UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16 of
the Securities Exchange Act of 1934
For the month of September 2023
Commission File Number: 001-36622
PROQR THERAPEUTICS N.V.
Zernikedreef 9
2333 CK Leiden
The Netherlands
Tel: +31 88 166 7000
(Address, Including Zip Code, and Telephone
Number,
Including Area Code, of Registrant’s Principal Executive Offices)
Indicate by check mark whether the registrant
files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F
x Form 40-F ¨
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ¨
Indicate
by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ¨
On September 27, 2023,
ProQR Therapeutics N.V. (“ProQR”) issued a press release titled, “ProQR Therapeutics Provides Update on Ophthalmic Assets.”
A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
ProQR hereby incorporates
by reference the information contained herein into ProQR’s registration statements on Form F-3 (File No. 333-270943, File
No. 333-263166, File No. 333-260775 and File No. 333-248740).
SIGNATURES
Pursuant to the requirements
of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto
duly authorized.
| |
PROQR THERAPEUTICS N.V. |
| |
|
| Date: September 27, 2023 |
By: |
/s/ René Beukema |
| |
|
René Beukema |
| |
|
Chief Corporate Development Officer and General Counsel |
INDEX TO EXHIBITS
Exhibit 99.1
ProQR Therapeutics Provides Update on
Ophthalmic Assets
LEIDEN, Netherlands &
CAMBRIDGE, Mass., September 27, 2023 – ProQR Therapeutics N.V. (Nasdaq: PRQR) (ProQR), a company dedicated to changing lives
through transformative RNA therapies today provided an update that its previously announced agreement to divest the Company’s late-stage
ophthalmic assets to Laboratoires Théa S.A.S. (Théa) has been terminated.
The completion of the
transaction was conditional on Théa entering into employment agreements with a number of key ophthalmology personnel from ProQR.
Some of these individuals have now elected not to proceed with employment at Théa, therefore that closing condition for the transaction
cannot be fulfilled and Théa has terminated the agreement.
“While we are
disappointed that the sale of these ophthalmology programs to Théa will not proceed, our primary focus at ProQR remains on our
Axiomer® RNA editing platform,” said Daniel A. de Boer, Founder and Chief Executive Officer of ProQR. “We
look forward to advancing our wholly owned pipeline with an initial focus on targets for cholestatic and cardiovascular diseases, in
addition to our partnership with Lilly, as we work to develop a new class of therapies for patients with high unmet need.”
About ProQR
ProQR Therapeutics is
dedicated to changing lives through the creation of transformative RNA therapies. ProQR is pioneering a next-generation RNA technology
called Axiomer®, which uses a cell’s own editing machinery called ADAR to make specific single nucleotide edits
in RNA to reverse a mutation or modulate protein expression and could potentially yield a new class of medicines for both rare and prevalent
diseases with unmet need. Based on our unique proprietary RNA repair platform technologies we are growing our pipeline with patients
and loved ones in mind.
Learn more about ProQR at www.proqr.com.
About Axiomer®
ProQR is pioneering
a next-generation RNA base editing technology called Axiomer®, which could potentially yield a new class of medicines for diverse
types of diseases. Axiomer® “Editing Oligonucleotides”, or EONs, mediate single nucleotide changes to RNA in a highly
specific and targeted way using molecular machinery that is present in human cells called ADAR (Adenosine Deaminase Acting on RNA). Axiomer®
EONs are designed to recruit and direct endogenously expressed ADARs to change an Adenosine (A) to an Inosine (I) in the RNA
– an Inosine is translated as a Guanosine (G) – correcting an RNA with a disease-causing mutation back to a normal (wild
type) RNA, modulating protein expression, or altering a protein so that it will have a new function that helps prevent or treat disease.
Forward Looking Statements
This press release contains forward-looking
statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms
such as "anticipate," "believe," "could," "estimate," "expect," "goal," "intend,"
"look forward to", "may," "plan," "potential," "predict," "project," "should,"
"will," "would" and similar expressions. Such forward-looking statements include, but are not limited to, statements
regarding the termination of the sale of our ophthalmology programs, as well as the potential of our technologies and product candidates.
Forward-looking statements are based on management's beliefs and assumptions and on information available to management only as of the
date of this press release. Our actual results could differ materially from those anticipated in these forward-looking statements for
many reasons, including, without limitation, the risks, uncertainties and other factors in our filings made with the Securities and Exchange
Commission, including certain sections of our annual report filed on Form 20-F. These risks and uncertainties include, among others,
the cost, timing and results of preclinical studies and other development activities by us and our collaborative partners whose operations
and activities may be slowed or halted shortage and pressure on supply and logistics on the global market; our reliance on contract manufacturers
or suppliers to supply materials for research and development and the risk of supply interruption or delays from suppliers or contract
manufacturers; the ability to secure, maintain and realize the intended benefits of collaborations with partners, including the collaboration
with Eli Lilly and Company; the possible impairment of, inability to obtain, and costs to obtain intellectual property rights; possible
safety or efficacy concerns that could emerge as new data are generated in research and development; and general business, operational,
financial and accounting risks, and risks related to litigation and disputes with third parties. Given these risks, uncertainties and
other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking
statements, even if new information becomes available in the future, except as required by law.
For ProQR Therapeutics
N.V.
Investor contact:
Sarah Kiely
ProQR Therapeutics N.V.
T: +1 617 599 6228
skiely@proqr.com
or
Hans Vitzthum
LifeSci Advisors
T: +1 617 430 7578
hans@lifesciadvisors.com
Media contact:
Robert Stanislaro
FTI Consulting
T: +1 212 850 5657
robert.stanislaro@fticonsulting.com
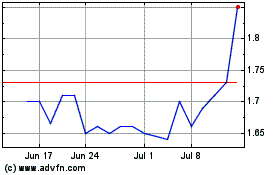
ProQR Therapeutics NV (NASDAQ:PRQR)
Historical Stock Chart
From Mar 2024 to Apr 2024
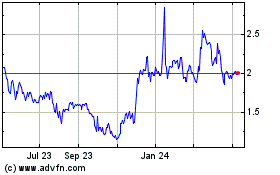
ProQR Therapeutics NV (NASDAQ:PRQR)
Historical Stock Chart
From Apr 2023 to Apr 2024