0001579428false00015794282023-12-072023-12-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): December 07, 2023 |
Axsome Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
001-37635 |
45-4241907 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
One World Trade Center, 22nd Floor |
|
New York, New York |
|
10007 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (212) 332-3241 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, Par Value $0.0001 Per Share |
|
AXSM |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On December 7, 2023, Axsome Therapeutics, Inc. (the “Company”) held its “Solriamfetol Investor Day Event.” A copy of the press release dated December 7, 2023 announcing this event is filed as Exhibit 99.1 hereto and a copy of the materials used in connection with this event are filed as Exhibit 99.2 hereto, and both are incorporated by reference herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
Axsome Therapeutics, Inc. |
|
|
|
|
Date: |
December 7, 2023 |
By: |
/s/ Herriot Tabuteau, M.D. |
|
|
Name: Title: |
Herriot Tabuteau, M.D.
President and Chief Executive Officer |

Axsome Therapeutics Hosts Solriamfetol Virtual Investor Event with Expert Thought Leaders Today
NEW YORK, Dec. 7, 2023 (Globe Newswire) – Axsome Therapeutics, Inc. (NASDAQ: AXSM), a biopharmaceutical company developing and delivering novel therapies for the management of central nervous system (CNS) disorders, is hosting a virtual investor event and conference call today from 8 a.m. – 10 a.m. Eastern Time to provide an update on solriamfetol, a dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) and trace amine-associated receptor 1 (TAAR1) agonist.
At the event, invited physician thought leaders will discuss current and potential future indications. The Axsome senior leadership team will provide an overview of clinical development plans. The presenters will be available to answer questions at the end of the presentations. To access the event, please click here.
Expert thought leaders presenting at the event include:
•Craig Chepke, MD, Medical Director of Excel Psychiatric Associates in Huntersville, N.C. and Adjunct Associate Professor of Psychiatry for Atrium Health. Dr. Chepke will provide an overview of TAAR1 signaling and major depressive disorder (MDD).
•Andrew Cutler, MD, Clinical Associate Professor of Psychiatry SUNY Upstate Medical University. Dr. Cutler will provide an overview of attention deficit hyperactivity disorder (ADHD).
•Susan McElroy, MD, Chief Research Officer and Director of Psychopharmacology Research at the Lindner Center of HOPE at the University of Cincinnati. Dr. McElroy will provide an overview of binge eating disorder (BED).
•Richard Bogan, MD, Associate Clinical Professor at the University of South Carolina School of Medicine and at the Medical University of South Carolina in Charleston, S.C., and is Principal of Bogan Sleep Consultants. Dr. Bogan will provide an overview of the SHARP study of solriamfetol in patients with excessive daytime sleepiness (EDS) associated with obstructive sleep apnea (OSA), and impaired cognitive function.
•Charles Czeisler, MD, PhD, Baldino Professor of Sleep Medicine, Director of the Division of Sleep Medicine at Harvard Medical School and Chief of the Division of Sleep Medicine in the Department of Medicine at Brigham and Women’s Hospital in Boston. Dr. Czeisler will provide an overview of shift work disorder (SWD).
Webcast and Conference Call Information
A live webcast of the event can be accessed on the “Webcasts & Presentations” page of the “Investors” section of the Company’s website at www.axsome.com. To participate in the live conference call, please dial (877) 405-1239 (toll-free domestic). A replay of the webcast will be available for approximately 30 days following the live event.
About Solriamfetol
Solriamfetol is a dual-acting dopamine and norepinephrine reuptake inhibitor and trace amine-associated receptor 1 (TAAR1) agonist. Solriamfetol is protected by a robust patent estate with expiries out to 2042.
About Axsome Therapeutics, Inc.
Axsome Therapeutics, Inc. is a biopharmaceutical company developing and delivering novel therapies for central nervous system (CNS) conditions that have limited treatment options. Through development of therapeutic options with novel mechanisms of action, we are transforming the approach to treating CNS conditions. At Axsome, we are committed to developing products that meaningfully improve the lives of patients and provide new therapeutic options for physicians. For more information, please visit the Company’s website at axsome.com. The Company may occasionally disseminate material, nonpublic information on the company website.
1
Forward Looking Statements
Certain matters discussed in this press release are “forward-looking statements”. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the continued commercial success of our Sunosi® and Auvelity® products and the success of our efforts to obtain any additional indication(s) with respect to solriamfetol and/or AXS-05; the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected revenues or expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials, and the number or type of studies or nature of results necessary to support the filing of a new drug application (“NDA”) for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates; whether issues identified by FDA in the complete response letter may impact the potential approvability of the Company’s NDA for AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment for the MOMENTUM clinical trial; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s products and product candidates, if approved; the Company’s anticipated capital requirements, including the amount of capital required for the continued commercialization of Sunosi and Auvelity and for the Company’s commercial launch of its other product candidates, if approved, and the potential impact on the Company’s anticipated cash runway; unforeseen circumstances or other disruptions to normal business operations arising from or related to COVID-19; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this press release and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance.
Axsome Contacts:
Investors:
Mark Jacobson
Chief Operating Officer
Axsome Therapeutics, Inc.
One World Trade Center, 22nd Floor
New York, NY 10007
Tel: 212-332-3243
Email: mjacobson@axsome.com
www.axsome.com
Media:
Darren Opland
Director, Corporate Communications
Axsome Therapeutics, Inc.
One World Trade Center, 22nd Floor
New York, NY 10007
Tel: 929-837-1065
Email: dopland@axsome.com
www.axsome.com
2

Solriamfetol Investor Day December 7, 2023

Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the continued commercial success of our Sunosi® and Auvelity® products and the success of our efforts to obtain any additional indication(s) with respect to solriamfetol and/or AXS-05; the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected revenues or expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials, and the number or type of studies or nature of results necessary to support the filing of a new drug application (“NDA”) for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates; whether issues identified by FDA in the complete response letter may impact the potential approvability of the Company’s NDA for AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment for the MOMENTUM clinical trial; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s products and product candidates, if approved; the Company’s anticipated capital requirements, including the amount of capital required for the continued commercialization of Sunosi and Auvelity and for the Company’s commercial launch of its other product candidates, and the potential impact on the Company’s anticipated cash runway; unforeseen circumstances or other disruptions to normal business operations arising from or related to COVID-19; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this press release and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance.. This presentation contains statements regarding the Company’s observations based upon the reported clinical data. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about the Company's industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. Axsome, Auvelity, Sunosi, and MoSEIC, are trademarks or registered trademarks of Axsome Therapeutics, Inc. or its affiliates. Except as with respect to Auvelity and Sunosi for their approved indications, the development products referenced herein have not been approved by the FDA. Forward Looking Statements & Safe Harbor

Today’s KOL Speakers Andrew J. Cutler, MD Clinical Associate Professor of Psychiatry SUNY Upstate Medical University Susan McElroy, MD Chief Research Officer and Director of Psychopharmacology Research at the Lindner Center of HOPE at the University of Cincinnati Richard Bogan, MD Associate Clinical Professor at the University of South Carolina School of Medicine and Medical University of South Carolina in Charleston, South Carolina and Principal of Bogan Sleep Consultants Charles Czeisler, MD, PhD Baldino Professor of Sleep Medicine, Director of the Division of Sleep Medicine at Harvard Medical School and Chief of the Division of Sleep Medicine in the Department of Medicine at Brigham and Women’s Hospital Craig Chepke, MD, DFAPA Medical Director of Excel Psychiatric Associates Adjunct Associate Professor of Psychiatry for Atrium Health

Today’s Agenda Welcome Solriamfetol Overview TAAR1 MOA And Major Depressive Disorder (MDD) Attention Deficit Hyperactivity Disorder (ADHD) Q+A on TAAR1, MDD and ADHD Binge Eating Disorder (BED) Cognition Shift Work Disorder (SWD) Q+A on Cognition, BED, SWD Closing Remarks Mark Jacobson, COO Dr. Herriot Tabuteau, CEO Dr. Craig Chepke Dr. Andrew Cutler Drs. Chepke and Cutler Dr. Susan McElroy Dr. Richard Bogan Dr. Charles Czeisler Drs. McElroy, Bogan, and Czeisler Dr. Herriot Tabuteau, CEO

Solriamfetol Overview Herriot Tabuteau, MD
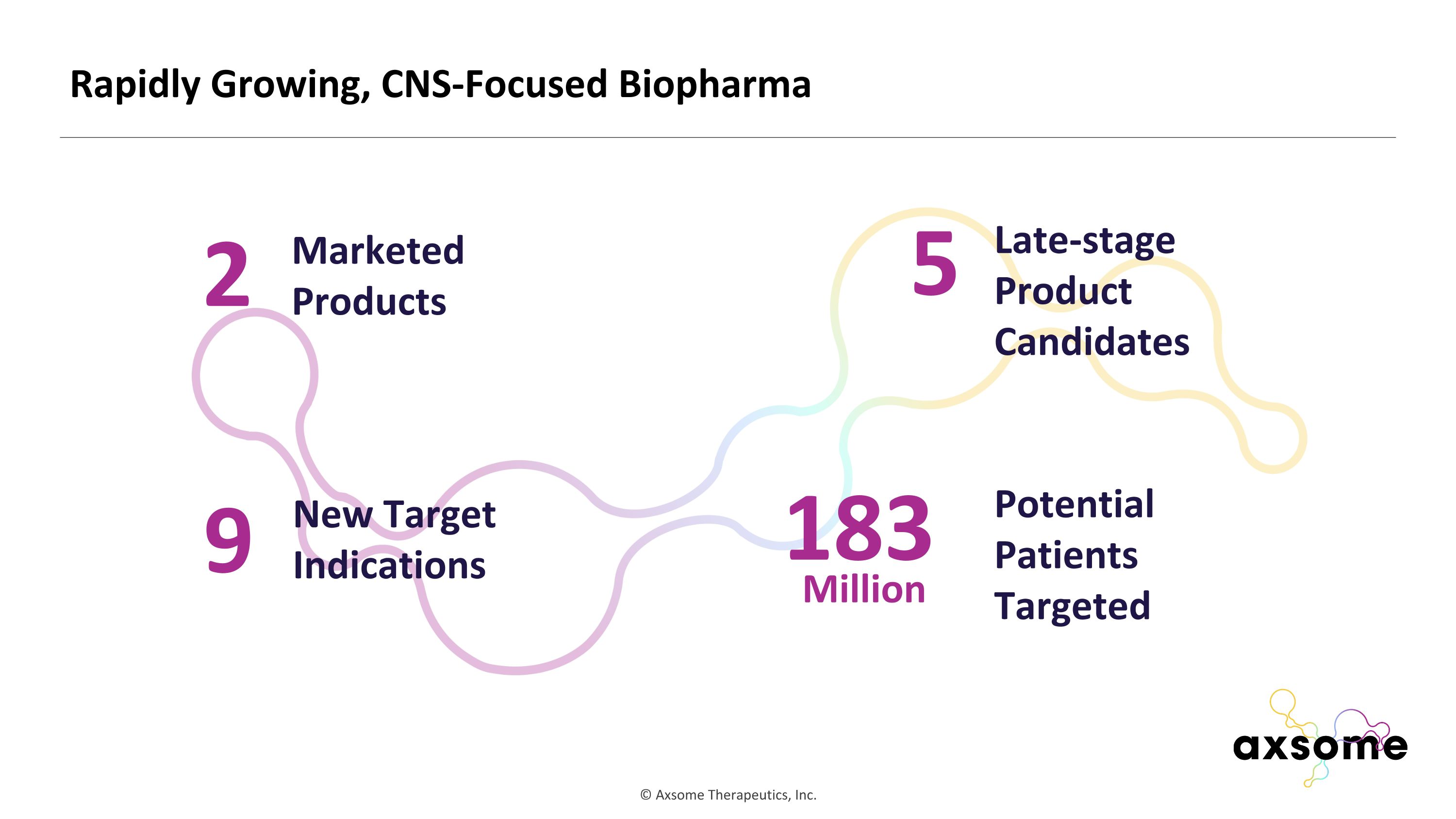
Rapidly Growing, CNS-Focused Biopharma 2 Marketed Products 9 New Target Indications 5 Late-stage Product Candidates 183 Potential Patients Targeted Million
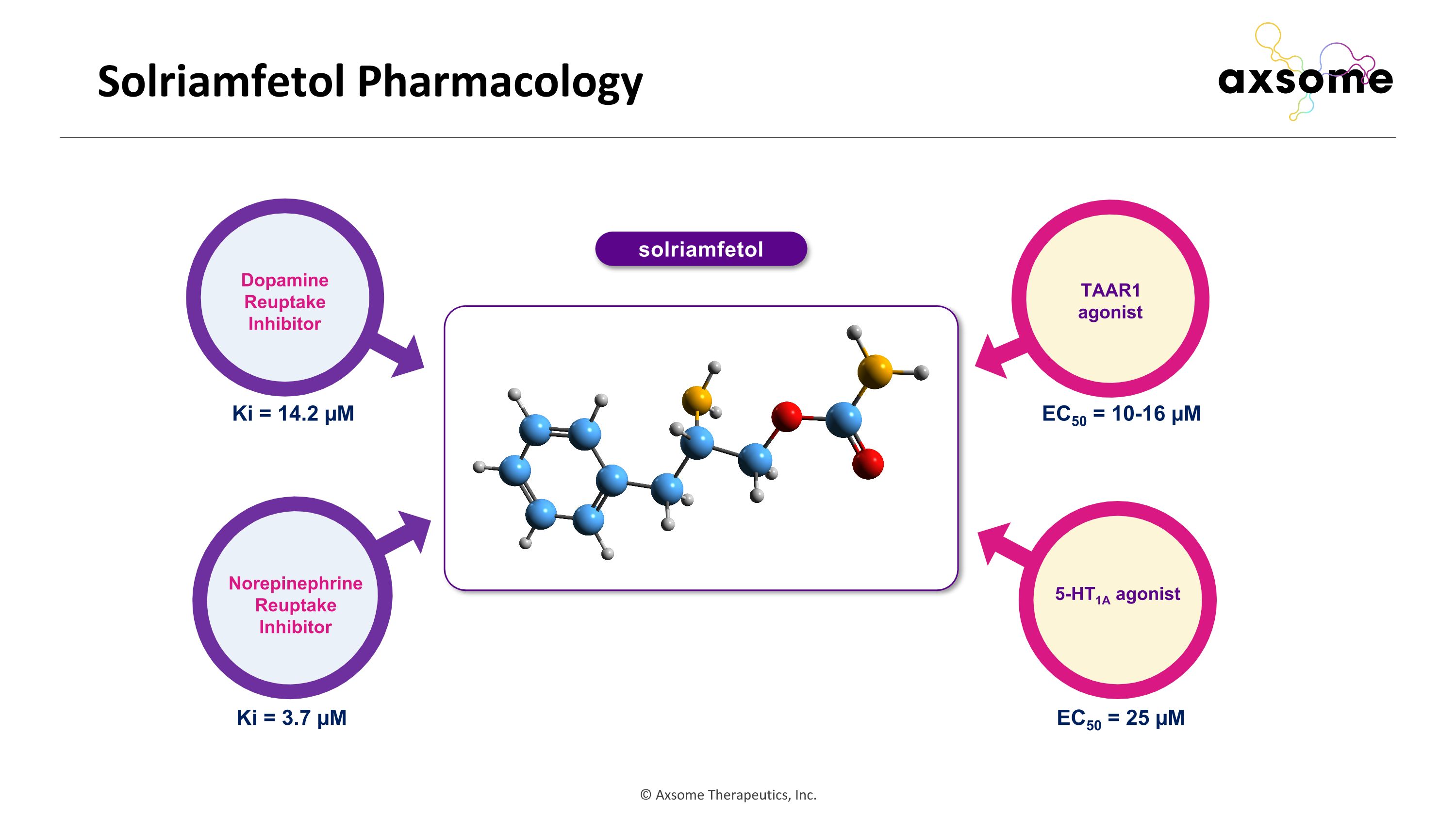
Solriamfetol Pharmacology Dopamine Reuptake Inhibitor Norepinephrine Reuptake Inhibitor TAAR1 agonist 5-HT1A agonist Ki = 3.7 µM Ki = 14.2 µM EC50 = 10-16 µM EC50 = 25 µM solriamfetol
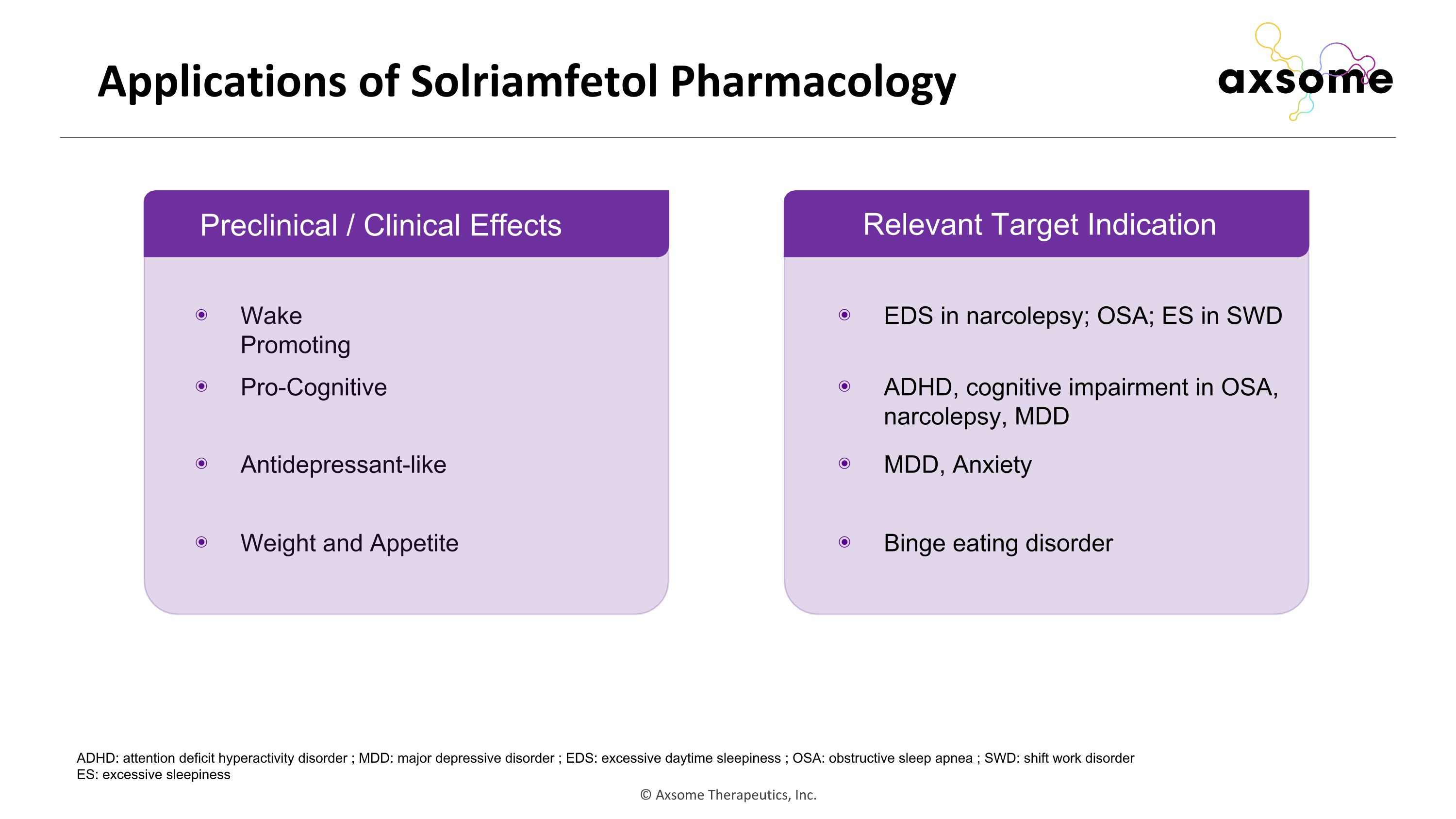
Applications of Solriamfetol Pharmacology Relevant Target Indication Wake Promoting Pro-Cognitive Antidepressant-like Weight and Appetite EDS in narcolepsy; OSA; ES in SWD ADHD, cognitive impairment in OSA, narcolepsy, MDD MDD, Anxiety Binge eating disorder ADHD: attention deficit hyperactivity disorder ; MDD: major depressive disorder ; EDS: excessive daytime sleepiness ; OSA: obstructive sleep apnea ; SWD: shift work disorder ES: excessive sleepiness Preclinical / Clinical Effects
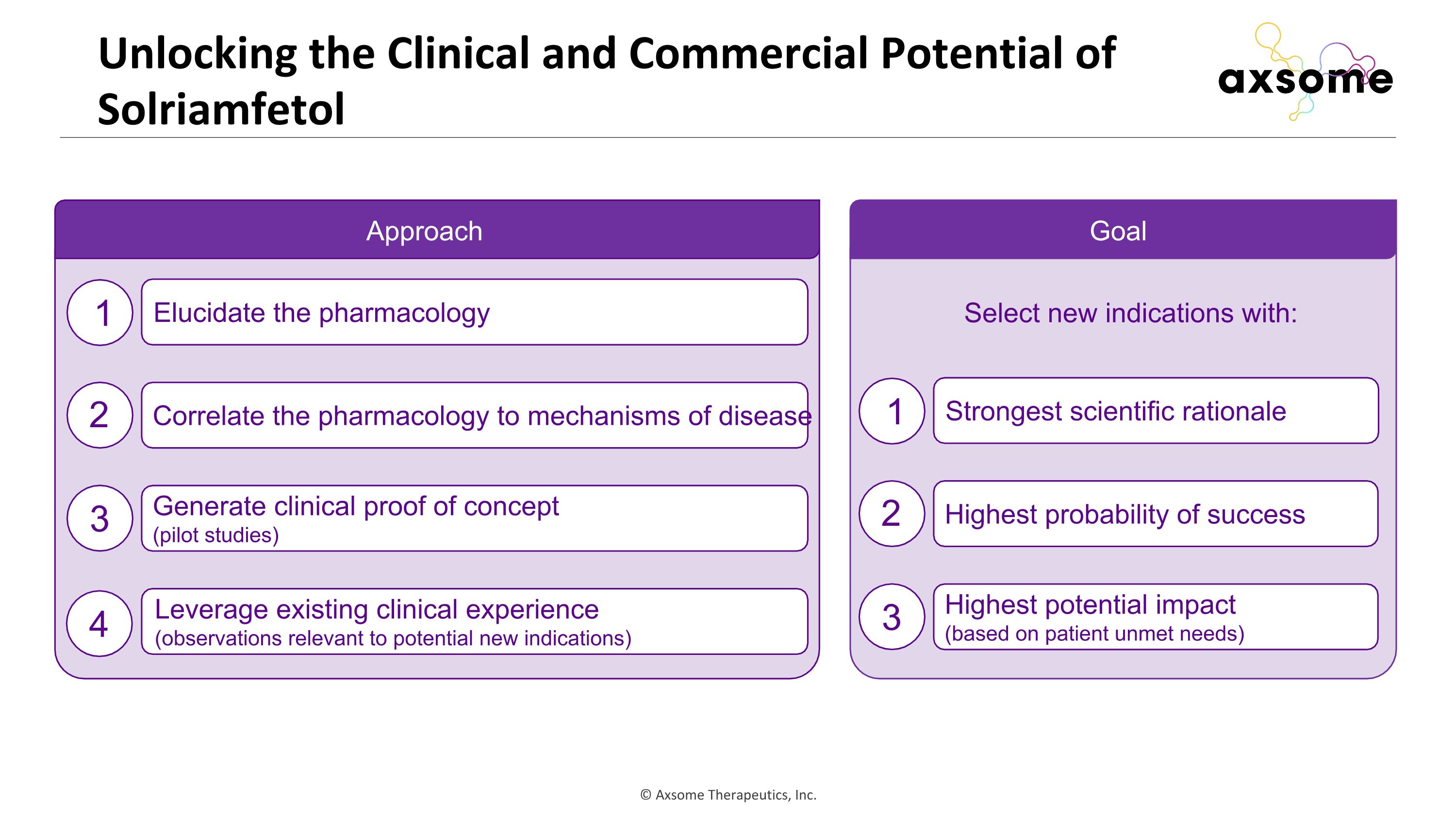
Unlocking the Clinical and Commercial Potential of Solriamfetol Elucidate the pharmacology Correlate the pharmacology to mechanisms of disease Generate clinical proof of concept (pilot studies) Leverage existing clinical experience (observations relevant to potential new indications) Approach 1 2 3 4 Goal Strongest scientific rationale Highest probability of success Highest potential impact (based on patient unmet needs) 1 2 3 Select new indications with:
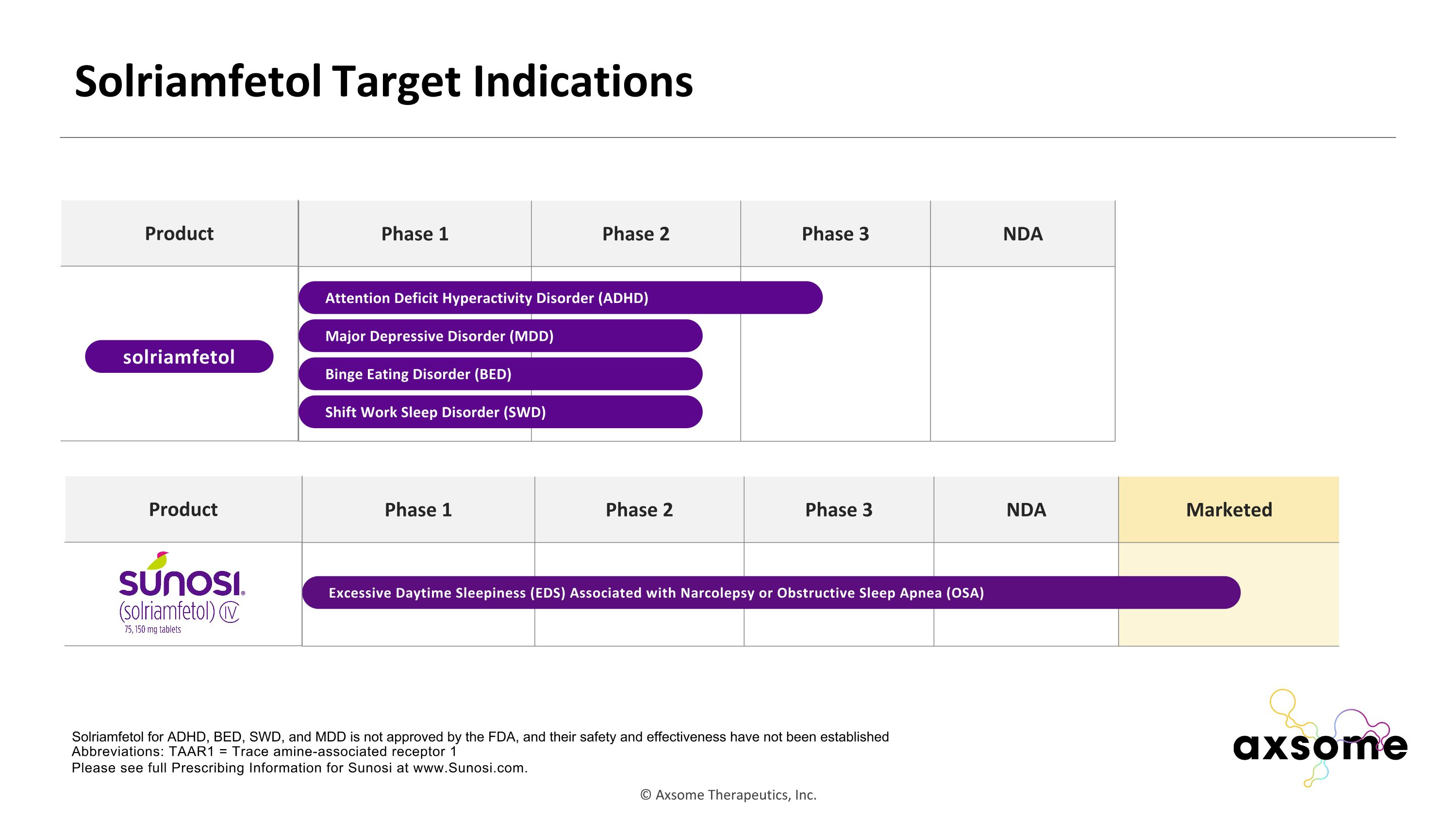
Phase 1 Phase 2 Phase 3 NDA Marketed Solriamfetol Target Indications Abbreviations: TAAR1 = Trace amine-associated receptor 1 Please see full Prescribing Information for Sunosi at www.Sunosi.com. Phase 1 Phase 2 Phase 3 NDA Attention Deficit Hyperactivity Disorder (ADHD) Solriamfetol for ADHD, BED, SWD, and MDD is not approved by the FDA, and their safety and effectiveness have not been established Binge Eating Disorder (BED) Product solriamfetol Shift Work Sleep Disorder (SWD) Major Depressive Disorder (MDD) Excessive Daytime Sleepiness (EDS) Associated with Narcolepsy or Obstructive Sleep Apnea (OSA) Product
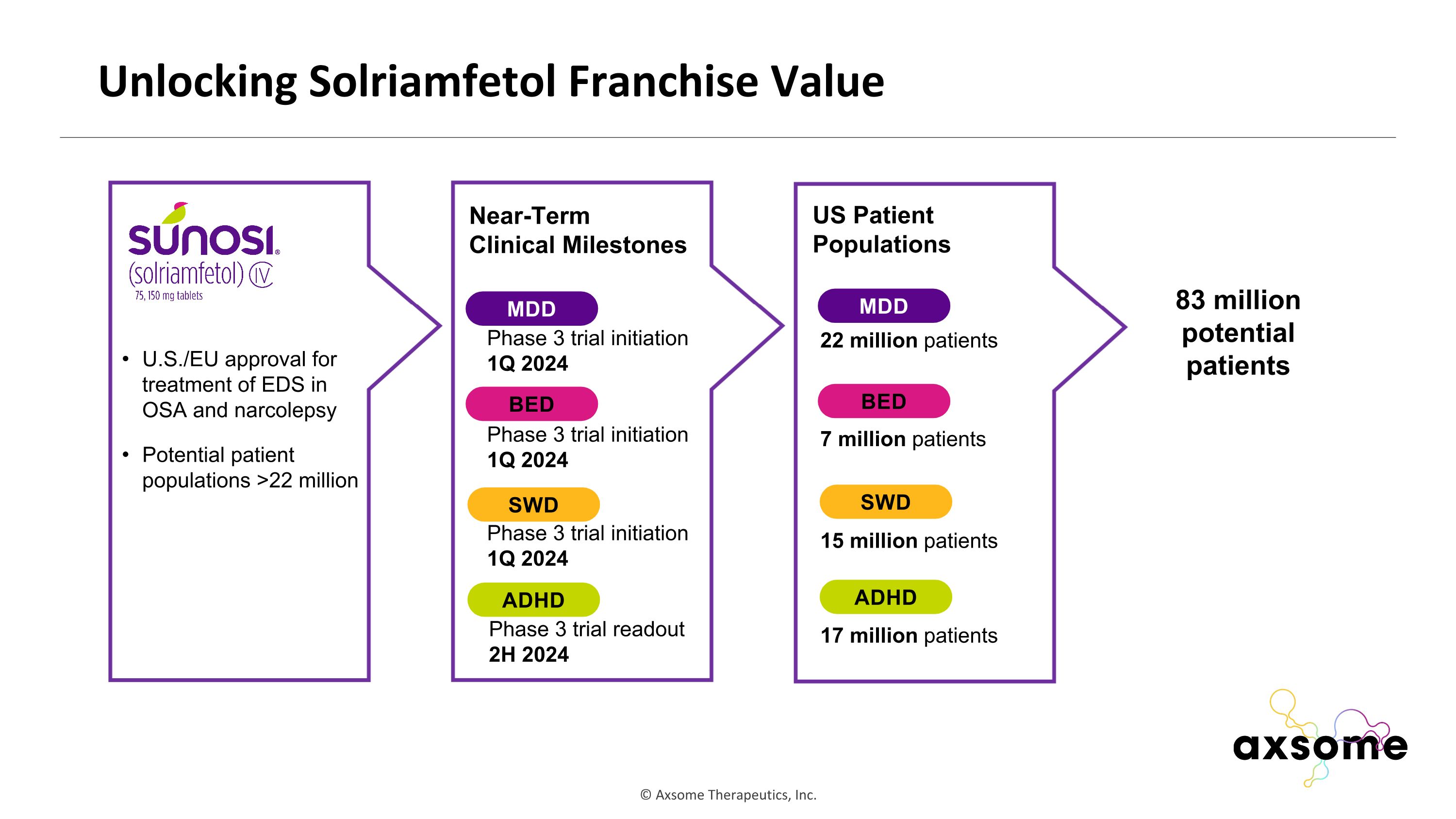
Unlocking Solriamfetol Franchise Value U.S./EU approval for treatment of EDS in OSA and narcolepsy Potential patient populations >22 million Near-Term Clinical Milestones ADHD MDD BED SWD Phase 3 trial readout 2H 2024 Phase 3 trial initiation 1Q 2024 Phase 3 trial initiation 1Q 2024 Phase 3 trial initiation 1Q 2024 ADHD MDD BED SWD US Patient Populations 22 million patients 7 million patients 15 million patients 17 million patients 83 million potential patients

