UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of July 2023 (Report No. 2)
Commission file number: 001-39957
NLS PHARMACEUTICS LTD.
(Translation of registrant’s name into English)
The Circle 6
8058 Zurich, Switzerland
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual
reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form
40-F ☐
CONTENTS
On July 7, 2023, the Registrant
made available a presentation on its website. A copy of the presentation is attached hereto as Exhibit 99.1.
EXHIBIT INDEX
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
NLS Pharmaceutics Ltd. |
| |
|
|
| Date: July 20, 2023 |
By: |
/s/ Alexander Zwyer |
| |
|
Name: |
Alexander Zwyer |
| |
|
Title: |
Chief Executive Officer |
2
Exhibit 99.1

Thank you for joining. The presentation will begin shortly.

NLS Pharmaceutics Company Update July 6, 2023 Re defining Central Nervous System Therapies

This presentation contains expressed or implied forward - looking statements pursuant to U.S. Federal securities laws. For example, NLS is using forward - looking statements when it discusses its clinical trials, the expected timing of our future clinical trials; its proposed product pipeline; the receipt of the results from clinical trials and obtaining regulatory approval for our products; its planned phase 3 trial in terms of timing and format; proposed trials that may occur in the future; the potential commercialization of its product candidates; compounds or product candidates that we may seek to develop or add to our pipeline; and the potential benefits and impact our product candidates could have on improving patient health care. These forward - looking statements and their implications are based on the current expectations of the management of NLS only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward - looking statements: changes in technology and market requirements; NLS may encounter delays or obstacles in launching and/or successfully completing its clinical trials; NLS' products may not be approved by regulatory agencies, NLS' technology may not be validated as it progresses further and its methods may not be accepted by the scientific community; NLS may be unable to retain or attract key employees whose knowledge is essential to the development of its products; unforeseen scientific difficulties may develop with NLS' process; NLS' products may wind up being more expensive than it anticipates; results in the laboratory may not translate to equally good results in real clinical settings; results of preclinical studies may not correlate with the results of human clinical trials; NLS' patents may not be sufficient; NLS' products may harm recipients; changes in legislation may adversely impact NLS; inability to timely develop and introduce new technologies, products and applications; and loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of NLS to differ materially from those contemplated in such forward - looking statements. Except as otherwise required by law, NLS undertakes no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. More detailed information about the risks and uncertainties affecting NLS is contained under the heading "Risk Factors" in NLS' annual report on Form 20 - F for the year ended December 31, 2022 filed with the Securities and Exchange Commission (SEC), which is available on the SEC's website, https://pr.report/CQyiRWYb" www.sec.gov, and in subsequent filings made by NLS with the SEC. Our logo and some of our trademarks and tradenames are used or incorporated by reference in this presentation. This presentation also includes trademarks, tradenames and service marks that are the property of other organizations. Solely for convenience, trademarks, tradenames and service marks referred to in this presentation may appear without the ®, TM and SM symbols, but those references are not intended to indicate in any way that we will not assert to the fullest extent under applicable law our rights or the rights of the applicable licensor to these trademarks, tradenames and service marks. We obtained the statistical data, market data and other industry data and forecasts described by reference in this presentation from market research, publicly available information and industry publications. Industry publications generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy and completeness of the information. Similarly, while we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data, and we do not make any representation as to the accuracy of the information. As used in this presentation, “the Company,” “we” and “our” refer to NLS Pharmaceutics Ltd. Forward - looking Statement

Alex Zwyer, MBA Chief Executive Officer & Co - Founder

VISION Awaken a brighter future for patients by overcoming rare and complex CNS diseases MISSION To develop better therapies to safeguard and empower the brain throughout all stages of life VALUES Put Patients First Think Inquisitive & Disruptively Act with Integrity & Scientific Honesty Collaborate Pragmatically & Respectfully

