0001360214
false
0001360214
2023-07-18
2023-07-18
0001360214
HROW:CommonStock0.001ParValuePerShareMember
2023-07-18
2023-07-18
0001360214
HROW:Sec8.625SeniorNotesDue2026Member
2023-07-18
2023-07-18
0001360214
HROW:Sec11.875SeniorNotesDue2027Member
2023-07-18
2023-07-18
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): July 18, 2023
HARROW
HEALTH, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-35814 |
|
45-0567010 |
(State
or other jurisdiction
of
incorporation) |
|
(Commission
File Number) |
|
(IRS Employer
Identification No.) |
| 102
Woodmont Blvd., Suite 610 |
|
|
| Nashville,
Tennessee |
|
37205 |
| (Address
of principal executive offices) |
|
(Zip
Code) |
Registrant’s
telephone number, including area code: (615) 733-4730
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
on exchange on which registered |
| Common
Stock, $0.001 par value per share |
|
HROW |
|
The
NASDAQ Stock Market LLC |
| 8.625%
Senior Notes due 2026 |
|
HROWL |
|
The
NASDAQ Stock Market LLC |
| 11.875%
Senior Notes due 2027 |
|
HROWM |
|
The
NASDAQ Stock Market LLC |
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions:
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2
of the Securities Act of 1934: Emerging growth company ☐
If
any emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item
1.01 Entry into a Material Definitive Agreement.
On
July 18, 2023, Harrow Health, Inc. (the “Company”) entered into the First Amendment to Credit Agreement and Guaranty
and Consent (the “Oaktree Amendment”) to the Credit Agreement and Guaranty (the “Oaktree Loan”) originally entered
into on March 27, 2023, with the lenders from time to time party thereto and Oaktree Fund Administration, LLC, as administrative agent
for the lenders (together, “Oaktree”). Under the Oaktree Amendment, the overall credit facility size was increased from $100,000,000
to $112,500,000, and the Company made other changes related to the Santen Products Acquisition (described further below under Item 8.01).
Upon satisfaction of certain conditions to funding, the Company will draw down a principal amount of $12,500,000 (the “Loan
Increase”) to fund the initial one-time payment associated with the Santen Products Acquisition and for other working capital
and general corporate purposes. No other material changes to the Oaktree Loan were provided in the Oaktree Amendment. Following entry
into the Oaktree Amendment and the funding of the Loan Increase upon closing of the Santen Products Acquisition, the Company will
have drawn down a total principal loan amount of $77,500,000 under the Oaktree Loan and an additional principal loan amount of up to
$35,000,000 remains available to the Company upon the commercialization of TRIESENCE.
The
foregoing description of the Oaktree Amendment does not purport to be complete and is qualified in its entirety by reference to the full
text of the Oaktree Amendment, which the Company expects to file as an exhibit to its Quarterly Report on Form 10-Q for the three months
ended June 30, 2023.
Item
2.02 Results of Operations and Financial Condition.
Management
expects the Company to record over $31,000,000 of total revenues and over $9,300,000 of Adjusted EBITDA (a non-GAAP measure) for the
three-month period ended June 30, 2023.
Management
utilizes Adjusted EBITDA, an unaudited financial measure that is not calculated in accordance with generally accepted accounting principles
(“GAAP”), to evaluate the Company’s financial results and performance and to plan and forecast future periods. Investors
are encouraged to review the Company’s complete results of operations and additional information provided in the Company’s
Annual Report on Form 10-K for the year ended December 31, 2022 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2023.
Management believes that Adjusted EBITDA reflects an additional way of viewing aspects of the Company’s operations that, when viewed
in conjunction with GAAP results, provides a more complete understanding of the Company’s results of operations and the factors
and trends affecting its business.
Although
the Company is providing management guidance on anticipated Adjusted EBITDA, management is unable to determine with reasonable certainty
the ultimate outcome of certain items necessary to calculate net income, the most directly comparable GAAP measure, without unreasonable
effort. These items include, but are not limited to, final calculation of investment related gains/losses, inventory reserves, profit
transfers, revenue discounts, returns, chargebacks and stock-based compensation. These items are uncertain, depend on various factors,
and could have a material impact on the GAAP reported results for the period. All estimates presented are subject to completion of the
applicable quarter-end closing procedures. The Company’s actual results for such period are not expected to be available until
early August 2023 and may vary from these estimates. In addition, estimated financial information is necessarily speculative in nature,
and it can be expected that some or all of the assumptions underlying the estimated financial information described above will not materialize
or will vary significantly from actual results. Accordingly, undue reliance should not be placed on this estimate. The preliminary estimate
is not necessarily indicative of any future period and should be read together with the sections titled “Risk Factors” and
“Special Note Regarding Forward-Looking Statements,” and under similar headings in the documents filed by the Company with
the Securities and Exchange Commission (“SEC”) as well as the financial statements, related notes and other financial information
included in the Company’s filings with the SEC.
The
foregoing guidance on anticipated results for the three months ended June 30, 2023 has not been reviewed by Company’s auditors,
is based on preliminary information as of the date hereof and is subject to material changes following completion of the quarter-end
review process and other adjustments that may be made before the Company’s financial results are finalized. In addition, these
preliminary unaudited results are not comprehensive financial results for the quarter ended June 30, 2023, should not be viewed as a
substitute for complete GAAP financial statements or more comprehensive financial information, and are not indicative of the results
for any future period.
A
copy of the press release announcing the second quarter 2023 management guidance is being furnished as Exhibit 99.1 to this Current
Report on Form 8-K.
Item
7.01 Regulation FD Disclosure.
On
July 18, 2023, the Company issued a press release announcing an offering of shares of the Company’s common stock, par value $0.001
(the “Common Stock”) (the “Offering”).
A
copy of the press release for the Offering is being furnished as Exhibit 99.2 to this Current Report on Form 8-K.
In
connection with the Offering, the Company will be making road show presentations to certain existing and potential securityholders of
the Company. The road show materials are being furnished as Exhibit 99.3 to this Current Report on Form 8-K.
The
information furnished under Items 2.02 and 7.01 of this Current Report on Form 8-K, including Exhibit 99.1, Exhibit
99.2, and Exhibit 99.3 shall not be deemed to be “filed” for the purposes of Section 18 of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section. The information
in Items 2.02 and 7.01, including Exhibit 99.1, Exhibit 99.2, and Exhibit 99.3 shall not be
deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent
it is specifically incorporated by reference but regardless of any general incorporation language in such filing.
Item
8.01 Other Items.
Acquisition
of VEVYETM U.S. and Canadian Commercial Rights
On
July 18, 2023, the Company announced that it had acquired commercial rights of VEVYE (cyclosporine
ophthalmic solution) 0.1%, an ophthalmic drug product, for the U.S. and Canadian markets (the “VEVYE
Acquisition”). VEVYE, which is dispensed topically in a unique ten microliter per
one drop and is labeled for twice-daily (BID) dosing, is the first and only cyclosporine-based product indicated for the
treatment of both signs and symptoms of dry eye disease (DED). VEVYE was approved on May 30, 2023 by the Food and Drug
Administration. The Company acquired the commercial rights to VEVYE by entering into a license agreement with Novaliq GmbH
(“Novaliq”). As consideration, the Company will make initial payments to Novaliq totaling $8,000,000 and will pay low
double-digit royalties on net sales of VEVYE along with potential commercial milestone payments.
A
copy of the press release announcing the VEVYE Acquisition is being filed as Exhibit 99.4 to this Current Report on Form
8-K.
Acquisition
of Certain U.S. and Canadian Commercial Rights to Santen and Eyevance Products
On
July 18, 2023, the Company entered into an Asset Purchase Agreement with Eyevance
Pharmaceuticals, LLC and a License Agreement with Santen S.A.S. (collectively, the “Santen Agreements”), each a
subsidiary of Santen Pharmaceuticals Co., Ltd. (collectively, “Santen”). Pursuant to the Santen Agreements, we will be
acquiring the exclusive commercial rights to assets associated with certain ophthalmic products identified in the Santen Agreements
and described in the press release issued on July 18, 2023 (collectively, the “Santen
Products”):
The
transactions pursuant to the Santen Agreements are referred to in this Current Report on Form 8-K as the “Santen Products Acquisition.”
