false 0001061027 0001061027 2023-10-04 2023-10-04
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 4, 2023
VIRACTA THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
000-51531 |
|
94-3295878 |
(State or Other Jurisdiction
of Incorporation) |
|
(Commission File Number) |
|
(IRS Employer
Identification No.) |
|
|
|
|
|
| 2533 S. Coast Hwy. 101, Suite 210 |
|
|
|
|
| Cardiff, California |
|
|
|
92007 |
| (Address of Principal Executive Offices) |
|
|
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (858) 400-8470
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share |
|
VIRX |
|
The NASDAQ Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
As previously announced, the Company will host a R&D Day today (October 4, 2023) highlighting, among other things, updates on Nana-val’s development programs. Also on October 4, 2023, the Company issued a press release related to the updates that will be presented at its R&D Day. The press release is attached hereto as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The Company is filing an updated corporate deck in connection with the R&D Day, which is attached hereto as Exhibit 99.2 and incorporated herein by reference.
Forward-Looking Statements
Certain of the statements made in this report are forward looking, such as those, among others, relating to the Company’s development pipeline; the details, timeline and expected progress for the Company’s ongoing trials; the expected ability of the Company to undertake certain activities and accomplish certain goals with respect to its clinical program in EBV+ lymphoma, EBV+ solid tumors, other virus-associated malignancies or its programs; expectations regarding future therapeutic and commercial potential with respect to the Company’s clinical program in EBV+ lymphoma, EBV+ solid tumors or other virus-associated malignancies; the ability of the Company to support multiple new drug application filings and approvals from the NAVAL-1 trial; the Company’s plans to meet with the FDA to discuss preliminary results from the NAVAL-1 trial, amending the NAVAL-1 protocol to add patients as necessary to enable registration and provide other program updates; the Company’s cash projections and the sufficiency of its cash and cash equivalents to fund operations into late 2024; the future availability of capital under the Company’s credit facility; the expected future milestones and key upcoming events and their significance; and other statements that are not historical facts. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. More information about the risks and uncertainties faced by the Company is contained under the caption “Risk Factors” in the Company’s Quarterly Report on Form 10-Q filed with the SEC on August 14, 2023. You are cautioned not to place undue reliance on forward-looking statements which are current only as of the date hereof. Except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
| Item 9.01. |
Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
Viracta Therapeutics, Inc. |
|
|
|
|
| Date: October 4, 2023 |
|
|
|
By: |
|
/s/ Daniel Chevallard |
|
|
|
|
|
|
Daniel Chevallard |
|
|
|
|
|
|
Chief Operating Officer and Chief Financial Officer |
Exhibit 99.1

