FALSE000171127900017112792023-07-262023-07-26
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 26, 2023
KRYSTAL BIOTECH, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | | | | |
| Delaware | | 001-38210 | | 82-1080209 |
(State or other jurisdiction
of incorporation) | | (Commission
File Number) | | (IRS Employer
Identification Number) |
2100 Wharton Street, Suite 701
Pittsburgh, Pennsylvania 15203
(Address of principal executive offices, including Zip Code)
Registrant’s telephone number, including area code: (412) 586-5830
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| | | | | |
☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| | | | | |
☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| | | | | |
☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock | | KRYS | | Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
On July 26, 2023, Krystal Biotech, Inc. (the “Company”) announced it has expanded its R&D pipeline to oncology and that the U.S. Food and Drug Administration has accepted its Investigational New Drug application of its lead oncology drug candidate, KB707 for the treatment of locally advanced or metastatic solid tumor malignancies. In addition, the Company will host an investor conference call at 8:00 a.m. ET on July 27, 2023, to discuss the KB707 program. For purposes of the call, the Company will provide an investor slide presentation (the “Investor Slide Presentation”), which is available on the “Investors” section of the Company’s website at www.krystalbio.com. Copies of the press release and the Investor Slide Presentation are attached hereto as Exhibit 99.1 and Exhibit 99.2, respectively, and are incorporated by reference herein. For those unable to listen to the live conference call, a replay will be available on the “Investors” section of the Company’s website at www.krystalbio.com.
The information contained in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 and Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, or incorporated by reference in any filing with the Securities and Exchange Commission by the Company under the Exchange Act or the Securities Act of 1933, as amended, whether made before or after the date hereof, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| | | | | | | | |
Exhibit
No. | | Description |
| |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data file (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | | | | | | | | | | | | | | | | | | | |
| Date: July 26, 2023 | | | | KRYSTAL BIOTECH, INC. |
| | | |
| | | | By: | | /s/ Krish S. Krishnan |
| | | | Name: | | Krish S. Krishnan |
| | | | Title: | | Chairman and Chief Executive Officer |
Krystal Biotech Announces Pipeline Expansion into Oncology
and FDA Acceptance of IND Application for
Lead Oncology Candidate KB707
PITTSBURGH, July 26, 2023 (GLOBE NEWSWIRE) – Krystal Biotech, Inc. (the “Company”) (NASDAQ: KRYS), a commercial-stage biotechnology company focused on the discovery, development and commercialization of genetic medicines to treat diseases with high unmet medical needs, announced today that it has expanded its R&D pipeline to oncology and that the US Food and Drug Administration (FDA) has accepted its Investigational New Drug (IND) application of its lead oncology drug candidate KB707 for the treatment of locally advanced or metastatic solid tumor malignancies. The Company will host an investor conference call and webcast, Thursday, July 27, 2023, at 8:00 am ET, to discuss the KB707 program. To join the investor conference call, please see the instructions below. The presentation for the investor conference call is attached to the Company’s Form 8-K.
“The KB707 program leverages our learnings and clinical experience in two tissue areas, the skin and the lung, and underscores the broader potential of our HSV-1 platform to deliver all types of exogenous genetic material and improve outcomes for patients with debilitating diseases,” said Krish S. Krishnan, Chairman and CEO of Krystal Biotech.
KB707 is a modified HSV-1 vector designed to deliver genes encoding both human IL-12 and IL-2 to the tumor microenvironment and promote systemic immune-mediated tumor clearance. Two formulations of KB707 are in development, a solution formulation for transcutaneous injection and an inhaled (nebulized) formulation for lung delivery.
“We believe KB707 is a unique and highly differentiated drug candidate with the potential to unlock the capabilities of cytokine-based immunotherapy,” said Suma Krishnan, President of Research & Development at Krystal Biotech. “By enabling localized and sustained cytokine expression within a treated tumor, KB707 has the potential to maximize therapeutic efficacy while avoiding the tolerability challenges of systemic cytokine treatments.”
The FDA has accepted the Company’s IND to evaluate intratumoral KB707 in patients with solid tumors accessible by transcutaneous injection, and the Company expects to initiate a Phase 1 study in the second half of 2023. The Company is planning to file an amendment to the KB707 IND in the second half of 2023 to evaluate inhaled KB707 in a clinical trial in the first half of 2024.
Interleukin-2 (IL-2) and interleukin-12 (IL-12) are secreted cytokines with complementary functions promoting cell-mediated immunity in humans. Both IL-2 and IL-12 have been shown to elicit anti-tumor immune responses in preclinical or clinical models and have been extensively studied for their potential in cancer immunotherapy. Despite promising signs of efficacy, it has proven difficult to effectively harness IL-2 and IL-12 for therapeutic benefit, as systemic administration is often poorly tolerated, and their inherently short half-lives necessitate high dose levels and extremely frequent dose intervals. KB707 leverages the Company’s modified HSV-1 vector – and its ability to efficiently deliver a durable DNA payload without active replication and minimal cytotoxicity – to drive local and sustained cytokine expression within the tumor microenvironment and maximize the therapeutic window and benefit of IL-2 and IL-12.
“There remains an urgent unmet need for new therapies in cutaneous oncology, including for patients that do not respond to current first-line options and for the many who eventually progress on available therapy,” said Jason Luke, MD, Associate Professor of Medicine in the Division of Hematology/Oncology and Director of the Cancer Immunotherapeutic Center within UPMC Hillman Cancer Center Immunology and Immunotherapy Program in Pittsburgh, PA. “As the lead investigator on multiple practice changing immunotherapy trials, I have seen first-hand the benefits that can be realized through effective immune
modulation and am excited about the potential of Krystal's approach for localized, sustained cytokine delivery.”
In preclinical studies, KB707 has been shown to efficiently transduce mammalian cells in vitro leading to the secretion of bioactive IL-2 and IL-12 and can drive localized, durable cytokine expression in mouse skin after intradermal injection. Furthermore, in stringent checkpoint inhibitor refractory ‘cold’ syngeneic mouse models, HSV-1 vector based delivery of murine equivalent IL2 and IL12 elicited robust antitumor responses and survival benefits, including via intratumoral injection in single and dual flank B16F10 melanoma models, as well as via intratracheal delivery in a metastatic K7M2 osteosarcoma model, with evidence of protection from tumor rechallenge in both models suggestive of prolonged adaptive immunity.
The intratumoral KB707 Phase 1/Opal 1 study is an open-label, multi-center, monotherapy, dose escalation and expansion study, enrolling patients with locally advanced or metastatic solid tumors, who relapsed or are refractory to standard of care, with at least one measurable and injectable tumor accessible by transcutaneous route. The primary objective of the study is to evaluate safety and tolerability of KB707. Efficacy will also be assessed by multiple measures including overall response rate, progression free survival, and overall survival, and the immune effects of KB707 monotherapy will be assessed in tumor tissue, lymph nodes, and blood.
Investor Conference Call, Webcast and Presentation Information
The Company will host an investor conference call and webcast, Thursday, July 27, at 8:00 am ET, to discuss the KB707 program.
The conference call will include management’s overview of the Company’s expanded pipeline and research and development focus in oncology and discuss potential target indications as well as a summary of preclinical data and clinical development plans. External speakers will include Samuel Broder, M.D., former Director of the National Cancer Institute where he oversaw the development of numerous anti-cancer therapeutic agents, and Jason Luke, M.D., F.A.C.P., Associate Professor of Medicine in the Division of Hematology/Oncology and Director of the Cancer Immunotherapeutic Center within UPMC Hillman Cancer Center Immunology and Immunotherapy Program in Pittsburgh, PA.
To register and join the conference call, please go to: https://www.netroadshow.com/events/login?show=6a3175e6&confId=53637
For those unable to listen to the live conference call, a replay will be available on the Investor’s section of the Company’s website at www.krystalbio.com.
About Krystal Biotech, Inc.
Krystal Biotech, Inc. (NASDAQ: KRYS) is a commercial-stage biotechnology company focused on the discovery, development and commercialization of genetic medicines to treat diseases with high unmet medical needs. VYJUVEKTM is the Company’s first commercial product, the first-ever redosable gene therapy, and the only medicine approved by the FDA for the treatment of dystrophic epidermolysis bullosa. The Company is rapidly advancing a robust preclinical and clinical pipeline of investigational genetic medicines in respiratory, oncology, dermatology, ophthalmology, and aesthetics. Krystal Biotech is headquartered in Pittsburgh, Pennsylvania. For more information, please visit http://www.krystalbio.com, and follow @KrystalBiotech on LinkedIn and Twitter.
Forward Looking Statements
Any statements in this press release about future expectations, plans and prospects for Krystal Biotech, Inc., including statements about the potential of the Company’s proprietary HSV-1 platform, the Company’s beliefs about the clinical utility of KB707 and its potential therapeutic capabilities, the Company expectations regarding the timing of a Phase 1 study of the transcutaneous injection formulation of KB707, the Company’s plans to file an amendment to the KB707 IND in the second half of 2023 to evaluate inhaled KB707 in a clinical trial in the first half of 2024, and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “likely,” “will,” “would,” “could,” “should,” “continue,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of product candidates including KB707, the sufficiency of cash resources and need for additional financing and such other important factors as are set forth under the caption “Risk Factors” in the Company’s annual and quarterly reports on file with the U.S. Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company’s views as of the date of this release. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this release.
Disclosures
Dr. Jason Luke is a consultant for Krystal Biotech, Inc.
CONTACT
Investors and Media:
Meg Dodge
Krystal Biotech
mdodge@krystalbio.com

© Copyright 2023 Krystal Biotech, Inc. All rights reserved. Research & Development Oncology Program Announcement July 2023
Krystal | 2 Speakers Suma Krishnan President, Research & Development Trevor Parry, PhD VP, Research and Scientific Affairs David Chien, MD SVP, Clinical Development – Oncology Jason J. Luke, MD, FACP • Associate Professor of Medicine in the Division of Hematology/Oncology at the University of Pittsburgh and UPMC Hillman Cancer Center • Associate Director for Clinical Research and the Director of the Immunotherapy and Drug Development Center (Phase I) at UPMC • Leading investigator in immunotherapeutics, having led trials of checkpoint inhibitors, bispecifics, metabolism modifiers, innate agonists, oncolytic viruses, and cellular therapies; over 150 publications • Leadership roles for the Melanoma Committees of ASCO, Society for Melanoma Research, AACR and Board Member at SITC Krystal R&D Leadership Samuel Broder, MD • Former Director of the National Cancer Institute where he oversaw the development of numerous anti-cancer therapeutic agents, such as TAXOL® and helped launch a number of large-scale clinical trials related to the prevention, diagnosis, and treatment of cancer, and he inaugurated the highly successful SPORE Program • Authored over 340 scientific publications and is an inventor on many patents • Elected to the National Academy of Medicine in 1991

Krystal | 3 Forward-Looking Statements and Disclosures Forward-Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties and are based on the current expectations and beliefs of Krystal Biotech, Inc. (the “Company”). Any statements in this presentation about future expectations, plans and prospects for the Company, including but not limited to statements about the Company’s technology platform; the Company’s oncology program, including the therapeutic approach, target indications for KB707, market opportunities, preclinical safety and efficacy of KB707, the design, conduct, and timeline of the planned KB707 clinical program, and the clinical utility of KB707 and expected timing of clinical updates constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Words such as “anticipate”, “believe”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict”, “project”, “target”, “potential”, “likely”, “will”, “would”, “could”, “should”, “continue” and similar expressions or the negative of these terms or other comparable terminology are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are neither forecasts, promises nor guarantees, and are based on the beliefs of the Company's management as well as assumptions made by and information currently available to the Company. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, risks and uncertainties, including: the content and timing of decisions made by regulatory authorities; the uncertainties inherent in the initiation and conduct of clinical trials; availability and timing of data from clinical trials; whether results of early clinical trials or studies in different disease indications will be indicative of the results of ongoing or future trials; uncertainties associated with regulatory review of clinical trials and applications for marketing approvals; the availability or commercial potential of product candidates; the ability to retain and hire key personnel; the sufficiency of cash resources and need for additional financing; and such other important factors as are set forth in the Company’s annual and quarterly reports and other filings on file with the U.S. Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak to the Company’s current beliefs and expectations only as of the date this presentation is given. Except as required by law, the Company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation in the event of new information, future developments or otherwise. No representation is made as to the safety or effectiveness of the product candidates for the therapeutic use for which such product candidates are being studied. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its own internal research is reliable, such research has not been verified by any independent source. Disclosures Dr. Jason Luke is a paid consultant of Krystal Biotech, Inc. The views expressed by Dr. Jason Luke in this presentation are his own views and not those of the University of Pittsburgh or UPMC.