Under
the terms of the Santen Agreements, the Company is required to make an initial one-time payment of $8,000,000. In addition, the agreements
provide for various one-time milestone payments associated with certain manufacturing-related events as well as low-double digit royalty
payments on net sales of Verkazia and high-single digit royalty payments on net sales of Cationorm Plus. Under the Santen Agreements,
the Company also assumed certain obligations associated with other third parties that require mid-single digit royalties on sales of
Freshkote and Zerviate. Immediately following the closing and subject to certain conditions, for a period that the Company expects to
last approximately four months, and prior to the transfer of the Santen Products NDAs and other marketing authorizations to the Company,
Santen will continue to sell the Santen Products on the Company’s behalf and transfer the net profit from the sale of the Santen
Products to the Company.
A
copy of the press release announcing the Santen Products Acquisition is being filed as Exhibit 99.5 to this Current Report
on Form 8-K.
Cautionary
Note Regarding Forward-Looking Statements
This
Current Report on Form 8-K (and the exhibits attached hereto) may contain “forward-looking” statements as defined by the
Private Securities Litigation Reform Act of 1995 or by the SEC in its rules, regulations and releases. These statements include, but
are not limited to, the Company’s plans, objectives, expectations and intentions regarding the performance of its business, statements
regarding the Company’s anticipated results for the second quarter of 2023, the terms and conditions and timing of the Offering,
the intended use of proceeds of the Offering and other non-historical statements. These statements can be identified by the use of words
such as “believes,” “anticipates,” “expects,” “intends,” “plans,” “continues,”
“estimates,” “predicts,” “projects,” “forecasts,” and similar expressions. All forward
looking statements are based on management’s current expectations and beliefs only as of the date of this report and are subject
to risks, uncertainties and assumptions that could cause actual results to differ materially from those discussed in, or implied by,
the forward-looking statements, including the risks identified and discussed from time to time in the Company’s reports filed with
the SEC, including the Company’s Annual Report on Form 10-K for the year ended December 31, 2022 and Quarterly Report on Form 10-Q
for the three months ended March 31, 2023. Readers are strongly encouraged to review carefully the full cautionary statements described
in these reports. Except as required by law, the Company undertakes no obligation to revise or update publicly any forward-looking statements
to reflect events or circumstances after the date of this report, or to reflect the occurrence of unanticipated events or circumstances.
Item
9.01. Financial Statements and Exhibits
(d)
Exhibits
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
HARROW
HEALTH, INC. |
| |
|
|
| Dated:
July 18, 2023 |
By: |
/s/
Andrew R. Boll |
| |
|
Andrew
R. Boll |
| |
|
Chief
Financial Officer |
Exhibit
99.1

Harrow
Provides Select Preliminary Second Quarter 2023 Financial Guidance
NASHVILLE,
Tenn., July 18, 2023 – Harrow (Nasdaq: HROW), a leading U.S. eyecare pharmaceutical company, today announced the following
select preliminary second quarter 2023 financial guidance:
| |
● |
Second
quarter 2023 revenues in excess of $31.0 million compared with prior-year second quarter revenues of $23.3 million |
| |
● |
Second
quarter 2023 Adjusted EBITDA (a non-GAAP measure) in excess of $9.3 million |
Management
expects to release full second quarter 2023 financial results on August 9, 2023.
About
Harrow
Harrow
Health, Inc. (Nasdaq: HROW) is a leading U.S. eyecare pharmaceutical company engaged in the discovery, development, and commercialization
of innovative ophthalmic prescription therapies that are accessible and affordable. Harrow owns U.S. commercial rights to ten branded
FDA-approved ophthalmic pharmaceutical products. Harrow also owns and operates ImprimisRx, a leading U.S. ophthalmic-focused pharmaceutical
compounding business, which also serves as a mail-order pharmacy licensed to ship prescription medications in all 50 states. Harrow has
non-controlling equity positions in Surface Ophthalmics, Inc. and Melt Pharmaceuticals, Inc., companies that began as subsidiaries
of Harrow. Harrow also owns royalty rights in four late-stage drug candidates being developed by Surface and Melt.
Non-GAAP
Measures
Management
utilizes Adjusted EBITDA, an unaudited financial measure that is not calculated in accordance with General Accepted Accounting Principles
(“GAAP”), to evaluate the Company’s financial results and performance and to plan and forecast future periods. Investors
are encouraged to review the Company’s complete results of operations and additional information provided in the Company’s
Annual Report on Form 10-K for the year ended December 31, 2022 and Quarterly Report on Form 10-Q for the quarter ended March 31, 2023.
Management believes that Adjusted EBITDA reflects an additional way of viewing aspects of the Company’s operations that, when viewed
in conjunction with GAAP results, provides a more complete understanding of the Company’s results of operations and the factors
and trends affecting its business.
Although
we are providing management guidance on anticipated Adjusted EBITDA, we are unable to determine with reasonable certainty the ultimate
outcome of certain items necessary to calculate net income, the most directly comparable GAAP measure, without unreasonable effort. These
items include, but are not limited to, final calculation of investment related gains/losses, inventory reserves, profit transfers, revenue
discounts, returns, chargebacks and stock-based compensation. These items are uncertain, depend on various factors, and could have a
material impact on the GAAP reported results for the period. All estimates presented are subject to completion of the applicable quarter-end
closing procedures. Our actual results for such period are not expected to be available until early August 2023 and may vary from these
estimates. In addition, estimated financial information is necessarily speculative in nature, and it can be expected that some or all
of the assumptions underlying the estimated financial information described above will not materialize or will vary significantly from
actual results. Accordingly, undue reliance should not be placed on this estimate. The preliminary estimate is not necessarily indicative
of any future period and should be read together with the sections titled “Risk Factors” and “Special Note Regarding
Forward-Looking Statements,” and under similar headings in the documents filed by the Company with the U.S. Securities and Exchange
Commission (“SEC”) as well as our financial statements, related notes and other financial information included in the Company’s
filings with the SEC.
-MORE-
HROW
Provides Select Preliminary Second Quarter 2023 Financial Guidance
Page
2
July
18, 2023
The
foregoing guidance on anticipated results for the three months ended June 30, 2023, has not been reviewed by our auditors, is based on
preliminary information as of the date hereof and is subject to material changes following completion of the quarter-end review process
and other adjustments that may be made before our financial results are finalized. In addition, these preliminary unaudited results are
not comprehensive financial results for the quarter ended June 30, 2023, should not be viewed as a substitute for complete GAAP financial
statements or more comprehensive financial information, and are not indicative of the results for any future period.
Forward-Looking
Statements
This
press release contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act
of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.”
Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties which may
cause results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties
that could cause actual results to differ from those predicted include, among others, risks related to completion of applicable quarter-end
closing procedures. Additional risks and uncertainties are more fully described in Harrow’s filings with the Securities and Exchange
Commission (“SEC”), including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Such documents may be
read free of charge on the SEC’s web site at sec.gov. Undue reliance should not be placed on forward-looking statements,
which speak only as of the date they are made. Except as required by law, Harrow undertakes no obligation to update any forward-looking
statements to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated
events.
Contact:
Jamie
Webb
Director
of Communications and Investor Relations
jwebb@harrowinc.com
615-733-4737
-END-
Exhibit 99.2

Harrow
Announces Proposed Public Offering of Common Stock
NASHVILLE,
Tenn., July 18, 2023 – Harrow (Nasdaq: HROW), a leading U.S. eyecare pharmaceutical company, today announced a proposed underwritten
registered public offering of its common stock, subject to market and certain other conditions. Harrow expects to grant the underwriters
a 30-day option to purchase additional shares of its common stock in connection with the offering.
The
Company expects to use the net proceeds from the sale of the common stock to fund the initial amount payable for an acquisition, with
the remaining net proceeds available for general corporate purposes, including funding future strategic product acquisitions and related
investments, making capital expenditures, and funding working capital and other cash needs, including tax withholding obligations in
connection with the settlement of outstanding equity awards vesting as a result of the achievement of stock price targets.
B.
Riley Securities, Inc. is acting as book-running manager for this offering. Lake Street Capital Markets, LLC is acting as lead-manager
and Ladenburg Thalmann & Co. Inc. is acting as co-manager for this offering.
