false 0001806952 0001806952 2023-09-11 2023-09-11
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): September 11, 2023
Lyell Immunopharma, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-40502 |
|
83-1300510 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
|
|
| 201 Haskins Way |
|
|
|
|
| South San Francisco, California |
|
|
|
94080 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: 650 695-0677
(Former Name or Former Address, if Changed Since Last Report)
Not Applicable
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, $0.0001 par value per share |
|
LYEL |
|
NASDAQ Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Lyell Immunopharma, Inc. (the “Company”) hereby furnishes the investor presentation the Company will present to analysts and investors on or after September 11, 2023 (the “Investor Presentation”). The slides of the Investor Presentation are attached hereto as Exhibit 99.1 and will be available on the Company’s website at https://ir.lyell.com/news-events/presentations. The information contained in the Investor Presentation is summary information that is intended to be considered in the context of the Company’s Securities and Exchange Commission (“SEC”) filings and other public announcements that the Company may make, by press release or otherwise, from time to time. The Company undertakes no duty or obligation to publicly update or revise the information contained in this presentation, although it may do so from time to time. Any such updating may be made through the filing of other reports or documents with the SEC, through press releases or through other public disclosure.
The information in this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is being furnished and shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing made by the Company under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing. The furnishing of this information hereby shall not be deemed an admission as to the materiality of any such information.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
Lyell Immunopharma, Inc. |
|
|
|
|
| Date: September 11, 2023 |
|
|
|
By: |
|
/s/ Matthew Lang |
|
|
|
|
|
|
Matthew Lang |
|
|
|
|
|
|
Chief Business Officer |
Exhibit 99.1 DRAFT JPM22 Updated 12-14-21 Lyell Immunopharma September
11, 2023

Forward-looking statements Certain matters discussed in this
presentation are “forward-looking statements” of Lyell Immunopharma, Inc, Inc. (hereinafter referred to as the “Company,” “we,” “us,” or “our”) within the meaning of the Private Securities
Litigation Reform Act of 1995 (the “PSLRA”). All such written or oral statements made in this presentation, other than statements of historical fact, are forward-looking statements and are intended to be covered by the safe harbor for
forward-looking statements provided by the PSLRA. Without limiting the foregoing, we may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,”
“anticipates,” “expects,” plans“,” “intends,” “forecast,” “guidance,” “outlook,” “may,” “could,” “might,” “will,”
“should” or other words that convey uncertainty of future events or outcomes and are intended to identify forward-looking statements. Forward-looking statements are based on assumptions and assessments made in light of management’s
experience and perception of historical trends, current conditions, expected future developments and other factors believed to be appropriate. Forward looking statements in this presentation are made as of the date of this presentation, and we
undertake no duty to update or revise any such statements, whether as a result of new information, future events or otherwise. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and other
factors, many of which are outside of our control, that may cause actual results, levels of activity, performance, achievements, timelines and developments to be materially different from those expressed in or implied by these forward-looking
statements. Important factors that could cause actual results, developments and business decisions to differ materially from forward-looking statements are described in the sections titled “Risk Factors“ in our filings with the
Securities and Exchange Commission (the “SEC”), and include, but are not limited to, the following substantial known and unknown risks and uncertainties inherent in our business related to: the effects of geopolitical instability;
macroeconomic conditions and the lingering effects of the COVID-19 pandemic; our ability to submit planned INDs or initiate or progress clinical trials on the anticipated timelines, if at all; our limited experience as a company in enrolling,
conducting or completing clinical trials; our ability to manufacture and supply our product candidates for our clinical trials; the nonclinical profiles of our product candidates not translating in clinical trials; the potential for results from
clinical trials to differ from nonclinical, early clinical, preliminary or expected results; significant adverse events, toxicities or other undesirable side effects associated with our product candidates; the significant uncertainty associated with
our product candidates ever receiving any regulatory approvals; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; implementation of our strategic plans for our business and product
candidates; the sufficiency of our capital resources and the need for additional capital to achieve our goals; other risks, including general economic conditions and regulatory developments, not within our control; and those risks described under
the heading “Risk Factors” in our SEC filings, including our Quarterly Report on Form 10-Q for the quarter ended June 30, 2023 and subsequent filings with the SEC. 2 PROPRIETARY
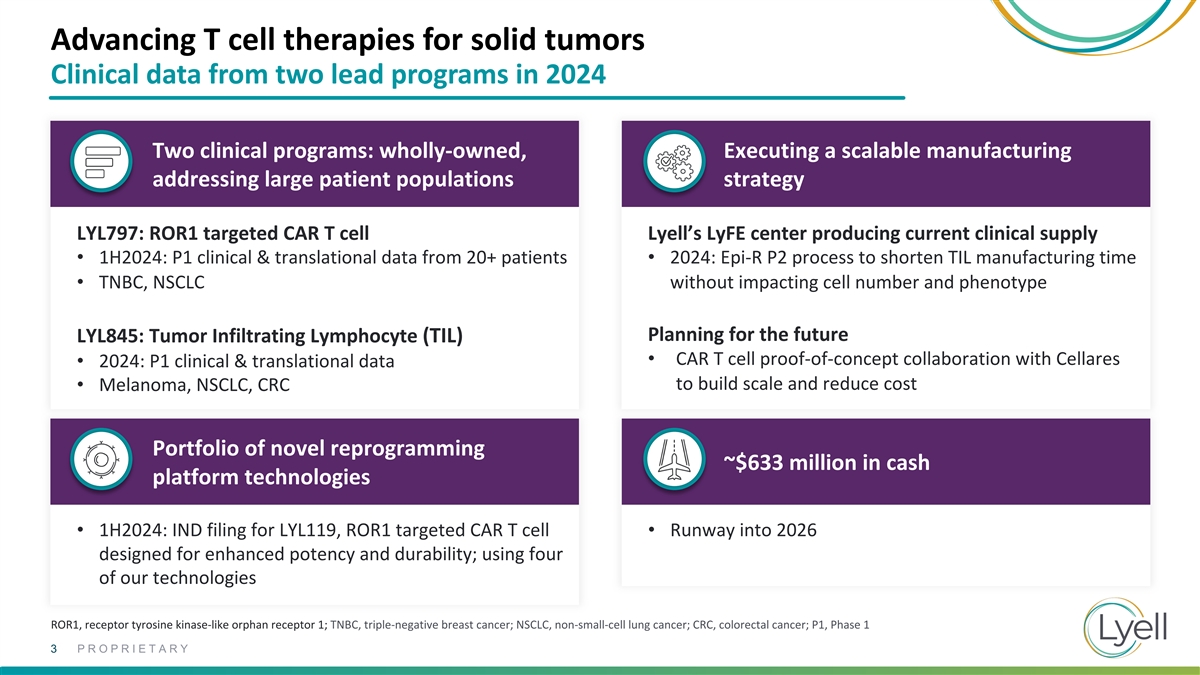
Advancing T cell therapies for solid tumors Clinical data from two lead
programs in 2024 Two clinical programs: wholly-owned, Executing a scalable manufacturing addressing large patient populations strategy LYL797: ROR1 targeted CAR T cell Lyell’s LyFE center producing current clinical supply • 1H2024: P1
clinical & translational data from 20+ patients • 2024: Epi-R P2 process to shorten TIL manufacturing time • TNBC, NSCLC without impacting cell number and phenotype Planning for the future LYL845: Tumor Infiltrating Lymphocyte (TIL)
• CAR T cell proof-of-concept collaboration with Cellares • 2024: P1 clinical & translational data to build scale and reduce cost • Melanoma, NSCLC, CRC Portfolio of novel reprogramming ~$633 million in cash platform
technologies • 1H2024: IND filing for LYL119, ROR1 targeted CAR T cell • Runway into 2026 designed for enhanced potency and durability; using four of our technologies ROR1, receptor tyrosine kinase-like orphan receptor 1; TNBC,
triple-negative breast cancer; NSCLC, non-small-cell lung cancer; CRC, colorectal cancer; P1, Phase 1 3 PROPRIETARY