Craig Chepke, MD, DFAPA Adjunct Associate Professor of Psychiatry, Atrium Health Medical Director, Excel Psychiatric Associates Trace Amine Associated Receptor Signaling and Psychiatric Disorders
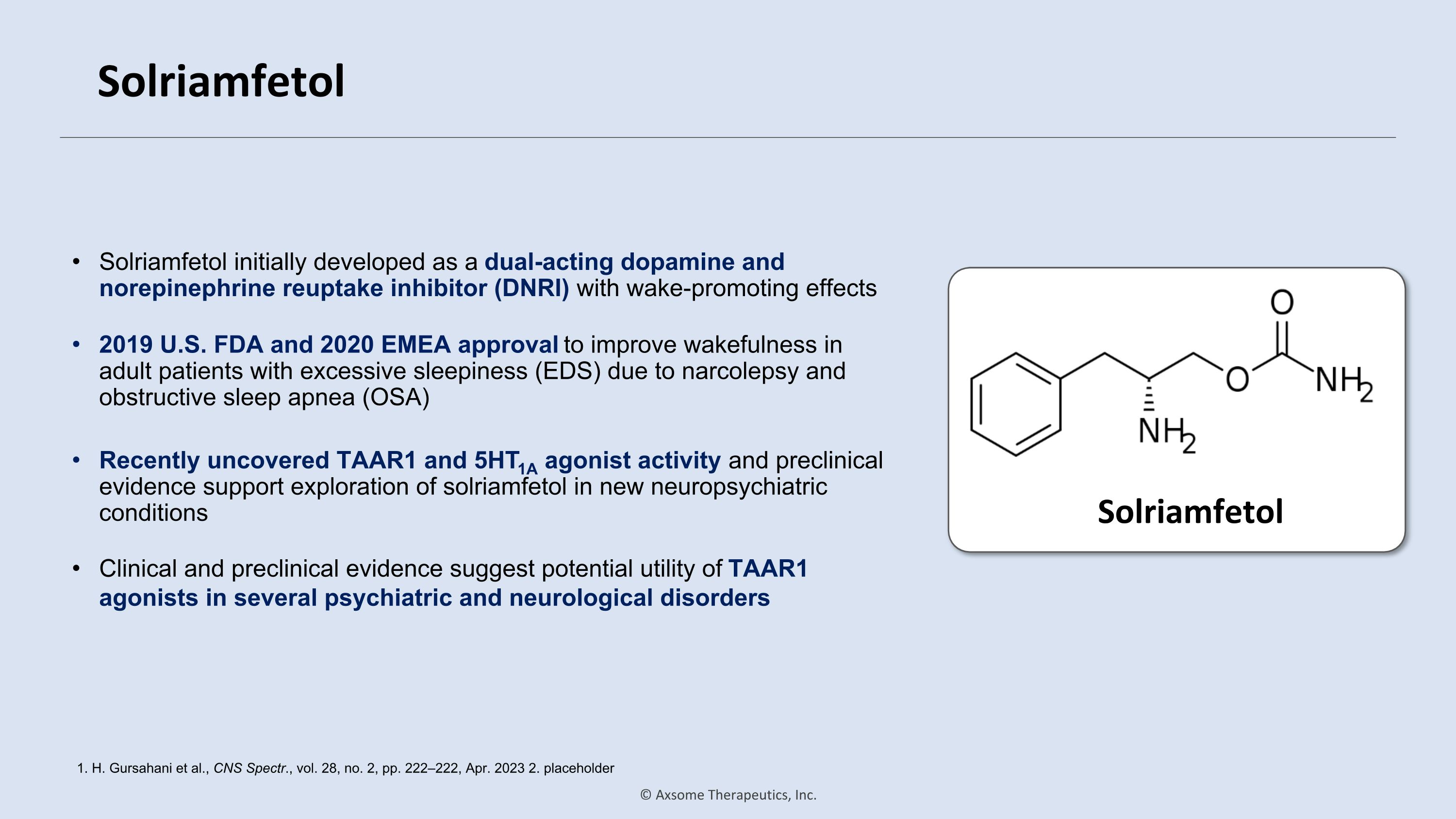
Solriamfetol Solriamfetol initially developed as a dual-acting dopamine and norepinephrine reuptake inhibitor (DNRI) with wake-promoting effects 1. H. Gursahani et al., CNS Spectr., vol. 28, no. 2, pp. 222–222, Apr. 2023 2. placeholder Solriamfetol 2019 U.S. FDA and 2020 EMEA approval to improve wakefulness in adult patients with excessive sleepiness (EDS) due to narcolepsy and obstructive sleep apnea (OSA) Recently uncovered TAAR1 and 5HT1A agonist activity and preclinical evidence support exploration of solriamfetol in new neuropsychiatric conditions Clinical and preclinical evidence suggest potential utility of TAAR1 agonists in several psychiatric and neurological disorders
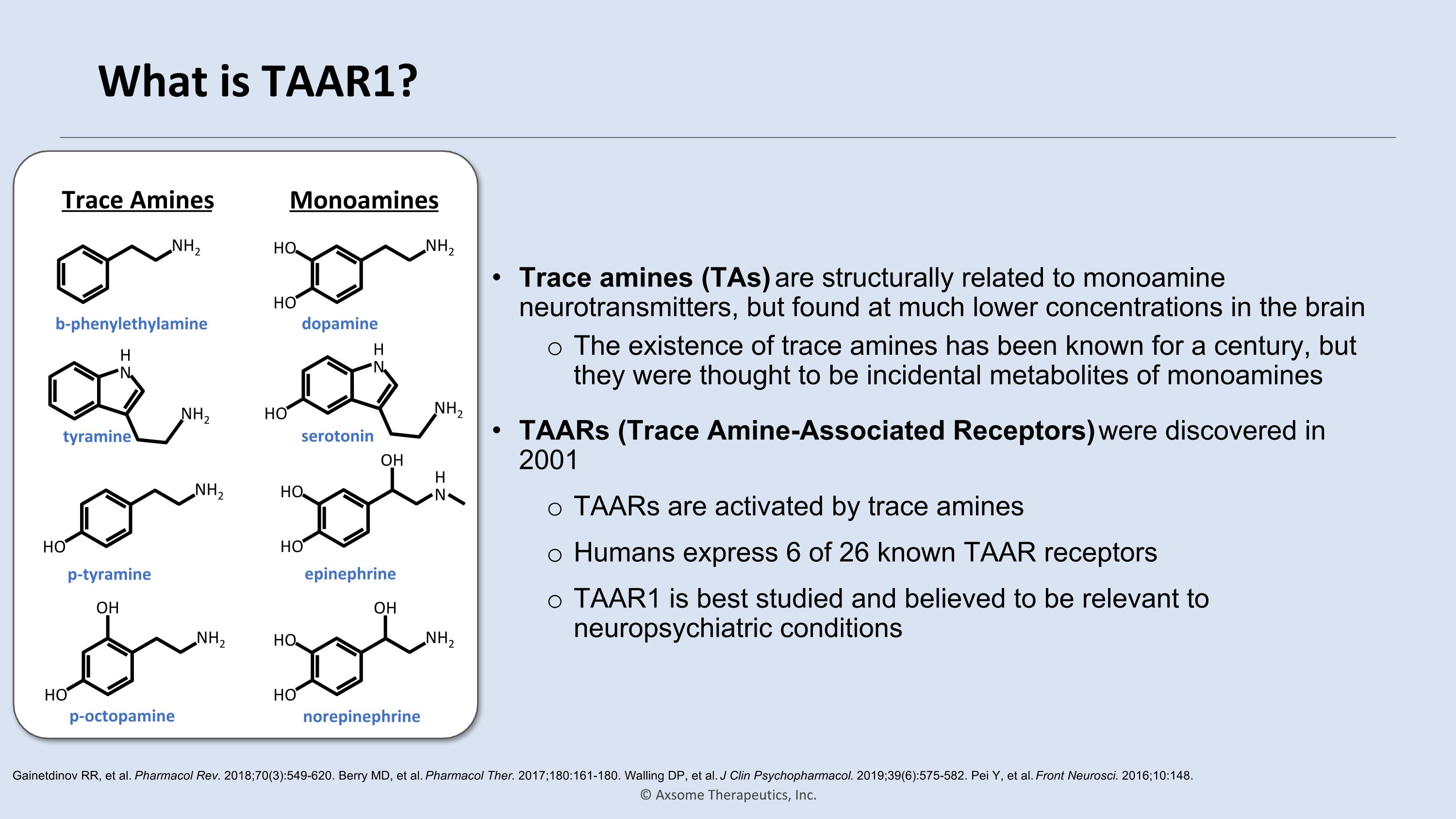
What is TAAR1? Trace amines (TAs) are structurally related to monoamine neurotransmitters, but found at much lower concentrations in the brain The existence of trace amines has been known for a century, but they were thought to be incidental metabolites of monoamines Gainetdinov RR, et al. Pharmacol Rev. 2018;70(3):549-620. Berry MD, et al. Pharmacol Ther. 2017;180:161-180. Walling DP, et al. J Clin Psychopharmacol. 2019;39(6):575-582. Pei Y, et al. Front Neurosci. 2016;10:148. TAARs (Trace Amine-Associated Receptors) were discovered in 2001 TAARs are activated by trace amines Humans express 6 of 26 known TAAR receptors TAAR1 is best studied and believed to be relevant to neuropsychiatric conditions NH2 b-phenylethylamine NH2 HO OH p-octopamine NH2 HO p-tyramine NH2 HO HO dopamine NH2 HO HO OH norepinephrine NH2 HO H�N serotonin NH2 H�N tyramine H�N HO HO OH epinephrine Trace Amines Monoamines
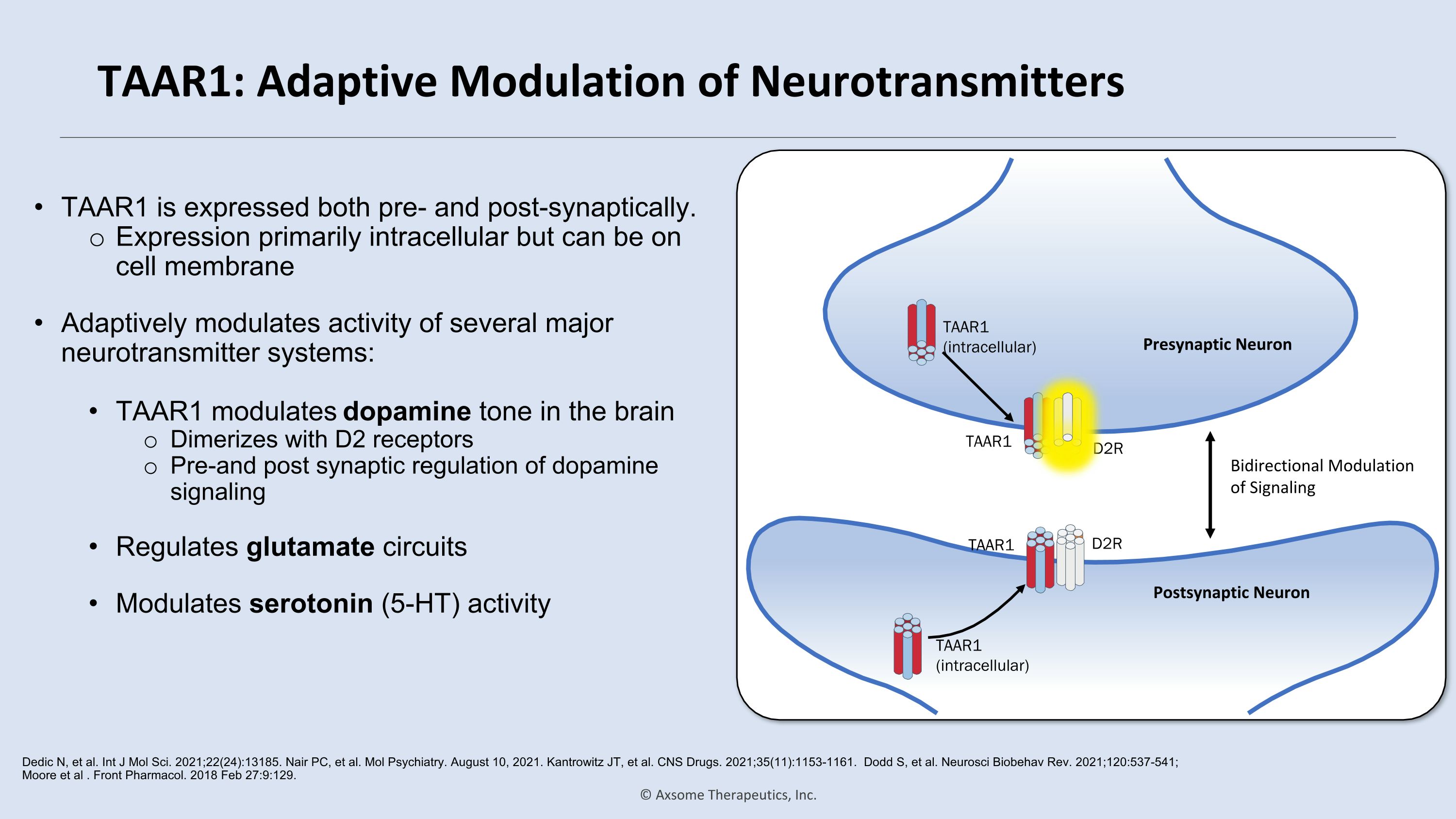
TAAR1: Adaptive Modulation of Neurotransmitters TAAR1 is expressed both pre- and post-synaptically. Expression primarily intracellular but can be on cell membrane Adaptively modulates activity of several major neurotransmitter systems: TAAR1 modulates dopamine tone in the brain Dimerizes with D2 receptors Pre-and post synaptic regulation of dopamine signaling Regulates glutamate circuits Modulates serotonin (5-HT) activity Dedic N, et al. Int J Mol Sci. 2021;22(24):13185. Nair PC, et al. Mol Psychiatry. August 10, 2021. Kantrowitz JT, et al. CNS Drugs. 2021;35(11):1153-1161. Dodd S, et al. Neurosci Biobehav Rev. 2021;120:537-541; Moore et al . Front Pharmacol. 2018 Feb 27:9:129. TAAR1 (intracellular) TAAR1 (intracellular) TAAR1 D2R TAAR1 D2R Bidirectional Modulation of Signaling Postsynaptic Neuron Presynaptic Neuron
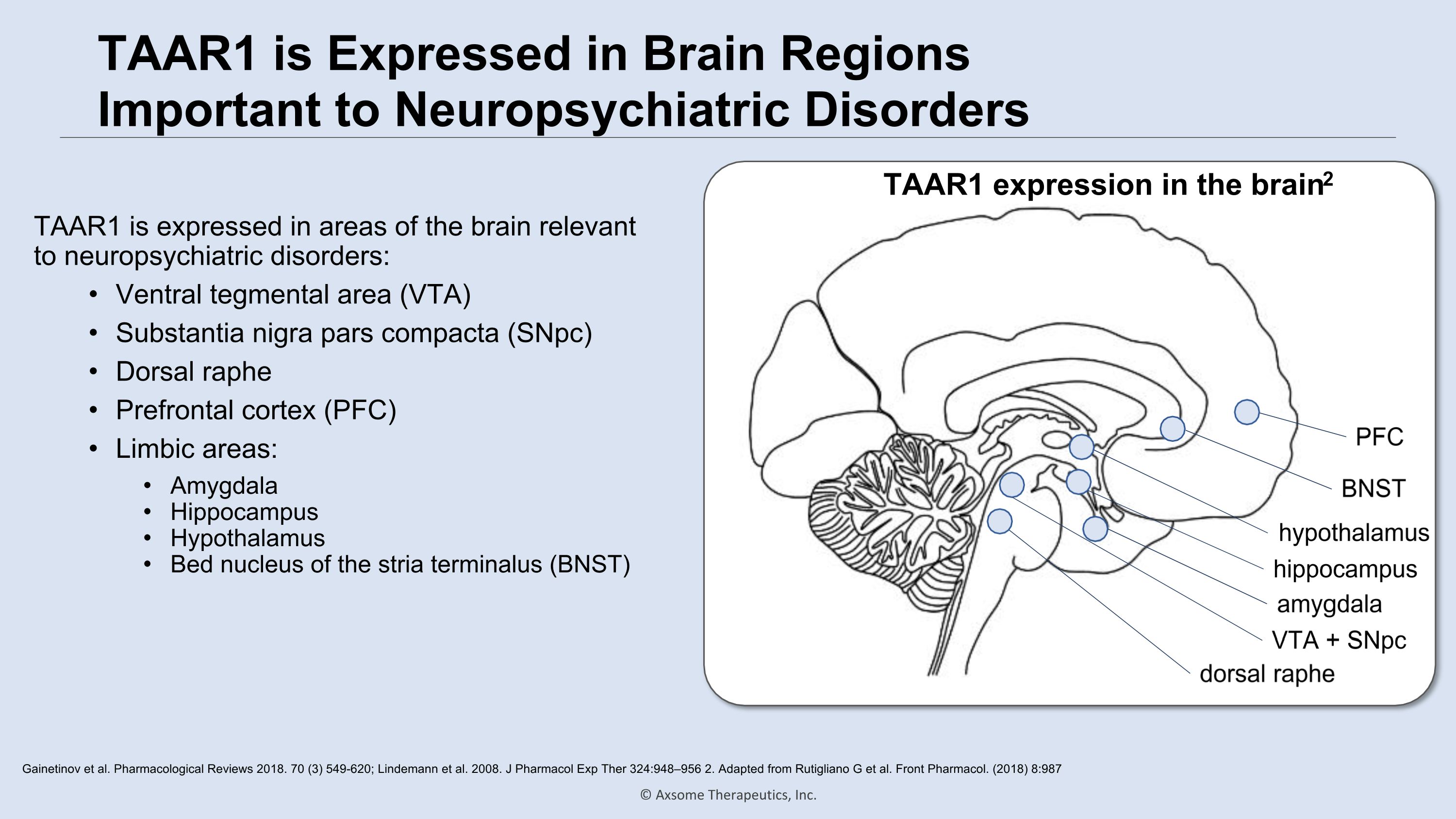
TAAR1 is Expressed in Brain Regions Important to Neuropsychiatric Disorders TAAR1 is expressed in areas of the brain relevant to neuropsychiatric disorders: Ventral tegmental area (VTA) Substantia nigra pars compacta (SNpc) Dorsal raphe Prefrontal cortex (PFC) Limbic areas: Amygdala Hippocampus Hypothalamus Bed nucleus of the stria terminalus (BNST) Gainetinov et al. Pharmacological Reviews 2018. 70 (3) 549-620; Lindemann et al. 2008. J Pharmacol Exp Ther 324:948–956 2. Adapted from Rutigliano G et al. Front Pharmacol. (2018) 8:987 VTA + SNpc dorsal raphe amygdala hypothalamus hippocampus BNST PFC TAAR1 expression in the brain2
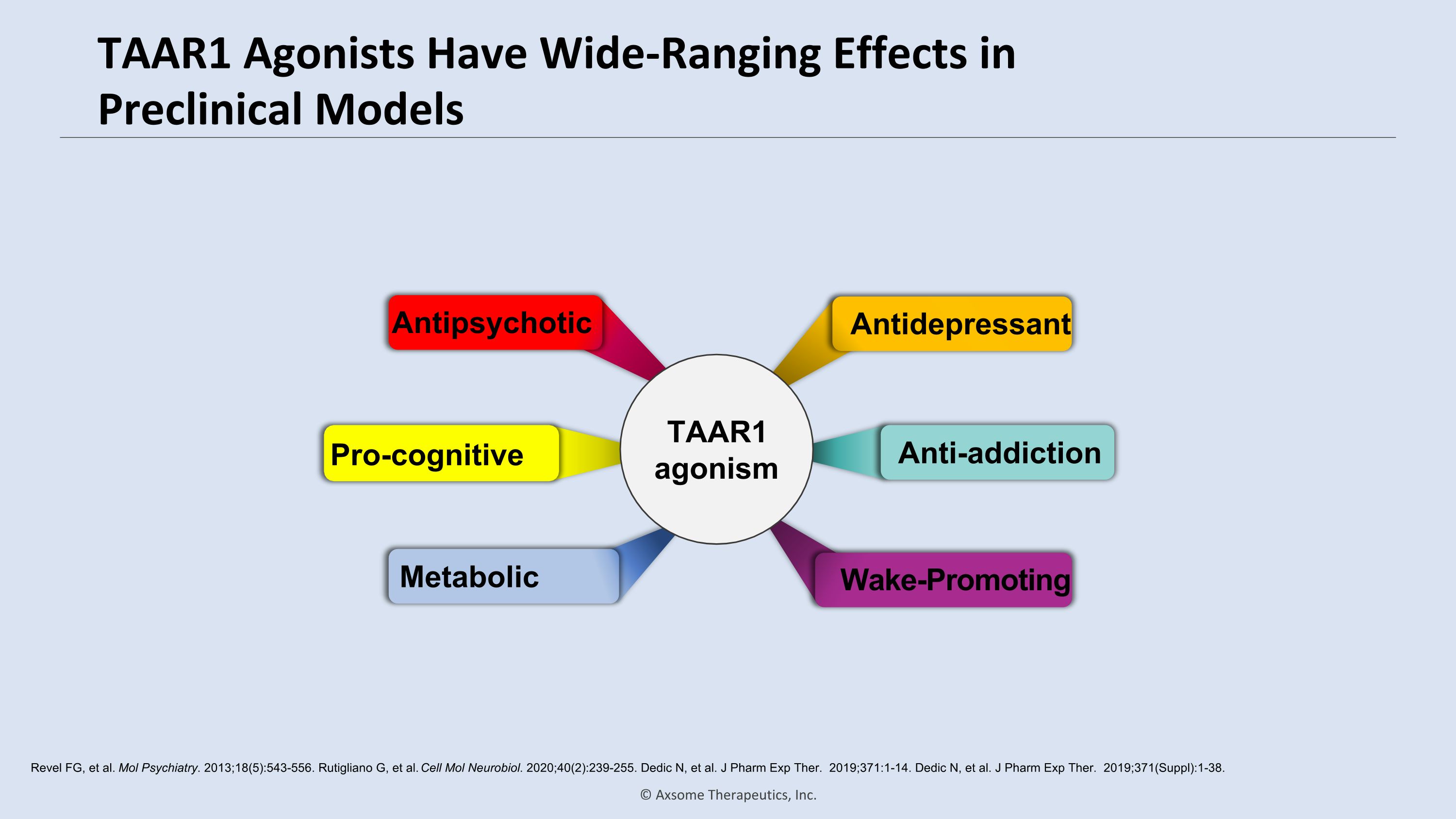
TAAR1 Agonists Have Wide-Ranging Effects in Preclinical Models Revel FG, et al. Mol Psychiatry. 2013;18(5):543-556. Rutigliano G, et al. Cell Mol Neurobiol. 2020;40(2):239-255. Dedic N, et al. J Pharm Exp Ther. 2019;371:1-14. Dedic N, et al. J Pharm Exp Ther. 2019;371(Suppl):1-38. Antidepressant Pro-cognitive Antipsychotic Wake-Promoting Metabolic Anti-addiction TAAR1 agonism
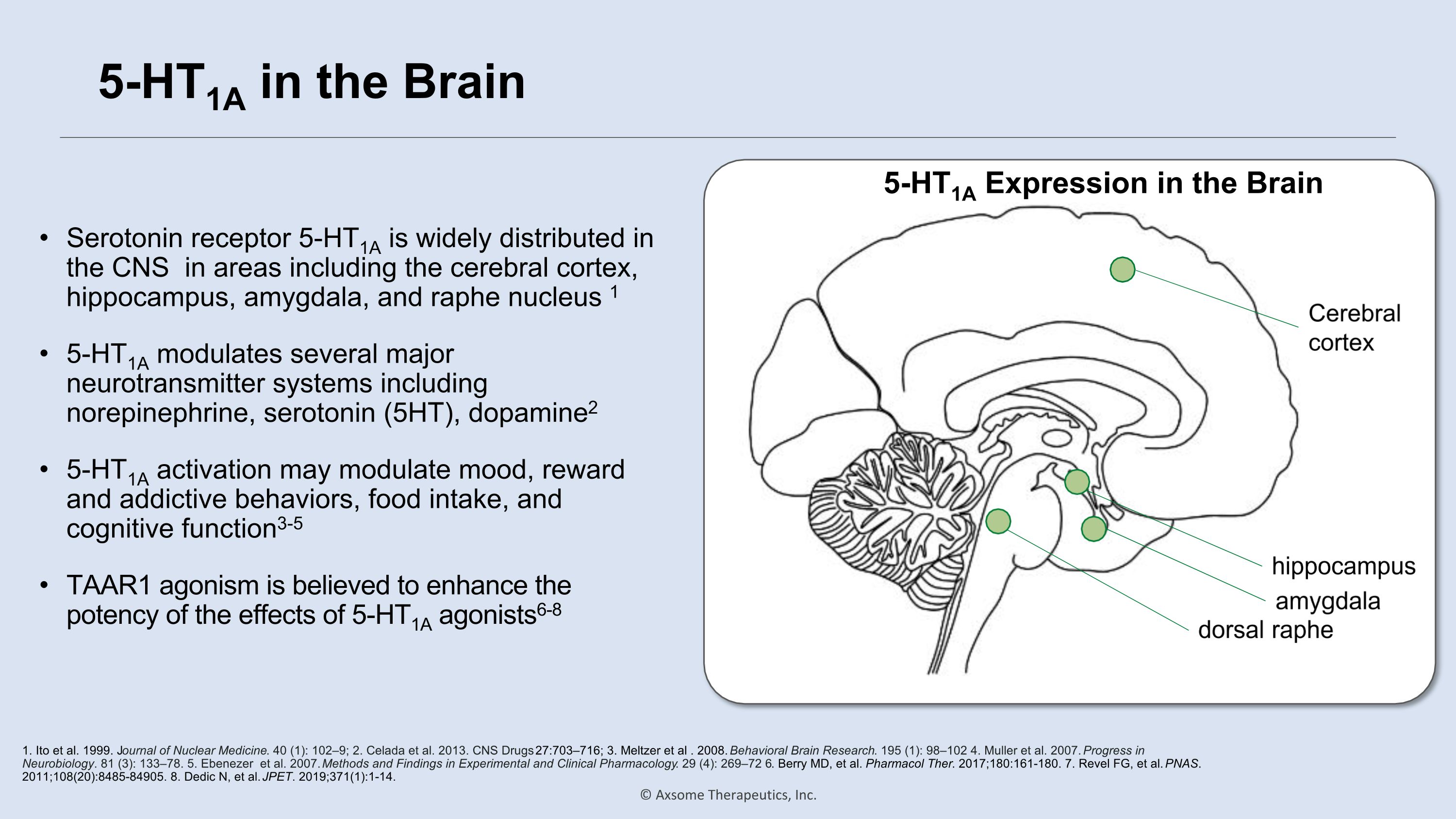
5-HT1A in the Brain Serotonin receptor 5-HT1A is widely distributed in the CNS in areas including the cerebral cortex, hippocampus, amygdala, and raphe nucleus 1 5-HT1A modulates several major neurotransmitter systems including norepinephrine, serotonin (5HT), dopamine2 5-HT1A activation may modulate mood, reward and addictive behaviors, food intake, and cognitive function3-5 TAAR1 agonism is believed to enhance the potency of the effects of 5-HT1A agonists6-8 1. Ito et al. 1999. Journal of Nuclear Medicine. 40 (1): 102–9; 2. Celada et al. 2013. CNS Drugs 27:703–716; 3. Meltzer et al . 2008. Behavioral Brain Research. 195 (1): 98–102 4. Muller et al. 2007. Progress in Neurobiology. 81 (3): 133–78. 5. Ebenezer et al. 2007. Methods and Findings in Experimental and Clinical Pharmacology. 29 (4): 269–72 6. Berry MD, et al. Pharmacol Ther. 2017;180:161-180. 7. Revel FG, et al. PNAS. 2011;108(20):8485-84905. 8. Dedic N, et al. JPET. 2019;371(1):1-14. 5-HT1A Expression in the Brain dorsal raphe amygdala hippocampus Cerebral cortex

