0001540159false--09-30FY20230false002380280238028000001011720000400000005609845365195323.940000000002300000000015401592022-10-012023-09-300001540159us-gaap:SubsequentEventMemberus-gaap:RevolvingCreditFacilityMember2023-10-310001540159us-gaap:CurrencySwapMember2022-10-012023-09-300001540159edsa:OntarioSubsidaryMember2022-10-012023-09-300001540159edsa:OntarioSubsidaryMember2023-09-300001540159edsa:UniitedStatesMember2023-09-300001540159edsa:UniitedStatesMemberedsa:TwentyFortyTwoMember2023-09-300001540159edsa:UniitedStatesMemberedsa:TwentyFortyOneMember2023-09-300001540159edsa:UniitedStatesMemberedsa:TwentyFortyMember2023-09-300001540159edsa:UniitedStatesMemberedsa:TwentyThirtyNineMember2023-09-300001540159edsa:CanadasMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyFortyTwoMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyFourtyThreeMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyFourtyOneMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyFortyMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyNineMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyEightMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtySevenMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtySixMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyFiveMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyFourMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyThreeMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyTwoMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyOneMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyThirtyMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyTwentyNineMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyTwentyEightMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyTwentyFiveMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyTwentySixMember2023-09-300001540159edsa:CanadasMemberedsa:TwentyTwentySevenMember2023-09-300001540159edsa:SIFAgreementMemberedsa:FebruaryTwoThousandTwentyOneMember2021-02-012021-02-280001540159edsa:SIFAgreementMemberedsa:FebruaryTwoThousandTwentyOneMember2022-10-012023-09-300001540159edsa:SIFAgreementMemberedsa:OctoberTwoThousandTwentyThreeMember2023-10-012023-10-310001540159edsa:AffiliatedDesigneesMember2022-03-012022-03-240001540159edsa:NovemberTwoTwoThousandTwentyTwoMember2022-11-012022-11-020001540159edsa:ClassBWarrantsMember2022-11-012022-11-020001540159edsa:EmployeesMember2023-09-300001540159edsa:AffiliatedDesigneesMember2022-03-240001540159edsa:PreFundedWarrantsMember2023-09-300001540159edsa:PreFundedWarrantsMember2022-03-240001540159edsa:MarchTwoFourTwoThousandTwentyTwoMember2022-03-240001540159edsa:ClassAWarrantsMember2022-11-020001540159edsa:ClassBWarrantsMember2022-11-020001540159edsa:MarchTwoFourTwoThousandTwentyTwoMember2022-03-012022-03-240001540159edsa:BlackScholesOptionMember2022-10-012023-09-300001540159edsa:WarrantsAdditionalPaidInCapitalMember2022-03-012022-03-240001540159edsa:ClassAWarrantsMember2022-11-012022-11-020001540159edsa:PreFundedWarrantsMember2022-03-012022-03-240001540159edsa:NovemberTwoTwoThousandTwentyTwoMember2022-11-012022-11-2200015401592023-03-012023-03-270001540159edsa:EmployeesMember2022-10-012023-09-300001540159edsa:TwoThousandNineteenPlanMember2022-10-012023-09-300001540159edsa:RestrictedShareUnitsOneMember2022-10-012023-09-300001540159edsa:RestrictedShareUnitsMember2023-09-300001540159edsa:RestrictedShareUnitsMember2021-10-012022-09-300001540159edsa:RestrictedShareUnitsMember2022-10-012023-09-300001540159edsa:RestrictedShareUnitsMember2021-09-300001540159edsa:RestrictedShareUnitsMember2022-09-300001540159srt:MaximumMemberedsa:StockOptionSevenMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionSevenMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionSixMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionSixMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionFiveMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionFiveMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionFourMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionFourMember2022-10-012023-09-300001540159edsa:StockOptionTwoMember2022-10-012023-09-300001540159edsa:StockOptionThreeMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionTwoMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionThreeMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionOneMember2022-10-012023-09-300001540159srt:MinimumMemberedsa:StockOptionOneMember2022-10-012023-09-300001540159srt:MaximumMemberedsa:StockOptionSevenMember2023-09-300001540159srt:MinimumMemberedsa:StockOptionSevenMember2023-09-300001540159srt:MaximumMemberedsa:StockOptionSixMember2023-09-300001540159srt:MinimumMemberedsa:StockOptionSixMember2023-09-300001540159srt:MaximumMemberedsa:StockOptionFiveMember2023-09-300001540159srt:MinimumMemberedsa:StockOptionFiveMember2023-09-300001540159srt:MaximumMemberedsa:StockOptionFourMember2023-09-300001540159srt:MinimumMemberedsa:StockOptionFourMember2023-09-300001540159srt:MaximumMemberedsa:StockOptionOneMember2023-09-300001540159srt:MinimumMemberedsa:StockOptionOneMember2023-09-300001540159edsa:StockOptionSevenMember2023-09-300001540159edsa:StockOptionSixMember2023-09-300001540159edsa:StockOptionFiveMember2023-09-300001540159edsa:StockOptionFourMember2023-09-300001540159edsa:StockOptionThreeMember2023-09-300001540159edsa:StockOptionTwoMember2023-09-300001540159edsa:StockOptionOneMember2023-09-300001540159edsa:PurchaseCommonShareOptionsMember2023-09-300001540159edsa:PurchaseCommonShareOptionsMember2021-10-012022-09-300001540159edsa:PurchaseCommonShareOptionsMember2022-10-012023-09-300001540159edsa:PurchaseCommonShareOptionsMember2021-09-300001540159edsa:PurchaseCommonShareOptionsMember2022-09-300001540159edsa:Pre-fundedWarrantSharesMember2022-10-012023-09-300001540159edsa:ClassBWarrantsMember2021-10-012022-09-300001540159edsa:ClassAWarrantsMember2021-10-012022-09-300001540159edsa:ClassBWarrantsMember2022-10-012023-09-300001540159edsa:ClassAWarrantsMember2022-10-012023-09-300001540159edsa:WarrantSevenMember2022-10-012023-09-300001540159edsa:WarrantSixMember2022-10-012023-09-300001540159edsa:WarrantFourMember2022-10-012023-09-300001540159edsa:WarrantFiveMember2022-10-012023-09-300001540159edsa:WarranttThreeMember2022-10-012023-09-300001540159edsa:WarrantTwoMember2022-10-012023-09-300001540159edsa:WarrantOneMember2022-10-012023-09-300001540159edsa:WarrantSevenMember2023-09-300001540159edsa:WarrantSixMember2023-09-300001540159edsa:WarrantFiveMember2023-09-300001540159edsa:WarrantFourMember2023-09-300001540159edsa:WarranttThreeMember2023-09-300001540159edsa:WarrantTwoMember2023-09-300001540159edsa:WarrantOneMember2023-09-3000015401592022-06-300001540159edsa:TwoThousandTwentyOneMemberedsa:LicenseCommitmentsMember2021-10-012022-09-300001540159edsa:TwoThousandSixteenMemberedsa:LicenseCommitmentsMember2022-10-012023-09-300001540159edsa:TwoThousandTwentyOneMemberedsa:LicenseCommitmentsAgreementMember2022-10-012023-09-300001540159edsa:TwoThousandTwentyOneMemberedsa:LicenseCommitmentsMember2022-10-012023-09-3000015401592023-06-300001540159us-gaap:GeneralAndAdministrativeExpenseMember2021-10-012022-09-300001540159us-gaap:GeneralAndAdministrativeExpenseMember2022-10-012023-09-300001540159us-gaap:ComputerEquipmentMember2023-09-300001540159us-gaap:ComputerEquipmentMember2022-09-300001540159us-gaap:FurnitureAndFixturesMember2022-09-300001540159us-gaap:FurnitureAndFixturesMember2023-09-300001540159edsa:IntangibleAssetsMember2022-10-012023-09-300001540159edsa:FurnitureAndEquipmentMember2022-10-012023-09-300001540159us-gaap:ComputerEquipmentMember2022-10-012023-09-300001540159edsa:EquityDistributionAgreementWithCanaccordMember2022-10-012023-09-300001540159edsa:EquityDistributionAgreementWithCanaccordMember2023-03-012023-03-310001540159us-gaap:PrivatePlacementMember2022-11-012022-11-300001540159us-gaap:PrivatePlacementMember2022-03-012022-03-310001540159us-gaap:RetainedEarningsMember2023-09-300001540159us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-09-300001540159us-gaap:AdditionalPaidInCapitalMember2023-09-300001540159edsa:CommonStocksMember2023-09-300001540159us-gaap:RetainedEarningsMember2022-10-012023-09-300001540159us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-10-012023-09-300001540159us-gaap:AdditionalPaidInCapitalMember2022-10-012023-09-300001540159edsa:CommonStocksMember2022-10-012023-09-300001540159us-gaap:RetainedEarningsMember2022-09-300001540159us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-09-300001540159us-gaap:AdditionalPaidInCapitalMember2022-09-300001540159edsa:CommonStocksMember2022-09-300001540159us-gaap:RetainedEarningsMember2021-10-012022-09-300001540159us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-10-012022-09-300001540159us-gaap:AdditionalPaidInCapitalMember2021-10-012022-09-300001540159edsa:CommonStocksMember2021-10-012022-09-300001540159us-gaap:RetainedEarningsMember2021-09-300001540159us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-09-300001540159us-gaap:AdditionalPaidInCapitalMember2021-09-300001540159edsa:CommonStocksMember2021-09-3000015401592021-09-3000015401592021-10-012022-09-3000015401592022-09-3000015401592023-09-3000015401592023-12-1300015401592023-11-24iso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:pureiso4217:CAD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended September 30, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-37619
EDESA BIOTECH, INC. |
(Exact name of registrant as specified in its charter) |
British Columbia, Canada | | N/A |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
| | |
100 Spy Court, Markham, ON, Canada L3R 5H6 | | (289) 800-9600 |
(Address of principal executive offices and zip code) | | (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | | Trading Symbol | | Name of each exchange on which registered |
Common Shares, without par value | | EDSA | | The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
| | Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to Section 240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
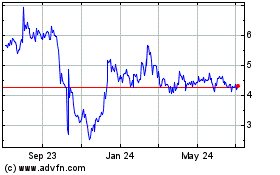
As of March 31, 2023, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s outstanding common shares held by nonaffiliates was approximately $14,782,585 which was calculated based on 2,865,524 common shares outstanding as of that date, of which 2,315,314 common shares were held by nonaffiliates at the closing price of the registrant’s common shares on The Nasdaq Capital Market on such date. These amounts reflect the one-for-seven reverse split of the registrant’s outstanding common shares effected October 11, 2023.
As of December 13, 2023, the registrant had 3,164,722 common shares issued and outstanding.
/s/ MNP LLP
Toronto, Canada
DOCUMENTS INCORPORATED BY REFERENCE: NONE
EDESA BIOTECH, INC.
ANNUAL REPORT ON FORM 10-K
Year Ended September 30, 2023
Table of Contents
FORWARD-LOOKING STATEMENTS AND OTHER MATTERS
This Annual Report on Form 10-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act) and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act) and, as such, may involve known and unknown risks, uncertainties and assumptions. Forward-looking statements are based upon our current expectations, speak only as of the date hereof, are subject to change and include statements about, among other things: the status, progress and results of our clinical programs; our ability to obtain regulatory approvals for or successfully commercialize any of our product candidates; our business plans, strategies and objectives, including plans to pursue collaboration, licensing or other similar arrangements or transactions; our expectations regarding our liquidity and performance, including our expense levels, sources of capital and ability to maintain our operations; the competitive landscape of our industry; and general market, economic and political conditions.
Forward-looking statements are those that predict or describe future events or trends and that do not relate solely to historical matters. You can generally identify forward-looking statements as those statements containing the words “anticipate,” “believe,” “plan,” “estimate,” “expect,” “intend,” “may,” “will,” “would,” “could,” “should,” “might,” “potential,” “continue” or other similar expressions. You should not rely on our forward-looking statements as they are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate because the matters they describe are subject to assumptions, known and unknown risks, uncertainties and other unpredictable factors, many of which are beyond our control.
Our actual results could differ materially and adversely from those expressed in any forward-looking statements as a result of various factors, some of which are discussed in this report in the Part I, Item 1A. Risk Factors and elsewhere in this report. Risks and uncertainties include, among others:
| · | our ability to obtain funding for our operations; |
| · | our estimates regarding our expenses, revenues, anticipated capital requirements and our needs for additional financing; |
| · | the timing of the commencement, progress and receipt of data from any of our preclinical and clinical trials; |
| · | the expected results of any preclinical or clinical trial and the impact on the likelihood or timing of any regulatory approval; |
| · | the therapeutic benefits, effectiveness and safety of our product candidates; |
| · | the timing or likelihood of regulatory filings and approvals; |
| · | changes in our strategy or development plans; |
| · | the volatility of our common share price; |
| · | the rate and degree of market acceptance and clinical utility of any future products; |
| · | the effect of competition; |
| · | our ability to protect our intellectual property as well as comply with the terms of license agreements with third parties; |
| · | our ability to identify, develop and commercialize additional products or product candidates; |
| · | reliance on key personnel; and |
| · | general changes in economic or business conditions. |
Except as required by law, we undertake no obligation to update forward-looking statements. You should review the factors and risks and other information we describe in the reports we will file from time to time with the SEC.
As used in this Annual Report on Form 10-K, “Edesa,” “the Company,” “we,” “us,” and “our” refer to Edesa Biotech, Inc. and our consolidated subsidiaries, except where the context otherwise requires.
Our logo and other trademarks or service marks of Edesa Biotech, Inc. appearing in this Annual Report on Form 10-K are the property of Edesa Biotech, Inc. This Annual Report on Form 10-K contains additional trade names, trademarks and service marks of other companies. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply relationships with, or endorsement or sponsorship of us by, these other companies.
PART 1
Item 1. BUSINESS.
Overview
We are a biopharmaceutical company developing innovative ways to treat inflammatory and immune-related diseases.
Our approach is to acquire, develop and commercialize drug candidates based on mechanisms of action that have demonstrated proof-of-concept in human subjects. We prioritize our efforts on disease indications where there is compelling scientific rationale, no approved therapies or where there are unmet medical needs, and where there are large addressable market opportunities, among other factors. We have multiple late-stage product candidates in our development pipeline.
Our most advanced drug candidate is EB05 (paridiprubart). Paridiprubart represents a new class of emerging therapies called Host-Directed Therapeutics (HDTs) that are designed to modulate the body’s own immune response when confronted with infectious diseases or even chemical agents. Importantly, these therapies are designed to work across multiple infectious diseases and threats, and could be stockpiled preemptively ahead of outbreaks. Because they are threat agnostic, HDTs like paridiprubart have the potential to become standard of care in Intensive Care Units (ICUs) and critical countermeasures for both pandemic preparedness and biodefense. We are currently evaluating EB05 as a potential treatment for Acute Respiratory Distress Syndrome (ARDS), a life-threatening form of respiratory failure. Recruitment in a Phase 3 study is ongoing.
In addition to EB05, we are developing product candidates for a number of chronic dermatological and inflammatory conditions. In November 2023, we reported final results from a Phase 2b clinical study evaluating multiple concentrations of our drug candidate, EB01 (daniluromer), as a monotherapy for moderate-to-severe chronic Allergic Contact Dermatitis (ACD), a common occupational skin condition. Among the findings, 1.0% EB01 cream demonstrated statistically significant improvement over placebo for the primary endpoint and a key secondary endpoint. For our EB06 monoclonal candidate, we have received regulatory approval by Health Canada to conduct a future Phase 2 study in patients with moderate to severe nonsegmental vitiligo, a common autoimmune disorder that causes skin to lose its color in patches. We are also preparing an investigational new drug application (IND) in the United States (U.S.) for our EB07 product candidate to conduct a future Phase 2 study in patients with fibrotic diseases such as systemic sclerosis.
Competitive Strengths
We believe that we possess a number of competitive strengths that position us to become a leading biopharmaceutical company focused on inflammatory and immune-related diseases, including:
| · | Validated technology and drug development capabilities. We believe that the strength of our technologies has been validated by our favorable clinical data and progress to date, more than C$37 million in competitive government grant and funding awards, and our multiple arrangements with third parties to develop and commercialize their clinical-stage drug candidates. |
| | |
| · | Innovative pipeline addressing large underserved markets. Our product candidates include novel clinical-stage compounds and antibodies that have significant scientific rationale for effectiveness. By initially targeting large markets that have significant unmet medical needs, we believe that we can drive adoption of new products and improve our competitive position. For example, ARDS is associated with approximately 10% of ICU admissions globally, impacts millions of people, and costs billions of dollars annually. |
| | |
| · | Intellectual property protection and market exclusivity. We have opportunities to develop our competitive position through patents, trade secrets, technical know-how and continuing technological innovation. We have exclusive license rights in our target indications to multiple patents and pending patent applications in the U.S. and in various foreign jurisdictions. In addition to patent protection, we intend to utilize trade secrets and market exclusivity afforded to new chemical entities and biologics, where applicable, to enhance or maintain our competitive position. |
| | |
| · | Experienced leadership. Our leadership team possesses core capabilities in dermatology, infectious diseases, gastrointestinal medicine, drug development and commercialization, chemistry, manufacturing and controls, and finance. Our founder and Chief Executive Officer, Pardeep Nijhawan, MD, FRCPC, AGAF, is a board-certified gastroenterologist and hepatologist with a successful track record of building life science businesses, including Exzell Pharma Inc., which was sold to BioLab Pharma in 2022, and Medical Futures, Inc., which was sold to Tribute Pharmaceuticals in 2015. In addition to our internal capabilities, we have also established a network of key opinion leaders, contract research organizations, contract manufacturing organizations and consultants. As a result, we believe we are well positioned to efficiently develop novel treatments for inflammatory and immune-related diseases. |
Our Business Strategy
Our business strategy is to develop and commercialize innovative drug products that address unmet medical needs for large, underserved markets with limited competition. Key elements of our strategy include:
| · | Prioritize the development and commercialization of later-stage product candidates. Our goal is to obtain regulatory approval and commercialize multiple clinical assets in our pipeline. We seek to expedite development, in part, through the use of innovative trial designs, including adaptive design protocols, as well as by focusing on disease indications that we believe have clear regulatory pathways and interest from potential licensing or development partners. We also plan to evaluate opportunities to apply, as applicable, for expedited regulatory review and orphan drug programs, which could potentially lead to accelerated clinical development and commercialization timelines for our product candidates. |
| | |
| · | Maximize our current portfolio opportunity by expanding use across multiple indications. We aim to identify clinical-stage assets that have the potential to treat multiple diseases. Our assets are designed to modulate pathways that are implicated across a number of immune and inflammatory/allergic conditions. For example, we believe that our monoclonal antibody candidates have potential utility in additional indications, including chronic conditions like systemic sclerosis and vitiligo. |
| | |
| · | Maximize the commercial potential of our product candidates via direct marketing or strategic arrangements. If our product candidates are successfully developed and approved, we plan to either build commercial infrastructure capable of directly marketing the products, or alternatively, outsource the sales and marketing of our products. We also plan to evaluate strategic licensing or partnering arrangements with pharmaceutical companies for the further development or commercialization of our drugs, where applicable, such as in areas or regions outside North America where a partner may contribute additional resources, infrastructure and expertise. |
| | |
| · | In-license promising product candidates. We are applying our cost-effective development approach to advance and expand our pipeline. Our current product candidates are in-licensed from academic institutions or other biopharmaceutical companies, and, from time to time, we plan to identify, evaluate and potentially obtain rights to and develop additional assets. Our objective is to maintain a well-balanced portfolio with product candidates across various stages of development. We do not currently intend to invest significant capital in basic research, which can be expensive and time-consuming. |
Acute Respiratory Distress Syndrome
ARDS is a life-threatening form of respiratory failure characterized by an exaggerated and dysfunctional immune response, rapid onset of widespread inflammation in the lungs, and hypoxia (an absence of enough oxygen in the tissues to sustain bodily functions). ARDS can be precipitated by a number of conditions including viral and bacterial pneumonia, sepsis, chest injury and even mechanical ventilation, among other causes. ARDS has historically accounted for 10% of ICU admissions, representing more than 3 million patients globally each year. Based on the prevalence data of ARDS, we estimate that there are as many as 600,000 ARDS-related admissions to ICUs each year in the seven major markets (U.S, UK, Germany, France, Spain, Italy, Japan) and Canada. According to medical literature, ICU stays for ARDS patients in the U.S. range from 7 to 21 days on average, at an average cost of more than $100,000 per patient.
For moderate to severe cases of ARDS, treatments remain limited and patients suffer high mortality rates. Countering the exaggerated innate immune response in ARDS patients has been a key area of interest among researchers. One of the most studied targets has been Toll-like receptor 4 (TLR4) - a key component of the innate immune system and an important mediator of inflammation. Since TLR4 detects molecules found in pathogens and also binds to endogenous molecules produced as a result of injury, it is a key receptor on which both infectious and noninfectious stimuli converge to induce a proinflammatory response. Specifically, TLR4 signaling activates leukocytes to secrete proinflammatory cytokines (i.e., CXCL10, IL-6, IFN-b, IL-1b, TNF-α), which under certain circumstances can result in a “cytokine storm” - a severe immune reaction in which the body releases too many cytokines into the blood too quickly.
Such upregulation of TLR4 and its associated cytokines has been observed in respiratory infections such as influenza and SARS-CoV-2. In multiple third-party studies, high serum levels of alarmins, such as calprotectin (S100A8/A9) and HMGB1(high mobility group protein B1), that bind to and activate TLR4 are associated with poor outcomes and disease progression in ARDS patients. In addition, TLR4 inhibition (antagonism) prevents cytokine production at a very early stage and has been shown to have a protective effect. For example, in preclinical studies in mice, it was demonstrated that administration of a TLR4 antagonist blocked influenza-induced lethality and ameliorated virus-induced acute lung injury. Antagonism of TLR4 has also been shown to modulate the secretion of proinflammatory cytokines (IL-6, CRP, IFNb, TNF-a, CXCL-10, IL8 and MIP-1b). Based on these data as well as previous clinical results, we believe that the modulation of TLR4 provides a compelling opportunity to treat ARDS.
EB05 (paridiprubart)
Overview
EB05 is an intravenous formulation of paridiprubart, a first-in-class monoclonal antibody (mAb) that has been engineered to alter inflammatory signaling by binding to and blocking the activation of TLR4. Specifically, paridiprubart dampens TLR4 signaling by blocking receptor dimerization (and subsequent intracellular signaling cascades). The drug has demonstrated the ability to block signaling irrespective of the presence or concentration of the various molecules that frequently bind with TLR4, known as ligands. Based on this broad mechanism of action, we believe that paridiprubart could ameliorate TLR4-mediated inflammation cascades in ARDS patients, thereby reducing lung injury, ventilation rates and mortality.
Phase 2 Results of Phase 2/Phase 3 Study
In September 2022, we reported final results for the Phase 2 part of an international Phase 2/3 clinical study evaluating the safety and efficacy of EB05 as a therapy for adult hospitalized Covid-19 patients.
The Phase 2 part of the Phase 2/3 study was primarily exploratory and designed to refine patient stratification and statistical powering for the Phase 3 study. The study included hospitalized Covid-19 patients, ranging from Level 3 (hospitalized, not requiring supplemental oxygen) on the nine-point WHO Covid-19 Severity Scale (WCSS) to WCSS Level 7 (hospitalized, requiring intubation plus additional organ support such as ECMO). Enrollment in the study as well as the analysis was stratified according to baseline WCSS level into patients with mild Covid-19, defined as WCSS level ≤4, or severe Covid-19, defined as WCSS level ≥5, or critically ill, defined as WCSS level 7. Following a single intravenous infusion of EB05 or placebo, patients were evaluated for disease progression, mortality, side effects and other critical care measurements. Standard-of-care Covid-19 treatment was given to all patients.
In the Phase 2 study, EB05 demonstrated a statistically significant and clinically meaningful trend for 28-day mortality for all randomized subjects in the critically ill cohort (the intent to treat, or ITT, population). The 28-day death rate in the EB05 plus standard of care (SOC) arm was 7.7% versus 40% in the placebo + SOC arm in critically severe patients on ECMO therapy (extracorporeal membrane oxygenation) or Invasive Mechanical Ventilation (IMV) plus organ support with ARDS at baseline (p=0.04). The Survival Analysis using Cox’s Proportional Hazard Model also demonstrated that patients treated with EB05 + SOC had an 84% reduction in the risk of dying when compared to placebo + SOC at 28 days. To our knowledge, no other study has demonstrated a result of this magnitude in this population. The 60-day mortality rate was 23.1% (3/13) in the EB05 + SOC arm versus 45% (9/20) in the placebo + SOC arm for this same population (p=0.20). The Survival Analysis using Cox’s Proportional Hazard Model showed that the patients treated with EB05 + SOC had a 61% reduction in the risk of dying when compared to placebo + SOC at 60 days.
In addition to the critically ill population, the analysis of the full Phase 2 dataset revealed other efficacy signals. For severe Covid-19 patients at WCSS Level 5 and 6 (99% of patients had ARDS at baseline), there were clinically meaningful differences with respect to the proportion of patients who were alive without any need for oxygen support at Day 28 (the Phase 2 study’s primary endpoint). From the ITT analysis of this population, 45.8% in the EB05 + SOC arm versus 36.1% in the placebo + SOC arm achieved the primary endpoint (p=0.16). Similarly positive efficacy signals were also demonstrated in this same population for the proportion of patients who achieved at least a 2-point improvement on the WCSS. From the ITT analysis of this population, 46.7% in the EB05 + SOC arm versus 36.1% in the placebo + SOC arm achieved at least a 2-point improvement in on the WCSS (p=0.12). For mild Covid-19 patients at WCSS Level ≤4, the study did not detect meaningful clinical differences between the arms for these endpoints, which is likely the result of the baseline severity score being too close to the endpoint (WCSS of 3 or less) on these scoring scales. The Phase 2 study demonstrated that EB05 appears to be well-tolerated and consistent with the observed safety profile to date.
Phase 3 of a Phase 2/Phase 3 Study
In December 2022, the U.S. Food and Drug Administration (FDA) granted Fast Track designation to EB05 as a treatment for ARDS in critically ill Covid-19 patients. The Fast Track program provides us with the opportunity for more frequent communication with the agency to discuss the development path for EB05 as a treatment for ARDS in critically ill Covid-19 patients. Investigational drugs that receive Fast Track designation are also eligible for rolling review of their marketing application as well as potential pathways for accelerated regulatory approval. To receive this designation, drug candidates must both treat a serious disease and have non-clinical or clinical data that demonstrate the potential to address an unmet medical need.
The Phase 3 part of our Phase 2/3 study is designed to assess the efficacy and safety of EB05 among hospitalized patients with severe and critical disease, for whom there continues to be limited treatment options and high mortality rates. In March 2023, we announced that the Company and the FDA agreed on the primary endpoint and population for a Phase 3 study evaluating EB05 as a therapy for hospitalized Covid-19 patients with ARDS. Under the amended protocol design, we will evaluate a single cohort of severely ill patients on invasive mechanical ventilation, both with and without additional organ support such as ECMO. The protocol calls for approximately 600 evaluable hospitalized subjects to be enrolled. The primary endpoint will be the mortality rate at 28 days. In October 2023, Canadian regulators approved an amendment that harmonized the previously approved Canadian protocol with the U.S. protocol.
Based on current hospitalization trends and our recruitment experience, we believe that Covid-19-related hospitalization patterns have become more predictable and seasonal in nature, similar to those of influenza, with increased hospitalizations and deaths anticipated in the fall/winter and among populations and geographies with low booster/vaccination rates. As a result, we believe that the pace of future enrollment will be more closely linked to the number and location of investigational sites we activate rather than the unpredictable waves of the pandemic. We plan to increase the number of investigational centers from 23 to up to 60 hospitals in the U.S. and Canada. We have the flexibility to adjust the timing of these and other clinical trial expenditures to manage our working capital.
In addition to Covid-19 induced ARDS, we are also exploring various approaches to evaluate our EB05 drug candidate in a general, all-cause ARDS population. Given the broader pool of patients, we believe a general, all-cause ARDS study could increase efficiency and expedite development timelines as well as validate the broader potential utility of EB05. Any changes we make to our clinical study protocol may impact how previously enrolled subjects are categorized and/or included in the study’s results. As of the date of this filing, recruitment is ongoing in the U.S. and Canada.
Previous Phase 1 and Phase 2 Clinical Studies
Paridiprubart has demonstrated the potential ability to regulate inflammation and resolve fever and stabilize heart and breathing rates in human subjects that were injected with lipopolysaccharide (LPS) - a potent inducer of acute systemic inflammation. In previous Phase 1 and Phase 2 clinical studies, paridiprubart has demonstrated favorable safety and tolerability profiles.
In a previous Phase 1 study, paridiprubart was administered in healthy volunteers (HV) as a single intravenous infusion using a single ascending pharmacokinetic/pharmacodynamic dose design. Paridiprubart was administered at different dose levels and was followed by in vivo LPS challenges. The study demonstrated that paridiprubart inhibited the release of various pro-inflammatory cytokines (IL-6, TNF-α and CXCL10) and stabilized certain other vital signs for up to 22 days after the infusion of paridiprubart. The study further demonstrated that cytokine response and baseline vital signs were restored at 40 days after paridiprubart infusion.
Paridiprubart demonstrated a favorable safety profile in the Phase 1 study in healthy volunteers as well as in a multiple-infusion Phase 2 study in subjects with rheumatoid arthritis (RA). In the Phase 1 study, doses ranged from 0.001 mg/kg up to 15mg/kg. The Phase 2 RA study was a multiple dose study where patients received one dose of paridiprubart 5 mg/kg every two weeks for 12 weeks. In the Phase 1 study, a total of 60 subjects received paridiprubart, and in the Phase 2 RA study, 61 patients were randomized to the paridiprubart group. There were no meaningful differences observed between the placebo and paridiprubart treatment groups with respect to the incidence of treatment emergent serious and non-serious adverse events in either of these studies.
Federal Funding from the Government of Canada
In October 2023, our wholly owned subsidiary Edesa Biotech Research, Inc. (Edesa Biotech Research) entered into a multi-year contribution agreement (the 2023 SIF Agreement) with the Canadian government’s Strategic Innovation Fund, or SIF. Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding toward (i) conducting and completing a Phase 3 clinical study of our investigational therapy EB05 in critical-care patients with ARDS caused by Covid-19 or other infectious agents, and (ii) submitting EB05 for governmental approvals and manufacturing scale-up, following, and subject to, completing the Phase 3 study and (iii) conducting two non-clinical safety studies to assess the potential long-term impact of EB05 exposure. Of the C$23 million committed by SIF, up to C$5.75 million is not repayable. The remaining C$17.25 million is conditionally repayable starting in 2029 only if and when we earn gross revenue. Edesa Biotech Research has agreed to complete the project by December 31, 2025. In the event that we or Edesa Biotech Research breach our obligations under the 2023 SIF Agreement, subject to applicable cure, the SIF may exercise a number of remedies, including suspending or terminating funding under the 2023 SIF Agreement, demanding repayment of funding previously received and/or terminating the 2023 SIF Agreement. The performance obligations of Edesa Biotech Research under the 2023 SIF Agreement are guaranteed by us.
Our previously completed Phase 2 study of EB05 was also funded, in part, by SIF. Under a February 2021 agreement (the 2021 SIF Agreement), the Government of Canada committed C$14.1 million in nonrepayable funding for an international Phase 2 study and certain pre-clinical experiments. In the event that we or Edesa Biotech Research breach our obligations under the 2021 SIF Agreement, subject to applicable cure, the SIF may exercise a number of remedies, including demanding repayment of funding previously received and/or terminating the agreement. The performance obligations of Edesa Biotech Research under the contribution agreement are guaranteed by us. All potential funding available under the 2021 SIF Agreement has been received. As of the date of this filing, we have met all of our performance and reporting requirements under the 2021 SIF Agreement.
Vitiligo
Vitiligo is a chronic autoimmune disease that causes the loss of skin pigmentation in patches. It occurs when melanocytes, the pigment-producing skin cells, die or stop producing melanin. The extent of color loss from vitiligo is unpredictable and can affect the skin on any part of the body. It is estimated that vitiligo prevalence is between 0.5 to 2% of the global population. Vitiligo patients are not born with lesioned skin. Rather, unpigmented spots appear over time, with about 50% of patients having symptom onset before 20 years of age. There are two main forms of vitiligo: segmental, where depigmentation is limited to one area and side of the body, and nonsegmental (generalized), where patches of pale skin occur on both sides of the body, often symmetrically. Nonsegmental vitiligo is the most common type of vitiligo.
At present, there is only one FDA-approved therapeutic indicated for repigmentation in vitiligo, a Janus Kinase (JAK) inhibitor cream (ruxolitinib); however, there is an increased risk of serious infections and malignancies associated with ruxolitinib. Similarly, off-label non-surgical therapies tend to be time-consuming, expensive, or prone to causing side effects. Common treatments include topical drugs, phototherapies and surgical interventions. Based on the availability and limitations of current treatments, we believe there is a significant need for well targeted and systemic immunotherapies.
EB06
Overview and Status
EB06 is a monoclonal antibody candidate that binds specifically and selectively to chemokine ligand 10 (CXCL10) and inhibits the interaction of CXCL10 with its receptor(s). We believe that there is significant scientific rationale for the potential utility of this mechanism of action to reduce disease symptoms and progression in vitiligo patients. CXCL10 is highly expressed in vitiligo patients, and has been shown to play both a key role in the trafficking of anti-melanocytic T-cells to the epidermis as well as in inducing apoptosis (death) of melanocytes. Furthermore, neutralization of CXCL10 has been demonstrated to both prevent and reverse depigmentation in animal models. EB06 is currently formulated for intravenous administration, with future plans for a potential subcutaneous formulation.
We have approval from Health Canada to conduct a Phase 2 study of EB06 in moderate to severe nonsegmental vitiligo patients, and we are currently evaluating potential funding options to initiate this project, which may include both drug manufacturing and clinical activities.
Previous Clinical Experience
EB06 has demonstrated a favorable safety and tolerability profile in three previous clinical studies of 65 subjects in total. The first Phase 1 study was a double-blind, placebo-controlled, ascending, single-dose study in 20 healthy subjects. Participants received single intravenous doses of EB06, ranging from 0.1 to 20 mg/kg. No deaths or serious adverse events (AEs) were reported. EB06 was generally safe and well tolerated at doses up to 20 mg/kg. A second Phase 1 study evaluated the effect of single doses of EB06 to generate proof-of-principle data on the neutralization of CXCL10 in an inflammatory setting in humans using an experimentally nickel-induced allergic contact dermatitis model. For this double-blind, placebo-controlled study, 16 subjects were exposed to single intravenous doses of 180 and 720 mg of EB06. No deaths or serious AEs were reported, and EB06 was generally safe and well tolerated.
A third, open-label, single-arm Phase 2 study investigating multiple administrations of EB06 in patients with primary biliary cirrhosis with an incomplete response to ursodeoxycholic acid (UDCA) was also completed. A total of 29 patients were treated with 10 mg/kg intravenous doses of EB06 every two weeks, for a total of 6 doses. No serious treatment-related AEs were reported.
In addition, in a variety of pre-clinical in vitro and in vivo experiments, EB06 demonstrated the ability to neutralize the biological activity of CXCL10. In animal toxicology studies, EB06 was well-tolerated.
Allergic Contact Dermatitis
Contact dermatitis is a common occupational and work-related skin condition. The disease can be either irritant contact dermatitis or ACD. Based on market research, we believe that together these conditions cost up to $2 billion annually in the U.S. as a result of lost work, reduced productivity, medical care and disability payments. Based on the prevalence data of contact allergy in the general population, which we sourced from scientific literature and market reports, we estimate that there are as many as 30 million people in the seven major markets (US, UK, Germany, France, Spain, Italy, Japan) and Canada with ACD, and of these, we estimate that 40% have chronic exposure or frequent recurring exposure to a causative allergen. Based on the mechanism of action and topical delivery, we believe that the total addressable patient population for EB01 is as high as five million people in the seven major markets and Canada.
ACD is caused by an allergen interacting with skin and usually occurs on areas of the body that are open to the environment, with a high prevalence on the hands and face. Common allergens associated with ACD include plants, metals, plastics and resins, rubber additives, dyes, biocides, and various cosmetics. The disease is characterized by inflammation, erythema (redness), pruritus (itchiness), and blistering of the skin. Inflammation can vary from mild irritation and redness to open sores, depending on the type of irritant, the body part affected and the degree of sensitivity. ACD can become chronic if not treated or if the causative allergen is not removed. In many chronic cases, the causative allergen is unknown or difficult to avoid (as an example, the allergen is present in the workplace).
The immune mechanisms involved in ACD are well documented. During the initial contact with the offending allergen, the immune system is sensitized. Upon subsequent contact, a delayed-type hypersensitivity reaction (Type IV) occurs at the point of contact between the skin and the allergen. As a cell-mediated response, the immune reaction primarily involves the interaction of T cells with antigens rather than an antibody response. More specifically, ACD involves an exogenous substance binding a cell surface protein to form a hapten that is recognized as a foreign antigen by the immune system. Haptens are known to signal through toll-like receptors, a family of receptors involved in the innate immune system, which leads to the induction of pro-inflammatory cytokines such as interleukin (IL)-1b. EB01 has been shown in preclinical studies to inhibit the production of pro-inflammatory cytokines induced via toll-like receptor signaling (IL-1b, IL-6, IL-8, MIP-1a, and TNF-α), suggesting that EB01 may address the underlying disease mechanism of ACD.
Generally, dermatologists view chronic ACD from both a duration and recurrence perspective, considering how often and how long symptoms persist. Chronic disease affects patients over a prolonged period, typically greater than six months or even years. These chronic patients have either frequent intermittent exposure or continuous exposure. Since inflammation in ACD is driven by external exposure to an allergen, the severity of ACD does not necessarily correlate with body surface area, as is often the case with other dermatological diseases.
Current treatment plans begin by attempting to identify and remove exposure to the causative allergen. However, the causative allergen(s) is frequently not identified, and even when it is, avoiding exposure is often not possible (e.g., it is present in the workplace), according to our market research. To our knowledge, there are no drug treatment options specifically indicated for ACD. As such, physicians must utilize agents approved for other dermatological conditions. Topical corticosteroids are the most commonly used therapeutic intervention for ACD but cannot be used continuously since they have well-known side-effects including skin thinning, stretch marks, acne, stinging, burning and dryness. Other topical treatments for ACD include topical immunomodulators such as topical calcineurin inhibitors. However, these are less efficacious than topical corticosteroids and have an FDA “black box warning” for risk of malignancies. Systemic corticosteroids can be used for acute control of severe cases of ACD but have safety concerns including hypothalamic-pituitary-adrenal axis suppression, growth suppression and loss of bone-density, thereby limiting the utility of steroids for treating chronic disease. Finally, patients may be treated with systemic immunomodulators, which have “black box warnings” and associated safety issues. Systemic therapies also need to be tapered off each time the physician wants to patch test allergens to identify the source of a patient’s ACD.
EB01 (daniluromer)
Overview and Status
EB01 is a potential first-in-class, topical vanishing cream containing a novel, non-steroidal anti-inflammatory compound. Daniluromer exerts its anti-inflammatory activity through the inhibition of certain pro-inflammatory enzymes known as secretory phospholipase 2, or sPLA2. These enzymes are secreted by immune cells upon their activation and produce arachidonic acid via phospholipid hydrolysis, which, in turn, initiates a broad inflammatory cascade. The sPLA2 enzyme family plays a key role in initiating inflammation associated with many diseases, and we believe that targeting the sPLA2 enzyme family with enzyme inhibitors will have a superior anti-inflammatory therapeutic effect because the inflammatory process will be inhibited at its inception rather than after inflammation has occurred.
Phase 2b Clinical Results of EB01
In November 2023, we reported final results from a Phase 2b clinical study evaluating multiple concentrations of our drug candidate, EB01, as a monotherapy for chronic moderate-to-severe ACD. The double-blind, placebo-controlled trial evaluated the safety and efficacy of EB01 in approximately 200 subjects, who were treated for 28 days with either EB01 cream (2.0%, 1.0% or 0.2%) or a placebo/vehicle cream. The primary efficacy outcome measurement was the mean percent improvement in symptoms from baseline at day 29 on the Contact Dermatitis Severity Index (CDSI). A key secondary efficacy measurement was the success rate of subjects achieving a score of "clear" or "almost clear" with at least a 2-point improvement from baseline after treatment at day 29 on the Investigator's Static Global Assessment (ISGA) scale.
The 1.0% EB01 cream demonstrated statistically significant improvement over placebo. For the primary endpoint, patients with 1.0% EB01-treated lesions demonstrated a 60% average improvement in symptoms from baseline at day 29 on the CDSI versus 40% for placebo/vehicle (p=0.027). For the ISGA secondary efficacy endpoint, 53% of patients with 1.0% EB01-treated lesions achieved a score of "clear" or "almost clear" with at least a 2-point improvement from baseline after treatment at day 29 (p=0.048). Only 29% of patients in the placebo group reached the same endpoint. No serious treatment-related adverse events were reported across all concentrations. The 2.0% and 0.2% formulations did not show significant differences compared to placebo. We are currently evaluating potential partnerships and funding opportunities for the continued development of this drug candidate.
Previous Clinical Results of EB01
EB01 has demonstrated efficacy for the treatment of ACD in two separate clinical trials. Both studies were double-blind, placebo/vehicle-controlled bilateral comparison studies to assess the safety, tolerability and efficacy of EB01 cream applied twice daily for the treatment of ACD of the hand and forearm as determined by the CDSI, a physician’s visual assessment. The CDSI is a composite endpoint, which grades each symptom of the disease (dryness, scaling, redness, pruritus, and fissures) scored from 0 (none) to 3 (severe), with a maximum total severity score of 15. A diagnosis of ACD was confirmed by a positive patch test deemed to be clinically relevant by the investigator.
The first Phase 2 study (n=11) was a double-blind, placebo/vehicle-controlled clinical study to assess the safety and efficacy of topical 1% EB01 cream for the treatment of ACD. Subjects selected for inclusion had bilateral ACD. Prior to randomization, subjects were patch tested. The study was bilateral in design with one lesion treated with 1% EB01 cream twice daily, while a comparable lesion was treated with placebo cream. Disease severity was assessed before treatment (Day 0) and at Day 30 by the investigator using the CDSI. For each individual patient, the change in disease score in the drug-treated hand was compared to that in the placebo-treated hand, thus making the latter an internal control for each patient. The mean change from baseline for 1% EB01 cream treated lesions was 69.9%, compared to 36.5% in the placebo cream lesions (p=0.0024).
A second Phase 2 study was a larger (n=30) bilateral study was conducted to assess 2% EB01 cream applied twice daily for 21 consecutive days in connection with the treatment of ACD. To be included in the study, patients had to have bilateral ACD with a CDSI score of at least 10 on each side, with no more than a 1-point difference between lesions. At Day 21, EB01-treated lesions had a mean improvement from baseline of 56%, compared to 24% for those treated with placebo cream (p<0.001). Efficacy of the 2% EB01 cream was maintained through Day 42 (21-days after ending treatment) with a 49% decrease in total CDSI score for 2% EB01 cream-treated hands, compared to 15% in the placebo/vehicle-treated hands (p<0.001). Within the total CDSI score, EB01 demonstrated statistically significant reductions for each of the individual CDSI components (dryness, scaling, redness, pruritus, and fissures).
Total clinical experience with daniluromer, including the current Phase 2b study, has involved approximately 270 subjects. No serious adverse events have been encountered to date.
Pre-Clinical Results
Daniluromer has demonstrated anti-inflammatory activity in a variety of in vitro and in vivo preclinical pharmacology models. Using a model for hapten signaling indicative of ACD, lipopolysaccharide-stimulated peripheral blood mononuclear cells were treated with daniluromer and shown to inhibit pro-inflammatory cytokines including IL-1b, IL-6, IL-8, MIP-1a, and TNF-α at the protein and mRNA expression levels. Additionally, in several Good Laboratory Practice animal toxicology studies, daniluromer was well-tolerated and systemic exposure was negligible (below the limit of detection). No genotoxicity was demonstrated in bacterial reverse mutation and micronucleus testing.
Other Future Product Candidates
We are seeking to advance additional product candidates as well as add new disease indications for current product candidates, and from time to time we may request approval from regulators in various jurisdictions to initiate new clinical studies or amend the scope of current clinical studies. In addition, we plan to continue to identify, evaluate and potentially obtain rights to and develop additional clinical assets across various stages of development, focusing primarily on inflammatory and immune-related diseases.
Among our activities, we are preparing IND in the U.S. for our EB07 (paridiprubart) product candidate to conduct a future study in patients with fibrotic diseases such as systemic sclerosis. This project represents a potential additional use for our anti-TLR4 monoclonal antibody candidate in chronic diseases with limited treatment options and high mortality and morbidity. In addition, our EB02 (daniluromer) drug candidate represents a potential extension of our sPLA2 anti-inflammatory technology. Based on our analysis of clinical data in dermatitis, we believe that EB02, which is currently formulated as a cream, may be effective in treating the erythema, swelling and exudation associated with hemorrhoids disease (HD). We have received approval from Health Canada for an exploratory Phase 2a clinical study of EB02 as a potential treatment for patients with grade I-III internal hemorrhoids. In light of our focus on the development of other product candidates, we are currently evaluating the timing for the initiation of this planned study of EB02. Initiating recruitment in the EB07 and EB02 studies is subject to, among other limitations, funding, regulatory approvals, drug manufacturing and activation of clinical investigational sites.
Intellectual Property and Key Licenses
We have an exclusive license from Yissum Research Development Company, the technology transfer company of Hebrew University of Jerusalem Ltd. (Yissum), for patents and patent applications that cover our product candidates EB01 and EB02 in the U.S., Canada, Australia and various countries in Europe. Method of use patents, for which we hold an inbound license from Yissum and an affiliate of Yissum, have been issued for use in dermatologic and gastrointestinal conditions and infections that will expire in 2024. We expect to seek patent term extension in the U.S. related to time under IND, which could add up to three to five years of additional protection. Additional patents subject to the license agreement have been filed by Yissum which we believe, if issued, could potentially prevent generic substitution until after 2033.
We also hold an exclusive license from NovImmune SA, for patents and patent applications that cover our product candidates that utilize our anti-TLR4 and anti-CXCL10 monoclonal antibody technology in the U.S., Canada and various other countries. Composition of matter patents, for which we hold an inbound license from NovImmune, have been issued that will expire as late as 2033 and 2028, respectively. We expect to seek patent term extension in the U.S. related to time under IND, which could extend protection. We have also filed additional method of use patent applications which we believe, if issued, could potentially prevent biosimilar substitution until as late as 2041. We have also filed provisional patent applications for use of these monoclonal antibody technologies in vitiligo (EB06) and systemic sclerosis (EB07).
In the event we are successful in commercializing a new drug candidate, we believe we would be eligible for data/market exclusivity, in addition to exclusivity rights granted through patent protection. We would be eligible for up to five years of exclusivity for EB01 and EB02 and up to 12 years of exclusivity for EB05 and EB06 after approval in the U.S., and, for any of these drug products, eight years of exclusivity after approval in Canada and ten years of exclusivity after approval in the European Union (EU).
We expect patents and other proprietary intellectual property rights to be an essential element of our business. We intend to protect our proprietary positions by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements. We also rely on trade secrets, know-how, continuing technological innovation and other in-licensing opportunities to develop and maintain our proprietary position. Our success will depend, in part, on our ability to obtain and maintain proprietary protection for our product candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights.
License Agreement with NovImmune SA
In April 2020, through Edesa Biotech Research, we entered into an exclusive license agreement (the NovImmune License Agreement) with NovImmune SA (NovImmune), which operates under the brand Light Chain Bioscience, whereby we obtained exclusive rights throughout the world to certain know-how, patents and data relating to the monoclonal antibodies targeting TLR4 and CXCL10 (the Constructs). We will use the exclusive rights to develop products containing these Constructs (the Licensed Products) for therapeutic, prophylactic and diagnostic applications in humans and animals. Unless earlier terminated, the term of the NovImmune License Agreement will remain in effect for 25 years from the date of first commercial sale of Licensed Products. Subsequently, the NovImmune License Agreement will automatically renew for 5-year periods unless either party terminates the agreement in accordance with its terms.
Under the NovImmune License Agreement, we are exclusively responsible, at our expense, for the research, development manufacture, marketing, distribution and commercialization of the Constructs and Licensed Products and to obtain all necessary licenses and rights. We are required to use commercially reasonable efforts to develop and commercialize the Constructs in accordance with the terms of a development plan established by the parties. In exchange for the exclusive rights to develop and commercialize the Constructs, we issued to NovImmune $2.5 million of newly designated Series A-1 Convertible Preferred Shares (all of which were subsequently converted into common shares) pursuant to the terms of a securities purchase agreement entered into between the parties concurrently with the NovImmune License Agreement. In addition, we are committed to payments of various amounts to NovImmune upon meeting certain development, approval and commercialization milestones as outlined in the NovImmune License Agreement up to an aggregate amount of $356 million. We also have a commitment to pay NovImmune a royalty based on net sales of Licensed Products in countries where we directly commercialize Licensed Products and a percentage of sublicensing revenue received by us in the countries where we do not directly commercialize Licensed Products.
The NovImmune License Agreement provides that NovImmune will remain the exclusive owner of existing intellectual property in the Constructs and that we will be the exclusive owner of all intellectual property resulting from the exploitation of the Constructs pursuant to the license. Subject to certain limitations, we are responsible for prosecuting, maintaining and enforcing all intellectual property relating to the Constructs. During the term of the agreement, we also have the option to purchase the licensed patents and know-how at a price to be negotiated by the parties. If we default or fail to perform any of the terms, covenants, provisions or its obligations under the NovImmune License Agreement, NovImmune has the option to terminate the NovImmune License Agreement, subject to providing us with an opportunity to cure such default. The NovImmune License Agreement is also terminable by NovImmune upon the occurrence of certain bankruptcy related events pertaining to us.