Lyell is developing two types of personalized cell therapy: Focused on
getting the T-cells right OUR GOAL: Reprogram T cells to defeat solid tumors Resist exhaustion Durable cytotoxicity CAR T cells Self-renewal Persistence Metastatic Durable cytotoxicity Tumor- cancer Maintain polyclonality infiltrating Right
phenotype lymphocytes Hot and cold tumors CAR, chimeric antigen receptor 4 PROPRIETARY

Lyell’s T-cell reprogramming technologies are designed to address
primary barriers to success in solid tumors TO ACHIEVE SUCCESS IN SOLID TUMORS, CELL THERAPY MUST: RESIST EXHAUSTION, Maintain cancer cell killing in the immunosuppressive tumor microenvironment RETAIN FUNCTION ENHANCE DURABLE Increase ability to
self-renew and persist to drive durable tumor cytotoxicity STEMNESS 5 PROPRIETARY

Solid tumors drive T cells down a path to exhaustion Solid Hematologic
Autologous Infusion Autologous Infusion tumor malignancy CAR T cell CAR T cell Adequate cell Inadequate expansion, expansion, persistence, driven to exhaustion, and tumor killing and lack of durability Tumor cell Lack of T-cell clearance function;
cancer cells persist Riddell et al, Keystone, 2020 6 PROPRIETARY
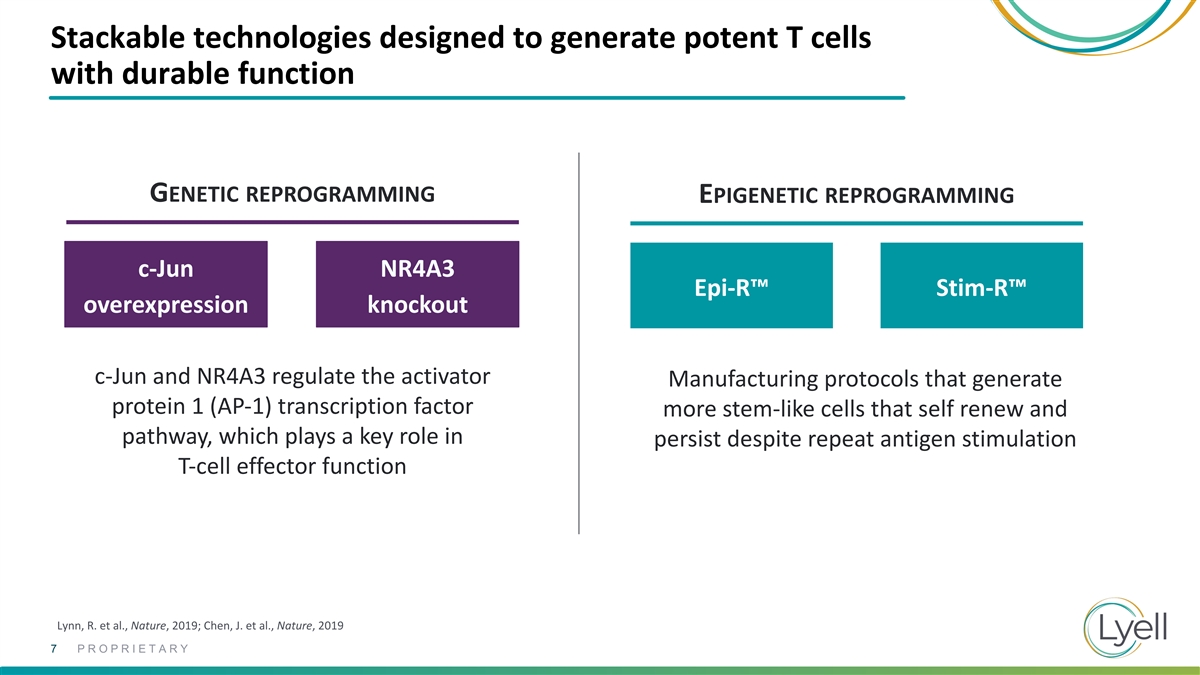
Stackable technologies designed to generate potent T cells with durable
function GENETIC REPROGRAMMING EPIGENETIC REPROGRAMMING c-Jun NR4A3 Epi-R™ Stim-R™ overexpression knockout c-Jun and NR4A3 regulate the activator Manufacturing protocols that generate protein 1 (AP-1) transcription factor more stem-like
cells that self renew and pathway, which plays a key role in persist despite repeat antigen stimulation T-cell effector function Lynn, R. et al., Nature, 2019; Chen, J. et al., Nature, 2019 7 PROPRIETARY

A clinical-stage company with a growing pipeline of novel therapies for
solid tumors Genetic Epigenetic Product Reprogramming Reprogramming Target Phase 2 / Next Expected Candidate/ Target Preclinical Phase 1 Indications Pivotal Milestone Modality c-Jun NR4A3 Epi-R™ Stim-R™ TNBC LYL797 Initial data in NSCLC
ROR1 √ √ CAR T Cell 1H 2024 Other Solid Tumors ROR1+ LYL119 Submit IND in ROR1 CAR T Cell √ √ √ √ 1H 2024 Solid Tumors Melanoma LYL845 Multiple Initial data in CRC, NSCLC antigens √ Other Solid TIL 2024
Tumors 2nd Multiple Genetic and Epigenetic Solid Tumors Generation antigens Reprogramming TIL ROR1, receptor tyrosine kinase-like orphan receptor 1; IND, investigational new drug; CAR, chimeric antigen receptor; NSCLC, non-small cell lung cancer;
TNBC, triple-negative breast cancer; TIL, tumor infiltrating lymphocytes; CRC, colorectal cancer 8 PROPRIETARY

People with cancer need better therapies 90% <2 <3 Years Cancer
Years most deaths before metastatic cancer caused by cancer progresses solid patients tumors live after diagnosis seer.cancer.gov; Deaths (Estimated 2021); Survival Rates by Time Since Diagnosis, 2000-2017 CDC Nat’l Ctr for Health Statistics,
Mortality in the US, 2020 9 PROPRIETARY

Lyell product candidates target large unmet needs ~500K new cases and
~180K US deaths annually TRIPLE-NEGATIVE NON-SMALL CELL MELANOMA COLORECTAL BREAST CANCER LUNG CANCER CANCER • 15% of breast cancer • 84% of new lung • 80% of all skin • 3rd most common diagnoses in the US cancer diagnoses
cancer-related deaths form of cancer each year each year • ~40,000 new cases • ~200,000 new cases • ~100,000 new cases • ~150,000 new cases • ~10,000 deaths • ~110,000 deaths • ~8,000 deaths • ~53,000
deaths LYL797 LYL797 & LYL845 LYL845 LYL845 National Cancer Institute and the American Cancer Society and are based on US cases. (2022) 10 PROPRIETARY

