0001676163
false
0001676163
2023-12-04
2023-12-04
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
Pursuant to Section 13 or 15(d) of The Securities
Exchange Act of 1934
Date of report (Date of earliest event reported):
December 4, 2023
SS INNOVATIONS INTERNATIONAL, INC.
(Exact name of registrant as specified in its charter)
| Florida |
|
333-216054 |
|
47-3478854 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
1600 SE 15th Street, #512
Fort Lauderdale, Florida |
|
33316 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s telephone number, including
area code: (954) 478-1410
(Former name or former address, if changed since
last report)
Check the appropriate box below if the Form 8-K
filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General
Instruction A.2. below):
| ☐ | Written communications pursuant
to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant
to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications
pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications
pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b)
of the Act:
| Title of each Class |
|
Trading Symbol |
|
Name of each exchange on which registered |
| None |
|
N/A |
|
N/A |
Indicate by check mark whether the registrant
is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the
Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act.
As used in this Current Report on Form 8-K (this
“Report”), and unless otherwise indicated, the terms “SSII,” “the Company,” “we,”
“us” and “our” refer to SS Innovations International, Inc.
Item 7.01 Regulation FD Disclosure.
At 8:55 A.M. Eastern
Time on Monday, December 4, 2023, Vishwa Srivastava, M.D., our President, will be making a presentation on “The Money Show.”
A copy of Dr. Srivastava’s presentation
is attached as Exhibits 99.1 to this Report.
The foregoing information,
including the investor presentation attached as Exhibit 99.1, is being furnished pursuant to Item 7.01 of
this Current Report and shall not be deemed “filed” for the purposes of Section 18 of the Securities and Exchange
Act of 1934, as amended or incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly set
forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. |
|
Description |
| 99.1* |
|
Investor Presentation |
| |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
| * | Furnished but not filed. |
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: December 4, 2023 |
SS INNOVATIONS INTERNATIONAL, INC. |
| |
|
|
| |
By: |
/s/ Sudhir Srivastava |
| |
|
Sudhir Srivastava, M.D.
Chairman and Chief Executive Officer |
2
Exhibit
99.1

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 1 SS INNOVATIONS PRODUCT PRESENTATION SUDHIR SRIVASTAVA INNOVATIONS PVT. LTD. - CONFIDENTIAL

DISCLAIMERS 2 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N No Offering of Securities No offer is made by this Investor Presentation (this “ Presentation ”) to invest in SS Innovations International, Inc. (the “ Company ”) or to purchase any of its securities. Any offer to make such an investment or purchase any of its securities will be made only pursuant to definitive offering documentation furnished by the Company. Forward - Looking Statements This Presentation contains forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 , as amended and Section 21 E of the Securities Exchange Act of 1934 , as amended . These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by our forward - looking statements . Examples of forward - looking statements include projected financial information, statements of our plans and objectives for future operations and statements concerning proposed new products and services . In some cases, you can identify forward - looking statements by the use of terminology such as “may,” “will,” “should,” “could,” “expects,” “plans,” “intends,” “anticipates,” “believes,” “estimates,” “predicts,” “potential” and other comparable terminology . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee future results, performance or achievements . Actual events or results may differ materially . We undertake no obligation to update any of the forward - looking statements after the date of this Presentation to conform them to actual results . Projected Financial Information The projected financial information included in this Presentation has been prepared by Company management and is subject to a high degree of uncertainty . It is based upon estimates of future events and circumstances that might or might not ultimately prove to be accurate . All of the assumptions upon which the projections are based, and which would be material, are not presented . No representation or warranty can be made as to the accuracy of any of these assumptions . There can be no assurance that the projections will be realized, and actual results may differ materially from those set forth in the projections . The assumptions underlying the projected financial information are inherently uncertain and are subject to significant business, economic, and competitive risks and uncertainties that would cause actual results to differ materially from those projected . No opinion or report on the projected financial information was received from any independent accountants . If the projected results are not achieved, the Company’s business and financial performance could be adversely affected . Because of the above limitations on these projections, you are cautioned about placing undue reliance on them .

“To create a new, technologically advanced system that would surpass the existing surgical robotic systems and be very cost effective to benefit many more patients around the world ” 3 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N SSI Vision

Sudhir Prem Srivastava, MD 4 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N Founder, Chairman and CEO SS Innovations Group Companies Recognizing the global need for an affordable surgical robotic system, Dr. Srivastava, a world renown cardiac surgeon having accumulated more than 1400 robotic cardiac cases, made it his mission to lead a team of engineers to develop an advanced robotic surgical system with more and better features, while being the most cost - effective system globally.

