UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of August 2023
Commission File Number: 001-40086
Portage Biotech Inc.
(Translation of registrant’s name into English)
Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, British
Virgin Islands, VG1110
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
EXHIBITS
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934,
the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: August 29, 2023
PORTAGE BIOTECH INC.
| By: |
/s/ Allan Shaw |
|
| |
Allan Shaw |
|
| |
Chief Financial Officer |
|
0001095435
false
6-K
--03-31
2024
Q1
0001095435
2023-04-01
2023-06-30
0001095435
2023-06-30
0001095435
2023-03-31
0001095435
2022-04-01
2022-06-30
0001095435
ifrs-full:IssuedCapitalMember
2023-03-31
0001095435
ptgef:StockOptionReserveMember
2023-03-31
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2023-03-31
0001095435
ifrs-full:RetainedEarningsMember
2023-03-31
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2023-03-31
0001095435
ifrs-full:NoncontrollingInterestsMember
2023-03-31
0001095435
ifrs-full:IssuedCapitalMember
2022-03-31
0001095435
ptgef:StockOptionReserveMember
2022-03-31
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2022-03-31
0001095435
ifrs-full:RetainedEarningsMember
2022-03-31
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2022-03-31
0001095435
ifrs-full:NoncontrollingInterestsMember
2022-03-31
0001095435
2022-03-31
0001095435
ifrs-full:IssuedCapitalMember
2023-04-01
2023-06-30
0001095435
ptgef:StockOptionReserveMember
2023-04-01
2023-06-30
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2023-04-01
2023-06-30
0001095435
ifrs-full:RetainedEarningsMember
2023-04-01
2023-06-30
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2023-04-01
2023-06-30
0001095435
ifrs-full:NoncontrollingInterestsMember
2023-04-01
2023-06-30
0001095435
ifrs-full:IssuedCapitalMember
2022-04-01
2022-06-30
0001095435
ptgef:StockOptionReserveMember
2022-04-01
2022-06-30
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2022-04-01
2022-06-30
0001095435
ifrs-full:RetainedEarningsMember
2022-04-01
2022-06-30
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2022-04-01
2022-06-30
0001095435
ifrs-full:NoncontrollingInterestsMember
2022-04-01
2022-06-30
0001095435
ifrs-full:IssuedCapitalMember
2023-06-30
0001095435
ptgef:StockOptionReserveMember
2023-06-30
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2023-06-30
0001095435
ifrs-full:RetainedEarningsMember
2023-06-30
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2023-06-30
0001095435
ifrs-full:NoncontrollingInterestsMember
2023-06-30
0001095435
ifrs-full:IssuedCapitalMember
2022-06-30
0001095435
ptgef:StockOptionReserveMember
2022-06-30
0001095435
ifrs-full:AccumulatedOtherComprehensiveIncomeMember
2022-06-30
0001095435
ifrs-full:RetainedEarningsMember
2022-06-30
0001095435
ifrs-full:EquityAttributableToOwnersOfParentMember
2022-06-30
0001095435
ifrs-full:NoncontrollingInterestsMember
2022-06-30
0001095435
2022-06-30
0001095435
ptgef:SalvaRxLimitedMember
2018-08-13
0001095435
2021-09-01
2021-09-30
0001095435
ifrs-full:BottomOfRangeMember
2021-09-01
2021-09-30
0001095435
ifrs-full:TopOfRangeMember
2021-09-01
2021-09-30
0001095435
2023-07-31
0001095435
ptgef:CountryOfBritishVirginIslandsMember
2023-04-01
2023-06-30
0001095435
ptgef:CountryOfDelawareMember
2023-04-01
2023-06-30
0001095435
ptgef:SubsidiariesTwoMember
2023-04-01
2023-06-30
0001095435
ptgef:StimunitySAMember
2023-04-01
2023-06-30
0001095435
ptgef:StimunitySAMember
2022-04-01
2023-03-31
0001095435
ptgef:StimunitySAMember
2022-04-01
2022-06-30
0001095435
2023-07-12
2023-07-13
0001095435
2022-09-11
2022-09-12
0001095435
2022-04-01
2023-03-31
0001095435
ptgef:IntensityMember
ptgef:AcquisitionOfSalvaRxMember
2023-04-01
2023-06-30
0001095435
ptgef:IntensityMember
ptgef:AcquisitionOfSalvaRxMember
2023-06-30
0001095435
ptgef:IntensityMember
ptgef:AcquisitionOfIntensityHoldingLimitedMember
2019-07-11
0001095435
2022-10-31
0001095435
2022-11-30
0001095435
2022-12-31
0001095435
2023-04-30
0001095435
ifrs-full:BottomOfRangeMember
2023-04-30
0001095435
ifrs-full:TopOfRangeMember
2023-04-30
0001095435
ptgef:OCIMember
2022-04-01
2023-03-31
0001095435
ptgef:OCIMember
2023-03-31
0001095435
ptgef:IntensityMember
2023-07-04
2023-07-05
0001095435
ptgef:IntensityMember
2023-04-01
2023-06-30
0001095435
ptgef:IntensityMember
2023-07-06
2023-07-07
0001095435
ptgef:IntensityMember
2023-07-07
0001095435
ptgef:TarusAcquisitionMember
2023-04-01
2023-06-30
0001095435
2022-07-01
0001095435
ptgef:TarusAcquisitionMember
2023-06-30
0001095435
ptgef:TarusAcquisitionMember
2022-04-01
2022-06-30
0001095435
ptgef:IMM60IOXMelanomaLungCancersMember
2023-06-30
0001095435
ptgef:IMM60IOXMelanomaLungCancersMember
2023-03-31
0001095435
ptgef:IMM65IOXMelanomaLungCancersMember
2023-06-30
0001095435
ptgef:IMM65IOXMelanomaLungCancersMember
2023-03-31
0001095435
ptgef:IOXMember
2023-06-30
0001095435
ptgef:IOXMember
2023-03-31
0001095435
ptgef:OncomerSaugatuckDNAAptamersMember
2023-06-30
0001095435
ptgef:OncomerSaugatuckDNAAptamersMember
2023-03-31
0001095435
ptgef:TarusAdenosineReceptorsMember
2023-06-30
0001095435
ptgef:TarusAdenosineReceptorsMember
2023-03-31
0001095435
ptgef:TarusAdenosineReceptors1Member
2023-06-30
0001095435
ptgef:TarusAdenosineReceptors1Member
2023-03-31
0001095435
ptgef:TarusAdenosineReceptors2Member
2023-06-30
0001095435
ptgef:TarusAdenosineReceptors2Member
2023-03-31
0001095435
ptgef:TarusMember
2023-06-30
0001095435
ptgef:TarusMember
2023-03-31
0001095435
ptgef:IoxIPRAndDMember
ifrs-full:TopOfRangeMember
2023-04-01
2023-06-30
0001095435
ptgef:IoxIPRAndDMember
ifrs-full:BottomOfRangeMember
2023-04-01
2023-06-30
0001095435
ptgef:TrusIPRAndDMember
ifrs-full:TopOfRangeMember
2023-04-01
2023-06-30
0001095435
ptgef:TrusIPRAndDMember
ifrs-full:BottomOfRangeMember
2023-04-01
2023-06-30
0001095435
ptgef:IOXMember
2023-06-30
0001095435
ptgef:IOXMember
2023-03-31
0001095435
2021-04-01
2022-03-31
0001095435
ptgef:UnitedStateMember
2023-06-30
0001095435
ptgef:BVIMember
2023-06-30
0001095435
ptgef:CountryOfUnitedKingdomMember
2023-06-30
0001095435
ptgef:UnitedStateMember
2022-06-30
0001095435
ptgef:BVIMember
2022-06-30
0001095435
ptgef:CountryOfUnitedKingdomMember
2022-06-30
0001095435
2022-07-15
2022-07-18
0001095435
2022-07-18
0001095435
2022-07-05
2022-07-06
0001095435
ptgef:ATMMember
2022-04-01
2023-03-31
0001095435
ptgef:LincolnMember
2022-04-01
2023-03-31
0001095435
ptgef:ATMMember
2023-04-01
2023-06-30
0001095435
ptgef:N2021EquityIncentivePlanMember
2021-01-13
0001095435
ptgef:BoardOfDirectorsMember
2021-01-13
0001095435
ptgef:ConsultantsMember
2021-01-13
0001095435
ptgef:RestrictedStockUnitsMember
2021-01-12
2021-01-13
0001095435
ptgef:RestrictedStockUnitsMember
2020-04-01
2021-03-31
0001095435
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-01-18
2022-01-19
0001095435
ptgef:TwoMembersMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-01-18
2022-01-19
0001095435
ptgef:EmployeesMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-01-18
2022-01-19
0001095435
ptgef:DirectorsMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-01-18
2022-01-19
0001095435
ptgef:DirectorsMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-02-14
2022-02-15
0001095435
ptgef:ExecutiveMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-06-07
2022-06-08
0001095435
ptgef:BoardOfDirectorsMember
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2022-07-26
2022-07-27
0001095435
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2023-03-25
2023-03-30
0001095435
2023-03-25
2023-03-30
0001095435
ptgef:VestedOptionsMember
2023-06-30
0001095435
ptgef:VestedOptionsMember
2023-03-31
0001095435
ptgef:UnvestedOptionsMember
2023-06-30
0001095435
ptgef:UnvestedOptionsMember
2023-03-31
0001095435
ptgef:AmendedAndRestated2021EquityIncentivePlanMember
2023-04-01
2023-06-30
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2023-03-31
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2022-03-31
0001095435
ptgef:StockOptionsTwoMember
2023-03-31
0001095435
ptgef:StockOptionsTwoMember
2022-03-31
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2023-04-01
2023-06-30
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2022-04-01
2022-06-30
0001095435
ptgef:StockOptionsTwoMember
2023-04-01
2023-06-30
0001095435
ptgef:StockOptionsTwoMember
2022-04-01
2022-06-30
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2023-06-30
0001095435
ptgef:PBI2021EquityIncentivePlanMember
2022-06-30
0001095435
ptgef:StockOptionsTwoMember
2023-06-30
0001095435
ptgef:StockOptionsTwoMember
2022-06-30
0001095435
ifrs-full:ShareOptionsMember
2023-04-01
2023-06-30
0001095435
ifrs-full:ShareOptionsMember
2022-04-01
2022-06-30
0001095435
ifrs-full:RestrictedShareUnitsMember
2023-04-01
2023-06-30
0001095435
ifrs-full:RestrictedShareUnitsMember
2022-04-01
2022-06-30
0001095435
2022-07-13
0001095435
ptgef:LincolnMember
2022-07-05
2022-07-06
0001095435
ptgef:SalvaRxMember
2021-09-07
2021-09-08
0001095435
2021-12-14
2021-12-15
0001095435
2022-12-18
2022-12-19
0001095435
2022-12-25
2022-12-31
0001095435
ptgef:AmortizedCostMember
2023-06-30
0001095435
ptgef:FairValueToOtherComprehensiveIncomeMember
2023-06-30
0001095435
ptgef:FairValueThroughProfitOrLossMember
2023-06-30
0001095435
ptgef:AmortizedCostMember
2023-03-31
0001095435
ptgef:FairValueToOtherComprehensiveIncomeMember
2023-03-31
0001095435
ptgef:FairValueThroughProfitOrLossMember
2023-03-31
0001095435
ptgef:TarusMember
2023-04-01
2023-06-30
0001095435
ptgef:IOXMember
2023-04-01
2023-06-30
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2023-03-31
0001095435
ptgef:IOXMember
2023-04-01
2023-06-30
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2023-04-01
2023-06-30
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2023-06-30
0001095435
ptgef:IOXMember
2022-03-31
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2022-03-31
0001095435
ptgef:IOXMember
2022-04-01
2022-06-30
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2022-04-01
2022-06-30
0001095435
ptgef:IOXMember
2022-06-30
0001095435
ptgef:SaugatuckAndSubsidiaryMember
2022-06-30
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
iso4217:EUR
iso4217:EUR
xbrli:shares
Exhibit 99.1
Portage Biotech Inc.
Condensed Consolidated Interim Financial Statements
For the Three Months Ended June 30, 2023
(Unaudited – Prepared by Management as of August 29, 2023)
(U.S. Dollars)
Portage Biotech Inc.
Condensed Consolidated Interim Financial Statements
| Index |
|
Page |
| |
|
|
| Notice to Reader |
|
F-1 |
| |
|
|
Condensed Consolidated Interim Statements of Financial Position
As of June 30, 2023 (Unaudited) and March 31, 2023 |
|
F-2 |
| |
|
|
Condensed Consolidated Interim Statements of Operations and Other Comprehensive Income (Loss) (Unaudited)
Three months ended June 30, 2023 and 2022 |
|
F-3 |
| |
|
|
Condensed Consolidated Interim Statements of Changes in Shareholders’ Equity (Unaudited)
Three months ended June 30, 2023 and 2022 |
|
F-4 |
| |
|
|
Condensed Consolidated Interim Statements of Cash Flows (Unaudited)
Three months ended June 30, 2023 and 2022 |
|
F-5 |
| |
|
|
| Notes to Condensed Consolidated Interim Financial Statements |
|
F-6 to F-37 |
NOTICE TO READER OF CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS
The condensed consolidated interim financial statements of Portage Biotech Inc. are comprised
of the condensed consolidated statements of financial position as of June 30, 2023 and March 31, 2023, the condensed consolidated interim
statements of operations and other comprehensive income (loss) for the three months ended June 30, 2023 and 2022 and the statements of
equity and cash flows for each of the three months ended June 30, 2023 and 2022 and are the responsibility of Portage Biotech Inc.’s
management.
The condensed consolidated interim financial statements of Portage Biotech Inc. have been prepared
by Portage Biotech Inc.’s management and include the selection of appropriate accounting principles, judgments and estimates necessary
to prepare these condensed consolidated interim financial statements in accordance with International Financial Reporting Standards.
| /s/ Allan Shaw |
/s/ Ian Walters |
| Allan Shaw, CFO |
Ian Walters, MD, Chairman of the Board and Chief Executive Officer |
| |
|
| DATE: August 29, 2023 |
|
Portage Biotech Inc.
Condensed Consolidated Interim Statements of Financial Position
(U.S. Dollars in thousands)
(Unaudited – see Notice to Reader dated August 29, 2023)
| | |
| |
| | | |
| | |
| | |
Notes | |
June 30,
2023 | |
March 31,
2023 |
| | |
| |
| | | |
| (Audited) | |
| Assets | |
| |
| | | |
| | |
| Current assets | |
| |
| | | |
| | |
| Cash and cash equivalents | |
| |
$ | 7,698 | | |
$ | 10,545 | |
| Prepaid expenses and other receivables | |
5 | |
| 2,752 | | |
| 2,689 | |
| Convertible note receivable | |
6 | |
| 442 | | |
| 442 | |
| Total current assets | |
| |
| 10,892 | | |
| 13,676 | |
| Non-current assets | |
| |
| | | |
| | |
| Investment in associate | |
6 | |
| 756 | | |
| 806 | |
| Investment in public company | |
7 | |
| 3,855 | | |
| 2,087 | |
| In-process research and development | |
9, 10 | |
| 81,683 | | |
| 81,683 | |
| Deferred commitment fee | |
15 | |
| 839 | | |
| 839 | |
| Right to use asset | |
8 | |
| 293 | | |
| - | |
| Other assets, including equipment, net | |
| |
| 51 | | |
| 38 | |
| Total non-current assets | |
| |
| 87,477 | | |
| 85,453 | |
| Total assets | |
| |
$ | 98,369 | | |
$ | 99,129 | |
| | |
| |
| | | |
| | |
| Liabilities and Equity | |
| |
| | | |
| | |
| Current liabilities | |
| |
| | | |
| | |
| Accounts payable and accrued liabilities | |
| |
$ | 2,591 | | |
$ | 1,865 | |
| Lease liability - current, including interest | |
8 | |
| 47 | | |
| - | |
| Total current liabilities | |
| |
| 2,638 | | |
| 1,865 | |
| Non-current liabilities | |
| |
| | | |
| | |
| Lease liability - non-current | |
8 | |
| 249 | | |
| - | |
| Deferred tax liability | |
10, 11 | |
| 10,416 | | |
| 10,564 | |
| Deferred purchase price payable - Tarus | |
9, 17 | |
| 7,864 | | |
| 7,179 | |
| Deferred obligation - iOx milestone | |
16, 17 | |
| 4,552 | | |
| 4,126 | |
| Total non-current liabilities | |
| |
| 23,081 | | |
| 21,869 | |
| Total liabilities | |
| |
| 25,719 | | |
| 23,734 | |
| | |
| |
| | | |
| | |
| Shareholders’ Equity | |
| |
| | | |
| | |
| Capital stock | |
12 | |
| 219,425 | | |
| 218,782 | |
| Stock option reserve | |
13 | |
| 21,973 | | |
| 21,204 | |
| Accumulated other comprehensive loss | |
| |
| (2,556 | ) | |
| (4,325 | ) |
| Accumulated deficit | |
| |
| (165,535 | ) | |
| (159,616 | ) |
| Total equity attributable to owners of the Company | |
| |
| 73,307 | | |
| 76,045 | |
| Non-controlling interest | |
19 | |
| (657 | ) | |
| (650 | ) |
| Total equity | |
| |
| 72,650 | | |
| 75,395 | |
| Total liabilities and equity | |
| |
$ | 98,369 | | |
$ | 99,129 | |
| Commitments and Contingent Liabilities (Note 15) | |
| |
| - | | |
| - | |
The accompanying notes are an integral part of these condensed consolidated
interim financial statements.
Portage Biotech Inc.
Condensed Consolidated Interim Statements of Operations and Other Comprehensive
Income (Loss)
(U.S. Dollars in thousands, except per share amounts)
(Unaudited – see Notice to Reader dated August 29, 2023)
| | |
| |
| | | |
| | |
| | |
Note | |
Three Months Ended June 30, |
| | |
| |
2023 | |
2022 |
| Expenses | |
| |
| | | |
| | |
| Research and development | |
| |
| 3,627 | | |
| 1,876 | |
| General and administrative expenses | |
| |
| 1,370 | | |
| 2,211 | |
| Loss from operations | |
| |
| (4,997 | ) | |
| (4,087 | ) |
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone | |
9, 16, 17 | |
| (1,111 | ) | |
| - | |
| Share of loss in associate accounted for using equity method | |
6 | |
| (50 | ) | |
| (60 | ) |
| Change in fair value of warrant liability | |
| |
| - | | |
| 1 | |
| Foreign exchange transaction gain (loss) | |
11 | |
| 18 | | |
| (52 | ) |
| Depreciation expense | |
| |
| (11 | ) | |
| - | |
| Interest income, net | |
| |
| 80 | | |
| 21 | |
| Loss before (provision) benefit for income taxes | |
| |
| (6,071 | ) | |
| (4,177 | ) |
| Income tax benefit | |
11 | |
| 145 | | |
| 2,552 | |
| Net loss | |
| |
| (5,926 | ) | |
| (1,625 | ) |
| Other comprehensive income (loss) | |
| |
| | | |
| | |
| Net unrealized gain on investments | |
6, 7 | |
| 1,769 | | |
| - | |
| Total comprehensive loss for period | |
| |
$ | (4,157 | ) | |
$ | (1,625 | ) |
| | |
| |
| | | |
| | |
| Net (loss) income attributable to: | |
| |
| | | |
| | |
| Owners of the Company | |
| |
$ | (5,919 | ) | |
$ | (1,729 | ) |
| Non-controlling interest | |
19 | |
| (7 | ) | |
| 104 | |
| Net loss | |
| |
$ | (5,926 | ) | |
$ | (1,625 | ) |
| | |
| |
| | | |
| | |
| Comprehensive (loss) income attributable to: | |
| |
| | | |
| | |
| Owners of the Company | |
| |
$ | (4,150 | ) | |
$ | (1,729 | ) |
| Non-controlling interest | |
19 | |
| (7 | ) | |
| 104 | |
| Total comprehensive loss for period | |
| |
$ | (4,157 | ) | |
$ | (1,625 | ) |
| | |
| |
| | | |
| | |
| Loss per share | |
14 | |
| | | |
| | |
| Basic and diluted | |
| |
$ | (0.33 | ) | |
$ | (0.13 | ) |
| | |
| |
| | | |
| | |
| Weighted average shares outstanding | |
14 | |
| | | |
| | |
| Basic and diluted | |
| |
| 17,701 | | |
| 13,351 | |
The accompanying notes are an integral part of these condensed consolidated
interim financial statements.
Portage Biotech Inc.
Condensed Consolidated Interim Statements of Changes in Shareholders’
Equity
For the Three Months Ended June 30, 2023 and 2022
(U.S. Dollars)
(Unaudited – see Notice to Reader dated August 29, 2023)
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
| |
| |
| |
Accumulated | |
| |
Equity | |
| |
|
| | |
Number | |
| |
Stock | |
Other | |
| |
Attributable | |
Non- | |
|
| | |
of | |
Capital | |
Option | |
Comprehensive | |
(Accumulated | |
to Owners | |
Controlling | |
Total |
| | |
Shares | |
Stock | |
Reserve | |
Income (Loss) | |
Deficit) | |
of Company | |
Interest | |
Equity |
| Balance, April 1, 2023 | |
| 17,606 | | |
| 218,782 | | |
| 21,204 | | |
| (4,325 | ) | |
| (159,616 | ) | |
| 76,045 | | |
| (650 | ) | |
| 75,395 | |
| Share-based compensation expense | |
| - | | |
| - | | |
| 769 | | |
| - | | |
| - | | |
| 769 | | |
| - | | |
| 769 | |
| Shares issued under ATM | |
| 171 | | |
| 632 | | |
| - | | |
| - | | |
| - | | |
| 632 | | |
| - | | |
| 632 | |
| Share issuance costs | |
| - | | |
| (19 | ) | |
| - | | |
| - | | |
| - | | |
| (19 | ) | |
| - | | |
| (19 | ) |
| Shares issued or accrued for services | |
| 9 | | |
| 30 | | |
| - | | |
| - | | |
| - | | |
| 30 | | |
| - | | |
| 30 | |
| Net unrealized gain on investments | |
| - | | |
| - | | |
| - | | |
| 1,769 | | |
| - | | |
| 1,769 | | |
| - | | |
| 1,769 | |
| Net loss for period | |
| - | | |
| - | | |
| - | | |
| - | | |
| (5,919 | ) | |
| (5,919 | ) | |
| (7 | ) | |
| (5,926 | ) |
| Balance, June 30, 2023 | |
| 17,786 | | |
| 219,425 | | |
| 21,973 | | |
| (2,556 | ) | |
| (165,535 | ) | |
| 73,307 | | |
| (657 | ) | |
| 72,650 | |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance, April 1, 2022 | |
| 13,349 | | |
| 158,324 | | |
| 16,928 | | |
| 958 | | |
| (55,005 | ) | |
| 121,205 | | |
| 44,229 | | |
| 165,434 | |
| Share-based compensation expense | |
| - | | |
| - | | |
| 1,176 | | |
| - | | |
| - | | |
| 1,176 | | |
| - | | |
| 1,176 | |
| Shares issued or accrued for services | |
| 4 | | |
| 30 | | |
| - | | |
| - | | |
| - | | |
| 30 | | |
| - | | |
| 30 | |
| Net (loss) income for period | |
| - | | |
| - | | |
| - | | |
| - | | |
| (1,729 | ) | |
| (1,729 | ) | |
| 104 | | |
| (1,625 | ) |
| Balance, June 30, 2022 | |
| 13,353 | | |
$ | 158,354 | | |
$ | 18,104 | | |
$ | 958 | | |
$ | (56,734 | ) | |
$ | 120,682 | | |
$ | 44,333 | | |
$ | 165,015 | |
The accompanying notes are an integral part of these condensed consolidated
interim financial statements.
Portage Biotech Inc.
Condensed Consolidated Interim Statements of Cash Flows
For the Three Months Ended June 30, 2023 and 2022
(U.S. Dollars in thousands)
(Unaudited – see Notice to Reader dated August 29, 2023)
| | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss for the period | |
$ | (5,926 | ) | |
$ | (1,625 | ) |
| Adjustments for non-cash items: | |
| | | |
| | |
| Share-based compensation expense | |
| 769 | | |
| 1,176 | |
| Change in fair value of deferred purchase price payable – Tarus and deferred obligation – iOx milestone | |
| 1,111 | | |
| - | |
| Decrease in deferred tax liability | |
| (148 | ) | |
| (2,552 | ) |
| Share of loss in associate | |
| 50 | | |
| 60 | |
| Fair value of shares issued for services | |
| 30 | | |
| 30 | |
| Depreciation | |
| 11 | | |
| - | |
| Change in fair value of warrant liability | |
| - | | |
| (1 | ) |
| Changes in operating working capital: | |
| | | |
| | |
| Accounts receivable | |
| (17 | ) | |
| (44 | ) |
| Prepaid expenses and other receivables | |
| (50 | ) | |
| (408 | ) |
| Other assets | |
| (10 | ) | |
| - | |
| Accounts payable and accrued liabilities | |
| 726 | | |
| 1,188 | |
| Other | |
| 1 | | |
| - | |
| Net cash used in operating activities | |
| (3,453 | ) | |
| (2,176 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from shares issued under ATM and Committed Purchase Agreement | |
| 632 | | |
| - | |
| Share issuance costs | |
| (19 | ) | |
| - | |
| Repayment of lease liability | |
| (7 | ) | |
| - | |
| Net cash provided by financing activities | |
| 606 | | |
| - | |
| | |
| | | |
| | |
| Decrease in cash and cash equivalents during period | |
| (2,847 | ) | |
| (2,176 | ) |
| Cash and cash equivalents at beginning of period | |
| 10,545 | | |
| 23,352 | |
| Cash and cash equivalents at end of period | |
$ | 7,698 | | |
$ | 21,176 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Net unrealized gain on investment in Intensity | |
$ | 1,769 | | |
$ | - | |
| Cash paid for interest | |
$ | 3 | | |
$ | - | |
| | |
| | | |
| | |
| Supplemental disclosure of non-cash investing and financing activities: | |
| | | |
| | |
| Right to use asset acquired | |
$ | 303 | | |
$ | - | |
| Lease liability incurred | |
$ | 303 | | |
$ | - | |
The accompanying notes are an integral part of these condensed consolidated
interim financial statements.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 1. NATURE OF OPERATIONS
Portage Biotech Inc. (the “Company” or “Portage”)
is incorporated in the British Virgin Islands (“BVI”) with its registered office located at Clarence Thomas Building, P.O.
Box 4649, Road Town, Tortola, BVI. Its USA agent, Portage Development Services Inc. (“PDS”), is located at 61 Wilton Road,
Westport, CT, 06880, USA.
The Company is a foreign private issuer under the Securities and Exchange
Commission (the “SEC”) rules. It is also a reporting issuer under the securities legislation of the provinces of Ontario and
British Columbia. Its ordinary shares were listed on the Canadian Securities Exchange (“CSE”) under the symbol “PBT.U”.
On February 25, 2021, the ordinary shares of the Company began trading on the Nasdaq Capital Market (“Nasdaq”) under the symbol
“PRTG”. As the principal market for the Company’s ordinary shares is Nasdaq, the Company voluntarily delisted from the
CSE on April 23, 2021.
Portage is a clinical-stage immuno-oncology company advancing therapies
that target known checkpoint resistance pathways to improve long-term treatment response and quality of life in patients with invasive
cancers. Portage’s access to next-generation technologies coupled with a deep understanding of biological mechanisms enable the
identification of clinical therapies and product development strategies that accelerate these medicines through the translational pipeline.
Portage’s portfolio consists of four diverse platforms, with lead programs consisting of invariant natural killer T-cell (“iNKT”)
engagers and a suite of treatments targeting the adenosine pathway. Additional programs leverage delivery by intratumorals, nanoparticles,
liposomes, aptamers, and virus-like particles. Within these four platforms, Portage has 9 product candidates currently in development
with multiple clinical readouts expected through the end of calendar year 2024.
On August 13, 2018, the Company reached a definitive agreement to acquire
100% of SalvaRx Limited (“SalvaRx”) in exchange for 8,050,701 ordinary shares of the Company (the “SalvaRx Acquisition”).
The SalvaRx Acquisition was completed on January 8, 2019 (the “Acquisition Date”) upon receiving shareholder and regulatory
approval. In connection with the SalvaRx Acquisition, the Company acquired interests in SalvaRx’s five research and development
invested entities and subsidiaries: iOx Therapeutics Ltd. (“iOx”) (60.49% interest), Nekonal Oncology Limited (“Nekonal”),
Intensity Therapeutics, Inc. (“Intensity”), Saugatuck Therapeutics, Ltd. (“Saugatuck”) and Rift Biotherapeutics
Inc. The Company also acquired an option in Nekonal SARL, a Luxembourg-based company holding intellectual property rights for therapeutics
and diagnostics in the field of autoimmune disorders and oncology, to participate in the funding of its autoimmune programs.
In September 2021, the Company, through SalvaRx, exchanged certain notes,
accrued interest, warrants and receivables in exchange for shares of iOx representing 60.49% of the outstanding shares of iOx. As a result
of this exchange, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%. On July 18, 2022, the Company purchased
the remaining non-controlling interest of iOx. See Note 16, “Related Party Transactions –
Share Exchange Agreement – iOx,” for a further discussion.
NOTE 2. GOING CONCERN
As of June 30, 2023, the Company had cash and cash equivalents of approximately
$7.7 million and total current liabilities of approximately $2.6 million. For the three months ended June 30, 2023, the Company is reporting
a net loss of approximately $5.9 million and cash used in operating activities of approximately $3.5 million. As of July 31, 2023, the
Company had approximately $6.4 million of cash and cash equivalents on hand.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 2. GOING CONCERN (Cont'd)
The Company’s cash and cash equivalents balance is decreasing, and
the Company will not generate positive cash flows from operations for the fiscal year ending March 31, 2024.
The Company may have to delay, scale-back, or eliminate certain of its
activities and other aspects of its operations until such time as the Company is successful in securing additional funding. The Company
is exploring various dilutive and non-dilutive sources of funding, including equity and debt financings, strategic alliances, business
development and other sources. The future success of the Company is dependent upon its ability to obtain additional funding. There can
be no assurance, however, that the Company will be successful in obtaining such funding in sufficient amounts, on terms acceptable to
the Company, or at all. As of the date of this filing, the Company currently anticipates that current cash and cash equivalents, excluding
any potential proceeds from its ATM program and Committed Purchase Agreement with Lincoln Park, access to which are generally limited
based on the Company’s Nasdaq trading volume, will be sufficient to meet its anticipated cash requirements through the end of October
2023. These factors raise significant doubt about the Company’s ability to continue as a going concern within one year after the
date that these financial statements are issued.
The Company has incurred significant operating losses since inception
and expects to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result
primarily from its conduct of research and development activities.
The Company historically has funded its operations principally from proceeds
from issuances of equity and debt securities. The Company will require significant additional capital to make the investments it needs
to execute its longer-term business plan, beyond the potential proceeds that could be reasonably generated from its ATM program and Committed
Purchase Agreement with Lincoln Park given the Company’s current trading volume on Nasdaq. The Company's ability to successfully
raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, future
equity issuances would result in dilution to existing stockholders and any future debt securities may contain covenants that limit the
Company's operations or ability to enter into certain transactions.
NOTE 3. BASIS OF PRESENTATION
Statement of Compliance and Basis of Presentation
These condensed consolidated interim financial statements have been prepared
in accordance with the International Financial Reporting Standards (“IFRS”) issued by the International Accounting Standards
Board (“IASB”), IAS 34 Interim Financial Reporting and interpretations of the International Financial Reporting Interpretations
Committee. These condensed consolidated interim financial statements do not include all of the information required for full annual financial
statements and should be read in conjunction with the audited consolidated financial statements of the Company for the year ended March
31, 2023.
These condensed consolidated interim financial statements have been prepared
on an historical cost basis except for items disclosed herein at fair value (see Note 17, “Financial Instruments and Risk Management”).
In addition, these condensed consolidated interim financial statements have been prepared using the accrual basis of accounting, except
for cash flow information.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 3. BASIS OF PRESENTATION (Cont'd)
The Company has only one reportable operating segment.
These condensed consolidated interim financial statements were approved
and authorized for issuance by the Audit Committee and Board of Directors (the “Board”) on August 29, 2023.
Consolidation
The condensed consolidated interim financial statements include the accounts
of the Company and:
| (a) | SalvaRx, a wholly-owned subsidiary, incorporated on May 6, 2015 in the British Virgin Islands; |
| (b) | iOx, a wholly-owned subsidiary incorporated in the U.K. on February 10, 2015. In September 2021, the Company, through SalvaRx, exchanged
certain notes, accrued interest, warrants and receivables in exchange for shares of iOx representing 60.49% of the outstanding shares
of iOx. As a result of this exchange, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%. On July 18,
2022, the Company purchased the remaining non-controlling interest of iOx. See Note 16, “Related
Party Transactions – Share Exchange Agreement – iOx,” for a further discussion; |
| (c) | Saugatuck, a 70% owned subsidiary incorporated in the British Virgin Islands. Saugatuck and subsidiary refers to Saugatuck and Saugatuck
Rx LLC; |
| (d) | PDS, a 100% owned subsidiary incorporated in Delaware, which provides human resources, and other services to each operating subsidiary
via a shared services agreement; |
| (e) | SalvaRx LLC, a wholly-owned subsidiary through SalvaRx; |
| (f) | Saugatuck Rx LLC, a wholly-owned subsidiary of Saugatuck; and |
| (g) | Tarus Therapeutics, LLC (“Tarus”), a wholly-owned subsidiary of Portage. |
All inter-company balances and transactions have been eliminated in consolidation.
Non-controlling interest in the equity of a subsidiary
is accounted for and reported as a component of stockholders’ equity. As of June 30, 2023, non-controlling interest represents the
30% shareholder ownership interest in Saugatuck and subsidiary, which is consolidated by the Company. See Note 16, “Related Party
Transactions – Share Exchange Agreement – iOx” for a discussion of the Company’s purchase of the balance of the
non-controlling interest in iOx.
Functional and Presentation Currency
The Company’s functional and presentation currency is the U.S. Dollar.
Use of Estimates and Judgments
The preparation of the condensed consolidated interim financial statements
in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies
and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 3. BASIS OF PRESENTATION (Cont'd)
Estimates and underlying assumptions are reviewed on an ongoing basis.
Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial
instruments (including the Stimunity Convertible Note (as defined below), deferred tax assets and liabilities, research and development
costs, fair value used for acquisition of intangible assets, contingent consideration assumed and measurement of share-based compensation.
Significant areas where critical judgments are applied include assessment of impairment of investments, goodwill and in-process research
and development and the determination of the accounting acquirer and acquiree in the business combination accounting.
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES
The accounting policies are set out in Note 4 to the Company’s audited
consolidated financial statements for the fiscal year ended March 31, 2023 (“Fiscal 2023”). These policies have been applied
consistently to all periods presented in these condensed consolidated interim financial statements.
Recent Accounting Pronouncements
IFRS Pronouncements Issued
Impact of Adoption of Significant New IFRS Standards in Fiscal 2023
| (a) | Annual Improvements to IFRS Standards 2018-2020 |
The annual improvements process addresses issues in the 2018-2020 reporting
cycles including changes to IFRS 9, “Financial Instruments,” IFRS 1, “First Time Adoption of IFRS,” IFRS 16, “Leases,”
and IAS 41, “Biological Assets”.
| i) | The amendment to IFRS 9 addresses which fees should be included in the 10% test for derecognition of financial liabilities. |
| ii) | The amendment to IFRS 1 allows a subsidiary adopting IFRS at a later date than its parent to also measure cumulative translation differences
using the amounts reported by the parent based on the parent’s date of transition to IFRS. |
| iii) | The amendment to IFRS 16’s illustrative example 13 removes the illustration of payments from the lessor related to leasehold
improvements. |
These amendments were effective for annual periods beginning on or after
January 1, 2022. The adoption of these amendments did not have a material effect on the Company’s annual consolidated financial
statements or the condensed consolidated interim financial statements for the three months ended June 30, 2023.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES (Cont'd)
New Accounting Standards, Interpretations and Amendments
Standards issued but not yet effective up to the date of issuance of the
Company’s condensed consolidated interim financial statements are listed below. This listing is of standards and interpretations
issued, which the Company reasonably expects to be applicable at a future date. The Company intends to adopt those standards when they
become effective.
| (a) | IAS 1: Presentation of Financial Statements |
The amendment to IAS 1 clarifies how to classify debt and other liabilities
as either current or non-current. The amendment will be effective for annual periods beginning on or after January 1, 2024. The Company
is currently evaluating the new guidance and impacts on its consolidated financial statements.
| (b) | Amendments to IFRS 10 and IAS 28: Sale or Contribution of Assets between an Investor and Its Associate or Joint Venture |
The amendment addresses the conflict between IFRS 10, “Consolidated
Financial Statements,” and IAS 28, “Investments in Associates and Joint Ventures,” in dealing with the loss of control
of a subsidiary that is sold or contributed to an associate or joint venture. The amendments clarify that the gain or loss resulting from
the sale or contribution of assets that constitute a business, as defined in IFRS 3, “Business Combinations,” between an investor
and its associate or joint venture, is recognized in full. Any gain or loss resulting from the sale or contribution of assets that do
not constitute a business, however, is recognized only to the extent of unrelated investors' interests in the associate or joint venture.
The IASB has deferred the effective date of these amendments indefinitely, but an entity that early adopts the amendments must apply them
prospectively. The Company is evaluating whether the adoption of the above amendment will have a material impact on its financial statements.
NOTE 5. PREPAID EXPENSES AND OTHER RECEIVABLES
| Schedule of prepaid expenses and other receivables | |
| | | |
| | |
| (In thousands) | |
As of
June 30, 2023 | |
As of
March 31, 2023 |
| | |
| |
|
| Prepaid clinical research costs | |
$ | 1,783 | | |
$ | 1,653 | |
| Prepaid insurance | |
| 446 | | |
| 621 | |
| Research & development tax credits | |
| 186 | | |
| 169 | |
| Other prepaid expenses | |
| 151 | | |
| 56 | |
| Tax deposits | |
| 115 | | |
| 119 | |
| Other receivables | |
| 71 | | |
| 71 | |
| Total prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | 2,689 | |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 6. INVESTMENT IN ASSOCIATE
Details of the Company’s associate, Stimunity S.A. (“Stimunity”),
as of June 30, 2023 and March 31, 2023 are as follows:
| Schedule of investment associate | |
| |
| |
| |
|
| Name | |
Principal Activity | |
Place of Incorporation and
Principal Place of Business | |
Voting Rights Held as
of June 30, 2023 | |
Voting Rights Held as
of March 31, 2023 |
| Associate: Stimunity S.A. | |
Biotechnology | |
Paris, France | |
44.0% | |
44.0% |
The following table is a roll-forward of the Company’s investment
in Stimunity as of and for the three months ended June 30, 2023 and 2022:
| Schedule of investment in Stimunity S.A. | |
| | | |
| | |
| | |
As of and for the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Balance, beginning of period | |
$ | 806 | | |
$ | 1,673 | |
| Share of loss | |
| (50 | ) | |
| (60 | ) |
| Balance, end of period | |
$ | 756 | | |
$ | 1,613 | |
The Company accounts for its investment in Stimunity under the equity
method and, accordingly, records its share of Stimunity’s earnings or loss based on its ownership percentage. The Company recorded
loss in equity in Stimunity of $50,000 and $60,000 for the three months ended June 30, 2023 and 2022, respectively.
Under the Shareholders’ Agreement entered into on June 1, 2020,
Portage has (i) a preferential subscription right to maintain its equity interest in Stimunity in the event of a capital increase from
the issuance of new securities by Stimunity, except for issuances of new securities for stock options, under a merger plan or for an acquisition,
and (ii) the right to vote against any (a) issuances of additional securities that would call for Portage to waive its preferential subscription
right, or (b) any dilutive issuance.
On July 13, 2022, the Company entered into a commitment with Stimunity
to provide €600,000 under a convertible note (the “Stimunity Convertible Note”) with a maturity date of September 1,
2023 (the “Maturity Date”). The Stimunity Convertible Note provides for simple interest at 7% per annum. The Stimunity Convertible
Note is automatically converted into Series A shares of Stimunity upon Stimunity completing a Series A round for at least €20 million.
If such subscription round is completed prior to the Maturity Date, the Company will be entitled to convert the Stimunity Convertible
Note into Series A shares of Stimunity at the subscription share price less 15%. Additionally, if Stimunity completes a financing with
a new category of shares (other than Common Shares or Series A shares) for at least €5 million (the “Minimum Raise”),
the Company will have the right to convert the Stimunity Convertible Note and the historical Series A shares of Stimunity owned into the
new category of shares. In the event that Stimunity does not close a financing prior to the Maturity Date or raises less than the Minimum
Raise, the Company will have the right to convert the Stimunity Convertible Note into Series A shares at €363.00 per share or the
raise price less 15%, whichever is lower. The Stimunity Convertible Note was funded on September 12, 2022. See Note 15, “Commitments
and Contingent Liabilities – Stimunity Convertible Note,” for a further discussion.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 6. INVESTMENT IN ASSOCIATE (Cont’d)
The Stimunity Convertible Note was initially recorded at $0.614 million
to record the translated value of the Stimunity Convertible Note on September 12, 2022. The Company recognized an unrealized gain of $0.039
million through other comprehensive income (“OCI”) in Fiscal 2023 to reflect the change in translation rate for the Stimunity
Convertible Note settleable in euros, increasing the carrying value of the Stimunity Convertible Note to $0.653 million.
As of March 31, 2023, the Company determined that there were indications
of impairment of both the investment in associate and the Stimunity Convertible Note receivable, based upon the inability of Stimunity
to obtain financing. The Company performed an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current
market conditions, the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity. The Company
recorded provisions of impairment of $0.607 million and $0.211 million, with respect to the investment in associate and the Stimunity
Convertible Note receivable, respectively, decreasing the carrying value of the investment in associate and the Stimunity Convertible
Note to $0.806 million and $0.442 million, respectively, as of March 31, 2023.
During the three months ended June 30, 2023, the Company recorded a
loss of $0.05
million to recognize its share of Stimunity’s results of operations and recorded a marginal gain through OCI to reflect the
translation rate effect on the Stimunity Convertible Note settleable in euros. The Company determined that there were no
significant changes in the market or Stimunity’s outlook from March 31, 2023 to June 30, 2023, and, accordingly, determined that
the carrying value at June 30, 2023 was not further impaired.
NOTE 7. INVESTMENT IN PUBLIC COMPANY
The following is a discussion of the Company’s investment in Intensity
Therapeutics, Inc. (“Intensity”) as of June 30, 2023 and March 31, 2023.
Intensity Therapeutics, Inc.
In connection with the SalvaRx Acquisition in fiscal 2019, the Company
acquired a $4.5 million interest in Intensity, a private clinical stage biotechnology company, of 1.0 million shares, which represented
a 7.5% equity interest in Intensity. The investment was recorded at fair value (which approximates cost) at the acquisition date. The
investment in Intensity has been irrevocably designated as a financial asset recorded at fair value with gains and losses recorded through
OCI. Upon Intensity’s IPO discussed below, effective June 30, 2023, the fair value of the asset is determined by quoted market price.
On July 11, 2019, the Company entered into an agreement with Fast Forward
Innovations Limited (“Fast Forward”) to purchase Intensity Holdings Limited (“IHL”), a wholly-owned subsidiary
of Fast Forward. The Company paid $1.3 million for IHL through the issuance of 129,806 ordinary shares of the Company. The sole asset
of IHL consists of 288,458 shares of Intensity. This transaction increased the Company’s ownership of Intensity to 1,288,458 shares.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 7. INVESTMENT IN PUBLIC COMPANY (Cont’d)
On October 28, 2021, Intensity filed a Form S-1 Registration Statement
with the SEC to register shares for an initial public offering (“IPO”), which was declared effective by the SEC, but subsequently
withdrawn prior to closing.
In October and November of 2022, Intensity filed amendments to its Form
S-1 Registration Statement, which reflected a proposed offering price in the range of $4.00 - $5.00 per share, which is less than the
Company’s carrying value, which was an external indication of impairment. Accordingly, the Company performed an IAS 36, “Impairment
of Assets,” fair value analysis and determined a fair value of $3.363 million, which was $4.046 million less than the then carrying
value at December 31, 2022. Intensity continued to seek a successful IPO during the fourth quarter of fiscal year ended March 31, 2023.
At March 31, 2023, the Company undertook an IAS 36 fair value analysis based on the continued existence of external indications of impairment.
The analysis included evaluating the likelihood of a successful IPO and the timing of such an event, as well as the then lack of marketability
of the shares and the continued uncertainty surrounding an IPO, or any type of financing.
In April 2023, Intensity completed a 1:2 reverse stock split, which
reduced the Company’s holdings to 644,229
shares. As the offering was priced at $4.00
to $5.00
per share, the Company determined the fair value of its interest to be $2.087
million. In total, the Company recorded an unrealized loss of $5.322
million with respect to Intensity for the fiscal year ended March 31, 2023, which was recognized through OCI, reducing the
Company’s carrying value in Intensity to $2.087 million as of March 31, 2023.
