0000827871
false
0000827871
2023-09-27
2023-09-27
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
FORM 8-K
CURRENT REPORT
Pursuant to Section
13 or 15(d)
of the Securities
Exchange Act of 1934
Date of Report (Date of
earliest event reported): September 27, 2023
Eagle Pharmaceuticals, Inc.
(Exact name of registrant
as specified in its charter)
| Delaware |
001-36306 |
20-8179278 |
| (State
or other jurisdiction of |
(Commission File Number) |
(IRS Employer Identification No.) |
| incorporation) |
|
|
50 Tice Boulevard, Suite 315
Woodcliff Lake, NJ |
|
07677 |
| (Address of principal executive offices) |
|
(Zip Code) |
Registrant’s
telephone number, including area code: (201) 326-5300
Check the appropriate box below if the Form 8-K filing is intended
to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
¨ Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
¨ Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
¨ Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities
registered pursuant to Section 12(b) of the Act:
| Title of each class |
|
Trading
Symbol |
|
Name
of each exchange on which registered |
| Common Stock (par value $0.001 per share) |
|
EGRX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging
growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR §230.405) or Rule 12b-2 of the Securities Exchange
Act of 1934 (17 CFR §240.12b-2).
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards
provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure. |
On September 27,
2023, Eagle Pharmaceuticals, Inc., or the Company, released an investor presentation relating to the Company’s business,
products, product candidates and certain financial information and guidance, which the Company will use from time to time in
meetings with investors.
A copy of the above-referenced
presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference. The information
furnished pursuant to Item 7.01 of this current report, including Exhibit 99.1, shall not be deemed “filed” for purposes of
Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities of that
section, and shall not be deemed incorporated by reference into any of the Company’s filings under the Securities Act of 1933,
as amended, or the Exchange Act, whether made before or after the date hereof, regardless of any general incorporation language in
such filing, except as shall be expressly set forth by specific reference in such filing. The furnishing of the information in this Current
Report on Form 8-K is not intended to, and does not, constitute a determination or admission by the Company that the information in this
Current Report on Form 8-K is material or complete, or that investors should consider this information before making an investment decision
with respect to any security of the Company.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
SIGNATURES
Pursuant to the requirements of the Securities
Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Dated: September
27, 2023 |
EAGLE PHARMACEUTICALS, INC. |
| |
|
| |
By: |
/s/ Scott Tarriff |
| |
|
Scott Tarriff |
| |
|
Chief Executive Officer |
Exhibit 99.1

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 1 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Company Overview September 2023

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 2 Forward - Looking Statements This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 19 95, as amended, and other securities law All statements other than statements of historical fact contained in this presentati on are forward - looking statements. In some cases, you can identify forward - looking statements by terminology such as “may,” “could,” “will,” “would,” “ should,” “expect,” “plan,”, “anticipate,” “believe,” “estimate,” “intend,” “predict,” “seek,” “contemplate,” “project,” “cont inu e,” “potential,” “ongoing,” “prospects,” “outlook,” “goal” or the negative of these terms or other comparable terminology, although not all fo rwa rd - looking statements contain these identifying words. These forward - looking statements include, but are not limited to, stateme nts with respect to: Eagle Pharmaceuticals, Inc.’s (“Eagle” or the “Company”) ability to achieve earnings growth and support research and developm ent , and its capability for further expansion and improve margin and contribution of key products; expectations with respect to the Company’s financial results, including projected estimated financial information, and expectations with respect to anticipated future p rod uct revenue and profits for fiscal year 2023; expectations with respect to potential exit run rates, revenues, market share, com mercial opportunity, expected pricing of drugs and future royalties; expectations with respect to Enalare , including any potential further investments by Eagle in Enalare , the potential exercise of Eagle’s option to acquire the outstanding shares of Enalare upon the achievement of certain milestones, Enalare’s development programs and expectations with respect to the achievement of milestones by Enalare , including the timing thereof; the Company’s development programs, products and pipeline; the potential for the Company to t ran sition into a diversified pharmaceutical company with a portfolio of branded, first - in - class assets and to utilize legacy products; the Company's clinical development plan for its product candidates, including the number and timing of development initiatives or new indications fo r the Company’s product candidates; the development of, potential therapeutic and economic benefits of and expected regulatory activities and ma tters with respect to the product candidates of the Company and Enalare ; potential commercial opportunities, addressable markets, patient populations and settings for the Company’s and Enalare’s products and product candidates; CAL02’s ability to neutralize virulence factors produced by bacteria that are commonly assoc ia ted severe pneumonia; the potential of CAL02 to be a first - in - class broad - spectrum anti - virulence agent for the treatment of severe community - acquired bacterial pneumonia; the Company’s expectations for the design an d timing of the CAL02 Phase 2 study, including with respect to enrollment and the timing thereof; the potential of landiolol’s to provide short - term reduction of ventricular rate in patients with supraventricular tachycardia, including atrial fibrillation and atrial fl utt er and potential for regulatory approval; the timeline for the fentanyl toxicology study, initiation of Phase 2 enrollment an d a vailability of Phase 2 topline data for ENA - 001 in post - op respiratory depression; the Company and Enalare’s expectations for the design, enrollment and timing of the planned Phase 1 community drug overdose study for ENA - 001; the design of future animal studies and clinical pathway for ENA - 001 for apnea of prematurity; the Company’s marketing, product development, partnering and growth strategy, including relating to the co mmercialization of Barhemsys and Byfavo and its other products; expectations with respect to the Company’s ability to potentially acquire additional assets; the timing, scope or likelihood and timing of regulatory filings and approvals from the U.S. Food and Drug Ad ministration (“FDA”) for product candidates and the ability to maintain regulatory approval of products and product candidate s; clinical development plans for product candidates; the success of the Company's collaborations with its strategic partners and the tim ing and results of these partners’ preclinical studies and clinical trials, and the Company’s potential earnings potential throug h such collaborations; the Company's plans and ability to advance the product candidate in its pipeline; potential opportunities for, and the Company’s abi lity to complete acquisitions or business development transactions, in a timely manner, on favorable terms to the Company, or at all; the sufficiency of the Company’s cash flows and capital resources and expectations with respect to deployment of cash resources; and the Comp any ’s ability to achieve expected future financial performance and results. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the Company’s control, that could cause actual results to differ mater ial ly from those expressed in, or implied or projected by, the forward - looking information and statements. Such risks and uncertainties include, but are not limited to: the risk that the anticipated benefits of the Company’s acquisi tio n of Acacia are not realized; the impacts of the post - COVID - 19 environment and geopolitical factors such as the conflict in Ukr aine, including disruption or impact in the sales of the Company's marketed products, interruptions or other adverse effects to clinical tria ls, delays in regulatory review, manufacturing and supply chain interruptions, adverse effects on healthcare systems, disruption in the operations of the Company's third party partners and disruption of the global economy or other events on the Company's business, financial cond iti on and results of operations; macroeconomic conditions, including rising inflation and interest rates, uncertain credit and f ina ncial markets and recent and potential disruptions in banking systems; whether the Company will incur unforeseen expenses or liabilities or oth er market factors; whether the Company will successfully implement its development plan for its product candidates; the rate and de gree of market acceptance of our products; delay in or failure to obtain regulatory approval of the Company's or its partners’ product candi dat es; whether the Company can successfully market and commercialize its product candidates; the success of the Company's relati ons hips with its partners; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of its products or that may have an impact on any of our products; successful compliance with the FDA and other governmental reg ul ations applicable to product approvals, manufacturing facilities, products and/or businesses; the strength and enforceability of the Company's int ell ectual property rights or the rights of third parties; competition from other pharmaceutical and biotechnology companies and the potential for competition from generic entrants into the market; the risks inherent in the early stages of drug development and in conducti ng clinical trials; our expectations regarding anticipated future costs, operating expenses and capital requirements; any unanti cip ated factors in addition to the foregoing that may impact the Company’s financial and business projections and guidance that may cause the Company’s a ctu al results and outcomes to materially differ from its projections and guidance; and those risks and uncertainties identified in the “Risk Factors” sections of the Company's Annual Report on Form 10 - K for the year ended December 31, 2022, filed with the Securities and Exchang e Commission (the “SEC”) on March 23, 2023, and its other subsequent filings with the SEC, including the Company’s Quarterly Rep orts on Form 10 - Q for the quarters ended March 31, 2023 and June 30, 2023, filed with the SEC on May 9, 2023 and August 8, 2023, respect ively. Readers are cautioned not to place undue reliance on these forward - looking statements. All forward - looking statements con tained in this presentation speak only as of the date on which they were made. Except to the extent required by law, the Company undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they we re made. This presentation includes statistical and other industry and market data that the Company obtained from industry publication s a nd research, surveys and studies conducted by third parties or us. Industry publications and third - party research, surveys and s tudies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the ac cur acy or completeness of such information. All of the market data used in this presentation involves a number of assumptions an d l imitations, and you are cautioned not to give undue weight to such estimates. While the Company believes these industry publications and third - party research, surveys and studies are reliable, the Company has not independently verified such data. The industry in which the C om pany operates is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ mat eri ally from those expressed in the estimates made by the independent parties and by the Company.