In connection with the NovImmune License Agreement and pursuant to a purchase agreement entered into by the parties in April 2020, we acquired from NovImmune its inventory of the TLR4 antibody for an aggregate purchase price of $5.0 million.
License and Development Agreement with Pendopharm
In August 2017, Edesa Biotech Research entered into an exclusive license and development agreement with Pendopharm, a division of Pharmascience Inc. (the Pendopharm License Agreement). Pursuant to the Pendopharm License Agreement, we granted to Pendopharm an exclusive license throughout Canada to certain know-how, patents and data for the sole purpose of obtaining regulatory approval for certain pharmaceutical products to allow Pendopharm to distribute, market and sell the licensed products for human therapeutic use in certain gastrointestinal conditions. If Pendopharm elects not to seek regulatory approval of the applicable product, the applicable product will be removed from the license rights granted to Pendopharm and will revert to us. If Pendopharm elects to seek regulatory approval in Canada for the sale and marketing of the applicable product, Pendopharm will be responsible for obtaining regulatory approval for the applicable licensed product in Canada. In exchange for the exclusive rights to market, import, distribute, and sell the pharmaceutical products, Pendopharm is required to pay us a royalty in respect of aggregate annual net sales for each pharmaceutical product sold in Canada. Unless earlier terminated, the term of the Pendopharm License Agreement will expire, on a licensed product by licensed product basis, on the later to occur of (i) the date that is 13 years after the first commercial sale of the licensed product in Canada; (ii) the date of expiry of the last valid licensed patent in Canada relating to the licensed product; or (iii) the date of expiry of any period of exclusivity granted to the licensed product by a regulatory authority in Canada. The Pendopharm License Agreement shall also terminate upon the termination of certain other license agreements that we have with third parties. Pendopharm also has the right to terminate the Pendopharm License Agreement for convenience upon 120 days’ notice to us.
License Agreements with Yissum and Inventor
In June 2016, Edesa Biotech Research, entered into an exclusive license agreement with Yissum, which was subsequently amended in April 2017, May 2017 and October 2022 (collectively, the Yissum License Agreement). Pursuant to the Yissum License Agreement, as amended, we obtained exclusive rights throughout the world to certain know-how, patents and data relating to a pharmaceutical product for the following fields of use: therapeutic, prophylactic and diagnostic uses in topical dermal applications and anorectal applications. Unless earlier terminated, the term of the Yissum License Agreement will expire on a country by country basis on the later of (i) the date of expiry of the last valid licensed patent in such country; (ii) the date of expiry of any period of exclusivity granted to a product by a regulatory authority in such country or (iii) the date that is 15 years after the first commercial sale of a product in such country.
Under the Yissum License Agreement, we are exclusively responsible, at our expense, for the development of the product, including conducting clinical trials and seeking regulatory approval for the product, and once regulatory approval has been obtained, for the commercialization of the product. We are required to use our commercially reasonable efforts to develop and commercialize the product in accordance with the terms of a development plan established by the parties. Subject to certain conditions, we are permitted to engage third parties to perform our activities or obligations under the agreement. In exchange for the exclusive rights to develop and commercialize the product for topical dermal applications and anorectal applications, we are committed to payments of various amounts to Yissum upon meeting certain milestones outlined in the Yissum License Agreement up to an aggregate amount of $18.4 million. In addition, in the event of a divestiture of substantially all of our assets, we are obligated to pay Yissum a percentage of the valuation of the licensed technology sold as determined by an external objective expert. We also have a commitment to pay Yissum a royalty based on net sales of the product in countries where we, or an affiliate of ours, directly commercializes the product and a percentage of sublicensing revenue received by us and our affiliates in the countries where we do not directly commercialize the product.
The Yissum License Agreement provides that Yissum shall remain the exclusive owner of the licensed technology and that we are responsible for preparing, filing, prosecuting and maintaining the patents on the licensed technology in Yissum’s name. Notwithstanding the foregoing, we will be the exclusive owner of all patents and other intellectual property that is made by, or on our behalf, after the date of the agreement, including all improvements to the licensed technology. If we default or fail to perform any of the terms, covenants, provisions or our obligations under the Yissum License Agreement, Yissum has the option to terminate the Yissum License Agreement, subject to providing us with an opportunity to cure such default. We have the right to terminate the Yissum License Agreement if we determine that the development and commercialization of the product is no longer commercially viable. Subject to certain exceptions, we have undertaken to indemnify Yissum against any liability, including product liability, damage, loss or expense derived from the use, development, manufacture, marketing, sale or sublicensing of the licensed product and technology.
In March 2021, through Edesa Biotech Research, we entered into a license agreement with the inventor of the same pharmaceutical product, which was subsequently amended in September 2023 (together, the Inventor License Agreement), to acquire global rights for all fields of use beyond those named under the Yissum License Agreement. As a result of the Inventor License Agreement, we now hold exclusive global rights to the pharmaceutical product that forms the basis of our EB01 and EB02 drug candidates for all fields of use in humans and animals. We are required to use commercially reasonable efforts to develop and commercialize the product in accordance with the terms of a development plan established by the parties. We are exclusively responsible, at our expense, for the development of the product. We are committed to remaining payments of up to an aggregate amount of $69.1 million, primarily relating to future potential commercial approval and sales milestones. In addition, if we fail to file an IND application or foreign equivalent for the product within a certain period of time following the date of the agreement, we are required to remit to the inventor a fixed license fee on a quarterly basis as long as the requirement to file an IND remains unfulfilled. We also have a commitment to pay the inventor a royalty based on net sales of the product in countries where we, or an affiliate, directly commercialize the product and a percentage of sublicensing revenue received by us and our affiliates in the countries where we do not directly commercialize the product. Unless earlier terminated, the term of the Inventor License Agreement will expire on a country by country basis on the later of (i) the date of expiry of the last valid licensed patent in such country or (ii) the date that is 15 years after the first commercial sale of a product in such country. If we default or fail to perform any of the terms, covenants, provisions or our obligations under the Inventor License Agreement, the inventor has the option to terminate the Inventor License Agreement, subject to providing us with an opportunity to cure such default. We have the right to terminate the Inventor License Agreement if we determine that the development and commercialization of the product is no longer commercially viable. Subject to certain exceptions, we have undertaken to indemnify the inventor against any liability, including product liability, damage, loss or expense derived from the use, development, manufacture, marketing, sale or sublicensing of the licensed product and technology.
Manufacturing and Marketing
We rely, and expect to continue to rely for the foreseeable future, on third-party contract manufacturing organizations, or CMOs, to produce both our synthetic chemical and biological product candidates for clinical testing, as well as for commercial manufacture if our product candidates receive marketing approval. Additional contract manufacturers are used to fill, label, package and distribute investigational drug products. We believe that this strategy will enable us to direct operational and financial resources to the development of our product candidates rather than diverting resources to establishing manufacturing infrastructure. Our current arrangements with our manufacturers are subject to customary industry terms and conditions, and manufacturing is performed on an as-requested basis. While we have not experienced significant shortages of raw materials to date, as a result of increased industry demand, CMOs have generally reported that supplies of raw materials and critical components necessary for manufacturing processes have been more challenging and expensive to obtain, and longer lead times may be required for scheduling future production runs. We believe that we have sufficient supplies on hand to complete the Phase 3 clinical study for EB05.
To supply future clinical studies and potential commercialization of our product candidates, we are engaged in discussions with various CMOs regarding long-term supply agreements. These supply agreements typically require significant financial commitments, including upfront amounts prior to commencement of manufacturing, progress payments through the course of the manufacturing process as well as payments for technology transfer and other start-up costs. Based on our discussions with CMOs and industry announcements regarding future expansion plans, we believe there will be sufficient supplies of raw materials and manufacturing capacity to service our near-term and future product needs.
Because we are focused on the discovery and development of drugs, we do not have any marketing or distribution capabilities, nor are we at a stage where we would have any customers for our investigational medicines. If we receive marketing approval or emergency use authorization in the U.S., Canada or Europe for a product candidate, we plan to either build the capabilities to commercialize the product candidate in the applicable region with our own focused, specialized sales force, or alternatively, outsource the sales and marketing infrastructure necessary to market and sell our products. We also plan to utilize strategic licensing, collaboration, distribution or other marketing arrangements with third parties for the further development or commercialization of our products and product candidates, where applicable, such as in areas or regions outside North America where a partner may contribute additional resources, infrastructure and expertise.
Competition
The pharmaceutical and biotechnology industry is highly competitive, and the development and commercialization of new drugs is influenced by rapid technological developments and innovation. We face competition from companies developing and commercializing products that will be competitive with our drug candidates, including large pharmaceutical and smaller biotechnology companies, many of which have greater financial and commercial resources than we do. For our EB01 and EB02 product candidates, our potential competitors include, among others, Aclaris Therapeutics, Inc., Citius Pharmaceuticals Inc., Dermavant Sciences, Inc., Fresh Tracks Therapeutics, Inc. (formerly Brickell), Incyte Corporation, Leo Pharma A/S, Pfizer Inc., Sanofi S.A., and Sun Pharmaceutical Industries Ltd. For our EB05 product candidate, there are numerous competing therapies, including prophylactic vaccines for the SARS-Cov2 virus, experimental stem cell therapies, novel therapeutics and repurposed commercial drugs. Our potential competitors include, among others: Aqualung Therapeutics Corporation, Eli Lilly and Company, Enzychem Lifesciences Corp., Merck & Co, Inc., Mesoblast Limited, Pfizer Inc., Regeneron Pharmaceuticals, Inc., Roche Holding AG and Veru Inc. For any future product for vitiligo or fibrotic diseases, potential competitors, include, among others: Bausch Health, Eli Lilly and Company, Galderma Laboratories, LP, Incyte Corporation, Boehringer Ingelheim AG, Chemomab Therapeutics Ltd., F. Hoffmann-La Roche AG, GlaxoSmithKline plc., Leo Pharma A/S, Merck & Co., Inc., Mitsubishi Tanabe Pharma Corporation, and Sanofi S.A. Some of the competing product development programs may be based on scientific approaches that are similar to our approach, and others may be based on entirely different approaches. Potential competitors also include new entrants to the market, academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization of products similar to ours or that otherwise target indications that we are pursuing. Key factors affecting the success of any approved product will be its efficacy, safety profile, drug interactions, method of administration, pricing, reimbursement and level of promotional activity relative to those of competing drugs. We believe that our product candidates will compete favorably with respect to such factors. However, we may not be able to maintain our competitive position against current and potential competitors.
Government Regulation
We plan to conduct clinical studies and seek approvals for our product candidates in the U.S., Canada, EU and other jurisdictions. Therefore, we currently are, and may in the future be, subject to a variety of national and regional regulations governing clinical trials as well as commercial sales and distribution of our products, if approved.
To conduct clinical trials for our product candidates, we rely on third parties, such as contract research organizations, medical institutions and clinical investigators. Although we have entered into agreements with these third parties, we continue to be responsible for confirming that each of our clinical trials is conducted in accordance with our investigational plan or research protocol, as well as International Conference on Harmonization Good Clinical Practices, or GCP, which include guidelines for conducting, recording and reporting the results of clinical trials.
The FDA in the U.S., Health Canada in Canada, the European Medicines Agency (EMA) in the European Union and comparable regulatory agencies in foreign countries impose substantial requirements on the clinical development, manufacture and marketing of pharmaceutical products and product candidates. These agencies and other federal, state, provincial and local entities regulate research and development activities and the testing, manufacture, packaging, importing, distribution, quality control, safety, effectiveness, labeling, storage, record-keeping, approval and promotion of our products and product candidates. All of our product candidates will require regulatory approval before commercialization. In particular, therapeutic product candidates for human use are subject to rigorous preclinical and clinical testing and other statutory and regulatory requirements of the U.S., Canada, the EU and foreign countries. Obtaining these marketing approvals and subsequently complying with ongoing statutory and regulatory requirements require substantial time, effort and financial resources.
U.S. Regulations
In the U.S., the FDA regulates drugs under the federal Food, Drug and Cosmetic Act as well as the Public Health Service (PHS) Act for biological drugs. The process required by the FDA before our product candidates may be marketed in the U.S. generally involves the following:
| · | Pre-clinical testing. Drug developers complete extensive pre-clinical laboratory tests, animal studies and formulation studies, performed in accordance with the FDA’s Good Laboratory Practice regulations and other applicable requirements. These studies typically assess efficacy, toxicology and pharmacokinetics. |
| · | Submission to the FDA of an IND, which must become effective before human clinical trials may begin. As part of an IND application to the FDA, trial sponsors submit the results of pre- clinical tests, together with manufacturing information and analytical data. The IND automatically becomes effective 30-days after receipt by the FDA, unless the FDA, within the 30-day time frame, has questions or concerns about the proposed study. In such a case, the IND sponsor and the FDA must resolve any outstanding items before the clinical trial can begin. A separate submission to an existing IND must also be made for each successive phase of a clinical trial conducted during product development. |
| | |
| · | Approval by a central or institutional review board (IRB), or ethics committee at each clinical trial site before each trial may be initiated. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completion. There are also requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. |
| | |
| · | Multiple Phases of Human Clinical Trials. Drug developers conduct adequate and well-controlled human clinical trials that establish the safety and efficacy of the product candidate for the intended use, typically in the following three stages, which are often sequential but may overlap: |
| o | Phase 1: The clinical trials are initially conducted in a limited population to test the product candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion in healthy human volunteers or, on occasion, in patients, such as cancer patients. Phase 1 clinical trials can be designed to evaluate the impact of the product candidate in combination with currently approved drugs. |
| | |
| o | Phase 2: These clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, to determine the efficacy of the product candidate for specific targeted indications and to determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials may be conducted by the sponsor to obtain information before beginning a larger and more expensive Phase 3 clinical trial. |
| | |
| o | Phase 3: These clinical trials are commonly referred to as pivotal clinical trials. If the Phase 2 clinical trials demonstrate that a dose range of the product candidate is effective and has an acceptable safety profile, Phase 3 clinical trials are then undertaken in large patient populations to further evaluate dosage, to provide substantial evidence of clinical efficacy and to further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial sites. |
| · | Product Candidate Chemistry, Controls and Manufacturing. Concurrent with clinical trials, companies typically complete additional animal and laboratory studies, develop additional information about the chemistry and physical characteristics of the drug, and finalize a process for manufacturing the product in commercial quantities in accordance with FDA’s current Good Manufacturing Practices (cGMP) requirements. The manufacturing process must consistently produce quality batches of the drug, and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate the effectiveness of the packaging and that the compound does not undergo unacceptable deterioration over its shelf life. |
| | |
| · | U.S. Review and Approval Processes |
| o | After the completion of clinical trials of an investigational drug or biologic product, an NDA or BLA is prepared and submitted to the FDA. FDA approval must be obtained before commercial marketing and distribution of the product may begin in the U.S. The NDA or BLA must include results of product development, laboratory, and animal studies, human trials, information on the manufacture and composition of the product, proposed labeling and other relevant information. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will file the NDA or BLA and, even if filed, that any approval will be granted on a timely basis, if at all. |
| o | Under the Prescription Drug User Fee Act, as amended (PDUFA), each NDA or BLA must be accompanied by a significant user fee. The FDA adjusts the PDUFA user fees on an annual basis. PDUFA also imposes annual program fees on prescription drugs, including biologics. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. No user fees are assessed on NDAs or BLAs for products designated as orphan drugs, unless the application also includes a non-orphan indication. |
| | |
| o | Within 60 days following submission, the FDA reviews the NDA or BLA to determine if it is substantially complete before the agency files it. The FDA may request additional information or may refuse to file any NDA or BLA that it deems incomplete or not properly reviewable at the time of submission. In this event, the NDA or BLA must be resubmitted with the additional information. The resubmitted application also is subject to an initial filing review before the FDA files it. Once the submission is filed, the FDA begins an in-depth substantive review of the NDA or BLA. Under PDUFA, FDA has agreed to performance goals to review 90% of original standard NDAs or BLAs within 10 months of the 60-day filing date and 90% of original priority NDAs or BLAs within 6 months of the 60-day filing date, whereupon a review decision is to be made. The FDA does not always meet its PDUFA goal dates for standard and priority NDAs and BLAs. The review process and the PDUFA goal date may be extended by three months if the FDA requests or the NDA/BLA sponsor otherwise provides additional information or clarification regarding information already provided in the NDA or BLA submission. The FDA reviews the NDA or BLA to determine, among other things, whether the proposed product is safe and potent, or effective, for its intended use, and has an acceptable purity profile, and whether the product is being manufactured in accordance with GMP to assure and preserve the product’s identity, safety, strength, quality, potency and purity. |
| | |
| o | The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations when making decisions. During the product approval process, the FDA also will determine whether a Risk Evaluation and Mitigation Strategy (REMS) is necessary to assure that the benefits of the biologic outweigh the potential risks of the product to patients. A REMS can include medication guides, communication plans for healthcare professionals, and elements to assure a product’s safe use (ETASU). An ETASU can include, but is not limited to, special training or certification for prescribing or dispensing the product, dispensing the product only under certain circumstances, special monitoring, and the use of patient-specific registries. If the FDA concludes that a REMS is needed, the sponsor of the BLA must submit a proposed REMS; the FDA will not approve the BLA without a REMS, if required. |
| | |
| o | Before approving an NDA or BLA, the FDA will typically inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in substantial compliance with GMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites, to assure that the clinical trials were conducted in compliance with GCP requirements. To assure GMP, GLP and GCP compliance, an applicant must incur significant expenditure of time, money, and effort in the areas of training, record keeping, production, and quality control. |
| | |
| o | Notwithstanding the submission of relevant data and information, the FDA may ultimately decide that the NDA or BLA does not satisfy its regulatory criteria for approval and deny approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently from how we interpret the same data. If the agency decides not to approve the NDA or BLA in its present form, the FDA will issue a complete response letter that usually describes all of the specific deficiencies identified by the FDA. The deficiencies identified may be minor, for example, requiring labeling changes, or major, for example, requiring additional clinical trials. Additionally, the complete response letter may include recommended actions that the applicant might take to place the application in a condition for approval. If a complete response letter is issued, the applicant may resubmit, addressing all of the deficiencies identified in the letter, withdraw the application, or request a hearing. |
| o | If a product candidate receives regulatory approval, the FDA will issue an approval letter. The approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings, or precautions be included in the product labeling. The FDA may impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval. In addition, the FDA may require post marketing clinical trials, sometimes referred to as Phase 4 clinical trials, designed to assess further a biological product’s safety and effectiveness, and testing and surveillance programs to monitor the safety of approved products that have been commercialized. |
Satisfaction of FDA regulations and requirements or similar requirements of state, local and foreign regulatory agencies typically take several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product or disease. Typically, if a product candidate is intended to treat a chronic disease, as is the case with some of our product candidates, safety and efficacy data must be gathered over an extended period.
Orphan Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 individuals in the U.S., or a patient population greater than 200,000 individuals in the U.S. and when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the U.S. will be recovered from sales in the U.S. for that drug or biologic. Orphan drug designation must be requested before submitting a BLA or NDA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product marketing exclusivity, which means that the FDA may not approve any other applications, including a full BLA, to market the same biologic for the same use or indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA or NDA application user fee.
A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the U.S. may be lost if the FDA later determines that the request for designation was materially defective or, as noted above, if the second applicant demonstrates that its product is clinically superior to the approved product with orphan exclusivity or the manufacturer of the approved product is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Expedited Development and Review Programs
The FDA has several programs intended to facilitate and expedite development and review of new drugs to address unmet medical needs in the treatment of serious or life-threatening diseases or conditions. These programs include Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval.
A new drug is eligible for Fast Track designation if it is intended to treat a serious or life-threatening disease or condition and demonstrates the potential to address unmet medical needs for such disease or condition. Fast Track designation provides increased opportunities for sponsor interactions with the FDA during preclinical and clinical development, in addition to the potential for rolling review once a marketing application is filed. Rolling review means that the agency may review portions of the marketing application before the sponsor submits the complete application.
In addition, a new drug may be eligible for Breakthrough Therapy designation if it is intended to treat a serious or life- threatening disease or condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough Therapy designation provides all the features of Fast Track designation in addition to intensive guidance on an efficient drug development program beginning as early as Phase 1, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced review staff in a cross-disciplinary review, where appropriate.
Any product submitted to the FDA for approval, including a product with Fast Track or Breakthrough Therapy designation, may also be eligible for additional FDA programs intended to expedite the review and approval process, including Priority Review designation and Accelerated Approval. A product is eligible for Priority Review, once an NDA or BLA is submitted, if the drug that is the subject of the marketing application has the potential to provide a significant improvement in safety or effectiveness in the treatment, diagnosis or prevention of a serious disease or condition. Under priority review, the FDA’s goal date to take action on the marketing application is six months compared to ten months for a standard review. Products are eligible for Accelerated Approval if they can be shown to have an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or an effect on a clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality.
Accelerated Approval is usually contingent on a sponsor’s agreement to conduct additional post-approval studies to verify and describe the product’s clinical benefit. The FDA may withdraw approval of a drug or an indication approved under Accelerated Approval if, for example, the confirmatory trial fails to verify the predicted clinical benefit of the product. In addition, the FDA generally requires, as a condition for Accelerated Approval, that all advertising and promotional materials intended for dissemination or publication within 120 days of marketing approval be submitted to the agency for review during the pre-approval review period. After the 120-day period has passed, all advertising and promotional materials must be submitted at least 30 days prior to the intended time of initial dissemination or publication.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or the time period for FDA review or approval may not be shortened. Furthermore, Fast Track designation, Breakthrough Therapy designation, Priority Review and Accelerated Approval do not change the scientific or medical standards for approval or the quality of evidence necessary to support approval, though they may expedite the development or review process.
Emergency Use Authorizations
While, in most cases, a therapeutic must be approved by FDA before the product may be sold, when there is a public health emergency involving chemical, biological, radiological, or nuclear agents, including infectious diseases like Covid-19, new therapeutics may be distributed pursuant to an Emergency Use Authorization, or EUA. Under an EUA, FDA may authorize the emergency use of an unapproved medical product or an unapproved use of an approved product for certain emergency circumstances to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, and after the Secretary of the Department of Health and Human Services has issued a declaration of emergency or threat justifying emergency use.
To receive an EUA, the product sponsor must demonstrate that the product “may be effective” in the prevention, diagnosis, or treatment of an applicable disease or condition. Additionally, FDA must determine that the product’s known and potential benefits outweigh the known and potential risks. Further there must be no adequate, approved, and available alternative product for the indication. Potential alternative products may be unavailable if there are insufficient supplies to meet the emergency need. FDA may establish additional conditions on an EUA that are necessary to protect public health, including conditions related to information that must be disseminated to health care providers and patients, the monitoring and reporting of adverse events, and record keeping. Conditions may also relate to how a product is distributed and administered and how a product is advertised. Importantly, EUAs are not full marketing approvals. Rather, EUAs are only effective for the duration of the applicable EUA declaration. Full approval of the product under applicable standards would be necessary to continue to distribute the product absent an EUA. EUAs may also be revised or revoked by FDA at any time.
U.S. Patent Term Restoration
Depending upon the timing, duration, and specifics of the FDA approval of the use of our current and potential product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Amendments). The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
Biosimilars and Exclusivity
The Biologics Price Competition and Innovation Act of 2009 (BPCIA) created an abbreviated approval pathway for biological products shown to be highly similar to, or interchangeable with, an FDA-licensed reference biological product. The FDA has issued several guidance documents outlining an approach to review and approval of biosimilars.
Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
The BPCIA includes, among other provisions:
| ● | A 12-year exclusivity period from the date of first licensure, or BLA approval, of the reference product, during which approval of a 351(k) application referencing that product may not be made effective; |
| ● | A 4-year exclusivity period from the date of first licensure of the reference product, during which a 351(k) application referencing that product may not be submitted; and |
| ● | An exclusivity period for certain biological products that have been approved through the 351(k) pathway as interchangeable biosimilars. |
The BPCIA also establishes procedures for identifying and resolving patent disputes involving applications submitted under section 351(k) of the PHSA.
The BPCIA is complex and its interpretation and implementation by the FDA remains unpredictable. In addition, government proposals have sought to reduce the 12-year reference product exclusivity period. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. As a result, the ultimate effect, implementation, and meaning of the BPCIA is subject to uncertainty.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA-regulated products, including biological products, are required to register and disclose certain clinical trial information on the website www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, trial sites and investigators, and other aspects of a clinical trial are then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of clinical trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of clinical development programs as well as clinical trial design.
Pediatric Information
Under the Pediatric Research Equity Act (PREA), BLAs or supplements to BLAs must contain data to assess the safety and effectiveness of the biological product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the biological product is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any biological product with orphan product designation except a product with a new active ingredient that is a molecularly targeted cancer product intended for the treatment of an adult cancer and directed at a molecular target determined by FDA to be substantially relevant to the growth or progression of a pediatric cancer that is subject to a BLA submitted on or after August 18, 2020.
The Best Pharmaceuticals for Children Act (BPCA) provides a six-month extension of any non-patent exclusivity for a biologic if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new drug or biologic in the pediatric population may produce health benefits in that population, the FDA making a written request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
FDA Post-Approval Requirements
Once an NDA or BLA is approved, maintaining post-approval compliance with applicable federal, state, and local statutes and regulations requires the expenditure of substantial time and financial resources. Manufacturers and other entities involved in the manufacture and distribution of approved products are required to register the establishments where the approved products are made with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with GMP and other laws. Rigorous and extensive FDA regulation of products continues after approval, particularly with respect to GMP. We rely, and expect to continue to rely, on third parties for the production and distribution of clinical and commercial quantities of any products that we may commercialize. Manufacturers of our products are required to comply with applicable requirements in the GMP regulations, including quality control and quality assurance and maintenance of records and documentation. Other post-approval requirements include reporting of GMP deviations that may affect the identity, potency, purity and overall safety of a distributed product, record-keeping requirements, reporting of adverse effects, reporting updated safety and efficacy information, and complying with electronic record and signature requirements. After a BLA is approved, the product also may be subject to official lot release. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA also may perform certain confirmatory tests on lots of some products before releasing the lots for distribution by the manufacturer. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain GMP compliance. Discovery of problems with a product after approval may result in restrictions on a product, manufacturer, or holder of an approved NDA or BLA, including withdrawal of the product from the market. In addition, changes to the manufacturing process or facility generally require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval.
We also must comply with the FDA’s advertising and promotion requirements, such as those related to direct-to-consumer advertising, the prohibition on promoting products for uses or in patient populations that are not described in the product’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet. Discovery of previously unknown problems or the failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market as well as possible civil or criminal sanctions.
Failure to comply with the applicable U.S. requirements after approval may subject an applicant or manufacturer to administrative or judicial civil or criminal sanctions and adverse publicity. FDA sanctions could include refusal to approve pending applications, withdrawal of an approval, clinical hold, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, mandated corrective advertising or communications with doctors, debarment, restitution, disgorgement of profits, or civil or criminal penalties.
Other Regulatory Requirements
The federal anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. This statute has been broadly interpreted to apply to manufacturer arrangements with prescribers, purchasers and pharmacy benefit managers, among others. Several other countries, including the United Kingdom, have enacted similar anti-kickback laws and regulations.
The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program or for knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
The federal Physician Payments Sunshine Act requirements under the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010, referred to together as the Affordable Care Act, require manufacturers of FDA-approved drugs, devices, biologics and medical supplies covered by Medicare or Medicaid to report to the Department of Health and Human Services information related to payments and other transfers of value made to or at the request of covered recipients, such as physicians and teaching hospitals, and physician ownership and investment interests in such manufacturers. Among other payments, the law requires payments made to physicians and teaching hospitals for clinical trials be disclosed.
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to future potential sales or marketing arrangements and claims involving healthcare items or services reimbursed by nongovernmental third-party payors, including private insurers. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines, or the relevant compliance guidance promulgated by the federal government, in addition to requiring drug manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures to the extent that those laws impose requirements that are more stringent than the Physician Payments Sunshine Act. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Canada Regulations
Health Canada is the Canadian federal authority that regulates, evaluates and monitors the safety, effectiveness, and quality of drugs, medical devices, and other therapeutic products available to Canadians. Health Canada’s regulatory process for review, approval and regulatory oversight of products is similar to the regulatory process conducted by the FDA. To initiate clinical testing of a drug candidate in human subjects in Canada, a Clinical Trial Application (CTA) must be filed with and approved by Health Canada. In addition, all federally regulated trials must be approved and monitored by research ethics boards. The review boards study and approve study-related documents and monitor trial data.
Prior to being given market authorization for a drug product, a manufacturer must present substantive scientific evidence of a product’s safety, efficacy and quality as required by the Food and Drugs Act (Canada) and its associated regulations, including the Food and Drug Regulations. This information is usually submitted in the form of a New Drug Submission (NDS). Health Canada reviews the submitted information, sometimes using external consultants and advisory committees, to evaluate the potential benefits and risks of a drug. If after the review, the conclusion is that the patient benefits outweigh the risks associated with the drug, the drug is issued a Drug Identification Number (DIN), followed by a Notice of Compliance (NOC), which permits the market authorization holder (i.e., the NOC and DIN holder) to market the drug in Canada. Drugs granted an NOC may be subject to additional postmarket surveillance and reporting requirements.
All establishments engaged in the fabrication, packaging/labeling, importation, distribution, and wholesale of drugs and operation of a testing laboratory relating to drugs are required to hold a Drug Establishment License to conduct one or more of the licensed activities unless expressly exempted under the Food and Drug Regulations. The basis for the issuance of a Drug Establishment License is to ensure the facility complies with cGMP as stipulated in the Food and Drug Regulations and as determined by cGMP inspection conducted by Health Canada. An importer of pharmaceutical products manufactured at foreign sites must also be able to demonstrate that the foreign sites comply with cGMP, and such foreign sites are included on the importer’s Drug Establishment License.
Regulatory obligations and oversight continue following the initial market approval of a pharmaceutical product. For example, every market authorization holder must report any new information received concerning adverse drug reactions, including timely reporting of serious adverse drug reactions that occur in Canada and any serious unexpected adverse drug reactions that occur outside of Canada. The market authorization holder must also notify Health Canada of any new safety and efficacy issues that it becomes aware of after the launch of a product.
Employees
As of the date of this filing, we have 16 full-time employees: ten employees are primarily engaged in research and development, and six employees are engaged in management, administration, business development and finance. All employees are located in Canada or the U.S. None of our employees are members of any labor unions.
We take pride in the diversity of our workforce and being an equal opportunity employer. As a growth-oriented company focused on innovation, we strive to foster diversity and inclusion. As of the date of this filing, women represented more than 50% of all employees, and individuals from underrepresented racial or ethnic groups, or who are foreign born, represented more than 50% of our employees.
Corporate Information
We are a British Columbia, Canada corporation founded in 2007 and operate through our wholly owned subsidiaries, Edesa Biotech Research, Inc., an Ontario, Canada corporation and Edesa Biotech USA, Inc., a California, USA corporation. In June 2019, we acquired the Ontario corporation through a reverse acquisition and changed our name to Edesa Biotech, Inc.
Our executive offices are located at 100 Spy Court, Markham, Ontario, L3R 5H6, Canada. Our phone number is 289-800- 9600. Our registered and records office is 2900 - 550 Burrard Street, Vancouver, British Columbia, V6C 0A3, Canada. Our website address is www.edesabiotech.com. The contents of our website or social media postings are not part of our Securities and Exchange Commission (SEC) reports for any purpose or otherwise incorporated by reference. Any references to website addresses contained in this report are intended to be inactive textual references only.
Available Information
We file or furnish periodic reports and amendments thereto, including our annual reports on Form 10-K, our quarterly reports on Form 10-Q and current reports on Form 8-K, proxy statements and other information with the SEC. Such reports and other information filed or furnished by us with the SEC are available free of charge on our website at www.edesabiotech.com/investors/sec-filings as soon as reasonably practicable after such reports are available on the SEC’s website at www.sec.gov. Our filings are also available at the Canadian Securities Administrators’ SEDAR website at www.sedar.com. Investors and other interested parties should note that we may also use our website and our social media channels to publish information about us that may be deemed material to investors. We encourage investors and other interested parties to review the information we may publish through our website and social media channels.
Smaller Reporting Company
We are currently a “smaller reporting company” as defined by Rule 12b-2 of the Exchange Act, and are thus allowed to provide simplified executive compensation disclosures in our filings, are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that an independent registered public accounting firm provide an attestation report on the effectiveness of internal control over financial reporting and have certain other reduced disclosure obligations with respect to our SEC filings.
Item 1A. RISK FACTORS.
Certain factors may have a material adverse effect on our business, prospects, financial condition and results of operations. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this Annual Report on Form 10-K, including our financial statements and the related notes, before deciding to invest in our securities. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business and cause the market price of our securities to decline. In addition, many of the following risk factors could be exacerbated by any worsening of the global business and economic environment or the resurgence of Covid-19 or other public health threats. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects could be materially and adversely affected. In addition, we cannot assure investors that our assumptions and expectations will prove to be correct. Important factors could cause our actual results to differ materially from those indicated or implied by forward-looking statements. See “Forward-Looking Statements And Other Matters” for a discussion of some of the forward-looking statements that are qualified by these risk factors.
Summary of Risks
The following summarizes key risks and uncertainties that could materially adversely affect us. You should read this summary together with the more detailed description of each risk factor contained below.
● | We are a late-stage biopharmaceutical company with no products approved for commercial sale, and we have incurred significant losses since our inception and expect to continue to incur losses and may never generate profits from operations or maintain profitability. |
● | We will need substantial additional funding to finance our operations through regulatory approval of one or more of our product candidates. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs, commercialization efforts or other operations. |
● | We depend heavily on the success of our drug product candidates. If we are unable to obtain regulatory approval or commercialize one or more of these experimental treatments, or experience significant delays in doing so, our business will be materially harmed. We cannot give any assurance that we will receive regulatory approval for such product candidates or any other product candidates, which is necessary before they can be commercialized. |
● | We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively. |
● | A successful sPLA2, anti-TLR4 or anti-CXCL10 drug has not been developed to date and we can provide no assurances that we will be successful or that there will be no adverse side effects. |
● | Even if one of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success. |
● | If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and any of our other current or future product candidates, we may not be successful in commercializing the applicable product candidate if it receives marketing approval. |
● | Even if we are able to commercialize one of our product candidates, the product may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business. |
● | We face substantial competition, which may result in others discovering, developing or commercializing products to treat our target indications or markets before or more successfully than we do. |
● | We will be dependent on third parties for manufacturing, including optimization, technology transfers and scaling up of clinical scale quantities of all of our product candidates. |
● | The manufacturing of our monoclonal antibody candidates is complex and subject to a multitude of risks. These manufacturing risks could substantially increase our costs and limit supply of these drug candidates for clinical development, and commercialization. |
● | We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such clinical trials. |
● | Even if we complete the necessary clinical trials, the marketing approval process is expensive, time consuming and uncertain. If we are not able to obtain, or if there are delays in obtaining, required marketing approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired. |
● | Even if we obtain marketing approval for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products, and compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue. |
● | If we are unable to obtain and maintain patent protection for our licensed technology and products, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our licensed technology and products may be adversely affected. |
● | The ownership of our common shares is highly concentrated, which may prevent you and other shareholders from influencing significant corporate decisions and may result in conflicts of interest that could cause our common shares price to decline. |
Risks Related to Our Business, Financial Position and Capital Requirements
We are a late-stage biopharmaceutical company with no products approved for commercial sale, and we have incurred significant losses since our inception and expect to continue to incur losses and may never generate profits from operations or maintain profitability.
Since inception, we have incurred significant operating losses. At September 30, 2023, we had an accumulated deficit of $52.4 million. We have historically financed operations primarily through issuances of common shares, the exercise of common share purchase warrants, convertible preferred shares, convertible loans, government grants and tax incentives. We have devoted substantially all of our efforts to research and development, including clinical trials, and have not completed the development of any of our drug candidates.
We expect to continue to incur significant expenses and operating losses without corresponding revenue for the foreseeable future as we continue the development of, and seek marketing approvals for our product candidates, prepare for and begin the commercialization of any approved products, and add infrastructure and personnel to support our product development efforts and operations as a public company in the U.S. and Canada. The net losses we incur may fluctuate significantly from quarter to quarter and year to year.
Based on our current plans, we do not expect to generate significant revenue unless and until we or a current or potential future licensee obtains marketing approval for, and commercializes, one or more of our product candidates, which may require several years. Neither we nor a licensee may ever succeed in obtaining marketing approval for, or commercializing our product candidates and, even if marketing approval is obtained, we may never generate revenues that are significant enough to generate profits from operations. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable would impair our ability to sustain operations and adversely affect the price of our securities and our ability to raise capital.
We will need substantial additional funding to finance our operations through regulatory approval of one or more of our product candidates. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs, commercialization efforts or other operations.
We expect our research and development expenses to increase substantially in the future, particularly for any drug candidates beyond Phase 2 clinical development or if we expand the number of drug candidates in clinical studies. In addition, if we obtain marketing approval for any of our product candidates that are not then subject to licensing, collaboration or similar arrangements with third parties, we expect to incur significant commercialization expenses related to product sales, marketing, distribution and manufacturing.
We cannot be certain that additional funding will be available on acceptable terms, or at all. In addition, future debt financing into which we may enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, redeem our shares, make certain investments and engage in certain merger, consolidation or asset sale transactions.
If we are unable to raise additional capital when required or on acceptable terms, we may be required to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates, restrict our operations or obtain funds by entering into agreements on unattractive terms, which would likely have a material adverse effect on our business, share price and our relationships with third parties with whom we have business relationships, at least until additional funding is obtained. If we do not have sufficient funds to continue operations, we could be required to seek bankruptcy protection or other alternatives that would likely result in our securityholders losing some or all of their investment in us. In addition, our ability to achieve profitability or to respond to competitive pressures would be significantly limited.
Raising additional capital may cause dilution to our investors, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, licensing, collaboration or similar arrangements, grants and debt financings. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our common shares. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our existing shareholders. If we raise additional funds through licensing, collaboration or similar arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research and development programs or product candidates or to grant licenses on terms that may not be favorable to us. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue the development of our product candidates.
To continue to grow our business over the longer term, we plan to commit substantial resources to research and development, clinical trials of our product candidates, and other operations and potential product acquisitions and in-licensing. We have evaluated and expect to continue to evaluate a wide array of strategic transactions as part of our plan to acquire or in license and develop additional products and product candidates to augment our internal development pipeline. Strategic transaction opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur additional indebtedness, seek equity capital or both. In addition, we may pursue development, acquisition or in-licensing of approved or development products in new or existing therapeutic areas or continue the expansion of our existing operations. Accordingly, we expect to continue to opportunistically seek access to additional capital to license or acquire additional products, product candidates or companies to expand our operations, or for general corporate purposes. Strategic transactions may require us to raise additional capital through one or more public or private debt or equity financings or could be structured as a collaboration or partnering arrangement. We have no arrangements, agreements, or understandings in place at the present time to enter into any acquisition, in-licensing or similar strategic business transaction.
We partially rely on government grants to contribute to our EB05 (paridiprubart) development program. If we are unable to satisfy our contractual obligations and manage our covenants or meet expected under both SIF agreements, the development of EB05 may be extended, delayed, modified, or terminated and we may be required to repay all or part of the grant earlier than expected.
In February 2021, we and Edesa Biotech Research signed the 2021 SIF Agreement whereby the Government of Canada agreed to contribute C$14.1 million in nonrepayable funding for an international Phase 2 study and certain pre-clinical experiments. In the event that we or Edesa Biotech Research breach our obligations under the 2021 SIF Agreement, subject to applicable cure, the SIF may exercise a number of remedies, including demanding repayment of funding previously received and/or terminating the agreement. The performance obligations of Edesa Biotech Research under the contribution agreement are guaranteed by us. All potential funding available under the 2021 SIF Agreement has been received. As of the date of this filing, we have met all of our performance and reporting requirements under the 2021 SIF Agreement.
On October 12, 2023, we and Edesa Biotech Research signed the 2023 SIF Agreement whereby the Government of Canada agreed to contribute up to C$23 million from the SIF in partially repayable funding toward of the development and commercialization of our investigational therapy EB05. Under the 2023 SIF Agreement, we agreed to complete the project, to be conducted exclusively in Canada except as permitted otherwise under certain circumstances, on or before December 31, 2025. We also have agreed to certain financial and non-financial covenants and other obligations in relation to EB05, including the achievement of certain headcount requirements in Canada, the maintenance of a collaboration with a Canadian research institute or post-secondary institutions, and the maintenance of certain research and development expenditures in Canada. In an event of default, such as our breach of our covenants and obligations under either the 2023 SIF Agreement or the 2021 SIF Agreement, the Government of Canada may suspend or terminate its contribution to the project, or require repayment. As a result, if we default on our obligations under the SIF agreements, we may not have sufficient funds available to continue the Phase 3 clinical study of our investigational therapy EB05, and we cannot be certain that we will be able to obtain additional capital to fund the program. We are currently not in default of our obligations per the terms of either SIF agreement.
Our existing and any future indebtedness could adversely affect our ability to operate our business.
In October 2023, we entered into a credit agreement with Pardeep Nijhawan Medicine Professional Corporation, an entity controlled by Dr. Nijhawan, our Chief Executive Officer, Secretary and member of our board of directors, providing for an unsecured revolving credit facility in the principal amount of up to $10 million. Such credit facility combined with our other financial obligations and contractual commitments, including any future indebtedness, could have adverse consequences, including:
| · | requiring us to dedicate a portion of our cash resources to the payment of interest and principal, thereby reducing money available to fund working capital, capital expenditures, product development and other general corporate purposes; |
| · | increasing our vulnerability to adverse changes in general economic, industry and market conditions; |
| · | subjecting us to restrictive covenants that may reduce our ability to take certain corporate actions or obtain further debt or equity financing; and |
| · | limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we compete. |
We depend heavily on the success of our drug product candidates. If we are unable to obtain regulatory approval or commercialize one or more of these experimental treatments, or experience significant delays in doing so, our business will be materially harmed. We cannot give any assurance that we will receive regulatory approval for such product candidates or any other product candidates, which is necessary before they can be commercialized.
We have not completed development of and/or obtained regulatory approval for any of our product candidates. Development will require the commitment of substantial financial resources, extensive product candidate development, and clinical trials. This process takes years of effort without any assurance of ultimate success.
Our ability to generate product revenues, which may not occur for multiple years, if at all, will depend heavily on the successful development and commercialization of our drug product candidates. The success of our product candidates will depend on a number of factors, including, but not limited to:
| · | our ability to obtain additional capital from potential future licensing, collaboration or similar arrangements or from any future offering of our debt or equity securities; |
| · | our ability to identify and enter into potential future licenses or other collaboration arrangements with third parties and the terms of the arrangements; |
| · | our timing to obtain applicable regulatory approvals; |
| · | successful completion of clinical development; |
| · | the ability to provide acceptable evidence demonstrating a product candidates’ safety and efficacy; |
| · | receipt of marketing approvals from applicable regulatory authorities and similar foreign regulatory authorities; |
| · | the availability of raw materials to produce our product candidates; |
| · | obtaining and maintaining commercial manufacturing arrangements with third-party manufacturers or establishing commercial-scale manufacturing capabilities; |
| · | obtaining and maintaining patent and trade secret protection and regulatory exclusivity; |
| · | establishing sales, marketing and distribution capabilities; |
| · | generating commercial sales of the product candidate, if and when approved, whether alone or in collaboration with others; |
| · | acceptance of the product candidate, if and when approved, by patients, the medical community and third-party payors; |
| · | effectively competing with other therapies; and |
| · | maintaining an acceptable safety profile of the product candidate following approval. |
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize any of our product candidates, which would materially harm our business. Many of these factors are beyond our control. Accordingly, we may never be able to generate revenues through the license or sale of any of our product candidates.
Our product development efforts with respect to our product candidates may fail for many reasons, including but not limited to:
| · | the failure of the product candidate in clinical studies; |
| · | adverse patient reactions to the product candidate or indications of other safety concerns; |
| · | insufficient clinical trial data to support the effectiveness or superiority of the product candidate; |
| · | the inability to manufacture sufficient quantities of the product candidate for development or commercialization activities in a timely and cost-efficient manner; and |
| · | changes in the regulatory environment, including pricing and reimbursement, that make development of a new product or of an existing product for a new indication no longer attractive. |
Deterioration in general economic conditions in the U.S., Canada and globally, including the effect of prolonged periods of inflation on our suppliers, third-party service providers and potential partners, could harm our business and results of operations.
Our business and results of operations could be adversely affected by changes in national or global economic conditions. These conditions include but are not limited to inflation, rising interest rates, availability of capital markets, energy availability and costs, the negative impacts caused by pandemics and public health crises, negative impacts resulting from the military conflict between Russia and the Ukraine, and the effects of governmental initiatives to manage economic conditions. Impacts of such conditions could be passed on to our business in the form of higher costs for labor and materials, higher investigator fees, possible reductions in pharmaceutical industry-wide spending on research and development and acquisitions and higher costs of capital.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our primarily operating entity, Edesa Biotech Research, Inc., was formed in July 2015. To date, our operations have been limited to organization and staffing, developing and securing our technology, entering into licensing arrangements, raising capital and undertaking preclinical studies and clinical trials of our product candidates. We have not yet demonstrated our ability to successfully complete development of any product candidate, obtain marketing approval, manufacture a commercial-scale product, or arrange for a third-party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Assuming we obtain marketing approval for any of our product candidates, we will need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We may encounter unforeseen expenses, difficulties, complications and delays and may not be successful in such a transition. Any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history.
We are exposed to risks related to currency exchange rates.
We conduct a significant portion of our operations outside of the U.S. Because our financial statements are presented in U.S. dollars, changes in currency exchange rates have had and could have in the future a significant effect on our operating results when our operating results are translated into U.S. dollars.
We are subject to anti-corruption laws, as well as export control laws, customs laws, sanctions laws, privacy laws and other laws governing our operations. If we fail to comply with these laws, it could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our operations are subject to anti-corruption laws, including the U.S. Foreign Corrupt Practices Act (FCPA), and other anti-corruption laws that apply in countries where we do business and may do business in the future. We are also subject to other laws and regulations governing our international operations, including regulations administered by the government of the U.S. and authorities in the EU, including applicable export control regulations, economic sanctions on countries and persons, customs requirements and currency exchange regulations. There is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws. If we are not in compliance, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Similarly, compliance with global privacy and data security requirements could result in additional costs and liabilities to us or inhibit our ability to collect and process data globally, and our failure to comply with data protection laws and regulations could lead to government enforcement actions, which would cause our business and reputation to suffer.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations. Our operations may involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations produce hazardous waste products. We expect to contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants and collaborators, including intentional failures to comply with FDA or Office of Inspector General regulations or similar regulations of comparable non-U.S. regulatory authorities, provide accurate information to the FDA or comparable non-U.S. regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable non-U.S. regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. Misconduct by these parties could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. Such actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a material and adverse impact on our business, financial condition, results of operations and prospects including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, imprisonment, loss of eligibility to obtain marketing approvals from the FDA, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional reporting requirements if subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with any of these laws, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our operating results.
We expect to expand our capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs, finance and administration and, potentially, sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. We may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. If we are not able to effectively manage our growth and expand our organization, we might be unable to implement successfully the tasks necessary to execute effectively on our planned research, development and commercialization activities and, accordingly, might not achieve our research, development and commercialization goals.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Dr. Pardeep Nijhawan, our CEO and Secretary; and Michael Brooks, our President; as well as other principal members of our management and scientific teams. Although we have employment agreements with each of our executives, these agreements do not prevent our executives from terminating their employment at any time. The unplanned loss of the services of any of these persons could materially impact the achievement of our research, development, financial and commercialization objectives. If we lose the services of any of these individuals, we might not be able to find suitable replacements on a timely basis or at all, and our business could be harmed as a result.
Recruiting and retaining qualified personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous biotechnology and pharmaceutical companies for similar personnel. We could have difficulty attracting experienced personnel to our Company and may be required to expend significant financial resources in our employee recruitment and retention efforts. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will harm our ability to implement our business strategy and achieve our business objectives.
In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. These advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our computer systems and those of third parties with which we contract are vulnerable to damage, including damage from cyberattacks, ransomware attacks, computer viruses, unauthorized access, human error and technological errors, natural disasters and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations, and could result in a material disruption of our clinical and commercialization activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our product research, development and commercialization efforts could be delayed.
Risks Related to Clinical Development, Regulatory Approval and Commercialization
If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of the FDA, Health Canada (HC) or the European Medicines Agency (EMA), or do not otherwise produce favorable results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization our product candidates.
In connection with obtaining marketing approval from regulatory authorities for the sale of any drug candidate, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical trials are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials. In particular, the small number of subjects and patients in early clinical trials of our product candidates may make the results of these clinical trials less predictive of the outcome of later clinical trials. The design of a clinical trial can determine whether our results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. There is no assurance that we will be able to design and execute a clinical trial to support marketing approval. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products. Pre-clinical studies or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional pre-clinical studies or clinical trials, or to discontinue clinical trials altogether. Ultimately, we may be unable to complete the development and commercialization of any of our product candidates.
Interim results, top-line, initial data may not accurately reflect the complete results of a particular study or trial.
We may publicly disclose interim, top-line or initial data from time to time that is based on a preliminary analysis of then- available efficacy and safety data, and the results and related findings and conclusions are subject to change following a more comprehensive review of the data related to the particular study or trial. We also make assumptions, estimates, calculations and conclusions as part of our analyses of data, and we may not have received or had the opportunity to fully evaluate all data. Interim, top-line and initial data should be viewed with caution until the final data are available. In addition, the information we may publicly disclose regarding a particular preclinical or clinical study is based on what is typically extensive information, and you or others may not agree with what we determine is the material or otherwise appropriate information to include in our disclosure, and any information we determine not to disclose may ultimately be deemed significant regarding a particular drug, drug candidate or our business. If the interim, top-line or initial data that we report differ from actual results, or if others, including regulatory authorities, disagree with the conclusions reached, our ability to obtain approval for, and commercialize, our product candidates may be harmed or delayed, which could harm our business, financial condition, operating results or prospects.