Viracta Therapeutics to Host R&D Day Highlighting Nana-val in Epstein-Barr Virus (EBV)-Associated
Cancers
Preliminary clinical data from patients with relapsed/refractory EBV+
peripheral T-cell lymphoma in the pivotal NAVAL-1 trial demonstrated overall and complete response rates of 40%; follow-up from
the Phase 1b/2 study demonstrated median duration of response extended to 17.3 months
Additional response and durability data in
patients with relapsed/refractory EBV+ diffuse large B-cell lymphoma in the Phase 1b/2 study demonstrated sustained overall response rate of 67%; median
duration of response not yet reached, with several patients continuing on study treatment in ongoing response out to 42 months
Partial responses observed in recurrent/metastatic EBV+ nasopharyngeal carcinoma in
Phase 1b dose escalation study suggestive of dose response without any dose-limiting toxicities observed
Discussion to feature Key
Opinion Leaders Pierluigi Porcu, M.D. and Robert A. Baiocchi, M.D., Ph.D.
today at 8:00 a.m. EDT
SAN DIEGO October 4, 2023 – Viracta Therapeutics, Inc. (Nasdaq: VIRX), a clinical-stage precision oncology company focused on the treatment
and prevention of virus-associated cancers that impact patients worldwide, today announced that it plans to highlight new preliminary clinical and preclinical data from studies of nanatinostat and valganciclovir (Nana-val), its all-oral investigational therapy targeting Epstein-Barr virus (EBV)-associated cancers, during an R&D Day today, Wednesday, October 4, 2023, at 8:00 a.m. EDT.
“We are pleased by the growing clinical data that we believe underscores the therapeutic potential of Nana-val’s innovative ‘Kick and
Kill’ approach to target EBV-positive cancer cells and address the adverse survival outcomes seen with most EBV-associated cancers,” said Mark Rothera,
President and Chief Executive Officer of Viracta. “The clinical responses and favorable safety profile observed in multiple relapsed or refractory EBV-positive lymphoma patient populations continue to be
encouraging. New Stage 1 clinical data from patients in the PTCL cohort of the NAVAL-1 trial demonstrated preliminary overall and complete response rates of 40%, which are consistent with our previous Phase
1b/2 study data. Importantly, the combination of response rates and duration of response observed to date in these studies exceeds the current standard of care in this relapsed/refractory patient population. We are on track to complete Stage 2 of
the PTCL cohort, targeting to engage with FDA in 2024 on additional requirements for a potential accelerated approval. In addition, we are excited about the emerging signal of dose response in patients with recurrent or metastatic EBV-positive nasopharyngeal carcinoma, now with responses observed at the higher dose levels without dose-limiting toxicities. We look forward to the evaluation of our novel
go-forward dosing regimen in patients with advanced EBV-positive solid tumors based on compelling preclinical data.”
The R&D Day will feature presentations by members of Viracta’s senior management team focusing on its highest priority EBV+ lymphoma indications in the pivotal NAVAL-1 trial, namely, peripheral T-cell lymphoma (PTCL) and diffuse large B-cell lymphoma (DLBCL), as well as its advanced EBV+ solid tumor program in patients with recurrent or metastatic (R/M) EBV+ nasopharyngeal carcinoma (NPC). In addition, the R&D Day will feature presentations by expert key opinion leaders who will discuss the high unmet medical needs of
EBV-associated lymphomas.
External speakers will include:
| |
• |
|
Pierluigi Porcu, M.D., Professor of Medical Oncology, Director of the Division of Hematologic Malignancies and
Hematopoietic Stem Cell Transplantation, Department of Medical Oncology at Thomas Jefferson University |
Page | 1
| |
• |
|
Robert A. Baiocchi, M.D., Ph.D., Professor of Internal Medicine, Associate Director for Translational and
Clinical Science in the Division of Hematology at The Ohio State University |
Key R&D Day Topics and Highlights
Initial preliminary data from the pivotal NAVAL-1 clinical trial of Nana-val in patients with relapsed or refractory
(R/R) EBV+ lymphoma
| |
• |
|
As of the data cutoff date of June 30, 2023, initial results from the first five patients with R/R EBV+ PTCL treated with Nana-val showed an overall response rate (ORR) and complete response rate (CRR) of 40%. |
| |
• |
|
The EBV+ PTCL cohort met the efficacy threshold for
expansion into Stage 2 of the study, which was based upon having achieved two objective responses within the first five of 10 patients to be enrolled in Stage 1 of the study. |
| |
• |
|
Median duration of response (DoR) has not yet been reached. |
| |
• |
|
Anticipated upcoming 2024 milestones: |
| |
• |
|
Completion of enrollment into Stage 2 of the R/R EBV+ PTCL
cohort, |
| |
• |
|
Engagement with FDA on additional requirements for accelerated approval, |
| |
• |
|
Presentation of Stage 2 data. |
Additional response and durability assessments from the Phase 1b/2 trial (Study 201) of Nana-val in patients with R/R EBV+ lymphoma as of the May 4, 2023 data cutoff date
| |
• |
|
Median DoR for patients with R/R EBV+ PTCL was 17.3 months
with an ORR/CRR of 50%/38% (n=8). |
| |
• |
|
In patients with R/R EBV+ DLBCL, additional response
assessments from a formulation pharmacokinetics bridging substudy included two additional responders, one complete response (CR) and one partial response (PR), resulting in an ORR/CRR of 67%/33% (n=9). |
| |
• |
|
Median DoR in the R/R EBV+ DLBCL cohort has not yet been
reached, with three patients remaining in response and on continued study treatment with DoRs of 11.1 months (CR), 36.8 months (PR), and 41.9 months (CR). |
| |
• |
|
Additional follow-up further demonstrated that Nana-val was generally
well tolerated with manageable, if not reversible, low-grade toxicities; the most commonly observed treatment-emergent adverse events were hematologic or gastrointestinal in nature as well as low-grade creatinine elevations. |
New interim clinical data in Phase 1b/2 study of Nana-val in
advanced EBV+ solid tumors (Study 301) highlight the opportunity to dose escalate further with an innovative dosing regimen supported by new preclinical data to potentially drive additional
responses in this patient population
| |
• |
|
Enrollment completed through the fifth dose level of the Phase 1b dose escalation portion of the trial without
any dose-limiting toxicities reported. |
| |
• |
|
Best responses to date included two PRs (one ongoing for more than seven months) at the higher dose levels plus
five stable diseases in 17 patients with R/M EBV+ NPC. |
| |
• |
|
In a preclinical murine EBV+ gastric cancer xenograft model,
split daily Nana-val dosing had superior anti-tumor activity than intermittent (four days on/three days off) once-daily dosing, which supports the evaluation of this split daily dosing (SDD) regimen in patients with advanced EBV+ solid tumors. |
| |
• |
|
Anticipated upcoming 2024 milestones: |
| |
• |
|
Up to three additional dose levels are planned with Nana-val on an SDD schedule to select a recommended Phase 2
dose, |
| |
• |
|
Initiation of the clinical trial’s randomized Phase 2 expansion cohort designed to evaluate Nana-val at the
RP2D with or without pembrolizumab in patients with R/M EBV+ NPC, |
Page | 2
| |
• |
|
Initiation of the clinical trial’s exploratory Phase 1b expansion cohort designed to evaluate Nana-val at
the RP2D in patients with other advanced EBV+ solid tumors, including gastric carcinoma, leiomyosarcoma, and lymphoepithelioma. |
R&D Day Webcast Information
A live video webcast of
the presentation will be available on the Investors section of the Viracta website under “Events and Webcasts”. A replay of the presentation will be available approximately one hour after the presentation and will be archived and
available for at least 30 days following the event at the same location.
About NAVAL-1
NAVAL-1 (NCT05011058) is a global, multicenter, clinical trial of Nana-val in patients with relapsed or refractory
(R/R) Epstein-Barr virus-positive (EBV+) lymphoma. This trial employs a Simon two-stage design where, in Stage 1, participants are enrolled into one of
three prioritized indication cohorts based on EBV+ lymphoma subtype. If a pre-specified antitumor activity threshold is reached within a lymphoma subtype in
Stage 1 (n=10), then additional patients will be enrolled in Stage 2 for a total of 21 patients. EBV+ lymphoma subtypes demonstrating promising antitumor activity in Stage 2 may be further
expanded following discussion with regulators to potentially support registration.
About the Phase 1b/2 Study of Nana-val in R/M EBV+ NPC and Other EBV+ Solid Tumors
This Phase 1b/2
trial (NCT05166577) is an open-label, multinational clinical trial evaluating Nana-val alone and in combination with pembrolizumab. The Phase 1b dose escalation part is designed to evaluate safety and to determine the recommended Phase 2 dose (RP2D)
of Nana-val in patients with recurrent or metastatic (R/M) Epstein-Barr virus-positive (EBV+) nasopharyngeal carcinoma (NPC). In Phase 2, up to 60 patients with R/M EBV+ NPC will be randomized to receive Nana-val at the RP2D with or without pembrolizumab to further evaluate antitumor activity, safety and tolerability, pharmacokinetics, and potential pharmacodynamic
biomarkers. Additionally, patients with other advanced EBV+ solid tumors will be enrolled to receive Nana-val at the RP2D in a Phase 1b dose expansion cohort.
About Nana-val (Nanatinostat and Valganciclovir)
Nanatinostat is an orally available histone deacetylase (HDAC) inhibitor being developed by Viracta. Nanatinostat is selective for specific isoforms of
Class I HDACs, which are key to inducing viral genes that are epigenetically silenced in Epstein-Barr virus (EBV)-associated malignancies. Nanatinostat is currently being investigated in combination with the antiviral agent valganciclovir as an
all-oral combination therapy, Nana-val, in various subtypes of EBV-associated malignancies. Ongoing trials include a pivotal, global, multicenter, open-label Phase 2
basket trial in multiple subtypes of relapsed or refractory (R/R) EBV+ lymphoma (NAVAL-1) as well as a multinational Phase 1b/2 clinical trial in patients
with recurrent or metastatic (R/M) EBV+ NPC and other EBV+ solid tumors.
About EBV-Associated Cancers
Approximately 90% of the world’s adult population is infected with EBV. Infections are commonly asymptomatic or associated with mononucleosis. Following
infection, the virus remains latent in a small subset of cells for the duration of the patient’s life. Cells containing latent virus are increasingly susceptible to malignant transformation. Patients who are immunocompromised are at an
increased risk of developing EBV-positive (EBV+) lymphomas. EBV is estimated to be associated with approximately 2% of the global cancer burden including
lymphoma, nasopharyngeal carcinoma (NPC), and gastric cancer.
Page | 3
About Viracta Therapeutics, Inc.
Viracta is a clinical-stage precision oncology company focused on the treatment and prevention of virus-associated cancers that impact patients worldwide.
Viracta’s lead product candidate is an all-oral combination therapy of its proprietary investigational drug, nanatinostat, and the antiviral agent valganciclovir (collectively referred to as Nana-val).
Nana-val is currently being evaluated in multiple ongoing clinical trials, including a pivotal, global, multicenter, open-label Phase 2 basket trial for the treatment of multiple subtypes of relapsed or refractory (R/R) Epstein-Barr virus-positive
(EBV+) lymphoma (NAVAL-1), as well as a multinational, open-label Phase 1b/2 clinical trial for the treatment of patients with recurrent or metastatic (R/M)
EBV+ nasopharyngeal carcinoma (NPC) and other advanced EBV+ solid tumors. Viracta is also pursuing the application of its “Kick and
Kill” approach in other virus-related cancers.
For additional information, please visit www.viracta.com.
Forward-Looking Statements
This communication contains
“forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, without limitation, statements regarding: the details, timeline and expected progress for Viracta’s ongoing and
anticipated clinical trials and updates regarding the same, the Company’s expectations of the significance and implications of the preliminary interim data from its clinical trials and preclinical studies disclosed herein, the Company’s
expectations related to the FDA submission process and timelines and expectations regarding our target patient populations. Risks and uncertainties related to Viracta that may cause actual results to differ materially from those expressed or implied
in any forward-looking statement include, but are not limited to: Viracta’s ability to successfully enroll patients in and complete its ongoing and planned clinical trials; Viracta’s plans to develop and commercialize its product
candidates, including all oral combinations of nanatinostat and valganciclovir; the timing of initiation of Viracta’s planned clinical trials; the timing of the availability of data from Viracta’s clinical trials; previous preclinical and
clinical results may not be predictive of future clinical results; the timing of any planned investigational new drug application or new drug application; Viracta’s plans to research, develop, and commercialize its current and future product
candidates; the clinical utility, potential benefits, and market acceptance of Viracta’s product candidates and Viracta’s ability to manufacture or supply nanatinostat, valganciclovir, and pembrolizumab for clinical testing.
If any of these risks materialize or underlying assumptions prove incorrect, actual results could differ materially from the results implied by these
forward-looking statements. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and
elsewhere in Viracta’s reports and other documents that Viracta has filed, or will file, with the SEC from time to time and available at www.sec.gov.
The forward-looking statements included in this communication are made only as of the date hereof. Viracta assumes no obligation and does not intend to update
these forward-looking statements, except as required by law or applicable regulation.
Investor Relations Contact:
Ashleigh Barreto
Head of Investor Relations & Corporate
Communications
Viracta Therapeutics, Inc.
abarreto@viracta.com
SOURCE Viracta Therapeutics, Inc.
Page | 4