Reprogramming T cells to target aggressive cancers LYL797: A
genetically and epigenetically reprogrammed ROR1 CAR T cell product candidate designed for differentiated potency and durability

LYL797 CAR T cell Phase 1 trial design • Patient population
CLINICAL TRIAL DESIGN (mTPI-2) – Relapsed/Refractory TNBC patients who have Dose Escalation Dose Expansion failed two lines of therapy The RP2D – Relapsed/Refractory NSCLC patients who have Dose Level 4 moves failed one line of therapy
forward to LYL797 TNBC expansion – ROR1 positive Dose Level 3 cohort (N = ~15) • Study objectives LYL797 NSCLC Dose Level 2 – Patient safety and tolerability (N = ~15) – Assessment of cytotoxicity and duration of Dose Level 1
T-cell function – Overall response rate and durability De-escalation if required – Recommended phase 2 dose Dose Level -1 – CAR T cell pharmacokinetics Potential to expand into additional tumor types NCT05274451 Spigel et al, ESMO
2022 mTPI-2, modified toxicity probability interval 2; NSCLC, non-small-cell lung cancer; ROR1, receptor tyrosine kinase–like orphan receptor 1; TNBC, triple-negative breast cancer; RP2D, recommended Phase 2 dose 12 PROPRIETARY

ROR1 is highly expressed in many human cancers and correlates with a
poor prognosis ROR1 expression ~60% ~40% Triple-negative Non-small cell breast cancer lung cancer ~50% ~95% Chronic Ovarian cancer lymphocytic leukemia In previous clinical trials and a non-human primate ROR1 CAR T cell toxicity study, no on-target
off-tumor toxicity from ROR1-targeted therapies have been reported ROR1, receptor tyrosine kinase-like orphan receptor 1 Jeong, Medicina, 2022; Chien, Virchows Arch 2016; Zhang, PNAS, 2014; Wang, NEJM Evidence, 2022; Berger, Cancer Immunol Res,
2015; Choi, Cell Stem Cell, 2018 13 PROPRIETARY

Lyell’s ROR1 assay and screening program support current and
future clinical trials Screening data with Lyell’s assay consistent with ROR1 expression in the literature TRIPLE-NEGATIVE BREAST CANCER 53% ROR1+ N=77 • 15% of breast cancer diagnoses/year • ~40,000 new cases / ~10,000 deaths 33%
ROR1+ NON-SMALL CELL LUNG CANCER N=18 • 84% of new lung cancer diagnoses/year • ~200,000 new cases / ~110,000 deaths ROR1, receptor tyrosine kinase-like orphan receptor 1; National Cancer Institute and the American Cancer Published
literature: Balakrishnan et al, Clin Cancer Res. 2017, Society and are based on US cases (2022) TNBC ~60%, NSCLC ~40% 14 PROPRIETARY

LYL797 clinical program supported by robust preclinical data ROR1 CAR T
cell + c-Jun + Epi-R Key differentiators • Tumor reduction, enhanced cytokine production and tumor infiltration in aggressive NSCLC syngeneic animal model with c-Jun • Stem-like phenotype, durability and enhanced cytotoxicity with Epi-R
technology • Prolonged survival by combining c-Jun and Epi-R technologies (LYL797) in xenograft NSCLC animal model 15 PROPRIETARY

Superior preclinical efficacy demonstrated with c-Jun overexpressing
ROR1 CAR T cells in aggressive NSCLC model Lymphodepletion and • Syngeneic Kras/p53 model that recapitulates human NSCLC NSCLC tumor-bearing mice CAR T cell +/- c-JUN • Also recapitulates the barriers in treating human NSCLC with the
ROR1 CAR T cells Day 15 21 30 • Extremely difficult model in which to achieve tumor regression Enhanced intratumoral function Enhanced infiltration Tumor control in 50% of mice IFN�� CAR T cell frequency in tumor Tumor volume WT
ROR1 CAR T cells WT ROR1 CAR T cells WT CAR T cell-treated mice c-Jun ROR1 CAR T cells c-Jun ROR1 CAR T cells c-Jun ROR1 CAR T cell-treated mice 6/12 progressing 6/12 stable/ regressing Pre-infusion 4 weeks * is p<.05 ** is p<.01 *** is
p<.001 **** is p<.0001 Riddell Lab, Fred Hutch, unpublished data 16 PROPRIETARY %CAR+ of PE-CD8+ mm^3

