UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF
FOREIGN PRIVATE ISSUER
PURSUANT TO
RULE 13a-16 OR 15d-16
UNDER THE
SECURITIES EXCHANGE ACT OF 1934
For the month of September 2023
Commission File Number: 001-40618
Stevanato Group S.p.A.
(Translation of registrant’s name into English)
Via Molinella
17
35017 Piombino Dese – Padua
Italy
(Address of
principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of
Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
EXHIBIT INDEX
The following exhibits are furnished as part of this Form 6-K:
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Stevanato Group S.p.A. |
|
|
|
|
| Date: September 28, 2023 |
|
|
|
By: |
|
/s/ Franco Moro |
|
|
|
|
Name: |
|
Franco Moro |
|
|
|
|
Title: |
|
Chief Executive Officer |

Exhibit 99.1

Lisa Miles x Investor Relations, Senior Vice President Welcome Capital
Markets Day – September 27, 2023

Safe Harbor Statement Forward-Looking Statements This presentation
contains certain forward-looking statements w ithin the meaning of the Private Securities Litigation Reform Act of 1995 that reflect the current views of Stevanato Group S.p.A. (“we”, “our”, “us”, “Stevanato
Group” or the “Company”). These forward-looking statements include, or may include, words such as increasing, sets,” expanding, drive, build, driving, growth, strong, sustainable, expected, future, potential, can provide,
positioned, ongoing, poised, projected, well positioned,“ “should,” “promising,” “expect,” “expand,” “accelerate,” favorable, increasingly, are seeing, creates, targeting,
reiterating, growing, and other similar terminology. Forw ard-looking statements contained in this presentation include, but are not limited to, statements about: our future financial performance, including our revenue, operating expenses and our
ability to maintain profitability and operational and commercial capabilities; our expectations regarding the development of our industry and the competitive environment in w hich we operate; the expansion of our plants and our expectations to
increase production capacity; the global supply chain and our committed orders; the continued global response to COVID-19 and our role in it; our geographical and industrial footprint; and our goals, strategies and investment plans. These statements
are neither promises nor guarantees but involve known and unknown risks, uncertainties and other important factors and circumstances that may cause Stevanato Group’s actual results, performance or achievements to be materially different from
its expectations expressed or implied by the forward-looking statements, including conditions in the U.S. capital markets, negative global economic conditions, inflation, potential negative developments in the COVID-19 pandemic, the impact of the
conflict between Russia and Ukraine, supply chain challenges and other negative developments in Stevanato Group’s business or unfavorable legislative or regulatory developments. The follow ing are some of the factors that could cause our
actual results to differ materially from those expressed in or underlying our forward-looking statements: (i) our product offerings are highly complex, and, if our products do not satisfy applicable quality criteria, specifications and performance
standards, we could experience lost sales, delayed or reduced market acceptance of our products, increased costs and damage to our reputation; (ii) we must develop new products and enhance existing products, adapt to significant technological and
innovative changes and respond to introductions of new products by competitors to remain competitive; (iii) our backlog might not accurately predict our future revenue, and we might not realize all or any part of the anticipated revenue reflected in
our backlog; (iv) if we fail to maintain and enhance our brand and reputation, our business, results of operations and prospects may be materially and adversely affected; (v) we are highly dependent on our management and employees. Competition for
our employees is intense, and we may not be able to attract and retain the highly skilled employees that we need to support our business and our intended future growth; (vi) our business, financial condition and results of operations depend upon
maintaining our relationships w ith suppliers and service providers; (vii) our business, financial condition and results of operations depend upon the availability and price of high-quality materials and energy supply and our ability to contain
production costs; (viii) the current conflict between Russia and Ukraine and the financial and economic sanctions imposed by the European Union, the U.S., the United Kingdom and other countries and organizations against officials, individuals,
regions, and industries in Russia and Belarus may negatively impact our ability to source gas at commercially reasonable terms or at all and could have a material adverse effect on our operations; (ix) significant interruptions in our operations
could harm our business, financial condition and results of operations; (x) as a consequence of the COVID-19 pandemic, sales of syringes and vials to and for vaccination programs globally increased resulting in a revenue growth acceleration. The
demand for such products may shrink, if the need for COVID-19 related solutions declines; (xi) our manufacturing facilities are subject to operating hazards which may lead to production curtailments or shutdowns and have an adverse effect on our
business, results of operations, financial condition or cash flows; (xii) we may face significant competition in implementing our strategies for revenue growth in light of actions taken by our competitors; (xiii) our global operations are subject to
international market risks that may have a material effect on our liquidity, financial condition, results of operations and cash flows; (xiv) we are required to comply w ith a w ide variety of laws and regulations and are subject to regulation by
various federal, state and foreign agencies; (xv) if relations between China and the United States deteriorate, our business in the United States and China could be materially and adversely affected; and (xvi) Cyber security risks and the failure to
maintain the confidentiality, integrity and availability of our computer hardware, software and internet applications and related tools and functions, could result in damage to our reputation, data integrity and/or subject us to costs, fines or
lawsuits under data privacy or other laws or contractual requirements. This list is not exhaustive. We caution you therefore against relying on these forward-looking statements and we qualify all of our forward-looking statements by these cautionary
statements. These forward-looking statements speak only as at their dates. The Company undertakes no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made
or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible to predict all of these factors. Further, the Company cannot assess the impact of each such factor on our business or the extent to w
hich any factor, or combination of factors, may cause actual results to be materially different from those contained in any forward-looking statements. For a description of certain additional factors that could cause the Company’s future
results to differ from those expressed in any such forward-looking statements, refer to the risk factors discussed in our most recent Annual Report on Form 20-F, filed w ith the U.S. Securities and Exchange Commission. Non-GAAP Financial Information
This presentation contains non-GAAP measures. Please refer to the tables included in this presentation for a reconciliation of non-GAAP measures. Management monitors and evaluates our operating and financial performance using several non-GAAP
financial measures, including Constant Currency Revenue, EBITDA, Adjusted EBITDA, Adjusted EBITDA Margin, Adjusted Operating Profit, Adjusted Operating Profit Margin, Adjusted Net Profit, Adjusted Diluted EPS, Capital Employed, Net Cash, Free Cash
Flow and CAPEX. We believe that these non-GAAP financial measures provide useful and relevant information regarding our performance and improve our ability to assess our financial condition. While similar measures are widely used in the industry in
which we operate, the financial measures we use may not be comparable to other similarly titled measures used by other companies, nor are they intended to be substitutes for measures of financial performance or financial position as prepared in
accordance with IFRS. Capital Markets Day – September 27, 2023 3
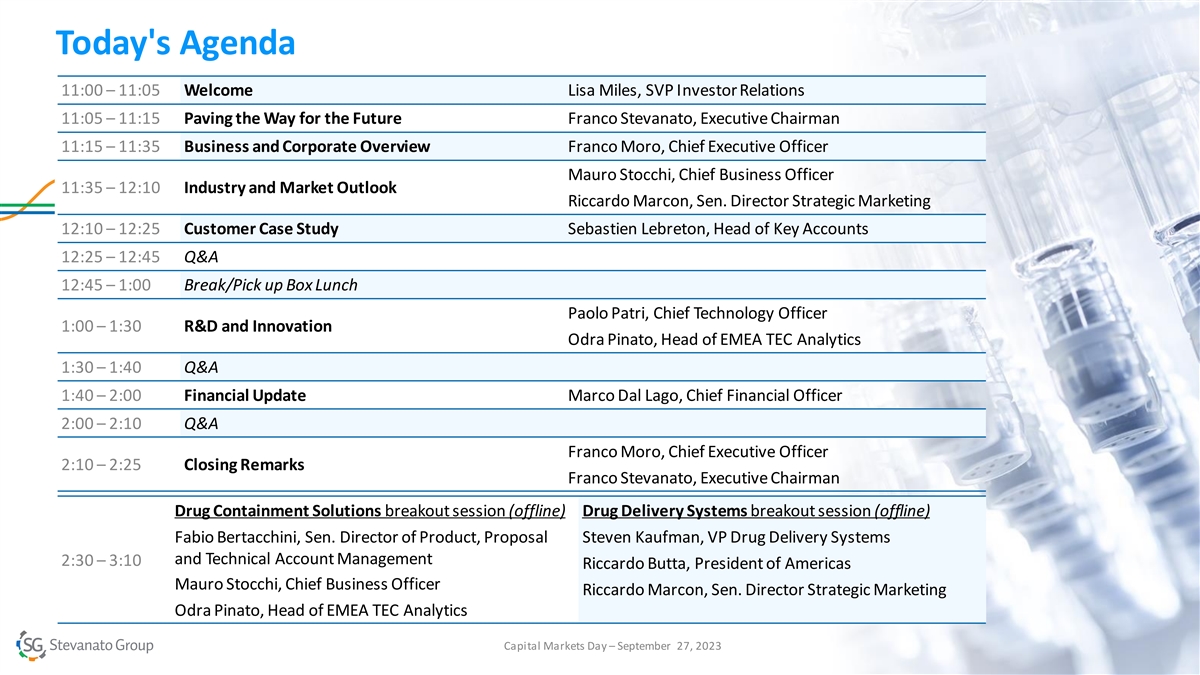
Today's Agenda 11:00 – 11:05 Welcome Lisa Miles, SVP Investor
Relations 11:05 – 11:15 Paving the Way for the Future Franco Stevanato, Executive Chairman 11:15 – 11:35 Business and Corporate Overview Franco Moro, Chief Executive Officer Mauro Stocchi, Chief Business Officer 11:35 – 12:10
Industry and Market Outlook Riccardo Marcon, Sen. Director Strategic Marketing 12:10 – 12:25 Customer Case Study Sebastien Lebreton, Head of Key Accounts 12:25 – 12:45 Q&A 12:45 – 1:00 Break/Pick up Box Lunch Paolo Patri, Chief
Technology Officer 1:00 – 1:30 R&D and Innovation Odra Pinato, Head of EMEA TEC Analytics 1:30 – 1:40 Q&A 1:40 – 2:00 Financial Update Marco Dal Lago, Chief Financial Officer 2:00 – 2:10 Q&A Franco Moro, Chief
Executive Officer 2:10 – 2:25 Closing Remarks Franco Stevanato, Executive Chairman Drug Containment Solutions breakout session (offline) Drug Delivery Systems breakout session (offline) Fabio Bertacchini, Sen. Director of Product, Proposal
Steven Kaufman, VP Drug Delivery Systems and Technical Account Management 2:30 – 3:10 Riccardo Butta, President of Americas Mauro Stocchi, Chief Business Officer Riccardo Marcon, Sen. Director Strategic Marketing Odra Pinato, Head of EMEA TEC
Analytics Capital Markets Day – September 27, 2023

Franco Stevanato x Executive Chairman Paving the Way for the Future
Capital Markets Day – September 27, 2023
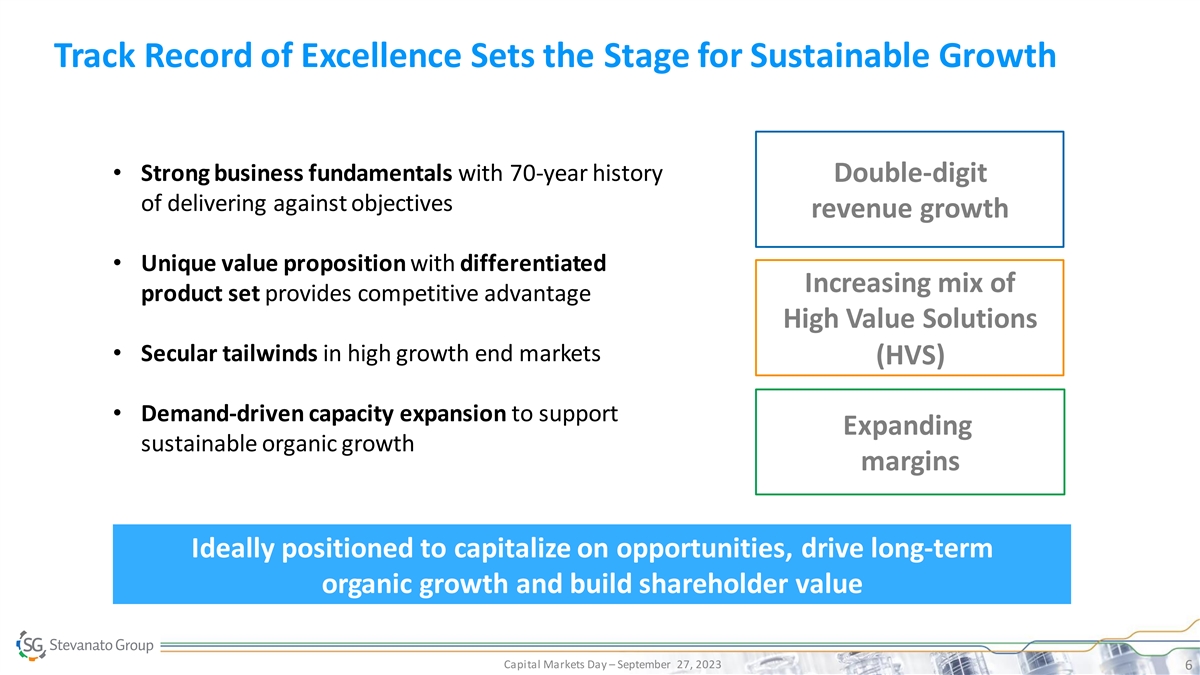
Track Record of Excellence Sets the Stage for Sustainable Growth •
Strong business fundamentals with 70-year history Double-digit of delivering against objectives revenue growth • Unique value proposition with differentiated Increasing mix of product set provides competitive advantage High Value Solutions
• Secular tailwinds in high growth end markets (HVS) • Demand-driven capacity expansion to support Expanding sustainable organic growth margins Ideally positioned to capitalize on opportunities, drive long-term organic growth and build
shareholder value Capital Markets Day – September 27, 2023 6
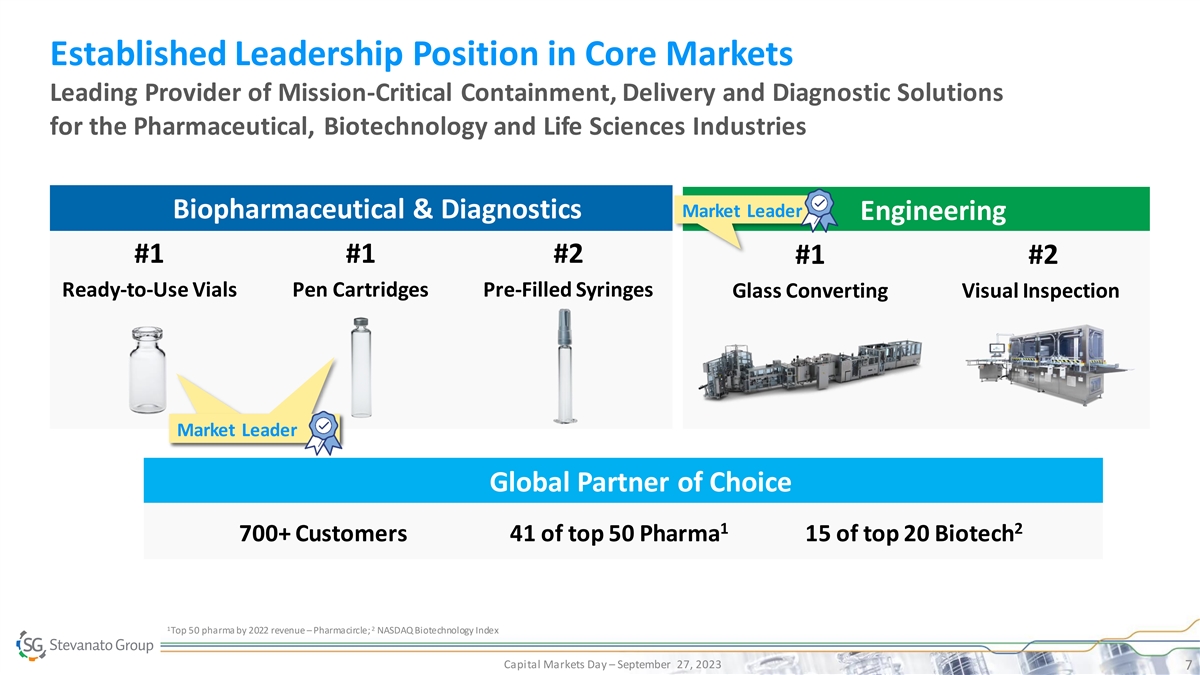
Established Leadership Position in Core Markets Leading Provider of
Mission-Critical Containment, Delivery and Diagnostic Solutions for the Pharmaceutical, Biotechnology and Life Sciences Industries Market Leader Biopharmaceutical & Diagnostics Engineering #1 #1 #2 #1 #2 Ready-to-Use Vials Pen Cartridges
Pre-Filled Syringes Glass Converting Visual Inspection Market Leader Global Partner of Choice 1 2 700+ Customers 41 of top 50 Pharma 15 of top 20 Biotech 1 2 Top 50 pharma by 2022 revenue – Pharmacircle; NASDAQ Biotechnology Index Capital
Markets Day – September 27, 2023 7
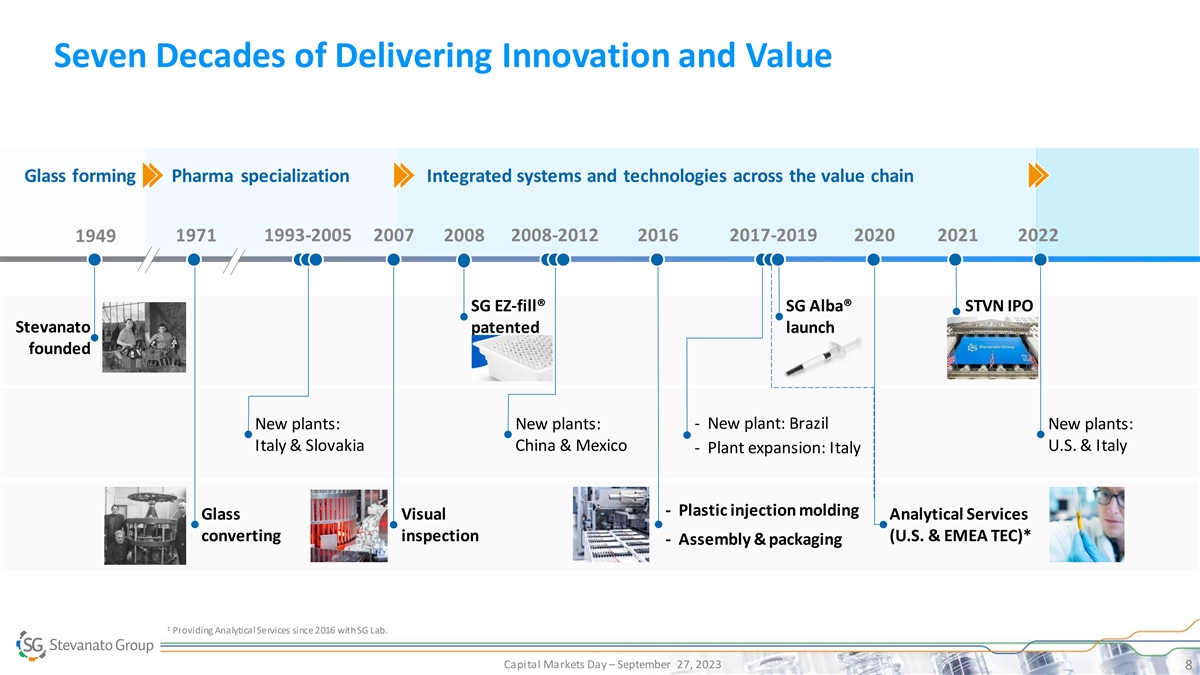
Seven Decades of Delivering Innovation and Value Glass forming Pharma
specialization Integrated systems and technologies across the value chain 1971 1993-2005 2007 2008 2008-2012 2016 2017-2019 2020 2021 2022 1949 SG EZ-fill® SG Alba® STVN IPO Stevanato patented launch founded - New plant: Brazil New plants:
New plants: New plants: Italy & Slovakia China & Mexico U.S. & Italy - Plant expansion: Italy - Plastic injection molding Glass Visual Analytical Services converting inspection (U.S. & EMEA TEC)* - Assembly & packaging 1
Providing Analytical Services since 2016 with SG Lab. Capital Markets Day – September 27, 2023 8
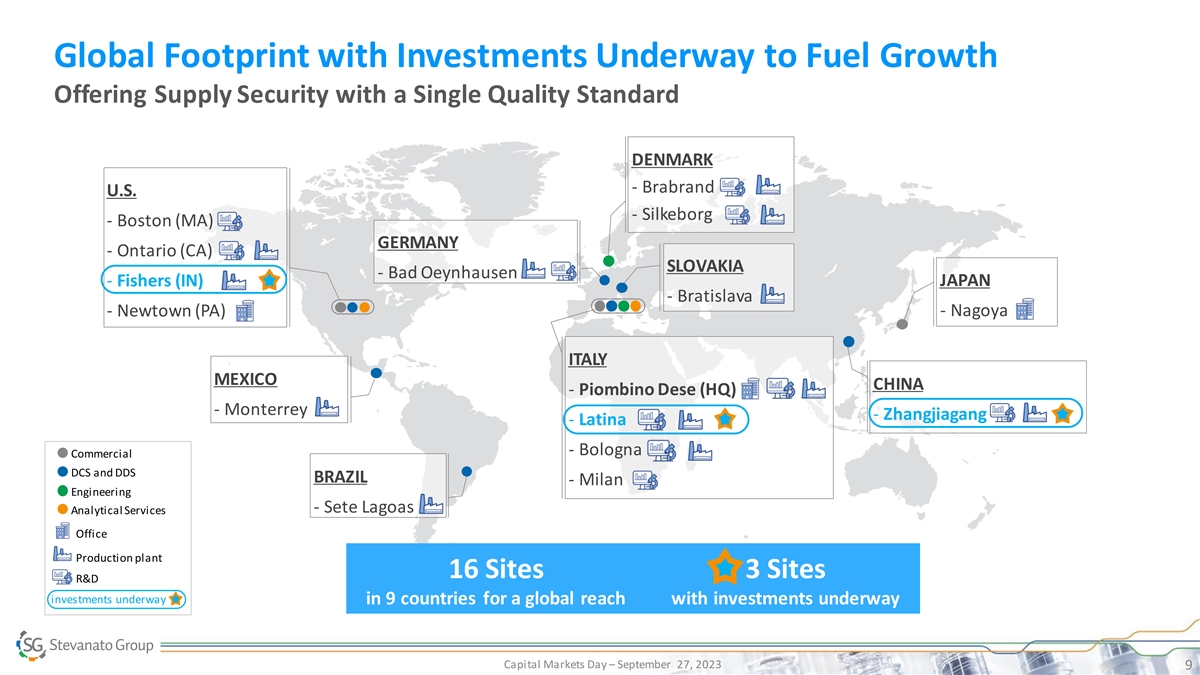
Global Footprint with Investments Underway to Fuel Growth Offering
Supply Security with a Single Quality Standard DENMARK - Brabrand U.S. - Silkeborg - Boston (MA) GERMANY - Ontario (CA) SLOVAKIA - Bad Oeynhausen - Fishers (IN) JAPAN - Bratislava - Newtown (PA) - Nagoya ITALY MEXICO CHINA - Piombino Dese (HQ) -
Monterrey - Zhangjiagang - Latina - Bologna Commercial DCS and DDS BRAZIL - Milan Engineering - Sete Lagoas Analytical Services Office Production plant 16 Sites 3 Sites R&D investments underway in 9 countries for a global reach with investments
underway Capital Markets Day – September 27, 2023 9
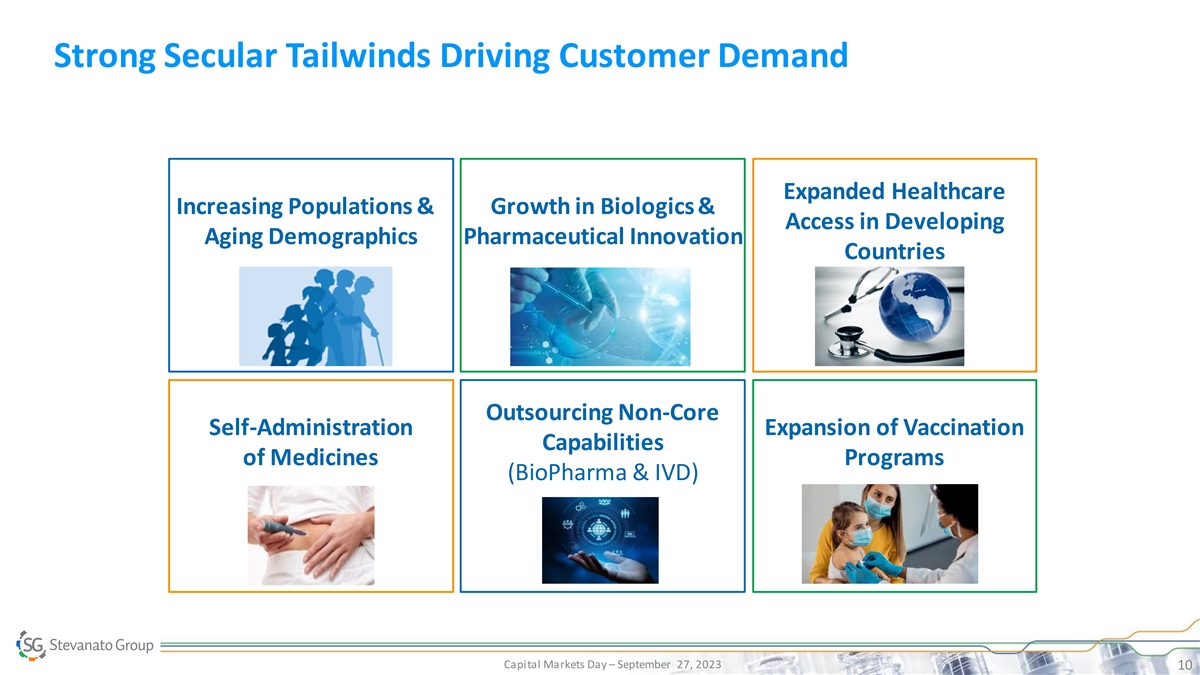
Strong Secular Tailwinds Driving Customer Demand Expanded Healthcare
Increasing Populations & Growth in Biologics & Access in Developing Aging Demographics Pharmaceutical Innovation Countries Outsourcing Non-Core Self-Administration Expansion of Vaccination Capabilities of Medicines Programs (BioPharma &
IVD) Capital Markets Day – September 27, 2023 10

…with a Clear Ambition to Further Fuel Growth Global partner of
choice to biopharma customers, positioned to meet increasing demand for end-to-end solutions from drug development through life-cycle management Capital Markets Day – September 27, 2023 11

Franco Moro x Chief Executive Officer Business and Corporate Overview
Capital Markets Day – September 27, 2023