Exhibit 99.2 Viracta Therapeutics, Inc. October 2023

Forward Looking Statements This communication contains forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995, which are based on current expectations, estimates and projections based on information currently available to management of Viracta Therapeutics, Inc.
(“Viracta” or the “Company”), including, without limitation, statements regarding: Viracta’s development pipeline; the details, timeline and expected progress for Viracta’s ongoing trials; the expected ability of
Viracta to undertake certain activities and accomplish certain goals with respect to its + + clinical program in EBV lymphoma, EBV solid tumors, other virus-associated malignancies or its programs; expectations regarding future + + therapeutic and
commercial potential with respect to Viracta’s clinical program in EBV lymphoma, EBV solid tumors or other virus-associated malignancies; the ability of Viracta to support multiple new drug application filings and approvals from the NAVAL-1
trial; Viracta’s plans to meet with the FDA to discuss preliminary results from the NAVAL-1 trial, amending the NAVAL-1 protocol to add patients as necessary to enable registration and provide other program updates; Viracta's cash projections
and the sufficiency of its cash and cash equivalents to fund operations into late 2024; the future availability of capital under Viracta’s credit facility; the expected future milestones and key upcoming events and their significance; and
other statements that are not historical facts. Risks and uncertainties related to Viracta that may cause actual results to differ materially from those expressed or implied in any forward-looking statement include, but are not limited to:
Viracta’s ability to successfully enroll patients in and complete its ongoing and planned clinical trials; Viracta's plans to develop and commercialize its product candidates, including all oral combinations of nanatinostat and valganciclovir;
the timing of initiation of Viracta's planned clinical trials; the timing of the availability of data from Viracta's clinical trials; the possibility that previous preclinical and clinical results may not be predictive of future clinical results;
the timing of any planned investigational new drug application or new drug application; Viracta's plans to research, develop and commercialize its current and future product candidates; the clinical utility, potential benefits and market acceptance
of Viracta's product candidates; Viracta's ability to identify additional products or product candidates with significant commercial potential; developments and projections relating to Viracta's competitors and its industry; the impact of government
laws and regulations; Viracta's ability to protect its intellectual property position; and Viracta's estimates regarding future expenses, capital requirements and need for additional financing. These risks and uncertainties may be amplified by the
COVID-19 pandemic, which has caused significant economic uncertainty. If any of these risks materialize or underlying assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements.
Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” and elsewhere in Viracta’s
most recent filings with the SEC and any subsequent reports on Form 10-K, Form 10-Q or Form 8-K filed with the SEC from time to time and available at www.sec.gov. The forward-looking statements included in this communication are made only as of the
date hereof. Viracta assumes no obligation and does not intend to update these 2 forward-looking statements, except as required by law or applicable regulation.

Experienced Leadership Team Mark Rothera Daniel Chevallard, CPA Darrel
P. Cohen, MD, PhD Chief Operating Officer & Chief Financial President and Chief Executive Chief Medical Officer Officer Officer Patric Nelson, MBA Ayman Elguindy, PhD Senior VP, Business Dev. & Chief Scientific Officer Corporate Strategy
(~25 yrs. studying the role of viruses in cancer) 3

A Clinical-Stage Precision Oncology Company Focused on the Treatment and
Prevention of Virus-Associated Cancers that Impact Patients Worldwide Adverse survival outcomes are seen with most Epstein-Barr virus (EBV)-associated cancers; need for targeted therapies EBV-associated cancer incidence >300,000 per annum (~2% of
global cancer burden) + Nana-val, an all-oral combination approach targeting EBV cancers with potential tumor agnostic MOA Novel “Kick & Kill” Mechanism of Action + + Pivotal NAVAL-1 study in multiple R/R EBV lymphoma subtypes; R/R
EBV PTCL cohort advancing to Stage 2 Completion of Stage 2 enrollment in the PTCL cohort and engagement with FDA on additional requirements for regulatory approval anticipated in 2024 + Phase 1b/2 study in advanced EBV solid tumors; evaluating new
dosing regimen based on compelling preclinical data Emerging evidence of dose response at higher doses with no dose-limiting toxicities observed to date Strong balance sheet with cash runway into late 2024 and IP out to 2041 Lean operating model,
efficient use of capital; multiple ODDs issued in US and EU MOA: Mechanism of action, R/R: Relapsed or Refractory, PTCL: Peripheral T-cell lymphoma, DLBCL: Diffuse large B-cell lymphoma, PTLD: Post-transplant lymphoproliferative Disorders, ODD:
Orphan drug 4 designation