Epi-R™ technology produces transcriptionally distinct populations
of T cells that resist exhaustion and maintain cytotoxicity Distinct gene expression profile of Epi-R expanded cells Epi-R expanded cells demonstrate prolonged vs. standard preparation cytotoxicity after removal from Epi-R conditions Sequential cell
killing assay (ROR1 CAR T cells) Standard preparation Epi-R Mock Mock Standard Standard Preparation preparation Epi-R Epi-R UMAP_1 Time (Hours) Park et al., AACR 2022 Tumor target: H1975 17 PROPRIETARY UMAP_2 Relative target cell fluorescence
(%)
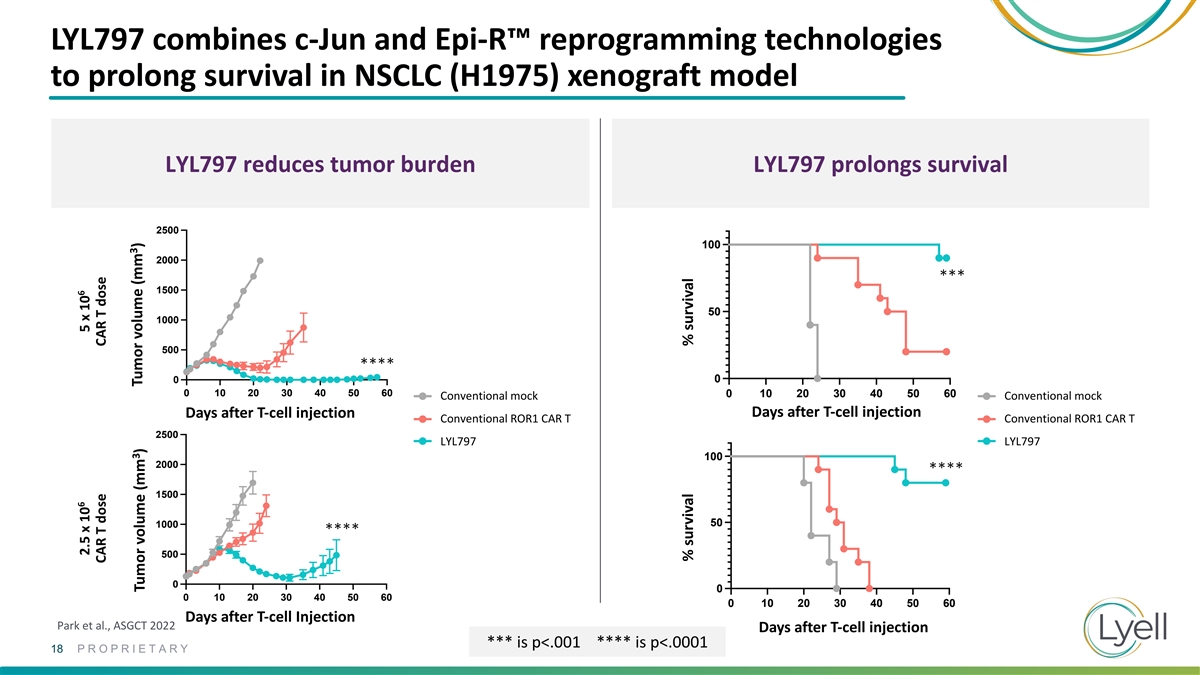
LYL797 combines c-Jun and Epi-R™ reprogramming technologies to
prolong survival in NSCLC (H1975) xenograft model LYL797 reduces tumor burden LYL797 prolongs survival 2500 100 2000 *** 1500 50 1000 500 **** 0 0 2500 2500 0 10 20 30 40 50 60 0 10 20 30 40 50 60 Conventional Mock Conventional mock Conventional
Mock Conventional mock Days after T-cell injection Days after T-cell injection Conventional ROR1 CAR T Conventional ROR1 CAR T Conventional ROR1 CAR T Conventional ROR1 CAR T 2000 2000 2500 LY LYL7 L7 9 9 77 LY LYL7 L7 9 9 77 1500 1500 100 2000 ****
1000 1000 1500 50 1000 500 500 **** 500 0 0 0 10 20 30 40 50 60 0 10 20 30 40 50 60 0 0 0 10 20 30 40 50 60 0 10 20 30 40 50 60 Days after T-cell Injection Park et al., ASGCT 2022 Days after T-cell injection *** is p<.001 **** is p<.0001 18
PROPRIETARY 6 6 2.5 x 10 5 x 10 CAR T dose CAR T dose 3 3 Tumor volume (mm ) Tumor volume (mm ) % survival % survival

Novel stackable technologies designed to improve potency and durability
LYL119: Innovative ROR1 CAR T cell product candidate designed for enhanced cytotoxicity