On July 5, 2023, Intensity completed an IPO of its common stock
selling 3,900,000
shares at a price of $5.00
per share generating net proceeds of approximately $16.2
million. In connection with the offering, Intensity’s common stock began trading on Nasdaq on June 30, 2023, under the ticker
symbol “INTS.” The Intensity shares closed at a price of $5.96
on June 30, 2023. The Company received an additional 2,659
shares in connection with the offering pursuant to certain anti-dilution rights. Intensity sold its overallotment shares totaling 585,000
shares, which closed on July 7, 2023. At that date, the Company owned approximately 4.7%
of the issued and outstanding shares of Intensity. The Company recorded unrealized gain of $1.768
million through OCI to reflect the difference between the market value of the Company’s ownership interest and its then
carrying value, increasing the Company’s carrying value in Intensity to $3.855
million as of June 30, 2023. There was no
unrealized gain or loss with respect to the Company’s investment in Intensity during the three months ended June 30, 2022.
NOTE 8. LEASE
The Company entered into a lease of office space, which commenced on May
1, 2023. The lease provides for an original term of two years with an option to renew the lease for an additional term of three years.
The Company has included the extension option in the lease analysis under IFRS 16, based upon management’s intentions. The Company
calculated the lease liability using its incremental borrowing rate of 13%. The Company provided a $0.013 million security deposit. The
lease liability is payable as follows (in thousands):
| Schedule of lease
liability | |
| | |
| Twelve Months Ended June 30, | |
Amount |
| 2024 | |
$ | 79 | |
| 2025 | |
| 81 | |
| 2026 | |
| 83 | |
| 2027 | |
| 84 | |
| 2028 | |
| 71 | |
| Total | |
| 398 | |
| Less: interest | |
| (102 | ) |
| Total lease liability | |
| 296 | |
| Lease liability - current | |
| 47 | |
| Lease liability - non-current | |
$ | 249 | |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 9. ACQUISITION OF TARUS
On July 1, 2022, the Company, its wholly-owned subsidiary, Portage Merger
Sub I, Inc., its wholly-owned subsidiary, Portage Merger Sub II, LLC and Tarus Therapeutics, Inc., a Delaware corporation advancing adenosine
receptor antagonists for the treatment of solid tumors, entered into an Agreement and Plan of Merger and Reorganization (the “Merger
Agreement”). Under the structure of the Merger Agreement, Tarus Therapeutics, Inc. was ultimately merged into Portage Merger Sub
II, LLC of the Company with the surviving entity renamed Tarus Therapeutics, LLC. The Tarus merger entitles the Company to the rights,
know-how and/or ownership related to the assets developed by Tarus (the “Adenosine Compounds”), including:
| 1. | All rights and obligations related to the License Agreement between Tarus and Impetis Biosciences Limited, dated October 29, 2019
(“Tarus License Agreement”), and the Call Option under the Tarus License Agreement, which was exercised on November 5, 2020; |
| 2. | All intellectual property and related documents owned or controlled by Tarus, including issued or pending patents, patent applications
and trade secrets. Additionally, any draft submissions and/or correspondence with patent authorities; |
| 3. | All documents and supplies related to Adenosine Compounds (as defined in the Tarus License Agreement) including inventory, reagents,
data, assays, reports, vendor agreements and other information related to the preclinical development; |
| 4. | All clinical supplies, manufacturing know-how, batch records, regulatory documents pertaining to the Adenosine
Compounds, certain reservations for manufacturing campaigns and any related agreements; |
| 5. | All regulatory documents and correspondence pertaining to the Adenosine Compounds; |
| 6. | All Contract Research Organization (“CRO”) agreements and protocol related documents for Adenosine
Compounds; |
| 7. | All current documents related to market research, forecasting, budgets and competitive intelligence; and |
| 8. | Rights to the use of Tarus Therapeutics’ name for regulatory purposes. |
As consideration for Tarus, the Company issued to former
Tarus shareholders an aggregate of 2,425,999 ordinary shares of Portage, calculated on the basis of $18 million divided by the 60-day
volume weighted average price per share of ordinary shares of Portage. Such ordinary shares have not been registered with the SEC and
were subject to lock-up agreements for terms ranging from six to twelve months, which expired on February 1, 2023 and July 1, 2023, respectively.
Additionally, the ordinary shares that were subject to a twelve month lock-up period, are also subject to a three month dribble-out period,
which commenced July 1, 2023. During the dribble out period, each holder may not sell more than 10% of the average trading volume of the
Company’s ordinary shares for the rolling three month period prior to the date on which the holder executes a trade of the Company’s
ordinary shares without its prior written consent (which the Company is permitted to withhold at its sole discretion). Additionally, milestone
payments of up to $32 million in cash or Portage ordinary shares (at the discretion of the Company) would be triggered upon achievement
of future development and sales milestones, as described below. As a result of the transaction:
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 9. ACQUISITION OF TARUS (Cont’d)
| · | The Company also assumed $2 million in short-term debt held by Tarus and deferred license milestones obligations
($1 million plus interest). The short-term debt was repaid by the Company in July 2022. |
| · | Upon enrolling the first patient in a Phase 2 clinical trial utilizing Tarus’s adenosine receptor antagonists,
the Company will pay an additional one-time milestone payment of $15 million to the former Tarus shareholders. Payment will be in the
form of cash or Portage ordinary shares (at the discretion of the Company). The remaining $17 million milestone is based on targeted commercial
sales. |
In connection with the acquisition of Tarus, the Company performed a fair
value analysis of the assets acquired and liabilities assumed. The Company based the analysis on its clinical plan and timing of development
events, and the probabilities of success determined primarily based upon empirical third party data and Company experience as well as
the relevant cost of capital. In its fair value analysis, the Company used the Multi-Period Excess Earnings Method for PORT-6 and PORT-7
and the Replacement Cost Method for PORT-8 and PORT-9, determined based upon the maturity of the assets and the availability of sufficient
data to measure fair value. The Company recorded the ordinary shares issued at $17.2 million, which represented the aggregate market value
of the ordinary shares issued on July 1, 2022. The Company followed the guidance of IAS 3 and IAS 32 and recorded a deferred purchase
price payable - Tarus of $8.538 million, which reflected the estimated acquisition date fair value of contractual milestone obligations
incurred. The principal assumptions for determining the fair value include the timing of development events, the probabilities of success
and the discount rate used. The Company recorded the obligation as a non-current liability, in accordance with the provisions IAS 32 with
respect to the classification of financial assets and financial liabilities.
The Company will determine the fair value of the shares issuable upon
achievement of future development and sales milestones at each balance sheet date. Any change to the fair value will be recorded in the
Company’s statements of operations and other comprehensive income (loss).
The following table summarizes the purchase price allocation to the fair
value of assets acquired and liabilities assumed for Tarus:
| Schedule of fair
value of assets acquired and liabilities assumed | |
| | |
| Assets: | |
(In thousands) |
| Identifiable intangible assets | |
$ | 28,200 | |
| Goodwill | |
| 538 | |
| Total assets | |
$ | 28,738 | |
| Consideration: | |
| | |
| Fair value of shares issued | |
$ | 17,200 | |
| Liabilities assumed | |
| 3,000 | |
| Deferred purchase consideration at fair value | |
| 8,538 | |
| Total liabilities | |
$ | 28,738 | |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 9. ACQUISITION OF TARUS (Cont’d)
Pro forma Information
Summary unaudited pro forma condensed
results of operations for the three months ended June 30, 2022, assuming the Tarus acquisition had occurred at the beginning of the earliest
period presented, are as follows:
| Schedule of Pro forma Information | |
| | |
| (In thousands) | |
Three Months Ended
June 30, 2022 |
| Loss from operations | |
$ | (3,788 | ) |
| Loss before provision for income taxes | |
$ | (3,893 | ) |
| Net loss | |
$ | (1,341 | ) |
| Total comprehensive loss for period | |
$ | (1,341 | ) |
| Loss per share | |
$ | (0.09 | ) |
These pro forma results are not
necessarily indicative of what would have occurred if the acquisition had been in effect for the period presented, and they may not be
indicative of results expected in the future.
NOTE 10. IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY
In-process research and development (“IPR&D”) consists
of the following projects (in thousands):
| Schedule of in-process research and development | |
| |
| | | |
| | |
| | |
| |
Value as of |
| Project # | |
Description | |
June 30, 2023 | |
March 31, 2023 |
| iOx: | |
| |
| | | |
| | |
| PORT 2 (IMM60) | |
Melanoma & Lung Cancers | |
$ | 36,181 | | |
$ | 36,181 | |
| PORT 3 (IMM65) | |
Ovarian/Prostate Cancers | |
| 21,709 | | |
| 21,709 | |
| | |
| |
| 57,890 | | |
| 57,890 | |
| | |
| |
| | | |
| | |
| Oncomer/Saugatuck | |
DNA Aptamers | |
| 178 | | |
| 178 | |
| | |
| |
| | | |
| | |
| Tarus: | |
| |
| | | |
| | |
| PORT 6 & PORT 7 | |
Adenosine Receptors | |
| 22,723 | | |
| 22,723 | |
| PORT 8 | |
Adenosine Receptors | |
| 420 | | |
| 420 | |
| PORT 9 | |
Adenosine Receptors | |
| 472 | | |
| 472 | |
| | |
| |
| 23,615 | | |
| 23,615 | |
| | |
| |
| | | |
| | |
| In-process research and development | |
| |
$ | 81,683 | | |
$ | 81,683 | |
| | |
| |
| | | |
| | |
| Deferred tax liability | |
| |
$ | 13,510 | | |
$ | 13,195 | |
At the end of each reporting period, the Company is required to assess
whether there is any indication that an asset may be impaired. Pursuant to IAS 36, the Company evaluated the then-current capital markets,
the increasing costs of capital, and the delays in the timing of asset development and concluded that provisions for impairment were required
during the year ended March 31, 2023 with respect to the iOx IPR&D and the Tarus IPR&D. The Company recognized an impairment of
$59.320 million with respect to the iOx assets, reducing the Company’s carrying value from $117.210 million to $57.890 million and
an impairment of $4.585 million with respect to the Tarus assets, reducing the Company’s carrying value from $28.2 million to $23.615
million. The deferred tax liability in the U.K. was reduced as a result of the IPR&D impairment loss recognized by iOx for financial
statement purposes.
Deferred tax liability represents iOx’s estimated tax on the difference
between book and tax basis of the IPR&D, which is taxable in the U.K, and the effect of usable net operating loss carryforwards.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 11. INCOME TAXES
The Company is a BVI business company. The BVI government does not, under
existing legislation, impose any income or corporate tax on corporations.
PDS is a U.S. corporation and is subject to U.S. federal, state and local
income taxes, as applicable.
iOx is subject to U.K. taxes.
The (expense) benefit from income taxes consists of the following for
the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
| Schedule of income taxes benefit | |
| | | |
| | |
| | |
For the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Current: | |
| | | |
| | |
| Federal | |
$ | (3 | ) | |
$ | - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| - | | |
| - | |
| Total current | |
| (3 | ) | |
| - | |
| | |
| | | |
| | |
| Deferred: | |
| | | |
| | |
| Federal | |
| - | | |
| - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| 148 | | |
| 2,552 | |
| Total deferred | |
| 148 | | |
| 2,552 | |
| Benefit from income taxes | |
$ | 145 | | |
$ | 2,552 | |
The following is a reconciliation of the U.S. taxes to the effective income
tax rates for the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
| Schedule of reconciliation income tax rates | |
| |
|
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (Loss) income on ordinary activities before tax | |
$ | (605 | ) | |
$ | 34 | |
| Statutory U.S. income tax rate | |
| 21.0 | % | |
| 21.0 | % |
| Income tax benefit (expense) at statutory income tax rate | |
| 127 | | |
| (7 | ) |
| Share-based compensation expense recognized for financial statement purposes | |
| (142 | ) | |
| - | |
| Other losses recognized | |
| - | | |
| 7 | |
| Utilization of losses not previously benefitted | |
| 12 | | |
| - | |
| Income tax (expense) | |
$ | (3 | ) | |
$ | - | |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 11. INCOME TAXES (Cont’d)
As of June 30, 2023, the Company had $0.6 million of federal net operating
losses, which carryforward indefinitely but are limited to 80% of taxable income when utilized and $0.4 million of items deducted for
financial statements but not tax, excluding share-based compensation. As of each of June 30, 2023 and March 31, 2023, the Company had
U.S. deferred tax assets of $0.2 million.
The following is a reconciliation of the U.K. taxes to the effective income
tax rates for the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
Research and development credit
receivables of $0.2 million were included in prepaid expenses and other receivables on the condensed consolidated interim statements of
financial position as of each of June 30, 2023 and March 31, 2023. The receivable was collected in July 2023.
The following is a reconciliation of financial statement income (loss)
to tax basis income (loss) (in thousands):
| Schedule of reconciliation of financial statement loss to tax basis loss | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended |
| | |
June 30, 2023 | |
June 30, 2022 |
| | |
United
States | |
BVI | |
United
Kingdom | |
Total | |
United
States | |
BVI | |
Foreign | |
Total |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Pre-tax loss | |
$ | (609 | ) | |
$ | (3,886 | ) | |
$ | (1,576 | ) | |
$ | (6,071 | ) | |
$ | 34 | | |
$ | (2,468 | ) | |
$ | (1,743 | ) | |
$ | (4,177 | ) |
| Share-based compensation expense for financial statement purposes for which no benefit was taken | |
| 683 | | |
| - | | |
| - | | |
| 683 | | |
| - | | |
| - | | |
| - | | |
| - | |
| Losses not subject to tax | |
| - | | |
| 3,886 | | |
| - | | |
| 3,886 | | |
| - | | |
| 2,468 | | |
| - | | |
| 2,468 | |
| Utilization of losses not previously benefitted | |
| (59 | ) | |
| - | | |
| - | | |
| (59 | ) | |
| (34 | ) | |
| - | | |
| - | | |
| (34 | ) |
| Taxable income (loss) | |
$ | 15 | | |
$ | - | | |
$ | (1,576 | ) | |
$ | (1,561 | ) | |
$ | - | | |
$ | - | | |
$ | (1,743 | ) | |
$ | (1,743 | ) |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 11. INCOME TAXES (Cont’d)
As of June 30, 2023 and March 31,
2023, the Company’s deferred tax assets and liabilities in the U.K. consisted of the effects of temporary differences attributable
to the following (U.S. Dollars in thousands):
| Schedule of deferred tax assets and liabilities | |
| | | |
| | |
| | |
As of June 30, | |
As of March 31, |
| | |
2023 | |
2023 |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss | |
$ | (4,594 | ) | |
$ | (4,131 | ) |
| Deferred tax asset (unrecognized) | |
| 1,500 | | |
| 1,500 | |
| Deferred tax asset | |
| (3,094 | ) | |
| (2,631 | ) |
| | |
| | | |
| | |
| Deferred tax liabilities: | |
| | | |
| | |
| In-process research and development | |
| 13,510 | | |
| 13,195 | |
| Deferred tax liability | |
| 13,510 | | |
| 13,195 | |
| | |
| | | |
| | |
| Net deferred tax liability | |
$ | 10,416 | | |
$ | 10,564 | |
iOx generated no research and development cash credits recorded for the
three months ended June 30, 2023.
As of June 30, 2023 and March 31, 2023, iOx had a net deferred tax liability
of approximately $10.4 million and approximately $10.6 million, respectively. On January 8, 2019, the Company originally recognized a
$19.8 million deferred tax liability, reflecting the then prevailing U.K. tax rate of 17% on the difference between the book and income
tax basis of IPR&D acquired as part of the SalvaRx Acquisition. In the fiscal 2022, the Company recorded a $7.0 million increase in
deferred income taxes to reflect a future change in the U.K. income tax rate to 25% effective April 1, 2023 and recognized $0.7 million
of current year losses and $0.8 million of prior year losses. The Company also recognized a $1.1 million decrease in deferred tax liability
in fiscal 2022 to reflect the effect of the change in exchange rates on the liability settleable in British pound sterling. For the year
ended March 31, 2023, the Company recognized an aggregate reduction in net deferred tax liability of $17.9 million, comprised of $11.3
million to recognized the deferred tax effect of loss on impairment recognized with respect to the iOx IPR&D, $0.7 million related
to other current year losses, $3.8 million to reflect the change related to the future U.K. tax rates and $2.1 million to reflect the
effect of the change in exchange rates on the liability settleable in British pound sterling. For the three months ended June 30, 2023,
the Company recognized a net decrease in the deferred tax liability of $0.1 million comprised of $0.3 million to reflect the effect of
the change in exchange rates on the liability in the period and recognized $0.4 million of current period losses.
There is no expiration date for accumulated tax losses in the U.K. entities.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 12. CAPITAL STOCK
| (a) | Authorized ordinary shares: Unlimited number of Portage ordinary shares without par value. |
| (b) | The following is a roll-forward of Portage ordinary shares for the years ended June 30, 2023 and 2022: |
| Schedule of unlimited number
of common shares without par value | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| | |
Ordinary
Shares | |
Amount | |
Ordinary
Shares | |
Amount |
| | |
In 000’ | |
In 000’$ | |
In 000’ | |
In 000’$ |
| Balance, beginning of period | |
| 17,606 | | |
$ | 218,782 | | |
| 13,349 | | |
$ | 158,324 | |
| Shares issued under public offering and ATM, net of issue costs | |
| 171 | | |
| 613 | | |
| - | | |
| - | |
| Shares issued or accrued for services | |
| 9 | | |
| 30 | | |
| 4 | | |
| 30 | |
| Balance, end of period | |
| 17,786 | | |
$ | 219,425 | | |
| 13,353 | | |
$ | 158,354 | |
Portage filed a shelf registration statement with the SEC under which
it may sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on
March 8, 2021 (“Registration Statement”). In connection with the Registration Statement, Portage has filed with the SEC:
| · | a base prospectus, which covers the offering, issuance and sale by Portage of up to $200,000,000 in the aggregate of the securities
identified above from time to time in one or more offerings; |
| · | a prospectus supplement, which covers the offer, issuance and sale by Portage in an “at-the-market” (“ATM”)
offering of up to a maximum aggregate offering price of $50,000,000 of Portage’s ordinary shares that may be issued and sold from
time to time under a Controlled Equity Offering Sales Agreement, dated February 24, 2021 (the “Sales Agreement”), with Cantor
Fitzgerald & Co., the sales agent (“Cantor Fitzgerald”); |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by Portage of 1,150,000 ordinary shares for gross proceeds
of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; and |
| · | a prospectus supplement dated August 19, 2022, for the resale of up to $30,000,000 in ordinary shares that Portage may sell from time
to time to Lincoln Park Capital Fund, LLC (“Lincoln”) and an additional 94,508 shares that were issued to Lincoln. |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 12. CAPITAL STOCK (Cont’d)
The Sales Agreement permits the Company to sell in an ATM program up to
$50,000,000 of ordinary shares from time to time, the amount of which is included in the $200,000,000 of securities that may be offered,
issued and sold by the Company under the base prospectus. The sales under the prospectus will be deemed to be made pursuant to an ATM
program as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended. Upon termination of the Sales Agreement,
any portion of the $50,000,000 included in the Sales Agreement prospectus that is not sold pursuant to the Sales Agreement will be available
for sale in other offerings pursuant to the base prospectus.
During Fiscal 2022, the Company sold 90,888 ordinary shares under the
ATM program, generating gross proceeds of approximately $2.6 million ($2.5 million, net of commissions).
The Company has issued 2,425,999 ordinary shares in connection with the
acquisition of Tarus Therapeutics, Inc. and in connection with the Tarus Therapeutics, Inc.’s acquisition it may issue additional
ordinary shares. See Note 9, “Acquisition of Tarus,” for a further discussion.
On July 18, 2022, the Company entered into the iOx Share Exchange Agreement
under which it exchanged 1,070,000 ordinary shares of the Company for the remaining minority interest of 21.68% of iOx. See
Note 16, “Related Party Transactions – Share Exchange Agreement – iOx,” for a further discussion.
On July 6, 2022, the Company entered into a Purchase Agreement (the “Committed
Purchase Agreement”) with Lincoln, under which it may require Lincoln to purchase ordinary shares of the Company having an aggregate
value of up to $30 million (the “Purchase Shares”) over a period of 36 months. Upon execution of the Committed Purchase Agreement,
the Company issued to Lincoln 94,508 ordinary shares, representing a 3% commitment fee. Pursuant to the Committed Purchase Agreement,
Lincoln will be obligated to purchase the Purchase Shares in three different scenarios that are based on various market criteria and share
amounts. The Company has the right to terminate the Committed Purchase Agreement for any reason, effective upon one business day prior
written notice to Lincoln. Lincoln has no right to terminate the Committed Purchase Agreement. The requirement that Lincoln must make
a purchase will be suspended based on various criteria such as there not being an effective registration statement for Lincoln to be able
to resell the ordinary shares it is committed to purchase and market criteria such as the Company continuing to be Depository Trust Company
eligible, among other things. The Committed Purchase Agreement does not impose any financial or business covenants on the Company, and
there are no limitations on the use of proceeds. The Company may raise capital from other sources in its sole discretion; provided, however,
that the Company shall not enter into any similar agreement for the issuance of variable priced equity-like securities until the three-year
anniversary of the date of the Committed Purchase Agreement, excluding, however, an ATM transaction with a registered broker-dealer, which
includes any sales under the Sales Agreement with Cantor Fitzgerald.
As discussed in Note 2, “Going
Concern,” the Company’s access to the ATM program and the Committed Purchase Agreement is generally limited based on the Company’s
trading volume on Nasdaq. See Note 15, “Commitments and Contingent Liabilities – Committed Purchase Agreement,”
for a further discussion.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 12. CAPITAL STOCK (Cont’d)
In Fiscal 2023, the Company sold 166,145 ordinary shares under the ATM
program, generating net proceeds of approximately $0.9 million. Separately, in Fiscal 2023, the Company sold 480,000 ordinary shares to
Lincoln under the Committed Purchase Agreement for net proceeds totaling approximately $2.0 million. From April 1, 2023 through June 30,
2023, the Company sold 171,504 ordinary shares under the ATM program, generating net proceeds of approximately $0.6 million.
NOTE 13. STOCK OPTION RESERVE
| (a) | The following table provides the activity for the Company’s stock option reserve for the three months ended June 30, 2023 and
2022: |
| Schedule
of terms stock option reserve explanatory | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (In thousands) | |
Non-Controlling Interest | |
Stock Option Reserve | |
Non-Controlling Interest | |
Stock Option Reserve |
| Balance, beginning of period | |
$ | - | | |
$ | 21,204 | | |
$ | 11,659 | | |
$ | 16,928 | |
| Share-based compensation expense | |
| - | | |
| 769 | | |
| - | | |
| 1,176 | |
| Balance, end of period | |
$ | - | | |
$ | 21,973 | | |
$ | 11,659 | | |
$ | 18,104 | |
Stock Options
On June 25, 2020, at the annual meeting of shareholders, the Company’s
incentive stock option plan (the “2020 Stock Option Plan”) was approved, which authorized the Company’s directors to
fix the option exercise price and to issue stock options under the plan as appropriate. The Company’s 2020 Stock Option Plan was
a 10% rolling stock option plan under which the Company’s directors were authorized to grant up to a maximum of 10% of the issued
and outstanding ordinary shares on the date of grant.
Effective January 13, 2021, the
Company amended and restated its 2020 Stock Option Plan to permit the grant of additional types of equity compensation securities, including
restricted stock units (“RSUs”) and dividend equivalent rights (the “2021 Equity Incentive Plan”). The aggregate
number of equity securities, which may be issued under the 2021 Equity Incentive Plan has not been changed. Pursuant to the 2021
Equity Incentive Plan, on January 13, 2021, the Company granted an aggregate of 868,000 stock options exercisable at a price of $17.75
per share, representing the closing price of the shares on the day immediately preceding the grant date, which expire on January 13, 2031
to various directors, officers and consultants of the Company. 350,000 options granted to members of the Board vest 1/3 on grant date,
1/3 on the first anniversary of the grant and 1/3 on the second anniversary of the grant. 518,000 options granted to consultants (one
of whom is also a director of the Company) vest 1/3 on each of the first three anniversaries of the grant date.
Additionally, the Company granted
243,000 RSUs on January 13, 2021, with a fair value of $17.75 per share, which was the closing price on the day immediately preceding
the grant date. The RSUs vested on the date of grant, but underlying shares cannot be sold until one of four of the following conditions
are met: (1) a Change in Control (as defined in the Amended and Restated 2021 Equity Incentive Plan (defined below)), (2) the participant’s
Separation from Service (as defined in the Amended and Restated 2021 Equity Incentive Plan), (3) the participant’s death, or (4)
the participant’s Disability (as defined in the Amended and Restated 2021 Equity Incentive Plan). In accordance with IFRS
2, “Share-based Payment,” the Company recognized compensation expense of $4.3 million in the year ended March 31, 2021, in
connection with the RSU grants.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 13. STOCK OPTION RESERVE (Cont’d)
Amended and Restated 2021 Equity Incentive Plan and Grants of Stock Options and Restricted
Stock Units
On January 19, 2022, the Board unanimously approved the Amended and Restated
2021 Equity Incentive Plan (the “Amended and Restated 2021 Equity Incentive Plan”). The Amended and Restated 2021 Equity Incentive
Plan provides for:
| (1) | An increase of aggregate number of ordinary shares available for awards to 2,001,812, which is equal to 15% of the issued and outstanding
ordinary shares of the Company as of January 19, 2022 subject to discretionary annual increases (on a cumulative basis) as may be approved
by the Board in future years by a number of ordinary shares not to exceed an additional 5% of the aggregate number of shares then outstanding; |
| (2) | The authorization of incentive stock options under the Amended and Restated 2021 Equity Incentive Plan; and |
| (3) | The provision of dividend equivalent rights to be issued when authorized. |
Pursuant to the Amended and Restated 2021 Equity Incentive Plan, on January
19, 2022, the Company granted an aggregate of 302,000 stock options exercisable at a price of $10.22 per share, representing the average
price of the Company’s ordinary shares on the grant date, which expire on January 19, 2032, to various directors, officers and consultants
of the Company. A total of 13,800 of the 302,000 stock options were granted to two members of the Board and vest on the first anniversary
of the grant date. The balance of 288,200 stock options was granted to employees (one of whom is also a director of the Company), and
a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date.
Additionally, the Company granted
135,740 RSUs to employees (one of whom is also a director of the Company) on January 19, 2022, with a fair value of $10.22 per share,
representing the average price of the shares on the grant date. The RSUs were fully vested and nonforfeitable as of the grant
date and will expire on January 19, 2032.
On February 15, 2022, James Mellon, Linda Kozick and Mark Simon were appointed
to the Board. Mr. Mellon owned approximately 23.9% of the Company’s outstanding shares at that date. Additionally, Mr. Mellon had
previously served as a member of the Board from 2016 to August 14, 2020. On February 15, 2022, in connection with the appointments, each
of these directors was granted 13,800 non-qualified stock options, which vest ratably monthly over a three-year period. The options have
an exercise price of $8.59 per share, representing the average price of the shares on the grant date, and will expire, if unexercised,
on February 15, 2032.
On June 8, 2022, the Company granted 50,000 options to purchase shares
to an executive of the Company. The options have an exercise price of $11.00, the average price of the stock on that date, vest ratably
on each of the first four anniversaries of the grant date and will expire, if unexercised, on June 8, 2032.
On July 27, 2022, the Company granted 15,900 options to purchase shares
to a member of the Board. The options have an exercise price of $10.06, the average price of the stock on that date, vest ratably on each
monthly anniversary of the grant date in the three year period following the grant date and will expire, if unexercised, on July 27, 2032.
On March 30, 2023, the Board unanimously approved to increase the maximum
number of ordinary shares reserved for issuance under the Amended and Restated 2021 Equity Incentive Plan. The aggregate number of shares
available for awards under the Amended and Restated 2021 Equity Incentive Plan was increased to 2,880,992, which represented a 5% increase
(or 879,180 shares) based on ordinary shares outstanding on March 29, 2023, which is equal to 16% of the issued and outstanding ordinary
shares in the capital of the Company as of this date.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 13. STOCK OPTION RESERVE (Cont’d)
On March 30, 2023, the Company granted an aggregate of 746,120 stock options
exercisable at a price of $2.92 per share, representing the average price of the shares on the grant date, which expire on March 30, 2033,
to various directors, officers and a consultant of the Company. 14,600 options to purchase ordinary shares, totaling 87,600, were granted
to each non-executive member of the Board and vest on the first anniversary of the grant date. A total of 651,020 stock options were granted
to employees (including Mr. Walters, who is Chairman of the Board of Directors), and a consultant, and such stock options vest ratably
on each of the first four annual anniversaries of the grant date. The balance of 7,500 stock options were also granted to a consultant,
which was fully vested as of the grant date.
| (b) | The changes in the number of options issued for the three months ended June 30, 2023 and 2022 were: |
| Schedule of outstanding stock options | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Balance, beginning of period | |
| 1,963,420 | | |
| 1,151,400 | | |
| - | | |
| 1,275 | |
| Granted | |
| - | | |
| 50,000 | | |
| - | | |
| - | |
| Expired or forfeited | |
| - | | |
| - | | |
| - | | |
| (1,275 | ) |
| Balance, end of year | |
| 1,963,420 | | |
| 1,201,400 | | |
| - | | |
| - | |
| Exercisable, end of period | |
| 764,438 | | |
| 410,600 | | |
| - | | |
| - | |
| (c) | The following is the weighted average exercise price and the remaining contractual life for outstanding options by plan as of June
30, 2023 and 2022: |
| Schedule of weighted average exercise price and remaining contractual life | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Weighted average exercise price | |
$ | 10.53 | | |
| 15.26 | | |
$ | - | | |
$ | - | |
| Weighted average remaining contractual life (in years) | |
| 8.61 | | |
| 8.90 | | |
| - | | |
| - | |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 13. STOCK OPTION RESERVE (Cont’d)
The vested options can be exercised at any time in accordance with the
applicable option agreement. The exercise price was greater than the market price for all options outstanding as of June 30, 2023 and
March 31, 2023, except 7,500 vested options and 738,620 unvested options as of each date.
The Company recorded approximately $0.8 million and $1.2 million of share-based
compensation expense with respect to the Amended and Restated 2021 Equity Incentive Plan in the three months ended June 30, 2023 and 2022,
respectively. The Company expects to record additional share-based compensation expense of approximately $3.4 million through March 2027
with respect to the Amended and Restated 2021 Equity Incentive Plan.
As of June 30, 2022, the Company’s iOx stock option plan was fully
vested. The iOx stock option plan expired on May 5, 2022 and all outstanding stock option awards issued under the iOx stock option plan
expired.
NOTE 14. (LOSS) PER SHARE
Basic earnings per share (“EPS”) is calculated by dividing
the net income (loss) attributable to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding
during the period.
Diluted EPS is calculated by dividing the net income (loss) attributable
to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding during the period plus the weighted
average number of ordinary shares that would be issued on conversion of all the dilutive potential ordinary shares into ordinary shares.
The following table reflects the loss and share data used in the basic
and diluted EPS calculations (U.S. Dollars in thousands, except per share amounts):
| Schedule of basic and diluted EPS calculations | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| Numerator (in 000’$) | |
2023 | |
2022 |
| | |
| |
|
| Net loss attributable to owners of the Company | |
$ | (5,919 | ) | |
$ | (1,729 | ) |
| Denominator (in 000’) | |
| | | |
| | |
| Weighted average number of shares – Basic and Diluted | |
| 17,701 | | |
| 13,351 | |
| Basic and diluted (loss) per share | |
$ | (0.33 | ) | |
$ | (0.13 | ) |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 14. (LOSS) PER SHARE (Cont’d)
The inclusion of the Company’s stock options, RSUs and share purchase
warrants in the computation of diluted loss per share would have an anti-dilutive effect on loss per share and are therefore excluded
from the computation. Consequently, there is no difference between basic loss per share and diluted loss per share for the three months
ended June 30, 2023 and 2022. The following table reflects the Company's outstanding securities by year that would have an anti-dilutive
effect on loss per share and, accordingly, were excluded from the calculation.
| Schedule of anti-dilutive share | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 |
| Stock options | |
| 1,963,420 | | |
| 1,201,400 | |
| Restricted stock units | |
| 378,740 | | |
| 378,740 | |
NOTE 15. COMMITMENTS AND CONTINGENT LIABILITIES
Effective March 15, 2022, iOx entered into a Master Services Agreement
(the “MSA”) with Parexel International (IRE) Limited (“Parexel”) under which Parexel agreed to act as clinical
service provider (CRO) pursuant to a work order (“Work Order”) effective June 1, 2022. Pursuant to such Work Order, Parexel
will operate a Phase 2 trial of IMM60 and pembrolizumab in advanced melanoma and non-small lung cancer (“NSCLC”). The MSA
provides for a five-year term, and the Work Order provides for a term to be ended upon the completion of the services required. The budget
provides for service fees and pass-through expenses and clinical sites totaling $11.5 million. During Fiscal 2023, the Company executed
two change orders resulting in a $0.6 million increase in the overall estimated budgeted costs.
On March 1, 2023, the Company, through Tarus, entered into a clinical
service agreement with Fortrea Inc. (formerly Labcorp Drug Development Inc.), a third-party CRO. The term of the agreement is through
the earlier of August 14, 2025 or the completion of provision of services and the payment of contractual obligations. The budgeted costs
for the services to be provided is approximately $12.1 million.
Stimunity Convertible Note
On July 13, 2022, the Company entered into a commitment with Stimunity
to provide €600,000 under the Stimunity Convertible Note. The Stimunity Convertible Note provides for simple interest at 7% per annum.
The Stimunity Convertible Note is automatically converted into Series A shares of Stimunity upon Stimunity completing a Series A round
for at least €20 million. If such subscription round is completed prior to the Maturity Date, the Company will be entitled to convert
the Stimunity Convertible Note into Series A shares of Stimunity at the subscription share price less 15%. Additionally, if Stimunity
completes a financing with a new category of shares (other than Common Shares or Series A shares of Stimunity) for at least €5 million
(the “Minimum Raise”), the Company will have the right to convert the Stimunity Convertible Note and the historical Series
A shares of Stimunity owned into the new category of shares. In the event that Stimunity does not close a financing prior to the Maturity
Date or raises less than the Minimum Raise, the Company will have the right to convert the Stimunity Convertible Note into Series A shares
of Stimunity at €363.00 per share or the raise price less 15%, whichever is lower. The Stimunity Convertible Note was funded by the
Company on September 12, 2022. In addition, the Company has not reflected the interest earned on the Stimunity Convertible Note in reporting
its consolidated financial results. See Note 6, “Investment in Associate,” for a further discussion.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 15. COMMITMENTS AND CONTINGENT LIABILITIES (Cont’d)
Committed Purchase Agreement
On July 6, 2022 (the “Signing Date”), the Company entered
into the Committed Purchase Agreement with Lincoln, pursuant to which the Company may require Lincoln to purchase ordinary shares having
an aggregate value of up to $30 million over a period of 36 months. Pursuant to the Committed Purchase Agreement, Lincoln will be obligated
to purchase the Company’s ordinary shares in three different scenarios that are based on various market criteria and share amounts.
Upon execution of the Committed Purchase Agreement, the Company issued
to Lincoln 94,508 ordinary shares, representing a 3% commitment fee valued at $0.9 million. The Company has the right to terminate the
Committed Purchase Agreement for any reason, effective upon one business day prior written notice to Lincoln. Lincoln has no right to
terminate the Committed Purchase Agreement. The Company is accounting for the commitment fee as a deferred commitment fee on the consolidated
statement of financial position as of June 30, 2023 and March 31, 2023 and will amortize it pro-rata against equity sold under the Committed
Purchase Agreement. Any unamortized balance will be written-off to operations at the expiration of the commitment.
The Committed Purchase Agreement does not impose any financial or business
covenants on the Company and there are no limitations on the use of proceeds received by the Company from Lincoln. The Company may raise
capital from other sources in its sole discretion; provided, however, that the Company shall not enter into any similar agreement for
the issuance of variable priced equity-like securities until the three-year anniversary of the Signing Date, excluding, however, an at-the-market
transaction with a registered broker-dealer.
In connection with the Committed Purchase Agreement, the Company and Lincoln
entered into a Registration Rights Agreement, dated July 6, 2022 (the “Registration Rights Agreement”). Pursuant to the Registration
Rights Agreement, the Company agreed to file with the SEC the prospectus supplement to the Company’s shelf registration statement
pursuant to Rule 424(b) for the purpose of registering for resale the ordinary shares to be issued to Lincoln under the Committed Purchase
Agreement. The prospectus supplement was filed on August 19, 2022.
The Company is obligated under the Tarus Merger Agreement
and the iOx Share Exchange Agreement to pay certain third party earnouts based on the achievement of certain milestones. See Note 9, “Acquisition
of Tarus,” and Note 16, “Related Party Transactions – Share Exchange Agreement
– iOx,” respectively, for further discussions.
NOTE 16. RELATED PARTY TRANSACTIONS
SalvaRx Acquisition
Two of the Company’s directors are also directors of SalvaRx Group
plc, a company which owns approximately 4.1% of the Company’s issued and outstanding ordinary shares as of June 30, 2023.
Investments
The Company has entered into related party transactions and certain services
agreements with its investees. Key management personnel of the Company have also entered into related party transactions with investees.
Key management personnel are those persons having the authority and responsibility for planning, directing and controlling the activities
of the Company, including directors and senior management of the Company.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 16. RELATED PARTY TRANSACTIONS (Cont’d)
The following subsidiaries and associates are considered related parties:
| (a) | Stimunity. The CEO of Portage is one of three members of the board of directors of Stimunity (see Note 6, “Investment
in Associate,” and Note 15, “Commitments and Contingent Liabilities – Stimunity Convertible Note”). |
| (b) | iOx. Upon execution of the iOx Share Exchange on July 18, 2022, the non-Portage director resigned from the iOx board leaving
two Portage insiders as directors. The CEO of Portage is also the CEO of iOx, and the management team of Portage comprises the management
team of iOx. See below for a discussion of the Company’s purchase of the non-controlling interest in iOx through its wholly-owned
subsidiary SalvaRx. |
| (c) | Saugatuck. One of the three directorships on the board of directors of Saugatuck is controlled by Portage. Additionally, the
CEO of Portage is also the CEO of Saugatuck, and the management team of Portage comprises the management team of Saugatuck. |
| (d) | Intensity. The CEO of Portage previously served as a part-time officer of Intensity until becoming a consultant in 2023. Additionally,
Intensity provided services (primarily rent) to Portage through April 2023. |
| (e) | Portage Development Services Inc. PDS provides human resources and other services to each operating subsidiary of Portage through
a shared services agreement. |
The following are related party balances and transactions other than those
disclosed elsewhere in the condensed consolidated interim financial statements:
Transactions between the parent company and its subsidiaries, which are
related parties, have been eliminated in consolidation and are not disclosed in this note.
On September 8, 2021, the Company,
through SalvaRx, completed a settlement of loans (including interest) to and receivables from iOx for services rendered in exchange for
23,772 ordinary shares of iOx at a price of £162. Simultaneously, the Company entered into an agreement with OSI, the holder of
$0.15 million notes plus accrued interest under which OSI exchanged the notes plus accrued interest for 820 shares of iOx.
The Company followed the guidance provided by an IFRS Discussion Group Public Meeting dated November 29, 2016, following the general tenets
of IAS 39, “Financial Instruments: Recognition and Measurement,” and IFRIC 19, “Extinguishing Financial Liabilities
with Equity Instruments,” and recorded the exchange at historical cost. Additionally, no profit or loss was recorded in connection
with the exchange. As a result of these transactions, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%.
Share Exchange Agreement – iOx
On July 18, 2022, the Company and SalvaRx entered into a Share Exchange
Agreement (the “Share Exchange Agreement”) with each of the minority shareholders of iOx (the “Sellers”) resulting
in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the iNKT engager
platform. The Company followed IFRS 3, “Business Combinations,” and IAS 27, “Separate Financial Statements,” (which
substantially replaced IAS 3) to account for this transaction. The Company achieved control of iOx, as defined, on January 8, 2019 upon
the completion of the SalvaRx Acquisition. Further transactions whereby the parent entity acquires further equity interests from non-controlling
interests, or disposes of equity interests but without losing control, are accounted for as equity transactions (i.e., transactions with
owners in their capacity as owners). As such:
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 16. RELATED PARTY TRANSACTIONS (Cont’d)
| · | the carrying amounts of the controlling and non-controlling interests are adjusted to reflect the changes in their relative interests
in the subsidiary; |
| · | any difference between the amount by which the non-controlling interests is adjusted and the fair value of the consideration paid
or received is recognized directly in equity and attributed to the owners of the parent; and |
| · | there is no consequential adjustment to the carrying amount of goodwill, and no gain or loss is recognized in profit or loss. |
The Company now owns the worldwide rights to its small molecule iNKT engagers,
including lead programs PORT-2 and PORT-3. Under the terms of the Share Exchange Agreement, each Seller sold to the Company, and the Company
acquired from each Seller, legal and beneficial ownership of the number of iOx shares held by each Seller, free and clear of any share
encumbrances, in exchange for the issuance in an aggregate of 1,070,000 Portage ordinary shares to be allocated among the Sellers based
upon their relative ownership. As a result of the Share Exchange Agreement, the Company owns 100% of the issued and outstanding shares
of iOx through its wholly-owned subsidiary, SalvaRx.
As additional consideration for the sale of the iOx
shares to the Company under the Share Exchange Agreement, the Sellers shall have the contingent right to receive additional shares (“Earnout
Shares”) from the Company having an aggregate value equal to $25 million calculated at the Per Share Earnout Price, as defined in
the Share Exchange Agreement, upon the achievement of certain milestones defined as the dosing of the first patient in a Phase 3 clinical
trial for either PORT-2 (IMM60 iNKT cell activator/engager) or PORT-3 (PLGA-nanoparticle formulation of IMM60 combined with a NY-ESO-1
peptide vaccine). The Company shall have the option, in its sole and absolute discretion, to settle the Earnout Shares in cash. The Company
followed IFRS 3 and IAS 32, “Financial Instruments: Presentation,” to account for the fair value of the Earnout Shares. The
principal assumptions for determining the fair value include the timing of development events, the probabilities of success and the discount
rate used. The fundamental principle of IAS 32 is that a financial instrument should be classified as either a financial liability or
an equity instrument according to the substance of the contract, not its legal form, and the definitions of financial liability and equity
instrument. A financial instrument is an equity instrument if, and only if, both conditions (a) and (b) below are met:
| (a) | the instrument includes no contractual obligation to deliver cash or another financial asset to another entity,
and |
| (b) | if the instrument will or may be settled in the Company’s own equity instruments, it is either: |
| (i) | a non-derivative that includes no contractual obligation for the Company to deliver a variable number of
its own equity instruments; or |
| (ii) | a derivative that will be settled only by the issuer exchanging a fixed amount of cash or another financial
asset for a fixed number of its own equity instruments. |
When a derivative financial instrument gives one party a choice over how
it is settled (for instance, the Company or the holder can choose settlement net in cash or by exchanging shares for cash), it is a financial
asset or a financial liability unless all of the settlement alternatives would result in it being an equity instrument. The financial
instrument includes the exclusive right of the Company to settle the obligation with cash or equity and, accordingly, accounted for the
fair value of the Earnout Shares as a non-current liability.
The Company recorded $5.478 million as the fair value estimate of the
Earnout Shares, which is reflected as deferred obligation - iOx milestone on the condensed consolidated interim statements of financial
position included herein. The Company will determine the fair value of the Earnout Shares at each balance sheet date. Any change to the
fair value will be recorded in the Company’s statements of operations and other comprehensive income (loss). The Company recorded
a (loss) from the change (increase) in fair value of the liability of $0.685 million for the three months ended June 30, 2023.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 16. RELATED PARTY TRANSACTIONS (Cont’d)
Employment Agreements
PDS entered into a Services Agreement with the Company’s CEO effective
December 15, 2021 (the “CEO Services Agreement”). The CEO Services Agreement originally provided for a base salary of $618,000,
plus cost-of-living increases. On December 19, 2022, the Compensation Committee approved the CEO’s compensation of $642,700 for
Fiscal 2024. The CEO Services Agreement provides for annual increases based upon the review of the base salary by the Board prior to the
anniversary of the CEO Services Agreement provided that the annual increase cannot be less than the cost-of-living increase. The CEO Services
Agreement also provides that the CEO is eligible to receive an annual performance-based bonus targeted at 59% of the applicable year’s
base salary, which bonus is earned based on the achievement of performance targets, as determined annually by the Board and communicated
to the CEO in the first quarter of the year. Any annual bonus, to the extent earned, is to be paid no later than March 15 of the following
year. The CEO Services Agreement is for an initial term of three years, after which it will automatically renew annually unless terminated
in accordance with the CEO Services Agreement.
Under the CEO Services Agreement, the CEO may terminate his employment
with PDS at any time for Good Reason (as defined in the CEO Services Agreement). PDS may terminate the CEO’s employment immediately
upon his death, upon a period of disability or without Just Cause (as defined in the CEO Services Agreement). In the event that the CEO’s
employment is terminated due to his death or Disability (as defined in the CEO Services Agreement), for Good Reason or without Just Cause,
he will be entitled to accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses).
