0001082038false00010820382023-11-072023-11-07
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 8-K
Current Report
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
November 7, 2023
Date of Report
(Date of earliest event reported)
DURECT CORPORATION
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
Delaware |
|
000-31615 |
|
94-3297098 |
(State or other jurisdiction of incorporation or organization) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
10260 Bubb Road
Cupertino, CA 95014
(Address of principal executive offices) (Zip code)
(408) 777-1417
(Registrant’s telephone number, including area code)
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which Registered |
Common Stock $0.0001 par value per share Preferred Share Purchase Rights |
DRRX |
The NASDAQ Stock Market LLC (The Nasdaq Capital Market) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Item 8.01 Other Events
On November 7, 2023, DURECT Corporation issued a press release announcing topline results from its AHFIRM trial, a Phase 2b randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of larsucosterol in 307 patients with severe alcohol-associated hepatitis.
DURECT Corporation will also be conducting a webcast scheduled to be held at 2:00 p.m. Pacific Time on November 7, 2023, presenting the topline results from its AHFIRM trial.
Copies of the press release and the materials to be used in the presentation are filed hereto as Exhibit 99.1 and Exhibit 99.2, respectively, to this Form 8-K, and are incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits
(d) Exhibits
2
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
DURECT Corporation |
|
|
|
|
|
Date: November 7, 2023 |
|
By: |
|
/s/ James E. Brown |
|
|
|
|
James E. Brown President and Chief Executive Officer |
|
|
|
|
|
3
Exhibit 99.1
DURECT Corporation Announces Topline Results from Phase 2b AHFIRM Trial
of Larsucosterol in Alcohol-Associated Hepatitis with Promising Effect on Mortality
Compelling efficacy signal in favor of larsucosterol in the key secondary endpoint of mortality at 90 days. Clinically relevant reduction in 90-day mortality of 41% for 30 mg dose and 35% for 90 mg dose compared with standard of care (SOC)
Numerical improvement in primary endpoint of mortality or transplant at 90 days did not achieve statistical significance
More pronounced effect in the U.S. trial population of 232 patients, representing 76% of the trial population, with a clinically meaningful 90-day mortality reduction of 57% for 30 mg dose and 58% for 90 mg dose compared with SOC
Larsucosterol was well-tolerated and both 30 mg and 90 mg dose groups had numerically fewer adverse events than SOC
Strong rationale for advancing larsucosterol in a Phase 3 registration trial in alcohol-associated hepatitis with reduction in 90-day mortality as the primary endpoint
DURECT will host a conference call and webcast at 5 p.m. ET today
CUPERTINO, Calif. – November 7, 2023 – DURECT Corporation (Nasdaq: DRRX), a late-stage biopharmaceutical company pioneering the development of epigenetic therapies to transform the treatment of serious and life-threatening conditions, including acute organ injury and cancer, today announced topline results from its AHFIRM trial, a Phase 2b randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of larsucosterol in 307 patients with severe alcohol-associated hepatitis (AH). Topline data from AHFIRM showed:
•Both the 30 mg and 90 mg larsucosterol doses demonstrated a compelling and clinically meaningful trend in reduction of mortality at 90 days, the key secondary endpoint, with mortality reductions of 41% (p=0.070) in the 30 mg arm and 35% (p=0.126) in the 90 mg arm compared with SOC.
•The numerical improvement in the primary endpoint of mortality or transplant at 90 days did not achieve statistical significance for either dose of larsucosterol.
•Both doses of larsucosterol showed a more pronounced reduction in mortality in patients enrolled in the U.S., representing 76% of patients enrolled in the trial. The reductions in mortality at 90 days were 57% (p=0.014) for the 30 mg arm and 58% (p=0.008) for the 90 mg arm compared with SOC.
•Larsucosterol was safe and well tolerated. There were fewer treatment-emergent adverse events (TEAEs) in the larsucosterol arms compared with SOC.
DURECT intends to have an End of Phase 2 (EOP2) meeting with the U.S. Food and Drug Administration (FDA) to discuss the trial results and the Phase 3 registration trial design in the first quarter of 2024. DURECT also intends to present the results of AHFIRM at an upcoming medical meeting.
“The topline results from AHFIRM provide compelling evidence that administration of larsucosterol can reduce mortality at 90 days in this devastating disease,” said James E. Brown, D.V.M., President and CEO of DURECT. “We have strong rationale to advance larsucosterol into a Phase 3 registration trial designed with adequate power to detect a statistically significant result using 90-day mortality as the primary endpoint. We look forward to meeting with the FDA to discuss next steps. Based on the strength of the clinical data generated to date, if approved, larsucosterol could save many patient lives. We extend our thanks to all the patients, families, clinical trial investigators, and staff across the multiple sites globally who have worked with the DURECT team to bring larsucosterol to this advanced stage.”
Craig McClain, M.D., AGAF, FACG, FAASLD, FACN, Professor of Medicine and Pharmacology & Toxicology at University of Louisville School of Medicine, commented, “In my practice, I treat AH patients frequently and can personally attest to the frustration of the hepatology community at the lack of effective treatment options for these critically ill patients. The
AHFIRM trial results represent the most promising data set I have seen on new therapy for severe AH with no important toxicity and a trend toward reducing mortality.”
Norman Sussman, M.D., FAASLD, Chief Medical Officer at DURECT, added, “Patients with alcohol-associated hepatitis are extremely ill and have a high mortality in the three months following hospital admission. The AHFIRM trial provides strong evidence that larsucosterol has the potential to reduce 90-day mortality and has demonstrated an excellent safety profile to date. We are continuing to analyze the AHFIRM data to fully understand the results and to inform future trials and our discussion with the FDA.”
Key AHFIRM trial results:
Mortality or Liver Transplantation at 90 Days
The primary endpoint for the AHFIRM trial was the reduction in mortality or liver transplantation at 90 days. The endpoint was analyzed using a hierarchical assessment of patient outcomes to calculate a win probability for each of the 30 mg and 90 mg dose of larsucosterol compared with SOC. The results for the primary endpoint were not statistically significant for either the 30 mg or 90 mg doses compared with SOC, though a numerical improvement was observed.
Patient Outcomes
|
|
|
|
|
SOC |
Larsucosterol 30 mg |
Larsucosterol 90 mg |
Number of patients randomized |
103 |
102 |
102 |
Number of patients with 90-day outcome data |
102 |
99 |
101 |
|
|
|
|
Deaths (%) |
25 (24.5%) |
15 (15.2%) |
17 (16.8%) |
Transplants (%) |
4 (3.9%) |
6 (6.1%) |
9 (8.9%) |
Alive & Transplant-free (%) |
73 (71.6%) |
78 (78.8%) |
75 (74.3%) |
Win Probability Analysis
|
|
|
|
|
|
|
Larsucosterol 30 mg vs. SOC |
|
Larsucosterol 90 mg vs. SOC |
|
SOC |
30 mg |
|
SOC |
90 mg |
Win Probability %1 |
15.8% |
23.6% |
|
19.2% |
23.1% |
p-value |
|
0.196 |
|
|
0.533 |
1 Win probability was calculated based on the hierarchy of alive and transplant-free being superior to transplant and death and transplant being superior to death. Comparisons of the same outcome were included in the denominator as ties.
Mortality at 90 Days
Mortality at 90 Days was a key secondary endpoint for the AHFIRM trial. In this analysis, the 30 mg and 90 mg doses of larsucosterol showed numerical trends toward a clinically meaningful survival benefit with 90-day mortality reductions of 41% and 35%, respectively, when compared to SOC, although these results were not statistically significant.
|
|
|
|
|
Group |
Mortality at 90 Days |
% Reduction vs. SOC |
Difference vs. SOC |
p-value |
Larsucosterol 30 mg (n=102) |
15.3% |
-40.7% |
-10.5% |
0.070 |
SOC (n=103) |
25.8% |
|
|
|
|
|
|
|
|
Larsucosterol 90 mg (n=102) |
16.2% |
-34.9% |
-8.7% |
0.126 |
SOC (n=103) |
24.9% |
|
|
|
Mortality at 90 Days (U.S. patients)
When further analyzed by geography, both the 30 mg and 90 mg doses showed an enhanced survival benefit at 90 days with reductions in 90-day mortality of 57% and 58%, respectively, in patients enrolled in the U.S., which represented 76% of the total patients enrolled.
|
|
|
|
|
Group |
Mortality at 90 Days |
% Reduction vs. SOC |
Difference vs. SOC |
p-value |
Larsucosterol 30 mg (n=76) |
12.3% |
-56.8% |
-16.1% |
0.014 |
SOC (n=78) |
28.5% |
|
|
|
|
|
|
|
|
Larsucosterol 90 mg (n=78) |
11.7% |
-58.1% |
-16.2% |
0.008 |
SOC (n=78) |
27.9% |
|
|
|
Safety and Tolerability
Both the 30 mg and 90 mg doses of larsucosterol were well tolerated. There were fewer TEAEs in the larsucosterol arms compared with SOC.
|
|
|
|
|
SOC |
Larsucosterol 30 mg |
Larsucosterol 90 mg |
Number of TEAEs |
721 |
545 |
567 |
Dial-In and Webcast Information, 5pm ET Today
Toll Free: 1-877-407-4018
International: 1-201-689-8471
Conference ID: 13742589
Call me™: click here
Participants can use guest dial-in numbers above to reach an operator or they can click the Call me™ link for instant telephone access to the event (dial-out). The Call me™ link will be made active 15 minutes prior to the scheduled start time.