Krystal | 4 Agenda Introduction Krish Krishnan; Chairman and CEO Krystal Oncology Program Therapeutic Approach & Target Indications Preclinical Overview Clinical Program Suma Krishnan, MS, MBA; President, Research & Development Samuel Broder, MD Trevor Parry, PhD; VP, Research and Scientific Affairs David Chien, MD; SVP, Clinical Development Lead Investigator’s Perspective Jason J. Luke, MD, FACP Q&A All Speakers Closing Krish Krishnan, Chairman and CEO

Krystal Oncology Program Therapeutic Approach & Target Indications
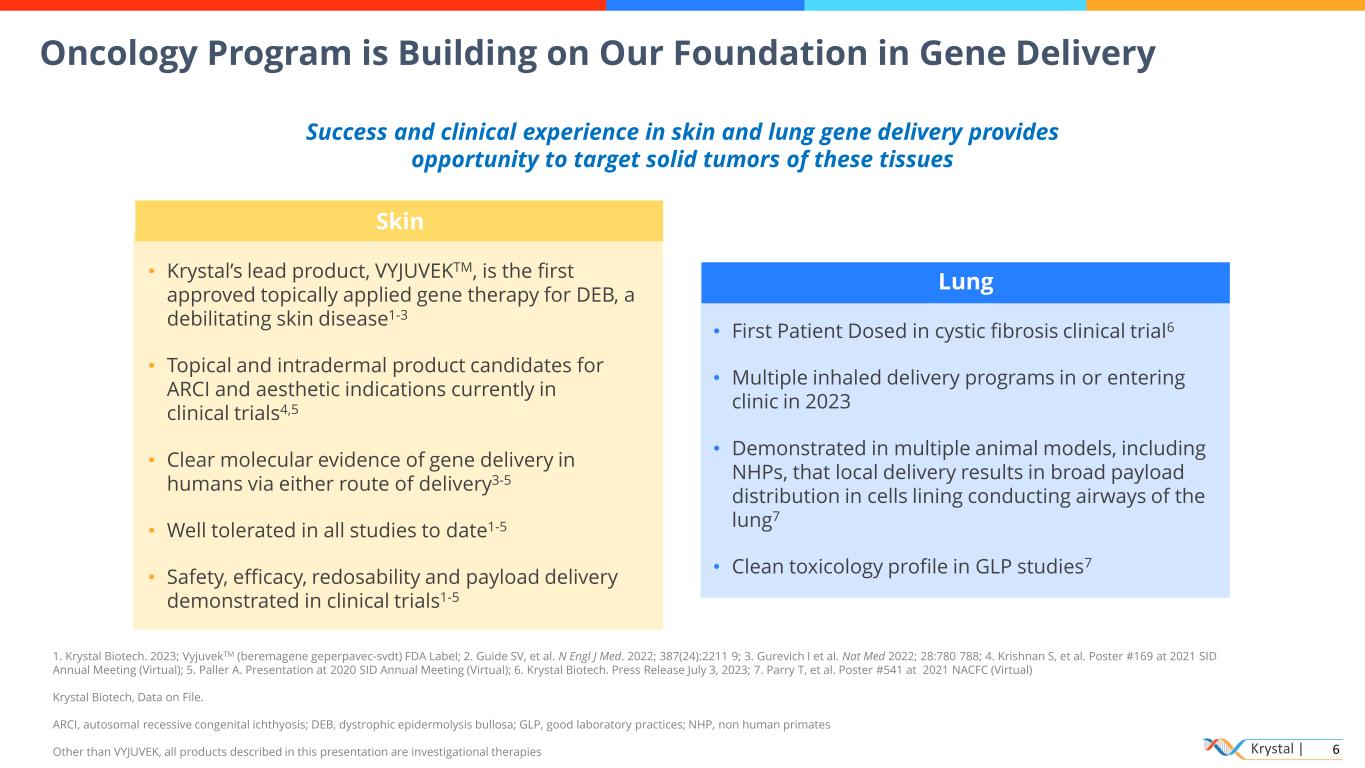
Krystal | 6 Oncology Program is Building on Our Foundation in Gene Delivery Lung • First Patient Dosed in cystic fibrosis clinical trial6 • Multiple inhaled delivery programs in or entering clinic in 2023 • Demonstrated in multiple animal models, including NHPs, that local delivery results in broad payload distribution in cells lining conducting airways of the lung7 • Clean toxicology profile in GLP studies7 Skin • Krystal’s lead product, VYJUVEKTM, is the first approved topically applied gene therapy for DEB, a debilitating skin disease1-3 • Topical and intradermal product candidates for ARCI and aesthetic indications currently in clinical trials4,5 • Clear molecular evidence of gene delivery in humans via either route of delivery3-5 • Well tolerated in all studies to date1-5 • Safety, efficacy, redosability and payload delivery demonstrated in clinical trials1-5 Success and clinical experience in skin and lung gene delivery provides opportunity to target solid tumors of these tissues 1. Krystal Biotech. 2023; VyjuvekTM (beremagene geperpavec-svdt) FDA Label; 2. Guide SV, et al. N Engl J Med. 2022; 387(24):2211 9; 3. Gurevich I et al. Nat Med 2022; 28:780 788; 4. Krishnan S, et al. Poster #169 at 2021 SID Annual Meeting (Virtual); 5. Paller A. Presentation at 2020 SID Annual Meeting (Virtual); 6. Krystal Biotech. Press Release July 3, 2023; 7. Parry T, et al. Poster #541 at 2021 NACFC (Virtual) Krystal Biotech, Data on File. ARCI, autosomal recessive congenital ichthyosis; DEB, dystrophic epidermolysis bullosa; GLP, good laboratory practices; NHP, non human primates Other than VYJUVEK, all products described in this presentation are investigational therapies