Business and Corporate Overview Agenda for the session • Business
Overview • Update to Strategic & Operational Priorities • Injectables Market Trends Capital Markets Day – September 27, 2023
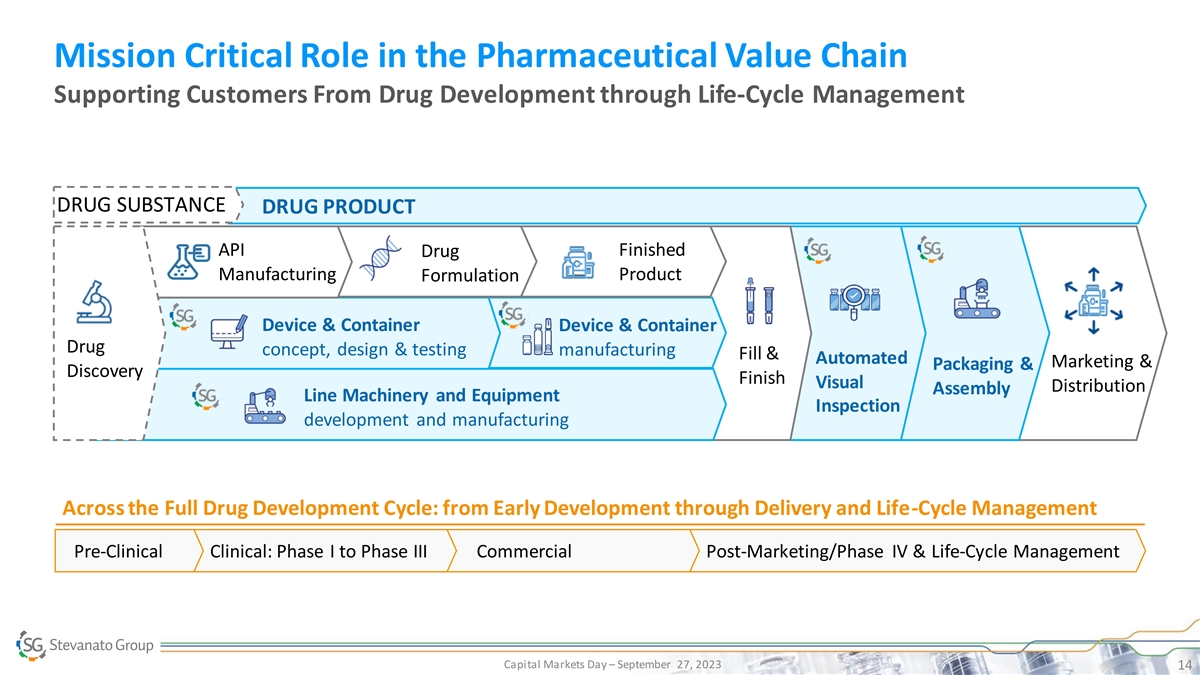
Mission Critical Role in the Pharmaceutical Value Chain Supporting
Customers From Drug Development through Life-Cycle Management DRUG SUBSTANCE DRUG PRODUCT API Finished Drug Manufacturing Product Formulation Device & Container Device & Container Drug concept, design & testing manufacturing Fill &
Automated Marketing & Packaging & Discovery Finish Visual Distribution Assembly Line Machinery and Equipment Inspection development and manufacturing Across the Full Drug Development Cycle: from Early Development through Delivery and
Life-Cycle Management Pre-Clinical Clinical: Phase I to Phase III Commercial Post-Marketing/Phase IV & Life-Cycle Management Capital Markets Day – September 27, 2023 14
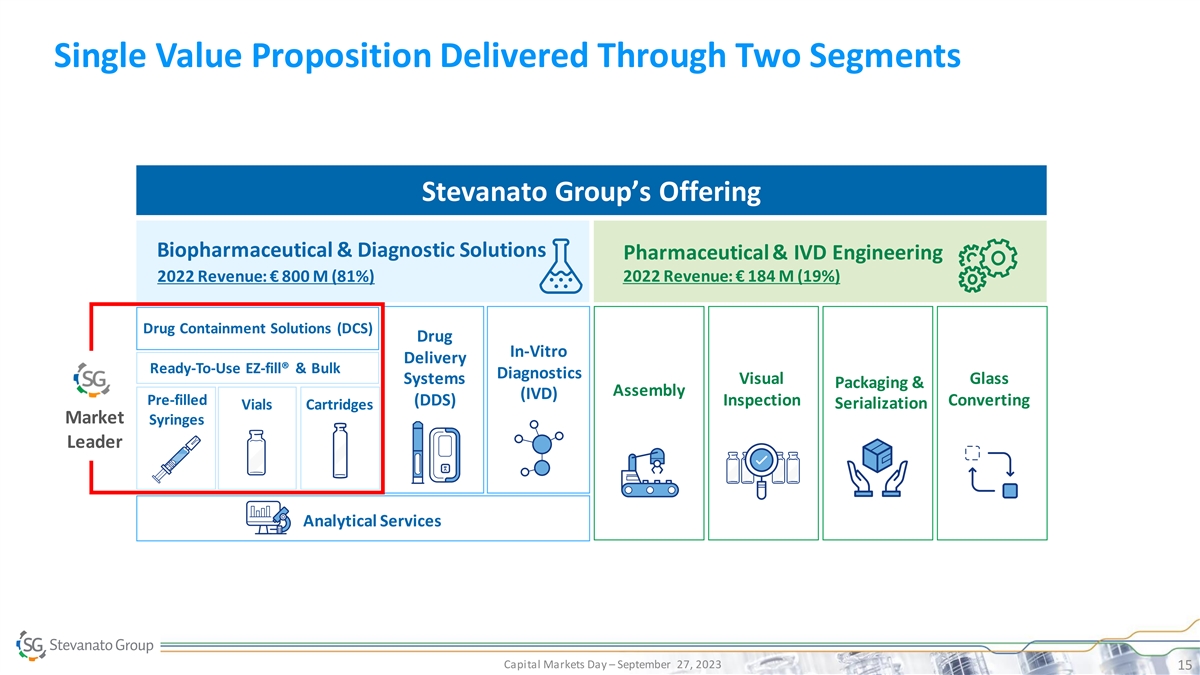
Single Value Proposition Delivered Through Two Segments Stevanato
Group’s Offering Biopharmaceutical & Diagnostic Solutions Pharmaceutical & IVD Engineering 2022 Revenue: € 800 M (81%) 2022 Revenue: € 184 M (19%) Drug Containment Solutions (DCS) Drug In-Vitro Delivery Ready-To-Use
EZ-fill® & Bulk Diagnostics Systems Visual Glass Packaging & Assembly (IVD) Pre-filled (DDS) Inspection Converting Serialization Vials Cartridges Market Syringes Leader Analytical Services Capital Markets Day – September 27, 2023
15

Leveraging Engineering to Power SG Product & Services Portfolio
Benefits to Customer Benefits to Stevanato CUSTOMER • Higher quality containment Glass • Optimizes industrial setup: higher solutions yield and greater flexibility Converting • Shorter lead-times BioPharma • Easy single
supplier management & Diagnostic • Combined expertise to drive Assembly, Solutions • Optimizes processing parameters continued product innovation Packaging which streamlines scale-up, lowers • Higher commercial visibility on
risk and supports future expansion and Visual client manufacturing lines and Inspection • Supports custom & tailored investments solutions Upside for Product Offerings (DCS & DDS) Upside for Services Offerings Capital Markets Day
– September 27, 2023 16

Unique Integrated Offering Delivers High Value to Customers Key
Differentiator and Competitive Advantage Merck-Serono Customer Case Study 2 Products • Developed custom testing protocols 1 2 • Provided high-quality containment solution: SG NEXA® 3 • Developed state-of-the-art automated
assembly equipment to manufacture three 3 different pen injector configurations 3 1 ...and since, providing SG's integrated offering Services Processes More info: https://www.ondrugdelivery.com/meeting-quality-demands-
through-integrated-products-and-services/ Capital Markets Day – September 27, 2023 17

Near-Term Strategic and Operational Priorities to Capitalize on Strong
Secular Tailwinds Global HVS R&D Multi-Year Expansion Growth Innovation Pipeline Capital Markets Day – September 27, 2023 18
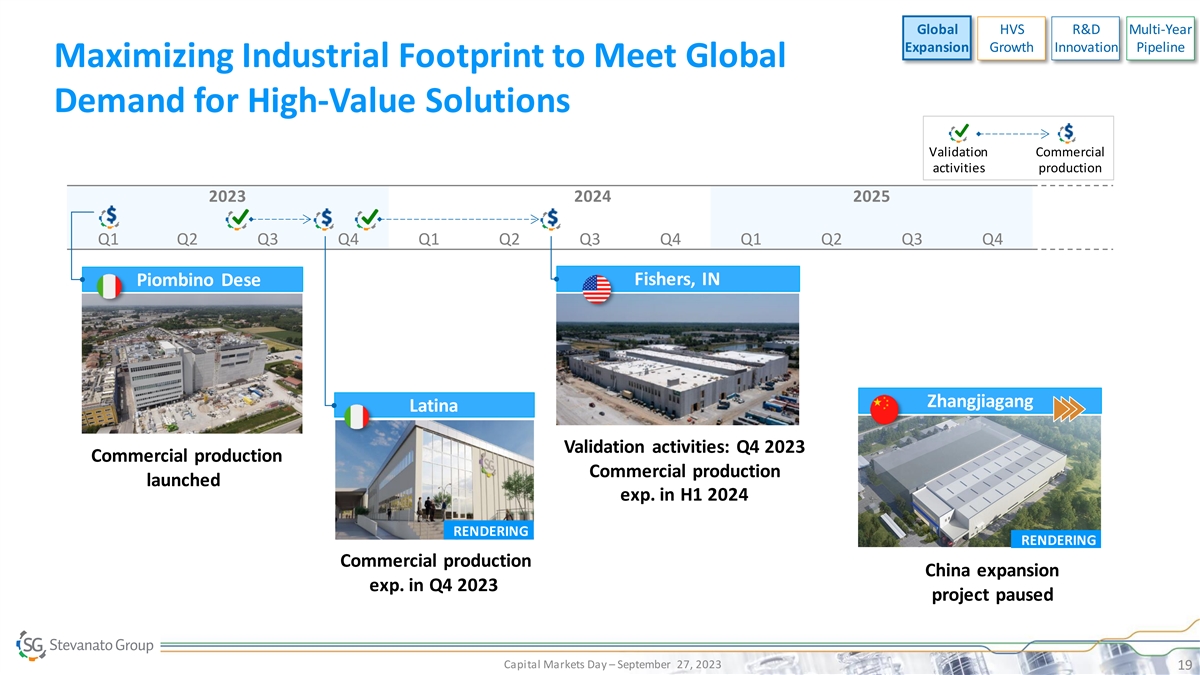
Global HVS R&D Multi-Year Expansion Growth Innovation Pipeline
Maximizing Industrial Footprint to Meet Global Demand for High-Value Solutions Validation Commercial activities production 2023 2024 2025 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Fishers, IN Piombino Dese Zhangjiagang Latina Validation activities: Q4
2023 Commercial production Commercial production launched exp. in H1 2024 RENDERING RENDERING Commercial production China expansion exp. in Q4 2023 project paused Capital Markets Day – September 27, 2023 19
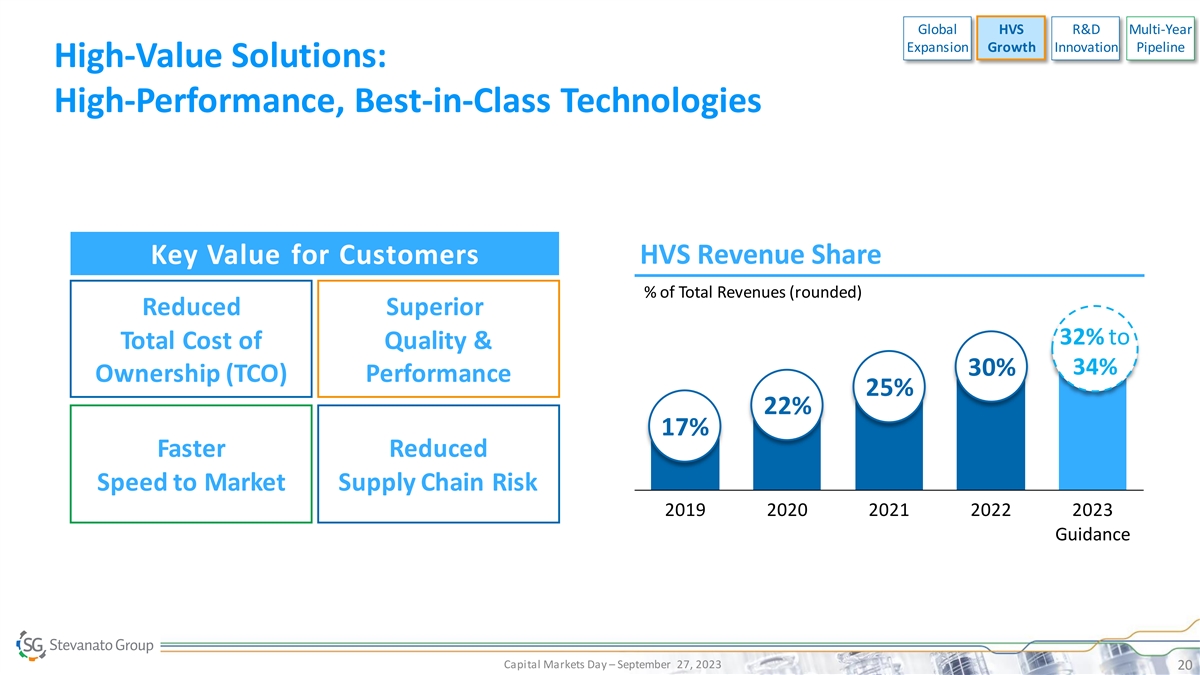
Global HVS R&D Multi-Year Expansion Growth Innovation Pipeline
High-Value Solutions: High-Performance, Best-in-Class Technologies Key Value for Customers HVS Revenue Share % of Total Revenues (rounded) Reduced Superior 32% to Total Cost of Quality & 34% 30% Ownership (TCO) Performance 25% 22% 17% Faster
Reduced Speed to Market Supply Chain Risk 2019 2020 2021 2022 2023 Guidance Capital Markets Day – September 27, 2023 20
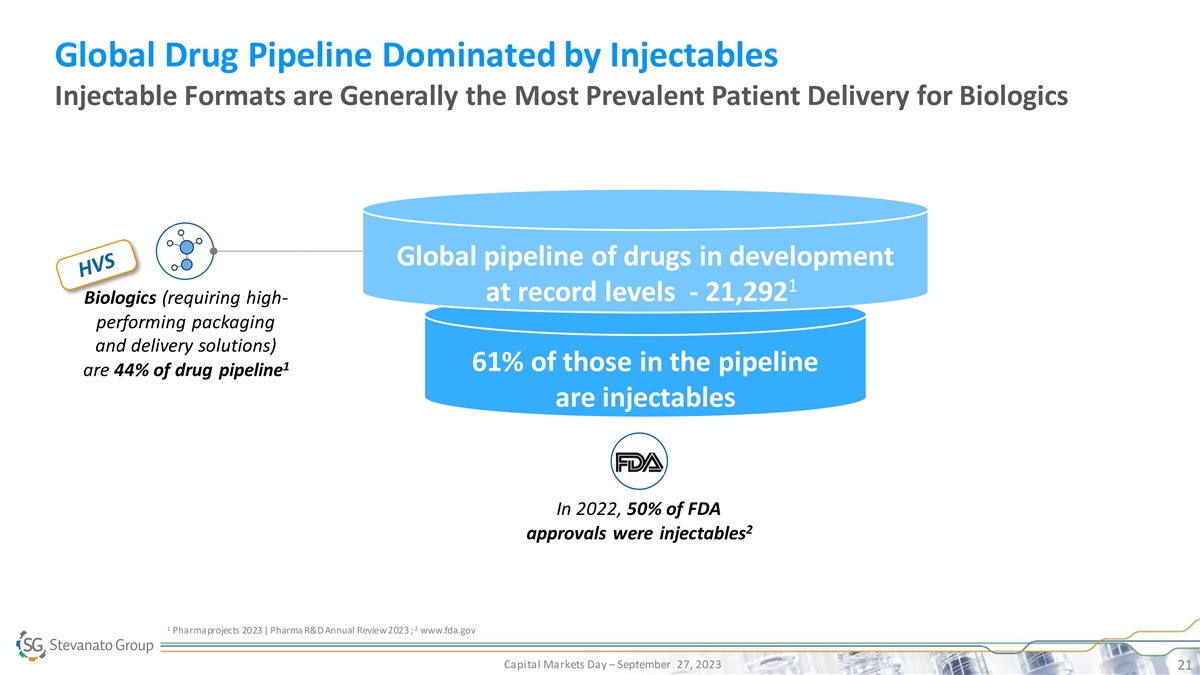
Global Drug Pipeline Dominated by Injectables Injectable Formats are
Generally the Most Prevalent Patient Delivery for Biologics Global pipeline of drugs in development 1 at record levels - 21,292 Biologics (requiring high- performing packaging and delivery solutions) 1 61% of those in the pipeline are 44% of drug
pipeline are injectables In 2022, 50% of FDA 2 approvals were injectables 1 2 Pharmaprojects 2023 | Pharma R&D Annual Review 2023 ; www.fda.gov Capital Markets Day – September 27, 2023 21
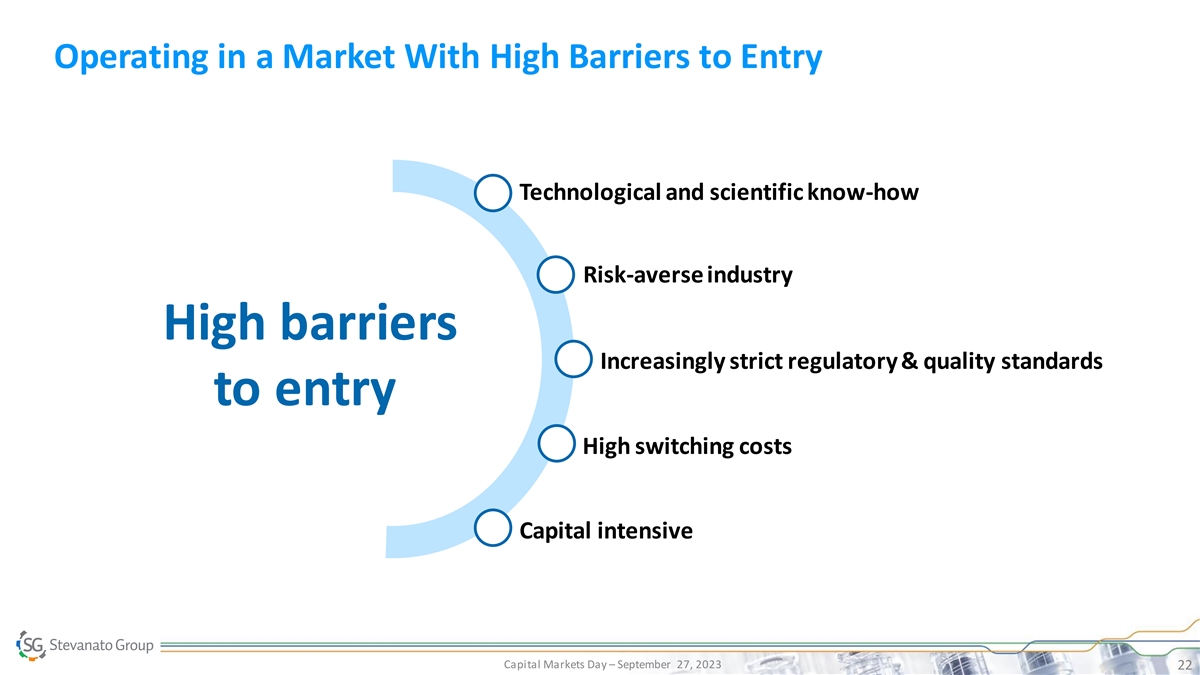
Operating in a Market With High Barriers to Entry Technological and
scientific know-how Risk-averse industry High barriers Increasingly strict regulatory & quality standards to entry High switching costs Capital intensive Capital Markets Day – September 27, 2023 22

Setting the Stage for Sustainable Long-Term Growth • Mission
critical role in Pharmaceutical supply chain • Unique integrated value proposition to support customers at every step • Concentrated market with high barriers to entry • Clear operational priorities to capitalize on secular macro
tailwinds • Demand-driven expansion in HVS to support sustainable long-term organic growth Capital Markets Day – September 27, 2023 23

Mauro Stocchi Chief Business Officer Riccardo Marcon Strategic
Marketing, Alliances and M&A, Senior Director Industry and Market Outlook Capital Markets Day – September 27, 2023

Industry and Market Outlook Agenda for the session • End-market
Pharmaceutical Demand Trends • Supply Chain and Regulatory Trends Capital Markets Day – September 27, 2023
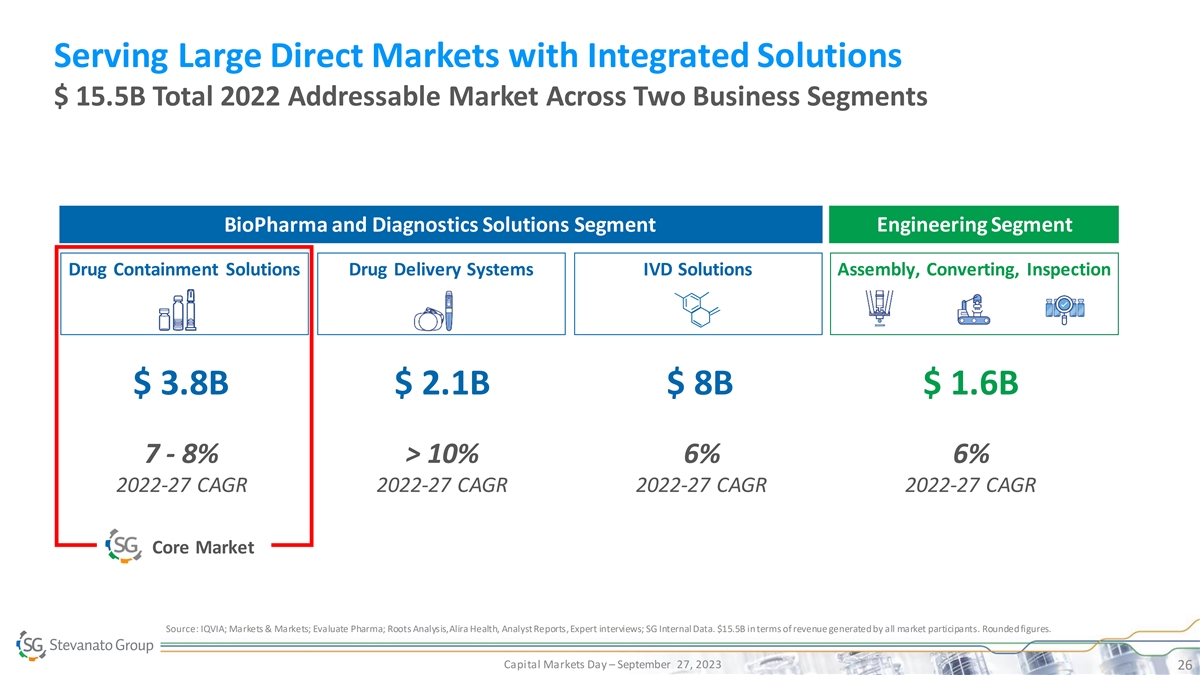
Serving Large Direct Markets with Integrated Solutions $ 15.5B Total
2022 Addressable Market Across Two Business Segments Engineering Segment BioPharma and Diagnostics Solutions Segment Drug Containment Solutions Drug Delivery Systems IVD Solutions Assembly, Converting, Inspection $ 3.8B $ 2.1B $ 8B $ 1.6B 7 - 8%
> 10% 6% 6% 2022-27 CAGR 2022-27 CAGR 2022-27 CAGR 2022-27 CAGR Core Market Source: IQVIA; Markets & Markets; Evaluate Pharma; Roots Analysis, Alira Health, Analyst Reports, Expert interviews; SG Internal Data. $15.5B in terms of revenue
generated by all market participants. Rounded figures. Capital Markets Day – September 27, 2023 26
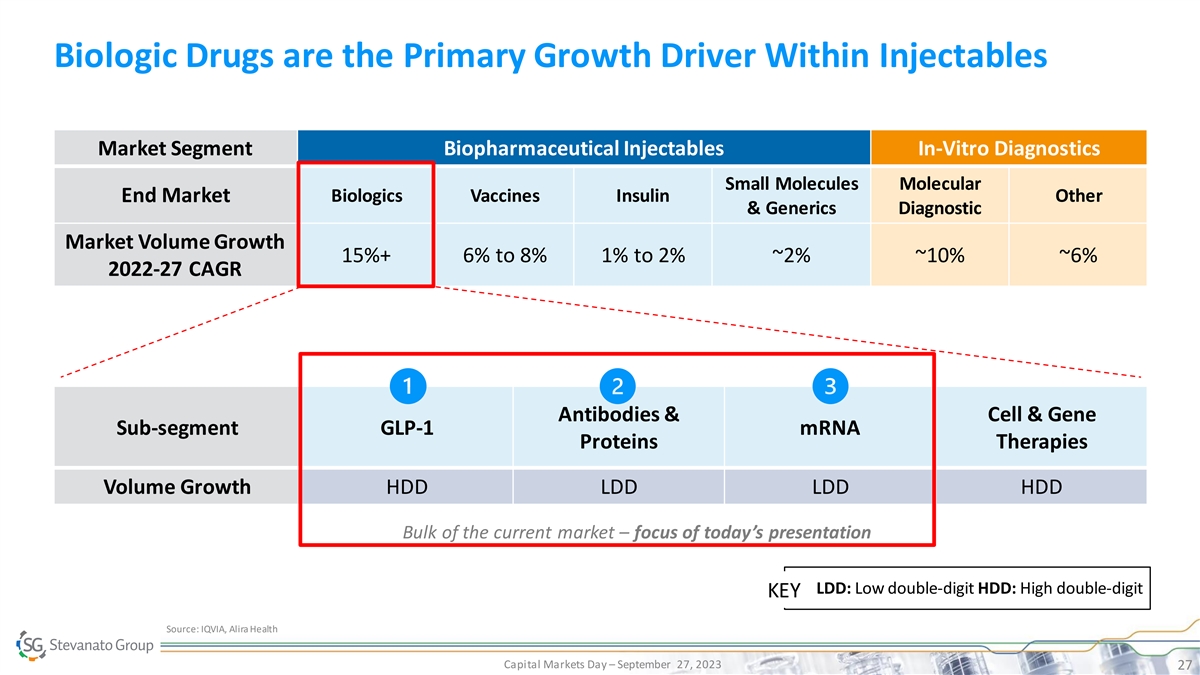
Biologic Drugs are the Primary Growth Driver Within Injectables Market
Segment Biopharmaceutical Injectables In-Vitro Diagnostics Small Molecules Molecular End Market Biologics Vaccines Insulin Other & Generics Diagnostic Market Volume Growth 15%+ 6% to 8% 1% to 2% ~2% ~10% ~6% 2022-27 CAGR Antibodies & Cell
& Gene Sub-segment GLP-1 mRNA Proteins Therapies Volume Growth HDD LDD LDD HDD Bulk of the current market – focus of today’s presentation LDD: Low double-digit HDD: High double-digit KEY Source: IQVIA, Alira Health Capital Markets
Day – September 27, 2023 27

Stevanato Group Has the Commercial Footprint to Capture Expected Market
Growth, Comprised Mainly of Commercial-Stage BioPharma Exp. Biologics Growth by Customer Status SG Current Presence in Exp. Biologics Market Growth Exp. Sales of Biologics by volume in the 7 Major Markets Exp. Sales of Biologics by volume in the 7
Major Markets (U.S., UK, Germany, France, Italy, Spain, Japan) (U.S., UK, Germany, France, Italy, Spain, Japan) Commercial-stage BioPharma SG already present as supplier Emerging BioPharma SG not present as supplier ~70% SG is already supporting 98%
Biologics growth is exp. to BioPharma companies be delivered by commercial- expecting to deliver ~70% of stage BioPharma the growth in Biologics ~30% 2% 2022-27 Growth 2022-27 Growth Source: IQVIA, SG internal data. Note: Emerging biopharma
companies - defined as those with less than $200M in R&D spending and less than $500M per year in annual sales. Capital Markets Day – September 27, 2023 28

…and With Several Levers That Offer Multi-Year Visibility of
Future Customers’ Demand Multi-Year Commercial Agreements provide security and flexibility: • Minimum volume commitments 3 to 12 1. Standalone purchase order months • Take-or-pay SG Key Accounts are • Price adjustment clauses
1 to 2 commonly under multi-year 2. Rolling commercial forecast years commercial agreements … coupled with transparency and effective negotiation with our long-term 3 to 5 customers 3. Multi-year Commercial Agreements years over 3 4. Customer
investments for manufacturing capacity (SG Engineering division) years over 5 5. Early-stage customer’s pipeline discussion and support for R&D and development activity years Capital Markets Day – September 27, 2023 29

Biologics Growth is Driving Demand for High Value Solutions SG Offers
Full Suite of High-performance Products to Address Scientific and Therapeutic Need BioPharma and Diagnostics Solutions Segment Drug Containment Solutions Drug Delivery Systems IVD Solutions • EZ-fill® ALBA® PFS Proprietary Devices
• Bulk NEXA® Vials and • EZ-fill® NEXA® PFS • Highly-complex Cartridges • Alina® Pen Injector High • EZ-fill® NEXA Flex™ PFS Molecular • Bulk LDP Vials • Aidaptus® Auto
Inj. Diagnostic Systems • EZ-fill® ITC PFS TM • MicroVials • Vertiva OBDS • EZ-fill® Vials & Cartridges • Contract • Bulk Std. Vials, • Contract Manufacturing on Cartridges, PFS Standard
• EZ-fill® Std. PFS Manufacturing on Immunoassay and pharma IP • Ampoules Other IVD devices Bulk Ready-To-Use Capital Markets Day – September 27, 2023 30 Product Quality, SG IP or Know-How