LYL119 incorporates novel stackable technologies designed to improve
potency and durability ROR1 CAR T cell + c-Jun + NR4A3 KO + Epi-R + Stim-R Key Differentiators • Combining NR4A3 knockout and c-Jun overexpression further reduces T cell exhaustion and enhances cytotoxicity – Reducing NR4A expression
enhances T-cell function associated with increased expression of AP-1–regulated genes – NR4A family transcription factors may contribute to T-cell exhaustion by restraining c-Jun activity • Stim-R CAR T cells demonstrate prolonged
persistence and enhanced cytotoxicity in response to serial antigen stimulation Lam et al., SITC, 2022 Li et al., SITC 2022 Chen J, et al. Nature. 2019; Tirosh I, et al. Science. 2016; Liu X, et al. Nature. 2019; Odagiu I, et al. Proc Natl Acad Sci
USA 20 PROPRIETARY
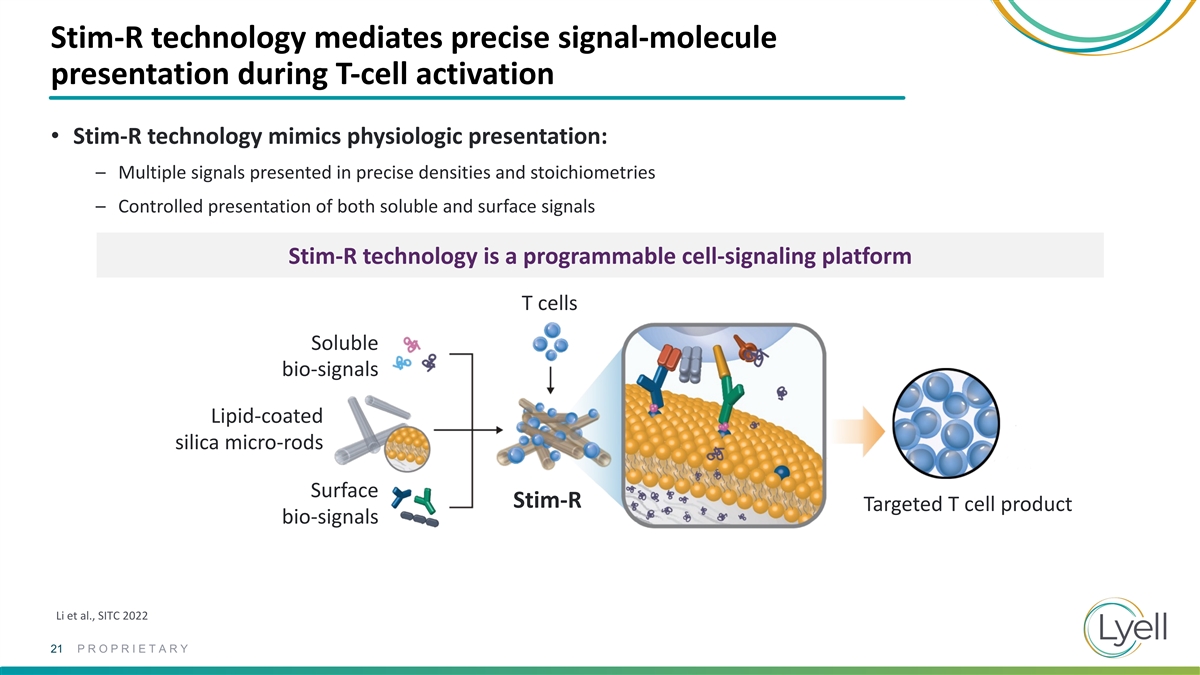
Stim-R technology mediates precise signal-molecule presentation during
T-cell activation • Stim-R technology mimics physiologic presentation: – Multiple signals presented in precise densities and stoichiometries – Controlled presentation of both soluble and surface signals Stim-R technology is a
programmable cell-signaling platform T cells Soluble bio-signals Lipid-coated silica micro-rods Surface Stim-R Targeted T cell product bio-signals Li et al., SITC 2022 21 PROPRIETARY
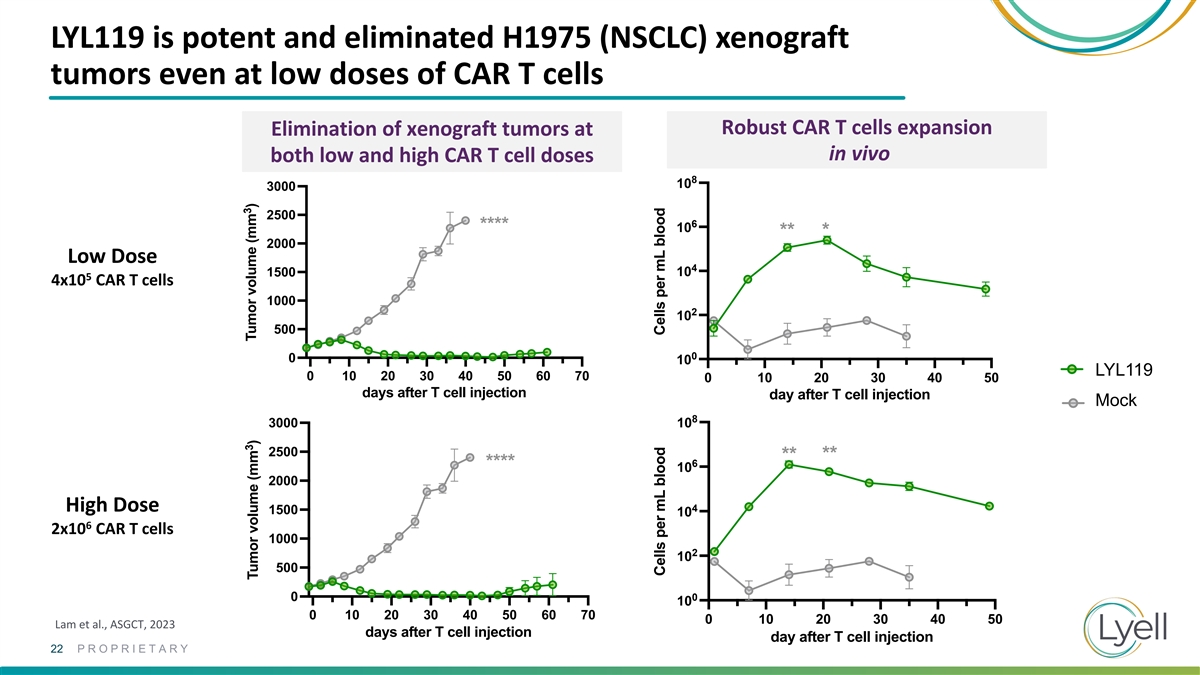
