Filed Pursuant to Rule 424(b)(5)
Registration No. 333-255743
PROSPECTUS SUPPLEMENT
(To Prospectus dated May 11, 2021)
Timber Pharmaceuticals, Inc.
21,325,000 Shares of Common Stock
Pre-funded Warrants to Purchase
up to 2,112,500 Shares of Common Stock
Warrants to Purchase up
to 23,437,500 Shares of Common Stock
We are offering up to
21,325,000 shares of our common stock and warrants to purchase one shares of common stock. Each share of our common stock is
being sold with one warrant to purchase one share of our common stock, at the combined offering price of $0.64 per share of common
stock and accompanying warrant to purchase one share of common stock, representing an offering price of $0.63 per share of common stock and $0.01 per accompanying
warrant. The warrants are exercisable from and after the date of their issuance and expire on the fifth anniversary of such date, at
an exercise price of $0.70 per share of common stock. The shares of common stock and warrants will be issued separately.
We are also offering 2,112,500
pre-funded warrants, or the Pre-funded Warrants (and the shares of common stock issuable from time to time upon exercise of the Pre-funded
Warrants), to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with
its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding
shares of common stock following the consummation of this offering in lieu of the shares of common stock that would result in such excess
ownership. Each Pre-funded Warrant will be exercisable for one share of common stock at an exercise price of $0.001 per shares of common
stock. The public offering price is $0.639 per Pre-funded Warrant and accompanying warrant, which is equal to the public offering price
per share and accompanying warrant less $0.001. Each Pre-funded Warrant will be exercisable upon issuance and will expire when exercised
in full. There is no established public trading market for the Pre-funded Warrants, and we do not expect a market to develop. We do not
intend to apply for listing of the Pre-funded Warrants on any securities exchange or nationally recognized trading system. Without an
active trading market, the liquidity of the Pre-funded Warrants will be extremely limited.
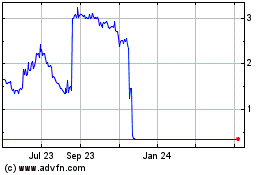
Our common stock is listed
on The NYSE American, LLC (the “NYSE American”) under the symbol “TMBR”. On November 2, 2021, the last reported
sales price of our common stock on the NYSE American was $0.80 per share. Our common stock has recently experienced extreme volatility
in price and trading volume. For example, on March 4, 2021 and March 11, 2021, the closing price of our common stock on the NYSE American
was $1.66 and $3.55, respectively, and daily trading volume on these days was approximately 3.5 million and 143.7 million shares, respectively.
During this period, there were no recent changes in our financial condition or results of operations that were consistent with the change
in our stock price. Investors that purchase shares of our common stock may lose a significant portion of their investments if the price
of our common stock declines. Please see the section of this prospectus supplement titled “Risk Factors.” You are urged to
obtain current market quotations of our common stock. We have no preferred stock, warrants, subscription rights or units listed on any
market.
The offering is being underwritten
on a firm commitment basis. The underwriters may offer the shares of common stock and Pre-funded Warrants from time to time to purchasers
directly or through agents, or through brokers in brokerage transactions on the NYSE American, or to dealers in negotiated transactions
or in a combination of such methods of sale, or otherwise, at fixed price or prices, which may be changed, or at market prices prevailing
at the time of sale, at prices related to such prevailing market prices.
Investing in our common
stock involves risks. Before buying any shares, you should read the discussion of material risks of investing in our common stock in “Risk
Factors” beginning on page S-8 of this prospectus supplement, on page 4 of the accompanying prospectus and in the documents incorporated
by reference in this prospectus supplement.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
supplement is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share and
Warrant
|
|
|
Per Pre-Funded
Warrant and
Warrant
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
0.64
|
|
|
$
|
0.639
|
|
|
$
|
14,997,887.50
|
|
|
Underwriting discounts and commissions(1)
|
|
$
|
0.0384
|
|
|
$
|
0.0384
|
|
|
$
|
900,000.00
|
|
|
Proceeds, before expenses and fees, to us
|
|
$
|
0.6016
|
|
|
$
|
0.6006
|
|
|
$
|
14,097.887.50
|
|
|
|
(1)
|
In addition, we have agreed to pay a management fee to the underwriters equal to 1.0% of the aggregate
gross proceeds in the offering and to reimburse certain expenses of the underwriters in connection with this offering. See “Underwriting”
for additional disclosure regarding underwriting compensation.
|
We have granted the underwriters an option for
a period of up to 30 days from the date of this prospectus supplement to purchase up to 3,515,625 additional shares of our common stock
at the public offering price per share, and/or warrants to purchase up to 3,515,625 shares of our common stock at the public offering
price per warrant, less the underwriting discounts and commissions. If the underwriters exercise the option in full, the total underwriting
discounts and commissions payable by us will be $1,035,000 and the total proceeds to us, before expenses, will be $16,212,887.50.
Delivery of the shares of our common stock, Pre-funded
Warrants and warrants being offered pursuant to this prospectus supplement and the accompanying prospectus is expected to be made on or
about November 5, 2021, subject to satisfaction of customary closing conditions.
H.C. Wainwright & Co.
The date of this prospectus supplement is November
2, 2021.
TABLE OF CONTENTS
ABOUT
THIS PROSPECTUS SUPPLEMENT
On
May 4, 2021, we filed with the Securities and Exchange Commission, or SEC, a registration statement on Form S-3 (File No. 333-255743)
utilizing a “shelf” registration process relating to the securities described in this prospectus supplement, which registration
statement was declared effective on May 11, 2021. Under this shelf registration process, we may offer and sell, either individually or
in combination, in one or more offerings, any of the securities described in the accompanying prospectus, for total gross proceeds of
up to $100,000,000.
This prospectus supplement
describes the specific terms of this offering and also adds to and updates information contained in the accompanying prospectus and the
documents incorporated by reference herein and into the accompanying prospectus. The second part, the accompanying prospectus, provides
more general information. If the information in this prospectus supplement or any relevant free writing prospectus we may authorize for
use in connection with this offering is inconsistent with the accompanying prospectus or any document incorporated by reference therein
filed prior to the date of this prospectus supplement or such free writing prospectus, you should rely on the information in this prospectus
supplement or such free writing prospectus.
We and the underwriters have
not authorized anyone to provide you with any information or to make any representations other than those included or incorporated by
reference in this prospectus supplement and the accompanying prospectus and any relevant free writing prospectus we may authorize for
use in connection with this offering. If you receive any information not authorized by us, we and the underwriters take no responsibility
for, and can provide no assurance as to the reliability of, such information. We are not making an offer to sell the securities offered
hereby in any jurisdiction where the offer or sale is not permitted. You should not assume that the information contained or incorporated
by reference in this prospectus supplement or the accompanying prospectus or any relevant free writing prospectus we may authorize for
use in connection with this offering is accurate as of any date other than its respective date, regardless of its time of delivery or
any sale of the securities covered hereby. Our business, financial condition, results of operations and prospects may have changed since
that date.
It is important for you to
read and consider all of the information contained or incorporated by reference in this prospectus supplement, the accompanying prospectus
and any relevant free writing prospectus we may authorize for use in connection with this offering in making your investment decision.
This prospectus supplement contains summaries of certain provisions contained in some of the documents described herein, but reference
is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents.
We include cross-references in this prospectus supplement and the accompanying prospectus to captions in these materials where you can
find additional related discussions. The table of contents in this prospectus supplement provides the pages on which these captions are
located. You should read both this prospectus supplement and the accompanying prospectus, together with the additional information described
in the sections entitled “Where You Can Find Additional Information” on page S-18 and “Incorporation of Documents by
Reference” on page S-19 of this prospectus supplement, before investing in our common stock.
We are offering to sell, and
seeking offers to buy, the securities offered hereby only in jurisdictions where offers and sales are permitted. The distribution of this
prospectus supplement, the accompanying prospectus and any relevant free writing prospectus we may authorize for use in connection with
this offering and the offering of the securities offered hereby in certain jurisdictions may be restricted by law. Persons outside the
United States who come into possession of this prospectus supplement, the accompanying prospectus and any relevant free writing prospectus
we may authorize for use in connection with this offering must inform themselves about, and observe any restrictions relating to, the
offering of the securities offered hereby and the distribution of this prospectus supplement, the accompanying prospectus and any relevant
free writing prospectus we may authorize for use in connection with this offering outside the United States. This prospectus supplement,
the accompanying prospectus and any relevant free writing prospectus we may authorize for use in connection with this offering do not
constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by
this prospectus supplement, the accompanying prospectus and any relevant free writing prospectus we may authorize for use in connection
with this offering by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
Timber Pharmaceuticals, Inc.
and its consolidated subsidiaries are referred to herein as “Timber,” “the Company,” “we,” “us”
and “our,” unless the context indicates otherwise.
This prospectus supplement
and the accompanying prospectus contain, or incorporate by reference, trademarks, tradenames, service marks and service names of Timber
Pharmaceuticals, Inc. and its subsidiaries.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement,
including the documents that we incorporate by reference, contains forward-looking statements as that term is defined in the federal securities
laws. The events described in forward-looking statements contained in this prospectus supplement, including the documents that we incorporate
by reference, may not occur. Generally, these statements relate to our business plans or strategies, projected or anticipated benefits
or other consequences of our plans or strategies, financing plans, projected or anticipated benefits from acquisitions that we may make,
or projections involving anticipated revenues, earnings or other aspects of our operating results or financial position, and the outcome
of any contingencies. Any such forward-looking statements are based on current expectations, estimates and projections of management.
We intend for these forward-looking statements to be covered by the safe-harbor provisions for forward-looking statements. Words such
as “may,” “expect,” “believe,” “anticipate,” “project,” “plan,”
“intend,” “estimate,” and “continue,” and their opposites and similar expressions are intended to
identify forward-looking statements. We caution you that these statements are not guarantees of future performance or events and are subject
to a number of uncertainties, risks and other influences, many of which are beyond our control that may influence the accuracy of the
statements and the projections upon which the statements are based. Factors that may affect our results include, but are not limited to,
the risks and uncertainties discussed in the “Risk Factors” section on page S-8 of this prospectus supplement and on
page 4 of the accompanying prospectus, in our Annual Report on Form 10-K for the fiscal year ended December 31, 2020 or in other
reports we file with the Securities and Exchange Commission.
Any one or more of these uncertainties,
risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately
prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these
forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information,
future events or otherwise.
You should rely only on the
information in this prospectus supplement and accompanying base prospectus. We have not authorized any other person to provide you with
different information. If anyone provides you with different or inconsistent information, you should not rely upon it.
PROSPECTUS
SUPPLEMENT SUMMARY
The following summary highlights
some information from this prospectus supplement. It is not complete and does not contain all of the information that you should consider
before making an investment decision. You should read this entire prospectus, including the “Risk Factors” section on page S-8
and the accompanying prospectus on page 4, the consolidated financial statements and related notes and the other more detailed information
appearing elsewhere or incorporated by reference into this prospectus supplement.
The Company
We are a clinical-stage biopharmaceutical
company focused on the development and commercialization of treatments for orphan dermatologic diseases. Our investigational therapies
have proven mechanisms-of-action backed by decades of clinical experience and well-established CMC (chemistry, manufacturing and control)
and safety profiles. We are initially focused on developing non-systemic treatments for rare dermatologic diseases including congenital
ichthyosis, facial angiofibromas (“FAs”) in tuberous sclerosis complex (“TSC”), and other sclerotic skin diseases.
Our lead programs are TMB-001, TMB-002 and TMB-003.
TMB-001
TMB-001, a patented topical
formulation of isotretinoin was recently evaluated in a Phase 2b clinical trial for the treatment of moderate to severe subtypes
of congenital ichthyosis (“CI”), a group of rare genetic keratinization disorders that lead to dry, thickened, and scaling
skin. A prior Phase 1/2 study involving 19 patients with CI demonstrated safety and a signal of preliminary efficacy of TMB-001, as well
as minimal systemic absorption. In 2018, the FDA awarded us the first tranche of a $1.5 million grant in the amount of $500,000 to support
clinical trials evaluating TMB-001 through its Orphan Products Grant program. In March 2020 and March 2021, the FDA awarded
us the second and third tranches of the grant, respectively, each in the amount of $500,000.
On
July 1, 2020, we announced that all 11 sites across the United States and Australia in the Phase 2b CONTROL study evaluating TMB-001
in patients with moderate to severe CI were enrolling patients. As of December 31, 2020, all sites participating in a Phase 2b clinical
trial evaluating TMB-001 were opened and enrolling patients. On May 31, 2021, we completed patient enrollment in the Phase 2b clinical
trial with 34 patients randomized. On October 7, 2021, we announced top line data from the TMB-001 Phase 2b trial. The data demonstrated
a reduction in targeted and overall severity of CI in patients treated with topical IPEG™ TMB-001 (topical isotretinoin).
|
|
·
|
In
the per protocol population (the “PP population”), 100 percent (nominal p = 0.04 ) and 40 percent (nominal p=
ns ) of patients treated with TMB-001 0.05% and 0.1%, respectively, achieved VIIS-50 compared to 40 percent in the vehicle group.
|
|
|
·
|
In
the ITT population, 64 percent (nominal p =0.17 ) and 40 percent (nominal p =ns ) of patients treated with TMB-001
0.05% and 0.1%, respectively, achieved VIIS-50 compared to 33 percent in the vehicle group.
|
|
|
·
|
In
the PP population, 100 percent (nominal p =0.002 ) and 60 percent (nominal p =ns ) of patients treated with TMB-001
0.05% and 0.1%, respectively, achieved a ≥2 point improvement in the IGA at week 12 compared to 10 percent in the vehicle group.
|
|
|
·
|
In
the ITT population, 55 percent (nominal p =0.02 ) and 40 percent (nominal p =ns ) of patients treated with TMB-001
0.05% and 0.1%, respectively, achieved a ≥2 point improvement in the IGA at week 12 compared to 8 percent in the vehicle group.
|
|
|
·
|
TMB-001
was generally well tolerated with a similar incidence of adverse events (AEs) across treatment groups. The most frequent AEs were local
adverse effects common for such topical treatments. There were no treatment-related serious adverse events (SAE).
|
On
December 15, 2020, we announced that we had received a notice of allowance from the U.S. Patent and Trademark Office (USPTO) for a Company
patent application covering TMB-001 (U.S. Patent Application No.: 15/772,456) and the application subsequently issued on February 10,
2021 as US 10,933,018.
On
April 28, 2021, we announced that the Japanese Patent Office had decided to grant a patent (No. 2018542677) for TMB-001.
On
May 18, 2021, we received notice that the Australian Patent Office had decided to grant a patent (No. 2016346203) for TMB-001.
TMB-002
TMB-002, a proprietary topical
formulation of rapamycin, is currently being evaluated in a Phase 2b clinical trial for the treatment of FAs in TSC, a multisystem genetic
disorder resulting in the growth of hamartomas in multiple organs. TSC results from dysregulation in the mTOR pathway, and as a topical
mTOR inhibitor, TMB-002 may address FAs in TSC without the systemic absorption of an oral agent.
