UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of
the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
October 23, 2023
Citius Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
Nevada
(State or other jurisdiction of incorporation)
| 001-38174 |
|
27-3425913 |
| (Commission File Number) |
|
(IRS Employer
Identification No.) |
|
11 Commerce Drive, 1st Floor,
Cranford, NJ |
|
07016 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code (908) 967-6677
Check the appropriate box
below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following
provisions:
| |
☒ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
|
| |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
|
| |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
|
| |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common stock, $0.001 par value |
|
CTXR |
|
The Nasdaq Capital Market |
Indicate by check mark whether
the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule
12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01. Entry into a Material Definitive
Agreement.
On October 23, 2023, Citius
Pharmaceuticals, Inc. (“Citius Pharma”) and Citius Oncology, Inc. (“SpinCo”), a wholly owned subsidiary of Citius
Pharma, entered into an agreement and plan of merger and reorganization (the “Merger Agreement”) with TenX Keane Acquisition,
a Cayman Islands exempted company (“TenX”), and TenX Merger Sub Inc., a Delaware corporation and a wholly owned subsidiary
of TenX (“Merger Sub”).
The Merger Agreement provides,
among other things, (i) on the terms and subject to the conditions set forth therein, that Merger Sub will merge with and into SpinCo,
with SpinCo surviving as a wholly owned subsidiary of TenX (the “Merger”), and (ii) that prior to the effective time of the
Merger (the “Effective Time”), TenX will migrate to and domesticate as a Delaware corporation in accordance with Section 388
of the General Corporation Law of the State of Delaware and the Cayman Islands Companies Act (As Revised) (the “Domestication”).
Upon the closing of the Merger (the “Closing”), it is anticipated that TenX will change its name to “Citius Oncology,
Inc.” (“New Citius Oncology”). The Domestication, Merger and the other transactions contemplated by the Merger Agreement
are referred to herein as the “Business Combination”. Shares of TenX common stock following the Domestication are referred
to herein as “New Citius Oncology Shares”. The date on which the Closing actually occurs is referred to herein as the “Closing
Date”.
The Merger Agreement, Business
Combination and the transactions contemplated thereby were unanimously approved by the boards of directors of each of Citius Pharma, SpinCo
and TenX.
Consideration and Structure
In accordance with the terms
and subject to the conditions of the Merger Agreement, at the Effective Time of the Merger and following the Domestication: (i) each share
of common stock of SpinCo issued and outstanding immediately prior to the Effective Time will be cancelled and automatically converted
into the right to receive, without interest, a number of New Citius Oncology Shares equal to the Base Exchange Ratio (as defined below);
and (ii) each option to purchase a share of SpinCo common stock that is then outstanding will be converted into the right to receive an
option to purchase a number of New Citius Oncology Shares as determined by the Base Exchange Ratio with substantially the same terms and
conditions as are in effect with respect to such option immediately prior to the Effective Time, with the exercise price thereof adjusted
by the Base Exchange Ratio. TenX and Citius Pharma negotiated and set the Base Exchange Ratio based on their assumptions about the value
of New Citius Oncology following the Merger. The “Base Exchange Ratio” is the quotient of (a) 67,500,000, divided by (b) the
aggregate number of shares of SpinCo Common Stock outstanding as of immediately prior to the Effective Time.
The Business Combination is
expected to close in the first half of 2024, subject to the closing conditions, as further described below.
Representations and Warranties; Covenants
The parties to the Merger
Agreement have agreed to customary representations and warranties for transactions of this type. In addition, the parties to the Merger
Agreement have agreed to be bound by certain customary covenants for transactions of this type, including, among others, covenants with
respect to the conduct of Citius Pharma, SpinCo, TenX and Merger Sub during the period between execution of the Merger Agreement and the
Closing. The representations, warranties, agreements and covenants of the parties set forth in the Merger Agreement will terminate at
the Closing, except for those covenants and agreements that, by their terms, contemplate performance after the Closing. Each of the parties
to the Merger Agreement has agreed to use its reasonable best efforts to take or cause to be taken all actions and things necessary, proper
or advisable to consummate the Business Combination.
Conditions to Closing
Under the Merger Agreement,
the obligations of each of Citius Pharma, SpinCo, TenX and Merger Sub to consummate the Merger are subject to the satisfaction or waiver
of certain customary closing conditions of the respective parties, including, among others: (i) all requisite regulatory approvals, including
under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 and the rules and regulations promulgated thereunder, have been obtained
and remain in full force and effect and all statutory waiting periods in respect thereof have expired or been terminated; (ii) the registration
statement and proxy statement/prospectus to be filed by TenX relating to the issuance of New Citius Oncology Shares pursuant to the Merger
Agreement (the “Registration Statement”) becoming effective under the Securities Act of 1933, as amended (the “Securities
Act”) and no actual or threatened stop order suspending the effectiveness of the Registration Statement having been issued; (iii)
the approval and adoption of the Merger Agreement and the transactions contemplated thereby by the requisite vote of TenX’s shareholders
(the “Parent Shareholder Approval”) and the requisite approval of Citius Pharma, as SpinCo’s sole shareholder; (iv)
no existence of any order, statute, rule or regulation enjoining or prohibiting the consummation of the Business Combination; and (v)
TenX having at least $5,000,001 in net tangible assets (as determined in accordance with Rule 3a51(g)(1) of the Securities Exchange Act
of 1934, as amended (the “Exchange Act”)) at or prior to the Effective Time.
The obligation of Citius Pharma
and SpinCo to consummate the Merger is also subject to the satisfaction or waiver of certain other closing conditions, including, among
others: (i) the absence of a Parent Material Adverse Effect (as defined in the Merger Agreement) since the date of the Merger Agreement;
(ii) the Domestication having been completed; (iii) TenX having not received any written notice from The Nasdaq Stock Market LLC (“Nasdaq”)
that it has failed, or would reasonably be expected to fail to meet the Nasdaq initial or continued listing requirements as of the Closing
Date for any reason, where such notice has not been subsequently withdrawn by Nasdaq or the underlying failure appropriately remedied
or satisfied; and (v) the Sponsor (as defined below) having paid all Estimated Parent Transaction Expenses (as defined in the Merger Agreement)
in excess of $500,000, if any.
The obligation of TenX and
the Merger Sub to consummate the Business Combination is also subject to the satisfaction or waiver of certain other closing conditions,
including, among others, the absence of a SpinCo Material Adverse Effect (as defined in the Merger Agreement) since the date of the Merger
Agreement.
Termination
The Merger Agreement may be
terminated under certain customary and limited circumstances at any time prior to the Closing, including, but not limited to: (i) by the
mutual written consent of Citius Pharma and TenX; (ii) by either Citius Pharma or TenX if prior to the Closing the FDA issues a complete
response letter in response to any resubmission of Citius Pharma’s biologics license application for LymphirTM (denileukin
diftitox) to the U.S. Food and Drug Administration (iii) by written notice from Citius Pharma or TenX, if the Closing has not occurred
on or prior to September 30, 2024 (the “Outside Date”); (iv) by either Citius Pharma or TenX, if any law or order prohibiting,
restraining or making illegal the consummation of the Merger is in effect and has become final and non-appealable; (v) by TenX, subject
to certain exceptions, if Citius Pharma or SpinCo breach of any representation, warranty, covenant or agreement or any such representation
and warranty becomes untrue or inaccurate after the date of the Merger Agreement, such that certain conditions would not be satisfied
at the Effective Time, and which, (i) is not cured by the Citius Pharma or SpinCo by the earlier of: (x) 15 days after receipt of written
notice thereof; or (y) the Outside Date, or (ii) is incapable of being cured prior to the Outside Date; (vi) by Citius Pharma, subject
to certain exceptions, if TenX or Merger Sub breach of any representation, warranty, covenant or agreement or any such representation
and warranty becomes untrue or inaccurate after the date of the Merger Agreement, such that certain conditions would not be satisfied
at the Effective Time, and which, (i) is not cured by TenX or Merger Sub by the earlier of: (x) 15 days after receipt of written notice
thereof; or (y) the Outside Date, or (ii) is incapable of being cured prior to the Outside Date; (vii) by Citius Pharma or TenX, if the
Parent Shareholder Approval is not obtained or if TenX shareholders do not approve amending TenX’s governing documents to extend
the final date on which TenX must consummate a business combination; and (viii) by Citius Pharma, prior to February 29, 2024, in order
to accept an Acquisition Proposal (as defined in the Merger Agreement), provided, that Citius Pharma pays a $5,000,000 Termination Fee
(as defined in the Merger Agreement) to TenX.
If the Merger Agreement is
validly terminated, none of the parties to the Merger Agreement will have any liability or any further obligation under the Merger Agreement,
other than customary confidentiality obligations, except with respect to (i) liabilities for any Actual Fraud or Willful Breach (each,
as defined in the Merger Agreement) (ii) any due but unpaid portion of any applicable Extension Fee (as defined in the Merger Agreement)
owed by Citius Pharma, or (iii) the Termination Fee, as applicable, and as described above.
The foregoing description
of the Merger Agreement and the Business Combination does not purport to be complete and is qualified in its entirety by reference to
the Merger Agreement, a copy of which is filed with this Current Report on Form 8-K as Exhibit 2.1 and is incorporated herein by reference.
The Merger Agreement contains representations, warranties and covenants that the respective parties made to each other as of the date
of the Merger Agreement or other specific dates. The assertions embodied in those representations, warranties and covenants were made
for purposes of the contract among the respective parties and are subject to important qualifications and limitations agreed to by the
parties in connection with negotiating the Merger Agreement. The Merger Agreement is being filed to provide investors with information
regarding its terms. It is not intended to provide any other factual information about the parties to the Merger Agreement. In particular,
the representations, warranties, covenants and agreements contained in the Merger Agreement, which were made only for purposes of the
Merger Agreement and as of specific dates, were solely for the benefit of the parties to the Merger Agreement, may be subject to limitations
agreed upon by the contracting parties (including being qualified by confidential disclosures made for the purposes of allocating contractual
risk between the parties to the Merger Agreement instead of establishing these matters as facts) and may be subject to standards of materiality
applicable to the contracting parties that differ from those applicable to investors, security holders and reports and documents filed
with the SEC. Investors and security holders are not third-party beneficiaries under the Merger Agreement and should not rely on the representations,
warranties, covenants and agreements or any descriptions thereof, as characterizations of the actual state of facts or condition of any
party to the Merger Agreement. In addition, the representations, warranties, covenants and agreements and other terms of the Merger Agreement
may be subject to subsequent waiver or modification by the parties.
Other Agreements
The Merger Agreement contemplates
the execution of various additional agreements and instruments, on or before the Closing Date, including, among others, the following:
Sponsor Support Agreement
Concurrently with the execution
of the Merger Agreement, 10XYZ Holdings LP, a Delaware limited partnership (the “Sponsor”),
entered into a support agreement with TenX, Citius Pharma and SpinCo (the “Sponsor Support Agreement”), pursuant to which
the Sponsor agreed to, among other things: (i) vote in favor of the Transaction Proposals (as defined in the Merger Agreement) and in
favor of any proposal in respect of an Extension Amendment (as defined in the Merger Agreement); (ii) vote against (or withhold written
consent of) any business combination or any proposal relating thereto (in each case, other than as contemplated by the Merger Agreement);
(iii) vote against (or withhold written consent of) any merger agreement or merger, consolidation, combination, sale of substantial assets,
reorganization, recapitalization, dissolution, liquidation or winding up of or by TenX (other than the Merger Agreement and the transactions
contemplated thereby); (iv) vote against (or withhold written consent of) any change in the business, management or board of directors
of TenX (other than as contemplated by the Merger Agreement); (v) vote against (or withhold written consent of) any proposal, action
or agreement that would (A) impede, frustrate, prevent or nullify any provision of the Sponsor Support Agreement or the Merger Agreement
or any of the transactions contemplated thereby, (B) result in a breach in any respect of any covenant, representation, warranty or any
other obligation or agreement of TenX or Merger Sub under the Merger Agreement, (C) result in any of the conditions set forth in Section
8.1(c), Section 8.1(f) or Section 8.2 of the Merger Agreement not being fulfilled or (D) change in any manner the dividend policy or
capitalization of TenX, including the voting rights of any class of capital stock of TenX; and (vi) pay in full any Parent Estimated
Transaction Expenses in excess of $500,000, if any.
Additionally, the Sponsor
Support Agreement provides that, following the Closing, New Citius Oncology will reimburse the Sponsor for amounts outstanding under the
promissory notes TenX issued to the Sponsor evidencing the Sponsor’s deposits into the Trust Account (as defined in the Investment
Management Trust Agreement, dated as of October 13, 2022, between TenX and American Stock Transfer & Trust Company, LLC, as trustee
(the “Trust Agreement”)) to extend the timeline to complete a business combination (the “Extension Promissory Notes”).
New Citius Oncology will be obligated to make such reimbursements only if, following payment in respect of any redemptions by TenX’s
shareholders, at least $2,000,000 remains of the liquidated Trust Account Property (as defined in the Trust Agreement) and New Citius
Oncology has reimbursed Citius Pharma pursuant to Section 7.16(b)(iii) of the Merger Agreement. New Citius Oncology’s reimbursement
of amounts outstanding under the Extension Promissory Notes may only be made using amounts remaining of the liquidated Trust Account Property.
To the extent the Extension Promissory Notes are not reimbursed in full, such amounts outstanding will convert into New Citius Oncology
common stock.
The foregoing description
of the Sponsor Support Agreement does not purport to be complete and is qualified in its entirety by the terms and conditions of the Sponsor
Support Agreement, a copy of which is attached as Exhibit 10.1 hereto, and the terms of which are incorporated herein by reference.
Amended and Restated Registration Rights Agreement
The Merger Agreement contemplates
that, at or prior to the Closing, TenX (as New Citius Oncology), the Sponsor and certain of their respective affiliates and certain stockholders
of Citius Oncology will enter into an Amended and Restated Registration Rights Agreement (the “Registration Rights Agreement”),
which, among other things, will govern the registration of certain New Citius Oncology Shares for resale and be effective as of the Closing.
Additionally, the Sponsor
and certain stockholders of Citius Oncology who are a party to the agreement have restrictions on transferring New Citius Oncology Shares
(or any security convertible into, or exercisable or exchangeable for New Citius Oncology Shares) beginning at Closing until the date
that is six months after Closing; provided that the restrictions may be lifted early if (a) the price of the New Citius Oncology Shares
equals or exceeds $12.00 per share for any 20 trading days within any 30-day trading period, or (b) New Citius Oncology completes a transaction
that results in public shareholders having the right to exchange their New Citius Oncology Shares for cash, securities or other property.
The foregoing description
of the Registration Rights Agreement does not purport to be complete and is qualified in its entirety by the terms and conditions of the
Registration Rights Agreement, a form of which is attached as Exhibit 10.2 hereto, and the terms of which are incorporated herein by reference.
Amended and Restated Shared Services Agreement
The Merger Agreement contemplates
that, at the Closing, Citius Pharma and New Citius Oncology will enter into an amended and restated shared services agreement (the “Shared
Services Agreement”), which, among other things, will govern certain management and scientific services that Citius Pharma will
provide New Citius Oncology and will be effective as of the Closing.
The foregoing description
of the Shared Services Agreement does not purport to be complete and is qualified in its entirety by the terms and conditions of the Shared
Services Agreement, a form of which is attached as Exhibit 10.3 hereto, and the terms of which are incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
On October 24, 2023, Citius
Pharma issued a press release announcing that on October 23, 2023, it executed the Merger Agreement with SpinCo, TenX and Merger Sub.
A copy of the press release is furnished hereto as Exhibit 99.1 and incorporated herein by reference.
On October 23, 2023, Citius
Pharma posted an updated Corporate Presentation on its website. A copy of the Corporate Presentation is attached hereto as Exhibit 99.2
and incorporated herein by reference.
The information in this Item
7.01, as well as Exhibit 99.1 and Exhibit 99.2 attached hereto, shall not be deemed “filed” for purposes of Section 18 of
the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing
under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Additional Information
In connection with the Business
Combination, TenX intends to file with the SEC a Registration Statement on Form S-4, which will include a preliminary prospectus and preliminary
proxy statement (the “TenX Registration Statement”). TenX will mail a definitive proxy statement/final prospectus and other
relevant documents to its shareholders. This communication is not a substitute for the TenX Registration Statement, the definitive proxy
statement/final prospectus or any other document that TenX will send to its shareholders in connection with the Business Combination.
Investors and security holders of TenX are advised to read, when available, the proxy statement/prospectus in connection with TenX’s
solicitation of proxies for its extraordinary meeting of shareholders to be held to approve the Business Combination (and related matters)
because the proxy statement/prospectus will contain important information about the Business Combination and the parties to the Business
Combination. The definitive proxy statement/final prospectus will be mailed to shareholders of TenX as of a record date to be established
for voting on the Business Combination. Shareholders will also be able to obtain copies of the proxy statement/prospectus, without charge,
once available, at the SEC’s website at www.sec.gov or by directing a request to: TenX Keane Acquisition, c/o Taylor Zhang, 420
Lexington Ave., Suite 2446, New York, New York 10170.
Participants in the Solicitation
This communication is not
a solicitation of a proxy from any investor or security holder. However, Citius Pharma, SpinCo, TenX and certain of their respective directors,
executive officers and other members of management and employees may be deemed to be participants in the solicitation of proxies from
shareholders of TenX in connection with the proposed Business Combination under the rules of the SEC. Information regarding the persons
who are, under the rules of the SEC, participants in the solicitation of the stockholders of TenX in connection with the proposed transaction,
including a description of their direct or indirect interests, by security holdings or otherwise, will be set forth in the proxy statement/prospectus
when it is filed with the SEC. Information about the directors and executive officers of TenX may be found in its Annual Report on Form
10-K filed with the SEC on April 17, 2023. Information about the directors and executive officers of Citius Pharma may be found in its
definitive proxy statement relating to its 2023 Annual Meeting of Stockholders filed with the SEC on December 22, 2022. These documents
can be obtained free of charge from the sources indicated above. Other information regarding the participants in the proxy solicitation
and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the TenX Registration
Statement, prospectus and proxy statement and other relevant materials to be filed with the SEC when they become available.
Forward-Looking Statements
This Current Report on Form
8-K contains forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,”
“estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,”
“potential,” “predict,” “project,” “should,” “would” and similar expressions
may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. All statements
other than statements of historical fact contained in this Current Report on Form 8-K, including statements regarding the expected timing
and structure of the Business Combination and the ability of the parties to complete the Business Combination are forward-looking statements.
These forward-looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown
risks, uncertainties, assumptions and other important factors, many of which are outside the control of Citius Pharma, SpinCo and TenX,
that could cause actual results or outcomes to differ materially from those discussed in the forward-looking statements. The forward-looking
statements contained in this Current Report on Form 8-K are based on current expectations and beliefs concerning future developments and
their potential effects on Citius Pharma, SpinCo or TenX. There can be no assurance that future developments affecting Citius Pharma,
SpinCo or TenX will be those that Citius Pharma, SpinCo or TenX have anticipated. These forward-looking statements involve a number of
risks, uncertainties (some of which are beyond the control of Citius Pharma, SpinCo and TenX) or other assumptions that may cause actual
results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and
uncertainties include, but are not limited to, the following: the risk that the Business Combination may not be completed in a timely
manner or at all, which may adversely affect the price of Citius Pharma’s common stock; the risk that the Business Combination may
not be completed by TenX’s business combination deadline and the potential failure to obtain an extension of the business combination
deadline if sought by TenX; the failure to satisfy the conditions to the consummation of the Business Combination, including the adoption
of the Merger Agreement by the stockholders of TenX; the satisfaction of the minimum trust account amount following redemptions by TenX’s
public stockholders; the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement;
the effect of the announcement or pendency of the transaction on Citius Pharma’s business relationships, performance, and business
generally; risks that the proposed business combination disrupts current plans or operations of Citius Pharma; the outcome of any legal
proceedings that may be instituted against Citius Pharma or TenX related to the Merger Agreement or the proposed Business Combination;
the ability to maintain the listing of TenX’s securities (which would be New Citius Oncology Shares) on Nasdaq after the closing
of the Business Combination; after the closing of the Business Combination, the price of the New Citius Oncology Shares may be volatile
due to a variety of factors, including changes in the competitive and highly regulated industries in which Citius Oncology will operate,
variations in performance across competitors, changes in laws and regulations affecting Citius Oncology’s business and changes in
its capital structure; the ability to implement business plans, forecasts, and other expectations after the completion of the proposed
business combination, and identify and realize additional opportunities provided by the business combination; the cost and timing of the
resubmission of the BLA for LYMPHIR; the FDA may not approve our BLA for LYMPHIR; our need for substantial additional funds; the estimated
markets for our product candidates and the acceptance thereof by any market; our ability to commercialize our products if approved by
the FDA; our dependence on third-party suppliers; the ability of our product candidates to impact the quality of life of our target patient
populations; our ability to successfully undertake and complete clinical and non-clinical trials and the results from those trials for
our product candidates; risks relating to the results of research and development activities, including those from existing and new pipeline
assets; uncertainties relating to preclinical and clinical testing; the early stage of products under development; market and other conditions;
our ability to attract, integrate, and retain key personnel; risks related to our growth strategy; patent and intellectual property matters;
our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability to identify, acquire,
close and integrate product candidates and companies successfully and on a timely basis; our ability to procure cGMP commercial-scale
supply; government regulation; competition; as well as other risks described in our SEC filings. These risks have been and may be further
impacted by Covid-19 and global geopolitical events, such as the war in Ukraine and the Middle East; as well as other risks detailed from
time to time in Citius Pharma’s and TenX’s reports filed with the SEC, including Citius Pharma’s and TenX’s Annual
Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other documents filed with the SEC. The foregoing
list of important factors is not exclusive.
Any forward-looking statements
speak only as of the date of this report. None of Citius Pharma, SpinCo or TenX undertakes, and each party expressly disclaims, any obligation
to update any forward-looking statements, whether as a result of new information or development, future events or otherwise, except as
required by law. Readers are cautioned not to place undue reliance on any of these forward-looking statements.
No Offer or Solicitation
This communication is for
informational purposes only and is neither an offer to purchase, nor a solicitation of an offer to sell, subscribe for or buy any securities
or the solicitation of any vote in any jurisdiction pursuant to the Business Combination or otherwise, nor shall there be any sale, issuance
or transfer of securities in any jurisdiction in contravention of applicable law. No offer of securities shall be made except by means
of a prospectus meeting the requirements of Section 10 of the Securities Act.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| 2.1* |
Agreement and Plan of Merger, dated as of October 23, 2023, by and among Citius Pharmaceuticals, Inc., Citius Oncology, Inc., TenX Keane Acquisition, and TenX Merger Sub Inc. |
| 10.1* |
Sponsor Support Agreement, dated as of October 23, 2023, by and among 10XYZ Holdings LP, TenX Keane Acquisition, Citius Pharmaceuticals, Inc. and Citius Oncology, Inc. |
| 10.2 |
Form of Amended and Restated Registration Rights Agreement. |
| 10.3* |
Form of Amended and Restated Shared Services Agreement. |
| 99.1 |
Press Release, dated October 24, 2023. |
| 99.2 |
Corporate Presentation of October 2023. |
| 104 |
Cover Page Interactive Data File, formatted in Inline Extensible Business Reporting Language (iXBRL). |
| * | Certain of the exhibits and schedules to this exhibit have
been omitted in accordance with Regulation S-K Item 601(b)(2) or 601(a)(5), as applicable. Citius Pharma agrees to furnish supplementally
a copy of all omitted exhibits and schedules to the SEC upon its request. |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| |
CITIUS PHARMACEUTICALS, INC. |
| |
|
|
|
| Date: October 24, 2023 |
By: |
/s/ Leonard Mazur |
| |
|
Name: |
Leonard Mazur |
| |
|
Chairman and Chief Executive Officer |
8
Exhibit 2.1
Execution Version
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
by and among
CITIUS
PHARMACEUTICALS, INC.,
CITIUS
ONCOLOGY, INC.,
TENX KEANE
ACQUISITION
and
TENX MERGER SUB, INC.
Dated as of October 23, 2023
THIS DOCUMENT IS INTENDED SOLELY TO FACILITATE DISCUSSIONS AMONG THE
PARTIES. THIS DOCUMENT IS NOT INTENDED TO CREATE, NOR WILL IT BE DEEMED TO CREATE, A LEGALLY BINDING OR ENFORCEABLE OFFER OR AGREEMENT
OF ANY TYPE OR NATURE, UNLESS AND UNTIL AGREED TO AND EXECUTED BY THE PARTIES.
TABLE OF CONTENTS
| |
|
Page |
| Article I - DEFINITIONS |
3 |
| |
1.1 |
Definitions |
3 |
| |
1.2 |
Interpretation |
3 |
| Article II - THE MERGER |
5 |
| |
2.1 |
The Merger |
5 |
| |
2.2 |
Closing |
5 |
| |
2.3 |
Closing Deliverables; Effective Time |
5 |
| |
2.4 |
Certificate of Incorporation and Bylaws of the Surviving Corporation; Directors and Officers of the Surviving Corporation |
7 |
| |
2.5 |
Governance Matters |
7 |
| |
2.6 |
Tax Treatment of the Domestication and the Merger |
8 |
| |
2.7 |
Adjustment to Parent Transaction Expenses |
8 |
| Article III - CONVERSION OF SHARES |
10 |
| |
3.1 |
Effect on Capital Stock and SpinCo Options |
10 |
| |
3.2 |
Exchange Fund |
11 |
| |
3.3 |
Appraisal Rights |
11 |
| Article IV - REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
12 |
| |
4.1 |
Organization of the Company |
12 |
| |
4.2 |
Due Authorization |
12 |
| |
4.3 |
Governmental Consents |
12 |
| |
4.4 |
No Conflict |
13 |
| |
4.5 |
Litigation and Proceedings |
13 |
| |
4.6 |
Brokers’ Fees |
13 |
| |
4.7 |
Internal Controls |
14 |
| Article V - |
REPRESENTATIONS AND WARRANTIES OF THE COMPANY AND SPINCO |
15 |
| |
5.1 |
Organization of SpinCo |
15 |
| |
5.2 |
Due Authorization |
15 |
| |
5.3 |
Capitalization of SpinCo |
16 |
| |
5.4 |
Subsidiaries |
16 |
| |
5.5 |
Governmental Consents |
16 |
| |
5.6 |
No Conflict |
17 |
| |
5.7 |
Sufficiency of the SpinCo Assets |
17 |
| |
5.8 |
Financial Statements |
18 |
| |
5.9 |
No Undisclosed Liabilities |
18 |
| |
5.10 |
Litigation and Proceedings |
18 |
| |
5.11 |
Real Property |
19 |
| |
5.12 |
Tax Matters |
19 |
| |
5.13 |
Absence of Changes |
20 |
| |
5.14 |
Material Contracts |
21 |
| |
5.15 |
Employment Matters |
23 |
| |
5.16 |
Compliance with Law; Permits |
25 |
| |
5.17 |
Benefit Plans |
26 |
| |
5.18 |
Intellectual Property |
27 |
| |
5.19 |
Environmental Matters |
29 |
| |
5.20 |
Affiliate Matters |
30 |
| |
5.21 |
Brokers’ Fees |
30 |
| |
5.22 |
Proxy Statement; Registration Statement |
30 |
| |
5.23 |
Board and Shareholder Approval |
30 |
| |
5.24 |
Healthcare Regulatory Matters |
30 |
| |
5.25 |
Data Privacy |
31 |
| |
5.26 |
Anti-Bribery, Anti-Corruption and Anti-Money Laundering |
32 |
| |
5.27 |
Sanctions, Import, and Export Controls |
33 |
| |
5.28 |
No Other Representations and Warranties |
33 |
| Article VI - REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB |
33 |
| |
6.1 |
Organization of Parent and Merger Sub |
33 |
| |
6.2 |
Due Authorization |
34 |
| |
6.3 |
Capital Stock and Other Matters |
35 |
| |
6.4 |
Governmental Consents |
36 |
| |
6.5 |
No Conflict |
36 |
| |
6.6 |
Internal Controls; Listing; Financial Statements |
37 |
| |
6.7 |
No Undisclosed Liabilities |
38 |
| |
6.8 |
Litigation and Proceedings |
38 |
| |
6.9 |
Tax Matters |
38 |
| |
6.10 |
Absence of Changes |
40 |
| |
6.11 |
Brokers’ Fees |
41 |
| |
6.12 |
Proxy Statement; Registration Statement |
41 |
| |
6.13 |
SEC Filings |
41 |
| |
6.14 |
Trust Account |
42 |
| |
6.15 |
Investment Company Act; JOBS Act |
42 |
| |
6.16 |
Indebtedness |
42 |
| |
6.17 |
Stock Market Quotation |
43 |
| |
6.18 |
Business Activities |
43 |
| |
6.19 |
Section 280G |
44 |
| |
6.20 |
Sanctions, Import, and Export Controls |
44 |
| |
6.21 |
No Other Representations and Warranties |
44 |
| Article VII - COVENANTS |
45 |
| |
7.1 |
Conduct of Business by Parent and Merger Sub Pending the Merger |
45 |
| |
7.2 |
Conduct of the Company and SpinCo Business Pending the Merger |
46 |
| |
7.3 |
Tax Matters |
49 |
| |
7.4 |
Preparation of the Registration Statement and Proxy Statement; Parent Shareholders Meeting |
50 |
| |
7.5 |
Reasonable Efforts |
53 |
| |
7.6 |
Access to Information |
55 |
| |
7.7 |
Permitted Activities and Exclusivity |
55 |
| |
7.8 |
Public Announcements |
57 |
| |
7.9 |
Section 16 Matters |
57 |
| |
7.10 |
Control of Other Party’s Business |
58 |
| |
7.11 |
Domestication |
58 |
| |
7.12 |
NASDAQ Listing |
58 |
| |
7.13 |
Takeover Statutes |
59 |
| |
7.14 |
Sole Shareholder Approvals |
59 |
| |
7.15 |
Financial Information |
59 |
| |
7.16 |
Extension Proposal |
60 |
| |
7.17 |
Equity Incentive Plan |
61 |
| |
7.18 |
Transfer of Certain Intellectual Property to SpinCo |
61 |
| |
7.19 |
Insurance |
62 |
| |
7.20 |
BLA Resubmission |
62 |
| Article VIII - CONDITIONS TO THE MERGER |
62 |
| |
8.1 |
Conditions to the Obligations of SpinCo, the Company, Parent and Merger Sub to Effect the Merger |
62 |
| |
8.2 |
Additional Conditions to the Obligations of the Company and SpinCo |
63 |
| |
8.3 |
Additional Conditions to the Obligations of Parent and Merger Sub |
64 |
| Article IX - TERMINATION |
65 |
| |
9.1 |
Termination |
65 |
| |
9.2 |
Effect of Termination |
67 |
| |
9.3 |
Termination Fee |
67 |
| |
9.4 |
Fees and Expenses |
67 |
| Article X - MISCELLANEOUS |
68 |
| |
10.1 |
Trust Account |
68 |
| |
10.2 |
Non-Survival of Representations, Warranties and Agreements |
68 |
| |
10.3 |
Governing Law; Jurisdiction |
68 |
| |
10.4 |
Notices |
69 |
| |
10.5 |
Headings |
70 |
| |
10.6 |
Entire Agreement |
70 |
| |
10.7 |
Amendments and Waivers |
70 |
| |
10.8 |
Assignment; Parties in Interest; Non-Parties |
70 |
| |
10.9 |
Specific Performance |
71 |
| |
10.10 |
WAIVER OF JURY TRIAL |
72 |
| |
10.11 |
Severability |
72 |
| |
10.12 |
Counterparts |
72 |
| |
10.13 |
Disclosure Schedules |
72 |
EXHIBITS
| Exhibit A | |
Form of Parent Charter |
| Exhibit B | |
Form of Parent Bylaws |
| Exhibit C | |
Form of A&R Registration Rights Agreement |
| Exhibit D | |
Form of Parent Equity Incentive Plan |
| Exhibit E | |
Form of Amended and Restated Shared Services Agreement |
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
This AGREEMENT AND PLAN OF
MERGER AND REORGANIZATION, dated as of October 23, 2023 (this “Agreement”), is entered into by and among Citius
Pharmaceuticals, Inc., a Nevada corporation (the “Company”), Citius
Oncology, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“SpinCo”), TenX
Keane Acquisition, a Cayman Islands exempted company (which will migrate to and domesticate as a Delaware corporation prior to
the Closing (as defined below)) (“Parent”), and TenX Merger Sub, Inc., a Delaware corporation and wholly owned Subsidiary
of Parent (“Merger Sub”). Each of the foregoing parties is referred to herein as a “Party” and collectively
as the “Parties.”
RECITALS
A.
Parent is a blank check company incorporated as a Cayman Islands exempted company for the purpose of effecting a merger, share
exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses.
B.
At least one day prior to the Closing Date (as defined below), as the first step in the consummation of the transactions contemplated
herein and subject to the conditions set forth in this Agreement, Parent shall migrate to and domesticate as a Delaware corporation (the
“Domestication”) in accordance with Section 388 of the Delaware General Corporation Law, as amended (the “DGCL”),
and the Cayman Islands Companies Act (As Revised) (the “CICA”).
C.
Concurrently with the Domestication, Parent shall file a certificate of incorporation with the Secretary of State of the State
of Delaware and adopt bylaws, substantially in the forms attached as Exhibits A and B hereto, respectively, with such changes
as may be agreed in writing by Parent and the Company.
D.
In connection with the Domestication, (i) each then-issued and outstanding ordinary share, par value $0.0001 each per share, in
the capital of Parent (the “Parent Common Stock”) shall convert automatically, on a one-for-one basis, into one share
of common stock, par value $0.0001 per share, of Parent after its domestication as a corporation incorporated in the State of Delaware
(the “Domesticated Parent Common Stock”); (ii) each then-issued and outstanding right to receive two-tenths of one
share of Parent Common Stock (the “Parent Rights”) shall convert automatically into a right to receive two-tenths of
one share of Domesticated Parent Common Stock (each, a “Domesticated Parent Right”); and (iii) each then-issued and
outstanding unit of Parent (the “Parent Units”) shall be cancelled and entitle the holder to one share of Domesticated
Parent Common Stock and a Domesticated Parent Right.
E.
Following the Domestication, (i) the Parties will effect the merger of Merger Sub with and into SpinCo, with SpinCo continuing
as the surviving corporation and changing its name to one as selected by SpinCo in its discretion (the “Merger”), upon
the terms and subject to the conditions set forth herein, and (ii) Parent will change its name to “Citius Oncology, Inc.”
F.
Pursuant to the Merger, shares of SpinCo Common Stock will be exchanged for shares of Domesticated Parent Common Stock, on the
terms and subject to the conditions set forth herein.
G.
The board of directors of Parent (the “Parent Board”) unanimously has (i) determined that it is in the best
interests of Parent and the shareholders of Parent, and declared it advisable, to enter into this Agreement providing for the Domestication
and the Merger in accordance with the DGCL and CICA, (ii) approved this Agreement and the Transactions, including the Domestication and
the Merger, on the terms and subject to the conditions of this Agreement, and (iii) adopted a resolution recommending that the Domestication
be approved, and the plan of merger set forth in this Agreement be adopted by the shareholders of Parent (the “Parent Board Recommendation”).
H.
The board of directors of Merger Sub has determined that the Merger and this Agreement are advisable, has approved this Agreement
and the Transactions, including the Merger, and has recommended the approval of this Agreement and the Merger to the sole stockholder
of Merger Sub.
I.
Parent, as the sole stockholder of Merger Sub, promptly following the execution and delivery of this Agreement, will approve and
adopt this Agreement and the Transactions.
J.
The board of directors of the Company (the “Company Board”) unanimously has approved this Agreement and the
Transactions.
K.
The board of directors of SpinCo has determined that the Merger and this Agreement are advisable, has approved this Agreement and
the Transactions, including the Merger, and has recommended the approval of this Agreement and the Merger to the sole stockholder of SpinCo.
L.
The Company, as the sole stockholder of SpinCo, promptly following the execution and delivery of this Agreement, will approve and
adopt this Agreement and the Transactions.
M.
In accordance with the terms of this Agreement, Parent shall provide an opportunity to holders of the Parent Common Stock to have
their outstanding shares redeemed on the terms and subject to the conditions set forth in this Agreement and Parent’s Governing
Documents in connection with obtaining the Parent Shareholder Approval.
N.
At the Effective Time, Parent, the Sponsor, and the other Persons named as parties therein shall amend and restate that certain
Registration Rights Agreement, dated October 13, 2022, by and among Parent, the Sponsor and the other Persons party thereto, substantially
in the form attached hereto as Exhibit C (as so amended and restated, and with such further changes as may be agreed in writing
by Parent and the Company, the “A&R Registration Rights Agreement”), to, among other things, include a six (6)
month lockup on shares issued to directors and officers of the Company and SpinCo.
O.
As a material inducement to the Company’s and SpinCo’s willingness to enter into this Agreement, concurrently with
the execution and delivery of this Agreement, Sponsor and Parent are entering into a Sponsor Support Agreement with the Company and SpinCo
(the “Sponsor Support Agreement”).
P.
It is the intention of the Parties that, for U.S. federal income Tax (as defined below) purposes, the Domestication qualify as
a “reorganization” under Section 368(a)(1)(F) of the Code, the Merger qualify as a “reorganization” within the
meaning of Section 368(a)(1)(A) of the Code, the Company, Merger Sub and Parent be parties to such reorganization (within the meaning
of Section 368(b) of the Code) under Section 368(a)(1)(A), and this Agreement constitutes a “plan of reorganization” within
the meaning of the regulations promulgated under the Code.
AGREEMENT
In consideration of the premises and mutual covenants
contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties
agree as follows:
Article
I
DEFINITIONS
1.1
Definitions. Unless otherwise provided herein, terms with initial capital letters used in this Agreement will have the meanings
ascribed to such terms in Annex A attached hereto, which is incorporated herein and made a part hereof.
1.2
Interpretation.
(a)
Unless the context of this Agreement otherwise requires:
(i)
(A) words of any gender include each other gender and neuter form and the use herein shall not limit any provision of this Agreement;
(B) words using the singular or plural number also include the plural or singular number, respectively; (C) derivative forms of defined
terms will have correlative meanings; (D) the terms “hereof,” “herein,” “hereby,” “hereto,”
“herewith,” “hereunder” and derivative or similar words refer to this entire Agreement; (E) the terms “Article,”
“Section,” “Annex,” “Exhibit,” “Schedule,” and “Disclosure Schedule” refer
to the specified Article, Section, Annex, Exhibit, Schedule or Disclosure Schedule of this Agreement and references to “paragraphs”
or “clauses” shall be to separate paragraphs or clauses of the Section or subsection in which the reference occurs; and (F)
the words “include,” “includes” and “including” shall be deemed to be followed by the phrase “without
limitation”.
(ii)
any Law defined or referred to in this Agreement or in any agreement or instrument that is referred to herein means such Law as
from time to time amended, modified or supplemented, in whole or in part, including (in the case of statutes) by succession of comparable
successor Laws and the related regulations, or enforcement procedures thereunder and published interpretations thereof, and references
to any Contract or instrument (excluding any Contracts, documents or instruments disclosed in the Disclosure Schedules) are to that Contract
or instrument as from time to time amended, modified, supplemented, or the terms thereof waived to the extent permitted by, and in accordance
with, the terms thereof; provided, that for purposes of any representations and warranties contained in this Agreement that are
made as of a specific date or dates, references to any Law shall be deemed to refer to such Law, as amended, and the related regulations,
or enforcement procedures thereunder and published interpretations thereof, in each case, as of such date or dates.
(iii)
references to any federal, state, local, or foreign statute or Law shall include all regulations promulgated thereunder; and
(iv)
references to any Person include references to such Person’s successors and permitted assigns, and in the case of any Governmental
Authority, to any Person succeeding to its functions and capacities.
(b)
The language used in this Agreement shall be deemed to be the language chosen by the Parties to express their mutual intent. The
Parties acknowledge that each Party and its attorney has reviewed and participated in the drafting of this Agreement and that any rule
of construction to the effect that any ambiguities are to be resolved against the drafting Party, or any similar rule operating against
the drafter of an agreement, shall not be applicable to the construction or interpretation of this Agreement.
(c)
No reference in the Disclosure Schedules to or disclosure of a possible breach or violation of any Contract or Law shall be construed
as an admission by any Party or any of its Affiliates, in any Action, that such Party or any such Affiliate, or any third party, is or
is not in breach or violation of, or in default in, the performance or observance of any term or provisions of any Contract or any Law.
(d)
Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified.
If any action is to be taken or given on or by a particular calendar day, and such calendar day is not a Business Day, then such action
may be deferred until the next Business Day.
(e)
When calculating the period of time before which, within which or following which any act is to be done or step taken pursuant
to this Agreement, the date that is the reference date in calculating such period shall be excluded and if the last day of such period
is not a Business Day, the period shall end on the next succeeding Business Day.
(f)
The phrase “to the extent” shall mean the degree to which a subject or other thing extends, and such phrase shall not
mean simply “if”, and the word “or” shall be disjunctive but not exclusive.
(g)
The term “writing,” “written” and comparable terms refer to printing, typing and other means of reproducing
words (including electronic media) in a visible form.
(h)
All accounting terms used herein and not expressly defined herein shall have the meanings given to them under GAAP, unless the
context otherwise requires.
(i)
All monetary figures shall be in United States dollars unless otherwise specified.
(j)
No reference in this Agreement to dollar amount thresholds shall be deemed to be evidence of a SpinCo Material Adverse Effect,
Company Material Adverse Effect or Parent Material Adverse Effect, as applicable.
(k)
The phrases “furnished,” “provided,” “delivered” or “made available” to a Party,
or similar formulations, when used with respect to information or documents means that such information or documents (i) have been physically
or electronically delivered directly to such Party or its legal counsel or financial advisors or made available to such Party (without
material redactions) in the electronic data room hosted by the providing Party in connection with the Transactions, or (ii) are Parent
SEC Filings or Company SEC Documents and have been made publicly available on the SEC’s EDGAR database by Parent or the Company,
as applicable, and in each of clause (i) and (ii), not later than forty-eight (48) hours prior to the execution of this Agreement (and
continuously available to such Party and its legal counsel and financial advisors through the date hereof).
Article
II
THE MERGER
2.1
The Merger. On the Closing Date, upon the terms and subject to the conditions of this Agreement, Parent and Merger Sub shall
cause Merger Sub to be merged with and into SpinCo in accordance with the applicable provisions of the DGCL. The Merger shall become effective
at the time the Certificate of Merger is duly filed with the Secretary of State of the State of Delaware, or such later time as Parent
and SpinCo shall agree and specify in the Certificate of Merger (such time as the Merger becomes effective being the “Effective
Time”). At the Effective Time, the separate corporate existence of Merger Sub shall cease, and SpinCo shall continue as the
surviving corporation after the Merger (sometimes referred to herein as the “Surviving Corporation”). The Merger will
have the effects set forth in this Agreement and the applicable provisions of the DGCL. Without limiting the generality of, and subject
to, the immediately preceding sentence, from and after the Effective Time, all property, rights, privileges, immunities, powers, franchises,
licenses, and authority of SpinCo and the Merger Sub will vest in the Surviving Corporation, and all debts, liabilities, obligations,
restrictions, and duties of each of SpinCo and the Merger Sub will become the debts, liabilities, obligations, restrictions, and duties
of the Surviving Corporation. As a result of the Merger, SpinCo shall become a direct, wholly owned Subsidiary of Parent. References herein
to “SpinCo” with respect to the period from and after the Effective Time shall be deemed to be references to the Surviving
Corporation. At the Effective Time, the effects of the Merger shall be as provided in this Agreement, the Certificate of Merger, and the
applicable provisions of the DGCL.
2.2
Closing. Unless the Transactions have been abandoned and this Agreement terminated pursuant to Section 9.1, then
upon the terms and subject to the conditions set forth in this Agreement, the closing of the Merger (the “Closing”)
shall take place by electronic exchange of documents and signatures on the third (3rd) Business Day after the conditions set
forth in Article VIII (other than those that are to be satisfied at or immediately prior to the Closing, but subject to the satisfaction
or, to the extent permitted by applicable Law, waiver of such conditions at the Closing) have been satisfied or, to the extent permitted
by applicable Law, waived, unless another date, time or place is agreed to in writing by the Company and Parent. The date on which the
Closing actually occurs is hereinafter referred to as the “Closing Date.”
2.3
Closing Deliverables; Effective Time.
(a)
At the Closing, the Company or SpinCo, as applicable, will deliver or cause to be delivered to Parent or Merger Sub:
(i)
a certificate signed by an authorized officer of the Company, dated as of the Closing Date, certifying that, to the knowledge and
belief of such authorized officer, the conditions specified in Section 8.3(a), (b), (c) and (e) have been
satisfied;
(ii)
the A&R Registration Rights Agreement, duly executed by the parties set forth on Section 2.3(a)(ii) of the Company Disclosure
Schedule;
(iii)
duly executed counterparts to each of the other Transaction Documents to be entered into by the Company or SpinCo, as applicable;
(iv)
an IRS Form W-9, duly executed by the Company;
(v)
copies of resolutions and actions taken by the Company’s and SpinCo’s board of directors in connection with the approval
of this Agreement and the Transactions;
(vi)
the Amended and Restated Shared Services Agreement, duly executed by the Company and SpinCo;
(vii)
all other documents, instruments or certificates required to be delivered by the Company at or prior to the Closing pursuant to
Section 8.3; and
(viii)
such other documents or certificates as is reasonably determined by Parent and its legal counsel to be required in order to consummate
the Transactions.
(b)
At the Closing, Parent will deliver or cause to be delivered:
(i)
to the Exchange Agent, the shares of Domesticated Parent Common Stock to be paid in respect of shares of SpinCo Common Stock in
accordance with Section 3.1(a);
(ii)
to the Company, a certificate signed by an authorized officer of Parent, dated the Closing Date, certifying that, to the knowledge
and belief of such authorized officer, the conditions specified in Section 8.1(f) and Section 8.2(a), (b), (c),
(e) and (f) have been satisfied;
(iii)
to the Company, the A&R Registration Rights Agreement, and other Transaction Documents to be entered into by the Parent and
the Sponsor, as applicable, duly executed by a duly authorized representative of Parent and the Sponsor;
(iv)
to the Company, the written resignations of all of the directors and officers of Parent (other than those Persons identified as
the initial directors and officers, respectively, of Parent after the Effective Time, in accordance with the provisions of Section
2.5), effective as of the Effective Time;
(v)
to the Company, copies of resolutions and actions taken by Parent’s and Merger Sub’s board of directors and stockholders
in connection with the approval of this Agreement and the Transactions;
(vi)
to the Company, all other documents, instruments or certificates required to be delivered by Parent at or prior to the Closing
pursuant to Section 8.2; and
(vii)
such other documents or certificates as is reasonably determined by the Company and its legal counsel to be required in order to
consummate the Transactions.
(c)
At least two (2) Business Days prior to the Closing, Parent will deliver to the Company a written statement setting forth Parent’s
good faith estimate of (i) all Parent Transaction Expenses as of the Closing (“Parent Estimated Transaction Expenses”)
(in each case with reasonable detail reports including the respective amounts and wire transfer instructions for the payment thereof,
together with corresponding invoices), and (ii) the resulting amount, if any, Sponsor would be obligated to pay to Parent assuming such
Parent Estimated Transaction Expenses were the final Parent Transaction Expenses for purposes of Section 2.7.
(d)
On the Closing Date, in connection with the Effective Time, Parent will pay or cause to be paid, by wire transfer of immediately
available funds, all Parent Estimated Transaction Expenses, and Parent shall cause the Sponsor to pay in full, by wire transfer of immediately
available funds to Parent, any Parent Estimated Transaction Expenses in excess of $500,000.
(e)
On the Closing Date, SpinCo and Merger Sub shall file a certificate of merger relating to the Merger (the “Certificate
of Merger”) with the Secretary of State of the State of Delaware in accordance with the relevant provisions of the DGCL and
shall make all other filings or recordings required under the DGCL.
(f)
On the Closing Date, in connection with the Effective Time, the Company will pay or cause to be paid, by wire transfer of immediately
available funds to Parent, $10,000,000 as a capital contribution to Parent for purposes of funding working capital of the Surviving Corporation.
(g)
The Closing and the Effective Time shall occur no sooner than the date that is the day after the completion of the Domestication.
2.4
Certificate of Incorporation and Bylaws of the Surviving Corporation; Directors and Officers of the Surviving Corporation.
(a)
The certificate of incorporation of Merger Sub in effect immediately prior to the Effective Time shall be the certificate of incorporation
of the Surviving Corporation until amended in accordance with applicable Law, except the name of the Surviving Corporation shall be as
provided in Section 2.4(b) and the reference to the incorporator shall be deleted.
(b)
The bylaws of Merger Sub in effect immediately prior to the Effective Time shall be the bylaws of the Surviving Corporation until
amended in accordance with applicable Law, except the name of the Surviving Corporation shall be such name as selected by SpinCo in its
absolute discretion.
(c)
From and after the Effective Time, until successors are duly elected or appointed and qualified in accordance with applicable Law,
(i) the directors of SpinCo as of immediately prior to the Effective Time shall be the directors of the Surviving Corporation and (ii)
the officers of SpinCo as of immediately prior to the Effective Time shall be the officers of the Surviving Corporation.
2.5
Governance Matters.
(a)
The Parties shall use commercially reasonable efforts to ensure that the individuals listed on Section 2.5(a) of the Company
Disclosure Schedule are nominated and elected as directors of Parent effective immediately after the Closing.
(b)
Subject to the terms of Parent’s Governing Documents, Parent shall take all such action within its power as may be necessary
or appropriate such that immediately following the Effective Time (i) the Parent Board shall have a majority of “independent”
directors for purposes of NASDAQ and (ii) the initial officers of Parent shall be as set forth on Section 2.5(b) of the Company Disclosure
Schedule, in each case, each of whom shall serve in such capacity in accordance with the terms of Parent’s Governing Documents
following the Effective Time.
2.6
Tax Treatment of the Domestication and the Merger. It is the intention by the Parties that, for U.S. federal income Tax
purposes, the Domestication qualify as a “reorganization” under Section 368(a)(1)(F) of the Code, the Merger qualify as a
“reorganization” within the meaning of Section 368(a) of the Code, and SpinCo, Merger Sub and Parent be parties to such Merger
reorganization within the meaning of Section 368(b) of the Code. The Parties hereby adopt this Agreement as a “plan of reorganization”
within the meaning of Sections 1.368-2(g) and 1.368-3(a) of the Treasury Regulations. All of the Parties agree to cooperate and use their
best efforts in order to qualify the Merger as a reorganization under Section 368(a)(1)(A) of the Code, to not take any action that could
reasonably be expected to cause the Merger to fail to so qualify, and to report the Merger for federal, state and any local income Tax
purposes in a manner consistent with such characterization.
2.7
Adjustment to Parent Transaction Expenses.
(a)
Within ninety (90) days following the Closing Date, the Surviving Corporation will prepare and deliver to Sponsor a statement (the
“Closing Statement”) containing the actual amount of all Parent Transaction Expenses (including those invoiced following
the Closing). The Surviving Corporation shall provide Sponsor with reasonable access to its books and records as may be reasonably requested
by Sponsor to verify the information contained in the Closing Statement.
(b)
Dispute Resolution.
(i)
Within thirty (30) days following Sponsor’s receipt of the Closing Statement, Sponsor will deliver written notice to the
Surviving Corporation of any dispute with respect to the Closing Statement, setting forth such disputed item in reasonable detail (a “Closing
Statement Dispute”). If the Sponsor does not notify the Surviving Corporation of any Closing Statement Dispute within such thirty
(30)-day period, then the Closing Statement and the determinations and calculations of the Parent Transaction Expenses set forth therein
will be final, conclusive and binding on the Parties. If Sponsor delivers to the Surviving Corporation a Closing Statement Dispute, then
Sponsor and the Surviving Corporation will negotiate in good faith to resolve all disputed matters set forth in the Closing Statement
Dispute. If Sponsor and the Surviving Corporation, notwithstanding such good faith effort, fail to resolve the Closing Statement Dispute
within thirty (30) days (or longer, as mutually agreed to by such Parties in writing) after Sponsor delivers to the Surviving Corporation
notice of the Closing Statement Dispute, then Sponsor and the Surviving Corporation will mutually agree on and jointly engage an independent
auditor that is experienced in such matters (the “Independent Auditor”), to promptly resolve any and all unresolved
matters of the Closing Statement Dispute.
(ii)
The Independent Auditor shall consider only those items and amounts set forth in the Closing Statement Dispute that are identified
by either Sponsor or the Surviving Corporation as being items that Sponsor and the Surviving Corporation are unable to resolve. As promptly
as practicable thereafter, Sponsor and the Surviving Corporation will each prepare and submit a written presentation to the Independent
Auditor (each of which will be shared with the other party, but not until such time that both Parties have submitted their presentations)
and will use commercially reasonable efforts to cause the Independent Auditor to make a final determination with respect to the Parties’
respective positions based upon the applicable language, definitions and Exhibits of this Agreement, and the presentations by Sponsor
and the Surviving Corporation.
(iii)
In resolving any disputed item, the Independent Auditor will be bound by the terms of this Agreement, will serve as an expert and
not an arbitrator, and will not assign a value to any item greater than the greatest value for such item claimed by either party or less
than the smallest value for such item claimed by either Party. Except as Sponsor and the Surviving Corporation may otherwise agree, all
communications between any party or its respective Representatives, on the one hand, and the Independent Auditor, on the other hand, will
be in writing with copies simultaneously delivered to the non-communicating Party (except in such cases where both Parties are submitting
a presentation). The fees, costs and expenses of the Independent Auditor will be borne by Sponsor and the Surviving Corporation in inverse
proportion, as determined by the Independent Auditor, as they may prevail on the matter resolved by the Independent Auditor. Absent fraud,
all determinations made by the Independent Auditor will be final, conclusive and binding on the Parties. The Parties agree that judgment
may be entered upon the determination of the Independent Auditor in any court having jurisdiction over the party against which such determination
is to be enforced.
(iv)
If the Parent Transaction Expenses (as finally determined pursuant to Section 2.7(b)) is greater than the Parent Estimated
Transaction Expenses and greater than $500,000, then Sponsor will pay by wire transfer of immediately available funds to Parent, an amount
in cash equal to (A) the amount by which the Parent Transaction Expenses exceeds $500,000 minus (B) the amount, if any, that Sponsor
paid at Closing in respect of Parent Estimated Transaction Expenses.
(v)
Any payment required pursuant to this Section 2.7(b) will be made within five (5) Business Days after the date of final
determination of the Parent Transaction Expenses in accordance with Section 2.7(b).
(vi)
Each party will reasonably cooperate with and make available to the other party and its respective accountants and other Representatives
all information, records, data and working papers, and will permit access to its records, facilities and personnel, as may be reasonably
requested in connection with this Section 2.7(b). including the resolution of any matters or disputes hereunder.
Article
III
CONVERSION OF SHARES
3.1
Effect on Capital Stock and SpinCo Options. At the Effective Time, by virtue of the Merger and without any action on the
part of any party to this Agreement or any holder of the capital stock of the Company, SpinCo, Merger Sub or Parent:
(a)
SpinCo Common Stock and Merger Sub Common Stock.
(i)
Each share of SpinCo Common Stock issued and outstanding and held by the Company as of immediately prior to the Effective Time
(the “Outstanding SpinCo Shares”) shall be automatically converted into the right to receive a number of fully paid
and non-assessable shares of Domesticated Parent Common Stock equal to the Base Exchange Ratio, subject to adjustment in accordance with
Section 3.1(a)(iii), with any fractional shares of Domesticated Parent Common Stock rounded down to the nearest whole share for
no additional consideration (the “Merger Consideration”). Each Outstanding SpinCo Share, when converted in accordance
with this Section 3.1(a)(i), shall no longer be outstanding and shall automatically be canceled and shall cease to exist, and the
holder thereof shall cease to have any rights with respect thereto, except the right to receive the Merger Consideration and any dividends
or distributions and other amounts payable in accordance with Section 3.2(b).
(ii)
Each share of SpinCo Common Stock held by SpinCo as treasury stock as of immediately prior to the Effective Time shall automatically
be canceled and shall cease to exist and no stock or other consideration shall be issued or delivered in exchange therefor or in respect
thereof.
(iii)
The Aggregate Parent Common Stock Consideration and the resulting Base Exchange Ratio shall be adjusted to the extent appropriate
to reflect the effect of any stock split, split-up, reverse stock split, stock dividend or distributions of Parent Common Stock, or securities
convertible into any such securities, reorganization, recapitalization, reclassification or other like change with respect to Parent Common
Stock having a record date occurring on or after the date of this Agreement and prior to the Effective Time; provided, that nothing
in this Section 3.1(a)(iii) shall be construed to permit Parent to take or to permit any of its Subsidiaries to take any action
with respect to its securities that is prohibited by the terms of this Agreement.
(iv)
At the Effective Time, all of the shares of common stock, par value $0.01 per share, of Merger Sub (“Merger Sub Common
Stock”) issued and outstanding immediately prior to the Effective Time shall be automatically converted into one fully paid
and nonassessable share of common stock, par value $0.001 per share, of the Surviving Corporation.
(b)
SpinCo Options. As of the Effective Time, Parent shall assume each SpinCo Option that is outstanding (whether vested or
unvested) as of the Effective Time (such assumed options are referred to as the “Domesticated Parent Options”). The
Domesticated Parent Options will continue to have, and be subject to, the same terms and conditions (including with respect to vesting
and termination-related provisions) set forth in the applicable option documents (including the SpinCo Equity Incentive Plan and stock
option agreement or other document evidencing such SpinCo Option) immediately prior to the Effective Time, except that (i) each SpinCo
Option will be exercisable from and after the Effective Time for that whole number of shares of Domesticated Parent Common Stock (rounded
down to the nearest whole share) equal to the number of shares of SpinCo Common Stock subject to such SpinCo Option, multiplied by the
Base Exchange Ratio, and (ii) the exercise price per share for each such share of Domesticated Parent Common Stock shall be equal to the
exercise price per share of such SpinCo Option in effect immediately prior to the Effective Time, divided by the Base Exchange Ratio (the
exercise price per share, as so determined, being rounded up to the nearest full cent). The assumption of the SpinCo Options by Parent
is intended to satisfy the requirements of Treasury Regulations Section 1.424-1 (to the extent such SpinCo Options were intended to qualify
as incentive stock options within the meaning of Code Section 422) and of Treasury Regulations Section 1.409A-1(b)(5)(v)(D) (for SpinCo
Options not so qualifying).
(c)
Parent Securities. Each share of Domesticated Parent Common Stock, each Domesticated Parent Right and each Domesticated
Parent Unit that is issued and outstanding immediately prior to and at the Effective Time shall remain outstanding immediately following
the Effective Time, except to the extent as otherwise provided in the Parent Governing Documents or Rights Agreement (including in respect
of automatic conversion of the Domesticated Parent Rights or redemption of the Domesticated Parent Common Stock).
3.2
Exchange Fund.
(a)
At or substantially concurrently with the Effective Time, Parent shall issue, and shall deposit, or shall cause to be deposited,
with the Exchange Agent, for the benefit of the holders of Outstanding SpinCo Shares, for exchange in accordance with this Section
3.2, the number of Domesticated Parent Common Stock in book-entry form sufficient to represent the Merger Consideration (the “Exchange
Fund”). Parent shall cause the Exchange Agent to distribute, immediately following the Effective Time, pursuant to irrevocable
instructions from the Parent, the Merger Consideration out of the Exchange Fund. The Exchange Fund shall not be used for any other purpose.
(b)
Distributions After the Effective Time. Subject to the following sentence, no dividends or other distributions declared
after the Effective Time with respect to Domesticated Parent Common Stock shall be paid with respect to any shares of Domesticated Parent
Common Stock that are not able to be delivered by the Exchange Agent promptly after the Effective Time, whether due to a legal impediment
to such delivery or otherwise. Subject to the effect of abandoned property, escheat, Tax or other applicable Laws, following the delivery
of any such previously undelivered shares of Domesticated Parent Common Stock, there shall be paid to the record holder of such shares
of Domesticated Parent Common Stock, without interest, at the time of delivery, to the extent not previously paid, the amount of dividends
or other distributions with a record date after the Effective Time theretofore paid with respect to such shares of Domesticated Parent
Common Stock.
(c)
No Liability. None of the Company, the Surviving Corporation, Parent, Merger Sub, the Exchange Agent or any other Person
shall be liable for shares of Domesticated Parent Common Stock (or dividends or distributions with respect thereto or with respect to
SpinCo Common Stock) or cash properly delivered to a public official pursuant to any applicable abandoned property, escheat or similar
Law.
(d)
Closing of Transfer Books. From and after the Effective Time, the stock transfer books of SpinCo shall be closed, and no
transfer shall be made of any shares of capital stock of SpinCo that were outstanding as of immediately prior to the Effective Time.
(e)
Tax Withholding. Parent, the Company, SpinCo, Merger Sub and the Exchange Agent shall each be entitled to deduct and withhold
from the consideration otherwise payable pursuant to this Agreement such amounts as are required to be deducted and withheld with respect
to the making of such payment under the Code, or under any provision of state, local or foreign Tax Law. To the extent that amounts are
so deducted or withheld and paid over to the appropriate Governmental Authority, such deducted or withheld amounts will be treated for
all purposes of this Agreement as having been paid to the Person in respect of which such deduction or withholding was made.
3.3
Appraisal Rights. In consideration of the benefits to be received by the Company pursuant to this Agreement, the Company
waives any rights to appraisal that may otherwise be available under the DGCL in connection with the Merger.
Article
IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
As an inducement to Parent
and Merger Sub to enter into this Agreement, except as otherwise disclosed or identified in the Company Disclosure Schedule, the Company
hereby represents and warrants to Parent and Merger Sub as follows:
4.1
Organization of the Company. The Company has been duly incorporated and is validly existing and in good standing as a Nevada
corporation. The Company has all requisite power and authority to own, lease and operate its properties and assets in the manner in which
such assets and properties are now owned, leased and operated and to conduct its business as it is now being conducted. The Company has
made available to Parent and Merger Sub true and complete copies of the Governing Documents of the Company as in effect on the date hereof.
The Company is duly licensed or qualified and in good standing (or equivalent status as applicable) in each jurisdiction in which the
properties and assets owned or leased by it or the character of its activities require it to be so licensed or qualified or in good standing
(or equivalent status as applicable).
4.2
Due Authorization. The Company has all requisite power and authority to execute and deliver this Agreement and the Transaction
Documents to which it is or will be a party, to carry out its obligations hereunder and thereunder, and to consummate the Transactions.
The execution and delivery by the Company of this Agreement and the Transaction Documents to which it is or will be a party as of the
Effective Time and the consummation of the Transactions have been duly authorized by all necessary and proper action on its part, and
no other action on the part of the Company is necessary to authorize this Agreement or the Transaction Documents to which it is or will
be a party as of the Effective Time or consummate the Transactions. Each of this Agreement and the Transaction Documents to which the
Company is or will be a party as of the Effective Time has been or will be duly and validly executed and delivered by it and (assuming
that this Agreement or such other applicable Transaction Documents to which each of Parent and Merger Sub is or will be a party as of
the Effective Time constitutes a legal, valid and binding obligation of each of Parent and Merger Sub (as applicable)), constitutes or
will when executed and delivered constitute the legal, valid and binding obligation of the Company, enforceable against it in accordance
with its terms, subject to applicable bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and similar Laws affecting
creditors’ rights generally and subject to general principles of equity (regardless of whether considered in a proceeding at law
or in equity) (collectively, the “Remedies Exception”).
4.3
Governmental Consents. No Consent of, with or to any Governmental Authority is required to be obtained or made by the Company
in connection with the execution or delivery by the Company of this Agreement or the Transaction Documents to which it is or will be a
party or the consummation by the Company of the Transactions, except for or in compliance with (a) any Premerger Notification and Report
Form required under and in compliance with the HSR Act or other filings in connection therewith; (b) the filing of the Certificate of
Merger and the Parent Charter with the Secretary of State of the State of Delaware pursuant to the provisions of the DGCL; (c) the rules
and regulations of NASDAQ; (d) applicable requirements of state securities or “blue sky” Laws, the Securities Act and the
Exchange Act; and (e) Consents described in Section 5.5 and Consents set forth on Section 4.3 of the Company Disclosure Schedule.
4.4
No Conflict. Subject to the receipt of the Consents set forth in Section 4.3, the execution and delivery by the Company
of this Agreement and the Transaction Documents to which it is or will be a party as of the Effective Time and the consummation by the
Company of the Transactions do not and will not as of the Closing Date, (a) violate any provision of, or result in a conflict with or
the breach of, any Law applicable to the Company or by which any of its assets or properties is bound; (b) with or without lapse of time
or the giving of notice or both, require a consent or approval under, conflict with, result in a violation or breach of, or give to others
any rights of amendment, suspension, revocation of, constitute a default under, result in the acceleration of, create in any party the
right to accelerate, terminate, amend, suspend, revoke, or cancel any Contract to which the Company is a party or by which its assets
or properties are bound, or result in the creation of any Lien on any assets or properties of the Company; or (c) breach or violate any
provision of the Governing Documents of the Company, except, in the case of each of clauses (a) and (b), to the extent that such conflicts,
breaches, defaults or other matters would not (i) materially and adversely affect the ability of the Company to carry out its obligations
under, and to consummate the transactions contemplated by, this Agreement and the Transaction Documents or (ii) materially and adversely
affect the ability of the Company to conduct the SpinCo Business.
4.5
Litigation and Proceedings. Other than disclosed in the Company SEC Documents there are no Actions pending or, to the Knowledge
of the SpinCo Group, threatened, by or against the Company or any of its Subsidiaries (other than SpinCo or with respect to the SpinCo
Assets) that, individually or in the aggregate, would reasonably be expected to result in a Company Material Adverse Effect. Neither the
Company nor any of its Subsidiaries (other than SpinCo or with respect to the SpinCo Assets) is subject to any Order nor, to the Knowledge
of the SpinCo Group, are there any such Orders threatened to be imposed by any Governmental Authority that, in each case, would reasonably
be expected to result, individually or in the aggregate, in a Company Material Adverse Effect.
4.6
Brokers’ Fees. Except for Maxim Group LLC, no broker, investment banker, or other Person is entitled to any brokerage
fee, finders’ fee or other similar commission in connection with the transactions contemplated by this Agreement, including for
which Parent or any of its Subsidiaries, including Merger Sub or the Surviving Corporation, would be liable, based on arrangements made
on behalf of the Company or any of its Affiliates. The Company is solely responsible for the fees and expenses of Maxim Group LLC arising
from the Company’s engagement with Maxim Group LLC.
4.7
Internal Controls. (a) The Company has established and maintains a system of internal controls over financial reporting
(as defined in Rule 13a-l5(f) or 15d-15(f), as applicable, under the Exchange Act (collectively, “Internal Controls”))
that are designed to provide reasonable assurances that (i) transactions are executed in accordance with management’s general or
specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with
GAAP and to maintain accountability for assets, (iii) access to assets is permitted only in accordance with management’s general
or specific authorization and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals
and appropriate action is taken with respect to any differences. The Internal Controls are overseen by the audit committee of the Company
Board (the “Company Audit Committee”). Since September 30, 2019, the Company’s principal executive officer and
its principal financial officer have disclosed to the Company’s independent auditor and the Company Audit Committee (a) any significant
deficiency or material weakness in the Company’s Internal Controls and (b) any fraud involving management or other employees who
have a significant role in the Company’s Internal Controls. Since September 30, 2019, neither the Company nor any of its Subsidiaries
has received any written complaint, allegation, assertion or claim regarding the impropriety of any accounting or auditing practices,
procedures, methodologies or methods of the Company or any of its Subsidiaries or their respective internal accounting controls.
(b)
The financial statements and notes contained or incorporated by reference in the Company SEC Documents (the “Company Financial
Statements”) (i) fairly present in all material respects the financial condition and the results of operations, changes in stockholders’
equity and cash flows of the Company and its Subsidiaries as at the respective dates of, and for the periods referred to, in such financial
statements, all in accordance with GAAP, and (ii) comply in all material respects with the applicable accounting requirements and with
the rules and regulations of Regulation S-X or Regulation S-K, as applicable, subject, in the case of interim financial statements, to