Webcast: https://viavid.webcasts.com/starthere.jsp?ei=1642726&tp_key=3921e455c2
A replay of the webcast will be available on the Investor section of the DURECT website at https://www.durect.com/investors/ after the call.
About the AHFIRM trial
AHFIRM was a Phase 2b randomized, double-blind, placebo-controlled, international, multi-center study designed in subjects with severe alcohol-associated hepatitis (AH) to evaluate the saFety and effIcacy of laRsucosterol treatMent (AHFIRM). The study was comprised of three arms comprising 307 total patients, with approximately 100 patients in each arm: (1) SOC, which consists of placebo plus supportive care, with or without methylprednisolone capsules at the investigators’ discretion; (2) larsucosterol (30 mg); and (3) larsucosterol (90 mg). Patients in the larsucosterol arms received the same supportive care without steroids. In order to maintain blinding, patients in the two active arms received matching placebo capsules if the investigator prescribed steroids. The primary outcome measure was the 90-Day incidence of mortality or liver transplantation for patients treated with larsucosterol compared to those treated with SOC. The Company enrolled patients at clinical trial sites across the U.S., EU, U.K., and Australia. Reflecting the life-threatening nature of AH and the lack of therapeutic options, the U.S. Food and Drug Administration (FDA) granted larsucosterol Fast Track Designation for the treatment of AH. For more information, refer to ClinicalTrials.gov Identifier: NCT04563026.
About Alcohol-associated Hepatitis (AH)
AH is an acute form of alcohol-associated liver disease (ALD), associated with long-term heavy intake of alcohol and often occurs after a recent period of increased alcohol consumption (i.e., a binge). AH is typically characterized by severe inflammation and destruction of liver tissue (i.e., necrosis), potentially leading to life-threatening complications including liver failure, acute kidney injury and multi-organ failure. There are no FDA approved therapies for AH and a retrospective analysis of 77 studies published between 1971 and 2016, which included data from a total of 8,184 patients, showed the overall mortality from AH was 26% at 28 days, 29% at 90 days and 44% at 180 days. A subsequent global study published in December 2021, which included 85 tertiary centers in 11 countries across 3 continents, prospectively enrolled 2,581 AH patients with a median Model of End-Stage Liver Disease (MELD) score of 23.5, reported mortality at 28 and 90 days of approximately 20% and 31%, respectively. Stopping alcohol consumption is necessary, but frequently not sufficient for recovery in many moderate (defined as MELD scores of 11-20) and severe (defined as MELD scores >20) patients and therapies that reduce liver inflammation, such as corticosteroids, are limited by contraindications, have not been shown to improve survival at 90 days or one year, and have demonstrated an increased risk of infection. While liver transplantation is becoming more common for ALD patients, including AH patients, the total number of such transplants is still relatively small. Average charges for a liver transplant exceed $875,000, and patients require lifelong immunosuppressive therapy to prevent organ rejection.
About Larsucosterol
Larsucosterol is an endogenous sulfated oxysterol and an epigenetic modulator. Epigenetic regulators are compounds that regulate patterns of gene expression without modifying the DNA sequence. DNA hypermethylation, an example of epigenetic dysregulation, results in transcriptomic reprogramming and cellular dysfunction, and has been found to be associated with many acute (e.g., AH) or chronic diseases (e.g., NASH). As an inhibitor of DNA methyltransferases (DNMT1, DNMT3a and 3b), larsucosterol inhibits DNA methylation, which subsequently modulates expression of genes that are involved in cell signaling pathways associated with stress responses, cell death and survival, and lipid biosynthesis. This may ultimately lead to improved cell survival, reduced inflammation, and decreased lipotoxicity. As an epigenetic modulator, the proposed mechanism of action provides further scientific rationale for developing larsucosterol for the treatment of acute organ injury and certain chronic diseases.
About DURECT Corporation
DURECT is a late-stage biopharmaceutical company pioneering the development of epigenetic therapies that target dysregulated DNA methylation to transform the treatment of serious and life-threatening conditions, including acute organ injury and cancer. Larsucosterol, DURECT’s lead drug candidate, binds to and inhibits the activity of DNA methyltransferases (DNMTs), epigenetic enzymes that are elevated and associated with hypermethylation found in alcohol-associated hepatitis (AH) patients. Larsucosterol is in clinical development for the potential treatment of AH, for which FDA has granted a Fast Track Designation; non-alcoholic steatohepatitis (NASH) is also being explored. In addition, POSIMIR® (bupivacaine solution) for infiltration use, a non-opioid analgesic utilizing the innovative SABER® platform technology, is FDA-approved and is exclusively licensed to Innocoll Pharmaceuticals for sale and distribution in the
United States. For more information about DURECT, please visit www.durect.com and follow us on X (formerly Twitter) at https://twitter.com/DURECTCorp.
DURECT Forward-Looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, relating to: the potential for larsucosterol to demonstrate a reduction in mortality or liver transplant in patients with AH and to save lives, our plans to meet with the FDA and other regulatory agencies to review the results of AHFIRM trial, the potential FDA or other regulatory approval of larsucosterol for the treatment of AH, the commercialization of POSIMIR by Innocoll, the potential to develop larsucosterol for AH, NASH or other indications, and the potential benefits, if any, of our product candidates. Actual results may differ materially from those contained in the forward-looking statements contained in this press release, and reported results should not be considered as an indication of future performance. The potential risks and uncertainties that could cause actual results to differ from those projected include, among other things, the risk that future clinical trials of larsucosterol do not confirm the results from subset analyses of the AHFIRM trial, including geographic or other segmentation, or of earlier clinical or pre-clinical trials, or do not demonstrate the safety or efficacy of larsucosterol in a statistically significant manner, the risk that the FDA or other government agencies may require additional clinical trials for larsucosterol before approving it for the treatment of AH, risks that Innocoll may not commercialize POSIMIR successfully, and risks related to the sufficiency of our cash resources, our anticipated capital requirements and capital expenditures, our need or desire for additional financing, our ability to obtain capital to fund our operations and expenses and our ability to continue to operate as a going concern. Further information regarding these and other risks is included in DURECT's most recent Securities and Exchange Commission (SEC) filings, including its annual report on Form 10-K for the year ended December 31, 2022 and quarterly report on Form 10-Q for the quarter ended September 30, 2023, when filed, under the heading “Risk Factors.” These reports are available on our website www.durect.comunder the “Investors” tab and on the SEC’s website at www.sec.gov. All information provided in this press release and in the attachments is based on information available to DURECT as of the date hereof, and DURECT assumes no obligation to update this information as a result of future events or developments, except as required by law.
NOTE: POSIMIR® is a trademark of Innocoll Pharmaceuticals, Ltd. in the U.S. and a trademark of DURECT Corporation outside of the U.S. SABER® is a trademark of DURECT Corporation. Other referenced trademarks belong to their respective owners. Larsucosterol is an investigational drug candidate under development and has not been approved for commercialization by the U.S. Food and Drug Administration or other health authorities for any indication.