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 5 Robotic Surgery Global Landscape
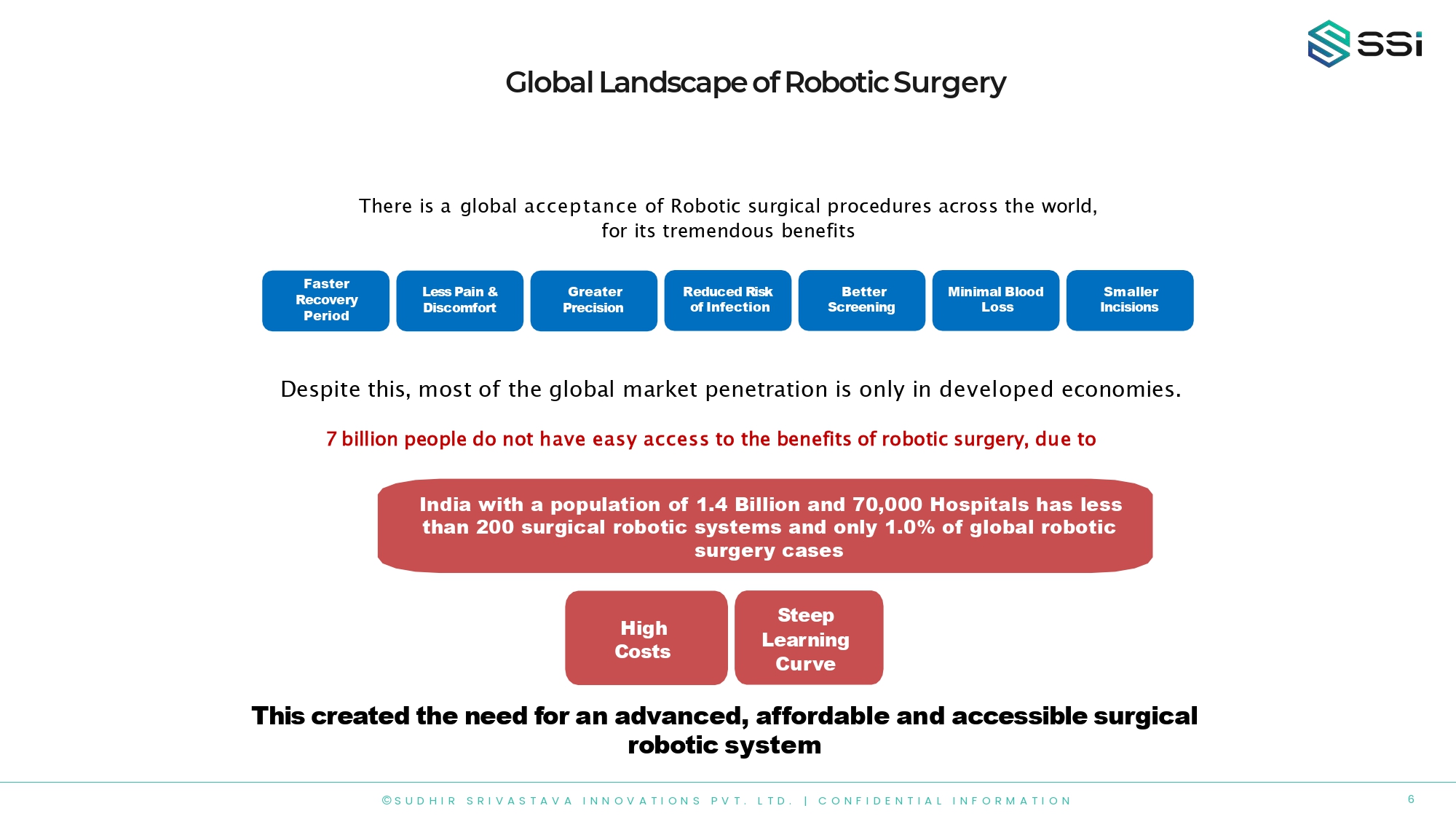
Global Landscape of Robotic Surgery There is a global acceptance of Robotic surgical procedures across the world, for its tremendous benefits Faster Recovery Period Greater Precision Better Screening Less Pain & Discomfort Reduced Risk of Infection Minimal Blood Loss Smaller Incisions Despite this, most of the global market penetration is only in developed economies. 7 billion people do not have easy access to the benefits of robotic surgery, due to High Costs Steep Learning Curve This created the need for an advanced, affordable and accessible surgical robotic system India with a population of 1.4 Billion and 70,000 Hospitals has less than 200 surgical robotic systems and only 1.0% of global robotic surgery cases 6 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