Any product candidate we advance into and through clinical trials may cause unacceptable adverse events or have other properties that may delay or prevent their regulatory approval or commercialization or limit their commercial potential.
Unacceptable adverse events caused by our product candidates in clinical trials could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications and markets. This, in turn, could prevent us from commercializing the affected product candidate and generating revenues from its sale. We have not yet completed testing of any of our product candidates for the treatment of the indications for which we intend to seek product approval in humans, and we currently do not know the extent of adverse events, if any, that will be observed in patients who receive any of our product candidates. If any of our product candidates cause unacceptable adverse events in clinical trials, we may not be able to obtain regulatory approval or commercialize such product or, if such product candidate is approved for marketing, future adverse events could cause us to withdraw such product from the market.
If clinical trials for our product candidates are prolonged or delayed, we may incur additional costs, and may not be able to commercialize our product candidates on a timely basis or at all.
We cannot predict whether we will encounter problems with any of our ongoing or planned clinical trials that will cause us or any regulatory authority to delay or suspend those clinical trials. A number of events, including any of the following, could delay the completion of our ongoing and planned clinical trials and negatively impact our ability to obtain regulatory approval for, and to market and sell, a particular product candidate:
| · | conditions imposed by the FDA or any foreign regulatory authority regarding the scope or design of our clinical trials; |
| · | delays in obtaining, or the inability to obtain, required approvals from institutional review boards, or IRBs, or other reviewing entities at clinical sites selected for participation in our clinical trials; |
| · | insufficient supply or deficient quality of product candidates supply or materials to produce our product candidates or other materials necessary to conduct our clinical trials; |
| · | delays in obtaining regulatory agreement for the conduct of the clinical trials; |
| · | lower than anticipated enrollment and retention rate of subjects in clinical trials; |
| · | serious and unexpected drug-related side effects experienced by patients in clinical trials; |
| · | failure of third-party contractors to meet their contractual obligations in a timely manner; |
| · | pre-clinical or clinical trials may produce negative or inconclusive results, which may require us or any potential future collaborators to conduct additional pre-clinical or clinical testing or to abandon projects that we expect to be promising; |
| · | even if pre-clinical or clinical trial results are positive, the FDA or foreign regulatory authorities could nonetheless require unanticipated additional clinical trials; |
| · | regulators or institutional review boards may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; |
| · | delays in establishing the appropriate dosage levels; |
| · | product candidates may not have the desired effects; and |
| · | the lack of adequate funding to continue clinical trials. |
Additionally, changes in standard of care or regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Such amendments may require us to resubmit our clinical trial protocols to IRBs for re-examination, which may impact the cost, timing or successful completion of a clinical trial. Such changes may also require us to reassess the viability of the program in question.
We do not know whether our clinical trials will begin or continue as planned, will need to be restructured or will be completed on schedule, if at all. Delays in clinical trials will result in increased development costs for our product candidates. In addition, if we experience delays in completion of, or if we terminate, any of our clinical trials, the commercial prospects for our product candidates may be affected and our ability to generate product revenues will be delayed. Furthermore, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate.
The clinical trial designs, endpoints and outcomes that will be required to obtain marketing approval for our drug candidates are uncertain. We may never receive marketing approval for our drug candidates.
To our knowledge, there are currently no FDA-approved drug treatment options specifically approved for many of the disease indications we are targeting with our drug candidates. Accordingly, there may not be well-established development paths and outcomes. The FDA, Health Canada or any other regulatory authority may determine that the designs or endpoints of any trial that we conduct, or that the outcome shown on any particular endpoint in any trial that we conduct, are not sufficient to establish a clinically meaningful benefit for our drug candidates, or otherwise, to support approval, even if the primary endpoint(s) of the trial is met with statistical significance. If this occurs, our business could be materially harmed. Moreover, if the regulatory authorities require us to conduct additional clinical trials beyond the ones that we currently contemplate, our finances and results from operations will be adversely impacted. If our clinical studies meet their respective primary endpoints, we plan to seek marketing approval. We cannot predict whether each of these regulatory agencies will agree that our study data and information will be sufficient to meet the requirements for filing a marketing application or the standards for approval. If the regulatory agencies determine that more data and information are needed, it could delay and/or negatively impact our ability to obtain regulatory approval to market and sell a particular product candidate.
If the commercial opportunity in chronic ACD, ARDS, vitiligo or fibrotic diseases like systemic sclerosis (SSc) is smaller than we anticipate, our future revenue from our drug candidates will be adversely affected and our business will suffer.
It is critical to our ability to grow and become profitable that we successfully identify patients with chronic ACD, ARDS, vitiligo or SSc. Our estimates of the number of people who have these conditions as well as the subset who have the potential to benefit from treatment with EB01, EB05, EB06 or EB07, are based on a variety of sources, including third-party estimates and analyses in the scientific literature, and may prove to be incorrect. Further, new information may emerge that changes our estimate of the prevalence of these diseases or the number of patient candidates for these drug candidates. The effort to identify patients for our other potential target indications is at an early stage, and we cannot accurately predict the number of patients for whom treatment might be possible. Additionally, the potentially addressable patient population for our drug candidates may be limited or may not be amenable to treatment with our drug candidates, and new patients may become increasingly difficult to identify or access. If the commercial opportunity for these conditions is smaller than we anticipate, our future financial performance may be adversely impacted.
While we have chosen to test our product candidates in specific clinical indications based in part on our understanding of their mechanisms of action, our understanding may be incorrect or incomplete and, therefore, our product candidates may not be effective against the diseases tested in our clinical trials.
Our rationale for selecting the particular therapeutic indications for each of our product candidates is based in part on our understanding of the mechanism of action of these product candidates. However, our understanding of the product candidates’ mechanism of action may be incomplete or incorrect, or the mechanism may not be clinically relevant to the diseases treated. In such cases, our product candidates may prove to be ineffective in the clinical trials for treating those diseases, and adverse clinical trial results would likely negatively impact our business and results from operations.
A successful sPLA2, anti-TLR4 or anti-CXCL10 drug has not been developed to date and we can provide no assurances that we will be successful or that there will be no adverse side effects.
Our sPLA2, anti-TLR4 and anti-CXCL10 product candidates employ novel mechanisms of action. To our knowledge no drug companies have successfully commercialized an sPLA2 inhibitor, an anti-TLR4 antibody or an anti-CXCL10 antibody and as a result the efficacy and long-term side effects are not known. There is no guarantee that we will successfully develop and/or commercialize any of these therapies, and/or that our product candidates will have no adverse side effects.
Even if one of our product candidates receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success.
If any product candidate receives marketing approval, the approved product may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. If an approved product does not achieve an adequate level of acceptance, we may not generate significant product revenues or any profits from operations. Our ability to negotiate, secure and maintain third-party coverage and reimbursement for our product candidates may be affected by political, economic and regulatory developments in the U.S., Canada, the EU and other jurisdictions. Governments continue to impose cost containment measures, and third-party payors are increasingly challenging prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. These and other similar developments could significantly limit the degree of market acceptance of any of our future product candidates that receive marketing approval.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and any of our other current or future product candidates, we may not be successful in commercializing the applicable product candidate if it receives marketing approval.
We do not have a sales or marketing infrastructure and have no experience as a company in the sale or marketing of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales and marketing organization or outsource these functions to third parties. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel. If we enter into arrangements with third parties to perform sales and marketing services, our product revenues or the profitability of these product revenues to us could be lower than if we were to market and sell any products that we develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to sell and market our product candidates or may be unable to do so on terms that are acceptable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products to treat our target indications or markets before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates and any products we may seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. Competitors may also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization. Many of our competitors have significantly greater financial resources and expertise than we do. Our commercial opportunities could be reduced or eliminated if our competitors develop and commercialize products that are more effective, safer, have fewer or less severe side effects, are approved for broader indications or patient populations, or are more convenient or less expensive than any products that we develop and commercialize. Our competitors may also obtain marketing approval for their products more rapidly than we may obtain approval for our products, which could result in our competitors establishing a strong market position before we are able to enter the market. If approved, our product candidates will compete for a share of the existing market with numerous other products being used to treat ACD, ARDS, vitiligo, SSc or any other indications for which we may receive government approval.
Even if we are able to commercialize one of our product candidates, the product may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drug products vary widely from country to country. Current and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted and, in some markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize EB01, EB05, EB06, EB07 or any other product candidate successfully also will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and other third- party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could cause us to incur substantial liabilities and limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. The amount of insurance that we currently hold may not be adequate to cover all liabilities that we may incur. We will need to increase our insurance coverage when and if we begin conducting more expansive clinical development of our product candidates, and we may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise. If any of our product candidates are approved for commercial sale, we will be highly dependent upon consumer perceptions of us and the safety and quality of our products. We could be adversely affected if we are subject to negative publicity. We could also be adversely affected if any of our products or any similar products manufactured and distributed by other companies prove to be, or are asserted to be, harmful to patients. Because of our dependence upon consumer perceptions, any adverse publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our financial condition or results of operations.
We will be dependent on third parties for manufacturing, including optimization, technology transfers and scaling up of clinical scale quantities of all of our product candidates.
We have no direct experience in manufacturing any of our product candidates, and currently lack the resources or capability to manufacture any of our product candidates on a clinical or commercial scale. As a result, we will be dependent on third parties for manufacturing, including optimization, technology transfers and scaling up of clinical scale quantities of all our product candidates. We believe that this strategy will enable us to direct operational and financial resources to the development of our product candidates rather than diverting resources to establishing manufacturing infrastructure; however our use of third parties to manufacture our product candidates may increase the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not currently have any agreements with third-party manufacturers for the long-term clinical or commercial supply of any of our product candidates and may in the future be unable to scale-up and/or conclude agreements for commercial supply with commercial third-party manufacturers on acceptable terms, or at all. Even if we are able to establish and maintain arrangements with third-party manufacturers, they may encounter difficulties in achieving volume production, laboratory testing, quality control or quality assurance or suffer shortages of qualified personnel, any of which could result in our inability to manufacture sufficient quantities to meet clinical timelines for a particular product candidate, to obtain marketing approval for the product candidate or to commercialize the product candidate. We may compete with other companies for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
If the third parties that we contract to manufacture product for our preclinical tests and clinical trials cease to continue to do so for any reason or if we elect to change suppliers, we likely would experience delays in advancing these clinical trials while we identify and qualify replacement suppliers and we may be unable to obtain replacement suppliers on terms that are favorable to us. In addition, if we are not able to obtain adequate supplies of our product candidates or the drug substances used to manufacture them, it will be more difficult for us to develop our product candidates and compete effectively. Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that receive marketing approval on a timely and competitive basis.
The manufacturing of our monoclonal antibody candidates is complex and subject to a multitude of risks. These manufacturing risks could substantially increase our costs and limit supply of these drug candidates for clinical development, and commercialization.
The manufacture of our monoclonal antibody candidates requires processing steps that are more complex than those required for most small molecule drugs. As a result of the complexities in manufacturing biologics, the cost to manufacture biologics in general, and our monoclonal antibody candidates in particular, is generally higher than traditional small molecule chemical compounds, and the manufacturing processes are less reliable and are more difficult to reproduce. Although we are working with third parties to develop reproducible and commercially viable manufacturing processes for our product candidates, doing so is a difficult and uncertain task, and there are risks associated with scaling to the level required for advanced clinical trials or commercialization, including, among others, cost overruns, potential problems with process scale-out, process reproducibility, stability issues, lot consistency, and timely availability of reagents or raw materials.
We may make changes as we continue to evolve the manufacturing processes for our product candidates for advanced clinical trials and commercialization, and we cannot be sure that even minor changes in these processes will not cause our product candidates to perform differently and affect the results of our ongoing clinical trials, future clinical trials, or the performance of the product once commercialized. In some circumstances, changes in manufacturing operations, including to our protocols, processes, materials or facilities used, may require us to perform additional preclinical or comparability studies, or to collect additional clinical data from patients prior to undertaking additional clinical studies or filing for regulatory approval for a product candidate. These requirements may lead to delays in our clinical development and commercialization plans for our product candidates, and may increase our development costs substantially.
We may also decide to transfer certain manufacturing process know-how and certain intermediates to other contract manufacturing organizations. Transferring manufacturing testing and processes and know-how is complex and involves review and incorporation of both documented and undocumented processes that may have evolved over time. We and any CMOs or third parties that we engage for manufacturing our product candidates will need to conduct significant development work to transfer these processes and manufacture each of our product candidates for clinical trials and commercialization. In addition, we may be required to demonstrate the comparability of material generated by any CMO or third parties that we engage for manufacturing our product candidates with material previously produced and used in testing. The inability to manufacture comparable drug product by us or our CMO could delay the continued development of our product candidates.
We also must develop satisfactory methods for testing the identity, strength, quality and purity of the final drug. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate the effectiveness of the packaging and that the compound does not undergo unacceptable deterioration over its shelf life. If we fail at any of these tasks, we may not be able to obtain approval or successfully commercialize our product candidates.
We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such clinical trials.
We do not independently conduct clinical trials for our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions, drug distributers and clinical investigators, to perform this function. Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it would delay our product development activities. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. Our product development costs will increase if we experience delays in testing or obtaining marketing approvals. Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. For example, we remain responsible for ensuring that each of our clinical trials complies with standards, commonly referred to as Good Clinical Practice, and is conducted in accordance with the general investigational plan and protocols for the trial.
If we are not able to establish additional collaborations, we may have to alter our development and commercialization plans.
We may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of our product candidates. Collaborations are complex and time-consuming to negotiate and document and we face significant competition in seeking appropriate collaborators. We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of a product candidate, reduce or delay our development program or one or more of our other development programs, delay our potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund development or commercialization activities on our own, we would likely need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
Even if we complete the necessary clinical trials, the marketing approval process is expensive, time consuming and uncertain. If we are not able to obtain, or if there are delays in obtaining, required marketing approvals, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA, Health Canada and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market EB01, EB05, EB06, EB07 or any other Edesa product candidate from regulatory authorities in any jurisdiction.
Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to regulatory authorities for each therapeutic indication to establish the product candidate’s safety and effectiveness. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Regulatory authorities may determine that EB01, EB05, EB06, EB07 or any of our other product candidates is not effective, is only moderately effective or has undesirable or unintended side effects, toxicities, safety profiles or other characteristics that preclude us from obtaining marketing approval or that prevent or limit commercial use. Regulatory authorities have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical studies, clinical trials or other trials. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable. If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
Even if we obtain marketing approval for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products, and compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue.
Even if marketing approval of a product candidate is granted, an approved product and our manufacturer and marketer are subject to ongoing review and extensive regulation, including the possible requirement to implement a risk evaluation and mitigation strategy or to conduct costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product. We must also comply with requirements concerning advertising and promotion for any of our product candidates for which we obtain marketing approval. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product’s approved labeling. Thus, we will not be able to promote any products we develop for indications or uses for which they are not approved. In addition, manufacturers of approved products and those manufacturers’ facilities are required to ensure that quality control and manufacturing procedures conform to cGMP, which include requirements relating to quality control, quality assurance and documentation. Accordingly, assuming we receive marketing approval for one or more of our product candidates, we and our contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control. If we are not able to comply with post-approval regulatory requirements, we could have the marketing approvals for our products withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
We may not qualify for or ultimately benefit from various expedited regulatory review programs or other special designations.
We have obtained a Fast Track designation in the U.S. for EB05 as a treatment for ARDS in critically ill Covid-19 patients, and we may seek additional designations for EB05 or our other product candidates; however, we may never receive such designations. If we believe we meet eligibility requirements, we intend to apply for various regulatory incentives in the U.S., such as breakthrough therapy designation, fast track designation, accelerated approval and priority review, where available, that provide for expedited review and/or other benefits, and we may also seek similar designations elsewhere in the world. Similarly, we may seek orphan drug designation in the U.S. and other jurisdictions for our product candidates, but we may be unable to obtain such designation or to obtain or maintain the benefits associated with orphan drug designation, including market exclusivity. Often, regulatory agencies have broad discretion in determining whether or not product candidates qualify for such regulatory incentives and benefits and we cannot guarantee we would be successful in obtaining beneficial regulatory designations by FDA or other regulatory agencies. Even if approved, expedited designations may not result in faster development processes, reviews or approvals compared to drugs considered for approval under conventional FDA procedures. In addition, the FDA may later decide that any of our development programs no longer meet the conditions for qualification or decide that the time period for FDA review or approval will not be shortened. If we are not able to obtain or maintain such designations for EB05 or other product candidates, it could delay and/or negatively impact our ability to obtain regulatory approval.
Regulators have broad discretion regarding emergency use authorizations for medical products, and such authorizations may only be valid during a public health emergency.
While, in most cases, a therapeutic must be approved by FDA before the product may be sold, when a public health emergency is declared, subject to certain conditions, FDA may authorize the emergency use of an unapproved medical product under an Emergency Use Authorization (EUA). Similar systems are in place in Canada and the EU. In the event that our clinical study of EB05 is successful, and if we believe we meet eligibility requirements, we intend to submit an application with the regulators for emergency use. Regulators typically do not have review deadlines with respect to such submissions and, therefore, the timing of any potential approval of an emergency use submission would be uncertain. Regulators may refuse to approve our application. In addition, even if granted, the regulators may revoke an emergency use where it is determined that the underlying health emergency no longer exists or warrants such authorization. If we are unsuccessful in obtaining an EUA, or if any granted EUA is revoked after a short period of time, it could have a material adverse effect on our future business, financial condition and operating results.
Increasing use of social media platforms could give rise to liability, breaches of data security and privacy laws, or reputational damage.
We believe that our potential patient population is active on social media. Social media practices in the pharmaceutical and biotechnology industries are evolving, which creates uncertainty and risk of noncompliance with regulations applicable to our business. For example, patients may use social media platforms to comment on the effectiveness of, or adverse experiences with, a product candidate, which could result in reporting obligations. In addition, there is a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us or our product candidates on any social networking website.
In addition, our employees or third parties with whom we contract, such as our CROs or CMOs, may knowingly or inadvertently make use of social media in a manner that may give rise to liability, lead to the loss of trade secrets or other intellectual property or result in public exposure of personal information of our employees, clinical trial patients, customers and others or information regarding our product candidates or clinical trials. Any of these events could have a material adverse effect on our business, prospects, operating results and financial condition and could adversely affect the price of our common shares.
Risks Related to Our Intellectual Property
We are dependent on license relationships with third parties for our key drug development programs.
In 2016, we entered into the Yissum License Agreement to obtain exclusive rights to certain know-how, patents and data relating to a pharmaceutical product. We are using the exclusive rights to develop the product for therapeutic, prophylactic and diagnostic uses in topical dermal applications and anorectal applications, including for the development of EB01 to treat ACD and EB02 to treat HD. In 2021, we also entered into the Inventor License Agreement to acquire global rights for all fields of use beyond those named under the Yissum License Agreement. If we default or fail to perform any of the terms, covenants, provisions or our obligations under the Yissum License Agreement, Yissum has the option to terminate the Yissum License Agreement, subject to advance notice to cure such default. Any termination of this license agreement would have a materially adverse impact on our business and results from operations.
In April 2020, we entered into the NovImmune License Agreement to obtain exclusive rights throughout the world to certain know-how, patents and data relating to the monoclonal antibodies targeting TLR4 and CXCL10. We are using these rights to develop EB05 as a potential treatment for ARDS and other disease indications. If we default or fail to perform any of the terms, covenants, provisions or our obligations under the NovImmune License Agreement, including milestone payments, NovImmune has the option to terminate the NovImmune License Agreement, subject to advance notice to cure such default. Any termination of this license agreement would have a materially adverse impact on our business and results from operations.
If we are unable to obtain and maintain patent protection for our licensed technology and products, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our licensed technology and products may be adversely affected.
Our success will partially depend on our ability to obtain and maintain patent protection in the U.S. and other countries with respect to our proprietary technology and products. We intend to protect our proprietary position by filing patent applications in the U.S., in Europe and in certain additional jurisdictions related to our novel technologies and product candidates that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, if we license technology or product candidates from third parties in the future, these license agreements may not permit us to control the preparation, filing and prosecution of patent applications, or to maintain or enforce the patents, covering the licensed technology or product candidates.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of any patents issued to us will likely be highly uncertain. Patent applications that we file may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the U.S. and other countries may also diminish the value of patents issued to us, narrow the scope of our patent protection or make enforcement more difficult or uncertain.
We may become involved in lawsuits or other enforcement proceedings to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and potentially unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file claims, which can be expensive and time consuming to prosecute. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property or that our patent and other intellectual property rights are invalid or unenforceable, including for antitrust reasons. As a result, in a patent infringement proceeding, a court or administrative body may decide that a patent of ours is invalid or unenforceable, in whole or in part, or may construe the patent’s claims narrowly and so refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the competitor technology in question. Even if we are successful in a patent infringement action, the unsuccessful party may subsequently raise antitrust issues and bring a follow-on action thereon. Antitrust issues may also provide a bar to settlement or constrain the permissible settlement terms.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the intellectual property and other proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology. The risks of being involved in such litigation and office proceedings may also increase as our product candidates approach commercialization, and as our business gains greater visibility. Third parties may assert infringement claims against us based on existing or future intellectual property rights and to restrict our freedom to operate. Third parties may also seek injunctive relief against us, whereby they would attempt to prevent us from practicing our technologies altogether pending outcome of any litigation against us. We may not be aware of all such intellectual property rights potentially relating to our product candidates prior to their assertion against us. If we are found to infringe a third party’s intellectual property rights, we could incur substantial monetary damages. A finding of infringement could also prevent us from commercializing our product candidates, lose market exclusivity, require substantial license payments, or force us to cease some of our business operations, which could materially harm our business.
Intellectual property litigation could cause us to spend substantial resources and could distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and likely would distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments that could have a substantial adverse effect on the price of our securities. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development, sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Accordingly, costs and lost management time, as well as uncertainties resulting from the initiation and continuation of patent litigation or other proceedings, could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
We partially rely on trade secrets and know-how, including unpatented know-how, technology and other proprietary and confidential information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into nondisclosure and confidentiality agreements with parties who have access to them. However, we cannot guarantee that we have executed these agreements with each party that may have or have had access to our trade secrets or that the agreements we have executed will provide adequate protection. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary or confidential information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets, particularly unpatented know-how, were to be obtained or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Owning Our Securities
The price of our common shares may continue to be volatile.
Market prices for securities of clinical-stage pharmaceutical, biotechnology and other life sciences companies have historically been particularly volatile, and the market price of our common shares has been subject to significant fluctuations. This volatility can be exacerbated by low trading volume. Some of the factors that may cause the market price of our shares to fluctuate include:
| · | sales or potential sales of substantial amounts of our common shares; |
| · | announcements about us or our competitors, including funding announcements, corporate or business updates, updates on manufacturing of our products, clinical trial results, regulatory approvals or new product introductions; |
| · | developments concerning our product manufacturers; |
| · | litigation and other developments relating to our licensed patents or other proprietary rights or those of our competitors; |
| · | governmental regulation and legislation; |
| · | change in securities analysts’ estimates of our performance, or failure to meet analysts’ expectations; |
| · | the terms and timing of any future collaborative, licensing or other arrangements that we may establish; |
| · | our ability to raise additional capital to carry through with our development plans and current and future operations; |
| · | the timing of achievement of, or failure to achieve, our manufacturing, pre-clinical, clinical, regulatory and other milestones, such as the commencement of clinical development, the completion of a clinical trial or the receipt of regulatory approval; |
| · | actions taken by regulatory agencies with respect to our product candidates; |
| · | uncontemplated problems in the supply of the raw materials used to produce our product candidates; |
| · | introductions or announcements of technological innovations or new products candidates by us, our potential future collaborators, or our competitors, and the timing of these introductions or announcements; |
| · | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
| · | actual or anticipated fluctuations in our results of operations; |
| · | hedging or arbitrage trading activity that may develop regarding our common shares; |
| · | regional or worldwide recession; |
| · | sales of our common shares by our executive officers, directors and significant shareholders; |
| · | changes in accounting principles; and |
| · | the loss of any of our key scientific or management personnel. |
Moreover, the stock markets in general have experienced substantial volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations may also adversely affect the trading price of our common shares. In the past, following periods of volatility in the market price of a company’s securities, shareholders have often instituted class action securities litigation. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our profitability and reputation.
If we fail to meet all applicable Nasdaq Capital Market requirements and Nasdaq determines to delist our common shares, the delisting could adversely affect the market liquidity of our common shares and the market price of our common shares could decrease and our ability to access the capital markets could be negatively impacted.
Our common shares are listed on The Nasdaq Capital Market. We must satisfy the continued listing requirements of Nasdaq, to maintain the listing of our common shares on The Nasdaq Capital Market.
As previously reported, on June 22, 2023, we received notice from Nasdaq’s Listing Qualifications Staff indicating that, based upon the closing bid price of our common shares for the prior 30 consecutive business days, we were not in compliance with the requirement to maintain a minimum bid price of $1.00 per share for continued listing on Nasdaq, as set forth in Nasdaq Listing Rule 5550(a)(2) (the Bid Price Rule). We had 180 days, or through December 19, 2023, to regain compliance with the Bid Price Rule. On October 11, 2023, we effected a one-for-seven reverse split of our common shares. By letter dated October 25, 2023, Nasdaq advised us that we had regained compliance with the Bid Price Rule.
There can be no assurance that we will be able to continue to maintain compliance with the Nasdaq continued listing requirements, and if we are unable to maintain compliance with the continued listing requirements, including the Bid Price Rule, our securities may be delisted from Nasdaq, which could reduce the liquidity of our common shares materially and result in a corresponding material reduction in the price of our common shares. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees, suppliers and business development opportunities. Such a delisting likely would impair your ability to sell or purchase our common shares when you wish to do so. Further, if we were to be delisted from Nasdaq, our common shares may no longer be recognized as a “covered security” and we would be subject to regulation in each state in which we offer our securities. Thus, delisting from Nasdaq could adversely affect our ability to raise additional financing through the public or private sale of equity securities, would significantly impact the ability of investors to trade our securities and would negatively impact the value and liquidity of our common shares.
We do not currently intend to pay dividends on our common shares in the foreseeable future, and consequently, any gains from an investment in our common shares will likely depend on appreciation in the price of our common shares.
We have never declared or paid cash dividends on our common shares and do not anticipate paying any cash dividends to holders of our common shares in the foreseeable future. Consequently, investors must rely on sales of their common shares and warrants after price appreciation, which may never occur, as the only way to realize any future gains on their investments. There is no guarantee that our common shares will appreciate in value or even maintain the price at which the shareholders have purchased their shares.
A sale of a substantial number of our common shares in the public market could cause the market price of our common shares to drop significantly, even if our business is doing well.
The price of our common shares could decline as a result of sales of a large number of our common shares or the perception that these sales could occur. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
In addition, in the future, we may issue additional common shares, warrants or other equity or debt securities convertible into common shares in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. Any such issuance could result in substantial dilution to our existing shareholders and could cause the price of our common shares to decline.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common shares, the price of our common shares could decline.
The trading market for our common shares relies in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts. The price of our common shares could decline if one or more equity analysts downgrade our common shares or if analysts issue other unfavorable commentary or cease publishing reports about us or our business.
Our Articles allow for our board of directors to create new series of preferred shares without further approval by the shareholders, which could adversely affect the rights of the holders of our common shares.
As previously approved by our shareholders, our board of directors has the authority to authorize up to an unlimited number of a new series of our preferred shares and to fix and determine the special rights and restrictions of that series without further shareholder approval, subject to the terms set out in the Articles and unless otherwise required by the Business Corporations Act (British Columbia). As a result, our board of directors could authorize the creation of a series of our preferred shares that would grant to holders of the preferred shares a right to our assets upon liquidation before a distribution to the holders of our common shares. In addition, our board of directors could authorize the creation of a new series of our preferred shares that is convertible into our common shares, which could result in dilution to existing shareholders.
Failure to maintain effective internal control over financial reporting could have a material adverse effect on our share price.
Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC require an annual management assessment of the effectiveness of our internal control over financial reporting. As a smaller reporting company as defined in Rule 12b-2 under the Exchange Act, we are currently exempt from the auditor attestation requirement of Section 404(b). If we lose this eligibility, we will incur increased personnel and audit fees in connection with the additional audit requirements.
If we fail to maintain the adequacy of our internal control over financial reporting, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting once that firm begins its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common shares could decline, and we could be subject to sanctions or investigations by Nasdaq, the SEC, or other regulatory authorities. Failure to remedy any material weakness or significant deficiencies in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also adversely affect investor confidence in the reliability of our financial reports and restrict our future access to the capital markets.
The ownership of our common shares is highly concentrated, which may prevent you and other shareholders from influencing significant corporate decisions and may result in conflicts of interest that could cause our common shares price to decline.
The ownership of our common shares is highly concentrated among insiders and affiliates. Accordingly, these shareholders will have substantial influence over the outcome of corporate actions requiring shareholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of the Company’s assets or any other significant corporate transaction. These shareholders may also delay or prevent a change of control of the Company, even if such a change of control would benefit the other shareholders of the Company. The significant concentration of share ownership may adversely affect the trading price of our common shares due to investors’ perception that conflicts of interest may exist or arise.
We may be deemed a passive foreign investment company, and as a result, U.S. shareholders may be subject to special taxation rules that restrict capital gains treatment, unless the shareholders make a timely tax election to treat the company as a qualified electing fund.
A special set of U.S. federal income tax rules applies to a foreign corporation that is deemed a passive foreign investment company (PFIC) for U.S. federal income tax purposes. Based on our audited financial statements, income tax returns, and relevant market and shareholder data, we believe that we likely will not be classified as a PFIC in the September 30, 2023 taxable year. There can be no assurance, however, that we will not be considered to be a PFIC for any particular year in the future because PFIC status is factual in nature, depends upon factors not wholly within our control, generally cannot be determined until the close of the taxable year in question, and is determined annually. If we are deemed to be a PFIC during the current or a future taxable year, U.S. shareholders would be subject to special taxation rules related to gain on sale or disposition of our shares and excess distributions unless they make a timely election to treat our shares as a qualified electing fund (QEF election). A QEF election cannot be made unless we provide U.S. shareholders the information and computations needed to report income and gains pursuant to a QEF election. Without a QEF election, U.S. shareholders may not be able to use capital gains tax treatment and may be subject to potentially adverse tax consequences. Given the complexities of the PFIC and QEF election rules, U.S. shareholders may need to incur the time and expense of consulting a tax adviser about these rules.
Item 1B. UNRESOLVED STAFF COMMENTS.
None.
Item 1C. CYBERSECURITY.
Not applicable.
Item 2. PROPERTIES.
We currently lease approximately 2,800 square feet of office space for our executive offices in Markham, Ontario, from 1968160 Ontario Inc., an entity affiliated with Dr. Nijhawan. Pursuant to the lease, as amended and extended on December 31, 2022, the term of the lease expires on December 31, 2024. We believe our current offices are sufficient to meet our needs. We may seek to negotiate new leases or evaluate additional or alternate space to accommodate operations. We believe that appropriate alternative space is readily available on commercially reasonable terms.
Item 3. LEGAL PROCEEDINGS.
From time to time, we may be involved in legal proceedings, claims and litigation arising in the ordinary course of business, including contract disputes, employment matters and intellectual property disputes. We are not currently a party to any material legal proceedings or claims outside the ordinary course of business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 4. MINE SAFETY DISCLOSURES.
Not applicable.
PART II
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common shares trade on The Nasdaq Capital Market in the United States under the symbol “EDSA”.
Holders
As of December 13, 2023, we had 3,164,722 common shares outstanding, with 13 shareholders of record. The number of record shareholders was determined from the records of our stock transfer agent and does not reflect persons or entities that hold their shares in nominee or “street” name through various brokerage firms.
Securities Authorized for Issuance Under Equity Compensation Plans
See Part III, Item 12 “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” of this report.
Dividends
We have not declared any dividends on our common shares since our incorporation and do not anticipate that we will do so in the foreseeable future. Our present policy is to retain future earnings, if any, for use in our operations and the expansion of our business. Any future determination to pay dividends will be made at the discretion of our board of directors.
Item 6. RESERVED.
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
This discussion contains forward-looking statements that involve risks and uncertainties that could cause actual results or events to differ materially from those expressed or implied by such forward-looking statements as a result of many important factors, including those set forth in Part I of this Annual Report on Form 10-K under the caption “Risk Factors.” Please see “Forward-Looking Statements and Other Matters” in Part I above. We do not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Annual Report.
Overview
We are a biopharmaceutical company developing innovative ways to treat inflammatory and immune-related diseases.
Our approach is to acquire, develop and commercialize drug candidates based on mechanisms of action that have demonstrated proof-of-concept in human subjects. We prioritize our efforts on disease indications where there is compelling scientific rationale, no approved therapies or where there are unmet medical needs, and where there are large addressable market opportunities, among other factors. We have multiple late-stage product candidates in our development pipeline.
Our most advanced drug candidate is EB05. EB05 represents a new class of emerging therapies called Host-Directed Therapeutics (HDTs) that are designed to modulate the body’s own immune response when confronted with infectious diseases or even chemical agents. Importantly, these therapies are designed to work across multiple infectious diseases and threats, and could be stockpiled preemptively ahead of outbreaks. Because they are threat agnostic, HDTs like EB05 have the potential to become standard of care in ICUs and critical countermeasures for both pandemic preparedness and biodefense. We are currently evaluating EB05 as a potential treatment for ARDS, a life-threatening form of respiratory failure. Recruitment in a Phase 3 study is ongoing.
In addition to EB05, we are developing product candidates for a number of chronic dermatological and inflammatory conditions. In November 2023, we reported final results from a Phase 2b clinical study evaluating multiple concentrations of our drug candidate, EB01, as a monotherapy for moderate-to-severe chronic ACD, a common occupational skin condition. Among the findings, 1.0% EB01 cream demonstrated statistically significant improvement over placebo for the primary endpoint and a key secondary endpoint. For our EB06 monoclonal candidate, we have received regulatory approval by Health Canada to conduct a future Phase 2 study in patients with moderate to severe nonsegmental vitiligo, a common autoimmune disorder that causes skin to lose its color in patches. We are also preparing an IND in the U.S. for our EB07 product candidate to conduct a future Phase 2 study in patients with fibrotic diseases such as systemic sclerosis.
Operating and Financial Review and Prospects
Our financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP) and include the accounts of the Company and our wholly owned subsidiaries, Edesa Biotech Research, Inc. and Edesa Biotech USA, Inc.
Our operations have been funded primarily through issuances of common shares, exercises of common share purchase warrants, convertible preferred shares, convertible loans, government grants and tax incentives. We have devoted substantially all of our efforts to research and development, including clinical trials, and have not completed the development of any of our drug candidates. We believe our cash and cash equivalents on hand, including net proceeds from our equity distribution agreement with Canaccord Genuity LLC (Canaccord), advances under the credit facility and reimbursements of eligible research and development expenses under our contribution agreements with the Canadian government are sufficient to support the Company’s operations for at least the next 12 months.
As a clinical-stage biopharmaceutical company, we expect to continue to incur significant expenses and operating losses for the foreseeable future as we continue the development of, and seek marketing approvals for our product candidates, prepare for and begin the commercialization of any approved products, and add infrastructure and personnel to support our product development efforts and operations as a public company in the United States and Canada. To fund operations, we may seek additional financing through the sale of equity, government grants, debt financings or other capital sources, including potential future licensing, collaboration or similar arrangements with third parties or other strategic transactions.
Results of Operations
Fiscal Year Ended September 30, 2023 Compared to the Fiscal Year Ended September 30, 2022
Our total operating expenses decreased by $9.2 million to $9.2 million for the year ended September 30, 2023 compared to $18.4 million for the prior year:
| · | Research and development (R&D) expenses decreased by $8.5 million to $4.8 million for the year ended September 30, 2023 compared to $13.3 million for the prior year primarily due to lower external R&D expenses related to our ongoing clinical studies and manufacturing of our investigational drugs, which included the purchase of $2.5 million in bulk drug product in the prior year. Our R&D expenses consist primarily of employee-related expenses, including salaries, benefits, taxes, travel, and share-based compensation expense for personnel in R&D functions; expenses related to process development and production of product candidates paid to contract manufacturing organizations, including the cost of acquiring, developing, and manufacturing research material; costs associated with clinical activities, including expenses for contract research organizations; and clinical trials and activities related to regulatory filings for our product candidates, including regulatory consultants. |
| | |
| · | General and administrative (G&A) expenses decreased by $0.6 million to $4.4 million for the year ended September 30, 2023 compared to $5.0 million for the prior year primarily due to a decrease in noncash share-based compensation. Our G&A expenses consist primarily of salaries and related costs for our employees in administrative, executive and finance functions. G&A expenses also include professional fees for legal, accounting, audit, tax and consulting services, insurance, office, and travel expenses. |
Total other income was unchanged at $0.8 million for the years ended September 30, 2023 and September 30, 2022 and was composed of the following:
| · | Grant income decreased by $0.2 million to $0.6 million for the year ended September 30, 2023 compared to $0.8 million for the year ended September 30, 2022, reflecting a decrease in grant income associated with the completion activities under the 2021 SIF funding Agreement, which was partially offset by the initiation of reimbursable expenses under the 2023 SIF Agreement. |
| · | Interest income increased by $0.2 million to $0.3 million for the year ended September 30, 2023 compared to $0.1 million for the prior year primarily due to higher cash balances and an increase in interest rates. |
| · | Foreign exchange loss was unchanged at $21,000 for both the year ended September 30, 2023 and September 30, 2022. |
For the year ended September 30, 2023, our net loss was $8.4 million, or $2.93 per common share, compared to a net loss of $17.6 million, or $8.37 per common share, for the year ended September 30, 2022.
Capital Expenditures
Our capital expenditures primarily consist of computer and office equipment. There were no significant capital expenditures for the years ended September 30, 2023 and 2022.
Liquidity and Capital Resources
As a clinical-stage company we have not generated significant revenue, and we expect to incur operating losses as we continue our efforts to acquire, develop, seek regulatory approval for and commercialize product candidates and execute on our strategic initiatives. Our operations have historically been funded through issuances of common shares, exercises of common share purchase warrants, convertible preferred shares, convertible loans, government grants and tax incentives.
Our primary use of cash is to fund our operating expenses, which consist of R&D and G&A expenditures. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in accounts payable and accrued expenses. Net cash used in operating activities was $6.6 million and $12.3 million for the years ended September 30, 2023 and 2022, respectively. We incurred net losses of $8.4 million and $17.5 million for those same years.
In October 2023, we entered into the 2023 SIF Agreement with the Canadian Government’s SIF. Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding. Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by us. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when we earn gross revenue. In February 2021, we entered into the 2021 SIF Agreement, pursuant to which we were eligible to receive cash reimbursements up to C$14.1 million in the aggregate for certain R&D expenses related to our EB05 clinical development program. All potential funding available under the 2021 SIF Agreement has been received. For the years ended September 30, 2023 and 2022, we recorded grant income of $0.6 million and $0.8 million respectively related to both the 2023 SIF Agreement and the 2021 SIF Agreement.
In October 2023, we entered into $10.0 million revolving credit agreement with Pardeep Nijhawan Medicine Professional Corporation, an entity controlled by Dr. Pardeep Nijhawan, MD, our Chief Executive Officer and Secretary and member of our board of directors (Credit Agreement), providing an unsecured revolving credit facility, with a credit limit of $3.5 million (Credit Limit) which was available immediately. The line of credit bears interest at the Canadian Imperial Bank of Commerce US Base-Interest Rate plus 3% per annum and has a maturity date of March 31, 2026, unless terminated earlier by either party with 90 days’ notice. Advances under the line of credit are tied to a borrowing base (Borrowing Base) consisting of eligible grant receivables from SIF, future potential license fee receivables and any other accounts receivable. At no time shall the aggregate principal amount of all advances outstanding exceed the lesser of (i) the Credit Limit and (ii) an amount equal to 85% of the Borrowing Base. We have not drawn any funds from the Credit Agreement.
In August 2022, we filed a $150.0 million shelf registration statement. In March 2023, we entered into an equity distribution agreement with Canaccord, as sales agent, pursuant to which we may offer and sell, from time to time, common shares through an at-the-market equity offering program for up to $20 million in gross proceeds, subject to certain offering limitations that currently allow us to offer and sell common shares having an aggregate gross sales price of up to $8.4 million (Canaccord ATM). There was approximately $7.1 million of available capacity on the Canaccord ATM as of September 30, 2023.We have no obligation to sell any of the common shares and may at any time suspend sales or terminate the equity distribution agreement in accordance with its terms. For the fiscal year ended September 30, 2023, we sold a total of 196,401 common shares pursuant to the agreement for net proceeds of $1.1 million after deducting commissions and costs of $0.2. Subsequent to September 30, 2023, we sold a total of 89,241 common shares pursuant to the agreement for net proceeds of $0.3 million after deducting sales agent commissions.
In November 2022, we completed a private placement of units consisting of 384,475 common shares, 12-month warrants to purchase up to an aggregate of 192,248 common shares and 3-year warrants to purchase up to an aggregate of 192,248 common shares. The gross proceeds from this offering were approximately $3.0 million, before offering expenses.
In March 2022, we completed a registered direct offering of 220,000 common shares and pre-funded warrants to purchase up to an aggregate of 171,390 common shares. In a concurrent private placement, we issued common share purchase warrants to purchase an aggregate of up to 391,390 common shares. After deducting the placement agent fees and offering expenses, net proceeds to us were approximately $9.0 million.
In November 2021, we entered into an equity distribution agreement with RBC Capital Markets, LLC (RBCCM), as sales agent, which was subsequently terminated in March 2022. Pursuant to the terms of the agreement, as amended, the Company could offer and sell, from time to time, common shares through an at-the-market offering program for up to $15.4 million in gross cash proceeds. During the term of the agreement, we sold a total of 89,558 common shares. After deducting commissions and direct costs, net proceeds totaled approximately $2.6 million.
At September 30, 2023, we had an accumulated deficit of $52.4 million and working capital of $4.6 million, including $5.4 million in cash and cash equivalents. We plan to finance company operations over the course of the next twelve months with cash and cash equivalents on hand, including net proceeds from the Canaccord ATM, advances under the Credit Facility and reimbursements of eligible R&D expenses under the 2023 SIF Agreement with the Canadian government. Management has flexibility to adjust this timeline by making changes to planned expenditures related to, among other factors, the size and timing of clinical trial expenditures and manufacturing campaigns, staffing levels, and the acquisition or in-licensing of new product candidates. To help fund our operations and meet our obligations in the future, we plan to seek additional financing through the sale of equity, government grants, debt financings or other capital sources, including potential future licensing, collaboration or similar arrangements with third parties or other strategic transactions. If we raise additional funds by issuing equity securities, our shareholders will experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our existing shareholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us. Adequate funding may not be available to us on acceptable terms, or at all. If we fail to raise capital or enter into such agreements as and when needed, we may have to significantly delay, scale back or discontinue the development of our product candidates.
We expect to continue to incur substantial additional operating losses for at least the next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval, the eventual commercialization of our product candidates. If we obtain marketing approval for our product candidates, we will incur significant sales, marketing and outsourced manufacturing expenses. In addition, we expect to incur additional expenses to add operational, financial and information systems and personnel, including personnel to support our planned product commercialization efforts. We also expect to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to us as a public company. To continue to grow our business over the longer term, we plan to commit substantial resources to research and development, clinical trials of our product candidates, and other operations and potential product acquisitions and in licensing. We have evaluated and expect to continue to evaluate a wide array of strategic transactions as part of our plan to acquire or in license and develop additional products and product candidates to augment our internal development pipeline. Strategic transaction opportunities that we may pursue could materially affect our liquidity and capital resources and may require us to incur additional indebtedness, seek equity capital or both. In addition, we may pursue development, acquisition or in licensing of approved or development products in new or existing therapeutic areas or continue the expansion of our existing operations. Accordingly, we expect to continue to opportunistically seek access to additional capital to license or acquire additional products, product candidates or companies to expand our operations, or for general corporate purposes. Strategic transactions may require us to raise additional capital through one or more public or private debt or equity financings or could be structured as a collaboration or partnering arrangement. We have no arrangements, agreements, or understandings in place at the present time to enter into any acquisition, in licensing or similar strategic business transaction.
Cash Flows
Net cash used in operating activities
Net cash used in operating activities was $6.6 million for the year ended September 30, 2023 compared to $12.3 million for the year ended September 30, 2022 primarily due to a decrease in R&D expenses of $8.5 million, partially offset by a reduction in the recovery of working capital of $2.3 million in the current year compared to a $2.9 million recovery of working capital in the comparative year.
Net cash used in investing activities
Net cash used in investing activities was $5,700 for the year ended September 30, 2022. There was no cash used in investing activities for the year ended September 30, 2023. In the comparative year, we purchased a nominal amount of computer equipment.
Net cash provided by financing activities
Net cash provided by financing activities was $4.8 million for the year ended September 30, 2023 as compared to $11.6 million for the year ended September 30, 2022. In the current year, we received proceeds of $3.0 million from a private placement completed in November 2022, $1.3 million from the Canaccord ATM and $0.8 million from the exercise of warrants, partially offset by issuance costs of $0.3 million. In the comparative year, we received proceeds of $11.9 million and incurred issuance costs of $0.3 million for net proceeds of $11.6 million. The net proceeds relate to $9.0 million from a registered direct offering in March 2022 and $2.6 million was from the equity distribution agreement with RBCCM.
Research and Development
Our primary business is the development of innovative therapeutics for inflammatory and immune-related diseases with clear unmet medical needs. We focus our resources on R&D activities, including the conduct of clinical studies and product development, and expense such costs as they are incurred.
R&D expenses, which have historically varied based on the level of activity in our clinical programs, are significantly influenced by study initiation expenses and patient recruitment rates, and as a result are expected to continue to fluctuate, sometimes substantially. Our R&D expenses were $4.8 million and $13.3 million for the years ended September 30, 2023 and 2022, respectively. The decrease was due primarily to lower external research expenses related to our ongoing clinical studies and investigational drug product manufacturing expenses, which were partially offset by increased personnel expenses.
Foreign Exchange Risk
Our exposure to foreign exchange risk is primarily related to fluctuations between the Canadian dollar and the U.S. dollar. We have balances in Canadian dollars which are subject to foreign currency fluctuations relating to the impact of translating to U.S. dollars for financial statements presentation. We also periodically exchange U.S. dollars for Canadian dollars since most operating expenses are incurred in Canadian dollars. The fluctuation of the U.S. dollar in relation to the Canadian dollar will have an impact upon our profitability and may also affect the value of our assets and the amount of shareholders’ equity. We have not entered into any agreements or purchased any instruments to hedge possible currency risks. At September 30, 2023, we had assets denominated in Canadian dollars of approximately C$3.0 million and the U.S. dollar exchange rate as at this date was equal to 1.3581 Canadian dollars. Based on the exposure at September 30, 2023, a 10% annual change in the Canadian/U.S. exchange rate would impact our net loss and other comprehensive loss by $0.2 million.
Concentration of Credit Risk
We are potentially subject to financial instrument concentration of credit risk through our cash and cash equivalents, and accounts and other receivable. We place our cash and cash equivalents in money market mutual funds of U.S. government securities or financial institutions believed to be credit worthy and perform periodic evaluations of their relative credit standing.
Accounts and other receivable include Harmonized Sales Tax (HST) refunds receivable from the Canada Revenue Agency and reimbursements receivable from the Canadian government’s SIF. We assess the collectability of our accounts receivable through a review of our current aging and payment terms, as well as an analysis of our historical collection rate, general economic conditions and credit status of the government agencies. As of September 30, 2023 and 2022, all outstanding accounts and other receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
Significant Accounting Policies and Estimates
Our consolidated financial statements, which are indexed under Item 15 of this Annual Report on Form 10-K, have been prepared in accordance with accounting principles generally accepted in the United States, which require that the management make certain assumptions and estimates and, in connection therewith, adopt certain accounting policies. Our significant accounting policies are set forth in Note 3 in the Notes to Consolidated Financial Statements. Of those policies, we believe that the policies discussed below may involve a higher degree of judgment or may otherwise be more relevant to our financial condition and results of operations.
Accounts and other receivable
Accounts and other receivable include HST refunds receivable and reimbursements receivable from the Canadian government’s SIF. As of September 30, 2023, all outstanding accounts, grants and HST refunds receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
Intangible assets
Intangible assets represent the exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights, acquired by entering into the NovImmune License Agreement. Unless earlier terminated, the term of the NovImmune License Agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the NovImmune License Agreement will automatically renew for 5-year periods unless either party terminates the agreement in accordance with its terms. We recognize intangible assets at their historical cost, amortized on a straight-line basis over their expected useful lives, which is 25 years, and subject to impairment review at the end of each reporting period.
Right-of-Use assets
We recognize operating lease right-of-use (ROU) assets and operating lease liabilities on the balance sheet for operating leases with terms longer than 12 months. We follow the ongoing practical expedient not to recognize operating lease right-of-use assets and operating lease liabilities for short-term leases. The ROU assets are initially measured at cost and amortized using the straight-line method through the end of the lease term. The lease liabilities are initially measured at the present value of the lease payments that are not paid at the commencement date, discounted using our incremental borrowing rate.
Share-based compensation
We have equity incentive plans under which various types of equity-based awards including share options, restricted shares and restricted share unit awards may be granted to employees, non-employee directors and non-employee consultants and warrants that may be granted as compensation to non-employees.
We measure the cost of equity-settled transactions by reference to the fair value of the equity instruments at the date at which they are granted since the fair value of the goods or services received by us cannot be reliably estimated.
We recognize compensation expense for all share-based awards based on the estimated grant-date fair values. For restricted share unit awards to employees, the fair value is based on the 5-day volume weighted average price (VWAP) of our common shares up to the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period.
The fair value of share options is determined using the Black-Scholes option pricing model. We utilize a dividend yield of zero based on the fact that we have never paid cash dividends and have no current intention of paying cash dividends. We elected an accounting policy to record forfeitures as they occur. See Note 8 for a discussion of the assumptions used by us in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the share option activity under our share-based compensation plan for all years presented.
