false 0001708493 0001708493 2023-10-23 2023-10-23
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): October 23, 2023
Harpoon Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
| Delaware |
|
001-38800 |
|
47-3458693 |
| (State or Other Jurisdiction of Incorporation) |
|
(Commission
File Number) |
|
(IRS Employer Identification No.) |
|
|
|
| 611 Gateway Boulevard, Suite 400 South San Francisco, California |
|
94080 |
| (Address of Principal Executive Offices) |
|
(Zip Code) |
(650) 443-7400
(Registrant’s Telephone Number, Including Area Code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instructions A.2. below):
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
| Title of each class |
|
Trading
Symbol(s) |
|
Name of each exchange
on which registered |
| Common stock, par value $0.0001 per share |
|
HARP |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
On October 23, 2023, Harpoon Therapeutics, Inc., a Delaware corporation (the “Company”), presented updated interim monotherapy data from the Company’s Phase 1/2 clinical trial evaluating HPN328 in small cell lung cancer and other neuroendocrine tumor types at the European Society of Medical Oncology Congress (ESMO) 2023.
A copy of this presentation is being filed as Exhibit 99.1 hereto and incorporated by reference herein. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
| Item 9.01 |
Financial Statements and Exhibits. |
|
|
|
| Exhibit
No. |
|
Description |
|
|
| 99.1 |
|
ESMO Presentation |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934 the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Harpoon Therapeutics, Inc. |
|
|
|
|
| Date: October 23, 2023 |
|
|
|
By: |
|
/s/ Julie Eastland |
|
|
|
|
|
|
Julie Eastland |
|
|
|
|
|
|
President and Chief Executive Officer |

Spearheading Immunotherapies ESMO 2023
– HPN328 Investor webcast october 23, 2023 Exhibit 99.1

This presentation and accompanying
oral commentary contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,”
“target,” "goal," “estimate” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward-looking statements. These forward-looking
statements are based on Harpoon's expectations and assumptions as of the date of this presentation. Each of these forward-looking statements involves risks and uncertainties that could cause Harpoon's clinical development programs, future results or