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 3 3 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Eagle’s profit and gross margin continue to exceed internal projections. We expect further growth as our portfolio of products and underlying business continue to strengthen.
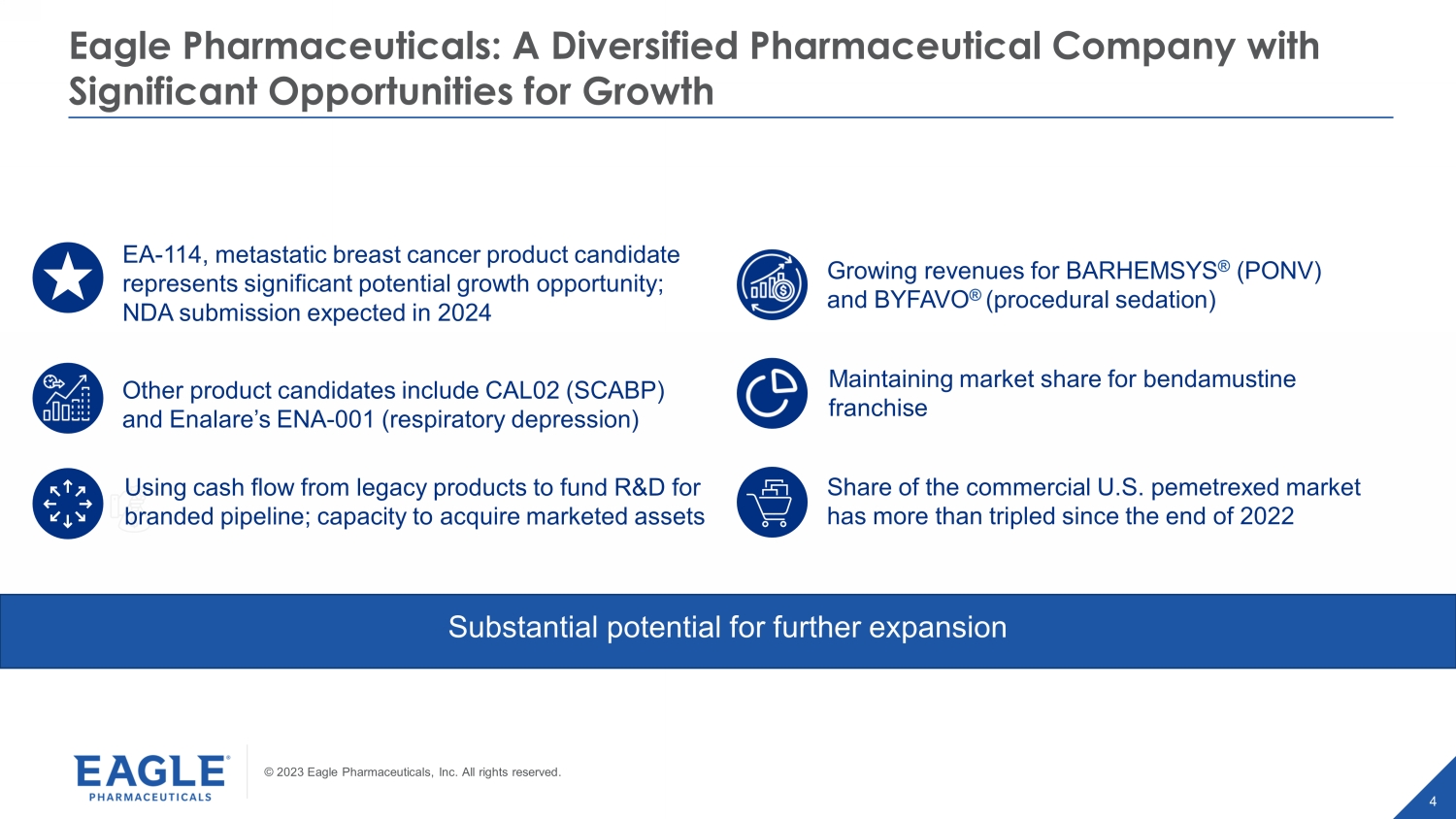
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 4 Eagle Pharmaceuticals: A Diversified Pharmaceutical Company with Significant Opportunities for Growth Substantial potential for further expansion Maintaining market share for bendamustine franchise Share of the commercial U.S. pemetrexed market has more than tripled since the end of 2022 Growing revenues for BARHEMSYS ® (PONV) and BYFAVO ® (procedural sedation) EA - 114, metastatic breast cancer product candidate represents significant potential growth opportunity; NDA submission expected in 2024 Other product candidates include CAL02 (SCABP) and Enalare’s ENA - 001 (respiratory depression) Using cash flow from legacy products to fund R&D for branded pipeline; capacity to acquire marketed assets