Additionally, he may be entitled to Severance Benefits (as defined in the CEO Services Agreement), which include his then current base
salary and the average of his annual bonus for the prior two completed performance years, paid over 12 monthly installments. Additionally,
the CEO will be entitled to life insurance benefits and medical and dental benefits for a period of 12 months at the same rate the CEO
and PDS shared such costs during his period of employment.
Additionally, all stock options (and any other unvested equity incentive
award) held by the CEO relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined
in the CEO Services Agreement), and the exercise period for such stock options will be increased by a period of two years from the Termination
Date.
If the CEO’s employment by PDS is terminated by PDS or any successor
entity without Just Cause (not including termination by virtue of the CEO’s death or Disability) or by the CEO for Good Reason within
12 months following the effective date of a Change in Control (as defined in the CEO Services Agreement), then, in addition to paying
or providing the CEO with the Accrued Obligations (as defined in the CEO Services Agreement), the Company will provide the following Change
in Control Severance Benefits (as defined in the CEO Services Agreement):
| (1) | PDS will pay the base salary continuation benefit for 18 months; |
| (2) | PDS will pay the life insurance benefit for 18 months; |
| (3) | PDS will pay an additional amount equivalent to the CEO’s target annual bonus calculated using the bonus percentage for the
performance year in which the CEO’s termination occurs. This bonus will be paid in 12 equal installments commencing on the first
payroll date that is more than 60 days following the date of termination of the CEO’s employment, with the remaining installments
occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the CEO with continued medical and dental benefits, as described above, for 18 months; and |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 16. RELATED PARTY TRANSACTIONS (Cont’d)
| (5) | All stock options (and any other unvested equity incentive award) held by the CEO relating to shares of PDS or the Company will be
deemed fully vested and exercisable on the Termination Date, as defined, and the exercise period for such stock options will be increased
by a period of two years from the Termination Date. |
PDS entered into services agreements (individually, an “Executive
Service Agreement,” and collectively, the “Executive Service Agreements”) with each of the Company’s five other
members of senior management (individually, “Executive” and collectively, “Executives”), three of which are dated
as of December 1, 2021, one of which is dated December 15, 2021 and one of which is dated June 1, 2022. Each of the Executive Services
Agreements provides for an initial term of two years that is automatically renewed for one-year periods (except two of the Executive Services
Agreement, which provides for an initial term of one year and that is automatically renewed for one-year periods). The Executive Services
Agreements initially provided for annual base salaries ranging from $175,000 to $348,000 (pro-rated for services rendered) and annual
bonus targets ranging from 30% to 40%. They also provide for long-term incentives in the form of equity awards from time to time under
the Portage Biotech Inc. Amended and Restated 2021 Equity Incentive Plan.
On December 19, 2022, the Compensation Committee approved executive compensation
for Fiscal 2024 for annual base salaries ranging from $183,750 to $469,000 (pro-rated for services rendered) and annual bonus targets
ranging from 30% to 40%.
The Executive Services Agreements can be terminated by PDS without Just
Cause, by death or Disability, or by the Executive (except one) for Good Reason (each as defined in the respective Executive Services
Agreements). In such instances, the Executive Services Agreements provide for the payment of accrued obligations (accrued unpaid portion
of base salary, accrued unused vacation time and any unpaid expenses). Additionally, the Executives (except two) are entitled to 50% of
base salary plus 50% of average annual bonus earned over the prior two performance years, as well as prevailing life insurance benefits
for a period of six months and medical and dental benefits for a period of six months at the prevailing rate PDS and the Executive were
sharing such expenses.
Additionally, all stock options (and any other unvested equity incentive
award) held by the Executives relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as
defined in the respective Executive Services Agreements), and the exercise period for such stock options will be increased by a period
of two years from the Termination Date.
If an Executive’s employment by PDS is terminated by the Company
or any successor entity without Just Cause (not including termination by virtue of the Executive’s death or Disability) or by the
Executive (except one) for Good Reason within 12 months following the effective date of a Change in Control (as defined in the respective
Executive Services Agreements), then, in addition to paying or providing the Executive with the Accrued Obligations (as defined in the
respective Executive Services Agreements), the Company will provide the following Change in Control Severance Benefits (as defined in
the respective Executive Services Agreements), except in two cases in which the Executive is entitled to Item (5) and 50% of Items (1)
and (3) below:
| (1) | PDS will pay the Base Salary continuation benefit for 12 months; |
| (2) | PDS will pay the life insurance benefit for 12 months; |
| (3) | The Company will pay an additional amount equivalent to the Executive’s target annual bonus calculated using the bonus percentage
for the performance year in which the Executive’s termination occurs. This bonus will be payable in 12 equal installments commencing
on the first payroll date that is more than 60 days following the date of termination of the Executive’s employment, with the remaining
installments occurring on the first day of the month for the 11 months thereafter; |
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 16. RELATED PARTY TRANSACTIONS (Cont’d)
| (4) | PDS will provide the Executive with continued medical and dental benefits, as described above, for 12 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the Executive relating to shares of PDS or the Company will
be deemed fully vested and exercisable on the Termination Date and the exercise period for such stock options will be increased by a period
of two years from the Termination Date. |
The Executive Services Agreements also include customary confidentiality,
as well as provisions relating to assignment of inventions. The Executive Services Agreements also includes non-competition and non-solicitation of
employees and customers provision that run during the Executive’s employment with PDS and for a period of one year after termination
of employment.
Bonuses & Board Compensation Arrangements
In December 2022, the Board approved
executive performance bonuses, as recommended by the Compensation Committee, totaling $0.6 million, which is equivalent to 73.5% of original
annual targets established by the Board in December 2021. The bonuses were approved based upon the original performance targets established.
The Board further approved a payment structure of 25% of approved bonuses, which were paid in January 2023, with the balance of amounts
due payable upon a new financing. The accrued, unpaid amount of approximately $0.4 million is included in accounts payable and accrued
expenses on the condensed consolidated statements of financial position as of each of June 30, 2023 and March 31, 2023.
Effective January 1, 2022, each non-employee Board member are entitled
to receive cash Board fees of $40,000 per annum, payable quarterly in arrears. Additionally, each non-employee Board member is entitled
to an annual grant of 6,900 options to purchase Portage ordinary shares, which would vest the first annual anniversary of the grant date.
The Company incurred Board fees totaling $82,500 and $75,000 during the three months ended June 30, 2023 and 2022, respectively.
Non-employee Board chairpersons are entitled to an annual cash fee of
$30,000, payable quarterly in arrears. In lieu of a non-executive chairperson, the lead director is entitled to an annual cash fee of
$20,000 per annum paid quarterly in arrears. Additionally, the chairperson of each of the Audit Committee, Compensation Committee and
Nominating Committee is entitled to annual fees of $15,000, $12,000 and $8,000, respectively, payable quarterly in arrears. Members of
those committees will be entitled to annual fees of $7,500, $6,000 and $4,000, respectively, payable quarterly in arrears.
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
The Company’s financial instruments recognized in the Company’s
condensed consolidated interim statements of financial position consist of the following:
Fair value estimates are made at a specific point in time, based on relevant
market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of
significant judgment; and therefore, these estimates cannot be determined with precision. Changes in assumptions could significantly affect
these estimates.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont’d)
The following table summarizes the Company’s financial instruments
as of June 30, 2023 and March 31, 2023:
| Schedule of financial instruments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTOCI | |
FVTPL | |
Amortized
Cost | |
FVTOCI | |
FVTPL |
| | |
| |
| |
| |
| |
| |
|
| Financial assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 7,698 | | |
$ | - | | |
$ | - | | |
$ | 10,545 | | |
$ | - | | |
$ | - | |
| Prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | - | | |
$ | - | | |
$ | 2,689 | | |
$ | - | | |
$ | - | |
| Convertible note receivable, including accrued interest, net of impairment | |
$ | - | | |
$ | - | | |
$ | 442 | | |
$ | - | | |
$ | - | | |
$ | 442 | |
| Investment in associate | |
$ | - | | |
$ | - | | |
$ | 756 | | |
$ | - | | |
$ | - | | |
$ | 806 | |
| Investment in public company | |
$ | - | | |
$ | 3,855 | | |
$ | - | | |
$ | - | | |
$ | 2,087 | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTPL | |
Amortized
Cost | |
FVTPL |
| Financial liabilities | |
| | | |
| | | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
$ | 2,591 | | |
$ | - | | |
$ | 1,865 | | |
$ | - | |
| Lease liability - current | |
$ | 47 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Lease liability - non-current | |
$ | 249 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Deferred purchase price payable - Tarus | |
$ | - | | |
$ | 7,864 | | |
$ | - | | |
$ | 7,179 | |
| Deferred obligation - iOx milestone | |
$ | - | | |
$ | 4,552 | | |
$ | - | | |
$ | 4,126 | |
A summary of the Company’s risk exposures as it relates to financial
instruments are reflected below.
Fair value of Financial Instruments
The Company’s financial assets and liabilities are comprised of
cash and cash equivalents, receivables and investments in equities and private and public entities, accounts payable, lease liability,
deferred purchase price payable, deferred obligation and unsecured notes payable.
The Company classifies the fair value of these transactions according
to the following fair value hierarchy based on the amount of observable inputs used to value the instrument:
| · | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of
the reporting date. |
| · | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which
can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of
the reporting date. |
| · | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified
as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value
measurement requires judgment and may affect the placement within the fair value hierarchy.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont’d)
Management has assessed that the fair values of cash and cash equivalents,
other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
The following methods and assumptions were used to estimate their fair
values:
Investment in Associate: The fair value of the Stimunity investment
was determined based on an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current market conditions,
the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity (Level 3). The Company recorded
a provision of impairment of $0.607 million with respect to the investment in associate decreasing the carrying value of the investment
in associate to $0.806 million as of March 31, 2023. There was no provision for impairment recorded in the three months ended June 30,
2023. See Note 6, “Investment in Associate,” for a further discussion.
Convertible Note Receivable: The fair value of the Stimunity Convertible
Note receivable denominated in euros at initial recognition is the transaction price for the instrument adjusted for the effect of the
currency translation rate on the reporting date (Level 3) (see Note 15, “Commitments and Contingent Liabilities – Stimunity
Convertible Note”). The Stimunity Convertible Note was initially recorded at $0.614 million to record the translated value of the
Stimunity Convertible Note on September 12, 2022. The Company recognized an unrealized gain of $0.039 million through OCI in Fiscal 2023
to reflect the change in translation rate for the Stimunity Convertible Note settleable in euros, increasing the carrying value of the
Stimunity Convertible Note to $0.653 million. As of March 31, 2023, the Company determined that there were indications of impairment,
based upon the inability of Stimunity to obtain financing. The Company performed an IAS 36 fair value analysis evaluating the likelihood
of various scenarios given the then-current market conditions, the increasing cost of capital and development delays associated with Stimunity’s
lack of liquidity. The Company recorded an impairment of $0.211 million resulting from the impairment analysis decreasing the carrying
value of the Stimunity Convertible Note to $0.442 million as of March 31, 2023. The Company recorded a nominal unrealized gain in the
three months ended June 30, 2023 with respect to the Stimunity Convertible Note.
Investment in Public Company: Upon Intensity’s IPO effective
June 30, 2023, the fair value of the investment is determined by the quoted market price (Level 1).
Accrued Equity Issuable: The fair value is estimated based on the
average of the quoted market prices for the period in which the shares were earned (Level 1).
Lease Liability - Current: The lease liability - current represents
the present value of the lease payments due over the next twelve months discounted at the Company’s incremental borrowing rate (Level
2).
Lease Liability - Non-Current: The lease liability - non-current
represents the present value of the lease payments due under the lease less the current portion of such payments discounted at the Company’s
incremental borrowing rate (Level 2).
Deferred Purchase Price Payable - Tarus: The fair value is the
estimated value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 at acquisition
date, adjusted at each reporting date for any change in fair value (Level 3) (see Note 9, “Acquisition of Tarus”). The fair
value was determined using the Income Approach and was based upon the analysis on the Tarus clinical plan, the timing of development events
and the probabilities of success determined primarily based upon empirical third party data and Company experience, as well as the relevant
cost of capital. The Company recorded a (loss) from the change (increase) in fair value of the liability of $0.685 million for the three
months ended June 30, 2023.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont’d)
Deferred Obligation - iOx Milestone: The fair value is the estimated
value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 as of July 18, 2022, the
date of the Share Exchange Agreement, adjusted at each reporting date for any change in fair value (Level 3) (see Note 16, “Related
Party Transactions – Share Exchange Agreement – iOx”). The fair value was determined using the Income Approach and based
on factors including the clinical plan, the timing of development events and the probabilities of success determined primarily based upon
empirical third party data and Company experience, as well as the relevant cost of capital. The Company recorded a (loss) from the change
(increase) in fair value of the liability of $0.426 million for the three months ended June 30, 2023.
Fair Value Hierarchy
The investment in public company (Intensity)
was transferred from Level 3 to Level 1 of the fair value hierarchy for the three months ended June 30, 2023 as the result of Intensity’s
IPO. For the year ended March 31, 2023, the fair value of the investment was determined based on an IAS 36 impairment analysis after
determining there were external indications of impairment (Level 3). See Note 7, “Investment in Public Company.”
The Company’s financial instruments are exposed to certain financial
risks: Credit Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s
inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit
risk is limited to the carrying value as reflected in the Company’s condensed consolidated interim statements of financial position.
Cash and cash equivalents. Cash and cash equivalents comprise
cash on hand and amounts invested in underlying Treasury and money market funds that are readily convertible to a known amount of cash
with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As
of June 30, 2023 and March 31, 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the
date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of
loss is minimal.
Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty
in satisfying financial obligations as they become due.
The Company’s approach to managing liquidity is to ensure, as far
as possible, that it will have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions without
incurring unacceptable losses or risking harm to the Company’s reputation. The Company holds sufficient cash and cash equivalents
to satisfy current obligations under accounts payable and accruals.
The Company monitors its liquidity position regularly to assess whether
it has the funds necessary to meet its operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant
internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available
to the Company, or that actual drug development expenditures may exceed those planned. The current uncertainty in global markets could
have an impact on the Company’s future ability to access capital on terms that are acceptable to the Company. There can be no assurance
that required financing will be available to the Company. See Note 2, “Going Concern,” and Note 12, “Capital Stock,”
for a discussion of the Company’s share offering and Note 15, “Commitments and Contingent Liabilities – Committed Purchase
Agreement,” for a further discussion.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Cont’d)
Foreign Currency Risk
While the Company operates in various jurisdictions, substantially all
of the Company’s transactions are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British
pound sterling and the Stimunity Convertible Note receivable settleable in euros.
NOTE 18. CAPITAL DISCLOSURES
The Company considers the items included in shareholders’
equity as capital. The Company had accounts payable and accrued liabilities of approximately $2.6
million and lease liability - current of $0.047
million as of June 30, 2023 (accounts payable and accrued liabilities of approximately $1.9
million as of March 31, 2023) and current assets of approximately $10.9
million as of June 30, 2023 (approximately $13.7
million as of March 31, 2023). The Company’s objectives when managing capital are to safeguard the Company’s ability to
continue as a going concern in order to pursue new business opportunities and to maintain a flexible capital structure, which
optimizes the costs of capital at an acceptable risk.
The Company manages the capital structure and makes adjustments to it
in light of changes in economic conditions and the risk characteristics of the underlying assets.
As of June 30, 2023, shareholders’ equity attributable to the owners
of the company was approximately $73.3 million (approximately $76.0 million as of March 31, 2023).
The Company is not subject to any externally imposed capital requirements
and does not presently utilize any quantitative measures to monitor its capital. There have been no changes to the Company’s approach
to capital management during the three months ended June 30, 2023 and 2022.
Portage Biotech Inc.
Notes to Condensed Consolidated Interim Financial Statements
(U.S. Dollars)
(Unaudited – See Notice to Reader dated August 29, 2023)
NOTE 19. NON-CONTROLLING INTEREST
| Schedule of non-controlling interest | |
| | | |
| | | |
| | |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2023 | |
$ | - | | |
$ | (650 | ) | |
$ | (650 | ) |
| Net loss attributable to non-controlling interest | |
| - | | |
| (7 | ) | |
| (7 | ) |
| Non-controlling interest as of June 30, 2023 | |
$ | - | | |
$ | (657 | ) | |
$ | (657 | ) |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2022 | |
$ | 44,701 | | |
$ | (472 | ) | |
$ | 44,229 | |
| Net income (loss) attributable to non-controlling interest | |
| 175 | | |
| (71 | ) | |
| 104 | |
| Non-controlling interest as of June 30, 2022 | |
$ | 44,876 | | |
$ | (543 | ) | |
$ | 44,333 | |
On September 8, 2021, the Company, through SalvaRx, completed a settlement
of loans (including interest) to and receivables from iOx for services rendered in exchange for 23,772 ordinary shares of iOx at a price
of £162. On July 18, 2022, the Company completed the acquisition of the remaining non-controlling interest in iOx, by issuing 1,070,000
shares of its ordinary shares and assuming certain milestone obligations. See Note 16, “Related Party Transactions – Share
Exchange Agreement – iOx” for a discussion of the Company’s purchase of the balance of the non-controlling interest
in iOx.
Saugatuck and subsidiary includes Saugatuck and its wholly-owned subsidiary,
Saugatuck Rx LLC.
F-37
Exhibit 99.2
PORTAGE BIOTECH INC.
THREE MONTHS ENDED JUNE 30, 2023
MANAGEMENT’S DISCUSSION AND ANALYSIS
Prepared as of August 29, 2023
TABLE OF CONTENTS
| |
Page No. |
| Forward-Looking Statements |
3 |
| Nature of Operations and Overview |
4 |
| Summary of Results |
8 |
| Number of Ordinary Shares |
8 |
| Business Environment – Risk Factors |
9 |
| Our Programs and Technology – Recent Developments |
9 |
| Results of Operations |
15 |
| Liquidity and Capital Resources |
17 |
| Key Contractual Obligations |
21 |
| Off-balance Sheet Arrangements |
24 |
| Transactions with Related Parties |
24 |
| Financial and Derivative Instruments |
24 |
| Use of Estimates and Judgments |
27 |
| New Accounting Standards, Interpretations and Amendments |
27 |
| Internal Control over Financial Reporting |
28 |
| Public Securities Filings |
28 |
Management Discussion and Analysis
The following discussion and analysis by management of the financial condition
and financial results for Portage Biotech Inc. for the three months ended June 30, 2023, should be read in conjunction with the unaudited
condensed consolidated interim financial statements for the three months ended June 30, 2023, together with the related Management’s
Discussion and Analysis and audited consolidated financial statements for the year ended March 31, 2023, and the annual report on Form
20-F (“Annual Report”) for the year ended March 31, 2023.
Forward-Looking Statements
This document includes “forward-looking statements.” All statements,
other than statements of historical facts, included herein or incorporated by reference herein, including without limitation, statements
regarding our business strategy, plans and objectives of management for future operations and those statements preceded by, followed by
or that otherwise include the words “believe,” “expects,” “anticipates,” “intends,” “estimates,”
“will,” “may,” “should,” “could,” “targets,” “projects,” “predicts,”
“plans,” “potential,” or “continue,” or similar expressions or variations on such expressions are
forward-looking statements. We can give no assurances that such forward-looking statements will prove to be correct.
Each forward-looking statement reflects our current view of future events
and is subject to risks, uncertainties and other factors that could cause actual results to differ materially from any results expressed
or implied by our forward-looking statements.
Risks and uncertainties include, but are not limited to:
| · | our need for financing and our estimates regarding our capital requirements and future revenues and profitability; |
| · | our plans and ability to develop and commercialize product candidates and the timing of these development programs; |
| · | clinical development of our product candidates, including the timing for availability and release of results of current and future
clinical trials; |
| · | our expectations regarding regulatory communications, submissions or approvals; |
| · | the potential functionality, capabilities, benefits and risks of our product candidates as compared to others; |
| · | our maintenance and establishment of intellectual property rights in our product candidates; |
| · | our estimates of the size of the potential markets for our product candidates; and |
| · | our selection and licensing of product candidates. |
Our business focus is that of being primarily a pharmaceutical development
business subject to all of the risks of a pharmaceutical development business. We do not anticipate directly engaging in the commercialization
of the product candidates we develop.
These statements are based on assumptions and analyses made by us in light
of our experience and our perception of historical trends, current conditions and expected future developments based on the focus of our
business activities on biotechnology, as well as other factors we believe are appropriate in particular circumstances. However, whether
actual results and developments will meet our expectations and predictions depends on a number of risks and uncertainties, which could
cause actual results to differ materially from our expectations, including the risks set forth in “Item 3 - Key Information - Risk
Factors” in our Annual Report on Form 20-F for the year ended March 31, 2023.
Consequently, all of the forward-looking statements made in this Management’s
Discussion and Analysis are qualified by these cautionary statements. We cannot assure you that the actual results or developments anticipated
by us will be realized or, even if substantially realized, that they will have the expected effect on us or our business or operations.
Unless the context indicates otherwise the terms “Portage Biotech
Inc.,” “the Company,” “our Company,” “Portage,” “we,” “us” or “our”
are used interchangeably in this Management’s Discussion and Analysis and mean Portage Biotech Inc. and its subsidiaries. Capitalized
terms used but not defined herein have the meaning ascribed to such terms in our unaudited condensed consolidated interim financial statements
for the three months ended June 30, 2023.
Nature of Operations and Overview
Portage is a clinical stage immune-oncology company advancing treatments
it believes will be first-in-class therapies that target known checkpoint resistance pathways to improve long-term treatment response
and quality of life in patients with invasive cancers. Our access to next-generation technologies coupled with a deep understanding of
biological mechanisms enables the identification of clinical therapies and product development strategies that accelerate these medicines
through the translational pipeline. We currently are working on 9 immuno-oncology assets, of which five are pre-clinical and four of which
are clinical stage. This excludes backup compounds. We source, nurture and develop the creation of early- to mid-stage treatments that
we believe will be first-in-class therapies for a variety of cancers, by funding, implementing viable, cost effective product development
strategies, clinical counsel/trial design, shared services, financial and project management to enable efficient, turnkey execution of
commercially informed development plans. Our drug development pipeline portfolio encompasses product candidates or technologies based
on biology addressing known resistance pathways/mechanisms of current checkpoint inhibitors with established scientific rationales, including
intratumoral delivery, nanoparticles, liposomes, aptamers, and virus-like particles.
The Portage Approach
Our mission is to advance and grow a portfolio
of innovative, early-stage oncology assets based on the latest scientific breakthroughs focused on overcoming immune resistance and expanding
the addressable patient population. Given these foundations, we manage capital allocation and risk as much as we oversee drug development.
By focusing our efforts on translational medicine and pipeline diversification, we seek to mitigate overall exposure to many of the inherent
risks of drug development.
Our approach is guided by the following core
elements:
| · | Portfolio diversification to mitigate risk and maximize optionality; |
| · | Capital allocation based on risk-adjusted potential, including staged funding to pre-specified scientific and clinical results; |
| · | Virtual infrastructure and key external relationships to maintain a lean operating base; |
| · | Internal development capabilities complemented by external business development; |
| · | Rigorous asset selection for broad targets with disciplined ongoing evaluation; |
| · | Focus on translational medicine and therapeutic candidates with single agent activity; |
| · | Conduct randomized trials early and test non-overlapping mechanisms of action; and |
| · | Improve potential outcomes for patients with invasive cancers. |
Our execution is achieved, in part,
through our internal core team and our large network of experts, contract labs, and academic partners.
Our Science Strategy
Our goal is to develop immuno-oncology therapeutics
that will dramatically improve the standard-of-care for patients with cancer. The key elements of our scientific strategy are to:
| · | Build a pipeline of differentiated oncology therapeutic candidates that are diversified by mechanism, broad targets, therapeutic approach,
modality, stage of development, leading to a variety of deal types that can be executed with partners; |
| · | Expand our pipeline through research collaborations, business development and internally designed programs; |
| · | Continue to advance and evolve our pipeline with a goal of advancing one therapeutic candidate into the clinic and one program into
Investigational New Drug (“IND”)-enabling studies each year; and |
| · | Evaluate strategic opportunities to accelerate development timelines and maximize the value of our portfolio. |
Our Pipeline
We have built a pipeline of immuno-oncology therapeutic
candidates and programs that are diversified by mechanism, therapeutic approach, modality and stage of development. On an ongoing basis,
we rigorously assess each of our programs using internally defined success criteria to justify continued investment and determine proper
capital allocation. When certain programs do not meet our de-risking criteria for advancement, we look to monetize or terminate those
programs and preserve our capital and resources to invest in programs with greater potential. As a result, our pipeline will continue
to be dynamic.
The charts below set forth, as of August 29, 2023, the current state
of our immuno-oncology therapeutic candidates and programs. The chart contains forward-looking information and projections based
on management’s current estimates. The chart information is based on and subject to many assumptions, as determined by management
and not verified by any independent third party, which may change or may not occur as modeled. We undertake no obligation to update or
revise any forward-looking statements, whether as a result of new information, future events or otherwise. Before you make an investment
decision regarding us, you should make your own analysis of forward-looking statements and our projections about candidate and program
development and results.
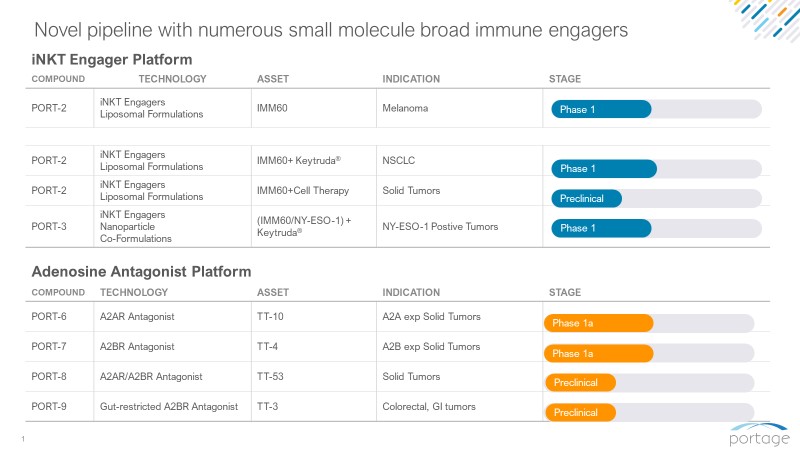

Our Business Model
We are a development organization that is structured
to facilitate flexibility in financing and ease of partnering, licensing, and merger/acquisition of individual assets and or technology
platforms. The intellectual property (“IP”) for each platform is held in separate private entities. Our employees
and consultants work across the pipeline of assets and we believe that this can (i) enhance operational efficiency, (ii) maintain an optimal
cost structure, (iii) attract leading collaborators, and (iv) promote asset flexibility, as further described below.
| · | Enhance operational efficiency: We allocate resources while empowering managers to make program-level decisions in order to
increase productivity and speed. We believe this model enables a flexible organizational structure that can achieve scale through the
addition of programs without increasing burdensome bureaucracy or redundant infrastructure. |
| · | Maintain an optimal cost structure: We have a relatively small number of employees and have partnered with a number of service
providers to leverage their infrastructure and expertise as needed instead of embarking on capital-intensive lab, manufacturing, and equipment
expenditures. By reducing overhead costs, we believe we can increase the likelihood that we can generate a return on invested capital. |
| · | Attract leading collaborators and licensors: Our pipeline is comprised of therapies we believe will be first-in-class therapies
for a variety of cancers sourced via our extensive industry contacts and relationships (including academia and pharmaceutical industry
executives). On preclinical programs/technology, we initially established development structures enabling us to keep licensors economically
incentivized at the program level. We believe that our experienced drug development leadership team and approach to resource allocation
differentiate us from other potential licensees. |
| · | Leverage the commoditized checkpoint marketplace and explore the potential to further enhance long-term clinical benefits for patients
with cancer and also expand the eligible population to include those who do not currently receive anti-PD-1 therapy: Presently there
are multiple approved checkpoint therapeutics that lack differentiation, resulting in a competitive market dynamic, which will favor combination
therapy. There is substantial opportunity for potential expansion in the PD-1 market with our iNKT engagers and adenosine antagonists.
Studies show that 70-80% of patients do not respond or have a limited response to existing monotherapies, such as PD-1 checkpoint inhibitors.
We see potential for our unique approach of using iNKT engagers and adenosine antagonists to initiate an immune response in tumors that
have become refractory to checkpoint therapy or to increase the number of front-line patients achieving more durable responses. Combinations
can improve this but often come at the cost of significant additional toxicity. The market is saturated with 14 approved PD-1 antibodies,
and every major pharmaceutical company competes in this space. One illustrative example of potentially expanding eligible patients is
with iNKT engagers upregulating expression of PD-L1. Patient populations that are typically not good candidates for PD-1 antibodies due
to their lack or low expression of PD-L1 may be able to utilize iNKTs to sensitize tumors to PD-1 agents. Extending the use of PD-1 antibodies
represents a significant potential upside for one of these companies competing for market share, should they choose to partner with Portage. |
| · | Promote asset flexibility: Our structure is designed to maximize flexibility and cost efficiency. This allows us to efficiently
pursue various subsidiary-level transactions, such as stock or asset sales, licensing transactions, strategic partnerships and/or co-development
arrangements. It also provides us with the flexibility to terminate programs with minimal costs if results do not meet our de-risking
criteria for advancement. |
We are a BVI business company incorporated under the BVI Act with our
registered office located at Clarence Thomas Building, P.O. Box 4649, Road Town, Tortola, British Virgin Islands, VG1110. Our U.S. agent,
Portage Development Services Inc. (“PDS”), is located at 61 Wilton Road, Westport, CT 06880.
We currently are a foreign private issuer under the United States Securities
and Exchange Commission (“SEC”) rules. We are also a reporting issuer under the securities legislation of the provinces of
Ontario and British Columbia. Our ordinary shares were listed on the Canadian Securities Exchange (“CSE”) under the symbol
“PBT.U”. On February 25, 2021, our ordinary shares began trading on the Nasdaq Capital Market under the symbol “PRTG”.
As the principal market for our ordinary shares is Nasdaq, we voluntarily delisted from the CSE on April 23, 2021.
Summary of Results
The following table summarizes financial information for the quarter ended
June 30, 2023, and the preceding eight quarters (all amounts in 000’US$ except net loss per share, which are actual amounts). All
share and per share amounts reflect the 1:100 reverse stock split effected June 5, 2020.
| | |
June 30, | |
Mar. 31, | |
Dec. 31, | |
Sept. 30 | |
June 30, | |
Mar. 31, | |
Dec. 31, | |
Sept. 30, | |
June 30, |
| Quarter ended | |
2023 | |
2023 | |
2022 | |
2022 | |
2022 | |
2022 | |
2021 | |
2021 | |
2021 |
| | |
|
| Net loss attributable to owners of the Company | |
| (5,919 | ) | |
| (94,448 | ) | |
| (7,485 | ) | |
| (949 | ) | |
| (1,729 | ) | |
| (7,317 | ) | |
| (3,512 | ) | |
| (2,975 | ) | |
| (3,066 | ) |
| Comprehensive loss attributable to the owners of the Company | |
| (4,150 | ) | |
| (95,714 | ) | |
| (11,502 | ) | |
| (949 | ) | |
| (1,729 | ) | |
| (7,317 | ) | |
| (3,512 | ) | |
| (2,975 | ) | |
| (3,066 | ) |
| Working capital (1) to (8) | |
| 8,254 | | |
| 11,811 | | |
| 13,110 | | |
| 15,737 | | |
| 21,138 | | |
| 24,049 | | |
| 25,639 | | |
| 27,301 | | |
| 28,106 | |
| Equity attributable to owners of the Company | |
| 73,307 | | |
| 76,045 | | |
| 168,945 | | |
| 178,434 | | |
| 120,682 | | |
| 121,205 | | |
| 125,789 | | |
| 127,140 | | |
| 127,711 | |
| Net loss per share - Basic | |
| (0.33 | ) | |
| (5.45 | ) | |
| (0.44 | ) | |
| (0.06 | ) | |
| (0.13 | ) | |
| (0.55 | ) | |
| (0.26 | ) | |
| (0.22 | ) | |
| (0.25 | ) |
| Net loss per share - Diluted | |
| (0.33 | ) | |
| (5.45 | ) | |
| (0.44 | ) | |
| (0.06 | ) | |
| (0.13 | ) | |
| (0.55 | ) | |
| (0.26 | ) | |
| (0.22 | ) | |
| (0.25 | ) |
| (1) | September 30, 2022 working capital is net of warrant liability of $8 settleable on a non-cash basis. |
| (2) | June 30, 2022 working capital is net of warrant liability of $32 settleable on a non-cash basis. |
| (3) | March 31, 2022 working capital is net of warrant liability of $33 settleable on a non-cash basis. |
| (4) | December 31, 2021 working capital is net of warrant liability of $159 settleable on a non-cash basis. |
| (5) | September 30, 2021 working capital is net of warrant liability of $535 settleable on a non-cash basis. |
| (6) | June 30, 2021 working capital is net of warrant liability of $751 settleable on a non-cash basis. |
| (7) | March 31, 2021 working capital is net of warrant liability of $1,120 settleable on a non-cash basis. |
| (8) | December 31, 2020 working capital is net of warrant liability of $771 settleable on a non-cash basis. |
Number of Ordinary Shares
The following table summarizes the number of our ordinary shares issued
and outstanding at June 30, 2023 and August 29, 2023:
| As of, | |
June 30, 2023 | |
August 29, 2023 |
| Shares issued and outstanding (a) (b) | |
| 17,786,290 | | |
| 17,801,390 | |
| (a) | This amount excludes an aggregate of 243,000 restricted stock units granted to our executive chairman and an employee on January 13,
2021, which vested immediately on the date of grant and are subject to certain restrictions and 135,740 restricted stock units granted
to employees (one of whom is also a director) on January 19, 2022, which vested immediately
on the date of grant and are subject to certain restrictions. |
| (b) | The June 30, 2023 amount includes 9,038 shares earned for services rendered from April 1, 2023 to June 30, 2023, accrued at June 30,
2023 for financial statement purposes and issued in July 2023. The August 29, 2023 amount excludes 3,019 shares earned for services rendered
in July 2023, accrued at July 31, 2023 but not yet issued. |
Business Environment – Risk Factors
Please refer to the Annual Report on Form 20-F for the year ended March
31, 2023 for detailed information as the economic and industry factors that are substantially unchanged as of the date hereof.
Our Programs and Technology – Recent Developments
Invariant Natural Killer T-cells (iNKT cells) Platform
iNKT cells play an important role in anti-tumor immune responses and are
a distinct class of T lymphocyte displaying a limited diversity of T-cell receptors. They recognize lipid antigens on the surface of tumor
cells and produce large amounts of cytokines within hours of stimulation without the need for clonal expansion. Furthermore, iNKT cells
activate multiple immune system components, including dendritic cells (“DC”), T-cells and B-cells and stimulate an antigen-specific
expansion of these cells. Our operating subsidiary, iOx Therapeutics Ltd. (“iOx”), holds an exclusive license (with the right
to sub-license) from the Ludwig Institute for Cancer Research (the "Ludwig Institute") to use, research, develop and commercialize
iNKT cell engagers, for the treatment of various forms of human disease, including cancer, under the Ludwig Institute’s intellectual
property and know-how.
PORT-2 (IMM60)
PORT-2 is an iNKT cell engager formulated in a liposome with a six-member
carbon head structure that has been shown to activate both human and murine iNKT cells, resulting in DC maturation and the priming of
Ag-specific T and B cells.
In animal models, PORT-2 enhanced the frequency of tumor specific immune
responses. iNKT cells are unique lymphocytes defined by their co-expression of surface markers associated with NK cells along with a T-cell
antigen receptor. They recognize amphipathic ligands such as glycolipids or phospholipids presented in the context of the non-polymorphic,
MHC class I-like molecule CD1d. Activated iNKT cells rapidly produce IFN-gamma and IL-4 and induce DC maturation and IL-12 production.
In August 2021, we dosed the first patient in the IMP-MEL PORT-2 clinical
trial, a Phase 1/2 dose escalation and randomized expansion trial. The PORT-2 trial is expected to enroll up to 88 patients with melanoma
or non-small cell lung carcinoma (“NSCLC”) in order to evaluate safety and efficacy. In November 2022, we announced that we
had entered into a clinical trial collaboration with Merck to evaluate PORT-2 in combination with pembrolizumab for patients with NSCLC.
Under the terms of the collaboration, Merck will supply pembrolizumab for our Phase 1/2 trial of PORT-2 in patients with NSCLC and melanoma.
Preliminary Phase 1 data, presented at the American Society of Clinical
Oncology (“ASCO”) Annual Meeting in June 2023, suggests PORT-2 was well tolerated when administered as a monotherapy, with
no related severe or serious adverse events. All possibly related adverse events were mild to moderate and did not limit dosing. This
enabled the opening of the combination safety cohort with pembrolizumab, in parallel with the ongoing high dose monotherapy cohort. As
of August 2023, two patients have received the combination with pembrolizumab, and no related severe or serious adverse events were reported.
The adverse event profile was consistent with pembrolizumab. Previously reported biomarker data confirmed the mechanism of action (i.e.,
both activation of the innate and adaptive arms of the immune system). The following figure illustrates the different lesion responses.
Although these are preliminary results, several lesions showed shrinkage, and the responses in liver metastases were encouraging.
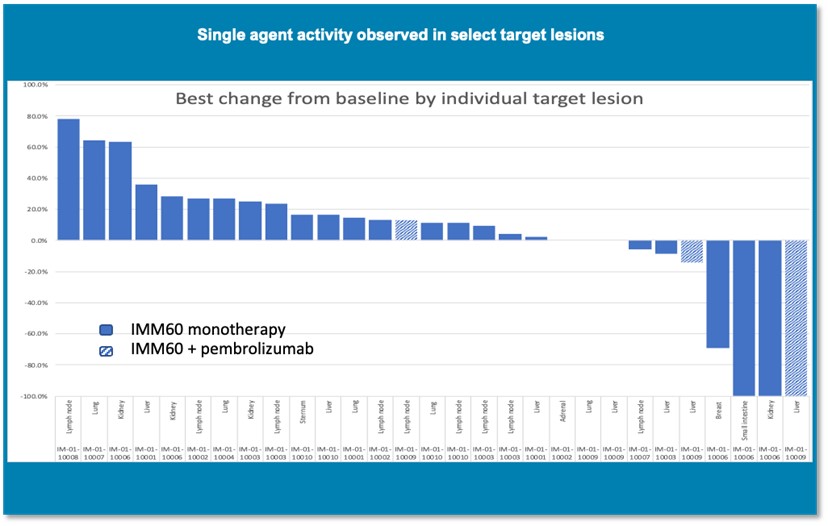
We are encouraged by the growing patient data set that supports proof
of concept for using an iNKT engager in cancer treatment. Preliminary Phase 1 data suggests that PORT-2 has a favorable safety and tolerability
profile as a monotherapy at all doses tested to date (as noted above), has demonstrated evidence of single agent activity, and biomarkers
confirm mechanistic potential of PORT-2 to activate both the adaptive and innate immune systems.
To accelerate development, we have transitioned sponsorship and have expanded
the PORT-2 trial beyond the U.K. while addressing COVID-19 headwinds. The Clinical Trial Agreement with CRO Parexel, our clinical research
organization in the U.K., has been transferred from the University of Oxford to us through our iOx subsidiary and the trial is being converted
to a program sponsored by iOx. Sites in the U.K. will continue to enroll, as we bring on sites in the U.S. and in Spain. Should there
be delays in recruiting patients, this could result in increasing overall costs of program administration and ultimately, slow down the
completion of the trial and achievement of results.
Given the safety data shown at the highest planned doses, the trial protocol
is being amended to escalate patient dosing to include one additional higher dose, and we anticipate the Phase 2 portion of the trial
to commence in the first half of calendar 2024.
PORT-3 (IMM65)
PORT-3 is a poly(lactide-co-glycolide) (“PLGA”)-nanoparticle
formulation of PORT-2 (IMM60) combined with a NY-ESO-1 peptide vaccine. Biodegradable PLGA-nanoparticles function as a delivery platform
for immunomodulators and tumor antigens to induce a specific anti-tumor immune response. PLGA has minimal (systemic) toxicity and is used
in various drug-carrying platforms as an encapsulating agent. Furthermore, co-formulating an iNKT engager with a peptide vaccine in a
particle has shown to be approximately five times more potent in killing cancer cells and generating an antigen-specific CD8 T-cell response
than giving the two agents individually.
NY-ESO-1 is a cancer-testis antigen expressed during embryogenesis and
in the testis, an immune privileged site. Furthermore, NY-ESO-1 expression is observed in several advanced cancers: Lung (2-32%), melanoma
(40%), bladder (32-35%), prostate (38%), ovarian (30%), esophageal (24-33%), and gastric cancers (8-12%). Clinical trials have shown the
safety and tolerability of Good Manufacturing Practices (“GMP”)-grade NY-ESO-1 peptides in patients with cancer.
The first patient was dosed in 2021 and patients continue to enroll in
the PRECIOUS Phase 1 trial of PORT-3 in patients with solid tumors. The Phase 1 portion of the trial is expected to enroll 15 patients.
The trial was having difficulty identifying tumors that expressed NY-ESO-1, so the trial protocol was amended to include all solid tumors
regardless of expression to facilitate assessment of safety. This platform is designed to demonstrate proof of concept. The combination
of NY-ESO-1 and IMM-60 is being evaluated to determine its ability to prime and boost an anti-tumor immune response. Our patent position
extends to other known tumor antigens, and we are prepared to rapidly launch other assets into the clinic if we see strong activity of
this formulation. Preliminary safety data for repeat dosing of PORT-3 in the PRECIOUS Phase 1 trial shows a favorable safety profile.
The investigator trial has paused while we await more data. It is our understanding that the investigators with who we work with have
continued to explore next generation targeted nanoparticles.
Adenosine Receptor Antagonist Platform
A critical mechanism of cancer immune evasion is the generation of high
levels of immunosuppressive adenosine within the tumor microenvironment (“TME”). Research suggests that the TME has significantly
elevated concentrations of extracellular adenosine. Engagement with adenosine receptors A2A and A2B triggers a dampening effect on the
immune response, suppressing effector cell function and stabilizing immunosuppressive regulatory cells. Over-expression of the A2A and
A2B receptors leads to a poor prognosis in multiple cancers, including prostate cancer, colorectal cancer and lung adenocarcinoma, driven
by a reduced ability to generate an immune response against the tumor.
These findings have made A2A and A2B high-priority targets for immunotherapeutic
intervention. We are advancing four adenosine antagonists that we believe to be first-in-class, which together represent a broad suite
of adenosine-targeting approaches and are expected to enable a comprehensive exploration of how targeting the adenosine pathway could
potentially improve response in multiple cancer and non-cancer indications. By modulating the adenosine pathway in four different ways,
we expect to determine the optimal approach to maximize the impact of the mechanism of action on different tumors.
We have designed the ADPORT-601 clinical trial to evaluate the activity
and safety of PORT-6 and PORT-7 alone and in combination. This trial will adapt over time and also include safety cohorts for these two
agents with other immune activating agents including others from our internal pipeline. Depending on the data, it can be expanded to evaluate
either agent as monotherapy or a randomized comparison of either agent plus standard of care versus standard of care alone.
PORT-6 (TT-10)
PORT-6 is an adenosine receptor type 2A (“A2A”) antagonist
being studied for the treatment of A2A expressing solid tumors. We believe PORT-6 is more potent, more durable and more selective than
other clinical stage A2A agents.
The ADPORT-601 portion of the Phase 1 trial for PORT-6, dosed its first
patient in June 2023. We have activated 6 sites in the U.S. to complete the Phase 1 including:
MD Anderson Cancer Center, UC San Francisco, University of Southern California, Thomas Jefferson University, Virginia Cancer Specialists
and Sarah Canon Research Institute.
PORT-7 (TT-4)
PORT-7 is an adenosine receptor type 2B (“A2B”) antagonist
that we expect to study for the treatment of solid tumors. PORT-7 has a very selective profile that focuses on A2B. We expect, based upon
available liquidity, to commence a Phase 1 trial in late calendar 2023 or early calendar 2024.
PORT-8 (TT-53)
PORT-8 is a dual antagonist of adenosine receptors 2A and 2B (A2A/A2B)
that we expect to study for the treatment of solid tumors. We have the ability to combine these two adenosine receptors to titrate the
levels of A2A and A2B or has the ability to give the dual antagonist (PORT-08). The PORT-8 program is a pre-clinical stage program.
PORT-9 (TT-3)
PORT-9 is an A2B antagonist to treat colorectal and gastrointestinal cancers.
In connection with the adenosine programs, we will focus on solid tumor
types with high adenosine expression of receptors A2A and A2B and enrich for patients that have high adenosine expression and therefore
have potential to benefit most from treatment. The PORT-9 program is currently in the pre-clinical stage.