The provisions of our share-based compensation plans do not require us to settle any options or restricted share units by transferring cash or other assets, and therefore we classify the awards as equity.
Translation of foreign currency transactions
Our reporting currency is the U.S. dollar. The financial statements of our wholly owned Canadian subsidiary is measured using the Canadian dollar as the functional currency. Assets and liabilities of the Canadian operation have been translated at year-end exchange rates and related revenue and expenses have been translated at average exchange rates for the year. Accumulated gains and losses resulting from the translation of the financial statements of the Canadian operation are included as part of accumulated other comprehensive loss, a separate component of shareholders' equity.
For other transactions denominated in currencies other than our functional currency, the monetary assets and liabilities are translated at the year-end rates. Revenue and expenses are translated at rates of exchange prevailing on the transaction dates. Non-monetary balance sheet and related income statement accounts are remeasured into U.S. dollar using historical exchange rates. All of the exchange gains or losses resulting from these other transactions are recognized in the statements of operations and comprehensive loss.
Recent Accounting Pronouncements
Recent accounting pronouncements are contained in Note 3 to the financial statements, which are indexed under Item 15 of this Annual Report on Form 10-K.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are a smaller reporting company and are not required to provide disclosure under this item.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
The financial statements and related financial information required to be filed hereunder are indexed under Item 15 of this Annual Report on Form 10-K and are incorporated herein by reference.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
Not applicable.
Item 9A. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining disclosure controls and procedures to provide reasonable assurance that material information related to our Company, including our consolidated subsidiaries, is made known to senior management, including our Chief Executive Officer and the Chief Financial Officer, by others within those entities on a timely basis so that appropriate decisions can be made regarding public disclosure.
We carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer and our Principal Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of September 30, 2023. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Our Chief Executive Officer and Chief Financial Officer concluded that the disclosure controls and procedures as of September 30, 2023, were effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for designing, establishing and maintaining a system of internal controls over financial reporting (as defined in Exchange Act Rule 13a-15(f)) to provide reasonable assurance that the financial information prepared by us for external purposes is reliable and has been recorded, processed and reported in an accurate and timely manner in accordance with accounting principles generally accepted in the United States. Our board of directors is responsible for ensuring that management fulfills its responsibilities. The audit committee of our board of directors fulfills its role of ensuring the integrity of the reported information through its review of the interim and annual financial statements. Management reviewed the results of their assessment with our audit committee.
Management has used the criteria issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in “Internal Control - Integrated Framework (2013)” to evaluate the effectiveness of our internal control over financial reporting. Management has assessed the effectiveness of our internal control over financial reporting and concluded that such internal control over financial reporting was effective as of September 30, 2023.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Attestation Report of Our Registered Public Accounting Firm
Because we are a non-accelerated filer, this Annual Report does not include an attestation report from our independent registered public accounting firm. We are not required to provide an attestation report on our internal control over financial reporting until such time as we are an accelerated filer or large accelerated filer.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the year ended September 30, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. OTHER INFORMATION.
Not applicable.
Item 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors
Our directors and their ages as of the date of this filing are set forth below. Each director is elected annually to serve until the next annual meeting of shareholders, or until his or her successor is duly elected.
Name | | Age | | Position(s) Held | | Director Since |
Joan Chypyha (1) (2) | | 57 | | Director | | May 23, 2023 |
Sean MacDonald | | 47 | | Director | | June 7, 2019 |
Patrick Marshall (1) (3) | | 52 | | Director | | May 23, 2023 |
Pardeep Nijhawan, MD | | 53 | | Director, Chief Executive Officer and Corporate Secretary | | June 7, 2019 |
Frank Oakes (2) (3) | | 73 | | Director | | April 9, 2010 |
Charles Olson, DSc (2) (3) | | 66 | | Director | | May 23, 2023 |
Carlo Sistilli, CPA, CMA (1) | | 67 | | Board Chair | | June 7, 2019 |
(1) Member of Audit Committee.
(2) Member of Compensation Committee.
(3) Member of Nominating and Corporate Governance Committee.
There are no family relationships between any of our directors or executive officers.
Biographies and Qualifications
The biographies of our directors and certain information regarding each director’s experience, attributes, skills and/or qualifications that led to the conclusion that the director should be serving as a director of our Company are as follows:
Joan Chypyha has more than 30 years of experience in the pharmaceutical industry including executive and operational positions in business development, sales and marketing, and general management. She is the President of Alto Pharmaceuticals, Ltd., a specialty pharmaceutical company focused on dermatology, women's health and elder care, which she founded in 2009. Alto is also a major shareholder in Pepper and Pink Inc., a manufacturer of brand and private label personal care products for major retailers in Canada. From July 2015 to June 2017, she was the President of Cipher Pharmaceuticals, Inc., having previously served as Vice President of Marketing and Sales. Ms. Chypyha’s professional career also includes executive positions at Rhei Pharmaceuticals Ltd. and Barrier Therapeutics Canada, Inc., following sixteen years at Hoffman-La Roche, where she held progressively senior positions. Since February 2018, she has served as a director and a member of the audit committee of Ovation Science Inc., a research and development company that develops topical and transdermal consumer products. She is a past advisory board member of Up Cannabis Inc (from August 2017 to January 2019). Ms. Chypyha currently served as the President of the Canadian Dermatology Industry Association, from 2015 to 2023, and is a Co-Chair of DiTiDE (Dermatology Industry Taskforce on Inclusiveness, Diversity & Equity), a group she co-founded in 2020. She has also previously served on boards of other non-profits and business organizations, including the Canadian Healthcare Licensing Association. Ms. Chypyha earned her Bachelor’s Degree from the University of Toronto and a Master’s Degree in Business Administration from Queen's University. Ms. Chypyha’s qualifications to serve on our board of directors include her extensive operational experience in founding, managing and building companies, previous board experience and her extensive experience in the dermatology industry.
Sean MacDonald has been a member of our Board since June 2019, and served as Chairman of the Board from June 2019 until May 2023. He previously served on the board of our principal operating subsidiary, Edesa Biotech Research, from September 2017 to June 2019. In his career, he has led and closed multiple licensing transactions, financings, acquisitions and divestments, and led corporate strategy for several pharmaceutical and biotechnology companies. Mr. MacDonald is currently an advisor to investors and biotechnology companies, including Domain Therapeutics Inc. and Raya Therapeutic Inc., a role he has held since April 2022. From August 2021 to April 2022, he was the Chief Business Officer of iOnctura SA, a Swiss clinical-stage oncology company. From April 2019 to August 2021, he was the Head of Business Development for Cosmo Pharmaceuticals NV, a European gastroenterology focused pharmaceutical company; and from October 2018 to August 2021 he was the chief executive of Corbin Therapeutics, a Montreal-based biotech company focused on treating neuroinflammation. Mr. MacDonald held various operational and executive leadership roles from October 2012 to October 2018 at Pharmascience Inc., one of Canada's largest pharmaceutical companies, including Vice President of Business Development and Corporate Development. He received his BSc in Molecular Biology and MBA from the University of Ottawa. Mr. MacDonald's qualifications to serve on our board of directors include his extensive operational experience and background in the pharmaceutical/biotechnology industry.
Patrick Marshall has more than 20 years of experience raising capital, building and launching new products and services, developing strategy and completing mergers and acquisitions to support growth in private and public companies. Since 2010 he has been a managing director at VRG Capital, having previously held various executive roles in several of the firm’s portfolio companies since 2000, including Wheels Group Inc., a North American third-party logistics company acquired by Radiant Logistics, Inc. in 2015, and Thomas International Ltd., a global provider of psychometric and aptitude tests acquired by Palamon Capital Partners in 2018. Mr. Marshall currently serves as President and board member of Adrem Brands Inc., a privately held Canadian company focused on the over-the-counter and nutritional supplements markets, a position he has held since January 2016. Prior to 2000, he held fundraising, business development and strategy roles for various international enterprises and non-governmental organizations. Mr. Marshall is a cofounder and board member of Together Project (since 2016) and a trustee of Lakefield College School (since 2012). He received his Bachelor of Arts in Sociology from Queen's University and Master of Business Administration from the University of Exeter. Mr. Marshall’s qualifications to serve on our board of directors include his experience managing and building companies, strategic transactions, raising capital and prior board experience.
Pardeep Nijhawan, MD, FRCPC, AGAF has served as our Chief Executive Officer, Corporate Secretary and a member of our board of directors since June 2019, having previously founded and led our principal operating subsidiary, Edesa Biotech Research, since January 2015. Dr. Nijhawan is a seasoned pharmaceutical entrepreneur with more than 20 years of experience in cross-functional leadership roles in finance, marketing, corporate strategy and business development. In 2002 Dr. Nijhawan founded Medical Futures Inc., and served as its CEO. He sold Medical Futures to Tribute Pharmaceuticals in 2015. In 2014, he founded Exzell Pharma, a specialty Canadian-based pharmaceutical organization that markets and commercializes approved products. He sold Exzell Pharma to BioLab Pharma in 2022. Dr. Nijhawan also founded Digestive Health Clinic in 2000 and led it to become one of Canada’s largest provider of private endoscopy services. He continues to serve on the Board of Digestive Health Clinic. Since January 2021, he has served on the advisory board of Private Debt Partners, a Canadian alternative asset management firm. Dr. Nijhawan received his MD from the University of Ottawa and completed his internship at Yale University, and his internal medicine residency and fellowship at the Mayo Clinic. Dr. Nijhawan’s qualifications to serve on our board of directors include his extensive executive leadership and experience in the life sciences industry and his knowledge of our business as its chief executive.
Frank Oakes has more than 40 years of executive leadership experience. He has been a member of our board of directors since April 2010 and served as the Chairman of the Board until June 2019. From 1999 to 2019, he also served as the President and Chief Executive Officer of our legacy operating subsidiary, which he founded. Prior to founding Stellar Biotechnologies, Inc., he was the Chief Executive Officer of The Abalone Farm, Inc., where he led the company through the research and development, capitalization, and commercialization phases of development to become the largest abalone producer in the United States at the time. Mr. Oakes has consulted and lectured around the world. He received his BS degree from California State Polytechnic University, San Luis Obispo and is a graduate of the Los Angeles Regional Technology Alliance University’s management program. Mr. Oakes qualifications to serve on our board of directors include his extensive operational experience building companies and management teams and leading a U.S. and Canadian publicly listed life science company.
Charles Olson, DSc is a CMC consultant with more than 40 years of biotech experience. From September 2021 to April 2023 he was Chief Operating Officer at Dendreon Corporation, where he was responsible for the commercial manufacturing of Provenge, a commercial cell-based product for prostate cancer, overseeing multiple sites and several hundred employees. From September 2017 to August 2021, he was a senior Vice President of Operations at Applied Molecular Transport. From April 2010 to August 2017, Dr. Olson held various leadership roles at Anthera Pharmaceuticals Inc., including Chief Technology Officer. He has also been a Principal Biotechnology Consultant for Compass Biotechnology LLC since 2006. Dr. Olson previously held senior and executive management positions at NGM Biopharmaceuticals Inc., Coherus BioSciences Inc., Nexbio Inc., Cell Genesys, Inc., Biomarin Pharmaceuticals, Inc, and Onyx Pharmaceuticals, Inc. From December 2016 to June 2019, Dr. Olson served on the board of directors of Edesa Biotech, Inc. (then operating as Stellar Biotechnologies, Inc.), having previously served on Stellar’s scientific advisory board. After graduate school, Dr. Olson was a Research Scientist at Kaiser Hospitals, followed by Scientist and Senior Scientist positions at Genentech and Bayer, respectively. He holds a B.A. in biology and chemistry from Westmont College, an M.A. in chemistry from the University of California at Santa Barbara and a D.Sc. in biochemistry. Dr. Olson qualifications to serve on our board of directors include his extensive scientific, manufacturing operations, process development and senior management and board experience in the biopharmaceutical industry.
Carlo Sistilli, CPA, CMA has more than 35 years of financial experience and has held a variety of executive positions in accounting and finance during his career. He has been a member of our board of directors since June 2019, having previously served as a board observer of our principal operating subsidiary, Edesa Biotech Research, since September 2017. Mr. Sistilli has served as the Chief Financial Officer of Arista Homes since March 2003 to present. Prior to Arista, Mr. Sistilli was a founder and served as CFO and a board member of an Internet start-up company in the automotive sector, and played a key role in taking the company public on the Alberta Ventures Exchange. Earlier in his career, Mr. Sistilli was the Controller and a member of the senior management team of a major regional trust company, which Mr. Sistilli helped sell to Manulife Financial. Since January 2021, he has served on the board of directors and audit committee of Aleafia Health Inc. In addition to his professional career, Mr. Sistilli is an officer and a member of the board of directors of Mother of Mercy Centre. Mr. Sistilli holds a Bachelor of Arts from York University, with a major in economics, Certified Management Accountant Designation and a Chartered Professional Accountant Designation. Mr. Sistilli’s qualifications to serve on our board of directors include his knowledge of Edesa’s business and his background in accounting and finance.
Executive Officers
Set forth below is certain information with respect to the names, ages, and positions of our executive officers as of the date of this filing. Biographical information pertaining to Dr. Nijhawan, who is a director and an executive officer, may be found in the above section entitled “Directors.” The executive officers serve at the pleasure of our Board of Directors.
Name | | Age | | Position(s) Held | | Date of Appointment |
Pardeep Nijhawan, MD | | 53 | | Director, Chief Executive Officer and Corporate Secretary | | June 7, 2019 |
Stephen Lemieux, CPA | | 48 | | Chief Financial Officer | | July 15, 2023 |
Michael Brooks, PhD | | 45 | | President | | June 7, 2019 |
Stephen Lemieux, CPA was appointed Chief Financial Officer in July 2023. He is a veteran of the healthcare and biopharmaceutical sectors, with more than 20 years of experience in financial planning and analysis, licensing and mergers & acquisitions. Prior to joining the Company, Mr. Lemieux held senior financial leadership positions at healthcare and biopharmaceutical biotechnology companies, where he guided financial strategies, optimized capital structures and supported significant corporate transactions and sales growth. From July 2021 until June 2023, Mr. Lemieux served as CFO of Titan Medical Inc., and from April 2019 to July 2021 as CFO and Secretary of NeuPath Health. Previously, he was the CFO and Secretary of Cipher Pharmaceuticals (TSX: CPH) from September 2016 to March 2019 and during his tenure served as Interim-CEO from November 2016 to April 2017. Prior to Cipher, he was CFO at Nuvo Pharmaceuticals and Crescita Therapeutics. Mr. Lemieux is a Chartered Professional Accountant and holds a Master of Management & Professional Accounting degree from the University of Toronto.
Michael Brooks, PhD was appointed President of Edesa in June 2019, having served as Vice President of Corporate Development and Strategy for our principal operating subsidiary, Edesa Biotech Research, since January 2015. Prior to joining Edesa, Dr. Brooks held positions of increasing responsibility at Cipher Pharmaceuticals Inc from 2010 to 2015 and served most recently as the company’s Director of Business Development. Prior to joining Cipher, Dr. Brooks was a Postdoctoral fellow at the University of Toronto. Dr. Brooks holds a Hons B.Sc. degree in Microbiology and a PhD in Molecular Genetics from the University of Toronto. Dr. Brooks received his MBA degree from the Rotman School of Management where he was a Canadian Institute for Health Research (CIHR) Science-to- Business Scholar.
Code of Ethics
We have adopted a Code of Ethics and Business Conduct that applies to all of our directors, officers, and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A copy of our Code of Ethics and Business Conduct is available on the Investor Relations section of our website at edesabiotech.com/investors/governance, in the Corporate Governance section, under the Governance Documents section. We intend to satisfy the SEC’s disclosure requirements regarding amendments to, or waivers of, our Code of Ethics and Business Conduct that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions by posting such information on our website identified above. Copies of our Code of Ethics and Business Conduct may be obtained, free of charge, by writing to our Corporate Secretary, Edesa Biotech, Inc., 100 Spy Court, Markham, ON Canada L3R 5H6.
Information about our Board Committees
Our Board of Directors has appointed an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. The Board of Directors has determined that each director who serves on these committees is “independent,” as that term is defined by the listing rules of Nasdaq and rules of the SEC. The Board of Directors has adopted written charters for its Audit Committee, Compensation Committee, and Nominating and Corporate Governance Committee. Copies of these charters are available on our website at www.edesabiotech.com/investors/governance.
Audit Committee
Our Audit Committee is composed of Joan Chypyha, Patrick Marshall and Carlo Sistilli (chair). The purpose of the Audit Committee is to oversee our accounting and financial reporting processes and the audits of our financial statements. In that regard, the Audit Committee assists the Board in monitoring: (a) the integrity of our financial statements; (b) our independent auditor’s qualifications, independence, and performance; (c) the performance of our system of internal controls, financial reporting, and disclosure controls; and (d) our compliance with legal and regulatory requirements. To fulfill this obligation and perform its duties, the Audit Committee maintains effective working relationships with the Board, management, and our independent auditor.
Carlo Sistilli is the Chair of our Audit Committee and has extensive financial experience. He holds a Bachelor of Arts from York University, with a major in economics, Certified Management Accountant Designation and a Chartered Professional Accountant Designation. He has held a variety of executive positions in accounting and finance during the past 35 years. The Board has determined that Mr. Sistilli is an “audit committee financial expert” as defined in Item 407(d)(5)(ii) of Regulation S-K.
Compensation Committee
Our Compensation Committee is composed of Joan Chypyha (chair), Frank Oakes and Charles Olson. The purpose of the Compensation Committee is to assist the Board’s oversight relating to compensation of our Chief Executive Officer and our other Named Executive Officers. It has responsibility for evaluating and recommending to the independent members of the Board for approval, our compensation plans, policies and programs as such plans, policies and programs affect executive officers.
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee is composed of Patrick Marshall, Frank Oakes (chair) and Charles Olson. The purpose of the Nominating and Corporate Governance Committee is to identify individuals qualified to become Board members; recommend to the Board individuals to serve as directors; advise the Board with respect to Board composition, procedures and committees; lead the Board in its annual review of the Board and management’s performance; develop, recommend to the Board and annually review a set of corporate governance principles applicable to the Company; and oversee any related matters required by the federal securities laws.
Item 11. EXECUTIVE COMPENSATION.
Executive Compensation
Our named executive officers for the year ended September 30, 2023 were Pardeep Nijhawan, MD, Director, Chief Executive Officer and Corporate Secretary; Stephen Lemieux, CPA, Chief Financial Officer; Michael Brooks, PhD, President and Kathi Niffenegger, CPA, Former Chief Financial Officer.
Summary Compensation Table
The following table sets forth information regarding the compensation awarded to, earned by or paid to the named executive officers for the years ended September 30, 2023 and September 30, 2022.
Name and Principal Position | | Fiscal Year | | Salary ($) | | | Bonus ($) | | | Stock Awards ($) (1) | | | Options Awards ($) (1) | | | All Other Compensation ($) | | | Total ($) | |
Pardeep Nijhawan, MD | | 2023 | | $ | 341,391 | | | $ | 99,340 | (2) | | $ | - | | | $ | 74,229 | | | $ | 34,601 | (3) | | $ | 549,561 | |
Director, Chief Executive Officer and Corporate Secretary | | 2022 | | | 325,861 | | | | 89,600 | | | | - | | | | 123,152 | | | | 63,118 | (3) | | | 601,731 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Stephen Lemieux, CPA | | 2023 | | | 82,500 | | | | - | | | | 13,922 | (4) | | | 46,396 | | | | 13,126 | (5) | | | 155,944 | |
Chief Financial Officer | | 2022 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Michael Brooks, PhD | | 2023 | | | 320,054 | | | | 93,160 | (6) | | | - | | | | 49,488 | | | | 26,060 | (7) | | | 488,762 | |
President | | 2022 | | | 305,495 | | | | 96,000 | | | | - | | | | 109,329 | | | | 41,908 | (7) | | | 552,731 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Kathi Niffenegger, CPA (8) | | 2023 | | | 253,391 | | | | 102,080 | (9) | | | - | | | | 49,488 | | | | 48,414 | (10) | | | 453,373 | |
Former Chief Financial Officer | | 2022 | | | 295,075 | | | | 87,000 | | | | - | | | | 109,329 | | | | 50,546 | (10) | | | 541,950 | |
| (1) | The amounts shown in these columns represent the aggregate grant date fair value of the restricted share units and share option awards computed in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification 718, not the actual amounts paid to or realized by the named executive officers during the covered fiscal year. The assumptions used in determining grant date fair value of these awards are set forth in Note 8 to our audited consolidated financial statements for the year ended September 30, 2023 included in this Annual Report. |
| (2) | Includes 14,186 restricted share units with an aggregate grant date fair value of $79,440 issued as partial payment of the bonus with the balance paid in cash. |
| (3) | Represents (i) $32,415 in car allowance and (ii) $2,186 in health insurance in 2023. Represents (i) $32,415 in car allowance, (ii) $2,186 in health insurance and (iii) $28,517 in vacation payout in 2022. All compensation to Dr. Nijhawan was paid in Canadian dollars and was converted from US dollars using the average foreign exchange rate for each bi-weekly pay period of the year from oanada.com. |
| (4) | Represents the aggregate grant date fair value of 2,486 restricted share units issued as partial payment on consulting invoices for services prior to Mr. Lemieux’s appointment as Chief Financial Officer. |
| (5) | Represents (i) $5,000 in car allowance and (ii) $8,126 in consulting fees for services prior to Mr. Lemieux’s appointment as Chief Financial Officer in 2023. All compensation to Mr. Lemieux was paid in Canadian dollars and was converted from US dollars using the average foreign exchange rate for the year from oanda.com. |
| (6) | Includes 13,315 restricted share units with an aggregate grant date fair value of $74,560 as partial payment of the bonus with the balance paid in cash. |
| (7) | Represents (i) $24,000 in car allowance and (ii) $2,060 in health insurance in 2023. Represents (i) $24,000 in car allowance (ii) $2,788 in health insurance and (iii) $15,120 in vacation payout in 2022. All compensation to Dr. Brooks was paid in Canadian dollars and was converted from US dollars using the average foreign exchange rate for each bi-weekly pay period of the year from oanda.com. |
| (8) | Ms. Niffenegger served as Chief Financial Officer through July 15, 2023. |
| (9) | Includes 10,943 restricted share units with an aggregate grant date fair value of $61,280 as partial payment of the bonus with the balance paid in cash. |
| (10) | Represents (i) $21,273 in health insurance, (ii) $8,167 in 401(k) Company contributions and (iii) $18,974 in non-executive salary following Ms. Niffenegger’s tenure as Chief Financial Officer in 2023. Represents (i) $19,751 in health insurance, (ii) $9,150 in 401(k) Company contributions and (iii) $21,645 in vacation pay out in 2022. |
Narrative Disclosure to Summary Compensation Table Employment Agreements
Amended and Restated Employment Agreement with Pardeep Nijhawan effective as of August 4, 2023, as amended December 7, 2023
On August 4, 2023, the Company entered into an amended and restated employment agreement with Pardeep Nijhawan, the Company’s Chief Executive Officer that superseded prior employment agreements (as amended, the Nijhawan Employment Agreement).
Pursuant to the Nijhawan Employment Agreement, Dr. Nijhawan serves as the Company’s Chief Executive Officer as well as Chief Executive Officer of each of the Company’s subsidiaries, Edesa Biotech Research and Edesa Biotech USA, Inc. and a director of Edesa Biotech Research. Dr. Nijhawan’s employment will continue for an indefinite term until terminated in accordance with the Nijhawan Employment Agreement.
Pursuant to the Nijhawan Employment Agreement, Dr. Nijhawan is entitled to a base salary of $357,700 per year effective May 13, 2023 and is eligible to receive a target annual bonus of 40% of his base salary, subject to the achievement of corporate and personal targets as determined by the Company and the Board of Directors. Dr. Nijhawan’s base salary is subject to annual review by the Board of Directors. Dr. Nijhawan is also entitled to an automobile allowance of $2,701.50 per month and is eligible to participate in the Company’s group insured benefits program, as may be in effect from time-to-time for employees generally, and executive employees specifically. Dr. Nijhawan is eligible for equity-based awards pursuant to the Company’s Equity Incentive Compensation Plan, as determined by the Board of Directors or Compensation Committee, commensurate with Dr. Nijhawan’s position and any business milestones that may be established by the Company.
If Dr. Nijhawan’s employment is terminated for “Cause” (as such term is defined in the Nijhawan Employment Agreement), subject to applicable law, Dr. Nijhawan is entitled to his base salary and vacation pay earned through the date of termination, and all of Dr. Nijhawan’s non-vested equity-based awards will be automatically extinguished. All vested equity-based awards shall be subject to the terms of the Company’s Equity Incentive Compensation Plan.
If Dr. Nijhawan is terminated without “Cause”, subject to Dr. Nijhawan signing a general release of claims, Dr. Nijhawan is entitled to: (i) a lump sum payment equal to Dr. Nijhawan’s then current base salary for 12 months plus one additional month for every completed year of service since August 1, 2017 (the Nijhawan Severance Period) which shall not exceed 24 months, inclusive of, and not in addition to, his notice and severance entitlements, if any, pursuant to applicable law, (ii) a lump sum payment of the annual bonus to which Dr. Nijhawan is entitled for the calendar year immediately preceding the date of termination, if such bonus has not already been paid, (iii) a lump sum payment equal to Dr. Nijhawan’s annual bonus entitlement, prorated over Dr. Nijhawan’s length of service in the calendar year in which his employment is terminated, calculated in accordance with the terms of the Nijhawan Employment Agreement, (iv) payment of Dr. Nijhawan’s annual bonus entitlement during the full Nijhawan Severance Period, calculated in accordance with the terms of the Nijhawan Employment Agreement, (v) continuation of Dr. Nijhawan’s benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Nijhawan Employment Agreement, and (vi) subject to applicable law, all vested equity-based awards granted to Dr. Nijhawan shall be exercisable in accordance with the terms of the applicable Equity Incentive Compensation Plan.
In the event that Dr. Nijhawan is terminated or constructively terminated, which includes a material change in Dr. Nijhawan’s title, responsibilities, authority or status or a material reduction of his compensation, without “Cause” upon or within a 12-month period following a “Change of Control” (as such term is defined in the Nijhawan Employment Agreement), Dr. Nijhawan is entitled to (i) a change of control payment equal to 24 months of the value of Dr. Nijhawan’s then current base salary as of the date of termination, (ii) a lump sum payment of the annual bonus to which Dr. Nijhawan is entitled for the calendar year immediately preceding the date of termination, if such bonus has not already been paid, (iii) a lump sum payment equal to Dr. Nijhawan’s annual bonus entitlement, prorated over Dr. Nijhawan’s length of service in the calendar year in which his employment is terminated, calculated in accordance with the terms of the Nijhawan Employment Agreement, (iv) payment of Dr. Nijhawan’s annual bonus entitlement during the full Nijhawan Severance Period, calculated in accordance with the terms of the Nijhawan Employment Agreement, (v) continuation of Dr. Nijhawan’s benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Nijhawan Employment Agreement, and (vi) subject to applicable law, all vested equity-based awards granted to Dr. Nijhawan shall be exercisable in accordance with the terms of the applicable Equity Incentive Compensation Plan.
Dr. Nijhawan may resign from his employment at any time by providing the Company with a minimum of 60 days advance notice, in writing. Dr. Nijhawan’s notice may be waived by the Company, subject only to providing Dr. Nijhawan with payment of his base salary and continuation of benefits until the end of the notice period. If Dr. Nijhawan resigns from his employment, subject to applicable law, (i) all non-vested equity based awards held by Dr. Nijhawan shall be automatically extinguished and (ii) Dr. Nijhawan shall not be entitled to any bonus or pro rata bonus payment not already awarded on or before the date of termination. All vested equity-based awards shall be subject to the terms of the applicable Equity Incentive Compensation Plan.
During the term of Dr. Nijhawan’s employment and for 12 months following the cessation of Dr. Nijhawan’s employment, Dr. Nijhawan is prohibited from competing with the business of the Company in North America. In addition, for 24 months following the cessation of Dr. Nijhawan’s employment, Dr. Nijhawan is prohibited from soliciting customers or prospective customers for any purpose competitive with the business of the Company, encouraging any customer to cease doing business with the Company and soliciting the employment or engagement of certain of Company’s employees.
Prior Employment Agreement with Pardeep Nijhawan effective as of June 7, 2019 and amended March 19, 2021 and April 12, 2022
Prior to the Nijhawan Employment Agreement, on June 14, 2019 but effective as of June 7, 2019, we entered into an employment agreement with Pardeep Nijhawan (as amended, the Nijhawan Prior Employment Agreement). Pursuant to the Nijhawan Prior Employment Agreement, Dr. Nijhawan agreed to serve as our Chief Executive Officer for an indefinite term until Dr. Nijhawan’s employment was terminated in accordance with the agreement. As compensation for his services to us, Dr. Nijhawan received a base salary of $320,000 per year effective for the period January 1, 2021 to March 23, 2022 and a base salary of $331,200 per year effective for the period March 24, 2022 to May 12, 2023 and was eligible to receive a target annual bonus of 40% of his base salary, subject to achieving corporate and personal targets to be determined by us. Dr. Nijhawan also received an automobile allowance of approximately $2,700 per month and was eligible to participate in our group insured benefits program, as may be in effect from time-to-time for our employees generally, and executive employees specifically. Dr. Nijhawan was eligible for future share and/or option grants, as may have been determined by our Compensation Committee, commensurate with Dr. Nijhawan’s position and any business milestones which may have been established by the Compensation Committee and subject to availability of shares and/or options for grant under our Equity Incentive Compensation Plan.
Under the Nijhawan Prior Employment Agreement, if Dr. Nijhawan’s employment with us was terminated for “Cause” (as such term is defined in the Nijhawan Prior Employment Agreement), subject to applicable law, our only obligation would have been to provide Dr. Nijhawan with his base salary and vacation pay earned through the date of termination and all of Dr. Nijhawan’s vested or non-vested share options which had not been exercised by Dr. Nijhawan as of the date of termination would have been automatically extinguished. If Dr. Nijhawan was terminated by us without “Cause”, our obligation would have been to provide Dr. Nijhawan with (i) a lump sum payment equal to Dr. Nijhawan’s then current base salary for twenty-four months (the Nijhawan Prior Severance Period), (ii) a lump sum payment of the annual bonus to which Dr. Nijhawan would have been entitled for the fiscal year immediately preceding the date of termination, if such bonus had not already been paid, (iii) a lump sum payment equal to Dr. Nijhawan’s annual bonus entitlement, prorated over Dr. Nijhawan’s length of service in the fiscal year in which his employment was terminated, calculated in accordance with the terms of the Nijhawan Prior Employment Agreement, (iv) payment of Dr. Nijhawan’s annual bonus entitlement during the full Nijhawan Prior Severance Period, calculated in accordance with the terms of the Nijhawan Prior Employment Agreement, (v) continuation of Dr. Nijhawan’s benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Nijhawan Prior Employment Agreement and (vi) subject to applicable law, all share options granted to Dr. Nijhawan would have been exercisable in accordance with the terms of the applicable share option plan. Dr. Nijhawan could have resigned from his employment at any time by providing us with a minimum of sixty days advance notice, in writing. Dr. Nijhawan’s notice could have been waived by us, subject only to providing Dr. Nijhawan with payment of his base salary and continuation of benefits until the end of the notice period. If Dr. Nijhawan had resigned from his employment, subject to applicable law, (i) all non-vested share options and all vested share options held by Dr. Nijhawan which had not been exercised by Dr. Nijhawan as of the date of termination would have been automatically extinguished and (ii) Dr. Nijhawan would not have been entitled to any bonus or pro rata bonus payment not already paid on or before the date of termination.
During the term of Dr. Nijhawan’s employment with us and for twelve months following the cessation of Dr. Nijhawan’s employment with us, Dr. Nijhawan was prohibited from competing with our business in North America. In addition, for twenty-four months following the cessation of Dr. Nijhawan’s employment with us, Dr. Nijhawan was prohibited from soliciting customers or prospective customers for any purpose competitive with our business, encouraging any customer to cease doing business with us and soliciting the employment or engagement of certain of our employees.
Employment Agreement with Stephen Lemieux effective as of July 15, 2023
On June 26, 2023 but effective as of July 15, 2023, we entered into an employment agreement with Stephen Lemieux (the Lemieux Employment Agreement). Pursuant to the Lemieux Employment Agreement, Mr. Lemieux will serve as our Chief Financial Officer for an indefinite term until Mr. Lemieux’s employment is terminated in accordance with the agreement. As compensation for his services to us, Mr. Lemieux will receive a base salary of $330,000 per year and is eligible to receive a target annual bonus of 40% of his base salary, subject to achieving corporate and personal targets to be determined by us. Mr. Lemieux also receives an automobile allowance of $2,000 per month and is eligible to participate in our group insured benefits program, as may be in effect from time-to-time for our employees generally, and executive employees specifically. Mr. Lemieux is eligible for future equity-based awards, as determined by our Compensation Committee, commensurate with Mr. Lemieux’s position and any business milestones which may be established by the Compensation Committee and subject to availability of shares and/or options for grant under our Equity Incentive Compensation Plan.
If Mr. Lemieux’s employment with the Company is terminated for “Cause” (as such term is defined in the Lemieux Employment Agreement), subject to applicable law, the Company’s only obligation shall be to provide Mr. Lemieux with his base salary and vacation pay earned through the date of termination and all of Mr. Lemieux’s non-vested equity-based awards as of the date of termination will be automatically extinguished. All vested equity-based awards will be subject to the terms of the applicable equity incentive compensation plan. If Mr. Lemieux is terminated by the Company without “Cause”, subject to Mr. Lemieux executing a general release of claims in a form reasonably required by the Company, the Company’s obligation shall be to provide Mr. Lemieux with (i) a lump sum payment equal to Mr. Lemieux’s then current base salary for twelve months plus one additional month for every completed year of service since July 15, 2023, not to exceed an aggregate of twenty- four months (the Lemieux Severance Period), (ii) a lump sum payment of the annual bonus to which Mr. Lemieux is entitled for the calendar year immediately preceding the date of termination, if such bonus has not already been paid, (iii) a lump sum payment equal to Mr. Lemieux’s annual bonus entitlement, prorated over Mr. Lemieux’s length of service in the calendar year in which his employment is terminated, calculated in accordance with the terms of the Lemieux Employment Agreement, (iv) payment of Mr. Lemieux’s annual bonus entitlement during the full Lemieux Severance Period, calculated in accordance with the terms of the Lemieux Employment Agreement, (v) continuation of Mr. Lemieux’s benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Lemieux Employment Agreement and (vi) subject to applicable law, any and all vested equity-based awards shall be exercisable in accordance with the terms of the applicable equity incentive compensation plan. If Mr. Lemieux’s employment is terminated or “constructively terminated” (as such term is defined in the Lemieux Employment Agreement) by the Company without “Cause” upon or within a twelve month period following a Change of Control (as such term is defined in the Lemieux Employment Agreement), Mr. Lemieux shall be entitled to the payments and benefits provided as described in clauses (ii) to (v) above, plus a change of control payment equal to twenty-four months of his then current base salary. Mr. Lemieux may resign from his employment at any time by providing the Company with a minimum of sixty days advance notice, in writing. Mr. Lemieux’s notice may be waived by the Company, subject only to providing Mr. Lemieux with payment of his base salary and continuation of benefits until the end of the notice period. If Mr. Lemieux resigns from his employment, subject to applicable law, (i) all non-vested equity-based awards held by Mr. Lemieux as of the date of termination shall be automatically extinguished and all vested equity-based awards will be subject to the terms of the applicable equity incentive compensation plan and (ii) Mr. Lemieux shall not be entitled to any bonus or pro rata bonus payment not already awarded on or before the date of termination.
During the term of Mr. Lemieux’s employment with us and for twelve months following the cessation of Mr. Lemieux’s employment with us, Mr. Lemieux is prohibited from competing with our business in North America. In addition, for twenty-four months following the cessation of Mr. Lemieux’s employment with us, Mr. Lemieux is prohibited from soliciting customers or prospective customers for any purpose competitive with our business, encouraging any customer to cease doing business with us and soliciting the employment or engagement of certain of our employees.
Prior Consulting Agreement with Stephen Lemieux effective December 22, 2022, terminated July 15, 2023
Prior to the Lemieux Employment Agreement, on December 21, 2022, the Company entered into a consulting agreement with Stephen Lemieux to provide, as an independent contractor, advice and services related to finance, accounting, financial reporting, financial planning and analysis and similar services as requested by the Company from time to time at a rate of C$135 per hour. The agreement contained customary confidentiality, nondisclosure and proprietary information provisions. The consulting agreement was terminated upon Mr. Lemieux’s appointment as Chief Financial Officer.
Amended and Restated Employment Agreement with Michael Brooks effective as of August 4, 2023
On August 4, 2023, the Company entered into an amended and restated employment agreement with Michael Brooks, the Company’s President, that superseded prior employment agreements (the Brooks Employment Agreement).
Pursuant to the Brooks Employment Agreement, Dr. Brooks serves as the Company’s President as well as President and a director of the Company’s subsidiary, Edesa Biotech Research. Dr. Brooks’ employment will continue for an indefinite term until terminated in accordance with the Brooks Employment Agreement.
Pursuant to the Brooks Employment Agreement, Dr. Brooks is entitled to a base salary of $335,340 per year effective May 13, 2023 and is eligible to receive a target annual bonus of 40% of his base salary, subject to the achievement of corporate and personal targets as determined by the Company and the Board of Directors. Dr. Brooks’ base salary is subject to annual review by the Board of Directors. Dr. Brooks is also entitled to an automobile allowance of $2,000 per month and is eligible to participate in the Company’s group insured benefits program, as may be in effect from time-to-time for employees generally, and executive employees specifically. Dr. Brooks is eligible for equity-based awards pursuant to the Company’s Equity Incentive Compensation Plan, as determined by the Board of Directors or Compensation Committee, commensurate with Dr. Brooks’ position and any business milestones that may be established by the Company.
If Dr. Brooks’ employment is terminated for “Cause” (as such term is defined in the Brooks Employment Agreement), subject to applicable law, Dr. Brooks is entitled to his base salary and vacation pay earned through the date of termination, and all of Dr. Brooks’ non-vested equity-based awards will be automatically extinguished. All vested equity-based awards shall be subject to the terms of the Company’s Equity Incentive Compensation Plan.
If Dr. Brooks is terminated without “Cause”, subject to Dr. Brooks signing a general release of claims, Dr. Brooks is entitled to: (i) a lump sum payment equal to Dr. Brooks’ then current base salary for 12 months plus one additional month for every completed year of service since September 1, 2015 (the Brooks Severance Period) which shall not exceed 24 months, inclusive of, and not in addition to, his notice and severance entitlements, if any, pursuant to applicable law, (ii) a lump sum payment of the annual bonus to which Dr. Brooks is entitled for the calendar year immediately preceding the date of termination, if such bonus has not already been paid, (iii) a lump sum payment equal to Dr. Brooks’ annual bonus entitlement, prorated over Dr. Brooks’ length of service in the calendar year in which his employment is terminated, calculated in accordance with the terms of the Brooks Employment Agreement, (iv) payment of Dr. Brooks’ annual bonus entitlement during the full Brooks Severance Period, calculated in accordance with the terms of the Brooks Employment Agreement, (v) continuation of Dr. Brooks’ benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Brooks Employment Agreement, and (vi) subject to applicable law, all vested equity-based awards granted to Dr. Brooks shall be exercisable in accordance with the terms of the applicable Equity Incentive Compensation Plan.
In the event that Dr. Brooks is terminated or constructively terminated, which includes a material change in Dr. Brooks’ title, responsibilities, authority or status or a material reduction of the Employee’s compensation, without cause upon or within a 12-month period following a “Change of Control” (as such term is defined in the Brooks Employment Agreement), Dr. Brooks is entitled to (i) a change of control payment equal to 24 months of the value of Dr. Brooks’ then current base salary as of the date of termination, (ii) a lump sum payment of the annual bonus to which Dr. Brooks is entitled for the calendar year immediately preceding the date of termination, if such bonus has not already been paid, (iii) a lump sum payment equal to Dr. Brooks’ annual bonus entitlement, prorated over Dr. Brooks’ length of service in the calendar year in which his employment is terminated, calculated in accordance with the terms of the Brooks Employment Agreement, (iv) payment of Dr. Brooks’ annual bonus entitlement during the full Brooks Severance Period, calculated in accordance with the terms of the Brooks Employment Agreement, (v) continuation of Dr. Brooks’ benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Brooks Employment Agreement, and (vi) subject to applicable law, all vested equity-based awards granted to Dr. Brooks shall be exercisable in accordance with the terms of the applicable Equity Incentive Compensation Plan.
Dr. Brooks may resign from his employment at any time by providing the Company with a minimum of 60 days advance notice, in writing. Dr. Brooks’ notice may be waived by the Company, subject only to providing Dr. Brooks with payment of his base salary and continuation of benefits until the end of the notice period. If Dr. Brooks resigns from his employment, subject to applicable law, (i) all non-vested equity based awards held by Dr. Brooks shall be automatically extinguished and (ii) Dr. Brooks shall not be entitled to any bonus or pro rata bonus payment not already awarded on or before the date of termination. All vested equity-based awards shall be subject to the terms of the applicable Equity Incentive Compensation Plan.
During the term of Dr. Brooks’ employment and for 12 months following the cessation of Dr. Brooks’ employment, Dr. Brooks is prohibited from competing with the business of the Company in North America. In addition, for 24 months following the cessation of Dr. Brooks’ employment, Dr. Brooks is prohibited from soliciting customers or prospective customers for any purpose competitive with the business of the Company, encouraging any customer to cease doing business with the Company and soliciting the employment or engagement of certain of Company’s employees.
Prior Employment Agreement with Michael Brooks effective as of June 7, 2019 and amended March 19, 2021 and April 12, 2022
Prior to the Brooks Employment Agreement, on June 14, 2019 but effective as of June 7, 2019, we entered into an employment agreement with Michael Brooks, PhD (as amended, the Brooks Prior Employment Agreement). Pursuant to the Brooks Prior Employment Agreement, Dr. Brooks agreed to serve as our President for an indefinite term until Dr. Brooks’ employment was terminated in accordance with the agreement. As compensation for his services to us, Dr. Brooks received a base salary of $300,000 per year effective for the period January 1, 2021 to March 23, 2022 and a base salary of $310,500 per year effective for the period March 24, 2022 to May 12, 2023 and was eligible to receive a target annual bonus of 40% of his base salary, subject to achieving corporate and personal targets to be determined by us. Dr. Brooks also received an automobile allowance of $2,000 per month and was eligible to participate in our group insured benefits program, as may be in effect from time-to-time for our employees generally, and executive employees specifically. Dr. Brooks was eligible for future share and/or option grants, as may have been determined by our Compensation Committee, commensurate with Dr. Brooks’ position and any business milestones which may have been established by the Compensation Committee and subject to availability of shares and/or options for grant under our Equity Incentive Compensation Plan.
If Dr. Brooks’ employment with us was terminated for “Cause” (as such term is defined in the Brooks Prior Employment Agreement), subject to applicable law, our only obligation would have been to provide Dr. Brooks with his base salary and vacation pay earned through the date of termination and all of Dr. Brooks’ vested or non-vested share options which had not been exercised by Dr. Brooks as of the date of termination would have been automatically extinguished. If Dr. Brooks was terminated by us without “Cause”, our obligation would have been to provide Dr. Brooks with (i) a lump sum payment equal to Dr. Brooks’ then current base salary for months plus one additional month for every completed year of service since September 2015, not to exceed an aggregate of twenty-four months (the Brooks Prior Severance Period), (ii) a lump sum payment of the annual bonus to which Dr. Brooks was entitled for the fiscal year immediately preceding the date of termination, if such bonus had not already been paid, (iii) a lump sum payment equal to Dr. Brooks’ annual bonus entitlement, prorated over Dr. Brooks’ length of service in the fiscal year in which his employment was terminated, calculated in accordance with the terms of the Brooks Prior Employment Agreement, (iv) payment of Dr. Brooks’ annual bonus entitlement during the full Brooks Prior Severance Period, calculated in accordance with the terms of the Brooks Prior Employment Agreement, (v) continuation of Dr. Brooks’ benefits and car allowance and any other benefit required to be maintained by law in accordance with the terms of the Brooks Prior Employment Agreement and (vi) subject to applicable law, all share options granted to Dr. Brooks would have been exercisable in accordance with the terms of the applicable share option plan. If Dr. Brooks’ employment was terminated or “constructively terminated” (as such term is defined in the Brooks Prior Employment Agreement) by us without “Cause” upon or within a twelve month period following a Change of Control (as such term is defined in the Brooks Prior Employment Agreement), Dr. Brooks would have been entitled to the payments and benefits provided as described in clauses (ii) to (v) above, plus a change of control payment equal to twenty-four months of the his then current base salary. Dr. Brooks could have resigned from his employment at any time by providing us with a minimum of sixty days advance notice, in writing. Dr. Brooks’ notice could have been waived by us, subject only to providing Dr. Brooks with payment of his base salary and continuation of benefits until the end of the notice period. If Dr. Brooks had resigned from his employment, subject to applicable law, (i) all non-vested share options and all vested share options held by Dr. Brooks which had not been exercised by Dr. Brooks as of the date of termination would have been automatically extinguished and (ii) Dr. Brooks would not have been entitled to any bonus or pro rata bonus payment not already paid on or before the date of termination.
During the term of Dr. Brooks’ employment with us and for twelve months following the cessation of Dr. Brooks' employment with us, Dr. Brooks was prohibited from competing with our business in North America. In addition, for twenty-four months following the cessation of Dr. Brooks' employment with us, Dr. Brooks was prohibited from soliciting customers or prospective customers for any purpose competitive with our business, encouraging any customer to cease doing business with us and soliciting the employment or engagement of certain of our employees.
Prior Employment Agreement with Kathi Niffenegger effective as of December 1, 2020 and amended March 19, 2021 and April 12, 2022, which terminated July 15, 2023
Prior to the Non-Executive Employment Agreement (as defined below), on December 1, 2020, we entered into an employment agreement with Ms. Niffenegger (as amended, the Niffenegger Employment Agreement) that superseded prior employment agreements. Pursuant to the Niffenegger Employment Agreement, Ms. Niffenegger had agreed to continue to serve as our Chief Financial Officer. Both Ms. Niffenegger and we had the right to terminate the employment relationship at any time, with or without cause. As compensation for her services to us, Ms. Niffenegger received a base salary of $290,000 per year effective for the period January 1, 2021 to March 23, 2022 and a base salary of $300,150 per year effective for the period March 24, 2022 to July 15, 2023 when Ms. Niffenegger ended her tenure as Chief Financial Officer. She was eligible to receive a discretionary bonus in an amount up to 40% of her base salary based on her performance and the Company’s performance and such other employee benefits as are generally provided to similarly situated employees of the Company. Ms. Niffenegger was eligible for future share and/or option grants in accordance with our executive compensation policy as in effect from time to time as determined by the independent members of our Board of Directors subject to availability of shares and/or options for grant under our Equity Incentive Compensation Plan.
If Ms. Niffenegger’s employment with us was terminated for “Cause” (as defined in the Niffenegger Employment Agreement) or if Ms. Niffenegger resigned from her employment at any time, our only obligation would have been to provide Ms. Niffenegger with: (i) her accrued salary and accrued unused vacation pay through and including her last day of employment (the Separation Date); (ii) reimbursement of any reimbursable expenses properly incurred through and including the Separation Date; and (iii) any benefit required under applicable law. If Ms. Niffenegger was terminated by us without “Cause”, our obligations would have been (a) to provide Ms. Niffenegger with the same payments and benefits as would be provided if we had terminated her employment for Cause; and (b) subject to Ms. Niffenegger’s execution of a release in our favor, Ms. Niffenegger would have also been paid, as severance (the Prior Severance Payment), (i) a lump sum payment equal to twelve months of Ms. Niffenegger’s then current base salary, plus one additional month of base salary for every completed year of service since June 7, 2019, not to exceed an aggregate of twenty-four months, (ii) a lump sum payment of any discretionary bonus for the prior calendar year already determined by our Board of Directors, if such bonus had not yet been paid; and (iii) a lump sum payment equal to Ms. Niffenegger’s potential discretionary bonus for the calendar year in which the Separation Date occurred, prorated over Ms. Niffenegger’s length of service in the calendar year in which her employment was terminated, calculated in accordance with the terms of the Niffenegger Employment Agreement. If Ms. Niffenegger’s employment was terminated or “constructively terminated” (as such term is defined in the Niffenegger Employment Agreement) by us without “Cause” upon or within a twelve month period following a Change of Control (as such term is defined in the Niffenegger Employment Agreement), Ms. Niffenegger would have been entitled to the Prior Severance Payment described above, except that the portion of the Prior Severance Payment established by (b)(i) would be equal to twenty four months of Ms. Niffenegger’s base salary.
The Niffenegger Employment Agreement provided that during the term of Ms. Niffenegger’s employment with us and for a period of one year thereafter, Ms. Niffenegger was prohibited from soliciting for employment certain of our employees. The Agreement also provided that both during and after Ms. Niffenegger’s employment with us, she was prohibited from (i) making use of our trade secrets to solicit on behalf of Ms. Niffenegger or any other person business from any of our customers and (ii) inducing or attempting to induce any person to sever any existing contractual relationship they have with us.
Non-executive Employment Agreement with Kathi Niffenegger effective as of July 15, 2023
On July 16, 2023 following Ms. Niffenegger’s tenure as Chief Financial Officer, Edesa Biotech USA, Inc. entered into a non-executive employment agreement with Ms. Niffenegger (the Non-Executive Employment Agreement) that superseded the Niffenegger Employment Agreement described above. Pursuant to the Non-Executive Employment Agreement, both Ms. Niffenegger and we have the right to terminate the employment relationship at any time, with or without cause. As compensation for her services to us. Ms. Niffenegger received a base salary of $100,000 per year effective July 16, 2023 and a one-time retention bonus of $10,000. She is eligible to receive a discretionary bonus in an amount up to 40% of her prorated base salary under the current and prior employment agreements based on her performance and the Company’s performance and such other employee benefits as are generally provided to similarly situated employees of the Company. Ms. Niffenegger is eligible for future share and/or option grants commensurate with her current position in accordance with our non-executive compensation policy as in effect from time to time as determined by the independent members of our Board of Directors subject to availability of shares and/or options for grant under our Equity Incentive Compensation Plan.