The
common stock will be offered by Harrow under its shelf registration statement on Form S-3, which was declared effective by the Securities
and Exchange Commission (the “SEC”) on June 6, 2022. The offering of the common stock will be made solely by means of a prospectus
supplement and accompanying base prospectus, which will be filed with the SEC. Copies of the prospectus supplement and the accompanying
base prospectus may be obtained on the SEC’s website at www.sec.gov, or by contacting B. Riley Securities by phone at (703)
312-9580, or by emailing prospectuses@brileyfin.com.
This
press release shall not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation
or sale in any jurisdiction in which such offer, solicitation or sale is unlawful.
About
Harrow
Harrow
Health, Inc. (Nasdaq: HROW) is a leading U.S. eyecare pharmaceutical company engaged in the discovery, development, and commercialization
of innovative ophthalmic prescription therapies that are accessible and affordable. Harrow owns U.S. commercial rights to ten branded
FDA-approved ophthalmic pharmaceutical products. Harrow also owns and operates ImprimisRx, a leading U.S. ophthalmic-focused pharmaceutical
compounding business, which also serves as a mail-order pharmacy licensed to ship prescription medications in all 50 states. Harrow has
non-controlling equity positions in Surface Ophthalmics, Inc. and Melt Pharmaceuticals, Inc., companies that began as subsidiaries
of Harrow. Harrow also owns royalty rights in four late-stage drug candidates being developed by Surface and Melt.
HROW Announces Proposed Public Offering of Common Stock |
| Page 2 |
July 18, 2023 |
Forward-Looking
Statements
This
press release contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act
of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.”
Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties which may
cause results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties
that could cause actual results to differ from those predicted include, among others, risks related to: liquidity or results of operations;
our ability to successfully implement our business plan, develop and commercialize our products, product candidates and proprietary formulations
in a timely manner or at all, identify and acquire additional products, manage our pharmacy operations, service our debt, obtain financing
necessary to operate our business, recruit and retain qualified personnel, manage any growth we may experience and successfully realize
the benefits of our previous acquisitions and any other acquisitions and collaborative arrangements we may pursue; competition from pharmaceutical
companies, outsourcing facilities and pharmacies; general economic and business conditions, including inflation and supply chain challenges;
regulatory and legal risks and uncertainties related to our pharmacy operations and the pharmacy and pharmaceutical business in general;
and physician interest in and market acceptance of our current and any future formulations and compounding pharmacies generally. These
and additional risks and uncertainties are more fully described in Harrow’s filings with the Securities and Exchange Commission
(“SEC”), including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Such documents may be read free
of charge on the SEC’s web site at sec.gov. Undue reliance should not be placed on forward-looking statements, which speak
only as of the date they are made. Except as required by law, Harrow undertakes no obligation to update any forward-looking statements