TAAR1 agonists in clinical development Solriamfetol (Axsome): DNRI with TAAR1 and 5-HT1A agonist activity Only commercially-available TAAR1 agonist (for treatment of excessive daytime sleepiness in obstructive sleep apnea or narcolepsy) Ralmitaront (Roche): TAAR1 partial agonist Evaluated in two Phase 2 studies (schizophrenia and schizoaffective disorder) that were terminated due to inadequate efficacy Ulotaront (Sumitomo/Otsuka): TAAR1 agonist with 5-HT1A agonist activity Currently under clinical development for treatment of schizophrenia, generalized anxiety disorder and adjunctive major depressive disorder Other TAAR1 agonists have been studied preclinically but none have reached clinical trials Dedic N, et al. Int J Mol Sci. 2021;22(24):13185
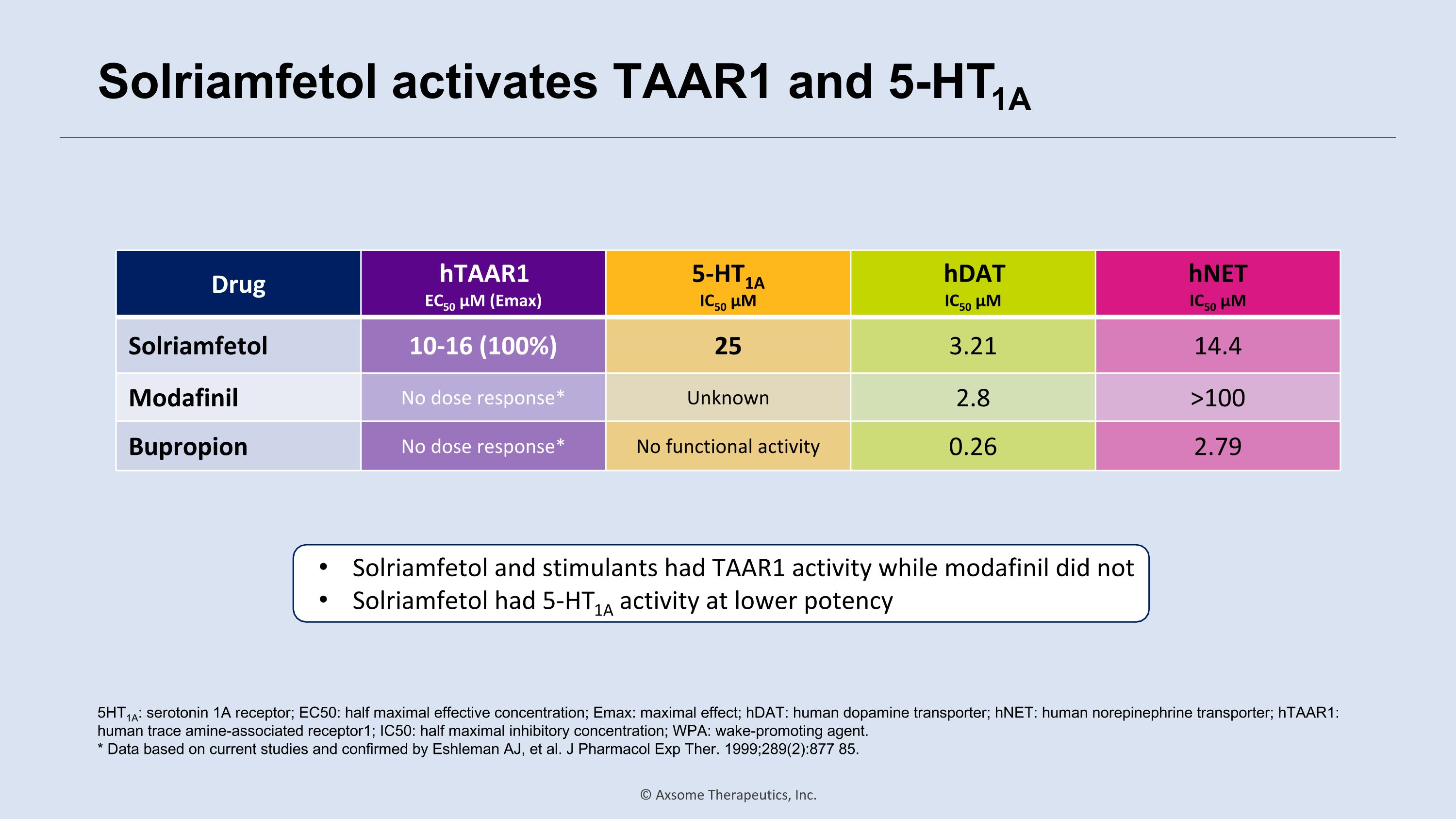
Solriamfetol activates TAAR1 and 5-HT1A Drug hTAAR1 EC50 µM (Emax) 5-HT1A IC50 µM hDAT IC50 µM hNET IC50 µM Solriamfetol 10-16 (100%) 25 3.21 14.4 Modafinil No dose response* Unknown 2.8 >100 Bupropion No dose response* No functional activity 0.26 2.79 5HT1A: serotonin 1A receptor; EC50: half maximal effective concentration; Emax: maximal effect; hDAT: human dopamine transporter; hNET: human norepinephrine transporter; hTAAR1: human trace amine-associated receptor1; IC50: half maximal inhibitory concentration; WPA: wake-promoting agent. * Data based on current studies and confirmed by Eshleman AJ, et al. J Pharmacol Exp Ther. 1999;289(2):877 85. Solriamfetol and stimulants had TAAR1 activity while modafinil did not Solriamfetol had 5-HT1A activity at lower potency
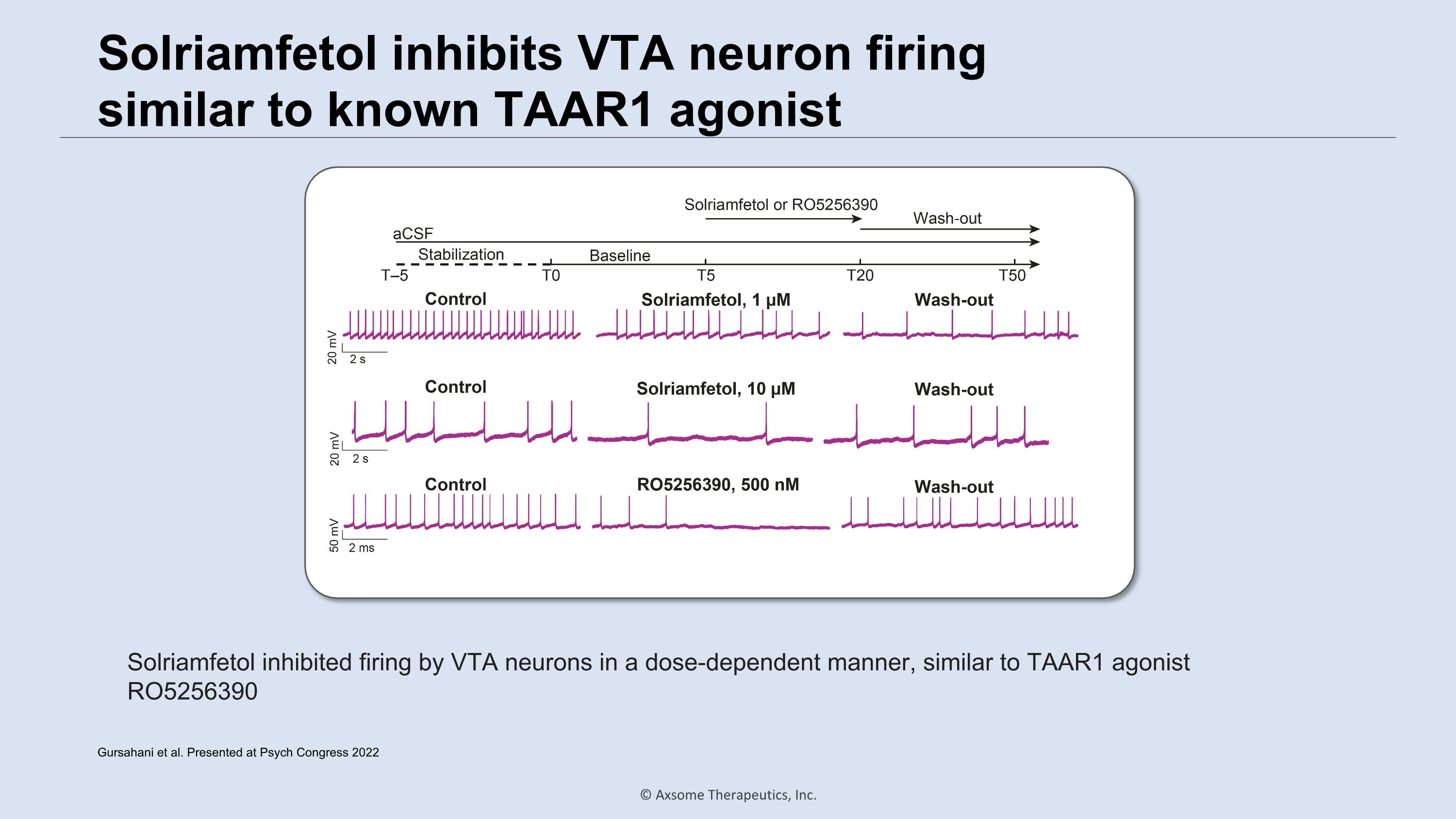
Solriamfetol inhibits VTA neuron firing �similar to known TAAR1 agonist Solriamfetol inhibited firing by VTA neurons in a dose-dependent manner, similar to TAAR1 agonist RO5256390 Gursahani et al. Presented at Psych Congress 2022
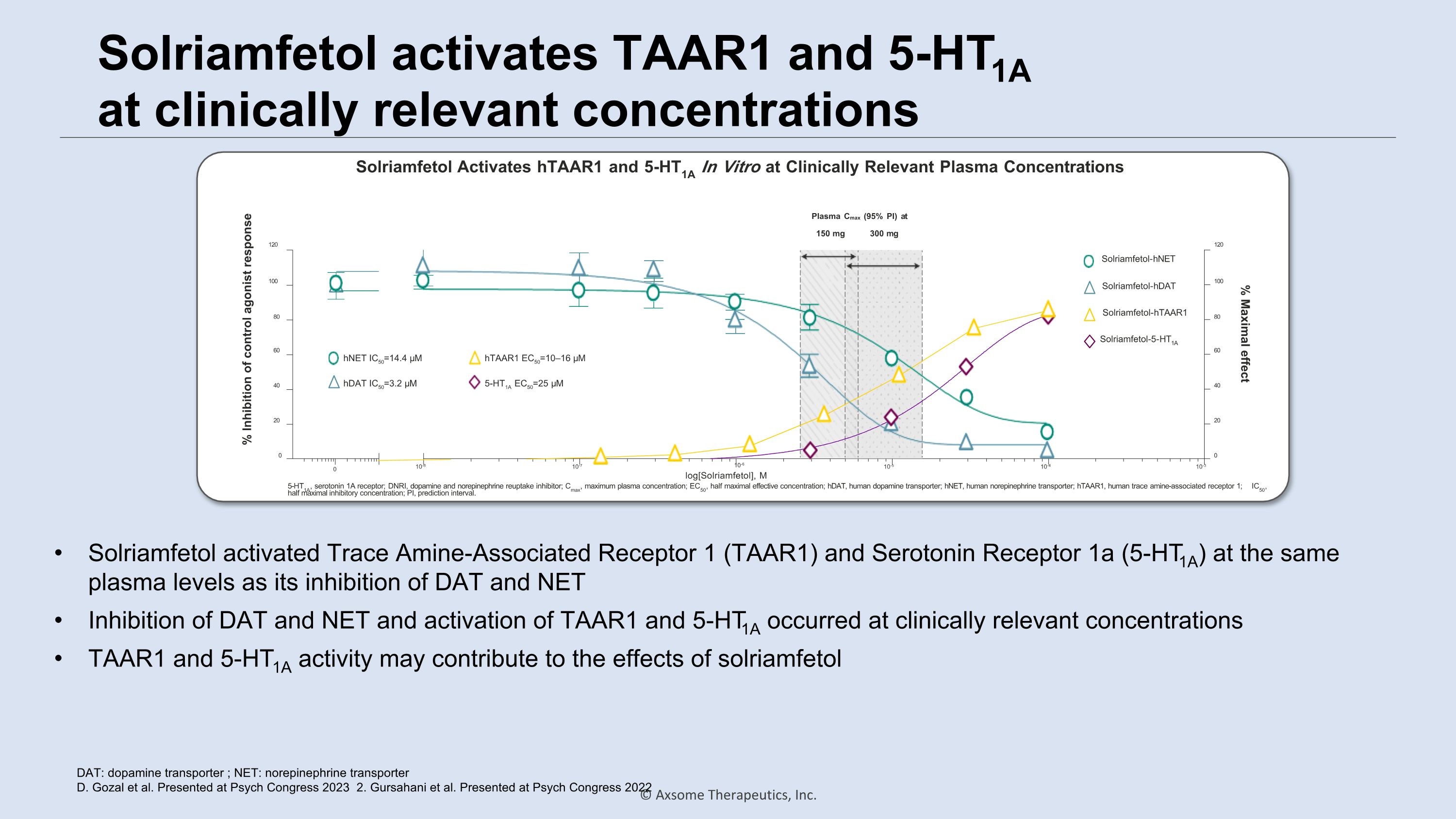
Solriamfetol activates TAAR1 and 5-HT1A �at clinically relevant concentrations DAT: dopamine transporter ; NET: norepinephrine transporter D. Gozal et al. Presented at Psych Congress 2023 2. Gursahani et al. Presented at Psych Congress 2022 0 0 10-8 10-7 10-5 10-4 10-3 20 40 60 80 100 120 0 20 40 60 80 100 120 % Inhibition of control agonist response % Maximal effect 10-6 log[Solriamfetol], M 150 mg 300 mg hNET IC50=14.4 µM hDAT IC50=3.2 µM Solriamfetol-hNET Solriamfetol-5-HT1A Solriamfetol-hDAT Solriamfetol-hTAAR1 hTAAR1 EC50=10–16 µM 5-HT1A EC50=25 µM Plasma Cmax (95% PI) at 5-HT1A, serotonin 1A receptor; DNRI, dopamine and norepinephrine reuptake inhibitor; Cmax, maximum plasma concentration; EC50, half maximal effective concentration; hDAT, human dopamine transporter; hNET, human norepinephrine transporter; hTAAR1, human trace amine-associated receptor 1; IC50, half maximal inhibitory concentration; PI, prediction interval. Solriamfetol Activates hTAAR1 and 5-HT1A In Vitro at Clinically Relevant Plasma Concentrations Solriamfetol activated Trace Amine-Associated Receptor 1 (TAAR1) and Serotonin Receptor 1a (5-HT1A) at the same plasma levels as its inhibition of DAT and NET Inhibition of DAT and NET and activation of TAAR1 and 5-HT1A occurred at clinically relevant concentrations TAAR1 and 5-HT1A activity may contribute to the effects of solriamfetol
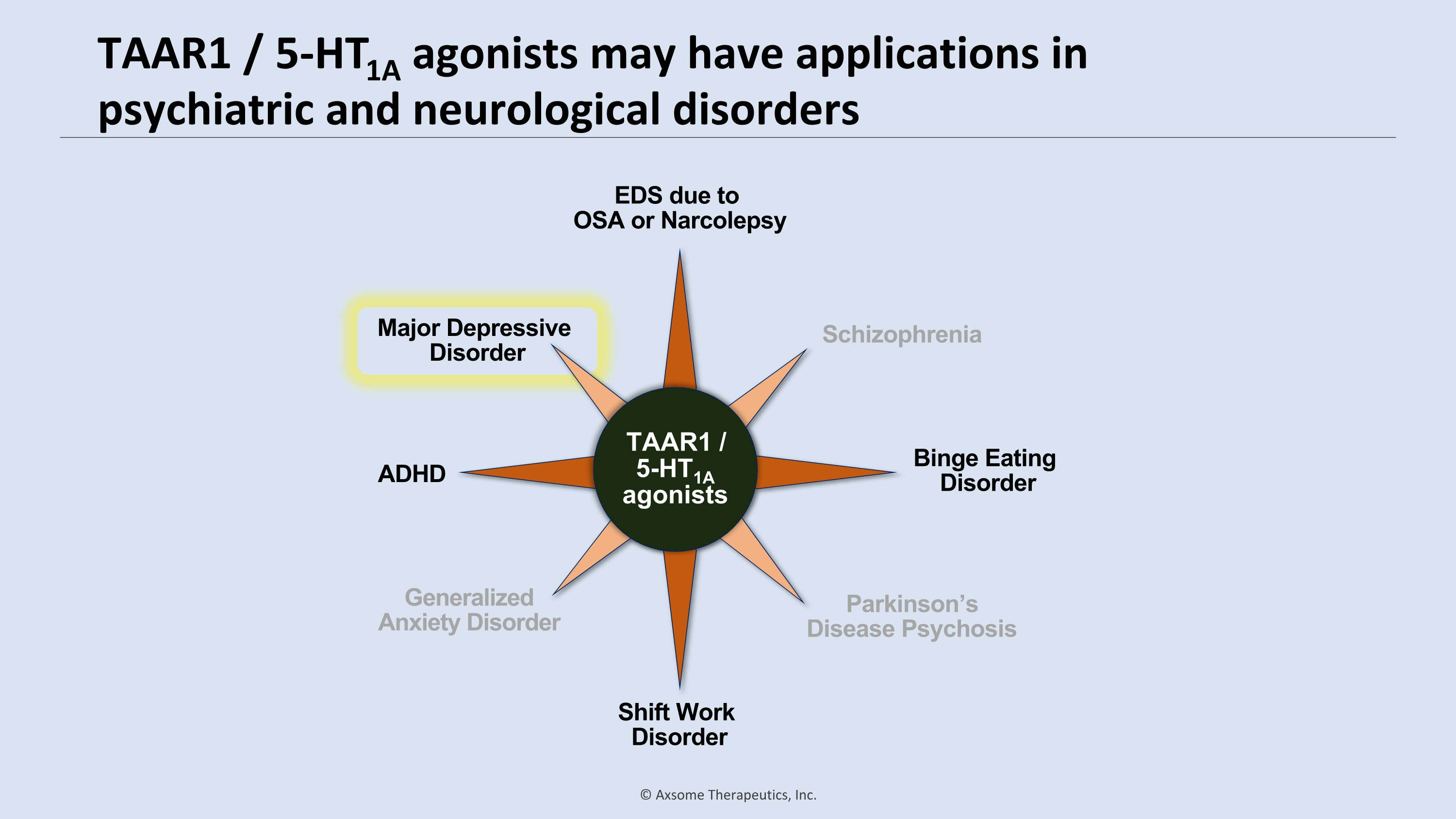
TAAR1 / 5-HT1A agonists may have applications in psychiatric and neurological disorders Schizophrenia Generalized Anxiety Disorder Parkinson’s Disease Psychosis Shift Work Disorder EDS due to �OSA or Narcolepsy Binge Eating Disorder ADHD Major Depressive Disorder Schizophrenia Generalized Anxiety Disorder Parkinson’s Disease Psychosis Major Depressive Disorder Major Depressive Disorder TAAR1 / 5-HT1A agonists

Craig Chepke, MD, DFAPA Adjunct Associate Professor of Psychiatry, Atrium Health Medical Director, Excel Psychiatric Associates Major Depressive Disorder (MDD)
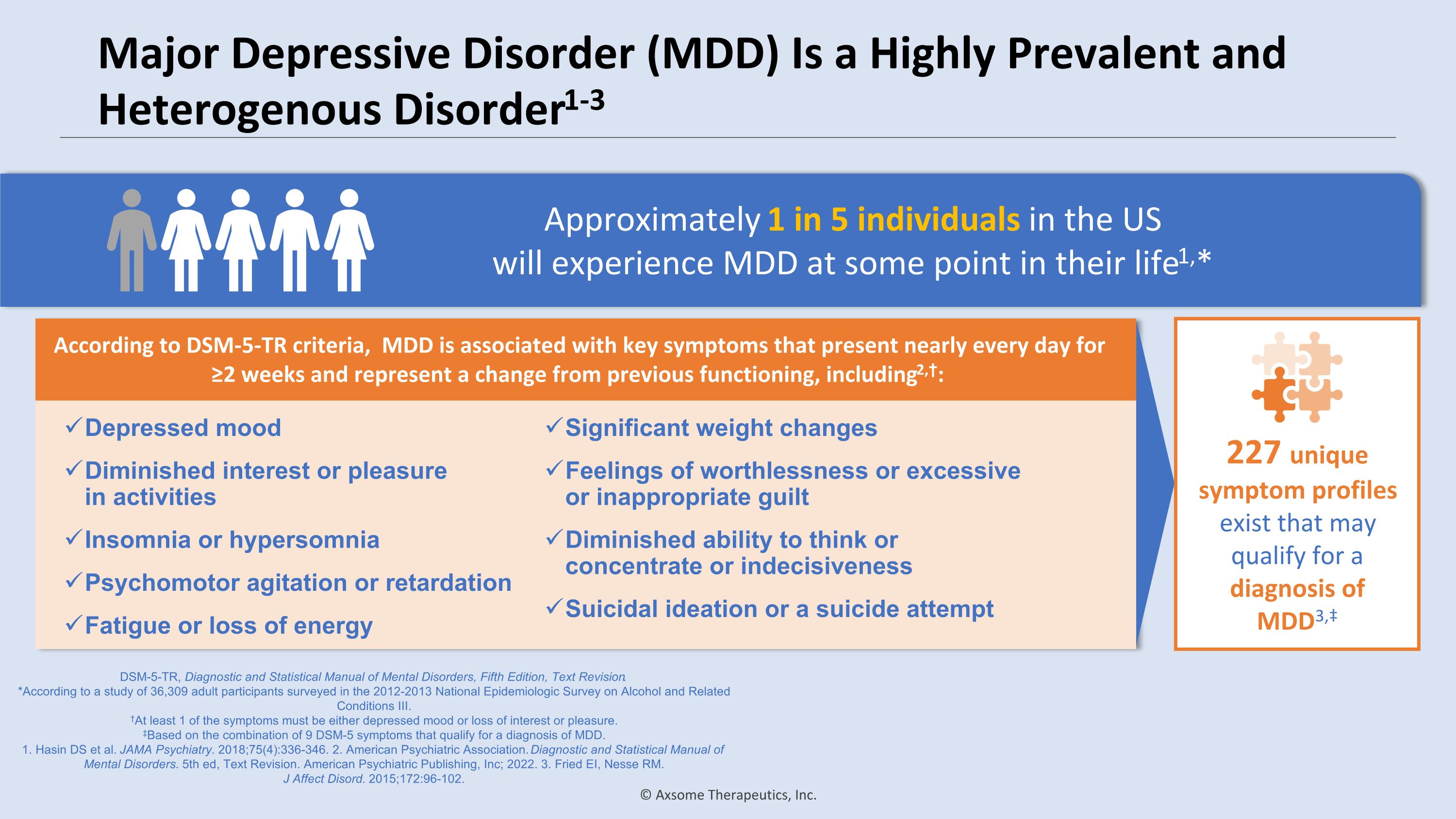
Major Depressive Disorder (MDD) Is a Highly Prevalent and Heterogenous Disorder1-3 DSM-5-TR, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision. *According to a study of 36,309 adult participants surveyed in the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III. †At least 1 of the symptoms must be either depressed mood or loss of interest or pleasure. ‡Based on the combination of 9 DSM-5 symptoms that qualify for a diagnosis of MDD. 1. Hasin DS et al. JAMA Psychiatry. 2018;75(4):336-346. 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, Text Revision. American Psychiatric Publishing, Inc; 2022. 3. Fried EI, Nesse RM. �J Affect Disord. 2015;172:96-102. Approximately 1 in 5 individuals in the US �will experience MDD at some point in their life1,* According to DSM-5-TR criteria, MDD is associated with key symptoms that present nearly every day for ≥2 weeks and represent a change from previous functioning, including2,†: Depressed mood Diminished interest or pleasure �in activities Insomnia or hypersomnia Psychomotor agitation or retardation Fatigue or loss of energy Significant weight changes Feelings of worthlessness or excessive or inappropriate guilt Diminished ability to think or concentrate or indecisiveness Suicidal ideation or a suicide attempt 227 unique symptom profiles exist that may qualify for a diagnosis of MDD3,‡
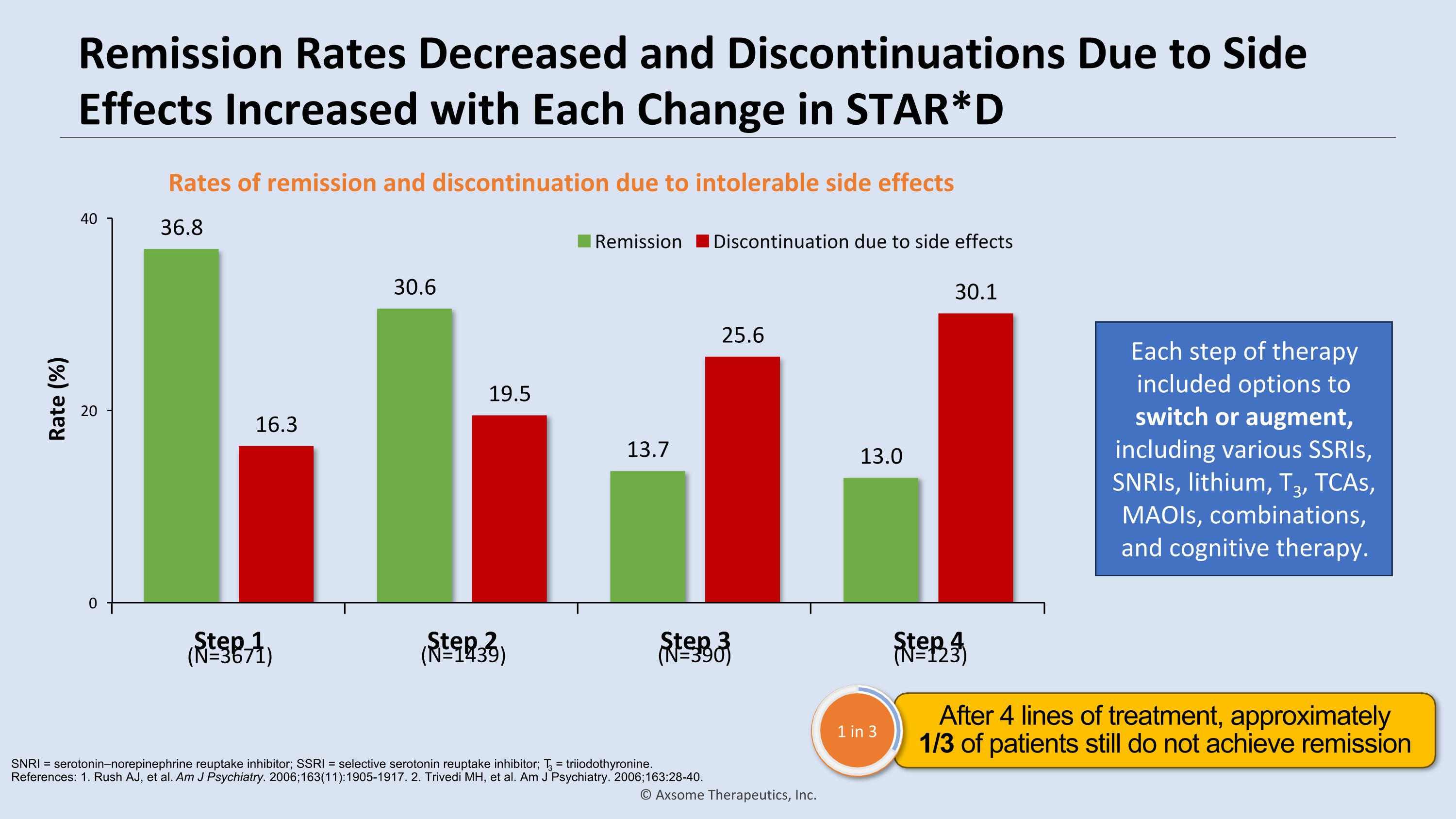
Remission Rates Decreased and Discontinuations Due to Side Effects Increased with Each Change in STAR*D Rates of remission and discontinuation due to intolerable side effects Each step of therapy included options to switch or augment, �including various SSRIs, SNRIs, lithium, T3, TCAs, MAOIs, combinations, �and cognitive therapy. (N=3671) (N=1439) (N=390) (N=123) SNRI = serotonin–norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor; T3 = triiodothyronine. �References: 1. Rush AJ, et al. Am J Psychiatry. 2006;163(11):1905-1917. 2. Trivedi MH, et al. Am J Psychiatry. 2006;163:28-40. After 4 lines of treatment, approximately �1/3 of patients still do not achieve remission 1 in 3
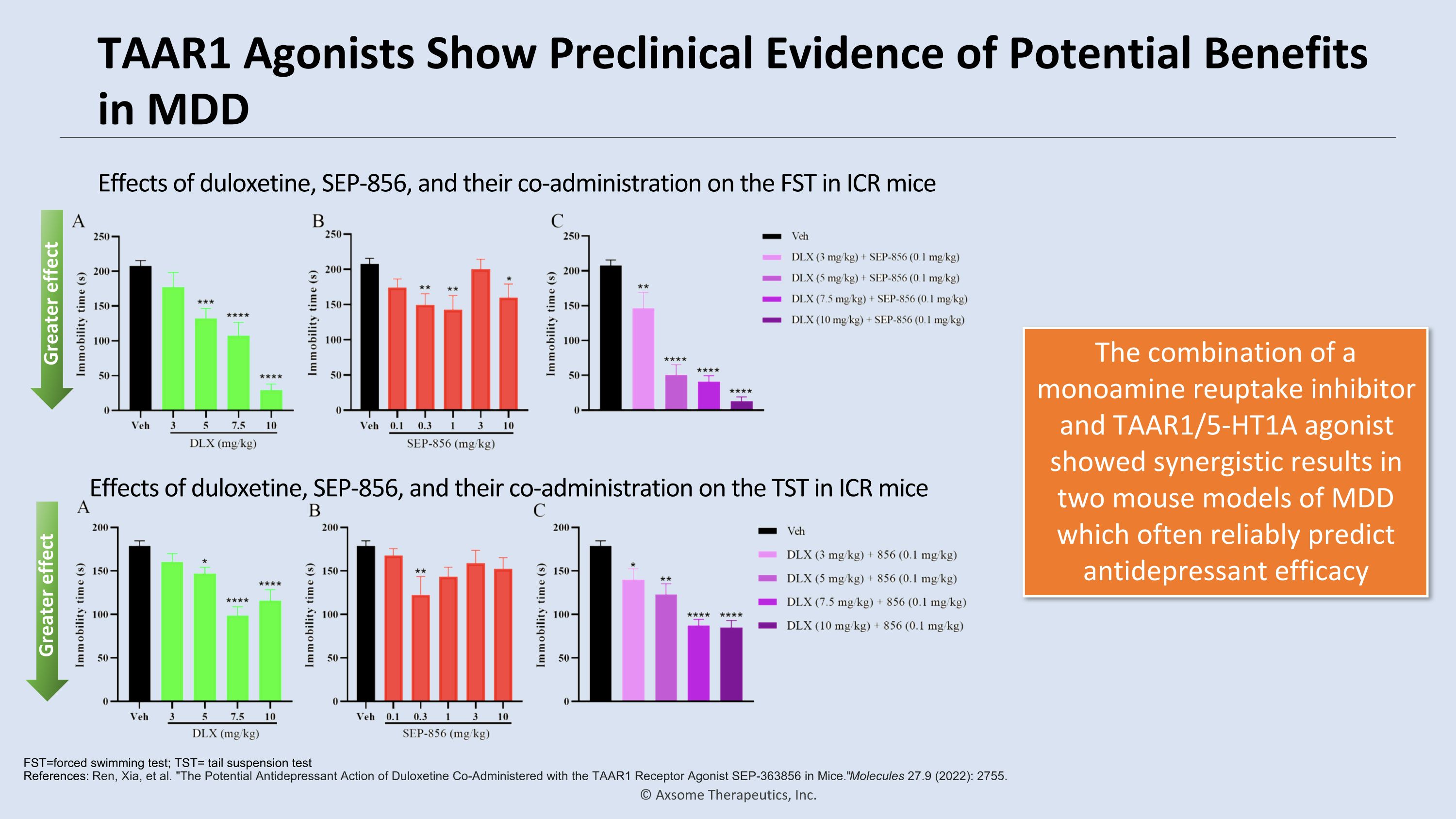
TAAR1 Agonists Show Preclinical Evidence of Potential Benefits in MDD The combination of a monoamine reuptake inhibitor and TAAR1/5-HT1A agonist showed synergistic results in two mouse models of MDD which often reliably predict antidepressant efficacy FST=forced swimming test; TST= tail suspension test�References: Ren, Xia, et al. "The Potential Antidepressant Action of Duloxetine Co-Administered with the TAAR1 Receptor Agonist SEP-363856 in Mice." Molecules 27.9 (2022): 2755. Effects of duloxetine, SEP-856, and their co-administration on the FST in ICR mice Effects of duloxetine, SEP-856, and their co-administration on the TST in ICR mice Greater effect Greater effect
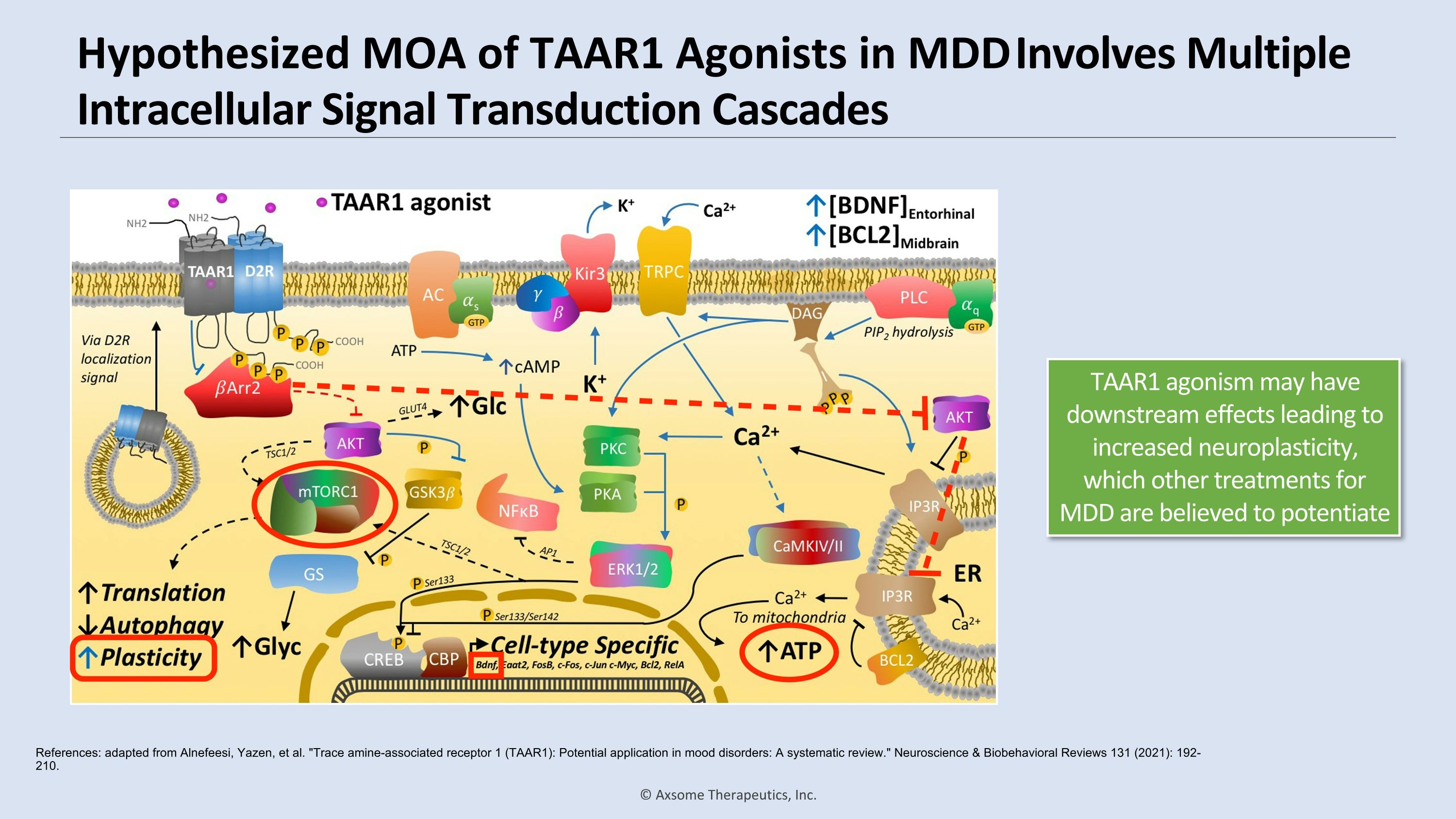
Hypothesized MOA of TAAR1 Agonists in MDD Involves Multiple Intracellular Signal Transduction Cascades References: adapted from Alnefeesi, Yazen, et al. "Trace amine-associated receptor 1 (TAAR1): Potential application in mood disorders: A systematic review." Neuroscience & Biobehavioral Reviews 131 (2021): 192-210. TAAR1 agonism may have downstream effects leading to increased neuroplasticity, which other treatments for MDD are believed to potentiate
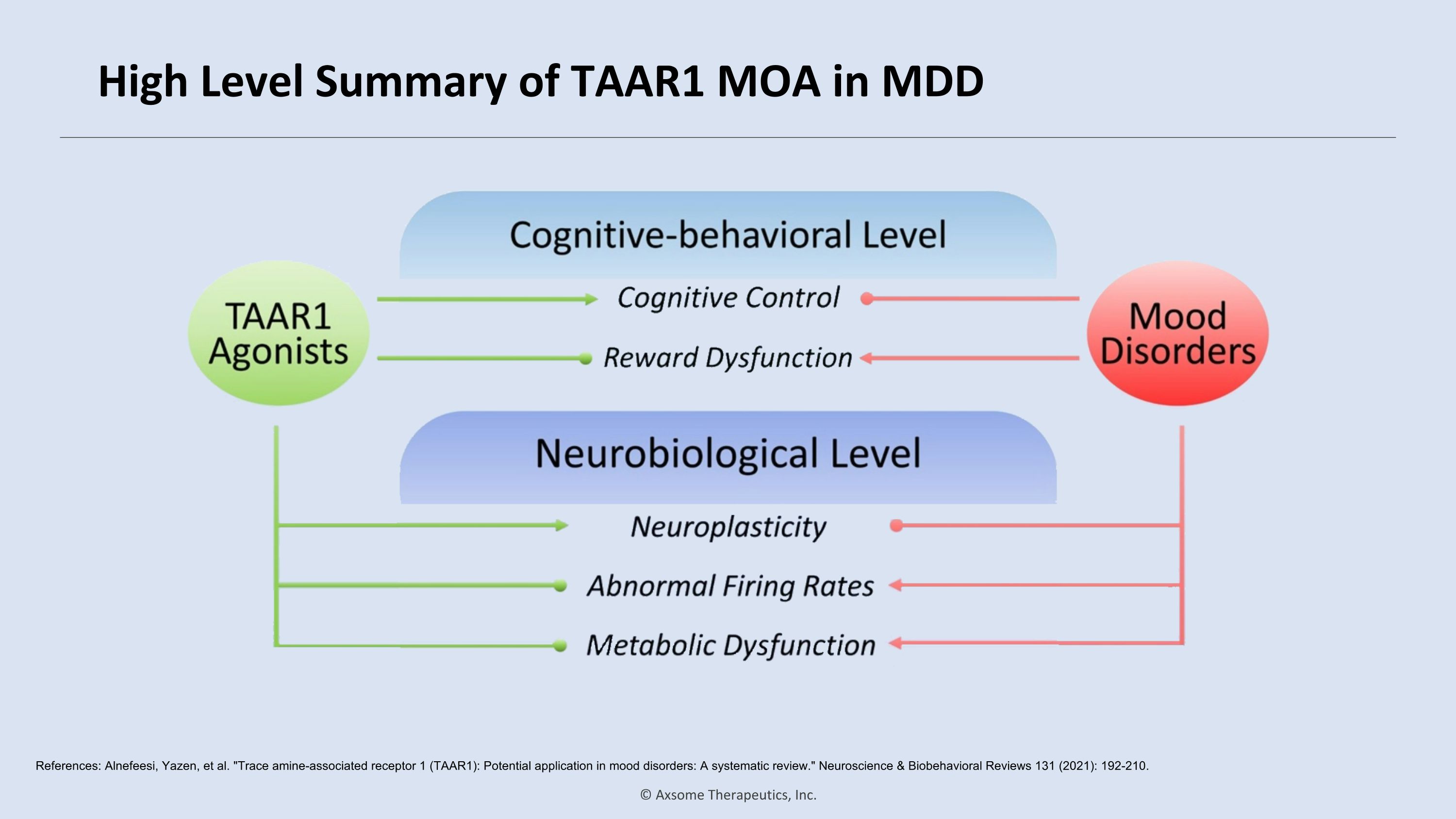
High Level Summary of TAAR1 MOA in MDD References: Alnefeesi, Yazen, et al. "Trace amine-associated receptor 1 (TAAR1): Potential application in mood disorders: A systematic review." Neuroscience & Biobehavioral Reviews 131 (2021): 192-210.
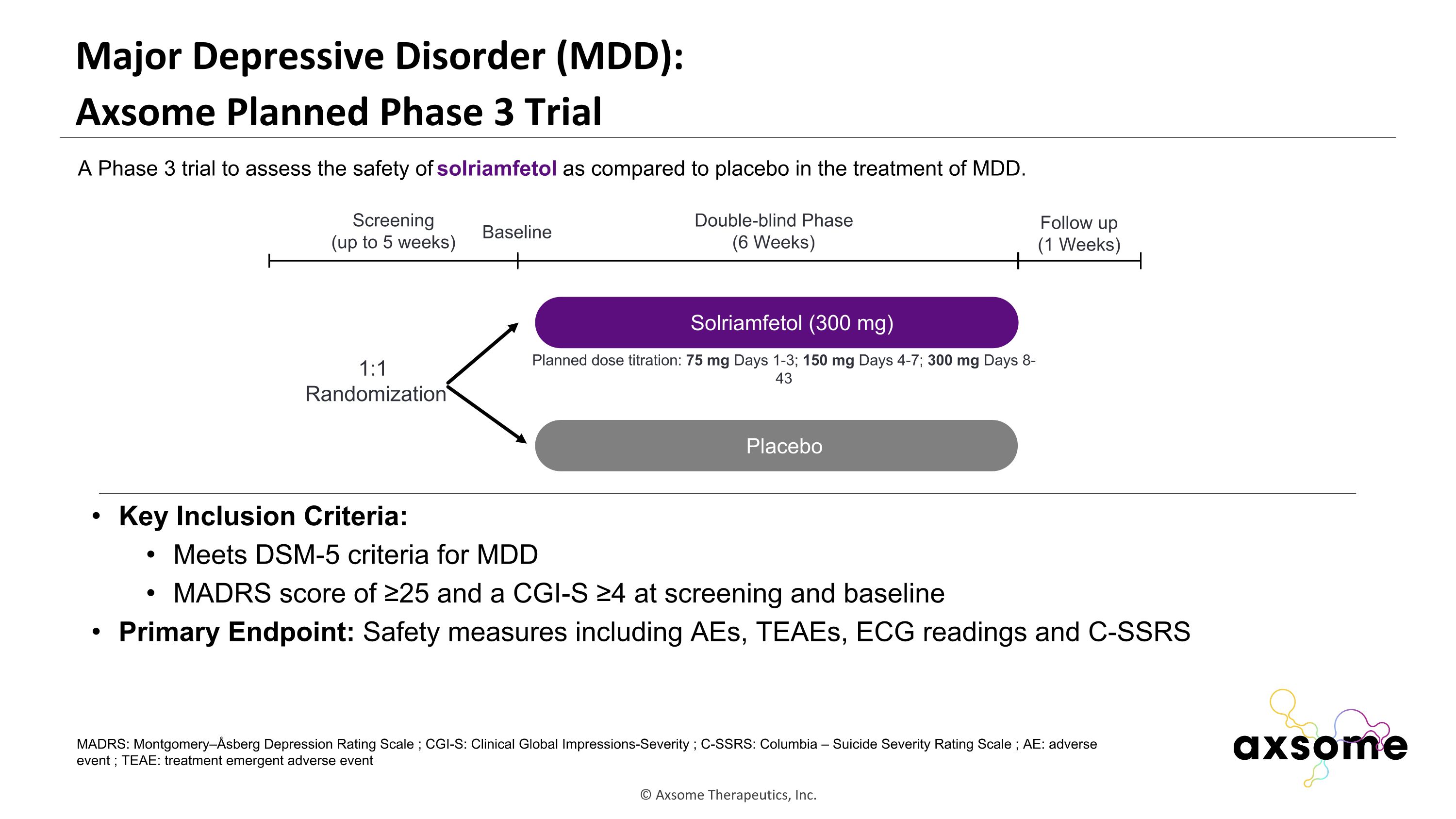
Major Depressive Disorder (MDD): �Axsome Planned Phase 3 Trial Key Inclusion Criteria: Meets DSM-5 criteria for MDD MADRS score of ≥25 and a CGI-S ≥4 at screening and baseline Primary Endpoint: Safety measures including AEs, TEAEs, ECG readings and C-SSRS AXS-05 twice daily Placebo Double-blind Phase (6 Weeks) Screening (up to 5 weeks) 1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess the safety of solriamfetol as compared to placebo in the treatment of MDD. Solriamfetol (300 mg) MADRS: Montgomery–Åsberg Depression Rating Scale ; CGI-S: Clinical Global Impressions-Severity ; C-SSRS: Columbia – Suicide Severity Rating Scale ; AE: adverse event ; TEAE: treatment emergent adverse event Planned dose titration: 75 mg Days 1-3; 150 mg Days 4-7; 300 mg Days 8-43
Key Takeaways for TAAR1 MOA in MDD TAAR1 and 5-HT1A agonists as compelling drug development target in neuropsychiatry Agents with TAAR1 and 5-HT1A agonist activity have shown potential in neuropsychiatry Solriamfetol, the only commercially available TAAR1 agonist, has potential in multiple new target indications because of its recently uncovered TAAR1/ 5-HT1A agonist activity Major Depressive Disorder (MDD) Cognitive deficits are a major driver of disability in MDD, and based on its MOA solriamfetol could have cognitive benefits in MDD. The multiple MOAs (NRI, DRI, 5-HT1A agonism, TAAR1 agonism) may be beneficial for a broad range of patients, including potentially patients not well served by traditional antidepressants Solriamfetol tolerability profile is well-characterized and is devoid of the adverse reactions often considered the most bothersome for people with MDD: weight gain, sexual dysfunction, and sedation