normal recurring year-end adjustments (the effect of which will not, individually or in the aggregate, be material) and the omission of
notes to the extent permitted by Regulation S-X or Regulation S-K, as applicable. The Company has no off-balance sheet arrangements (as
defined by Regulation S-K) that are not disclosed in the Company SEC Documents.
(c) Neither
the Company (including any employee thereof) nor the Company’s independent auditors has identified or been made aware of (i) any
significant deficiency or material weakness in the system of internal accounting controls utilized by the Company, (ii) any Actual Fraud,
whether or not material, that involves the Company’s management or other employees who have a role in the preparation of financial
statements or the internal accounting controls utilized by the Company or (iii) any claim or allegation regarding any of the foregoing.
4.8
Company SEC Filings. Since September 30, 2020, the Company
has timely filed or furnished all statements, prospectuses, registration statements, forms, reports and documents required to be filed
by it with the SEC pursuant to the Exchange Act or the Securities Act. Each of the Company’s SEC filings since September 30, 2020,
as of the respective date of such filing (or if amended or superseded by a filing prior to the date of this Agreement or the Closing Date,
then on the date of such filing), complied in all material respects with the applicable requirements of the Securities Act, the Exchange
Act, the Sarbanes-Oxley Act and any rules and regulations promulgated thereunder applicable to such Company SEC filings. As of the respective
date of its filing (or if amended or superseded by a filing prior to the date of this Agreement or the Closing Date, then on the date
of such filing), no Company SEC filings since September 30, 2020 contained any untrue statement of a material fact or omitted to state
a material fact required to be stated therein or necessary to make the statements made therein, in light of the circumstances under which
they were made, not misleading. As of the date hereof, there are no outstanding or unresolved comments in comment letters received from
the SEC with respect to the Company SEC filings. To the Knowledge of the Company, none of the Company SEC filings filed on or prior to
the date hereof is subject to ongoing SEC review or investigation.
Article
V
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
AND SPINCO
As an inducement to Parent
and Merger Sub to enter into this Agreement, except as otherwise disclosed or identified in (a) with respect to Sections 5.8 and
5.9 the Company SEC Documents filed and publicly available on the SEC’s EDGAR database at least two (2) Business Days prior
to the date hereof (excluding any disclosures of factors or risks contained or references therein under the captions “Risk Factors”
or “Forward-Looking Statements” to the extent they are forward-looking statements and any other similar general, predictive
or cautionary statements) or (b) the corresponding section or subsection of the SpinCo Disclosure Schedule, the Company and SpinCo hereby
represent and warrant as follows:
5.1
Organization of SpinCo. SpinCo has been duly incorporated and is validly existing and in good standing as a Delaware corporation
and has all requisite power and authority to own, lease and operate its assets in the manner in which such assets are now owned, leased
or operated and to conduct its business as it has been and is now being conducted. SpinCo has made available to Parent and Merger Sub
true and complete copies of the Governing Documents of SpinCo. SpinCo is duly licensed or qualified and in good standing (or equivalent
status as applicable) in each jurisdiction in which the properties and assets owned or leased by it or the character of its activities
require it to be so licensed or qualified or in good standing (or equivalent status as applicable).
5.2
Due Authorization. SpinCo has all requisite power and authority to execute and deliver this Agreement and the Transaction
Documents to which it is or will be a party, to carry out its obligations hereunder and thereunder, and to consummate the Transactions
(subject, in the case of the Merger, to the SpinCo Shareholder Approval). The execution and delivery by SpinCo of this Agreement and the
Transaction Documents to which it is or will be a party as of the Effective Time and the consummation by SpinCo of the Transactions have
been or will be duly and validly authorized and approved by all necessary and proper action and, except for the SpinCo Shareholder Approval,
no other action on the part of SpinCo is necessary to authorize this Agreement or the Transaction Documents to which it is or will be
a party at the Effective Time. Each of this Agreement and the Transaction Documents to which it is or will be a party at the Effective
Time has been, or when executed and delivered will be, duly and validly executed and delivered by SpinCo and (assuming that this Agreement
or such other applicable Transaction Document to which Parent or Merger Sub is or will be a party at the Effective Time constitutes a
legal, valid and binding obligation of Parent or Merger Sub (as applicable)) constitutes or will constitute a legal, valid and binding
obligation of SpinCo, enforceable against SpinCo in accordance with its terms, subject to the Remedies Exception.
5.3
Capitalization of SpinCo.
(a)
(i) The authorized capital stock of SpinCo consists of 100,000,000 shares of SpinCo Common Stock, (ii) the issued and outstanding
shares of capital stock of SpinCo consists of 67,500,000 shares of SpinCo Common Stock, and (iii) no shares of SpinCo Common Stock are
being held by SpinCo in its treasury. All of the issued and outstanding shares of SpinCo Common Stock are owned, of record and beneficially,
by the Company and have been duly authorized and validly issued, are fully paid and nonassessable, free and clear of all Liens (other
than Liens imposed by applicable securities Laws) and have not been issued in violation of any preemptive or similar rights.
(b)
Except as set forth in Section 5.3(b) of the SpinCo Disclosure Schedule, there are no (i) outstanding options, warrants,
rights or other securities convertible into or exchangeable or exercisable for shares of capital stock of SpinCo, or any other commitments
or agreements providing for the issuance, sale, repurchase or redemption of shares of capital stock of SpinCo, (ii) obligations of any
kind which may require SpinCo to issue, purchase, redeem or otherwise acquire any of its shares of capital stock or to provide funds to,
or make any investment (in the form of a loan, capital contribution or otherwise) in, any other Person or (iii) voting trusts, proxies
or other agreements or understandings with respect to the voting shares of capital stock of SpinCo.
(c)
The books and records of SpinCo contain a true, correct and complete listing of: (i) the name and address of each Person owning
SpinCo Common Stock and (ii) the certificate number of each certificate evidencing shares of capital stock issued by SpinCo, the number
of shares evidenced by each such certificate, the date of issuance thereof and, in the case of cancellation, the date of cancellation.
5.4
Subsidiaries. SpinCo has no Subsidiaries. Without limiting
the generality of the foregoing, SpinCo does not control, own or possess, directly or indirectly, or have any interest or participation
(direct or indirect) in, any other Person.
5.5
Governmental Consents. No Consent of, with or to
any Governmental Authority is or will be required to be obtained or made by SpinCo in connection with the execution or delivery by SpinCo
of this Agreement or the Transaction Documents to which SpinCo is or will be a party at the Effective Time or the consummation by SpinCo
of the Transactions, except for or in compliance with: (a) any Premerger Notification and Report Form required under and in compliance
with the HSR Act or other filings in connection therewith, (b) the filing of the Certificate of Merger with the Secretary of State of
the State of Delaware pursuant to the provisions of the DGCL; (c) the rules and regulations of NASDAQ; (d) applicable requirements of
state securities or “blue sky” Laws, the Securities Act and the Exchange Act; and (e) Consents described in Section 4.3
and Consents set forth on Section 5.5 of the SpinCo Disclosure Schedule.
5.6
No Conflict. Subject to the receipt of the Consents described
in Section 5.5, the execution and delivery by SpinCo of this Agreement and the Transaction Documents to which SpinCo is or will
be a party at the Effective Time and the consummation by SpinCo of the Transactions do not and will not: (a) violate any provision of,
or result in a conflict with, or the breach of, any Law applicable to SpinCo or by which any of its assets or properties is bound; (b)
with or without lapse of time or the giving of notice or both, require a consent or approval under, conflict with, result in a violation
or breach of, or give to others any rights of amendment, suspension, revocation of, constitute a default under, result in the acceleration
of, create in any party the right to accelerate, terminate, amend, suspend, revoke, or cancel any Contract to which SpinCo is a party
or by which its assets or properties are bound; (c) result in the creation of any Lien upon any of the properties or assets of SpinCo;
or (d) violate any provision of the Governing Documents of SpinCo, except, in the case of clauses (a), (b) and (c), to the extent that
such conflicts, breaches, defaults or other matters would not (i) materially and adversely affect the ability of the SpinCo to carry out
its obligations under, and to consummate the transactions contemplated by, this Agreement and the Transaction Documents or (ii) materially
and adversely affect the ability of the SpinCo to conduct the SpinCo Business.
5.7
Sufficiency of the SpinCo Assets.
(a)
Except as set forth in Section 5.7 of the SpinCo Disclosure Schedule, on the Closing Date, assuming receipt of all consents,
approvals and authorizations relating to the matters set forth in Section 4.3 and Section 5.5, the SpinCo Assets, taking
into account the Shared Services Agreement, the Amended and Restated Shared Services Agreement, and the license granted pursuant to Section
7.18(c), constitute all the properties, assets and rights forming a part of, used, held or intended to be used in, the SpinCo Business,
and constitute all of the assets, properties and rights reasonably necessary for Parent and the Surviving Corporation to conduct the SpinCo
Business immediately following the Closing in all material respects and in substantially the same manner as it is conducted on the date
hereof.
(b)
The Company has caused the SpinCo Assets that are material tangible assets or personal property (“Tangible Personal Property”)
to be maintained in accordance with good business and industry practice, and such SpinCo Assets are in good operating condition and repair
and are suitable for the purposes for which they are used and intended to be used, ordinary wear and tear excepted.
(c)
Except for Permitted Liens, SpinCo has good, valid and marketable title to, or a valid leasehold in, license to or other legal
right to use, as the case may be, all of the SpinCo Assets, free and clear of any Liens other than Permitted Liens.
(d)
The operation of the SpinCo Business as it is now conducted is not dependent upon the right to use the Tangible Personal Property
of Persons other than the Company or its Subsidiary, except for such Tangible Personal Property that is owned by, leased, licensed, or
otherwise duly and validly contracted to the Company or one of its Subsidiaries for use in connection with the SpinCo Business. Any leases
related to the Tangible Personal Property are valid and binding against the Company or one of its Subsidiaries, as applicable, and to
the Knowledge of the SpinCo Group, the counterparty thereto, and are in full force and effect and are enforceable in accordance with their
terms, subject to the Remedies Exception. No event has occurred which (whether with or without notice, lapse of time or both or the happening
or occurrence of any other event) would constitute a material default on the part of the Company or its respective Subsidiary under any
lease related to the Tangible Personal Property, and to the Knowledge of the SpinCo Group, no event has occurred which (whether with or
without notice, lapse of time or both or the happening or occurrence of any other event) would constitute a material default by any other
party under any such lease, and none of the Company or its Subsidiaries has received written notice of any such condition. Neither the
Company nor any of its Subsidiaries has waived any material rights under any license or lease related to the Tangible Personal Property.
To the Knowledge of the SpinCo Group, no event has occurred which either entitles, or, on notice or lapse of time or both, would entitle
the other party to any license or lease related to the Tangible Personal Property with the Company or any of its Subsidiaries to declare
a default or to accelerate, or which does accelerate, the maturity of any obligations of the Company or its Subsidiaries under any such
license or lease.
5.8
Financial Statements.
(a)
Set forth on Section 5.8(a) of the SpinCo Disclosure Schedule are copies of the SpinCo Financial Statements. Each of the
SpinCo Financial Statements fairly presents, and each of the Subsequent Period SpinCo Financial Statements will fairly present, in all
material respects, the financial condition and results of operations and cash flows of the SpinCo Business, as of the dates indicated
therein and for the periods referred to therein; provided, that the SpinCo Financial Statements, the Subsequent Period SpinCo Financial
Statements and the representations and warranties in this Section 5.8 are qualified by the fact that (i) the SpinCo Business has
not operated on a separate standalone basis and has historically been reported within the Company Financial Statements, and (ii) the SpinCo
Financial Statements assume, and the Subsequent Period SpinCo Financial Statements will assume, certain allocated charges and credits,
which do not necessarily reflect amounts that would have resulted from arm’s-length transactions or that the SpinCo Business would
incur on a standalone basis, including after the Closing. The SpinCo Financial Statements were prepared in accordance with GAAP and were
derived from the financial reporting systems and the Company Financial Statements.
(b)
There are no outstanding loans or other extensions of credit made by SpinCo to any executive officer (as defined in Rule 3b-7 under
the Exchange Act) or director of SpinCo. SpinCo has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
5.9
No Undisclosed Liabilities. There are no Liabilities of the Company or any of its Subsidiaries (excluding SpinCo) (in each
case primarily with respect to the SpinCo Business) and SpinCo (excluding any Liabilities related or attributable to Taxes as set forth
on Section 5.9 of the SpinCo Disclosure Schedule) whether or not of a type required to be reflected or reserved for on a consolidated
balance sheet of the Company or related to the SpinCo Business or in the notes thereto prepared in accordance with GAAP, except for (a)
Liabilities reflected or reserved for in the Company Financial Statements or the SpinCo Financial Statements or disclosed in any notes
thereto; (b) Liabilities that have arisen since the Balance Sheet Date in the ordinary course of the operations of the SpinCo Business
and consistent with past practice; (c) Liabilities arising out of or in connection with this Agreement, the Transaction Documents and
the Transactions; (d) Liabilities set forth in Section 5.9 of the SpinCo Disclosure Schedule; (e) Liabilities that are executory
obligations arising in the ordinary course of business under any SpinCo Material Contract (and not as a result of any breach thereof)
or (f) Liabilities that, individually or in the aggregate, would not reasonably be expected to be material to SpinCo.
5.10
Litigation and Proceedings. There are no Actions pending or, to the Knowledge of the SpinCo Group, threatened against SpinCo
or the Company with respect to the SpinCo Business or affecting any of the SpinCo Assets. Neither SpinCo nor the Company (solely in respect
of the SpinCo Assets) is subject to any Order (nor, to the Knowledge of the SpinCo Group, are there any such Orders threatened to be imposed
by any Governmental Authority) which has materially and adversely affected SpinCo or the SpinCo Business or would reasonably be expected
to materially or adversely affect the legality, validity or enforceability of this Agreement, any Transaction Document or the consummation
of the Transactions contemplated hereby or thereby.
5.11
Real Property. SpinCo does not own, and at no time previously has owned, any real property. With respect to the Company
Real Property, (i) the Company or its applicable Subsidiary has good and valid title (or the applicable local equivalent) or a valid and
enforceable leasehold interest, as applicable, in such Company Real Property, free and clear of any Liens, subject to the Remedies Exception
and other than Permitted Liens, and (ii) neither the Company nor any of its Subsidiaries has received (A) written notice of any pending
condemnation, expropriation, eminent domain or similar Action affecting all or any portion of such Company Real Property (to the extent
relating to or affecting the SpinCo Business) or (B) written notice of default or breach of any Company Leased Property. To the Knowledge
of the SpinCo Group, no event has occurred which, with notice, lapse of time or both, would constitute a material default or breach of
any Company Leased Property by the Company or its Subsidiaries. A complete and correct list of the Company Real Property is provided in
Section 5.11 of the SpinCo Disclosure Schedule.
5.12
Tax Matters.
(a)
Except as would not, individually or in the aggregate, have a SpinCo Material Adverse Effect:
(i)
(A) All Tax Returns required to be filed by the Company and SpinCo or with respect to SpinCo or the SpinCo Business have been filed
(taking into account applicable extensions), (B) all such Tax Returns are true, correct and complete, and (C) all Taxes, whether or not
shown as due on such Tax Returns, in respect of SpinCo and the SpinCo Business have been paid, in the case of each of clauses (A) through
(C), except to the extent adequate reserves therefor in accordance with GAAP have been provided on the SpinCo Financial Statements;
(ii)
The Company and SpinCo have each made available to Parent and Merger Sub true, correct and complete copies of all Tax Returns filed
by or with respect to the SpinCo Business for tax years ending on or after December 31, 2021.
(iii)
(A) No Governmental Authority has asserted any written claim, assessment or deficiency for Taxes against the Company, SpinCo or
the SpinCo Business, except for deficiencies or proposed deficiencies that have been satisfied by payment, settled or withdrawn, and (B)
no claim, audit or other proceeding by any Governmental Authority is pending or threatened in writing with respect to any Taxes of the
Company, SpinCo or the SpinCo Business;
(iv)
The Company and SpinCo have no outstanding waivers or extensions of any applicable statute of limitations to assess any amount
of Taxes. There are no outstanding requests by the Company or SpinCo for any extension of time within which to file any material Tax Return
or within which to pay any Taxes shown to be due on any Tax Return;
(v)
SpinCo has no Liability for Taxes of any other Person (other than the Company or any of its Subsidiaries) whether under Treasury
Regulations Section 1.1502-6, as a transferee or successor, or by contract (other than commercial contracts the primary purpose of which
is not Taxes), or by operation of Law or otherwise;
(vi)
Within the past two (2) years, SpinCo has not constituted either a “distributing corporation” or a “controlled
corporation” (within the meaning of Section 355(a)(1)(A) of the Code) in a distribution of stock intended to qualify for tax-free
treatment under Section 355 of the Code;
(vii)
SpinCo has not participated in, and is currently not participating in, a “listed transaction” within the meaning of
Treasury Regulations Section 1.6011-4(b)(2);
(viii)
There are no Liens for Taxes (other than Permitted Liens) upon the assets of SpinCo or the SpinCo Business;
(ix)
SpinCo is not a party to or bound by any Tax indemnity agreement, Tax sharing agreement or Tax allocation agreement or similar
agreement, arrangement or practice with respect to Taxes (including advance pricing agreement, closing agreement or other agreement relating
to Taxes with any taxing authority), except for any commercial contracts the primary purpose of which is not Taxes; and
(x)
SpinCo has not requested and is not the subject of or bound by any private letter ruling, technical advice memorandum, closing
agreement or similar ruling, memorandum, or agreement with any taxing authority with respect to any material Taxes of SpinCo, nor is any
such request outstanding.
(b)
Neither the Company nor any of its Subsidiaries has taken or agreed to take any action or knows of any fact, agreement, plan or
other circumstance that could reasonably be expected to prevent or impede the Tax-Free Status.
(c)
The representations and warranties set forth in this Section 5.12 and, to the extent relating to Tax matters, Section
5.17, constitute the sole and exclusive representations and warranties of the Company and SpinCo regarding Tax matters.
5.13
Absence of Changes.
(a)
Since the Balance Sheet Date until the date of this Agreement there has not been any SpinCo Material Adverse Effect;
(b)
Except in connection with the Transaction Process or as contemplated by this Agreement and the other Transaction Documents, since
the Balance Sheet Date and through the date hereof, the Company and its Subsidiaries, including SpinCo, have, in all material respects,
conducted the SpinCo Business in the ordinary course of business and consistent with past practice.
(c)
Except as disclosed on Section 5.13(c) of the SpinCo Disclosure Schedule, since the Balance Sheet Date, the Company has
not, nor has any of its Subsidiaries, including SpinCo, taken any action that, if taken during the period from the date of this Agreement
through the Closing, would require the consent of Parent or Merger Sub pursuant to Section 7.2.
5.14
Material Contracts.
(a)
Section 5.14(a) of the SpinCo Disclosure Schedule sets forth a true, correct and complete list of, and the Company or SpinCo
has made available to the Parent and the Merger Sub, true, correct and complete copies of, each Contract in effect to which SpinCo is
a party or to which the Company or any of its Subsidiaries is a party to the extent it relates primarily to the SpinCo Business or the
SpinCo Assets or by which the SpinCo Business or the SpinCo Assets are bound (each, a “SpinCo Material Contract”):
(i)
any Contract that relates to the purchase or sale of goods or services pursuant to which the SpinCo Business has received more
than $500,000 or paid more than $500,000 in the past twelve (12) months;
(ii)
(A) any Contract to which SpinCo is a party, that by its terms calls for aggregate payments by the Company or any of its Subsidiaries
under such Contract of more than $500,000 per year or $1,200,000 in the aggregate over the length of the Contract, and (B) any Contract
to which the Company or its Subsidiary (other than SpinCo) is a party, that by its terms calls for aggregate payments by the Company or
any of its Subsidiaries under such contract of more than $500,000 per year or $1,200,000 in the aggregate over the length of the contract,
which payments solely relate to the SpinCo Business;
(iii)
any Contract that contains covenants that materially limit the ability of SpinCo (A) to compete in any line of business or with
any Person or in any geographic area or to sell, or provide any service or product or solicit any Person, including any non-competition
covenants, exclusivity restrictions, rights of first refusal or (B) to purchase or acquire an interest in any other Person;
(iv)
any Contract that limits or purports to limit in any material respect the ability of the SpinCo Business to compete with any Person
or in any line of business or in any geographic region in the world;
(v)
any Contract that grants exclusive rights to a customer or a supplier or (to the extent material to the SpinCo Business) any other
commercial counterparty that will relate to or affect the SpinCo Business after the Closing;
(vi)
any Contract that requires any future capital expenditures by the SpinCo Business in excess of $500,000 that will not be paid prior
to the Closing;
(vii)
any Contract that requires any continuing indemnification obligations or milestone, earn out or similar payments to be made by
the SpinCo Business in excess of $1,000,000 in the aggregate that will not be paid prior to the Closing;
(viii)
any Contract that relates to the creation, incurrence, assumption or guarantee of any indebtedness for borrowed money or any bonds,
debentures, notes or similar instruments, in each case, in excess of $500,000;
(ix)
any Contract pursuant to which (A) any Person grants to SpinCo or, with respect to the SpinCo Business, to the Company or any of
its Subsidiaries other than SpinCo, any license, right, permission, consent, non-assertion or release with respect to any Intellectual
Property that is material to the SpinCo Business, other than (1) non-exclusive click-wrap, shrink-wrap or off-the-shelf software licenses
that are commercially available on standard and reasonable terms to the public generally with license, maintenance, support and other
fees of less than $200,000 in any twelve (12)-month period, (2) non-disclosure agreements entered into in the ordinary course of business
consistent with past practice and (3) non-exclusive licenses granted by any suppliers or service providers to SpinCo in the ordinary course
of business consistent with past practice solely for the receipt of services from such supplier or service provider, and solely where
such licenses are ancillary to the primary purpose of such Contract, or (B) the Company or any of its Subsidiaries (excluding SpinCo)
(in each case solely with respect to the SpinCo Business) or SpinCo grants any license, right, permission, consent, non-assertion or release
with respect to any Intellectual Property that is material to the SpinCo Business, other than (1) non-exclusive licenses granted to customers
of SpinCo in the ordinary course of business consistent with past practice, (2) non-exclusive licenses granted to any suppliers or service
providers by SpinCo in the ordinary course of business consistent with past practice and (3) non-disclosure agreements entered into in
the ordinary course of business consistent with past practice;
(x)
any Contract to which the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo
Business) or SpinCo is a party with any Governmental Authority or any university, college, research institute, or other educational institution
that provides for the provision of funding by or to SpinCo or the Company or any of its other Subsidiaries, in each case, for any research
or development activities involving the invention, creation, conception or development of any Intellectual Property that is material to
the SpinCo Business;
(xi)
any lease, sublease, occupancy agreement or license for real property;
(xii)
any Contract that contains “most favored nation” pricing provisions for the benefit of the relevant counterparty that
will relate to or affect the SpinCo Business after the Closing;
(xiii)
any joint venture, strategic alliance, joint development, partnership or similar arrangement;
(xiv)
any Contract relating to the acquisition or disposal or divestiture of, or investment in, any joint venture, partnership or similar
arrangement or any material assets or businesses;
(xv)
any Contract providing for (or providing an option for) any merger, consolidation or other business combination with any other
Person or the acquisition or disposition of any other entity or its business or material assets or the sale of the Company, SpinCo, their
material assets or the SpinCo Business;
(xvi)
any Contract that is between SpinCo and any of its directors, executive officers, shareholders or Affiliates, including the Company,
including all non-competition, severance and indemnification agreements;
(xvii)
any Contract that provides another Person with a power of attorney;
(xviii)
any prime contract, subcontract, purchase order, task order, delivery order, teaming agreement, joint venture agreement, strategic
alliance agreement, basic ordering agreement, pricing agreement, letter contract or other similar arrangement of any kind where the counterparty
or the ultimate customer is, or the work performed under such contract was funded by, a Governmental Authority; and
(xix)
any Contract not otherwise described in any other subsection of this Section 5.14(a) that would be required to be filed
by SpinCo as a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K of the SEC) if SpinCo were
subject to the reporting requirements of the Exchange Act as of the date hereof.
(b)
Except as disclosed in Section 5.14(b) of the SpinCo Disclosure Schedule, (i) each SpinCo Material Contract is valid and
binding on the Company or its applicable Subsidiary, including SpinCo and, to the Knowledge of the SpinCo Group, the counterparty thereto,
and is in full force and effect and enforceable in accordance with its terms, subject to the Remedies Exception; (ii) neither the Company
nor its applicable Subsidiary, including SpinCo is, and to the Knowledge of the SpinCo Group, no counterparty thereto is, in breach of,
or default under, any SpinCo Material Contract in any material respect; (iii) to the knowledge of the SpinCo Group, as applicable, no
event has occurred that with the passage of time or giving of notice or both would constitute such a breach or default by a counterparty
to a SpinCo Material Contract, or permit termination or acceleration by the Company or SpinCo, under such SpinCo Material Contract; (iv)
no other party to a SpinCo Material Contract has notified the Company or SpinCo in writing that it is terminating or considering terminating
its business with the Company (solely in respect of the SpinCo Business) or SpinCo or is planning to materially reduce its future business
with the Company (solely in respect of the SpinCo Business) or SpinCo; and (v) neither the Company nor SpinCo has waived any material
rights under a SpinCo Material Contract.
5.15
Employment Matters.
(a)
Since their respective dates of formation, neither the Company nor any of its Subsidiaries, including SpinCo, is or has been a
party to any agreement with any trade union, works council, employee representative body or labor organization (covered by the National
Labor Relations Act) that represents (or that otherwise governs or relates to the employment of) any employees of the Company or its Subsidiaries,
including SpinCo. To the Knowledge of the SpinCo Group, (i) no petition for recognition of a labor organization or other body for the
representation of the employees of the Company and its Subsidiaries is pending or threatened, and (ii) there has not during the last three
(3) years been any (or threat of any), and there are no pending and no Person has threatened to commence any, strike, slowdown, work stoppage,
lockout, picketing, labor dispute, union organizing activity, or any similar activity or dispute, in each case affecting the SpinCo Business
or SpinCo.
(b)
There are no pending, or to the Knowledge of the SpinCo Group, anticipated or threatened, unfair labor or other employment-related
practice charges, complaints or other grievances or Actions by or before any Governmental Authority, arising under any applicable Law
governing labor or employment, in each case in respect of which SpinCo or the SpinCo Business will have any material Liability at the
Effective Time.
(c)
There are no Actions pending or, or to the Knowledge of the SpinCo Group, threatened involving the Company or any of its Subsidiaries
(excluding SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo, or any of their respective employees or former
employees (with respect to their status as an employee or former employee, as applicable) including any harassment, discrimination, retaliatory
act, or similar claim.
(d)
Since its formation, SpinCo has had no employees and all employee or consulting services necessary for the operation of the SpinCo
Business have been provided by the Company or one of its Subsidiaries pursuant to the Shared Services Agreement. For the past three (3)
years, the Company has been in compliance in all material respects with all Laws relating to terms and conditions of employment, employment
practices, employment discrimination and harassment, civil rights, the Worker Adjustment and Retraining Notification Act and any similar
state or local plant closures and mass layoffs Laws, wages (including minimum wage and overtime), hours of work, withholdings and deductions,
classification and payment of employees, independent contractors and consultants, employment equity, occupational health and safety, workers’
compensation, immigration, and workforce reduction with respect to any employee or independent contractor providing services to the SpinCo
Business or in respect of which SpinCo will have any material Liability at the Effective Time.
(e)
Except as would not reasonably be expected to result in material Liability to the SpinCo Business (taken as a whole), neither the
Company nor any of its Subsidiaries, including SpinCo, has incurred or is liable for, and no circumstances exist under which the Company
or any of its Subsidiaries would reasonably be expected to incur or be liable for with respect to any Person that provides services to
SpinCo as an employee or independent contractor, any liability arising from (i) the failure to pay wages (including overtime wages), (ii)
the misclassification of employees as independent contractors or (iii) the misclassification of employees as exempt from the requirements
of the Fair Labor Standards Act or similar state Laws.
(f)
Section 5.15(f) of the SpinCo Disclosure Schedule sets forth a complete and accurate list of all current employees of the
Company or any of its Subsidiaries who provide services to SpinCo or the SpinCo Business, showing each employee’s name, job title
or description, employer, location, and salary level (including any bonus, commission, deferred compensation or other remuneration payable,
and detailing the extent of their allocation to SpinCo or the SpinCo Business). Except as set forth on Section 5.15(f) of the Company
Disclosure Schedule, (A) no such employee is a party to a written employment agreement or contract with the Company or any of its
Subsidiaries and each is employed “at will”, and (B) the Company, or one of its Subsidiaries, has paid in full to all such
employees all wages, salaries, commission, bonuses and other compensation due to such employees, including overtime compensation, and
there are no severance payments which are or could become payable by the Company, or the Subsidiary, to any such employees under the terms
of any written or oral agreement, or commitment or any Law, custom, trade or practice. Each such employee has entered into the Company’s
or one of its Subsidiary’s standard form of employee non-disclosure, inventions and restrictive covenants agreement with the Company
or such Subsidiary, a copy of which has been provided to the Parent by the Company. The Company has provided to Parent and Merger Sub
true and complete copies of each employment agreement with such employee.
(g)
Section 5.15(g) of the SpinCo Disclosure Schedule contains a list of all independent contractors (including consultants)
currently engaged by the Company who provide services to SpinCo or the SpinCo Business, along with the position, the entity engaging such
Person, date of retention and rate of remuneration, and details of the extent of their allocation to SpinCo or the SpinCo Business for
each such Person. All of such independent contractors are a party to a written agreement or contract with the Company or one of its Subsidiaries.
Each such independent contractor has entered into customary covenants regarding confidentiality and assignment of inventions and copyrights
in such Person’s agreement with the Company or one of its Subsidiaries, a copy of which has been provided to the Parent by the Company.
For the purposes of applicable Law, including the Internal Revenue Code of 1986, as amended, all independent contractors who are currently,
or within the last three (3) years have been, engaged by the Company or one of its Subsidiaries are bona fide independent contractors
and not employees of the Company or such Subsidiary. Except as set forth on Section 5.15(g) of the SpinCo Disclosure Schedule,
each independent contractor is terminable on fewer than thirty (30) days’ notice, without any obligation of the Company or SpinCo
to pay severance or a termination fee. The Company has provided to Parent and Merger Sub true and complete copies of each service or other
agreement with such independent contractor.
5.16
Compliance with Law; Permits.
(a)
Except as set forth on Section 5.16(a) of the Company Disclosure Schedule, and for Environmental Laws (which are addressed
exclusively as set forth in Section 5.19(a)), the Company and its Subsidiaries (excluding SpinCo) (in each case solely with respect
to the SpinCo Business) and SpinCo are, and, during the past two (2) years, have (i) been in compliance with all applicable Laws, except
where noncompliance has not been and would not reasonably be expected to materially and adversely affect SpinCo or the SpinCo Business
and (ii) not received notice from any Governmental Authority alleging any material non-compliance with or material violation of any applicable
Law or that the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo
is subject to any inspection, investigation, survey, audit or other review.
(b)
Except as set forth on Section 5.16(b) of the Company Disclosure Schedule and except with respect to Permits required under
applicable Environmental Laws (which are addressed exclusively in Section 5.19(a)), (i) the Company and its Subsidiaries (excluding
SpinCo) (in each case solely with respect to the SpinCo Business) and SpinCo hold all Permits necessary to conduct the SpinCo Business
consistent with past practice and in compliance with applicable Law (the “SpinCo Permits”) and (ii) the SpinCo Permits
are valid and in full force and effect and the Company or its applicable Subsidiary is in compliance with the terms thereof.
(c)
Section 5.16(c) of the SpinCo Disclosure Schedule sets forth a list of each SpinCo Permit.
5.17
Benefit Plans.
(a)
Except as set forth on Section 5.17(a) of the SpinCo Disclosure Schedule, there are no SpinCo Benefit Plans. No Company
Benefit Plan or SpinCo Benefit Plan is (i) an employee benefit plan subject to Section 302 or Title IV of ERISA or Section 412, 430 or
4971 of the Code; (ii) an employee benefit plan for which SpinCo could incur liability under Section 4069 of ERISA in the event such plan
has been or were to be terminated or (iii) a plan in respect of which SpinCo or the Company could incur liability under Section 4212(c)
of ERISA. There are no other employee benefit plans, including any multiemployer plan (within the meaning of Section 3(37) or 4001(a)(3)
of ERISA) or single employer pension plan (within the meaning of Section 4001(a)(15) of ERISA), for which SpinCo could incur liability
under Section 4063 or 4064 of ERISA. Neither SpinCo nor the Company have any express or implied commitment, (1) to create, incur liability
with respect to, or cause to exist, any other SpinCo Benefit Plan, or (2) to modify, change or terminate any SpinCo Benefit Plan, other
than with respect to a modification, change or termination required by applicable Law. SpinCo does not have any express or implied commitment
to enter into any contract or agreement to provide compensation or benefits to any individual.
(b)
With respect to each SpinCo Benefit Plan, the Company has made available to Parent true and complete copies of, to the extent applicable,
(i) the current plan document (including all amendments thereto), (ii) the most recent summary plan description including any summary
of material modifications provided to participants, (iii) the last filed Form 5500 series and all schedules thereto, (iv) the most recent
determination, opinion or advisory letter issued by the IRS and (v) any material, non-routine communications with any Governmental Authority
in the past three (3) years.
(c)
Each SpinCo Benefit Plan has been operated, funded and administered in all material respects in accordance with its terms and in
material compliance with applicable Law, including ERISA and the Code. Except as set forth on Section 5.17(c) of the SpinCo Disclosure
Schedule, there are no pending or threatened Actions or audits (other than routine claims for benefits) involving any SpinCo Benefit
Plan.
(d)
With respect to each SpinCo Benefit Plan (i) all required contributions have been made or properly accrued, (ii) there have been
no “prohibited transactions” (as that term is defined in Section 406 of ERISA or Section 4975 of the Code) and (iii) all reports,
returns, and similar documents required to be filed with any Governmental Authority or distributed to any SpinCo Benefit Plan participant
have been timely filed or distributed. SpinCo has complied with its obligations under any plan, program or arrangement that is sponsored,
maintained or administered by any Governmental Authority.
(e)
Except as set forth on Section 5.17(e) of the SpinCo Disclosure Schedule the consummation of the Transactions shall not,
either alone or in combination with another event: (i) entitle any current or former employee of or consultant to SpinCo, including those
operating the SpinCo Business pursuant to the Shared Services Agreement, to severance pay, unemployment compensation or any other benefits
or payments; or (ii) accelerate the time of payment, funding or vesting, or increase the amount of any payments or benefits due to any
employee of or consultant to SpinCo, including those operating the SpinCo Business pursuant to the Shared Services Agreement.
(f)
Except as set forth in Section 5.17(f) of the SpinCo Disclosure Schedule, no SpinCo Benefit Plan provides for, and SpinCo
does not have any current or contingent Liability in respect of, post-retirement or other postemployment health or welfare benefits.
(g)
Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated hereby or by the Transaction
Documents shall, either alone or in combination with any other event(s), result in the payment of any amount to any current or former
employee, director, officer or independent contractor of SpinCo that could, individually or in combination with any other such payment,
constitute an “excess parachute payment” as defined in Section 280G(b)(1) of the Code.
(h)
Each SpinCo Benefit Plan that is a “nonqualified deferred compensation plan” (as such term is defined in Section 409A(d)(1)
of the Code and the guidance thereunder) is in material compliance in both form and operation with Section 409A of the Code, and no Taxes
are owed (or will be owed, as applicable) under Section 409A(a)(1) for any such plan or arrangement. SpinCo is not under any obligation
to reimburse or gross up any employer or other service provider in respect of any Taxes, including under Section 409A of the Code or Sections
280G or 4999 of the Code.
5.18
Intellectual Property.
(a)
Section 5.18(a) of the SpinCo Disclosure Schedule sets forth a list of, as of the date hereof, all SpinCo Owned Intellectual
Property that is the subject of any registration, issuance, or application for registration or issuance, with any Governmental Authority
or Internet domain name registrar (specifying for each such item (i) the record owner (and, if different from the record owner, the beneficial
owner), (ii) the jurisdiction in which such item has been issued, registered or filed, (iii) the issuance, registration or application
date and (iv) the issuance, registration or application number) (any Intellectual Property set forth or required to be set forth on Section
5.18(a) of the SpinCo Disclosure Schedule, collectively, the “SpinCo Registered Intellectual Property”).
(b)
All SpinCo Registered Intellectual Property that is material to the SpinCo Business is subsisting, and, to the Knowledge of the
SpinCo Group, valid and enforceable. Except as scheduled in Section 5.18(b) of the SpinCo Disclosure Schedule, to the Knowledge
of the SpinCo Group, none of the SpinCo Registered Intellectual Property has been or is subject to any interference, derivation, reexamination,
including ex parte reexamination, inter partes reexamination, inter partes review or post grant review, cancellation or opposition proceeding.
(c)
SpinCo solely and exclusively owns all rights, title and interest in and to the SpinCo Owned Intellectual Property, in each case,
free and clear of all Liens (other than Permitted Liens and the license granted pursuant to Section 7.18(c)) and to the Knowledge
of the SpinCo Group, SpinCo has valid and enforceable rights to use and exploit, pursuant to a written SpinCo Contract, all other Intellectual
Property (except for such other Intellectual Property in the public domain for which no license is necessary) used or practiced by the
SpinCo Business that is material to the SpinCo Business. The SpinCo Intellectual Property constitutes all Intellectual Property (except
for such other Intellectual Property in the public domain for which no license is necessary) used in, and necessary and sufficient for,
the conduct and operation of the SpinCo Business, as currently conducted; provided, that the foregoing representation shall not in any
way be construed as a representation of non-infringement or other violation of the Intellectual Property rights of any Person.
(d)
To the Knowledge of the SpinCo Group, in the past three (3) years, none of the Company or any of its Subsidiaries (excluding SpinCo)
(in each case solely with respect to the SpinCo Business) or SpinCo, the conduct of the SpinCo Business, or any SpinCo Owned Intellectual
Property has infringed, misappropriated (or constituted or resulted from a misappropriation of), or otherwise violated, or is infringing,
misappropriating (or constitutes or results from the misappropriation of), or otherwise violating any Intellectual Property of any Person.
To the Knowledge of the SpinCo Group, none of the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect
to the SpinCo Business) or SpinCo has received from any Person in the past three (3) years any written notice charge, complaint, claim
or other assertion: (i) of any infringement, misappropriation or other violation of any Intellectual Property of any Person or (ii) challenging
the ownership, use, validity or enforceability of any SpinCo Owned Intellectual Property, in each case of clauses (i) and (ii) that is
material to the SpinCo Business as currently conducted.
(e)
To the Knowledge of the SpinCo Group, no other Person has infringed, misappropriated, diluted or violated, or is infringing, misappropriating,
diluting or violating, any SpinCo Owned Intellectual Property or any SpinCo Licensed Intellectual Property exclusively licensed to the
Company or any of its Subsidiaries, in each case, that is material to the SpinCo Business. No such claims have been made in writing or,
to the Knowledge of the SpinCo Group, otherwise made against any Person by the Company or any of its Subsidiaries (excluding SpinCo) (in
each case solely with respect to the SpinCo Business) or SpinCo in the past three (3) years.
(f)
To the Knowledge of the SpinCo Group, the Company and its Subsidiaries (excluding SpinCo) (in each case solely with respect to
the SpinCo Business) and SpinCo have taken and currently take reasonably adequate and commercially reasonable steps to maintain the secrecy
and confidentiality of all Trade Secrets included in the SpinCo Owned Intellectual Property and all Trade Secrets of any Person to whom,
the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo, has a confidentiality
obligation with respect to such Trade Secrets. No Trade Secret material to the SpinCo Business has been authorized by SpinCo, the Company
or any of its Subsidiaries, to be disclosed (or, to the Knowledge of the SpinCo Group, has been disclosed) to any Person other than (i)
pursuant to a written agreement reasonably restricting the disclosure and use of such Trade Secret or (ii) to a Person who otherwise has
a duty to protect such Trade Secret.
(g)
Each of the past and present employees of, and individuals acting on a consultant or independent contractor basis for, SpinCo,
the Company or any of its Subsidiaries who has been or is engaged in inventing, creating, conceiving or developing any Intellectual Property
that is material to the SpinCo Business as currently conducted for the Company or any of its Subsidiaries (excluding SpinCo) (in each
case solely with respect to the SpinCo Business) or SpinCo, has executed and delivered to the Company or such Subsidiary or SpinCo, as
applicable, a written agreement, pursuant to which such Person (x) agreed to hold all confidential information of the SpinCo Business
in confidence both during and after such Person’s employment or retention, as applicable, and (y) assigned to SpinCo, the Company
or such Subsidiary, as applicable, all of such Person’s rights, title and interest in and to all Intellectual Property invented,
created, conceived or developed in the course of such Person’s employment or engagement thereby (each, a “Personnel IP
Contract”). To the Knowledge of the SpinCo Group, there is no uncured breach by any such Person with respect to any Intellectual
Property that is material to the SpinCo Business as currently conducted under any such Personnel IP Contract.
(h)
No funding, facilities or personnel of any Governmental Authority or any university, college, research institute or other educational
institution has been or is being used to invent, create, conceive or develop, in whole or in part, (i) any SpinCo Owned Intellectual Property
or (ii) to the Knowledge of the SpinCo Group, any SpinCo Licensed Intellectual Property exclusively licensed to SpinCo, the Company or
any of its Subsidiaries, in each case of clauses (i) and (ii), that is material to the SpinCo Business as currently conducted, except
for any such funding or use of facilities or personnel that (A) does not result in such Governmental Authority, university, college, research
institute or other educational institution obtaining or retaining, or having the right to obtain or retain ownership of, or use rights
to (except for use rights during the term of the applicable SpinCo Contract with such Governmental Authority, university, college, research
institute or other educational institution), any SpinCo Owned Intellectual Property, or (B) does not require or otherwise obligate SpinCo,
the Company or any of its Subsidiaries to grant or offer to any Governmental Authority, university, college, research institute or other
educational institution any license or other right to, or covenant not to assert with respect to, any SpinCo Owned Intellectual Property
(except for use rights during the term of the applicable SpinCo Contract with such Governmental Authority, university, college, research
institute or other educational institution).
(i)
Neither the execution of this Agreement or any of the other Transaction Documents nor the consummation of the Transactions will
result in the loss or impairment of, or any Lien on, the payment of any additional consideration, or the reduction of any amount(s) payable
in connection with, any material SpinCo Intellectual Property.
5.19
Environmental Matters.
(a)
Except as would not otherwise reasonably be expected to have, individually or in the aggregate, a SpinCo Material Adverse Effect:
(i)
With respect to the operation of SpinCo and the SpinCo Business, the Company and its Subsidiaries are, and during the past three
(3) years the Company and its Subsidiaries have been, in compliance with applicable Environmental Laws, which compliance includes obtaining,
maintaining, and complying with all Permits required under Environmental Laws for the operation of the SpinCo Business, all of which Permits
are in full force and effect;
(ii)
With respect to the operation of SpinCo and the SpinCo Business, the Company and its Subsidiaries have not received written notice
from any Governmental Authority or Person alleging any non-compliance with or Liability under any applicable Environmental Law by the
Company or any of its Subsidiaries;
(iii)
No Actions pursuant to any Environmental Law to the extent affecting SpinCo, the SpinCo Business or any SpinCo Assets are pending
or threatened in writing against the Company or any of its Subsidiaries; and
(b)
Neither the Company nor any of its Subsidiaries has Released Hazardous Materials at, on, upon, into or from any Company Real Property
at concentrations or under conditions that would result in the Company or any Subsidiary incurring any Liability that is material to SpinCo
under Environmental Laws.
(c)
Except for the representations and warranties expressly set forth in this Section 5.19, none of the Company, SpinCo, or
any other Person makes any other express or implied representation or warranty with respect to Environmental Laws or Permits thereunder,
and none of the other representations and warranties contained in this Agreement or in any certificate, document or instrument delivered
pursuant to this Agreement will be deemed to be given in relation to Environmental Laws Permits thereunder.
5.20
Affiliate Matters. Except for Contracts set forth in Section 5.20 of the SpinCo Disclosure Schedule, and Contracts
for employment, compensation or benefit agreements or arrangements with directors, officers and employees made in the ordinary course
of business or pursuant to SpinCo Benefit Plans or Company Benefit Plans, SpinCo is not a party to any SpinCo Affiliate Contract.
5.21
Brokers’ Fees. Except for Maxim Group LLC, no broker, finder, investment banker or other Person is entitled to any
brokerage fee, finders’ fee or other similar commission, for which Parent, Merger Sub or SpinCo would be liable in connection with
the transactions contemplated by this Agreement or any other Transaction Document.
5.22
Proxy Statement; Registration Statement. None of the information regarding any of the Company or any of its Subsidiaries
(including SpinCo), the SpinCo Business, or the Transactions to be provided by the Company or SpinCo specifically for inclusion in, or
incorporation by reference into, the Proxy Statement or the Parent Registration Statement will, in the case of the Proxy Statement or
any amendment or supplement thereto, at the time of the first mailing of the Proxy Statement and of any amendment or supplement thereto,
or, in the case of the Parent Registration Statement, at the time such registration statement becomes effective, on the date of the Parent
Shareholders Meeting, or at the Effective Time, contain any untrue statement of a material fact or omit to state any material fact required
to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they are made,
not false or misleading.
5.23
Board and Shareholder Approval. Each of the Company Board and the board of directors of SpinCo (the “SpinCo Board”),
at a meeting duly called and held or by written consent, has by unanimous vote of all directors present or unanimous consent, approved
this Agreement and the other Transaction Documents and authorized and approved the execution, delivery and performance hereof and thereof
and the consummation of the Transactions, including the Merger. Each of the Company and the SpinCo Board at a meeting duly called and
held or by written consent, has by unanimous vote of all directors present or unanimous consent, declared this Agreement advisable. The
SpinCo Shareholder Approval is the only approval of the shareholders of SpinCo required to approve the Transactions.
5.24
Healthcare Regulatory Matters.
(a)
The Company and SpinCo are, and have been since their formation, in compliance with all applicable Healthcare Laws, except where
such failure to so comply would not reasonably be expected to materially and adversely impact the SpinCo Business. Neither the Company
nor SpinCo has received any written notice from any Regulatory Authority alleging any material violation of any applicable Healthcare
Law. To the Knowledge of the SpinCo Group, there are no Actions pending or threatened against the Company or any of its Subsidiaries (excluding
SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo or any of the SpinCo Products or alleging any material violation
by the Company, SpinCo, the SpinCo Business or the SpinCo Products of any such applicable Healthcare Law.
(b)
Except as set forth in Section 5.24(b) of the SpinCo Disclosure Schedule, (i) SpinCo does not hold any Regulatory Authorization,
and (ii) SpinCo does not have any application for a Regulatory Authorization pending with the FDA or any other applicable Regulatory Authority.
(c)
Except as set forth in Section 5.24(c) of the SpinCo Disclosure Schedule, (i) the Company does not hold any Regulatory Authorization
in relation to the SpinCo Business, and (ii) the Company, in relation to the SpinCo Business, does not have any application for a Regulatory
Authorization pending with the FDA or any other applicable Regulatory Authority.
(d)
None of the Company and its Subsidiaries or any person acting on behalf of any of the Company and its Subsidiaries has with respect
to any SpinCo Product: (i) been subject to a shutdown or import or export prohibition imposed by any Regulatory Authority; or (ii) received
any FDA Form 483, or other written notice of inspectional observations, “warning letters,” “untitled letters”
or any similar written correspondence from any Regulatory Authority in respect of such Entity or its business operations, alleging or
asserting material noncompliance with any applicable Healthcare Law or Regulatory Authorization. No Regulatory Authority has threatened
such action.
(e)
To the Knowledge of the SpinCo Group, neither SpinCo nor the Company has (i) made an untrue statement of a material fact or a fraudulent
claim or statement to any Regulatory Authority with respect to the SpinCo Business or the SpinCo Products, (ii) failed to disclose a material
fact required to be disclosed to any Regulatory Authority with respect to the SpinCo Business or the SpinCo Products or (iii) committed
an act, made a disclosure, or failed to commit an act or make a disclosure, including with respect to any scientific data or information,
that, at the time of such action, failure to act, disclosure or failure to disclose (as applicable), would reasonably be expected to provide
a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities”,
set forth in 56 Fed. Reg. 46191 (September 10, 1991), and any amendments thereto, or for the FDA or any other Regulatory Authority to
invoke any similar policy, in each case with respect to the SpinCo Business or the SpinCo Products. None of the Company, SpinCo nor, to
the Knowledge of the SpinCo Group, any of their officers, employees or agents has been convicted of any crime or engaged in any conduct
that has resulted in, or would reasonably be expected to result in, debarment from participation in any program related to pharmaceutical
products pursuant to 21 U.S.C. Section 335a (a) or (b) or exclusion from participation in any federal health care program.
5.25
Data Privacy.
(a)
The Company and its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) and SpinCo are and
have for the past three (3) years been, in material compliance with all Privacy Requirements. During the past three (3) years, neither
the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo has received
any written notice of any claims, charges, investigations, or regulatory inquiries related to or alleging the violation of any Privacy
Requirements.
(b)
The Company and its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) and SpinCo, have
(i) implemented, and for the past three (3) years have maintained, commercially reasonable technical and organizational safeguards to
protect Personal Information in its possession or under its control, and (ii) taken commercially reasonable steps to ensure that any third
party with access to any Personal Information collected by or on behalf of the Company or any of its Subsidiaries (excluding SpinCo) (in
each case solely with respect to the SpinCo Business) or SpinCo has implemented and maintained commercially reasonable safeguards with
respect to such third party’s processing of Personal Information.
(c)
During the past three (3) years, to the Knowledge of the SpinCo Group: (i) there have been no material breaches, security incidents,
misuse of or unauthorized access to or disclosure of any Personal Information (“Security Incident”) maintained by the
Company or its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) or SpinCo; nor (ii) has any Person
processing Personal Information on behalf of the Company or any of its Subsidiaries (excluding SpinCo) (in each case solely with respect
to the SpinCo Business) or SpinCo experienced any Security Incidents with respect to such Personal Information. The Company and its Subsidiaries
(excluding SpinCo) (in each case solely with respect to the SpinCo Business) and SpinCo have implemented reasonable disaster recovery
and business continuity plans.
(d)
To the Knowledge of the SpinCo Group, the transfer of Personal Information in connection with the Transactions will not violate
in any material respect any Privacy Requirements.
5.26
Anti-Bribery, Anti-Corruption and Anti-Money Laundering. None of the Company or its Subsidiaries (excluding SpinCo) (in
each case solely with respect to the SpinCo Business) or SpinCo or, to the Knowledge of the SpinCo Group, any of their respective directors,
officers, employees, agents, or any other Person acting for or on behalf of the SpinCo Business has, directly or indirectly (a) made,
offered, or promised to make or offer any payment, loan, or transfer of anything of value including any reward, advantage or benefit of
any kind, to or for the benefit of any Government Official, candidate for public office, political party, or political campaign, for the
purpose of (i) influencing any act or decision of such Government Official, candidate, party or campaign, (ii) inducing such Government
Official, candidate, party or campaign to do or omit to do any act in violation of a lawful duty, (iii) obtaining or retaining business
for or with any Person, (iv) expediting or securing the performance of official acts of a routine nature, or (v) otherwise securing any
improper advantage; (b) paid, offered, or promised to pay or offer any bribe, payoff, influence payment, kickback, unlawful rebate, or
other similar unlawful payment of any nature; (c) made, offered or promised to make or offer any contributions, gifts, entertainment,
or other unlawful expenditures; (d) established or maintained any fund of corporate monies or other properties; (e) created or caused
the creation of any false or inaccurate books and records of the Company or its Subsidiaries (excluding SpinCo) (in each case solely with
respect to the SpinCo Business) or SpinCo; or (f) otherwise violated any provision of the Foreign Corrupt Practices Act of 1977, 15 U.S.C.
§§ 78dd-1, et seq., the Money Laundering Control Act, the Currency and Foreign Transactions Reporting Act, The Uniting and Strengthening
America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001, or any other Laws relating to corruption,
bribery, or money laundering, in each case of clauses (a)-(e), in a manner that would result in a violation of any of the Laws described
in clause (f). None of the Company or its Subsidiaries (excluding SpinCo) (in each case solely with respect to the SpinCo Business) or
SpinCo has made any disclosure to any Governmental Authority relating to corruption, bribery, or money laundering Laws, been the subject
of any investigation or inquiry regarding compliance with such Laws; or been assessed any fine or penalty under such Laws.
5.27
Sanctions, Import, and Export Controls. None of the Company or its Subsidiaries (excluding SpinCo) (in each case solely
with respect to the SpinCo Business) or SpinCo or, to the Knowledge of the SpinCo Group, any of their respective directors, officers,
employees, agents, or any other Person acting for or on behalf of the SpinCo Business or SpinCo (a) is a Person with whom transactions
are prohibited or limited under any economic sanctions Laws, including those administered by the U.S. government (including, without limitation,
the Department of the Treasury’s Office of Foreign Assets Control, the Department of State, or the Department of Commerce), the
United Nations Security Council, the European Union, or His Majesty’s Treasury, or (b) has violated any Laws relating to economic
sanctions within the last five (5) years. Within the past five (5) years, none of the Company or its Subsidiaries (excluding SpinCo) (in
each case solely with respect to the SpinCo Business) or SpinCo has violated any Laws related to or made any disclosure to any Governmental
Authority relating to sanctions, customs, import, or export control Laws; to the Knowledge of the SpinCo Group, been the subject of any
investigation or inquiry regarding compliance with such Laws; or been assessed any fine or penalty under such Laws.
5.28
No Other Representations and Warranties.
(a)
Except as expressly set forth in Article IV and this Article V, neither the Company nor any of its Affiliates (including
SpinCo) is making, and each of the Company and its Affiliates (including SpinCo) expressly disclaims, any representation or warranty,
express or implied, with respect to the Company, its Affiliates (including SpinCo) or the SpinCo Business or with respect to any other
information provided, or made available, to Parent or any of its Affiliates or Representatives in connection with the Transactions, including
information, documents, projections, forecasts or other material made available to Parent, its Affiliates or Representatives in any “data
rooms,” management presentations or otherwise in connection with the Transactions.
(b)
Each of the Company and SpinCo acknowledges that it is not relying on, and that Parent and its Affiliates have not made, any representation
or warranty except as specifically set forth in Article VI.
Article
VI
REPRESENTATIONS AND WARRANTIES OF PARENT AND
MERGER SUB
As an inducement to the Company
and SpinCo to enter into this Agreement, except as otherwise disclosed or identified in the corresponding section or subsection of the
Parent Disclosure Schedule, Parent and Merger Sub hereby represent and warrant to the Company and SpinCo as follows:
6.1
Organization of Parent and Merger Sub.
(a)
Parent has been duly incorporated and is validly existing and in good standing as a Cayman Islands exempted company and has all
requisite power and authority to own, lease and operate its assets in the manner in which such assets are now owned, leased and operated
and to conduct its business as it is now being conducted. Parent has made available to the Company true and complete copies of the Governing
Documents of Parent. Parent is duly licensed or qualified and in good standing (or equivalent status as applicable) in each jurisdiction
in which the assets owned or leased by it or the character of its activities require it to be so licensed or qualified or in good standing
(or equivalent status as applicable).
(b)
Merger Sub is a corporation duly incorporated, validly existing and in good standing under the Laws of Delaware. Merger Sub is
a wholly owned Subsidiary of Parent. The copies of the Governing Documents of Merger Sub which were previously furnished or made available
to the Company are true and complete copies of such documents as in effect on the date of this Agreement.
6.2
Due Authorization.
(a)
Each of Parent and Merger Sub has all requisite power and authority to execute and deliver this Agreement and the Transaction Documents
to which it is or will be a party at the Effective Time and (subject to the receipt of the Consents described in Section 6.4, the
Parent Shareholder Approval and the Merger Sub Shareholder Approval) to consummate the Transactions. The execution and delivery by each
of Parent and Merger Sub of this Agreement and the Transaction Documents to which it is or will be a party at the Effective Time and the
consummation by each of Parent and Merger Sub of the Transactions have been duly and validly authorized and approved by all necessary
and proper corporate action on its part, and, except for the Parent Shareholder Approval and the Merger Sub Shareholder Approval, no other
corporate action on the part of Parent or Merger Sub is necessary to authorize this Agreement or the Transaction Documents to which it
is or will be a party at the Effective Time. Each of this Agreement and the Transaction Documents to which it is or will be a party at
the Effective Time has been, or when executed and delivered will be, duly and validly executed and delivered by Parent and (assuming that
this Agreement or such other applicable Transaction Documents to which each of the Company or SpinCo is or will be a party at the Effective
Time constitutes a legal, valid and binding obligation of each of the Company and SpinCo (as applicable)) constitutes or will constitute
a legal, valid and binding obligation of Parent and Merger Sub (as applicable), enforceable against Parent and Merger Sub (as applicable)
in accordance with its terms, subject to the Remedies Exception.
(b)
Assuming that a quorum (as determined pursuant to Parent’s Governing Documents) is present:
(i)
each of those Transaction Proposals identified in clauses (A), (B) and (C) of Section 7.4(e)(ii) shall
require approval by a special resolution under the CICA (being the affirmative vote of the holders of at least two-thirds of the ordinary
shares who, being present and entitled to vote at the Parent Shareholders Meeting, vote at the Parent Shareholders Meeting);
(ii)
each of those Transaction Proposals identified in clauses (D), (E), (F) and (I), of Section 7.4(e)(ii),
in each case, shall require approval by an ordinary resolution (being the affirmative vote of the holders of a majority of the ordinary
shares who, being present and entitled to vote at the Parent Shareholders Meeting, vote at the Parent Shareholders Meeting); and
(iii)
each of those Transaction Proposals identified in clauses (G) and (H), of Section 7.4(e)(ii), in each case,
shall require approval by the number of holders of Parent Common Stock required to approve such Transaction Proposals under applicable
Law and the Governing Documents of Parent.
(c)
The foregoing votes are the only votes of any of Parent’s share capital necessary in connection with entry into this Agreement
by Parent and Merger Sub and the consummation of the Transactions, including the Closing.
(d)
At a meeting duly called and held, or by written resolutions of the Parent Board signed by all directors of the Parent in lieu
of a meeting, the Parent Board has unanimously approved the Transactions as a Business Combination.
6.3
Capital Stock and Other Matters.
(a)
As of the date of this Agreement, the authorized share capital of Parent is 151,000,000 shares, divided into (i) 150,000,000 shares
of Parent Common Stock, 8,941,000 of which are issued and outstanding as of the date of this Agreement, of which the ownership of the
Sponsor and the directors, officers and five (5) percent equity holders of the Parent is set forth in Section 6.3(a) of the Parent
Disclosure Schedule, and (ii) 1,000,000 preferred shares of par value $0.0001 each, of which no shares are issued and outstanding
as of the date of this Agreement ((i) and (ii) collectively, the “Parent Securities”). The foregoing represents all
of the issued and outstanding Parent Securities as of the date of this Agreement. All issued and outstanding Parent Securities (i) have
been duly authorized and validly issued and are fully paid and non-assessable; (ii) have been offered, sold and issued in compliance with
applicable Law, including federal and state securities Laws, and all requirements set forth in (1) Parent’s Governing Documents,
and (2) any other applicable Contracts governing the issuance of such securities; and (iii) are not subject to, nor have they been issued
in violation of, any purchase option, call option, right of first refusal, preemptive right, subscription right or any similar right under
any provision of any applicable Law, Parent’s Governing Documents or any Contract to which Parent is a party or otherwise bound.
(b)
As of the date of this Agreement, 6,994,000 Parent Rights are issued and outstanding, of which the ownership of the Sponsor, and
the directors, officers and five (5) percent equity holders of the Parent are set forth in Section 6.3(b) of the Parent Disclosure
Schedule. As of immediately following the Domestication, all Parent Rights will convert into 6,994,000 Domesticated Parent Rights.
All outstanding Parent Rights have been or are (i) duly authorized and validly issued and constitute valid and binding obligations of
Parent, enforceable against Parent in accordance with their terms, subject to the Remedies Exemption; (ii) offered, sold and issued in
compliance with applicable Law, including federal and state securities Laws, and all requirements set forth in (1) Parent’s Governing
Documents and (2) any other applicable Contracts governing the issuance of such securities; and (iii) not subject to, nor have they been
(or will be) issued in violation of, any purchase option, call option, right of first refusal, preemptive right, subscription right or
any similar right under any provision of any applicable Law, Parent’s Governing Documents or any Contract to which Parent is a party
or otherwise bound. Except for Parent’s Governing Documents and this Agreement, there are no outstanding Contracts of Parent to
repurchase, redeem or otherwise acquire any Parent Securities. Subject to the terms of conditions of the Rights Agreement, at the Closing,
each Domesticated Parent Right will automatically convert into two-tenths (2/10) of one share of Domesticated Parent Common Stock.
(c)
Except as disclosed in the Parent SEC Filings, as set forth in Section 6.3(c) of the Parent Disclosure Schedule, or as contemplated
by this Agreement or the other documents contemplated hereby, Parent has not granted any outstanding options, stock appreciation rights,
warrants, rights or other securities convertible into or exchangeable or exercisable for Parent Securities, or any other commitments or
agreements providing for the issuance of additional shares, the sale of treasury shares, for the repurchase or redemption of any Parent
Securities or the value of which is determined by reference to the Parent Securities, and there are no Contracts of any kind which may
obligate Parent to issue, purchase, redeem or otherwise acquire any of its Parent Securities. Parent is not a party to any voting trusts,
voting agreements, proxies, shareholder agreements or other agreements with respect to the voting or transfer of Parent Common Stock or
any of the equity interests or other securities of Parent.
(d)
Subject to obtaining the Parent Shareholder Approval, the shares of Domesticated Parent Common Stock comprising the Merger Consideration,
when issued in accordance with the terms hereof, shall be duly authorized and validly issued, fully paid and non-assessable and issued
in compliance with all applicable state and federal securities Laws and not subject to, and not issued in violation of, any Lien, purchase,
option, call option, right of first refusal, preemptive right, subscription right or any similar right under any provision of applicable
Law, Parent’s Governing Documents, or any Contract to which Parent is a party or otherwise bound.
(e)
Parent has no Subsidiaries apart from Merger Sub, and does not own, directly or indirectly, any Interest or other interest or investment
(whether equity or debt) in any Person, whether incorporated or unincorporated (each, an “Investment”). Parent is not
party to any Contract that obligates Parent to invest money in, loan money to or make any capital contribution to any other Person.
6.4
Governmental Consents. No consent, waiver, approval or authorization of, or designation, declaration or filing with, or
notification to, any Governmental Authority or other Person is required on the part of Parent or Merger Sub with respect to Parent’s
or Merger Sub’s execution or delivery of this Agreement or the consummation of the Transactions, except for or in compliance with
(a) any Premerger Notification and Report Form required under and compliance with the HSR Act; (b) the filing of the Certificate of Merger
with the Secretary of State of the State of Delaware pursuant to the provisions of the DGCL; (c) the rules and regulations of NASDAQ;
(d) applicable requirements of state securities or “blue sky” Laws, the Securities Act and the Exchange Act; (e) in connection
with the Domestication, the filing of a certificate of domestication and the Parent Charter with the Secretary of State of the State of
Delaware pursuant to the provisions of the DGCL and the applicable requirements and required approval of the Cayman Registrar; and (f)
as otherwise disclosed on Section 6.4 of the Parent Disclosure Schedule.
6.5
No Conflict. Subject to the receipt of the Consents described in Section 6.4 and the Parent Shareholder Approval,
the execution and delivery by each of Parent and Merger Sub of this Agreement and the other Transaction Documents to which it is or will
be a party at the Effective Time and the consummation by Parent and Merger Sub of the Transactions do not and will not as of the Closing
Date: (a) violate any provision of, or result in the material breach of, any Law applicable to Parent and the Parent Subsidiaries or by
which any of its assets or properties is bound; (b) with or without lapse of time or the giving of notice or both, require a consent or
approval under, conflict with, result in a violation or breach of, or constitute a default under, result in the acceleration of, or create
in any party the right to accelerate, terminate or cancel any Parent Material Contract; (c) result in the creation of any Lien upon any
of the properties or assets of Parent or Merger Sub; or (d) violate any provision of the Governing Documents of Parent, or Merger Sub,
except, in the case of clauses (a) and (b), to the extent that such conflicts, breaches, defaults or other matters would not materially
and adversely affect the ability of Parent and Merger Sub to carry out their respective obligations under, and to consummate the transactions
contemplated by, this Agreement and the Transaction Documents. None of Parent, Merger Sub, or Sponsor is a “foreign person”
in which a “foreign government” has a “substantial interest,” as such terms are defined in 31 C.F.R. Part 800.
6.6
Internal Controls; Listing; Financial Statements.
(a)
Except as not required in reliance on exemptions from various reporting requirements by virtue of Parent’s status as an “emerging
growth company” within the meaning of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (“JOBS
Act”), Parent has established and maintains disclosure controls and procedures (as defined in Rule 13a-15 under the Exchange
Act). Such disclosure controls and procedures are designed to ensure that material information relating to Parent, including its consolidated
Subsidiaries, if any, is made known to Parent’s principal executive officer and its principal financial officer by others within
those entities as appropriate to allow timely decisions regarding required disclosure and to make the certifications required pursuant
to Sections 302 and 906 of the Sarbanes-Oxley Act, particularly during the periods in which the periodic reports required under the Exchange
Act are being prepared. To the Parent’s Knowledge, such disclosure controls and procedures are effective in timely alerting Parent’s