2023 NLS Strategic Priorities Focus on Rare and Complex CNS Disorders • Leading edge science • Seasoned team with track record of success in CNS • Multiple shots on goal • Flexible & nimble business model Develop Mazindol • Initiate Phase 3 program for treatment in adult patients with narcolepsy • Expedited approval pathway • Promising data across multiple CNS indications Advance Pipeline • Progress NLS - 4 for the treatment of chronic fatigue and other hypersomnolence disorders • Progress portfolio of compounds for further study in rare CNS Disorders

Marianne Lambertson, MSc, MSW Corporate Communications and Investor Relations Harvey Kushner Biostatistics Tom Curatolo, MBA US Commercial Operations Beat Widler, PhD Quality Hervé Girsault, MBA Business Development & Licensing Christina Allgeier US GAAP Controller Pelin Vatanacan Finance Operations Jennifer Franco Scientific Excellence Jeff Bernier Clinical Operations Sharon Tan, MBA Program Management & Strategy Bao Le, MS Regulatory Affairs Experienced Team Assembled Andreas Fischer Supply Chain Sam Salamone, MS, MBA, RAC CMC Elena Thyen Finance, Controlling, and Human Resources Jim Herman Toxicology Alex Zwyer, MBA Chief Executive Officer Co - Founder George Apostal, MD, MS Chief Medical Officer Eric Konofal, MD, PhD Chief Scientific Officer Co - Founder Keith Dewedoff Chief Financial Officer

NLS Pipeline Overview Phase 3 Phase 2 IND enabling Preclinical Compound Indication Therapeutic Area Mazindol ER Narcolepsy NT1 Narcolepsy NT2 Idiopathic Hypersomnia Sleep Disorders NLS - 4 ⇞ Idiopathic Hypersomnia NLS - 11* Kleine - Levin Syndrome NLS - 4 ⇞ CFS/ME Long COVID CNS/Immunology NLS - 8* Alzheimer’s Disease Neurodegenerative Diseases NLS - 12* Lewy Body Disease NLS - 1 ADHD Opioid Use Disorder Psychiatry/ Addiction NLS - 3 ADHD Autism Spectrum Disorder Substance Use Disorder Over 100 patents in over 140 countries including technology, mechanism of action and application for: • Sleep Disorders including Narcolepsy, Idiopathic Hypersomnia, and Kleine Levin Syndrome • ADHD • Neurodegenerative diseases with Lewy Body Disease, Parkinson’s Disease and/or Alzheimer's Disease • CNS diseases treatment associated with sleep disorders July 2023 *Patents published but not granted ⇞ New Chemical Entity

NT1 IH Cataplexy Refreshing naps Disrupted sleep Sleep paralysis Sleep - related hallucinations No cataplexy Excessive daytime sleepiness (e.g., ESS >10) MSLT sleep latency <8 min Sleep inertia May have long sleep (> 11 h/day) May remit spontaneously Circadian dysrhythmia NT1, Narcolepsy type - 1; NT2, Narcolepsy type - 2; IH, Idiopathic Hypersomnia; KLS, Kleine - Levin Syndrome; CSF, Cerebrospinal Fluid; ESS, Epworth Sleepiness Scale; MSLT, multiple sleep latency test; PSG, polysomnography; REM, rapid eye movement; SOREM, Sleep Onset Rapid Eye Movement;; CDH, Central Disorders of Hypersomnolence. 9 Altered perception NT2 KLS MAZINDOL NLS - 3 Unrefreshing naps Cognitive impairment Ultradian dysrhythmia May have long sleep (several weeks with >18h/day) Eating disorders (anorexia or hyperphagia) Disinhibited behaviors (e.g., hypersexuality) NLS - 4 NLS - 11 Central Disorders of Hypersomnolence (CDH)

George Apostol, MD, MS Chief Medical Officer

Multifaceted Impact on Patients • Worsened HRQoL • Impaired social life and isolation • Memory Problems • Impaired critical thinking Sleep Disruption Excessive Daytime Sleepiness Cataplexy Hypnogogic Hallucinations Sleep Paralysis • Increase risk of accidents while driving • Poor work/academic performance • Strain on relationships • Brain Fog “Living with narcolepsy is a daily struggle” “I never feel like a normal person” “I started withdrawing from social situations, I stopped doing things one by one, until I found myself at home alone not doing much of anything.”