Krystal | 7 Major Unmet Needs in Checkpoint Inhibitor (CPI) Refractory Solid Tumors 1. NCI SEER. 2023; https://seer.cancer.gov/statfacts/html/common.html [accessed July 20, 2023], combined estimates for incident cases and deaths from cancers of the anus, bladder, bone and joint, brain and nervous system, breast, cervix uteri, colon and rectum, eosophagus, kidney and renal pelvis, larynx, liver and intrahepatic bile duct, lung and bronchus, melanoma, oral cavity and pharynx, ovary, pancreas, prostate, small intestine, stomach, testis, thyroid, uterus, and vulva SEER; Surveillance, Epidemiology, and End Results Program; US, United States Solid Tumor Incidence and Mortality in US 2023 SEER Estimates1 New Solid Tumor Cases Per Year 1.6M+ 450K+ Solid Tumor Deaths Per Year
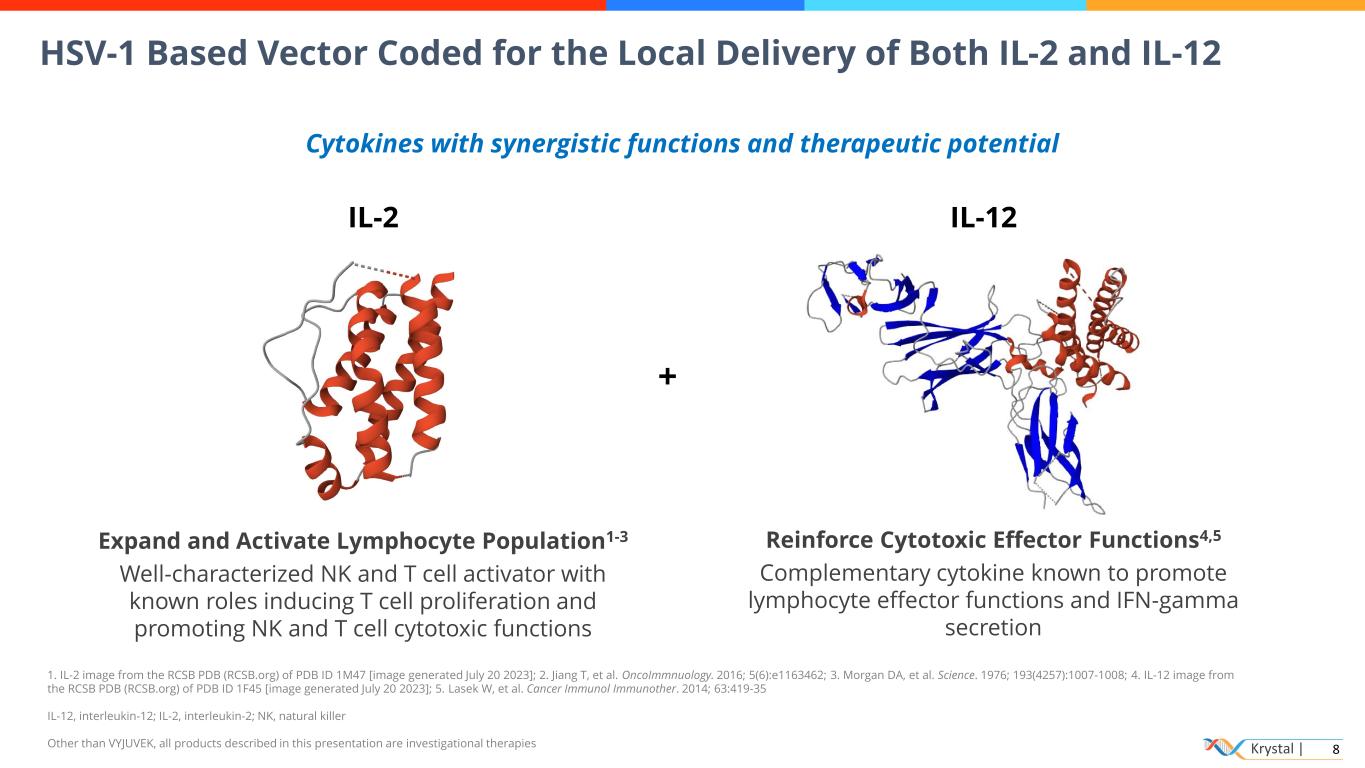
Krystal | 8 HSV-1 Based Vector Coded for the Local Delivery of Both IL-2 and IL-12 Cytokines with synergistic functions and therapeutic potential IL-2 IL-12 Reinforce Cytotoxic Effector Functions4,5 Complementary cytokine known to promote lymphocyte effector functions and IFN-gamma secretion + Expand and Activate Lymphocyte Population1-3 Well-characterized NK and T cell activator with known roles inducing T cell proliferation and promoting NK and T cell cytotoxic functions 1. IL-2 image from the RCSB PDB (RCSB.org) of PDB ID 1M47 [image generated July 20 2023]; 2. Jiang T, et al. OncoImmnuology. 2016; 5(6):e1163462; 3. Morgan DA, et al. Science. 1976; 193(4257):1007-1008; 4. IL-12 image from the RCSB PDB (RCSB.org) of PDB ID 1F45 [image generated July 20 2023]; 5. Lasek W, et al. Cancer Immunol Immunother. 2014; 63:419-35 IL-12, interleukin-12; IL-2, interleukin-2; NK, natural killer Other than VYJUVEK, all products described in this presentation are investigational therapies
Krystal | 9 Advantages of Replication-Defective HSV-1 Based Cytokine Delivery Platform well suited to accomplish dual goals of targeted but sustained delivery of IL-2 and IL-12 to the tumor Optimal vector platform to maximize cytokine expression and immune activation ✓ Efficiently transduces a wide variety of cell types maximizing reach within tumor ✓ DNA payload persists in transduced cells extending the window of cytokine expression ✓ Lack of replication avoids premature lytic cell death or host cell shutdown ✓ Redosability to further boost local cytokine expression ✓ Safety profile suitable for both inhaled or intratumoral administration Krystal Biotech, Data on File. Other than VYJUVEK, all products described in this presentation are investigational therapies
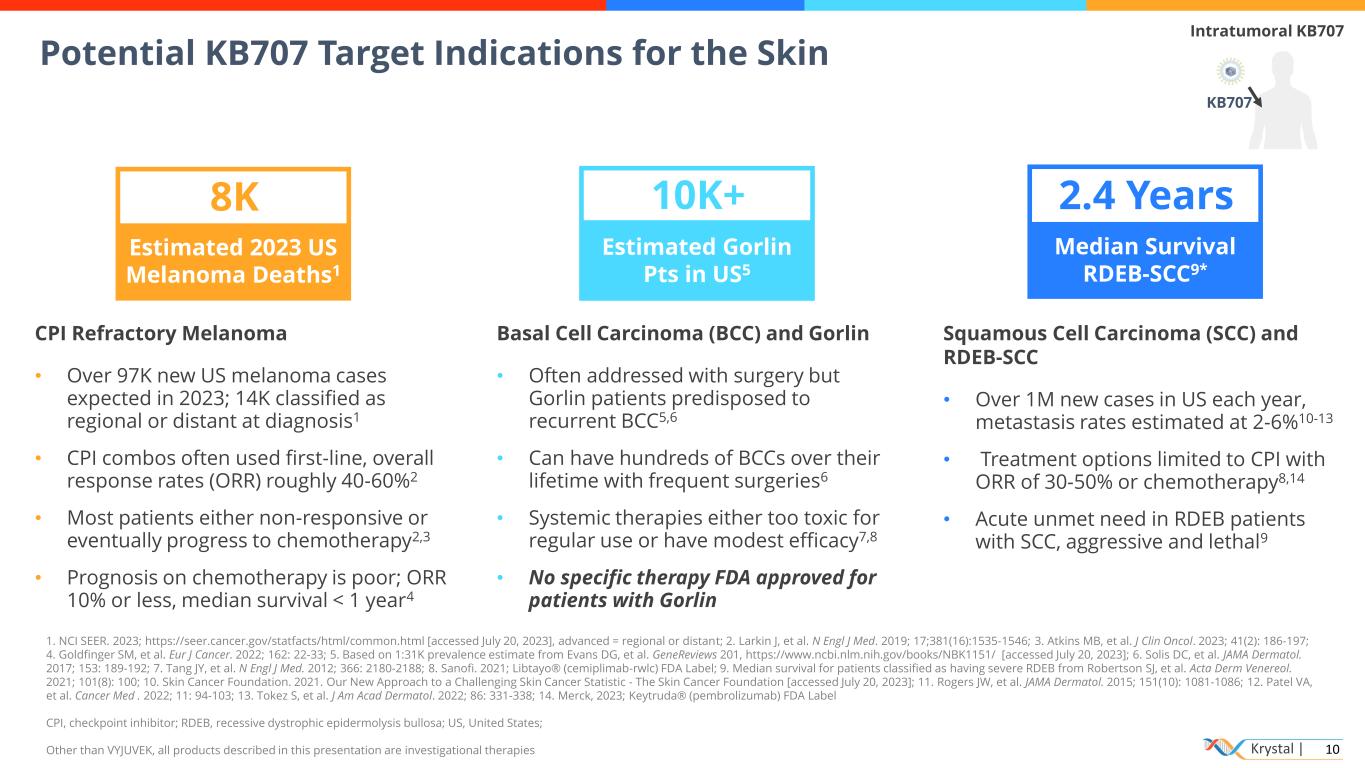
Krystal | 10 Potential KB707 Target Indications for the Skin KB707 Intratumoral KB707 Estimated 2023 US Melanoma Deaths1 8K Estimated Gorlin Pts in US5 10K+ Median Survival RDEB-SCC9* 2.4 Years CPI Refractory Melanoma • Over 97K new US melanoma cases expected in 2023; 14K classified as regional or distant at diagnosis1 • CPI combos often used first-line, overall response rates (ORR) roughly 40-60%2 • Most patients either non-responsive or eventually progress to chemotherapy2,3 • Prognosis on chemotherapy is poor; ORR 10% or less, median survival < 1 year4 Basal Cell Carcinoma (BCC) and Gorlin • Often addressed with surgery but Gorlin patients predisposed to recurrent BCC5,6 • Can have hundreds of BCCs over their lifetime with frequent surgeries6 • Systemic therapies either too toxic for regular use or have modest efficacy7,8 • No specific therapy FDA approved for patients with Gorlin Squamous Cell Carcinoma (SCC) and RDEB-SCC • Over 1M new cases in US each year, metastasis rates estimated at 2-6%10-13 • Treatment options limited to CPI with ORR of 30-50% or chemotherapy8,14 • Acute unmet need in RDEB patients with SCC, aggressive and lethal9 1. NCI SEER. 2023; https://seer.cancer.gov/statfacts/html/common.html [accessed July 20, 2023], advanced = regional or distant; 2. Larkin J, et al. N Engl J Med. 2019; 17;381(16):1535-1546; 3. Atkins MB, et al. J Clin Oncol. 2023; 41(2): 186-197; 4. Goldfinger SM, et al. Eur J Cancer. 2022; 162: 22-33; 5. Based on 1:31K prevalence estimate from Evans DG, et al. GeneReviews 201, https://www.ncbi.nlm.nih.gov/books/NBK1151/ [accessed July 20, 2023]; 6. Solis DC, et al. JAMA Dermatol. 2017; 153: 189-192; 7. Tang JY, et al. N Engl J Med. 2012; 366: 2180-2188; 8. Sanofi. 2021; Libtayo® (cemiplimab-rwlc) FDA Label; 9. Median survival for patients classified as having severe RDEB from Robertson SJ, et al. Acta Derm Venereol. 2021; 101(8): 100; 10. Skin Cancer Foundation. 2021. Our New Approach to a Challenging Skin Cancer Statistic - The Skin Cancer Foundation [accessed July 20, 2023]; 11. Rogers JW, et al. JAMA Dermatol. 2015; 151(10): 1081-1086; 12. Patel VA, et al. Cancer Med . 2022; 11: 94-103; 13. Tokez S, et al. J Am Acad Dermatol. 2022; 86: 331-338; 14. Merck, 2023; Keytruda® (pembrolizumab) FDA Label CPI, checkpoint inhibitor; RDEB, recessive dystrophic epidermolysis bullosa; US, United States; Other than VYJUVEK, all products described in this presentation are investigational therapies
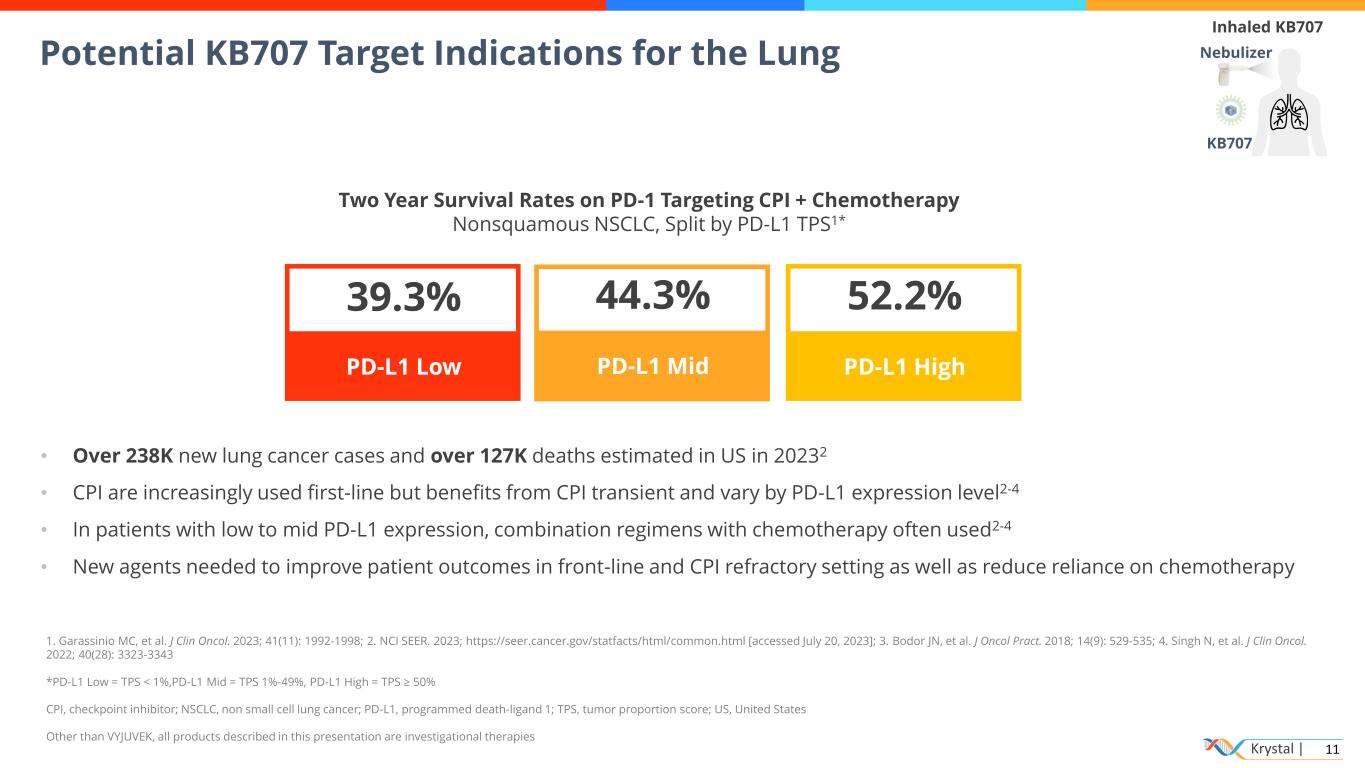
Krystal | 11 Potential KB707 Target Indications for the Lung Inhaled KB707 Nebulizer KB707 44.3% PD-L1 Mid • Over 238K new lung cancer cases and over 127K deaths estimated in US in 20232 • CPI are increasingly used first-line but benefits from CPI transient and vary by PD-L1 expression level2-4 • In patients with low to mid PD-L1 expression, combination regimens with chemotherapy often used2-4 • New agents needed to improve patient outcomes in front-line and CPI refractory setting as well as reduce reliance on chemotherapy 52.2% PD-L1 High 39.3% PD-L1 Low Two Year Survival Rates on PD-1 Targeting CPI + Chemotherapy Nonsquamous NSCLC, Split by PD-L1 TPS1* 1. Garassinio MC, et al. J Clin Oncol. 2023; 41(11): 1992-1998; 2. NCI SEER. 2023; https://seer.cancer.gov/statfacts/html/common.html [accessed July 20, 2023]; 3. Bodor JN, et al. J Oncol Pract. 2018; 14(9): 529-535; 4. Singh N, et al. J Clin Oncol. 2022; 40(28): 3323-3343 *PD-L1 Low = TPS < 1%,PD-L1 Mid = TPS 1%-49%, PD-L1 High = TPS ≥ 50% CPI, checkpoint inhibitor; NSCLC, non small cell lung cancer; PD-L1, programmed death-ligand 1; TPS, tumor proportion score; US, United States Other than VYJUVEK, all products described in this presentation are investigational therapies