principal executive officer and principal financial officer to material information required to be included in Parent’s periodic
reports required under the Exchange Act. Parent has established and maintained a system of internal controls over financial reporting
(as defined in Rule 13a-15 under the Exchange Act) that are designed to and, to the Parent’s Knowledge, are sufficient to provide,
reasonable assurance regarding the reliability of Parent’s financial reporting and the preparation of Parent’s financial statements
for external purposes in accordance with GAAP, including policies and procedures sufficient to provide reasonable assurance: (i) that
Parent maintains records that in reasonable detail fairly reflect, in all material respects, its transactions and dispositions of assets
of Parent; (ii) that transactions are recorded as necessary to permit the preparation of financial statements in conformity with GAAP;
(iii) that receipts and expenditures are being made only in accordance with authorizations of management and Parent Board; and (iv) regarding
prevention or timely detection of unauthorized acquisition, use or disposition of its assets that could have a material effect on its
financial statements. Parent has not received written notice from any Governmental Authority or Person alleging, and to the Parent’s
Knowledge there have been no, significant deficiencies or material weakness in Parent’s internal control over financial reporting
(whether or not remediated). Since June 30, 2023, there has been no change in Parent’s control over financial reporting that has
materially affected, or is reasonably likely to materially affect, Parent’s internal control over financial reporting.
(b)
As of the date hereof, each director and executive officer of Parent has filed with the SEC on a timely basis all statements required
by Section 16(a) of the Exchange Act and the rules and regulations promulgated thereunder. The Parent has not taken any action prohibited
by Section 402 of the Sarbanes-Oxley Act.
(c)
The financial statements and notes contained or incorporated by reference in the Parent SEC Filings (the “Parent Financial
Statements”) (i) accurately and fairly present in all material respects the financial condition and the results of operations,
changes in stockholders’ equity and cash flows of Parent as at the respective dates of, and for the periods referred to, in such
financial statements, all in accordance with GAAP, and (ii) comply in all material respects with the applicable accounting requirements
and with the rules and regulations of Regulation S-X or Regulation S-K, as applicable, subject, in the case of interim financial statements,
to normal recurring year-end adjustments (the effect of which will not, individually or in the aggregate, be material) and the omission
of notes to the extent permitted by Regulation S-X or Regulation S-K, as applicable. Parent has no off-balance sheet arrangements (as
defined by Regulation S-K) that are not disclosed in the Parent SEC Filings. No financial statements other than those of Parent are required
by GAAP to be included in the Parent Financial Statements.
(d)
There are no outstanding loans or other extensions of credit made by Parent to any executive officer (as defined in Rule 3b-7 under
the Exchange Act) or director of Parent. Parent has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
(e)
Neither Parent (including any employee thereof) nor Parent’s independent auditors has identified or been made aware of (i)
any significant deficiency or material weakness in the system of internal accounting controls utilized by Parent, (ii) any Actual Fraud,
whether or not material, that involves Parent’s management or other employees who have a role in the preparation of the Parent Financial
Statements or the internal accounting controls utilized by Parent or (iii) any claim or allegation regarding any of the foregoing.
6.7
No Undisclosed Liabilities. Except for the Parent Transaction Expenses, there is no liability, debt or obligation of or
claim or judgment against Parent or Merger Sub (whether direct or indirect, absolute or contingent, accrued or unaccrued, known or unknown,
liquidated or unliquidated, or due or to become due), except for liabilities and obligations (i) reflected or reserved for on the financial
statements or disclosed in the notes thereto included in Parent SEC Filings, (ii) that have arisen since the date of the most recent balance
sheet included in the Parent SEC Filings in the ordinary course of business of Parent and Merger Sub, or (iii) which, individually or
in the aggregate, would not be, or would not reasonably be expected to be, material to Parent.
6.8
Litigation and Proceedings. As of the date of this Agreement, there are no pending or, to the Knowledge of Parent, threatened
Actions against Parent or Merger Sub, their respective properties or assets, or, to the Knowledge of Parent, any of their respective directors,
managers, officers or employees (in their capacity as such). As of the date of this Agreement, there are no investigations or other inquiries
pending or, to the Knowledge of Parent, threatened by any Governmental Authority, against Parent or Merger Sub, their respective properties
or assets, or, to the Knowledge of Parent, any of their respective directors, managers, officers or employees (in their capacity as such).
As of the date of this Agreement, there is no outstanding Order imposed upon Parent or Merger Sub, nor are any assets of Parent or Merger
Sub’s respective businesses bound or subject to any Order the violation of which would, individually or in the aggregate, reasonably
be expected to have a Parent Material Adverse Effect. From their respective dates of inception to the date of this Agreement, Parent and
Merger Sub have not received any written notice of or been charged with the violation of any Laws, except where such violation has not
had, or would not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect.
6.9
Tax Matters.
(a)
Except as would not, individually or in the aggregate, have a Parent Material Adverse Effect:
(i)
(A) All Tax Returns required to be filed by Parent or Merger Sub have been timely filed (taking into account applicable extensions),
(B) all such Tax Returns are true, correct and complete, and (C) all Taxes, whether or not shown as due on such Tax Returns, have been
paid; in the case of each of clauses (A) through (C), except to the extent adequate reserves therefor in accordance with GAAP have been
provided on the Parent Financial Statements.
(ii)
(A) No Governmental Authority has asserted any written claim, assessment or deficiency for Taxes against Parent or any Parent Subsidiary
(and, to the Knowledge of Parent, no such claim, assessment or deficiency has been threatened or proposed in writing), except for deficiencies
or proposed deficiencies that have been satisfied by payment, settled or withdrawn, and (B) no claim, audit or other proceeding by any
Governmental Authority is pending or threatened in writing with respect to any Taxes of Parent or Merger Sub;
(iii)
Parent and Merger Sub have no outstanding waivers or extensions of any applicable statute of limitations to assess any material
amount of Taxes. There are no outstanding requests by Parent or Merger Sub for any extension of time within which to file any Tax Return
or within which to pay any Taxes shown to be due on any Tax Return;
(iv)
Neither Parent nor any Parent Subsidiary has any Liability for Taxes of any other Person (other than Parent or any Parent Subsidiary)
under Treasury Regulations Section 1.1502-6 (or any similar provision of state, local or non-U.S. Law), as a transferee or successor or
by contract or operation of Law or otherwise;
(v)
Within the past two (2) years, neither Parent nor any Parent Subsidiary has constituted either a “distributing corporation”
or a “controlled corporation” (within the meaning of Section 355(a)(1)(A) of the Code) in a distribution of stock intended
to qualify for tax-free treatment under Section 355 of the Code;
(vi)
Neither Parent nor any Parent Subsidiary has participated in, or is currently participating in, a “listed transaction”
within the meaning of Treasury Regulations Section 1.6011-4(b)(2);
(vii)
There are no Liens for Taxes (other than Permitted Liens) upon the assets of Parent or Merger Sub;
(viii)
Neither Parent nor Merger Sub will be required to include any material item of income in, or exclude any material item of deduction
from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method
of accounting for a taxable period ending on or prior to the Closing Date under Section 481(c) of the Code (or any corresponding or similar
provision of state, local or foreign income Tax Law); (ii) “closing agreement” as described in Section 7121 of the Code (or
any corresponding or similar provision of state, local or foreign income Tax Law) executed on or prior to the Closing Date; or (iii) installment
sale made on or prior to the Closing Date;
(ix)
Neither Parent nor Merger Sub has been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal,
state, local or foreign income Tax Return;
(x)
Neither Parent nor Merger Sub has any request for a ruling in respect of Taxes, technical advice memorandum, closing agreement
or similar ruling, memorandum, or agreement pending between Parent or Merger Sub, on the one hand, and any Tax authority, on the other
hand; nor is any such request outstanding; and
(xi)
Neither Parent nor Merger Sub is a party to, is bound by or has an obligation under any Tax sharing agreement, Tax indemnification
agreement, Tax allocation agreement or similar contract or arrangement (including any agreement, contract or arrangement providing for
the sharing or ceding of credits or losses, any advance pricing agreement, closing agreement or other agreement relating to Taxes with
any taxing authority) or has a potential liability or obligation to any person as a result of or pursuant to any such agreement, contract,
arrangement or commitment other than an agreement, contract, arrangement or commitment the primary purpose of which does not relate to
Taxes and which is not entered into with any affiliate or direct or indirect owner of Parent.
(b)
Neither Parent nor Merger Sub has taken or agreed to take any action or knows of any fact, agreement, plan or other circumstance
that could reasonably be expected to (i) prevent or impede (A) the Domestication from qualifying as a “reorganization” within
the meaning of Section 368(a)(1)(F), or (B) the Tax-Free Status; or (ii) cause there to be any Tax imposed on any of the Parties by reason
of the Domestication.
(c)
As of the date of this Agreement it is the present intention, and as of the day of the Effective Time it will be the present intention,
of Parent to continue, either through Parent or through a member of Parent’s “qualified group” within the meaning of
Treasury Regulations Section 1.368-1(d)(4)(ii) (the “Qualified Group”), at least one significant historic business
line of SpinCo, or to use at least a significant portion of SpinCo‘s historic business assets in a business, in each case within
the meaning of Treasury Regulations Section 1.368-1(d). As of the date of this Agreement and as of the date of the Effective Time, neither
Parent nor any “related person” (as defined in Treasury Regulations Section 1.368-1(e)(4)) to Parent has or will have any
plan or intention to redeem or reacquire, either directly or indirectly, any of the Domesticated Parent Common Stock issued to any holder
of SpinCo Common Stock in connection with the Merger. As of the date of this Agreement and as of the date of the Effective Time, Parent
does not have and will not have any plan or intention to sell or otherwise dispose of any of the assets of SpinCo acquired in the Merger,
except for dispositions made in the ordinary course of business or transfers described in Section 368(a)(2)(C) of the Code or described
and permitted in Treasury Regulations Section 1.368-2(k).
(d)
Merger Sub was formed solely for the purpose of engaging in the Merger, and does not have any assets and has not engaged in any
business activities or conducted any operations other than in connection with the Merger; and
(e)
The representations and warranties set forth in this Section 6.9 or in Section 6.19 constitute the sole and exclusive
representations and warranties of Parent regarding Tax matters.
6.10
Absence of Changes
(a)
Since June 30, 2023, until the date of this Agreement, (a) there has not been any event or occurrence that has had, or would not
reasonably be expected to have, individually or in the aggregate a Parent Material Adverse Effect, and (b) except as set forth in Section
6.10 of the Parent Disclosure Schedule or as contemplated by this Agreement and the other Transaction Documents, Parent and Merger
Sub have, in all material respects, conducted their business and operated their properties in the ordinary course of business consistent
with past practice.
(b)
Except as disclosed in Parent SEC Filings that were filed or furnished on or after June 30, 2023, Parent has not taken any action
that, if taken during the period from the date of this Agreement through the Closing, would require the consent of the Company or SpinCo
pursuant to Section 7.1.
6.11
Brokers’ Fees. No broker, finder, investment banker or other Person is entitled to any brokerage fee,
finders’ fee or other similar commission, for which Parent, Merger Sub or SpinCo would be liable in connection with the transactions
contemplated by this Agreement based upon arrangements made by Parent or any Parent Subsidiary.
6.12
Proxy Statement; Registration Statement. None of the information regarding Parent, Merger Sub or the Transactions
to be provided by Parent specifically for inclusion in, or incorporation by reference into, the Proxy Statement or the Parent Registration
Statement will, in the case of the Proxy Statement or any amendment or supplement thereto, at the time of the first mailing of the Proxy
Statement and of any amendment or supplement thereto, or, in the case of the Parent Registration Statement, at the time the registration
statement becomes effective, on the date of the Parent Shareholders Meeting, and at the Effective Time, contain any untrue statement of
a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein,
in the light of the circumstances under which they are made, not false or misleading. The Proxy Statement and the Parent Registration
Statement will comply as to form in all material respects with the provisions of the Securities Act and the Exchange Act, as the case
may be, except that no representation is made by Parent with respect to information provided by the Company or SpinCo specifically for
inclusion in, or incorporation by reference into, the Proxy Statement or the Parent Registration Statement.
6.13
SEC Filings. Parent has timely filed or furnished all statements, prospectuses, registration statements, forms, reports
and documents required to be filed by it with the SEC pursuant to the Exchange Act or the Securities Act (collectively, as they have
been amended since the time of their filing through the date hereof, the “Parent SEC Filings”). Each of the Parent
SEC Filings, as of the respective date of its filing (or if amended or superseded by a filing prior to the date of this Agreement or
the Closing Date, then on the date of such filing), complied in all material respects with the applicable requirements of the Securities
Act, the Exchange Act, the Sarbanes-Oxley Act and any rules and regulations promulgated thereunder applicable to the Parent SEC Filings.
As of the respective date of its filing (or if amended or superseded by a filing prior to the date of this Agreement or the Closing Date,
then on the date of such filing), the Parent SEC Filings did not contain any untrue statement of a material fact or omit to state a material
fact required to be stated therein or necessary to make the statements made therein, in light of the circumstances under which they were
made, not misleading. As of the date hereof, there are no outstanding or unresolved comments in comment letters received from the SEC
with respect to the Parent SEC Filings. To the Knowledge of Parent, none of the Parent SEC Filings filed on or prior to the date hereof
is subject to ongoing SEC review or investigation as of the date hereof.
6.14
Trust Account. As of the date of this Agreement, Parent has at least $70,929,382
in the Trust Account, such monies invested in United States government securities or money market funds meeting certain conditions under
Rule 2a-7 promulgated under the Investment Company Act pursuant to the Investment Management Trust Agreement, dated as of October 13,
2022, between Parent and American Stock Transfer & Trust Company, LLC, as trustee (the “Trustee”) (the “Trust
Agreement”). The Trust Agreement has not been amended or modified, other than to permit any Parent Share Redemptions, and is
valid and in full force and effect and is enforceable in accordance with its terms, and no termination, repudiation, rescission, amendment,
supplement or modification is contemplated. There are no separate Contracts, side letters or other arrangements or understandings (whether
written or unwritten, express or implied) that would cause the description of the Trust Agreement in the Parent SEC Filings to be inaccurate
or that would entitle any Person (other than the Parent Shareholders holding shares of Parent Common Stock sold in Parent’s initial
public offering who elect to redeem their shares of Parent Common Stock pursuant to Parent’s Governing Documents and the underwriters
of Parent’s initial public offering with respect to deferred underwriting commissions) to any portion of the proceeds in the Trust
Account. Prior to the Closing, none of the funds held in the Trust Account may be released other than (i) to pay Taxes, (ii) to make
payments with respect to all Parent Share Redemptions or (iii) to commence liquidation in accordance with and as required by the Trust
Agreement (taking into account any amendments to the Trust Agreement providing for a longer period of time before the Trust Account is
required to be liquidated, including, as applicable, the Second Extension, Third Extension, and Fourth Extension). There are no Actions
pending or, to the Knowledge of Parent, threatened with respect to the Trust Account. Parent has performed all material obligations required
to be performed by it to date under, and is not in material default, breach or delinquent in performance or any other respect (claimed
or actual) in connection with, the Trust Agreement, as it may be amended in accordance with the terms of this Agreement, and no event
has occurred which, with due notice or lapse of time or both, would constitute such a default or breach thereunder. As of the Effective
Time, the obligations of Parent to dissolve or liquidate pursuant to Parent’s Governing Documents shall terminate, and as of the
Effective Time, Parent shall have no obligation whatsoever pursuant to Parent’s Governing Documents to dissolve and liquidate the
assets of Parent by reason of the consummation of the Transactions. To Parent’s Knowledge, as of the date hereof, following the
Effective Time, no shareholder of Parent shall be entitled to receive any amount from the Trust Account except to the extent such shareholder
of Parent is exercising a Parent Share Redemption. As of the date hereof, assuming the accuracy of the representations and warranties
of the Company contained herein and the compliance by the Company and SpinCo with its obligations hereunder and under the other Transaction
Documents, neither Parent nor Merger Sub has any reason to believe that any of the conditions to the use of funds in the Trust Account
will not be satisfied or funds available in the Trust Account will not be available to Parent and Merger Sub on the Closing Date.
6.15
Investment Company Act; JOBS Act. Parent is not an “investment company” or a Person directly or indirectly “controlled”
by or acting on behalf of an “investment company”, in each case within the meaning of the Investment Company Act. Parent constitutes
an “emerging growth company” within the meaning of the JOBS Act.
6.16
Indebtedness. Section 6.16 of the Parent Disclosure Schedule sets forth the principal amount of all of the outstanding
indebtedness, as of the date hereof, of Parent and Merger Sub.
6.17
Stock Market Quotation. As of the date hereof, the Parent Common Stock is registered pursuant to Section 12(b) of the Exchange
Act and is listed for trading on NASDAQ under the symbol “TENK”. As of the date hereof, the Parent Rights are registered pursuant
to Section 12(b) of the Exchange Act and are listed for trading on NASDAQ under the symbol “TENKR.” As of the date hereof,
the Parent Units are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on NASDAQ under the symbol “TENKU.”
There is no Action, Order, or proceeding pending or, to the Knowledge of Parent, threatened against Parent by NASDAQ or the SEC with respect
to any intention by such entity to deregister the Parent Common Stock or Parent Rights or terminate the listing of Parent Common Stock
or Parent Rights on NASDAQ. None of Parent, Merger Sub or their respective Affiliates has taken any action in an attempt to terminate
the registration of the Parent Common Stock or Parent Rights under the Exchange Act except as contemplated by this Agreement. Parent has
not received written notice from any Governmental Authority or Person alleging any non-compliance with the applicable listing and corporate
governance rules and regulations of NASDAQ. As of the date hereof, to the Knowledge of Parent, Parent is in compliance in all material
respects with the applicable listing and corporate governance rules and regulations of NASDAQ.
6.18
Business Activities.
(a)
Since formation, neither Parent nor Merger Sub have conducted any business activities other than activities related to Parent’s
initial public offering or directed toward the accomplishment of a Business Combination. Except as set forth in Parent’s Governing
Documents or as otherwise contemplated by this Agreement or the Transaction Documents and the Transactions and thereby, there is no agreement,
commitment, or Order binding upon Parent or Merger Sub or to which Parent or Merger Sub is a party which has or would reasonably be expected
to have the effect of prohibiting or impairing any business practice of Parent or Merger Sub or any acquisition of property by Parent
or Merger Sub or the conduct of business by Parent or Merger Sub as currently conducted or as contemplated to be conducted as of the Closing,
other than such effects which have not been and would not reasonably be expected to materially and adversely affect Parent.
(b)
Except for Merger Sub and the Transactions, Parent does not own or have a right to acquire, directly or indirectly, any Investment
in any corporation, partnership, joint venture, business, trust or other Entity. Except for this Agreement and the Transaction Documents
and the Transactions contemplated hereby and thereby, Parent has no material interests, rights, obligations or Liabilities with respect
to, and is not party to, bound by or has its assets or property subject to, in each case whether directly or indirectly, any Contract
or transaction which is, or would reasonably be interpreted as constituting, a Business Combination. Except for the Transactions, Merger
Sub does not own or have a right to acquire, directly or indirectly, any Investment in any corporation, partnership, joint venture, business,
trust or other Entity.
(c)
Merger Sub was formed solely for the purpose of effecting the Transactions and has not engaged in any business activities or conducted
any operations other than incident to the Transactions and has no, and at all times prior to the Effective Time, except as expressly contemplated
by this Agreement, the Transaction Documents and the other documents and Transactions, will have no, assets, liabilities or obligations
of any kind or nature whatsoever other than those incident to its formation.
(d)
As of the date hereof and except for this Agreement, the Transaction Documents and the Transactions (other than with respect to
expenses and fees incurred in connection therewith and the Business Combination), neither Parent nor Merger Sub is party to any Contract
with any other Person that would require payments by Parent or any of its Subsidiaries after the date hereof in excess of $100,000 in
the aggregate with respect to any individual Contract.
(e)
Section 6.18 of the Parent Disclosure Schedule lists all material Contracts, oral or written to which Parent or Merger Sub
is a party or by which Parent’s or Merger Sub’s assets is bound as of the date hereof, other than those Contracts that are
included in the Parent SEC Filings and are available in full without redaction on the SEC’s EDGAR database.
6.19
Section 280G. Neither the execution and delivery of this Agreement nor the consummation of the Transactions contemplated
hereby or by the Transaction Documents, either alone or in connection with any other event(s), will result in the payment of any amount
to any current or former employee, officer, director or independent contractor of Parent or other Person that could, individually or in
the aggregate, or in combination with any other such payment, constitute an “excess parachute payment” as defined in Section
280G(b)(1) of the Code.
6.20
Sanctions, Import, and Export Controls. None of Parent or its Subsidiaries or any of their respective directors, officers,
employees, agents, or any other Person acting for or on behalf of Parent or its Subsidiaries (a) is a Person with whom transactions are
prohibited or limited under any economic sanctions Laws, including those administered by the U.S. government (including, without limitation,
the Department of the Treasury’s Office of Foreign Assets Control, the Department of State, or the Department of Commerce), the
United Nations Security Council, the European Union, or His Majesty’s Treasury, or (b) has violated any Laws relating to economic
sanctions within the last five (5) years. Within the past five (5) years, none of Parent or its Subsidiaries has violated any Laws related
to or made any disclosure to any Governmental Authority relating to sanctions, customs, import, or export control Laws; to the Knowledge
of Parent, been the subject of any investigation or inquiry regarding compliance with such Laws; or been assessed any fine or penalty
under such Laws.
6.21
No Other Representations and Warranties.
(a)
Except as for the representations and warranties set forth in this Article VI neither Parent nor any of its Affiliates is making,
and Parent expressly disclaims, any representation or warranty, express or implied, with respect to Parent or its Affiliates, or their
respective businesses with respect to any other information provided, or made available, to the Company or any of its Affiliates or Representatives
in connection with the Transactions, including information, documents, projections, forecasts or other material made available to the
Company, its Affiliates or Representatives in any “data rooms,” management presentations or otherwise in connection with the
Transactions.
(b)
Each of Parent and Merger Sub acknowledges that it is not relying on, and that the Company and its Affiliates have not made, any
representation or warranty except as specifically set forth in Article IV and Article V.
Article
VII
COVENANTS
7.1
Conduct of Business by Parent and Merger Sub Pending the Merger.
(a)
From the date hereof and prior to the Effective Time (or the earlier termination of this Agreement) (the “Interim Period”),
except (i) as required or otherwise expressly contemplated by this Agreement (including as set forth in Section 7.1 of the Parent
Disclosure Schedule), the Transaction Documents, or in connection with the Domestication, (ii) as consented to by the Company in writing
(which consent shall not be unreasonably withheld, conditioned, delayed or denied) or (iii) as required by applicable Law, Order or other
directive by a Governmental Authority (including any COVID-19 Measures), Parent shall, and shall cause its Subsidiaries (including Merger
Sub), to conduct its operations in the ordinary course of business in all material respects. During the Interim Period, Parent shall,
and shall cause its Subsidiaries (including Merger Sub) to comply with, and continue performing under, as applicable, the Governing Documents
of Parent, the Trust Agreement and all other agreements or Contracts to which Parent or its Subsidiaries may be a party. In addition to,
and not in limitation of, the restrictions set forth in this Section 7.1, from the date hereof through the Effective Time, Parent
shall remain a “blank check company” as defined under the Securities Act, shall not conduct any business operations other
than in connection with this Agreement and ordinary course operations to maintain its status as a Nasdaq-listed special purpose acquisition
company pending the completion of the Transactions.
(b)
Without limiting the generality of Section 7.1(a), during the Interim Period, except (x) as required or otherwise expressly
contemplated by this Agreement, the Transaction Documents, or in connection with the Domestication, (y) as consented to by the Company
in writing (which consent shall not be unreasonably withheld, conditioned, delayed or denied, other than with respect to subsection (b)(ii),
with respect to which consent may be withheld at the Company’s sole discretion) or (z) as required by applicable Law, Order or other
directive by a Governmental Authority (including any COVID-19 Measures), Parent shall not, and shall cause its Subsidiaries, including
Merger Sub, as applicable, not to:
(i)
seek any approval from the Parent Shareholders to amend, modify, restate, waive, rescind or otherwise change the Trust Agreement
or the Governing Documents of Parent or Merger Sub (other than as contemplated by the Transaction Proposals, the extensions provided for
under Section 7.16(a), and to permit any Parent Share Redemptions);
(ii)
(A) make or declare any dividend or distribution to the Parent Shareholders or make any other distributions in respect of any of
Parent’s equity interests or Merger Sub capital stock, share capital or equity interests, (B) split, combine, reclassify or otherwise
amend any terms of any shares or series of Parent’s equity interests or Merger Sub capital stock or equity interests, or (C) purchase,
repurchase, redeem or otherwise acquire any issued and outstanding share capital, outstanding shares of capital stock, share capital or
membership interests, warrants or other equity interests of Parent or Merger Sub, other than a redemption of shares of Parent Common Stock
made as part of the Parent Share Redemptions;
(iii)
(A) make, change or revoke any material Tax election or (B) settle or compromise any material Tax liability;
(iv)
amend the Trust Agreement or any other agreement related to the Trust Account (other than in relation to the extensions provided
for under Section 7.16(a) and to permit any Parent Share Redemptions), or enter into, renew or amend in any material respect, any
transaction or Contract with the Sponsor or an Affiliate of the Sponsor, solely to the extent such transaction or Contract directly relates,
or would relate, to the Transactions or any Transaction Document;
(v)
incur or assume any indebtedness or Liability or guarantee any indebtedness or Liability of another Person (other than indebtedness,
not to exceed $500,000, that is incurred by Parent or Merger Sub as Parent Transaction Expenses, and, to the extent outstanding as of
the Closing Date, payable as Parent Transaction Expenses), issue or sell any debt securities or warrants or other rights to acquire any
debt securities of the Company or any of the Company’s Subsidiaries or guaranty any debt securities of another Person, other than
any indebtedness;
(vi)
(A) issue any Parent Securities or securities exercisable for or convertible into Parent Securities, other than issuances contemplated
by the Transactions, (B) grant any options, warrants or other equity-based awards with respect to Parent Securities not outstanding on
the date hereof or (C) amend, modify or waive any of the material terms or rights set forth in any Parent Right or the Rights Agreement;
(vii)
grant or provide any change-in-control, severance, termination, retention, success-based payment, or other payments or benefits
to any employee of or consultant to Parent; or
(viii)
enter into any agreement to do any action prohibited under this Section 7.1.
7.2
Conduct of the Company and SpinCo Business Pending the Merger.
(a)
During the Interim Period (solely with respect to the SpinCo Business), except (i) as required or otherwise expressly contemplated
by this Agreement (including as set forth in Section 7.2 of the SpinCo Disclosure Schedule) or the Transaction Documents, (ii)
as consented to by Parent in writing (which consent shall not be unreasonably withheld, conditioned or delayed other than with respect
to Section 7.2(b)(vi) below, with respect to which consent may be withheld at the Parent’s sole discretion), (iii) as required
by applicable Law, Order or other directive by a Governmental Authority (including, any COVID-19 Measures), or (iv) as undertaken substantially
consistent with the Commercialization Plan, the Company and SpinCo shall (x) use commercially reasonable efforts to conduct the SpinCo
Business in the ordinary course of business and consistent with past practices in all material respects, (y) use commercially reasonable
efforts to manage the SpinCo Business’ working capital and maintain the books and records related to the SpinCo Business consistent
with past practice and (z) use commercially reasonable efforts to maintain their respective relations and goodwill with all material suppliers,
material customers and other material commercial counterparties and Governmental Authorities (in each case, as related to the SpinCo Business).
(b)
Without limiting the generality of Section 7.2(a), during the Interim Period (solely with respect the SpinCo Business),
except (A) as required or contemplated by this Agreement (including as set forth in Section 7.2 of the SpinCo Disclosure Schedule)
or the Transaction Documents, (B) as consented to by Parent in writing (which consent shall not be unreasonably withheld, conditioned
or delayed) or (C) as required by applicable Law, Order or other directive by a Governmental Authority (including any COVID-19 Measures),
the Company (solely with respect to the SpinCo Business) and SpinCo shall not:
(i)
amend, modify, restate, waive, rescind or otherwise change the Governing Documents of SpinCo;
(ii)
(A) split, combine, subdivide, reduce, or reclassify shares of SpinCo Common Stock or issue or authorize or propose the issuance
of any other securities in respect of, in lieu of, or in substitution for, shares of SpinCo Common Stock, or (B) redeem, repurchase or
otherwise acquire shares of SpinCo Common Stock (including any securities convertible or exchangeable into SpinCo Common Stock);
(iii)
issue, sell, pledge, dispose of, grant, transfer or encumber, any shares of capital stock of SpinCo or securities convertible into,
or exchangeable or exercisable for, any shares of such capital stock in SpinCo, or any options, warrants, stock units, or other rights
of any kind to acquire any shares of capital stock or other Interests or such convertible or exchangeable securities, or any other ownership
interest (including any such interest represented by Contract right), or any “phantom” stock, “phantom” stock
rights, stock appreciation rights or stock-based performance rights, in each case, of SpinCo, other than the issuance of capital stock
or other Interests upon the exercise, vesting or settlement of any equity awards of the Company outstanding as of the date hereof and,
in each case, in accordance with their respective terms as in effect as of the date hereof;
(iv)
sell, assign, transfer, convey, lease, license, abandon, mortgage, pledge or permit any Lien on (other than a Permitted Lien) or
otherwise dispose of any SpinCo Assets (excluding Intellectual Property, which is the subject of Section 7.2(b)(v) below);
(v)
purchase, sell, license, sublicense, lease, pledge, covenant not to assert, assign, transfer, abandon, cancel, let lapse or expire,
or otherwise dispose, transfer or grant any other rights in or with respect to any SpinCo Owned Intellectual Property or SpinCo Licensed
Intellectual Property (other than with respect to (A) immaterial or obsolete SpinCo Owned Intellectual Property or (B) the grant of non-exclusive
licenses of SpinCo Owned Intellectual Property in the ordinary course of business consistent with past practice);
(vi)
merge, combine or consolidate (pursuant to a plan of merger or otherwise) SpinCo with any Person or adopt a plan of complete or
partial liquidation or resolutions providing for a complete or partial liquidation, dissolution, restructuring, recapitalization or other
reorganization of SpinCo;
(vii)
repurchase, repay, prepay, refinance or incur any indebtedness for borrowed money, issue any debt securities, engage in any securitization
transactions or similar arrangements or assume, guarantee or endorse, or otherwise as an accommodation become responsible for (whether
directly, contingently or otherwise), the obligations of any Person for borrowed money other than in the ordinary course of business consistent
with past practice;
(viii)
make any material loans to, material capital contributions or material investments in, or advances of money to, in each case, in
excess of $250,000 individually or $500,000 in the aggregate, any Person, in each case except for (A) advances to employees or officers
of SpinCo for expenses incurred in the ordinary course of the SpinCo Business consistent with past practice or (B) extended payment terms
for customers in the ordinary course of the SpinCo Business consistent with past practice;
(ix)
(A) amend or modify in any material respect, terminate (excluding any expiration in accordance with its terms), or waive any material
right, benefit or remedy under, any SpinCo Material Contract or Company Contract, solely to the extent such Company Contract (x) relates
to the SpinCo Business or the Transactions, or (y) as a result of such amendment or modification, would relate to the SpinCo Business
and would be required to be listed on Section 5.14(a) of the SpinCo Disclosure Schedule, or (B) enter into any Contract (other
than in the ordinary course of business) that if entered into prior to the date hereof would be required to be listed on Section 5.14(a)
of the SpinCo Disclosure Schedule;
(x)
except as contemplated by the Transaction Documents or Contract in effect as of the date hereof, grant or provide any change-in-control,
severance, termination, retention or similar payments or benefits to any employee of or consultant to SpinCo;
(xi)
hire or engage, or make an offer to hire or engage, any officer, employee, service provider or individual independent contractor
of SpinCo or the Company (solely for purposes of the Company, if such Person is hired or engaged with the intent that such Person would
provide services to SpinCo under the Shared Services Agreement prior to the Closing or the Amended and Restated Shared Services Agreement
post-Closing) whose annual base pay exceeds $250,000;
(xii)
except as required by GAAP, make any change to any financial accounting principles, methods or practices of SpinCo or with respect
to the SpinCo Business;
(xiii)
waive, release, settle, compromise or otherwise resolve any Action, litigation or other proceedings, except where such waivers,
releases, settlements or compromises involve only the payment of monetary damages in an amount less than $250,000 in the aggregate;
(xiv)
(A) make any material Tax election inconsistent with prior practice or change or revoke any material Tax election in respect of
the SpinCo Business, or (B) settle or compromise any material Tax liability for which SpinCo or the SpinCo Business would be responsible
post-Closing;
(xv)
make or commit to make any capital expenditures, on an annualized basis, in the aggregate, in excess of $500,000, in the aggregate;
(xvi)
enter into any collective bargaining agreement or other similar Contract with a labor union, works’ council, employee representative
body or labor organization;
(xvii)
terminate without replacement or fail to use reasonable best efforts to maintain any license or permit that is material to the
conduct of the business of SpinCo;
(xviii)
(A) limit the right of SpinCo to engage in any line of business or in any geographic area, to develop, market or sell products
or services, or to compete with any Person or (B) grant any exclusive or similar rights to any Person, in each case, except where such
limitation or grant does not, and would not be reasonably likely to, individually or in the aggregate, materially and adversely affect,
or materially disrupt, the ordinary course operation of the SpinCo Business; or
(xix)
authorize or enter into any Contract to do any of the foregoing or otherwise agree or make any commitment to do any of the foregoing.
7.3
Tax Matters.
(a)
From and after the date of this Agreement and until the Effective Time, each Party (i) shall use its commercially reasonable efforts
to ensure that (A) the Merger will qualify as a “reorganization” within the meaning of Section 368(a) of the Code (the “Tax-Free
Status”) and (B) the Domestication will qualify as a “reorganization” within the meaning of Section 368(a)(1)(F),
and (ii) shall not take any action, cause or permit any action to be taken, fail to take any action, or cause any action to fail to be
taken, which action or failure to act could prevent (A) the Domestication from qualifying as a “reorganization” within the
meaning of Section 368(a)(1)(F) or (B) the Tax-Free Status. Following the Effective Time, none of the Company, Parent or any of their
respective Affiliates shall take any action, cause or permit any action to be taken, fail to take any action or cause any action to fail
to be taken, which action or failure to act could prevent the Tax-Free Status.
(b)
At and after the Effective Time, each of Parent and the Surviving Corporation covenants and agrees that it:
(i)
will maintain all books and records and file all federal, state, and local income Tax Returns and schedules thereto of Parent,
the Surviving Corporation, and Merger Sub in a manner consistent with the Merger’s being qualified as a reorganization and nontaxable
exchange under Section 368(a)(1)(A) of the Code (and comparable provisions of any applicable state or local Tax laws);
(ii)
will, either directly or through a member of Parent’s Qualified Group, continue at least one significant historic business
line of SpinCo, or use at least a significant portion of the historic business assets of SpinCo in a business, in each case within the
meaning of Treasury Regulations Section 1.368-1(d);
(iii)
in connection with the Merger, will not reacquire, and will not permit any Person that is a “related person” (as defined
in Treasury Regulations Section 1.368-1(e)(4)) to Parent to acquire, any of the Domesticated Parent Common Stock issued in connection
with the Merger; and
(iv)
will not sell or otherwise dispose of any of SpinCo assets acquired via the Merger, except for dispositions made in the ordinary
course of business or transfers described in Section 368(a)(2)(C) of the Code or described and permitted in Treasury Regulations Section
1.368-2(k).
(c)
If, in connection with the preparation and filing of the Parent Registration Statement, the SEC requires that tax opinions related
to the tax effects of the Merger be prepared and submitted, the Company and SpinCo, on the one hand, and Parent, on the other hand, shall
cooperate with one another in obtaining, and shall use their respective reasonable best efforts to obtain, a written opinion of the Company’s
Tax counsel (the “Company Merger Tax Opinion”), in the case of the Company and SpinCo, and a written opinion of Parent’s
Tax counsel (the “Parent Merger Tax Opinion”), in the case of Parent, reasonably satisfactory in form and substance
to the Company and Parent, respectively, dated as of the Closing Date, to the effect that, on the basis of the facts, customary representations
and assumptions set forth or referred to in such opinion, the Merger will be treated as a “reorganization” within the meaning
of Section 368(a) of the Code. In delivering the Company Merger Tax Opinion and the Parent Merger Tax Opinion, Tax counsel may rely upon
reasonable representations of an officer of SpinCo and the Parent, respectively, dated as of the Closing Date, in form and substance reasonably
satisfactory to tax counsel and delivered to tax counsel.
(d)
If, in connection with the preparation and filing of the Parent Registration Statement, the SEC requires that tax opinions related
to the tax effects of the Domestication be prepared and submitted, Parent shall use its reasonable best efforts to obtain a written opinion
of Parent’s Tax counsel (the “Parent Domestication Tax Opinion”) reasonably satisfactory in form and substance
to Parent and the Company, dated as of the Closing Date, to the effect that, on the basis of the facts, customary representations and
assumptions set forth or referred to in such opinion, the Domestication will qualify as a “reorganization” under Section 368(a)(1)(F)
of the Code. In delivering the Parent Domestication Tax Opinion, Tax counsel may rely upon reasonable representations of an officer of
Parent dated as of the Closing Date, in form and substance reasonably satisfactory to tax counsel.
7.4
Preparation of the Registration Statement and Proxy Statement; Parent Shareholders Meeting.
(a)
As promptly as practicable after the execution of this Agreement to the extent such filings are required by Law in connection with
the transactions contemplated by this Agreement: (i) Parent, the Company and SpinCo shall jointly prepare and Parent shall file with the
SEC the Parent Registration Statement; (ii) Parent, the Company and SpinCo shall jointly prepare and Parent shall file with the SEC the
Proxy Statement (which Proxy Statement may form a part of the Parent Registration Statement); and (iii) the Parties shall jointly prepare
and cause to be filed such other filings required under applicable securities Laws in connection with the Transactions.
(b)
Each of Parent, the Company and SpinCo shall use its reasonable best efforts to have the Parent Registration Statement declared
effective as promptly as practicable after such filing (including by responding to comments of the SEC) and after the delivery of any
financial statements pursuant to Section 7.16(b) that are required to be included in the Parent Registration Statement, and to
keep such registration statement effective for as long as is necessary to consummate the Transactions, and, prior to the effective date
of the Parent Registration Statement, each of Parent, the Company and SpinCo shall take all action reasonably required (other than qualifying
to do business in any jurisdiction in which it is not now so qualified or filing a general consent to service of process in any such jurisdiction)
to be taken under any applicable securities Laws in connection with the Parent Share Issuance. As promptly as practicable after the Parent
Registration Statement becomes effective, Parent shall cause the Proxy Statement to be mailed or made available to the Parent’s
stockholders and the final prospectus contained in the Registration Statement to the Company pursuant to applicable Law. No filing of,
or amendment or supplement to, the Parent Registration Statement or the Proxy Statement will be made by Parent without providing the Company
and SpinCo with a reasonable opportunity to review and comment thereon (and such comments shall be reasonably considered by Parent in
good faith). Parent will use its commercially reasonable efforts to cause the Parent Registration Statement to comply in all material
respects with the applicable requirements of U.S. federal securities Laws.
(c)
Each of Parent, the Company and SpinCo shall ensure that none of the information supplied by or on its behalf for inclusion or
incorporation by reference in (A) the Parent Registration Statement will, at the time filed with the SEC, at each time at which it is
amended and at the time it becomes effective under the Securities Act, contain any untrue statement of a material fact or omit to state
any material fact required to be stated therein or necessary to make the statements therein, not misleading, or (B) the Proxy Statement
will, at the date it is first mailed or made available to the Parent’s shareholders and at the time of the Parent Shareholders’
Meeting, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary
in order to make the statements therein, in light of the circumstances under which they are made, not misleading.
(d)
If, at any time prior to the Effective Time, any information relating to Parent, the Company or SpinCo, or any of their respective
Affiliates, directors or officers, should be discovered by Parent, the Company or SpinCo, which should be set forth in an amendment or
supplement to the Parent Registration Statement or the Proxy Statement, so that any such document would not include any misstatement of
a material fact or omit to state any material fact necessary to make the statements therein, in light of the circumstances under which
they were made, not misleading, the Party that discovers such information shall promptly notify the other Party and an appropriate amendment
or supplement describing such information shall be promptly filed with the SEC, and, to the extent required by Law, disseminated to the
shareholders of Parent. Parent shall notify the Company promptly of the time when the Parent Registration Statement has become effective
and of the issuance of any stop order or suspension of the qualification of the shares of Domesticated Parent Common Stock issuable pursuant
to the Merger for offering or sale in any jurisdiction. Parent agrees to provide the Company and its counsel promptly with copies of any
written comments or requests for amendments or supplements, and shall promptly inform the Company of any oral comments or requests for
amendments or supplements, that Parent or its counsel may receive from time to time from the SEC with respect to the Parent Registration
Statement or the Proxy Statement promptly after receipt of such comments, and shall provide the Company with copies of any written or
oral responses or correspondence between it or its Affiliates and the SEC related thereto. The Company and its counsel shall be given
a reasonable opportunity to review in advance any such written responses and to participate in any discussions or oral material communications
with the SEC, and Parent shall reasonably consider in good faith the additions, deletions, comments or changes suggested thereto by the
Company and its counsel.
(e)
Parent Shareholders Meeting.
(i)
Parent shall call, give notice of, convene and hold a general meeting (the “Parent Shareholders Meeting”) in
accordance with Parent’s Governing Documents and applicable Law as promptly as reasonably practicable following the date on which
the Parent Registration Statement is declared effective, and in any case, no later than thirty (30) Business Days thereafter, for the
purpose of obtaining the Parent Shareholder Approval; provided, that subject to the requirements of any applicable Law, Parent
may (and, in the case of clause (C) on up to two (2) occasions upon the reasonable request of the Company (and for no more than five (5)
Business Days each) shall) postpone or adjourn the Parent Shareholders Meeting (A) if a quorum has not been established; (B) after consultation
with the Company, to allow reasonable additional time for the filing and mailing of any supplement or amendment to the Proxy Statement
as may be required under applicable Law and for such supplement or amendment to be disseminated and reviewed by Parent’s shareholders
sufficiently in advance of the Parent Shareholders Meeting; (C) to allow reasonable additional time to solicit additional proxies, if
and to the extent the requisite Parent Shareholder Approval would not otherwise be obtained; (D) after consultation with the Company,
if otherwise required by applicable Law; or (E) with the prior written consent of the Company; provided, that the Parent Shareholders’
Meeting will not be adjourned to a date that is more than thirty (30) days after the date for which the Parent Shareholders’ Meeting
was originally scheduled (excluding any adjournments required by applicable Law). Parent shall advise the Company upon request on a daily
basis during each of the last five (5) Business Days prior to the date of the Parent Shareholders Meeting as to the aggregate tally of
proxies received by Parent with respect to the Parent Shareholder Approval and at additional times upon the reasonable request of the
Company. Parent agrees that it shall provide the holders of shares of Parent Common Stock the opportunity to elect redemption of such
shares of Parent Common Stock in connection with the Parent Shareholders’ Meeting, as required by Parent’s Governing Documents.
(ii)
Subject to Section 7.4(e)(iii), Parent, through the Parent Board, shall unanimously recommend to its shareholders (A) adoption
and approval of the Domestication and change in the jurisdiction of incorporation of Parent from the Cayman Islands to the State of Delaware,
(B) amendment and restatement of Parent’s existing Governing Documents in connection with the Domestication, including any separate
or unbundled proposals as are required to implement the foregoing and the change of Parent’s name to Citius Oncology, Inc. as set
forth in the Parent Charter, (C) adoption and approval of this Agreement, the Transaction Documents and the Transactions contemplated
hereby and thereby, in each case, in accordance with applicable Law and exchange rules and regulations, (D) approval of the issuance of
shares of Domesticated Parent Common Stock in connection with the Merger, (E) approval of the adoption by Parent of an incentive equity
plan with a share reserve equal to 15% of the issued and outstanding shares of Parent immediately following the Effective Time (in substantially
the form attached hereto as Exhibit D, “Parent Equity Incentive Plan”), (F) election of directors to the Parent
Board effective as of the Closing as contemplated by this Agreement, (G) adoption and approval of any other proposals as the SEC (or staff
thereof) may indicate are necessary in its comments to the Proxy Statement or the Parent Registration Statement or correspondence related
thereto, (H) adoption and approval of any other proposals as reasonably agreed by Parent and the Company to be necessary or appropriate
in connection with the Transactions and (I) adjournment of the Parent Shareholders Meeting, if necessary, to permit further solicitation
of proxies because there are not sufficient votes to constitute a quorum or approve and adopt any of the foregoing (such proposals in
(A) through (I), together, the “Transaction Proposals”), and include such Parent Board Recommendation in the Proxy
Statement. Parent shall use its commercially reasonable efforts to (1) solicit from its shareholders proxies in favor of the approval
of the proposals required under the Parent Shareholder Approval, and (2) take all other action necessary or advisable to secure the Parent
Shareholder Approval. Subject to Section 7.4(e)(iii), neither the Parent Board nor any committee thereof shall withdraw, amend,
qualify or modify its recommendation to the Parent Shareholders that they vote in favor of the Transaction Proposals.
(iii)
Notwithstanding anything in this Section 7.4 to the contrary, if, at any time prior to obtaining the Parent Shareholder
Approval, the Parent Board determines, in good faith, after consultation with its outside legal counsel of the applicable jurisdiction,
that an Intervening Event has occurred and that, as a result thereof, a failure to withdraw or modify a Parent Board Recommendation would
be inconsistent with the Parent Board’s fiduciary duties under applicable Law, then the Parent Board may withdraw or modify such
Parent Board Recommendation; provided that the Parent shall not withdraw or modify such Parent Board Recommendation unless (i) Parent
first delivers to the Company a written notice advising the Company that the Parent Board proposes to take such action and containing
the material facts underlying the Parent Board’s determination that an Intervening Event has occurred and that a failure to withdraw
or modify a Parent Board Recommendation would constitute a breach by the Parent Board of its fiduciary obligations under applicable Law
(an “Intervening Event Notice”), and (ii) at or after 5:00 p.m. Eastern Time, on the fourth (4th) Business Day immediately
following the day on which Parent delivered the Intervening Event Notice (such period from the time the Intervening Event Notice is provided
until 5:00 p.m. Eastern Time on the fourth (4th) Business Day immediately following the day on which Parent delivered the Intervening
Event Notice (it being understood that any material development with respect to an Intervening Event shall require a new notice, but with
an additional three (3) Business Day (instead of four (4) Business Day) period from the date of such notice), the “Intervening
Event Notice Period”), the Parent Board reaffirms in good faith (after consultation with its outside legal counsel) that a failure
to withdraw or modify such Parent Board Recommendation would be inconsistent with the Parent Board’s fiduciary duties under applicable
Law. If requested by the Company, Parent shall, and shall use its reasonable best efforts to cause its Representatives to, during the
Intervening Event Notice Period, engage in good faith negotiations with the Company and its Representatives to make such adjustments in
the terms and conditions of this Agreement so as to obviate the need for any withdrawal or modification of such Parent Board Recommendation.
7.5
Reasonable Best Efforts.
(a)
Each of Parent, the Company and their respective Subsidiaries shall use its reasonable best efforts to promptly take, or cause
to be taken, all actions, and to promptly do, or cause to be done, and to assist and cooperate with the other in doing, all things reasonably
necessary, proper or advisable under applicable Laws to consummate and make effective the Merger and the other transactions contemplated
by this Agreement and the other Transaction Documents, as promptly as practicable and in any event prior to the Outside Date, including
(i) the obtaining of all necessary actions or nonactions, waivers, consents, clearances, approvals, and expirations or terminations of
waiting periods, from Governmental Authorities and the making of all necessary registrations and filings in connection therewith, and
(ii) using its reasonable best efforts to obtain all necessary consents, approvals or waivers from third parties; provided, that
in no event shall the Company, Parent or their respective Subsidiaries be required to pay any fee, penalty or other consideration to any
third party for any consent or approval required for the consummation of the transactions contemplated by this Agreement under any Contract.
(b)
The Company and Parent shall (i) as reasonably practicable and advisable file (or cause to be filed) any and all required pre-merger
notification and report forms under the HSR Act with respect to the Merger, and (ii) make, as promptly as practicable and advisable, any
appropriate filings with other Governmental Authorities, if necessary or advisable, pursuant to any other Antitrust Law. The Company and
Parent shall (and, to the extent required, shall cause its Affiliates to) request early termination of any applicable waiting periods
under the Antitrust Laws (if available) and shall respectively use their reasonable best efforts to cause the expiration or termination
of such waiting periods, and shall supply to the Antitrust Division of the United States Department of Justice or the United States Federal
Trade Commission as promptly as reasonably practicable and advisable any additional information or documents that may be requested pursuant
to any Law or by any of them. No party hereto shall take any action without the other party’s consent that could reasonably be expected
to adversely affect or materially delay (including by entering into a timing agreement), and each party hereto shall diligently pursue,
the approval of any Governmental Authority of any required filings or applications under Antitrust Laws.
(c)
In furtherance of the covenants of the parties contained in this Section 7.5 (i) if any administrative or judicial action
or proceeding, including any proceeding by a private party, is instituted (or threatened to be instituted) challenging the Merger as violative
of any Antitrust Law, each of the parties hereto shall use reasonable best efforts to contest and resist any such action or proceeding
and to have vacated, lifted, reversed or overturned any decree, judgment, injunction, or other order, whether temporary, preliminary or
permanent, that results from such action or proceeding and that prohibits, prevents or restricts consummation of the Merger on or before
the Outside Date and (ii) Parent and the Company each shall use reasonable best efforts to take such further action as may be necessary
to avoid or eliminate promptly each impediment under any Antitrust Law so as to enable the Closing to occur as promptly as practicable
(and in any event no later than the Outside Date); provided, that neither Parent nor the Company shall be required to take any
action under this Section 7.5 that would materially impact Parent’s or the Company’s expected benefits resulting from
the Transactions. Notwithstanding anything in this Agreement to the contrary, the Company and its Subsidiaries shall not be obligated
to take or agree or commit to take any action (A) that is not conditioned on the Closing, or (B) that relates to any retained business
or assets of the Company.
(d)
Parent and the Company shall cooperate and consult with each other in connection with the making of all filings, notifications,
communications, submissions, and any other actions pursuant to this Section 7.5, and, subject to applicable legal limitations and
the instructions of any Governmental Authority, Parent and the Company shall keep each other apprised on a current basis of the status
of matters relating to the completion of the Transactions, including promptly furnishing the other with copies of notices or other communications
received by Parent and the Company, as the case may be, or any of their respective Subsidiaries or Affiliates, from any third party or
any Governmental Authority with respect to such Transactions. Subject to applicable Law relating to the exchange of information, Parent
and the Company shall permit counsel for the other party reasonable opportunity to review in advance, and consider in good faith the views
of the other party in connection with, any proposed notifications or filings and any substantive written communications or submissions
to any Governmental Authority; provided, that materials may be redacted (i) to remove references concerning the valuation of SpinCo
or information concerning the Transaction Process, or proposals from third parties with respect thereto, (ii) as necessary to comply with
contractual agreements, and (iii) as necessary to address reasonable privilege or confidentiality concerns. Parent and the Company agree
not to participate in any pre-scheduled meeting or discussion, either in person, by video conference, or by telephone, with any Governmental
Authority in connection with the Transactions unless it consults with the other party in advance and, to the extent not prohibited by
such Governmental Authority, gives the other party a reasonable opportunity to attend and participate.
7.6
Access to Information.
(a)
From the date of this Agreement until the Effective Time or the earlier termination of this Agreement, the Company and Parent shall
(and shall cause their respective Subsidiaries to): (i) provide to the other Party (and the other Party’s Representatives) reasonable
access during normal business hours and upon reasonable prior notice to the officers, employees, agents, properties, offices and other
facilities of such party and its subsidiaries and to the books and records thereof, except that such access shall not include any unreasonably
invasive or intrusive investigations or other testing, sampling or analysis of any properties, facilities or equipment of the Company
without the prior written consent of the Company; and (ii) furnish promptly to the other party such information concerning the business,
properties, contracts, assets, liabilities, personnel and other aspects of such party and its subsidiaries as the other party or its Representatives
may reasonably request. Notwithstanding the immediately preceding sentence, neither the Company nor Parent shall be required to provide
access to or disclose information to the extent such party has been advised by legal counsel that the access or disclosure would (x) violate
its obligations of confidentiality or similar legal restrictions with respect to such information, (y) jeopardize the protection of attorney-client
privilege or (z) contravene applicable Law (it being agreed that the parties shall use their commercially reasonable efforts to cause
such information to be provided in a manner that would not result in such inconsistency, conflict, jeopardy or contravention).
(b)
The Parties hereby agree that the provisions of the Confidentiality Agreement shall apply to all information and material furnished
by any Party or its Representatives thereunder and hereunder. The Confidentiality Agreement shall survive any termination of this Agreement.
7.7
Permitted Activities and Exclusivity.
(a)
At any time during the Specified Period, the Company may:
(i)
solicit, initiate, knowingly encourage or knowingly facilitate (including by way of furnishing information that has not been previously
publicly disseminated) any proposal from or on behalf of a third party relating to, directly or indirectly, any acquisition (whether by
merger, purchase of Interests, purchase of assets or otherwise), exclusive license, joint venture, partnership, recapitalization, liquidation,
dissolution or other transaction involving any portion of the business or assets of the Company and any of its Subsidiaries (including
SpinCo and LYMPHIRTM (denileukin diftitox)) (any of the foregoing, an “Acquisition Proposal”), or any inquiry,
proposal or offer which would reasonably be expected to lead to an Acquisition Proposal;
(ii)
engage in any discussions or negotiations regarding, or furnish to any Person any nonpublic information relating to, the Company
or any of its Subsidiaries in connection with any Acquisition Proposal or any inquiry, proposal, effort or attempt related to or that
could reasonably be expected to lead to an Acquisition Proposal;
(iii)
adopt, approve or recommend, or publicly propose to adopt, approve or recommend, any Acquisition Proposal; or
(iv)
approve or authorize, or cause or permit the Company or any of its Subsidiaries to enter into, any merger agreement, acquisition
agreement, reorganization agreement, letter of intent, memorandum of understanding, agreement in principle, option agreement, joint venture
agreement, partnership agreement or similar agreement or document relating to, or providing for, any Acquisition Proposal.
(b)
Notwithstanding anything to the contrary set forth in this Section 7.7, at any time prior to the termination of the Specified
Period, the Company may elect to terminate this Agreement pursuant to and subject to the terms of Section 9.1(h) and Section
9.3 in order to accept an Acquisition Proposal and enter into a definitive agreement with respect to an Acquisition Proposal. Prior
to accepting an Acquisition Proposal and terminating this Agreement pursuant to and subject to the terms of Section 9.1(h) and
Section 9.3, the Company must have delivered written notice to the Parent of its intention to enter into such definitive agreement
at least five (5) Business Days prior to taking such action by the Company, which notice must include the terms and conditions of such
Acquisition Proposal. During the five (5)-Business Day period and prior to entering into such definitive agreement, Parent may offer to
amend the terms and conditions of this Agreement to match the Acquisition Proposal, and the Company shall negotiate in good faith with
Parent regarding such matching offer.
(c)
As of the termination of the Specified Period, the Company shall cease and cause SpinCo and their respective Representatives to
cease any discussions or negotiations with any Person (other than Parent and its affiliates) that may be ongoing with respect to (i) an
Acquisition Proposal that is related to SpinCo or LYMPHIRTM (denileukin diftitox) only or (ii) an Acquisition Proposal other
than a Permitted Acquisition Proposal (each of (i) and (ii) a “SpinCo Proposal”), or any inquiry, proposal or offer
that would reasonably be expected to lead to a SpinCo Proposal.
(d)
From the date of the termination of the Specified Period until the earlier to occur of (i) the termination of this Agreement pursuant
to Article IX and (ii) the Effective Time, the Company will not, and will use commercially reasonable efforts to cause its Representatives
not to:
(i)
solicit, initiate, encourage or facilitate (including by way of furnishing information that has not been previously publicly disseminated)
any proposal from or on behalf of a third party relating to, directly or indirectly, any SpinCo Proposal, or any inquiry, proposal or
offer that would reasonably be expected to lead to a SpinCo Proposal;
(ii)
engage in any discussions or negotiations regarding, or exchange with any Person any nonpublic information in connection with,
any SpinCo Proposals or any inquiry, proposal, effort or attempt related to or that would reasonably be expected to lead to, a SpinCo
Proposal;
(iii)
adopt, approve or recommend, or publicly propose to adopt, approve or recommend, any SpinCo Proposal; or
(iv)
approve or authorize or cause or permit the Company or SpinCo to enter into, any merger agreement, acquisition agreement, reorganization
agreement, letter of intent, memorandum of understanding, agreement in principle, option agreement, joint venture agreement, partnership
agreement or similar agreement or document relating to, or providing for, any SpinCo Proposal.
The restrictions set forth in this Section
7.7(d) shall not limit the Company’s ability to pursue or engage in any transaction relating to a Permitted Acquisition Proposal.
(e)
Parent shall cease and shall cause its Representatives to cease, any discussions or negotiations with any Person (other than the
Company or its Affiliates) that may be ongoing with respect to a Parent Business Combination Proposal, or any inquiry, proposal or offer
that would reasonably be expected to lead to a Parent Business Combination Proposal. From the date hereof until the earlier to occur of
(i) the termination of this Agreement pursuant to Article IX and (ii) the Effective Time, Parent shall not and shall use commercially
reasonable efforts to cause its Representatives not to: (w) solicit, initiate, encourage or facilitate (including by way of furnishing
information that has not been previously publicly disseminated) any proposal from or on behalf of a third party relating to, directly
or indirectly, any Business Combination, or any inquiry, proposal or offer which would reasonably be expected to lead to a Parent Business
Combination Proposal, (x) engage in any discussions or negotiations regarding, or exchange with any Person any nonpublic information in
connection with, any Parent Business Combination Proposal or any inquiry, proposal, effort or attempt related to or that would reasonably
be expected to lead to, a Parent Business Combination Proposal, (y) adopt, approve or recommend, or publicly propose to adopt, approve
or recommend, any Parent Business Combination Proposal or (z) approve or authorize, or cause or permit Parent to enter into, any merger
agreement, acquisition agreement, reorganization agreement, letter of intent, memorandum of understanding, agreement in principle, option
agreement, joint venture agreement, partnership agreement or similar agreement or document relating to, or providing for, any Parent Business
Combination Proposal.
7.8
Public Announcements. Except (a) as otherwise expressly contemplated by this Agreement, (b) in connection with any press
release, public statement or filing to be issued or made by Parent, and (c) for the separate or joint press releases to be issued by the
Parties in the forms agreed by the Parties (or any public statement or disclosure that contains or reflects only such information previously
disclosed in press releases or other public disclosures made in accordance with this Section 7.8), neither Parent nor the Company
will, and each of Parent and the Company will cause its Subsidiaries not to, issue any press release or otherwise make any public statements
or disclosure with respect to the Transactions without the prior written consent of the other Party. Notwithstanding the foregoing, to
the extent such disclosure is required by applicable Law or the rules of any stock exchange, the Party seeking to make such disclosure
will promptly notify the other Party thereof and the Party making such statement will use efforts reasonable under the circumstances to
consult in good faith with the other Party thereto prior to making such disclosure in order to allow a mutually agreeable release or announcement
to be issued. Notwithstanding the foregoing, any Party may make statements that are consistent with previous public releases made by such
Party in compliance with this Section 7.8.
7.9
Section 16 Matters. Prior to the Effective Time, each of Parent, the Company and SpinCo shall take all such steps as may
be required (to the extent permitted by applicable Law) to cause any dispositions of SpinCo Common Stock (including derivative securities
with respect to SpinCo Common Stock) or acquisitions of Parent Common Stock or Domesticated Parent Common Stock resulting from the Transactions,
directly or indirectly, by each individual, if any, who is subject to Section 16(a) of the Exchange Act with respect to Parent as an officer
or director thereof to be exempt under Rule 16b-3 promulgated under the Exchange Act, such steps to be taken in accordance with (and to
the extent permitted by) applicable SEC rules and regulations and interpretations of the SEC staff.
7.10
Control of Other Party’s Business. Nothing contained in this Agreement shall give the Company or SpinCo, directly
or indirectly, the right to control or direct Parent’s operations prior to the Effective Time. Nothing contained in this Agreement
shall give Parent, directly or indirectly, the right to control or direct the operations of the Company, including the SpinCo Business,
prior to the Effective Time. Prior to the Effective Time, each of the Company, SpinCo and Parent shall exercise, consistent with the terms
and conditions of this Agreement, complete control and supervision over its respective operations.
7.11
Domestication. Subject to receipt of the Parent Shareholder Approval, no later than the date that is one day prior to the
Effective Time, Parent shall cause the Domestication to become effective, including by (a) filing with the Secretary of State of the State
of Delaware a Certificate of Domestication with respect to the Domestication, in form and substance reasonably acceptable to Parent and
the Company, together with the Parent Charter substantially in the form attached as Exhibit A hereto (with such changes as may
be agreed in writing by Parent and the Company, the “Parent Charter”), in each case, in accordance with the provisions
thereof and applicable Law, (b) completing, making and procuring all those filings required to be made with the Cayman Registrar in connection
with the Domestication and (c) obtaining a certificate of de-registration from the Cayman Registrar. In accordance with applicable Law,
the Domestication shall provide that at the effective time of the Domestication, by virtue of the Domestication, and without any action
on the part of any shareholder of Parent, (i) each then issued and outstanding share of Parent Common Stock shall convert automatically,
on a one-for-one basis, into a share of Domesticated Parent Common Stock; (ii) each then issued and outstanding Parent Right shall convert
automatically into a Domesticated Parent Right, pursuant to the Rights Agreement; (iii) each then issued and outstanding Parent Unit shall
convert automatically into one Domesticated Parent Unit; and (iv) Parent’s bylaws from and after the effective time of the Domestication
shall be substantially in the form attached as Exhibit B hereto (with such changes as may be agreed in writing by Parent and the
Company, the “Parent Bylaws”).
7.12 NASDAQ
Listing. (a) From the date hereof through the Effective Time, Parent shall use its commercially reasonable efforts to maintain
the listing of the Parent Common Stock, Parent Units, and Parent Rights on NASDAQ and maintain all applicable initial and continuing
listing requirements of NASDAQ. Parent shall prepare and submit to NASDAQ a listing application, if required under NASDAQ rules,
covering the shares of Domesticated Parent Common Stock issuable in the Merger, and shall use its commercially reasonable efforts to
cause the shares of Domesticated Parent Common Stock issuable pursuant to the Transactions to be approved for listing on the NASDAQ,
subject to official notice of issuance, as promptly as practicable after the date of this Agreement, and in any event prior to the
Effective Time.
(b)
The Company and Parent shall cooperate and consult with each other in connection with ensuring that the total number of round lot
holders of Domesticated Parent Common Stock is sufficient for the Domesticated Parent Common Stock to satisfy the applicable NASDAQ initial
listing requirement related to round lot holders as of the Closing. Such actions potentially could include, for example, the Company authorizing
the distribution of a number of shares of Domesticated Parent Common Stock that the Company will receive as a result of the Merger to
the Company’s shareholders.
7.13
Takeover Statutes. If any “fair price,” “moratorium,” “control share acquisition,” “business
combination” or other form of antitakeover Law shall become applicable to the Transactions, Parent, Merger Sub and their respective
boards of directors shall use all reasonable efforts to grant such approvals and take such actions as are reasonably necessary so that
the Transactions may be consummated as promptly as practicable on the terms contemplated hereby and otherwise act to eliminate or minimize
the effects of such statute or regulation on the Transactions.
7.14
Sole Shareholder Approvals. Promptly after the execution of this Agreement, (a) the Company will approve and adopt, as SpinCo’s
sole shareholder, this Agreement and the Transaction Documents and the Transactions, including the Merger (the “SpinCo Shareholder
Approval”) and deliver the SpinCo Shareholder Approval to Parent, and (b) Parent, as the sole shareholder of Merger Sub, acting
by written consent, will adopt this Agreement and approve the consummation of the Transactions, upon the terms and subject to the conditions
stated herein and in accordance with the applicable provisions of the DGCL (the “Merger Sub Shareholder Approval”)
and deliver a copy of the Merger Sub Shareholder Approval to the Company.
7.15
Financial Information.
(a)
The Company shall, from the date hereof until the Closing Date, prepare and deliver to Parent, (i) as promptly as reasonably practicable
and no later than seventy-five (75) calendar days after the end of any fiscal quarter, the unaudited balance sheet of SpinCo as of the
end of such fiscal quarter and the related unaudited statements of income and cash flows of SpinCo for such fiscal quarter, together with
