FDA: Abbott Recall of Some Ellipse Defibrillators Categorized as Class I
August 05 2019 - 7:41PM
Dow Jones News
By Josh Beckerman
The U.S. Food and Drug Administration has identified an Abbott
Laboratories (ABT) June recall of certain Ellipse Implantable
Cardioverter Defibrillators as a Class I recall.
The FDA said 108 devices were recalled in the U.S. because of a
faulty manufacturing process that caused some wires to be partially
exposed.
Abbott isn't aware of any reports of device failure in implanted
devices, the FDA said.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
August 05, 2019 19:26 ET (23:26 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
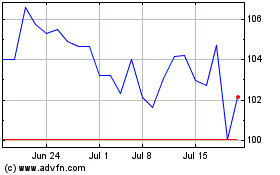
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Mar 2024 to Apr 2024
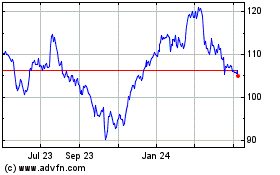
Abbott Laboratories (NYSE:ABT)
Historical Stock Chart
From Apr 2023 to Apr 2024