The Narcolepsy Patient Journey Increasing Prevalence In 2022, it was estimated that ~165 K people had narcolepsy in the U.S., likely growing to ~224 K by 2035 Median time to diagnosis 22 months • More than 50% of patients experience a delay >1 year between report of symptoms and diagnosis • Nearly 20% had a delay >5 years Sources Carter et al. (2014) Patients' Journeys to a Narcolepsy Diagnosis: A Physician Survey and Retrospective Chart Review LifeSci Consulting Market Research 2022 Bogan,R., Thorpy, M., & Weaver, T. (2022) Achieving Optimal Outcomes in Patients with Narcolepsy: Aligning Treatment Goals with Patients 2018. National Know Narcolepsy Survey. https://knownarcolepsy.com/hcp/impact Misdiagnosis is reported in more than half of patients (59.9%) • Depression 31.3% • Insomnia 17.9% • Obstructive Sleep Apnea 13.1% • EDS (not narcolepsy) 12.7% • ADHS 6.0% Majority of patients (66.8%) saw multiple health care providers Onerous diagnostic testing • Overnight PSG 82% • Full Day MSLT 75% Limited Treatment Options Often Polytherapy with Safety Considerations and Drug - Drug Interactions • Modafinil • Stimulants • Xyrem/Xywav • Wakix Unmet Needs in Treatment • Only 12% think their narcolepsy symptoms are completely or mostly under control • 93% expressed frustration with current treatment options • 94% stated that new treatment options are needed

Currently FDA Approved Treatment Safety Considerations Common AE’s Dose MOA Medicine May reduce effectiveness of oral contraceptives; may increase HR and BP Anxiety, back pain, diarrhea, dizziness, dyspepsia, headache, insomnia, and nausea 100 - 400mg/ 50 - 250mg Dopamine reuptake inhibitor Modafinil/ Armodafinil High potential for misuse; serious cardiovascular events Dry mouth, upset stomach, loss of appetite, weight loss, headache, dizziness, tremors, tachycardia, elevated BP, insomnia, and mood changes Varies Sympathomimetic; enhance dopamine, norepinephrine, serotonin Amphetamines/ Methylphenidate Precautions regarding BP & HR Anxiety, decreased appetite, headache, insomnia, and nausea 75 - 150 mg Dopamine - norepinephrine reuptake inhibitor Solriamfetol May reduce effectiveness of oral contraceptives; may increase QTc Anxiety, insomnia, and nausea 8.9 - 35.6mg Histamine H3 antagonist/inverse agonist Pitolisant SXB contraindicated in patients at risk for CVD events Anxiety, decreased appetite, diarrhea, dizziness, headache, hyperhidrosis, parasomnia, and vomiting 4.5 - 9.0g (twice nightly dosing) GABA B agonist Sodium Oxibate (SXB/LXB)

• Narcolepsy is a chronic neurodegenerative disease caused by a deficiency of orexin - producing neurons in the lateral hypothalamus 1 • No commercially available products target this immune - mediated neuronal destruction; most patients remain unsatisfied with their treatment 2 1. www.ncbi.nlm.nih.gov/pmc/articles/PMC6492289/ 2. 2018 National Know Narcolepsy Survey. https://knownarcolepsy.com/hcp/impact Narcolepsy Debilitating Disorder; Large and Underserved Population 3 Million people suffer worldwide Source: www.narcolepsynetwork.org