The Non-Executive Employment Agreement provides that during the term of Ms. Niffenegger’s employment with us and for a period of one year thereafter, Ms. Niffenegger is prohibited from soliciting for employment certain of our employees. The Non-Executive Employment Agreement also provides that both during and after Ms. Niffenegger’s employment with us, she is prohibited from (i) making use of our trade secrets to solicit on behalf of Ms. Niffenegger or any other person business from any of our customers and (ii) inducing or attempting to induce any person to sever any existing contractual relationship they have with us.
Outstanding Equity Awards at September 30, 2023
| | | | Option Awards | |
Name | | Award grant date | | Number of securities underlying unexercised options (#) exercisable | | | Number of securities underlying unexercised options (#) unexercisable (1) | | | Option exercise prices | | | Option expiration date | |
Pardeep Nijhawan, MD | | 9/26/17 | | | 6,785 | | | | - | | | C$ | 15.12 | | | 9/26/27 | |
| | 12/28/18 | | | 232 | | | | - | | | C$ | 15.12 | | | 12/28/28 | |
| | 10/13/20 | | | 8,572 | | | | - | | | $ | 52.08 | | | 10/13/30 | |
| | 4/22/21 | | | 14,287 | | | | 2,856 | (2) | | $ | 38.15 | | | 4/22/31 | |
| | 2/28/22 | | | 3,896 | | | | 3,104 | (2) | | $ | 25.97 | | | 2/28/32 | |
| | 7/20/23 | | | 1,435 | | | | 15,708 | (2) | | $ | 5.79 | | | 7/20/33 | |
| | | | | | | | | | | | | | | | | |
Stephen Lemieux, CPA | | 7/20/23 | | | 881 | | | | 9,837 | (2) | | $ | 5.79 | | | 7/20/33 | |
| | | | | | | | | | | | | | | | | |
Michael Brooks, PhD | | 8/28/17 | | | 19,488 | | | | - | | | C$ | 15.12 | | | 8/28/27 | |
| | 9/26/17 | | | 3,472 | | | | - | | | C$ | 15.12 | | | 9/26/27 | |
| | 12/28/18 | | | 232 | | | | - | | | C$ | 15.12 | | | 12/28/28 | |
| | 2/12/20 | | | 9,856 | | | | - | | | $ | 22.12 | | | 2/12/30 | |
| | 10/13/20 | | | 7,143 | | | | - | | | $ | 52.08 | | | 10/13/30 | |
| | 4/22/21 | | | 9,527 | | | | 1,902 | (2) | | $ | 38.18 | | | 4/22/31 | |
| | 2/28/22 | | | 3,447 | | | | 2,768 | (2) | | $ | 25.97 | | | 2/28/32 | |
| | 7/20/23 | | | 968 | | | | 10,461 | (2) | | $ | 5.79 | | | 7/20/33 | |
| | | | | | | | | | | | | | | | | |
Kathi Niffenegger, CPA (3) | | 12/20/16 | | | 34 | | | | - | | | $ | 596.82 | | | 12/20/23 | |
Former Chief Financial Officer | | 3/12/18 | | | 119 | | | | - | | | $ | 246.93 | | | 3/12/25 | |
| | 2/12/20 | | | 12,672 | | | | - | | | $ | 22.12 | | | 2/12/30 | |
| | 10/13/20 | | | 7,143 | | | | - | | | $ | 52.08 | | | 10/13/30 | |
| | 4/22/21 | | | 9,527 | | | | 1,902 | (2) | | $ | 38.18 | | | 4/22/31 | |
| | 2/28/22 | | | 3,447 | | | | 2,768 | (2) | | $ | 25.97 | | | 2/28/32 | |
| | 7/20/23 | | | 968 | | | | 10,461 | (2) | | $ | 5.79 | | | 7/20/33 | |
| 1) | Our options vesting policy is described in the Outstanding Equity Awards Narrative Disclosure section. |
| 2) | The option will vest over a period of three years, with monthly vesting on a pro-rata basis beginning on the date of grant. |
| 3) | Ms. Niffenegger served as Chief Financial Officer through July 15, 2023. |
Outstanding Equity Awards Narrative Disclosure
Equity Incentive Compensation Plan
We adopted an Equity Incentive Compensation Plan in 2019 (the 2019 Plan) which amended and restated prior plans. Under the 2019 Plan, we are authorized to grant options, restricted shares and restricted share units (RSUs) to any of our officers, directors, employees, and consultants and those of our subsidiaries and other designated affiliates. The number of shares available for issuance under the 2019 Plan is 575,737, including shares available for the exercise of outstanding options and RSUs under the 2019 Plan and prior plans. The purpose of the 2019 Plan is to advance the interests of the Company by encouraging equity participation through the acquisition of common shares of the Company. The 2019 Plan is to be administered by the Compensation Committee of our Board of Directors, except to the extent (and subject to the limitations set forth in the 2019 Plan) the Board elects to administer the 2019 Plan, in which case the 2019 Plan shall be administered by only those members of the Board who are “independent” members of the Board. The administrator of the 2019 Plan has the power to, among other things:
| · | allot common shares for issuance in connection with the exercise of options; |
| · | grant options, restricted shares or restricted share units; |
| · | amend, suspend, terminate or discontinue the plan; and |
| · | delegate all or a portion of its administrative powers as it may determine to one or more committees. |
Options to purchase 420,615 common shares at prices ranging from C$15.12 and $5.79 to $596.82 are outstanding at September 30, 2023. RSUs eligible for conversion to 33,045 common shares are outstanding at September 30, 2023.
Options granted during the year ended September 30, 2023 to directors, officers and employees under the 2019 Plan totaled 118,579 options to purchase common shares at exercise prices ranging from $5.79 to $10.01. Options granted during the year ended September 30, 2022 to directors, officers and employees under the 2019 Plan totaled 71,451 options to purchase common shares at exercise prices ranging from $20.58 to $25.97. There were 46,602 RSUs granted during the year ended September 30, 2023. These RSUs were immediately vested and were for payment of bonuses or consulting fees to the current CFO. There were no RSUs granted during the year ended September 30, 2022.
Options Vesting Policy
Vesting requirements for option awards are determined by the independent members of the Board of Directors. Options granted by the Company during the year ended September 30, 2023 and 2022 generally had monthly vesting for directors in equal proportions over 12 months beginning on the grant date, monthly vesting for officers and current employees in equal proportions over 36 months beginning on the grant date and monthly vesting for new employees in equal proportions over 36 months beginning on the monthly anniversary of the grant date following 90 days of employment.
Retirement Benefits
Executive officers and employees of our California subsidiary are eligible to receive the Company’s non-elective contribution of 3% of eligible compensation under a 401(k) plan to provide retirement benefits. Any Company contributions we made to the plan for our named executive officers are reflected in the “All Other Compensation” column of the Summary Compensation Table above.
Other than the funds contributed under our 401(k) plan, no other funds were set aside or accrued by us during the years ended September 30, 2023 and 2022 to provide pension, retirement or similar benefits for our named executive officers.
Director Compensation
The following table sets forth information regarding the compensation of our non-employee directors for the year ended September 30, 2023.
Name | | Fees Earned or Paid in Cash ($) | | | Option Awards ($) (1) | | | Total ($) | |
Joan Chypyha | | $ | 18,437 | (2) | | $ | 12,375 | | | $ | 30,812 | |
Sean MacDonald | | | 55,632 | (2) | | | 12,375 | | | | 68,007 | |
Patrick Marshall | | | 16,826 | (2) | | | 12,375 | | | | 29,201 | |
Frank Oakes | | | 43,990 | | | | 12,375 | | | | 56,365 | |
Charles Olson, DSc | | | 15,752 | | | | 12,375 | | | | 28,127 | |
Carlo Sistilli, CPA, CMA | | | 55,450 | (2) | | | 12,375 | | | | 67,825 | |
| 1) | The amounts shown in this column represent the aggregate grant date fair value of the share option awards computed in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification 718, not the actual amounts paid to or realized by the directors during the covered fiscal year. The assumptions used in determining grant date fair value of these awards are set forth in Note 8 to our audited consolidated financial statements for the year ended September 30, 2023 included in this Annual Report. As of September 30, 2023, (i) Ms. Chypyha, Mr. Marshall and Dr. Olson each held 2,858 share options, (ii) Mr. MacDonald and Mr. Sistilli each held 11,773 share options and Mr. Oakes held 11,909 share options. |
| 2) | The compensation was paid in Canadian dollars and was converted from US dollars using the average foreign exchange rate for each month of the year from oanda.com. |
Narrative to Director Compensation Table
Non-Employee Director Compensation Policy
The board adopted a compensation policy effective June 7, 2019 and amended it effective March 24, 2022. As compensation for their services on the Board of Directors, each non-executive board member received annual remuneration as noted below and prorated during the effective periods. The Chief Executive Officer does not receive any additional compensation for his services on the Board of Directors.
From March 24, 2022 through September 30, 2023, each non-executive director received annual base remuneration of $35,000 and the Board Chair received annual remuneration of $65,000, inclusive of compensation for his services on committees of the Board of Directors. Each member of the Company’s Audit Committee received annual remuneration of $7,500, and the Chair of the Audit Committee received $15,000 annually for his services. Each member of the Company’s Compensation Committee and Nominating and Corporate Governance Committee received annual remuneration of $4,500 for each committee on which they serve, and the Chairs of each of the Compensation Committee and Nominating and Corporate Governance Committee received $9,000 annually for their services.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Equity Compensation Plan Information
The following table provides certain information as of September 30, 2023 about our common shares that may be issued under our equity compensation plans, which consists of our 2019 Equity Incentive Compensation Plan in effect at September 30, 2023:
Plan Category | | Number of securities to be issued upon exercise of outstanding options and rights | | | Weighted-average exercise price of outstanding options and rights | | | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |
| | (a) | | | (b) | | | (c) | |
Equity compensation plans approved by security holders | | | 453,660 | (1) | | $ | 25.60 | (2) | | | 81,765 | |
Equity compensation plans not approved by security holders | | | N/A | | | | N/A | | | | N/A | |
Total | | | 453,660 | | | $ | 25.60 | | | | 81,765 | |
(1) | Includes 422,615 common shares issuable upon the exercise of outstanding options and 33,045 common shares issuable upon the conversion of outstanding RSUs. |
| |
(2) | The weighted-average exercise price does not consider shares issuable upon the conversion of outstanding RSUs, which have no exercise price. |
Warrants and other equity held by directors, officers and employees outside of the compensation plans are not included in the table above.
Security Ownership of Certain Beneficial Owners and Management
The following tables sets forth certain information as of December 13, 2023, with respect to the beneficial ownership of our common shares by: (1) all of our directors; (2) our named executive officers listed in the Summary Compensation Table; (3) all of directors and executive officers as a group; and (4) each person known by us to beneficially own more than 5% of our outstanding common shares.
We have determined beneficial ownership in accordance with the rules of the SEC, based on a review of filings with the SEC and information known to us. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power with respect to all common shares that they beneficially own, subject to applicable community property laws.
Common shares subject to options, warrants or restricted share units currently exercisable or exercisable within 60 days of December 13, 2023 are deemed outstanding for computing the share ownership and percentage of the person holding such options, warrants and restricted share units, but are not deemed outstanding for computing the percentage of any other person. The percentage ownership of our common shares of each person or entity named in the following table is based on 3,164,722 common shares outstanding as of December 13, 2023.
Directors and Officers
Name and Address of Beneficial Owner (1) | | Number of Shares Beneficially Owned | | | Percentage of Shares Beneficially Owned | |
| | | | | | |
Joan Chypyha | | | 1,695 | (2) | | * | |
Sean MacDonald | | | 12,634 | (3) | | * | |
Patrick Marshall | | | 2,131 | (4) | | * | |
Pardeep Nijhawan, MD | | | 658,568 | (5) | | | 20.1 | % |
Frank Oakes | | | 10,717 | (6) | | * | |
Charles Olson, DSc | | | 1,666 | (7) | | * | |
Carlo Sistilli, CPA, CMA | | | 11,103 | (8) | | * | |
Stephen Lemieux, CPA | | | 4,572 | (9) | | * | |
Michael Brooks, PhD | | | 75,615 | (10) | | | 2.3 | % |
Kathi Niffenegger, CPA | | | 50,861 | (11) | | | 1.6 | % |
| | | | | | | | |
All directors and named executive officers as a group (10 persons) | | | 829,562 | (12) | | | 25.4 | % |
* Percentage of shares beneficially owned does not exceed one percent.
| 1) | Unless otherwise indicated, the address of each beneficial owner is c/o Edesa Biotech, Inc., 100 Spy Court, Markham, ON Canada L3R 5H6. |
| 2) | Consists of (i) 29 common shares and (ii) 1,666 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023. |
| 3) | Consists of (i) 2,053 common shares and (ii) 10,581 common shares issuable upon exercise of options exercisable within sixty days December 13, 2023. |
| 4) | Consists of (i) 1,666 common shares issuable upon exercise of options exercisable within sixty days December 13, 2023 held by Patrick Marshall and.(ii) 465 common shares held by Quidnet Inc. for which Patrick Marshall has sole voting and dispositive power over all such shares. |
| 5) | Consists of (A)(i) 84,973 common shares, (ii) 39,761 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023 and (iii) 14,186 common shares issuable upon the conversion of restricted share units held by Pardeep Nijhawan; (B)(i) 336,702 common shares and (ii) 32,610 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 held by Pardeep Nijhawan Medicine Professional Corporation for which Pardeep Nijhawan has sole voting and dispositive power over all such shares; (C) 32,013 common shares held by The Digestive Health Clinic Inc. for which Pardeep Nijhawan has sole voting and dispositive power over all such shares; (D) 53,104 common shares held by 1968160 Ontario Inc. for which Pardeep Nijhawan has sole voting and dispositive power over all such shares and (E)(i) 32,609 common shares and (ii) 32,610 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 held by The New Nijhawan Family Trust 2015 for which each of Pardeep Nijhawan and Nidhi Nijhawan, as trustees, have voting and dispositive power over all such shares. |
| 6) | Consists of 10,717 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023. |
| 7) | Consists of 1,666 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023. |
| 8) | Consists of (i) 10,581 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023 held by Carlo Sistilli and (ii) 522 Common Shares held by York-Cav Enterprises Inc. for which Carlo Sistilli, as President and Director, has sole voting and dispositive power over all such shares. |
| 9) | Consists of (i) 2,086 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023 and (ii) 2,486 common shares issuable upon the conversion of restricted share units. |
| 10) | Consists of (i) 4,354 common shares, (ii) 57,340 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023, (iii) 606 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 and (iv) 13,315 common shares issuable upon conversion of restricted share units. |
| 11) | Consists of (A)(i) 10,943 common shares and (ii) 37,117 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023 held by Kathi Niffenegger and (B)(i) 1,531 common shares and (ii) 1,270 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 held by the Kathi Niffenegger Trust for which Kathi Niffenegger, as trustee, has sole voting and dispositive power over all such shares. |
| 12) | Consists of (i) 559,298 common shares, (ii) 173,181 common shares issuable upon exercise of options exercisable within sixty days of December 13, 2023, (iii) 67,096 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 and (iv) 29,987 common shares issuable upon conversion of restricted share units. |
Shareholders Known by Us to Own 5% or More of Our Common Shares
Name and Address of Beneficial Owner | | Number of Shares Beneficially Owned | | | Percentage of Shares Beneficially Owned | |
| | | | | | |
Lumira Capital II, L.P. and Lumira Capital II (International), L.P. (1) | | | 234,786 | (1) | | | 7.5 | % |
Velan Capital Partners LP (2) | | | 253,968 | (2) | | | 7.9 | % |
| 1) | Consists of (i) 214,913 common shares held by Lumira Capital II, L.P. and (ii) 19,873 common shares held by Lumira Capital II (International), L.P. and beneficially owned by affiliates of Lumira Capital II, L.P. and Lumira Capital II (International), L.P. The address of both entities is 141 Adelaide Street West, Suite 770, Toronto, Ontario, Canada M5H 3L5. We relied in part on the SEC Schedule 13D/A filed with the SEC on January 13, 2023 for this information. |
| 2) | Consists of (i) 190,476 common shares and (ii) 63,492 common shares issuable upon exercise of warrants exercisable within sixty days of December 13, 2023 held by Velan Capital Partners LP and beneficially owned by its affiliates. The address is 1055b Powers Place, Alpharetta, GA 30009. We relied in part on the SEC Schedule 13G filed with the SEC on January 6, 2023 for this information. |
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Related Party Transactions
The following is a description of transactions since October 1, 2021 to which we have been a participant in which the amount involved exceeded or will exceed the lesser of (i) $120,000 and (ii) one percent of the average of our total assets at year end for the last two completed fiscal years in which any of our directors, executive officers or holders of more than 5% of our voting securities, or any members of their immediate family, had or will have a direct or indirect material interest, other than compensation arrangements.
Right-of-Use Lease Agreement
In January 2017, Edesa Biotech Research entered into a right-of-use lease agreement with 1968160 Ontario Inc., a company related to Dr. Nijhawan, our Chief Executive Officer, for office space that serves as our principal executive office. The original lease expired on December 31, 2022 and we executed a two-year term extension through December 31, 2024. Monthly rents during the term ranged from C$8,320 to C$9,020 plus HST. Rents of approximately $82,000 and $81,000 were incurred during the years ended September 30, 2023 and 2022, respectively. Rents of approximately $15,000 and $22,000 were payable at September 30, 2023 and September 30, 2022, respectively.
Credit Agreement
On October 20, 2023, the Company entered into the Credit Agreement with Pardeep Nijhawan Medicine Professional Corporation, an entity controlled by Dr. Nijhawan, our Chief Executive Officer, providing for the Line of Credit in the principal amount of up to $10 million, with the Credit Limit of $3.5 million, which was available immediately upon the execution of the Credit Agreement. Subject to the terms of the Credit Agreement, the Credit Limit may be increased by the lender upon request from the Company in an amount not to exceed $10 million.
The Line of Credit bears interest at the Canadian Imperial Bank of Commerce US Base-Interest Rate plus 3% per annum and has a maturity date of March 31, 2026, unless terminated earlier by either party with 90 days’ notice. The Company has the right at any time, and from time to time, to prepay all or any portion of each advance without premium or penalty.
Additionally, the Company agreed to pay a monthly standby fee for the term of the Credit Agreement, calculated as of the last business day of each month, on the difference between the Credit Limit at such time and the principal amount of outstanding advances, based on an annual interest rate of 1.5%.
As of December 13, 2023, the entire $3.5 million Credit Limit was available on the Line of Credit and the Company accrued $0.01 million in monthly standby fees.
Director Independence
In evaluating the independence of our Board members and the composition of the committees of our Board of Directors, the Board of Directors utilizes the definition of “independence” as that term is defined by the Exchange Act and the Nasdaq Listing Rules. Using this standard, the Board of Directors has determined that Joan Chypyha, Sean MacDonald, Patrick Marshall, Frank Oakes, Charles Olson and Carlo Sistilli are “independent directors.” Accordingly, our Board of Directors is composed of a majority of independent directors as required by the rules of Nasdaq. Pardeep Nijhawan is not an independent director due to his position as our Chief Executive Officer. We have established an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee, each of which are composed of independent directors.
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The following table shows the aggregate fees billed for audit and other services provided for the years ended September 30, 2023 and 2022 rendered by MNP LLP.
Principal Accountant Fees and Services
Type of Service | | Year Ended 2023 | | | Year Ended 2022 | |
Audit Fees | | $ | 129,318 | | | $ | 139,189 | |
Audit-related Fees | | | 108,294 | | | | 73,382 | |
Tax Fees | | | 10,076 | | | | 14,771 | |
| | | | | | | | |
Total | | $ | 247,688 | | | $ | 227,342 | |
Audit Fees
Audit fees consisted of fees incurred for professional services rendered for audits and interim reviews of the years ended September 30, 2023 and 2022. Audit-related fees include assurance and related services that were incurred for procedures related to registrations and offerings.
Tax Fees
Tax fees consisted of fees incurred for professional services rendered for tax compliance related to tax returns during the years ended September 30, 2023 and 2022.
Pre-Approval Policies and Procedures
The Audit Committee is directly responsible for the appointment, compensation and oversight of our auditors. It has established procedures for the receipt, retention, and treatment of complaints received by us regarding accounting, internal accounting controls, or auditing matters, and the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters. The Audit Committee also has the authority and the funding to engage independent counsel and other outside advisors.
The Audit Committee pre-approves all audit and permissible non-audit services provided by the independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year, and any pre-approval is detailed as to the particular service or category of services and is generally subject to an amount or range of estimated fees. All proposed engagements of the auditor for audit and permitted non-audit services are submitted to the Audit Committee for approval prior to the beginning of any such services. Our auditors are required to periodically report to the Audit Committee regarding the extent of services provided by the independent registered public accounting firm in accordance with the pre-approval, and the fees for the services performed to date. The Audit Committee may also pre-approve particular services on a case-by-case basis. The Audit Committee pre-approved 100% of the audit and non-audit services performed by our independent registered public accounting firm for the years ended September 30, 2023 and 2022.
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
| (a) | The following documents are filed as a part of this Annual Report: |
The list of consolidated financial statements and notes required by this Item 15 (a) (1) is set forth in the “Index to Financial Statements” on page F-1 of this Annual Report.
| (2) | Financial Statement Schedules |
All schedules have been omitted because the required information is included in the financial statements or notes thereto.
The exhibits listed on the Exhibit Index below are filed as part of this Annual Report.
EXHIBIT INDEX
Exhibit No. | | Description |
2.1* | | Share Exchange Agreement, dated as of March 7, 2019, by and between Stellar Biotechnologies Inc., Edesa Biotech Inc. and the Edesa Shareholders (included as Exhibit 2.1 to the Company's Current Report on Form 8-K filed on March 8, 2019, and incorporated herein by reference). |
| | |
3.1 | | Certificate of Incorporation of the Company, dated June 12, 2007 (included as Exhibit 1(a) to the Company's Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). |
| | |
3.2 | | Certificate of Amendment of the Company, dated April 15, 2008 (included as Exhibit 1(b) to the Company's Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). |
| | |
3.3 | | Certificate of Continuation of the Company, dated November 25, 2009 (included as Exhibit 1(c) to the Company's Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). |
| | |
3.4 | | Certificate of Change of Name of the Company, dated April 7, 2010 (included as Exhibit 1(f) to the Company’s Registration Statement on Form 20-F filed on February 3, 2012, and incorporated herein by reference). |
| | |
3.5 | | Certificate of Change of Name of the Company, dated June 7, 2019 (included as Exhibit 3.6 to the Company's Annual Report on Form 10-K filed on December 12, 2019, and incorporated herein by reference). |
| | |
3.6 | | Amended and Restated Articles of Edesa Biotech, Inc. (included as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on April 23, 2020, and incorporated herein by reference). |
| | |
3.7 | | Notice of Articles of Edesa Biotech, Inc. (included as Exhibit 3.7 to the Company’s Registration Statement on Form S-1 filed on April 11, 2022, and incorporated herein by reference). |
| | |
4.1 | | Specimen of common share certificate (included as Exhibit 4.1 to the Company’s Registration Statement on Form S-3 filed on August 30, 2019, and incorporated herein by reference). |
| | |
4.2 | | Form of Class A Purchase Warrant issued to investors (included as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on January 6, 2020, and incorporated herein by reference). |
| | |
4.3 | | Form of Warrant issued to Brookline Capital Markets, a division of Arcadia Securities, LLC (included as Exhibit 4.3 to the Company’s Current Report on Form 8-K filed on January 6, 2020, and incorporated herein by reference). |
| | |
4.4 | | Form of Warrant (included as Exhibit 4.2 to the Company's Registration Statement on Form S-1 filed on May 8, 2018, and incorporated herein by reference). |
4.5 | | Form of Underwriter Warrant (included as Exhibit 4.1 to the Company’s Current Report on Form 8-K/A filed on February 26, 2021, and incorporated herein by reference). |
| | |
4.6 | | Form of Pre-Funded Warrant (included as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 23, 2022, and incorporated herein by reference). |
| | |
4.7 | | Form of Private Placement Warrant (included as Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on March 23, 2022, and incorporated herein by reference). |
| | |
4.8 | | Form of Placement Agent Warrant (included as Exhibit 4.3 to the Company’s Current Report on Form 8-K filed on March 23, 2022, and incorporated herein by reference). |
| | |
4.9 | | Form of Class A Warrant (included as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on November 3, 2022, and incorporated herein by reference). |
| | |
4.10 | | Form of Class B Warrant (included as Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on November 3, 2022, and incorporated herein by reference). |
| | |
4.11 | | Description of Securities (filed herewith). |
| | |
4.12 | | Form of Common Share Purchase Warrant issued to H.C. Wainwright & Co., Inc. designees on June 7, 2019 (filed herewith). |
| | |
10.1 | | Advance Notice Policy, adopted October 31, 2013 (included as Exhibit 10.14 to the Company's Annual Report on Form 10-K filed on November 14, 2014, and incorporated herein by reference). |
| | |
10.2@ | | Employment Agreement by and between the Company and Pardeep Nijhawan, dated June 14, 2019 (included as Exhibit 10.2 to the Company's Current Report on Form 8-K/A filed on June 20, 2019, and incorporated herein by reference). |
| | |
10.3@ | | Employment Agreement by and between the Company and Michael Brooks, dated June 14, 2019 (included as Exhibit 10.3 to the Company's Current Report on Form 8-K/A filed on June 20, 2019, and incorporated herein by reference). |
| | |
10.4@ | | Form of Indemnification Agreement, by and between the Company and each of its directors and executive officers (included as Exhibit 10.4 to the Company's Current Report on Form 8-K/A filed on June 20, 2019, and incorporated herein by reference). |
10.5@ | | 2019 Equity Incentive Compensation Plan (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 25, 2019, and incorporated herein by reference). |
| | |
10.6@ | | Amendment No. 1 to Edesa Biotech, Inc. 2019 Equity Incentive Compensation Plan (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 23, 2021, and incorporated herein by reference). |
| | |
10.7 | | Lease, dated as of January 1, 2017, by and between the Registrant and 1968160 Ontario Inc. (included as Exhibit 10.1 to the Company's Current Report on Form 8-K filed on August 30, 2019, and incorporated herein by reference). |
| | |
10.8+ | | Exclusive License Agreement, dated as of June 29, 2016, by and between the Registrant and Yissum Research Development Company (included as Exhibit 10.2 to the Company's Current Report on Form 8-K filed on August 30, 2019, and incorporated herein by reference). |
| | |
10.9 | | First Amendment to Exclusive License Agreement, dated April 3, 2017, by and between the Registrant and Yissum Research Development Company (included as Exhibit 10.3 to the Company's Current Report on Form 8-K filed on August 30, 2019, and incorporated herein by reference). |
| | |
10.10 | | Second Amendment to Exclusive License Agreement, dated May 7, 2017, by and between the Registrant and Yissum Research Development Company (included as Exhibit 10.4 to the Company's Current Report on Form 8-K filed on August 30, 2019, and incorporated herein by reference). |
| | |
10.11+ | | Third Amendment to Exclusive License Agreement, dated October 26, 2022, by and between the Registrant and Yissum Research Development Company (included as Exhibit 10.11 to the Company’s Annual Report on Form 10-K filed on December 16, 2022 and incorporated herein by reference). |
| | |
10.12+ | | License and Development Agreement, dated as of August 27, 2017, by and between the Registrant and Pendopharm, a division of Pharmascience Inc. (included as Exhibit 10.6 to the Company's Current Report on Form 8-K filed on August 30, 2019, and incorporated herein by reference). |
| | |
10.13+ | | License Agreement by and between Edesa Biotech Research, Inc. and NovImmune SA dated April 17, 2020 (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 23, 2020, and incorporated herein by reference). |
| | |
10.14+ | | Purchase Agreement by and between Edesa Biotech Research, Inc. and NovImmune SA dated April 17, 2020 (included as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on April 23, 2020, and incorporated herein by reference). |
| | |
10.15@ | | Employment Agreement by and between the Company and Kathi Niffenegger, dated December 1, 2020 (included as Exhibit 10.21 to the Company’s Annual Report on Form 10-K filed on December 7, 2020, and incorporated herein by reference). |
| | |
10.16+ | | Strategic Innovation Fund Agreement among Edesa Biotech Research, Inc., Edesa Biotech, Inc., and her Majesty the Queen in right of Canada as represented by the Minister of Industry, dated February 2, 2021 (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 3, 2021, and incorporated herein by reference). |
10.17+ | | Exclusive License Agreement, dated as of March 16, 2021, by and between Edesa Biotech Research, Inc. and Dr. Saul Yedgar (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 22, 2021, and incorporated herein by reference). |
| | |
10.18@ | | Amendment to Employment Agreement, entered into on March 19, 2021, by and between Par Nijhawan and Edesa Biotech, Inc. (included as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on May 14, 2021, and incorporated herein by reference). |
| | |
10.19@ | | Amendment to Employment Agreement, entered into on March 19, 2021, by and between Kathi Niffenegger and Edesa Biotech USA, Inc. (included as Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on May 14, 2021, and incorporated herein by reference). |
| | |
10.20@ | | Amendment to Employment Agreement, entered into on March 19, 2021, by and between Michael Brooks and Edesa Biotech, Inc. (included as Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on May 14, 2021, and incorporated herein by reference). |
| | |
10.21 | | Form of Securities Purchase Agreement, dated March 21, 2022, by and between the Company and the Purchaser (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 23, 2022, and incorporated herein by reference). |
| | |
10.22@ | | Amendment to Employment Agreement, entered into on April 12, 2022, by and between Par Nijhawan and Edesa Biotech, Inc. (included as Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on May 13, 2022, and incorporated herein by reference). |
| | |
10.23@ | | Amendment to Employment Agreement, entered into on April 12, 2022, by and between Kathi Niffenegger and Edesa Biotech USA, Inc. (included as Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on May 13, 2022, and incorporated herein by reference). |
| | |
10.24@ | | Amendment to Employment Agreement, entered into on April 12, 2022, by and between Michael Brooks and Edesa Biotech USA, Inc. (included as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on May 13, 2022, and incorporated herein by reference). |
| | |
10.25 | | Form of Non-U.S. Subscription Agreement (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 3, 2022, and incorporated herein by reference). |
| | |
10.26 | | Form of U.S. Subscription Agreement (included as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 3, 2022, and incorporated herein by reference). |
10.27 | | Lease Extending and Amending Agreement dated as of December 31, 2022 by and between Edesa Biotech Research, Inc. and 1968160 Ontario, Inc. (included as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on February 10, 2023 and incorporated herein by reference). |
| | |
10.28 | | Equity Distribution Agreement, dated as of March 27, 2023, by and between Edesa Biotech, Inc. and Canaccord Genuity LLC (included as Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on March 27, 2023, and incorporated herein by reference). |
| | |
10.29@ | | Amendment No. 2 to Edesa Biotech, Inc. 2019 Equity Incentive Compensation Plan (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on May 24, 2023, and incorporated herein by reference). |
| | |
10.30@ | | Employment Agreement by and between the Company and Stephen Lemieux, dated June 26, 2023 (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on June 27, 2023, and incorporated herein by reference). |
| | |
10.31@ | | Amended and Restated Employment Agreement, by and between the Company and Pardeep Nijhawan, dated August 4, 2023 (included as Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed on August 9, 2023, and incorporated herein by reference). |
| | |
10.32@ | | Amended and Restated Employment Agreement, by and between the Company and Michael Brooks, dated August 4, 2023 (included as Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q filed on August 9, 2023, and incorporated herein by reference). |
| | |
10.33+ | | Strategic Innovation Fund Agreement, dated October 12, 2023, by and among Edesa Biotech Research, Inc., Edesa Biotech, Inc., and his Majesty the King in right of Canada as represented by the Minister of Industry (filed herewith). |
| | |
10.34 | | Credit Agreement, effective as of October 20, 2023, by and between the Company and Pardeep Nijhawan Medicine Professional Corporation (included as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on October 23, 2023, and incorporated herein by reference). |
| | |
10.35+ | | First Amendment to Exclusive License Agreement, dated as of September 21, 2023, by and between Edesa Biotech Research, Inc. and Dr. Saul Yedgar (filed herewith). |
| | |
10.36@ | | First Amendment to Amended and Restated Employment Agreement, by and between the Company and Pardeep Nijhawan, dated December 7, 2023 (filed herewith). |
| | |
21 | | Subsidiaries of Edesa Biotech, Inc. (included as Exhibit 21 to the Company’s Annual Report on Form 10-K filed on December 7, 2020, and incorporated herein by reference). |
| | |
23.1 | | Consent of MNP LLP (filed herewith). |
| | |
24.1 | | Power of Attorney (included on signature page). |
| | |
31.1 | | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities and Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| | |
31.2 | | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities and Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith). |
| | |
32.1** | | Certification of the Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
* All schedules and exhibits to the Share Exchange Agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request.
** The information in this exhibit is furnished and deemed not filed with the SEC for purposes of section 18 of the Exchange Act, and is not to be incorporated by reference into any filing of Edesa Biotech, Inc. under the Securities Act, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
@ Management contract or compensatory plan or arrangement.
+ Portions of this exhibit have been omitted pursuant to Rule 601(b)(10)(iv) of Regulation S-K.
Item 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | EDESA BIOTECH, INC. | |
| | | |
Date: December 15, 2023 | | /s/ Pardeep Nijhawan | |
| | Pardeep Nijhawan, MD | |
| | Director, Chief Executive Officer and Corporate Secretary (Principal Executive Officer) | |
| | | |
Date: December 15, 2023 | | /s/ Stephen Lemieux | |
| | Stephen Lemieux | |
| | Chief Financial Officer (Principal Financial Officer) | |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Pardeep Nijhawan and Stephen Lemieux, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution for him or her, and in his or her name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ Pardeep Nijhawan | | Director, Chief Executive Officer, and | | December 15, 2023 |
Pardeep Nijhawan | | Corporate Secretary (Principal Executive Officer) | | |
| | | | |
/s/ Stephen Lemieux | | Chief Financial Officer | | December 15, 2023 |
Stephen Lemieux | | (Principal Financial and Accounting Officer) | | |
| | | | |
/s/ Joan Chypyha | | Director | | December 15, 2023 |
Joan Chypyha | | | | |
| | | | |
/s/ Sean MacDonald | | Director | | December 15, 2023 |
Sean MacDonald | | | | |
| | | | |
/s/ Patrick Marshall | | Director | | December 15, 2023 |
Patrick Marshall | | | | |
| | | | |
/s/ Frank Oakes | | Director | | December 15, 2023 |
Frank Oakes | | | | |
| | | | |
/s/ Charles Olson | | Director | | December 15, 2023 |
Charles Olson | | | | |
| | | | |
/s/ Carlo Sistilli | | Chairman of the Board of Directors | | December 15, 2023 |
Carlo Sistilli | | | | |
EDESA BIOTECH, INC.
INDEX TO FINANCIAL STATEMENTS

EDESA BIOTECH, INC.
Consolidated Balance Sheets
| | | | | | |
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Assets: | | | | | | |
| | | | | | |
Current assets: | | | | | | |
Cash and cash equivalents | | $ | 5,361,397 | | | $ | 7,090,919 | |
Accounts and other receivable | | | 626,543 | | | | 1,255,451 | |
Prepaid expenses and other current assets | | | 448,912 | | | | 745,543 | |
| | | | | | | | |
Total current assets | | | 6,436,852 | | | | 9,091,913 | |
| | | | | | | | |
Non-current assets: | | | | | | | | |
Property and equipment, net | | | 8,702 | | | | 12,694 | |
Long-term deposits | | | 173,490 | | | | 171,464 | |
Intangible asset, net | | | 2,180,020 | | | | 2,281,192 | |
Right-of-use assets | | | 91,373 | | | | 18,465 | |
| | | | | | | | |
Total assets | | $ | 8,890,437 | | | $ | 11,575,728 | |
| | | | | | | | |
Liabilities and shareholders' equity: | | | | | | | | |
| | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable and accrued liabilities | | $ | 1,747,150 | | | $ | 2,121,802 | |
Short-term right-of-use lease liabilities | | | 74,714 | | | | 18,975 | |
| | | | | | | | |
Total current liabilities | | | 1,821,864 | | | | 2,140,777 | |
| | | | | | | | |
Non-current liabilities: | | | | | | | | |
| | | | | | | | |
Long-term payables | | | - | | | | 43,662 | |
Long-term right-of-use lease liabilities | | | 19,773 | | | | - | |
| | | | | | | | |
Total liabilities | | | 1,841,637 | | | | 2,184,439 | |
| | | | | | | | |
Commitments (Note 7) | | | | | | | | |
| | | | | | | | |
Shareholders' equity: | | | | | | | | |
Capital shares | | | | | | | | |
Authorized unlimited common and preferred shares without par value | | | | | | | | |
Issued and outstanding: 3,075,473 common shares (September 30, 2022 - 2,380,280) | | | 46,643,151 | | | | 42,473,099 | |
Additional paid-in capital | | | 13,039,265 | | | | 11,176,345 | |
Accumulated other comprehensive loss | | | (214,648 | ) | | | (213,602 | ) |
Accumulated deficit | | | (52,418,968 | ) | | | (44,044,553 | ) |
| | | | | | | | |
Total shareholders' equity | | | 7,048,800 | | | | 9,391,289 | |
| | | | | | | | |
Total liabilities and shareholders' equity | | $ | 8,890,437 | | | $ | 11,575,728 | |
The accompanying notes are an integral part of these consolidated financial statements.
EDESA BIOTECH, INC.
Consolidated Statements of Operations and Comprehensive Loss
| | Years Ended | |
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Expenses: | | | | | | |
Research and development | | $ | 4,794,549 | | | $ | 13,335,334 | |
General and administrative | | | 4,428,209 | | | | 5,035,456 | |
| | | | | | | | |
Loss from operations | | | (9,222,758 | ) | | | (18,370,790 | ) |
| | | | | | | | |
Other income (loss): | | | | | | | | |
Reimbursement grant income | | | 581,039 | | | | 780,257 | |
Interest income | | | 289,846 | | | | 63,523 | |
Foreign exchange loss | | | (21,742 | ) | | | (21,114 | ) |
| | | | | | | | |
| | | 849,143 | | | | 822,666 | |
| | | | | | | | |
Loss before income taxes | | | (8,373,615 | ) | | | (17,548,124 | ) |
| | | | | | | | |
Income tax expense | | | 800 | | | | 800 | |
| | | | | | | | |
Net loss | | | (8,374,415 | ) | | | (17,548,924 | ) |
| | | | | | | | |
Exchange differences on translation | | | (1,046 | ) | | | (8,340 | ) |
| | | | | | | | |
Net comprehensive loss | | $ | (8,375,461 | ) | | $ | (17,557,264 | ) |
| | | | | | | | |
Weighted average number of common shares | | | 2,858,929 | | | | 2,096,446 | |
| | | | | | | | |
Loss per common share - basic and diluted | | $ | (2.93 | ) | | $ | (8.37 | ) |
The accompanying notes are an integral part of these consolidated financial statements.
EDESA BIOTECH, INC.
Consolidated Statements of Cash Flows
| | Years Ended | |
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Cash flows from operating activities: | | | | | | |
Net loss | | $ | (8,374,415 | ) | | $ | (17,548,924 | ) |
Adjustments for: | | | | | | | | |
Depreciation and amortization | | | 183,471 | | | | 118,188 | |
Share-based compensation | | | 1,246,457 | | | | 2,260,634 | |
Changes in working capital items: | | | | | | | | |
Accounts and other receivable | | | 562,770 | | | | 2,027,454 | |
Prepaid expenses and other current assets | | | 301,504 | | | | (19,497 | ) |
Accounts payable and accrued liabilities | | | (556,270 | ) | | | 882,843 | |
| | | | | | | | |
Net cash used in operating activities | | | (6,636,483 | ) | | | (12,279,302 | ) |
| | | | | | | | |
Cash flows from investing activities: | | | | | | | | |
Purchase of property and equipment | | | - | | | | (5,656 | ) |
| | | | | | | | |
Net cash used in investing activities | | | - | | | | (5,656 | ) |
| | | | | | | | |
Cash flows from financing activities: | | | | | | | | |
Proceeds from issuance of common shares and warrants | | | 4,345,017 | | | | 11,957,687 | |
Proceeds from exercise of warrants | | | 770,532 | | | | - | |
Payments for issuance costs of common shares and warrants | | | (285,438 | ) | | | (328,059 | ) |
| | | | | | | | |
Net cash provided by financing activities | | | 4,830,111 | | | | 11,629,628 | |
| | | | | | | | |
Effect of exchange rate changes on cash and cash equivalents | | | 76,850 | | | | (93,010 | ) |
| | | | | | | | |
Net change in cash and cash equivalents | | | (1,729,522 | ) | | | (748,340 | ) |
Cash and cash equivalents, beginning of year | | | 7,090,919 | | | | 7,839,259 | |
| | | | | | | | |
Cash and cash equivalents, end of year | | $ | 5,361,397 | | | $ | 7,090,919 | |
| | | | | | | | |
Supplemental Disclosure of Noncash Financing Activities: | | | | | | | | |
Issuance costs withheld from gross proceeds from issuance of common shares and warrants | | $ | - | | | $ | 393,461 | |
Fair value of placement agent warrants | | | - | | | | 408,059 | |
The accompanying notes are an integral part of these consolidated financial statements.
EDESA BIOTECH, INC.
Consolidated Statements of Changes in Shareholders’ Equity
| | Shares # | | | Common Shares | | | Additional Paid-in Capital | | | Accumulated Other Comprehensive Loss | | | Accumulated Deficit | | | Total Shareholders' Equity | |
Balance - September 30, 2021 | | | 1,899,333 | | | $ | 34,887,721 | | | $ | 4,871,461 | | | $ | (205,262 | ) | | $ | (26,495,629 | ) | | $ | 13,058,291 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common shares and warrants in equity offering | | | 309,558 | | | | 6,239,181 | | | | 6,702,293 | | | | - | | | | - | | | | 12,941,474 | |
Issuance costs including fair value of placement agent warrants | | | - | | | | (863,227 | ) | | | (448,739 | ) | | | - | | | | - | | | | (1,311,966 | ) |
Issuance of common shares upon exercise of pre-funded warrants, net of costs | | | 171,389 | | | | 2,209,424 | | | | (2,209,304 | ) | | | - | | | | - | | | | 120 | |
Share-based compensation | | | - | | | | - | | | | 2,260,634 | | | | - | | | | - | | | | 2,260,634 | |
Net loss and comprehensive loss | | | - | | | | - | | | | - | | | | (8,340 | ) | | | (17,548,924 | ) | | | (17,557,264 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance - September 30, 2022 | | | 2,380,280 | | | $ | 42,473,099 | | | $ | 11,176,345 | | | $ | (213,602 | ) | | $ | (44,044,553 | ) | | $ | 9,391,289 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Issuance of common shares and warrants in equity offering | | | 580,876 | | | | 3,400,191 | | | | 944,828 | | | | - | | | | - | | | | 4,345,019 | |
Issuance of common shares upon exercise of warrants | | | 100,760 | | | | 994,618 | | | | (224,087 | ) | | | - | | | | - | | | | 770,531 | |
Issuance of common shares upon exercise of restricted share units | | | 13,557 | | | | 75,920 | | | | (75,920 | ) | | | - | | | | - | | | | - | |
Issuance costs | | | - | | | | (300,677 | ) | | | (28,358 | ) | | | - | | | | - | | | | (329,035 | ) |
Share-based compensation | | | - | | | | - | | | | 1,246,457 | | | | - | | | | - | | | | 1,246,457 | |
Net loss and comprehensive loss | | | - | | | | - | | | | - | | | | (1,046 | ) | | | (8,374,415 | ) | | | (8,375,461 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance - September 30, 2023 | | | 3,075,473 | | | $ | 46,643,151 | | | $ | 13,039,265 | | | $ | (214,648 | ) | | $ | (52,418,968 | ) | | $ | 7,048,800 | |
The accompanying notes are an integral part of these consolidated financial statements.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
1. Nature of operations
Edesa Biotech, Inc. (the Company or Edesa) is a biopharmaceutical company focused on acquiring, developing and commercializing clinical stage drugs for inflammatory and immune-related diseases with clear unmet medical needs. The Company is organized under the laws of British Columbia, Canada and is headquartered in Markham, Ontario. It operates under its wholly owned subsidiaries, Edesa Biotech Research, Inc., an Ontario, Canada corporation, and Edesa Biotech USA, Inc., a California, USA corporation.
The Company’s common shares trade on The Nasdaq Capital Market in the United States under the symbol “EDSA”.
Liquidity
The Company’s operations have historically been funded through issuances of common shares, exercises of common share purchase warrants, convertible preferred shares, convertible loans, government grants and tax incentives.
At September 30, 2023, the Company had an accumulated deficit of $52.4 million and working capital of $4.6 million, including $5.4 million in cash and cash equivalents. In August 2022 the Company filed a $150.0 million shelf registration statement, under which the Company entered into an equity distribution agreement with Canaccord for $20.0 million in gross proceeds, subject to certain offering limitations that currently allows the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million (Canaccord ATM). There was approximately $7.1 million of available capacity on the Canaccord ATM as of September 30, 2023.
The Company’s primary use of cash and cash equivalents is to fund our operating expenses, which consist of research and development (R&D) and general and administrative (G&A) expenditures. Cash used to fund operating expenses is impacted by the timing of when the Company pays these expenses, as reflected in the change in accounts payable and accrued expenses. Net cash used in operating activities was $6.6 million and $12.3 million for the years ended September 30, 2023 and 2022, respectively. The Company incurred net losses of $8.4 million and $17.6 million for the years ended September 30, 2023 and 2022.
Subsequent to the year end, in October 2023, the Company entered into a multi-year contribution agreement (2023 SIF Agreement) with the Canadian Government’s Strategic Innovation Fund (SIF). Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding. Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue. See Note 9. In February 2021, the Company signed a contribution agreement with the Canadian government’s SIF (2021 SIF Agreement), the Company was eligible to receive cash reimbursements up to C$14.1 million in the aggregate for certain R&D expenses related to our EB05 clinical development program. All potential funding available under the 2021 SIF Agreement has been received. For the years ended September 30, 2023 and 2022, the Company recorded grant income of $0.6 million and $0.8 million respectively related to both the 2023 SIF Agreement and the 2021 SIF Agreement.
Subsequent to the year end, in October 2023, the Company entered into $10.0 million revolving credit agreement with Pardeep Nijhawan Medicine Professional Corporation (Credit Agreement), providing an unsecured revolving credit facility, with a credit limit of $3.5 million (Credit Limit) which is available immediately. The line of credit bears interest at the Canadian Imperial Bank of Commerce US Base-Interest Rate plus 3% per annum and has a maturity date of March 31, 2026, unless terminated earlier by either party with 90 days’ notice. Advances under the line of credit are tied to a borrowing base (Borrowing Base) consisting of eligible grant receivables from SIF, future potential license fee receivables and any other accounts receivable. At no time shall the aggregate principal amount of all advances outstanding exceed the lesser of (i) the Credit Limit and (ii) an amount equal to 85% of the Borrowing Base. No amounts have been drawn upon from the Credit Agreement.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
In March 2023, the Company entered into an equity distribution agreement with Canaccord, as sales agent, pursuant to which the Company may offer and sell, from time to time, common shares through an at-the-market equity offering program for up to $20 million in gross proceeds, subject to certain offering limitations that currently allows the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million. At September 30, 2023, the Company sold a total of 196,401 common shares pursuant to the agreement for gross proceeds of approximately $1.3 million.
In November 2022, the Company completed a private placement of units consisting of 384,475 common shares, 12-month warrants to purchase up to an aggregate of 192,248 common shares and 3-year warrants to purchase up to an aggregate of 192,248 common shares. The gross proceeds from this offering are approximately $3.0 million, before offering expenses.
In March 2022, the Company completed a registered direct offering of 220,000 common shares, no par value, and pre-funded warrants to purchase up to an aggregate of 171,390 common shares. In a concurrent private placement, the Company issued common share purchase warrants to purchase an aggregate of up to 391,390 common shares. Net proceeds to the Company were approximately $9.0 million.
During the year ended September 30, 2022, the Company sold a total of 89,558 common shares for net proceeds of $2.6 million, under an at-the-market equity offering program.
The Company plans to finance operations for at least the next twelve months with cash and cash equivalents on hand, utilization of the Canaccord ATM, drawing upon the Credit Agreement and reimbursements of eligible R&D expenses under the Company’s 2023 SIF Agreement.
1. Basis of preparation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) and include the accounts of the Company and its wholly owned subsidiaries, Edesa Biotech Research, Inc. and Edesa Biotech USA, Inc. All intercompany balances and transactions have been eliminated upon consolidation.
2. Significant accounting policies
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the period or year. Actual results could differ from those estimates. Areas where significant judgment is involved in making estimates are valuation of accounts and other receivable; valuation and useful lives of property and equipment; intangible assets; right-of-use assets; deferred income taxes; the determination of fair value of share-based compensation; the determination of fair value of warrants in order to allocate proceeds from equity issuances; and forecasting future cash flows for assessing the going concern assumption.
Functional and reporting currencies
The consolidated financial statements of the Company are presented in U.S. dollars, unless otherwise stated, which is the Company’s and its wholly owned subsidiary’s, Edesa Biotech USA, Inc., functional currency. The functional currency of the Company’s wholly owned subsidiary, Edesa Biotech Research, Inc., as determined by management, is Canadian dollars.
Cash and cash equivalents
Cash and cash equivalents consist of demand deposits with financial institutions held in checking, savings and money market mutual funds and highly liquid investments which are readily convertible into cash with maturities of three months or less when purchased. The carrying amount of cash and cash equivalents approximates its fair value due to its short-term nature.