New Weekly GLP1s for Obesity are Driving Market Expansion GLP-1
Selected Examples of Injectables Obesity Products Obesity Market Forecast PRODUCT DDS DCS (USD Bn.) Syringe/ Wegovy (semaglutide) Cartridge Auto-injector & Pen 56 Cagrisema Syringe (semaglutide + Auto-injector cagrilintide) +36% Saxenda
Cartridge (liraglutide) Pen injector Mounjaro Syringe (tirzepatide) Auto-injector 12 Syringe Retatrutide Auto-injector 2023 2028 N/A N/A AMG-133 Source: IQVIA, January 2023, Weight Management Drugs Only; Pharmacircle Capital Markets Day –
September 27, 2023 31
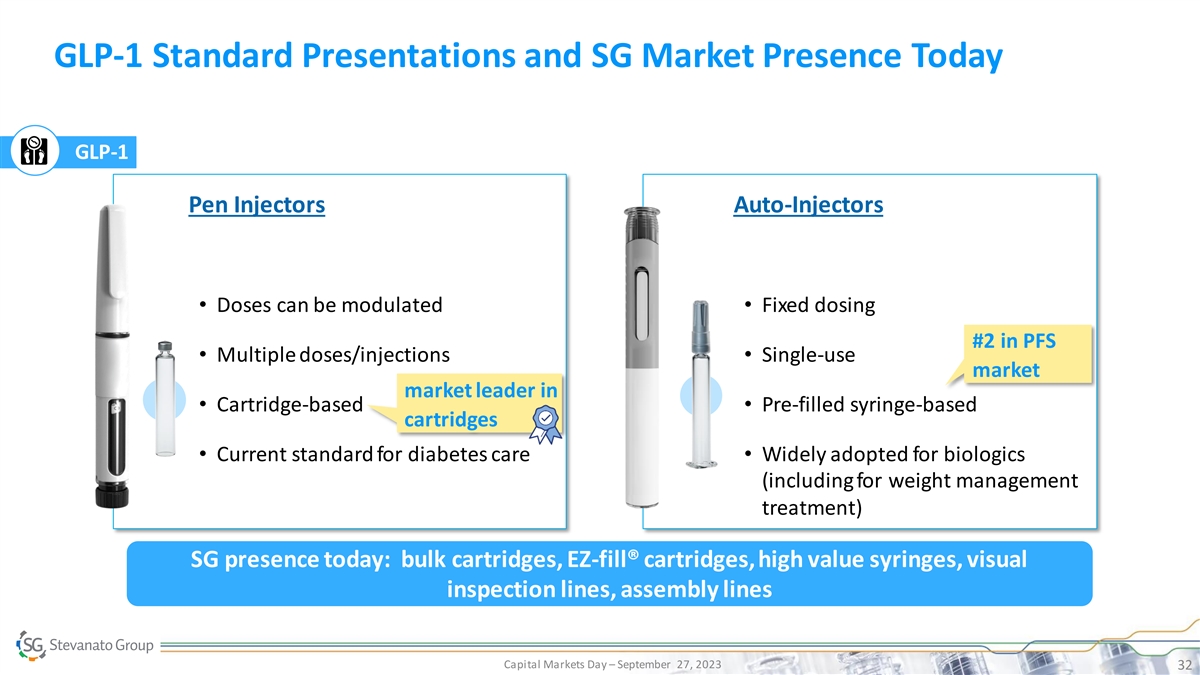
GLP-1 Standard Presentations and SG Market Presence Today GLP-1 Pen
Injectors Auto-Injectors • Doses can be modulated• Fixed dosing #2 in PFS • Multiple doses/injections• Single-use market market leader in • Cartridge-based• Pre-filled syringe-based cartridges • Current
standard for diabetes care• Widely adopted for biologics (including for weight management treatment) SG presence today: bulk cartridges, EZ-fill® cartridges, high value syringes, visual inspection lines, assembly lines Capital Markets Day
– September 27, 2023 32

Building on Our “Core” BDS Products to Exploit the GLP1
Opportunity Over the Next Decade GLP-1 Obesity/GLP-1 “Runway” for SG: Incremental Revenue Potential Upside 3: Proprietary Device: Aidaptus® AI / Alina® Pen ILLUSTRATIVE Upside 2: Device Contract Manufacturing Upside 1:
EZ-fill® NEXA® Cartridges Inspection, Assembly, Due to GLP1s multiple presentations, and Packaging Equipment pharma manufacturers have shown concrete interest in EZ-fill® Nexa® EZ-fill® Nexa® PFS cartridges, enabling
flexibility between PFS and cartridges on the same filling Bulk Cartridges line 2010 2023* 2032 Wave 1 : Wave 2 : *Currently supplying: Bulk & EZ-fill® cartridges, EZ-fill® Nexa® PFS “Initial innovators launch”
“Biosimilars’ entry” (BDS Segment) and Visual Inspection & Assembly lines (Engineering) Capital Markets Day – September 27, 2023 33
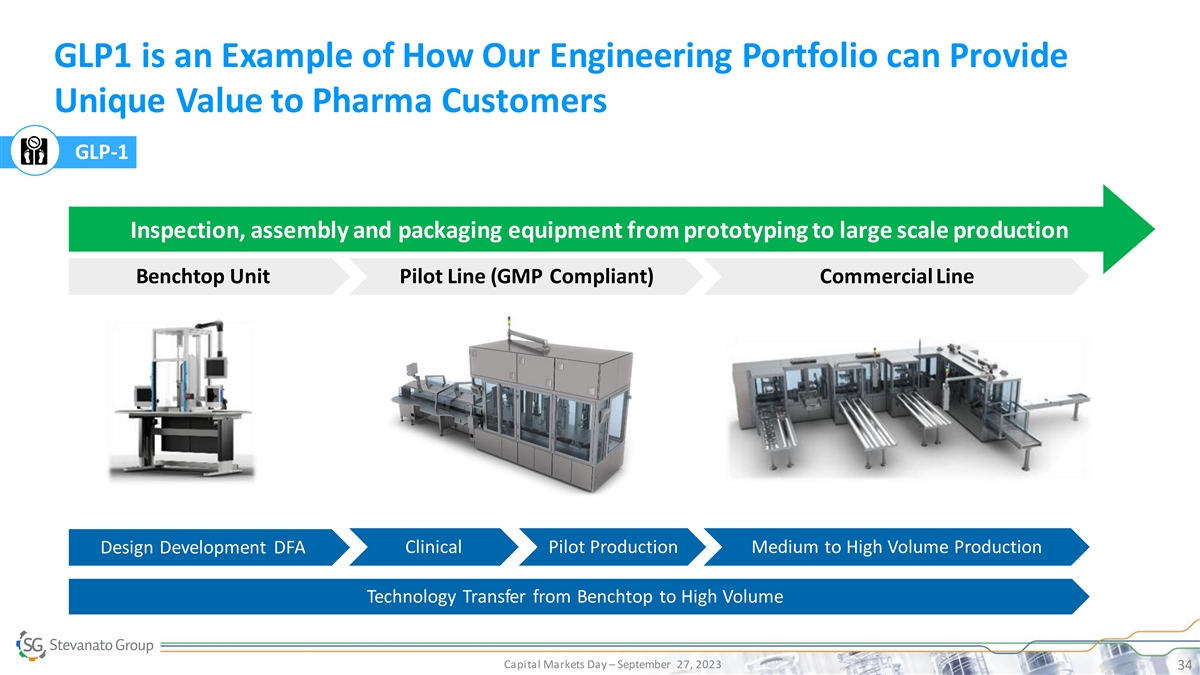
GLP1 is an Example of How Our Engineering Portfolio can Provide Unique
Value to Pharma Customers GLP-1 Inspection, assembly and packaging equipment from prototyping to large scale production Benchtop Unit Pilot Line (GMP Compliant) Commercial Line Design Development DFA Clinical Pilot Production Medium to High Volume
Production Technology Transfer from Benchtop to High Volume Capital Markets Day – September 27, 2023 34
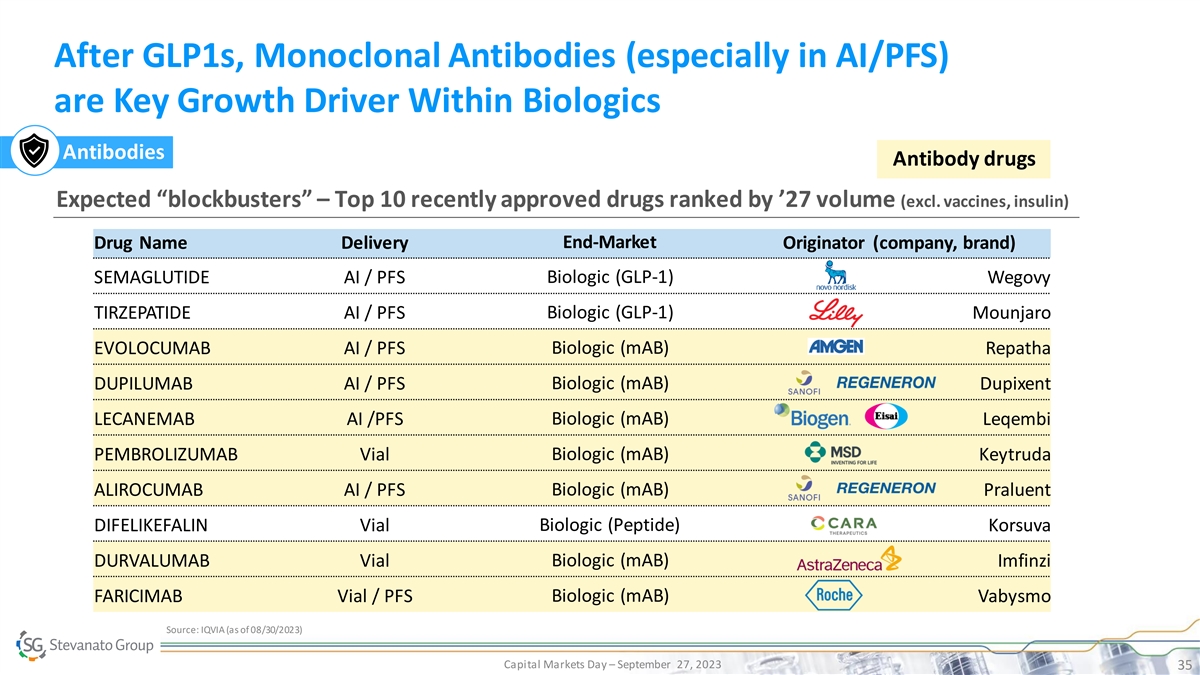
After GLP1s, Monoclonal Antibodies (especially in AI/PFS) are Key
Growth Driver Within Biologics Antibodies Antibody drugs Expected “blockbusters” – Top 10 recently approved drugs ranked by ’27 volume (excl. vaccines, insulin) Drug Name Delivery End-Market Originator (company, brand)
SEMAGLUTIDE AI / PFS Biologic (GLP-1) Wegovy TIRZEPATIDE AI / PFS Biologic (GLP-1) Mounjaro EVOLOCUMAB AI / PFS Biologic (mAB) Repatha DUPILUMAB AI / PFS Biologic (mAB) Dupixent LECANEMAB AI /PFS Biologic (mAB) Leqembi Biologic (mAB) PEMBROLIZUMAB
Vial Keytruda ALIROCUMAB AI / PFS Biologic (mAB) Praluent DIFELIKEFALIN Vial Biologic (Peptide) Korsuva Biologic (mAB) DURVALUMAB Vial Imfinzi FARICIMAB Vial / PFS Biologic (mAB) Vabysmo Source: IQVIA (as of 08/30/2023) Capital Markets Day –
September 27, 2023 35

SG Offers Wide Portfolio of HVS, Specifically Addressing the Most
Challenging Antibodies Requirements Antibodies • ALBA® PFS SG containment and delivery• NEXA® Cartridges • NEXA® Vials (both • NEXA® PFS TM Bulk and EZ-fill®) • Vertiva OBDS high-value solutions
• Aidaptus® AI Container compatibility with delivery device ● ● Tungsten residues, increasing oxidation risk ● Main antibodies Silicone oil, leading to protein aggregation ● ● challenges Multiple contact
materials, increasing reducing drug ● ● ● extractables and leachables shelf life and posing risks for Delamination, improving particles generation ● patients Mechanical resistance, increasing breakage risk ● ●
● Capital Markets Day – September 27, 2023 36
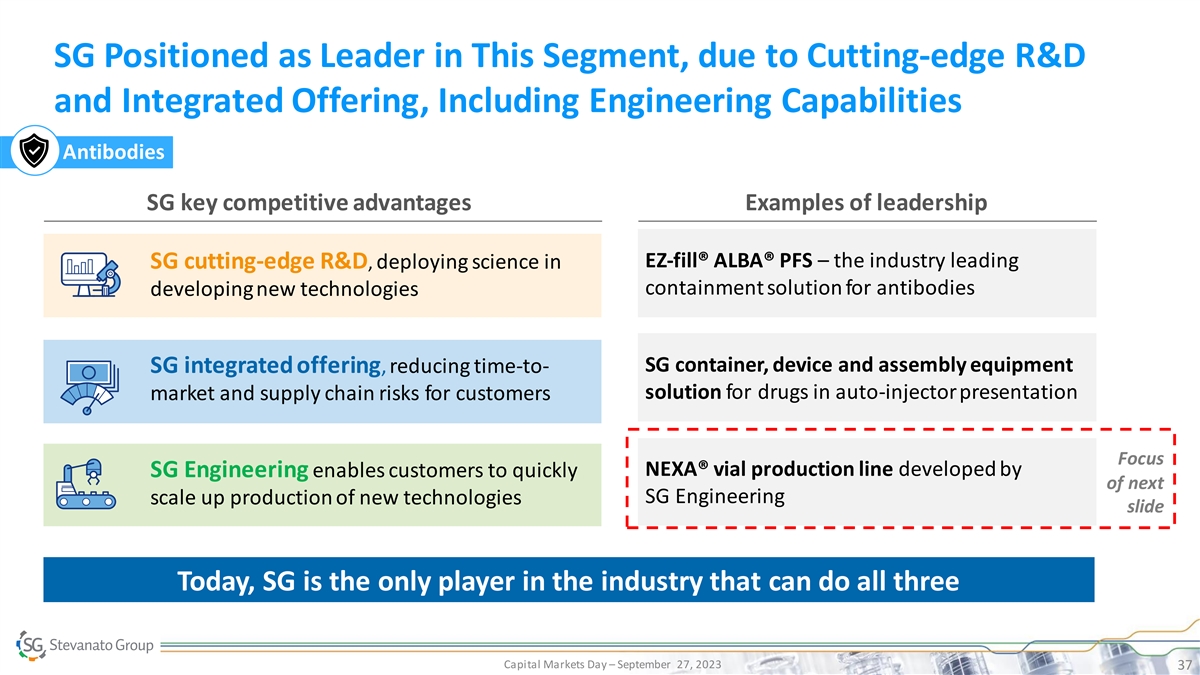
SG Positioned as Leader in This Segment, due to Cutting-edge R&D
and Integrated Offering, Including Engineering Capabilities Antibodies SG key competitive advantages Examples of leadership EZ-fill® ALBA® PFS – the industry leading SG cutting-edge R&D, deploying science in containment solution
for antibodies developing new technologies SG container, device and assembly equipment SG integrated offering, reducing time-to- solution for drugs in auto-injector presentation market and supply chain risks for customers Focus NEXA® vial
production line developed by SG Engineering enables customers to quickly of next SG Engineering scale up production of new technologies slide Today, SG is the only player in the industry that can do all three Capital Markets Day – September
27, 2023 37

SG NEXA® Vial: Proprietary Technology Where Industrialization Was
Possible Thanks to SG Engineering Antibodies Vials 1 - Proprietary Forming Process • No glass-to-glass • No metal-to-glass • Upgraded forming stations with lower contact forces during 1 forming of glass • Optimized flame
control for bottom forming 2 3 2 - Annealing Oven 3 - Dedicated Inspection System • Re-designed handling and • Multiple cameras transport system • Automatic inspection of several • No buffer station surface of vials (no
glass-to-glass) (typical defect dimension: 200μm) Capital Markets Day – September 27, 2023 38
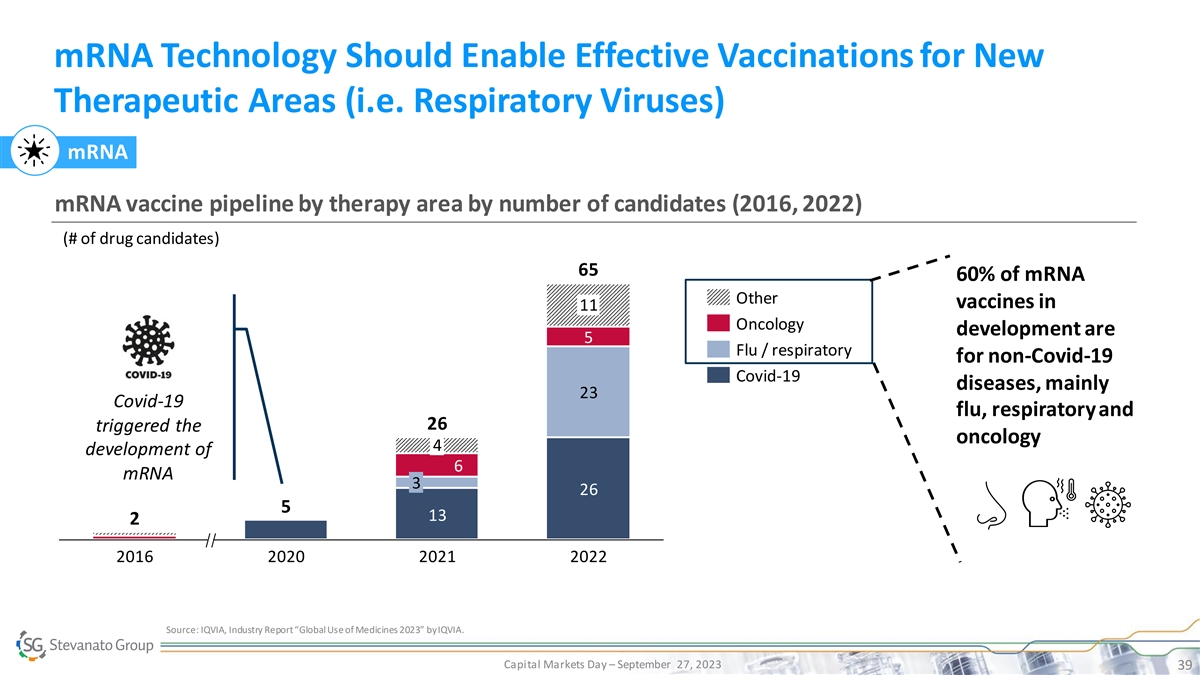
mRNA Technology Should Enable Effective Vaccinations for New
Therapeutic Areas (i.e. Respiratory Viruses) mRNA mRNA vaccine pipeline by therapy area by number of candidates (2016, 2022) (# of drug candidates) 65 60% of mRNA Other vaccines in 11 Oncology development are 5 Flu / respiratory for non-Covid-19
Covid-19 diseases, mainly 23 Covid-19 flu, respiratory and 26 triggered the oncology 4 development of 6 mRNA 3 26 5 13 2 2016 2020 2021 2022 Source: IQVIA, Industry Report “Global Use of Medicines 2023” by IQVIA. Capital Markets Day
– September 27, 2023 39

mRNA Vaccines Require Deep Cold-Storage and Pose Challenges to Drug
Product Stability and Container Closure Integrity (CCI) to PFS mRNA mRNA challenges moving from multi-dose vials to single-dose PFS SG HVS Solutions EZ-fill® Alba® ITC PFS The intrinsic nature of mRNA/Lipid (glass) Nanoparticles pose
specific challenges to maximize the product stability and drug delivery inside a pre-filled syringe The impact of deep-freezing temperatures on Container Closure Integrity (CCI) and EZ-fill® Nexa Flex™ PFS also on the functionality of the
syringes (polymer) are key elements to be verified Thanks to recent partnership with Transcoject Capital Markets Day – September 27, 2023 40

Industry and Market Outlook Agenda for the session • End-market
Pharmaceutical Demand Trends • Supply Chain and Regulatory Trends Capital Markets Day – September 27, 2023
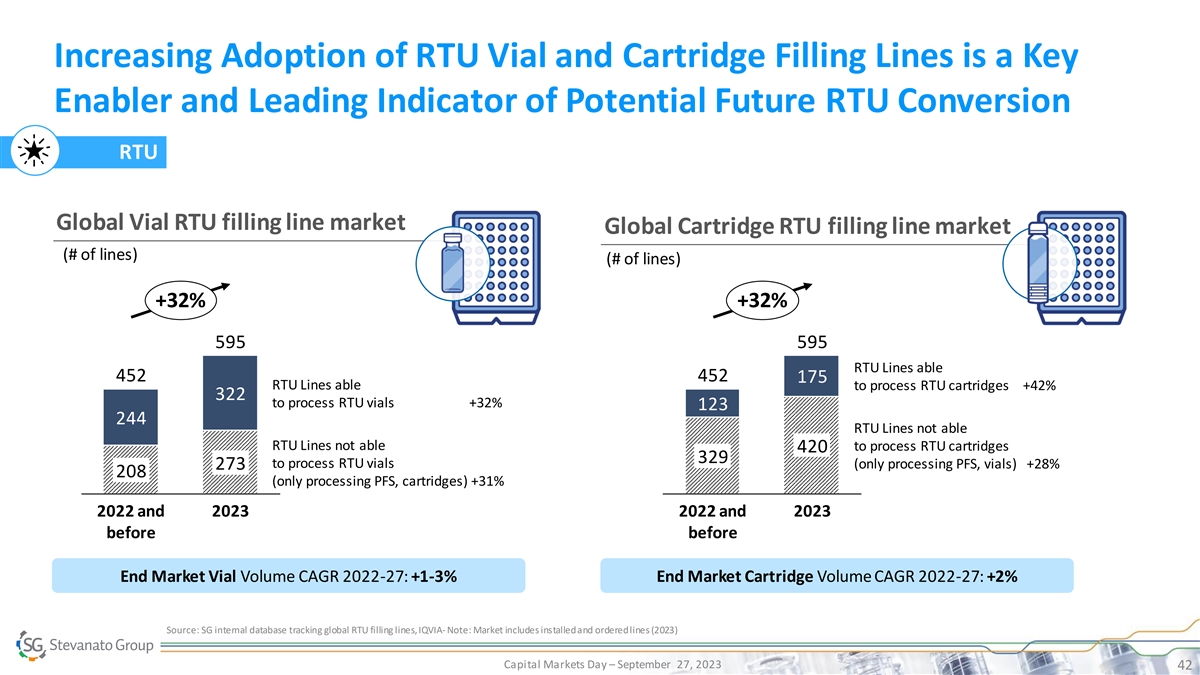
Increasing Adoption of RTU Vial and Cartridge Filling Lines is a Key
Enabler and Leading Indicator of Potential Future RTU Conversion RTU Global Vial RTU filling line market Global Cartridge RTU filling line market (# of lines) (# of lines) +32% +32% 595 595 RTU Lines able 452 452 175 RTU Lines able to process RTU
cartridges +42% 322 to process RTU vials +32% 123 244 RTU Lines not able RTU Lines not able to process RTU cartridges 420 329 to process RTU vials (only processing PFS, vials) +28% 273 208 (only processing PFS, cartridges) +31% 2022 and 2023 2022
and 2023 before before End Market Vial Volume CAGR 2022-27: +1-3% End Market Cartridge Volume CAGR 2022-27: +2% Source: SG internal database tracking global RTU filling lines, IQVIA - Note: Market includes installed and ordered lines (2023) Capital
Markets Day – September 27, 2023 42

Adoption of RTU Containers by Biopharma Manufacturers Helps Simplify
Compliance and Reduce Burden of EU-GMP Annex 1 Regulation RTU Revised Principles And Main Impacts By ANNEX 1 RTU containers simplify compliance to ANNEX 1 Impacted by RTU containers Out-of-scope for RTU containers Optimized investments to align
existing filling lines Premises and Barriers Systems with new regulation Reduced risk of particle generation during F&F process Quality Risk Management (QRM) Contamination Control Strategy (CCS) Simplifying QRM; RTU containers prevent
contamination Pre-use Post-Sterilization Integrity Testing Externalizing part of the Contamination Control Strategy (CCS) responsibilities to RTU suppliers Container Closure Integrity Testing Source:
https://www.pda.org/pda-letter-portal/home/full-article/pda-annex-1-workshop-six-key-takeaways-from-the-final-annex-1 Capital Markets Day – September 27, 2023 43
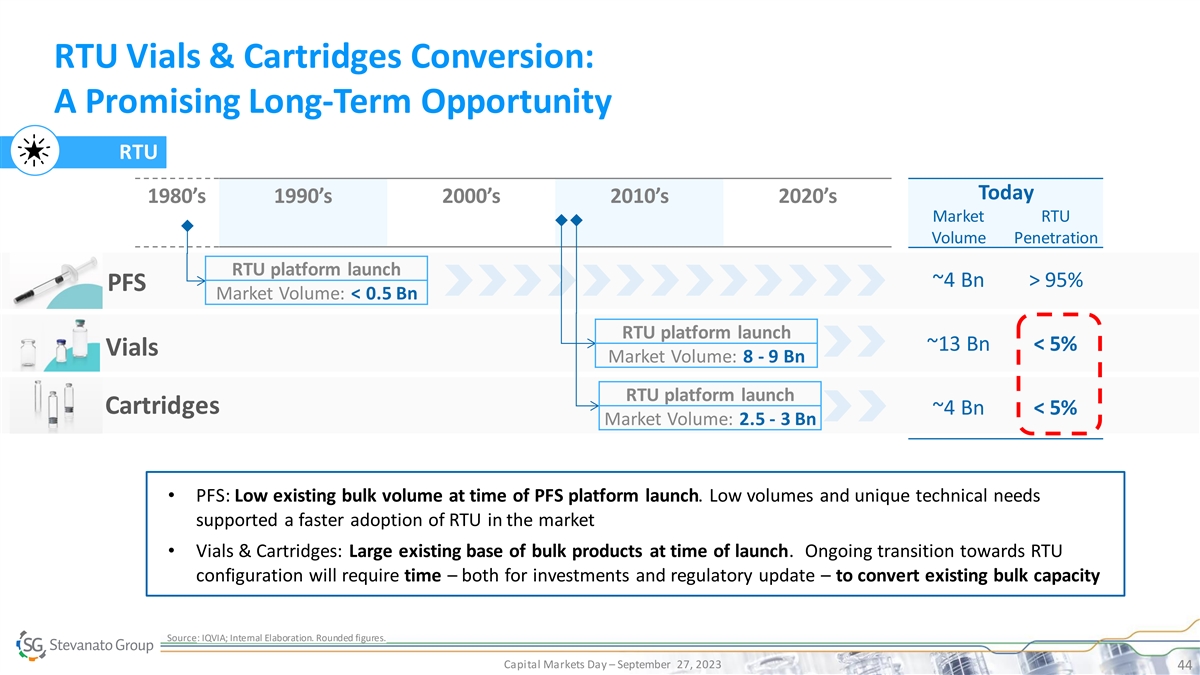
RTU Vials & Cartridges Conversion: A Promising Long-Term
Opportunity RTU Today 1980’s 1990’s 2000’s 2010’s 2020’s Market RTU Volume Penetration RTU platform launch ~4 Bn > 95% PFS Market Volume: < 0.5 Bn RTU platform launch ~13 Bn < 5% Vials Market Volume: 8 - 9 Bn
RTU platform launch Cartridges ~4 Bn < 5% Market Volume: 2.5 - 3 Bn • PFS: Low existing bulk volume at time of PFS platform launch. Low volumes and unique technical needs supported a faster adoption of RTU in the market • Vials &
Cartridges: Large existing base of bulk products at time of launch. Ongoing transition towards RTU configuration will require time – both for investments and regulatory update – to convert existing bulk capacity Source: IQVIA; Internal
Elaboration. Rounded figures. Capital Markets Day – September 27, 2023 44
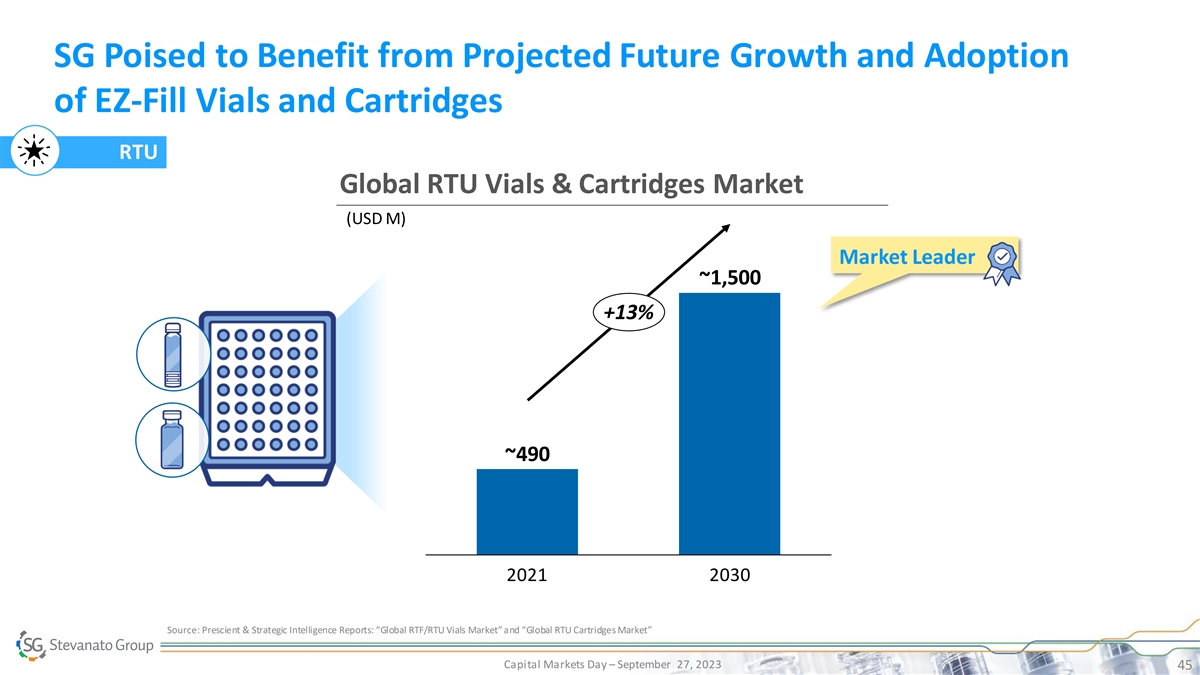
SG Poised to Benefit from Projected Future Growth and Adoption of
EZ-Fill Vials and Cartridges RTU Global RTU Vials & Cartridges Market (USD M) Market Leader ~1,500 +13% ~490 2021 2030 Source: Prescient & Strategic Intelligence Reports: “Global RTF/RTU Vials Market” and “Global RTU
Cartridges Market” Capital Markets Day – September 27, 2023 45