Other Programs and Investee Programs in Development
We are focused on delivering clinical data with the iNKT and adenosine
programs and prioritizing the allocation of financial resources to these programs. Developmental work continues on some of the other developmental
assets, through collaborations such as that with the U.S. National Cancer Institute (“NCI”) and other academic groups, as
further described below. These developmental assets may be re-evaluated at a future point depending on market conditions, ongoing data,
funding priorities and status.
Amphiphilic platform
DfuseRx SM, identifies combinations of anti cancer agents with amphiphilic
diffuse enhancers that can passively enter into cancer cells. These novel formulations with unique IP can be directly injected into
any solid tumors, and the payloads will diffuse across the membrane and disperse throughout the tumor, while sparing healthy cells. Once
inside the cells, the technology is diluted away and the payloads are stuck inside the cells. The payloads are able to disperse to areas
of the tumor that do not have blood supply and hence oral or IV drugs will not reach.
PORT-1 (INT230-6)
Intensity Therapeutics, Inc., (“Intensity”), which we have
an investment in, is developing INT230-6 (“PORT-1”) as a fixed dose formulation of cisplatin, vinblastine and a penetration
enhancer. In animal models, the drug has shown efficacy in the majority of the animals, by a combination of direct killing of the
cancer cells, and also a CD4 and CD8 T-cell response. Interim safety and survival data from the Phase 1/2 IT-01 trial exploring
the safety and efficacy of PORT-1 in patients with refractory or metastatic cancers presented at ASCO Annual Meeting in 2021 demonstrated
that both PORT-1 monotherapy and combination therapy with immune checkpoint drugs are well-tolerated. The mechanism of action includes
direct tumor-killing effects, as well as responses generated in non-injected tumors (abscopal responses) resulting from antigen presentation
and immune activation. The specific rapid local killing in the normal three-dimensional environment inside the body we believe is critical
for robust antigen presentation and immune activation. Animal studies also showed synergy when combined with checkpoint inhibition.
PORT-1 has shown proof of concept in humans in that the vast majority of the drug has been shown to stay in the tumor, and a dose equivalent
to three times the approved dose of the cytotoxic agent was well tolerated without the typical chemotherapy side effects. The most
common adverse event related to the treatment was pain at the injection site. As a result, PORT-1 is being studied in nine Phase
2 trials including seven clinical collaborations with the two largest immuno-oncology drug manufacturers, Bristol Myers Squibb (“BMS”)
and Merck, in combination with their respective checkpoints in high unmet need medical types (including pancreatic, gall bladder, sarcoma,
non-microsatellite unstable colorectal).
Intensity has also launched a randomized Phase 2 trial of PORT-1 for the
treatment in early stage breast cancer for patients who are ineligible or chose not to have presurgical chemotherapy (compared to no treatment,
which is the standard of care) (the “INVINCIBLE Trial”) and has expanded its collaboration efforts with the INVINCIBLE Trial,
conducted by the Ottawa Hospital and the Ontario Institute for Cancer Research. The INVINCIBLE Trial suggests that one treatment with
PORT-1, can result in near complete necrosis of breast tumors greater than 3 cm with an influx of key immune cells to process the dying
tumor. Intensity presented clinical data from the PORT-1 INVINCIBLE Trial at the ASCO Annual Meeting in June 2023,
which demonstrated significant necrosis of presurgical breast cancer tumors in the majority of patients injected with PORT-1 in the window
period from diagnosis to surgery and a pathway enrichment analysis that demonstrated changes in T-cell activation, lymphocyte activation
and inflammatory response from the INVINCIBLE Trial.
On July 5, 2023, Intensity completed an IPO of its common stock selling
3,900,000 shares at a price of $5.00 per share generating net proceeds of approximately $16.2 million. In connection with the offering,
Intensity’s common stock began trading on Nasdaq on June 30, 2023, under the ticker symbol “INTS.” The Intensity shares
closed at a price of $5.96 on June 30, 2023. We received an additional 2,659 shares in connection with the offering pursuant to certain
anti-dilution rights. Intensity sold its overallotment shares totaling 585,000 shares, which closed on July 7, 2023. As of that date,
we owned approximately 4.7% of the issued and outstanding shares of Intensity.
PORT-4, Nanolipogel (“NLG”) co-formulation Platform
Scientists are interested in novel ways to deliver multiple signals to
the immune system in order to better activate an anti-tumor response. We have been impressed with a platform from Yale University
that allows different types of agents to be packaged together and will concentrate them in tumors. We have licensed the platform
for delivery of DNA aptamers and certain aptamer-small molecule-based combination products. In order to have multiple proprietary agents
with known mechanisms of action, we have licensed rights to create DNA aptamers for immune-oncology targets and the first one developed
is a proprietary PD1 aptamer, which has been placed in the NLG formulation. Early testing has shown the formulation properly modulates
PD1 signaling in vitro similar to a PD1 antibody I. In non-clinical, in vivo experiments, the NLG-PD1 performed favorably compared
to a mouse PD1 antibody. The current level of funding is expected to support exploration of multiple PD1 based co-formulations with
small molecules and other DNA aptamers. We have conducted further research with the technology licensed from Yale University to co-deliver
a PD1 blocking signal with a small molecule vascular endothelial growth factor inhibitor.
As of June 30, 2023, we owned approximately 70% of the outstanding shares
of Saugatuck Therapeutics, Ltd. (“Saugatuck”), the subsidiary on which the PORT-4 platform is managed.
PORT-5, STING Agonist Platform
Proprietary immune priming and boosting technology (using a STING agonist
delivered in a virus-like particle) has shown proof of concept in animal models and Stimunity S.A. (“Stimunity”) is beginning
to progress its lead asset towards the clinic. This platform offers multiple ways to target immune stimulation towards the cancer,
as well as to co-deliver multiple signals in a single product. The PORT-5 STING platform provides distinct advantages over chemical
intratumoral approaches by offering a potent immune priming and boosting pathway within a virus-like particle to enable convenient systemic
administration and traffic to the correct targets. This technology preferentially targets dendritic cells, which is differentiated from
other chemical STING approaches. Stimunity is progressing this project towards clinical trials as well as developing next generation
compounds. To that end, Stimunity has received grant funding to study this technology with any COVID-19 vaccine to evaluate if it
is possible to boost the immune response for immunocompromised or elderly patients. During April 2022, the American Association for Cancer
Research showcased PORT-5 preclinical data at a late-breaking session that shows that one or more targeted immunotherapy agents could
be packaged within a virus-like particle to increase potency, while enabling a selective immune activation. Advancing the program to an
IND for PORT-5 is subject to Stimunity securing additional financing.
As of June 30, 2023, we owned approximately 44% of the outstanding shares
of Stimunity, the subsidiary on which the PORT-5 platform is managed.
Early-Stage Research and Development Collaborations
We continue to evaluate and test new antibody targets. Our interest
here lies in the suppressive tumor microenvironment, and how we can down regulate or remove MDSC, TAMs, Tregs and other signals that impede
the immune response from clearing cancer cells.
| · | We are collaborating with Dr. Robert Negrin and his team at Stanford University in an IST study to evaluate the use of PORT-2 with
iNKT cell therapies in animals. This work will evaluate if an engager co-administered with expanded or transformed iNKT cells can further
activate the transplanted and endogenous cells inside the patient. The Stanford collaboration will also study the impact iNKT engagers
have on driving an adaptive immune response and correcting the suppressive tumor microenvironment. |
| · | We have entered into a Cooperative Research and Development Agreement (“CRADA”) with the NCI. We and NCI will advance
preclinical and potential clinical development of STING agonists and anti-RAGE agents for cancer vaccines. We and NCI will develop agents
to enhance the efficacy of proprietary cancer vaccines and mouse model cancer vaccines developed by NCI. After the Tarus acquisition,
we amended the CRADA to include exploration of the different adenosine compounds. |
Three Months Ended June 30, 2023 Compared to the Three Months Ended
June 30, 2022
(All Amounts in 000’$)
Results of Operations
The following details major expenses for the three months ended June 30,
2023, compared to the three months ended June 30, 2022:
| Three months ended June 30, | |
2023 | |
2022 |
| | |
In 000’$ | |
In 000’$ |
| Operating expenses | |
$ | (4,997 | ) | |
$ | (4,087 | ) |
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone | |
| (1,111 | ) | |
| - | |
| Share of loss in associate accounted for using equity method | |
| (50 | ) | |
| (60 | ) |
| Change in fair value of warrant liability | |
| - | | |
| 1 | |
| Foreign exchange transaction gain (loss) | |
| 18 | | |
| (52 | ) |
| Depreciation expense | |
| (11 | ) | |
| - | |
| Interest income, net | |
| 80 | | |
| 21 | |
| Loss before (provision) benefit for income taxes | |
| (6,071 | ) | |
| (4,177 | ) |
| Income tax benefit | |
| 145 | | |
| 2,552 | |
| Net loss | |
| (5,926 | ) | |
| (1,625 | ) |
| Other comprehensive income (loss) | |
| | | |
| | |
| Net unrealized gain on investments | |
| 1,769 | | |
| - | |
| Total comprehensive loss for period | |
$ | (4,157 | ) | |
$ | (1,625 | ) |
| | |
| | | |
| | |
| Comprehensive (loss) income attributable to: | |
| | | |
| | |
| Owners of the Company | |
$ | (4,150 | ) | |
$ | (1,729 | ) |
| Non-controlling interest | |
| (7 | ) | |
| 104 | |
| Total comprehensive loss for period | |
$ | (4,157 | ) | |
$ | (1,625 | ) |
Results of Operations for the Three Months Ended June 30, 2023 Compared
to the Three Months Ended June 30, 2022
We incurred a net loss of approximately $5.9 million and total comprehensive
loss of approximately $4.2 million during the three months ended June 30, 2023 (the “Fiscal 2024 Quarter”), which include
approximately $1.7 million of non-cash expenses, net, compared to a net loss and total comprehensive loss of approximately $1.6 million
during the three months ended June 30, 2022 (the “Fiscal 2023 Quarter”), an increase in net loss of $4.3 million and an increase
in total comprehensive loss of $2.6 million, quarter-over-quarter.
The components of the change in net loss and total comprehensive loss
are as follows:
| · | Operating expenses, which include R&D and general and administrative (“G&A”) expenses, were $5.0 million in the
Fiscal 2024 Quarter, compared to $4.1 million in the Fiscal 2023 Quarter, an increase of $0.9 million, which is discussed more fully below. |
| · | Our other items of income and expense were substantially non-cash in nature and aggregated approximately $1.074 million net loss in
the Fiscal 2024 Quarter, compared to approximately $0.090 million net loss in the Fiscal 2023 Quarter, a change in other items of income
and expense of approximately $0.984 million, quarter-over-quarter. The primary reason for the quarter-over-quarter difference in other
items of income and expense were (a) the non-cash loss from the change (increase) in fair value of the deferred purchase price payable
to the former Tarus shareholders and (b) the deferred obligation - iOx milestone totaling $1.111 million, partially offset by the increase
in our net interest income from investments in short-term investments in the Fiscal 2024 Quarter. The deferred purchase price payable
- Tarus and the deferred obligation - iOx milestone were incurred subsequent to the prior year period. |
| · | Additionally, we reflected a non-cash net deferred income tax benefit of $0.1 million in the Fiscal 2024 Quarter, compared to a net
deferred income tax benefit of $2.6 million in the Fiscal 2023 Quarter. For the Fiscal 2024 Quarter, we recognized an increase in net
deferred tax liability of $0.3 million to reflect the effect of the change in exchange rates on the liability during the period and the
recognition $0.4 million of current period losses in the U.K. The Fiscal 2023 Quarter includes the foreign currency effect on deferred
tax liability balance settleable in British pound sterling of $2.2 million and the recognition of current period losses in the U.K. of
$0.4 million. |
Operating Expenses
Total operating expenses are comprised of the following:
| Three months ended June 30, | |
2023 | |
2022 |
| | |
In 000’$ | |
In 000’$ |
| Research and development | |
$ | 3,627 | | |
$ | 1,876 | |
| General and administrative expenses | |
| 1,370 | | |
| 2,211 | |
| Total operating expenses | |
$ | 4,997 | | |
$ | 4,087 | |
Research and Development Costs
R&D costs are comprised of the following:
| Three months ended June 30, | |
2023 | |
2022 |
| | |
In 000’$ | |
In 000’$ |
| Research and development – Clinical | |
$ | 1,496 | | |
$ | 642 | |
| Contractual milestone | |
| 500 | | |
| - | |
| Payroll-related expenses | |
| 480 | | |
| 406 | |
| Share-based compensation expense | |
| 423 | | |
$ | 552 | |
| Manufacturing costs | |
| 345 | | |
| - | |
| Consulting fees | |
| 213 | | |
| 24 | |
| Licensing fees | |
| 112 | | |
| 24 | |
| Research and development – CRADA | |
| 31 | | |
| 69 | |
| Research and development services and storage | |
| 19 | | |
| 119 | |
| Legal regarding patents’ registration | |
| 8 | | |
| 40 | |
| Total research and development costs | |
$ | 3,627 | | |
$ | 1,876 | |
R&D costs increased by approximately $1.7 million, or
approximately 89%, from approximately $1.9 million in the Fiscal 2023 Quarter, to approximately $3.6 million in the Fiscal 2024
Quarter. The increase was primarily attributable to an overall increase in clinical trial costs of $0.8 million, part of $1.4 million
of start-up and manufacturing costs associated with the adenosine assets (PORT-6 and PORT-7) acquired in the Tarus acquisition, and
the clinical trial costs and other R&D costs associated with the iNKT clinical trial for PORT-2 totaling $1.7 million.
Additionally, we incurred a contractual milestone obligation of $0.5 million upon the dosing of the first patients under the
adenosine program. These increases reflect the increase in clinical activity and manufacturing costs related to accelerating the
development of our adenosine and iNKT programs. Non-cash share-based compensation expense aggregated $0.4 million in the Fiscal 2024
Quarter (allocated equally to each of the adenosine and iNKT programs), as compared to approximately $0.6 million in the Fiscal 2023
quarter, due to the continued vesting of options, as well as recent grants having a lower grant date fair value.
General and Administrative Expenses
Key components of G&A expenses are
the following:
| Three months ended June 30, | |
2023 | |
2022 |
| | |
In 000’$ | |
In 000’$ |
| Professional fees | |
$ | 469 | | |
$ | 903 | |
| Share-based compensation expense | |
| 347 | | |
| 624 | |
| Payroll-related expenses | |
| 225 | | |
| 251 | |
| D&O insurance | |
| 175 | | |
| 307 | |
| Directors fees | |
| 83 | | |
| 75 | |
| Office and general expenses | |
| 68 | | |
| 51 | |
| Consulting fees | |
| 3 | | |
| - | |
| Total general and administrative expenses | |
$ | 1,370 | | |
$ | 2,211 | |
G&A expenses decreased by approximately $0.8 million, or
approximately 36%, from approximately $2.2 million in the Fiscal 2023 Quarter, to approximately $1.4 million in the Fiscal 2024
Quarter. Professional fees decreased by $0.4 million, primarily attributable to legal fees associated with the Tarus acquisition in
the Fiscal 2023 Quarter. Additionally, non-cash share-based compensation expense decreased by $0.3 million attributable to the
vesting of certain stock options granted in prior years, lower fair value associated with more recent grants and D&O insurance
expense decreased by $0.1 million due to the decrease in the D&O premium market year-over-year.
Liquidity and Capital Resources
Capital Resources
We filed a Registration statement with the SEC under which we may sell
ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on March 8, 2021.
In connection with the Registration Statement, we have filed with the SEC:
| · | a base prospectus, which covers the offering, issuance and sale by us of up to $200,000,000 in the aggregate of the securities identified
above from time to time in one or more offerings; |
| · | a prospectus supplement, which covers the offer, issuance and sale by us in an “at-the-market” (“ATM”) offering
program of up to a maximum aggregate offering price of $50,000,000 of our ordinary shares that may be issued and sold from time to time
under a Controlled Equity Offering Sales Agreement, dated February 24, 2021, with Cantor Fitzgerald & Co., the sales agent; |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by us of 1,150,000 ordinary shares for gross proceeds
of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; and |
| · | a prospectus supplement dated August 19, 2022, for the resale of up to $30,000,000 in ordinary shares that we may sell from time to
time to Lincoln and an additional 94,508 shares that were issued to Lincoln. |
The Sales Agreement permits us to sell in an ATM program up to $50,000,000
of ordinary shares from time to time, the amount of which is included in the $200,000,000 of securities that may be offered, issued and
sold by us under the base prospectus. The sales under the prospectus will be deemed to be made pursuant to an ATM program as defined in
Rule 415(a)(4) promulgated under the Securities Act. Upon termination of the Sales Agreement, any portion of the $50,000,000 included
in the Sales Agreement prospectus that is not sold pursuant to the Sales Agreement will be available for sale in other offerings pursuant
to the base prospectus.
On June 24, 2021, we completed the sale of 1,150,000 ordinary shares,
including the underwriters’ option, at a price of $23.00 per share, which generated gross proceeds of approximately $26.5 million
and net proceeds of approximately $25.0 million, and was settled June 28, 2021.
On July 6, 2022 (the “Signing Date”), we entered into the
Committed Purchase Agreement with Lincoln, pursuant to which we may require Lincoln to purchase our ordinary shares having an aggregate
value of up to $30 million over a period of 36 months. Pursuant to the Committed Purchase Agreement, Lincoln will be obligated to purchase
the Purchase Shares in three different scenarios as described below.
Regular Purchase – At any time after the Closing Date (as defined
below) and provided that the closing sale price of the ordinary shares is not less than $0.25 per share, from time to time on any business
day selected by us (the “Purchase Date”), we shall have the right, but not the obligation, to require Lincoln to purchase
up to 30,000 ordinary shares (the “Regular Purchase Amount”) at the Purchase Price (as defined below) per purchase notice
(each such purchase, a “Regular Purchase”). Lincoln’s committed obligation under each Regular Purchase shall not exceed
$1,500,000; provided, that the parties may mutually agree at any time to increase the dollar amount of any Regular Purchase on any Purchase
Date above and beyond the forgoing amounts that Lincoln is committed to purchase. The purchase price for Regular Purchases (the “Purchase
Price”) shall be equal to the lesser of: (i) the lowest sale price of the ordinary shares during the Purchase Date, and (ii) the
average of the three (3) lowest closing sale prices of the ordinary shares during the ten (10) business days prior to the Purchase Date.
We shall have the right to submit a Regular Purchase notice to Lincoln as often as every business day. “Closing Date” shall
mean the date that customary conditions to closing have been satisfied, including that our shelf registration statement for the ordinary
shares to be issued pursuant to the Committed Purchase Agreement is effective and available for use and any listing application and/or
exchange approvals, to the extent applicable, have been approved.
| · | Accelerated Purchase – In addition to Regular Purchases and provided that we have directed a Regular Purchase in full, we in
our sole discretion may require Lincoln on each Purchase Date to purchase on the following business day (“Accelerated Purchase Date”)
up to the lesser of (i) three (3) times the number of ordinary shares purchased pursuant to such Regular Purchase, and (ii) 25% of the
trading volume on the Accelerated Purchase Date at a purchase price equal to the lesser of 97% of (i) the closing sale price on the Accelerated
Purchase Date, and (ii) the Accelerated Purchase Date’s volume weighted average price (the “Accelerate Purchase Price”).
The parties may mutually agree to increase the number of ordinary shares sold to Lincoln on any Accelerated Purchase Date at the Accelerated
Purchase Price. We shall have the right in our sole discretion to set a minimum price threshold for each Accelerated Purchase in the notice
provided with respect to such Accelerated Purchase and we may direct multiple Accelerated Purchases in a day; provided, that delivery
of ordinary shares has been completed with respect to any prior Regular Purchases and Accelerated Purchases Lincoln has purchased. |
| · | Tranche Purchase – In addition to Regular Purchases and Accelerated Purchases and provided that the closing price of the ordinary
shares is not below $0.25, at any time beginning five (5) business days from the Closing Date, we shall have the option to require Lincoln
to purchase up to $3,000,000 in separate purchases of up to $1,000,000 of ordinary shares for each purchase (the “Tranche Purchases”,
and with Regular Purchases and Accelerated Purchases, the “Committed Purchases”). The purchase price for each Tranche Purchase
shall be equal to 90% of the Purchase Price. We may deliver notice to Lincoln for a Tranche Purchase so long as at least twenty (20) business
days have passed since any Tranche Purchase was completed. |
Upon execution of the Committed Purchase Agreement, we issued to Lincoln
94,508 ordinary shares, representing a 3% commitment fee. We have the right to terminate the Committed Purchase Agreement for any reason,
effective upon one (1) business day prior written notice to Lincoln. Lincoln has no right to terminate the Committed Purchase Agreement.
Committed Purchases shall be suspended if any of the following occur:
(i) the shelf registration statement is not available for the sale of all of the ordinary shares issued pursuant to the Committed Purchase
Agreement for ten (10) consecutive trading days or for a total of thirty (30) trading days out of the preceding 365 days; (ii) the ordinary
shares cease to be DTC authorized and participating in the D.W.A.C./F.A.S.T. systems; (iii) suspension of the ordinary shares from trading
for one (1) trading day; (iv) any breach of the representations and warranties or covenants contained in any related agreements with Lincoln
which has or which could have a material adverse effect on us, Lincoln or the value of the ordinary shares, subject to reasonable cure
periods to be agreed upon for curable breaches of covenants; (v) if we are listed on a national exchange or market (excluding the OTC
Markets, OTC Bulletin Board or comparable market), at any time prior to shareholder approval of the Committed Purchase Agreement more
than 19.99% of our aggregate ordinary shares, determined as of the Signing Date, would be issuable to Lincoln in violation of the principal
securities exchange or market rules; (vi) if the ordinary shares cease to be eligible for trading on the Nasdaq Capital Market, our principal
market, and is not immediately thereafter trading on the Nasdaq Global Select Market, the Nasdaq Global Market, the NYSE, the NYSE American,
or the OTC Markets; or (vii) our insolvency or our participation or threatened participation in insolvency or bankruptcy proceedings by
or against us. The Committed Purchases may resume following the resolution of any of the forgoing events.
The Committed Purchase Agreement does not impose any financial or business
covenants on us and there are no limitations on the use of proceeds received by us from Lincoln. We may raise capital from other sources
in our sole discretion; provided, however, that we shall not enter into any similar agreement for the issuance of variable priced equity-like
securities until the three-year anniversary of the Signing Date, excluding, however, an ATM transaction with a registered broker-dealer,
which includes any sales under the Sales Agreement with Cantor Fitzgerald.
In connection with the Committed Purchase Agreement, we and Lincoln entered
into a Registration Rights Agreement (the “Registration Rights Agreement”), dated July 6, 2022. Pursuant to the Registration
Rights Agreement, we agreed, within the time required under Rule 424(b) under the Securities Act, to file with the SEC an initial prospectus
supplement to our shelf registration statement pursuant to Rule 424(b) for the purpose of registering for resale the ordinary shares to
be issued to Lincoln under the Committed Purchase Agreement. All reasonable expenses of ours incurred through the registration of the
ordinary shares under the Committed Purchase Agreement shall be paid by us.
In October 2022, we began selling shares pursuant to the ATM program and
the Sales Agreement. From October 2022 through March 31, 2023, we sold 166,145 ordinary shares under the ATM program, generating net proceeds
of approximately $0.9 million. Separately, between October 2022 and March 31, 2023, we sold 480,000 ordinary shares to Lincoln under the
Committed Purchase Agreement for net proceeds totaling approximately $2.0 million. From April 1, 2023 through June 30, 2023, we sold 171,504
ordinary shares under the ATM program, generating net proceeds of approximately $0.6 million.
On March 1, 2023, we, through Tarus, entered into a clinical service agreement
with a third-party service provider. The term of the agreement is through the earlier of August 14, 2025 or the completion of provision
of services and the payment of contractual obligations. The budgeted costs for the services to be provided is approximately $12.1 million.
Going Concern
The accompanying condensed consolidated interim financial statements for
the three months ended June 30, 2023 have been prepared on a basis that assumes that we will continue as a going concern and that contemplates
the continuity of operations, the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
Accordingly, the accompanying condensed consolidated interim financial statements for the three months ended June 30, 2023 do not include
any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might result
from the outcome of this uncertainty.
As of June 30, 2023, we had cash and cash equivalents of approximately
$7.7 million and total current liabilities of approximately $2.6 million. For the three months ended June 30, 2023, we are reporting a
net loss of approximately $5.9 million and cash used in operating activities of approximately $3.5 million. As of July 31, 2023, we had
approximately $6.4 million of cash and cash equivalents on hand.
Our cash and cash equivalents balance is decreasing and we will not generate
positive cash flows from operations for the fiscal year ending March 31, 2024.
We have and may continue to delay, scale-back, or eliminate certain of
our activities and other aspects of our operations until such time as we are successful in securing additional funding. We are exploring
various dilutive and non-dilutive sources of funding, including equity and debt financings, strategic alliances, business development
and other sources. Our future success is dependent upon our ability to obtain additional funding. There can be no assurance, however,
that we will be successful in obtaining such funding in sufficient amounts, on terms acceptable to us, or at all. As of the date of this
filing, we currently anticipate that current cash and cash equivalents, excluding any potential proceeds from our ATM program and Committed
Purchase Agreement with Lincoln, will be sufficient to meet our anticipated cash requirements through the end of October 2023. Access
to our Committed Purchase Agreement with Lincoln is generally limited based on, among other things, our trading volume. Furthermore, under
General Instruction I.B.5 to Form F-3 (the “Baby Shelf Rule”), the amount of funds we can raise through primary public offerings
of securities in any 12-month period using our registration statement on Form F-3 is limited to one-third of the aggregate market value
of the ordinary shares held by our non-affiliates, which limitation may change over time based on our stock price, number of ordinary
shares outstanding and the percentage of ordinary shares held by non-affiliates. We therefore are limited by the Baby Shelf Rule as of
the filing of this Form 6-K, until such time as our non-affiliate public float exceeds $75 million. These factors raise significant doubt
about our ability to continue as a going concern within one year after the date that the financial statements are issued.
We have incurred significant operating losses since inception and expect
to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result primarily
from our conduct of research and development activities.
We historically have funded our operations principally from proceeds from
issuances of equity and debt securities. We will require significant additional capital to make the investments we need to execute our
longer-term business plan. Our ability to successfully raise sufficient funds through the sale of debt or equity securities when needed
is subject to many risks and uncertainties and, future equity issuances would result in dilution to existing stockholders and any future
debt securities may contain covenants that limit our operations or ability to enter into certain transactions.
Cash Flows From Operating Activities
During the Fiscal 2024 Quarter, we used cash of approximately $3.5 million
to fund operating activities, compared to approximately $2.2 million used during the Fiscal 2023 Quarter. Operations in both the Fiscal
2024 Quarter and the Fiscal 2023 Quarter were funded by cash raised from the ATM program, the public offering in June 2021 and the ordinary
shares issued to Lincoln under the Committed Purchase Agreement, described above under “Capital Resources.”
Our continuing operations are dependent upon any one of:
| 1. | the development and identification of economically recoverable therapeutic solutions; |
| 2. | the ability of us to obtain the necessary financing to complete the research and development; or |
| 3. | the future profitable production, or proceeds, from the disposition of intellectual property. |
We have incurred significant operating losses since inception due to significant
R&D spending and corporate overhead, and we expect to continue to incur significant operating losses for the foreseeable future and
may never become profitable. As of June 30, 2023, we had cash and cash equivalents of approximately $7.7 million, working capital of approximately
$8.3 million (including prepaid expenses of $2.8 million) and an accumulated deficit of approximately $165.5 million. We have funded
our operations primarily from proceeds from the sale of equity and debt securities. We will require significant additional capital to
make the investments that we need to execute our longer-term business plan. Our ability to successfully raise sufficient funds through
the sale of debt or equity securities when needed is subject to many risks and uncertainties and, even if it were successful, future equity
issuances would result in dilution to our existing stockholders and any future debt securities may contain covenants that limit our operations
or ability to enter into certain transactions.
Cash Flows From Investing Activities
There were no cash flows from investing activities during both the Fiscal
2024 Quarter and the Fiscal 2023 Quarter.
Cash Flows From Financing Activities
During the Fiscal 2024 Quarter, we generated net cash of $0.6 million
from financing activities. During the Fiscal 2023 Quarter, there were no financing cash flow activities.
In October 2022, we began selling shares pursuant to the ATM program and
the Sales Agreement. From April 1, 2023 through June 30, 2023, we sold 171,504 ordinary shares under the ATM program, generating net proceeds
of approximately $0.6 million. Additionally, the Fiscal 2024 Quarter reflects a small repayment of the lease liability.
Key Contractual Obligations
Details of contractual obligations, commitments and contingent liabilities
are provided in Note 15, “Commitments and Contingent Liabilities,” to the unaudited condensed consolidated interim financial
statements for the three months ended June 30, 2023.
Acquisition of Tarus
On July 1, 2022, we, our wholly-owned subsidiary, Portage Merger Sub I,
Inc., our wholly-owned subsidiary, Portage Merger Sub II, LLC and Tarus Therapeutics, Inc., a Delaware corporation advancing adenosine
receptor antagonists for the treatment of solid tumors, entered into an Agreement and Plan of Merger and Reorganization (the “Merger
Agreement”). Under the structure of the Merger Agreement, Tarus Therapeutics, Inc. was ultimately merged into Portage Merger Sub
II, LLC with the surviving entity renamed Tarus Therapeutics, LLC (“Tarus”).
As consideration for Tarus, we issued to the former Tarus shareholders
an aggregate of 2,425,999 ordinary shares of Portage, calculated on the basis of $18 million divided by the 60-day volume weighted average
price per share of ordinary shares of Portage. Such ordinary shares have not been registered with the SEC and were subject to lock-up
agreements for terms ranging from six to twelve months, which expired on February 1, 2023 and July 1, 2023, respectively. Additionally,
the ordinary shares that were subject to a twelve month lock-up period, are also subject to a three month dribble-out period which commenced
July 1, 2023. During the dribble out period, each holder may not sell more than 10% of the average trading volume of our ordinary shares
for the rolling three month period prior to the date on which the holder executes a trade of our ordinary shares without our prior written
consent (which we are permitted to withhold at our sole discretion). Additionally, payments of up to $32 million in cash or our ordinary
shares (at our discretion) would be triggered upon achievement of future development and sales milestones, as described below. As a result
of the transaction:
| · | We also assumed $2 million in short-term debt held by Tarus and deferred license milestones obligations ($1 million plus interest),
for an aggregate of $3 million in liabilities. We repaid the short-term debt in July 2022. |
| · | Upon enrolling the first patient in a Phase 2 clinical trial utilizing Tarus’s adenosine receptor antagonists,
we will pay an additional one-time payment of $15 million to the former Tarus shareholders. Payment will be in the form of cash or our
ordinary shares (at our discretion). The remaining $17 million milestone is based on target commercial sales. |
Additionally, in connection with the acquisition of Tarus, we initially
recorded a deferred purchase price payable of $8.538 million, which reflected the estimated acquisition date fair value of contractual
milestone obligations incurred.
We recognized a loss of $0.685 million in the Fiscal 2024 Quarter to reflect
the estimated fair value of the obligation at June 30, 2023 and the carrying value of the liability of $4.552 million is reflected on
the condensed consolidated interim statements of financial position as of June 30, 2023.
Stimunity Convertible Note
On July 13, 2022, we entered into a commitment with Stimunity to provide
€600,000 under a convertible note (the “Stimunity Convertible Note”) with a maturity date of September 1, 2023 (the “Maturity
Date”). The Stimunity Convertible Note provides for interest at 7% per annum. The Stimunity Convertible Note is automatically converted
into Series A shares upon Stimunity completing a Series A round for at least €20 million. If such subscription round is completed
prior to the Maturity Date, we will be entitled to convert the Stimunity Convertible Note into Series A shares of Stimunity at the subscription
share price less 15%. Additionally, if Stimunity completes a financing with a new category of shares (other than Common Shares or Series
A shares) for at least €5 million (the “Minimum Raise”), we will have the right to convert the Stimunity Convertible
Note and the historical Series A shares owned into the new category of shares of Stimunity. In the event that Stimunity does not close
a financing prior to the Maturity Date or raises less than the Minimum Raise, we will have the right to convert the Stimunity Convertible
Note into Series A shares at €363.00 per share or the raise price less 15%, whichever is lower. The Stimunity Convertible Note was
funded by us on September 12, 2022 by existing cash and cash provided under the Committed Purchase Agreement described above.
iOx Share Exchange Agreement
On July 18, 2022, we and our wholly-owned subsidiary, SalvaRx, entered
into a Share Exchange Agreement (the “Share Exchange Agreement”) with each of the minority shareholders of iOx (the “Sellers”)
resulting in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the
iNKT engager platform. We followed IFRS 3, “Business Combinations,” and IAS 27, “Separate Financial Statements,”
(which substantially replaced IAS 3) to account for this transaction. We achieved control of iOx on January 8, 2019 upon the completion
of our acquisition of SalvaRx. See our Annual Report on Form 20-F for the year ended March 31, 2023 for additional information. Further
transactions whereby we acquire further equity interests from non-controlling interests, or disposes of equity interests but without losing
control, are accounted for as equity transactions (i.e., transactions with owners in their capacity as owners). As such:
| · | the carrying amounts of the controlling and non-controlling interests are adjusted to reflect the changes in their relative interests
in the subsidiary; |
| · | any difference between the amount by which the non-controlling interests is adjusted and the fair value of the consideration paid
or received is recognized directly in equity and attributed to us; and |
| · | there is no consequential adjustment to the carrying amount of goodwill, and no gain or loss is recognized in profit or loss. |
We now own the worldwide rights to iOx’s small molecule iNKT engagers,
including lead programs PORT-2 and PORT-3. Under the terms of the Share Exchange Agreement, each Seller sold to us, and we acquired from
each Seller, legal and beneficial ownership of the number of iOx shares held by each Seller, free and clear of any share encumbrances,
in exchange for the issuance in an aggregate of 1,070,000 of our ordinary shares to be allocated among the Sellers based upon their relative
ownership. As a result of the Share Exchange Agreement, we own 100% of the issued and outstanding shares of iOx through our wholly-owned
subsidiary, SalvaRx.
As additional consideration for the sale of the iOx shares to us under
the Share Exchange Agreement, the Sellers shall have the contingent right to receive additional shares (“Earnout Shares”)
from us having an aggregate value equal to $25 million calculated at the Per Share Earnout Price, as defined in the Share Exchange Agreement,
upon the achievement of certain milestones defined as the dosing of the first patient in a Phase 3 clinical trial for either PORT-2 (IMM60
iNKT cell activator/engager) or PORT-3 (PLGA-nanoparticle formulation of IMM60 combined with a NY-ESO-1 peptide vaccine). We shall have
the option, in our sole and absolute discretion, to settle the Earnout Shares in cash.
We initially recorded $5.478 million as the fair value estimate of the
Earnout Shares, which is reflected as deferred obligation - iOx milestone. We recognized a loss of $0.426 million in the Fiscal 2024 Quarter
to reflect the estimated fair value of the obligation as of June 30, 2023 and the carrying value of the liability of $4.552 million is
reflected on the condensed consolidated interim statements of financial position as of June 30, 2023.
Master Services Agreement
Effective March 15, 2022, through iOx, we entered into a Master Services
Agreement (the “MSA”) with Parexel International (IRE) Limited (“Parexel”) under which Parexel agreed to act as
clinical service provider (CRO) pursuant to a work order (“Work Order”) effective June 1, 2022. Pursuant to such Work Order,
Parexel will operate a Phase 2 trial of IMM60 and pembrolizumab in advanced melanoma and non-small lung cancer (“NSCLC”).
The MSA provides for a five-year term, and the Work Order provides for a term to be ended upon the completion of the services required.
The budget provides for service fees and pass-through expenses and clinical sites totaling $11.5 million. During Fiscal 2023, we executed
two change orders resulting in a $0.6 million increase in the overall estimated budgeted costs.
Clinical Service Agreement
On March 1, 2023, we, through Tarus, entered into a clinical service agreement
with Fortrea Inc. (formerly Labcorp Drug Development Inc.), a third-party CRO. The term of the agreement is through the earlier of August
14, 2025, or the completion of provision of services and the payment of contractual obligations. The budgeted costs for the services to
be provided is approximately $12.1 million.
iOx License
On July 1, 2015, iOx entered into a licensing agreement with Ludwig Institute
for Cancer Research Ltd. (“LICR”), which covers certain technology, intellectual property and know-how and development with
respect to iNKT cell agonists to treat human diseases. Under the terms of the licensing agreement (“LICR License”), LICR granted
to iOx an exclusive worldwide license, with the right to grant sublicenses, under the Licensed Patent and Licensed Technology, each as
defined in the LICR License, in each case, to development, make, have made, use, sell, offer for sale and import Licensed Products, as
defined in the LICR License, subject to certain rights retained by LICR for academic and research purposes. The LICR License provides
for a royalty term of ten years after the first commercial sale, on a Licensed Product by Licensed Product, country by country basis.
Upon the expiration of the applicable royalty term, the license with respect to such Licensed Product in such country will convert to
a non-exclusive, fully paid-up license.
LICR is entitled to 15,000 GBP as an annual license fee on each annual
anniversary of the effective date of the LICR License until royalties become duly payable and 15,000 GBP as a patent reimbursement fee
until LICR has been fully reimbursed for all patent costs incurred prior to the LICR License.
Additionally, LICR is entitled to milestone payments totaling up to 20.45
million GBP based upon the first Licensed Product achieving specific clinical, regulatory and sales based milestones. LICR is also entitled
to milestone payment totaling up to 10.25 million GBP based upon a second Licensed Product achieving specific clinical, regulatory and
sales based milestones.
Finally, LICR is entitled to a low-single digit royalty on net sales of
Licensed Products that marginally escalates upon sales levels all determined by territory. LICR is also entitled to a percentage of any
sublicensing income that gradually decreases based on the stage of development of the most advanced Licensed Product that is the subject
of the applicable sublicense agreement.
Pursuant to the terms and conditions of the LICR License, LICR is responsible
for managing the preparation, filing, prosecution and maintenance of all Licensed Patent Rights, as defined in the LICR License. iOx will
reimburse LICR for all reasonable patent costs it incurs after the effective date of the LICR License. Further, the LICR License provides
that both parties have the right to termination for material breach or default in the performance of obligations under the LICR License
by the other party and in the event of insolvency of the other party.
Tarus License
On July 1, 2022, we acquired Tarus Therapeutics, Inc. Pursuant to the
license agreement entered into by Tarus Therapeutics, Inc. and Impetis Biosciences Limited (“Impetis”) dated October 29, 2019
(“Impetis License”), Impetis granted to Tarus an exclusive sublicensable worldwide license to develop and commercialize the
adenosine receptor antagonists for all indications and certain other assets which were granted upon exercise of a call option on November
5, 2020.
Under the terms of the Impetis License, Impetis is eligible to receive
payments totaling up to $38 million on an Impetis Compound (as defined in the Impetis License) based upon achievement of certain clinical
and commercial milestones. Milestone payments due in the amount of USD $1 million for achievement of certain regulatory milestones were
paid in July 2022.
Additionally, commencing upon the First Commercial Sale (as defined in
the Impetis License) of a Licensed Product (as defined in the Impetis License), Impetis is entitled to royalties on worldwide net sales
that begin in the mid-single digits and escalate through multiple tiers, with net sales over $1 billion receiving low double digit royalties.
Pursuant to the terms and conditions of the Impetis License, Tarus has
exclusive and full authority to manage all intellectual property (whether licensed or not) underlying the assets covered by the Impetis
License and any other aspects related to exploitation, development and commercialization thereof at its own cost, and Impetis must provide
Tarus reasonable assistance as requested at Tarus’ cost and expense. Further, the Impetis License provides that both parties have
the right to termination for material breach by the other party and in the event that the other party undergoes certain events such as
a voluntary winding-up, a liquidation or entry into receivership.
Off-balance Sheet Arrangements
As of June 30, 2023 and March 31, 2023, we did not have any off-balance
sheet arrangements, including any relationships with unconsolidated entities or financial partnership to enhance perceived liquidity.
Transactions with Related Parties
Significant related party transactions are detailed in Note 16, “Related
Party Transactions,” to the unaudited condensed consolidated interim financial statements for the three months ended June 30, 2023.
Financial and Derivative Instruments
Our financial instruments recognized in our condensed consolidated interim
statements of financial position consist of the following:
Fair value estimates are made at a specific point in time, based on relevant
market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of
significant judgment; and therefore, these estimates cannot be determined with precision. Changes in assumptions could significantly affect
these estimates.
The following table summarizes our financial instruments as of June 30,
2023 and March 31, 2023:
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTOCI | |
FVTPL | |
Amortized
Cost | |
FVTOCI | |
FVTPL |
| | |
| |
| |
| |
| |
| |
|
| Financial assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 7,698 | | |
$ | - | | |
$ | - | | |
$ | 10,545 | | |
$ | - | | |
$ | - | |
| Prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | - | | |
$ | - | | |
$ | 2,689 | | |
$ | - | | |
$ | - | |
| Convertible note receivable, including accrued interest, net of impairment | |
$ | - | | |
$ | - | | |
$ | 442 | | |
$ | - | | |
$ | - | | |
$ | 442 | |
| Investment in associate | |
$ | - | | |
$ | - | | |
$ | 756 | | |
$ | - | | |
$ | - | | |
$ | 806 | |
| Investment in public company | |
$ | - | | |
$ | 3,855 | | |
$ | - | | |
$ | - | | |
$ | 2,087 | | |
$ | - | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTPL | |
Amortized
Cost | |
FVTPL |
| Financial liabilities | |
| | | |
| | | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
$ | 2,591 | | |
$ | - | | |
$ | 1,865 | | |
$ | - | |
| Lease liability - current | |
$ | 47 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Lease liability - non-current | |
$ | 249 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Deferred purchase price payable - Tarus | |
$ | - | | |
$ | 7,864 | | |
$ | - | | |
$ | 7,179 | |
| Deferred obligation - iOx milestone | |
$ | - | | |
$ | 4,552 | | |
$ | - | | |
$ | 4,126 | |
A summary of our risk exposures as it relates to financial instruments
are reflected below.
Fair value of Financial Instruments
Our financial assets and liabilities are comprised of cash and cash equivalents,
receivables and investments in equities and private and public entities, accounts payable, lease liability, deferred purchase price payable,
deferred obligation and unsecured notes payable.
We classify the fair value of these transactions according to the following
fair value hierarchy based on the amount of observable inputs used to value the instrument:
| · | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of
the reporting date. |
| · | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which
can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of
the reporting date. |
| · | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified
as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value
measurement requires judgment and may affect the placement within the fair value hierarchy.
Management has assessed that the fair values of cash and cash equivalents,
other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
The following methods and assumptions were used to estimate their fair
values:
Investment in Associate: The fair value of the Stimunity investment
was determined based on an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current market conditions,
the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity (Level 3). We recorded a provision
of impairment of $0.607 million with respect to the investment in associate decreasing the carrying value of the investment in associate
to $0.806 million as of March 31, 2023. There was no provision for impairment recorded in the three months ended June 30, 2023.
Convertible Note Receivable: The fair value of the Stimunity Convertible
Note receivable denominated in euros at initial recognition is the transaction price for the instrument adjusted for the effect of the
currency translation rate on the reporting date (Level 3). The Stimunity Convertible Note was initially recorded at $0.614 million to
record the translated value of the Stimunity Convertible Note on September 12, 2022. We recognized an unrealized gain of $0.039 million
through other comprehensive income in fiscal 2023 to reflect the change in translation rate for the Stimunity Convertible Note settleable
in euros, increasing the carrying value of the Stimunity Convertible Note to $0.653 million. As of March 31, 2023, we determined that
there were indications of impairment, based upon the inability of Stimunity to obtain financing. We performed an IAS 36 fair value analysis
evaluating the likelihood of various scenarios given the then-current market conditions, the increasing cost of capital and development
delays associated with Stimunity’s lack of liquidity. We recorded an impairment of $0.211 million resulting from the impairment
analysis decreasing the carrying value of the Stimunity Convertible Note to $0.442 million as of March 31, 2023. We recorded a nominal
unrealized gain in the three months ended June 30, 2023 with respect to the Stimunity Convertible Note.
Investment in Public Company: Upon Intensity’s IPO effective
June 30, 2023, the fair value of the investment is determined by the quoted market price (Level 1).
Accrued Equity Issuable: The fair value is estimated based on the
average of the quoted market prices for the period in which the shares were earned (Level 1).
Lease Liability - Current: The lease liability - current represents
the present value of the lease payments due over the next twelve months discounted at the Company’s incremental borrowing rate (Level
2).
Lease Liability - Non-Current: The lease liability - non-current
represents the present value of the lease payments due under the lease less the current portion of such payments discounted at the Company’s
incremental borrowing rate (Level 2).
Deferred Purchase Price Payable - Tarus: The fair value is the
estimated value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 at acquisition
date, adjusted at each reporting date for any change in fair value (Level 3). The fair value was determined using the Income Approach
and was based upon the analysis on the Tarus clinical plan, the timing of development events and the probabilities of success determined
primarily based upon empirical third party data and our experience, as well as the relevant cost of capital. We recorded a (loss) from
the change (increase) in fair value of the liability of $0.685 million for the three months ended June 30, 2023.