Accounts and other receivable
The Company assesses the collectability of its accounts receivable through a review of its current aging and payment terms, as well as an analysis of its historical collection rate, general economic conditions and credit status of the government agencies. Accounts and other receivable include reimbursement grant income for the Company’s federal grant with the Canadian government’s SIF and Harmonized Sales Tax (HST) refunds receivable. As of September 30, 2023, all outstanding accounts, grants and HST refunds receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
Property and equipment
Property and equipment are recorded at historical cost less accumulated depreciation and any accumulated impairment losses. Depreciation is recorded to write off the cost of assets less their residual values over their useful lives, using the declining balance and straight-line methods. Maintenance and repair expenditures that do not improve or extend the life are expensed in the period incurred. Any gain or loss arising on the disposal or retirement of an item of property and equipment is recognized as the difference between the sales proceeds and the carrying amount of the asset. The estimated useful lives, residual values and depreciation methods are reviewed at the end of each year, with the effect of any changes in estimate accounted for on a prospective basis.
The depreciation policy for the principal asset categories are calculated as follows:
| · | Computer equipment 30% declining balance method or straight line 3 years |
| · | Furniture and equipment 20% declining balance method |
Intangible assets
Intangible assets represent the exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights, acquired by entering into a license agreement with a pharmaceutical development company. Unless earlier terminated, the term of the license agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the license agreement will automatically renew for five-year periods unless either party terminates the agreement in accordance with its terms. Intangible assets are stated at their historical cost, amortized on a straight-line basis over their expected useful lives, which is 25 years, and subject to impairment review at the end of each reporting period.
Impairment of long-lived assets
Long-lived assets are tested for impairment when indicators of impairment exist. When a significant change in the expected timing or amount of the future cash flows of the financial asset is identified, the carrying amount of the financial asset is reduced and the amount of the write-down is recognized as a loss. A previously recognized impairment loss may be reversed to the extent of the improvement, provided it is not greater than the amount that would have been reported at the date of the reversal had the impairment not been recognized previously, and the amount of the reversal is recognized in net income (loss).
Right-of-Use assets and liabilities
The Company recognizes right-of-use (ROU) assets and liabilities on the balance sheet for operating leases with terms longer than 12 months. The Company follows the ongoing practical expedient not to recognize ROU assets and liabilities for short-term leases. The ROU assets are initially measured at cost and amortized using the straight-line method through the end of the lease term. The ROU liabilities are initially measured at the present value of the lease payments that are not paid at the commencement date, discounted using the Company's incremental borrowing rate.
Fair value measurement
The Company uses the fair value measurement framework for valuing financial assets and liabilities. See Note 11.
Revenue recognition
Reimbursement grant income is recognized based on the reimbursement rate included in the government contribution agreement when allowable expenses have been incurred.
Research and development
Research and development expenses principally consist of (i) contract research organizations for clinical trial management services, (ii) contract manufacturing organizations for manufacturing the drug compound(s) for use in clinical trials and (iii) salaries of employees directly involved in research and development efforts. Research and development costs are expensed as incurred.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
Share-based compensation
The Company has equity incentive plans under which various types of equity-based awards including share options, restricted shares and restricted share unit awards may be granted to employees, non-employee directors and non-employee consultants and warrants that may be granted as compensation to non-employees.
The Company measures the cost of equity-settled transactions by reference to the fair value of the equity instruments at the date at which they are granted since the fair value of the goods or services received by the Company cannot be reliably estimated.
The Company recognizes compensation expense for all share-based awards based on the estimated grant-date fair values. For restricted share unit awards to employees, the fair value is based on the 5-day volume weighted average price (VWAP) of the Company’s common shares up to the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period.
The fair value of share options is determined using the Black-Scholes option pricing model. The Company utilizes a dividend yield of zero based on the fact that the Company has never paid cash dividends and has no current intention of paying cash dividends. The Company elected an accounting policy to record forfeitures as they occur. See Note 8 for a discussion of the assumptions used by the Company in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the share option activity under the Company’s share-based compensation plan for all years presented.
The provisions of the Company’s share-based compensation plans do not require the Company to settle any options or restricted share units by transferring cash or other assets, and therefore the Company classifies the awards as equity.
Translation of foreign currency transactions
The Company’s reporting currency is the U.S. dollar. The financial statements of the wholly owned Canadian subsidiary is measured using the Canadian dollar as the functional currency. Assets and liabilities of the Canadian operation have been translated at year-end exchange rates and related revenue and expenses have been translated at average exchange rates for the year. Accumulated gains and losses resulting from the translation of the financial statements of the Canadian operation are included as part of accumulated other comprehensive loss, a separate component of shareholders’ equity.
For other transactions denominated in currencies other than the Company’s functional currency, the monetary assets and liabilities are translated at the year-end rates. Revenue and expenses are translated at rates of exchange prevailing on the transaction dates. Non-monetary balance sheet and related income statement accounts are remeasured into U.S. dollar using historical exchange rates. All of the exchange gains or losses resulting from these other transactions are recognized in the statements of operations and comprehensive loss.
Income taxes
Deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the tax bases of assets and liabilities and their financial statement reported amounts using enacted tax rates and laws in effect in the year in which the differences are expected to reverse. A valuation allowance is provided against deferred tax assets when it is determined to be more likely than not that the deferred tax asset will not be realized.
The Company assesses the likelihood of the financial statement effect of a tax position that should be recognized when it is more likely than not that the position will be sustained upon examination by a taxing authority based on the technical merits of the tax position, circumstances, and information available as of the reporting date. The Company is subject to examination by taxing authorities in Canada and the U.S. Management does not believe that there are any uncertain tax positions that would result in an asset or liability for taxes being recognized in the accompanying financial statements. The Company recognizes tax-related interest and penalties, if any, as a component of income tax expense.
The Company accounts for income taxes on a tax jurisdictional basis. The Company files income tax returns in Canada, the provinces of British Columbia and Ontario, the U.S. and the state of California.
Earnings (loss) per share
Basic earnings (loss) per share is calculated by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding during the year.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
The computation of diluted earnings (loss) per share assumes the conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on earnings (loss) per share. The dilutive effect of convertible securities would be reflected in diluted earnings per share by application of the “if converted” method. The dilutive effect of outstanding share options and warrants and their equivalents would be reflected in diluted earnings per share by application of the treasury stock method. However, conversion of outstanding share options and warrants would have an antidilutive effect on loss per share for the years ended September 30, 2023 and 2022 and are therefore excluded from the computation of diluted loss per share. See Note 8 for share options and warrants at September 30, 2023 and 2022.
Segmented Information
The Company’s operations comprise a single reportable segment engaged in the research and development, manufacturing and commercialization of innovative pharmaceutical products. As the operations comprise a single reportable segment, amounts disclosed in the consolidated financial statements for net loss, comprehensive loss, depreciation and total assets also represent segmented amounts.
Adoption of Recent Accounting Pronouncements
On October 1, 2022, the Company adopted Accounting Standards Update ASU 2021-10 Disclosure by Business Entities About Government Assistance, modifying ASC Topic 832, Government Assistance. The amendments in ASU 2021-10 require disclosure of information about certain types of government assistance received. The Company expanded its disclosures related to government assistance.
Future accounting pronouncements
In November 2023, the FASB issued Accounting Standards Update ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure of incremental segment information on an interim and annual basis. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal periods beginning after December 15, 2024, and requires retrospective application to all prior periods presented in the financial statements. The Company is currently evaluating the impact of the guidance on the consolidated financial statements and disclosures.
4. Property and equipment
Property and equipment, net consisted of the following:
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Computer equipment | | $ | 46,945 | | | $ | 46,674 | |
Furniture and equipment | | | 5,603 | | | | 5,538 | |
| | | | | | | | |
| | | 52,548 | | | | 52,212 | |
Less: accumulated depreciation | | | (43,846 | ) | | | (39,518 | ) |
| | | | | | | | |
Total property and equipment, net | | $ | 8,702 | | | $ | 12,694 | |
Depreciation expense amounted to $4,328 and $6,991 for the years ended September 30, 2023 and 2022, respectively.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
5. Intangible assets
Acquired License
In April 2020, the Company entered into a license agreement with a pharmaceutical development company to obtain exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights. Unless earlier terminated, the term of the license agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the license agreement will automatically renew for five-year periods unless either party terminates the agreement in accordance with its terms.
Under the license agreement, the Company is exclusively responsible, at its expense, for the research, development manufacture, marketing, distribution and commercialization of the Constructs and licensed products and to obtain all necessary licenses and rights. The Company is required to use commercially reasonable efforts to develop and commercialize the Constructs in accordance with the terms of a development plan established by the parties.
The Company has determined that the license has multiple alternative future uses in research and development projects and sublicensing in other countries or for other disease indications. The value of the acquired license is recorded as an intangible asset with amortization over the estimated useful life of 25 years and evaluation for impairment at the end of each reporting period.
The required upfront license payment of $2.5 million was paid by issuance of Series A-1 Convertible Preferred Shares, which have been fully converted to common shares. The value of the license includes acquisition legal costs. See Note 7 for license commitments.
Intangible assets, net consisted of the following:
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
The Constructs | | $ | 2,529,483 | | | $ | 2,529,483 | |
| | | | | | | | |
Less: accumulated amortization | | | (349,463 | ) | | | (248,291 | ) |
| | | | | | | | |
Total intangible assets, net | | $ | 2,180,020 | | | $ | 2,281,192 | |
Amortization expense amounted to $101,172 for each of the years ended September 30, 2023, and 2022, respectively.
Total estimated future amortization of intangible assets for each fiscal year is as follows:
Year Ending | | | |
September 30, 2024 | | | 101,172 | |
September 30, 2025 | | | 101,172 | |
September 30, 2026 | | | 101,172 | |
September 30, 2027 | | | 101,172 | |
September 30, 2028 | | | 101,172 | |
Thereafter | | | 1,674,160 | |
| | | | |
| | $ | 2,180,020 | |
6. Right-of-Use Asset and Liabilities
Related party ROU asset and liability
The Company leases a facility used for executive offices from a related company. The original lease expired in December 2022 and the Company executed a two-year term extension through December 31, 2024.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
The components of lease cost were as follows:
| | September 30, 2023 | | | September 30, 2022 | |
Right-of-use lease cost, included in general and administrative on the Statements of Operations | | $ | 82,358 | | | $ | 18,465 | |
Lease terms and discount rates were as follows:
| | September 30, 2023 | | | September 30, 2022 | |
Remaining lease term (months): | | | 15 | | | | 3 | |
Estimated incremental borrowing rate: | | | 9.2 | % | | | 6.5 | % |
The approximate future minimum lease payments under operating leases at September 30, 2023 were as follows:
Year Ending | | | |
September 30, 2024 | | $ | 79,697 | |
September 30, 2025 | | | 19,924 | |
| | | | |
Total lease payments | | | 99,621 | |
Less imputed interest | | | 5,134 | |
| | | | |
Present value of right-of-use lease liabilities | | | 94,487 | |
Present value included in current liabilities | | | 74,714 | |
| | | | |
Present value included in long-term liabilities | | $ | 19,773 | |
Cash flow information was as follows:
| | Years Ended | |
| | September 30, 2023 | | | September 30, 2022 | |
Cash paid for amounts included in the measurement of right-of-use lease liabilities, included in accounts payable and accrued liabilities on the Statements of Cash Flow. | | $ | 79,222 | | | $ | 80,377 | |
7. Commitments
Research and other commitments
The Company has commitments for contracted research organizations who perform clinical trials for the Company’s ongoing clinical studies and other service providers. Aggregate future contractual payments at September 30, 2023 are as follows:
Year Ending | | | |
| | | |
September 30, 2024 | | | 1,798,000 | |
September 30, 2025 | | | 49,000 | |
September 30, 2026 | | | 36,000 | |
September 30, 2027 | | | 41,000 | |
September 30, 2028 | | | - | |
| | | | |
| | $ | 1,924,000 | |
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
License and royalty commitments
In April 2020, through its Ontario subsidiary, the Company entered into a license agreement with a third party to obtain exclusive world-wide rights to certain know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights. An intangible asset for the acquired license has been recognized. See Note 5 for intangible assets. Under the license agreement, the Company is committed to payments of up to an aggregate amount of $356 million contingent upon meeting certain milestones outlined in the license agreement, primarily relating to future potential commercial approval and sales milestones. The Company also has a commitment to pay royalties based on any net sales of products containing the Constructs in the countries where the Company directly commercializes the products containing the Constructs and a percentage of any sublicensing revenue received by the Company and its affiliates in the countries where it does not directly commercialize the products containing the Constructs. No milestone, royalty or sublicensing payments were made to the third party during the years ended September 30, 2023 and 2022. In connection with this license agreement and pursuant to a purchase agreement entered into in April 2020, the Company acquired drug substance of one of the Constructs for an aggregate purchase price of $5.0 million. The Company recorded an expense of $2.5 million for the second installment payment during the year ended September 30, 2022. No expense was recorded during the year ended September 30, 2023.
In 2016, through its Ontario subsidiary, the Company entered into a license agreement with a third party to obtain exclusive rights to certain know-how, patents and data relating to a pharmaceutical product. The Company will use the exclusive rights to develop the product for therapeutic, prophylactic and diagnostic uses in topical dermal applications and anorectal applications. No intangible assets have been recognized under the license agreement with the third party. Under the license agreement, the Company is committed to payments of various amounts to the third party upon meeting certain milestones outlined in the license agreement, up to an aggregate amount of $18.4. Upon divestiture of substantially all of the assets of the Company, the Company shall pay the third party a percentage of the valuation of the licensed technology sold as determined by an external objective expert. The Company also has a commitment to pay the third party a royalty based on net sales of the product in countries where the Company, or an affiliate, directly commercializes the product and a percentage of sublicensing revenue received by the Company and its affiliates in the countries where it does not directly commercialize the product. No license or royalty payments were made to the third party during the years ended September 30, 2023 and 2022, respectively.
In March 2021, through its Ontario subsidiary, the Company entered into a license agreement with the inventor of the same pharmaceutical product to acquire global rights for all fields of use beyond those named under the 2016 license agreement. For the years ended September 30, 2023 and 2022, the Company recorded expenses of $50,000 and $25,693, respectively, as a result of meeting milestones outlined in the 2021 license agreement. The Company is committed to remaining payments of up to an aggregate amount of $68.9 million, primarily relating to future potential commercial approval and sales milestones. In addition, if the Company fails to file an investigational new drug application or foreign equivalent (IND) for the product within a certain period of time following the date of the agreement, the Company is required to remit to the inventor a fixed license fee quarterly as long as the requirement to file an IND remains unfulfilled.
Retirement savings plan 401(k) contributions
Executive officers and employees of our California subsidiary are eligible to receive the Company’s non-elective safe harbor employer contribution of 3% of eligible compensation under a 401(k) plan to provide retirement benefits. Employees are 100% vested in employer contributions and in any voluntary employee contributions. Contributions to the 401(k) plan were $16,872 and $19,740 during the years ended September 30, 2023 and 2022, respectively.
8. Capital shares
Equity offerings
On November 2, 2022, the Company completed a private placement of units consisting of 384,475 common shares, Class A warrants to purchase up to an aggregate of 192,248 common shares and Class B warrants to purchase up to an aggregate of 192,248 common shares. Net proceeds from the offering were $2.9 million, which were allocated between the relative fair values of the common shares (using a fair value of $2.7 million) and the common share purchase warrants (using a total fair value of $1.2 million). The warrants became exercisable December 23, 2022. The Class A warrants have an exercise price of $10.50 per share and will expire on December 23, 2025. The Class B warrants have an exercise price of $7.00 per share and will expire on December 23, 2023. The warrants are considered contracts on the Company’s own shares and are classified as equity.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
On March 24, 2022, the Company completed a registered direct offering of 220,000 common shares, no par value, and pre-funded warrants to purchase up to an aggregate of 171,390 common shares. In a concurrent private placement, the Company issued common share purchase warrants to purchase an aggregate of up to 391,390 common shares. Net proceeds from the offering were $9.0 million. The common share purchase warrants were immediately exercisable at an exercise price of $24.64 per share and will expire on September 24, 2027. The pre-funded warrants were immediately exercisable at an exercise price of $0.0007 per share and do not expire. The warrants are considered contracts on the Company’s own shares and are classified as equity. The Company allocated gross proceeds with $5.9 million as the value of common shares and pre-funded warrants and $4.1 million as the value of common share purchase warrants under additional paid-in capital on a relative fair value basis. In connection with the offering, the Company issued warrants to purchase an aggregate of 27,397 common shares to certain affiliated designees of the placement agent as part of the placement agent’s compensation. The placement agent warrants are exercisable on or after March 24, 2022, at an exercise price of $31.9375 per share and will expire on March 21, 2027 with a fair value of $0.4 million.
Equity distribution agreements
On March 27, 2023, the Company entered into the Canaccord ATM, pursuant to which the Company may offer and sell, from time to time, common shares through an at-the-market equity offering program for up to $20 million in gross proceeds, subject to certain offering limitations that currently allow the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million. The Company has no obligation to sell any of the common shares and may at any time suspend sales or terminate the equity distribution agreement in accordance with its terms. During the year ended September 30, 2023, the Company sold a total of 196,401 common shares pursuant to the agreement for gross proceeds of approximately $1.3 million.
From November 22, 2021 until terminated on March 21, 2022, the Company had an equity distribution agreement for an at-the-market equity offering program with another sales agent. During the year ended September 30, 2022, the Company sold a total of 89,558 common shares pursuant to the agreement for net proceeds of $2.6 million.
Black-Scholes option valuation model
The Company uses the Black-Scholes option valuation model to determine the fair value of share-based compensation for share options and compensation warrants granted and the fair value of warrants issued. Option valuation models require the input of highly subjective assumptions including the expected price volatility. The Company calculates expected volatility based on historical volatility of the Company’s share price. When there is insufficient data available, the Company uses a peer group that is publicly traded to calculate expected volatility. The Company adopted interest-free rates by reference to the U.S. treasury yield rates. The Company calculated the fair value of share options granted based on the expected life of 5 years considering expected forfeitures during the option term of 10 years. Expected life of warrants is based on warrant terms. The Company did not and is not expected to declare any dividends. Changes in the subjective input assumptions can materially affect the fair value estimates, and therefore the existing models do not necessarily provide a reliable single measure of the fair value of the Company’s warrants and share options.
Warrants
A summary of the Company’s warrants activity is as follows:
| | Number of Warrant Shares (#) | | | Weighted Average Exercise Price | |
| | | | | | |
Balance - September 30, 2021 | | | 102,929 | | | $ | 39.83 | |
| | | | | | | | |
Issued | | | 418,789 | | | | 25.13 | |
| | | | | | | | |
September 30, 2022 | | | 521,718 | | | $ | 28.00 | |
| | | | | | | | |
Issued | | | 384,496 | | | | 8.75 | |
Exercised | | | (100,760 | ) | | | 7.65 | |
Expired | | | (84,545 | ) | | | 37.29 | |
| | | | | | | | |
September 30, 2023 | | | 720,909 | | | $ | 19.51 | |
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
The weighted average contractual life remaining on the outstanding warrants at September 30, 2023 is 35 months.
The following table summarizes information about the warrants outstanding at September 30, 2023:
Number of Warrants (#) | | | Exercise Prices | | | Expiry Dates | |
| 110,122 | | | $ | 7.00 | | | December 2023 | |
| 1,070 | | | $ | 33.67 | | | June 2024 | |
| 1,687 | | | $ | 22.40 | | | January 2025 | |
| 173,614 | | | $ | 10.50 | | | December 2025 | |
| 15,627 | | | $ | 56.00 | | | February 2026 | |
| 27,399 | | | $ | 31.94 | | | March 2027 | |
| 391,390 | | | $ | 24.64 | | | September 2027 | |
| 720,909 | | | | | | | | |
The fair value of warrants issued during the years ended September 30, 2023 and 2022 was estimated using the Black-Scholes option valuation model using the following assumptions:
| | Year Ended September 30, 2023 | | | Year Ended September 30, 2022 | |
| | Class A Warrants | | | Class B Warrants | | | Common Warrants | | | Placement Agent Warrants | |
| | | | | | | | | | | | |
Risk free interest rate | | | 4.54 | % | | | 4.76 | % | | | 2.37 | % | | | 2.37 | % |
Expected life | | 3.14 years | | | 1.14 years | | | 5.5 years | | | 5 years | |
Expected share price volatility | | | 90.73 | % | | | 89.70 | % | | | 87.09 | % | | | 87.09 | % |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % |
Pre-funded Warrants
A summary of the Company’s pre-funded warrant activity is as follows:
| | Number of Pre-funded Warrant Shares (#) | |
Balance - September 30, 2021 | | | - | |
| | | | |
Issued | | | 171,389 | |
Exercised | | | (171,389 | ) |
| | | | |
Balance - September 30, 2022 | | | - | |
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
Share Options
The Company adopted an Equity Incentive Compensation Plan in 2019 (the 2019 Plan) administered by the independent members of the Board of Directors, which amended and restated prior plans. Options, restricted shares and restricted share units are eligible for grant under the 2019 Plan. The total number of shares available for issuance under the terms of the 2019 Plan is 575,737. The remaining number of shares available to grant at September 30, 2023 is 81,765.
The Company’s 2019 Plan allows options to be granted to directors, officers, employees and certain external consultants and advisers. Under the 2019 Plan, the option term is not to exceed 10 years and the exercise price of each option is determined by the independent members of the Board of Directors.
Options have been granted under the 2019 Plan allowing the holders to purchase common shares of the Company as follows:
| | Number of Options (#) | | | Weighted Average Exercise Price | | | Weighted Average Grant Date Fair Value | |
| | | | | | | | | |
Balance - September 30, 2021 | | | 253,777 | | | $ | 35.42 | | | $ | 26.53 | |
| | | | | | | | | | | | |
Granted | | | 71,451 | | | | 25.62 | | | | 17.36 | |
Forfeited | | | (3,851 | ) | | | 45.92 | | | | 34.79 | |
Expired | | | (6,524 | ) | | | 56.35 | | | | 45.36 | |
| | | | | | | | | | | | |
Balance - September 30, 2022 | | | 314,853 | | | $ | 32.62 | | | $ | 23.94 | |
| | | | | | | | | | | | |
Granted | | | 118,579 | | | | 7.47 | | | | 5.23 | |
Forfeited | | | (12,779 | ) | | | 22.76 | | | | 16.23 | |
Expired | | | (38 | ) | | | 1,973.20 | | | | 1,973.20 | |
| | | | | | | | | | | | |
Balance - September 30, 2023 | | | 420,615 | | | $ | 25.60 | | | $ | 18.84 | |
There were no options exercised during the years ended September 30, 2023 or September 30, 2022 and there was no intrinsic value of options outstanding at September 30, 2023.
The weighted average contractual life remaining on the outstanding options at September 30, 2022 is 85 months.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
The following table summarizes information about the options under the 2019 Plan outstanding and exercisable at September 30, 2023:
Number of Options (#) | | | Exercisable at September 30, 2023 (#) | | | Range of Exercise Prices | | | Expiry Dates | |
| 497 | | | | 497 | | | $ | 246.96 - 596.82 | | | Dec 2023 - Mar 2025 | |
| 42,348 | | | | 42,348 | | | C$ | 15.12 | | | May 2024 - Dec 2028 | |
| 46,285 | | | | 46,285 | | | $ | 22.12 | | | May 2024 - Feb 2030 | |
| 56,722 | | | | 56,702 | | | $ | 52.08 - 56.49 | | | May 2024 - Oct 2030 | |
| 93,344 | | | | 81,048 | | | $ | 36.75 - 40.18 | | | Apr 2024 - Sep 2031 | |
| 68,777 | | | | 44,441 | | | $ | 20.58 - 25.97 | | | Apr 2024 - Feb 2032 | |
| 112,642 | | | | 18,334 | | | $ | 5.79 - 10.01 | | | Apr 2024 - Jul 2033 | |
| 420,615 | | | | 289,655 | | | | | | | | |
The options exercisable at September 30, 2023 had a weighted average exercise price of $31.03, no intrinsic value and a weighted average remaining life of 74 months. There were 130,960 options at September 30, 2023 that had not vested with a weighted average exercise price of $13.61 no intrinsic value and a weighted average remaining life of 111 months.
The fair value of options granted during the years ended September 30, 2023 and 2022 was estimated using the Black-Scholes option valuation model using the following assumptions:
| | Years Ended | |
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Risk free interest rate | | 3.62% - 4.18% | | | 1.71% - 2.54% | |
Expected life | | 5 years | | | 5 years | |
Expected share price volatility | | 95.3% - 97.34% | | | 85.91% - 86.59% | |
Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
The Company recorded $1.2 million and $2.3 million of share-based compensation expenses for the years ended September 30, 2023 and 2022, respectively.
As of September 30, 2023, the Company had approximately $0.5 million of unrecognized share-based compensation expense, which is expected to be recognized over a period of 31 months.
Restricted Share Units
The Company's 2019 Plan allows restricted share units (RSUs) to be granted to directors, officers, employees and certain external consultants and advisers. Under the 2019 Plan, the RSU term is not to exceed 10 years. The fair value is based on the 5-day VWAP of the Company's common shares up to the date of grant.
The following is a summary of changes in the status of RSUs from October 1, 2021 through September 30, 2023:
| | Number of RSU (#) | | | Weighted Average Grant Date Fair Value | |
| | | | | | | | |
Balance - September 30, 2021 and 2022 | | | - | | | $ | - | |
| | | | | | | | |
Granted | | | 46,602 | | | | 5.60 | |
Converted to common shares | | | (13,557 | ) | | | 5.60 | |
| | | | | | | | |
Balance - September 30, 2023 | | | 33,045 | | | $ | 5.60 | |
The following table summarizes information about the RSUs under the 2019 Plan outstanding and exercisable at September 30, 2023:
| | | Number of RSU(#) | | Expiry Date | |
Fully-vested RSUs | | | 33,045 | | August 4, 2033 | |
The RSUs that were granted in the current year were in lieu of cash bonuses for certain employees and in lieu of payments on consulting invoices for services prior to the appointment of the new Chief Financial Officer. All RSUs that were granted in the current year vested immediately upon the grant date. The outstanding RSUs can be converted to common shares by the holder at any time prior to the expiry date.
There is no future unrecorded compensation expense for the RSUs.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
9. Government Contributions
Reimbursement grant income for the Company’s federal grant with the Canadian government’s SIF is recorded based on the claim period of eligible costs.
In February 2021, the Company entered into the 2021 SIF Agreement with the Canadian Government. Under the 2021 SIF Agreement, the Government of Canada committed up to C$14.1 million in nonrepayable funding which was intended to support research and development related to our EB05 clinical program. Under the February 2021 SIF Agreement the Company recorded grant income of $0.8 million for the year ended September 30, 2022. No grant income was recorded under the 2021 SIF Agreement during the year ended September 30, 2023. No further funding will be received from the 2021 SIF Agreement.
In October 2023, the Company entered into the 2023 SIF Agreement with the Canadian Government. Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding toward (i) conducting and completing the Company’s Phase 3 clinical study of its experimental drug EB05 in critical-care patients with Acute Respiratory Distress Syndrome (ARDS) caused by COVID-19 or other infectious agents, (ii) submitting EB05 for governmental approvals and manufacturing scale-up, following, and subject to, completing the Phase 3 study and (iii) conducting two non-clinical safety studies to assess the potential long-term impact of EB05 exposure (the Project). Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue. The repayable portion would be payable over fifteen (15) years based on a percentage rate of the Company’s annual revenue growth. The maximum amount repayable under the Agreement is 1.4 times the original repayable amount. In addition, the Company is entitled to partial reimbursement of certain eligible expenses under the Agreement.
Under the Agreement, the Company agreed to certain financial and non-financial covenants and other obligations in relation to the Project. Pursuant to the Agreement, certain customary events of default, such as the Company’s or Edesa Biotech Research’s breach of their covenants and obligations under the Agreement, their insolvency, winding up or dissolution, and other similar events, may permit the Government of Canada to declare an event of default under the Agreement. Upon an event of default, subject to applicable cure, the Government of Canada may exercise a number of remedies, including suspending or terminating funding under the Agreement, demanding repayment of funding previously received and/or terminating the Agreement.
The funding and any associated conditional repayments are not secured by any assets of Edesa Biotech Research or the Company.
The Agreement will expire on the later of December 31, 2042 or the date of the last repayment, unless earlier terminated, subject to certain provisions that extend three (3) years beyond the term or early termination of the Agreement.
Under the October 2023 SIF Agreement the Company recorded grant income of $0.6 million for the year ended September 30, 2023. No grant income was recorded under the 2023 SIF Agreement during the year ended September 30, 2022.
10. Income Tax
The reconciliation of the combined Canadian federal and provincial statutory income tax rate to the approximate effective tax rate is as follows:
| | Years Ended | |
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Net loss before recovery of income taxes | | $ | (8,374,000 | ) | | $ | (17,548,000 | ) |
Canadian federal and provincial statutory income tax rate | | | 26.5 | % | | | 26.5 | % |
| | | | | | | | |
Expected income tax recovery | | $ | (2,219,000 | ) | | $ | (4,650,000 | ) |
Effect of foreign currency and foreign tax rate differences | | | (207,200 | ) | | | 976,800 | |
Permanent differences | | | 339,000 | | | | 650,000 | |
Share issuance cost booked through equity or capitalization | | | (89,000 | ) | | | (449,000 | ) |
Non-capital losses limitation - U.S. | | | 899,000 | | | | - | |
Other | | | (94,000 | ) | | | - | |
Change in valuation allowance | | | 1,372,000 | | | | 3,473,000 | |
| | | | | | | | |
Income tax (recovery) expense | | $ | 800 | | | $ | 800 | |
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
Components of the net deferred tax asset or liability
Deferred taxes are provided as a result of temporary differences that arise due to the difference between the income tax values and the carrying amount of assets and liabilities. Approximate deferred tax assets and liabilities are as follows:
| | September 30, 2023 | | | September 30, 2022 | |
| | | | | | |
Non-capital losses carried forward - Canada | | $ | 13,943,000 | | | $ | 11,740,000 | |
Non-capital losses carried forward - U.S. | | | 731,000 | | | | 1,631,000 | |
Research and development tax credits | | | 1,371,000 | | | | 1,052,000 | |
Share issuance and financing costs | | | 585,000 | | | | 686,000 | |
Right-of-use lease liabilities | | | 25,000 | | | | 5,000 | |
Other temporary differences | | | 43,000 | | | | 15,000 | |
| | | | | | | | |
Subtotal | | $ | 16,699,000 | | | $ | 15,129,000 | |
Less: valuation allowance | | | (16,466,000 | ) | | | (15,093,000 | ) |
| | | | | | | | |
Total net deferred tax assets | | $ | 233,000 | | | $ | 36,000 | |
| | | | | | | | |
Property and equipment | | $ | (3,000 | ) | | $ | (15,000 | ) |
Right-of-use assets | | | (24,000 | ) | | | (5,000 | ) |
Grant Income receivable | | | (153,000 | ) | | | - | |
Deferred share issuance costs | | | (53,000 | ) | | | (16,000 | ) |
| | | | | | | | |
Total deferred tax liabilities | | $ | (233,000 | ) | | $ | (36,000 | ) |
| | | | | | | | |
Net deferred taxes | | $ | - | | | $ | - | |
Realization of the deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. It is more likely than not that a tax benefit will not be realized. Accordingly, net deferred tax assets have been fully offset by a valuation allowance.
Non-capital losses, capital losses, and research and development credits generated by Edesa Biotech USA, Inc. prior to changes in share ownership that occurred as a result of the reverse acquisition are substantially limited. It is unlikely that tax losses totaling $29.6 million and credits totaling $0.6 million will be utilized to offset potential future taxable income before expiration and they are excluded from deferred tax assets above.
The approximate Canadian non-capital losses carried forward at September 30, 2023 expire as follows:
2025 | | C$ | 21,000 | |
2026 | | | 56,000 | |
2027 | | | 114,000 | |
2028 | | | 233,000 | |
2029 | | | 688,000 | |
2030 | | | 860,000 | |
2031 | | | 685,000 | |
2032 | | | 673,000 | |
2033 | | | 107,000 | |
2034 | | | 1,941,000 | |
2035 | | | 2,207,000 | |
2036 | | | 2,216,000 | |
2037 | | | 2,123,000 | |
2038 | | | 3,500,000 | |
2039 | | | 1,732,000 | |
2040 | | | 7,992,000 | |
2041 | | | 12,675,000 | |
2042 | | | 22,387,000 | |
2043 | | | 10,765,000 | |
| | | | |
| Total | | C$ | 70,975,000 | |
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
Share issuance and financing costs will be fully amortized in 2026.
The U.S. non-capital losses carried forward at September 30, 2023 totaled approximately $3.4 million, which do not expire for federal taxes. The U.S. state research and development tax credits carried forward at September 30, 2023 totaled approximately $0.6 million, which do not expire for state taxes. The approximate U.S. state non-capital losses carried forward at September 30, 2023 expire as follows:
2039 | | $ | 70,000 | |
2040 | | | 150,000 | |
2041 | | | 68,000 | |
2042 | | | 6,000 | |
| | | | |
Total | | $ | 294,000 | |
11. Financial instruments
(a) Fair values
The Company uses the fair value measurement framework for valuing financial assets and liabilities measured on a recurring basis in situations where other accounting pronouncements either permit or require fair value measurements.
The Company follows the fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Observable inputs are inputs that reflect assumptions market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances.
There are three levels of inputs that may be used to measure fair value:
| · | Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| · | Level 2 - Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets and liabilities in markets that are not active. |
| · | Level 3 - Unobservable inputs for the asset or liability that are supported by little or no market activity. |
The carrying value of certain financial instruments such as cash and cash equivalents, accounts and other receivable, accounts payable and accrued liabilities approximates fair value due to the short-term nature of such instruments.
(b) Interest rate and credit risk
Interest rate risk is the risk that the value of a financial instrument might be adversely affected by a change in interest rates. The Company does not believe that the results of operations or cash flows would be affected to any significant degree by a significant change in market interest rates, relative to interest rates on cash and cash equivalents due to the short-term nature of these balances.
The Company is also exposed to credit risk at period end from the carrying value of its cash and cash equivalents and accounts and other receivable. The Company manages this risk by maintaining bank accounts with Canadian Chartered Banks, U.S. banks believed to be credit worthy and money market mutual funds of U.S. government securities. The Company’s cash is not subject to any external restrictions. The Company assesses the collectability of accounts receivable through a review of the current aging and terms, as well as an analysis of historical collection rates, general economic conditions and credit status of government agencies. Credit risk for the reimbursement grant and HST refunds receivable are not considered significant since amounts are due from the Canadian government’s SIF and the Canada Revenue Agency.
EDESA BIOTECH, INC.
Notes to Consolidated Financial Statements
For the Years Ended September 30, 2023 and 2022
(c) Foreign exchange risk
The Company and its subsidiary have balances in Canadian dollars that give rise to exposure to foreign exchange (FX) risk relating to the impact of translating certain non-U.S. dollar balance sheet accounts as these statements are presented in U.S. dollars. A strengthening U.S. dollar will lead to a FX loss while a weakening U.S. dollar will lead to a FX gain. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks. At September 30, 2023, the Company and its Canadian subsidiary had assets denominated in Canadian dollars of approximately C$3.0 million and the U.S. dollar exchange rate at this date was equal to 1.3581 Canadian dollars. Based on the exposure at September 30, 2023, a 10% annual change in the Canadian/U.S. exchange rate would impact the Company’s loss and other comprehensive loss by approximately $0.2 million.
(d) Liquidity risk
Liquidity risk is the risk that the Company will encounter difficulty raising liquid funds to meet commitments as they fall due. In meeting its liquidity requirements, the Company closely monitors its forecasted cash requirements with expected cash drawdown.
12. Loss per Share
The Company had securities outstanding which could potentially dilute basic earnings per share in the future but were excluded from the computation of diluted loss per share in the periods presented, as their effect would have been anti-dilutive.
13. Related party transactions
During each of the years ended September 30, 2023 and 2022, the Company paid cash of $82,000 and $81,000, respectively, for a ROU lease from a company controlled by the Company's CEO. These transactions are in the normal course of operations and are measured at the exchange amount, which is the amount of consideration established and agreed to by both parties. On December 31, 2022, the Company executed a two-year lease extension through December 31, 2024 in accordance with the terms of the original lease agreement. Rents of approximately $15,000 and $22,000 were payable at September 30, 2023 and September 30, 2022, respectively.
14. Subsequent events
Subsequent to the year end, equity sales under the Company’s at-the-market offering program have resulted in the issuance of 89,241 common shares and receipt of net cash proceeds of $0.3 million after deducting sales agent commissions.
In October 2023, the Company entered into $10.0 million revolving credit agreement with a company controlled by the Company's CEO, providing an unsecured revolving credit facility, with a credit limit of $3.5 million. No amounts have been drawn on the credit agreement subsequent to the year end.
nullnullnullnullnullnullnullnullnullnullnull
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPCAOB issued Audit Firm Identifier Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorFirmId |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:nonemptySequenceNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorLocation |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_AuditorName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:internationalNameItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an annual report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentAnnualReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates whether any of the financial statement period in the filing include a restatement due to error correction. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-K
-Number 229
-Section 402
-Subsection w
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 4: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_DocumentFinStmtErrorCorrectionFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.
| Name: |
dei_EntityPublicFloat |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
| Name: |
dei_EntityVoluntaryFilers |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 405
| Name: |
dei_EntityWellKnownSeasonedIssuer |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- ReferencesReference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-K
-Number 249
-Section 310
Reference 2: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 20-F
-Number 249
-Section 220
-Subsection f
Reference 3: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_IcfrAuditorAttestationFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Balance Sheets - USD ($)
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 5,361,397
|
$ 7,090,919
|
| Accounts and other receivable |
626,543
|
1,255,451
|
| Prepaid expenses and other current assets |
448,912
|
745,543
|
| Total current assets |
6,436,852
|
9,091,913
|
| Non-current assets: |
|
|
| Property and equipment, net |
8,702
|
12,694
|
| Long-term deposits |
173,490
|
171,464
|
| Intangible asset, net |
2,180,020
|
2,281,192
|
| Right-of-use assets |
91,373
|
18,465
|
| Total assets |
8,890,437
|
11,575,728
|
| Current liabilities: |
|
|
| Accounts payable and accrued liabilities |
1,747,150
|
2,121,802
|
| Short-term right-of-use lease liabilities |
74,714
|
18,975
|
| Total current liabilities |
1,821,864
|
2,140,777
|
| Non-current liabilities: |
|
|
| Long-term payables |
0
|
43,662
|
| Long-term right-of-use lease liabilities |
19,773
|
0
|
| Total liabilities |
1,841,637
|
2,184,439
|
| Shareholders' equity: |
|
|
| Capital shares Authorized unlimited common and preferred shares without par value Issued and outstanding: 3,075,473 common shares (September 30, 2022 - 2,380,280) |
46,643,151
|
42,473,099
|
| Additional paid-in capital |
13,039,265
|
11,176,345
|
| Accumulated other comprehensive loss |
(214,648)
|
(213,602)
|
| Accumulated deficit |
(52,418,968)
|
(44,044,553)
|
| Total shareholders' equity |
7,048,800
|
9,391,289
|
| Total liabilities and shareholders' equity |
$ 8,890,437
|
$ 11,575,728
|
| X |
- DefinitionAmount, after allowance, receivable from customers, clients, or other third-parties, and receivables classified as other due within one year or the normal operating cycle, if longer.
| Name: |
us-gaap_AccountsAndOtherReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of capital units or capital shares. This element is relevant to issuers of face-amount certificates and registered investment companies.
| Name: |
us-gaap_CapitalUnits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value of amounts transferred to third parties for security purposes that are expected to be returned or applied towards payment after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DepositsAssetsNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesNoncurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying value as of the balance sheet date of loans payable (with maturities initially due after one year or beyond the operating cycle if longer), excluding current portion. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.22)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LongTermLoansPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PrepaidExpenseAndOtherAssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Balance Sheets (Parenthetical) - $ / shares
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Consolidated Balance Sheets |
|
|
| Capital Shares, Par Value |
$ 0
|
$ 0
|
| Capital Shares, Issued |
3,075,473
|
2,380,280
|
| Capital Shares, Outstanding |
2,380,280
|
2,380,280
|
| X |
- DefinitionFace amount per share of no-par value common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockNoParValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Operations and Comprehensive Loss - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Expenses: |
|
|
| Research and development |
$ 4,794,549
|
$ 13,335,334
|
| General and administrative |
4,428,209
|
5,035,456
|
| Loss from operations |
(9,222,758)
|
(18,370,790)
|
| Other income (loss): |
|
|
| Reimbursement grant income |
581,039
|
780,257
|
| Interest income |
289,846
|
63,523
|
| Foreign exchange loss |
(21,742)
|
(21,114)
|
| Total other income loss |
849,143
|
822,666
|
| Loss before income taxes |
(8,373,615)
|
(17,548,124)
|
| Income tax expense |
800
|
800
|
| Net loss |
(8,374,415)
|
(17,548,924)
|
| Exchange differences on translation |
(1,046)
|
(8,340)
|
| Net comprehensive loss |
$ (8,375,461)
|
$ (17,557,264)
|
| Weighted average number of common shares |
2,858,929
|
2,096,446
|
| Loss per common share - basic and diluted |
$ (2.93)
|
$ (8.37)
|
| X |
- References
+ Details
| Name: |
edsa_EarningPerShareBasicAndDiluted |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ReimbursementGrantIncome |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageNumberOfCommonSharesOutstanding |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized and unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 35
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482014/830-20-35-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481839/830-10-45-17
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossBeforeTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income earned from interest bearing assets classified as other.
| Name: |
us-gaap_InterestIncomeOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Consolidated Statements of Cash Flows - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Cash flows from operating activities: |
|
|
| Net loss |
$ (8,374,415)
|
$ (17,548,924)
|
| Adjustments for: |
|
|
| Depreciation and amortization |
183,471
|
118,188
|
| Share-based compensation |
1,246,457
|
2,260,634
|
| Changes in working capital items: |
|
|
| Accounts and other receivable |
562,770
|
2,027,454
|
| Prepaid expenses and other current assets |
301,504
|
(19,497)
|
| Accounts payable and accrued liabilities |
(556,270)
|
882,843
|
| Net cash used in operating activities |
(6,636,483)
|
(12,279,302)
|
| Cash flows from investing activities: |
|
|
| Purchase of property and equipment |
0
|
(5,656)
|
| Net cash used in investing activities |
0
|
(5,656)
|
| Cash flows from financing activities: |
|
|
| Proceeds from issuance of common shares and warrants |
4,345,017
|
11,957,687
|
| Proceeds from exercise of warrants |
770,532
|
0
|
| Payments for issuance costs of common shares and warrants |
(285,438)
|
(328,059)
|
| Net cash provided by financing activities |
4,830,111
|
11,629,628
|
| Effect of exchange rate changes on cash and cash equivalents |
76,850
|
(93,010)
|
| Net change in cash and cash equivalents |
(1,729,522)
|
(748,340)
|
| Cash and cash equivalents, beginning of year |
7,090,919
|
7,839,259
|
| Cash and cash equivalents, end of year |
5,361,397
|
7,090,919
|
| Supplemental Disclosure of Noncash Financing Activities: |
|
|
| Issuance costs withheld from gross proceeds from issuance of common shares and warrants |
0
|
393,461
|
| Fair value of placement agent warrants |
$ 0
|
$ 408,059
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceCostsWithheldFromGrossProceedsFromIssuanceOfCommonSharesAndWarrants |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe current period expense charged against earnings on long-lived, physical assets not used in production, and which are not intended for resale, to allocate or recognize the cost of such assets over their useful lives; or to record the reduction in book value of an intangible asset over the benefit period of such asset; or to reflect consumption during the period of an asset that is not used in production. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_DepreciationAndAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 230
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount due from customers for the credit sale of goods and services; includes accounts receivable and other types of receivables. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsAndOtherReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in prepaid expenses, and assets classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for cost incurred directly with the issuance of an equity security. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfStockIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquirePropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from holders exercising their stock warrants. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromWarrantExercises |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-19
Reference 16: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 235
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-05(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479557/942-235-S99-1
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 33: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4J
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4J
Reference 34: http://www.xbrl.org/2003/role/exampleRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 4K
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481175/810-10-55-4K
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-2
Reference 38: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
Reference 39: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1A
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-1A
| Name: |
us-gaap_ProfitLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Condensed Interim Consolidated Statements of Changes in Shareholders' Equity - USD ($)
|
Total |
Additional Paid-In Capital |
Accumulated other comprehensive loss |
Retained Earnings (Accumulated Deficit) |
Common Shares |
| Balance, shares at Sep. 30, 2021 |
|
|
|
|
1,899,333
|
| Balance, amount at Sep. 30, 2021 |
$ 13,058,291
|
$ 4,871,461
|
$ (205,262)
|
$ (26,495,629)
|
$ 34,887,721
|
| Issuance of common shares and warrants in equity offering, shares |
|
|
|
|
309,558
|
| Issuance of common shares and warrants in equity offering, amount |
$ 12,941,474
|
$ 6,702,293
|
0
|
0
|
$ 6,239,181
|
| Issuance costs including fair value of placement agent warrants, shares |
0
|
0
|
|
|
0
|
| Issuance costs including fair value of placement agent warrants, amount |
$ (1,311,966)
|
$ (448,739)
|
0
|
0
|
$ (863,227)
|
| Issuance of common shares upon exercise of pre-funded warrants, net of costs, shares |
|
|
|
|
171,389
|
| Issuance of common shares upon exercise of pre-funded warrants, net of costs, amount |
120
|
(2,209,304)
|
0
|
0
|
$ 2,209,424
|
| Share-based compensation |
2,260,634
|
2,260,634
|
0
|
0
|
0
|
| Net loss and comprehensive loss |
(17,557,264)
|
0
|
(8,340)
|
(17,548,924)
|
0
|
| Net loss and comprehensive loss |
(17,557,264)
|
|
|
|
|
| Balance, amount at Sep. 30, 2022 |
9,391,289
|
11,176,345
|
(213,602)
|
(44,044,553)
|
$ 42,473,099
|
| Balance shares at Sep. 30, 2022 |
|
|
|
|
2,380,280
|
| Issuance of common shares and warrants in equity offering, shares |
|
|
|
|
580,876
|
| Issuance of common shares and warrants in equity offering, amount |
4,345,019
|
944,828
|
0
|
0
|
$ 3,400,191
|
| Share-based compensation |
1,246,457
|
1,246,457
|
0
|
0
|
$ 0
|
| Issuance of common shares upon exercise of warrants, shares |
|
|
|
|
100,760
|
| Issuance of common shares upon exercise of warrants, amount |
770,531
|
(224,087)
|
0
|
0
|
$ 994,618
|
| Issuance of common shares upon exercise of restricted stock share, shares |
|
|
|
|
13,557
|
| Issuance of common shares upon exercise of restricted stock share, amount |
0
|
(75,920)
|
0
|
0
|
$ 75,920
|
| Issuance costs, amount |
(329,035)
|
(28,358)
|
0
|
0
|
300,677
|
| Net loss and comprehensive loss |
(8,375,461)
|
0
|
(1,046)
|
(8,374,415)
|
0
|
| Balance, amount at Sep. 30, 2023 |
$ 7,048,800
|
$ 13,039,265
|
$ (214,648)
|
$ (52,418,968)
|
$ 46,643,151
|
| Balance shares at Sep. 30, 2023 |
|
|
|
|
3,075,473
|
| X |
- References
+ Details
| Name: |
edsa_ComprehensiveIncomeNetOfTax1 |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceCostsAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceCostsIncludingFairValueOfPlacementAgentWarrantsAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceCostsIncludingFairValueOfPlacementAgentWarrantsshares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesAndWarrantsInEquityOfferingAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesAndWarrantsInEquityOfferingShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesUponExerciseOfPreFunded |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesUponExerciseOfRestrictedStockUnitsAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesUponExerciseOfRestrictedStockUnitsShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesUponExerciseOfWarrantsAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_IssuanceOfCommonSharesUponExerciseOfWarrantsShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WarrantsNetOfCostsAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(24))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(26))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-5
| Name: |
us-gaap_ComprehensiveIncomeNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued as of the balance sheet date, including shares that had been issued and were previously outstanding but which are now held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
| Name: |
us-gaap_SharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Nature of operations
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Nature of operations |
|
| Nature Of Operations |
1. Nature of operations Edesa Biotech, Inc. (the Company or Edesa) is a biopharmaceutical company focused on acquiring, developing and commercializing clinical stage drugs for inflammatory and immune-related diseases with clear unmet medical needs. The Company is organized under the laws of British Columbia, Canada and is headquartered in Markham, Ontario. It operates under its wholly owned subsidiaries, Edesa Biotech Research, Inc., an Ontario, Canada corporation, and Edesa Biotech USA, Inc., a California, USA corporation. The Company’s common shares trade on The Nasdaq Capital Market in the United States under the symbol “EDSA”. Liquidity The Company’s operations have historically been funded through issuances of common shares, exercises of common share purchase warrants, convertible preferred shares, convertible loans, government grants and tax incentives. At September 30, 2023, the Company had an accumulated deficit of $52.4 million and working capital of $4.6 million, including $5.4 million in cash and cash equivalents. In August 2022 the Company filed a $150.0 million shelf registration statement, under which the Company entered into an equity distribution agreement with Canaccord for $20.0 million in gross proceeds, subject to certain offering limitations that currently allows the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million (Canaccord ATM). There was approximately $7.1 million of available capacity on the Canaccord ATM as of September 30, 2023. The Company’s primary use of cash and cash equivalents is to fund our operating expenses, which consist of research and development (R&D) and general and administrative (G&A) expenditures. Cash used to fund operating expenses is impacted by the timing of when the Company pays these expenses, as reflected in the change in accounts payable and accrued expenses. Net cash used in operating activities was $6.6 million and $12.3 million for the years ended September 30, 2023 and 2022, respectively. The Company incurred net losses of $8.4 million and $17.6 million for the years ended September 30, 2023 and 2022. Subsequent to the year end, in October 2023, the Company entered into a multi-year contribution agreement (2023 SIF Agreement) with the Canadian Government’s Strategic Innovation Fund (SIF). Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding. Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue. See Note 9. In February 2021, the Company signed a contribution agreement with the Canadian government’s SIF (2021 SIF Agreement), the Company was eligible to receive cash reimbursements up to C$14.1 million in the aggregate for certain R&D expenses related to our EB05 clinical development program. All potential funding available under the 2021 SIF Agreement has been received. For the years ended September 30, 2023 and 2022, the Company recorded grant income of $0.6 million and $0.8 million respectively related to both the 2023 SIF Agreement and the 2021 SIF Agreement. Subsequent to the year end, in October 2023, the Company entered into $10.0 million revolving credit agreement with Pardeep Nijhawan Medicine Professional Corporation (Credit Agreement), providing an unsecured revolving credit facility, with a credit limit of $3.5 million (Credit Limit) which is available immediately. The line of credit bears interest at the Canadian Imperial Bank of Commerce US Base-Interest Rate plus 3% per annum and has a maturity date of March 31, 2026, unless terminated earlier by either party with 90 days’ notice. Advances under the line of credit are tied to a borrowing base (Borrowing Base) consisting of eligible grant receivables from SIF, future potential license fee receivables and any other accounts receivable. At no time shall the aggregate principal amount of all advances outstanding exceed the lesser of (i) the Credit Limit and (ii) an amount equal to 85% of the Borrowing Base. No amounts have been drawn upon from the Credit Agreement. In March 2023, the Company entered into an equity distribution agreement with Canaccord, as sales agent, pursuant to which the Company may offer and sell, from time to time, common shares through an at-the-market equity offering program for up to $20 million in gross proceeds, subject to certain offering limitations that currently allows the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million. At September 30, 2023, the Company sold a total of 196,401 common shares pursuant to the agreement for gross proceeds of approximately $1.3 million. In November 2022, the Company completed a private placement of units consisting of 384,475 common shares, 12-month warrants to purchase up to an aggregate of 192,248 common shares and 3-year warrants to purchase up to an aggregate of 192,248 common shares. The gross proceeds from this offering are approximately $3.0 million, before offering expenses. In March 2022, the Company completed a registered direct offering of 220,000 common shares, no par value, and pre-funded warrants to purchase up to an aggregate of 171,390 common shares. In a concurrent private placement, the Company issued common share purchase warrants to purchase an aggregate of up to 391,390 common shares. Net proceeds to the Company were approximately $9.0 million. During the year ended September 30, 2022, the Company sold a total of 89,558 common shares for net proceeds of $2.6 million, under an at-the-market equity offering program. The Company plans to finance operations for at least the next twelve months with cash and cash equivalents on hand, utilization of the Canaccord ATM, drawing upon the Credit Agreement and reimbursements of eligible R&D expenses under the Company’s 2023 SIF Agreement.