Oncology Program KB707 Preclinical Update

Krystal | 13 Preclinical Research Objectives Research program built on stringent preclinical models to support clinical development Confirm expression, secretion, and bioactivity of vector-produced cytokines❑ ❑ ❑ Demonstrate safety and efficacy of repeated dosing of cytokine-expressing vectors in checkpoint refractory ‘cold’ syngeneic murine models of solid tumors KB707 Replication-defective HSV-1 vector containing functional human IL2 and IL12 Assess durability of expression following local delivery in healthy immunocompetent animals IL-12/IL12, interleukin-12; IL-2/IL2, interleukin-2 Other than VYJUVEK, all products described in this presentation are investigational therapies
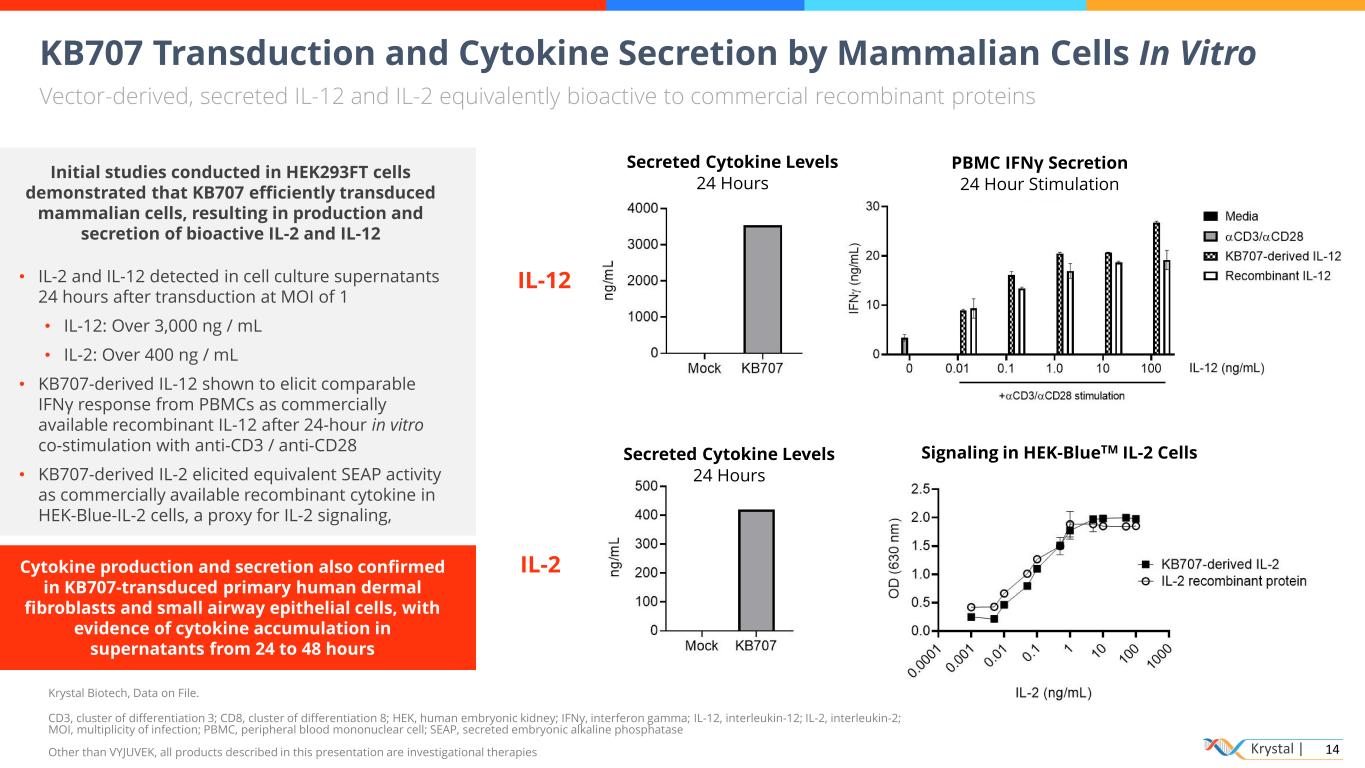
Krystal | 14 KB707 Transduction and Cytokine Secretion by Mammalian Cells In Vitro Vector-derived, secreted IL-12 and IL-2 equivalently bioactive to commercial recombinant proteins Krystal Biotech, Data on File. CD3, cluster of differentiation 3; CD8, cluster of differentiation 8; HEK, human embryonic kidney; IFNy, interferon gamma; IL-12, interleukin-12; IL-2, interleukin-2; MOI, multiplicity of infection; PBMC, peripheral blood mononuclear cell; SEAP, secreted embryonic alkaline phosphatase Other than VYJUVEK, all products described in this presentation are investigational therapies IL-12 Secreted Cytokine Levels 24 Hours PBMC IFNγ Secretion 24 Hour Stimulation IL-2 Secreted Cytokine Levels 24 Hours Signaling in HEK-BlueTM IL-2 Cells Initial studies conducted in HEK293FT cells demonstrated that KB707 efficiently transduced mammalian cells, resulting in production and secretion of bioactive IL-2 and IL-12 • IL-2 and IL-12 detected in cell culture supernatants 24 hours after transduction at MOI of 1 • IL-12: Over 3,000 ng / mL • IL-2: Over 400 ng / mL • KB707-derived IL-12 shown to elicit comparable IFNγ response from PBMCs as commercially available recombinant IL-12 after 24-hour in vitro co-stimulation with anti-CD3 / anti-CD28 • KB707-derived IL-2 elicited equivalent SEAP activity as commercially available recombinant cytokine in HEK-Blue-IL-2 cells, a proxy for IL-2 signaling, Cytokine production and secretion also confirmed in KB707-transduced primary human dermal fibroblasts and small airway epithelial cells, with evidence of cytokine accumulation in supernatants from 24 to 48 hours
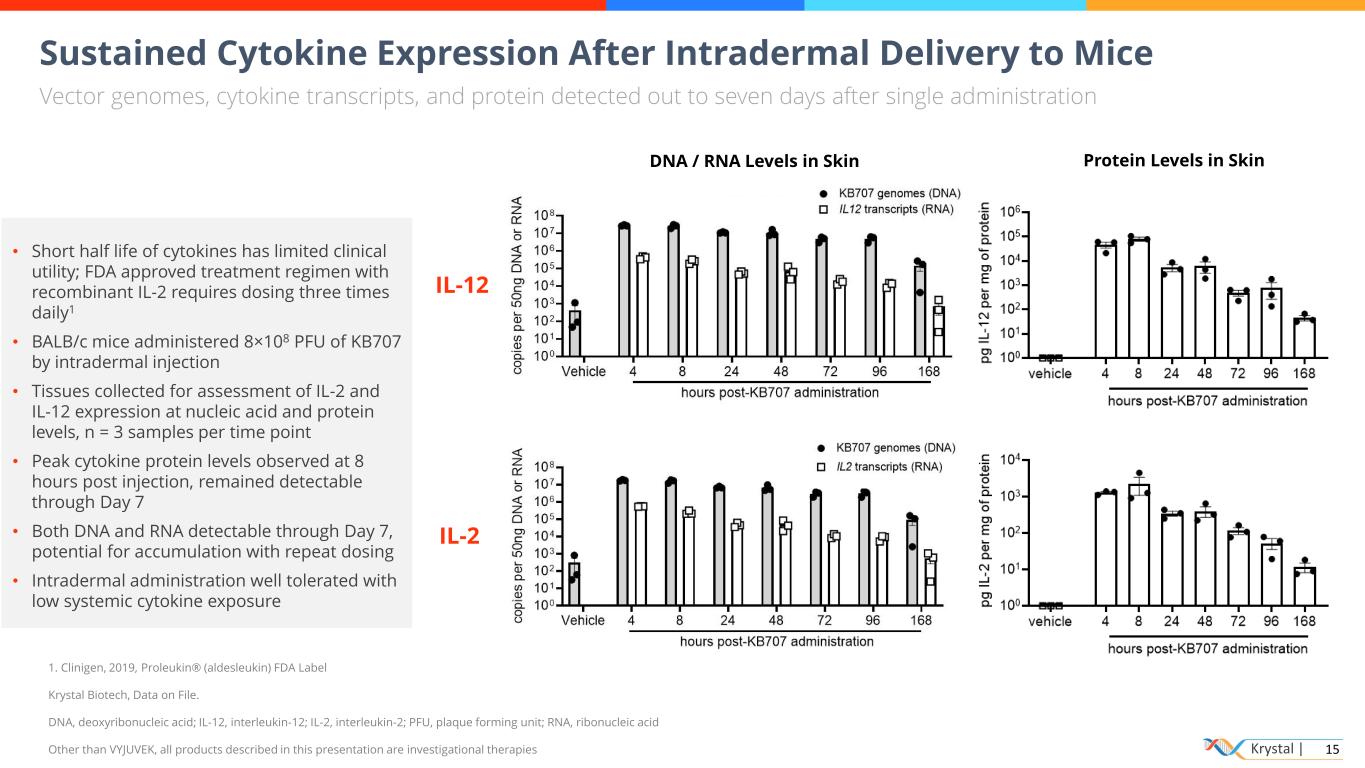
Krystal | 15 Sustained Cytokine Expression After Intradermal Delivery to Mice Vector genomes, cytokine transcripts, and protein detected out to seven days after single administration 1. Clinigen, 2019, Proleukin® (aldesleukin) FDA Label Krystal Biotech, Data on File. DNA, deoxyribonucleic acid; IL-12, interleukin-12; IL-2, interleukin-2; PFU, plaque forming unit; RNA, ribonucleic acid Other than VYJUVEK, all products described in this presentation are investigational therapies • Short half life of cytokines has limited clinical utility; FDA approved treatment regimen with recombinant IL-2 requires dosing three times daily1 • BALB/c mice administered 8×108 PFU of KB707 by intradermal injection • Tissues collected for assessment of IL-2 and IL-12 expression at nucleic acid and protein levels, n = 3 samples per time point • Peak cytokine protein levels observed at 8 hours post injection, remained detectable through Day 7 • Both DNA and RNA detectable through Day 7, potential for accumulation with repeat dosing • Intradermal administration well tolerated with low systemic cytokine exposure IL-12 IL-2 Protein Levels in SkinDNA / RNA Levels in Skin