As of April 30, 2021, all
sites participating in a Phase 2b clinical trial evaluating TMB-002 were opened and are currently enrolling patients. Site activation
and patient enrollment and data collection have been impacted by the COVID-19 pandemic in the larger and longer TMB-002 study, especially
at our contracted test sites in Eastern Europe. Currently, we can confirm that recruitment has been finalized on the TMB-002 Phase 2b
trial with a total of 114 consented (108 randomized) patients. We expect to review top line results from this trial in the third quarter
of 2022.
On March 17, 2021, we announced
that AFT Pharmaceuticals Limited (“AFT”), one of our development partners, entered into a license and supply agreement with
Desitin Arzneimittel GmbH (“Desitin”) for Pascomer® (TMB-002 topical rapamycin) for the treatment of FAs associated with
TSC in Europe. Pursuant to the AFT Licensing and Development Agreement, we are entitled to receive a significant percentage of the economics
(royalties and milestones) in any licensing transaction that AFT executes outside of North America, Australia, New Zealand, and Southeast
Asia. The current transaction with Desitin is included in the scope of this provision and as such in the third quarter of 2021 the Company
has received €250,000 related to an upfront milestone payment paid to AFT by Desitin during the quarter ended September 30, 2021
and has recorded approximately $0.3 million in our financial statements.
TMB-003
The product in its earliest
stage in our pipeline is TMB-003, a proprietary formulation of Sitaxsentan, a new chemical entity in the U.S., which is a selective endothelin-A
receptor antagonist. It is currently in preclinical development as a locally applied formulation for the treatment of fibrotic and sclerotic
skin disorders, such as scleroderma, a rare connective tissue disorder characterized by abnormal thickening of the skin.
On January 12, 2021, we announced
that the FDA has granted orphan drug designation for TMB-003, our locally delivered formulation of Sitaxsentan, for the treatment of systemic
sclerosis. We are planning to pursue additional orphan drug designations in other indications.
BPX-01 and BPX-04
Pursuant
to the Merger Agreement (as defined below), BPX-01 (Topical Minocycline, 2%) and BPX-04 (Topical Minocycline, 1%) were added to our portfolio.
BPX-01 and BPX-04 are assets currently in development for acne vulgaris and papulopustular rosacea, respectively. On July 22, 2020, we
announced that we had received notice from the European Patent Office that it intends to grant a patent for the Company’s topical
composition of pharmaceutical tetracycline (including minocycline) for dermatological use (European Patent Application No. 16714168.8)
and the application subsequently issued on December 16, 2020 as EP 3273940. Patents covering the BPX-01 and BPX-04 assets have previously
been granted in the United States, Japan, South Africa, and Australia. We are currently evaluating our strategic options regarding these
assets.
On
September 15, 2020, we announced that we had received a notice of allowance from the USPTO for a Company patent application covering BPX-01
and BPX-04 (U.S. Patent Application No.: 16/514,459) and the application subsequently issued on January 5, 2021 as US 10,881,672.
The Merger, Reverse Stock Split and Name Change
On
May 18, 2020, we completed our business combination with Timber Sub, in accordance with the terms of the Agreement and Plan of Merger
and Reorganization, dated as of January 28, 2020, as amended, by and among Timber, Timber Sub and BITI Merger Sub Inc., a wholly-owned
subsidiary of Timber (“Merger Sub”) (the “Merger Agreement”), pursuant to which Merger Sub merged with and into
Timber Sub, with Timber Sub surviving as a wholly owned subsidiary of Timber (the “Merger”).
In
connection with, and immediately prior to the completion of, the Merger, we effected a reverse stock split of our common stock, at a ratio
of 1-for-12 (the “Reverse Stock Split”). Under the terms of the Merger Agreement, after taking into account the Reverse Stock
Split, we issued shares of common stock to Timber Sub’s unitholders at an exchange rate of 629.57 shares of common stock for each
Timber Sub common unit outstanding immediately prior to the Merger. In connection with the Merger, we changed our name from “BioPharmX
Corporation” to “Timber Pharmaceuticals, Inc.,” and the business conducted by the Company became the business conducted
by Timber Sub.
Private Placement of Common Stock and Warrants
On
May 18, 2020, Timber and Timber Sub completed a private placement transaction (the “Pre-Merger Financing”) with the Investors
pursuant to the Securities Purchase Agreement for an aggregate purchase price of approximately $25.0 million (comprised of (i) approximately
$5 million credit with respect to the senior secured notes issued in connection with the bridge loan that certain of the Investors made
to Timber Sub at the time of the execution of the Merger Agreement and (ii) approximately $20 million in cash from the Investors).
Pursuant
to the Pre-Merger Financing, (i) Timber Sub issued and sold to the Investors common units of Timber Sub which converted pursuant
to the exchange ratio in the Merger into an aggregate of approximately 4,137,509 shares (the “Converted Shares”) of common
stock; and (ii) the Company agreed to issue to each Investor, on the tenth trading day following the consummation of the Merger,
(A) Series A Warrants representing the right to acquire shares of common stock equal to 75% of the sum of (a) the number
of Converted Shares issued to the Investor, without giving effect to any limitation on delivery contained in the Securities Purchase Agreement,
and (b) the number of shares of common stock underlying the Series B Warrants issued to the Investor (the “Series B
Warrants”) and (B) the Series B Warrants. On June 2, 2020, pursuant to the terms of the Securities Purchase Agreement,
the Company issued the Series A Warrants and the Series B Warrants.
In
addition, pursuant to the terms of the Securities Purchase Agreement, dated as of January 28, 2020 between Timber Sub and several
of the Investors, the Company issued to such purchasers, on May 22, 2020, warrants to purchase 413,751 shares of our common stock
(the “Bridge Warrants”) which have an exercise price of $2.2362 per share, subject to full ratchet anti-dilution and adjustment
if shares are sold for a price less than the exercise price of the warrants.
Pursuant
to the Waiver Agreements we entered into with the Selling Stockholders on November 19, 2020, (A) the exercise price of the Series A
Warrants was definitively set at $1.16 per share, (B) the number of shares underlying all of the Series A Warrants was definitively
set at 20,178,214 and (C) the number of shares underlying all of the Series B Warrants was definitively set at 22,766,776. As
of March 4, 2021, all Series B Warrants had been exercised in full.
Recent Developments
Preliminary Operating
Results for the Three and Nine Months Ended September 30, 2021 and Estimated Financial Condition Information as of September 30, 2021
Preliminary unaudited
operating results for the quarter ended September 30, 2021 and certain preliminary financial condition information as of September 30,
2021 are as follows:
|
|
·
|
Net
operating loss for the three and nine months ended September 30, 2021 is expected to be approximately $3.0 million and $7.8
million, respectively.
|
|
|
·
|
The
Company ended the third quarter with approximately $3.4 million in cash and common shares outstanding of 36,659,685 at September
30, 2021.
|
The
above information is preliminary financial information for the third quarter of 2021 and subject to completion. The unaudited, estimated
results for the third quarter of 2021 are preliminary and were prepared by our management, based upon our estimates, a number of assumptions
and currently available information, and are subject to revision based upon, among other things, quarter-end closing procedures and/or
adjustments, the completion of our interim consolidated financial statements and other operational procedures. This preliminary financial
information is the responsibility of management and has been prepared in good faith on a consistent basis with prior periods. However,
we have not completed our financial closing procedures for the quarter ended September 30, 2021, and our actual results could be materially
different from this preliminary financial information, which preliminary information should not be regarded as a representation by us,
our management, or the underwriters as to our actual results for quarter ended September 30, 2021. In addition, KPMG LLP, our independent
registered public accounting firm, has not audited, reviewed, compiled, or performed any procedures with respect to this preliminary financial
information and does not express an opinion or any other form of assurance with respect to this preliminary financial information. During
the course of the preparation of our financial statements and related notes as of and for the quarter ended September 30, 2021, we may
identify items that would require us to make material adjustments to this preliminary financial information. As a result, prospective
investors should exercise caution in relying on this information and should not draw any inferences from this information. This preliminary
financial information should not be viewed as a substitute for full financial statements prepared in accordance with United States generally
accepted accounting principles and reviewed by our auditors. See “Risk Factors” and “Cautionary Note Regarding Forward-Looking
Statements.”
Our
financial statements and related notes as of and for the quarter ended September 30, 2021 may not be filed with the SEC until after this
offering is completed. We currently expect to file our Quarterly Report on Form 10-Q including our financial statements for the quarter
ended September 30, 2021 on or about November 15, 2021.
Corporate Information
Our principal offices are
located at 110 Allen Road, Suite 401, Basking Ridge, NJ, and our telephone number is (908) 636-7160. Our website address is www.timberpharma.com.
Our website and the information contained on, or that can be accessed through, our website shall not be deemed to be incorporated by reference
in, and are not considered part of, this prospectus supplement. You should not rely on any such information in making your decision whether
to purchase our common stock.
THE
OFFERING
|
Securities offered by us
|
|
21,325,000 shares of common stock, warrants to purchase an aggregate of 23,437,500 shares of common stock and Pre-funded Warrants to purchase an aggregate of 2,112,500 shares of common stock.
|
|
|
|
|
|
Warrants offered
|
|
Warrants to purchase up to
23,437,500 shares of common stock, which will be exercisable during the period commencing on the date of their issuance and ending
five years from such date at an exercise price of $0.70 per share of common stock. This prospectus also relates to the offering of
the shares of common stock issuable upon exercise of the warrants. There is no established public trading market for the warrants,
and we do not expect a market to develop. In addition, we do not intend to apply for listing of the warrants on any national
securities exchange or other nationally recognized trading system
|
|
|
|
|
|
Description of Pre-funded Warrants
|
|
If the issuance of shares of common stock to a purchaser in this offering would result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock following the consummation of this offering, then such purchaser may purchase, if they so choose, in lieu of the shares of common stock that would result in such excess ownership, a Pre-funded Warrant to purchase shares of common stock for a purchase price per share of common stock subject to such Pre-funded Warrant equal to the per share public offering price for the shares of common stock in this offering less $0.001. Each Pre-funded Warrant will have an exercise price of $0.001 per share of common stock, will be exercisable upon issuance and will expire when exercised in full. This prospectus supplement also relates to the offering of shares of common stock issuable upon exercise of these Pre-funded Warrants.
|
|
|
|
|
|
Option to purchase additional shares and/or warrants
|
|
We have granted the underwriter a 30-day option to purchase up to 3,515,625 additional shares of
common stock and/or warrants to purchase up to 3,515,625 shares of our common stock at the public offering price per share of common
stock or warrant, respectively, less underwriting discounts and commissions.
|
|
|
|
|
|
Common stock to be outstanding immediately after this offering
|
|
60,097,185 shares
of common stock (or 63,612,810 shares if the underwriters exercise in full their option to purchase additional shares), assuming
the exercise in full of Pre-funded Warrants issued in this offering.
|
|
|
|
|
Use of proceeds
|
|
We intend to use the net proceeds from this offering for general corporate purposes, including, but not limited to, ongoing research and pre-clinical studies, clinical trials, the development of new biological and pharmaceutical technologies, investing in or acquiring companies that are synergistic with or complementary to our technologies, and licensing activities related to our current and future product candidates, including a payment of $4.0 million due to Patagonia Pharmaceuticals LLC (“Patagonia”) on the initiation of a Phase 3 pivotal trial for TMB-001, and working capital. See “Use of Proceeds” on page S-10.
|
|
|
|
|
Risk factors
|
|
This investment involves a high degree of risk. You should read the description of risks set forth under “Risk Factors” beginning on page S-8 of this prospectus supplement or otherwise incorporated by reference in this prospectus supplement for a discussion of factors to consider before deciding to purchase our securities.
|
|
|
|
|
NYSE American symbol
|
|
Our common stock is listed on the NYSE American under the symbol “TMBR”. There is no established trading market for the Pre-funded Warrants, and we do not expect a trading market to develop. We do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the Pre-funded Warrants will be extremely limited.
|
The number of shares of our common stock to be
outstanding immediately after this offering is based on 36,659,685 shares outstanding as of November 1, 2021, and excludes, as
of that date, the following:
|
|
●
|
2,657,640 shares of common stock issuable upon the exercise of outstanding vested and unvested stock options under our 2020 Omnibus Equity Incentive Plan at a weighted-average exercise price of $1.10 per share, 15,781 shares of common stock underlying legacy BioPharmX stock options at a weighted-average exercise price of $75.27 per share, and 367,670 shares of common stock issuable upon the exercise of outstanding value appreciation rights (“VARs”);
|
|
|
|
|
|
|
●
|
17,329,621 shares of common stock issuable upon the exercise of outstanding warrants, having a weighted-average exercise price of $2.25 per share, including (i) 16,701,824 Series A Warrants with an exercise price of $1.16, (ii) 213,992 BioPharmX legacy warrants with a weighted-average exercise price of $87.21, and (iii) 413,751 Bridge Warrants with an exercise price of $2.2362 that are subject to potential anti-dilution adjustment as a result of this offering;
|
|
|
|
|
|
|
●
|
100,753 shares of common stock issuable upon the conversion of outstanding Series A Preferred Stock;
|
|
|
|
|
|
|
●
|
183,360 shares of treasury stock; and
|
|
|
|
|
|
|
●
|
the shares of common stock
issuable upon exercise of the warrants issued in this offering.
|
RISK
FACTORS
Before deciding whether to
invest in our common stock, you should carefully consider the risk factors set forth below and incorporated by reference in this prospectus
supplement from our Annual Report on Form 10-K for the fiscal year ended December 31, 2020 and any subsequent updates described
in our Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as the risks, uncertainties and additional information
set forth in our SEC reports on Forms 10-K, 10-Q and 8-K and in the other documents incorporated by reference in this prospectus supplement.
For a description of these reports and documents, and information about where you can find them, see “Where You Can Find Additional
Information” and “Incorporation of Certain Information By Reference”. Additional risks not presently known or that we
presently consider to be immaterial could subsequently materially and adversely affect our financial condition, results of operations,
business and prospects.
Risks Related to this Offering and our Common
Stock
We have broad discretion
in the use of the net proceeds from this offering and our existing cash and may not use them effectively.
Our management will have broad
discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled
“Use of Proceeds,” as well as our existing cash and cash equivalents, and you will be relying on the judgment of our management
regarding such application. You will not have the opportunity, as part of your investment decision, to assess whether the net proceeds
are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this
offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply the net proceeds
or our existing cash in ways that ultimately increase the value of your investment. If we do not invest or apply the net proceeds from
this offering or our existing cash and cash equivalents in ways that enhance stockholder value, we may fail to achieve expected business
and financial results, which could cause our stock price to decline. Pending their use, we may invest the net proceeds from this offering
in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders.
You will experience
immediate dilution in the book value per share of the securities you purchase in this offering.
Because
the price per share of our common stock being offered is substantially higher than the net tangible book value per share of
our common stock after giving effect to this offering, you will suffer substantial dilution in the as adjusted net tangible
book value of the common stock you purchase in this offering. If you purchase shares of common stock in this offering, you will suffer
immediate and substantial dilution of $0.33 per share in the as adjusted net tangible book value of the common stock you
purchase. Any exercise of outstanding stock options, warrants or other equity awards will result in further dilution. See “Dilution”
for a more detailed discussion of the dilution you will incur if you purchase our securities in this offering.
The issuance or
sale of shares in the future to raise money or for strategic purposes could reduce the market price of our common stock.
