Corporate Presentation NASDAQ:BDRX December 2023 Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333-274895 December 7, 2023

Disclaimer & cautionary language regarding forward - looking statements The following presentation, including a hard copy of these slides/the talks given by the presenters, the information communic ate d during any delivery of the presentation and any question and answer session and any document or material distributed at or in connection with the pr esentation (together, the "Presentation"), has been prepared by Biodexa Pharmaceuticals PLC (the "Company"). This Presentation is being made available on a strictly confidential basis and is for information purposes only, and may not be reproduced or redistributed or otherwise disclosed (in whole or in part) to any other person, or used in whole or in part for any other pur pos e. By accepting delivery of this presentation, you agree to these restrictions. The Presentation does not purport to contain all the information relating to the Company. The information in the Presentation is provided as at the date of the Presentation (unless stated otherwise) and is subject to updating, completion, revision and further verification. In furnishing the Presentation, the Company does not undertake or agree to any obligation to provide the recipient with access to any additiona l i nformation or to update the Presentation or to correct any inaccuracies in, or omissions from the Presentation which may become apparent. Neither the Company nor any of its directors, officers, employees, advisors or representatives (collectively the "Representatives") makes any represe nta tion or warranty of any sort as to the accuracy or completeness of the information contained in this Presentation or in any other model or informatio n m ade available in connection with this Presentation or the reasonableness of the assumptions on which any such information is based. No person sha ll have any right of action against the Company, its Representatives or any other person in relation to the accuracy or completeness of any such i nfo rmation. No reliance may be placed for any purpose whatsoever on the information or opinions contained or expressed in the Presentation or on the acc uracy, completeness or fairness of such information and opinions. This Presentation is not intended for distribution to, or use by any person or entity in any jurisdiction or country where su ch distribution or use would be contrary to local law or regulation or which would require any registration or licensing within such jurisdiction. Nothing in the Presentation is, or should be relied on as, a promise or representation as to the future. This Presentation co nta ins certain forward - looking statements within the meaning of legislation in the United Kingdom and/or United States relating to the business, fin anc ial performance and results of the Company and/or the industry in which it operates. Forward - looking statements concern future circumstances, not hi storical facts and are sometimes identified by the words such as "may," "could," "expect," "intend," "plan," "seek," "anticipate," "believe," "potential," "estimate," "aim," "foresee," "target," "predict," "potential," "project," or "continue" or the negative of these terms or other comparable term ino logy . 2

Disclaimer & cautionary language regarding forward - looking statements (cont’d..) The forward - looking statements contained in this Presentation (including assumptions, opinions and views of the Company or opini ons cited from third party sources) are subject to risks, uncertainties and other factors that may cause actual events to differ materially from a ny anticipated development. None of the Company nor any of its Representatives provides any assurance that the assumptions underlying such forward - looking s tatements are free from errors, nor does any of them accept any responsibility for the future accuracy of the opinions expressed in this Present ati on or the actual occurrence of the forecasted developments described herein. No representations or warranties of any kind are made by any pers on as to the accuracy of such statements, estimates or projections, or that any of the events expressed or implied in any such statements, estimate s o r projections will actually occur. All subsequent written and oral forward - looking statements by or concerning the Company are expressly qualified in their entiret y by the cautionary statements above. T he Company is not under any obligation, and expressly disclaims any intention, to update or revise any such statements, estimates or projections. No statement in the Presentation is intended as a profit forecast or a profit estimate. AN INVESTMENT IN THE COMPANY INVOLVES RISK. SEVERAL FACTORS COULD CAUSE THE ACTUAL RESULTS, PERFORMANCE OR ACHIEVEMENTS OF TH E COMPANY TO BE MATERIALLY DIFFERENT FROM ANY FUTURE RESULTS, PERFORMANCE OR ACHIEVEMENTS THAT MAY BE PREDICTED OR IMPLIED BY STATEMENTS AND INFORMATION IN THIS PRESENTATION. SHOULD ONE OR MORE OF THESE RISKS OR UNCERTAINTIES MATERIALIZE, OR SHOULD UNDERLYING ASSUMPTIONS PROVE INCORRECT, THE ACTUAL RESULTS OF THE COMPANY MAY VARY MATERIALLY FROM THOSE FORECASTED IN THE PRESENTATION. ANY INDICATION IN THIS PRESENTATION OF THE PRICE AT WHICH ORDINA RY SHARES OR ADSS HAVE BEEN BOUGHT OR SOLD IN THE PAST CANNOT BE RELIED UPON AS A GUIDE TO FUTURE PERFORMANCE. THE PRICE OF SHARES AND ANY INCOME EXPECTED FROM THEM MAY GO DOWN AS WELL AS UP AND INVESTORS MAY NOT GET BACK THE FULL AMOUN T INVESTED UPON DISPOSAL OF THE SHARES. PAST PERFORMANCE IS NO GUIDE TO FUTURE PERFORMANCE . OTHER FACTORS THAT COULD ADVERSELY AFFECT THE COMPANY’S BUSINESS AND PROSPECTS ARE DESCRIBED IN THE FILINGS MADE BY THE COMPANY WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION (“ SEC”), INCLUDING ITS MOST RECENT ANNUAL REPORT ON FORM 20 - F. Registered trademarks referred to in this presentation are the property of their respective owners. 3