Epstein-Barr Virus (EBV): A High Global Cancer Priority + EBV
malignancies account for ~2% of all new cancer cases globally 1, 2,3 EBV positivity, by lymphoma subtype 4 EBV positivity, by solid tumor subtype * Peripheral T-cell lymphoma (PTCL) 40-65% Nasopharyngeal carcinoma (NPC) 75-95% Diffuse large B-cell
lymphoma (DLBCL) 5-15% Gastric cancer (GC) 8-10% Post-transplant lymphoproliferative 60-80% disorders (PTLD) ~90% of the adult population Latency confers resistance to >300,000 new cases/year Responsible for + are infected with EBV anti-viral
therapies and of EBV lymphomas and solid ~180,000 cancer 5 5 facilitates evasion of immune tumors deaths/year detection The incidence of EBV-associated cancers is likely greater, impacting more cancer types 1 2 Haverkos BM, et al. Int J Cancer.
2017; 140:1899-1906; Dupuis J et al. Blood. 2006;108:4163–9; Swerdlow SH et al. (2017) WHO classification of Tumours of the Haematopoietic and Lymphoid Tissues; 3 4 5 EBV positivity varies by geography; Wong Y, et al. Journal of Cancer
Research and Clinical Oncology (2022) 148:31–46 Exp. Therapeutic Med. 15: 3687, 2018; Kahn,G, et al. BMJ 10:1136, 2020. *Includes 5 Peripheral T-cell lymphoma, NOS and Angioimmunoblastic T-cell lymphoma

Viracta is Developing a Precision Medicine to Treat Unique Subsets of +
EBV Lymphoma with Adverse Survival Outcomes Currently limited or no targeted therapy options for EBV-associated cancers 2 1 Diffuse Large B-cell Lymphoma Peripheral T-cell Lymphoma (Progression-Free Survival) (Overall Survival) Addressing patient
populations with high unmet medical need + + PTCL EBV Rate: 40-65% DLBCL EBV Rate: 5-15% 6 1 2 Haverkos BM, et al. Int J Cancer. 2017; 140:1899-1906; Dupuis J et al. Blood. 2006;108:4163–9, Lu TX et al. Sci Rep. 2015;5:1-14

+ Nana-val: a Unique Approach to Targeting and Killing EBV Cancer Cells
+ Nanatinostat sensitizes EBV tumors to the cytotoxic effects of ganciclovir LATENCY THE KICK THE KILL EBV is latent in cancer cells. Nanatinostat potently induces Activated GCV inhibits DNA Valganciclovir, antiviral & cytotoxic pro- expression
of EBV protein kinase replication leading to apoptosis of + drug of ganciclovir (GCV), is inactive in (PK), which activates GCV into its EBV cancer cells the absence of EBV protein kinase (PK) cytotoxic form cancer cells 7

Nana-val + R/R EBV Lymphoma Program

+ Nana-val Study “201”: Phase 1b/2 Trial in R/R EBV Lymphoma
+ § Open-label, dose escalation study of Nana-val in patients with R/R EBV lymphoma + § Eligibility: R/R EBV lymphoma (any histology), ≥1 prior therapy with no curative options per Investigator § Endpoints: Response rate (ORR by
PET-CT; Lugano 2014), response duration, safety, clinical benefit rate (CBR) § Enrollment: n=55 patients, Phase 1b (n=25): Determined recommended Phase 2 dose (RP2D); Phase 2: Expansion at the RP2D (n=30) § Recommended Phase 2 Dose: §
nanatinostat capsule 20 mg orally daily, 4 days/week + valganciclovir 900 mg orally daily § Published in Blood Advances (2023), a journal of the American Society of Hematology § Completed enrollment in a tablet formulation bridging
sub-study (n=9) in 2022 for use in NAVAL-1 and Study 301 • PK consistent between capsule and tablet; improved blend uniformity and stability with tablet • Enables optimized formulation for future commercialization (potential Fixed Dose
Combination tablet) • Potential for incremental IP 9 R/R: Relapsed or Refractory, ORR: Overall Response Rate,

Extended Follow-up and Additional Patients Continues to Build Upon
Previously Published Clinical Outcomes Data Heavily pretreated patient population with all major subtypes of aggressive lymphomas represented Key Subtype Data All Subtypes* PTCL DLBCL PTLD Study 201 Capsule + Study 201 Capsule + Study 201 Capsule +
Study 201 Capsule + 2 2 Tablet Data Tablet Data Tablet Data Tablet Data (n=64) (n=13) (n=10) (n=5) 1 1 1 1 Evaluable Patients Evaluable Patients Evaluable Patients Evaluable Patients (n=50) (n=8) (n=9) (n=3) Response ORR 19 (38%) 4 (50%) 6 (67%) 1
(33%) CR 9 (18%) 3 (38%) 3 (33%) 1 (33%) PR 10 (20%) 1 3 0 SD 8 1 1 1** PD 23 3 2 1 Clinical Benefit Rate 27 (54%) 5 (63%) 7 (78%) 1 (33%) Median duration of response out to ~42 months and ongoing (as of May 4, 2023) 1 2 Data cutoff: May 4, 2023,
Evaluable patients: EBER-ISH+ with ≥1 post-treatment response assessment, Data submitted to HEMO 2023, *Lymphoma subtypes included: Cutaneous T-cell lymphoma, HIV, Other B-NHL, Extranodal NK/T-Cell Lymphoma, Peripheral T-cell lymphoma,
Angioimmunoblastic T-cell lymphoma, Immunodeficiency-associated lymphoproliferative disorders, Diffuse large B-cell lymphoma, **SD 10 was < 6 months ORR: Overall Response Rate, CR: Complete Response, PR: Partial Response, SD: Stable Disease, PD:
Progressive Disease, Clinical Benefit Rate = CR + PR + SD ≥ 6 months

Expanded and Extended Safety Data Demonstrated Nana-val Regimen was
Generally Well-Tolerated Treatment-Emergent Adverse Events Treatment-Emergent Serious Adverse Events Occurred in 23 of 64 (36%) Patients Reported in >16 (>25%) Patients Study 201 Capsule + Tablet (N=64) § Treatment-emergent serious
adverse events occurring in more than 1 patient (n=2 each): Any G3 G4 · febrile neutropenia · atrial fibrillation Thrombocytopenia 27 (42%) 8 (13%) 6 (10%) · sepsis · pneumonia (pneumonia and viral pneumonia) Neutropenia 25 (39%)
10 (16%) 11 (17%) · dyspnea Nausea 25 (39%) 2 (3%) 0· acute kidney injury · pyrexia Anemia 24 (38%) 12 (19%) 1 (2%) Fatigue 22 (34%) 4 (6%) 0 § There were no study treatment-related deaths Constipation 19 (30%) 1 (2%) 0 Diarrhea
19 (30%) 1 (2%) 0 Creatinine Increased 17 (27%) 1 (2%) 0 Safety profile suggests potential for combining with other chemo- and/or immunotherapies 11 Data cutoff date: May 4, 2023