In the future, we may issue
securities to raise cash for operations, to pay down then existing indebtedness, as consideration for the acquisition of assets, as consideration
for receipt of goods or services, and to pay for the development of our product candidates. We have and in the future may issue securities
convertible into our common stock. Any of these events may dilute stockholders’ ownership interests in our company and have an adverse
impact on the price of our common stock.
In addition, sales of a substantial
amount of our common stock in the public market, or the perception that these sales may occur, could reduce the market price of our common
stock. This could also impair our ability to raise additional capital through the sale of our securities.
Any actual or
anticipated sales of shares by our stockholders may cause the trading price of our common stock to decline. The sale of a
substantial number of shares of our common stock by our stockholders, or anticipation of such sales, could make it more difficult
for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect
sales.
Issuance of our
common stock upon exercise of convertible securities may depress the price of our common stock.
As of November 1, 2021, we
had 36,659,685 shares of common stock issued and outstanding, warrants to purchase 17,329,567 shares of common stock at a weighted-average
exercise price of $2.25, including (i) 16,701,824 Series A Warrants with an exercise price of $1.16, and (ii) 413,751 Bridge Warrants
with an exercise price of $2.2362 that are subject to potential anti-dilution as a result of this offering, (iii) 213,992 BioPharmX legacy
warrants with a weighted-average exercise price of $87.21, and (iv) outstanding stock options to purchase 2,657,640 shares of common stock
under our 2020 Omnibus Equity Incentive Plan at a weighted-average exercise price of $1.10 per share, 15,781 shares of common stock issuable
upon the exercise of outstanding and vested legacy BioPharmX stock options at a weighted-average exercise price of $75.27 and outstanding
VARs convertible into an aggregate of 367,670 shares of common stock. All warrants, and stock options are convertible, or exercisable
into, one share of common stock. The issuance of shares of our common stock upon the exercise of outstanding convertible securities could
result in substantial dilution to our stockholders, which may have a negative effect on the price of our common stock.
There is no public market for the Pre-funded Warrants
being offered by us in this offering.
There is no established public
trading market for the Pre-funded Warrants being offered in this offering, and we do not expect a market to develop. In addition,
we do not intend to apply to list the Pre-Funded warrants on any national securities exchange or other nationally recognized
trading system, including the NYSE American. Without an active market, the liquidity of the Pre-funded Warrants will be limited.
Holders of the Pre-funded Warrants
offered hereby will have no rights as common stockholders with respect to the common stock underlying the Pre-funded Warrants
until such holders exercise their Pre-funded Warrants and acquire our common stock, except as otherwise provided in the Pre-funded Warrants.
Until holders of the Pre-funded Warrants
acquire our common stock upon exercise thereof, such holders will have no rights with respect to the common stock underlying such Pre-funded Warrants,
except to the extent that holders of such Pre-Funded warrants will have certain rights to participate in distributions or dividends
paid on our common stock as set forth in the Pre-funded Warrants. Upon exercise of the Pre-funded Warrants, the holders
will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise
date.
There is no public
market for the warrants to purchase common stock being offered in this offering.
There is no established public
trading market for the warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend
to apply for listing of the warrants on any securities exchange. Without an active market, the liquidity of the warrants will be limited.
Holders
of our warrants will have no rights as a common stockholder until such holders exercise their warrants and acquire
our common stock, except as provided in the warrants.
Until holders of warrants
acquire shares of our common stock upon exercise of the warrants, holders of warrants will have no rights with respect to the shares of
our common stock underlying such warrants, except as provided in the warrants. Upon exercise of the warrants, the holders thereof will be entitled to exercise the rights
of a common stockholder only as to matters for which the record date occurs after the exercise date.
USE
OF PROCEEDS
We estimate that the net
proceeds from the offering will be approximately $13.5 million (or approximately $15.6 million if the underwriters exercise in full
their option to purchase up to 3,515,625 additional shares of common stock and/or warrants to purchase 3,515,625 additional shares
of common stock), after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net
proceeds from the offering for general corporate purposes, which includes, without limitation, ongoing research and pre-clinical
studies, clinical trials, the development of new biological and pharmaceutical technologies, investing in or acquiring companies
that are synergistic with or complementary to our technologies, licensing activities related to our current and future product
candidates (including a payment of $4.0 million due to Patagonia on the initiation of a Phase 3 pivotal trial for TMB-001), the development of emerging technologies, investing in or acquiring companies that are developing emerging
technologies, licensing activities, or the acquisition of other businesses and working capital. The amounts and timing of these
expenditures will depend on numerous factors, including the development of our current business initiatives. We have no specific
acquisition contemplated at this time. As of the date of this prospectus supplement, we cannot specify with certainty all of the
particular uses for the net proceeds from this offering. The amounts and timing of our actual expenditures will depend on numerous
factors, including factors described under “Risk Factors” in this prospectus supplement, the accompanying base
prospectus and the documents incorporated by reference herein and therein.
DILUTION
If you invest in our securities
in this offering, your interest will be diluted immediately to the extent of the difference between the public offering price paid by
the purchasers of the shares of common stock and warrants sold in this offering and the as-adjusted net tangible book value per shares
of common stock after this offering.
The net tangible book value
of our common stock as of June 30, 2021 was approximately $4.8 million, or approximately $0.13 per shares of common stock.
Net tangible book value per share represents the amount of our total tangible assets less total liabilities divided by the total number
of our shares of common stock outstanding as of June 30, 2021.
After giving effect to
the sale by us in this offering of 21,325,000 shares of common stock, 2,112,500 Pre-Funded Warrants and 23,437,500 warrants at
a price per share and related warrant of $0.64 after deducting estimated underwriting discounts and commissions and estimated
offering expenses payable by us, and assuming the exercise in full of Pre-funded Warrants issued in this offering, our as
adjusted net tangible book value as of June 30, 2021 would have been approximately
$18.3 million, or approximately
$0.31 per share of common stock. This represents an
immediate increase in net tangible book value of approximately
$0.18 per share of common stock to our existing
security holders and an immediate dilution in as adjusted net tangible book value of approximately
$0.33 per share of common stock to purchasers of
common stock in this offering, as illustrated by the following table:
|
Public offering price per share of common stock and related warrant
|
|
|
|
|
|
$
|
0.64
|
|
|
Consolidated net tangible book value per share as of June 30, 2021
|
|
$
|
0.13
|
|
|
|
|
|
|
Increase in consolidated net tangible book value per share of common stock attributable to the offering
|
|
$
|
0.18
|
|
|
|
|
|
|
As adjusted consolidated net tangible book value per share of common stock as of June 30, 2021
|
|
$
|
0.31
|
|
|
|
|
|
|
Dilution per share of common stock to new investors participating in this offering
|
|
|
|
|
|
$
|
0.33
|
|
If the underwriters exercise
their option to purchase up to 3,515,625 additional shares of common stock and/or warrants to purchase 3,515,625 additional shares in
full, the as adjusted net tangible book value will increase to $0.32
per share, representing an immediate increase in net tangible book value of $0.19
per share of our common stock to existing stockholders and an immediate dilution of $0.32 per share to purchasers of our common stock
in this offering.
The figures above are based
on 36,659,685 shares outstanding as of June 30, 2021, and excludes as of such date:
|
|
●
|
184,456 shares of common stock issuable upon the exercise of outstanding vested and unvested stock options under our 2020 Omnibus Equity Incentive Plan at a weighted-average exercise price of $2.87 per share (excluding aggregate of 2,473,184 shares of common stock issuable upon the exercise of outstanding options with a weighted-average exercise price of $0.96 granted in the third quarter of 2021), 15,781 shares of common stock issuable upon the exercise of outstanding and vested legacy BioPharmX stock options at a weighted-average exercise price of $75.27, and 367,670 shares of common stock issuable upon the exercise of outstanding vested and unvested VARs;
|
|
|
|
|
|
|
●
|
17,335,503 shares of common stock issuable upon the exercise of outstanding warrants, having a weighted-average exercise price of $2.25 per share, including (i) 16,701,824 Series A Warrants with an exercise price of $1.16, (ii) 214,046 BioPharmX legacy warrants with a weighted-average exercise price of $87.25, and (iii) 413,751 Bridge Warrants with an exercise price of $2.2362 that are subject to potential anti-dilution adjustment as a result of this offering;
|
|
|
|
|
|
|
●
|
100,753 shares of common stock issuable upon the conversion of outstanding Series A Preferred Stock;
|
|
|
|
|
|
|
●
|
183,360 shares of treasury stock; and
|
|
|
|
|
|
|
●
|
the shares of common stock issuable upon exercise of the warrants issued in this offering.
|
To the extent that outstanding
exercisable options or warrants are exercised, including the warrants issued in this offering, you may experience further dilution. In addition, we
may need to raise additional capital and to the extent that we raise additional capital by issuing equity or convertible debt securities
your ownership will be further diluted.
DESCRIPTION OF SECURITIES OFFERED
We are offering 21,325,000 shares
of our common stock and warrants to purchase up to 23,437,500 shares of common stock. We are also offering 2,112,500 Pre-funded Warrants
to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates
and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares
of common stock following the consummation of this offering in lieu of the shares of common stocks that would result in such excess ownership.
Each Pre-funded Warrant will be exercisable for one share of common stock. No warrant for fractional shares of common stock will be issued,
rather warrants will be issued only for whole shares of common stock. We are also registering the shares of common stock issuable from
time to time upon exercise of the Pre-funded Warrants and warrants offered hereby.
Common Stock
The descriptions of our common
stock included in the accompanying prospectus on page 7 under the heading “Description of Capital Stock” are incorporated
herein by reference.
Warrants
The
following is a summary of all material terms and provisions of the warrants that are being offered hereby, the form of which will
be filed as an exhibit to a Current Report on Form 8 K in connection with this offering and incorporated by reference into the registration
statement of which this prospectus supplement forms a part. Prospective investors should carefully review the terms and provisions of
the form of warrant for a complete description of the terms and conditions of the warrants.
Duration and Exercise Price
Each warrant offered hereby
will have an exercise price equal to $0.70. The warrants will be immediately exercisable and may be exercised until the fifth anniversary
of the issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment
in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The
warrants will be issued separately from the common stock or pre-funded warrants, respectively, and may be transferred separately immediately
thereafter. Warrants will be issued in certificated form only.
Exercisability
The warrants will be
exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment
in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed
below). A holder (together with its affiliates) may not exercise any portion of such holder’s warrants to the extent that
the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’
prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s
warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as
such percentage ownership is determined in accordance with the terms of the warrants.
Cashless Exercise
If, at the time a holder exercises
its warrants, a registration statement registering the issuance or resale of the shares of common stock underlying the warrants
under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise
contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon
such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the
warrant. The warrants will be automatically exercised on a cashless basis on the expiration date.
Fundamental Transactions
In the event of any fundamental
transaction, as described in the warrants and generally including any merger with or into another entity, sale of all or substantially
all of our assets, tender offer or exchange offer, or reclassification of our common stock, then upon any subsequent exercise of a warrant,
the holder will have the right to receive as alternative consideration, for each share of our common stock that would have been issuable
upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor
or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as
a result of such transaction by a holder of the number of shares of our common stock for which the warrant is exercisable immediately
prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the warrants have the right
to require us or a successor entity to redeem the warrants for cash in the amount of the Black-Scholes Value (as defined in each warrant)
of the unexercised portion of the warrants concurrently with or within 30 days following the consummation of a fundamental transaction.
However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our
board of directors, the holders of the warrants will only be entitled to receive from us or our successor entity, as of the date of consummation
of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the
unexercised portion of the warrant that is being offered and paid to the holders of our common stock in connection with the fundamental
transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of
our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
Transferability
Subject to applicable laws,
a warrant may be transferred at the option of the holder upon surrender of the warrant to us together with the appropriate
instruments of transfer.
Fractional Shares
No fractional shares of common
stock will be issued upon the exercise of the warrants. Rather, the number of shares of common stock to be issued will, at our
election, either be rounded up to the nearest whole number or we will pay a cash adjustment in respect of such final fraction in an amount
equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading
market for the warrants, and we do not expect an active trading market to develop. We do not intend to apply to list the
warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the warrants will be extremely
limited.
Right as a Stockholder
Except as otherwise provided
in the warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of warrants does
not have the rights or privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s
warrants.
Waivers and Amendments
No term of the warrants
may be amended or waived without the written consent of the majority of the holders of the warrants purchased in this offering.
Pre-funded Warrants
The following summary of certain
terms and provisions of the Pre-funded Warrants that are being offered hereby is not complete and is subject to, and qualified
in its entirety by, the provisions of the Pre-funded Warrant, the form of which will be filed with the SEC as an exhibit to
a Current Report on Form 8-K in connection with this offering and incorporated by reference into the registration statement
of which this prospectus supplement forms a part. Prospective investors should carefully review the terms and provisions of the form of Pre-funded Warrant
for a complete description of the terms and conditions of the Pre-funded Warrants.
Duration and Exercise Price
Each Pre-funded Warrant
offered hereby will have an initial exercise price per share of common stock equal to $0.001. The Pre-funded Warrants will be
immediately exercisable and will expire when exercised in full. The exercise price and number of shares of common stock issuable upon
exercise is subject to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting
our shares of common stock and the exercise price. Subject to the rules and regulations of the applicable trading market, we may at any
time during the term of the Pre-funded Warrant, subject to the prior written consent of the holders, reduce the then current
exercise price to any amount and for any period of time deemed appropriate by our board of directors.
Exercisability
The Pre-funded Warrants
will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied
by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as
discussed below). A holder (together with its affiliates) may not exercise any portion of the Pre-funded Warrant to the extent
that the holder would own more than 4.99% of the outstanding shares of common stock immediately after exercise, except that upon at least
61 days’ prior notice from the holder to us, the holder may increase the amount of beneficial ownership of outstanding shares
after exercising the holder’s Pre-funded Warrants up to 9.99% of the number of our shares of common stock outstanding
immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-funded Warrants.
Purchasers of Pre-funded Warrants in this offering may also elect prior to the issuance of the Pre-funded Warrants
to have the initial exercise limitation set at 9.99% of our outstanding shares of common stock.
Cashless Exercise
In lieu of making the cash
payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead
to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula
set forth in the Pre-funded Warrants.
Fractional Shares
No fractional shares of common
stock will be issued upon the exercise of the Pre-funded Warrants. Rather, at the Company’s election, the number of shares
of common stock to be issued will be rounded up to the nearest whole number or the Company will pay a cash adjustment in an amount equal
to such fraction multiplied by the exercise price.
Transferability
Subject to applicable laws,
a Pre-funded Warrant may be transferred at the option of the holder upon surrender of the Pre-funded Warrant to us
together with the appropriate instruments of transfer.
Trading Market
There is no trading market
available for the Pre-funded Warrants on any securities exchange or nationally recognized trading system, and we do not expect
a trading market to develop. We do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized
trading market. Without a trading market, the liquidity of the Pre-funded Warrants will be extremely limited. The shares of
common stock issuable upon exercise of the Pre-funded Warrants are currently traded on the NYSE American.
Right as a Shareholder
Except as otherwise provided
in the Pre-funded Warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the Pre-funded Warrants
do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their Pre-funded Warrants.