Deferred Obligation - iOx Milestone: The fair value is the estimated
value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 as of July 18, 2022, the
date of the Share Exchange Agreement, adjusted at each reporting date for any change in fair value (Level 3). The fair value was determined
using the Income Approach and based on factors including the clinical plan, the timing of development events and the probabilities of
success determined primarily based upon empirical third party data and our experience, as well as the relevant cost of capital. We recorded
a (loss) from the change (increase) in fair value of the liability of $0.426 million for the three months ended June 30, 2023.
Fair Value Hierarchy
The investment in public company (Intensity)
was transferred from Level 3 to Level 1 of the fair value hierarchy for the three months ended June 30, 2023 as the result of Intensity’s
IPO. For the year ended March 31, 2023, the fair value of the investment was determined based on an IAS 36 impairment analysis after
determining there were external indications of impairment (Level 3).
Our financial instruments are exposed to certain financial risks: Credit
Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s
inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit
risk is limited to the carrying value as reflected in our condensed consolidated interim statements of financial position.
Cash and cash equivalents. Cash and cash equivalents comprise
cash on hand and amounts invested in underlying Treasury and money market funds that are readily convertible to a known amount of cash
with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As
of June 30, 2023 and March 31, 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the
date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of
loss is minimal.
Liquidity Risk
Liquidity risk is the risk that we will encounter difficulty in satisfying
financial obligations as they become due.
Our approach to managing liquidity is to ensure, as far as possible, that
we will have sufficient liquidity to meet our liabilities when due, under both normal and stressed conditions without incurring unacceptable
losses or risking harm to our reputation. We hold sufficient cash and cash equivalents to satisfy current obligations under accounts payable
and accruals.
We monitor our liquidity position regularly to assess whether we have
the funds necessary to meet our operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant
internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available
to us, or that actual drug development expenditures may exceed those planned. The current uncertainty in global markets could have an
impact on our future ability to access capital on terms that are acceptable to us. There can be no assurance that required financing will
be available to us.
Foreign Currency Risk
While we operate in various jurisdictions, substantially all of our transactions
are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British pound sterling and the Stimunity
Convertible Note receivable settleable in euros.
Use of Estimates and Judgments
The preparation of the condensed consolidated interim financial statements
in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies
and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis.
Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial
instruments (including the Stimunity Convertible Note, deferred tax assets and liabilities, R&D costs, fair value used for acquisition
of intangible assets, contingent consideration assumed and measurement of share-based compensation. Significant areas where critical judgments
are applied include assessment of impairment of investments, goodwill and in-process research and development and the determination of
the accounting acquirer and acquiree in the business combination accounting.
New Accounting Standards, Interpretations and Amendments
We are also unaware of any applicable but not-yet-adopted standards that
are expected to materially affect our financial statements for future periods.
Internal Control Over Financial Reporting
The management of the Company, including the Chief Executive Officer and
Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as defined
in Rule 13a-15(f) under the Securities Exchange Act of 1934, as amended). The Company’s internal control system was designed to
provide reasonable assurance to the Company’s management and the Company’s Board of Directors regarding the reliability of
financial reporting and preparation and fair presentation of published financial statements for external purposes in accordance with IFRS.
Internal control over financial reporting includes those policies and procedures that:
| 1. | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of
the assets of the Company; |
| 2. | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance
with IFRS, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and the
directors of the Company; and |
| 3. | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s
assets that could have a material effect on the financial statements. |
All internal control systems, no matter how well designed, have inherent
limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial
statement preparation and presentation. Because of its inherent limitations, internal control over financial reporting may not prevent
or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company’s internal
control over financial reporting as of June 30, 2023. In making this assessment, it used the criteria established in Internal Control
- Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on the evaluation
under these criteria, management identified material weaknesses in the Company’s internal control over financial reporting and,
as a result, management concluded that the Company’s internal control over financial reporting was not effective as of June 30,
2023.
Management identified the following material weaknesses in our internal
control over financial reporting.
| · | Management was unable to perform an effective risk assessment or monitor internal controls
over financial reporting; |
| · | Management lacks the number of skilled persons that it requires given the complexity of the
reporting requirements that it has to make, which more specifically include the staff and expertise to (i) properly segregate duties
and perform oversight of work performed and to perform compensating controls over the finance and accounting functions, (ii) establish
and perform fair value estimates or subsequently monitor fluctuations in fair value estimates, and (iii) apply complex accounting principles,
including those relating to business combination accounting, income taxes and fair value estimates; and |
| · | There are insufficient written policies and procedures in place to ensure the correct application
of accounting and financial reporting with respect to the current requirements of IFRS and SEC disclosure requirements, some of which
specifically relate to investment accounting and fair value measures, assessment of in-process R&D assets, share-based payments,
carrying amounts of goodwill and intangible assets and business combination accounting. |
Public Securities Filings
Additional information, including our annual information in the Annual
Report on Form 20-F, is filed with the Canadian Securities Administrators at www.sedar.com and with the SEC at www.edgar.com.
28
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 3 such as an Office Park
| Name: |
dei_EntityAddressAddressLine3 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Statements of Financial Position (Unaudited) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Mar. 31, 2023 |
| Current assets |
|
|
| Cash and cash equivalents |
$ 7,698
|
$ 10,545
|
| Prepaid expenses and other receivables |
2,752
|
2,689
|
| Convertible note receivable |
442
|
442
|
| Total current assets |
10,892
|
13,676
|
| Non-current assets |
|
|
| Investment in associate |
756
|
806
|
| Investment in public company |
3,855
|
2,087
|
| In-process research and development |
81,683
|
81,683
|
| Deferred commitment fee |
839
|
839
|
| Right to use asset |
293
|
|
| Other assets, including equipment, net |
51
|
38
|
| Total non-current assets |
87,477
|
85,453
|
| Total assets |
98,369
|
99,129
|
| Current liabilities |
|
|
| Accounts payable and accrued liabilities |
2,591
|
1,865
|
| Lease liability - current, including interest |
47
|
|
| Total current liabilities |
2,638
|
1,865
|
| Non-current liabilities |
|
|
| Lease liability - non-current |
249
|
|
| Deferred tax liability |
10,416
|
10,564
|
| Deferred purchase price payable - Tarus |
7,864
|
7,179
|
| Deferred obligation - iOx milestone |
4,552
|
4,126
|
| Total non-current liabilities |
23,081
|
21,869
|
| Total liabilities |
25,719
|
23,734
|
| Shareholders’ Equity |
|
|
| Capital stock |
219,425
|
218,782
|
| Stock option reserve |
21,973
|
21,204
|
| Accumulated other comprehensive loss |
(2,556)
|
(4,325)
|
| Accumulated deficit |
(165,535)
|
(159,616)
|
| Total equity attributable to owners of the Company |
73,307
|
76,045
|
| Non-controlling interest |
(657)
|
(650)
|
| Total equity |
72,650
|
75,395
|
| Total liabilities and equity |
98,369
|
99,129
|
| Commitments and Contingent Liabilities (Note 15) |
|
|
| X |
- DefinitionThe amount of accumulated items of income and expense (including reclassification adjustments) that are not recognised in profit or loss as required or permitted by other IFRSs. [Refer: IFRSs [member]; Other comprehensive income] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AccumulatedOtherComprehensiveIncome |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of a present economic resource controlled by the entity as a result of past events. Economic resource is a right that has the potential to produce economic benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_a&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_c&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_b&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_e&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Assets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of cash on hand and demand deposits, along with short-term, highly liquid investments that are readily convertible to known amounts of cash and that are subject to an insignificant risk of changes in value. [Refer: Cash; Cash equivalents] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B13
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B13_a&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashAndCashEquivalents |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of assets that the entity (a) expects to realise or intends to sell or consume in its normal operating cycle; (b) holds primarily for the purpose of trading; (c) expects to realise within twelve months after the reporting period; or (d) classifies as cash or cash equivalents (as defined in IAS 7) unless the asset is restricted from being exchanged or used to settle a liability for at least twelve months after the reporting period. [Refer: Assets] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_i&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CurrentAssetsAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of liabilities that: (a) the entity expects to settle in its normal operating cycle; (b) the entity holds primarily for the purpose of trading; (c) are due to be settled within twelve months after the reporting period; or (d) the entity does not have the right at the end of the reporting period to defer settlement for at least twelve months after the reporting period. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_iii&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 69
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_69&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CurrentLiabilitiesAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amounts of income taxes payable in future periods in respect of taxable temporary differences. [Refer: Temporary differences [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph o
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_o&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 56
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_56&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DeferredTaxLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of residual interest in the assets of the entity after deducting all its liabilities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_a&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_i&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_a&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_e&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Equity |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_EquityAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of the entity's equity and liabilities. [Refer: Equity; Liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_EquityAndLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of equity attributable to the owners of the parent. This specifically excludes non-controlling interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph r
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_r&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_EquityAttributableToOwnersOfParent |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of property (land or a building - or part of a building - or both) held (by the owner or by the lessee as a right-of-use asset) to earn rentals or for capital appreciation or both, rather than for: (a) use in the production or supply of goods or services or for administrative purposes; or (b) sale in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 40
-IssueDate 2023-01-01
-Paragraph 76
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=40&code=ifrs-tx-2023-en-r&anchor=para_76&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 40
-IssueDate 2023-01-01
-Paragraph 79
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=40&code=ifrs-tx-2023-en-r&anchor=para_79_d&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_InvestmentProperty |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of investments in subsidiaries, joint ventures and associates in an entity's separate financial statements. [Refer: Associates [member]; Joint ventures [member]; Subsidiaries [member]; Investments in subsidiaries reported in separate financial statements] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 10
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_10&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_InvestmentsInSubsidiariesJointVenturesAndAssociates |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe nominal value of capital issued. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IssuedCapital |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of a present obligation of the entity to transfer an economic resource as a result of past events. Economic resource is a right that has the potential to produce economic benefits. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_a&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_b&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_e&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_d&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Liabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of equity in a subsidiary not attributable, directly or indirectly, to a parent. [Refer: Subsidiaries [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 12
-Subparagraph f
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_12_f&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 10
-IssueDate 2023-01-01
-Paragraph 22
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=10&code=ifrs-tx-2023-en-r&anchor=para_22&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph q
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_q&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncontrollingInterests |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of assets that do not meet the definition of current assets. [Refer: Current assets] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_ii&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncurrentAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_NoncurrentAssetsAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of non-current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of liabilities that do not meet the definition of current liabilities. [Refer: Current liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_iv&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 69
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_69&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncurrentLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_NoncurrentLiabilitiesAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of assets that the entity does not separately disclose in the same statement or note. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_OtherAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionA component of equity representing reserves within equity, not including retained earnings. [Refer: Retained earnings] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_OtherReserves |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionA component of equity representing the entity's cumulative undistributed earnings or deficit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph IG6
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_IG6&doctype=Implementation%20Guidance
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_RetainedEarnings |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of assets that represent a lessee's right to use an underlying asset for the lease term that do not meet the definition of investment property. Underlying asset is an asset that is the subject of a lease, for which the right to use that asset has been provided by a lessor to a lessee. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 53
-Subparagraph j
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_53_j&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_RightofuseAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current trade payables and current other payables. [Refer: Current trade payables; Other current payables] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph k
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_k&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_TradeAndOtherCurrentPayables |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current trade receivables and current other receivables. [Refer: Current trade receivables; Other current receivables] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph h
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_h&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_TradeAndOtherCurrentReceivables |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_CommitmentAndContingencies |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ConvertibleNoteReceivable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredCommitmentFee |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredObligationIoxMilestone |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredPurchasePricePayableTarus |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_InprocessResearchAndDevelopment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
Condensed Consolidated Interim Statements of Operations and Other Comprehensive Income (Loss) (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Expenses |
|
|
| Research and development |
$ 3,627
|
$ 1,876
|
| General and administrative expenses |
1,370
|
2,211
|
| Loss from operations |
(4,997)
|
(4,087)
|
| Change in fair value of deferred purchase price payable - Tarus and deferred obligation - iOx milestone |
(1,111)
|
|
| Share of loss in associate accounted for using equity method |
(50)
|
(60)
|
| Change in fair value of warrant liability |
|
1
|
| Foreign exchange transaction gain (loss) |
18
|
(52)
|
| Depreciation expense |
(11)
|
|
| Interest income, net |
80
|
21
|
| Loss before (provision) benefit for income taxes |
(6,071)
|
(4,177)
|
| Income tax benefit |
145
|
2,552
|
| Net loss |
(5,926)
|
(1,625)
|
| Other comprehensive income (loss) |
|
|
| Net unrealized gain on investments |
1,769
|
|
| Total comprehensive loss for period |
(4,157)
|
(1,625)
|
| Net (loss) income attributable to: |
|
|
| Owners of the Company |
(5,919)
|
(1,729)
|
| Non-controlling interest |
(7)
|
104
|
| Comprehensive (loss) income attributable to: |
|
|
| Owners of the Company |
(4,150)
|
(1,729)
|
| Non-controlling interest |
$ (7)
|
$ 104
|
| X |
- DefinitionThe amount of change in equity resulting from transactions and other events, other than those changes resulting from transactions with owners in their capacity as owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_c&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause ix
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_ix&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ComprehensiveIncome |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_ComprehensiveIncomeAttributableToAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of comprehensive income attributable to non-controlling interests. [Refer: Comprehensive income; Non-controlling interests] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81B
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81B_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ComprehensiveIncomeAttributableToNoncontrollingInterests |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of comprehensive income attributable to owners of the parent. [Refer: Comprehensive income] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81B
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81B_b_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ComprehensiveIncomeAttributableToOwnersOfParent |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of depreciation and amortisation expense. Depreciation and amortisation are the systematic allocations of depreciable amounts of assets over their useful lives. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_e&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23_e&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B13
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B13_d&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 102
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_102&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 99
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_99&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 104
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_104&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DepreciationAndAmortisationExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense relating to general and administrative activities of the entity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_OtherComprehensiveIncomeAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe total of income less expenses from continuing and discontinued operations, excluding the components of other comprehensive income. [Refer: Other comprehensive income] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 18
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_18_b&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 17
-IssueDate 2023-01-01
-Paragraph 113
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=17&code=ifrs-tx-2023-en-r&anchor=para_113_b&doctype=Standard
-URIDate 2023-03-23
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_a&doctype=Standard
-URIDate 2023-03-23
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_ProfitLossAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_ProfitLossAttributableToAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) from continuing and discontinued operations attributable to non-controlling interests. [Refer: Profit (loss); Non-controlling interests] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 12
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_12_e&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81B
-Subparagraph a
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81B_a_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossAttributableToNoncontrollingInterests |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) from continuing and discontinued operations attributable to owners of the parent. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81B
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81B_a_ii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossAttributableToOwnersOfParent |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) before tax expense or income. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 5
-IssueDate 2023-01-01
-Paragraph 33
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=5&code=ifrs-tx-2023-en-r&anchor=para_33_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 103
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_103&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 102
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_102&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossBeforeTax |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) from operating activities of the entity. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 32
-IssueDate 2023-01-01
-Paragraph IE33
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=32&code=ifrs-tx-2023-en-r&anchor=para_IE33&doctype=Illustrative%20Examples
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 85
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_85&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossFromOperatingActivities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expenditure directly attributable to research or development activities, recognised in profit or loss. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 38
-IssueDate 2023-01-01
-Paragraph 126
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=38&code=ifrs-tx-2023-en-r&anchor=para_126&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of income arising from interest. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23_c&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_e&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B13
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B13_e&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_RevenueFromInterest |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe entity's share of the profit (loss) of associates accounted for using the equity method. [Refer: Associates [member]; Investments accounted for using equity method; Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 85
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_85&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ShareOfProfitLossOfAssociatesAccountedForUsingEquityMethod |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ChangeInFairValueOfDeferredPurchasePricePayableTarusAndDeferredObligationIoxMilestone |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ForeignExchangeTransactionGainLoss |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_GainsLossesOnChangeInFairValueOfWarrants |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NetUnrealizedGainOnInvestments |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe amount of profit (loss) attributable to ordinary equity holders of the parent entity (the numerator) divided by the weighted average number of ordinary shares outstanding during the period (the denominator). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 67
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_67&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_BasicEarningsLossPerShare |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of profit (loss) attributable to ordinary equity holders of the parent entity (the numerator), divided by the weighted average number of ordinary shares outstanding during the period (the denominator), both adjusted for the effects of all dilutive potential ordinary shares. [Refer: Ordinary shares [member]; Weighted average [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 67
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_67&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DilutedEarningsLossPerShare |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_IncomeStatementAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_WeightedAverageSharesOutstandingBasic |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_WeightedAverageSharesOutstandingDiluted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Statements of Changes in Shareholders' Equity (Unaudited) - USD ($)
$ in Thousands |
Issued capital [member] |
Stock Option Reserve [Member] |
Accumulated other comprehensive income [member] |
Retained earnings [member] |
Equity attributable to owners of parent [member] |
Non-controlling interests [member] |
Total |
| Beginning balance, value at Mar. 31, 2022 |
$ 158,324
|
$ 16,928
|
$ 958
|
$ (55,005)
|
$ 121,205
|
$ 44,229
|
$ 165,434
|
| Beginning balance, shares at Mar. 31, 2022 |
13,349
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
| Share-based compensation expense |
|
1,176
|
|
|
1,176
|
|
1,176
|
| Shares issued or accrued for services |
$ 30
|
|
|
|
30
|
|
30
|
| Shares issued or accrued for services, shares |
4
|
|
|
|
|
|
|
| Net (loss) income for period |
|
|
|
(1,729)
|
(1,729)
|
104
|
(1,625)
|
| Ending balance, value at Jun. 30, 2022 |
$ 158,354
|
18,104
|
958
|
(56,734)
|
120,682
|
44,333
|
165,015
|
| Ending balance, shares at Jun. 30, 2022 |
13,353
|
|
|
|
|
|
|
| Beginning balance, value at Mar. 31, 2023 |
$ 218,782
|
21,204
|
(4,325)
|
(159,616)
|
76,045
|
(650)
|
75,395
|
| Beginning balance, shares at Mar. 31, 2023 |
17,606
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
| Share-based compensation expense |
|
769
|
|
|
769
|
|
769
|
| Shares issued under ATM |
$ 632
|
|
|
|
632
|
|
632
|
| Shares issued under ATM, shares |
171
|
|
|
|
|
|
|
| Share issuance costs |
$ (19)
|
|
|
|
(19)
|
|
(19)
|
| Shares issued or accrued for services |
$ 30
|
|
|
|
30
|
|
30
|
| Shares issued or accrued for services, shares |
9
|
|
|
|
|
|
|
| Net unrealized gain on investments |
|
|
1,769
|
|
1,769
|
|
1,769
|
| Net (loss) income for period |
|
|
|
(5,919)
|
(5,919)
|
(7)
|
(5,926)
|
| Ending balance, value at Jun. 30, 2023 |
$ 219,425
|
$ 21,973
|
$ (2,556)
|
$ (165,535)
|
$ 73,307
|
$ (657)
|
$ 72,650
|
| Ending balance, shares at Jun. 30, 2023 |
17,786
|
|
|
|
|
|
|
| X |
- DefinitionThe amount of residual interest in the assets of the entity after deducting all its liabilities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_a&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_i&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_a&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 13
-IssueDate 2023-01-01
-Paragraph 93
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=13&code=ifrs-tx-2023-en-r&anchor=para_93_e&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Equity |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe increase (decrease) in equity resulting from share-based payment transactions. [Refer: Equity] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d_iii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IncreaseDecreaseThroughSharebasedPaymentTransactions |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares that have been authorised and issued, reduced by treasury shares held. [Refer: Treasury shares] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 79
-Subparagraph a
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_79_a_iv&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NumberOfSharesOutstanding |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe total of income less expenses from continuing and discontinued operations, excluding the components of other comprehensive income. [Refer: Other comprehensive income] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 18
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_18_b&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 17
-IssueDate 2023-01-01
-Paragraph 113
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=17&code=ifrs-tx-2023-en-r&anchor=para_113_b&doctype=Standard
-URIDate 2023-03-23
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_a&doctype=Standard
-URIDate 2023-03-23
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NetUnrealizedGainOnInvestment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ShareIssuanceCosts |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedOrAccruedForServices |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedOrAccruedForServicesShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedUnderAtm |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedUnderAtmShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Statements of Cash Flows (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Cash flows from operating activities: |
|
|
| Net loss for the period |
$ (5,926)
|
$ (1,625)
|
| Adjustments for non-cash items: |
|
|
| Share-based compensation expense |
769
|
1,176
|
| Change in fair value of deferred purchase price payable – Tarus and deferred obligation – iOx milestone |
1,111
|
|
| Decrease in deferred tax liability |
(148)
|
(2,552)
|
| Share of loss in associate |
50
|
60
|
| Fair value of shares issued for services |
30
|
30
|
| Depreciation |
11
|
|
| Change in fair value of warrant liability |
|
(1)
|
| Changes in operating working capital: |
|
|
| Accounts receivable |
(17)
|
(44)
|
| Prepaid expenses and other receivables |
(50)
|
(408)
|
| Other assets |
(10)
|
|
| Accounts payable and accrued liabilities |
726
|
1,188
|
| Other |
1
|
|
| Net cash used in operating activities |
(3,453)
|
(2,176)
|
| Cash flows from financing activities: |
|
|
| Proceeds from shares issued under ATM and Committed Purchase Agreement |
632
|
|
| Share issuance costs |
(19)
|
|
| Repayment of lease liability |
(7)
|
|
| Net cash provided by financing activities |
606
|
|
| Decrease in cash and cash equivalents during period |
(2,847)
|
(2,176)
|
| Cash and cash equivalents at beginning of period |
10,545
|
23,352
|
| Cash and cash equivalents at end of period |
7,698
|
21,176
|
| Supplemental disclosure of cash flow information: |
|
|
| Net unrealized gain on investment in Intensity |
1,769
|
|
| Cash paid for interest |
3
|
|
| Supplemental disclosure of non-cash investing and financing activities: |
|
|
| Right to use asset acquired |
303
|
|
| Lease liability incurred |
$ 303
|
|
| X |
- DefinitionAdjustments for decrease (increase) in other assets to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Other assets; Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForDecreaseIncreaseInOtherAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments for the decrease (increase) in prepaid expenses to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Current prepaid expenses; Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForDecreaseIncreaseInPrepaidExpenses |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments for depreciation and amortisation expense to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Depreciation and amortisation expense; Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForDepreciationAndAmortisationExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments for increase (decrease) in other liabilities to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Other liabilities; Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForIncreaseDecreaseInOtherLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments for increase (decrease) in trade accounts payable to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForIncreaseDecreaseInTradeAccountPayable |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_AdjustmentsForReconcileProfitLossAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAdjustments for share-based payments to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForSharebasedPayments |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of cash on hand and demand deposits, along with short-term, highly liquid investments that are readily convertible to known amounts of cash and that are subject to an insignificant risk of changes in value. [Refer: Cash; Cash equivalents] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B13
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B13_a&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashAndCashEquivalents |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash flows from (used in) financing activities, which are activities that result in changes in the size and composition of the contributed equity and borrowings of the entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 10
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_10&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 50
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_50_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashFlowsFromUsedInFinancingActivities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CashFlowsFromUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash flows from (used in) operating activities, which are the principal revenue-producing activities of the entity and other activities that are not investing or financing activities. [Refer: Revenue] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 10
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_10&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 50
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_50_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashFlowsFromUsedInOperatingActivities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CashFlowsFromUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) in cash and cash equivalents after the effect of exchange rate changes on cash and cash equivalents held in foreign currencies. [Refer: Cash and cash equivalents; Effect of exchange rate changes on cash and cash equivalents] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IncreaseDecreaseInCashAndCashEquivalents |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) in a deferred tax liability (asset). [Refer: Deferred tax liability (asset)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IncreaseDecreaseInDeferredTaxLiabilityAsset |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for share issue costs. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 17
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_17&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_PaymentsForShareIssueCosts |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe total of income less expenses from continuing and discontinued operations, excluding the components of other comprehensive income. [Refer: Other comprehensive income] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 18
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_18_b&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 17
-IssueDate 2023-01-01
-Paragraph 113
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=17&code=ifrs-tx-2023-en-r&anchor=para_113_b&doctype=Standard
-URIDate 2023-03-23
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_a&doctype=Standard
-URIDate 2023-03-23
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CashPaidForInterest |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ChangeInFairValueOfDeferredsPurchasePricePayableTarusAndDeferredObligationIoxMilestone |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_FairValueOfStockIssuedForServices |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IncomeOnFairValueOfWarrantLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IncreaseDecreaseInShareOfLossInAssociate |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IncreasingDecreasingAccountsReceivable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_LeaseLiabilityIncurred |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NetChangeInWorkingCapitalComponentsAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NetUnrealizedGainOnInvestmentInIntensity |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NoncashInvestingItemsAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ProceedsFromSharesIssuedUnderAtmAndCommittedPurchaseAgreement |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_RepaymentOfLeaseLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_RightToUseAssetAcquired |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
NATURE OF OPERATIONS
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Nature Of Operations |
|
| NATURE OF OPERATIONS |
NOTE 1. NATURE OF OPERATIONS
Portage Biotech Inc. (the “Company” or “Portage”)
is incorporated in the British Virgin Islands (“BVI”) with its registered office located at Clarence Thomas Building, P.O.
Box 4649, Road Town, Tortola, BVI. Its USA agent, Portage Development Services Inc. (“PDS”), is located at 61 Wilton Road,
Westport, CT, 06880, USA.
The Company is a foreign private issuer under the Securities and Exchange
Commission (the “SEC”) rules. It is also a reporting issuer under the securities legislation of the provinces of Ontario and
British Columbia. Its ordinary shares were listed on the Canadian Securities Exchange (“CSE”) under the symbol “PBT.U”.
On February 25, 2021, the ordinary shares of the Company began trading on the Nasdaq Capital Market (“Nasdaq”) under the symbol
“PRTG”. As the principal market for the Company’s ordinary shares is Nasdaq, the Company voluntarily delisted from the
CSE on April 23, 2021.
Portage is a clinical-stage immuno-oncology company advancing therapies
that target known checkpoint resistance pathways to improve long-term treatment response and quality of life in patients with invasive
cancers. Portage’s access to next-generation technologies coupled with a deep understanding of biological mechanisms enable the
identification of clinical therapies and product development strategies that accelerate these medicines through the translational pipeline.
Portage’s portfolio consists of four diverse platforms, with lead programs consisting of invariant natural killer T-cell (“iNKT”)
engagers and a suite of treatments targeting the adenosine pathway. Additional programs leverage delivery by intratumorals, nanoparticles,
liposomes, aptamers, and virus-like particles. Within these four platforms, Portage has 9 product candidates currently in development
with multiple clinical readouts expected through the end of calendar year 2024.
On August 13, 2018, the Company reached a definitive agreement to acquire
100% of SalvaRx Limited (“SalvaRx”) in exchange for 8,050,701 ordinary shares of the Company (the “SalvaRx Acquisition”).
The SalvaRx Acquisition was completed on January 8, 2019 (the “Acquisition Date”) upon receiving shareholder and regulatory
approval. In connection with the SalvaRx Acquisition, the Company acquired interests in SalvaRx’s five research and development
invested entities and subsidiaries: iOx Therapeutics Ltd. (“iOx”) (60.49% interest), Nekonal Oncology Limited (“Nekonal”),
Intensity Therapeutics, Inc. (“Intensity”), Saugatuck Therapeutics, Ltd. (“Saugatuck”) and Rift Biotherapeutics
Inc. The Company also acquired an option in Nekonal SARL, a Luxembourg-based company holding intellectual property rights for therapeutics
and diagnostics in the field of autoimmune disorders and oncology, to participate in the funding of its autoimmune programs.
In September 2021, the Company, through SalvaRx, exchanged certain notes,
accrued interest, warrants and receivables in exchange for shares of iOx representing 60.49% of the outstanding shares of iOx. As a result
of this exchange, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%. On July 18, 2022, the Company purchased
the remaining non-controlling interest of iOx. See Note 16, “Related Party Transactions –
Share Exchange Agreement – iOx,” for a further discussion.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureNatureOfOperationsAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NatureOfOperationsExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
GOING CONCERN
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| GOING CONCERN |
NOTE 2. GOING CONCERN
As of June 30, 2023, the Company had cash and cash equivalents of approximately
$7.7 million and total current liabilities of approximately $2.6 million. For the three months ended June 30, 2023, the Company is reporting
a net loss of approximately $5.9 million and cash used in operating activities of approximately $3.5 million. As of July 31, 2023, the
Company had approximately $6.4 million of cash and cash equivalents on hand.
The Company’s cash and cash equivalents balance is decreasing, and
the Company will not generate positive cash flows from operations for the fiscal year ending March 31, 2024.
The Company may have to delay, scale-back, or eliminate certain of its
activities and other aspects of its operations until such time as the Company is successful in securing additional funding. The Company
is exploring various dilutive and non-dilutive sources of funding, including equity and debt financings, strategic alliances, business
development and other sources. The future success of the Company is dependent upon its ability to obtain additional funding. There can
be no assurance, however, that the Company will be successful in obtaining such funding in sufficient amounts, on terms acceptable to
the Company, or at all. As of the date of this filing, the Company currently anticipates that current cash and cash equivalents, excluding
any potential proceeds from its ATM program and Committed Purchase Agreement with Lincoln Park, access to which are generally limited
based on the Company’s Nasdaq trading volume, will be sufficient to meet its anticipated cash requirements through the end of October
2023. These factors raise significant doubt about the Company’s ability to continue as a going concern within one year after the
date that these financial statements are issued.
The Company has incurred significant operating losses since inception
and expects to continue to incur significant operating losses for the foreseeable future and may never become profitable. The losses result
primarily from its conduct of research and development activities.
The Company historically has funded its operations principally from proceeds
from issuances of equity and debt securities. The Company will require significant additional capital to make the investments it needs
to execute its longer-term business plan, beyond the potential proceeds that could be reasonably generated from its ATM program and Committed
Purchase Agreement with Lincoln Park given the Company’s current trading volume on Nasdaq. The Company's ability to successfully
raise sufficient funds through the sale of debt or equity securities when needed is subject to many risks and uncertainties and, future
equity issuances would result in dilution to existing stockholders and any future debt securities may contain covenants that limit the
Company's operations or ability to enter into certain transactions.
|
| X |
- DefinitionThe disclosure of the entity's ability to continue as a going concern. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 10
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_10_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfGoingConcernExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
BASIS OF PRESENTATION
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| BASIS OF PRESENTATION |
NOTE 3. BASIS OF PRESENTATION
Statement of Compliance and Basis of Presentation
These condensed consolidated interim financial statements have been prepared
in accordance with the International Financial Reporting Standards (“IFRS”) issued by the International Accounting Standards
Board (“IASB”), IAS 34 Interim Financial Reporting and interpretations of the International Financial Reporting Interpretations
Committee. These condensed consolidated interim financial statements do not include all of the information required for full annual financial
statements and should be read in conjunction with the audited consolidated financial statements of the Company for the year ended March
31, 2023.
These condensed consolidated interim financial statements have been prepared
on an historical cost basis except for items disclosed herein at fair value (see Note 17, “Financial Instruments and Risk Management”).
In addition, these condensed consolidated interim financial statements have been prepared using the accrual basis of accounting, except
for cash flow information.
The Company has only one reportable operating segment.
These condensed consolidated interim financial statements were approved
and authorized for issuance by the Audit Committee and Board of Directors (the “Board”) on August 29, 2023.
Consolidation
The condensed consolidated interim financial statements include the accounts
of the Company and:
| (a) | SalvaRx, a wholly-owned subsidiary, incorporated on May 6, 2015 in the British Virgin Islands; |
| (b) | iOx, a wholly-owned subsidiary incorporated in the U.K. on February 10, 2015. In September 2021, the Company, through SalvaRx, exchanged
certain notes, accrued interest, warrants and receivables in exchange for shares of iOx representing 60.49% of the outstanding shares
of iOx. As a result of this exchange, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%. On July 18,
2022, the Company purchased the remaining non-controlling interest of iOx. See Note 16, “Related
Party Transactions – Share Exchange Agreement – iOx,” for a further discussion; |
| (c) | Saugatuck, a 70% owned subsidiary incorporated in the British Virgin Islands. Saugatuck and subsidiary refers to Saugatuck and Saugatuck
Rx LLC; |
| (d) | PDS, a 100% owned subsidiary incorporated in Delaware, which provides human resources, and other services to each operating subsidiary
via a shared services agreement; |
| (e) | SalvaRx LLC, a wholly-owned subsidiary through SalvaRx; |
| (f) | Saugatuck Rx LLC, a wholly-owned subsidiary of Saugatuck; and |
| (g) | Tarus Therapeutics, LLC (“Tarus”), a wholly-owned subsidiary of Portage. |
All inter-company balances and transactions have been eliminated in consolidation.
Non-controlling interest in the equity of a subsidiary
is accounted for and reported as a component of stockholders’ equity. As of June 30, 2023, non-controlling interest represents the
30% shareholder ownership interest in Saugatuck and subsidiary, which is consolidated by the Company. See Note 16, “Related Party
Transactions – Share Exchange Agreement – iOx” for a discussion of the Company’s purchase of the balance of the
non-controlling interest in iOx.
Functional and Presentation Currency
The Company’s functional and presentation currency is the U.S. Dollar.
Use of Estimates and Judgments
The preparation of the condensed consolidated interim financial statements
in conformity with IFRS requires management to make judgments, estimates and assumptions that affect the application of accounting policies
and the reported amounts of assets, liabilities, income and expenses. Actual results may differ from these estimates.
Estimates and underlying assumptions are reviewed on an ongoing basis.
Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Significant areas where estimates are made include valuation of financial
instruments (including the Stimunity Convertible Note (as defined below), deferred tax assets and liabilities, research and development
costs, fair value used for acquisition of intangible assets, contingent consideration assumed and measurement of share-based compensation.
Significant areas where critical judgments are applied include assessment of impairment of investments, goodwill and in-process research
and development and the determination of the accounting acquirer and acquiree in the business combination accounting.
|
| X |
- DefinitionThe disclosure of the basis used for consolidation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 10
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_10_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfBasisOfConsolidationExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
SIGNIFICANT ACCOUNTING POLICIES
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Significant Accounting Policies |
|
| SIGNIFICANT ACCOUNTING POLICIES |
NOTE 4. SIGNIFICANT ACCOUNTING POLICIES
The accounting policies are set out in Note 4 to the Company’s audited
consolidated financial statements for the fiscal year ended March 31, 2023 (“Fiscal 2023”). These policies have been applied
consistently to all periods presented in these condensed consolidated interim financial statements.
Recent Accounting Pronouncements
IFRS Pronouncements Issued
Impact of Adoption of Significant New IFRS Standards in Fiscal 2023
| (a) | Annual Improvements to IFRS Standards 2018-2020 |
The annual improvements process addresses issues in the 2018-2020 reporting
cycles including changes to IFRS 9, “Financial Instruments,” IFRS 1, “First Time Adoption of IFRS,” IFRS 16, “Leases,”
and IAS 41, “Biological Assets”.
| i) | The amendment to IFRS 9 addresses which fees should be included in the 10% test for derecognition of financial liabilities. |
| ii) | The amendment to IFRS 1 allows a subsidiary adopting IFRS at a later date than its parent to also measure cumulative translation differences
using the amounts reported by the parent based on the parent’s date of transition to IFRS. |
| iii) | The amendment to IFRS 16’s illustrative example 13 removes the illustration of payments from the lessor related to leasehold
improvements. |
These amendments were effective for annual periods beginning on or after
January 1, 2022. The adoption of these amendments did not have a material effect on the Company’s annual consolidated financial
statements or the condensed consolidated interim financial statements for the three months ended June 30, 2023.
New Accounting Standards, Interpretations and Amendments
Standards issued but not yet effective up to the date of issuance of the
Company’s condensed consolidated interim financial statements are listed below. This listing is of standards and interpretations
issued, which the Company reasonably expects to be applicable at a future date. The Company intends to adopt those standards when they
become effective.
| (a) | IAS 1: Presentation of Financial Statements |
The amendment to IAS 1 clarifies how to classify debt and other liabilities
as either current or non-current. The amendment will be effective for annual periods beginning on or after January 1, 2024. The Company
is currently evaluating the new guidance and impacts on its consolidated financial statements.
| (b) | Amendments to IFRS 10 and IAS 28: Sale or Contribution of Assets between an Investor and Its Associate or Joint Venture |
The amendment addresses the conflict between IFRS 10, “Consolidated
Financial Statements,” and IAS 28, “Investments in Associates and Joint Ventures,” in dealing with the loss of control
of a subsidiary that is sold or contributed to an associate or joint venture. The amendments clarify that the gain or loss resulting from
the sale or contribution of assets that constitute a business, as defined in IFRS 3, “Business Combinations,” between an investor
and its associate or joint venture, is recognized in full. Any gain or loss resulting from the sale or contribution of assets that do
not constitute a business, however, is recognized only to the extent of unrelated investors' interests in the associate or joint venture.
The IASB has deferred the effective date of these amendments indefinitely, but an entity that early adopts the amendments must apply them
prospectively. The Company is evaluating whether the adoption of the above amendment will have a material impact on its financial statements.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfSummaryOfSignificantAccountingPoliciesExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureSignificantAccountingPoliciesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
PREPAID EXPENSES AND OTHER RECEIVABLES
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Prepaid Expenses And Other Receivables |
|
| PREPAID EXPENSES AND OTHER RECEIVABLES |
NOTE 5. PREPAID EXPENSES AND OTHER RECEIVABLES
| Schedule of prepaid expenses and other receivables | |
| | | |
| | |
| (In thousands) | |
As of
June 30, 2023 | |
As of
March 31, 2023 |
| | |
| |
|
| Prepaid clinical research costs | |
$ | 1,783 | | |
$ | 1,653 | |
| Prepaid insurance | |
| 446 | | |
| 621 | |
| Research & development tax credits | |
| 186 | | |
| 169 | |
| Other prepaid expenses | |
| 151 | | |
| 56 | |
| Tax deposits | |
| 115 | | |
| 119 | |
| Other receivables | |
| 71 | | |
| 71 | |
| Total prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | 2,689 | |
|
| X |
- DefinitionThe disclosure of other assets. [Refer: Other assets] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 10
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_10_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfOtherAssetsExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_PrepaidExpensesAndOtherReceivablesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INVESTMENT IN ASSOCIATE
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Investment In Associate |
|
| INVESTMENT IN ASSOCIATE |
NOTE 6. INVESTMENT IN ASSOCIATE
Details of the Company’s associate, Stimunity S.A. (“Stimunity”),
as of June 30, 2023 and March 31, 2023 are as follows:
| Schedule of investment associate | |
| |
| |
| |
|
| Name | |
Principal Activity | |
Place of Incorporation and
Principal Place of Business | |
Voting Rights Held as
of June 30, 2023 | |
Voting Rights Held as
of March 31, 2023 |
| Associate: Stimunity S.A. | |
Biotechnology | |
Paris, France | |
44.0% | |
44.0% |
The following table is a roll-forward of the Company’s investment
in Stimunity as of and for the three months ended June 30, 2023 and 2022:
| Schedule of investment in Stimunity S.A. | |
| | | |
| | |
| | |
As of and for the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Balance, beginning of period | |
$ | 806 | | |
$ | 1,673 | |
| Share of loss | |
| (50 | ) | |
| (60 | ) |
| Balance, end of period | |
$ | 756 | | |
$ | 1,613 | |
The Company accounts for its investment in Stimunity under the equity
method and, accordingly, records its share of Stimunity’s earnings or loss based on its ownership percentage. The Company recorded
loss in equity in Stimunity of $50,000 and $60,000 for the three months ended June 30, 2023 and 2022, respectively.
Under the Shareholders’ Agreement entered into on June 1, 2020,
Portage has (i) a preferential subscription right to maintain its equity interest in Stimunity in the event of a capital increase from
the issuance of new securities by Stimunity, except for issuances of new securities for stock options, under a merger plan or for an acquisition,
and (ii) the right to vote against any (a) issuances of additional securities that would call for Portage to waive its preferential subscription
right, or (b) any dilutive issuance.
On July 13, 2022, the Company entered into a commitment with Stimunity
to provide €600,000 under a convertible note (the “Stimunity Convertible Note”) with a maturity date of September 1,
2023 (the “Maturity Date”). The Stimunity Convertible Note provides for simple interest at 7% per annum. The Stimunity Convertible
Note is automatically converted into Series A shares of Stimunity upon Stimunity completing a Series A round for at least €20 million.
If such subscription round is completed prior to the Maturity Date, the Company will be entitled to convert the Stimunity Convertible
Note into Series A shares of Stimunity at the subscription share price less 15%. Additionally, if Stimunity completes a financing with
a new category of shares (other than Common Shares or Series A shares) for at least €5 million (the “Minimum Raise”),
the Company will have the right to convert the Stimunity Convertible Note and the historical Series A shares of Stimunity owned into the
new category of shares. In the event that Stimunity does not close a financing prior to the Maturity Date or raises less than the Minimum
Raise, the Company will have the right to convert the Stimunity Convertible Note into Series A shares at €363.00 per share or the
raise price less 15%, whichever is lower. The Stimunity Convertible Note was funded on September 12, 2022. See Note 15, “Commitments
and Contingent Liabilities – Stimunity Convertible Note,” for a further discussion.
The Stimunity Convertible Note was initially recorded at $0.614 million
to record the translated value of the Stimunity Convertible Note on September 12, 2022. The Company recognized an unrealized gain of $0.039
million through other comprehensive income (“OCI”) in Fiscal 2023 to reflect the change in translation rate for the Stimunity
Convertible Note settleable in euros, increasing the carrying value of the Stimunity Convertible Note to $0.653 million.
As of March 31, 2023, the Company determined that there were indications
of impairment of both the investment in associate and the Stimunity Convertible Note receivable, based upon the inability of Stimunity
to obtain financing. The Company performed an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current
market conditions, the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity. The Company
recorded provisions of impairment of $0.607 million and $0.211 million, with respect to the investment in associate and the Stimunity
Convertible Note receivable, respectively, decreasing the carrying value of the investment in associate and the Stimunity Convertible
Note to $0.806 million and $0.442 million, respectively, as of March 31, 2023.
During the three months ended June 30, 2023, the Company recorded a
loss of $0.05
million to recognize its share of Stimunity’s results of operations and recorded a marginal gain through OCI to reflect the
translation rate effect on the Stimunity Convertible Note settleable in euros. The Company determined that there were no
significant changes in the market or Stimunity’s outlook from March 31, 2023 to June 30, 2023, and, accordingly, determined that
the carrying value at June 30, 2023 was not further impaired.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInvestmentInAssociateAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_InvestmentInAssociateDisclosureTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INVESTMENT IN PUBLIC COMPANY
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Investment In Public Company |
|
| INVESTMENT IN PUBLIC COMPANY |
NOTE 7. INVESTMENT IN PUBLIC COMPANY
The following is a discussion of the Company’s investment in Intensity
Therapeutics, Inc. (“Intensity”) as of June 30, 2023 and March 31, 2023.
Intensity Therapeutics, Inc.
In connection with the SalvaRx Acquisition in fiscal 2019, the Company
acquired a $4.5 million interest in Intensity, a private clinical stage biotechnology company, of 1.0 million shares, which represented
a 7.5% equity interest in Intensity. The investment was recorded at fair value (which approximates cost) at the acquisition date. The
investment in Intensity has been irrevocably designated as a financial asset recorded at fair value with gains and losses recorded through
OCI. Upon Intensity’s IPO discussed below, effective June 30, 2023, the fair value of the asset is determined by quoted market price.
On July 11, 2019, the Company entered into an agreement with Fast Forward
Innovations Limited (“Fast Forward”) to purchase Intensity Holdings Limited (“IHL”), a wholly-owned subsidiary
of Fast Forward. The Company paid $1.3 million for IHL through the issuance of 129,806 ordinary shares of the Company. The sole asset
of IHL consists of 288,458 shares of Intensity. This transaction increased the Company’s ownership of Intensity to 1,288,458 shares.
On October 28, 2021, Intensity filed a Form S-1 Registration Statement
with the SEC to register shares for an initial public offering (“IPO”), which was declared effective by the SEC, but subsequently
withdrawn prior to closing.
In October and November of 2022, Intensity filed amendments to its Form
S-1 Registration Statement, which reflected a proposed offering price in the range of $4.00 - $5.00 per share, which is less than the
Company’s carrying value, which was an external indication of impairment. Accordingly, the Company performed an IAS 36, “Impairment
of Assets,” fair value analysis and determined a fair value of $3.363 million, which was $4.046 million less than the then carrying
value at December 31, 2022. Intensity continued to seek a successful IPO during the fourth quarter of fiscal year ended March 31, 2023.
At March 31, 2023, the Company undertook an IAS 36 fair value analysis based on the continued existence of external indications of impairment.
The analysis included evaluating the likelihood of a successful IPO and the timing of such an event, as well as the then lack of marketability
of the shares and the continued uncertainty surrounding an IPO, or any type of financing.
