Q103-31false00014441920001444192us-gaap:EmployeeSeveranceMember2023-04-012023-06-3000014441922023-08-090001444192us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300001444192acst:StockOptionPlanMemberacst:ConsultantMember2023-04-012023-06-300001444192acst:AtTheMarketOfferingMember2022-04-012022-06-300001444192acst:TaxYear2035Member2023-06-300001444192us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-04-012022-06-300001444192us-gaap:RetainedEarningsMember2023-04-012023-06-300001444192acst:ReverseStockSplitMember2023-06-292023-06-2900014441922023-06-300001444192acst:TaxYear2041Member2023-06-300001444192us-gaap:SellingAndMarketingExpenseMember2023-04-012023-06-300001444192us-gaap:GeneralAndAdministrativeExpenseMember2022-04-012022-06-300001444192acst:WarrantsIssuedDecember272017Member2023-06-300001444192acst:CommonClassDAndCommonClassEMembersrt:MinimumMember2023-04-012023-06-300001444192us-gaap:OtherRestructuringMember2023-04-012023-06-3000014441922023-05-080001444192acst:StockOptionPlanMemberus-gaap:RelatedPartyMember2023-04-012023-06-300001444192acst:TaxYearTwoThousandFortyThreeMember2023-06-300001444192us-gaap:RetainedEarningsMember2023-06-3000014441922020-06-2900014441922022-03-142022-03-140001444192us-gaap:CommonClassCMember2023-06-300001444192us-gaap:EmployeeSeveranceMember2023-03-310001444192acst:WarrantsIssuedDecember272017Member2022-06-300001444192us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-300001444192acst:RKOSupplyAgreementMember2019-10-250001444192us-gaap:RetainedEarningsMember2022-06-300001444192acst:WarrantsIssuedInMay2018Member2022-06-300001444192acst:CanadianDepositsMember2023-06-300001444192acst:TaxYear2033Member2023-06-3000014441922022-03-140001444192acst:AtTheMarketOfferingMember2020-06-292020-06-290001444192acst:TaxYear2032Member2023-06-300001444192acst:AtTheMarketOfferingMember2023-04-012023-06-300001444192us-gaap:AdditionalPaidInCapitalMember2022-03-310001444192acst:WarrantsIssuedInMay2018Member2023-06-300001444192acst:EquityIncentivePlanMember2023-06-3000014441922020-06-292020-06-2900014441922023-03-310001444192us-gaap:RetainedEarningsMember2023-03-310001444192acst:StockOptionPlanMember2023-04-012023-06-300001444192us-gaap:CommonClassAMember2023-06-300001444192acst:RKOSupplyAgreementMember2023-06-300001444192us-gaap:AdditionalPaidInCapitalMember2023-06-300001444192us-gaap:OtherRestructuringMember2023-03-310001444192acst:TaxYear2037Member2023-06-300001444192us-gaap:CommonClassBMember2023-04-012023-06-300001444192acst:EquityIncentivePlanMember2022-04-012022-06-300001444192acst:TaxYear2040Member2023-06-300001444192us-gaap:OtherRestructuringMember2023-06-300001444192us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-03-310001444192acst:CanadianDepositsMember2023-03-310001444192us-gaap:SubsequentEventMemberacst:AssetPurchaseAgreementMember2023-07-030001444192acst:TaxYear2030Member2023-06-300001444192acst:EquityIncentivePlanMember2022-06-300001444192us-gaap:ResearchAndDevelopmentExpenseMember2022-04-012022-06-300001444192us-gaap:CommonStockMember2022-03-3100014441922022-06-300001444192acst:StockOptionPlanMember2023-06-300001444192us-gaap:AdditionalPaidInCapitalMember2022-04-012022-06-3000014441922023-04-012023-06-300001444192acst:WarrantsIssuedInMay2018Member2023-06-300001444192us-gaap:AdditionalPaidInCapitalMember2023-04-012023-06-300001444192acst:TaxYear2036Member2023-06-300001444192us-gaap:SubsequentEventMemberacst:AssetPurchaseAgreementMember2023-07-032023-07-030001444192acst:TaxYear2029Member2023-06-300001444192us-gaap:CommonStockMember2023-06-300001444192acst:TaxYear2042Member2023-06-300001444192acst:TaxYear2031Member2023-06-300001444192us-gaap:SubsequentEventMember2023-07-142023-07-140001444192us-gaap:RetainedEarningsMember2022-03-310001444192acst:StockOptionPlanMember2023-03-310001444192acst:TaxYear2034Member2023-06-300001444192us-gaap:CommonClassCMember2023-04-012023-06-300001444192us-gaap:CommonStockMember2023-03-310001444192us-gaap:AdditionalPaidInCapitalMember2022-06-300001444192us-gaap:EmployeeSeveranceMember2023-06-300001444192us-gaap:CommonStockMember2022-06-3000014441922022-04-012022-06-300001444192us-gaap:SellingAndMarketingExpenseMember2022-04-012022-06-300001444192acst:TaxYear2038Member2023-06-300001444192acst:CommonClassDAndCommonClassEMembersrt:MaximumMember2023-04-012023-06-300001444192us-gaap:CommonClassBMember2023-06-300001444192acst:CommonClassDAndCommonClassEMember2023-06-300001444192acst:TaxYear2039Member2023-06-300001444192us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310001444192acst:EquityIncentivePlanMember2023-04-012023-06-300001444192us-gaap:CommonStockMember2022-04-012022-06-300001444192us-gaap:AdditionalPaidInCapitalMember2023-03-310001444192us-gaap:RetainedEarningsMember2022-04-012022-06-3000014441922022-03-3100014441922021-11-100001444192us-gaap:ResearchAndDevelopmentExpenseMember2023-04-012023-06-300001444192us-gaap:GeneralAndAdministrativeExpenseMember2023-04-012023-06-30iso4217:CADxbrli:sharesxbrli:purexbrli:sharesacst:Voteiso4217:USD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________
FORM 10-Q
______________________________________
(Mark One)
|
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2023
or
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-35776
______________________________________
Acasti Pharma Inc.
(Exact name of registrant as specified in its charter)
______________________________________
|
|
Québec, Canada |
98-1359336 |
(State or other jurisdiction of
incorporation or organization) |
(I.R.S. Employer
Identification Number) |
2572 boul. Daniel-Johnson, 2nd Floor
Laval, Québec, Canada H7T 2R3
(Address of principal executive offices, including zip code)
450-686-4555
(Registrant’s telephone number, including area code)
______________________________________
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Shares, no par value per share |
ACST |
NASDAQ Stock Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☐ |
|
Non-accelerated filer |
☒ |
|
Smaller reporting company |
☒ |
|
Emerging growth company |
☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of outstanding common shares of the registrant, no par value per share, as of August 9, 2023, was 7,448,033.
ACASTI PHARMA INC.
QUARTERLY REPORT ON FORM 10-Q
For the Quarter Ended June 30, 2023
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This quarterly report contains information that may be forward-looking information within the meaning of Canadian securities laws and forward-looking statements within the meaning of U.S. federal securities laws, both of which we refer to in this quarterly report as forward-looking information. Forward- looking information can be identified by the use of terms such as “may”, “will”, “should”, “expect”, “plan”, “anticipate”, “believe”, “intend”, “estimate”, “predict”, “potential”, “continue” or other similar expressions concerning matters that are not statements about the present or historical facts.
Although the forward-looking statements in this quarterly report are based upon what we believe are reasonable assumptions, you should not place undue reliance on those forward-looking statements since actual results may vary materially from them.
In addition, the forward-looking statements in this quarterly report are subject to a number of known and unknown risks, uncertainties and other factors many of which are beyond our control, that could cause our actual results and developments to differ materially from those that are disclosed in or implied by the forward-looking statements, including, among others:
•We are heavily dependent on the success of our lead drug candidate, GTX-104.
•Clinical development is a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results. Failure can occur at any stage of clinical development.
•We are subject to uncertainty relating to healthcare reform measures and reimbursement policies which, if not favorable to our drug candidates, could hinder or prevent our drug candidates’ commercial success.
•If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug products, if approved, we may be unable to generate any revenue.
•If we are unable to differentiate our drug products from branded reference drugs or existing generic therapies for similar treatments, or if the U.S. Food and Drug Administration (“FDA”) or other applicable regulatory authorities approve products that compete with any of our drug products, our ability to successfully commercialize our drug products would be adversely affected.
2
•Our success depends in part upon our ability to protect our intellectual property for our drug candidates.
•Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
•We do not have internal manufacturing capabilities, and if we fail to develop and maintain supply relationships with various third-party manufacturers, we may be unable to develop or commercialize our drug candidates.
•The design, development, manufacture, supply, and distribution of our drug candidates are highly regulated and technically complex.
•If we fail to meet applicable listing requirements, the Nasdaq Stock Market may delist our common shares from trading, in which case the liquidity and market price of our common shares could decline.
•The other risks and uncertainties identified in Item 1A. Risk Factors included in our Annual Report on Form 10-K for the year ended March 31, 2023.
All of the forward-looking statements in this quarterly report are qualified by this cautionary statement. There can be no guarantee that the results or developments that we anticipate will be realized or, even if substantially realized, that they will have the consequences or effects on our business, financial condition, or results of operations that we anticipate. As a result, you should not place undue reliance on the forward-looking statements. Except as required by applicable law, we do not undertake to update or amend any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements are made as of the date of this quarterly report.
We express all amounts in this quarterly report in U.S. dollars, except where otherwise indicated. References to “$” and “U.S.$” are to U.S. dollars and references to “C$” or “CAD$” are to Canadian dollars.
Except as otherwise indicated, references in this quarterly report to “Acasti,” “the Corporation,” “we,” “us” and “our” refer to Acasti Pharma Inc. and its consolidated subsidiaries.
3
PART I. FINANCIAL INFORMATION
Item 1: Financial Information
Unaudited Condensed Consolidated Interim Financial Statements
4
ACASTI PHARMA INC.
Condensed Consolidated Interim Balance Sheet
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
March 31,
2023 |
(Expressed in thousands of U.S. dollars except share data) |
|
Notes |
|
$ |
|
$ |
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
21,633 |
|
27,875 |
Short-term investments |
|
5 |
|
15 |
|
15 |
Receivables |
|
4 |
|
837 |
|
802 |
Prepaid expenses |
|
|
|
1,127 |
|
598 |
Total current assets |
|
|
|
23,612 |
|
29,290 |
|
|
|
|
|
|
|
Operating lease right of use asset |
|
|
|
71 |
|
463 |
Equipment |
|
|
|
84 |
|
104 |
Intangible assets |
|
|
|
41,128 |
|
41,128 |
Goodwill |
|
|
|
8,138 |
|
8,138 |
Total assets |
|
|
|
73,033 |
|
79,123 |
|
|
|
|
|
|
|
Liabilities and Shareholders’ equity |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Trade and other payables |
|
7 |
|
1,886 |
|
3,336 |
Operating lease liability |
|
8 |
|
80 |
|
75 |
Total current liabilities |
|
|
|
1,966 |
|
3,411 |
|
|
|
|
|
|
|
Operating lease liability |
|
|
|
— |
|
410 |
Deferred tax liability |
|
|
|
7,057 |
|
7,347 |
Total liabilities |
|
|
|
9,023 |
|
11,168 |
|
|
|
|
|
|
|
Shareholders’ equity: |
|
|
|
|
|
|
Common shares, no par value per share; unlimited shares authorized as of June 30, 2023 and March 31, 2023; 7,435,533 shares issued and outstanding as of June 30, 2023 and March 31, 2023 |
|
9(a) |
|
258,294 |
|
258,294 |
Additional paid-in capital |
|
|
|
14,043 |
|
13,965 |
Accumulated other comprehensive loss |
|
|
|
(6,038) |
|
(6,038) |
Accumulated deficit |
|
|
|
(202,289) |
|
(198,266) |
Total shareholders' equity |
|
|
|
64,010 |
|
67,955 |
|
|
|
|
|
|
|
Commitments and contingencies |
|
14 |
|
|
|
|
Total liabilities and shareholders’ equity |
|
|
|
73,033 |
|
79,123 |
See accompanying notes to unaudited interim financial statements.
5
ACASTI PHARMA INC.
Condensed Consolidated Interim Statements of Loss and Comprehensive Loss
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
(Expressed in thousands of U.S dollars, except share and per share data) |
|
Notes |
|
$ |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
Research and development expenses, net of government assistance |
|
6 |
|
|
(1,095 |
) |
|
|
(2,590 |
) |
General and administrative expenses |
|
|
|
|
(1,763 |
) |
|
|
(1,919 |
) |
Sales and marketing |
|
|
|
|
(111 |
) |
|
|
(221 |
) |
Restructuring cost |
|
15 |
|
|
(1,485 |
) |
|
|
— |
|
Loss from operating activities |
|
|
|
|
(4,454 |
) |
|
|
(4,730 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign exchange gain (loss) |
|
|
|
|
8 |
|
|
|
(78 |
) |
Change in fair value of warrant liabilities |
|
|
|
|
— |
|
|
|
10 |
|
Interest income and other expense |
|
|
|
|
134 |
|
|
|
32 |
|
Total other income (loss), net |
|
|
|
|
142 |
|
|
|
(36 |
) |
Loss before income tax recovery |
|
|
|
|
(4,312 |
) |
|
|
(4,766 |
) |
|
|
|
|
|
|
|
|
|
Income tax recovery |
|
|
|
|
289 |
|
|
|
242 |
|
|
|
|
|
|
|
|
|
|
Net loss and total comprehensive loss |
|
|
|
|
(4,023 |
) |
|
|
(4,524 |
) |
|
|
|
|
|
|
|
|
|
Basic and diluted loss per share |
|
11 |
|
|
(0.54 |
) |
|
|
(0.61 |
) |
|
|
|
|
|
|
|
|
|
Weighted average number of shares outstanding |
|
|
|
|
7,435,533 |
|
|
|
7,388,065 |
|
See accompanying notes to unaudited interim financial statements
6
ACASTI PARMA INC.
Condensed Consolidated Interim Statements of Shareholders' Equity
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands of U.S. dollars except share data) |
|
Notes |
|
|
Number |
|
|
Dollar |
|
|
Additional
paid-in
capital |
|
|
Accumulated
other
comprehensive
loss |
|
|
Deficit |
|
|
Total |
|
|
|
|
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Balance, March 31, 2023 |
|
|
|
|
|
7,435,533 |
|
|
|
258,294 |
|
|
|
13,965 |
|
|
|
(6,038 |
) |
|
|
(198,266 |
) |
|
|
67,955 |
|
Net loss and total comprehensive loss for the period |
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,023 |
) |
|
|
(4,023 |
) |
Stock-based compensation |
|
|
10 |
|
|
|
— |
|
|
|
— |
|
|
|
78 |
|
|
|
— |
|
|
|
— |
|
|
|
78 |
|
Balance at June 30, 2023 |
|
|
|
|
|
7,435,533 |
|
|
|
258,294 |
|
|
|
14,043 |
|
|
|
(6,038 |
) |
|
|
(202,289 |
) |
|
|
64,010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Shares |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands of US dollars except for share data) |
|
Notes |
|
Number |
|
|
Dollar |
|
|
Additional
paid-in
capital |
|
|
Accumulated
other
comprehensive
loss |
|
|
Deficit |
|
|
Total |
|
|
|
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Balance, March 31, 2022 |
|
|
|
|
7,381,425 |
|
|
|
257,990 |
|
|
|
12,154 |
|
|
|
(6,037 |
) |
|
|
(155,837 |
) |
|
|
108,270 |
|
Net loss and total comprehensive loss for the period |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4,524 |
) |
|
|
(4,524 |
) |
Cumulative translation adjustment |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2 |
) |
|
|
— |
|
|
|
(2 |
) |
Stock-based compensation |
|
10 |
|
|
— |
|
|
|
— |
|
|
|
464 |
|
|
|
— |
|
|
|
— |
|
|
|
464 |
|
Net proceeds from shares issued under the at-the-market (ATM) program |
|
|
|
|
34,335 |
|
|
|
195 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
195 |
|
Balance at June 30, 2022 |
|
|
|
|
7,415,760 |
|
|
|
258,185 |
|
|
|
12,618 |
|
|
|
(6,039 |
) |
|
|
(160,361 |
) |
|
|
104,403 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7
ACASTI PHARMA INC.
Condensed Consolidated Interim Statements of Cash Flows
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
(Expressed in thousands of U.S. dollars) |
|
Notes |
|
$ |
|
|
$ |
|
Cash flows used in operating activities: |
|
|
|
|
|
|
|
|
Net loss for the period |
|
|
|
|
(4,023 |
) |
|
|
(4,524 |
) |
Adjustments: |
|
|
|
|
|
|
|
|
Depreciation of equipment |
|
|
|
|
7 |
|
|
|
167 |
|
Stock-based compensation |
|
10 |
|
|
78 |
|
|
|
464 |
|
Change in fair value of warrant liabilities |
|
|
|
|
— |
|
|
|
(10 |
) |
Income tax recovery |
|
|
|
|
(289 |
) |
|
|
(242 |
) |
Unrealized foreign exchange (gain) loss |
|
|
|
|
— |
|
|
|
(10 |
) |
Write-off of equipment |
|
|
|
|
13 |
|
|
|
— |
|
Changes in operating assets and liabilities |
|
12 |
|
|
(2,026 |
) |
|
|
(1,271 |
) |
Net cash used in operating activities |
|
|
|
|
(6,240 |
) |
|
|
(5,426 |
) |
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
Acquisition of equipment |
|
|
|
|
— |
|
|
|
(7 |
) |
Acquisition of short-term investments |
|
|
|
|
— |
|
|
|
(16 |
) |
Maturity of short-term investment |
|
|
|
|
— |
|
|
|
13,281 |
|
Net cash from investing activities |
|
|
|
|
— |
|
|
|
13,258 |
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
Net proceeds from issuance under the at-the-market (ATM) program |
|
(9a) |
|
|
— |
|
|
|
195 |
|
Net cash from financing activities |
|
|
|
|
— |
|
|
|
195 |
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate fluctuations on cash and cash equivalents |
|
|
|
|
(2 |
) |
|
|
11 |
|
|
|
|
|
|
|
|
|
|
Net (decrease) increase in cash and cash equivalents |
|
|
|
|
(6,242 |
) |
|
|
8,038 |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period |
|
|
|
|
27,875 |
|
|
|
30,339 |
|
Cash and cash equivalents, end of period |
|
|
|
|
21,633 |
|
|
|
38,377 |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents are comprised of: |
|
|
|
|
|
|
|
|
Cash |
|
|
|
|
5,413 |
|
|
|
38,377 |
|
Cash equivalents |
|
|
|
|
16,220 |
|
|
|
— |
|
See accompanying notes to unaudited interim financial statements.
8
ACASTI PHARMA INC.
Notes to Condensed Consolidated Interim Financial Statements
(Unaudited)
(Expressed in thousands of U.S. dollars except share data)
1. Nature of operation
Acasti Pharma Inc. (“Acasti” or the “Corporation”) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 2572 boul. Daniel-Johnson, 2nd Floor Laval, Québec, Canada H7T 2R3.
The Corporation’s shares are listed on the Nasdaq Capital Market (the "Nasdaq"), and through March 27, 2023 the Corporation's shares were also listed on the TSX Venture Exchange ("TSXV"), in each case, under the symbol "ACST". On March 13, 2023 the Corporation received approval to voluntarily delist from the TSXV. Effective as at the close of trading on March 27, 2023, the Corporation's common shares are no longer listed and posted for trading on the TSXV.
In August 2021, the Corporation completed the acquisition via a share-for-share merger of Grace Therapeutics, Inc. (“Grace”), a privately held emerging biopharmaceutical company focused on developing innovative drug delivery technologies for the treatment of rare and orphan diseases. The post-merger Corporation is focused on building a late-stage specialty pharmaceutical company specializing in rare and orphan diseases and developing and commercializing products that improve clinical outcomes using its novel drug delivery technologies. The Corporation seeks to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by the Corporation for further development may be already approved in the target indication or could be repurposed for use in new indications.
The Corporation has incurred operating losses and negative cash flows from operations in each year since its inception. The Corporation expects to incur significant expenses and continued operating losses for the foreseeable future.
In May 2023, the Corporation implemented a strategic realignment plan to enhance shareholder value that resulted in the Corporation engaging a new management team, streamlining its research and development activities to concentrate on its lead product, GTX 104, and greatly reducing its workforce. Moving forward, the Corporation plans to build a smaller, more focused organization in the United States. Further development of GTX-102 and GTX-101 will occur at such time as additional funding is obtained or strategic partnerships are entered into. This strategic realignment is expected to significantly reduce administrative and research and development expenses and enable the Corporation to extend its available cash resources to the second calendar quarter of 2025.
The Corporation will require additional capital to fund our daily operating needs beyond that time. The Corporation does not expect to generate revenue from product sales unless and until it successfully completes drug development and obtains regulatory approval, which the Corporation expects will take several years and is subject to significant uncertainty. To date, the Corporation has financed its operations primarily through public offerings and private placements of its common shares, warrants and convertible debt and the proceeds from research tax credits. Until such time that the Corporation can generate significant revenue from drug product sales, if ever, it will require additional financing, which is expected to be sourced from a combination of public or private equity or debt financing or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties. Arrangements with collaborators or others may require the Corporation to relinquish certain rights related to its technologies or drug product candidates. Adequate additional financing may not be available to the Corporation on acceptable terms, or at all. The Corporation’s inability to raise capital as and when needed could have a negative impact on its financial condition and its ability to pursue its business strategy. The Corporation plans to raise additional capital prior to that time in order to maintain adequate liquidity. Negative results from studies, if any, and depressed prices of the Corporation’s stock could impact the Corporation’s ability to raise additional financing. Raising additional equity capital is subject to market conditions not within the Corporation’s control. If the Corporation does not raise additional funds in this time period, the Corporation may not be able to realize our assets and discharge our liabilities in the normal course of business.
The Corporation remains subject to risks similar to other development stage companies in the biopharmaceutical industry, including compliance with government regulations, protection of proprietary technology, dependence on third-party contractors and consultants and potential product liability, among others. Please refer to the risk factors included in Part 1, Item 1A of the Corporation’s annual report on Form 10-K for the year ended March 31, 2023, filed with the SEC on June 23, 2023 (the “Annual Report”).
Reverse stock split
On June 29, 2023, the Board of Directors of the Corporation approved an amendment to the Corporation's Articles of Incorporation to implement a reverse stock split of the Corporation's Class A common shares, no par value per share, at a ratio of 1-for-6 (the “Reverse Stock Split”). On July 4, 2023, the Corporation filed Articles of Amendment to its Articles of Incorporation with the Registraire des entreprises du Québec, to implement the Reverse Stock Split. All references in these financial statements to number of common shares, warrants and
9
options, price per share and weighted average number of shares outstanding have been adjusted to reflect the Reverse Stock Split, which became effective on July 10, 2023.
2. Summary of significant accounting policies:
Basis of presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X under the Securities Exchange Act of 1934. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements as of and for the year ended March 31, 2023, and, in the opinion of management, reflect all adjustments, consisting of normal recurring adjustments, necessary for the fair presentation of the Corporation’s consolidated financial position as of June 30, 2023, the consolidated results of its operations for the three months ended June 30, 2023 and 2022, its statements of shareholders’ equity for the three months ended June 30, 2023 and 2022 and its consolidated cash flows for the three months ended June 30, 2023 and 2022.
These unaudited condensed consolidated financial statements should be read in conjunction with the Corporation’s audited consolidated financial statements and the accompanying notes for the year ended March 31, 2023 included in the Corporation’s Annual Report. The condensed consolidated balance sheet data as of March 31, 2023 presented for comparative purposes was derived from the Corporation’s audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. The results for the three months ended June 30, 2023 are not necessarily indicative of the operating results to be expected for the full year or for any other subsequent interim period.
The Corporation’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report. There have been no changes to the Corporation's significant accounting policies since the date of the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report.
Use of estimates
The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based compensation, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date, and determining which research and development expenses qualify for research and development tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized.
3. Recent accounting pronouncements
The Corporation has considered recent accounting pronouncements and concluded that they are either not applicable to the business or that the effect is not expected to be material to the consolidated financial statements as a result of future adoption.
4. Receivables
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
March 31,
2023 |
|
|
Notes |
|
$ |
|
$ |
Sales tax receivables |
|
|
|
426 |
|
338 |
Government assistance |
|
6 |
|
361 |
|
412 |
Interest receivable |
|
|
|
50 |
|
52 |
Total receivables |
|
|
|
837 |
|
802 |
10
5. Short-term investments
The Corporation holds various marketable securities, with maturities greater than 3 months at the time of purchase, as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31,
2023 |
|
|
|
$ |
|
|
$ |
|
Term deposits issued in CAD currency earning interest at 3% and maturing on March 29, 2024 |
|
|
15 |
|
|
|
15 |
|
Total short-term investments |
|
|
15 |
|
|
|
15 |
|
6. Government assistance
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31, 2023 |
|
|
|
$ |
|
|
$ |
|
Investment tax credit |
|
|
361 |
|
|
|
412 |
|
Government assistance is comprised of research and development investment tax credits from the Québec provincial government, which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. For the three months ended June 30, 2023 and 2022, the Corporation recorded $(51) and $41, respectively, as an increase and a reduction of research and development expenses in the Statement of Loss and Comprehensive Loss.
Unrecognized Canadian federal tax credits may be used to reduce future Canadian federal income tax and expire as follows:
|
|
|
|
|
|
|
$ |
|
2029 |
|
9 |
|
2030 |
|
|
23 |
|
2031 |
|
|
36 |
|
2032 |
|
|
345 |
|
2033 |
|
|
353 |
|
2034 |
|
|
348 |
|
2035 |
|
|
415 |
|
2036 |
|
|
229 |
|
2037 |
|
|
252 |
|
2038 |
|
|
259 |
|
2039 |
|
|
355 |
|
2040 |
|
|
226 |
|
2041 |
|
|
146 |
|
2042 |
|
|
312 |
|
2043 |
|
|
642 |
|
|
|
|
3,950 |
|
7. Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31, 2023 |
|
|
|
$ |
|
|
$ |
|
Trade payables |
|
|
607 |
|
|
|
1,242 |
|
Accrued liabilities and other payables |
|
|
994 |
|
|
|
946 |
|
Employee salaries and benefits payable |
|
|
285 |
|
|
|
1,148 |
|
Total trade and other payables |
|
|
1,886 |
|
|
|
3,336 |
|
8. Leases
The Corporation has historically entered into lease arrangements for its research and development and quality control laboratory facility located in Sherbrooke, Québec. As of June 30, 2023, the Corporation had one operating lease with required future minimum payments. On
11
March 14, 2022, the Corporation renewed the lease agreement effective April 1, 2022, resulting in a commitment of $556 over a 24 months base lease term and 48 months additional lease renewal term. In April 2023, the Corporation elected not to renew the additional 48 months lease renewal term with the lease expected to terminate March 31, 2024. The Corporation accounted for the change in lease term as a lease modification under ASC 842. Due to the modification in lease term, the Corporation remeasured the lease liability and right-of-use asset associated with the lease. As of the effective date of modification, the Corporation recorded an adjustment to the right-of-use asset and lease liability in the amount of $369 based on the net present value of lease payments discounted using an estimate incremental borrowing rate of 4.3%.
The following table contains a summary of the lease costs recognized under ASC 842 and other information pertaining to the Corporation’s operating lease for the three month period ended June 30, 2023:
|
|
|
|
|
Operating cash flows for operating lease |
|
$ |
24 |
|
Weighted-average remaining lease term (in years) |
|
|
0.75 |
|
Weighted-average discount rate |
|
|
4.3 |
% |
As the Corporation's lease do not provide an implicit rate, the Corporation utilized its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Corporation could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment.
Future minimum lease payments under the Corporation’s operating lease as of June 30, 2023 were as follows:
|
|
|
|
|
|
|
June 30, 2023 |
|
|
|
$ |
|
2024 |
|
|
81 |
|
2025 and thereafter |
|
|
- |
|
Total lease payments |
|
|
81 |
|
Less: interest |
|
|
(1 |
) |
Total lease liability |
|
|
80 |
|
9. Capital and other components of equity
a. Common Shares
Authorized capital stock
Unlimited number of shares
•Class A shares (Common Shares), voting (one vote per share), participating and without par value. As of June 30, 2023, there were 7,435,533 Class A shares issued and outstanding.
•Class B shares, voting (ten votes per share), non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class B shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class B shares are redeemable at the holder’s discretion for CAD $4.80 per share, subject to certain conditions. As of June 30, 2023, there were no Class B shares issued and outstanding.
•Class C shares, non-voting, non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class C shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class C shares are redeemable at the holder’s discretion for CAD $1.20 per share, subject to certain conditions. As of June 30, 2023, there were no Class C shares issued and outstanding.
•Class D and E shares, they are non-voting, non-participating, without par value and maximum monthly non-cumulative dividend between 0.5% and 2% on the amount paid per share. Class D and E shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class D and E shares are redeemable at the holder’s discretion, subject to certain conditions. As of June 30, 2023, there were no Class D or E shares issued and outstanding.