• Long history of the active ingredient (mazindol) used to treat narcolepsy (“Compassionate Use” Programs) • Novel dual mechanism of action with partial Orexin - 2 receptor activation & triple monoamine reuptake inhibition • Positive Phase 2 results: met most endpoints with high statistical significance and favorable safety / tolerability • Once - daily formulation • Quick Onset of Action • Low drug - drug interaction potential • Low abuse potential: Schedule IV • Potential as monotherapy treatment of narcolepsy Mazindol ER dual SNDRI/OX2R partial agonist

Mazindol: History & Mechanism of Action Wakefulness Release of Dopamine Nucleus accumbens Tuberomammilary nucleus (histamine) Ventral tegmental area (dopamine) Raphe nuclei serotonin Locus coeruleus norepinephrine • Mazindol has been commercialized in the US and in Europe during the 1970’s - 2000’s as an appetite suppressant under the brand name Sanorex® • For decades, it was used off - label in Europe for treatment of narcolepsy with very good efficacy and safety profile • The presumed mechanism of action is triple monoamine reuptake inhibition (dopamine, serotonin, norepinephrine) • Subsequently it was discovered that it also has orexin - 2 receptor (OX 2 R) partial agonist activity • Currently investigated by NLS Pharmaceutics as once - daily treatment for narcolepsy • Results from the Phase 2 clinical trial are presented in the subsequent slides Hypocretin/Orexin (hypothalamus)
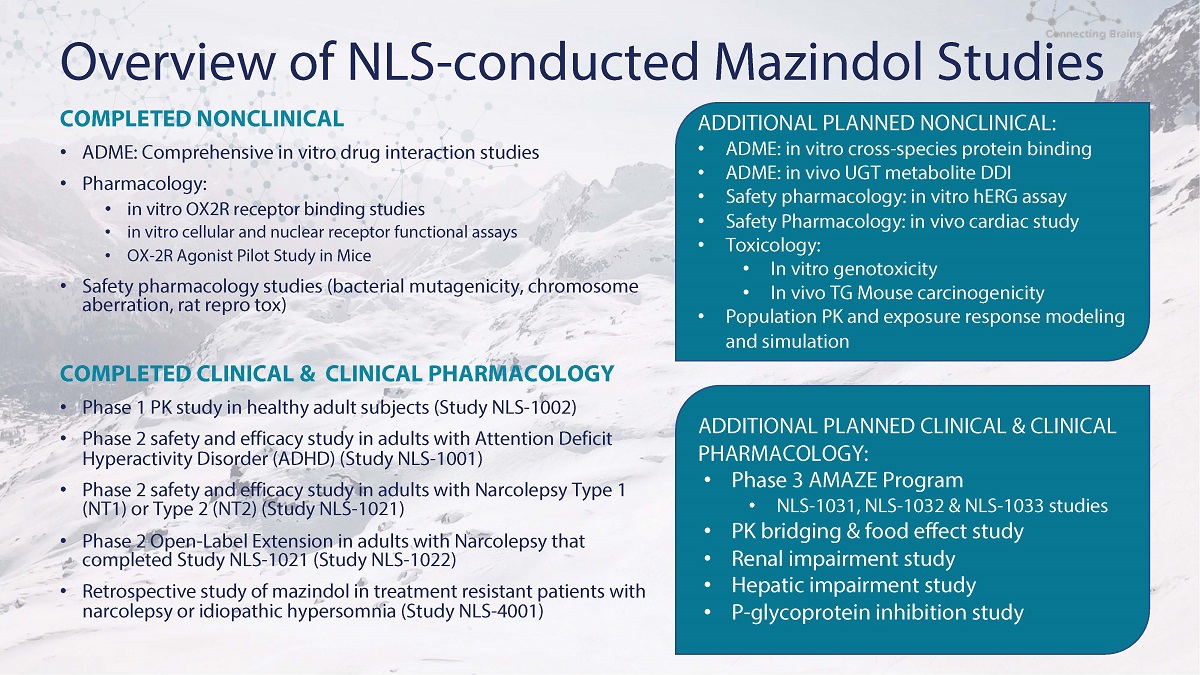
Overview of NLS - conducted Mazindol Studies COMPLETED NONCLINICAL • ADME: Comprehensive in vitro drug interaction studies • Pharmacology: • in vitro OX2R receptor binding studies • in vitro cellular and nuclear receptor functional assays • OX - 2R Agonist Pilot Study in Mice • Safety pharmacology studies (bacterial mutagenicity, chromosome aberration, rat repro tox) COMPLETED CLINICAL & CLINICAL PHARMACOLOGY • Phase 1 PK study in healthy adult subjects (Study NLS - 1002) • Phase 2 safety and efficacy study in adults with Attention Deficit Hyperactivity Disorder (ADHD) (Study NLS - 1001) • Phase 2 safety and efficacy study in adults with Narcolepsy Type 1 (NT1) or Type 2 (NT2) (Study NLS - 1021) • Phase 2 Open - Label Extension in adults with Narcolepsy that completed Study NLS - 1021 (Study NLS - 1022) • Retrospective study of mazindol in treatment resistant patients with narcolepsy or idiopathic hypersomnia (Study NLS - 4001) ADDITIONAL PLANNED NONCLINICAL: • ADME: in vitro cross - species protein binding • ADME: in vivo UGT metabolite DDI • Safety pharmacology: in vitro hERG assay • Safety Pharmacology: in vivo cardiac study • Toxicology: • In vitro genotoxicity • In vivo TG Mouse carcinogenicity • Population PK and exposure response modeling and simulation ADDITIONAL PLANNED CLINICAL & CLINICAL PHARMACOLOGY: • Phase 3 AMAZE Program • NLS - 1031, NLS - 1032 & NLS - 1033 studies • PK bridging & food effect study • Renal impairment study • Hepatic impairment study • P - glycoprotein inhibition study