Free Writing Prospectus This presentation highlights basic information about the Company and the offering. Because it is a summary that has been prep are d solely for informational purposes, it does not contain all of the information that you should consider before investing in our Company. Exc ept as otherwise indicated, this presentation speaks only as of the date hereof. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall th ere be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qua lification under the securities laws of any such state or jurisdiction. Neither the SEC nor any other regulatory body has approved or disapproved of our securities or passed upon the accuracy or adequacy of this presentation. Any representation to the contrary is a criminal offense. This presentation includes industry and market data that we obtained from industry publications and journals, third - party studie s and surveys, internal company studies and surveys, and other publicly available information. Industry publications and surveys generally s tat e that the information contained therein has been obtained from sources believed to be reliable. Although we believe the industry and ma rke t data to be reliable as of the date of this presentation, this information could prove to be inaccurate. Industry and market data could b e w rong because of the method by which sources obtained their data and because information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncert ain ties. In addition, we do not know all of the assumptions that were used in preparing the forecasts from the sources relied upon or cited herein. A registration statement on Form F - 1 (File No. 333 - 274895), as amended, including a preliminary prospectus, relating to the offe ring of securities has been filed with the SEC. The registration statement has not yet become effective. Before you invest, you should read the pre liminary prospectus in the registration statement, and, when available, the final prospectus relating to the offering, for more comple te information about the Company and the offering. An electronic copy of the preliminary prospectus relating to the offering is available, and a c opy of the final prospectus relating to the offering will be available, on the website of the SEC at www.sec.gov. Copies of the preliminary pr osp ectus and final prospectus relating to the offering, when available, may be obtained by contacting Ladenburg Thalmann & Co. Inc. by written r equ est addressed to Syndicate Department, 640 5th Avenue, 4th Floor, New York, NY 10019 (telephone number 1 - 800 - 573 - 2541) or by emailing prospectus@ladenburq.com. 4