Stevanato Group is Well Positioned to Capitalize on Secular Tailwinds
& Favorable Demand Trends • Biologics (and injectables) is a key growth market for Stevanato Group • SG has a broad and integrated portfolio able to capture the growth coming from GLP1s, antibodies and mRNA technologies •
Combining Engineering and Services is a key differentiation for SG to deliver cutting-edge solutions to customers • Regulatory and supply chain trends are expected to accelerate growth in EZ-fill® vials & cartridges Capital Markets
Day – September 27, 2023 46

Sebastien Lebreton x Head of Global Key Accounts Customer Case Study
Capital Markets Day – September 27, 2023

Customer Paths into High Value Solutions Demand for high- Other HVS
Adoption to RTU performance products and configuration containers services Focus of this session Capital Markets Day – September 27, 2023 48
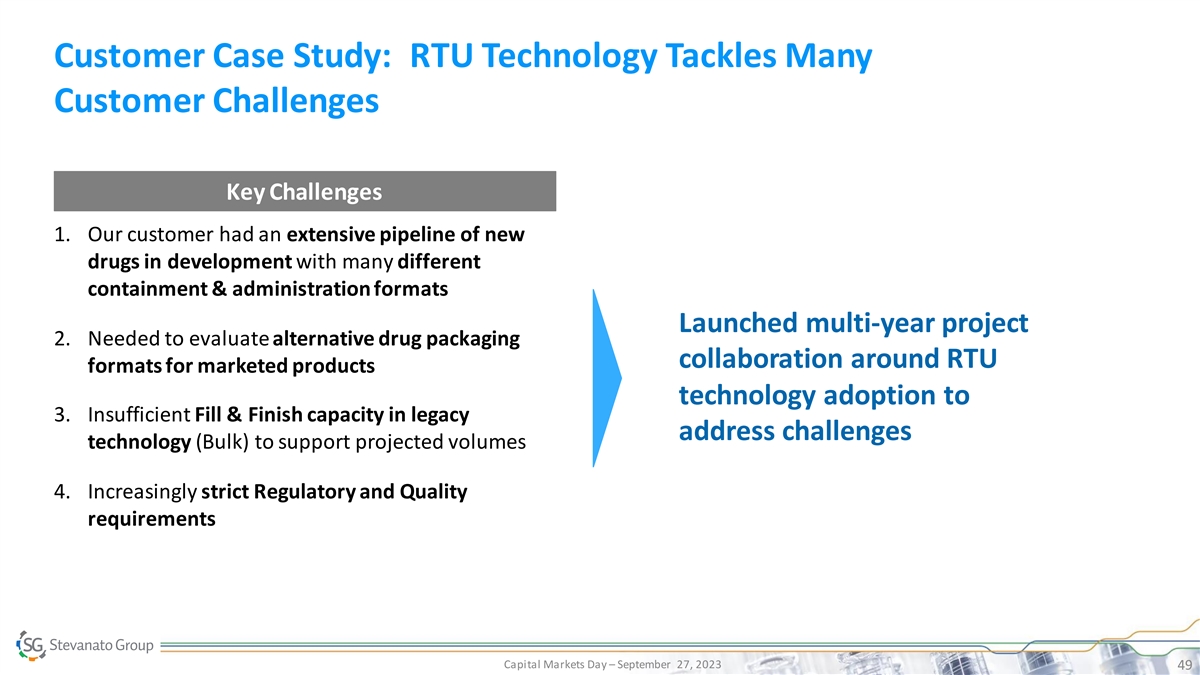
Customer Case Study: RTU Technology Tackles Many Customer Challenges
Key Challenges 1. Our customer had an extensive pipeline of new drugs in development with many different containment & administration formats Launched multi-year project 2. Needed to evaluate alternative drug packaging collaboration around RTU
formats for marketed products technology adoption to 3. Insufficient Fill & Finish capacity in legacy address challenges technology (Bulk) to support projected volumes 4. Increasingly strict Regulatory and Quality requirements Capital Markets
Day – September 27, 2023 49

R&D and scientific collaboration Multi-Year, Multi-Project
Collaboration to Small-batches product supply Large-batches product supply Support RTU (EZ-fill®) Adoption 2016 2017 2018 2019 2020 2021 2022 2023 Technology evaluation High-Value PFS SG first ever customer supplier in PFS custom development
Improved E&L profile for longer shelf life → SG TEC bypass PFS RTU Cartridges RTU custom development to optimize customer proprietary design RTU custom development accommodating RTU Vials customer proprietary design Support in scouting and
assessing best-performing (i) RTU filling equipment and (ii) CMOs Other Collaborations processing RTU Cartridges, to build internal (including retrofitting) and third-party capacity Capital Markets Day – September 27, 2023 50 V&C
Conversion to RTU

The Adoption of RTU Addresses Customer Challenges Key Challenges
Solutions 1. Our customer had an extensive pipeline of new Flexibility to accommodate any future product drugs in development with many different presentation and packaging requirement containment & administration formats 2. Needed to evaluate
alternative drug packaging Processing different formats allowing for efficient formats for marketed products (faster and cheaper) drug packaging change 3. Insufficient Fill & Finish capacity in legacy Additional capacity with higher-yield
technology (Bulk) to support projected volumes Higher quality processing standards and 4. Increasingly strict Regulatory and Quality regulation compliance requirements Reducing customer TCO and delivering superior quality Capital Markets Day –
September 27, 2023 51
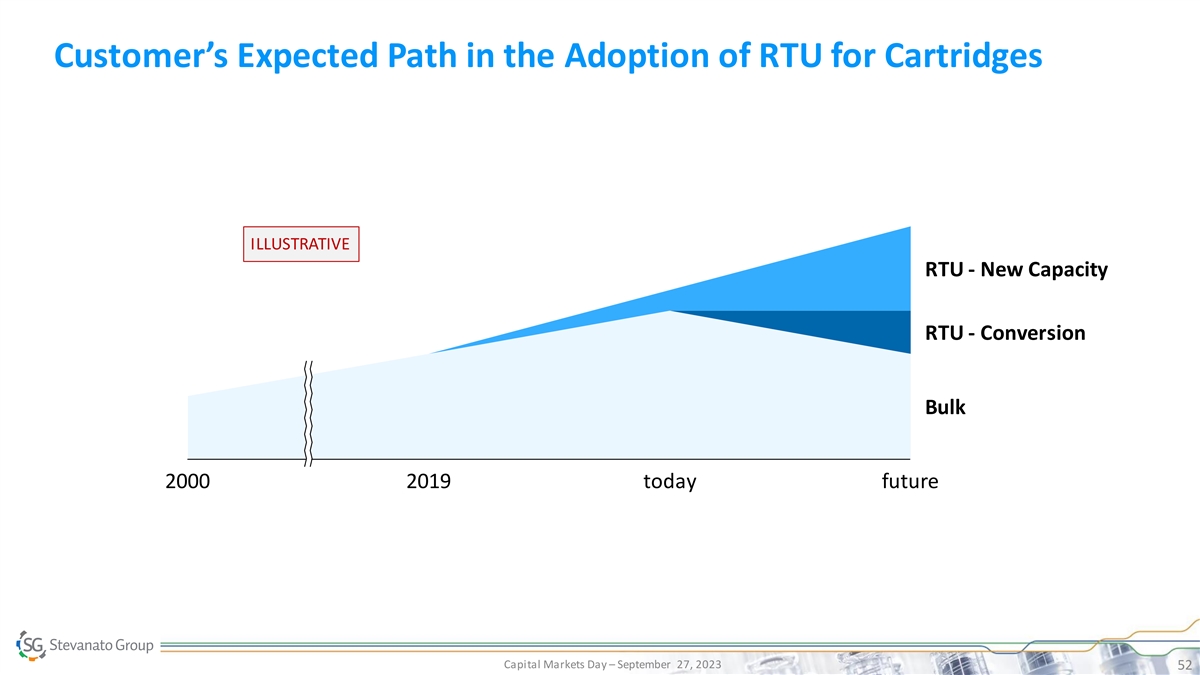
Customer’s Expected Path in the Adoption of RTU for Cartridges
ILLUSTRATIVE RTU - New Capacity RTU - Conversion Bulk 2000 2019 today future Capital Markets Day – September 27, 2023 52

Customers are Increasingly Dependent on Reliable External Partners to
Support Successful Pathway for Lifecycle Management • Supply chain needs higher flexibility to anticipate forecast variability, to Adoption of RTU answer to future needs of drugs in pipeline, and to de-risk investment configuration • The
complexity BioPharma is facing is increasingly higher High-performance • High number of non-core variables to manage (e.g., DCS + DDS) container, delivery • Increasingly strict standards required by sensitive drugs and system and
services complex regulations Unique integrated • Fragmented supply chain with multiple suppliers (on non-core value proposition capabilities) increase further complexity to manage Customers understand the key advantages of our HVS offering and
are increasingly receptive of our unique integrated value proposition Capital Markets Day – September 27, 2023 53

Q&A Capital Markets Day – September 27, 2023

Break See you at 1:00pm Capital Markets Day – September 27,
2023
Paolo Patri Chief Technology Officer Odra Pinato Head of EMEA
Technology Excellence Center R&D and Innovation Capital Markets Day – September 27, 2023

R&D and Innovation Agenda for the session • Strategy and
Overview • TEC: Technology Excellence Centers Capital Markets Day – September 27, 2023
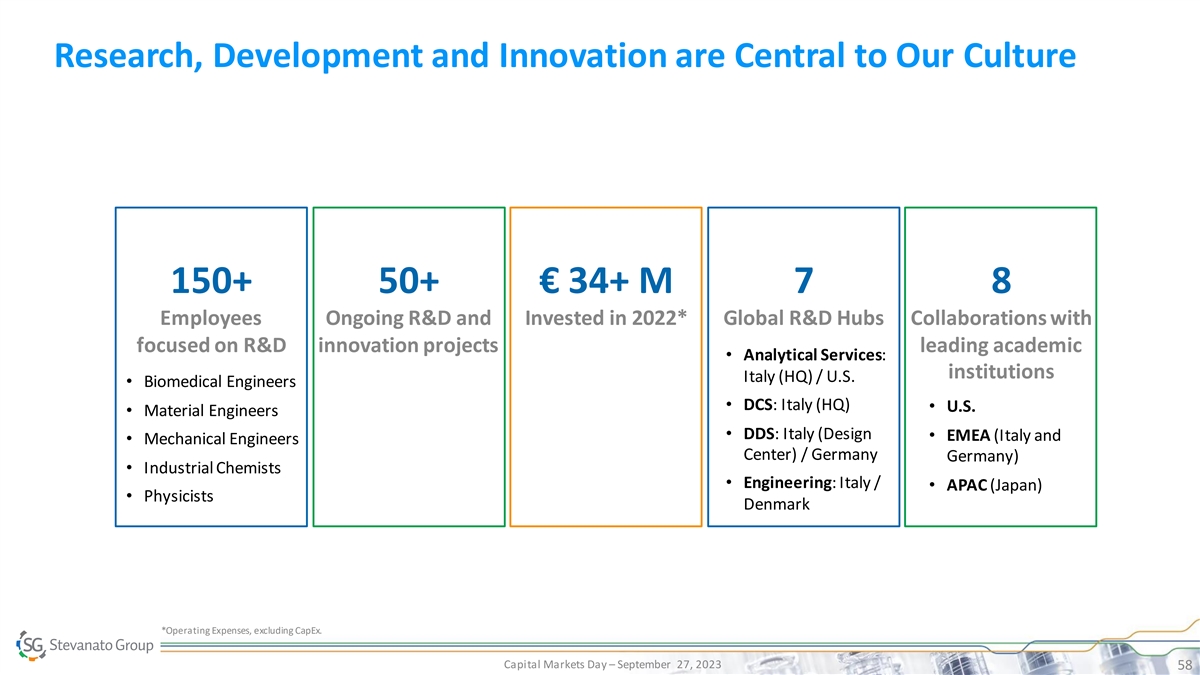
Research, Development and Innovation are Central to Our Culture 150+
50+ € 34+ M 7 8 Employees Ongoing R&D and Invested in 2022* Global R&D Hubs Collaborations with focused on R&D innovation projects leading academic • Analytical Services: institutions Italy (HQ) / U.S. • Biomedical
Engineers • DCS: Italy (HQ) • U.S. • Material Engineers • DDS: Italy (Design • EMEA (Italy and • Mechanical Engineers Center) / Germany Germany) • Industrial Chemists • Engineering: Italy / •
APAC (Japan) • Physicists Denmark *Operating Expenses, excluding CapEx. Capital Markets Day – September 27, 2023 58

R&D Strategy Designed to Meet Customers' Evolving Needs Investments
Centered on Three Key Pillars Process Drug Drug Delivery Excellence & Containment Systems Digitalization Solutions Innovate SG and customers’ Focus on patient-centricity, Develop solutions to manufacturing processes to sustainability and
maximize drug stability, deliver improved outputs and digitalization potency and purity reduced waste and risks Embedding sustainability across business operations and R&D strategy Decarbonization Bio-circular Alternate Re-usable DCS & Green
glass strategy and materials (carbon sterilization DDS concepts forming processes ISCC+ certification neutral plastic) Capital Markets Day – September 27, 2023 59

Process DCS DDS Excellence Helping Customers Bring Life Changing
Medicine to Market Pillar 1: Drug Containment Solutions • Development of DCS with lowest particle generation, reduced or no extractable release, and metal-free option • Strengthen deep freeze / dead volume properties of solutions to
support most stringent requirements of new applications • Advanced coatings methods and alternative materials to answer all needs, including from specific niche drugs Stevanato solutions are central to customers' regulatory, scientific &
technical data packages Capital Markets Day – September 27, 2023 60
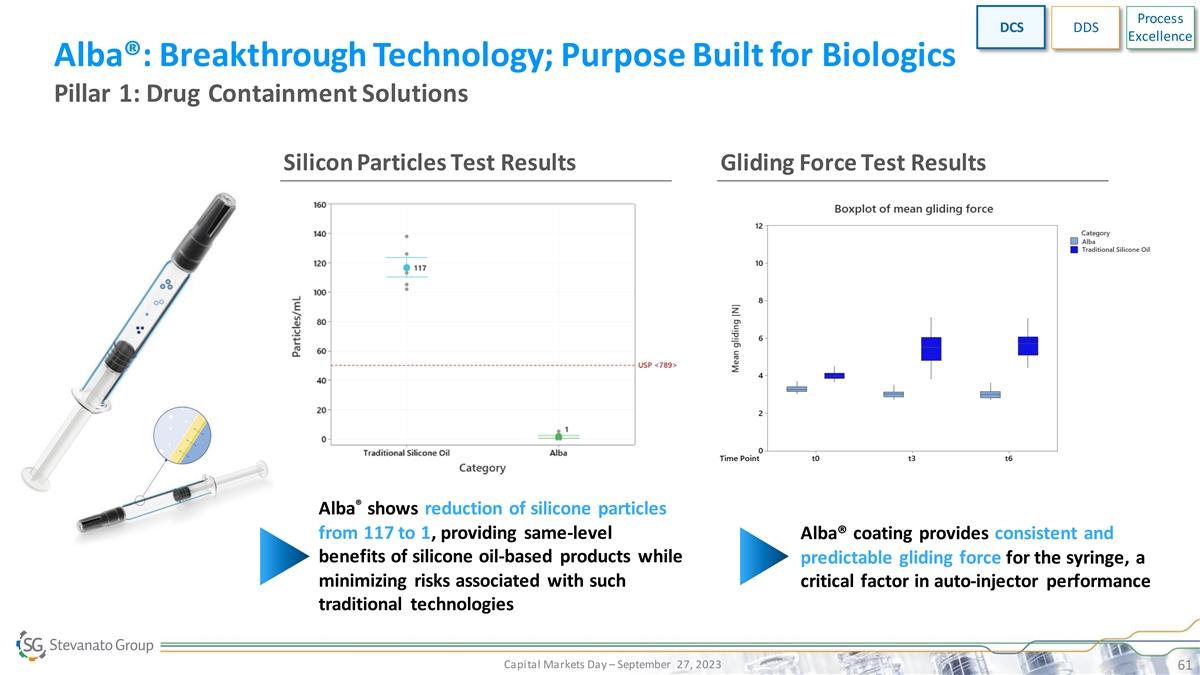
Process DCS DDS Excellence Alba®: Breakthrough Technology; Purpose
Built for Biologics Pillar 1: Drug Containment Solutions Silicon Particles Test Results Gliding Force Test Results ® Alba shows reduction of silicone particles from 117 to 1, providing same-level Alba® coating provides consistent and
benefits of silicone oil-based products while predictable gliding force for the syringe, a minimizing risks associated with such critical factor in auto-injector performance traditional technologies Capital Markets Day – September 27, 2023
61

Process DCS DDS Excellence Patient-centricity, Sustainability and
Digitalization Pillar 2: Drug Delivery Systems • Complete development of proprietary device platform portfolio: pen-injector, auto-injector, wearable device • Develop reusable and digital / connected concepts extending existing
injectable device platform • Collaborate with customer development teams to provide scientific data related to product functional performance of customers’ drugs alongside Stevanato delivery systems Capital Markets Day – September
27, 2023 62
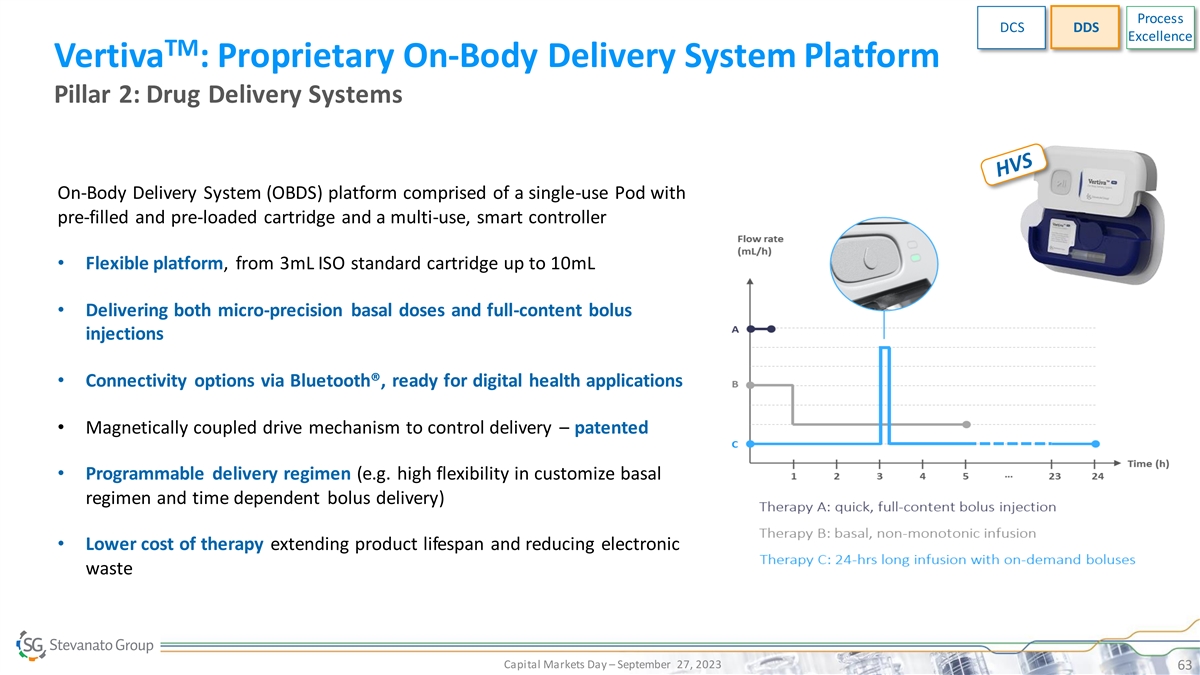
Process DCS DDS Excellence TM Vertiva : Proprietary On-Body Delivery
System Platform Pillar 2: Drug Delivery Systems On-Body Delivery System (OBDS) platform comprised of a single-use Pod with pre-filled and pre-loaded cartridge and a multi-use, smart controller • Flexible platform, from 3mL ISO standard
cartridge up to 10mL • Delivering both micro-precision basal doses and full-content bolus injections • Connectivity options via Bluetooth®, ready for digital health applications • Magnetically coupled drive mechanism to
control delivery – patented • Programmable delivery regimen (e.g. high flexibility in customize basal regimen and time dependent bolus delivery) • Lower cost of therapy extending product lifespan and reducing electronic waste
Capital Markets Day – September 27, 2023 63

Process DCS DDS Excellence Process Innovation Driving Increased
Efficiency in Manufacturing Processes Pillar 3 : Process Excellence & Digitalization • Virtual prototyping accelerating development process of DCS, DDs and equipment, taking lead time from months to weeks and reducing time-to-market
• Embed Artificial Intelligence to improve visual inspection inside biopharma operations to reduce drug product waste and mitigate medicine shortages Capital Markets Day – September 27, 2023 64
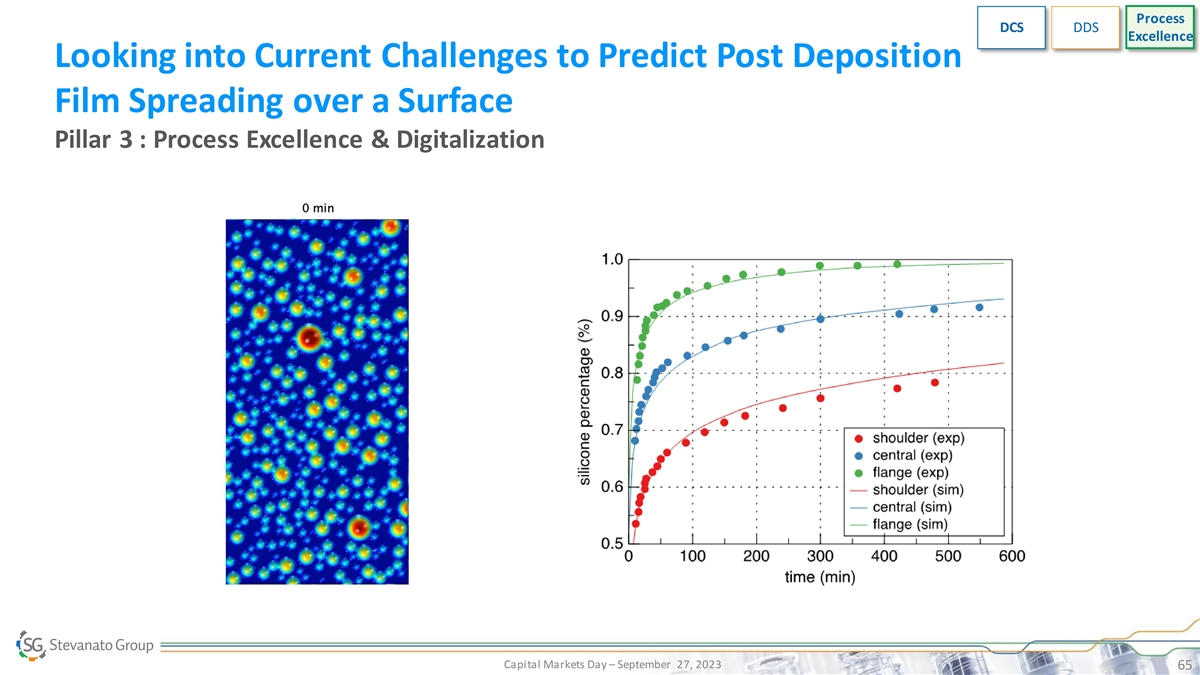
Process DCS DDS Excellence Looking into Current Challenges to Predict
Post Deposition Film Spreading over a Surface Pillar 3 : Process Excellence & Digitalization Capital Markets Day – September 27, 2023 65

Collaborations Complement Our Product & Service Portfolio 2021 2022
Extractable and leachables / TM Nexa Flex : Polymer* pre-fillable syringe characterization studies 2022 2023 End-to-end supply chain solution for Aidaptus®: Single-use auto-Injector proprietary on-body delivery system *Nexa Flex™ is
available both in COP (Cyclic Olefin Polymer) and in COC (Cyclic Olefin Copolymer). Capital Markets Day – September 27, 2023 66

R&D and Innovation Agenda for the session • Strategy and
Overview • TEC: Technology Excellence Centers Capital Markets Day – September 27, 2023
Supporting Customer Development with Early Analytics and Testing
Cutting-Edge Technology Excellence Centers (TEC) • Expert hub partnering with customers at an early stage to support their scientific and technical needs in drug product development • Wide breadth of scientific and technical expertise
leveraging 70+ years of glass technology and science • Providing technical and analytical services since 2016 (SG Lab) U.S. TEC, Boston EMEA TEC, Piombino Dese Capital Markets Day – September 27, 2023 68

Technology Excellence Centers Capital Markets Day – September 27,
2023 69
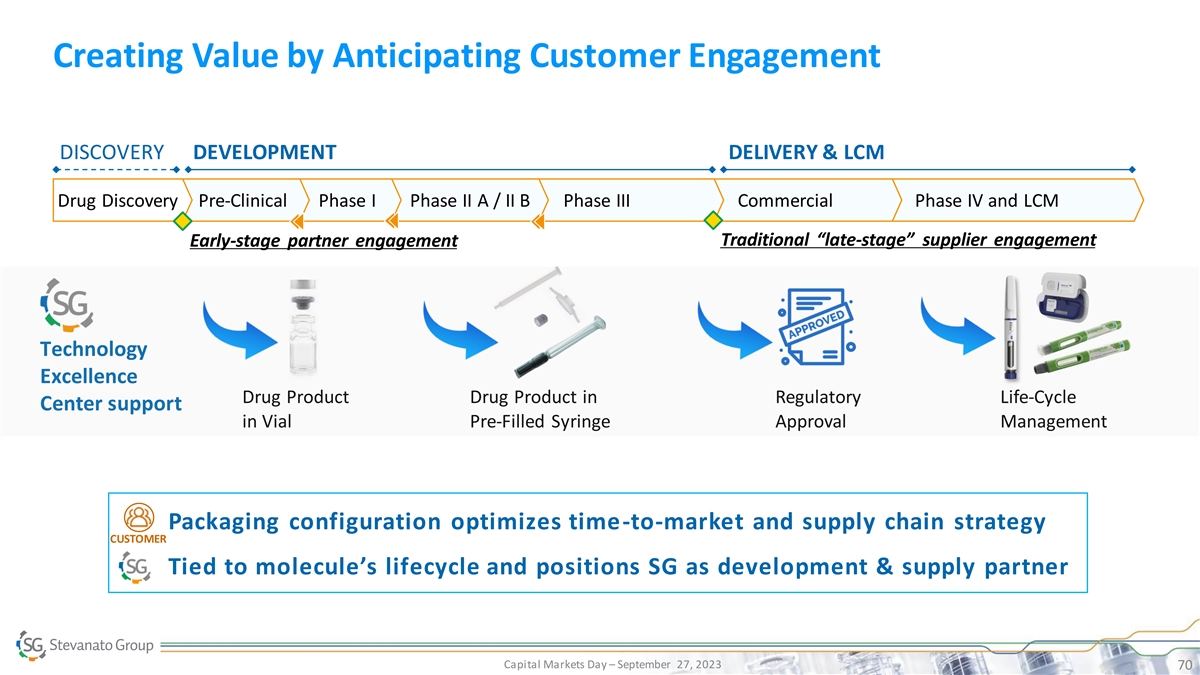
Creating Value by Anticipating Customer Engagement DISCOVERY
DEVELOPMENT DELIVERY & LCM Drug Discovery Pre-Clinical Phase I Phase II A / II B Phase III Commercial Phase IV and LCM Traditional “late-stage” supplier engagement Early-stage partner engagement Technology Excellence Drug Product
Drug Product in Regulatory Life-Cycle Center support in Vial Pre-Filled Syringe Approval Management • Packaging configuration optimizes time-to-market and supply chain strategy CUSTOMER • Tied to molecule’s lifecycle and positions
SG as development & supply partner Capital Markets Day – September 27, 2023 70
Analytical and Testing Services Help Enhance the Integrity of Medicine
Focus on Quality, Continuous Innovation and Value-added Services Surface Characterization Chemical Analysis to detect Container Performance (e.g., (e.g., silicone layer Small-batch Fill & Finish risk of chemical interaction gliding force test,
sub-visible distribution, surface defects Services with drugs particles release, leakage) evaluation) Consultancy Container Interaction to Tailored Analytical Services preserve and protect (e.g., statistical analysis, (e.g., regulatory, QA / QC,
…expanding offering with drug integrity fluid dynamics studies) stability & compatibility test) complementary set of drug product technical capabilities Capital Markets Day – September 27, 2023 71