POLARIS: Mazindol ER Development Program in Narcolepsy The Mazindol ER development program POLARIS consisted of two US clinical trials: • NLS - 1021 Phase 2, A four - week double - blind, placebo controlled, randomized, US multi - center study of Mazindol ER 3 mg once daily vs. placebo (1:1) • NLS - 1022 Open Label Extension, An Open Label Extension (OLE) Study available for individuals following completion of the four - week NLS 1021 study. This OLE study offered participants the opportunity to receive Mazindol ER once daily in the morning for up to six months.

19 Placebo Double - Blind study Open Label Extension study NLS - 1022 Reduction of Weekly Cataplexy Episodes Combined DB and OLE studies: 4 & 24 weeks Mazindol ER 2.0 0.0 4.0 6.0 8.0 10.0 12.0 14.0 16.0 18.0 DB Baseline (Day 1) DB Final (Day 28) OLE Week 4 OLE Week 8 OLE Week 12 OLE Week 16 OLE Week 20 OLE Week 24 81.9% reduction Weekly Cataplexy Episodes • Mazindol ER demonstrated rapid, consistent, and clinically meaningful efficacy in the treatment of cataplexy as well as sleepiness (ESS decreases of 44% from baseline) in patients with narcolepsy • This efficacy was noticeably perceived by both patients and clinicians on the Global Impressions scales and with majority of patients choosing to enroll in the open - label extension study • 6 - month OLE showed good subject participation (87%) and retention (71%), despite exclusion of all narcolepsy, depression, anxiolytic medications and oral contraceptives