Peripheral blood CD3+CAR+ numbers LYL119 is potent and eliminated H1975
(NSCLC) xenograft Peripheral blood CD3+CAR+ numbers 0.4e6 CAR T cells 8 10 0.4e6 CAR T cells 8 tumors even at low doses of CAR T cells 10 6 ** * 10 ** * 6 ** * Robust CAR T cells expansion10 Elimination of xenograft tumors at ** * 4 in vivo both low
and high CAR T cell doses 10 4 8 10 10 3000 2 10 2500 6 2 **** 10 10 ** * 2000 0 10 Low Dose 4 1500 10 0 5 0 10 20 30 40 10 4x10 CAR T cells day after T cell injection 0 10 20 30 40 1000 2 10 day after T cell injection NR4A3 KO g4 + c-Jun R12 CAR
500 NR4A3 KO g4 + c-Jun R12 CAR NR4A3 KO g47 + c-Jun R12 CAR 0 0 10 NR4A3 KO g47 + c-Jun R12 CAR LYL119 0 10 20 30 40 50 60 70 Control + c-Jun R12 CAR 0 10 20 30 40 50 days after T cell injection day after T cell injection Control + c-Jun R12 CAR
Mock Mock 8 3000 10 Mock 2500 ** ** 6 **** 10 2000 High Dose 4 1500 10 6 2x10 CAR T cells 1000 2 10 500 0 0 10 0 10 20 30 40 50 60 70 0 10 20 30 40 50 Lam et al., ASGCT, 2023 days after T cell injection day after T cell injection 22 PROPRIETARY 3 3
Tumor volume (mm ) Tumor volume (mm ) Cells per mL blood Cells per mL blood Cells per mL Cells per mL blood blood
Harnessing tumor-infiltrating lymphocytes to fight cancer LYL845: A
novel epigenetically reprogrammed TIL product candidate designed for differentiated potency and durability