Risk factors Investing in our securities includes a high degree of risk . You should consider carefully the specific factors discussed below, together with all of the other information contained in our SEC filings, including the list of risk factors in the Risk Factors section of the Company's most recent Form 20 - F and Form F - 1 , as amended . If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects would likely be materially and adversely affected . This could cause the market price of our securities to decline and could cause you to lose all or part of your investment . Risks include but are not limited to : • There can be no assurance that the transactions between Biodexa and Adhera Therapeutics, Inc . (“ Adhera ”) and Melior Pharmaceuticals I Inc . (“ Melior ”) will be consummated . The announcement and pendency of the transactions, or the failure of the transactions to be consummated, could have an adverse effect on our stock price, business, financial condition, results of operations or prospects . • We have incurred significant losses since our inception and anticipate that we will continue to incur losses in the future . • Our requirement for additional financing in the short - term represents a material uncertainty that raises substantial doubt about our ability to continue as a going concern . • Public health crises, such as the COVID - 19 pandemic, have had, and could in the future have, a negative effect on our business . • We are a “foreign private issuer” under the rules and regulations of the SEC and, as a result, are exempt from a number of rules under the Exchange Act and are permitted to file less information with the SEC than a company incorporated in the United States . • Our operations are in early - stage development with no sources of recurring revenue and there is no assurance that we will successfully develop and license our product candidates or ever become profitable . • We are exposed to political, regulatory, social and economic risk relating to the United Kingdom’s exit from the European Union . • In 2020 , our license agreement related to panobinostat , the active pharmaceutical ingredient in our MTX 110 product, was terminated by Secura Bio, Inc . , or Secura Bio . Because of this, we believe that the relevant Secura Bio patents may delay a launch of MTX 110 , which could have a material adverse effect on our business, financial condition and results of operations . • Our future success is dependent on product development and the ability to successfully license our product candidates to partners who can seek regulatory approval and commercialization of our product candidates . • Clinical drug development involves a risky, lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results . • 5

Risk factors (cont’d..) Investing in our securities includes a high degree of risk . You should consider carefully the specific factors discussed below, together with all of the other information contained in our SEC filings, including the list of risk factors in the Risk Factors section of the Company's most recent Form 20 - F and Form F - 1 , as amended . If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects would likely be materially and adversely affected . This could cause the market price of our securities to decline and could cause you to lose all or part of your investment . Risks include but are not limited to : • We expect to seek to establish agreements with potential licensing partners and collaborators and, if we are not able to establish them on commercially reasonable terms, we may have to alter our development and commercialization plans . • Recently enacted and future legislation in the United States and other countries may affect the prices we may obtain for our product candidates and increase the difficulty and cost for us to commercialize our product candidates . • Our success depends in part on our ability to protect rights in our intellectual property, which cannot be assured . • We rely on third parties to conduct our preclinical and clinical trials . If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed . • If we cannot meet NASDAQ's continued listing requirements, NASDAQ may delist our American Depositary Shares (" ADSs "), which could have an adverse impact on the liquidity and market price of our ADSs . • The liquidity of our Depositary Shares may have an adverse effect on share price . • Shareholder ownership interests in the Company may be diluted as a result of, among other things, future financings and/or additional acquisitions, and may have a material negative effect on the market price of our securities . • You will incur immediate and substantial dilution as a result of this offering . • Because our management will have broad discretion and flexibility in how the net proceeds from this offering are used, our management may use the net proceeds in ways with which you disagree or which may not prove effective . • There is no public market for the warrants being offered in this offering . 6

Indication Preclinical Phase 1 Phase2 Next Milestone MTX 110 ( panobinostat ) Diffuse Midline Glioma IND for DMG Phase II submission Q3 - 2024 MTX 110 ( panobinostat ) Glioblastoma Initiation of cohort B Q4 - 2023 Tolimidone (Lyn Kinase activator) Type 1 Diabetes Initiation of Phase Ib dose - ranging study MTX 110 ( panobinostat ) Medulloblastoma Phase I safety data H2 - 2024 MTD217 (panobinostat + OXPHOS inhibitor) Leptomeningeal Disease Pre - clinical efficacy data in orthotopic LMD model Q1 - 2024 INITIAL FOCUS Biodexa Expanded Clinical Pipeline 7