Andrew Cutler, MD SUNY Upstate Medical University and Neuroscience Education Institute, Syracuse, NY Overview of Attention Deficit Hyperactivity Disorder (ADHD)
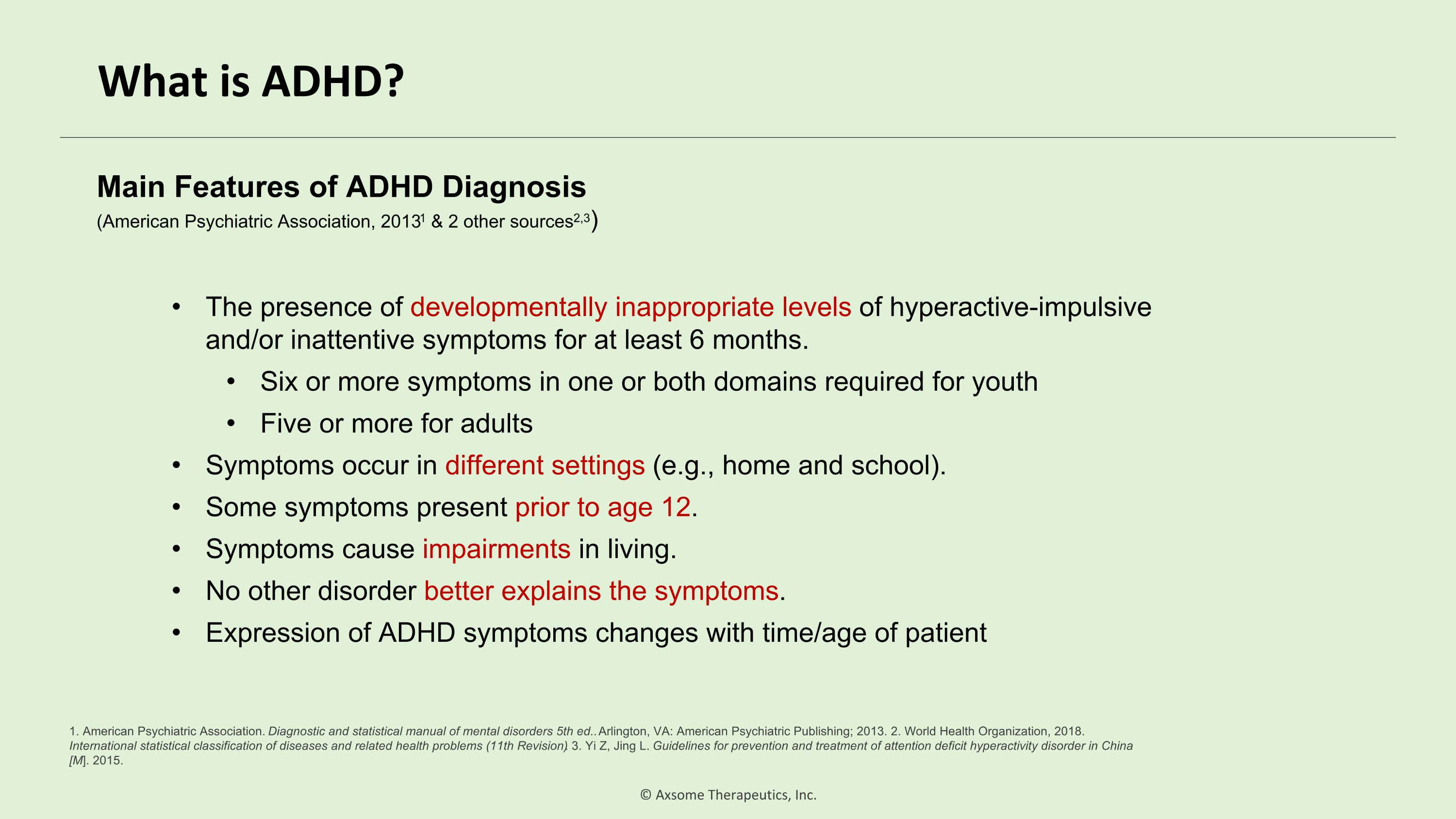
What is ADHD? The presence of developmentally inappropriate levels of hyperactive-impulsive and/or inattentive symptoms for at least 6 months. Six or more symptoms in one or both domains required for youth Five or more for adults Symptoms occur in different settings (e.g., home and school). Some symptoms present prior to age 12. Symptoms cause impairments in living. No other disorder better explains the symptoms. Expression of ADHD symptoms changes with time/age of patient Main Features of ADHD Diagnosis�(American Psychiatric Association, 20131 & 2 other sources2,3) 1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders 5th ed.. Arlington, VA: American Psychiatric Publishing; 2013. 2. World Health Organization, 2018. International statistical classification of diseases and related health problems (11th Revision). 3. Yi Z, Jing L. Guidelines for prevention and treatment of attention deficit hyperactivity disorder in China [M]. 2015.

ADHD is a Common Disorder in Childhood and Adulthood An estimated 17.4 million people in the U.S. are affected by ADHD1,2 The prevalence of ADHD has not changed over the past three decades that have data available3 No significant differences in prevalence between North America and Europe, Asia, Africa, South America, and Oceania3 17.4 million Americans with ADHD 6 million Youth1 11.4 million Adults2 1. Bitsko RH, et al. 2013–2019. MMWR Suppl. 2022;71(2):1-48. 2. Kessler RC, et al. Am J Psychiatry. 2006 Apr;163(4):716-23. 3. Polanczyk GV, et al. Int J Epidemiol. 2014;43(2):434-442.
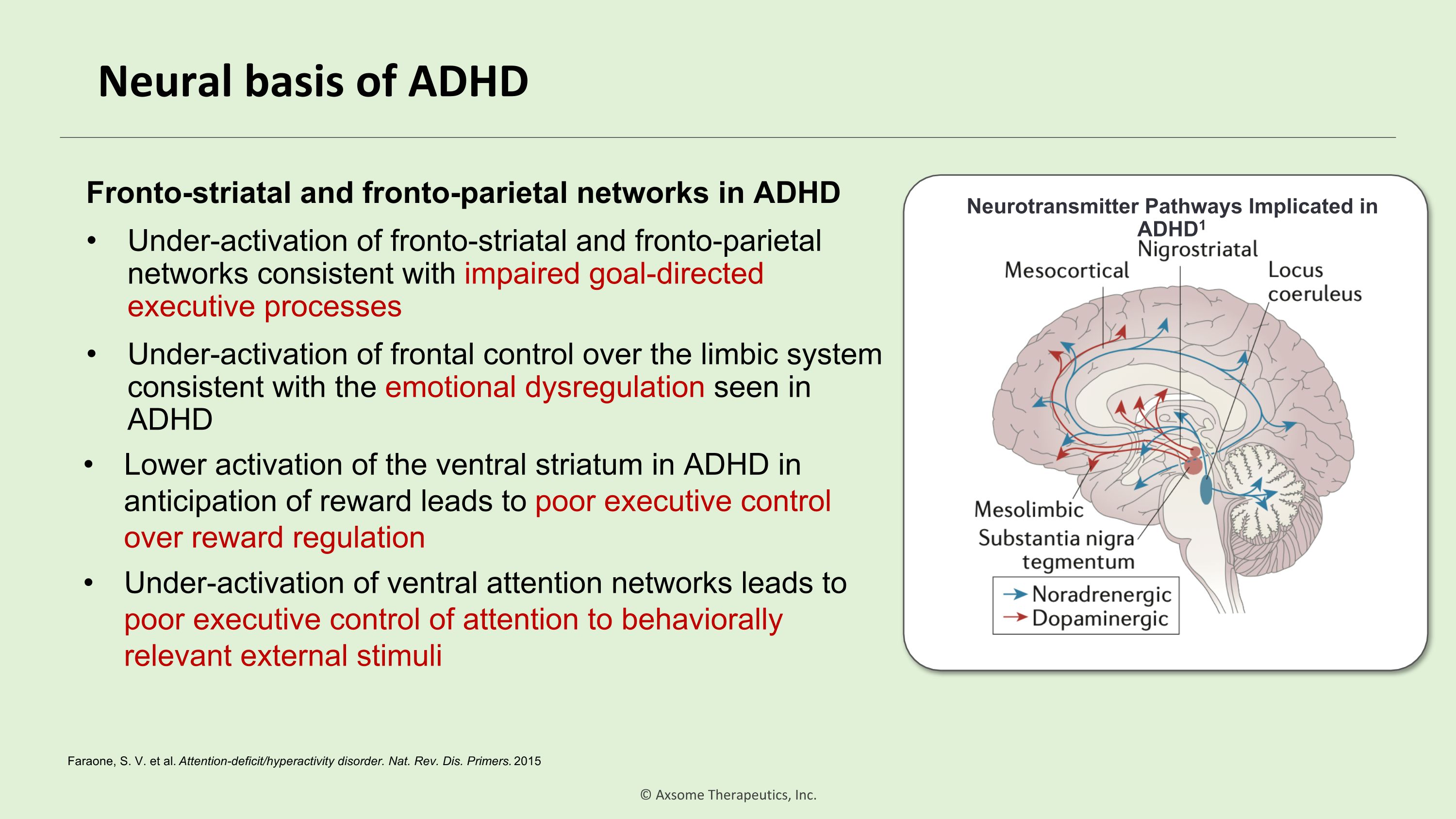
Neural basis of ADHD Neurotransmitter Pathways Implicated in ADHD1 Fronto-striatal and fronto-parietal networks in ADHD Under-activation of fronto-striatal and fronto-parietal networks consistent with impaired goal-directed executive processes Under-activation of frontal control over the limbic system consistent with the emotional dysregulation seen in ADHD Lower activation of the ventral striatum in ADHD in anticipation of reward leads to poor executive control over reward regulation Under-activation of ventral attention networks leads to poor executive control of attention to behaviorally relevant external stimuli Faraone, S. V. et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015
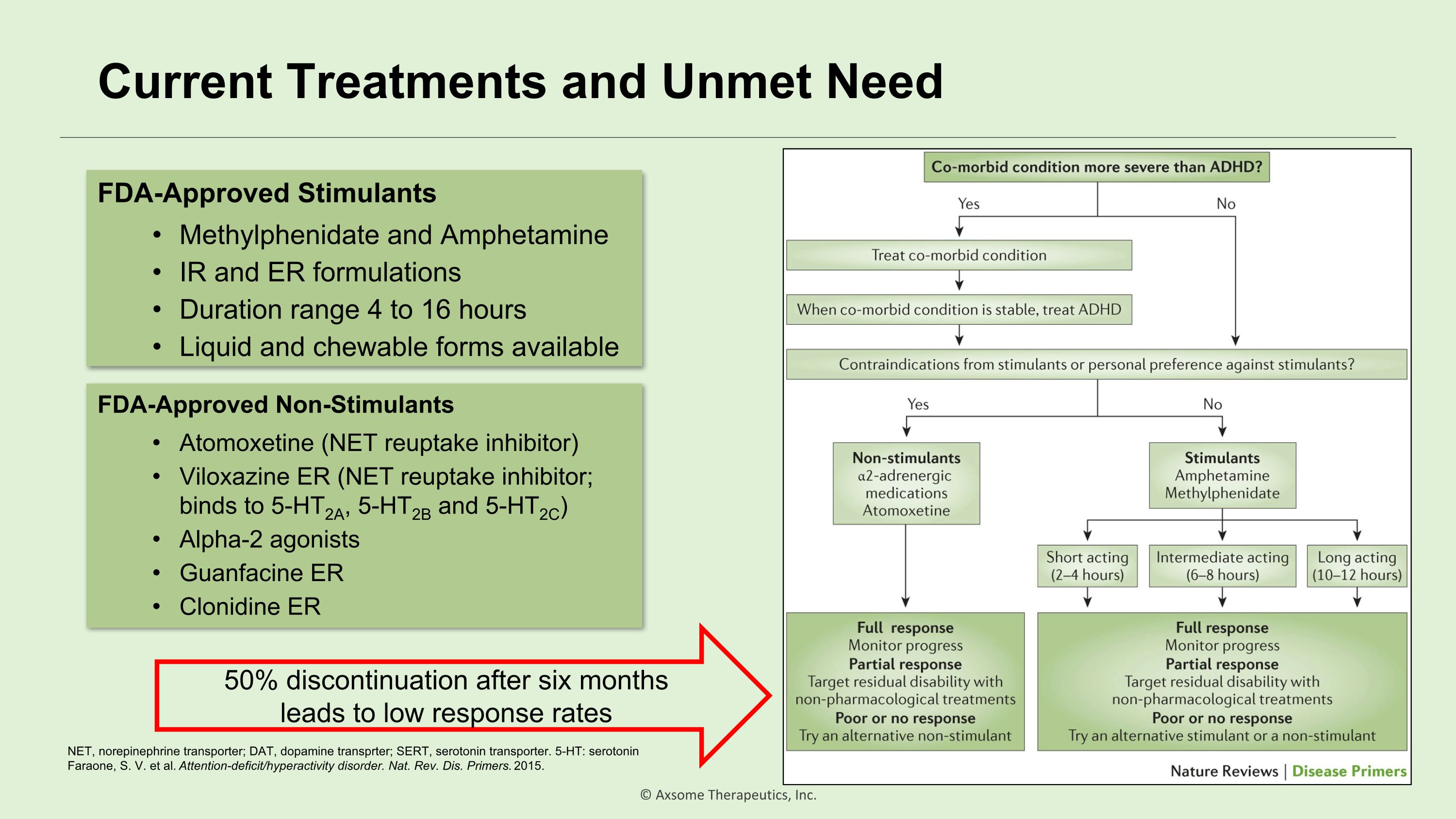
Current Treatments and Unmet Need FDA-Approved Stimulants Methylphenidate and Amphetamine IR and ER formulations Duration range 4 to 16 hours Liquid and chewable forms available FDA-Approved Non-Stimulants Atomoxetine (NET reuptake inhibitor) Viloxazine ER (NET reuptake inhibitor; binds to 5-HT2A, 5-HT2B and 5-HT2C) Alpha-2 agonists Guanfacine ER Clonidine ER 50% discontinuation after six months leads to low response rates NET, norepinephrine transporter; DAT, dopamine transprter; SERT, serotonin transporter. 5-HT: serotonin Faraone, S. V. et al. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers. 2015.
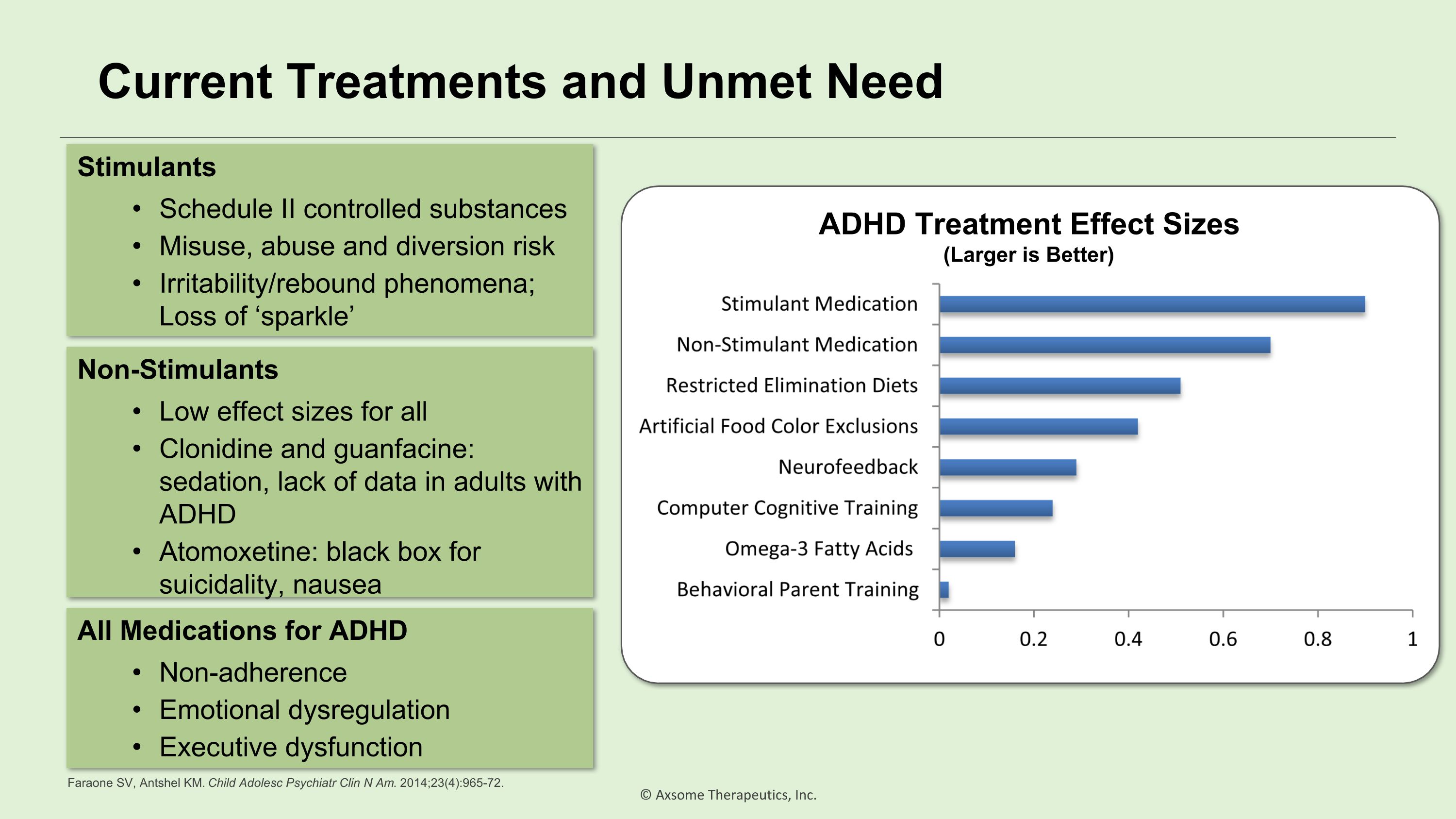
Current Treatments and Unmet Need Stimulants Schedule II controlled substances Misuse, abuse and diversion risk Irritability/rebound phenomena; Loss of ‘sparkle’ Non-Stimulants Low effect sizes for all Clonidine and guanfacine: sedation, lack of data in adults with ADHD Atomoxetine: black box for suicidality, nausea All Medications for ADHD Non-adherence Emotional dysregulation Executive dysfunction ADHD Treatment Effect Sizes (Larger is Better) Faraone SV, Antshel KM. Child Adolesc Psychiatr Clin N Am. 2014;23(4):965-72.
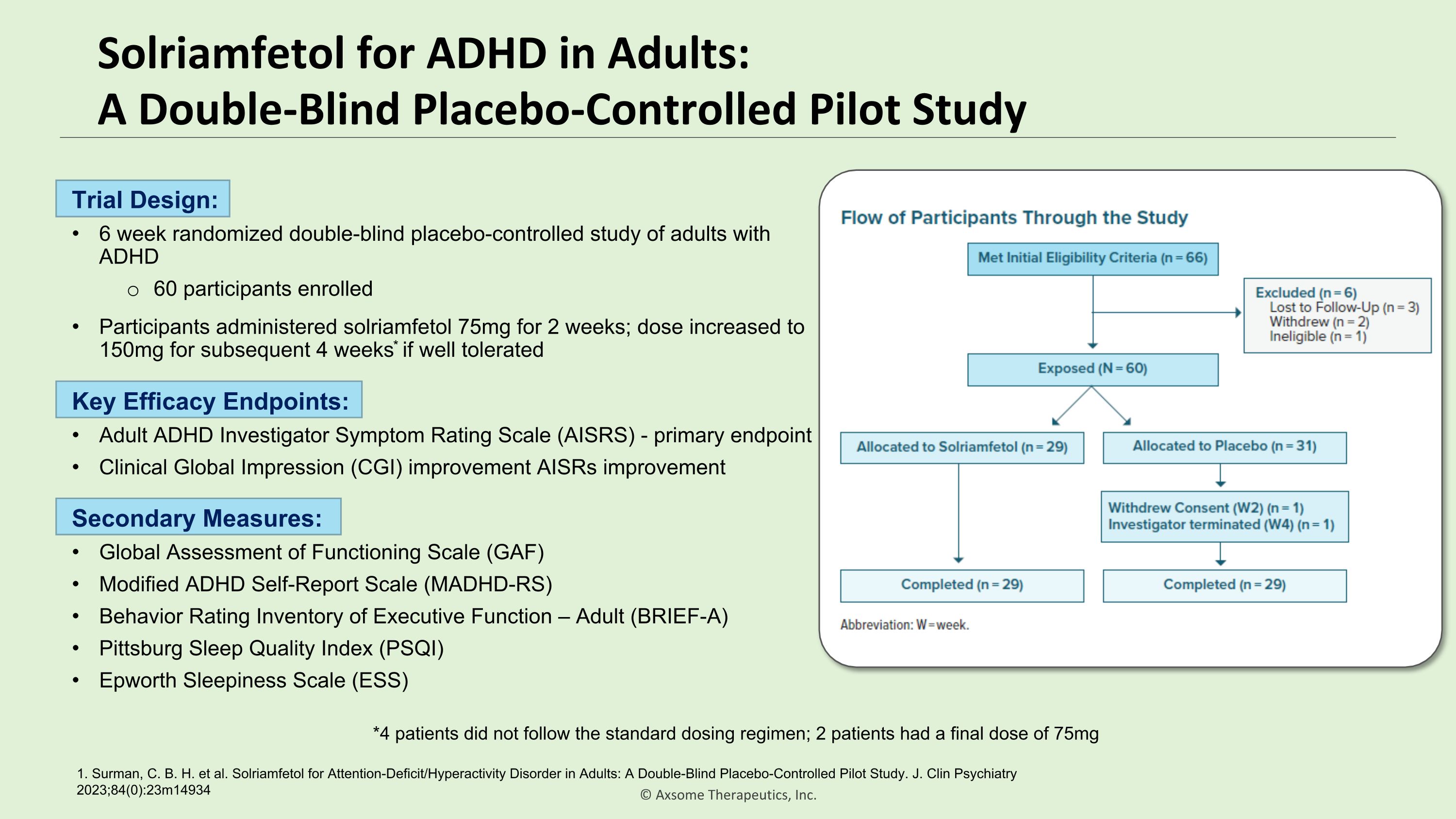
Solriamfetol for ADHD in Adults:�A Double-Blind Placebo-Controlled Pilot Study 1. Surman, C. B. H. et al. Solriamfetol for Attention-Deficit/Hyperactivity Disorder in Adults: A Double-Blind Placebo-Controlled Pilot Study. J. Clin Psychiatry 2023;84(0):23m14934 Trial Design: 6 week randomized double-blind placebo-controlled study of adults with ADHD 60 participants enrolled Participants administered solriamfetol 75mg for 2 weeks; dose increased to 150mg for subsequent 4 weeks* if well tolerated *4 patients did not follow the standard dosing regimen; 2 patients had a final dose of 75mg Key Efficacy Endpoints: Adult ADHD Investigator Symptom Rating Scale (AISRS) - primary endpoint Clinical Global Impression (CGI) improvement AISRs improvement Secondary Measures: Global Assessment of Functioning Scale (GAF) Modified ADHD Self-Report Scale (MADHD-RS) Behavior Rating Inventory of Executive Function – Adult (BRIEF-A) Pittsburg Sleep Quality Index (PSQI) Epworth Sleepiness Scale (ESS)
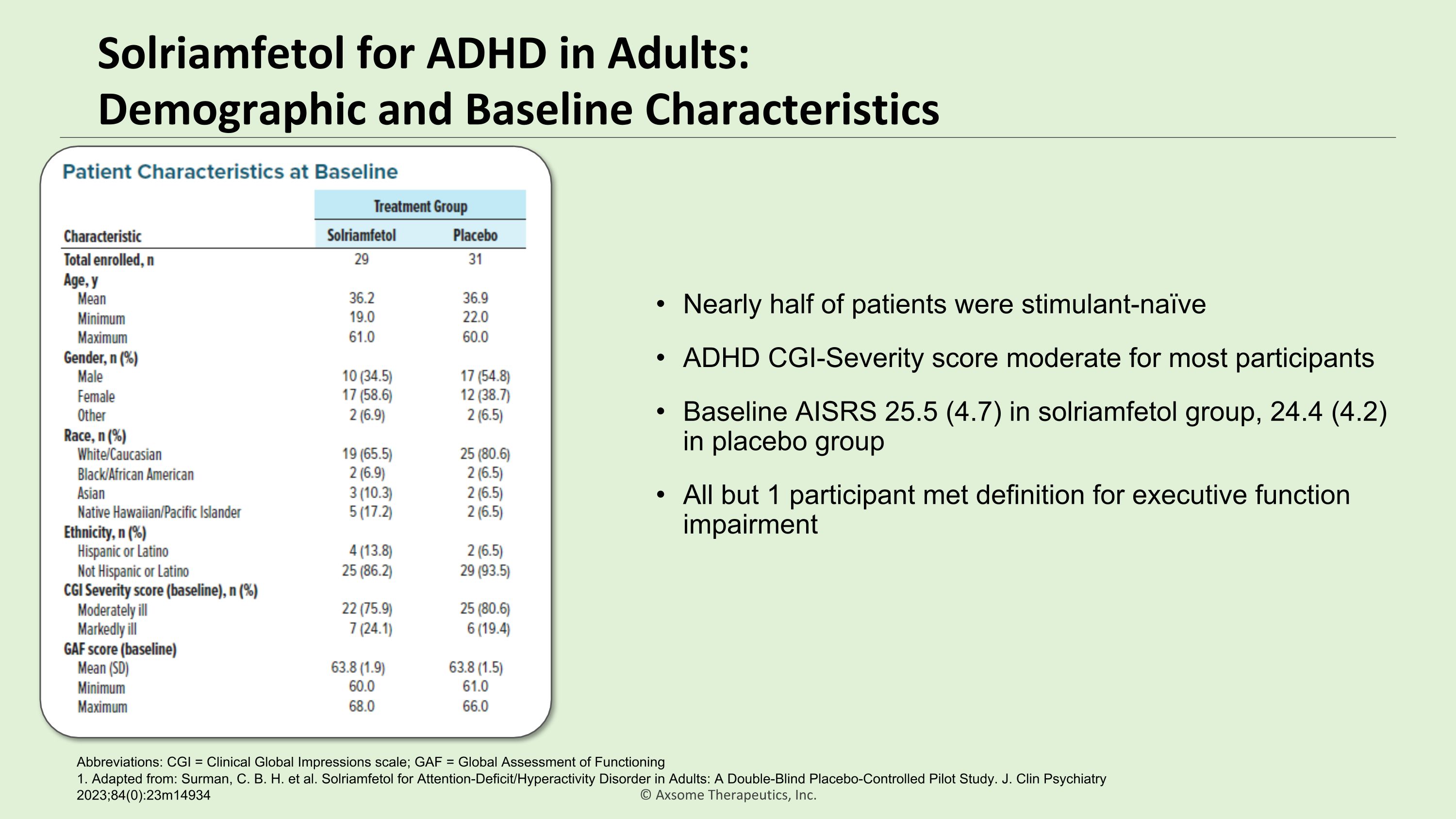
Solriamfetol for ADHD in Adults:�Demographic and Baseline Characteristics 1. Adapted from: Surman, C. B. H. et al. Solriamfetol for Attention-Deficit/Hyperactivity Disorder in Adults: A Double-Blind Placebo-Controlled Pilot Study. J. Clin Psychiatry 2023;84(0):23m14934 Abbreviations: CGI = Clinical Global Impressions scale; GAF = Global Assessment of Functioning Nearly half of patients were stimulant-naïve ADHD CGI-Severity score moderate for most participants Baseline AISRS 25.5 (4.7) in solriamfetol group, 24.4 (4.2) in placebo group All but 1 participant met definition for executive function impairment

Solriamfetol for ADHD in Adults:�A Double-Blind Placebo-Controlled Pilot Study For Weeks 3-6, mean improvement in total AISRS ratings was significantly greater for solriamfetol-treated individuals than for placebo-treated The mean total improvement in AISRS score by Week 6 was –7.6 for active study drug participants, and –2.1 for individuals on placebo (P = .0012; effect size = 1.09) By week 6, significantly more individuals on solriamfetol met remission AISRS total score definitions of 12 (24% vs 3% on placebo; P = .0517) or 18 (59% vs 21% on placebo; P = .0067) 1. Surman, C. B. H. et al. Solriamfetol for Attention-Deficit/Hyperactivity Disorder in Adults: A Double-Blind Placebo-Controlled Pilot Study. J. Clin Psychiatry 2023;84(0):23m14934
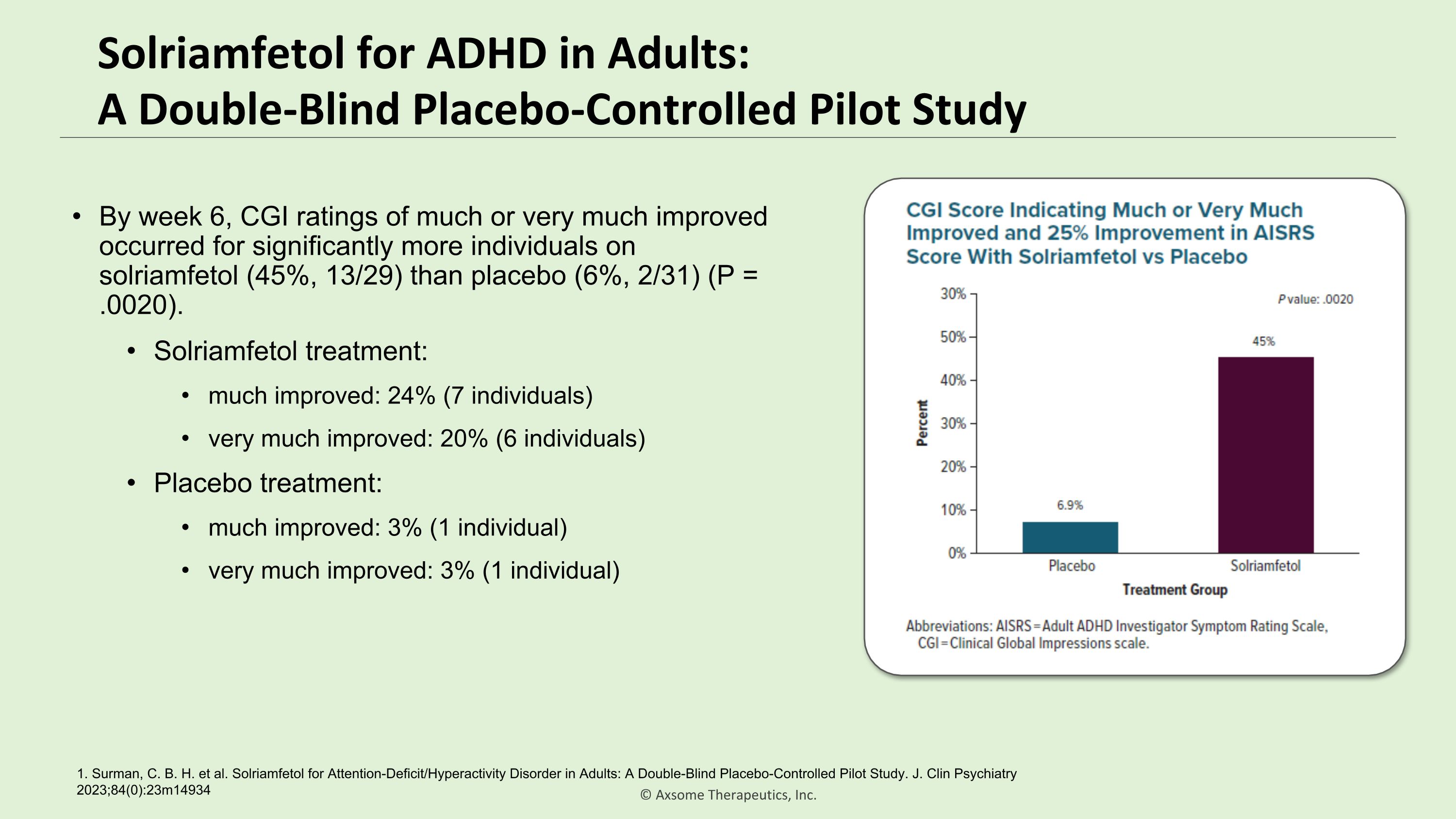
Solriamfetol for ADHD in Adults:�A Double-Blind Placebo-Controlled Pilot Study By week 6, CGI ratings of much or very much improved occurred for significantly more individuals on solriamfetol (45%, 13/29) than placebo (6%, 2/31) (P = .0020). Solriamfetol treatment: much improved: 24% (7 individuals) very much improved: 20% (6 individuals) Placebo treatment: much improved: 3% (1 individual) very much improved: 3% (1 individual) 1. Surman, C. B. H. et al. Solriamfetol for Attention-Deficit/Hyperactivity Disorder in Adults: A Double-Blind Placebo-Controlled Pilot Study. J. Clin Psychiatry 2023;84(0):23m14934

