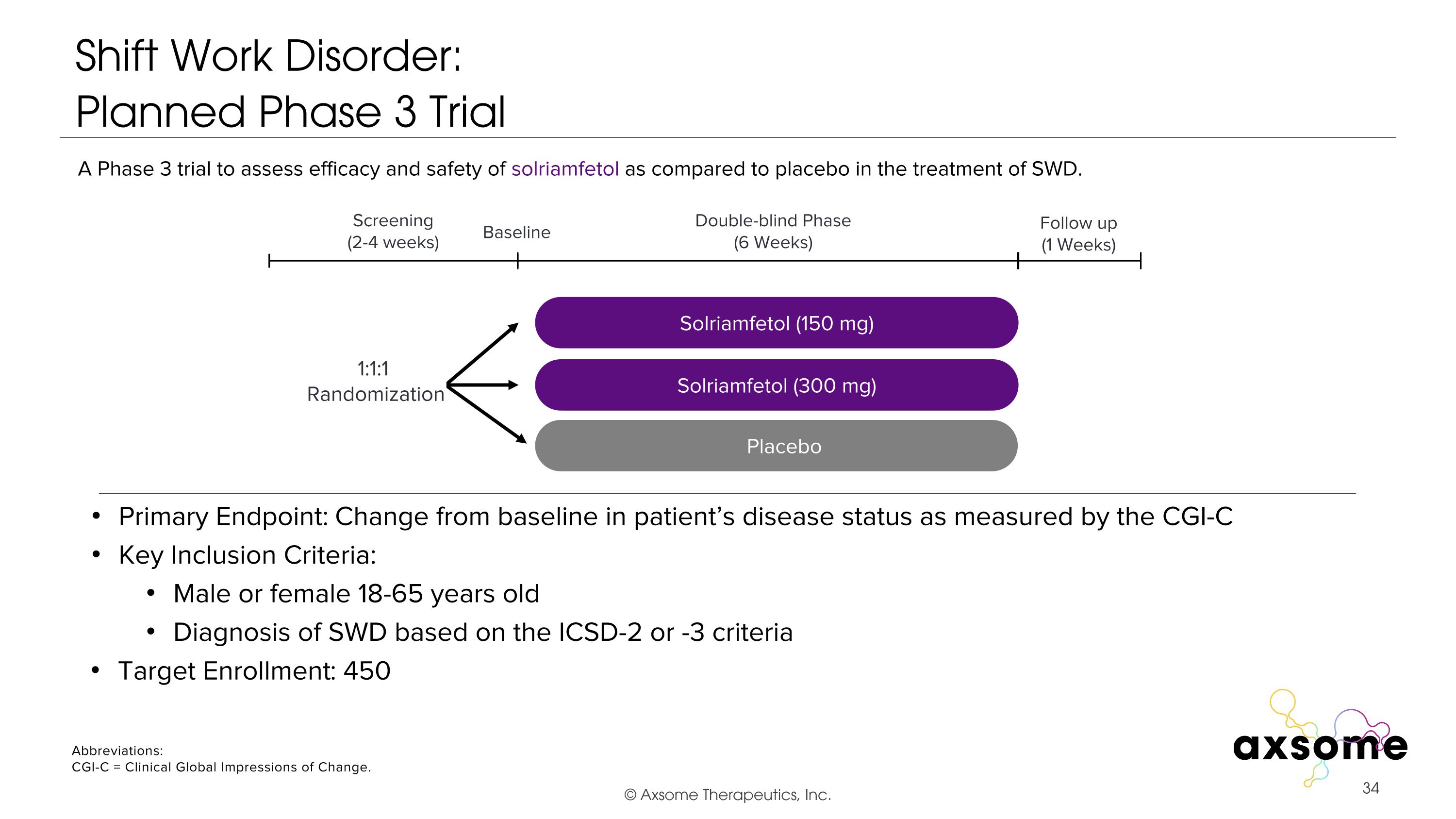
Shift Work Disorder: �Planned Phase 3 Trial Primary Endpoint: Change from baseline in patient’s disease status as measured by the CGI-C Key Inclusion Criteria: Male or female 18-65 years old Diagnosis of SWD based on the ICSD-2 or -3 criteria AXS-05 twice daily Solriamfetol (150 mg) Placebo Double-blind Phase (6 Weeks) Screening (2-4 weeks) 1:1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of SWD. Solriamfetol (300 mg) Abbreviations: CGI-C = Clinical Global Impressions of Change. Target Enrollment: 450
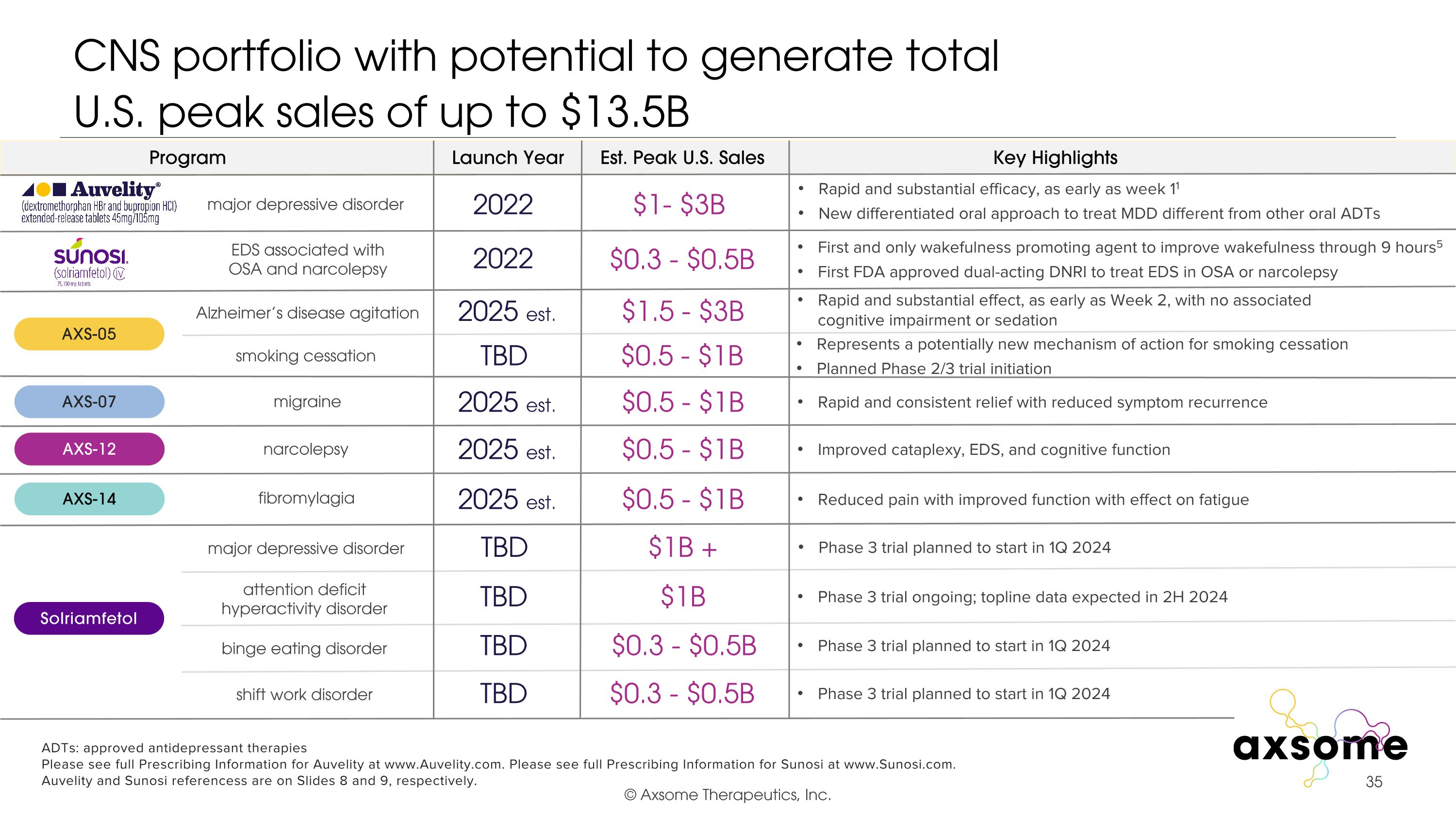
CNS portfolio with potential to generate total U.S. peak sales of up to $13.5B EDS associated with OSA and narcolepsy $1- $3B $0.5 - $1B $0.5 - $1B $0.5 - $1B $1.5 - $3B 2022 2025 est. 2025 est. 2025 est. 2025 est. 2022 Rapid and substantial efficacy, as early as week 11 New differentiated oral approach to treat MDD different from other oral ADTs First and only wakefulness promoting agent to improve wakefulness through 9 hours5 First FDA approved dual-acting DNRI