performance to differ significantly from those expressed or implied by the forward-looking statements. Forward-looking statements contained in this presentation and accompanying oral commentary include, but are not limited to, statements about the
progress, timing, scope, design and anticipated results of clinical trials, the timing of the presentation of data, the association of data with potential treatment outcomes, the development and advancement of platforms and product candidates, the
timing of development milestones for platforms and product candidates and Harpoon’s cash sufficiency and runway. Many factors may cause differences between current expectations and actual results, including unexpected safety or efficacy data
observed during clinical studies, clinical trial site activation or enrollment rates that are lower than expected, changes in expected or existing competition, our ability to enter into strategic arrangements and collaborations and uncertainties in
the success of such arrangements, changes in the regulatory environment, the uncertainties and timing of the regulatory approval process, the risk that initial or interim results from a clinical trial may not be predictive of the final results of
the trial or the results of future trials, the risk that trials may be delayed and may not have satisfactory outcomes, and unexpected litigation or other disputes that impede clinical trial progress. Other factors that may cause Harpoon's actual
results to differ from those expressed or implied in the forward-looking statements in this presentation and accompanying oral commentary are discussed in Harpoon's filings with the U.S. Securities and Exchange Commission (SEC) including under
“Risk Factors” in Harpoon Therapeutics’ quarterly report on Form 10-Q for the quarter ended June 30, 2023, filed with the SEC on August 9, 2023 and our other filings from time to time. Except as required by law, Harpoon assumes no
obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. Certain information contained in this presentation and statements made orally during this
presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and Harpoon’s own internal estimates and research. While Harpoon believes these third-party studies, publications, surveys
and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In
addition, no independent source has evaluated the reasonableness or accuracy of Harpoon’s internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such
internal estimates and research. Forward-looking Statements
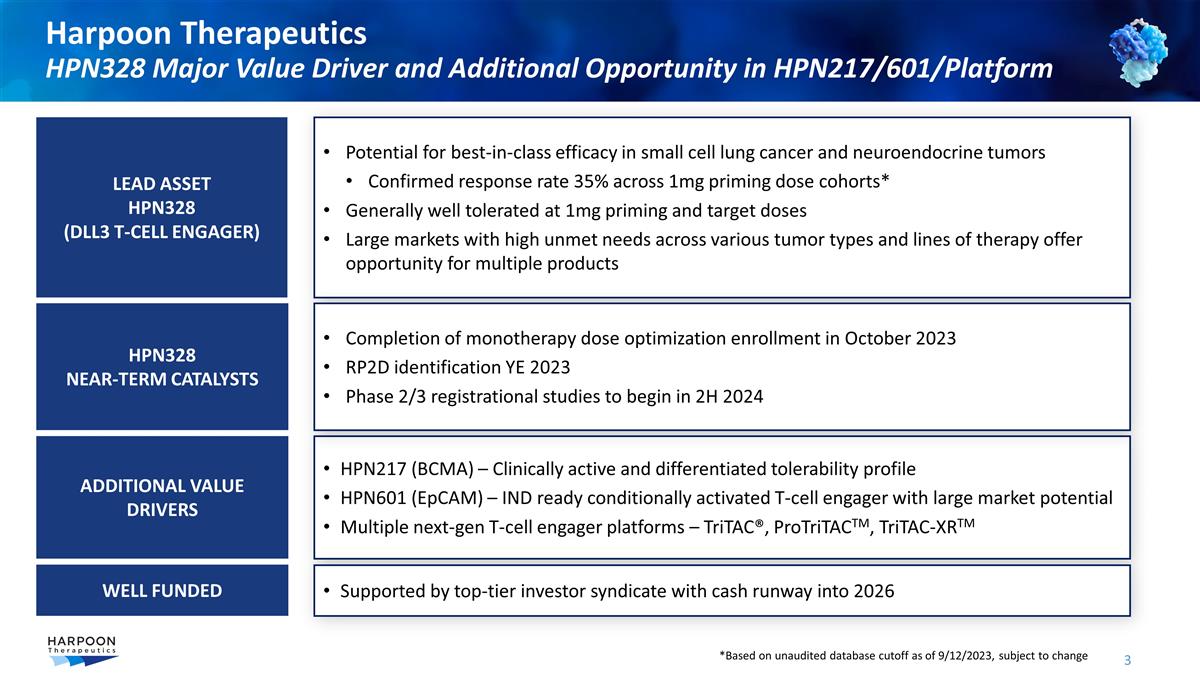
Harpoon Therapeutics HPN328 Major
Value Driver and Additional Opportunity in HPN217/601/Platform HPN217 (BCMA) – Clinically active and differentiated tolerability profile HPN601 (EpCAM) – IND ready conditionally activated T-cell engager with large market potential
Multiple next-gen T-cell engager platforms – TriTAC®, ProTriTACTM, TriTAC-XRTM Potential for best-in-class efficacy in small cell lung cancer and neuroendocrine tumors Confirmed response rate 35% across 1mg priming dose cohorts* Generally
well tolerated at 1mg priming and target doses Large markets with high unmet needs across various tumor types and lines of therapy offer opportunity for multiple products Completion of monotherapy dose optimization enrollment in October 2023 RP2D
identification YE 2023 Phase 2/3 registrational studies to begin in 2H 2024 Supported by top-tier investor syndicate with cash runway into 2026 ADDITIONAL VALUE DRIVERS LEAD ASSET HPN328 (DLL3 T-CELL ENGAGER) HPN328 NEAR-TERM CATALYSTS WELL FUNDED
*Based on unaudited database cutoff as of 9/12/2023, subject to change
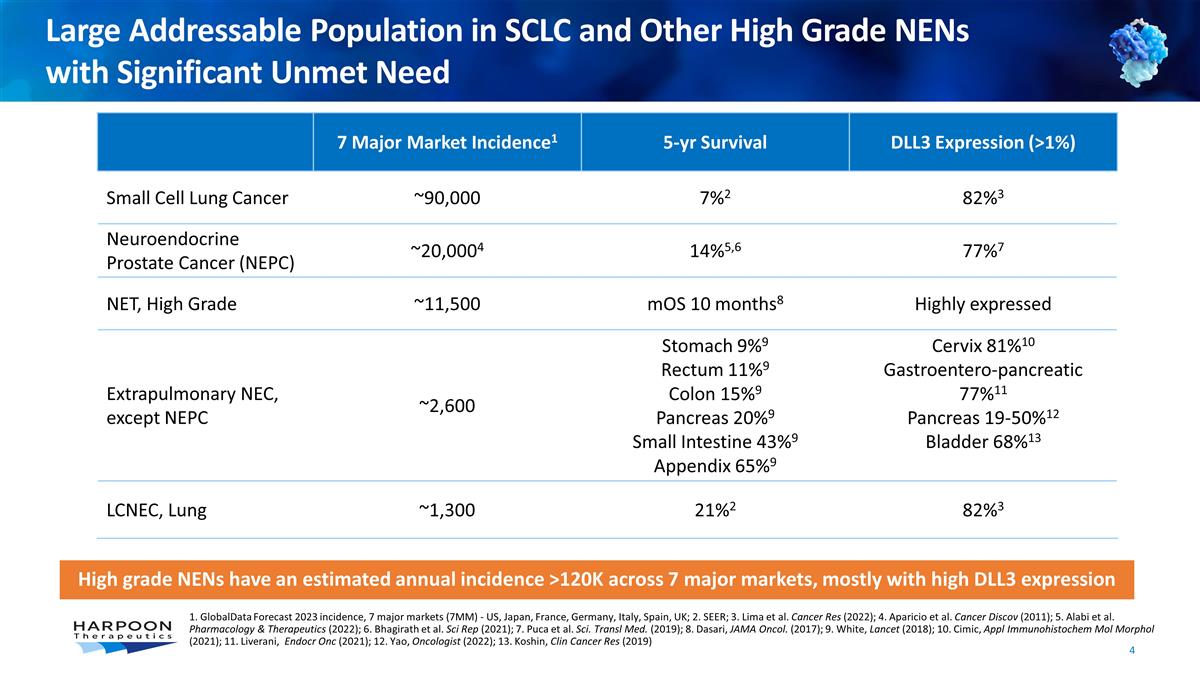
Large Addressable Population in SCLC
and Other High Grade NENs with Significant Unmet Need 7 Major Market Incidence1 5-yr Survival DLL3 Expression (>1%) Small Cell Lung Cancer ~90,000 7%2 82%3 Neuroendocrine Prostate Cancer (NEPC) ~20,0004 14%5,6 77%7 NET, High Grade ~11,500 mOS 10
months8 Highly expressed Extrapulmonary NEC, except NEPC ~2,600 Stomach 9%9 Rectum 11%9 Colon 15%9 Pancreas 20%9 Small Intestine 43%9 Appendix 65%9 Cervix 81%10 Gastroentero-pancreatic 77%11 Pancreas 19-50%12 Bladder 68%13 LCNEC, Lung ~1,300 21%2
82%3 High grade NENs have an estimated annual incidence >120K across 7 major markets, mostly with high DLL3 expression 1. GlobalData Forecast 2023 incidence, 7 major markets (7MM) - US, Japan, France, Germany, Italy, Spain, UK; 2. SEER; 3. Lima