Krystal | 16 Intratumoral IL-12 and IL-2 Effective in Cold Syngeneic Mouse Tumor Model Clear antitumor effect and survival benefit in checkpoint inhibitor refractory B16F10 tumor model Single Flank B16F10 Melanoma Model • B16F10 is a subclone of the B16 cancer cell line originally derived from the skin of a C57BL/6 mouse with melanoma • B16F10 tumors are highly aggressive and minimally responsive to immunotherapy, including refractory to PD-1 targeting CPI • Among the most stringent melanoma cell lines for the evaluation of candidate immunotherapeutics Study Design C57BL/6 mice 2×105 B16F10 SC ~108 PFU each of KB703 / KB704† or Vehicle Intratumoral Injection Days 7, 15, 21, 42 Survival, Weights, Tumor Size to Day 70 0 10 20 30 40 50 60 70 0 50 100 150 200 250 300 days post-tumor inoculation tu m o r a re a ( m m 2 ) Vehicle control KB703/KB704 ✱✱✱ 0 10 20 30 40 50 60 70 0 20 40 60 80 100 days post-tumor inoculation % s u rv iv a l Vehicle control KB703/KB704 ✱✱✱ Injected Tumor Size Survival 3 KB7 † ehi l C rol KB704† ehi l C rol Krystal Biotech, Data on File. † KB703 encodes murine IL-12, KB704 encodes murine IL-2, and KB703 + KB704 is murine equivalent to KB707 IL-12, interleukin-12; IL-2, interleukin-2; PFU, plaque forming unit; SC, subcutaneous Other than VYJUVEK, all products described in this presentation are investigational therapies Test Groups • Vehicle Control (n = 10) • KB703 + KB704 † (n =9) ***p<0.001
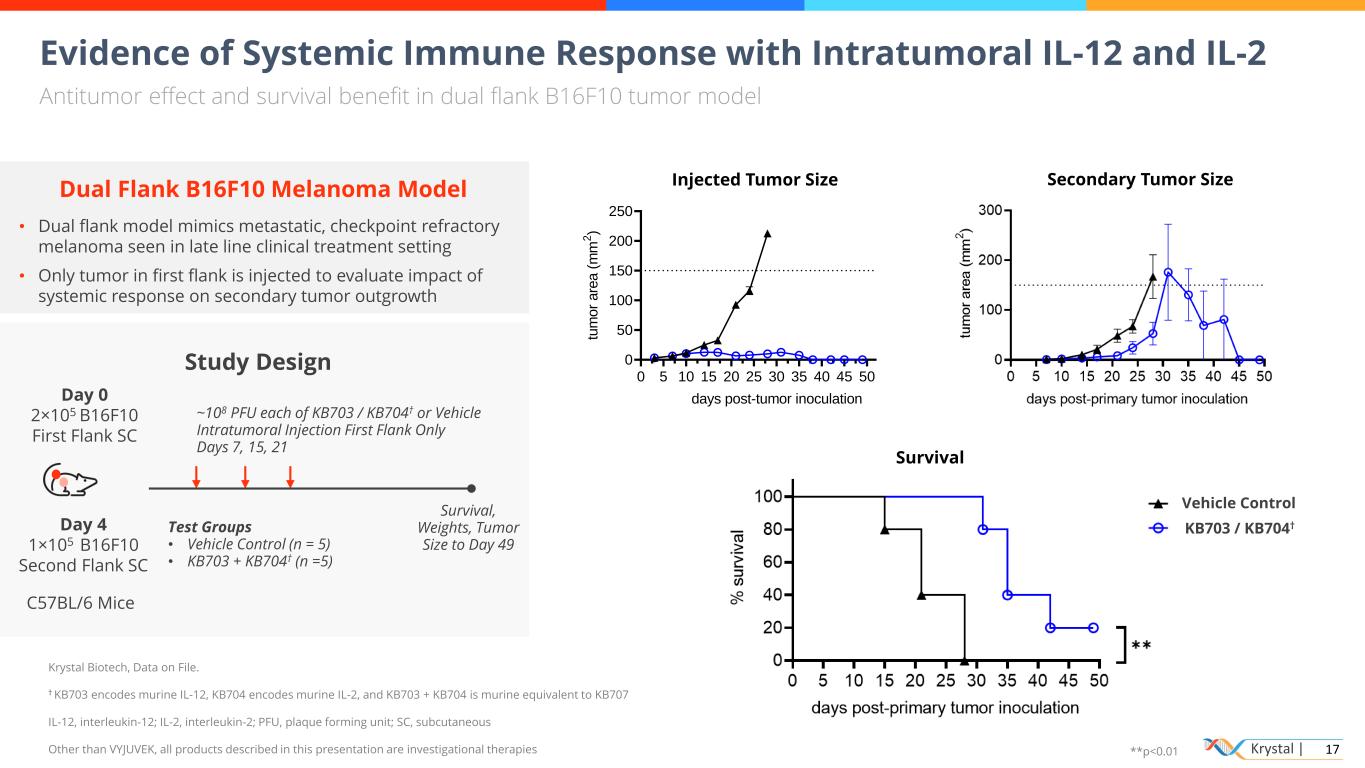
Krystal | 17 Evidence of Systemic Immune Response with Intratumoral IL-12 and IL-2 Antitumor effect and survival benefit in dual flank B16F10 tumor model Peak systemic exposure lower than recombinant Dual Flank B16F10 Melanoma Model • Dual flank model mimics metastatic, checkpoint refractory melanoma seen in late line clinical treatment setting • Only tumor in first flank is injected to evaluate impact of systemic response on secondary tumor outgrowth Study Design C57BL/6 Mice Day 0 2×105 B16F10 First Flank SC ~108 PFU each of KB703 / KB704† or Vehicle Intratumoral Injection First Flank Only Days 7, 15, 21 Survival, Weights, Tumor Size to Day 49 Day 4 1×105 B16F10 Second Flank SC 0 5 10 15 20 25 30 35 40 45 50 55 60 0 50 100 150 200 250 days post-tumor inoculation tu m o r a re a ( m m 2 ) B16-F10 day 4 - vehicle B16-F10 day 4- KB703/KB704 Injected Tumor Size Survival Secondary Tumor Size KB703 / KB704† Vehicle Control Krystal Biotech, Data on File. † KB703 encodes murine IL-12, KB704 encodes murine IL-2, and KB703 + KB704 is murine equivalent to KB707 IL-12, interleukin-12; IL-2, interleukin-2; PFU, plaque forming unit; SC, subcutaneous Other than VYJUVEK, all products described in this presentation are investigational therapies Test Groups • Vehicle Control (n = 5) • KB703 + KB704† (n =5) **p<0.01
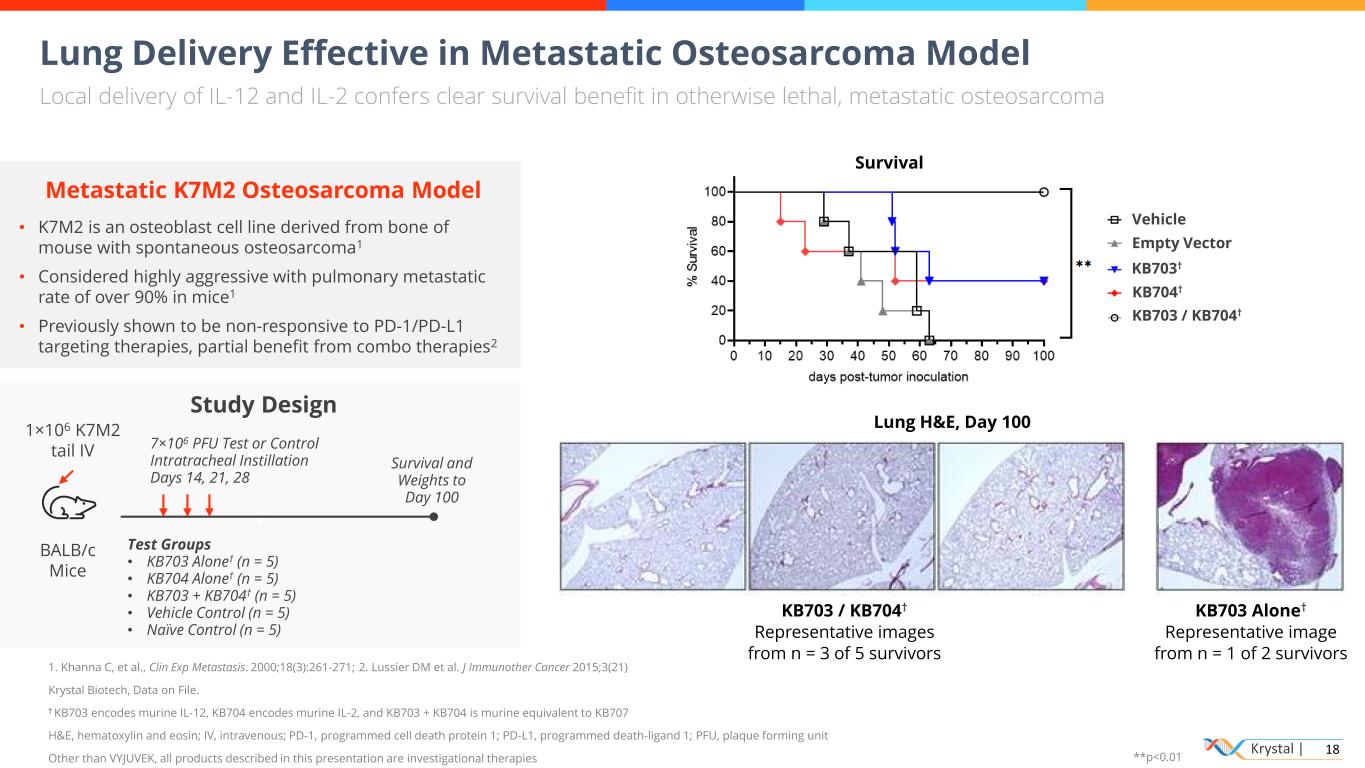
Krystal | 18 v Lung Delivery Effective in Metastatic Osteosarcoma Model Local delivery of IL-12 and IL-2 confers clear survival benefit in otherwise lethal, metastatic osteosarcoma 1. Khanna C, et al., Clin Exp Metastasis. 2000;18(3):261-271; 2. Lussier DM et al. J Immunother Cancer 2015;3(21) Krystal Biotech, Data on File. † KB703 encodes murine IL-12, KB704 encodes murine IL-2, and KB703 + KB704 is murine equivalent to KB707 H&E, hematoxylin and eosin; IV, intravenous; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFU, plaque forming unit Other than VYJUVEK, all products described in this presentation are investigational therapies Metastatic K7M2 Osteosarcoma Model • K7M2 is an osteoblast cell line derived from bone of mouse with spontaneous osteosarcoma1 • Considered highly aggressive with pulmonary metastatic rate of over 90% in mice1 • Previously shown to be non-responsive to PD-1/PD-L1 targeting therapies, partial benefit from combo therapies2 Study Design Peak systemic exposure lower than recombinant BALB/c Mice 1×106 K7M2 tail IV 7×106 PFU Test or Control Intratracheal Instillation Days 14, 21, 28 Survival and Weights to Day 100 Test Groups • KB703 Alone† (n = 5) • KB704 Alone† (n = 5) • KB703 + KB704† (n = 5) • Vehicle Control (n = 5) • Naïve Control (n = 5) KB703 / KB704† Representative images from n = 3 of 5 survivors Survival Lung H&E, Day 100 KB703 Alone† Representative image from n = 1 of 2 survivors KB703 / KB704† Vehicle Empty Vector KB703† KB704† **p<0.01
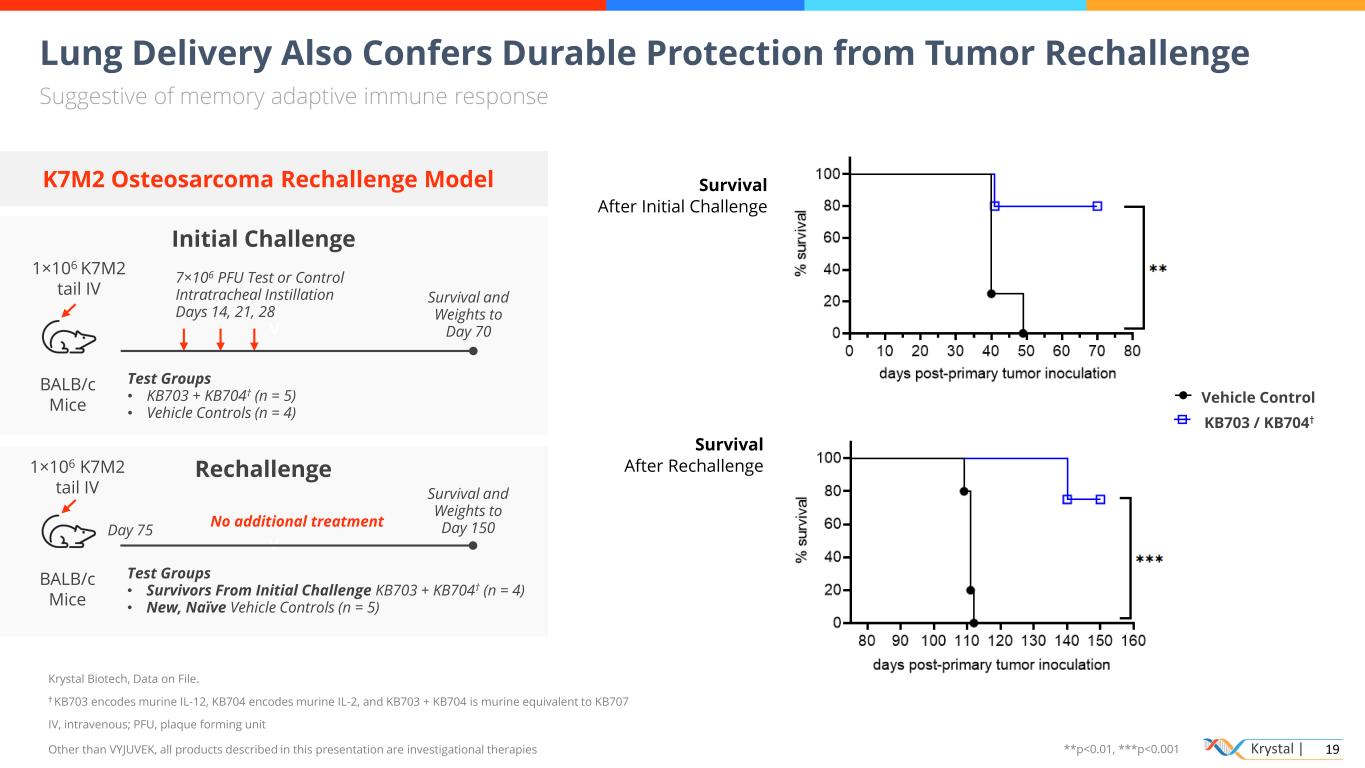
Krystal | 19 v Lung Delivery Also Confers Durable Protection from Tumor Rechallenge Suggestive of memory adaptive immune response Krystal Biotech, Data on File. † KB703 encodes murine IL-12, KB704 encodes murine IL-2, and KB703 + KB704 is murine equivalent to KB707 IV, intravenous; PFU, plaque forming unit Other than VYJUVEK, all products described in this presentation are investigational therapies Initial Challenge BALB/c Mice 1×106 K7M2 tail IV 7×106 PFU Test or Control Intratracheal Instillation Days 14, 21, 28 Survival and Weights to Day 70 Test Groups • KB703 + KB704† (n = 5) • Vehicle Controls (n = 4) Survival After Initial Challenge Survival After Rechallenge 0 1 0 2 0 3 0 4 0 5 0 6 0 7 0 8 0 0 2 0 4 0 6 0 8 0 1 0 0 % s u rv iv a l Vehicle control KB703/KB704 days post-primary tumor inoculation KB703 / KB704† icle Control K7M2 Osteosarcoma Rechallenge Model v Rechallenge BALB/c Mice 1×106 K7M2 tail IV Survival and Weights to Day 150 Test Groups • Survivors From Initial Challenge KB703 + KB704† (n = 4) • New, Naïve Vehicle Controls (n = 5) No additional treatment Day 75 **p<0.01, ***p<0.001
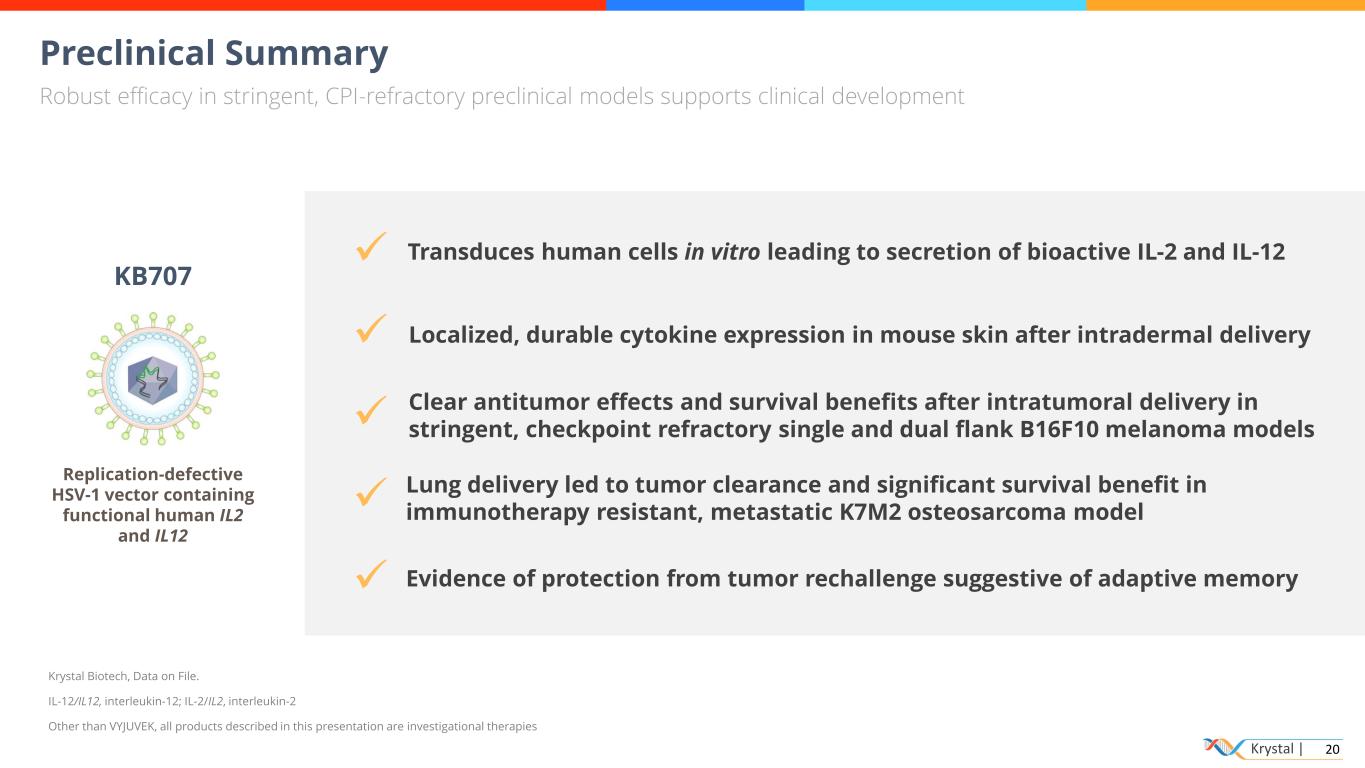
Krystal | 20 Preclinical Summary Robust efficacy in stringent, CPI-refractory preclinical models supports clinical development Transduces human cells in vitro leading to secretion of bioactive IL-2 and IL-12 ✓ ✓ ✓ ✓ ✓ Localized, durable cytokine expression in mouse skin after intradermal delivery Clear antitumor effects and survival benefits after intratumoral delivery in stringent, checkpoint refractory single and dual flank B16F10 melanoma models KB707 Replication-defective HSV-1 vector containing functional human IL2 and IL12 Krystal Biotech, Data on File. IL-12/IL12, interleukin-12; IL-2/IL2, interleukin-2 Other than VYJUVEK, all products described in this presentation are investigational therapies Lung delivery led to tumor clearance and significant survival benefit in immunotherapy resistant, metastatic K7M2 osteosarcoma model Evidence of protection from tumor rechallenge suggestive of adaptive memory

