Blue Water Vaccines to Present Update on Novel, Live Attenuated, Intranasally Delivered Streptococcus pneumoniae Vaccine to Prevent Acute Otitis Media, Pneumococcal Pneumonia, and Invasive Pneumococcal Disease at the World Vaccine & Immunotherapy Congress
December 01 2022 - 8:30AM

Blue Water Vaccines Inc. (“BWV” or “Blue Water Vaccines” or the
“Company”), today announced that Ali Fattom, Ph.D., will present at
the World Vaccine & Immunotherapy Congress West Coast 2022 in
San Diego, California on Thursday, December 1, 2022. Dr. Fattom
will present an overview and current progress of BWV-201, a live
attenuated, intranasally delivered, serotype independent
Streptococcus pneumoniae vaccine candidate for the prevention of
acute otitis media (“AOM”), pneumococcal pneumonia, and invasive
pneumococcal disease.
Session details are as follows:
|
Date: |
Thursday, December 1, 2022 |
| Time: |
1:55 p.m. Eastern Daylight Time
(EDT) |
| Title: |
A Combinatorial Live Attenuated
Vaccine Strategy Against Pneumonia and Otitis Media |
| BWV
Participant: |
Ali Fattom, Ph.D. |
“This program has made significant clinical development progress
this year, and we are very excited to showcase our technology at
such a prestigious event,” said Joseph Hernandez, Chairman and
Chief Executive Officer of Blue Water Vaccines. “Although our
primary targets of this vaccine remain AOM and pneumococcal
pneumonia, data from the original publication for this vaccine
suggests that BWV-201 may also protect against invasive
pneumococcal disease. We look forward to sharing our strategy with
all colleagues in attendance and further advancing this program
into clinical trials.”
About Blue Water Vaccines
Blue Water Vaccines Inc. is a biopharmaceutical company focused
on developing transformational vaccines to address significant
health challenges globally. Headquartered in Cincinnati, OH, the
company holds the rights to proprietary technology developed at the
University of Oxford, Cincinnati Children's Hospital Medical
Center, St. Jude Children's Hospital, and The University of Texas
Health San Antonio. The Company is developing a universal flu
vaccine that will provide protection from all virulent strains in
addition to licensing a novel norovirus (NoV) S&P nanoparticle
versatile virus-like particle (VLP) vaccine platform from
Cincinnati Children’s to develop vaccines for multiple infectious
diseases, including norovirus/rotavirus and malaria, among others.
Additionally, Blue Water Vaccines is developing a Streptococcus
pneumoniae (pneumococcus) vaccine candidate, designed to
specifically prevent the highly infectious middle ear infections,
known as Acute Otitis Media (AOM), in children. The Company is also
developing a Chlamydia vaccine candidate with UT Health San Antonio
to prevent infection and reduce the need for antibiotic treatment
associated with contracting Chlamydia disease. For more
information, visit www.bluewatervaccines.com.
Forward-Looking Statements
Certain statements in this press release are forward-looking
within the meaning of the Private Securities Litigation Reform Act
of 1995. These statements may be identified by the use of
forward-looking words such as “anticipate,” “believe,” “forecast,”
“estimate,” “expect,” and “intend,” among others. These
forward-looking statements are based on BWV’s current expectations
and actual results could differ materially. There are a number of
factors that could cause actual events to differ materially from
those indicated by such forward-looking statements. These factors
include, but are not limited to, risks related to the development
of BWV’s vaccine candidates; the failure to obtain FDA clearances
or approvals and noncompliance with FDA regulations; delays and
uncertainties caused by the global COVID-19 pandemic; risks related
to the timing and progress of clinical development of our product
candidates; our need for additional financing; uncertainties of
patent protection and litigation; uncertainties of government or
third party payor reimbursement; limited research and development
efforts and dependence upon third parties; and substantial
competition. As with any vaccine under development, there are
significant risks in the development, regulatory approval and
commercialization of new products. BWV does not undertake an
obligation to update or revise any forward-looking statement.
Investors should read the risk factors set forth in BWV’s
Registration Statement on Form S-1, filed with the Securities and
Exchange Commission (the “SEC”) on August 29, 2022 and periodic
reports filed with the SEC on or after the date thereof. All of
BWV’s forward-looking statements are expressly qualified by all
such risk factors and other cautionary statements. The information
set forth herein speaks only as of the date thereof.
Media Contact Information:Blue Water Media
RelationsTelephone: (646) 942-5591 Email:
Nic.Johnson@russopartnersllc.com
Investor Contact Information:Blue Water Investor RelationsEmail:
investors@bluewatervaccines.com
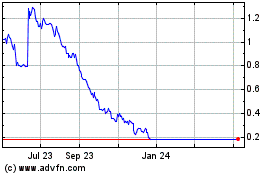
Blue Water Biotech (NASDAQ:BWV)
Historical Stock Chart
From Apr 2024 to May 2024
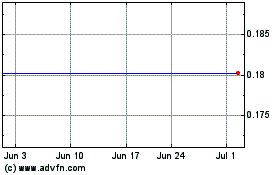
Blue Water Biotech (NASDAQ:BWV)
Historical Stock Chart
From May 2023 to May 2024