At-the-Market (“ATM”) Program
On June 29, 2020, the Corporation entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley FBR, Inc. ("B.Riley"), Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend the Corporation’s existing ATM program. Under the terms of the Sales Agreement, which had a three-year term, the Corporation could issue and sell from time-to-time common shares having aggregate gross proceeds of up to $75,000,000 through the Agents. Subject to the terms and conditions of the
12
Sales Agreement, the Agents would use their commercially reasonable efforts to sell the common shares from time to time, based upon the Corporation’s instructions. The Corporation had no obligation to sell any of the common shares and could, at any time, suspend sales under the Sales Agreement. The Corporation and the Agents could terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, the Corporation provided the Agents with customary indemnification rights and the Agents were entitled to compensation at a commission rate equal to 3.0% of the gross proceeds from each sale of the common shares. The Sales Agreement expired pursuant to its terms on June 29, 2023 and the Corporation plans to revisit the renewal of a facility in the coming months.
On November 10, 2021, the Corporation filed a prospectus supplement relating to its at-the-market program, expiring July 7, 2023, with B. Riley, Oppenheimer& Co. Inc. and H.C. Wainwright & Co., LLC acting as agents. Under the terms of the ATM Sales Agreement and the prospectus supplement, the Corporation may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the agents; however, our use of the shelf registration statement on Form S-3 will be limited for so long as we are subject to General Instruction I.B.6 of Form S-3, which limits the amounts that we may sell under the registration statement and in accordance with the ATM agreement. The common shares will be distributed at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The volume and timing of sales under the ATM program, if any, will be determined at the sole discretion of the Corporation’s board of directors and management.
During the three months ended June 30, 2023, no common shares were sold under the ATM program. During the three months ended June 30, 2022, 34,335 common shares were sold for total net proceeds of approximately $195 with commissions, legal expenses and costs related to the share sale amounting to $6. The common shares were sold at the prevailing market prices, which resulted in an average price of approximately $5.82 per share.
b. Warrants
During the three month period ended June 30, 2023, the remaining 137,370 warrants to acquire one common share at an exercise price of CAD $62.88 expired on May 9, 2023.
10. Stock-based compensation
At June 30, 2023, the Corporation had in place a stock option plan for directors, officers, employees, and consultants of the Corporation (“Stock Option Plan”).
The Stock Option Plan continues to provide for the granting of options to purchase common shares. Under the terms of the Stock Option Plan, the exercise price of the stock options granted under the Stock Option Plan may not be lower than the closing price of the Corporation’s common shares on the Nasdaq Capital Market at the close of such market the day preceding the grant. The maximum number of common shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 20% of the aggregate number of issued and outstanding shares of the Corporation as of July 28, 2022. The terms and conditions for acquiring and exercising options are set by the Corporation’s Board of Directors, subject to, among others, the following limitations: the term of the options cannot exceed ten years and (i) all options granted to a director will be vested evenly on a monthly basis over a period of at least twelve (12) months, and (ii) all options granted to an employee will be vested evenly on a quarterly basis over a period of at least thirty-six (36) months.
The total number of options issued to any one consultant within any twelve-month period cannot exceed 2% of the Corporation’s total issued and outstanding common shares (on a non-diluted basis). The Corporation is not authorized to grant within any twelve-month period such number of options under the Stock Option Plan that could result in a number of common shares issuable pursuant to options granted to (a) related persons exceeding 2% of the Corporation’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve-month period exceeding 2% of the Corporation’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted.
The following table summarizes information about activities within the Stock Option Plan for the three month period ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
options |
|
|
Weighted average
exercise price |
|
|
Weighted average
grant date
fair value |
|
Outstanding, March 31, 2023 |
|
|
740,957 |
|
|
|
13.60 |
|
|
|
11.23 |
|
Forfeited/Cancelled |
|
|
(267,797 |
) |
|
|
7.72 |
|
|
|
6.21 |
|
Outstanding, June 30, 2023 |
|
|
473,178 |
|
|
|
16.93 |
|
|
|
14.07 |
|
Exercisable, June 30, 2023 |
|
|
402,247 |
|
|
|
18.62 |
|
|
|
15.51 |
|
Forfeited and cancelled options were as a result of the Corporations restructuring that occurred during the three months ended June 30, 2023. No options were granted or exercised during the three month period ended June 30, 2023.
13
Compensation expense recognized under the stock option plan is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
|
|
$ |
|
|
$ |
|
Research and development expenses |
|
|
2 |
|
|
|
158 |
|
General and administrative expenses |
|
|
60 |
|
|
|
282 |
|
Sales and marketing expenses |
|
|
16 |
|
|
|
24 |
|
|
|
|
78 |
|
|
|
464 |
|
As of June 30, 2023, there was $130 of total unrecognized compensation cost, related to non-vested stock options, which is expected to be recognized over a remaining weighted average vesting period of 0.97 years.
Corporation equity incentive plan
The Corporation established an equity incentive plan (the “Equity Incentive Plan”) for employees, directors, and consultants. The Equity Incentive Plan provides for the issuance of restricted share units (RSUs), performance share units, restricted shares, deferred share units and other stock-based awards, subject to restricted conditions as may be determined by the Board of Directors. There were no such awards outstanding as of June 30, 2023, and June 30, 2022, and no stock-based compensation was recognized for the period ended June 30, 2023 and June 30, 2022 under the Equity Incentive Plan.
11. Loss per share
Diluted loss per share was the same amount as basic loss per share, as the effect of options, and warrants would have been anti-dilutive, as the Corporation has incurred losses in each of the periods presented. All currently outstanding options could potentially be dilutive in the future.
The Corporation excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
|
|
|
|
June 30, 2023 |
|
June 30, 2022 |
Options outstanding |
|
473,178 |
|
704,585 |
May 2018 public offering warrants |
|
— |
|
137,370 |
December 2017 public offering warrants |
|
— |
|
147,354 |
12. Supplemental cash flow disclosure
Changes in operating assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
|
$ |
|
|
$ |
|
Receivables |
|
|
(35 |
) |
|
|
(434 |
) |
Prepaid expenses |
|
|
(529 |
) |
|
|
(839 |
) |
Trade and other payables |
|
|
(1,449 |
) |
|
|
2 |
|
Write-off of operating lease right of use asset |
|
|
(13 |
) |
|
|
— |
|
Total changes in operating assets and liabilities |
|
|
(2,026 |
) |
|
|
(1,271 |
) |
14
13. Financial instruments
a. Concentration of credit risk
Financial instruments that potentially subject the Corporation to a concentration of credit risk consist primarily of cash and cash equivalents and investments. Cash and cash equivalents and investments are all invested in accordance with the Corporation’s Investment Policy with the primary objective being the preservation of capital and the maintenance of liquidity, which risk is managed by dealing only with highly rated Canadian institutions. The carrying amount of financial assets, as disclosed in the consolidated balance sheets, represents the Corporation’s credit exposure at the reporting date.
b. Foreign currency risk
The Corporation is exposed to financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of the Corporation's business transactions denominated in currencies other than the Corporation's functional currency of the U.S. dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in the Corporation's operating results. The Corporation does not use derivative instruments to hedge exposure to foreign exchange risk. The fluctuation of the Canadian dollar in relation to the U.S. dollar and other foreign currencies will consequently have an impact upon the Corporation’s net loss.
c. Liquidity risk
Liquidity risk is the risk that the Corporation will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Corporation manages liquidity risk through the management of its capital structure and financial leverage. It also manages liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves the Corporation's operating budgets, and reviews material transactions outside the normal course of business. The Corporation currently does not have long-term debt nor arranged committed sources of financing and is operating via use of existing cash and short-term investment balances. Refer to Note 1 – Nature of Operations.
The Corporation’s financial liabilities obligations include trade and other payables, which fall due within the next 12 months.
14. Commitments and contingencies
Research and development contracts and contract research organizations agreements
The Corporation utilize contract manufacturing organizations ("CMOs") for the development and production of clinical materials and contract research organizations (“CROs”) to perform services related to its clinical trials. Pursuant to the agreements with these CMOs and CROs, the Corporation has either the right to terminate the agreements without penalties or under certain penalty conditions.
Raw krill oil supply contract
On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antarctic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre, one of the Corporation’s former drug candidates, for a total fixed value of $3.1 million. As at June 30, 2023, the remaining balance of the commitment with Aker amounts to $2.8 million. During the second calendar quarter of 2022, Aker informed the Corporation that Aker believed it had satisfied the terms of the supply agreement as to their obligation to deliver the remaining balance of raw krill oil product, and that the Corporation was therefore required to accept the remaining product commitment and to pay Aker the $2.8 million balance. The Corporation disagrees with Aker’s position and believes that Aker is not entitled to further payment under the supply agreement. Accordingly, no liability has been recorded. The dispute was unresolved as of June 30, 2023, and remains unresolved. There is uncertainty as to whether the Corporation will be required to make further payment to Aker in connection with the dispute. Additionally, in the event the Corporation is required to accept delivery from Aker of the remaining balance of raw krill oil product under the supply agreement, there is uncertainty as to whether the Corporation can recover value from the product, which may result in the Corporation incurring a loss on the supply agreement in the near term.
15
Legal proceedings and disputes
In the ordinary course of business, the Corporation is at times subject to various legal proceedings and disputes. The Corporation assesses its liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that the Corporation will incur a loss and the amount of the loss can be reasonably estimated, the Corporation records a liability in its consolidated financial statements. These legal contingencies may be adjusted to reflect any relevant developments. Where a loss is not probable or the amount of loss is not estimable, the Corporation does not accrue legal contingencies. While the outcome of legal proceedings is inherently uncertain, based on information currently available, management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on the Corporation’s financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to the Corporation’s financial position, results of operations, or cash flows. No reserves or liabilities have been accrued as of June 30, 2023.
15. Restructuring Costs
On May 8, 2023, the Corporation communicated its decision to terminate a substantial amount of its workforce as part of a plan that intended to align the Corporation’s organizational and management cost structure to prioritize resources to GTX-104 and reduce losses to improve cash flow and extend available cash resources. The Corporation incurred $1,485 of costs primarily consisting of employee severance costs. Unpaid Liabilities associated with the restructuring costs are recorded in trade and other payables on the consolidated balance sheets.
The Corporation’s restructuring charges and payments are comprised of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee |
|
|
|
|
|
|
|
|
|
severance |
|
|
Legal |
|
|
Total |
|
Expenses incurred |
|
|
1,447 |
|
|
|
38 |
|
|
|
1,485 |
|
Payments made |
|
|
(1,212 |
) |
|
|
- |
|
|
|
(1,212 |
) |
Balance at June 30, 2023 |
|
|
235 |
|
|
|
38 |
|
|
|
274 |
|
16. Subsequent events
On July 3, 2023, the Corporation entered into an asset purchase agreement to sell its lab equipment with a net book value of $54 as of June 30, 2023 for $109 in net proceeds.
On July 14, 2023, the Corporation's Board of Directors approved the grant of 446,502 stock options at an exercise price of $2.64 under the Corporation's Stock Option Plan.
On July 19, 2023, the Corporation entered into a new short term lease for its new headquarters located at 2572 boul. Daniel-Johnson, 2nd Floor Laval, Québec, Canada H7T 2R3. On July 24, 2023, the Corporation terminated it's lease for premises located at 3009 boul. de la Concorde East, Suite 102, Laval, Québec, Canada H7E 2B5.
16
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operation
This management’s discussion and analysis (“MD&A”) is presented in order to provide the reader with an overview of the financial results and changes to our consolidated balance sheets as at June 30, 2023, and for the three month period then ended. This MD&A also explains the material variations in our results of operations, consolidated balance sheets and cash flows as of and for the three months ended June 30, 2023 and 2022.
Market data, and certain industry data and forecasts included in this MD&A were obtained from internal Corporation surveys and market research conducted by third parties hired by us, publicly available information, reports of governmental agencies and industry publications, and independent third-party surveys. We have relied upon industry publications as our primary sources for third-party industry data and forecasts. Industry surveys, publications and forecasts generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of that information is not guaranteed. We have not independently verified any of the data from third-party sources or the underlying economic assumptions they have made. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s or contracted third parties’ knowledge of our industry, have not been independently verified. Our estimates involve risks and uncertainties, including assumptions that may prove not to be accurate, and these estimates and certain industry data are subject to change based on various factors, including those discussed in this quarterly report and in our most recently filed Annual Report on Form 10-K, filed with the Securities and Exchange Commission (the “SEC”) on June 23, 2023 (the “Annual Report”). This MD&A contains forward-looking information. You should review our important note about forward-looking statements presented at the beginning of this quarterly report.
This MD&A should be read in conjunction with our unaudited condensed consolidated interim financial statements for the three months ended June 30, 2023 and 2022 included elsewhere in this quarterly report. Our interim financial statements were prepared in accordance with U.S. GAAP.
All amounts appearing in this MD&A for the period-by-period discussions are in thousands of U.S. dollars, except share and per share amounts or unless otherwise indicated.
Business Overview
We are focused on developing and commercializing products for rare and orphan diseases that have the potential to improve clinical outcomes by using our novel drug delivery technologies. We seek to apply new proprietary formulations to approved and marketed pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient drug delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients used in the drug candidates under development by Acasti may be already approved in a target indication or could be repurposed for use in new indications.
The existing well understood efficacy and safety profiles of these marketed compounds provides the opportunity for us to utilize the Section 505(b)(2) regulatory pathway under the Federal Food, Drug and Cosmetic Act for the development of our reformulated versions of these drugs, and therefore may provide a potentially shorter path to regulatory approval. Under Section 505(b)(2), if sufficient support of a product’s safety and efficacy either through previous U.S. Food and Drug Administration ("FDA") experience or sufficiently within the existing and accepted scientific literature, can be established, it may eliminate the need to conduct some of the pre-clinical studies and clinical trials that new drug candidates might otherwise require.
Our therapeutic pipeline consists of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio of more than 40 granted and pending patents in various jurisdictions worldwide. These drug candidates aim to improve clinical outcomes in the treatment of rare and orphan diseases by applying proprietary formulation and drug delivery technologies to existing pharmaceutical compounds to achieve improvements over the current standard of care, or to provide treatment for diseases with no currently approved therapies.
We believe that rare disorders represent an attractive area for drug development, and there remains an opportunity for us to utilize already approved drugs that have established safety profiles and clinical experience to potentially address significant unmet medical needs. A key advantage of pursuing therapies for rare disorders is the potential to receive orphan drug designation (“ODD”) from the FDA. Our three drug candidates currently in clinical development have received ODD status, provided certain conditions are met at new drug application ("NDA") approval. ODD provides for seven years of marketing exclusivity in the United States post-launch, provided certain conditions are met, and the potential for faster regulatory review. ODD status can also result in tax credits of up to 50% of clinical development costs conducted in the United States upon marketing approval and a waiver of the NDA fees, which we estimate can translate into savings of approximately $3.2 million for our lead drug candidate, GTX-104. Developing drugs for rare diseases can often allow for clinical trials that are more manageably scaled and may require a smaller, more targeted commercial infrastructure.
The specific diseases targeted for drug development by us are well understood, although the patient populations suffering from such diseases may remain poorly served by available therapies or in some cases, approved therapies do not yet exist. We aim to effectively treat debilitating symptoms that result from these underlying diseases.
17
Our lead drug candidate:
•GTX-104 is a clinical stage, novel, injectable formulation of nimodipine being developed for intravenous infusion (IV) in aneurysmal subarachnoid hemorrhage (aSAH) patients to address significant unmet medical needs. The unique nanoparticle technology of GTX-104 facilitates aqueous formulation of insoluble nimodipine for a standard peripheral IV infusion. GTX-104 provides a convenient IV delivery of nimodipine in the Intensive Care Unit eliminating the need for nasogastric tube administration in unconscious or dysphagic patients. Intravenous delivery of GTX-104 also has the potential to lower food effects, drug-to-drug interactions, and eliminate potential dosing errors. Further, GTX-104 has the potential to better manage hypotension in aSAH patients. GTX-104 has been administered in over 150 healthy volunteers and was well tolerated with significantly lower inter- and intra-subject pharmacokinetic variability compared to oral nimodipine. The pivotal PK bridging study was successfully completed in May 2022.
Other pipeline drug candidates:
•GTX-102, an oral-mucosal betamethasone spray for the treatment of Ataxia Telangiectasia (“A-T”), a complex orphan pediatric genetic neurodegenerative disorder usually diagnosed in young children, for which no FDA approved treatment currently exists.
•GTX-101, a topical bioadhesive film-forming bupivacaine spray for Postherpetic Neuralgia (“PHN”), which can be persistent and often causes debilitating pain following infection by the shingles virus. We believe that GTX-101 could be administered to patients with PHN to treat pain associated with the disease.
In May 2023, we announced the strategic decision to prioritize development of GTX-104 with a goal to advance the product candidate to commercialization, while conserving resources as much as possible to complete development efficiently. We estimate that the deferral of GTX-102 and GTX-101 clinical development could be at least three years given the timeline to complete the development and potential commercial launch of GTX-104. Further development of GTX-102 and GTX-101 will occur at such time as we obtain additional funding or enter into strategic partnerships for license or sale with third parties.
The decision to defer further development of GTX-102 and GTX-101 triggered a comprehensive impairment review of our intangible assets as of March 31, 2023. Given the extended timeline, we increased the discount rates used to value the related assets in order to recognize additional risks related to prioritizing one asset over the others, financing the projects given limited available resources and the need to preserve cash to advance GTX-104 as far as possible, potential competitor advances that could arise over three years, and the general market depression affecting small cap development companies like us and the prohibitively high dilution and expense of available funding in the capital markets. Increasing the discount rates significantly reduced the discounted cash flow values for each of the programs deferred. Accordingly, in the quarter ended March 31, 2023 we booked impairment charges related to GTX-102 and GTX-101 of $22.7 million and $6.0 million respectively, together with further adjustments made to deferred taxes and goodwill directly related to those assets. The impairment charge overall amounted to $33.5 million. We continue to believe that GTX-102 and GTX-101 may eventually provide significant value when development resumes and, if approved, commercialized successfully.
Our management team possesses significant experience in drug formulation and drug delivery research and development, clinical and pharmaceutical development and manufacturing, regulatory affairs, and business development, as well as being well-versed in late-stage drug development and commercialization. Importantly, our team is comprised of industry professionals with deep expertise and knowledge, including a world-renowned practicing neurosurgeon-scientist and respected authority in aSAH, as well as product development, chemistry, manufacturing and controls (“CMC”), planning, implementation, management, and execution of global Phase 2 and Phase 3 trials for a drug candidate for aSAH.
GTX-104 Overview
Nimodipine was granted FDA approval in 1988, and is the only approved drug that has been clinically shown to improve neurological outcomes in aSAH patients. It is only available in the United States as a generic oral capsule and as a branded oral liquid solution called NYMALIZE™, which is manufactured and sold by Arbor Pharmaceuticals (acquired in September 2021 by Azurity Pharmaceuticals). Nimodipine has poor water solubility and high permeability characteristics as a result of its high lipophilicity. Additionally, orally administered nimodipine has dose-limiting side-effects such as hypotension, poor absorption and low bioavailability resulting from high first-pass metabolism, and a narrow administration window as food effects lower bioavailability significantly. Due to these issues, blood levels of orally administered nimodipine can be highly variable, making it difficult to manage blood pressure in aSAH patients. Nimodipine capsules are also difficult to administer, particularly to unconscious patients or those with impaired ability to swallow. Concomitant use with CYP3A inhibitors is contraindicated (NIMODIPINE Capsule PI).
NIMOTOP™ is an injectable form of nimodipine that is manufactured by Bayer Healthcare. It is approved in Europe and in other regulated markets (but not in the United States). It has limited utility for aSAH patients because of its high organic solvent content, namely 23.7% ethanol and 17% polyethylene glycol 400 (NIMOTOP SmPC).
18
•GTX-104 is a clinical stage, novel formulation of nimodipine for IV infusion in aSAH patients. It uses surfactant micelles as the drug carrier to solubilize nimodipine. This unique nimodipine injectable formulation is composed of a nimodipine base, an effective amount of polysorbate 80, a non-ionic hydrophilic surfactant, and a pharmaceutically acceptable carrier for injection. GTX-104 is supplied as an aqueous concentrate that upon dilution with saline, dextrose or lactated ringer, is a ready-to-use infusion solution, which is stable and clear.
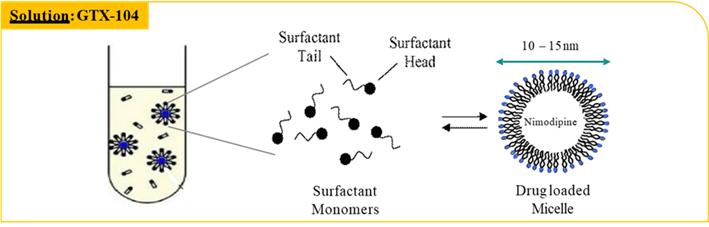
Key Potential Benefits:
•Novel nanoparticle technology facilitates aqueous formulation of insoluble nimodipine for a safe, standard peripheral IV infusion:
•Better control of blood pressure and improved management of hypotension
•Eliminates food effects that impact the absorption of the oral form of nimodipine
•Lower inter and intra-subject variability as compared to oral nimodipine
GTX-104 could provide a more convenient mode of administration as compared to generic nimodipine capsules or NYMALIZE™, GTX-104 is administered as and intravenous infusion compared to oral administration via a nasogastric tube in unconscious patients every four hours for both nimodipine capsules and NYMALIZE™. Therefore, GTX-104 could make a major contribution to patient care by potentially reducing the dosing associated nursing burden. More convenient continuous, and consistent dosing can also reduce the risk of medication errors. In addition, as depicted in the charts below, two PK studies have shown that GTX-104 has the potential to provide improved bioavailability and show reduced inter- and intra-subject variability compared to oral nimodipine, which is hypothesized to limit the risk of hypotension and to better achieve a desired therapeutic concentration. The variability was observed higher following the capsule administration as compared to IV infusion administration (nimodipine exposure variability at steady state observed 37.5% following oral capsule administration versus 15.5%, following GTX-104 IV infusion) Because of its IV formulation, we also expect GTX-104 to reduce certain drug-drug interactions and food effects.
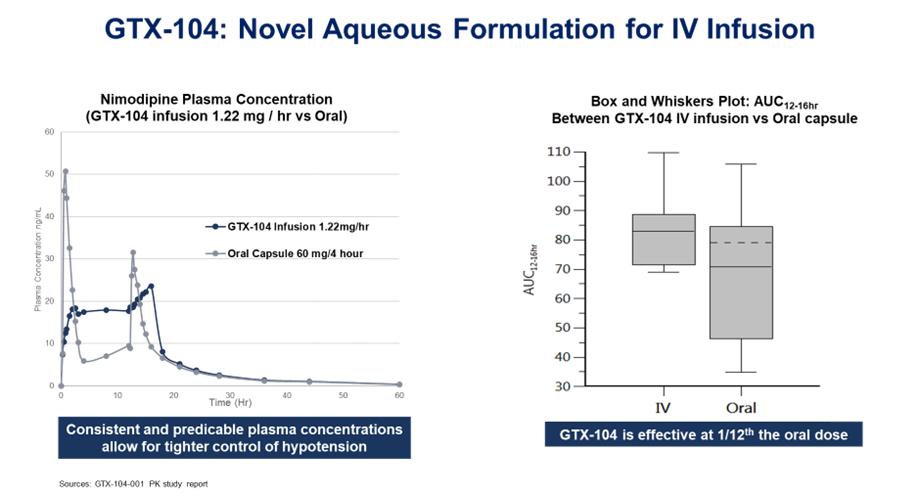
19
Despite the positive impact it has on recovery, physicians often must discontinue their patients from oral nimodipine, primarily as a result of hypotensive episodes that cannot be controlled by titrating the oral form of drug. Such discontinuation could potentially be avoided by administering GTX-104, which because of its IV administration, may reduce the complexity associated with the need for careful attention to the timing of nimodipine administration at least one hour before or two hours after a meal. Also, unconscious patients will likely receive more consistent concentrations of nimodipine when delivered via the IV route as compared to oral gavage or a nasogastric tube. More consistent dosing is expected to result in a reduction of vasospasm and a better, more consistent management of hypotension. As summarized in the table below, we also anticipate reduced use of rescue therapies, such as vasopressors, and expensive hospital resources, such as the angiography suite, are possible by more effectively managing blood pressure with GTX-104. Reduced incidences of vasospasm could result in shorter length of stay and better outcomes.
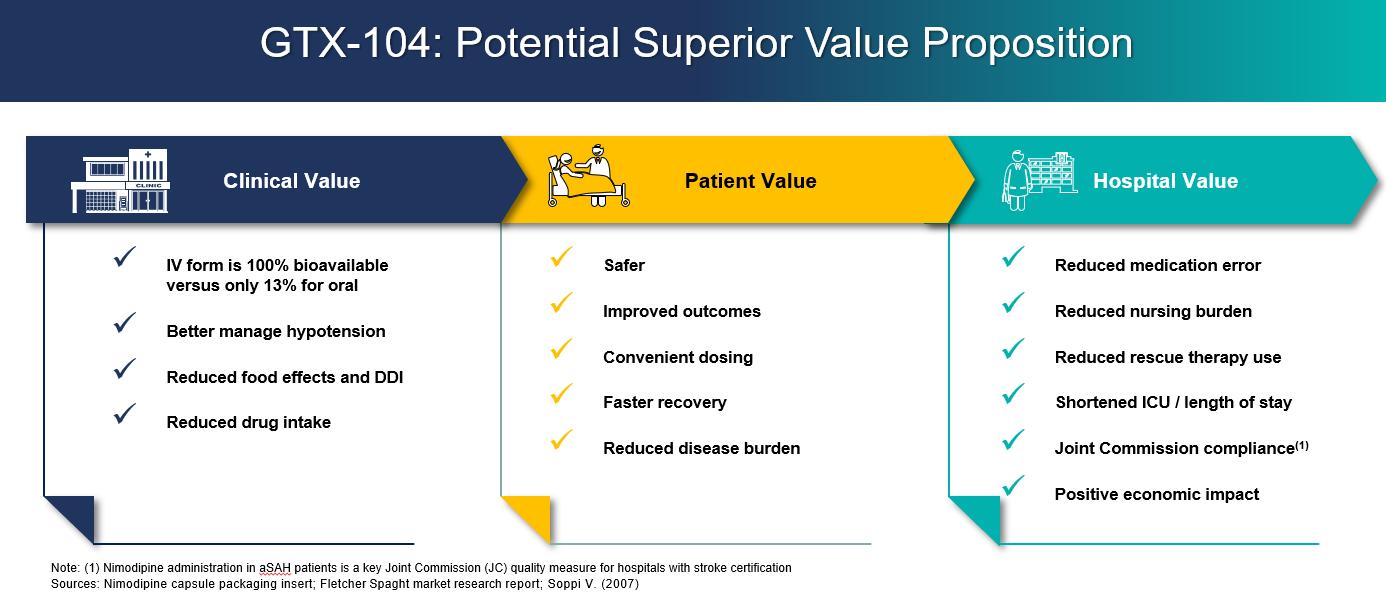
About aneurysmal Subarachnoid Hemorrhage (aSAH)
aSAH is bleeding over the surface of the brain in the subarachnoid space between the brain and the skull, which contains blood vessels that supply the brain. A primary cause of such bleeding is rupture of an aneurysm. The result is a relatively uncommon type of stroke that accounts for about 5% of all strokes and has an incidence of six per 100,000 person years.
In contrast to more common types of stroke in elderly individuals, aSAH often occurs at a relatively young age, with approximately half the affected patients younger than 60 years old. Approximately 10% to 15% of aneurysmal SAH (“aSAH”) patients die before reaching the hospital, and those who survive the initial hours post hemorrhage are admitted or transferred to tertiary care centers with high risk of complications, including rebleeding and delayed cerebral ischemia (“DCI”). Systemic manifestations affecting cardiovascular, pulmonary, and renal function are common and often complicate management of DCI. Approximately 70% of aSAH patients experience death or a permanent dependence on family members, and half die within one month after the hemorrhage. Of those who survive the initial month, half remain permanently dependent on a caregiver to maintain daily living.
We estimate that approximately 50,000 individuals experience aSAH each year in the U.S. based on third-party market research, and that total addressable market for SAH is approximately $300 million in the U.S. There are an estimated 150,000 aSAH patients each year in China and approximately 55,000 patients in the European Union based on annual inpatient admissions and the average length-of-stay.
GTX-104 Recent Activities & Near Term Milestones: Conduct Phase 3 Safety Trial
In September 2021, we initiated our pivotal PK bridging trial to evaluate the relative bioavailability of GTX-104 compared to currently marketed oral nimodipine capsules in approximately 50 healthy subjects. The PK trial was the next required step in our proposed 505(b)(2) regulatory pathway for GTX-104.
Final results from this pivotal PK trial were reported on May 18, 2022, and showed that the bioavailability of GTX-104 compared favorably with the oral formulation of nimodipine in all subjects, and no serious adverse events were observed for GTX-104.
20
All three endpoints indicated that statistically there was no difference in exposures between GTX-104 and oral nimodipine over the defined time periods for both maximum exposure and total exposure. Plasma concentrations obtained following IV administration showed significantly less variability between subjects as compared to oral administration of capsules, since IV administration is not as sensitive to some of the physiological processes that affect oral administration, such as taking the drug with and without meals, variable gastrointestinal transit time, variable drug uptake from the gastrointestinal tract into the systemic circulation, and variable hepatic blood flow and hepatic first pass metabolism. Previous studies have shown these processes significantly affect the oral bioavailability of nimodipine, and therefore cause oral administration to be prone to larger inter- and intra-subject variability.