to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
Contact:
Jamie
Webb
Director
of Communications and Investor Relations
jwebb@harrowinc.com
615-733-4737
Exhibit 99.3



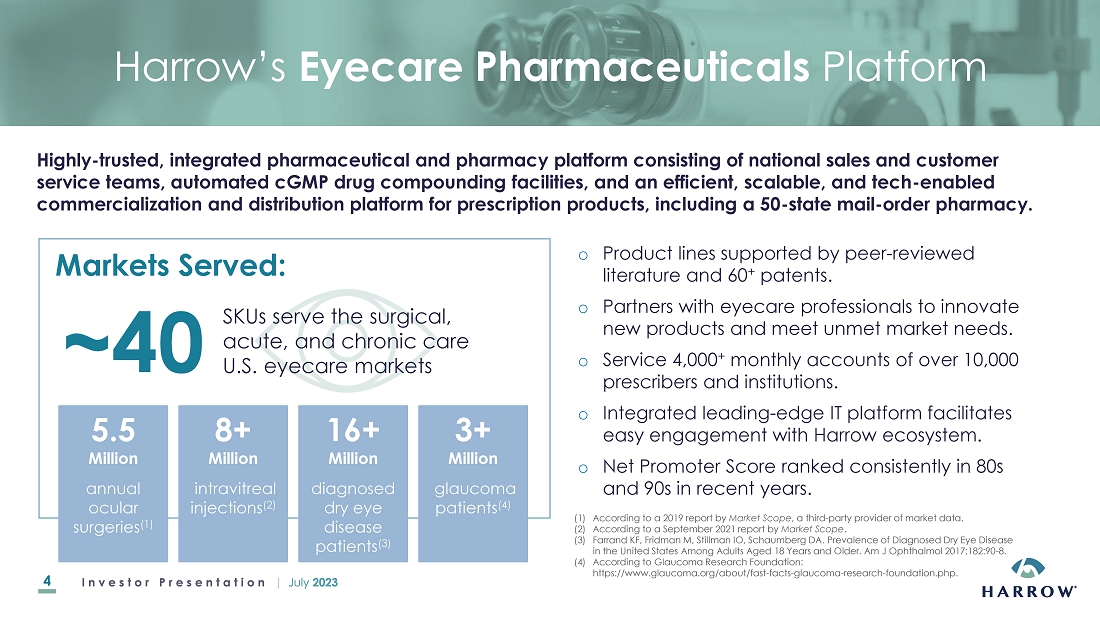
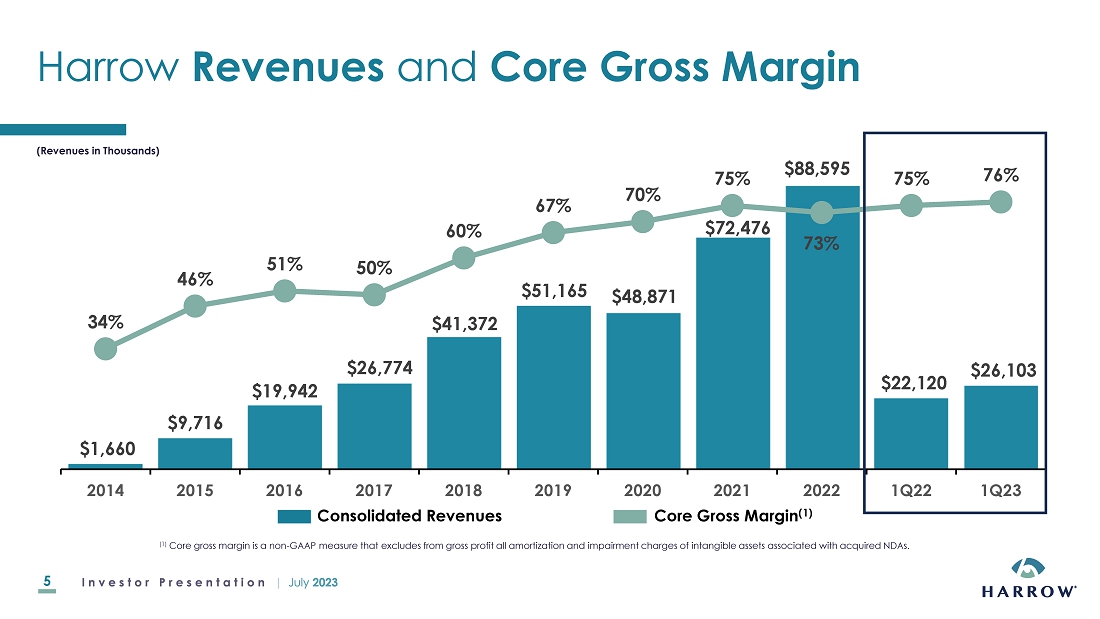

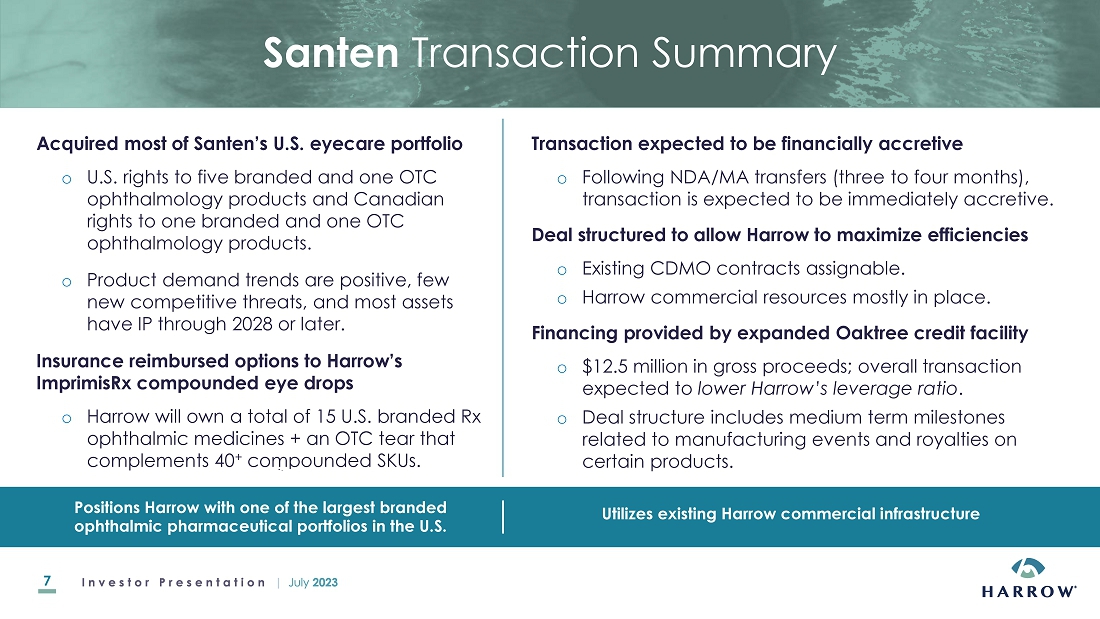

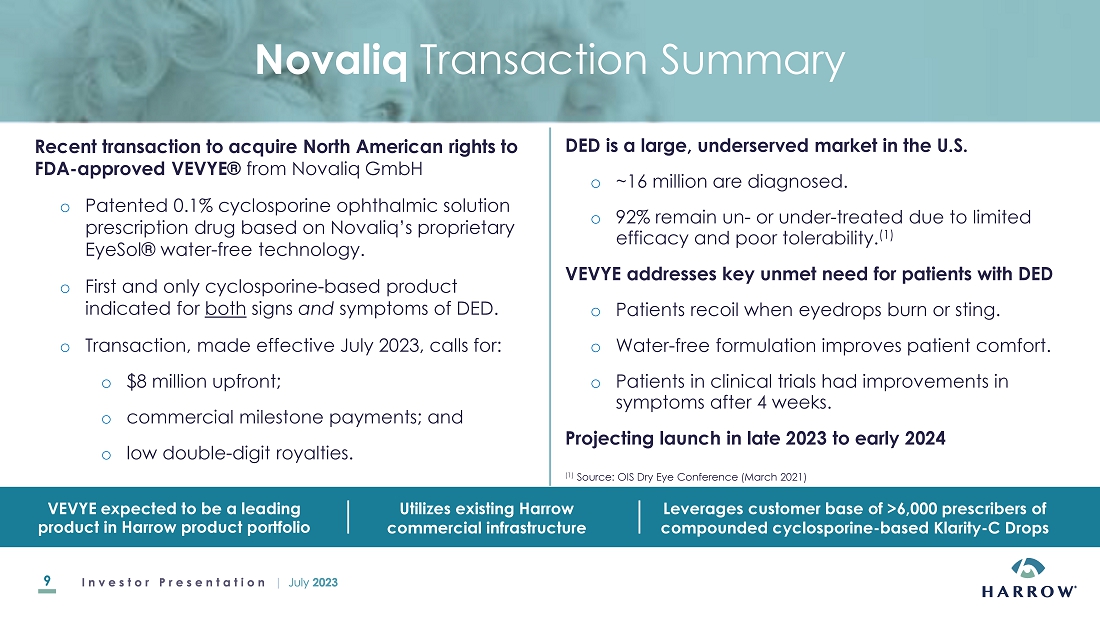

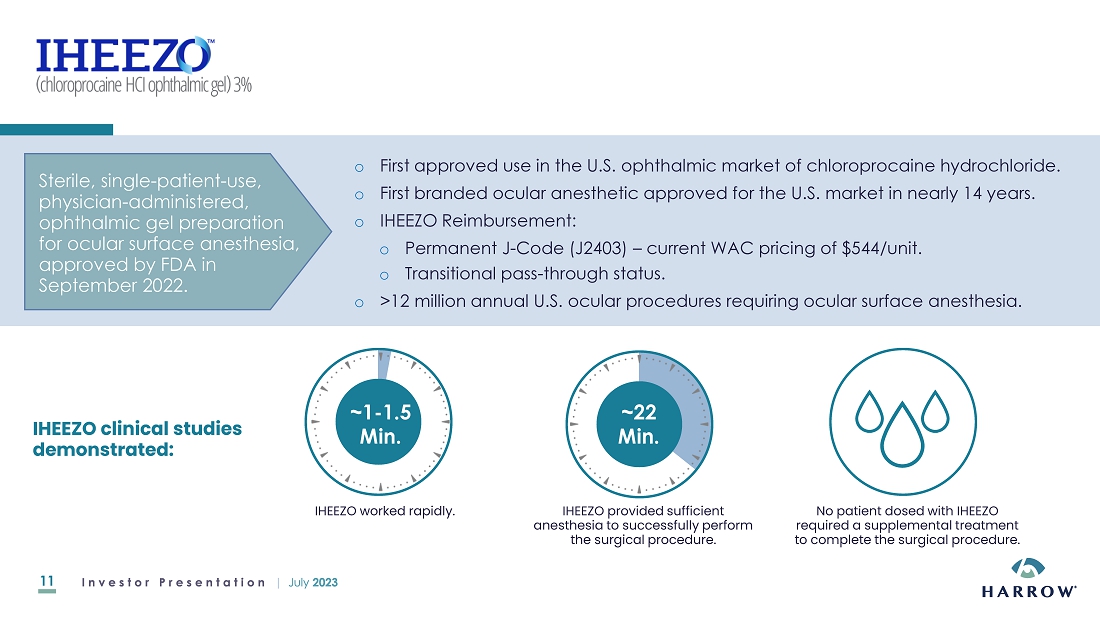

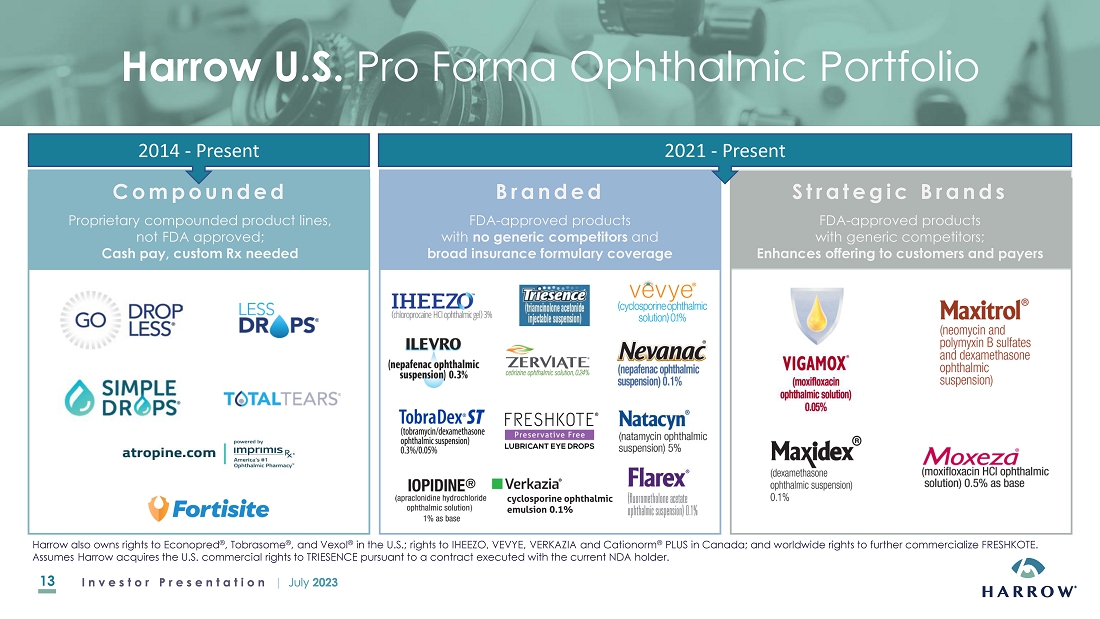
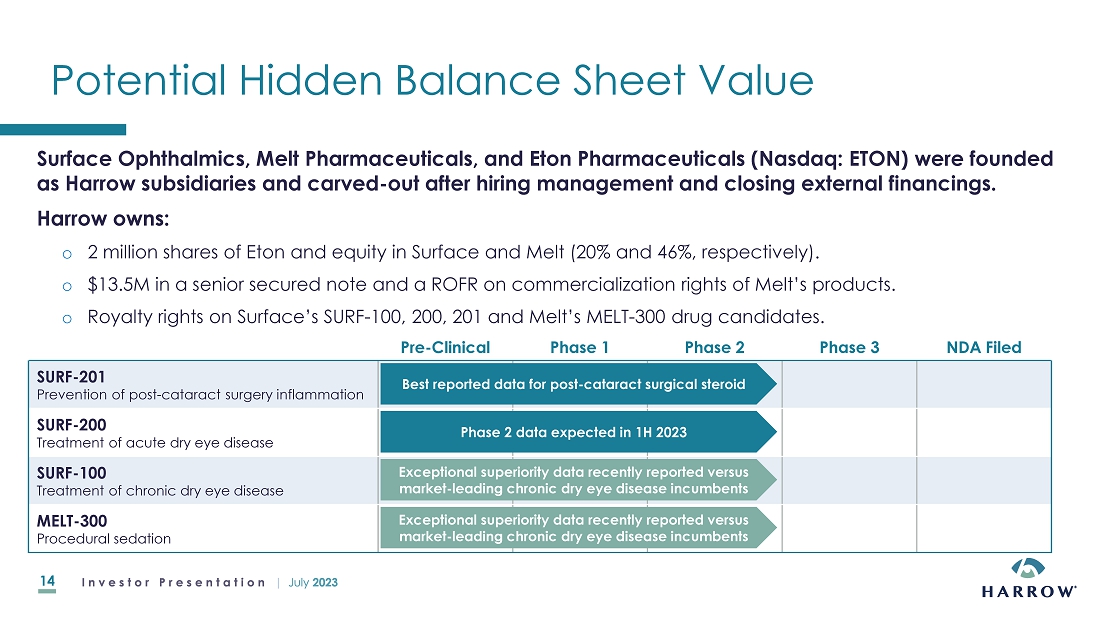

Exhibit 99.4
Harrow
Acquires U.S. and Canadian Commercial Rights to VEVYE®
(Cyclosporine
Ophthalmic Solution) 0.1% from Novaliq
VEVYE®
is the First and Only Cyclosporine-Based Product Indicated for the Treatment
of
Both Signs and Symptoms of Dry Eye Disease with Efficacy Demonstrated After Four Weeks
VEVYE® is
the Only Water-Free Ophthalmic Product with Convenient Twice-Daily (BID) Dosing
NASHVILLE,
Tenn. And HEIDELBERG, Germany, July 18, 2023 – Harrow (Nasdaq: HROW), a leading U.S. eyecare pharmaceutical company, and Novaliq
GmbH, a German biopharmaceutical company focusing on first- and best-in-class ocular therapeutics, today announced an agreement under
which Harrow will acquire the U.S. and Canadian commercial rights for VEVYE® (cyclosporine ophthalmic solution) 0.1%, a patented,
non-preserved, ophthalmic solution prescription drug based on Novaliq’s proprietary EyeSol® water-free technology. VEVYE, which
is dispensed topically in a unique 10 microliter per one drop and is labeled for twice-daily (BID) dosing, is the first
and only cyclosporine-based product indicated for the treatment of both signs and symptoms of dry eye disease (DED). VEVYE was approved
on May 30, 2023, by the U.S. Food and Drug Administration (FDA).
In
commenting on the transaction, Mark L. Baum, Chairman and Chief Executive Officer of Harrow, said, “The acquisition of the U.S.
and Canadian commercial rights to VEVYE demonstrates
our commitment to the highly underserved dry eye and ocular surface inflammation markets. We are particularly excited about adding VEVYE
to our portfolio because of our strong belief that the U.S. DED market is in need of a cyclosporine-based product that is generally well
tolerated, improves both the signs and symptoms of DED and, critically, reduces the time it takes for patients to experience relief from
this all-too-common and, in many cases, debilitating disease. VEVYE not only feels better in the eye, but it performs differently, and
we believe it addresses the numerous unmet needs in the large and growing U.S. DED market. We look forward to making VEVYE available
in the U.S. later this year.”
“There’s
good news for dry eye patients and for our colleagues,” commented Laura M. Periman, M.D., Director of Dry Eye Services and Clinical
Research, Periman Eye Institute, in Seattle, Washington. “VEVYE, which is expected to be available soon, is a unique cyclosporine
formulation indicated for treatment of the signs and symptoms of DED. The rapid onset and magnitude of improvements on ocular surface
epithelial damage, combined with the tolerability of the non-aqueous vehicle, are key differentiators to existing cyclosporin formulations.
These features represent an exciting advancement in addressing the medical needs of dry eye patients and clinicians.”
“For
patients with chronic and symptomatic dry eye disease, the tolerability profile of the medication can be critical for compliance and
treatment success,” said Paul Karpecki, O.D., director, Cornea and External Disease, Kentucky Eye Institute,