comparable financial statements for the corresponding periods of the prior fiscal years, in each case, to the extent required to be included
or incorporated by reference in the Parent Registration Statement (including the Proxy Statement), (collectively, the “Subsequent
Unaudited SpinCo Financial Statements”) and (ii) if necessary, as promptly as reasonably practicable and no later than one-hundred
(100) calendar days after the end of any fiscal year, the audited balance sheet of SpinCo as of the end of such fiscal year of SpinCo
and the related audited statements of income and cash flows of SpinCo for such fiscal year, together with comparable financial statements
for the prior fiscal years, in each case, to the extent required to be included or incorporated by reference in the Parent Registration
Statement (including the Proxy Statement) (collectively, the “Subsequent Audited Annual SpinCo Financial Statements”
and, together with the Subsequent Unaudited SpinCo Financial Statements, the “Subsequent Period SpinCo Financial Statements”).
The Subsequent Period SpinCo Financial Statements shall be prepared from the books and records of the Company and SpinCo and in accordance
with GAAP applied on a consistent basis throughout the periods involved (except as may otherwise be required under GAAP) and the applicable
rules and regulations of the SEC, including the requirements of Regulation S-X. When delivered, the Subsequent Period SpinCo Financial
Statements shall present fairly in all material respects the financial position and results of operations of SpinCo as of the dates and
for the periods shown therein.
(b)
During the Interim Period and from and after the Closing, the Company shall use its reasonable best efforts, in connection with
the filing of any applicable SEC filings, to cooperate with Parent to prepare pro forma financial statements that comply with the rules
and regulations of the SEC to the extent required for SEC filings, including the requirements of Regulation S-X.
7.16
Extension; Payment of Extension Fees.
(a)
Extensions of the Trust Agreement:
(i) The
Parent and the Sponsor may extend, in accordance with the terms of the Governing Documents of Parent and the Trust Agreement, the
date by which Parent must consummate a Business Combination, for an additional three (3) months after October 18, 2023 (the
“Second Extension”).
(ii) In
the event that Parent and the Company determine that it is reasonably likely that the Closing will not be consummated prior to
January 18, 2024, then Parent and the Sponsor may extend, in accordance with the terms of the Governing Documents of Parent and the
Trust Agreement, the date by which Parent must consummate a Business Combination, for an additional three (3) months (the
“Third Extension”).
(iii) In the event that
it is reasonably determined by Parent and the Company on or before February 14, 2024 (or such other date that is agreed to in
writing by Parent and the Company) that it is reasonably likely that the Merger will not be consummated by April 17, 2024, then upon
the written request of Parent or the Company to the other party, Parent and the Company shall (i) reasonably cooperate with respect
to the preparation, filing and mailing of a proxy statement and any other materials necessary to solicit proxies from Parent
Shareholders to vote, at an extraordinary general meeting of Parent to be called and held for purpose of such vote, in favor of (A)
amending Parent’s Governing Documents (such amendment, the “Extension Amendment”) to extend the final date
in respect of which Parent must consummate a Business Combination thereunder to October 17, 2024 or such other date that is mutually
agreed to by the Company and Parent in writing (such extension, the “Fourth Extension,” and such date, the
“Extension Date”) and (B) such other matters as the Company and Parent shall mutually determine to be necessary
or appropriate in order to effect the Extension Amendment; and (ii) execute and deliver such other documents and take such other
actions, as may reasonably be necessary to effectuate the Extension Amendment. Notwithstanding anything to the contrary, the right
to make a written request pursuant to the preceding sentence shall not be available to a party if the potential failure of the
Merger to be consummated by April 17, 2024 was due to such party’s breach of or failure to perform in any material respect any
of its covenants or agreements set forth in this Agreement.
(b)
Parent, Sponsor, and the Company will pay the extension fees the Sponsor is required to deposit in the Trust Account in connection
with the extension of the date by which Parent must complete a Business Combination (each, an “Extension Fee”) as follows:
(i)
The Company will pay a portion of each Extension Fee as follows:
A. The
Company will pay $125,000 of the Extension Fee related to the Second Extension directly to the Sponsor or its designee by wire
transfer of immediately available funds within two (2) Business Days after the date hereof;
B. The
Company will pay $200,000 of the Extension Fee related to the Third Extension into the Trust Account; and
C. The
Company will pay $200,000 of the Extension Fee related to the Fourth Extension into the Trust Account.
(ii)
Sponsor will deposit into the Trust Account the remainder of the respective Third and Fourth Extension Fees in excess of the Company’s
portion of such respective Extension Fees. The Company will be obligated to pay a portion of the Third and Fourth Extension Fees only
if this Agreement has not been terminated at the time of the Third Extension, or Fourth Extension, as applicable. If the applicable Extension
Fee is less than the respective amount specified in Section 7.16(b)(i), the Company will be obligated to pay only such lesser amount
of the actual Extension Fee. The Company’s obligation to pay a portion of the Extension Fees will terminate upon the termination
of this Agreement in accordance with Article IX, except that if the Company’s portion of an applicable Extension Fee is due
but not yet paid at the time of such termination, the Company’s obligation to pay such amount will not terminate upon the termination
of this Agreement.
(iii)
Upon the Closing, the Company will be repaid by Parent an amount equal to the portion of the Extension Fees that the Company paid
either to the Sponsor (or its designee) or into the Trust Account, but only if at the time of the Closing the balance of the Trust Account
equals or exceeds $2,000,000.
(c)
As promptly as reasonably practicable following the time at which the proxy statement contemplated by Section 7.16(a)(iii)
is cleared by the SEC, Parent shall establish the record date for, duly call, give notice of, and duly convene and hold, the applicable
extraordinary general meeting.
(d)
Without limiting the foregoing and for the avoidance of doubt, Parent shall be permitted to take any other means allowed under
Parent’s Governing Documents to extend the time in respect of which Parent must consummate a Business Combination.
7.17
Equity Incentive Plan. Prior to the Effective Time, Parent shall adopt and approve the Parent Equity Incentive Plan.
7.18
Transfer of Certain Intellectual Property to SpinCo.
(a)
Prior to the Closing, the Company and SpinCo shall enter into a transfer and assignment agreement, in a form reasonably satisfactory
to Parent and the Company, pursuant to which the Company will transfer the LYMPHIRTM (denileukin diftitox) trademark or trademark
application, as applicable, and SpinCo shall accept such transfer and assignment with regard to the LYMPHIRTM (denileukin diftitox)
trademark, which assignment and transfer will be automatically effective for all purposes as of the effective date of the Company FDA
Letter and the SpinCo FDA Letter; provided, however, that upon any termination of this Agreement in accordance with the
terms of Article IX such transfer and assignment agreement will terminate automatically.
(b)
Promptly following the Company’s receipt of the Notice of Approval, but in no event later than five (5) Business Days after
the Company’s receipt of the Notice of Approval, (i) the Company shall deliver to the FDA an executed Company FDA Letter, and (ii)
SpinCo (or if the Notice of Approval occurs after the Effective Time, the Surviving Corporation) shall deliver to the FDA an executed
SpinCo FDA Letter.
(c)
To the extent permitted under any SpinCo Material Contract and applicable Law, SpinCo hereby grants to the Company a fully-paid,
nonexclusive, worldwide, royalty-free license under, and to use, the SpinCo Intellectual Property for the purpose of performing Company’s
obligations under, and seeking and obtaining FDA approval of, the BLA (or any amended form thereof), which license shall include a right
of reference to any regulatory filings, applications, approvals, or clearances held, owned, or controlled by SpinCo. To the extent permitted
under any Contract to which the Company is a party and applicable Law, the Company hereby grants to SpinCo a fully-paid, nonexclusive,
worldwide, royalty-free, sublicensable license under the Intellectual Property owned, controlled, or licensed by the Company to use, research,
develop, make, have made, commercialize, and import any products that are subject to the BLA, including LYMPHIRTM (denileukin
diftitox), which rights shall include a right of reference to the BLA and IND. The foregoing licenses will terminate automatically upon
the earlier of the termination of this Agreement in accordance with the terms of Article IX or the effectiveness of the transfer
of the BLA to SpinCo pursuant to Section 7.18(b).
7.19
Insurance. The Company shall use commercially reasonable efforts, and the Parent and Merger Sub shall provide assistance
as the Company may reasonably request, to obtain for SpinCo insurance policies that are reasonable and customary for a company such as
SpinCo to be effective as of the Effective Time.
7.20
BLA Resubmission. The Company will use its reasonable best efforts to resubmit to the FDA prior to February 1, 2024 the
BLA for LYMPHIRTM (denileukin diftitox) in accordance with Company’s plan to address the FDA’s complete response
letter received by the Company on July 28, 2023, and with any additional guidance provide by the FDA to the Company. The immediately preceding
sentence notwithstanding, the form, substance, and timing of any resubmission to the FDA will be in the Company’s absolute discretion.
Article
VIII
CONDITIONS TO THE MERGER
8.1
Conditions to the Obligations of SpinCo, the Company, Parent and Merger Sub to Effect the Merger. The respective obligations
of each Party to consummate the Merger shall be subject to the fulfillment (or, to the extent permitted by applicable Law, waiver by the
Company and Parent) at or prior to the Closing of the following conditions:
(a)
(i) any waiting periods (including any extensions thereof) under the HSR Act with respect to the Transactions must have expired
or been terminated, and any other applicable waiting periods (or any extension thereof), filings or approvals under any applicable Laws
must have expired, been terminated, been made or been obtained, and (ii) there shall not be in effect any voluntary agreement between
the Parent or the Company and any Governmental Authority pursuant to which Parent or the Company has agreed not to consummate the Transactions
for any period of time;
(b)
the Parent Registration Statement shall have become effective in accordance with the Securities Act and shall not be the subject
of any stop order by the SEC or actual or threatened proceedings by a Governmental Authority seeking such a stop order;
(c)
the Parent Shareholder Approval shall have been obtained;
(d)
the SpinCo Shareholder Approval shall have been obtained;
(e)
no Governmental Authority of competent jurisdiction shall have enacted, issued or granted any Law (whether temporary, preliminary
or permanent), in each case that is in effect and which has the effect of restraining, enjoining or prohibiting the consummation of the
Transactions; and
(f)
Parent shall have at least $5,000,001 of net tangible assets (as determined in accordance with Rule 3a51-1(g)(1) of the Exchange
Act).
8.2
Additional Conditions to the Obligations of the Company and SpinCo. The obligation of the Company and SpinCo to consummate
the Merger shall be subject to the fulfillment (or, to the extent permitted by applicable Law, waiver by the Company) at or prior to the
Closing of the following additional conditions:
(a)
Parent and Merger Sub shall each have performed and complied in all material respects with the obligations, covenants and agreements
required by this Agreement to be performed or complied with by it at or prior to the Effective Time;
(b)
all representations and warranties made by Parent and Merger Sub set forth in Article VI (other than the representations
and warranties referenced in the second and third sentences of this Section 8.2(b)), without giving effect to materiality, Parent
Material Adverse Effect or similar qualifications, shall be true and correct in all respects at and as of the date hereof and as of the
Closing Date as though such representations and warranties were made at and as of the Closing Date (except in the case of any representation
or warranty that by its terms addresses matters only as of another specified date, which shall be so true and correct only as of such
specified date), except to the extent the failure of such representations and warranties to be true and correct (without giving effect
to materiality, Parent Material Adverse Effect or similar qualifications) would not have, individually or in the aggregate, a Parent Material
Adverse Effect. The representations and warranties made by Parent set forth in Section 6.1, Section 6.2, Section 6.3,
Section 6.10(a), and Section 6.11 shall be true and correct in all material respects at and as of the date hereof and as
of the Closing Date as though such representations and warranties were made at and as of the Closing Date (except in the case of any representation
or warranty that by its terms addresses matters only as of another specified date, which shall be so true and correct only as of such
specified date);
(c)
No Parent Material Adverse Effect shall have occurred between the date of this Agreement and the Closing Date;
(d)
Parent shall have delivered to the Company the certificate referenced in Section 2.3(b)(ii) dated as of the Closing Date
signed by an authorized officer of Parent certifying that each of the conditions set forth in Section 8.1(f) and Section 8.2(a),
(b), (c), and (e) have been satisfied;
(e)
Parent and Merger Sub shall have executed and delivered the applicable Transaction Documents, and to the extent applicable, performed
and complied with the obligations, covenants and agreements thereunder required to be performed by them prior to the Effective Time in
all material respects, and each such agreement shall be in full force and effect;
(f)
the Domestication shall have been completed as provided in Section 7.11 and a time-stamped copy of the certificate issued
by the Secretary of State of the State of Delaware in relation thereto shall have been delivered to the Company, and the shares of Domesticated
Parent Common Stock issuable pursuant to the Transactions shall have been approved for listing on NASDAQ, subject to official notice of
issuance;
(g)
As of the Closing Date, Parent shall not have received any written notice from NASDAQ that it has failed, or would reasonably be
expected to fail to meet the Nasdaq initial or continued listing requirements as of the Closing Date for any reason where such notice
has not been subsequently withdrawn by NASDAQ or the underlying failure appropriately remedied or satisfied;
(h)
other than those Persons identified as continuing directors on Section 8.2(h) of the Company Disclosure Schedule, all members
of the Parent Board and all executive officers of Parent shall have executed and delivered written resignations effective as of the Effective
Time, and the directors designated by the Company shall have been appointed to the board of directors of the Parent, effective as of the
Closing; and
(i)
Sponsor shall have paid or caused to be paid in full, by wire transfer of immediately available funds, all Parent Estimated Transaction
Expenses in excess of $500,000 (if any).
8.3
Additional Conditions to the Obligations of Parent and Merger Sub. The obligation of Parent and Merger Sub to consummate
the Merger shall be subject to the fulfillment (or, to the extent permitted by applicable Law, waiver by Parent) at or prior to the Closing
of the following additional conditions:
(a)
Each of SpinCo and the Company shall each have performed and complied in all material respects with the obligations, covenants
and agreements required by this Agreement to be performed or complied with by it at or prior to the Effective Time;
(b)
all representations and warranties made by the Company set forth in Article IV and Article V (other than the representations
and warranties referenced in the second and third sentences of this Section 8.3(b)), without giving effect to materiality, “Company
Material Adverse Effect”, “SpinCo Material Adverse Effect” or similar qualifications, shall be true and correct in all
respects at and as of the date hereof and as of the Closing Date as though such representations and warranties were made at and as of
the Closing Date (except in the case of any representation or warranty that by its terms addresses matters only as of another specified
date, which shall be so true and correct only as of such specified date), except to the extent the failure of such representations and
warranties to be true and correct (without giving effect to materiality, “Company Material Adverse Effect”, “SpinCo
Material Adverse Effect” or similar qualifications) would not have, individually or in the aggregate, a SpinCo Material Adverse
Effect, solely with respect to the representations and warranties set forth in Article V, or Company Material Adverse Effect, solely
with respect to the representations and warranties set forth in Article IV. The representations and warranties set forth in the
first sentence of Section 4.1, Section 4.2, Section 4.6, the first sentence of Section 5.1, Section 5.2,
Section 5.3, Section 5.13(a), and Section 5.21 shall be true and correct in all material respects at and as of the
date hereof and as of the Closing Date as though such representations and warranties were made at and as of the Closing Date (except in
the case of any representation or warranty that by its terms addresses matters only as of another specified date, which shall be so true
and correct only as of such specified date);
(c)
No SpinCo Material Adverse Effect shall have occurred between the date of this Agreement and the Closing Date;
(d)
The Company shall have delivered to Parent the certificate referenced in Section 2.3(a)(i) dated as of the Closing Date
signed by an authorized officer of the Company certifying that each of the conditions set forth in Section 8.3(a), (b),
(c) and (e) have been satisfied; and
(e)
SpinCo and the Company (or such other applicable Subsidiary of the Company) shall have executed and delivered each of the applicable
Transaction Documents, and to the extent applicable, performed and complied with the obligations, covenants and agreements to be performed
thereunder by them prior to the Effective Time in all material respects, and each such agreement shall be in full force and effect.
Article
IX
TERMINATION
9.1
Termination. This Agreement may be terminated and the Transactions may be abandoned at any time prior to the Effective Time,
whether before or after the Parent Shareholder Approval:
(a)
by mutual written agreement of the Company and Parent;
(b)
by written notice from the Company or Parent if, prior to the Closing, the FDA issues a complete response letter to the Company
in response to the Company’s resubmsission of its BLA;
(c)
by written notice from the Company or Parent, if the Closing shall not have occurred on or prior to September 30, 2024 (the “Outside
Date”); provided, that the right to terminate this Agreement pursuant to this Section 9.1(c) shall not be available
to any Party whose action or failure to comply with its obligations under this Agreement or any of the other Transaction Documents has
been the primary cause of, or has primarily resulted in, the failure of the Closing to occur on or prior to such date; and provided,
further, that if the FDA sets a PDUFA Date that is after the Outside Date, the Parties may hold meetings to discuss and consider extending
the Outside Date to a date after the PDUFA Date;
(d)
by written notice from the Company or Parent, if any Law or Order is promulgated, entered, enforced, enacted or issued and in effect
or is deemed to be applicable to the Merger or the other Transactions, by any Governmental Authority of competent jurisdiction that permanently
prohibits, restrains or makes illegal the consummation of the Merger or the other Transactions, and such Law or Order becomes final and
non-appealable; provided, that the right to terminate this Agreement pursuant to this Section 9.1 shall not be available
to any Party whose action or failure to perform any of its obligations under this Agreement or any of the Transaction Documents is the
primary cause of, or primarily resulted in, the enactment or issuance of any such Law or Order;
(e)
by Parent upon written notice to the Company, in the event of a breach of any representation, warranty, covenant or agreement on
the part of the Company or SpinCo or any such representation and warranty becomes untrue or inaccurate after the date of this Agreement,
such that the conditions specified in Section 8.3(a) or Section 8.3(b) would not be satisfied at the Closing, and which,
(i) with respect to any such breach that is capable of being cured, is not cured by the Company or SpinCo by the earlier of: (x) fifteen
(15) days after receipt of written notice thereof; or (y) the Outside Date, or (ii) is incapable of being cured prior to the Outside Date;
provided, that Parent shall not have the right to terminate this Agreement pursuant to this Section 9.1(e) if Parent or
Merger Sub is then in breach of any of its representations, warranties, covenants or agreements set forth in this Agreement to the extent
such breach or breaches would give rise to the failure of a condition set forth in Section 8.2(a) or Section 8.2(b);
(f)
by the Company upon written notice to Parent, in the event of a breach of any representation, warranty, covenant or agreement contained
in this Agreement on the part of Parent or Merger Sub or any such representation and warranty becomes untrue or inaccurate after the date
of this Agreement, such that the conditions specified in Section 8.2(a) or Section 8.2(b) would not be satisfied at the
Closing, and which, (i) with respect to any such breach that is capable of being cured, is not cured by Parent by the earlier of: (x)
fifteen (15) days after receipt of written notice thereof; or (y) the Outside Date, or (ii) is incapable of being cured prior to the Outside
Date; provided, that the Company shall not have the right to terminate this Agreement pursuant to this Section 9.1(f) if
the Company or SpinCo is then in breach of any of its representations, warranties, covenants or agreements set forth in this Agreement
to the extent such breach or breaches would give rise to the failure of a condition set forth in Section 8.3(a) or Section 8.3(b);
(g)
by the Company or Parent upon written notice, if (i) the Parent Shareholder Approval shall not have been obtained upon a vote taken
thereon at the Parent Shareholders Meeting, duly convened therefor, or at any adjournment or postponement thereof or (ii) if a vote is
taken on the Extension Amendment in accordance with Section 7.16(a)(iii) and the shareholders of Parent shall not have approved
the Extension Amendment; provided, that the right to terminate this Agreement pursuant to this Section 9.1(g) shall not
be available to Parent if Parent’s actions or failure to perform any of its obligations under this Agreement is the primary cause
of, or primarily resulted in, the failure to obtain such approval; and
(h)
by the Company, prior to the termination of the Specified Period, in order to accept an Acquisition Proposal and enter into, promptly
following such termination, a binding and definitive written Contract with respect to such Acquisition Proposal; provided, that
(i) the Company has complied in all material respects with its covenants and agreements under Section 7.7(a), (c) and (d)
and in all respects with its covenants and agreements under Section 7.7(b), and (ii) the Company pays the Termination Fee to Parent
in accordance with Section 9.3(a).
9.2
Effect of Termination. In the event of termination of this Agreement pursuant to Section 9.1, this Agreement shall
forthwith become null and void and have no effect, without any Liability on the part of any Party; provided, that no such termination
shall relieve any Party of any liability or damages resulting from Actual Fraud or Willful Breach; provided, further, that
Section 7.6(b), Section 7.16(b)(ii), this Section 9.2, Section 9.3, Section 9.4 and Article X
hereof shall survive any termination of this Agreement. The Confidentiality Agreement shall not be affected by any termination of this
Agreement.
9.3
Termination Fee.
(a)
In the event that this Agreement is validly terminated pursuant to Section 9.1(h), at or prior to such termination, the
Company shall pay Parent or its designee(s) a termination fee of $5,000,000 (such amount, the “Termination Fee”), by
wire transfer of immediately available funds to an account designated by Parent in writing.
(b)
Notwithstanding anything to the contrary set forth in this Agreement, except in the case of Actual Fraud or Willful Breach, if
the Termination Fee is paid pursuant to Section 9.3(a), such payment(s) shall constitute the sole and exclusive remedy of Parent,
Merger Sub, any of their respective Subsidiaries or any of their respective former, current or future general or limited partners, shareholders,
Representatives or assignees against the Company, SpinCo, any of their respective Subsidiaries and any of their respective former, current
or future general or limited partners, shareholders, Representatives or assignees (together with the Company, collectively, the “Company
Related Parties”) for all losses and damages suffered as a result of the failure of the Transactions to be consummated or for
a breach or failure to perform hereunder or otherwise, and none of the Company Related Parties shall have any further liability or obligation
relating to or arising out of this Agreement or the Transactions.
(c)
If the Company fails to pay promptly any amount due under this Section 9.3, as applicable, and in order to obtain such payment,
the Parent commences an Action that results in a judgment against the Company for any amount owed thereby under this Section 9.3,
as applicable, the Company shall reimburse Parent for its reasonable and documented costs and expenses (including reasonable and documented
attorneys’ fees) in connection with such Action.
(d)
Each of the Parties acknowledges that (i) the agreements contained in this Section 9.3 are an integral part of the Transactions,
(ii) without these agreements, the Parties would not enter into this Agreement and (iii) the Termination Fee does not constitute a penalty,
but rather is liquidated damages in a reasonable amount that will compensate Parent for the efforts and resources expended and opportunities
foregone while negotiating this Agreement and in reliance on this Agreement and on the expectation of the consummation of the Transactions,
which amount would otherwise be impossible to calculate with precision.
9.4
Fees and Expenses. Except (a) for the filing fee incurred in connection with the filings with any Governmental Authorities
pursuant to Section 7.5(b), which will be borne by the Company, or (b) as otherwise provided in this Agreement, all fees and expenses
incurred by the Parties shall be borne solely by the Party that has incurred such fees and expenses, whether or not the Merger is consummated.
Article
X
MISCELLANEOUS
10.1
Trust Account. As of the Effective Time, the obligations of Parent to dissolve or liquidate within a specified time period
as contained in Parent’s Certificate of Incorporation will be terminated and Parent shall have no obligation whatsoever to dissolve
and liquidate the assets of Parent by reason of the consummation of the Merger or otherwise, and no stockholder of Parent shall be entitled
to receive any amount from the Trust Account. At least 48 hours prior to the Effective Time, Parent shall provide notice to the Trustee
in accordance with the Trust Agreement and shall deliver any other documents, opinions or notices required to be delivered to the Trustee
pursuant to the Trust Agreement and cause the Trustee prior to the Effective Time to, and the Trustee shall thereupon be obligated to,
transfer all funds held in the Trust Account to Parent (to be held as available cash on the balance sheet of Parent, and to be used for
working capital and other general corporate purposes of the business following the Closing) and thereafter shall cause the Trust Account
and the Trust Agreement to terminate.
10.2
Non-Survival of Representations, Warranties and Agreements. The obligations, covenants and agreements that by their terms
are to be performed following the Closing pursuant to any Transaction Document, or this Agreement shall survive the Effective Time in
accordance with their terms and all other obligations, covenants and agreements herein and therein shall terminate and shall not survive
the Closing, except that this Article X shall survive the Closing indefinitely. None of the representations or warranties in this
Agreement or in any certificate or instrument delivered pursuant to this Agreement shall survive the Effective Time. Effective as of the
Closing, there are no remedies available to the Parties with respect to any breach of the representations, warranties, covenants or agreements
of the Parties, except in the case of fraud, and except, with respect to those covenants and agreements contained herein that by their
terms apply or are to be performed in whole or in part after the Closing, and the remedies that may be available under Section 10.9.
The Confidentiality Agreement shall survive the execution and delivery of this Agreement and any termination of this Agreement, and the
provisions of the Confidentiality Agreement shall apply to all information and material furnished by any Party or its Representatives
thereunder or hereunder; provided, that, following the Effective Time, Parent shall have no obligations under the Confidentiality
Agreement with respect to information related solely to SpinCo, the SpinCo Business or the SpinCo Assets.
10.3
Governing Law; Jurisdiction.
(a)
This Agreement, and all claims, disputes, controversies or causes of action (whether in contract, tort, equity or otherwise) that
may be based upon, arise out of or relate to this Agreement (including any schedule or exhibit hereto) or the negotiation, execution or
performance of this Agreement (including any claim, dispute, controversy or cause of action based upon, arising out of or related to any
representation or warranty made in or in connection with this Agreement or as an inducement to enter into this Agreement), shall be governed
by and construed in accordance with the Laws of the State of Delaware, without regard to any choice or conflict of law provision or rule
(whether of the State of Delaware or any other jurisdiction) that would cause the application of the Laws of any jurisdiction other than
the State of Delaware; provided, that the Domestication shall be effected in accordance with both the DGCL and the CICA (as applicable),
without giving effect to principles or rules of conflict of laws to the extent such principles or rules would require or permit the application
of Laws of another jurisdiction.
(b)
Each of the Parties agrees that any Action related to this agreement shall be brought exclusively in the Court of Chancery of the
State of Delaware or, if under applicable Law, exclusive jurisdiction over such matter is vested in the federal courts, any federal court
in the State of Delaware and any appellate court from any thereof (the “Chosen Courts”). By executing and delivering
this Agreement, each of the Parties irrevocably: (i) accepts generally and unconditionally submits to the exclusive jurisdiction of the
Chosen Courts for any Action relating to this Agreement; (ii) waives any objections which such party may now or hereafter have to the
laying of venue of any such Action contemplated by this Section 10.3 and hereby further irrevocably waives and agrees not to plead
or claim that any such Action has been brought in an inconvenient forum; (iii) agrees that it will not attempt to deny or defeat the personal
jurisdiction of the Chosen Courts by motion or other request for leave from any such court; (iv) agrees that it will not bring any Action
contemplated by this Section 10.3 in any court other than the Chosen Courts; (v) agrees that service of all process, including
the summons and complaint, in any Action may be made by registered or certified mail, return receipt requested, to such party at their
respective addresses provided in accordance with Section 10.4 or in any other manner permitted by Law; and (vi) agrees that service
as provided in the preceding clause (v) is sufficient to confer personal jurisdiction over such party in the Action, and otherwise constitutes
effective and binding service in every respect. Each of the parties hereto agrees that a final judgment in any Action in a Chosen Court
as provided above may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by Law, and each party
further agrees to the non-exclusive jurisdiction of the Chosen Courts for the enforcement or execution of any such judgment.
10.4
Notices. All notices and other communications among the Parties shall be in writing and shall be deemed to have been duly
given (a) when delivered in person, (b) when delivered after posting in the national mail having been sent registered or certified mail
return receipt requested, postage prepaid, (c) when delivered by FedEx or other internationally recognized overnight delivery service
or (d) when delivered by facsimile (solely if receipt is confirmed) or email (so long as the sender of such email does not receive an
automatic reply from the recipient’s email server indicating that the recipient did not receive such email), addressed as follows:
if to the Company or SpinCo, to:
Citius Pharmaceuticals, Inc.
11 Commerce Drive, First Floor
Cranford, NJ 07016
Attention: Jaime Bartushak
Email: jbartushak@citiuspharma.com
with a copy (which shall not constitute notice) to:
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suite 300
Raleigh, North Carolina 27607
| |
Attention: |
David Creekman |
| |
|
Alec Donaldson |
| |
Email: |
dcreekman@wyrick.com |
| |
|
adonaldson@wyrick.com |
if to Parent, to:
TenX Keane Acquisition
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Taylor Zhang |
| |
Email: |
tzhang@ascendantga.com |
with a copy (which shall not constitute notice) to:
The Crone Law Group
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Tammara Fort |
| |
|
Samara Thomas |
| |
Email: |
tfort@cronelawgroup.com |
| |
|
sthomas@cronelawgroup.com |
or to such other address or addresses as the Parties may from time
to time designate in writing by like notice.
10.5
Headings. The headings contained in this Agreement are inserted for convenience only and shall not be considered in interpreting
or construing any of the provisions contained in this Agreement.
10.6
Entire Agreement. This Agreement (including the Exhibits and Schedules hereto), the Confidentiality Agreement and the Transaction
Documents constitute the entire agreement between the Parties with respect to the subject matter hereof and thereof and supersede all
prior agreements and understandings between the Parties with respect to such subject matter; except that this Agreement shall not supersede
the terms and provisions of the Confidentiality Agreement, which shall survive and remain in effect until expiration or termination thereof
in accordance with its respective terms (subject to Section 10.2).
10.7
Amendments and Waivers.
(a)
Any Party may, at any time prior to the Closing, by action taken by its board of directors, or officers thereunto duly authorized,
waive any inaccuracies in the representations and warranties of the other Party contained herein or in any document, certificate or writing
delivered pursuant hereto and any of the terms or conditions of this Agreement or (without limiting Section 10.7(b)) agree to an
amendment or modification to this Agreement by an agreement in writing executed in the same manner (but not necessarily by the same Persons)
as this Agreement. No waiver by any of the Parties of any breach hereunder shall be deemed to extend to any prior or subsequent breach
hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence. No waiver by any of the Parties
of any of the provisions hereof shall be effective unless explicitly set forth in writing and executed by the Party sought to be charged
with such waiver.
(b)
This Agreement may be amended or modified, in whole or in part, only by a duly authorized agreement in writing executed by the
Parties in the same manner (but not necessarily by the same Persons) as this Agreement, and which makes reference to this Agreement.
10.8
Assignment; Parties in Interest; Non-Parties.
(a)
No Party may assign its rights or delegate its duties under this Agreement without the prior written consent of the other Parties.
Any attempted assignment or delegation in breach of this Section 10.8 shall be null and void. This Agreement shall be binding upon
and inure to the benefit of the Parties and their respective permitted successors and assigns. Nothing expressed or implied in this Agreement
is intended or shall be construed to confer upon or give any Person, other than the Parties, any rights or remedies under or by reason
of this Agreement, except as provided in Section 10.8(b) (which is intended to be for the benefit of the Persons covered thereby
and may be enforced by such Persons).
(b)
Notwithstanding anything to the contrary in this Agreement, it is hereby agreed and acknowledged that this Agreement may only be
enforced against, and any claims of action that may be based upon, arise out of or relate to this Agreement or the negotiation, execution
or performance of this Agreement may only be made against, the Parties hereto, and no former, current or future Affiliates, officers,
directors, managers, employees, equityholders, lenders, financing sources, managers, members, partners, agents or representatives of any
Party, in each case, who is not a Party to this Agreement, shall have any liability for any obligations of the Parties hereto or for any
claim based on, in respect of, or by reason of, the Transactions.
10.9
Specific Performance.
(a)
The Parties agree and acknowledge that the failure to perform under this Agreement will cause an actual, immediate and irreparable
harm and injury and that the Parties would not have any adequate remedy at law in the event that any of the provisions of this Agreement
were not performed in accordance with their specific terms (including failing to take such actions as are required of them hereunder to
consummate the Transactions) or were otherwise breached. Accordingly, it is agreed that, (i) each of the Parties shall be entitled to
seek an injunction or injunctions, specific performance or other equitable relief, each without proof of damages prior to the valid termination
of this Agreement in accordance with Section 9.1, to prevent breaches or threatened breaches of this Agreement by any other Party
and to specifically enforce the terms and provisions of this Agreement, this being in addition to any other remedy to which they are entitled
under this Agreement, and (ii) prior to the Closing or any termination of this Agreement in accordance with Section 9.1, damages
shall be awarded only in a case where a court of competent jurisdiction determines that, notwithstanding the Parties’ intention
for specific performance to be the applicable remedy prior to termination or the Closing, such specific performance is not available or
otherwise will not be granted as a remedy.
(b)
The Parties further agree that (i) by seeking the remedies provided for in this Section 10.9, a Party shall not in any respect
waive its right to seek any other form of relief that may be available to a party under this Agreement, including monetary damages, subject
to the terms hereof, (ii) nothing contained in this Section 10.9 shall require any Party to institute any proceeding for (or limit
any Party’s right to institute any proceeding for) specific performance under this Section 10.9 before exercising any termination
right under Section 9.1 (and pursuing damages after such termination), nor shall the commencement of any Action pursuant to this
Section 10.9 or anything contained in this Section 10.9 restrict or limit any Party’s right to terminate this Agreement
in accordance with the terms of Section 9.1 or to pursue any other remedies under this Agreement that may be available then or
thereafter; (iii) the right of specific enforcement is an integral part of the Transactions and without that right, none of the Parties
would have entered into this Agreement; and (iv) no Person shall be required to obtain, furnish or post any bond or similar instrument
in connection with or as a condition to obtaining any remedy referred to in this Section 10.9, and each Party irrevocably waives
any right it may have to require the obtaining, furnishing or posting of any such bond or similar instrument.
(c)
To the extent either party hereto brings any Action to enforce specifically the performance of the terms and provisions of this
Agreement in accordance with this Section 10.9, the Outside Date shall automatically be extended by (i) the amount of time during
which such Action is pending, plus twenty (20) Business Days, or (ii) such other time period established by the court presiding over such
Action.
10.10
WAIVER OF JURY TRIAL. THE PARTIES HEREBY UNCONDITIONALLY AND IRREVOCABLY WAIVE (AND SHALL CAUSE THEIR SUBSIDIARIES AND AFFILIATES
TO WAIVE) THEIR RIGHT TO TRIAL BY JURY IN ANY JUDICIAL PROCEEDING IN ANY COURT RELATING TO ANY DISPUTE, CONTROVERSY OR CLAIM ARISING OUT
OF, RELATING TO OR IN CONNECTION WITH THIS AGREEMENT, THE TRANSACTIONS, OR ANY TRANSACTION DOCUMENT (INCLUDING ANY SCHEDULE OR EXHIBIT
HERETO AND THERETO) OR THE BREACH, TERMINATION OR VALIDITY OF SUCH AGREEMENTS OR THE NEGOTIATION, EXECUTION OR PERFORMANCE OF SUCH AGREEMENTS.
NO PARTY TO THIS AGREEMENT SHALL SEEK A JURY TRIAL IN ANY LAWSUIT, PROCEEDING, COUNTERCLAIM OR ANY OTHER LITIGATION PROCEDURE BASED UPON,
OR ARISING OUT OF, THIS AGREEMENT, THE TRANSACTIONS, ANY TRANSACTION DOCUMENT, OR ANY RELATED INSTRUMENTS. NO PARTY WILL SEEK TO CONSOLIDATE
ANY SUCH ACTION IN WHICH A JURY TRIAL HAS BEEN WAIVED WITH ANY OTHER ACTION IN WHICH A JURY TRIAL CANNOT BE OR HAS NOT BEEN WAIVED. EACH
PARTY TO THIS AGREEMENT CERTIFIES THAT IT HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT OR INSTRUMENT BY, AMONG OTHER THINGS, THE MUTUAL
WAIVERS AND CERTIFICATIONS SET FORTH ABOVE IN THIS SECTION 10.10. NO PARTY OR REPRESENTATIVE OF ANY PARTY HAS IN ANY WAY AGREED
WITH OR REPRESENTED TO ANY OTHER PARTY THAT THE PROVISIONS OF THIS SECTION 10.10 WILL NOT BE FULLY ENFORCED IN ALL INSTANCES.
10.11
Severability. If any provision of this Agreement or any Transaction Document, or the application of any such provision to
any Person or circumstance, shall be held invalid, illegal or unenforceable in any respect by a court of competent jurisdiction, such
invalidity, illegality or unenforceability shall not affect any other provision hereof. The Parties further agree that if any provision
contained herein is, to any extent, held invalid or unenforceable in any respect under the Laws governing this Agreement, they shall take
any actions necessary to render the remaining provisions of this Agreement valid and enforceable to the fullest extent permitted by Law
and, to the extent necessary, shall amend or otherwise modify this Agreement to replace any provision contained herein that is held invalid
or unenforceable with a valid and enforceable provision giving effect to the intent of the Parties.
10.12
Counterparts. This Agreement may be executed in two or more counterparts (including by electronic or .pdf transmission),
each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Delivery of any signature
page by facsimile, electronic or .pdf transmission shall be binding to the same extent as an original signature page.
10.13 Disclosure
Schedules. The Company Disclosure Schedule, the SpinCo Disclosure Schedule and the Parent Disclosure Schedule (each, a
“Disclosure Schedule” and, collectively, the “Disclosure Schedules”) (including, in each case,
any section thereof) referenced herein are a part of this Agreement as if fully set forth herein. All references herein to the
Company Disclosure Schedule, SpinCo Disclosure Schedule and Parent Disclosure Schedule (including, in each case, any section
thereof) shall be deemed references to such parts of this Agreement, unless the context shall otherwise require. Certain information
set forth in the Disclosure Schedules is included solely for informational purposes and may not be required to be disclosed pursuant
to this Agreement. The disclosure of any information in any Disclosure Schedule shall not be deemed to constitute in itself an
acknowledgment that such information is required to be disclosed in connection with this Agreement, nor shall such information be
deemed to establish a standard of materiality.
[Signature page follows.]
IN WITNESS WHEREOF, the Parties have caused this
Agreement and Plan of Merger and Reorganization to be duly executed by their respective authorized officers as of the day and year first
above written.
| |
CITIUS PHARMACEUTICALS, INC. |
| |
|
|
|
| |
By: |
/s/
Leonard Mazur |
| |
|
Name: |
Leonard Mazur |
| |
|
Title: |
Chief Executive Officer
and Chairman of the Board |
| |
|
|
|
| |
CITIUS ONCOLOGY, INC. |
| |
|
|
|
| |
By: |
/s/
Leonard Mazur |
| |
|
Name: |
Leonard Mazur |
| |
|
Title: |
Chief Executive Officer
and Chairman of the Board |
| |
|
|
|
| |
TENX KEANE ACQUISITION |
| |
|
|
|
| |
By: |
/s/
Xiaofeng Yuan |
| |
|
Name: |
Xiaofeng Yuan |
| |
|
Title: |
Chief Executive Officer and Chairman of the Board |
| |
|
|
|
| |
TENX MERGER SUB, INC. |
| |
|
|
|
| |
By: |
/s/
Xiaofeng Yuan |
| |
|
Name: |
Xiaofeng Yuan |
| |
|
Title: |
Chief Executive Officer and Chairman of the Board |
ANNEX A
Defined Terms
Terms with initial capitalized
letters used in the Agreement to which this Annex A is attached will have the following respective meanings, and all references
to Sections, Schedules or Annexes in the following definitions will refer to Sections, Schedules or Annexes of or to such Agreement:
“Action”
means any claim, action, suit, demand, litigation, arbitration, mediation, inquiry, investigation, hearing, inquest, or other proceeding,
in each case, by any Person or Governmental Authority, in each case, before, heard by or otherwise involving any Governmental Authority.
“Actual Fraud”
means, with respect to a Party, an actual and intentional fraud with respect to the making of the representations and warranties pursuant
to Article IV, Article V or Article VI (as applicable).
“Affiliate”
means, with respect to any Person, any other Person that, directly or indirectly, controls, is controlled by, or is under common control
with, such Person, through one or more intermediaries or otherwise; provided, that, for the purposes of this definition, “control”
(including, with correlative meanings, the terms “controlled by” and “under common control with”), as used with
respect to any Person, means the possession, directly or indirectly, of the power to direct or cause the direction of the management and
policies of such Person, whether through the ownership of voting securities, by Contract or otherwise. Following the Effective Time, Affiliates
of Parent shall include SpinCo.
“Aggregate Consideration”
means Six Hundred and Seventy-Five Million Dollars ($675,000,000).
“Aggregate Parent
Common Stock Consideration” means the quotient of (i) the Aggregate Consideration divided by (ii) $10.
“Agreement”
means this Agreement and Plan of Merger and Reorganization, including all Annexes, Exhibits and Schedules hereto (including the SpinCo
Disclosure Schedule, the Company Disclosure Schedule, and the Parent Disclosure Schedule), as it may be amended, restated, modified or
supplemented from time to time in accordance with its terms.
“Amended and Restated
Shared Services Agreement” means the Amended and Restated Shared Services Agreement to be entered into at the Closing, in substantially
the form attached as Exhibit E hereto, between the Company and the Surviving Corporation which amends and restates in its entirety
the Shared Services Agreement.
“Antitrust Laws”
means the Sherman Act, as amended, the Clayton Act, as amended, the Federal Trade Commission Act, as amended, the HSR Act and all other
applicable Laws issued by a Governmental Authority that are designed or intended to prohibit, restrict or regulate actions having the
purpose or effect of monopolization or restraint of trade or lessening of competition through merger or acquisition.
“Base Exchange Ratio”
means the quotient of (a) the Aggregate Parent Common Stock Consideration, divided by (b) the aggregate number of shares of SpinCo Common
Stock outstanding as of immediately prior to the Effective Time.
“Balance Sheet Date”
means June 30, 2023.
“Benefit Plan”
means each employment, compensation, benefits, severance or termination, consulting, bonus, deferred compensation, equity, phantom-equity,
or equity-based award, retention, relocation, vacation, change in control, transaction bonus, salary continuation, hospitalization, medical,
dental, vision, life insurance, disability or sick leave benefit, profit-sharing, pension or retirement or other fringe benefit or compensatory
plan, program, agreement or arrangement, whether or not in writing and whether or not funded, including any “employee benefit plan”
(within the meaning of Section 3(3) of ERISA, whether or not subject to ERISA) but excluding any plan or program sponsored by a Governmental
Authority.
“BLA” means
a biologics license application for LYMPHIRTM (denileukin diftitox), submitted to the FDA pursuant to 42 U.S.C. §
262 and 21 C.F.R. Part 601, and all supplements, amendments, variations, extensions and renewals thereto (and including correspondence
and reports submitted to or received from FDA with respect to any of the foregoing (including minutes and official contact reports relating
to any communications with FDA)).
“Business Combination”
has the meaning set forth in Parent’s Governing Documents as in effect on the date hereof.
“Business Day”
means any day that is not a Saturday, a Sunday, or another day on which banking institutions in New York, New York or Governmental Authorities
in the Cayman Islands (for so long as Parent remains domiciled in the Cayman Islands) are authorized or obligated by Law to be closed.
“Cayman Registrar”
means the Registrar of Companies of the Cayman Islands.
“Code”
means the U.S. Internal Revenue Code of 1986, as amended.
“Company Benefit
Plan” means any Benefit Plan sponsored, maintained, or contributed to (or required to be contributed to) by the Company or any
of its Subsidiaries that (a) is or has been maintained, sponsored, contributed to or entered into by the Company or any of its Subsidiaries
for the benefit of any Person that provides services to SpinCo as an employee or independent contractor or for which SpinCo could have
any Liability, and (b) that is not a SpinCo Benefit Plan.
“Commercialization
Plan” means the PowerPoint document titled “Lymphir Commercial Gantt Chart for Launch/Lymphir Launch Timeline” delivered
by the Company to Parent on October 18, 2023.
“Company Disclosure
Schedule” means the Disclosure Schedule delivered by the Company to Parent on the date hereof and identified as such.
“Company FDA Letter”
means the letter from the Company to the FDA, duly executed by the Company, to be filed with the FDA, which letter notifies the FDA that
all ownership rights of the BLA and IND will transfer from the Company to SpinCo (or the Surviving Corporation, if applicable) effective
as of the date of such letter.
“Company Material
Adverse Effect” means any Effect that (a) has, or would reasonably be expected to have, individually or in the aggregate, a
material adverse effect on the business, results of operations, condition (financial or otherwise), or assets of the Company and its Subsidiaries,
taken as a whole, provided, that none of the following shall be deemed in themselves, either alone or in combination, to constitute,
and none of the following shall be taken into account in determining whether there has been or would reasonably be expected to be, individually
or in the aggregate, a Company Material Adverse Effect for purposes of clause (a): (i) any changes resulting from general market, economic,
financial, capital markets or regulatory conditions, (ii) any general changes in the credit, debt, financial or capital markets or changes
in interest or exchange rates, (iii) any changes in applicable Law or GAAP (or, in each case, authoritative interpretations thereof),
(iv) any changes resulting from any natural disaster, including any hurricane, storm, flood, tornado, volcanic eruption, earthquake, other
weather-related events, or other comparable events, or any worsening thereof, (v) any changes resulting from local, national or international
political conditions, including the outbreak or escalation of any military conflict, declared or undeclared war, armed hostilities, acts
of foreign or domestic terrorism or civil unrest, (vi) any changes generally affecting the industries in which the Company conducts its
businesses, (vii) any changes directly resulting from the execution of this Agreement or the Transaction Documents or the announcement
or the pendency of the Merger or the Transactions but not the consummation of the Transactions contemplated hereunder, including actions
of suppliers, landlords, distributors, partners or Governmental Authorities (provided, that this clause (vii) shall not apply to
any representation or warranty to the extent the purpose of such representation or warranty is to address, as applicable, the consequences
resulting from the execution of this Agreement or the announcement or the pendency of the Merger), (viii) any changes resulting from any
action required to be taken by the terms of this Agreement or at the explicit request or direction of the Company, (ix) the failure to
meet any internal or analysts’ expectations, projections or results of operations (but not, in each case, the underlying cause of
any such changes, unless such underlying cause would otherwise be excepted by another clause of this definition), or (x) any changes resulting
from any epidemics, pandemics or disease (including COVID-19 or any COVID-19 Measures or any change in COVID-19 Measures following the
date hereof) provided, that in the case of clauses (i), (ii), (iii), (iv), (v), (vi) and (x), if such Effect disproportionately
impacts the Company as compared to other participants in similar industries to the industries in which the Company operates, the incremental
disproportionate impact thereof shall be taken into account in determining whether a Company Material Adverse Effect has occurred or would
reasonably be expected to occur; or (b) has impaired or delayed, individually or in the aggregate, or would reasonably be expected to
impair or delay, individually or in the aggregate, the ability of the Company to timely perform its obligations hereunder or under the
other Transaction Documents or to consummate the Transactions on a timely basis, including the Merger, or prevent it from performing such
obligations or consummating such Transactions.
“Company Real Property”
means any real property owned, leased, subleased, licensed or otherwise occupied by SpinCo pursuant to the Shared Services Agreement.
“Company SEC Documents”
means all forms, reports, Schedules, statements and other documents required to be filed or furnished by the Company with the SEC since
August 23, 2021.
“Confidentiality
Agreement” means that certain Confidentiality Agreement, by and between Parent and the Company, dated as of April 25, 2023.
“Consent”
means any consent, clearance, expiration or termination of a waiting period, approval, exemption, waiver, authorization, filing, registration
or notification.
“Contract”
means any legally binding contract, agreement, understanding, arrangement, note, bond, indenture, lease, purchase order, sublicense or
license or other instrument.
“COVID-19”
means SARS-CoV-2 or COVID-19, and any evolutions or mutations thereof or related or associated epidemics, pandemics or disease outbreaks.
“COVID-19 Measures”
means any quarantine, “shelter in place,” “stay at home,” social distancing, shut down, closure, sequester, workplace
safety or similar Law, directive, guidelines or recommendations promulgated by any industry group or any Governmental Authority, including
the Centers for Disease Control and Prevention and the World Health Organization, in each case, in connection with or in response to COVID-19,
including the CARES Act and Families First Coronavirus Response Act.
“Effect”
means any change, circumstance, fact, event, development, condition, occurrence or effect.
“Entity”
means any Person that is a legal entity; provided, that when used in reference to Parent, “Entity” means Parent together
with its Subsidiaries, taken as a whole.
“Environmental Law”
means any Law relating to pollution or protection of the environment (including air quality, surface water, groundwater, soils, subsurface
strata, sediments, drinking water), natural resources, or human health and safety (to the extent related to exposure to Hazardous Materials
or hazardous conditions) or the use, registration, management, generation, storage, treatment, recycling, transportation, Release, threatened
Release, investigation or remediation of Hazardous Materials.
“ERISA”
means the U.S. Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated and rulings issued thereunder.
“Exchange Act”
means the U.S. Securities Exchange Act of 1934, as amended.
“Exchange Agent”
means American Stock Transfer & Trust Company.
“FDA” means
the U.S. Food and Drug Administration.
“FDCA”
means the U.S. Food, Drug and Cosmetic Act, 21 U.S.C. § 301 et seq., as amended.
“GAAP”
means generally accepted accounting principles in the United States.
“Governing Documents”
means the legal document(s) by which any Person (other than an individual) establishes its legal existence or which govern its internal
affairs. For example, the “Governing Documents” of a corporation are its certificate of incorporation and by-laws,
the “Governing Documents” of a limited partnership are its limited partnership agreement and certificate of limited
partnership, the “Governing Documents” of a limited liability company are its operating agreement and certificate of
formation and the “Governing Documents” of an exempted company are its memorandum and articles of association (in each
case, as amended, restated, amended and restated or otherwise modified from time to time).
“Governmental Authority”
means any federal, state, local, transnational, supranational or foreign government, any Person exercising executive, legislative, judicial,
regulatory or administrative function of or pertaining to government or Law, including any regulatory, self-regulatory or quasi-regulatory
authority, agency, commission, body, department or other instrumentality, and any court, arbitral body or tribunal of competent jurisdiction.
“Government Official”
means any officer or employee of a Governmental Authority or any department, agency, or instrumentality thereof, including any political
subdivision thereof or any corporation or other Person owned or controlled in whole or in part by any Governmental Authority or any sovereign
wealth fund, or of a public international organization, or any Person acting in an official capacity for or on behalf of any such government
or department, agency, or instrumentality, or for or on behalf of any such public international organization, or any political party,
party official, or candidate thereof.
“Hazardous Material”
means any toxic, reactive, corrosive, ignitable or flammable chemical or chemical compound, or hazardous or toxic substance, material
or waste, or any pollutant or contaminant, whether solid, liquid or gas, or any other substance, material or waste that is subject to
regulation, control or remediation or for which liability or standards of care are imposed under any Environmental Law, including petroleum
(including crude oil or any fraction thereof), radon, asbestos, radioactive materials, per- and polyfluoroalkyl substances and polychlorinated
biphenyls.
“Healthcare Laws”
means (a) the FDCA, and the regulations promulgated thereunder, (b) the Public Health Service Act (42 U.S.C. 201 et seq.), and the regulations
promulgated thereunder, (c) all federal and state fraud and abuse Laws, including the Federal Anti-Kickback Statute, the civil False Claims
Act, the Anti-Inducement Law, the exclusion Laws, and the regulations promulgated pursuant to such statutes, (d) the Health Insurance
Portability and Accountability Act of 1996, and the regulations promulgated thereunder, and comparable state Laws, (e) Titles XVIII and
XIX of the Social Security Act, and the regulations promulgated thereunder and (f) all other applicable healthcare Laws, rules and regulations,
ordinances, judgments, decrees, orders, writs and injunctions administered by Regulatory Authorities, each of clause (a) through (f),
as may be amended from time to time.
“HSR Act”
means the U.S. Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended, and the rules and regulations promulgated thereunder.
“IND” means
an investigational new drug application related to denileukin diftitox filed with the FDA for authorization to commence clinical studies,
and all supplements and amendments that may be filed with respect to the foregoing.
“Intellectual Property”
means all intellectual property rights and related priority rights protected, created or arising under the Laws of the United States or
any other jurisdiction or under any international convention, including all (a) patents and patent applications, industrial designs and
design patent rights, including any continuations, divisionals, continuations-in-part and provisional applications and statutory invention
registrations, and any patents issuing on any of the foregoing and any revisions, reissues, reexaminations, substitutes, supplementary
protection certificates, or extensions of any of the foregoing; (b) trademarks, service marks, trade names, service names, brand names,
trade dress rights, logos, Internet domain names, social media accounts, corporate names and other source or business identifiers, together
with the goodwill associated with any of the foregoing, and all applications, registrations, extensions and renewals of any of the foregoing;
(c) copyrights and works of authorship and computer software, database and design rights, and rights in data collections, mask work rights
and moral rights, whether or not registered or published, and all registrations, applications, renewals, extensions and reversions of
any of any of the foregoing; (d) trade secrets, know-how and confidential and proprietary information, whether or not patentable, including
invention disclosures, inventions, formulae, designs, discoveries, processes, research and development information, technical information,
methods, techniques, procedures, specifications, operating and maintenance manuals, methods, and engineering drawings (collectively, “Trade
Secrets”); and (e) any other intellectual or proprietary rights protectable, arising under or associated with any of the foregoing,
including those protected by any Law (including under international treaties or conventions) anywhere in the world.
“Interests”
means shares, partnership interests, limited liability company interests or any other equity interest in any Person that is an Entity.
“Intervening Event”
means an Effect first arising after the date of this Agreement that is materially adverse to the business, condition (financial or otherwise),
assets, liabilities or results of operations of the SpinCo Business or SpinCo, taken as a whole; provided, that none of the following
shall be deemed in themselves, either alone or in combination, to constitute, and none of the following shall be taken into account in
determining whether there has been or would reasonably be expected to be, individually or in the aggregate, an Intervening Event:
(a) any Contract, proposal, offer or indication of interest in any form, written or oral, relating any Business Combination with respect
to Parent, (b) clearance (or the absence of clearance) of the Transaction by any Governmental Authority, including Effects relating to
actions taken pursuant to or required to be taken pursuant to Section 7.5, (c) any change in the price or trading volume of
Parent Common Stock, (d) the Company’s, SpinCo’s or any of their respective Subsidiaries’ meeting, failing to meet or
exceeding projections (in and of itself, but not the underlying causes thereof), (e) any changes resulting from general market, economic,
financial, capital markets or regulatory conditions, (f) any general changes in the credit, debt, financial or capital markets or changes
in interest or exchange rates, (g) any changes in applicable Law or GAAP (or, in each case, authoritative interpretations thereof), (h)
any changes resulting from any natural disaster, including any hurricane, storm, flood, tornado, volcanic eruption, earthquake, other
weather-related events, or other comparable events, or any worsening thereof, (i) any changes resulting from local, national or international
political conditions, including the outbreak or escalation of any military conflict, declared or undeclared war, armed hostilities, acts
of foreign or domestic terrorism or civil unrest, (j) any changes generally affecting the industries in which SpinCo conducts its business,
(k) any changes directly resulting from the execution of this Agreement or the announcement or the pendency of the Merger (but not the
consummation of the Transactions contemplated hereunder), including actions of suppliers, landlords, distributors, partners or Governmental
Authorities (provided, that this clause (k) shall not apply to any representation or warranty to the extent the purpose of such representation
or warranty is to address, as applicable, the consequences resulting from the execution of this Agreement or the announcement or pendency
of the Merger), (l) any changes resulting from any action required to be taken by the terms of this Agreement or at the explicit request
or direction of Parent or Merger Sub, (m) the failure to meet any internal or analysts’ expectations, projections or results of
operations (but not, in each case, the underlying cause of any such changes, unless such underlying cause would otherwise be excepted
by another clause of this definition), or (n) any changes resulting from any epidemics, pandemics or disease (including COVID-19 or any
COVID-19 Measures or any change in COVID-19 Measures following the date hereof); provided, that in the case of clause (e), (f),
(g), (i), (j) and (n), if such Effect disproportionately impacts SpinCo or the SpinCo Business as compared to other participants in the
industry in which SpinCo or the SpinCo Business operates, the incremental disproportionate impact thereof shall be taken into account
in determining whether an Intervening Event has occurred or would reasonably be expected to occur; provided, further, that
in the event the BLA for LYMPHIRTM (denileukin diftitox) is not approved by the FDA such event and the Effects of such event
shall be automatically deemed an Intervening Event.
“Investment Company Act” means
the U.S. Investment Company Act of 1940, as amended.
“IRS” means the U.S. Internal
Revenue Service.
“Knowledge” or any other similar
knowledge qualification, means (a) with respect to the SpinCo Group, the actual knowledge of the persons set forth in Section 1.1(a)
of the SpinCo Disclosure Schedule, after reasonable due inquiry and (b) with respect to Parent, the actual knowledge of the persons
set forth in Section 1.1(a) of the Parent Disclosure Schedule, after reasonable due inquiry.
“Law” means any federal, national,
foreign, supranational, state, provincial or local law, statute, code, ordinance, order, decree, award, directive, judgment, ruling, rule,
regulation or similar requirement or rule of law (including common law) issued, promulgated, enforced or enacted by or under the authority
of a Governmental Authority.
“Liability” means any liability
or obligation (whether known or unknown, fixed or variable, determined or determinable, whether asserted or unasserted, whether absolute
or contingent, whether accrued or unaccrued, whether liquidated or unliquidated, whether direct or indirect, and whether due or to become
due).
“Lien” means with respect to
any property or asset, any mortgage, deed of trust, deed to secure debt, pledge, charge, hypothecation, encumbrance, recorded or unrecorded
easement, encroachment, license, option, right of pre-emption or first refusal, security interest or other lien or restriction of any
kind or nature whatsoever, and any agreement to create any of the foregoing.
“NASDAQ” means the U.S. Nasdaq
Capital Market.
“Notice of Approval” means a
notice issued by the FDA to the Company approving the BLA and authorizing the commercialization and sale of LYMPHIRTM (denileukin
diftitox).
“Order” means any judgment,
order, injunction, decree, writ, permit or license of any Governmental Authority or any arbitrator.
“Parent Business Combination Proposal”
means any inquiry, offer, proposal or indication of interest (whether written or oral, binding or non-binding, and other than an inquiry,
offer, proposal or indication of interest made or submitted by Parent to the Company or by the Company to Parent) contemplating or otherwise
relating to any potential Business Combination (other than the Merger or the other Transactions).
“Parent Disclosure Schedule”
means the Disclosure Schedule delivered by Parent to the Company and SpinCo on the date hereof and identified as such.
“Parent Material
Adverse Effect” means any Effect that (a) has, or would reasonably be expected to have, individually or in the aggregate with
any other Effects, a material adverse effect on the business, properties, assets, liabilities, or financial condition of the Parent or
Merger Sub, taken as a whole; provided, that none of the following shall be deemed in themselves, either alone or in combination,
to constitute, and none of the following shall be taken into account in determining whether there has been or would reasonably be expected
to be, individually or in the aggregate, a Parent Material Adverse Effect for purposes of this clause (a): (i) any changes resulting from
general market, economic, financial, capital markets or regulatory conditions, (ii) any general changes in the credit, debt, financial
or capital markets or changes in interest or exchange rates, (iii) any changes in applicable Law or GAAP (or, in each case, authoritative
interpretations thereof), (iv) any changes resulting from any natural disaster, including any hurricane, storm, flood, tornado, volcanic
eruption, earthquake, other weather-related events, or other comparable events, or any worsening thereof, (v) any changes resulting from
local, national or international political conditions, including the outbreak or escalation of any military conflict, declared or undeclared
war, armed hostilities, acts of foreign or domestic terrorism or civil unrest, (vi) any changes generally affecting the industries in
which the Parent conducts its businesses, (vii) any changes directly resulting from the execution of this Agreement or the Transaction
Documents or the announcement or the pendency of the Merger or the Transactions (but not the consummation of the Transactions contemplated
hereunder), including actions of suppliers, landlords, distributors, partners or Governmental Authorities (provided, that this
clause (vii) shall not apply to any representation or warranty to the extent the purpose of such representation or warranty is to address,
as applicable, the consequences resulting from the execution of this Agreement or the announcement or the pendency of the Merger), (viii)
any changes resulting from any action required to be taken by the terms of this Agreement or at the explicit request or direction of the
Company, (ix) the failure to meet any internal or analysts’ expectations, projections or results of operations (but not, in each
case, the underlying cause of any such changes, unless such underlying cause would otherwise be excepted by another clause of this definition),
(x) any changes resulting from any epidemics, pandemics or disease (including COVID-19 or any COVID-19 Measures or any change in COVID-19
Measures following the date hereof) or (xi) elections to redeem shares of Parent Common Stock in connection with the Parent Shareholders
Meeting as required by Parent’s Governing Documents; provided, that in the case of clauses (i), (ii), (iii), (iv), (v), (vi)
and (x), if such Effect disproportionately impacts the Parent or Merger Sub, taken as a whole, as compared to other participants in similar
industries to the industries in which Parent operates, the incremental disproportionate impact thereof shall be taken into account in
determining whether a Parent Material Adverse Effect has occurred or would reasonably be expected to occur; or (b) has impaired or delayed,
individually or in the aggregate, or would reasonably be expected to impair or delay, individually or in the aggregate, the ability of
Parent to timely perform its obligations hereunder or under the other Transaction Documents, or to consummate the Transactions on a timely
basis, including the Merger, or prevent it from performing such obligations or consummating the Transactions.
“Parent Registration Statement”
means the registration statement on Form S-4 to be filed by Parent with the SEC (as amended and supplemented from time to time) to effect
the registration under the Securities Act of the issuance of (a) the shares of Domesticated Parent Common Stock that will be issued to
the Company pursuant to the Merger, and (b) the shares of Domesticated Parent Common Stock, the Domesticated Parent Rights and the Domesticated
Parent Units that will be issued to holders of Parent Common Stock, Parent Rights and Parent Units in the Domestication and the shares
of Domesticated Parent Common Stock underlying such Domesticated Parent Rights and Domesticated Parent Units.
“Parent Share Issuance” means
the issuance of shares of Domesticated Parent Common Stock pursuant to the Domestication and the Merger.
“Parent Share Redemption” means
the election of an eligible holder of Parent Common Stock (as determined in accordance with Parent’s Governing Documents) to redeem
all or a portion of the shares of Parent Common Stock held by such holder at a per-share price, payable in cash, equal to the pro rata
share of the aggregate amount on deposit in the Trust Account (including any interest earned on the funds held in the Trust Account) represented
by such redeemed shares (as determined in accordance with Parent’s Governing Documents and the Trust Agreement, as may be amended
to effect a Parent Share Redemption).
“Parent Shareholder
Approval” means the approval of (a) those Transaction Proposals identified in clauses (A), (B) and (C) of Section 7.4(e)(ii),
in each case, by a special resolution under the CICA (being the affirmative vote of the holders of at least two-thirds (2/3) of the ordinary
shares who, being present and entitled to vote at the Parent Shareholders Meeting, vote at the Parent Shareholders Meeting), (b) those
Transaction Proposals identified in clauses (D), (E), (F) and (I) of Section 7.4(e)(ii), in each case, by an ordinary resolution
under the CICA (being the affirmative vote of the holders of a majority of the ordinary shares who, being present and entitled to vote
at the Parent Shareholders Meeting, vote at the Parent Shareholders Meeting) and (c) those Transaction Proposals identified in clauses
(G) and (H) of Section 7.4(e)(ii), in each case, by an affirmative vote of the number of holders of Parent Common Stock required
to approve such Transaction Proposals under applicable Law and the Governing Documents of Parent.
“Parent Shareholders”
means holders of Parent’s capital stock.
“Parent Subsidiaries”
means all direct and indirect Subsidiaries of Parent. For the avoidance of doubt, following the Effective Time, the Parent Subsidiaries
shall include SpinCo.
“Parent Transaction
Expenses” means any and all fees and expenses of Parent incurred in connection with the transactions contemplated by this Agreement
and the Transaction Documents, including (a) any fees or expenses incurred in connection with the preparation and filing of Parent SEC
Filings, any Extension Amendment and any other actions contemplated by Section 7.16, and any fees and expenses incurred in connection
with the Parent Shareholders Meeting, (b) all brokers’ or finders’ fees and fees and expenses of counsel, investment bankers,
accountants and other advisers, and directors’ and officers’ tail insurance, (c) any change-in-control, transaction bonus,
retention, severance or other similar payments to any current or former employee, consultant, independent contractor, officer, or director
of Parent arising from or incurred in connection with the transactions contemplated by this Agreement and the Transaction Documents, and
(d) the employer portion of any Taxes arising from or incurred in connection with the payments described in clause (c) or otherwise payable
with respect to or in connection with the transactions contemplated by this Agreement and the Transaction Documents, in each case of clauses
(a) through (d) including any such fees or expenses incurred or invoiced following the Closing Date. “Parent Transaction Expenses”
excludes the value of common stock issuable to Newbridge Securities in connection with the transactions contemplated by this Agreement.
“Permits”
means licenses, franchises, permits, certificates, approvals and authorizations from Governmental Authorities.
“Permitted Acquisition
Proposal” means an Acquisition Proposal that relates to any transaction involving the Company so long as such transaction would
not prevent or materially impair or materially delay the Company’s ability to comply with its obligations under this Agreement or
to consummate the transactions contemplated by this Agreement with Parent.
“Permitted Liens”
means (a) requirements and restrictions of zoning, licensing, permitting, building and other similar land-use Laws which are not violated
by the present use or occupancy of the real property subject thereto; (b) Liens for Taxes or mechanics’, materialmen’s and
similar Liens arising or incurred in the ordinary course of business consistent with past practice and with respect to any amounts, in
each case (i) not yet due and payable or (ii) that are being contested in good faith by appropriate proceedings and for which adequate
reserves have been established in accordance with GAAP; (c) all encroachments, overlaps, overhangs, variations in area or measurement,
rights of parties in possession, servitudes or easements (including conservation easements and public trust easements, rights-of-way,
road use Contracts, covenants, conditions, restrictions, reservations, licenses, Contracts and other similar non-monetary matters) of
public record or any other similar matters not of record which would be disclosed by an accurate survey or physical inspection of the