Oncology Program KB707 Clinical Program
Krystal | 22 KB707 Clinical Program Overall Phase 1/2 Approach Deliver response to all solid tumor patients with immunotherapy Phase 1 first-in-human study with intratumoral administration • Evaluate the safety and tolerability of monotherapy in ascending dose • Demonstrate single agent anti-tumor activity • Patients with solid tumors that progressed on SOC • Data will support further assessment in disease-specific indications Inhaled administration • Advantage in delivery to respiratory tract cancers (e.g., Lung, H&N), cancer metastasized to the lungs • Staggered start to intratumoral administration leverages safety, tolerability, and pharmacodynamic data H&N, head and neck; IL-12/IL12, interleukin-12; IL-2/IL2, interleukin-2; SOC, standard of care Other than VYJUVEK, all products described in this presentation are investigational therapies
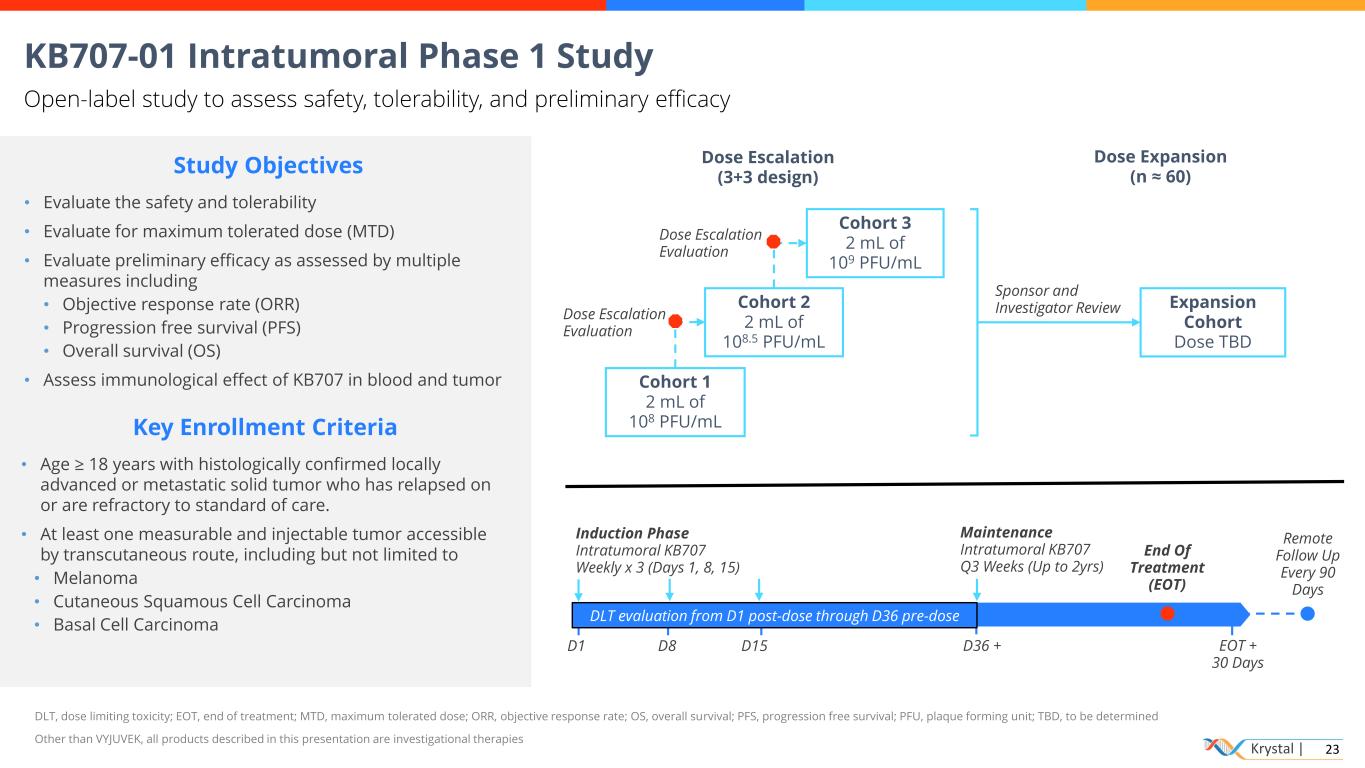
Krystal | 23 KB707-01 Intratumoral Phase 1 Study Open-label study to assess safety, tolerability, and preliminary efficacy DLT, dose limiting toxicity; EOT, end of treatment; MTD, maximum tolerated dose; ORR, objective response rate; OS, overall survival; PFS, progression free survival; PFU, plaque forming unit; TBD, to be determined Other than VYJUVEK, all products described in this presentation are investigational therapies D15 Cohort 3 2 mL of 109 PFU/mL Cohort 2 2 mL of 108.5 PFU/mL Cohort 1 2 mL of 108 PFU/mL Induction Phase Intratumoral KB707 Weekly x 3 (Days 1, 8, 15) Key Enrollment Criteria • Age ≥ 18 years with histologically confirmed locally advanced or metastatic solid tumor who has relapsed on or are refractory to standard of care. • At least one measurable and injectable tumor accessible by transcutaneous route, including but not limited to • Melanoma • Cutaneous Squamous Cell Carcinoma • Basal Cell Carcinoma D1 Sponsor and Investigator Review End Of Treatment (EOT) D8 Dose Escalation Evaluation Maintenance Intratumoral KB707 Q3 Weeks (Up to 2yrs) Dose Escalation (3+3 design) Expansion Cohort Dose TBD Dose Expansion (n ≈ 60) D36 + EOT + 30 Days Remote Follow Up Every 90 Days Study Objectives • Evaluate the safety and tolerability • Evaluate for maximum tolerated dose (MTD) • Evaluate preliminary efficacy as assessed by multiple measures including • Objective response rate (ORR) • Progression free survival (PFS) • Overall survival (OS) • Assess immunological effect of KB707 in blood and tumor Dose Escalation Evaluation DLT evaluation from D1 post-dose through D36 pre-dose
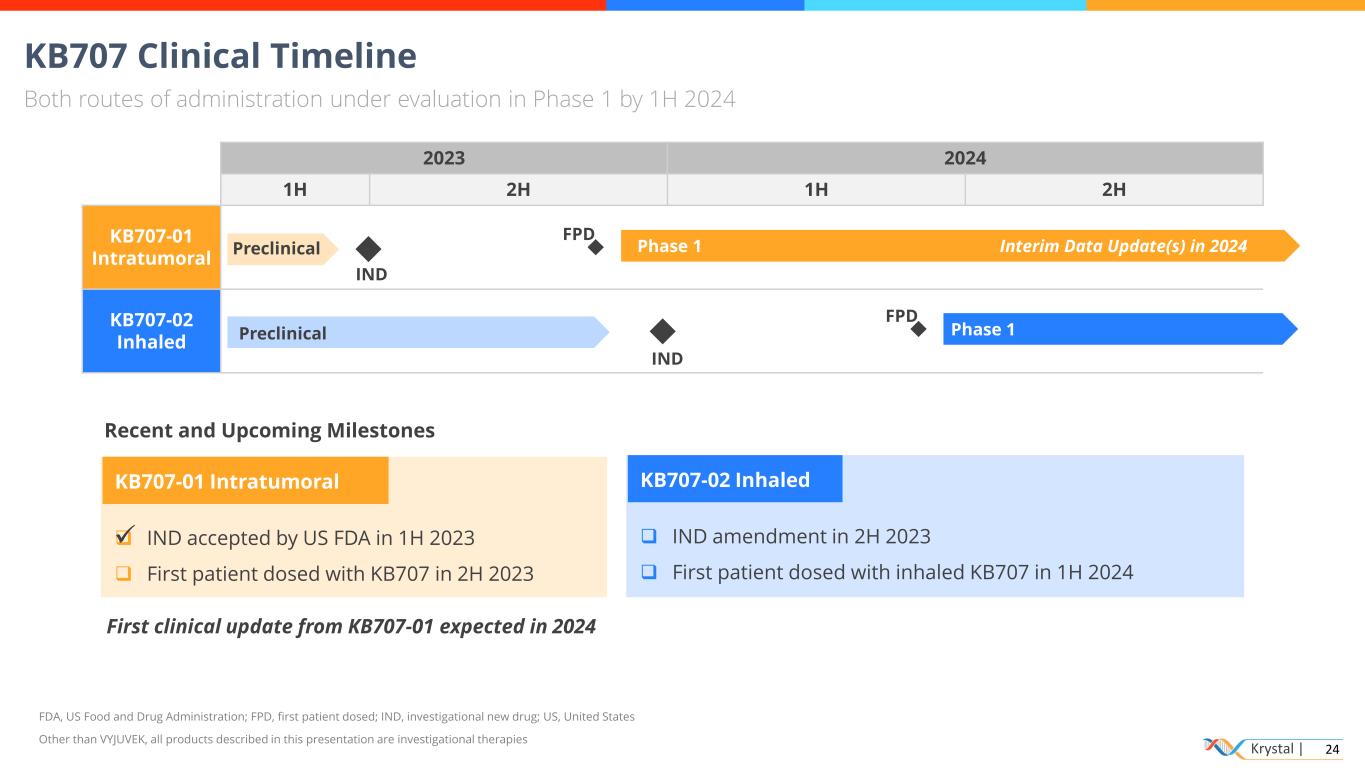
Krystal | 24 2023 2024 1H 2H 1H 2H KB707-01 Intratumoral KB707-02 Inhaled KB707 Clinical Timeline Both routes of administration under evaluation in Phase 1 by 1H 2024 FDA, US Food and Drug Administration; FPD, first patient dosed; IND, investigational new drug; US, United States Other than VYJUVEK, all products described in this presentation are investigational therapies KB707-01 Intratumoral ❑ IND accepted by US FDA in 1H 2023 ❑ First patient dosed with KB707 in 2H 2023 IND FPD Interim Data Update(s) in 2024Phase 1 Preclinical Phase 1 Preclinical IND FPD Recent and Upcoming Milestones KB707-02 Inhaled ❑ IND amendment in 2H 2023 ❑ First patient dosed with inhaled KB707 in 1H 2024 First clinical update from KB707-01 expected in 2024 ✓