20 Tolerability Total (n = 52) MZD MZD (n = 24) PBO MZD (n = 28) TEAE 7 (13.5%) 3 (12.5%) 4 (14.3%) Dry Mouth 5 (9.6%) 2 (8.3%) 3 (10.7%) Covid - 19 4 (7.7%) 0 4 (14.3%) Constipation 4 (7.7%) 3 (12.5%) 1 (3.6%) Urinary Tract Infection 3 (5.8%) 1 (4.2%) 2 (7.1%) Nausea Total (n = 52) MZD MZD (n = 24) PBO MZD (n = 28) Treatment Emergent Adverse Events (TEAEs) 32 (61.5%) 15 (62.5%) 17 (60.7%) TEAEs 18 (34.6%) 8 (33.3%) 10 (35.7%) Treatment - Related TEAEs 2 (3.8%) 1 (4.2%) 1 (3.6%) Severe TEAEs 2 (3.8%) 1 (4.2%) 1 (3.6%) Serious TEAEs 0 0 0 ⎼ Any Treatment - Related Serious TEAE 1 (1.9%) 0 1 (3.6%) ⎼ Hypersensitivity 1 (1.9%) 1 (4.2%) 0 ⎼ Cellulitis • Mazindol ER was well - tolerated with even the most common AEs (dry mouth, nausea, decreased appetite) being benign in nature and occurring in less than one in five patients • Slight increases in heart rate and diastolic blood pressure were in line with those previously described with stimulants in ADHD

Summary of SLEEP 2023 • Oral presentation: • Clinician and Patient Global Impression in a Phase 2 Study of Mazindol (NLS - 1021) in Adults with Narcolepsy Type 1 & Type 2 • Additional Poster Presentations: • Functional Outcomes of Sleep Questionnaire in a phase 2 study of mazindol ER in narcolepsy • Long - term efficacy on cataplexy attacks and excessive daytime sleepiness in open - label extension study (NLS - 1022) of mazindol ER • A four - week randomized, double - blind, placebo - controlled, phase 2 study of mazindol ER in the treatment of narcolepsy • Effects of NLS - 4 (Lauflumide) and modafinil in a rat model of circadian rhythm and chronic severe fatigue • Symposium: “ Mazindol ER: Pioneering the Combination of OX2R and SNDRI in the Treatment of Narcolepsy” • 100+ participants in person and online • 500+ booth contacts • 480 Narcolepsy books distributed • Multiple KOL and Patient Advocacy Organization meetings • “Sleeping Around” podcast with NLS’s CEO and CMO

Phase 3 Program NLS - 1031, NLS - 1032 and NLS - 1033

Phase 3 Narcolepsy Program Two identical placebo - controlled Phase 3 trials investigating Mazindol ER vs placebo, plus a 12 - month extension study NLS - 1033 • Open - label, one - year extension for participants completing NLS - 1031 and NLS - 1032 NLS - 1031 NLS - 1032 Summer 2023 Fall 2023 20 - 25 US clinical sites ~50 narcolepsy type 1 (NT1) participants Primary Endpoint: change in cataplexy attacks, measured via e - diary Secondary Endpoints: ESS and PROMIS - SRI

Parallel Design Studies • Population: ~50 NT1 participants treated with Mazindol ER or PBO for 8 weeks (1:1) • Dose: 3 mg once daily, no titration • Primary endpoint: Weekly Cataplexy Episodes from baseline to week 8 • An electronic diary will be completed daily for the week preceding the study visits • Excessive Daytime Sleepiness will be a secondary objective (PROMIS - SRI and ESS) • Study visits: Screening, Baseline, Weeks 1, 2, 5, 8, and Follow - up (or directly into NLS - 1033) • Designed to be participant - and site - friendly (no overnight stays, light on procedures) Placebo (NLS - 1031 and NLS - 1032 will be identical) 8 - Weeks Screening & Baseline ~50 NT1 patients R Mazindol ER 3 mg 3 - week Safety Follow - up 12 - month Open - Label Extension Study

12 - Month OLE Study (NLS - 1033) • Study Design: A 52 - week, open - label, multi - center study of Mazindol ER 3 mg once daily • Study population: ~100 participants (Completers from studies NLS - 1031 and NLS - 1032) • Rescue narcolepsy/psychiatric medications allowed in the study • Primary objective: Long - term safety and tolerability of Mazindol ER • Secondary objective: Long - term therapeutic response to Mazindol ER in the treatment of Cataplexy and EDS • Study visits: Baseline, Weeks 2, 4, 12, 26, 40, 52, Follow up NLS - 1032 52 - Week Open - Label Treatment Period Mazindol ER 3 mg 3 - week Safety Follow - up NLS - 1031 ~100 NT1 patients