Q1’ 24 Q2’ 24 Q3’ 24 Anticipated catalysts 8 Q4’ 23 MTX110 Initiation of Cohort B of Magic - G1 study in rGBM Q4’ 24 CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION Tolimidone Initiation of Phase Ib dose - ranging study in T1D Multiple pre - clinical data expected from MTD217 program Tolimidone Initiation of phase II study in T1D MTD217 Submission of IND for LMD MTX110 Submission of IND for DMG MTD217 Readout of in vivo preclinical data in LMD model MTX110 Expected Progression Free Survival data from MAGIC - G1 study MTX110 DSMB approval to proceed with Cohort B of Magic - G1 study in rGBM

Tolimidone in Type 1 Diabetes The potential to restore β - Cell Function

Type 1 vs Type 2 Diabetes Type 1 Diabetes • Primarily juvenile onset • An autoimmune process destroying pancreatic - cells • Reduced to no insulin production • Presence of autoantibodies • Not preventable • 10% of all DM patients Type 2 Diabetes • Usually late adult onset • (Partially) functional pancreas • (Some) insulin produced • Insulin resistance is an underlying mechanism • Preventable • 90% of all DM patients 10
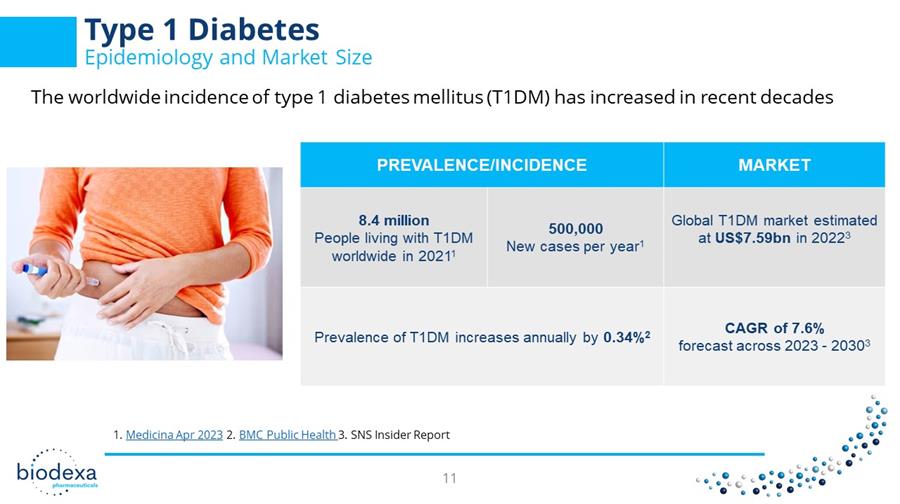
Type 1 Diabetes Epidemiology and Market Size PREVALENCE/INCIDENCE MARKET 8.4 million People living with T1DM worldwide in 2021 1 500,000 New cases per year 1 Global T1DM market estimated at US$7.59bn in 2022 3 Prevalence of T1DM increases annually by 0.34% 2 CAGR of 7.6% forecast across 2023 - 2030 3 1. Medicina Apr 2023 2. BMC Public Health 3. SNS Insider Report The worldwide incidence of type 1 diabetes mellitus (T1DM) has increased in recent decades 11

Lyn kinase as target in T1D • Lyn is a tyrosine kinase signaling protein which modulates key intracellular functions such as proliferation, differentiation, apoptosis, migration and metabolism • In fat cells it increases utilization of insulin and thus decreases blood sugar without having an effect on insulin production • In pancreatic islets, activation of lyn kinase promotes β - cell survival whereas its inhibition leads to cell death and precipitates diabetes 12