Investor Relations Contact:
Ashley R. Robinson
Managing Director | LifeSci Advisors, LLC
E: arr@lifesciadvisors.com
Media Contact
Mollie Godbout
LifeSci Communications
E: mgodbout@lifescicomms.com

Phase 2b AHFIRM Topline Results November 2023 Exhibit 99.2

Disclaimer This presentation and various remarks we make during this presentation contain forward-looking statements of DURECT Corporation ("DURECT," the "Company," "we," "our" or "us") and its collaborative partners within the meaning of applicable securities laws and regulations, which are subject to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements relating to the potential for larsucosterol to demonstrate a reduction in mortality or liver transplant in patients with alcohol-associated hepatitis ("AH") and to save lives, DURECT's plans to meet with the FDA and other regulatory agencies to review the results of AHFIRM trial, the potential for a Phase 3 trial of larsucosterol to show a statistically significant improvement in the treatment of AH over standard of care, the potential FDA or other regulatory approval of larsucosterol for the treatment of AH, anticipated product benefits and other potential uses of larsucosterol, anticipated product markets and potential sales, and clinical trial results and plans. Actual results may differ materially from those contained in the forward-looking statements contained in this presentation, and reported results should not be considered as an indication of future performance. These forward-looking statements involve risks and uncertainties that can cause actual results to differ materially from those in such forward-looking statements. Potential risks and uncertainties include, but are not limited to, the risk that future clinical trials of larsucosterol do not confirm the results from subset analyses of the AHFIRM trial, including geographic or other segmentation, or of earlier clinical or pre-clinical trials, or do not demonstrate the safety or efficacy of larsucosterol in a statistically significant manner, the risk that the FDA or other government agencies may require additional clinical trials for larsucosterol before approving it for the treatment of AH, and risks related to the sufficiency of our cash resources, our anticipated capital requirements and capital expenditures, our need or desire for additional financing, our ability to obtain capital to fund our operations and expenses and our ability to continue to operate as a going concern. Further information regarding these and other risks is included in DURECT's most recent U.S. Securities and Exchange Commission ("SEC") filings, including its Annual and Quarterly Report on Form 10-K or 10-Q, respectively, filed with the SEC under the heading “Risk Factors.” DURECT is under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Subsequent events and developments may cause DURECT’s expectations and beliefs to change. This presentation does not constitute an offer to sell or a solicitation of an offer to buy any securities of the Company. Any offer of securities will only be made pursuant