et al. Cancer Res (2022); 4. Aparicio et al. Cancer Discov (2011); 5. Alabi et al. Pharmacology & Therapeutics (2022); 6. Bhagirath et al. Sci Rep (2021); 7. Puca et al. Sci. Transl Med. (2019); 8. Dasari, JAMA Oncol. (2017); 9. White, Lancet
(2018); 10. Cimic, Appl Immunohistochem Mol Morphol (2021); 11. Liverani, Endocr Onc (2021); 12. Yao, Oncologist (2022); 13. Koshin, Clin Cancer Res (2019)

Unaudited database as of 9/12/2023
Data subject to change HPN328 Phase 1 Interim Update
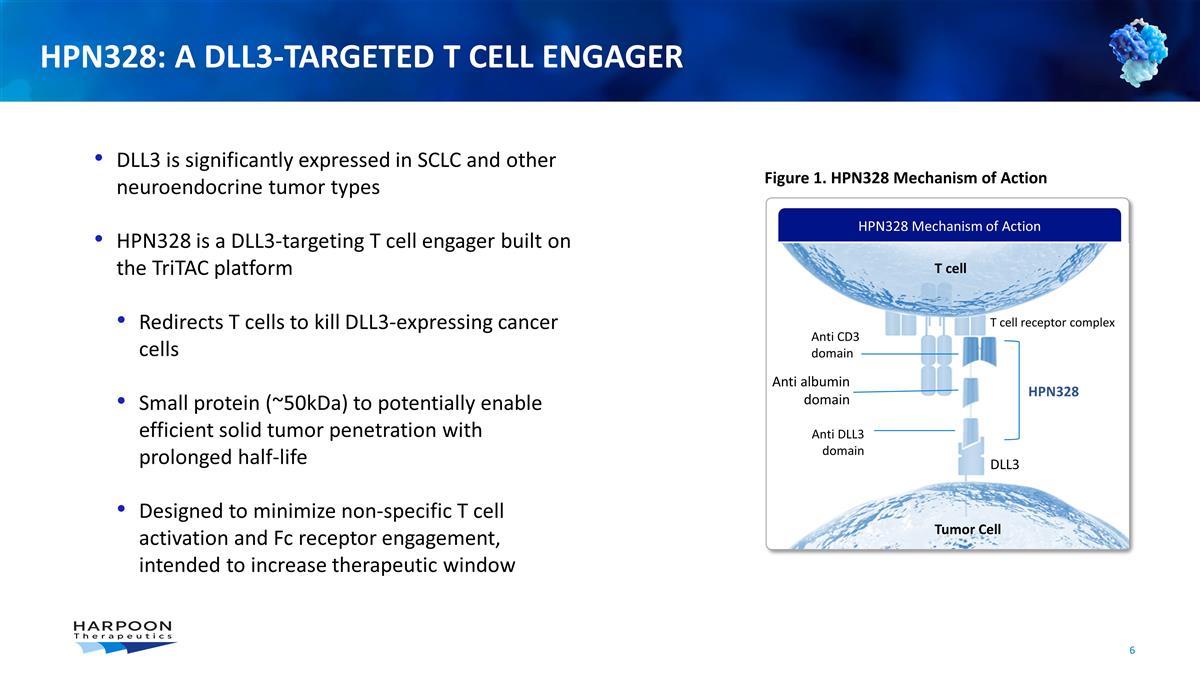
Figure 1. HPN328 Mechanism of Action
HPN328 Mechanism of Action T cell receptor complex DLL3 HPN328 Tumor Cell T cell Anti CD3 domain Anti albumin domain Anti DLL3 domain DLL3 is significantly expressed in SCLC and other neuroendocrine tumor types HPN328 is a DLL3-targeting T cell
engager built on the TriTAC platform Redirects T cells to kill DLL3-expressing cancer cells Small protein (~50kDa) to potentially enable efficient solid tumor penetration with prolonged half-life Designed to
minimize non-specific T cell activation and Fc receptor engagement, intended to increase therapeutic window HPN328: A DLL3-Targeted T Cell Engager

HPN328 Phase 1 Trial YE 2022 Planned
Targets: patients enrolled Optimization of priming dose for step-dosing regimens: 1 – 6 mg step-dose cohort opened Planned 2023 Events: Q1 2023: Start enrollment in atezolizumab combo cohorts Start enrollment in Q2W dosing
cohorts for HPN328 monotherapy Mid-2023: Complete enrollment in monotherapy and combination therapy dose escalation cohorts: ~100 patients enrolled (including backfill) by End of Q2 Planned interim data update with potential data maturity at: End of
Q1: anticipate up to 30 pts with ≥1 scan End of Q2: anticipate up to 70 pts with ≥1 scan 2H 2023: Complete total study enrollment of ~130 patients Target Population Extensive stage SCLC relapsed after platinum chemotherapy
Neuroendocrine prostate cancer and other DLL3 expressing tumors with high grade neuroendocrine features relapsed/refractory to standard therapy Trial Design Assess safety and tolerability at increasing dose levels PK and pharmacodynamic data
Evaluate preliminary anti-tumor activity Dosing & Administration IV infusion with weekly and Q2W administration schedules Monotherapy cohorts for all tumor types, and combination cohorts with atezolizumab for SCLC Premedication and step