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 5 Eagle Pharmaceuticals: Strong Financial Position Q2 2023 revenue of $64.6M and adjusted non - GAAP EBITDA of $20.7M † Gross margin of 74% and adjusted non - GAAP gross margin of 83% * † Oncology gross margin of 80% and adjusted non - GAAP gross margin of 84% * † Working Capital of $100.6M † Cash + Receivables = $130.5M † 13.2M shares outstanding on a fully diluted basis † *For a description and reconciliation of all non - GAAP financial measure to its most comparable GAAP financial measure, please see the appendix at the end of this presentation. †For the quarter ended 6/30/2023 ‡Diluted adjusted non - GAAP earnings per share, is a non - GAAP financial measures. For descriptions and reconciliations of these non - GAAP financial measures to their most comparable GAAP financial measures, please see the appendix at the end of this presentation. Raised 2023 diluted adjusted non - GAAP EPS guidance to $4.40 - $4.70 ‡ Resumed stock buybacks of $4.0M to date in 2023 under share repurchase program
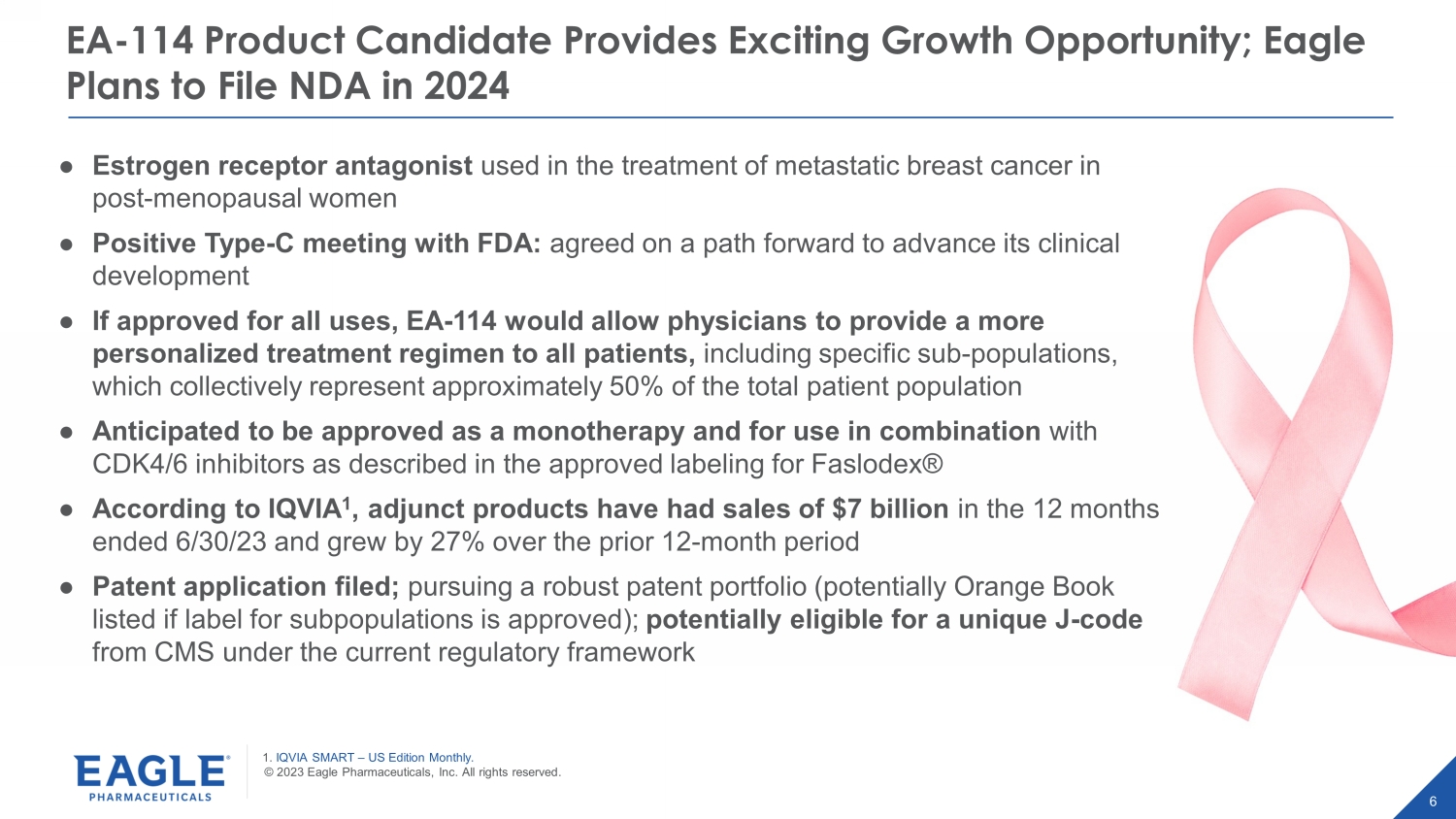
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 6 EA - 114 Product Candidate Provides Exciting Growth Opportunity; Eagle Plans to File NDA in 2024 1. IQVIA SMART – US Edition Monthly. ● Estrogen receptor antagonist used in the treatment of metastatic breast cancer in post - menopausal women ● Positive Type - C meeting with FDA: agreed on a path forward to advance its clinical development ● If approved for all uses, EA - 114 would allow physicians to provide a more personalized treatment regimen to all patients, including specific sub - populations, which collectively represent approximately 50% of the total patient population ● Anticipated to be approved as a monotherapy and for use in combination with CDK4/6 inhibitors as described in the approved labeling for Faslodex® ● According to IQVIA 1 , adjunct products have had sales of $7 billion in the 12 months ended 6/30/23 and grew by 27% over the prior 12 - month period ● Patent application filed; pursuing a robust patent portfolio (potentially Orange Book listed if label for subpopulations is approved); potentially eligible for a unique J - code from CMS under the current regulatory framework

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 7 7 Earnings Timeline – Actuals and Guidance 2020 2021 2022 Previous 2023E Range Revised 2023E Range 2 Adjusted EBITDA (US$M) 1 $64.7 $28.2 $132.1 $74.0 - $80.0 $78.0 - $84.0 Diluted Adjusted Non - GAAP EPS 1 $3.54 $1.68 $7.79 $4.20 - $4.53 $4.40 - $4.70 Adjusted EBITDA Multiple 1,3 10x 24x 3x 3x 3x 3 - Year CAGR (Diluted Adjusted Non - GAAP EPS) -- -- -- 6% - 9% 8% - 10% 1. Adjusted EBITDA, diluted adjusted non - GAAP earnings per share, adjusted EBITDA multiple, and diluted adjusted non - GAAP earnings per share CAGR are non - GAAP financial measures. For descriptions and reconciliations of these non - GAAP financial measures to the ir most comparable GAAP financial measures, please see the appendix at the end of this presentation. 2. Expected 2023 Adjusted EBITDA and Adjusted non - GAAP EPS and related measures based on internal estimates. 3. Adjusted EBITDA multiple is calculated as Eagle’s market capitalization divided by Adjusted EBITDA for the corresponding 12 - mont h period using y ear end share price 2020 - 2022, 30 - day moving average 8/2 1/2023. Key Financial Metrics: Strong Performance and Raised Guidance

