UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
July 6, 2023
(Commission File No. 001-38475)
ASLAN PHARMACEUTICALS LIMITED
(REG. NO. 289175)
(Translation of registrant’s name into English)
CAYMAN ISLANDS
(Jurisdiction of incorporation or organization)
3 Temasek Avenue
Level 18 Centennial Tower
Singapore 039190
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes ☐ No ☒
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes ☐ No ☒
Announcement of Positive Topline Data from Phase 2b Study of eblasakimab in Moderate-to-Severe Atopic Dermatitis
On July 6, 2023, ASLAN Pharmaceuticals Limited (the “Company”) announced positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis (AD), the TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study.
Eblasakimab met the primary endpoint of percent change from baseline in the Eczema Area and Severity Index (EASI) score at week 16 versus placebo, with statistical significance in three dosing arms: 600mg dosed once every 4 weeks (600mg Q4W), which was numerically the best performing arm, 400mg dosed once every 2 weeks (400mg Q2W) and 300mg dosed once every 2 weeks (300mg Q2W).
Eblasakimab is the first biologic in moderate-to-severe AD to demonstrate a competitive efficacy profile with once-monthly dosing from initiation comparable to regimens dosing once every two weeks, supporting advancement to Phase 3.
600mg eblasakimab dosed once every four weeks in the TREK-AD study met the primary endpoint with statistical significance, with 62.7% of patients achieving EASI-75, 34.1% reaching EASI-90 and 31.2% achieving validated Investigator Global Assessment of Atopic Dermatitis (vIGA-AD) of 0 or 1.
Regimens dosing once every two weeks also met the primary endpoint with statistical significance, as well as meeting key secondary endpoints.
Eblasakimab’s unique loading dose regimen was observed to deliver rapid onset of action with statistically significant improvement in EASI score by week 4.
Eblasakimab was generally well-tolerated at all dose levels, with low rates of conjunctivitis and injection site reactions supporting the potential for a differentiated safety profile.
Key study results are set out below:
•Patients treated with eblasakimab 600mg Q4W, 400mg Q2W and 300mg Q2W saw a rapid onset of action in the first few weeks of treatment, with a statistically significant improvement in EASI score by week 4. Clinically meaningful improvements were achieved in other key efficacy measures compared to placebo (n=57) after 16 weeks of treatment, including:
oEblasakimab 600mg Q4W (n=59)
•62.7% of eblasakimab treated patients achieved a reduction of at least 75% from baseline (EASI-75), compared to 30.7% on placebo (p=0.0041).
•34.1% of eblasakimab treated patients achieved a reduction of at least 90% from baseline (EASI-90), compared to 10.1% on placebo (p=0.0088).
•31.2% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1 (clear or nearly clear skin), compared to 15.1% with placebo (p=0.0502).
•73.0% least squares (LS) mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0010).
oEblasakimab 400mg Q2W (n=56)
•54.5% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0322).
•32.6% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0139).
•32.6% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0380).
•65.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0294).
oEblasakimab 300mg Q2W (n=58)
•60.7% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0064).
•37.7% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0033).
•33.1% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0327).
•69.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0050).
oEblasakimab 400mg Q4W (n=59)
The eblasakimab 400mg Q4W dosing arm (n=59) did not meet the primary or secondary endpoints with statistical significance.
•51.9% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0556).
•24.3% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0949).
•15.0% of eblasakimab-treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.7457).
•61.9% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.1054).
•Overall, discontinuation rates were comparable between the treatment arms and higher in the placebo arm. No new safety signals were seen, and the frequency of adverse events (AEs) was comparable between treatment and placebo arms. The most frequently observed AEs across all treatment arms were nasopharyngitis (13.4% across all treatment arms compared to 8.8% for placebo), atopic dermatitis (8.6% compared to 7.0% for placebo), headache (6.9% compared to 7.0% for placebo) and upper respiratory tract infection (6.5% compared to 5.3% for placebo). Rates of conjunctivitis (5.2% compared to 1.8% for placebo), injection site reactions (4.7% compared to 1.8% for placebo) and herpes infections (3.0% compared to 5.3% for placebo) were low.
Further information is set out in the press release attached hereto as Exhibit 99.1 and which is incorporated by reference herein.