dosing to manage cytokine release syndrome (CRS)
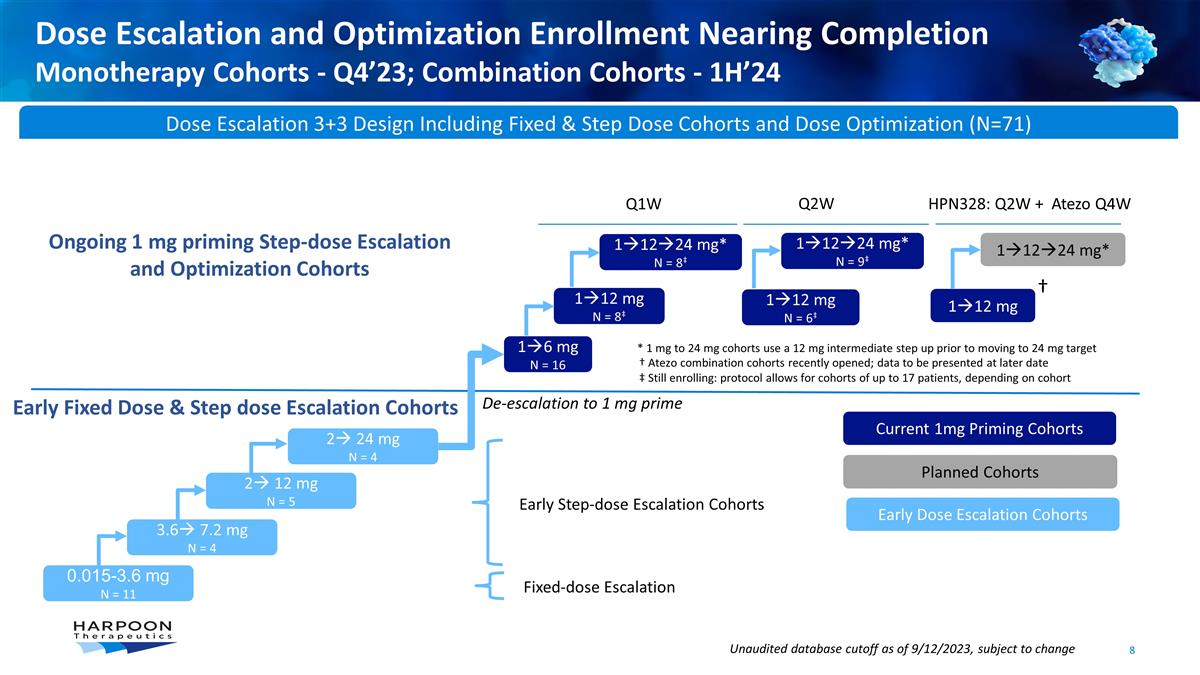
Dose Escalation and Optimization
Enrollment Nearing Completion Monotherapy Cohorts - Q4’23; Combination Cohorts - 1H’24 Unaudited database cutoff as of 9/12/2023, subject to change Dose Escalation 3+3 Design Including Fixed & Step Dose Cohorts and Dose Optimization
(N=71) 0.015-3.6 mg N = 11 3.6à 7.2 mg N = 4 2à 12 mg N = 5 2à 24 mg N = 4 1à12 mg N = 6‡ 1à12à24 mg* N = 9‡ Q2W 1à12 mg 1à12à24 mg* 1à12 mg N = 8‡ Q1W 1à12à24 mg* N =
8‡ Fixed-dose Escalation 1à6 mg N = 16 HPN328: Q2W + Atezo Q4W De-escalation to 1 mg prime Ongoing 1 mg priming Step-dose Escalation and Optimization Cohorts Early Fixed Dose & Step dose Escalation Cohorts Early Dose Escalation
Cohorts Current 1mg Priming Cohorts Planned Cohorts Early Step-dose Escalation Cohorts † * 1 mg to 24 mg cohorts use a 12 mg intermediate step up prior to moving to 24 mg target † Atezo combination cohorts recently opened; data to be
presented at later date ‡ Still enrolling: protocol allows for cohorts of up to 17 patients, depending on cohort
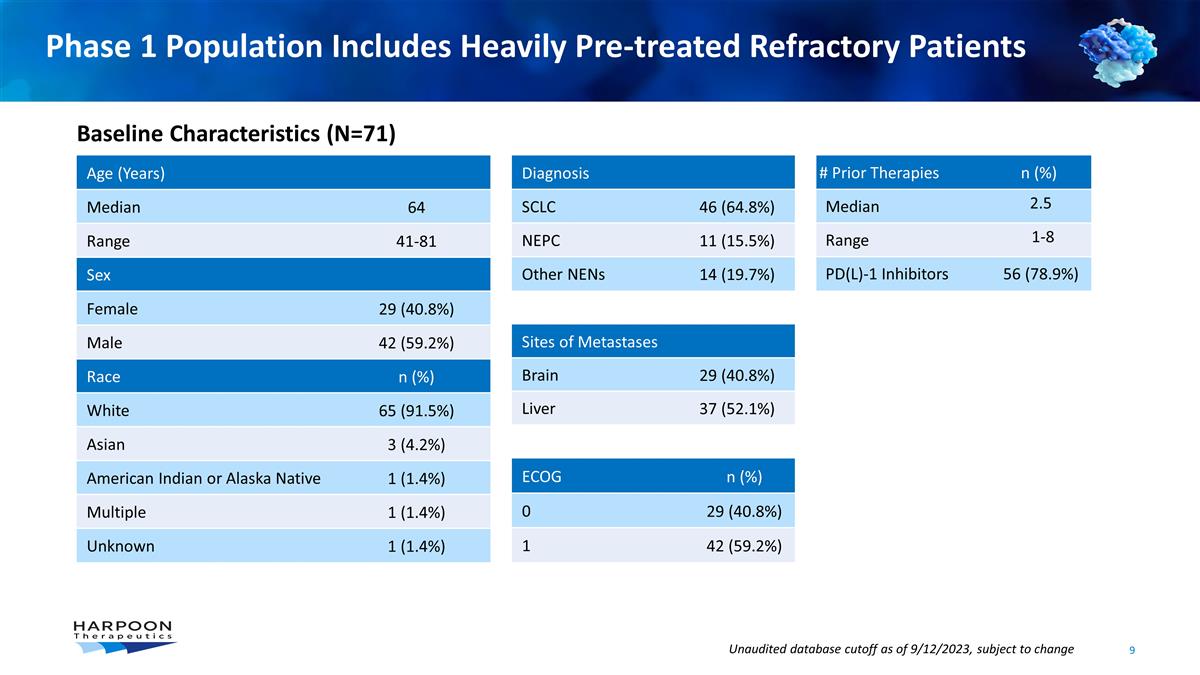
Phase 1 Population Includes Heavily
Pre-treated Refractory Patients Age (Years) Median 64 Range 41-81 Sex Female 29 (40.8%) Male 42 (59.2%) Race n (%) White 65 (91.5%) Asian 3 (4.2%) American Indian or Alaska Native 1 (1.4%) Multiple 1 (1.4%) Unknown 1 (1.4%) # Prior
Therapies n (%) Median 2.5 Range 1-8 PD(L)-1 Inhibitors 56 (78.9%) Diagnosis SCLC 46 (64.8%) NEPC 11 (15.5%) Other NENs 14 (19.7%) Sites of Metastases Brain 29 (40.8%) Liver 37 (52.1%) ECOG n (%) 0 29 (40.8%) 1 42 (59.2%) Baseline
Characteristics (N=71) Unaudited database cutoff as of 9/12/2023, subject to change
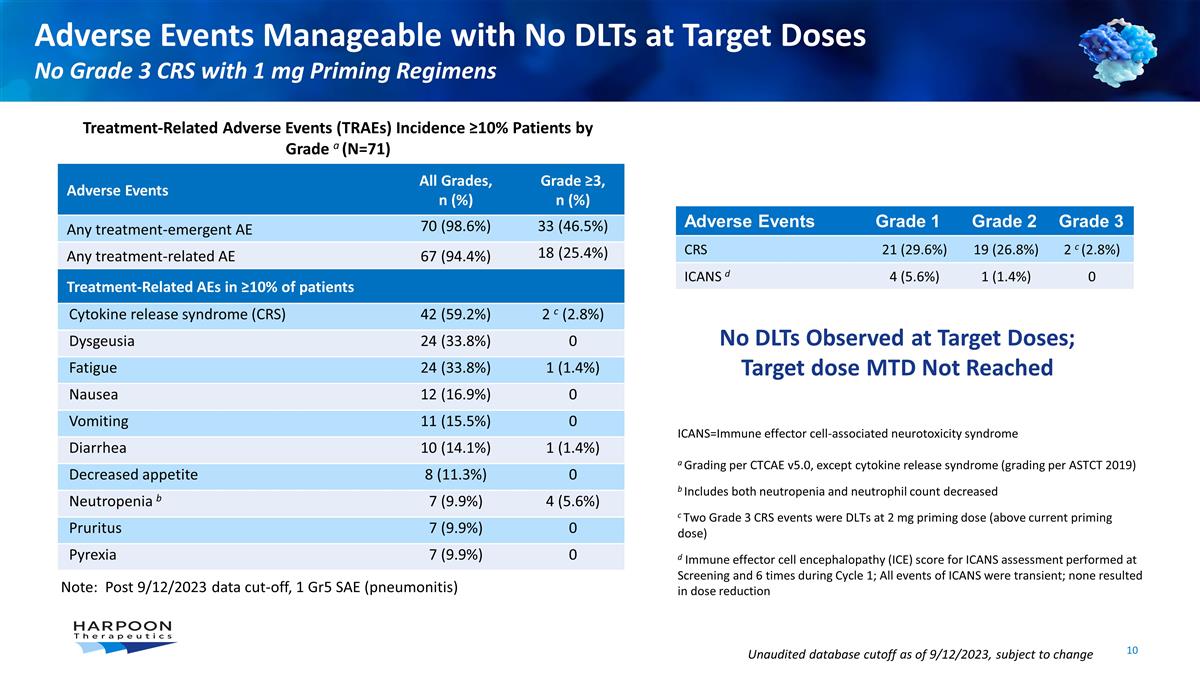
Adverse Events Manageable with No
DLTs at Target Doses No Grade 3 CRS with 1 mg Priming Regimens Unaudited database cutoff as of 9/12/2023, subject to change ICANS=Immune effector cell-associated neurotoxicity syndrome a Grading per CTCAE v5.0, except cytokine release syndrome
(grading per ASTCT 2019) b Includes both neutropenia and neutrophil count decreased c Two Grade 3 CRS events were DLTs at 2 mg priming dose (above current priming dose) d Immune effector cell encephalopathy (ICE) score for ICANS assessment performed