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 8 Oncology & Acute Care Contribution (US$M) 1. Adjusted non - GAAP EBITDA, adjusted non - GAAP Gross Profit, adjusted non - GAAP R&D expense and adjusted non - GAAP SG&A expense are non - GAAP financial measures. For descriptions and reconciliations of these non - GAAP financial measures to their most comparable GAAP financial measures, please see the appendix at the end of this presentation. 2. Acute care and oncology R&D expense and non - GAAP R&D expense allocation estimates includes directly allocable expense paid to 3 rd parties related to specific product candidates and products. These allocations are estimates made by company. 3. Acute care and oncology SG&A expense and non - GAAP SG&A expense allocation estimates includes directly allocable expense paid to 3 rd parties related to the commercialization of specific products and the estimated allocation of sales force and marketing headcount expense at approximately 70% to acute care and 30% to oncology. These allocations are estimates made by company. 4. Adjusted non - GAAP EBITDA contribution estimate is calculated by subtracting non - GAAP R&D and non - GAAP SG&A expense from Adjusted non - GAAP Gross Profit. 1H 2023 Revenue Acute Care Oncology Unallocated Total Bendeka 39.4 39.4 Treakisym 2.3 2.3 Royalty revenue 0.0 41.7 0.0 41.7 Pemfexy 42.3 42.3 Ryanodex 18.9 18.9 Belrapzo 13.2 13.2 Bendeka 6.2 6.2 Vasopressin 4.5 4.5 Treakisym 2.1 2.1 Barhemsys 1.7 1.7 Byfavo 0.4 0.4 Product sales, net 25.5 63.8 0.0 89.3 Total Revenue 25.5 105.5 0.0 131.0 1H 2023 Profit Acute Care Oncology Unallocated Total Gross Profit 11.5 85.3 0 96.8 Gross Margin % 45% 81% -- 74% Adjusted Non-GAAP Gross Profit(1) 19.4 89.0 0 108.4 Adjusted nonGAAP Gross Margin % 76% 84% -- 83% Operating Expense Allocation Est Acute Care(2) Oncology(3) Unallocated Total Research & Development 8.7 1.0 9.4 19.1 Research & Development - Non-GAAP(1) 8.7 1.0 8.0 17.8 Selling, general and administrative 16.5 5.3 33.8 55.6 Selling, general and administrative - Non-GAAP(1) 16.0 5.1 26.6 47.6 Adjusted Non-GAAP EBITDA Contribution Est(4) -5.2 82.9 -34.6 43.0
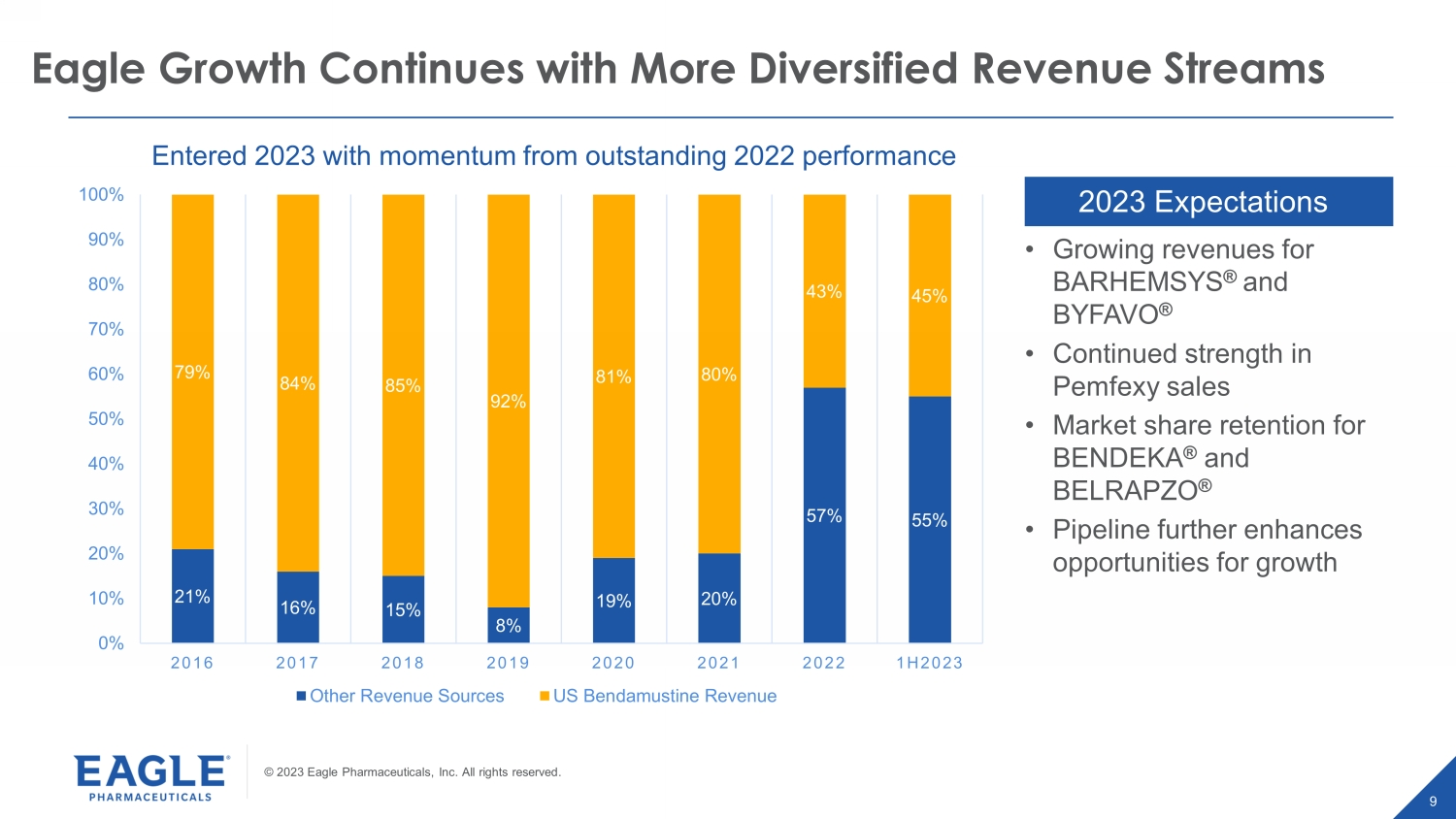
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 9 9 Eagle Growth Continues with More Diversified Revenue Streams 2023 Expectations 21% 16% 15% 8% 19% 20% 57% 55% 79% 84% 85% 92% 81% 80% 43% 45% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 2016 2017 2018 2019 2020 2021 2022 1H2023 Other Revenue Sources US Bendamustine Revenue • Growing revenues for BARHEMSYS ® and BYFAVO ® • Continued strength in Pemfexy sales • Market share retention for BENDEKA ® and BELRAPZO ® • Pipeline further enhances opportunities for growth Entered 2023 with momentum from outstanding 2022 performance
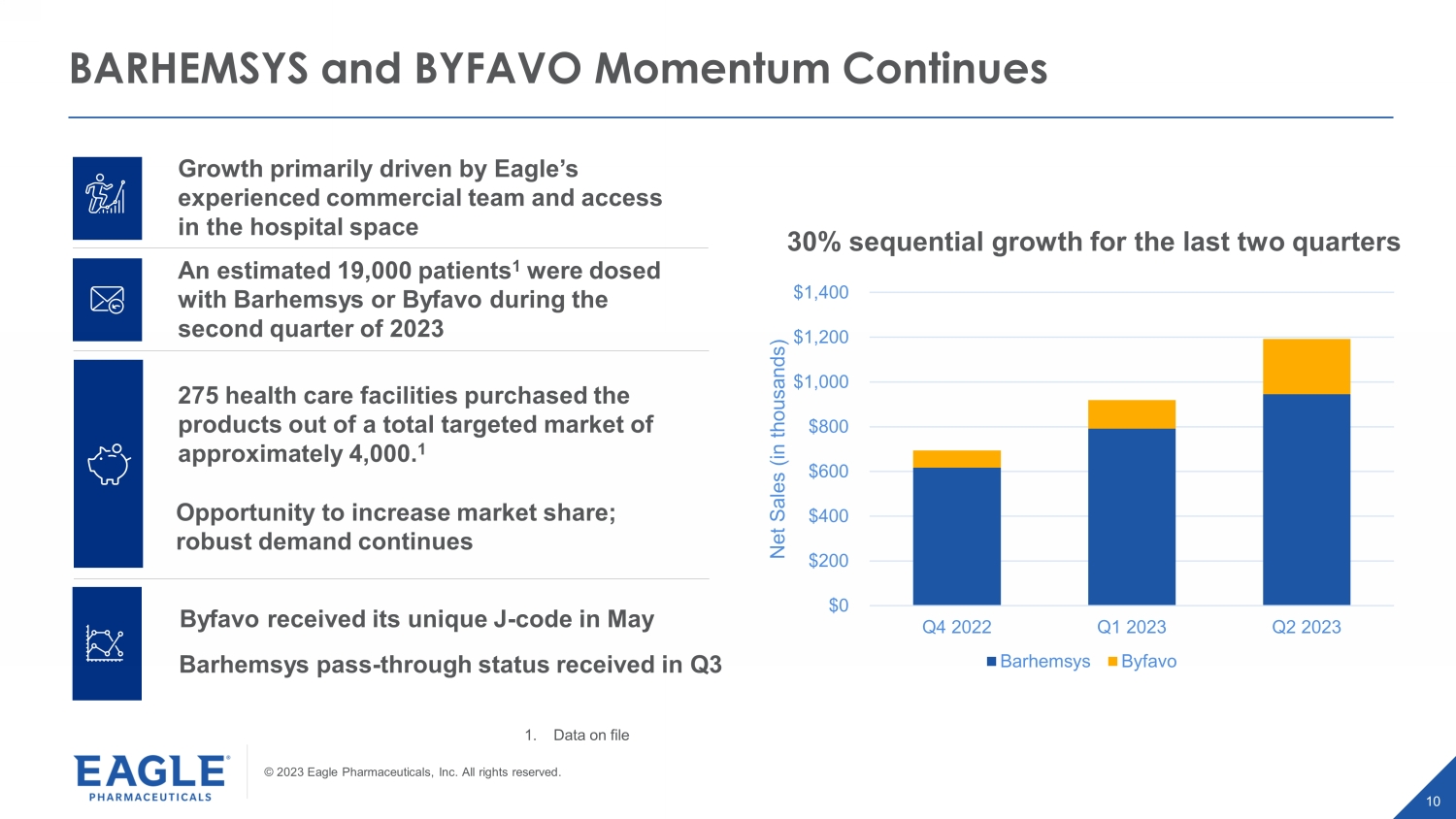
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 10 Growth primarily driven by Eagle’s experienced commercial team and access in the hospital space An estimated 19,000 patients 1 were dosed with Barhemsys or Byfavo during the second quarter of 2023 275 health care facilities purchased the products out of a total targeted market of approximately 4,000. 1 BARHEMSYS and BYFAVO Momentum Continues $0 $200 $400 $600 $800 $1,000 $1,200 $1,400 Q4 2022 Q1 2023 Q2 2023 Net Sales (in thousands) Barhemsys Byfavo 30% sequential growth for the last two quarters Byfavo received its unique J - code in May Barhemsys pass - through status received in Q3 Opportunity to increase market share; robust demand continues 1. Data on file