Solriamfetol for ADHD in Adults:�Safety and Tolerability Safety and tolerability of solriamfetol was in line with previous clinical studies
Solriamfetol Pilot ADHD Trial Conclusions In this single-center pilot trial conducted at Massachusetts General Hospital in Boston, solriamfetol was associated with significantly greater improvement in ADHD symptoms compared to placebo on the AISRS (primary endpoint). Results on secondary endpoints including both clinician- and patient-reported measures were consistent with the findings on the primary endpoint. Solriamfetol was well tolerated in the study with a safety profile consistent with prior experience. 1. Surman, C. B. H. et al. Solriamfetol for Attention-Deficit/Hyperactivity Disorder in Adults: A Double-Blind Placebo-Controlled Pilot Study. J. Clin Psychiatry 2023;84(0):23m14934
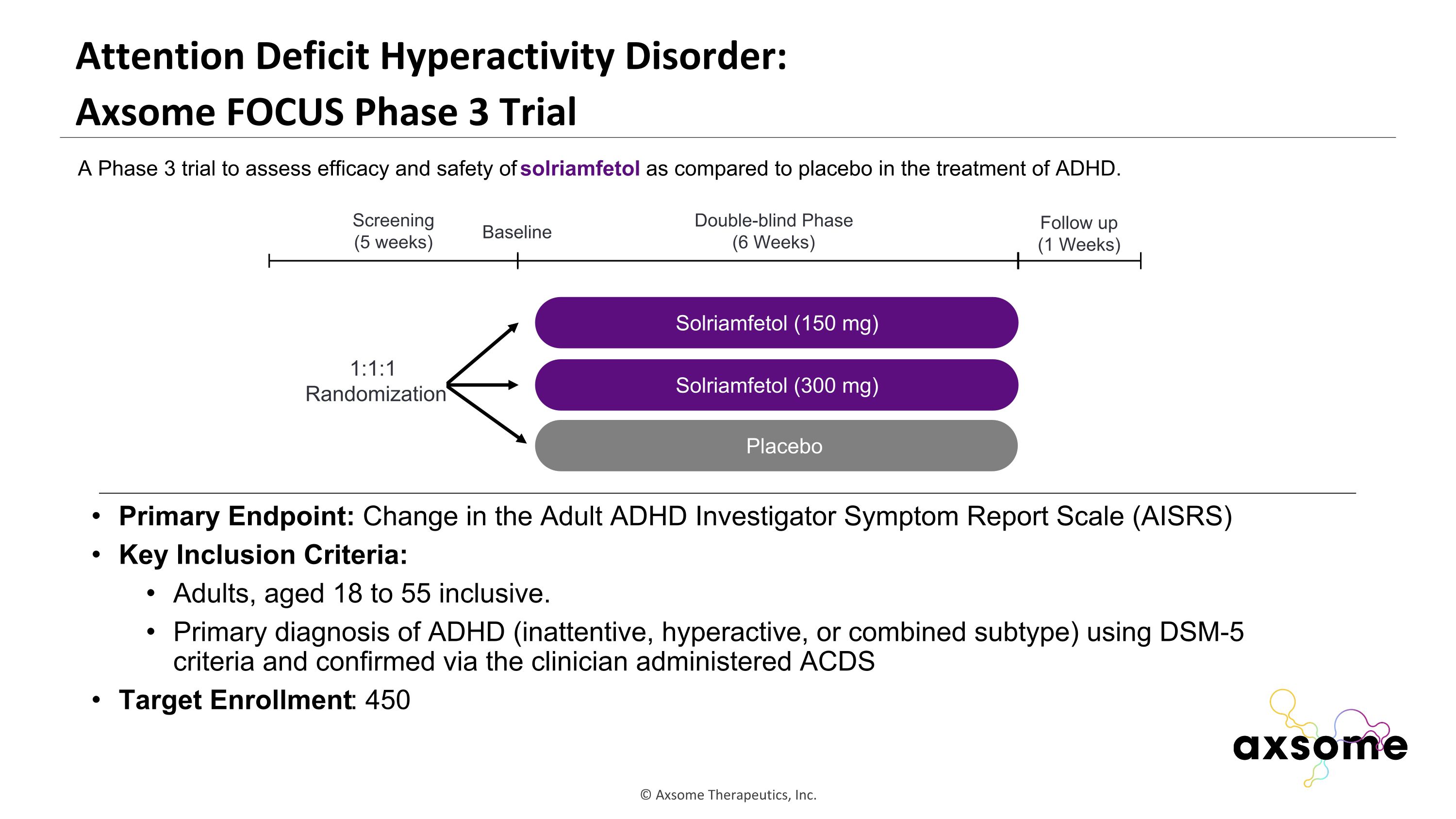
Attention Deficit Hyperactivity Disorder: �Axsome FOCUS Phase 3 Trial Primary Endpoint: Change in the Adult ADHD Investigator Symptom Report Scale (AISRS) Key Inclusion Criteria: Adults, aged 18 to 55 inclusive. Primary diagnosis of ADHD (inattentive, hyperactive, or combined subtype) using DSM-5 criteria and confirmed via the clinician administered ACDS Target Enrollment: 450 AXS-05 twice daily Solriamfetol (150 mg) Placebo Double-blind Phase (6 Weeks) Screening (5 weeks) 1:1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of ADHD. Solriamfetol (300 mg)
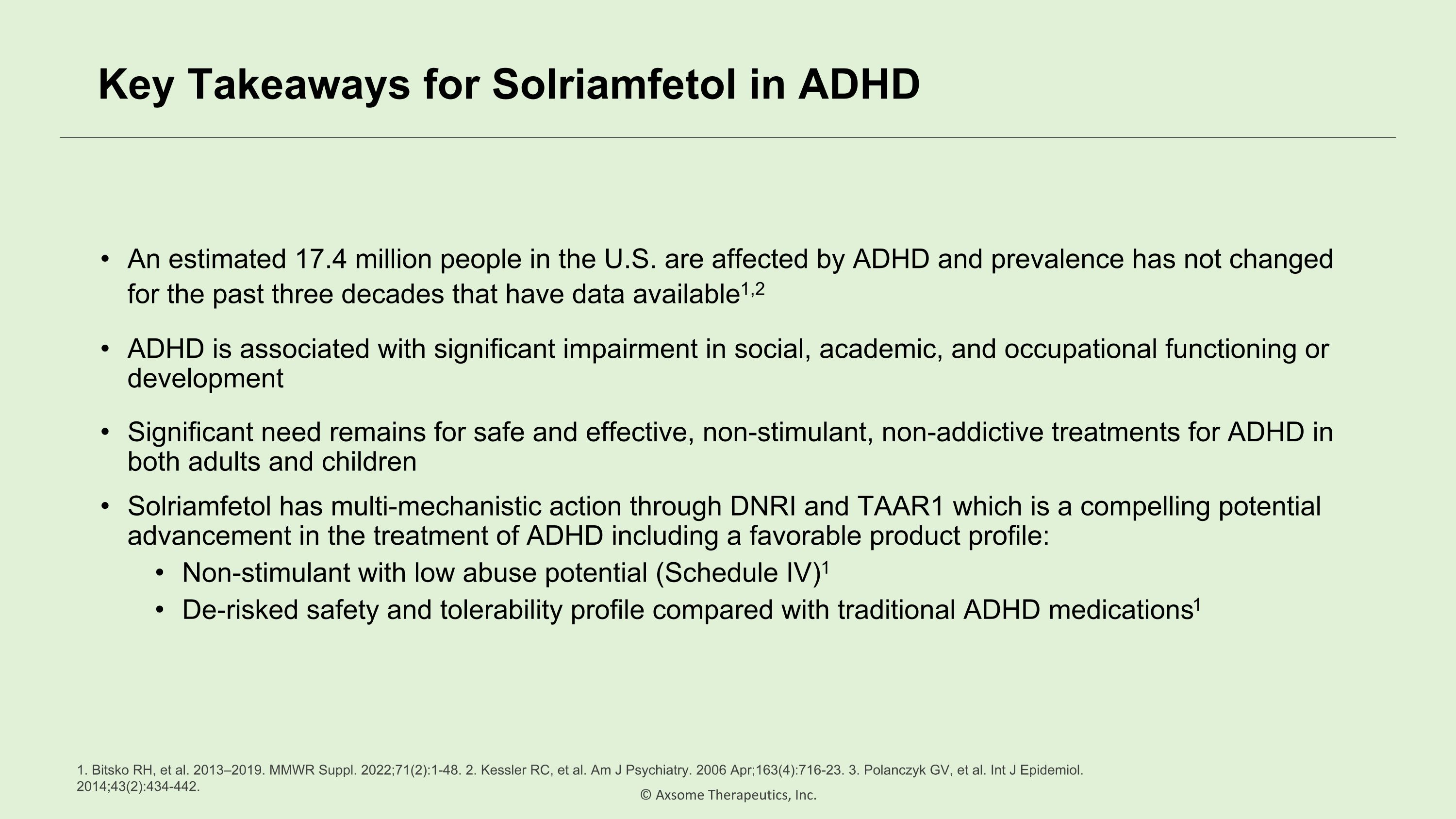
Key Takeaways for Solriamfetol in ADHD An estimated 17.4 million people in the U.S. are affected by ADHD and prevalence has not changed for the past three decades that have data available1,2 ADHD is associated with significant impairment in social, academic, and occupational functioning or development Significant need remains for safe and effective, non-stimulant, non-addictive treatments for ADHD in both adults and children Solriamfetol has multi-mechanistic action through DNRI and TAAR1 which is a compelling potential advancement in the treatment of ADHD including a favorable product profile: Non-stimulant with low abuse potential (Schedule IV)1 De-risked safety and tolerability profile compared with traditional ADHD medications1 1. Bitsko RH, et al. 2013–2019. MMWR Suppl. 2022;71(2):1-48. 2. Kessler RC, et al. Am J Psychiatry. 2006 Apr;163(4):716-23. 3. Polanczyk GV, et al. Int J Epidemiol. 2014;43(2):434-442.

on TAAR1, MDD, and ADHD Q+A Andrew J. Cutler, MD Craig Chepke, MD, DFAPA

Susan L. McElroy, MD Chief Research Officer, Lindner Center of HOPE Linda & Harry Fath Professor of Psychiatry & Behavioral Neuroscience University of Cincinnati College of Medicine Cincinnati, Ohio Solriamfetol in Binge Eating Disorder

Disclosures 2023 Principal/Co-Investigator Allergan, Avanir, Brainsway, Marriott Foundation, Myriad, Neurocrine, Novo Nordisk, Shire, Sunovion Consultant/Ad Board Allergan, Avanir, Bracket, F. Hoffman-La Roche Ltd., Idorsia, Mitsubishi Tanabe, Myriad, Shire, Sunovian Patents Inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and, along with the patent’s assignee, University of Cincinnati, Cincinnati, Ohio has received payments from Johnson & Johnson Pharmaceutical Research & Development, L.L.C., which has exclusive rights under the patent.

Outline Brief overview of Binge Eating Disorder (BED) Brief overview of treatment of BED Overview for rationale of solriamfetol in BED

Definition of a “Binge” Eating, in a discrete period of time (e.g., within any 2-hour period), an amount of food that is definitely larger than most people would eat during a similar period of time and under similar circumstances A sense of lack of control over eating during the episode (e.g., a feeling that one cannot stop eating or control what or how much one is eating)

Binge Eating Disorder (BED) Binge Eating: Eating in a discrete period of time an amount of food larger than most people would eat in a similar amount of time under similar circumstances AND a sense of lack of control overeating Binge Eating Disorder: BED broadly defined as recurrent binge eating without inappropriate compensatory behaviors BED is the most common eating disorder More common than anorexia nervosa (AN) and bulimia nervosa (BN) combined 1. Citrome L. CNS Spectr. 2015;20 Suppl 1:44-50.
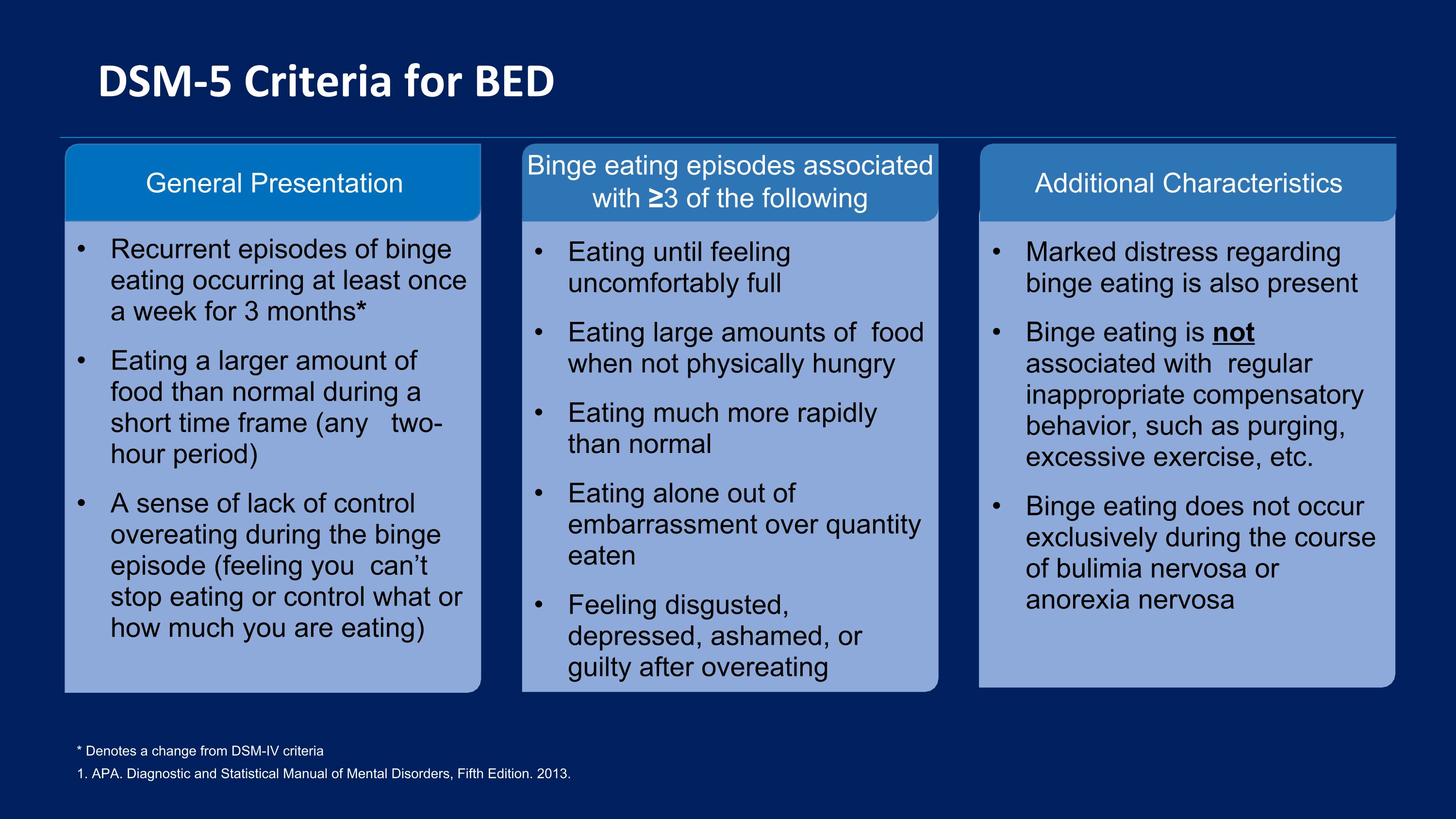
DSM-5 Criteria for BED 1. APA. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 2013. * Denotes a change from DSM-IV criteria General Presentation Recurrent episodes of binge eating occurring at least once a week for 3 months* Eating a larger amount of food than normal during a short time frame (any two-hour period) A sense of lack of control overeating during the binge episode (feeling you can’t stop eating or control what or how much you are eating) Binge eating episodes associated with ≥3 of the following Eating until feeling uncomfortably full Eating large amounts of food when not physically hungry Eating much more rapidly than normal Eating alone out of embarrassment over quantity eaten Feeling disgusted, depressed, ashamed, or guilty after overeating Additional Characteristics Marked distress regarding binge eating is also present Binge eating is not associated with regular inappropriate compensatory behavior, such as purging, excessive exercise, etc. Binge eating does not occur exclusively during the course of bulimia nervosa or anorexia nervosa

BED Epidemiology/Course BED is the most common eating disorder 2.8% (US adults), 1.6% (adolescents), 1.9% worldwide Prevalence for other eating disorders: Anorexia Nervosa 0.6%, Bulimia Nervosa 1% BED affects women(3.5%) more often than men (2.0%) BED is more common in men than other eating disorders: AN, BN 3x more common in women than men BED 1.75x more common in women than men Frequently presents in young to middle adulthood Course may be remitting, recurrent, or chronic Swanson SA, et al. Arch Gen Psychiatry. 2011;68:714-23. Pope H.G. et al,, AmJ Psychiatry. 206; 163:2181-3. Hudson JI, et al. Biol Psych. 2007; 61:348-58.

BED is Associated With… Overeating (night eating, grazing, LOC) and weight gain Obesity, including severe obesity (BMI ≥40), & obesity-related conditions Mood, anxiety, substance use, impulse control (e.g., ADHD) disorders 80% have ≥1 comorbid disorder Reduced quality of life, impairment in functioning (comparable to BN), & increased health care use and costs Marked distress - shame, guilt, self disgust BED is NOT a trivial disorder Hudson JI et al. Biol Psych. 2007;61:348. ; Preti A et al. J Psychiatric Res. 2009;43:1125. ; Swanson SA et al. Arch Gen Psychiatry. 2011;68:714. Kessler RC et al. Biol Psych. 2013;73:904.
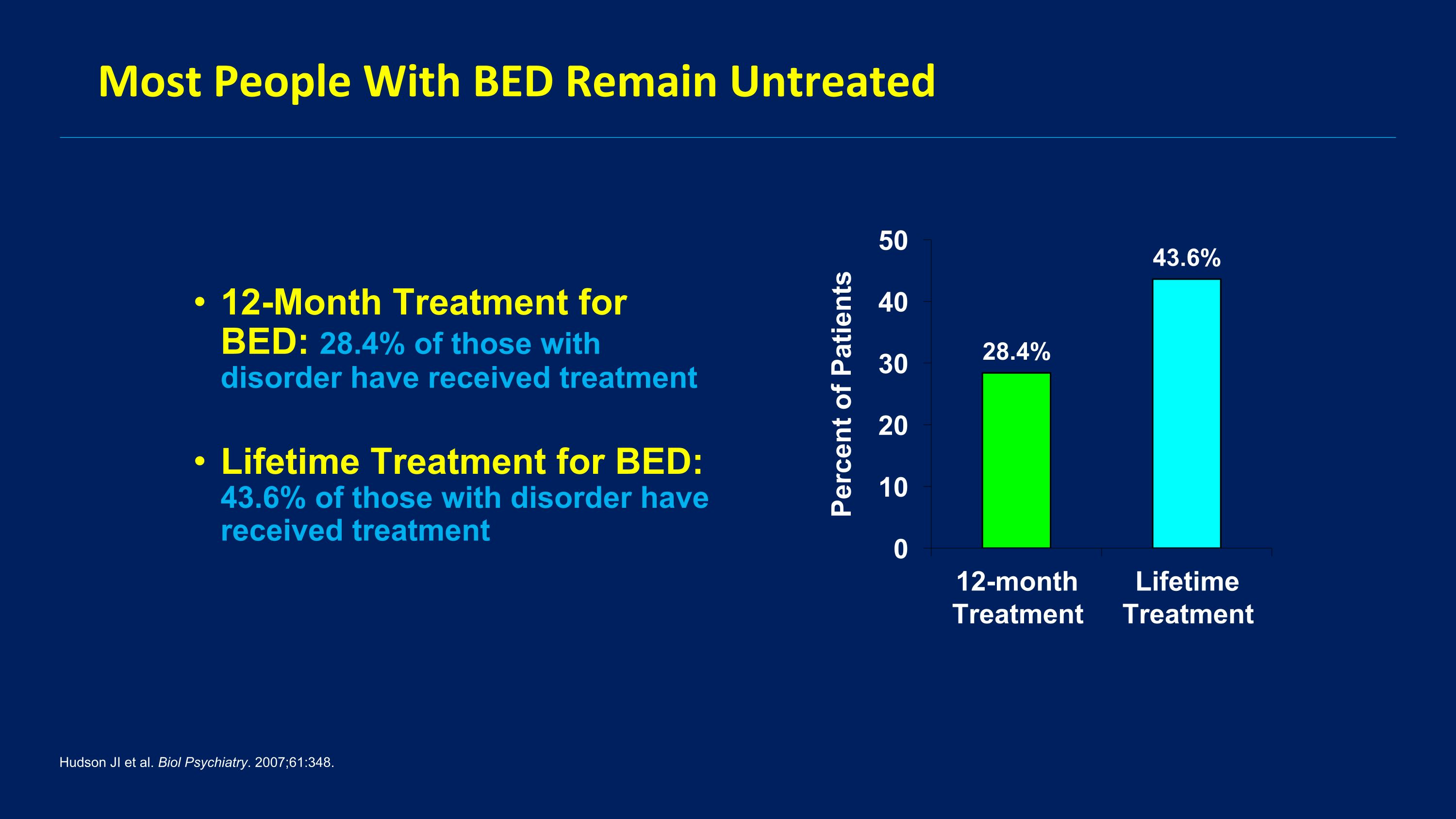
Most People With BED Remain Untreated 12-Month Treatment for BED: 28.4% of those with disorder have received treatment Lifetime Treatment for BED: 43.6% of those with disorder have received treatment Hudson JI et al. Biol Psychiatry. 2007;61:348.

Standard of Care for BED not yet clearly defined Psychoeducation, self-help strategies Behavioral weight loss treatment Empirically based psychotherapies (ex.CBT-E) Pharmacotherapy Obesity surgery Combination therapy McElroy SL et al. Ther Clin Risk Manag. 2012;8:219.

Pharmacotherapy of BED Only 1 drug with FDA approval for BED: Lisdexamfetamine for BED Fluoxetine for BN Dasotraline development for BED halted despite 2 positive RCTs Problems with lisdexamfetamine: Addiction (schedule 2) Cardiovascular side effects Can be mood destabilizing Not everyone responds or tolerates Need new medications with novel MOAs

Dasotraline Selective DA-NE reuptake inhibitor Was being developed for ADHD and BED 2 positive registration trials for BED Navia B et al, 2017. APA 170th Meeting
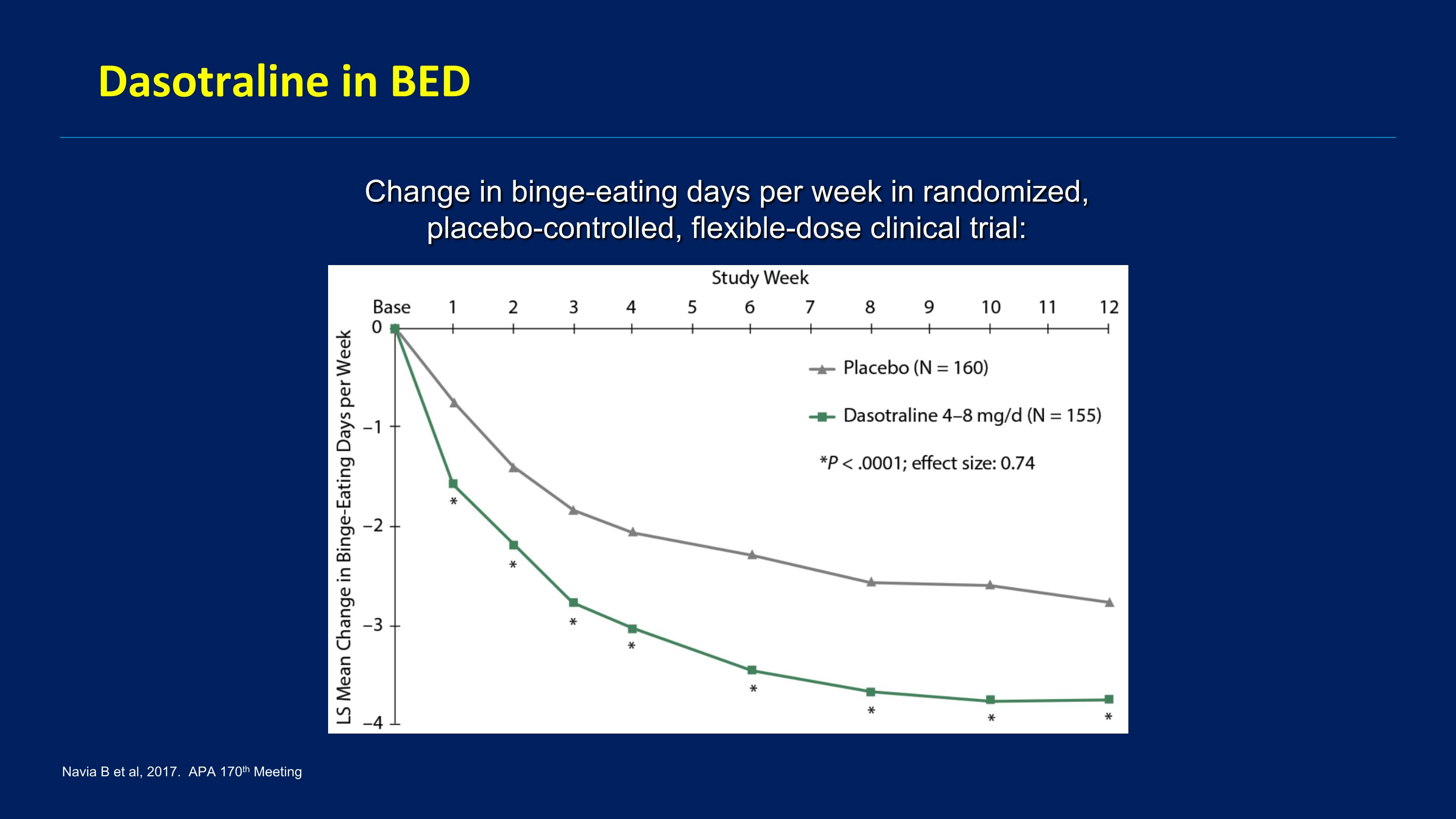
Dasotraline in BED Change in binge-eating days per week in randomized, placebo-controlled, flexible-dose clinical trial: Navia B et al, 2017. APA 170th Meeting