and associate professor, University of Pikeville, Kentucky College of Optometry. “Most patients are not comfortable
with drops in their eyes that cause burning or stinging. As a water-free drug product, VEVYE does not require potentially irritating
ingredients, such as preservatives, oils or surfactants, and has demonstrated in clinical trials a high patient satisfaction rate. Having
a new treatment option with a favorable comfort and tolerability profile is a significant advancement for the dry eye patient, especially
those who experience burning and stinging with topical eye medications.”
Christian
Roesky, Ph.D., Chief Executive Officer of Novaliq, stated, “We are excited to partner with Harrow, one of the fastest growing and
most dynamic ophthalmic pharmaceutical companies in the U.S., to commercialize VEVYE in the U.S. and Canadian markets. Harrow and its
commercial team have a distinguished track record for successfully commercializing new and clinically important pharmaceutical products
in the U.S. market, and they specifically have many years of experience successfully marketing cyclosporine-based formulations to U.S.
eyecare professionals. The Novaliq team looks forward to supporting Harrow during the launch of VEVYE, a truly unique and powerful new
treatment option for U.S. eyecare professionals and the more than 16 million Americans who have been diagnosed with DED.”
HROW Acquires U.S. and Canadian Rights to VEVYE® (Cyclosporine Ophthalmic
Solution) 0.1% |
| Page 2 |
July 18, 2023 |
VEVYE
Clinical Data
The
safety and efficacy of VEVYE (development name: CyclASol®) for the treatment of dry eye disease were assessed in a total of 1,369
patients with dry eye disease, of which 738 received VEVYE.
Study
CYS-001 (NCT02113293) was the first-in-human study and was conducted to investigate the safety, tolerability, and pharmacokinetics
(PK) in healthy volunteers. In this study, VEVYE was shown to be safe, and no systemic exposure of cyclosporin was observed after ocular
administration.
Study
CYS-002 (NCT02617667, Wirta et al 2019) demonstrated that VEVYE-dosed patients showed a statistically significant early and clinically
meaningful increase in Schirmer’s tear test score at Day 29 compared to vehicle. Additionally, VEVYE showed greater improvement
in corneal and conjunctival staining compared to (i) vehicle and (ii) Restasis® over the four-month treatment period. The favorable
safety and tolerability profile of VEVYE was confirmed.
Study
CYS-003 (ESSENCE-1; NCT03292809, Sheppard et al 2021) confirmed the effects seen in CYS-002. Compared to vehicle at the end of treatment,
there was a statistically significant higher percentage of patients with increases of ≥10 mm from baseline in Schirmer’s tear
test score at Day 85. Notably, the study demonstrated statistically significant reduction in total, central corneal fluorescein and conjunctival
staining scores favoring VEVYE at all time points, in addition to VEVYE meeting the primary endpoint of the study. 52.9% of patients
responded within four weeks with a clinically meaningful improvement of ≥3 grades in total corneal staining, which was significantly
higher compared to vehicle. Responders showed statistically significant improvements in a variety of symptoms compared to non-responders.
VEVYE was safe, well tolerated, and comfortable over the three-month treatment duration.
Study
CYS-004 (ESSENCE-2; NCT04523129, Akpek et al 2023) was designed to replicate CYS-003 and met the primary corneal staining endpoint.