+ NAVAL-1: Pivotal Phase 2 Trial in R/R EBV Lymphomas Global study,
with an adaptive Simon 2-stage design, focused on the largest EBV-positive lymphoma patient populations, with high unmet medical need and positive Study 201 clinical data + EBV PTCL cohort achieved ORR threshold in Q2 2023 to advance into Stage 2
Stage 2 (n=11) Potential Stage 2 Patient population: Stage 1 (n=10) (Stage 1 + Stage 2 = 21) Expansion + • R/R EBV lymphoma with ≥2 prior + EBV PTCL (2L) therapies and no curative options Nana-val arm, advancing to Stage 2 •
(≥1 prior therapy for PTCL) + EBV PTCL (2L) Expand lymphoma Nstat monotherapy arm, • Further expansion of closing at 10 patients subtype(s) from Primary endpoint: promising lymphoma Stage 1 meeting • Objective response rate (ORR)
by subtype(s) with ORR threshold + EBV DLBCL (3L) additional patients independent central review may support • Potential to further expand registration + EBV PTLD (3L) indications with promising antitumor activity after Stage 2 Other 12 R/R:
Relapsed or Refractory; 2L Second-Line; 3L:Third-Line

+ R/R EBV PTCL: T-cell lymphoma with high unmet medical need

* PTCL: Patient Journey and Treatment Options are Suboptimal No
established second-line treatment for PTCL 1 1L Patients R/R PTCL Patients Key Considerations § PTCL is highly aggressive with limited No Current SoC treatment options • 5-year survival rate: Nana-val o PTCL, NOS ~25% overall (2L Initial
positioning) + o EBV PTCL ~11% Diagnosis § No current standard of care (SoC) for Combination Salvage Chemotherapy R/R PTCL Chemotherapy § Single agent For non-HCT candidates, chemotherapy is 1L, combination e.g. CHOP, CHOEP Combination
regimen regimens preferred § In R/R patients, single-agent chemotherapy is preferred to limit Other agents toxicity HDAC inhibitors § In R/R patients, other agents may be CD30 Antibody used guided by the subtype of PTCL and their toxicity
profile Clinical trial 1 14 *Transplant Ineligible; Trinity market research; HCT: Hematopoietic cell transplant; HDAC: Histone deacetylase

+ EBV PTCL: Initial Data from NAVAL-1 is Consistent with Study 201
Complete responses achieved ORR threshold to advance PTCL indication into Stage 2 New New Study 201 Capsule NAVAL-1 1 + Tablet Data (n=5*) (n=13) 2 Evaluable Patients ITT (n=8) (n=5) Response ORR 4 (50%) 2 (40%) CR 3 (38%) 2 (40%) PR 1 0 SD 1 0 PD 3
2 Clinical Benefit Rate 5 (63%) 2 (40%) Data Cutoff May 4, 2023 June 30, 2023 § Median duration of response (DoR) for Study 201 is 17.3 months as of May 4, 2023 • Median DoR not yet reached in NAVAL-1 1 2 Data submitted to HEMO 2023;
Evaluable patients: EBER-ISH+ with ≥1 post-treatment response assessment; *One patient withdrew from study due to an AE, PTCL includes: Peripheral T-cell lymphoma and 15 Angioimmunoblastic T-cell lymphoma, ITT: Intention-to-Treat, ORR: Overall
Response Rate, CR: Complete Response, PR: Partial Response, SD: Stable Disease, PD: Progressive Disease, Clinical Benefit Rate = CR + PR + SD ≥ 6 months

+ Nana-val is Well Positioned for Potential Accelerated Approval in R/R
EBV PTCL Anticipate engagement with FDA in 2024 to align on accelerated registration pathway + Accelerated Approval Criteria Nana-val: R/R EBV PTCL Program + Unmet medical need population No approved therapies for R/R EBV PTCL Rarity of the serious
life-threatening disease without alternate + EBV PTCL 5-year survival rate of ~11%* available treatment options Magnitude of the response rate observed ORR of 30% - 45%+ Duration of response (DoR) 17.3 months median DoR observed in Phase 1b/2 study
Favorability of the safety profile Generally well-tolerated + Base Case Assumption: ~60-90 total R/R EBV PTCL patients may be required in the NAVAL-1 trial for potential accelerated approval 16 *Trinity market research

+ EBV DLBCL: A distinct and unique subtype

+ - EBV DLBCL Has a Significantly Worse Prognosis Compared to EBV DLBCL
Recognized as a unique subtype of DLBCL with its own classification by the World Health Organization § DLBCL is the most common lymphoma (~25% of all NHLs) • ~5-15% of DLBCL cases are associated with EBV • 5-year relative survival
rate of ~64% overall Diffuse Large B-cell Lymphoma • Poor survival in R/R disease, current treatments offer modest response in 3L (Progression-Free Survival) + § EBV DLBCL is a clinically more aggressive subtype of DLBCL - •
Survival rate is significantly less compared to EBV disease • Poor response/survival with standard immuno-chemotherapy • Associated with distinct biologic features and mutational landscape + • Currently, no approved treatment
options specifically targeting EBV DLBCL 18 NHL: Non-Hodgkin's lymphoma, Source: Charles River Associates Primary Research 2023

+ R/R EBV DLBCL Lymphoma: Expanded Clinical Response Data Early data
suggests Nana-val delivers a compelling combination of ORR and DoR New New Study 201 Capsule 1 + Tablet Data (n=10) 2 Evaluable Patients (n=9) Response ORR 6 (67%) CR 3 (33%) PR 3 SD 1 PD 2 Clinical Benefit Rate 7 (78%) Data Cutoff May 4, 2023
§ Median Duration of Response (DoR) not yet reached § 3 responding patients remain on study treatment with DoR times of ~11 months (CR), ~37 months (PR), and ~42 months (CR) (as of May 2023) 1 2 Data cutoff: May 4, 2023, Data submitted to
HEMO 2023; Evaluable patients: EBER-ISH+ with ≥1 post-treatment response assessment; ORR: Overall Response Rate, CR: Complete Response, PR: Partial 19 Response, SD: Stable Disease, PD: Progressive Disease, Clinical Benefit Rate = CR + PR + SD
≥ 6 months