Vial Comparative Study for Customer Monoclonal Antibodies Platform
Technology Excellence Center: Customer Case Study Customer Need & Objective Strong value for customer and SG Vial selection for high-value mABs platform → de-risking of primary container selection to reduce Secured early stage (clinical)
mABs potential stability and purity issues (container-related) CUSTOMER platform de-risking container-related issues Qualified as vial supplier for customer's SG TEC role mABs platform Scientific partner providing analytical TEC recognized as
leading scientific partner services assessing glass delamination and for customer's current and future protein adsorption (head-to-head study including different vial suppliers) development needs and studies Capital Markets Day – September 27,
2023 72

R&D is a Cornerstone in Driving Real Value to Customers • We
are a Science & Technology driven company • We gain competitive advantages in developing and industrializing market-leading innovative technologies • Pharma innovation is driving an increasing need for strong scientific support at
early stage in the product development cycle • Working alongside our customers from early development creates added value – both to customers and to Stevanato – positioning us as a long-term strategic partner Capital Markets Day
– September 27, 2023 73

Q&A Capital Markets Day – September 27, 2023

Marco Dal Lago x Chief Financial Officer Financial Update Capital
Markets Day – September 27, 2023
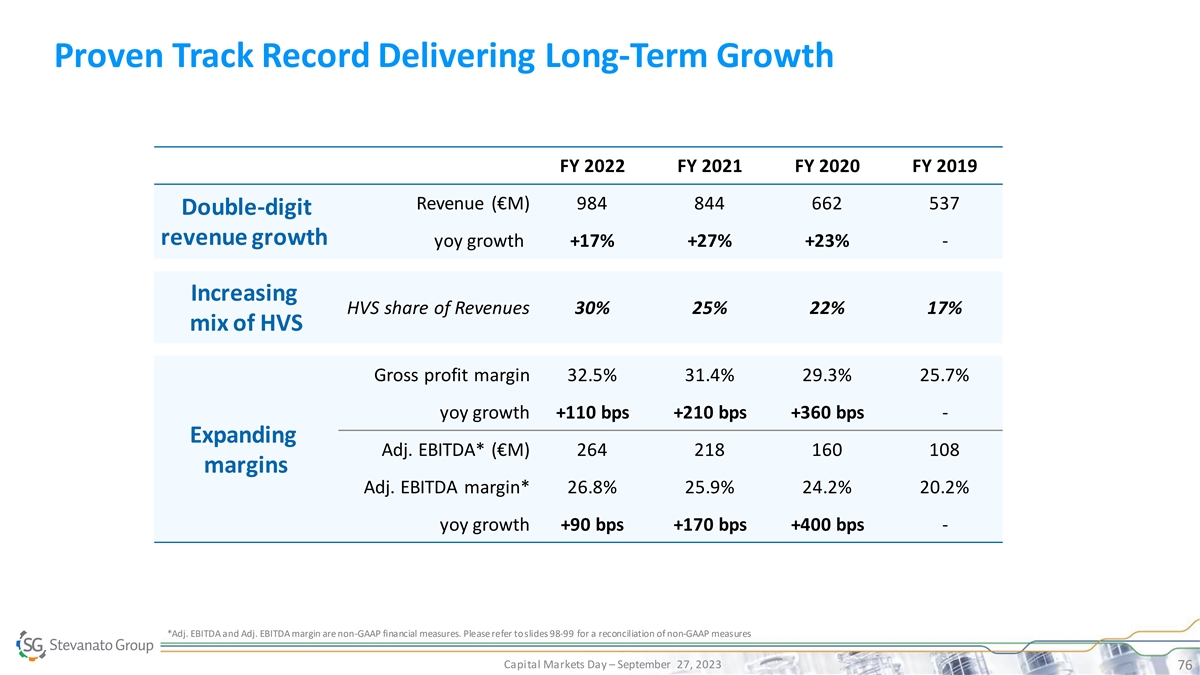
Proven Track Record Delivering Long-Term Growth FY 2022 FY 2021 FY 2020
FY 2019 Revenue (€M) 984 844 662 537 Double-digit revenue growth yoy growth +17% +27% +23% - Increasing HVS share of Revenues 30% 25% 22% 17% mix of HVS Gross profit margin 32.5% 31.4% 29.3% 25.7% yoy growth +110 bps +210 bps +360 bps -
Expanding Adj. EBITDA* (€M) 264 218 160 108 margins Adj. EBITDA margin* 26.8% 25.9% 24.2% 20.2% yoy growth +90 bps +170 bps +400 bps - *Adj. EBITDA and Adj. EBITDA margin are non-GAAP financial measures. Please refer to slides 98-99 for a
reconciliation of non-GAAP measures Capital Markets Day – September 27, 2023 76

Reiterating 2023 Guidance; Updating Mid-Term Outlook to 2027 2023 prior
outlook outlook to 2027 Revenue € 1.085B to € 1.115B - Revenue Growth 10% to 13% HSD to LDD 2022to 2026 LDD 2024 to 2027 HVS Share of Revenue 32% to 34% High 30% in 2026 40% to 45% € 291.8M to € Adjusted EBITDA* - - 303.8M
Adjusted EBITDA Margin* 27.1% (midpoint) High 20% in 2026 ~30% Adjusted DEPS* € 0.58 to € 0.62 - *Adj. EBITDA, Adj. EBITDA margin and Adj. DEPS are non-GAAP financial measures. Please refer to slides 98-99 for a reconciliation of
non-GAAP measures Capital Markets Day – September 27, 2023 77
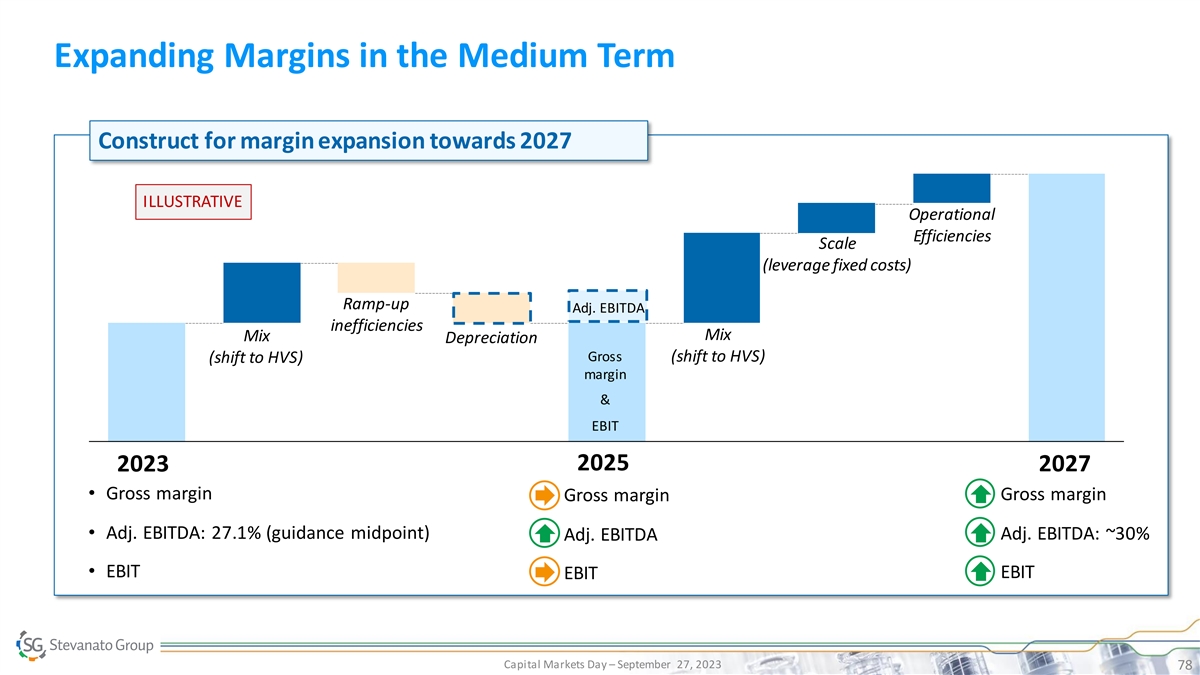
Expanding Margins in the Medium Term Construct for margin expansion
towards 2027 ILLUSTRATIVE Operational Efficiencies Scale (leverage fixed costs) Ramp-up Adj. EBITDA inefficiencies Mix Mix Depreciation Gross (shift to HVS) (shift to HVS) margin & EBIT 2025 2023 2027 • Gross margin• Gross margin
• Gross margin • Adj. EBITDA: 27.1% (guidance midpoint) • Adj. EBITDA: ~30% • Adj. EBITDA • EBIT • EBIT • EBIT Capital Markets Day – September 27, 2023 78

Mid-Term Clear Capital Allocation Priorities Towards 2027 ORGANIC
Growth R&D M&A Dividend Expand global capacity in Invest in R&D to maintain Opportunistic M&A to Expect to continue HVS to drive sustainable and accelerate HVS complement our existing distributing comparable low and accretive growth
pipeline book of business and dividend level, in line with accelerate growth plan recent years Focus of following slides Capital Markets Day – September 27, 2023 79
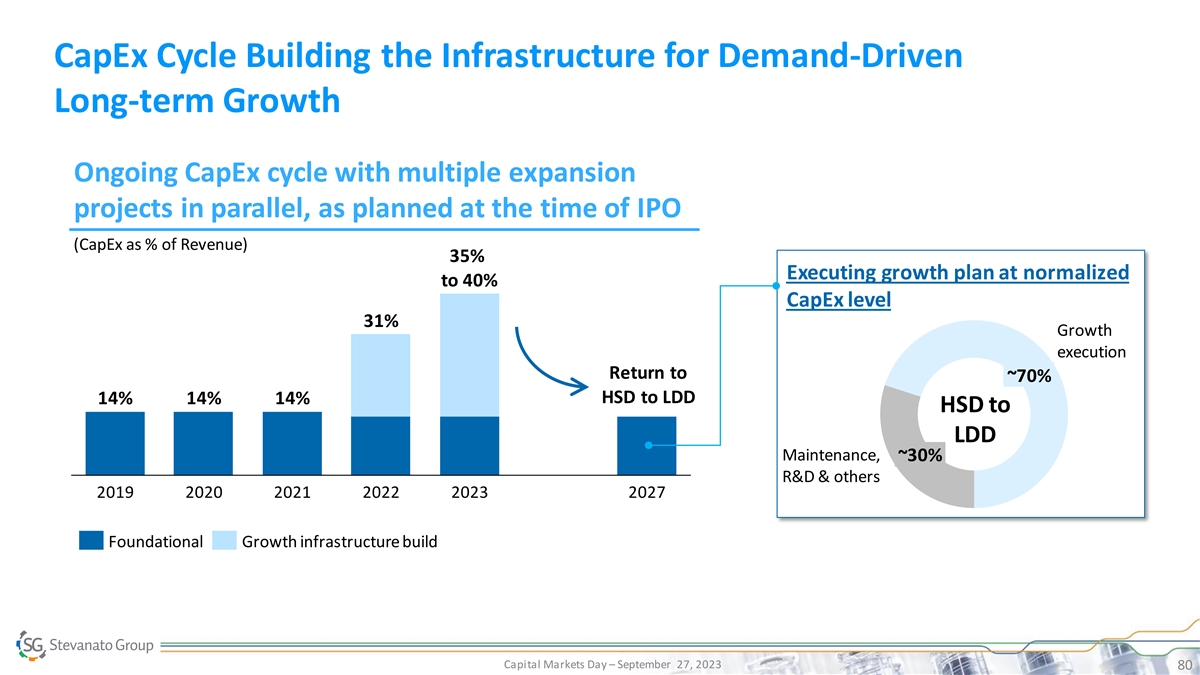
CapEx Cycle Building the Infrastructure for Demand-Driven Long-term
Growth Ongoing CapEx cycle with multiple expansion projects in parallel, as planned at the time of IPO (CapEx as % of Revenue) 35% Executing growth plan at normalized to 40% CapEx level 31% Growth execution Return to ~70% HSD to LDD 14% 14% 14% HSD
to LDD Maintenance, ~30% R&D & others 2019 2020 2021 2022 2023 2027 Foundational Growth infrastructure build Capital Markets Day – September 27, 2023 80

Attractive Return Profile with Carefully Managed Investment Risk Exp.
metrics from our EZ-fill® facilities Risk-mitigating levers • Strong commercial visibility on future demand € 1 € 1 • Anchor customers Invested CapEx Revenue Generated • Wide set of commercial opportunities; (fully
ramped-up) low customer and therapeutic area concentration • Margin-accretive Revenue • Differentiated product portfolio • Project Internal Rate of Return (IRR): over 20% • Modular approach to investments (consistent with
Piombino Dese, Italy expansion project) Capital Markets Day – September 27, 2023 81

Backing our Investments with Long-Term Demand Visibility Multi-year
Commercial Agreements provide security and flexibility: 3 to 12 1. Standalone purchase order SG Key Accounts are • Minimum volume commitments months commonly under multi- 1 to 2 • Take-or-pay 2. Rolling commercial forecast year
commercial years agreements • Price adjustment clauses … coupled with transparency and 3 to 5 3. Multi-year Commercial Agreements effective negotiation with our long-term years customers Purchase order Purchase order Purchase order over
3 4. Customer investments for manufacturing capacity (SG Engineering division) years over 5 5. Early-stage customer pipeline discussion and support for R&D and development activity years Backlog and order Intake include only purchase orders, and
do not capture the entire value of multi-year commercial agreements Capital Markets Day – September 27, 2023 82
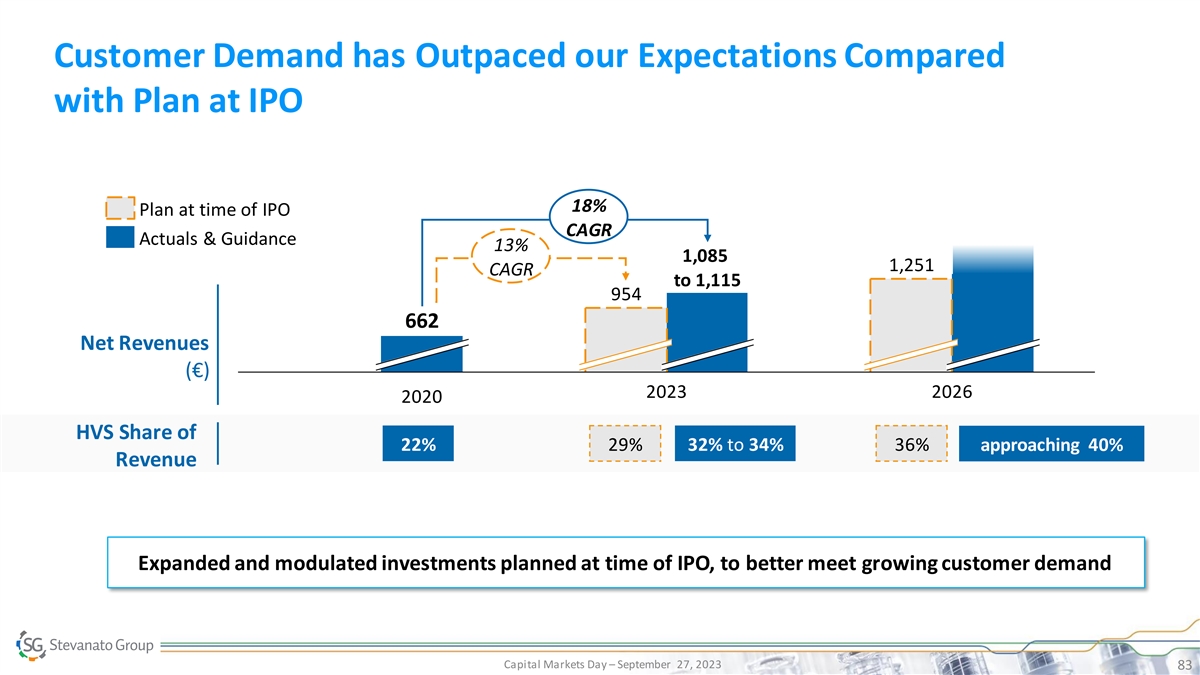
Customer Demand has Outpaced our Expectations Compared with Plan at IPO
18% Plan at time of IPO CAGR Actuals & Guidance 13% 1,085 1,251 CAGR to 1,115 954 662 Net Revenues (€) 2023 2026 2020 HVS Share of 22% 29% 32% to 34% 36% approaching 40% Revenue Expanded and modulated investments planned at time of IPO, to
better meet growing customer demand Capital Markets Day – September 27, 2023 83

Free Cash Flow Driven by Growth CapEx Cycle Free Cash Flow Trajectory
ILLUSTRATIVE 35% to 45% of adj. EBITDA Positive (with current allocations assumptions) Approaching Negative break-even (peak) 2023 2024 2025 2027 Capital Markets Day – September 27, 2023 84
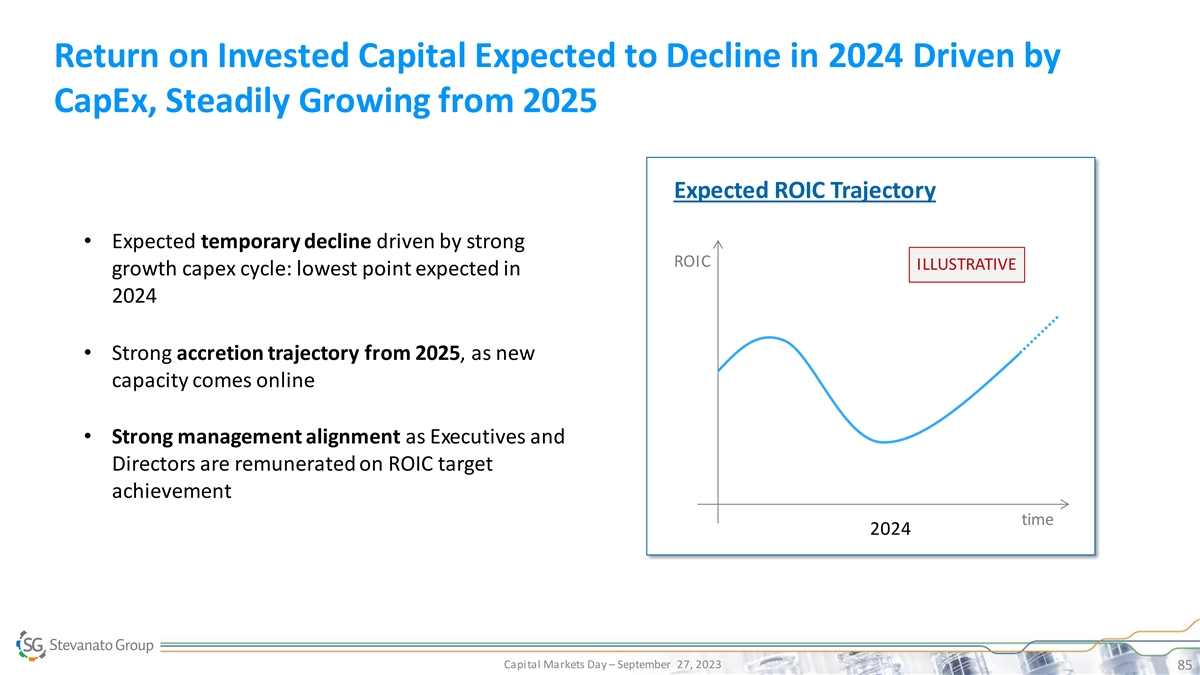
Return on Invested Capital Expected to Decline in 2024 Driven by CapEx,
Steadily Growing from 2025 Expected ROIC Trajectory • Expected temporary decline driven by strong ROIC ILLUSTRATIVE growth capex cycle: lowest point expected in 2024 • Strong accretion trajectory from 2025, as new capacity comes online
• Strong management alignment as Executives and Directors are remunerated on ROIC target achievement time 2024 Capital Markets Day – September 27, 2023 85
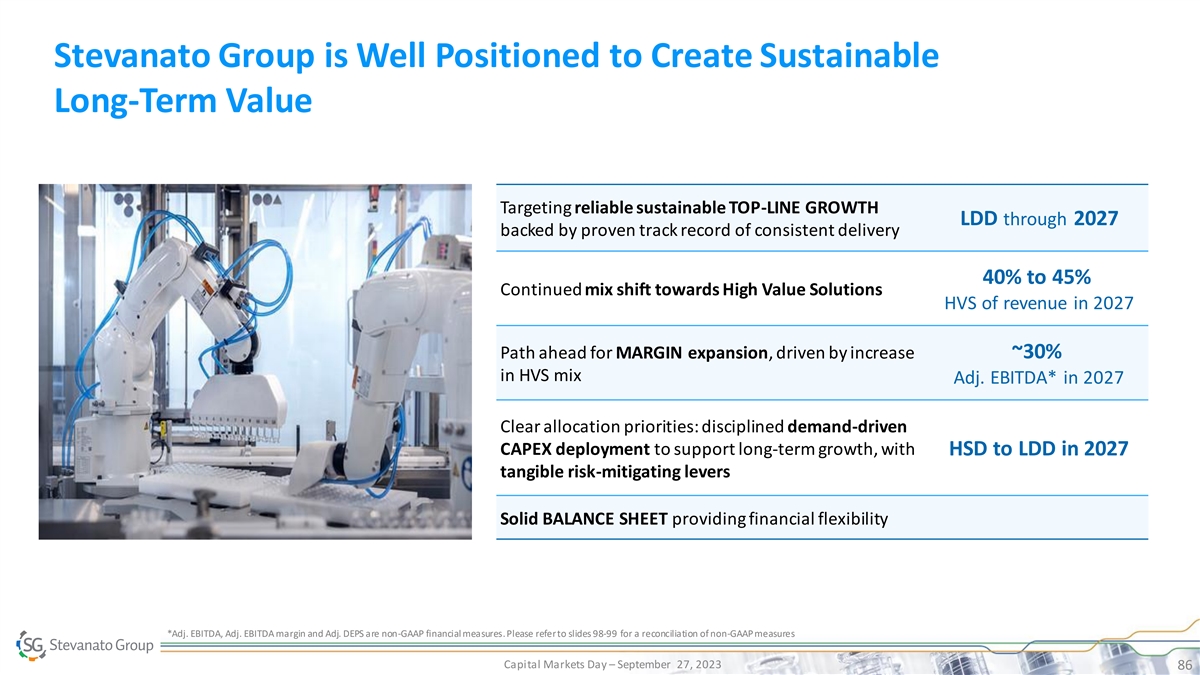
Stevanato Group is Well Positioned to Create Sustainable Long-Term
Value Targeting reliable sustainable TOP-LINE GROWTH LDD through 2027 backed by proven track record of consistent delivery 40% to 45% Continued mix shift towards High Value Solutions HVS of revenue in 2027 Path ahead for MARGIN expansion, driven by
increase ~30% in HVS mix Adj. EBITDA* in 2027 Clear allocation priorities: disciplined demand-driven CAPEX deployment to support long-term growth, with HSD to LDD in 2027 tangible risk-mitigating levers Solid BALANCE SHEET providing financial
flexibility *Adj. EBITDA, Adj. EBITDA margin and Adj. DEPS are non-GAAP financial measures. Please refer to slides 98-99 for a reconciliation of non-GAAP measures Capital Markets Day – September 27, 2023 86

Q&A Capital Markets Day – September 27, 2023

Franco Stevanato Executive Chairman Franco Moro Chief Executive Officer
Closing Remarks Capital Markets Day – September 27, 2023

Paving the Way for the Future Global partner of choice to biopharma
customers, positioned to meet increasing demand for end-to-end solutions from drug development through life-cycle management Capital Markets Day – September 27, 2023 89

Thank You

Meet the Stevanato Team Franco Stevanato, Executive Chairman Franco
Moro, Chief Executive Officer Marco Dal Lago, Chief Financial Officer Marco Dal Lago brings more than 25 years of experience in With more than 30 years of experience managing global API (Active Franco tevanato was appointed Executive Chairman in
2021, controlling, finance, administration, compliance, and risk Pharmaceutical Ingredient) manufacturing operations, Franco Moro following his decade-long tenure as Chief Executive Officer. Prior to management in multinational industrial companies
and joined Stevanato Group in 2018 as Chief Operating Officer and was that he held a variety of management positions within the Group, coordinating multi-year planning, mergers, and acquisitions promoted to Chief Executive Officer in 2021. including
sales and business development. Prior to Stevanato, processes. Franco started his career in sales at Saint Gobain in France. As CEO, Franco oversees the Company’s long-term growth strategy Marco joined Stevanato Group in 2020 as the
Company’s Chief and global industrial operations and leads 6,000 team members to Franco is the driving force behind the Group’s long-term vision and is Financial Officer where he leads the global finance, controlling, tax, drive our
mission and commitment to be the best, objective- responsible for the Company’s global expansion and development of administrative and sustainability teams. focused partner in the research and delivery of innovative solutions its robust
product portfolio. As CEO, he implemented enhanced to support the success of our customers. He is a member of the managerial processes, and structural changes to drive continuous Prior to joining Stevanato Group, Marco served as CFO of Gruppo board
of directors. innovation embedded in science and technology. He led the board Mastrotto, one of the largest leather manufacturer in Europe, with refreshment strategy to enhance corporate governance, while 13 plants worldwide. He spent more than 15
years with Nidec ASI Prior to joining Stevanato Group, Franco served in management building a management team with extensive experience in S.p.A in a variety of executive financial management positions positions in operations and chemical
engineering with Fabbrica pharmaceutical manufacturing. including group CFO. Italiana Sintetici (FIS) where he most recently served as CEO for nearly a decade. Franco holds an MBA from SDA Bocconi in Milan Franco holds a Bachelor’s Degree in
Political Science from the He graduated with a Bachelor’s Degree in Business Administration and graduated with a Bachelor’s Degree in Chemical Engineering University of Trieste and completed the advanced management from the Ca’
Foscari University of Venice. from the University of Padua. program at the Kellogg School of Management at Northwestern University. Capital Markets Day – September 27, 2023 91

Meet the Stevanato Team Riccardo Butta, President of the Americas Paolo
Patri, Chief Technology Officer Mauro Stocchi, Chief Business Officer With more than 25 years on industry experience, Riccardo Paolo Patri joined Stevanato Group in 2018 and serves as our Chief With more than 25 years of extensive business
development Butta joined Stevanato Group in February 2022 as President Technology officer. He is responsible for all aspects of R&D including experience, Mauro Stocchi joined Stevanato Group in 2004 of the Americas where he is responsible for
operations, strategic planning, R&D investments, specialized customer research and has held a variety of key management positions expansion and growth within the region. and analytical projects, and other activities supporting the Group’s
throughout his tenure, including Corporate General Manger vision. and Chief Financial Officer. Riccardo was previously Senior Vice President of Flex Health Solutions, where led the global commercial organization of a Paolo brings over 25 years of
experience in the pharmaceutical Mauro currently serves as Chief Business Officer of Stevanato Flex business unit providing contract design, manufacturing, industry, both in production and in the development of Group where he oversees all strategic
business development and logistics services with a focus on medical devices, drug pharmaceuticals and biotech, gaining a significant track record of activities including sales, product management, corporate delivery solutions, diagnostics and life
sciences equipment. achieving global regulatory approvals for both large and small development and strategic marketing, as well as supply chain During his tenure at Flex, Riccardo led the European device molecules, and combination medicinal products
through standard management. development organization, and launched and managed the and accelerated programs. Prior to Stevanato, Paolo was Chief device design center in Milan. Manufacturing Officer at Dompe Farmaceutici, and previously he He
previously spent a decade with Siemens Group and prior spent a decade as Global Head of Chemistry, Manufacturing and to that he commenced his career at De Longhi S.p.A. Riccardo earned a Master’s Degree in Mechanical Controls at Chiesi
Farmaceutici. He has held various management Engineering from the Politecnico in Milan, and a Bachelor’s positions at Cambrex Profarmaco, and Janseen-Cilag, a Johnson & Mauro earned an MBA from SDA Bocconi in Milan and holds Degree in
Business Management and Innovation from MIP in Johnson Company. a Bachelor’s Degree from Ca’ Foscari University of Venice. Milan. He successfully completed the Executive Leadership Paolo earned a Master's Degree in Chemistry from the
University of Development Program from Stanford University. Milan. Capital Markets Day – September 27, 2023 92