to treat EDS in OSA or narcolepsy Rapid and substantial effect, as early as Week 2, with no associated cognitive impairment or sedation Rapid and consistent relief with reduced symptom recurrence Improved cataplexy, EDS, and cognitive function Reduced pain with improved function with effect on fatigue AXS-05 AXS-07 AXS-12 AXS-14 Alzheimer’s disease agitation migraine narcolepsy fibromylagia major depressive disorder TBD $1B Phase 3 trial ongoing; topline data expected in 2H 2024 attention deficit hyperactivity disorder smoking cessation Represents a potentially new mechanism of action for smoking cessation Planned Phase 2/3 trial initiation TBD $0.5 - $1B ADTs: approved antidepressant therapies Please see full Prescribing Information for Auvelity at www.Auvelity.com. Please see full Prescribing Information for Sunosi at www.Sunosi.com. Auvelity and Sunosi referencess are on Slides 8 and 9, respectively. TBD $0.3 - $0.5B Phase 3 trial planned to start in 1Q 2024 binge eating disorder TBD Phase 3 trial planned to start in 1Q 2024 shift work disorder $0.3 - $0.5B $0.3 - $0.5B TBD Phase 3 trial planned to start in 1Q 2024 Solriamfetol major depressive disorder $1B + Program Launch Year Est. Peak U.S. Sales Key Highlights
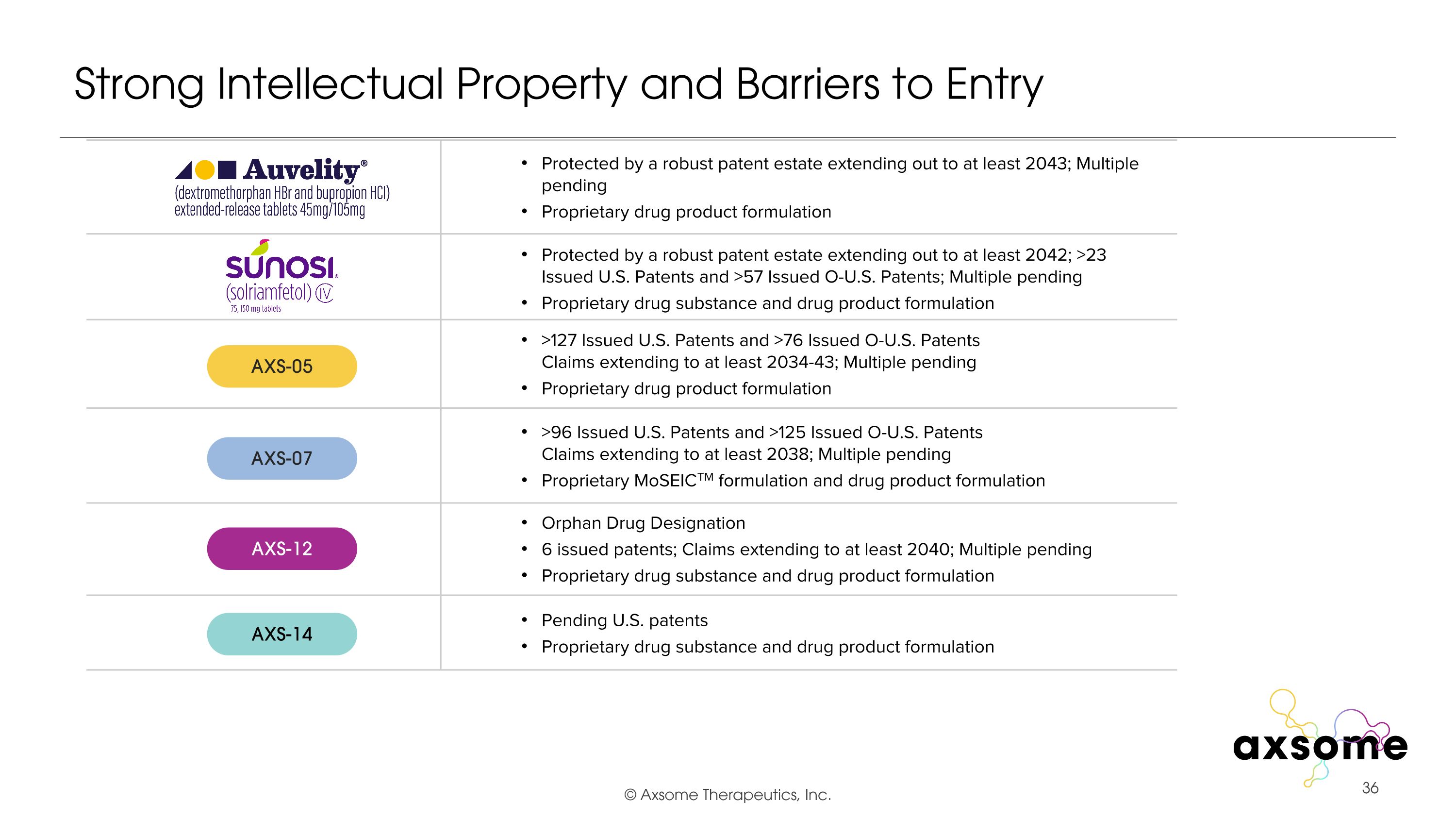
Strong Intellectual Property and Barriers to Entry >127 Issued U.S. Patents and >76 Issued O-U.S. Patents�Claims extending to at least 2034-43; Multiple pending Proprietary drug product formulation >96 Issued U.S. Patents and >125 Issued O-U.S. Patents�Claims extending to at least 2038; Multiple pending Proprietary MoSEICTM formulation and drug product formulation Orphan Drug Designation 6 issued patents; Claims extending to at least 2040; Multiple pending Proprietary drug substance and drug product formulation Pending U.S. patents Proprietary drug substance and drug product formulation Protected by a robust patent estate extending out to at least 2043; Multiple pending Proprietary drug product formulation Protected by a robust patent estate extending out to at least 2042; >23 Issued U.S. Patents and >57 Issued O-U.S. Patents; Multiple pending Proprietary drug substance and drug product formulation AXS-05 AXS-07 AXS-12 AXS-14