to a registration statement (including a base prospectus) and prospectus supplement filed with the SEC, copies of which may be obtained for free on our website at www.durect.com under the "Investors" tab or by visiting EDGAR on the SEC website at www.sec.gov. All information provided in this presentation is based on information available to DURECT as of November 7, 2023, and DURECT assumes no obligation to update this information as a result of future events or developments, except as required by law.
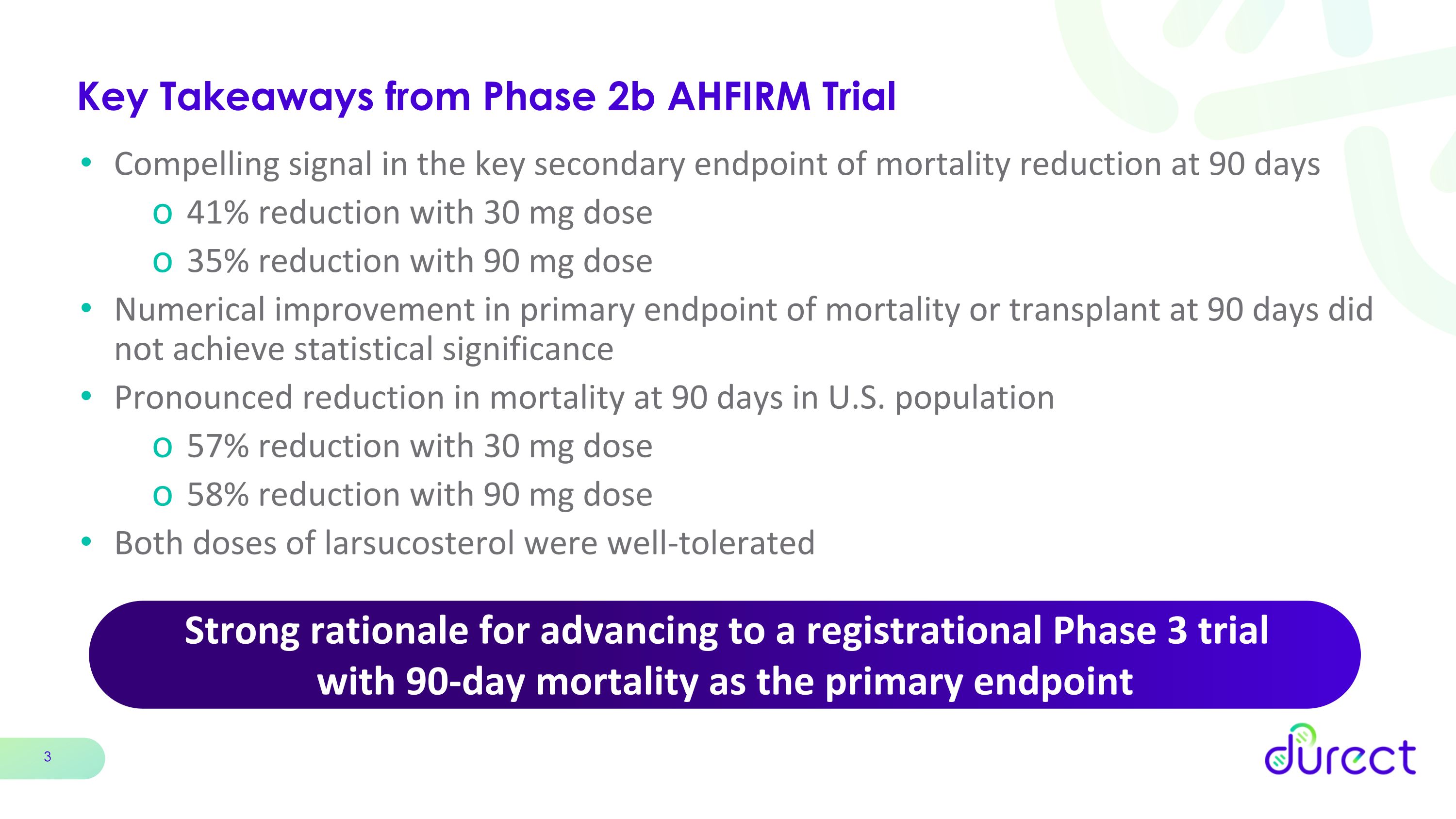
Strong rationale for advancing to a registrational Phase 3 trial �with 90-day mortality as the primary endpoint Key Takeaways from Phase 2b AHFIRM Trial Compelling signal in the key secondary endpoint of mortality reduction at 90 days 41% reduction with 30 mg dose 35% reduction with 90 mg dose Numerical improvement in primary endpoint of mortality or transplant at 90 days did not achieve statistical significance Pronounced reduction in mortality at 90 days in U.S. population 57% reduction with 30 mg dose 58% reduction with 90 mg dose Both doses of larsucosterol were well-tolerated
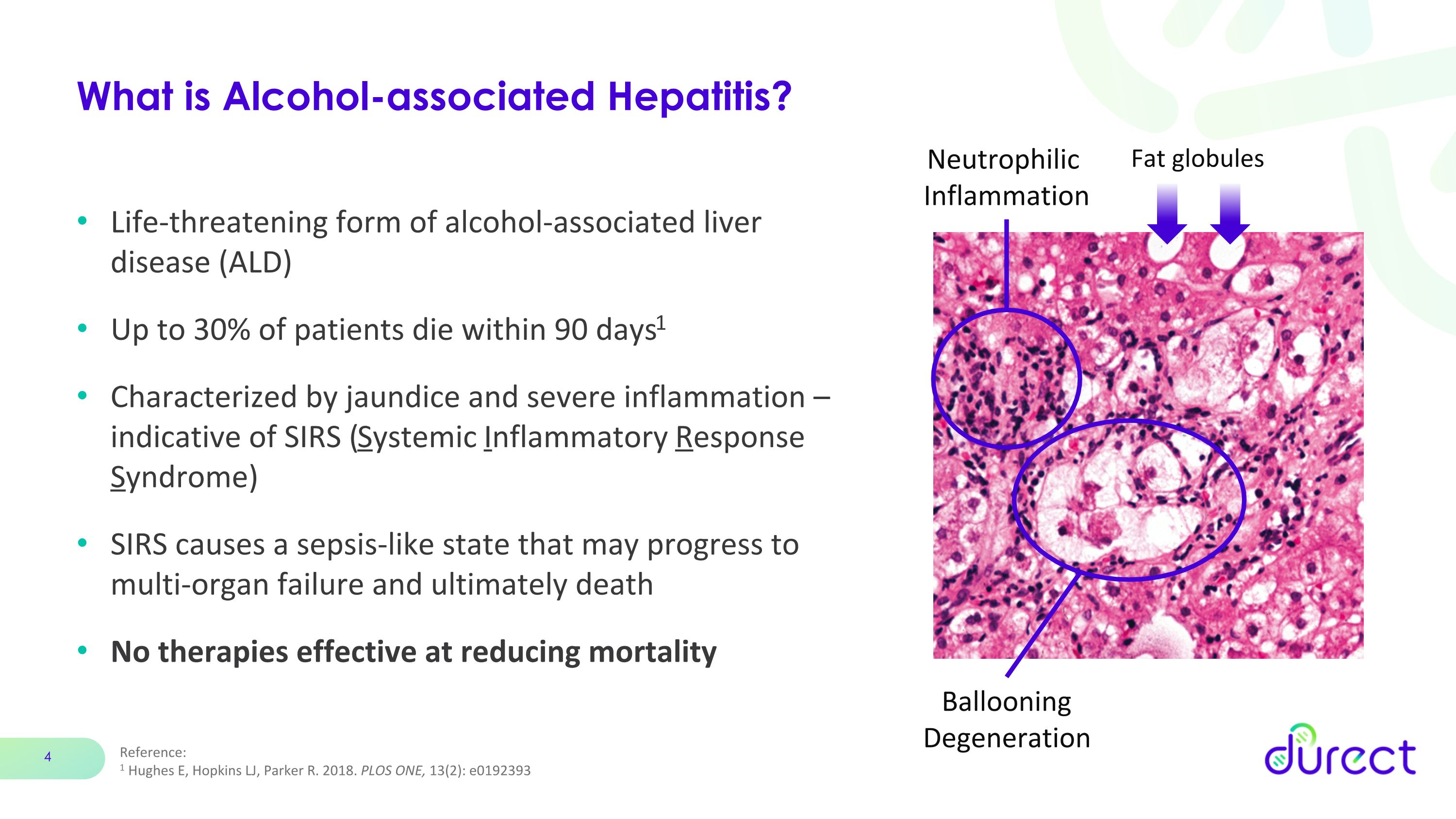
Reference: 1 Hughes E, Hopkins LJ, Parker R. 2018. PLOS ONE, 13(2): e0192393 What is Alcohol-associated Hepatitis? Life-threatening form of alcohol-associated liver disease (ALD) Up to 30% of patients die within 90 days1 Characterized by jaundice and severe inflammation – indicative of SIRS (Systemic Inflammatory Response Syndrome) SIRS causes a sepsis-like state that may progress to multi-organ failure and ultimately death No therapies effective at reducing mortality Fat globules Ballooning Degeneration Neutrophilic Inflammation