Meet the Stevanato Team Fabio Bertacchini, Senior Director of Product,
Doug Bruno, Senior Vice Sharon DeWolf, Marketing and Proposal and Technical Account Management President & General Counsel Communications, Americas Region Fabio Bertacchini joined Stevanato Group in 2014 and currently With more than 20 years of
experience, Doug Bruno joined Sharon DeWolfe joined Stevanato Group in 2022 Stevanato Group in 2022 as Senior Vice President, General serves as our Senior Director of Product, Proposal and Technical supporting the Americas in operational planning
and Counsel & Corporate Secretary. Account Management where he is responsible for product marketing execution of external communication plans in the region. throughout the product lifecycle including Company’s core EZ-fill® She brings
more than 20 years of experience in marketing He previously spent 11 years at West Pharmaceutical product portfolio. He serves as our project lead for the Company’s communications and event planning. Previously, she held Services, Inc.,
holding the position of Vice President and next generation EZ-fill Smart™ products, which are set for Associate General Counsel. Before West, Doug spent the position of Marketing Services Events Manager at commercial production launch (vials)
in 2024. His team also leads nearly 10 years practicing corporate law in the Philadelphia Dentsply Sirona and spent 13 years at Jacobsen, A Textron customer transformations from bulk to ready-to-use products. region, including as an Associate in the
Business & Finance Company, as their Marketing Communications Events Departments at the law firms Ballard Spahr LLP and Drinker Specialist. Prior to his current role, Fabio held management positions in Biddle & Reath LLP. strategic
marketing, M&A and finance within Stevanato Group. He Sharon earned a Bachelor’s Degree from Winthrop joined Stevanato from PwC and started his career with KPMG. He earned his J.D. with honors from Villanova University University and is a
certified LEAN Six Sigma Green Belt and School of Law, where he was managing editor of the silver level Certified Trade Show Marketer (CTSM). Fabio earned a Bachelor’s Degree in Economics and Finance from the Villanova Law Review, and holds a
Bachelor’s degree in Università degli Studi di Parma and he’s a 2024 Executive MBA Economics from the University of Pennsylvania. candidate from SDA Bocconi in Milan. Capital Markets Day – September 27, 2023 93

Meet the Stevanato Team Giacomo Guiducci, Investor Relations, Latoya
Greve, Senior Director Steven Kaufman, Vice President Strategic Alliances & M&A HR, Americas Region Drug Delivery Systems Giacomo Guiducci joined Stevanato Group in 2021 and brings Latoya Greve brings over 20 years of industry experience in
HR Steven Kaufman joined Stevanato Group in 2018 as Vice more than a decade of experience in the life sciences and operations to Stevanato Group. She joined Stevanato in 2022 as President Drug Delivery Systems where he is responsible for
pharmaceutical industry. Giacomo currently holds a dual role in Senior Director of HR for the Americas Region where she is business development, product management and strategic Investor Relations, and Strategic Alliances and M&A at responsible
for supporting the business operations in workforce initiatives in the group’s DDS business. Stevanato Group. planning, organizational development, and design and Steven brings 20 years of industry experience working with effectiveness, as
well as performance and change management. Previously, Giacomo worked in Strategic Marketing and Business leading multinational biopharmaceutical companies to Development for DBV Technologies, a French biopharmaceutical Prior to Stevanato Group,
Latoya spent 12 years at Beckton provide pen injectors, auto-injectors, and on-body delivery firm. Prior to that he served on the investment team at Innovest Dickenson, most recently as Senior Director HR for North systems, as well as test
equipment, assembly equipment, with a focus on business planning, valuation, and market America. She previously spent ten years in HR with Carl Zeiss and final device assembly services. Before joining strategies mainly in life sciences. He started
his career as a Sales Vision and LifeCare solutions. Stevanato, Steven served as Global Business Development and Business Development analyst at Alcon. Lead at Bespak and spent more than a decade at SHL. Latoya earned her Master’s Degree in
Human Resource Giacomo earned a Masters in Management and International Management from the University of Southern California and her Steven completed his Master’s Degree in Marketing & Economics from ESCP Europe and from London City
University, Bachelor’s Degree in Business Administration & Human Resource International Business from National Chengchi University in and he holds a Bachelor’s Degree in Finance from Universita Management. Taiwan and holds a
Bachelor's Degree with Honors from Bocconi. Western University in Canada. Capital Markets Day – September 27, 2023 94

Meet the Stevanato Team Sebastien Lebreton, Riccardo Marcon, Senior
Lisa Miles, Senior Vice President of Head of Global Key Accounts Director Alliances & M&A Investor Relations Sebastian Lebreton currently serves as Head of Global Key Riccardo Marcon joined Stevanato Group in 2017 and serves as With over 25
years of experience, Lisa Miles joined Stevanato Group in 2021 as Senior Vice President of Account for Stevanato Group where he maintains Senior Director of Strategic Alliances and M&A where he is Investor Relations. responsibility for sales,
customer service and collaboration responsible for strategic growth initiatives such as licensing and for the Company’s major global pharmaceutical and biotech M&A. He served as the Company’s lead for its recent strategic Previously,
Lisa spent 18 years at Maximus in a variety of customers. partnerships with Owen Mumford, Haselmeier, Transcoject, and management positions. In her most recent role, she served ThermoFisher. Riccardo also supports strategic planning, as well as
Senior Vice President of Investor Relations & Corporate Seb joined Stevanato Group in 2009 as an account manager as market and competitive intelligence for Stevanato. Communications for nearly a decade where she was and was promoted to his
current role in 2019. Prior to responsible for investor relations, global marketing and Prior to Stevanato Group, Riccardo held senior management Stevanato Group, Seb held sales and business development branding, media relations, and employee
communications. consulting positions with Bain & Company, Accenture and positions with Mecaplast. Gartner Group. Named to the 2017 and 2018 Institutional Investor’s All Seb earned a Master’s Degree in International Marketing Riccardo
earned his Master of Science and Bachelor of Science in America Executive Team for Midcap Investor Relations, Lisa and Negotiation from NEGOCIA (Paris Chamber of Electronics Engineering from the University of Padua in Italy. was ranked third for
Best Midcap IR Professionals in the Commerce & Industry), and he holds a Bachelor’s Degree in Riccardo also holds a Certificate in Technology Entrepreneurship Business, Education, and Professional Services sector. She Mechanical and
Production Engineering from University of from Santa Clara University, and a post-degree certificate in holds a Bachelor’s Degree in Communications from Paris XI. Business Administration through the Fulbright Program. Pennsylvania State
University. Capital Markets Day – September 27, 2023 95

Meet the Stevanato Team Anthony Vico, Head of U.S. Odra Pinato, Head of
EMEA Technology Technology Excellence Center Excellence Center, Lab Analytics Odra Pinato, Ph.D., joined Stevanato Group in 2014 and currently Anthony Vico joined Stevanato Group in 2011 and currently leads leads the Company’s EMEA Technology
Excellence Center Analytics our U.S. Technology Excellence Center (TEC) in Boston where he teams, where she leads our advanced laboratory focused on is responsible for R&D and analytical lab services in support of analytical chemistry, material
properties, physical and mechanical pharmaceutical and biotech customers throughout North performances testing on pharmaceutical packaging and drug America. delivery systems. With more than ten years of experience in designing, testing, Odra is a
pharmaceutical biotechnologist, with a focus on protein validating, and industrializing glass drug containment system biochemistry including a two-year post-doctoral experience in solutions for injectable drugs, Anthony has held a variety of roles
biophysics of nucleic acids and pharmaceutical chemistry. Before in technical and quality assurance for Stevanato Group. joining Stevanato, she previously served as an analytical chemist Anthony earned his Master’s Degree in Mechanical
Engineering specializing in analytical method development and validation of and Bachelor’s Degree in Mechanical Engineering from the drug products according to GMP/GLP requirements at Merieux University of Padua. NutriSciences Italy. Odra
earned her Ph.D. from the School of Biochemistry and Biotechnology, University of Padova and holds a Bachelor’s Degree in Pharmaceutical Biotechnology from the University of Padua. Capital Markets Day – September 27, 2023 96

Appendix Capital Markets Day – September 27, 2023

Reconciliation Tables (1/2) Reconciliation of 2019, 2020, 2021 and 2022
Adjusted EBITDA (Amounts in € millions, except per share data) (Unaudited) 2022 2021 2020 2019 Reported EBITDA 257.3 218.6 157.2 108.4 Adjusting items: Restructuring and related charges 0.1 1.2 - - Incentive Plans Settlement - (9.9) - - IPO
costs - 0.8 0.2 - Out-of-cycle bonus to personnel - 6.5 - - Start-up costs new plant 6.2 1.1 - - Litigation costs - - 2.8 - Adjusted EBITDA 263.6 218.3 160.2 108.4 Capital Markets Day – September 27, 2023 98
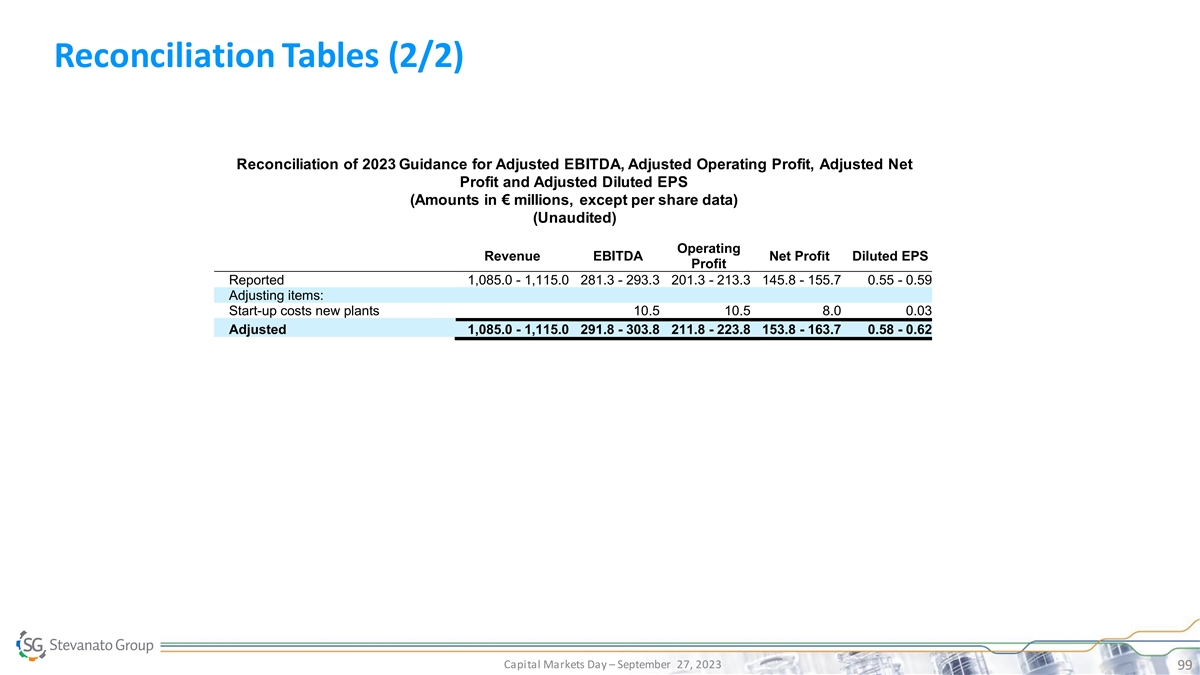
Reconciliation Tables (2/2) Reconciliation of 2023 Guidance for
Adjusted EBITDA, Adjusted Operating Profit, Adjusted Net Profit and Adjusted Diluted EPS (Amounts in € millions, except per share data) (Unaudited) Operating Revenue EBITDA Net Profit Diluted EPS Profit Reported 1,085.0 - 1,115.0 281.3 - 293.3
201.3 - 213.3 145.8 - 155.7 0.55 - 0.59 Adjusting items: Start-up costs new plants 10.5 10.5 8.0 0.03 Adjusted 1,085.0 - 1,115.0 291.8 - 303.8 211.8 - 223.8 153.8 - 163.7 0.58 - 0.62 Capital Markets Day – September 27, 2023 99

Glossary API Active Pharmaceutical Ingredient BDS BioPharmaceutical
and Diagnostic Solutions Segment CDMO Contract Development and Manufacturing Organization CMC Chemistry, Manufacturing, and Controls CMO Contract Manufacturing Organization DCS Drug Containment Solutions DDS Drug Delivery Systems FCF Free Cash Flow
HVS High Value Solutions IVD In-Vitro Diagnostic mABs Monoclonal Antibodies PFS Pre-Fillable Syringe QA Quality Assurance QC Quality Control QMS Quality Management System R&D Research and Development RTU Ready-to-Use SG Stevanato Group TCO Total
Cost of Ownership Capital Markets Day – September 27, 2023 100

Thank You

Breakout Session – Drug Containment Solutions

Fabio Bertacchini x Product, Proposal and Technical Account
Management, Senior Director Drug Containment Solutions Product Portfolio Capital Markets Day – September 27, 2023
Drug Containment Solutions – Product Portfolio Agenda for the
session • Overview • Performance • EZ-fill® Configuration Capital Markets Day – September 27, 2023

Drug Containment Solutions Overview Mission Critical Components in the
Production of Pharmaceutical and Biotechnology Products; SG's Solutions are Complex and Rely on Multiple Sophisticated Industrial Processes to Form, Treat, Inspect and Package these Products Syringe Platform Vial Platform Cartridge Platform (glass
and polymer) & Microvials Capital Markets Day – September 27, 2023 105
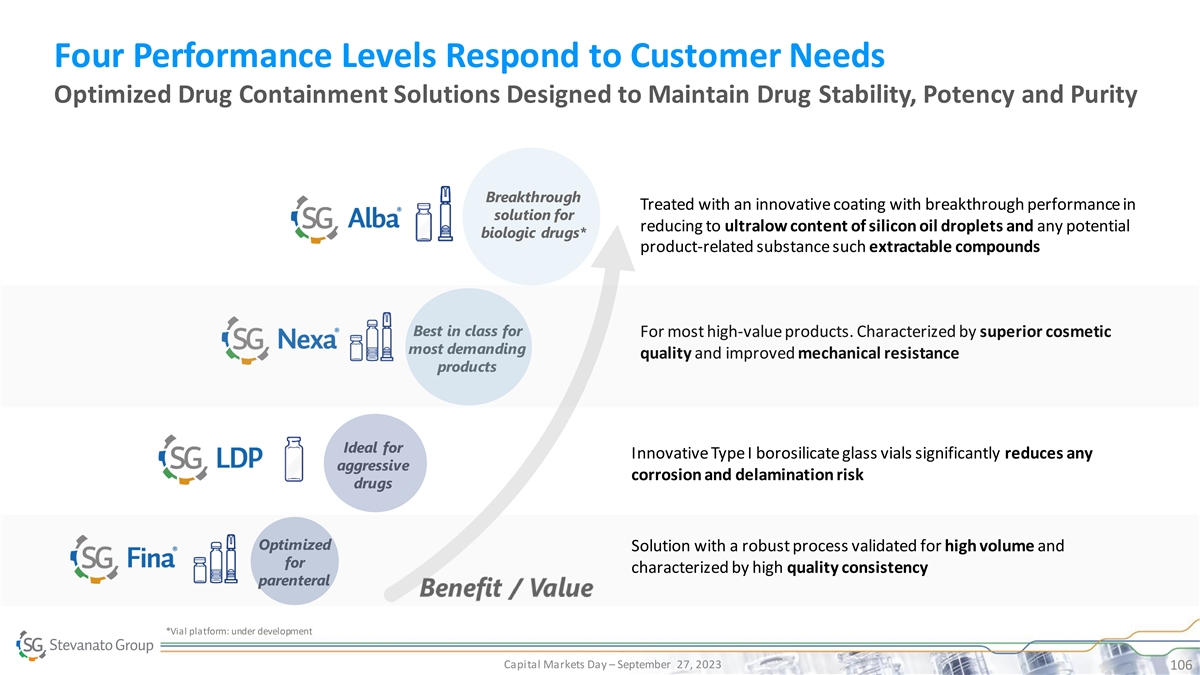
Four Performance Levels Respond to Customer Needs Optimized Drug
Containment Solutions Designed to Maintain Drug Stability, Potency and Purity Breakthrough Treated with an innovative coating with breakthrough performance in solution for reducing to ultralow content of silicon oil droplets and any potential
biologic drugs* product-related substance such extractable compounds Best in class for For most high-value products. Characterized by superior cosmetic most demanding quality and improved mechanical resistance products Ideal for Innovative Type I
borosilicate glass vials significantly reduces any aggressive corrosion and delamination risk drugs Optimized Solution with a robust process validated for high volume and for characterized by high quality consistency parenteral *Vial platform: under
development Capital Markets Day – September 27, 2023 106
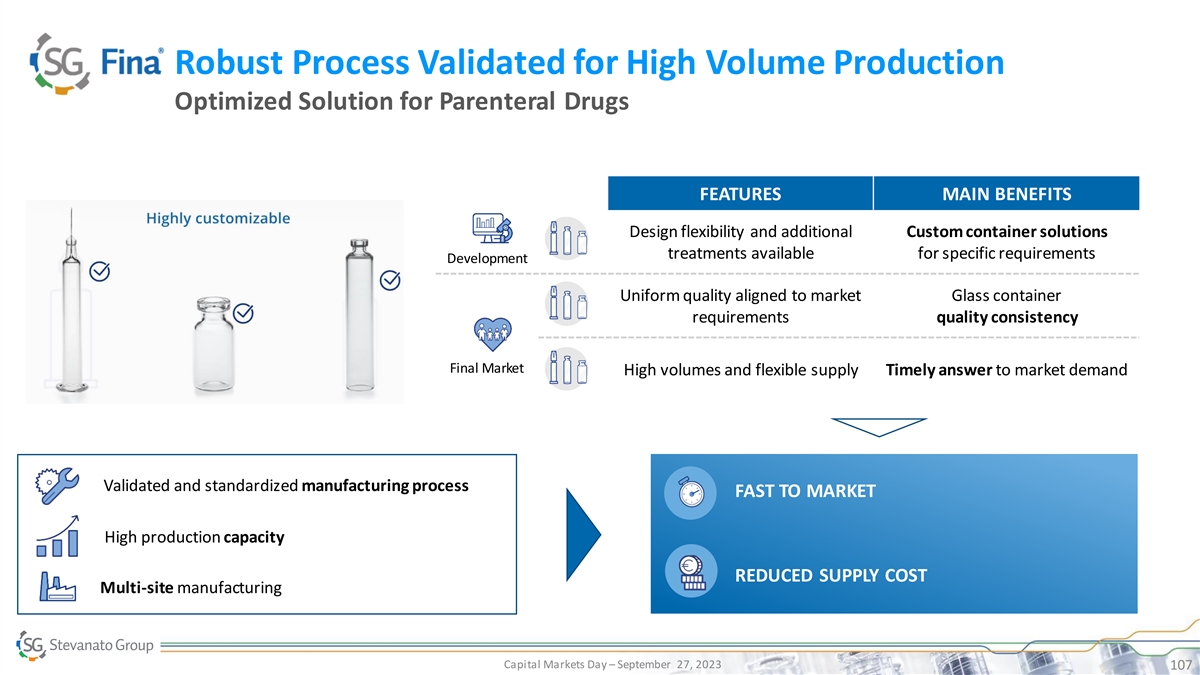
Robust Process Validated for High Volume Production Optimized Solution
for Parenteral Drugs FEATURES MAIN BENEFITS Design flexibility and additional Custom container solutions treatments available for specific requirements Development Uniform quality aligned to market Glass container requirements quality consistency
Final Market High volumes and flexible supply Timely answer to market demand Validated and standardized manufacturing process FAST TO MARKET High production capacity REDUCED SUPPLY COST Multi-site manufacturing Capital Markets Day – September
27, 2023 107
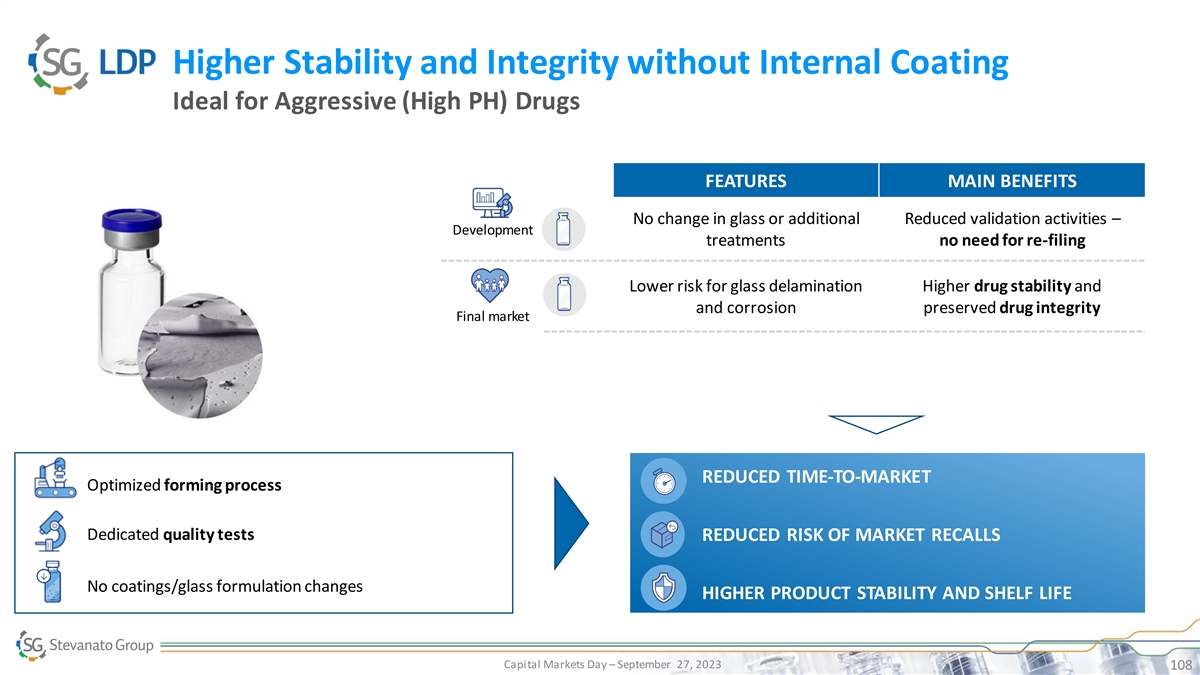
Higher Stability and Integrity without Internal Coating Ideal for
Aggressive (High PH) Drugs FEATURES MAIN BENEFITS No change in glass or additional Reduced validation activities – Development treatments no need for re-filing Lower risk for glass delamination Higher drug stability and and corrosion preserved
drug integrity Final market REDUCED TIME-TO-MARKET Optimized forming process Dedicated quality tests REDUCED RISK OF MARKET RECALLS No coatings/glass formulation changes HIGHER PRODUCT STABILITY AND SHELF LIFE Capital Markets Day – September
27, 2023 108
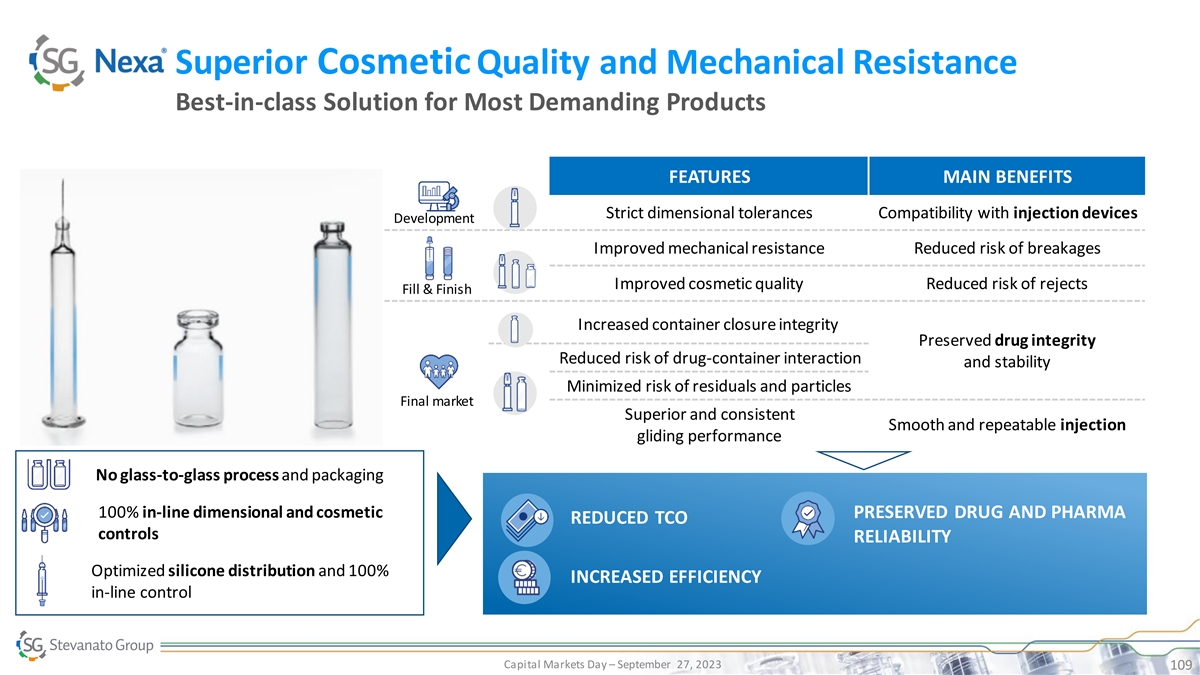
Superior Cosmetic Quality and Mechanical Resistance Best-in-class
Solution for Most Demanding Products FEATURES MAIN BENEFITS Strict dimensional tolerances Compatibility with injection devices Development Improved mechanical resistance Reduced risk of breakages Improved cosmetic quality Reduced risk of rejects
Fill & Finish Increased container closure integrity Preserved drug integrity Reduced risk of drug-container interaction and stability Minimized risk of residuals and particles Final market Superior and consistent Smooth and repeatable injection
gliding performance No glass-to-glass process and packaging 100% in-line dimensional and cosmetic PRESERVED DRUG AND PHARMA REDUCED TCO controls RELIABILITY Optimized silicone distribution and 100% INCREASED EFFICIENCY in-line control Capital
Markets Day – September 27, 2023 109
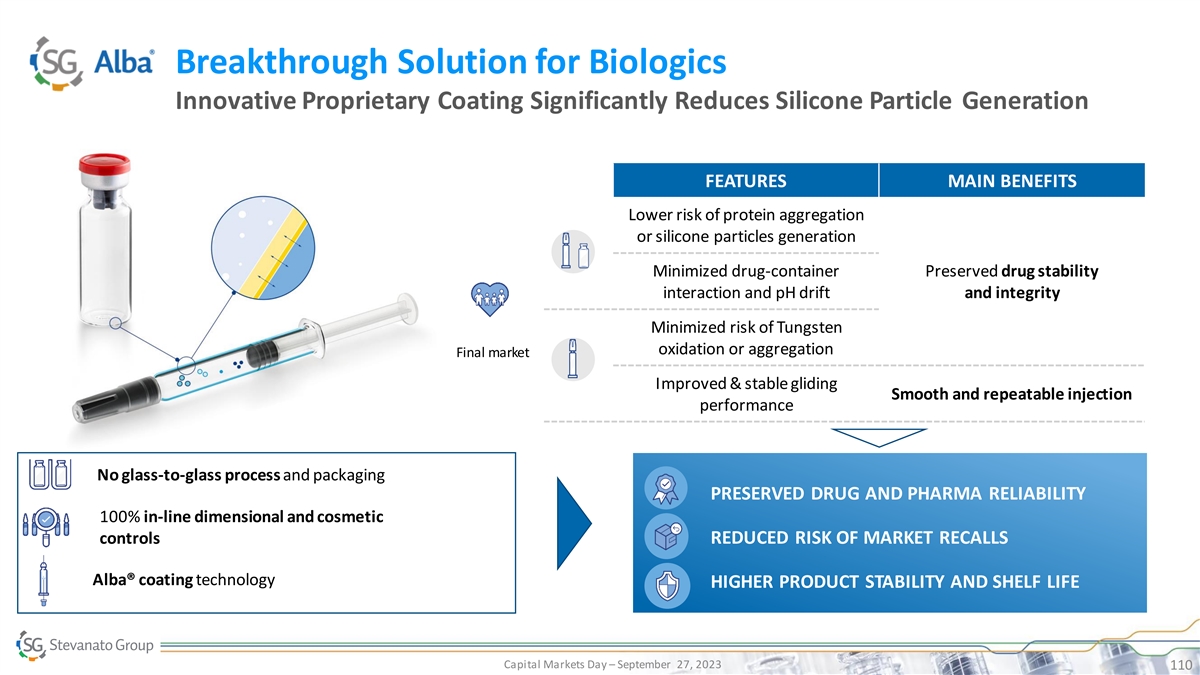
Breakthrough Solution for Biologics Innovative Proprietary Coating
Significantly Reduces Silicone Particle Generation FEATURES MAIN BENEFITS Lower risk of protein aggregation or silicone particles generation Minimized drug-container Preserved drug stability interaction and pH drift and integrity Minimized risk of
Tungsten oxidation or aggregation Final market Improved & stable gliding Smooth and repeatable injection performance No glass-to-glass process and packaging PRESERVED DRUG AND PHARMA RELIABILITY PRESERVED DRUG AND PHARMA 100% in-line dimensional
and cosmetic REDUCED TCO RELIABILITY controls REDUCED RISK OF MARKET RECALLS INCREASED EFFICIENCY Alba® coating technology HIGHER PRODUCT STABILITY AND SHELF LIFE SUCCESSFUL AND TIMELY LAUNCH Capital Markets Day – September 27, 2023
110