Clinical Trial At - A - Glance • Who can participate? People who are aged 18 and above who have narcolepsy and are experiencing cataplexy • What medical conditions are being studied? Narcolepsy • What is the clinical trial testing? Mazindol ER, Placebo • How many participants are being enrolled? 100 • Are placebos part of the clinical trial? Yes • When is the clinical trial being conducted? July 2023 – December 2024 • How long is participation in the clinical trial? Participants will be in this study for 8 weeks with the option to participate in a 12 - month open - label extension (OLE) amaze@nls - pharma.com https://amaze.nlspharma.com/ Would you like to participate in our Phase 3 studies? Scan this QR Code :

If approved, Mazindol ER May Represent a Convenient Once - Daily Monotherapy Option for Narcolepsy Simple, ONCE - DAILY, MONOTHERAPY , 30 - minutes before breakfast Effective on BOTH Cataplexy and Excessive Sleepiness as well as other symptoms of Narcolepsy RAPID onset of action Favorable drug - drug interaction potential (e.g., contraceptives) Schedule IV: low potential for abuse, misuse & diversion Mazindol ER is an investigational agent available for clinical trial use only. 3mg QD FIRST IN CLASS , dual mechanism of action addressing the main narcolepsy symptoms Well characterized and manageable safety & tolerability profile TMRI OX2R

Alex Zwyer, MBA Chief Executive Officer & Co - Founder Tom Curatolo, MBA Head of US Commercial

*Timelines are subject to change. There is no guarantee as to the success of any clinical trial. In addition, there is inherent risk and variability in the overall regulatory process. Approval by the FDA may not be granted, or the FDA may require different study parameters from those that are intended to be included in the submission. Anticipated Development Timeline * 2026 Q2 2025 Q4 2024 Q3 2023 Q3 2022 Q2 2022 Sept. 2021 Commercial NDA Submission AMAZE Initiate AMAZE Data Readout for Interim topline Initiated Launch Completed Phase 3 Pivotal POLARIS DB results at World POLARIS (Results) Trials (FPI) Phase 2 trial Sleep 2022 Phase 2 trial 2024 2025 2026 2023 2021 2022 Q1 2023 End of Phase 2 FDA Meeting Q2 2022 Initiate Early Access IH Program

2025 2023 2024 Pre - Pivotal Data (Present through 2024) • Research: Forecasting, Market Access evaluation, Value Proposition • Advocacy Engagement: Collaborating on disease - state outreach and elevating company insight into patient unmet needs • Physician Mapping: Systematically analyzing the market to understand KOLs, Key Institutions, Large Sleep Practices, etc. • Pre - commercial Outreach: Establishing corporate reputation, early HCP engagement on MOA and Ph 2 data, Disease State campaign development, building anticipation for pivotal program • Collaboration with Medical: Delivering consistent scientific messaging, Launching MSLs, Physician and Patient conferences • Organizational and Launch Planning Message Development Branding HCP Promotional Materials Launch Anticipation Communications Market Access Pricing and Contracting Strategy Payer Value Proposition Dev. Payer Contracting Outreach Commercial Operations HCP Targeting Analysis Sales Force Configuration Sales Force Head of Sales Sales Managers Professional Sales Representatives Commercialization Plan Q3 2023 Initiate AMAZE Q4 2024 Complete AMAZE Q2 2025 Submit NDA 2026 Commercial Launch 2026 Post - Pivotal Data (2025 – Approval) Marketing Disease State Campaign In - market Timelines are subject to change. There is no guarantee as to the success of any clinical trial. In addition, there is inherent risk and variability in the overall regulatory process. Approval by the FDA may not be granted, or the FDA may require different study parameters from the those that are intended to be included in the submission.