DLBCL Treatment Landscape Emerging data and high unmet medical need
could enable earlier-line positioning 1L Patients 2L R/R DLBCL Patients 3L R/R DLBCL Patients Antibody-based therapy Nana-val (3L Initial positioning) Chemotherapy Diagnosis Chemotherapy +/- Gemcitabine-based regimen Antibody-based therapy
radiotherapy Platinum-based regimen e.g. R-CHOP Chemotherapy CAR-T Clinical trial HCT 20 HCT: Hematopoietic cell transplant, Source: Charles River Associates Primary Research 2023

+ EBV PTLD: Post-transplant complication highly associated with
EBV

2 Main Risk Factors for Developing PTLD: EBV Status and
Immunosuppression Patients with these risk factors are the most likely to receive regular EBV monitoring EBV Status Immunosuppression (IS) § EBV-negative patients are at the highest risk § Patients with higher levels of IS are at higher
risk of developing PTLD due to introduction of EBV of developing EBV viremia and PTLD due to from donor decreased T-cell number / function § Higher incidence of PTLD in EBV-naïve children; the risk of PTLD among pediatric Factors which
drive requirements for greater IS: transplant patients is greater than in adults Transplant type (e.g., solid HLA mismatch / unrelated organ vs. stem cell) donor + § ~ 60-80% of PTLD is EBV , and the disease typically develops in <1 year
Previous cancer treatment Younger recipient age 22 Source: Charles River Associates Primary Research 2023

+ R/R EBV PTLD Lymphoma: Initial Clinical Response Data Promising early
signal from Phase 1b/2 study Study 201 Capsule Study 201 Capsule + 1 Data Tablet Data (n=4) (n=5) 2 2 Evaluable Patients Evaluable Patients (n=3) (n=3) Response ORR 1 (33%) 1 (33%) CR 1 (33%) 1 (33%) PR 0 0 SD 0 1* PD 2 1 Clinical Benefit 1 (33%) 1
(33%) Rate Data Cutoff October 28, 2021 May 4, 2023 § Duration of Response of CR was ~6 months as of May 4, 2023 1 2 Data published in Blood Advances 2023; Evaluable patients: EBER-ISH+ with ≥1 post-treatment response assessment; *SD was
< 6 months; ORR: Overall Response Rate, CR: Complete Response, PR: 23 Partial Response, SD: Stable Disease, PD: Progressive Disease, Clinical Benefit Rate = CR + PR + SD ≥ 6 months

R/R PTLD Patients Generally Enrolled in Clinical Trials or Receive
Intensive Chemotherapy Regimens PTLD Treatment Algorithm (R/R, transplant ineligible) Insights Reduce immunosuppression § Initial approach to treating PTLD is EBV PCR remains elevated lowering the dose of immunosuppression Rituximab §
Rituximab-based regimens are 1L systemic therapy § Response rates in R/R patient Chemotherapy population is very poor, 2L+ patients CHOP, CHOEP are difficult to treat § After progression, patients receive chemotherapy, but risk of causing
Nana-val transplant failure must be balanced (3L Initial positioning) § Potential opportunity for Nana-val Clinical trial Tabelecleucel in 2L, particularly in post-HCT setting 24 HCT: Hematopoietic cell transplant, Source: Charles River
Associates Primary Research 2023 2L 3L 1L

Nana-val: Lymphoma Market Opportunity

+ US Incidence Estimates for EBV Hematological Malignancies Incidence
and % EBV Positivity by Lymphoma Subtype Annual EBV Subtype R/R Total (newly diagnosed) Positivity Peripheral T-cell ~1,100 ~3,600 40%-65% ~2,600 lymphoma (PTCL)* Diffuse large B-cell ~13,800 ~41,500 5%-15% ~27,700 lymphoma (DLBCL) PTLD ~1,300 ~700
~2,000 60%-80% We believe the diagnosed incidence of EBV-associated hematological malignancies is likely understated, given inconsistent testing due to the absence of a targeted and actionable therapy 26 Tessellon epi estimates 2022: US. *PTCL
includes AITL and PTCL,NOS

+ Global Incidence Estimates for Priority EBV Hematological
Malignancies + Nana-val has the potential to address other EBV hematological malignancies Incidence and % EBV Positivity by Lymphoma Subtype Annual EBV Subtype R/R Total (newly diagnosed) Positivity Peripheral T-cell ~15,200 ~6,300 ~21,500 40%-65%
lymphoma (PTCL)* Diffuse large B-cell ~113,000 ~56,000 ~169,000 5%-15% lymphoma (DLBCL) PTLD ~9,100 ~4,600 ~13,700 60%-80% We believe the diagnosed incidence of EBV-associated hematological malignancies is likely underestimated, given inconsistent
testing due to the absence of a targeted and actionable therapy 27 Source: Tessellon epi estimates 2022: US, Canada, EU5, Benelux, Brazil, China, South Korea, Indonesia, Taiwan, Singapore, Thailand *PTCL includes AITL and PTCL,NOS

Summary of Key Growth Drivers for Nana-val in Lymphoma High unmet need,
targeted treatment, compelling efficacy and duration data will drive strong value PTCL DLBCL PTLD + No standard of care for second line Separate EBV WHO classification Pricing Considerations Low 5-year survival (11%) Low 5-year survival Ebvallo may
set a high price (Unmet Need) Strong preliminary Nana-val data Strong preliminary Nana-val data Line of Therapy Potential 2L à 1L Potential 3L à 2L Potential 3L à 2L DoR >12 months considered clinically meaningful** Current
therapies: 4-8 months** Duration of Response DoR for Nana-val not yet calculated, multiple DoR TBD, early data encouraging (DOR) patients still on long-term therapy (ranging from 11- Nana-val: 17.3 months* 42 months)* Market Penetration Effective,
well tolerated, targeted, easy to use product (outpatient oral therapy). Can drive high penetration EBV Testing Rate ++ ++++ ++++ Today** Opportunity to drive awareness Viracta’s clinical development footprint is designed to support global
registration and market access 28 *Submitted to HEMO 2023, Juliana Pereira, **Charles River Associates Primary Research 2023

Nana-val: + EBV Solid Tumor Program

+ Nana-val Study “301”: Phase 1b/2 Trial in Advanced EBV
Solid Tumors Open-label, multicenter study to evaluate the safety, tolerability, PK, and preliminary antitumor activity + of Nana-val in patients with advanced EBV solid tumors Endpoints: Phase 1b dose escalation + EBV Recurrent/Metastatic NPC
§ Primary: • Phase 1b: Incidence of dose-limiting toxicities • Phase 2: Overall response rate by RECIST v1.1 Establish Phase 2 Dose § Key Secondary: • Incidence and severity of AEs • Duration of response Phase 2
• Progression-free survival Phase 1b dose expansion + + EBV R/M NPC • Other EBV solid tumors Pharmacokinetic parameters (gastric cancer, lymphoepithelioma, N = 60 patients leiomyosarcoma) 1:1 randomization N ≤ 10 patients Nana-val
± anti-PD-1 Nana-val NCT05166577 30 RECIST: Response Evaluation Criteria in Solid Tumors; AE: Adverse Event