Characterization of lyn kinase as a target In mice with ‘switched off’ lyn kinase ( lyn knock - out mice) increased β - cell death is observed via apoptosis staining (shown in red below) At the same time, staining for active β - cells demonstrates decrease in their mass in lyn knock - out animals Data here and in further slides are from unpublished but publicly presented earlier elsewhere materials of prof. Jean Buteau laboratory (University of Alberta, Canada) 13
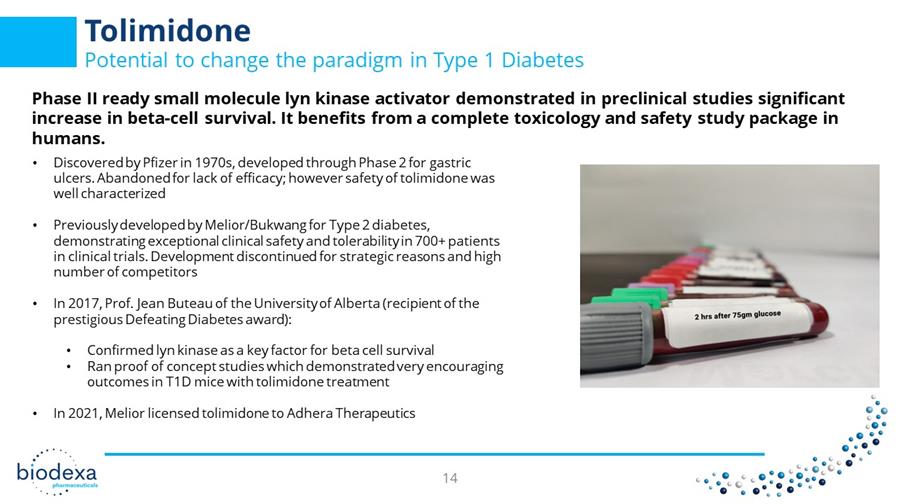
Tolimidone Potential to change the paradigm in Type 1 Diabetes Phase II ready small molecule lyn kinase activator demonstrated in preclinical studies significant increase in beta - cell survival. It benefits from a complete toxicology and safety study package in humans. 14 • Discovered by Pfizer in 1970s, developed through Phase 2 for gastric ulcers. Abandoned for lack of efficacy; however safety of tolimidone was well characterized • Previously developed by Melior / Bukwang for Type 2 diabetes, demonstrating exceptional clinical safety and tolerability in 700+ patients in clinical trials. Development discontinued for strategic reasons and high number of competitors • In 2017, Prof. Jean Buteau of the University of Alberta (recipient of the prestigious Defeating Diabetes award): • Confirmed lyn kinase as a key factor for beta cell survival • Ran proof of concept studies which demonstrated very encouraging outcomes in T1D mice with tolimidone treatment • In 2021, Melior licensed tolimidone to Adhera Therapeutics

Tolimidone effects in T1D mouse model • Tolimidone increases β - cell mass by promoting their survival as demonstrated by insulin staining in the images below • β - cell function preservation may be due to a direct protective activity of tolimidone on the cells, or reduction of glucotoxicity Tolimidone Tolimidone 15

Improved glucose control and improvement in islet health markers Improved glucose control in non - obese mouse model (NOS model) with preserved effect after treatment termination – protective effect of tolimidone Improvement in markers of islet health confirm protective effect of tolimidone Tolimidone Tolimidone TMD TMD Ctrl Ctrl Lean Vehicle Discontinue Tolimidone administration 16

Clinical development of tolimidone Phase Ib : • Phase I efficacy dose selection study to confirm Phase II dose. Safety data to be derived from earlier Pfizer/ Melior studies • N ≈ 12 - 15 patients, 3 - 4 dose/escalations • Runs in parallel with preparations for Phase II Phase II: • Aiming to open recruitment within 12 months • Double - blind, placebo - controlled study in adult patients with confirmed T1D, diagnosed btw >=1 year and <=5 years at the time of randomization • Adequate C - peptide level at baseline (clinical marker of insulin production) • Treatment duration: up to 6 months • Primary objective: changes in AUC of C - peptide after a 2 - hour MMTT test over the course of treatment 17