The information contained in this Form 6-K is hereby incorporated by reference into the Company’s Registration Statement on Form F-3 (File No. 333-252575), Registration Statement on Form F-3 (File No. 333-254768), Registration Statement on Form F-3 (File No. 333-270835), Registration Statement on Form F-3 (File No. 333-270837), Registration Statement on Form S-8 (File No. 333-252118), Registration Statement on Form S-8 (File No. 333-263843) and Registration Statement on Form S-8 (File No 333-270832).
Forward Looking Statements
This release contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of the Company. These forward-looking statements may include, but are not limited to, statements regarding the Company’s business strategy and clinical development plans; the Company’s plans to develop and commercialize eblasakimab; the safety and efficacy of eblasakimab; the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for eblasakimab; and the potential for eblasakimab as a first-in-class treatment for AD. The Company’s estimates, projections and other forward-looking statements are based on management’s current assumptions and expectations of future events and trends, which affect or may affect the Company’s business, strategy, operations or financial performance, and inherently involve significant known and unknown risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation, unexpected safety or efficacy data observed during preclinical or clinical studies; the fact that results of earlier studies and trials may not be predictive of future trial results; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic or the ongoing conflict between Ukraine and Russia and bank failures on the Company’s business and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Other factors that may cause actual results to differ from those expressed or implied in such forward-looking statements are described in the Company’s Securities and Exchange Commission (“SEC”) filings and reports (Commission File No. 001-38475), including the Company’s Annual Report on Form 20-F filed with the SEC on March 24, 2023. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify estimates, projections and other forward-looking statements. Estimates, projections and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, the Company undertakes no obligation to update or review any estimate, projection or forward-looking statement.
Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
|
|
|
|
ASLAN PHARMACEUTICALS LIMITED (Registrant) |
|
|
By: |
|
/s/ Kiran Kumar Asarpota |
Name: |
|
Kiran Kumar Asarpota |
Title: |
|
Chief Operating Officer |
Date: July 6, 2023

Exhibit 99.1
PRESS RELEASE
EBLASAKIMAB MONTHLY DOSING SHOWS POTENTIAL FOR BEST-IN-CLASS THERAPY IN POSITIVE PHASE 2B STUDY IN ATOPIC DERMATITIS
-Eblasakimab is the first biologic in moderate-to-severe atopic dermatitis to demonstrate a competitive efficacy profile with once-monthly dosing from initiation comparable to once every two weeks, supporting advancement to Phase 3.
-600mg eblasakimab dosed once every four weeks in the TREK-AD study met the primary endpoint with statistical significance, with 62.7% of patients achieving EASI-75, 34.1% reaching EASI-90 and 31.2% achieving vIGA-AD of 0 or 1.
-Regimens dosing once every two weeks also met the primary endpoint with statistical significance, as well as meeting key secondary endpoints.
-Unique loading dose regimen observed to deliver rapid onset of action with statistically significant improvement in EASI score by week 4.
-Eblasakimab was generally well-tolerated at all dose levels, with low rates of conjunctivitis and injection site reactions supporting the potential for a differentiated safety profile.
-Management to host webcast today, July 6, at 8:30 a.m. ET / 8:30 p.m. SGT.
San Mateo, California, and Singapore, July 6, 2023 – ASLAN Pharmaceuticals (NASDAQ: ASLN), a clinical-stage, immunology-focused biopharmaceutical company developing innovative treatments to transform the lives of patients, today announced positive topline data from its Phase 2b dose-ranging study of eblasakimab in adult patients with moderate-to-severe atopic dermatitis (AD), the TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study. Eblasakimab met the primary endpoint of percent change from baseline in the Eczema Area and Severity Index (EASI) score at week 16 versus placebo with statistical significance in three dosing arms: 600mg dosed once every 4 weeks (600mg Q4W), which was numerically the best performing arm, 400mg dosed once every 2 weeks (400mg Q2W) and 300mg dosed once every 2 weeks (300mg Q2W).
Eblasakimab is a novel monoclonal antibody targeting the IL‑13 receptor subunit of the Type 2 receptor, a key pathway driving several allergic inflammatory diseases. Eblasakimab’s unique mechanism of action has been observed to enable specific blockade of the Type 2 receptor, preventing signaling through both interleukin 4 (IL‑4) and interleukin 13 (IL‑13) – the key drivers of inflammation in AD, while sparing the Type 1 receptor.