The bioavailability of oral nimodipine capsules observed was only 8% compared to 100% for GTX-104. Consequently, about one-twelfth the amount of nimodipine is delivered with GTX-104 to achieve the same blood levels as with the oral capsules.
No serious adverse events and no adverse events leading to withdrawal were reported during the trial.
Next Steps – Initiate Phase 3 Safety Trial for GTX-104
In April 2023, we received a Type C written meeting response and clarifying feedback from the FDA on our proposed Phase 3 safety trial for GTX-104. The FDA provided additional comments on our development plan that, pending submission of the final clinical protocol and FDA approval of same, will allow us to proceed with the initiation of a Phase 3 safety clinical trial in aSAH patients. On July 5, 2023, we announced the alignment with the U.S. Food and Drug Administration on our GTX-104 pivotal Phase 3 safety trial protocol.
The FDA concurred with the suitability of the 505(b)(2) regulatory pathway with the selected Reference Listed Drug NIMOTOP oral capsules (NDA 018869), and that our GTX-104-002 PK trial may have met the criteria for a scientific bridge.
Based on the proposed design of our Phase 3 trial, which we have titled STRIVE-ON (Safety, Tolerability, Randomized, IV and Oral Nimodipine), the clinical trial will be a prospective, open-label, randomized (1:1 ratio), parallel group trial of GTX-104 compared with oral nimodipine, in patients hospitalized for aSAH. Key trial design features include:
•Approximately 100 patients will be enrolled at an estimated 25 hospitals in the U.S.
•The primary endpoint is safety and will be measured as comparative adverse events, including hypotension, between the two groups.
•GTX-104 will be administered as a continuous IV infusion of 0.15 mg/hour, and a 30-minute IV bolus of 4 mg every 4 hours. Oral nimodipine will be administered as 60 mg (two 30 mg capsules) every 4 hours.
•Both groups will receive their assigned GTX-104 or oral nimodipine for up to 21 consecutive days and will be evaluated from commencement of patient treatment through a 90-day follow-up period.
We expect the first patient to be enrolled during the fourth quarter of calendar year of 2023. The trial is expected to take approximately 18 months to complete from the time the first patient is enrolled, and we expect this safety trial to be the final clinical step required to seek FDA approval under the 505(b)(2) regulatory pathway. Before submitting an NDA, we plan to hold a pre-NDA meeting with the FDA.
GTX-102 Overview
GTX-102 is a novel, concentrated oral-mucosal spray of betamethasone intended to improve neurological symptoms of A-T for which there are currently no FDA-approved therapies. GTX-102 is a stable, concentrated oral spray formulation comprised of the gluco-corticosteroid betamethasone that together with other excipients can be sprayed conveniently over the tongue of the A-T patient and is rapidly absorbed.
About Ataxia Telangiectasia
A-T is a rare genetic progressive autosomal recessive neurodegenerative disorder that affects children, with the hallmark symptoms of cerebellar ataxia and other motor dysfunction, and dilated blood vessels (telangiectasia) that occur in the sclera of the eyes. A-T is caused by mutations in the ataxia telangiectasia gene, which is responsible for modulating cellular response to stress, including breaks in the double strands of DNA.
21
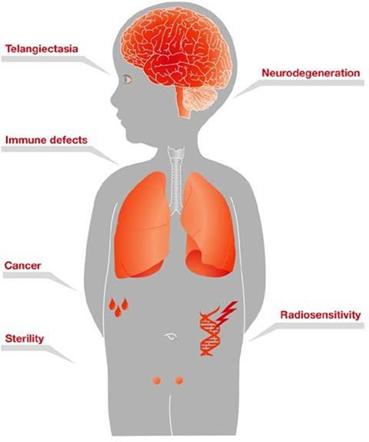
Children with A-T begin to experience balance and coordination problems when they begin to walk (toddler age), and ultimately become wheelchair-bound in their second decade of life. In pre-adolescence (between ages 5 and 8), patients experience oculomotor apraxia, dysarthria, and dysphagia. They also often develop compromised immune systems and are at increased risk of developing respiratory tract infections and cancer (typically lymphomas and leukemia).
A-T is diagnosed through a combination of clinical assessment (especially neurologic and oculomotor deficits), laboratory analysis, and genetic testing. There is no known treatment to slow disease progression, and treatments that are used are strictly aimed at controlling the symptoms (e.g., physical, occupational or speech therapy for neurologic issues), or conditions secondary to the disease (e.g., antibiotics for lung infections, chemotherapy for cancer, etc.). There are no FDA-approved therapeutic options currently available. Patients typically die by age 25 from complications of lung disease or cancer. According to a third-party report we commissioned, A-T affects approximately 4,300 patients per year in the United States and has a potential total addressable market of $150 million, based on the number of treatable patients in the United States.
GTX-102 - R&D and Clinical Trials to Date
We have licensed the data from the multicenter, double-blinded, randomized, placebo-controlled crossover trial from Azienda Ospedaliera Universitaria Senese, Siena, Italy, where Dr. Zannolli et. al. studied the effect of oral liquid solution of betamethasone to reduce ataxia symptoms in patients with A-T. This oral liquid solution is not marketed in the United States, and therefore is not available for clinical use; currently, betamethasone is only available in the United States as an injectable or as a topical cream. This license gives us the right to reference the trial’s data in our NDA filing. On November 12, 2015, we submitted the data from the Zannolli trial to the FDA’s Division of Neurology at a pre-Investigational New Drug (“IND”) meeting and received guidance from the agency on the regulatory requirements to seek approval.
In a multicenter, double-blind, randomized, placebo-controlled crossover trial conducted in Italy, Dr. Zannolli et al. studied the effect of an oral liquid solution of betamethasone on the reduction of ataxia symptoms in 13 children (between ages 2 to 8 years) with A-T. The primary outcome measure was the reduction in ataxia symptoms as assessed by the International Cooperative Ataxia Rating Scale (“ICARS”).
In the trial, oral liquid betamethasone reduced the ICARS total score by a median of 13 points in the intent-to-treat population and 16 points in the per-protocol population (the median percent decreases of ataxia symptoms of 28% and 31%, respectively). Adverse events in the trial were minimal, with no compulsory withdrawals and only minor side effects that did not require medical intervention. Clinical trial results in A-T patients administered oral betamethasone indicated that betamethasone significantly reduced ICARS total score relative to placebo (P = 0.01). The median ICARS change score (change in score with betamethasone minus change in score with placebo) was -13 points (95% confidence interval for the difference in medians was -19 to -5.5 points).
Based on the Zannolli data, we believe that our GTX-102 concentrated oral spray has the potential to provide clinical benefits in decreasing A-T symptoms, including assessments of posture and gait disturbance and kinetic, speech and oculomotor functions. In addition, GTX-102 may ease drug administration for patients experiencing A-T given its application of 1-3x/day of 140µL of concentrated betamethasone liquid sprayed onto the tongue using a more convenient metered dose delivery system, as these A-T patients typically have difficulty swallowing.
22
GTX-102 PK Data to Date:
GTX-102 administered as a concentrated oral spray achieves similar blood levels at only 1/70th the volume of an oral solution of betamethasone. This more convenient mode of administration will be important for A-T patients who have difficulties swallowing large volumes of liquids.
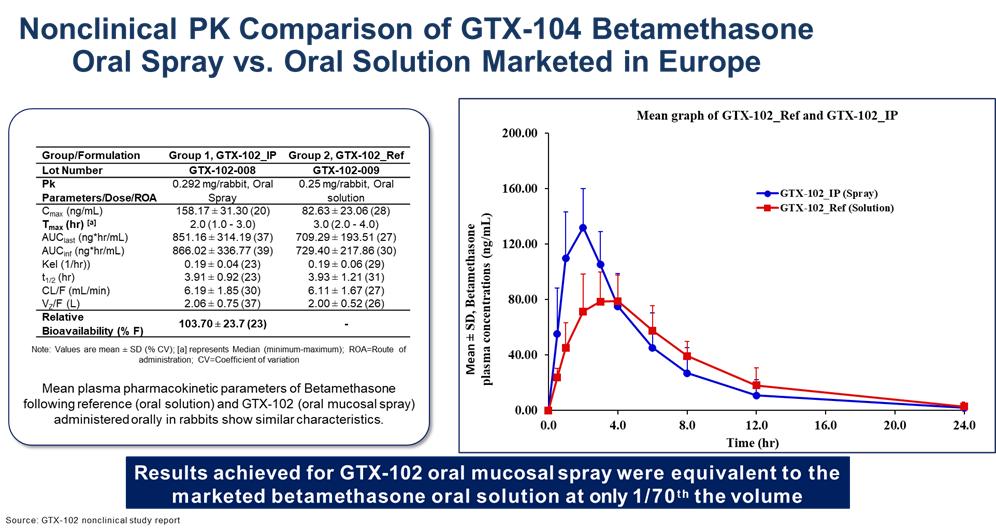
We initiated a PK bridging trial of GTX-102 as compared to the oral liquid solution of betamethasone used in the Zannolli trial and against the injectable form of betamethasone that is approved in the U.S. in the third calendar quarter of 2022. The primary objectives of the PK bridging trial were to evaluate the bioavailability, pharmacokinetics and safety of GTX-102. On December 28, 2022, we reported that the topline results of this trial met all primary outcome measures.
Results showed that GTX-102 betamethasone blood concentrations were highly predictable and consistent based on AUC (the area under the concentration time curve up to 72 hours post-dose, extrapolated to infinity) and Cmax (the maximum concentration occurring between 0 hour to 72 hours after trial drug administration), indicating good linearity and dose-proportionality. GTX-102 betamethasone blood concentrations were within the same range of exposure as IM betamethasone, based on AUC. This IM formulation will serve as a bridge for GTX-102 in the context of the proposed 505(b)(2) regulatory pathway. GTX-102 betamethasone blood concentrations were also within the same range of exposure as Oral Solution (OS), based on AUC. This OS formulation was used by Zannolli and may serve as a clinical comparator for further clinical development. Furthermore, statistically there was no significant difference (p>0.05) between GTX-102 administered at a fast rate (each spray immediately following the preceding one) vs. a slow rate (1 spray/minute), as indicated by Cmax and AUC. We believe this result is important because being able to use the fast or the slow rate of administration may provide greater flexibility for patients and caregivers. The Cmax of GTX-102 was within the same range of exposure as the OS, but the Cmax for the IM formulation was lower than both GTX-102 and the OS, as well as what has been reported previously for the IM in industry publications. It is important to note that achieving bioequivalence with the IM was not an objective of this trial, nor was it expected. Finally, of the 48 healthy adult subjects, no serious adverse events (AE) were reported, and the most frequent drug-related adverse effect was mild headache (4 cases).
The further development of GTX-102 has been deprioritized in favor of our focus on development of GTX-104. Pending additional funding for GTX-102 or the signing of a strategic partnership, we will work with our clinical experts and the FDA to determine the best final dosing regimen for GTX-102 to incorporate into our Phase 3 trial design. Based on previous discussions with the FDA, we plan to conduct a
23
confirmatory Phase 3 safety and efficacy trial in A-T patients, and plan to seek guidance from the FDA on the trial design at a Type B meeting if and when development of GTX-102 resumes. It is also possible that we may out-license or sell our GTX-102 drug candidate.
GTX-101 Overview
GTX-101 is a non-narcotic, topical bio-adhesive film-forming bupivacaine spray designed to ease the symptoms of patients suffering with postherpetic neuralgia (“PHN”). GTX-101 is administered via a metered-dose of bupivacaine spray and forms a thin bio-adhesive topical film on the surface of the patient’s skin, which enables a touch-free, non-greasy application. It also comes in convenient, portable 30 ml plastic bottles. Unlike oral gabapentin and lidocaine patches, we believe that the biphasic delivery mechanism of GTX-101 has the potential for rapid onset of action and continuous pain relief for up to eight hours. No skin sensitivity was reported in a Phase 1 trial.
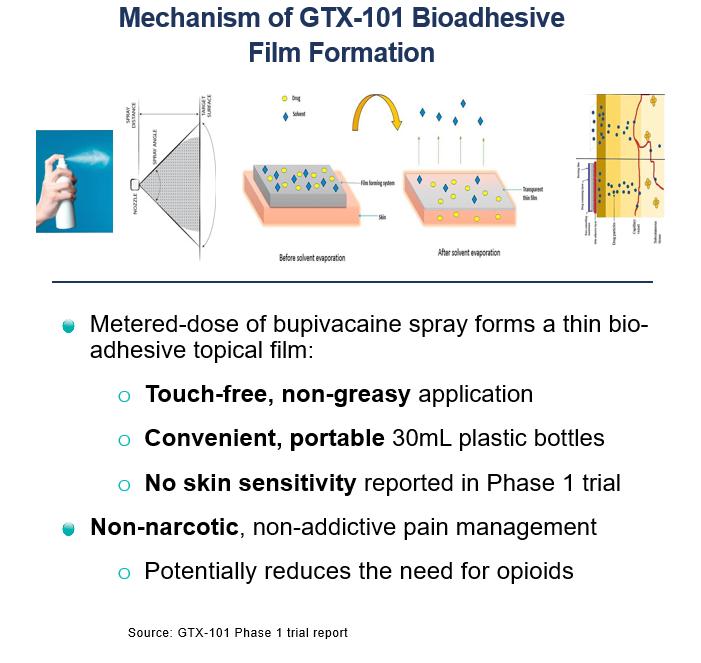
About Postherpetic Neuralgia (PHN)
PHN is neuropathic pain due to damage caused by the varicella zoster virus (“VZV”). Infection with VZV causes two distinct clinical conditions. Primary VZV infection causes varicella (i.e., chickenpox), a contagious rash illness that typically occurs among young children. Secondary VZV can reactivate clinically, decades after initial infection, to cause herpes zoster (“HZ”), otherwise known as shingles. Acute HZ arises when dormant virus particles, persisting within an affected sensory ganglion from the earlier, primary infection with VZV become reactivated when cellular immunity to varicella decreases. Viral particles replicate and may spread to the dorsal root, into the dorsal horn of the spinal cord, and through peripheral sensory nerve fibers down to the level of the skin. Viral particles also may circulate in the blood. This reactivation is accompanied by inflammation of the skin, immune response, hemorrhage, and destruction of peripheral and central neurons and their fibers. Following such neural degeneration, distinct types of pathophysiological mechanisms involving both the central and peripheral nervous systems may give rise to the severe nerve pain associated with PHN.
While the rash associated with HZ typically heals within two to four weeks, the pain may persist for months or even years, and this PHN manifestation is the most common and debilitating complication of HZ. There is currently no consensus definition for PHN, but it has been suggested by the Centers for Disease Control and Prevention (“CDC”) that PHN is best defined as pain lasting at least three months after resolution of the rash.
PHN is associated with significant loss of function and reduced quality of life, particularly in the elderly. It has a detrimental effect on all aspects of a patient's quality of life. The nature of PHN pain varies from mild to severe, constant, intermittent, or triggered by trivial stimuli. Approximately half of patients with PHN describe their pain as “horrible” or “excruciating,” ranging in duration from a few minutes to constant on a daily or almost daily basis. The pain can disrupt sleep, mood, work, and activities of daily living, adversely impacting the
24
quality of life and leading to social withdrawal and depression. PHN is the number-one cause of intractable, debilitating pain in the elderly, and has been cited as the leading cause of suicide in chronic pain patients over the age of 70.
Current treatment of PHN most often consists of oral gabapentin (first line) and prescription lidocaine patches or antidepressants (second line), and refractory cases may be prescribed opioids to address persistent pain. Gabapentin and opioid abuse have continued to proliferate, and lidocaine patches are suboptimal for many reasons. An independent third-party market research firm we commissioned interviewed more than 250 physicians who regularly treat PHN patients, and found that approximately 40% of patients using lidocaine patches experience insufficient pain relief. Lidocaine patches are difficult to use, fall off, and look unsightly with possible skin sensitivity and irritation. Additionally, lidocaine patches can only be used for 12 hours on and then need to be removed for 12 hours before being reapplied. Prescription lidocaine patches are only approved for PHN, and the market is currently made up of both branded and generic offerings. It is estimated that PHN affects approximately 120,000 patients per year in the United States. According to a third-party report we commissioned, the total addressable market for GTX-101 could be as large as $2.5 billion, consisting of approximately $200 million for PHN pain and $2.3 billion for non-PHN pain indications.
GTX-101 R&D History and Clinical Trials Completed to Date
To date, we have conducted four Phase I trials in healthy volunteers to assess the PK, safety and tolerability of GTX-101 and to determine the plasma levels of bupivacaine HCl administered as a single dose in various concentrations between 30 mg (three sprays) and 2100 mg (twenty sprays).
These trials confirmed that bupivacaine delivered as a topical spray (GTX-101) is well absorbed through the skin, as demonstrated in the graph below, while very little is absorbed systemically.
In all four trials, the administration of GTX-101 to healthy volunteers was safe and well tolerated. In addition, no evidence of skin irritation was observed at the application site following the spray administrations. The data below is from two separate trials of GTX-101 and the Lidoderm patch superimposed on each other.
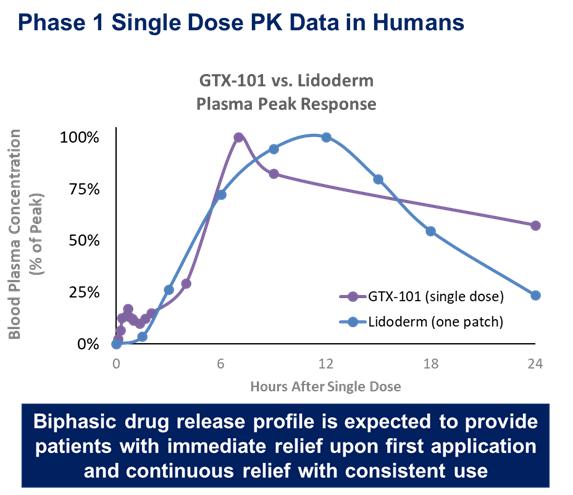
GTX-101 recent activities:
We believe that the PHN pain market will continue to grow, and non-opioid products like GTX-101 that can relieve PHN pain more quickly and in a sustained manner by means of a more efficient delivery system, will be an attractive therapy option for patients and physicians. GTX-101 is administered by spraying our proprietary bupivacaine formulation over the affected area, which we believe has the potential to provide several advantages over currently marketed products such as the lidocaine patch, including faster onset of action, sustained pain relief, possibly lower dosing requirements and improved dosing convenience, all which could lead to increased patient satisfaction and compliance.
The data from the single dose Phase 1 clinical trial for GTX-101 was submitted to the FDA’s Division of Anesthesiology and feedback was received at a pre-IND meeting on April 18, 2018, that informed the design of pre-clinical toxicology studies and a clinical and regulatory pathway to approval under section 505(b)(2). We completed a minipig skin sensitivity study in the second calendar quarter of 2022, and we
25
initiated a single dose PK trial in healthy human volunteers in July 2022. Topline results from this single dose PK trial were reported on December 23, 2022 and the results met all primary outcome measures.
The median Tmax (the time of maximum concentration between 0 hour and 240 hours after study drug administration) of bupivacaine in plasma following GTX-101 single-dose topical applications ranged between 18 to 24 hours depending on dose, while the median Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 23 minutes. This result suggests that bupivacaine delivered by GTX-101 remains in the skin for a long period of time, potentially inducing prolonged analgesic effect in the sprayed area. The exposure to bupivacaine based on Cmax (the maximum concentration occurring at Tmax between 0 hour and 240 hours after study drug administration) and AUC (the area under the concentration time curve, extrapolated to infinity) following GTX-101 topical application as a single-dose increased with increasing dose.
The systemic exposure to bupivacaine following a 200mg dose of GTX-101 was approximately 29-fold less than a single subcutaneous dose of 10mg of bupivacaine based on Cmax and approximately 6-fold less than a single subcutaneous dose of 10mg of bupivacaine based on AUC. We predict these lower blood levels will correspond to an increased safety margin for GTX-101 with regards to toxicity risk. Mean half-life (T half) following GTX-101 single-dose topical applications ranged between 24 to 37 hours depending on dose, suggesting a slow elimination and potentially long duration of effect, while mean Tmax following the subcutaneous injection of 10 mg of bupivacaine was only 8 hours.
There were only two adverse events judged as related to the study drug by the investigator for each of GTX-101 and the bupivacaine subcutaneous injection. Following GTX-101 topical application: headache (1 event = 3%) and numbness (1 event = 3%) at the sprayed area following bupivacaine subcutaneous injection: dizziness (1 event = 8%) and nausea (1 event = 8%).
The further development of GTX-101 has been deprioritized in favor of our focus on development of GTX-104. Pending additional funding for GTX-101 or the signing of a strategic partnership, we plan to follow this successful PK trial with a multiple ascending dose trial in 2023. Results from these non-clinical studies and clinical trials are required before the initiation of our Phase 2 program in PHN patients. It is also possible that we may out-license or sell our GTX-101 drug candidate.
26
Overall Commercialization Strategy
We have worldwide commercialization rights for all our pipeline drug candidates and plan to maximize the value of each asset. Currently, we have prioritized the development of GTX-104 and de-emphasized the development of GTX-102 and GTX-101. If we receive regulatory approval for GTX-104 in the US, we may look to out-license commercialization or consider self-commercialization including outsourcing sales to ensure efficient commercial management and maximize market penetration and financial returns. We may seek commercial partnerships to fully exploit the market potential of GTX-104 in territories outside the US. It is possible that we may out-license or sell GTX-102 and/or GTX-101 for the US and/or global markets.
Recent Developments
Announcement of compliance with the Nasdaq minimum bid price requirement
On July 24, 2023, we received written notice (the “Notification Letter”) from The Nasdaq Stock Market LLC notifying us that we had regained compliance with the minimum bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on the Nasdaq Capital Market. The Notification Letter was sent following the implementation of a 1-for-6 reverse split of our Class A common shares (the “Reverse Stock Split”), which became effective on July 10, 2023.
Announcement of alignment with U.S. Food and Drug Administration
On July 5, 2023, we announced the alignment with the U.S. Food and Drug Administration on our GTX-104 pivotal Phase 3 safety trial protocol.
Reverse stock split
On June 29, 2023, our Board of Directors approved an amendment to our Articles of Incorporation to implement the Reverse Stock Split. On July 4, 2023, we filed Articles of Amendment to our Articles of Incorporation with the Registraire des entreprises du Québec, to implement the Reverse Stock Split. All applicable references in this MD&A to number of common shares, warrants and options, price per share and weighted average number of shares outstanding prior to the Reverse Stock Split have been adjusted to reflect the Reverse Stock Split, which was made effective on July 10, 2023.
Announcement of successful submission of pivotal GTX-104 Phase 3 safety study protocol with FDA and implementation of strategic realignment plan
On May 8, 2023, we announced the successful submission to the FDA of GTX-104's full protocol of our pivotal Phase 3 safety trial and implementation of a strategic realignment plan to maximize shareholder value. The realignment follows a comprehensive strategic review by Prashant Kohli, our recently appointed Chief Executive Officer, and the Board of Directors.
Key strategies being implemented are:
•Prioritizing resources to GTX-104. We submitted the full pivotal Phase 3 safety trial protocol for GTX-104 to the FDA with all supporting documentation. On July 5, 2023, we announced alignment with the FDA on our GTX-104 pivotal Phase 3 safety trial protocol. The first patient, first dose for the pivotal Phase 3 safety trial is expected in calendar Q4 2023.
•Strategic transformation of our operating model to an agile biopharma reflecting our complete focus on GTX-104. In alignment with our new operating model, we brought on a highly experienced new management team with deep subject matter knowledge and direct, hands-on clinical trial experience in aSAH.
•Significant extension of our cash runway expected to be sufficient to fund our operations through calendar Q2 2025, which we expect to facilitate the achievement of critical value inflection milestones, including a potential NDA filing for GTX-104.
•Evaluation of strategic alternatives to maximize value of de-prioritized pipeline assets, GTX-102 and GTX-101.
In connection with the transformation of our operating model, we have appointed the following industry experts to our senior management team:
•Dr. R. Loch Macdonald, MD, PhD, as Chief Medical Officer. A world-renowned practicing neurosurgeon-scientist and respected authority in SAH, Dr. Macdonald is the former founder of a clinical-stage biotechnology company focused on subarachnoid hemorrhage.
•Carrie D’Andrea, as VP Clinical Operations. Ms. D’Andrea is a highly experienced professional who has built and led the planning, implementation, management, and execution of global Phase 2 and Phase 3 trials for a drug candidate for subarachnoid hemorrhage.
27
•Amresh Kumar, PhD, as VP Program Management. Mr. Kumar is an experienced drug development, CMC, and program management expert. Mr. Kumar was the former product leader of GTX-104 while at Grace Therapeutics, Inc. ("Grace”) (which was acquired by us).
As a result of this strategic realignment, we are, over time, discontinuing our operations in Canada, and have proceeded to lay off substantially all our workforce, which is expected to allow our new management team to begin to rebuild a leaner organization in the United States.
Announcement of appointment of Prashant Kohli as CEO
On April 4, 2023, we announced the appointment of Prashant Kohli as our new Chief Executive Officer, succeeding Jan D'Alvise. The parties mutually agreed to part ways, and in connection with her resignation as Chief Executive Officer, Ms. D'Alvise stepped down from her position on the Board of Directors.
Announcement of intention to proceed with Phase 3 clinical safety study for GTX-104 following FDA feedback
On April 4, 2023 we announced that we received a Type C written meeting response and clarifying feedback from the FDA on our proposed Phase 3 safety trial for GTX-104. The FDA provided additional comments on our development plan that, subject to submission of the final clinical protocol and FDA approval of same, will allow us to proceed with the initiation of a Phase 3 safety clinical trial in aneurysmal aSAH patients.
Basis of Presentation of the Financial Statements
Our condensed consolidated interim financial statements, which include the accounts of our wholly owned subsidiaries, Acasti Pharma U.S., and Acasti Innovations AG, have been prepared in accordance with U.S. GAAP and the rules and regulations of the SEC related to quarterly reports filed on Form 10-Q. All intercompany transactions and balances are eliminated on consolidation.
Our assets as of June 30, 2023, include cash and cash equivalents and short-term investments totaling $21.6 million and intangible assets and goodwill totaling $49.3 million. Our current liabilities total $2.0 million as at June 30, 2023 and are comprised primarily of amounts due to or accrued for creditors.
28
Comparative Financial Information for the Three months ended June 30, 2023 and 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
Increase (Decrease) |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Net loss |
|
|
(4,023 |
) |
|
|
(4,524 |
) |
|
|
(501 |
) |
Basic and diluted loss per share |
|
|
(0.54 |
) |
|
|
(0.61 |
) |
|
|
0.07 |
|
Total assets |
|
|
73,033 |
|
|
|
124,931 |
|
|
|
(51,898 |
) |
Working capital1 |
|
|
21,646 |
|
|
|
37,541 |
|
|
|
(15,895 |
) |
Total non-current liabilities |
|
|
7,057 |
|
|
|
174 |
|
|
|
6,883 |
|
Total shareholders’ equity |
|
|
64,010 |
|
|
|
104,403 |
|
|
|
(40,393 |
) |
|
|
|
|
|
|
|
|
|
|
1 Working capital is calculated by subtracting current liabilities from current assets. Because there is no standard method endorsed by U.S. GAAP requirements, the results may not be comparable to similar measurements presented by other public companies.
Results of Operations for the Three months ended June 30, 2023 and 2022
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
Increase (Decrease) |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses |
|
|
|
|
|
|
|
|
|
Research and development expenses, net of government assistance |
|
|
1,095 |
|
|
|
2,590 |
|
|
|
(1,495 |
) |
General and administrative expenses |
|
|
1,763 |
|
|
|
1,919 |
|
|
|
(156 |
) |
Sales and marketing expenses |
|
|
111 |
|
|
|
221 |
|
|
|
(110 |
) |
Restructuring costs |
|
|
1,485 |
|
|
|
— |
|
|
|
1,485 |
|
Loss from operating activities |
|
|
(4,454 |
) |
|
|
(4,730 |
) |
|
|
(276 |
) |
|
|
|
|
|
|
|
|
|
|
Foreign exchange gain (loss) |
|
|
8 |
|
|
|
(78 |
) |
|
|
86 |
|
Change in fair value of warrant liabilities |
|
|
— |
|
|
|
10 |
|
|
|
(10 |
) |
Interest income and other expense |
|
|
134 |
|
|
|
32 |
|
|
|
102 |
|
Income tax recovery |
|
|
289 |
|
|
|
242 |
|
|
|
47 |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
|
(4,023 |
) |
|
|
(4,524 |
) |
|
|
(501 |
) |
|
|
|
|
|
|
|
|
|
|
Net Loss
The net loss of $4,023 or $0.54 per share for the three months ended June 30, 2023 decreased by $501 from the net loss of $4,524 or $0.61 per share for the three months ended June 30, 2022.
Research and development expenses
Research and development expenses consist primarily of:
•fees paid to external service providers such as contract research organizations ("CROs") and contract manufacturing organizations ("CMOs") related to clinical trials, including contractual obligations for clinical development, clinical sites, manufacturing and scale-up, and formulation of clinical drug supplies;
•fees paid to contract service providers related to drug discovery efforts including chemistry and biology services; and
•salaries and related expenses for research and development personnel, including expenses related to stock options.