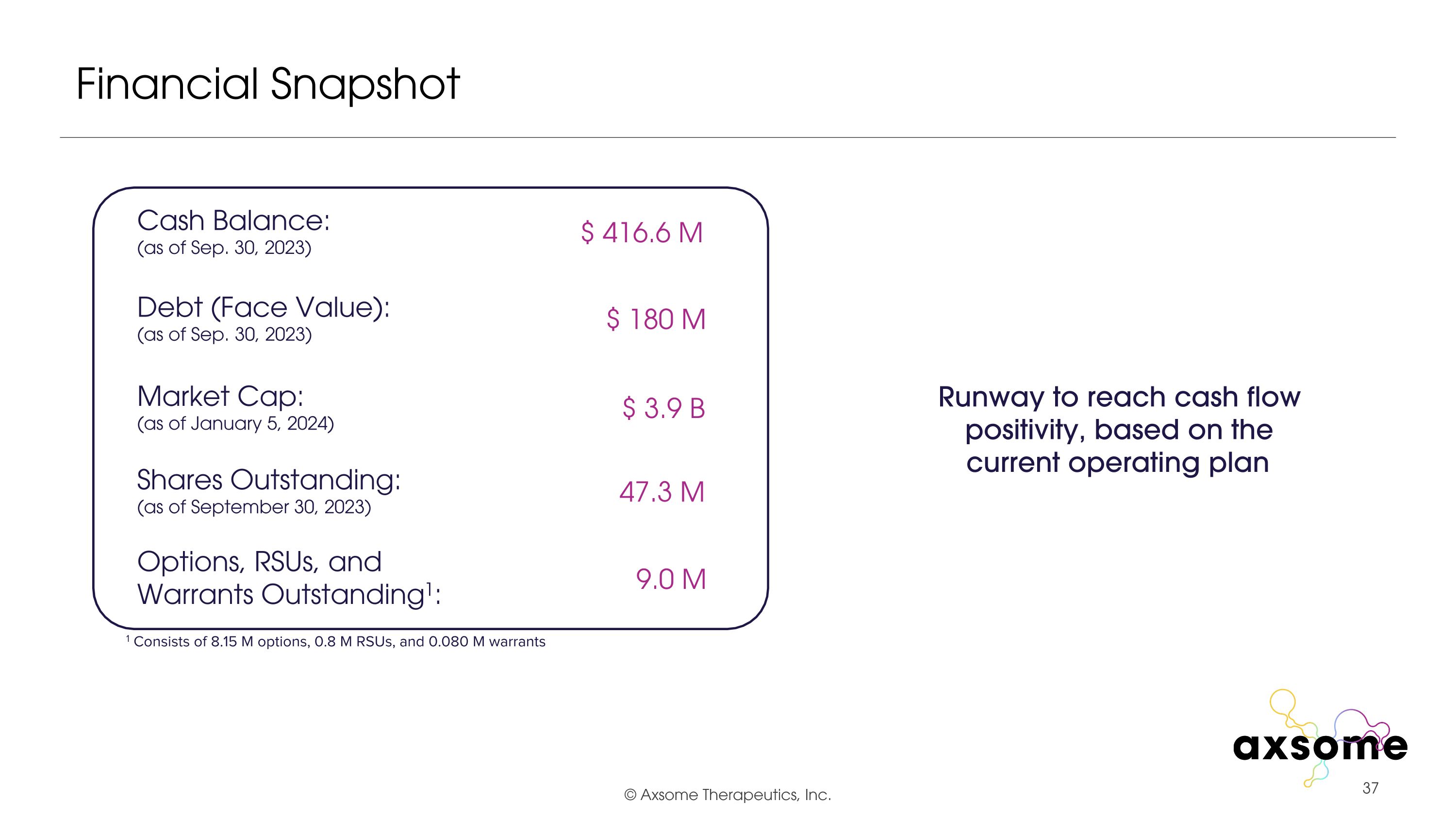
Financial Snapshot Cash Balance: (as of Sep. 30, 2023) Debt (Face Value): (as of Sep. 30, 2023) Market Cap: (as of January 5, 2024) Shares Outstanding: (as of September 30, 2023) Options, RSUs, and Warrants Outstanding1: 1 Consists of 8.15 M options, 0.8 M RSUs, and 0.080 M warrants $ 416.6 M $ 180 M $ 3.9 B 9.0 M 47.3 M Runway to reach cash flow positivity, based on the current operating plan
Leadership Team Roger Jeffs, PhD�CEO�Liquidia Corporation�Former President, Co-CEO, Director United Therapeutics Corp. Prior positions at Amgen and Burroughs Wellcome Mark Saad�Former CFO Bird Rock Bio, Inc.�Former COO of the Global Healthcare Group at UBS Mark Coleman, MD�Director of Clinical Services National Spine and Pain Centers�Diplomat of the American Board of Anesthesiology Susan Mahony, PhD Former SVP of Eli Lilly and President Lilly Oncology Prior positions at BMS, Amgen and Shering-Plough Herriot Tabuteau, MD�Chairman