Rationale for Solriamfetol in BED DNRI like dasotraline Decreased appetite and weight loss Link between narcolepsy and BED
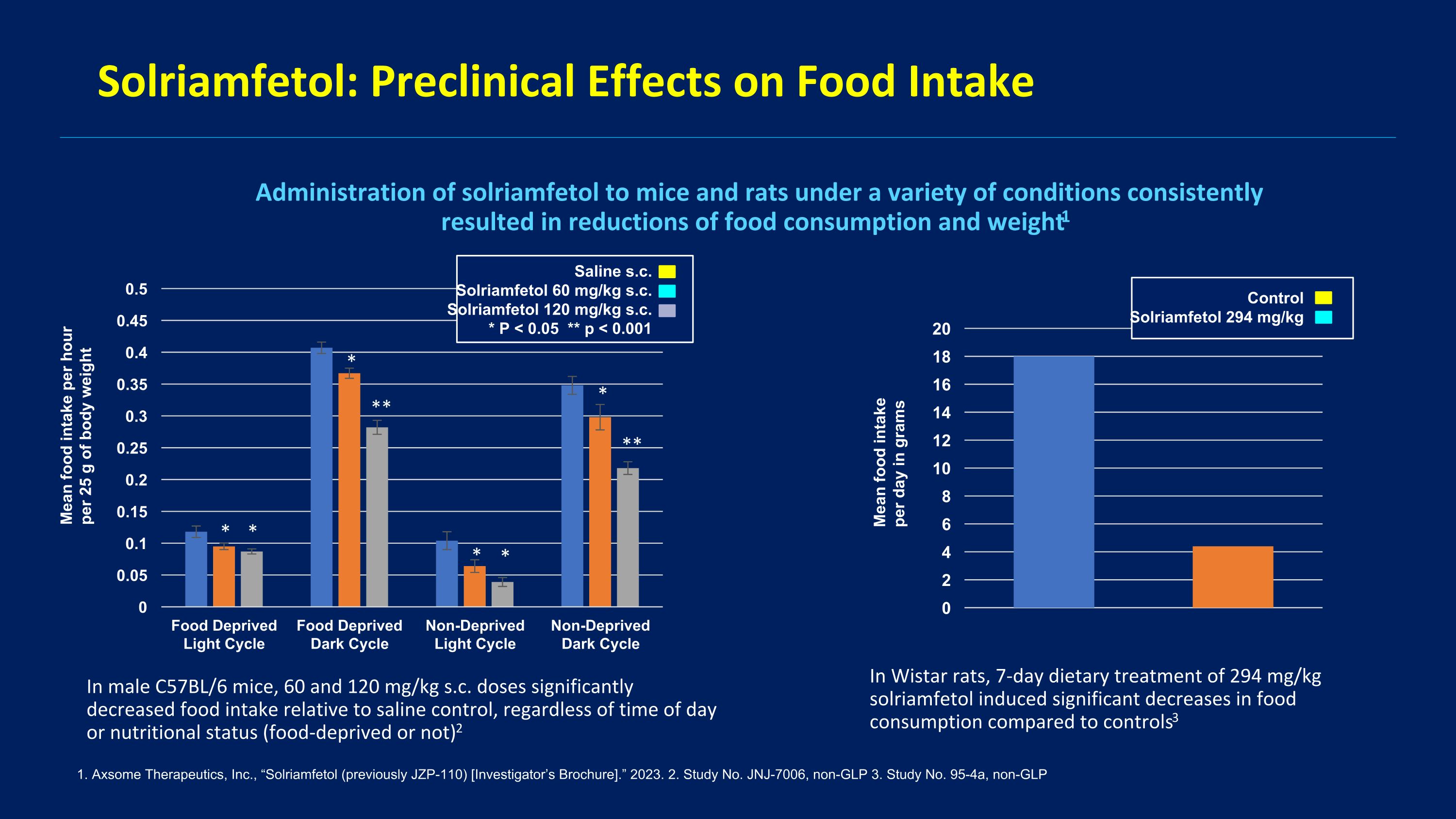
Solriamfetol: Preclinical Effects on Food Intake 1. Axsome Therapeutics, Inc., “Solriamfetol (previously JZP-110) [Investigator’s Brochure].” 2023. 2. Study No. JNJ-7006, non-GLP 3. Study No. 95-4a, non-GLP Administration of solriamfetol to mice and rats under a variety of conditions consistently resulted in reductions of food consumption and weight1 In male C57BL/6 mice, 60 and 120 mg/kg s.c. doses significantly decreased food intake relative to saline control, regardless of time of day or nutritional status (food-deprived or not)2 In Wistar rats, 7-day dietary treatment of 294 mg/kg solriamfetol induced significant decreases in food consumption compared to controls3 Mean food intake per hour per 25 g of body weight Mean food intake per day in grams Saline s.c. Solriamfetol 60 mg/kg s.c. Solriamfetol 120 mg/kg s.c. * P < 0.05 ** p < 0.001 Control Solriamfetol 294 mg/kg * * * ** * * * **
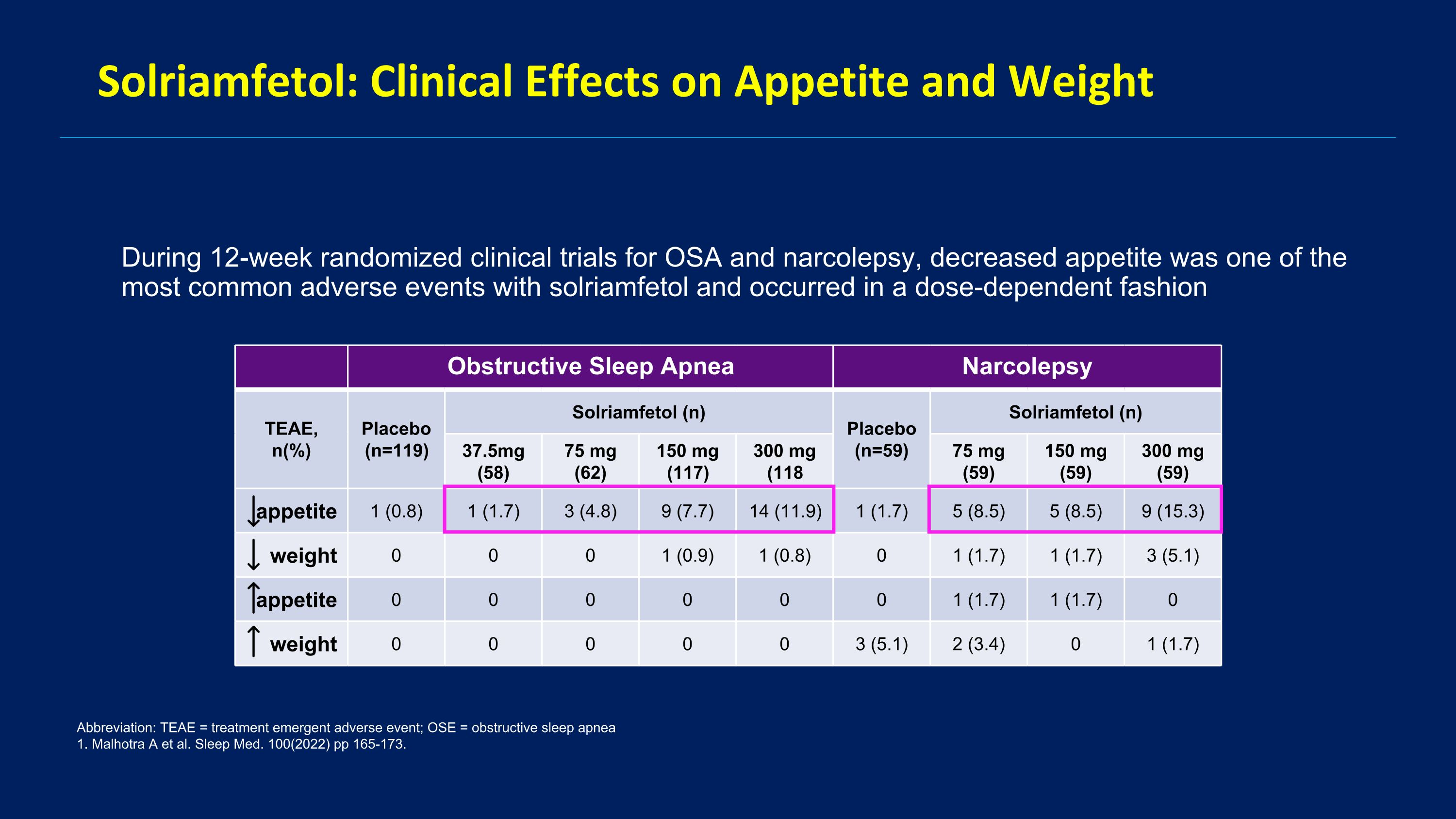
Solriamfetol: Clinical Effects on Appetite and Weight During 12-week randomized clinical trials for OSA and narcolepsy, decreased appetite was one of the most common adverse events with solriamfetol and occurred in a dose-dependent fashion Abbreviation: TEAE = treatment emergent adverse event; OSE = obstructive sleep apnea 1. Malhotra A et al. Sleep Med. 100(2022) pp 165-173. Obstructive Sleep Apnea Narcolepsy TEAE, n(%) Placebo (n=119) Solriamfetol (n) Placebo (n=59) Solriamfetol (n) 37.5mg (58) 75 mg (62) 150 mg (117) 300 mg (118 75 mg (59) 150 mg (59) 300 mg (59) appetite 1 (0.8) 1 (1.7) 3 (4.8) 9 (7.7) 14 (11.9) 1 (1.7) 5 (8.5) 5 (8.5) 9 (15.3) weight 0 0 0 1 (0.9) 1 (0.8) 0 1 (1.7) 1 (1.7) 3 (5.1) appetite 0 0 0 0 0 0 1 (1.7) 1 (1.7) 0 weight 0 0 0 0 0 3 (5.1) 2 (3.4) 0 1 (1.7)
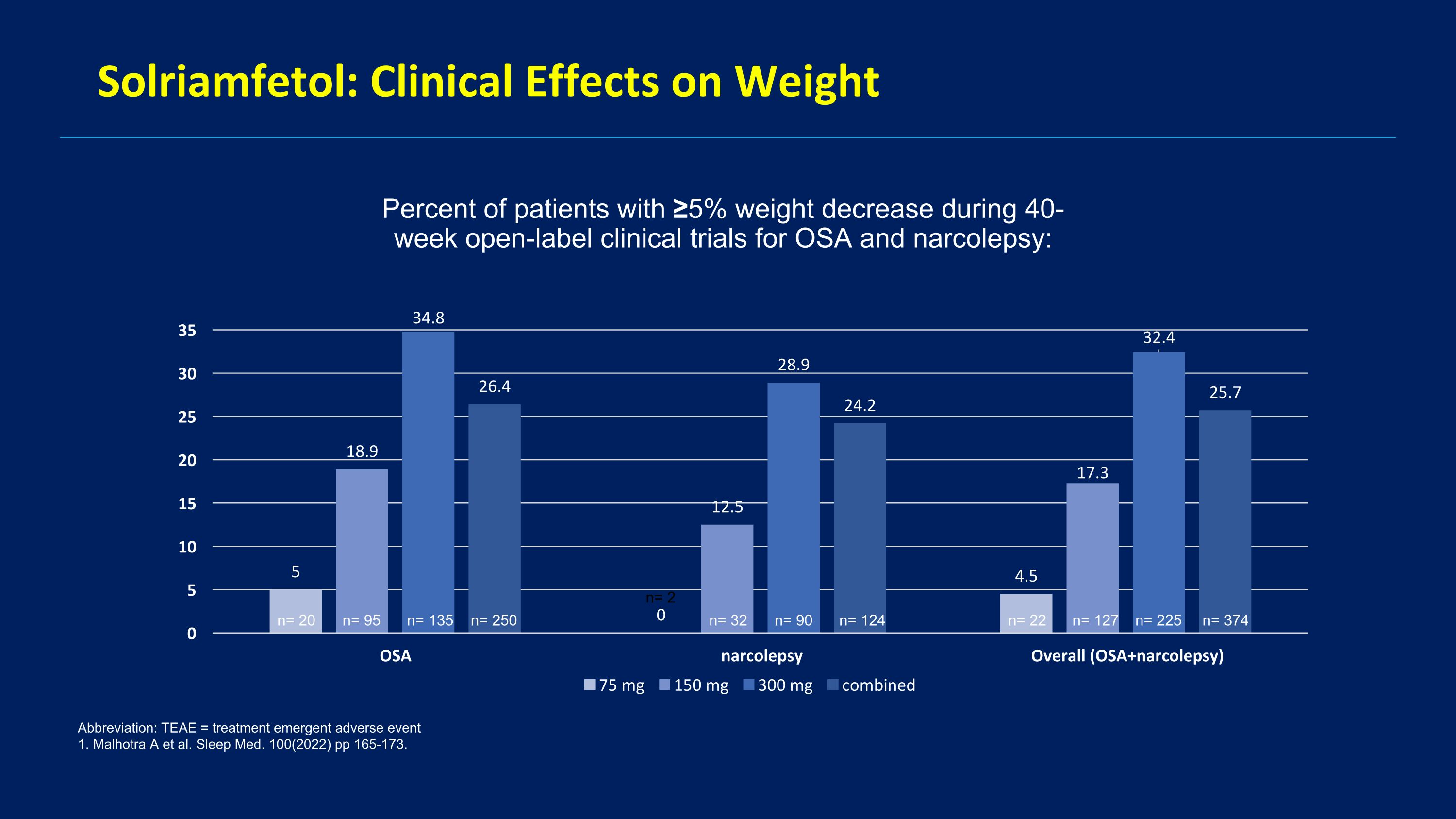
Solriamfetol: Clinical Effects on Weight Percent of patients with ≥5% weight decrease during 40-week open-label clinical trials for OSA and narcolepsy: Abbreviation: TEAE = treatment emergent adverse event 1. Malhotra A et al. Sleep Med. 100(2022) pp 165-173. n= 374 n= 225 n= 127 n= 22 n= 124 n= 90 n= 32 n= 2 n= 250 n= 135 n= 95 n= 20
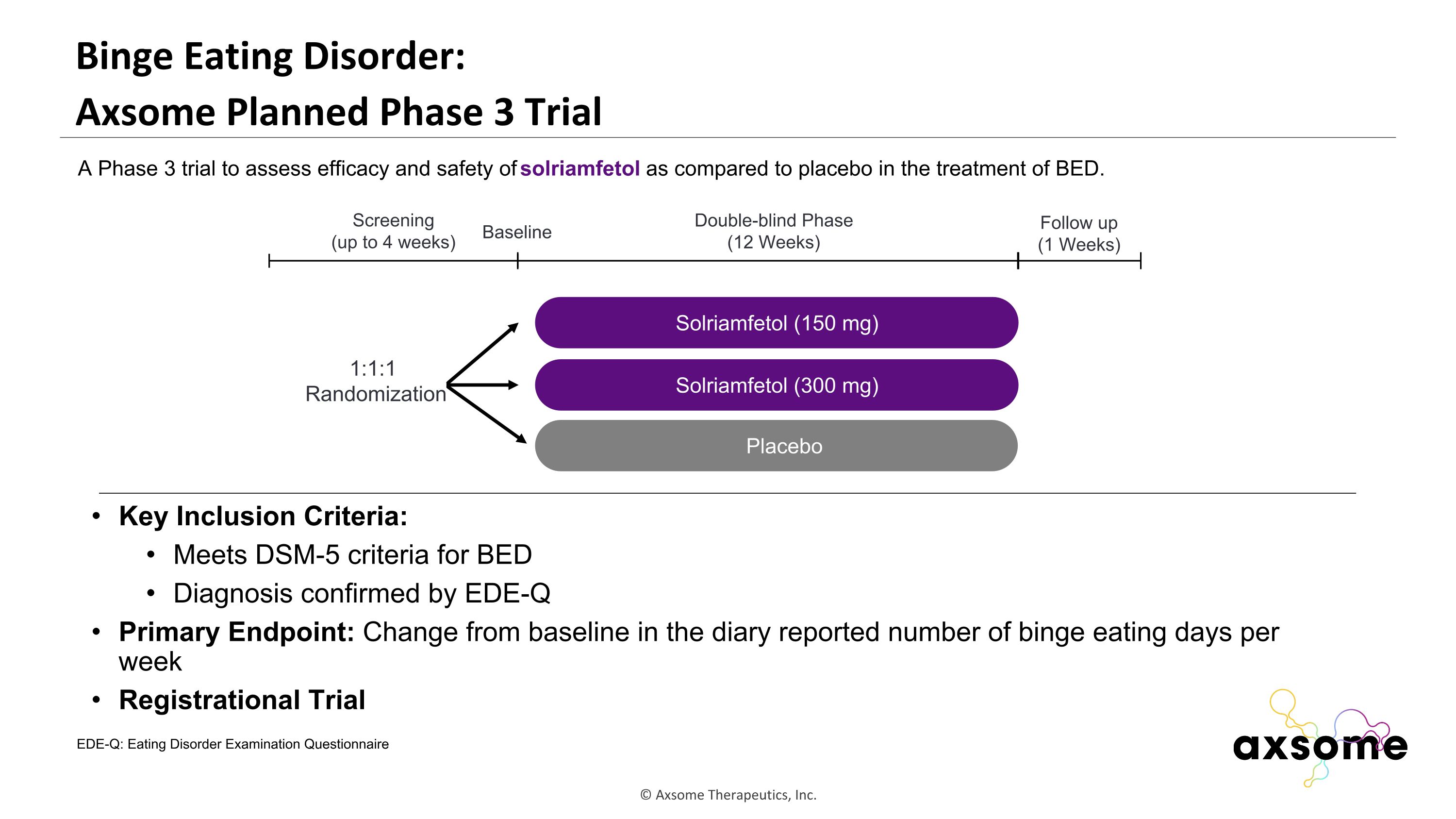
Binge Eating Disorder: �Axsome Planned Phase 3 Trial Key Inclusion Criteria: Meets DSM-5 criteria for BED Diagnosis confirmed by EDE-Q Primary Endpoint: Change from baseline in the diary reported number of binge eating days per week Registrational Trial AXS-05 twice daily Solriamfetol (150 mg) Placebo Double-blind Phase (12 Weeks) Screening (up to 4 weeks) 1:1:1 Randomization Follow up (1 Weeks) Baseline A Phase 3 trial to assess efficacy and safety of solriamfetol as compared to placebo in the treatment of BED. Solriamfetol (300 mg) EDE-Q: Eating Disorder Examination Questionnaire

Key Takeaways for Solriamfetol in Binge Eating Disorder BED is the most common eating disorder, affecting almost 3% of the U.S. population1 BED is associated with a 2- to 3-fold increased risk of psychiatric and medical comorbidities2 Despite high prevalence and burden, there is currently only one FDA-approved medication for BED Evaluation of solriamfetol in BED is supported by solriamfetol pharmacology, preclinical effects, and clinical effects on appetite and weight observed in OSA and narcolepsy patients. References: Hudson JI, et al. Biol. Psychiatry, vol. 61, no. 3, pp. 348–358, Feb. 2007 McElroy SL et al. J. Clin. Psychiatry, vol. 81, no. 5, Sep. 2020

Richard Bogan, M.D. Associate Clinical Professor at the University of South Carolina School of Medicine and Medical University of South Carolina Principal of Bogan Sleep Consultants SHARP Study:�Effects of Solriamfetol on Cognitive Function in Participants�With Cognitive Impairment Associated With Excessive Daytime�Sleepiness in Obstructive Sleep Apnea
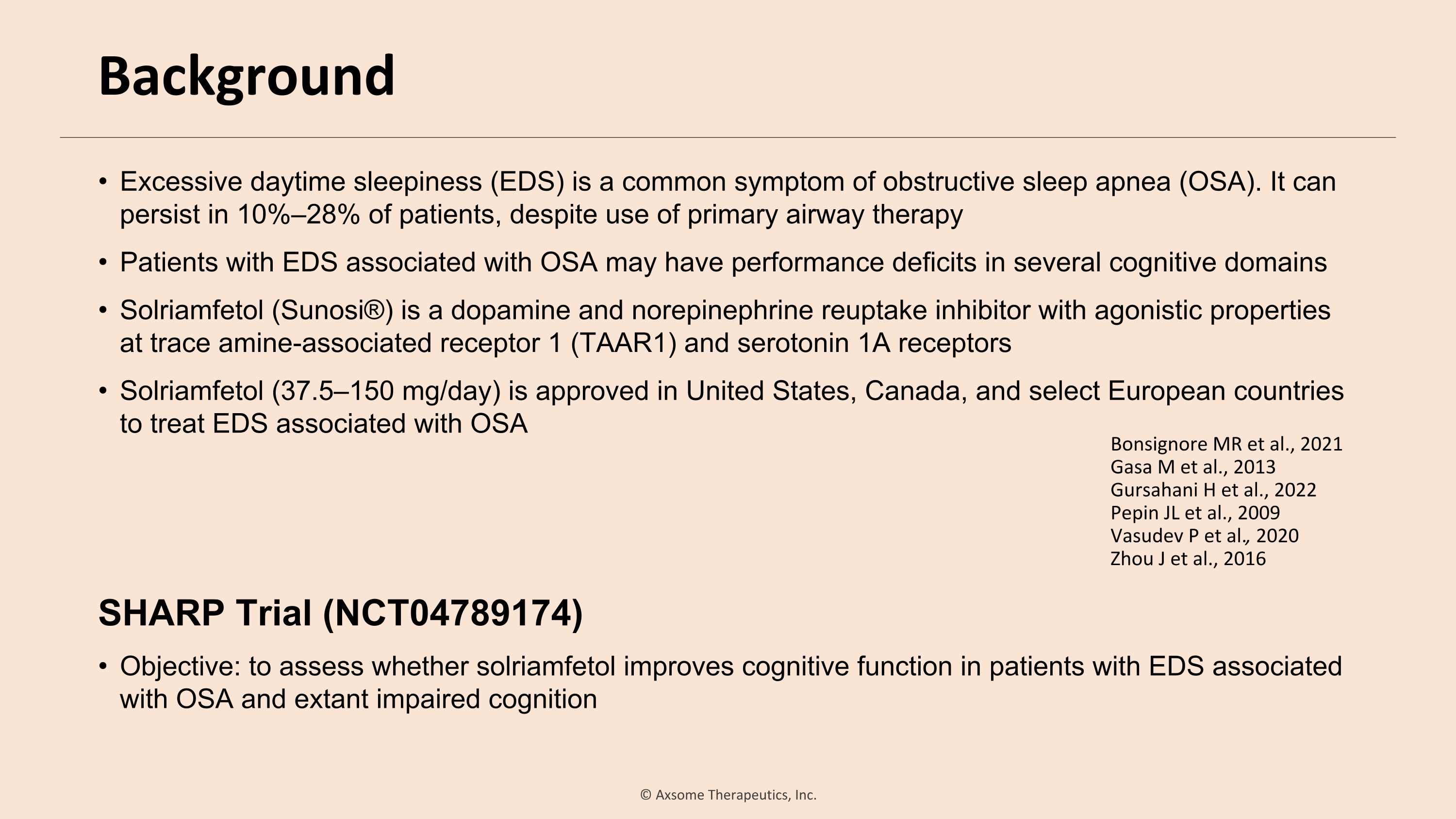
Background Excessive daytime sleepiness (EDS) is a common symptom of obstructive sleep apnea (OSA). It can persist in 10%–28% of patients, despite use of primary airway therapy Patients with EDS associated with OSA may have performance deficits in several cognitive domains Solriamfetol (Sunosi®) is a dopamine and norepinephrine reuptake inhibitor with agonistic properties at trace amine-associated receptor 1 (TAAR1) and serotonin 1A receptors Solriamfetol (37.5–150 mg/day) is approved in United States, Canada, and select European countries to treat EDS associated with OSA SHARP Trial (NCT04789174) Objective: to assess whether solriamfetol improves cognitive function in patients with EDS associated with OSA and extant impaired cognition Bonsignore MR et al., 2021 Gasa M et al., 2013 Gursahani H et al., 2022 Pepin JL et al., 2009 Vasudev P et al., 2020 Zhou J et al., 2016
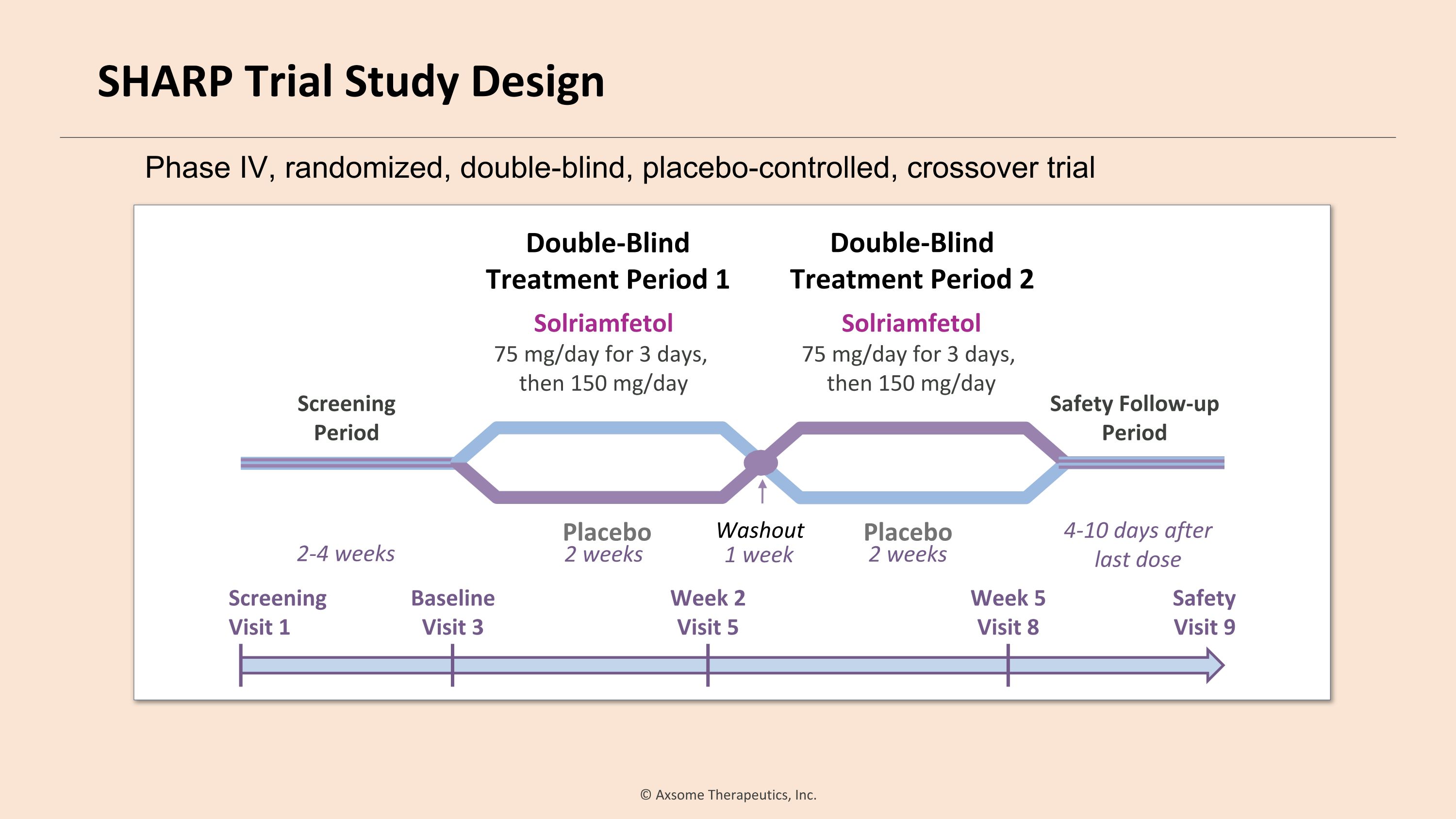
Baseline Visit 3 Double-Blind �Treatment Period 1 Safety Follow-up Period Placebo 2-4 weeks Double-Blind �Treatment Period 2 4-10 days after last dose Safety Visit 9 Screening Visit 1 Week 2 Visit 5 Week 5 Visit 8 2 weeks 1 week Solriamfetol 75 mg/day for 3 days, then 150 mg/day Screening�Period Solriamfetol 75 mg/day for 3 days, then 150 mg/day Placebo Washout 2 weeks Phase IV, randomized, double-blind, placebo-controlled, crossover trial SHARP Trial Study Design
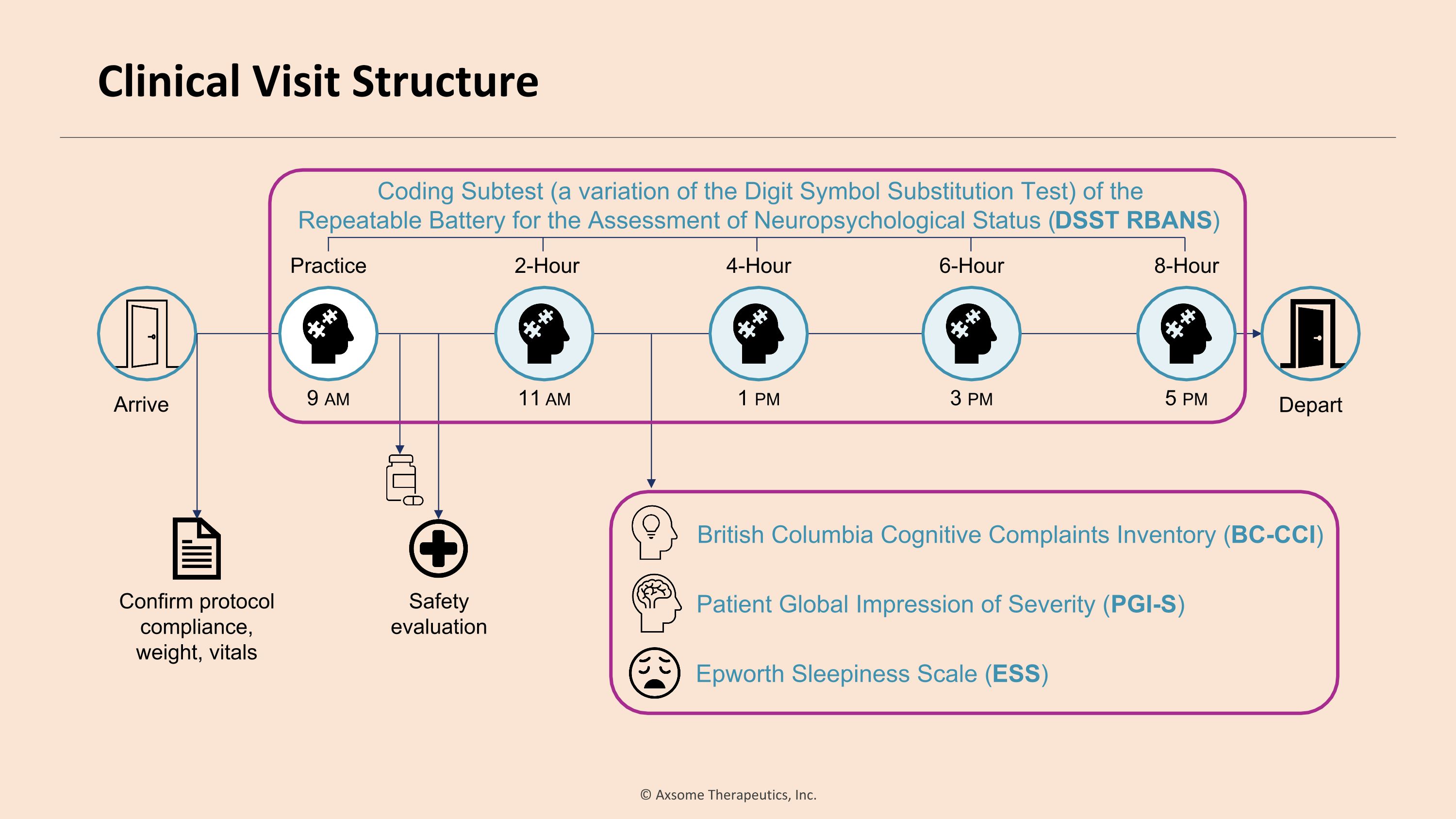
Arrive Depart Safety evaluation Confirm protocol �compliance, weight, vitals Patient Global Impression of Severity (PGI-S) Epworth Sleepiness Scale (ESS) British Columbia Cognitive Complaints Inventory (BC-CCI) Coding Subtest (a variation of the Digit Symbol Substitution Test) of the �Repeatable Battery for the Assessment of Neuropsychological Status (DSST RBANS) 9 am Practice 11 am 2-Hour 1 pm 4-Hour 3 pm 6-Hour 5 pm 8-Hour Clinical Visit Structure
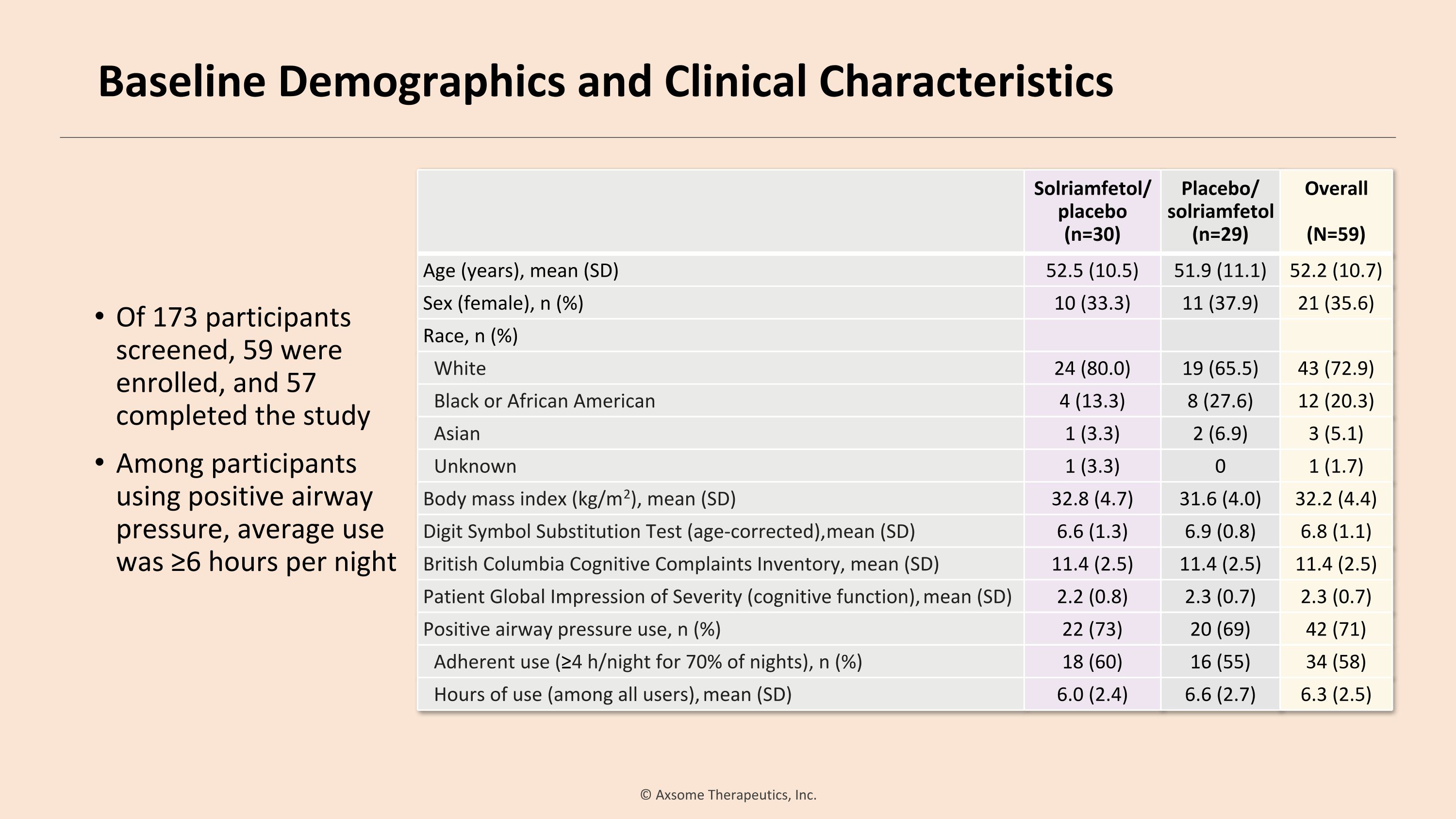
Of 173 participants screened, 59 were enrolled, and 57 completed the study Among participants using positive airway pressure, average use was ≥6 hours per night Solriamfetol/ placebo (n=30) Placebo/ solriamfetol (n=29) Overall (N=59) Age (years), mean (SD) 52.5 (10.5) 51.9 (11.1) 52.2 (10.7) Sex (female), n (%) 10 (33.3) 11 (37.9) 21 (35.6) Race, n (%) White 24 (80.0) 19 (65.5) 43 (72.9) Black or African American 4 (13.3) 8 (27.6) 12 (20.3) Asian 1 (3.3) 2 (6.9) 3 (5.1) Unknown 1 (3.3) 0 1 (1.7) Body mass index (kg/m2), mean (SD) 32.8 (4.7) 31.6 (4.0) 32.2 (4.4) Digit Symbol Substitution Test (age-corrected), mean (SD) 6.6 (1.3) 6.9 (0.8) 6.8 (1.1) British Columbia Cognitive Complaints Inventory, mean (SD) 11.4 (2.5) 11.4 (2.5) 11.4 (2.5) Patient Global Impression of Severity (cognitive function), mean (SD) 2.2 (0.8) 2.3 (0.7) 2.3 (0.7) Positive airway pressure use, n (%) 22 (73) 20 (69) 42 (71) Adherent use (≥4 h/night for 70% of nights), n (%) 18 (60) 16 (55) 34 (58) Hours of use (among all users), mean (SD) 6.0 (2.4) 6.6 (2.7) 6.3 (2.5) Baseline Demographics and Clinical Characteristics
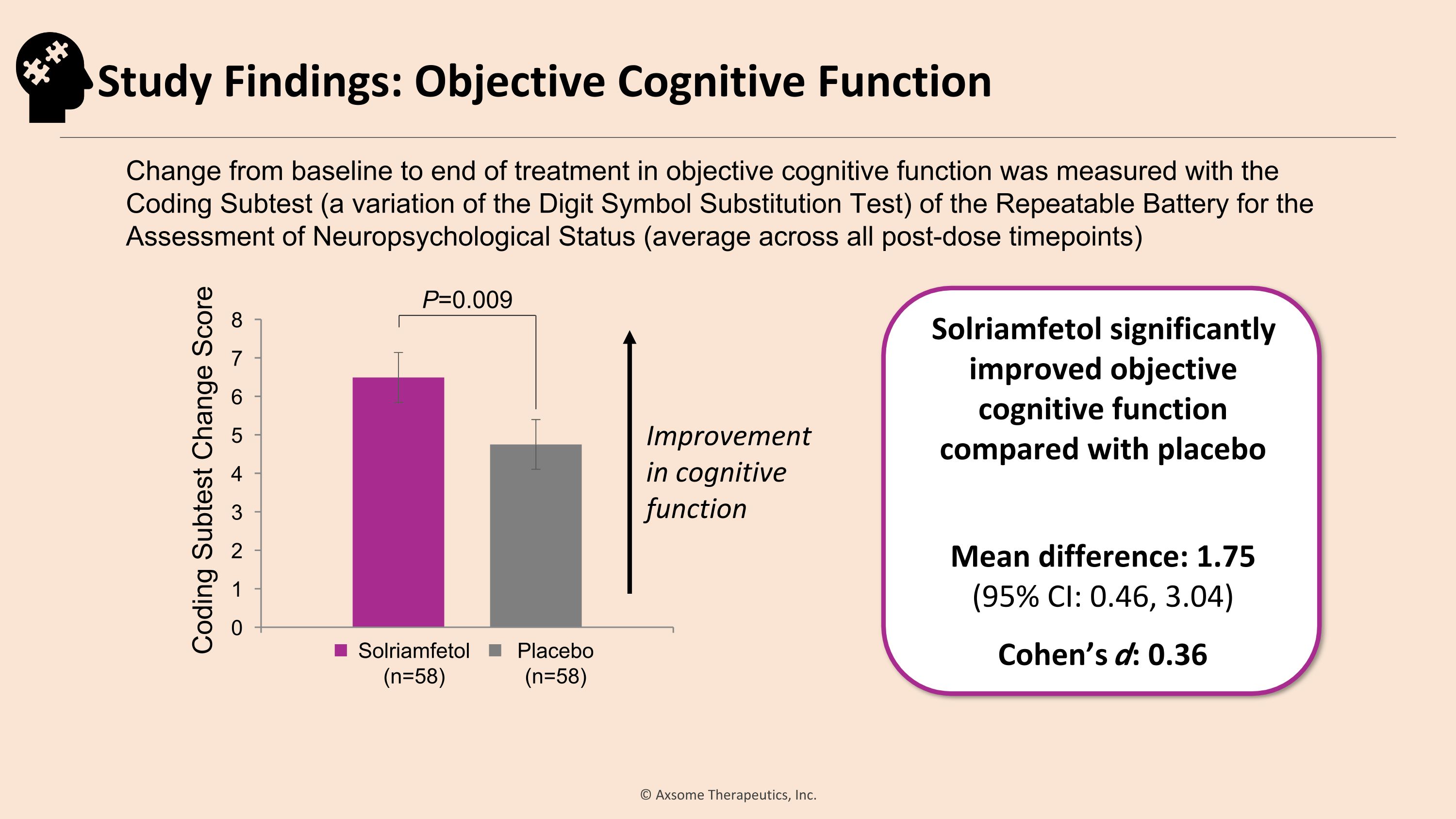
Improvement in cognitive function P=0.009 Solriamfetol significantly improved objective cognitive function compared with placebo Mean difference: 1.75�(95% CI: 0.46, 3.04) Cohen’s d: 0.36 Change from baseline to end of treatment in objective cognitive function was measured with the �Coding Subtest (a variation of the Digit Symbol Substitution Test) of the Repeatable Battery for the Assessment of Neuropsychological Status (average across all post-dose timepoints) Coding Subtest Change Score Solriamfetol (n=58) Placebo (n=58) Study Findings: Objective Cognitive Function
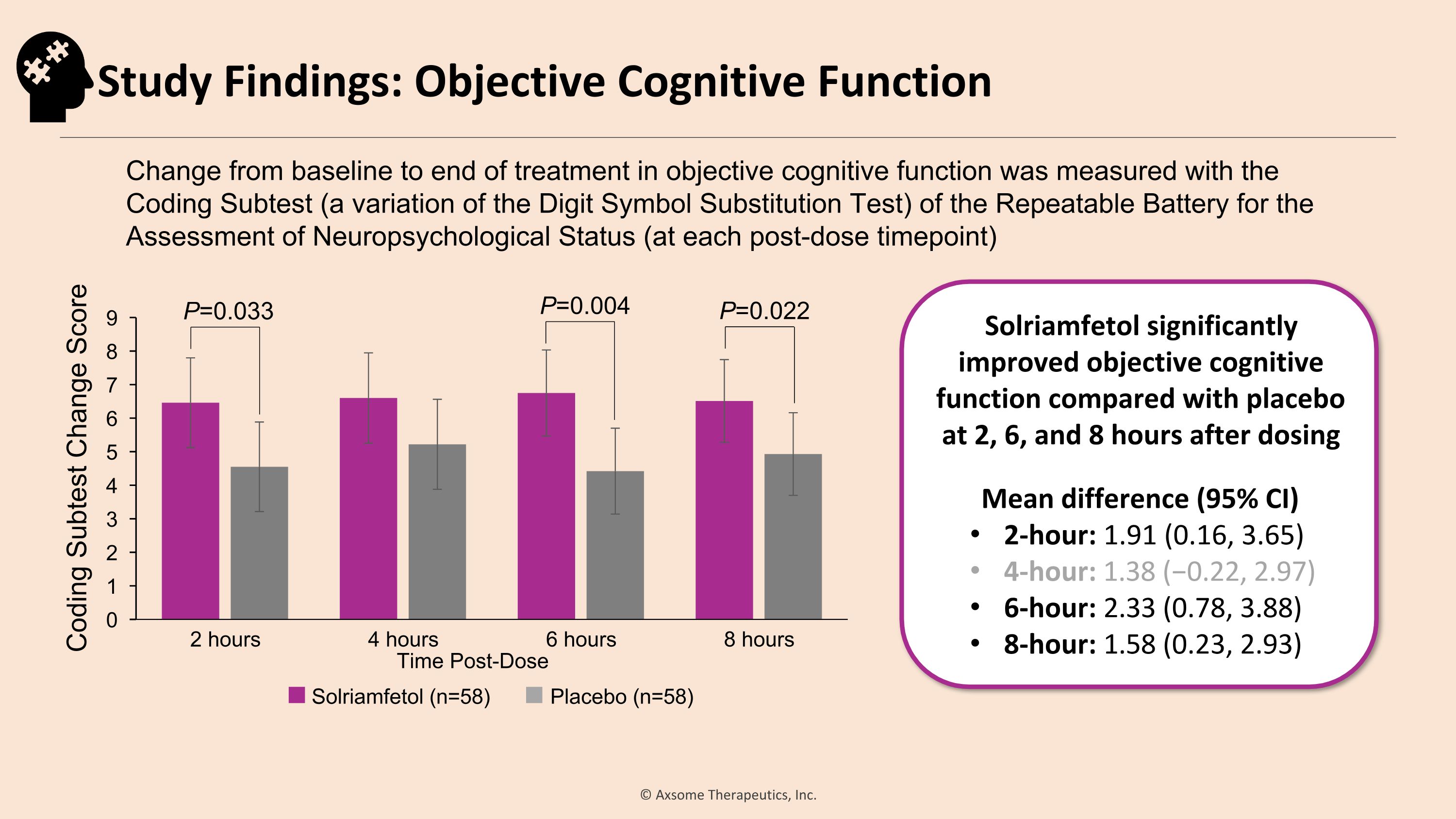
Solriamfetol (n=58) Placebo (n=58) P=0.033 P=0.022 Solriamfetol significantly improved objective cognitive function compared with placebo at 2, 6, and 8 hours after dosing Mean difference (95% CI) 2-hour: 1.91 (0.16, 3.65) 4-hour: 1.38 (−0.22, 2.97) 6-hour: 2.33 (0.78, 3.88) 8-hour: 1.58 (0.23, 2.93) Change from baseline to end of treatment in objective cognitive function was measured with the �Coding Subtest (a variation of the Digit Symbol Substitution Test) of the Repeatable Battery for the Assessment of Neuropsychological Status (at each post-dose timepoint) Coding Subtest Change Score P=0.004 Study Findings: Objective Cognitive Function
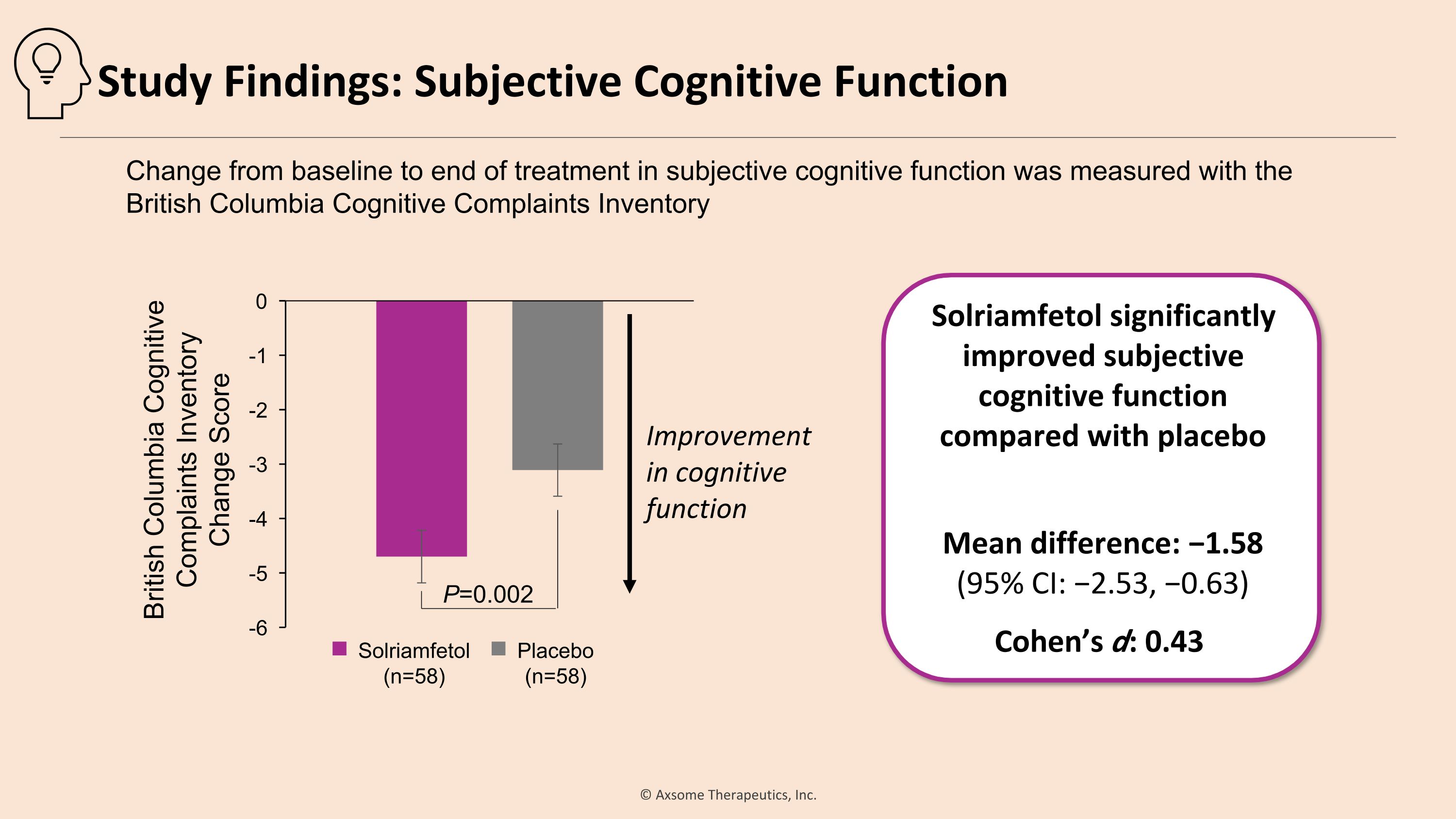
Solriamfetol significantly improved subjective cognitive function compared with placebo Mean difference: −1.58 �(95% CI: −2.53, −0.63) Cohen’s d: 0.43 P=0.002 Improvement in cognitive function Change from baseline to end of treatment in subjective cognitive function was measured with the �British Columbia Cognitive Complaints Inventory Solriamfetol (n=58) Placebo (n=58) British Columbia Cognitive Complaints Inventory �Change Score Study Findings: Subjective Cognitive Function
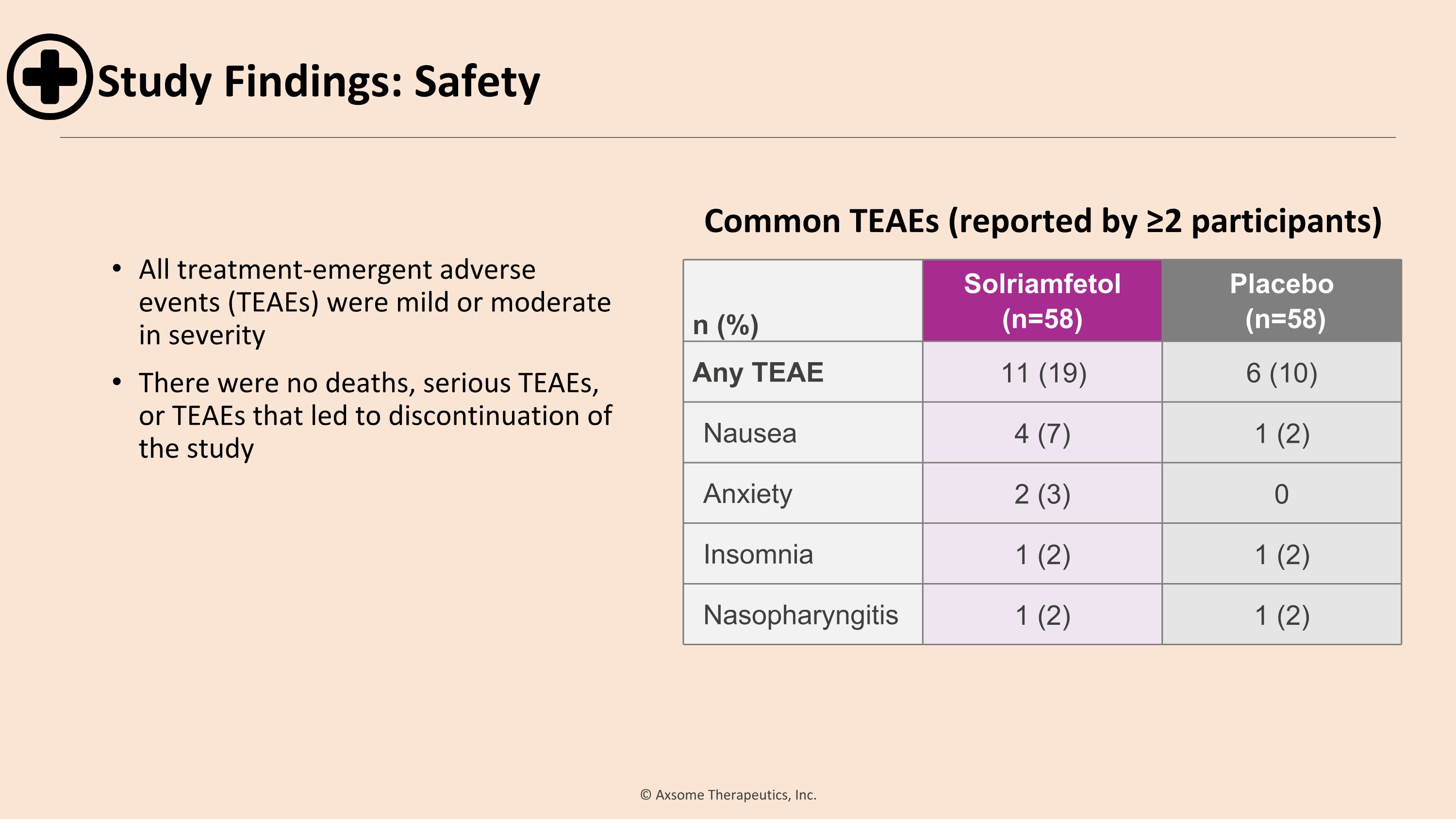
All treatment-emergent adverse events (TEAEs) were mild or moderate in severity There were no deaths, serious TEAEs, or TEAEs that led to discontinuation of the study n (%) Solriamfetol (n=58) Placebo (n=58) Any TEAE 11 (19) 6 (10) Nausea 4 (7) 1 (2) Anxiety 2 (3) 0 Insomnia 1 (2) 1 (2) Nasopharyngitis 1 (2) 1 (2) Common TEAEs (reported by ≥2 participants) Study Findings: Safety
Conclusions Solriamfetol provided sustained improvement in objective cognition, reduced participants’ perceived severity of cognitive impairment, and reduced subjective sleepiness The adverse event profile and high study completion rate suggest solriamfetol was well tolerated Solriamfetol has the potential to improve cognitive function in patients with cognitive impairment associated with OSA and EDS