We record research and development expenses as incurred.
Our research and development during the three months ended June 30, 2023, was focused primarily on our clinical development programs for our GTX-104 drug candidate. Research and development expenses during the three months ended June 30, 2022 were focused primarily on our clinical development programs GTX-104, GTX-102, and GTX-101 drug candidates, which were acquired in the Grace merger on August 27, 2021.
29
The following table summarizes our research and development expenses for the periods presented:
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development expenses |
|
|
|
|
|
|
|
|
|
|
|
Three months |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
Increase (Decrease) |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Third-party contract research expenses: |
|
|
|
|
|
|
|
|
|
Clinical development programs: |
|
|
|
|
|
|
|
|
|
GTX-104 |
|
|
299 |
|
|
|
323 |
|
|
|
(24 |
) |
GTX-102 |
|
|
103 |
|
|
|
567 |
|
|
|
(464 |
) |
GTX-101 |
|
|
34 |
|
|
|
637 |
|
|
|
(603 |
) |
Other third-party contract research expenses |
|
|
31 |
|
|
|
154 |
|
|
|
(123 |
) |
Professional fees |
|
|
309 |
|
|
|
243 |
|
|
|
66 |
|
Other research and development costs |
|
|
24 |
|
|
|
88 |
|
|
|
(64 |
) |
Government grants & tax credits |
|
|
51 |
|
|
|
(190 |
) |
|
|
241 |
|
Total third-party research and development expenses 1 |
|
|
851 |
|
|
|
1,822 |
|
|
|
(971 |
) |
Salaries and benefits |
|
|
225 |
|
|
|
489 |
|
|
|
(264 |
) |
Research and development expense before stock-based compensation and depreciation |
|
|
1,076 |
|
|
|
2,311 |
|
|
|
(1,235 |
) |
Stock-based compensation |
|
|
2 |
|
|
|
158 |
|
|
|
(156 |
) |
Depreciation and write-off of equipment |
|
|
17 |
|
|
|
121 |
|
|
|
(104 |
) |
Total |
|
|
1,095 |
|
|
|
2,590 |
|
|
|
(1,495 |
) |
|
|
|
|
|
|
|
|
|
|
1 Total third-party research and development expenses are calculated before salaries and benefits, depreciation, write-off of equipment and stock-based compensation. Because there is no standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
Total third-party research and development expenses before salaries and benefits, depreciation, write-off of equipment and stock-based compensation expenses for the three months ended June 30, 2023, totaled $851 compared to $1,822 for the three months ended June 30, 2022. This decrease of $971 related mostly to the restructuring to align our organizational and management cost structure to prioritize resources to GTX-104 and reduce losses and cash flow.
Third-party contract research expenses related to GTX-104 amounted to $299 for the three months ended June 30, 2023, as our PK bridging study wound down. Third party contract research expenses related to GTX-102 amounted to $103 for the three months ended June 30, 2023, and are mostly related to the initiation of the PK bridging study and for clinical trial materials. Third party contract research expenses related to GTX-101 amounted to $34 for the three months ended June 30, 2023 were mostly related to the planning and initiation of the Phase 1 single dose study. We expect third-party contract research expenses related to GTX-102 and GTX-101 to continue to decrease as we shift our development priority to GTX-104. Other third-party contract research expenses of $31 for the three months ended June 30, 2023 decreased by $123, from $154, for the three months ended June 30, 2022, related to other third-party contract research expenses for non-clinical outside services.
Professional fees of $309 for the three months ended June 30, 2023, increased by $66 from $243 for the three months ended June 30, 2022, which was related to increased specialized clinical and regulatory consultants supporting our clinical program for GTX-104.
For the three months ended June 30, 2023, total third-party research and development expenses were increased by $51 respectively, related to government credit eligible research activities related to our clinical programs for GTX-104, GTX-102 and GTX-101.
Salaries and benefits of $225 for the three months ended June 30, 2023, decreased by $264 compared to $489 for the three months ended June 30, 2022. The decrease relates to a reduction in research and development headcount due to the restructuring as we prioritize resources to GTX-104.
30
General and administrative expenses
General and administrative expenses consist primarily of salaries and related benefits, including share-based compensation, related to our executive, finance, legal, and support functions, including professional fees for auditing, tax, consulting, rent and utilities and insurance.
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses |
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
Increase (Decrease) |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Salaries and benefits |
|
|
342 |
|
|
|
532 |
|
|
|
(190 |
) |
Professional fees |
|
|
945 |
|
|
|
603 |
|
|
|
342 |
|
Other |
|
|
413 |
|
|
|
456 |
|
|
|
(43 |
) |
General and administrative expense before stock-based compensation and depreciation 1 |
|
|
1,700 |
|
|
|
1,591 |
|
|
|
109 |
|
Stock-based compensation |
|
|
60 |
|
|
|
282 |
|
|
|
(222 |
) |
Depreciation |
|
|
3 |
|
|
|
46 |
|
|
|
(43 |
) |
Total |
|
|
1,763 |
|
|
|
1,919 |
|
|
|
(156 |
) |
|
|
|
|
|
|
|
|
|
|
1 General and administrative sub-total expenses are calculated before stock-based compensation and depreciation. Because there is no standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
General and administrative expenses totaled $1,700 before stock-based compensation and depreciation expense for the three months ended June 30, 2023, an increase of $109 from $1,591 for the three months ended June 30, 2022. The increase was primarily a result of increase legal, tax, accounting and other professional fees. Stock-based compensation of $60 for the three months ended June 30, 2023, decreased by $222 compared to $282 for the three months ended June 30, 2022. The decrease relates to a reduction in general and administrative headcount due to our restructuring and reorganization of our management structure.
Sales and marketing expenses
Sales and marketing expenses consist primarily of salaries and benefits, including share-based compensation, related to our commercial functions.
|
|
|
|
|
|
|
|
|
|
|
|
|
Sales and marketing expenses |
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
Increase (Decrease) |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Salaries and benefits |
|
|
15 |
|
|
|
182 |
|
|
|
(167 |
) |
Professional fees |
|
|
20 |
|
|
|
5 |
|
|
|
15 |
|
Other |
|
|
60 |
|
|
|
10 |
|
|
|
50 |
|
Sales and Marketing expenses before stock-based compensation1 |
|
|
95 |
|
|
|
197 |
|
|
|
(102 |
) |
Stock-based compensation |
|
|
16 |
|
|
|
24 |
|
|
|
(8 |
) |
Total |
|
|
111 |
|
|
|
221 |
|
|
|
(110 |
) |
|
|
|
|
|
|
|
|
|
|
1 Sales and marketing sub-total expenses are calculated before stock-based compensation. Because there is no standard method endorsed by U.S. GAAP, the results may not be comparable to similar measurements presented by other public companies.
Sales and marketing expenses before stock-based compensation expense totaled $95 for the three months ended June 30, 2023 compared to $197 for the three months ended June 30, 2022. The decrease of $102, was mostly due to the reduction of headcount due to our restructuring and reorganization of our management structure.
Aggregate stock-based compensation expense decreased by $8 to $16 for the three months ended June 30, 2023 as compared to $24 for the three months ended June 30, 2022. This decrease was due to the reduction of headcount due to our restructuring and reorganization of our management structure.
31
Restructuring Costs
On May 8, 2023, we announced our decision to terminate a substantial amount of our workforce as part of a plan that intended to align our organizational and management cost structure to prioritize resources to GTX-104 and reduce losses to improve cash flow and extend available cash resources. We incurred $1,485 of cost primarily consisting of employee severance costs.
Interest income and other expense
Interest income was $134 and $32 for the three months ended June 30, 2023 and 2022, respectively, which related to interest earned on our cash, cash equivalents, and short-term investment balances. The increase in our interest income was due to higher interest rates earned on average balances of cash, cash equivalents and short-term investments.
Liquidity and Capital Resources
Share capital structure
Our authorized share capital consists of an unlimited number of Class A, Class B, Class C, Class D and Class E common shares, without par value. Issued and outstanding fully paid shares, stock options, and warrants, were as follows for the periods indicated (after giving effect to the Reverse Stock Split, which became effective on July 10, 2023):
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31,
2023 |
|
|
|
Number
outstanding |
|
|
Number
outstanding |
|
Class A common shares, voting, participating and without par value |
|
|
7,435,533 |
|
|
|
7,435,533 |
|
Stock options granted and outstanding |
|
|
473,178 |
|
|
|
740,957 |
|
May 2018 Canadian public offering of warrants exercisable at CAD$62.88 until May 9, 2023 |
|
|
— |
|
|
|
137,370 |
|
December 2017 U.S. public offering of warrants exercisable at US$60.48 expired December 19, 2022 |
|
|
— |
|
|
|
— |
|
December 2017 U.S. public offering broker warrants exercisable at US$60.60 expired December 27, 2022 |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
Total fully diluted shares |
|
|
7,908,711 |
|
|
|
8,313,860 |
|
Cash flows and financial condition for the three months ended June 30, 2023 and 2022
Summary
We do not expect to generate revenue from product sales unless and until we successfully complete drug development and obtain regulatory approval, which we expect will take several years and is subject to significant uncertainty. To date, we have financed our operations primarily through public offerings and private placements of our common shares, warrants and convertible debt and with the proceeds from research tax credits. Until such time that we can generate significant revenue from drug product sales, if ever, we will require additional financing, which we expect to be sourced from a combination of public or private equity offerings or debt financings or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties.
As of June 30, 2023, cash and cash equivalents totaled $21,633, a decrease of $6,242 compared to cash and cash equivalents totaling $27,875 at March 31, 2023 primarily due to ongoing research and development activities, and funding the restructuring expense.
Net cash used in operating activities
32
Net cash used in operating activities for the three months ended June 30, 2023 was $6,240, compared to $5,426 for the three months ended June 30, 2022, an increase of $814. Cash used in operating activities during the three months ended June 30, 2023 primarily related to our net loss of $4,023, adjusted for non-cash items such as stock-based compensation of $78, income tax recovery of $289 and changes in our operating assets and liabilities of $2,026. Cash used in operating activities during the three months ended June 30, 2022 primarily related to our net loss of $4,524, adjusted for non-cash items such as change in depreciation of $167, stock-based compensation of $464, income tax recovery $242, and changes in our operating assets and liabilities of $1,271.
Net cash used in investing activities
For the three months ended June 30, 2023, we had no investing activities compared to cash generated from investing activities of $13,258 for the three months ended June 30, 2022. Cash generated was a function of an increase in proceeds from maturity of short-term investments.
Net cash used in financing activities
Net cash provided by financing activities for the three months ended June 30, 2023, totaled nil compared to cash generated of $195 during the three months ended June 30, 2022 due to proceeds from the sale of common shares under our ATM program. The decrease in net cash provided by financing activities was primarily due to our reduced utilization of our ATM program.
At-the-Market (“ATM”) program
On June 29, 2020, we entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”). Under the terms of the Sales Agreement, which had a three-year term, we could issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. Subject to the terms and conditions of the Sales Agreement, the Agents would use their commercially reasonable efforts to sell the common shares from time to time, based upon our instructions. We had no obligation to sell any of the common shares and could, at any time, suspend sales under the Sales Agreement. We and the Agents could terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, we provided the Agents with customary indemnification rights and the Agents were entitled to compensation at a commission rate equal to 3.0% of the gross proceeds from each sale of the common shares. The Sales Agreement expired pursuant to its terms on June 29, 2023. We intend to examine our financing strategies on a go-forward basis and may consider entering into a new ATM program in the future.
On November 10, 2021, we filed a prospectus supplement relating to our ATM program to restore available capacity to $75,000,000, with B. Riley, Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC continuing to act as Agents. Under the terms of the Sales Agreement and the prospectus supplement, we may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the Agents. The common shares will be distributed at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The volume and timing of sales under the ATM program, if any, will be determined at the sole discretion of our board of directors and management.
During the three months ended June 30, 2023, there were no common shares sold under the ATM program. During the three months ended June 30, 2022, 34,335 common shares were sold for total net proceeds of approximately $195 with commissions, legal expenses and costs related to the share sale amounting to $6. The common shares were sold at the prevailing market prices, which resulted in an average price of approximately $5.82 per share.
The Sales Agreement expired June 29, 2023 and the Corporation plans to revisit the renewal of a facility in the coming months.
33
Financial position
The following table details the significant changes to our consolidated balance sheets as of June 30, 2023, compared to the prior fiscal year end at March 31, 2023:
|
|
|
|
|
|
|
Accounts |
|
Increase
(Decrease) $ |
|
|
Comments |
Cash and cash equivalents |
|
|
(6,242 |
) |
|
See cash flow statement |
Receivables |
|
|
35 |
|
|
Timing of reimbursement of sales taxes |
Prepaid expenses |
|
|
529 |
|
|
Renewal of insurance contract and other prepaid expenses (advances to US vendors) |
Right of use asset |
|
|
(392 |
) |
|
Adjustment to the net present value of lease contract for Sherbrooke due to lease modification |
Equipment |
|
|
(20 |
) |
|
Depreciation of equipment and write off of equipment from restructuring |
Trade and other payables |
|
|
(1,450 |
) |
|
Timing of payments net of accruals |
Lease liability |
|
|
(405 |
) |
|
Adjustment to the net present value of lease contract for Sherbrooke due to lease modification |
Deferred tax liability |
|
|
(290 |
) |
|
Related to recovery expense |
See the consolidated statements of shareholders' equity in our financial statements for details of changes to our equity accounts during the three months ended June 30, 2023 and 2022.
Treasury Operations
Our treasury policy is to invest cash that is not required immediately into instruments with an investment strategy based on capital preservation. Cash equivalents and marketable securities are primarily in guaranteed investment certificates, term deposits and high-interest savings accounts, which are issued and held with Canadian chartered banks, highly rated promissory notes issued by government bodies and commercial paper. We hold cash denominated in both U.S. and Canadian dollars. Funds received in U.S. dollars from equity financings are invested as per our treasury policy in U.S. dollar investments and converted to Canadian dollars as appropriate to fulfill operational requirements and funding.
Intangible Assets
On August 27, 2021, we completed the Grace merger.
In connection with the share-for-share noncash transaction, Grace was merged with a new wholly owned subsidiary of Acasti and became a subsidiary of Acasti. As a result, we acquired Grace’s entire therapeutic pipeline consisting of three unique clinical stage and multiple pre-clinical stage assets supported by an intellectual property portfolio consisting of various granted and pending patents in various jurisdictions worldwide. Under the terms of the acquisition, each issued and outstanding share of Grace common stock was automatically converted into the right to receive Acasti common shares equal to the equity exchange ratio set forth in the merger agreement.
Intangible assets of $69,810 relate to the value of in-process research and development (“IPR&D”), related to Grace’s therapeutic pipeline, consisting of three unique clinical stage programs/assets supported by intellectual property, the value of which has been attributed as follows:
|
|
|
|
|
|
|
|
$ |
$ |
$ |
$ |
|
|
GTX-104 |
GTX-102 |
GTX-101 |
Total |
Intangible assets – in-process research and development |
|
|
|
|
|
Balance, April 1, 2022 |
|
27,595 |
31,908 |
10,307 |
69,810 |
Impairment |
|
— |
(22,712) |
(5,970) |
(28,682) |
Balance, March 31, 2023 |
|
27,595 |
9,196 |
4,337 |
41,128 |
|
|
|
|
|
|
|
|
$ |
$ |
$ |
$ |
|
|
GTX-104 |
GTX-102 |
GTX-101 |
Total |
Intangible assets – in-process research and development |
|
|
|
|
|
Balance, April 1, 2023 |
|
27,595 |
9,196 |
4,337 |
41,128 |
Impairment |
|
— |
— |
— |
— |
Balance, June 30, 2023 |
|
27,595 |
9,196 |
4,337 |
41,128 |
In April 2023, we announced the strategic decision to prioritize development of GTX-104 with a goal to advance to commercialization, while conserving resources as much as possible to complete development efficiently. We estimate that the deferral of the GTX-102 and GTX-101 clinical programs could be at least three years given the timeline to complete the development and potential commercial launch
34
of GTX-104. Further development of GTX-102 and GTX-101 will occur at such time as we obtain additional funding or enter into strategic partnerships for license or sale with third parties. The decision to defer further development triggered a comprehensive impairment review of our intangible assets in March 2023. Given the extended timeline, we increased the discount rates used to value the assets in order to recognize additional risks related to prioritizing one asset over the others, financing the projects given limited available resources and the need to preserve cash to advance GTX-104 as far as possible, potential competitor advances that could arise over three years, and the general market depression affecting small cap development companies like us and the prohibitively high dilution and expense of available funding in the capital markets. Increasing the discount rates significantly reduced the discounted cash flow values for each of the programs deferred. Accordingly, an impairment of intangible assets of $28,682 resulted in the year ended March 31, 2023. In addition, an impairment of $4,826 of goodwill resulted in the year ended March 31, 2023. We determined there was no triggering event in the for the three months ended June 30, 2023 that would have required us to perform a quantitative impairment test.
Contractual Obligations and Commitments
Our contractual obligations and commitments include trade payables, operating lease obligations, CMO and CRO agreements, and the RKO supply agreement, as described below.
Research and development contracts and contract research organizations agreements:
We utilize CMOs, for the development and production of clinical materials and CROs to perform services related to our clinical trials. Pursuant to the agreements with CMOs and CROs, we have either the right to terminate the agreements without penalties or under certain penalty conditions.
Raw krill oil supply contract
On October 25, 2019, we signed a supply agreement with Aker Biomarine Antarctic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre, one of our former drug candidates, for a total fixed value of $3.1 million. As of June 30, 2023, the remaining balance of the commitment with Aker amounts to $2.8 million. During the second calendar quarter of 2022, Aker informed us that Aker believed it had satisfied the terms of the supply agreement as to their obligation to deliver the remaining balance of raw krill oil product, and that we were therefore required to accept the remaining product commitment and to pay Aker the $2.8 million balance. We disagree with Aker’s position and believe that Aker is not entitled to further payment under the supply agreement. Accordingly, no liability has been recorded. The dispute was unresolved as of June 30, 2023 and remains unresolved. There is uncertainty as to whether we will be required to make further payment to Aker in connection with the dispute. Additionally, in the event we are required to accept delivery from Aker of the remaining balance of raw krill oil product under the supply agreement, there is uncertainty as to whether we can recover value from the product, which may result in us incurring a loss on the supply agreement in the near term.
Contingencies
We evaluate contingencies on an ongoing basis and establish loss provisions for matters in which losses are probable and the amount of the loss can be reasonably estimated.
Use of Estimates and Measurement of Uncertainty
The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates.
Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected.
Estimates and assumptions include the measurement of stock-based compensation, accruals for research and development contracts and contract organizations agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date, and determining which research and development expenses qualify for research and development tax credits and in what amounts. We recognize tax credits once it has reasonable assurance that they will be realized.
Critical Accounting Policies
During the three months ended June 30, 2023, there were no material changes to our critical accounting policies from those described in our Annual Report for the year ended March 31, 2023.
Future Accounting Changes
35
We have considered recent accounting pronouncements and concluded that they are either not applicable to our business or that the effect is not expected to be material to our consolidated financial statements as a result of future adoption.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
A smaller reporting company is not required to provide the information required by this Item.
Item 4. Controls and Procedures
Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report, our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures within the meaning of Rules 13a-15(e) and 15d-15(e) of the Exchange Act. Based upon this evaluation, our management has concluded that, as of June 30, 2023, our existing disclosure controls and procedures were effective. It should be noted that while our Chief Executive Officer and Chief Financial Officer believe that our disclosure controls and procedures provide a reasonable level of assurance that they are effective, they do not expect the disclosure controls and procedures to be capable of preventing all errors and fraud. A control system, no matter how well conceived or operated, can provide only reasonable, but not absolute, assurance that the objectives of the control system are met.
Changes in Internal Control over Financial Reporting
No changes were made to our internal controls over financial reporting that occurred during the quarter ended June 30, 2023 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
In the ordinary course of business, we are at times subject to various legal proceedings and disputes. We assess our liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that we will incur a loss and the amount of the loss can be reasonably estimated, we record a liability in our consolidated financial statements. These legal reserves may be increased or decreased to reflect any relevant developments on a quarterly basis. Where a loss is not probable or the amount of loss is not estimable, we do not accrue legal reserves. While the outcome of legal proceedings is inherently uncertain, based on information currently available and available insurance coverage, our management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on our financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to our financial position, results of operations, or cash flows. We are not currently a party to any legal proceedings that, in the opinion of management, are likely to have a material adverse effect on our business.
Item 1A. Risk Factors
There have been no material changes from the risk factors disclosed in our Annual Report.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
Item 6. Exhibits
36
* Filed or furnished herewith
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: August 11, 2023
|
|
|
|
|
ACASTI PHARMA INC. |
|
|
|
|
By: |
/s/ Prashant Kohl |
|
|
|
Name: Prashant Kohl |
|
|
Title: Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
By: |
/s/ Brian Ford |
|
|
|
Name: Brian Ford |
|
|
Title: Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
37
Exhibit 3.4
GENERAL BY-LAW 2020-1
OF
ACASTI PHARMA INC.
(the “Corporation”)
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
TABLE OF CONTENTS
|
|
|
1 - DEFINITIONS |
1 |
1.1 |
Definitions |
1 |
1.2 |
Interpretation |
1 |
1.3 |
Execution in Counterpart, by Facsimile and by Electronic Signature |
1 |
2 - GENERAL BUSINESS |
2 |
2.1 |
Head Office |
2 |
2.2 |
Establishment |
2 |
2.3 |
Seal |
2 |
2.4 |
Fiscal Year |
2 |
2.5 |
Execution of Instruments |
2 |
2.6 |
Banking Arrangements |
2 |
2.7 |
Voting Rights in Other Bodies Corporate |
3 |
3 - DIRECTORS |
3 |
3.1 |
Duties and Powers |
3 |
3.2 |
Delegation |
3 |
3.3 |
Qualifications of Directors |
3 |
3.4 |
Number of Directors |
3 |
3.5 |
Quorum |
4 |
3.6 |
Election and Term |
4 |
3.7 |
Removal of Directors |
4 |
3.8 |
Cessation of Office |
5 |
3.9 |
Resignation |
5 |
3.10 |
Vacancies |
5 |
3.11 |
Meetings by Telephone, Electronic or other Communication Facility |
5 |
3.12 |
Attendance |
5 |
3.13 |
Place of Meetings |
5 |
3.14 |
Calling of Meetings |
5 |
3.15 |
Notice of Meetings |
6 |
3.16 |
First Meeting of New Board |
6 |
3.17 |
Adjourned Meeting |
6 |
3.18 |
Votes to Govern |
6 |
3.19 |
Dissent |
6 |
3.20 |
Resolution in Writing |
7 |
3.21 |
Chairperson and Secretary |
7 |
- ii -
|
|
|
3.22 |
Remuneration and Expenses |
7 |
3.23 |
Duty of Loyalty and Conflict of Interest |
7 |
3.24 |
Contracts or Transactions - Disclosure of Interest |
8 |
3.25 |
Contracts or Transactions - Votes |
8 |
4 - COMMITTEES |
9 |
4.1 |
Committees of the Board |
9 |
4.2 |
Procedure |
9 |
5 - OFFICERS |
9 |
5.1 |
Appointment of Officers |
9 |
5.2 |
Agents and Attorneys |
9 |
5.3 |
Disclosure of Interest |
10 |
5.4 |
End of Mandate |
10 |
6 - PROTECTION OF DIRECTORS AND OFFICERS |
10 |
6.1 |
Indemnity of Directors and Officers |
10 |
6.2 |
Insurance |
11 |
7 - MEETINGS OF SHAREHOLDERS |
11 |
7.1 |
General Business |
11 |
7.2 |
Annual Meetings |
11 |
7.3 |
Special Meetings |
12 |
7.4 |
Place of Meetings |
12 |
7.5 |
Participation in Meetings by Electronic Means |
12 |
7.6 |
Notice of Meetings |
12 |
7.7 |
Waiver of Notice |
13 |
7.8 |
Record Date for Notice |
13 |
7.9 |
Chair and Secretary |
13 |
7.10 |
Procedure |
14 |
7.11 |
Persons Entitled to be Present |
14 |
7.12 |
Quorum |
14 |
7.13 |
Right to Vote |
14 |
7.14 |
Proxies and Representatives |
14 |
7.15 |
Joint Shareholders |
15 |
7.16 |
Votes to Govern |
15 |
7.17 |
Casting Vote |
15 |
7.18 |
Show of Hands |
15 |
7.19 |
Ballots |
15 |
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- iii -
|
|
|
7.20 |
Adjournment |
15 |
7.21 |
Storage of Ballots and Proxies |
16 |
8 - SHARES AND CERTIFICATES |
16 |
8.1 |
Issuance of Shares |
16 |
8.2 |
Payment of Shares |
16 |
8.3 |
Unpaid Shares |
16 |
8.4 |
Securities Register |
16 |
8.5 |
Register of Transfer |
17 |
8.6 |
Registration of Transfer |
17 |
8.7 |
Registered Ownership |
18 |
8.8 |
Share Certificates |
18 |
8.9 |
Certificated Shares |
18 |
8.10 |
Uncertificated Shares |
18 |
8.11 |
Replacement of Share Certificates |
19 |
8.12 |
Joint Shareholders |
19 |
8.13 |
Deceased Shareholders |
19 |
8.14 |
Delegation |
19 |
9 - DIVIDENDS AND RIGHTS |
19 |
9.1 |
Dividends |
19 |
9.2 |
Dividend Cheques |
20 |
9.3 |
Non-receipt or Loss of Cheques |
20 |
9.4 |
Record Date for Dividends and Rights |
20 |
9.5 |
Unclaimed Dividends |
20 |
10 - NOTICES |
20 |
10.1 |
Method of Giving Notices |
20 |
10.2 |
Notice to Joint Shareholders |
21 |
10.3 |
Undelivered Notices |
21 |
10.4 |
Omissions and Errors |
21 |
10.5 |
Persons Entitled by Death or Operation of Law |
21 |
10.6 |
Waiver of Notice |
21 |
11 - MISCELLANEOUS |
22 |
11.1 |
Declarations to the Enterprise Register |
22 |
11.2 |
Enactment, Repeal and Amendment of the By-Law |
22 |
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- iv -
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
1 - DEFINITIONS
1.1 Definitions
In this By-law, and all other By-laws of the Corporation, unless the context indicates otherwise:
a) “Act” means the Business Corporations Act (Quebec), or any statute which may be substituted therefor, including the regulations made thereunder as amended from time to time;
b) “Articles” shall mean the articles of the Corporation and includes any amendments thereto;
c) “Board” means the board of directors of the Corporation;
d) “By-laws” means the administrative By-laws of the Corporation, as well as all other administrative by-laws of the Corporation in force from time to time, including those referred to in section 726 of the Act, and any amendments which may be made to such By-laws from time to time;
e) “Director” means a member of the Board;
f) “Person” includes an individual, a sole proprietorship, a partnership, an association, a labour organization, an organization, a trust, a body corporate and all individuals acting as a trustee, executor, curator or as any other legal representative;
g) “Reporting Issuer” means a reporting issuer as defined in the Act; and
h) “Shareholders Meeting” means an annual shareholders meeting or a special meeting of shareholders.
1.2 Interpretation
a) words importing the singular number also include the plural and vice-versa; words importing the masculine gender include the feminine and vice-versa;
b) the headings used in this By-law are for ease of reference only and do not form part of it;
c) all words used in this By-law and defined in the Act shall have the meanings given to such words in the Act or in the related parts thereof;
d) this By-law is adopted pursuant to the Act, and is subject to, and must be read in conjunction with the Act. In the event of an inconsistency between a provision of this By-law and a provision of the Act, the latter shall prevail.
1.3 Execution in Counterpart, by Facsimile and by Electronic Signature
Subject to the Act, any notice, resolution, requisition, statement or other document required or permitted to be executed for the purposes of the Act, may be signed by way of electronic signature,
- 2 -
by way of a facsimile signature or by way of signing several similar documents by one or more Persons, and those documents, when duly signed by all Persons required or permitted to sign, as appropriate, shall constitute a single document for the purposes of the Act.
2 - GENERAL BUSINESS
2.1 Head Office
The head office of the Corporation must be permanently located in Quebec. The Corporation may relocate its head office in accordance with the Act.
2.2 Establishment
In addition to its head office, the Corporation may establish and maintain other establishments, offices, places of business and branches both within and outside Quebec, as the Board may determine from time to time.
2.3 Seal
The Corporation may have a seal, which shall be adopted and may be changed by the Board. The absence of a seal on a document of the Corporation does not render the document invalid.
2.4 Fiscal Year
The fiscal year end of the Corporation shall be March 31 or be as determined from time to time by the Board.
2.5 Execution of Instruments
Deeds, transfers, assignments, contracts, obligations, certificates and other instruments shall be signed on behalf of the Corporation by any Director or officer of the Corporation. In addition, the Board may from time to time direct the manner in which, and the Person or Persons by whom, any particular instrument or class of instruments may or shall be signed.