applicable real property; provided, that such Liens, individually or in the aggregate, do not or would not reasonably be expected
to materially impair or interfere with the operation or use of such real property in the ordinary course operation of the respective businesses
of SpinCo or Parent and its Subsidiaries, as applicable, in each case, as currently conducted thereon; (d) with respect to any real property,
(i) the interests and rights of the respective lessors with respect thereto, including any statutory landlord liens and any Lien on the
lessor’s interest therein and (ii) any Liens encumbering the underlying fee title of the real property; provided, that such
Liens, individually or in the aggregate, do not or would not reasonably be expected to materially impair or interfere with the operation
or use of such real property in the ordinary course operation of the respective businesses of SpinCo or Parent and its Subsidiaries, as
applicable, in each case, as currently conducted thereon; (e) any real property Liens that do not, individually or in the aggregate, result
in a Parent Material Adverse Effect or SpinCo Material Adverse Effect, as applicable; (f) reversionary rights in favor of landlords under
any real property leases with respect to any of the buildings or other improvements owned by SpinCo, Parent or any of its Subsidiaries,
as applicable; provided, that such Liens, individually or in the aggregate, do not or would not reasonably be expected to materially
impair or interfere with the operation or use of such real property in the ordinary course operation of the respective businesses of SpinCo
or Parent and its Subsidiaries, as applicable, in each case, as currently conducted thereon; (g) purchase money Liens and Liens securing
rental payments under capital lease agreements; (h) pledges or deposits made in the ordinary course of business consistent with past practice
in connection with workers’ compensation, unemployment insurance and other types of social security (other than pursuant to Section
303(k) or 4068 of ERISA or Section 430(k) of the Code) or to secure the performance of tenders, statutory obligations, surety and appeal
bonds, bids, performance and return of money bonds and similar obligations; (i) liens arising under conditional sales Contracts and equipment
leases with third parties entered into in the ordinary course of business consistent with past practice to the extent not subject to any
default; (j) pledges or deposits to secure public or statutory obligations unrelated to any default or violation of any Law; (k) Liens
arising under or created by this Agreement or any Transaction Document (other than as a result of a breach or default under such Contracts);
(l) Liens described on Section 1.1(b) of the SpinCo Disclosure Schedule or Section 1.1(b) of the Parent Disclosure Schedule,
as applicable; and (m) Liens that do not, individually or in the aggregate, materially affect or disrupt the ordinary course operation
of the respective businesses of SpinCo or Parent and its Subsidiaries, as applicable, in each case, taken as a whole.
“Person”
means any individual, state, trust or trustee on behalf of a trust, firm, corporation, partnership, limited liability company, incorporated
or unincorporated association, joint venture, joint stock company, Governmental Authority or other organization or Entity of any kind.
“Personal Information”
means all information in any form or media that identifies or could be used to identify an individual person (including any current, prospective,
or former customer, end user or employee), including any information defined as “personal information” or any similar term
provided by applicable Law or by the Company or SpinCo in any of their public-facing privacy policies or notices, or in any contracts
to which SpinCo is bound as of the date hereof (e.g., “personal data,” “personally identifiable information” or
“PII”).
“Privacy Laws”
means applicable Laws, and self-regulatory guidelines (including of any applicable foreign jurisdiction) relating to the receipt, collection,
compilation, use, storage, processing, sharing, safeguarding, security (technical, physical or administrative), disposal, destruction,
disclosure or transfer (including cross-border) of any Personal Information, including where and to the extent applicable the Federal
Trade Commission Act, California Consumer Privacy Act (CCPA), Payment Card Industry Data Security Standard (PCI-DSS), EU General Data
Protection Regulation (GDPR), any and all applicable Laws relating to breach notification, the use of biometric identifiers, and the use
of Personal Information for marketing purposes.
“Privacy Requirements”
means (i) all applicable Privacy Laws and (ii) all of the Company’s and SpinCo’s public-facing policies and notices, and contractual
obligations to which SpinCo is bound as of the date hereof, relating to the receipt, collection, compilation, use, storage, processing,
sharing, safeguarding, security (technical, physical and administrative), disposal, destruction, disclosure, or transfer (including cross-border)
of Personal Information.
“Proxy Statement”
means the proxy statement to be mailed to the shareholders of Parent relating to the Parent Shareholders Meeting, including any amendments
or supplements thereto.
“Regulatory Authority”
means the FDA or any other comparable Governmental Authority.
“Regulatory Authorizations”
means any approvals, clearances, authorizations, registrations, certifications, licenses, exemptions and permits granted by any Regulatory
Authority, including FDA approval of the BLAs.
“Release”
means any spilling, leaking, pumping, pouring, emitting, emptying, discharging, injecting, depositing, escaping, leaching or migration,
disposing or dumping into or through the environment.
“Representative”
means, with respect to any Person, such Person’s directors, managers, members, officers, employees, agents, partners, attorneys,
financial advisors, financing sources, consultants, advisors or other Persons acting on behalf of such Person.
“Rights Agreement”
means the Rights Agreement dated as of October 13, 2022, between Parent and American Stock Transfer & Trust Company, LLC.
“SEC” means
the U.S. Securities and Exchange Commission.
“Securities Act”
means the Securities Act of 1933, as amended.
“Shared Services
Agreement” means the Shared Services Agreement, dated as of June 22, 2022, and effective as of April 1, 2022, by and between
Citius Acquisition Corp. and the Company.
“Specified Period”
means period of time from the date of this Agreement until the later of (i) the date the Parent Registration Statement first becomes publicly
filed on SEC’s EDGAR database, or (ii) February 29, 2024.
“SpinCo Affiliate
Contract” means any Contract (a) between SpinCo, on the one hand, and any present or former officer or director of SpinCo or
“immediate family member” thereof (as defined in Rule 16a-1 under the Exchange Act), on the other hand, or (b) between SpinCo,
on the one hand, and the Company and/or any of its Subsidiaries (other than SpinCo), on the other hand.
“SpinCo Assets”
means all rights, title and ownership interests in and to all properties, claims, Contracts, businesses, or assets (including goodwill),
which constitute all of the interests in real and tangible personal property owned, leased or licensed by SpinCo and are required for
the continued operation of the SpinCo Business as currently conducted, wherever located, of every kind, character and description, whether
real, personal, or mixed, tangible or intangible, whether accrued, contingent or otherwise, in each case, whether or not recorded or reflected
on the books and records or financial statements of any Person, which includes the SpinCo Owned Intellectual Property.
“SpinCo Benefit Plans”
means any Benefit Plan sponsored, maintained or contributed to exclusively by SpinCo.
“SpinCo Business
means the business of developing and commercializing of the SpinCo Products, which shall include the businesses and operations of SpinCo
conducted prior to the Closing and conducted through the use of the SpinCo Assets.
“SpinCo Common Stock”
means the common stock, par value $0.001 per share, of SpinCo.
“SpinCo Equity Incentive
Plan” means the Citius Oncology, Inc. 2023 Omnibus Stock Incentive Plan.
“SpinCo Disclosure
Schedule” means the Disclosure Schedule delivered by the Company and SpinCo to Parent on the date hereof and identified as such.
“SpinCo FDA Letter”
means the letter from SpinCo (or, if applicable, the Surviving Corporation) to the FDA, duly executed by SpinCo (or, if applicable, the
Surviving Corporation), to be filed with the FDA, which letter notifies the FDA that all ownership rights of the BLA and IND will transfer
from the Company to SpinCo (or the Surviving Corporation, if applicable), effective as of the date of such letter.
“SpinCo Financial
Statements” means, collectively, the audited balance sheet of SpinCo as of September 30, 2022 and the related audited statements
of income and cash flows of SpinCo for such fiscal year and the unaudited balance sheet of SpinCo as of the quarter ended June 30, 2023
and the related unaudited statements of income and cash flows of SpinCo for such quarter, each as attached to Section 5.8(a) of the
SpinCo Disclosure Schedule.
“SpinCo Group”
means collectively, SpinCo and the Company.
“SpinCo Intellectual
Property” means, collectively, SpinCo Owned Intellectual Property and SpinCo Licensed Intellectual Property.
“SpinCo Licensed
Intellectual Property” means all Intellectual Property that (x) is licensed from a third party pursuant to a written Contract
to (i) SpinCo or (ii) as of the date hereof, the Company or any of its Subsidiaries (in each case solely with respect to the SpinCo Business),
and (y) is used, practiced or held for use or practice by or on behalf of, as of the date hereof, the Company or any of its Subsidiaries
(in each case solely with respect to the SpinCo Business), including any Intellectual Property that might be included in the BLA.
“SpinCo Material
Adverse Effect” means any Effect that (a) has, or would reasonably be expected to have, individually or in the aggregate with
any other Effects, a material adverse effect on the business, financial condition, properties, assets, liabilities, or results of operations
of SpinCo or the SpinCo Business, taken as a whole; provided, that none of the following shall be deemed in themselves, either
alone or in combination, to constitute, and none of the following shall be taken into account in determining whether there has been or
would reasonably be expected to be, individually or in the aggregate, a SpinCo Material Adverse Effect for purposes of this clause (a):
(i) any changes resulting from general market, economic, financial, capital markets or regulatory conditions, (ii) any general changes
in the credit, debt, financial or capital markets or changes in interest or exchange rates, (iii) any changes in applicable Law or GAAP
(or, in each case, authoritative interpretations thereof), (iv) any changes resulting from any natural disaster, including any hurricane,
storm, flood, tornado, volcanic eruption, earthquake, other weather-related events, or other comparable events, or any worsening thereof,
(v) any changes resulting from local, national or international political conditions, including the outbreak or escalation of any military
conflict, declared or undeclared war, armed hostilities, acts of foreign or domestic terrorism or civil unrest, (vi) any changes generally
affecting the industries in which SpinCo conducts its business, (vii) any changes directly resulting from the execution of this Agreement
or the announcement or the pendency of the Merger (but not the consummation of the Transactions contemplated hereunder), including actions
of suppliers, landlords, distributors, partners or Governmental Authorities (provided, that this clause (vii) shall not apply to any representation
or warranty to the extent the purpose of such representation or warranty is to address, as applicable, the consequences resulting from
the execution of this Agreement or the announcement or pendency of the Merger), (viii) any changes resulting from any action required
to be taken by the terms of this Agreement or at the explicit request or direction of Parent or Merger Sub, (ix) the failure to meet any
internal or analysts’ expectations, projections or results of operations (but not, in each case, the underlying cause of any such
changes, unless such underlying cause would otherwise be excepted by another clause of this definition), or (x) any changes resulting
from any epidemics, pandemics or disease (including COVID-19 or any COVID-19 Measures or any change in COVID-19 Measures following the
date hereof); provided, that in the case of clauses (i), (ii), (iii), (iv), (v), (vi) and (x), if such Effect disproportionately
impacts SpinCo or the SpinCo Business as compared to other participants in the industry in which SpinCo or the SpinCo Business operates,
the incremental disproportionate impact thereof shall be taken into account in determining whether a SpinCo Material Adverse Effect has
occurred or would reasonably be expected to occur; or (b) has impaired, individually or in the aggregate, or would reasonably be expected
to impair or delay, individually or in the aggregate, the ability of SpinCo to timely perform its obligations hereunder or under the other
Transaction Documents, or to consummate the Transactions on a timely basis, including the Merger, or prevent it from performing such obligations
or consummating the Transactions.
“SpinCo Option”
means each option to purchase any SpinCo Common Stock that is unexpired, unexercised and outstanding as of immediately prior to the Effective
Time.
“SpinCo Owned Intellectual
Property” means all Intellectual Property owned or purported to be owned by SpinCo.
“SpinCo Products”
means I/ONTAK, or LYMPHIRTM (denileukin diftitox), a late-stage oncology immunotherapy for the treatment of cutaneous T-cell
lymphoma, a rare form of non-Hodgkin lymphoma.
“Sponsor”
means 10XYZ Holdings LP, a Delaware limited partnership.
“Subsidiary”
means, with respect to any Person, a corporation or other entity of which more than 50% of the voting power of the equity securities or
Interests that by their terms have ordinary voting power to elect a majority of the board of directors or other similar body is owned
or controlled, directly or indirectly, by such Person, or any organization of which such Person or any of its Subsidiaries is, directly
or indirectly, a general partner or managing member or holds a similar role.
“Tax” or
“Taxes” means any and all federal, state, provincial, territorial, local, foreign and other net income tax, alternative
or add-on minimum tax, franchise tax, gross income, adjusted gross income or gross receipts tax, employment related tax (including employee
withholding or employer payroll tax) ad valorem, transfer, franchise, license, excise, severance, stamp, occupation, premium, personal
property, real property, escheat or unclaimed property, capital stock, profits, disability, registration, value added, estimated, customs
duties, and sales or use tax, or other tax or like assessment or charge of any kind whatsoever imposed by a Governmental Authority in
the nature of a tax, together with any interest, penalty, addition to tax or additional amount imposed with respect thereto by a Governmental
Authority, whether disputed or not, and including any secondary Liability for any of the aforementioned.
“Tax Return”
means any return, report, statement, refund, claim, declaration, information return, statement, estimate or other document filed or required
to be filed with a Governmental Authority in respect of Taxes, including any schedule or attachment thereto and including any amendments
thereof.
“Transaction Documents”
means the Sponsor Support Agreement, the A&R Registration Rights Agreement, the Amended and Restated Shared Services Agreement, and
any certificate or other instrument delivered by any Party to any other Party pursuant to this Agreement or any of the foregoing.
“Transaction Process”
means all matters relating to the review of strategic alternatives with respect to SpinCo, including matters relating to (a) the solicitation
of proposals from and negotiations with third parties in connection with the disposition or sale of SpinCo or (b) the drafting, negotiation
or interpretation of any of the provisions of this Agreement or the other Transaction Documents.
“Transactions”
shall mean the Domestication, the Merger and the other transactions contemplated by this Agreement and the Transaction Documents.
“Treasury Regulations”
means the final or temporary regulations promulgated by the U.S. Department of the Treasury under the Code.
“Trust Account”
means the segregated trust account established and governed by the Trust Agreement, as such agreement may be amended from time to time
in accordance with the terms of this Agreement.
“U.S.”
or “United States” means and refers to the United States of America.
“Willful Breach”
means, with respect to any obligation, covenant or agreement of a Party in this Agreement, any material breach of or material failure
to perform such obligation, covenant or agreement that such Party intentionally takes (or intentionally fails to take or perform) with
actual knowledge that such action or omission or failure to perform would, or would reasonably be expected to, cause or result in a material
breach of this Agreement.
Cross References
Each of the following terms is defined in the Section set forth opposite
such term:
| Term |
|
Section |
| A&R Registration Rights Agreement |
|
Recitals |
| Acquisition Proposal |
|
Section 7.1(a)(i) |
| Certificate of Merger |
|
Section 2.3(e) |
| Chosen Courts |
|
Section 10.3(b) |
| CICA |
|
Recitals |
| Closing |
|
Section 2.2 |
| Closing Date |
|
Section 2.2 |
| Company |
|
Preamble |
| Company Audit Committee |
|
Section 4.7 |
| Company Board |
|
Recitals |
| Company Merger Tax Opinion |
|
Section 7.3(c) |
| Company Related Parties |
|
Section 9.3(c) |
|
Closing Statement
|
|
Section 2.7(a) |
| Closing Statement Dispute |
|
Section 2.7(b) |
| DGCL |
|
Recitals |
| Disclosure Schedules |
|
Section 10.13 |
| Domesticated Parent Common Stock |
|
Recitals |
|
Domesticated Parent Option
|
|
Section 3.1(b) |
| Domesticated Parent Right |
|
Recitals |
| Domesticated Parent Unit |
|
Recitals |
| Domestication |
|
Recitals |
| DPA |
|
Section 6.5 |
| Effective Time |
|
Section 2.1 |
| Exchange Fund |
|
Section 3.2(a) |
| Extension Amendment |
|
Section 7.16(a)(iii) |
| Extension Date |
|
Section 7.16(a)(iii) |
| Extension Fee |
|
Section 7.16(b) |
| Fourth Extension |
|
Section 7.16(a)(iii) |
| Interim Period |
|
Section 7.1 |
| Internal Controls |
|
Section 4.7 |
|
Intervening Event Notice
|
|
Section 7.4(e)(iii)
|
| Intervening Event Notice Period |
|
Section 7.4(e)(iii) |
| Investment |
|
Section 6.3(e) |
| JOBS Act |
|
Section 6.6(a) |
| Merger |
|
Recitals |
| Merger Consideration |
|
Section 3.1(a)(i) |
| Merger Sub |
|
Preamble |
| Merger Sub Common Stock |
|
Section 3.1(a)(iv) |
| Merger Sub Shareholder Approval |
|
Section 7.15 |
| Outside Date |
|
Section 9.1(b) |
| Outstanding SpinCo Shares |
|
Section 3.1(a)(i) |
| Parent |
|
Preamble |
| Term |
|
Section |
| Parent Board |
|
Recitals |
| Parent Board Recommendation |
|
Recitals |
| Parent Bylaws |
|
Section 7.11 |
| Parent Charter |
|
Section 7.11 |
| Parent Common Stock |
|
Recitals |
| Parent Domestication Tax Opinion |
|
Section 7.3(d) |
|
Parent Equity Incentive Plan
|
|
Section 7.4(e)(ii)
|
| Parent Estimated Transaction Expenses |
|
Section 2.3(c) |
| Parent Financial Statements |
|
Section 6.6(c) |
| Parent Merger Tax Opinion |
|
Section 7.3(c) |
| Parent Rights |
|
Recitals |
| Parent SEC Filings |
|
Section 6.13 |
| Parent Securities |
|
Section 6.3(a) |
| Parent Shareholders Meeting |
|
Section 7.4(e)(i) |
| Parent Units |
|
Recitals |
| Party |
|
Preamble |
| Personnel IP Contract |
|
Section 5.18(g) |
| PDUFA Date |
|
Section 7.16(b)(i)(C) |
| Qualified Group |
|
Section 6.9(c) |
| Remedies Exception |
|
Section 4.2 |
| Second Extension |
|
Section 7.16(a)(i) |
| Security Incident |
|
Section 5.25(c) |
| SpinCo |
|
Preamble |
| SpinCo Board |
|
Section 5.23 |
| SpinCo Material Contracts |
|
Section 5.14(a) |
| SpinCo Proposal |
|
Section 7.7(c) |
| SpinCo Registered Intellectual Property |
|
Section 5.18(a) |
| SpinCo Shareholder Approval |
|
Section 7.15 |
| Sponsor Support Agreement |
|
Recitals |
|
Subsequent Period SpinCo Financial Statements
|
|
Section 7.16(a) |
| Subsequent Unaudited SpinCo Financial Statements |
|
Section 7.16(a) |
| Surviving Corporation |
|
Section 2.1 |
| Tangible Personal Property |
|
Section 5.7 (a) |
| Tax-Free Status |
|
Section 7.3(a) |
| Termination Fee |
|
Section 9.3(a) |
| Third Extension |
|
Section 7.16(a)(ii) |
| Transaction Proposals |
|
Section 7.4(e)(ii) |
| Trust Agreement |
|
Section 6.14 |
| Trustee |
|
Section 6.14 |
88
Exhibit
10.1
EXECUTION
VERSION
sponsor support
AGREEMENT
THIS SPONSOR SUPPORT AGREEMENT (this “Agreement”)
is made and entered into as of October 23, 2023, by and among 10XYZ Holdings LP, a Delaware limited partnership (“Sponsor”),
TenX Keane Acquisition, a Cayman Islands exempted company (which will migrate to and domesticate as a Delaware corporation prior to the
Closing) (“Parent”), Citius Pharmaceuticals, Inc., a Nevada corporation (the “Company”), and Citius
Oncology, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“SpinCo”, and following the
Closing, the “Surviving Corporation”). Sponsor, Parent, the Company and SpinCo are sometimes referred to herein collectively
as the “Parties” and individually as a “Party.” Capitalized terms used and not defined herein shall
have the respective meanings ascribed to such terms in the Merger Agreement (as defined below).
RECITALS:
A. As
of the date hereof, Sponsor is the holder of record and the “beneficial owner” (within the meaning of Rule 13d-3 under the
Exchange Act) of 2,044,000 shares of Parent Common Stock and 394,000 Parent Rights.
B. Contemporaneously
with the execution and delivery of this Agreement, Parent, TenX Merger Sub Inc., a Delaware corporation and wholly owned subsidiary of
Parent (“Merger Sub”), the Company, and SpinCo have entered into an Agreement and Plan of Merger and Reorganization
(as amended or modified from time to time, the “Merger Agreement”), dated as of the date hereof.
C. Upon
the terms and subject to the conditions set forth therein and in accordance with the applicable provisions of the DGCL, following the
Domestication, Merger Sub will merge with and into SpinCo (the “Merger”), and SpinCo will continue as the surviving
company in the Merger.
D. As
an inducement to Parent, the Company and SpinCo to enter into the Merger Agreement and to consummate the transactions contemplated therein,
the Parties desire to agree to certain matters as set forth herein.
NOW, THEREFORE, in consideration of the foregoing
and the mutual agreements contained herein, and intending to be legally bound hereby, the Parties hereby agree as follows:
1. Binding
Effect of Merger Agreement. Sponsor hereby acknowledges that it has read the Merger Agreement and this Agreement and has had the opportunity
to consult with its tax and legal advisors. Sponsor agrees not to, directly or indirectly, take any action, or authorize or knowingly
permit any of its Affiliates or representatives to take any action on its behalf, that would be a breach of Section 7.7(e) (Permitted
Activities and Exclusivity) or Section 7.8 (Public Announcements) of the Merger Agreement if such action were taken by Parent.
2. No
Transfer. During the period commencing on the date hereof and ending on the earlier of (a) the Effective Time and (b) such date and
time as the Merger Agreement shall be terminated in accordance with Article IX thereof (the earlier of clauses (a) and (b), the “Expiration
Time”), Sponsor shall not (i) sell, offer to sell, contract or agree to sell, hypothecate, pledge, grant any option to purchase,
make any short sale or otherwise dispose of or agree to dispose of, directly or indirectly, file (or participate in the filing of) a registration
statement with the SEC (other than the Parent Registration Statement) or establish or increase a put equivalent position or liquidate
or decrease a call equivalent position within the meaning of Section 16 of the Exchange Act, with respect to any Parent Common Stock,
Parent Rights or any other shares of capital stock or warrants of Parent that Sponsor is the holder of record and the “beneficial
owner” (within the meaning of Rule 13d-3 under the Exchange Act) to which Sponsor has voting rights (collectively, “Subject
Securities”), (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic
consequences of ownership of any Subject Securities, or (iii) publicly announce any intention to effect any transaction specified in clause
(i) or (ii).
3. New
Shares. In the event that, including in respect of the Domestication, (a) any Parent Common Stock, Parent Unit, Parent Right, Domesticated
Parent Common Stock, Domesticated Parent Right or other equity securities of Parent are issued to Sponsor after the date of this Agreement
pursuant to (i) any stock dividend, stock split, recapitalization, reclassification, combination or exchange of Parent Common Stock or
Parent Rights of, on, or affecting the Parent Common Stock or Parent Rights owned by Sponsor or otherwise, or (ii) by conversion pursuant
to the terms of the promissory notes issued, or to be issued, to the Sponsor by Parent evidencing the Sponsor’s deposits of up to
and aggregate of $2,115,000 into the Trust Account to extend the timeline to complete a business combination (each an “Extension
Promissory Note” and, collectively, the “Extension Promissory Notes”); provided, that such conversion
of the Extension Promissory Notes shall occur only to the extent any amounts remain outstanding under the Extension Promissory Notes after
taking into account any reimbursements paid to the Sponsor in respect of outstanding amounts under the Extension Promissory Notes pursuant
to Section 7 below, (b) Sponsor purchases or otherwise acquires beneficial ownership of any Parent Common Stock, Parent Rights
or other equity securities of Parent after the date of this Agreement, or (c) Sponsor acquires the right to vote or share in the voting
of any Parent Common Stock or other equity securities of Parent after the date of this Agreement (such Parent Common Stock, Parent Units,
Parent Rights or other equity securities of Parent, collectively the “New Securities”), then such New Securities acquired
or purchased by Sponsor shall be subject to the terms of this Agreement to the same extent as if they constituted the Parent Common Stock
or Parent Rights owned by Sponsor as of the date hereof.
4. Support
Agreements.
(a) At any
meeting of the shareholders of Parent, however called, or at any adjournment or postponement thereof, or in any other circumstance in
which the vote, consent or other approval of the shareholders of Parent is sought, Sponsor shall (i) appear at each such meeting or otherwise
cause all of its Parent Common Stock to be counted as present thereat for purposes of calculating a quorum and (ii) vote (or cause to
be voted), or execute and deliver a written consent (or cause a written consent to be executed and delivered) covering, all of its Subject
Securities:
(i)
in favor of each of the Transaction Proposals and in favor of any proposal in respect of an Extension Amendment;
(ii) against
(or otherwise withhold written consent of, as applicable) any Business Combination or any proposal relating to a Business Combination
(in each case, other than as contemplated by the Merger Agreement);
(iii) against
(or otherwise withhold written consent of, as applicable) any merger agreement or merger, consolidation, combination, sale of substantial
assets, reorganization, recapitalization, dissolution, liquidation or winding up of or by Parent (other than the Merger Agreement and
the transactions contemplated thereby);
(iv) against
(or otherwise withhold written consent of, as applicable) any change in the business, management or board of directors of Parent (other
than in connection with the Merger Agreement and the transactions contemplated thereby); and
(v) against
(or otherwise withhold written consent of, as applicable) any proposal, action or agreement that would (A) impede, frustrate, prevent
or nullify any provision of this Agreement or the Merger Agreement or any of the transactions contemplated hereby or thereby, (B) result
in a breach in any respect of any covenant, representation, warranty or any other obligation or agreement of Parent or Merger Sub under
the Merger Agreement, (C) result in any of the conditions set forth in Section 8.1(c), Section 8.1(f), or Section 8.2
of the Merger Agreement not being fulfilled or (D) change in any manner the dividend policy or capitalization of, including the voting
rights of any class of capital stock of, Parent.
Sponsor hereby agrees that it shall not commit or
agree to take any action inconsistent with the foregoing, and shall not deposit any of its Parent Common Stock in a voting trust, grant
any proxy or power of attorney with respect to any of its Parent Common Stock or subject any of its Parent Common Stock to any arrangement
or agreement with respect to the voting of such Parent Common Stock unless specifically requested to do so by the Company and Parent in
writing in connection with the Merger Agreement, the Transaction Documents or the transactions contemplated thereby.
(b) Sponsor
shall comply with, and fully perform all of its obligations, covenants and agreements set forth in, that certain Letter Agreement, dated
as of October 13, 2022, by and among Sponsor, Parent and its directors and officers (the “Sponsor Letter”).
(c) Sponsor
agrees that, if Parent seeks shareholder approval of the transactions contemplated by the Merger Agreement or any Transaction Documents,
Sponsor shall not redeem any Subject Securities owned by it in conjunction with such shareholder approval or the transactions contemplated
thereby.
(d) During
the period commencing on the date hereof and ending on the Expiration Time, Sponsor shall not modify or amend any Contract between or
among Sponsor or any of its Affiliates (other than Parent or any of its Subsidiaries), on the one hand, and Parent or any of Parent’s
Subsidiaries, on the other hand.
5. Payment
Obligations of Parent Transaction Expenses at Closing. On the Closing Date, in connection with the Effective Time, Sponsor shall pay
in full, by wire transfer of immediately available funds, any Parent Estimated Transaction Expenses in excess of $500,000, in accordance
with the Payment Schedule.
6. Payment
Obligations of Parent Transaction Expenses post-Closing.
(a) Within
ninety (90) days following the Closing Date, the Surviving Corporation will prepare and deliver to Sponsor a statement (the “Closing
Statement”) containing the actual amount of all Parent Transaction Expenses (including those invoiced following the Closing).
The Surviving Corporation shall provide Sponsor with reasonable access to its books and records as may be reasonably requested by Sponsor
to verify the information contained in the Closing Statement.
(b) Dispute
Resolution.
(i) Within
thirty (30) days following Sponsor’s receipt of the Closing Statement, Sponsor will deliver written notice to the Surviving Corporation
of any dispute with respect to the Closing Statement, setting forth such disputed item in reasonable detail (a “Closing Statement
Dispute”). If Sponsor does not notify the Surviving Corporation of any Closing Statement Dispute within such thirty (30)-day
period, then the Closing Statement and the determinations and calculations of the Parent Transaction Expenses set forth therein will be
final, conclusive and binding on the Parties. If Sponsor delivers to the Surviving Corporation a Closing Statement Dispute, then Sponsor
and the Surviving Corporation will negotiate in good faith to resolve all disputed matters set forth in the Closing Statement Dispute.
If Sponsor and the Surviving Corporation, notwithstanding such good faith effort, fail to resolve the Closing Statement Dispute within
thirty (30) days (or longer, as mutually agreed to by such Parties in writing) after Sponsor delivers to the Surviving Corporation notice
of the Closing Statement Dispute, then Sponsor and the Surviving Corporation jointly will mutually agree on and jointly engage an independent
auditor that is experienced in such matters (the “Independent Auditor”) to promptly resolve any and all unresolved
matters of the Closing Statement Dispute.
(ii) The
Independent Auditor shall consider only those items and amounts set forth in the Closing Statement Dispute that are identified by either
Sponsor or the Surviving Corporation as being items that Sponsor and the Surviving Corporation are unable to resolve. As promptly as practicable
thereafter, Sponsor and the Surviving Corporation will each prepare and submit a written presentation to the Independent Auditor (each
of which will be shared with the other party, but not until such time that both Parties have submitted their presentations) and will use
commercially reasonable efforts to cause the Independent Auditor to make a final determination with respect to the Parties’ respective
positions based upon the applicable language, definitions and Exhibits of the Merger Agreement, and the presentations by Sponsor and the
Surviving Corporation.
(iii) In
resolving any disputed item, the Independent Auditor will be bound by the terms of this Agreement and the Merger Agreement, will serve
as an expert and not an arbitrator, and will not assign a value to any item greater than the greatest value for such item claimed by either
Party or less than the smallest value for such item claimed by either Party. Except as Sponsor and the Surviving Corporation may otherwise
agree, all communications between any Party or its respective Representatives, on the one hand, and the Independent Auditor, on the other
hand, will be in writing with copies simultaneously delivered to the non-communicating Party (except in such cases where both Parties
are submitting a presentation). The fees, costs and expenses of the Independent Auditor will be borne by Sponsor and the Surviving Corporation
in inverse proportion, as determined by the Independent Auditor, as they may prevail on the matter resolved by the Independent Auditor.
Absent fraud, all determinations made by the Independent Auditor will be final, conclusive and binding on the Parties. The Parties agree
that judgment may be entered upon the determination of the Independent Auditor in any court having jurisdiction over the party against
which such determination is to be enforced.
(iv) If
the Parent Transaction Expenses (as finally determined pursuant to this Section 6(b)) is greater than the Parent Estimated
Transaction Expenses and greater than $500,000, then Sponsor will pay by wire transfer of immediately available funds to Parent, an amount
in cash equal to (A) the amount by which the Parent Transaction Expenses exceeds $500,000 minus (B) the amount, if any, that Sponsor
paid at Closing in respect of Parent Estimated Transaction Expenses pursuant to Section 5 above.
(v) Any
payment required pursuant to this Section 6(b) will be made within five (5) Business Days after the date of final determination
of the Parent Transaction Expenses in accordance with this Section 6(b).
(vi) Each
party will reasonably cooperate with and make available to the other party and its respective accountants and other Representatives all
information, records, data and working papers, and will permit access to its records, facilities and personnel, as may be reasonably requested
in connection with this Section 6(b), including the resolution of any matters or disputes hereunder.
(vii) Unless
otherwise required by applicable Law, the Parties agree that any payments made pursuant to this Section 6(b) will be treated
as an adjustment to the Merger Consideration for all Tax purposes.
7. Payment
Obligations of Parent after the Closing.
(a) After
the Closing and upon the liquidation of the Trust Account in accordance Section 1(i) of the Trust Agreement, Parent shall pay or
cause to be paid to the Sponsor the amounts due and payable under the Extension Promissory Notes, by wire transfer of immediately available
funds within five (5) business days after the Closing, as provided in this Section 7. Parent’s obligation to pay or cause
to be paid to the Sponsor such amounts only will arise if each of the following conditions has been satisfied:
(i) the
Parent Common Stock (or Domesticated Parent Common Stock, as the case may be) must have redeemed from each Public Shareholder (as such
term is defined in the Trust Agreement) that properly elected to have his, her, or its Parent Common Stock redeemed;
(ii) at
least $2,000,000 or more of the liquidated Trust Account Property (as such term is defined in the Trust Agreement) must remain after payment
in full for all such redemptions described in Section 7(a)(i); and
(iii) Parent
must have reimbursed the Company pursuant to Section 7.16(b)(iii) of the Merger Agreement (the “Company Extension Fee
Reimbursement”).
(b) If each
of the conditions specified in Sections 7(a) are satisfied, then the Parent will reimburse the Sponsor the amounts due and payable
under the Extension Promissory Notes to the extent any amounts remain of the liquidated Trust Account Property after giving effect to
the payments from the liquidated Trust Account Property specified in Sections 7(a)(i) and Section 7(a)(iii). If (i) the
amount remaining in the liquidated Trust Account Property after payment in full of the amounts required to redeem Parent Common Stock
(or Domesticated Parent Common Stock, as the case may be) as described in Section 7(a)(i) is less than $2,000,000 or (ii) the Company
Extension Fee Reimbursement is not paid in full, Parent will not be obligated to make any payment to Sponsor in respect of the Extension
Promissory Notes, and all amounts remaining under the Extension Promissory Notes will be convertible into Domesticated Parent Common Stock
in accordance with Section 3(a)(ii) above and the terms of the Extension Promissory Notes. If, after the Company Extension Fee
Reimbursement has been paid in full, the amount of liquidated Trust Account Property remaining is not sufficient to repay the total amount
due and payable under the Extension Promissory Notes, Parent shall pay such remaining amount of the liquidated Trust Account Property
to the Sponsor, and any unpaid portion remaining under the Extension Promissory Notes will be convertible into Domesticated Parent Common
Stock in accordance with Section 3(a)(ii) above and the terms of the Extension Promissory Notes. The remaining liquidated Trust
Account Property will serve as the only source of funds for purposes of any payments to the Sponsor under this Section 7.
8. No
Actions. Sponsor hereby agrees not to commence or participate in any claim, derivative or otherwise, against the Company, Parent or
any of their respective Affiliates (a) challenging the validity of, or seeking to enjoin the operation of, any provision of this Agreement
or (b) alleging a breach of any fiduciary duty of the board of directors of Parent in connection with this Agreement, the Transaction
Proposals, the Merger Agreement or the transactions contemplated thereby.
9. Permitted
Disclosure. Sponsor hereby authorizes each of Parent, the Company, and their respective Subsidiaries to publish and disclose, in any
announcement, filing or disclosure required to be made by any Order or other applicable Law or the rules of any national securities exchange
or as requested by the SEC, Sponsor’s identity and ownership of equity securities of Parent and Sponsor’s obligations under
this Agreement.
10. Anti-Dilution
Waiver. Sponsor hereby agrees that Sponsor shall waive, and hereby does waive, any and all anti-dilution or similar rights (if any)
that may otherwise be available under applicable Law or pursuant to any Contract between or among Sponsor or any Affiliate of Sponsor
(other than Parent or any of its Subsidiaries), on the one hand, and Parent or any of Parent’s Subsidiaries, on the other hand,
with respect to the transactions contemplated by the Merger Agreement and that it shall not take any action in furtherance of exercising
any such rights.
11. Stop
Orders. Parent hereby agrees to (a) place a revocable stop order on Sponsor’s Parent Common Stock, including those which may
be covered by a registration statement, and (b) notify Parent’s transfer agent in writing of such stop order and the restrictions
on such Parent Common Stock and direct Parent’s transfer agent not to process any attempts by Sponsor to transfer any Parent Common
Stock; for the avoidance of doubt, the obligations of Parent under this Section 11 shall be deemed to be satisfied by the existence
of any similar stop order and restrictions currently existing on Sponsor’s Parent Common Stock.
12. No
Inconsistent Agreement. Sponsor hereby agrees and represents and covenants that Sponsor has not entered into, and shall not enter
into, any agreement that would restrict, limit or interfere with the performance of Sponsor’s obligations hereunder.
13. Further
Assurances. Sponsor shall execute and deliver such documents and take such action necessary to consummate the Merger and the other
transactions contemplated by the Merger Agreement on the terms and subject to the conditions set forth therein and herein.
14. A&R
Registration Rights Agreement. On the Closing Date, Sponsor shall deliver to Parent and the Company a duly executed copy of the A&R
Registration Rights Agreement.
15. Representations
and Warranties of Sponsor. Sponsor represents and warrants to Parent and the Company as follows:
(a) Organization;
Due Authorization. Sponsor is duly organized, validly existing and in good standing under the Laws of the jurisdiction in which it
is incorporated, formed, organized or constituted, and the execution, delivery and performance of this Agreement and the consummation
of the transactions contemplated hereby are within Sponsor’s corporate, limited liability company or organizational powers and have
been duly authorized by all necessary corporate, limited liability company or organizational actions on the part of Sponsor. This Agreement
has been duly executed and delivered by Sponsor and, assuming due authorization, execution and delivery by the other parties to this Agreement,
this Agreement constitutes a legally valid and binding obligation of Sponsor, enforceable against Sponsor in accordance with the terms
hereof, subject to applicable bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and similar Laws affecting creditors’
rights generally and subject, as to enforceability, to general principles of equity. If this Agreement is being executed in a representative
or fiduciary capacity, the Person signing this Agreement has full power and authority to enter into this Agreement on behalf of Sponsor.
(b) Ownership.
Sponsor is the record and “beneficial owner” (within the meaning of Rule 13d-3 under the Exchange Act) of, and has good title
to, all of its Parent Common Stock and Parent Rights, and there exist no Liens or any other limitation or restriction (including any restriction
on the right to vote, sell or otherwise dispose of such Parent Common Stock and Parent Rights (other than transfer restrictions under
the Securities Act)) affecting any such Parent Common Stock or Parent Rights, or after the Domestication, any Domesticated Parent Common
Stock or Domesticated Parent Right, other than Liens pursuant to (i) this Agreement, (ii) Parent’s and Sponsor’s Governing
Documents, (iii) the Merger Agreement, (iv) the agreements entered into by Sponsor with Parent in connection with Parent’s initial
public offering or (v) any applicable securities Laws. Sponsor’s Parent Common Stock and Parent Rights are the only equity securities
in Parent owned of record or beneficially by Sponsor on the date of this Agreement, and none of Sponsor’s Parent Common Stock or
Parent Rights are subject to any proxy, voting trust or other agreement or arrangement with respect to the voting of such Parent Common
Stock or Parent Rights, except as provided hereunder and pursuant to the Sponsor Letter. Other than the Parent Rights and the Parent Common
Stock, Sponsor does not hold or own any rights to acquire (directly or indirectly) any equity securities of Parent or any equity securities
convertible into, or which can be exchanged for, equity securities of Parent.
(c) No
Conflicts. The execution and delivery of this Agreement by Sponsor does not, and the performance by Sponsor of its obligations hereunder
will not, (i) conflict with or result in a violation of the Governing Documents of Sponsor or (ii) require any consent or approval that
has not been given or other action that has not been taken by any Person (including under any Contract binding upon Sponsor or Sponsor’s
Parent Common Stock or Parent Rights), in each case, to the extent such consent, approval or other action would prevent, enjoin or materially
delay the performance by Sponsor of its, his or her obligations under this Agreement.
(d) Litigation.
There are no Actions pending against Sponsor, or to the knowledge of Sponsor threatened against Sponsor, before (or, in the case of threatened
Actions, that would be before) any arbitrator or any Governmental Authority, which in any manner challenges or seeks to prevent, enjoin
or materially delay the performance by Sponsor of its, his or her obligations under this Agreement. Sponsor has not instigated an action
regarding the transactions contemplated in the Merger Agreement.
(e) Brokerage
Fees. No broker, finder, investment banker or other Person is entitled to any brokerage fee, finders’ fee or other commission
in connection with the transactions contemplated by the Merger Agreement based upon arrangements made by Sponsor, for which Parent or
any of its Affiliates may become liable.
(f) Affiliate
Arrangements. Except as set forth on Schedule 2.1(f) hereto, neither Sponsor nor, to the knowledge of Sponsor, any Person in
which Sponsor has a direct or indirect legal, contractual or beneficial ownership of 5% or more is party to, or has any rights with respect
to or arising from, any Contract with Parent or its Subsidiaries.
(g) Acknowledgment.
Sponsor understands and acknowledges that each of Parent, the Company and SpinCo is entering into the Merger Agreement in reliance upon
Sponsor’s execution and delivery of this Agreement.
16. Termination.
This Agreement and all of its provisions shall terminate and be of no further force or effect upon the earlier of (a) the Expiration Time
and (b) the written agreement of Sponsor, Parent and the Company. Upon such termination of this Agreement, all obligations of the parties
under this Agreement will terminate, without any liability or other obligation on the part of any party hereto to any Person in respect
hereof or the transactions contemplated hereby, and no party hereto shall have any claim against another (and no person shall have any
rights against such party), whether under contract, tort or otherwise, with respect to the subject matter hereof; provided, however, that
the termination of this Agreement shall not relieve any Party from liability arising in respect of any breach of this Agreement prior
to such termination. Sections 15 through 25 shall survive the termination of this Agreement.
17. Amendment.
This Agreement cannot be amended, except by a writing signed by each of the Parties, and cannot be terminated orally or by course of conduct.
No provision hereof can be waived, except by a writing signed by the Party against whom such waiver is to be enforced, and any such waiver
shall apply only in the particular instance in which such waiver shall have been given.
18. Assignment.
No Party may assign any right or delegate any obligation hereunder, including by merger, consolidation, operation of law, or otherwise,
without the written consent of the other Parties. Any purported assignment or delegation without such consent shall be void, in addition
to constituting a material breach of this Agreement.
19. Governing
Law. This Agreement shall be construed in accordance with and governed by the laws of the State of Delaware, without giving effect
to the conflict of laws principles thereof.
20. Jurisdiction.
Any Action based upon, arising out of or related to this Agreement or the transactions contemplated hereby must be brought in the Court
of Chancery of the State of Delaware (or, to the extent such court does not have subject matter jurisdiction, the Superior Court of the
State of Delaware), or, if it has or can acquire jurisdiction, in the United States District Court for the District of Delaware, and each
of the parties irrevocably (a) submits to the exclusive jurisdiction of each such court in any such proceeding or Action, (b) waives any
objection it may now or hereafter have to personal jurisdiction, venue or to convenience of forum, (c) agrees that all claims in respect
of the proceeding or Action shall be heard and determined only in any such court, and (d) agrees not to bring any proceeding or Action
arising out of or relating to this Agreement or the transactions contemplated hereby in any other court. Nothing herein contained shall
be deemed to affect the right of any party to serve process in any manner permitted by Law or to commence any Action or otherwise proceed
against any other party in any other jurisdiction, in each case, to enforce judgments obtained in any Action brought pursuant to this
Section 20.
21. Specific
Performance. The Parties agree that irreparable damage may occur in the event that any of the provisions of this Agreement were not
performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the Parties shall be entitled
to seek an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this
Agreement in the chancery court or any other state or federal court within the State of Delaware, this being in addition to any other
remedy to which such party is entitled at Law or in equity.
22. Notices.
Any notice hereunder shall be sent in writing, addressed as specified below, and shall be deemed given: (a) if by hand or recognized courier
service on the date of delivery; (b) if by fax or email, on the date that transmission is confirmed electronically; or (c) five (5) days
after mailing by certified or registered mail, return receipt requested. Notices shall be addressed to the respective parties as follows
(excluding telephone numbers, which are for convenience only), or to such other address as a party shall specify to the others in accordance
with these notice provisions:
(a) if to
the Company or SpinCo, to:
Citius Pharmaceuticals, Inc.
11 Commerce Drive, First Floor
Cranford, NJ 07016
| |
Attention: |
Jaime Bartushak |
| |
Email: |
jbartushak@citiuspharma.com |
with a copy (which shall not constitute notice) to:
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suit 300
Raleigh, North Carolina 27607
| |
Attention: |
David Creekman |
| |
|
Alec Donaldson |
| |
Email: |
dcreekman@wyrick.com |
| |
|
adonaldson@wyrick.com |
(b) if to
Parent, to:
TenX Keane Acquisition
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Taylor Zhang |
| |
Email: |
tzhang@ascendantga.com |
with a copy (which shall not constitute notice) to:
The Crone Law Group
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Tammara Fort |
| |
|
Samara Thomas |
| |
Email: |
tfort@cronelawgroup.com |
| |
|
sthomas@cronelawgroup.com |
(c) if to
Sponsor, to:
10XYZ Holdings LP, a Delaware limited partnership
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Taylor Zhang |
| |
Email: |
tzhang@ascendantga.com |
with a copy (which shall not constitute notice) to:
The Crone Law Group
420 Lexington Avenue, Suite 2446
New York, New York 10170
| |
Attention: |
Tammara Fort |
| |
|
Samara Thomas |
| |
Email: |
tfort@cronelawgroup.com |
| |
|
sthomas@cronelawgroup.com |
23. Counterparts;
Facsimile Signatures. This Agreement may be executed in counterparts, each of which shall constitute an original, but all of which
shall constitute one agreement. This Agreement shall become effective upon delivery to each Party of an executed counterpart or the earlier
delivery to each Party of original, photocopied, or electronically transmitted signature pages that together (but need not individually)
bear the signatures of all other parties.
24. Entire
Agreement. This Agreement together with the agreements referenced herein set forth the entire agreement of the parties with respect
to the subject matter hereof and supersedes all prior and contemporaneous understandings and agreements related hereto (whether written
or oral).
25. Severability.
A determination by a court or other legal authority that any provision that is not of the essence of this Agreement is legally invalid
shall not affect the validity or enforceability of any other provision hereof. The Parties shall cooperate in good faith to substitute
(or cause such court or other legal authority to substitute) for any provision so held to be invalid a valid provision, as alike in substance
to such invalid provision as is lawful.
26. Waiver
of Jury Trial; Exemplary Damages.
(a) THE
PARTIES TO THIS AGREEMENT HEREBY KNOWINGLY, VOLUNTARILY AND IRREVOCABLY WAIVE ANY RIGHT EACH SUCH PARTY MAY HAVE TO TRIAL BY JURY IN ANY
ACTION OF ANY KIND OR NATURE, IN ANY COURT IN WHICH AN ACTION MAY BE COMMENCED, ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT OR
ANY TRANSACTION DOCUMENT, OR BY REASON OF ANY OTHER CAUSE OR DISPUTE WHATSOEVER BETWEEN OR AMONG ANY OF THE PARTIES TO THIS AGREEMENT
OF ANY KIND OR NATURE. NO PARTY SHALL BE AWARDED PUNITIVE OR OTHER EXEMPLARY DAMAGES RESPECTING ANY DISPUTE ARISING UNDER THIS AGREEMENT.
(b) Each
Party acknowledges that each has been represented in connection with the signing of this waiver by independent legal counsel selected
by the respective Party and that such Party has discussed the legal consequences and import of this waiver with legal counsel. Each Party
further acknowledges that such Party has read and understands the meaning of this waiver and grants this waiver knowingly, voluntarily,
without duress and only after consideration of the consequences of this waiver with legal counsel.
[SIGNATURE
PAGES FOLLOW]
In
Witness Whereof, Sponsor, Parent, the Company and SpinCo have each caused this Sponsor Support Agreement to be duly executed as
of the date first written above.
| |
SPONSOR: |
| |
|
| |
10XYZ Holdings LP |
| |
|
| |
By: |
/s/
Taylor Zhang |
| |
Name: |
Taylor Zhang |
| |
Title: |
Manager |
| |
|
| |
PARENT: |
| |
|
| |
TenX Keane Acquisition |
| |
|
| |
By: |
/s/ Xiaofeng
Yuan |
| |
Name: |
Xiaofeng Yuan |
| |
Title: |
Chief Executive Officer and Chairman |
| |
|
| |
COMPANY: |
| |
|
| |
Citius Pharmaceuticals, Inc. |
| |
|
| |
By: |
/s/ Leonard
Mazur |
| |
Name: |
Leonard Mazur |
| |
Title: |
Chief Executive Officer and Chairman of the Board |
| |
|
| |
SPINCO: |
| |
|
| |
Citius Oncology, Inc. |
| |
|
| |
By: |
/s/ Leonard
Mazur |
| |
Name: |
Leonard Mazur |
| |
Title: |
Chief Executive Officer and Chairman of the Board |
12
Exhibit 10.2
FORM OF
AMENDED AND RESTATED REGISTRATION RIGHTS AGREEMENT
THIS AMENDED AND RESTATED
REGISTRATION RIGHTS AGREEMENT (this “Agreement”), dated as of [_____ __], 2023, is made and entered into by
and among (i) Citius Oncology, Inc., a Delaware corporation, formerly known as TenX Keane Acquisition, a Cayman Islands exempted company
(the “Company”), (ii) the equityholders designated as Sponsor Equityholders on the signature page hereto (collectively,
the “Sponsor Equityholders”); and (iii) Citius Pharmaceuticals, Inc. (the “Legacy Citius Oncology
Equityholder” and, together with the Sponsor Equityholders and any person or entity who hereafter becomes a party to this
Agreement pursuant to Section 6.2 of this Agreement, the “Holders” and individually, a “Holder”).
Capitalized terms used but not otherwise defined in this Agreement shall have the meanings ascribed to such terms in the Merger Agreement
(as defined below).
RECITALS
WHEREAS, the Company
and the Sponsor Equityholders are parties to that certain Registration Rights Agreement, dated as of October 13, 2022 (the “Prior
Agreement”);
WHEREAS, the Company,
TenX Merger Sub, Inc., a Delaware corporation (“Merger Sub”), and [New Citius Oncology Name], an entity formerly
known as Citius Oncology, Inc., a Delaware corporation (“Legacy Citius Oncology”), are parties to that certain
Agreement and Plan of Merger, dated as of [_____ __], 2023 (as amended or restated from time to time, the “Merger Agreement”),
pursuant to which, on the date hereof, Merger Sub merged (the “Merger”) with and into Legacy Citius Oncology,
with Legacy Citius Oncology surviving the Merger as a wholly owned subsidiary of the Company;
WHEREAS, the Legacy
Citius Oncology Equityholder is receiving shares of common stock, par value $0.0001 per share, of the Company (the “Common
Stock”) on or about the date hereof, pursuant to the Merger Agreement (the “Merger Shares”);
WHEREAS, in connection
with the consummation of the Merger, the parties to the Prior Agreement desire to amend and restate the Prior Agreement in its entirety
as set forth herein, and the parties hereto desire to enter into this Agreement pursuant to which the Company shall grant the Holders
certain registration rights with respect to the Registrable Securities (as defined below) on the terms and conditions set forth in this
Agreement, effective as of the Closing; and
WHEREAS, pursuant
to Section 5.5 of the Prior Agreement, no amendment, modification or termination of the Prior Agreement shall be binding upon any
party unless executed in writing by such party.
NOW, THEREFORE,
in consideration of the representations, covenants and agreements contained herein, and certain other good and valuable consideration,
the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE I
DEFINITIONS
1.1 Definitions. Terms
used, but not otherwise defined, shall have the meaning ascribed to them in the Merger Agreement. The terms defined in this Article
I shall, for all purposes of this Agreement, have the respective meanings set forth below:
“Adverse Disclosure”
shall mean any public disclosure of material non-public information, which disclosure, in the good faith judgment of the Chief Executive
Officer or Chief Financial Officer of the Company, after consultation with counsel to the Company, (i) would be required to be made in
any Registration Statement or Prospectus in order for the applicable Registration Statement or Prospectus not to contain any untrue statement
of a material fact or omit to state a material fact necessary to make the statements contained therein (in the case of any prospectus
and any preliminary prospectus, in the light of the circumstances under which they were made) not misleading, (ii) would not be required
to be made at such time if the Registration Statement were not being filed, declared effective or used, as the case may be, and (iii)
the Company has a bona fide business purpose for not making such information public.
“Agreement”
shall have the meaning given in the Preamble.
“Block Trade”
shall mean an offering and/or sale of Registrable Securities by any Holder on a block trade or underwritten basis (whether firm commitment
or otherwise) without substantial marketing efforts prior to pricing, including, without limitation, a same day trade, overnight trade
or similar transaction.
“Board”
shall mean the Board of Directors of the Company.
“Change
in Control” shall mean any transfer (whether by tender offer, merger, stock purchase, consolidation or other similar transaction),
in one transaction or a series of related transactions, to a person or group of affiliated persons of the Company’s voting securities
if, after such transfer, such person or group of affiliated persons would hold more than 50% of outstanding voting securities of the Company
(or surviving entity) or would otherwise have the power to control the Board or to direct the operations of the Company.
“Code”
shall have the meaning given in subsection 4.2.13.
“Commission”
shall mean the Securities and Exchange Commission.
“Common Stock”
shall have the meaning given in the Recitals.
“Company”
shall have the meaning given in the Preamble.
“Demand Registration”
shall have the meaning given in subsection 2.1.1.
“Demanding Holder”
shall have the meaning given in subsection 2.1.1.
“EDGAR”
shall have the meaning given in subsection 3.1.3.
“Exchange Act”
shall mean the Securities Exchange Act of 1934, as it may be amended from time to time.
“Form S-1”
shall have the meaning given in subsection 2.1.1.
“Form S-3”
shall have the meaning given in subsection 2.3.
“Founder Shares”
shall mean the 1,650,000 shares of ordinary shares of the Company, which subsequently converted into 1,650,000 shares of Common Stock,
issued to its initial stockholders prior to the Company’s initial public offering.
“Holder Information”
shall have the meaning given in subsection 5.1.2.
“Holders”
shall have the meaning given in the Preamble, for so long as such person or entity holds any Registrable Securities.
“Legacy Citius
Oncology” shall have the meaning given in the Recitals.
“Lock-up”
shall have the meaning given in Section 4.1.
“Lock-up Party”
shall have the meaning given in Section 4.1.
“Lock-up Period”
shall have the meaning given in Section 4.1.
“Maximum Number
of Securities” shall have the meaning given in subsection 2.1.4
“Merger”
shall have the meaning given in the Recitals.
“Merger Agreement”
shall have the meaning given in the Recitals.
“Merger Shares”
shall have the meaning given in the Recitals.
“Merger Sub”
shall have the meaning given in the Recitals.
“Misstatement”
shall mean an untrue statement of a material fact or an omission to state a material fact required to be stated in a Registration Statement
or Prospectus, or necessary to make the statements in a Registration Statement or Prospectus (in the case of a Prospectus, in the light
of the circumstances under which they were made) not misleading.
“Permitted Transferees”
shall mean a person or entity to whom a Holder of Registrable Securities is permitted to transfer such Registrable Securities prior to
the expiration of the Lock-up Period under this Agreement, and any other applicable agreement between such Holder and the Company, and
to any transferee thereafter.
“Piggyback Registration”
shall have the meaning given in subsection 2.2.1.
“Prior Agreement”
shall have the meaning given in the Recitals.
“Private Placement
Rights” shall mean the 394,000 rights to receive two-tenths (2/10) of one ordinary share issued by the Company that were
part of the Private Placement Units which (i) subsequently converted into a right to receive two-tenths (2/10) of a share of Common Stock
in connection with the Domestication and in accordance with the Merger Agreement and (ii) were automatically converted into whole shares
of Common Stock at the Closing.
“Private Placement
Shares” shall mean the 394,000 ordinary shares issued by the Company as part of the Private Placement Units and which subsequently
converted into 394,000 shares of Common Stock in connection with the Domestication.
“Private Placement
Units” shall mean the 394,000 units issued by the Company that were privately purchased simultaneously with the consummation
of the Company’s initial public offering and for which each unit was comprised of one Private Placement Share and one Private Placement
Right.
“Pro Rata”
shall have the meaning given in subsection 2.1.4.
“Prospectus”
shall mean the prospectus included in any Registration Statement, as supplemented by any and all prospectus supplements and as amended
by any and all post-effective amendments and including all material incorporated by reference in such prospectus.
“Registrable
Security”1 shall mean (a) the Merger Shares, (b) the Founder Shares, (c) the shares of Common Stock issued upon
the conversion of the Private Placement Rights at the Closing,2
(d) the Private Placement Shares, (e) any outstanding Common Stock or any other equity security (including the shares of
Common Stock issued or issuable upon the exercise of any other equity security) of the Company held by a Holder as of the date of this
Agreement, (f) any equity securities (including the Common Stock issued or issuable upon the exercise of any such equity security) of
the Company issuable upon conversion of any working capital loans in an amount up to $1,500,000 made to the Company by a Holder, and
(g) any other equity security of the Company issued or issuable with respect to any such Common Stock by way of a share dividend or share
split or in connection with a combination of shares, recapitalization, merger, consolidation or reorganization; provided, however,
that, as to any particular Registrable Security, such securities shall cease to be Registrable Securities upon the earliest to occur
of: (A) a Registration Statement with respect to the sale of such securities shall have become effective under the Securities Act and
such securities shall have been sold, transferred, disposed of or exchanged in accordance with such Registration Statement; (B) such
securities shall have been otherwise transferred (other than to a Permitted Transferee), new certificates for such securities not bearing
(or book entry positions not subject to) a legend restricting further transfer shall have been delivered by the Company and subsequent
public distribution of such securities shall not require registration under the Securities Act; (C) such securities shall have ceased
to be outstanding; (D) such securities may be sold without registration pursuant to Rule 144 promulgated under the Securities Act (or
any successor rule promulgated thereafter by the Commission) (but with no volume or other restrictions or limitations); or (E) such securities
have been sold to, or through, a broker, dealer or underwriter in a public distribution or other public securities transaction.
| 1 | NTD: TenX to provide list of all shares
and rights that will be outstanding immediately following the merger, which should be included
as “Registrable Securities”. All TenX Rights will be converted into their
underlying shares Common Stock at the Closing/Effective Time and there will be none outstanding
post-Closing. |
“Registration”
shall mean a registration effected by preparing and filing a registration statement or similar document in compliance with the requirements
of the Securities Act, and the applicable rules and regulations promulgated thereunder, and such registration statement becoming effective.
“Registration
Expenses” shall mean the out-of-pocket expenses of a Registration, including, without limitation, the following:
(A) all registration and
filing fees (including fees with respect to filings required to be made with the Financial Industry Regulatory Authority, Inc.) and any
securities exchange on which the Common Stock is then listed;
(B) fees and expenses of
compliance with securities or blue sky laws (including reasonable fees and disbursements of counsel for the Underwriters in connection
with blue sky qualifications of Registrable Securities);
(C) printing, messenger,
telephone and delivery expenses;
(D) reasonable fees and disbursements
of counsel for the Company;
(E) reasonable fees and disbursements
of all independent registered public accountants of the Company incurred specifically in connection with such Registration; and
(F) in an Underwritten Offering,
reasonable fees and expenses of one (1) legal counsel selected by the majority-in-interest of the Demanding Holders (not to exceed $50,000
without prior written consent of the Company).
“Registration
Statement” shall mean any registration statement filed by the Company with the Commission that covers the Registrable Securities
pursuant to the provisions of this Agreement, including the Prospectus included in such registration statement, amendments (including
post-effective amendments) and supplements to such registration statement, and all exhibits to and all material incorporated by reference
in such registration statement.
“Regulations”
shall have the meaning given in subsection 4.2.13.
“Requesting Holder”
shall have the meaning given in subsection 2.1.1.
“Securities Act”
shall mean the Securities Act of 1933, as amended from time to time.
“Shelf”
shall mean the Form S-1 Shelf, the Form S-3 Shelf or any subsequent Shelf Registration.
“Shelf Registration”
shall mean a shelf registration of securities pursuant to a Registration Statement on Form S-1 or Form S-3 filed with the Commission in
accordance with and pursuant to Rule 415 promulgated under the Securities Act (or any successor rule then in effect).
“Sponsor Equityholders”
shall have the meaning given in the Preamble.
“Transfer”
shall mean the (a) the sale or assignment of, offer to sell, contract or agreement to sell, grant of any option to purchase or otherwise
dispose of or agreement to dispose of, directly or indirectly, or establishment or increase of a put equivalent position or liquidation
with respect to or decrease of a call equivalent position within the meaning of Section 16 of the Exchange Act with respect to, any security,
(b) entry into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership
of any security, whether any such transaction is to be settled by delivery of such securities, in cash or otherwise, or (c) public announcement
of any intention to effect any transaction specified in clause (a) or (b).
“Underwriter”
shall mean a securities dealer who purchases any Registrable Securities as principal in an Underwritten Offering and not as part of such
dealer’s market-making activities.
“Underwritten
Registration” or “Underwritten Offering” shall mean a Registration in which securities of the
Company are sold to an Underwriter in a firm commitment underwriting for distribution to the public.
ARTICLE II
REGISTRATIONS
2.1 Demand Registration.
2.1.1 Request for Registration.
Subject to the provisions of subsection 2.1.4 and Section 2.4 hereof, the Holders of at least a majority in interest of
the then-outstanding number of Registrable Securities (the “Demanding Holders”) may make a written demand for
Registration under the Securities Act of all or part of their Registrable Securities, which written demand shall describe the amount and
type of securities to be included in such Registration and the intended method(s) of distribution thereof (such written demand a “Demand
Registration”). The Company shall, within ten (10) days of the Company’s receipt of the Demand Registration, notify,
in writing, all other Holders of Registrable Securities of such demand, and each Holder of Registrable Securities who thereafter wishes
to include all or a portion of such Holder’s Registrable Securities in a Registration pursuant to a Demand Registration (each such
Holder that includes all or a portion of such Holder’s Registrable Securities in such Registration, a “Requesting Holder”)
shall so notify the Company, in writing, within five (5) days after the receipt by the Holder of the notice from the Company. Upon receipt
by the Company of any such written notification from a Requesting Holder(s) to the Company, such Requesting Holder(s) shall be entitled
to have their Registrable Securities included in a Registration pursuant to a Demand Registration and the Company shall effect, as soon
thereafter as practicable, but not more than sixty (60) days immediately after the Company’s receipt of the Demand Registration,
the Registration of all Registrable Securities requested by the Demanding Holders and Requesting Holders pursuant to such Demand Registration.
2.1.2 Effective Registration.
Notwithstanding the provisions of subsection 2.1.1 above or any other part of this Agreement, a Registration pursuant to a Demand
Registration shall not count as a Registration unless and until (i) the Registration Statement filed with the Commission with respect
to a Registration pursuant to a Demand Registration has been declared effective by the Commission and (ii) the Company has complied with
all of its obligations under this Agreement with respect thereto; provided, further, that if, within six months after such
Registration Statement has been declared effective, an offering of Registrable Securities in a Registration pursuant to a Demand Registration
is subsequently interfered with by any stop order or injunction of the Commission, federal or state court or any other governmental agency
the Registration Statement with respect to such Registration shall be deemed not to have been declared effective, unless and until, (i)
such stop order or injunction is removed, rescinded or otherwise terminated, and (ii) a majority-in-interest of the Demanding Holders
initiating such Demand Registration thereafter affirmatively elect to continue with such Registration and accordingly notify the Company
in writing, but in no event later than five (5) days, of such election; and provided, further, that the Company shall not
be obligated or required to file another Registration Statement until the Registration Statement that has been previously filed with respect
to a Registration pursuant to a Demand Registration becomes effective or is subsequently terminated.
2.1.3 Underwritten Offering.
Subject to the provisions of subsection 2.1.4 and Section 2.4 hereof, if a majority-in-interest of the Demanding Holders
so advise the Company as part of their Demand Registration that the offering of the Registrable Securities pursuant to such Demand Registration
shall be in the form of an Underwritten Offering, then the right of such Demanding Holder or Requesting Holder (if any) to include its
Registrable Securities in such Registration shall be conditioned upon such Holder’s participation in such Underwritten Offering
and the inclusion of such Holder’s Registrable Securities in such Underwritten Offering to the extent provided herein. All such
Holders proposing to distribute their Registrable Securities through an Underwritten Offering under this subsection 2.1.3 shall
enter into an underwriting agreement in customary form with the Underwriter(s) selected for such Underwritten Offering by the majority-in-interest
of the Demanding Holders initiating the Demand Registration.
2.1.4 Reduction of Underwritten
Offering. If the managing Underwriter or Underwriters in an Underwritten Registration pursuant to a Demand Registration, in good faith,
advises the Company, the Demanding Holders and the Requesting Holders (if any) in writing that the dollar amount or number of Registrable
Securities that the Demanding Holders and the Requesting Holders (if any) desire to sell, taken together with all other shares of Common
Stock or other equity securities that the Company desires to sell and shares of Common Stock, if any, as to which a Registration has been
requested pursuant to separate written contractual piggy-back registration rights held by any other stockholders who desire to sell, exceeds
the maximum dollar amount or maximum number of equity securities that can be sold in the Underwritten Offering without adversely affecting
the proposed offering price, the timing, the distribution method, or the probability of success of such offering (such maximum dollar
amount or maximum number of such securities, as applicable, the “Maximum Number of Securities”), then the Company
shall include in such Underwritten Offering, as follows: (i) first, the Registrable Securities of the Demanding Holders and the Requesting
Holders (if any) (pro rata based on the respective number of Registrable Securities that each Demanding Holder and Requesting Holder (if
any) has requested be included in such Underwritten Registration and the aggregate number of Registrable Securities that the Demanding
Holders and Requesting Holders have requested be included in such Underwritten Registration (such proportion is referred to herein as
“Pro Rata”)) that can be sold without exceeding the Maximum Number of Securities; (ii) second, to the extent
that the Maximum Number of Securities has not been reached under the foregoing clause (i), the Registrable Securities of Holders (Pro
Rata, based on the respective number of Registrable Securities that each Holder has so requested) exercising their rights to register
their Registrable Securities pursuant to subsection 2.2.1 hereof, without exceeding the Maximum Number of Securities; (iii) third,
to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (i) and (ii), the shares of Common
Stock or other equity securities that the Company desires to sell, which can be sold without exceeding the Maximum Number of Securities;
and (iv) fourth, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (i), (ii) and (iii),
the Common Stock or other equity securities of other persons or entities that the Company is obligated to register in a Registration pursuant
to separate written contractual arrangements with such persons and that can be sold without exceeding the Maximum Number of Securities.
2.1.5 Demand Registration
Withdrawal. A majority-in-interest of the Demanding Holders initiating a Demand Registration or a majority-in-interest of the Requesting
Holders (if any), pursuant to a Registration under subsection 2.1.1 shall have the right to withdraw from a Registration pursuant
to such Demand Registration for any or no reason whatsoever upon written notification to the Company and the Underwriter or Underwriters
(if any) of their intention to withdraw from such Registration prior to the effectiveness of the Registration Statement filed with the
Commission with respect to the Registration of their Registrable Securities pursuant to such Demand Registration. Notwithstanding anything
to the contrary in this Agreement, the Company shall be responsible for the Registration Expenses incurred in connection with a Registration
pursuant to a Demand Registration as provided in Section 3.3 prior to its withdrawal under this subsection 2.1.5. If withdrawn,
a Demand Registration shall constitute a demand for an Underwritten Offering by the withdrawing Demanding Holder for purposes of Section
2.1.1.
2.2 Piggyback Registration.
2.2.1 Piggyback Rights.
If the Company proposes to file a Registration Statement under the Securities Act with respect to an offering of equity securities, or
securities or other obligations exercisable or exchangeable for, or convertible into equity securities, for its own account or for the
account of stockholders of the Company (or by the Company and by the stockholders of the Company including, without limitation, pursuant
to Section 2.1 hereof), other than a Registration Statement (i) filed in connection with any employee stock option or other benefit
plan, (ii) for an exchange offer or offering of securities solely to the Company’s existing stockholders, (iii) for an offering
of debt that is convertible into equity securities of the Company, (iv) pursuant to a Registration Statement on Form S-4 (or similar form
that relates to a transaction subject to Rule 145 under the Securities Act or any successor rule thereto), (v) for a dividend reinvestment
plan, or (vi) for a Block Trade, then the Company shall give written notice of such proposed filing to all of the Holders of Registrable
Securities as soon as practicable but not less than ten (10) days before the anticipated filing date of such Registration Statement or,
in the case of an Underwritten Offering pursuant to a Shelf Registration, the applicable “red herring” prospectus or prospectus
supplement used for marketing such offering, which notice shall (A) describe the amount and type of securities to be included in such
offering, the intended method(s) of distribution, and the name of the proposed managing Underwriter or Underwriters, if any, in such offering,
and (B) offer to all of the Holders of Registrable Securities the opportunity to register the sale of such number of Registrable Securities
as such Holders may request in writing within five (5) days after receipt of such written notice (such Registration a “Piggyback
Registration”). Subject to Section 2.2.2, the Company shall, in good faith, cause such Registrable Securities to
be included in such Piggyback Registration and, if applicable, shall use its commercially reasonable efforts to cause the managing Underwriter