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 11 Eagle’s Business Development Strategy and Select Capital Spend Value OpEx Size Portfolio x Pursuing accretive acquisition opportunities x Leverage infrastructure x Opportunity for synergies / expense reductions x Potentially a ble to finance with balance sheet or supplement with additional debt financing x Quickly pay down debt x Targeting one or two product company * Total purchase price of €94.7 million in equity and €25 million in debt **Direct R&D includes expenditures to 3rd parties directly allocable to the products; does not include an allocation of internal expense, such as headcount and facilities costs $38 $130 $25 $0 $50 $100 $150 $200 $250 2022 - 2023 Capital Spend ($ millions) Enalare equity stake & option to acquire Acacia shares + debt* Direct R&D for EA-114 & CAL02**

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 12 Eagle Product Portfolio Is Supported by 80 - Person Commercial Team RYANODEX ® For treatment of malignant hyperthermia BARHEMSYS ® For prevention of PONV*, and treatment of PONV in patients who received antiemetic prophylaxis with an agent of a different class or have not received prophylaxis BYFAVO ® For the induction and maintenance of procedural sedation in adults undergoing procedures lasting 30 minutes or less Acute Care Hospital ONCOLOGY BENDEKA ® BELRAPZO ® Treatment of chronic lymphocytic leukemia (CLL) and non - Hodgkin lymphoma (NHL) Treatment of nonsquamous non - small cell lung cancer and mesothelioma PEMFEXY ®† * PONV Post operative nausea and vomiting †Launched 2/1/22 ‡Eagle’s bendamustine franchise TREAKISYM ® Japan ‡ Treatment of CLL, NHL and diffuse large B - cell lymphoma (DLBCL) Rapid infusion (RI) (50ml) liquid formulation approved and launched in 2022

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 13 CAL02 Has the Potential to Elevate the SOC for SCABP W ithout Contributing to Antibiotic Resistance ● CAL02 1 is a novel first - in - class broad - spectrum anti - virulence agent being developed for the treatment of severe community - acquired bacterial pneumonia ● Global Phase 2 study underway ‒ Approx. 276 patients expected ‒ Approx. 100 centers in 22 countries expected ● FDA granted Qualified Infectious Disease Product (QIDP) Designation and Fast Track Designation Eagle believes CAL02 qualifies as a new chemical entity, which would result in five years of marketing exclusivity upon approval or three years without NCE designation. In total, CAL02 may be eligible for a total of eight or ten years of marketing exclusivity upon approval. ● Patent protection through September 2035 , with filed patent applications that would extend into 2037 or later and may qualify for up to five additional years of patent term exclusivity as a new chemical entity, up to 2040 ● Interim analyses: Depending upon recruitment rates, Eagle anticipates having its 50% interim report around the first half of 2024 1. Eagle Pharmaceuticals. Press Release, November 14, 2022. https://investor.eagleus.com/news - releases/news - release - details/eagle - pharmaceuticals - announces - fda - acceptance - investigational . Using cash flow from legacy products to fund R&D for branded pipeline Capacity to acquire marketed assets

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 14 ENA - 001: An NCE with a Unique Mechanism of Action for Acute Respiratory Depression ● ENA - 001 1 is an investigational new chemical entity being developed by Enalare as an agnostic respiratory stimulant for multiple patient populations experiencing respiratory depression ● Post - op respiratory depression (Fast - Track status) ‒ Enalare commenced fentanyl tox study ~ in early 2023 ‒ Expect to start Phase 2 enrollment ~ as early as 3Q23 ● Community Drug Overdose (BARDA and NIH funding) ‒ Executing toxicology studies with intramuscular formulation (IM) ‒ Expect Phase 1 enrollment as soon as mid - year 2023 ● Apnea of Prematurity (Rare Pediatric Disease and Orphan Drug designations) ‒ Completed animal proof of concept ‒ Designing next set of animal studies and clinical pathway Using cash flow from legacy products to fund R&D for branded pipeline Capacity to acquire marketed assets 1. In August 2022, Eagle acquired a 17% equity stake in Enalare , with an option to purchase the remaining shares of Enalare .