Notwithstanding the foregoing, the secretary or any other officer or any Director may sign certificates and similar instruments (other than share certificates) on the Corporation’s behalf with respect to any factual matters relating to the Corporation’s business and affairs, including certificates verifying copies of the Articles, By-laws, resolutions and minutes of meetings of the Corporation.
2.6 Banking Arrangements
The banking business of the Corporation, or any part or division of the Corporation, shall be transacted with such bank, trust company or other firm or body corporate as the Board may designate, appoint or authorize from time to time and all such banking business, or any part thereof, shall be transacted on the Corporation’s behalf by such one or more officers or other
Persons as the Board may designate, direct or authorize from time to time and to the extent thereby provided.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 3 -
2.7 Voting Rights in Other Bodies Corporate
Except as otherwise provided by the Board, any Director or officer has the full power to represent the Corporation, and more particularly to vote all of the shares or other securities carrying voting rights of any other entity held from time to time by the Corporation, at any and all meetings of shareholders, bondholders, debentureholders or holders of other securities (as the case may be) of such other entity and exercise all other rights attached to the said shares or securities as if he were the owner thereof. The Board may, from time to time, appoint any other Person for the same purpose.
3 - DIRECTORS
3.1 Duties and Powers
The Board exercises all the powers necessary to manage or supervise the management of the business and affairs of the Corporation. Subject to the Act, the Board shall exercise its powers by or pursuant to a resolution passed at a meeting of the Board at which a quorum is present or approved in writing by all Directors in office.
Without limiting the foregoing, the Board may, on behalf of the Corporation:
a) borrow money;
b) issue, reissue, sell or hypothecate its debt obligations;
c) enter into a suretyship to secure performance of an obligation of any Person; and
d) hypothecate all or any of its property, owned or subsequently acquired, to secure any obligation.
3.2 Delegation
Subject to the Act, the Articles and any By-laws, the Board may from time to time delegate to a Director, a committee of the Board or an officer or such other person or persons so designated by the Board all or any of the powers conferred on the Board by the Act to such extent and in such manner as the Board shall determine at the time of each such delegation.
3.3 Qualifications of Directors
Any natural person may be a Director of the Corporation unless such a person is less than eighteen (18) years of age, is under guardianship or curatorship, is of unsound mind and has been so found by a court in Canada or elsewhere, is a person for whom the court prohibits the exercise of this function, or has the status of bankrupt. A Director is not required to hold shares of the Corporation.
3.4 Number of Directors
The Board of Directors of the Corporation shall be made up of a minimum and a maximum number of Directors as indicated in the Articles of the Corporation as amended from time to time. The exact number of Directors shall be established from time to time by resolution of the Board.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 4 -
3.5 Quorum
A majority of the Directors in office constitutes a quorum at any meeting of the Board. In the absence of a quorum within the first fifteen (15) minutes following the start of the meeting, the Directors may only deliberate on the meeting’s adjournment. A quorum of Directors may exercise all the powers of the Board despite any vacancy on the Board.
3.6 Election and Term
Directors shall be elected by the shareholders at the first Shareholders Meeting and at each subsequent annual meeting at which an election of Directors is required, by an ordinary resolution adopted by a majority of the votes cast by shareholders able to vote on such resolution, and shall hold office until the next annual Shareholders Meeting or, if elected for an expressly stated term, for a term expiring no later than three (3) years following the election. The election need not be by ballot unless a ballot is demanded by any shareholder or required by the chairperson in accordance with section 7.19. If an election of Directors is not held at an annual Shareholders Meeting at which such election is required, the incumbent Directors shall continue in office until their resignation, replacement or removal.
If shareholders holding a certain class or series of shares have an exclusive right to elect one or more Directors, such number of Directors shall be elected by the majority of votes cast by the holders of such class or series of shares.
If permitted by the articles, the Directors may appoint one or more additional Directors to hold office for a term expiring not later than the close of the next annual Shareholders Meeting, provided the total number of Directors so appointed does not exceed one-third (1/3) of the number of Directors elected at the annual Meeting of Shareholders preceding their appointment.
3.7 Removal of Directors
Subject to the Act, the shareholders may, by ordinary resolution passed by a majority of votes cast at a special Shareholders Meeting duly called for that purpose, remove any Director or Directors. If holders of any class or series of shares have an exclusive right to elect one or more Directors, a Director so elected may only be removed by ordinary resolution of such holders.
A Director whose removal is to be proposed at a Shareholders Meeting must be informed of the time and place of the meeting within the same delays as those prescribed for the calling of such meeting. Such Director may attend the meeting and be heard or, if not in attendance, may explain, in a written statement read by the person presiding over the meeting or made available to the shareholders before or at the meeting, why he opposes the resolution proposing his removal.
Any vacancy created by the removal of a Director may be filled by a resolution of the shareholders at the Shareholders Meeting at which the Director is removed or, if it is not, at a subsequent meeting of the Board. If the holders of any class or series of shares have an exclusive right to elect one or more Directors and a vacancy occurs among these Directors, the vacancy may be filled by the holders of that class or series of shares by ordinary resolution at the Shareholders Meeting at which the Director is removed or, if it is not, by the remaining Directors elected by the holders of that class or series of shares, if there are such remaining Directors.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 5 -
3.8 Cessation of Office
A Director ceases to hold office when he dies, resigns, is removed, becomes disqualified from holding office or otherwise no longer meets the requirements to hold office as specified by the Act.
3.9 Resignation
A Director may resign from office by delivering or sending a written notice to the Corporation and such resignation becomes effective at the time the Director’s written resignation is received by the Corporation or at the time specified in the notice, whichever is later.
3.10 Vacancies
Subject to the Act or to the Articles, a quorum of Directors may fill a vacancy on the Board.
If there is no quorum of Directors, or if there has been a failure to elect the number or minimum number of Directors required by the Articles, the Directors then in office must without delay call a special Shareholders Meeting to fill the vacancies on the Board. If the Directors refuse or fail to call a meeting or if there are no Directors then in office, the meeting may be called by any shareholder.
A Director appointed or elected to fill a vacancy holds office for the unexpired term of his predecessor and remains in office until his successor is elected or nominated.
3.11 Meetings by Telephone, Electronic or other Communication Facility
A Director may participate in a meeting of the Board or of a committee of the Board by means of a telephonic, electronic or other communication facility that permits all participants to communicate adequately with each other during the meeting. A Director who participates in such meeting by such means is deemed to be present at that meeting.
3.12 Attendance
In addition to the Directors having to attend meetings of the Board, other Persons may also attend as needed, with the authorization of the chairperson of the meeting or the majority of the Directors present at that meeting.
3.13 Place of Meetings
Meetings of the Board are held at the registered office of the Corporation or at any other place within or outside of Quebec.
3.14 Calling of Meetings
Meetings of the Board shall be held from time to time at such place, on such day and at such time as the Board, the chairperson of the Board, the president, the secretary or any two Directors may determine. Meetings are called by the chairperson of the Board, the president or two Directors or
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 6 -
by the secretary upon being asked to call such a meeting by the chairperson of the Board, the president or two Directors.
3.15 Notice of Meetings
The notice stating the time and place of the meeting and specifying any matter to be dealt with relating to powers which the Board may not delegate, shall be given to each Director at least forty-eight (48) hours before the meeting is to occur. In the event of an emergency, such time limit shall be shortened to twenty-four (24) hours. This notice does not have to be given in writing.
Any Director may waive a notice of a meeting of the Board. Attendance of a Director at a meeting of the Board constitutes a waiver of notice of such meeting unless the Director attends such meeting for the sole purpose of objecting to the holding of the meeting on the grounds that it was not duly called.
3.16 First Meeting of New Board
Provided a quorum of Directors is present, each newly elected Board may without notice hold its first meeting following the Shareholders Meeting at which such Board is elected.
3.17 Adjourned Meeting
Whether or not there is quorum, any meeting of the Board may be adjourned from time to time by a vote of a majority of the Directors who are present and subsequently resumed without the requirement that a new notice be given, if the time and place of the adjourned meeting is announced at the same time as the adjournment.
At the adjourned meeting, the Board may validly transact business in accordance with the terms established at the time of the adjournment provided that there is a quorum. The Directors who constituted a quorum at the original meeting do not have to constitute the quorum at the adjourned meeting. If there is no quorum at the adjourned meeting, the meeting is deemed to have ended immediately after the adjournment.
3.18 Votes to Govern
Subject to the Act, at all meetings of the Board, any question shall be decided by a majority of the votes cast on the question and, in the case of an equality of votes, the chairperson of the meeting shall not be entitled to a second or casting vote. Any question at a meeting of the Board shall be decided by a show of hands unless a ballot is required or demanded.
3.19 Dissent
A Director who is present at a meeting of the Board or a committee of the Board is deemed to have consented to any resolution passed at the meeting unless:
a) the Director’s dissent has been entered in the minutes;
b) the Director sends a written dissent to the secretary of the meeting before the meeting is adjourned; or
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 7 -
c) the Director delivers a written dissent to the chairperson of the Board, sends it to the chairperson by any means providing proof of the date of receipt or delivers it to the head office of the Corporation immediately after the meeting is adjourned.
A Director is not entitled to dissent after voting for or consenting to a resolution.
A Director who was not present at a meeting at which a resolution was passed is deemed to have consented to the resolution unless he delivers a written dissent to the chairperson of the Board, sends it to the chairperson of the Board by any means providing proof of the date of receipt or delivers it to the head office of the Corporation within seven (7) days after becoming aware of the resolution.
3.20 Resolution in Writing
A resolution in writing, signed by all the Directors entitled to vote thereon is as valid as if it had been passed at a meeting of the Board or, as the case may be, of a committee of the Board. A copy of the resolution must be kept with the minutes of the meetings and the resolutions of the Board and its committees.
3.21 Chairperson and Secretary
The chairperson of the Board or, in the chairperson’s absence, the president or, in the president’s absence, a vice-president, shall be chairperson of any meeting of the Board. If none of these officers are present, the Directors present shall choose one of their number to be chairperson. The secretary of the Corporation shall act as secretary at any meeting of the Board and, if the secretary of the Corporation is absent, the chairperson of the meeting shall appoint a Person, who need not be a Director, to act as secretary of the meeting.
3.22 Remuneration and Expenses
The Directors shall be paid such remuneration for their services as Directors as the Board may from time to time authorize. In addition, the Board may authorize, by resolution, a special remuneration to a Director who executes specific or additional duties on behalf of the Corporation. The Directors shall also be entitled to be paid in respect of travelling and other expenses properly incurred by them in attending meetings of the Board or any committee thereof or in otherwise serving the Corporation. Nothing herein contained shall preclude any Director from serving the Corporation in any other capacity and receiving remuneration therefor.
3.23 Duty of Loyalty and Conflict of Interest
Subject to the Act, the Directors are bound by the same obligations as are imposed by the Civil Code of Quebec (Quebec) on any Director of a legal person. Consequently, in the exercise of their functions, the Directors are duty-bound toward the Corporation to act with prudence and diligence, honesty and loyalty and in the interest of the Corporation.
In particular, but without limiting the generality of the foregoing:
a) a Director may not mingle the property of the Corporation with his own property nor may he use for his own profit or that of a third Person any property of the
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 8 -
Corporation or any information he obtains by reason of his duties, unless he is expressly authorized to do so by the shareholders of the Corporation;
b) unless he has obtained the express consent of the Board, a Director must keep confidential the deliberations of the Board, any internal document and any other information to which he has access in the performance of his duties which is not publicly known and which has not been publicly disclosed by the Corporation;
c) a Director shall avoid placing himself in any situation where his personal interests would be in conflict with his obligations as a Director of the Corporation;
d) a director must disclose to the Corporation any interest he has in a business or association that may place him in a situation of conflict of interest and of any right he may set up against it, indicating their nature and value, where applicable.
3.24 Contracts or Transactions - Disclosure of Interest
A Director must disclose the nature and value of any interest he has in a contract or transaction to which the Corporation is a party. “Interest” means any financial stake in a contract or transaction that may reasonably be considered likely to influence decision-making. Furthermore, a proposed contract or a proposed transaction, including related negotiations, is considered a contract or transaction.
A Director must also disclose any contract or transaction to which the Corporation and any of the following are a party:
a) an associate of the Director or officer;
b) a group of which the Director or officer is a Director or officer; or.
c) a group in which the Director or officer or an associate of the Director or officer has an interest.
The Director satisfies the requirement if he discloses, in a case specified in subparagraph b) above, the Directorship or office held within the group or, in a case specified in subparagraph c) above, the nature and value of the interest he or his associate has in the group.
Unless it is recorded in the minutes of the first meeting of the Board at which the contract or transaction is discussed, the disclosure of an interest, contract or transaction must be made in writing to the Board as soon as the Director becomes aware of the interest, contract or transaction.
The disclosure must be made even in the case of a contract or transaction that does not require approval by the Board.
3.25 Contracts or Transactions - Votes
No Director may vote on a resolution to approve, amend or terminate a contract or transaction described in section 3.24 or be present during deliberations concerning the approval, amendment or termination of such a contract or transaction, unless the contract or transaction:
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 9 -
a) relates primarily to the remuneration of the Director or an associate of the Director as a Director of the Corporation or an affiliate of the Corporation;
b) relates primarily to the remuneration of the Director or an associate of the Director as an officer, employee or mandatary of the Corporation or an affiliate of the Corporation, if the Corporation is not a Reporting Issuer;
c) is for indemnity or liability insurance; or
d) is with an affiliate of the Corporation, and the sole interest of the Director is as a Director or officer of the affiliate.
If no quorum exists for the purpose of voting on a resolution to approve a contract or transaction only because a Director is not permitted to be present during deliberations, the other Directors present are deemed to constitute a quorum for the purpose of voting on the resolution.
If all the Directors are required to abstain from voting, the contract or transaction may be approved solely by the shareholders entitled to vote, by ordinary resolution. The disclosure required by section 3.24 must be made to the shareholders in a sufficiently clear manner before the contract or transaction is approved.
4 - COMMITTEES
4.1 Committees of the Board
The Board may, by resolution, create one or more committees comprised of Directors and, subject to the limitations prescribed by the Act, from time to time set the mandate and the number of Directors of any such committee.
4.2 Procedure
Subject to the Act and unless otherwise determined by a resolution of the Board, each committee shall have the power to fix its quorum at not less than a majority of its members, to elect its chairperson and to regulate its procedure. Each committee must provide the Board with a report concerning its activities if the Board makes such a request. The Board may cancel or modify any decision made by the committee.
5 - OFFICERS
5.1 Appointment of Officers
The Board may appoint any officers and any other mandataries as it deems appropriate and determine their titles, functions, powers, employment conditions and remuneration. An officer may but need not be a Director or a shareholder and any person may hold more than one office.
The Board may, in accordance with this By-law and subject to the Act, delegate to such officers powers to manage, or supervise the management of, the business and affairs of the Corporation.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 10 -
5.2 Agents and Attorneys
The Board shall have the power from time to time to appoint agents or attorneys for the Corporation in or out of the Province of Quebec with such powers of management or otherwise (including the power to sub-delegate) as the Board may determine.
5.3 Disclosure of Interest
The officers are mandataries of the Corporation. In this capacity, in the exercise of their functions, the officers are bound, among other things, toward the Corporation to act with prudence and diligence, honesty and loyalty and in the interest of the Corporation.
An officer must disclose the nature and value of any interest he h s in a contract or transaction to which the Corporation is a party, in the same way that a Director must disclose such an interest pursuant to section 3.24. In the case of an officer who is not a Director, disclosure must be made as soon as:
a) the officer becomes an officer;
b) the officer becomes aware that the contract or transaction is to be discussed or has been discussed at a meeting of the Board; or
c) the officer or the officer’s associate acquires an interest in the contract or transaction, if it was entered into earlier.
The disclosure must be made even in the case of a contract or transaction that does not require approval by the Board.
5.4 End of Mandate
An officer may resign at any time. The resignation of an officer takes effect on the date the Corporation receives the written notice he gives or on the later date indicated therein.
The Board may, at its own discretion, remove an officer of the Corporation at all times and the reason for the removal is not required to be given.
6 - PROTECTION OF DIRECTORS AND OFFICERS
6.1 Indemnity of Directors and Officers
Subject to the following, the Corporation must indemnify a Director or officer of the Corporation, a former Director or officer of the Corporation, a mandatary, any other person who acts or acted at the Corporation’s request as a Director or officer of another group, as well as their heirs, legatees, liquidators, assignees, authorized representatives or beneficiaries, against all costs, charges and expenses reasonably incurred in the exercise of their functions, including an amount paid to settle an action or satisfy a judgment, or arising from any investigative or other proceeding in which the person is involved if:
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 11 -
a) the person acted with honesty and loyalty in the interest of the Corporation or, as the case may be, in the interest of the other group for which the person acted as Director or officer or in a similar capacity at the Corporation’s request; and
b) in the case of a proceeding that is enforced by a monetary penalty, the person had reasonable grounds for believing that his conduct was lawful.
The Corporation must also advance moneys to such a person for the costs, charges and expenses of a proceeding referred to in the above paragraph.
However, in the event that a court or any other competent authority judges that the conditions set out in subparagraphs a) and b) above are not fulfilled or that the person committed an intentional or gross fault, the Corporation may not indemnify the person and the person must repay to the Corporation any moneys advanced.
The Corporation may, with the approval of the court, in respect of an action by or on behalf of the Corporation or other group referred to above, against a person referred to above, advance the necessary monies to the person or indemnify the person against all costs, charges and expenses reasonably incurred by the person in connection with the action, if the person fulfills the conditions set out above.
The provisions of this section 6.1 shall not, to the extent permitted by law, operate to affect or otherwise restrict the scope of any indemnification contractually agreed by or in favour of the Corporation or otherwise applicable under previous provisions of the law or any by-law of the Corporation of which a Director or an officer may avail himself.
6.2 Insurance
The Corporation may purchase and maintain insurance for the benefit of its Directors, officers and other mandataries against any liability they may incur as such or in their capacity as Directors, officers or mandataries of another group, if they act or acted in that capacity at the Corporation’s request.
7 - MEETINGS OF SHAREHOLDERS
7.1 General Business
The Corporation must hold an annual shareholders meeting; if necessary, the Corporation may also hold one or more special shareholder meetings.
7.2 Annual Meetings
An annual Shareholders Meeting entitled to vote at such a meeting must be held not later than eighteen (18) months after the Corporation is constituted and, subsequently, not later than fifteen (15) months after the last preceding annual shareholders meeting, for the purpose of:
a) considering the financial statements of the Corporation for the fiscal year ending within six (6) months preceding the date of such meeting and the auditor’s report thereon, if any;
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 12 -
b) considering any other financial information presentation of which is required by the Articles or the By-laws;
c) electing Directors;
d) appointing the auditor; and
e) deliberating with respect to all other matters which may be presented at the meeting.
The Board calls the annual Shareholders Meeting. Otherwise, the meeting may be called by the shareholders in accordance with the Act or with section 7.3 below.
7.3 Special Meetings
The Board may at any time call a special Shareholders Meeting.
The holders of not less than ten percent (10%) of the issued shares that carry the right to vote at a Shareholders Meeting sought to be held may requisition the Board to call a Shareholders Meeting for the purposes stated in the requisition.
The requisition, signed by at least one shareholder, must state the business to be transacted at the meeting and must be sent to each Director and to the head office of the Corporation.
On receiving the requisition, the Board calls a Shareholders Meeting to transact the business stated in the requisition. If the Board does not within twenty-one (21) days after receiving the requisition call a meeting, any shareholder who signed the requisition may call the meeting.
Unless the shareholders otherwise resolve at a meeting called by shareholders, the Corporation must reimburse the shareholders for the expenses reasonably incurred by them in requisitioning, calling and holding the meeting.
7.4 Place of Meetings
Subject to the Articles, Shareholders Meetings must be held in Quebec at the place determined by the Board. If the Articles so allow, or in the absence of such a provision, if all the shareholders entitled to vote at the meeting agree, the meeting may be held at a place outside of Quebec.
7.5 Participation in Meetings by Electronic Means
A meeting may be held solely by means of equipment enabling all participants to communicate directly with one another.
In addition, any Person entitled to attend a Shareholders Meeting may participate in the meeting by means of any equipment enabling all participants to communicate directly with one another. A Person participating in a meeting by such means is deemed present at the meeting.
Any shareholder participating in a Shareholders Meeting by means of equipment enabling all participants to communicate directly with one another may vote by any means enabling votes to be cast in a way that allows them to be verified afterwards and protects the secrecy of the vote when a secret ballot has been requested.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 13 -
7.6 Notice of Meetings
A notice of a Shareholders Meeting specifying the time and place of the meeting, as well as the business to be transacted, must be sent, in writing and by any means providing proof of the date of receipt, to each Person entitled to vote at the meeting not less than twenty-one (21) days and not more than sixty (60) days before the meeting. It must also specify the time before which the Corporation must receive the proxies of the shareholders who wish to be represented at the Shareholders Meeting, which time must not exceed forty-eight (48) hours preceding the Shareholders Meeting or the resumption of a Shareholders Meeting after an adjournment, excluding Saturdays and holidays.
Notice of a Shareholders Meeting at which special business is to be transacted shall state the nature of that business in sufficient detail to permit the shareholder to form a reasoned judgment thereon, and contain the text of any special resolution to be submitted to the meeting. All business transacted at a special meeting of the shareholders and all business transacted at an annual shareholders meeting, except consideration of the financial statements and auditor’s report, the appointment of the auditor and the election of Directors, is deemed to be special business.
If a Director or a shareholder entitled to vote at a Shareholders Meeting gives written notice not less than ten (10) days before the meeting to the auditor or a former auditor of the Corporation, the auditor or former auditor attends the meeting at the Corporation’s expense and answers any question relating to their duties as auditor.
Irregularities in the notice of the Shareholders Meeting or in its sending will not affect the validity of the Shareholders Meeting. Similarly, the unintentional failure to send a notice of Shareholders Meeting to a person entitled to it, or the failure to receive it by a person entitled to the notice, does not invalidate the resolutions passed at such meeting.
7.7 Waiver of Notice
A shareholder or Director may waive notice of a Shareholder Meeting; the waiver may be given either before or after the meeting. Their attendance at the meeting is a waiver of notice of the meeting unless they attend the meeting for the sole purpose of objecting to the holding of the meeting on the grounds that it was not lawfully called or held.
7.8 Record Date for Notice
The Board may set, in conformity with applicable securities law requirements, a date prior to the date on which a meeting is to be called or held as the record date for the purpose of determining shareholders entitled to receive notice of or to vote at the meeting, and only those registered shareholders registered on the date so set shall be so entitled, notwithstanding any transfer of shares in the registers of the Corporation between the record date and the date on which the meeting is called or held. The record date must be not less than twenty-one (21) days and not more than sixty (60) days before the meeting.
7.9 Chair and Secretary
The chairperson of the Board or, in the chairperson’s absence, the president or, in the president’s absence, a vice-president shall be chairperson of any meeting of shareholders. If none of these
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 14 -
officers are present within fifteen (15) minutes after the time appointed for holding the meeting, the Persons present and entitled to vote shall choose a chairperson from amongst themselves. The secretary of the Corporation shall act as secretary at any Shareholders Meeting or, if the secretary of the Corporation is absent, the chairperson of the meeting shall appoint some person, who need not be a shareholder, to act as secretary of the meeting. If desired, one or more scrutineers, who need not be shareholders, may be appointed by resolution or by the chairperson with the consent of the meeting in accordance with the procedure set out in section 7.16.
7.10 Procedure
The chairperson of the meeting directs the meeting and ensures its orderly conduct. His decisions, including those relating to the validity of proxies, are final and binding on all the shareholders.
7.11 Persons Entitled to be Present
The only persons entitled to be present at a Shareholders Meeting shall be those entitled to vote thereat, the Directors and auditors of the Corporation and others who, although not entitled to vote, are entitled or required under any provision of the Act or the Articles or By-laws to be present at the meeting. Any other person may be admitted only on the invitation of the chairperson of the meeting or with the consent of the meeting in accordance with the procedure set out in section 7.17.
7.12 Quorum
A quorum of shareholders is present at a meeting of shareholders, provided that a quorum shall not be less than two persons, if the holders of at least thirty-three and one-third percent (33 1/3%) of the shares of the Corporation entitled to vote at the meeting are present in person or represented by proxy. A quorum need not be present throughout the meeting provided a quorum is present at the opening of the meeting.
7.13 Right to Vote
Subject to a record date established in accordance with section 7.8, at a Shareholders Meeting, the shareholders registered on the securities register of the Corporation are entitled to exercise the voting rights attached to the shares in their name.
7.14 Proxies and Representatives
Every shareholder entitled to vote at a Shareholders Meeting may, by means of a proxy, appoint a proxyholder, or one or more alternate proxyholders, who need not be shareholders, to attend and act at the meeting in the manner and to the extent authorized and with the authority conferred by the proxy. A proxy shall be signed in writing or by electronic signature by the shareholder or the shareholder’s representative authorized in writing or by electronic signature.
Unless otherwise indicated, a proxy lapses one year after the date it is given. It may be revoked at anytime.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 15 -
A proxyholder has the same rights as the shareholder represented to speak at a Shareholders Meeting in respect of any matter and to vote at the meeting. However, a proxyholder who has conflicting instructions from more than one shareholder may not vote by a show of hands.
7.15 Joint Shareholders
If two or more Persons hold shares jointly, one of those holders present at a Shareholders Meeting may in the absence of the others vote the share, but if two or more of those Persons who are present, in person or by proxy, vote, they shall vote as one on the shares jointly held by them.
7.16 Votes to Govern
Except as otherwise required by the Act and the Articles, all questions proposed for the consideration of shareholders at a Shareholders Meeting shall be determined by a majority of the votes cast by all who are entitled to vote.
7.17 Casting Vote
In case of an equality of votes at any meeting of shareholders, regardless of the manner of voting, the chairperson of the meeting shall not be entitled to a second or casting vote.
7.18 Show of Hands
Any question at a Shareholders Meeting shall be decided by a show of hands, unless a ballot thereon is demanded by a shareholder entitled to vote at the Shareholders Meeting as hereinafter provided. Every Person who is present and entitled to vote thereon shall have one vote. Whenever a vote by any means other than by ballot is taken, a declaration by the chairperson of the meeting that the vote upon the question has been carried or carried by a particular majority or not carried and an entry to that effect in the minutes of the meeting shall be prima facie evidence of the fact without proof of the number or proportion of the votes recorded in favour of or against any resolution or other proceeding in respect of the said question, and the result of the vote so taken shall be the decision of the shareholders upon the said question.
7.19 Ballots
On any question proposed for consideration at a meeting of shareholders, and whether or not a show of hands has been taken thereon, the chairperson may require, or any shareholder or proxyholder entitled to vote at the meeting may demand, a ballot. A ballot so required or demanded shall be taken in such manner as the chairperson shall direct. A requirement or demand for a ballot may be withdrawn at any time prior to the taking of the ballot. If a ballot is taken each Person present shall be entitled, in respect of the shares which the Person is entitled to vote at the meeting upon the question, to that number of votes provided by the Act or the Articles, and the result of the ballot so taken shall be the decision of the shareholders upon the said question.
7.20 Adjournment
Whether or not there is quorum, the chairperson of the Shareholders Meeting may, with the consent of the shareholders present or represented by proxy and following the procedure set at section 7.16,
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 16 -
adjourn any Shareholders Meeting. The chairperson of the Shareholders Meeting may also adjourn a meeting ex officio if he believes it is impossible to conduct it in an orderly manner.
If a Shareholders Meeting is adjourned for less than thirty (30) days, it is not necessary to give notice of the adjourned meeting other than by announcement at the original meeting. If a Shareholders Meeting is adjourned by one or more adjournments for an aggregate of thirty (30) days or more, notice of the adjourned meeting must be given as for an original meeting.
The Shareholders Meeting is validly resumed if it is held on the date and at the time and place announced and if there is quorum. In the absence of quorum at the adjourned meeting, the original meeting is deemed to have terminated immediately after its adjournment.
7.21 Storage of Ballots and Proxies
The Corporation must, for at least three (3) months after a Shareholders Meeting, keep at its head office the ballots cast and the proxies presented at the meeting. Any shareholder or proxyholder who was entitled to vote at the meeting may, without charge, inspect the ballots and proxies kept by the Corporation.
8 - SHARES AND CERTIFICATES
8.1 Issuance of Shares
Subject to any pre-emptive right granted to shareholders, shares may be issued at the times, to the Persons, including Directors and officers, and for the consideration that the Board determines. The Board may, by resolution, accept subscriptions, issue and allot unissued shares from the Corporation’s share capital and grant exchange rights, options or acquisition rights with respect to those shares.
8.2 Payment of Shares
Shares may be issued whether or not they are fully paid. However, shares may only be considered paid if consideration equal to the issue price determined by the Board has been paid to the Corporation.
Consideration for the shares issued by the Corporation is payable in money, or in property or past services determined by the Board to be the fair equivalent of the money consideration, considering all the circumstances.
A promissory note or a promise to pay made by a Person to whom shares are issued, or a Person who does not deal at arm’s length, within the meaning of that expression in the Taxation Act (Quebec), with a Person to whom shares are issued does not constitute consideration for the shares.
8.3 Unpaid Shares
Unless the terms of payment for shares are determined by contract, the Board may call for payment of all or part of the unpaid amounts on shares subscribed or held by the shareholders, the whole as provided by the Act.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 17 -
8.4 Securities Register
The securities register of the Corporation must contain the following information with respect to its shares:
a) the names, in alphabetical order, and the addresses of present and past shareholders;
b) the number of shares held by each such shareholder;
c) the date and details of the issue and transfer of each share; and
d) any amount due on any share.