NLS Focused Growth in 2023/24 Continue to build an organization dedicated to rare and complex central nervous system disorders Solidify opportunity for lead product Mazindol ER through rigorous Phase 3 program Realize mazindol franchise full potential across additional rare sleep disorders Progress pipeline of innovative products to meet the unmet needs and transform lives of patients with rare diseases NLS Pharmaceutics is a clinical - stage pharmaceutical company focused on the discovery and development of innovative therapies for patients with rare and complex central nervous system, or CNS, disorders, who have unmet medical needs.

Keith Dewedoff Chief Financial Officer

AGM 2023 Highlights • The Board of Directors received the highest voting approval in the Company’s history of 99.5% of votes cast in favor of the proposals • 64% percent of the shares entitled to votes were represented • Shareholders approved the financial statements, the compensation report, and the balance sheet results of the Company for the fiscal year 2022 • Shareholders also approved the total compensation budgets for NLS Pharmaceutics’ Board of Directors and Executive Management for the financial year 2024 • PricewaterhouseCoopers AG was re - elected as NLS’ independent auditors for another term lasting until the next Annual General Meeting • Election of Audrey Greenberg and Dr. Anthony Walsh to the Board of Directors

New Board Members Audrey Greenberg, MBA Co - Founder and Chief Business Officer, Center for Breakthrough Medicines Audrey is the co - founder, Executive Managing Director and Board Member of the Discovery Labs Center for Breakthrough Medicines, an integrated life science innovation hub helping to streamline the path to approval by supporting manufacturing, funding, equipment, processes, and people. Audrey serves as a subject matter expert in building regional bioinnovation hubs and has spoken at over 50 global conferences and is quoted in over 100 articles in the past two years. BSBA in Accounting & Finance from the University of Arizona and MBA from the Wharton School of the University of Pennsylvania Anthony Walsh, PhD Chief Business Officer, Ability Biologics Previous roles: RA Capital, Mission BioCapital (partner) Anthony led a number of high - profile investments in both public and private companies including Vedere Bio (acquired by Novartis in 2020 for up to $280M), QurAlis, Tune Therapeutics, Tidal Therapeutics (acquired by Sanofi in 2021 for up to $470M), Comet Therapeutics (acquired by Vectiv Bio in 2021), Biohaven Pharmaceuticals (acquired by Pfizer for $11.6B in 2022) as well as an early - stage investment in Sage Therapeutics. BA in Biochemistry from Trinity College, Dublin, and a Ph.D. in Biophysics from Oxford University.

Key Financials YE 2022 Cash And Cash Equivalents $8.9M Total OPEX FY 2022 $15.9M Shares Outstanding as of 12 / 31 / 22 32,428,893 Employees as of 6/30/23 20 Institutional Ownership as of 12/ 31 / 22 19.67% $NLSP Financials

Capital Markets Partnerships Venture Debt BD Assessing multiple paths to advance Pipeline NLSP Financial Strategy

Why invest in NLS? Focused Growth • Experienced management team across pharmaceuticals, neuroscience, R&D and capital markets with a proven track record of bringing multiple drugs to market • Multiple innovative drug programs targeting rare and complex central nervous system disorders with high unmet medical needs • Growing IP portfolio across 6 patent families to support clinical trials, M&A, and IP strategies of innovative compounds • Solid opportunity for lead product mazindol ER through rigorous Phase 3 program • Partnerships with world - class scientists and Contract Research Organizations supporting R&D programs and further validating portfolio potential

Redefining Central Nervous System Therapies

Questions for Panel

• • • • • • • •• ••• . ,; . ... • • • Nl I S P - HarmaceutiCs , · - Connecting Brains j:; ,. f

This concludes our presentation. Thank you for joining.
NLS Pharmaceutics (NASDAQ:NLSP)
Historical Stock Chart
From Apr 2024 to May 2024
NLS Pharmaceutics (NASDAQ:NLSP)
Historical Stock Chart
From May 2023 to May 2024