Cutaneous oncology landscape for refractory disease Jason J. Luke, MD, FACP Associate Professor Director of the Immunotherapy and Drug Development Center Associate Director for Clinical Research

Treatment flow chart across melanoma stages @jasonlukemd
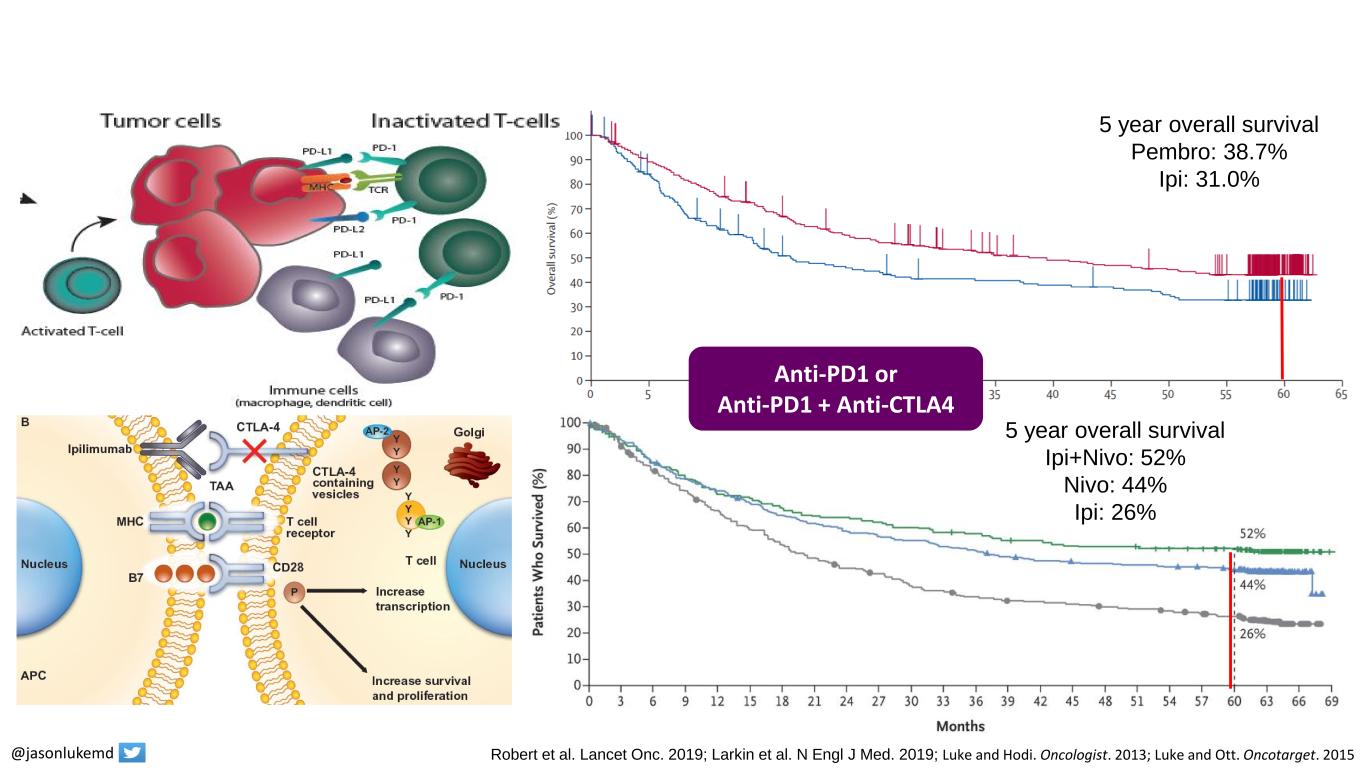
5-year survival with anti-PD1 +/- CTLA4 in melanoma 5 year overall survival Pembro: 38.7% Ipi: 31.0% 5 year overall survival Ipi+Nivo: 52% Nivo: 44% Ipi: 26% Anti-PD1 or Anti-PD1 + Anti-CTLA4 Robert et al. Lancet Onc. 2019; Larkin et al. N Engl J Med. 2019; Luke and Hodi. Oncologist. 2013; Luke and Ott. Oncotarget. 2015@jasonlukemd
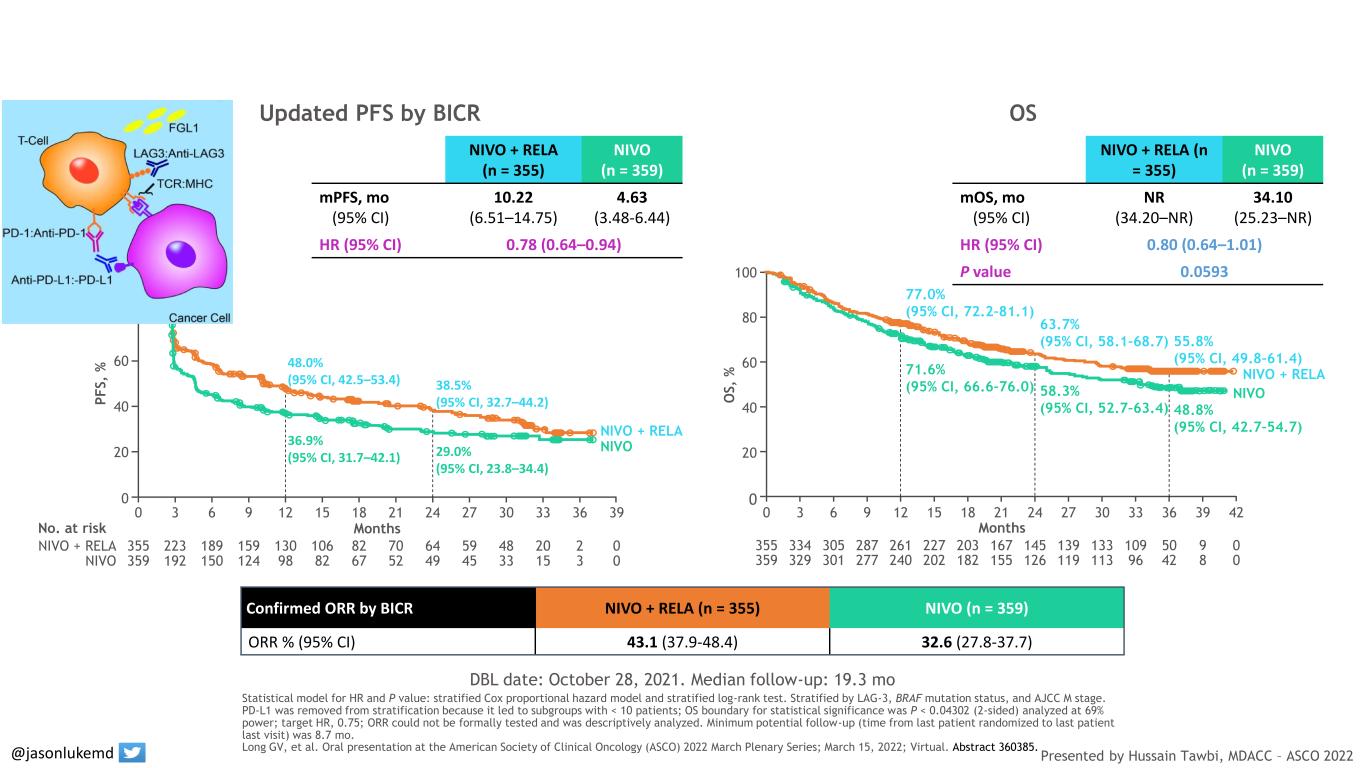
Confirmed ORR by BICR NIVO + RELA (n = 355) NIVO (n = 359) ORR % (95% CI) 43.1 (37.9-48.4) 32.6 (27.8-37.7) DBL date: October 28, 2021. Median follow-up: 19.3 mo Updated PFS by BICR OS NIVO + RELA (n = 355) NIVO (n = 359) mPFS, mo (95% CI) 10.22 (6.51–14.75) 4.63 (3.48-6.44) HR (95% CI) 0.78 (0.64–0.94) NIVO + RELA (n = 355) NIVO (n = 359) mOS, mo (95% CI) NR (34.20–NR) 34.10 (25.23–NR) HR (95% CI) 0.80 (0.64–1.01) P value 0.0593 Months NIVO + RELA NIVO No. at risk 355 0223 189 159 130 106 82 70 64 59 48 20 2 359 0192 150 124 98 82 67 52 49 45 33 15 3 P F S , % 0 100 80 60 40 20 0 393 6 9 12 15 18 21 24 27 30 33 36 48.0% (95% CI, 42.5–53.4) 36.9% (95% CI, 31.7–42.1) 38.5% (95% CI, 32.7–44.2) 29.0% (95% CI, 23.8–34.4) NIVO NIVO + RELA 359 0329 301 277 240 202 182 155 126 119 113 96 42 8 355 0334 305 287 261 227 203 167 145 139 133 109 50 9 O S , % 0 100 80 60 40 20 Months 0 423 6 9 12 15 18 21 24 27 30 33 36 39 NIVO NIVO + RELA 77.0% (95% CI, 72.2–81.1) 71.6% (95% CI, 66.6–76.0) 63.7% (95% CI, 58.1–68.7) 58.3% (95% CI, 52.7–63.4) 55.8% (95% CI, 49.8–61.4) 48.8% (95% CI, 42.7–54.7) Statistical model for HR and P value: stratified Cox proportional hazard model and stratified log-rank test. Stratified by LAG-3, BRAF mutation status, and AJCC M stage. PD-L1 was removed from stratification because it led to subgroups with < 10 patients; OS boundary for statistical significance was P < 0.04302 (2-sided) analyzed at 69% power; target HR, 0.75; ORR could not be formally tested and was descriptively analyzed. Minimum potential follow-up (time from last patient randomized to last patient last visit) was 8.7 mo. Long GV, et al. Oral presentation at the American Society of Clinical Oncology (ASCO) 2022 March Plenary Series; March 15, 2022; Virtual. Abstract 360385. Presented by Hussain Tawbi, MDACC – ASCO 2022 LAG3-PD1 Combination improves progression-free survival and response in melanoma @jasonlukemd