The register must contain, if applicable, the same information with respect to the Corporation’s debentures, bonds, notes and other securities, with the necessary modifications.
8.5 Register of Transfer
The Corporation shall cause to be kept a register of transfers in which all transfers of securities issued by the Corporation in registered form and the date and other particulars of each transfer shall be set out.
Subject to the Act, the transfer of shares is governed by the Act respecting the transfer of securities and the establishment of security entitlements (Quebec).
8.6 Registration of Transfer
If an endorsed share certificate in registered form is presented to the Corporation with a request to register a transfer of the certificated share or an instruction is presented to the Corporation with a request to register a transfer of an uncertificated share, the Corporation registers the transfer as requested if:
a) under the terms of the share, the purchaser is eligible to have the share registered in that Person’s name;
b) the endorsement or instruction is made by the appropriate Person or by that Person’s representative;
c) reasonable assurance is given that the endorsement or instruction is neither forged nor counterfeited and is authorized;
d) any applicable fiscal law that imposes duties on the Corporation at the time of the transfer has been complied with;
e) the transfer does not violate any restriction on transfer imposed by the Corporation that is enforceable against the purchaser or imposed by law; and
f) the transfer is rightful or is to a protected purchaser, pursuant to the Act respecting the transfer of securities and the establishment of security entitlements (Quebec).
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 18 -
Shares that are not fully paid but for which no instalment is payable may only be transferred with the authorization of the Board. The Directors must reasonably verify the acquirer’s ability to pay for the shares before authorizing the transfer.
A share may not be transferred until all instalments payable up to the time of transfer have been fully paid.
8.7 Registered Ownership
Subject to the Act, the Corporation may treat the registered owner of a share as the Person exclusively entitled to vote, to receive notices, to receive any dividend or other payments in respect thereof and otherwise to exercise all the rights and powers of an owner of a share.
8.8 Share Certificates
A share issued by the Corporation may be a certificated share or an uncertificated share. A certificated share is represented by a paper certificate in registered form, and an uncertificated share is represented by an entry in the securities register in the name of the shareholder.
Unless otherwise provided in the Articles, shares are issued as certificated shares unless the Board determines, by resolution, that the shares of any class or series or certain shares of a class or series are to be issued as uncertificated shares.
The Board may also, by resolution, determine that a certificated share becomes an uncertificated share as soon as the paper certificate is surrendered to the Corporation.
Inversely, the Board may, by resolution, determine that an uncertificated share becomes a certificated share on delivery to the shareholder of a certificate in the shareholder’s name or, in the case of a control agreement under the Act respecting the transfer of securities and the establishment of security entitlements (Quebec), on delivery to the purchaser, within the meaning of the Act respecting the transfer of securities and the establishment of security entitlements (Quebec), of a certificate in the purchaser’s name, unless there are provisions inconsistent with such a control agreement, in which case those provisions apply. The Board must give notice of the resolution to the shareholders of the classes or series of shares concerned.
8.9 Certificated Shares
In the case of certificated shares, the Corporation must issue to the shareholder, without charge, a certificate in registered form.
Share certificates shall be in such form as the Board may from time to time approve in accordance with the requirements of the Act.
Subject to any resolution of the Board providing otherwise, the share certificates of the Corporation must be signed by any of the Directors or officers or by a person acting in their name. The signature may be affixed by an automatic device or electronic process.
In the absence of any evidence to the contrary, the certificate is proof of the shareholder’s title to the shares represented by the certificate.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 19 -
Share certificates need not be under corporate seal.
8.10 Uncertificated Shares
In the case of uncertificated shares, the Corporation must send the shareholder a written notice containing the information required under the Act.
8.11 Replacement of Share Certificates
If the shareholder of a certificated share claims that the certificate has been lost, wrongfully taken or destroyed, the Corporation must issue a new certificate if the shareholder:
a) so requests before the Corporation has notice that the lost, wrongfully taken or allegedly destroyed certificate has been delivered to a protected purchaser, as such term is defined in the Act respecting the transfer of securities and the establishment of security entitlements (Quebec);
b) provides security sufficient in the Corporation’s judgment to protect the Corporation from any loss that the Corporation may suffer by issuing a new certificate; and
c) satisfies any other reasonable requirements imposed by the Corporation.
8.12 Joint Shareholders
If two or more Persons are registered as joint holders of any share, the Corporation shall not be bound to issue more than one certificate in respect thereof, and delivery of such certificate to one of such Persons shall be sufficient delivery to all of them. Any one of such Persons may give effectual receipts for the certificate issued in respect thereof or for any dividend, bonus, return of capital or other money payable or warrant issuable in respect of such share.
8.13 Deceased Shareholders
In the event of the death of a holder, or of one of the joint holders, of any share, the Corporation shall not be required to make any entry in the securities register in respect thereof or to make payment of any dividends thereon except upon production of all such documents as may be required by the Act and upon compliance with the reasonable requirements of the Corporation or it transfer agent.
8.14 Delegation
Subject to the limits of the Act, the Board may delegate the powers and duties provided for in this section 8 inter alia, to the corporate secretary of the Corporation or to a transfer agent or any other agent responsible for keeping, in whole or in part, the securities register.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 20 -
9 - DIVIDENDS AND RIGHTS
9.1 Dividends
Subject to the provisions of the Act and the Articles, the Board may from time to time declare dividends payable to· the shareholders according to their respective rights and interests in the Corporation. Dividends may be paid, in whole or in part, in money or property or by issuing fully paid shares or options or rights to acquire fully paid shares of the Corporation.
If shares of the Corporation are issued in payment of a dividend, the Corporation may add all or part of the value of those shares to the appropriate issued and paid-up share capital account.
The Corporation may not declare and pay a dividend, except by issuing shares or options or rights to acquire shares, if there are reasonable grounds for believing that the Corporation is, or would after the payment be, unable to pay its liabilities as they become due.
The Corporation may deduct from the dividends payable to a shareholder any amount due to the Corporation by the shareholder, on account of calls for payment or otherwise.
9.2 Dividend Cheques
A dividend payable in cash may be paid by cheque drawn on the Corporation’s banks or by electronic means to the order of each registered holder of shares of the class or series in respect of which it has been declared. Cheques may be sent by prepaid ordinary mail to such registered holder at such holder’s address recorded in the Corporation’s securities register, unless in each case such holder otherwise directs. In the case of joint holders the cheque shall, unless such joint holders otherwise direct, be made payable to the order of all of such joint holders and, if more than one address is recorded in the Corporation’s securities register in respect of such joint holding, the cheque shall be mailed to the first address so appearing. The mailing of such cheque, in such manner, unless the cheque is not paid on due presentation, shall satisfy and discharge the liability for the dividend to the extent of the sum represented thereby plus the amount of any tax which the Corporation is required to and does withhold.
9.3 Non-receipt or Loss of Cheques
In the event of non-receipt or loss of any dividend cheque by the Person to whom it is sent, the Corporation shall issue to such Person a replacement cheque for a like amount on such terms as to indemnity, reimbursement of expenses and evidence of non-receipt or loss and of title as the Board may from time to time prescribe, whether generally or in any particular case.
9.4 Record Date for Dividends and Rights
The Board may fix, in advance, in accordance with applicable securities law requirements, a record date for the determination of the shareholders entitled to receive dividends.
9.5 Unclaimed Dividends
Any dividend unclaimed after a period of two (2) years from the date on which the dividend has been declared to be payable shall be forfeited and shall revert to the Corporation.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 21 -
10 - NOTICES
10.1 Method of Giving Notices
Any notice, communication or document (“notice”) to be given or sent pursuant to the Act, the Articles, the By-laws or otherwise to a shareholder, Director, officer or auditor shall be sufficiently given or sent if given or sent by prepaid mail, prepaid transmitted, recorded, or electronic communication capable of providing a written copy of such notice, or delivered Personally to such Person’s latest address as shown on the securities register of the Corporation or, in the case of a Director, if more current, the address as shown in the most recent declaration filed under the Act Respecting the Legal Publicity of Enterprises (Quebec). A notice shall be deemed to have been received on the date when it is delivered Personally, or on the fifth (5th) day after mailing, or on the date of dispatch of a transmitted or recorded electronic communication. The secretary may change or cause to be changed the recorded address of any shareholder, Director, officer or auditor in accordance with any information believed by the secretary to be reliable.
10.2 Notice to Joint Shareholders
Subject to the Securities Act (Quebec) and applicable regulations in securities laws, if two or more Persons are registered as joint holders of any share, any notice shall be addressed to all of such joint holders but notice to one of such Persons shall be sufficient notice to all of them.
10.3 Undelivered Notices
If any notice given to a shareholder pursuant to section 10.1 is returned on two consecutive occasions because the shareholder cannot be found, the Corporation shall not be required to give any further notice to such shareholder until such shareholder informs the Corporation in writing of the shareholder’s new address.
10.4 Omissions and Errors
The accidental omission to give or send any notice to any shareholder, Director, officer or auditor, or the non-receipt of any notice by any such Person or any error in any notice not affecting the substance thereof, shall not invalidate any action taken at any meeting held pursuant to such notice or otherwise based thereon.
10.5 Persons Entitled by Death or Operation of Law
Every Person who, by operation of law, transfer, death of a shareholder or any other means whatsoever, shall become entitled to any share, shall be bound by every notice in respect of such share which shall have been duly given or sent to the shareholder from whom the Person derives title to such share prior to that Person’s name and address being entered on the securities register (whether such notice was given or sent before or after the happening of the event upon which that Person becomes so entitled) and prior to that Person furnishing to the Corporation the proof of authority or evidence of entitlement prescribed by the Act.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
- 22 -
10.6 Waiver of Notice
Any shareholder (or shareholder’s duly appointed proxyholder), Director, officer or auditor may at any time waive the giving or sending of any notice, or waive or abridge the time for any notice, required to be given to that Person under any provision of the Act, the Articles, the By laws or otherwise and such waiver or abridgement shall cure any default in the giving or sending or in the time of such notice, as the case may be. Any such waiver or abridgement shall be in writing or given by electronic signature except a waiver of notice of a Shareholders Meeting or of the Board which may be given in any manner.
11 - MISCELLANEOUS
11.1 Declarations to the Enterprise Register
A Director, officer or any authorized person signs the declarations which must be sent by the Corporation to the enterprise registrar under the Act respecting the legal publicity of enterprises (Quebec).
11.2 Enactment, Repeal and Amendment of the By-Law
The Directors may from time to time amend the present By-law, repeal the provisions thereof in whole or in part or add thereto by adopting any other administrative by-law or any other by-law dealing with any other applicable matter. Subject to the applicable provisions of the Act, any such amendment, repeal or addition is effective as of the date of the resolution of the Board adopting it. It must be submitted to the shareholders for approval at the next Shareholders Meeting, and the shareholders may, by ordinary resolution, ratify, amend or reject it. It ceases to be effective at the close of the Shareholders’ Meeting if it is rejected by or not submitted to the shareholders. However, By-law amendments relating to procedural matters with respect to Shareholders Meetings take effect only once they have received shareholder approval.
The Board is authorized to make any clerical change to the By-law to correct typographical errors or to clarify the meaning of a particular provision without requiring the approval of the shareholders.
*****
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:62166140.2
Exhibit 10.1
ACASTI PHARMA INC.
STOCK OPTION PLAN AS AMENDED SEPTEMBER 28, 2022
OPTION AGREEMENT
(the “Option Agreement”)
Acasti Pharma Inc. (the “Company”) hereby grants the following Options to you subject to the terms and conditions of this Option Agreement, together with the provisions of the Company’s Stock Option Plan as amended September 28, 2022 (the “Plan”), all the terms of which Plan are hereby incorporated into this Option Agreement:
Name of Option Holder: ____________________________________________________
Grant Date: _____________________________________________
Number of Options Granted: ________________________________________________
Exercise Price:
Expiry Date:
The Options are intended to be incentive stock options qualifying under Section 422 of the Code, although the Company makes no representation or guarantee that the Options will qualify as ISOs.
1.The terms and conditions of the Plan are hereby incorporated by reference as terms and conditions of this Option Agreement and all capitalized terms used herein, unless expressly defined in a different manner, have the meanings ascribed thereto in the Plan.
2.The Options are only exercisable before they expire and then only with respect to the vested portion of the Options. The Options shall vest in accordance with the vesting schedule set forth on the Vesting Schedule attached hereto (the “Schedule”); provided, however, that for purposes of vesting, fractional numbers of Shares shall be rounded to the nearest whole number, and the number of Shares that shall vest on the final vesting date shall be rounded up or down as necessary such that the total number of Shares that vest pursuant to the vesting schedule shall be equal to the number of Shares covered by the Options as set forth on the Schedule.
3.The Option Holder consents to and authorizes the use of the Option Holder’s personal information required by the Company in order to administer the Plan, the disclosure of such personal information to any custodian appointed in respect of the Plan and other third parties, and to the disclosure of such personal information to such persons (including persons located outside my jurisdiction of residence) in connection with the administration of the Plan. The Option Holder acknowledges that jurisdictions outside the Option Holder’s jurisdiction of residence may not provide the same statutory protection for the personal information as the Option Holder’s jurisdiction of residence.
4.Each notice relating to the Options must be in writing and signed by the Option Holder or the Option Holder’s legal representative. All notices to the Company must be delivered personally, e-mail or mail, postage prepaid and must be addressed to [ ]. All notices to the Option Holder will be addressed to the principal address of the Option Holder on file with the Company. Either the Option Holder or the Company may designate a different address by written notice to the other. Any notice
- 2 -
given by either the Option Holder or the Company is not binding on the recipient thereof until received.
5.Nothing in the Plan, in this Option Agreement, or as a result of the grant of Options to the Option Holder, will affect the Company’s right, or that of any Subsidiary of the Corporation, to terminate the Option Holder’s employment at any time for any reason whatsoever. Upon such termination, the Option Holder’s rights with respect to the Option will be subject to restrictions and time limits, complete details of which are set out in the Plan.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
- 3 -
VESTING SCHEUDLE TO OPTION AGREEMENT
Vesting Schedule: [ ]
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
- 4 -
UNDERSTANDING, ACKNOWLEDGEMENT AND ACCEPTANCE:
I acknowledge that I have received a copy of the Plan. I have read this Option Agreement and the Plan and accept the Options in accordance with and subject to the terms and conditions of this Option Agreement and the Plan.
6.For the avoidance of any doubt:
(a)I confirm that I have read and understood Section 4.2 of the Plan, including the definition of “Termination Date”.
(b)I understand that Section 4.2 of the Plan governs my rights pursuant to the Options upon the termination of my employment or other service engagement, as applicable, with the Company or a Subsidiary of the Corporation.
7.I hereby waive the right to assert or argue that either (i) I did not read the Option Agreement and/or the Plan, or (ii) I did not understand the consequences of Section 4.2 of the Plan. I understand that I may at any time ask questions about the Plan (including the consequences of Section 4.2 of the Plan), by contacting [ ] of the Company or such person’s designate.
8.I acknowledge and agree that I have received good and sufficient consideration for entering into this Option Agreement, including the grant of the Options which is fully conditional on me entering into this Option Agreement. I agree to be bound by the terms and conditions of the Plan governing these Options.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
Exhibit 10.2
ACASTI PHARMA INC.
STOCK OPTION PLAN AS AMENDED SEPTEMBER 28, 2022
OPTION AGREEMENT
(the “Option Agreement”)
Acasti Pharma Inc. (the “Company”) hereby grants the following Options to you subject to the terms and conditions of this Option Agreement, together with the provisions of the Company’s Stock Option Plan as amended September 28, 2022 (the “Plan”), all the terms of which Plan are hereby incorporated into this Option Agreement:
Name of Option Holder: ____________________________________________________
Grant Date: ___________________________________________
Number of Options Granted: ________________________________________________
Exercise Price:
Expiry Date:
Options issued to directors will be considered Non-Qualified Stock Options as they do not qualify under Section 422 of the Code as Incentive Stock Options. We recommend that the US resident Directors consult tax counsel about the tax implications when exercising options in future.
1.The terms and conditions of the Plan are hereby incorporated by reference as terms and conditions of this Option Agreement and all capitalized terms used herein, unless expressly defined in a different manner, have the meanings ascribed thereto in the Plan.
2.The Options are only exercisable before they expire and then only with respect to the vested portion of the Options. The Options shall vest in accordance with the vesting schedule set forth on the Vesting Schedule attached hereto (the “Schedule”); provided, however, that for purposes of vesting, fractional numbers of Shares shall be rounded to the nearest whole number, and the number of Shares that shall vest on the final vesting date shall be rounded up or down as necessary such that the total number of Shares that vest pursuant to the vesting schedule shall be equal to the number of Shares covered by the Options as set forth on the Schedule.
3.The Option Holder consents to and authorizes the use of the Option Holder’s personal information required by the Company in order to administer the Plan, the disclosure of such personal information to any custodian appointed in respect of the Plan and other third parties, and to the disclosure of such personal information to such persons (including persons located outside my jurisdiction of residence) in connection with the administration of the Plan. The Option Holder acknowledges that jurisdictions outside the Option Holder’s jurisdiction of residence may not provide the same statutory protection for the personal information as the Option Holder’s jurisdiction of residence.
4.Each notice relating to the Options must be in writing and signed by the Option Holder or the Option Holder’s legal representative. All notices to the Company must be delivered personally, e-mail or mail, postage prepaid and must be addressed to [ ]. All notices to the Option Holder will be addressed to the principal address of the Option Holder on file with the Company. Either the Option Holder or the Company may designate a different address by written notice to the other. Any notice
- 2 -
given by either the Option Holder or the Company is not binding on the recipient thereof until received.
5.Nothing in the Plan, in this Option Agreement, or as a result of the grant of Options to the Option Holder, will affect the Company’s right, or that of any Subsidiary of the Corporation, to terminate the Option Holder’s employment at any time for any reason whatsoever. Upon such termination, the Option Holder’s rights with respect to the Option will be subject to restrictions and time limits, complete details of which are set out in the Plan.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
- 3 -
VESTING SCHEUDLE TO OPTION AGREEMENT
Vesting Schedule: [ ]
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
- 4 -
UNDERSTANDING, ACKNOWLEDGEMENT AND ACCEPTANCE:
I acknowledge that I have received a copy of the Plan. I have read this Option Agreement and the Plan and accept the Options in accordance with and subject to the terms and conditions of this Option Agreement and the Plan.
6.For the avoidance of any doubt:
(a)I confirm that I have read and understood Section 4.2 of the Plan, including the definition of “Termination Date”.
(b)I understand that Section 4.2 of the Plan governs my rights pursuant to the Options upon the termination of my employment or other service engagement, as applicable, with the Company or a Subsidiary of the Corporation.
7.I hereby waive the right to assert or argue that either (i) I did not read the Option Agreement and/or the Plan, or (ii) I did not understand the consequences of Section 4.2 of the Plan. I understand that I may at any time ask questions about the Plan (including the consequences of Section 4.2 of the Plan), by contacting [ ] of the Company or such person’s designate.
8.I acknowledge and agree that I have received good and sufficient consideration for entering into this Option Agreement, including the grant of the Options which is fully conditional on me entering into this Option Agreement. I agree to be bound by the terms and conditions of the Plan governing these Options.
DOCPROPERTY "DocsID" * MERGEFORMAT LEGAL_1:71361924.1
Exhibit 31.1
CERTIFICATION
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Prashant Kohli, certify that:
1. I have reviewed this quarterly report on Form 10-Q of Acasti Pharma Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: August 11, 2023
|
|
/s/ Prashant Kohli |
|
Chief Executive Officer |
Exhibit 31.2
CERTIFICATION
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Brian Ford, certify that:
1. I have reviewed this quarterly report on Form 10-Q of Acasti Pharma Inc.;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: August 11, 2023
|
|
/s/ Brian Ford |
|
Chief Financial Officer |
Exhibit 32.1
SECTION 906 CERTIFICATION
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code) in connection with the quarterly report on Form 10-Q of Acasti Pharma Inc. for the quarterly period ended June 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned officer hereby certifies, to such officer’s knowledge, that:
|
|
(1) |
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
|
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Acasti Pharma Inc. |
|
|
|
/s/ Prashant Kohli |
|
Name: |
Prashant Kohli |
Title: |
Chief Executive Officer |
Date: |
August 11, 2023 |
|
This certification accompanies the Report pursuant to §906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by the Sarbanes-Oxley Act of 2002, be deemed “filed” by Acasti Pharma Inc. for purposes of §18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section.
Exhibit 32.2
SECTION 906 CERTIFICATION
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code) in connection with the quarterly report on Form 10-Q of Acasti Pharma Inc. for the quarterly period ended June 30, 2023 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned officer hereby certifies, to such officer’s knowledge, that:
|
|
(1) |
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and |
|
|
(2) |
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of Acasti Pharma Inc. |
|
|
|
/s/ Brian Ford |
|
Name: |
Brian Ford |
|
Title: |
Chief Financial Officer |
Date: |
August 11, 2023 |
|
|
|
This certification accompanies the Report pursuant to §906 of the Sarbanes-Oxley Act of 2002 and shall not, except to the extent required by the Sarbanes-Oxley Act of 2002, be deemed “filed” by Acasti Pharma Inc. for purposes of §18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liability of that section.
v3.23.2
Document And Entity Information - shares
|
3 Months Ended |
|
Jun. 30, 2023 |
Aug. 09, 2023 |
| Cover [Abstract] |
|
|
| Entity Central Index Key |
0001444192
|
|
| Entity Registrant Name |
Acasti Pharma Inc.
|
|
| Amendment Flag |
false
|
|
| Current Fiscal Year End Date |
--03-31
|
|
| Document Fiscal Period Focus |
Q1
|
|
| Document Fiscal Year Focus |
2023
|
|
| Document Type |
10-Q
|
|
| Document Quarterly Report |
true
|
|
| Document Period End Date |
Jun. 30, 2023
|
|
| Document Transition Report |
false
|
|
| Securities Act File Number |
001-35776
|
|
| Entity Incorporation, State or Country Code |
Z4
|
|
| Entity Tax Identification Number |
98-1359336
|
|
| Entity Address, Address Line One |
2572 boul. Daniel-Johnson, 2nd Floor
|
|
| Entity Address, City or Town |
Laval
|
|
| Entity Address, State or Province |
QC
|
|
| Entity Address, Country |
CA
|
|
| Entity Address, Postal Zip Code |
H7T 2R3
|
|
| City Area Code |
450
|
|
| Local Phone Number |
686-4555
|
|
| Title of 12(b) Security |
Common Shares, no par value per share
|
|
| Trading Symbol |
ACST
|
|
| Security Exchange Name |
NASDAQ
|
|
| Entity Current Reporting Status |
Yes
|
|
| Entity Interactive Data Current |
Yes
|
|
| Entity Filer Category |
Non-accelerated Filer
|
|
| Entity Small Business |
true
|
|
| Entity Emerging Growth Company |
false
|
|
| Entity Shell Company |
false
|
|
| Entity Common Stock, Shares Outstanding |
|
7,448,033
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEnd date of current fiscal year in the format --MM-DD.
| Name: |
dei_CurrentFiscalYearEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gMonthDayItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.
| Name: |
dei_DocumentFiscalPeriodFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fiscalPeriodItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThis is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.
| Name: |
dei_DocumentFiscalYearFocus |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:gYearItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as an quarterly report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 10-Q
-Number 240
-Section 308
-Subsection a
| Name: |
dei_DocumentQuarterlyReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true only for a form used as a transition report. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Forms 10-K, 10-Q, 20-F
-Number 240
-Section 13
-Subsection a-1
| Name: |
dei_DocumentTransitionReport |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.
| Name: |
dei_EntityCommonStockSharesOutstanding |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionIndicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.
| Name: |
dei_EntityCurrentReportingStatus |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Regulation S-T
-Number 232
-Section 405
| Name: |
dei_EntityInteractiveDataCurrent |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:yesNoItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityShellCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Balance Sheet (Unaudited) - USD ($)
$ in Thousands |
Jun. 30, 2023 |
Mar. 31, 2023 |
| Current assets: |
|
|
| Cash and cash equivalents |
$ 21,633
|
$ 27,875
|
| Short-term investments |
15
|
15
|
| Receivables |
837
|
802
|
| Prepaid expenses |
1,127
|
598
|
| Total current assets |
23,612
|
29,290
|
| Operating lease right of use asset |
71
|
463
|
| Equipment |
84
|
104
|
| Intangible assets |
41,128
|
41,128
|
| Goodwill |
8,138
|
8,138
|
| Total assets |
73,033
|
79,123
|
| Current liabilities: |
|
|
| Total trade and other payables |
1,886
|
3,336
|
| Operating lease liability |
80
|
75
|
| Total current liabilities |
1,966
|
3,411
|
| Operating lease liability |
0
|
410
|
| Deferred tax liability |
7,057
|
7,347
|
| Total liabilities |
9,023
|
11,168
|
| Shareholders' equity: |
|
|
| Common shares, no par value per share; unlimited shares authorized as of June 30, 2023 and March 31, 2023; 7,435,533 shares issued and outstanding as of June 30, 2023 and March 31, 2023 |
258,294
|
258,294
|
| Additional paid-in capital |
14,043
|
13,965
|
| Accumulated other comprehensive loss |
(6,038)
|
(6,038)
|
| Accumulated deficit |
(202,289)
|
(198,266)
|
| Total shareholder’s equity |
64,010
|
67,955
|
| Commitments and contingencies |
|
|
| Total liabilities and shareholders’ equity |
$ 73,033
|
$ 79,123
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14A
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14A
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 220
-SubTopic 10
-Section 45
-Paragraph 14
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-14
| Name: |
us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 26: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.17)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.25)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount after accumulated impairment loss of an asset representing future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482548/350-20-55-24
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(15))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482598/350-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 350
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482573/350-20-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(10)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_Goodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 50
-Paragraph 2
-Subparagraph ((a)(1),(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482665/350-30-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 350
-SubTopic 30
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482686/350-30-45-1
| Name: |
us-gaap_IntangibleAssetsNetExcludingGoodwill |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionSum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 22: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19-26)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481203/810-10-50-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-5
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481404/852-10-50-7
Reference 21: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.21)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accumulated depreciation of tangible personal property used to produce goods and services, including, but is not limited to, tools, dies and molds, computer and office equipment. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 360
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_MachineryAndEquipmentGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset related to consideration paid in advance for costs that provide economic benefits within a future period of one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482955/340-10-05-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 340
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483032/340-10-45-1
| Name: |
us-gaap_PrepaidExpenseCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe total amount due to the entity within one year of the balance sheet date (or one operating cycle, if longer) from outside sources, including trade accounts receivable, notes and loans receivable, as well as any other types of receivables, net of allowances established for the purpose of reducing such receivables to an amount that approximates their net realizable value. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
| Name: |
us-gaap_ReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480990/946-20-50-11
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of investments including trading securities, available-for-sale securities, held-to-maturity securities, and short-term investments classified as other and current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(8))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_ShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Balance Sheet (Unaudited) (Parenthetical) - $ / shares
|
Jun. 30, 2023 |
Mar. 31, 2023 |
| Statement of Financial Position [Abstract] |
|
|
| Common shares, par value |
$ 0
|
$ 0
|
| Common shares, issued |
7,435,533
|
7,435,533
|
| Common shares, outstanding |
7,435,533
|
7,435,533
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StatementOfFinancialPositionAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Statements of Loss and Comprehensive Loss (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Operating expenses |
|
|
| Research and development expenses, net of government assistance |
$ (1,095)
|
$ (2,590)
|
| General and administrative expenses |
(1,763)
|
(1,919)
|
| Sales and marketing |
(111)
|
(221)
|
| Restructuring cost |
(1,485)
|
0
|
| Loss from operating activities |
(4,454)
|
(4,730)
|
| Foreign exchange gain (loss) |
8
|
(78)
|
| Change in fair value of warrant liabilities |
0
|
10
|
| Interest income and other expense |
134
|
32
|
| Total other income (loss), net |
142
|
(36)
|
| Loss before income tax recovery |
(4,312)
|
(4,766)
|
| Income tax recovery |
289
|
242
|
| Net loss and total comprehensive loss |
$ (4,023)
|
$ (4,524)
|
| Loss per share, Basic |
$ (0.54)
|
$ (0.61)
|
| Loss per share, Diluted |
$ (0.54)
|
$ (0.61)
|
| Weighted Average Number of Shares Outstanding, Basic |
7,435,533
|
7,388,065
|
| Weighted Average Number of Shares Outstanding, Diluted |
7,435,533
|
7,388,065
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-15
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-7
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482635/260-10-55-52
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of realized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossRealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount before accretion (amortization) of purchase discount (premium) of interest income on nonoperating securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_InvestmentIncomeInterest |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.7)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate costs incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, including the costs of developing computer software up to the point in time of achieving technological feasibility, and costs allocated in accounting for a business combination to in-process projects deemed to have no alternative future use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482916/730-10-50-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482517/912-730-25-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 985
-SubTopic 20
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481283/985-20-50-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total amount of expenses directly related to the marketing or selling of products or services.