MTX110 18
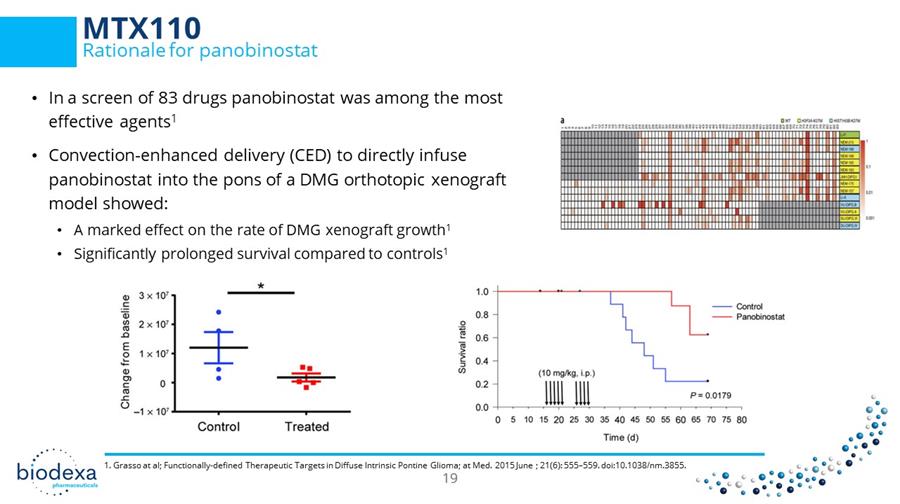
• In a screen of 83 drugs panobinostat was among the most effective agents 1 • Convection - enhanced delivery ( CED ) to directly infuse panobinostat into the pons of a DMG orthotopic xenograft model showed: • A marked effect on the rate of DMG xenograft growth 1 • Significantly prolonged survival compared to controls 1 1. Grasso at al; Functionally - defined Therapeutic Targets in Diffuse Intrinsic Pontine Glioma; at Med. 2015 June ; 21(6): 555 – 55 9. doi:10.1038/nm.3855. CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION MTX110 Rationale for panobinostat 19 19

Delivered directly into tumor Drug bypasses blood brain barrier Re - fillable implanted pump Optimized Therapeutic Dose with Minimal Potential for Systemic Exposure MTX110 Product concept 20
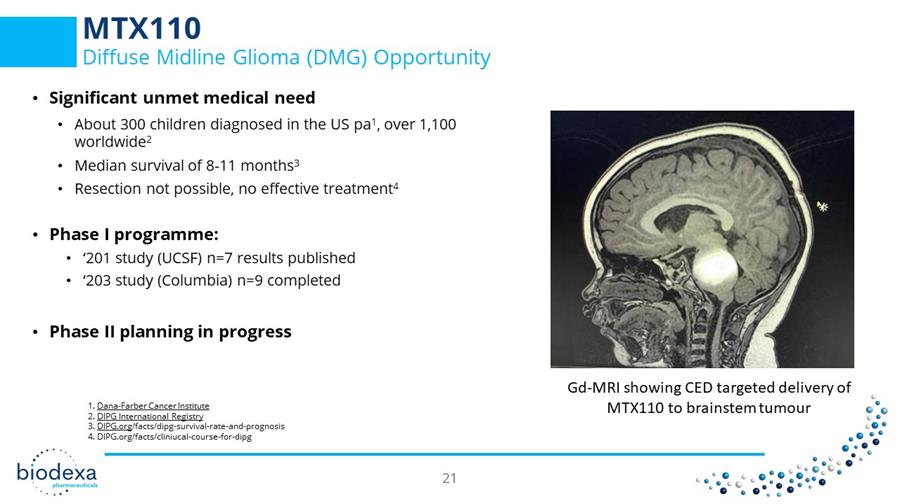
MTX110 Diffuse Midline Glioma (DMG) Opportunity • Significant unmet medical need • About 300 children diagnosed in the US pa 1 , over 1,100 worldwide 2 • Median survival of 8 - 11 months 3 • Resection not possible, no effective treatment 4 • Phase I programme: • ‘201 study (UCSF) n=7 results published • ‘203 study (Columbia) n=9 completed • Phase II planning in progress Gd - MRI showing CED targeted delivery of MT X110 to brainstem tumour 1. Dana - Farber Cancer Institute 2. DIPG International Registry 3. DIPG.org /facts/ dipg - survival - rate - and - prognosis 4. DIPG.org/facts/ cliniucal - course - for - dipg CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION 21 21