Luke et al. Lancet. 2022; Weber et al. NEJM 2021; Long et al. NEJM 2021; Patel et al. ESMO. 2022 Majority of patients who progress to stage IV now eligible for peri- operative systemic therapy @jasonlukemd BRAF-MEK Stage IIB/C – anti-PD1 Stage III – anti-PD1 Stage IIIB/C/IV – anti-PD1

Melanoma Vignette • Patient with a 3.2 mm melanoma on the right leg undergoes resection and nodal evaluation identifying 2 nodes involved. Tumor is BRAF WT • Adjuvant treatment with nivolumab is given for eight months with progression in new nodes and lung. • Nivo + ipi vs Nivo + rela are considered but ipi combo is chosen. • Patient has obvious new lesions within 1.5 months and rising LDH. • What treatment to choose then? @jasonlukemd
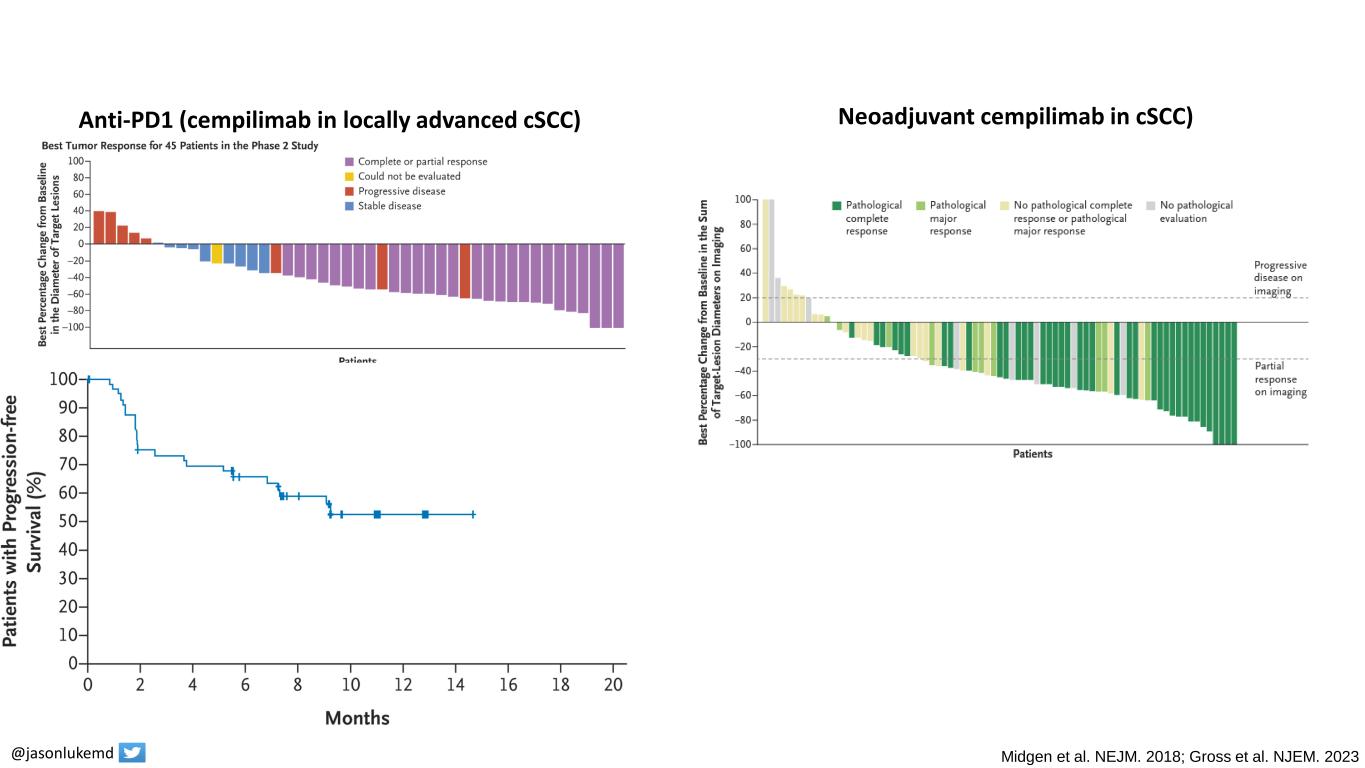
@jasonlukemd Midgen et al. NEJM. 2018; Gross et al. NJEM. 2023 Anti-PD1 in cutaneous squamous cell carcinoma Anti-PD1 (cempilimab in locally advanced cSCC) Neoadjuvant cempilimab in cSCC)
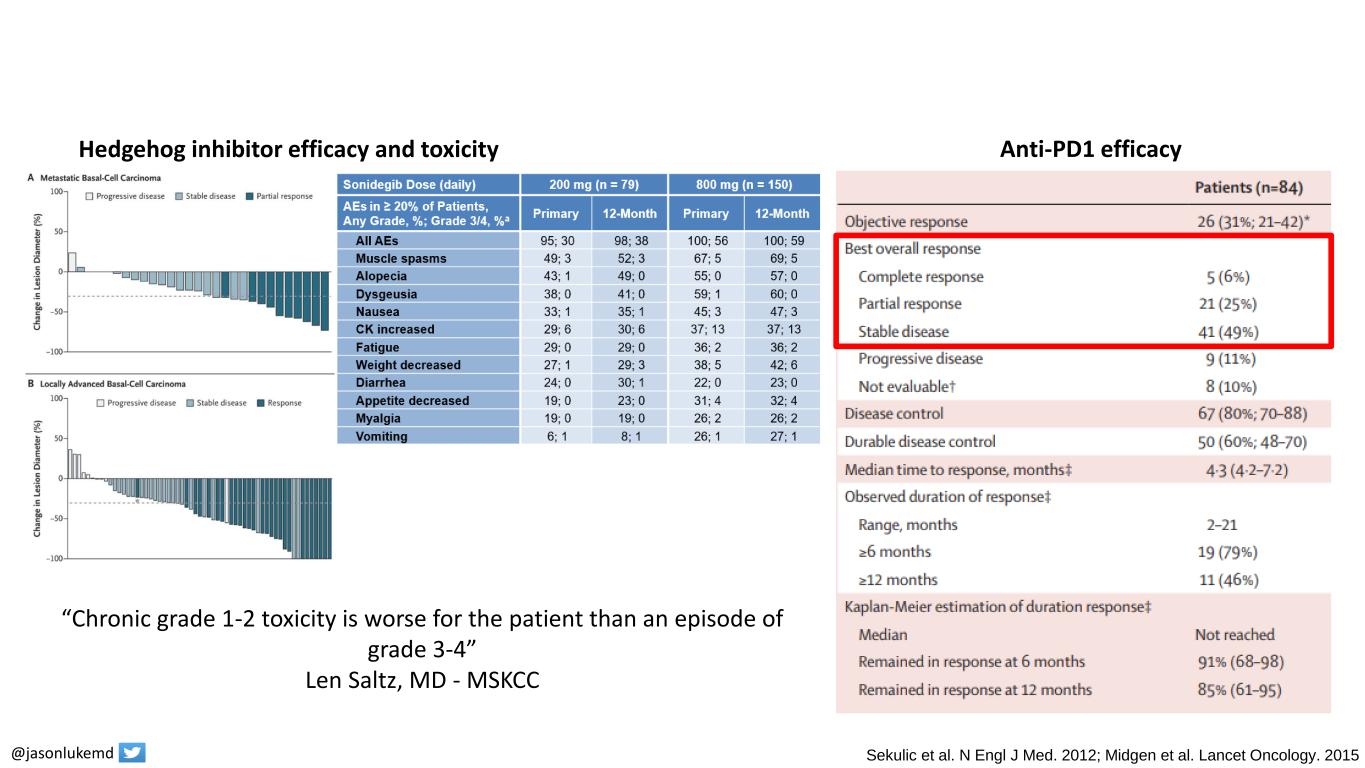
Basal cell carcinoma treatment options @jasonlukemd Sekulic et al. N Engl J Med. 2012; Midgen et al. Lancet Oncology. 2015 “Chronic grade 1-2 toxicity is worse for the patient than an episode of grade 3-4” Len Saltz, MD - MSKCC Hedgehog inhibitor efficacy and toxicity Anti-PD1 efficacy

cSCC Vignette • Patient with history of multiple early stage non-melanoma skin cancers develops a bleeding ulcer on the scalp. • Moh’s procedure removes the lesion but within 4 months the skin graft erodes and the ulcer returns • A second resection attempt is considered but aborted when margins are positive • Anti-PD1 with cemiplimab is initiated but the lesion continue to grow. • What treatment to consider? @jasonlukemd

Conclusions • In melanoma, unmet needs remains after progression on anti-PD1 • KB707 has high upside potential for combinations with anti-PD1 in earlier lines of therapy given arming with IL2 and IL12 • Therapeutic landscape open in non-melanoma skin cancers • Anti-PD1 is SOC but 50% do not respond in cSCC • FDA has greenlighted development of therapeutics in BCC despite activity of HHi due to toxicity of those agents • KB707 is well positioned to overcome previous cytokine therapy challenges by leveraging unique molecular biology and field leading cytokine combinations @jasonlukemd

Krystal | 35 Q&A Panelists Suma Krishnan President, Research & Development Trevor Parry, PhD Vice President, Research and Scientific Affairs Jason J. Luke, MD, FACP David Chien, MD Senior Vice President, Clinical Development – Oncology Krish Krishnan Chairman and CEO Samuel Broder, MD

© Copyright 2023 Krystal Biotech, Inc. All rights reserved.
v3.23.2
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Krystal Biotech (NASDAQ:KRYS)
Historical Stock Chart
From Oct 2024 to Nov 2024
Krystal Biotech (NASDAQ:KRYS)
Historical Stock Chart
From Nov 2023 to Nov 2024