at Screening and 6 times during Cycle 1; All events of ICANS were transient; none resulted in dose reduction Adverse Events Grade 1 Grade 2 Grade 3 CRS 21 (29.6%) 19 (26.8%) 2 c (2.8%) ICANS d 4 (5.6%) 1 (1.4%) 0 Adverse Events All Grades, n (%)
Grade ≥3, n (%) Any treatment-emergent AE 70 (98.6%) 33 (46.5%) Any treatment-related AE 67 (94.4%) 18 (25.4%) Treatment-Related AEs in ≥10% of patients Cytokine release syndrome (CRS) 42 (59.2%) 2 c (2.8%) Dysgeusia 24 (33.8%) 0 Fatigue
24 (33.8%) 1 (1.4%) Nausea 12 (16.9%) 0 Vomiting 11 (15.5%) 0 Diarrhea 10 (14.1%) 1 (1.4%) Decreased appetite 8 (11.3%) 0 Neutropenia b 7 (9.9%) 4 (5.6%) Pruritus 7 (9.9%) 0 Pyrexia 7 (9.9%) 0 Treatment-Related Adverse Events (TRAEs) Incidence
≥10% Patients by Grade a (N=71) No DLTs Observed at Target Doses; Target dose MTD Not Reached Note: Post 9/12/2023 data cut-off, 1 Gr5 SAE (pneumonitis)
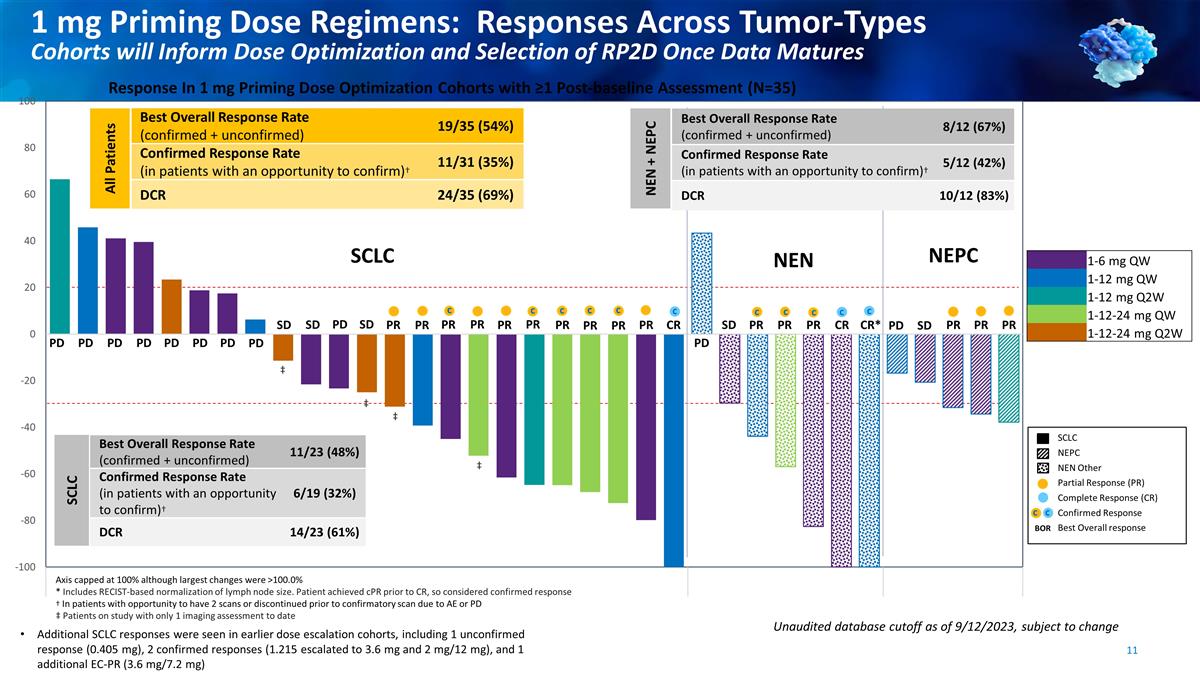
SCLC NEN NEPC PD PD PD PD PD PD PD
SD PD SD PR PR c PR PR PR c PR PR PR c CR c PD PR c PR c PR c CR CR* c PD SD PR PR PR 1-6 mg QW 1-12 mg QW 1-12 mg Q2W 1-12-24 mg QW 1-12-24 mg Q2W SD PR c c SD PD PR Axis capped at 100% although largest changes
were >100.0% * Includes RECIST-based normalization of lymph node size. Patient achieved cPR prior to CR, so considered confirmed response † In patients with opportunity to have 2 scans or discontinued prior to confirmatory scan due to AE or
PD ‡ Patients on study with only 1 imaging assessment to date Response In 1 mg Priming Dose Optimization Cohorts with ≥1 Post-baseline Assessment (N=35) Additional SCLC responses were seen in earlier dose escalation cohorts, including 1
unconfirmed response (0.405 mg), 2 confirmed responses (1.215 escalated to 3.6 mg and 2 mg/12 mg), and 1 additional EC-PR (3.6 mg/7.2 mg) ‡ ‡ ‡ ‡ Best Overall Response Rate (confirmed + unconfirmed) 11/23 (48%) Confirmed
Response Rate (in patients with an opportunity to confirm)† 6/19 (32%) DCR 14/23 (61%) Best Overall Response Rate (confirmed + unconfirmed) 19/35 (54%) Confirmed Response Rate (in patients with an opportunity to confirm)† 11/31 (35%) DCR