7 Robotic Surgeries: Changing Dynamics 230 Million Conventional Surgeries 133 Million Minimally Invasive Surgeries Estimated Global Surgery Count 2020 363 Million Total Surgeries 1.6 Million Robotic Surgeries 253 Million Conventional Surgeries 236 Million Minimally Invasive Surgeries Projected Global Surgery Count 2030 489 Million Total Surgeries Robotic surgery as a percentage of overall surgeries is expected to increase from 0.4% in 2020 to 1.3 % by 2030 CAGR ~6% CAGR ~15% 6.2 Million Robotic Surgeries CAGR ~1% Source: BIS Global Surgical Robotics Market Report 2020, https://www.robsurgical.com/market - trends / Total Number of Surgeries Conducted Using Robotic Systems Globally is Expected to Become ~4X By 2030 7 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

BREAKUP OF GLOBAL SURGICAL ROBOTICS MARKET BY COMPONENT TYPE 5,460 16,774 GLOBAL SURGICAL ROBOTICS MARKET IN US$ Millions The three broad revenue segments of surgical robotics by component type : 2020 2031 Surgical Robotics Revenue by geography (US$ Millions) 907 1,737 2,816 CAGR 8% 2,116 CAGR 12% 9,536 Service System Instruments & accessories 2020 2031 Instruments and accessories as a component currently contributes to ~52% & is expected to increase to ~57% by 2031 • Surgical systems • Instruments and accessories (used per procedure) • Service (annual maintenance and upgrades) CAGR 10% 5,122 Global surgical robotics market is expected to grow at a CAGR of 11% from ~5.5 Bn USD in 2020 to ~17 Bn USD by 2031 8 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N CAGR 2031 2020 Geography 8.40% 8,374 3,463 America 13.00% 3,830 998 Europe 15.00% 4,343 936 Asia - Pacific 12.30% 228 63 Rest of the world

SSII Value Drivers 9 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N Clinically Validated: Two successful clinical trials completed. Over 500 successful surgeries performed using the SSi Mantra surgical robotic system . The SSi Mantra is clinically validated to perform more than 40 types of surgeries. Innovative Product Offering: Developed by a team of world - class medical and engineering professionals: the SSi Mantra surgical robotic system, SSi Mudra surgical instruments, and the SSi Maya, an XR - based surgical robotic training platform. Competitive Cost Advantage: SSi Mantra surgical robotic system offers a wide range of specialty applications at approximately 30% the cost of the market leader. Attractive Market Opportunity: The global addressable market for surgical robotics is expected to reach US$17 billion by 2031. The Indian surgical robotics market alone is anticipated to reach US $313 million in 2024. An Underserved Population: The current Robotic Systems cover mostly developed economies that accounts for almost 93% penetration, leaving only 7% penetration for almost 7 Billion people globally. India has approximately 70,000 hospitals and only 150 surgical robotic machines. Early Adoption: Seventeen systems currently installed, eleven additional purchase orders received, and International sales have begun. Regulatory Approvals: Indian Medical Device regulatory approval (CDSCO) secured for the SSi Mantra; FDA and CE approvals expected in 2024/2025. Global Access: Access to 120 countries with minimal regulatory approvals or Indian Regulatory Approval acceptance. Strong Patent Protection: An impressive IP portfolio of 88 patents, Copyrights and Trademarks, of which 26 granted.