Microvials: Optimized Containers for Nasal Delivery Devices Microvials
Primary Packaging can be Easily Integrated into Drug Delivery Devices MICROVIALS FEATURES MAIN BENEFITS Design approved by main nasal device manufacturer Easy integration within nasal device systems and enhanced functionality Optimized small shape
for device integration Final market High cosmetic quality Design flexibility Available in Nest & Tub configuration PRESERVED DRUG AND PHARMA MEETS NEW MARKET NEEDS REDUCED TCO RELIABILITY Single and bidose applications INCREASED FLEXIBILITY
INCREASED EFFICIENCY SUCCESSFUL AND TIMELY LAUNCH 100% dimensional and cosmetic controls Capital Markets Day – September 27, 2023 111
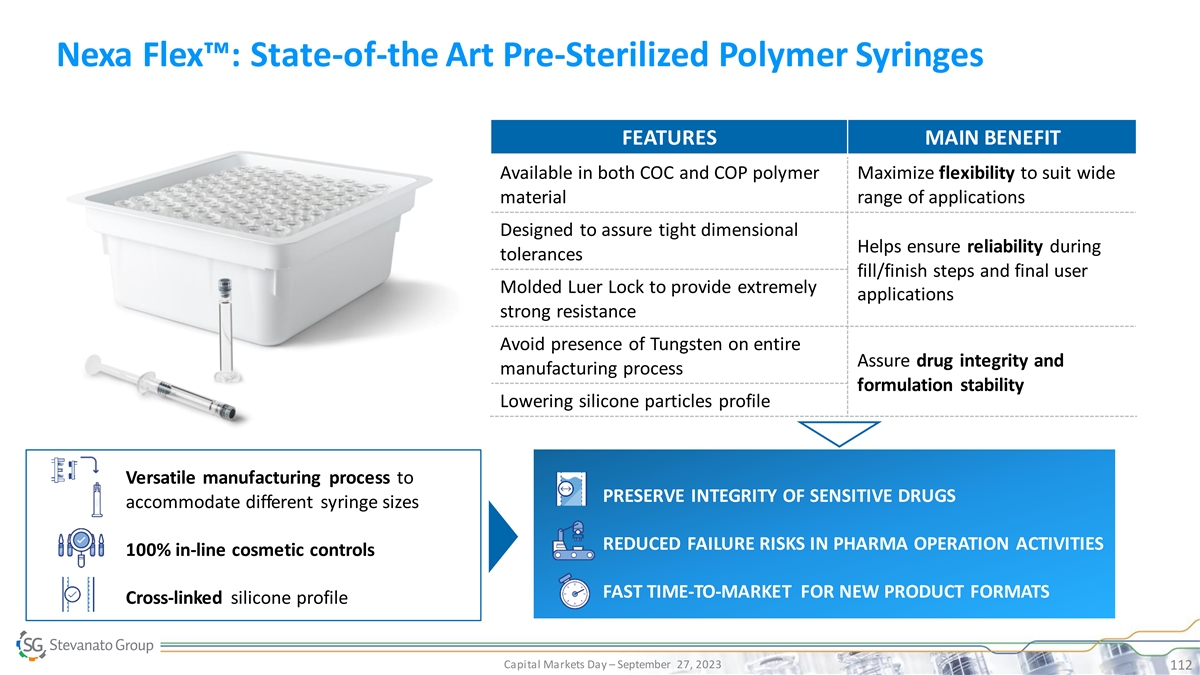
Nexa Flex™: State-of-the Art Pre-Sterilized Polymer Syringes
FEATURES MAIN BENEFIT Available in both COC and COP polymer Maximize flexibility to suit wide material range of applications Designed to assure tight dimensional Helps ensure reliability during tolerances fill/finish steps and final user Molded Luer
Lock to provide extremely applications strong resistance Avoid presence of Tungsten on entire Assure drug integrity and manufacturing process formulation stability Lowering silicone particles profile Versatile manufacturing process to PRESERVE
INTEGRITY OF SENSITIVE DRUGS accommodate different syringe sizes PRESERVED DRUG AND PHARMA REDUCED TCO RELIABILITY REDUCED FAILURE RISKS IN PHARMA OPERATION ACTIVITIES 100% in-line cosmetic controls INCREASED EFFICIENCY SUCCESSFUL AND TIMELY LAUNCH
FAST TIME-TO-MARKET FOR NEW PRODUCT FORMATS Cross-linked silicone profile Capital Markets Day – September 27, 2023 112
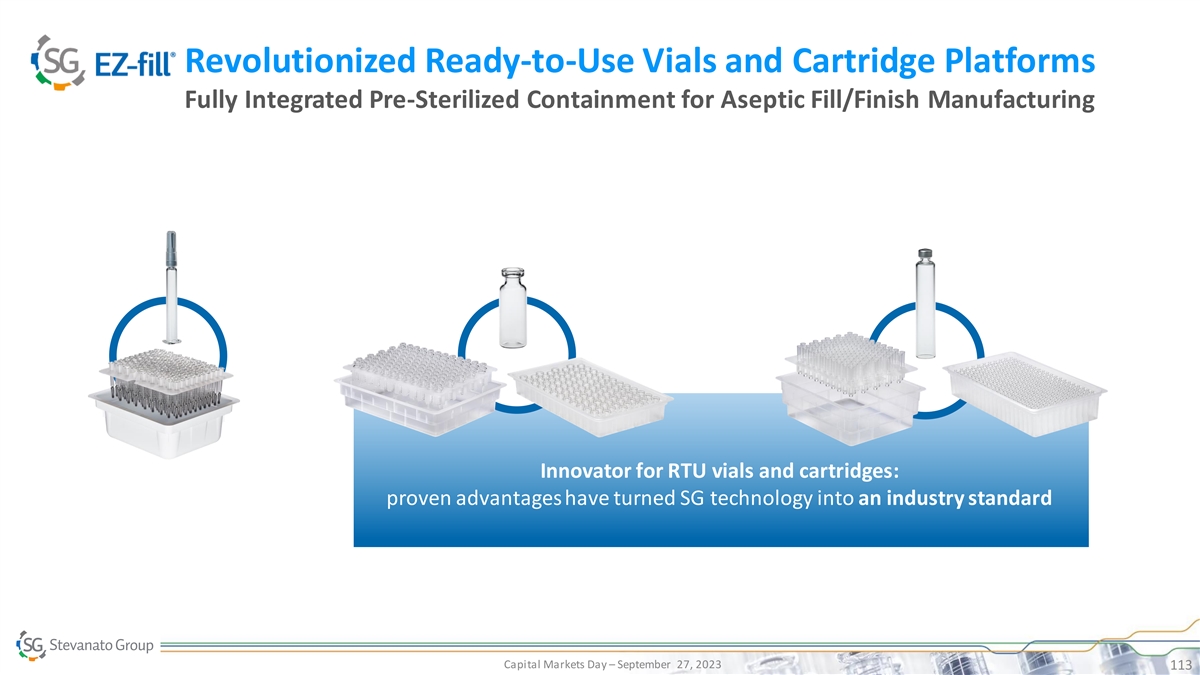
Revolutionized Ready-to-Use Vials and Cartridge Platforms Fully
Integrated Pre-Sterilized Containment for Aseptic Fill/Finish Manufacturing Innovator for RTU vials and cartridges: proven advantages have turned SG technology into an industry standard PRESERVED DRUG AND PHARMA RELIABILITY SUCCESSFUL AND TIMELY
LAUNCH Capital Markets Day – September 27, 2023 113
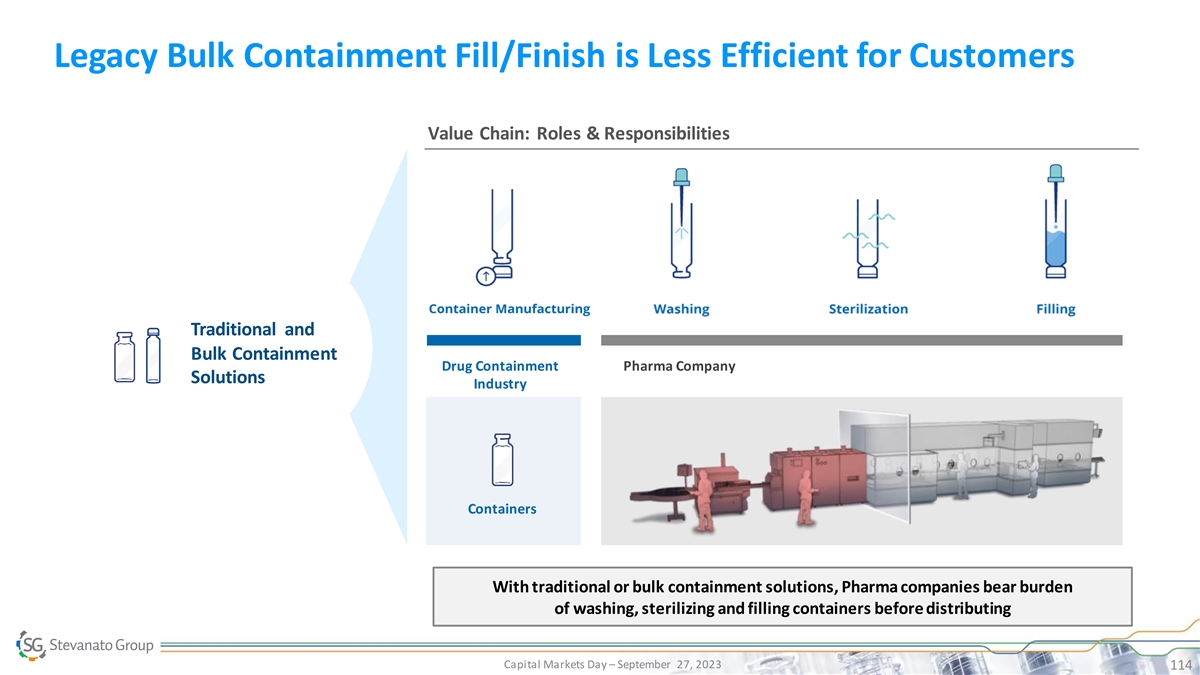
Legacy Bulk Containment Fill/Finish is Less Efficient for Customers
Value Chain: Roles & Responsibilities Traditional and Bulk Containment Drug Containment Pharma Company Solutions Industry Containers PRESERVED DRUG AND PHARMA REDUCED TCO RELIABILITY INCREASED EFFICIENCY SUCCESSFUL AND TIMELY LAUNCH With
traditional or bulk containment solutions, Pharma companies bear burden of washing, sterilizing and filling containers before distributing Capital Markets Day – September 27, 2023 114
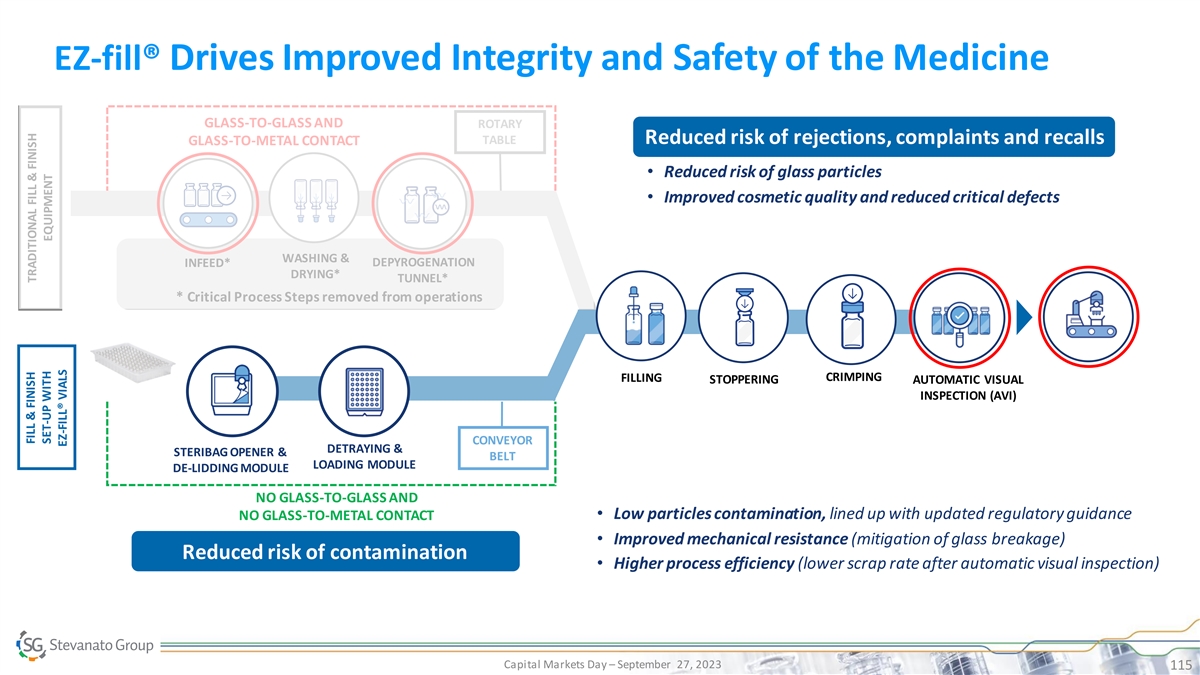
EZ-fill® Drives Improved Integrity and Safety of the Medicine
GLASS-TO-GLASS AND ROTARY TABLE Reduced risk of rejections, complaints and recalls GLASS-TO-METAL CONTACT • Reduced risk of glass particles • Improved cosmetic quality and reduced critical defects WASHING & DEPYROGENATION INFEED*
DRYING* TUNNEL* * Critical Process Steps removed from operations CRIMPING FILLING STOPPERING AUTOMATIC VISUAL INSPECTION (AVI) CONVEYOR DETRAYING & STERIBAG OPENER & BELT LOADING MODULE DE-LIDDING MODULE NO GLASS-TO-GLASS AND • Low
particles contamination, lined up with updated regulatory guidance NO GLASS-TO-METAL CONTACT • Improved mechanical resistance (mitigation of glass breakage) Reduced risk of contamination • Higher process efficiency (lower scrap rate
after automatic visual inspection) Capital Markets Day – September 27, 2023 115 FILL & FINISH TRADITIONAL FILL & FINISH SET-UP WITH EQUIPMENT EZ-FILL® VIALS
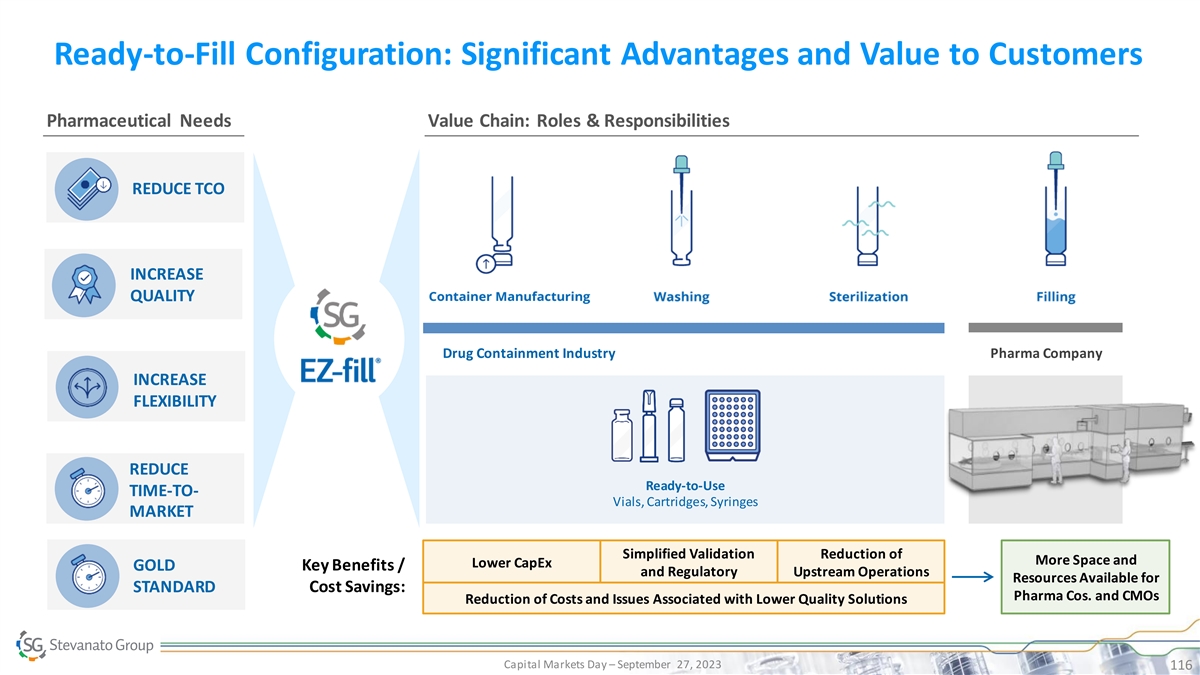
Ready-to-Fill Configuration: Significant Advantages and Value to
Customers Pharmaceutical Needs Value Chain: Roles & Responsibilities REDUCE TCO INCREASE QUALITY Drug Containment Industry Pharma Company INCREASE FLEXIBILITY REDUCE Ready-to-Use TIME-TO- Vials, Cartridges, Syringes PRESERVED DRUG AND PHARMA
MARKET REDUCED TCO RELIABILITY Simplified Validation Reduction of More Space and Lower CapEx GOLD Key Benefits / and Regulatory Upstream Operations INCREASED EFFICIENCY Resources Available for SUCCESSFUL AND TIMELY LAUNCH STANDARD Cost Savings:
Pharma Cos. and CMOs Reduction of Costs and Issues Associated with Lower Quality Solutions Capital Markets Day – September 27, 2023 116
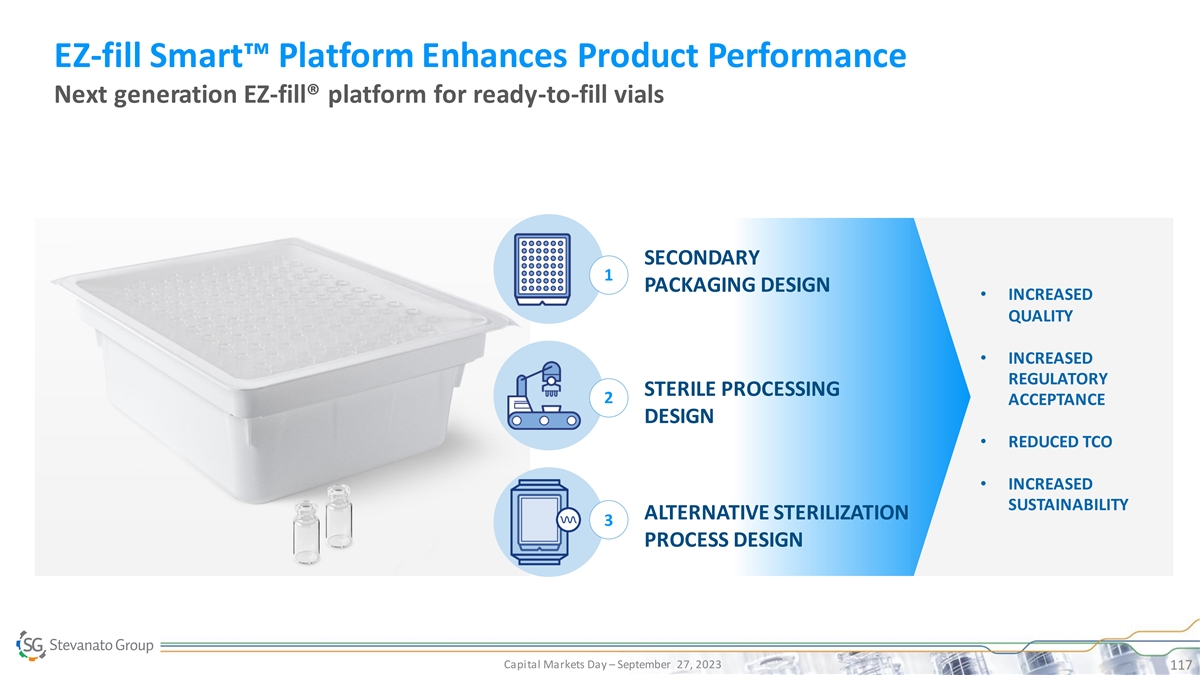
EZ-fill Smart™ Platform Enhances Product Performance Next
generation EZ-fill® platform for ready-to-fill vials SECONDARY 1 PACKAGING DESIGN • INCREASED QUALITY • INCREASED REGULATORY STERILE PROCESSING 2 ACCEPTANCE DESIGN • REDUCED TCO • INCREASED SUSTAINABILITY PRESERVED DRUG
AND PHARMA ALTERNATIVE STERILIZATION REDUCED TCO 3 RELIABILITY PROCESS DESIGN INCREASED EFFICIENCY SUCCESSFUL AND TIMELY LAUNCH Capital Markets Day – September 27, 2023 117

Key Takeaways Drug Containment Solutions • We are continually
innovating across our Containment Solution platform • We have developed multiple performance levels to meet current and future market needs • We are accelerating the shift to ready-to-use vials and cartridges across the industry Capital
Markets Day – September 27, 2023 118

Q&A Capital Markets Day – September 27, 2023

Thank You

Breakout Session – Drug Delivery Systems
Riccardo Butta President of Americas Steven Kaufman Vice President,
Drug Delivery Systems Drug Delivery Systems Product Portfolio Capital Markets Day – September 27, 2023

Drug Delivery Systems – Product Portfolio Agenda for the Session
• Value Proposition • Go-to-Market Strategy • SG Drug Delivery Device Portfolio Capital Markets Day – September 27, 2023
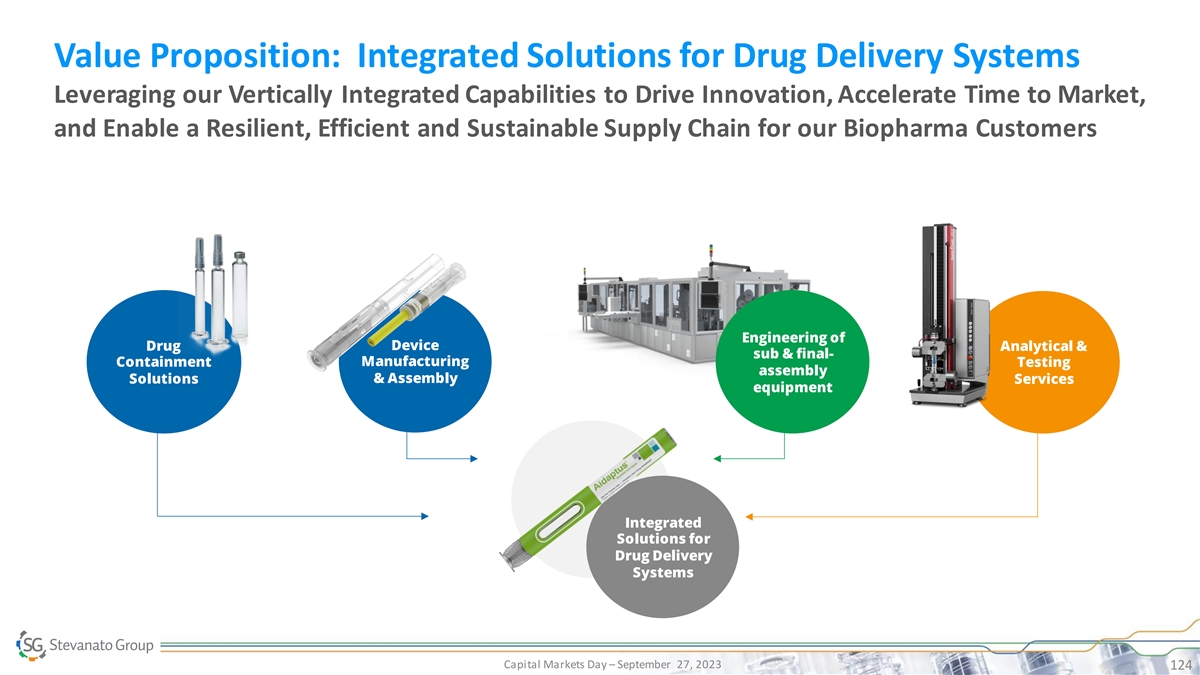
Value Proposition: Integrated Solutions for Drug Delivery Systems
Leveraging our Vertically Integrated Capabilities to Drive Innovation, Accelerate Time to Market, and Enable a Resilient, Efficient and Sustainable Supply Chain for our Biopharma Customers Engineering of Device Drug Analytical & sub & final-
Manufacturing Containment Testing assembly Solutions & Assembly Services equipment Integrated Solutions for Drug Delivery Systems Capital Markets Day – September 27, 2023 124 September 2022
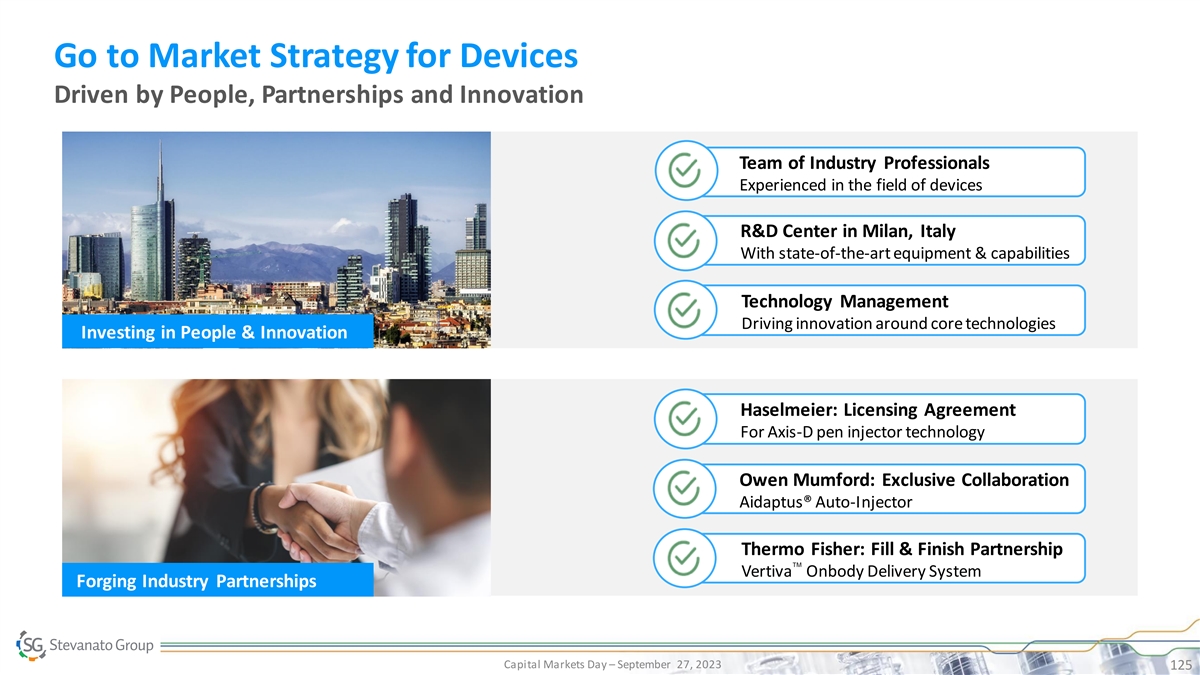
Go to Market Strategy for Devices Driven by People, Partnerships and
Innovation Team of Industry Professionals Experienced in the field of devices R&D Center in Milan, Italy With state-of-the-art equipment & capabilities Technology Management Driving innovation around core technologies Investing in People
& Innovation Haselmeier: Licensing Agreement For Axis-D pen injector technology Owen Mumford: Exclusive Collaboration Aidaptus® Auto-Injector Thermo Fisher: Fill & Finish Partnership ™ Vertiva Onbody Delivery System Forging
Industry Partnerships Capital Markets Day – September 27, 2023 125
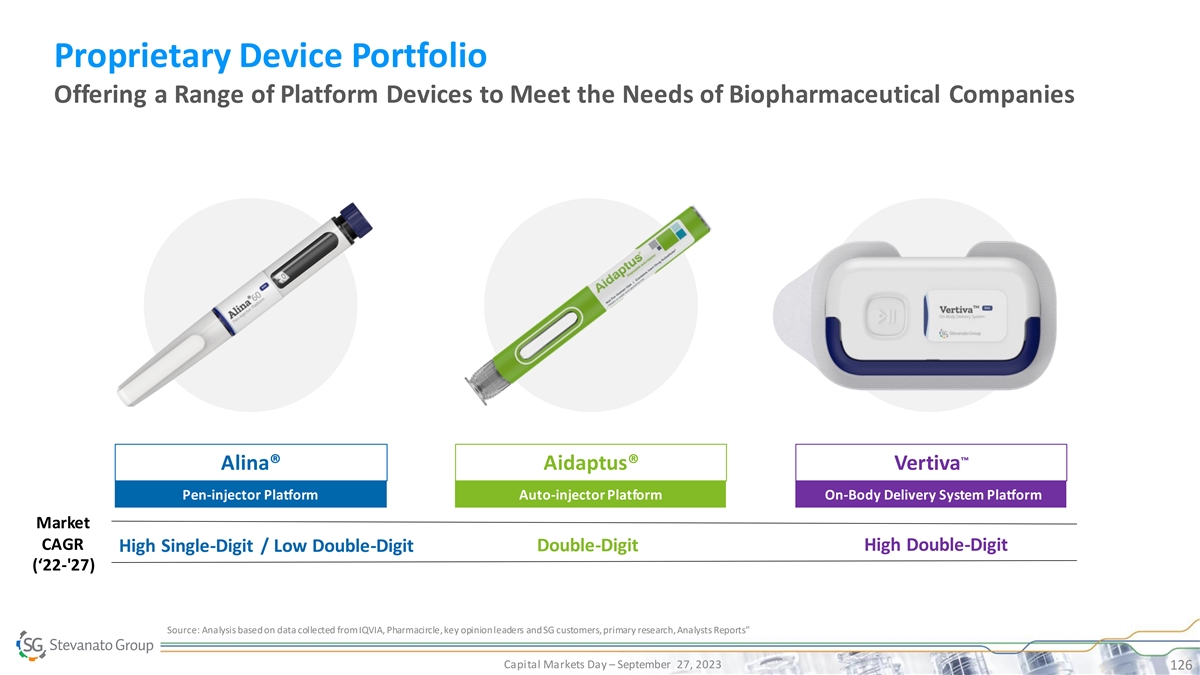
Proprietary Device Portfolio Offering a Range of Platform Devices to
Meet the Needs of Biopharmaceutical Companies ™ Alina® Aidaptus® Vertiva Pen-injector Platform Auto-injector Platform On-Body Delivery System Platform Market CAGR Double-Digit High Double-Digit High Single-Digit / Low Double-Digit
(‘22-'27) Source: Analysis based on data collected from IQVIA, Pharmacircle, key opinion leaders and SG customers, primary research, Analysts Reports” Capital Markets Day – September 27, 2023 126
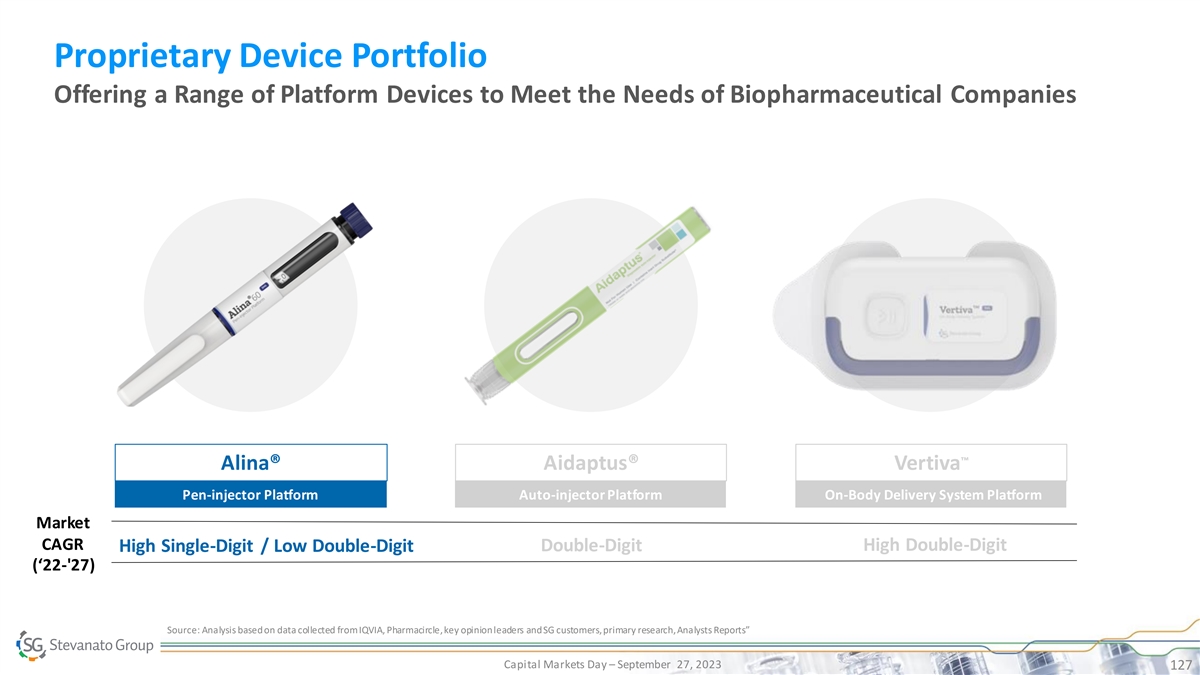
Proprietary Device Portfolio Offering a Range of Platform Devices to
Meet the Needs of Biopharmaceutical Companies ™ Alina® Aidaptus® Vertiva Pen-injector Platform Auto-injector Platform On-Body Delivery System Platform Market CAGR Double-Digit High Double-Digit High Single-Digit / Low Double-Digit
(‘22-'27) Source: Analysis based on data collected from IQVIA, Pharmacircle, key opinion leaders and SG customers, primary research, Analysts Reports” Capital Markets Day – September 27, 2023 127