+ R/M EBV NPC Responses to Date and Phase 1b Dose Escalation Schedule
Emerging evidence of dose response at higher doses suggesting promise of split dosing approach New Dose Nstat Oral Dose Best VGCV Oral Dose N Level (Days 1-4/wk) Additional Response responses at 30 mg split dose of Nstat 5 20 mg / 10 mg split dose
900 mg BID x 21 d, then QD 4 4 10 mg split dose 900 mg BID x 21 d, then QD 3 First response at 3 40 mg QD 900 mg QD 3 NE 40 mg Nstat 900 mg QD 2 30 mg QD 4 1 20 mg QD 900 mg QD 3 Started at RP2D + for R/R EBV lymphoma Partial responses observed at
Dose Level 3 and Dose Level 5* • Partial Response Dose Level 3 response is ongoing and durable, >6.9 months on treatment** • Stable Disease No dose-limiting toxicities reported across 5 dose levels (n=17 patients) • Progressive
Disease 31 *Dose Level 5 awaiting confirmation, **As of July 28, 2023, NE: Not evaluable for response, R/M: Recurrent/Metastatic, R/R: Relapsed or Refractory; QD: Once a day; BID: Twice a day

Nana-val has been Generally Well-Tolerated at all Dose Levels
Preliminary safety data support continued dose escalation to determine RP2D New Treatment-Related Adverse Events in ≥3 Patients Dose Level 1 = RP2D for R/R Lymphoma Safety: Dose Level Dose Level Dose Level Dose Level Dose Level 1 2 3 4 5 No
dose-limiting toxicities • (n=3) (n=4) (n=3) (n=3) (n=4) reported G1-2 G3-4 G1-2 G3-4 G1-2 G3-4 G1-2 G3-4 G1-2 G3-4 Majority of treatment- • Nausea 1 2 2 1 1 related adverse events Decreased appetite 1 1 1 2 2 were mild to moderate in
severity Creatinine 1 2 2 increased Fatigue 1 2 1 1 Anemia 1 1 1 Lymphopenia 1 1 1 Vomiting 2 1 32 Submitted to ESMO Asia 2023; RP2D: Recommended Phase 2 Dose; R/R: Relapsed or Refractory

New Eight Hours Exposure to Nanatinostat Required for EBV Lytic
Reactivation in Solid Tumors Expression of EBV protein kinase (BGLF4) was markedly higher in animals treated with split dose Nstat Single Dose e.g., 40 mg Vehicle Split Dose e.g., 20+20 mg Single Threshold for EBV lytic Dose reactivation
Nanatinostat by Nstat Split Dose 0 4 8 Nanatinostat Time (h) 33 Data on file Nstat Concentration (mg/day)

New Split Dosing Augments the Anti-Tumor Activity of Nana-val in
Xenograft Model Anti-tumor activity of SNU-719 subcutaneous xenograft model in B-NDG mice 1400 1200 Vehicle 1000 Group 1 Vehicle 800 Group 2 Ganciclovir Ganciclovir, 600 **** p = 0.0001 50ul/mice Group 5 400 Nanatinostat (20 mg QD) + Nstat+GCV
Ganciclovir ,20mg/kg 200 +50ul/mice Group 6 0 Nanatinostat (10 mg split) Nstat+GCV 0 5 10 15 20 25 30 + Ganciclovir ,10mg/kg Days after the start of treatment +50ul/mice 34 Data on file Tumor Volume (mm3)

New Dosing Schedule of 4 Days ON/3 Days OFF Allows Solid Tumor Regrowth
but Daily Dosing Renders Potent Anti-Tumor Activity in Xenograft Model 4 Days ON/3 Days OFF Daily Dosing 35 Data on file

Rationale for Split Daily Dosing (SDD) of Nanatinostat in Combination
with Valganciclovir Compelling preclinical data provides supporting evidence to evaluate a new dosing regimen Split dose Split dose (2-4 hours apart) increases expression of EBV protein Significantly increased the anti-tumor activity of Nana-val in
+ kinase, BGLF4 murine EBV gastric cancer xenograft model Higher doses Daily dosing Safety data suggest patients with NPC can withstand higher Enables increased anti-tumor activity relative to 4 days on doses of nanatinostat compared to lymphoma
patients 3 days off New SDD of Nanatinostat offers a potential to extend Nana-val patent portfolio with differentiated strategy from lymphoma; US provisional application(s) have been filed 36
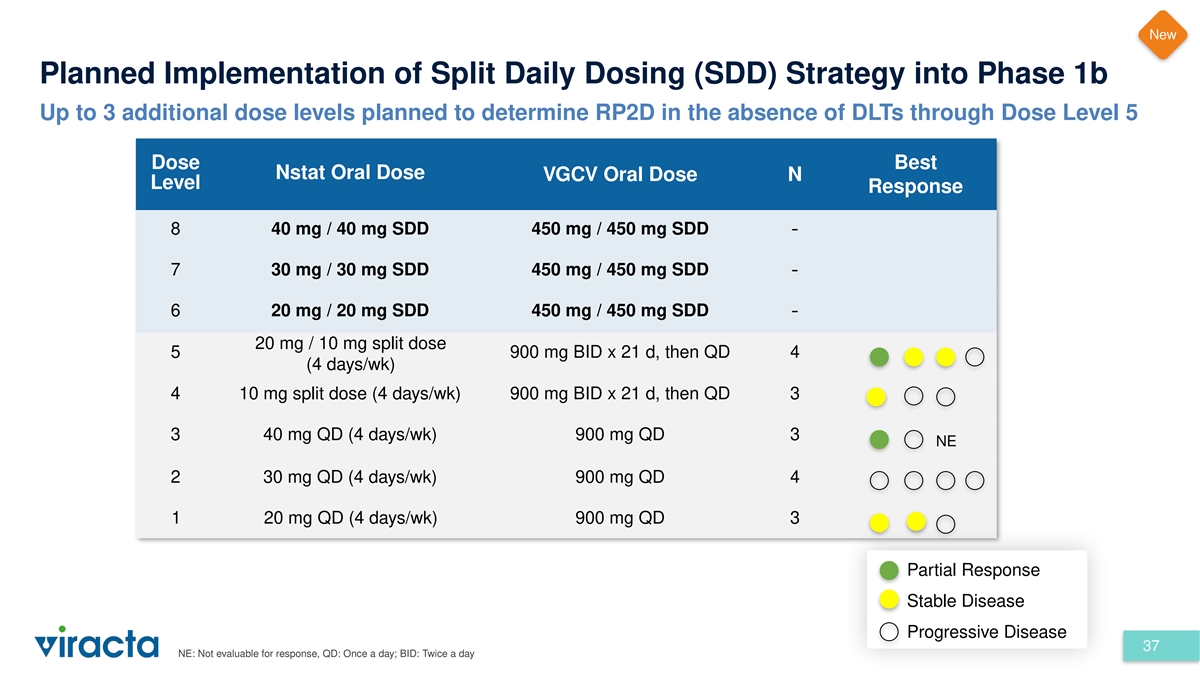
New Planned Implementation of Split Daily Dosing (SDD) Strategy into
Phase 1b Up to 3 additional dose levels planned to determine RP2D in the absence of DLTs through Dose Level 5 Dose Best Nstat Oral Dose VGCV Oral Dose N Level Response 8 40 mg / 40 mg SDD 450 mg / 450 mg SDD - 7 30 mg / 30 mg SDD 450 mg / 450 mg SDD
- 6 20 mg / 20 mg SDD 450 mg / 450 mg SDD - 20 mg / 10 mg split dose 5 900 mg BID x 21 d, then QD 4 (4 days/wk) 4 10 mg split dose (4 days/wk) 900 mg BID x 21 d, then QD 3 3 40 mg QD (4 days/wk) 900 mg QD 3 NE 2 30 mg QD (4 days/wk) 900 mg QD 4 1 20
mg QD (4 days/wk) 900 mg QD 3 Partial Response Stable Disease Progressive Disease 37 NE: Not evaluable for response, QD: Once a day; BID: Twice a day