LYL845 TIL Phase 1 trial design • Patient population CLINICAL
TRIAL DESIGN (mTPI-2) – Relapsed and/or refractory metastatic or Dose Expansion* Dose Escalation locally advanced solid tumors: (Melanoma) – Melanoma RP2D moves LYL845 Melanoma – Non-small cell lung cancer forward to Dose Level 2
(N = ~ 15) expansion – Colorectal cancer cohort • Study objectives LYL845 NSCLC Dose Level 1 (N = ~ 15) – Patient safety and tolerability De-escalation if required – Overall response rate and durability LYL845 Colorectal Dose
Level -1 – Recommended Phase 2 dose (N = ~ 15) – Evaluation of expansion, phenotype, clonal diversity and persistence NCT05573035 *Potential to expand into additional tumor types mTPI-2, modified toxicity probability interval 2; NSCLC,
non-small-cell lung cancer; RP2D, recommended Phase 2 dose 24 PROPRIETARY

LYL845: A novel and differentiated TIL product candidate Control TIL
Key differentiators Standard TIL expansion protocol • Phenotypes (stemness markers and cytotoxic cells) associated with clinical responses LYL845 Tumor Lyell Epi-R protocol comprises: tissue • Proprietary media • Preserved
polyclonal tumor reactive cells • Optimized cytokine compositions • Well-defined cell activation and expansion protocols • Robust TIL expansion across both hot and cold tumors Long-lived Short-lived effector cells stem-like cells
25 PROPRIETARY

LYL845 is enriched for cells with characteristics associated with
improved clinical outcomes Increased % of cytotoxic cells Increased % of stem-like T cells Control LYL845 Krishna et al., Science Dec. 2020 Patel et al., SITC 2022 26 PROPRIETARY * is p<.05 ** is p<.01 *** is p<.001 **** is p<.0001 %
CD8+ T cells % CD8+CD27+ T cells % CD8+CD39-CD69- T cells

LYL845 TIL preserve ~94% of predicted tumor reactive clones to enable
targeting of heterogeneous solid tumors LYL845 T-Cell preparations LYL845 TIL clinical scale runs Malignant tumor tissue 100 75 50 HIGH Bioinformatics EXHAUSTED FREQUENCY 25 analysis CLONES CLONES 0 Donor Check for preservation of predicted tumor
Predicted tumor Melanoma NSCLC CRC reactive clones in reactive clones LYL845 products Harris et al., SITC 2022 Pasetto et al., CIR 2016, Lowrey et al., Science 2022, Oliveira et al., Nature 2021 27 PROPRIETARY % of predicted tumor reactive
clones