Alina® Pen-Injector Platform In-licensed Patented Technology to
Address Growing Demand for Self-administration Alina® is a variable and fixed dose, disposable, pen-injector platform based on proven technology • Platform offering with multiple variants to support a range of therapeutic applications
• Targeting generics & biosimilars in diabetes space, licensed for additional therapies • Optimized processes and equipment for Focus of enhanced time-to-market next slide Capital Markets Day – September 27, 2023 128

Final Assembly Equipment Streamlining Production through Innovative,
Efficient, Reliable Automation Technologies Offering for Alina® includes final assembly equipment solutions for installation at customer, as well as Contract Manufacturing Organization (CMO) fill & finish locations • Designed and
built using modular, flexible platforms that scale according to production demands • Leveraging internal expertise in high-speed, automated equipment for sub-assembly, final assembly, inspection, packaging & serialization • Trusted
by leading pharmaceutical companies 129 Capital Markets Day – September 27, 2023 129 DDS Overview of SG Q2 2023 Confidential Capabilities
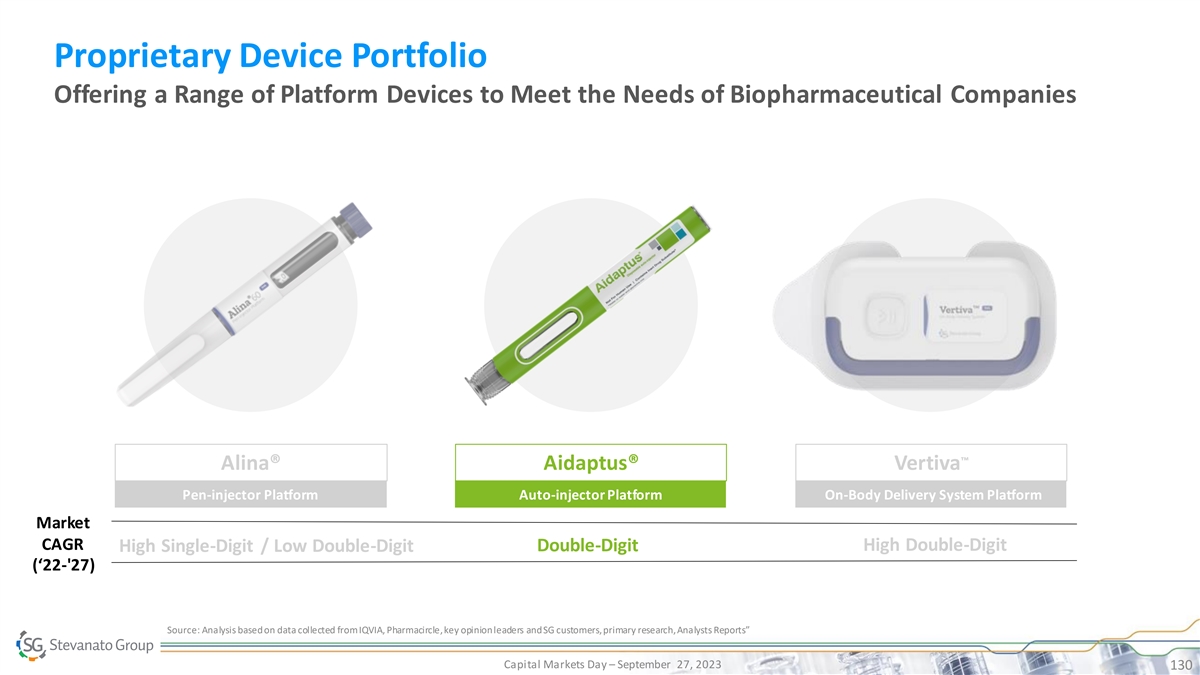
Proprietary Device Portfolio Offering a Range of Platform Devices to
Meet the Needs of Biopharmaceutical Companies ™ Alina® Aidaptus® Vertiva Pen-injector Platform Auto-injector Platform On-Body Delivery System Platform Market CAGR Double-Digit High Double-Digit High Single-Digit / Low Double-Digit
(‘22-'27) Source: Analysis based on data collected from IQVIA, Pharmacircle, key opinion leaders and SG customers, primary research, Analysts Reports” Capital Markets Day – September 27, 2023 130
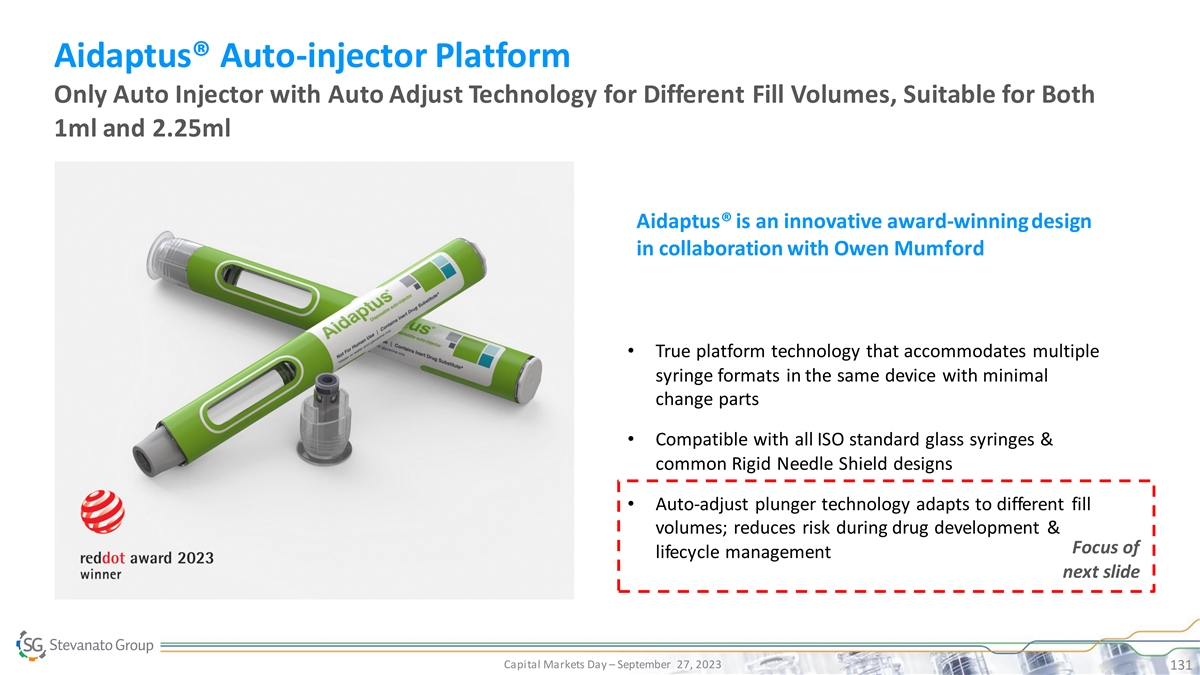
Aidaptus® Auto-injector Platform Only Auto Injector with Auto
Adjust Technology for Different Fill Volumes, Suitable for Both 1ml and 2.25ml Aidaptus® is an innovative award-winning design in collaboration with Owen Mumford • True platform technology that accommodates multiple syringe formats in the
same device with minimal change parts • Compatible with all ISO standard glass syringes & common Rigid Needle Shield designs • Auto-adjust plunger technology adapts to different fill volumes; reduces risk during drug development
& Focus of lifecycle management next slide Capital Markets Day – September 27, 2023 131
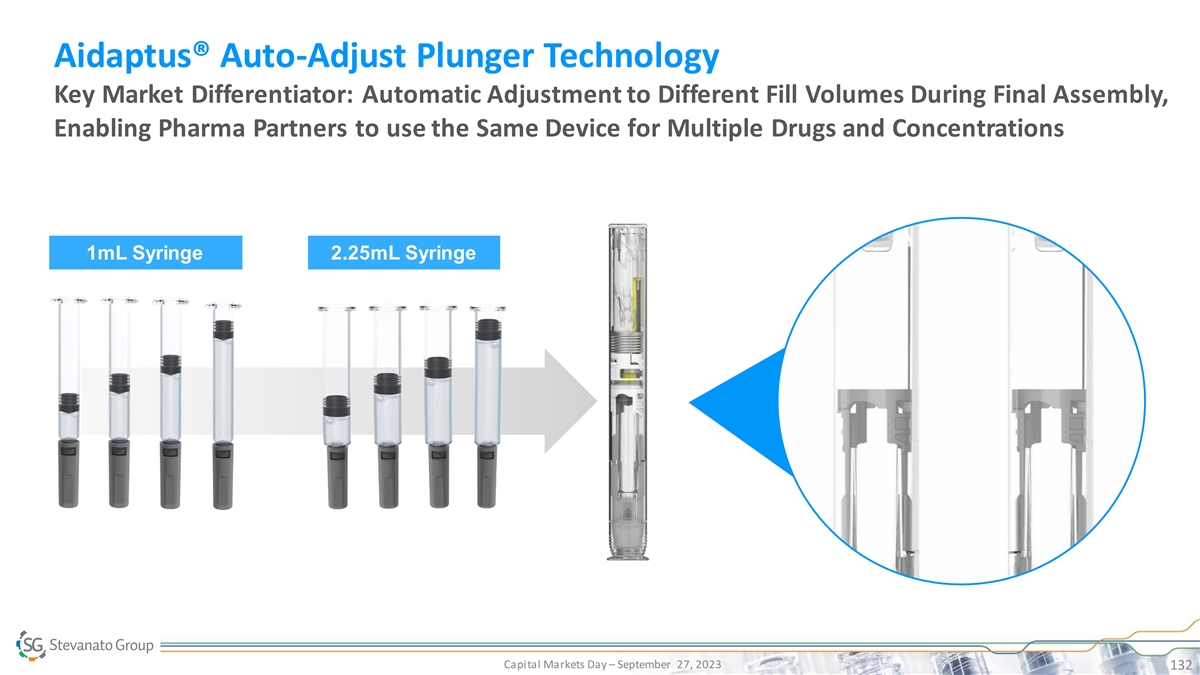
Aidaptus® Auto-Adjust Plunger Technology Key Market
Differentiator: Automatic Adjustment to Different Fill Volumes During Final Assembly, Enabling Pharma Partners to use the Same Device for Multiple Drugs and Concentrations 1mL Syringe 2.25mL Syringe Capital Markets Day – September 27, 2023
132
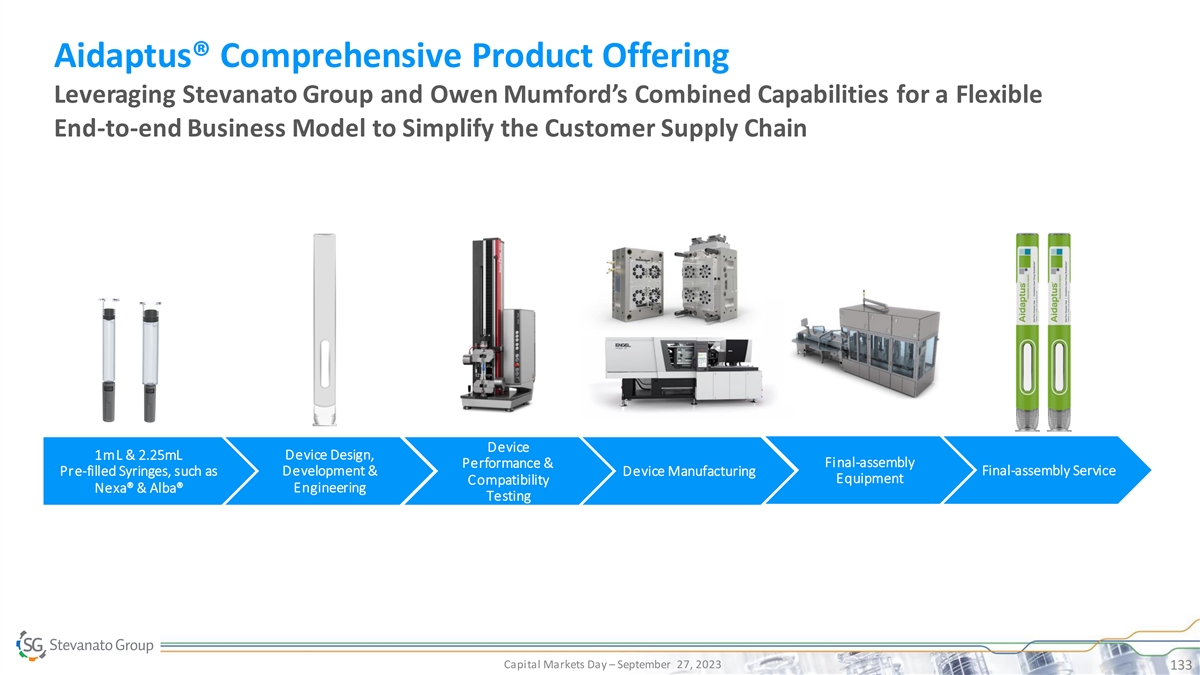
Aidaptus® Comprehensive Product Offering Leveraging Stevanato
Group and Owen Mumford’s Combined Capabilities for a Flexible End-to-end Business Model to Simplify the Customer Supply Chain Device 1mL & 2.25mL Device Design, Performance & Final-assembly Development & Final-assembly Service
Pre-filled Syringes, such as Device Manufacturing Equipment Compatibility Nexa® & Alba® Engineering Testing Capital Markets Day – September 27, 2023 133
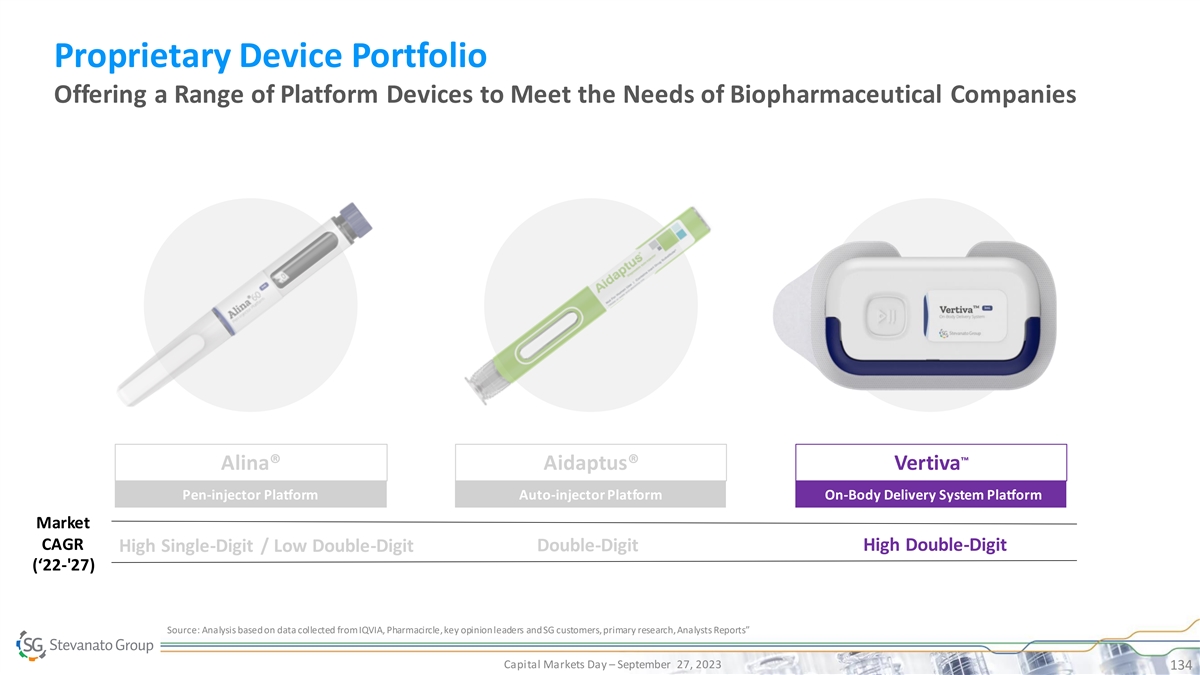
Proprietary Device Portfolio Offering a Range of Platform Devices to
Meet the Needs of Biopharmaceutical Companies ™ Alina® Aidaptus® Vertiva Pen-injector Platform Auto-injector Platform On-Body Delivery System Platform Market CAGR Double-Digit High Double-Digit High Single-Digit / Low Double-Digit
(‘22-'27) Source: Analysis based on data collected from IQVIA, Pharmacircle, key opinion leaders and SG customers, primary research, Analysts Reports” Capital Markets Day – September 27, 2023 134
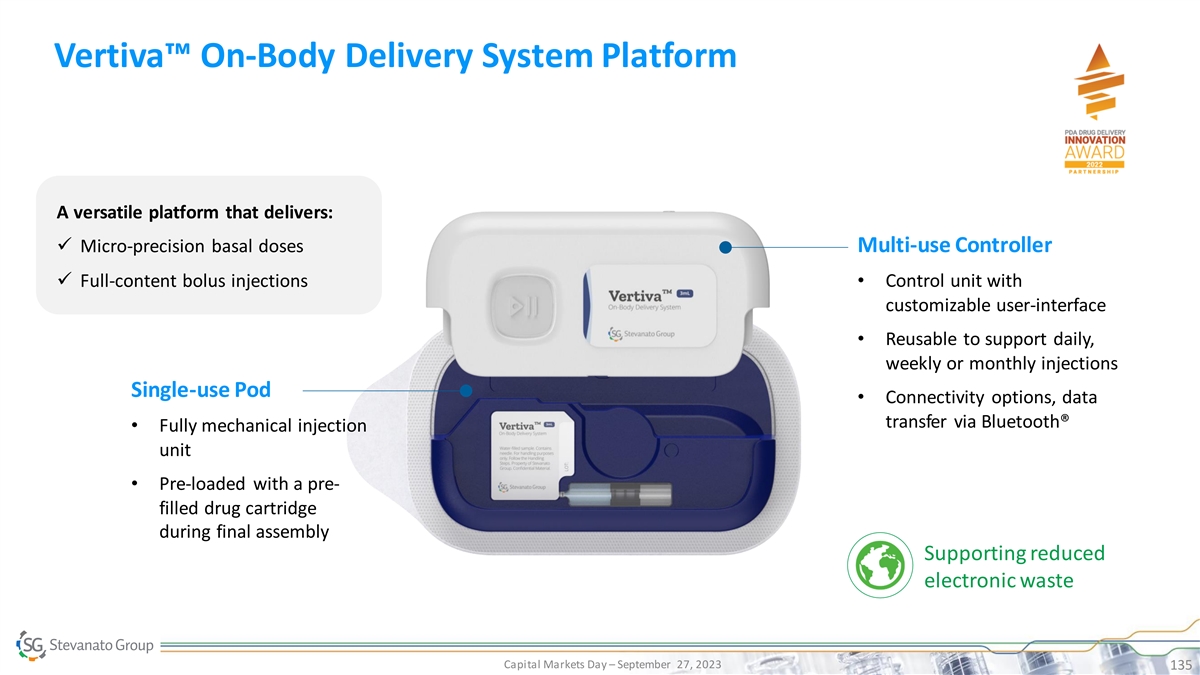
Vertiva™ On-Body Delivery System Platform A versatile platform
that delivers: ✓ Micro-precision basal doses Multi-use Controller ✓ Full-content bolus injections• Control unit with customizable user-interface • Reusable to support daily, weekly or monthly injections Single-use Pod
• Connectivity options, data transfer via Bluetooth® • Fully mechanical injection unit • Pre-loaded with a pre- filled drug cartridge during final assembly Supporting reduced electronic waste Capital Markets Day –
September 27, 2023 135
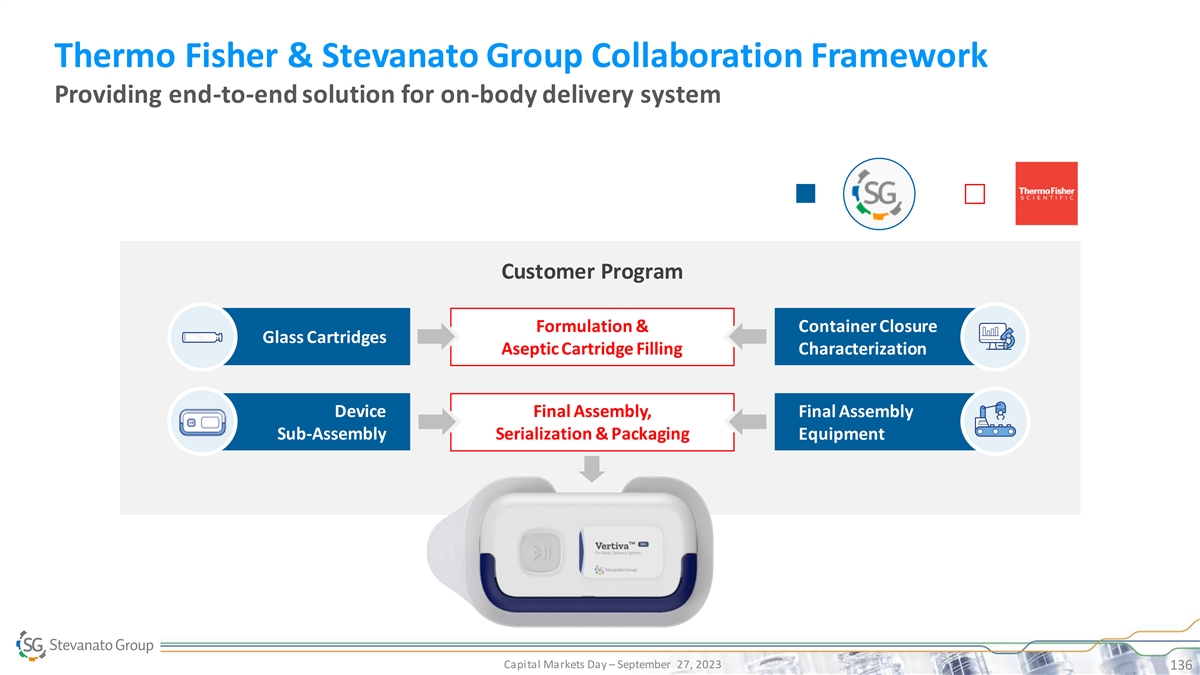
Thermo Fisher & Stevanato Group Collaboration Framework Providing
end-to-end solution for on-body delivery system Customer Program Formulation & Container Closure Glass Cartridges Aseptic Cartridge Filling Characterization Device Final Assembly, Final Assembly Sub-Assembly Serialization & Packaging
Equipment Capital Markets Day – September 27, 2023 136

Innovative Approach: People, Partnerships & Innovation Empowering
patients with next-generation drug-delivery solutions • Supporting biotech and pharmaceutical customers to bring their combination products to market quickly and efficiently • Built a global team of device professionals • Industry
collaboration & partnerships • Ongoing R&D investment Capital Markets Day – September 27, 2023 137

Q&A Capital Markets Day – September 27, 2023

Thank You

Reference videos Highlights of our in-house capabilities &
automation experience for medical device projects
Q2 2023 Confidential DDS Overview of SG Capabilities

Q2 2023 Confidential DDS Overview of SG Capabilities

Alina® Pen Injector Video 143 Copyright Stevanato Group
2023

Vertiva Pen Injector Video 144 Copyright Stevanato Group
2023

Stevanato (NYSE:STVN)
Historical Stock Chart
From Apr 2024 to May 2024
Stevanato (NYSE:STVN)
Historical Stock Chart
From May 2023 to May 2024