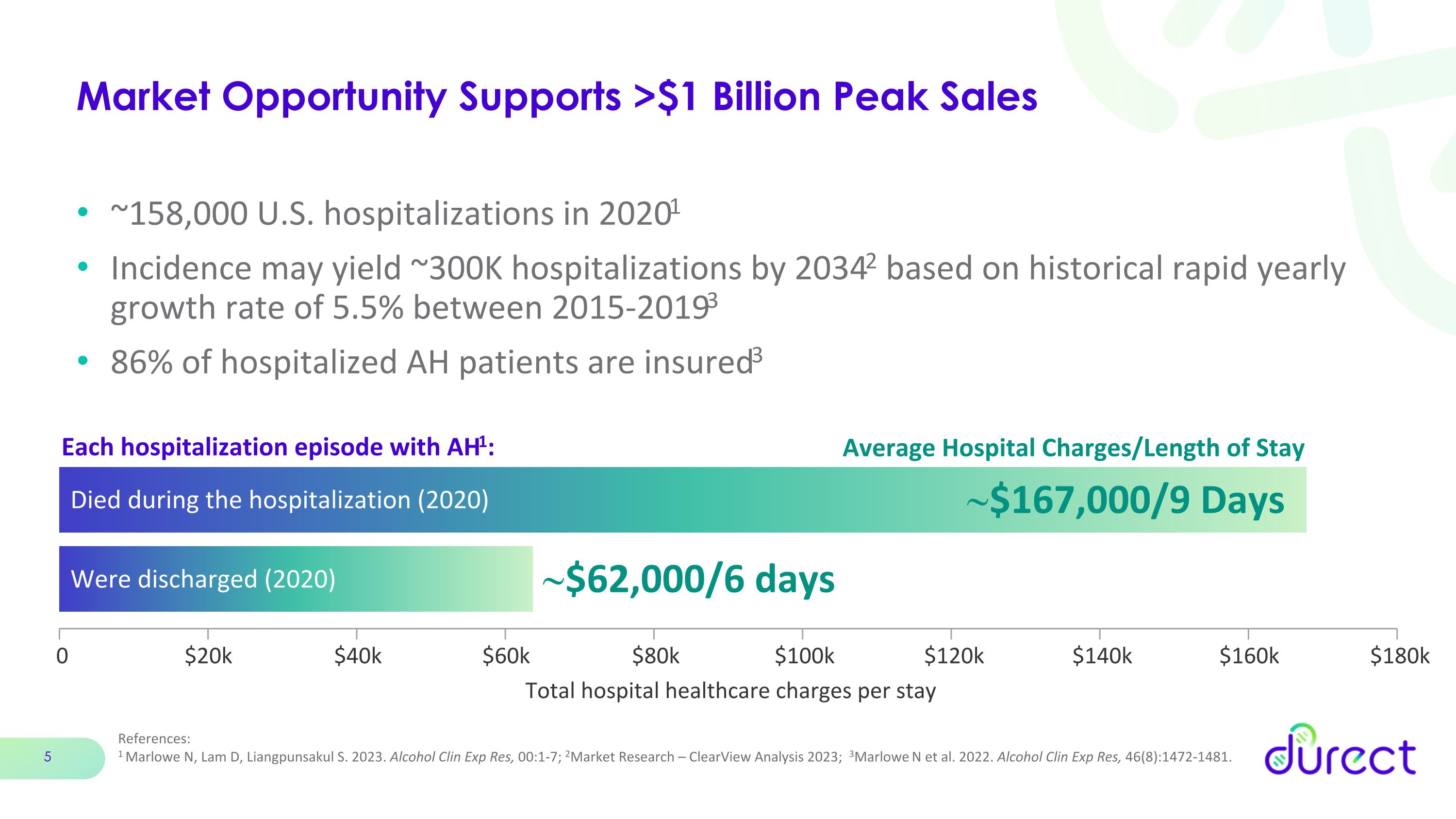
Market Opportunity Supports >$1 Billion Peak Sales ~158,000 U.S. hospitalizations in 20201 Incidence may yield ~300K hospitalizations by 20342 based on historical rapid yearly growth rate of 5.5% between 2015-20193 86% of hospitalized AH patients are insured3 Each hospitalization episode with AH1: Died during the hospitalization (2020) Total hospital healthcare charges per stay ~$167,000/9 Days ~$62,000/6 days 0 $20k $40k $60k $80k $100k $120k $140k $160k $180k References: 1 Marlowe N, Lam D, Liangpunsakul S. 2023. Alcohol Clin Exp Res, 00:1-7; 2Market Research – ClearView Analysis 2023; 3Marlowe N et al. 2022. Alcohol Clin Exp Res, 46(8):1472-1481. Average Hospital Charges/Length of Stay Were discharged (2020)
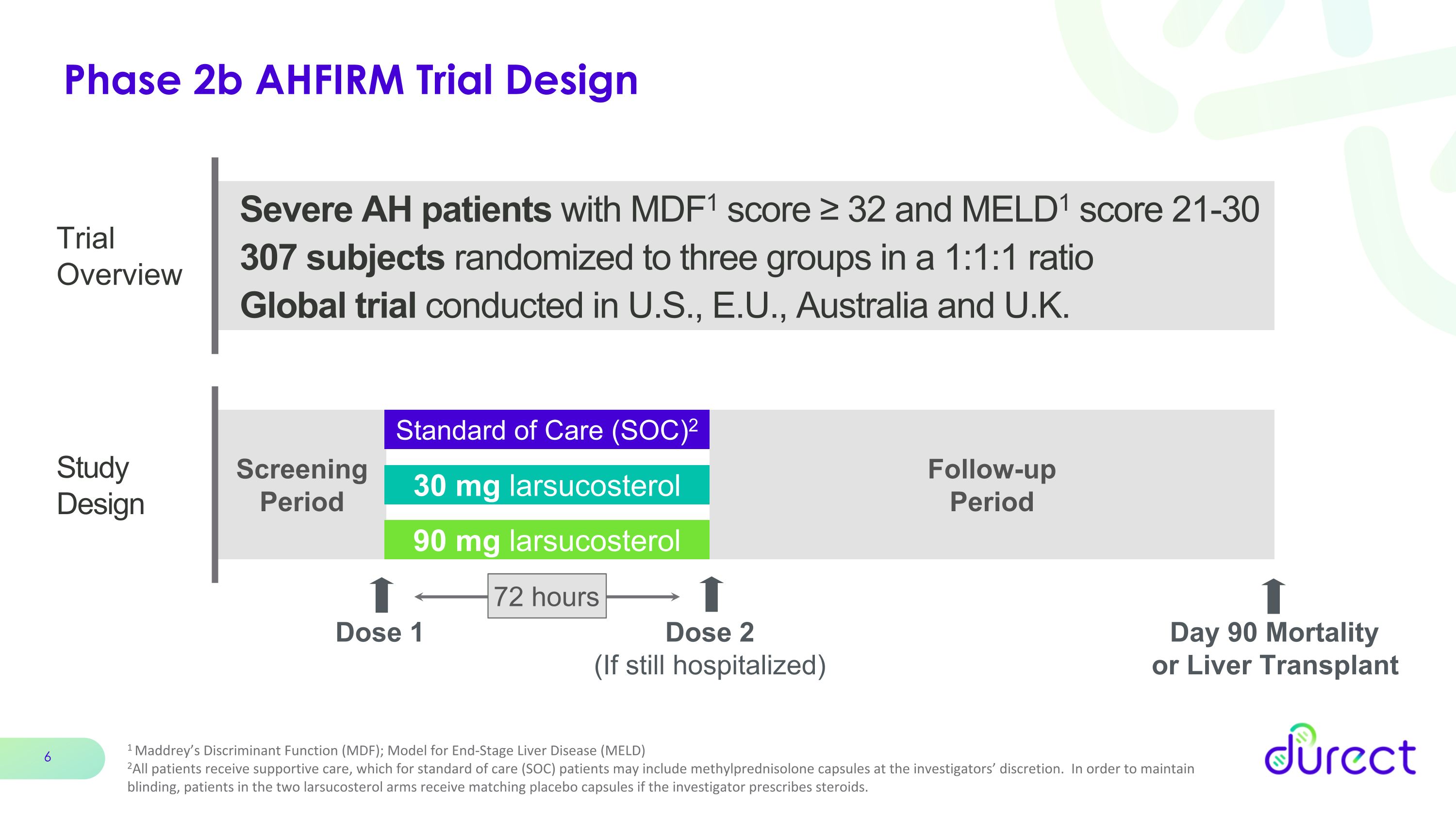
Phase 2b AHFIRM Trial Design Study Design Screening Period 30 mg larsucosterol 90 mg larsucosterol Standard of Care (SOC)2 Severe AH patients with MDF1 score ≥ 32 and MELD1 score 21-30 307 subjects randomized to three groups in a 1:1:1 ratio Global trial conducted in U.S., E.U., Australia and U.K. Dose 1 Trial Overview Follow-up Period Day 90 Mortality �or Liver Transplant 1 Maddrey’s Discriminant Function (MDF); Model for End-Stage Liver Disease (MELD) 2All patients receive supportive care, which for standard of care (SOC) patients may include methylprednisolone capsules at the investigators’ discretion. In order to maintain blinding, patients in the two larsucosterol arms receive matching placebo capsules if the investigator prescribes steroids. 72 hours Dose 2 (If still hospitalized)