24/35 (69%) SCLC NEPC NEN Other Partial Response (PR) Complete Response (CR) Confirmed Response Best Overall response BOR c c c All Patients SCLC Best Overall Response Rate (confirmed + unconfirmed) 8/12 (67%) Confirmed Response Rate (in patients
with an opportunity to confirm)† 5/12 (42%) DCR 10/12 (83%) NEN + NEPC Unaudited database cutoff as of 9/12/2023, subject to change 1 mg Priming Dose Regimens: Responses Across Tumor-Types Cohorts will Inform Dose Optimization and Selection of
RP2D Once Data Matures
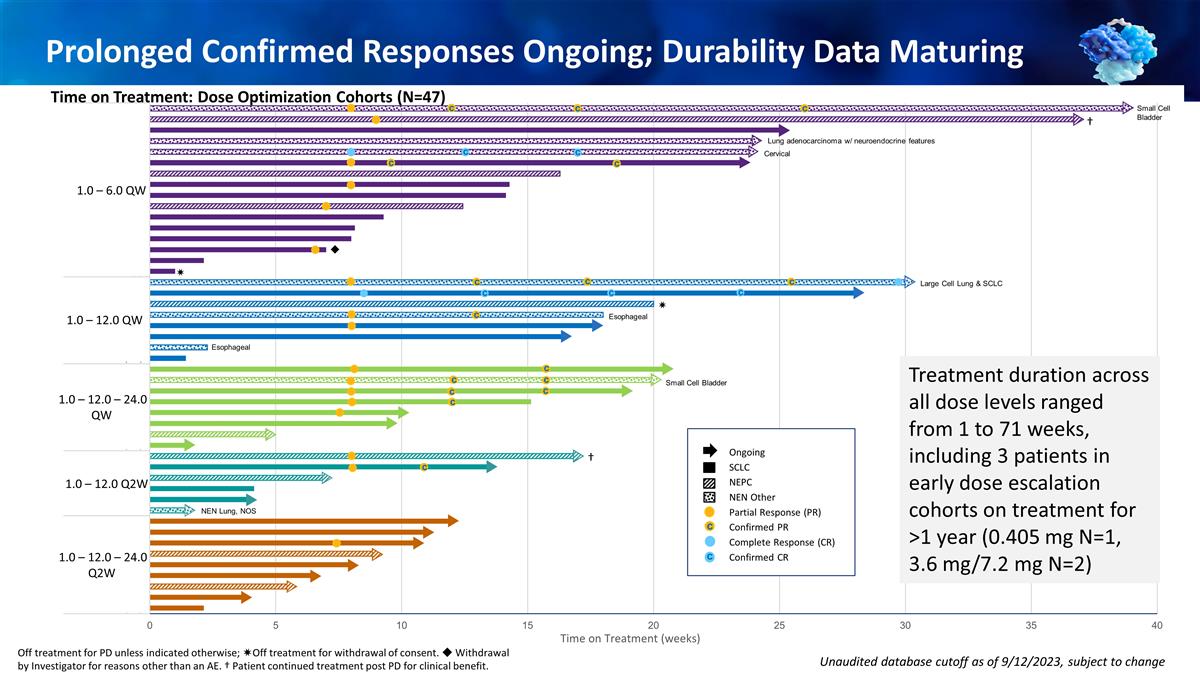
Prolonged Confirmed Responses
Ongoing; Durability Data Maturing Time on Treatment: Patients Treated With 1 mg Priming Dose (N=47) Unaudited database cutoff as of 9/12/2023, subject to change Ongoing SCLC NEPC NEN Other Partial Response (PR) Confirmed PR Complete Response (CR)
Confirmed CR c c c c c Cervical c c c c ◆ ✷ Large Cell Lung & SCLC c c c c Esophageal Esophageal c c Small Cell Bladder c Lung adenocarcinoma w/ neuroendocrine features c c c c NEN Lung, NOS c c c ✷ 1.0 – 6.0 QW 1.0
– 12.0 QW 1.0 – 12.0 – 24.0 QW 1.0 – 12.0 Q2W 1.0 – 12.0 – 24.0 Q2W † Small Cell Bladder Treatment duration across all dose levels ranged from 1 to 71 weeks, including 3 patients in early dose escalation
cohorts on treatment for >1 year (0.405 mg N=1, 3.6 mg/7.2 mg N=2) Off treatment for PD unless indicated otherwise; ✷Off treatment for withdrawal of consent. ◆ Withdrawal by Investigator for reasons other than an AE. † Patient
continued treatment post PD for clinical benefit. Time on Treatment: Dose Optimization Cohorts (N=47)
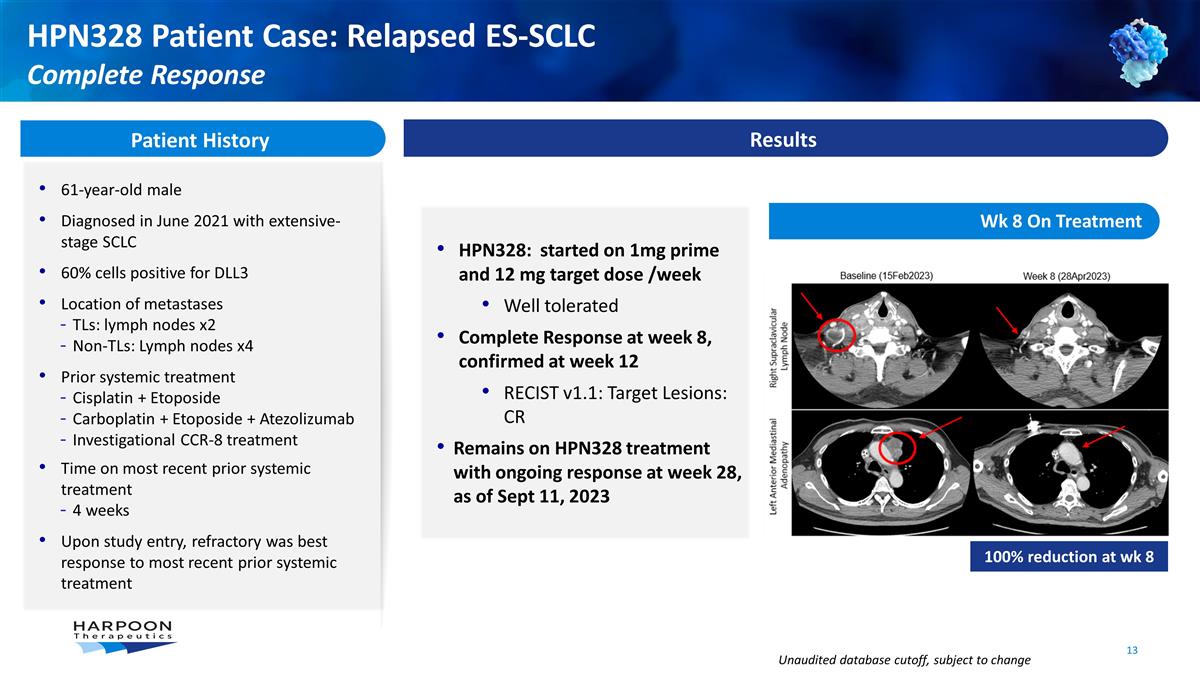
HPN328 Patient Case: Relapsed
ES-SCLC Complete Response Patient History 61-year-old male Diagnosed in June 2021 with extensive-stage SCLC 60% cells positive for DLL3 Location of metastases TLs: lymph nodes x2 Non-TLs: Lymph nodes x4 Prior systemic treatment Cisplatin + Etoposide
Carboplatin + Etoposide + Atezolizumab Investigational CCR-8 treatment Time on most recent prior systemic treatment 4 weeks Upon study entry, refractory was best response to most recent prior systemic treatment Results HPN328: started on 1mg
prime and 12 mg target dose /week Well tolerated Complete Response at week 8, confirmed at week 12 RECIST v1.1: Target Lesions: CR Remains on HPN328 treatment with ongoing response at week 28, as of Sept 11, 2023 Wk 8 On Treatment 100% reduction at