The Pre-funded Warrants will provide that holders have the right to participate in distributions or dividends paid on our shares
of common stock.
Fundamental Transaction
In the event of a fundamental
transaction, as described in the Pre-funded Warrants and generally including any reorganization, recapitalization or reclassification
of our shares of common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation
or merger with or into another person, the acquisition of more than 50% of our outstanding shares of common stock, or any person or group
becoming the beneficial owner of 50% of the voting power represented by our outstanding shares of common stock, the holders of the Pre-funded Warrants
will be entitled to receive upon exercise of the Pre-funded Warrants the kind and amount of securities, cash or other property
that the holders would have received had they exercised the Pre-funded Warrants immediately prior to such fundamental transaction
on a net exercise basis.
UNDERWRITING
Pursuant to the underwriting
agreement with H.C. Wainwright & Co., LLC (the “Representative”), as Representative of the several underwriters in this
offering, we have agreed to issue and sell, and each of the underwriters have agreed to purchase, the number of shares of common stock, warrants
and Pre-funded Warrants listed opposite its name below, less the underwriting discounts and commissions, on the closing date, subject
to the terms and conditions contained in the underwriting agreement. The underwriting agreement provides that the obligations of the underwriters
are subject to certain customary conditions precedent, representations and warranties contained therein.
|
Underwriter
|
|
Number of
Shares
|
|
|
Number of
Warrants
|
|
|
Number of
Pre-funded
Warrants
|
|
|
H.C. Wainwright & Co., LLC
|
|
|
21,325,000
|
|
|
|
23,437,500
|
|
|
|
2,112,500
|
|
|
Total
|
|
|
21,325,000
|
|
|
|
23,437,500
|
|
|
|
2,112,500
|
|
Pursuant to the
underwriting agreement, the underwriters have agreed to purchase all of the shares and warrants sold under the underwriting
agreement if any of these shares and warrants are purchased, other than those shares and warrants covered by the underwriters’
option to purchase additional shares of common stock described below. The underwriters have advised us that they do not intend to
confirm sales to any account over which they exercise discretionary authority.
Discount, Commissions and Expenses
The underwriters may
offer the shares of common stock and warrants from time to time to purchasers directly or through agents, or through brokers in
brokerage transactions on the NYSE American, or to dealers in negotiated transactions or in a combination of such methods of sale,
or otherwise, at a fixed price or prices, which may be changed, or at market prices prevailing at the time of sale, at prices
related to such prevailing market prices or at negotiated prices, subject to receipt and acceptance by them and subject to their
right to reject any order in whole or in part. The difference between the price at which the underwriters purchase shares and
warrants from us and the price at which the underwriters resell such shares may be deemed underwriting compensation. If the
underwriters effect such transactions by selling shares of common stock and warrants to or through dealers, such dealers may receive
compensation in the form of discounts, concessions or commissions from the underwriters and/or purchasers of shares of common stock
and warrants for whom they may act as agents or to whom they may sell as principal.
While the underwriters
intend to offer the shares of common stock at the price set forth on the cover of this prospectus supplement, the underwriters may
offer the shares of common stock from time to time to purchasers directly or through agents, or through brokers in brokerage
transactions on NYSE American, or to dealers in negotiated transactions or in a combination of such other methods of sale, or
otherwise, at a fixed price or prices, which may be changed, or at market prices prevailing at the time of sale, at prices related
to such prevailing market price or at negotiated prices. Any shares and warrants sold by the underwriters to securities dealers will
be sold at the public offering price less a selling concession not in excess of $0.0288 per share and related warrant.
We have granted to the Representative
an option, exercisable for 30 days from the date of this prospectus supplement, to purchase up to 3,515,625 additional shares of common
stock and/or warrants to purchase up to 3,515,625 shares of our common stock at the public offering price per share of common stock or
warrant, respectively, less underwriting discounts and commissions.
The following table shows
the public offering price, underwriting discounts and commissions and proceeds, before expenses, to us.
|
|
|
Per Share and
Related
Warrant
|
|
|
Per Pre-funded
Warrant and
Related Warrant
|
|
Total Without
Option
|
|
|
Total With
Option
|
|
|
Public offering price
|
|
$
|
0.64
|
|
|
$
|
0.639
|
|
$
|
14,997,887.50
|
|
|
$
|
17,247,887.50
|
|
|
Underwriting discounts and commissions
|
|
$
|
0.0384
|
|
|
$
|
0.0384
|
|
$
|
900,000.00
|
|
|
$
|
1,035,000.00
|
|
|
Proceeds, before expenses and fees, to us
|
|
$
|
0.6016
|
|
|
$
|
0.6006
|
|
$
|
14,097,887.50
|
|
|
$
|
16,212,887.50
|
|
We have agreed to pay
the legal fees and expenses of the underwriters, in the sum of up to $100,000 in connection with this offering, $50,000 for
non-accountable expenses and clearing fees of $15,950. We have also agreed to pay the underwriters a management fee equal to 1.0% of
the aggregate gross proceeds in this offering. We estimate that the total expenses of the offering, excluding the underwriting
discounts and commissions, will be approximately $410,000 and are payable by us.
Right of First Refusal
We have granted the Representative
a six-month right of first refusal to act as sole book-running manager, sole underwriter, or sole placement agent if we or any of our
subsidiaries raise funds by means of a public offering or a private placement or any other capital-raising financing of equity or equity-linked
securities using an underwriter or placement agent. If the Representative or an affiliate of the Representative accept any such engagement,
the agreement governing such engagement will contain, among other things, provisions for customary fees and terms for transactions of
similar size and nature, including indemnification, which are appropriate to such a transaction.
Tail
If an offering is not consummated
by November 15, 2021, we shall pay the Representative the cash compensation provided above on the gross proceeds provided to us by investors
that were brought over-the-wall or introduced to the Company by the Representative during our engagement of the Representative in a public
or private offering or other financing or capital-raising transaction within six months following the termination of our engagement of
the Representative.
Indemnification
We have agreed to indemnify
the underwriters against certain liabilities, including civil liabilities under the Securities Act, or to contribute to payments that
the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
We have agreed to not sell
any shares of our common stock, or any securities convertible into or exercisable or exchangeable into shares of common stock, subject to
certain exceptions, for a period of 90 days after the closing of this offering unless we obtain prior written consent of the underwriters.
This consent may be given at any time without public notice, and the underwriters may consent in their sole discretion. The exceptions
to the restriction include, among other things, the issuance of any shares of our capital stock or securities convertible into shares
of our capital stock that are issued (i) pursuant to a stock or option plan, (ii) pursuant to the exercise or exchange of or conversion
of any securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date of this
prospectus supplement, (iii) as consideration in an acquisition, merger or similar strategic transaction approved by a majority of the
disinterested directors, provided that such securities are issued as “restricted securities” as defined in Rule 144 and carry
no registration rights that require or permit the filing of any registration statement in connection therewith within 90 days following
the closing of this offering, and provided that any such issuance shall only be to a person providing us business synergies and additional
benefits in addition to the investment of funds, but shall not include a transaction in which we are issuing securities primarily for
the purpose of raising capital or to an entity whose primary business is investing in securities.
In addition, each of our directors
and executive officers has entered into a lock-up agreement with the Representative. Under the lock-up agreements, subject to certain
limited circumstances, our directors and officers may not, directly or indirectly, sell, offer to sell, contract to sell, or grant any
option for the sale (including any short sale), grant any security interest in, pledge, hypothecate, hedge, establish an open “put
equivalent position” (within the meaning of Rule 16a-1(h) under the Exchange Act), or otherwise dispose of, or enter into any transaction
which is designed to or could be expected to result in the disposition of, any shares of our common stock or securities convertible into
or exchangeable for shares of our common stock, or publicly announce any intention to do any of the foregoing, unless such directors and
executive officers obtain prior written consent of the Representative for a period of 90 days from the date of this prospectus supplement.
This consent may be given at any time without public notice, and the Representative may consent in its sole discretion.
Price Stabilization, Short Positions and Penalty
Bids
In connection with this offering,
the underwriters may engage in stabilizing transactions, syndicate covering transactions and penalty bids in connection with our common
stock.
Stabilizing transactions permit
bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum.
Syndicate covering transactions
involve purchases of common stock in the open market after the distribution has been completed in order to cover syndicate short positions.
Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created
if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing
that could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriters
to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a
stabilizing or syndicate covering transaction to cover syndicate short positions. These stabilizing transactions, syndicate covering transactions
and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline
in the market price of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise
be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the
transactions described above may have on the price of our common stock. These transactions may be effected on the NYSE American, in the
over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
In connection with this offering,
the underwriters also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period
before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the
distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that
security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific
purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might
otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Electronic Distribution
A prospectus supplement and
the accompanying prospectus in electronic format may be made available on the websites maintained by the underwriters, if any, participating
in this offering and the underwriters may distribute such prospectuses electronically. Other than the prospectus supplement and the accompanying
prospectus in electronic format, the information on these websites is not part of this prospectus supplement and the accompanying prospectus
or the registration statement of which this prospectus supplement and the accompanying prospectus form a part, has not been approved or
endorsed by us or the underwriters, and should not be relied upon by investors.
Other Relationships
From time to time, the underwriters
and their affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services
to us in the ordinary course of business, for which it has received and may continue to receive customary fees and commissions.
Transfer Agent
The transfer agent for our
common stock is Computershare Trust Company, N.A. The transfer agent address is 520 Pike Street Suite 1305, Seattle, WA 98101, (206) 674-3050.
Listing on the NYSE American
Our common stock is
listed on the NYSE American under the symbol “TMBR”. We do not plan to list the warrants or the Pre-funded
Warrants on the NYSE American or any other securities exchange or trading market.
LEGAL
MATTERS
The validity of the common
stock being offered will be passed upon for us by Lowenstein Sandler LLP, Roseland, New Jersey. Ellenoff Grossman & Schole LLP is
counsel for the underwriters in connection with this offering.
EXPERTS
The consolidated financial
statements of Timber Pharmaceuticals, Inc. as of December 31, 2020 and 2019, and for the year ended December 31, 2020 and
the period from February 26, 2019 (Inception) through December 31, 2019, have been incorporated by reference herein and in the
registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference
herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2020
consolidated financial statements contains an explanatory paragraph that states that the Company's recurring losses from operations raise
substantial doubt about the entity's ability to continue as a going concern. The consolidated financial statements do not include any
adjustments that might result from the outcome of that uncertainty.
WHERE
YOU CAN FIND ADDITIONAL INFORMATION
This prospectus supplement
is part of a registration statement on Form S-3 that we have filed with the SEC relating to our securities being offered
hereby. This prospectus does not contain all of the information in the registration statement and its exhibits. The registration statement,
its exhibits and the documents incorporated by reference in this prospectus supplement and their exhibits, all contain information that
is material to the offering of the securities hereby. Whenever a reference is made in this prospectus supplement to any of our contracts
or other documents, the reference may not be complete. You should refer to the exhibits that are a part of the registration statement
in order to review a copy of the contract or documents. The registration statement and the exhibits are available at the SEC’s Public
Reference Room or through its website.
We file annual, quarterly
and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet site at http://www.sec.gov that
contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically with
the SEC. Additionally, you may access our filings with the SEC through our website at www.timberpharma.com. We have included our website
address as an inactive textual reference only and our website and the information contained on, or that can be accessed through, our website
will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus supplement.
We will provide you without
charge, upon your oral or written request, with an electronic or paper copy of any or all reports, proxy statements and other documents
we file with the SEC, as well as any or all of the documents incorporated by reference in this prospectus supplement (other than exhibits
to such documents unless such exhibits are specifically incorporated by reference into such documents). Requests for such copies should
be directed to:
Timber Pharmaceuticals, Inc.
Attn: John Koconis, Chief Executive Officer and
Chairman of the Board
110 Allen Road, Suite 401 Basking Ridge, NJ
07920
Telephone number: (908) 636-7160
You should rely only on the
information in this prospectus supplement and the additional information described above and under the heading “Incorporation of
Certain Information by Reference” below. We have not authorized any other person to provide you with different information. If anyone
provides you with different or inconsistent information, you should not rely upon it. We are not making an offer to sell these securities
in any jurisdiction where such offer or sale is not permitted. You should assume that the information in this prospectus supplement was
accurate on the date of the front cover of this prospectus supplement only. Our business, financial condition, results of operations and
prospects may have changed since that date.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate
by reference” information that we file with it into this prospectus supplement, which means that we can disclose important information
to you by referring you to those documents. The information incorporated by reference is an important part of this prospectus supplement.
The information incorporated by reference is considered to be a part of this prospectus supplement, and information that we file later
with the SEC will automatically update and supersede information contained in this prospectus supplement.
We incorporate by reference
the documents listed below that we have previously filed with the SEC:
|
|
●
|
our Annual Report on Form 10-K for the year ended December 31,
2020, filed with the SEC on March 23, 2021, as amended on Form 10-K/A, filed with the SEC on May 17, 2021;
|
|
|
|
|
|
|
●
|
our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2021, filed with the SEC on May
11, 2021 and June 30, 2021, filed with the SEC on August 10, 2021;
|
|
|
|
|
|
|
●
|
our Current Reports on Form 8-K dated January 12, 2021, January 19, 2021, February 4, 2021 (other than any portions thereof deemed furnished and not filed), March 16, 2021, March 17, 2021,
April 16, 2021, April 23, 2021, April 28, 2021, May 26, 2021, June 3, 2021, July 1, 2021 (including Item 7.01), July 2, 2021, August 24, 2021, September 13, 2021, September 16, 2021, September 29, 2021, and October 7, 2021;
|
|
|
|
|
|
|
●
|
our definitive Proxy Statement on Schedule 14A, filed with the SEC on April 23, 2021 (solely with respect to those portions incorporated by reference into our Annual Report on Form 10-K for the fiscal year ended
December 31, 2020), as supplemented by the Proxy Supplement on Schedule 14A, filed with the SEC on April 29, 2021; and
|
|
|
|
|
|
|
●
|
the description of the Company’s common stock contained in the Company’s Registration Statement on Form 8-A filed with the SEC on June 1, 2015 under Section 12 of the Exchange Act, including any amendment or report filed for the purpose of updating such description.
|
All reports and other documents
(other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed in such forms that are related to such
items unless such Form 8-K expressly provides to the contrary) we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the
Exchange Act, prior to the termination of this offering, will also be incorporated by reference in this prospectus supplement and deemed
to be part of this prospectus supplement from the date of the filing of such reports and documents.
Any statement contained in
a document incorporated or deemed to be incorporated by reference in this prospectus supplement shall be deemed modified, superseded or
replaced for purposes of this prospectus supplement to the extent that a statement contained in this prospectus supplement, or in any
subsequently filed document that also is deemed to be incorporated by reference in this prospectus supplement, modifies, supersedes or
replaces such statement. Any statement so modified, superseded or replaced shall not be deemed, except as so modified, superseded or replaced,
to constitute a part of this prospectus supplement. None of the information that we disclose under Items 2.02 or 7.01 of any Current Report
on Form 8-K or any corresponding information, either furnished under Item 9.01 or included as an exhibit therein, that we may from time
to time furnish to the SEC will be incorporated by reference into, or otherwise included in, this prospectus supplement, except as otherwise
expressly set forth in the relevant document. Subject to the foregoing, all information appearing in this prospectus supplement is qualified
in its entirety by the information appearing in the documents incorporated by reference.