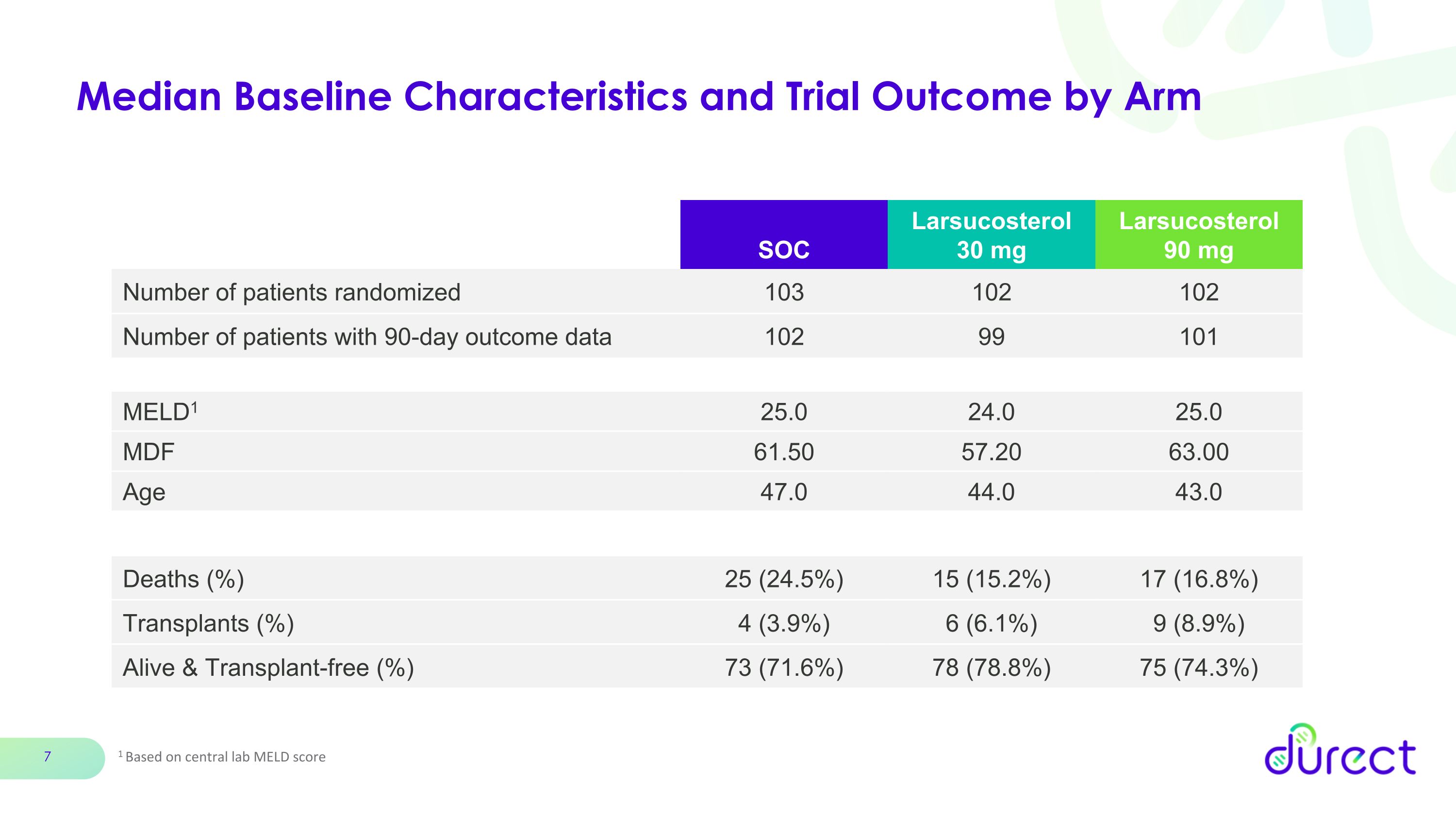
Median Baseline Characteristics and Trial Outcome by Arm 1 Based on central lab MELD score SOC Larsucosterol 30 mg Larsucosterol 90 mg Number of patients randomized 103 102 102 Number of patients with 90-day outcome data 102 99 101 MELD1 25.0 24.0 25.0 MDF 61.50 57.20 63.00 Age 47.0 44.0 43.0 Deaths (%) 25 (24.5%) 15 (15.2%) 17 (16.8%) Transplants (%) 4 (3.9%) 6 (6.1%) 9 (8.9%) Alive & Transplant-free (%) 73 (71.6%) 78 (78.8%) 75 (74.3%)
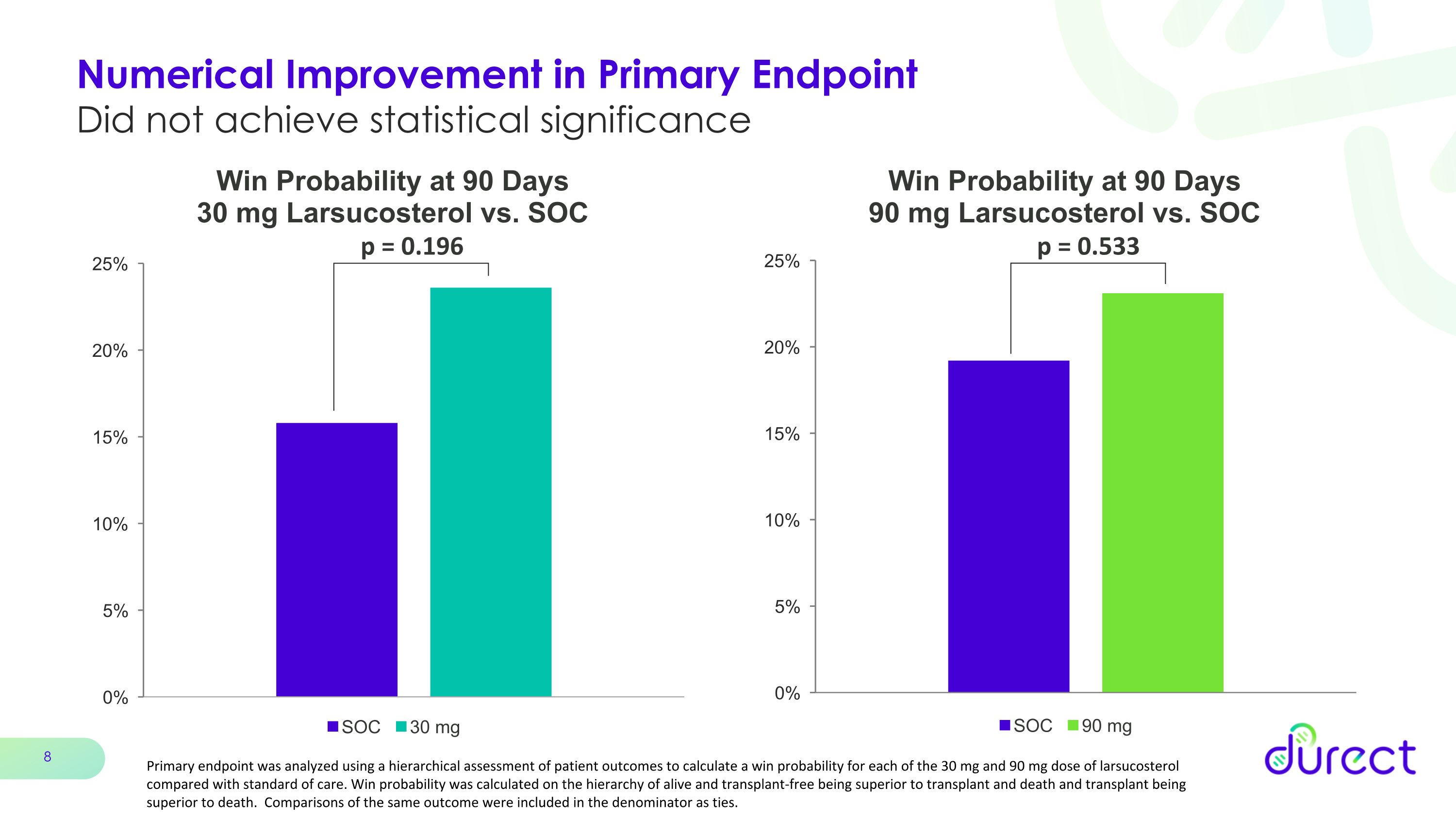
Numerical Improvement in Primary Endpoint �Did not achieve statistical significance p = 0.533 p = 0.196 Primary endpoint was analyzed using a hierarchical assessment of patient outcomes to calculate a win probability for each of the 30 mg and 90 mg dose of larsucosterol compared with standard of care. Win probability was calculated on the hierarchy of alive and transplant-free being superior to transplant and death and transplant being superior to death. Comparisons of the same outcome were included in the denominator as ties.
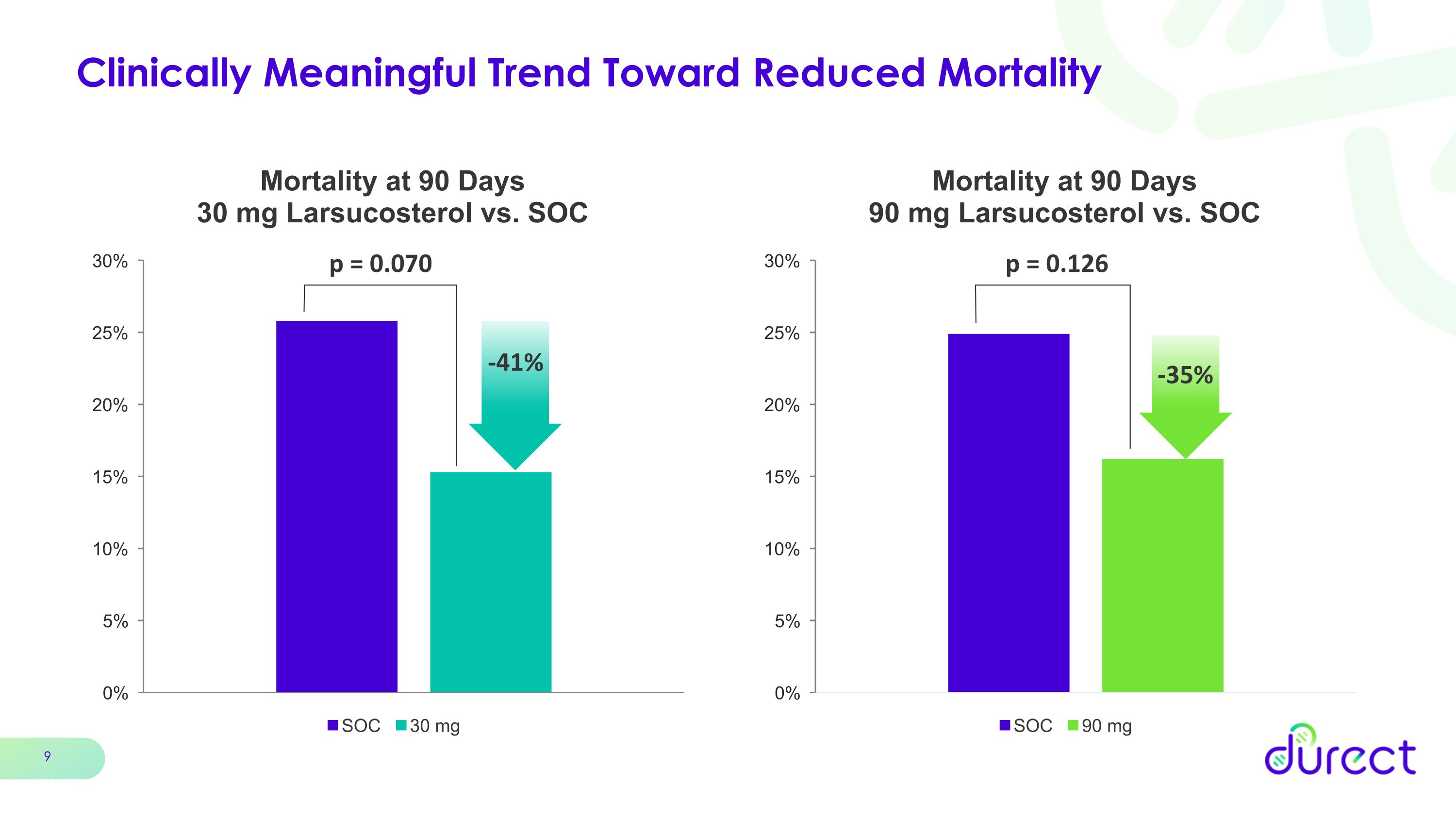
Clinically Meaningful Trend Toward Reduced Mortality p = 0.126 p = 0.070 -41% -35%
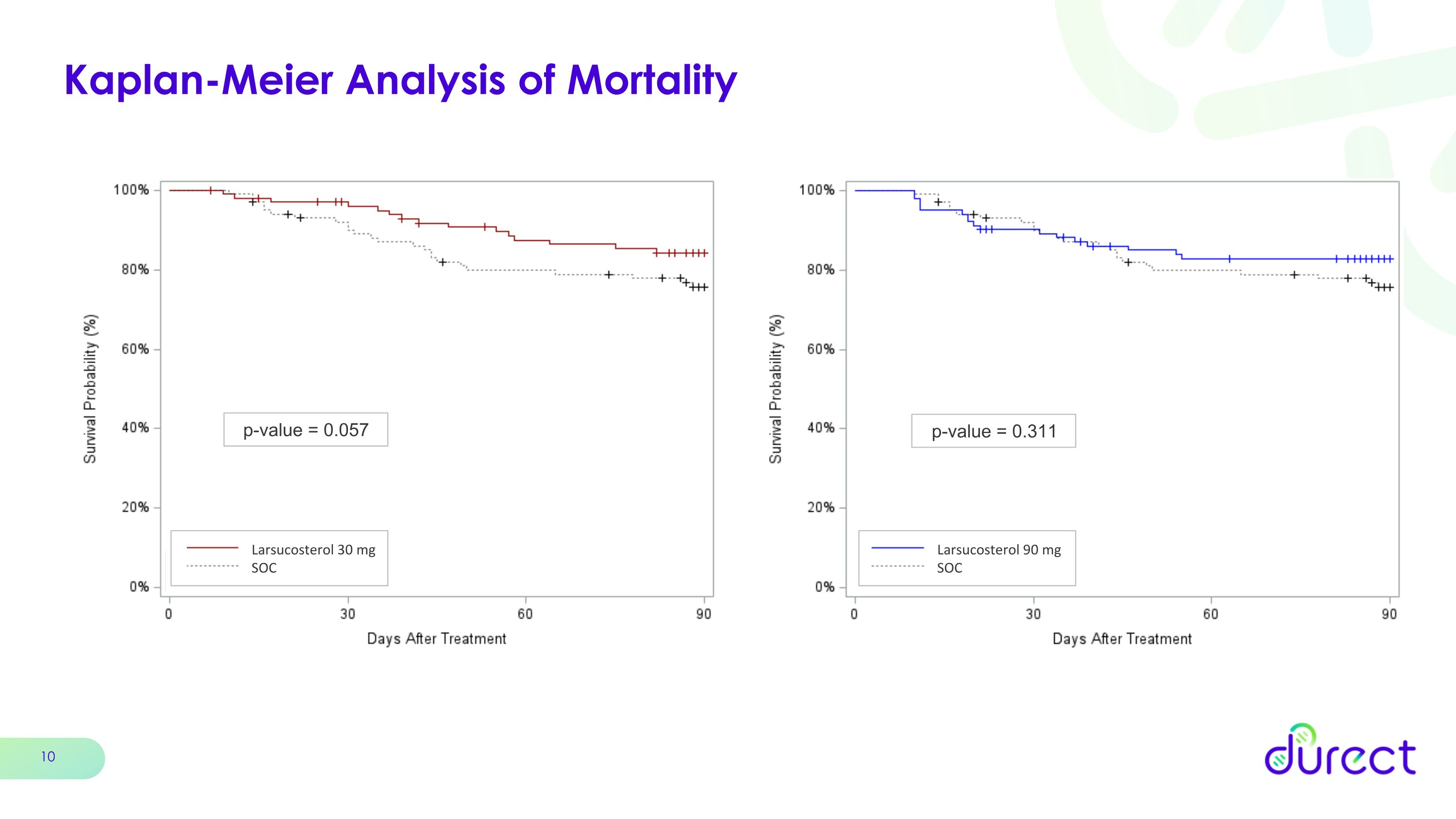
Kaplan-Meier Analysis of Mortality p-value = 0.057 Larsucosterol 30 mg SOC p-value = 0.311 Larsucosterol 90 mg SOC