□ Current Installations : 17 (including 3 systems under evaluation with 3 of the large hospital chains in India and one system at Johns Hopkins, Baltimore for research and training purposes) □ First export of SSI Mantra system to Aster, Dubai (UAE) □ Planned Deliveries this month Dec 23 : 11 Systems □ Additional Orders expected by end of Dec 23 : ~ 10 - 15 systems □ Total installations expected by end of Dec 23 : 28 systems 10 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

SSI India Facility 11 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N R&D, Manufacturing & Assembly, and Operations in Gurgaon, Delhi NCR (India) Easy proximity to Delhi International airport and excellent connectivity with all parts of the world. 55,000 sq ft space spread over two floors Current facility has capacity for assembly of 20 systems per month Current manpower: 180+ SSI plans to have in - house CNC and Tooling machines with larger funding within next 12 months

SSI New Facility 12 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 13 Surgical Robotic System

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 14 Advanced . Affordable . Accessible Robotic Surgery

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 15 Technology Attributes Large 32 inch 3D 4K Monitor with Head Tracking Safety Feature Large 23 inch 2D touch monitor for system controls and DICOM applications Active visibility of the hand controls, Foot Controls and the entire Operating Room Surgeon Console Open Faced Console for ergonomic surgeon position

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 16 Patient Side Robotic Arm Carts © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N Modular Robotic Carts Freedom of Patient Docking 3 / 4 / 5 Patient Cart Connectivity Absolute Stability Parking Locks Smaller Individual cart footprint Advanced touch screen controls

NADI AUTOMATED CORONARY ANASTOMOTIC CONNECTOR Robotic Automated Anastomotic Connector 17 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N SS INNOVATIONS PRODUCT PRESENTATION Manual Anastomotic Connector

SSi Multi - Fire Clip Applier 18 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

SSI Mixed Reality Headset • Head mounted with peripheral view • 1080P 3D HD vision • 32” image projection for a 1m depth perception • Two separate Left and Right eye video signals projected through an optical engine onto an opaque micro - LED screen • Natural reconstruction of 3D image by human brain Collaborating Medical Diagnostics with the Metaverse, Our state - of - the - art Mixed reality platform can help in : Holographic Anatomy Virtual Surgery Training OR Tele - mentoring AI Based DICOM 19 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

Ergonomic Surgeon Console: The Surgeon is able to sit straight and visualise both hands and feet thereby reducing the learning curve. Ergonomic hand controls are extremely precise and designed to produce the least amount of strain during the surgeon’s movements. 4K 3D Resolution for the Entire Surgical Team: The Surgeon Console and Vision Cart both have a large 32 - inch 3D 4K monitor with up to 10 times magnification, resulting in the best vision possible today for the surgeon and support team. Tele - Mentoring Capabilities: With our bult in live streaming platform, Remote Proctoring is possible to make teaching and training available in the most efficient and cost - effective way. Modular Design: The robotic arms and carts mounted individually allow the flexibility of 3 to 5 arms and better placement in relation to the procedure to avoid collision. Multispecialty Use including Cardiac Surgery: The system can be used in all surgical specialties including the complete spectrum of cardiac surgeries. Cost Effectiveness: The SSI Mantra is the most cost - effective surgical robotic system available globally. Per procedure costs and maintenance charges are 1/3 that of the competition. 20 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N SSI Mantra Key Advantages

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 21 Clinical Experience

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 22 Clinical Validation The SSI Mantra has been clinically validated in 500+ Surgical procedures .

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 23 Current Status of SSI Mantra 16 Systems Installed, 5 more in process 2023 450+ Surgical Procedures Successfully Completed (No Device Related Adverse Effects) • Radical Prostatectomy with B/L PNLD • Right Radical Nephrectomy • Right Partial Nephrectomy • Radical Cysto - Prostatectomy with neo bladder • Radical Cysto - Prostatectomy with ileal conduit • Vesicovaginal Fistula • Radical Prostatectomy with B/L PNLD + Left inguinal hernia • Left Radical Nephrectomy • Left Partial Nephrectomy • Partial Cystectomy /Cholecystectomy/ Radical Cystectomy • Anterior Exenteration with Ileal conduit • Radical Hysterectomy/Hysterectomy • Ureteric reimplantation • B/L / LT/ RT Inguinal hernia • Thymectomy • LIMA take down • Uretero - ureterostomy • Esophagectomy • Inter - sphincteric resection • Pancreatic Whipple • Pyeloplasty • Sacro - Colopexy • Radical Adrenalectomy • Radical Cysto - prostatectomy with ileal conduit+ Radical left Nephrectomy • Right Nephroureterectomy • Robot Assisted CABG

© S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N 24 SSI India and Overseas Market Approvals Current Status

Regulatory Approvals Approved/Certified In Process of Approval/Certification 25 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