In April 2023, Intensity completed a 1:2 reverse stock split, which
reduced the Company’s holdings to 644,229
shares. As the offering was priced at $4.00
to $5.00
per share, the Company determined the fair value of its interest to be $2.087
million. In total, the Company recorded an unrealized loss of $5.322
million with respect to Intensity for the fiscal year ended March 31, 2023, which was recognized through OCI, reducing the
Company’s carrying value in Intensity to $2.087 million as of March 31, 2023.
On July 5, 2023, Intensity completed an IPO of its common stock
selling 3,900,000
shares at a price of $5.00
per share generating net proceeds of approximately $16.2
million. In connection with the offering, Intensity’s common stock began trading on Nasdaq on June 30, 2023, under the ticker
symbol “INTS.” The Intensity shares closed at a price of $5.96
on June 30, 2023. The Company received an additional 2,659
shares in connection with the offering pursuant to certain anti-dilution rights. Intensity sold its overallotment shares totaling 585,000
shares, which closed on July 7, 2023. At that date, the Company owned approximately 4.7%
of the issued and outstanding shares of Intensity. The Company recorded unrealized gain of $1.768
million through OCI to reflect the difference between the market value of the Company’s ownership interest and its then
carrying value, increasing the Company’s carrying value in Intensity to $3.855
million as of June 30, 2023. There was no
unrealized gain or loss with respect to the Company’s investment in Intensity during the three months ended June 30, 2022.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInvestmentInPublicCompanyAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfPublicCompaniesExplanatoryTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
LEASE
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| LEASE |
NOTE 8. LEASE
The Company entered into a lease of office space, which commenced on May
1, 2023. The lease provides for an original term of two years with an option to renew the lease for an additional term of three years.
The Company has included the extension option in the lease analysis under IFRS 16, based upon management’s intentions. The Company
calculated the lease liability using its incremental borrowing rate of 13%. The Company provided a $0.013 million security deposit. The
lease liability is payable as follows (in thousands):
| Schedule of lease
liability | |
| | |
| Twelve Months Ended June 30, | |
Amount |
| 2024 | |
$ | 79 | |
| 2025 | |
| 81 | |
| 2026 | |
| 83 | |
| 2027 | |
| 84 | |
| 2028 | |
| 71 | |
| Total | |
| 398 | |
| Less: interest | |
| (102 | ) |
| Total lease liability | |
| 296 | |
| Lease liability - current | |
| 47 | |
| Lease liability - non-current | |
$ | 249 | |
|
| X |
- DefinitionThe entire disclosure for leases. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Section Presentation
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&doctype=Standard&dita_xref=IFRS16_g47-50_TI
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Section Disclosure
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&doctype=Standard&dita_xref=IFRS16_g51-60_TI
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfLeasesExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
ACQUISITION OF TARUS
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Acquisition Of Tarus |
|
| ACQUISITION OF TARUS |
NOTE 9. ACQUISITION OF TARUS
On July 1, 2022, the Company, its wholly-owned subsidiary, Portage Merger
Sub I, Inc., its wholly-owned subsidiary, Portage Merger Sub II, LLC and Tarus Therapeutics, Inc., a Delaware corporation advancing adenosine
receptor antagonists for the treatment of solid tumors, entered into an Agreement and Plan of Merger and Reorganization (the “Merger
Agreement”). Under the structure of the Merger Agreement, Tarus Therapeutics, Inc. was ultimately merged into Portage Merger Sub
II, LLC of the Company with the surviving entity renamed Tarus Therapeutics, LLC. The Tarus merger entitles the Company to the rights,
know-how and/or ownership related to the assets developed by Tarus (the “Adenosine Compounds”), including:
| 1. | All rights and obligations related to the License Agreement between Tarus and Impetis Biosciences Limited, dated October 29, 2019
(“Tarus License Agreement”), and the Call Option under the Tarus License Agreement, which was exercised on November 5, 2020; |
| 2. | All intellectual property and related documents owned or controlled by Tarus, including issued or pending patents, patent applications
and trade secrets. Additionally, any draft submissions and/or correspondence with patent authorities; |
| 3. | All documents and supplies related to Adenosine Compounds (as defined in the Tarus License Agreement) including inventory, reagents,
data, assays, reports, vendor agreements and other information related to the preclinical development; |
| 4. | All clinical supplies, manufacturing know-how, batch records, regulatory documents pertaining to the Adenosine
Compounds, certain reservations for manufacturing campaigns and any related agreements; |
| 5. | All regulatory documents and correspondence pertaining to the Adenosine Compounds; |
| 6. | All Contract Research Organization (“CRO”) agreements and protocol related documents for Adenosine
Compounds; |
| 7. | All current documents related to market research, forecasting, budgets and competitive intelligence; and |
| 8. | Rights to the use of Tarus Therapeutics’ name for regulatory purposes. |
As consideration for Tarus, the Company issued to former
Tarus shareholders an aggregate of 2,425,999 ordinary shares of Portage, calculated on the basis of $18 million divided by the 60-day
volume weighted average price per share of ordinary shares of Portage. Such ordinary shares have not been registered with the SEC and
were subject to lock-up agreements for terms ranging from six to twelve months, which expired on February 1, 2023 and July 1, 2023, respectively.
Additionally, the ordinary shares that were subject to a twelve month lock-up period, are also subject to a three month dribble-out period,
which commenced July 1, 2023. During the dribble out period, each holder may not sell more than 10% of the average trading volume of the
Company’s ordinary shares for the rolling three month period prior to the date on which the holder executes a trade of the Company’s
ordinary shares without its prior written consent (which the Company is permitted to withhold at its sole discretion). Additionally, milestone
payments of up to $32 million in cash or Portage ordinary shares (at the discretion of the Company) would be triggered upon achievement
of future development and sales milestones, as described below. As a result of the transaction:
| · | The Company also assumed $2 million in short-term debt held by Tarus and deferred license milestones obligations
($1 million plus interest). The short-term debt was repaid by the Company in July 2022. |
| · | Upon enrolling the first patient in a Phase 2 clinical trial utilizing Tarus’s adenosine receptor antagonists,
the Company will pay an additional one-time milestone payment of $15 million to the former Tarus shareholders. Payment will be in the
form of cash or Portage ordinary shares (at the discretion of the Company). The remaining $17 million milestone is based on targeted commercial
sales. |
In connection with the acquisition of Tarus, the Company performed a fair
value analysis of the assets acquired and liabilities assumed. The Company based the analysis on its clinical plan and timing of development
events, and the probabilities of success determined primarily based upon empirical third party data and Company experience as well as
the relevant cost of capital. In its fair value analysis, the Company used the Multi-Period Excess Earnings Method for PORT-6 and PORT-7
and the Replacement Cost Method for PORT-8 and PORT-9, determined based upon the maturity of the assets and the availability of sufficient
data to measure fair value. The Company recorded the ordinary shares issued at $17.2 million, which represented the aggregate market value
of the ordinary shares issued on July 1, 2022. The Company followed the guidance of IAS 3 and IAS 32 and recorded a deferred purchase
price payable - Tarus of $8.538 million, which reflected the estimated acquisition date fair value of contractual milestone obligations
incurred. The principal assumptions for determining the fair value include the timing of development events, the probabilities of success
and the discount rate used. The Company recorded the obligation as a non-current liability, in accordance with the provisions IAS 32 with
respect to the classification of financial assets and financial liabilities.
The Company will determine the fair value of the shares issuable upon
achievement of future development and sales milestones at each balance sheet date. Any change to the fair value will be recorded in the
Company’s statements of operations and other comprehensive income (loss).
The following table summarizes the purchase price allocation to the fair
value of assets acquired and liabilities assumed for Tarus:
| Schedule of fair
value of assets acquired and liabilities assumed | |
| | |
| Assets: | |
(In thousands) |
| Identifiable intangible assets | |
$ | 28,200 | |
| Goodwill | |
| 538 | |
| Total assets | |
$ | 28,738 | |
| Consideration: | |
| | |
| Fair value of shares issued | |
$ | 17,200 | |
| Liabilities assumed | |
| 3,000 | |
| Deferred purchase consideration at fair value | |
| 8,538 | |
| Total liabilities | |
$ | 28,738 | |
Pro forma Information
Summary unaudited pro forma condensed
results of operations for the three months ended June 30, 2022, assuming the Tarus acquisition had occurred at the beginning of the earliest
period presented, are as follows:
| Schedule of Pro forma Information | |
| | |
| (In thousands) | |
Three Months Ended
June 30, 2022 |
| Loss from operations | |
$ | (3,788 | ) |
| Loss before provision for income taxes | |
$ | (3,893 | ) |
| Net loss | |
$ | (1,341 | ) |
| Total comprehensive loss for period | |
$ | (1,341 | ) |
| Loss per share | |
$ | (0.09 | ) |
These pro forma results are not
necessarily indicative of what would have occurred if the acquisition had been in effect for the period presented, and they may not be
indicative of results expected in the future.
|
| X |
- References
+ Details
| Name: |
ptgef_AcquisitionsOfTarusExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureAcquisitionOfTarusAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| In-process Research And Development And Deferred Tax Liability |
|
| IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY |
NOTE 10. IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY
In-process research and development (“IPR&D”) consists
of the following projects (in thousands):
| Schedule of in-process research and development | |
| |
| | | |
| | |
| | |
| |
Value as of |
| Project # | |
Description | |
June 30, 2023 | |
March 31, 2023 |
| iOx: | |
| |
| | | |
| | |
| PORT 2 (IMM60) | |
Melanoma & Lung Cancers | |
$ | 36,181 | | |
$ | 36,181 | |
| PORT 3 (IMM65) | |
Ovarian/Prostate Cancers | |
| 21,709 | | |
| 21,709 | |
| | |
| |
| 57,890 | | |
| 57,890 | |
| | |
| |
| | | |
| | |
| Oncomer/Saugatuck | |
DNA Aptamers | |
| 178 | | |
| 178 | |
| | |
| |
| | | |
| | |
| Tarus: | |
| |
| | | |
| | |
| PORT 6 & PORT 7 | |
Adenosine Receptors | |
| 22,723 | | |
| 22,723 | |
| PORT 8 | |
Adenosine Receptors | |
| 420 | | |
| 420 | |
| PORT 9 | |
Adenosine Receptors | |
| 472 | | |
| 472 | |
| | |
| |
| 23,615 | | |
| 23,615 | |
| | |
| |
| | | |
| | |
| In-process research and development | |
| |
$ | 81,683 | | |
$ | 81,683 | |
| | |
| |
| | | |
| | |
| Deferred tax liability | |
| |
$ | 13,510 | | |
$ | 13,195 | |
At the end of each reporting period, the Company is required to assess
whether there is any indication that an asset may be impaired. Pursuant to IAS 36, the Company evaluated the then-current capital markets,
the increasing costs of capital, and the delays in the timing of asset development and concluded that provisions for impairment were required
during the year ended March 31, 2023 with respect to the iOx IPR&D and the Tarus IPR&D. The Company recognized an impairment of
$59.320 million with respect to the iOx assets, reducing the Company’s carrying value from $117.210 million to $57.890 million and
an impairment of $4.585 million with respect to the Tarus assets, reducing the Company’s carrying value from $28.2 million to $23.615
million. The deferred tax liability in the U.K. was reduced as a result of the IPR&D impairment loss recognized by iOx for financial
statement purposes.
Deferred tax liability represents iOx’s estimated tax on the difference
between book and tax basis of the IPR&D, which is taxable in the U.K, and the effect of usable net operating loss carryforwards.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInprocessResearchAndDevelopmentAndDeferredTaxLiabilityAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_InProcessResearchAndDevelopmentAndDeferredTaxLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INCOME TAXES
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| INCOME TAXES |
NOTE 11. INCOME TAXES
The Company is a BVI business company. The BVI government does not, under
existing legislation, impose any income or corporate tax on corporations.
PDS is a U.S. corporation and is subject to U.S. federal, state and local
income taxes, as applicable.
iOx is subject to U.K. taxes.
The (expense) benefit from income taxes consists of the following for
the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
| Schedule of income taxes benefit | |
| | | |
| | |
| | |
For the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Current: | |
| | | |
| | |
| Federal | |
$ | (3 | ) | |
$ | - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| - | | |
| - | |
| Total current | |
| (3 | ) | |
| - | |
| | |
| | | |
| | |
| Deferred: | |
| | | |
| | |
| Federal | |
| - | | |
| - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| 148 | | |
| 2,552 | |
| Total deferred | |
| 148 | | |
| 2,552 | |
| Benefit from income taxes | |
$ | 145 | | |
$ | 2,552 | |
The following is a reconciliation of the U.S. taxes to the effective income
tax rates for the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
| Schedule of reconciliation income tax rates | |
| |
|
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (Loss) income on ordinary activities before tax | |
$ | (605 | ) | |
$ | 34 | |
| Statutory U.S. income tax rate | |
| 21.0 | % | |
| 21.0 | % |
| Income tax benefit (expense) at statutory income tax rate | |
| 127 | | |
| (7 | ) |
| Share-based compensation expense recognized for financial statement purposes | |
| (142 | ) | |
| - | |
| Other losses recognized | |
| - | | |
| 7 | |
| Utilization of losses not previously benefitted | |
| 12 | | |
| - | |
| Income tax (expense) | |
$ | (3 | ) | |
$ | - | |
As of June 30, 2023, the Company had $0.6 million of federal net operating
losses, which carryforward indefinitely but are limited to 80% of taxable income when utilized and $0.4 million of items deducted for
financial statements but not tax, excluding share-based compensation. As of each of June 30, 2023 and March 31, 2023, the Company had
U.S. deferred tax assets of $0.2 million.
The following is a reconciliation of the U.K. taxes to the effective income
tax rates for the three months ended June 30, 2023 and 2022 (U.S. Dollars in thousands):
Research and development credit
receivables of $0.2 million were included in prepaid expenses and other receivables on the condensed consolidated interim statements of
financial position as of each of June 30, 2023 and March 31, 2023. The receivable was collected in July 2023.
The following is a reconciliation of financial statement income (loss)
to tax basis income (loss) (in thousands):
| Schedule of reconciliation of financial statement loss to tax basis loss | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended |
| | |
June 30, 2023 | |
June 30, 2022 |
| | |
United
States | |
BVI | |
United
Kingdom | |
Total | |
United
States | |
BVI | |
Foreign | |
Total |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Pre-tax loss | |
$ | (609 | ) | |
$ | (3,886 | ) | |
$ | (1,576 | ) | |
$ | (6,071 | ) | |
$ | 34 | | |
$ | (2,468 | ) | |
$ | (1,743 | ) | |
$ | (4,177 | ) |
| Share-based compensation expense for financial statement purposes for which no benefit was taken | |
| 683 | | |
| - | | |
| - | | |
| 683 | | |
| - | | |
| - | | |
| - | | |
| - | |
| Losses not subject to tax | |
| - | | |
| 3,886 | | |
| - | | |
| 3,886 | | |
| - | | |
| 2,468 | | |
| - | | |
| 2,468 | |
| Utilization of losses not previously benefitted | |
| (59 | ) | |
| - | | |
| - | | |
| (59 | ) | |
| (34 | ) | |
| - | | |
| - | | |
| (34 | ) |
| Taxable income (loss) | |
$ | 15 | | |
$ | - | | |
$ | (1,576 | ) | |
$ | (1,561 | ) | |
$ | - | | |
$ | - | | |
$ | (1,743 | ) | |
$ | (1,743 | ) |
As of June 30, 2023 and March 31,
2023, the Company’s deferred tax assets and liabilities in the U.K. consisted of the effects of temporary differences attributable
to the following (U.S. Dollars in thousands):
| Schedule of deferred tax assets and liabilities | |
| | | |
| | |
| | |
As of June 30, | |
As of March 31, |
| | |
2023 | |
2023 |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss | |
$ | (4,594 | ) | |
$ | (4,131 | ) |
| Deferred tax asset (unrecognized) | |
| 1,500 | | |
| 1,500 | |
| Deferred tax asset | |
| (3,094 | ) | |
| (2,631 | ) |
| | |
| | | |
| | |
| Deferred tax liabilities: | |
| | | |
| | |
| In-process research and development | |
| 13,510 | | |
| 13,195 | |
| Deferred tax liability | |
| 13,510 | | |
| 13,195 | |
| | |
| | | |
| | |
| Net deferred tax liability | |
$ | 10,416 | | |
$ | 10,564 | |
iOx generated no research and development cash credits recorded for the
three months ended June 30, 2023.
As of June 30, 2023 and March 31, 2023, iOx had a net deferred tax liability
of approximately $10.4 million and approximately $10.6 million, respectively. On January 8, 2019, the Company originally recognized a
$19.8 million deferred tax liability, reflecting the then prevailing U.K. tax rate of 17% on the difference between the book and income
tax basis of IPR&D acquired as part of the SalvaRx Acquisition. In the fiscal 2022, the Company recorded a $7.0 million increase in
deferred income taxes to reflect a future change in the U.K. income tax rate to 25% effective April 1, 2023 and recognized $0.7 million
of current year losses and $0.8 million of prior year losses. The Company also recognized a $1.1 million decrease in deferred tax liability
in fiscal 2022 to reflect the effect of the change in exchange rates on the liability settleable in British pound sterling. For the year
ended March 31, 2023, the Company recognized an aggregate reduction in net deferred tax liability of $17.9 million, comprised of $11.3
million to recognized the deferred tax effect of loss on impairment recognized with respect to the iOx IPR&D, $0.7 million related
to other current year losses, $3.8 million to reflect the change related to the future U.K. tax rates and $2.1 million to reflect the
effect of the change in exchange rates on the liability settleable in British pound sterling. For the three months ended June 30, 2023,
the Company recognized a net decrease in the deferred tax liability of $0.1 million comprised of $0.3 million to reflect the effect of
the change in exchange rates on the liability in the period and recognized $0.4 million of current period losses.
There is no expiration date for accumulated tax losses in the U.K. entities.
|
v3.23.2
CAPITAL STOCK
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Capital Stock |
|
| CAPITAL STOCK |
NOTE 12. CAPITAL STOCK
| (a) | Authorized ordinary shares: Unlimited number of Portage ordinary shares without par value. |
| (b) | The following is a roll-forward of Portage ordinary shares for the years ended June 30, 2023 and 2022: |
| Schedule of unlimited number
of common shares without par value | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| | |
Ordinary
Shares | |
Amount | |
Ordinary
Shares | |
Amount |
| | |
In 000’ | |
In 000’$ | |
In 000’ | |
In 000’$ |
| Balance, beginning of period | |
| 17,606 | | |
$ | 218,782 | | |
| 13,349 | | |
$ | 158,324 | |
| Shares issued under public offering and ATM, net of issue costs | |
| 171 | | |
| 613 | | |
| - | | |
| - | |
| Shares issued or accrued for services | |
| 9 | | |
| 30 | | |
| 4 | | |
| 30 | |
| Balance, end of period | |
| 17,786 | | |
$ | 219,425 | | |
| 13,353 | | |
$ | 158,354 | |
Portage filed a shelf registration statement with the SEC under which
it may sell ordinary shares, debt securities, warrants and units in one or more offerings from time to time, which became effective on
March 8, 2021 (“Registration Statement”). In connection with the Registration Statement, Portage has filed with the SEC:
| · | a base prospectus, which covers the offering, issuance and sale by Portage of up to $200,000,000 in the aggregate of the securities
identified above from time to time in one or more offerings; |
| · | a prospectus supplement, which covers the offer, issuance and sale by Portage in an “at-the-market” (“ATM”)
offering of up to a maximum aggregate offering price of $50,000,000 of Portage’s ordinary shares that may be issued and sold from
time to time under a Controlled Equity Offering Sales Agreement, dated February 24, 2021 (the “Sales Agreement”), with Cantor
Fitzgerald & Co., the sales agent (“Cantor Fitzgerald”); |
| · | a prospectus supplement dated June 24, 2021, for the offer, issuance and sale by Portage of 1,150,000 ordinary shares for gross proceeds
of approximately $26.5 million in a firm commitment underwritten public offering with Cantor Fitzgerald; and |
| · | a prospectus supplement dated August 19, 2022, for the resale of up to $30,000,000 in ordinary shares that Portage may sell from time
to time to Lincoln Park Capital Fund, LLC (“Lincoln”) and an additional 94,508 shares that were issued to Lincoln. |
The Sales Agreement permits the Company to sell in an ATM program up to
$50,000,000 of ordinary shares from time to time, the amount of which is included in the $200,000,000 of securities that may be offered,
issued and sold by the Company under the base prospectus. The sales under the prospectus will be deemed to be made pursuant to an ATM
program as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended. Upon termination of the Sales Agreement,
any portion of the $50,000,000 included in the Sales Agreement prospectus that is not sold pursuant to the Sales Agreement will be available
for sale in other offerings pursuant to the base prospectus.
During Fiscal 2022, the Company sold 90,888 ordinary shares under the
ATM program, generating gross proceeds of approximately $2.6 million ($2.5 million, net of commissions).
The Company has issued 2,425,999 ordinary shares in connection with the
acquisition of Tarus Therapeutics, Inc. and in connection with the Tarus Therapeutics, Inc.’s acquisition it may issue additional
ordinary shares. See Note 9, “Acquisition of Tarus,” for a further discussion.
On July 18, 2022, the Company entered into the iOx Share Exchange Agreement
under which it exchanged 1,070,000 ordinary shares of the Company for the remaining minority interest of 21.68% of iOx. See
Note 16, “Related Party Transactions – Share Exchange Agreement – iOx,” for a further discussion.
On July 6, 2022, the Company entered into a Purchase Agreement (the “Committed
Purchase Agreement”) with Lincoln, under which it may require Lincoln to purchase ordinary shares of the Company having an aggregate
value of up to $30 million (the “Purchase Shares”) over a period of 36 months. Upon execution of the Committed Purchase Agreement,
the Company issued to Lincoln 94,508 ordinary shares, representing a 3% commitment fee. Pursuant to the Committed Purchase Agreement,
Lincoln will be obligated to purchase the Purchase Shares in three different scenarios that are based on various market criteria and share
amounts. The Company has the right to terminate the Committed Purchase Agreement for any reason, effective upon one business day prior
written notice to Lincoln. Lincoln has no right to terminate the Committed Purchase Agreement. The requirement that Lincoln must make
a purchase will be suspended based on various criteria such as there not being an effective registration statement for Lincoln to be able
to resell the ordinary shares it is committed to purchase and market criteria such as the Company continuing to be Depository Trust Company
eligible, among other things. The Committed Purchase Agreement does not impose any financial or business covenants on the Company, and
there are no limitations on the use of proceeds. The Company may raise capital from other sources in its sole discretion; provided, however,
that the Company shall not enter into any similar agreement for the issuance of variable priced equity-like securities until the three-year
anniversary of the date of the Committed Purchase Agreement, excluding, however, an ATM transaction with a registered broker-dealer, which
includes any sales under the Sales Agreement with Cantor Fitzgerald.
As discussed in Note 2, “Going
Concern,” the Company’s access to the ATM program and the Committed Purchase Agreement is generally limited based on the Company’s
trading volume on Nasdaq. See Note 15, “Commitments and Contingent Liabilities – Committed Purchase Agreement,”
for a further discussion.
In Fiscal 2023, the Company sold 166,145 ordinary shares under the ATM
program, generating net proceeds of approximately $0.9 million. Separately, in Fiscal 2023, the Company sold 480,000 ordinary shares to
Lincoln under the Committed Purchase Agreement for net proceeds totaling approximately $2.0 million. From April 1, 2023 through June 30,
2023, the Company sold 171,504 ordinary shares under the ATM program, generating net proceeds of approximately $0.6 million.
|
| X |
- References
+ Details
| Name: |
ptgef_CapitalStockTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureCapitalStockAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
STOCK OPTION RESERVE
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| STOCK OPTION RESERVE |
NOTE 13. STOCK OPTION RESERVE
| (a) | The following table provides the activity for the Company’s stock option reserve for the three months ended June 30, 2023 and
2022: |
| Schedule
of terms stock option reserve explanatory | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (In thousands) | |
Non-Controlling Interest | |
Stock Option Reserve | |
Non-Controlling Interest | |
Stock Option Reserve |
| Balance, beginning of period | |
$ | - | | |
$ | 21,204 | | |
$ | 11,659 | | |
$ | 16,928 | |
| Share-based compensation expense | |
| - | | |
| 769 | | |
| - | | |
| 1,176 | |
| Balance, end of period | |
$ | - | | |
$ | 21,973 | | |
$ | 11,659 | | |
$ | 18,104 | |
Stock Options
On June 25, 2020, at the annual meeting of shareholders, the Company’s
incentive stock option plan (the “2020 Stock Option Plan”) was approved, which authorized the Company’s directors to
fix the option exercise price and to issue stock options under the plan as appropriate. The Company’s 2020 Stock Option Plan was
a 10% rolling stock option plan under which the Company’s directors were authorized to grant up to a maximum of 10% of the issued
and outstanding ordinary shares on the date of grant.
Effective January 13, 2021, the
Company amended and restated its 2020 Stock Option Plan to permit the grant of additional types of equity compensation securities, including
restricted stock units (“RSUs”) and dividend equivalent rights (the “2021 Equity Incentive Plan”). The aggregate
number of equity securities, which may be issued under the 2021 Equity Incentive Plan has not been changed. Pursuant to the 2021
Equity Incentive Plan, on January 13, 2021, the Company granted an aggregate of 868,000 stock options exercisable at a price of $17.75
per share, representing the closing price of the shares on the day immediately preceding the grant date, which expire on January 13, 2031
to various directors, officers and consultants of the Company. 350,000 options granted to members of the Board vest 1/3 on grant date,
1/3 on the first anniversary of the grant and 1/3 on the second anniversary of the grant. 518,000 options granted to consultants (one
of whom is also a director of the Company) vest 1/3 on each of the first three anniversaries of the grant date.
Additionally, the Company granted
243,000 RSUs on January 13, 2021, with a fair value of $17.75 per share, which was the closing price on the day immediately preceding
the grant date. The RSUs vested on the date of grant, but underlying shares cannot be sold until one of four of the following conditions
are met: (1) a Change in Control (as defined in the Amended and Restated 2021 Equity Incentive Plan (defined below)), (2) the participant’s
Separation from Service (as defined in the Amended and Restated 2021 Equity Incentive Plan), (3) the participant’s death, or (4)
the participant’s Disability (as defined in the Amended and Restated 2021 Equity Incentive Plan). In accordance with IFRS
2, “Share-based Payment,” the Company recognized compensation expense of $4.3 million in the year ended March 31, 2021, in
connection with the RSU grants.
Amended and Restated 2021 Equity Incentive Plan and Grants of Stock Options and Restricted
Stock Units
On January 19, 2022, the Board unanimously approved the Amended and Restated
2021 Equity Incentive Plan (the “Amended and Restated 2021 Equity Incentive Plan”). The Amended and Restated 2021 Equity Incentive
Plan provides for:
| (1) | An increase of aggregate number of ordinary shares available for awards to 2,001,812, which is equal to 15% of the issued and outstanding
ordinary shares of the Company as of January 19, 2022 subject to discretionary annual increases (on a cumulative basis) as may be approved
by the Board in future years by a number of ordinary shares not to exceed an additional 5% of the aggregate number of shares then outstanding; |
| (2) | The authorization of incentive stock options under the Amended and Restated 2021 Equity Incentive Plan; and |
| (3) | The provision of dividend equivalent rights to be issued when authorized. |
Pursuant to the Amended and Restated 2021 Equity Incentive Plan, on January
19, 2022, the Company granted an aggregate of 302,000 stock options exercisable at a price of $10.22 per share, representing the average
price of the Company’s ordinary shares on the grant date, which expire on January 19, 2032, to various directors, officers and consultants
of the Company. A total of 13,800 of the 302,000 stock options were granted to two members of the Board and vest on the first anniversary
of the grant date. The balance of 288,200 stock options was granted to employees (one of whom is also a director of the Company), and
a consultant, and such stock options vest ratably on each of the first four annual anniversaries of the grant date.
Additionally, the Company granted
135,740 RSUs to employees (one of whom is also a director of the Company) on January 19, 2022, with a fair value of $10.22 per share,
representing the average price of the shares on the grant date. The RSUs were fully vested and nonforfeitable as of the grant
date and will expire on January 19, 2032.
On February 15, 2022, James Mellon, Linda Kozick and Mark Simon were appointed
to the Board. Mr. Mellon owned approximately 23.9% of the Company’s outstanding shares at that date. Additionally, Mr. Mellon had
previously served as a member of the Board from 2016 to August 14, 2020. On February 15, 2022, in connection with the appointments, each
of these directors was granted 13,800 non-qualified stock options, which vest ratably monthly over a three-year period. The options have
an exercise price of $8.59 per share, representing the average price of the shares on the grant date, and will expire, if unexercised,
on February 15, 2032.
On June 8, 2022, the Company granted 50,000 options to purchase shares
to an executive of the Company. The options have an exercise price of $11.00, the average price of the stock on that date, vest ratably
on each of the first four anniversaries of the grant date and will expire, if unexercised, on June 8, 2032.
On July 27, 2022, the Company granted 15,900 options to purchase shares
to a member of the Board. The options have an exercise price of $10.06, the average price of the stock on that date, vest ratably on each
monthly anniversary of the grant date in the three year period following the grant date and will expire, if unexercised, on July 27, 2032.
On March 30, 2023, the Board unanimously approved to increase the maximum
number of ordinary shares reserved for issuance under the Amended and Restated 2021 Equity Incentive Plan. The aggregate number of shares
available for awards under the Amended and Restated 2021 Equity Incentive Plan was increased to 2,880,992, which represented a 5% increase
(or 879,180 shares) based on ordinary shares outstanding on March 29, 2023, which is equal to 16% of the issued and outstanding ordinary
shares in the capital of the Company as of this date.
On March 30, 2023, the Company granted an aggregate of 746,120 stock options
exercisable at a price of $2.92 per share, representing the average price of the shares on the grant date, which expire on March 30, 2033,
to various directors, officers and a consultant of the Company. 14,600 options to purchase ordinary shares, totaling 87,600, were granted
to each non-executive member of the Board and vest on the first anniversary of the grant date. A total of 651,020 stock options were granted
to employees (including Mr. Walters, who is Chairman of the Board of Directors), and a consultant, and such stock options vest ratably
on each of the first four annual anniversaries of the grant date. The balance of 7,500 stock options were also granted to a consultant,
which was fully vested as of the grant date.
| (b) | The changes in the number of options issued for the three months ended June 30, 2023 and 2022 were: |
| Schedule of outstanding stock options | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Balance, beginning of period | |
| 1,963,420 | | |
| 1,151,400 | | |
| - | | |
| 1,275 | |
| Granted | |
| - | | |
| 50,000 | | |
| - | | |
| - | |
| Expired or forfeited | |
| - | | |
| - | | |
| - | | |
| (1,275 | ) |
| Balance, end of year | |
| 1,963,420 | | |
| 1,201,400 | | |
| - | | |
| - | |
| Exercisable, end of period | |
| 764,438 | | |
| 410,600 | | |
| - | | |
| - | |
| (c) | The following is the weighted average exercise price and the remaining contractual life for outstanding options by plan as of June
30, 2023 and 2022: |
| Schedule of weighted average exercise price and remaining contractual life | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Weighted average exercise price | |
$ | 10.53 | | |
| 15.26 | | |
$ | - | | |
$ | - | |
| Weighted average remaining contractual life (in years) | |
| 8.61 | | |
| 8.90 | | |
| - | | |
| - | |
The vested options can be exercised at any time in accordance with the
applicable option agreement. The exercise price was greater than the market price for all options outstanding as of June 30, 2023 and
March 31, 2023, except 7,500 vested options and 738,620 unvested options as of each date.
The Company recorded approximately $0.8 million and $1.2 million of share-based
compensation expense with respect to the Amended and Restated 2021 Equity Incentive Plan in the three months ended June 30, 2023 and 2022,
respectively. The Company expects to record additional share-based compensation expense of approximately $3.4 million through March 2027
with respect to the Amended and Restated 2021 Equity Incentive Plan.
As of June 30, 2022, the Company’s iOx stock option plan was fully
vested. The iOx stock option plan expired on May 5, 2022 and all outstanding stock option awards issued under the iOx stock option plan
expired.
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 44
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_44&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfSharebasedPaymentArrangementsExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
(LOSS) PER SHARE
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Profit or loss [abstract] |
|
| (LOSS) PER SHARE |
NOTE 14. (LOSS) PER SHARE
Basic earnings per share (“EPS”) is calculated by dividing
the net income (loss) attributable to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding
during the period.
Diluted EPS is calculated by dividing the net income (loss) attributable
to ordinary equity holders of the Company by the weighted average number of ordinary shares outstanding during the period plus the weighted
average number of ordinary shares that would be issued on conversion of all the dilutive potential ordinary shares into ordinary shares.
The following table reflects the loss and share data used in the basic
and diluted EPS calculations (U.S. Dollars in thousands, except per share amounts):
| Schedule of basic and diluted EPS calculations | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| Numerator (in 000’$) | |
2023 | |
2022 |
| | |
| |
|
| Net loss attributable to owners of the Company | |
$ | (5,919 | ) | |
$ | (1,729 | ) |
| Denominator (in 000’) | |
| | | |
| | |
| Weighted average number of shares – Basic and Diluted | |
| 17,701 | | |
| 13,351 | |
| Basic and diluted (loss) per share | |
$ | (0.33 | ) | |
$ | (0.13 | ) |
The inclusion of the Company’s stock options, RSUs and share purchase
warrants in the computation of diluted loss per share would have an anti-dilutive effect on loss per share and are therefore excluded
from the computation. Consequently, there is no difference between basic loss per share and diluted loss per share for the three months
ended June 30, 2023 and 2022. The following table reflects the Company's outstanding securities by year that would have an anti-dilutive
effect on loss per share and, accordingly, were excluded from the calculation.
| Schedule of anti-dilutive share | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 |
| Stock options | |
| 1,963,420 | | |
| 1,201,400 | |
| Restricted stock units | |
| 378,740 | | |
| 378,740 | |
|
| X |
- DefinitionThe disclosure of earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_EarningsPerShareExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_IncomeStatementAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
COMMITMENTS AND CONTINGENT LIABILITIES
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| COMMITMENTS AND CONTINGENT LIABILITIES |
NOTE 15. COMMITMENTS AND CONTINGENT LIABILITIES
Effective March 15, 2022, iOx entered into a Master Services Agreement
(the “MSA”) with Parexel International (IRE) Limited (“Parexel”) under which Parexel agreed to act as clinical
service provider (CRO) pursuant to a work order (“Work Order”) effective June 1, 2022. Pursuant to such Work Order, Parexel
will operate a Phase 2 trial of IMM60 and pembrolizumab in advanced melanoma and non-small lung cancer (“NSCLC”). The MSA
provides for a five-year term, and the Work Order provides for a term to be ended upon the completion of the services required. The budget
provides for service fees and pass-through expenses and clinical sites totaling $11.5 million. During Fiscal 2023, the Company executed
two change orders resulting in a $0.6 million increase in the overall estimated budgeted costs.
On March 1, 2023, the Company, through Tarus, entered into a clinical
service agreement with Fortrea Inc. (formerly Labcorp Drug Development Inc.), a third-party CRO. The term of the agreement is through
the earlier of August 14, 2025 or the completion of provision of services and the payment of contractual obligations. The budgeted costs
for the services to be provided is approximately $12.1 million.
Stimunity Convertible Note
On July 13, 2022, the Company entered into a commitment with Stimunity
to provide €600,000 under the Stimunity Convertible Note. The Stimunity Convertible Note provides for simple interest at 7% per annum.
The Stimunity Convertible Note is automatically converted into Series A shares of Stimunity upon Stimunity completing a Series A round
for at least €20 million. If such subscription round is completed prior to the Maturity Date, the Company will be entitled to convert
the Stimunity Convertible Note into Series A shares of Stimunity at the subscription share price less 15%. Additionally, if Stimunity
completes a financing with a new category of shares (other than Common Shares or Series A shares of Stimunity) for at least €5 million
(the “Minimum Raise”), the Company will have the right to convert the Stimunity Convertible Note and the historical Series
A shares of Stimunity owned into the new category of shares. In the event that Stimunity does not close a financing prior to the Maturity
Date or raises less than the Minimum Raise, the Company will have the right to convert the Stimunity Convertible Note into Series A shares
of Stimunity at €363.00 per share or the raise price less 15%, whichever is lower. The Stimunity Convertible Note was funded by the
Company on September 12, 2022. In addition, the Company has not reflected the interest earned on the Stimunity Convertible Note in reporting
its consolidated financial results. See Note 6, “Investment in Associate,” for a further discussion.
Committed Purchase Agreement
On July 6, 2022 (the “Signing Date”), the Company entered
into the Committed Purchase Agreement with Lincoln, pursuant to which the Company may require Lincoln to purchase ordinary shares having
an aggregate value of up to $30 million over a period of 36 months. Pursuant to the Committed Purchase Agreement, Lincoln will be obligated
to purchase the Company’s ordinary shares in three different scenarios that are based on various market criteria and share amounts.
Upon execution of the Committed Purchase Agreement, the Company issued
to Lincoln 94,508 ordinary shares, representing a 3% commitment fee valued at $0.9 million. The Company has the right to terminate the
Committed Purchase Agreement for any reason, effective upon one business day prior written notice to Lincoln. Lincoln has no right to
terminate the Committed Purchase Agreement. The Company is accounting for the commitment fee as a deferred commitment fee on the consolidated
statement of financial position as of June 30, 2023 and March 31, 2023 and will amortize it pro-rata against equity sold under the Committed
Purchase Agreement. Any unamortized balance will be written-off to operations at the expiration of the commitment.
The Committed Purchase Agreement does not impose any financial or business
covenants on the Company and there are no limitations on the use of proceeds received by the Company from Lincoln. The Company may raise
capital from other sources in its sole discretion; provided, however, that the Company shall not enter into any similar agreement for
the issuance of variable priced equity-like securities until the three-year anniversary of the Signing Date, excluding, however, an at-the-market
transaction with a registered broker-dealer.
In connection with the Committed Purchase Agreement, the Company and Lincoln
entered into a Registration Rights Agreement, dated July 6, 2022 (the “Registration Rights Agreement”). Pursuant to the Registration
Rights Agreement, the Company agreed to file with the SEC the prospectus supplement to the Company’s shelf registration statement
pursuant to Rule 424(b) for the purpose of registering for resale the ordinary shares to be issued to Lincoln under the Committed Purchase
Agreement. The prospectus supplement was filed on August 19, 2022.
The Company is obligated under the Tarus Merger Agreement
and the iOx Share Exchange Agreement to pay certain third party earnouts based on the achievement of certain milestones. See Note 9, “Acquisition
of Tarus,” and Note 16, “Related Party Transactions – Share Exchange Agreement
– iOx,” respectively, for further discussions.
|
| X |
- DefinitionThe disclosure of commitments and contingent liabilities. [Refer: Contingent liabilities [member]] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 10
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_10_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfCommitmentsAndContingentLiabilitiesExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
RELATED PARTY TRANSACTIONS
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Related party transactions [abstract] |
|
| RELATED PARTY TRANSACTIONS |
NOTE 16. RELATED PARTY TRANSACTIONS
SalvaRx Acquisition
Two of the Company’s directors are also directors of SalvaRx Group
plc, a company which owns approximately 4.1% of the Company’s issued and outstanding ordinary shares as of June 30, 2023.
Investments
The Company has entered into related party transactions and certain services
agreements with its investees. Key management personnel of the Company have also entered into related party transactions with investees.
Key management personnel are those persons having the authority and responsibility for planning, directing and controlling the activities
of the Company, including directors and senior management of the Company.
The following subsidiaries and associates are considered related parties:
| (a) | Stimunity. The CEO of Portage is one of three members of the board of directors of Stimunity (see Note 6, “Investment
in Associate,” and Note 15, “Commitments and Contingent Liabilities – Stimunity Convertible Note”). |
| (b) | iOx. Upon execution of the iOx Share Exchange on July 18, 2022, the non-Portage director resigned from the iOx board leaving
two Portage insiders as directors. The CEO of Portage is also the CEO of iOx, and the management team of Portage comprises the management
team of iOx. See below for a discussion of the Company’s purchase of the non-controlling interest in iOx through its wholly-owned
subsidiary SalvaRx. |
| (c) | Saugatuck. One of the three directorships on the board of directors of Saugatuck is controlled by Portage. Additionally, the
CEO of Portage is also the CEO of Saugatuck, and the management team of Portage comprises the management team of Saugatuck. |
| (d) | Intensity. The CEO of Portage previously served as a part-time officer of Intensity until becoming a consultant in 2023. Additionally,
Intensity provided services (primarily rent) to Portage through April 2023. |
| (e) | Portage Development Services Inc. PDS provides human resources and other services to each operating subsidiary of Portage through
a shared services agreement. |
The following are related party balances and transactions other than those
disclosed elsewhere in the condensed consolidated interim financial statements:
Transactions between the parent company and its subsidiaries, which are
related parties, have been eliminated in consolidation and are not disclosed in this note.
On September 8, 2021, the Company,
through SalvaRx, completed a settlement of loans (including interest) to and receivables from iOx for services rendered in exchange for
23,772 ordinary shares of iOx at a price of £162. Simultaneously, the Company entered into an agreement with OSI, the holder of
$0.15 million notes plus accrued interest under which OSI exchanged the notes plus accrued interest for 820 shares of iOx.
The Company followed the guidance provided by an IFRS Discussion Group Public Meeting dated November 29, 2016, following the general tenets
of IAS 39, “Financial Instruments: Recognition and Measurement,” and IFRIC 19, “Extinguishing Financial Liabilities
with Equity Instruments,” and recorded the exchange at historical cost. Additionally, no profit or loss was recorded in connection
with the exchange. As a result of these transactions, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%.
Share Exchange Agreement – iOx
On July 18, 2022, the Company and SalvaRx entered into a Share Exchange
Agreement (the “Share Exchange Agreement”) with each of the minority shareholders of iOx (the “Sellers”) resulting
in the acquisition of the outstanding non-controlling ownership interest (approximately 22%) of iOx, which is developing the iNKT engager
platform. The Company followed IFRS 3, “Business Combinations,” and IAS 27, “Separate Financial Statements,” (which
substantially replaced IAS 3) to account for this transaction. The Company achieved control of iOx, as defined, on January 8, 2019 upon
the completion of the SalvaRx Acquisition. Further transactions whereby the parent entity acquires further equity interests from non-controlling
interests, or disposes of equity interests but without losing control, are accounted for as equity transactions (i.e., transactions with
owners in their capacity as owners). As such:
| · | the carrying amounts of the controlling and non-controlling interests are adjusted to reflect the changes in their relative interests
in the subsidiary; |
| · | any difference between the amount by which the non-controlling interests is adjusted and the fair value of the consideration paid
or received is recognized directly in equity and attributed to the owners of the parent; and |
| · | there is no consequential adjustment to the carrying amount of goodwill, and no gain or loss is recognized in profit or loss. |
The Company now owns the worldwide rights to its small molecule iNKT engagers,
including lead programs PORT-2 and PORT-3. Under the terms of the Share Exchange Agreement, each Seller sold to the Company, and the Company
acquired from each Seller, legal and beneficial ownership of the number of iOx shares held by each Seller, free and clear of any share
encumbrances, in exchange for the issuance in an aggregate of 1,070,000 Portage ordinary shares to be allocated among the Sellers based
upon their relative ownership. As a result of the Share Exchange Agreement, the Company owns 100% of the issued and outstanding shares
of iOx through its wholly-owned subsidiary, SalvaRx.
As additional consideration for the sale of the iOx
shares to the Company under the Share Exchange Agreement, the Sellers shall have the contingent right to receive additional shares (“Earnout
Shares”) from the Company having an aggregate value equal to $25 million calculated at the Per Share Earnout Price, as defined in
the Share Exchange Agreement, upon the achievement of certain milestones defined as the dosing of the first patient in a Phase 3 clinical
trial for either PORT-2 (IMM60 iNKT cell activator/engager) or PORT-3 (PLGA-nanoparticle formulation of IMM60 combined with a NY-ESO-1
peptide vaccine). The Company shall have the option, in its sole and absolute discretion, to settle the Earnout Shares in cash. The Company
followed IFRS 3 and IAS 32, “Financial Instruments: Presentation,” to account for the fair value of the Earnout Shares. The
principal assumptions for determining the fair value include the timing of development events, the probabilities of success and the discount
rate used. The fundamental principle of IAS 32 is that a financial instrument should be classified as either a financial liability or
an equity instrument according to the substance of the contract, not its legal form, and the definitions of financial liability and equity
instrument. A financial instrument is an equity instrument if, and only if, both conditions (a) and (b) below are met:
| (a) | the instrument includes no contractual obligation to deliver cash or another financial asset to another entity,
and |
| (b) | if the instrument will or may be settled in the Company’s own equity instruments, it is either: |
| (i) | a non-derivative that includes no contractual obligation for the Company to deliver a variable number of
its own equity instruments; or |
| (ii) | a derivative that will be settled only by the issuer exchanging a fixed amount of cash or another financial
asset for a fixed number of its own equity instruments. |
When a derivative financial instrument gives one party a choice over how
it is settled (for instance, the Company or the holder can choose settlement net in cash or by exchanging shares for cash), it is a financial
asset or a financial liability unless all of the settlement alternatives would result in it being an equity instrument. The financial
instrument includes the exclusive right of the Company to settle the obligation with cash or equity and, accordingly, accounted for the
fair value of the Earnout Shares as a non-current liability.