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Basis of presentation
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Basis of presentation |
|
| Basis Of Presentation |
1. Basis of preparation The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (U.S. GAAP) and include the accounts of the Company and its wholly owned subsidiaries, Edesa Biotech Research, Inc. and Edesa Biotech USA, Inc. All intercompany balances and transactions have been eliminated upon consolidation.
|
| X |
- References
+ Details
| Name: |
edsa_BasisOfPresentationAbstract |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the business description and basis of presentation concepts. Business description describes the nature and type of organization including but not limited to organizational structure as may be applicable to holding companies, parent and subsidiary relationships, business divisions, business units, business segments, affiliates and information about significant ownership of the reporting entity. Basis of presentation describes the underlying basis used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 275
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//205/tableOfContent
| Name: |
us-gaap_BusinessDescriptionAndBasisOfPresentationTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Significant accounting policies
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Significant accounting policies |
|
| Significant accounting policies |
2. Significant accounting policies Use of estimates The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the period or year. Actual results could differ from those estimates. Areas where significant judgment is involved in making estimates are valuation of accounts and other receivable; valuation and useful lives of property and equipment; intangible assets; right-of-use assets; deferred income taxes; the determination of fair value of share-based compensation; the determination of fair value of warrants in order to allocate proceeds from equity issuances; and forecasting future cash flows for assessing the going concern assumption. Functional and reporting currencies The consolidated financial statements of the Company are presented in U.S. dollars, unless otherwise stated, which is the Company’s and its wholly owned subsidiary’s, Edesa Biotech USA, Inc., functional currency. The functional currency of the Company’s wholly owned subsidiary, Edesa Biotech Research, Inc., as determined by management, is Canadian dollars. Cash and cash equivalents Cash and cash equivalents consist of demand deposits with financial institutions held in checking, savings and money market mutual funds and highly liquid investments which are readily convertible into cash with maturities of three months or less when purchased. The carrying amount of cash and cash equivalents approximates its fair value due to its short-term nature. Accounts and other receivable The Company assesses the collectability of its accounts receivable through a review of its current aging and payment terms, as well as an analysis of its historical collection rate, general economic conditions and credit status of the government agencies. Accounts and other receivable include reimbursement grant income for the Company’s federal grant with the Canadian government’s SIF and Harmonized Sales Tax (HST) refunds receivable. As of September 30, 2023, all outstanding accounts, grants and HST refunds receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded. Property and equipment Property and equipment are recorded at historical cost less accumulated depreciation and any accumulated impairment losses. Depreciation is recorded to write off the cost of assets less their residual values over their useful lives, using the declining balance and straight-line methods. Maintenance and repair expenditures that do not improve or extend the life are expensed in the period incurred. Any gain or loss arising on the disposal or retirement of an item of property and equipment is recognized as the difference between the sales proceeds and the carrying amount of the asset. The estimated useful lives, residual values and depreciation methods are reviewed at the end of each year, with the effect of any changes in estimate accounted for on a prospective basis. The depreciation policy for the principal asset categories are calculated as follows: | · | Computer equipment 30% declining balance method or straight line 3 years | | · | Furniture and equipment 20% declining balance method |
Intangible assets Intangible assets represent the exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights, acquired by entering into a license agreement with a pharmaceutical development company. Unless earlier terminated, the term of the license agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the license agreement will automatically renew for five-year periods unless either party terminates the agreement in accordance with its terms. Intangible assets are stated at their historical cost, amortized on a straight-line basis over their expected useful lives, which is 25 years, and subject to impairment review at the end of each reporting period. Impairment of long-lived assets Long-lived assets are tested for impairment when indicators of impairment exist. When a significant change in the expected timing or amount of the future cash flows of the financial asset is identified, the carrying amount of the financial asset is reduced and the amount of the write-down is recognized as a loss. A previously recognized impairment loss may be reversed to the extent of the improvement, provided it is not greater than the amount that would have been reported at the date of the reversal had the impairment not been recognized previously, and the amount of the reversal is recognized in net income (loss). Right-of-Use assets and liabilities The Company recognizes right-of-use (ROU) assets and liabilities on the balance sheet for operating leases with terms longer than 12 months. The Company follows the ongoing practical expedient not to recognize ROU assets and liabilities for short-term leases. The ROU assets are initially measured at cost and amortized using the straight-line method through the end of the lease term. The ROU liabilities are initially measured at the present value of the lease payments that are not paid at the commencement date, discounted using the Company's incremental borrowing rate. Fair value measurement The Company uses the fair value measurement framework for valuing financial assets and liabilities. See Note 11. Revenue recognition Reimbursement grant income is recognized based on the reimbursement rate included in the government contribution agreement when allowable expenses have been incurred. Research and development Research and development expenses principally consist of (i) contract research organizations for clinical trial management services, (ii) contract manufacturing organizations for manufacturing the drug compound(s) for use in clinical trials and (iii) salaries of employees directly involved in research and development efforts. Research and development costs are expensed as incurred. Share-based compensation The Company has equity incentive plans under which various types of equity-based awards including share options, restricted shares and restricted share unit awards may be granted to employees, non-employee directors and non-employee consultants and warrants that may be granted as compensation to non-employees. The Company measures the cost of equity-settled transactions by reference to the fair value of the equity instruments at the date at which they are granted since the fair value of the goods or services received by the Company cannot be reliably estimated. The Company recognizes compensation expense for all share-based awards based on the estimated grant-date fair values. For restricted share unit awards to employees, the fair value is based on the 5-day volume weighted average price (VWAP) of the Company’s common shares up to the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period. The fair value of share options is determined using the Black-Scholes option pricing model. The Company utilizes a dividend yield of zero based on the fact that the Company has never paid cash dividends and has no current intention of paying cash dividends. The Company elected an accounting policy to record forfeitures as they occur. See Note 8 for a discussion of the assumptions used by the Company in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the share option activity under the Company’s share-based compensation plan for all years presented. The provisions of the Company’s share-based compensation plans do not require the Company to settle any options or restricted share units by transferring cash or other assets, and therefore the Company classifies the awards as equity. Translation of foreign currency transactions The Company’s reporting currency is the U.S. dollar. The financial statements of the wholly owned Canadian subsidiary is measured using the Canadian dollar as the functional currency. Assets and liabilities of the Canadian operation have been translated at year-end exchange rates and related revenue and expenses have been translated at average exchange rates for the year. Accumulated gains and losses resulting from the translation of the financial statements of the Canadian operation are included as part of accumulated other comprehensive loss, a separate component of shareholders’ equity. For other transactions denominated in currencies other than the Company’s functional currency, the monetary assets and liabilities are translated at the year-end rates. Revenue and expenses are translated at rates of exchange prevailing on the transaction dates. Non-monetary balance sheet and related income statement accounts are remeasured into U.S. dollar using historical exchange rates. All of the exchange gains or losses resulting from these other transactions are recognized in the statements of operations and comprehensive loss. Income taxes Deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the tax bases of assets and liabilities and their financial statement reported amounts using enacted tax rates and laws in effect in the year in which the differences are expected to reverse. A valuation allowance is provided against deferred tax assets when it is determined to be more likely than not that the deferred tax asset will not be realized. The Company assesses the likelihood of the financial statement effect of a tax position that should be recognized when it is more likely than not that the position will be sustained upon examination by a taxing authority based on the technical merits of the tax position, circumstances, and information available as of the reporting date. The Company is subject to examination by taxing authorities in Canada and the U.S. Management does not believe that there are any uncertain tax positions that would result in an asset or liability for taxes being recognized in the accompanying financial statements. The Company recognizes tax-related interest and penalties, if any, as a component of income tax expense. The Company accounts for income taxes on a tax jurisdictional basis. The Company files income tax returns in Canada, the provinces of British Columbia and Ontario, the U.S. and the state of California. Earnings (loss) per share Basic earnings (loss) per share is calculated by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding during the year. The computation of diluted earnings (loss) per share assumes the conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on earnings (loss) per share. The dilutive effect of convertible securities would be reflected in diluted earnings per share by application of the “if converted” method. The dilutive effect of outstanding share options and warrants and their equivalents would be reflected in diluted earnings per share by application of the treasury stock method. However, conversion of outstanding share options and warrants would have an antidilutive effect on loss per share for the years ended September 30, 2023 and 2022 and are therefore excluded from the computation of diluted loss per share. See Note 8 for share options and warrants at September 30, 2023 and 2022. Segmented Information The Company’s operations comprise a single reportable segment engaged in the research and development, manufacturing and commercialization of innovative pharmaceutical products. As the operations comprise a single reportable segment, amounts disclosed in the consolidated financial statements for net loss, comprehensive loss, depreciation and total assets also represent segmented amounts. Adoption of Recent Accounting Pronouncements On October 1, 2022, the Company adopted Accounting Standards Update ASU 2021-10 Disclosure by Business Entities About Government Assistance, modifying ASC Topic 832, Government Assistance. The amendments in ASU 2021-10 require disclosure of information about certain types of government assistance received. The Company expanded its disclosures related to government assistance. Future accounting pronouncements In November 2023, the FASB issued Accounting Standards Update ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure of incremental segment information on an interim and annual basis. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal periods beginning after December 15, 2024, and requires retrospective application to all prior periods presented in the financial statements. The Company is currently evaluating the impact of the guidance on the consolidated financial statements and disclosures.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Property and equipment
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Property and equipment |
|
| Property and equipment |
4. Property and equipment Property and equipment, net consisted of the following: | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Computer equipment | | $ | 46,945 | | | $ | 46,674 | | Furniture and equipment | | | 5,603 | | | | 5,538 | | | | | | | | | | | | | | 52,548 | | | | 52,212 | | Less: accumulated depreciation | | | (43,846 | ) | | | (39,518 | ) | | | | | | | | | | Total property and equipment, net | | $ | 8,702 | | | $ | 12,694 | |
Depreciation expense amounted to $4,328 and $6,991 for the years ended September 30, 2023 and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//360/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-7
| Name: |
us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible assets
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Intangible assets |
|
| Intangible assets |
5. Intangible assets Acquired License In April 2020, the Company entered into a license agreement with a pharmaceutical development company to obtain exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights. Unless earlier terminated, the term of the license agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the license agreement will automatically renew for five-year periods unless either party terminates the agreement in accordance with its terms. Under the license agreement, the Company is exclusively responsible, at its expense, for the research, development manufacture, marketing, distribution and commercialization of the Constructs and licensed products and to obtain all necessary licenses and rights. The Company is required to use commercially reasonable efforts to develop and commercialize the Constructs in accordance with the terms of a development plan established by the parties. The Company has determined that the license has multiple alternative future uses in research and development projects and sublicensing in other countries or for other disease indications. The value of the acquired license is recorded as an intangible asset with amortization over the estimated useful life of 25 years and evaluation for impairment at the end of each reporting period. The required upfront license payment of $2.5 million was paid by issuance of Series A-1 Convertible Preferred Shares, which have been fully converted to common shares. The value of the license includes acquisition legal costs. See Note 7 for license commitments. Intangible assets, net consisted of the following: | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | The Constructs | | $ | 2,529,483 | | | $ | 2,529,483 | | | | | | | | | | | Less: accumulated amortization | | | (349,463 | ) | | | (248,291 | ) | | | | | | | | | | Total intangible assets, net | | $ | 2,180,020 | | | $ | 2,281,192 | |
Amortization expense amounted to $101,172 for each of the years ended September 30, 2023, and 2022, respectively. Total estimated future amortization of intangible assets for each fiscal year is as follows: Year Ending | | | | September 30, 2024 | | | 101,172 | | September 30, 2025 | | | 101,172 | | September 30, 2026 | | | 101,172 | | September 30, 2027 | | | 101,172 | | September 30, 2028 | | | 101,172 | | Thereafter | | | 1,674,160 | | | | | | | | | $ | 2,180,020 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all or part of the information related to intangible assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//350-30/tableOfContent
| Name: |
us-gaap_IntangibleAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Right-of-Use Asset and Liabilities
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Right-of-Use Asset and Liabilities |
|
| Right-of-Use Lease with Related Party |
6. Right-of-Use Asset and Liabilities Related party ROU asset and liability The Company leases a facility used for executive offices from a related company. The original lease expired in December 2022 and the Company executed a two-year term extension through December 31, 2024. The components of lease cost were as follows: | | September 30, 2023 | | | September 30, 2022 | | Right-of-use lease cost, included in general and administrative on the Statements of Operations | | $ | 82,358 | | | $ | 18,465 | |
Lease terms and discount rates were as follows: | | September 30, 2023 | | | September 30, 2022 | | Remaining lease term (months): | | | 15 | | | | 3 | | Estimated incremental borrowing rate: | | | 9.2 | % | | | 6.5 | % |
The approximate future minimum lease payments under operating leases at September 30, 2023 were as follows: Year Ending | | | | September 30, 2024 | | $ | 79,697 | | September 30, 2025 | | | 19,924 | | | | | | | Total lease payments | | | 99,621 | | Less imputed interest | | | 5,134 | | | | | | | Present value of right-of-use lease liabilities | | | 94,487 | | Present value included in current liabilities | | | 74,714 | | | | | | | Present value included in long-term liabilities | | $ | 19,773 | |
Cash flow information was as follows: | | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | Cash paid for amounts included in the measurement of right-of-use lease liabilities, included in accounts payable and accrued liabilities on the Statements of Cash Flow. | | $ | 79,222 | | | $ | 80,377 | |
|
| X |
- References
+ Details
| Name: |
edsa_RightOfUseAssetAndLiabilitiesabstract |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RightOfUseLeaseWithRelatedPartyDisclosureTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments |
|
| Commitments |
7. Commitments Research and other commitments The Company has commitments for contracted research organizations who perform clinical trials for the Company’s ongoing clinical studies and other service providers. Aggregate future contractual payments at September 30, 2023 are as follows: Year Ending | | | | | | | | September 30, 2024 | | | 1,798,000 | | September 30, 2025 | | | 49,000 | | September 30, 2026 | | | 36,000 | | September 30, 2027 | | | 41,000 | | September 30, 2028 | | | - | | | | | | | | | $ | 1,924,000 | |
License and royalty commitments In April 2020, through its Ontario subsidiary, the Company entered into a license agreement with a third party to obtain exclusive world-wide rights to certain know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights. An intangible asset for the acquired license has been recognized. See Note 5 for intangible assets. Under the license agreement, the Company is committed to payments of up to an aggregate amount of $356 million contingent upon meeting certain milestones outlined in the license agreement, primarily relating to future potential commercial approval and sales milestones. The Company also has a commitment to pay royalties based on any net sales of products containing the Constructs in the countries where the Company directly commercializes the products containing the Constructs and a percentage of any sublicensing revenue received by the Company and its affiliates in the countries where it does not directly commercialize the products containing the Constructs. No milestone, royalty or sublicensing payments were made to the third party during the years ended September 30, 2023 and 2022. In connection with this license agreement and pursuant to a purchase agreement entered into in April 2020, the Company acquired drug substance of one of the Constructs for an aggregate purchase price of $5.0 million. The Company recorded an expense of $2.5 million for the second installment payment during the year ended September 30, 2022. No expense was recorded during the year ended September 30, 2023. In 2016, through its Ontario subsidiary, the Company entered into a license agreement with a third party to obtain exclusive rights to certain know-how, patents and data relating to a pharmaceutical product. The Company will use the exclusive rights to develop the product for therapeutic, prophylactic and diagnostic uses in topical dermal applications and anorectal applications. No intangible assets have been recognized under the license agreement with the third party. Under the license agreement, the Company is committed to payments of various amounts to the third party upon meeting certain milestones outlined in the license agreement, up to an aggregate amount of $18.4. Upon divestiture of substantially all of the assets of the Company, the Company shall pay the third party a percentage of the valuation of the licensed technology sold as determined by an external objective expert. The Company also has a commitment to pay the third party a royalty based on net sales of the product in countries where the Company, or an affiliate, directly commercializes the product and a percentage of sublicensing revenue received by the Company and its affiliates in the countries where it does not directly commercialize the product. No license or royalty payments were made to the third party during the years ended September 30, 2023 and 2022, respectively. In March 2021, through its Ontario subsidiary, the Company entered into a license agreement with the inventor of the same pharmaceutical product to acquire global rights for all fields of use beyond those named under the 2016 license agreement. For the years ended September 30, 2023 and 2022, the Company recorded expenses of $50,000 and $25,693, respectively, as a result of meeting milestones outlined in the 2021 license agreement. The Company is committed to remaining payments of up to an aggregate amount of $68.9 million, primarily relating to future potential commercial approval and sales milestones. In addition, if the Company fails to file an investigational new drug application or foreign equivalent (IND) for the product within a certain period of time following the date of the agreement, the Company is required to remit to the inventor a fixed license fee quarterly as long as the requirement to file an IND remains unfulfilled. Retirement savings plan 401(k) contributions Executive officers and employees of our California subsidiary are eligible to receive the Company’s non-elective safe harbor employer contribution of 3% of eligible compensation under a 401(k) plan to provide retirement benefits. Employees are 100% vested in employer contributions and in any voluntary employee contributions. Contributions to the 401(k) plan were $16,872 and $19,740 during the years ended September 30, 2023 and 2022, respectively.
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Capital shares
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Capital shares |
|
| Capital Shares |
8. Capital shares Equity offerings On November 2, 2022, the Company completed a private placement of units consisting of 384,475 common shares, Class A warrants to purchase up to an aggregate of 192,248 common shares and Class B warrants to purchase up to an aggregate of 192,248 common shares. Net proceeds from the offering were $2.9 million, which were allocated between the relative fair values of the common shares (using a fair value of $2.7 million) and the common share purchase warrants (using a total fair value of $1.2 million). The warrants became exercisable December 23, 2022. The Class A warrants have an exercise price of $10.50 per share and will expire on December 23, 2025. The Class B warrants have an exercise price of $7.00 per share and will expire on December 23, 2023. The warrants are considered contracts on the Company’s own shares and are classified as equity. On March 24, 2022, the Company completed a registered direct offering of 220,000 common shares, no par value, and pre-funded warrants to purchase up to an aggregate of 171,390 common shares. In a concurrent private placement, the Company issued common share purchase warrants to purchase an aggregate of up to 391,390 common shares. Net proceeds from the offering were $9.0 million. The common share purchase warrants were immediately exercisable at an exercise price of $24.64 per share and will expire on September 24, 2027. The pre-funded warrants were immediately exercisable at an exercise price of $0.0007 per share and do not expire. The warrants are considered contracts on the Company’s own shares and are classified as equity. The Company allocated gross proceeds with $5.9 million as the value of common shares and pre-funded warrants and $4.1 million as the value of common share purchase warrants under additional paid-in capital on a relative fair value basis. In connection with the offering, the Company issued warrants to purchase an aggregate of 27,397 common shares to certain affiliated designees of the placement agent as part of the placement agent’s compensation. The placement agent warrants are exercisable on or after March 24, 2022, at an exercise price of $31.9375 per share and will expire on March 21, 2027 with a fair value of $0.4 million. Equity distribution agreements On March 27, 2023, the Company entered into the Canaccord ATM, pursuant to which the Company may offer and sell, from time to time, common shares through an at-the-market equity offering program for up to $20 million in gross proceeds, subject to certain offering limitations that currently allow the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million. The Company has no obligation to sell any of the common shares and may at any time suspend sales or terminate the equity distribution agreement in accordance with its terms. During the year ended September 30, 2023, the Company sold a total of 196,401 common shares pursuant to the agreement for gross proceeds of approximately $1.3 million. From November 22, 2021 until terminated on March 21, 2022, the Company had an equity distribution agreement for an at-the-market equity offering program with another sales agent. During the year ended September 30, 2022, the Company sold a total of 89,558 common shares pursuant to the agreement for net proceeds of $2.6 million. Black-Scholes option valuation model The Company uses the Black-Scholes option valuation model to determine the fair value of share-based compensation for share options and compensation warrants granted and the fair value of warrants issued. Option valuation models require the input of highly subjective assumptions including the expected price volatility. The Company calculates expected volatility based on historical volatility of the Company’s share price. When there is insufficient data available, the Company uses a peer group that is publicly traded to calculate expected volatility. The Company adopted interest-free rates by reference to the U.S. treasury yield rates. The Company calculated the fair value of share options granted based on the expected life of 5 years considering expected forfeitures during the option term of 10 years. Expected life of warrants is based on warrant terms. The Company did not and is not expected to declare any dividends. Changes in the subjective input assumptions can materially affect the fair value estimates, and therefore the existing models do not necessarily provide a reliable single measure of the fair value of the Company’s warrants and share options. Warrants A summary of the Company’s warrants activity is as follows: | | Number of Warrant Shares (#) | | | Weighted Average Exercise Price | | | | | | | | | Balance - September 30, 2021 | | | 102,929 | | | $ | 39.83 | | | | | | | | | | | Issued | | | 418,789 | | | | 25.13 | | | | | | | | | | | September 30, 2022 | | | 521,718 | | | $ | 28.00 | | | | | | | | | | | Issued | | | 384,496 | | | | 8.75 | | Exercised | | | (100,760 | ) | | | 7.65 | | Expired | | | (84,545 | ) | | | 37.29 | | | | | | | | | | | September 30, 2023 | | | 720,909 | | | $ | 19.51 | |
The weighted average contractual life remaining on the outstanding warrants at September 30, 2023 is 35 months. The following table summarizes information about the warrants outstanding at September 30, 2023: Number of Warrants (#) | | | Exercise Prices | | | Expiry Dates | | | 110,122 | | | $ | 7.00 | | | December 2023 | | | 1,070 | | | $ | 33.67 | | | June 2024 | | | 1,687 | | | $ | 22.40 | | | January 2025 | | | 173,614 | | | $ | 10.50 | | | December 2025 | | | 15,627 | | | $ | 56.00 | | | February 2026 | | | 27,399 | | | $ | 31.94 | | | March 2027 | | | 391,390 | | | $ | 24.64 | | | September 2027 | | | 720,909 | | | | | | | | |
The fair value of warrants issued during the years ended September 30, 2023 and 2022 was estimated using the Black-Scholes option valuation model using the following assumptions: | | Year Ended September 30, 2023 | | | Year Ended September 30, 2022 | | | | Class A Warrants | | | Class B Warrants | | | Common Warrants | | | Placement Agent Warrants | | | | | | | | | | | | | | | Risk free interest rate | | | 4.54 | % | | | 4.76 | % | | | 2.37 | % | | | 2.37 | % | Expected life | | 3.14 years | | | 1.14 years | | | 5.5 years | | | 5 years | | Expected share price volatility | | | 90.73 | % | | | 89.70 | % | | | 87.09 | % | | | 87.09 | % | Expected dividend yield | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % |
Pre-funded Warrants A summary of the Company’s pre-funded warrant activity is as follows: | | Number of Pre-funded Warrant Shares (#) | | Balance - September 30, 2021 | | | - | | | | | | | Issued | | | 171,389 | | Exercised | | | (171,389 | ) | | | | | | Balance - September 30, 2022 | | | - | |
Share Options The Company adopted an Equity Incentive Compensation Plan in 2019 (the 2019 Plan) administered by the independent members of the Board of Directors, which amended and restated prior plans. Options, restricted shares and restricted share units are eligible for grant under the 2019 Plan. The total number of shares available for issuance under the terms of the 2019 Plan is 575,737. The remaining number of shares available to grant at September 30, 2023 is 81,765. The Company’s 2019 Plan allows options to be granted to directors, officers, employees and certain external consultants and advisers. Under the 2019 Plan, the option term is not to exceed 10 years and the exercise price of each option is determined by the independent members of the Board of Directors. Options have been granted under the 2019 Plan allowing the holders to purchase common shares of the Company as follows: | | Number of Options (#) | | | Weighted Average Exercise Price | | | Weighted Average Grant Date Fair Value | | | | | | | | | | | | Balance - September 30, 2021 | | | 253,777 | | | $ | 35.42 | | | $ | 26.53 | | | | | | | | | | | | | | | Granted | | | 71,451 | | | | 25.62 | | | | 17.36 | | Forfeited | | | (3,851 | ) | | | 45.92 | | | | 34.79 | | Expired | | | (6,524 | ) | | | 56.35 | | | | 45.36 | | | | | | | | | | | | | | | Balance - September 30, 2022 | | | 314,853 | | | $ | 32.62 | | | $ | 23.94 | | | | | | | | | | | | | | | Granted | | | 118,579 | | | | 7.47 | | | | 5.23 | | Forfeited | | | (12,779 | ) | | | 22.76 | | | | 16.23 | | Expired | | | (38 | ) | | | 1,973.20 | | | | 1,973.20 | | | | | | | | | | | | | | | Balance - September 30, 2023 | | | 420,615 | | | $ | 25.60 | | | $ | 18.84 | |
There were no options exercised during the years ended September 30, 2023 or September 30, 2022 and there was no intrinsic value of options outstanding at September 30, 2023. The weighted average contractual life remaining on the outstanding options at September 30, 2022 is 85 months. The following table summarizes information about the options under the 2019 Plan outstanding and exercisable at September 30, 2023: Number of Options (#) | | | Exercisable at September 30, 2023 (#) | | | Range of Exercise Prices | | | Expiry Dates | | | 497 | | | | 497 | | | $ | 246.96 - 596.82 | | | Dec 2023 - Mar 2025 | | | 42,348 | | | | 42,348 | | | C$ | 15.12 | | | May 2024 - Dec 2028 | | | 46,285 | | | | 46,285 | | | $ | 22.12 | | | May 2024 - Feb 2030 | | | 56,722 | | | | 56,702 | | | $ | 52.08 - 56.49 | | | May 2024 - Oct 2030 | | | 93,344 | | | | 81,048 | | | $ | 36.75 - 40.18 | | | Apr 2024 - Sep 2031 | | | 68,777 | | | | 44,441 | | | $ | 20.58 - 25.97 | | | Apr 2024 - Feb 2032 | | | 112,642 | | | | 18,334 | | | $ | 5.79 - 10.01 | | | Apr 2024 - Jul 2033 | | | 420,615 | | | | 289,655 | | | | | | | | |
The options exercisable at September 30, 2023 had a weighted average exercise price of $31.03, no intrinsic value and a weighted average remaining life of 74 months. There were 130,960 options at September 30, 2023 that had not vested with a weighted average exercise price of $13.61 no intrinsic value and a weighted average remaining life of 111 months. The fair value of options granted during the years ended September 30, 2023 and 2022 was estimated using the Black-Scholes option valuation model using the following assumptions: | | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Risk free interest rate | | 3.62% - 4.18% | | | 1.71% - 2.54% | | Expected life | | 5 years | | | 5 years | | Expected share price volatility | | 95.3% - 97.34% | | | 85.91% - 86.59% | | Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
The Company recorded $1.2 million and $2.3 million of share-based compensation expenses for the years ended September 30, 2023 and 2022, respectively. As of September 30, 2023, the Company had approximately $0.5 million of unrecognized share-based compensation expense, which is expected to be recognized over a period of 31 months. Restricted Share Units The Company's 2019 Plan allows restricted share units (RSUs) to be granted to directors, officers, employees and certain external consultants and advisers. Under the 2019 Plan, the RSU term is not to exceed 10 years. The fair value is based on the 5-day VWAP of the Company's common shares up to the date of grant. The following is a summary of changes in the status of RSUs from October 1, 2021 through September 30, 2023: | | Number of RSU (#) | | | Weighted Average Grant Date Fair Value | | | | | | | | | | | Balance - September 30, 2021 and 2022 | | | - | | | $ | - | | | | | | | | | | | Granted | | | 46,602 | | | | 5.60 | | Converted to common shares | | | (13,557 | ) | | | 5.60 | | | | | | | | | | | Balance - September 30, 2023 | | | 33,045 | | | $ | 5.60 | |
The following table summarizes information about the RSUs under the 2019 Plan outstanding and exercisable at September 30, 2023: | | | Number of RSU(#) | | Expiry Date | | Fully-vested RSUs | | | 33,045 | | August 4, 2033 | |
The RSUs that were granted in the current year were in lieu of cash bonuses for certain employees and in lieu of payments on consulting invoices for services prior to the appointment of the new Chief Financial Officer. All RSUs that were granted in the current year vested immediately upon the grant date. The outstanding RSUs can be converted to common shares by the holder at any time prior to the expiry date. There is no future unrecorded compensation expense for the RSUs.
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Government Contributions
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Government Contributions |
|
| Government Contributions |
9. Government Contributions Reimbursement grant income for the Company’s federal grant with the Canadian government’s SIF is recorded based on the claim period of eligible costs. In February 2021, the Company entered into the 2021 SIF Agreement with the Canadian Government. Under the 2021 SIF Agreement, the Government of Canada committed up to C$14.1 million in nonrepayable funding which was intended to support research and development related to our EB05 clinical program. Under the February 2021 SIF Agreement the Company recorded grant income of $0.8 million for the year ended September 30, 2022. No grant income was recorded under the 2021 SIF Agreement during the year ended September 30, 2023. No further funding will be received from the 2021 SIF Agreement. In October 2023, the Company entered into the 2023 SIF Agreement with the Canadian Government. Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding toward (i) conducting and completing the Company’s Phase 3 clinical study of its experimental drug EB05 in critical-care patients with Acute Respiratory Distress Syndrome (ARDS) caused by COVID-19 or other infectious agents, (ii) submitting EB05 for governmental approvals and manufacturing scale-up, following, and subject to, completing the Phase 3 study and (iii) conducting two non-clinical safety studies to assess the potential long-term impact of EB05 exposure (the Project). Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue. The repayable portion would be payable over fifteen (15) years based on a percentage rate of the Company’s annual revenue growth. The maximum amount repayable under the Agreement is 1.4 times the original repayable amount. In addition, the Company is entitled to partial reimbursement of certain eligible expenses under the Agreement. Under the Agreement, the Company agreed to certain financial and non-financial covenants and other obligations in relation to the Project. Pursuant to the Agreement, certain customary events of default, such as the Company’s or Edesa Biotech Research’s breach of their covenants and obligations under the Agreement, their insolvency, winding up or dissolution, and other similar events, may permit the Government of Canada to declare an event of default under the Agreement. Upon an event of default, subject to applicable cure, the Government of Canada may exercise a number of remedies, including suspending or terminating funding under the Agreement, demanding repayment of funding previously received and/or terminating the Agreement. The funding and any associated conditional repayments are not secured by any assets of Edesa Biotech Research or the Company. The Agreement will expire on the later of December 31, 2042 or the date of the last repayment, unless earlier terminated, subject to certain provisions that extend three (3) years beyond the term or early termination of the Agreement. Under the October 2023 SIF Agreement the Company recorded grant income of $0.6 million for the year ended September 30, 2023. No grant income was recorded under the 2023 SIF Agreement during the year ended September 30, 2022.
|
| X |
- References
+ Details
| Name: |
edsa_GovernmentContributionsTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CompensationAndRetirementDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Tax
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Income Tax |
|
| Income Tax |
10. Income Tax The reconciliation of the combined Canadian federal and provincial statutory income tax rate to the approximate effective tax rate is as follows: | | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Net loss before recovery of income taxes | | $ | (8,374,000 | ) | | $ | (17,548,000 | ) | Canadian federal and provincial statutory income tax rate | | | 26.5 | % | | | 26.5 | % | | | | | | | | | | Expected income tax recovery | | $ | (2,219,000 | ) | | $ | (4,650,000 | ) | Effect of foreign currency and foreign tax rate differences | | | (207,200 | ) | | | 976,800 | | Permanent differences | | | 339,000 | | | | 650,000 | | Share issuance cost booked through equity or capitalization | | | (89,000 | ) | | | (449,000 | ) | Non-capital losses limitation - U.S. | | | 899,000 | | | | - | | Other | | | (94,000 | ) | | | - | | Change in valuation allowance | | | 1,372,000 | | | | 3,473,000 | | | | | | | | | | | Income tax (recovery) expense | | $ | 800 | | | $ | 800 | |
Components of the net deferred tax asset or liability Deferred taxes are provided as a result of temporary differences that arise due to the difference between the income tax values and the carrying amount of assets and liabilities. Approximate deferred tax assets and liabilities are as follows: | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Non-capital losses carried forward - Canada | | $ | 13,943,000 | | | $ | 11,740,000 | | Non-capital losses carried forward - U.S. | | | 731,000 | | | | 1,631,000 | | Research and development tax credits | | | 1,371,000 | | | | 1,052,000 | | Share issuance and financing costs | | | 585,000 | | | | 686,000 | | Right-of-use lease liabilities | | | 25,000 | | | | 5,000 | | Other temporary differences | | | 43,000 | | | | 15,000 | | | | | | | | | | | Subtotal | | $ | 16,699,000 | | | $ | 15,129,000 | | Less: valuation allowance | | | (16,466,000 | ) | | | (15,093,000 | ) | | | | | | | | | | Total net deferred tax assets | | $ | 233,000 | | | $ | 36,000 | | | | | | | | | | | Property and equipment | | $ | (3,000 | ) | | $ | (15,000 | ) | Right-of-use assets | | | (24,000 | ) | | | (5,000 | ) | Grant Income receivable | | | (153,000 | ) | | | - | | Deferred share issuance costs | | | (53,000 | ) | | | (16,000 | ) | | | | | | | | | | Total deferred tax liabilities | | $ | (233,000 | ) | | $ | (36,000 | ) | | | | | | | | | | Net deferred taxes | | $ | - | | | $ | - | |
Realization of the deferred tax assets is dependent upon the generation of future taxable income, the amount and timing of which are uncertain. It is more likely than not that a tax benefit will not be realized. Accordingly, net deferred tax assets have been fully offset by a valuation allowance. Non-capital losses, capital losses, and research and development credits generated by Edesa Biotech USA, Inc. prior to changes in share ownership that occurred as a result of the reverse acquisition are substantially limited. It is unlikely that tax losses totaling $29.6 million and credits totaling $0.6 million will be utilized to offset potential future taxable income before expiration and they are excluded from deferred tax assets above. The approximate Canadian non-capital losses carried forward at September 30, 2023 expire as follows: 2025 | | C$ | 21,000 | | 2026 | | | 56,000 | | 2027 | | | 114,000 | | 2028 | | | 233,000 | | 2029 | | | 688,000 | | 2030 | | | 860,000 | | 2031 | | | 685,000 | | 2032 | | | 673,000 | | 2033 | | | 107,000 | | 2034 | | | 1,941,000 | | 2035 | | | 2,207,000 | | 2036 | | | 2,216,000 | | 2037 | | | 2,123,000 | | 2038 | | | 3,500,000 | | 2039 | | | 1,732,000 | | 2040 | | | 7,992,000 | | 2041 | | | 12,675,000 | | 2042 | | | 22,387,000 | | 2043 | | | 10,765,000 | | | | | | | | Total | | C$ | 70,975,000 | |
Share issuance and financing costs will be fully amortized in 2026. The U.S. non-capital losses carried forward at September 30, 2023 totaled approximately $3.4 million, which do not expire for federal taxes. The U.S. state research and development tax credits carried forward at September 30, 2023 totaled approximately $0.6 million, which do not expire for state taxes. The approximate U.S. state non-capital losses carried forward at September 30, 2023 expire as follows: 2039 | | $ | 70,000 | | 2040 | | | 150,000 | | 2041 | | | 68,000 | | 2042 | | | 6,000 | | | | | | | Total | | $ | 294,000 | |
|
| X |
- DefinitionThe entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(h)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//740/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-14
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-21
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 270
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482526/740-270-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 6.I.5.Q1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 11.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479360/740-10-S99-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482603/740-30-50-2
| Name: |
us-gaap_IncomeTaxDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Financial instruments
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Financial instruments |
|
| Financial Instruments |
11. Financial instruments (a) Fair values The Company uses the fair value measurement framework for valuing financial assets and liabilities measured on a recurring basis in situations where other accounting pronouncements either permit or require fair value measurements. The Company follows the fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. Observable inputs are inputs that reflect assumptions market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. There are three levels of inputs that may be used to measure fair value: | · | Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. | | · | Level 2 - Inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly or indirectly. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets and liabilities in markets that are not active. | | · | Level 3 - Unobservable inputs for the asset or liability that are supported by little or no market activity. |
The carrying value of certain financial instruments such as cash and cash equivalents, accounts and other receivable, accounts payable and accrued liabilities approximates fair value due to the short-term nature of such instruments. (b) Interest rate and credit risk Interest rate risk is the risk that the value of a financial instrument might be adversely affected by a change in interest rates. The Company does not believe that the results of operations or cash flows would be affected to any significant degree by a significant change in market interest rates, relative to interest rates on cash and cash equivalents due to the short-term nature of these balances. The Company is also exposed to credit risk at period end from the carrying value of its cash and cash equivalents and accounts and other receivable. The Company manages this risk by maintaining bank accounts with Canadian Chartered Banks, U.S. banks believed to be credit worthy and money market mutual funds of U.S. government securities. The Company’s cash is not subject to any external restrictions. The Company assesses the collectability of accounts receivable through a review of the current aging and terms, as well as an analysis of historical collection rates, general economic conditions and credit status of government agencies. Credit risk for the reimbursement grant and HST refunds receivable are not considered significant since amounts are due from the Canadian government’s SIF and the Canada Revenue Agency. (c) Foreign exchange risk The Company and its subsidiary have balances in Canadian dollars that give rise to exposure to foreign exchange (FX) risk relating to the impact of translating certain non-U.S. dollar balance sheet accounts as these statements are presented in U.S. dollars. A strengthening U.S. dollar will lead to a FX loss while a weakening U.S. dollar will lead to a FX gain. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks. At September 30, 2023, the Company and its Canadian subsidiary had assets denominated in Canadian dollars of approximately C$3.0 million and the U.S. dollar exchange rate at this date was equal to 1.3581 Canadian dollars. Based on the exposure at September 30, 2023, a 10% annual change in the Canadian/U.S. exchange rate would impact the Company’s loss and other comprehensive loss by approximately $0.2 million. (d) Liquidity risk Liquidity risk is the risk that the Company will encounter difficulty raising liquid funds to meet commitments as they fall due. In meeting its liquidity requirements, the Company closely monitors its forecasted cash requirements with expected cash drawdown.
|
| X |
- References
+ Details
| Name: |
us-gaap_FairValueDisclosuresAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for financial instruments. This disclosure includes, but is not limited to, fair value measurements of short and long term marketable securities, international currencies forward contracts, and auction rate securities. Financial instruments may include hedging and non-hedging currency exchange instruments, derivatives, securitizations and securities available for sale at fair value. Also included are investment results, realized and unrealized gains and losses as well as impairments and risk management disclosures.
| Name: |
us-gaap_FinancialInstrumentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Loss per Share
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Loss per Share |
|
| Loss per Share |
12. Loss per Share The Company had securities outstanding which could potentially dilute basic earnings per share in the future but were excluded from the computation of diluted loss per share in the periods presented, as their effect would have been anti-dilutive.
|
| X |
- References
+ Details
| Name: |
us-gaap_EarningsPerShareAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Related party transactions
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Related party transactions |
|
| Related Party Transactions |
13. Related party transactions During each of the years ended September 30, 2023 and 2022, the Company paid cash of $82,000 and $81,000, respectively, for a ROU lease from a company controlled by the Company's CEO. These transactions are in the normal course of operations and are measured at the exchange amount, which is the amount of consideration established and agreed to by both parties. On December 31, 2022, the Company executed a two-year lease extension through December 31, 2024 in accordance with the terms of the original lease agreement. Rents of approximately $15,000 and $22,000 were payable at September 30, 2023 and September 30, 2022, respectively.
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Subsequent events
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Subsequent events |
|
| Subsequent events |
14. Subsequent events Subsequent to the year end, equity sales under the Company’s at-the-market offering program have resulted in the issuance of 89,241 common shares and receipt of net cash proceeds of $0.3 million after deducting sales agent commissions. In October 2023, the Company entered into $10.0 million revolving credit agreement with a company controlled by the Company's CEO, providing an unsecured revolving credit facility, with a credit limit of $3.5 million. No amounts have been drawn on the credit agreement subsequent to the year end.
|
| X |
- References
+ Details
| Name: |
us-gaap_SubsequentEventsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Significant accounting policies (Policies)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Significant accounting policies |
|
| Use Of Estimates |
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the period or year. Actual results could differ from those estimates. Areas where significant judgment is involved in making estimates are valuation of accounts and other receivable; valuation and useful lives of property and equipment; intangible assets; right-of-use assets; deferred income taxes; the determination of fair value of share-based compensation; the determination of fair value of warrants in order to allocate proceeds from equity issuances; and forecasting future cash flows for assessing the going concern assumption.
|
| Functional And Reporting Currencies |
The consolidated financial statements of the Company are presented in U.S. dollars, unless otherwise stated, which is the Company’s and its wholly owned subsidiary’s, Edesa Biotech USA, Inc., functional currency. The functional currency of the Company’s wholly owned subsidiary, Edesa Biotech Research, Inc., as determined by management, is Canadian dollars.
|
| Cash and cash equivalents |
Cash and cash equivalents consist of demand deposits with financial institutions held in checking, savings and money market mutual funds and highly liquid investments which are readily convertible into cash with maturities of three months or less when purchased. The carrying amount of cash and cash equivalents approximates its fair value due to its short-term nature.
|
| Accounts and other receivable |
The Company assesses the collectability of its accounts receivable through a review of its current aging and payment terms, as well as an analysis of its historical collection rate, general economic conditions and credit status of the government agencies. Accounts and other receivable include reimbursement grant income for the Company’s federal grant with the Canadian government’s SIF and Harmonized Sales Tax (HST) refunds receivable. As of September 30, 2023, all outstanding accounts, grants and HST refunds receivable were deemed to be fully collectible, and therefore, no allowance for doubtful accounts was recorded.
|
| Property and equipment |
Property and equipment are recorded at historical cost less accumulated depreciation and any accumulated impairment losses. Depreciation is recorded to write off the cost of assets less their residual values over their useful lives, using the declining balance and straight-line methods. Maintenance and repair expenditures that do not improve or extend the life are expensed in the period incurred. Any gain or loss arising on the disposal or retirement of an item of property and equipment is recognized as the difference between the sales proceeds and the carrying amount of the asset. The estimated useful lives, residual values and depreciation methods are reviewed at the end of each year, with the effect of any changes in estimate accounted for on a prospective basis. The depreciation policy for the principal asset categories are calculated as follows: | · | Computer equipment 30% declining balance method or straight line 3 years | | · | Furniture and equipment 20% declining balance method |
|
| Intangible assets |
Intangible assets represent the exclusive world-wide rights to know-how, patents and data relating to certain monoclonal antibodies (the Constructs), including sublicensing rights, acquired by entering into a license agreement with a pharmaceutical development company. Unless earlier terminated, the term of the license agreement will remain in effect for 25 years from the date of first commercial sale of licensed products containing the Constructs. Subsequently, the license agreement will automatically renew for five-year periods unless either party terminates the agreement in accordance with its terms. Intangible assets are stated at their historical cost, amortized on a straight-line basis over their expected useful lives, which is 25 years, and subject to impairment review at the end of each reporting period.
|
| Impairment of long-lived assets |
Long-lived assets are tested for impairment when indicators of impairment exist. When a significant change in the expected timing or amount of the future cash flows of the financial asset is identified, the carrying amount of the financial asset is reduced and the amount of the write-down is recognized as a loss. A previously recognized impairment loss may be reversed to the extent of the improvement, provided it is not greater than the amount that would have been reported at the date of the reversal had the impairment not been recognized previously, and the amount of the reversal is recognized in net income (loss).
|
| Right-of-Use assets |
The Company recognizes right-of-use (ROU) assets and liabilities on the balance sheet for operating leases with terms longer than 12 months. The Company follows the ongoing practical expedient not to recognize ROU assets and liabilities for short-term leases. The ROU assets are initially measured at cost and amortized using the straight-line method through the end of the lease term. The ROU liabilities are initially measured at the present value of the lease payments that are not paid at the commencement date, discounted using the Company's incremental borrowing rate.
|
| Fair value measurement |
The Company uses the fair value measurement framework for valuing financial assets and liabilities. See Note 11.
|
| Revenue recognition |
Reimbursement grant income is recognized based on the reimbursement rate included in the government contribution agreement when allowable expenses have been incurred.
|
| Research and development |
Research and development expenses principally consist of (i) contract research organizations for clinical trial management services, (ii) contract manufacturing organizations for manufacturing the drug compound(s) for use in clinical trials and (iii) salaries of employees directly involved in research and development efforts. Research and development costs are expensed as incurred.
|
| Share-based compensation |
The Company has equity incentive plans under which various types of equity-based awards including share options, restricted shares and restricted share unit awards may be granted to employees, non-employee directors and non-employee consultants and warrants that may be granted as compensation to non-employees. The Company measures the cost of equity-settled transactions by reference to the fair value of the equity instruments at the date at which they are granted since the fair value of the goods or services received by the Company cannot be reliably estimated. The Company recognizes compensation expense for all share-based awards based on the estimated grant-date fair values. For restricted share unit awards to employees, the fair value is based on the 5-day volume weighted average price (VWAP) of the Company’s common shares up to the date of grant. The value of the portion of the award that is ultimately expected to vest is recognized as expense on a straight-line basis over the requisite service period. The fair value of share options is determined using the Black-Scholes option pricing model. The Company utilizes a dividend yield of zero based on the fact that the Company has never paid cash dividends and has no current intention of paying cash dividends. The Company elected an accounting policy to record forfeitures as they occur. See Note 8 for a discussion of the assumptions used by the Company in determining the grant date fair value of options granted under the Black-Scholes option pricing model, as well as a summary of the share option activity under the Company’s share-based compensation plan for all years presented. The provisions of the Company’s share-based compensation plans do not require the Company to settle any options or restricted share units by transferring cash or other assets, and therefore the Company classifies the awards as equity.
|
| Translation of foreign currency transactions |
The Company’s reporting currency is the U.S. dollar. The financial statements of the wholly owned Canadian subsidiary is measured using the Canadian dollar as the functional currency. Assets and liabilities of the Canadian operation have been translated at year-end exchange rates and related revenue and expenses have been translated at average exchange rates for the year. Accumulated gains and losses resulting from the translation of the financial statements of the Canadian operation are included as part of accumulated other comprehensive loss, a separate component of shareholders’ equity. For other transactions denominated in currencies other than the Company’s functional currency, the monetary assets and liabilities are translated at the year-end rates. Revenue and expenses are translated at rates of exchange prevailing on the transaction dates. Non-monetary balance sheet and related income statement accounts are remeasured into U.S. dollar using historical exchange rates. All of the exchange gains or losses resulting from these other transactions are recognized in the statements of operations and comprehensive loss.
|
| Income taxes |
Deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the tax bases of assets and liabilities and their financial statement reported amounts using enacted tax rates and laws in effect in the year in which the differences are expected to reverse. A valuation allowance is provided against deferred tax assets when it is determined to be more likely than not that the deferred tax asset will not be realized. The Company assesses the likelihood of the financial statement effect of a tax position that should be recognized when it is more likely than not that the position will be sustained upon examination by a taxing authority based on the technical merits of the tax position, circumstances, and information available as of the reporting date. The Company is subject to examination by taxing authorities in Canada and the U.S. Management does not believe that there are any uncertain tax positions that would result in an asset or liability for taxes being recognized in the accompanying financial statements. The Company recognizes tax-related interest and penalties, if any, as a component of income tax expense. The Company accounts for income taxes on a tax jurisdictional basis. The Company files income tax returns in Canada, the provinces of British Columbia and Ontario, the U.S. and the state of California.