“This is the first time we’ve seen a once-a-month treatment option deliver competitive efficacy data, which would be a game-changer for patients with AD,” said Eric L. Simpson, M.D., Frances J. Storrs Professor of Medical Dermatology at the Oregon Health and Science University and Lead Investigator in the TREK-AD study. “We haven’t seen much in the way of advancement since the launch of dupilumab, and there remains a huge unmet burden of disease experienced by patients. These results support eblasakimab’s potential to be a leading therapy for the treatment of AD, if approved.”

“We are delighted to announce these positive topline data from the TREK-AD study, which support recent translational studies we have published showing eblasakimab’s unique mechanism of action may provide a more efficient and targeted approach to blocking Type 2 signaling, which is responsible for allergic inflammation. Based on these results, we believe that eblasakimab could be a leading treatment in AD, if approved, getting more patients to EASI-75 or EASI-90, with fewer unwanted side effects, a rapid onset of action, and – uniquely – once-monthly dosing convenience,” said Dr Carl Firth, Chief Executive Officer of ASLAN Pharmaceuticals. “We look forward to advancing quickly into a Phase 3 program in AD, and exploring the broad range of indications where we would expect this drug candidate to be successful. We are grateful to the patients, investigators, and our team who have made such an important contribution to the development of eblasakimab.”
Key study results
In the TREK-AD Phase 2b study, 289 patients were randomized and treated in the intent-to-treat (ITT) population across five dosing arms in a 1:1:1:1:1 ratio to receive one of four doses of eblasakimab (300mg Q2W, 400mg Q2W, 400mg Q4W and 600mg Q4W) or placebo for 16 weeks. Key efficacy endpoints, including EASI-75 and a validated Investigator Global Assessment of Atopic Dermatitis (vIGA-AD) score of 0/1, the relevant endpoints for regulatory approval in Europe and the US respectively, are shown below:
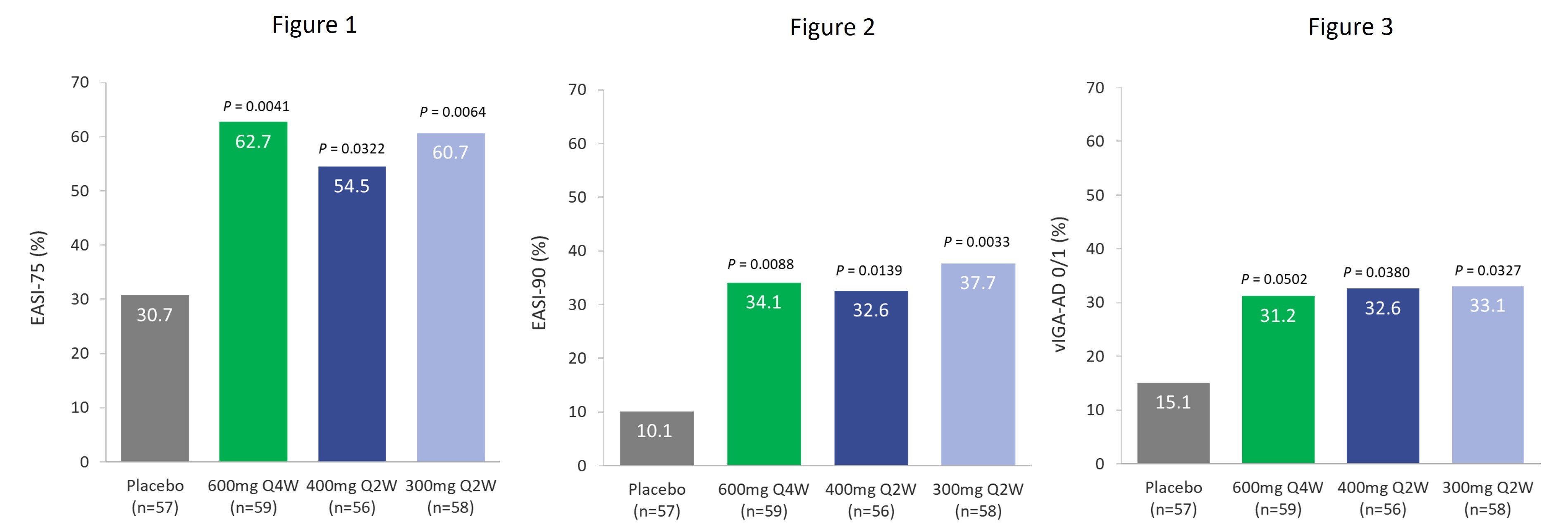
Patients treated with eblasakimab 600mg Q4W, 400mg Q2W and 300mg Q2W saw a rapid onset of action in the first few weeks of treatment, with a statistically significant improvement in EASI score by week 4. Clinically meaningful improvements were achieved in other key efficacy measures compared to placebo (n=57) after 16 weeks of treatment, including:
Eblasakimab 600mg Q4W (n=59)
•62.7% of eblasakimab treated patients achieved a reduction of at least 75% from baseline (EASI-75), compared to 30.7% on placebo (p=0.0041).