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 15 15 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Financial Appendix

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 16 Non - GAAP Financial Measures In addition to financial information prepared in accordance with U.S. GAAP, this presentation also contains non - GAAP financial m easures, including adjusted non - GAAP net income, adjusted non - GAAP EBITDA, adjusted non - GAAP earnings per share, adjusted EBITDA multiple, non - GAAP gross margin, and adjusted non - GAAP gross profit. The Company believes these measu res provide investors and management with supplemental information relating to operating performance and trends that facilitate comparisons between periods and with respect to projected information. Adjusted non - GAAP net income and related earnings per share information excludes amortization expense, stock - based compensation expense, depreciation expense, severance expense, non - cash interest expense, fair value adjustments on equity investment, fair value adjustments related to derivative instruments, foreign currency exchange gain or loss, gain on Euro debt, amortization of inventory step - up, acquisition related costs, legal settlement, convertible promissory note related adjustments, debt issuance costs, and the tax effect of these adjustments. Adjusted non - GAAP EBITDA excludes interest expense net of interest income, income tax provision, depreciation and amortization e xpense, stock - based compensation expense, fair value adjustments on equity investment, convertible promissory note related adjustments, fair value adjustments related to derivative instruments, foreign currency exchange gain or loss, gain on Euro debt, legal settlement, acquisition related costs, debt issuance costs, and severance expense. Adjusted EBITDA multiple is calculated as Eagle’s market capitalization divided by adjusted EBITDA for the corresponding 12 - mont h period. Adjusted non - GAAP gross profit excludes amortization expense and amortization of inventory step - up. Adjusted non - GAAP R&D expense excludes stock - based compensation expense, depreciation expense and severance expense. Adjusted non - GAAP SG&A expense excludes stock - based compensation expense, depreciation expense, severance expense, acquisition r elated costs, and legal settlement. The Company believes the use of non - GAAP financial measures helps indicate underlying trends in the Company’s business and is im portant in comparing current results with prior period results and understanding projected operating performance. Non - GAAP financial measures provide the Company and its investors with an indication of the Company’s baseline perf ormance before items that are considered by the Company not to be reflective of the Company’s ongoing results. See the reconciliation tables in this Financial Appendix of this presentation for details of the amounts excluded an d i ncluded to arrive at certain of the non - GAAP financial measures. Investors should note that reconciliations of the forward - looking or projected non - GAAP financial measures included in this pres entation to their most comparable GAAP financial measures cannot be provided because the Company cannot do so without unreasonable efforts due to the unavailability of information needed to calculate the reconciling items and the va ria bility, complexity, and limited visibility of comparable GAAP measures, and the reconciling items that would be excluded from the non - GAAP financial measures in the future. Likewise, the Company is unable to provide projected GAAP financial measures. GAAP projections and reconciliations of the components of projected adjusted non - GAAP EBITDA, adjusted non - GAAP earnings per share, adjusted EBITDA multiple and adjusted non - GAAP earnings per share CAGR to their mo st comparable GAAP financial measures are not provided because the quantification of projected GAAP net income, GAAP earnings per share, and GAAP earnings per share CAGR and the reconciling items between projected GAAP t o p rojected adjusted non - GAAP EBITDA, adjusted non - GAAP earnings per share, adjusted EBITDA multiple and adjusted non - GAAP earnings per share CAGR cannot be reasonably calculated or predicted at this time without unreaso nable efforts. For example, with respect to GAAP net income and R&D Expense, the Company is not able to calculate the favorable or unfavorable expenses related to the fair value adjustments on equity investments and derivative in struments primarily due to nature of these transactions. Such unavailable information could be significant such that actual GAAP net income, GAAP earnings per share and GAAP earnings per share CAGR would vary significantly from projected adju ste d non - GAAP EBITDA, adjusted non - GAAP earnings per share, adjusted EBITDA multiple and adjusted non - GAAP earnings per share CAGR. These non - GAAP financial measures should be considered in addition to, but not as a substitute for, the information prepared in accordance with U.S. GAAP. In addition, from time to time in the future there may be other items that the Company may exclude for purposes of its non - GAAP financial measures; and the Company has ceased, and may in the future cease, to exclude items that it has historically excluded for purposes of its non - GAAP financial measures. For example, commencing in 2023, the Company no longer excludes expense of acquired in - process research & development from the Compa ny’s adjusted non - GAAP net income or adjusted non - GAAP EBITDA, their line item components, and non - GAAP earnings per share. For purposes of comparability, non - GAAP adjusted financial measures for the three and six months en ded June 30, 2022 have been updated to reflect this change. Accordingly, such expenses are not excluded from the Company’s non - GAAP financial measures for the three and six months ended June 30, 2023 and 2022, as detailed in the rec onciliation tables that follow, or from 2023 non - GAAP adjusted net income and adjusted non - GAAP earnings per share guidance. Likewise, the Company may determine to modify the nature of its adjustments to arrive at its non - GA AP financial measures. The Company strongly encourages investors to review its consolidated financial statements and publicly - filed reports in their entirety and cautions investors that the non - GAAP financial measures used by the Company may differ from similar measures used by other companies, even when similar terms are used to identify such measures.