Charles A. Czeisler, PhD, MD, FRCP, FAPS Frank Baldino, Jr, PhD Professor of Sleep Medicine Professor of Medicine and Director, Division of Sleep Medicine, Harvard Medical School Chief, Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham & Women’s Hospital Shift Work Disorder Diagnostic Features, Prevalence, Adverse Health Outcomes, and Approved Treatments © 1994 NY Times Investor Conference December 7, 2023

Disclosures 1. Modafinil for Shift-Work Sleep-Wake Disorder1,3 2. Armodafinil for Shift-Work Sleep-Wake Disorder2 3. Tasimelteon for Non-24-Hour Sleep-Wake Disorder4,5 1. Czeisler CA et al. (2005). Modafinil for excessive sleepiness associated with shift work sleep disorder. N Engl J Med 353: 476-486. 2. Czeisler CA, Walsh JK, Wesnes KA, Arora S, Roth T. (2009). Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study. Mayo Clin Proc 84: 958-972. 3. Grady S, Aeschbach D, Wright KP, Jr., Czeisler CA. Effect of modafinil on impairments in neurobehavioral performance and learning associated with extended wakefulness and circadian misalignment. Neuropsychopharmacology 2010;35:1910-20. 4. Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, Klerman EB (2008). Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet 373(9662): 482-491. 5. Lockley SW, Dressman MA, Licamele L, Xiao C, Fisher DM, Flynn-Evans EE, Hull JT, Torres R, Lavedan C, Polymeropoulos MH. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet 386(10005): 1754-1764. *Dr. Czeisler reports grants and contracts to BWH from Dayzz Live Well, Delta Airlines, Jazz Pharmaceuticals PLC Inc, Puget Sound Pilots, Regeneron Pharmaceuticals/Sanofi; is/was a paid consultant to or speaker for Boston Celtics, Boston Red Sox, Cleveland Browns, Columbia River Bar Pilots, Ganésco, Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, M. Davis and Co, Physician's Seal, Samsung, State of Washington Board of Pilotage Commissioners, Tencent Holdings Ltd, Teva Pharma Australia, Vanda Pharmaceuticals Inc (in which Dr. Czeisler holds an equity interest), and With Deep, Inc. (in which he holds an equity interest); receives/received research/education support through BWH from Cephalon, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Harmony Biosciences LLC, Johnson & Johnson, NeuroCare, Inc., Philips Respironics Inc/Philips Homecare Solutions, Regional Home Care, Teva Pharmaceuticals Industries Ltd, Optum, ResMed, San Francisco Bar Pilots, Sanofi, Schneider, Simmons, Sysco, Philips, Vanda Pharmaceuticals; is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC, Amtrak; Casper Sleep Inc, C&J Energy Services, Complete General Construction Co, Dallas Police Association, Enterprise Rent-A-Car, Steel Warehouse, FedEx, Greyhound Lines, Palomar Health District, PAR Electrical Contractors, Product & Logistics Services LLC, Puckett Emergency Medical Services LLC, South Carolina Central Railroad Company LLC, Union Pacific Railroad, UPS, and Vanda Pharmaceuticals; serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc.; and receives royalties from McGraw Hill, and Philips Respironics for the Actiwatch-2 and Actiwatch Spectrum devices. Dr. Czeisler’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict-of-interest policies 3 FDA-approved treatments for circadian rhythm sleep-wake disorders I have contributed to:

Shift Work Disorder: Diagnostic Features Insomnia during daytime sleep or excessive sleepiness during night work, accompanied by reduced total sleep time Associated with work schedule that overlaps usual sleep time Symptoms cause clinically significant distress or impairment in important areas of functioning for at least 3 months Symptoms not better explained by another disorder Overnight and early morning work shifts (starting between 3:00 and 7:00 am) can also cause SWD Ref: International Classification of Sleep Disorders, 3rd Revision, 2014, American Academy of Sleep Medicine
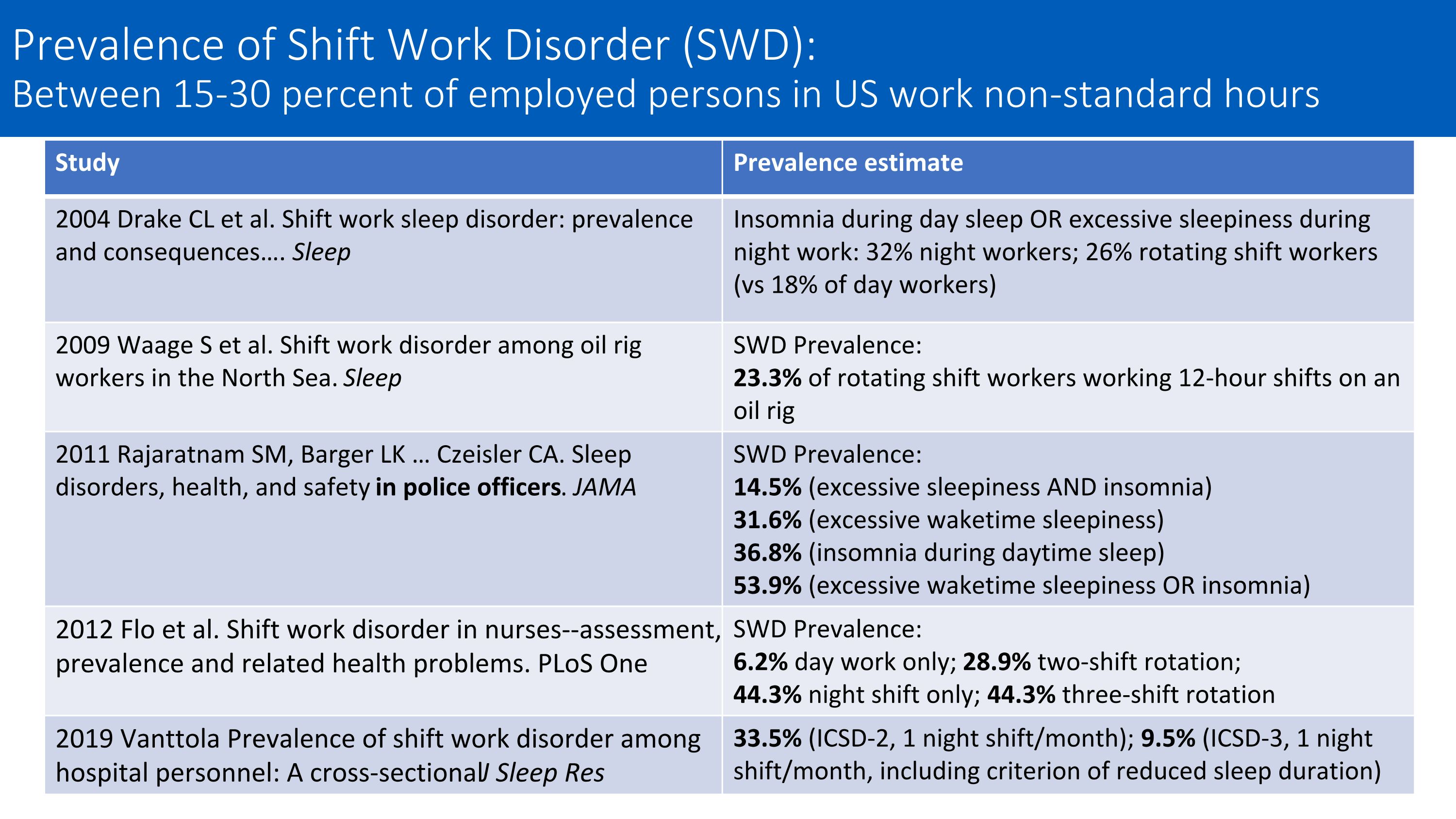
Study Prevalence estimate 2004 Drake CL et al. Shift work sleep disorder: prevalence and consequences…. Sleep Insomnia during day sleep OR excessive sleepiness during night work: 32% night workers; 26% rotating shift workers (vs 18% of day workers) 2009 Waage S et al. Shift work disorder among oil rig workers in the North Sea. Sleep SWD Prevalence: 23.3% of rotating shift workers working 12-hour shifts on an oil rig 2011 Rajaratnam SM, Barger LK … Czeisler CA. Sleep disorders, health, and safety in police officers. JAMA SWD Prevalence: 14.5% (excessive sleepiness AND insomnia) 31.6% (excessive waketime sleepiness) 36.8% (insomnia during daytime sleep) 53.9% (excessive waketime sleepiness OR insomnia) 2012 Flo et al. Shift work disorder in nurses--assessment, prevalence and related health problems. PLoS One SWD Prevalence: 6.2% day work only; 28.9% two-shift rotation; 44.3% night shift only; 44.3% three-shift rotation 2019 Vanttola Prevalence of shift work disorder among hospital personnel: A cross‐sectional J Sleep Res 33.5% (ICSD-2, 1 night shift/month); 9.5% (ICSD-3, 1 night shift/month, including criterion of reduced sleep duration) Prevalence of Shift Work Disorder (SWD): Between 15-30 percent of employed persons in US work non-standard hours
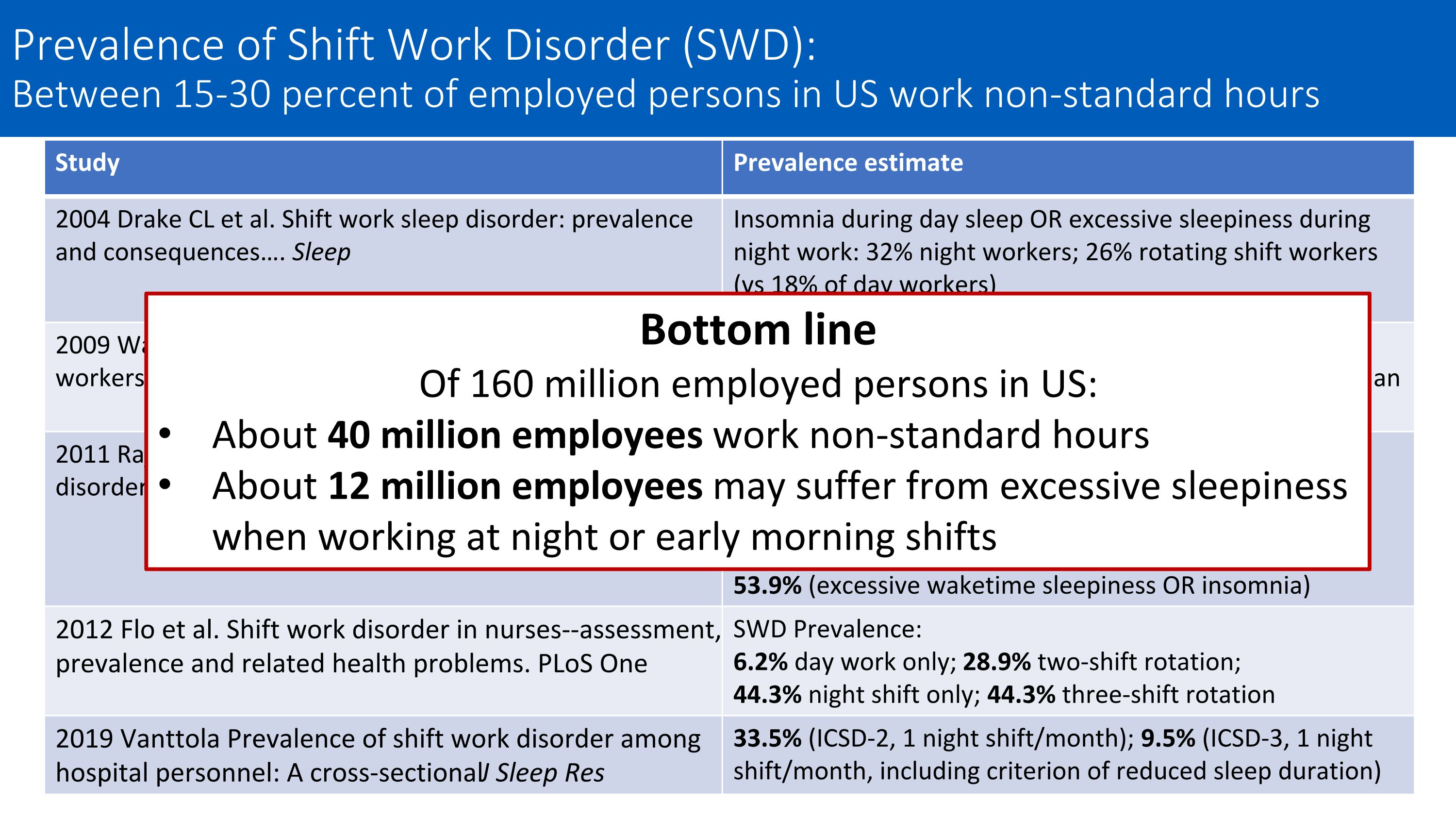
Study Prevalence estimate 2004 Drake CL et al. Shift work sleep disorder: prevalence and consequences…. Sleep Insomnia during day sleep OR excessive sleepiness during night work: 32% night workers; 26% rotating shift workers (vs 18% of day workers) 2009 Waage S et al. Shift work disorder among oil rig workers in the North Sea. Sleep SWD Prevalence: 23.3% of rotating shift workers working 12-hour shifts on an oil rig 2011 Rajaratnam SM, Barger LK … Czeisler CA. Sleep disorders, health, and safety in police officers. JAMA SWD Prevalence: 14.5% (excessive sleepiness AND insomnia) 31.6% (excessive waketime sleepiness) 36.8% (insomnia during daytime sleep) 53.9% (excessive waketime sleepiness OR insomnia) 2012 Flo et al. Shift work disorder in nurses--assessment, prevalence and related health problems. PLoS One SWD Prevalence: 6.2% day work only; 28.9% two-shift rotation; 44.3% night shift only; 44.3% three-shift rotation 2019 Vanttola Prevalence of shift work disorder among hospital personnel: A cross‐sectional J Sleep Res 33.5% (ICSD-2, 1 night shift/month); 9.5% (ICSD-3, 1 night shift/month, including criterion of reduced sleep duration) Bottom line Of 160 million employed persons in US: About 40 million employees work non-standard hours About 12 million employees may suffer from excessive sleepiness when working at night or early morning shifts Prevalence of Shift Work Disorder (SWD): Between 15-30 percent of employed persons in US work non-standard hours
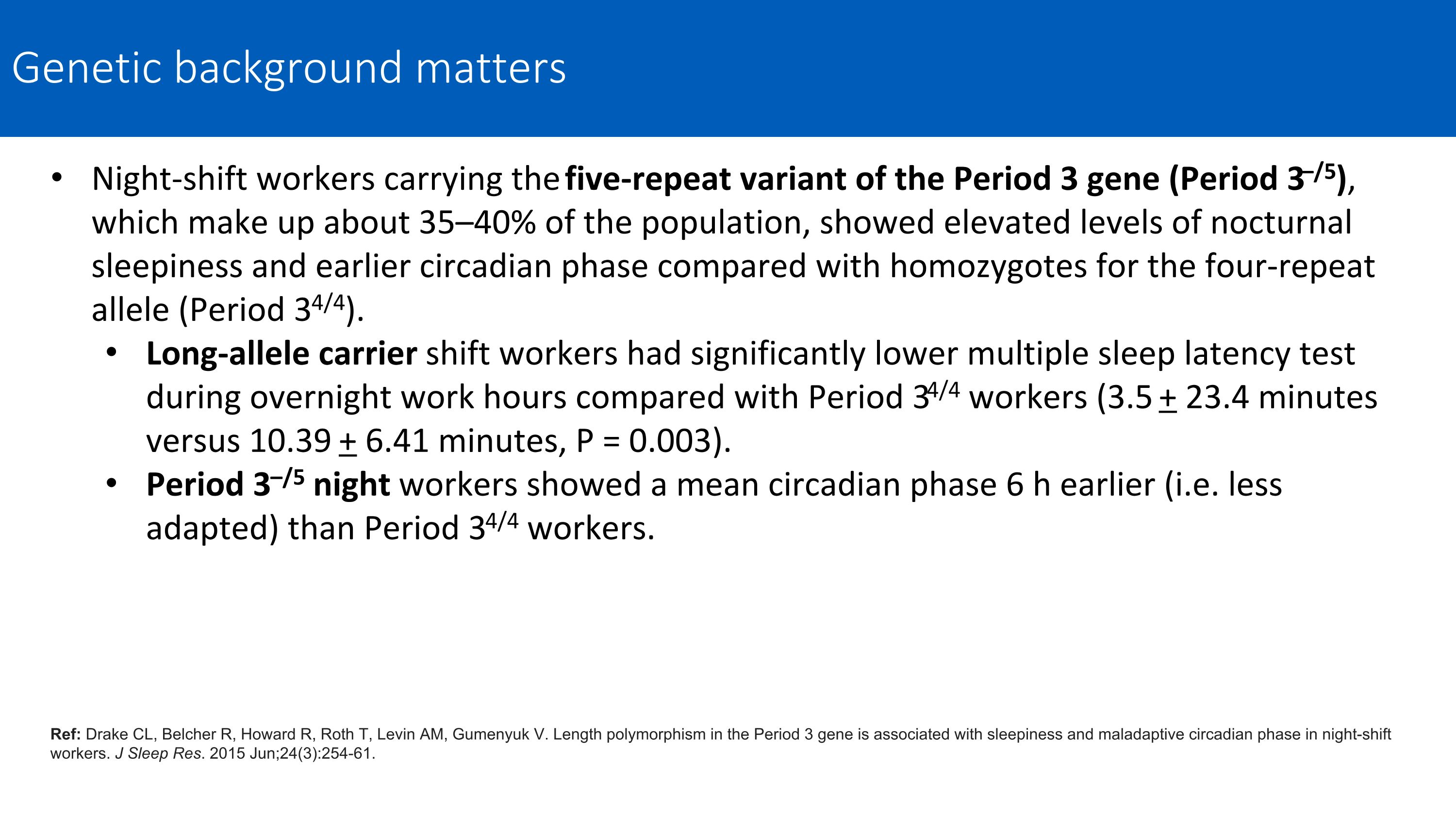
Night-shift workers carrying the five-repeat variant of the Period 3 gene (Period 3–/5), which make up about 35–40% of the population, showed elevated levels of nocturnal sleepiness and earlier circadian phase compared with homozygotes for the four-repeat allele (Period 34/4). Long-allele carrier shift workers had significantly lower multiple sleep latency test during overnight work hours compared with Period 34/4 workers (3.5 + 23.4 minutes versus 10.39 + 6.41 minutes, P = 0.003). Period 3–/5 night workers showed a mean circadian phase 6 h earlier (i.e. less adapted) than Period 34/4 workers. Ref: Drake CL, Belcher R, Howard R, Roth T, Levin AM, Gumenyuk V. Length polymorphism in the Period 3 gene is associated with sleepiness and maladaptive circadian phase in night-shift workers. J Sleep Res. 2015 Jun;24(3):254-61. Genetic background matters
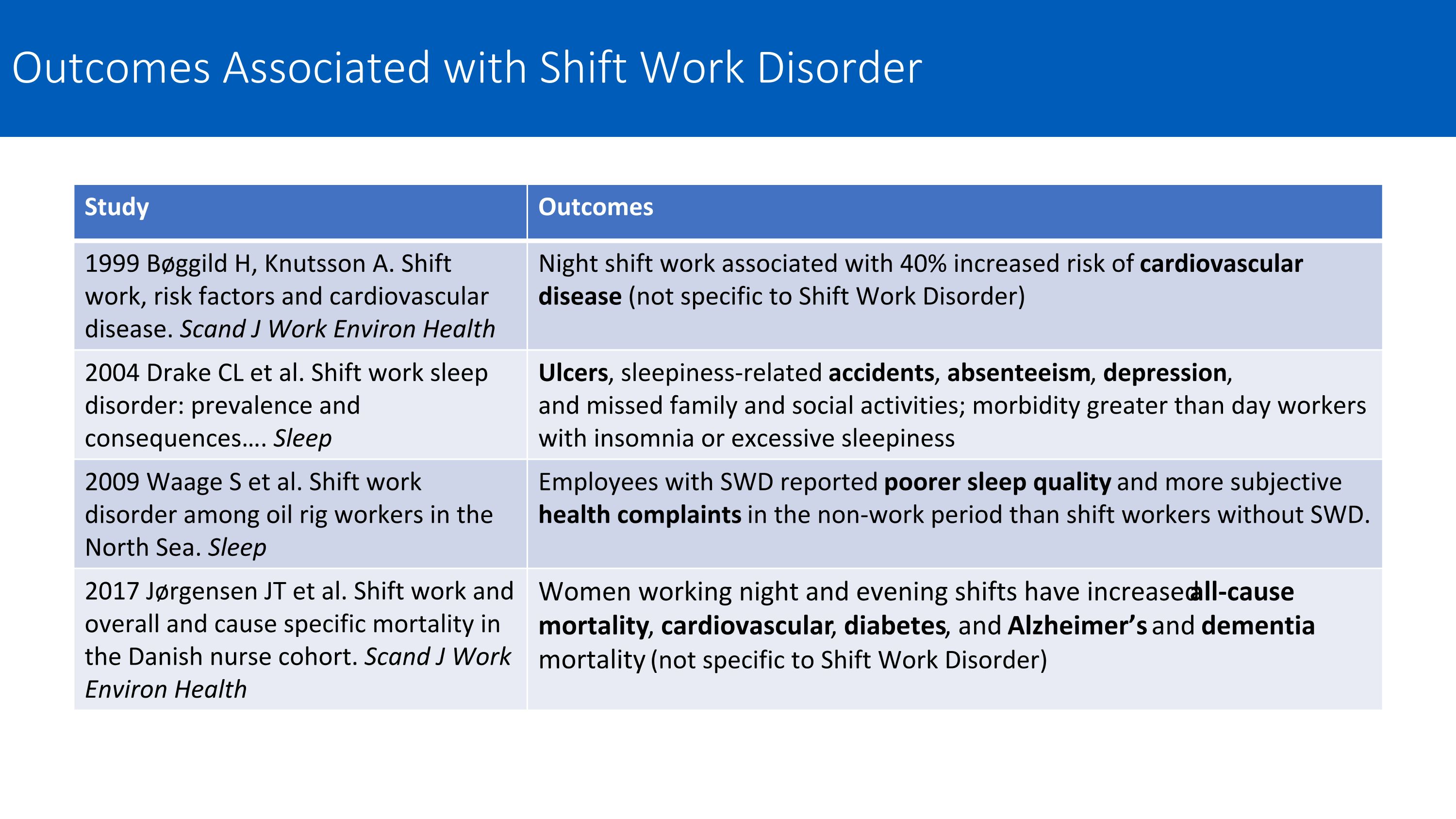
Study Outcomes 1999 Bøggild H, Knutsson A. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health Night shift work associated with 40% increased risk of cardiovascular disease (not specific to Shift Work Disorder) 2004 Drake CL et al. Shift work sleep disorder: prevalence and consequences…. Sleep Ulcers, sleepiness-related accidents, absenteeism, depression, and missed family and social activities; morbidity greater than day workers with insomnia or excessive sleepiness 2009 Waage S et al. Shift work disorder among oil rig workers in the North Sea. Sleep Employees with SWD reported poorer sleep quality and more subjective health complaints in the non-work period than shift workers without SWD. 2017 Jørgensen JT et al. Shift work and overall and cause specific mortality in the Danish nurse cohort. Scand J Work Environ Health Women working night and evening shifts have increased all-cause mortality, cardiovascular, diabetes, and Alzheimer’s and dementia mortality (not specific to Shift Work Disorder) Outcomes Associated with Shift Work Disorder
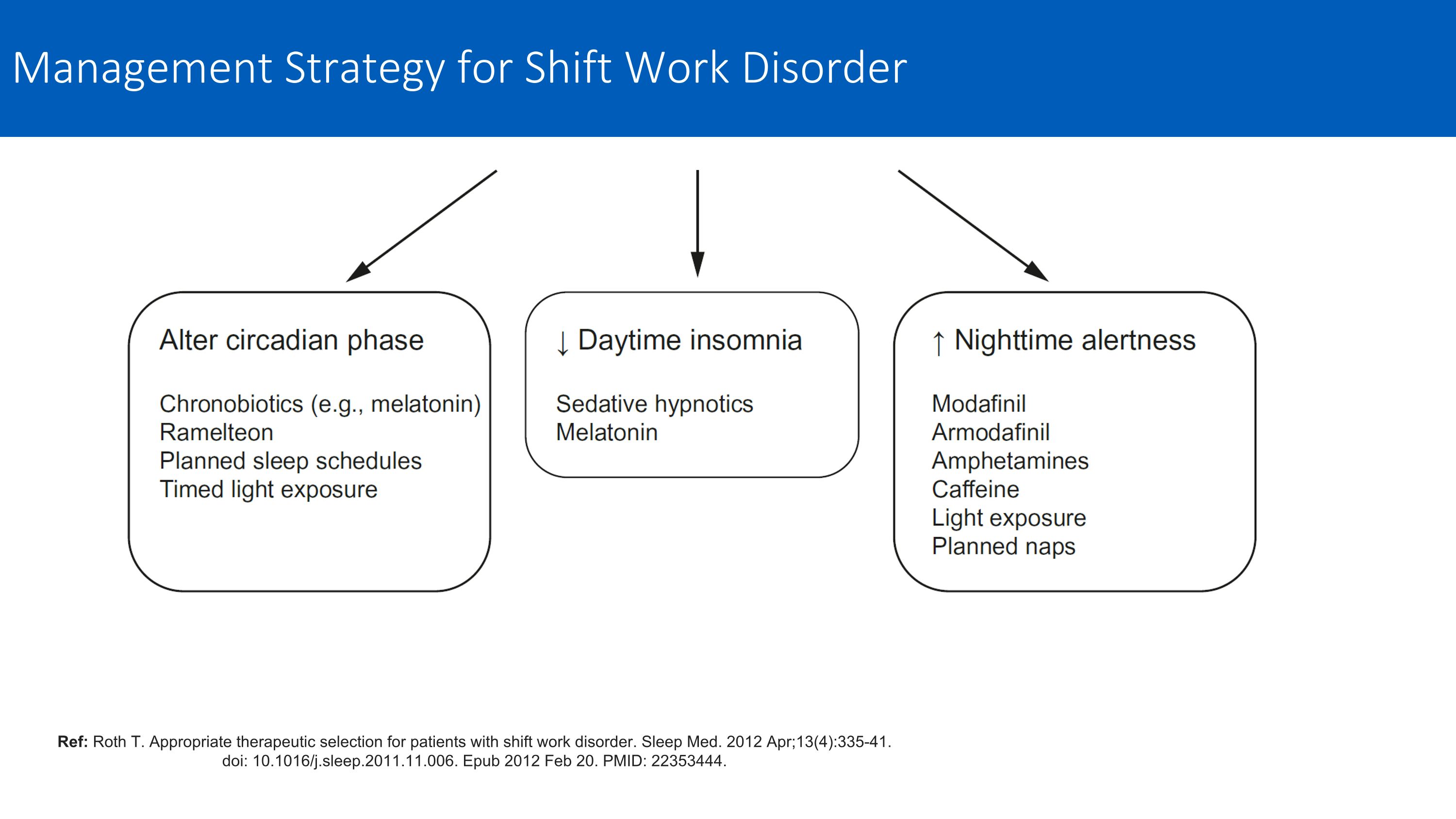
Ref: Roth T. Appropriate therapeutic selection for patients with shift work disorder. Sleep Med. 2012 Apr;13(4):335-41. doi: 10.1016/j.sleep.2011.11.006. Epub 2012 Feb 20. PMID: 22353444. Management Strategy for Shift Work Disorder