MTX110 Diffuse Midline Glioma (DMG) – clinical data from 201 study 22 CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION 1. Jansen et al, 2015. Neuro - Oncology 17(1):160 - 166 The median overall survival (OS) for cohort was 26.1 months (95% CI) Median survival based on a cohort of 316 cases was 10.0 months 1

MTX110 Glioblastoma (GBM) Opportunity • Most common and aggressive form of brain cancer in adults • Survival with standard of care treatment ranges from approximately 13 to 30 months depending on MGMT methylation 1 . • Standard of care: resection, radiation, limited drug options • Near 100% recurrence • More than 13,000 GBM diagnoses were expected in US in 2022 2 • Difficult to clearly define incidence as it varies on reports, but the range includes 3 - 4 per 100,000 3 • Global GBM treatment market valued at US$ 2.46 billion in 2022, expected to grow at 9.7% pa through 2030 4 1. Radke et al (2019). Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathologica Communications 1:89 2. National Brain Tumor Society 3 . Cancers | Free Full - Text | Epidemiology of Glioblastoma Multiforme–Literature Review (mdpi.com) 4 . Grand View Research Glioblastoma Multiforme Treatment Market Size, Share & Trends Analysis Report CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION 23 23

MTX110 Glioblastoma (GBM) preclinical data 24 • Problem: • Many drug failures due to poor BBB penetration • Potential Solution: • MTX110 is delivered intra - tumorally at high doses via CED, by - passing BBB • Compelling mechanism and preclinical evidence of efficacy in GBM models • Encouraging data from DIPG studies • Phase I (MAGIC – G1 study) recruiting : • Two centers: Duke and MD Anderson Baptist • Open label, dose escalation • First cohort (4 pts recruited), second cohort imminent • First data readout expected Q2 2024 Figure 1. Tumour volume data from IDH1 mutated tumour bearing mice resulted in a significant reduction in tumour growth compared to control. CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION Area of necrosis = treatment effect

Transaction overview Biodexa Pharmaceuticals PLC ( NASDAQ:BDRX ) has entered into conditional agreements with Melior Pharmaceuticals and Adhera Therapeutics Inc ( ATRX ) to acquire global rights to tolimidone - a Phase II ready asset for the treatment of Type 1 diabetes Building a portfolio of high value, clinical - stage assets Transaction Overview Structure: License Agreement (Melior) Assignment & Exchange Agreement (Adhera / Adhera Noteholders) Pro - Forma Ownership 1 : BDRX Melior Adhera Noteholders 2 37.3% 9.9% 52.8% Expected Timing: Q4 2023 25 1. Assumes Offering Price of $3.75 2. Assumes Adhera noteholoders subscribe $4MM in offering

1 Priced a t Offering Price, assumed to be $3.75 2 Assumes Adhera noteholoders subscribe $4MM in offering, otherwise $2MM 3. Priced at 10% discount to 5 - day VWAP or payable in cash Lock Up: 90 Days from Closing; Leak Out Agreement once resale registration statement declared effective. Consideration to Adhera , Adhera’s noteholders and Melior / Bukwang : At closing of transaction: Adhera $0.7m cash Adhera noteholders $5.0m ADSs 1,2 Melior / Bukwang $0.75m ADSs 1 Contingent consideration (payable to Adhera noteholders): Completion of successful Phase II study: $1.0m ADSs 1 First commercial sale: $3.0m ADSs 3 Contingent consideration payable to Melior / Bukwang : Tiered royalties on Net Sales Low – High single digit Transaction terms 26