or Underwriters of a proposed Underwritten Offering to permit the Registrable Securities requested by the Holders pursuant to this subsection
2.2.1 to be included in a Piggyback Registration on the same terms and conditions as any similar securities of the Company included
in such Registration and to permit the sale or other disposition of such Registrable Securities in accordance with the intended method(s)
of distribution thereof. All such Holders proposing to distribute their Registrable Securities through an Underwritten Offering under
this subsection 2.2.1 shall enter into an underwriting agreement in customary form with the Underwriter(s) selected for such Underwritten
Offering by the Company.
2.2.2 Reduction of Piggyback
Registration. If the managing Underwriter or Underwriters in an Underwritten Registration that is to be a Piggyback Registration,
in good faith, advises the Company and the Holders of Registrable Securities participating in the Piggyback Registration in writing that
the dollar amount or number of the shares of Common Stock or other equity securities that the Company desires to sell, taken together
with (i) the shares of Common Stock or other equity securities, if any, as to which Registration has been demanded pursuant to separate
written contractual arrangements with persons or entities other than the Holders of Registrable Securities hereunder, (ii) the Registrable
Securities as to which registration has been requested pursuant to Section 2.2 hereof, and (iii) the shares of Common Stock or
other equity securities, if any, as to which Registration has been requested pursuant to separate written contractual piggy-back registration
rights of other stockholders of the Company, exceeds the Maximum Number of Securities, then:
(a) If the Registration
is undertaken for the Company’s account, the Company shall include in any such Registration (A) first, the shares of Common Stock
or other equity securities that the Company desires to sell, which can be sold without exceeding the Maximum Number of Securities; (B)
second, to the extent that the Maximum Number of Securities has not been reached under the foregoing clause (A), the Registrable Securities
of Holders exercising their rights to register their Registrable Securities pursuant to subsection 2.2.1 hereof, Pro Rata, which
can be sold without exceeding the Maximum Number of Securities; and (C) third, to the extent that the Maximum Number of Securities has
not been reached under the foregoing clauses (A) and (B), the shares of Common Stock or other equity securities, if any, as to which Registration
has been requested pursuant to written contractual piggy-back registration rights of other stockholders of the Company, which can be sold
without exceeding the Maximum Number of Securities;
(b) If the Registration
is pursuant to a request by persons or entities other than the Holders of Registrable Securities, then the Company shall include in any
such Registration (A) first, the shares of Common Stock or other equity securities, if any, of such requesting persons or entities, other
than the Holders of Registrable Securities, which can be sold without exceeding the Maximum Number of Securities; (B) second, to the extent
that the Maximum Number of Securities has not been reached under the foregoing clause (A), the Registrable Securities of Holders exercising
their rights to register their Registrable Securities pursuant to subsection 2.2.1, pro rata based on the number of Registrable
Securities that each Holder has requested be included in such Underwritten Registration and the aggregate number of Registrable Securities
that the Holders have requested to be included in such Underwritten Registration, which can be sold without exceeding the Maximum Number
of Securities; (C) third, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses (A) and
(B), the shares of Common Stock or other equity securities that the Company desires to sell, which can be sold without exceeding the Maximum
Number of Securities; and (D) fourth, to the extent that the Maximum Number of Securities has not been reached under the foregoing clauses
(A), (B) and (C), the shares of Common Stock or other equity securities for the account of other persons or entities that the Company
is obligated to register pursuant to separate written contractual arrangements with such persons or entities, which can be sold without
exceeding the Maximum Number of Securities.
2.2.3 Piggyback Registration
Withdrawal. Any Holder of Registrable Securities shall have the right to withdraw from a Piggyback Registration for any or no reason
whatsoever upon written notification to the Company and the Underwriter or Underwriters (if any) of his, her or its intention to withdraw
from such Piggyback Registration prior to the effectiveness of the Registration Statement filed with the Commission with respect to such
Piggyback Registration or, in the case of a Piggyback Registration pursuant to a Shelf Registration, the filing of the applicable “red
herring” prospectus or prospectus supplement with respect to such Piggyback Registration used for marketing such transaction. The
Company (whether on its own good faith determination or as the result of a request for withdrawal by persons pursuant to separate written
contractual obligations) may withdraw or abandon a Registration Statement filed with the Commission or Shelf takedown in connection with
a Piggyback Registration at any time prior to the effectiveness of such Registration Statement. Notwithstanding anything to the contrary
in this Agreement, the Company shall be responsible for the Registration Expenses incurred in connection with the Piggyback Registration
as provided in Section 3.2 prior to its withdrawal under this subsection 2.2.3.
2.2.4 Limitations on Registration
Rights. Under no circumstances shall the Company be obligated to effect more than an aggregate of three (3) Registrations pursuant
to a Demand Registration under Section 2.1 with respect to any or all Registrable Securities, provided that a Piggyback
Registration under this Section 2.2 shall not be counted as a Registration pursuant to a Demand Registration effected under Section
2.1.
2.3 Registrations on Form
S-3. The Holders of Registrable Securities may at any time, and from time to time, request in writing that the Company, pursuant to
Rule 415 under the Securities Act (or any successor rule promulgated thereafter by the Commission), register the resale of any or all
of their Registrable Securities on Form S-3 or any similar short form registration statement that may be available at such time (“Form
S-3”); provided, however, that the Company shall not be obligated to effect such request through an Underwritten
Offering. Within five (5) days of the Company’s receipt of a written request from a Holder or Holders of Registrable Securities
for a Registration on Form S-3, the Company shall promptly give written notice of the proposed Registration on Form S-3 to all other Holders
of Registrable Securities, and each Holder of Registrable Securities who thereafter wishes to include all or a portion of such Holder’s
Registrable Securities in such Registration on Form S-3 shall so notify the Company, in writing, within ten (10) days after the receipt
by the Holder of the notice from the Company. As soon as practicable thereafter, but not more than sixty (60) days after the Company’s
initial receipt of such written request for a Registration on Form S-3, the Company shall register all or such portion of such Holder’s
Registrable Securities as are specified in such written request, together with all or such portion of Registrable Securities of any other
Holder or Holders joining in such request as are specified in the written notification given by such Holder or Holders; provided,
however, that the Company shall not be obligated to effect any such Registration pursuant to Section 2.3 hereof if (i) a
Form S-3 is not available for such offering; or (ii) the Holders of Registrable Securities, together with the Holders of any other equity
securities of the Company entitled to inclusion in such Registration, propose to sell the Registrable Securities and such other equity
securities (if any) at any aggregate price to the public of less than $10,000,000.
2.4 Restrictions on Registration
Rights. If (A) during the period starting with the date sixty (60) days prior to the Company’s good faith estimate of the date
of the filing of, and ending on a date one hundred and twenty (120) days after the effective date of, a Company initiated Registration
and provided that the Company has delivered written notice to the Holders prior to receipt of a Demand Registration pursuant to subsection
2.1.1 and it continues to actively employ, in good faith, all commercially reasonable efforts to cause the applicable Registration
Statement to become effective; (B) the Holders have requested an Underwritten Registration and the Company and the Holders are unable
to obtain the commitment of underwriters to firmly underwrite the offer; or (C) in the good faith judgment of the Board such Registration
would be seriously detrimental to the Company and the Board concludes as a result that it is essential to defer the filing of such Registration
Statement at such time, then in each case the Company shall furnish to such Holders a certificate signed by the Chairman of the Board
stating that in the good faith judgment of the Board it would be seriously detrimental to the Company for such Registration Statement
to be filed in the near future and that it is therefore essential to defer the filing of such Registration Statement. In such event, the
Company shall have the right to defer such filing for a period of not more than ninety (90) days.
ARTICLE III
COMPANY PROCEDURES
3.1 General Procedures.
If the Company is required to effect the Registration of Registrable Securities pursuant to this Agreement, the Company shall use its
commercially reasonable efforts to effect such Registration to permit the sale of such Registrable Securities in accordance with the intended
plan of distribution thereof, and pursuant thereto the Company shall:
3.1.1 prepare and file with
the Commission as soon as practicable a Registration Statement with respect to such Registrable Securities and use its commercially reasonable
efforts to cause such Registration Statement to become effective and remain effective until all Registrable Securities covered by such
Registration Statement have been sold or have ceased to be Registrable Securities;
3.1.2 prepare and file with
the Commission such amendments and post-effective amendments to the Registration Statement, and such supplements to the Prospectus, as
may be reasonably requested by the Holders or any Underwriter of Registrable Securities or as may be required by the rules, regulations
or instructions applicable to the registration form used by the Company or by the Securities Act or rules and regulations thereunder to
keep the Registration Statement effective until all Registrable Securities covered by such Registration Statement are sold in accordance
with the intended plan of distribution set forth in such Registration Statement or supplement to the Prospectus or have ceased to be Registrable
Securities;
3.1.3 not later than five
(5) days prior to filing a Registration Statement or prospectus, or any amendment or supplement thereto, furnish without charge to the
Underwriters, if any, and the Holders of Registrable Securities included in such Registration, and such Holders’ legal counsel,
copies of such Registration Statement as proposed to be filed, each amendment and supplement to such Registration Statement (in each case
including all exhibits thereto and documents incorporated by reference therein), the Prospectus included in such Registration Statement
(including each preliminary Prospectus), and such other documents as the Underwriters and the Holders of Registrable Securities included
in such Registration or the legal counsel for any such Holders may reasonably request in order to facilitate the disposition of the Registrable
Securities owned by such holders, provided, that the Company shall have no obligation to furnish any documents publicly filed or furnished
with the Commission pursuant to the Electronic Data Gathering Analysis and Retrieval System (“EDGAR”);
3.1.4 prior to any public
offering of Registrable Securities, use its commercially reasonable efforts to (i) register or qualify the Registrable Securities covered
by the Registration Statement under such securities or “blue sky” laws of such jurisdictions in the United States as the Holders
of Registrable Securities included in such Registration Statement (in light of their intended plan of distribution) may request (or provide
evidence reasonably satisfactory to such Holders that the Registrable Securities are exempt from such registration or qualification) and
(ii) take such action necessary to cause such Registrable Securities covered by the Registration Statement to be registered with or approved
by such other governmental authorities as may be necessary by virtue of the business and operations of the Company and do any and all
other acts and things that may be necessary or advisable to enable the Holders of Registrable Securities included in such Registration
Statement to consummate the disposition of such Registrable Securities in such jurisdictions; provided, however, that the
Company shall not be required to qualify generally to do business in any jurisdiction where it would not otherwise be required to qualify
or take any action to which it would be subject to general service of process or taxation in any such jurisdiction where it is not then
otherwise so subject;
3.1.5 use its commercially
reasonable efforts to cause all such Registrable Securities included in any registration to be listed on such exchanges or otherwise designated
for trading in the same manner as similar securities issued by the Company are then listed or designated or, if no such similar securities
are then listed or designated, in a manner satisfactory to the holders of a majority-in-interest of the Registrable Securities included
in such registration;
3.1.6 provide a transfer
agent as applicable, and registrar for all such Registrable Securities no later than the effective date of such Registration Statement;
3.1.7 advise each seller
of such Registrable Securities, promptly after it shall receive notice or obtain knowledge thereof, of the issuance of any stop order
by the Commission suspending the effectiveness of such Registration Statement or the initiation or threatening of any proceeding for such
purpose and promptly use its commercially reasonable efforts to prevent the issuance of any stop order or to obtain its withdrawal if
such stop order should be issued;
3.1.8 notify the Holders
at any time when a Prospectus relating to such Registration Statement is required to be delivered under the Securities Act, of the happening
of any event as a result of which the Prospectus included in such Registration Statement, as then in effect, includes a Misstatement,
and then to correct such Misstatement as set forth in Section 3.4 hereof;
3.1.9 permit a representative
of the Holders (such representative to be selected by a majority of the participating Holders), the Underwriters, if any, and any attorney
or accountant retained by such Holders or Underwriter to participate, at each such person’s own expense, in the preparation of the
Registration Statement, and cause the Company’s officers, directors and employees to supply all information reasonably requested
by any such representative, Underwriter, attorney or accountant in connection with the Registration; provided, however,
that such representatives or Underwriters enter into a confidentiality agreement, in form and substance reasonably satisfactory to the
Company, prior to the release or disclosure of any such information;
3.1.10 obtain a “comfort”
letter from the Company’s independent registered public accountants in the event of an Underwritten Registration which the participating
Holders may rely on, in customary form and covering such matters of the type customarily covered by “comfort” letters for
transactions of its type as the managing Underwriter may reasonably request, and reasonably satisfactory to a majority-in-interest of
the participating Holders;
3.1.11 on the date the Registrable
Securities are delivered for sale pursuant to such Registration, to the extent customary for a transaction of its type, obtain an opinion,
dated such date, of counsel representing the Company for the purposes of such Registration, addressed to the Holders, the placement agent
or sales agent, if any, and the Underwriters, if any, covering such legal matters with respect to the Registration in respect of which
such opinion is being given as the Holders, placement agent, sales agent, or Underwriter may reasonably request and as are customarily
included in such opinions and negative assurance letters;
3.1.12 in the event of any
Underwritten Offering, enter into and perform its obligations under an underwriting agreement, in usual and customary form, with the managing
Underwriter of such offering;
3.1.13 make available to
its security holders, as soon as reasonably practicable, an earnings statement covering the period of at least twelve (12) months beginning
with the first day of the Company’s first full calendar quarter after the effective date of the Registration Statement which satisfies
the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder (or any successor rule promulgated thereafter by
the Commission), and which requirement will be deemed to be satisfied if the Company timely files complete and accurate information on
Forms 10-Q, 10-K or 8-K under the Exchange Act and otherwise complies with Rule 158 under the Securities Act;
3.1.14 if the Registration
involves the Registration of Registrable Securities involving gross proceeds in excess of $60,000,000, use its commercially reasonable
efforts to make available senior executives of the Company to participate in customary “road show” presentations that may
be reasonably requested by the Underwriter in such Underwritten Offering; and
3.1.15 otherwise, in good
faith, cooperate reasonably with, and take such customary actions as may reasonably be requested by the Holders, consistent with the terms
of this Agreement, in connection with such Registration.
Notwithstanding the foregoing,
the Company shall not be required to provide any documents or information to an Underwriter, broker, sales agent or placement agent if
such Underwriter, broker, sales agent or placement agent has not then been named with respect to the applicable Underwritten Offering
or other offering involving a Registration as an Underwriter, broker, sales agent or placement agent, as applicable.
3.2 Registration Expenses.
Except as otherwise provided herein, the Registration Expenses of all Registrations shall be borne by the Company. It is acknowledged
by the Holders that the Holders shall bear all incremental selling expenses relating to the sale of Registrable Securities, such as Underwriters’
commissions and discounts, brokerage fees, Underwriter marketing costs and, other than as set forth in the definition of “Registration
Expenses,” all reasonable fees and expenses of any legal counsel representing the Holders.
3.3 Requirements for Participation
in Underwritten Offerings. Notwithstanding anything in this Agreement to the contrary, if any Holder does not provide the Company
with its requested Holder Information, the Company may exclude such Holder’s Registrable Securities from the applicable Registration
Statement or Prospectus if the Company determines, based on the advice of counsel, that it is necessary or advisable to review such information
prior to filing, or include such information in, the applicable Registration Statement or Prospectus and such Holder continues thereafter
to withhold such information. In addition, no person may participate in any Underwritten Offering for equity securities of the Company
pursuant to a Registration initiated by the Company hereunder unless such person (i) agrees to sell such person’s securities on
the basis provided in any underwriting arrangements approved by the Company and (ii) completes and executes all customary questionnaires,
powers of attorney, indemnities, lock-up agreements, underwriting agreements and other customary documents as may be reasonably required
under the terms of such underwriting arrangements.
3.4 Suspension of Sales;
Adverse Disclosure. Upon receipt of written notice from the Company that a Registration Statement or Prospectus contains a Misstatement,
each of the Holders shall forthwith discontinue disposition of Registrable Securities until it has received copies of a supplemented or
amended Prospectus correcting the Misstatement (it being understood that the Company hereby covenants to prepare and file such supplement
or amendment as soon as reasonably practicable after the time of such notice), or until it is advised in writing by the Company that the
use of the Prospectus may be resumed. If the filing, initial effectiveness or continued use of a Registration Statement in respect of
any Registration at any time would (a) require the Company to make an Adverse Disclosure, (b) require the inclusion in such Registration
Statement of financial statements that are unavailable to the Company for reasons beyond the Company’s control, or (c) in the good
faith judgment of the majority of the Board such Registration, would be seriously detrimental to the Company and the majority of the Board
concludes as a result that it is essential to defer such filing, initial effectiveness or continued use at such time, the Company may,
upon giving prompt written notice of such action to the Holders, delay the filing or initial effectiveness of, or suspend use of, such
Registration Statement for the shortest period of time reasonably practicable, but in no event more than ninety (90) days, determined
in good faith by the Company to be necessary for such purpose. In the event the Company exercises its rights under the preceding sentence,
the Holders agree to suspend, immediately upon their receipt of the notice referred to above, their use of the Prospectus relating to
any Registration in connection with any sale or offer to sell Registrable Securities until such Holder receives written notice from the
Company that such sales or offers of Registrable Securities may be resumed, and in each case maintain the confidentiality of such notice
and its contents. The Company shall as promptly as reasonably practicable notify the Holders of the expiration of any period during which
it exercised its rights under this Section 3.4.
3.5 Reporting Obligations.
As long as any Holder shall own Registrable Securities, the Company, at all times while it shall be a reporting company under the Exchange
Act, covenants to file timely (or obtain extensions in respect thereof and file within the applicable grace period) all reports required
to be filed by the Company after the date hereof pursuant to Sections 13(a) or 15(d) of the Exchange Act and to promptly
furnish the Holders with true and complete copies of all such filings; provided that any documents publicly filed or furnished with the
Commission pursuant to EDGAR shall be deemed to have been furnished or delivered to the Holders pursuant to this Section 3.5. The
Company further covenants that it shall take such further action as any Holder may reasonably request, all to the extent required from
time to time to enable such Holder to sell shares of Common Stock held by such Holder without registration under the Securities Act within
the limitation of the exemptions provided by Rule 144 promulgated under the Securities Act (or any successor rule promulgated thereafter
by the Commission), including providing any legal opinions. Upon the request of any Holder, the Company shall deliver to such Holder a
written certification of a duly authorized officer as to whether it has complied with such requirements.
ARTICLE IV
LOCK-UP
4.1 Lock-up.
4.1.1 Except as permitted by Section 4.2,
the Legacy Citius Oncology Equityholder and each Sponsor Equityholder (each, a “Lock-up Party”) shall not Transfer any
shares of Common Stock or any security convertible into or exercisable or exchanged for Common Stock beneficially owned or owned of record
by such Holder (the “Lock-up”) until the date that is the earlier of (A) six (6) months after the date hereof
or (B) if the last sale price of the Common Stock equals or exceeds $12.00 per share (as adjusted for share splits, share capitalizations,
rights issuances, subdivisions, reorganizations, recapitalizations and the like) for any 20 trading days within any 30-trading day period
commencing at least 150 days after the date hereof, or (y) the date following the date hereof on which the Company completes a liquidation,
merger, share exchange, reorganization or other similar transaction that results in all of its stockholders having the right to exchange
their shares of Common Stock for cash, securities or other property (the “Lock-up Period”).
4.2 Exceptions. The provisions of
Section 4.1 shall not apply to:
4.2.1 transactions relating to shares of Common
Stock or warrants acquired in open market transactions after the date hereof;
4.2.2 Transfers of shares of Common Stock or any
security convertible into or exercisable or exchangeable for Common Stock as a bona fide gift or charitable contribution;
4.2.3 Transfers of shares of Common Stock or any
security convertible into or exercisable or exchangeable for Common Stock to a trust, family limited partnership or other entity formed
for estate planning purposes for the primary benefit of the spouse, domestic partner, parent, sibling, child or grandchild of a Holder
or any other person with whom a Holder has a relationship by blood, marriage or adoption not more remote than first cousin and Transfers
to any such family member;
4.2.4 Transfers of shares
of Common Stock or any security convertible into or exercisable or exchangeable for Common Stock by will or intestate succession or the
laws of descent and distributions upon the death of a Holder (it being understood and agreed that the appointment of one or more executors,
administrators or personal representatives of the estate of a Holder shall not be deemed a Transfer hereunder to the extent
that such executors, administrators and/or personal representatives comply with the terms of this Article IV on
behalf of such estate);
4.2.5 Transfers of shares of Common Stock or any
security convertible into or exercisable or exchangeable for Common Stock pursuant to a qualified domestic order or in connection with
a divorce settlement;
4.2.6 if a Holder is a corporation, partnership
(whether general, limited or otherwise), limited liability company, trust or other business entity, (i) Transfers of shares of Common
Stock or any security convertible into or exercisable or exchangeable for Common Stock to another corporation, partnership, limited liability
company, trust or other business entity that controls, is controlled by or is under common control or management with a Holder (including,
for the avoidance of doubt, where such Holder is a partnership, to its general partner or a successor partnership or fund, or any other
funds managed by such partnership), or (ii) Transfers of shares of Common Stock or any security convertible into or exercisable or exchangeable
for Common Stock as part of a dividend, distribution, transfer or other disposition of shares of Common Stock to partners, limited liability
company members, direct or indirect stockholders or other equity holders of a Holder, including, for the avoidance of doubt, where such
Holder is a partnership, to its general partner or a successor partnership, fund or investment vehicle, or any other partnerships, funds
or investment vehicles controlled or managed by such partnership;
4.2.7 if the Holder is a trust, Transfers of shares
of Common Stock or any security convertible into or exercisable or exchangeable for Common Stock to a trustor or beneficiary of such trust
or to the estate of a beneficiary of such trust;
4.2.8 Transfers of shares of Common Stock or any
security convertible into or exercisable or exchangeable for Common Stock to the Company’s or the Holder’s officers, directors,
members, consultants or their affiliates;
4.2.9 pledges of shares of Common Stock or any
security convertible into or exercisable or exchangeable for Common Stock as security or collateral in connection with any borrowing or
the incurrence of any indebtedness by any Holder (provided such borrowing or incurrence of indebtedness is secured by a portfolio of assets
or equity interests issued by multiple issuers);
4.2.10 Transfers of shares of Common Stock or
any security convertible into or exercisable or exchangeable for Common Stock pursuant to a bona fide third-party tender
offer, merger, asset acquisition, stock sale, recapitalization, consolidation, business combination or other transaction or series of
related transactions involving a Change in Control of the Company, provided that in the event that such tender offer, merger,
asset acquisition, stock sale, recapitalization, consolidation, business combination or other such transaction is not completed, the securities
subject to this Agreement shall remain subject to this Agreement;
4.2.11 Transfers of shares of Common Stock or
any security convertible into or exercisable or exchangeable for Common Stock to the Company in connection with the liquidation or dissolution
of the Company by virtue of the laws of the state of the Company’s organization and the Company’s organizational documents;
4.2.12 the establishment of a trading plan pursuant
to Rule 10b5-1 promulgated under the Exchange Act, provided that such plan does not provide for the Transfer of
any shares of Common Stock or any security convertible into or exercisable or exchangeable for Common Stock during the Lock-up Period;
and
4.2.13 Transfers of shares of Common Stock or
any security convertible into or exercisable or exchangeable for Common Stock to satisfy any U.S. federal, state, or local income tax
obligations of the Lock-up Party (or its direct or indirect owners) arising from a change in the U.S. Internal Revenue Code
of 1986, as amended (the “Code”), or the U.S. Treasury Regulations promulgated thereunder (the “Regulations”)
after the date on which the Merger Agreement was executed by the parties, and such change prevents the Merger from qualifying as a “reorganization”
pursuant to Section 368 of the Code (and the Merger does not qualify for similar tax-free treatment pursuant to any successor
or other provision of the Code or Regulations taking into account such changes), in each case solely and to the extent necessary to cover
any tax liability as a direct result of the transaction; and
4.2.14 to the extent a waiver from the Lock-up
is required in order for the Company to meet the applicable Nasdaq initial or continued listing rules with respect to the minimum number
of unrestricted round lot holders, as determined by the Company in good faith.
4.3 Null and Void. If any Transfer of
shares of Common Stock prior to the end of the Lock-up Period is made or attempted contrary to the provisions of this Agreement,
such purported Transfer shall be null and void ab initio, and the Company shall refuse to recognize any such purported
transferee as one of its equityholders for any purpose.
ARTICLE V
INDEMNIFICATION AND CONTRIBUTION
5.1 Indemnification.
5.1.1 The Company agrees
to indemnify, to the extent permitted by law, each Holder of Registrable Securities, its officers and directors and each person who controls
such Holder (within the meaning of the Securities Act) against all losses, claims, damages, liabilities and out-of-pocket expenses (including
actual, reasonable and documented attorneys’ fees) caused by any untrue or alleged untrue statement of material fact contained in
any Registration Statement, Prospectus or preliminary Prospectus or any amendment thereof or supplement thereto or any omission or alleged
omission of a material fact required to be stated therein or necessary to make the statements therein not misleading, except insofar as
the same are caused by or contained in any information furnished in writing to the Company by such Holder expressly for use therein. The
Company shall indemnify the Underwriters, their officers and directors and each person who controls such Underwriters (within the meaning
of the Securities Act) to the same extent as provided in the foregoing with respect to the indemnification of the Holder.
5.1.2 In connection with
any Registration Statement in which a Holder of Registrable Securities is participating, such Holder shall furnish to the Company in writing
such information and affidavits as the Company reasonably requests for use in connection with any such Registration Statement or Prospectus
(the “Holder Information”) and, to the extent permitted by law, shall indemnify the Company, its directors and
officers and agents and each person who controls the Company (within the meaning of the Securities Act) against any losses, claims, damages,
liabilities and out-of-pocket expenses (including without limitation actual, reasonable and documented attorneys’ fees) resulting
from any untrue or alleged untrue statement of material fact contained in the Registration Statement, Prospectus or preliminary Prospectus
or any amendment thereof or supplement thereto or any omission or alleged omission of a material fact required to be stated therein or
necessary to make the statements therein not misleading, but only to the extent that such untrue statement is contained in (or not contained
in, in the case of an omission) any information or affidavit so furnished in writing by such Holder expressly for use therein; provided,
however, that the obligation to indemnify shall be several, not joint and several, among such Holders of Registrable Securities,
and the liability of each such Holder of Registrable Securities shall be in proportion to and limited to the net proceeds received by
such Holder from the sale of Registrable Securities pursuant to such Registration Statement. The Holders of Registrable Securities shall
indemnify the Underwriters, their officers, directors and each person who controls such Underwriters (within the meaning of the Securities
Act) to the same extent as provided in the foregoing with respect to indemnification of the Company.
5.1.3 Any person entitled
to indemnification herein shall (i) give prompt written notice to the indemnifying party of any claim with respect to which it seeks indemnification
(provided that the failure to give prompt notice shall not impair any person’s right to indemnification hereunder to the extent
such failure has not materially prejudiced the indemnifying party) and (ii) unless in such indemnified party’s reasonable judgment
a conflict of interest between such indemnified and indemnifying parties may exist with respect to such claim, permit such indemnifying
party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party. If such defense is assumed, the
indemnifying party shall not be subject to any liability for any settlement made by the indemnified party without its consent (but such
consent shall not be unreasonably withheld). An indemnifying party who is not entitled to, or elects not to, assume the defense of a claim
shall not be obligated to pay the fees and expenses of more than one counsel for all parties indemnified by such indemnifying party with
respect to such claim, unless in the reasonable judgment of any indemnified party a conflict of interest may exist between such indemnified
party and any other of such indemnified parties with respect to such claim. No indemnifying party shall, without the consent of the indemnified
party, consent to the entry of any judgment or enter into any settlement which cannot be settled in all respects by the payment of money
(and such money is so paid by the indemnifying party pursuant to the terms of such settlement) or which settlement does not include as
an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect
to such claim or litigation.
5.1.4 The indemnification
provided for under this Agreement shall remain in full force and effect regardless of any investigation made by or on behalf of the indemnified
party or any officer, director or controlling person of such indemnified party and shall survive the transfer of securities. The Company
and each Holder of Registrable Securities participating in an offering also agrees to make such provisions as are reasonably requested
by any indemnified party for contribution to such party in the event the Company’s or such Holder’s indemnification is unavailable
for any reason.
5.1.5 If the indemnification
provided under Section 5.1 hereof from the indemnifying party is unavailable or insufficient to hold harmless an indemnified party
in respect of any losses, claims, damages, liabilities and out-of-pocket expenses referred to herein, then the indemnifying party, in
lieu of indemnifying the indemnified party, shall contribute to the amount paid or payable by the indemnified party as a result of such
losses, claims, damages, liabilities and out-of-pocket expenses in such proportion as is appropriate to reflect the relative fault of
the indemnifying party and the indemnified party, as well as any other relevant equitable considerations. The relative fault of the indemnifying
party and indemnified party shall be determined by reference to, among other things, whether any action in question, including any untrue
or alleged untrue statement of a material fact or omission or alleged omission to state a material fact, was made by (or not made by,
in the case of an omission), or relates to information supplied by (or not supplied by, in the case of an omission), such indemnifying
party or indemnified party, and the indemnifying party’s and indemnified party’s relative intent, knowledge, access to information
and opportunity to correct or prevent such action; provided, however, that the liability of any Holder under this subsection
5.1.5 shall be limited to the amount of the net proceeds received by such Holder in such offering giving rise to such liability. The
amount paid or payable by a party as a result of the losses or other liabilities referred to above shall be deemed to include, subject
to the limitations set forth in subsections 5.1.1, 5.1.2 and 5.1.3 above, any legal or other fees, charges or expenses
reasonably incurred by such party in connection with any investigation or proceeding. The parties hereto agree that it would not be just
and equitable if contribution pursuant to this subsection 5.1.5 were determined by pro rata allocation or by any other method of
allocation, which does not take account of the equitable considerations referred to in this subsection 5.1.5. No person guilty
of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution pursuant
to this subsection 5.1.5 from any person who was not guilty of such fraudulent misrepresentation.
ARTICLE VI
MISCELLANEOUS
6.1 Notices. Any notice
or communication under this Agreement must be in writing and given by (i) deposit in the United States mail, addressed to the party to
be notified, postage prepaid and registered or certified with return receipt requested, (ii) delivery in person or by courier service
providing evidence of delivery, or (iii) transmission by hand delivery, electronic mail, telecopy, telegram or facsimile. Each notice
or communication that is mailed, delivered, or transmitted in the manner described above shall be deemed sufficiently given, served, sent,
and received, in the case of mailed notices, on the third business day following the date on which it is mailed and, in the case of notices
delivered by courier service, hand delivery, electronic mail, telecopy, telegram or facsimile, at such time as it is delivered to the
addressee or at such time as delivery is refused by the addressee upon presentation. Any notice or communication under this Agreement
must be addressed, if to the Company, to: 11 Commerce Drive, First Floor Cranford, NJ 07016, and, if to any Holder, at such Holder’s
address or facsimile number as set forth in the Company’s books and records. Any party may change its address for notice at any
time and from time to time by written notice to the other parties hereto, and such change of address shall become effective thirty (30)
days after delivery of such notice as provided in this Section 6.1.
6.2 Assignment; No Third
Party Beneficiaries.
6.2.1 This Agreement and
the rights, duties and obligations of the Company hereunder may not be assigned or delegated by the Company in whole or in part.
6.2.2 Prior to the expiration
of the Lock-up Period, no Holder may assign or delegate such Holder’s rights, duties or obligations under this Agreement, in whole
or in part, except in connection with a transfer of Registrable Securities by such Holder to a Permitted Transferee but only if such Permitted
Transferee agrees to become bound by the transfer restrictions set forth in this Agreement.
6.2.3 This Agreement and
the provisions hereof shall be binding upon and shall inure to the benefit of each of the parties and its successors and the permitted
assigns of the Holders, which shall include Permitted Transferees.
6.2.4 This Agreement shall
not confer any rights or benefits on any persons that are not parties hereto, other than as expressly set forth in this Agreement and
Section 6.2 hereof.
6.2.5 No assignment by any
party hereto of such party’s rights, duties and obligations hereunder shall be binding upon or obligate the Company unless and until
the Company shall have received (i) written notice of such assignment as provided in Section 6.1 hereof and (ii) the written agreement
of the assignee, in a form reasonably satisfactory to the Company, to be bound by the terms and provisions of this Agreement (which may
be accomplished by an addendum or certificate of joinder to this Agreement). Any transfer or assignment made other than as provided in
this Section 6.2 shall be null and void.
6.3 Counterparts.
This Agreement may be executed in multiple counterparts (including facsimile or PDF counterparts), each of which shall be deemed an original,
and all of which together shall constitute the same instrument, but only one of which need be produced.
6.4 Governing Law; Venue.
NOTWITHSTANDING THE PLACE WHERE THIS AGREEMENT MAY BE EXECUTED BY ANY OF THE PARTIES HERETO, THE PARTIES EXPRESSLY AGREE THAT (I) THIS
AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED UNDER THE LAWS OF THE STATE OF DELAWARE AS APPLIED TO AGREEMENTS AMONG DELAWARE RESIDENTS
ENTERED INTO AND TO BE PERFORMED ENTIRELY WITHIN DELAWARE, WITHOUT REGARD TO THE CONFLICT OF LAW PROVISIONS OF SUCH JURISDICTION AND (II)
THE VENUE FOR ANY ACTION TAKEN WITH RESPECT TO THIS AGREEMENT SHALL BE the Court of Chancery of
the State of Delaware or, if under applicable Law, exclusive jurisdiction over such matter is vested in the federal courts, any federal
court in the State of Delaware and any appellate court from any thereof.
6.5 Amendments and Modifications.
Upon the written consent of the Company and the Holders of at least a majority in interest of the Registrable Securities at the time in
question, compliance with any of the provisions, covenants and conditions set forth in this Agreement may be waived, or any of such provisions,
covenants or conditions may be amended or modified; provided, however, that notwithstanding the foregoing, any amendment
hereto or waiver hereof that adversely affects one Holder, solely in its capacity as a holder of the shares of the Company, in a manner
that is materially different from the other Holders (in such capacity) shall require the consent of the Holder so affected. No course
of dealing between any Holder or the Company and any other party hereto or any failure or delay on the part of a Holder or the Company
in exercising any rights or remedies under this Agreement shall operate as a waiver of any rights or remedies of any Holder or the Company.
No single or partial exercise of any rights or remedies under this Agreement by a party shall operate as a waiver or preclude the exercise
of any other rights or remedies hereunder or thereunder by such party.
6.6 Other Registration
Rights. The Company represents and warrants that no person, other than a Holder of Registrable Securities, has any right to require
the Company to register any securities of the Company for sale or to include such securities of the Company in any Registration filed
by the Company for the sale of securities for its own account or for the account of any other person. Further, the Company represents
and warrants that this Agreement supersedes any other registration rights agreement or agreement with similar terms and conditions and
in the event of a conflict between any such agreement or agreements and this Agreement, the terms of this Agreement shall prevail.
6.7 Term. This Agreement
shall terminate upon the earlier of (i) the fifth (5th) anniversary of the date of this Agreement or (ii) the date as of which
(A) all of the Registrable Securities have been sold pursuant to a Registration Statement (but in no event prior to the applicable period
referred to in Section 4(a)(3) of the Securities Act and Rule 174 thereunder (or any successor rule promulgated thereafter by the Commission))
or (B) the Holders of all Registrable Securities are permitted to sell the Registrable Securities under Rule 144 (or any similar provision)
under the Securities Act without limitation on the amount of securities sold or the manner of sale. The provisions of Section 3.5
and Article V shall survive any termination.
[SIGNATURE PAGES FOLLOW]
IN WITNESS WHEREOF,
the undersigned have caused this Agreement to be executed as of the date first written above.
|
COMPANY: |
| |
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
| |
|
|
| SPONSOR EQUITYHOLDERS: |
| |
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
| |
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
| |
|
|
| LEGACY CITIUS ONCOLOGY EQUITYHOLDER: |
| |
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
[Signature Page to Registration Rights Agreement]
Exhibit 10.3
FORM OF
AMENDED AND
RESTATED SHARED SERVICES AGREEMENT
THIS
AMENDED AND RESTATED SHARED SERVICES AGREEMENT (this “Agreement”) is made
as of [_____] [___], 2023, by and between Citius Oncology, Inc.,1
a Delaware corporation (the “Company”), and Citius Pharmaceuticals, Inc, a Delaware corporation (“Citius”)
(the Company and Citius may be referred to herein individually as a “Party” or collectively as the “Parties”).
WHEREAS, prior to the effectiveness of the Merger (as defined below) Citius owned 100% of the outstanding equity of the Company;
WHEREAS,
the Parties have entered into an Agreement and Plan of Merger and Reorganization by and among Citius, the Company, TenX Keane Acquisition
(“TenX”), and TENX MERGER SUB, INC. (“Merger Sub”) dated as of [_____], 2023 (the “Merger
Agreement”), pursuant to which Merger Sub will merge with and into the Company, with the Company surviving the merger as the
surviving corporation (the “Merger”);
WHEREAS,
as of time the Merger becomes effective pursuant to the Merger Agreement (the “Effective Time”), Citius will own at
least 80% of the outstanding equity of TenX (which will be renamed Citius Oncology, Inc.);
WHEREAS,
the Parties are entering into this Agreement concurrently with the closing of the Merger;
WHEREAS,
in connection with the closing of the Merger, the Company will be renamed [_____];
WHEREAS,
the Company does not have any employees, office space or operations of its own and does not expect to for the foreseeable future;
WHEREAS,
on the terms and subject to the conditions contained in this Agreement, the Company desires to obtain certain management and scientific
services from Citius, and Citius has agreed to perform such management and scientific services;
WHEREAS,
the Company has requested, and Citius has agreed, for Citius to provide administrative and scientific services to the Company, pursuant
to the terms of this Agreement; and
| 1 | Note to Draft: Name of parties to
be updated depending on timing of name changes. If necessary, recitals to be updated to reflect
the timing of name changes. |
WHEREAS,
this Agreement has been approved by the Company’s Board of Directors and by Citius’s Board of Directors.
NOW,
THEREFORE, in consideration of the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency
of which are hereby acknowledged, the Parties hereto agree as follows:
1. Effectiveness.
Effective as of the Effective Time, this Agreement hereby amends, restates, and replaces in its entirety the Shared Services
Agreement between Citius and the Company dated as of April 1, 2022 (the “Original Agreement”).
2.
Management and Scientific Services.
2.1 Services.
Subject to any limitations imposed by applicable law or regulation, Citius shall render or cause to be rendered management and
scientific services to the Company, which services may include advice and assistance concerning any and all aspects of the
operations, executive management, pre-clinical and clinical trials, regulatory development, manufacturing and the regulatory
approval process, accounting, financial planning and strategic transactions and financings of the Company and conducting relations
on behalf of the Company with accountants, attorneys, financial advisors and other professionals (collectively, the
“Services”). Exhibit A sets forth the services Citius will provide to the Company as of the Effective time, which
services are substantially similar to the services provided or procured by Citius and used in the operation of the business of the
Company immediately prior to the Effective time. Citius shall provide and devote to the performance of this Agreement such employees
and agents of Citius as Citius shall deem appropriate to the furnishing of the Services hereunder.
Citius
shall devote such time and efforts to the performance of Services contemplated hereby as Citius deems reasonably necessary or appropriate;
provided, however, that no minimum number of hours is required to be devoted by Citius on a weekly, monthly, annual or other
basis. The Company acknowledges that Citius’s Services are not exclusive to the Company and that Citius may render similar Services
to other persons and entities.
Citius
covenants that the Services will be performed in a diligent, timely, efficient, workmanlike and commercially reasonable manner and in
a fashion designed to support the business of the Company in substantially the same manner and to substantially the same standard as the
business is conducted prior to the Effective Time, and, with respect to specific Services, in accordance with the standards, if any, set
forth on Exhibit A with respect to such Services or as otherwise agreed. In performing the Services, Citius agrees that part of
its responsibilities shall be to review, to the extent applicable, the regulations and laws applicable to the Services to be provided
hereunder, and to reflect any such regulations, laws or requirements in the performance of Services to the Company.
Citius
may change operational aspects of the Services or the way in which they are provided, or substitute them with other services, so long
as the Services are provided or procured to substantially the same standards as they were provided immediately prior to the Effective
Time. If changes or substitutions are made, Citius shall use reasonable efforts so that:
| (i) | the Services are not disrupted; and |
| (ii) | the change or substitution does not result in an increase in the Service Fees, unless the Company has
agreed to the increase in advance. |
2.2
Freedom to Pursue Opportunities and Limitation on Liability.
2.2.1 Freedom
to Pursue Opportunities. In recognition that the Company and Citius and its wholly owned and partly owned subsidiaries engage
and may in the same or similar activities or lines of business and have an interest in the same general areas of corporate
opportunities, and in recognition of the benefits to be derived by the Company hereunder, each of the Company and Citius wish to
guide the conduct of certain affairs of the Company as they may involve Citius.
Except
as Citius may otherwise agree in writing after the date hereof:
(i) Citius will have the right: (A) to directly or indirectly engage in any business including, without limitation, any business activities or lines of business that are the same as the Company’s or its affiliates or similar to those pursued by the Company or its affiliates: or (B) to directly or indirectly do business with any client or customer of the Company or its affiliates: provided that Citius does not compete with the Company in the area of developing treatments for cutaneous T-cell lymphoma or peripheral T-cell lymphoma, immuno-oncology treatments and other oncology treatments
(ii)
Citius will have no duty (contractual or otherwise) to communicate or present any corporate opportunities to the Company that do not
violate this Section 2.2.1.
(iii)
Neither Citius nor any officer, director, employee, partner, member, stockholder, affiliate or associated entity thereof will be
liable to the Company or its affiliates for breach of any duty (contractual or otherwise) by reason of any activities or omissions
of the types referred to in this Section 2.2.1 or of any such person’s participation therein.
3. Term.
Citius shall provide the Services set forth in Section 2 above from the Effective Time until the earlier of (a) termination
of this Agreement by mutual agreement of Citius and the Company and (b) the 2nd anniversary of this Agreement; provided
that this Agreement shall be automatically extended for additional one-year periods unless Citius or the Company provides written
notice of its desire not to automatically extend the term of this Agreement to the other Parties hereto at least thirty (30) days
prior to such date (such period, the “Term”).
No
termination of this Agreement, whether pursuant to this Section 3 or otherwise, will affect the Company’s duty to pay any
Service Fee (as defined herein in Section 4) accrued, or to reimburse any cost or expense incurred pursuant to Section 5
hereof, prior to the effective date of such termination. Upon termination of this Agreement, Citius’s right to receive any further
Service Fee or reimbursement for costs and expenses that have not accrued or been incurred to the date of termination shall cease and
terminate. Additionally, the obligations of the Company under Section 5 (Expenses), Section 7 (Indemnification), the provisions
of Section 2.2.2 above (whether in respect of or relating to Services rendered prior to termination of this Agreement or in respect
of or relating to any Services provided after termination of this Agreement), the provisions of Section 9 (Confidentiality) (for
the term provided therein) and the provisions of Section 21 (Governing Law) will also survive any termination of this Agreement
to the maximum extent permitted under applicable law.
4.
Compensation.
4.1
The Company and Citius agree that the compensation set forth in this Agreement is being paid to Citius in consideration of the
Services provided and the substantial commitment and effort made by Citius hereunder, and that such fees have been negotiated at
arms’ length and are fair, reasonable and consistent with fair market value. Citius shall be paid a quarterly fee (the
“Service Fee”) as set forth on Exhibit A hereto, which may be amended from time to time upon mutual
agreement of the Parties.
4.2
Unless otherwise agreed by the Parties in writing, any payment pursuant to this Section 4 shall be made in cash by wire
transfer(s) of immediately available funds to or among one or more accounts as designated from time-to-time by Citius to the Company
in writing. [Citius’s Service Fee will be invoiced quarterly, in arrears, and the Company
shall pay all invoices within thirty (30) days of the date of such invoice.]2
4.3
It is hereby acknowledged that the Company shall have no payment obligations hereunder other than as set forth in this Section
4 and in Section 5 for the Services to be performed hereunder (including, without limitation, the Services as set forth
on Exhibit A attached hereto), and the Company’s indemnity obligations to Citius under Section 7.
4.4
In the event any Service is terminated other than at the end of a payment period under the applicable underlying arrangement, such
period’s associated costs shall be pro-rated based on the number of days in such period prior to the termination date.
5. Expenses.
Actual and direct out-of-pocket expenses reasonably incurred by Citius and its personnel in performing the Services shall be
reimbursed to Citius by the Company upon the delivery to the Company of an invoice, receipt or such other supporting data as the
Company reasonably shall require. Unless otherwise agreed by the Parties in writing, the Company shall reimburse Citius by wire
transfer of immediately available funds for any amount paid by Citius, which shall be in addition to any other amount payable to
Citius under this Agreement.
6. Independent
Contractor. Citius shall act solely as an independent contractor and shall have complete charge of its respective personnel engaged
in the performance of the Services under this Agreement. Neither Citius nor its officers, employees or agents will be considered employees
or agents of the Company as a result of this Agreement. As an independent contractor, Citius shall have authority only to act as an advisor
to the Company and shall have no authority to enter into any agreement or to make any representation, commitment or warranty binding upon
the Company or to obtain or incur any right, obligation or liability on behalf of the Company. Nothing contained in this Agreement shall
result in Citius or any of its affiliates or their respective directors, officers, employees or agents being a partner, joint venturer,
principal, agent, fiduciary or beneficiary of the Company.
| 2 | Note to Draft: Suggest clarifying
invoicing policy. |
7. Indemnification.
7.1 Indemnification.
Subject to the applicable limitations set forth in this Section 7, the Company shall indemnify Citius, its affiliates (other than
the Company or TenX) and their respective directors, officers, employees and agents (collectively, the “Citius Indemnified Party”),
to the fullest extent permitted by law, from and against any and all actions, causes of action, suits, claims, liabilities, losses, damages
and costs and expenses in connection therewith, including without limitation reasonable attorneys’ fees and expenses (“Indemnified
Liabilities”) to which the Citius Indemnified Party may become subject, directly or indirectly caused by, related to or arising
out of the Services or any other advice or Services contemplated by this Agreement or the engagement of Citius pursuant to, and the performance
by Citius of the Services contemplated by, this Agreement.
7.2 Subject
to the applicable limitations set forth in this Section 7, Citius shall indemnify the Company, its affiliates (other than
Citius) and their respective directors, officers and employees (collectively, the “Company Indemnified Party”), harmless
from and against any and all Indemnified Liabilities to which the Company Indemnified Party may become subject, directly or indirectly
caused by, related to or arising out based upon or related to the Services performed for the Company hereunder to the extent that any
such Indemnified Liabilities were the result of, arise out of, or are based upon, (i) a breach of the provisions of this Agreement caused
by the gross negligence or intentional misconduct of Citius, or (ii) any action or inaction of Citius, its affiliates or its third party
contractors providing the Services, or their respective directors, officers, employees, contractors or agents at the request, at the direction,
or with the consent, of the Company or any of its directors, officers, employees, representatives or agents.
7.3 An
indemnifying party shall promptly reimburse the respective Indemnified Party for Indemnified Liabilities as incurred, in connection with
the investigation of, preparation for or defense of any pending or threatened claim or any action or proceeding arising therefrom, whether
or not such Indemnified Party is a party and whether or not such claim, action or proceeding is initiated or brought by or on behalf of
the Company or Citius and whether or not resulting in any liability. If and to the extent that the foregoing undertaking may be unenforceable
for any reason, the Parties hereby agree to make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities
that is permissible under applicable law.
7.4 Limited
Liability. In no event shall either Party be liable under any provision of this Agreement to each or each other’s respective
affiliates, directors, officers, employees or agents for indirect, special, incidental, consequential (including, without limitation,
lost profits or savings, whether or not such damages are foreseeable) or punitive damages; provided, however, that this
limitation shall not apply to any indirect, incidental, consequential (including lost profits) or punitive damages asserted or awarded
to any third party for which Citius would otherwise be responsible under Section 7.2; provided, further, however,
that Citius’s aggregate liability for all claims brought by the Company hereunder shall be limited to the total fees paid to Citius
for the Services provided to the Company through the date of any claim, whether or not such claim arose under this Agreement or the Original
Agreement.
7.5 An
indemnifying party shall not be liable under the indemnification contained in Section 7.1 or 7.2, as applicable, hereof
with respect to an Indemnified Party to the extent that such Indemnified Liabilities are found in a final non-appealable judgment by a
court of competent jurisdiction to have resulted directly from the Indemnified Party’s willful misconduct or gross negligence. The
Parties further agree that no Indemnified Party shall have any liability (whether direct or indirect, in contract, tort or otherwise)
to such Indemnified Party’s securities holders or creditors related to or arising out of the engagement of Citius pursuant to, or
the performance by Citius of the Services contemplated by, this Agreement.
8. Employee
Matters. Citius shall at all times remain the employer of all of its employees performing the Services and Citius shall perform
all of the responsibilities of an employer under applicable federal, state, and local laws and regulations. Citius, as relates to
its employees performing Services, shall be responsible for: (i) selecting and hiring its employees legally, including compliance
with all applicable laws in connection therewith; (ii) the supervision, direction and control of its employees performing Services;
(iii) paying its employees’ wages and other benefits in accordance with applicable laws; (iv) paying or withholding all
required payroll taxes and mandated insurance premiums; (v) providing worker’s compensation coverage for employees as required
by law; (vi) fulfilling the employer’s obligations with respect to unemployment compensation; and (vii) any and all claims of
its employees and other personnel arising out of this Agreement or performance of the Services. Citius shall indemnify, defend and
hold harmless the Company from any third-party claim resulting from a breach by Citius of the foregoing obligations, including,
without limitation, any claims made by Citius’s personnel against the Company alleging rights or benefits as an employee of
the Company.
9. Confidentiality. Citius
or the Company (the “Disclosing Party”) may provide the other party (the “Receiving Party”)
with certain confidential and proprietary information (“Confidential Information”). Confidential Information of a
Party hereto includes, but is not limited to, its product specifications and technical data, product designs/ideas, market/sales
forecasts and information, proprietary materials, suppliers, tooling, all business trade secrets, financial and accounting data,
customers and prospective customers, pricing information, and know-how. However, “Confidential Information” does not
include information that (i) is publicly known at the time of its disclosure or becomes publicly known thereafter through no fault
of the Receiving Party, (ii) is lawfully received by the Receiving Party from a third party not under an obligation of
confidentiality to the Disclosing Party, (iii) is published or otherwise made known to the public by the Disclosing Party, or (iv)
was generated independently by the Receiving Party without the use of the Confidential Information provided by the Disclosing
Party.
The
Receiving Party will refrain from using the Disclosing Party’s Confidential Information for any purpose other than in connection
with providing the Services contemplated by this Agreement. The Receiving Party may only disclose the Disclosing Party’s Confidential
Information to the Receiving Party’s officers, directors, key employees, and financial and legal advisors (collectively, “Representatives”)
who have the need to know such Confidential Information in order for the Receiving Party to perform its obligations under this Agreement.
Such Representatives will be informed of and will agree to be bound by the provisions of this Section (or other terms and conditions that
are no less protective of the Discloser’s Confidential Information than the terms herein), and the Receiving Party will remain responsible
for any unauthorized use or disclosure of the Disclosing Party’s Confidential Information by its Representatives. The Receiving
Party may also disclose the Disclosing Party’s Confidential Information pursuant to the requirement or request of a governmental
agency, a court or administrative subpoena, or an order or other legal process or requirement of law so long as it shall (i) first notify
the Disclosing Party of such request or requirement, (ii) in the case of a required disclosure, furnish only such portion of the Disclosing
Party’s Confidential Information as it is advised in writing by counsel that it is legally required to disclose, and (iii) cooperate
with the Disclosing Party in its efforts to obtain an order or other reliable assurance that confidential treatment will be accorded to
that portion of the Disclosing Party’s Confidential Information that is required to be disclosed.
Within
seven (7) days after the termination or expiration of this Agreement or after written request of the Disclosing Party, the Receiving Party
shall promptly (i) return or destroy all Confidential Information of the Disclosing Party and all copies thereof, (ii) destroy all of
its files and memoranda prepared based on the Disclosing Party’s Confidential Information, and (iii) provide the Disclosing Party
with a written certification that all such information and materials have been returned or destroyed. Notwithstanding the foregoing, the
Receiving Party may retain archival copies of the Disclosing Party’s Confidential Information in accordance with policies and procedures
designed to comply with legal, regulatory, and professional requirements, solely to demonstrate compliance therewith. This Section
9 shall survive the expiration or termination of this Agreement for a period of three (3) years; provided, however,
that for any Confidential Information that constitutes a trade secret (as defined by applicable law), the obligations of this Section
9 shall survive until such Confidential Information is no longer a trade secret.
10. Intellectual
Property.
10.1 Intellectual
Property. Except as expressly set forth in Section 10.2, solely as between the Parties, all right, title and interest in and
to all intellectual property, including, without limitation, each and every invention, discovery, design, drawing, protocol, process,
technique, formula, trade secret, device, compound, substance, material, pharmaceutical, method, software program (including, without
limitation, object code, source code, flow charts, algorithms and related documentation), listing, routine, manual and specification,
whether or not patentable or copyrightable, that are made, developed, perfected, designed, conceived or first reduced to practice, either
solely or jointly with others in the performance of the Services conducted under this Agreement or the Original Agreement by Citius’s
employees, agents, consultants, subcontractors or other representatives, but specifically excluding Citius Background Technology (as defined
below), (collectively “Work Product”), will be owned solely by the Company, and Citius hereby assigns to the Company
any and all rights that Citius may have in the Work Product. Citius represents and warrants to the Company that each employee, agent,
consultant and subcontractor of Citius providing Services hereunder is obligated to assign all of his/her/its right, title and interest
in and to Work Product to Citius. Citius and all employees, agents, consultants and subcontractors of Citius shall execute and deliver
to the Company all writings and do all such things as may be necessary or appropriate to vest in the Company all right, title and interest
in and to Work Product. Citius shall disclose to the Company in a timely manner any Work Product arising under this Agreement or the Original
Agreement. Excluding Citius’s Background Technology (as defined in Section 10.2), the Company may, in its sole discretion,
file and prosecute in its own name and at its own expense, patent applications on any patentable inventions within the Work Product. Upon
the request of the Company, and at the Company’s expense, Citius will assist the Company in the preparation, filing and prosecution
of such patent applications and will execute and deliver any and all instruments necessary to effectuate the ownership of such patent
applications and to enable the Company to file and prosecute such patent applications in any country.
10.2 Citius
Background Technology. Notwithstanding anything to the contrary contained in this Agreement or the Original Agreement, Work Product
shall exclude (a) any Citius proprietary technology existing prior to April 1, 2022 or that is developed or acquired by Citius independent
of the Services performed pursuant to this Agreement or the Original Agreement and (b) any modifications, enhancements or improvements
to any of the foregoing that are or were developed by Citius in the course of performing the Services (collectively, “Citius
Background Technology”), and, as between the Parties, all Citius Background Technology shall be and remain the sole and exclusive
property of Citius. Citius hereby grants the Company a non-exclusive, perpetual, irrevocable, worldwide, royalty-free license, including
the right to sublicense through multiple tiers of sublicense, to use the Citius Background Technology that is embodied within the Work
Product solely if and to the extent necessary for the exploitation of the Work Product. For clarity, Work Product shall not include any
industry know-how that is created by Citius hereunder which is broadly applicable to the businesses of both Citius and the Company at
the time of creation, such as processes, techniques and methods.
10.3
Force Majeure. Neither Party shall be liable or responsible to the other Party, nor be deemed
to have defaulted under or breached this Agreement, for any failure or delay in fulfilling or performing any term of this Agreement (except
for any obligations to make payments to the other Party hereunder), when and to the extent such failure or delay is caused by or results
from acts beyond the impacted Party’s (“Impacted Party”) reasonable
control, including, without limitation, the following force majeure event (“Force Majeure”): (a) acts of God; (b)
flood, fire, earthquake, other potential disasters or catastrophes, such as epidemics, pandemics, or quarantines, or explosion; (c) war,
invasion, hostilities (whether war is declared or not), terrorist threats or acts, riot, or other civil unrest; (d) government order,
law, or actions; (e) embargoes, or blockades in effect on or after the date of this Agreement; (f) national or regional emergency; (g)
strikes, labor stoppages or slowdowns, or other industrial disturbances; (h) shortage of adequate power or transportation facilities;
and (i) any other similar events or circumstances beyond the reasonable control of the Impacted Party. Notwithstanding the foregoing,
the Impacted Party will use good faith efforts to complete performance or correct any default or breach upon removal of any Force Majeure
event that caused such delay in performance, default or breach.
11. Reports
and Records. Citius shall, upon request from the Company, and within a reasonable amount of time, provide the Company with a detailed
report of Services performed on its behalf. Citius shall, upon request from the Company, and within a reasonable amount of time, provide
the Company with copies of documents relevant to this Agreement and the Services and reasonably required or requested by the Company,
including without limitation books, records and accounts. The Parties shall maintain records of all costs and expenses incurred and shared
pursuant to this Agreement in a manner that satisfies the record keeping requirements of federal income tax regulations and generally
accepted accounting principles.
12. Non-solicitation.
The Parties agree that during the term of this Agreement and for a period of twenty-four (24) months following the termination or expiration
of this Agreement, neither Party shall directly or indirectly solicit, hire, recruit, or attempt to do so, any of the employees of the
other Party without written consent of the other Party; provided, however, this Section 13 shall not preclude either
Party from (A) making general or public solicitations not targeted at any employees of the other Party or (B) hiring any such employee
of the other Party who has ceased being an employee, consultant or independent contractor for at least twelve (12) months.
13. Covenants.
Each Party represents, warrants and covenants to the other Party that: (i) such Party has the full power and authority to enter into this
Agreement and to perform its obligations hereunder, without the need for any consents; and (ii) such Party’s execution of and performance
under this Agreement shall not breach any oral or written agreement with any third party or any obligation owed by the Party to any third
party.
14. Notices.
All notices, demands, or other communications to be given or delivered by reason of the provisions of this Agreement shall be in writing
and shall be deemed to have been given or made when (i) delivered personally to the recipient, (ii) telecopied to the recipient (with
a hard copy sent to the recipient by reputable overnight courier service (charges prepaid)) if telecopied before 5:00 p.m. Eastern Standard
Time on a business day, and otherwise on the next business day, (iii) one (1) business day after being sent to the recipient by reputable
overnight courier service (charges prepaid) or (iv) received via electronic mail by the recipient if received via electronic mail before
5:00 p.m. Eastern Standard Time on a business day, and otherwise on the next business day after such receipt. Such notices, demands and
other communications shall be sent to the address for such recipient indicated below or to such other address or to the attention of such
other person as the recipient party has specified by prior written notice to the sending party.
Notices
to Citius
11Commerce
Drive, 1st Floor
Cranford,
NJ 07016
Attn:
Leonard Mazur - CEO
lmazur@citiuspharma.com
Notices
to the Company:
11
Commerce Drive, 1st Floor
Cranford,
NJ 07016
Attn:
Jaime Bartushak - CFO
jbartushak@citiuspharma.com
15.
Severability. If any term, provision, covenant or restriction of this Agreement is held by
a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants
and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and
the Parties hereto shall use their best efforts to find and employ an alternative means to achieve the same or substantially the same
result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention
of the Parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any such terms,
provisions, covenants and restrictions which may be hereafter declared invalid, illegal, void or unenforceable.
16. Entire
Agreement. This Agreement and the Merger Agreement contain the entire understanding of the Parties with respect to the subject matter
hereof and supersede any prior communication or agreement with respect thereto.
17. Counterparts.
This Agreement may be executed in multiple counterparts, and any Party may execute any such counterpart, each of which when executed and
delivered will thereby be deemed to be an original and all of which counterparts taken together will constitute one and the same instrument.
The delivery of this Agreement may be effected by means of an exchange of facsimile or portable document format (.pdf) signatures.
18. Amendments
and Waiver. No amendment or waiver of any term, provision or condition of this Agreement will be effective, unless in writing and
executed by both the Company and Citius. No waiver on any one occasion will extend to, effect or be construed as a waiver of any right
or remedy on any future occasion. No course of dealing of any person nor any delay or omission in exercising any right or remedy will
constitute an amendment of this Agreement or a waiver of any right or remedy of any Party hereto.
19. Successors
and Assigns. All covenants and agreements contained in this Agreement by or on behalf of any of the Parties hereto will bind and inure
to the benefit of the respective successors and permitted assigns of the Parties hereto whether so expressed or not. Neither the Company
nor Citius may assign its rights or delegate its obligations hereunder without the prior written consent of the other Party, which consent
shall not be unreasonably withheld.
20. Governing
Law. This Agreement shall be governed by and construed in accordance with the substantive laws of the State of Delaware, without giving
effect to any choice of law or conflict of law provision or rule that would cause the application of the laws of any
jurisdiction other than the State of Delaware.
21. Waiver
of Jury Trial. To the extent not prohibited by applicable law which cannot be waived, each of the Parties hereto hereby waives, and
covenants that it will not assert (whether as plaintiff, defendant or otherwise), any right to trial by jury in any forum in respect of
any issue, claim, demand, cause of action, action, suit or proceeding arising out of or based upon this Agreement or the subject matter
hereof, in each case whether now existing or hereafter arising and whether in contract or tort or otherwise. Any of the Parties hereto
may file an original counterpart or a copy of this Agreement with any court as written evidence of the consent of each of the Parties
hereto to the waiver of its right to trial by jury.
22. Third
Party Beneficiaries. This Agreement is not intended to confer on any person or entity except
the Parties any rights or remedies hereunder.
23. No
Strict Construction. The Parties hereto have participated jointly in the negotiation and drafting of this Agreement. In the event
an ambiguity or question of intent or interpretation arises, this Agreement will be construed as if drafted jointly by the Parties hereto,
and no presumption or burden of proof will arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions
of this Agreement.
24. Headings:
Interpretation. The headings in this Agreement are for convenience and reference only and shall not limit or otherwise affect the
meaning hereof. The use of the word “including” in this Agreement will be by way of example rather than by limitation.
*
* * * * *
IN
WITNESS WHEREOF, the Parties hereto have executed this Amended and Restated Shared Services Agreement as of the date first written above.
| |
CITIUS ONCOLOGY, INC. |
| |
|
| |
By: |
|
| |
Name: |
[____________] |
| |
Title: |
[____________] |
| |
|
|
| |
CITIUS PHARMACEUTICALS, INC. |
| |
|
| |
By: |
|
| |
Name: |
[____________] |
| |
Title: |
[____________] |
[Signature
Page to Amended and Restated Shared Services Agreement]
EXHIBIT A
COMPENSATION
Exhibit 99.1