| Name: |
us-gaap_SellingAndMarketingExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Condensed Consolidated Interim Statements of Changes in Shareholder's Equity (Unaudited) - USD ($)
$ in Thousands |
Total |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
AOCI Attributable to Parent [Member] |
Retained Earnings [Member] |
| Beginning Balance (in shares) at Mar. 31, 2022 |
|
7,381,425
|
|
|
|
| Beginning Balance at Mar. 31, 2022 |
$ 108,270
|
$ 257,990
|
$ 12,154
|
$ (6,037)
|
$ (155,837)
|
| Net loss and total comprehensive loss for the period |
(4,524)
|
|
|
|
(4,524)
|
| Cumulative translation adjustment |
(2)
|
|
|
(2)
|
|
| Net proceeds from shares issued under the at-the-market (ATM) program |
195
|
$ 195
|
|
|
|
| Net proceeds from shares issued under the at-the-market (ATM) program (in shares) |
|
34,335
|
|
|
|
| Stock based compensation |
464
|
|
464
|
|
|
| Ending Balance (in shares) at Jun. 30, 2022 |
|
7,415,760
|
|
|
|
| Ending Balance at Jun. 30, 2022 |
104,403
|
$ 258,185
|
12,618
|
(6,039)
|
(160,361)
|
| Beginning Balance (in shares) at Mar. 31, 2023 |
|
7,435,533
|
|
|
|
| Beginning Balance at Mar. 31, 2023 |
67,955
|
$ 258,294
|
13,965
|
(6,038)
|
(198,266)
|
| Net loss and total comprehensive loss for the period |
(4,023)
|
|
|
|
(4,023)
|
| Stock based compensation |
78
|
|
78
|
|
|
| Ending Balance (in shares) at Jun. 30, 2023 |
|
7,435,533
|
|
|
|
| Ending Balance at Jun. 30, 2023 |
$ 64,010
|
$ 258,294
|
$ 14,043
|
$ (6,038)
|
$ (202,289)
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount after tax and reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 10A
-Subparagraph (a)
-SubTopic 10
-Topic 220
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-10A
| Name: |
us-gaap_OtherComprehensiveIncomeLossForeignCurrencyTransactionAndTranslationAdjustmentNetOfTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionEquity impact of the value of new stock issued during the period. Includes shares issued in an initial public offering or a secondary public offering. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-11
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 205
-Name Accounting Standards Codification
-Section 45
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480767/946-205-45-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 9: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 11: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 12: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 13: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Condensed Consolidated Interim Statements of Cash Flows (Unaudited) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Cash flows used in operating activities: |
|
|
| Net loss for the period |
$ (4,023)
|
$ (4,524)
|
| Adjustments: |
|
|
| Depreciation of equipment |
7
|
167
|
| Stock-based compensation |
78
|
464
|
| Change in fair value of warrant liabilities |
0
|
(10)
|
| Income tax recovery |
(289)
|
(242)
|
| Unrealized foreign exchange (gain) loss |
0
|
(10)
|
| Write off of equipment |
13
|
0
|
| Changes in operating assets and liabilities |
2,026
|
1,271
|
| Net cash used in operating activities |
(6,240)
|
(5,426)
|
| Cash flows from investing activities: |
|
|
| Acquisition of equipment |
0
|
(7)
|
| Acquisition of short-term investments |
|
(16)
|
| Maturity of short-term investment |
0
|
13,281
|
| Net cash from investing activities |
0
|
13,258
|
| Cash flows from financing activities: |
|
|
| Net proceeds from issuance under the at-the-market (ATM) program |
0
|
195
|
| Net cash from financing activities |
0
|
195
|
| Effect of exchange rate fluctuations on cash and cash equivalents |
(2)
|
11
|
| Net (decrease) increase in cash and cash equivalents |
(6,242)
|
8,038
|
| Cash and cash equivalents, beginning of period |
27,875
|
30,339
|
| Cash and cash equivalents, end of period |
21,633
|
38,377
|
| Cash and cash equivalents are comprised of: |
|
|
| Cash |
5,413
|
38,377
|
| Cash equivalents |
$ 16,220
|
$ 0
|
| X |
- DefinitionAmount of increase (decrease) from the effect of exchange rate changes on cash and cash equivalent balances held in foreign currencies.
| Name: |
acst_EffectOfTranslationEffectOnCashAndCashEquivalents |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-12
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 45
-Paragraph 21
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-21
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 20
-SubTopic 210
-Topic 946
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480555/946-210-45-20
| Name: |
us-gaap_Cash |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashAndCashEquivalentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481877/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479440/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 360
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482099/360-10-50-1
| Name: |
us-gaap_Depreciation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, before tax, of unrealized gain (loss) from foreign currency transaction. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 6
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-6
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(7)(c))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481956/830-20-45-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 830
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481926/830-20-50-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ForeignCurrencyTransactionGainLossUnrealized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in operating assets after deduction of operating liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInOtherOperatingCapitalNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481687/323-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482907/825-10-50-28
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482765/220-10-50-6
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-8
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-9
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-11
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-4
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480167/946-830-55-10
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483581/946-220-45-7
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483586/944-220-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-22
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-30
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482689/260-10-45-60B
Reference 32: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-31
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482810/280-10-50-32
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483499/205-20-50-7
Reference 35: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1A
Reference 37: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482790/220-10-45-1B
Reference 38: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
Reference 39: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483589/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net cash outflow or inflow from purchases, sales and disposals of property, plant and equipment and other productive assets, including intangibles.
| Name: |
us-gaap_PaymentsForProceedsFromProductiveAssets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash outflow for securities or other assets acquired, which qualify for treatment as an investing activity and are to be liquidated, if necessary, within the current operating cycle. Includes cash flows from securities classified as trading securities that were acquired for reasons other than sale in the short-term. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
| Name: |
us-gaap_PaymentsToAcquireShortTermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the additional capital contribution to the entity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfCommonStock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from maturities, prepayments, calls and collections of all investments, including securities and other assets, having ready marketability and intended by management to be liquidated, if necessary, within the current operating cycle. Includes cash flows from securities classified as trading securities that were acquired for reasons other than sale in the short-term. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-13
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-12
| Name: |
us-gaap_ProceedsFromMaturitiesPrepaymentsAndCallsOfShorttermInvestments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Note 1 - Nature of Operation
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Nature Of Operations |
1. Nature of operation Acasti Pharma Inc. (“Acasti” or the “Corporation”) is incorporated under the Business Corporations Act (Québec) (formerly Part 1A of the Companies Act (Québec)). The Corporation is domiciled in Canada and its registered office is located at 2572 boul. Daniel-Johnson, 2nd Floor Laval, Québec, Canada H7T 2R3. The Corporation’s shares are listed on the Nasdaq Capital Market (the "Nasdaq"), and through March 27, 2023 the Corporation's shares were also listed on the TSX Venture Exchange ("TSXV"), in each case, under the symbol "ACST". On March 13, 2023 the Corporation received approval to voluntarily delist from the TSXV. Effective as at the close of trading on March 27, 2023, the Corporation's common shares are no longer listed and posted for trading on the TSXV. In August 2021, the Corporation completed the acquisition via a share-for-share merger of Grace Therapeutics, Inc. (“Grace”), a privately held emerging biopharmaceutical company focused on developing innovative drug delivery technologies for the treatment of rare and orphan diseases. The post-merger Corporation is focused on building a late-stage specialty pharmaceutical company specializing in rare and orphan diseases and developing and commercializing products that improve clinical outcomes using its novel drug delivery technologies. The Corporation seeks to apply new proprietary formulations to existing pharmaceutical compounds to achieve enhanced efficacy, faster onset of action, reduced side effects, more convenient delivery and increased patient compliance; all of which could result in improved patient outcomes. The active pharmaceutical ingredients chosen by the Corporation for further development may be already approved in the target indication or could be repurposed for use in new indications. The Corporation has incurred operating losses and negative cash flows from operations in each year since its inception. The Corporation expects to incur significant expenses and continued operating losses for the foreseeable future. In May 2023, the Corporation implemented a strategic realignment plan to enhance shareholder value that resulted in the Corporation engaging a new management team, streamlining its research and development activities to concentrate on its lead product, GTX 104, and greatly reducing its workforce. Moving forward, the Corporation plans to build a smaller, more focused organization in the United States. Further development of GTX-102 and GTX-101 will occur at such time as additional funding is obtained or strategic partnerships are entered into. This strategic realignment is expected to significantly reduce administrative and research and development expenses and enable the Corporation to extend its available cash resources to the second calendar quarter of 2025. The Corporation will require additional capital to fund our daily operating needs beyond that time. The Corporation does not expect to generate revenue from product sales unless and until it successfully completes drug development and obtains regulatory approval, which the Corporation expects will take several years and is subject to significant uncertainty. To date, the Corporation has financed its operations primarily through public offerings and private placements of its common shares, warrants and convertible debt and the proceeds from research tax credits. Until such time that the Corporation can generate significant revenue from drug product sales, if ever, it will require additional financing, which is expected to be sourced from a combination of public or private equity or debt financing or other non-dilutive sources, which may include fees, milestone payments and royalties from collaborations with third parties. Arrangements with collaborators or others may require the Corporation to relinquish certain rights related to its technologies or drug product candidates. Adequate additional financing may not be available to the Corporation on acceptable terms, or at all. The Corporation’s inability to raise capital as and when needed could have a negative impact on its financial condition and its ability to pursue its business strategy. The Corporation plans to raise additional capital prior to that time in order to maintain adequate liquidity. Negative results from studies, if any, and depressed prices of the Corporation’s stock could impact the Corporation’s ability to raise additional financing. Raising additional equity capital is subject to market conditions not within the Corporation’s control. If the Corporation does not raise additional funds in this time period, the Corporation may not be able to realize our assets and discharge our liabilities in the normal course of business. The Corporation remains subject to risks similar to other development stage companies in the biopharmaceutical industry, including compliance with government regulations, protection of proprietary technology, dependence on third-party contractors and consultants and potential product liability, among others. Please refer to the risk factors included in Part 1, Item 1A of the Corporation’s annual report on Form 10-K for the year ended March 31, 2023, filed with the SEC on June 23, 2023 (the “Annual Report”). Reverse stock split On June 29, 2023, the Board of Directors of the Corporation approved an amendment to the Corporation's Articles of Incorporation to implement a reverse stock split of the Corporation's Class A common shares, no par value per share, at a ratio of 1-for-6 (the “Reverse Stock Split”). On July 4, 2023, the Corporation filed Articles of Amendment to its Articles of Incorporation with the Registraire des entreprises du Québec, to implement the Reverse Stock Split. All references in these financial statements to number of common shares, warrants and options, price per share and weighted average number of shares outstanding have been adjusted to reflect the Reverse Stock Split, which became effective on July 10, 2023.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//275/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
| Name: |
us-gaap_NatureOfOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 2 - Summary of Significant Accounting Policies
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Summary of significant accounting policies |
2. Summary of significant accounting policies: Basis of presentation The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X under the Securities Exchange Act of 1934. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements as of and for the year ended March 31, 2023, and, in the opinion of management, reflect all adjustments, consisting of normal recurring adjustments, necessary for the fair presentation of the Corporation’s consolidated financial position as of June 30, 2023, the consolidated results of its operations for the three months ended June 30, 2023 and 2022, its statements of shareholders’ equity for the three months ended June 30, 2023 and 2022 and its consolidated cash flows for the three months ended June 30, 2023 and 2022. These unaudited condensed consolidated financial statements should be read in conjunction with the Corporation’s audited consolidated financial statements and the accompanying notes for the year ended March 31, 2023 included in the Corporation’s Annual Report. The condensed consolidated balance sheet data as of March 31, 2023 presented for comparative purposes was derived from the Corporation’s audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. The results for the three months ended June 30, 2023 are not necessarily indicative of the operating results to be expected for the full year or for any other subsequent interim period. The Corporation’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report. There have been no changes to the Corporation's significant accounting policies since the date of the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report. Use of estimates The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates. Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. Estimates and assumptions include the measurement of stock-based compensation, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date, and determining which research and development expenses qualify for research and development tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized. |
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 3 - Recent Accounting Pronouncements
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Recent accounting pronouncements |
3. Recent accounting pronouncements The Corporation has considered recent accounting pronouncements and concluded that they are either not applicable to the business or that the effect is not expected to be material to the consolidated financial statements as a result of future adoption.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for change in accounting principle. Includes, but is not limited to, nature, reason, and method of adopting amendment to accounting standards or other change in accounting principle. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480016/944-40-65-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 5
-Subparagraph (SAB Topic 11.M.Q2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480530/250-10-S99-5
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (i)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480528/815-20-65-6
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 5
-Subparagraph (f)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479832/842-10-65-5
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 848
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483550/848-10-65-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 105
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479343/105-10-65-6
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 8
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482615/740-10-65-8
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 6
-Subparagraph (e)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482833/825-10-65-6
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 10
-Name Accounting Standards Codification
-Section 65
-Paragraph 4
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479654/326-10-65-4
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 926
-SubTopic 20
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (f)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483194/926-20-65-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480530/250-10-S99-6
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483443/250-10-50-1
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Topic 250
-Publisher FASB
-URI https://asc.fasb.org//250/tableOfContent
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480175/815-40-65-1
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(1)
-SubTopic 20
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
Reference 34: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (d)(2)
-SubTopic 20
-Topic 310
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481925/310-20-65-2
| Name: |
us-gaap_NewAccountingPronouncementsAndChangesInAccountingPrinciplesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 4 - Receivables
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Receivables |
4. Receivables
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
March 31,
2023 |
|
|
Notes |
|
$ |
|
$ |
Sales tax receivables |
|
|
|
426 |
|
338 |
Government assistance |
|
6 |
|
361 |
|
412 |
Interest receivable |
|
|
|
50 |
|
52 |
Total receivables |
|
|
|
837 |
|
802 |
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for claims held for amounts due a entity, excluding financing receivables. Examples include, but are not limited to, trade accounts receivables, notes receivables, loans receivables. Includes disclosure for allowance for credit losses. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//310-10/tableOfContent
| Name: |
us-gaap_LoansNotesTradeAndOtherReceivablesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Short-term Investments
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Short-term Investments |
5. Short-term investments The Corporation holds various marketable securities, with maturities greater than 3 months at the time of purchase, as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31,
2023 |
|
|
|
$ |
|
|
$ |
|
Term deposits issued in CAD currency earning interest at 3% and maturing on March 29, 2024 |
|
|
15 |
|
|
|
15 |
|
Total short-term investments |
|
|
15 |
|
|
|
15 |
|
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for investments in certain debt and equity securities. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//320/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 10
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-6B
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6B
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482106/820-10-50-6B
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Regulation S-K (SK)
-Number 229
-Section 1403
-Paragraph (b)
-Publisher SEC
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//946-320/tableOfContent
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 940
-SubTopic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//940-320/tableOfContent
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 320
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//942-320/tableOfContent
| Name: |
us-gaap_InvestmentsInDebtAndMarketableEquitySecuritiesAndCertainTradingAssetsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 6 - Government Assistance
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Government assistance |
6. Government assistance
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31, 2023 |
|
|
|
$ |
|
|
$ |
|
Investment tax credit |
|
|
361 |
|
|
|
412 |
|
Government assistance is comprised of research and development investment tax credits from the Québec provincial government, which relate to qualifiable research and development expenditures under the applicable tax laws. The amounts recorded as receivables are subject to a government tax audit and the final amounts received may differ from those recorded. For the three months ended June 30, 2023 and 2022, the Corporation recorded $(51) and $41, respectively, as an increase and a reduction of research and development expenses in the Statement of Loss and Comprehensive Loss. Unrecognized Canadian federal tax credits may be used to reduce future Canadian federal income tax and expire as follows:
|
|
|
|
|
|
|
$ |
|
2029 |
|
9 |
|
2030 |
|
|
23 |
|
2031 |
|
|
36 |
|
2032 |
|
|
345 |
|
2033 |
|
|
353 |
|
2034 |
|
|
348 |
|
2035 |
|
|
415 |
|
2036 |
|
|
229 |
|
2037 |
|
|
252 |
|
2038 |
|
|
259 |
|
2039 |
|
|
355 |
|
2040 |
|
|
226 |
|
2041 |
|
|
146 |
|
2042 |
|
|
312 |
|
2043 |
|
|
642 |
|
|
|
|
3,950 |
|
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for government assistance. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483507/832-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483507/832-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483507/832-10-50-4
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483507/832-10-50-4
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 832
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483507/832-10-50-4
| Name: |
us-gaap_GovernmentAssistanceTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 7 - Trade and other payables
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Trade and Other Payables |
7. Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31, 2023 |
|
|
|
$ |
|
|
$ |
|
Trade payables |
|
|
607 |
|
|
|
1,242 |
|
Accrued liabilities and other payables |
|
|
994 |
|
|
|
946 |
|
Employee salaries and benefits payable |
|
|
285 |
|
|
|
1,148 |
|
Total trade and other payables |
|
|
1,886 |
|
|
|
3,336 |
|
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for accounts payable and accrued liabilities at the end of the reporting period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a),20,24)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 8 - Leases
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Leases [Abstract] |
|
| Leases |
8. Leases The Corporation has historically entered into lease arrangements for its research and development and quality control laboratory facility located in Sherbrooke, Québec. As of June 30, 2023, the Corporation had one operating lease with required future minimum payments. On March 14, 2022, the Corporation renewed the lease agreement effective April 1, 2022, resulting in a commitment of $556 over a 24 months base lease term and 48 months additional lease renewal term. In April 2023, the Corporation elected not to renew the additional 48 months lease renewal term with the lease expected to terminate March 31, 2024. The Corporation accounted for the change in lease term as a lease modification under ASC 842. Due to the modification in lease term, the Corporation remeasured the lease liability and right-of-use asset associated with the lease. As of the effective date of modification, the Corporation recorded an adjustment to the right-of-use asset and lease liability in the amount of $369 based on the net present value of lease payments discounted using an estimate incremental borrowing rate of 4.3%. The following table contains a summary of the lease costs recognized under ASC 842 and other information pertaining to the Corporation’s operating lease for the three month period ended June 30, 2023:
|
|
|
|
|
Operating cash flows for operating lease |
|
$ |
24 |
|
Weighted-average remaining lease term (in years) |
|
|
0.75 |
|
Weighted-average discount rate |
|
|
4.3 |
% |
As the Corporation's lease do not provide an implicit rate, the Corporation utilized its incremental borrowing rate to discount lease payments, which reflects the fixed rate at which the Corporation could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment. Future minimum lease payments under the Corporation’s operating lease as of June 30, 2023 were as follows:
|
|
|
|
|
|
|
June 30, 2023 |
|
|
|
$ |
|
2024 |
|
|
81 |
|
2025 and thereafter |
|
|
- |
|
Total lease payments |
|
|
81 |
|
Less: interest |
|
|
(1 |
) |
Total lease liability |
|
|
80 |
|
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 9 - Capital and Other Components of Equity
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Capital and other components of equity |
9. Capital and other components of equity a. Common Shares Authorized capital stock Unlimited number of shares •Class A shares (Common Shares), voting (one vote per share), participating and without par value. As of June 30, 2023, there were 7,435,533 Class A shares issued and outstanding. •Class B shares, voting (ten votes per share), non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class B shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class B shares are redeemable at the holder’s discretion for CAD $4.80 per share, subject to certain conditions. As of June 30, 2023, there were no Class B shares issued and outstanding. •Class C shares, non-voting, non-participating, without par value and maximum annual non-cumulative dividend of 5% on the amount paid per share. Class C shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class C shares are redeemable at the holder’s discretion for CAD $1.20 per share, subject to certain conditions. As of June 30, 2023, there were no Class C shares issued and outstanding. •Class D and E shares, they are non-voting, non-participating, without par value and maximum monthly non-cumulative dividend between 0.5% and 2% on the amount paid per share. Class D and E shares are convertible, at the holder’s discretion, into Class A shares (Common Shares), on a one-for-one basis, and Class D and E shares are redeemable at the holder’s discretion, subject to certain conditions. As of June 30, 2023, there were no Class D or E shares issued and outstanding. At-the-Market (“ATM”) Program On June 29, 2020, the Corporation entered into an amended and restated sales agreement (the “Sales Agreement”) with B. Riley FBR, Inc. ("B.Riley"), Oppenheimer & Co. Inc. and H.C. Wainwright & Co., LLC (collectively, the “Agents”) to amend the Corporation’s existing ATM program. Under the terms of the Sales Agreement, which had a three-year term, the Corporation could issue and sell from time-to-time common shares having aggregate gross proceeds of up to $75,000,000 through the Agents. Subject to the terms and conditions of the Sales Agreement, the Agents would use their commercially reasonable efforts to sell the common shares from time to time, based upon the Corporation’s instructions. The Corporation had no obligation to sell any of the common shares and could, at any time, suspend sales under the Sales Agreement. The Corporation and the Agents could terminate the Sales Agreement in accordance with its terms. Under the terms of the Sales Agreement, the Corporation provided the Agents with customary indemnification rights and the Agents were entitled to compensation at a commission rate equal to 3.0% of the gross proceeds from each sale of the common shares. The Sales Agreement expired pursuant to its terms on June 29, 2023 and the Corporation plans to revisit the renewal of a facility in the coming months. On November 10, 2021, the Corporation filed a prospectus supplement relating to its at-the-market program, expiring July 7, 2023, with B. Riley, Oppenheimer& Co. Inc. and H.C. Wainwright & Co., LLC acting as agents. Under the terms of the ATM Sales Agreement and the prospectus supplement, the Corporation may issue and sell from time-to-time common shares having an aggregate offering price of up to $75,000,000 through the agents; however, our use of the shelf registration statement on Form S-3 will be limited for so long as we are subject to General Instruction I.B.6 of Form S-3, which limits the amounts that we may sell under the registration statement and in accordance with the ATM agreement. The common shares will be distributed at market prices prevailing at the time of the sale and, as a result, prices may vary between purchasers and during the period of distribution. The volume and timing of sales under the ATM program, if any, will be determined at the sole discretion of the Corporation’s board of directors and management. During the three months ended June 30, 2023, no common shares were sold under the ATM program. During the three months ended June 30, 2022, 34,335 common shares were sold for total net proceeds of approximately $195 with commissions, legal expenses and costs related to the share sale amounting to $6. The common shares were sold at the prevailing market prices, which resulted in an average price of approximately $5.82 per share. b. Warrants During the three month period ended June 30, 2023, the remaining 137,370 warrants to acquire one common share at an exercise price of CAD $62.88 expired on May 9, 2023.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for equity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481062/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-6
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480237/815-40-50-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(e)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//505/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-13
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 14
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-14
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 16
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-16
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-18
| Name: |
us-gaap_StockholdersEquityNoteDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Stock Based Compensation
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Stock-based compensation |
10. Stock-based compensation At June 30, 2023, the Corporation had in place a stock option plan for directors, officers, employees, and consultants of the Corporation (“Stock Option Plan”). The Stock Option Plan continues to provide for the granting of options to purchase common shares. Under the terms of the Stock Option Plan, the exercise price of the stock options granted under the Stock Option Plan may not be lower than the closing price of the Corporation’s common shares on the Nasdaq Capital Market at the close of such market the day preceding the grant. The maximum number of common shares that may be issued upon exercise of options granted under the amended Stock Option Plan shall not exceed 20% of the aggregate number of issued and outstanding shares of the Corporation as of July 28, 2022. The terms and conditions for acquiring and exercising options are set by the Corporation’s Board of Directors, subject to, among others, the following limitations: the term of the options cannot exceed ten years and (i) all options granted to a director will be vested evenly on a monthly basis over a period of at least twelve (12) months, and (ii) all options granted to an employee will be vested evenly on a quarterly basis over a period of at least thirty-six (36) months. The total number of options issued to any one consultant within any twelve-month period cannot exceed 2% of the Corporation’s total issued and outstanding common shares (on a non-diluted basis). The Corporation is not authorized to grant within any twelve-month period such number of options under the Stock Option Plan that could result in a number of common shares issuable pursuant to options granted to (a) related persons exceeding 2% of the Corporation’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted, or (b) any one eligible person in a twelve-month period exceeding 2% of the Corporation’s issued and outstanding common shares (on a non-diluted basis) on the date an option is granted. The following table summarizes information about activities within the Stock Option Plan for the three month period ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
options |
|
|
Weighted average
exercise price |
|
|
Weighted average
grant date
fair value |
|
Outstanding, March 31, 2023 |
|
|
740,957 |
|
|
|
13.60 |
|
|
|
11.23 |
|
Forfeited/Cancelled |
|
|
(267,797 |
) |
|
|
7.72 |
|
|
|
6.21 |
|
Outstanding, June 30, 2023 |
|
|
473,178 |
|
|
|
16.93 |
|
|
|
14.07 |
|
Exercisable, June 30, 2023 |
|
|
402,247 |
|
|
|
18.62 |
|
|
|
15.51 |
|
Forfeited and cancelled options were as a result of the Corporations restructuring that occurred during the three months ended June 30, 2023. No options were granted or exercised during the three month period ended June 30, 2023. Compensation expense recognized under the stock option plan is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
|
|
$ |
|
|
$ |
|
Research and development expenses |
|
|
2 |
|
|
|
158 |
|
General and administrative expenses |
|
|
60 |
|
|
|
282 |
|
Sales and marketing expenses |
|
|
16 |
|
|
|
24 |
|
|
|
|
78 |
|
|
|
464 |
|
As of June 30, 2023, there was $130 of total unrecognized compensation cost, related to non-vested stock options, which is expected to be recognized over a remaining weighted average vesting period of 0.97 years. Corporation equity incentive plan The Corporation established an equity incentive plan (the “Equity Incentive Plan”) for employees, directors, and consultants. The Equity Incentive Plan provides for the issuance of restricted share units (RSUs), performance share units, restricted shares, deferred share units and other stock-based awards, subject to restricted conditions as may be determined by the Board of Directors. There were no such awards outstanding as of June 30, 2023, and June 30, 2022, and no stock-based compensation was recognized for the period ended June 30, 2023 and June 30, 2022 under the Equity Incentive Plan.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 11 - Loss per share
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Loss per share |
11. Loss per share Diluted loss per share was the same amount as basic loss per share, as the effect of options, and warrants would have been anti-dilutive, as the Corporation has incurred losses in each of the periods presented. All currently outstanding options could potentially be dilutive in the future. The Corporation excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
|
|
|
|
June 30, 2023 |
|
June 30, 2022 |
Options outstanding |
|
473,178 |
|
704,585 |
May 2018 public offering warrants |
|
— |
|
137,370 |
December 2017 public offering warrants |
|
— |
|
147,354 |
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Supplemental Cash Flow Disclosure
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Supplemental cash flow disclosure |
12. Supplemental cash flow disclosure Changes in operating assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
|
$ |
|
|
$ |
|
Receivables |
|
|
(35 |
) |
|
|
(434 |
) |
Prepaid expenses |
|
|
(529 |
) |
|
|
(839 |
) |
Trade and other payables |
|
|
(1,449 |
) |
|
|
2 |
|
Write-off of operating lease right of use asset |
|
|
(13 |
) |
|
|
— |
|
Total changes in operating assets and liabilities |
|
|
(2,026 |
) |
|
|
(1,271 |
) |
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for supplemental cash flow activities, including cash, noncash, and part noncash transactions, for the period. Noncash is defined as information about all investing and financing activities of an enterprise during a period that affect recognized assets or liabilities but that do not result in cash receipts or cash payments in the period. "Part noncash" refers to that portion of the transaction not resulting in cash receipts or cash payments in the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//230/tableOfContent
| Name: |
us-gaap_CashFlowSupplementalDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 13 - Financial instruments
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Financial instruments |
13. Financial instruments a. Concentration of credit risk Financial instruments that potentially subject the Corporation to a concentration of credit risk consist primarily of cash and cash equivalents and investments. Cash and cash equivalents and investments are all invested in accordance with the Corporation’s Investment Policy with the primary objective being the preservation of capital and the maintenance of liquidity, which risk is managed by dealing only with highly rated Canadian institutions. The carrying amount of financial assets, as disclosed in the consolidated balance sheets, represents the Corporation’s credit exposure at the reporting date. b. Foreign currency risk The Corporation is exposed to financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates. Foreign currency risk is limited to the portion of the Corporation's business transactions denominated in currencies other than the Corporation's functional currency of the U.S. dollar. Fluctuations related to foreign exchange rates could cause unforeseen fluctuations in the Corporation's operating results. The Corporation does not use derivative instruments to hedge exposure to foreign exchange risk. The fluctuation of the Canadian dollar in relation to the U.S. dollar and other foreign currencies will consequently have an impact upon the Corporation’s net loss. c. Liquidity risk Liquidity risk is the risk that the Corporation will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. The Corporation manages liquidity risk through the management of its capital structure and financial leverage. It also manages liquidity risk by continuously monitoring actual and projected cash flows. The Board of Directors reviews and approves the Corporation's operating budgets, and reviews material transactions outside the normal course of business. The Corporation currently does not have long-term debt nor arranged committed sources of financing and is operating via use of existing cash and short-term investment balances. Refer to Note 1 – Nature of Operations. The Corporation’s financial liabilities obligations include trade and other payables, which fall due within the next 12 months.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for financial instruments. This disclosure includes, but is not limited to, fair value measurements of short and long term marketable securities, international currencies forward contracts, and auction rate securities. Financial instruments may include hedging and non-hedging currency exchange instruments, derivatives, securitizations and securities available for sale at fair value. Also included are investment results, realized and unrealized gains and losses as well as impairments and risk management disclosures.