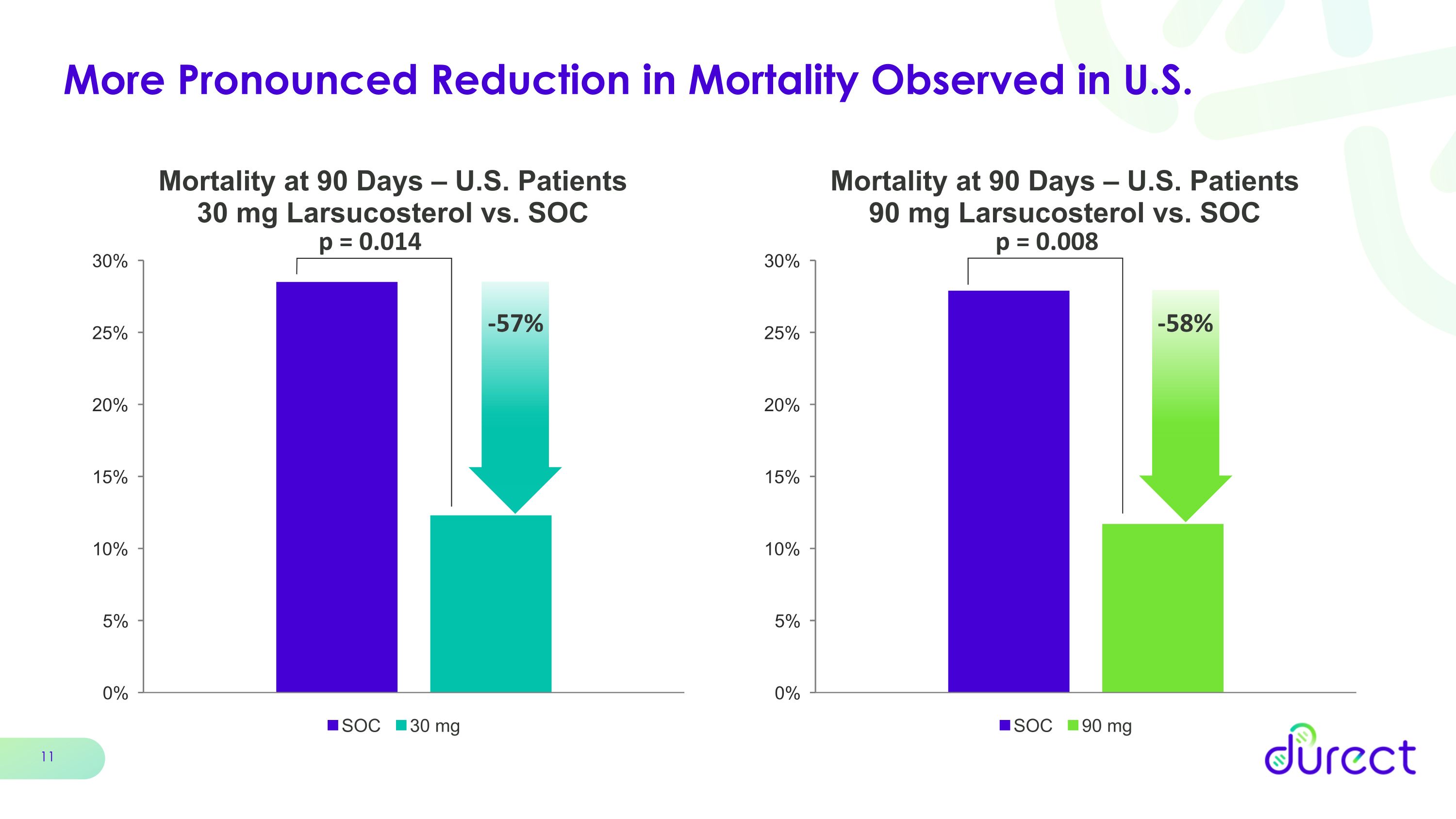
More Pronounced Reduction in Mortality Observed in U.S. p = 0.008 p = 0.014 -57% -58%
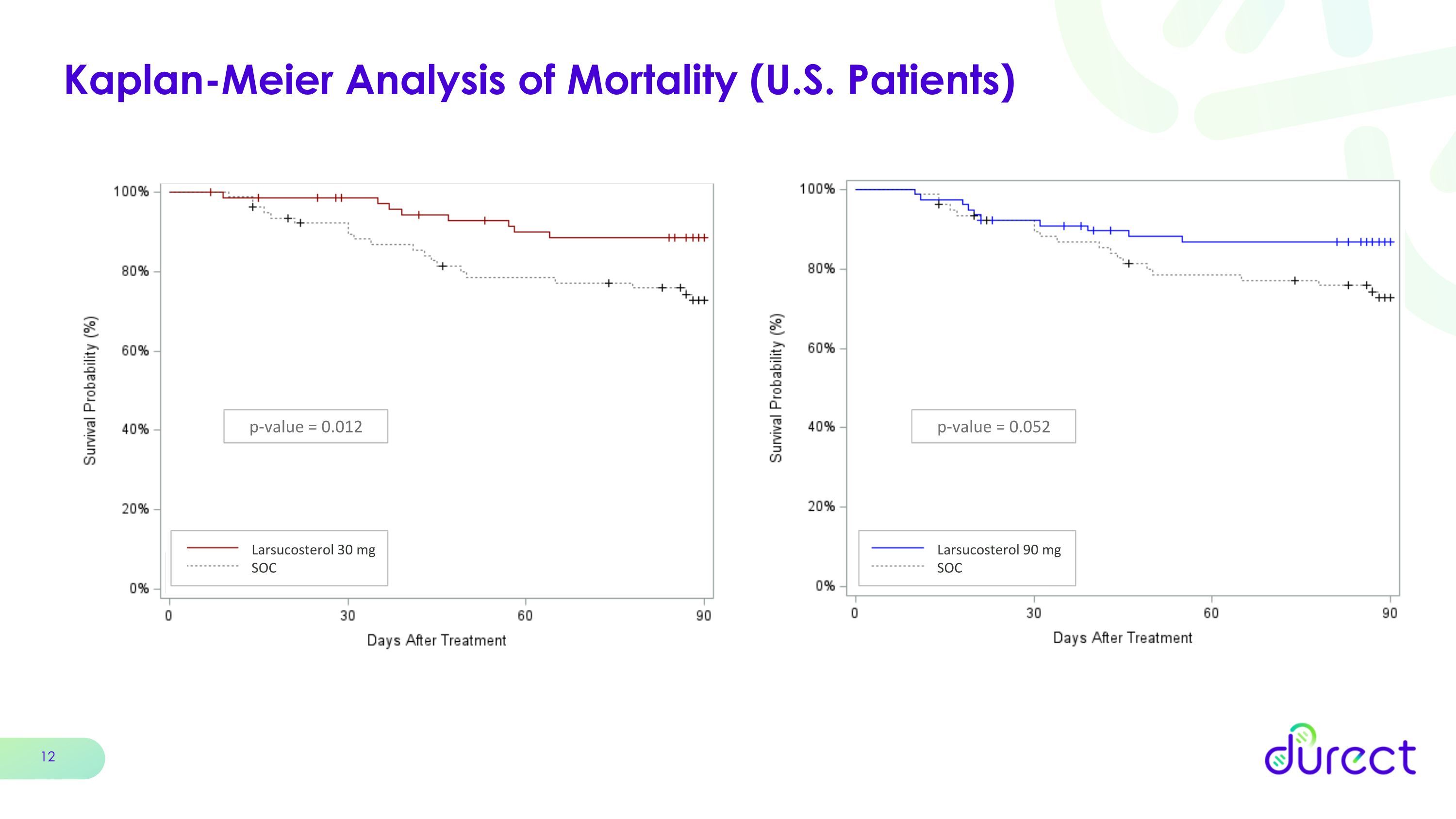
Kaplan-Meier Analysis of Mortality (U.S. Patients) p-value = 0.012 Larsucosterol 30 mg SOC p-value = 0.052 Larsucosterol 90 mg SOC
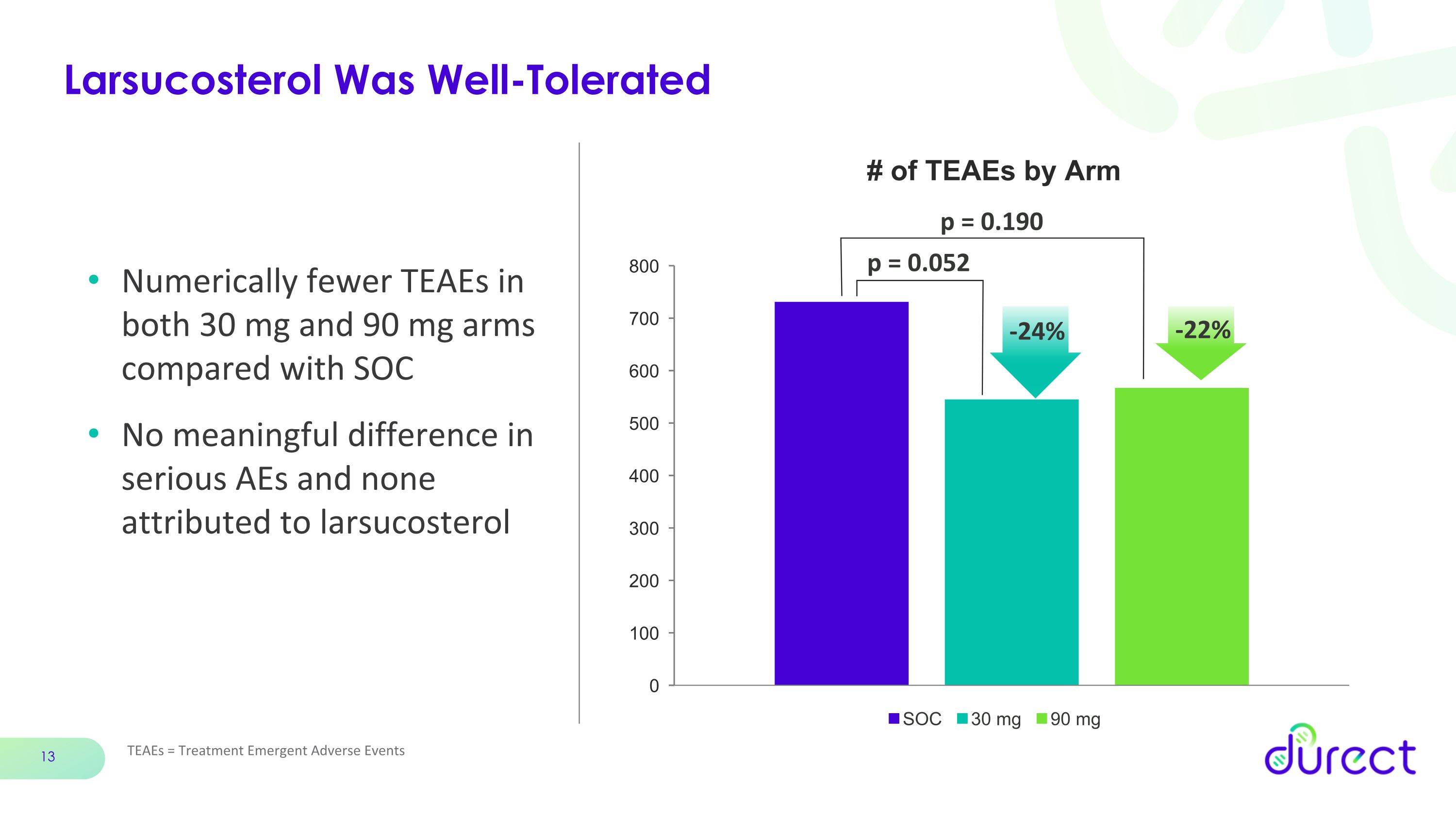
Larsucosterol Was Well-Tolerated TEAEs = Treatment Emergent Adverse Events Numerically fewer TEAEs in both 30 mg and 90 mg arms compared with SOC No meaningful difference in serious AEs and none attributed to larsucosterol p = 0.052 p = 0.190 -22% -24%
Conclusions and Next Steps for Larsucosterol in AH Compelling efficacy signal in favor of larsucosterol in key secondary endpoint of reduced mortality at 90 days; 41% for the 30 mg dose and 35% for the 90 mg dose compared with SOC In U.S. patients, larsucosterol treatment reduced mortality at 90 days by 57% for the 30 mg dose (p=0.014) and by 58% for the 90 mg dose (p=0.008) compared with SOC Larsucosterol was well-tolerated; both dose groups had numerically fewer adverse events than standard of care NEXT STEPS Discuss AHFIRM data with FDA in first quarter of 2024 Strong rationale for advancing larsucosterol to a registrational Phase 3 trial with 90-day mortality as the primary endpoint AHFIRM data to be presented at upcoming scientific meeting

Q&A
v3.23.3
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Durect (NASDAQ:DRRX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Durect (NASDAQ:DRRX)
Historical Stock Chart
From Apr 2023 to Apr 2024