In this study, 71.6% of patients responded within four weeks with a clinically meaningful improvement of ≥3 grades in total corneal
staining. Again, responders showed statistically significant improvements in a variety of symptoms compared to non-responders at Day
29. Subjects with high central corneal staining at baseline were shown to benefit from VEVYE with statistically significant improvements
in their blurred vision score compared to vehicle CYS-004 studies as shown in CYS-003. Schirmer’s tear test responses of ≥10
mm increase was statistically significantly higher in the VEVYE compared vehicle at Day 29. VEVYE was safe, well tolerated, and comfortable
over the one-month duration.
Study
CYS-005 (NCT04523142, Wirta et al 2023) was an open label extension study of CYS-004. VEVYE was shown to be safe and well tolerated
during long-term use over 12 months. Sign and symptom endpoints continued to improve over the course of the study demonstrating sustained
efficacy over 52 weeks of therapy in both signs and symptoms.
| 1. | Wirta
DL, Torkildsen GL, Moreira HR, Lonsdale JD, Ciolino JB, Jentsch G, Beckert M, Ousler GM,
Steven P, Krösser S. A Clinical Phase II Study to Assess Efficacy, Safety, and Tolerability
of Waterfree Cyclosporine Formulation for Treatment of Dry Eye Disease. Ophthalmology. 2019;
126:793-800 |
| 2. | Sheppard
JD, Wirta DL, McLaurin E, Boehmer BE, Ciolino CB, Meides AS, Schlüter T, Ousler GW,
Usner D, Krösser S. A Water-free 0.1% Cyclosporine A Solution for Treatment of Dry Eye
Disease: Results of the Randomized Phase II/III ESSENCE Study. Cornea. 2021; 40:1290-1297 |
| 3. | Akpek
EK, Wirta DL, Downing JE, Tauber J, Sheppard JD, Ciolino JB, Meides AS, Krösser S: Efficacy
and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate
to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial. JAMA Ophthalmology. 2023;
141(5):459-466. |
| 4. | Wirta
DL, Krösser S, Long -Term Safety and Efficacy of a Water-Free Cyclosporine Ophthalmic
Solution for the Treatment of Dry-Eye Disease: ESSENCE-2-OLE study. ASCRS 2023 paper presentation. |
HROW Acquires U.S. and Canadian Rights to VEVYE® (Cyclosporine Ophthalmic
Solution) 0.1% |
| Page 3 |
July 18, 2023 |
About
VEVYE® (cyclosporine ophthalmic solution) 0.1%
VEVYE
(cyclosporine ophthalmic solution) 0.1%, non-preserved, for topical ophthalmic use.
INDICATIONS
AND USAGE
VEVYE
is indicated for the treatment of the signs and symptoms of dry eye disease.
CONTRAINDICATIONS
None.
WARNINGS
AND PRECAUTIONS
Potential
for Eye Injury and Contamination. To avoid the potential for eye injury and/or contamination, patients should not touch the bottle
tip to the eye or other surfaces.
Use
with Contact Lenses. VEVYE should not be administered while wearing contact lenses. If contact lenses are worn, they should be removed
prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of VEVYE ophthalmic solution.
ADVERSE
REACTIONS
Clinical
Trials Experience. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the
clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed
in practice. In clinical trials with 738 subjects receiving at least 1 dose of VEVYE, the most common adverse reactions were instillation
site reactions (8%) and temporary decreases in visual acuity (3%).
USE
IN SPECIAL POPULATIONS
Pregnancy.
There are no adequate and well-controlled studies of VEVYE administration in pregnant women to inform a drug-associated risk.
Lactation.
Caution should be exercised when VEVYE is administered to a nursing woman.
For
additional information about VEVYE®, please see the Full Prescribing Information.
About
Novaliq
Novaliq
is a private biopharmaceutical company focusing on the development and commercialization of first- and best-in-class ocular therapeutics
based on EyeSol®, the worldwide first water-free technology. Novaliq GmbH is headquartered in Heidelberg, Germany, and Novaliq Inc.
has an office in Cambridge, MA, USA. The long-term shareholder is dievini Hopp BioTech holding GmbH & Co. KG, an active investor
in Life and Health Sciences companies. More on novaliq.com.
About
Harrow
Harrow
Health, Inc. (Nasdaq: HROW) is a leading U.S. eyecare pharmaceutical company engaged in the discovery, development, and commercialization
of innovative ophthalmic prescription therapies that are accessible and affordable. Harrow owns U.S. commercial rights to ten branded
FDA-approved ophthalmic pharmaceutical products. Harrow also owns and operates ImprimisRx, a leading U.S. ophthalmic-focused pharmaceutical
compounding business, which also serves as a mail-order pharmacy licensed to ship prescription medications in all 50 states. Harrow has
non-controlling equity positions in Surface Ophthalmics, Inc. and Melt Pharmaceuticals, Inc., companies that began as subsidiaries
of Harrow. Harrow also owns royalty rights in four late-stage drug candidates being developed by Surface and Melt.
HROW Acquires U.S. and Canadian Rights to VEVYE® (Cyclosporine Ophthalmic
Solution) 0.1% |
| Page 4 |
July 18, 2023 |
Harrow
Forward-Looking Statements
This
press release contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act
of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.”
Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties which may
cause results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties
that could cause actual results to differ from those predicted include, among others, risks related to: liquidity or results of operations;
our ability to successfully implement our business plan, develop and commercialize our products, product candidates and proprietary formulations
in a timely manner or at all, identify and acquire additional products, manage our pharmacy operations, service our debt, obtain financing
necessary to operate our business, recruit and retain qualified personnel, manage any growth we may experience and successfully realize
the benefits of our previous acquisitions and any other acquisitions and collaborative arrangements we may pursue; competition from pharmaceutical
companies, outsourcing facilities and pharmacies; general economic and business conditions, including inflation and supply chain challenges;
regulatory and legal risks and uncertainties related to our pharmacy operations and the pharmacy and pharmaceutical business in general;
and physician interest in and market acceptance of our current and any future formulations and compounding pharmacies generally. These
and additional risks and uncertainties are more fully described in Harrow’s filings with the Securities and Exchange Commission
(“SEC”), including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Such documents may be read free
of charge on the SEC’s web site at sec.gov. Undue reliance should not be placed on forward-looking statements, which speak
only as of the date they are made. Except as required by law, Harrow undertakes no obligation to update any forward-looking statements
to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
Contacts:
| Harrow: |
Novaliq: |
| |
|
| Investors |
Media |
| |
|
| Jamie
Webb |
Simone
Angstmann-Mehr |
| Director
of Communications and Investor Relations |
info@novaliq.com |
| jwebb@harrowinc.com |
|
| 615-733-4737 |
|
Media
Deb
Holliday
Holliday
Communications, Inc.
deb@hollidaycommunications.net
412-877-4519
Exhibit
99.5

Harrow
Acquires Santen’s Branded Ophthalmic Portfolio
Transaction
Includes U.S. and Canadian Commercial Rights to FLAREX®, NATACYN®, TOBRADEX® ST, VERKAZIA®, ZERVIATE®, and Non-Prescription
Brands FRESHKOTE® and Cationorm® PLUS
NASHVILLE,
Tenn., July 18, 2023 – Harrow (Nasdaq: HROW), a leading U.S. eyecare pharmaceutical company, today announced the signing of
agreements with affiliates of Santen Pharmaceutical Co., Ltd. (“Santen”) under which Harrow will acquire certain U.S. and
Canadian commercial rights for the following branded products from Santen:
U.S.