| Name: |
us-gaap_FinancialInstrumentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 14 - Commitments and Contingencies
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Commitments and Contingencies Disclosure [Text Block] |
14. Commitments and contingencies Research and development contracts and contract research organizations agreements The Corporation utilize contract manufacturing organizations ("CMOs") for the development and production of clinical materials and contract research organizations (“CROs”) to perform services related to its clinical trials. Pursuant to the agreements with these CMOs and CROs, the Corporation has either the right to terminate the agreements without penalties or under certain penalty conditions. Raw krill oil supply contract On October 25, 2019, the Corporation signed a supply agreement with Aker Biomarine Antarctic. (“Aker”) to purchase raw krill oil product for a committed volume of commercial starting material for CaPre, one of the Corporation’s former drug candidates, for a total fixed value of $3.1 million. As at June 30, 2023, the remaining balance of the commitment with Aker amounts to $2.8 million. During the second calendar quarter of 2022, Aker informed the Corporation that Aker believed it had satisfied the terms of the supply agreement as to their obligation to deliver the remaining balance of raw krill oil product, and that the Corporation was therefore required to accept the remaining product commitment and to pay Aker the $2.8 million balance. The Corporation disagrees with Aker’s position and believes that Aker is not entitled to further payment under the supply agreement. Accordingly, no liability has been recorded. The dispute was unresolved as of June 30, 2023, and remains unresolved. There is uncertainty as to whether the Corporation will be required to make further payment to Aker in connection with the dispute. Additionally, in the event the Corporation is required to accept delivery from Aker of the remaining balance of raw krill oil product under the supply agreement, there is uncertainty as to whether the Corporation can recover value from the product, which may result in the Corporation incurring a loss on the supply agreement in the near term. Legal proceedings and disputes In the ordinary course of business, the Corporation is at times subject to various legal proceedings and disputes. The Corporation assesses its liabilities and contingencies in connection with outstanding legal proceedings utilizing the latest information available. Where it is probable that the Corporation will incur a loss and the amount of the loss can be reasonably estimated, the Corporation records a liability in its consolidated financial statements. These legal contingencies may be adjusted to reflect any relevant developments. Where a loss is not probable or the amount of loss is not estimable, the Corporation does not accrue legal contingencies. While the outcome of legal proceedings is inherently uncertain, based on information currently available, management believes that it has established appropriate legal reserves. Any incremental liabilities arising from pending legal proceedings are not expected to have a material adverse effect on the Corporation’s financial position, results of operations, or cash flows. However, it is possible that the ultimate resolution of these matters, if unfavorable, may be material to the Corporation’s financial position, results of operations, or cash flows. No reserves or liabilities have been accrued as of June 30, 2023.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//450/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480327/954-440-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482648/440-10-50-4
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 15 - Restructuring
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Restructuring and Related Activities [Abstract] |
|
| Restructuring |
15. Restructuring Costs On May 8, 2023, the Corporation communicated its decision to terminate a substantial amount of its workforce as part of a plan that intended to align the Corporation’s organizational and management cost structure to prioritize resources to GTX-104 and reduce losses to improve cash flow and extend available cash resources. The Corporation incurred $1,485 of costs primarily consisting of employee severance costs. Unpaid Liabilities associated with the restructuring costs are recorded in trade and other payables on the consolidated balance sheets. The Corporation’s restructuring charges and payments are comprised of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employee |
|
|
|
|
|
|
|
|
|
severance |
|
|
Legal |
|
|
Total |
|
Expenses incurred |
|
|
1,447 |
|
|
|
38 |
|
|
|
1,485 |
|
Payments made |
|
|
(1,212 |
) |
|
|
- |
|
|
|
(1,212 |
) |
Balance at June 30, 2023 |
|
|
235 |
|
|
|
38 |
|
|
|
274 |
|
|
| X |
- DefinitionThe entire disclosure for restructuring and related activities. Description of restructuring activities such as exit and disposal activities, include facts and circumstances leading to the plan, the expected plan completion date, the major types of costs associated with the plan activities, total expected costs, the accrual balance at the end of the period, and the periods over which the remaining accrual will be settled. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//420/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(e))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_RestructuringAndRelatedActivitiesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 16 - Subsequent events
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Subsequent events |
16. Subsequent events On July 3, 2023, the Corporation entered into an asset purchase agreement to sell its lab equipment with a net book value of $54 as of June 30, 2023 for $109 in net proceeds. On July 14, 2023, the Corporation's Board of Directors approved the grant of 446,502 stock options at an exercise price of $2.64 under the Corporation's Stock Option Plan. On July 19, 2023, the Corporation entered into a new short term lease for its new headquarters located at 2572 boul. Daniel-Johnson, 2nd Floor Laval, Québec, Canada H7T 2R3. On July 24, 2023, the Corporation terminated it's lease for premises located at 3009 boul. de la Concorde East, Suite 102, Laval, Québec, Canada H7E 2B5.
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org//855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Significant Accounting Policies (Policies)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Accounting Policies [Abstract] |
|
| Basis of presentation |
Basis of presentation The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and with the instructions to Form 10-Q and Article 8 of Regulation S-X under the Securities Exchange Act of 1934. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and as amended by Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited annual consolidated financial statements as of and for the year ended March 31, 2023, and, in the opinion of management, reflect all adjustments, consisting of normal recurring adjustments, necessary for the fair presentation of the Corporation’s consolidated financial position as of June 30, 2023, the consolidated results of its operations for the three months ended June 30, 2023 and 2022, its statements of shareholders’ equity for the three months ended June 30, 2023 and 2022 and its consolidated cash flows for the three months ended June 30, 2023 and 2022. These unaudited condensed consolidated financial statements should be read in conjunction with the Corporation’s audited consolidated financial statements and the accompanying notes for the year ended March 31, 2023 included in the Corporation’s Annual Report. The condensed consolidated balance sheet data as of March 31, 2023 presented for comparative purposes was derived from the Corporation’s audited consolidated financial statements but does not include all disclosures required by U.S. GAAP. The results for the three months ended June 30, 2023 are not necessarily indicative of the operating results to be expected for the full year or for any other subsequent interim period. The Corporation’s significant accounting policies are disclosed in the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report. There have been no changes to the Corporation's significant accounting policies since the date of the audited consolidated financial statements for the year ended March 31, 2023 included in the Annual Report.
|
| Use of estimates |
Use of estimates The preparation of these financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, income, and expenses. Actual results may differ from these estimates. Estimates are based on management’s best knowledge of current events and actions that management may undertake in the future. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. Estimates and assumptions include the measurement of stock-based compensation, accruals for research and development contracts and contract organization agreements, and valuation of intangibles and goodwill. Estimates and assumptions are also involved in measuring the accrual of services rendered with respect to research and development expenditures at each reporting date, and determining which research and development expenses qualify for research and development tax credits and in what amounts. The Corporation recognizes the tax credits once it has reasonable assurance that they will be realized.
|
| X |
- References
+ Details
| Name: |
us-gaap_AccountingPoliciesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS).
| Name: |
us-gaap_BasisOfAccountingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the various types of trade accounts and notes receivable and for each the gross carrying value, allowance, and net carrying value as of the balance sheet date. Presentation is categorized by current, noncurrent and unclassified receivables. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.3,4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ScheduleOfAccountsNotesLoansAndFinancingReceivableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 5 - Short-term Investments (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Short-Term Investments [Abstract] |
|
| Marketable Securities |
The Corporation holds various marketable securities, with maturities greater than 3 months at the time of purchase, as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
March 31,
2023 |
|
|
|
$ |
|
|
$ |
|
Term deposits issued in CAD currency earning interest at 3% and maturing on March 29, 2024 |
|
|
15 |
|
|
|
15 |
|
Total short-term investments |
|
|
15 |
|
|
|
15 |
|
|
| X |
- DefinitionTabular disclosure of marketable securities. This may consist of investments in certain debt and equity securities, short-term investments and other assets.
| Name: |
us-gaap_MarketableSecuritiesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShortTermInvestmentsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe tabular disclosure for government assistance.
| Name: |
acst_ScheduleOfGovernmentAssistanceTableTextBlock |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of tax credit carryforwards available to reduce future taxable income, including amounts, expiration dates, limitations on use and the related deferred tax assets and valuation allowances. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_SummaryOfTaxCreditCarryforwardsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the (a) carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business (accounts payable); (b) other payables; and (c) accrued liabilities. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). An alternative caption includes accrued expenses.
| Name: |
us-gaap_ScheduleOfAccountsPayableAndAccruedLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 8 - Leases (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Leases [Abstract] |
|
| Schedule of Lease Costs Recognized for Operating Leases |
The following table contains a summary of the lease costs recognized under ASC 842 and other information pertaining to the Corporation’s operating lease for the three month period ended June 30, 2023:
|
|
|
|
|
Operating cash flows for operating lease |
|
$ |
24 |
|
Weighted-average remaining lease term (in years) |
|
|
0.75 |
|
Weighted-average discount rate |
|
|
4.3 |
% |
|
| Schedule of Future Minimum Lease Payments |
Future minimum lease payments under the Corporation’s operating lease as of June 30, 2023 were as follows:
|
|
|
|
|
|
|
June 30, 2023 |
|
|
|
$ |
|
2024 |
|
|
81 |
|
2025 and thereafter |
|
|
- |
|
Total lease payments |
|
|
81 |
|
Less: interest |
|
|
(1 |
) |
Total lease liability |
|
|
80 |
|
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 10 - Stock Based Compensation (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Share-Based Payment Arrangement, Noncash Expense [Abstract] |
|
| Schedule of Share-Based Payment Arrangement, Option, Activity |
information about activities within the Stock Option Plan for the three month period ended June 30, 2023:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
options |
|
|
Weighted average
exercise price |
|
|
Weighted average
grant date
fair value |
|
Outstanding, March 31, 2023 |
|
|
740,957 |
|
|
|
13.60 |
|
|
|
11.23 |
|
Forfeited/Cancelled |
|
|
(267,797 |
) |
|
|
7.72 |
|
|
|
6.21 |
|
Outstanding, June 30, 2023 |
|
|
473,178 |
|
|
|
16.93 |
|
|
|
14.07 |
|
Exercisable, June 30, 2023 |
|
|
402,247 |
|
|
|
18.62 |
|
|
|
15.51 |
|
|
| Schedule of Share-Based Payment Arrangement, Expensed and Capitalized, Amount |
Compensation expense recognized under the stock option plan is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
|
|
$ |
|
|
$ |
|
Research and development expenses |
|
|
2 |
|
|
|
158 |
|
General and administrative expenses |
|
|
60 |
|
|
|
282 |
|
Sales and marketing expenses |
|
|
16 |
|
|
|
24 |
|
|
|
|
78 |
|
|
|
464 |
|
|
| X |
- DefinitionTabular disclosure of allocation of amount expensed and capitalized for award under share-based payment arrangement to statement of income or comprehensive income and statement of financial position. Includes, but is not limited to, corresponding line item in financial statement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (h)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfEmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_ShareBasedCompensationAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 11 - Loss per share (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Notes To Financial Statements [Abstract] |
|
| Schedule of diluted net loss per share attributable to common shareholders |
The Corporation excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common shareholders for the periods indicated because including them would have had an anti-dilutive effect:
|
|
|
|
|
|
|
June 30, 2023 |
|
June 30, 2022 |
Options outstanding |
|
473,178 |
|
704,585 |
May 2018 public offering warrants |
|
— |
|
137,370 |
December 2017 public offering warrants |
|
— |
|
147,354 |
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482662/260-10-50-1
| Name: |
us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
Note 12 - Supplemental Cash Flow Disclosure (Tables)
|
3 Months Ended |
Jun. 30, 2023 |
|---|
| Supplemental Cash Flow Elements [Abstract] |
|
| Schedule of Changes in working capital items |
Changes in operating assets and liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three months ended |
|
|
|
June 30, 2023 |
|
|
June 30,
2022 |
|
|
|
$ |
|
|
$ |
|
Receivables |
|
|
(35 |
) |
|
|
(434 |
) |
Prepaid expenses |
|
|
(529 |
) |
|
|
(839 |
) |
Trade and other payables |
|
|
(1,449 |
) |
|
|
2 |
|
Write-off of operating lease right of use asset |
|
|
(13 |
) |
|
|
— |
|
Total changes in operating assets and liabilities |
|
|
(2,026 |
) |
|
|
(1,271 |
) |
|
| X |
- DefinitionTabular disclosure of the net increase (decrease) in operating capital in the operating section of the statement of cash flows, represents the entire footnote disclosure that provides details regarding the net change during the reporting period of all assets and liabilities used in operating activities.
| Name: |
us-gaap_CashFlowOperatingCapitalTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_SupplementalCashFlowElementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionTabular disclosure of costs incurred for restructuring including, but not limited to, exit and disposal activities, remediation, implementation, integration, asset impairment, and charges against earnings from the write-down of assets. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SAB TOPIC 5.P.3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 420
-SubTopic 10
-Section S99
-Paragraph 2
-Subparagraph (SAB TOPIC 5.P.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
| Name: |
us-gaap_ScheduleOfRestructuringAndRelatedCostsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionDescription of the reverse stock split arrangement. Also provide the retroactive effect given by the reverse split that occurs after the balance sheet date but before the release of financial statements. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 4
-Subparagraph (SAB Topic 4.C)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-4
| Name: |
us-gaap_StockholdersEquityReverseStockSplit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ConversionOfStockByUniqueDescriptionAxis=acst_ReverseStockSplitMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionGrantsAndInvestmentTaxCreditReceivable
| Name: |
acst_GrantsAndInvestmentTaxCreditReceivable |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of sales taxes previously overpaid to tax authorities (such as U.S. Federal, state and local tax authorities) representing refunds of overpayments or recoveries based on agreed-upon resolutions of disputes.
| Name: |
acst_SalesTaxReceivablesCurrent |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481990/310-10-45-9
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of current interest earned but not received. Also called accrued interest or accrued interest receivable. For classified balance sheets, represents the current amount receivable, that is amounts expected to be collected within one year or the normal operating cycle, if longer. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestReceivableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionAmount of investment in marketable security. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 210
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
| Name: |
us-gaap_MarketableSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=acst_CanadianDepositsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionRate of interest on investment. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-5
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionMaturity date of investment, in YYYY-MM-DD format. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section 55
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480493/946-210-55-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-5
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 320
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481800/320-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.12-12A(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.12-12(Column A)(Footnote 4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-12B(Column A)(Footnote 4)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 320
-Name Accounting Standards Codification
-Section S99
-Paragraph 6
-Subparagraph (SX 210.12-14(Column A)(Footnote 3))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480032/946-320-S99-6
| Name: |
us-gaap_InvestmentMaturityDate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_FinancialInstrumentAxis=acst_CanadianDepositsMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
acst_NotesToFinancialStatementsAbstract |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSum of the carrying values as of the balance sheet date of obligations incurred through that date and due within one year (or the operating cycle, if longer), including liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received, taxes, interest, rent and utilities, accrued salaries and bonuses, payroll taxes and fringe benefits. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19,20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccountsPayableAndAccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.19(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section 45
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483467/210-10-45-8
| Name: |
us-gaap_AccountsPayableTradeCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 210
-SubTopic 10
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02.20)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AccruedLiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
| X |
- DefinitionThe amount of government assistance recorded in earnings.
| Name: |
acst_GovernmentAssistance |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionInvestment Tax Credit Receivables
| Name: |
acst_InvestmentTaxCreditReceivables |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_ReceivablesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe amount of the tax credit carryforward, before tax effects, available to reduce future taxable income under enacted tax laws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 3
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-3
| Name: |
us-gaap_TaxCreditCarryforwardAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_TaxCreditCarryforwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2029Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2030Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2031Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2032Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2033Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2034Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2035Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2036Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2037Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2038Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2039Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2040Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2041Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYear2042Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TaxCreditCarryforwardAxis=acst_TaxYearTwoThousandFortyThreeMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
v3.23.2
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease due after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFive |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.23.2
Note 8 - Leases (Additional Information) (Details) - USD ($)
$ in Thousands |
|
3 Months Ended |
Mar. 14, 2022 |
Jun. 30, 2023 |
| Leases [Abstract] |
|
|
| Extension of lease term, description |
|
On March 14, 2022, the Corporation renewed the lease agreement effective April 1, 2022, resulting in a commitment of $556 over a 24 months base lease term and 48 months additional lease renewal term. In April 2023, the Corporation elected not to renew the additional 48 months lease renewal term with the lease expected to terminate March 31, 2024
|
| Right-of-use asset and lease liability |
$ 369
|
|
| Lease payments discounted incremental borrowing rate |
4.30%
|
|
| Payments for rent |
$ 556
|
|
| Lease term |
24 months
|
|
| Additional lease renewal term |
48 months
|
|
| Lease expected to terminate date |
March 31, 2024
|
|
| X |
- References
+ Details
| Name: |
us-gaap_LeasesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDiscount rate used by lessee to determine present value of operating lease payments. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseDiscountRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of terms and conditions of option to extend lessee's operating lease. Includes, but is not limited to, information about option recognized as part of right-of-use asset and lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseOptionToExtend |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionRemaining lease term of operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRemainingLeaseTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTerm of lessee's operating lease renewal, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRenewalTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of terms and conditions of option to terminate lessor's operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479773/842-30-50-3
| Name: |
us-gaap_LessorOperatingLeaseOptionToTerminate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCash payments to lessor's for use of assets under operating leases. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (g)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-25
| Name: |
us-gaap_PaymentsForRent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
v3.23.2
Note 9 - Capital and Other Components of Equity (Details Textual)
|
|
3 Months Ended |
|
|
|
Jun. 29, 2020
USD ($)
shares
|
Jun. 30, 2023
Vote
$ / shares
shares
|
Jun. 30, 2022
USD ($)
$ / shares
shares
|
Mar. 31, 2023
shares
|
Nov. 10, 2021
USD ($)
|
| Common Stock, Shares, Issued |
|
7,435,533
|
|
7,435,533
|
|
| Common Stock, Shares, Outstanding |
|
7,435,533
|
|
7,435,533
|
|
| At-the-market Sales Agreement, Common Stock, Maximum Amount | $ |
$ 75,000,000
|
|
|
|
$ 75,000,000
|
| At-the-market Sales Agreement, Underwriter Fees, Percentage of Sales |
3.00%
|
|
|
|
|
| Warrants Issued in May 2018 [Member] |
|
|
|
|
|
| Class of Warrant or Right, Number of Securities Called by Each Warrant or Right (in shares) |
|
137,370
|
|
|
|
| Class of Warrant or Right, Exercise Price of Warrants or Rights (in CAD per share) | $ / shares |
|
$ 62.88
|
|
|
|
| At-the-market Offering [Member] |
|
|
|
|
|
| Stock Issued During Period, Shares, New Issues (in shares) |
0
|
0
|
34,335
|
|
|
| Proceeds from Issuance of Common Stock, Net | $ |
|
|
$ 195,000
|
|
|
| Sale of Stock, Average Price Per Share (in dollars per share) | $ / shares |
|
|
$ 5.82
|
|
|
| Other Selling, General and Administrative Expense | $ |
|
|
$ 6,000
|
|
|
| Common Class A [Member] |
|
|
|
|
|
| Common Stock, Votes Per Share | Vote |
|
1
|
|
|
|
| Common Stock, Shares, Issued |
|
7,435,533
|
|
|
|
| Common Stock, Shares, Outstanding |
|
7,435,533
|
|
|
|
| Common Class B [Member] |
|
|
|
|
|
| Common Stock, Votes Per Share | Vote |
|
10
|
|
|
|
| Common Stock, Shares, Issued |
|
0
|
|
|
|
| Common Stock, Shares, Outstanding |
|
0
|
|
|
|
| Common Stock, Dividend Rate, Percentage |
|
5.00%
|
|
|
|
| Common Stock, Redemption Price Per Share (in CAD per share) | $ / shares |
|
$ 4.8
|
|
|
|
| Common Class C [Member] |
|
|
|
|
|
| Common Stock, Shares, Issued |
|
0
|
|
|
|
| Common Stock, Shares, Outstanding |
|
0
|
|
|
|
| Common Stock, Dividend Rate, Percentage |
|
5.00%
|
|
|
|
| Common Stock, Redemption Price Per Share (in CAD per share) | $ / shares |
|
$ 1.2
|
|
|
|
| Common Class D and Common Class E [Member] |
|
|
|
|
|
| Common Stock, Shares, Issued |
|
0
|
|
|
|
| Common Stock, Shares, Outstanding |
|
0
|
|
|
|
| Minimum [Member] | Common Class D and Common Class E [Member] |
|
|
|
|
|
| Common Stock, Dividend Rate, Percentage |
|
0.50%
|
|
|
|
| Maximum [Member] | Common Class D and Common Class E [Member] |
|
|
|
|
|
| Common Stock, Dividend Rate, Percentage |
|
2.00%
|
|
|
|
| X |
- DefinitionThe maximum amount of common stock that can be sold under the at-the-market sales agreement.
| Name: |
acst_AtthemarketSalesAgreementCommonStockMaximumAmount |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionThe percentage of each sale that is to be paid to the underwriter under the at-the-market sales agreement.
| Name: |
acst_AtthemarketSalesAgreementUnderwriterFeesPercentageOfSales |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe percentage rate used to calculate dividend payments on common stock.
| Name: |
acst_CommonStockDividendRatePercentage |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount to be paid per share at which the common stock of an entity is redeemed or may be called at.
| Name: |
acst_CommonStockRedemptionPricePerShare |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of votes per one share of common stock.
| Name: |
acst_CommonStockVotesPerShare |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe cash inflow from the issuance of common stock, net of issuance cost.
| Name: |
acst_ProceedsFromIssuanceOfCommonStockNet |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average price per share received by entity for each share of common stock issued or sold in the stock transaction.
| Name: |
acst_SaleOfStockAveragePricePerShare |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of securities into which each warrant or right may be converted. For example, but not limited to, each warrant may be converted into two shares.
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByEachWarrantOrRight |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479617/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of selling, general and administrative expense classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03.4)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherSellingGeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of new stock issued during the period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481112/505-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 505
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481004/946-505-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483575/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479886/946-10-S99-3
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480008/505-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesNewIssues |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_ClassOfWarrantOrRightAxis=acst_WarrantsIssuedInMay2018Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsidiarySaleOfStockAxis=acst_AtTheMarketOfferingMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassAMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassBMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=us-gaap_CommonClassCMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=acst_CommonClassDAndCommonClassEMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 10 - Stock Based Compensation (Details Textual) - USD ($)
$ in Thousands |
3 Months Ended |
Jun. 30, 2023 |
Jun. 30, 2022 |
| Unrecognized compensation cost related to non-vested share options |
$ 130
|
|
| Share-Based Payment Arrangement, Nonvested Award, Cost Not yet Recognized, Period for Recognition |
11 months 19 days
|
|
| Share-Based Payment Arrangement, Expense |
$ 78
|
$ 464
|
| Stock Option Plan [Member] |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period (Year) |
10 years
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Percentage of Outstanding Stock Maximum Per Person |
2.00%
|
|
| Stock Option Plan [Member] | Consultant [Member] |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Percentage of Outstanding Stock Maximum Per Person |
2.00%
|
|
| Stock Option Plan [Member] | Related Party [Member] |
|
|
| Share-based Compensation Arrangement by Share-based Payment Award, Percentage of Outstanding Stock Maximum Per Person |
2.00%
|
|
| Equity Incentive Plan [Member] |
|
|
| Share-Based Compensation Arrangement by Share-Based Payment Award, Non-Option Equity Instruments, Outstanding, Number, Ending Balance |
0
|
0
|
| Share-Based Payment Arrangement, Expense |
$ 0
|
$ 0
|
| X |
- DefinitionMaximum number of shares that may be issued to one person in accordance with the plan as a proportion of outstanding capital stock.
| Name: |
acst_ShareBasedCompensationArrangementByShareBasedPaymentAwardPercentageOfOutstandingStockMaximumPerPerson |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of equity instruments other than options outstanding, including both vested and non-vested instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNonOptionEquityInstrumentsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionPeriod from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acst_StockOptionPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_TitleOfIndividualAxis=acst_ConsultantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acst_EquityIncentivePlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
Note 10 - Stock Based Compensation - Activities Within the Stock Option Plan (Details) - Stock Option Plan [Member] - $ / shares
|
3 Months Ended |
|
Jun. 30, 2023 |
Mar. 31, 2023 |
| Outstanding, weighted average exercise price (in CAD per share) |
$ 13.6
|
|
| acst_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageFairValue |
14.07
|
$ 11.23
|
| Forfeited, weighted average fair value (in CAD per share) |
$ 6.21
|
|
| Outstanding, number of options (in shares) |
740,957
|
|
| Forfeited, weighted average exercise price (in CAD per share) |
$ 7.72
|
|
| Forfeited, number of options (in shares) |
(267,797)
|
|
| Outstanding, weighted average exercise price (in CAD per share) |
$ 16.93
|
|
| Outstanding, number of options (in shares) |
473,178
|
|
| Exercisable, weighted average fair value (in CAD per share) |
$ 15.51
|
|
| Exercisable, weighted average exercise price (in CAD per share) |
$ 18.62
|
|
| Exercisable, number of options (in shares) |
402,247
|
|
| X |
- DefinitionThe weighted-average fair value price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan.
| Name: |
acst_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value of options forfeited during the reporting period as calculated by applying the disclosed option pricing methodology.
| Name: |
acst_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageFairValue |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe weighted average fair value of options outstanding during the reporting period as calculated by applying the disclosed option pricing methodology.
| Name: |
acst_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageFairValue |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acst_StockOptionPlanMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionAmount of expense for award under share-based payment arrangement. Excludes amount capitalized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 14.F)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479830/718-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2
| Name: |
us-gaap_AllocatedShareBasedCompensationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_ResearchAndDevelopmentExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_SellingAndMarketingExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ImpairmentEffectsOnEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of non-vested options outstanding.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsNonvestedNumberOfShares |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acst_WarrantsIssuedInMay2018Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_PlanNameAxis=acst_WarrantsIssuedDecember272017Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionIncrease decrease in non-cash working capital.
| Name: |
acst_IncreaseDecreaseInNonCashWorkingCapital |
| Namespace Prefix: |
acst_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the amount of outstanding money paid in advance for goods or services that bring economic benefits for future periods. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInPrepaidExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the total amount due within one year (or one operating cycle) from all parties, associated with underlying transactions that are classified as operating activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInReceivables |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_SupplementalCashFlowElementsAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.23.2
| X |
- DefinitionThe tax effect as of the balance sheet date of the amount of the estimated future tax deductions arising from estimated policyholder reserves, which will be deductible from future taxable income when actual costs are incurred, and which can only be realized if sufficient tax-basis income is generated in future periods to enable the tax deduction to be taken. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-6
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 740
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482685/740-10-50-8
| Name: |
us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccrualsPolicyholderLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionMinimum amount to be expended to satisfy the terms of arrangements in which the entity has agreed to expend funds to procure goods or services, excluding long-term purchase commitments or unconditional purchase obligations. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479853/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_PurchaseCommitmentRemainingMinimumAmountCommitted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionMinimum amount of purchase arrangement in which the entity has agreed to expend funds to procure goods or services from a supplier.
| Name: |
us-gaap_PurchaseObligation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
us-gaap_PurchaseCommitmentExcludingLongtermCommitmentAxis=acst_RKOSupplyAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
v3.23.2
| X |
- DefinitionThe cash outflow for cost incurred in the modification of term of existing debt agreement in order for the entity to achieve some advantage. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-15
| Name: |
us-gaap_PaymentsOfDebtRestructuringCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(2))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(b)(1))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 5.P.4(d))
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479823/420-10-S99-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 420
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482017/420-10-50-1
| Name: |
us-gaap_RestructuringCostAndReserveLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_EmployeeSeveranceMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_RestructuringCostAndReserveAxis=us-gaap_OtherRestructuringMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.23.2
| X |
- DefinitionShare Based Compensation Arrangement by Share Based Payment Award Fair Value Assumption Exercise Price.
| Name: |
acst_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionExercisePrice |
| Namespace Prefix: |
acst_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from additional borrowings, net of cash paid to third parties in connection with debt origination. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromDebtNetOfIssuanceCosts |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow from exercise of option under share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 14
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147482740/230-10-45-14
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2A
-Subparagraph (a)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147480429/718-10-50-2A
| Name: |
us-gaap_ProceedsFromStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe net book value of the asset(s) sold in connection with the sale of the property to another party and lease back to the seller. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 40
-Name Accounting Standards Codification
-Section 55
-Paragraph 50
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481266/840-40-55-50
Reference 2: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 40
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481266/840-40-55-52
Reference 3: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 840
-SubTopic 40
-Name Accounting Standards Codification
-Section 55
-Paragraph 51
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481266/840-40-55-51
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147479741/842-40-50-2
| Name: |
us-gaap_SaleLeasebackTransactionNetBookValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org//1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_FinancialSupportToNonconsolidatedLegalEntityAxis=acst_AssetPurchaseAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Acasti Pharma (NASDAQ:ACST)
Historical Stock Chart
From Mar 2024 to Apr 2024
Acasti Pharma (NASDAQ:ACST)
Historical Stock Chart
From Apr 2023 to Apr 2024