Overseas Regulatory Registrations and Approvals – Current Status 26 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N Timelines Territory Registration Status Authority Distributor /Notified Body Country 9 Months All EU Countries Application initiated EU Commission Procedo International Certification from Slovakia Europe 3 Months Sri Lanka In Process, approval awaited National Medicines Regulatory Authority (NMRA) SIBA HOLDINGS (PRIVATE) LIMITED Sri Lanka 2 Months Africa Region In Process, approval awaited The Egyptian Drug Authority (EDA)/Ministry of Health (MOH) Surgical Lab Egypt 2 Months Argentina In Process, approval awaited Administración Nacional de Medicamentos, Alimentos y Tecnología Médica (ANMAT) NEWGENE BIOTECNOLOGÍA S.A Argentina 2 Months Armenia, Turkey, Azerbaijan, Georgia, Kazakhstan, Moldova, Mongolia, Turkmenistan, Ukraine, Uzbekistan, Israel and Kuwait In Process, approval awaited State Regulation Agency for Medical Activities, Ministry of Labour, Health and Social Affairs. Medea International Medical Association (MIMA) Georgia, (Republic of) 2 Months Indonesia Application initiated Ministry of Health (MOH) PT. NEURO MEDIKA SEJAHTERA Indonesia 3 Months Oman Application initiated Ministry of Health (MOH) Taiba Medserv LLc Oman

LIST OF 50 COUNTRIES WITHOUT MEDICAL REGULATORY APPROVAL/REGISTRATION PROCESS European Region (EUR) - 1/54 Americas (AMR) - 12/35 African Region (AFR) - 18/47 Andorra Antigua and Barbuda Botswana South - East Asia Region (SEAR) - 2/11 Bahamas Burundi Nepal Barbados Chad Timor - Leste Belize Equatorial Guinea Western Pacific Region (WPR) - 13/27 Dominica Eswatini (Swaziland) Brunei Darussalam Grenada Gabon Cambodia Guyana Gambia Cook Islands Haïti Guinea - Bissau Kiribati Saint Kitts and Nevis Lesotho Marshall Islands Saint Lucia Liberia Micronesia, Federated States of Saint Vincent and the Grenadines Madagascar Mongolia Suriname Malawi Nauru Eastern Mediterranean Region (EMR) - 4/21 Mauritius Niue Djibouti Mozambique Palau Kuwait Niger Papua New Guinea Sudan Sao Tome and Principe Tonga Syrian Arab Republic Seychelles Vanuatu - South Sudan SSI allowed to sell in 50 Countries without any Regulatory Approvals and 79 countries have minimal registration process. 27 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N SSI Global Regulatory Approval/Registration Strategy

2020 Mantra 1.0 Dev. Animal trials Cadaver Trials 2021 H1 Mantra 1.0 Clinical Trials MSME Registration Mantra 2.0 Dev. 2021 H2 ISO 13485 Certification UDI Registration Biocompatibility Studies 2022 H1 Mantra 2.0 Dev Clinical Trials Design Transfer CDSCO Test License 2022 H2 ISO 13485 Surveillance Audit 1st System Sale CDSCO Application 2023 H1 Obtained CDSCO License FSC License GeM Registration UAE Approval 2023 H2 510(k) Q Sub. Egypt, Sri Lanka, Guatemala Registration etc,. FDA Consultant App. CE MDR Remediation 13485 Surveillance Audit Market Entry - UAE, Sri Lanka, Bangladesh, Nepal and Egypt 2024 H1 Regulatory Registration Approvals in various other countries Obtain 510 (k) Approval CE Agent Appointment CE Application Submission MDSAP Application 2024 H2 Import License Approval from various countries Obtain MDSAP Approval Obtain CE Approval Canada, Brazil, AUS, Japan Application 2025 H1 Australia, Brazil and Canada Approvals Registration Approvals from Other Countries 2025 H2 Japan, Mexico Approvals China NMPA Initiation SSi Milestones and Future Roadmap 28 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N

29 © S U D H I R S R I V A S T A V A I N N O V A T I O N S P V T . L T D . | C O N F I D E N T I A L I N F O R M A T I O N
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
AVRA Medical Robotics (PK) (USOTC:AVMR)
Historical Stock Chart
From Mar 2024 to Apr 2024
AVRA Medical Robotics (PK) (USOTC:AVMR)
Historical Stock Chart
From Apr 2023 to Apr 2024