Moderna Expects to Report Vaccine Data for Young Children in March
January 13 2022 - 1:02AM
Dow Jones News
By Ronnie Harui
Moderna Inc. on Wednesday said it expects to report data from
its Covid-19 vaccine trial in children ages 2 to 5 in March.
If the data are supportive and, subject to regulatory
consultation, Moderna may proceed with regulatory filings for the
vaccine for young children thereafter, the U.S. pharmaceutical and
biotechnology company said.
Moderna said it is also evaluating a booster dose in 12- to
17-year-olds.
In early December, Moderna decided to evaluate the potential of
lower doses to meet regulatory guidance for immunogenicity in
children 6 to 11 years of age and in adolescents 12 to 17 years of
age in its continuing clinical trials, it said.
Write to Ronnie Harui at ronnie.harui@wsj.com
(END) Dow Jones Newswires
January 13, 2022 00:47 ET (05:47 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
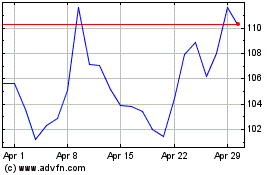
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
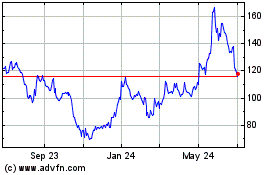
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Apr 2023 to Apr 2024