Products:
| ● | FLAREX®
(fluorometholone acetate ophthalmic suspension) 0.1%, a corticosteroid indicated for use
in the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar
conjunctiva, cornea, and anterior segment of the eye. |
| ● | NATACYN®
(natamycin ophthalmic suspension) 5%, a sterile antifungal indicated for the treatment of
fungal blepharitis, conjunctivitis, and keratitis caused by susceptible organisms, including
Fusarium solani keratitis. |
| ● | TOBRADEX®
ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05%, an antibiotic and corticosteroid
combination for steroid-responsive inflammatory ocular conditions for which a corticosteroid
is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular
infection exists. |
| ● | VERKAZIA®
(cyclosporine ophthalmic emulsion) 0.1%, a calcineurin inhibitor immunosuppressant indicated
for the treatment of vernal keratoconjunctivitis (VKC) in children and adults and holds orphan-drug
exclusivity. |
| ● | ZERVIATE®
(cetirizine ophthalmic solution) 0.24%, a histamine-1 (H1) receptor antagonist indicated
for treatment of ocular itching associated with allergic conjunctivitis. |
| ● | FRESHKOTE®,
used as a lubricant to reduce further irritation or to relieve dryness of the eye. |
Canadian
Products:
| ● | VERKAZIA®
(cyclosporine ophthalmic emulsion) 0.1%, a calcineurin inhibitor immunosuppressant indicated
for the treatment of vernal keratoconjunctivitis (VKC) in children from four years of age
through adolescence. |
| ● | Cationorm®
PLUS, a preservative-free emulsion for the treatment of dry eye symptoms and for the treatment
of signs and symptoms of ocular allergy. |
Please
see select Important Safety Information for these products and links to the Full Prescribing Information at the end of this release.
In
commenting on the transaction, Mark L. Baum, Chairman and Chief Executive Officer of Harrow, stated, “This acquisition furthers
Harrow’s goal of becoming a leader in the top tier of U.S. ophthalmic pharmaceutical companies, makes Harrow’s branded portfolio
one of the most comprehensive in the U.S. market, and is expected to be immediately financially accretive upon the transfer of the product
marketing authorizations. We are excited to add several high utility and trusted products that serve the ophthalmic surgical market,
a market in which we already have a strong presence, and significantly expand the breadth of our portfolio, which will now include the
only FDA-approved ophthalmic antifungal; a patented and ‘orphan-designated’ product for the nearly 50,000 Americans suffering
from the rare disease vernal keratoconjunctivitis (or VKC); a patented prescription drug to treat ocular itching associated with allergies;
and two patented non-prescription brands serving patients managing dry eye symptoms.”
| HROW Acquires Santen’s Branded Ophthalmic Portfolio |
| Page 2 |
| July 18, 2023 |
Richard
L. Lindstrom, M.D. added, “As an ophthalmic surgeon of nearly 50 years and an advisor to Mark and the Harrow leadership team for
many years, I am pleased to see Harrow step up and assemble not only a formidable posterior segment offering with products like IHEEZO®
and TRIESENCE®, but also an impressive array of innovative anterior segment products that U.S. ophthalmologists and optometrists
rely on to care for their patients. While some ophthalmic pharmaceutical companies have decided to place less emphasis on the anterior
segment despite the growing demand in this category of eyecare, with this acquisition, few companies, if any, can match the scope and
depth of Harrow’s ophthalmic product offerings, especially in the anterior segment. I believe this level of commitment to the eyecare
professional should further strengthen and expand the many relationships Harrow has been able to forge over the past 10 years.”
Financing
for the transaction was provided through the expansion of Harrow’s secured credit facility with funds managed by Oaktree Capital
Management, L.P. Harrow management expects the transaction to reduce the Company’s aggregate leverage ratio of adjusted EBITDA
to debt.
About
Harrow
Harrow
Health, Inc. (Nasdaq: HROW) is a leading U.S. eyecare pharmaceutical company engaged in the discovery, development, and commercialization
of innovative ophthalmic prescription therapies that are accessible and affordable. Harrow owns U.S. commercial rights to ten branded
FDA-approved ophthalmic pharmaceutical products. Harrow also owns and operates ImprimisRx, a leading U.S. ophthalmic-focused pharmaceutical
compounding business, which also serves as a mail-order pharmacy licensed to ship prescription medications in all 50 states. Harrow has
non-controlling equity positions in Surface Ophthalmics, Inc. and Melt Pharmaceuticals, Inc., companies that began as subsidiaries
of Harrow. Harrow also owns royalty rights in four late-stage drug candidates being developed by Surface and Melt.
Forward-Looking
Statements
This
press release contains “forward-looking statements” within the meaning of the U.S. Private Securities Litigation Reform Act
of 1995. Any statements in this release that are not historical facts may be considered such “forward-looking statements.”
Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties which may
cause results to differ materially and adversely from the statements contained herein. Some of the potential risks and uncertainties
that could cause actual results to differ from those predicted include, among others, risks related to: liquidity or results of operations;
our ability to successfully implement our business plan, develop and commercialize our products, product candidates and proprietary formulations
in a timely manner or at all, identify and acquire additional products, manage our pharmacy operations, service our debt, obtain financing
necessary to operate our business, recruit and retain qualified personnel, manage any growth we may experience and successfully realize
the benefits of our previous acquisitions and any other acquisitions and collaborative arrangements we may pursue; competition from pharmaceutical
companies, outsourcing facilities and pharmacies; general economic and business conditions, including inflation and supply chain challenges;
regulatory and legal risks and uncertainties related to our pharmacy operations and the pharmacy and pharmaceutical business in general;
and physician interest in and market acceptance of our current and any future formulations and compounding pharmacies generally. These
and additional risks and uncertainties are more fully described in Harrow’s filings with the Securities and Exchange Commission
(“SEC”), including its Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Such documents may be read free
of charge on the SEC’s web site at sec.gov. Undue reliance should not be placed on forward-looking statements, which speak
only as of the date they are made. Except as required by law, Harrow undertakes no obligation to update any forward-looking statements
to reflect new information, events, or circumstances after the date they are made, or to reflect the occurrence of unanticipated events.
Contacts:
| Investors |
Media |
| |
|
| Jamie Webb |
Deb Holliday |
| Director of Communications and Investor Relations |
Holliday Communications, Inc. |
| jwebb@harrowinc.com |
deb@hollidaycommunications.net |
| 615-733-4737 |
412-877-4519 |
| HROW Acquires Santen’s Branded Ophthalmic Portfolio |
| Page 3 |
| July 18, 2023 |
Information
for U.S. Products:
About
FLAREX® (fluorometholone acetate ophthalmic suspension) 0.1%
INDICATIONS
AND USAGE
FLAREX®
(fluorometholone acetate ophthalmic suspension) 0.1% is indicated for use in the treatment of steroid responsive inflammatory conditions
of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye.
CONTRAINDICATIONS
Contraindicated
in acute superficial herpes simplex keratitis, vaccinia, varicella, and most other viral diseases of cornea and conjunctiva; mycobacterial
infection of the eye; fungal diseases; acute purulent untreated infections, which like other diseases caused by microorganisms, may be
masked or enhanced by the presence of the steroid; and in those persons who have known hypersensitivity to any component of this preparation.
SELECT
WARNINGS
FOR
TOPICAL OPHTHALMIC USE. NOT FOR INJECTION. Use in the treatment of herpes simplex infection requires great caution. Prolonged use may
result in glaucoma, damage to the optic nerve, defect in visual acuity and visual field, cataract formation and/or may aid in the establishment
of secondary ocular infections from pathogens due to suppression of host response. Acute purulent infections of the eye may be masked
or exacerbated by presence of steroid medication. Topical ophthalmic corticosteroids may slow corneal wound healing. In those diseases
causing thinning of the cornea or sclera, perforation has been known to occur. If these products are used for 10 days or longer, intraocular
pressure (IOP) should be routinely monitored.
ADVERSE
REACTIONS
Glaucoma
with optic nerve damage, visual acuity and field defects, cataract formation, secondary ocular infection following suppression of host
response, and perforation of the globe may occur.
For
complete product information about FLAREX®, including important safety information, please visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=19918ea5-8568-44d6-b8ee-7b2197cee85c.
About
NATACYN® (natamycin ophthalmic suspension) 5%
INDICATIONS
AND USAGE
NATACYN®
(natamycin ophthalmic suspension) 5% is indicated for the treatment of fungal blepharitis, conjunctivitis, and keratitis caused by susceptible
organisms including Fusarium solani keratitis. As in other forms of suppurative keratitis, initial and sustained therapy of fungal
keratitis should be determined by the clinical diagnosis, laboratory diagnosis by smear and culture of corneal scrapings and drug response.
Whenever possible the in vitro activity of natamycin against the responsible fungus should be determined. The effectiveness of natamycin
as a single agent in fungal endophthalmitis has not been established.
CONTRAINDICATIONS
NATACYN®
(natamycin ophthalmic suspension) 5% is contraindicated in individuals with a history of hypersensitivity to any of its components.