The Company recorded $5.478 million as the fair value estimate of the
Earnout Shares, which is reflected as deferred obligation - iOx milestone on the condensed consolidated interim statements of financial
position included herein. The Company will determine the fair value of the Earnout Shares at each balance sheet date. Any change to the
fair value will be recorded in the Company’s statements of operations and other comprehensive income (loss). The Company recorded
a (loss) from the change (increase) in fair value of the liability of $0.685 million for the three months ended June 30, 2023.
Employment Agreements
PDS entered into a Services Agreement with the Company’s CEO effective
December 15, 2021 (the “CEO Services Agreement”). The CEO Services Agreement originally provided for a base salary of $618,000,
plus cost-of-living increases. On December 19, 2022, the Compensation Committee approved the CEO’s compensation of $642,700 for
Fiscal 2024. The CEO Services Agreement provides for annual increases based upon the review of the base salary by the Board prior to the
anniversary of the CEO Services Agreement provided that the annual increase cannot be less than the cost-of-living increase. The CEO Services
Agreement also provides that the CEO is eligible to receive an annual performance-based bonus targeted at 59% of the applicable year’s
base salary, which bonus is earned based on the achievement of performance targets, as determined annually by the Board and communicated
to the CEO in the first quarter of the year. Any annual bonus, to the extent earned, is to be paid no later than March 15 of the following
year. The CEO Services Agreement is for an initial term of three years, after which it will automatically renew annually unless terminated
in accordance with the CEO Services Agreement.
Under the CEO Services Agreement, the CEO may terminate his employment
with PDS at any time for Good Reason (as defined in the CEO Services Agreement). PDS may terminate the CEO’s employment immediately
upon his death, upon a period of disability or without Just Cause (as defined in the CEO Services Agreement). In the event that the CEO’s
employment is terminated due to his death or Disability (as defined in the CEO Services Agreement), for Good Reason or without Just Cause,
he will be entitled to accrued obligations (accrued unpaid portion of base salary, accrued unused vacation time and any unpaid expenses).
Additionally, he may be entitled to Severance Benefits (as defined in the CEO Services Agreement), which include his then current base
salary and the average of his annual bonus for the prior two completed performance years, paid over 12 monthly installments. Additionally,
the CEO will be entitled to life insurance benefits and medical and dental benefits for a period of 12 months at the same rate the CEO
and PDS shared such costs during his period of employment.
Additionally, all stock options (and any other unvested equity incentive
award) held by the CEO relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as defined
in the CEO Services Agreement), and the exercise period for such stock options will be increased by a period of two years from the Termination
Date.
If the CEO’s employment by PDS is terminated by PDS or any successor
entity without Just Cause (not including termination by virtue of the CEO’s death or Disability) or by the CEO for Good Reason within
12 months following the effective date of a Change in Control (as defined in the CEO Services Agreement), then, in addition to paying
or providing the CEO with the Accrued Obligations (as defined in the CEO Services Agreement), the Company will provide the following Change
in Control Severance Benefits (as defined in the CEO Services Agreement):
| (1) | PDS will pay the base salary continuation benefit for 18 months; |
| (2) | PDS will pay the life insurance benefit for 18 months; |
| (3) | PDS will pay an additional amount equivalent to the CEO’s target annual bonus calculated using the bonus percentage for the
performance year in which the CEO’s termination occurs. This bonus will be paid in 12 equal installments commencing on the first
payroll date that is more than 60 days following the date of termination of the CEO’s employment, with the remaining installments
occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the CEO with continued medical and dental benefits, as described above, for 18 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the CEO relating to shares of PDS or the Company will be
deemed fully vested and exercisable on the Termination Date, as defined, and the exercise period for such stock options will be increased
by a period of two years from the Termination Date. |
PDS entered into services agreements (individually, an “Executive
Service Agreement,” and collectively, the “Executive Service Agreements”) with each of the Company’s five other
members of senior management (individually, “Executive” and collectively, “Executives”), three of which are dated
as of December 1, 2021, one of which is dated December 15, 2021 and one of which is dated June 1, 2022. Each of the Executive Services
Agreements provides for an initial term of two years that is automatically renewed for one-year periods (except two of the Executive Services
Agreement, which provides for an initial term of one year and that is automatically renewed for one-year periods). The Executive Services
Agreements initially provided for annual base salaries ranging from $175,000 to $348,000 (pro-rated for services rendered) and annual
bonus targets ranging from 30% to 40%. They also provide for long-term incentives in the form of equity awards from time to time under
the Portage Biotech Inc. Amended and Restated 2021 Equity Incentive Plan.
On December 19, 2022, the Compensation Committee approved executive compensation
for Fiscal 2024 for annual base salaries ranging from $183,750 to $469,000 (pro-rated for services rendered) and annual bonus targets
ranging from 30% to 40%.
The Executive Services Agreements can be terminated by PDS without Just
Cause, by death or Disability, or by the Executive (except one) for Good Reason (each as defined in the respective Executive Services
Agreements). In such instances, the Executive Services Agreements provide for the payment of accrued obligations (accrued unpaid portion
of base salary, accrued unused vacation time and any unpaid expenses). Additionally, the Executives (except two) are entitled to 50% of
base salary plus 50% of average annual bonus earned over the prior two performance years, as well as prevailing life insurance benefits
for a period of six months and medical and dental benefits for a period of six months at the prevailing rate PDS and the Executive were
sharing such expenses.
Additionally, all stock options (and any other unvested equity incentive
award) held by the Executives relating to shares of the Company will be deemed fully vested and exercisable on the Termination Date (as
defined in the respective Executive Services Agreements), and the exercise period for such stock options will be increased by a period
of two years from the Termination Date.
If an Executive’s employment by PDS is terminated by the Company
or any successor entity without Just Cause (not including termination by virtue of the Executive’s death or Disability) or by the
Executive (except one) for Good Reason within 12 months following the effective date of a Change in Control (as defined in the respective
Executive Services Agreements), then, in addition to paying or providing the Executive with the Accrued Obligations (as defined in the
respective Executive Services Agreements), the Company will provide the following Change in Control Severance Benefits (as defined in
the respective Executive Services Agreements), except in two cases in which the Executive is entitled to Item (5) and 50% of Items (1)
and (3) below:
| (1) | PDS will pay the Base Salary continuation benefit for 12 months; |
| (2) | PDS will pay the life insurance benefit for 12 months; |
| (3) | The Company will pay an additional amount equivalent to the Executive’s target annual bonus calculated using the bonus percentage
for the performance year in which the Executive’s termination occurs. This bonus will be payable in 12 equal installments commencing
on the first payroll date that is more than 60 days following the date of termination of the Executive’s employment, with the remaining
installments occurring on the first day of the month for the 11 months thereafter; |
| (4) | PDS will provide the Executive with continued medical and dental benefits, as described above, for 12 months; and |
| (5) | All stock options (and any other unvested equity incentive award) held by the Executive relating to shares of PDS or the Company will
be deemed fully vested and exercisable on the Termination Date and the exercise period for such stock options will be increased by a period
of two years from the Termination Date. |
The Executive Services Agreements also include customary confidentiality,
as well as provisions relating to assignment of inventions. The Executive Services Agreements also includes non-competition and non-solicitation of
employees and customers provision that run during the Executive’s employment with PDS and for a period of one year after termination
of employment.
Bonuses & Board Compensation Arrangements
In December 2022, the Board approved
executive performance bonuses, as recommended by the Compensation Committee, totaling $0.6 million, which is equivalent to 73.5% of original
annual targets established by the Board in December 2021. The bonuses were approved based upon the original performance targets established.
The Board further approved a payment structure of 25% of approved bonuses, which were paid in January 2023, with the balance of amounts
due payable upon a new financing. The accrued, unpaid amount of approximately $0.4 million is included in accounts payable and accrued
expenses on the condensed consolidated statements of financial position as of each of June 30, 2023 and March 31, 2023.
Effective January 1, 2022, each non-employee Board member are entitled
to receive cash Board fees of $40,000 per annum, payable quarterly in arrears. Additionally, each non-employee Board member is entitled
to an annual grant of 6,900 options to purchase Portage ordinary shares, which would vest the first annual anniversary of the grant date.
The Company incurred Board fees totaling $82,500 and $75,000 during the three months ended June 30, 2023 and 2022, respectively.
Non-employee Board chairpersons are entitled to an annual cash fee of
$30,000, payable quarterly in arrears. In lieu of a non-executive chairperson, the lead director is entitled to an annual cash fee of
$20,000 per annum paid quarterly in arrears. Additionally, the chairperson of each of the Audit Committee, Compensation Committee and
Nominating Committee is entitled to annual fees of $15,000, $12,000 and $8,000, respectively, payable quarterly in arrears. Members of
those committees will be entitled to annual fees of $7,500, $6,000 and $4,000, respectively, payable quarterly in arrears.
|
v3.23.2
FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| FINANCIAL INSTRUMENTS AND RISK MANAGEMENT |
NOTE 17. FINANCIAL INSTRUMENTS AND RISK MANAGEMENT
The Company’s financial instruments recognized in the Company’s
condensed consolidated interim statements of financial position consist of the following:
Fair value estimates are made at a specific point in time, based on relevant
market information and information about financial instruments. These estimates are subject to and involve uncertainties and matters of
significant judgment; and therefore, these estimates cannot be determined with precision. Changes in assumptions could significantly affect
these estimates.
The following table summarizes the Company’s financial instruments
as of June 30, 2023 and March 31, 2023:
| Schedule of financial instruments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTOCI | |
FVTPL | |
Amortized
Cost | |
FVTOCI | |
FVTPL |
| | |
| |
| |
| |
| |
| |
|
| Financial assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 7,698 | | |
$ | - | | |
$ | - | | |
$ | 10,545 | | |
$ | - | | |
$ | - | |
| Prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | - | | |
$ | - | | |
$ | 2,689 | | |
$ | - | | |
$ | - | |
| Convertible note receivable, including accrued interest, net of impairment | |
$ | - | | |
$ | - | | |
$ | 442 | | |
$ | - | | |
$ | - | | |
$ | 442 | |
| Investment in associate | |
$ | - | | |
$ | - | | |
$ | 756 | | |
$ | - | | |
$ | - | | |
$ | 806 | |
| Investment in public company | |
$ | - | | |
$ | 3,855 | | |
$ | - | | |
$ | - | | |
$ | 2,087 | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTPL | |
Amortized
Cost | |
FVTPL |
| Financial liabilities | |
| | | |
| | | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
$ | 2,591 | | |
$ | - | | |
$ | 1,865 | | |
$ | - | |
| Lease liability - current | |
$ | 47 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Lease liability - non-current | |
$ | 249 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Deferred purchase price payable - Tarus | |
$ | - | | |
$ | 7,864 | | |
$ | - | | |
$ | 7,179 | |
| Deferred obligation - iOx milestone | |
$ | - | | |
$ | 4,552 | | |
$ | - | | |
$ | 4,126 | |
A summary of the Company’s risk exposures as it relates to financial
instruments are reflected below.
Fair value of Financial Instruments
The Company’s financial assets and liabilities are comprised of
cash and cash equivalents, receivables and investments in equities and private and public entities, accounts payable, lease liability,
deferred purchase price payable, deferred obligation and unsecured notes payable.
The Company classifies the fair value of these transactions according
to the following fair value hierarchy based on the amount of observable inputs used to value the instrument:
| · | Level 1 – Values are based on unadjusted quoted prices available in active markets for identical assets or liabilities as of
the reporting date. |
| · | Level 2 – Values are based on inputs, including quoted forward prices for commodities, time value and volatility factors, which
can be substantially observed or corroborated in the marketplace. Prices in Level 2 are either directly or indirectly observable as of
the reporting date. |
| · | Level 3 – Values are based on prices or valuation techniques that are not based on observable market data. Investments are classified
as Level 3 financial instrument. |
Assessment of the significance of a particular input to the fair value
measurement requires judgment and may affect the placement within the fair value hierarchy.
Management has assessed that the fair values of cash and cash equivalents,
other receivables and accounts payable approximate their carrying amounts largely due to the short-term maturities of these instruments.
The following methods and assumptions were used to estimate their fair
values:
Investment in Associate: The fair value of the Stimunity investment
was determined based on an IAS 36 fair value analysis evaluating the likelihood of various scenarios given the then-current market conditions,
the increasing cost of capital and development delays associated with Stimunity’s lack of liquidity (Level 3). The Company recorded
a provision of impairment of $0.607 million with respect to the investment in associate decreasing the carrying value of the investment
in associate to $0.806 million as of March 31, 2023. There was no provision for impairment recorded in the three months ended June 30,
2023. See Note 6, “Investment in Associate,” for a further discussion.
Convertible Note Receivable: The fair value of the Stimunity Convertible
Note receivable denominated in euros at initial recognition is the transaction price for the instrument adjusted for the effect of the
currency translation rate on the reporting date (Level 3) (see Note 15, “Commitments and Contingent Liabilities – Stimunity
Convertible Note”). The Stimunity Convertible Note was initially recorded at $0.614 million to record the translated value of the
Stimunity Convertible Note on September 12, 2022. The Company recognized an unrealized gain of $0.039 million through OCI in Fiscal 2023
to reflect the change in translation rate for the Stimunity Convertible Note settleable in euros, increasing the carrying value of the
Stimunity Convertible Note to $0.653 million. As of March 31, 2023, the Company determined that there were indications of impairment,
based upon the inability of Stimunity to obtain financing. The Company performed an IAS 36 fair value analysis evaluating the likelihood
of various scenarios given the then-current market conditions, the increasing cost of capital and development delays associated with Stimunity’s
lack of liquidity. The Company recorded an impairment of $0.211 million resulting from the impairment analysis decreasing the carrying
value of the Stimunity Convertible Note to $0.442 million as of March 31, 2023. The Company recorded a nominal unrealized gain in the
three months ended June 30, 2023 with respect to the Stimunity Convertible Note.
Investment in Public Company: Upon Intensity’s IPO effective
June 30, 2023, the fair value of the investment is determined by the quoted market price (Level 1).
Accrued Equity Issuable: The fair value is estimated based on the
average of the quoted market prices for the period in which the shares were earned (Level 1).
Lease Liability - Current: The lease liability - current represents
the present value of the lease payments due over the next twelve months discounted at the Company’s incremental borrowing rate (Level
2).
Lease Liability - Non-Current: The lease liability - non-current
represents the present value of the lease payments due under the lease less the current portion of such payments discounted at the Company’s
incremental borrowing rate (Level 2).
Deferred Purchase Price Payable - Tarus: The fair value is the
estimated value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 at acquisition
date, adjusted at each reporting date for any change in fair value (Level 3) (see Note 9, “Acquisition of Tarus”). The fair
value was determined using the Income Approach and was based upon the analysis on the Tarus clinical plan, the timing of development events
and the probabilities of success determined primarily based upon empirical third party data and Company experience, as well as the relevant
cost of capital. The Company recorded a (loss) from the change (increase) in fair value of the liability of $0.685 million for the three
months ended June 30, 2023.
Deferred Obligation - iOx Milestone: The fair value is the estimated
value of a future contingent obligation based upon a fair value analysis performed in accordance with IFRS 3 as of July 18, 2022, the
date of the Share Exchange Agreement, adjusted at each reporting date for any change in fair value (Level 3) (see Note 16, “Related
Party Transactions – Share Exchange Agreement – iOx”). The fair value was determined using the Income Approach and based
on factors including the clinical plan, the timing of development events and the probabilities of success determined primarily based upon
empirical third party data and Company experience, as well as the relevant cost of capital. The Company recorded a (loss) from the change
(increase) in fair value of the liability of $0.426 million for the three months ended June 30, 2023.
Fair Value Hierarchy
The investment in public company (Intensity)
was transferred from Level 3 to Level 1 of the fair value hierarchy for the three months ended June 30, 2023 as the result of Intensity’s
IPO. For the year ended March 31, 2023, the fair value of the investment was determined based on an IAS 36 impairment analysis after
determining there were external indications of impairment (Level 3). See Note 7, “Investment in Public Company.”
The Company’s financial instruments are exposed to certain financial
risks: Credit Risk, Liquidity Risk and Foreign Currency Risk.
Credit Risk
Credit risk is the risk of loss associated with a counterparty’s
inability to fulfill its payment obligations. The credit risk is attributable to various financial instruments, as noted below. The credit
risk is limited to the carrying value as reflected in the Company’s condensed consolidated interim statements of financial position.
Cash and cash equivalents. Cash and cash equivalents comprise
cash on hand and amounts invested in underlying Treasury and money market funds that are readily convertible to a known amount of cash
with three months or less from date of acquisition and are subject to an insignificant risk of change in value. As
of June 30, 2023 and March 31, 2023, cash equivalents was comprised of a money market account with maturities less than 90 days from the
date of purchase. Cash and cash equivalents are held with major international financial institutions and therefore the risk of
loss is minimal.
Liquidity Risk
Liquidity risk is the risk that the Company will encounter difficulty
in satisfying financial obligations as they become due.
The Company’s approach to managing liquidity is to ensure, as far
as possible, that it will have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions without
incurring unacceptable losses or risking harm to the Company’s reputation. The Company holds sufficient cash and cash equivalents
to satisfy current obligations under accounts payable and accruals.
The Company monitors its liquidity position regularly to assess whether
it has the funds necessary to meet its operating needs and needs for investing in new projects.
As a biotech company at an early stage of development and without significant
internally generated cash flows, there are inherent liquidity risks, including the possibility that additional financing may not be available
to the Company, or that actual drug development expenditures may exceed those planned. The current uncertainty in global markets could
have an impact on the Company’s future ability to access capital on terms that are acceptable to the Company. There can be no assurance
that required financing will be available to the Company. See Note 2, “Going Concern,” and Note 12, “Capital Stock,”
for a discussion of the Company’s share offering and Note 15, “Commitments and Contingent Liabilities – Committed Purchase
Agreement,” for a further discussion.
Foreign Currency Risk
While the Company operates in various jurisdictions, substantially all
of the Company’s transactions are denominated in the U.S. Dollar, except the deferred tax liability in the U.K. settleable in British
pound sterling and the Stimunity Convertible Note receivable settleable in euros.
|
| X |
- DefinitionThe entire disclosure for financial instruments. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 7
-IssueDate 2023-01-01
-Section Scope
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=7&code=ifrs-tx-2023-en-r&doctype=Standard&dita_xref=IFRS07_g3-5A_TI
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfFinancialInstrumentsExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
CAPITAL DISCLOSURES
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Capital Disclosures |
|
| CAPITAL DISCLOSURES |
NOTE 18. CAPITAL DISCLOSURES
The Company considers the items included in shareholders’
equity as capital. The Company had accounts payable and accrued liabilities of approximately $2.6
million and lease liability - current of $0.047
million as of June 30, 2023 (accounts payable and accrued liabilities of approximately $1.9
million as of March 31, 2023) and current assets of approximately $10.9
million as of June 30, 2023 (approximately $13.7
million as of March 31, 2023). The Company’s objectives when managing capital are to safeguard the Company’s ability to
continue as a going concern in order to pursue new business opportunities and to maintain a flexible capital structure, which
optimizes the costs of capital at an acceptable risk.
The Company manages the capital structure and makes adjustments to it
in light of changes in economic conditions and the risk characteristics of the underlying assets.
As of June 30, 2023, shareholders’ equity attributable to the owners
of the company was approximately $73.3 million (approximately $76.0 million as of March 31, 2023).
The Company is not subject to any externally imposed capital requirements
and does not presently utilize any quantitative measures to monitor its capital. There have been no changes to the Company’s approach
to capital management during the three months ended June 30, 2023 and 2022.
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureCapitalDisclosuresAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfCapitalManagementExplanatoryTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
NON-CONTROLLING INTEREST
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| NON-CONTROLLING INTEREST |
NOTE 19. NON-CONTROLLING INTEREST
| Schedule of non-controlling interest | |
| | | |
| | | |
| | |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2023 | |
$ | - | | |
$ | (650 | ) | |
$ | (650 | ) |
| Net loss attributable to non-controlling interest | |
| - | | |
| (7 | ) | |
| (7 | ) |
| Non-controlling interest as of June 30, 2023 | |
$ | - | | |
$ | (657 | ) | |
$ | (657 | ) |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2022 | |
$ | 44,701 | | |
$ | (472 | ) | |
$ | 44,229 | |
| Net income (loss) attributable to non-controlling interest | |
| 175 | | |
| (71 | ) | |
| 104 | |
| Non-controlling interest as of June 30, 2022 | |
$ | 44,876 | | |
$ | (543 | ) | |
$ | 44,333 | |
On September 8, 2021, the Company, through SalvaRx, completed a settlement
of loans (including interest) to and receivables from iOx for services rendered in exchange for 23,772 ordinary shares of iOx at a price
of £162. On July 18, 2022, the Company completed the acquisition of the remaining non-controlling interest in iOx, by issuing 1,070,000
shares of its ordinary shares and assuming certain milestone obligations. See Note 16, “Related Party Transactions – Share
Exchange Agreement – iOx” for a discussion of the Company’s purchase of the balance of the non-controlling interest
in iOx.
Saugatuck and subsidiary includes Saugatuck and its wholly-owned subsidiary,
Saugatuck Rx LLC.
|
| X |
- DefinitionThe entire disclosure for interests in other entities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 1
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_1&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfInterestsInOtherEntitiesExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
SIGNIFICANT ACCOUNTING POLICIES (Policies)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Significant Accounting Policies |
|
| Recent Accounting Pronouncements |
Recent Accounting Pronouncements
IFRS Pronouncements Issued
Impact of Adoption of Significant New IFRS Standards in Fiscal 2023
| (a) | Annual Improvements to IFRS Standards 2018-2020 |
The annual improvements process addresses issues in the 2018-2020 reporting
cycles including changes to IFRS 9, “Financial Instruments,” IFRS 1, “First Time Adoption of IFRS,” IFRS 16, “Leases,”
and IAS 41, “Biological Assets”.
| i) | The amendment to IFRS 9 addresses which fees should be included in the 10% test for derecognition of financial liabilities. |
| ii) | The amendment to IFRS 1 allows a subsidiary adopting IFRS at a later date than its parent to also measure cumulative translation differences
using the amounts reported by the parent based on the parent’s date of transition to IFRS. |
| iii) | The amendment to IFRS 16’s illustrative example 13 removes the illustration of payments from the lessor related to leasehold
improvements. |
These amendments were effective for annual periods beginning on or after
January 1, 2022. The adoption of these amendments did not have a material effect on the Company’s annual consolidated financial
statements or the condensed consolidated interim financial statements for the three months ended June 30, 2023.
New Accounting Standards, Interpretations and Amendments
Standards issued but not yet effective up to the date of issuance of the
Company’s condensed consolidated interim financial statements are listed below. This listing is of standards and interpretations
issued, which the Company reasonably expects to be applicable at a future date. The Company intends to adopt those standards when they
become effective.
| (a) | IAS 1: Presentation of Financial Statements |
The amendment to IAS 1 clarifies how to classify debt and other liabilities
as either current or non-current. The amendment will be effective for annual periods beginning on or after January 1, 2024. The Company
is currently evaluating the new guidance and impacts on its consolidated financial statements.
| (b) | Amendments to IFRS 10 and IAS 28: Sale or Contribution of Assets between an Investor and Its Associate or Joint Venture |
The amendment addresses the conflict between IFRS 10, “Consolidated
Financial Statements,” and IAS 28, “Investments in Associates and Joint Ventures,” in dealing with the loss of control
of a subsidiary that is sold or contributed to an associate or joint venture. The amendments clarify that the gain or loss resulting from
the sale or contribution of assets that constitute a business, as defined in IFRS 3, “Business Combinations,” between an investor
and its associate or joint venture, is recognized in full. Any gain or loss resulting from the sale or contribution of assets that do
not constitute a business, however, is recognized only to the extent of unrelated investors' interests in the associate or joint venture.
The IASB has deferred the effective date of these amendments indefinitely, but an entity that early adopts the amendments must apply them
prospectively. The Company is evaluating whether the adoption of the above amendment will have a material impact on its financial statements.
|
| X |
- References
+ Details
| Name: |
ptgef_DescriptionOfAccountingPolicyForAdoptionOfNewStandardsExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureSignificantAccountingPoliciesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
PREPAID EXPENSES AND OTHER RECEIVABLES (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Prepaid Expenses And Other Receivables |
|
| Schedule of prepaid expenses and other receivables |
| Schedule of prepaid expenses and other receivables | |
| | | |
| | |
| (In thousands) | |
As of
June 30, 2023 | |
As of
March 31, 2023 |
| | |
| |
|
| Prepaid clinical research costs | |
$ | 1,783 | | |
$ | 1,653 | |
| Prepaid insurance | |
| 446 | | |
| 621 | |
| Research & development tax credits | |
| 186 | | |
| 169 | |
| Other prepaid expenses | |
| 151 | | |
| 56 | |
| Tax deposits | |
| 115 | | |
| 119 | |
| Other receivables | |
| 71 | | |
| 71 | |
| Total prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | 2,689 | |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfDetailedInformationAboutPrepaidExpensesAndOtherReceivableTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_PrepaidExpensesAndOtherReceivablesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INVESTMENT IN ASSOCIATE (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Investment In Associate |
|
| Schedule of investment associate |
| Schedule of investment associate | |
| |
| |
| |
|
| Name | |
Principal Activity | |
Place of Incorporation and
Principal Place of Business | |
Voting Rights Held as
of June 30, 2023 | |
Voting Rights Held as
of March 31, 2023 |
| Associate: Stimunity S.A. | |
Biotechnology | |
Paris, France | |
44.0% | |
44.0% |
|
| Schedule of investment in Stimunity S.A. |
| Schedule of investment in Stimunity S.A. | |
| | | |
| | |
| | |
As of and for the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Balance, beginning of period | |
$ | 806 | | |
$ | 1,673 | |
| Share of loss | |
| (50 | ) | |
| (60 | ) |
| Balance, end of period | |
$ | 756 | | |
$ | 1,613 | |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInvestmentInAssociateAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfInvestmentAssociatetableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfInvestmentInStimunitySaTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe disclosure of a maturity analysis of operating lease payments. Operating lease is a lease that does not transfer substantially all the risks and rewards incidental to ownership of an underlying asset. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 97
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_97&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfMaturityAnalysisOfOperatingLeasePaymentsExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
ACQUISITION OF TARUS (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Acquisition Of Tarus |
|
| Schedule of fair value of assets acquired and liabilities assumed |
| Schedule of fair
value of assets acquired and liabilities assumed | |
| | |
| Assets: | |
(In thousands) |
| Identifiable intangible assets | |
$ | 28,200 | |
| Goodwill | |
| 538 | |
| Total assets | |
$ | 28,738 | |
| Consideration: | |
| | |
| Fair value of shares issued | |
$ | 17,200 | |
| Liabilities assumed | |
| 3,000 | |
| Deferred purchase consideration at fair value | |
| 8,538 | |
| Total liabilities | |
$ | 28,738 | |
|
| Schedule of Pro forma Information |
| Schedule of Pro forma Information | |
| | |
| (In thousands) | |
Three Months Ended
June 30, 2022 |
| Loss from operations | |
$ | (3,788 | ) |
| Loss before provision for income taxes | |
$ | (3,893 | ) |
| Net loss | |
$ | (1,341 | ) |
| Total comprehensive loss for period | |
$ | (1,341 | ) |
| Loss per share | |
$ | (0.09 | ) |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureAcquisitionOfTarusAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfFairValueOfAssetsAcquiredAndLiabilitiesAssumedTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfProformaInformationTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| In-process Research And Development And Deferred Tax Liability |
|
| Schedule of in-process research and development |
| Schedule of in-process research and development | |
| |
| | | |
| | |
| | |
| |
Value as of |
| Project # | |
Description | |
June 30, 2023 | |
March 31, 2023 |
| iOx: | |
| |
| | | |
| | |
| PORT 2 (IMM60) | |
Melanoma & Lung Cancers | |
$ | 36,181 | | |
$ | 36,181 | |
| PORT 3 (IMM65) | |
Ovarian/Prostate Cancers | |
| 21,709 | | |
| 21,709 | |
| | |
| |
| 57,890 | | |
| 57,890 | |
| | |
| |
| | | |
| | |
| Oncomer/Saugatuck | |
DNA Aptamers | |
| 178 | | |
| 178 | |
| | |
| |
| | | |
| | |
| Tarus: | |
| |
| | | |
| | |
| PORT 6 & PORT 7 | |
Adenosine Receptors | |
| 22,723 | | |
| 22,723 | |
| PORT 8 | |
Adenosine Receptors | |
| 420 | | |
| 420 | |
| PORT 9 | |
Adenosine Receptors | |
| 472 | | |
| 472 | |
| | |
| |
| 23,615 | | |
| 23,615 | |
| | |
| |
| | | |
| | |
| In-process research and development | |
| |
$ | 81,683 | | |
$ | 81,683 | |
| | |
| |
| | | |
| | |
| Deferred tax liability | |
| |
$ | 13,510 | | |
$ | 13,195 | |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInprocessResearchAndDevelopmentAndDeferredTaxLiabilityAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfInProcessResearchAndDevelopmentTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INCOME TAXES (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| Schedule of income taxes benefit |
| Schedule of income taxes benefit | |
| | | |
| | |
| | |
For the Three Months Ended June 30, |
| (In thousands) | |
2023 | |
2022 |
| | |
| |
|
| Current: | |
| | | |
| | |
| Federal | |
$ | (3 | ) | |
$ | - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| - | | |
| - | |
| Total current | |
| (3 | ) | |
| - | |
| | |
| | | |
| | |
| Deferred: | |
| | | |
| | |
| Federal | |
| - | | |
| - | |
| State and local | |
| - | | |
| - | |
| Foreign | |
| 148 | | |
| 2,552 | |
| Total deferred | |
| 148 | | |
| 2,552 | |
| Benefit from income taxes | |
$ | 145 | | |
$ | 2,552 | |
|
| Schedule of reconciliation income tax rates |
| Schedule of reconciliation income tax rates | |
| |
|
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (Loss) income on ordinary activities before tax | |
$ | (605 | ) | |
$ | 34 | |
| Statutory U.S. income tax rate | |
| 21.0 | % | |
| 21.0 | % |
| Income tax benefit (expense) at statutory income tax rate | |
| 127 | | |
| (7 | ) |
| Share-based compensation expense recognized for financial statement purposes | |
| (142 | ) | |
| - | |
| Other losses recognized | |
| - | | |
| 7 | |
| Utilization of losses not previously benefitted | |
| 12 | | |
| - | |
| Income tax (expense) | |
$ | (3 | ) | |
$ | - | |
|
| Schedule of effective income tax rates |
|
| Schedule of reconciliation of financial statement loss to tax basis loss |
| Schedule of reconciliation of financial statement loss to tax basis loss | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended |
| | |
June 30, 2023 | |
June 30, 2022 |
| | |
United
States | |
BVI | |
United
Kingdom | |
Total | |
United
States | |
BVI | |
Foreign | |
Total |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Pre-tax loss | |
$ | (609 | ) | |
$ | (3,886 | ) | |
$ | (1,576 | ) | |
$ | (6,071 | ) | |
$ | 34 | | |
$ | (2,468 | ) | |
$ | (1,743 | ) | |
$ | (4,177 | ) |
| Share-based compensation expense for financial statement purposes for which no benefit was taken | |
| 683 | | |
| - | | |
| - | | |
| 683 | | |
| - | | |
| - | | |
| - | | |
| - | |
| Losses not subject to tax | |
| - | | |
| 3,886 | | |
| - | | |
| 3,886 | | |
| - | | |
| 2,468 | | |
| - | | |
| 2,468 | |
| Utilization of losses not previously benefitted | |
| (59 | ) | |
| - | | |
| - | | |
| (59 | ) | |
| (34 | ) | |
| - | | |
| - | | |
| (34 | ) |
| Taxable income (loss) | |
$ | 15 | | |
$ | - | | |
$ | (1,576 | ) | |
$ | (1,561 | ) | |
$ | - | | |
$ | - | | |
$ | (1,743 | ) | |
$ | (1,743 | ) |
|
| Schedule of deferred tax assets and liabilities |
| Schedule of deferred tax assets and liabilities | |
| | | |
| | |
| | |
As of June 30, | |
As of March 31, |
| | |
2023 | |
2023 |
| Deferred tax assets: | |
| | | |
| | |
| Net operating loss | |
$ | (4,594 | ) | |
$ | (4,131 | ) |
| Deferred tax asset (unrecognized) | |
| 1,500 | | |
| 1,500 | |
| Deferred tax asset | |
| (3,094 | ) | |
| (2,631 | ) |
| | |
| | | |
| | |
| Deferred tax liabilities: | |
| | | |
| | |
| In-process research and development | |
| 13,510 | | |
| 13,195 | |
| Deferred tax liability | |
| 13,510 | | |
| 13,195 | |
| | |
| | | |
| | |
| Net deferred tax liability | |
$ | 10,416 | | |
$ | 10,564 | |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfDeferredTaxAssetsExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfIncomeTaxesBenefitTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfReconciliationEffectiveIncomeTaxratesTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfReconciliationOfFinancialStatementLossToTaxBasisLossTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
CAPITAL STOCK (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Capital Stock |
|
| Schedule of unlimited number of common shares without par value |
| Schedule of unlimited number
of common shares without par value | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| | |
Ordinary
Shares | |
Amount | |
Ordinary
Shares | |
Amount |
| | |
In 000’ | |
In 000’$ | |
In 000’ | |
In 000’$ |
| Balance, beginning of period | |
| 17,606 | | |
$ | 218,782 | | |
| 13,349 | | |
$ | 158,324 | |
| Shares issued under public offering and ATM, net of issue costs | |
| 171 | | |
| 613 | | |
| - | | |
| - | |
| Shares issued or accrued for services | |
| 9 | | |
| 30 | | |
| 4 | | |
| 30 | |
| Balance, end of period | |
| 17,786 | | |
$ | 219,425 | | |
| 13,353 | | |
$ | 158,354 | |
|
| X |
- References
+ Details
| Name: |
ptgef_CommonSharesUnlimitedNumberOfCommonSharesWithoutParValueTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureCapitalStockAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
STOCK OPTION RESERVE (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| Schedule of terms stock option reserve explanatory |
| Schedule
of terms stock option reserve explanatory | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 |
| (In thousands) | |
Non-Controlling Interest | |
Stock Option Reserve | |
Non-Controlling Interest | |
Stock Option Reserve |
| Balance, beginning of period | |
$ | - | | |
$ | 21,204 | | |
$ | 11,659 | | |
$ | 16,928 | |
| Share-based compensation expense | |
| - | | |
| 769 | | |
| - | | |
| 1,176 | |
| Balance, end of period | |
$ | - | | |
$ | 21,973 | | |
$ | 11,659 | | |
$ | 18,104 | |
|
| Schedule of outstanding stock options |
| Schedule of outstanding stock options | |
| | | |
| | | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Balance, beginning of period | |
| 1,963,420 | | |
| 1,151,400 | | |
| - | | |
| 1,275 | |
| Granted | |
| - | | |
| 50,000 | | |
| - | | |
| - | |
| Expired or forfeited | |
| - | | |
| - | | |
| - | | |
| (1,275 | ) |
| Balance, end of year | |
| 1,963,420 | | |
| 1,201,400 | | |
| - | | |
| - | |
| Exercisable, end of period | |
| 764,438 | | |
| 410,600 | | |
| - | | |
| - | |
|
| Schedule of weighted average exercise price and remaining contractual life |
| Schedule of weighted average exercise price and remaining contractual life | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 | |
2023 | |
2022 |
| | |
PBI Amended and Restated
2021 Equity Incentive Plan | |
iOx Option Plan
(Subsidiary Plan) |
| Weighted average exercise price | |
$ | 10.53 | | |
| 15.26 | | |
$ | - | | |
$ | - | |
| Weighted average remaining contractual life (in years) | |
| 8.61 | | |
| 8.90 | | |
| - | | |
| - | |
|
| X |
- DefinitionThe disclosure of the number and weighted average remaining contractual life of outstanding share options. [Refer: Weighted average [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DisclosureOfNumberAndWeightedAverageRemainingContractualLifeOfOutstandingShareOptionsExplanatory |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfShareBasedCompensationStockOptionsActivityExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfTermsStockOptionReserveExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
(LOSS) PER SHARE (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Profit or loss [abstract] |
|
| Schedule of basic and diluted EPS calculations |
| Schedule of basic and diluted EPS calculations | |
| | | |
| | |
| | |
Three Months Ended June 30, |
| Numerator (in 000’$) | |
2023 | |
2022 |
| | |
| |
|
| Net loss attributable to owners of the Company | |
$ | (5,919 | ) | |
$ | (1,729 | ) |
| Denominator (in 000’) | |
| | | |
| | |
| Weighted average number of shares – Basic and Diluted | |
| 17,701 | | |
| 13,351 | |
| Basic and diluted (loss) per share | |
$ | (0.33 | ) | |
$ | (0.13 | ) |
|
| Schedule of anti-dilutive share |
| Schedule of anti-dilutive share | |
| | | |
| | |
| | |
As of June 30, |
| | |
2023 | |
2022 |
| Stock options | |
| 1,963,420 | | |
| 1,201,400 | |
| Restricted stock units | |
| 378,740 | | |
| 378,740 | |
|
| X |
- References
+ Details
| Name: |
ifrs-full_IncomeStatementAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfAntiDilutiveEffectTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfEarningsPerShareTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| Schedule of financial instruments |
| Schedule of financial instruments | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTOCI | |
FVTPL | |
Amortized
Cost | |
FVTOCI | |
FVTPL |
| | |
| |
| |
| |
| |
| |
|
| Financial assets | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 7,698 | | |
$ | - | | |
$ | - | | |
$ | 10,545 | | |
$ | - | | |
$ | - | |
| Prepaid expenses and other receivables | |
$ | 2,752 | | |
$ | - | | |
$ | - | | |
$ | 2,689 | | |
$ | - | | |
$ | - | |
| Convertible note receivable, including accrued interest, net of impairment | |
$ | - | | |
$ | - | | |
$ | 442 | | |
$ | - | | |
$ | - | | |
$ | 442 | |
| Investment in associate | |
$ | - | | |
$ | - | | |
$ | 756 | | |
$ | - | | |
$ | - | | |
$ | 806 | |
| Investment in public company | |
$ | - | | |
$ | 3,855 | | |
$ | - | | |
$ | - | | |
$ | 2,087 | | |
$ | - | |
| | |
| | | |
| | | |
| | | |
| | |
| | |
As of June 30, 2023 | |
As of March 31, 2023 |
| | |
Amortized
Cost | |
FVTPL | |
Amortized
Cost | |
FVTPL |
| Financial liabilities | |
| | | |
| | | |
| | | |
| | |
| Accounts payable and accrued liabilities | |
$ | 2,591 | | |
$ | - | | |
$ | 1,865 | | |
$ | - | |
| Lease liability - current | |
$ | 47 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Lease liability - non-current | |
$ | 249 | | |
$ | - | | |
$ | - | | |
$ | - | |
| Deferred purchase price payable - Tarus | |
$ | - | | |
$ | 7,864 | | |
$ | - | | |
$ | 7,179 | |
| Deferred obligation - iOx milestone | |
$ | - | | |
$ | 4,552 | | |
$ | - | | |
$ | 4,126 | |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureOfFinancialAssetsAndFinancialLiabilitiesExplanatory |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
NON-CONTROLLING INTEREST (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes and other explanatory information [abstract] |
|
| Schedule of non-controlling interest |
| Schedule of non-controlling interest | |
| | | |
| | | |
| | |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2023 | |
$ | - | | |
$ | (650 | ) | |
$ | (650 | ) |
| Net loss attributable to non-controlling interest | |
| - | | |
| (7 | ) | |
| (7 | ) |
| Non-controlling interest as of June 30, 2023 | |
$ | - | | |
$ | (657 | ) | |
$ | (657 | ) |
| (In thousands) | |
iOx | |
Saugatuck
and subsidiary | |
Total |
| Non-controlling interest as of April 1, 2022 | |
$ | 44,701 | | |
$ | (472 | ) | |
$ | 44,229 | |
| Net income (loss) attributable to non-controlling interest | |
| 175 | | |
| (71 | ) | |
| 104 | |
| Non-controlling interest as of June 30, 2022 | |
$ | 44,876 | | |
$ | (543 | ) | |
$ | 44,333 | |
|
| X |
- References
+ Details
| Name: |
ptgef_ScheduleOfNoncontrollingInterestTableTextBlock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe percentage of voting equity interests acquired in a business combination. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_c&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_PercentageOfVotingEquityInterestsAcquired |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe proportion of ownership interest in a subsidiary attributable to the entity. [Refer: Subsidiaries [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 19B
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_19B_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProportionOfOwnershipInterestInSubsidiary |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ExchangeOfOrdinaryShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PercentageOfOutstandingShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_BottomOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_TopOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount of cash on hand and demand deposits. [Refer: Cash on hand] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Cash |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of cash held by the entity. This does not include demand deposits. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashOnHand |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_CashUsedInOperatingActivities |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NetLossIncome |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_TotalCurrentLiabilities |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe proportion of ownership interest in a subsidiary attributable to the entity. [Refer: Subsidiaries [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 19B
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_19B_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProportionOfOwnershipInterestInSubsidiary |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe proportion of ownership interests in a subsidiary held by non-controlling interests. [Refer: Subsidiaries [member]; Non-controlling interests] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 12
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_12_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProportionOfOwnershipInterestsHeldByNoncontrollingInterests |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_PercentageOfOutstandingShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_GeographicalAreasAxis=ptgef_CountryOfBritishVirginIslandsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_GeographicalAreasAxis=ptgef_CountryOfDelawareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_BottomOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_TopOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount of current advances made to suppliers before goods or services are received. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentAdvancesToSuppliers |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount recognised as a current asset for expenditures made prior to the period when the economic benefit will be realised. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentPrepaidExpenses |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current prepayments. [Refer: Prepayments] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentPrepayments |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current receivables from sale of properties. [Refer: Receivables from sale of properties] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentReceivablesFromSaleOfProperties |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current other receivables. [Refer: Other receivables] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 78
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_78_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_OtherCurrentReceivables |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PrepaidExpensesAndOtherReceivablesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ResearchAndDevelopmentCredit |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_TaxDeposits |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe country in which an associate of the entity is incorporated. [Refer: Associates [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 21
-Subparagraph a
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_21_a_iii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CountryOfIncorporationOrResidenceOfAssociate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe name of an associate. [Refer: Associates [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 21
-Subparagraph a
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_21_a_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NameOfAssociate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe principal place of business of an associate. [Refer: Principal place of business; Associates [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 21
-Subparagraph a
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_21_a_iii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_PrincipalPlaceOfBusinessOfAssociate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe proportion of the voting rights in an associate held by the entity. [Refer: Associates [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_17_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 16
-Subparagraph b
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_16_b_iii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 21
-Subparagraph a
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_21_a_iv&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProportionOfVotingPowerHeldInAssociate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_SignificantInvestmentsInAssociatesAxis=ptgef_StimunitySAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount of investments in subsidiaries, joint ventures and associates in an entity's separate financial statements. [Refer: Associates [member]; Joint ventures [member]; Subsidiaries [member]; Investments in subsidiaries reported in separate financial statements] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 27
-IssueDate 2023-01-01
-Paragraph 10
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=27&code=ifrs-tx-2023-en-r&anchor=para_10&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_InvestmentsInSubsidiariesJointVenturesAndAssociates |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of the unrecognised share of associates' losses if the entity has stopped recognising its share of losses when applying the equity method. [Refer: Associates [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 22
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_22_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_UnrecognisedShareOfLossesOfAssociates |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureInvestmentInAssociateAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INVESTMENT IN ASSOCIATE (Details Narrative) - USD ($)
$ in Thousands |
|
|
3 Months Ended |
12 Months Ended |
Jul. 13, 2023 |
Sep. 12, 2022 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Mar. 31, 2023 |
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
| Convertible Note description |
the Company entered into a commitment with Stimunity
to provide €600,000 under a convertible note (the “Stimunity Convertible Note”) with a maturity date of September 1,
2023 (the “Maturity Date”). The Stimunity Convertible Note provides for simple interest at 7% per annum. The Stimunity Convertible
Note is automatically converted into Series A shares of Stimunity upon Stimunity completing a Series A round for at least €20 million.