“Given the methodological diversity of the included studies, in terms of interventions, settings, and assessment tools, their limited reporting and the very low to low quality of the evidence they present, it is not possible to determine whether shift workers' sleepiness can be reduced or if their sleep length or quality can be improved with these interventions. We found 17 randomized controlled trials (with 556 participants) to include in this review. We rated the quality of evidence provided by most of the included studies to be between low and very low. The studies could be divided into three different types of interventions: (1) exposure to bright light; (2) a napping opportunity during the night shift; or (3) others, like physical activity or sleep education. We need better and adequately powered Randomized Clinical Trials of the effect of bright light, and naps, either on their own or together and other nonpharmacological interventions that also consider shift workers’ chronobiology on the investigated sleep parameters.” Refs: Slanger TE, Gross JV, Pinger A, Morfeld P, Bellinger M, Duhme AL, Reichardt Ortega RA, Costa G, Driscoll TR, Foster RG, Fritschi L, Sallinen M, Liira J, Erren TC. Person-directed, non-pharmacological interventions for sleepiness at work and sleep disturbances caused by shift work. Cochrane Database Syst Rev. 2016 Aug 23;2016(8):CD010641. doi: 10.1002/14651858.CD010641.pub2. PMID: 27549931; PMCID: PMC8406755. Efficacy of non-pharmacological interventions for sleepiness during night work not established
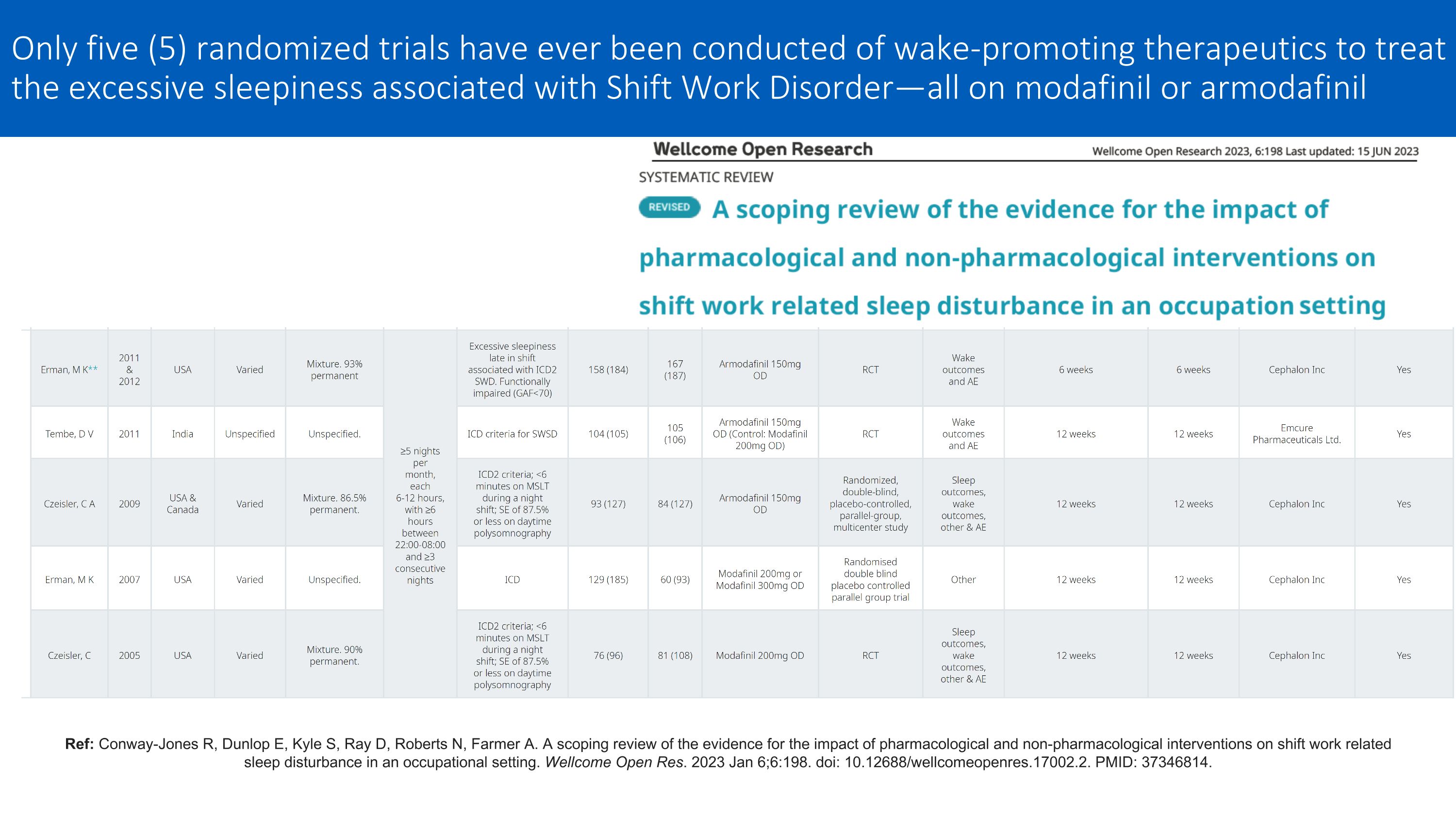
Ref: Conway-Jones R, Dunlop E, Kyle S, Ray D, Roberts N, Farmer A. A scoping review of the evidence for the impact of pharmacological and non-pharmacological interventions on shift work related sleep disturbance in an occupational setting. Wellcome Open Res. 2023 Jan 6;6:198. doi: 10.12688/wellcomeopenres.17002.2. PMID: 37346814. Only five (5) randomized trials have ever been conducted of wake-promoting therapeutics to treat the excessive sleepiness associated with Shift Work Disorder—all on modafinil or armodafinil

FDA Registration Trial for ES associated with a CRSWD
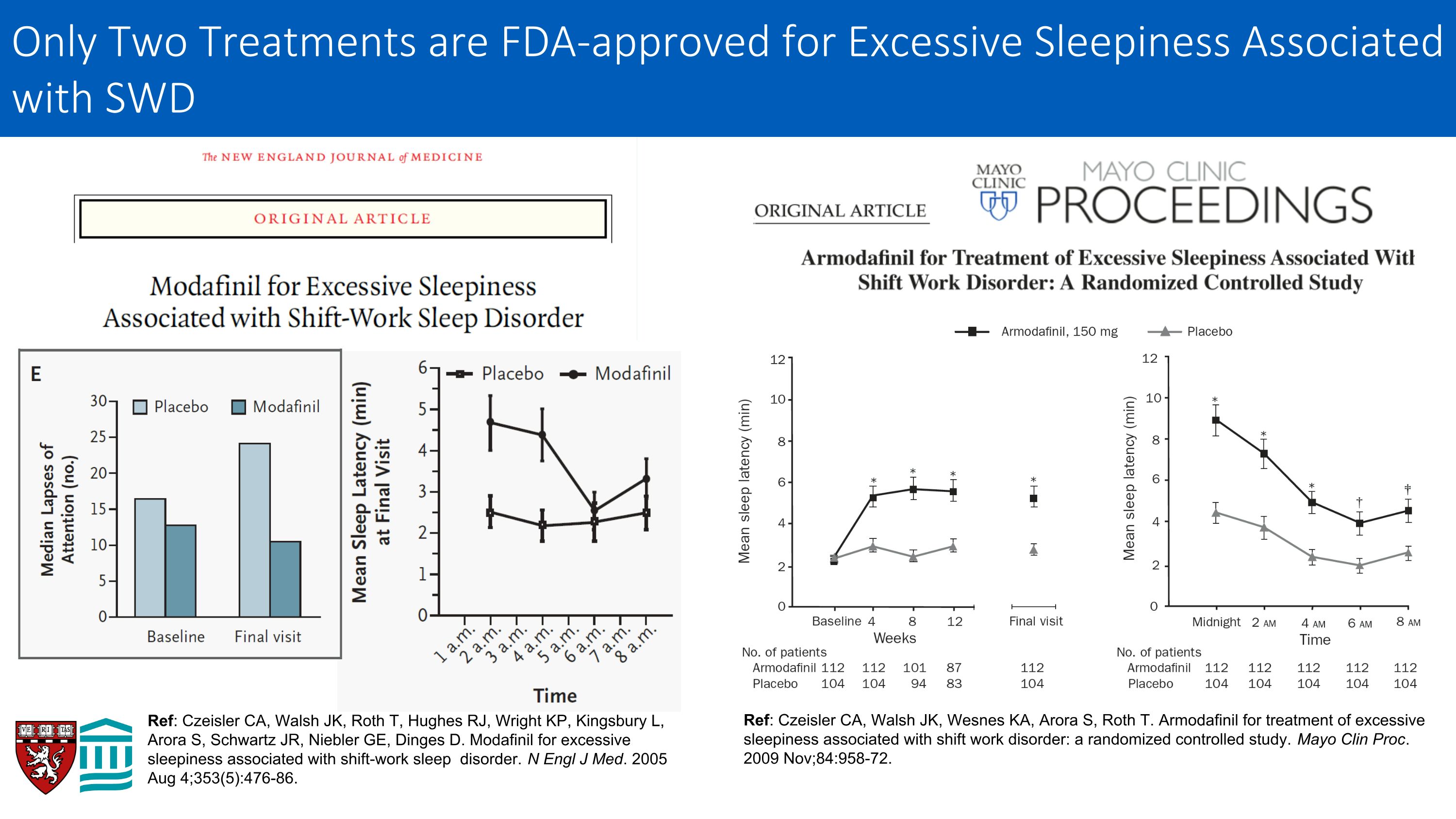
Ref: Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, Arora S, Schwartz JR, Niebler GE, Dinges D. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005 Aug 4;353(5):476-86. Ref: Czeisler CA, Walsh JK, Wesnes KA, Arora S, Roth T. Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study. Mayo Clin Proc. 2009 Nov;84:958-72. Only Two Treatments are FDA-approved for Excessive Sleepiness Associated with SWD
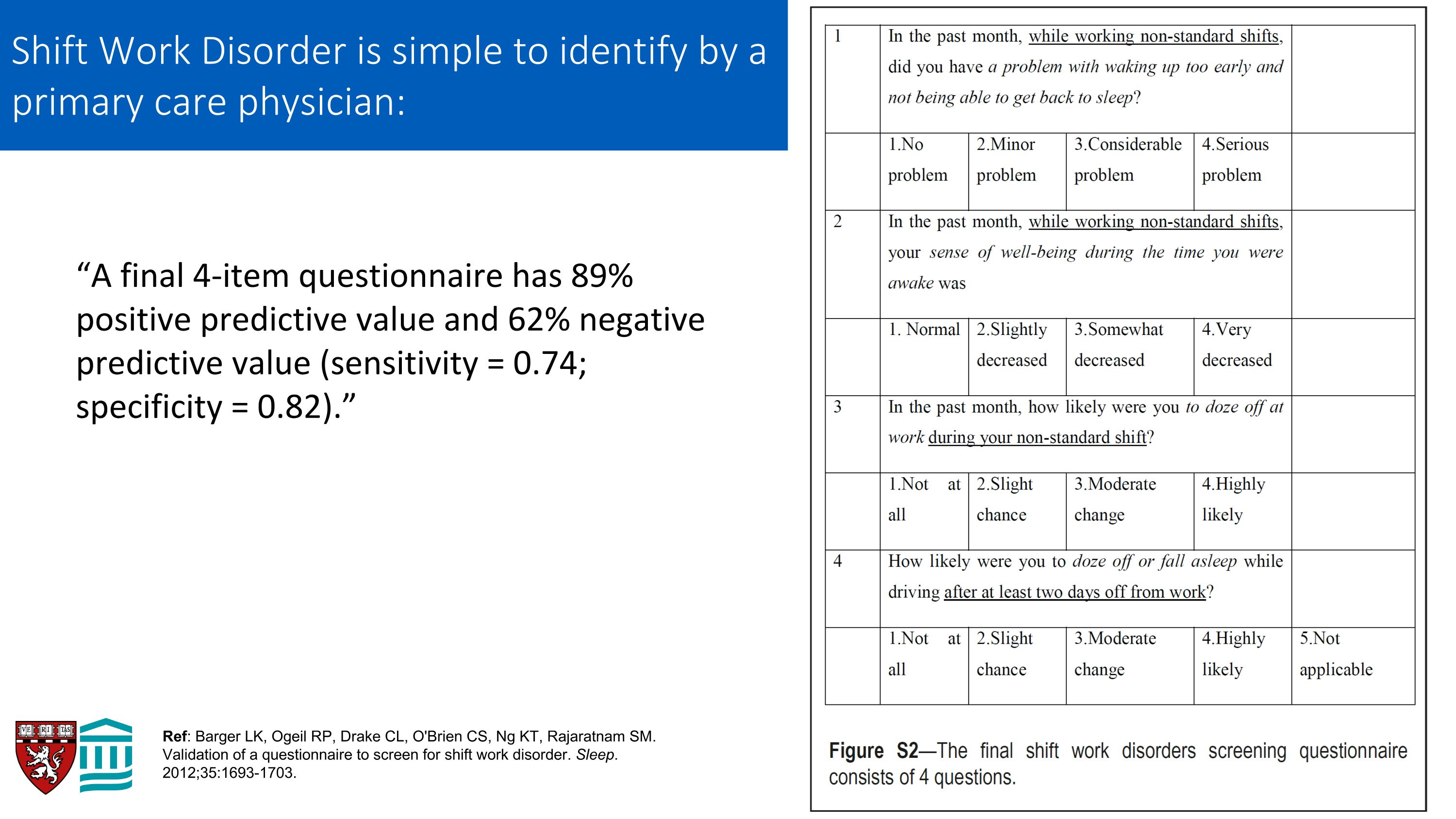
“A final 4-item questionnaire has 89% positive predictive value and 62% negative predictive value (sensitivity = 0.74; specificity = 0.82).” Ref: Barger LK, Ogeil RP, Drake CL, O'Brien CS, Ng KT, Rajaratnam SM. Validation of a questionnaire to screen for shift work disorder. Sleep. 2012;35:1693-1703. Shift Work Disorder is simple to identify by a primary care physician:
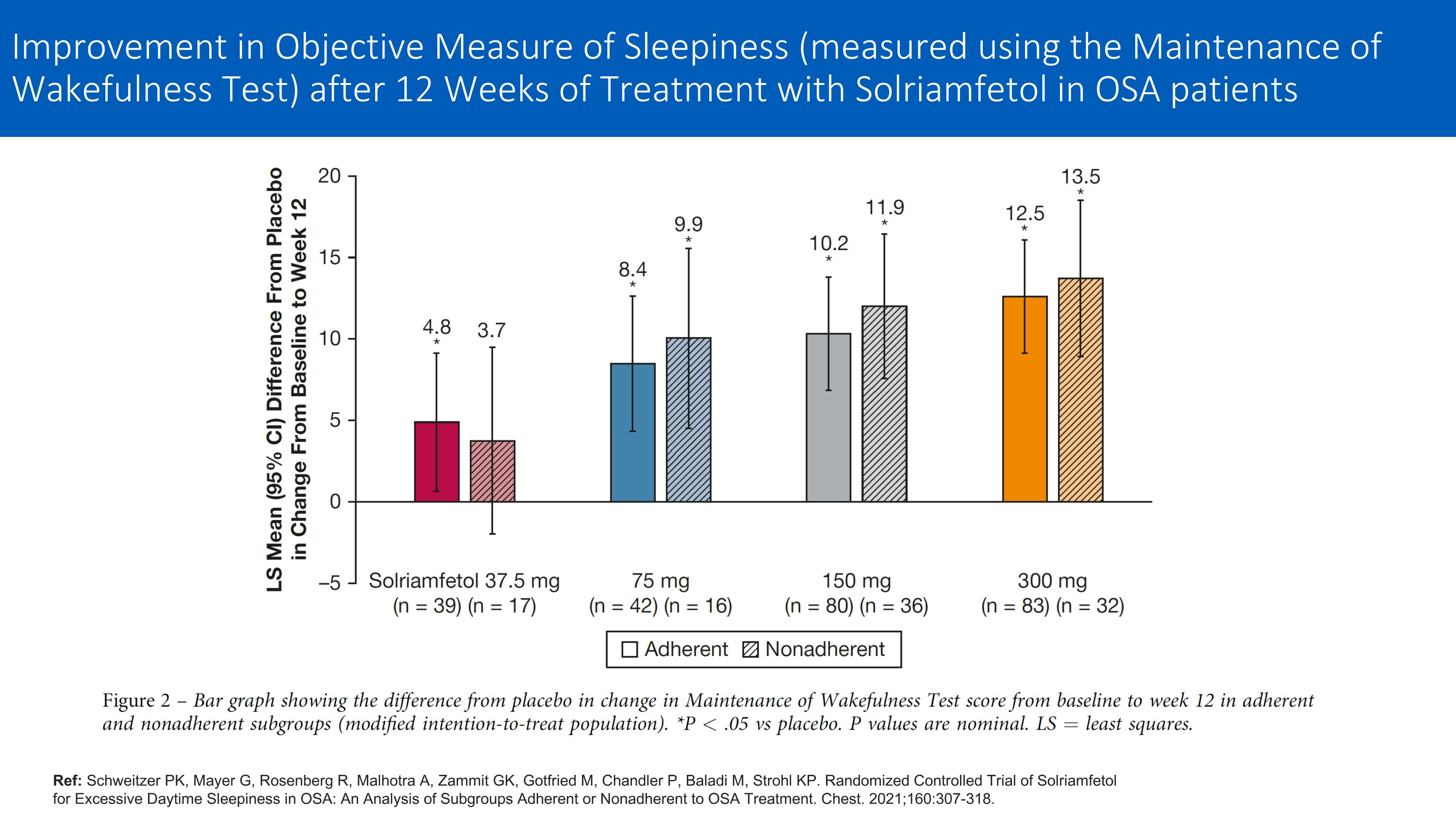
Ref: Schweitzer PK, Mayer G, Rosenberg R, Malhotra A, Zammit GK, Gotfried M, Chandler P, Baladi M, Strohl KP. Randomized Controlled Trial of Solriamfetol for Excessive Daytime Sleepiness in OSA: An Analysis of Subgroups Adherent or Nonadherent to OSA Treatment. Chest. 2021;160:307-318. Improvement in Objective Measure of Sleepiness (measured using the Maintenance of Wakefulness Test) after 12 Weeks of Treatment with Solriamfetol in OSA patients
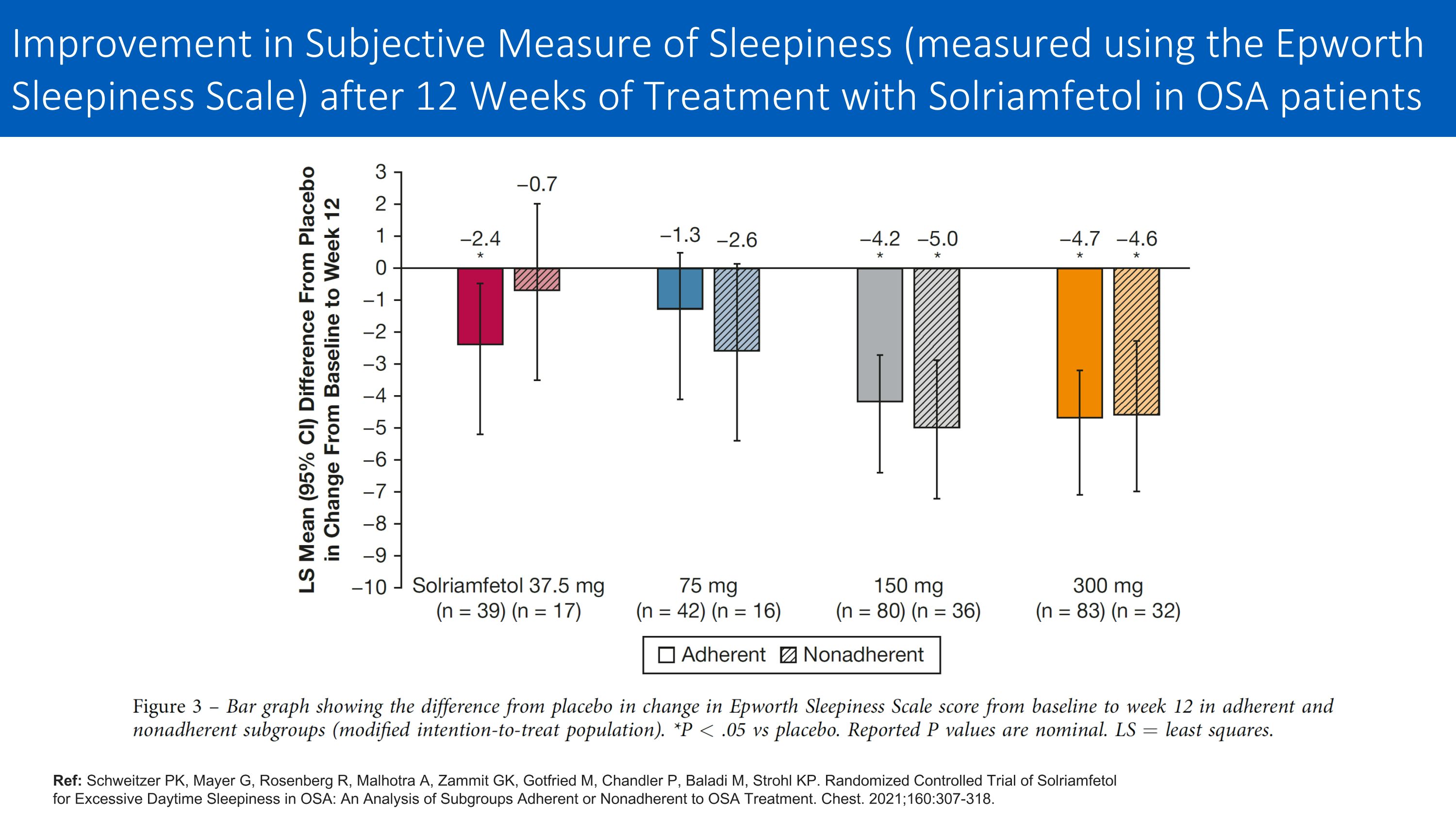
Ref: Schweitzer PK, Mayer G, Rosenberg R, Malhotra A, Zammit GK, Gotfried M, Chandler P, Baladi M, Strohl KP. Randomized Controlled Trial of Solriamfetol for Excessive Daytime Sleepiness in OSA: An Analysis of Subgroups Adherent or Nonadherent to OSA Treatment. Chest. 2021;160:307-318. Improvement in Subjective Measure of Sleepiness (measured using the Epworth Sleepiness Scale) after 12 Weeks of Treatment with Solriamfetol in OSA patients
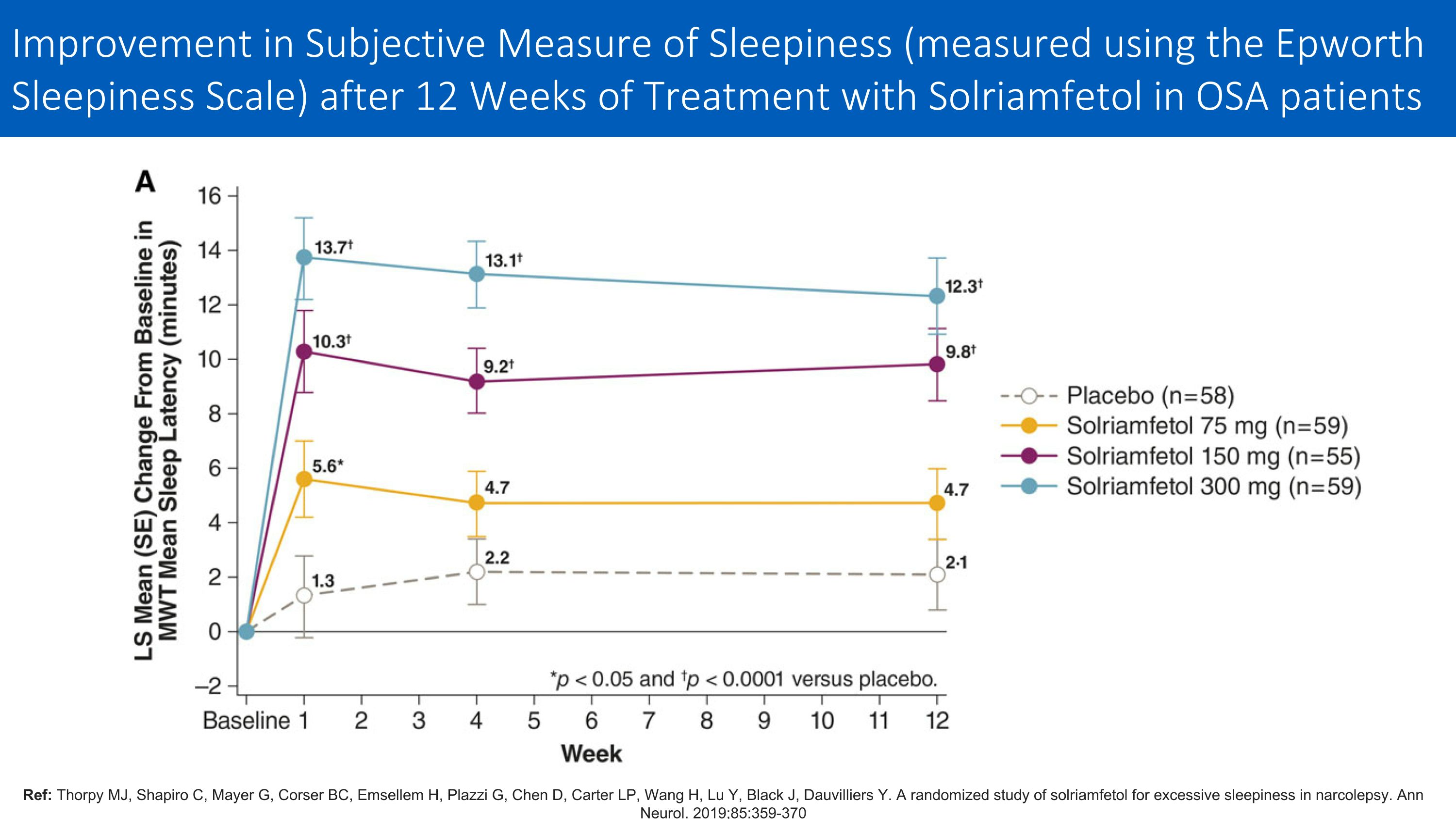
Ref: Thorpy MJ, Shapiro C, Mayer G, Corser BC, Emsellem H, Plazzi G, Chen D, Carter LP, Wang H, Lu Y, Black J, Dauvilliers Y. A randomized study of solriamfetol for excessive sleepiness in narcolepsy. Ann Neurol. 2019;85:359-370 Improvement in Subjective Measure of Sleepiness (measured using the Epworth Sleepiness Scale) after 12 Weeks of Treatment with Solriamfetol in OSA patients

Shift Work Disorder: Need for Treatment Shift work disorder affects about 12 million American workers Patients with shift work disorder are at increased risk of errors and accidents, including motor vehicle crashes, and adverse health outcomes Evidence for efficacy of commonly used non-pharmacological interventions for excessive sleepiness in SWD is weak Only two pharmacological treatments (modafinil and armodafinil) have been FDA-approved for shift work disorder, and EMA withdrew approval for modafinil after post-marketing severe skin reactions Solriamfetol effective in treating excessive sleepiness in OSA and narcolepsy
on Cognition and BED Q+A Richard Bogan, MD Susan McElroy, MD

Closing Remarks Herriot Tabuteau, MD

thank you
v3.23.3
Document And Entity Information
|
Dec. 07, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Dec. 07, 2023
|
| Entity Registrant Name |
Axsome Therapeutics, Inc.
|
| Entity Central Index Key |
0001579428
|
| Entity Emerging Growth Company |
false
|
| Entity File Number |
001-37635
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Tax Identification Number |
45-4241907
|
| Entity Address, Address Line One |
One World Trade Center, 22nd Floor
|
| Entity Address, City or Town |
New York
|
| Entity Address, State or Province |
NY
|
| Entity Address, Postal Zip Code |
10007
|
| City Area Code |
(212)
|
| Local Phone Number |
332-3241
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common Stock, Par Value $0.0001 Per Share
|
| Trading Symbol |
AXSM
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Axsome Therapeutics (NASDAQ:AXSM)
Historical Stock Chart
From Mar 2024 to Apr 2024
Axsome Therapeutics (NASDAQ:AXSM)
Historical Stock Chart
From Apr 2023 to Apr 2024