•34.1% of eblasakimab treated patients achieved a reduction of at least 90% from baseline (EASI-90), compared to 10.1% on placebo (p=0.0088).
•31.2% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1 (clear or nearly clear skin), compared to 15.1% with placebo (p=0.0502).
•73.0% least squares (LS) mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0010).

Eblasakimab 400mg Q2W (n=56)
•54.5% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0322).
•32.6% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0139).
•32.6% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0380).
•65.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0294).
Eblasakimab 300mg Q2W (n=58)
•60.7% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0064).
•37.7% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0033).
•33.1% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.0327).
•69.8% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.0050).
Eblasakimab 400mg Q4W (n=59)
The eblasakimab 400mg Q4W dosing arm did not meet the primary or secondary endpoints with statistical significance.
•51.9% of eblasakimab treated patients achieved EASI-75, compared to 30.7% on placebo (p=0.0556).
•24.3% of eblasakimab treated patients achieved EASI-90, compared to 10.1% on placebo (p=0.0949).
•15.0% of eblasakimab treated patients achieved vIGA-AD score of 0 or 1, compared to 15.1% with placebo (p=0.7457).
•61.9% LS mean reduction in EASI score from baseline, compared to 51.1% on placebo (p=0.1054).
Overall, discontinuation rates were comparable between the active treatment arms and higher in the placebo arm. No new safety signals were seen and the frequency of adverse events (AEs) was comparable between active treatment and placebo arms. The most frequently observed AEs across all active treatment arms were nasopharyngitis (13.4% compared to 8.8% for placebo), atopic dermatitis (8.6% compared to 7.0% for placebo), headache (6.9% compared to 7.0% for placebo) and upper respiratory tract infection (6.5% compared to 5.3% for placebo). Rates of conjunctivitis (5.2% compared to 1.8% for placebo), injection site reactions (4.7% compared to 1.8% for placebo) and herpes infections (3.0% compared to 3.5% for placebo) were low.
Further data from the Phase 2b study, including patient reported outcomes and biomarker data, is expected to be available in Q4 2023 and submitted for presentation at a future scientific congress. ASLAN plans to meet with the US Food and Drug Administration for an end-of-Phase 2 meeting and expects to initiate a Phase 3 clinical development program for eblasakimab in 2024.The Company is also conducting the TREK-DX (TRials in EblasaKimab in Dupilumab eXperienced AD patients) study of eblasakimab in dupilumab-experienced patients and expects to announce topline data from that study in 1Q 2024.

About the Phase 2b TREK-AD study
The TREK-AD (TRials with EblasaKimab in Atopic Dermatitis) study is a randomized, double-blind, placebo-controlled, dose-ranging clinical trial, designed to evaluate the efficacy and safety of eblasakimab as monotherapy in biologic-naïve adult patients with moderate-to-severe AD who are candidates for systemic therapy. Patients from the US, Europe and Asia were randomized in equal ratios to four active treatment arms and one placebo arm:
•Arm 1: Received a loading dose of 600mg of eblasakimab at weeks 0 and 1, followed by 300mg eblasakimab dosed every two weeks.
•Arm 2: Received a loading dose of 600mg of eblasakimab at weeks 0 and 1, followed by 400mg eblasakimab dosed every two weeks.
•Arm 3: Received a loading dose of 600mg of eblasakimab at weeks 0, 1 and 2, followed by 400mg eblasakimab dosed every four weeks.
•Arm 4: Received a loading dose of 600mg of eblasakimab at weeks 0, 1 and 2, followed by 600mg eblasakimab dosed every four weeks.
•Arm 5: Placebo dosed at weeks 0 and 1 and every two weeks thereafter.
The inclusion criteria for patients enrolled in the study included a clinical diagnosis of AD for at least one year, an EASI score of greater than or equal to 16, a vIGA-AD score of greater than or equal to 3 and a body surface area of AD involving at least 10% at screening and baseline. Following the end of the 16-week assessment period, patients were followed for an additional 12 weeks.