If such subscription round is completed prior to the Maturity Date, the Company will be entitled to convert the Stimunity Convertible
Note into Series A shares of Stimunity at the subscription share price less 15%. Additionally, if Stimunity completes a financing with
a new category of shares (other than Common Shares or Series A shares) for at least €5 million (the “Minimum Raise”),
the Company will have the right to convert the Stimunity Convertible Note and the historical Series A shares of Stimunity owned into the
new category of shares. In the event that Stimunity does not close a financing prior to the Maturity Date or raises less than the Minimum
Raise, the Company will have the right to convert the Stimunity Convertible Note into Series A shares at €363.00 per share or the
raise price less 15%, whichever is lower. The Stimunity Convertible Note was funded on September 12, 2022. See Note 15, “Commitments
and Contingent Liabilities – Stimunity Convertible Note,” for a further discussion.
|
|
|
|
|
| Translated value |
|
$ 614
|
|
|
|
| Unrealized gain |
|
|
|
|
$ 39
|
| Increasing the carrying value |
|
|
|
|
653
|
| Impairment analysis |
|
|
|
|
607
|
| Stimunity convertible note receivable |
|
|
|
|
211
|
| Decreasing the carrying value |
|
|
|
|
806
|
| Stimunity convertible note |
|
|
|
|
$ 442
|
| Loss on shares recognize |
|
|
$ 50
|
|
|
| Changes in the market |
|
|
0
|
|
|
| Stimunity S A [Member] |
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
| Equity in (loss) income |
|
|
$ 50,000
|
$ 60,000
|
|
| X |
- References
+ Details
| Name: |
ptgef_ChangesInMarket |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ConvertibleNoteDescription |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DecreasingCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_EquityInLossIncome |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ImpairmentAnalysis |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IncreasingCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_LossOnSharesRecognize |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_StimunityConvertibleNote |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_StimunityConvertibleNoteReceivable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_TranslatedValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_UnrealizedGain |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_SignificantInvestmentsInAssociatesAxis=ptgef_StimunitySAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
INVESTMENT IN PUBLIC COMPANY (Details Narrative) - USD ($)
$ / shares in Units, $ in Thousands |
|
|
3 Months Ended |
12 Months Ended |
|
|
|
|
|
Jul. 07, 2023 |
Jul. 05, 2023 |
Jun. 30, 2023 |
Jun. 30, 2022 |
Mar. 31, 2023 |
Apr. 30, 2023 |
Dec. 31, 2022 |
Nov. 30, 2022 |
Oct. 31, 2022 |
Jul. 11, 2019 |
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Per share price |
|
|
|
|
|
|
|
$ 5.00
|
$ 4.00
|
|
| Impairment asset fair value |
|
|
|
|
|
|
$ 3,363
|
|
|
|
| Impairment assets carring value |
|
|
|
|
|
|
$ 4,046
|
|
|
|
| Holding shares |
|
|
|
|
|
644,229
|
|
|
|
|
| Fair value interest |
|
|
$ 2,087
|
|
|
|
|
|
|
|
| Cash carrying value |
|
|
$ 3,855
|
|
|
|
|
|
|
|
| Number of shares sold |
|
|
2,425,999
|
|
|
|
|
|
|
|
| Unrealized gain or loss |
|
|
$ 1,768
|
$ 0
|
|
|
|
|
|
|
| O C I [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Impairment loss |
|
|
|
|
$ 5,322
|
|
|
|
|
|
| Cash carrying value |
|
|
|
|
$ 2,087
|
|
|
|
|
|
| Bottom of range [member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Offering price |
|
|
|
|
|
$ 4.00
|
|
|
|
|
| Top of range [member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Offering price |
|
|
|
|
|
$ 5.00
|
|
|
|
|
| Intensity [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares sold |
585,000
|
3,900,000
|
|
|
|
|
|
|
|
|
| Price per share |
|
5.00
|
5.96
|
|
|
|
|
|
|
|
| Proceeds from sales of biological assets |
|
$ 16,200
|
|
|
|
|
|
|
|
|
| Additional antidilution shares |
|
|
2,659
|
|
|
|
|
|
|
|
| Percentage of voting equity interests acquired |
4.70%
|
|
|
|
|
|
|
|
|
|
| Intensity [Member] | Acquisition Of Salva Rx [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Investment |
|
|
$ 4,500
|
|
|
|
|
|
|
|
| Number of shares purchased |
|
|
1,000.0
|
|
|
|
|
|
|
|
| Percentage of equity held |
|
|
7.50%
|
|
|
|
|
|
|
|
| Intensity [Member] | Acquisition Of Intensity Holding Limited [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Equity interests of acquirer |
|
|
|
|
|
|
|
|
|
$ 1,300
|
| Number of ordinary shares issued in acquisition |
|
|
|
|
|
|
|
|
|
129,806
|
| Number of private company share consists in sole asset |
|
|
|
|
|
|
|
|
|
288,458
|
| Number of shares outstanding |
|
|
|
|
|
|
|
|
|
1,288,458
|
| X |
- DefinitionThe fair value, at the acquisition date, of equity interests of the acquirer transferred as consideration in a business combination. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph f
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_f_iv&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_EquityInterestsOfAcquirer |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount recognised as a reduction of the carrying amount of an asset or cash-generating unit to its recoverable amount. [Refer: Carrying amount [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 130
-Subparagraph d
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_130_d_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 130
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_130_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ImpairmentLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of interest recognised as a receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_InterestReceivable |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares that have been authorised and issued, reduced by treasury shares held. [Refer: Treasury shares] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 79
-Subparagraph a
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_79_a_iv&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NumberOfSharesOutstanding |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe nominal value per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 79
-Subparagraph a
-Clause iii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_79_a_iii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ParValuePerShare |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe percentage of voting equity interests acquired in a business combination. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_c&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_PercentageOfVotingEquityInterestsAcquired |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe fair value of defined benefit plan assets. Plan assets comprise assets held by a long-term employee benefit fund and qualifying insurance policies. [Refer: At fair value [member]] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 19
-IssueDate 2023-01-01
-Paragraph 57
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=19&code=ifrs-tx-2023-en-r&anchor=para_57_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_PlanAssetsAtFairValue |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from sales of biological assets. [Refer: Biological assets] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 16
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_16&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProceedsFromSalesOfBiologicalAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_AdditionalAntidilutionShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CashCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ImpairmentAssetsCarringValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_InvestmentOwnedShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_InvestmentOwnershipPercentage |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfInstrumentsOrInterestsIssuedOrIssuables |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfPrivateCompanyShareConsistsInSoleAsset |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesSold |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_OfferingPerSharePrice |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PricePerShare |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_PrivateInvestmentsInterest |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ReverseStockSplitHoldingShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_UnrealizedGainOrLoss |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_ClassesOfIntangibleAssetsAndGoodwillAxis=ptgef_OCIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_BottomOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_RangeAxis=ifrs-full_TopOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_TypesOfInvestmentPropertyAxis=ptgef_IntensityMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_BusinessCombinationsAxis=ptgef_AcquisitionOfSalvaRxMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_BusinessCombinationsAxis=ptgef_AcquisitionOfIntensityHoldingLimitedMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount of current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of liabilities related to the entity's leases. Lease is a contract, or part of a contract, that conveys the right to use an underlying asset for a period of time in exchange for consideration. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_LeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of non-current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LeaseLiabilityInterest |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDueInRollingYearFive |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDueInRollingYearFour |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDueInRollingYearThree |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDueInRollingYearTwo |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_LesseeOperatingLeaseLiabilityPaymentDueNextRollingTwelveMonths |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe amount of long-term deposits held by the entity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_LongtermDeposits |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe fair value, at acquisition date, of the consideration transferred in a business combination. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph f
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_f&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_AcquisitiondateFairValueOfTotalConsiderationTransferred |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_AcquisitiondateFairValueOfTotalConsiderationTransferredAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_AssetsAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of contingent liabilities recognised in a business combination. [Refer: Contingent liabilities [member]; Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B67
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B67_c&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_ContingentLiabilitiesRecognisedInBusinessCombination |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount recognised as of the acquisition date for deferred tax liabilities assumed in a business combination. [Refer: Deferred tax liabilities; Business combinations [member]] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_i&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_DeferredTaxLiabilitiesRecognisedAsOfAcquisitionDate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of assets representing the future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognised. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 134
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_134_a&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 135
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_135_a&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B67
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B67_d&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_Goodwill |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount recognised as of the acquisition date for net identifiable assets acquired or liabilities assumed in a business combination. [Refer: Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_i&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph IE72
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_IE72&doctype=Illustrative%20Examples
-URIDate 2023-03-23
| Name: |
ifrs-full_IdentifiableAssetsAcquiredLiabilitiesAssumed |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount recognised as of the acquisition date for identifiable intangible assets acquired in a business combination. [Refer: Intangible assets other than goodwill; Business combinations [member]] Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_i&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph IE72
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_IE72&doctype=Illustrative%20Examples
-URIDate 2023-03-23
| Name: |
ifrs-full_IdentifiableIntangibleAssetsRecognisedAsOfAcquisitionDate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe fair value, at acquisition date, of other tangible or intangible assets (including a business or subsidiary of the acquirer) transferred as consideration in a business combination, that the entity does not separately disclose in the same note. [Refer: Intangible assets other than goodwill; Business combinations [member]; Subsidiaries [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 3
-IssueDate 2023-01-01
-Paragraph B64
-Subparagraph f
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=3&code=ifrs-tx-2023-en-r&anchor=para_B64_f_ii&doctype=Appendix&subtype=B
-URIDate 2023-03-23
| Name: |
ifrs-full_OtherTangibleOrIntangibleAssetsTransferred |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_TransactionsRecognisedSeparatelyFromAcquisitionOfAssetsAndAssumptionOfLiabilitiesInBusinessCombinationAxis=ptgef_TarusAcquisitionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
ACQUISITION OF TARUS (Details 1) - USD ($)
$ / shares in Units, $ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
| Loss before provision for income taxes |
$ (6,071)
|
$ (4,177)
|
| Net loss |
(5,926)
|
(1,625)
|
| Total comprehensive loss for period |
$ (4,157)
|
(1,625)
|
| Tarus Acquisition [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Loss from operations |
|
(3,788)
|
| Loss before provision for income taxes |
|
(3,893)
|
| Net loss |
|
(1,341)
|
| Total comprehensive loss for period |
|
$ (1,341)
|
| Loss per share |
|
$ (0.09)
|
| X |
- DefinitionThe amount of change in equity resulting from transactions and other events, other than those changes resulting from transactions with owners in their capacity as owners. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_c&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause ix
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_ix&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ComprehensiveIncome |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe total of income less expenses from continuing and discontinued operations, excluding the components of other comprehensive income. [Refer: Other comprehensive income] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 18
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_18_b&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 32
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_32_a_ii&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 1
-IssueDate 2023-01-01
-Paragraph 24
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=1&code=ifrs-tx-2023-en-r&anchor=para_24_b&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B10
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B10_b&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 17
-IssueDate 2023-01-01
-Paragraph 113
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=17&code=ifrs-tx-2023-en-r&anchor=para_113_b&doctype=Standard
-URIDate 2023-03-23
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_a&doctype=Standard
-URIDate 2023-03-23
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) before tax expense or income. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 5
-IssueDate 2023-01-01
-Paragraph 33
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=5&code=ifrs-tx-2023-en-r&anchor=para_33_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 103
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_103&doctype=Standard
-URIDate 2023-03-23
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 102
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_102&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossBeforeTax |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) from continuing operations. [Refer: Continuing operations [member]; Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 28
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_28_b&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B12
-Subparagraph b
-Clause vi
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B12_b_vi&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 8
-IssueDate 2023-01-01
-Paragraph 23
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=8&code=ifrs-tx-2023-en-r&anchor=para_23&doctype=Standard
-URIDate 2023-03-23
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81A
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81A_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossFromContinuingOperations |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_EarningLossPerShare |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_TransactionsRecognisedSeparatelyFromAcquisitionOfAssetsAndAssumptionOfLiabilitiesInBusinessCombinationAxis=ptgef_TarusAcquisitionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
ACQUISITION OF TARUS (Details Narrative) - USD ($)
$ in Thousands |
3 Months Ended |
|
Jun. 30, 2023 |
Jul. 01, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
| Number of share issued |
2,425,999
|
|
| Ordinary shares value |
$ 18,000
|
|
| Proceeds from offering |
32,000
|
|
| Short term debt |
2,000
|
|
| Milestone payment |
17,000
|
|
| Ordinary shares issued Value |
|
$ 17,200
|
| Deferred purchase price payable |
8,538
|
|
| Tarus Acquisition [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Milestone payment |
$ 15,000
|
|
| X |
- DefinitionThe cash inflow from the issuing of ordinary shares. [Refer: Ordinary shares [member]] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 17
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_17&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProceedsFromIssueOfOrdinaryShares |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of expense from employee benefits (other than termination benefits) that are expected to be settled wholly within twelve months after the end of the annual reporting period in which the employees render the related services. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ShorttermEmployeeBenefitsExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredPurchasePricePayable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_MilestonePayment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfShareIssued |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_OrdinarySharesIssuedValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_OrdinarySharesValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_TransactionsRecognisedSeparatelyFromAcquisitionOfAssetsAndAssumptionOfLiabilitiesInBusinessCombinationAxis=ptgef_TarusAcquisitionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
IN-PROCESS RESEARCH AND DEVELOPMENT AND DEFERRED TAX LIABILITY (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Mar. 31, 2023 |
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
$ 81,683
|
$ 81,683
|
| Deferred tax liability |
13,510
|
13,195
|
| I M M 60 I O X Melanoma Lung Cancers [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
36,181
|
36,181
|
| I M M 65 I O X Melanoma Lung Cancers [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
21,709
|
21,709
|
| I O X [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
57,890
|
57,890
|
| Oncomer Saugatuck D N A Aptamers [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
178
|
178
|
| Tarus Adenosine Receptors [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
22,723
|
22,723
|
| Tarus Adenosine Receptors 1 [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
420
|
420
|
| Tarus Adenosine Receptors 2 [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
472
|
472
|
| Tarus [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| In-process research and development |
$ 23,615
|
$ 23,615
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxesLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_InprocessResearchAndDevelopment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_IMM60IOXMelanomaLungCancersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_IMM65IOXMelanomaLungCancersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_IOXMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_OncomerSaugatuckDNAAptamersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TarusAdenosineReceptorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TarusAdenosineReceptors1Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TarusAdenosineReceptors2Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TarusMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
ptgef_CarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_Impairment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_RecognizedImpairment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_IoxIPRAndDMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_BorrowingsByNameAxis=ifrs-full_TopOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_BorrowingsByNameAxis=ifrs-full_BottomOfRangeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TrusIPRAndDMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionAdjustments for deferred tax expense to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Deferred tax expense (income); Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForDeferredTaxExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of income taxes payable (recoverable) in respect of the taxable profit (tax loss) for a period. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 80
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_80_a&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentTaxExpenseIncome |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CurrentTaxExpenseIncomeAndAdjustmentsForCurrentTaxOfPriorPeriodsAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_DeferredTaxExpenseIncomeAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
ptgef_IncomeLossOnOrdinaryActivitiesBeforeTax |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_OtherLossesRecognized |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharebasedCompensationExpenseRecognizedForFinancialStatementPurposes |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_UtilizationOfLossesNotPreviouslyBenefitted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe amount of profit (loss) for a period before deducting tax expense. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_ii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AccountingProfit |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe applicable income tax rate. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_ii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ApplicableTaxRate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount that represents the difference between the tax expense (income) and the product of the accounting profit multiplied by the applicable tax rate(s) that relates to tax losses. [Refer: Accounting profit] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_TaxEffectOfTaxLosses |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe product of the accounting profit multiplied by the applicable tax rate(s). [Refer: Accounting profit; Applicable tax rate] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_TaxExpenseIncomeAtApplicableTaxRate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ForeignCurrencyEffect |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_RecognitionOfDeferredTaxAssets |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
INCOME TAXES (Details 3) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Jun. 30, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
| Pre-tax loss |
$ (6,071)
|
$ (4,177)
|
| Share-based compensation expense for financial statement purposes for which no benefit was taken |
683
|
|
| Losses not subject to tax |
3,886
|
2,468
|
| Utilization of losses not previously benefitted |
(59)
|
(34)
|
| Taxable income (loss) |
(1,561)
|
(1,743)
|
| United State [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Pre-tax loss |
(609)
|
34
|
| Share-based compensation expense for financial statement purposes for which no benefit was taken |
683
|
|
| Losses not subject to tax |
|
|
| Utilization of losses not previously benefitted |
(59)
|
(34)
|
| Taxable income (loss) |
15
|
|
| B V I [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Pre-tax loss |
(3,886)
|
(2,468)
|
| Share-based compensation expense for financial statement purposes for which no benefit was taken |
|
|
| Losses not subject to tax |
3,886
|
2,468
|
| Utilization of losses not previously benefitted |
|
|
| Taxable income (loss) |
|
|
| United Kingdom [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Pre-tax loss |
(1,576)
|
(1,743)
|
| Share-based compensation expense for financial statement purposes for which no benefit was taken |
|
|
| Losses not subject to tax |
|
|
| Utilization of losses not previously benefitted |
|
|
| Taxable income (loss) |
$ (1,576)
|
$ (1,743)
|
| X |
- References
+ Details
| Name: |
ptgef_LossesNotSubjectToTax |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PretaxIncomeLoss |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PretaxLossSubjectToTax |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_SharebasedCompensationExpenseForFinancialStatementPurposesForWhichNoBenefitWasTaken |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_UtilizationOfLossNotPreviouslyBenefitted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_GeographicalAreasAxis=ptgef_UnitedStateMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_GeographicalAreasAxis=ptgef_BVIMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_GeographicalAreasAxis=ptgef_CountryOfUnitedKingdomMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
INCOME TAXES (Details 4) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Mar. 31, 2023 |
| Deferred tax assets: |
|
|
| Net operating loss |
$ (4,594)
|
$ (4,131)
|
| Deferred tax asset (unrecognized) |
1,500
|
1,500
|
| Deferred tax asset |
(3,094)
|
(2,631)
|
| Deferred tax liabilities: |
|
|
| In-process research and development |
13,510
|
13,195
|
| Deferred tax liability |
13,510
|
13,195
|
| Net deferred tax liability |
$ 10,416
|
$ 10,564
|
| X |
- DefinitionThe amount of deferred tax assets net of deferred tax liabilities, when the absolute amount of deferred tax assets is greater than the absolute amount of deferred tax liabilities. [Refer: Deferred tax assets; Deferred tax liabilities] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NetDeferredTaxAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of deferred tax liabilities net of deferred tax assets, when the absolute amount of deferred tax liabilities is greater than the absolute amount of deferred tax assets. [Refer: Deferred tax assets; Deferred tax liabilities] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NetDeferredTaxLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxAssetUnrecognized |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxAssetsAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxAssetsNetOperatingLossCarryforward |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxLiabilitiesInProcessRAndD |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxLiability1 |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxLiabiltiesAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
INCOME TAXES (Details Narrative) - USD ($)
$ in Thousands |
3 Months Ended |
12 Months Ended |
|
Jun. 30, 2023 |
Jun. 30, 2022 |
Mar. 31, 2022 |
Mar. 31, 2023 |
| IfrsStatementLineItems [Line Items] |
|
|
|
|
| Federal net operating losses |
$ 600
|
|
|
|
| Deferred tax assets |
200
|
|
|
$ 200
|
| Research and development credit receivables |
200
|
|
|
200
|
| Deferred tax liabilities |
10,416
|
|
|
10,564
|
| Deferred income taxes |
|
$ 7,000
|
|
|
| Current year losses |
700
|
|
|
|
| Increase (Decrease) in deferred tax liability |
|
|
$ 1,100
|
|
| Deferred tax liability |
100
|
|
|
|
| Change in exchange rates on the liability |
300
|
|
|
|
| Exchange rates losses |
400
|
|
|
|
| I O X [Member] |
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
| Deferred tax liabilities |
$ 10,400
|
|
|
$ 10,600
|
| X |
- DefinitionThe amounts of income taxes recoverable in future periods in respect of: (a) deductible temporary differences; (b) the carryforward of unused tax losses; and (c) the carryforward of unused tax credits. [Refer: Temporary differences [member]; Unused tax credits [member]; Unused tax losses [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph o
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_o&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 56
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_56&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DeferredTaxAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of tax expense (income) relating to changes in deferred tax liabilities and deferred tax assets. [Refer: Deferred tax assets; Deferred tax liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_ii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DeferredTaxExpenseIncome |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amounts of income taxes payable in future periods in respect of taxable temporary differences. [Refer: Temporary differences [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph g
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_g_i&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph o
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_o&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 56
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_56&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DeferredTaxLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe increase (decrease) in deferred tax liability (asset) resulting from business combinations. [Refer: Deferred tax liability (asset)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IncreaseDecreaseThroughBusinessCombinationsDeferredTaxLiabilityAsset |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount that represents the difference between the tax expense (income) and the product of the accounting profit multiplied by the applicable tax rate(s) that relates to changes in the tax rate. [Refer: Accounting profit] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 12
-IssueDate 2023-01-01
-Paragraph 81
-Subparagraph c
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=12&code=ifrs-tx-2023-en-r&anchor=para_81_c_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_TaxEffectFromChangeInTaxRate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CurrentYearLosses |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredTaxLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ExchangeRatesLosses |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_FederalNetOperatingLosses |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ResearchAndDevelopmentCreditReceivables |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
CAPITAL STOCK (Details) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Capital Stock |
|
|
| Beginning balance, Shares |
17,606
|
13,349
|
| Beginning balance, Amount |
$ 218,782
|
$ 158,324
|
| Shares issued under public offering and ATM, net of issue cost, shares |
171
|
|
| Shares issued under public offering and ATM, net of issue costs |
$ 613
|
|
| Shares issued or accrued for services, shares |
9
|
4
|
| Shares issued or accrued for services |
$ 30
|
$ 30
|
| Ending balance, Shares |
17,786
|
13,353
|
| Ending balance, Amount |
$ 219,425
|
$ 158,354
|
| X |
- DefinitionThe increase (decrease) in the number of ordinary shares issued. [Refer: Ordinary shares [member]] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_IncreaseDecreaseInNumberOfOrdinarySharesIssued |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase in equity resulting from the sale or issue of treasury shares. [Refer: Treasury shares] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_SaleOrIssueOfTreasuryShares |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CommonStockShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_CommonStockValues |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureCapitalStockAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedUnderPublicOfferingAndAtmNetOfIssueCostShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharesIssuedUnderPublicOfferingAndAtmNetOfIssueCosts |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
v3.23.2
CAPITAL STOCK (Details Narrative) - USD ($)
$ in Thousands |
|
|
3 Months Ended |
12 Months Ended |
Jul. 18, 2022 |
Jul. 06, 2022 |
Jun. 30, 2023 |
Mar. 31, 2023 |
Mar. 31, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
| Maximum aggregate offering price |
|
|
$ 50,000,000
|
|
|
| Issuance and sales, shares |
|
|
1,150,000
|
|
|
| Gross proceeds from stock |
|
|
$ 26,500
|
|
|
| Cash fund, description |
|
|
The Sales Agreement permits the Company to sell in an ATM program up to
$50,000,000 of ordinary shares from time to time, the amount of which is included in the $200,000,000 of securities that may be offered,
issued and sold by the Company under the base prospectus. The sales under the prospectus will be deemed to be made pursuant to an ATM
program as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended. Upon termination of the Sales Agreement,
any portion of the $50,000,000 included in the Sales Agreement prospectus that is not sold pursuant to the Sales Agreement will be available
for sale in other offerings pursuant to the base prospectus.
|
|
|
| Number of stock sold |
|
|
|
|
90,888
|
| Gross proceeds from stock sold |
|
|
|
|
$ 2,600
|
| Net proceeds from stock sold |
|
|
|
|
$ 2,500
|
| Number of shares sold |
|
|
2,425,999
|
|
|
| Number of shares exchanged |
1,070,000
|
|
|
|
|
| Minority interest, percentage |
21.68%
|
|
|
|
|
| Number of ordinary share issued |
|
94,508
|
|
|
|
| A T M [Member] |
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
| Number of shares sold |
|
|
171,504
|
166,145
|
|
| Net proceeds from sale |
|
|
$ 600
|
$ 900
|
|
| Lincoln [Member] |
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
| Number of shares sold |
|
|
|
480,000
|
|
| Net proceeds from sale |
|
|
|
$ 2,000
|
|
| X |
- DefinitionThe cash inflow from sales of biological assets. [Refer: Biological assets] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 16
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_16&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProceedsFromSalesOfBiologicalAssets |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CashFundDescription |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_GrossProceedsFromStock |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_GrossProceedsFromStockSold |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IssuanceAndSalesShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_MaximumAggregateOfferingPrice |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_MinorityInterestPercentage |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NetProceedsFromStockSold |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfOrdinaryShareIssued |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesExchanged |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesSold |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfStockSold |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_ATMMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_LincolnMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
STOCK OPTION RESERVE (Details) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
| Stock based compensation expense |
$ 769
|
$ 1,176
|
| Non-controlling interests [member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Begiining balance |
|
11,659
|
| Stock based compensation expense |
|
|
| Ending balance |
|
11,659
|
| Stock Option Reserve [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Begiining balance |
21,204
|
16,928
|
| Stock based compensation expense |
769
|
1,176
|
| Ending balance |
$ 21,973
|
$ 18,104
|
| X |
- DefinitionAdjustments for share-based payments to reconcile profit (loss) to net cash flow from (used in) operating activities. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 20
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_20_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_AdjustmentsForSharebasedPayments |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_StockOptionReserve |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_ComponentsOfEquityAxis=ifrs-full_NoncontrollingInterestsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_ComponentsOfEquityAxis=ptgef_StockOptionReserveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
STOCK OPTION RESERVE (Details 1) - shares
|
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Stock Options Two [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Beginning balance |
|
1,275
|
| Granted |
|
|
| Expired or forfeited |
|
(1,275)
|
| Ending balance |
|
|
| Exercisable as at end |
|
|
| P B I 2021 Equity Incentive Plan [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Beginning balance |
1,963,420
|
1,151,400
|
| Granted |
|
50,000
|
| Expired or forfeited |
|
|
| Ending balance |
1,963,420
|
1,201,400
|
| Exercisable as at end |
764,438
|
410,600
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfOutstandingShareOption |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfShareOptionsExercisableInSharebasedPaymentsArrangements |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfShareOptionsExpiredOrForfeitedInSharebasedPaymentsArrangement |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfShareOptionsGrantedInSharebasedPaymentsArrangement |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_TypesOfAntidilutiveInstrumentsAxis=ptgef_StockOptionsTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_DefinedBenefitPlansAxis=ptgef_PBI2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe weighted average exercise price of share options exercised in a share-based payment arrangement. [Refer: Weighted average [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph b
-Clause iv
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_b_iv&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_WeightedAverageExercisePriceOfShareOptionsExercisedInSharebasedPaymentArrangement2019 |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average remaining contractual life of outstanding share options. [Refer: Weighted average [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_WeightedAverageRemainingContractualLifeOfOutstandingShareOptions2019 |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_TypesOfAntidilutiveInstrumentsAxis=ptgef_StockOptionsTwoMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_DefinedBenefitPlansAxis=ptgef_PBI2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
STOCK OPTION RESERVE (Details Narrative)
$ / shares in Units, $ in Thousands |
|
|
|
|
|
|
3 Months Ended |
12 Months Ended |
|
|
Mar. 30, 2023
$ / shares
shares
|
Jul. 27, 2022
$ / shares
shares
|
Jun. 08, 2022
$ / shares
shares
|
Feb. 15, 2022
$ / shares
shares
|
Jan. 19, 2022
$ / shares
shares
|
Jan. 13, 2021
$ / shares
shares
|
Jun. 30, 2023
USD ($)
shares
|
Jun. 30, 2022
USD ($)
|
Mar. 31, 2021
USD ($)
|
Mar. 31, 2023
shares
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
746,120
|
|
|
|
|
|
|
|
|
|
| Exercisable price | $ / shares |
$ 2.92
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
879,180
|
|
|
|
|
|
|
|
|
|
| Expiration date |
Mar. 30, 2033
|
|
|
|
|
|
|
|
|
|
| Number of shares purchased |
14,600
|
|
|
|
|
|
|
|
|
|
| Number of shares available for granted |
87,600
|
|
|
|
|
|
|
|
|
|
| Total of stock options granted to employees |
651,020
|
|
|
|
|
|
|
|
|
|
| Stock options granted to consultant |
7,500
|
|
|
|
|
|
|
|
|
|
| Share based compensation expense | $ |
|
|
|
|
|
|
$ 800
|
$ 1,200
|
|
|
| Vested Options [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Options outstanding |
|
|
|
|
|
|
7,500
|
|
|
7,500
|
| Unvested Options [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Options outstanding |
|
|
|
|
|
|
738,620
|
|
|
738,620
|
| Board Of Directors [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Option issued |
|
|
|
|
|
350,000
|
|
|
|
|
| Consultants [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Option issued |
|
|
|
|
|
518,000
|
|
|
|
|
| N 2021 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Option issued |
|
|
|
|
|
868,000
|
|
|
|
|
| Exercise price | $ / shares |
|
|
|
|
|
$ 17.75
|
|
|
|
|
| Restricted Stock Units [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
|
|
|
243,000
|
|
|
|
|
| Fair value per share | $ / shares |
|
|
|
|
|
$ 17.75
|
|
|
|
|
| Compensation expense | $ |
|
|
|
|
|
|
|
|
$ 4,300
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
|
|
302,000
|
|
|
|
|
|
| Fair value per share | $ / shares |
|
|
|
|
$ 10.22
|
|
|
|
|
|
| Number of shares available for awards |
2,880,992
|
|
|
|
|
|
|
|
|
|
| Share based compensation expense | $ |
|
|
|
|
|
|
$ 3,400
|
|
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] | Board Of Directors [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
15,900
|
|
|
|
|
|
|
|
|
| Exercisable price | $ / shares |
|
$ 10.06
|
|
|
|
|
|
|
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] | Two Members [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
|
|
13,800
|
|
|
|
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] | Employees [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
|
|
288,200
|
|
|
|
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] | Directors [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
|
13,800
|
135,740
|
|
|
|
|
|
| Fair value per share | $ / shares |
|
|
|
$ 8.59
|
$ 10.22
|
|
|
|
|
|
| Amended And Restated 2021 Equity Incentive Plan [Member] | Executive [Member] |
|
|
|
|
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
|
|
|
|
| Number of shares available for awards |
|
|
50,000
|
|
|
|
|
|
|
|
| Exercisable price | $ / shares |
|
|
$ 11.00
|
|
|
|
|
|
|
|
| X |
- DefinitionThe amount of expense arising from communication. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 112
-Subparagraph c
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_112_c&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CommunicationExpense |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe exercise price of outstanding share options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ExercisePriceOfOutstandingShareOptions2019 |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of compensation to key management personnel in the form of share-based payments. [Refer: Key management personnel of entity or parent [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 24
-IssueDate 2023-01-01
-Paragraph 17
-Subparagraph e
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=24&code=ifrs-tx-2023-en-r&anchor=para_17_e&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_KeyManagementPersonnelCompensationSharebasedPayment |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of share options outstanding in a share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_d&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph b
-Clause i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_b_i&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 2
-IssueDate 2023-01-01
-Paragraph 45
-Subparagraph b
-Clause vi
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=2&code=ifrs-tx-2023-en-r&anchor=para_45_b_vi&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NumberOfOutstandingShareOptions |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:decimalItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares issued by the entity. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 106
-Subparagraph d
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_106_d&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NumberOfSharesIssued |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ExercisablePrice |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ExpirationDateOfGrantOfSharebasedPaymentArrangement |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_FairValuePerShare |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfShareOptionsAvailableInSharebasedPaymentArrangement |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesAvailableForGranted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesAvailableForGrants |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesAvailableForOptionGranted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfSharesPurchased |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_StockGranted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_MeasurementAxis=ptgef_VestedOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_MeasurementAxis=ptgef_UnvestedOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_BoardOfDirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_ConsultantsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_DefinedBenefitPlansAxis=ptgef_N2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_DefinedBenefitPlansAxis=ptgef_RestrictedStockUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_DefinedBenefitPlansAxis=ptgef_AmendedAndRestated2021EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TwoMembersMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_EmployeesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_DirectorsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_ExecutiveMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
(LOSS) PER SHARE (Details) - USD ($)
$ / shares in Units, $ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Numerator (in 000’$) |
|
|
| Net loss attributable to owners of the Company |
$ (5,919)
|
$ (1,729)
|
| Denominator (in 000’) |
|
|
| Weighted average number of shares, Basic |
17,701
|
13,351
|
| Weighted average number of shares, Diluted |
17,701
|
13,351
|
| Basic earnings (loss) per share |
$ (0.33)
|
$ (0.13)
|
| Diluted earnings (loss) per share |
$ (0.33)
|
$ (0.13)
|
| X |
- DefinitionThe amount of profit (loss) attributable to ordinary equity holders of the parent entity (the numerator) divided by the weighted average number of ordinary shares outstanding during the period (the denominator). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 67
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_67&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_BasicEarningsLossPerShare |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of profit (loss) attributable to ordinary equity holders of the parent entity (the numerator), divided by the weighted average number of ordinary shares outstanding during the period (the denominator), both adjusted for the effects of all dilutive potential ordinary shares. [Refer: Ordinary shares [member]; Weighted average [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 66
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_66&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 33
-IssueDate 2023-01-01
-Paragraph 67
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=33&code=ifrs-tx-2023-en-r&anchor=para_67&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_DilutedEarningsLossPerShare |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe profit (loss) from continuing and discontinued operations attributable to owners of the parent. [Refer: Profit (loss)] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 81B
-Subparagraph a
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_81B_a_ii&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ProfitLossAttributableToOwnersOfParent |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DenominatorAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumeratorAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_WeightedAverageNumberOfSharesBasic |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_WeightedAverageNumberOfSharesDiluted |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
ptgef_AntidilutiveEffectOnLossPerShare |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_TypesOfAntidilutiveInstrumentsAxis=ifrs-full_ShareOptionsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_TypesOfAntidilutiveInstrumentsAxis=ifrs-full_RestrictedShareUnitsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
COMMITMENTS AND CONTINGENT LIABILITIES (Details Narrative)
€ / shares in Units, $ in Thousands |
|
3 Months Ended |
|
|
Jul. 06, 2022
USD ($)
shares
|
Jun. 30, 2023
USD ($)
|
Jul. 13, 2022
EUR (€)
€ / shares
|
| IfrsStatementLineItems [Line Items] |
|
|
|
| Budget cost related to services | $ |
|
$ 12,100
|
|
| Convertible note | € |
|
|
€ 600,000
|
| Interest rate |
|
|
7.00%
|
| Conversion of debt | € |
|
|
€ 20,000,000
|
| Conversion of debt into shares | € |
|
|
€ 5,000,000
|
| Share price, per share | € / shares |
|
|
€ 363.00
|
| Number of ordinary shares issued | shares |
94,508
|
|
|
| Lincoln [Member] |
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
| Aggregate value | $ |
$ 30,000
|
|
|
| Commitment fee | $ |
$ 900
|
|
|
| X |
- DefinitionThe interest rate on borrowings. [Refer: Borrowings] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IFRS
-Number 7
-IssueDate 2023-01-01
-Paragraph 7
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=7&code=ifrs-tx-2023-en-r&anchor=para_7&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_BorrowingsInterestRate |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_AggregateValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CommitmentFee |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ConversionOfDebt |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ConversionOfDebtIntoShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ConvertibleNote |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_CostAndExpenses |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NumberOfOrdinaryShareIssued |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_SharePricePerShare |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_LincolnMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
RELATED PARTY TRANSACTIONS (Details Narrative) - USD ($)
|
|
|
|
|
3 Months Ended |
|
Dec. 31, 2022 |
Dec. 19, 2022 |
Dec. 15, 2021 |
Sep. 08, 2021 |
Jun. 30, 2023 |
Mar. 31, 2023 |
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
| Portage ordinary shares |
|
|
|
|
1,070,000
|
|
| Base salary |
|
$ 469,000
|
$ 618,000
|
|
|
|
| Annual base salary |
|
|
$ 348,000
|
|
|
|
| Compensation committee |
$ 600,000
|
|
|
|
|
|
| Original annual targets percentage |
73.50%
|
|
|
|
|
|
| Accounts payable and accrued expenses |
|
|
|
|
$ 400,000
|
$ 400,000
|
| Annual fees description |
|
|
|
|
Additionally, the chairperson of each of the Audit Committee, Compensation Committee and
Nominating Committee is entitled to annual fees of $15,000, $12,000 and $8,000, respectively, payable quarterly in arrears. Members of
those committees will be entitled to annual fees of $7,500, $6,000 and $4,000, respectively, payable quarterly in arrears.
|
|
| Salva Rx [Member] |
|
|
|
|
|
|
| IfrsStatementLineItems [Line Items] |
|
|
|
|
|
|
| Issuance of unsecured notes, description |
|
|
|
Company,
through SalvaRx, completed a settlement of loans (including interest) to and receivables from iOx for services rendered in exchange for
23,772 ordinary shares of iOx at a price of £162. Simultaneously, the Company entered into an agreement with OSI, the holder of
$0.15 million notes plus accrued interest under which OSI exchanged the notes plus accrued interest for 820 shares of iOx.
The Company followed the guidance provided by an IFRS Discussion Group Public Meeting dated November 29, 2016, following the general tenets
of IAS 39, “Financial Instruments: Recognition and Measurement,” and IFRIC 19, “Extinguishing Financial Liabilities
with Equity Instruments,” and recorded the exchange at historical cost. Additionally, no profit or loss was recorded in connection
with the exchange. As a result of these transactions, the Company, through SalvaRx, increased its ownership of iOx from 60.49% to 78.32%.
|
|
|
| X |
- DefinitionA class of employee benefits expense that represents wages and salaries. [Refer: Employee benefits expense] Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 19
-IssueDate 2023-01-01
-Paragraph 9
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=19&code=ifrs-tx-2023-en-r&anchor=para_9&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_WagesAndSalaries |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_AccountsPayableAndAccruedExpenses |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_AnnualBaseSalary |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_AnnualFeesdescription |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_CompensationCommittee |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IssuanceOfUnsecuredNotesDescription |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_OriginalAnnualTargetsPercentage |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_PortageOrdinaryShares |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (Details) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Mar. 31, 2023 |
Jun. 30, 2022 |
Mar. 31, 2022 |
| Financial assets |
|
|
|
|
| Cash and cash equivalents |
$ 7,698
|
$ 10,545
|
$ 21,176
|
$ 23,352
|
| Financial liabilities |
|
|
|
|
| Lease liability - current |
47
|
|
|
|
| Lease liability - non-current |
249
|
|
|
|
| Deferred purchase price payable - Tarus |
7,864
|
7,179
|
|
|
| Deferred obligation - iOx milestone |
4,552
|
4,126
|
|
|
| Amortized Cost [Member] |
|
|
|
|
| Financial assets |
|
|
|
|
| Cash and cash equivalents |
7,698
|
10,545
|
|
|
| Prepaid expenses and other receivables |
2,752
|
2,689
|
|
|
| Convertible note receivable, including accrued interest, net of impairment |
|
|
|
|
| Investment in associate |
|
|
|
|
| Investment in public company |
|
|
|
|
| Financial liabilities |
|
|
|
|
| Accounts payable and accrued liabilities |
2,591
|
1,865
|
|
|
| Lease liability - current |
47
|
|
|
|
| Lease liability - non-current |
249
|
|
|
|
| Deferred purchase price payable - Tarus |
|
|
|
|
| Deferred obligation - iOx milestone |
|
|
|
|
| Fair Value To Other Comprehensive Income [Member] |
|
|
|
|
| Financial assets |
|
|
|
|
| Cash and cash equivalents |
|
|
|
|
| Prepaid expenses and other receivables |
|
|
|
|
| Convertible note receivable, including accrued interest, net of impairment |
|
|
|
|
| Investment in associate |
|
|
|
|
| Investment in public company |
3,855
|
2,087
|
|
|
| Fair Value Through Profit Or Loss [Member] |
|
|
|
|
| Financial assets |
|
|
|
|
| Cash and cash equivalents |
|
|
|
|
| Prepaid expenses and other receivables |
|
|
|
|
| Convertible note receivable, including accrued interest, net of impairment |
442
|
442
|
|
|
| Investment in associate |
756
|
806
|
|
|
| Investment in public company |
|
|
|
|
| Financial liabilities |
|
|
|
|
| Accounts payable and accrued liabilities |
|
|
|
|
| Lease liability - current |
|
|
|
|
| Lease liability - non-current |
|
|
|
|
| Deferred purchase price payable - Tarus |
7,864
|
7,179
|
|
|
| Deferred obligation - iOx milestone |
$ 4,552
|
$ 4,126
|
|
|
| X |
- DefinitionThe amount of cash on hand and demand deposits, along with short-term, highly liquid investments that are readily convertible to known amounts of cash and that are subject to an insignificant risk of changes in value. [Refer: Cash; Cash equivalents] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 7
-IssueDate 2023-01-01
-Paragraph 45
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=7&code=ifrs-tx-2023-en-r&anchor=para_45&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph B13
-Subparagraph a
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_B13_a&doctype=Appendix&subtype=B
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph i
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_i&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CashAndCashEquivalents |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CategoriesOfFinancialAssetsAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ifrs-full_CategoriesOfFinancialLiabilitiesAbstract |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of current investments. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 55
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_55&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentInvestments |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of non-current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_AccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_ConvertibleNoteReceivableIncludingAccruedInterestNetOfImpairment |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredObligationIoxMilestone |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DeferredPurchasePricePayableTarus |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_InvestmentInPrivateCompany |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_PrepaidExpensesAndOtherReceivable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
ifrs-full_LevelsOfFairValueHierarchyAxis=ptgef_AmortizedCostMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_LevelsOfFairValueHierarchyAxis=ptgef_FairValueToOtherComprehensiveIncomeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_LevelsOfFairValueHierarchyAxis=ptgef_FairValueThroughProfitOrLossMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount recognised as an increase of the carrying amount of an asset or cash-generating unit to its recoverable amount when an impairment loss had been previously recognised. [Refer: Impairment loss] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 130
-Subparagraph d
-Clause ii
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_130_d_ii&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 36
-IssueDate 2023-01-01
-Paragraph 130
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=36&code=ifrs-tx-2023-en-r&anchor=para_130_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_ReversalOfImpairmentLoss |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DecreasingCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_DecreasingImpairmentCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ImpairmentAnalysis |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_IncreasingCarryingValue |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_LossOnDecreaseInFairValueOfLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_NotesReceivable |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_UnrealizedGains |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_TarusMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
ifrs-full_CounterpartiesAxis=ptgef_IOXMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionThe amount of current lease liabilities. [Refer: Lease liabilities] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 16
-IssueDate 2023-01-01
-Paragraph 47
-Subparagraph b
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=16&code=ifrs-tx-2023-en-r&anchor=para_47_b&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_CurrentLeaseLiabilities |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_AccountsPayableAndAccruedLiability |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_CurrentAsset |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_DisclosureCapitalDisclosuresAbstract |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
ptgef_ShareholdersEquityAttributableToOwners |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
NON-CONTROLLING INTEREST (Details) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| IfrsStatementLineItems [Line Items] |
|
|
| Non-controlling interest, at beginning |
$ (650)
|
$ 44,229
|
| Net (loss) attributable to non-controlling interest |
(7)
|
104
|
| Non-controlling interest, at ending |
(657)
|
44,333
|
| I O X [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Non-controlling interest, at beginning |
|
44,701
|
| Net (loss) attributable to non-controlling interest |
|
175
|
| Non-controlling interest, at ending |
|
44,876
|
| Saugatuck And Subsidiary [Member] |
|
|
| IfrsStatementLineItems [Line Items] |
|
|
| Non-controlling interest, at beginning |
(650)
|
(472)
|
| Net (loss) attributable to non-controlling interest |
(7)
|
(71)
|
| Non-controlling interest, at ending |
$ (657)
|
$ (543)
|
| X |
- DefinitionThe amount of equity in a subsidiary not attributable, directly or indirectly, to a parent. [Refer: Subsidiaries [member]] Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 12
-IssueDate 2023-01-01
-Paragraph 12
-Subparagraph f
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=12&code=ifrs-tx-2023-en-r&anchor=para_12_f&doctype=Standard
-URIDate 2023-03-23
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name IFRS
-Number 10
-IssueDate 2023-01-01
-Paragraph 22
-URI https://taxonomy.ifrs.org/xifrs-link?type=IFRS&num=10&code=ifrs-tx-2023-en-r&anchor=para_22&doctype=Standard
-URIDate 2023-03-23
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name IAS
-Number 1
-IssueDate 2023-01-01
-Paragraph 54
-Subparagraph q
-URI https://taxonomy.ifrs.org/xifrs-link?type=IAS&num=1&code=ifrs-tx-2023-en-r&anchor=para_54_q&doctype=Standard
-URIDate 2023-03-23
| Name: |
ifrs-full_NoncontrollingInterests |
| Namespace Prefix: |
ifrs-full_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
ptgef_NetLossAttributableToNoncontrollingInterest |
| Namespace Prefix: |
ptgef_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
Portage Biotech (NASDAQ:PRTG)
Historical Stock Chart
From Mar 2024 to Apr 2024
Portage Biotech (NASDAQ:PRTG)
Historical Stock Chart
From Apr 2023 to Apr 2024