PROSPECTUS
Timber Pharmaceuticals, Inc.
$100,000,000
Common Stock
Preferred Stock
Warrants
Subscription Rights
Units
We may offer, issue and sell
from time to time together or separately, in one or more offerings, any combination of (i) our common stock, (ii) our preferred
stock, which we may issue in one or more series, (iii) warrants, (iv) subscription rights and (v) units. The preferred
stock, warrants and subscription rights may be convertible into, or exercisable or exchangeable for, common or preferred stock or other
securities of ours. The units may consist of any combination of the securities listed above.
The aggregate public offering
price of the securities that we may offer will not exceed $100,000,000. We will offer the securities in an amount and on terms that market
conditions will determine at the time of the offering. Our common stock is presently listed on The NYSE American, LLC (the “NYSE
American”) under the symbol “TMBR”. On April 30, 2021, the last reported sale price of our Common Stock on the
NYSE American was $1.58 per share. Our common stock has recently experienced extreme volatility in price and trading volume. For
example, on March 4, 2021 and March 11, 2021, the closing price of our common stock on the NYSE American was $1.66 and $3.55,
respectively, and daily trading volume on these days was approximately 3.5 million and 143.7 million shares, respectively. During this
period, there were no recent changes in our financial condition or results of operations that were consistent with the recent change in
our stock price. Investors that purchase shares of our common stock may lose a significant portion of their investments if the price of
our common stock declines. Please see the section of this prospectus supplement titled “Risk Factors.” Each prospectus supplement
will indicate if the securities offered thereby will be listed on any securities exchange. You are urged to obtain current market quotations
of our common stock. We have no preferred stock, warrants, subscription rights or units listed on any market. Each prospectus supplement
will indicate if the securities offered thereby will be listed on any securities exchange.
Investing in our securities
involves risk. You should carefully consider the risks that we refer you to under the section captioned “Risk Factors” in
this prospectus on page 4 before buying our securities.
Should we offer any of the
securities described in this prospectus, we will provide you with the specific terms of the particular securities being offered in supplements
to this prospectus. You should read this prospectus and any supplement, together with additional information described under the headings
“Additional Information” and “Incorporation of Certain Information by Reference” carefully before you invest.
This prospectus may not be used to sell securities unless accompanied by a prospectus supplement.
We may sell these securities
directly to our stockholders or to other purchasers or through agents on our behalf or through underwriters or dealers as designated from
time to time. If any agents or underwriters are involved in the sale of any of these securities, the applicable prospectus supplement
will provide the names of the agents or underwriters and any applicable fees, commissions or discounts.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is May 11,
2021.
TABLE OF CONTENTS
Timber Pharmaceuticals, Inc. and its consolidated
subsidiaries are referred to herein as “Timber,” “the Company,” “we,” “us” and “our,”
unless the context indicates otherwise.
You may only rely on the information contained
in this prospectus or that we have referred you to. We have not authorized anyone to provide you with different information. This prospectus
does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the securities offered by this prospectus.
This prospectus and any future prospectus supplement do not constitute an offer to sell or a solicitation of an offer to buy any securities
in any circumstances in which such offer or solicitation is unlawful. Neither the delivery of this prospectus or any prospectus supplement
nor any sale made hereunder shall, under any circumstances, create any implication that there has been no change in our affairs since
the date of this prospectus or such prospectus supplement or that the information contained by reference to this prospectus or any prospectus
supplement is correct as of any time after its date.
ABOUT THIS PROSPECTUS
This prospectus is part of
a registration statement that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration process.
Under this shelf registration process, we may from time to time offer and sell, in one or more offerings, any or all of the securities
described in this prospectus, separately or together, up to an aggregate offering price of $100,000,000. This prospectus provides you
with a general description of our securities being offered. When we issue the securities being offered by this prospectus, we will provide
a prospectus supplement (which term includes, as applicable, the at-the-market sale agreement prospectus filed with the registration statement
of which this prospectus forms a part) that will contain specific information about the terms of that offering. The prospectus supplement
may also add, update or change information contained in this prospectus. You should read both this prospectus and any prospectus supplement
together with additional information described under the heading “Additional Information” and “Incorporation of Certain
Information by Reference.”
PROSPECTUS SUMMARY
The following summary highlights some information
from this prospectus. It is not complete and does not contain all of the information that you should consider before making an investment
decision. You should read this entire prospectus, including the “Risk Factors” section on page 4, the consolidated
financial statements and related notes and the other more detailed information appearing elsewhere or incorporated by reference into this
prospectus.
The Company
We are a clinical-stage biopharmaceutical
company focused on the development and commercialization of treatments for orphan dermatologic diseases. Our investigational therapies
have proven mechanisms-of-action backed by decades of clinical experience and well-established CMC (chemistry, manufacturing and control)
and safety profiles. We are initially focused on developing non-systemic treatments for rare dermatologic diseases including congenital
ichthyosis, facial angiofibromas (“FAs”) in tuberous sclerosis complex (“TSC”), and scleroderma. Our lead programs
are TMB-001, TMB-002 and TMB-003.
TMB-001
TMB-001, a patented
topical formulation of isotretinoin is currently being evaluated in a Phase 2b clinical trial for the treatment of moderate to
severe subtypes of congenital ichthyosis (“CI”), a group of rare genetic keratinization disorders that lead to dry,
thickened, and scaling skin. A prior Phase 1/2 study involving 19 patients with CI demonstrated safety and a signal of preliminary
efficacy of TMB-001, as well as minimal systemic absorption. In 2018, the FDA awarded us the first tranche of a $1.5 million grant
in the amount of $500,000 to support clinical trials evaluating TMB-001 through its Orphan Products Grant program. In
March 2020 and March 2021, the FDA awarded us the second and third tranches of the grant, respectively, each in the amount
of $500,000.
On July 1, 2020, we announced that
all 11 sites across the United States and Australia in the Phase 2b CONTROL study evaluating TMB-001 in patients with moderate to severe
CI were enrolling patients. As of December 31, 2020, all sites participating in a Phase 2b clinical trial evaluating TMB-001 were opened
and enrolling patients. On March 15, 2021, we announced that 50% of patients in the Phase 2b clinical trial have been enrolled. We continue
to anticipate topline data readout in Q3 for the Phase IIb TMB-001 trial. We are currently evaluating the sample size, including a potential
reduction, in order to maintain forward progress towards phase III.
On
December 15, 2020, we announced that we had received a notice of allowance from the U.S. Patent and Trademark Office (USPTO) for a
Company patent application covering TMB-001 (U.S. Patent Application No.: 15/772,456) and the application subsequently issued on
February 10, 2021 as US 10,933,018.
On April 28, 2021, we announced
that the Japanese Patent Office had decided to grant a patent (No. 2018542677) for TMB-001.
TMB-002
TMB-002, a proprietary topical formulation of rapamycin
is currently being evaluated in a Phase 2b clinical trial for the treatment of FAs in TSC, a multisystem genetic disorder resulting in
the growth of hamartomas in multiple organs. TSC results from dysregulation in the mTOR pathway, and as a topical mTOR inhibitor, TMB-002
may address FAs in TSC without the level of systemic absorption of an oral agent.
The impact of the worldwide spread
of a novel strain of coronavirus (“COVID-19”) has been unprecedented and unpredictable. Site activation and patient enrollment
have been impacted by the COVID-19 pandemic in the TMB-002 study, especially at our contracted test sites in Eastern Europe. We are expecting
that there will be some delay to the completion of recruitment and in the topline data readout for the TMB-002 program and we are currently
working with our partners and the sites to better qualify this situation. We fully expect recruitment to be completed by the end of Q3
2021. The Company is also continuing to assess the effect of COVID-19 on the TMB-002 study as well as the Company’s overall operations
by monitoring the spread of COVID-19 and the actions implemented to combat the virus throughout the world. The Company’s assessment
of the impact of COVID-19 may change in the future.
On March 17, 2021, we announced
that AFT Pharmaceuticals Limited (“AFT”), one of our development partners, entered into a license and supply agreement with
Desitin Arzneimittel GmbH (“Desitin”) for Pascomer® (TMB-002 topical rapamycin) for the treatment of FAs associated with
TSC in Europe. Pursuant to the AFT Licensing and Development Agreement, we are entitled to receive a significant percentage of the economics
(royalties and milestones) in any licensing transaction that AFT executes outside of North America, Australia, New Zealand, and Southeast
Asia for licensing rights to TMB-002. The current transaction with Desitin is included in the scope of this provision.
TMB-003
TMB-003, a proprietary formulation
of sitaxsentan, a new chemical entity in the U.S., which is a selective endothelin-A receptor antagonist, is currently in preclinical
development as a locally applied formulation for the treatment of scleroderma, a rare connective tissue disorder characterized by abnormal
thickening of the skin.
On January 12, 2021, we announced that the FDA has granted orphan drug designation for TMB-003, our locally delivered
formulation of Sitaxsentan, for the treatment of systemic sclerosis.
BPX-01 and BPX-04
Pursuant to the Merger Agreement
(as defined below), BPX-01 (Topical Minocycline, 2%) and BPX-04 (Topical Minocycline, 1%) were added to our portfolio. BPX-01 and BPX-04
are assets currently in development for acne vulgaris and papulopustular rosacea, respectively. On July 22, 2020, we announced that we
had received notice from the European Patent Office that it intends to grant a patent for the Company’s topical composition of pharmaceutical
tetracycline (including minocycline) for dermatological use (European Patent Application No. 16714168.8) and the application subsequently
issued on December 16, 2020 as EP 3273940. Patents covering the BPX-01 and BPX-04 assets have previously been granted in the United States,
South Africa, and Australia, among other countries. We are currently evaluating our strategic options regarding these assets.
On September 15, 2020, we announced
that we had received a notice of allowance from the USPTO for a Company patent application covering
BPX-01 and BPX-04 (U.S. Patent Application No.: 16/514,459) and the application subsequently issued on January 5, 2021 as US 10,881,672.
The Merger, Reverse Stock Split and Name Change
On
May 18, 2020, we completed our business combination with Timber Sub, in accordance with the terms of the Agreement and Plan of Merger
and Reorganization, dated as of January 28, 2020, as amended, by and among Timber, Timber Sub and BITI Merger Sub Inc., a wholly-owned
subsidiary of Timber (“Merger Sub”) (the “Merger Agreement”), pursuant to which Merger Sub merged with and into
Timber Sub, with Timber Sub surviving as a wholly owned subsidiary of Timber (the “Merger”).
In
connection with, and immediately prior to the completion of, the Merger, we effected a reverse stock split of our Common Stock, at a ratio
of 1-for-12 (the “Reverse Stock Split”). Under the terms of the Merger Agreement, after taking into account the Reverse Stock
Split, we issued shares of Common Stock to Timber Sub’s unitholders at an exchange rate of 629.57 shares of Common Stock for each
Timber Sub common unit outstanding immediately prior to the Merger. In connection with the Merger, we changed our name from “BioPharmX
Corporation” to “Timber Pharmaceuticals, Inc.,” and the business conducted by the Company became the business conducted
by Timber Sub.
Private Placement
of Common Stock and Warrants
On
May 18, 2020, Timber and Timber Sub completed a private placement transaction (the “Pre-Merger Financing”) with the Investors
pursuant to the Securities Purchase Agreement for an aggregate purchase price of approximately $25.0 million (comprised of (i) approximately
$5 million credit with respect to the senior secured notes issued in connection with the bridge loan that certain of the Investors made
to Timber Sub at the time of the execution of the Merger Agreement and (ii) approximately $20 million in cash from the Investors).
Pursuant
to the Pre-Merger Financing, (i) Timber Sub issued and sold to the Investors common units of Timber Sub which converted pursuant
to the exchange ratio in the Merger into an aggregate of approximately 4,137,509 shares (the “Converted Shares”) of Common
Stock; and (ii) the Company agreed to issue to each Investor, on the tenth trading day following the consummation of the Merger,
(A) Series A Warrants representing the right to acquire shares of Common Stock equal to 75% of the sum of (a) the number
of Converted Shares issued to the Investor, without giving effect to any limitation on delivery contained in the Securities Purchase Agreement,
and (b) the number of shares of Common Stock underlying the Series B Warrants issued to the Investor (the “Series B
Warrants”) and (B) the Series B Warrants. On June 2, 2020, pursuant to the terms of the Securities Purchase Agreement,
the Company issued the Series A Warrants and the Series B Warrants.
In
addition, pursuant to the terms of the Securities Purchase Agreement, dated as of January 28, 2020 between Timber Sub and several
of the Investors, the Company issued to such purchasers, on May 22, 2020, warrants to purchase 413,751 shares of Common Stock (the
“Bridge Warrants”) which have an exercise price of $2.2362 per share.
Pursuant
to the Waiver Agreements we entered into with the Selling Stockholders on November 19, 2020, (A) the exercise price of the Series A
Warrants was definitively set at $1.16 per share, (B) the number of shares underlying all of the Series A Warrants was definitively
set at 20,178,214 and (C) the number of shares underlying all of the Series B Warrants was definitively set at 22,766,776. As
of March 4, 2021, all Series B Warrants had been exercised in full.
Corporate Information
Our principal offices are
located at 110 Allen Road, Suite 401, Basking Ridge, NJ, and our telephone number is (908) 636-7160. Our website address is www.timberpharma.com.
Our website and the information contained on, or that can be accessed through, our website shall not be deemed to be incorporated by reference
in, and are not considered part of, this prospectus. You should not rely on any such information in making your decision whether to purchase
our Common Stock.
RISK
FACTORS
Before purchasing any of the
securities you should carefully consider the risk factors set forth below and incorporated by reference in this prospectus from our Annual
Report on Form 10-K for the fiscal year ended December 31, 2020 and any subsequent updates described in our Quarterly Reports
on Form 10-Q and Current Reports on Form 8-K, as well as the risks, uncertainties and additional information set forth in our
SEC reports on Forms 10-K, 10-Q and 8-K and in the other documents incorporated by reference in this prospectus. For a description of
these reports and documents, and information about where you can find them, see “Additional Information” and “Incorporation
of Certain Information By Reference.” Additional risks not presently known or that we presently consider to be immaterial could
subsequently materially and adversely affect our financial condition, results of operations, business and prospects.
The market price of
our common stock has been extremely volatile and may continue to be volatile due to numerous circumstances beyond our control.
The market price of our common
stock has fluctuated, and may continue to fluctuate, widely, due to many factors, some of which may be beyond our control. These factors
include, without limitation:
|
|
·
|
comments by securities analysts or other third parties, including blogs, articles, message boards and
social and other media;
|
|
|
·
|
large stockholders exiting their position in our common stock or an increase or decrease in the short
interest in our common stock;
|
|
|
·
|
actual or anticipated fluctuations in our financial and operating results;
|
|
|
·
|
risks and uncertainties associated with the ongoing COVID-19 pandemic;
|
|
|
·
|
the timing and allocations of new product candidates;
|
|
|
·
|
public perception of our product candidates and competitive products; and
|
|
|
·
|
overall general market fluctuations.
|
Stock markets in general and
our stock price in particular have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate
to the operating performance of those companies and our company. Our common stock has recently experienced extreme volatility in price
and trading volume. For example, on March 4, 2021 and March 11, 2021, the closing price of our common stock on the NYSE American
was $1.66 and $3.55, respectively, and daily trading volume on these days was approximately 3.5 million and 143.7 million shares, respectively.