About eblasakimab
Eblasakimab is a potential first-in-class monoclonal antibody targeting the IL‑13 receptor subunit of the Type 2 receptor, a key pathway driving several allergic inflammatory diseases. Eblasakimab’s unique mechanism of action enables specific blockade of the Type 2 receptor and has the potential to improve upon current biologics used to treat allergic disease. By blocking the Type 2 receptor, eblasakimab prevents signaling through both interleukin 4 (IL‑4) and interleukin 13 (IL‑13) – the key drivers of inflammation in AD.
Webcast
ASLAN’s management will host a webcast at 8:30 a.m. ET today, July 6, 2023, to discuss these data. The live webcast may be accessed by registering using this link: https://lifescievents.com/event/aslan/. A replay of the webcast will be available until October 4, 2023, using the information above after the live event.
About ASLAN Pharmaceuticals
ASLAN Pharmaceuticals (Nasdaq: ASLN) is a clinical-stage, immunology-focused biopharmaceutical company developing innovative treatments to transform the lives of patients. ASLAN is developing eblasakimab, a potential first-in-class antibody targeting the IL-13 receptor in moderate-to-severe atopic dermatitis (AD) with the potential to improve upon current biologics used to treat allergic disease, and has recently reported positive topline data from a Phase 2b dose ranging study in moderate-to-severe AD. ASLAN is also developing farudodstat, a potent oral inhibitor of the enzyme dihydroorotate dehydrogenase (DHODH) as a potential first-in-class treatment for alopecia areata (AA) in a Phase 2a proof-of-concept trial with an interim readout expected in 1Q 2024. ASLAN has teams in San Mateo, California, and in Singapore. For additional information please visit the website or follow ASLAN on LinkedIn.

Forward-looking statements
This release contains forward-looking statements. These statements are based on the current beliefs and expectations of the management of ASLAN Pharmaceuticals Limited and/or its affiliates (the "Company"). These forward-looking statements may include, but are not limited to statements regarding the Company’s business strategy and clinical development plans; the Company’s plans to develop and commercialize eblasakimab; the safety and efficacy of eblasakimab,including its potential to be best-in-class; the Company’s plans and expected timing with respect to clinical trials, clinical trial enrolment and clinical trial results for eblasakimab; the potential of eblasakimab as a treatment for atopic dermatitis. The Company’s estimates, projections and other forward-looking statements are based on management's current assumptions and expectations of future events and trends, which affect or may affect the Company’s business, strategy, operations, or financial performance, and inherently involve significant known and unknown risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of many risks and uncertainties, which include, without limitation, unexpected safety or efficacy data observed during preclinical or clinical studies; the fact that results of earlier studies and trials may not be predictive of future trial results; clinical site activation rates or clinical trial enrolment rates that are lower than expected; the impact of the COVID-19 pandemic or the ongoing conflict between Ukraine and Russia and bank failures on the Company’s business and the global economy; general market conditions; changes in the competitive landscape; and the Company’s ability to obtain sufficient financing to fund its strategic and clinical development plans. Other factors that may cause actual results to differ from those expressed or implied in such forward-looking statements are described in the Company’s US Securities and Exchange Commission filings and reports (Commission File No. 001- 38475), including the Company’s Annual Report on Form 20-F filed with the US Securities and Exchange Commission on March 24, 2023. All statements other than statements of historical fact are forward-looking statements. The words “believe,” “may,” “might,” “could,” “will,” “aim,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes are intended to identify estimates, projections, and other forward-looking statements. Estimates, projections, and other forward-looking statements speak only as of the date they were made, and, except to the extent required by law, the Company undertakes no obligation to update or review any estimate, projection, or forward-looking statement.
Ends
Media and IR contacts
|
|
Emma Thompson Spurwing Communications Tel: +65 6206 7350 Email: ASLAN@spurwingcomms.com |
Ashley R. Robinson LifeSci Advisors, LLC Tel: +1 (617) 430-7577 Email: arr@lifesciadvisors.com |
ASLAN Pharmaceuticals (NASDAQ:ASLN)
Historical Stock Chart
From Mar 2024 to Apr 2024
ASLAN Pharmaceuticals (NASDAQ:ASLN)
Historical Stock Chart
From Apr 2023 to Apr 2024