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 17 17 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Three Months Six Months Ended Ended 2022 2021 2020 Net income (loss) - GAAP 5,164$ 10,914$ 35,642$ (8,627)$ 11,989$ Add back: Interest expense, net of interest income 1,253 2,557 3,774 1,075 2,015 Income tax provision 4,134 8,615 25,791 4,079 10,688 Depreciation and amortization expense 5,984 11,856 12,570 3,760 3,538 Add back: Stock-based compensation expense 4,192 8,831 16,451 19,555 24,756 Fair value adjustments on equity investment (210) 193 4,457 6,170 5,300 Convertible promissory note related adjustments — — 4,242 758 — Fair value adjustments related to derivative instruments — (77) 7,965 (686) 2,962 Expense related to collaboration with Tyme — — — — 2,500 Foreign currency exchange gain (35) (125) (647) — — Gain on euro debt — — (264) — — Legal Settlement — — 300 — — Aquisition related costs — — 13,122 — — Debt issuance cost — — 258 — — Severance 198 241 8,451 2,084 924 Adjusted Non-GAAP EBITDA 20,680$ 43,005$ 132,112$ 28,168$ 64,672$ EAGLE PHARMACEUTICALS, INC. RECONCILIATION OF GAAP TO ADJUSTED NON-GAAP EBITDA (UNAUDITED) (In thousands) June 30, 2023 Twelve Months Ended December 31,
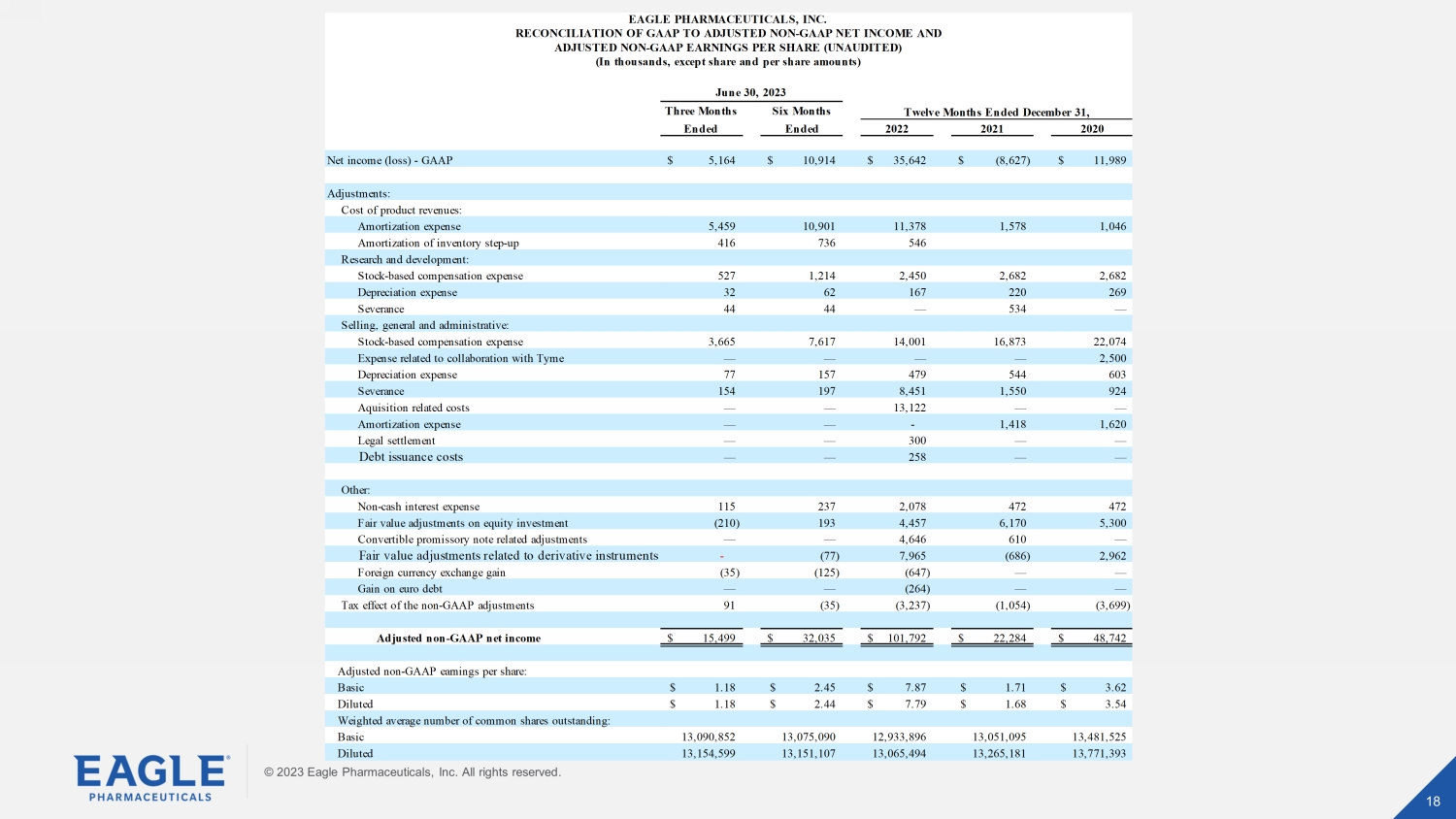
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 18 18 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Three Months Six Months Ended Ended 2022 2021 2020 Net income (loss) - GAAP 5,164$ 10,914$ 35,642$ (8,627)$ 11,989$ Adjustments: Cost of product revenues: Amortization expense 5,459 10,901 11,378 1,578 1,046 Amortization of inventory step-up 416 736 546 Research and development: Stock-based compensation expense 527 1,214 2,450 2,682 2,682 Depreciation expense 32 62 167 220 269 Severance 44 44 — 534 — Selling, general and administrative: Stock-based compensation expense 3,665 7,617 14,001 16,873 22,074 Expense related to collaboration with Tyme — — — — 2,500 Depreciation expense 77 157 479 544 603 Severance 154 197 8,451 1,550 924 Aquisition related costs — — 13,122 — — Amortization expense — — - 1,418 1,620 Legal settlement — — 300 — — Debt issuance costs — — 258 — — Other: Non-cash interest expense 115 237 2,078 472 472 Fair value adjustments on equity investment (210) 193 4,457 6,170 5,300 Convertible promissory note related adjustments — — 4,646 610 — Fair value adjustments related to derivative instruments - (77) 7,965 (686) 2,962 Foreign currency exchange gain (35) (125) (647) — — Gain on euro debt — — (264) — — Tax effect of the non-GAAP adjustments 91 (35) (3,237) (1,054) (3,699) Adjusted non-GAAP net income 15,499$ 32,035$ 101,792$ 22,284$ 48,742$ Adjusted non-GAAP earnings per share: Basic 1.18$ 2.45$ 7.87$ 1.71$ 3.62$ Diluted 1.18$ 2.44$ 7.79$ 1.68$ 3.54$ Weighted average number of common shares outstanding: Basic 13,090,852 13,075,090 12,933,896 13,051,095 13,481,525 Diluted 13,154,599 13,151,107 13,065,494 13,265,181 13,771,393 Twelve Months Ended December 31, EAGLE PHARMACEUTICALS, INC. RECONCILIATION OF GAAP TO ADJUSTED NON-GAAP NET INCOME AND ADJUSTED NON-GAAP EARNINGS PER SHARE (UNAUDITED) (In thousands, except share and per share amounts) June 30, 2023