During this period, there were no recent changes in our financial condition or results of operations that were consistent with the change
in our stock price. In particular, a proportion of our common stock has been and may continue to be traded by short sellers which may
put pressure on the supply and demand for our common stock, further influencing volatility in its market price. Additionally, these and
other external factors have caused and may continue to cause the market price and demand for our common stock to fluctuate, which may
limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our
common stock.
A “short squeeze”
due to a sudden increase in demand for shares of our common stock that largely exceeds supply could lead to extreme price volatility in
shares of our common stock.
Investors may purchase shares
of our common stock to hedge existing exposure or to speculate on the price of our common stock. Speculation on the price of our common
stock may involve long and short exposures. To the extent aggregate short exposure exceeds the number of shares of our common stock available
for purchase on the open market, investors with short exposure may have to pay a premium to repurchase shares of our common stock for
delivery to lenders of our common stock. Those repurchases may in turn, dramatically increase the price of our common stock until additional
shares of our common stock are available for trading or borrowing. This is often referred to as a “short squeeze.” A proportion
of our common stock has been and may continue to be traded by short sellers which may increase the likelihood that our common stock will
be the target of a short squeeze. A short squeeze could lead to volatile price movements in shares of our common stock that are unrelated
or disproportionate to our operating performance or prospectus and, once investors purchase the shares of our common stock necessary to
cover their short positions, the price of our common stock may rapidly decline. Investors that purchase shares of our common stock during
a short squeeze may lose a significant portion of their investment.
Enrollment and retention of patients in
clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors
outside our control.
We may encounter delays or difficulties in enrolling,
or be unable to enroll, a sufficient number of patients to complete any of our clinical trials on its current timelines, or at all, and
even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Enrollment in our clinical
trials may be slower than we anticipate, leading to delays in our development timelines. For example, due to the COVID-19 pandemic, we
have experienced delays to the completion of recruitment and in the topline data readout for the TMB-002 program and we are currently
working with our partners and the sites regarding the extent of such delays. We anticipate recruitment to be completed by the end of the
third quarter of 2021. Further, we may face additional difficulties enrolling or maintaining a sufficient number of patients in our clinical
trials due to the existing alternative treatments approved for the treatment of any of our targeted indications, such as topical corticosteroids
or topical steroid-free therapies for atopic dermatitis or psoriasis, as patients may decline to enroll or decide to withdraw from our
clinical trials due to the risk of receiving placebo. Patient enrollment and retention in clinical trials depends on many factors, including
the size of the patient population, the nature of the trial protocol, our ability to recruit clinical trial investigators with the appropriate
competencies and experience, the existing body of safety and efficacy data with respect to the study drug, the number and nature of competing
treatments and ongoing clinical trials of competing drugs for the same indication, the proximity of patients to clinical sites, the eligibility
criteria for the trial and the proportion of patients screened that meets those criteria, our ability to obtain and maintain patient consents,
and our ability to successfully complete prerequisite studies before enrolling certain patient populations.
Furthermore, any negative results or new safety
signals we may report in clinical trials of our product candidates may make it difficult or impossible to recruit and retain patients
in other clinical trials. Similarly, negative results reported by our competitors about their drug candidates may negatively affect patient
recruitment in our clinical trials. Also, marketing authorization of competitors in this same class of drugs may impair our ability to
enroll patients into our clinical trials, delaying or potentially preventing it from completing recruitment of one or more of our trials.
Delays or failures in planned patient enrollment
or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our product
candidates or could render further development impossible. In addition, we expect to rely on CROs and clinical trial sites to ensure proper
and timely conduct of our future clinical trials, and, while we intend to enter into agreements governing their services, we will be limited
in our ability to compel their actual performance.
FORWARD-LOOKING
STATEMENTS
This prospectus, including
the documents that we incorporate by reference, contains forward-looking statements as that term is defined in the federal securities
laws. The events described in forward-looking statements contained in this prospectus, including the documents that we incorporate by
reference, may not occur. Generally, these statements relate to our business plans or strategies, projected or anticipated benefits or
other consequences of our plans or strategies, financing plans, projected or anticipated benefits from acquisitions that we may make,
or projections involving anticipated revenues, earnings or other aspects of our operating results or financial position, and the outcome
of any contingencies. Any such forward-looking statements are based on current expectations, estimates and projections of management.
We intend for these forward-looking statements to be covered by the safe-harbor provisions for forward-looking statements. Words such
as “may,” “expect,” “believe,” “anticipate,” “project,” “plan,”
“intend,” “estimate,” and “continue,” and their opposites and similar expressions are intended to
identify forward-looking statements. We caution you that these statements are not guarantees of future performance or events and are subject
to a number of uncertainties, risks and other influences, many of which are beyond our control that may influence the accuracy of the
statements and the projections upon which the statements are based. Factors that may affect our results include, but are not limited to,
the risks and uncertainties discussed in the “Risk Factors” section on page 3 of this prospectus, in our Annual Report
on Form 10-K for the fiscal year ended December 31, 2020 or in other reports we file with the Securities and Exchange Commission.
Any one or more of these uncertainties,
risks and other influences could materially affect our results of operations and whether forward-looking statements made by us ultimately
prove to be accurate. Our actual results, performance and achievements could differ materially from those expressed or implied in these
forward-looking statements. We undertake no obligation to publicly update or revise any forward-looking statements, whether from new information,
future events or otherwise.
You should rely only on the
information in this prospectus. We have not authorized any other person to provide you with different information. If anyone provides
you with different or inconsistent information, you should not rely upon it.
USE
OF PROCEEDS
Unless we inform you otherwise
in the prospectus supplement relating to a particular offering of securities, we will use the net proceeds from the sale of the securities
offered by this prospectus and the exercise price from the exercise of any convertible securities, if any, for working capital and other
general corporate purposes, which may include funding acquisitions or investments in businesses, products or technologies that are complementary
to our own and reducing indebtedness.
When particular securities
are offered, the prospectus supplement relating to that offering will set forth our intended use of the net proceeds received from the
sale of those securities we sell. Pending the application of the net proceeds for these purposes, we expect to invest the proceeds in
short-term, interest-bearing instruments or other investment-grade securities.
THE
SECURITIES WE MAY OFFER
General
The descriptions of the securities
contained in this prospectus, together with the applicable prospectus supplements, summarize all of the material terms and provisions
of the various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities
the particular terms of the securities offered by that prospectus supplement. If we indicate in the applicable prospectus supplement,
the terms of the securities may differ from the terms we have summarized below. We may also include in the prospectus supplement information
about material United States federal income tax considerations relating to the securities, and the securities exchange, if any, on which
the securities will be listed.
We may sell from time to time,
in one or more offerings:
|
|
●
|
common stock;
|
|
|
|
|
|
|
●
|
preferred stock;
|
|
|
|
|
|
|
●
|
subscription rights to purchase shares of common stock or preferred stock;
|
|
|
|
|
|
|
●
|
warrants to purchase shares of common stock or preferred stock; and
|
|
|
|
|
|
|
●
|
units consisting of any combination of the securities listed above.
|
In this prospectus, we refer
to the common stock, preferred stock, subscription rights, warrants and units collectively as “securities.” The total dollar
amount of all securities that we may sell pursuant to this prospectus will not exceed $100,000,000.
This prospectus may not be
used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
DESCRIPTION
OF CAPITAL STOCK
General
We are authorized to issue
up to 460,000,000 shares of capital stock, including 450,000,000 shares of Common Stock, par value $0.001 per share, and 10,000,000 shares
of undesignated preferred stock, no par value, as to which 2,500 shares have been designated as Series A Preferred Stock. As of April 26,
2021, we had 36,659,685 shares of Common Stock and 1,819 shares of preferred stock issued and outstanding.
The additional shares of our
authorized stock available for issuance may be issued at times and under circumstances so as to have a dilutive effect on earnings per
share and on the equity ownership of the holders of our Common Stock. The ability of our board of directors to issue additional shares
of stock could enhance the board’s ability to negotiate on behalf of the stockholders in a takeover situation but could also be
used by the board to make a change-in-control more difficult, thereby denying stockholders the potential to sell their shares at a premium
and entrenching current management. The following description is a summary of the material provisions of our capital stock. You should
refer to our certificate of incorporation, as amended and bylaws, both of which are on file with the SEC as exhibits to previous SEC filings,
for additional information. The summary below is qualified by provisions of applicable law.
Common Stock
Subject to preferences
that may apply to any shares of preferred stock outstanding at the time, the holders of our Common Stock are entitled to receive
dividends out of funds legally available if our board of directors, in its discretion, determines to issue dividends and then only
at the times and in the amounts that our board of directors may determine. Holders of our Common Stock are entitled to one vote
for each share held on all matters submitted to a vote of stockholders. We have not provided for cumulative voting for any matter in
our certificate of incorporation. Accordingly, pursuant to our certificate of incorporation, holders of a majority of the shares of
our Common Stock will be able to elect all of our directors. Our Common Stock is not entitled to preemptive rights, and is not
subject to conversion, redemption or sinking fund provisions. Upon our liquidation, dissolution or winding-up, the assets legally
available for distribution to our stockholders would be distributable ratably among the holders of our Common Stock and any
participating preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and
the preferential rights of and the payment of liquidation preferences, if any, on any outstanding shares of preferred stock. As of
April 26, 2021, we had outstanding options to purchase an aggregate of 200,237 shares of our Common Stock, with a
weighted-average exercise price of $8.58 per share. As of April 26, 2021, there were Value Appreciation Rights payable in
367,670 shares of Common Stock. As of April 26, 2021, we had outstanding warrants to purchase an aggregate of 17,329,621
shares of our Common Stock, with a weighted-average exercise price of $2.25 per share.
In connection with our September 2016
public offering of warrants, or the 2016 Warrants, to Roth Capital Partners and certain designees of Rodman & Renshaw, a unit
of H.C. Wainwright & Co., LLC, the holders of Common Stock underlying such warrants were entitled to rights with respect to the
registration of such shares under the Securities Act. In January 2017, we issued additional warrants, or the 2017 Warrants, to Rodman &
Renshaw pursuant to a letter agreement. In August 2017, a shelf registration statement with respect to the 2016 Warrants and the
2017 Warrants was filed and declared effective by the SEC. We are required to use commercially reasonable efforts to cause such registration
statement to remain continuously effective for a period that will terminate upon the earlier of (i) the date on which all such Common
Stock has been disposed of pursuant to such registration statement, or (ii) the date on which all such Common Stock is sold in a
transaction that is exempt from registration pursuant to Rule 144 or a transaction in which such selling stockholders’ rights
under the registration rights agreement are not assigned; provided, however, that such requirement shall not apply during any period in
which all the shares of Common Stock then outstanding and held by selling stockholders may be sold under Rule 144 without restriction,
including volume limitations or manner of sale restrictions.
Preferred Stock
We are authorized to issue
up to 10,000,000 shares of preferred stock, as to which 2,500 is designated as Series A Preferred Stock and the remainder of which
are undesignated. As of April 26, 2021, 1,819 shares of our Series A Preferred Stock are issued, outstanding and currently redeemable
at the option of the Series A Preferred Stock holder and no such shares were subject to outstanding options or other rights to purchase
or acquire. Redemption is subject to certain limitations under Delaware law, so that our ability to pay the redemption price to such holder
is or may be limited. Our board of directors has the authority to issue preferred stock in one or more classes or series and to fix the
designations, powers, preferences and rights, and the qualifications, limitations or restrictions thereof, including dividend rights,
conversion right, voting rights, terms of redemption, liquidation preferences and the number of shares constituting any class or series,
without further vote or action by the stockholders. Although we have no present plans to issue any other shares of preferred stock, the
issuance of shares of preferred stock, or the issuance of rights to purchase such shares, could decrease the amount of earnings and assets
available for distribution to the holders of common stock, could adversely affect the rights and powers, including voting rights, of the
common stock, and could have the effect of delaying, deterring or preventing a change of control of us or an unsolicited acquisition proposal.
The preferred stock may provide for an adjustment of the conversion price in the event of an issuance or deemed issuance at a price less
than the applicable conversion price, subject to certain exceptions.
Future Issuance of Preferred Stock
If we offer a specific series
of preferred stock under this prospectus, we will describe the terms of the preferred stock in the prospectus supplement for such offering
and will file a copy of the certificate establishing the terms of the preferred stock with the SEC. To the extent required, this description
will include:
|
|
●
|
the title and stated value;
|
|
|
|
|
|
|
●
|
the number of shares offered, the liquidation preference per share and the purchase price;
|
|
|
|
|
|
|
●
|
the dividend rate(s), period(s) and/or payment date(s), or method(s) of calculation for such dividends;
|
|
|
|
|
|
|
●
|
whether dividends will be cumulative or non-cumulative and, if cumulative, the date from which dividends will accumulate;
|
|
|
●
|
the procedures for any auction and remarketing, if any;
|
|
|
|
|
|
|
●
|
the provisions for a sinking fund, if any;
|
|
|
|
|
|
|
●
|
the provisions for redemption, if applicable;
|
|
|
|
|
|
|
●
|
any listing of the preferred stock on any securities exchange or market;
|
|
|
|
|
|
|
●
|
whether the preferred stock will be convertible into our common stock, and, if applicable, the conversion price (or how it will be calculated) and conversion period;
|
|
|
|
|
|
|
●
|
voting rights, if any, of the preferred stock;
|
|
|
|
|
|
|
●
|
a discussion of any material and/or special U.S. federal income tax considerations applicable to the preferred stock;
|
|
|
|
|
|
|
●
|
the relative ranking and preferences of the preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs; and
|
|
|
|
|
|
|
●
|
any material limitations on issuance of any class or series of preferred stock ranking senior to or on a parity with the series of preferred stock as to dividend rights and rights upon liquidation, dissolution or winding up of our affairs.
|
Transfer Agent and Registrar for Preferred
Stock
The transfer agent and registrar
for any series or class of preferred stock will be set forth in each applicable prospectus supplement.
Anti-takeover Effects of Delaware Law and our
Certificate of Incorporation, as amended
The provisions of Delaware
law, our certificate of incorporation and our bylaws described below may have the effect of delaying, deferring or discouraging another
party from acquiring control of us.
Section 203 of the Delaware General Corporation
Law
Our Certificate of Incorporation,
as amended, and Bylaws, as amended, contain provisions that could have the effect of discouraging potential acquisition proposals or tender
offers or delaying or preventing a change of control. These provisions are as follows:
|
|
●
|
they provide that special meetings of stockholders may be called only by a majority of our board of directors or an officer instructed by the directors to call a special meeting, thus prohibiting a stockholder from calling a special meeting;
|
|
|
|
|
|
|
●
|
they authorize our board of directors to fill vacant directorships, including newly created seats;
|
|
|
|
|
|
|
●
|
they can be amended or repealed by unanimous written consent of our board of directors;
|
|
|
●
|
they do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in our board of directors; and
|
|
|
|
|
|
|
●
|
they allow us to issue, without stockholder approval, up to 10,000,000 shares of preferred stock that could adversely affect the rights and powers of the holders of our Common Stock.
|
We are subject to the provisions
of Section 203 of the Delaware General Corporation Law, an anti-takeover law. Subject to certain exceptions, the statute prohibits
a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder”
for a period of three years after the date of the transaction in which the person became an interested stockholder unless:
|
|
●
|
prior to such date, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
|
|
|
|
|
|
|
●
|
upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least eighty-five percent 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned (1) by persons who are directors and also officers and (2) by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
|
|
|
|
●
|
on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least sixty-six and two-thirds percent 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
|
Generally, for purposes of
Section 203, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial
benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates,
owns or, within three (3) years prior to the determination of interested stockholder status, owned fifteen percent (15%) or more
of a corporation’s outstanding voting securities.
