Emergent BioSolutions Stock Rises 17% on FDA Narcan Approval
March 29 2023 - 9:39AM
Dow Jones News
By Will Feuer
Shares of Emergent BioSolutions Inc. rose after the U.S. Food
and Drug Administration said the company's overdose reversal
medication Narcan could be sold over-the-counter for the first time
since the opioid crisis began.
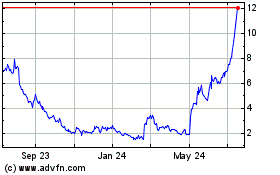
The stock climbed more than 17% to $10.45 in premarket
trading.
"Today's approval of OTC naloxone nasal spray will help improve
access to naloxone, increase the number of locations where it's
available and help reduce opioid overdose deaths throughout the
country," FDA Commissioner Robert Califf said. "We encourage the
manufacturer to make accessibility to the product a priority by
making it available as soon as possible and at an affordable
price."
Emergent said Narcan, the nasal-spray version of the medication
naloxone, would likely begin appearing on shelves by late summer.
Emergent is the first company to gain approval to sell naloxone
without a prescription.
Narcan nasal spray was first approved by the FDA in 2015 as a
prescription drug.
Write to Will Feuer at Will.Feuer@wsj.com
(END) Dow Jones Newswires
March 29, 2023 09:24 ET (13:24 GMT)
Copyright (c) 2023 Dow Jones & Company, Inc.
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
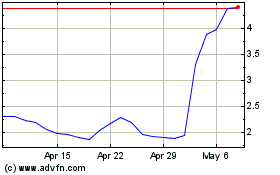
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024