|
| Earnings (loss) per share |
Basic earnings (loss) per share is calculated by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding during the year. The computation of diluted earnings (loss) per share assumes the conversion, exercise or contingent issuance of securities only when such conversion, exercise or issuance would have a dilutive effect on earnings (loss) per share. The dilutive effect of convertible securities would be reflected in diluted earnings per share by application of the “if converted” method. The dilutive effect of outstanding share options and warrants and their equivalents would be reflected in diluted earnings per share by application of the treasury stock method. However, conversion of outstanding share options and warrants would have an antidilutive effect on loss per share for the years ended September 30, 2023 and 2022 and are therefore excluded from the computation of diluted loss per share. See Note 8 for share options and warrants at September 30, 2023 and 2022.
|
| Segmented Information |
The Company’s operations comprise a single reportable segment engaged in the research and development, manufacturing and commercialization of innovative pharmaceutical products. As the operations comprise a single reportable segment, amounts disclosed in the consolidated financial statements for net loss, comprehensive loss, depreciation and total assets also represent segmented amounts.
|
| Adoption of Recent Accounting Pronouncements |
On October 1, 2022, the Company adopted Accounting Standards Update ASU 2021-10 Disclosure by Business Entities About Government Assistance, modifying ASC Topic 832, Government Assistance. The amendments in ASU 2021-10 require disclosure of information about certain types of government assistance received. The Company expanded its disclosures related to government assistance.
|
| Future accounting pronouncements |
In November 2023, the FASB issued Accounting Standards Update ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires disclosure of incremental segment information on an interim and annual basis. This ASU is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal periods beginning after December 15, 2024, and requires retrospective application to all prior periods presented in the financial statements. The Company is currently evaluating the impact of the guidance on the consolidated financial statements and disclosures.
|
| X |
- References
+ Details
| Name: |
edsa_FunctionalAndReportingCurrencies |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_FutureAccountingPronouncementsPolicy |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.
| Name: |
us-gaap_FairValueMeasurementPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//830/tableOfContent
| Name: |
us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for goodwill and intangible assets. This accounting policy also may address how an entity assesses and measures impairment of goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 30
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-1
| Name: |
us-gaap_GoodwillAndIntangibleAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for recognizing and measuring the impairment of long-lived assets. An entity also may disclose its accounting policy for long-lived assets to be sold. This policy excludes goodwill and intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.CC)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480091/360-10-S99-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 05
-Paragraph 4
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482338/360-10-05-4
| Name: |
us-gaap_ImpairmentOrDisposalOfLongLivedAssetsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-17
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-9
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-25
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-28
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-19
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-20
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for leasing arrangement entered into by lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-1
| Name: |
us-gaap_LesseeLeasesPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 360
-Topic 958
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480321/958-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for its research and development and computer software activities including the accounting treatment for costs incurred for (1) research and development activities, (2) development of computer software for internal use, (3) computer software to be sold, leased or otherwise marketed as a separate product or as part of a product or process and (4) in-process research and development acquired in a purchase business combination. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 4
-SubTopic 50
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482610/350-50-25-4
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 30
-Paragraph 1
-SubTopic 40
-Topic 350
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482633/350-40-30-1
| Name: |
us-gaap_ResearchDevelopmentAndComputerSoftwarePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-25
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 280
-SubTopic 10
-Section 50
-Paragraph 30
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
| Name: |
us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy election for determining cost for share-based payment arrangement by either estimating forfeiture expected to occur or by recognizing effect of forfeiture upon occurrence. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (m)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationForfeituresPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for accounts receivable. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481569/310-20-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (b)
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-11B
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-1
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-6
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Subparagraph (d)
-SubTopic 10
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481962/310-10-50-15
| Name: |
us-gaap_TradeAndOtherAccountsReceivablePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Property and equipment (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Property and equipment |
|
| Schedule of Property and equipment |
| | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Computer equipment | | $ | 46,945 | | | $ | 46,674 | | Furniture and equipment | | | 5,603 | | | | 5,538 | | | | | | | | | | | | | | 52,548 | | | | 52,212 | | Less: accumulated depreciation | | | (43,846 | ) | | | (39,518 | ) | | | | | | | | | | Total property and equipment, net | | $ | 8,702 | | | $ | 12,694 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of real estate properties and units in those properties that are included in the discussion of the nature of an entity's operations.
| Name: |
us-gaap_ScheduleOfRealEstatePropertiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible Assets (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Intangible assets |
|
| Intangible Assets |
| | September 30, 2023 | | | September 30, 2022 | | | | | | | | | The Constructs | | $ | 2,529,483 | | | $ | 2,529,483 | | | | | | | | | | | Less: accumulated amortization | | | (349,463 | ) | | | (248,291 | ) | | | | | | | | | | Total intangible assets, net | | $ | 2,180,020 | | | $ | 2,281,192 | |
|
| Estimated Future Amortization Of Intagible Assets |
Year Ending | | | | September 30, 2024 | | | 101,172 | | September 30, 2025 | | | 101,172 | | September 30, 2026 | | | 101,172 | | September 30, 2027 | | | 101,172 | | September 30, 2028 | | | 101,172 | | Thereafter | | | 1,674,160 | | | | | | | | | $ | 2,180,020 | |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of goodwill and intangible assets, which may be broken down by segment or major class. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
| Name: |
us-gaap_ScheduleOfIntangibleAssetsAndGoodwillTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the amount of amortization expense expected to be recorded in succeeding fiscal years for finite-lived intangible assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Right-of-Use Asset and Liabilities (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Right-of-Use Asset and Liabilities |
|
| Components of lease cost |
| | September 30, 2023 | | | September 30, 2022 | | Right-of-use lease cost, included in general and administrative on the Statements of Operations | | $ | 82,358 | | | $ | 18,465 | |
|
| Lease Terms And Discount Rates |
| | September 30, 2023 | | | September 30, 2022 | | Remaining lease term (months): | | | 15 | | | | 3 | | Estimated incremental borrowing rate: | | | 9.2 | % | | | 6.5 | % |
|
| Future Minimum Lease Payments |
Year Ending | | | | September 30, 2024 | | $ | 79,697 | | September 30, 2025 | | | 19,924 | | | | | | | Total lease payments | | | 99,621 | | Less imputed interest | | | 5,134 | | | | | | | Present value of right-of-use lease liabilities | | | 94,487 | | Present value included in current liabilities | | | 74,714 | | | | | | | Present value included in long-term liabilities | | $ | 19,773 | |
|
| Cash Paid-lease Liabilities |
| | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | Cash paid for amounts included in the measurement of right-of-use lease liabilities, included in accounts payable and accrued liabilities on the Statements of Cash Flow. | | $ | 79,222 | | | $ | 80,377 | |
|
| X |
- References
+ Details
| Name: |
edsa_LeaseTermAndDiscountRateTableTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RightOfUseAssetAndLiabilitiesabstract |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_SupplementalLeaseInformationTableTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Commitments |
|
| Future Contractual Payments |
Year Ending | | | | | | | | September 30, 2024 | | | 1,798,000 | | September 30, 2025 | | | 49,000 | | September 30, 2026 | | | 36,000 | | September 30, 2027 | | | 41,000 | | September 30, 2028 | | | - | | | | | | | | | $ | 1,924,000 | |
|
| X |
- DefinitionTabular disclosure of future minimum payments required in the aggregate and for each of the five succeeding fiscal years for operating leases having initial or remaining noncancelable lease terms in excess of one year and the total minimum rentals to be r
| Name: |
edsa_FutureContractualPaymentsTableTextblock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Capital Shares (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Capital shares |
|
| Warrant activity |
| | Number of Warrant Shares (#) | | | Weighted Average Exercise Price | | | | | | | | | Balance - September 30, 2021 | | | 102,929 | | | $ | 39.83 | | | | | | | | | | | Issued | | | 418,789 | | | | 25.13 | | | | | | | | | | | September 30, 2022 | | | 521,718 | | | $ | 28.00 | | | | | | | | | | | Issued | | | 384,496 | | | | 8.75 | | Exercised | | | (100,760 | ) | | | 7.65 | | Expired | | | (84,545 | ) | | | 37.29 | | | | | | | | | | | September 30, 2023 | | | 720,909 | | | $ | 19.51 | |
|
| Schedule of fair value of warrants black-Scholes option valuation table |
| | Year Ended September 30, 2023 | | | Year Ended September 30, 2022 | | | | Class A Warrants | | | Class B Warrants | | | Common Warrants | | | Placement Agent Warrants | | | | | | | | | | | | | | | Risk free interest rate | | | 4.54 | % | | | 4.76 | % | | | 2.37 | % | | | 2.37 | % | Expected life | | 3.14 years | | | 1.14 years | | | 5.5 years | | | 5 years | | Expected share price volatility | | | 90.73 | % | | | 89.70 | % | | | 87.09 | % | | | 87.09 | % | Expected dividend yield | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % | | | 0.00 | % |
|
| Warrants outstanding |
Number of Warrants (#) | | | Exercise Prices | | | Expiry Dates | | | 110,122 | | | $ | 7.00 | | | December 2023 | | | 1,070 | | | $ | 33.67 | | | June 2024 | | | 1,687 | | | $ | 22.40 | | | January 2025 | | | 173,614 | | | $ | 10.50 | | | December 2025 | | | 15,627 | | | $ | 56.00 | | | February 2026 | | | 27,399 | | | $ | 31.94 | | | March 2027 | | | 391,390 | | | $ | 24.64 | | | September 2027 | | | 720,909 | | | | | | | | |
|
| Pre-funded warrant activity |
| | Number of Pre-funded Warrant Shares (#) | | Balance - September 30, 2021 | | | - | | | | | | | Issued | | | 171,389 | | Exercised | | | (171,389 | ) | | | | | | Balance - September 30, 2022 | | | - | |
|
| Share options |
| | Number of Options (#) | | | Weighted Average Exercise Price | | | Weighted Average Grant Date Fair Value | | | | | | | | | | | | Balance - September 30, 2021 | | | 253,777 | | | $ | 35.42 | | | $ | 26.53 | | | | | | | | | | | | | | | Granted | | | 71,451 | | | | 25.62 | | | | 17.36 | | Forfeited | | | (3,851 | ) | | | 45.92 | | | | 34.79 | | Expired | | | (6,524 | ) | | | 56.35 | | | | 45.36 | | | | | | | | | | | | | | | Balance - September 30, 2022 | | | 314,853 | | | $ | 32.62 | | | $ | 23.94 | | | | | | | | | | | | | | | Granted | | | 118,579 | | | | 7.47 | | | | 5.23 | | Forfeited | | | (12,779 | ) | | | 22.76 | | | | 16.23 | | Expired | | | (38 | ) | | | 1,973.20 | | | | 1,973.20 | | | | | | | | | | | | | | | Balance - September 30, 2023 | | | 420,615 | | | $ | 25.60 | | | $ | 18.84 | |
|
| Summary of plan outstanding and exercisable |
Number of Options (#) | | | Exercisable at September 30, 2023 (#) | | | Range of Exercise Prices | | | Expiry Dates | | | 497 | | | | 497 | | | $ | 246.96 - 596.82 | | | Dec 2023 - Mar 2025 | | | 42,348 | | | | 42,348 | | | C$ | 15.12 | | | May 2024 - Dec 2028 | | | 46,285 | | | | 46,285 | | | $ | 22.12 | | | May 2024 - Feb 2030 | | | 56,722 | | | | 56,702 | | | $ | 52.08 - 56.49 | | | May 2024 - Oct 2030 | | | 93,344 | | | | 81,048 | | | $ | 36.75 - 40.18 | | | Apr 2024 - Sep 2031 | | | 68,777 | | | | 44,441 | | | $ | 20.58 - 25.97 | | | Apr 2024 - Feb 2032 | | | 112,642 | | | | 18,334 | | | $ | 5.79 - 10.01 | | | Apr 2024 - Jul 2033 | | | 420,615 | | | | 289,655 | | | | | | | | |
|
| Fair value Of warrant granted assumptions |
| | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Risk free interest rate | | 3.62% - 4.18% | | | 1.71% - 2.54% | | Expected life | | 5 years | | | 5 years | | Expected share price volatility | | 95.3% - 97.34% | | | 85.91% - 86.59% | | Expected dividend yield | | | 0.00 | % | | | 0.00 | % |
|
| Summary of changes in Restricted Share Units |
| | Number of RSU (#) | | | Weighted Average Grant Date Fair Value | | | | | | | | | | | Balance - September 30, 2021 and 2022 | | | - | | | $ | - | | | | | | | | | | | Granted | | | 46,602 | | | | 5.60 | | Converted to common shares | | | (13,557 | ) | | | 5.60 | | | | | | | | | | | Balance - September 30, 2023 | | | 33,045 | | | $ | 5.60 | |
|
| summary of information about the RSU |
| | | Number of RSU(#) | | Expiry Date | | Fully-vested RSUs | | | 33,045 | | August 4, 2033 | |
|
| X |
- References
+ Details
| Name: |
edsa_PreFundedWarrantActivity |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ScheduleOfFairValueOfWarrantsBlackScholesOptionValuationTableTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ScheduleOfPlanOutstandingAndExercisableTableTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ScheduleOfShareBasedCompensationWarrantsActivityTableTextBlock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareOptions |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_SummaryOfChangesInRestrictedShareUnits |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of warrants or rights issued. Warrants and rights outstanding are derivative securities that give the holder the right to purchase securities (usually equity) from the issuer at a specific price within a certain time frame. Warrants are often included in a new debt issue to entice investors by a higher return potential. The main difference between warrants and call options is that warrants are issued and guaranteed by the company, whereas options are exchange instruments and are not issued by the company. Also, the lifetime of a warrant is often measured in years, while the lifetime of a typical option is measured in months. Disclose the title of issue of securities called for by warrants and rights outstanding, the aggregate amount of securities called for by warrants and rights outstanding, the date from which the warrants or rights are exercisable, and the price at which the warrant or right is exercisable. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-1
| Name: |
us-gaap_ScheduleOfStockholdersEquityNoteWarrantsOrRightsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Income Tax (Tables)
|
12 Months Ended |
Sep. 30, 2023 |
|---|
| Income Tax |
|
| reconciliation of the combined Canadian federal to the approximate effective tax rate |
| | Years Ended | | | | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Net loss before recovery of income taxes | | $ | (8,374,000 | ) | | $ | (17,548,000 | ) | Canadian federal and provincial statutory income tax rate | | | 26.5 | % | | | 26.5 | % | | | | | | | | | | Expected income tax recovery | | $ | (2,219,000 | ) | | $ | (4,650,000 | ) | Effect of foreign currency and foreign tax rate differences | | | (207,200 | ) | | | 976,800 | | Permanent differences | | | 339,000 | | | | 650,000 | | Share issuance cost booked through equity or capitalization | | | (89,000 | ) | | | (449,000 | ) | Non-capital losses limitation - U.S. | | | 899,000 | | | | - | | Other | | | (94,000 | ) | | | - | | Change in valuation allowance | | | 1,372,000 | | | | 3,473,000 | | | | | | | | | | | Income tax (recovery) expense | | $ | 800 | | | $ | 800 | |
|
| Deferred taxes |
| | September 30, 2023 | | | September 30, 2022 | | | | | | | | | Non-capital losses carried forward - Canada | | $ | 13,943,000 | | | $ | 11,740,000 | | Non-capital losses carried forward - U.S. | | | 731,000 | | | | 1,631,000 | | Research and development tax credits | | | 1,371,000 | | | | 1,052,000 | | Share issuance and financing costs | | | 585,000 | | | | 686,000 | | Right-of-use lease liabilities | | | 25,000 | | | | 5,000 | | Other temporary differences | | | 43,000 | | | | 15,000 | | | | | | | | | | | Subtotal | | $ | 16,699,000 | | | $ | 15,129,000 | | Less: valuation allowance | | | (16,466,000 | ) | | | (15,093,000 | ) | | | | | | | | | | Total net deferred tax assets | | $ | 233,000 | | | $ | 36,000 | | | | | | | | | | | Property and equipment | | $ | (3,000 | ) | | $ | (15,000 | ) | Right-of-use assets | | | (24,000 | ) | | | (5,000 | ) | Grant Income receivable | | | (153,000 | ) | | | - | | Deferred share issuance costs | | | (53,000 | ) | | | (16,000 | ) | | | | | | | | | | Total deferred tax liabilities | | $ | (233,000 | ) | | $ | (36,000 | ) | | | | | | | | | | Net deferred taxes | | $ | - | | | $ | - | |
|
| Canadian non-capital losses |
2025 | | C$ | 21,000 | | 2026 | | | 56,000 | | 2027 | | | 114,000 | | 2028 | | | 233,000 | | 2029 | | | 688,000 | | 2030 | | | 860,000 | | 2031 | | | 685,000 | | 2032 | | | 673,000 | | 2033 | | | 107,000 | | 2034 | | | 1,941,000 | | 2035 | | | 2,207,000 | | 2036 | | | 2,216,000 | | 2037 | | | 2,123,000 | | 2038 | | | 3,500,000 | | 2039 | | | 1,732,000 | | 2040 | | | 7,992,000 | | 2041 | | | 12,675,000 | | 2042 | | | 22,387,000 | | 2043 | | | 10,765,000 | | | | | | | | Total | | C$ | 70,975,000 | |
|
| U.S. state non-capital losses |
2039 | | $ | 70,000 | | 2040 | | | 150,000 | | 2041 | | | 68,000 | | 2042 | | | 6,000 | | | | | | | Total | | $ | 294,000 | |
|
| X |
- References
+ Details
| Name: |
edsa_CanadianNonCapitalLosses |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_USStateNonCapitalLosses |
| Namespace Prefix: |
edsa_ |
| Data Type: |
nonnum:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Paragraph 12
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-12
| Name: |
us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Nature of operations (Details Narrative) - USD ($)
|
1 Months Ended |
12 Months Ended |
Mar. 31, 2023 |
Nov. 30, 2022 |
Mar. 31, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Accumulated deficit |
|
|
|
$ (52,400,000)
|
|
| Working capital |
|
|
|
4,600,000
|
|
| Cash and cash equivalents |
|
|
|
$ 5,400,000
|
|
| Description of shelf registration |
|
|
|
In August 2022 the Company filed a $150.0 million shelf registration statement, under which the Company entered into an equity distribution agreement with Canaccord for $20.0 million in gross proceeds, subject to certain offering limitations that currently allows the Company to offer and sell common shares having an aggregate gross sales price of up to $8.4 million (Canaccord ATM). There was approximately $7.1 million of available capacity on the Canaccord ATM as of September 30, 2023.
|
|
| Net Cash used in operating activities |
|
|
|
$ 6,600,000
|
$ 12,300,000
|
| Net losses |
|
|
|
$ (8,400,000)
|
(17,600,000)
|
| Description of multi year contribution agreement with canadian government |
|
|
|
Under the 2023 SIF Agreement, the Government of Canada committed up to C$23 million in partially repayable funding. Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue. See Note 9. In February 2021, the Company signed a contribution agreement with the Canadian government’s SIF (2021 SIF Agreement), the Company was eligible to receive cash reimbursements up to C$14.1 million in the aggregate for certain R&D expenses related to our EB05 clinical development program. All potential funding available under the 2021 SIF Agreement has been received. For the years ended September 30, 2023 and 2022, the Company recorded grant income of $0.6 million and $0.8 million respectively related to both the 2023 SIF Agreement and the 2021 SIF Agreement.
|
|
| Unsecured revolving credit facility, with a credit limit |
|
|
|
$ 3,500,000
|
|
| Sale of common shares |
|
|
|
89,558
|
|
| Net proceeds from sale of common shares |
|
|
|
$ 2,600,000
|
|
| Net proceeds from sale of common stock |
|
|
|
$ 4,345,017
|
$ 11,957,687
|
| Equity distribution agreement with Canaccord [Member] |
|
|
|
|
|
| Sale of common shares |
|
|
|
196,401
|
|
| Net proceeds from sale of common stock |
$ 20,000,000
|
|
|
|
|
| Aggregate gross sales price |
$ 8,400,000
|
|
|
$ 1,300,000
|
|
| Private Placement [Member] |
|
|
|
|
|
| Number of common share direct offer |
|
384,475
|
220,000
|
|
|
| Pre-funded warrants to purchase up to an aggregate number of common share |
|
192,248
|
171,390
|
|
|
| Purchase warrants to purchase an aggregate |
|
192,248
|
391,390
|
|
|
| Net proceeds from common stock |
|
$ 3,000,000
|
$ 9,000,000
|
|
|
| X |
- References
+ Details
| Name: |
edsa_AggregateGrossSalesPriceOfCommonShare |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NetCashUsedInOperatingActivities |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NetLosses |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_OfferOfCommonShareDirectOffer |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_PreFundedWarrantsToPurchaseNumberOfCommonShare |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ProceedsFromIssueOfWarrantsToPrchaseCommonStock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ProceedsFromSaleOfCommonStock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShelfRegistrationStatement |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_StrategicInnovationFundAgreementDescription |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WarrantsToPurchaseCommonShare |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WorkingCapital |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cumulative earnings (deficits) for relevant time periods.
| Name: |
us-gaap_CumulativeEarningsDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMaximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(b),22(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares issued or sold by the subsidiary or equity method investee per stock transaction.
| Name: |
us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=edsa_EquityDistributionAgreementWithCanaccordMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=us-gaap_PrivatePlacementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_FixedAssetsDepreciation |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_OperatingLeasesWithTerms |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_PropertyPlantAndEquipmentUsefulLifes |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=edsa_FurnitureAndEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=edsa_IntangibleAssetsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Property and Equipment (Details) - USD ($)
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Property and equipment, gross |
$ 52,548
|
$ 52,212
|
| Less: accumulated depreciation |
(43,846)
|
(39,518)
|
| Total property and equipment, net |
8,702
|
12,694
|
| Computer Equipment |
|
|
| Property and equipment, gross |
46,945
|
46,674
|
| Furniture and Equipment |
|
|
| Property and equipment, gross |
$ 5,603
|
$ 5,538
|
| X |
- DefinitionAmount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(13))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 360
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 360
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480842/942-360-50-1
| Name: |
us-gaap_PropertyPlantAndEquipmentNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_ComputerEquipmentMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PropertyPlantAndEquipmentByTypeAxis=us-gaap_FurnitureAndFixturesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_PropertyPlantAndEquipmentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intangible Assets (Details) - USD ($)
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Intangible assets |
|
|
| The Constructs |
$ 2,529,483
|
$ 2,529,483
|
| Less: Accumulated Amortization |
349,463
|
248,291
|
| Intangible Assets, Net |
$ 2,180,020
|
$ 2,281,192
|
| X |
- DefinitionAccumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 928
-SubTopic 340
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483147/928-340-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483154/926-20-50-5
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Intagible Assets (Details 1)
|
Sep. 30, 2023
USD ($)
|
| Intangible assets |
|
| September 30, 2024 |
$ 101,172
|
| September 30, 2025 |
101,172
|
| September30, 2026 |
101,172
|
| September 30, 2027 |
101,172
|
| September 30, 2028 |
101,172
|
| Thereafter |
1,674,160
|
| Total |
$ 2,180,020
|
| X |
- References
+ Details
| Name: |
edsa_FiniteLivedIntangibleAssetsAmortizationFutureExpense |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for asset, excluding financial asset and goodwill, lacking physical substance with finite life expected to be recognized after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFour |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 350
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_CapitalLeasesIndemnificationAgreementsDescriptions |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_FiniteLivedIntangibleAssetUsefulLifes |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
| Name: |
us-gaap_AmortizationOfIntangibleAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_RemainingLeaseTermMonths |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RightOfUseAssetAndLiabilitiesabstract |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.3
Right-of-Use Asset and Liabilities (Details 2) - USD ($)
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Year Ending |
|
|
| September 30, 2024 |
$ 79,697
|
|
| September 30, 2025 |
19,924
|
|
| Total Lease Payment |
99,621
|
|
| Less Imputed Interest |
5,134
|
|
| Present value of right-of-use lease liabilities |
94,487
|
|
| Present value included in current liabilities |
74,714
|
$ 18,975
|
| Present value included in long-term liabilities |
$ 19,773
|
$ 0
|
| X |
- References
+ Details
| Name: |
edsa_ImputedInterest |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingLeasesFutureMinimumPaymentsDueAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_RightOfUseAssetAndLiabilitiesabstract |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePaymentsUse |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.3
Commitments (Details)
$ in Millions |
Jun. 30, 2023
USD ($)
|
| Year Ending September 30, |
|
| September 30, 2028 |
$ 0
|
| September 30, 2024 |
1,798,000
|
| September 30, 2025 |
49,000
|
| September 30, 2026 |
36,000
|
| September 30, 2027 |
41,000
|
| Total |
$ 1,924,000
|
| X |
- References
+ Details
| Name: |
edsa_ContractualObligationDueInEightYear |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
edsa_ContractualObligationDueInSeventhYear |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
edsa_ContractualObligationDueInSixthYear |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of contractual obligation, including, but not limited to, long-term debt, lease obligation, purchase obligation, and other commitments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-3
| Name: |
us-gaap_ContractualObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of contractual obligation to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
us-gaap_ContractualObligationDueInFifthYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of contractual obligation to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).
| Name: |
us-gaap_ContractualObligationDueInFourthYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ContractualObligationFiscalYearMaturityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Commitments (Details Narrative) - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| 2021 [Member] | License Commitments Agreement [Member] |
|
|
| Payments to investors |
$ 68,900,000
|
|
| 2021 [Member] | License Commitments [Member] |
|
|
| Risk free interest rate |
100.00%
|
|
| Payments to investors |
$ 50,000
|
$ 25,693
|
| Payments to third party |
16,872
|
$ 19,740
|
| 2016 [Member] | License Commitments [Member] |
|
|
| Payments to investors |
18,400,000
|
|
| Payments to third party |
$ 40,000,000
|
|
| Common Shares |
|
|
| Description of aqquire drugs |
the Company acquired drug substance of one of the Constructs for an aggregate purchase price of $5.0 million. The Company recorded an expense of $2.5 million for the second installment payment during the year ended September 30, 2022. No expense was recorded during the year ended September 30, 2023
|
|
| Remaining payments of contingent |
$ 356,000,000
|
|
| X |
- References
+ Details
| Name: |
edsa_Descriptionofaqquiredrugs |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RemainingPaymentsOfContingent |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash payments made as the result of exit or disposal activities. Excludes payments associated with a discontinued operation or an asset retirement obligation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-17
| Name: |
us-gaap_PaymentsForRestructuring |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow associated with the purchase of all investments (debt, security, other) during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_TwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=edsa_LicenseCommitmentsAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=edsa_LicenseCommitmentsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_TwoThousandSixteenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=edsa_CommonStocksMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Capital Shares (Details) - $ / shares
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Capital shares |
|
|
| Number of warrants, beginning balance |
521,718
|
102,929
|
| Issued |
384,496
|
418,789
|
| Exercised |
(100,760)
|
|
| Expired |
(84,545)
|
|
| Number of warrants, ending balance |
720,909
|
521,718
|
| Weighted average exercise price, beginning balance |
$ 28.00
|
$ 39.83
|
| Issued |
8.75
|
25.13
|
| Exercised |
7.65
|
|
| Expired |
37.29
|
|
| Weighted average exercise price, ending balance |
$ 19.51
|
$ 28.00
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfWarrantsExercised |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfWarrantsExpired |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfWarrantsIssued |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageExercisePriceWarrantsExercised |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageExercisePriceWarrantsExpired |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageExercisePriceWarrantsIssued |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Capital Shares (Details 1) - $ / shares
|
12 Months Ended |
|
|
|
|
Sep. 30, 2023 |
Jun. 30, 2023 |
Sep. 30, 2022 |
Jun. 30, 2022 |
Sep. 30, 2021 |
| Number of warrants |
720,909
|
5,609,845
|
521,718
|
3,651,953
|
102,929
|
| Weighted average exercise price |
$ 19.51
|
|
$ 28.00
|
|
$ 39.83
|
| Warrant 3 [Member] |
|
|
|
|
|
| Number of warrants |
1,687
|
|
|
|
|
| Weighted average exercise price |
$ 22.40
|
|
|
|
|
| Expiry date |
January 2025
|
|
|
|
|
| Warrant 1 [Member] |
|
|
|
|
|
| Number of warrants |
110,122
|
|
|
|
|
| Weighted average exercise price |
$ 7.00
|
|
|
|
|
| Expiry date |
December 2023
|
|
|
|
|
| Warrant 2 [Member] |
|
|
|
|
|
| Number of warrants |
1,070
|
|
|
|
|
| Weighted average exercise price |
$ 33.67
|
|
|
|
|
| Expiry date |
June 2024
|
|
|
|
|
| Warrant 4 [Member] |
|
|
|
|
|
| Number of warrants |
173,614
|
|
|
|
|
| Weighted average exercise price |
$ 10.50
|
|
|
|
|
| Expiry date |
December 2025
|
|
|
|
|
| Warrant 5 [Member] |
|
|
|
|
|
| Number of warrants |
15,627
|
|
|
|
|
| Weighted average exercise price |
$ 56.00
|
|
|
|
|
| Expiry date |
February 2026
|
|
|
|
|
| Warrant 6 [Member] |
|
|
|
|
|
| Number of warrants |
27,399
|
|
|
|
|
| Weighted average exercise price |
$ 31.94
|
|
|
|
|
| Expiry date |
March 2027
|
|
|
|
|
| Warrant 7 [Member] |
|
|
|
|
|
| Number of warrants |
391,390
|
|
|
|
|
| Weighted average exercise price |
$ 24.64
|
|
|
|
|
| Expiry date |
September 2027
|
|
|
|
|
| X |
- References
+ Details
| Name: |
edsa_WarrantExpirationDate |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=edsa_WarranttThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantSixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_WarrantSevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_ClassAWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_ClassBWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Capital Shares (Details 3) - shares
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Number of warrants, beginning balance |
521,718
|
102,929
|
| Issued |
384,496
|
418,789
|
| Exercised |
(100,760)
|
|
| Number of warrants, ending balance |
720,909
|
521,718
|
| Pre-funded Warrant Shares |
|
|
| Issued |
171,389
|
|
| Exercised |
(171,389)
|
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfWarrantsExercised |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfWarrantsIssued |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_Pre-fundedWarrantSharesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Capital Shares (Details 4) - $ / shares
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Number of options, ending balance |
420,615
|
|
| Purchase Common Share Options [Member] |
|
|
| Number of options, beginning balance |
314,853
|
253,777
|
| Number of options granted |
118,579
|
71,451
|
| Number of options forfeited |
(12,779)
|
(3,851)
|
| Number of options expired |
(38)
|
(6,524)
|
| Number of options, ending balance |
420,615
|
314,853
|
| Weighted Average Exercise Price, beginning balance |
$ 32.62
|
$ 35.42
|
| Weighted average exercise price granted |
7.47
|
25.62
|
| Weighted average exercise price forfeited |
22.76
|
45.92
|
| Weighted average exercise price expired |
1,973.20
|
56.35
|
| Weighted Average Exercise Price, ending balance |
25.60
|
32.62
|
| Weighted Average Grant Date Fair Value, beginning balance |
23.94
|
26.53
|
| Weighted Average Grant Date Fair Value, Granted |
5.23
|
17.36
|
| Weighted Average Grant Date Fair Value, Forfeited |
16.23
|
34.79
|
| Weighted Average Grant Date Fair Value, expired |
1,973.20
|
45.36
|
| Weighted Average Grant Date Fair Value, ending balance |
$ 18.84
|
$ 23.94
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeitedInPeriod |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeitedInPeriodWeightedAverageForfeitedDateFairValue |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageForfeitedDateFairValue |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeitedInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueBeginningBalance |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueEndingBalance |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueExpired |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options or other stock instruments for which the right to exercise has lapsed under the terms of the plan agreements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options of the plan that expired. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExpirationsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=edsa_PurchaseCommonShareOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Capital Shares (Details 5)
|
12 Months Ended |
|
Sep. 30, 2023
$ / shares
shares
|
|---|
| Number of options |
420,615
|
| Options exercisable |
289,655
|
| Stock Option 1 |
|
| Number of options |
497
|
| Options exercisable |
497
|
| Stock Option 1 | Minumum |
|
| Weighted average exercise price | $ / shares |
$ 246.96
|
| Expiry dates |
Dec 2023
|
| Stock Option 1 | Maximum |
|
| Weighted average exercise price | $ / shares |
$ 596.82
|
| Expiry dates |
Mar 2025
|
| Stock Option 2 |
|
| Number of options |
42,348
|
| Options exercisable |
42,348
|
| Weighted average exercise price | $ / shares |
$ 15.12
|
| Expiry dates |
May 2024
|
| Stock Option 2 | Maximum |
|
| Expiry dates |
Dec 2028
|
| Stock Option 3 |
|
| Number of options |
46,285
|
| Options exercisable |
46,285
|
| Weighted average exercise price | $ / shares |
$ 22.12
|
| Expiry dates |
Feb 2030
|
| Stock Option 3 | Minumum |
|
| Expiry dates |
May 2024
|
| Stock Option 4 |
|
| Number of options |
56,722
|
| Options exercisable |
56,702
|
| Stock Option 4 | Minumum |
|
| Weighted average exercise price | $ / shares |
$ 52.08
|
| Expiry dates |
May 2024
|
| Stock Option 4 | Maximum |
|
| Weighted average exercise price | $ / shares |
$ 56.49
|
| Expiry dates |
Oct 2030
|
| Stock Option 5 |
|
| Number of options |
93,344
|
| Options exercisable |
81,048
|
| Stock Option 5 | Minumum |
|
| Weighted average exercise price | $ / shares |
$ 36.75
|
| Expiry dates |
Apr 2024
|
| Stock Option 5 | Maximum |
|
| Weighted average exercise price | $ / shares |
$ 40.18
|
| Expiry dates |
Sep 2031
|
| Stock Option 6 |
|
| Number of options |
68,777
|
| Options exercisable |
44,441
|
| Stock Option 6 | Minumum |
|
| Weighted average exercise price | $ / shares |
$ 20.58
|
| Expiry dates |
Apr 2024
|
| Stock Option 6 | Maximum |
|
| Weighted average exercise price | $ / shares |
$ 25.97
|
| Expiry dates |
Feb 2032
|
| Stock Option 7 Member |
|
| Number of options |
112,642
|
| Options exercisable |
18,334
|
| Stock Option 7 Member | Minumum |
|
| Weighted average exercise price | $ / shares |
$ 5.79
|
| Expiry dates |
Apr 2024
|
| Stock Option 7 Member | Maximum |
|
| Weighted average exercise price | $ / shares |
$ 10.01
|
| Expiry dates |
Jul 2033
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationDates |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionSixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_StockOptionSevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the maximum percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRateMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the minimum percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRateMinimum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe maximum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMaximum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe minimum risk-free interest rate assumption that is used in valuing an option on its own shares.
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRateMinimum |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityNoteAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.3
Capital Shares (Details 7) - $ / shares
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Number of options, ending balance |
420,615
|
|
| Restricted Share Units [Member] |
|
|
| Number of options, beginning balance |
0
|
0
|
| Number of RSU granted |
46,602
|
0
|
| Converted to common shares |
(13,557)
|
0
|
| Number of options, ending balance |
33,045
|
0
|
| Weighted Average Grant Date Fair Value, beginning balance |
$ 0
|
$ 0
|
| Weighted Average Grant Date Fair Value, Granted |
5.60
|
0
|
| Weighted Average Grant Date Fair Value, Converted To Common Shares |
5.60
|
0
|
| Weighted Average Grant Date Fair Value, ending balance |
$ 5.60
|
$ 0
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfRestrictedStockUnitConvertedToCommonShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageForfeitedDateFairValue |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueBeginningBalance |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueConvertedToCommonShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WeightedAverageGrantDateFairValueEndingBalance |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=edsa_RestrictedShareUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExpirationDates |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ShareBasedCompensationArrangementByShareBasedPaymentAwardRsuOutstandingNumber |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=edsa_RestrictedShareUnitsOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Capital Shares (Details Narrative) - USD ($)
$ / shares in Units, $ in Thousands |
|
1 Months Ended |
12 Months Ended |
|
Nov. 02, 2022 |
Mar. 27, 2023 |
Nov. 22, 2022 |
Mar. 24, 2022 |
Sep. 30, 2023 |
Sep. 30, 2022 |
Sep. 30, 2021 |
| Share-based compensation |
|
|
|
|
$ 12
|
$ 2,300
|
|
| Unrecognized share-based compensation |
|
|
|
|
$ 500
|
|
|
| Gross Proceeds |
|
$ 20,000
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
|
$ 19.51
|
$ 28.00
|
$ 39.83
|
| Aggregate gross sales price |
|
$ 84
|
|
|
$ 1,300
|
|
|
| Common share sold during period |
|
|
|
|
196,401
|
|
|
| Affiliated Designees [Member] |
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 31.9375
|
|
|
|
| Warrant expiry date |
|
|
|
Mar. 21, 2027
|
|
|
|
| Warrants to purchase common shares |
|
|
|
391,390
|
|
|
|
| Fair value |
|
|
|
$ 400
|
|
|
|
| Black Scholes Option [Member] |
|
|
|
|
|
|
|
| Expected term |
|
|
|
|
10 months
|
|
|
| Grant date |
|
|
|
|
5 months
|
|
|
| March 24, 2022 [Member] |
|
|
|
|
|
|
|
| Net proceeds from the offering |
|
|
|
$ 9,000
|
|
|
|
| Offering Common Shares |
|
|
|
220,000
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 24.64
|
|
|
|
| Warrant expiry date |
|
|
|
Sep. 24, 2027
|
|
|
|
| November 2, 2022[Member] |
|
|
|
|
|
|
|
| Gross Proceeds |
|
|
$ 2,600
|
|
|
|
|
| Warrant expiry date |
Dec. 23, 2025
|
|
|
|
|
|
|
| Units of Common Shares |
384,475
|
|
89,558
|
|
|
|
|
| Warrants Additional Paid In Capital [Member] |
|
|
|
|
|
|
|
| Gross Proceeds |
|
|
|
$ 4,100
|
|
|
|
| Employees [Member] |
|
|
|
|
|
|
|
| Granted option shares |
|
|
|
|
130,960
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
|
$ 31.03
|
|
|
| Expected Option Forfeitures Term |
|
|
|
|
111 years
|
|
|
| 2019 Plan [Member] |
|
|
|
|
|
|
|
| Weighted average contractual life remaining on outstanding options |
|
|
|
|
85 months
|
|
|
| Unrecognized share-based compensation recognition period |
|
|
|
|
35 months
|
|
|
| Number Of Shares Available For Issuance |
|
|
|
|
575,737
|
|
|
| Option Term |
|
|
|
|
10 years
|
|
|
| Remaining Number Of Options Available For Grant |
|
|
|
|
81,765
|
|
|
| Class A Warrants Member |
|
|
|
|
|
|
|
| Gross Proceeds |
$ 2,900
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
$ 10.50
|
|
|
|
|
|
|
| Warrants to purchase common shares |
192,248
|
|
|
|
|
|
|
| Class B Warrants [Member] |
|
|
|
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
$ 7.00
|
|
|
|
|
|
|
| Warrant expiry date |
Dec. 23, 2022
|
|
|
|
|
|
|
| Warrants to purchase common shares |
192,248
|
|
|
|
|
|
|
| Pre-Funded Warrants [Member] |
|
|
|
|
|
|
|
| Gross Proceeds |
|
|
|
$ 5,900
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights |
|
|
|
$ 0.0007
|
$ 13.61
|
|
|
| Warrants To Purchase Common Stock |
|
|
|
171,390
|
|
|
|
| X |
- References
+ Details
| Name: |
edsa_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensation |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_AggregateGrossSalesPrice |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ExpectedOptionForfeituresTerm |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_ExpectedTerm |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_GrantDate |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NumberOfSharesAvailableForIssuance |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_OfferingCommonShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_OptionTerm |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RemainingNumberOfOptionsAvailableForGrant |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_UnitsOfCommonShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WarrantExpiryDate |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WarrantsToPurchaseCommonShares |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_WarrantsToPurchaseCommonStock |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of cost not yet recognized for nonvested award under share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe fair value of assets acquired in noncash investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-4
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-3
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-5
| Name: |
us-gaap_FairValueOfAssetsAcquired |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow associated with the amount received from entity's first offering of stock to the public. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceInitialPublicOffering |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from debt classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromOtherDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNet number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares of stock issued attributable to transactions classified as other.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_BlackScholesOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_MarchTwoFourTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_NovemberTwoTwoThousandTwentyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=edsa_WarrantsAdditionalPaidInCapitalMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=edsa_TwoThousandNineteenPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_ClassAWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_ClassBWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=edsa_PreFundedWarrantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Government Contributions (Details Narrative) - USD ($)
|
1 Months Ended |
12 Months Ended |
Oct. 31, 2023 |
Feb. 28, 2021 |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Grant income |
|
|
|
$ 800,000
|
| Description of expiry of agreement |
|
|
The Agreement will expire on the later of December 31, 2042 or the date of the last repayment, unless earlier terminated, subject to certain provisions that extend three (3) years beyond the term or early termination of the Agreement
|
|
| SIF Agreement Member | February 2021 |
|
|
|
|
| Grant income |
|
|
$ 600,000
|
|
| Non repayable funding amount support R&D |
|
$ 14,100,000
|
|
|
| SIF Agreement Member | October 2023 |
|
|
|
|
| Repayable Funding Amount |
$ 23,000,000
|
|
|
|
| Description of SIF Agreement |
(i) conducting and completing the Company’s Phase 3 clinical study of its experimental drug EB05 in critical-care patients with Acute Respiratory Distress Syndrome (ARDS) caused by COVID-19 or other infectious agents, (ii) submitting EB05 for governmental approvals and manufacturing scale-up, following, and subject to, completing the Phase 3 study and (iii) conducting two non-clinical safety studies to assess the potential long-term impact of EB05 exposure (the Project). Of the C$23 million committed by SIF, up to C$5.8 million is not repayable by the Company. The remaining C$17.2 million is conditionally repayable starting in 2029 only if and when the Company earns gross revenue
|
|
|
|
| Repayable payment duration |
15 years
|
|
|
|
| X |
- References
+ Details
| Name: |
edsa_AgreementExpirationDescription |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_NonRepayableFundingAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RepayableFundingAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_RepayablePaymentPayableDuration |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_SIFAgreementDescription |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of revenue and income classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column E)(Footnote 6)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
| Name: |
us-gaap_OtherIncome |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=edsa_SIFAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_FebruaryTwoThousandTwentyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardDateAxis=edsa_OctoberTwoThousandTwentyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
Income Tax (Details) - USD ($)
|
12 Months Ended |
Sep. 30, 2023 |
Sep. 30, 2022 |
| Income Tax |
|
|
| Net loss before recovery of income taxes |
$ (8,374,000)
|
$ (17,548,000)
|
| Canadian federal and provincial statutory income tax rate |
26.50%
|
26.50%
|
| Expected income tax recovery |
$ (2,219,000)
|
$ (4,650,000)
|
| Effect of foreign currency and foreign tax rate differences |
(207,200)
|
976,800
|
| Permanent differences |
339,000
|
650,000
|
| Non-capital losses limitations - US |
899,000
|
0
|
| Other |
(94,000)
|
0
|
| Share issuance cost booked through equity or capitalization |
(89,000)
|
(449,000)
|
| Change in valuation allowance |
1,372,000
|
3,473,000
|
| Income tax (recovery) expense |
$ 800
|
$ 800
|
| X |
- References
+ Details
| Name: |
edsa_DeferredTaxAssetsOtherLosses |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.3
Income Tax (Details 1) - USD ($)
|
Sep. 30, 2023 |
Sep. 30, 2022 |
| Income Tax |
|
|
| Non-capital losses carried forward - Canada |
$ 13,943,000
|
$ 11,740,000
|
| Non-capital losses carried forward - US |
731,000
|
1,631,000
|
| Research and development tax credits |
1,371,000
|
1,052,000
|
| Share issuance and financing costs |
585,000
|
686,000
|
| Right-of-use lease liabilities |
25,000
|
5,000
|
| Other temporary differences |
43,000
|
15,000
|
| Subtotal |
16,699,000
|
15,129,000
|
| Less: valuation allowance |
(16,466,000)
|
(15,093,000)
|
| Total net deferred tax assets |
233,000
|
36,000
|
| Property and equipment |
(3,000)
|
(15,000)
|
| Right-of-use assets |
24,000
|
5,000
|
| Grant Income receivable |
(153,000)
|
0
|
| Deferred share issuance costs |
(53,000)
|
(16,000)
|
| Total deferred tax liabilities |
(233,000)
|
(36,000)
|
| Net deferred taxes |
$ 0
|
$ 0
|
| X |
- References
+ Details
| Name: |
edsa_DeferredTaxAssetsOperatingLeaseLiabilities |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
edsa_DeferredTaxLiabilitiesOperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsLiabilitiesNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible temporary differences, classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowance, of deferred tax asset attributable to deductible loss carryforwards, classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsOtherLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible foreign tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwardsForeign |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible research tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwardsResearch |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, before allocation of valuation allowances, of deferred tax asset attributable to deductible differences from reserves and accruals, compensation and benefit costs, and other provisions, reserves, and allowances. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deferred tax asset, of deferred tax liability attributable to taxable differences without jurisdictional netting. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 45
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482525/740-10-45-6
| Name: |
us-gaap_DeferredTaxLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from capitalized costs. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxLiabilitiesDeferredExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax liability attributable to taxable temporary differences from property, plant, and equipment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxLiabilitiesPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Income Tax (Details 2) - Sep. 30, 2023
|
CAD ($) |
USD ($) |
| Canada |
|
|
| Non-capital income tax losses |
$ 70,975,000
|
|
| Canada | 2027 |
|
|
| Non-capital income tax losses |
114,000
|
|
| Canada | 2026 |
|
|
| Non-capital income tax losses |
56,000
|
|
| Canada | 2025 |
|
|
| Non-capital income tax losses |
21,000
|
|
| Canada | 2028 |
|
|
| Non-capital income tax losses |
233,000
|
|
| Canada | 2029 |
|
|
| Non-capital income tax losses |
688,000
|
|
| Canada | 2030 |
|
|
| Non-capital income tax losses |
860,000
|
|
| Canada | 2031 |
|
|
| Non-capital income tax losses |
685,000
|
|
| Canada | 2032 |
|
|
| Non-capital income tax losses |
673,000
|
|
| Canada | 2033 |
|
|
| Non-capital income tax losses |
107,000
|
|
| Canada | 2034 |
|
|
| Non-capital income tax losses |
1,941,000
|
|
| Canada | 2035 |
|
|
| Non-capital income tax losses |
2,207,000
|
|
| Canada | 2036 |
|
|
| Non-capital income tax losses |
2,216,000
|
|
| Canada | 2037 |
|
|
| Non-capital income tax losses |
2,123,000
|
|
| Canada | 2038 |
|
|
| Non-capital income tax losses |
3,500,000
|
|
| Canada | 2039 |
|
|
| Non-capital income tax losses |
1,732,000
|
|
| Canada | 2040 |
|
|
| Non-capital income tax losses |
7,992,000
|
|
| Canada | 2041 |
|
|
| Non-capital income tax losses |
12,675,000
|
|
| Canada | 2042 |
|
|
| Non-capital income tax losses |
22,387,000
|
|
| Canada | 2043 |
|
|
| Non-capital income tax losses |
10,765,000
|
|
| United States |
|
|
| Non-capital income tax losses |
|
$ 294,000
|
| United States | 2039 |
|
|
| Non-capital income tax losses |
|
70,000
|
| United States | 2040 |
|
|
| Non-capital income tax losses |
|
150,000
|
| United States | 2042 |
|
|
| Non-capital income tax losses |
$ 6,000
|
|
| United States | 2041 |
|
|
| Non-capital income tax losses |
|
$ 68,000
|
| X |
- DefinitionAmount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_OperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyTwentySevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyTwentySixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyTwentyFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyTwentyEightMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyTwentyNineMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyFourMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyFiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtySixMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtySevenMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyEightMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyThirtyNineMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyFortyMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyFourtyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyFortyTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyFourtyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxPeriodAxis=edsa_TwentyFortyOneMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_CreditsTotaling |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_TaxLossesTotalingFutureIncome |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_TheUSStateResearchAndDevelopmentTaxCreditsCarriedForward |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
edsa_UsnoncapitalLossesCarriedForward |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
Financial Instruments (Details Narrative)
$ / shares in Units, $ in Millions |
12 Months Ended |
|
|
|
Sep. 30, 2023
USD ($)
$ / shares
|
Sep. 30, 2023
CAD ($)
|
Sep. 30, 2022
USD ($)
|
| Assets |
$ 8,890,437
|
|
$ 11,575,728
|
| USD to CAD [Member] |
|
|
|
| Currency exchange rate | $ / shares |
$ 1.3581
|
|
|
| Ontario Subsidary [Member] |
|
|
|
| Assets |
|
$ 3.0
|
|
| Currency exchange rate description |
Based on the exposure at September 30, 2023, a 10% annual change in the Canadian/U.S. exchange rate would impact the Company’s loss and other comprehensive loss by approximately $0.2 million
|
|
|
| X |
- References
+ Details
| Name: |
edsa_CurrencyExchangeRate |
| Namespace Prefix: |
edsa_ |
| Data Type: |
num:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
edsa_CurrencyExchangeRateDescription |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=us-gaap_CurrencySwapMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.3
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred through that date and payable for royalties. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 8
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedRoyaltiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.3
| X |
- References
+ Details
| Name: |
edsa_CommonSharesIssued |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
edsa_CreditLimitAmount |
| Namespace Prefix: |
edsa_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe carrying value as of the balance sheet date of the current and noncurrent portions of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_LineOfCredit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
| Name: |
us-gaap_ProceedsFromIssuanceOrSaleOfEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_CreditFacilityAxis=us-gaap_RevolvingCreditFacilityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Edesa Biotech (NASDAQ:EDSA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Edesa Biotech (NASDAQ:EDSA)
Historical Stock Chart
From Apr 2023 to Apr 2024