Potential Effects of Authorized but Unissued
Stock
We have shares of common stock
and preferred stock available for future issuance without stockholder approval. We may utilize these additional shares for a variety of
corporate purposes, including future public offerings to raise additional capital, to facilitate corporate acquisitions or payment as
a dividend on the capital stock.
The existence of unissued
and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management
or to issue preferred stock with terms that could render more difficult or discourage a third-party attempt to obtain control of us by
means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity of our management. In addition, the board
of directors has the discretion to determine designations, rights, preferences, privileges and restrictions, including voting rights,
dividend rights, conversion rights, redemption privileges and liquidation preferences of each series of preferred stock, all to the fullest
extent permissible under the Delaware General Corporation Law and subject to any limitations set forth in our certificate of incorporation.
The purpose of authorizing the board of directors to issue preferred stock and to determine the rights and preferences applicable to such
preferred stock is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of preferred stock, while
providing desirable flexibility in connection with possible financings, acquisitions and other corporate purposes, could have the effect
of making it more difficult for a third-party to acquire, or could discourage a third-party from acquiring, a majority of our outstanding
voting stock.
DESCRIPTION
OF STOCK WARRANTS
We summarize below some of
the provisions that will apply to the warrants unless the applicable prospectus supplement provides otherwise. This summary may not contain
all information that is important to you. The complete terms of the warrants will be contained in the applicable warrant certificate and
warrant agreement. These documents have been or will be included or incorporated by reference as exhibits to the registration statement
of which this prospectus is a part. You should read the warrant certificate and the warrant agreement. You should also read the prospectus
supplement, which will contain additional information and which may update or change some of the information below.
General
We may issue, together with
common or preferred stock as units or separately, warrants for the purchase of shares of our common or preferred stock. The terms of each
warrant will be discussed in the applicable prospectus supplement relating to the particular series of warrants. The form(s) of certificate
representing the warrants and/or the warrant agreement will be, in each case, filed with the SEC as an exhibit to a document incorporated
by reference in the registration statement of which this prospectus is a part on or prior to the date of any prospectus supplement relating
to an offering of the particular warrant. The following summary of material provisions of the warrants and the warrant agreements are
subject to, and qualified in their entirety by reference to, all the provisions of the warrant agreement and warrant certificate applicable
to a particular series of warrants.
The prospectus supplement
relating to any series of warrants that are offered by this prospectus will describe, among other things, the following terms to the extent
they are applicable to that series of warrants:
|
|
●
|
the procedures and conditions relating to the exercise of the warrants;
|
|
|
|
|
|
|
●
|
the number of shares of our common or preferred stock, if any, issued with the warrants;
|
|
|
|
|
|
|
●
|
the date, if any, on and after which the warrants and any related shares of our common or preferred stock will be separately transferable;
|
|
|
|
|
|
|
●
|
the offering price of the warrants, if any;
|
|
|
|
|
|
|
●
|
the number of shares of our common or preferred stock which may be purchased upon exercise of the warrants and the price or prices at which the shares may be purchased upon exercise;
|
|
|
|
|
|
|
●
|
the date on which the right to exercise the warrants will begin and the date on which the right will expire;
|
|
|
|
|
|
|
●
|
a discussion of the material United States federal income tax considerations applicable to the exercise of the warrants;
|
|
|
|
|
|
|
●
|
anti-dilution provisions of the warrants, if any;
|
|
|
|
|
|
|
●
|
call provisions of the warrants, if any; and
|
|
|
|
|
|
|
●
|
any other material terms of the warrants.
|
Each warrant may entitle the
holder to purchase for cash, or, in limited circumstances, by effecting a cashless exercise for, the number of shares of our common or
preferred stock at the exercise price that is described in the applicable prospectus supplement. Warrants will be exercisable during the
period of time described in the applicable prospectus supplement. After that period, unexercised warrants will be void. Warrants may be
exercised in the manner described in the applicable prospectus supplement.
A holder of a warrant will
not have any of the rights of a holder of our common or preferred stock before the stock is purchased upon exercise of the warrant. Therefore,
before a warrant is exercised, the holder of the warrant will not be entitled to receive any dividend payments or exercise any voting
or other rights associated with shares of our common or preferred stock which may be purchased when the warrant is exercised.
Transfer Agent and Registrar
The transfer agent and registrar,
if any, for any warrants will be set forth in the applicable prospectus supplement.
DESCRIPTION OF SUBSCRIPTION RIGHTS
We may issue subscription
rights to purchase our common stock or preferred stock. These subscription rights may be offered independently or together with any other
security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection
with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant
to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The prospectus supplement
relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating to the offering,
including some or all of the following:
|
|
●
|
the price, if any, for the subscription rights;
|
|
|
●
|
the exercise price payable for our common stock or preferred stock upon the exercise of the subscription rights;
|
|
|
|
|
|
|
●
|
the number of subscription rights to be issued to each stockholder;
|
|
|
|
|
|
|
●
|
the number and terms of our common stock or preferred stock which may be purchased per each subscription right;
|
|
|
|
|
|
|
●
|
the extent to which the subscription rights are transferable;
|
|
|
|
|
|
|
●
|
any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights;
|
|
|
|
|
|
|
●
|
the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire;
|
|
|
|
|
|
|
●
|
the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities or an over-allotment privilege to the extent the securities are fully subscribed; and
|
|
|
|
|
|
|
●
|
if applicable, the material terms of any standby underwriting or purchase arrangement which may be entered into by us in connection with the offering of subscription rights.
|
DESCRIPTION
OF UNITS
We may issue units comprised
of one or more of the other securities described in this prospectus in any combination. Each unit will be issued so that the holder of
the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of
a holder of each included security (but, to the extent convertible securities are included in the units, the holder of the units will
be deemed the holder of the convertible securities and not the holder of the underlying securities). The unit agreement under which a
unit is issued, if any, may provide that the securities included in the unit may not be held or transferred separately, at any time or
at any time before a specified date. The applicable prospectus supplement may describe:
|
|
●
|
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
|
|
|
|
●
|
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units;
|
|
|
●
|
the terms of the unit agreement governing the units;
|
|
|
|
|
|
|
●
|
United States federal income tax considerations relevant to the units; and
|
|
|
|
|
|
|
●
|
whether the units will be issued in fully registered global form.
|
This summary of certain general
terms of units and any summary description of units in the applicable prospectus supplement do not purport to be complete and are qualified
in their entirety by reference to all provisions of the applicable unit agreement and, if applicable, collateral arrangements and depositary
arrangements relating to such units. The forms of the unit agreements and other documents relating to a particular issue of units will
be filed with the SEC each time we issue units, and you should read those documents for provisions that may be important to you.
FORMS OF SECURITIES
Each warrant, subscription
right and unit, will be represented either by a certificate issued in definitive form to a particular investor or by one or more global
securities representing the entire issuance of securities. Certificated securities in definitive form and global securities will be issued
in registered form. Definitive securities name you or your nominee as the owner of the security, and in order to transfer or exchange
these securities or to receive payments other than interest or other interim payments, you or your nominee must physically deliver the
securities to the trustee, registrar, paying agent or other agent, as applicable. Global securities name a depositary or its nominee as
the owner of the warrants represented by these global securities. The depositary maintains a computerized system that will reflect each
investor’s beneficial ownership of the securities through an account maintained by the investor with its broker/dealer, bank, trust
company or other representative, as we explain more fully below.
Global Securities
Registered
Global Securities. We may issue the registered warrants, subscription rights and units, in the form of one or more fully registered
global securities that will be deposited with a depositary or its nominee identified in the applicable prospectus supplement and registered
in the name of that depositary or nominee. In those cases, one or more registered global securities will be issued in a denomination or
aggregate denominations equal to the portion of the aggregate principal or face amount of the securities to be represented by registered
global securities. Unless and until it is exchanged in whole for securities in definitive registered form, a registered global security
may not be transferred except as a whole by and among the depositary for the registered global security, the nominees of the depositary
or any successors of the depositary or those nominees.
If not described below, any
specific terms of the depositary arrangement with respect to any securities to be represented by a registered global security will be
described in the prospectus supplement relating to those securities. We anticipate that the following provisions will apply to all depositary
arrangements.
Ownership of beneficial interests
in a registered global security will be limited to persons, called participants, that have accounts with the depositary or persons that
may hold interests through participants. Upon the issuance of a registered global security, the depositary will credit, on its book-entry
registration and transfer system, the participants’ accounts with the respective principal or face amounts of the securities beneficially
owned by the participants. Any dealers, underwriters or agents participating in the distribution of the securities will designate the
accounts to be credited. Ownership of beneficial interests in a registered global security will be shown on, and the transfer of ownership
interests will be effected only through, records maintained by the depositary, with respect to interests of participants, and on the records
of participants, with respect to interests of persons holding through participants. The laws of some states may require that some purchasers
of securities take physical delivery of these securities in definitive form. These laws may impair your ability to own, transfer or pledge
beneficial interests in registered global securities.
So long as the depositary,
or its nominee, is the registered owner of a registered global security, that depositary or its nominee, as the case may be, will be considered
the sole owner or holder of the securities represented by the registered global security for all purposes under the applicable indenture
or warrant agreement. Except as described below, owners of beneficial interests in a registered global security will not be entitled to
have the securities represented by the registered global security registered in their names, will not receive or be entitled to receive
physical delivery of the securities in definitive form and will not be considered the owners or holders of the securities under the applicable
indenture or warrant agreement. Accordingly, each person owning a beneficial interest in a registered global security must rely on the
procedures of the depositary for that registered global security and, if that person is not a participant, on the procedures of the participant
through which the person owns its interest, to exercise any rights of a holder under the applicable indenture or warrant agreement. We
understand that under existing industry practices, if we request any action of holders or if an owner of a beneficial interest in a registered
global security desires to give or take any action that a holder is entitled to give or take under the applicable indenture or warrant
agreement, the depositary for the registered global security would authorize the participants holding the relevant beneficial interests
to give or take that action, and the participants would authorize beneficial owners owning through them to give or take that action or
would otherwise act upon the instructions of beneficial owners holding through them.
Any payments to holders with
respect to warrants represented by a registered global security registered in the name of a depositary or its nominee will be made to
the depositary or its nominee, as the case may be, as the registered owner of the registered global security. None of the Company, the
trustees, the warrant agents or any other agent of the Company, the trustees or the warrant agents will have any responsibility or liability
for any aspect of the records relating to payments made on account of beneficial ownership interests in the registered global security
or for maintaining, supervising or reviewing any records relating to those beneficial ownership interests.
We expect that the depositary
for any of the securities represented by a registered global security, upon receipt of any payment of principal, premium, interest or
other distribution of underlying securities or other property to holders on that registered global security, will immediately credit participants’
accounts in amounts proportionate to their respective beneficial interests in that registered global security as shown on the records
of the depositary. We also expect that payments by participants to owners of beneficial interests in a registered global security held
through participants will be governed by standing customer instructions and customary practices, as is now the case with the securities
held for the accounts of customers in bearer form or registered in “street name,” and will be the responsibility of those
participants.
If the depositary for any
of these securities represented by a registered global security is at any time unwilling or unable to continue as depositary or ceases
to be a clearing agency registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and a successor depositary
registered as a clearing agency under the Exchange Act is not appointed by us within 90 days, we will issue securities in definitive form
in exchange for the registered global security that had been held by the depositary. Any securities issued in definitive form in exchange
for a registered global security will be registered in the name or names that the depositary gives to the relevant trustee or warrant
agent or other relevant agent of ours or theirs. It is expected that the depositary’s instructions will be based upon directions
received by the depositary from participants with respect to ownership of beneficial interests in the registered global security that
had been held by the depositary.
PLAN
OF DISTRIBUTION
Initial Offering and Sale of Securities
Unless otherwise set forth
in a prospectus supplement accompanying this prospectus, we may sell the securities being offered hereby, from time to time, by one or
more of the following methods:
|
|
●
|
to or through underwriting syndicates represented by managing underwriters;
|
|
|
|
|
|
|
●
|
through one or more underwriters without a syndicate for them to offer and sell to the public;
|
|
|
|
|
|
|
●
|
through dealers or agents; and
|
|
|
|
|
|
|
●
|
to investors directly in negotiated sales or in competitively bid transactions.
|
Offerings of securities covered
by this prospectus also may be made into an existing trading market for those securities in transactions at other than a fixed price,
either:
|
|
●
|
on or through the facilities of the NYSE American or any other securities exchange or quotation or trading service on which those securities may be listed, quoted, or traded at the time of sale; and/or
|
|
|
|
|
|
|
●
|
to or through a market maker other than on the securities exchanges or quotation or trading services set forth above.
|
Those at-the-market offerings,
if any, will be conducted by underwriters acting as principal or agent of the Company, who may also be third-party sellers of securities
as described above. The prospectus supplement with respect to the offered securities will set forth the terms of the offering of the offered
securities, including:
|
|
●
|
the name or names of any underwriters, dealers or agents;
|
|
|
|
|
|
|
●
|
the purchase price of the offered securities and the proceeds to us from such sale;
|
|
|
●
|
any underwriting discounts and commissions or agency fees and other items constituting underwriters’ or agents’ compensation;
|
|
|
|
|
|
|
●
|
any initial public offering price and any discounts or concessions allowed or reallowed or paid to dealers;
|
|
|
|
|
|
|
●
|
any securities exchange on which such offered securities may be listed; and
|
|
|
|
|
|
|
●
|
any underwriter, agent or dealer involved in the offer and sale of any series of the securities.
|
The distribution of the securities
may be effected from time to time in one or more transactions:
|
|
●
|
at fixed prices, which may be changed;
|
|
|
|
|
|
|
●
|
at market prices prevailing at the time of the sale;
|
|
|
|
|
|
|
●
|
at varying prices determined at the time of sale; or
|
|
|
|
|
|
|
●
|
at negotiated prices.
|
Each prospectus supplement
will set forth the manner and terms of an offering of securities including:
|
|
●
|
whether that offering is being made to underwriters, through agents or directly to the public;
|
|
|
|
|
|
|
●
|
the rules and procedures for any auction or bidding process, if used;
|
|
|
|
|
|
|
●
|
the securities’ purchase price or initial public offering price; and
|
|
|
|
|
|
|
●
|
the proceeds we anticipate from the sale of the securities, if any.
|
In addition, we may enter
into derivative or hedging transactions with third parties, or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. The applicable prospectus supplement may indicate, in connection with such a transaction, that the third parties
may sell securities covered by and pursuant to this prospectus and an applicable prospectus supplement. If so, the third party may use
securities pledged by us or borrowed from us or others to settle such sales and may use securities received from us to close out any related
short positions. We may also loan or pledge securities covered by this prospectus and an applicable prospectus supplement to third parties,
who may sell the loaned securities or, in an event of default in the case of a pledge, sell the pledged securities pursuant to this prospectus
and the applicable prospectus supplement.