Citius Pharmaceuticals Executes Definitive
Agreement to
Merge Wholly Owned Subsidiary with TenX Keane Acquisition to Form Publicly Listed Citius Oncology, Inc.
Citius Pharmaceuticals, Inc. to receive $675
million in equity of Citius Oncology, Inc. and
retain approximately 90% majority control in publicly listed Citius Oncology, Inc. post
transaction
Transaction anticipated to close in the first
half of 2024
CRANFORD, N.J. and NEW YORK, N.Y., October
24, 2023 -- Citius Pharmaceuticals, Inc. (“Citius Pharma” or the “Company”) (Nasdaq: CTXR), a biopharmaceutical
company developing and commercializing first-in-class critical care products, and TenX Keane Acquisition (“TenX”) (NASDAQ:
TENKU), a publicly traded special purpose acquisition company (SPAC), today announced that they have entered into a definitive agreement,
dated October 23, 2023, for a proposed merger of TenX and Citius Pharma’s wholly owned oncology subsidiary that will continue as
a public company listed on the Nasdaq exchange. The newly combined public company will be named Citius Oncology, Inc. (“Citius Oncology”).
Upon closing, pursuant to the terms of the merger agreement, Citius Pharma would receive 67.5 million shares in Citius Oncology at $10
per share and retain majority ownership of approximately 90%. The transaction has been approved by the Board of Directors of both companies
and is expected to close in the first half of 2024.
CITIUS ONCOLOGY OVERVIEW
Citius Oncology will serve as a platform to develop
and commercialize novel targeted oncology therapies. The company is seeking approval from the U.S. Food and Drug Administration (FDA)
of LYMPHIR for an orphan indication in the treatment of persistent or recurrent cutaneous T-cell lymphoma (CTCL), a rare form of non-Hodgkin
lymphoma. Management estimates the initial market for LYMPHIR currently exceeds $400 million, is growing and is underserved by existing
therapies. If approved, LYMPHIR would be unique as the only IL-2 receptor targeted CTCL therapy, offering a novel option to patients cycling
through multiple treatments. Robust intellectual property protections that span orphan drug designation, complex technology, trade secrets
and pending patents for immuno-oncology use as a combination therapy with checkpoint inhibitors would further support Citius Oncology’s
competitive positioning.
Preparations are underway for a Biologics License
Application (BLA) resubmission in early 2024. If approved, LYMPHIR could be commercially available as early as the second half of 2024
for the treatment of CTCL. Additional value creating opportunities in larger markets include potential indications in peripheral T-cell
lymphoma or as a combination therapy with CAR-T and PD-1 inhibitors, and in markets outside the U.S. Currently, two investigator-initiated
trials are underway to explore LYMPHIR’s potential as an immuno-oncology combination therapy.
The transaction is expected to provide Citius
Oncology with improved access to the public equity markets and thereby facilitate the commercialization of LYMPHIR and position the company
to explore additional value creating opportunities more fully.
CITIUS PHARMA AND TENX COMMENTS
“We believe this transaction will allow
us to unlock the value of LYMPHIR, and solidly position Citius Pharma to advance our diversified pipeline. This transaction will enable
Citius Oncology, with access to the broader capital markets, to better support the successful commercialization of LYMPHIR, if approved,
and explore additional potential targeted oncology therapies. Our majority ownership position and shared services agreement ensures that
the Citius Pharma management team will remain fully engaged with the development and commercialization efforts at Citius Oncology. As
previously announced, the Company is in the process of formulating a plan of distribution of a portion of the shares of Citius Oncology
to its shareholders. At Citius Pharma, we intend to focus on completing the Mino-Lok trial and continuing to evaluate next steps with
our Halo-Lido program,” stated Leonard Mazur, Chairman and CEO of Citius Pharma.
“We are very pleased to announce the proposed
merger with Citius Oncology,” said Mr. Xiaofeng Yuan, Chairman and CEO of TenX. “After undertaking a comprehensive process
with external advisors to explore and evaluate numerous potential business combination targets, our board and management team believe
that this transaction with Citius Oncology represents the best opportunity to create substantial value for our stockholders. This business
combination, if consummated, will result in TenX investors owning an equity stake in a company that is focused on developing and commercializing
LYMPHIR to improve the lives of patients with CTCL and additional potential upside from combinations with other drugs as immuno-oncology
therapies with even larger addressable markets. We are thrilled to support Citius Oncology at an inflection point in its development and
to provide an avenue for Citius to expeditiously meet its development milestones.”
THE PROPOSED MERGER AGREEMENT
Pursuant to the proposed agreement, TenX will
acquire Citius Pharma’s wholly owned subsidiary via a merger, with the newly combined publicly traded company to be named Citius
Oncology, Inc. In the transaction, all shares of Citius Pharma’s wholly owned subsidiary would be converted into the right to receive
common stock of Citius Oncology. As a result, upon closing, Citius Pharma would receive 67.5 million shares of common stock of Citius
Oncology which, at an implied value of $10.00 per share, would be $675 million in equity of Citius Oncology, before fees and expenses.
As part of the transaction, Citius Pharma will contribute $10 million in cash to Citius Oncology. An additional 12.75 million existing
options will be assumed by Citius Oncology.
At closing, any cash remaining in TenX’s
trust account along with the cash provided by Citius Pharma will be contributed to Citius Oncology to support ongoing operations and planned
commercialization efforts. References to available cash from the TenX trust account and retained transaction proceeds are subject to any
redemptions by the public stockholders of TenX and payment of transaction fees and expenses.
Upon closing, Citius Oncology will operate under
a shared services agreement with Citius Pharma, with fees payable quarterly to Citius Pharma, for the services of several key members
of the Citius Pharma team, led by Leonard Mazur, Chief Executive Officer, Jaime Bartushak, Chief Financial Officer and Dr. Myron Czuczman,
Chief Medical Officer. Myron Holubiak will serve as Executive Vice Chairman of the Citius Oncology Board of Directors.
The transaction, which has been unanimously approved
by both Boards of Directors of Citius Pharma and TenX, is subject to approval by stockholders of TenX and other customary closing conditions.
Citius Pharma, as the sole holder of Citius Oncology common stock, has approved the transaction. The proposed business combination is
expected to be completed in the first half of 2024.
A more detailed description of the transaction
terms and a copy of the business combination agreement will be included in a Current Report on Form 8-K to be filed by each of Citius
Pharma and TenX with the United States Securities and Exchange Commission (“SEC”). In connection with the transaction, TenX
intends to file a registration statement (which will contain a proxy statement/prospectus) with the SEC.
This press release shall not constitute an offer
to sell or the solicitation of an offer to buy any of the securities described herein, nor shall there be any sale of these securities
in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under
the securities laws of any such state or jurisdiction.
CITIUS PHARMA AND TENX STOCKHOLDERS AND OTHER
INTERESTED PERSONS ARE ADVISED TO READ, ONCE AVAILABLE, THE REGISTRATION STATEMENT AND THE PRELIMINARY PROXY STATEMENT/PROSPECTUS AND
ANY AMENDMENTS THERETO AND, ONCE AVAILABLE, THE DEFINITIVE PROXY STATEMENT/PROSPECTUS IN CONNECTION WITH THE BUSINESS COMBINATION, BECAUSE
THESE DOCUMENTS WILL CONTAIN IMPORTANT INFORMATION ABOUT CITIUS PHARMA, TENX, CITIUS ONCOLOGY AND THE PROPOSED MERGER.
ADVISORS
Maxim Group LLC is acting as exclusive financial
advisor to Citius Pharma and Newbridge Securities Corporation is acting as exclusive financial advisor to TenX. Wyrick Robbins Yates &
Ponton LLP is acting as legal advisor to Citius Pharma. The Crone Law Group P.C. is acting as legal advisor to TenX.
IMPORTANT INFORMATION ABOUT THE PROPOSED BUSINESS
COMBINATION AND WHERE TO FIND IT
In connection with the proposed business combination,
TenX intends to file a registration statement on Form S-4 that will include a proxy statement of TenX and a prospectus of Citius Oncology.
The proxy statement/prospectus will be sent to all TenX stockholders. Before making any voting decision, securities holders of TenX are
urged to read the proxy statement/prospectus and all other relevant documents filed or that will be filed with the SEC in connection with
the proposed business combination as they become available because they will contain important information about the proposed business
combination and the parties to the proposed business combination.
Investors and securities holders will be able
to obtain free copies of the proxy statement/prospectus and all other relevant documents filed or that will be filed with the SEC by TenX
and Citius Pharma through the website maintained by the SEC at www.sec.gov. In addition, the documents filed by Citius Pharma may be obtained
free of charge from Citius Pharma’s website at www.citiuspharma.com, or by written request to Citius Pharmaceuticals, Inc., 11 Commerce
Drive, 1st Floor, Cranford, New Jersey 07016, Attention Chief Financial Officer. The documents filed by TenX may be obtained free of charge
by written request to TenX Keane Acquisition, 420 Lexington Avenue, Suite 2446, New York, New York 10170.
PARTICIPANTS IN THE SOLICITATION
Citius Pharma and Tenx and certain of their respective
directors, executive officers, and other members of management and employees may, under SEC rules, be deemed to be participants in the
solicitations of proxies from TenX’s shareholders in connection with the proposed transaction. Information regarding Citius Pharma’s
directors and executive officers is available in its definitive proxy statement on Schedule 14A for the 2023 annual meeting of stockholders,
which was filed with the SEC on December 22, 2022. Information about TenX’s directors and executive officers and their ownership
of TenX’s securities is set forth in TenX’s filings with the SEC, including TenX’s Annual Report on Form 10-K for the
fiscal year ended December 31, 2022, which was filed with the SEC on April 17, 2023. To the extent that holdings of TenX’s securities
have changed since the amounts printed in TenX’s Annual Report, such changes have been or will be reflected on Statements of Change
in Ownership on Form 4 filed with the SEC.
Additional information regarding the participants
in the proxy solicitation and a description of their direct and indirect interests will be included in the proxy statement/prospectus
when it becomes available. Shareholders, potential investors, and other interested persons in respect of Citius Pharma and TenX should
read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain
free copies of these documents from the sources indicated above.
About Citius Oncology, Inc.
Citius Oncology is a late-stage pharmaceutical
company focused on developing and commercializing targeted oncology therapies. Its strategy centers on achieving a market leading position
by advancing innovative therapies with reduced development and clinical risks, and leveraging competitive advantages supported by intellectual
property and regulatory exclusivity protection. This includes new formulations of previously approved drugs with substantial existing
safety and efficacy data or expanded indications for approved therapies.
Citius Oncology’s lead product candidate
is LYMPHIR, an engineered IL-2 diphtheria toxin fusion protein, for the treatment of patients with persistent or recurrent CTCL, a rare
form of non-Hodgkin lymphoma. Management believes the market for LYMPHIR for CTCL, estimated to exceed $400 million, is attractive, growing
and underserved by existing treatments. On July 28, 2023, the FDA issued a complete response letter (CRL) in response to the LYMPHIR BLA.
The FDA is requiring enhanced product testing and additional controls agreed to with the FDA during the market application review. There
were no concerns relating to the safety and efficacy of the clinical data package submitted with the BLA, or the proposed prescribing
information. In September 2023, Citius Pharma announced that the FDA has agreed with the plans to address the requirements outlined in
the CRL. This guidance has clarified the path forward in completing the necessary activities to support the resubmission of the BLA for
LYMPHIR. The BLA resubmission is anticipated in early 2024.
Citius Oncology was founded in August 2021 as
Citius Acquisition Corp., a Delaware corporation and wholly owned subsidiary of Citius Pharma and began operations in April 2022. The
corporate name was changed to Citius Oncology, Inc. in May 2023.
About Citius Pharmaceuticals, Inc.
Citius Pharma is a late-stage biopharmaceutical
company dedicated to the development and commercialization of first-in-class critical care products, with a focus on oncology, anti-infectives
in adjunct cancer care, unique prescription products, and stem cell therapies. The Company’s diversified pipeline includes two late-stage
product candidates. Mino-Lok®, an antibiotic lock solution for the treatment of patients with catheter-related bloodstream infections,
is enrolling patients in a Phase 3 Pivotal superiority trial and was granted Fast Track designation by the FDA. Citius Pharma is preparing
to resubmit the Biologics License Application for LYMPHIR, a novel IL-2R immunotherapy for an initial indication in CTCL, in early 2024.
LYMPHIR received orphan drug designation by the FDA for the treatment of CTCL and PTCL. At the end of March 2023, Citius Pharma completed
enrollment in its Phase 2b trial of CITI-002 (Halo-Lido), a topical formulation for the relief of hemorrhoids. For more information, please
visit www.citiuspharma.com.
About TenX Keane Acquisition
TenX Keane Acquisition is a blank check company,
also commonly referred to as a special purpose acquisition company (SPAC) formed for the purpose of effecting a merger, share exchange,
asset acquisition, share purchase, reorganization, or similar business combination with one or more businesses or entities. TenX is led
by Xiaofeng Yuan, Chairman and Chief Executive Officer, and Taylor Zhang, Chief Financial Officer, who are growth-oriented executives
with a long track record of value creation across industries.
Forward-Looking Statements
This press release may contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934,
including statements regarding the benefits of the transaction, the anticipated timing of the transaction, the products offered by Citius
Pharma and Citius Oncology and the markets in which each operates, and Citius Pharma and Citius Oncology’s projected future results.
These forward-looking statements generally are identified by the words “believe,” “project,” “expect,”
“anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,”
“plan,” “may,” “should,” “will,” “would,” “will be,” “will
continue,” “will likely result,” and similar expressions. Forward-looking statements are predictions, projections and
other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and
uncertainties. Many factors could cause actual future events to differ materially from the forward-looking statements in this document,
including, but not limited to: Such statements are made based on our expectations and beliefs concerning future events impacting Citius
Pharma. You can identify these statements by the fact that they use words such as “believe,” “anticipate,” “estimate,”
“expect,” “plan,” “would,” “should,” and “may” and other words and terms of similar
meaning or use of future dates. Forward-looking statements are based on management’s current expectations and are subject to risks and
uncertainties that could negatively affect our business, operating results, financial condition and stock price. Factors that could cause
actual results to differ materially from those currently anticipated are: the risk that the transaction may not be completed in a timely
manner or at all, which may adversely affect the price of Citius Pharma’s common stock; the risk that the transaction may not be
completed by TenX’s business combination deadline and the potential failure to obtain an extension of the business combination deadline
if sought by TenX; the failure to satisfy the conditions to the consummation of the transaction, including the adoption of the business
combination agreement by the stockholders of TenX; the satisfaction of the minimum trust account amount following redemptions by TenX’s
public stockholders; the occurrence of any event, change or other circumstance that could give rise to the termination of the business
combination agreement; the effect of the announcement or pendency of the transaction on Citius Pharma’s business relationships,
performance, and business generally; risks that the proposed business combination disrupts current plans or operations of Citius Pharma;
the outcome of any legal proceedings that may be instituted against Citius Pharma or TenX related to the business combination agreement
or the proposed business combination; the ability to maintain the listing of TenX’s securities (which would be Citius Oncology securities)
on Nasdaq after the closing of the transaction; after the closing of the transaction, the price of Citius Oncology’s securities
may be volatile due to a variety of factors, including changes in the competitive and highly regulated industries in which Citius Oncology
will operate, variations in performance across competitors, changes in laws and regulations affecting Citius Oncology’s business
and changes in its capital structure; the ability to implement business plans, forecasts, and other expectations after the completion
of the proposed business combination, and identify and realize additional opportunities provided by the business combination; the cost
and timing of the resubmission of the BLA for LYMPHIR; the FDA may not approve our BLA for LYMPHIR; our need for substantial additional
funds; the estimated markets for our product candidates and the acceptance thereof by any market; our ability to commercialize our products
if approved by the FDA; our dependence on third-party suppliers; the ability of our product candidates to impact the quality of life of
our target patient populations; our ability to successfully undertake and complete clinical and non-clinical trials and the results from
those trials for our product candidates; risks relating to the results of research and development activities, including those from existing
and new pipeline assets; uncertainties relating to preclinical and clinical testing; the early stage of products under development; market
and other conditions; our ability to attract, integrate, and retain key personnel; risks related to our growth strategy; patent and intellectual
property matters; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; our ability
to identify, acquire, close and integrate product candidates and companies successfully and on a timely basis; our ability to procure
cGMP commercial-scale supply; government regulation; competition; as well as other risks described in our SEC filings. These risks have
been and may be further impacted by Covid-19 and global geopolitical events, such as the war in Ukraine and the Middle East. Accordingly,
these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance
on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”)
filings which are available on the SEC’s website at www.sec.gov, including in our Annual Report on Form 10-K for the year ended
September 30, 2022, filed with the SEC on December 22, 2022 and updated by our subsequent filings with the SEC. These forward-looking
statements speak only as of the date hereof, and we expressly disclaim any obligation or undertaking to release publicly any updates or
revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions
or circumstances on which any such statement is based, except as required by law.
Citius Pharmaceuticals Investor Contact:
Ilanit Allen
ir@citiuspharma.com
Citius Pharmaceuticals Media Contact:
STiR-communications
Greg Salsburg
Greg@STiR-communications.com
TenX Contact
Taylor Zhang
target@TenXkeane.com
4
Exhibit 99.2