Nana-val: Milestones & IP Portfolio

* R&D Day** Data Presentation Key Update Anticipated Program
Milestones Indication 2023 2024 2025 NAVA L-1 + PTCL Initial Stage 1 Complete Meet with FDA & Initiate Present Registration Phase (EBV ORR DATA enrollment of align on potential Registration Stage 2 DATA LPI Lymph Stage 2 AA pathway Phase oma)
DLBCL 201: Expanded Potential Advancement Present Stage 1 Complete enrollment Meet with FDA & Present Stage 2 ORR & DoR DATA into Stage 2 DATA of Stage 2 align on potential DATA AA pathway PTLD Potential Advancement Present Stage 1 Complete
enrollment Meet with FDA & Present Stage 2 into Stage 2 DATA of Stage 2 align on potential DATA AA pathway Study 301 NPC Ph1b/ Present Disclose SDD Complete Phase 1b Determine Present final Initiate Phase 2 Meet with FDA & Present
preliminary 2 preliminary dosing strategy dose escalation RP2D Phase 1b DATA (+/- anti-PD-1) align on potential Phase 2 DATA + Phase 1b DATA AA pathway (EBV Solid Tumor GC s) Initiate Exploratory Present preliminary Initiate Phase 2 Phase 1b Study
Phase 1b GC DATA Study *Placement on calendar year does not denote specific timing, **R&D Day scheduled for October 4, 2023, AA: Accelerated Approval, LPI: Last Patient In, ORR: Overall Response Rate, DoR: Duration of 39 Response, SDD: Split
Daily Dosing, RP2D: Recommended Phase 2 Dose

Nana-val “Kick & Kill” Approach Protected by an
Expanding IP Portfolio Granted patents provide protection into 2041 2000 2005 2010 2015 2020 2025 2030 2035 2040 2045 Nanatinostat Composition of Matter Expiration January 2027 US 7,932,246 (Issued) (Including Patent Term Adjustment) Methods of
Treatment with Nana-val Expiration March 2031 10,857,152 (Issued) Nana-val Dosing Schedule (Lymphoma) Expiration May 2040 US 10,953,011 (Issued) Nanatinostat Polymorph New Expiration Dec 2040 (Notice of Allowance granted in August 2023)
Next-generation formulation of Nanatinostat New Expiration Oct 2041 (Notice of Allowance granted in July 2023) Additional intellectual property (IP) protection ▪ Additional pending applications for expanded patent protection in various
methods, doses and formulations ▪ Eligible for Patent Term Extension upon approval in the US ▪ Eligible for 7 years of market exclusivity upon approval in the US based on 4 granted Orphan Drug designations ▪ Eligible for 8+2+1
years data exclusivity in Europe ▪ Eligible for 6 months pediatric exclusivity, which is stackable to all other exclusivities 40

Adverse survival outcomes are seen with many EBV-associated cancers
Need for targeted therapies Well-tolerated, all-oral combination + approach to targeting EBV cancers Potential tumor agnostic MOA; strengthened clinical data Our focus is + Pivotal NAVAL-1 study in multiple R/R EBV maximizing the lymphoma subtypes
Nana-val Completion of Stage 2 enrollment in the PTCL and opportunity engagement with FDA anticipated in 2024 + Phase 1b/2 study in advanced EBV solid tumors Emerging evidence of dose response at higher doses with no DLTs observed to date Strategy
in place to access global markets Cash runway into late 2024 41

Thank you

Appendix

Blood Advances: Supplementary Figure 1 Patient Flowchart 44 Published
in Blood Advances, 2023

Study 201: Prior Therapies in Nana-val Responders NAVAL-1 prioritized
indications Time to Response Duration of Response Best Response Histology Prior Therapy for Nana-val for Nana-val to Nana-val (Days) (Days) PTCL (AITL) CHOP; ICE CR 53 1,491 PTCL (AITL) CHOP CR 45 839 PTCL (NOS) CHOP; romidepsin CR 58 175 PTCL (NOS)
CHOEP; romidepsin; BEAM, ASCT; romidepsin, XRT PR 51 199 DLBCL R-CHOP CR 113 1,276 (ongoing) DLBCL R-CHOP CR* 110 337 (ongoing) DLBCL R-CHOP CR 54 34 DLBCL R-EPOCH; XRT; R-GDP; PBR PR 57 1,121 (ongoing) DLBCL R-CHOP PR 162 122 DLBCL R-CHOP PR* 58 57
PTLD Rituximab CR 50 160 45 Data cutoff date: May 4, 2023, *Tablet

NAVAL-1: Enhancing Patient Enrollment § Global clinical trial
footprint established • Clinical trial sites established North America, Europe, Latin America, and Asia-Pacific • >70 sites now open; more study sites expected § Prioritization of PTCL, DLBCL and PTLD cohorts enables more
targeted engagement of sites § Medical Science Liaisons deployed in all key areas § Encouraging new data enhances investigator interest and motivation Study footprint aligns with clinical and regulatory strategy to enter US, EU, and Japan
46

v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Viracta Therapeutics (NASDAQ:VIRX)
Historical Stock Chart
From Apr 2024 to May 2024
Viracta Therapeutics (NASDAQ:VIRX)
Historical Stock Chart
From May 2023 to May 2024