wk 8 Unaudited database cutoff, subject to change
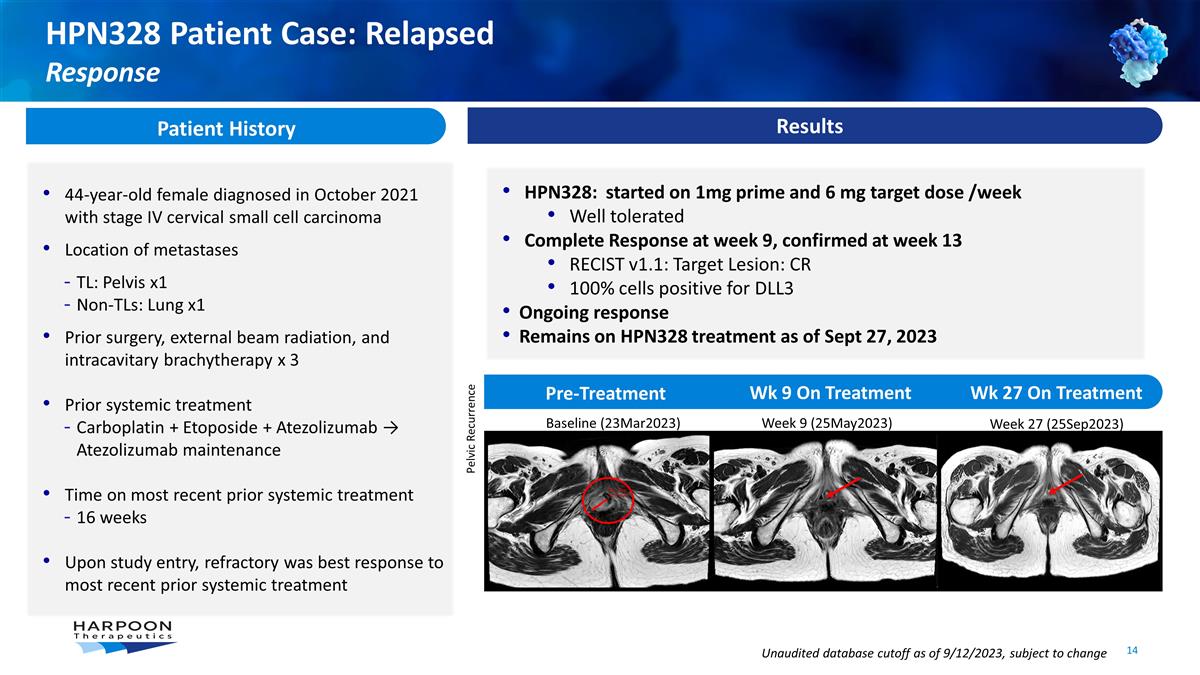
Pre-Treatment Wk 9 On Treatment Wk
27 On Treatment HPN328: started on 1mg prime and 6 mg target dose /week Well tolerated Complete Response at week 9, confirmed at week 13 RECIST v1.1: Target Lesion: CR 100% cells positive for DLL3 Ongoing response Remains on HPN328 treatment as of
Sept 27, 2023 Patient History 44-year-old female diagnosed in October 2021 with stage IV cervical small cell carcinoma Location of metastases TL: Pelvis x1 Non-TLs: Lung x1 Prior surgery, external beam radiation, and intracavitary brachytherapy x 3
Prior systemic treatment Carboplatin + Etoposide + Atezolizumab → Atezolizumab maintenance Time on most recent prior systemic treatment 16 weeks Upon study entry, refractory was best response to most recent prior systemic treatment HPN328
Patient Case: Relapsed Response Baseline (23Mar2023) Week 9 (25May2023) Week 27 (25Sep2023) Pelvic Recurrence Results Unaudited database cutoff as of 9/12/2023, subject to change
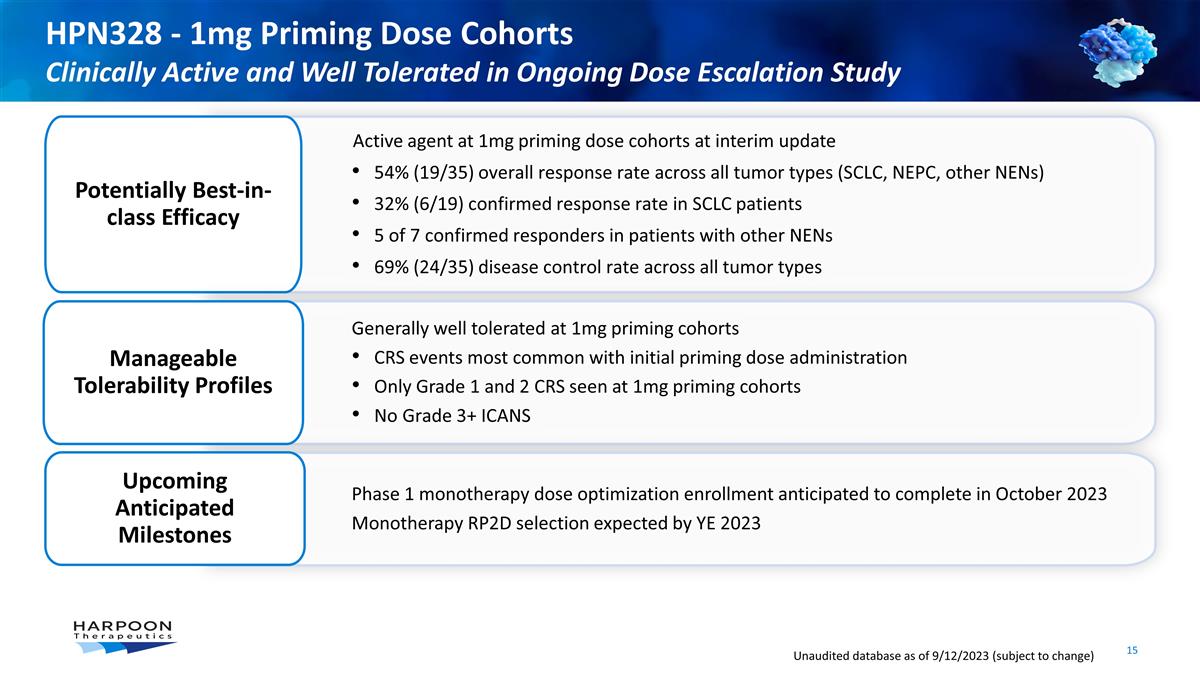
HPN328 - 1mg Priming Dose Cohorts
Clinically Active and Well Tolerated in Ongoing Dose Escalation Study Active agent at 1mg priming dose cohorts at interim update 54% (19/35) overall response rate across all tumor types (SCLC, NEPC, other NENs) 32% (6/19) confirmed response rate in
SCLC patients 5 of 7 confirmed responders in patients with other NENs 69% (24/35) disease control rate across all tumor types Potentially Best-in-class Efficacy Generally well tolerated at 1mg priming cohorts CRS events most common with initial
priming dose administration Only Grade 1 and 2 CRS seen at 1mg priming cohorts No Grade 3+ ICANS Phase 1 monotherapy dose optimization enrollment anticipated to complete in October 2023 Monotherapy RP2D selection expected by YE 2023 Upcoming
Anticipated Milestones Unaudited database as of 9/12/2023 (subject to change) Manageable Tolerability Profiles
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
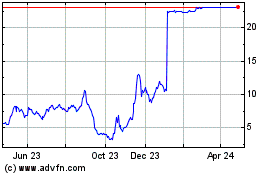
Harpoon Therapeutics (NASDAQ:HARP)
Historical Stock Chart
From Apr 2024 to May 2024
Harpoon Therapeutics (NASDAQ:HARP)
Historical Stock Chart
From May 2023 to May 2024