PRO - FORMA CAP TABLE 27

Pro - forma cap table Excluding F - 1 financing 28 BDRX has zero debt No Rachets or Resets 1. Assumes Adhera noteholders subscribe $4MM in offering at offer price of $3.75 per ADS 2. Melior / Bukwang to receive 9.9% of diluted in - the - money BDRX post F - 1 financing ADSs % WAV exercise price % Issued 941,254 37.3% 33.4% Adhera noteholders 1 1,333,333 52.8% 47.3% Melior / Bukwang 2 249,927 9.9% 8.9% Diluted - in-the-money 2,524,515 100.0% 89.6% Other Warrants 291,243 31.49$ 10.3% Stock options 303 2,021.20$ 0.0% Fully diluted 2,816,061 100.0%

Biodexa value cycle 29 Identify attractive opportunities In license late preclinical to early clinical stage assets Generate financing to enable development Complete proof of concept clinical studies to add value Out licence prior to late stage clinicals

30 Appendix

MTD217 31

MTD217 Leptomeningeal Disease (LMD) Background • Cancer in the cerebrospinal fluid and leptomeninges caused by metastases • Most commonly arises from breast and lung cancers • Approximately 5% of people who have cancer develop 1 LMD • No effective treatments currently exist for LMD • With treatment, survival is just three to six months after diagnosis 1 1. https://my.clevelandclinic.org/health/diseases/22737 - leptomeningeal - disease CONFIDENTIAL – NOT FOR PUBLIC DISTRIBUTION 32 32
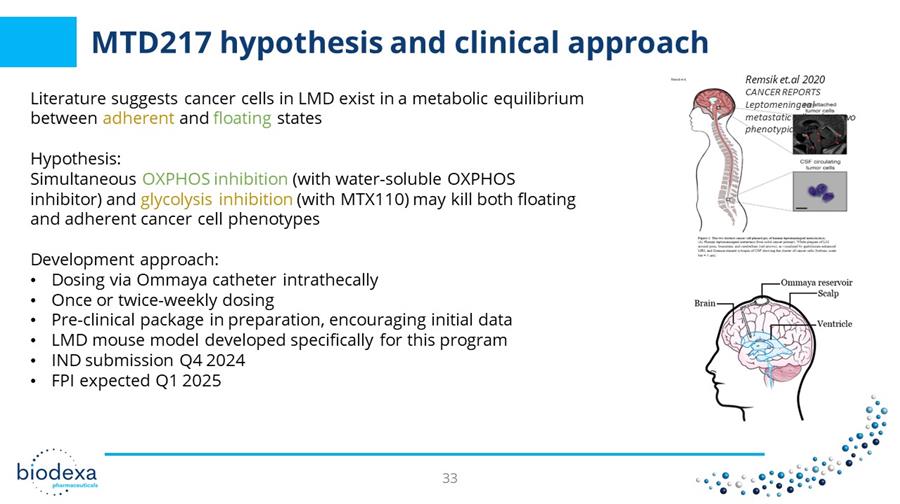
MTD217 hypothesis and clinical approach Literature suggests cancer cells in LMD exist in a metabolic equilibrium between adherent and floating states Hypothesis: Simultaneous OXPHOS inhibition (with water - soluble OXPHOS inhibitor) and glycolysis inhibition (with MTX110) may kill both floating and adherent cancer cell phenotypes Development approach: • Dosing via Ommaya catheter intrathecally • Once or twice - weekly dosing • Pre - clinical package in preparation, encouraging initial data • LMD mouse model developed specifically for this program • IND submission Q4 2024 • FPI expected Q1 2025 Remsik et.al 2020 CANCER REPORTS Leptomeningeal metastatic cells adopt two phenotypic states 33
Midatech Pharma (NASDAQ:MTP)
Historical Stock Chart
From Mar 2024 to Apr 2024
Midatech Pharma (NASDAQ:MTP)
Historical Stock Chart
From Apr 2023 to Apr 2024