Herriot Tabuteau, MD�Founder & CEO Nick Pizzie, CPA, MBA�Chief Financial Officer Mark Jacobson, MA�Chief Operating Officer Hunter Murdock, JD�General Counsel Ari Maizel�EVP, Head of Commercial Lori Englebert, MBA�EVP, Product Strategy Management Board of Directors

Anticipated Upcoming Clinical and Regulatory Milestones Regulatory and Commercial Clinical Trial Topline Results Clinical Trial Initiations AXS-05 Phase 2/3 trial in smoking cessation – 2024 AXS-12 SYMPHONY Phase 3 trial in narcolepsy – 1Q 2024 AXS-05 ADVANCE-2 Phase 3 trial in Alzheimer’s disease agitation – 1H 2024 AXS-07 Migraine NDA, planned resubmission – 1H 2024 AXS-14 Fibromyalgia NDA, planned submission – 1Q 2024 solriamfetol FOCUS Phase 3 trial in adult ADHD – 2H 2024 solriamfetol Phase 3 trial in binge eating disorder – 1Q 2024 solriamfetol Phase 3 trial in shift work disorder – 1Q 2024 solriamfetol Phase 3 trial in major depressive disorder – 1Q 2024
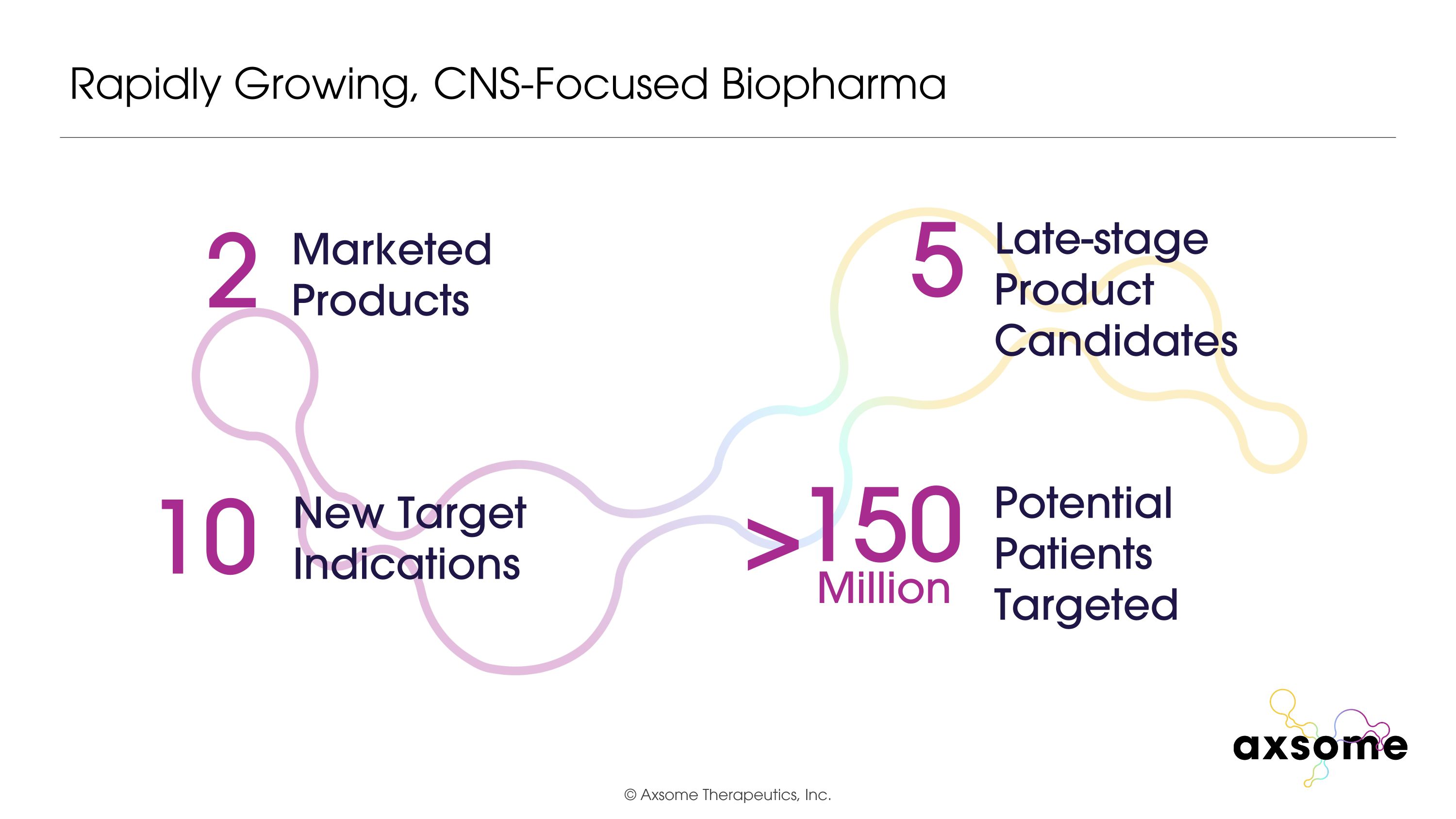
Rapidly Growing, CNS-Focused Biopharma 2 Marketed Products 10 New Target Indications 5 Late-stage Product Candidates Potential Patients Targeted 150 Million >

for more information, please contact: mark jacobson�chief operating officer 212-332-3243 mjacobson@axsome.com www.axsome.com thank you
v3.23.4
Document And Entity Information
|
Jan. 08, 2024 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jan. 08, 2024
|
| Entity Registrant Name |
Axsome Therapeutics, Inc.
|
| Entity Central Index Key |
0001579428
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37635
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
45-4241907
|
| Entity Address, Address Line One |
One World Trade Center, 22nd Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10007
|
| City Area Code |
(212)
|
| Local Phone Number |
332-3241
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, Par Value $0.0001 Per Share
|
| Trading Symbol |
AXSM
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Axsome Therapeutics (NASDAQ:AXSM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Axsome Therapeutics (NASDAQ:AXSM)
Historical Stock Chart
From Apr 2023 to Apr 2024