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 19 19 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Three Months Six Months Ended Ended Revenue: PEMFEXY TM 19,400$ 42,348$ BELRAPZO® 6,848 13,198 BENDEKA® 3,780 6,174 TREAKISYM 728 2,083 Oncology product sales, net 30,756$ 63,803$ BENDEKA® 20,485 39,380 TREAKISYM 1,168 2,357 Oncology royalty revenue 21,653$ 41,737$ Oncology Total Revenue 52,409$ 105,540$ Oncology cost of product sales 10,466 20,146 Oncology Gross Profit 41,943$ 85,394$ Adjustments: Oncology cost of product revenues: Oncology amortization expense 1,867 3,714 Adjusted Non-GAAP EBITDAAdjusted Oncology Non-GAAP Gross Profit 43,810$ 89,108$ RECONCILIATION OF GAAP ONCOLOGY GROSS PROFIT TO ADJUSTED ONCOLOGY NON-GAAP GROSS PROFIT (UNAUDITED) (In thousands) June 30, 2023 EAGLE PHARMACEUTICALS, INC. Three Months Six Months Ended Ended Revenue: Product sales, net 42,993$ 89,214$ Royalty revenue 21,653 41,737 Total Revenue 64,646 130,951 Cost of product sales 16,858 34,158 Gross Profit 47,788$ 96,793$ Adjustments: Cost of product revenues: Amortization expense 5,459 10,901 Amortization of inventory step-up 416 736 Adjusted Non-GAAP EBITDAAdjusted Non-GAAP Gross Profit 53,663$ 108,430$ EAGLE PHARMACEUTICALS, INC. RECONCILIATION OF GAAP GROSS PROFIT TO ADJUSTED NON-GAAP GROSS PROFIT (UNAUDITED) (In thousands) June 30, 2023 Three Months Six Months Ended Ended Revenue: RYANODEX® 10,026$ 18,780$ vasopressin 1,017 4,519 BARHEMSYS 945 1,737 BYFAVO 249 375 Acute Care product sales, net 12,237$ 25,411$ Acute Care cost of product sales 6,392 14,012 Acute Care Gross Profit 5,845$ 11,399$ Adjustments: Acute Care cost of product revenues: Amortization expense 3,592 7,187 Amortization of inventory step-up 416 736 Adjusted Non-GAAP EBITDAAdjusted Acute Care Non-GAAP Gross Profit 9,853$ 19,322$ June 30, 2023 EAGLE PHARMACEUTICALS, INC. RECONCILIATION OF GAAP ACUTE CARE GROSS PROFIT TO ADJUSTED ACUTE CARE NON-GAAP GROSS PROFIT (UNAUDITED) (In thousands)
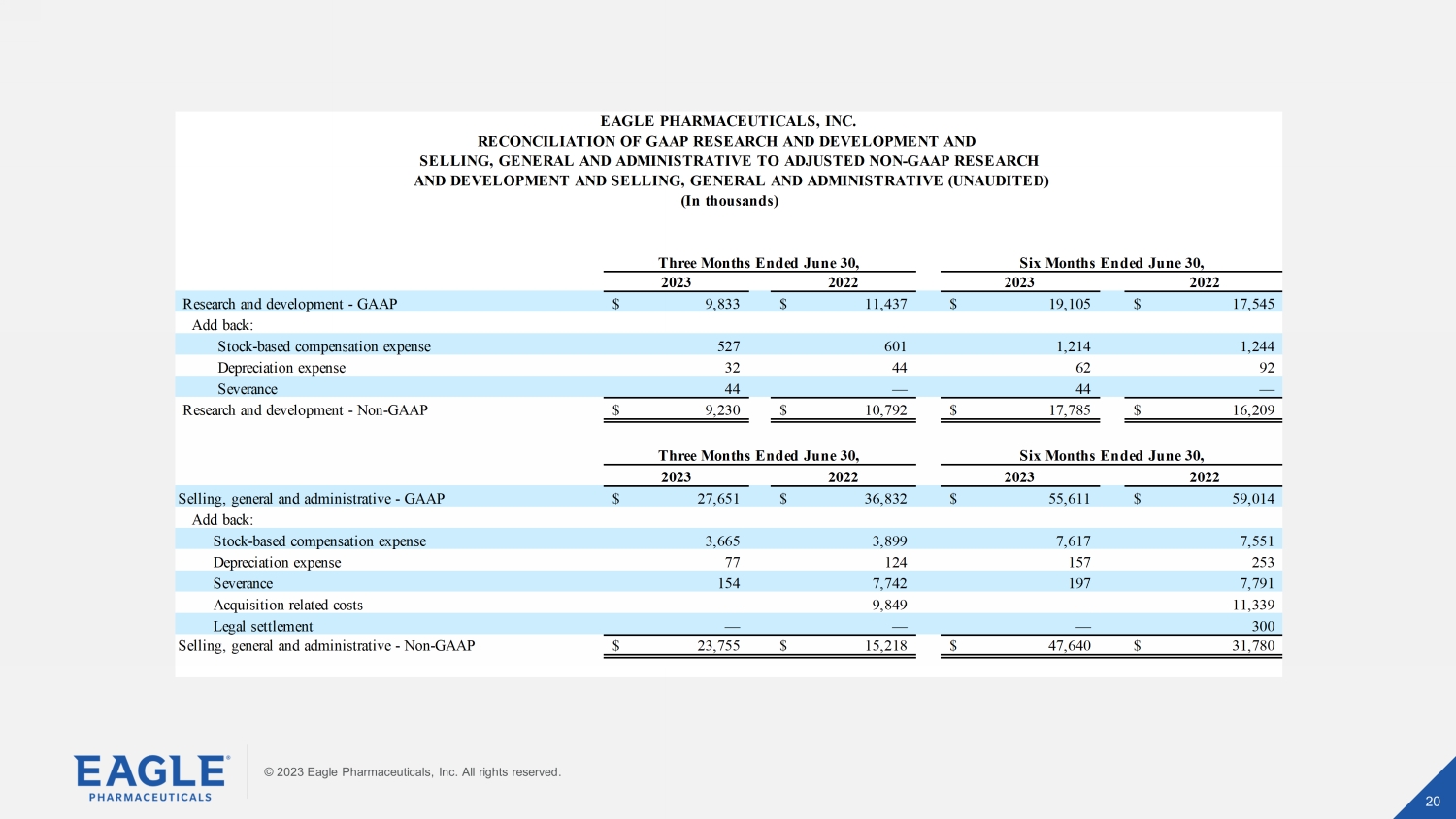
© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 20 20 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 2023 2022 2023 2022 Research and development - GAAP 9,833$ 11,437$ 19,105$ 17,545$ Add back: Stock-based compensation expense 527 601 1,214 1,244 Depreciation expense 32 44 62 92 Severance 44 — 44 — Research and development - Non-GAAP 9,230$ 10,792$ 17,785$ 16,209$ 2023 2022 2023 2022 Selling, general and administrative - GAAP 27,651$ 36,832$ 55,611$ 59,014$ Add back: Stock-based compensation expense 3,665 3,899 7,617 7,551 Depreciation expense 77 124 157 253 Severance 154 7,742 197 7,791 Acquisition related costs — 9,849 — 11,339 Legal settlement — — — 300 Selling, general and administrative - Non-GAAP 23,755$ 15,218$ 47,640$ 31,780$ Three Months Ended June 30, Six Months Ended June 30, Six Months Ended June 30, EAGLE PHARMACEUTICALS, INC. RECONCILIATION OF GAAP RESEARCH AND DEVELOPMENT AND SELLING, GENERAL AND ADMINISTRATIVE TO ADJUSTED NON-GAAP RESEARCH AND DEVELOPMENT AND SELLING, GENERAL AND ADMINISTRATIVE (UNAUDITED) (In thousands) Three Months Ended June 30,

© 2023 Eagle Pharmaceuticals, Inc. All rights reserved. 21 © 2023 Eagle Pharmaceuticals, Inc. All rights reserved. Thank You!
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Eagle Pharmaceuticals (NASDAQ:EGRX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Eagle Pharmaceuticals (NASDAQ:EGRX)
Historical Stock Chart
From Apr 2023 to Apr 2024