In vivo efficacy is superior with LYL845 TIL using our Epi-R process
compared to TIL using standard conditions in novel model In this study: No TIL 2500 • Two refractory melanoma donor Standard TIL samples were collected. 2000 • Samples were split processed to generate TIL products using either 1500 the
Epi-R (LYL845) or the standard process. No TIL Control Donor 1 Standard TIL 1000 • Mice were implanted with Donor 1 LYL845 TIL subcutaneous melanoma cell line Donor 2 Standard TIL on day -8. 500 Donor 2 LYL845 TIL • 4 million LYL845 or
standard TIL were dosed intravenously on day 0. 0 LYL845 TIL 0 10 20 30 40 Days Post treatment 28 PROPRIETARY & CONFIDENTIAL 3 Tumor Volume (mm )

Epi-R P2 is a new manufacturing process designed to shorten product
delivery time to patients • Compared to Epi-R, Epi-R P2: – Does not compromise yield, stemness phenotype or tumor reactive clones – Reliably produces 10+ billion cells in substantially shorter manufacturing time • Expect to
implement Epi-R P2 into our TIL manufacturing in 2024 Epi-R™ Epi-R™ P2 10+ billion cells ü ü Maintains stem-like qualities and tumor killing functionality ü ü Preserves tumor reactive clones ü ü Manufacture
time ~24 days ~16 days 29 PROPRIETARY

Epi-R and Epi-R P2 processes demonstrate comparable product profiles
across CD8 cells Comparable % of stem-like T cells Comparable % of cytotoxic cells NS Epi-R P1 Epi-R P2 Epi-R P1 Epi-R P2 Epi-R P1 Epi-R P2 N=21 include metastatic melanoma, lung cancer and colorectal cancers 30 PROPRIETARY % CD8+ T cells %
CD8+CD27+ T cells % CD8+CD39-CD69- T cells

Epi-R P2 preserves and allows expansion of tumor reactive TIL 100000 In
this study: • T-cell receptor (TCR) sequencing 10000 was performed across Day 0 tumor, 1000 and TIL samples to assess retention of tumor reactive clones in products 100 • T cell clones that are more highly 10 represented in the Day 0
tumor sample (e.g., top 50 or top 100 in 1 frequency) are more likely to be tumor reactive 0.1 • We also experimentally confirmed 0.01 several top frequency TCR clones to Day 0 Tumor Epi-R P1 Epi-R P2 be tumor reactive (shown in purple)
Confirmed tumor reactivity Top 50 Top 50-100 31 PROPRIETARY Clone size (Normalized to 30K TCR sequences)

Manufacturing

Advancing manufacturing strategy to deliver for the future •
Automated manufacturing processes to rapidly and cost- • Currently producing Phase 1 clinical supply effectively scale to meet anticipated patient demand for • Capabilities include CAR T cell, TIL and GMP vector our CAR T-cell product
candidates • Capacity for up to ~500 doses/year depending on • Proof-of-concept technology transfer for the manufacture product mix of LYL797 CAR T-cell therapy CAR T, chimeric antigen receptor T cell; TIL, tumor infiltrating
lymphocytes; GMP, good • Single Cell Shuttle capacity of up to ~800 LYL797 cell manufacturing practice doses/year 33 PROPRIETARY

Advancing T cell therapies for solid tumors Clinical data from two lead
programs in 2024 Two clinical programs: wholly-owned, Executing a scalable manufacturing addressing large patient populations strategy LYL797: ROR1 targeted CAR T cell Lyell’s LyFE center producing current clinical supply • 1H2024: P1
clinical & translational data from 20+ patients • 2024: Epi-R P2 process to shorten TIL manufacturing time • TNBC, NSCLC without impacting cell number and phenotype Planning for the future LYL845: Tumor Infiltrating Lymphocyte (TIL)
• CAR T cell proof-of-concept collaboration with Cellares • 2024: P1 clinical & translational data to build scale and reduce cost • Melanoma, NSCLC, CRC Portfolio of novel reprogramming ~$633 million in cash platform
technologies • 1H2024: IND filing for LYL119, ROR1 targeted CAR T cell • Runway into 2026 designed for enhanced potency and durability; using four of our technologies ROR1, receptor tyrosine kinase-like orphan receptor 1; TNBC,
triple-negative breast cancer; NSCLC, non-small-cell lung cancer; CRC, colorectal cancer; P1, Phase 1 34 PROPRIETARY

IT’S ALL ABOUT THE CELLS
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Lyell Immunopharma (NASDAQ:LYEL)
Historical Stock Chart
From Apr 2024 to May 2024
Lyell Immunopharma (NASDAQ:LYEL)
Historical Stock Chart
From May 2023 to May 2024