Sales Through Underwriters
If underwriters are used in
the sale of some or all of the securities covered by this prospectus, the underwriters will acquire the securities for their own account.
The underwriters may resell the securities, either directly to the public or to securities dealers, at various times in one or more transactions,
including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. The obligations
of the underwriters to purchase the securities will be subject to certain conditions. Unless indicated otherwise in a prospectus supplement,
the underwriters will be obligated to purchase all the securities of the series offered if any of the securities are purchased.
Any initial public offering
price and any concessions allowed or reallowed to dealers may be changed intermittently.
Sales Through Agents
Unless otherwise indicated
in the applicable prospectus supplement, when securities are sold through an agent, the designated agent will agree, for the period of
its appointment as agent, to use specified efforts to sell the securities for our account and will receive commissions from us as will
be set forth in the applicable prospectus supplement.
Securities bought in accordance
with a redemption or repayment under their terms also may be offered and sold, if so indicated in the applicable prospectus supplement,
in connection with a remarketing by one or more firms acting as principals for their own accounts or as agents for us. Any remarketing
firm will be identified and the terms of its agreement, if any, with us and its compensation will be described in the prospectus supplement.
Remarketing firms may be deemed to be underwriters in connection with the securities remarketed by them.
If so indicated in the applicable
prospectus supplement, we may authorize agents, underwriters or dealers to solicit offers by certain specified institutions to purchase
securities at a price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery
on a future date specified in the prospectus supplement. These contracts will be subject only to those conditions set forth in the applicable
prospectus supplement, and the prospectus supplement will set forth the commissions payable for solicitation of these contracts.
Direct Sales
We may also sell offered securities
directly to institutional investors or others. In this case, no underwriters or agents would be involved. The terms of such sales will
be described in the applicable prospectus supplement.
General Information
Broker-dealers, agents or
underwriters may receive compensation in the form of discounts, concessions or commissions from us and/or the purchasers of securities
for whom such broker-dealers, agents or underwriters may act as agents or to whom they sell as principal, or both. This compensation to
a particular broker-dealer might be in excess of customary commissions.
Underwriters, dealers and
agents that participate in any distribution of the offered securities may be deemed “underwriters” within the meaning of the
Securities Act of 1933, as amended, or the Securities Act, so any discounts or commissions they receive in connection with the distribution
may be deemed to be underwriting compensation. Those underwriters and agents may be entitled, under their agreements with us, to indemnification
by us against certain civil liabilities, including liabilities under the Securities Act, or to contribution by us to payments that they
may be required to make in respect of those civil liabilities. Certain of those underwriters or agents may be customers of, engage in
transactions with, or perform services for, us or our affiliates in the ordinary course of business. We will identify any underwriters
or agents, and describe their compensation, in a prospectus supplement. Any institutional investors or others that purchase offered securities
directly, and then resell the securities, may be deemed to be underwriters, and any discounts or commissions received by them from us
and any profit on the resale of the securities by them may be deemed to be underwriting discounts and commissions under the Securities
Act.
We will file a supplement
to this prospectus, if required, pursuant to Rule 424(b) under the Securities Act, if we enter into any material arrangement
with a broker, dealer, agent or underwriter for the sale of securities through a block trade, special offering, exchange distribution
or secondary distribution or a purchase by a broker or dealer. Such prospectus supplement will disclose:
|
|
●
|
the name of any participating broker, dealer, agent or underwriter;
|
|
|
|
|
|
|
●
|
the number and type of securities involved;
|
|
|
|
|
|
|
●
|
the price at which such securities were sold;
|
|
|
|
|
|
|
●
|
any securities exchanges on which such securities may be listed;
|
|
|
|
|
|
|
●
|
the commissions paid or discounts or concessions allowed to any such broker, dealer, agent or underwriter, where applicable; and
|
|
|
|
|
|
|
●
|
other facts material to the transaction.
|
In order to facilitate the
offering of certain securities under this prospectus or an applicable prospectus supplement, certain persons participating in the offering
of those securities may engage in transactions that stabilize, maintain or otherwise affect the price of those securities during and after
the offering of those securities. Specifically, if the applicable prospectus supplement permits, the underwriters of those securities
may over-allot or otherwise create a short position in those securities for their own account by selling more of those securities than
have been sold to them by us and may elect to cover any such short position by purchasing those securities in the open market.
In addition, the underwriters
may stabilize or maintain the price of those securities by bidding for or purchasing those securities in the open market and may impose
penalty bids, under which selling concessions allowed to syndicate members or other broker-dealers participating in the offering are reclaimed
if securities previously distributed in the offering are repurchased in connection with stabilization transactions or otherwise. The effect
of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail
in the open market. The imposition of a penalty bid may also affect the price of securities to the extent that it discourages resales
of the securities. No representation is made as to the magnitude or effect of any such stabilization or other transactions. Such transactions,
if commenced, may be discontinued at any time.
In order to comply with the
securities laws of certain states, if applicable, the securities must be sold in such jurisdictions only through registered or licensed
brokers or dealers. In addition, in certain states the securities may not be sold unless they have been registered or qualified for sale
in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Rule 15c6-1 under the
Exchange Act generally requires that trades in the secondary market settle in two business days, unless the parties to any such trade
expressly agree otherwise. Your prospectus supplement may provide that the original issue date for your securities may be more than two
scheduled business days after the trade date for your securities. Accordingly, in such a case, if you wish to trade securities on any
date prior to the second business day before the original issue date for your securities, you will be required, by virtue of the fact
that your securities initially are expected to settle in more than two scheduled business days after the trade date for your securities,
to make alternative settlement arrangements to prevent a failed settlement.
This prospectus, any applicable
prospectus supplement and any applicable pricing supplement in electronic format may be made available on the Internet sites of, or through
other online services maintained by, us and/or one or more of the agents and/or dealers participating in an offering of securities, or
by their affiliates. In those cases, prospective investors may be able to view offering terms online and, depending upon the particular
agent or dealer, prospective investors may be allowed to place orders online.
Other than this prospectus,
any applicable prospectus supplement and any applicable pricing supplement in electronic format, the information on our website or the
website of any agent or dealer, and any information contained in any other website maintained by any agent or dealer:
|
|
●
|
is not part of this prospectus, any applicable prospectus supplement or any applicable pricing supplement or the registration statement of which they form a part;
|
|
|
|
|
|
|
●
|
has not been approved or endorsed by us or by any agent or dealer in its capacity as an agent or dealer, except, in each case, with respect to the respective website maintained by such entity; and
|
|
|
|
|
|
|
●
|
should not be relied upon by investors.
|
There can be no assurance
that we will sell all or any of the securities offered by this prospectus.
This prospectus may also be
used in connection with any issuance of common stock or preferred stock upon exercise of a warrant if such issuance is not exempt from
the registration requirements of the Securities Act.
In addition, we may issue
the securities as a dividend or distribution or in a subscription rights offering to our existing securityholders. In some cases, we or
dealers acting with us or on our behalf may also purchase securities and reoffer them to the public by one or more of the methods described
above. This prospectus may be used in connection with any offering of our securities through any of these methods or other methods described
in the applicable prospectus supplement.
LEGAL
MATTERS
Unless otherwise indicated
in the applicable prospectus supplement, the validity of the securities offered hereby will be passed upon for us by Lowenstein Sandler
LLP, Roseland, New Jersey. If the validity of the securities offered hereby in connection with offerings made pursuant to this prospectus
are passed upon by counsel for the underwriters, dealers or agents, if any, such counsel will be named in the prospectus supplement relating
to such offering.
EXPERTS
The consolidated financial
statements of Timber Pharmaceuticals, Inc. as of December 31, 2020 and 2019, and for the year ended December 31, 2020 and
the period from February 26, 2019 (Inception) through December 31, 2019, have been incorporated by reference herein and in the
registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, incorporated by reference
herein, and upon the authority of said firm as experts in accounting and auditing. The audit report covering the December 31, 2020
consolidated financial statements contains an explanatory paragraph that states that the Company's recurring losses from operations raise
substantial doubt about the entity's ability to continue as a going concern. The consolidated financial statements do not include any
adjustments that might result from the outcome of that uncertainty.
DISCLOSURE
OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT
LIABILITIES
Section 145 of the Delaware
General Corporation Law provides that we may indemnify any person who was or is a party or is threatened to be made a party to any threatened,
pending or completed action, suit or proceeding, whether civil, criminal or investigative (other than an action by us or in our right)
by reason of the fact that he is or was our director, officer, employee or agent, or is or was serving at our request as a director, officer,
employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’
fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action,
suit or proceeding if he acted in good faith and in a manner he or she reasonably believed to be in or not opposed to our best interests,
and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful. Section 145
further provides that we similarly may indemnify any such person serving in any such capacity who was or is a party or is threatened to
be made a party to any threatened, pending or completed action or suit by is or in our right to procure judgment in our favor, against
expenses actually and reasonably incurred in connection with the defense or settlement of such action or suit if he or she acted in good
faith and in a manner he reasonably believed to be in or not opposed to our best interests and except that no indemnification shall be
made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to us unless and only to the
extent that the Delaware Court of Chancery or such other court in which such action or suit was brought shall determine upon application
that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled
to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Our certificate of incorporation,
as amended, limits the liability of our directors to the fullest extent permitted by Delaware law. In addition, we have entered into indemnification
agreements with certain of our directors and officers whereby we have agreed to indemnify those directors and officers to the fullest
extent permitted by law, including indemnification against expenses and liabilities incurred in legal proceedings to which the director
or officer was, or is threatened to be made, a party by reason of the fact that such director or officer is or was a director, officer,
employee or agent of the Company, provided that such director or officer acted in good faith and in a manner that the director or officer
reasonably believed to be in, or not opposed to, the best interests of the Company.
We have director and officer
liability insurance to cover liabilities our directors and officers may incur in connection with their services to us, including matters
arising under the Securities Act. Our certificate of incorporation and bylaws also provide that we will indemnify our directors and officers
who, by reason of the fact that he or she is one of our officers or directors of our company, is involved in any action, suit or proceeding,
whether civil, criminal, administrative or investigative, related to their board role with the company.
Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing
provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other
than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the
successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the
securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit
to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities
Act and will be governed by the final adjudication of such issue.
ADDITIONAL
INFORMATION
This prospectus is part of
a Registration Statement on Form S-3 that we have filed with the SEC relating to the shares of our securities being offered hereby.
This prospectus does not contain all of the information in the Registration Statement and its exhibits. The Registration Statement, its
exhibits and the documents incorporated by reference in this prospectus and their exhibits, all contain information that is material to
the offering of the securities hereby. Whenever a reference is made in this prospectus to any of our contracts or other documents, the
reference may not be complete. You should refer to the exhibits that are a part of the Registration Statement in order to review a copy
of the contract or documents. The Registration Statement and the exhibits are available at the SEC’s Public Reference Room or through
its website.
We file annual, quarterly
and current reports, proxy statements and other information with the SEC. The SEC maintains an Internet site at http://www.sec.gov that
contains reports, proxy and information statements, and other information regarding issuers, such as us, that file electronically with
the SEC. Additionally, you may access our filings with the SEC through our website at www.timberpharma.com. We have included our website
address as an inactive textual reference only and our website and the information contained on, or that can be accessed through, our website
will not be deemed to be incorporated by reference in, and are not considered part of, this prospectus.
We will provide you without
charge, upon your oral or written request, with an electronic or paper copy of any or all reports, proxy statements and other documents
we file with the SEC, as well as any or all of the documents incorporated by reference in this prospectus (other than exhibits to such
documents unless such exhibits are specifically incorporated by reference into such documents). Requests for such copies should be directed
to:
Timber Pharmaceuticals, Inc.
Attn: John Koconis, Chief Executive Officer and Chairman of the Board
110 Allen Road, Suite 401
Basking Ridge, NJ 07920
Telephone number: (908) 636-7160
You should rely only on the
information in this prospectus and the additional information described above and under the heading “Incorporation of Certain Information
by Reference” below. We have not authorized any other person to provide you with different information. If anyone provides you with
different or inconsistent information, you should not rely upon it. We are not making an offer to sell these securities in any jurisdiction
where such offer or sale is not permitted. You should assume that the information in this prospectus was accurate on the date of the front
cover of this prospectus only. Our business, financial condition, results of operations and prospects may have changed since that date.
INCORPORATION
OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate
by reference” information that we file with it into this prospectus, which means that we can disclose important information to you
by referring you to those documents. The information incorporated by reference is an important part of this prospectus. The information
incorporated by reference is considered to be a part of this prospectus, and information that we file later with the SEC will automatically
update and supersede information contained in this prospectus and any accompanying prospectus supplement.
We incorporate by reference
the documents listed below that we have previously filed with the SEC:
|
|
●
|
our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 23, 2021;
|
|
|
|
|
|
|
●
|
our Current Reports on Form 8-K dated January 12, 2021, January 19, 2021, February 4, 2021 (other than any portions thereof deemed furnished and not filed), March 16, 2021, March 17, 2021, April 16, 2021,
April 23, 2021 and April 28, 2021;
|
|
|
|
|
|
|
●
|
our definitive Proxy Statement on Schedule 14A, filed with the SEC on April 23, 2021 (solely with respect to those portions incorporated by reference
into our Annual Report on Form 10-K for the fiscal year ended December 31, 2020), as supplemented by the Proxy Supplement on Schedule 14A, filed with the SEC on April 29, 2021; and
|
|
|
|
|
|
|
●
|
the description of the Company’s Common Stock contained in the Company’s Registration Statement on Form 8-A filed with the SEC on June 1, 2015 under Section 12 of the Exchange Act, including any amendment or report filed for the purpose of updating such description.
|
All reports and other documents
that we file with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration
statement and prior to effectiveness of the registration statement, and after the date of this prospectus but before the termination of
the offering of the securities hereunder will also be considered to be incorporated by reference into this prospectus from the date of
the filing of these reports and documents, and will supersede the information herein; provided, however, that all reports, exhibits and
other information that we “furnish” to the SEC will not be considered incorporated by reference into this prospectus. We undertake
to provide without charge to each person (including any beneficial owner) who receives a copy of this prospectus, upon written or oral
request, a copy of all of the preceding documents that are incorporated by reference (other than exhibits, unless the exhibits are specifically
incorporated by reference into these documents). You may request a copy of these materials in the manner set forth under the heading “Additional
Information,” above.
TIMBER PHARMACEUTICALS, INC.
21,325,000 Shares of Common Stock
Pre-funded Warrants to Purchase up to 2,112,500
Shares of Common Stock
Warrants to Purchase up to 23,437,500 Shares
of Common Stock
PROSPECTUS SUPPLEMENT
H.C. Wainwright & Co.
November 2, 2021
Timber Pharmaceuticals (AMEX:TMBR)
Historical Stock Chart
From Aug 2024 to Sep 2024
Timber Pharmaceuticals (AMEX:TMBR)
Historical Stock Chart
From Sep 2023 to Sep 2024