Citius Oncology, Inc. SPAC Business Combination Overview October 2023

2 This presentation has been prepared by Citius Pharmaceuticals, Inc . (the “Company”) for informational purposes only and not for any other purpose . Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the Company or any director, employee, agent, or adviser of the Company . This presentation does not purport to be all - inclusive or to contain all of the information you may desire . The information contained in this presentation and the comments and remarks of the representatives of the Company made during any presentation to which this presentation relates are integrally related and, as such, are intended to be delivered and understood together . Information provided in this presentation speaks only as of the date hereof . The Company assumes no obligation to update any statement after the date of this presentation as a result of new information, subsequent events or any other circumstances . This presentation also includes express and implied forward - looking statements regarding the current expectations, including about the proposed business combination transaction with TenX Keane Acquisition and the resulting public company, Citius Oncology, Inc . , estimates, opinions and beliefs of the Company that are not historical facts . Such forward - looking statements may be identified by words such as “believes”, “expects”, “endeavors”, “anticipates”, “intends”, “plans”, “estimates”, “projects”, “would,” “should”, “objective” and variations of such words and similar words . The accuracy of such statements is dependent upon future events, and involves known and unknown risks, uncertainties and other factors beyond the Company’s control that may cause actual results to differ materially from what is presented herein . Investors are strongly encouraged to carefully review the Company’s SEC filings for a listing of the risks that could cause actual results to differ from these forward - looking statements . These forward - looking statements speak only as of the date of this presentation and should not be construed as statements of facts . FORWARD - LOOKING STATEMENTS
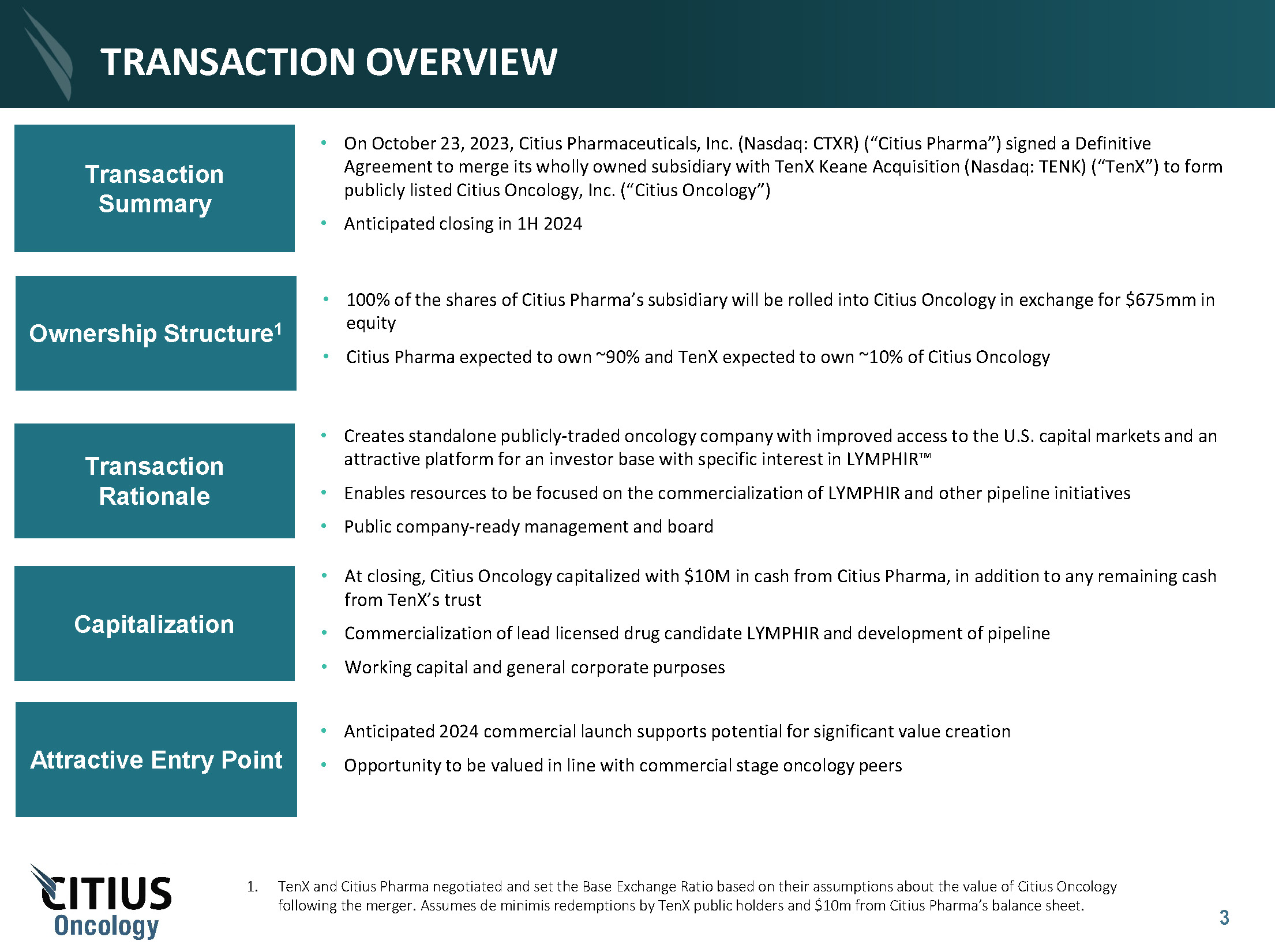
TRANSACTION OVERVIEW Ownership Structure 1 • 100% of the shares of Citius Pharma’s subsidiary will be rolled into Citius Oncology in exchange for $675mm in equity • Citius Pharma expected to own ~90% and TenX expected to own ~10% of Citius Oncology T r a n sac t i o n Rationale • Creates standalone publicly - traded oncology company with improved access to the U.S. capital markets and an attractive platform for an investor base with specific interest in LYMPHIR Ρ • Enables resources to be focused on the commercialization of LYMPHIR and other pipeline initiatives • Public company - ready management and board • Citius Pharmaceuticals, Inc. (Nasdaq: CTXR) (“Citius Pharma”) signed a Definitive Agreement to merge its wholly owned subsidiary with TenX Keane Acquisition (Nasdaq: TENK) (“TenX”) to form publicly listed Citius Oncology, Inc. (“Citius Oncology”) on October 23, 2023 • Anticipated closing in 1H 2024 T r a n sac t i o n Summary Capitalization • At closing, Citius Oncology capitalized with $10M in cash from Citius Pharma, in addition to any remaining cash from TenX’s trust • Commercialization of lead licensed drug candidate LYMPHIR and development of pipeline • Working capital and general corporate purposes Attractive Entry Point • Anticipated 2024 commercial launch supports potential for significant value creation • Opportunity to be valued in line with commercial stage oncology peers 1. TenX and Citius Pharma negotiated and set the Base Exchange Ratio based on their assumptions about the value of Citius Oncology following the merger. Assumes de minimis redemptions by TenX public holders and $10m from Citius Pharma’s balance sheet. 3

CITIUS ONCOLOGY HIGHLIGHTS Public Ready, Funded Opportunity Anticipated Commercial Launch in 2H 2024 Significant O ppo r t un i ty Robust IP Portfolio Seasoned M a n a g e me nt • LYMPHIR licensed by Citius Pharma in 2021; $40mm upfront payment and an additional $15mm funded to date • Upon closing of the business transaction, Citius Oncology to be funded with $10mm of cash by Citius Pharma, in addition to any cash remaining in TenX trust at closing • Anticipated positive cash flow commencing within first year of product launch • 2018 launch of competitive product Poteligeo by Kyowa Kirin indicates unmet need in CTCL given existing therapies are non curative • CTCL market estimated to be $300 - $400+ million • Potential for substantial growth via additional indications including PTCL and combinations with immunotherapy • Multiple potential future product in - licensing opportunities identified • Assuming approval, BLA exclusivity + Orphan drug designation + complex technology + trade secrets provide IP protections • Patents pending for immuno - oncology use as a combination therapy with checkpoint inhibitors • Citius Oncology management has extensive expertise in developing and marketing oncology products • Track record of successfully bringing pharmaceuticals to market • Services agreement between Citius Pharma and Citius Oncology to maintain continuity in execution of clinical and commercial program 4 • LYMPHIR formulation developed by Eisai and marketed in Japan as Remitoro® since 2021; Citius Oncology to control global marketing rights excluding certain East Asian countries • If approved, LYMPHIR offers a unique mechanism of action for the treatment of cutaneous T - cell lymphoma (CTCL) • Potential for follow up indication in Peripheral T - cell lymphoma (PTCL); Investigator - led studies ongoing in combination with CAR - T (Kymriah®) and PD - 1 Inhibitor (Keytruda®) Unique Oncology Platform

ATTRACTIVE PRODUCT PROFILE • LYMPHIR is a recombinant engineered fusion protein that combines interleukin - 2 and diphtheria toxin • Phase 3 Pivotal trial results are consistent with the prior formulation, and clinical profile supports the potential for rapid adoption • Filed as an original BLA, LYMPHIR may be eligible for reference product exclusivity (12 years marketing exclusivity) • Manufacturing & distribution agreements with leading global players 5 1. See slide 11, payment to be made by Citius Pharma (parent) prior to closing of the business combination.

OVERVIEW OF TENX KEANE ACQUISITION X I AO F E N G Y U AN CHA I RM A N & CE O T AY L O R Z HA NG CHI E F F I NANCI A L OFFICER CA T H Y J I ANG DIRECTOR BRIA N HA R TZ BA ND DIRECTOR J O E L M AYE R SO H N DIRECTOR Over 80+ years of global professional investing, capital markets, M&A, life sciences and operating experience TenX completed its $66M IPO in October 2022 6

CITIUS ONCOLOGY MANAGEMENT OVERVIEW - SHARED MANAGEMENT AGREEMENT WITH CTXR L E O NA R D M A Z UR CHA I RM A N & CE O J A I M E BA R T U S H AK EVP, C F O & C BO DR . M YR O N C Z UC Z M A N EVP, CH I EF M E D I C AL O FF I C ER KE LL Y CRE I G H T O N EVP, C M C CA T HE RI N E KESS L ER EVP, R EG U L A T O R Y A FF A I R S Key management personnel mitigate execution risk and leverage proven industry track record M YR O N H O L UB I A K EXE CU T I VE V I C E CH A I R M AN 7

CITIUS ONCOLOGY BOARD OF DIRECTORS L E O NA R D M A Z UR CHAIRMAN M YR O N H O L UB I A K EXE CU T I VE V I C E CH A I R M AN 8 S UREN DU T I A DIRECTOR J O E L M AYE R SO H N DIRECTOR DR . EU G E N E H O L UK A DIRECTOR CA R OL W EBB DIRECTOR DENNIS MC G RA T H DIRECTOR

ORIGINS OF ONTAK Accelerated FDA approval for denileukin diftitox (ONTAK) granted to Ligand Full FDA approval g r a nt ed t o O N T AK 1999 2014 2008 2011 9 A new formulation of denileukin diftitox (E7777) developed by Eisai in response to FDA guidance at time of accelerated approval Eisai Voluntarily Removed ONTAK from the Market • ONTAK was FDA approved and commercially available in the U . S . between 1999 – 2014 • LYMPHIR (E 7777 ) was developed by Eisai in 2011 as a purified and more bioactive formulation in response to a post marketing commitment agreed to with the FDA upon approval of ONTAK • Eisai voluntary removed ONTAK from the market in 2014

LYMPHIR LICENSING AND FDA PATHWAY Q3 2021: Citius Pharma Acquired the Exclusive License to Eisai’s E7777 in all Markets Except Japan and Parts of Asia from Dr. Reddy’s Q4 2021: E7777 Phase 3 Clinical Trial Completed (December) Q3 2021 Q3 2023 Q4 2021 Q3 2022 Q3 2022: Citius Pharma Filed BLA for New Formulation (E7777) in September CRL July 2023: remediation efforts underway • September 2021 • December 2021 • September 2022 • July 2023 Citius Pharma acquired the exclusive license to Eisai’s E7777 (LYMPHIR) Phase 3 clinical trial completed Citius Pharma submitted a BLA for E7777 (LYMPHIR) FDA issues complete response letter (CRL); Citius Pharma planning resubmission with remediation efforts underway 2024 2024: Anticipated commercial launch, if approved 10

LYMPHIR PIPELINE & OPPORTUNITIES BEYOND CTCL PHASE III PHASE II PHASE I PRECLINICAL INVESTIGATIONAL INDICATION PROGRAM P ERIPHERAL T - C ELL L YMPHOMA LYMPHIR - P 1 C OMBINATION WITH CAR - T (K YMRIAH ® ) 2 UNIVERSITY OF MINNESOTA, MASONIC CANCER CENTER C OMBINATION WITH PD - 1 I N HI B I T OR (K EYTR U D A ® ) 2 UNIVERSITY OF PITTSBURG MEDICAL CENTER, HILLMAN CANCER CENTER COLLABORATIONS AS IMMUNO - ONCOLOGY THERAPIES 1. We intend to explore a phase 3 clinical development pathway for an indication in PTCL. 2. KYMRIAH is a registered trademark of Novartis Pharmaceuticals Corporation. KEYTRUDA is a registered trademark of Merck & Co., Inc. Multiple potential future product in - licensing opportunities identified 11

LYMPHIR LICENSE AGREEMENT • Exclusive license of Eisai’s cancer immunotherapy LYMPHIR (E7777) • Initial indication in cutaneous T - cell lymphoma (CTCL) • Additional potential indications in peripheral T - cell lymphoma (PTCL) and immuno - oncology • Assigned exclusive license to Citius Pharma for all markets excluding China, Japan and certain parts of Asia; with option for India • Completed CTCL Pivotal Phase 3 trial work through BLA filing • $40 million upfront cash payment to Dr. Reddy’s (DRL) • Development and commercial milestone payments to DRL and Eisai including $33.5 million FDA approval milestone payment • Tiered royalties to DRL on net product sales 12

WHAT IS CUTANEOUS T - CELL LYMPHOMA (CTCL)? P l a que S t a ge T u m or S t a ge Considered to be incurable, CTCL is a general term for T - cell lymphoma that involves the skin, but may also involve the blood, lymph nodes, and internal organs CTCL accounts for approximately 4% of all non - Hodgkin lymphoma (NHL)* More prevalent in men than women and usually appears in patients in their 50s and 60s 60% 5% 37% M yc o s i s F u n go id e s S e zar y S y n d r o m e O t h e r C T C L Source: Company estimates. 13
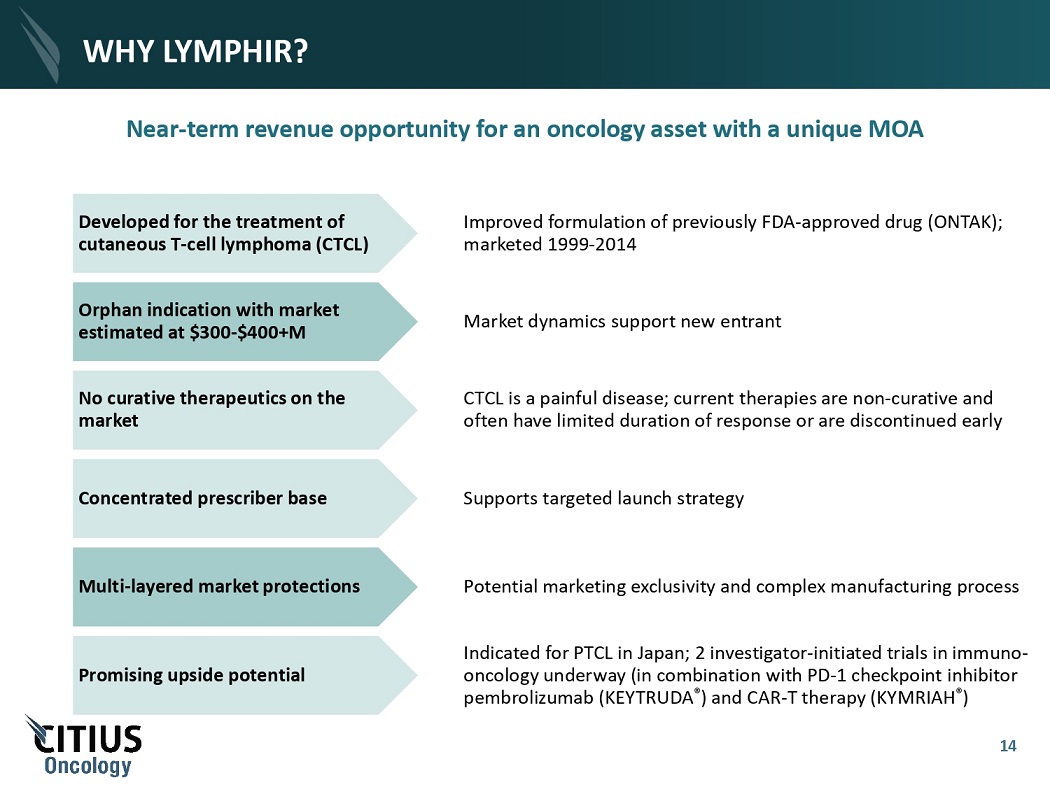
WHY LYMPHIR? Near - term revenue opportunity for an oncology asset with a unique MOA Improved formulation of previously FDA - approved drug (ONTAK); marketed 1999 - 2014 Developed for the treatment of cutaneous T - cell lymphoma (CTCL) Market dynamics support new entrant Orphan indication with market estimated at $300 - $400+M CTCL is a painful disease; current therapies are non - curative and often have limited duration of response or are discontinued early No curative therapeutics on the market Supports targeted launch strategy Concentrated prescriber base Potential marketing exclusivity and complex manufacturing process Multi - layered market protections Indicated for PTCL in Japan; 2 investigator - initiated trials in immuno - oncology underway (in combination with PD - 1 checkpoint inhibitor pembrolizumab (KEYTRUDA ® ) and CAR - T therapy (KYMRIAH ® ) Promising upside potential 14

DIFFERENTIATED MECHANISM OF ACTION (MOA) LYMPHIR (denileukin diftitox) is an engineered IL - 2 - diphtheria toxin fusion protein with a unique mechanism of action supporting two therapeutic effects Targets Malignant Cells Binds to IL - 2 receptors to deliver diphtheria toxin, killing tumor cells directly Eliminates Immunosuppressive Tregs Reduces number of Treg cells, subsequently enhancing anti - tumor immunity 15
PATIENT JOURNEY: TOPICAL TO SYSTEMIC TREATMENT Systemic Therapies (oncologists) Targeted • De n il e uk i n diftitox • Vorinostat • Romidepsin • Mogamulizumab • B r e n tu x imab vedotin Chemotherapy Biologics • bexarotene • interferons • e x t r a c or p o r eal photopheresis Allogeneic stem cell transplant Advanced CTCL, typically treated by oncologists, has a limited number of FDA - approved “targeted” therapies Skin - Directed Therapies (dermatologists) 16 Topical corticosteroids Topical chemotherapy Retinoids Phototherapy Involved - field Radiation therapy E l e c t r on b ea m therapy Early - stage CTCL commonly treated with “skin - directed” therapies by dermatologists

17 COMPETITIVE LANDSCAPE 1. CD30 - cell membrane protein of the tumor necrosis factor receptor superfamily (TNFRSF) and a tumor marker. 2. CCR4 - C - C chemokine receptor type 4 is a protein that in humans is encoded by the CCR4 gene. 3. HDAC - Histone deacetylase inhibitors are believed to induce death, apoptosis, and cell cycle arrest in cancer cells. • LYMPHIR’s differentiated MOA targeting the IL - 2 receptor reinforces rationale for inclusion among the current core therapeutic options in the U.S. market • CTCL treatments are non - curative, often have a limited duration of response and/or are discontinued early • Patients are put on multiple alternate therapies and cycle to 2nd line treatments within 5 months, on average • Key growth drivers expected to increase overall market size and facilitate market penetration • Evolving treatment paradigm; incremental therapeutic option for pre - treated patients • Historically, market growth has followed introduction of new therapeutics • Competitively priced • No other approved therapy since 2018 HDAC inhibitor 3 CCR4 targeted 2 CD30 antigen directed 1 N o v e mb e r 2009 August 2018 N o v e mb e r 2017 M OA App r o v al D a t e
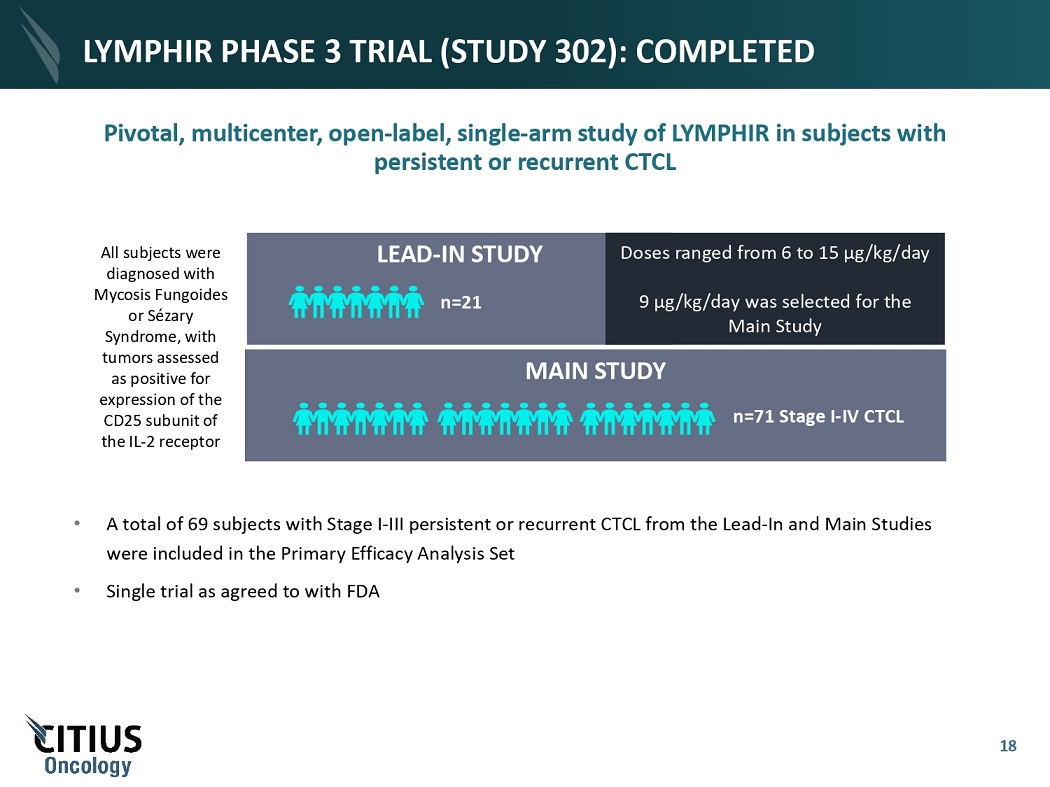
LYMPHIR PHASE 3 TRIAL (STUDY 302): COMPLETED Pivotal, multicenter, open - label, single - arm study of LYMPHIR in subjects with persistent or recurrent CTCL • A total of 69 subjects with Stage I - III persistent or recurrent CTCL from the Lead - In and Main Studies were included in the Primary Efficacy Analysis Set • Single trial as agreed to with FDA LEAD - IN STUDY n=21 MAIN STUDY n=71 Stage I - IV CTCL 18 All subjects were diagnosed with Mycosis Fungoides or Sézary Syndrome, with tumors assessed as positive for expression of the CD25 subunit of the IL - 2 receptor Doses ranged from 6 to 15 µg/kg/day 9 μg/kg/day was selected for the Main Study
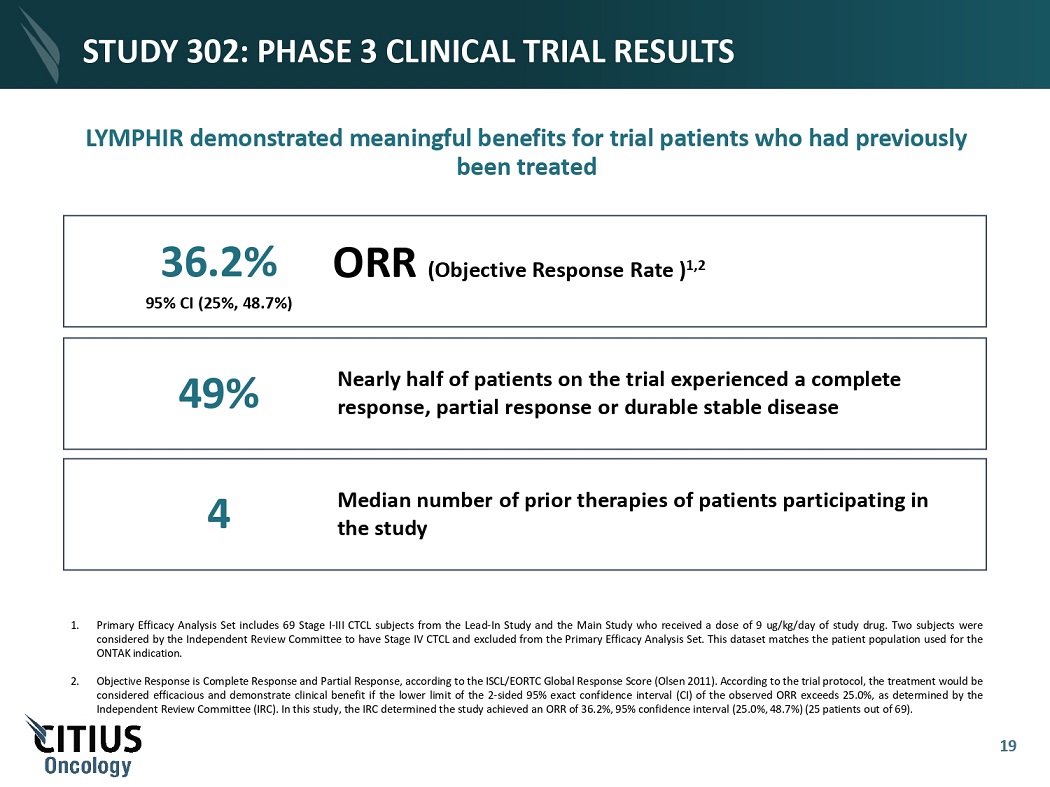
STUDY 302: PHASE 3 CLINICAL TRIAL RESULTS 1. Primary Efficacy Analysis Set includes 69 Stage I - III CTCL subjects from the Lead - In Study and the Main Study who received a dose of 9 ug/kg/day of study drug . Two subjects were considered by the Independent Review Committee to have Stage IV CTCL and excluded from the Primary Efficacy Analysis Set . This dataset matches the patient population used for the ONTAK indication . 2. Objective Response is Complete Response and Partial Response, according to the ISCL/EORTC Global Response Score (Olsen 2011 ) . According to the trial protocol, the treatment would be considered efficacious and demonstrate clinical benefit if the lower limit of the 2 - sided 95 % exact confidence interval (CI) of the observed ORR exceeds 25 . 0 % , as determined by the Independent Review Committee (IRC) . In this study, the IRC determined the study achieved an ORR of 36 . 2 % , 95 % confidence interval ( 25 . 0 % , 48 . 7 % ) ( 25 patients out of 69 ) . LYMPHIR demonstrated meaningful benefits for trial patients who had previously been treated 36.2% ORR (Objective Response Rate ) 1,2 95% CI (25%, 48.7%) 49% Nearly half of patients on the trial experienced a complete response, partial response or durable stable disease 4 Median number of prior therapies of patients participating in the study 19

MEANINGFUL RESPONSE IN CTCL PATIENTS 1. In the Primary Efficacy Analysis set, 84.4% (54/64) of skin evaluable subjects had a decrease in skin tumor burden, with 48.4% subjects with ≥50% reduction in skin tumor burden. Complete clearing of skin disease (skin CR) was observed in 12.5% (8/64) subjects. 2. The duration of response (DOR) was at least 6 months for 52% of responders and at least 12 months for 20% of responders (25/69 patients). RAPID RESPONSE TIME 1.4 months Median number of months to response among patients who experienced clinical benefit (complete or partial response) DURABLE RESPONSE 6.5 months Median months of controlled disease among patients who responded to E7777 2 Reduction in skin tumor burden among evaluable patients; 48.8% of patients with ≥50% reduction in skin tumor burden 1 REDUCED SKIN BURDEN 84.4% More than half of responders in the trial had at least six months of improved or controlled disease 20
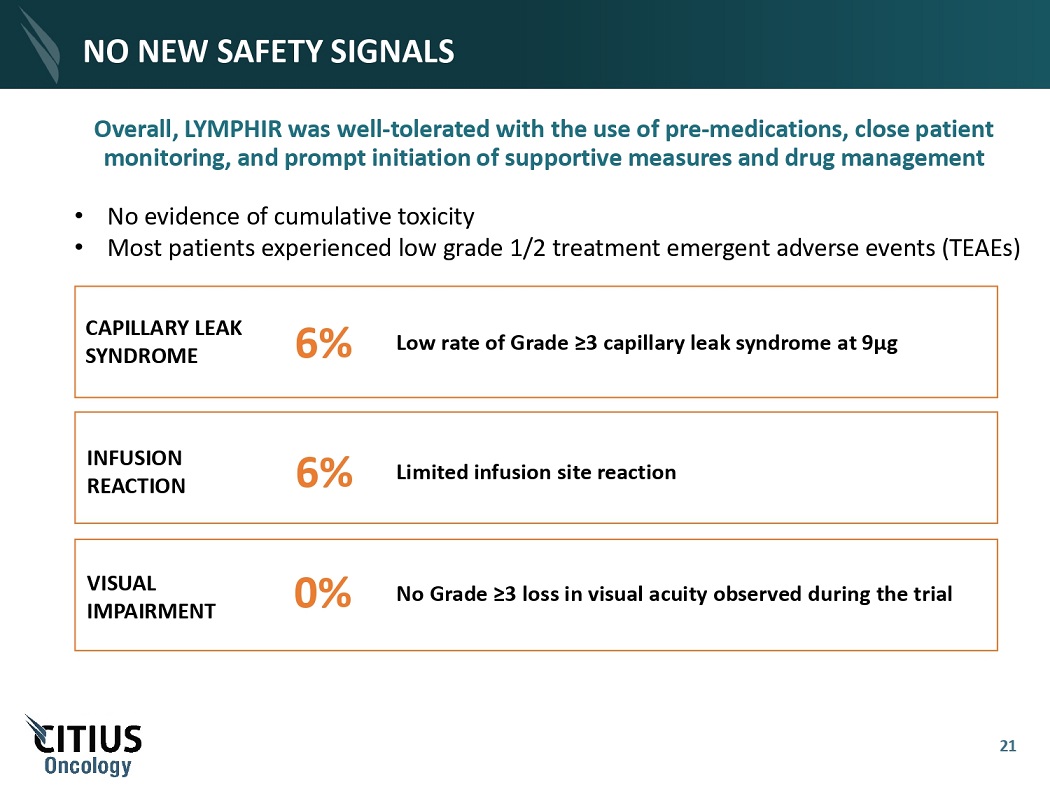
NO NEW SAFETY SIGNALS Low rate of Grade ≥3 capillary leak syndrome at 9µg 6% CAPILLARY LEAK SYNDROME Overall, LYMPHIR was well - tolerated with the use of pre - medications, close patient monitoring, and prompt initiation of supportive measures and drug management • No evidence of cumulative toxicity • Most patients experienced low grade 1/2 treatment emergent adverse events (TEAEs) 0% No Grade ≥3 loss in visual acuity observed during the trial VISUAL I M P A I R M ENT 6% Limited infusion site reaction INFUSION R E A C T I ON 21
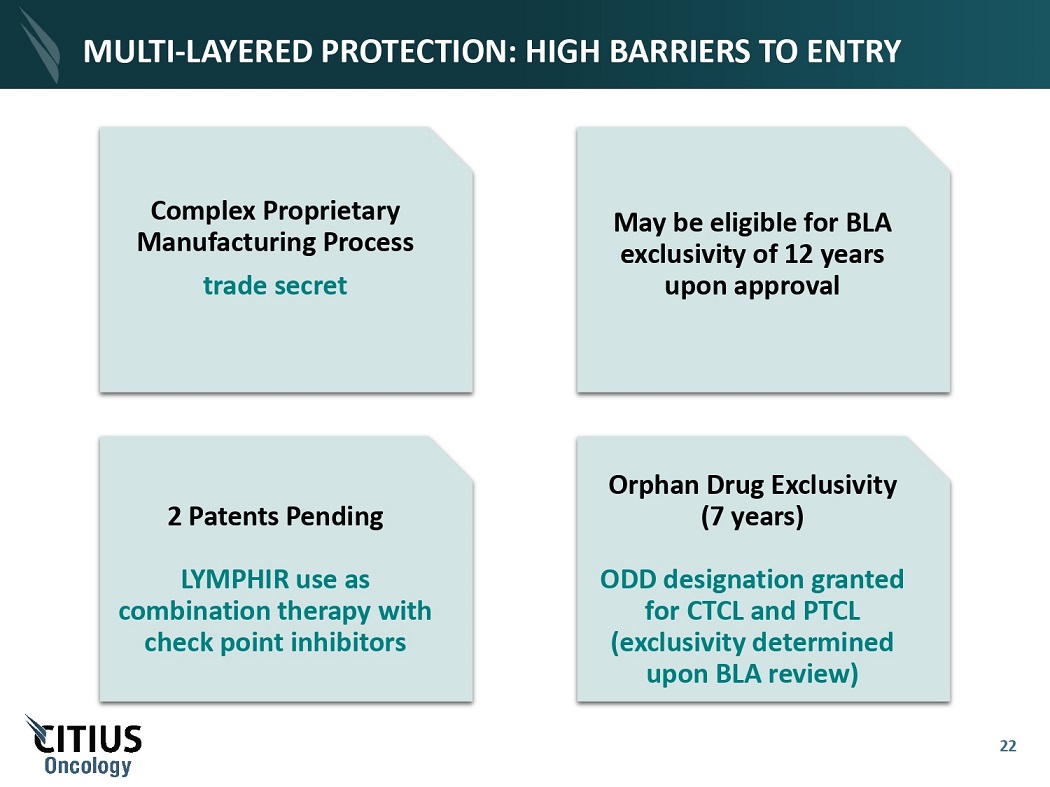
MULTI - LAYERED PROTECTION: HIGH BARRIERS TO ENTRY Complex Proprietary Manufacturing Process trade secret Orphan Drug Exclusivity (7 years) ODD designation granted for CTCL and PTCL (exclusivity determined upon BLA review) May be eligible for BLA exclusivity of 12 years upon approval 2 Patents Pending LYMPHIR use as combination therapy with check point inhibitors 22

ROBUST SUPPLY CHAIN Manufacturing and distribution strategic vendor relationships with leading global providers to support seamless product launch and ongoing commercialization needs BDS 1 Manufacturing BDS 1 Storage Drug Product M anu f ac t u ring Commercial P ac k a g ing & Labeling • Experts in cold chain - of - custody logistics • Commercial bi o r e p o s i t o r y w i th experience in management of domestic and international shipments 23 • Gl o ba l n etw o rk with major U . S . presence • Leading CDMO in biologics • 30+ years of mfg. expertise • Specialize in manufacturing cytotoxic drug pr o du cts f o r o n c o l o g y • Proven worldwide regulatory c om plian c e r ec o rd • World renown CDMO • Experts in c om m e r c ial packaging • Experienced in DSCSA compliance 1. BDS = bulk drug substance.
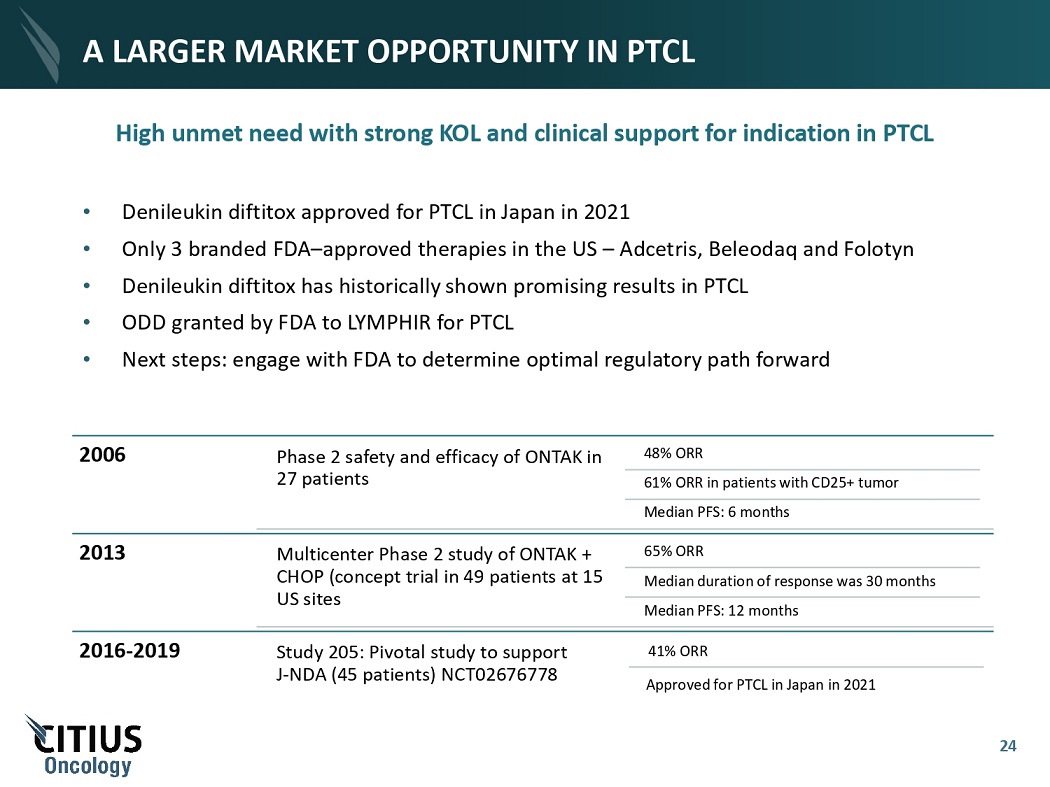
A LARGER MARKET OPPORTUNITY IN PTCL High unmet need with strong KOL and clinical support for indication in PTCL • Denileukin diftitox approved for PTCL in Japan in 2021 • Only 3 branded FDA – approved therapies in the US – Adcetris, Beleodaq and Folotyn • Denileukin diftitox has historically shown promising results in PTCL • ODD granted by FDA to LYMPHIR for PTCL • Next steps: engage with FDA to determine optimal regulatory path forward 2006 Phase 2 safety and efficacy of ONTAK in 27 patients 48% ORR 61% ORR in patients with CD25+ tumor Median PFS: 6 months 2013 Multicenter Phase 2 study of ONTAK + CHOP (concept trial in 49 patients at 15 US sites 65% ORR Median duration of response was 30 months Median PFS: 12 months 2016 - 2019 Study 205: Pivotal study to support J - NDA (45 patients) NCT02676778 Approved for PTCL in Japan in 2021 41% ORR 24

25 MORE OPPORTUNITIES FOR GROWTH KEYTRUDA® is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. KYMRIAH® is a registered trademark of Novartis AG, Basel, Switzerland. PTCL expanded indication potential Upside opportunity in immuno - oncology Collaboration with the University of Pittsburgh LYMPHIR in combination with KEYTRUDA® in patients with recurrent or metastatic solid tumors (NCT05200559) Collaboration with the University of Minnesota LYMPHIR given prior to lymphodepletion chemotherapy and CAR T therapies for the treatment of relapsed/refractory B - cell lymphomas considered at a high risk for failure from KYMRIAH® alone (NCT04855253) • Eisai’s E7777 is already approved for the treatment of Peripheral T - Cell Lymphoma (PTCL) in Japan (Remitoro®) • Would require clinical trial in U.S. designed as a single - arm pivotal study • Two investigator - initiated trials are underway to evaluate LYMPHIR for potential use as an immuno - oncology combination therapy

26 MERGER CONSIDERATION AND PRO FORMA OWNERSHIP • Citius Pharma to receive $675 million in equity of Citius Oncology, or 67.5 million shares at an implied value of $10 per share • An additional 12.75 million in equity options will be assumed by Citius Oncology • At closing, $10 million in cash provided by Citius Pharma and any cash remaining in TenX’s trust account would be contributed to Citius Oncology to support operations and planned commercialization efforts • Pro forma ownership of Citius Oncology 1 : • ~90% Citius Pharma • ~10% TenX public shareholders and founders 1. TenX and Citius Pharma negotiated and set the Base Exchange Ratio based on their assumptions about the value of Citius Oncology following the merger. Assumes de minimis redemptions by TenX public holders and $10m from Citius Pharma’s balance sheet.

27 ACCELERATED GROWTH AS A STANDALONE PUBLIC COMPANY Near - term revenue potential 2H 2024 Commercial Launch 1 High unmet need in CTCL implies rapid adoption Clear Market Opportunity 2 PTCL, combinations with CAR - T & PD - 1s and multiple potential future product in - licensing opportunities Drivers for Growth Beyond CTCL 3 Citius Oncology is primed for growth as a standalone public entity

CITIUS ONCOLOGY HIGHLIGHTS 28 A Unique Oncology Platform Anticipated Commercial Launch in 2H 2024 Significant Market Opportunity Robust IP Portfolio Seasoned M a n a g e m e n t Attractive Entry Point With Growth Potential

29 APPENDIX

TRIALS AND PUBLICATIONS 30 • A Clinical Study to Demonstrate Safety and Efficacy of LYMPHIR® in Persistent or Recurrent Cutaneous T - Cell Lymphoma Link to clinicaltrials.gov • Safety and Tolerability of LYMPHIR® (improved purity Denileukin diftitox [ONTAK]) in Patients with Relapsed or Refractory Cutaneous T T - cell Lymphoma: Results from Pivotal Study 302 View Poster

LYMPHIR SINGLE PIVOTAL TRIAL IN CTCL DESIGN P IVOTAL T RIAL DETAILS Global, multicenter, open label single arm pivotal clinical trial for the treatment of patients with persistent or recurrent CTCL Inclusion Criteria : - CTCL (MF or SS) - CD25+ - Stage IA - IVA - At least 1 prior CTCL therapy - ECOG PS ≥2 - Visceral metastasis and prior denileukin diftitox therapy excluded 2 P HASES Lead In: Completed FPI: May 2013 LPO: Aug 2015 Subjects: N=21 Dose finding by CRM: 6,9,12,15mcg/kg/d IV for 5 consecutive days in 21 day cycle PK and immunogenicity Pivotal Study: Completed FPI: Jun 2016 LPO: Dec 2021 Subjects: N=71 Dose: 9 mcg/kg/d IV for 5 consecutive days in 21 day cycle PK and immunogenicity O BJECTIVES 31 Primary: ORR (Olsen 2001) 1 Secondary: Overall DOR, TTR Skin RR, DOR, TTR ORR (Prince 2010) 2 PK and immunogenicity 1. Objective Response is Complete Response and Partial Response, according to the ISCL/EORTC Global Response Score (Olsen 2001). 2. To assess ORR for LYMPHIR® using the alternate response assessment criteria of Prince (2010).
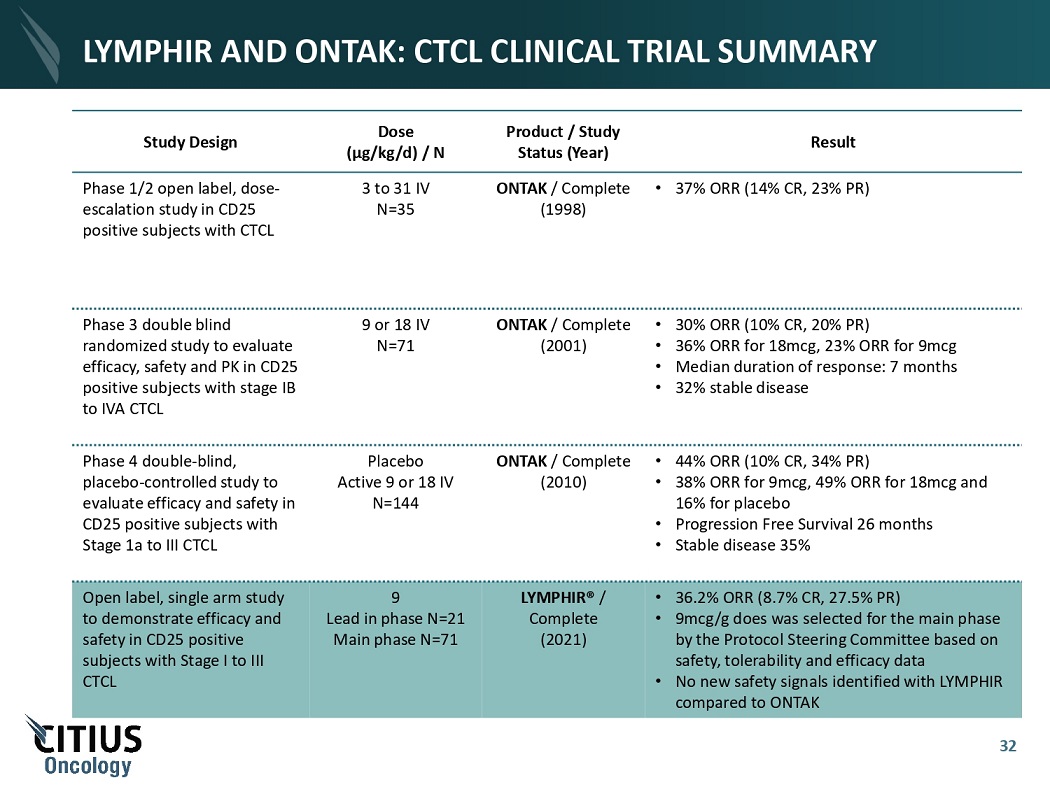
32 LYMPHIR AND ONTAK: CTCL CLINICAL TRIAL SUMMARY Result Product / Study Status (Year) Dose (µg/kg/d) / N Study Design • 37% ORR (14% CR, 23% PR) ONTAK / Complete (1998) 3 to 31 IV N=35 Phase 1/2 open label, dose - escalation study in CD25 positive subjects with CTCL • 30% ORR (10% CR, 20% PR) • 36% ORR for 18mcg, 23% ORR for 9mcg • Median duration of response: 7 months • 32% stable disease ONTAK / Complete (2001) 9 or 18 IV N=71 Phase 3 double blind randomized study to evaluate efficacy, safety and PK in CD25 positive subjects with stage IB to IVA CTCL • 44% ORR (10% CR, 34% PR) • 38% ORR for 9mcg, 49% ORR for 18mcg and 16% for placebo • Progression Free Survival 26 months • Stable disease 35% ONTAK / Complete (2010) Placebo Active 9 or 18 IV N=144 Phase 4 double - blind, placebo - controlled study to evaluate efficacy and safety in CD25 positive subjects with Stage 1a to III CTCL • 36 . 2 % ORR ( 8 . 7 % CR, 27 . 5 % PR) • 9 mcg/g does was selected for the main phase by the Protocol Steering Committee based on safety, tolerability and efficacy data • No new safety signals identified with LYMPHIR compared to ONTAK LYMPHIR® / C om p l e t e (2021) 9 Lead in phase N=21 Main phase N=71 Open label, single arm study to demonstrate efficacy and safety in CD25 positive subjects with Stage I to III CTCL

Citius Oncology, Inc. SPAC Business Combination Overview Copies of relevant documents filed or that will be filed with the SEC by Citius Oncology, Inc. may be obtained through the website maintained by the SEC at www.sec.gov or by contacting Citius Pharmaceuticals or TenX
Citius Pharmaceuticals (NASDAQ:CTXR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Citius Pharmaceuticals (NASDAQ:CTXR)
Historical Stock Chart
From Apr 2023 to Apr 2024