SELECT
PRECAUTIONS
General:
FOR TOPICAL OPHTHALMIC USE ONLY — NOT FOR INJECTION. Failure of improvement of keratitis following 7-10 days of administration
of the drug suggests that the infection may be caused by a microorganism not susceptible to natamycin.
| HROW Acquires Santen’s Branded Ophthalmic Portfolio |
| Page 4 |
| July 18, 2023 |
ADVERSE
REACTIONS
The
following events have been identified during post-marketing use of NATACYN ® (natamycin ophthalmic suspension) 5% in clinical practice:
allergic reaction, change in vision, chest pain, corneal opacity, dyspnea, eye discomfort, eye edema, eye hyperemia, eye irritation,
eye pain, foreign body sensation, paresthesia, and tearing.
For
complete product information about NATACYN®, including important safety information, please visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2818fcb8-5bac-41fb-864e-3b598308a428.
About
TOBRADEX® ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05%
INDICATIONS
AND USAGE
TOBRADEX®
ST ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and
where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
CONTRAINDICATIONS
Nonbacterial
Etiology: TOBRADEX® ST, as with other ophthalmic corticosteroids, is contraindicated in most viral diseases of the cornea and
conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial
infection of the eye and fungal diseases of ocular structures.
Hypersensitivity:
Hypersensitivity to a component of the medication.
SELECT
WARNINGS AND PRECAUTIONS
Intraocular
Pressure Increase: Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity
and fields of vision. If this product is used for 10 days or longer, intraocular pressure (IOP) should be monitored.
Aminoglycoside
Sensitivity: Sensitivity to topically applied aminoglycosides may occur.
Cataracts:
Use of corticosteroids may result in posterior subcapsular cataract formation.
Delayed
Healing: The use of steroids after cataract surgery may delay healing.
Bacterial
Infections: Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections.
Viral
Infections: Use in patients with a history of herpes simplex requires great caution as it may prolong the course and may exacerbate
the severity of many viral infections of the eye (including herpes simplex).
Fungal
Infections: Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application.
Vision
Blurred: Vision may be temporarily blurred following dosing with TOBRADEX ST. Care should be exercised in operating machinery or
driving a motor vehicle.
Risk
of Contamination: Do not touch the dropper tip of the bottle to any surface, as this may contaminate the contents.
Contact
Lens Use: TOBRADEX® ST contains benzalkonium chloride, an antimicrobial preservative, that may be absorbed by soft contact lenses.
Contact lenses should not be worn during the use of TOBRADEX ST.
| HROW Acquires Santen’s Branded Ophthalmic Portfolio |
| Page 5 |
| July 18, 2023 |
ADVERSE
REACTIONS
Clinical
Trials Experience: The most frequent adverse reactions to topical ocular tobramycin (TOBREX ®) are hypersensitivity and localized
ocular toxicity, including eye pain, eyelids pruritus, eyelid edema, and conjunctival hyperemia. These reactions occur in less than 4%
of patients.
For
complete product information about TOBRADEX® ST, including important safety information, please visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c2d7325e-4f58-5590-e053-2a95a90ace1b.
About
VERKAZIA® (cyclosporine ophthalmic emulsion) 0.1%
INDICATIONS
AND USAGE
VERKAZIA®
ophthalmic emulsion is indicated for the treatment of vernal keratoconjunctivitis (VKC) in children and adults.
ADVERSE
REACTIONS
The
most common adverse reactions reported in greater than 5% of patients were eye pain (12%) and eye pruritus (8%) which were usually transitory
and occurred during instillation.
For
complete product information about VERKAZIA®, including important safety information, please visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c795cd2f-89da-78e3-e053-2a95a90a9422.
About
ZERVIATE® (cetirizine ophthalmic solution) 0.24%
INDICATIONS
AND USAGE
ZERVIATE®
(cetirizine ophthalmic solution) 0.24% is indicated for the treatment of ocular itching associated with allergic conjunctivitis.
SELECT
WARNINGS AND PRECAUTIONS
Contamination
of Tip and Solution: As with any eye drop, care should be taken not to touch the eyelids or surrounding areas with the dropper tip
of the bottle or tip of the single-use container in order to avoid injury to the eye and to prevent contaminating the tip and solution.
Keep the multi-dose bottle closed when not in use. Discard the single-use container after using in each eye.
Contact
Lens Wear: Patients should be advised not to wear a contact lens if their eye is red.
ZERVIATE
should not be instilled while wearing contact lenses.
ADVERSE
REACTIONS
The
most commonly reported adverse reactions occurred in approximately 1–7% of patients treated with either ZERVIATE or vehicle. These
reactions were ocular hyperemia, instillation site pain, and visual acuity reduced.
For
complete product information about ZERVIATE®, including important safety information, please visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3e6fecc1-df71-4c01-a654-f55635617a7f
| HROW Acquires Santen’s Branded Ophthalmic Portfolio |
| Page 6 |
| July 18, 2023 |
Information
for Canadian Products
About
VERKAZIA® (cyclosporine topical ophthalmic emulsion) 0.1% w/v
Verkazia
(cyclosporine) is indicated for treatment of severe vernal keratoconjunctivitis in children from four years of age through adolescence.
For
complete Canadian product information about Verkazia, including important safety information, please visit: https://pdf.hres.ca/dpd_pm/00048991.PDF.
About
Cationorm® PLUS
Cationorm®
PLUS is an ophthalmic sterile preservative-free eye drop emulsion used for:
treatment
of dry eye symptoms: It helps to hydrate, lubricate and protect the ocular surface. It is recommended for the relief of dry eye symptoms
characterized by stinging, itching or burning eyes or by a foreign body sensation (sand, dust, etc.).
treatment
of signs and symptoms of ocular allergy: It is recommended for the relief of ocular allergy symptoms characterized by itching, tearing,
mucous discharge and photophobia, and the protection of the ocular surface (corneal staining improvement). Cationorm® PLUS can be
used in children from four years old.
Do
not use Cationorm® PLUS if you are allergic to any of the components of the product. This product is not intended for treating other
eye conditions. Please consult your doctor or pharmacist if you have any questions. If you currently use other eye drops, you should
wait at least 5 minutes between the administrations of each successive eye drop. It is recommended to use Cationorm® PLUS last.
Cationorm®
PLUS is compatible with all kinds of contact lenses.
In
very rare cases, a transient ocular discomfort such as: eye irritation, eye pain, eye redness, watery eyes, eye discharge, temporarily
blurred vision, eyelids inflammation, eyelids edema or transient discomfort at instillation can appear. These symptoms are also part
of typical symptoms of dry eye disease linked to the underlying existing medical conditions in the patient’s eyes suffering from
dry eye or ocular allergy.
v3.23.2
Cover
|
Jul. 18, 2023 |
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Jul. 18, 2023
|
| Entity File Number |
001-35814
|
| Entity Registrant Name |
HARROW
HEALTH, INC.
|
| Entity Central Index Key |
0001360214
|
| Entity Tax Identification Number |
45-0567010
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
102
Woodmont Blvd.
|
| Entity Address, Address Line Two |
Suite 610
|
| Entity Address, City or Town |
Nashville
|
| Entity Address, State or Province |
TN
|
| Entity Address, Postal Zip Code |
37205
|
| City Area Code |
(615)
|
| Local Phone Number |
733-4730
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Entity Emerging Growth Company |
false
|
| Common Stock, $0.001 par value per share |
|
| Title of 12(b) Security |
Common
Stock, $0.001 par value per share
|
| Trading Symbol |
HROW
|
| Security Exchange Name |
NASDAQ
|
| 8.625% Senior Notes due 2026 |
|
| Title of 12(b) Security |
8.625%
Senior Notes due 2026
|
| Trading Symbol |
HROWL
|
| Security Exchange Name |
NASDAQ
|
| 11.875% Senior Notes due 2027 |
|
| Title of 12(b) Security |
11.875%
Senior Notes due 2027
|
| Trading Symbol |
HROWM
|
| Security Exchange Name |
NASDAQ
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=HROW_CommonStock0.001ParValuePerShareMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=HROW_Sec8.625SeniorNotesDue2026Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementClassOfStockAxis=HROW_Sec11.875SeniorNotesDue2027Member |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Harrow (NASDAQ:HROW)
Historical Stock Chart
From Apr 2024 to May 2024
Harrow (NASDAQ:HROW)
Historical Stock Chart
From May 2023 to May 2024